Patents
Literature
41 results about "Lipid kinases" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
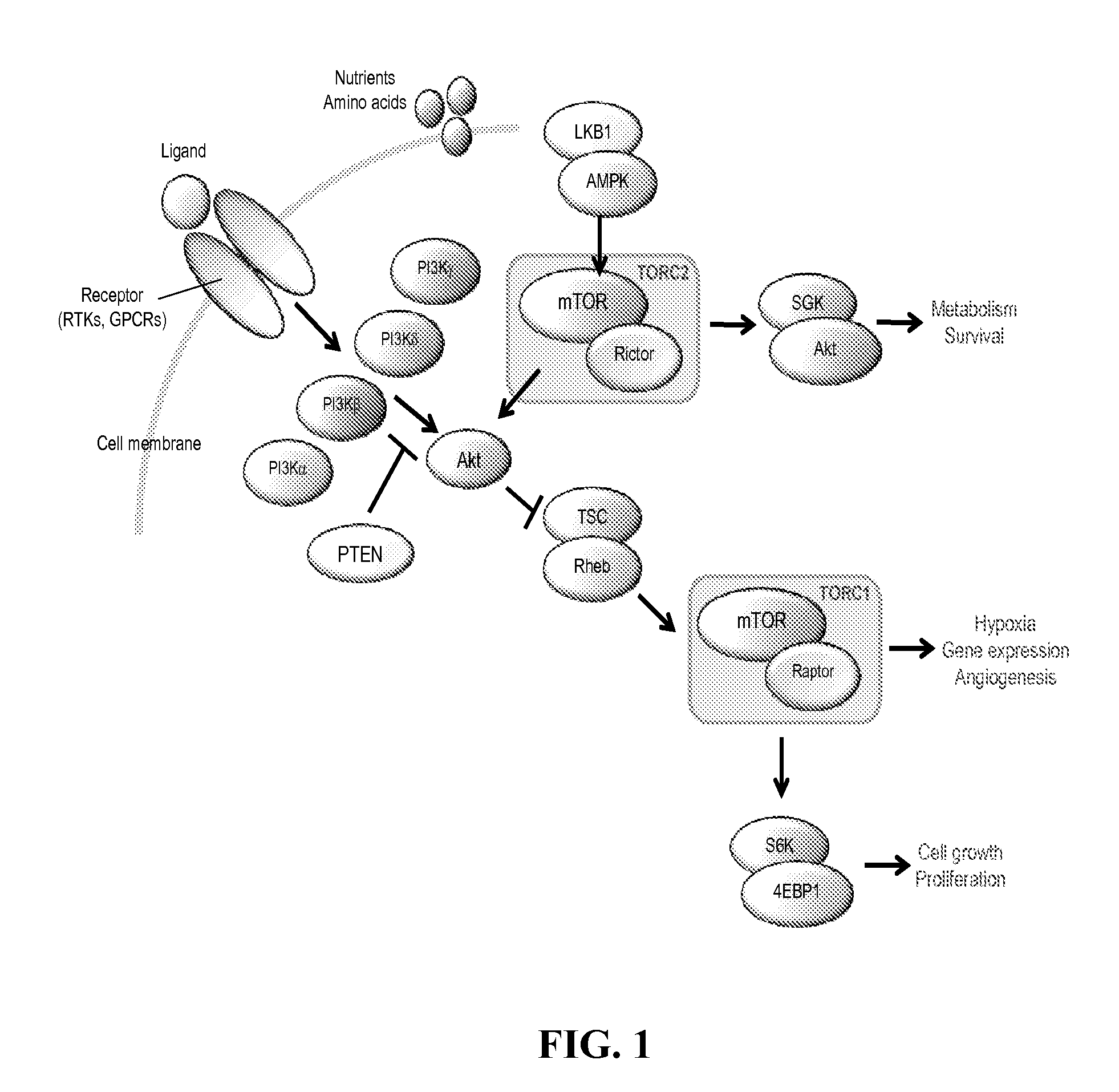
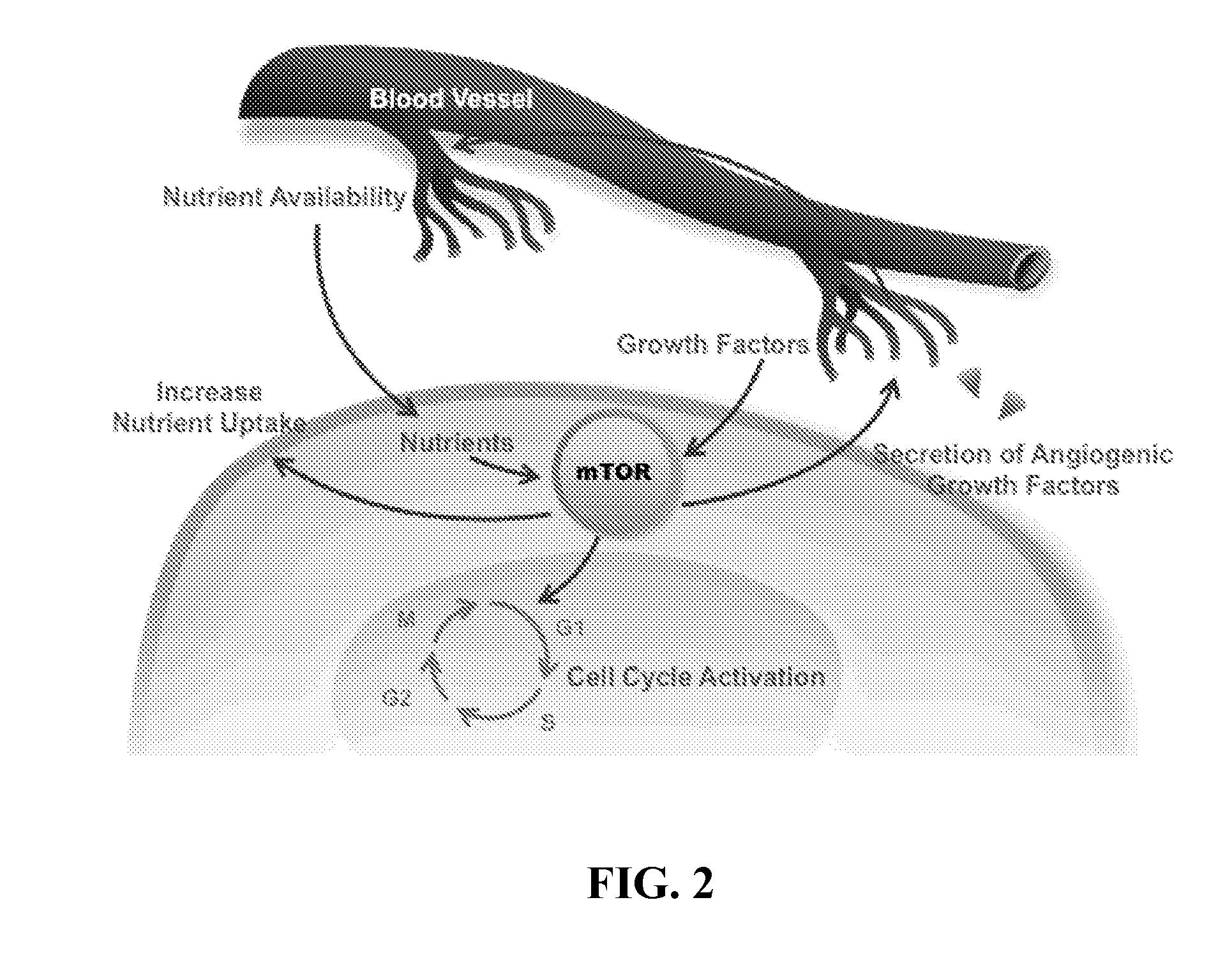
Lipid kinases are one of the most promising new classes of potential drug targets. PI-3 and sphingosine kinases regulate a wide variety of cellular functions, including cell growth, proliferation, differentiation, motility, intracellular trafficking, and survival. As a result, defects in lipid kinase function lead...
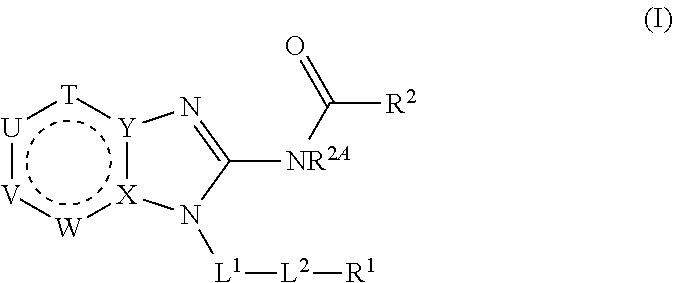
Substituted pyrazolo[3,4-D]pyrimidines as kinase antagonists
Owner:RGT UNIV OF CALIFORNIA
Methods and compositions for treatment of ophthalmic conditions
InactiveUS20110269779A1Relieve symptomsLower eye pressureBiocideSenses disorderKinase activityMedicine
The present invention provides chemical entities or compounds and pharmaceutical compositions thereof that are capable of modulating signal transduction by certain protein kinases such as mTor, tyrosine kinases, and / or lipid kinases such as PB kinase in an ocular tissue. Also provided in the present invention are methods of using these compositions to modulate activities of one or more of these kinases, especially for therapeutic applications.
Owner:INTELLIKINE
Novel kinases
InactiveUS20050125852A1Prevent hybridizationDecrease in proliferation and growth and differentiationSugar derivativesMicrobiological testing/measurementLipid kinasesProtein structure
The present invention relates to kinase polypeptides, nucleotide sequences encoding the kinase polypeptides, as well as various products and methods useful for the diagnosis and treatment of various kinase-related diseases and conditions. Through the use of a bioinformatics strategy, mammalian members of protein and lipid kinase families have been identified and their protein structure predicted.
Owner:SUGEN INC
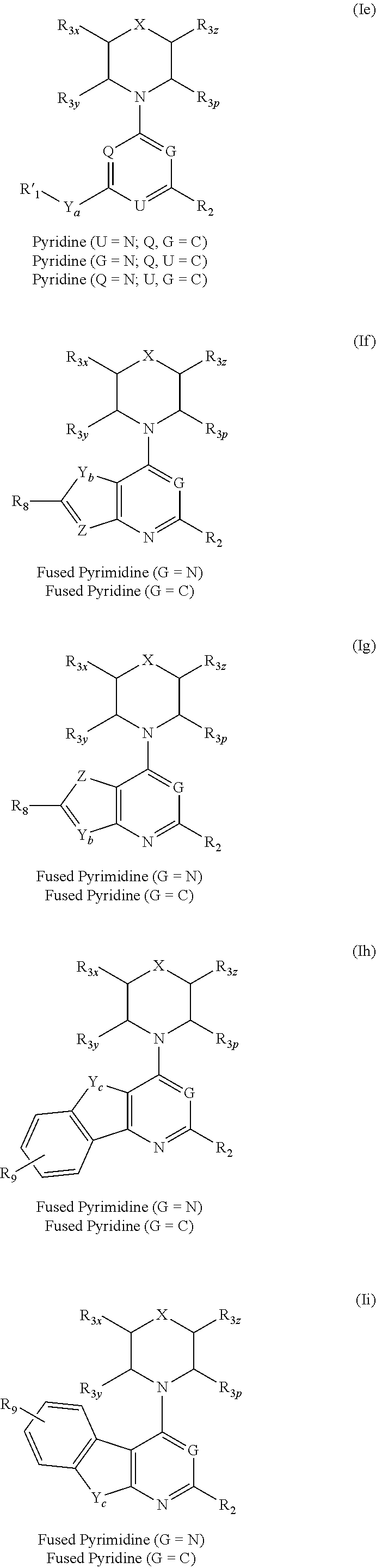
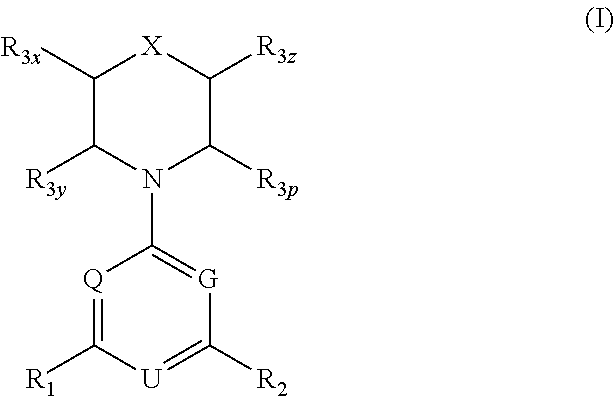
Triazine, pyrimidine and pyridine analogs and their use as therapeutic agents and diagnostic probes
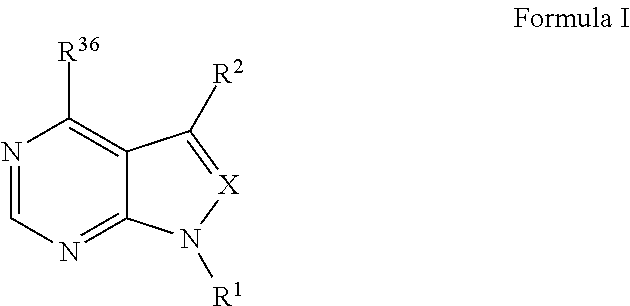
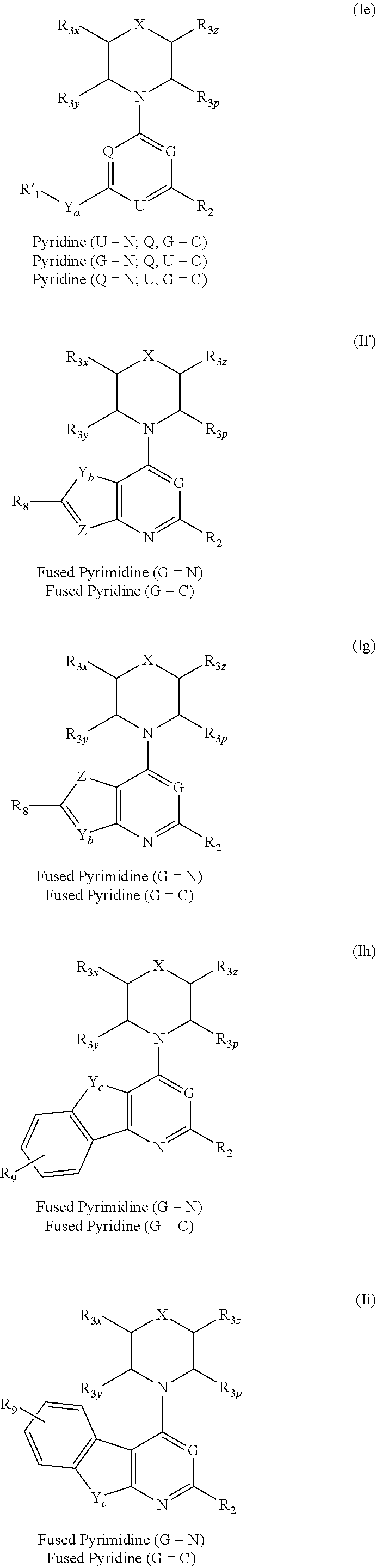
The invention relates to novel therapeutic agents and diagnostic probes. The invention also relates to phosphoinositide 3-kinase (PI3K) and mammalian target of rapamycin (mTOR) inhibitor triazine-, pyrimidine- and pyridine-based compoundŝ Formula (I), their stereoisomers, geometric isomers, tautomers, solvates, metabolites, N-oxide derivatives, pharmaceutically acceptable salts, and prodrugs thereof compositions of the new compounds; either alone or in combination with at least one additional therapeutic agent, with a pharmaceutically acceptable carrier; and uses of the new compounds, either alone or in combination with at least one additional therapeutic agent, for treating disorders mediated by lipid kinases. •Methods of using compounds of Formula (I) for in vitro, in situ, and in vivo diagnosis, prevention or treatment of such disorders in mammalian cells, or associated pathological conditions, are disclosed. (Formula I)
Owner:UNIVERSITY OF BASEL
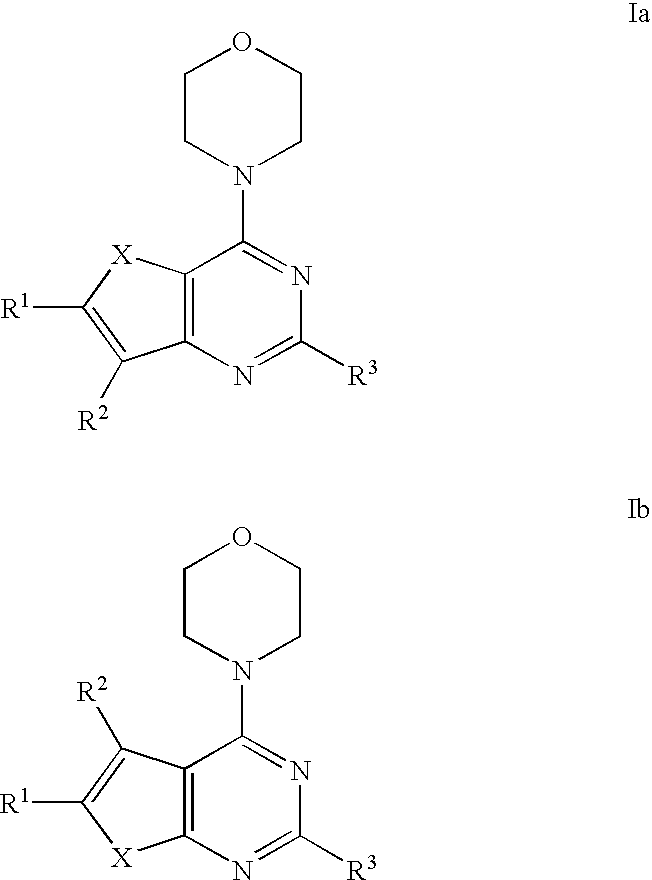
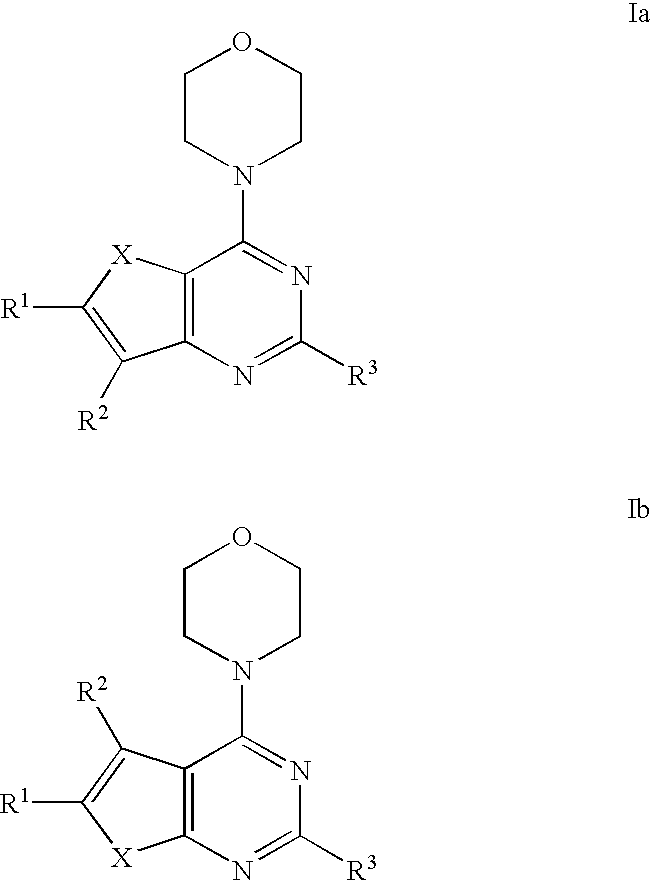
Phosphoinositide 3-kinase inhibitor compounds and methods of use
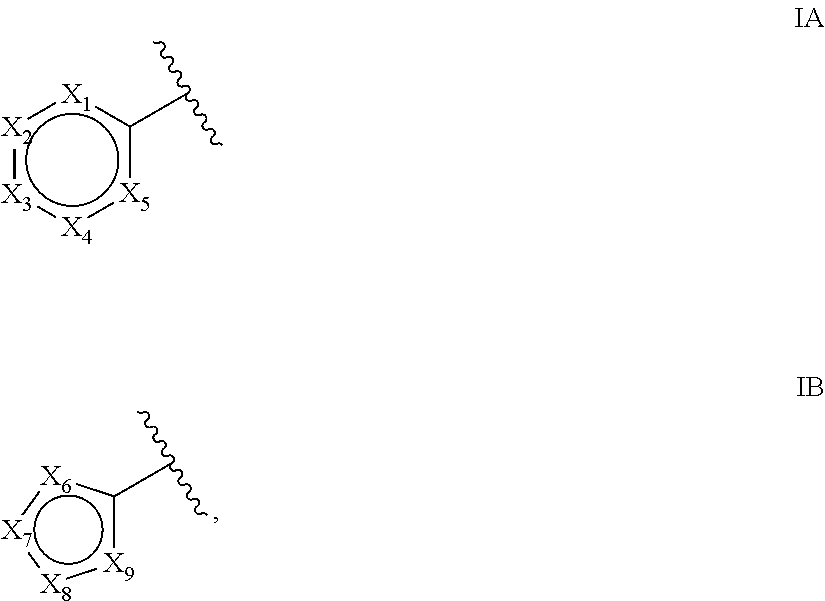
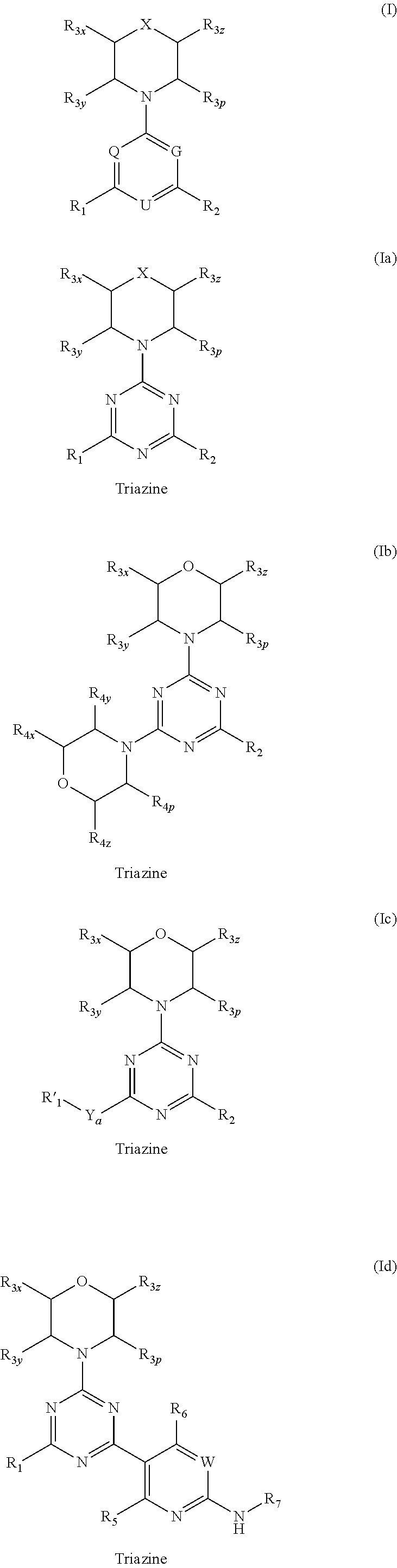
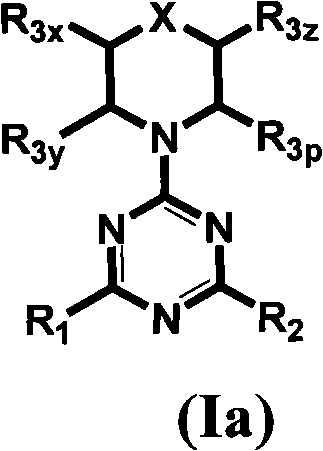
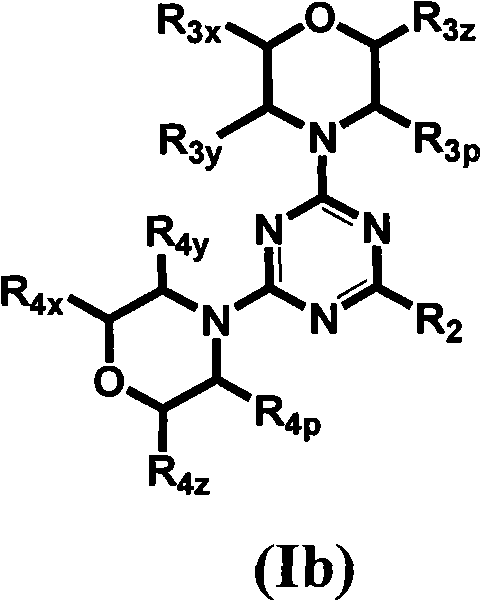
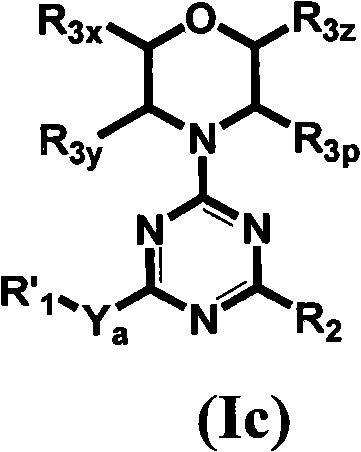
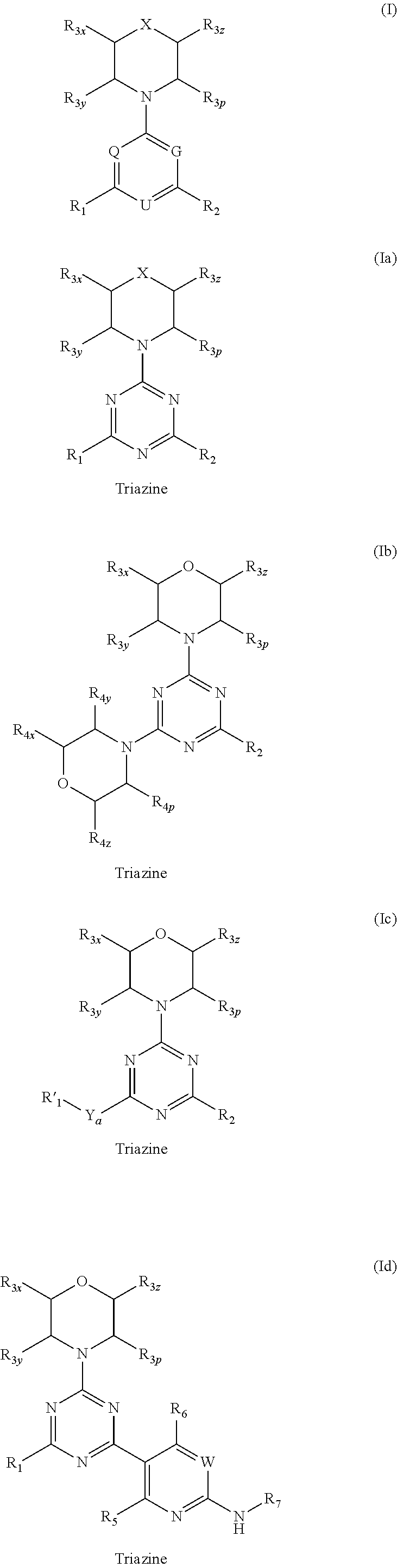
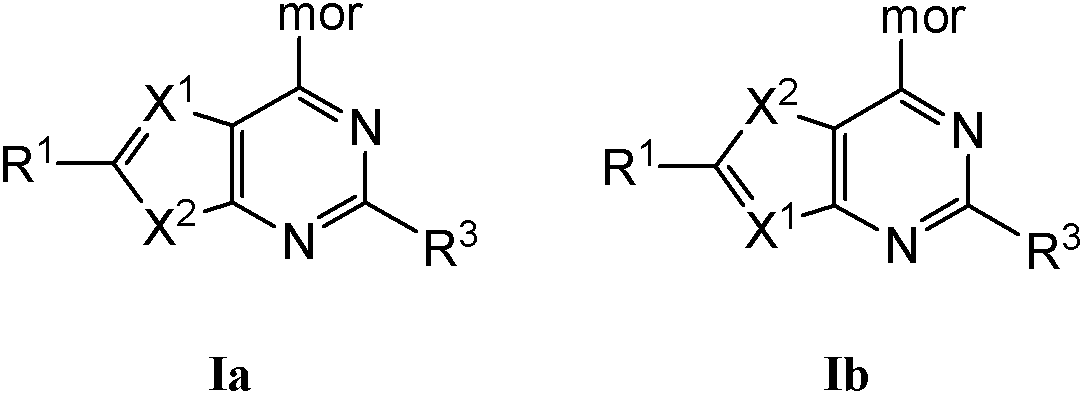
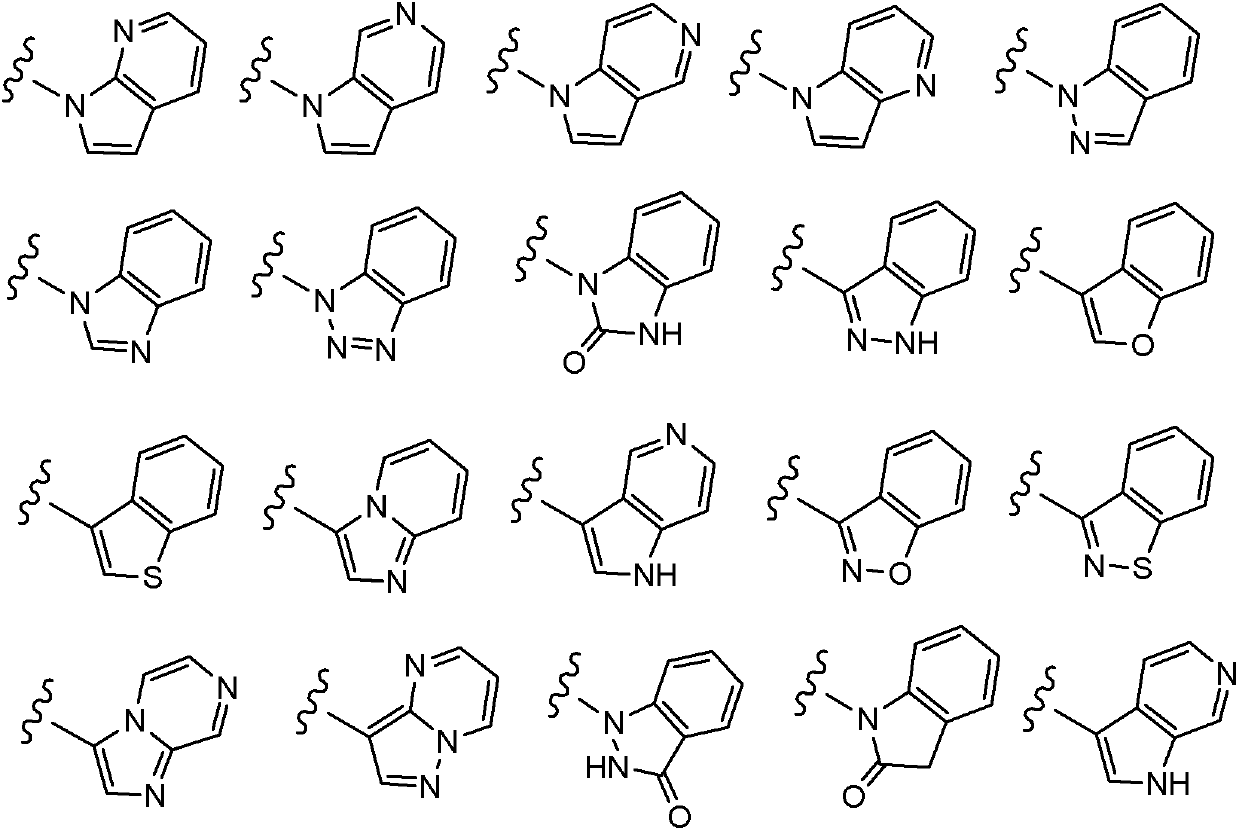
