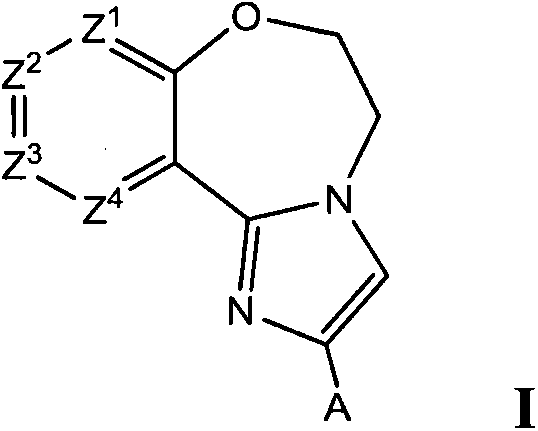
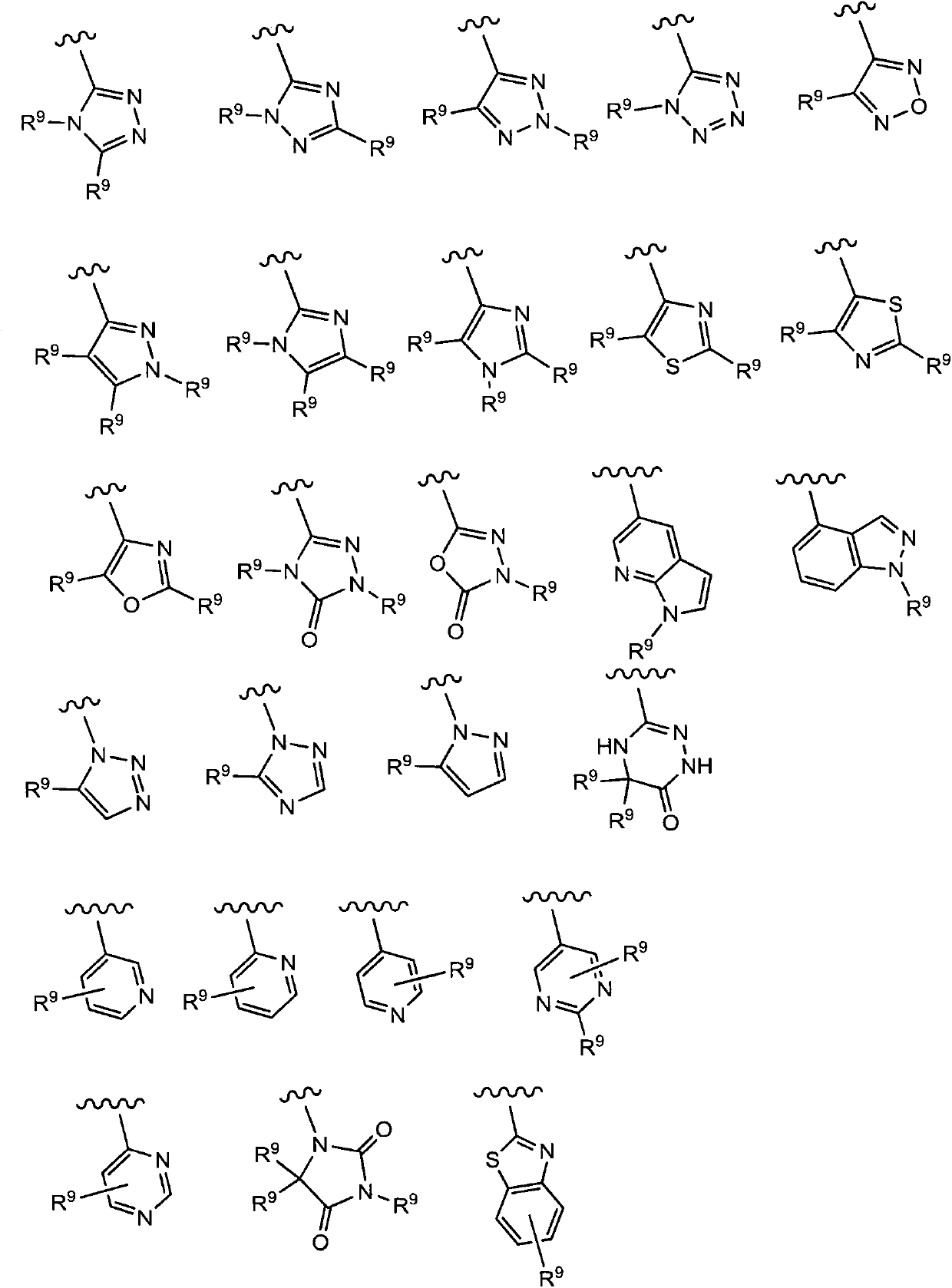
Benzoxazepin compounds selective for pi3k p110 delta and methods of use
A compound, the technology of C1-C12, is applied in the field of diagnosing or treating mammalian cells or related pathological diseases, and compounds that inhibit the activity of PI3 kinase, and can solve the problems of B cell proliferation, signal transduction loss, and signal transduction damage.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0527] Example 1 2-{4-[2-(2-isopropyl-2H-[1,2,4]triazol-3-yl)-4,5-dihydro-6-oxa-1,3a-diazepine Heterobenzo[e]azulene-9-ylsulfanyl]piperidin-1-yl}-2-methylpropan-1-ol
[0528]
[0529] Step 1: Methyl 2-(4-mercaptopiperidin-1-yl)-2-methylpropanoate
[0530]
[0531] H 2 S gas was bubbled through a solution of methyl 2-methyl-2-(4-oxopiperidin-1-yl)propanoate (1.28 g, 6.42 mmol) in isopropanol (13 mL) for 10 min. The reaction mixture was then closed and stirred at RT for 18 h, followed by standing at RT for 8 days. After this time, nitrogen was bubbled through the solution for 10 min, and then NaBH was added 4 (364 mg, 9.63 mmol). The reaction mixture was stirred at RT for 10 min, then heated at 80 °C for 2 h. After cooling to RT, the volatiles were removed under reduced pressure and the resulting residue was partitioned between diethyl ether and water. The aqueous layer was further extracted with ether, and the combined organic phases were washed with water, then b...
Embodiment 2
[0536] Example 2 2-(2-isopropyl-2H-[1,2,4]triazol-3-yl)-9-(piperidin-4-ylsulfanyl)-4,5-dihydro-6-oxo Hetero-1,3a-diazabenzo[e]azulene
[0537]
[0538] Step 1: 4-[2-(2-Isopropyl-2H-[1,2,4]triazol-3-yl)-4,5-dihydro-6-oxa-1,3a-diazepine Heterobenzo[e]azulene-9-ylsulfanyl]piperidine-1-carboxylate tert-butyl ester
