Patents
Literature
1490 results about "Nasal administration" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Nasal administration is a route of administration in which drugs are insufflated through the nose. It can be a form of either topical administration or systemic administration, as the drugs thus locally delivered can go on to have either purely local or systemic effects. Nasal sprays are locally acting drugs such as decongestants for cold and allergy treatment, whose systemic effects are usually minimal. Examples of systemically active drugs available as nasal sprays are migraine drugs, nicotine replacement, and hormone treatments.
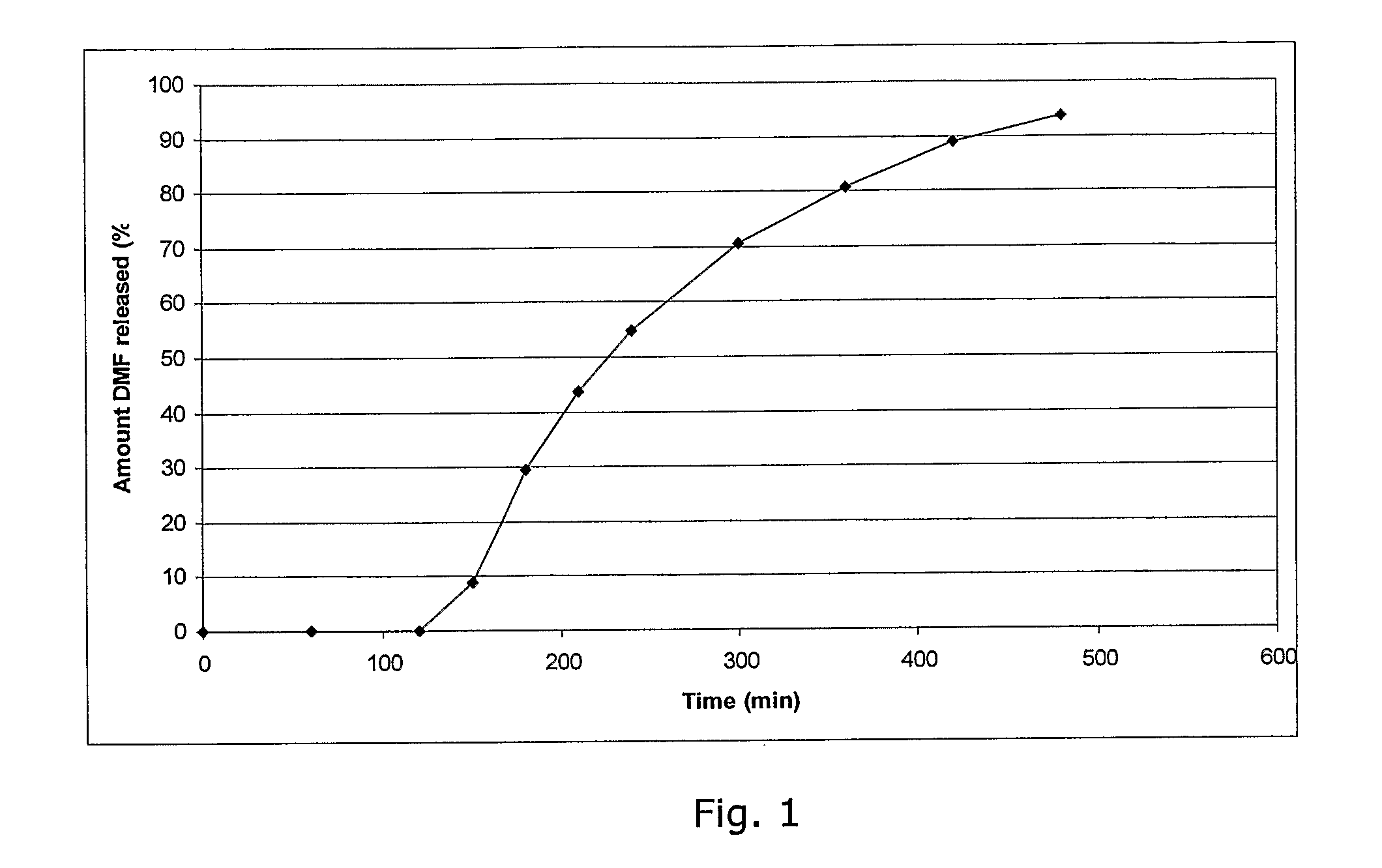
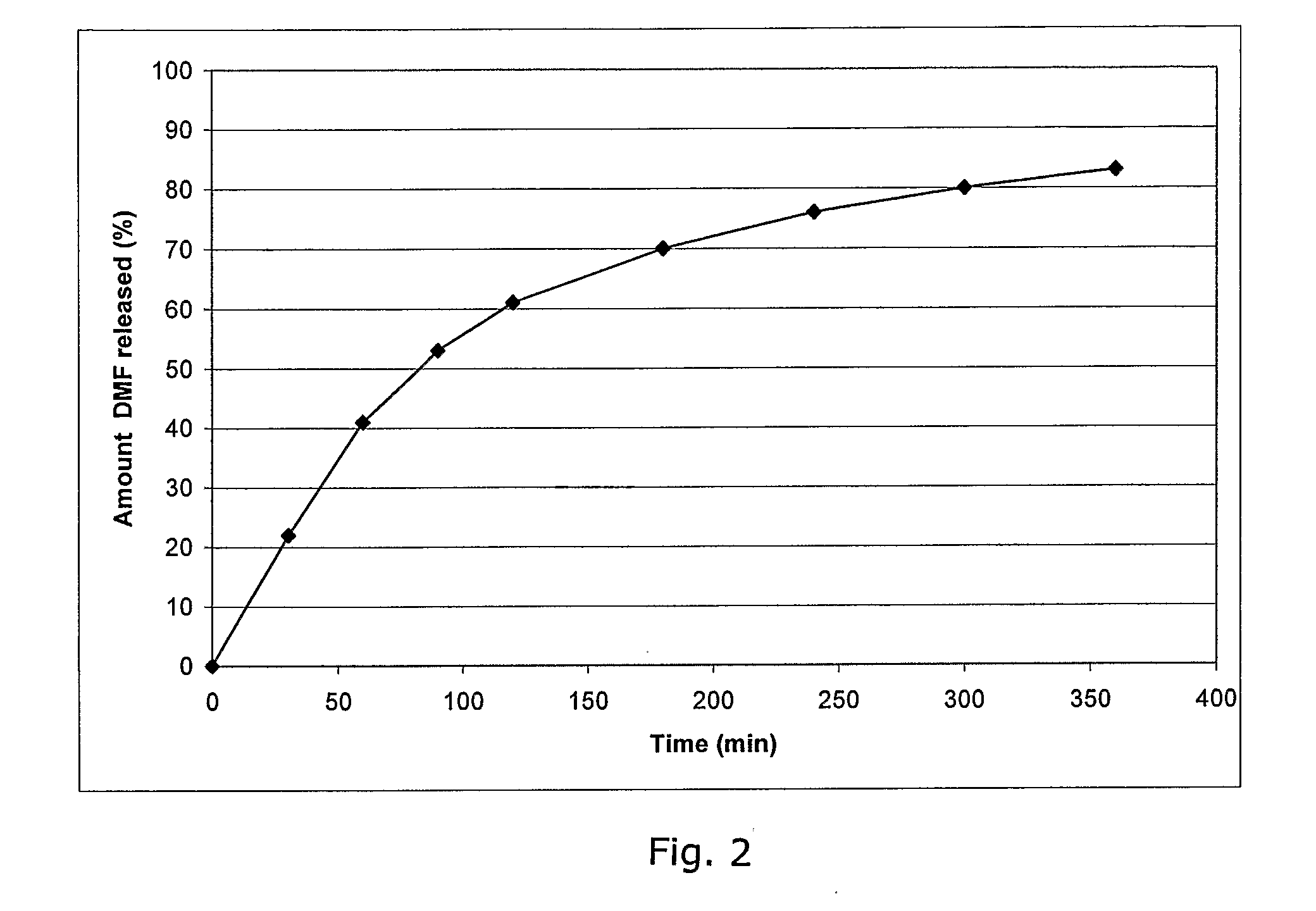
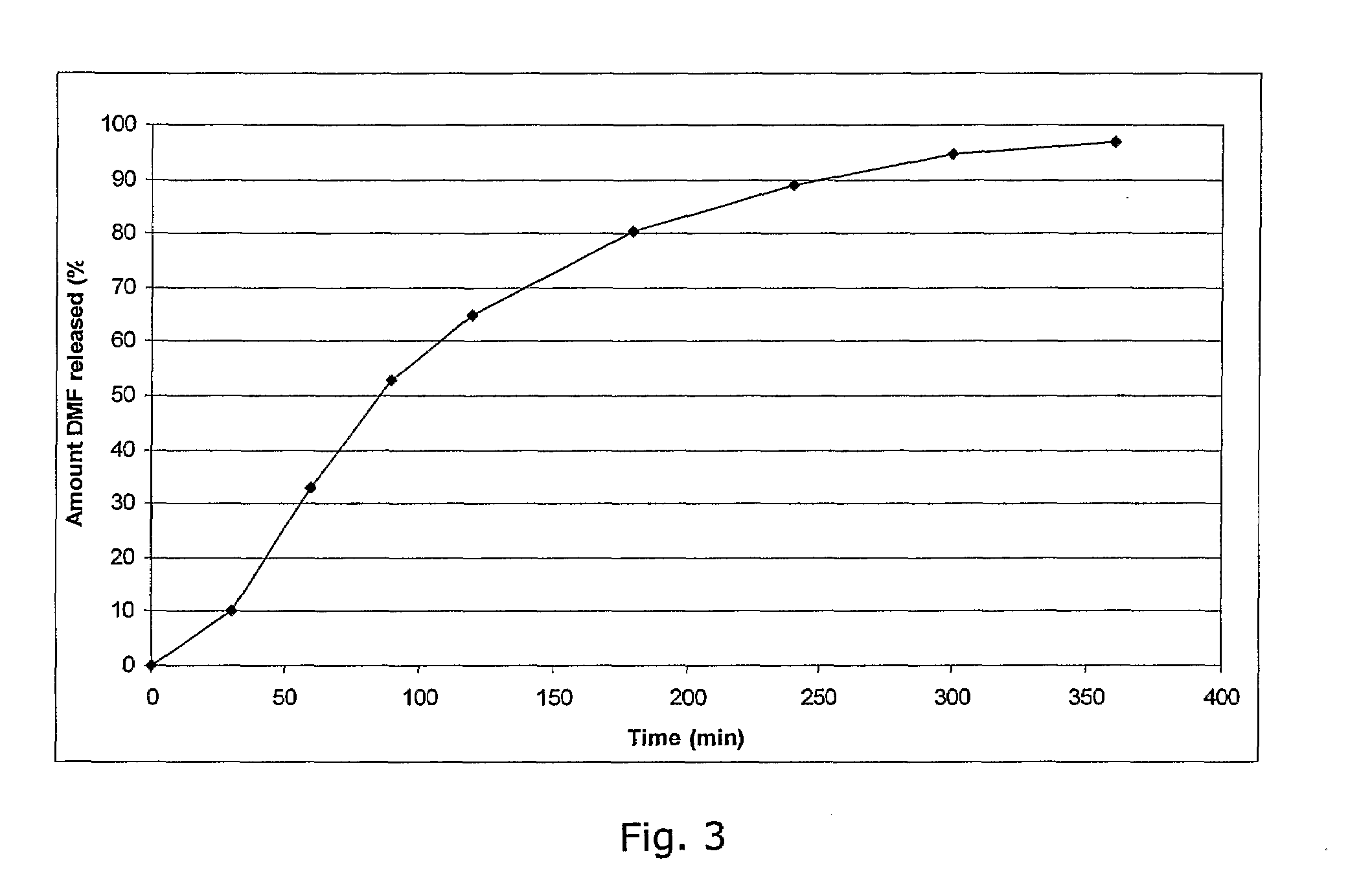
Compositions for nasal drug delivery, methods of making same, and methods of removing residual solvent from pharmaceutical preparations
InactiveUS6391452B1Taller in heightSlow down the mixing speedLiquid surface applicatorsPeptide/protein ingredientsNasal cavityNose
The present invention relates to pharmaceutical compositions for delivery of drugs intended to reside in the nose, compositions for nasal administration of drugs, e.g., antiviral agents, and particularly antiviral agents comprising the human major rhinovirus receptor, also known as intercellular adhesion molecule-1 (ICAM-1); to methods of making said nasal drug compositions, and to an improved process for the removal of residual solvent from pharmaceutical matrices.
Owner:BAYER PHARMA CORP
Pharmaceutical formulation containing opioid agonist,opioid antagonist and gelling agent
InactiveUS20030068371A1Reduce and eliminate effectInhibition effectBiocideNervous disorderOpioid antagonistOpioid Agonist
Disclosed in certain embodiments is an oral dosage form comprising a therapeutically effective amount of an opioid analgesic, an opioid antagonist and one or more pharmaceutically acceptable excipients; the dosage form further including a gelling agent in an effective amount to impart a viscosity unsuitable for administration selected from the group consisting of parenteral and nasal administration to a solubilized mixture formed when the dosage form is crushed and mixed with from about 0.5 to about 10 ml of an aqueous liquid.
