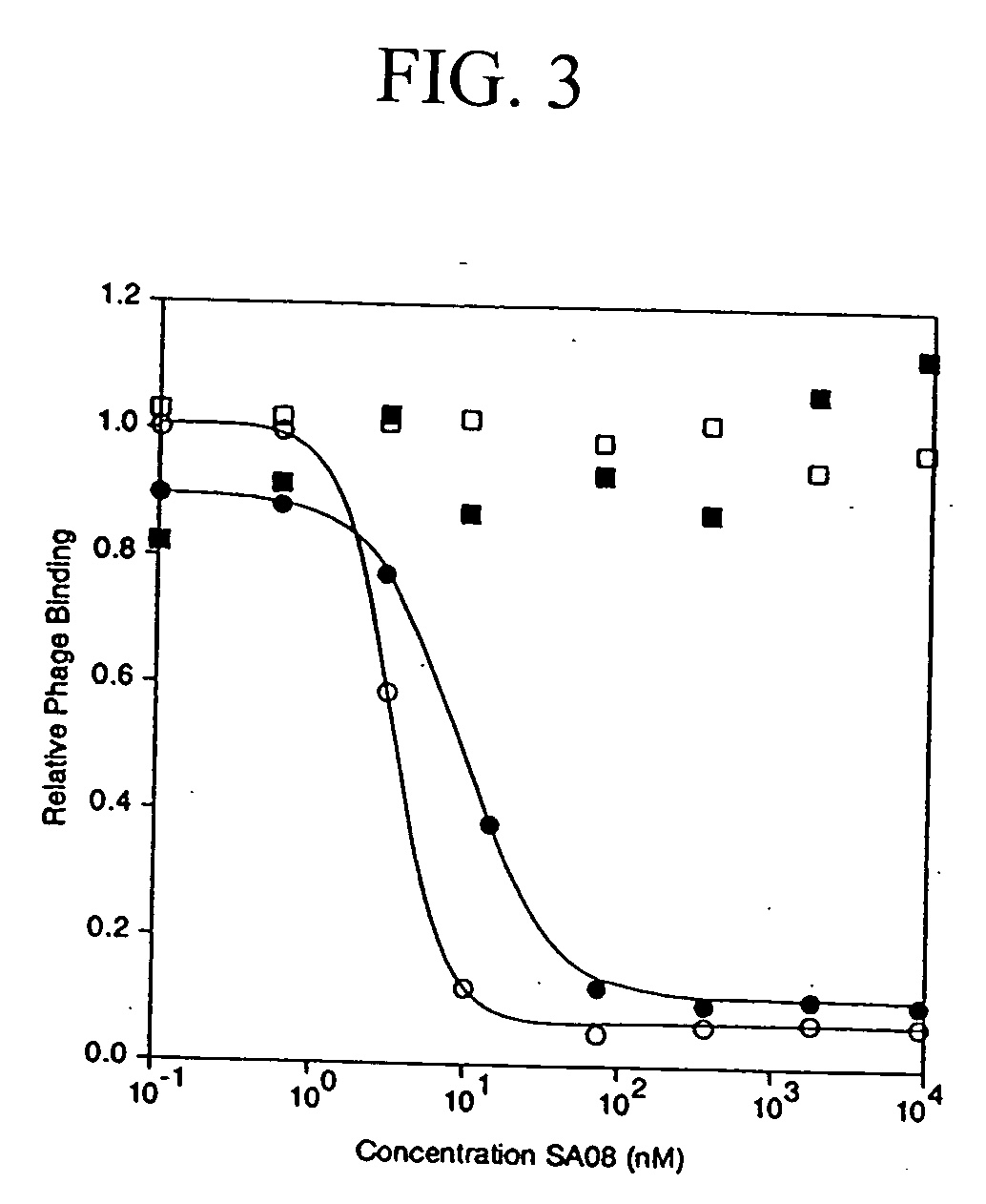
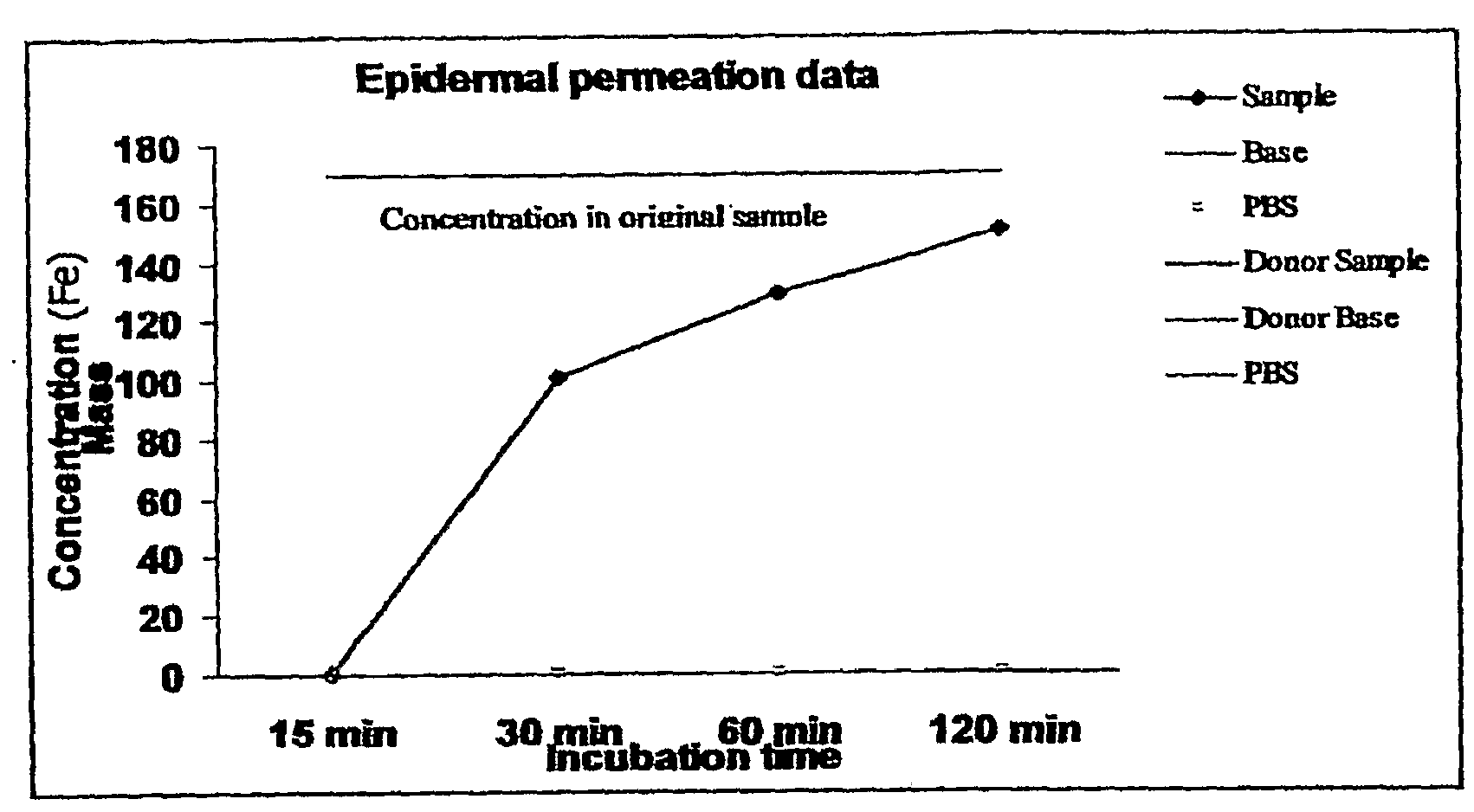
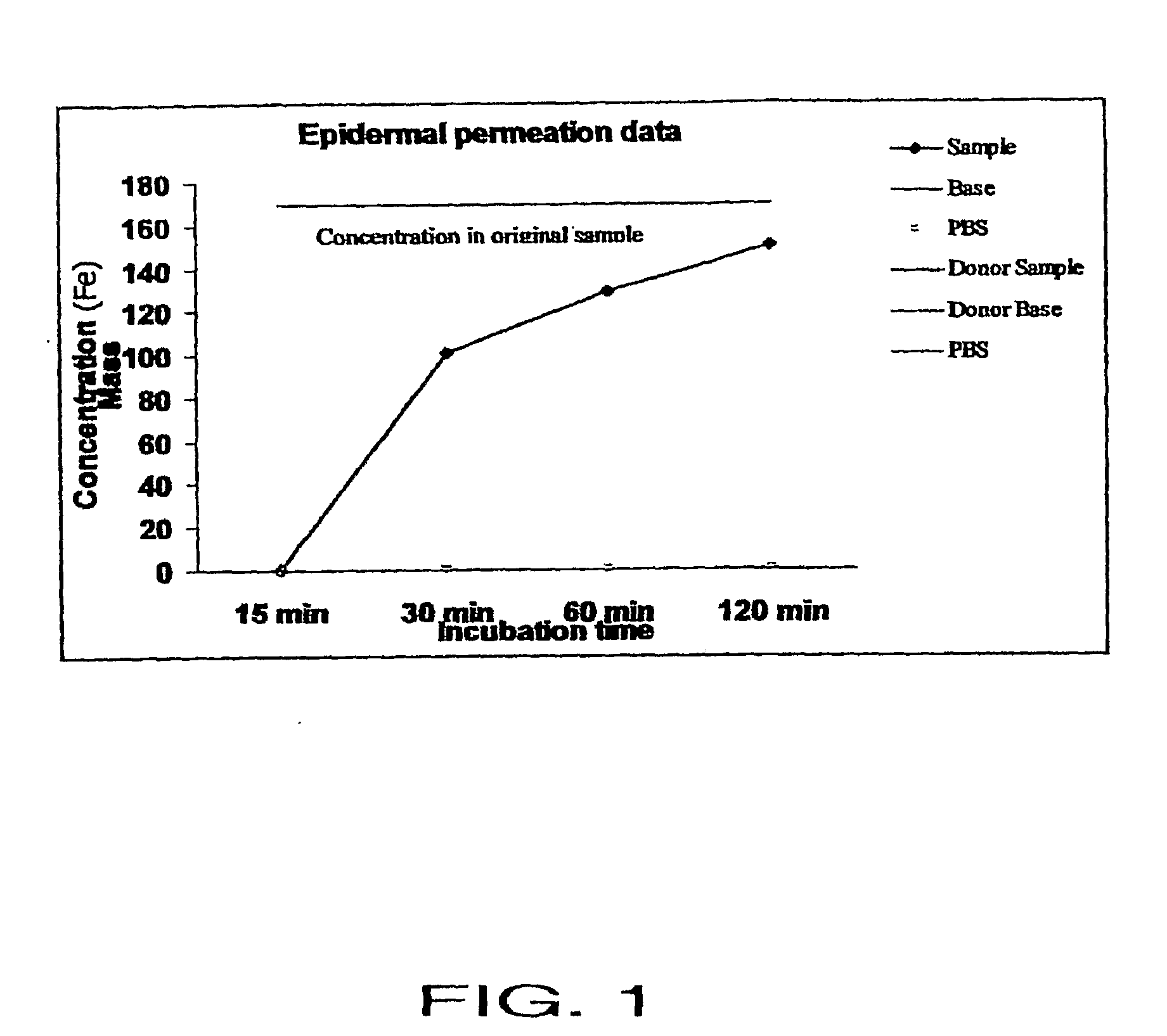
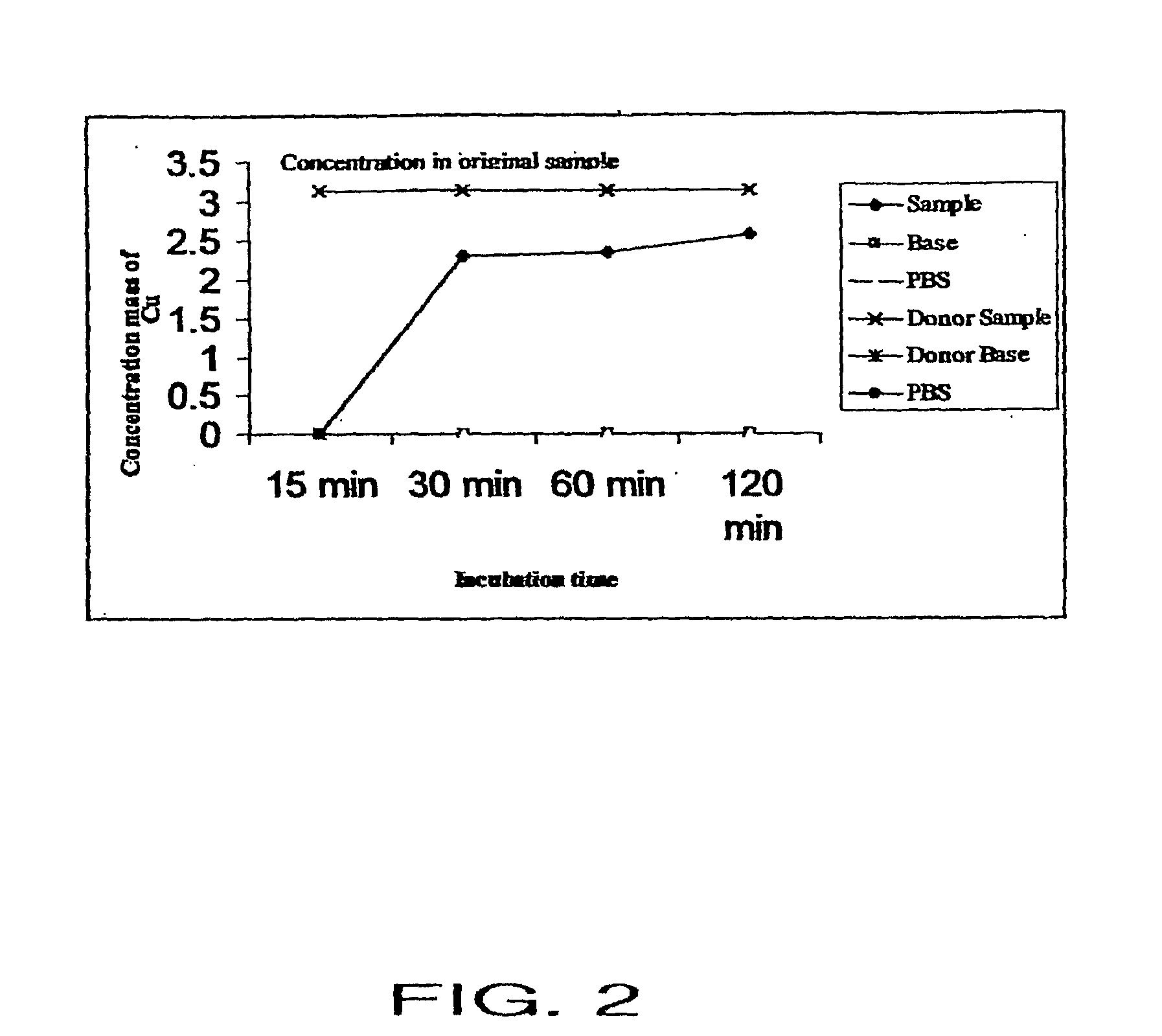
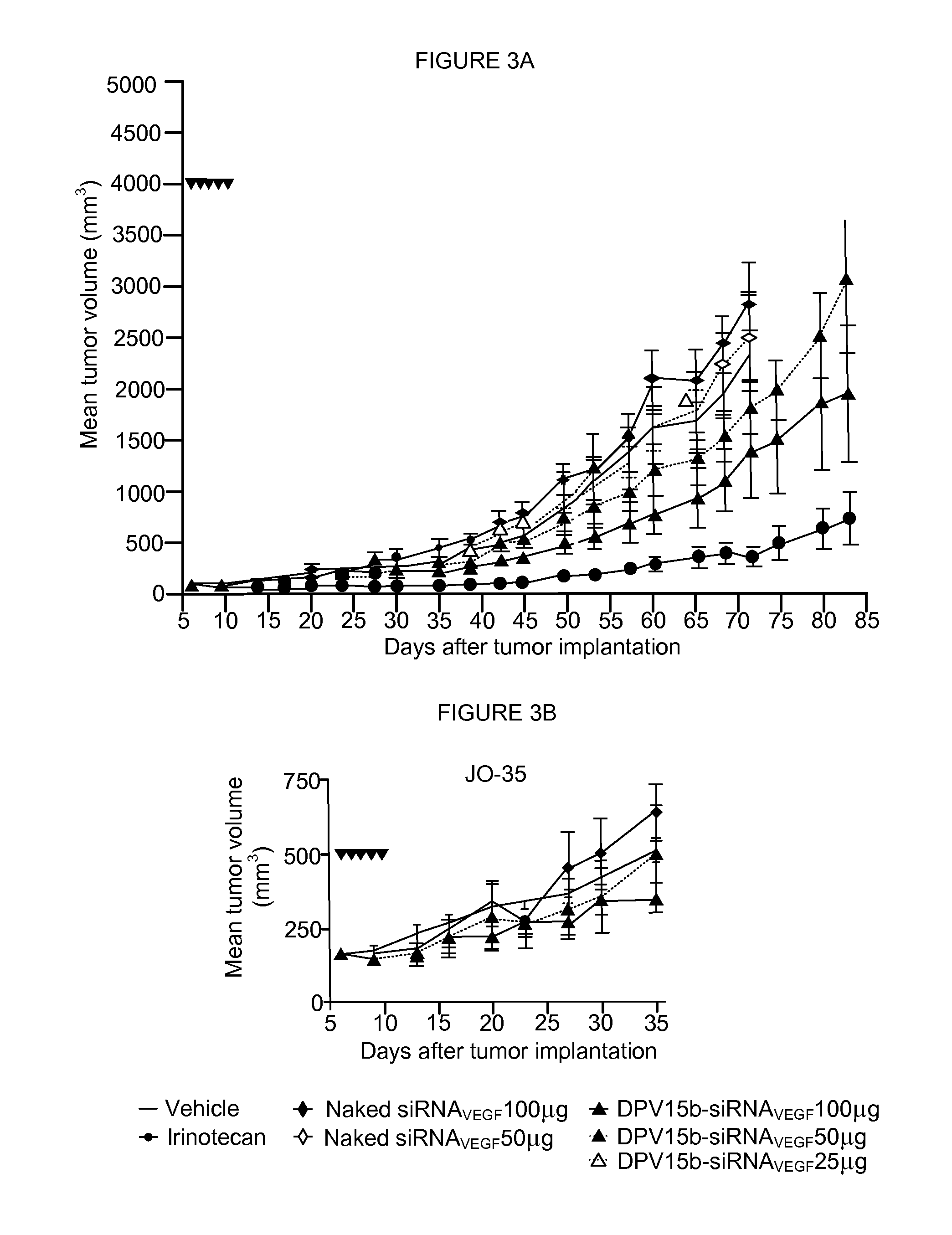
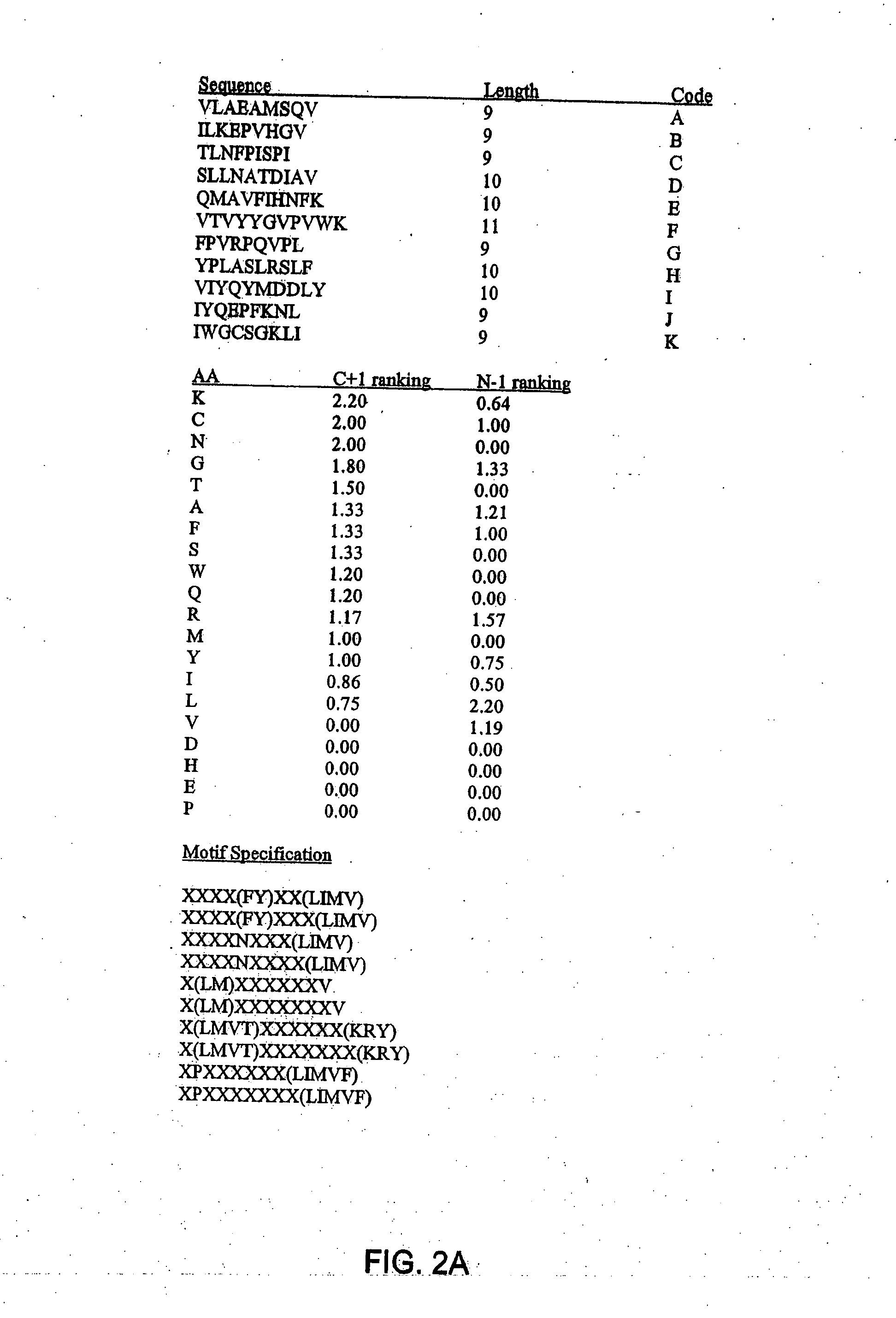
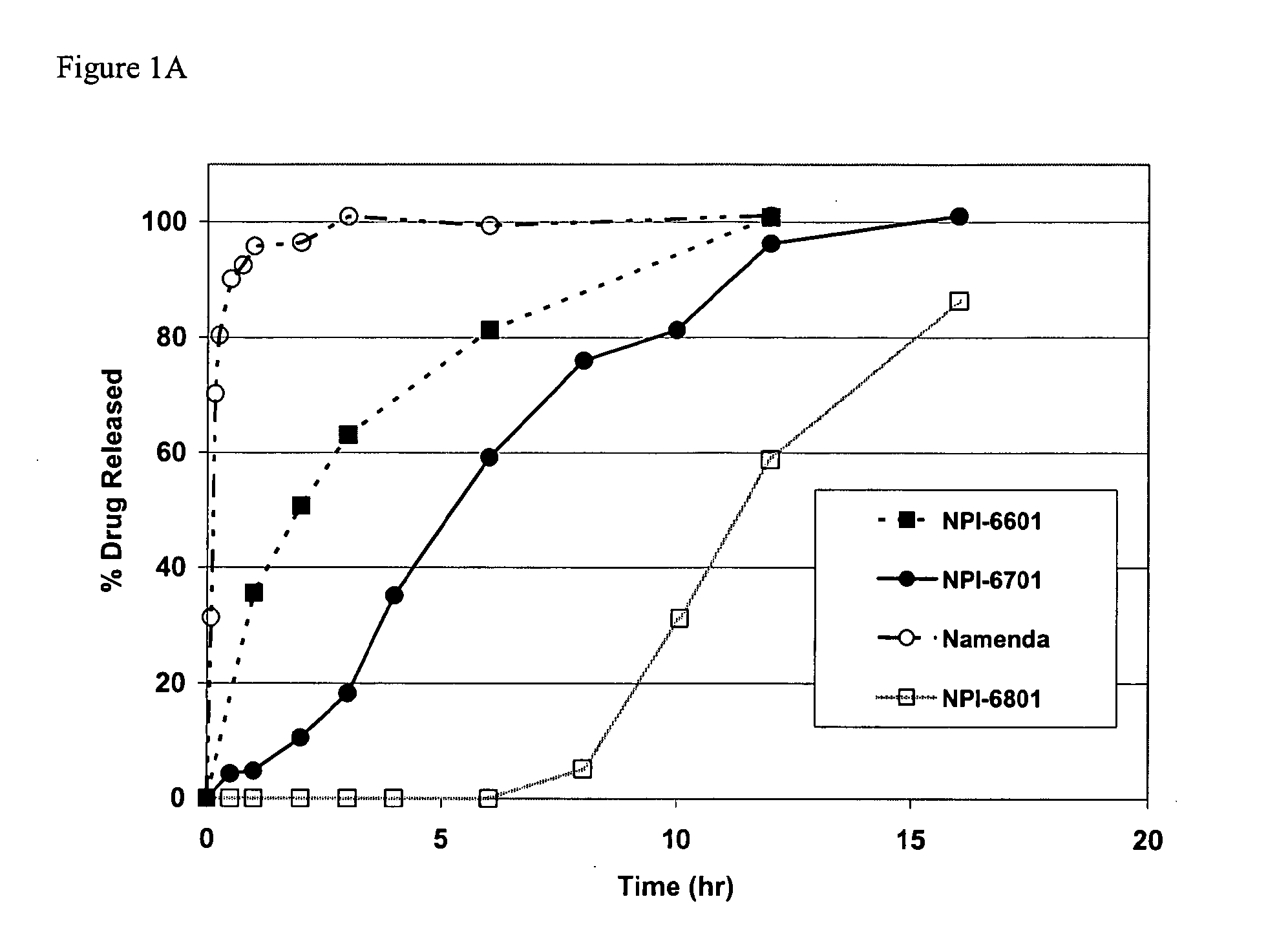
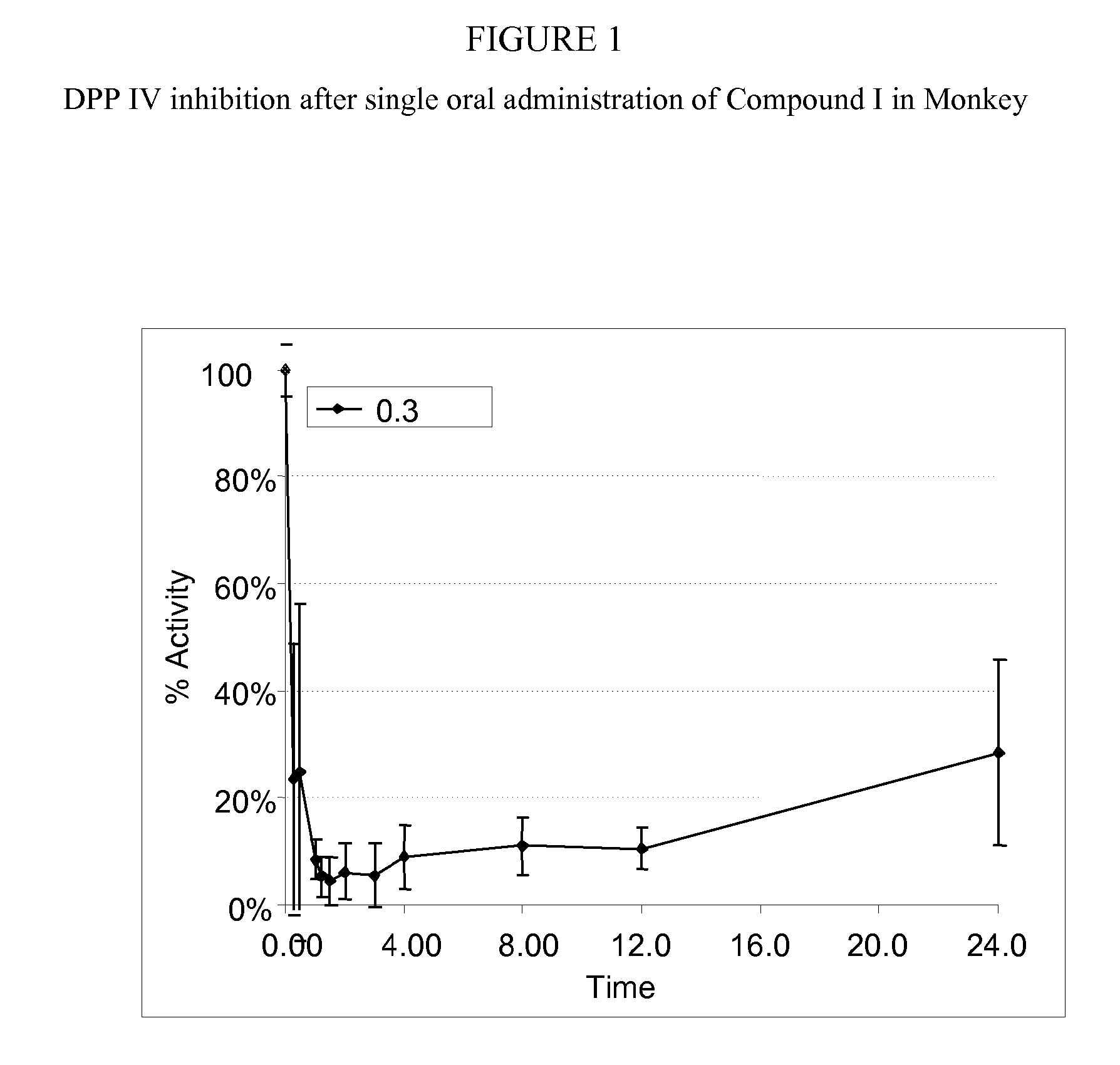Patents
Literature
4422results about How to "Eliminate side effects" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Methods and compositions useful for administration of chemotherapeutic agents
InactiveUS6096331AReduce morbidityLow toxicityPowder deliveryEchographic/ultrasound-imaging preparationsActive agentIn vivo
In accordance with the present invention, there are provided compositions and methods useful for the in vivo delivery of a pharmaceutically active agent, wherein the agent is associated with a polymeric biocompatible material.
Owner:ABRAXIS BIOSCI LLC
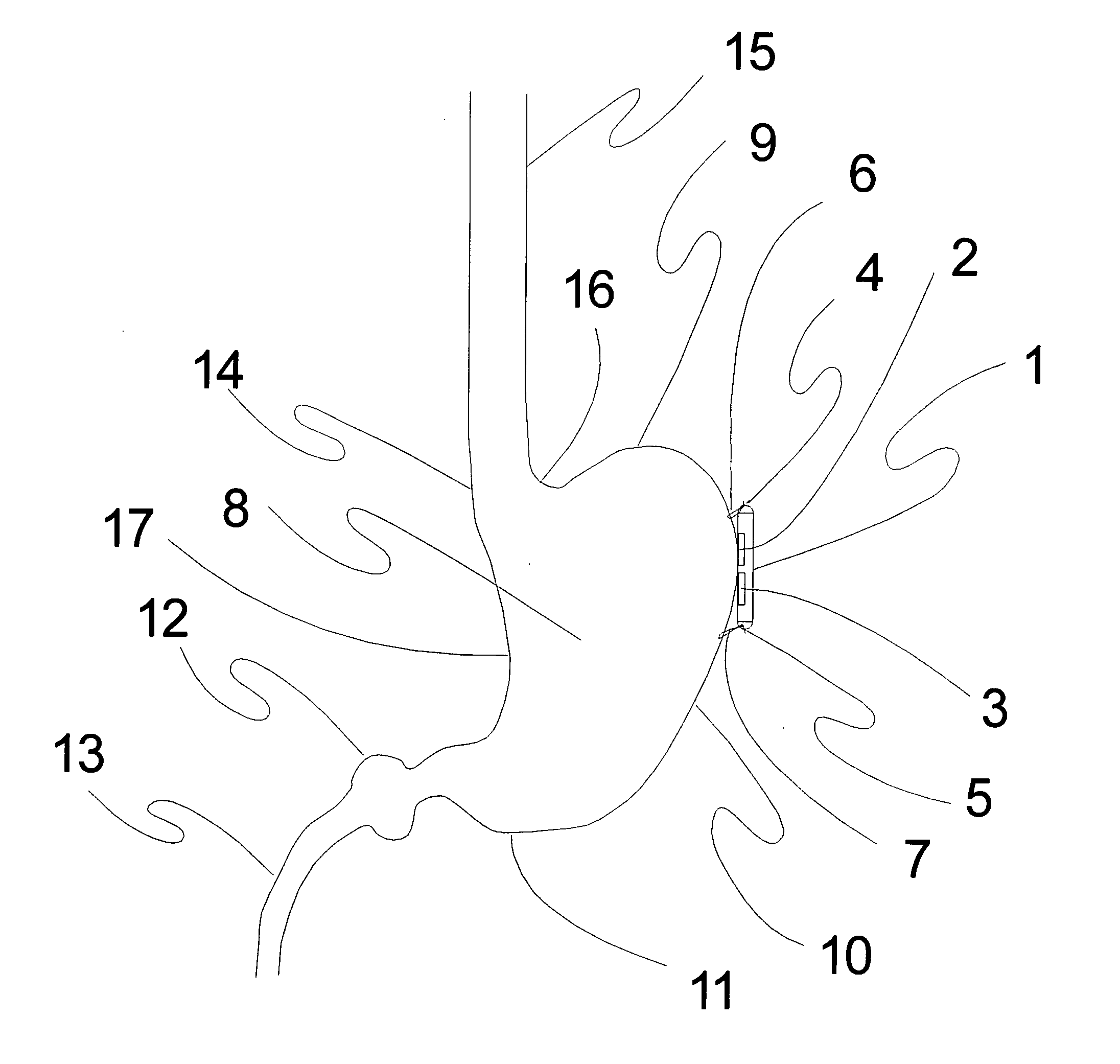
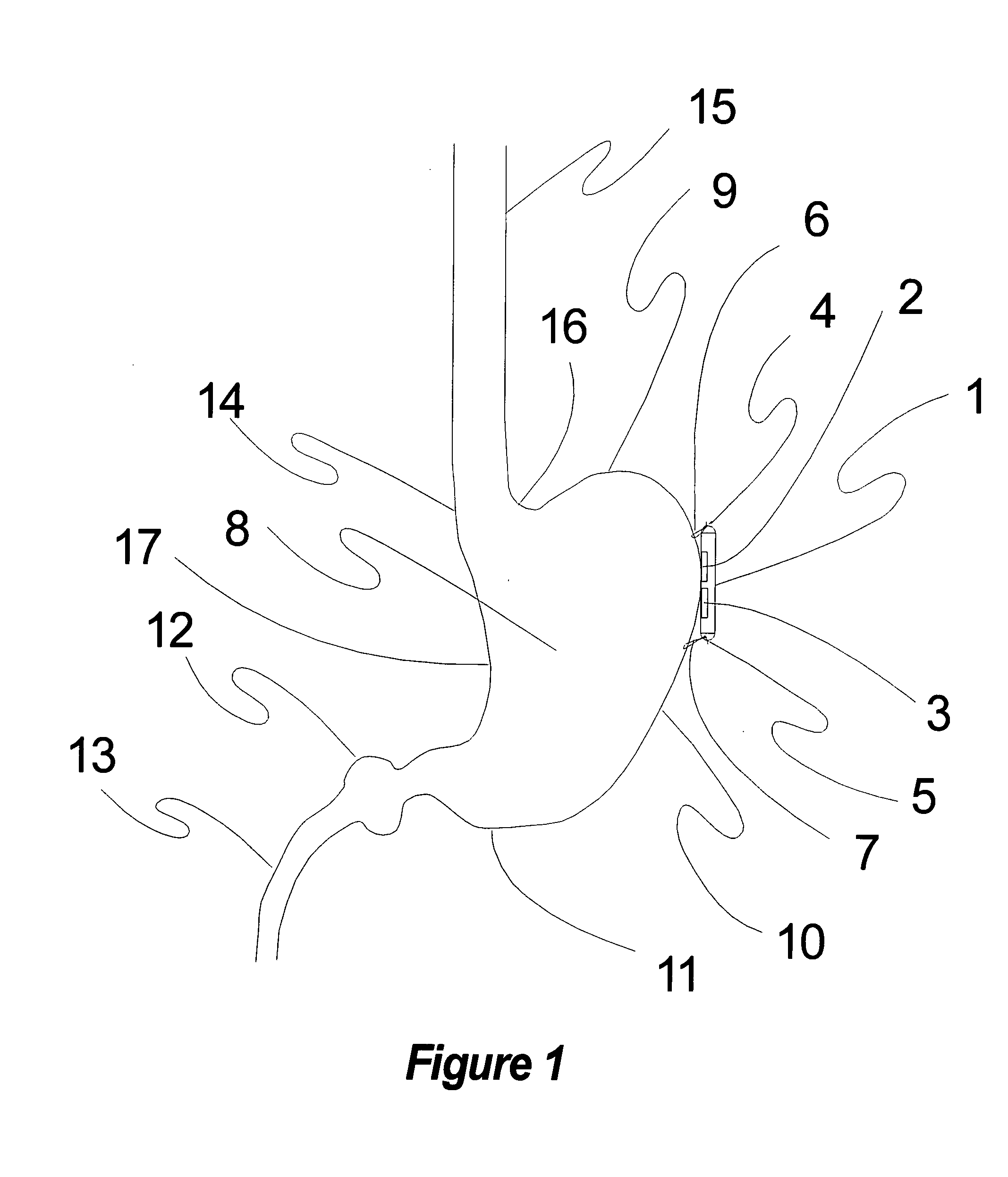
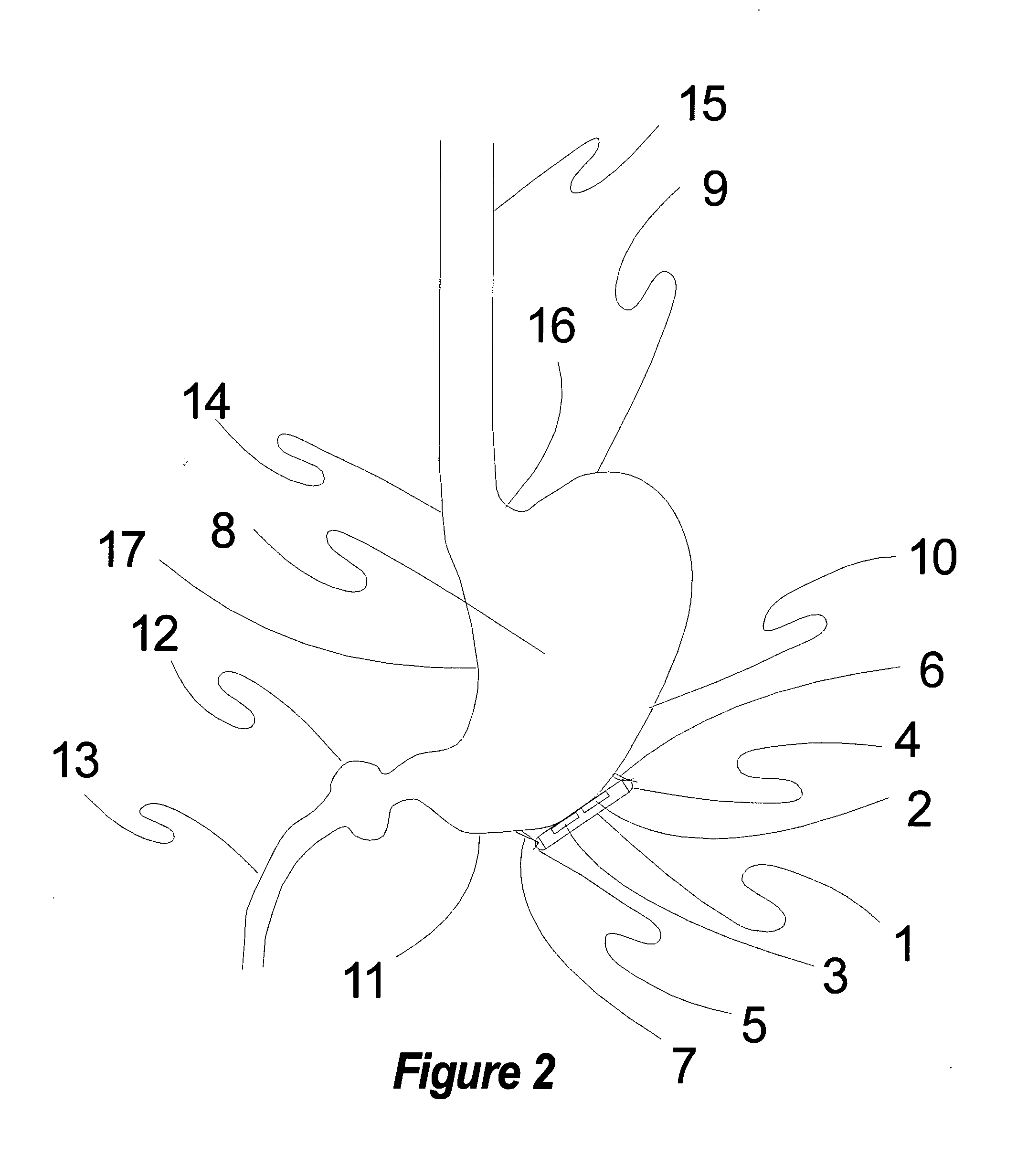
Method, apparatus, and surgical technique for autonomic neuromodulation for the treatment of disease
InactiveUS20060167498A1Reduce or prevent conditionReducing and preventing symptomSpinal electrodesSurgical needlesSplanchnic nervesDisease
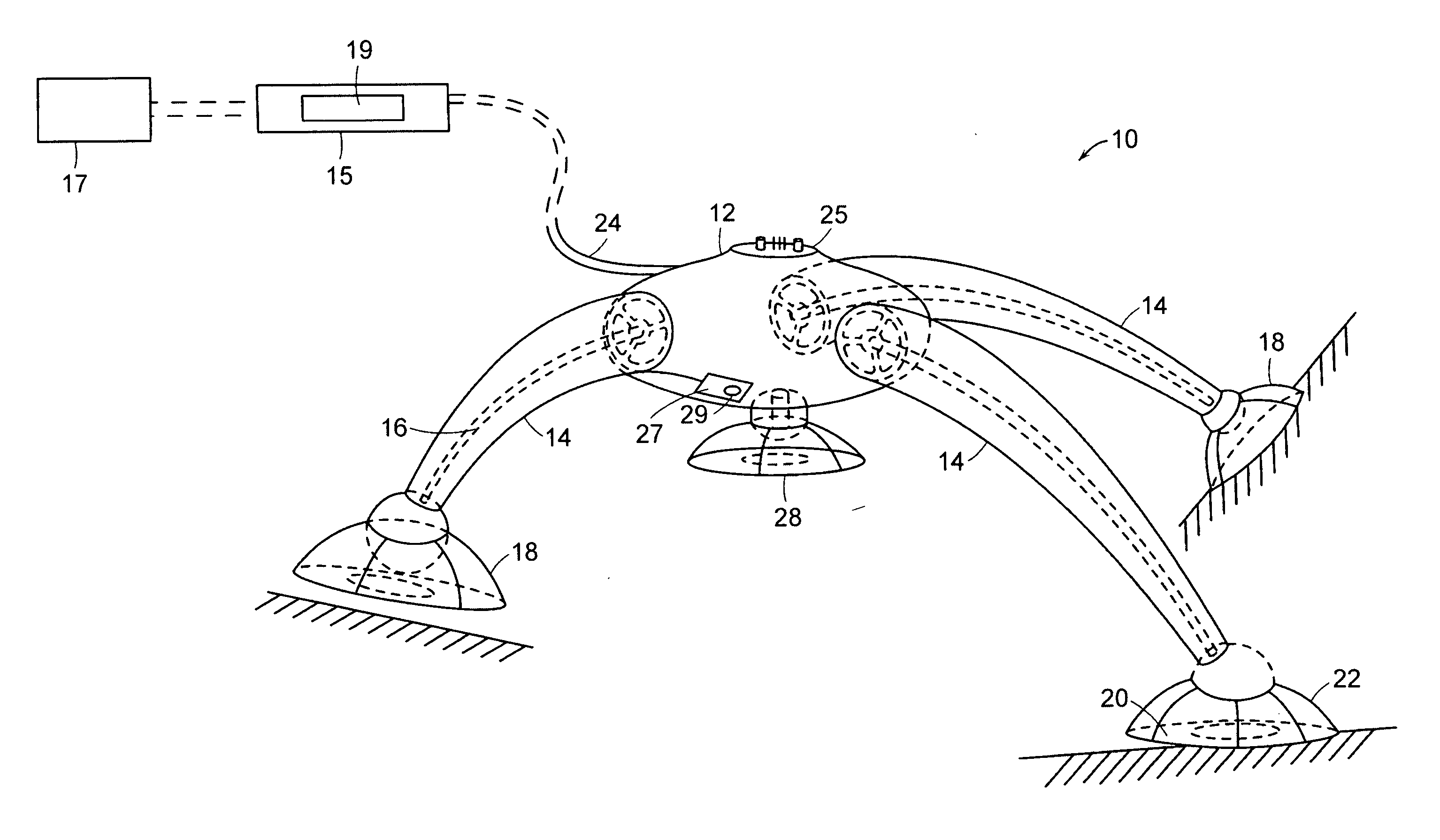
The present invention teaches a method and apparatus for physiological modulation, including neural and gastrointestinal modulation, for the purposes of treating several disorders, including obesity, depression, epilepsy, and diabetes. This includes chronically implanted neural and neuromuscular modulators, used to modulate the afferent neurons of the sympathetic nervous system to induce satiety. Furthermore, this includes neuromuscular stimulation of the stomach to effect baseline and intermittent smooth muscle contraction to increase gastric intraluminal pressure, which induces satiety, and stimulate sympathetic afferent fibers, including those in the sympathetic trunk, splanchnic nerves, and greater curvature of the stomach, to augment the perception of satiety.
Owner:DILORENZO BIOMEDICAL
Pharmaceutical formulation containing opioid agonist, opioid antagonist and irritant
ActiveUS20030068392A1Reduce and eliminate effectInhibition effectBiocideNervous disorderOpioid AgonistOpioid antagonist
Disclosed in certain embodiments is an oral dosage form comprising: a therapeutically effective amount of an opioid analgesic; an opioid antagonist; and an irritant in an effective amount to impart an irritating sensation to an abuser upon administration of the dosage form after tampering.