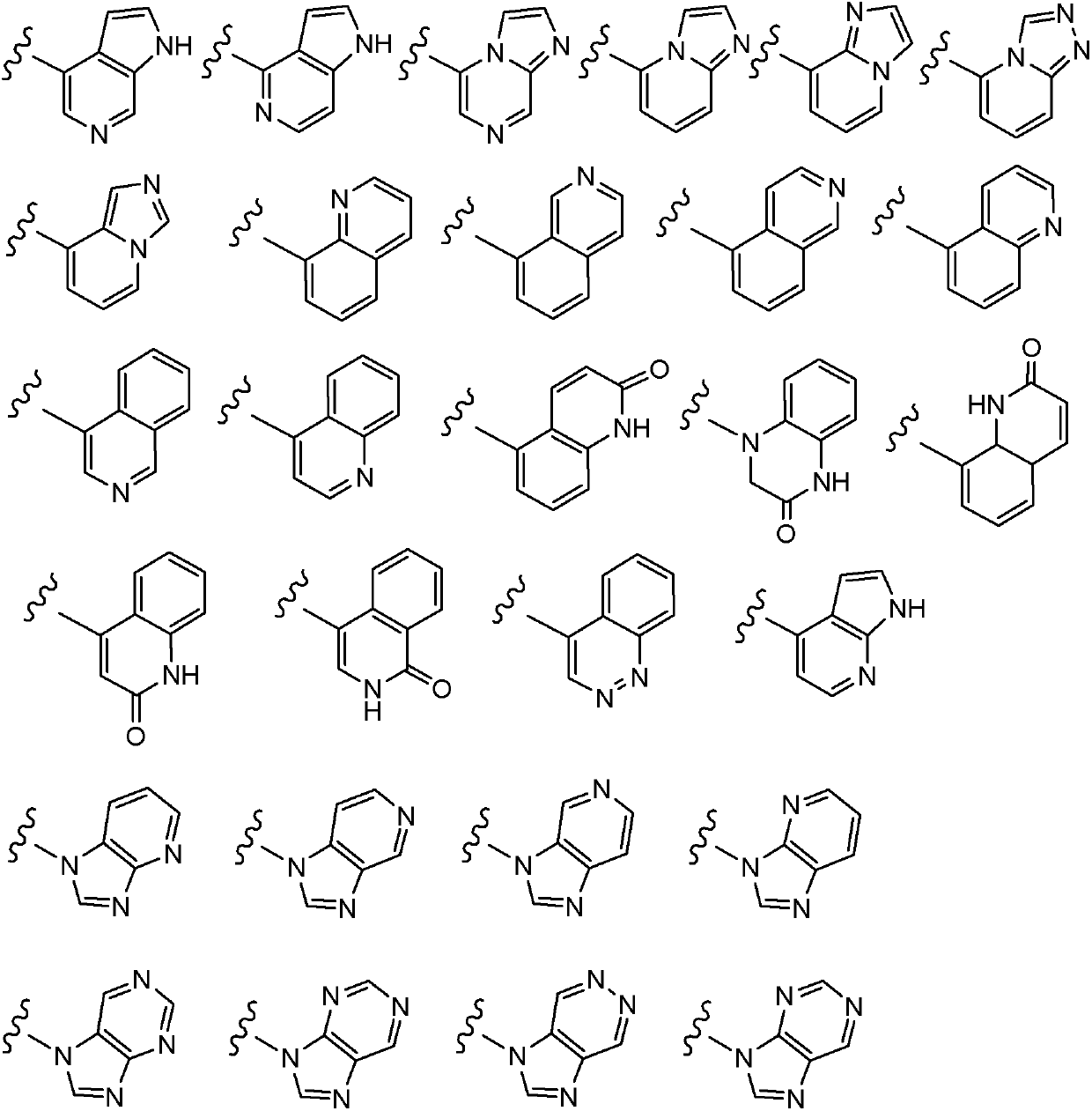
Compounds of Formulas Ia and Ib, and including stereoisomers, geometric isomers, tautomers, solvates, metabolites and pharmaceutically acceptable salts thereof, are useful for inhibiting lipid kinases including PI3K, and for treating disorders such as cancer mediated by lipid kinases. Methods of using compounds of Formula Ia and Ib for in vitro, in situ, and in vivo diagnosis, prevention or treatment of such disorders in mammalian cells, or associated pathological conditions, are disclosed.
Owner:GENENTECH INC +1
Triazine, pyrimidine and pyridine analogs and their use as therapeutic agents and diagnostic probes
The invention relates to novel therapeutic agents and diagnostic probes. The invention also relates to phosphoinositide 3-kinase (PI3K) and mammalian target of rapamycin (mTOR) inhibitor triazine-, pyrimidine- and pyridine-based compounds^ Formula (I), their stereoisomers, geometric isomers, tautomers, solvates, metabolites, N-oxide derivatives, pharmaceutically acceptable salts, and prodrugs thereof compositions of the new compounds; either alone or in combination with at least one additional therapeutic agent, with a pharmaceutically acceptable carrier; and uses of the new compounds, either alone or in combination with at least one additional therapeutic agent, for treating disorders mediated by lipid kinases.; DEG Methods of using compounds of Formula (I) for in vitro, in situ, and in vivo diagnosis,.prevention or treatment of such disorders in mammalian cells, or associated pathological conditions, are disclosed.
Owner:UNIVERSITY OF BASEL
Triazine, pyrimidine and pyridine analogs and their use as therapeutic agents and diagnostic probes
The invention relates to novel therapeutic agents and diagnostic probes. The invention also relates to phosphoinositide 3-kinase (PI3K) and mammalian target of rapamycin (mTOR) inhibitor triazine-, pyrimidine- and pyridine-based compounds^ Formula (I), their stereoisomers, geometric isomers, tautomers, solvates, metabolites, N-oxide derivatives, pharmaceutically acceptable salts, and prodrugs thereof compositions of the new compounds; either alone or in combination with at least one additional therapeutic agent, with a pharmaceutically acceptable carrier; and uses of the new compounds, either alone or in combination with at least one additional therapeutic agent, for treating disorders mediated by lipid kinases. •Methods of using compounds of Formula (I) for in vitro, in situ, and in vivo diagnosis, prevention or treatment of such disorders in mammalian cells, or associated pathological conditions, are disclosed. (Formula I)
Owner:UNIVERSITY OF BASEL
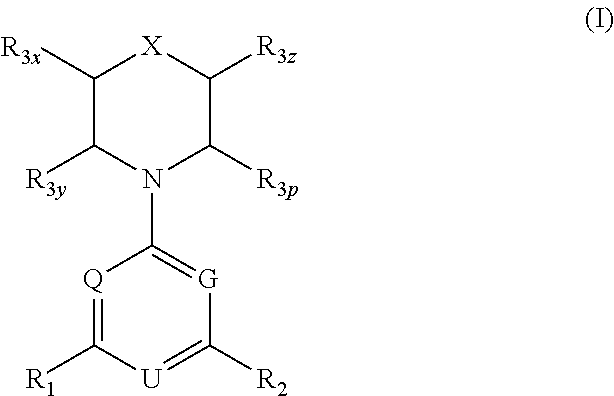
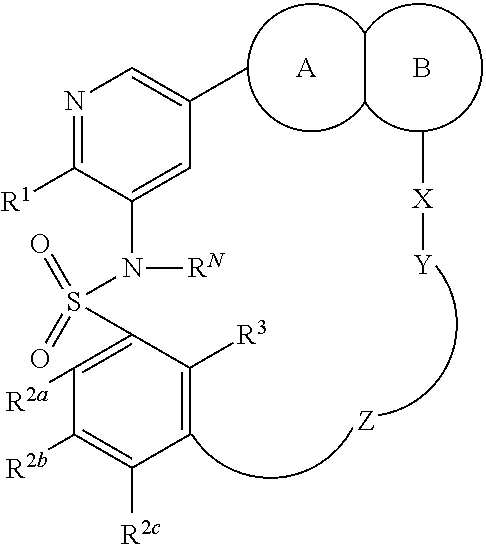
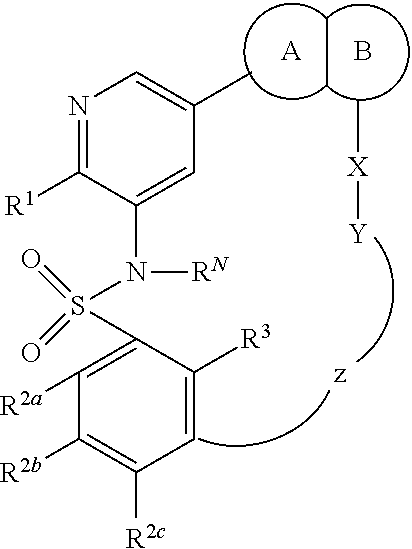
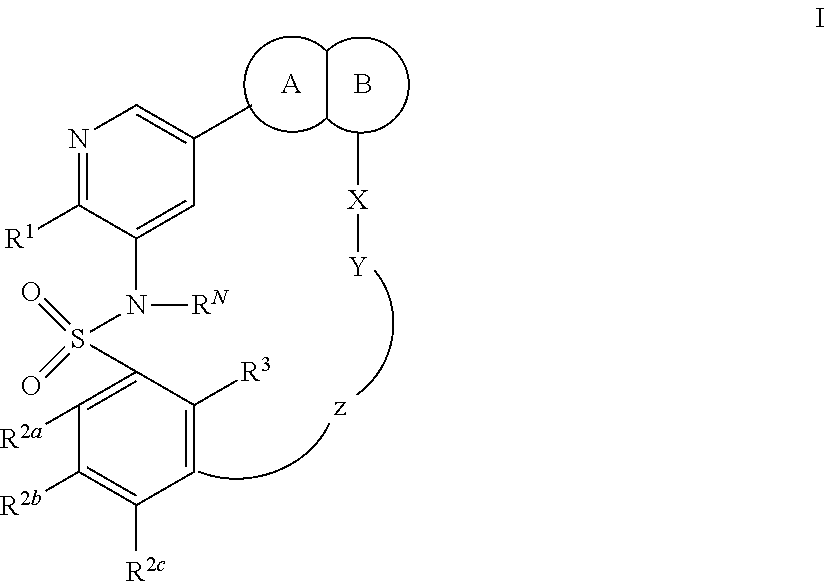
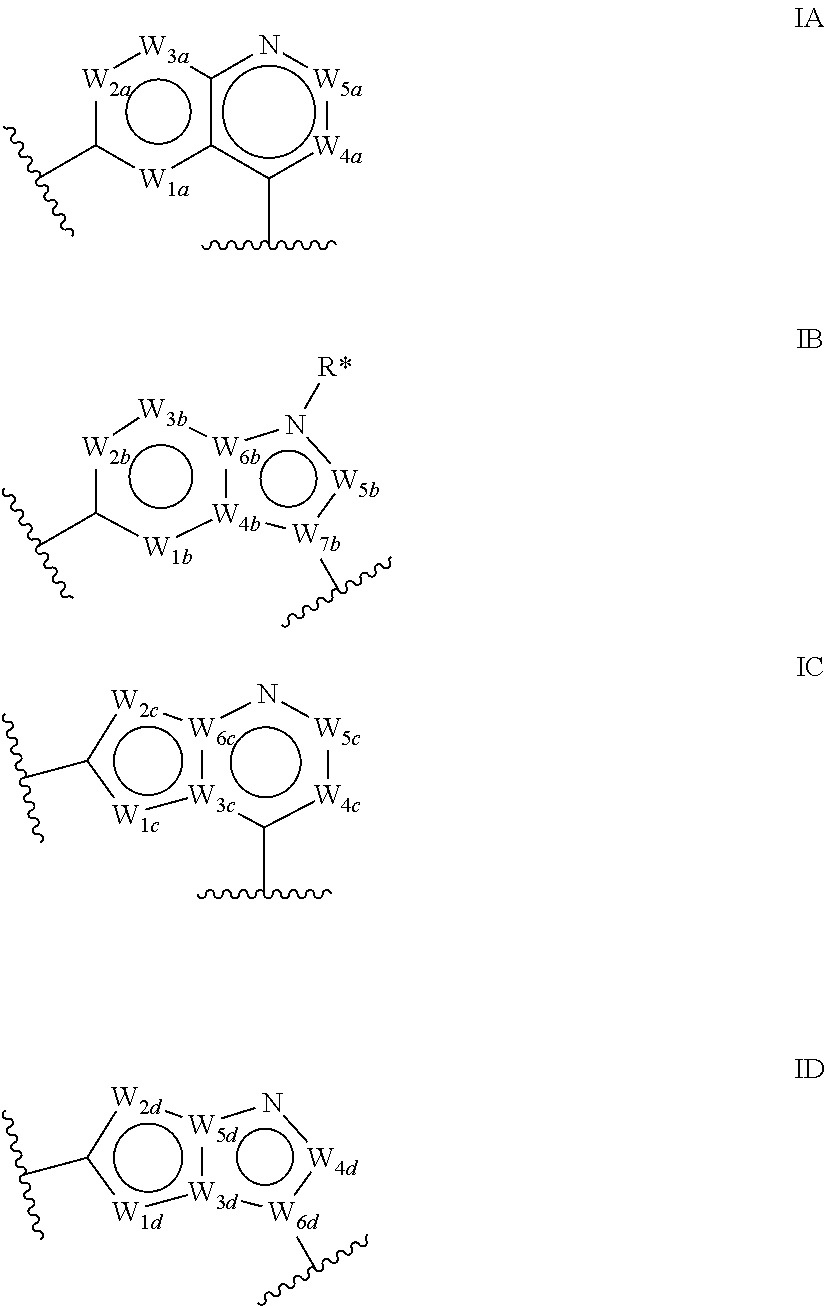
Macrocyclic compounds as protein kinase inhibitors
ActiveUS20140256717A1Ease of detectabilityEasy to prepareBiocideNervous disorderDiseasePTK Inhibitors
There is provided compounds of formula I, wherein R1, R2a, R2b, R2c, X, Y, Z, R3 and ring A / B have meanings given in the description, and pharmaceutically-acceptable esters, amides, solvates or salts thereof, which compounds are useful in the treatment of diseases in which inhibition of a protein or lipid kinase (e.g. PI3-K, particularly class I PI3K, PIM family kinase and / or mTOR) is desired and / or required, and particularly in the treatment of cancer. The invention also relates to combinations containing such compounds.