Owner:PURDUE PHARMA LP
Intrathecal administration of rituximab for treatment of central nervous system lymphomas
InactiveUS20020009444A1Prevent intermolecular disulfide formationPromote recoveryBiocidePeptide/protein ingredientsMeningesImmunocompromised patient
This invention describes methods of using anti-B cell antibodies, preferably anti-CD20 antibodies, and most preferably Rituximab, to treat B cell lymphomas of the brain, especially primary central nervous system lymphomas (PCNSLs), and to prevent meningeal relapse. The antibodies can be administered intrathecally alone, or in combination with other chemotherapeutics, such as methotrexate, or other anti-B cell antibodies to treat PCNSL in both immunocompromised and non-immunocompromised patients. These antibodies can also be used to diagnose patients with CNS lymphoma, especially in immunocompromised patients.
Owner:BIOGEN INC
Sustained release pharmaceutical compositions for the parenteral administration of hydrophilic compounds
InactiveUS7157099B2Easy to prepareOrganic active ingredientsPeptide/protein ingredientsParenteral nutritionBULK ACTIVE INGREDIENT
Owner:ITALFARMACO SPA
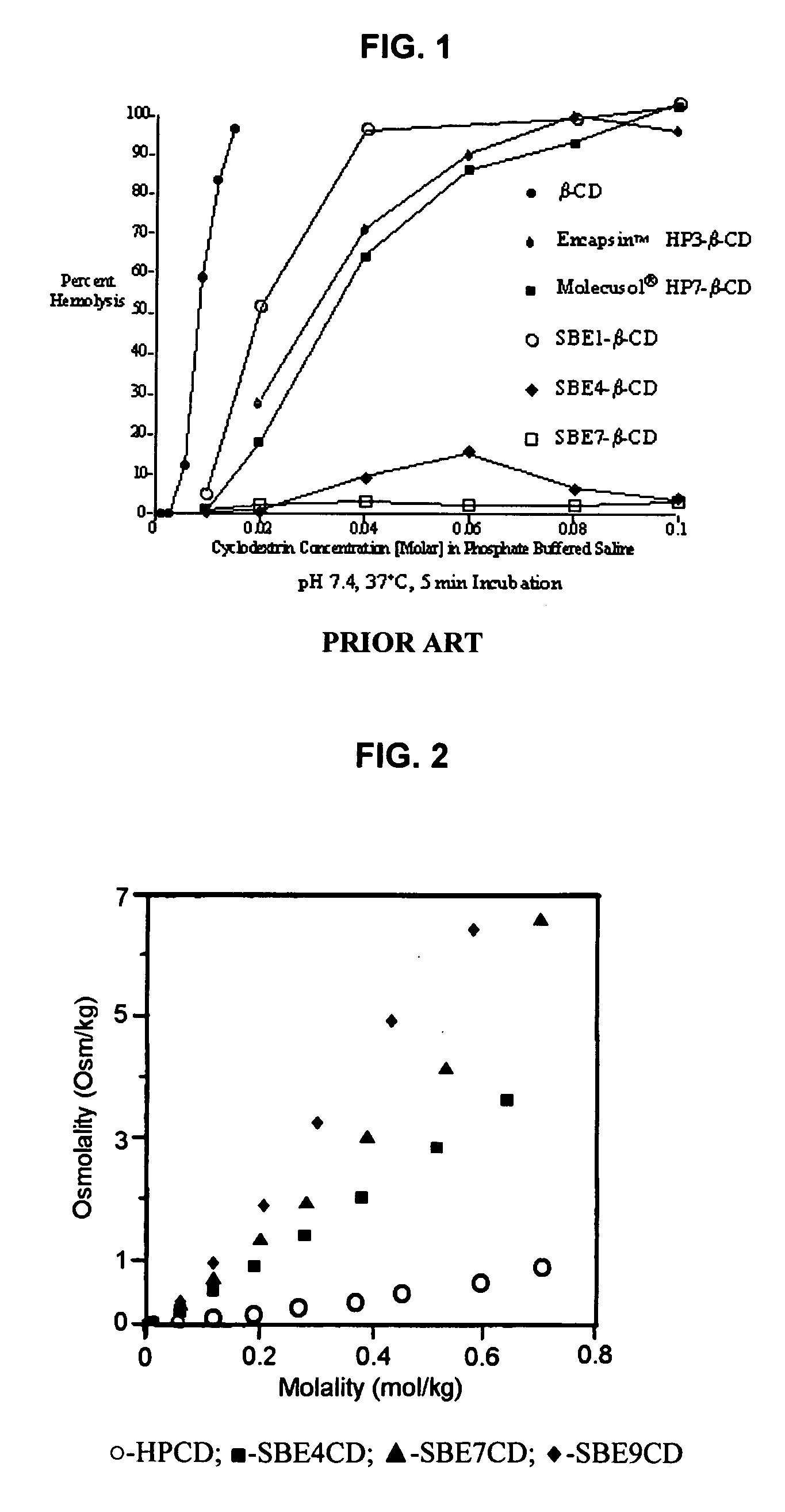
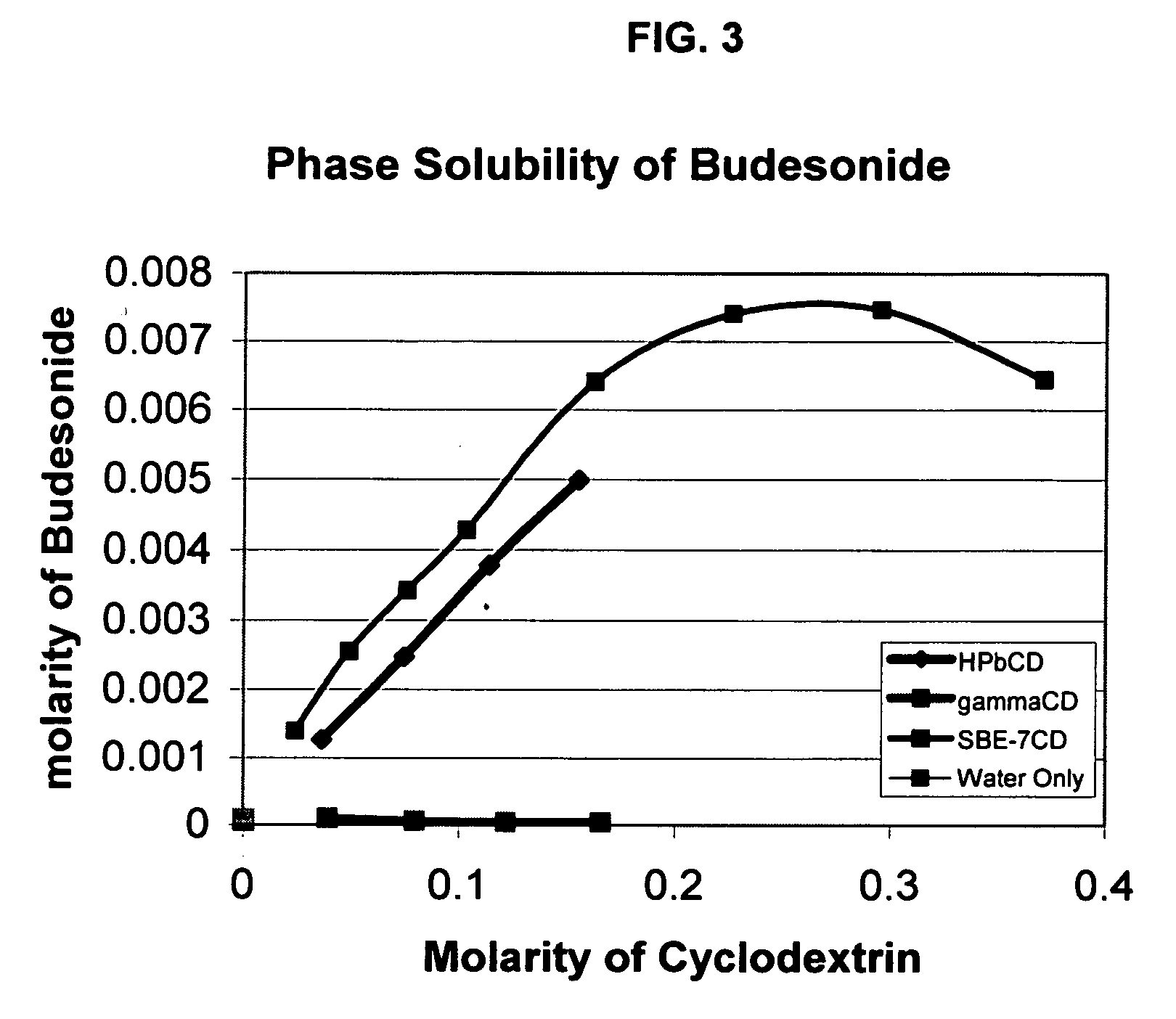
Inhalant formulation containing sulfoalkyl ether cyclodextrin and corticosteroid prepared from a unit dose suspension
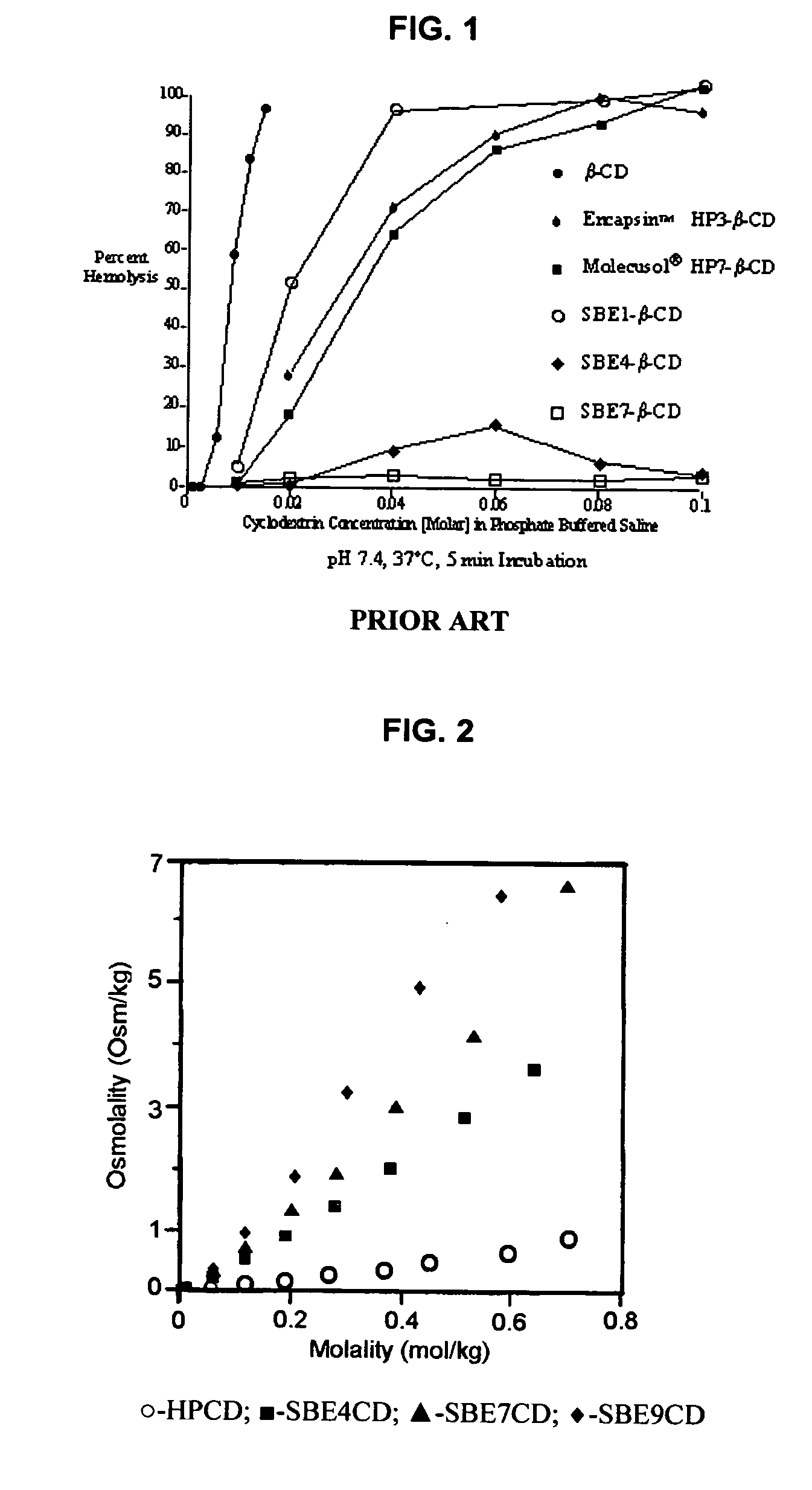
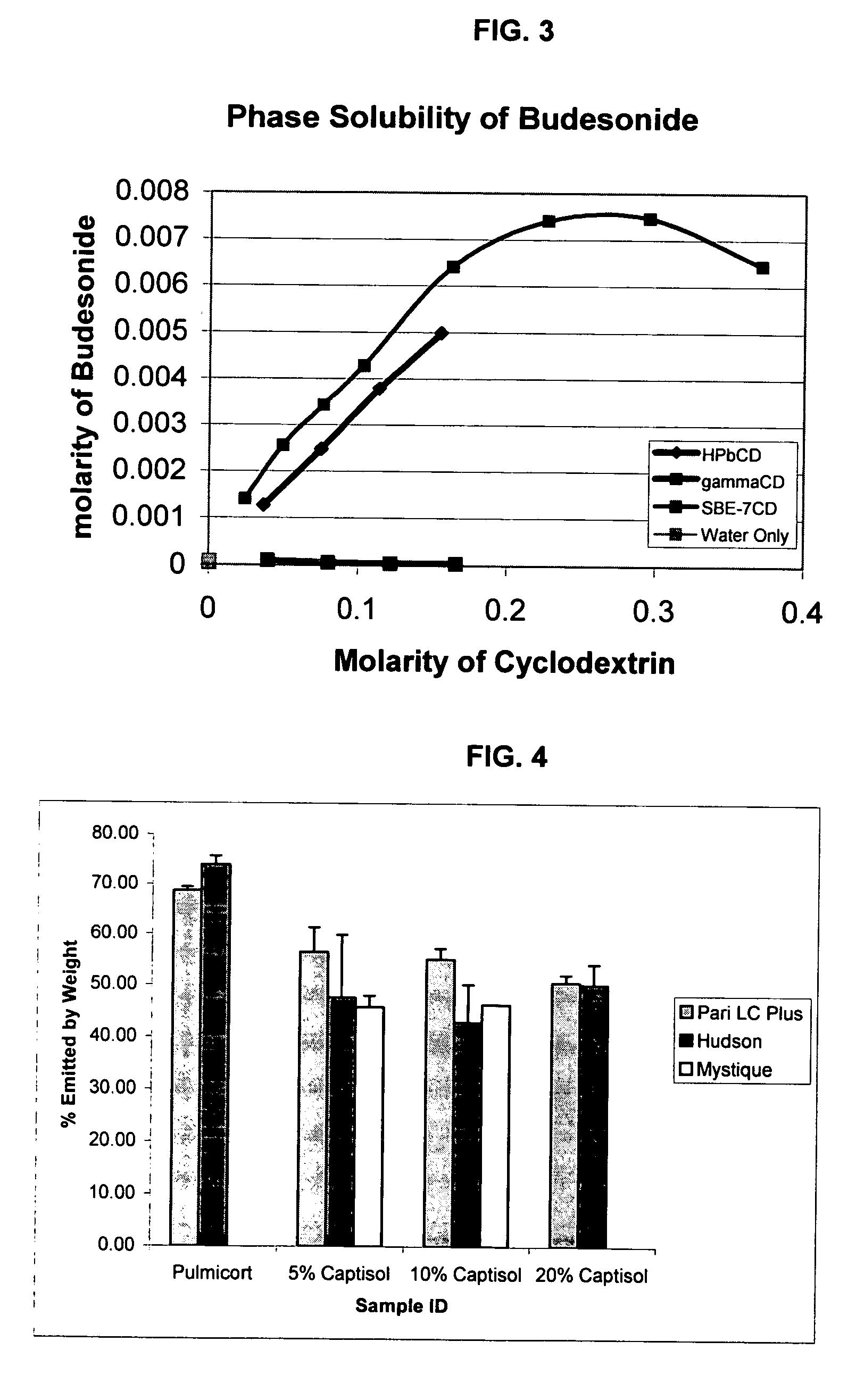
InactiveUS20070020196A1Reduce the degradation rateIncrease productivityBiocideDispersion deliveryNebulizerCyclodextrin
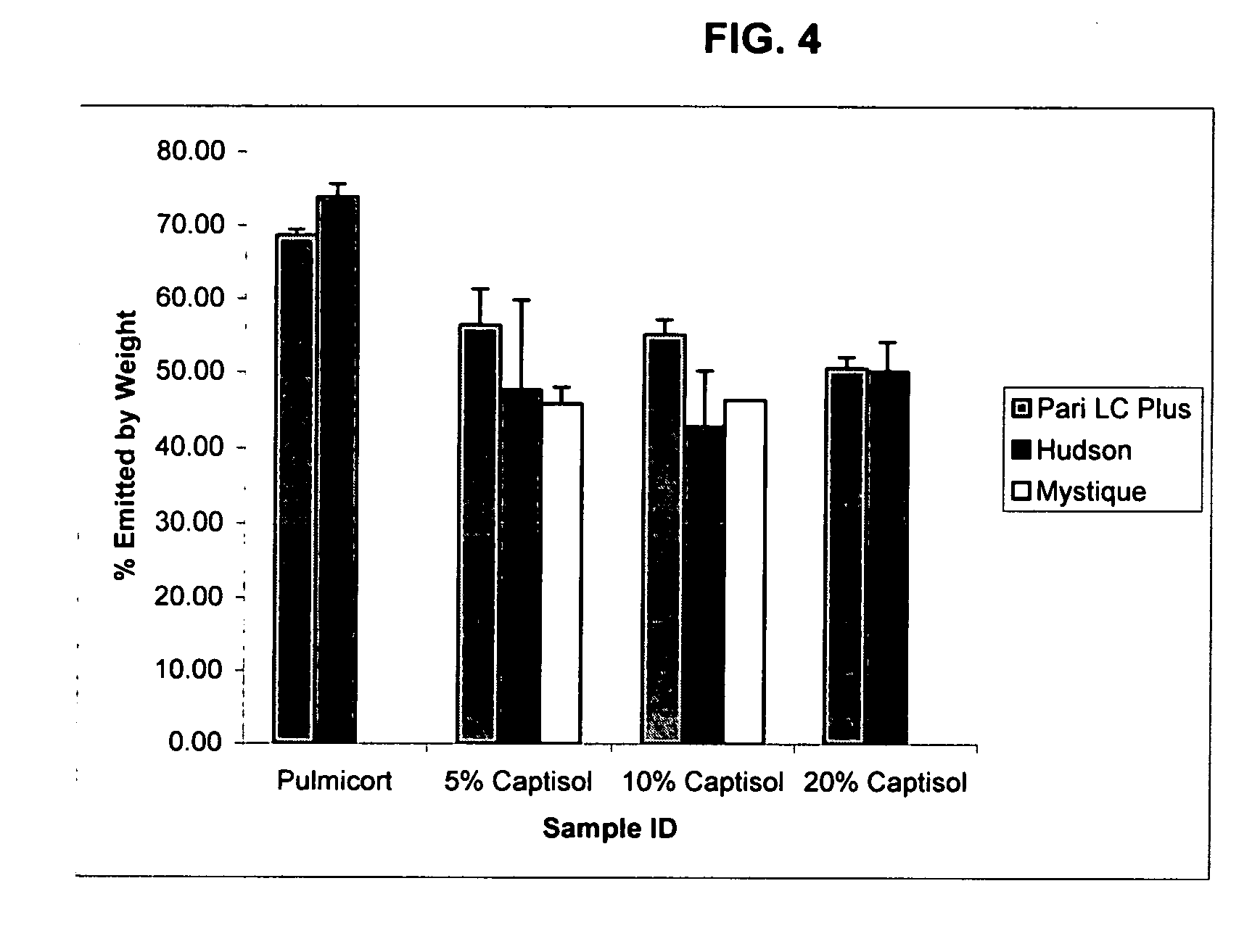
An inhalable unit dose liquid formulation containing SAE-CD and corticosteroid is provided. The formulation is adapted for administration to a subject by nebulization with any known nebulizer. The formulation can be included in a kit. The formulation is administered as an aqueous solution or concentrated composition. The formulation is employed in an improved nebulization system for administering corticosteroid by inhalation. SAE-CD present in the formulation significantly enhances the chemical stability of corticosteroid, such as budesonide. A method of administering the formulation by inhalation is provided. The formulation can also be administered by conventional nasal delivery apparatus. The formulation is prepared by mixing SAE-CD, in solid or liquid (dissolved) form, with an inhalable suspension-based unit dose formulation.
Owner:CYDEX PHARMACEUTICALS INC
Pharmaceutical formulation containing opioid agonist, opioid antagonist and gelling agent