Owner:PURDUE PHARMA LP
Pharmaceutical formulation containing opioid agonist, opioid antagonist and bittering agent
InactiveUS20030124185A1Analgesic and euphoric effect be reduce and eliminateCompromise integrityPowder deliveryPill deliveryOpioid antagonistDrug
Disclosed in certain embodiments is an oral dosage form comprising a therapeutically effective amount of an opioid analgesic; an opioid antagonist; and a bittering agent in an effective amount to impart a bitter taste to an abuser upon administration of the dosage form after tampering.
Owner:PURDUE PHARMA LP
Pharmaceutical formulation containing opioid agonist,opioid antagonist and gelling agent
InactiveUS20030068371A1Reduce and eliminate effectInhibition effectBiocideNervous disorderOpioid antagonistOpioid Agonist
Disclosed in certain embodiments is an oral dosage form comprising a therapeutically effective amount of an opioid analgesic, an opioid antagonist and one or more pharmaceutically acceptable excipients; the dosage form further including a gelling agent in an effective amount to impart a viscosity unsuitable for administration selected from the group consisting of parenteral and nasal administration to a solubilized mixture formed when the dosage form is crushed and mixed with from about 0.5 to about 10 ml of an aqueous liquid.
Owner:PURDUE PHARMA LP
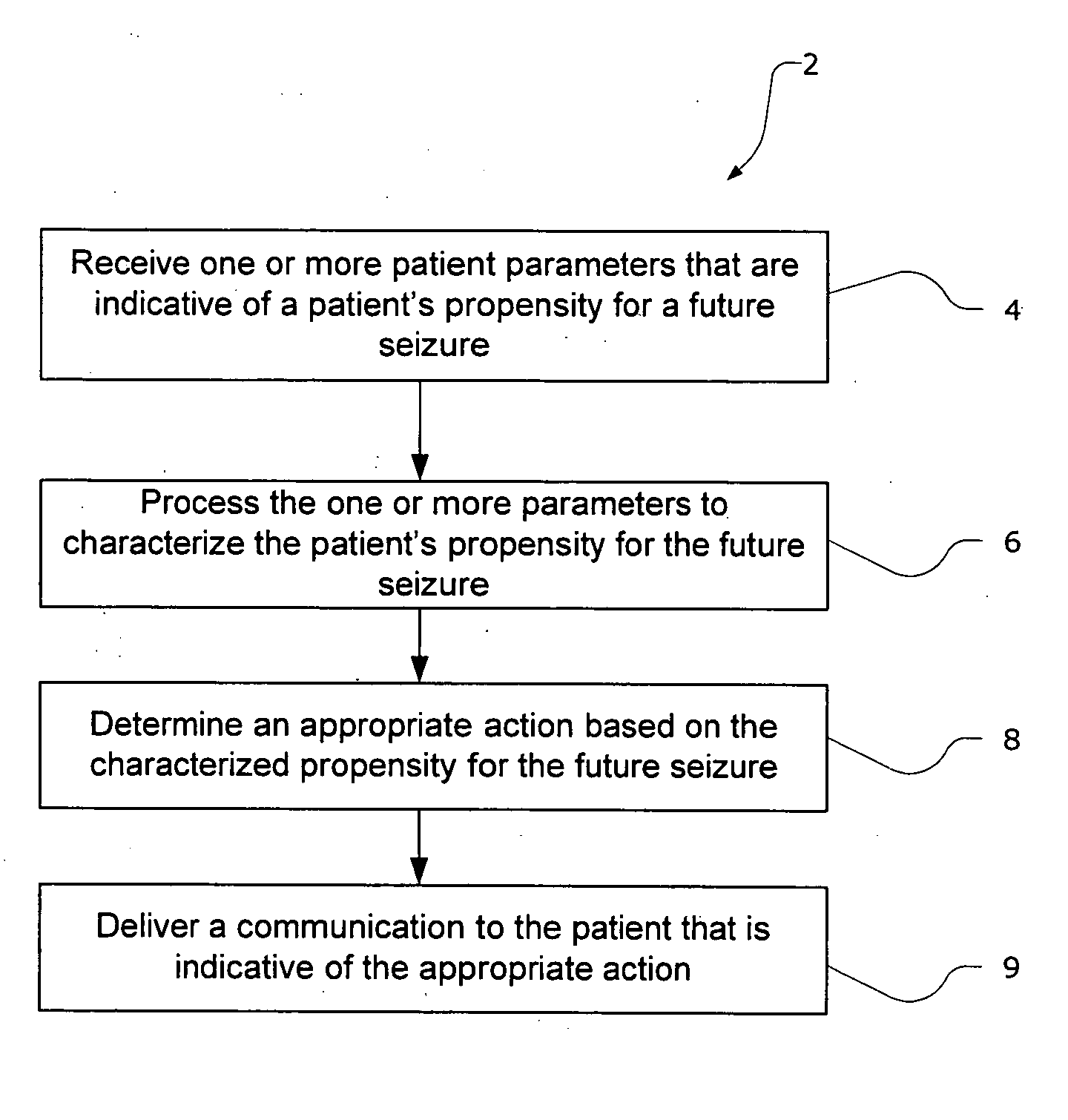
Methods and systems for recommending an appropriate action to a patient for managing epilepsy and other neurological disorders
ActiveUS20070150024A1Low profileIncrease probabilityElectroencephalographyPhysical therapies and activitiesNeurological disorderEpileptic seizure
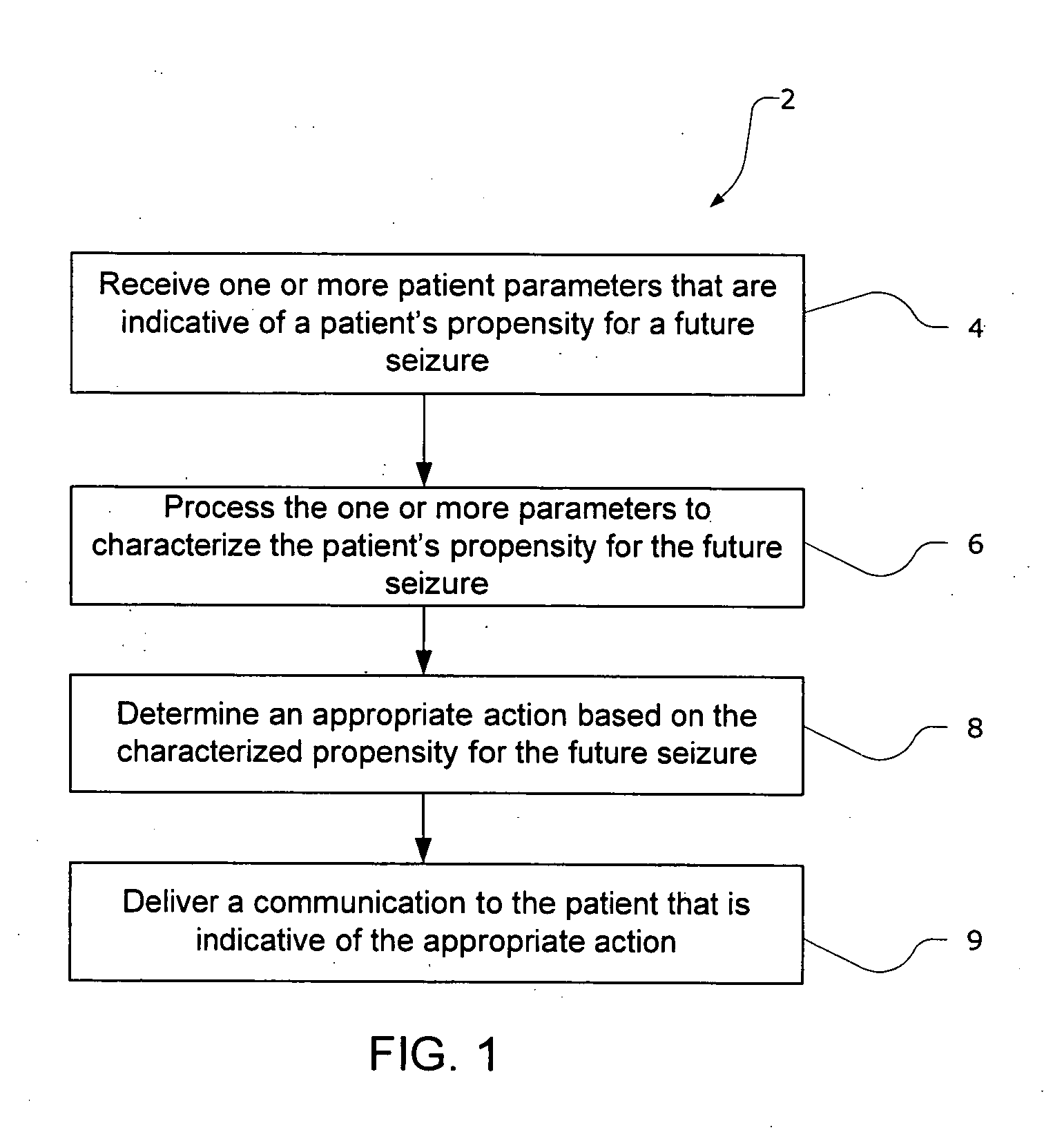
The present invention provides methods and system for managing neurological disorders such as epilepsy. In one embodiment, the method comprises measuring one or more signals from a patient and processing the one or more signals to characterize a patient's propensity for a future seizure. The characterized propensity for the seizure is thereafter used to determine an appropriate action for managing or treating the predicted seizure; and a recommendation is communicated to the patient that is indicative of the appropriate action.
Owner:CYBERONICS INC
Controlled-release compositions containing opioid agonist and antagonist
InactiveUS6716449B2Good curative effectPatient compliance is goodBiocideNervous disorderOpioid antagonistOpioid Agonist
Controlled-release dosage forms containing an opioid agonist; an opioid antagonist; and a controlled release material release during a dosing interval an analgesic or sub-analgesic amount of the opioid agonist along with an amount of the opioid antagonist effective to attenuate a side effect of the opioid agonist. The dosage form provides analgesia for at least about 8 hours when administered to human patients. In other embodiments, the dose of antagonist released during the dosing interval enhances the analgesic potency of the opioid agonist.
Owner:PURDUE PHARMA LP
Serum albumin binding peptides for tumor targeting
InactiveUS20050287153A1Altered pharmacodynamicsFacilitated DiffusionImmunoglobulins against blood coagulation factorsImmunoglobulins against cell receptors/antigens/surface-determinantsAbnormal tissue growthPeptide ligand
Peptide ligands having affinity for serum albumin are useful for tumor targeting. Conjugate molecules comprising a serum albumin binding peptide fused to a biologically active molecule demonstrate modified pharmacokinetic properties as compared with the biologically active molecule alone, including tissue (e.g., tumor) uptake, infiltration, and diffusion.
Owner:GENENTECH INC
Transdermal drug delivery compositions and topical compositions for application on the skin
InactiveUS20090053290A1Improve biological activityEliminate side effectsPowder deliveryCosmetic preparationsRadio frequencyOtic Agents
Transdermal delivery compositions and topical compositions for application to the skin are provided. The transdermal delivery composition includes at least two penetrants working synergistically but by disparate biochemical pathways. In one embodiment, the transdermal delivery system includes benzyl alcohol and lecithin organogel. The transdermal delivery compositions are used in a variety of topical compositions as a means of transdermally delivering and topically administering different drugs and agents, including compositions promoting collagen biosynthesis, retinoids and skin lighteners, chemical denervation agents such as BOTOX®, anti-fungal agents, anesthetics and non-steroidal anti-inflammatory drugs (NSAIDs). In addition, these topical compositions may be used in combination with non-ablative treatment modalities, such as microdermabrasion, laser-based skin remodeling and radio-frequency-based skin remodeling.
Owner:NUVIANCE
Degraded agonist antibody
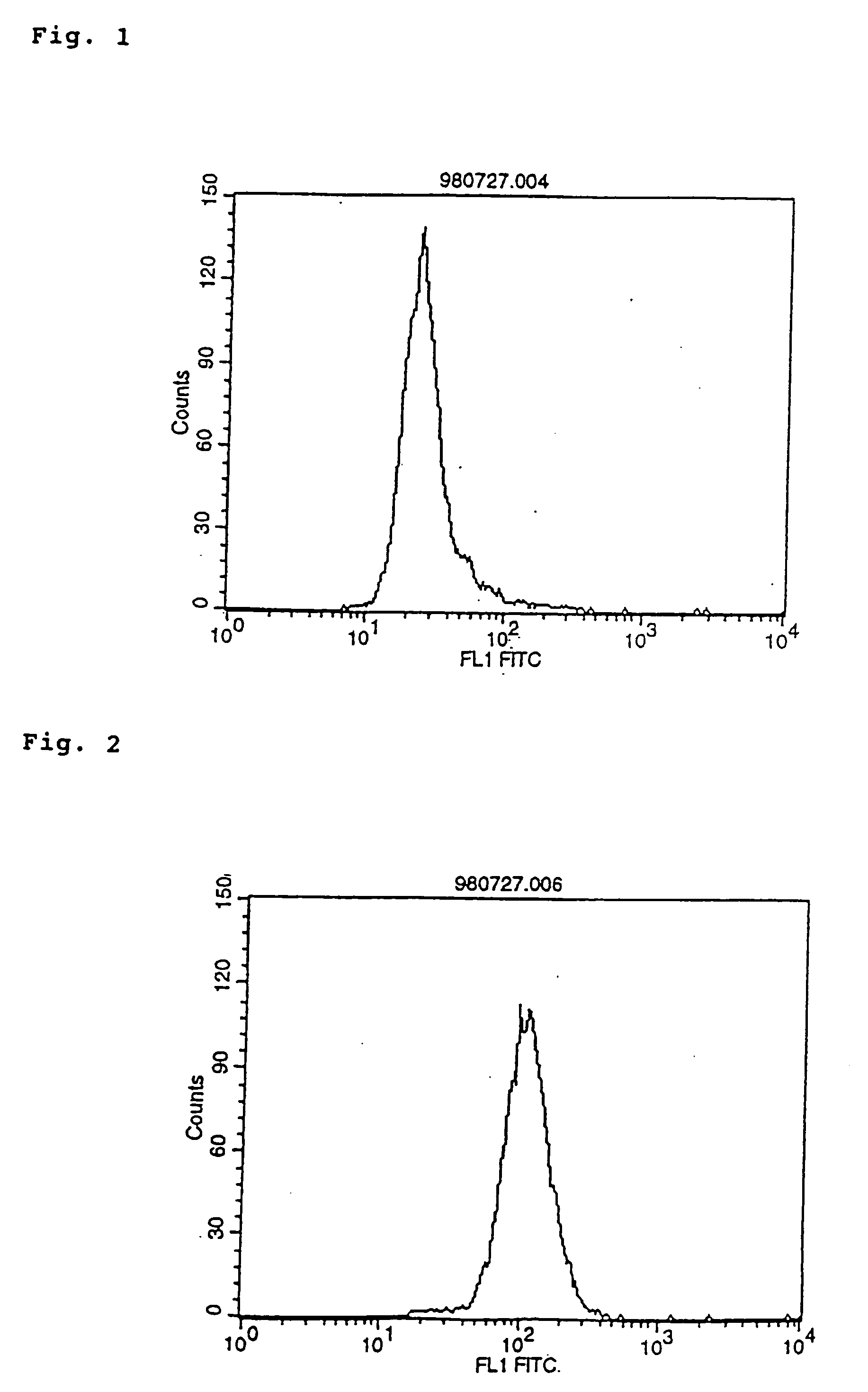
InactiveUS20040242847A1Excellent antigen-binding propertyExcellent agonist activityPeptide/protein ingredientsAntibody mimetics/scaffoldsDiseaseAntiendomysial antibodies
The invention relates to a modified antibody which contains two or more H chain V regions and two or more L chain V regions of monoclonal antibody and can transduce a signal into cells by crosslinking a cell surface molecule(s) to thereby serve as an agonist. The modified antibody can be used as a signal transduction agonist and, therefore, useful as a preventive and / or remedy for various diseases such as cancer, inflammation, hormone disorders and blood diseases.
Owner:CHUGAI PHARMA CO LTD
Robot for minimally invasive interventions
InactiveUS20070123748A1Reduce side effectsSafely transect the sac without harming the epicardiumEndoscopesSurgical instrument detailsDirect controlPhysician roles
The present invention relates to a miniature robotic device to be introduced, in the case of the heart, into the pericardium through a port, attach itself to the epicardial surface, and then, under the direct control of the user or physician, travel to the desired location for diagnosis or treatment.
Owner:ENHANCED MEDICAL SYST
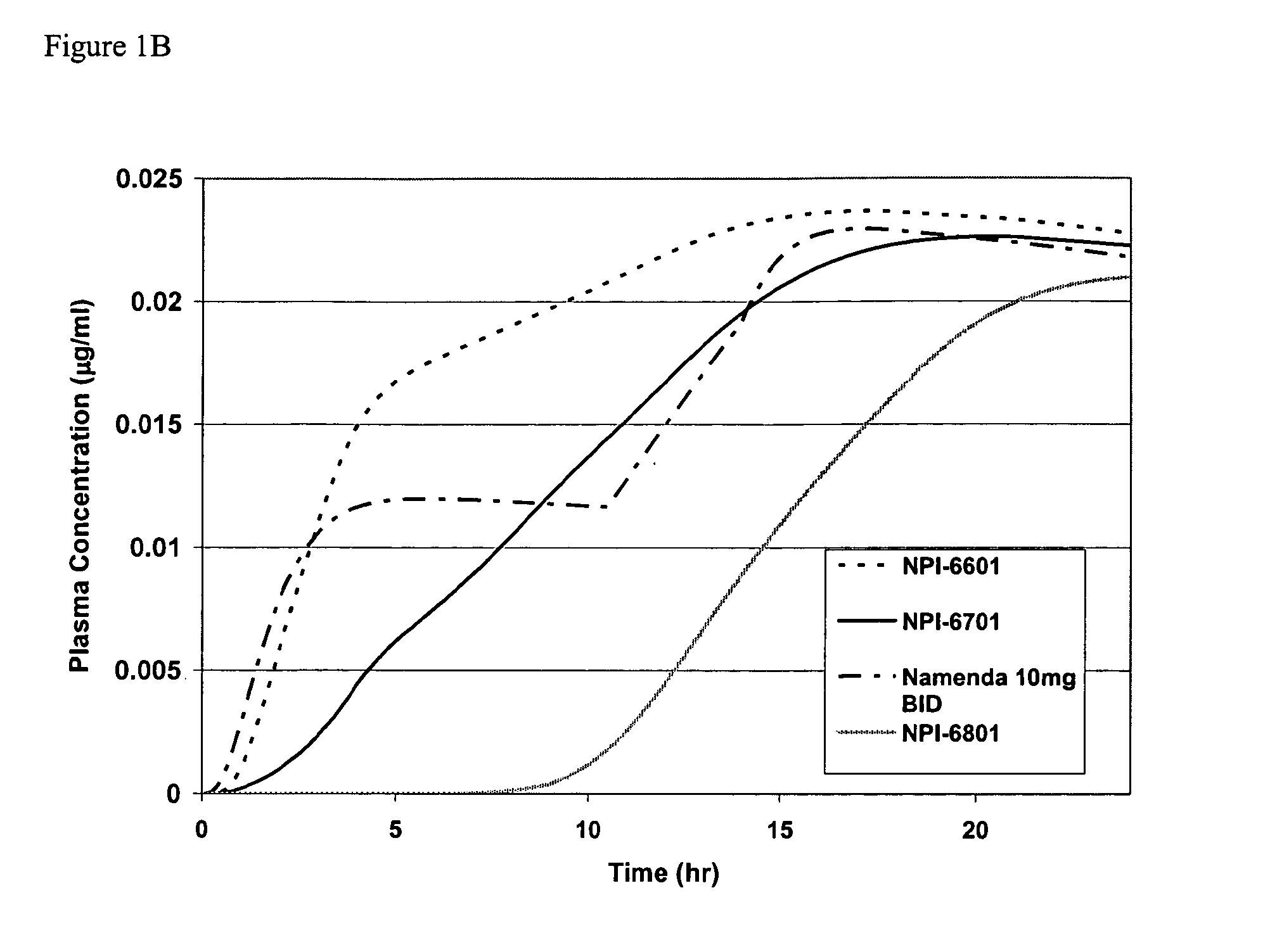
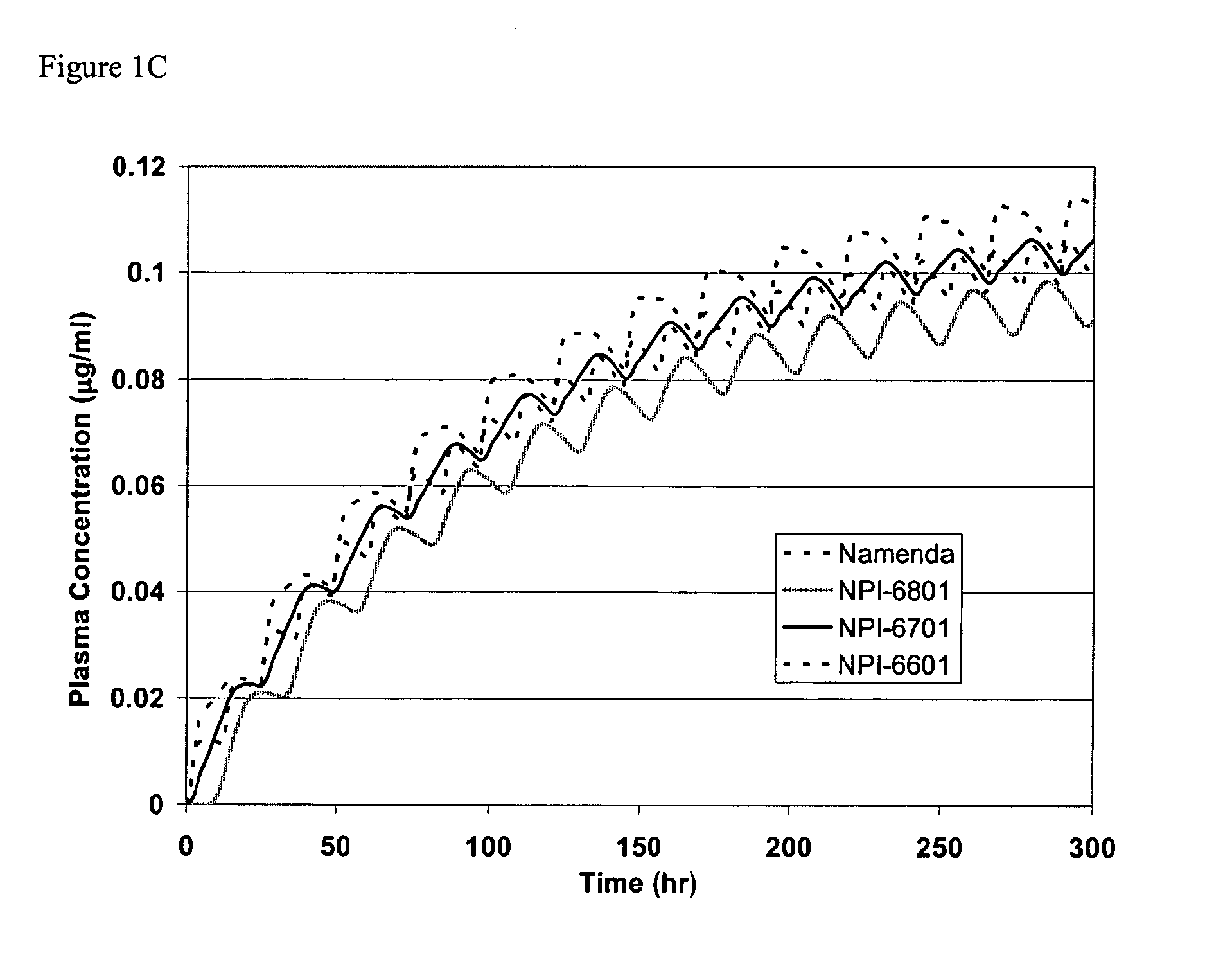
Methods and compositions for the treatment of psychiatric conditions
InactiveUS20050209218A1Low variabilityMaximizes therapeutic benefitBiocideNervous disorderDiseasePsychogenic disease
Owner:ADAMAS PHARMA INC
Topical administration of danazol
InactiveUS20080153789A1Diminished side effectReduce abundance and appearanceBiocideCosmetic preparationsEmulsionPharmaceutical preservatives
Pharmaceutical preparations for topical or local administration of drugs directly to the skin for treatment of disorders of the subcutaneous fatty tissue, in particular in cases of cellulite, are disclosed herein. In a preferred embodiment, the drug is danazol or gastrinone. In another embodiment, the drug is danazol in combination with an aromatase inhibitor or an estrogen compound. The preferred formulations contain drugs in the form of micro or nanoparticles, which may be formed of drug alone or in combination with an excipient or carrier. The excipient or carrier may modify the release rates or enhance absorption into the affected area. The drug formulation may be in the form of a cream, lotion, ointment, gel or emulsion, solution or foam.