Owner:FUNDACION CENT NACIONAL DE INVESTIGACIONES ONCOLOGICAS CARLOS III
Assaying apparatus, kit, and method for lipids and associated enzymes
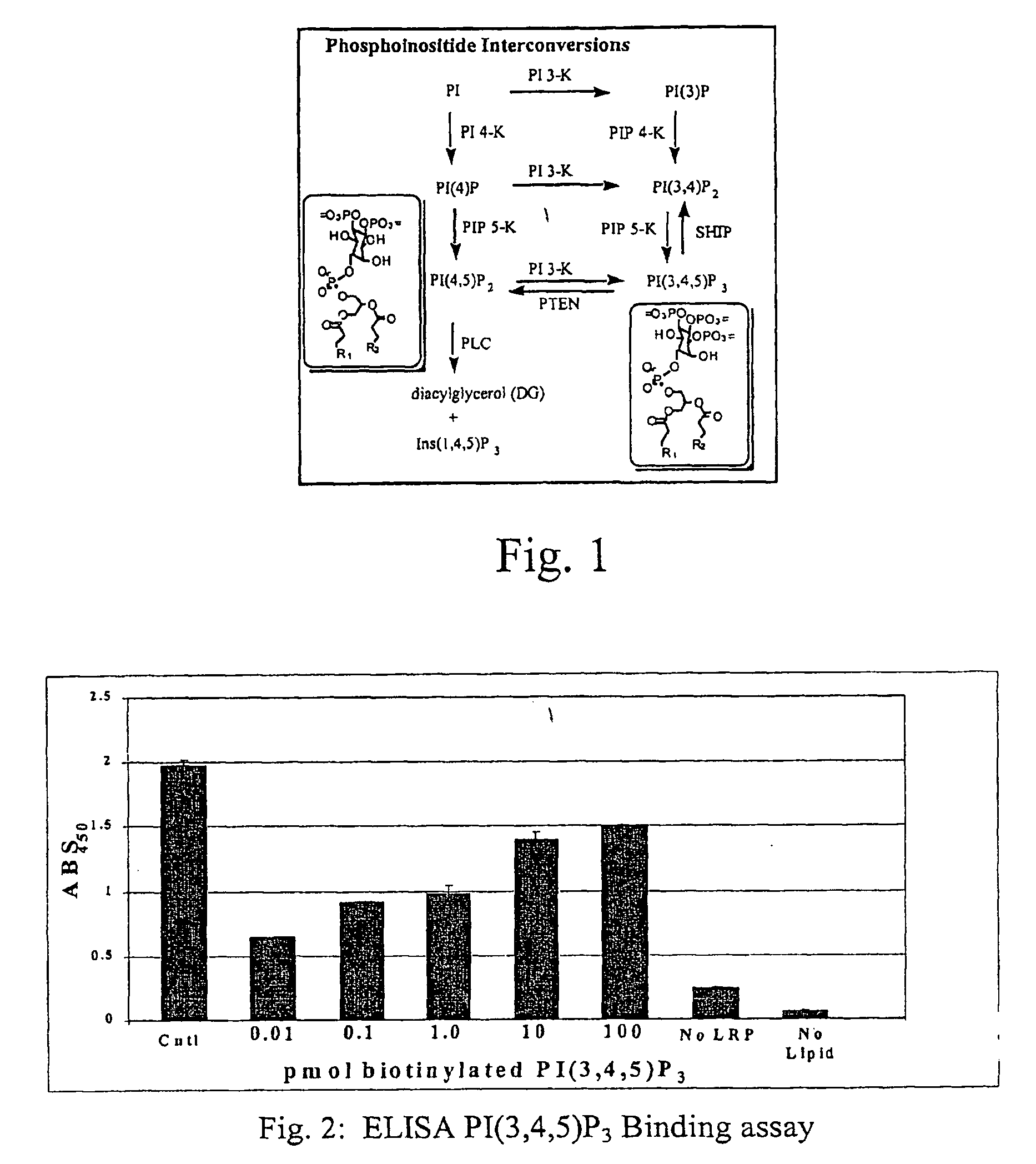
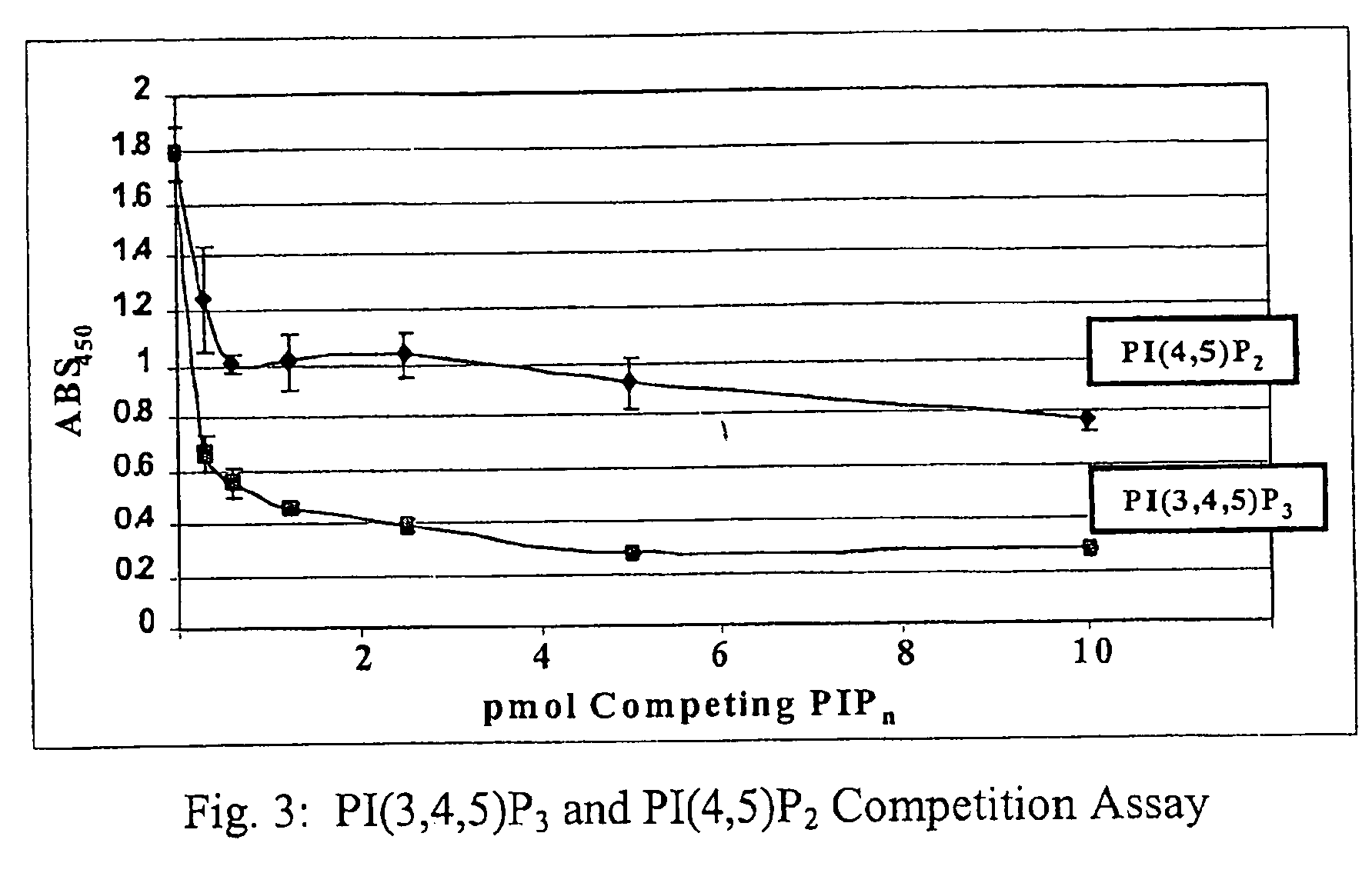
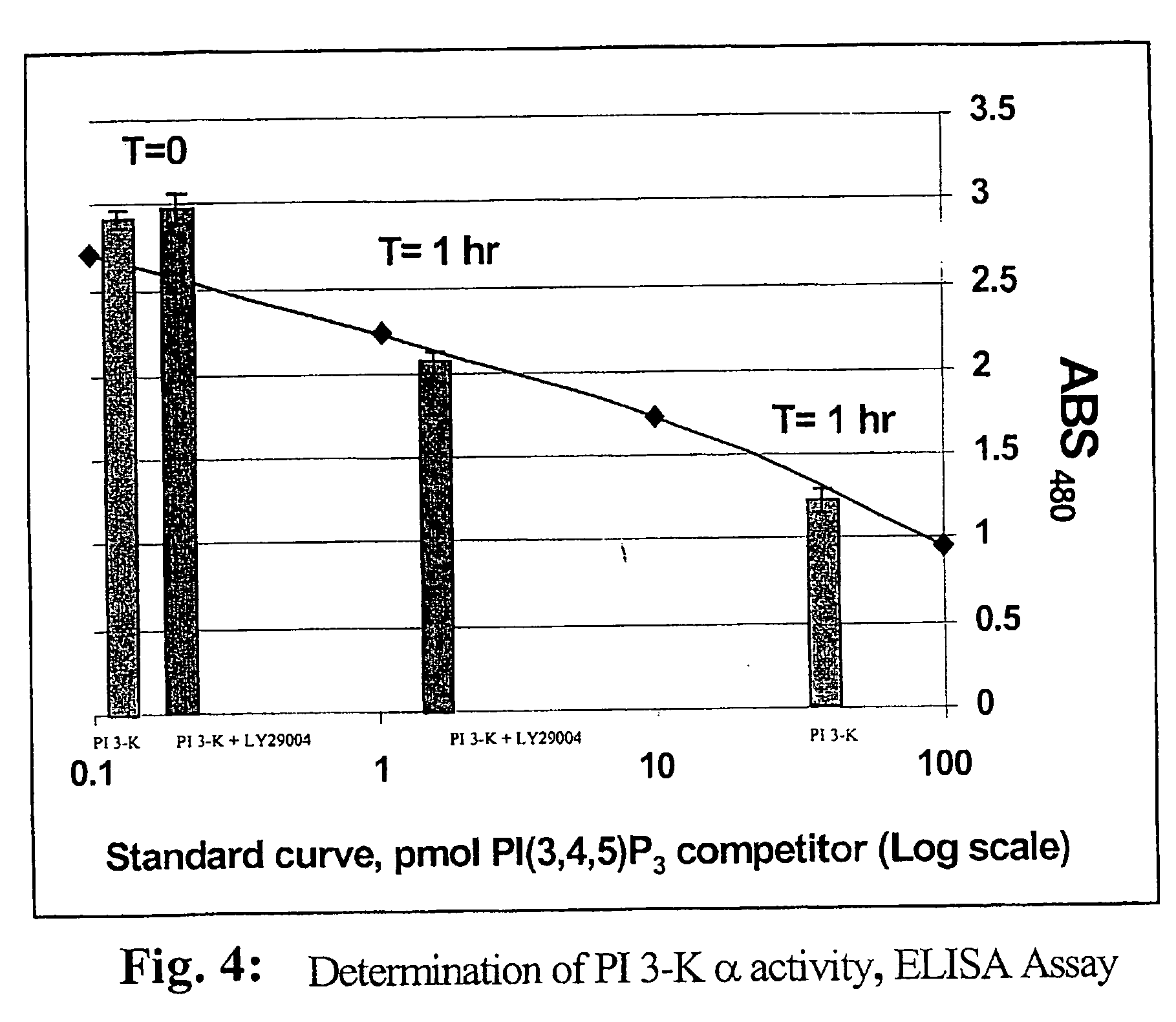
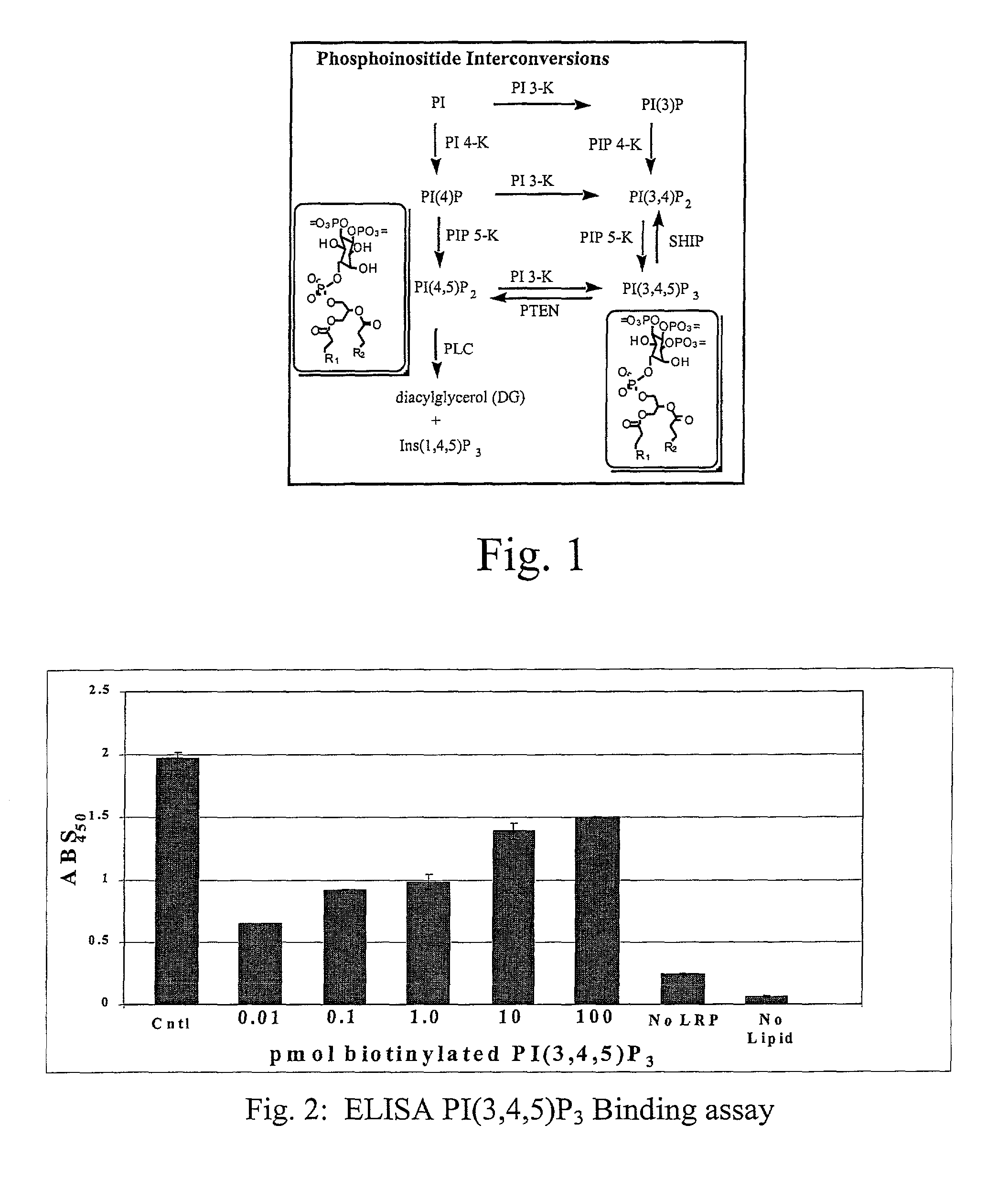
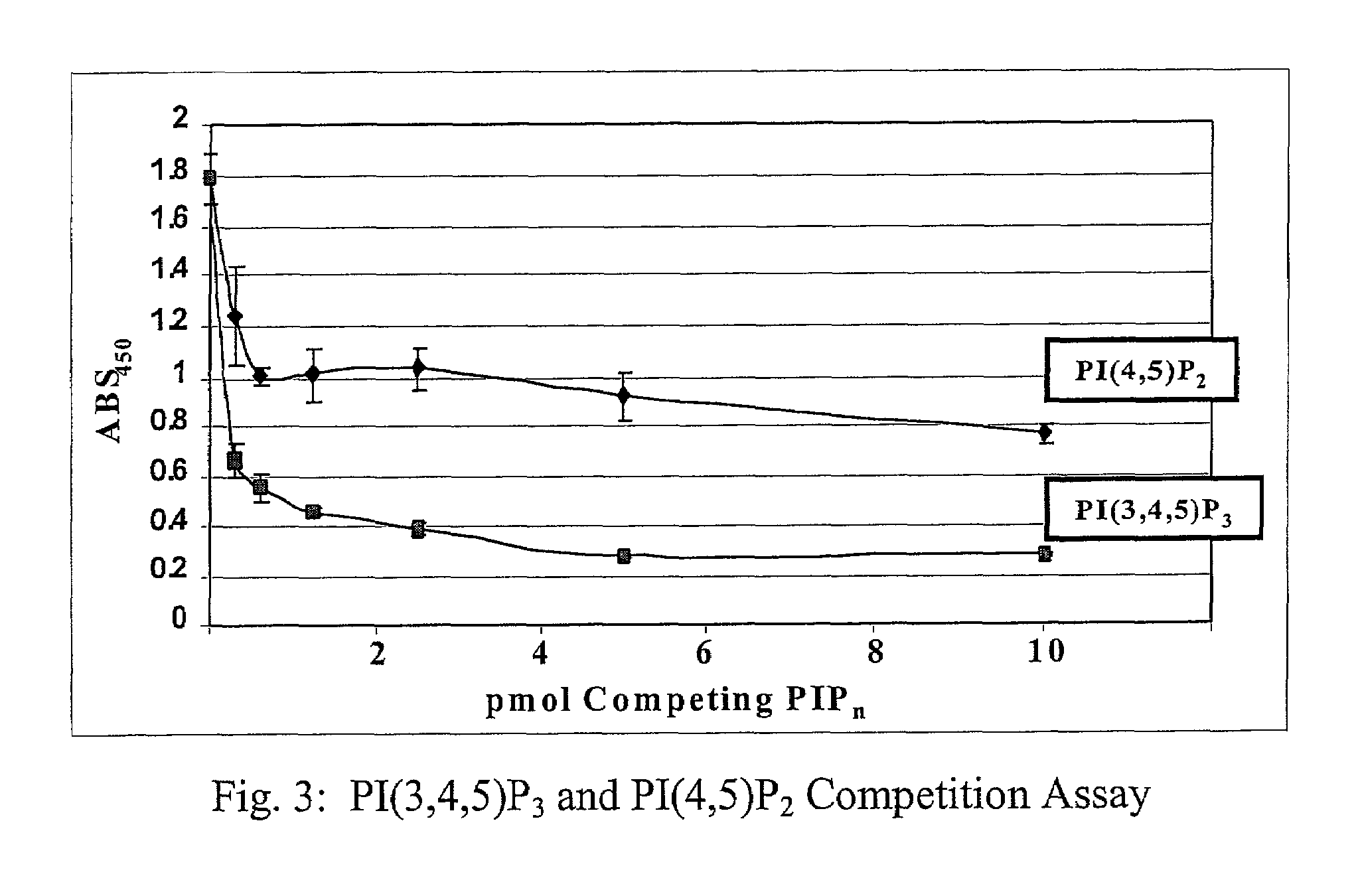
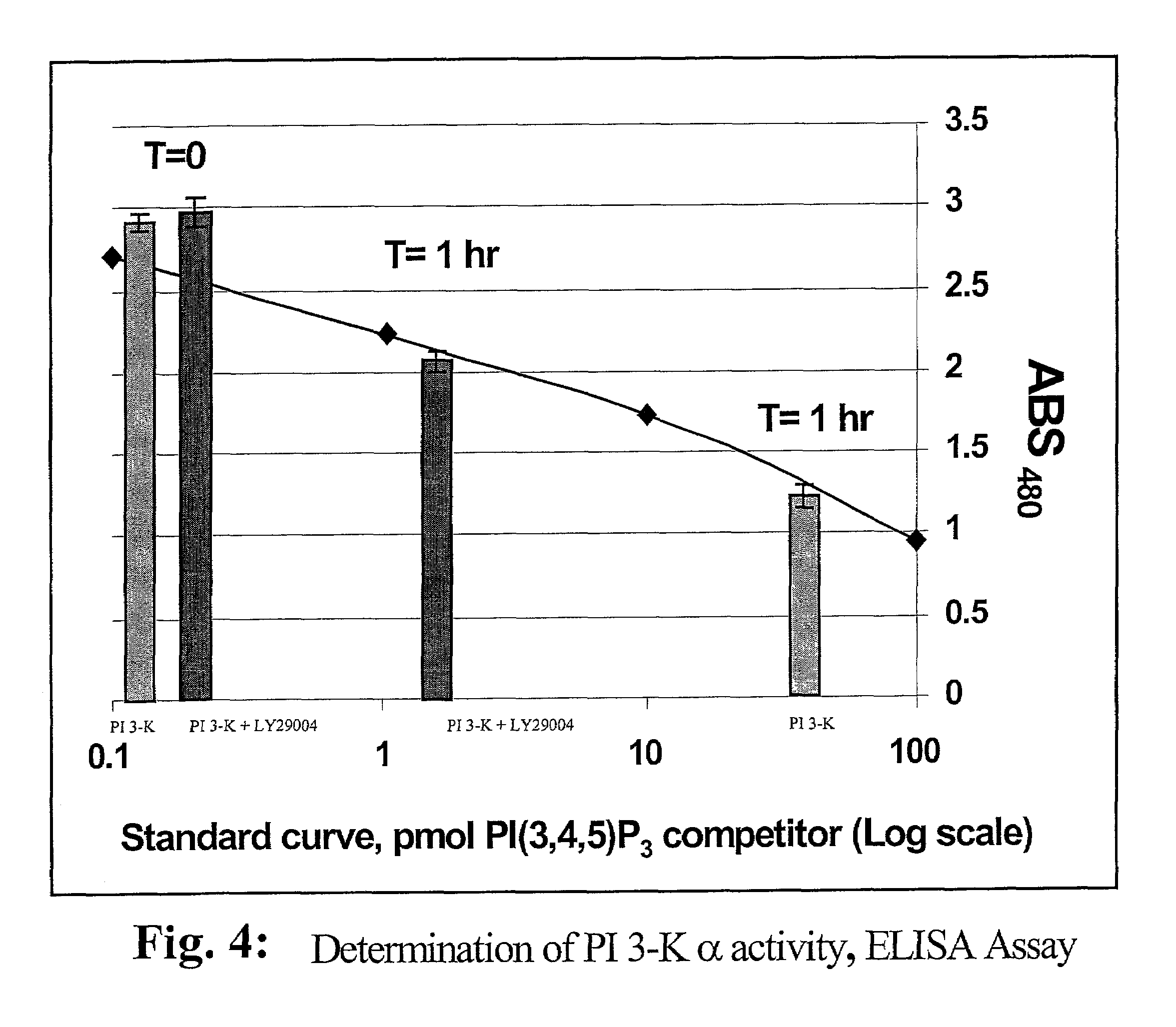
A lipid assay method, kit, and apparatus involving exposure of a protein, having a lipid recognition motif that interacts with a target lipid and a competing lipid, to a solution containing the competing lipid, and determining whether the target lipid is present in the solution. The target lipid has a stronger affinity to the lipid recognition motif than does the competing lipid. The lipid recognition motif is preferably a pleckstrin homology (PH) domain, with the target lipid being a phosphoinositide. The assay determines activity of a lipid kinase, the target lipid being a phosphorylation product of a reaction between the lipid kinase and a substrate lipid. The assay can be a cancer screening method for detection of cancer cells, where detection of certain levels of a PI(3,4,5)P3 target lipid is an indicator of a cancer cell.