InactiveUS7842307B2Reducing abuse potential of dosage formLower potentialBiocideNervous disorderOpioid antagonistOpioid Agonist
Disclosed in certain embodiments is an oral dosage form comprising a therapeutically effective amount of an opioid analgesic, an opioid antagonist and one or more pharmaceutically acceptable excipients; the dosage form further including a gelling agent in an effective amount to impart a viscosity unsuitable for administration selected from the group consisting of parenteral and nasal administration to a solubilized mixture formed when the dosage form is crushed and mixed with from about 0.5 to about 10 ml of an aqueous liquid.
Owner:PURDUE PHARMA LP
Omega-3 fatty acids and dyslipidemic agent for lipid therapy
InactiveUS20060211762A1Effective treatmentBiocideMetabolism disorderDyslipidemiaCoronary artery disease
A method and composition for blood lipid therapy by administering to the subject an effective amount of a dyslipidemic agent and omega-3 fatty acids. The method utilizes a single administration or a unit dosage of a combination of dyslipidemic agent and omega-3 fatty acids for the treatment of patients with hypertriglyceridemia, hypercholesterolemia, mixed dyslipidemia, coronary heart disease (CHD), vascular disease, artherosclerotic disease and related conditions, and the prevention or reduction of cardiovascular and vascular events.
Owner:RELIANT PHARMACEUTICALS INC +1
Method for directed intranasal administration of a composition
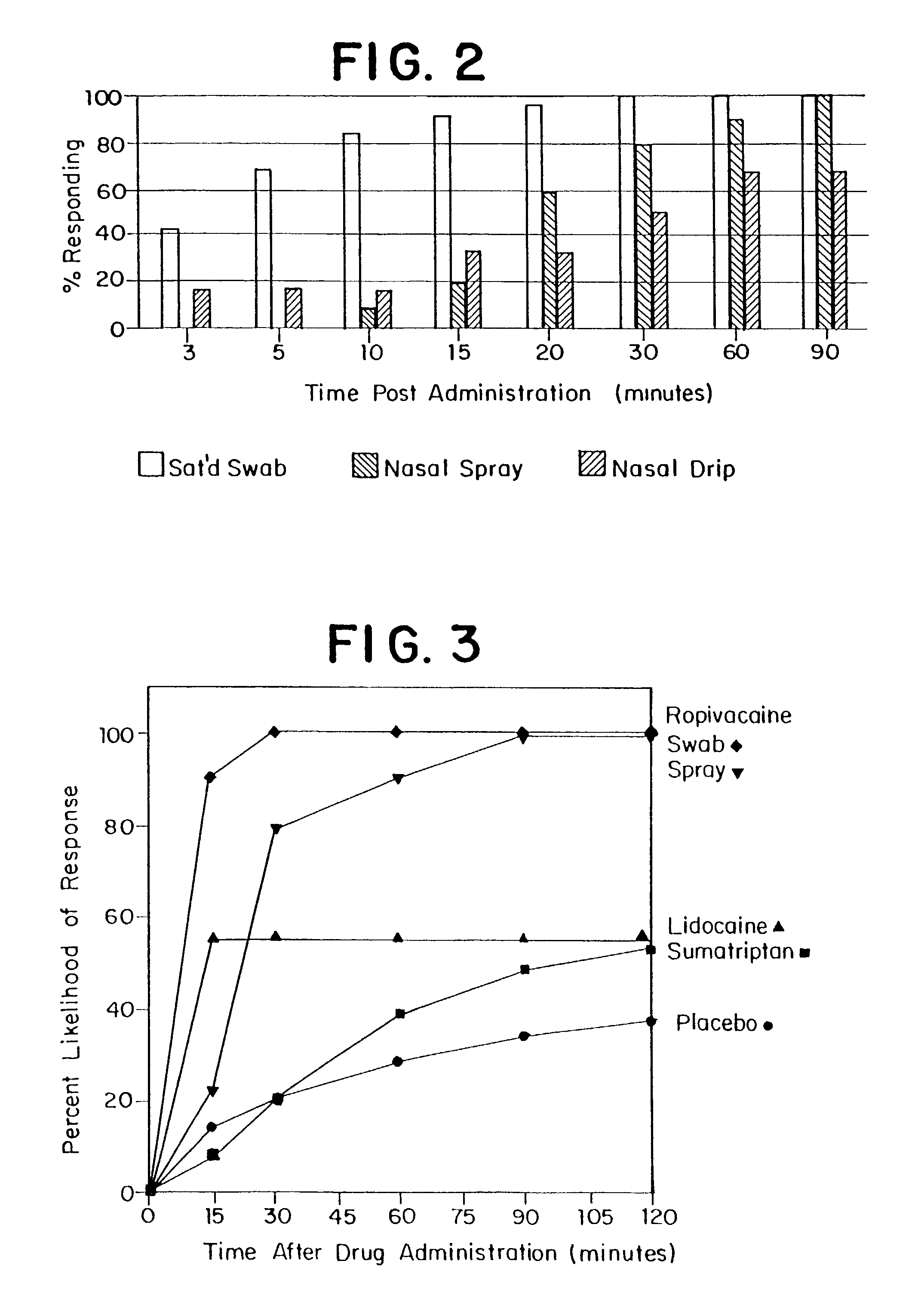
Methods, kits, apparatus, and compositions for inhibiting a cerebral neurovascular disorder, a muscular headache, or cerebral inflammation in a human patient are provided. The methods comprise intranasally administering to the patient a pharmaceutical composition comprising a local anesthetic, and preferably a long-acting local anesthetic ingredient. A composition useful for practicing the methods of the invention is described which comprises at least one local anesthetic in a pharmaceutically acceptable carrier, wherein the composition is formulated for intranasal delivery. Cerebral neurovascular disorders include migraine and cluster headache. Muscular headaches include tension headaches and muscle contraction headaches. A kit comprising the composition and an intranasal applicator and a method of systemically delivering a pharmaceutically active agent to an animal are also included in the invention. Apparatus for directed intranasal administration of the compositions of the invention and for performing the methods of the invention are also described.