Owner:FEMMEPHARMA HLDG CO INC
Administration of dipeptidyl peptidase inhibitors
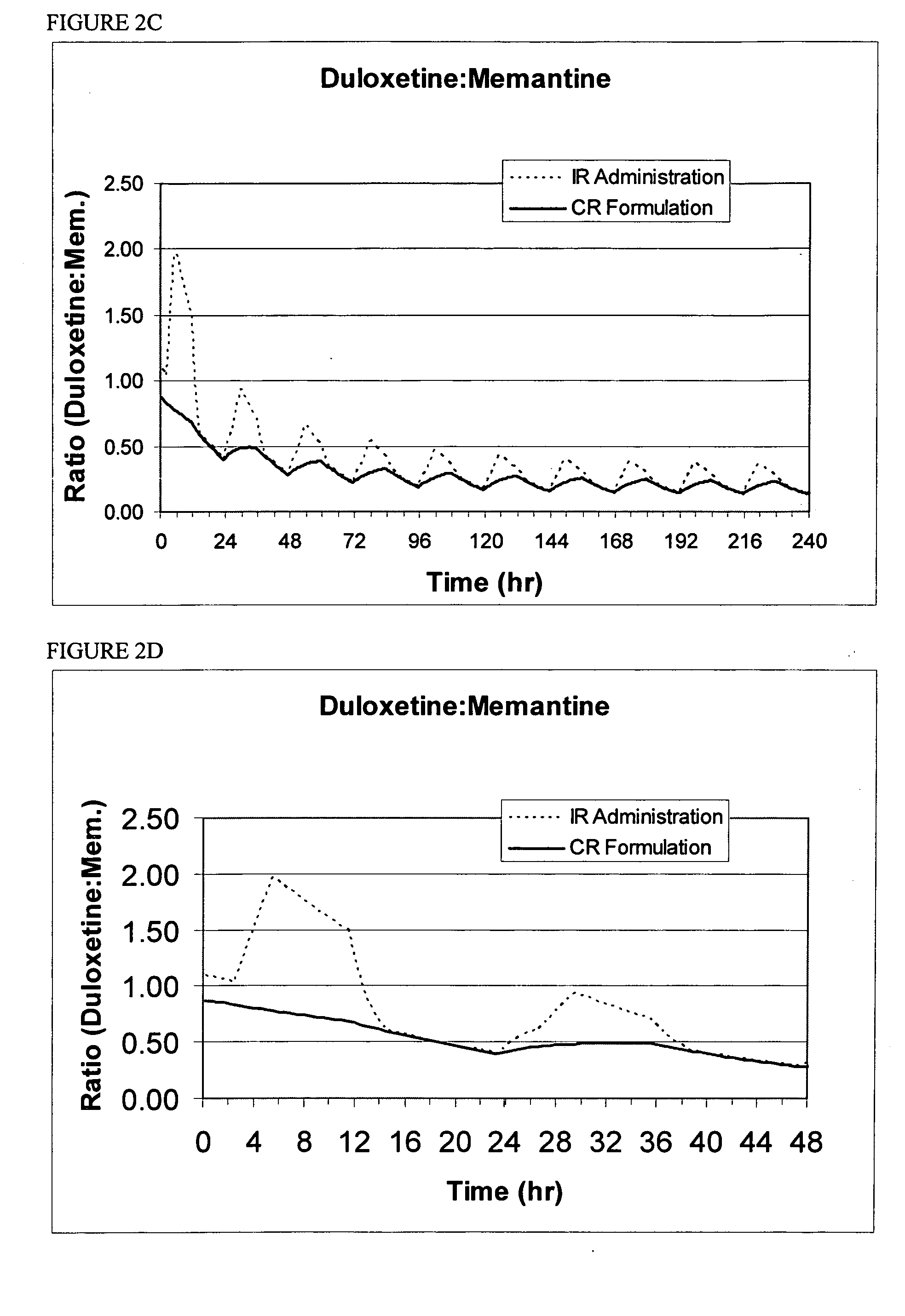
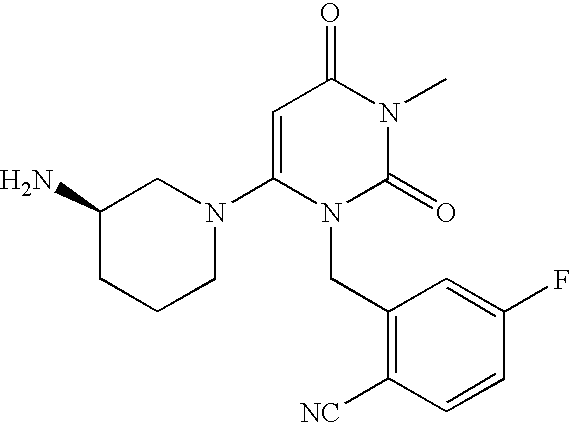
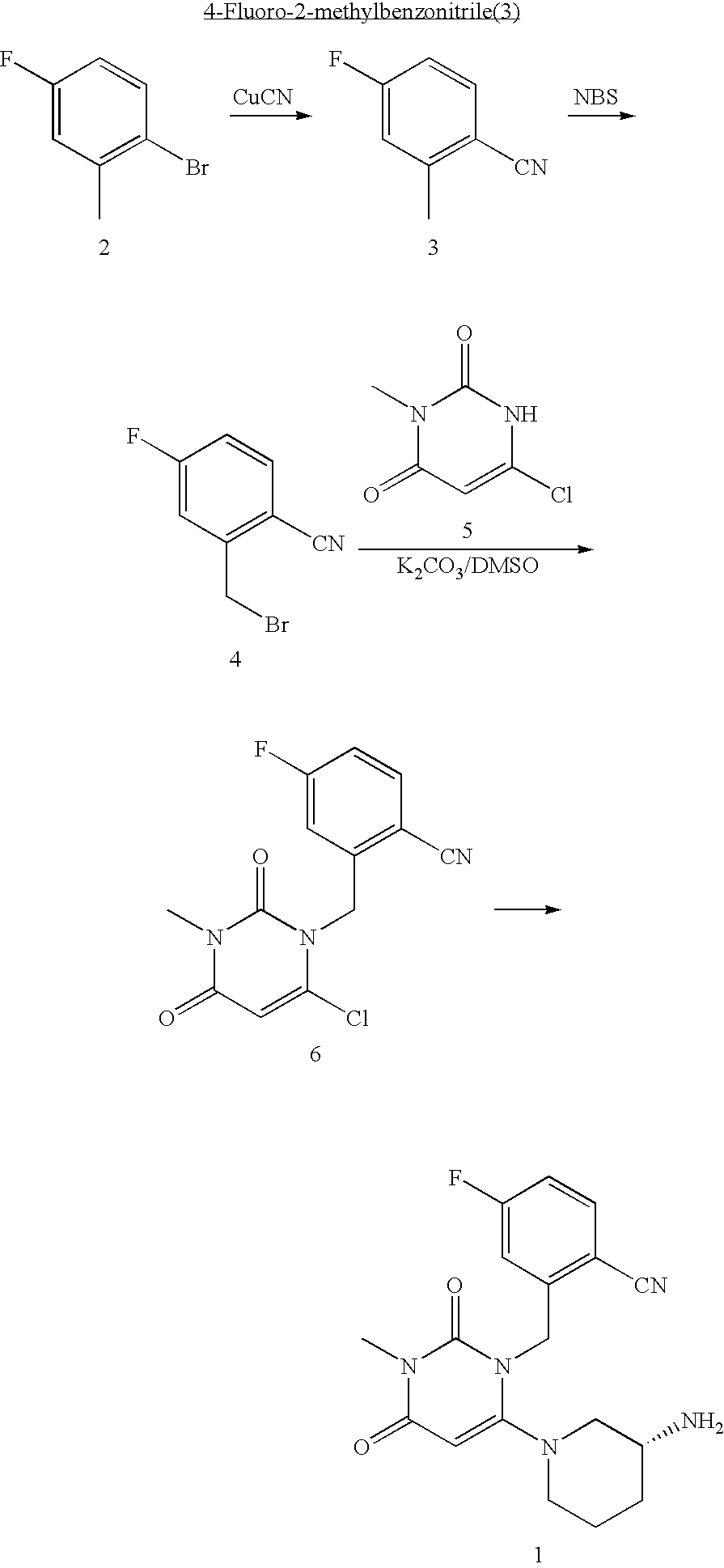
InactiveUS20070060530A1Convenient treatmentEliminate side effectsBiocidePeptide/protein ingredientsDipeptidyl peptidaseBenzonitrile
Pharmaceutical compositions comprising 2-[6-(3-Amino-piperidin-1-yl)-3-methyl-2,4-dioxo-3,4-dihydro-2H-pyrimidin-1-ylmethyl]-4-fluoro-benzonitrile and pharmaceutically acceptable salts thereof are provided as well as kits and articles of manufacture comprising the pharmaceutical compositions as well as methods of using the pharmaceutical compositions.
Owner:TAKEDA PHARMA CO LTD
Controlled-release compositions containing opioid agonist and antagonist
InactiveUS20020010127A1Increase efficacyPromote patient complianceBiocideNervous disorderOpioid antagonistSide effect
Controlled-release dosage forms containing an opioid agonist; an opioid antagonist; and a controlled release material release during a dosing interval an analgesic or sub-analgesic amount of the opioid agonist along with an amount of said opioid antagonist effective to attenuate a side effect of said opioid agonist. The dosage form provides analgesia for at least about 8 hours when administered to human patients. In other embodiments, the dose of antagonist released during the dosing interval enhances the analgesic potency of the opioid agonist.
Owner:PURDUE PHARMA LP
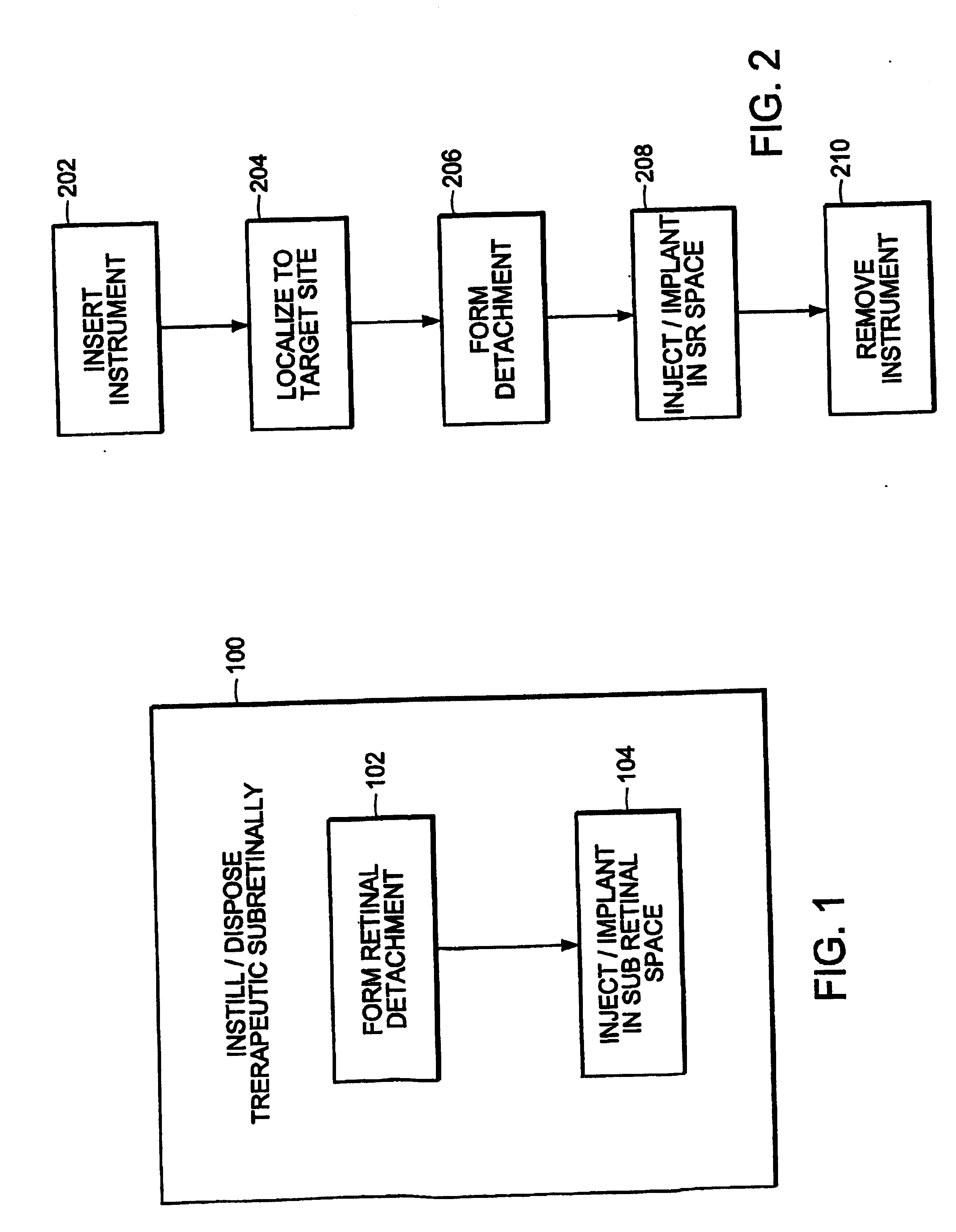
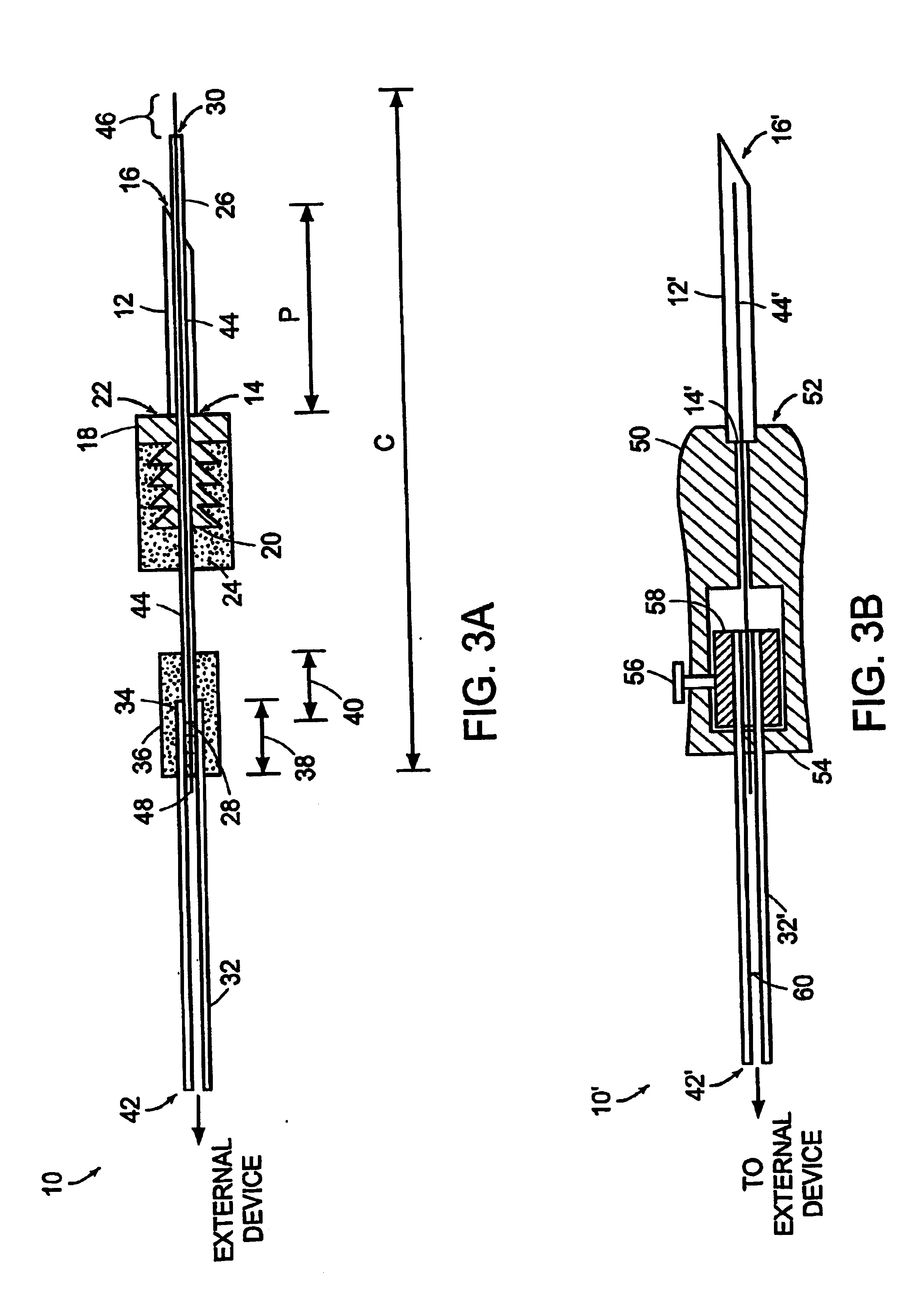
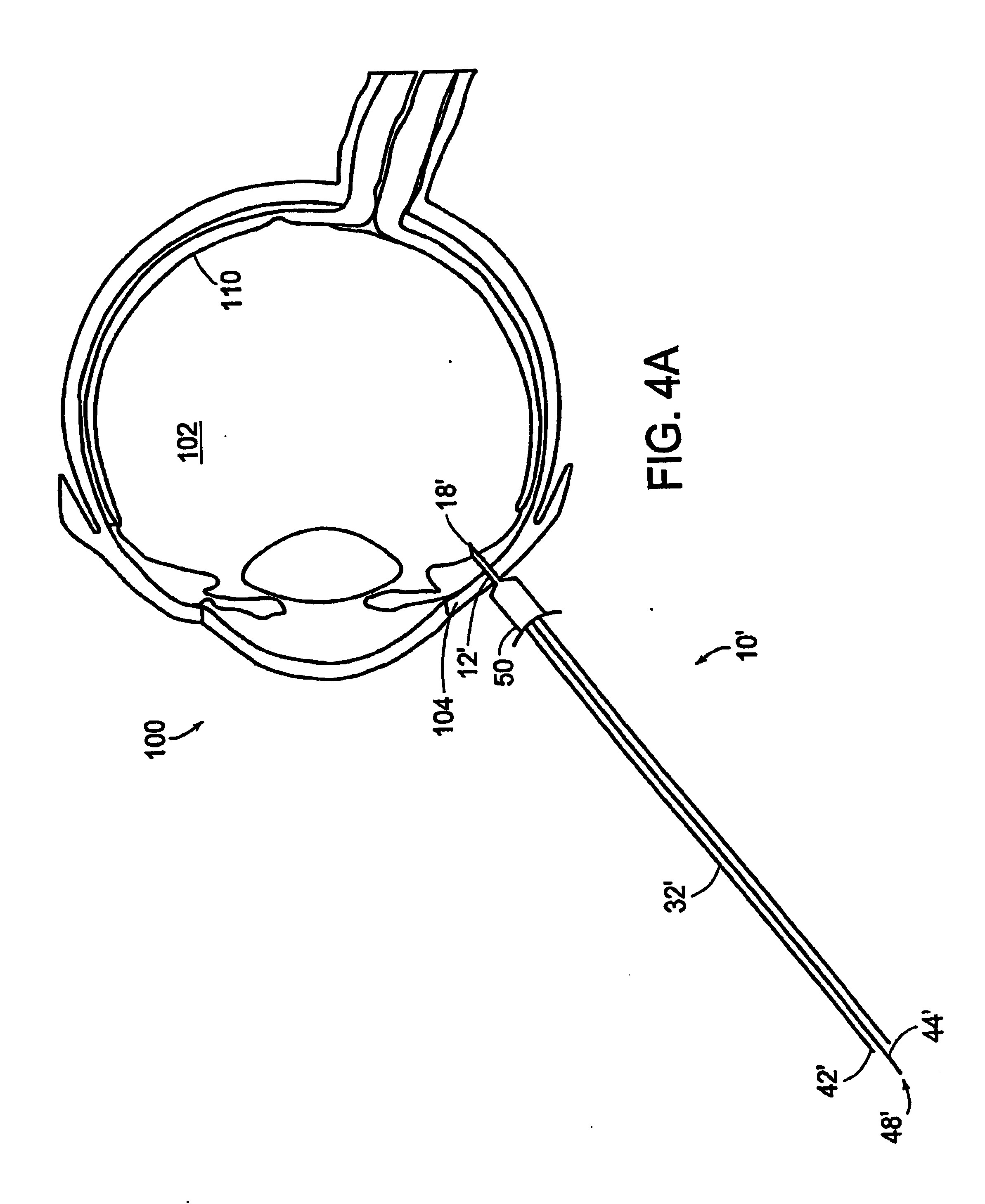
Method for subretinal administration of therapeutics including steroids; method for localizing pharmacodynamic action at the choroid of the retina; and related methods for treatment and/or prevention of retinal diseases
InactiveUS20050143363A1Inhibit progressEliminate side effectsOrganic active ingredientsSenses disorderDisease causeRetinal detachment
Featured is a methodology for administering a therapeutic medium to the posterior segment of an eye including instilling or disposing the therapeutic medium sub-retinally. In particular embodiments, the therapeutic medium is disposed in a sub retinal space. Such instillation being accomplished by one of injection or implantation of the therapeutic medium sub-retinally or the sub-retinal space. In other aspects, the methodology further includes forming a limited retinal detachment so as to define the sub-retinal space as well as methods for treating an eye by sub-retinally administering a therapeutic medium.