Owner:ECHELON BIOSCI
Bicyclic pyrimidine pi3k inhibitor compounds selective for p110 delta, and methods of use
Formula (I) ((Ia) and (Ib)) compounds wherein (i) X1 is N and X2 is S, (ii) X1 is CR7 and X2 is S, (iii) X1 is N and X2 is NR2, or (iv) X1 is CR7 and X2 is O, including stereoisomers, tautomers, metabolites and pharmaceutically acceptable salts thereof, are useful for inhibiting the delta isoform of PI3K, and for treating disorders mediated by lipid kinases such as inflammation, immunological, and cancer. Methods of using compounds of Formula (I) for in vitro, in situ, and in vivo diagnosis, prevention or treatment of such disorders in mammalian cells, or associated pathological conditions, are disclosed.
Owner:GENENTECH INC +1
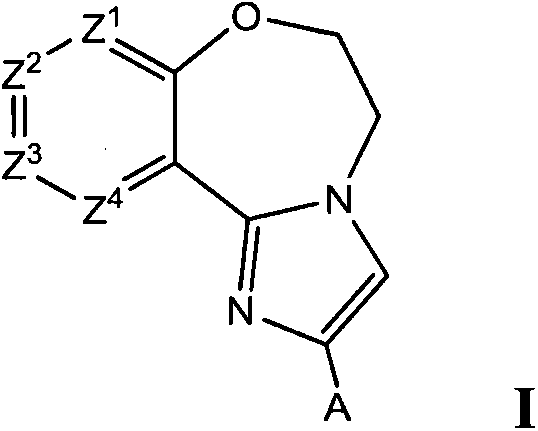
Benzoxazepin compounds selective for pi3k p110 delta and methods of use
Benzoxazepin Formula I compounds, including stereoisomers, geometric isomers, tautomers, metabolites and pharmaceutically acceptable salts thereof, are useful for inhibiting the delta isoform of PI3K, and for treating disorders mediated by lipid kinases such as inflammation, immunological disorders, and cancer. Methods of using compounds of Formula I for in vitro, in situ, and in vivo diagnosis, prevention or treatment of such disorders in mammalian cells, or associated pathological conditions, are disclosed.
Owner:F HOFFMANN LA ROCHE & CO AG
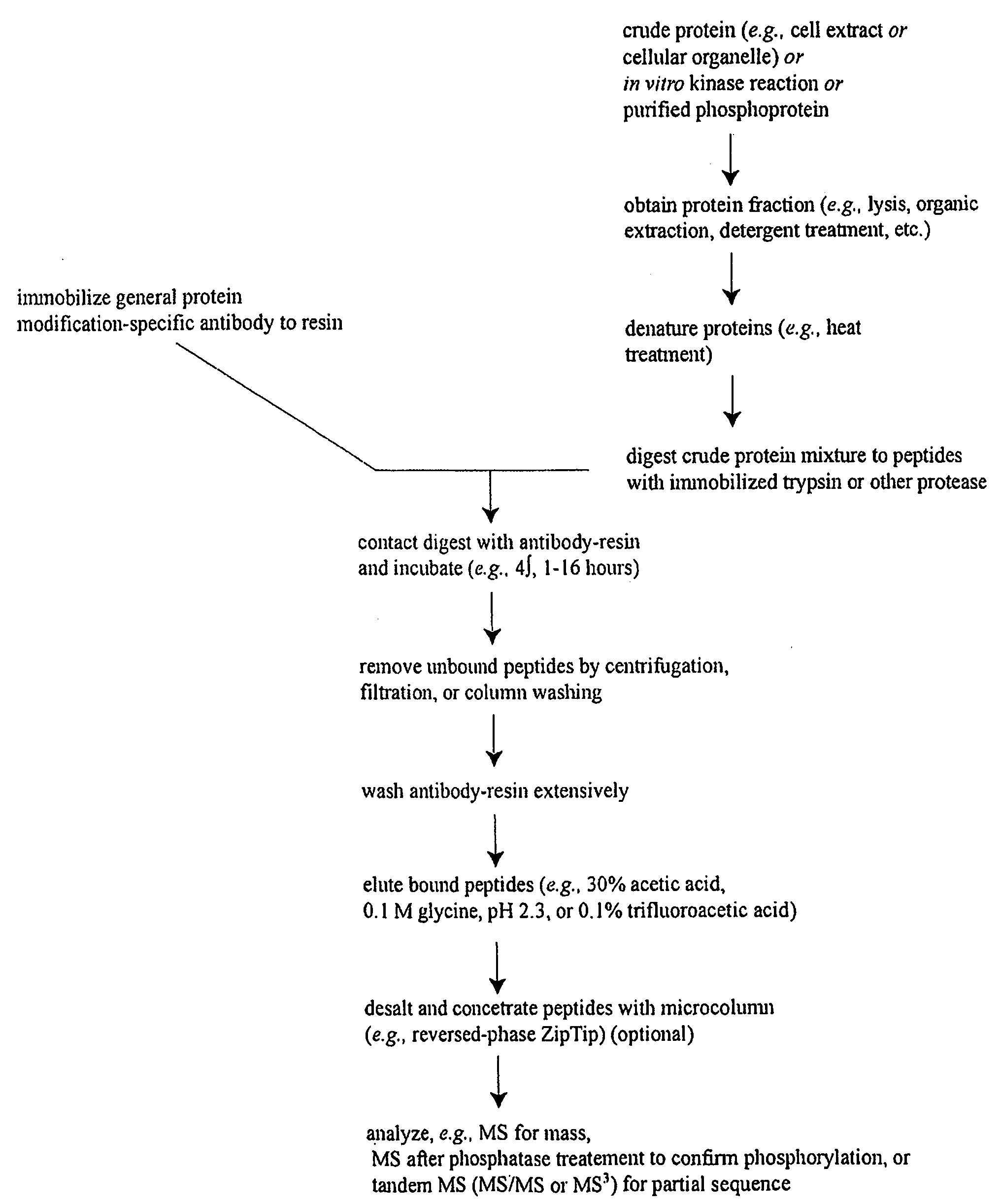
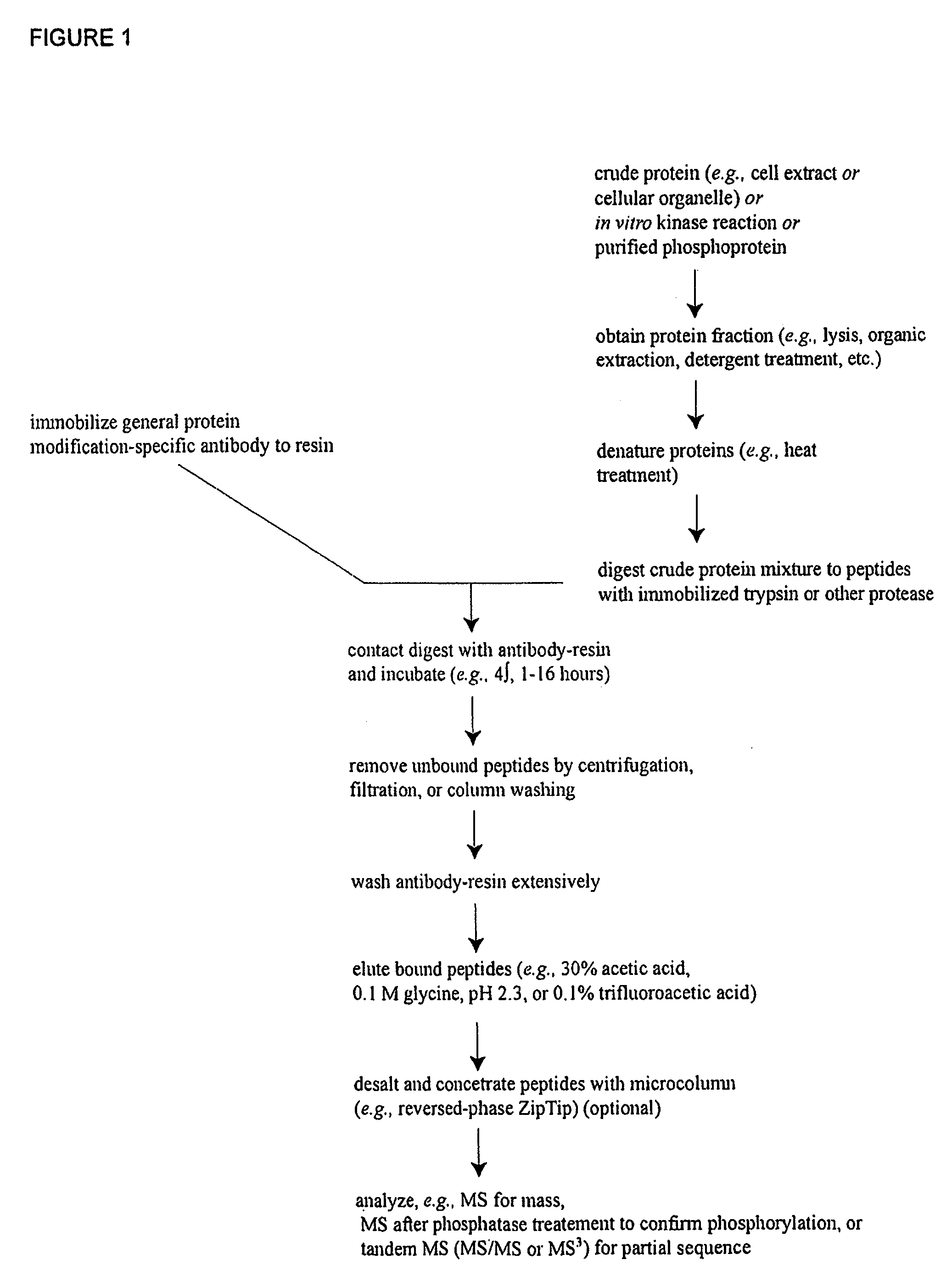
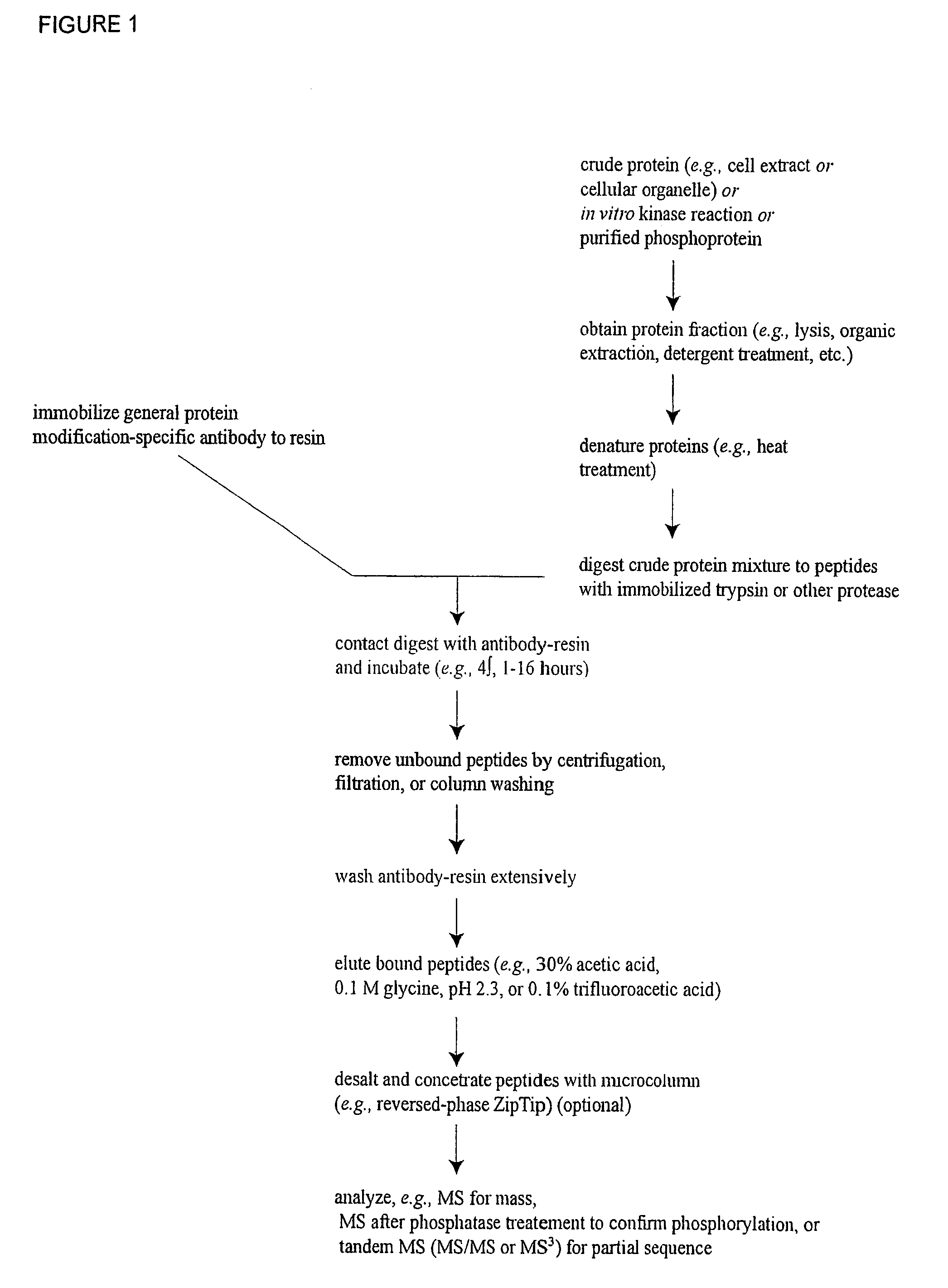
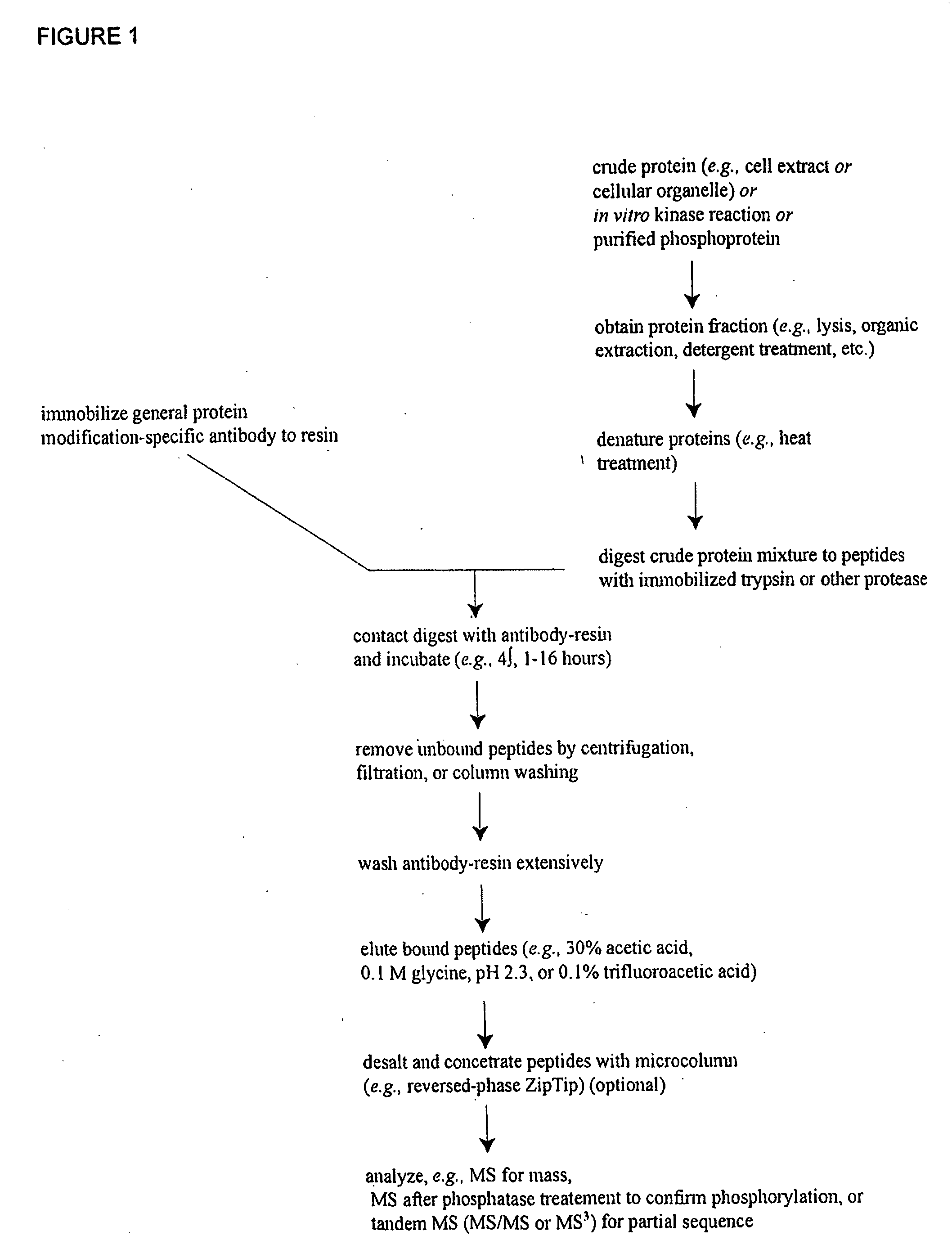
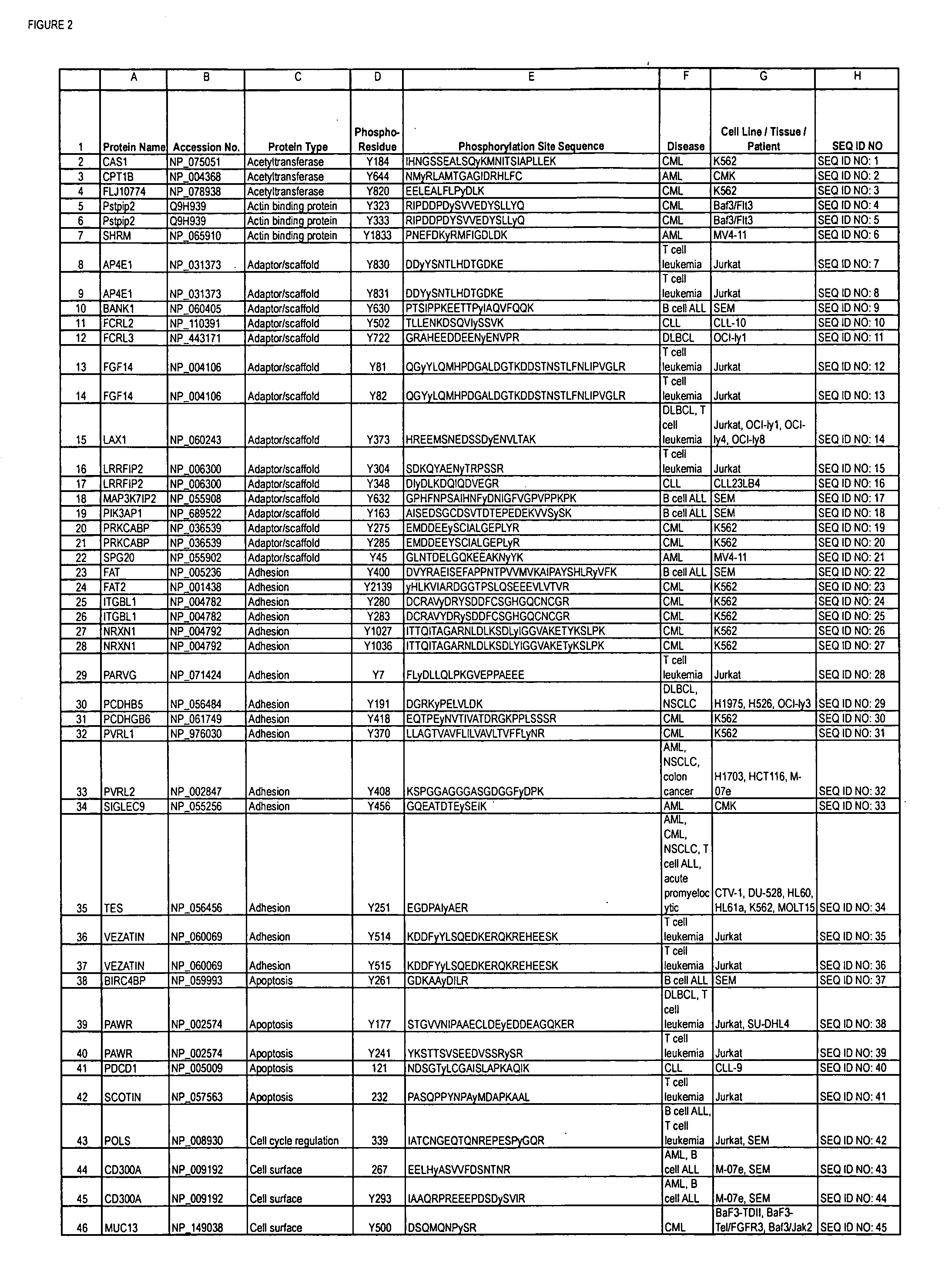
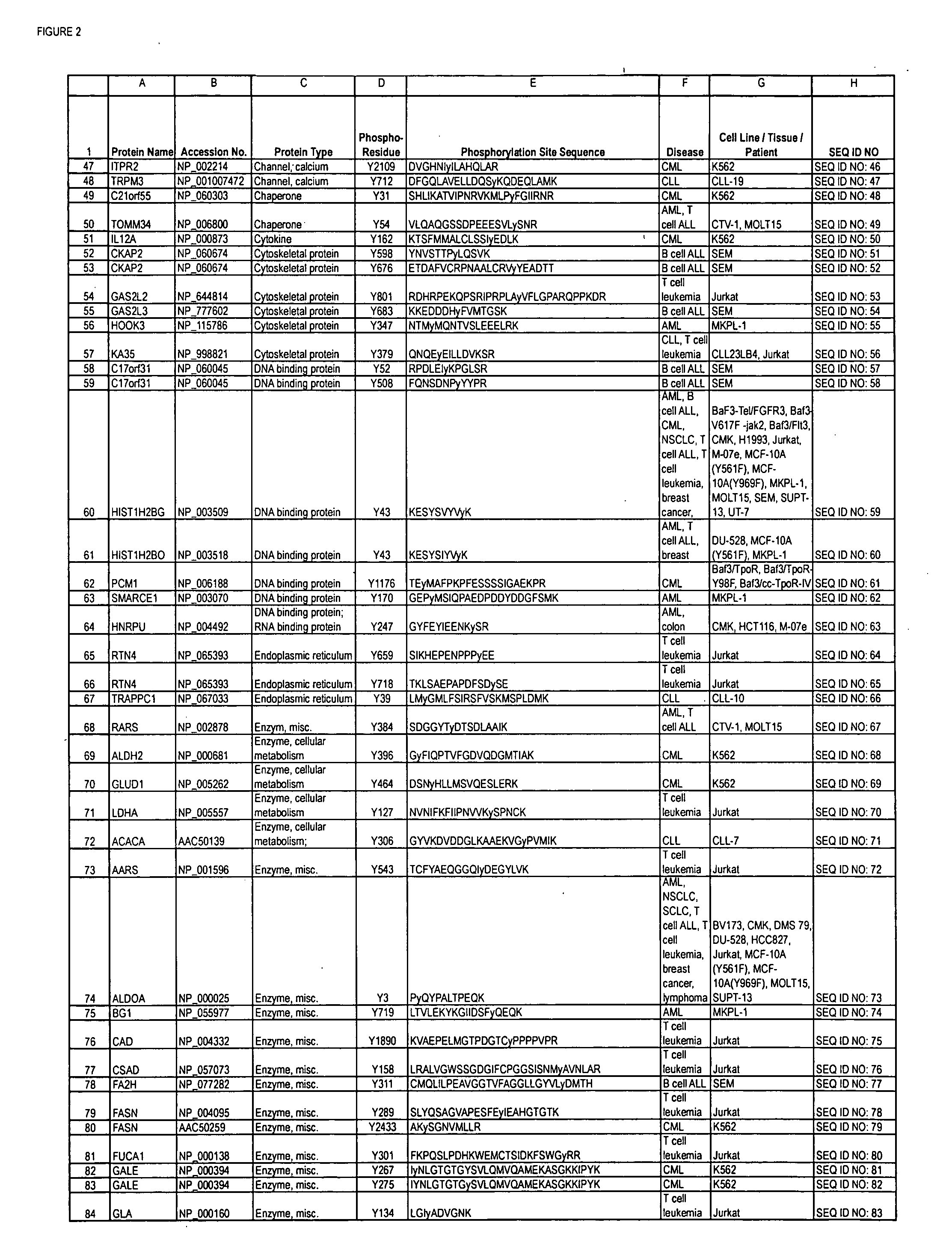
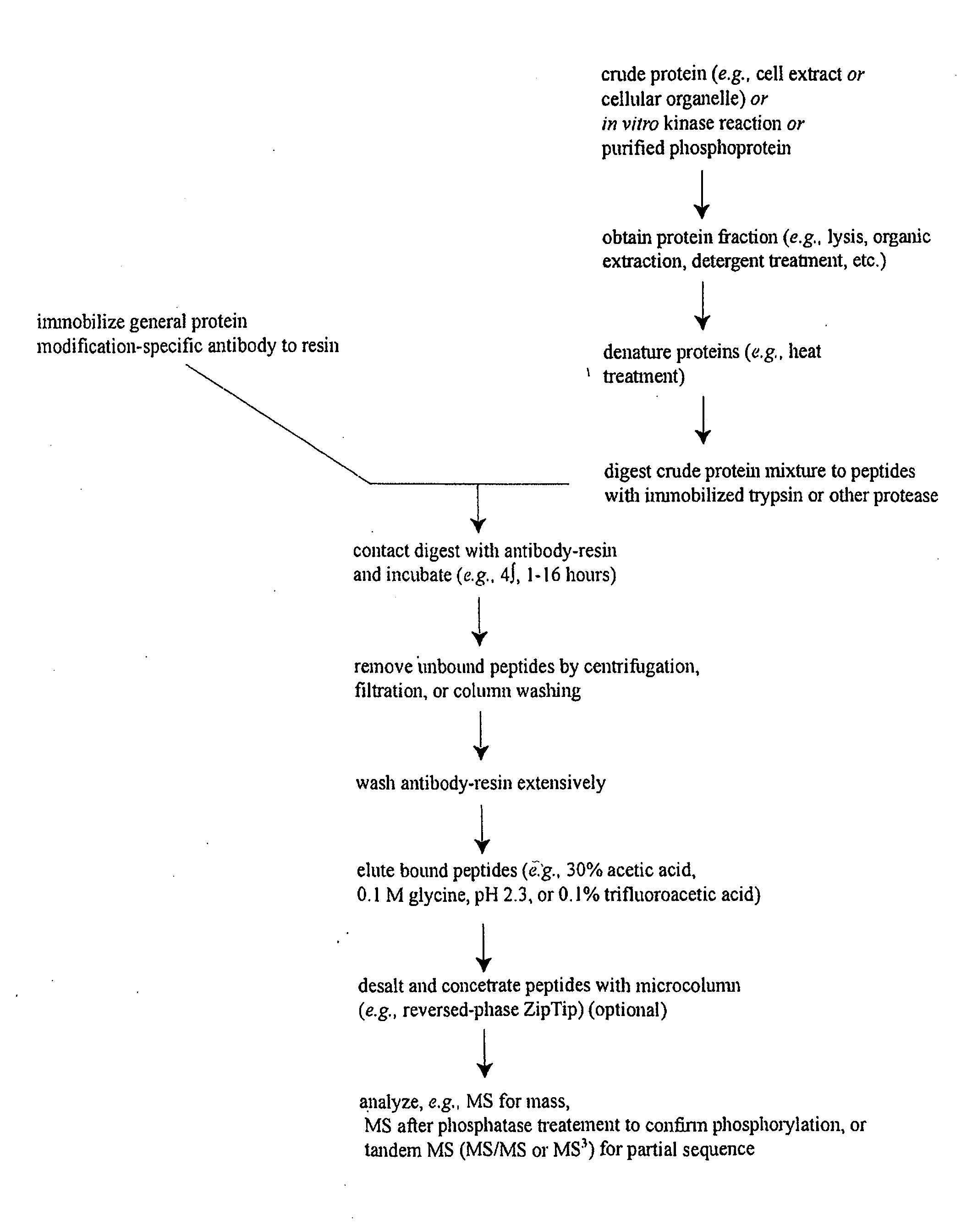
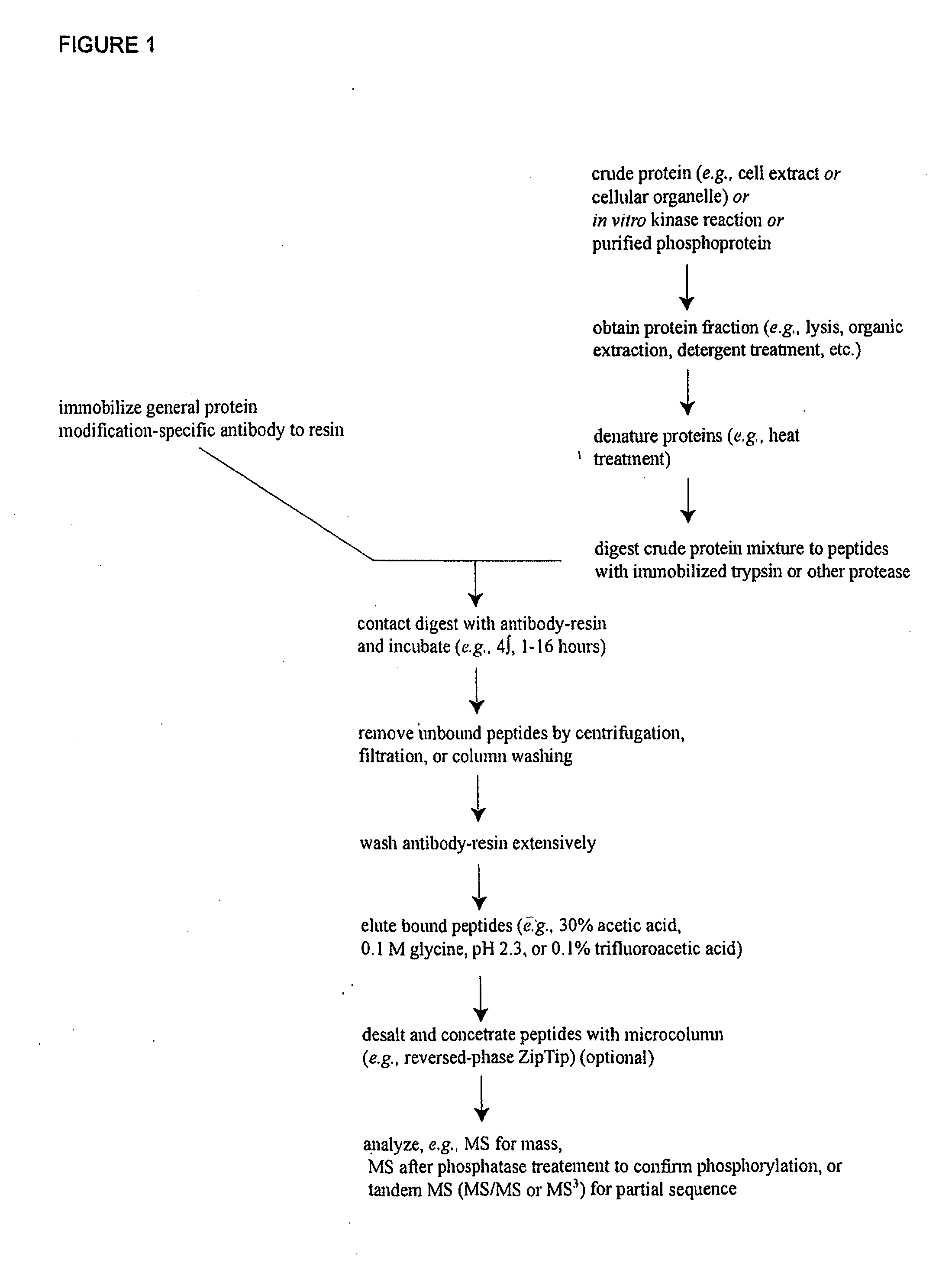
Reagents for the detection of protein phosphorylation in Leukemia signaling pathways