Owner:BHL PATENT HLDG
Omega-3 Fatty Acids and Dyslipidemic Agent for Lipid Therapy
InactiveUS20090012167A1Effective treatmentBiocideAnimal repellantsDyslipidemiaCoronary artery disease
Owner:RELIANT PHARMACEUTICALS INC +1
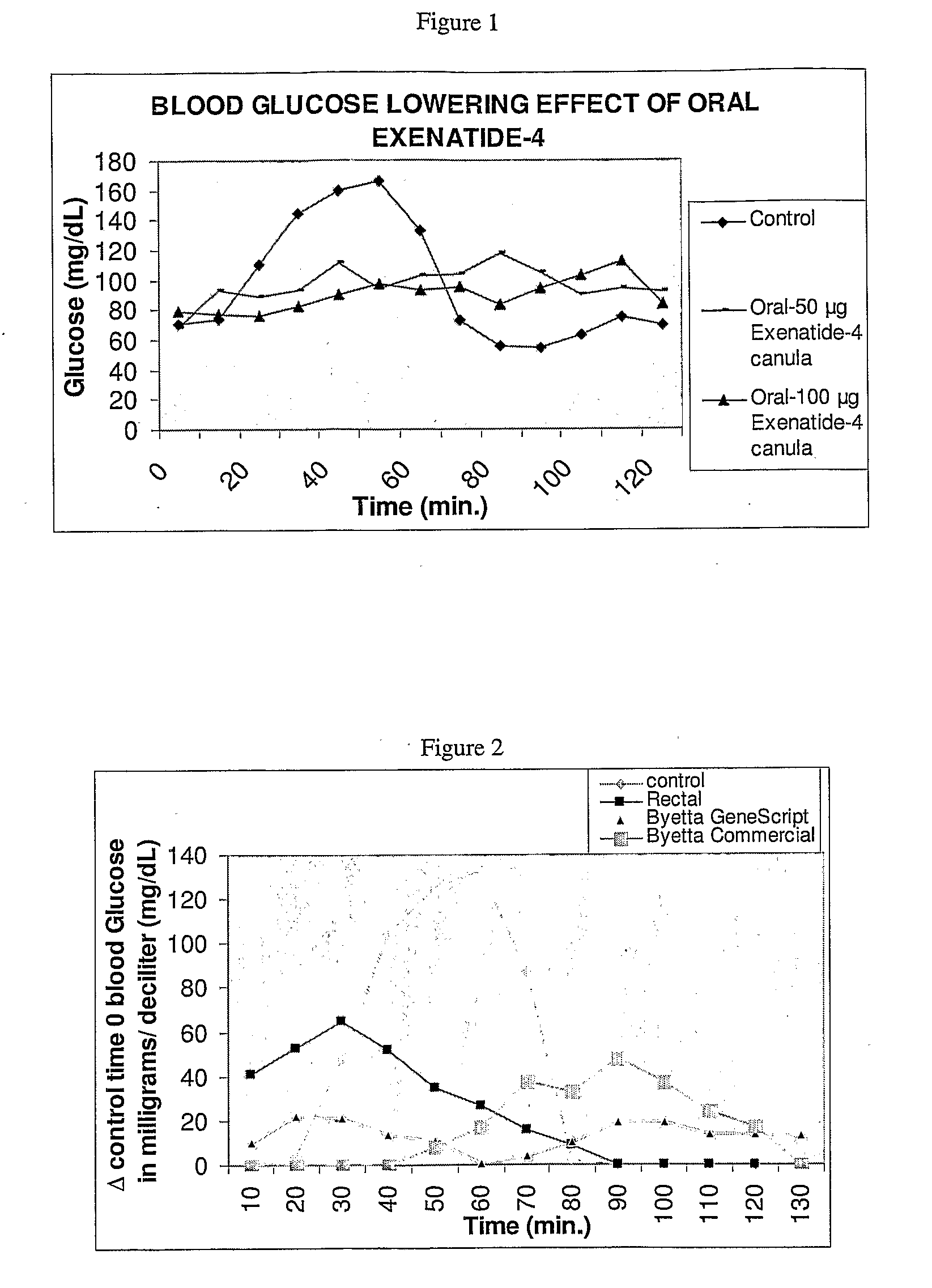
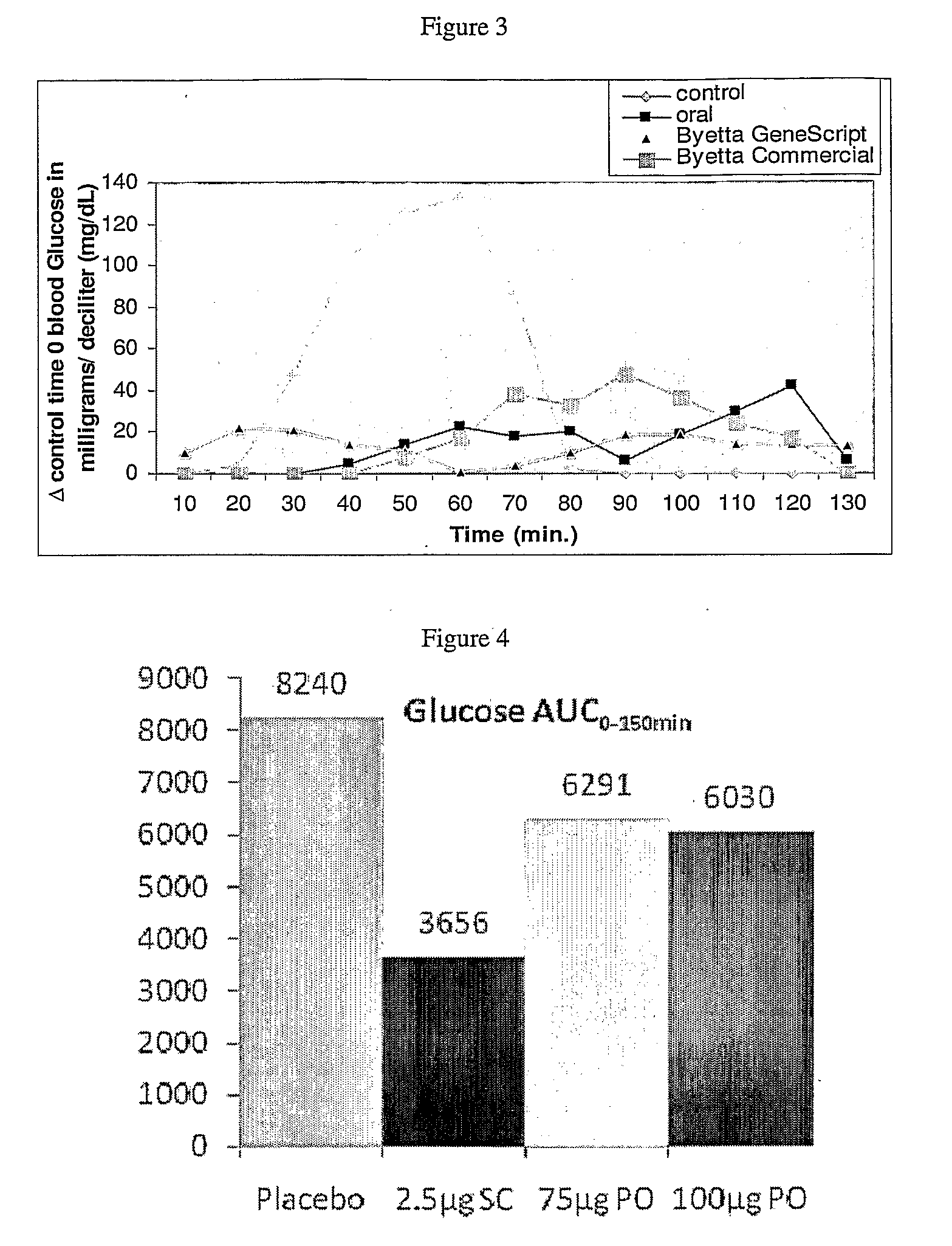
Methods and compositions for oral administration of exenatide
InactiveUS20110046053A1Reducing food intakeDecreased gastric motilityPeptide/protein ingredientsMetabolism disorderDiabetes mellitusOral medication
This invention provides compositions comprising a byetta, fish oil, and a protease inhibitor, method for treating diabetes mellitus, comprising administering same, and methods for oral or rectal administration of a byetta.