Owner:SURMODICS INC
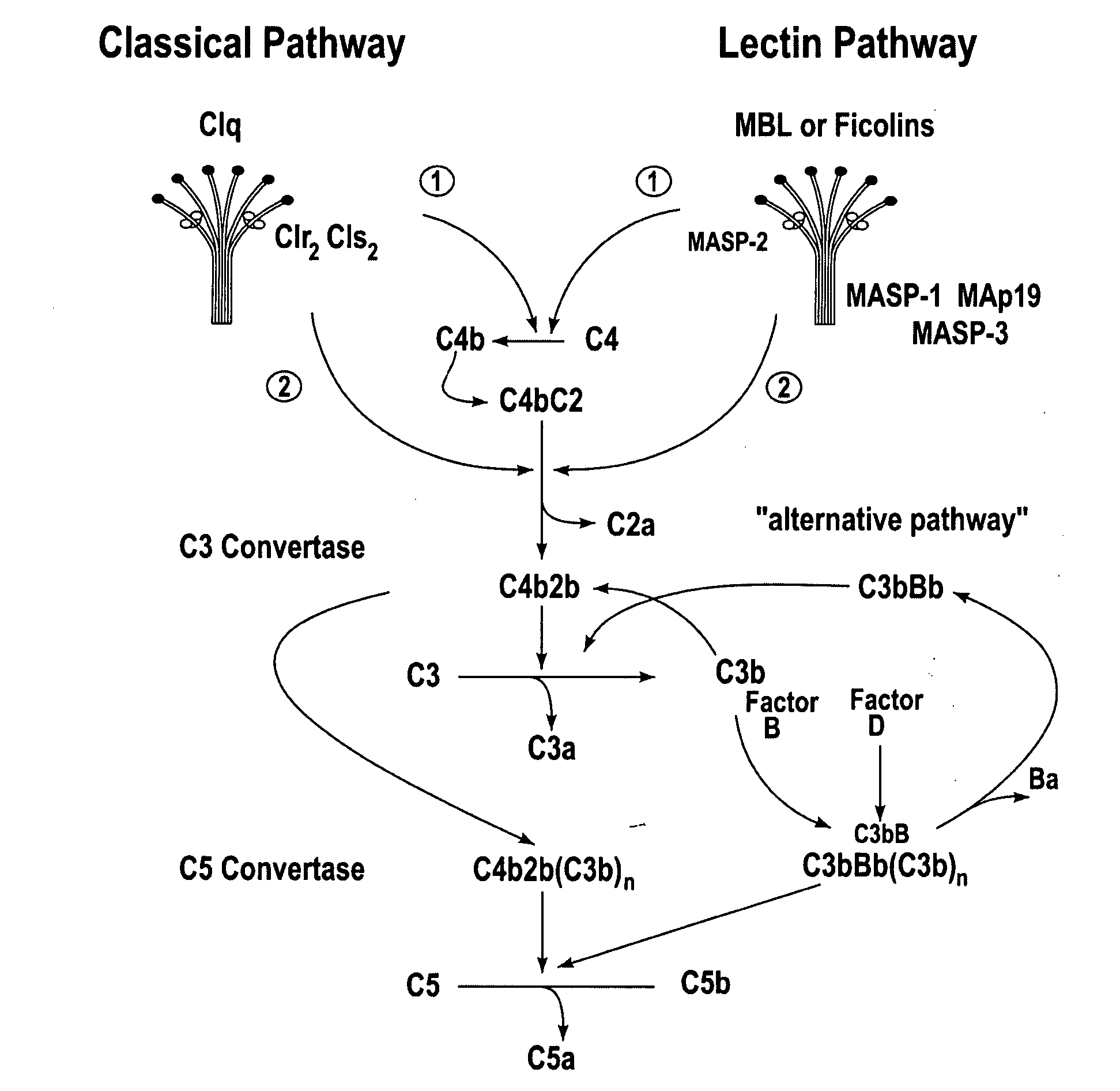
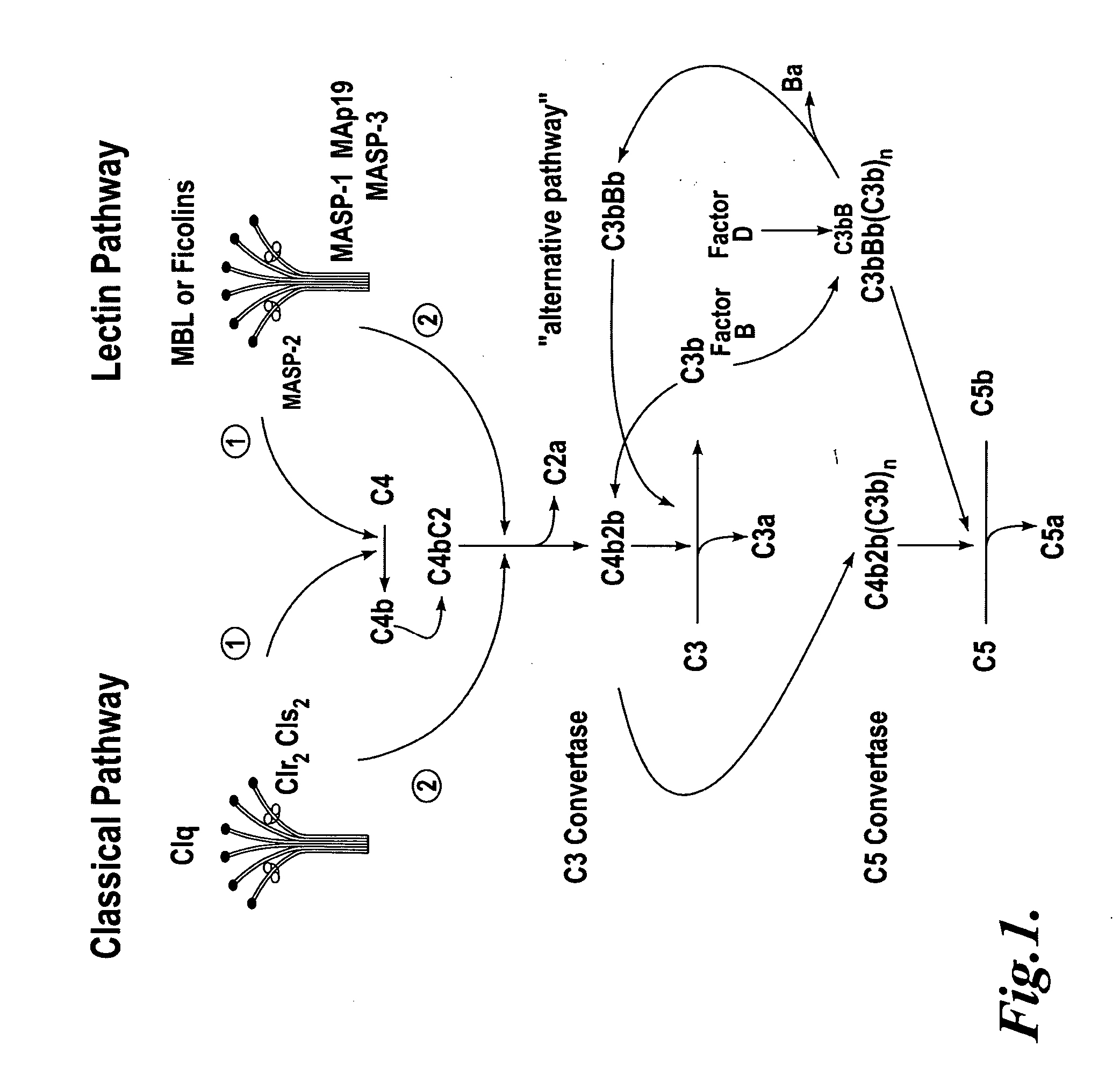
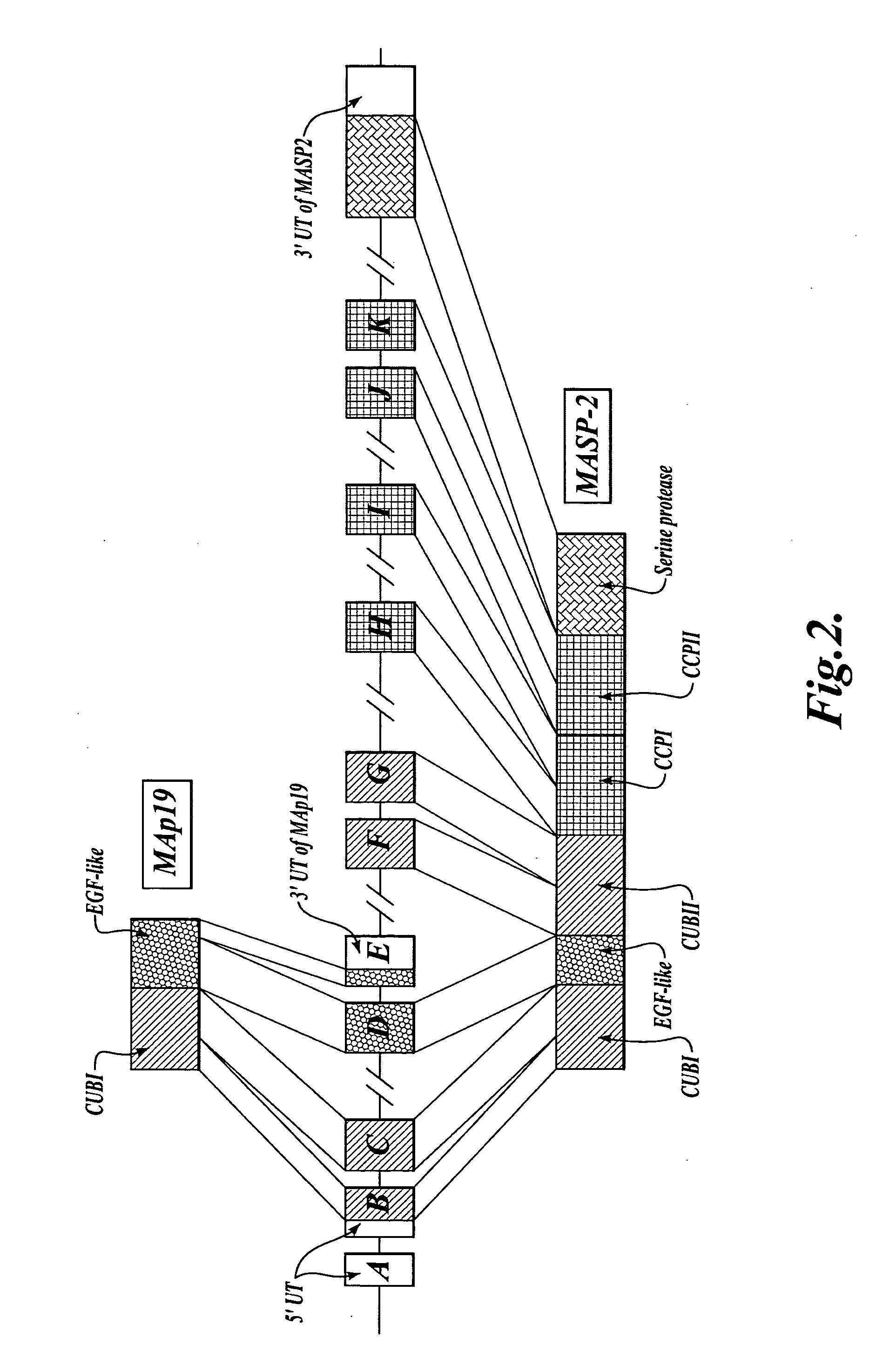
Methods for treating conditions associated with MASP-2 dependent complement activation
ActiveUS20070172483A1Eliminate side effectsInhibiting complement activationCompounds screening/testingAntibody ingredientsBiological activationAlternative complement pathway
In one aspect, the invention provides methods of inhibiting the effects of MASP-2-dependent complement activation in a living subject. The methods comprise the step of administering, to a subject in need thereof, an amount of a MASP-2 inhibitory agent effective to inhibit MASP-2-dependent complement activation. In some embodiments, the MASP-2 inhibitory agent inhibits cellular injury associated with MASP-2-mediated alternative complement pathway activation, while leaving the classical (C1q-dependent) pathway component of the immune system intact. In another aspect, the invention provides compositions for inhibiting the effects of lectin-dependent complement activation, comprising a therapeutically effective amount of a MASP-2 inhibitory agent and a pharmaceutically acceptable carrier.
Owner:OMEROS CORP +1
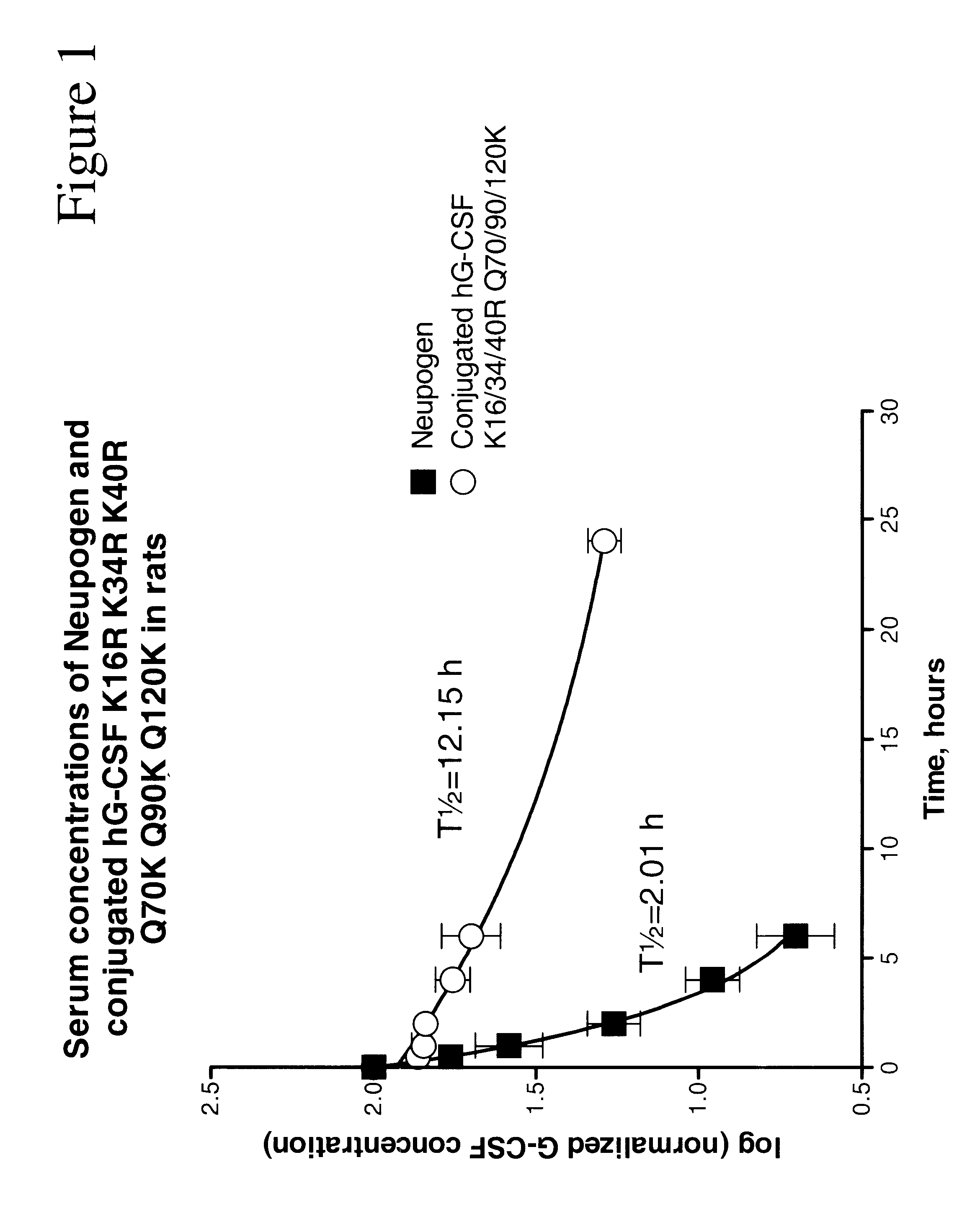
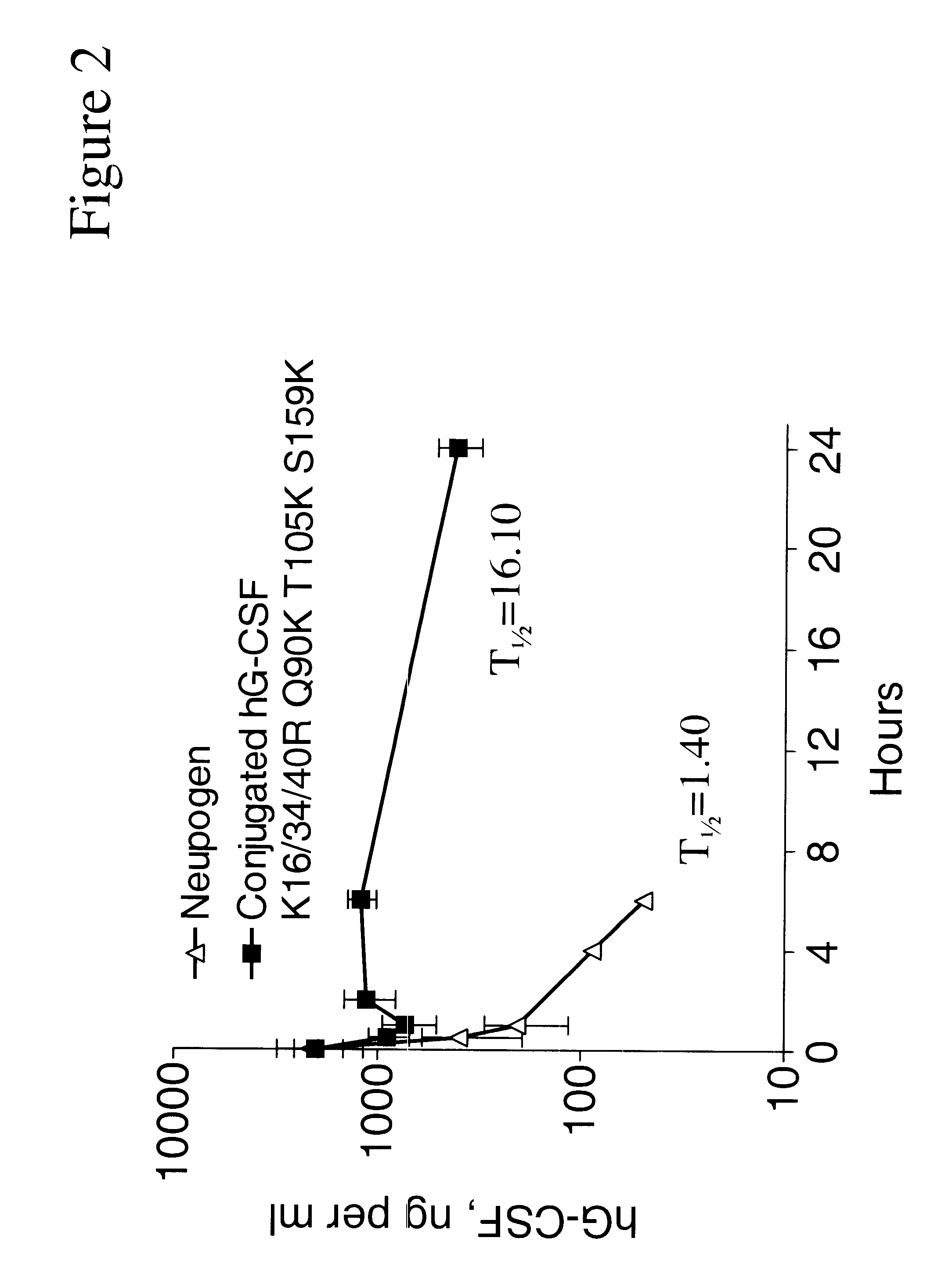
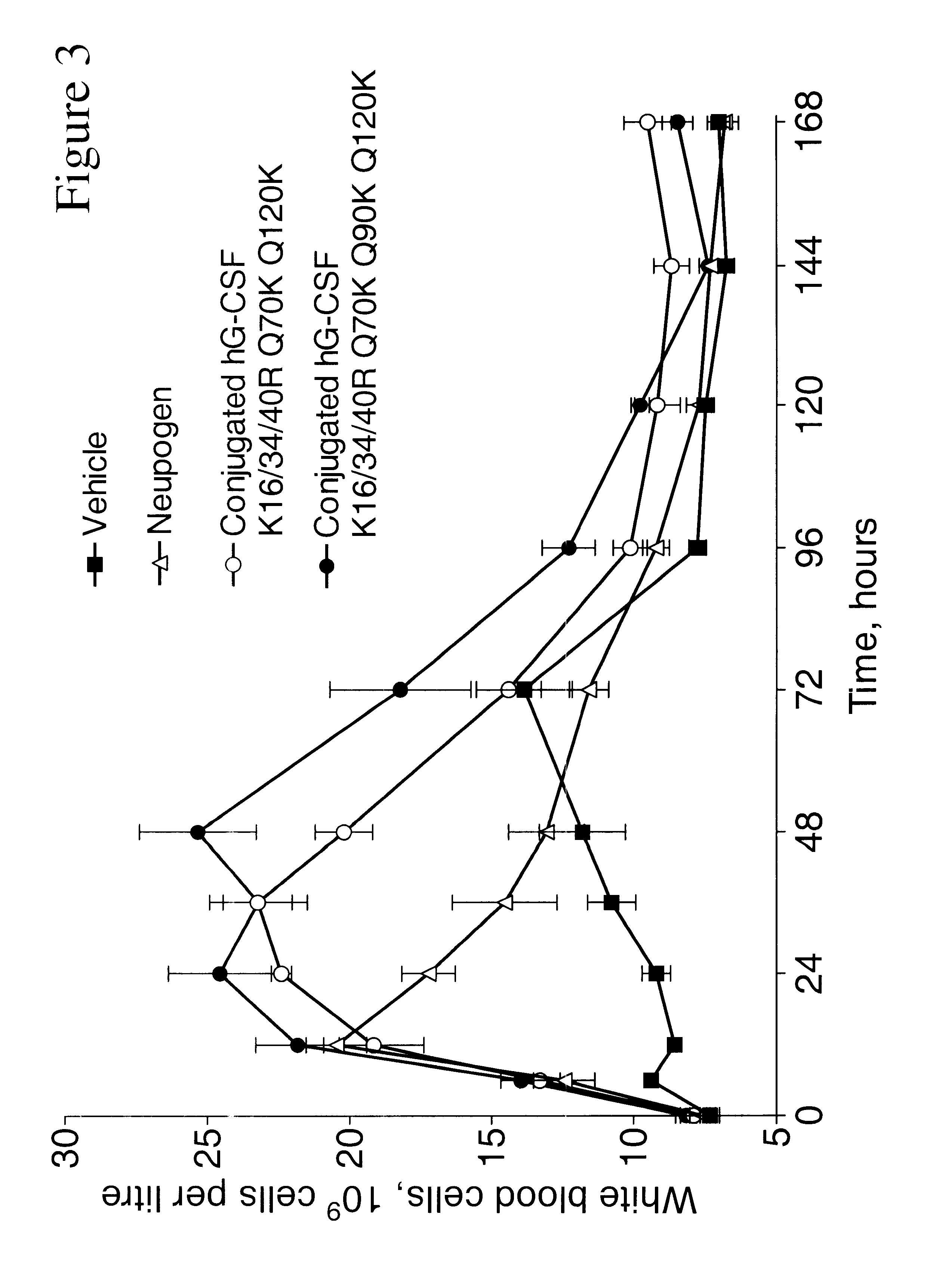
G-CSF conjugates
InactiveUS6555660B2Improved propertyReduced in vitroBiocidePeptide/protein ingredientsHalf-lifePolyethylene glycol
The invention relates to polypeptide conjugates comprising a polypeptide exhibiting G-CSF activity and having an amino acid sequence that differs from the amino acid sequence of human G-CSF in at least one specified introduced and / or removed amino acid residue comprising an attachment group for a non-polypeptide moiety, and having at least one non-polypeptide moiety attached to an attachment group of the polypeptide. The attachment group may e.g. be a lysine, cysteine, aspartic acid or glutamic acid residue or a glycosylation site, and the non-polypeptide moiety may e.g. be a polymer such as polyethylene glycol or an oligosaccharide. The conjugate, which has a reduced in vitro bioactivity compared to hG-CSF, has one or more improved properties such as increased biological half-life and increased stimulation of neutrophils.