ActiveUS20080248490A1Immunoglobulins against animals/humansBiological testingHuman leukemiaADAMTS Proteins
The invention discloses nearly 288 novel phosphorylation sites identified in signal transduction proteins and pathways underlying human Leukemia, and provides phosphorylation-site specific antibodies and heavy-isotope labeled peptides (AQUA peptides) for the selective detection and quantification of these phosphorylated sites / proteins, as well as methods of using the reagents for such purpose. Among the phosphorylation sites identified are sites occurring in the following protein types: Adaptor / Scaffold proteins, Cytoskeletal proteins, Cellular Metabolism enzymes, G Protein / GTPase Activating / Guanine Nucleotide Exchange Factor proteins, Immunoglobulin Superfamily proteins, Inhibitor proteins, Lipid Kinases, Nuclear DNA Repair / RNA Binding / Transcription proteins, Serine / Threonine Protein Kinases, Tyrosine Kinases, Protein Phosphatases, and Translation / Transporter proteins.
Owner:CELL SIGNALING TECHNOLOGY
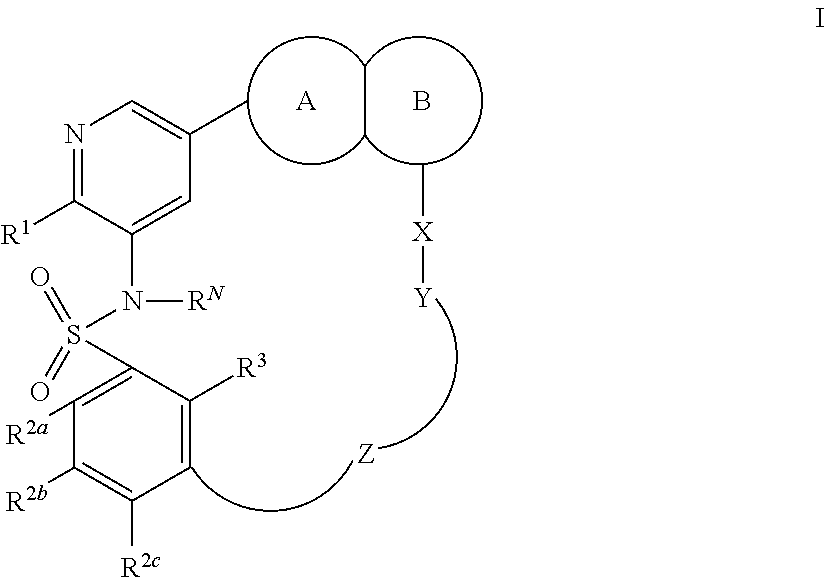
Macrocyclic compounds as protein kinase inhibitors
ActiveUS20160296528A1Effective inhibitorOrganic active ingredientsNervous disorderDiseasePTK Inhibitors
There is provided compounds of formula I, wherein R1, R2a, R2b, R2c, X, Y, Z, R3 and ring A / B have meanings given in the description, and pharmaceutically-acceptable esters, amides, solvates or salts thereof, which compounds are useful in the treatment of diseases in which inhibition of a protein or lipid kinase (e.g. PI3-K, particularly class I PI3K, PIM family kinase and / or mTOR) is desired and / or required, and particularly in the treatment of cancer. The invention also relates to combinations containing such compounds.
