Triazine, pyrimidine and pyridine analogs and their use as therapeutic agents and diagnostic probes
A pyridine, triazine technology, applied in the field of detection and/or modulation of kinase activity compounds, regulation, inhibition, can solve the problems of enhanced migration, harmful cells, uncontrolled proliferation, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0276] Preparation of compounds of the present invention
[0277] The compounds of the present invention may be synthesized by synthetic routes including methods analogous to those well known in the chemical arts, in particular according to the description contained herein. Starting materials are generally obtained from commercial sources, such as Aldrich Chemicals, or are readily prepared using methods well known to those skilled in the art (e.g., by methods generally described in: Louis F. Fieser and Mary Fieser, Reagents for Organic Synthesis , v.1-19, Wiley, N.Y. (1967-1999 editions), or Beilsteins Handbuch der organischen Chemie, 4, Aufl.ed. Springer-Verlag, Berlin, including its supplements (also available via the Beilstein online database)).
[0278] In certain embodiments, the compounds of the present invention can be readily prepared using well-known methods for the preparation of triazines and other heterocyclic rings, which are described in: Comprehensive Heterocycl...
specific Embodiment approach
[0624] The present invention will now be described in more detail with reference to specific embodiments and examples of the invention, which are intended to be illustrative rather than restrictive.
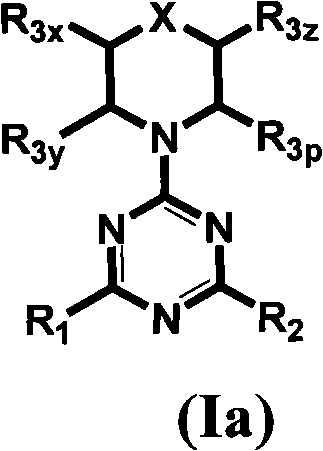
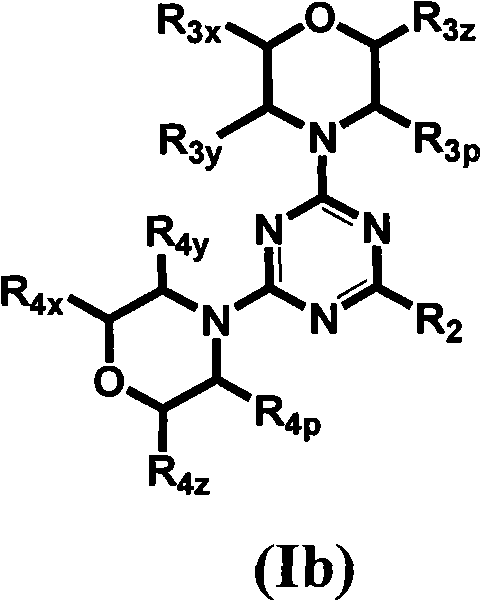
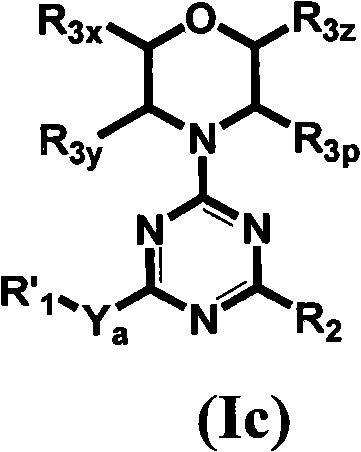
[0625] Table 1 gives the structures and corresponding IUPAC names of exemplary compound numbers 1-259 of formula (Ia), (Ib) or (Id) (using ChemDraw Ultra version 11.0.1 and its lower and higher software versions , CambridgeSoft Corp., Cambridge MA).
[0626] Table 1
[0627]
[0628]
[0629]
[0630]
[0631]
[0632]
[0633]
[0634]
[0635]
[0636]
[0637]
[0638]
[0639]
[0640]
[0641]
[0642]
[0643]
[0644]
[0645]
[0646]
[0647]
[0648]
[0649]
[0650]
[0651]
[0652]
[0653]
[0654]
[0655]
[0656]
[0657]
[0658]
[0659]
[0660]
[0661]
[0662]
[0663]
[0664]
[0665]
[0666]
[0667]
[0668]
[0669]
[06...
Embodiment P1
[0739]
[0740] 4,4'-(6-Chloro-1,3,5-triazine-2,4-diyl)dimorpholine:
[0741] Cyanuric chloride (1.00g, 5.42mmol, 1.0eq) was dissolved in DMF (5ml), and morpholine (2.11ml, 24.4mmol, 4.5eq) was slowly added to the reaction mixture at 0°C, and stirred at this temperature for 20 minutes, poured into water, filtered the colorless precipitate, washed with hexane and diethyl ether, and dried to afford the title compound as a colorless solid (860 mg, 56%).
[0742] analyze data:
[0743] 1 H-NMR (400MHz, CDCl 3 ): δ3.78-3.69 (16H, m).
[0744] 13 C-NMR (100MHz, CDCl 3 ): δ170.10, 164.88, 67.28, 66.98, 44.23.
[0745] ESI-MS (70eV, m / z): C 11 h 16 ClN 5 o 2 [M+H] + Calculated value: 286, measured 360.
[0746] X-ray analysis: The structure of 4,4'-(6-chloro-1,3,5-triazine-2,4-diyl)dimorpholine was confirmed by x-ray analysis.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com