Owner:ORAMED
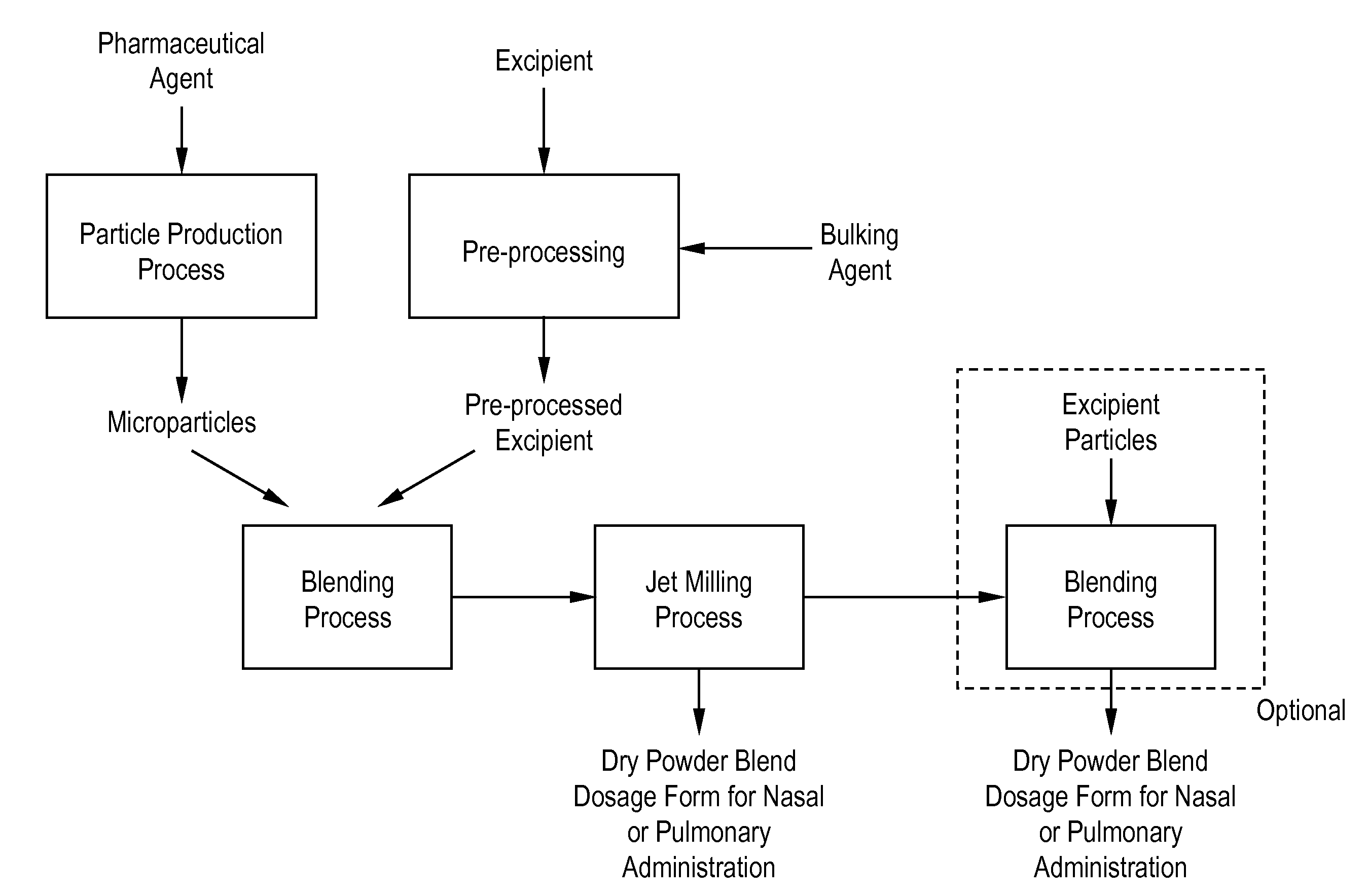
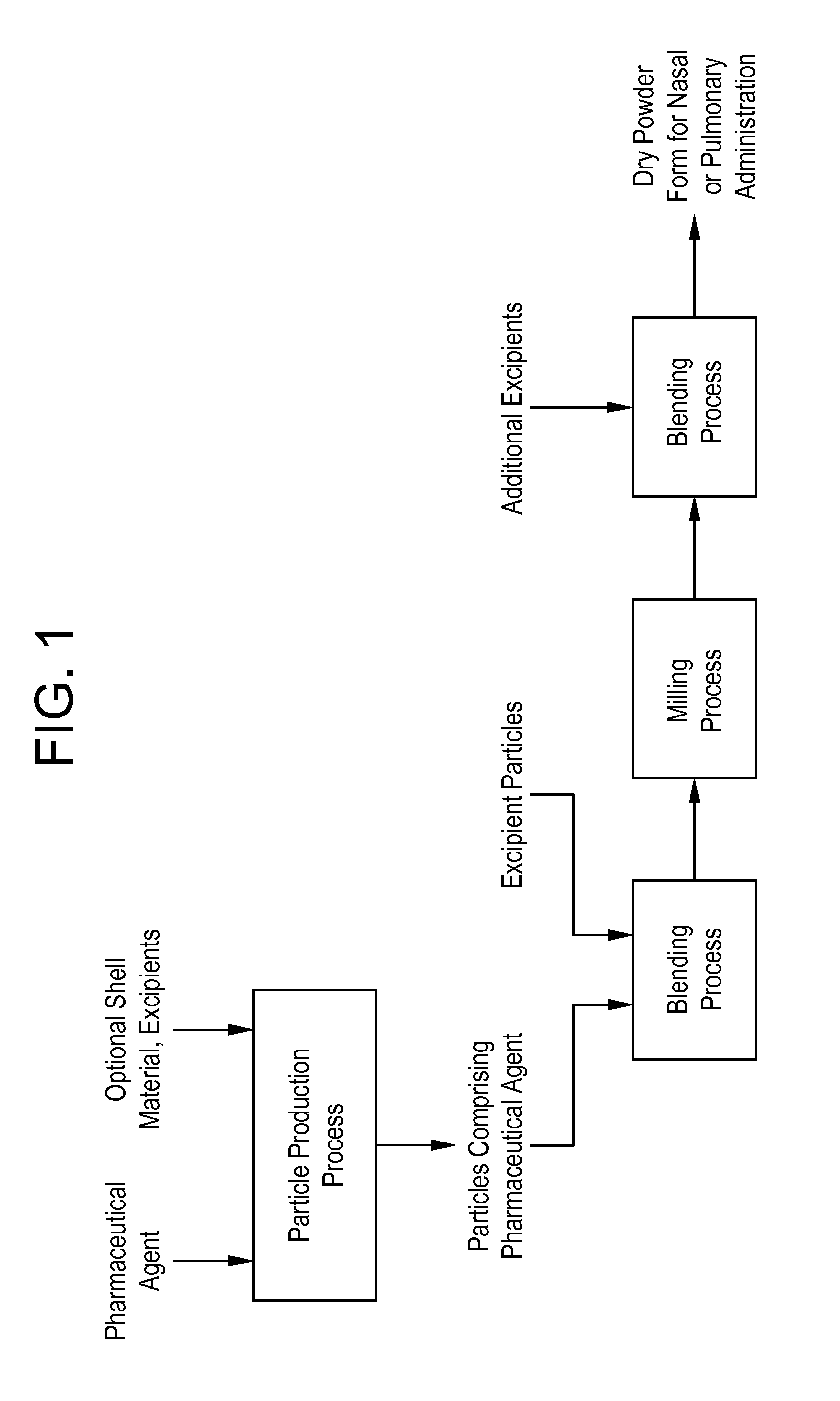
Processes for making particle-based pharmaceutical formulations for pulmonary or nasal administration
InactiveUS20070178166A1Improve stabilityStability storage conditionPowder deliverySpray deliveryPowder mixtureNanoparticle
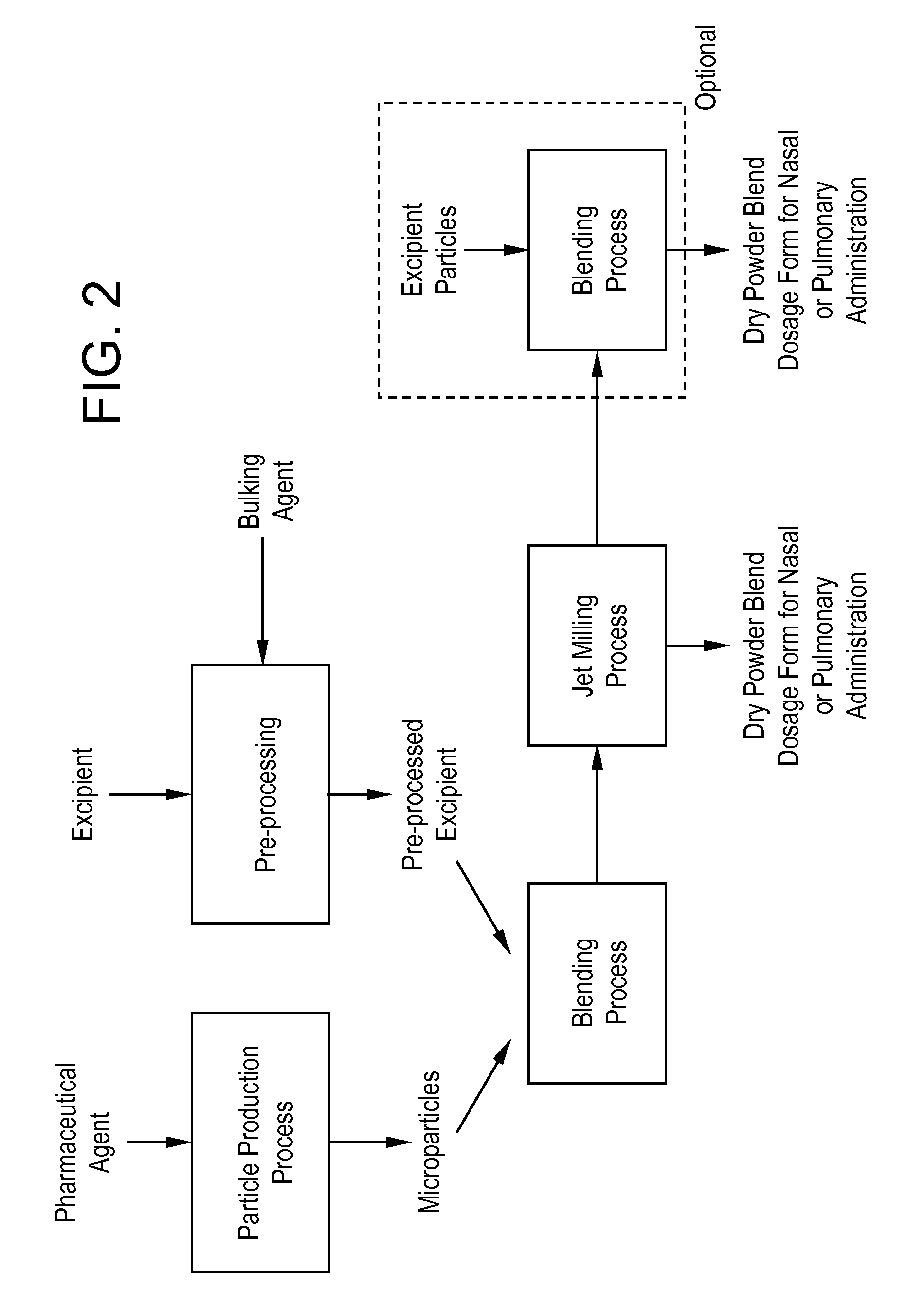
Dry powder pharmaceutical formulations for pulmonary or nasal administration are made to provide an improved respired dose. These formulations may be blends of milled blends and may include a phospholipid, alone or in combination with other excipient materials. In one case, the process includes the steps of (a) providing particles which comprise a pharmaceutical agent, (b) blending the particles with particles of at least one first excipient to form a first powder blend; (c) milling the first powder blend to form a milled blend which comprises microparticles or nanoparticles of the pharmaceutical agent; and (d) blending the milled blend with particles of a second excipient to form a blended dry powder blend pharmaceutical formulation suitable for pulmonary or nasal administration.
Owner:ACUSPHERE INC
Inhalant formulation containing sulfoalkyl ether cyclodextrin and corticosteroid
InactiveUS20070020299A1Reduce the degradation rateIncrease productivityBiocideOrganic active ingredientsNasal cavityNebulizer