Owner:FUNDACION CENT NACIONAL DE INVESTIGACIONES ONCOLOGICAS CARLOS III
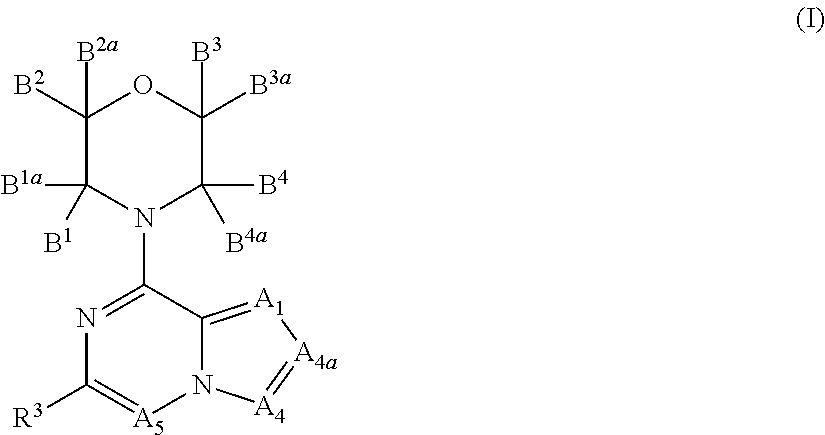
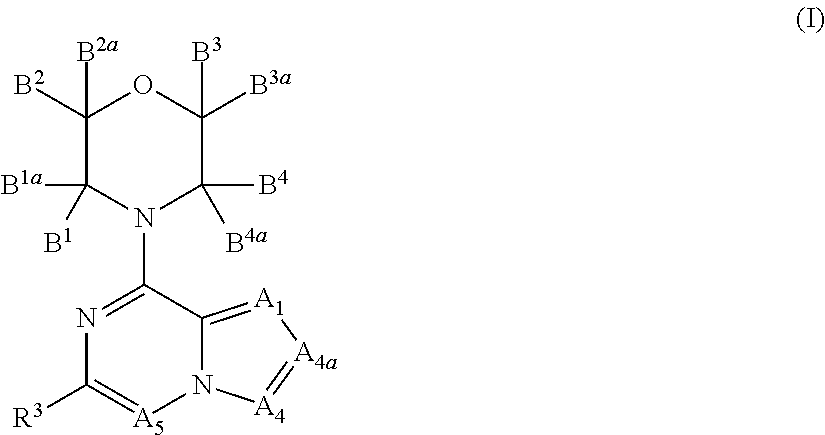
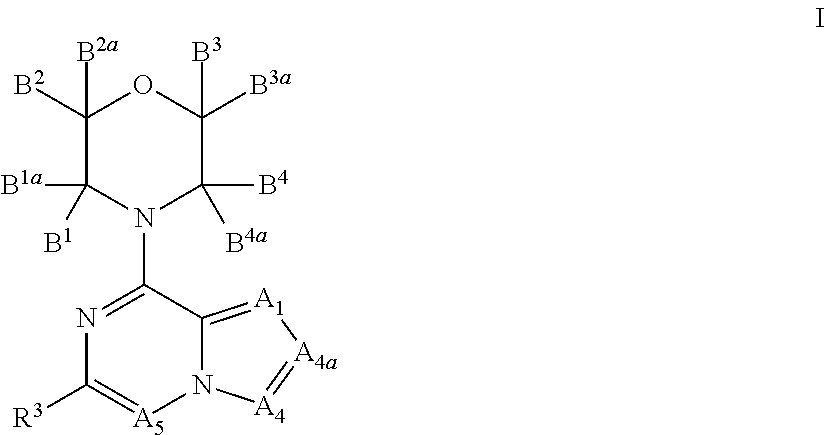
Inhibitors of pi3 kinase
ActiveUS20130053371A1Ease of detectabilityEasy to prepareBiocideNervous disorderLipid kinasesPI3-Kinase Inhibitors
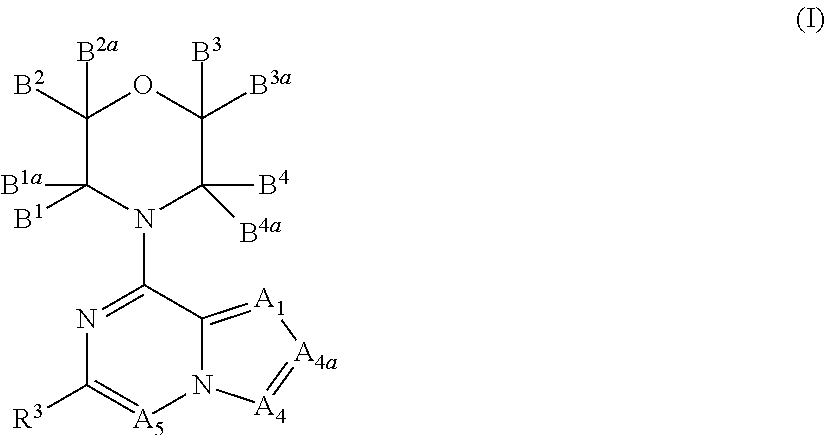
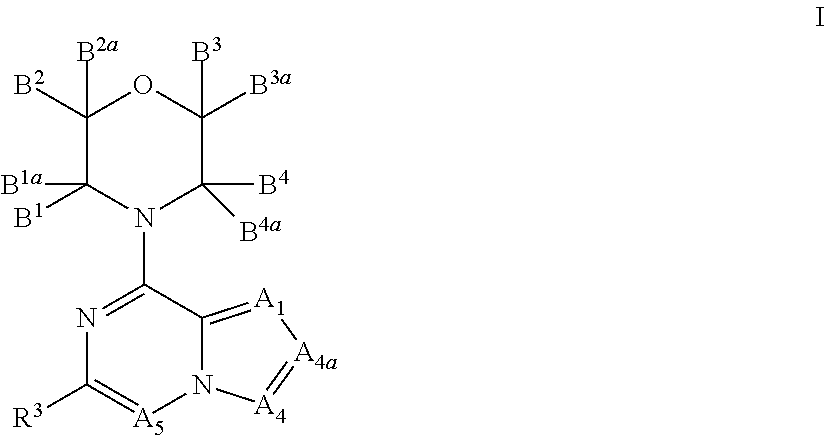
There is provided compounds of formula (I), wherein A1, A4, A4a, A5, B1, B1a, B2, B2a, B3, B3a, B4, B4a and R3 have meanings given in the description, and pharmaceutically-acceptable esters, amides, solvates or salts thereof, which compounds are useful in the treatment of diseases in which inhibition of a protein or lipid kinase (e.g. a PI3-K and / or mTOR) is desired and / or required, and particularly in the treatment of cancer or a proliferative disease.
Owner:FUNDACION CENT NACIONAL DE INVESTIGACIONES ONCOLOGICAS CARLOS III
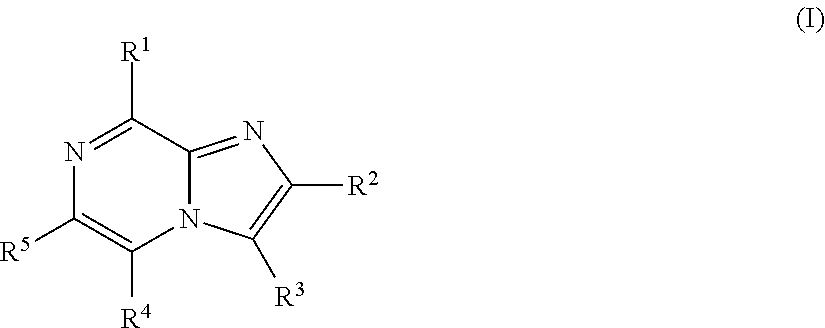
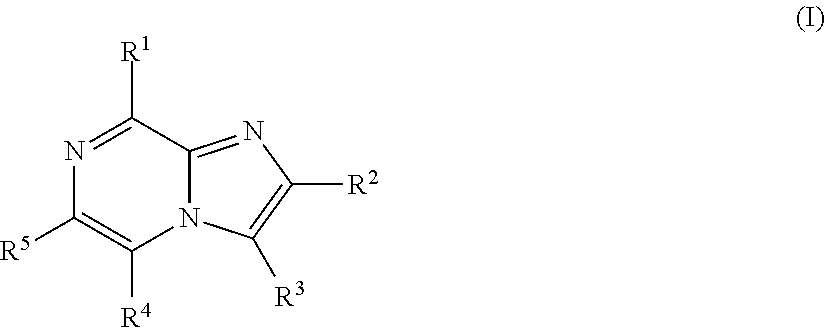
Imidazopyrazines for use as kinase inhibitors
ActiveUS20120083492A1Ease of detectabilityEasy to prepareAntibacterial agentsBiocideDiseaseLipid kinases
There is provided compounds of formula (I), wherein R1, R2, R3, R4 and R5 have meanings given in the description, and pharmaceutically-acceptable esters, amides, solvates or salts thereof, which compounds are useful in the treatment of diseases in which inhibition of a protein or lipid kinase (e.g. a PI3-K and / or mTOR) is desired and / or required, and particularly in the treatment of cancer or a proliferative disease.
Owner:FUNDACION CENT NACIONAL DE INVESTIGACIONES ONCOLOGICAS CARLOS III
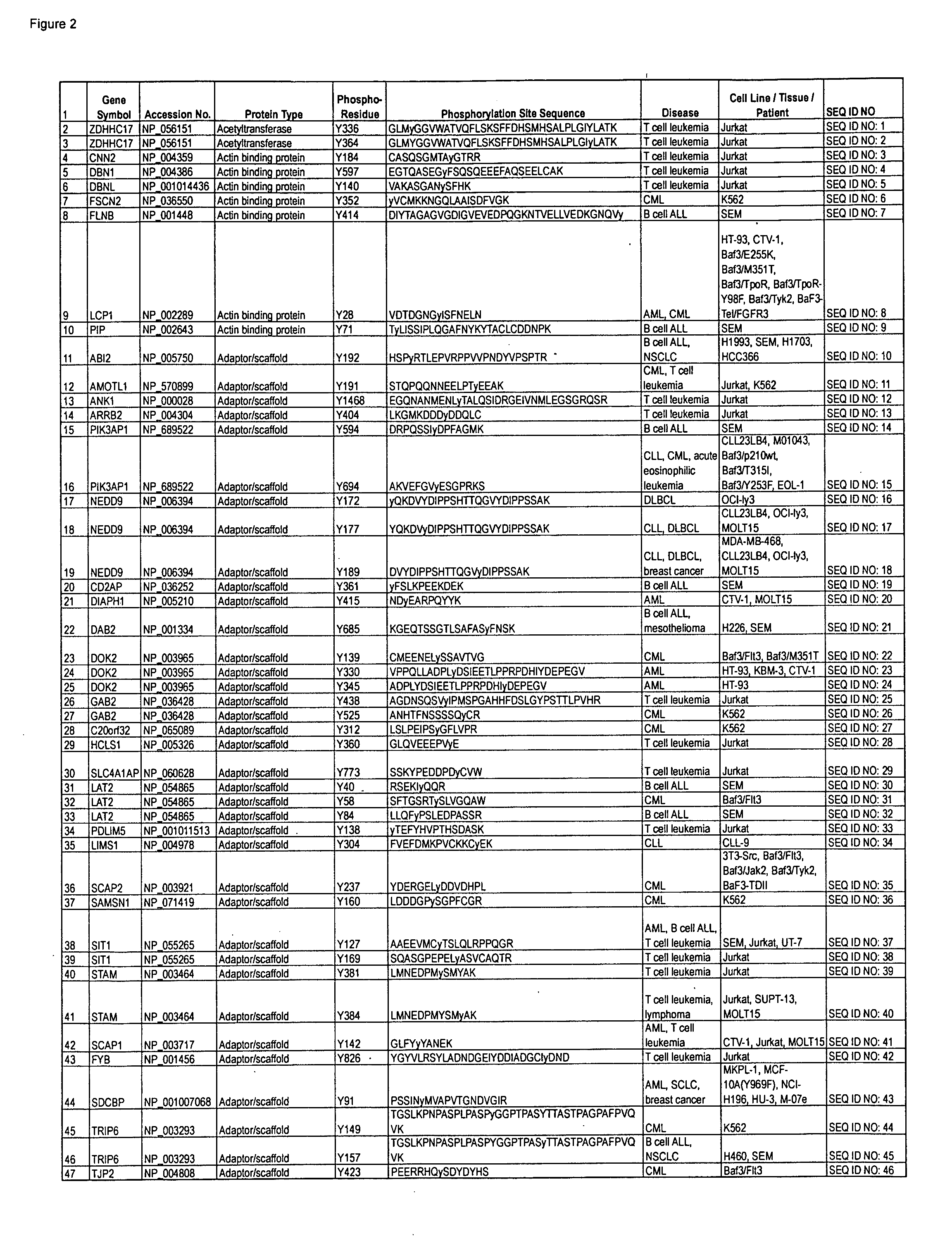
Reagents for the detection of protein phosphorylation in leukemia signaling pathways
InactiveUS20090220991A1Animal cellsImmunoglobulins against animals/humansPhosphodiesteraseLipid kinases
The invention discloses nearly 480 novel phosphorylation sites identified in signal transduction proteins and pathways underlying human Leukemia, and provides phosphorylation site specific antibodies and heavy-isotope labeled peptides (AQUA peptides) for the selective detection and quantification of these phosphorylated sites / proteins, as well as methods of using the reagents for such purpose. Among the phosphorylation sites identified are sites occurring in the following protein types: adaptor / scaffold proteins, acetyltransferases, actin binding proteins, adhesion proteins, apoptosis proteins, calcium-binding proteins, cell cycle regulation proteins, cell surface proteins, channel proteins, chaperone proteins, contractile proteins, cytokine proteins, cytoskeletal proteins, G protein regulators and GTPase activating proteins, guanine nucleotide exchange factors, helicase proteins, immunoglobulin superfamily proteins, inhibitor proteins, protein kinases, lipid kinases, ligases, lipid binding proteins, methytransferases, motor proteins, oxidoreductases, phosphotases, phosphodiesterases, phospholipases, proteases, receptor proteins, trascription factors, transferases, translation / transporter proteins, and ubiquitin conjugating system proteins.
Owner:CELL SIGNALING TECHNOLOGY
Imidazo [2, 1-B] [1, 3, 4] Thiadiazole Derivatives
ActiveUS20120094996A1Ease of detectabilityEasy to prepareBiocideNervous disorderDiseaseLipid kinases
There is provided compounds of formula (I): wherein R1, R2 and R3 have meanings given in the description, and pharmaceutically-acceptable esters, amides, solvates or salts thereof, which compounds are useful in the treatment of diseases in which inhibition of a protein or lipid kinase (e.g. PI3-K, particularly class I PI3K) is desired and / or required, and particularly in the treatment of cancer.
Owner:FUNDACION CENT NACIONAL DE INVESTIGACIONES ONCOLOGICAS CARLOS III
Substituted pyrido[2,3-b]pyrazine compounds as modulators of tyrosine kinases
The invention relates to pyrido[2,3-b]pyrazine compounds of formulae (Ia) and (Ib) which have activity to the modulation of misdirected cellular signal transduction processes such as tyrosine kinases, serine / threonine kinases and / or lipid kinases, their preparation and use as medicaments, especially for the treatment of malignant disorders and other disorders based on pathological cell proliferations:
Owner:AETERNA ZENTARIS GMBH
Triazolo [4, 5- B] Pyridin Derivatives
There is provided compounds of formula (I), wherein R1, R2, R3 and R4 have meanings given in the description, and pharmaceutically-acceptable esters, amides, solvates or salts thereof, which compounds are useful in the treatment of diseases in which inhibition of a protein or lipid kinase (e.g. a PIM family kinase, such as PIM-1, PIM-2 and / or PIM-3, and / or Flt3) is desired and / or required, and particularly in the treatment of cancer or a proliferative disease.
Owner:FUNDACION CENT NACIONAL DE INVESTIGACIONES ONCOLOGICAS CARLOS III
Conformationally restricted P13K and mTOR inhibitors
ActiveUS9556203B2Inhibit tumor growthShow activity activityOrganic active ingredientsOrganic chemistryArylLipid kinases
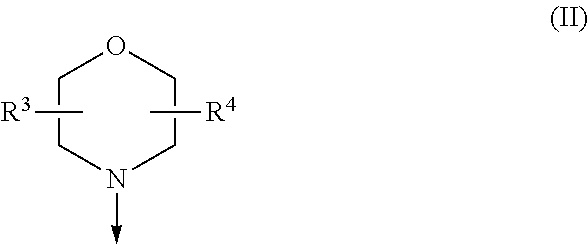
The invention relates to novel phosphoinositide 3-kinase (PI3K) and mammalian target of rapamycin (mTOR) inhibitor compounds of formula (I) and (II), which are conformationally restricted, and for which the meaning of the substituents are listed in the description. Preferred compounds are those wherein X isoxygen, R1 is morpholino and R2 is substituted phenyl or heteroaryl. These compounds are useful, either alone or in combination with further therapeutic agents, for treating disorders mediated by lipid kinases.
Owner:TORQUR AG +1
Imidazo [2, 1-B] [1, 3, 4] thiadiazole derivatives
There is provided compounds of formula (I): wherein R1, R2 and R3 have meanings given in the description, and pharmaceutically-acceptable esters, amides, solvates or salts thereof, which compounds are useful in the treatment of diseases in which inhibition of a protein or lipid kinase (e.g. PI3-K, particularly class I PI3K) is desired and / or required, and particularly in the treatment of cancer.
Owner:FUNDACION CENT NACIONAL DE INVESTIGACIONES ONCOLOGICAS CARLOS III
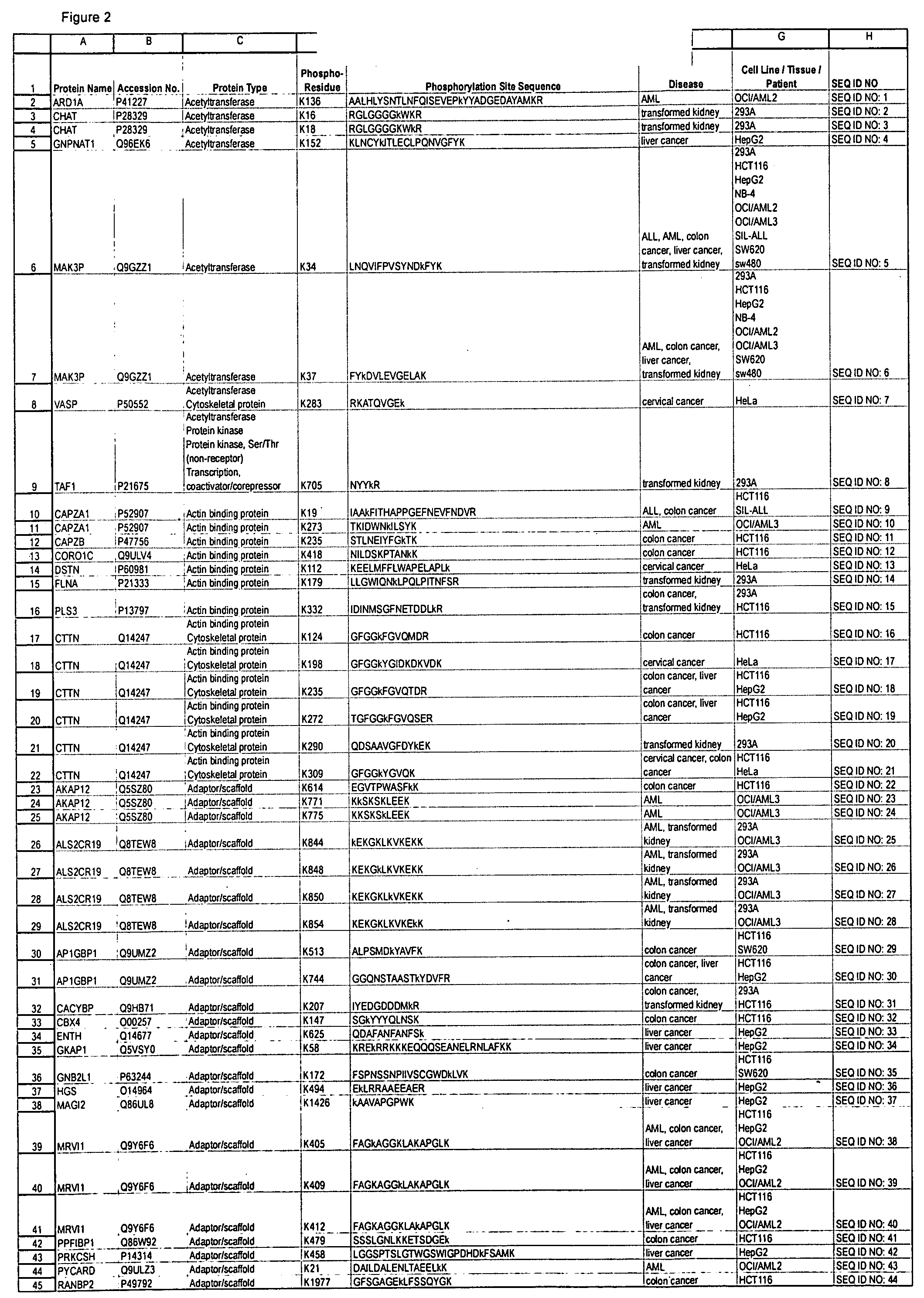
Reagens for the Detection of Protein Acetylation Signaling Pathways
InactiveUS20090124023A1Immunoglobulins against animals/humansBiological testingCell Surface ProteinsMetabolic enzymes
The invention discloses 432 novel acetylation sites identified in signal transduction proteins and pathways underlying human protein acetylation signaling pathways, and provides acetylation-site specific antibodies and heavy-isotope labeled peptides (AQUA peptides) for the selective detection and quantification of these acetylated sites / proteins, as well as methods of using the reagents for such purpose. Among the acetylation sites identified are sites occurring in the following protein types: Acetyltransferases, Adaptor / Scaffold proteins, Actin binding proteins, Adhesion proteins, Apoptosis proteins, Calcium-binding proteins, Cell Cycle Regulation proteins, Cell Surface proteins, DNA binding proteins, DNA replication proteins, Channel proteins, Chaperone proteins, Cellular Metabolism enzymes, Cytoskeletal proteins, DNA repair proteins, Endoplasmic reticulum proteins, Enzyme proteins, G protein and GTPase Activating proteins, Guanine Nucleotide Exchange Factors, Helicase proteins, Isomerase proteins, Extracelluar matrix proteins, Hydrolases, Ligase proteins, Lipid kinases, Inhibtor proteins, Lipid Binding proteins and Lyases.