Owner:MAXYGEN
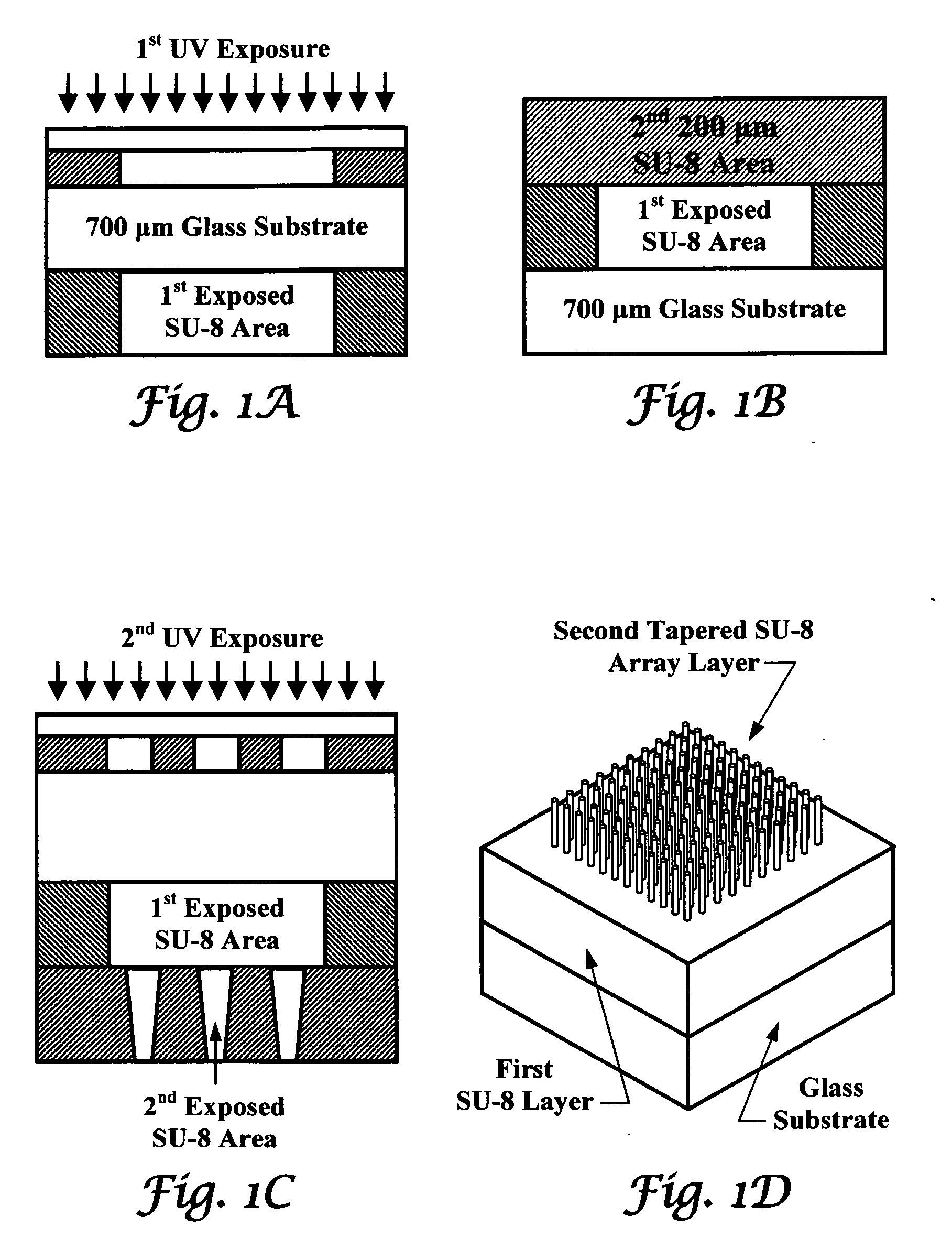
Tapered hollow metallic microneedle array assembly and method of making and using the same
InactiveUS20060084942A1Minimal and damageReduce riskLaminationPharmaceutical delivery mechanismBiomedical engineeringMultiple layer
The present invention includes device, system, method of using and making a microneedle array including the steps of forming one or more pins on a substrate, depositing one or more layers on the one or more pins and the substrate, exposing a portion of the one or more pins, and separating the one or more pins from the one or more layers to form the hollow microneedle array.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
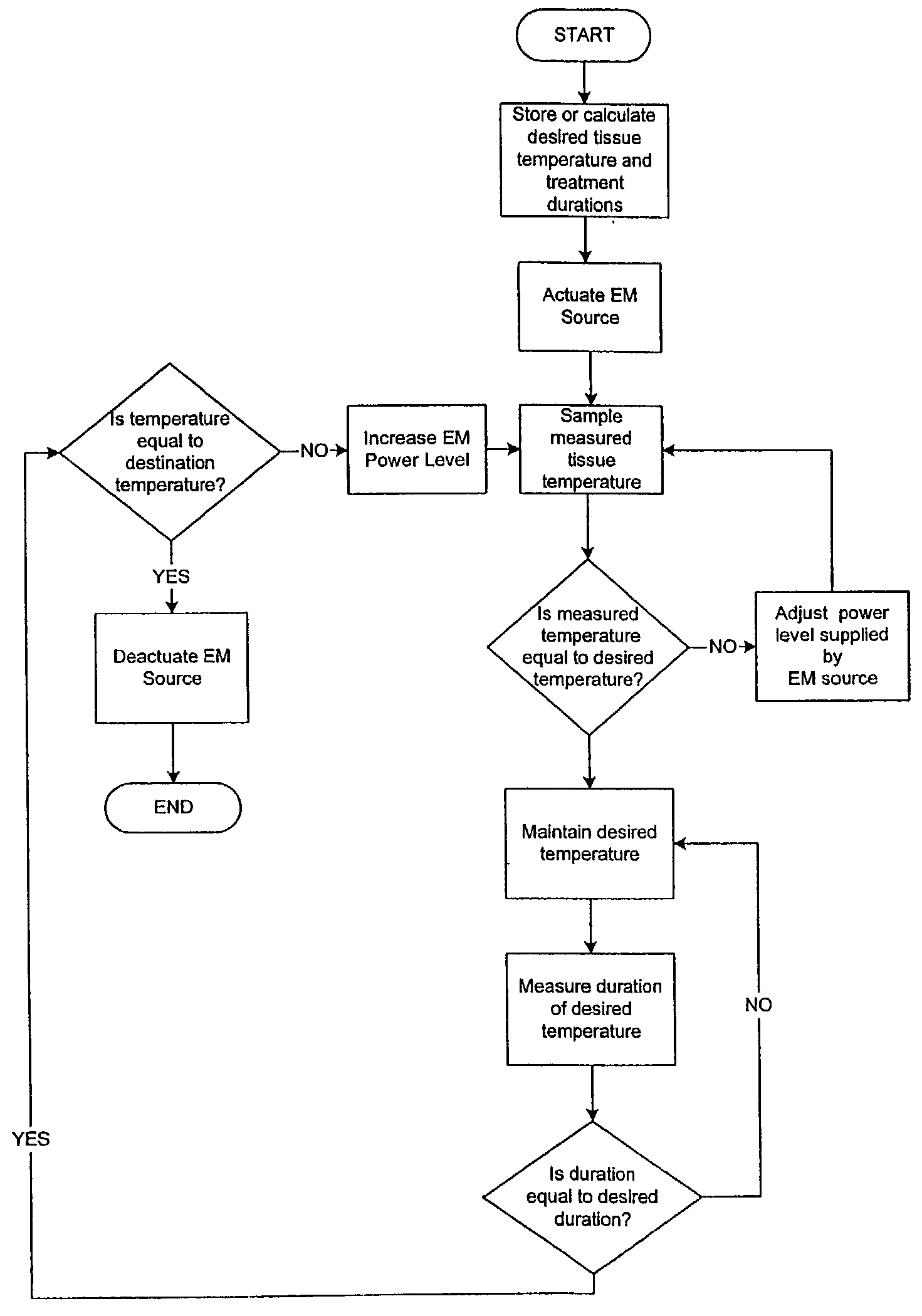
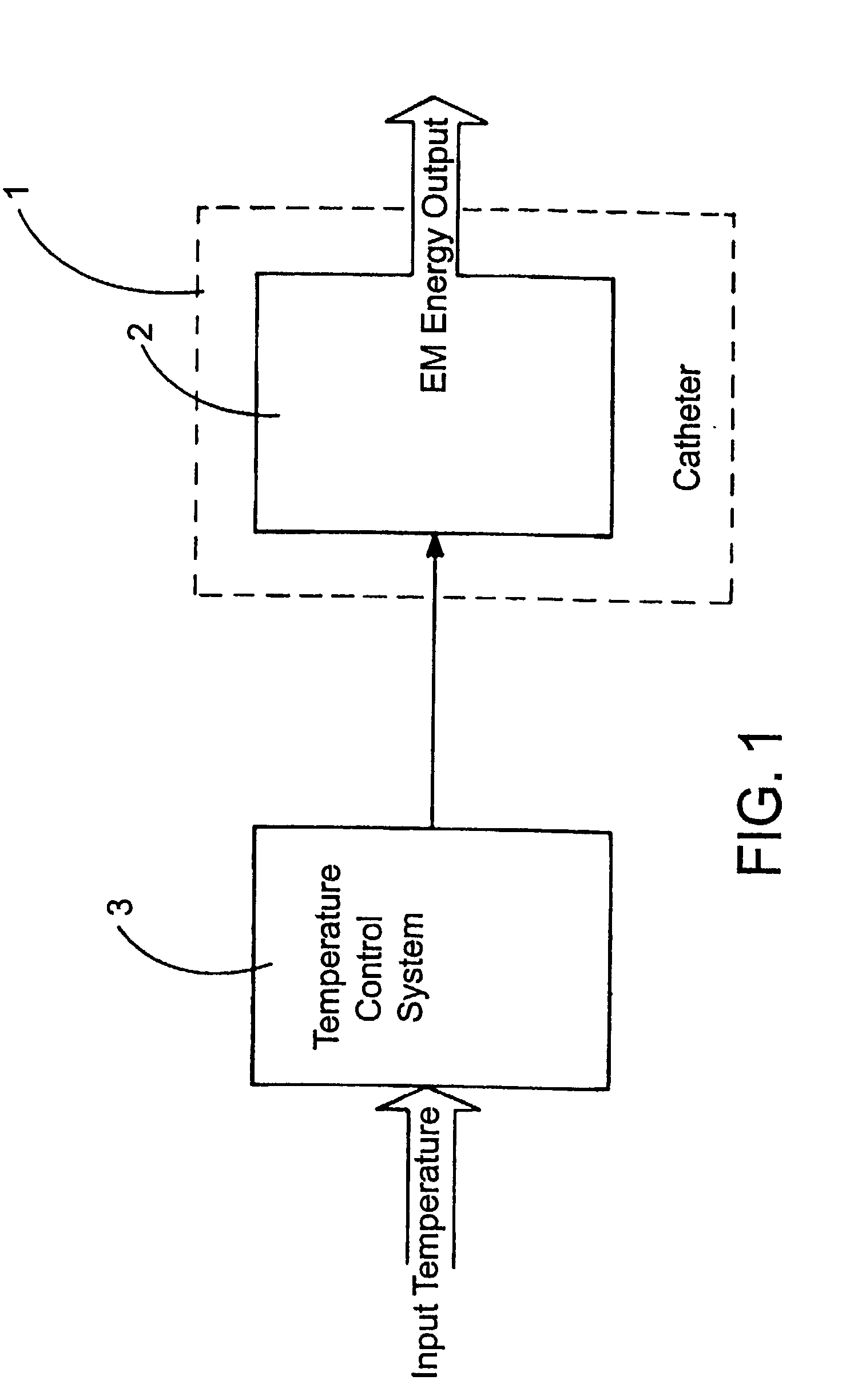
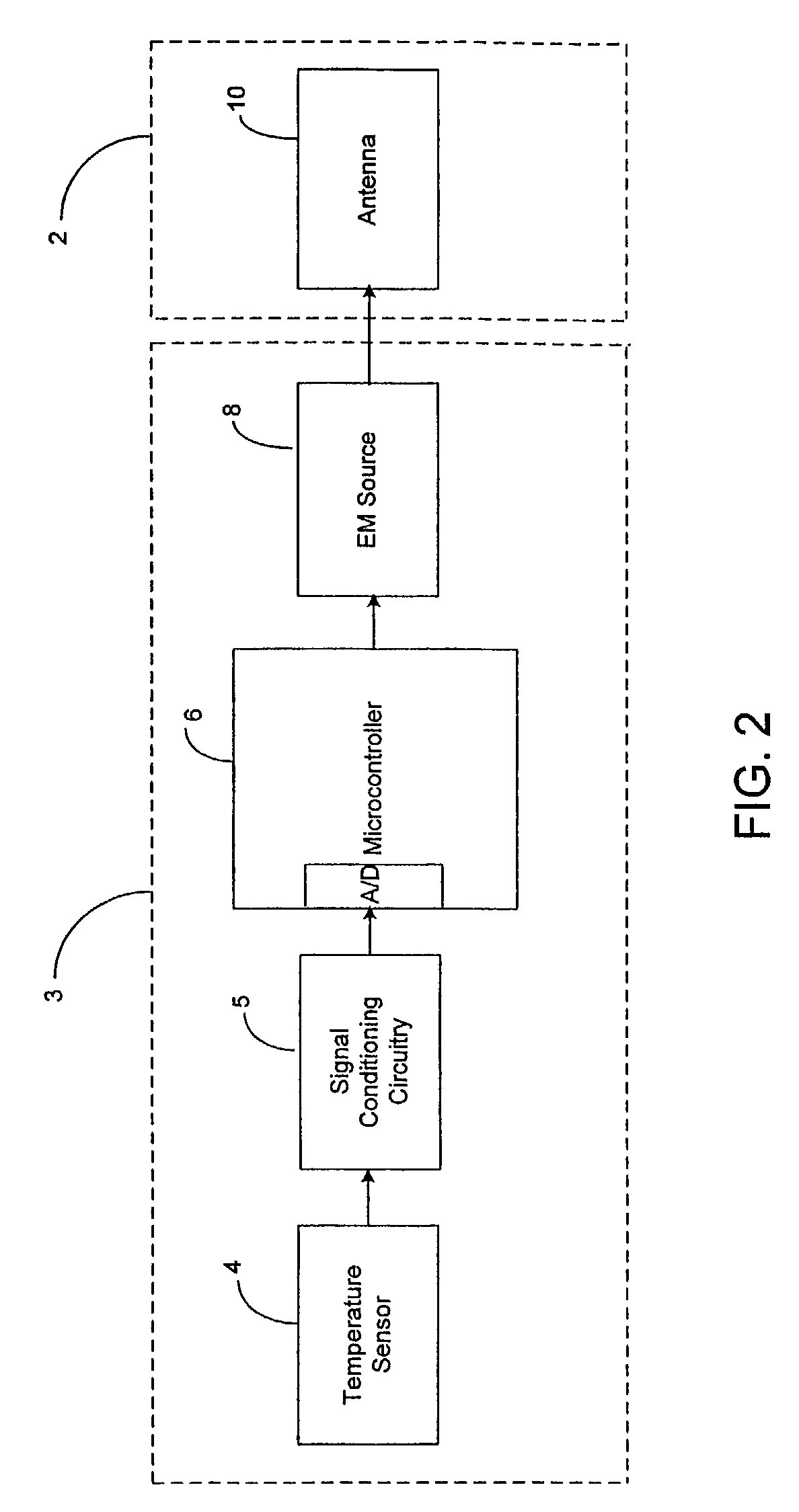
Thermal Ablation Design and Planning Methods
InactiveUS20090221999A1Improve efficiencyHigh success rateDiagnosticsSurgical systems user interfaceSimulation basedEngineering
Methods for simulation of heat transport phenomena applicable to the design of a near-field microwave ablation device, the design of such a device based on simulation and a patient planning and monitoring station using simulated thermal ablation of tissue are provided.
Owner:CALIFORNIA INST OF COMP ASSISTED SURGERY
Compound Having S1P Receptor Binding Potency and Use Thereof
InactiveUS20080207584A1Easy to optimizeEasy to separateBiocideNervous disorderAutoimmune conditionS1P Receptor
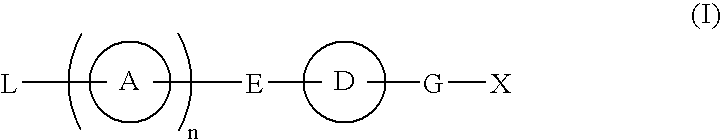
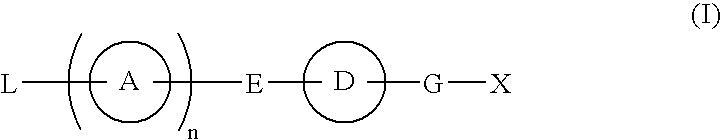
Provided are: a compound represented by formula (I):(wherein ring A and ring D each represent a cyclic group which may have a substituent(s); E and G each represent a bond or a spacer having 1 to 8 atoms in its main chain; L represents a hydrogen atom or a substituent; X represents amino which may have a substituent(s), or a heterocyclic group which contains at least one nitrogen atom and which may have a substituent(s); n represents 0 to 3, in which when n is 2 or more, a plurality of ring A's may be the same or different from one another); a salt thereof; an N-oxide form thereof; a solvate thereof, a prodrug thereof; and a medicament which includes those. The compound represented by formula (I) is capable of binding S1P receptors (in particular, EDG-1 and / or EDG-6), and useful for preventing and / or treating rejection in transplantation, autoimmune diseases, allergic diseases, etc.