An inhalable formulation containing SAE-CD and corticosteroid is provided. The formulation is adapted for administration to a subject by nebulization with any known nebulizer. The formulation can be included in a kit. The formulation is administered as an aqueous solution, however, it can be stored as a dry powder, ready-to-use solution, or concentrated composition. The formulation is employed in an improved nebulization system for administering corticosteroid by inhalation. SAE-CD present in the formulation significantly enhances the chemical stability of budesonide. A method of administering the formulation by inhalation is provided. The formulation can also be administered by conventional nasal delivery apparatus.
Owner:CYDEX PHARMACEUTICALS INC
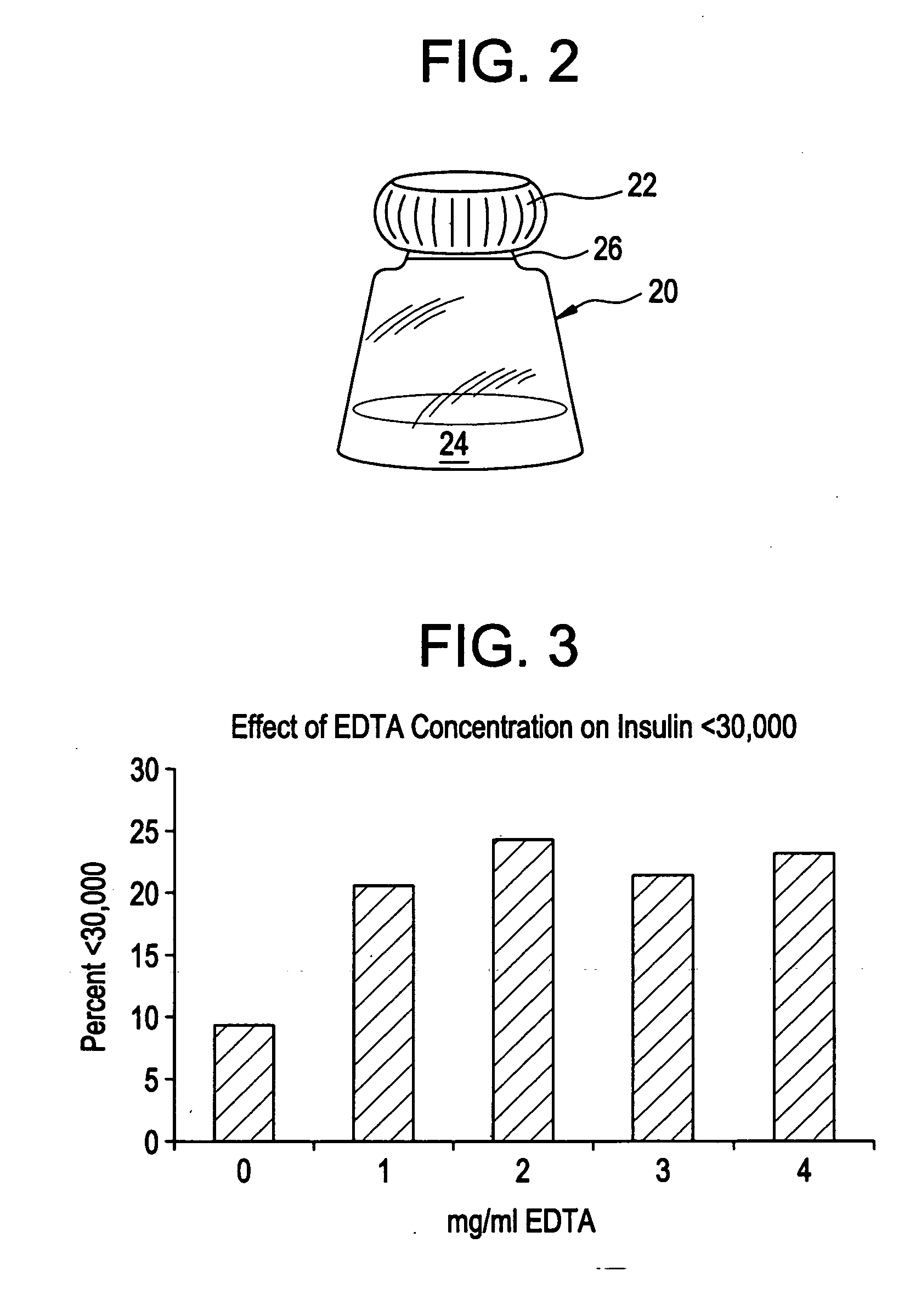
Rapid acting drug delivery compositions
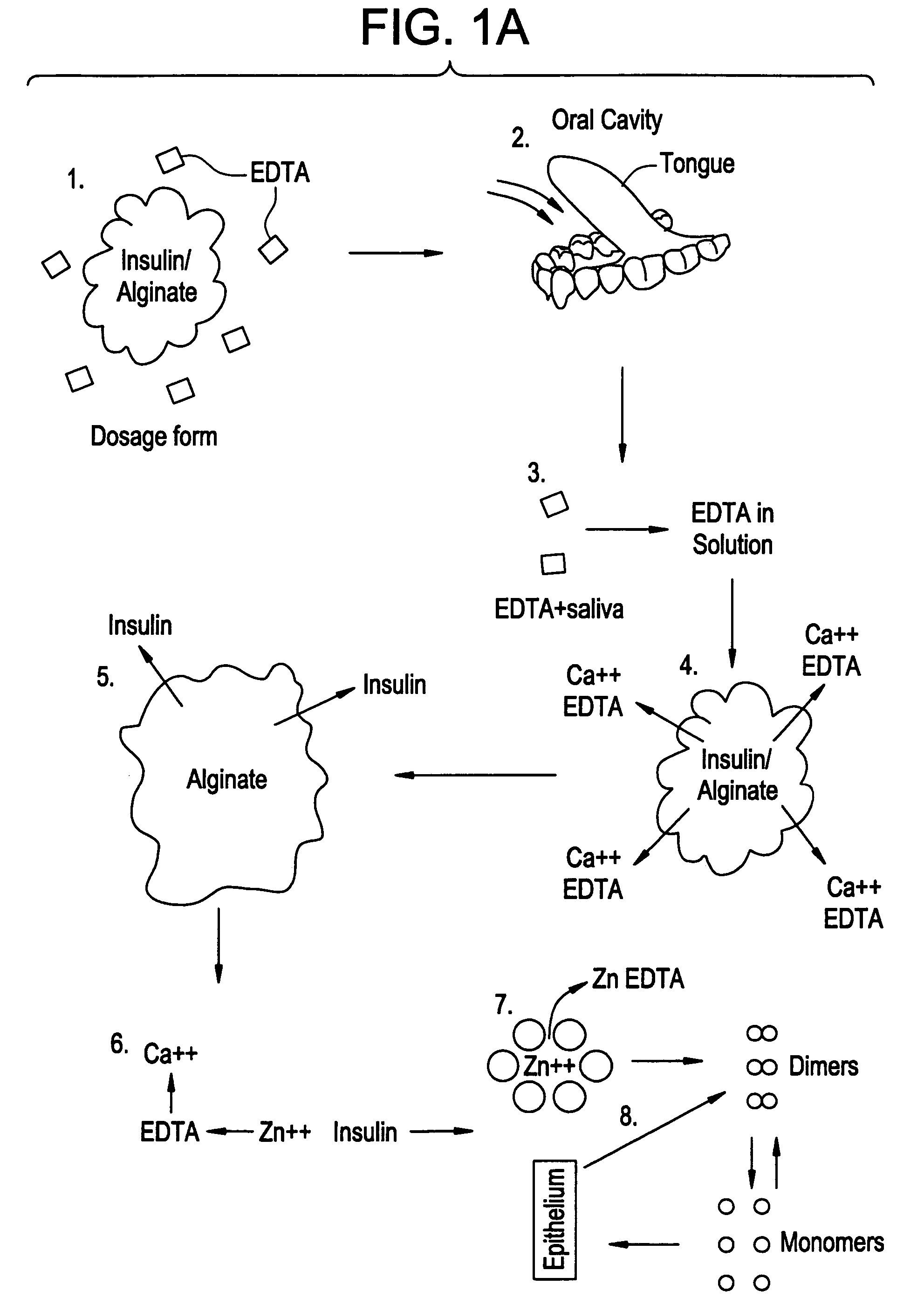
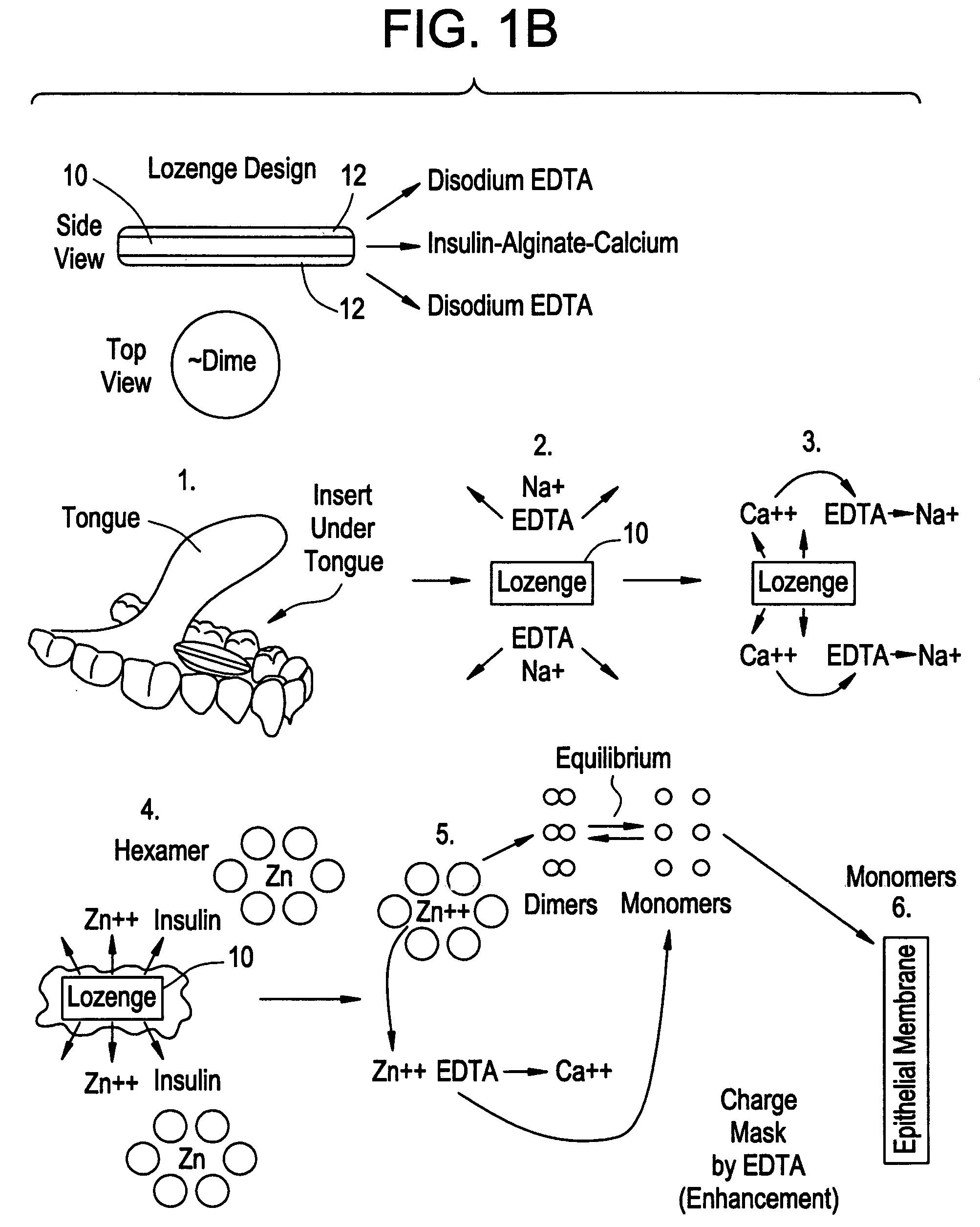
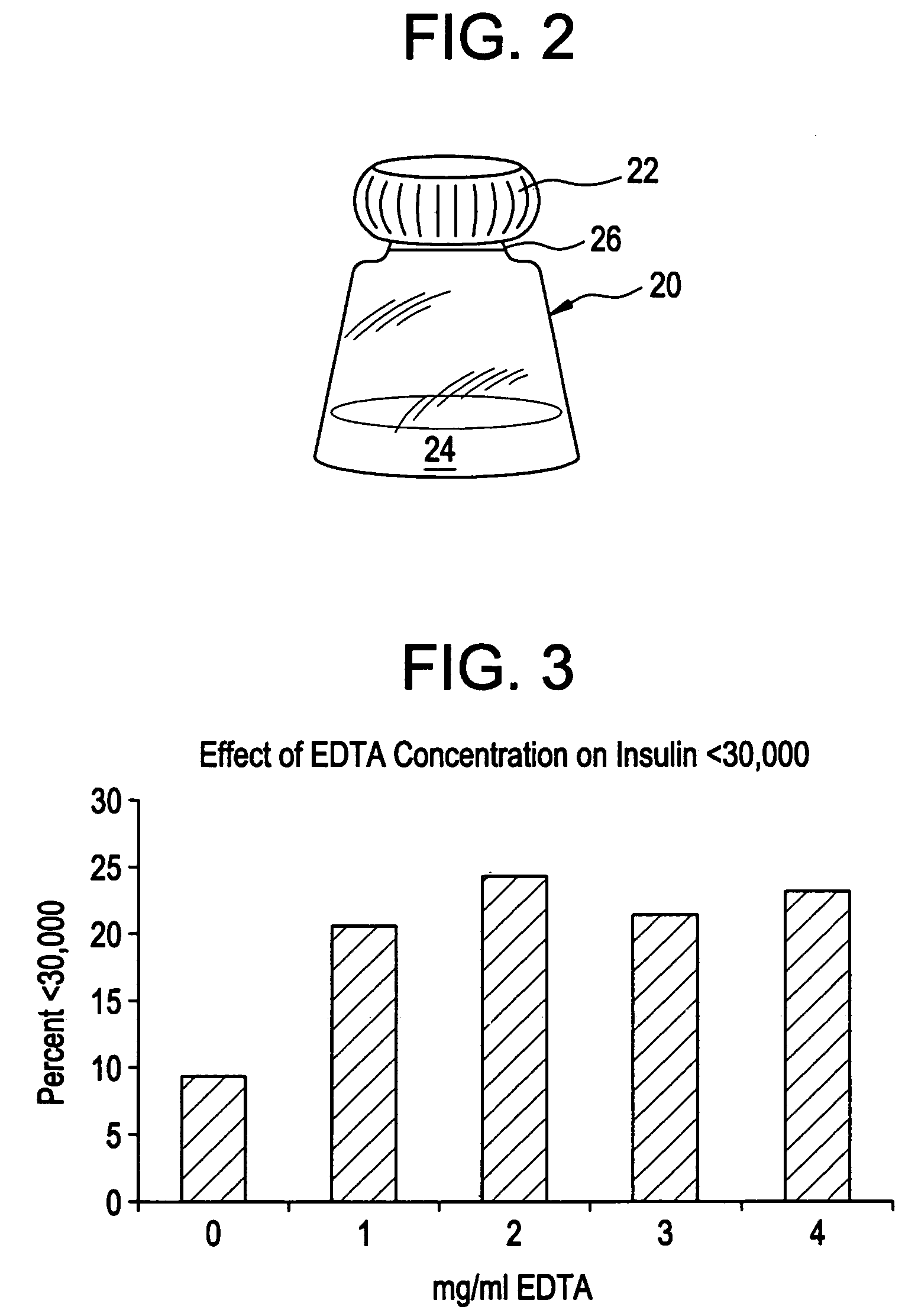
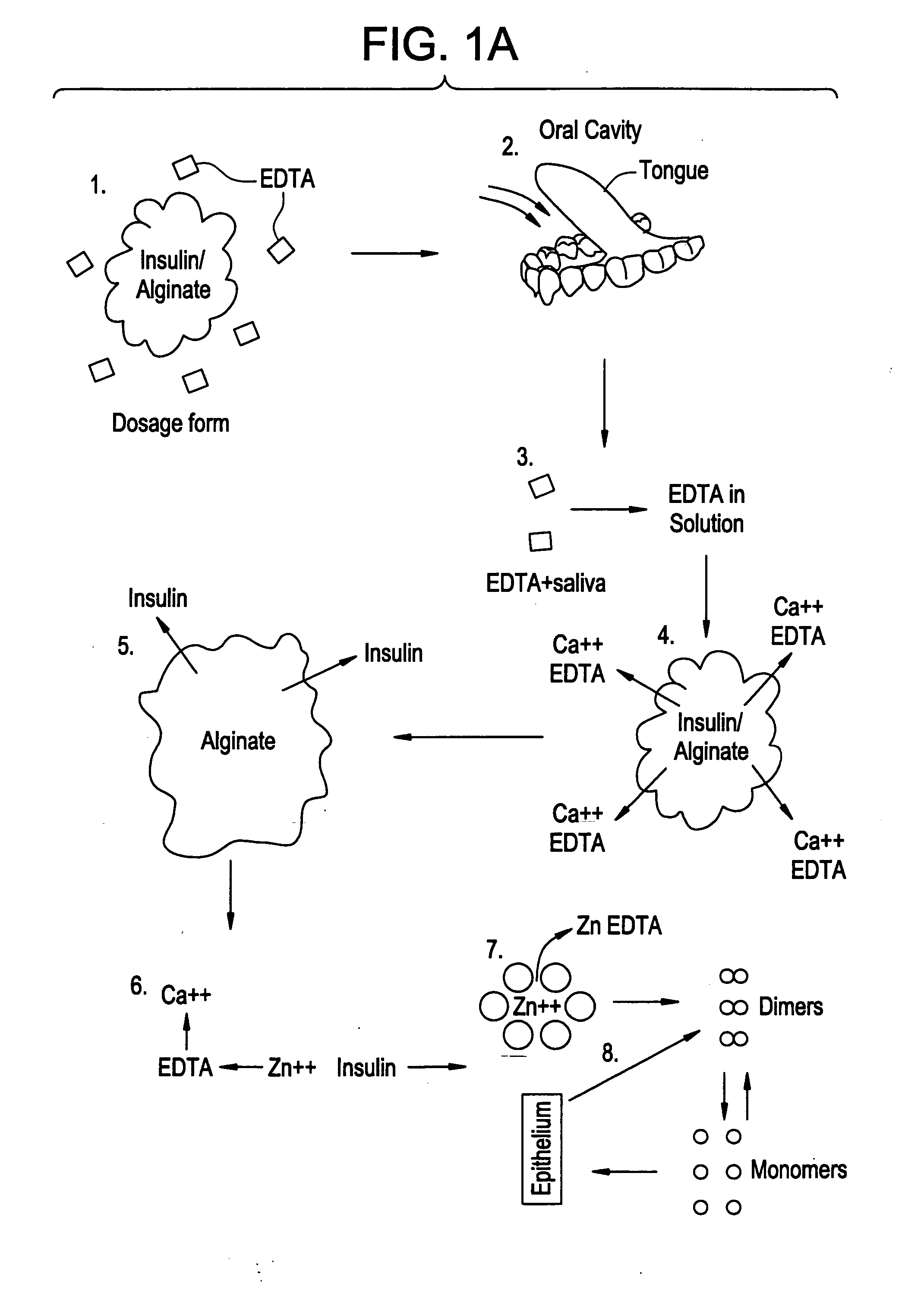
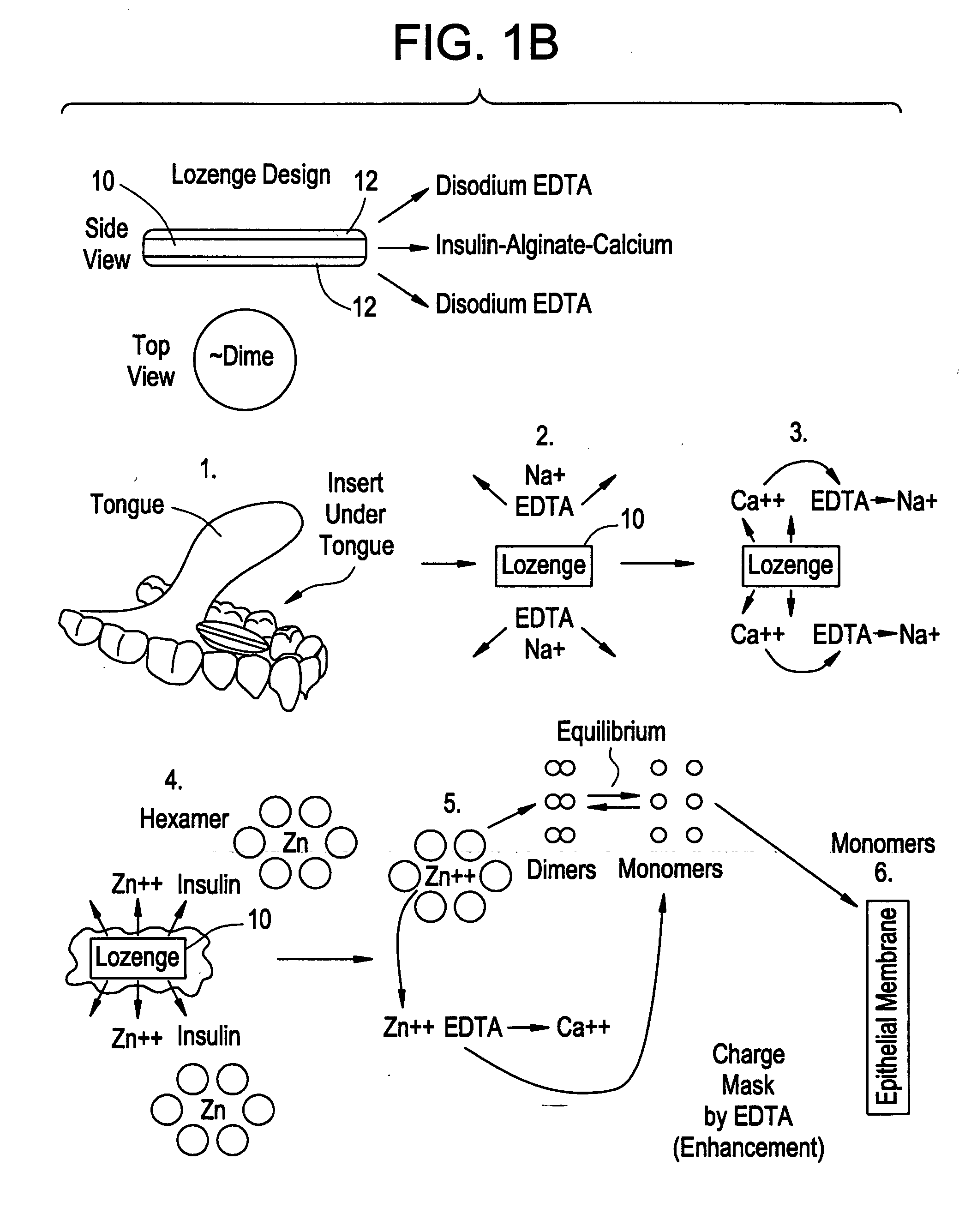
Drug formulations for systemic drug delivery with improved stability and rapid onset of action are described herein. The formulations may be administered via buccal administration, sublingual administration, pulmonary delivery, nasal administration, subcutaneous administration, rectal administration, vaginal administration, or ocular administration. In the preferred embodiments, the formulations are administered sublingually or via