Owner:CELL SIGNALING TECHNOLOGY
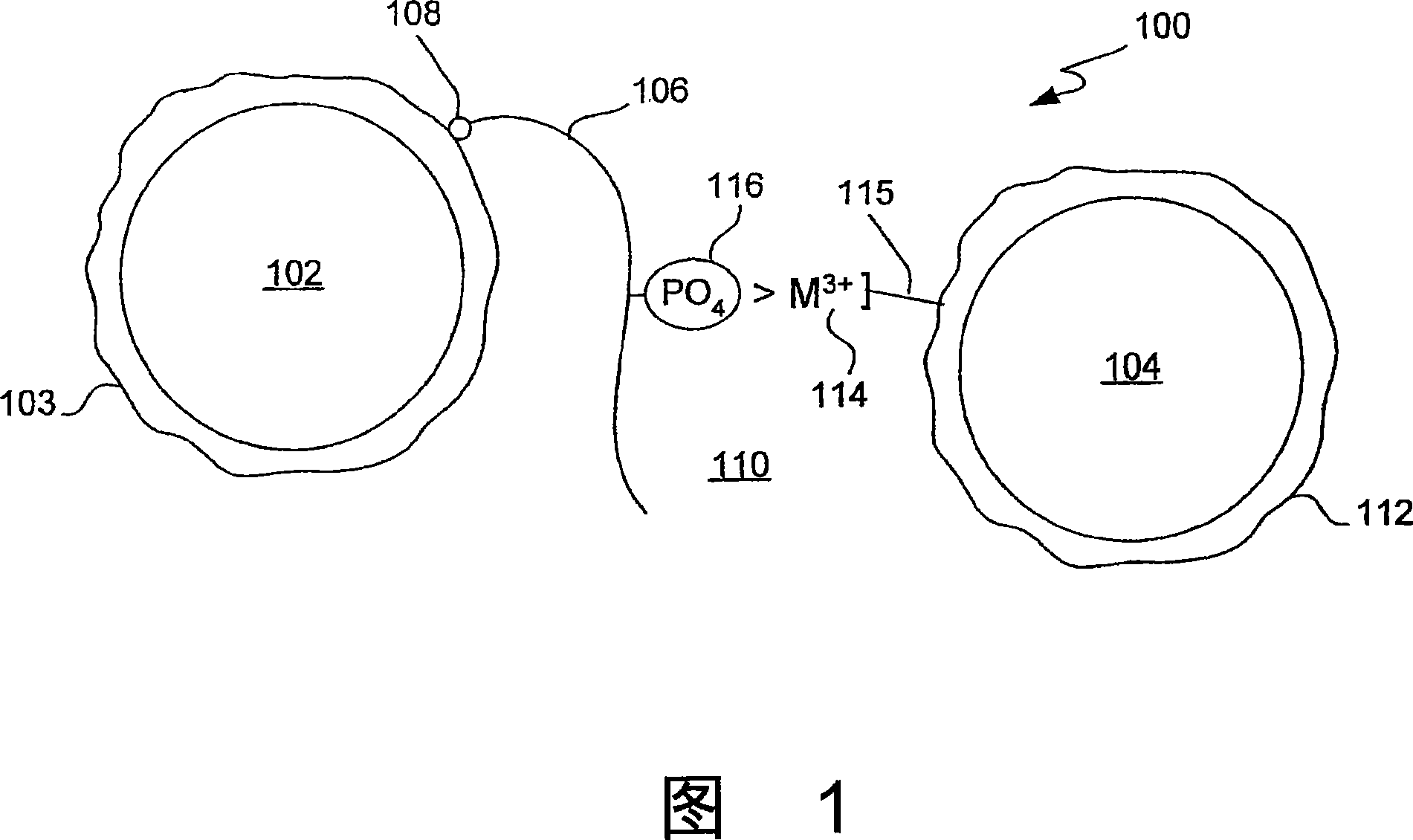
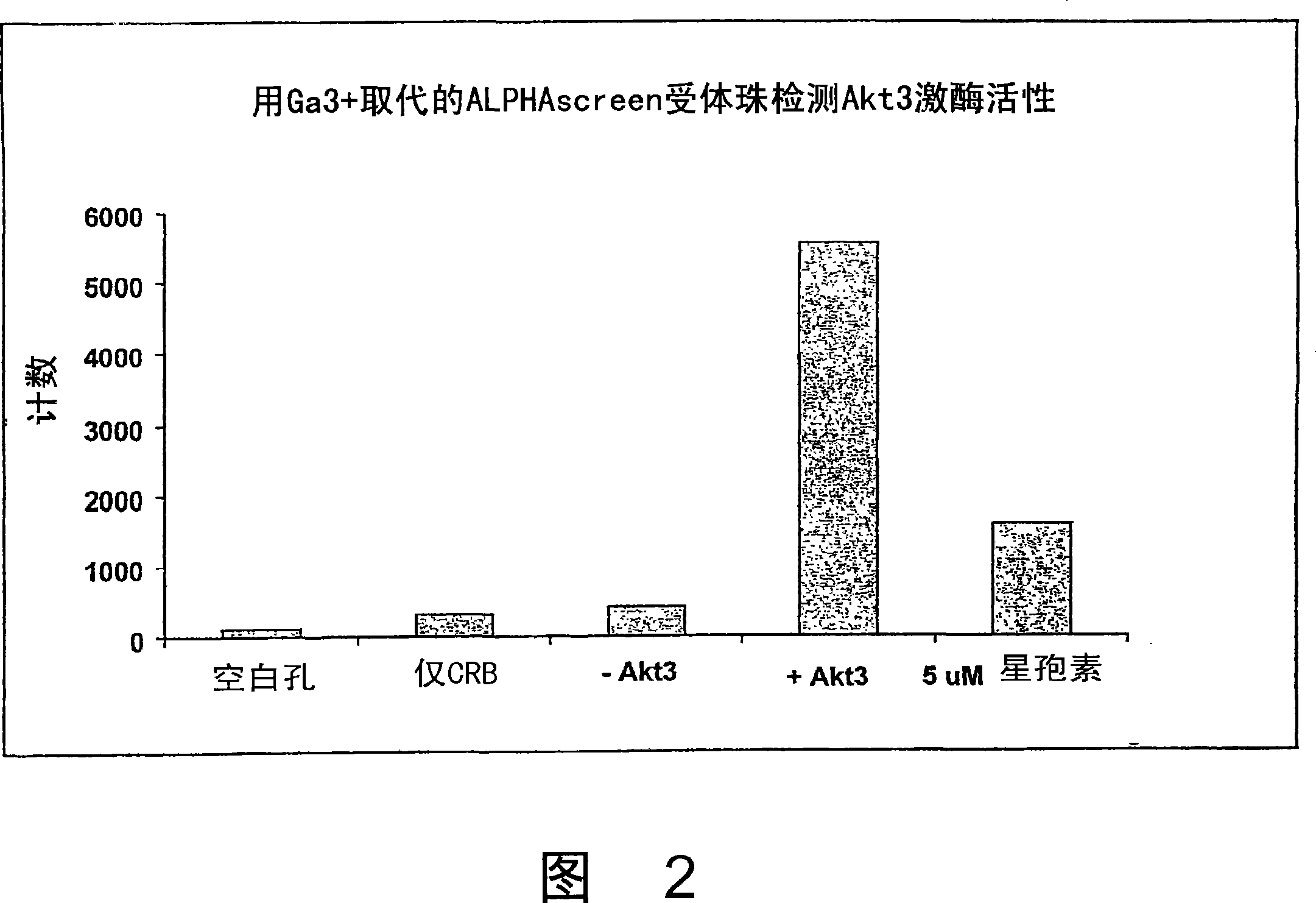
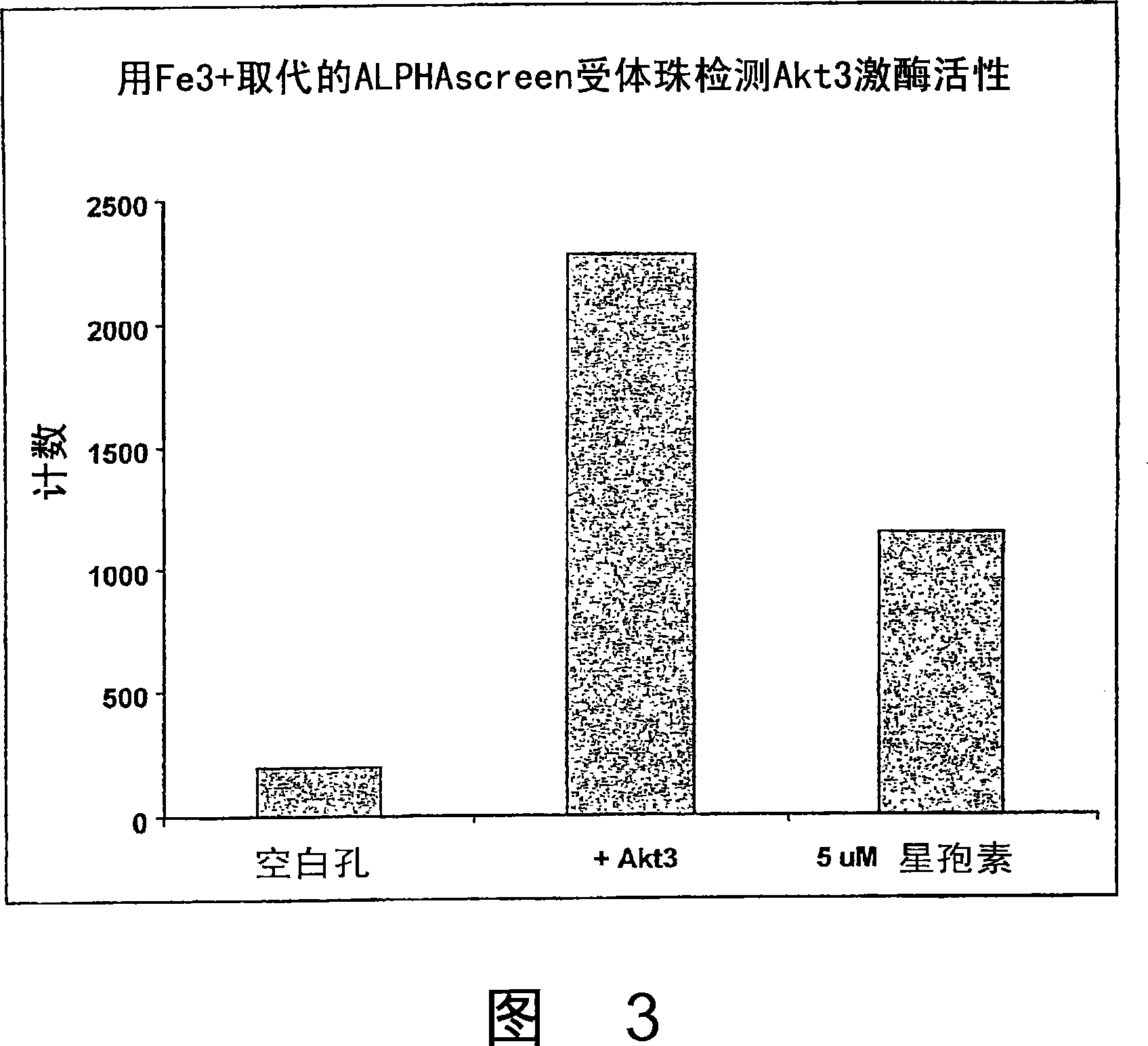
Trivalent metal mediated homogeneous luminescent proximity assay
An in vitro protein kinase assay technology that (1) exhibits a high assay signal to background ratio (S / B) and range (S-B); (2) is homogenous; (3) is non-radioactive; and (4) does not require a phospho-specific antibody involves complexing a trivalent metal ion (e.g. Ga<3+>, Fe<3+>, Al<3+>, In<3+>, Ru<3+>, Sc<3+>, Y<3+>) to the surface of amplified luminescent proximity assay acceptor or donor beads, e.g., via a suitable linker such as nitrilotriacetic acid (NTA; also referred to as carboxymethyl-lysine), iminodiacetic acid (IDA), or an appropriately substituted N-containing heterocycle, for example a triazoheterocycle, for example a triazocyclononaneononane, such as 1-propylamino-4-acetato-1,4,7-triazacyclononane. A protein (or constituent part) or other kinase substrate is bound to the surface of the other of an amplified luminescent proximity assay acceptor or donor bead and, if phosphorylated, brought into proximity with the trivalent metal ion-complexed acceptor bead to generate a luminescent signal. Presence of a kinase inhibitor inhibits phosphorylation and therefore signal generation and, in this way, is detectable. As the invention described herein recognizes the presence or absence of phosphate groups on a protein, (or constituent part), or other biological macromolecule (e.g., mono, di, or trinucleotides, cyclic nucleotides or phosphate substituted inositols), it is broadly applicable to any phosphorlylation or dephosphorylation reaction enzymes and provides a highly robust and flexible assay format for protein kinases and other enzyme classes, including lipid kinases, phosphatases, phosphodiesterases and others.
Owner:CHIRON CORP
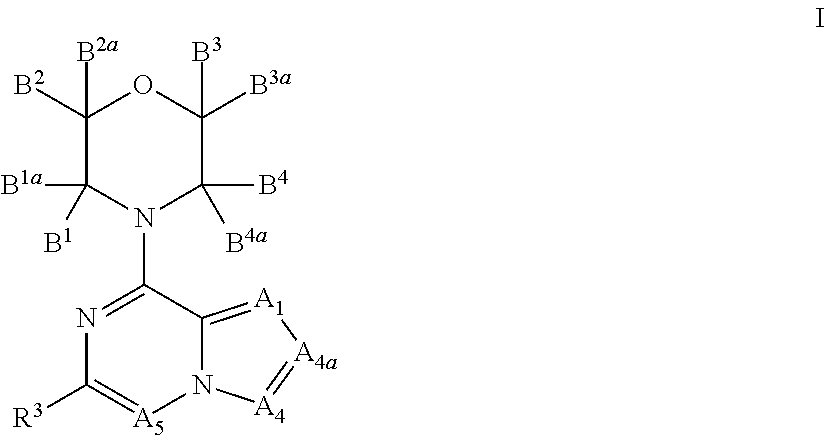
New bicyclic compounds as pi3-k and mtor inhibitors
InactiveUS20130131057A1Ease of detectabilityEasy to prepareOrganic active ingredientsNervous disorderLipid kinasesDiscovery and development of mTOR inhibitors
There is provided compounds of formula (I), wherein A1, A4, A4a, A5, B1, B1a, B2, B2a, B3, B3a, B4, B4a and R3 have meanings given in the description, and pharmaceutically-acceptable esters, amides, solvates or salts thereof, which compounds are useful in the treatment of diseases in which inhibition of a protein or lipid kinase (e.g. a PI3-K and / or mTOR) is desired and / or required, and particularly in the treatment of cancer or a proliferative disease.
Owner:FUNDACION CENT NACIONAL DE INVESTIGACIONES ONCOLOGICAS CARLOS III
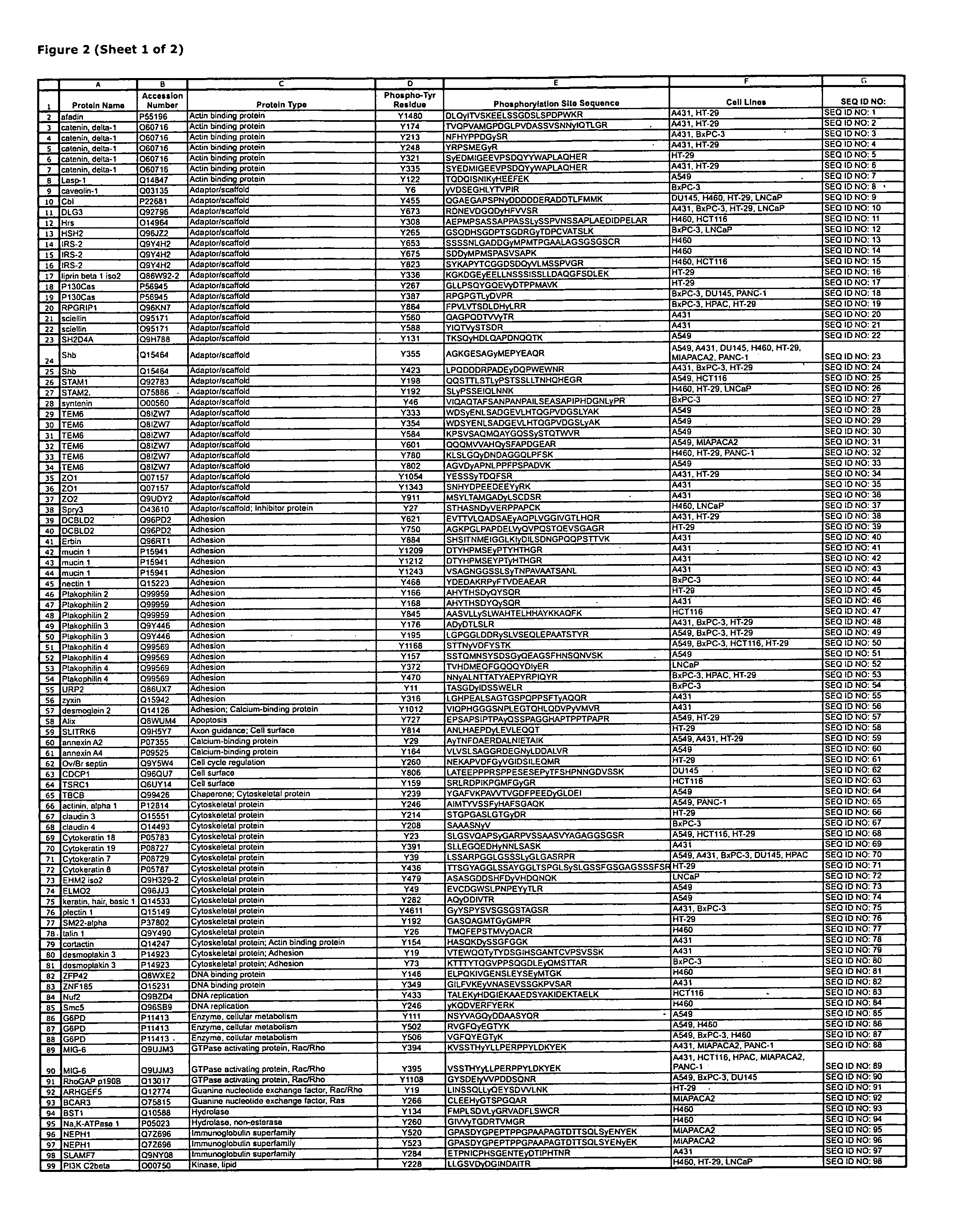
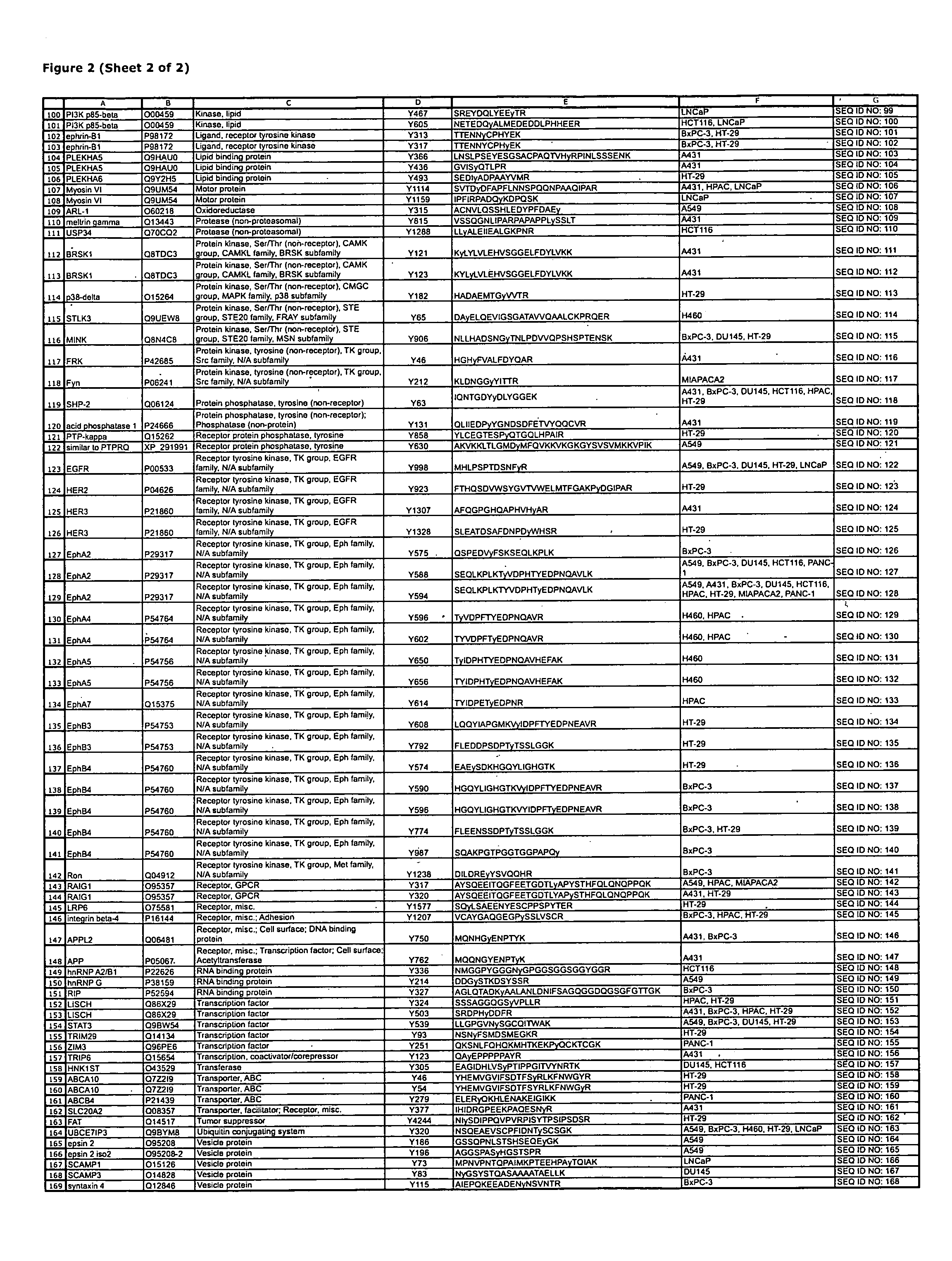
Reagents for the detection of protein phosphorylation in EGFR-signaling pathways
ActiveUS7807789B2Isotope introduction to peptides/proteinsImmunoglobulins against cell receptors/antigens/surface-determinantsADAMTS ProteinsLipid kinases
The invention discloses 168 novel phosphorylation sites identified in signal transduction proteins and pathways downstream of, and including, EGFR kinase, and provides phosphorylation-site specific antibodies and heavy-isotope labeled peptides (AQUA peptides) for the selective detection and quantification of these phosphorylated sites / proteins, as well as methods of using the reagents for such purpose. Among the phosphorylation sites identified are sites occurring in the following protein types: Actin Binding proteins, Adaptor / Scaffold proteins, Calcium-Binding Proteins, Cell Cycle Regulation proteins, Cytoskeletal proteins, DNA Binding and Replication Proteins, GTPase Activating proteins, Guanine Nucleotide Exchange Factor proteins, Lipid Kinases, Receptor Tyrosine Kinases, Receptor Tyrosine Kinase ligands, Protein Kinases, Receptor and Protein Phosphatases, Transcription Factor proteins, Tumor Suppressor proteins, and Vesicle proteins.