[0539]
[0540] 9-Bromo-2-(2-isopropyl-2H-[1,2,4]triazol-3-yl)-4,5-dihydro-6-oxa-1, 3a-diazabenzo[e]azulene (100mg, 0.267mmol), tert-butyl 4-mercaptopiperidine-1-carboxylate (87mg, 0.401mmol), Pd 2 (dba) 3 (13 mg, 5 mol%), XantPhos (15 mg, 10 mol%) and DIPEA (0.19 mL) in dioxane (3 mL) was purged with nitrogen and then heated at 120° C. for 1 h using microwave irradiation. Using 9-bromo-2-(2-isopropyl-2H-[1,2,4]triazol-3-yl)-4,5-dihydro-6-oxa-1,3a-diazepine Benzo[e]azulene (400mg, 1.068mmol), tert-butyl 4-mercaptopiperidine-1-carboxylate (348mg, 1.604mmol), Pd 2 (dba) 3 (50 mg, 5 mol%), a mixture of XantPhos (61 mg, 10 mol%) and DIPEA (0.745 mL) in dioxa...
Embodiment 3
[0545] Example 3 2-Methyl-2-[4-(2-pyridin-2-yl-4,5-dihydro-6-oxa-1,3a-diazabenzo[e]azulene-9-ylsulfane Base) piperidin-1-yl] propan-1-ol
[0546]
[0547] Step 1: 9-Bromo-2-pyridin-2-yl-4,5-dihydro-6-oxa-1,3a-diazabenzo[e]azulene
[0548]
[0549] 9-Bromo-2-iodo-4,5-dihydro-6-oxa-1,3a-diazabenzo[e]azulene (200mg, 0.512mmol), 2-tributylstannylpyridine (226g , 0.614mmol), Pd 2 (PPh 3 ) 2 Cl 2 (36 mg, 0.05 mmol) and copper iodide (29 mg, 0.15 mmol) in DMF (4 mL) was purged with nitrogen, then heated at 100 °C for 1 h using microwave irradiation. The reaction mixture was diluted with MeOH (20 mL), then loaded into SCX-2 cartridge, which was washed with MeOH, and the product was washed with 0.5M NH 3 / MeOH elution. Fractions containing product were combined and concentrated in vacuo. The resulting residue was purified by column chromatography (Si-PCC, gradient 0-8% MeOH in DCM, then Si-PCC, gradient 0-7% MeOH in EtOAc) to provide the title compound as a cream soli...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com