subcutaneous injection. The formulations contain an active agent and one or more excipients, selected to increase the rate of dissolution. In the preferred embodiment, the drug is insulin, and the excipients include a metal chelator such as EDTA and an acid such as citric acid. Following administration, these formulations are rapidly absorbed by the oral mucosa when administered sublingually and are rapidly absorbed into the blood stream when administered by subcutaneous injection. In one embodiment, the composition is in the form of a dry powder. In another embodiment, the composition is in the form of a film, wafer, lozenge, capsule, or tablet. In a third embodiment, a dry powdered insulin is mixed with a diluent containing a pharmaceutically acceptable carrier, such as water or saline, a metal chelator such as EDTA and an acid such as citric acid. Devices for storing and mixing these formulations are also described.
Owner:ELI LILLY & CO
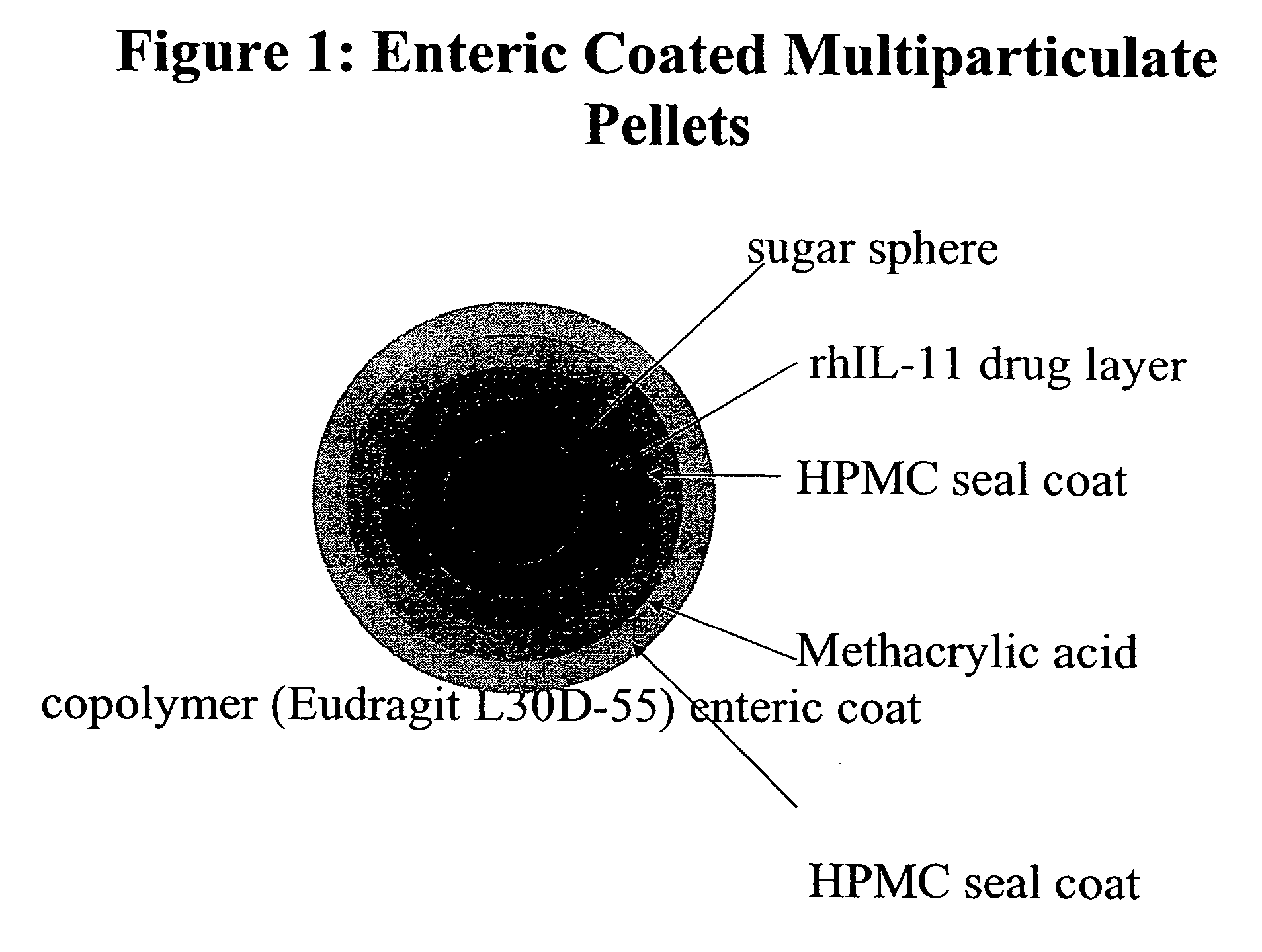
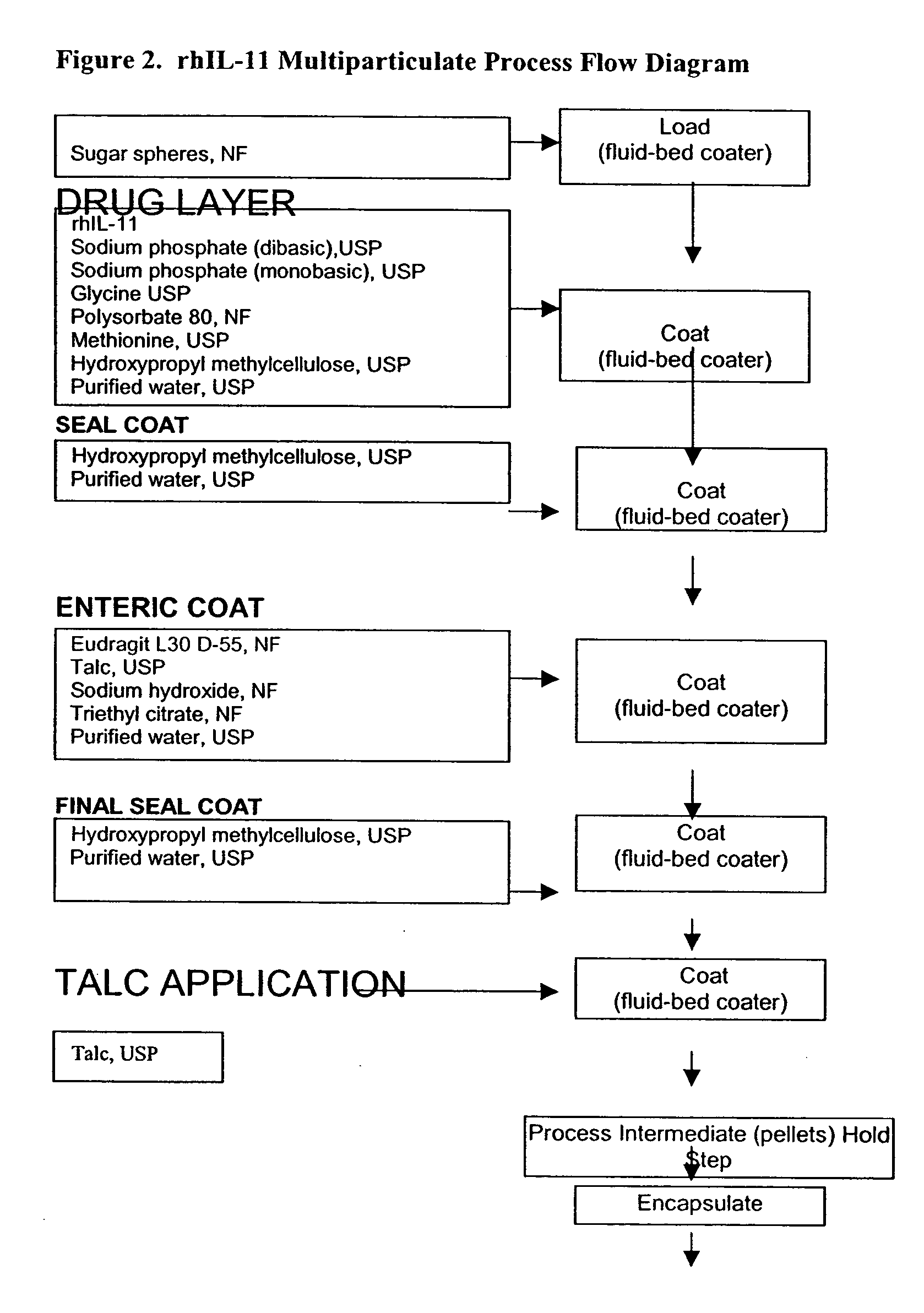
Delayed release formulations for oral administration of a polypeptide therapeutic agent and methods of using same
InactiveUS20040126358A1Increase ionic strengthReduced strengthAntipyreticAnalgesicsOral medicationWhite blood cell
The invention provides compositions containing polypeptides, including therapeutic polypeptides such as interleukin-11, that are suitable for oral administration.