Owner:CELL SIGNALING TECHNOLOGY
Inhibitors of PI3 kinase
There is provided compounds of formula (I), wherein A1, A4, A4a, A5, B1, B1a, B2, B2a, B3, B3a, B4, B4a and R3 have meanings given in the description, and pharmaceutically-acceptable esters, amides, solvates or salts thereof, which compounds are useful in the treatment of diseases in which inhibition of a protein or lipid kinase (e.g. a PI3-K and / or mTOR) is desired and / or required, and particularly in the treatment of cancer or a proliferative disease.
Owner:FUNDACION CENT NACIONAL DE INVESTIGACIONES ONCOLOGICAS CARLOS III
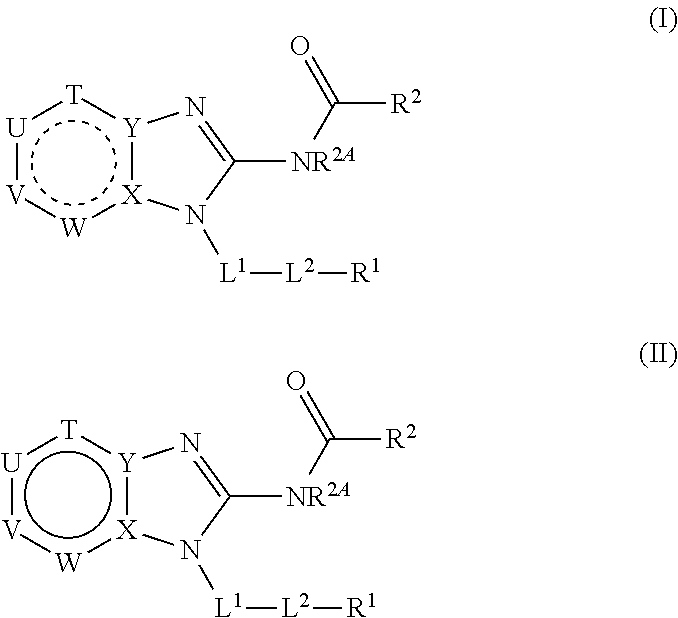
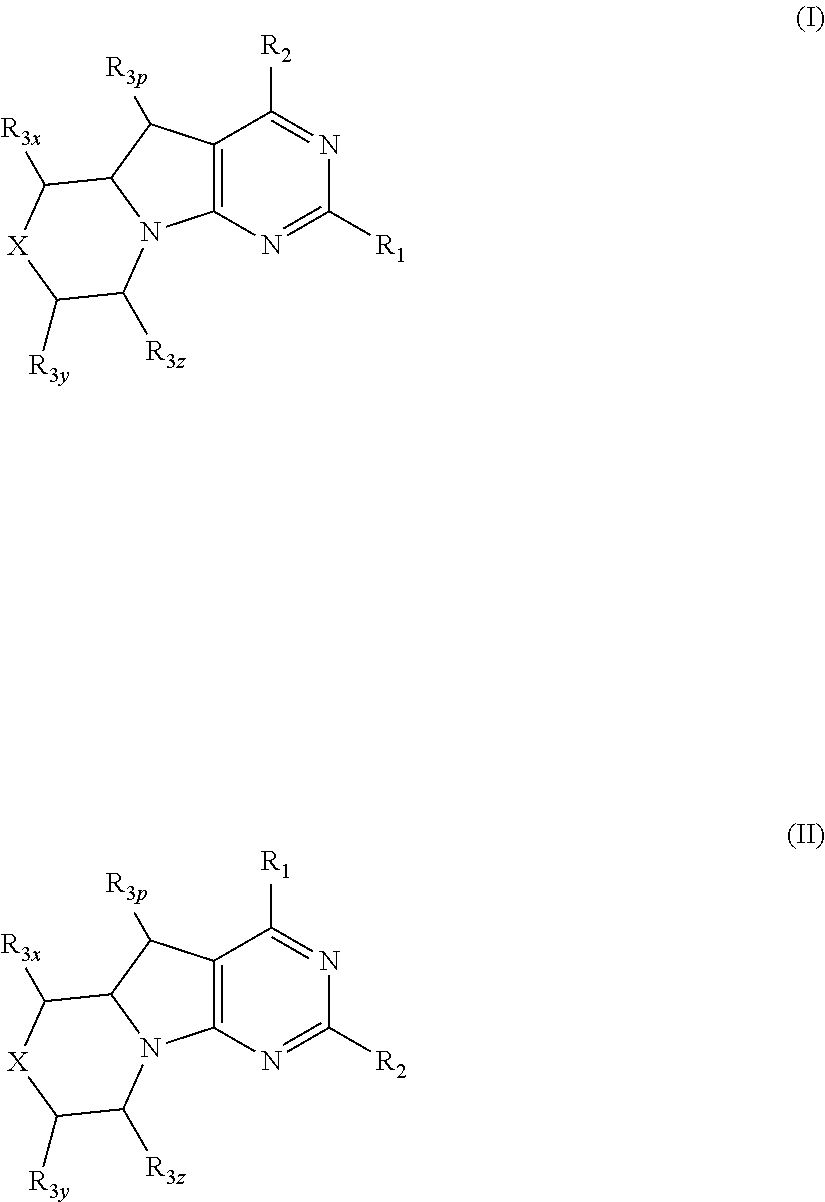
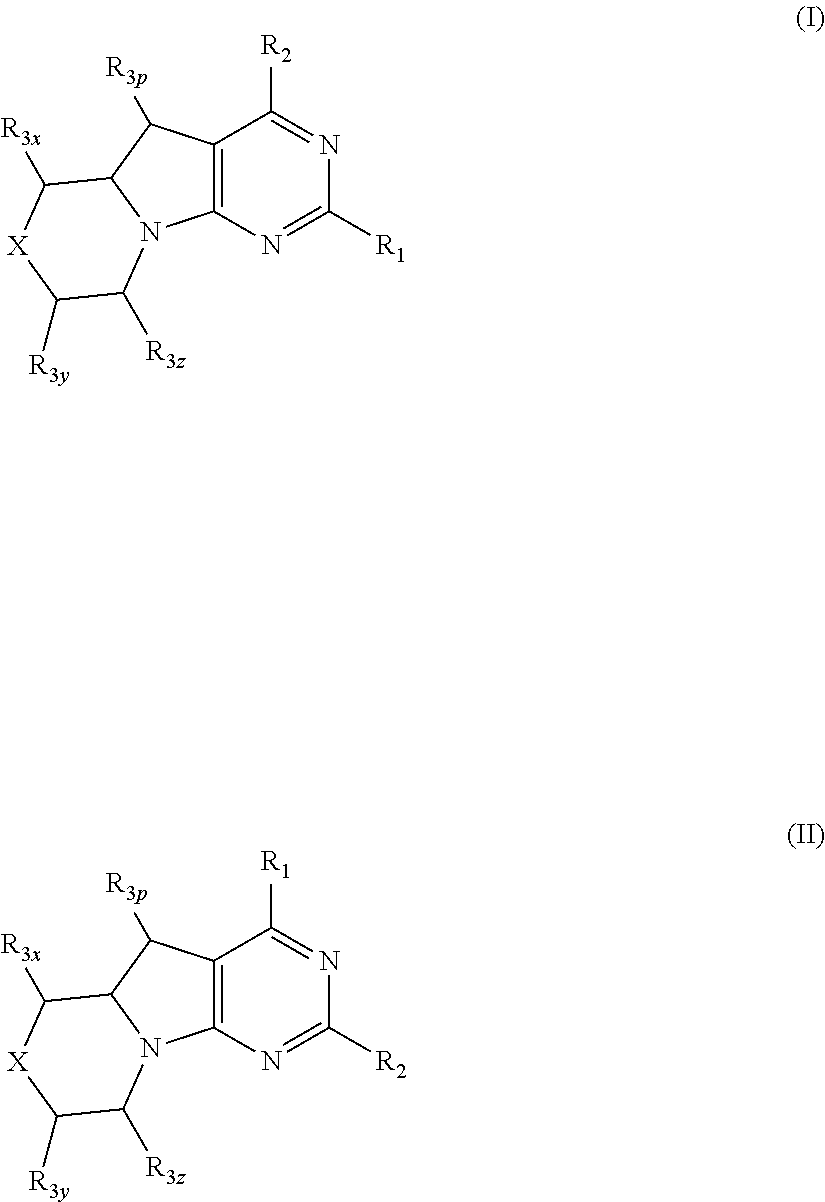
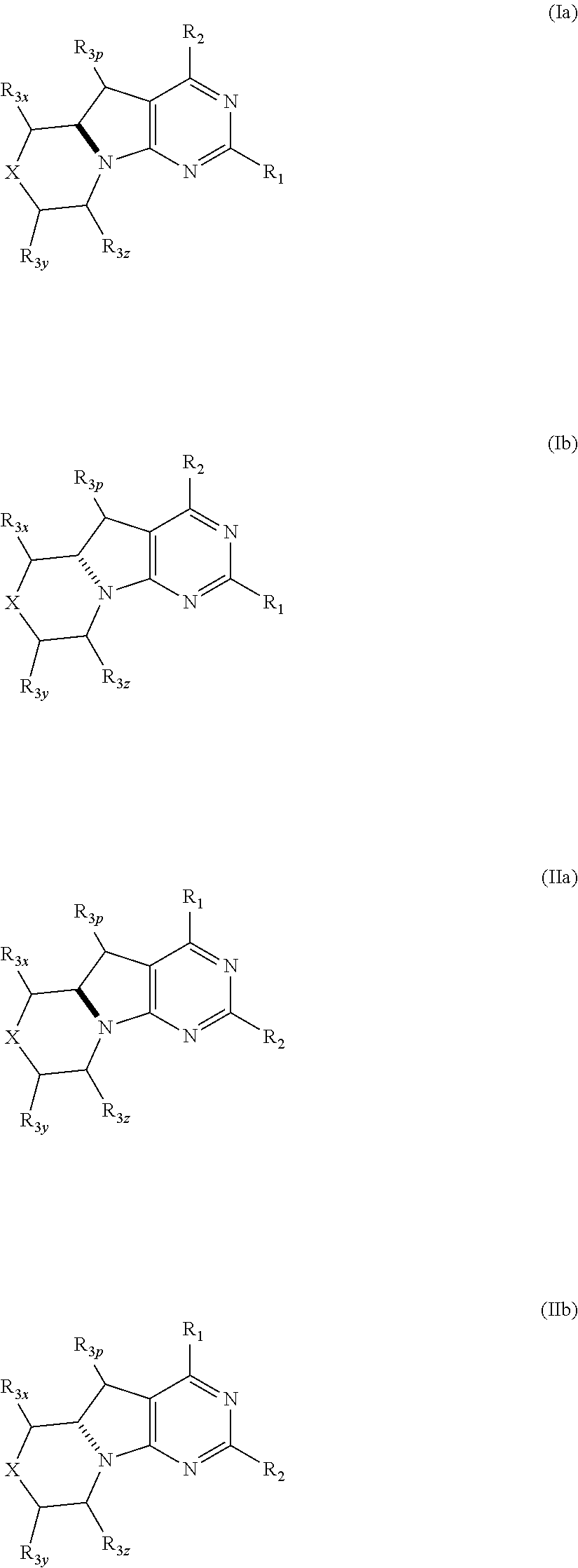
Benzimidazoles for use in the treatment of cancer and inflammatory diseases
Provided herein are methods for treating, preventing, or ameliorating one or more symptoms of a condition, disorder, or disease mediated by a lipid kinase or a protein kinase with benzimidazoles, for example, of Formula I or II, and pharmaceutical compositions thereof. Also provided herein are benzimidazoles, and pharmaceutical compositions thereof; and methods of their use for treating, preventing, or ameliorating one or more symptoms of a proliferative disease.
Owner:CAPELLA THERAPEUTICS
Benzimidazoles for use in the treatment of cancer and inflammatory diseases
Provided herein are methods for treating, preventing, or ameliorating one or more symptoms of a condition, disorder, or disease mediated by a lipid kinase or a protein kinase with benzimidazoles, for example, of Formula I or II, and pharmaceutical compositions thereof. Also provided herein are benzimidazoles, and pharmaceutical compositions thereof; and methods of their use for treating, preventing, or ameliorating one or more symptoms of a proliferative disease.
Owner:CAPELLA THERAPEUTICS
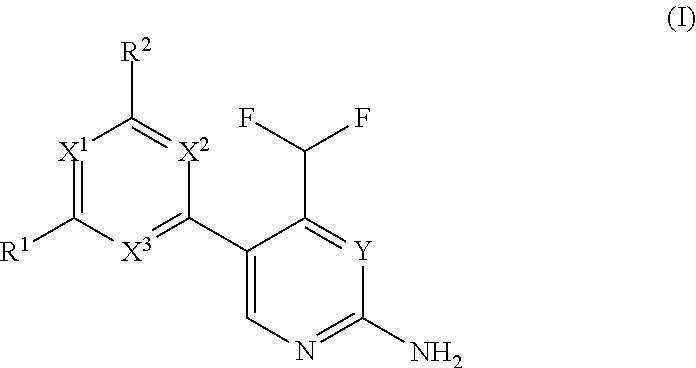
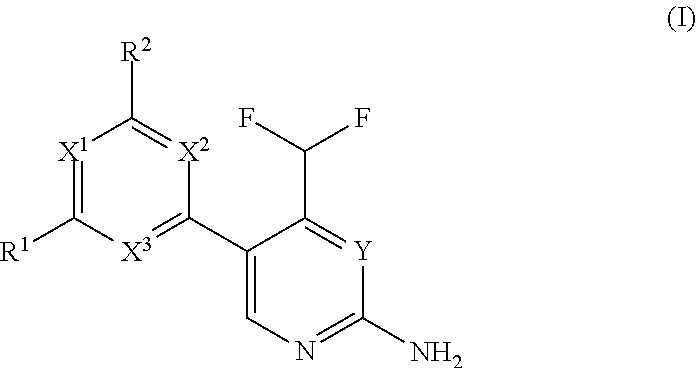
Difluoromethyl-aminopyridines and difluoromethyl-aminopyrimidines
The invention relates to novel phosphoinositide 3-kinase (PI3k), mammalian target of rapamycin (mTOR) and PI3k-related kinase (PIKKs) inhibitor compounds of formula (I),wherein X1, X2 and X3 are N or CH, with the proviso that at least two of X1, X2 and X3 are N; Y is N or CH, These compounds are useful, either alone or in combination with further therapeutic agents, for treating disorders mediated by lipid kinases.
Owner:UNIVERSITY OF BASEL +1
Assaying apparatus, kit, and method for lipids and associated enzymes
Lipid assay method, kit, and apparatus involving exposure of a protein, having a lipid recognition motif that interacts with a target lipid and a competing lipid, to a solution containing the competing lipid, and determining whether the target lipid is present in the solution. The target lipid has a stronger affinity to the lipid recognition motif than does the competing lipid. The lipid recognition motif is preferably a pleckstrin homology (PH) domain, with the target lipid being a phosphoinositide. The assay determines activity of a lipid kinase, the target lipid being a phosphorylation product of a reaction between the lipid kinase and a substrate lipid. The assay can be a cancer screening method for detection of cancer cells, where detection of certain levels of a PI(3,4,5)P3 target lipid is an indicator of a cancer cell.
Owner:ECHELON BIOSCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![Substituted pyrazolo[3,4-D]pyrimidines as kinase antagonists Substituted pyrazolo[3,4-D]pyrimidines as kinase antagonists](https://images-eureka.patsnap.com/patent_img/ab597247-dd6a-4821-a734-90c4dd7ae56f/US07585868-20090908-D00001.png)
![Substituted pyrazolo[3,4-D]pyrimidines as kinase antagonists Substituted pyrazolo[3,4-D]pyrimidines as kinase antagonists](https://images-eureka.patsnap.com/patent_img/ab597247-dd6a-4821-a734-90c4dd7ae56f/US07585868-20090908-D00002.png)
![Substituted pyrazolo[3,4-D]pyrimidines as kinase antagonists Substituted pyrazolo[3,4-D]pyrimidines as kinase antagonists](https://images-eureka.patsnap.com/patent_img/ab597247-dd6a-4821-a734-90c4dd7ae56f/US07585868-20090908-D00003.png)





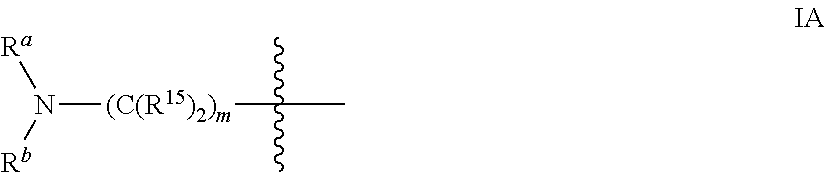
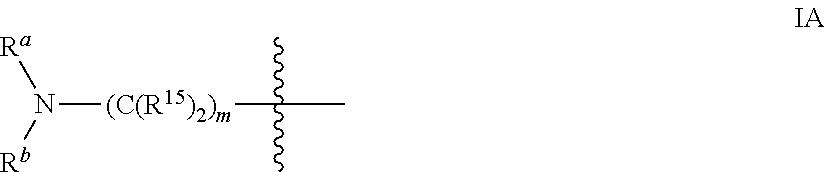
![Imidazo [2, 1-B] [1, 3, 4] Thiadiazole Derivatives Imidazo [2, 1-B] [1, 3, 4] Thiadiazole Derivatives](https://images-eureka.patsnap.com/patent_img/1be20692-6c9a-4292-98a2-ed5d84e440e4/US20120094996A1-20120419-C00001.png)
![Imidazo [2, 1-B] [1, 3, 4] Thiadiazole Derivatives Imidazo [2, 1-B] [1, 3, 4] Thiadiazole Derivatives](https://images-eureka.patsnap.com/patent_img/1be20692-6c9a-4292-98a2-ed5d84e440e4/US20120094996A1-20120419-C00002.png)
![Imidazo [2, 1-B] [1, 3, 4] Thiadiazole Derivatives Imidazo [2, 1-B] [1, 3, 4] Thiadiazole Derivatives](https://images-eureka.patsnap.com/patent_img/1be20692-6c9a-4292-98a2-ed5d84e440e4/US20120094996A1-20120419-C00003.png)
![Substituted pyrido[2,3-b]pyrazine compounds as modulators of tyrosine kinases Substituted pyrido[2,3-b]pyrazine compounds as modulators of tyrosine kinases](https://images-eureka.patsnap.com/patent_img/1d00d12d-6f15-44fd-86ad-da68e90571ab/US08202883-20120619-C00001.png)
![Substituted pyrido[2,3-b]pyrazine compounds as modulators of tyrosine kinases Substituted pyrido[2,3-b]pyrazine compounds as modulators of tyrosine kinases](https://images-eureka.patsnap.com/patent_img/1d00d12d-6f15-44fd-86ad-da68e90571ab/US08202883-20120619-C00002.png)
![Substituted pyrido[2,3-b]pyrazine compounds as modulators of tyrosine kinases Substituted pyrido[2,3-b]pyrazine compounds as modulators of tyrosine kinases](https://images-eureka.patsnap.com/patent_img/1d00d12d-6f15-44fd-86ad-da68e90571ab/US08202883-20120619-C00003.png)
![Triazolo [4, 5- B] Pyridin Derivatives Triazolo [4, 5- B] Pyridin Derivatives](https://images-eureka.patsnap.com/patent_img/9516906a-c6f1-42c2-82de-8ec4716318c6/US20130065883A1-20130314-C00001.png)
![Triazolo [4, 5- B] Pyridin Derivatives Triazolo [4, 5- B] Pyridin Derivatives](https://images-eureka.patsnap.com/patent_img/9516906a-c6f1-42c2-82de-8ec4716318c6/US20130065883A1-20130314-C00002.png)
![Triazolo [4, 5- B] Pyridin Derivatives Triazolo [4, 5- B] Pyridin Derivatives](https://images-eureka.patsnap.com/patent_img/9516906a-c6f1-42c2-82de-8ec4716318c6/US20130065883A1-20130314-C00003.png)
![Imidazo [2, 1-B] [1, 3, 4] thiadiazole derivatives Imidazo [2, 1-B] [1, 3, 4] thiadiazole derivatives](https://images-eureka.patsnap.com/patent_img/4db7aedd-ac7c-4c6e-b6cf-59e2e9307f4c/US08815918-20140826-C00001.png)
![Imidazo [2, 1-B] [1, 3, 4] thiadiazole derivatives Imidazo [2, 1-B] [1, 3, 4] thiadiazole derivatives](https://images-eureka.patsnap.com/patent_img/4db7aedd-ac7c-4c6e-b6cf-59e2e9307f4c/US08815918-20140826-C00002.png)
![Imidazo [2, 1-B] [1, 3, 4] thiadiazole derivatives Imidazo [2, 1-B] [1, 3, 4] thiadiazole derivatives](https://images-eureka.patsnap.com/patent_img/4db7aedd-ac7c-4c6e-b6cf-59e2e9307f4c/US08815918-20140826-C00003.png)