Owner:ONO PHARMA CO LTD
Battery charger for lithium based batteries
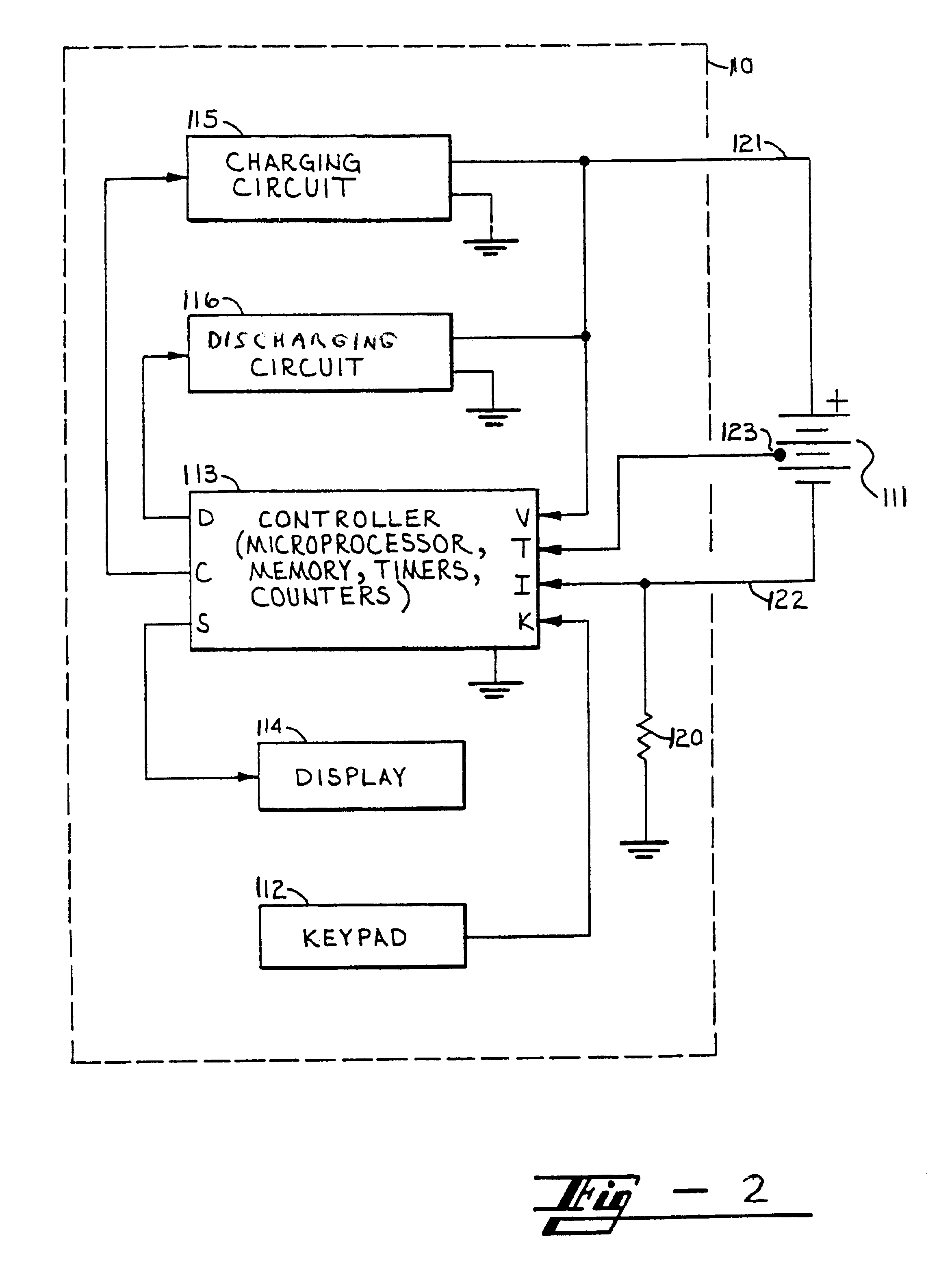
InactiveUS6366056B1Extend lifetime of battery packIncrease battery capacityBatteries circuit arrangementsSecondary cells charging/dischargingLoad circuitRest period
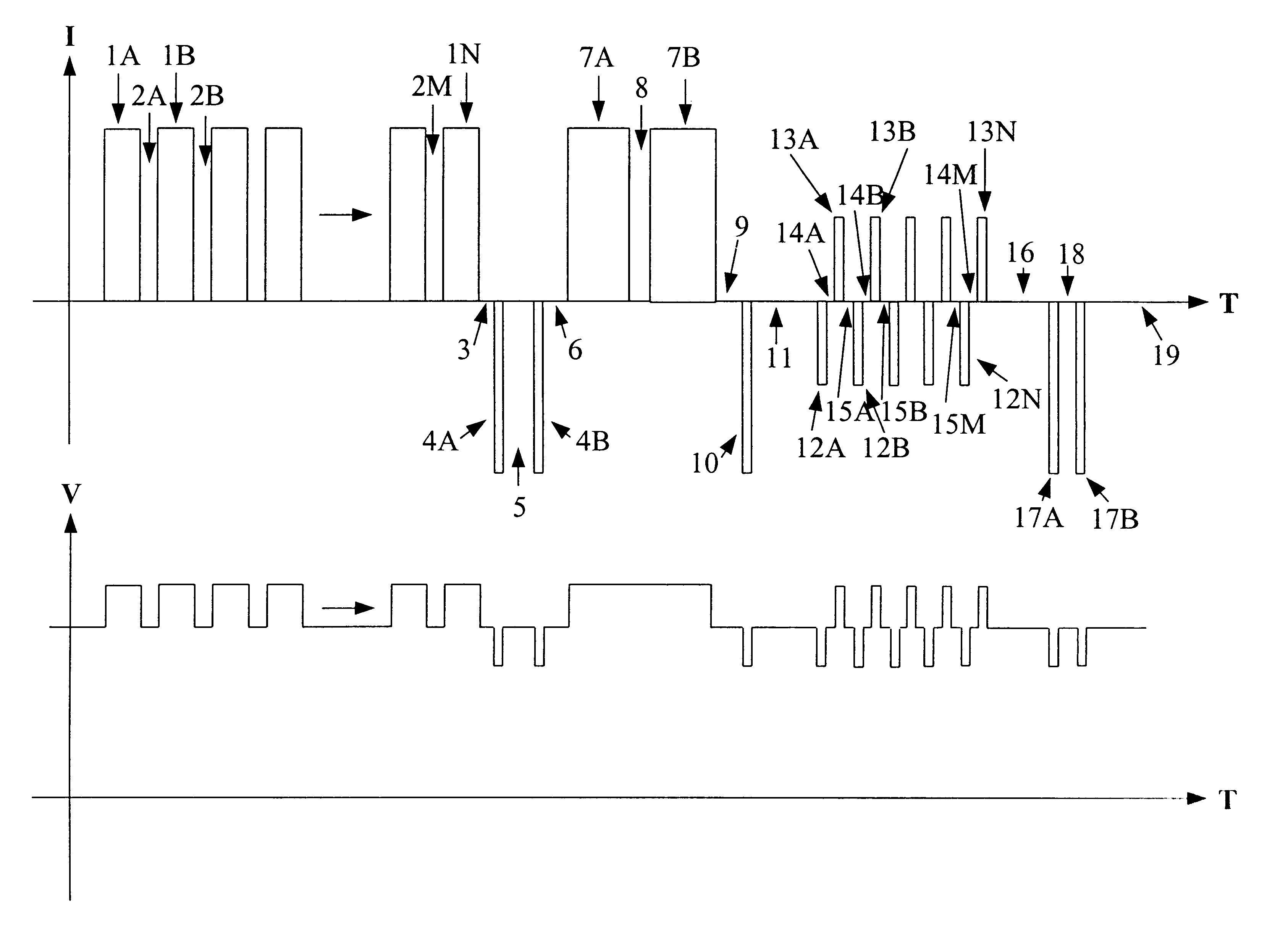
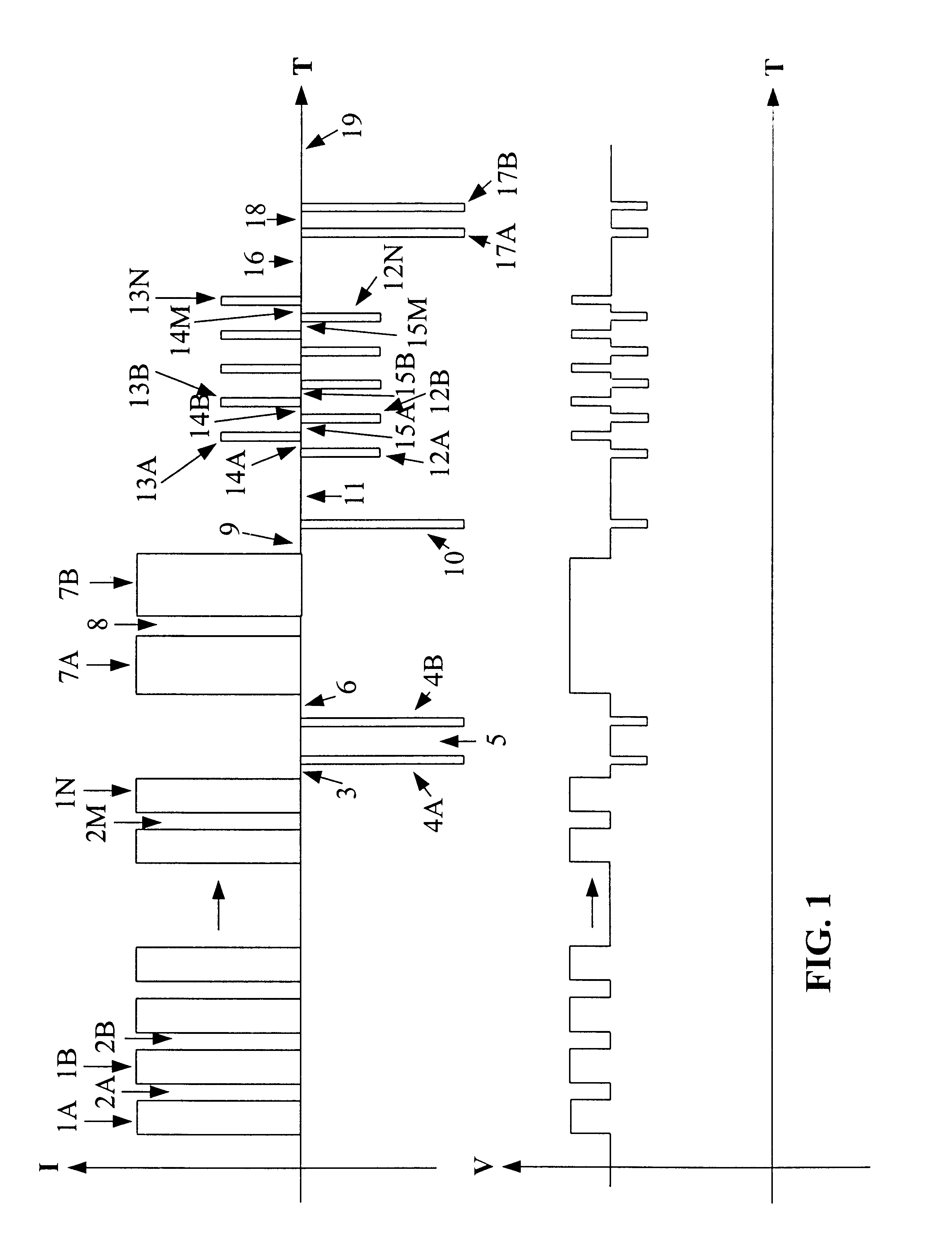
A method for charging a battery, such as a lithium based battery, which applies different charge pulses and discharge pulses to the battery, takes voltage measurements during those charge pulses, discharge pulses, and rest periods between the charge pulses and discharge pulses, and determines whether to terminate or to continue charging the battery. The full sequence of charge pulses, discharge pulses, and rest periods, includes a plurality of charge pulses (1), separated by rest periods (2) and followed by a rest period (3). This is then followed by a plurality of discharge pulses (4), separated by rest periods (5) and followed by a rest period (6). This is then followed by a plurality of extended charge pulses (7), separated by rest periods (8) and followed by a rest period (9). Then another discharge pulse (10) is applied, followed by a rest period (11). This is followed by a plurality of alternating charge pulses (13) and discharge pulses (12), separated by rest periods (13, 15) and followed by a rest period (16). Then another plurality of discharge pulses (17) is applied, separated by rest periods (18) and followed by a rest period (19). Open circuit voltage measurements taken during the rest periods, loaded circuit voltage measurements taken during the discharge pulses, and charge pulse voltage measurements taken during the charge pulses, are used to determine whether to continue or to terminate the charging of the battery.
Owner:ENREV
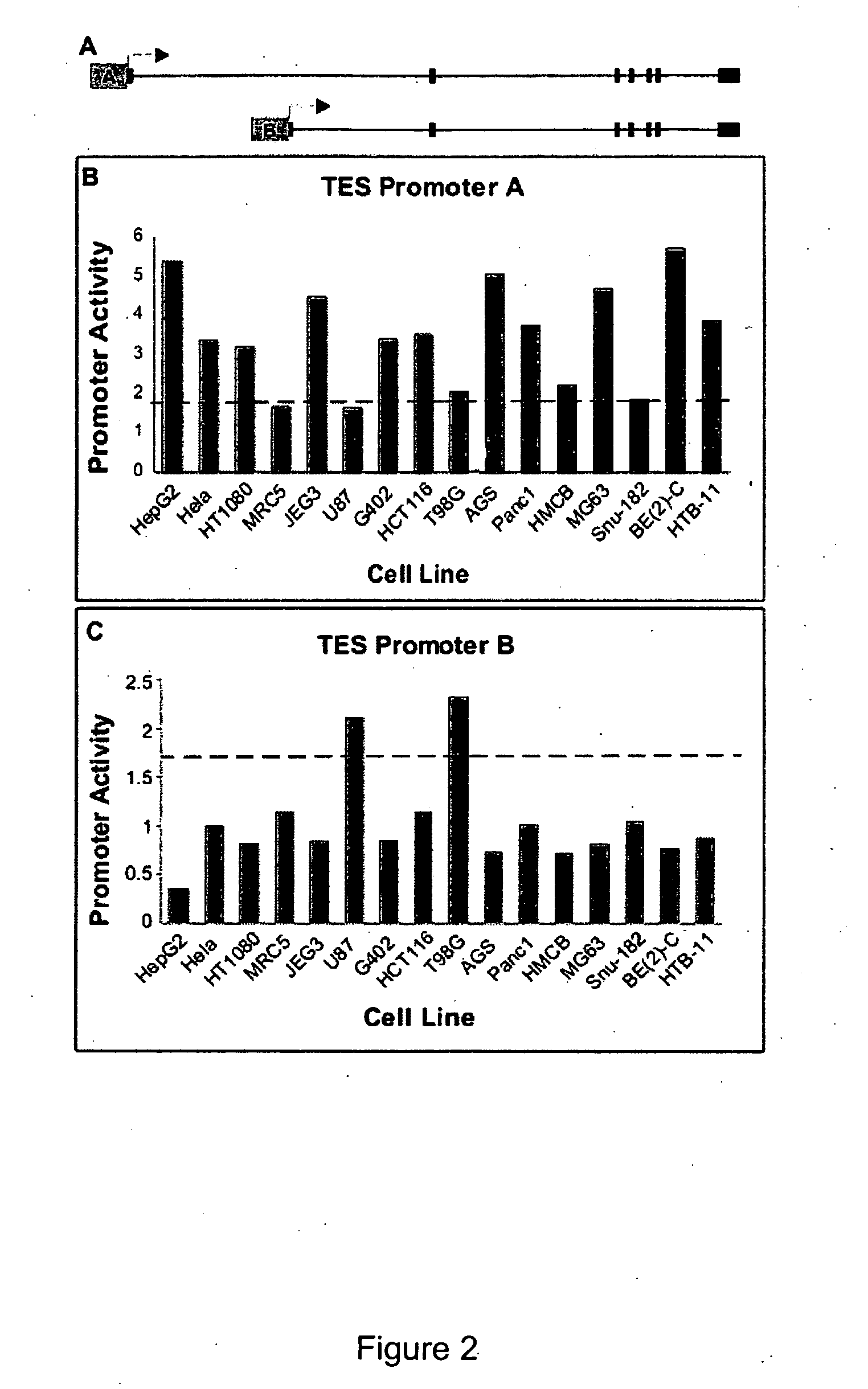
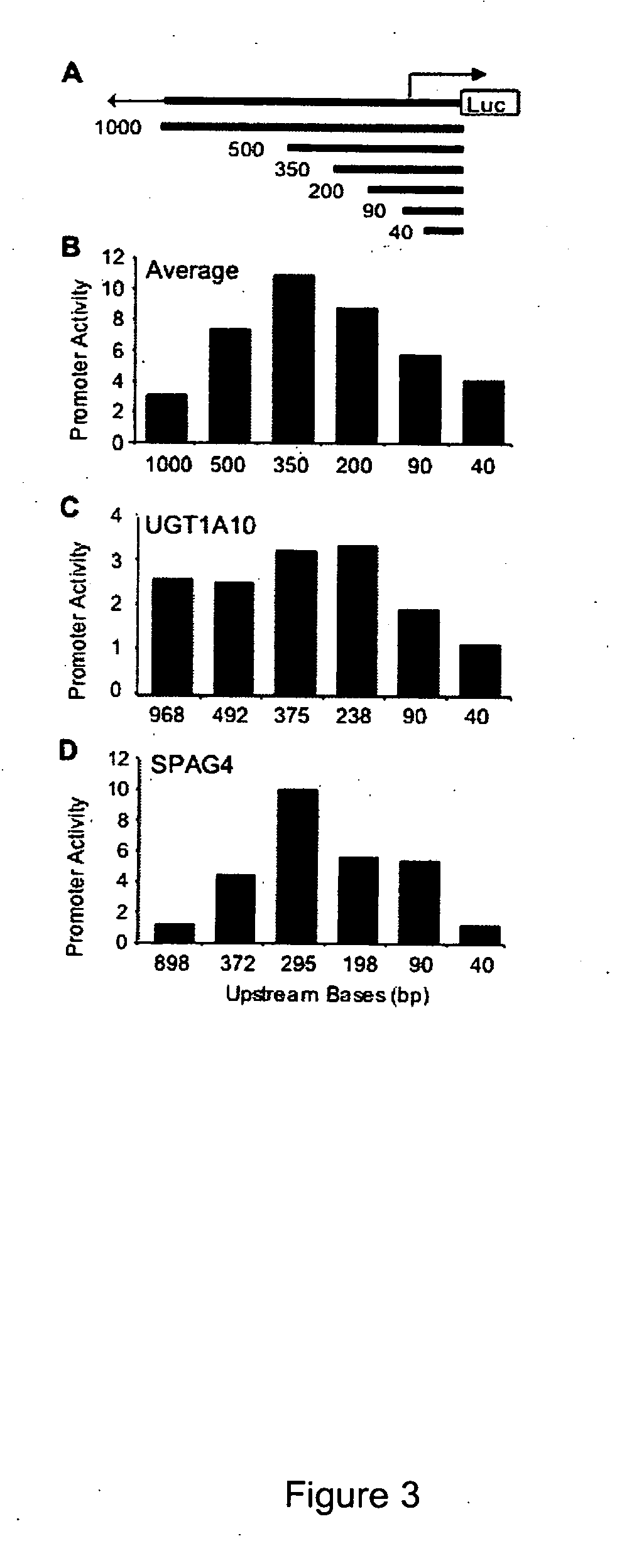
Functional arrays for high throughput characterization of gene expression regulatory elements
InactiveUS20070161031A1Good curative effectEliminate side effectsMicrobiological testing/measurementLibrary screeningGenomicsHuman DNA sequencing
The present invention provides compositions, kits, assemblies, libraries, arrays, and high throughput methods for large scale structural and functional characterization of gene expression regulatory elements in a genome of an organism, especially in a human genome. In one aspect of the invention, an array of expression constructs is provided, each of the expression constructs comprising: a nucleic acid segment operably linked with a reporter sequence in an expression vector such that expression of the reporter sequence is under the transcriptional control of the nucleic acid segment, the nucleic acid segment varying in the library and having a diversity of at least 50. The nucleic acid segments can be a large library of gene expression regulatory elements such as transcriptional promoters. The present invention can have a wide variety of applications such as in personalized medicine, pharmacogenomics, and correlation of polymorphisms with phenotypic traits.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Cell penetrating peptide conjugates for delivering of nucleic acids into a cell
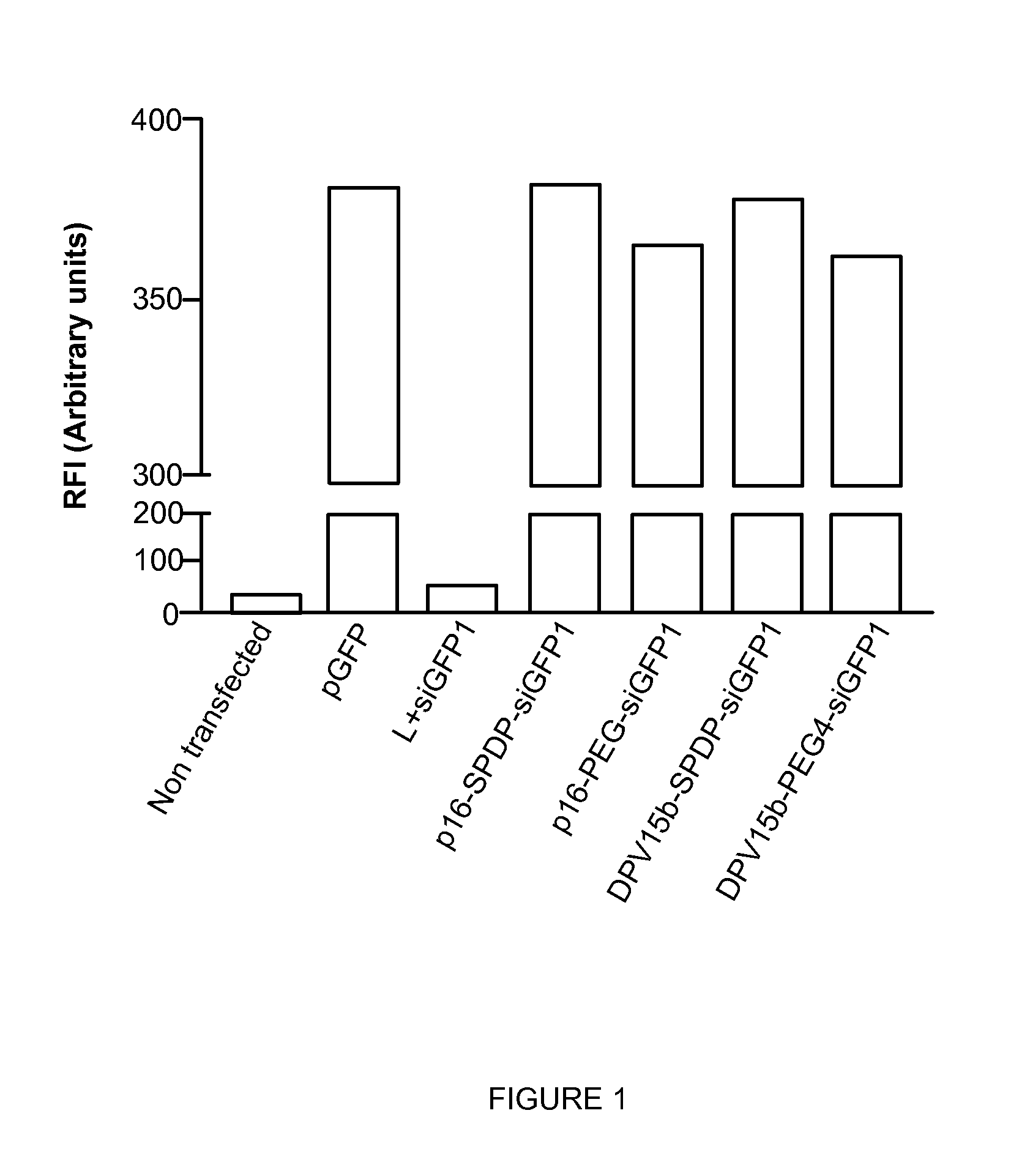
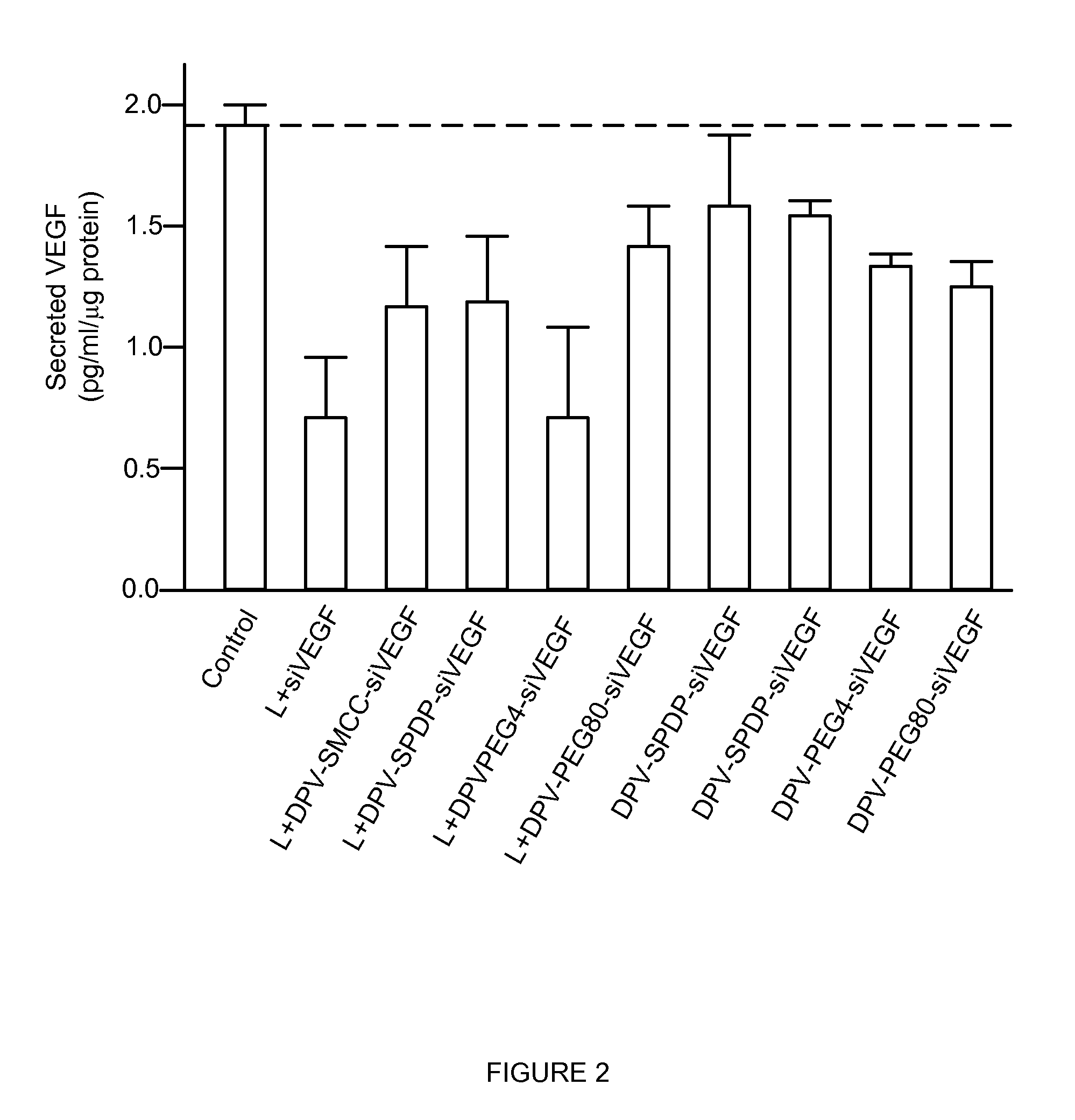
InactiveUS20130137644A1Good curative effectLarge indexNervous disorderAntipyreticHydrophilic polymersPolyethylene glycol
The invention provides cell penetrating peptide-nucleic acid conjugates having the formula P-L-N, wherein P is a cell penetrating peptide, N is a nucleic acid, preferably an oligonucleotide and more preferably a siRNA, and L is a hydrophilic polymer, preferably a polyethylene glycol (PEG)-based linker linking P and N together. Compositions, methods of use and methods for producing such conjugates are also disclosed.
Owner:CELLECTIS SA
Inducing cellular immune responses to human papillomavirus using peptide and nucleic acid compositions
InactiveUS20070014810A1Reduce the possibilityImproving immunogenicitySugar derivativesViral antigen ingredientsEpitopeT cell
This invention uses our knowledge of the mechanisms by which antigen is recognized by T cells to identify and prepare human papillomavirus (HPV) epitopes, and to develop epitope-based vaccines directed towards HPV. More specifically, this application communicates our discovery of pharmaceutical compositions and methods of use in the prevention and treatment of HPV infection.
Owner:GENIMMUNE NV +1
Methods and Compositions for Treating Migraine Pain
InactiveUS20100029665A1Benefit maximizationEliminate side effectsBiocideNervous disorderHeadache severeVascular headache
The present invention provides novel methods and compositions for the treatment and prevention of headaches, vascular headaches, migraine headaches, cluster headaches, and migraine. One of the headaches, vascular headaches, migraine headaches, cluster headaches, and migraine treated by the methods and compositions of the invention is migraine.
Owner:MEYERSON LAURENCE R +3
Method of hydration; infusion packet system(s), support member(s), delivery system(s), and method(s); with business model(s) and Method(s)
InactiveUS20020012689A1Constant deliveryUniform deliveryBiocideOrganic active ingredientsDiagnostic Radiology ModalityDietary supplement
Liquid activated infusion packet(s) / system, promoting hydration, containing active and / or inactive ingredients and / or a support member(s). Infusion Packet(s) / System is one or more individual compartments, and / or group(s), whereby the enveloping material(s) may be totally or partially dissolvable, edible, transparent, opaque, decorated, etc. Further, including of one or more: color(s), flavor(s), aroma(s), pharmaceutical(s), nutraceutical(s), dietary supplement(s), enzyme(s), pre / pro-biotic(s), amino-acid(s), soluble-fiber(s), diagnostic agent(s) etc. regardless of form, + / - effervescence, + / - uniform / controlled-release encapsulations into liquid for humans and / or animals. Enveloping material may be in whole and / or in combination; non-synthetic / porous, and / or synthetic porous / non-porous with deliberate perforations. Infusion Packet(s) / System + / - tag, support member for assistance, consumer compliance: promotion, advertising, education, entertainment, (toy / game), etc. Manual and / or power operated parts, lights, noise, etc. Additionally incorporated; unique business modalities with test market opportunities and / or the ability to provide income and / or esteem for the health challenged.
Owner:STILLMAN SUZANNE JAFFE
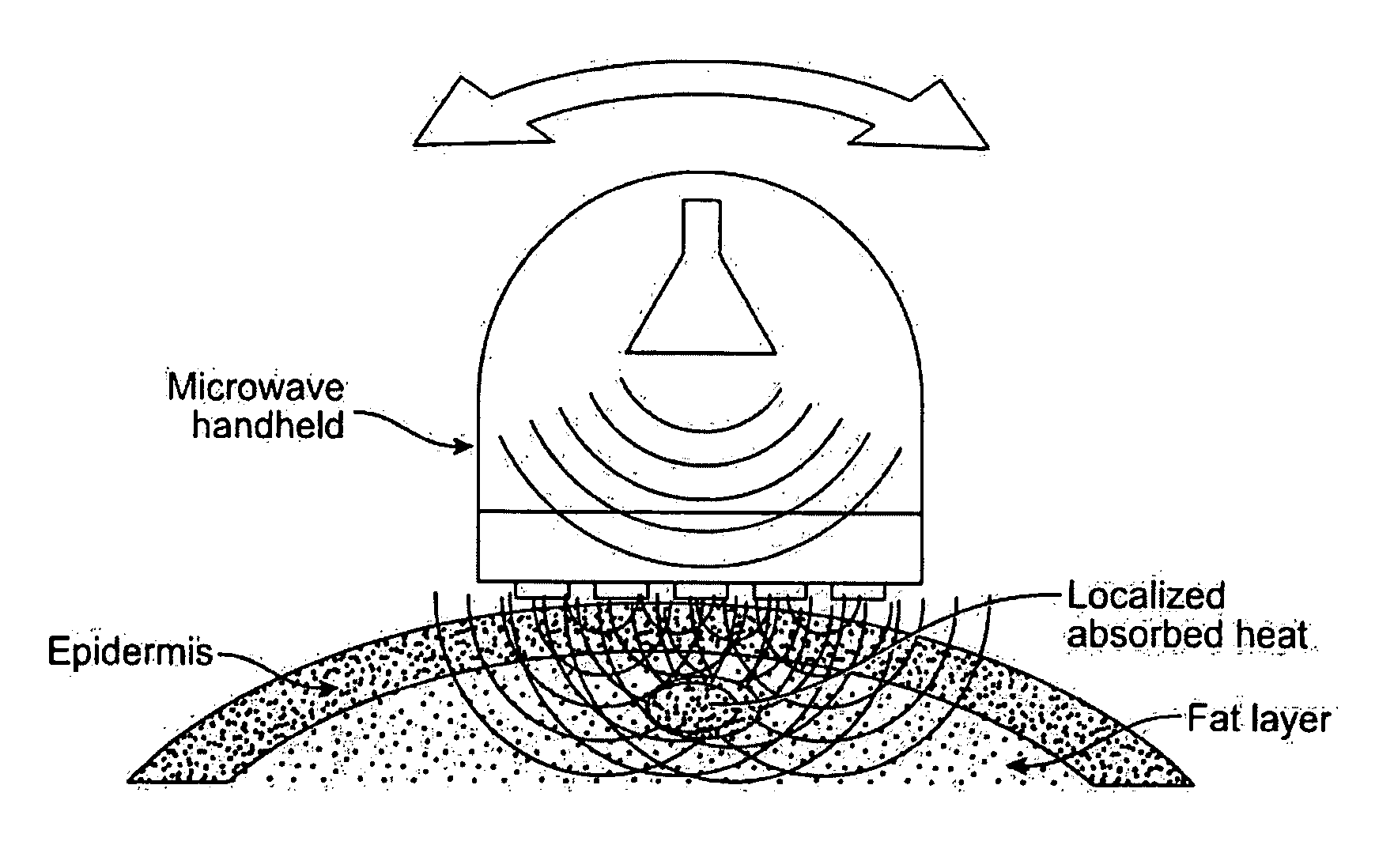
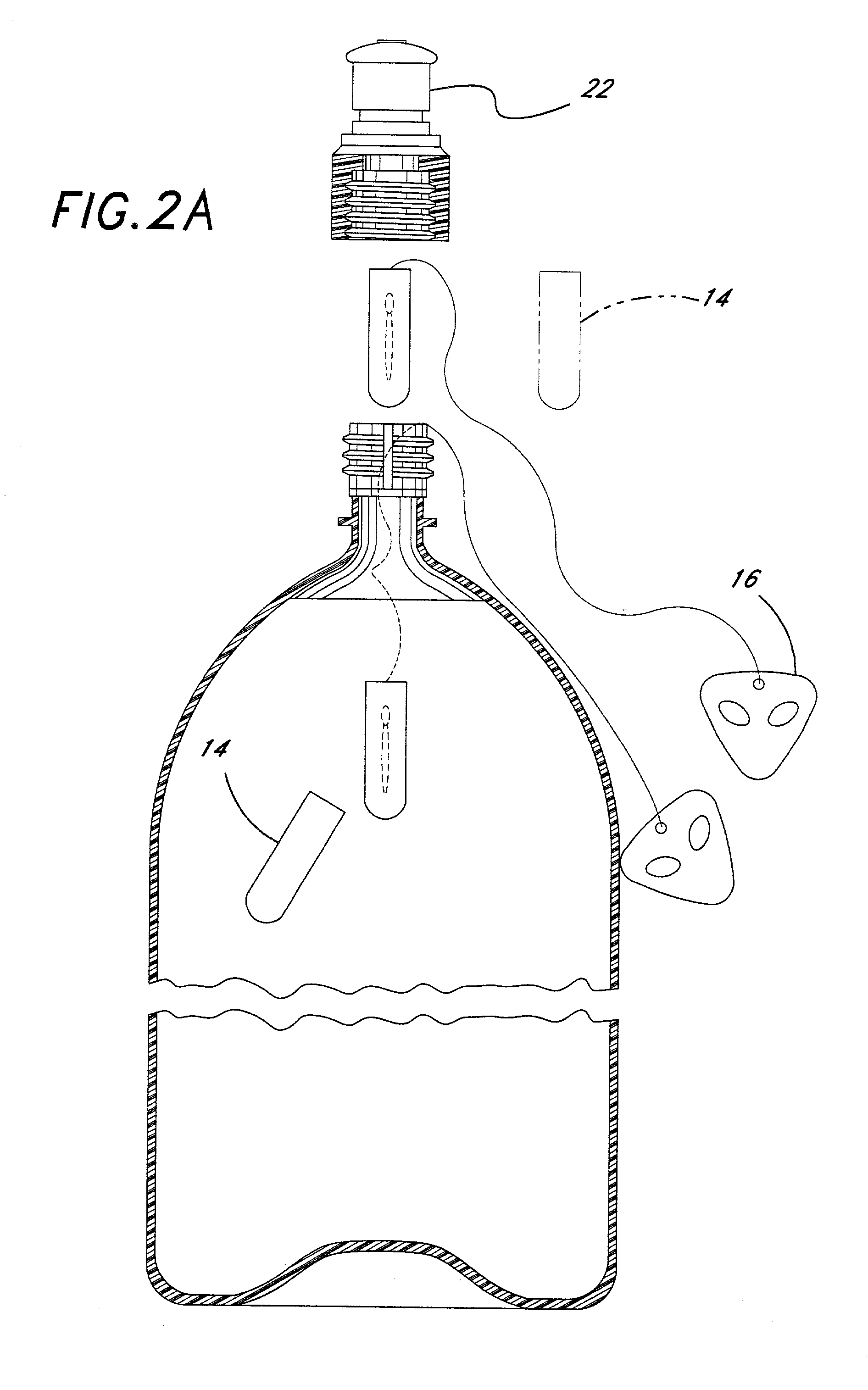
Therapeutic prostatic thermotherapy
InactiveUS7089064B2Reducing treatmentEliminate side effectsMicrowave therapySurgical instruments for heatingProstate thermotherapyIn patient
The present invention provides a method of treating a prostate in a patient in need thereof and a heating catheter, or an electromagnetic radiation applicator, system suitable for effecting the present inventive method. The present inventive method provides for substantial unexpected improvement in patient outcome by providing, inter alia, a preferred therapeutic temperature for thermotherapy of the prostate and a method of decreasing a patient's intolerance due to pain. The present inventive system provides for, inter alia, automatic implementation of the present inventive method.
Owner:BOSTON SCI SCIMED INC
Formulations for cell-schedule dependent anticancer agents
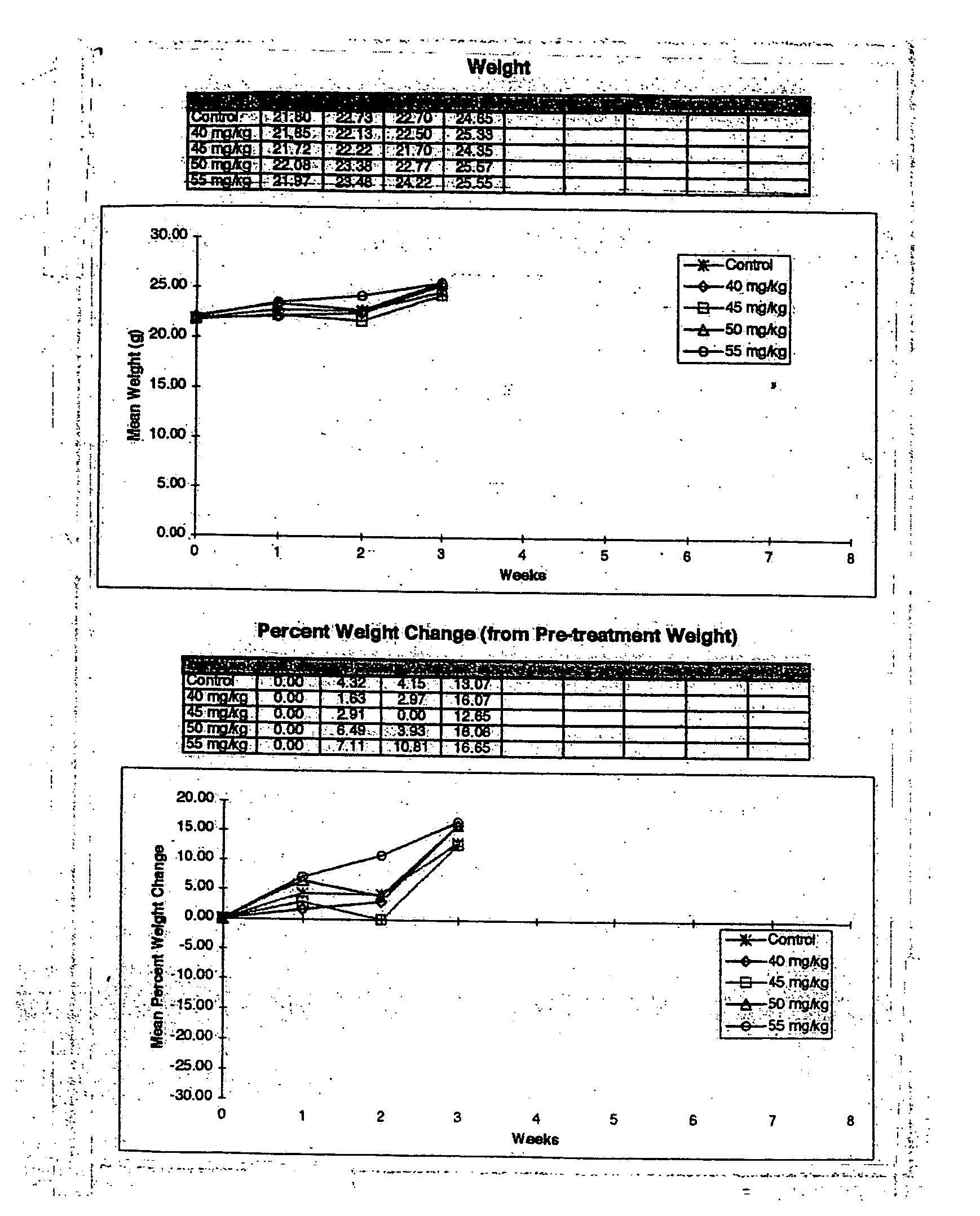
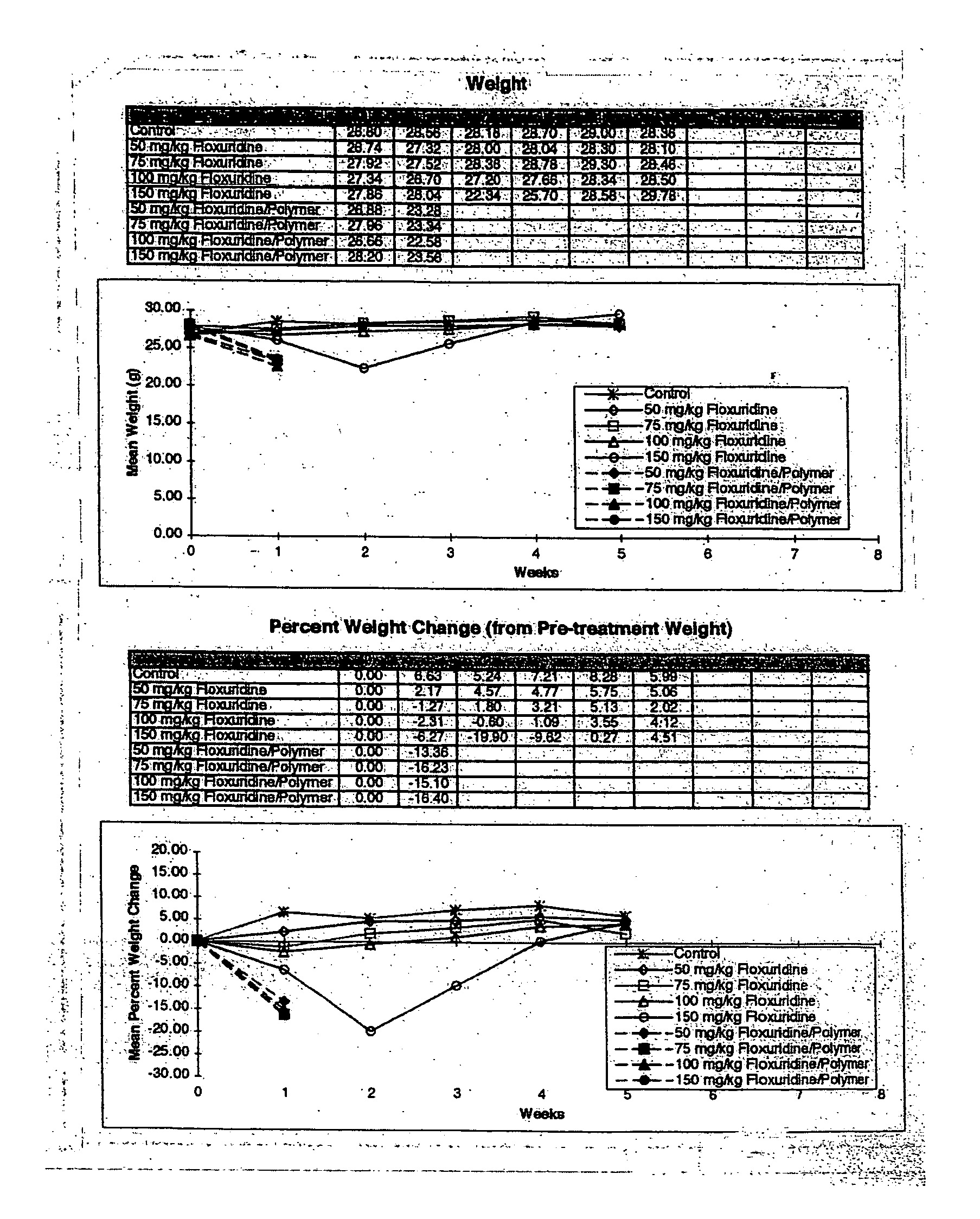
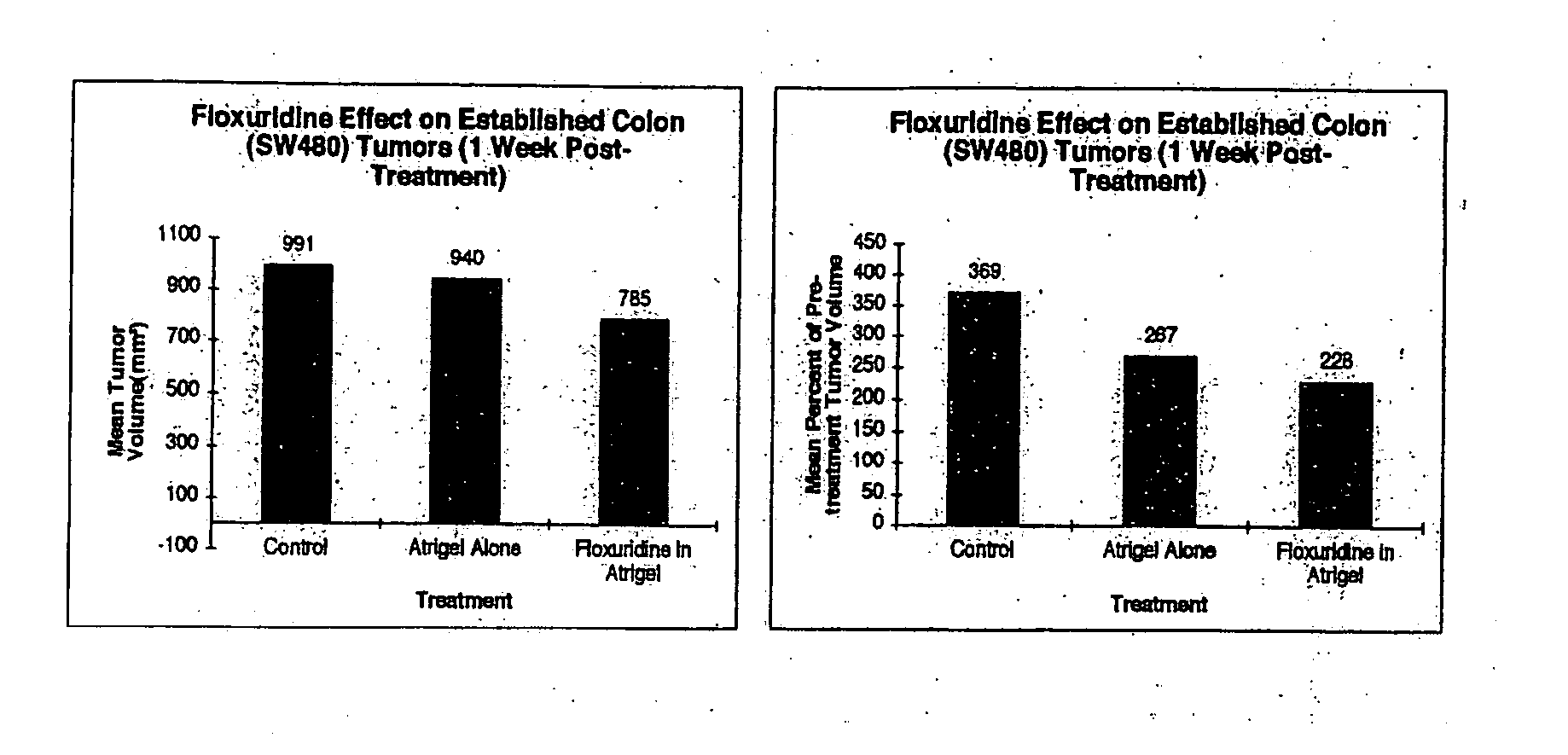
InactiveUS20060121085A1Strong specificityEliminate side effectsPowder deliveryBiocideMetaboliteAnticarcinogen
The present invention provides a flowable composition suitable for use as a controlled release implant. The composition includes: (a) a biodegradable, biocompatible thermoplastic polymer that is at least substantially insoluble in aqueous medium, water or body fluid; (b) a cell-cycle dependent biological agent, a schedule-dependent biological agent, a metabolite thereof, a pharmaceutically acceptable salt thereof, or a prodrug thereof; and (c) a biocompatible organic liquid, at standard temperature and pressure, in which the thermoplastic polymer is soluble. The present invention also provides a method of treating cancer in a mammal. The present invention also provides a method of blocking, impeding, or otherwise interfering with cell cycle progression at the G1-phase, G1 / S interphase, S-phase, G2 / M interface or M-phase of the cell cycle in a mammal. The methods includes administering to a mammal an effective amount of a flowable composition of the present invention.
Owner:QLT USA INC
Methods and devices for the treatment of ocular diseases in human subjects
InactiveUS20150258120A1Reduce in quantityReduce severityOrganic active ingredientsPowder deliveryDiseaseMicroparticle
Methods and devices are provided for targeted non-surgical administration of a drug formulation to the suprachoroidal space (SCS) of the eye of a human subject for the treatment of a posterior ocular disorder or a choroidal malady. In one embodiment, the method comprises inserting a hollow microneedle into the eye at an insertion site and infusing a drug formulation through the inserted microneedle and into the suprachoroidal space of the eye, wherein the infused drug formulation flows within the suprachoroidal space away from the insertion site during the infusion. In one embodiment, the fluid drug formulation comprises drug nanoparticles or microparticles.
Owner:CLEARSIDE BIOMEDICAL
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com