Owner:WYETH LLC
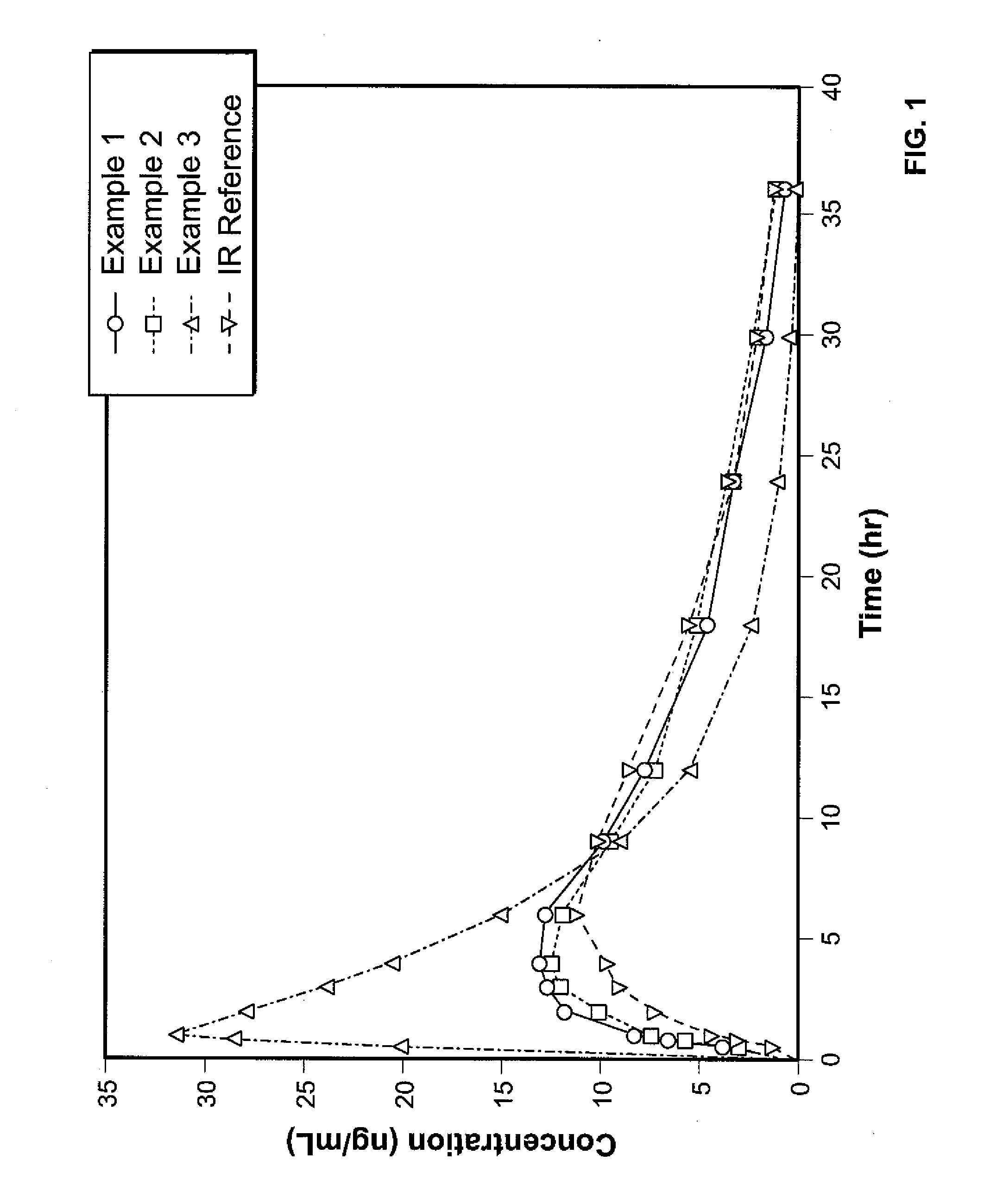
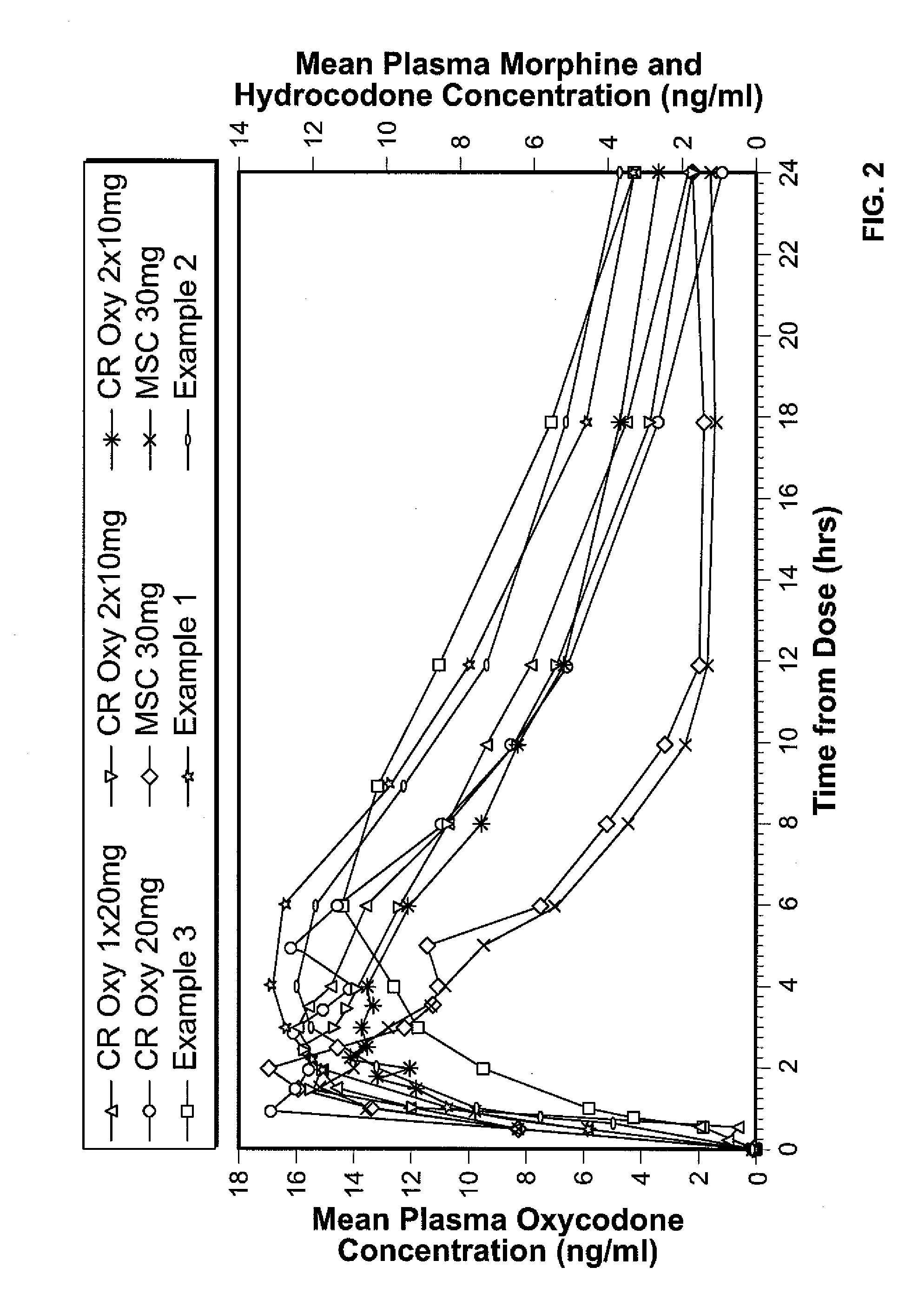
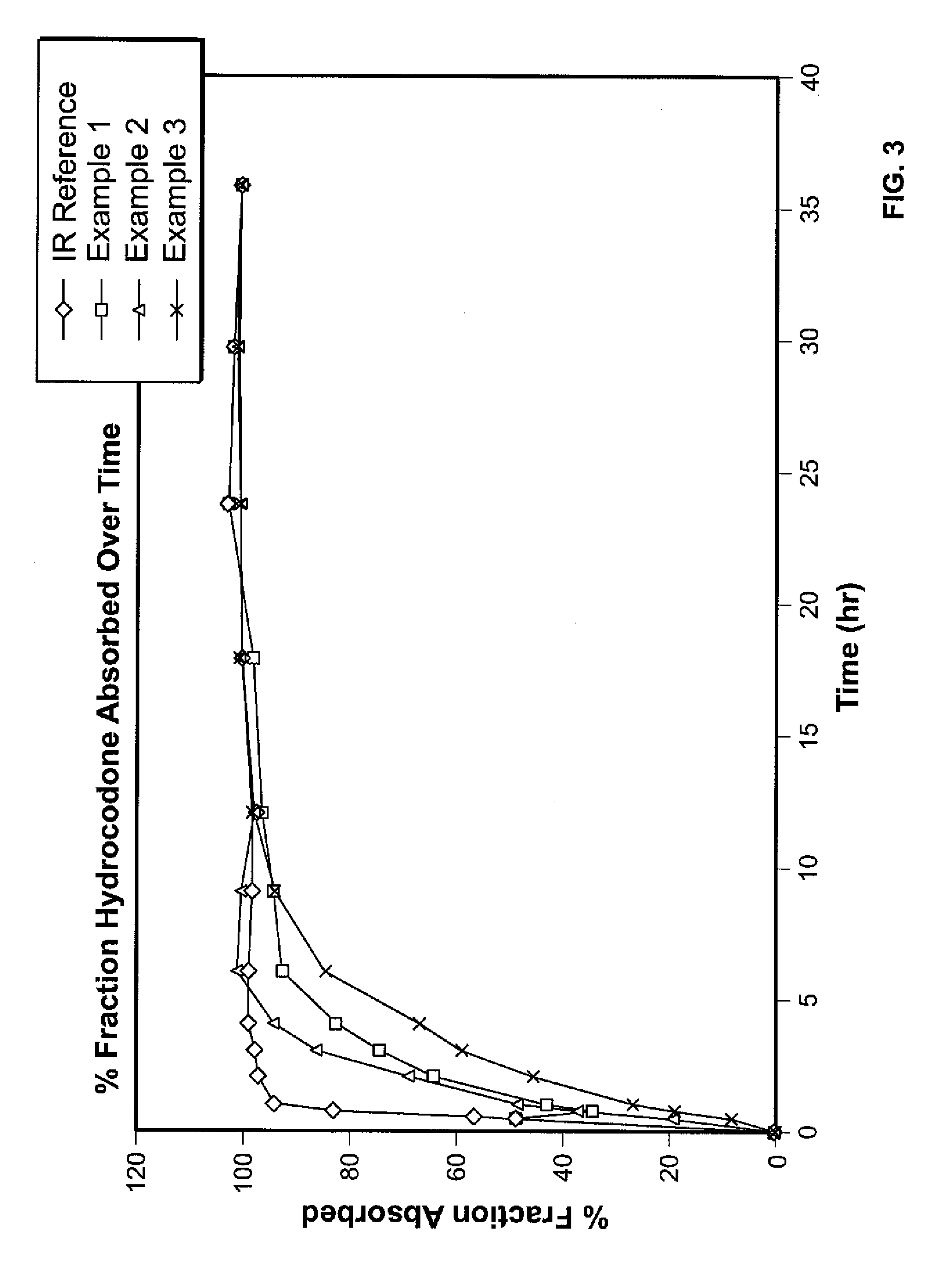
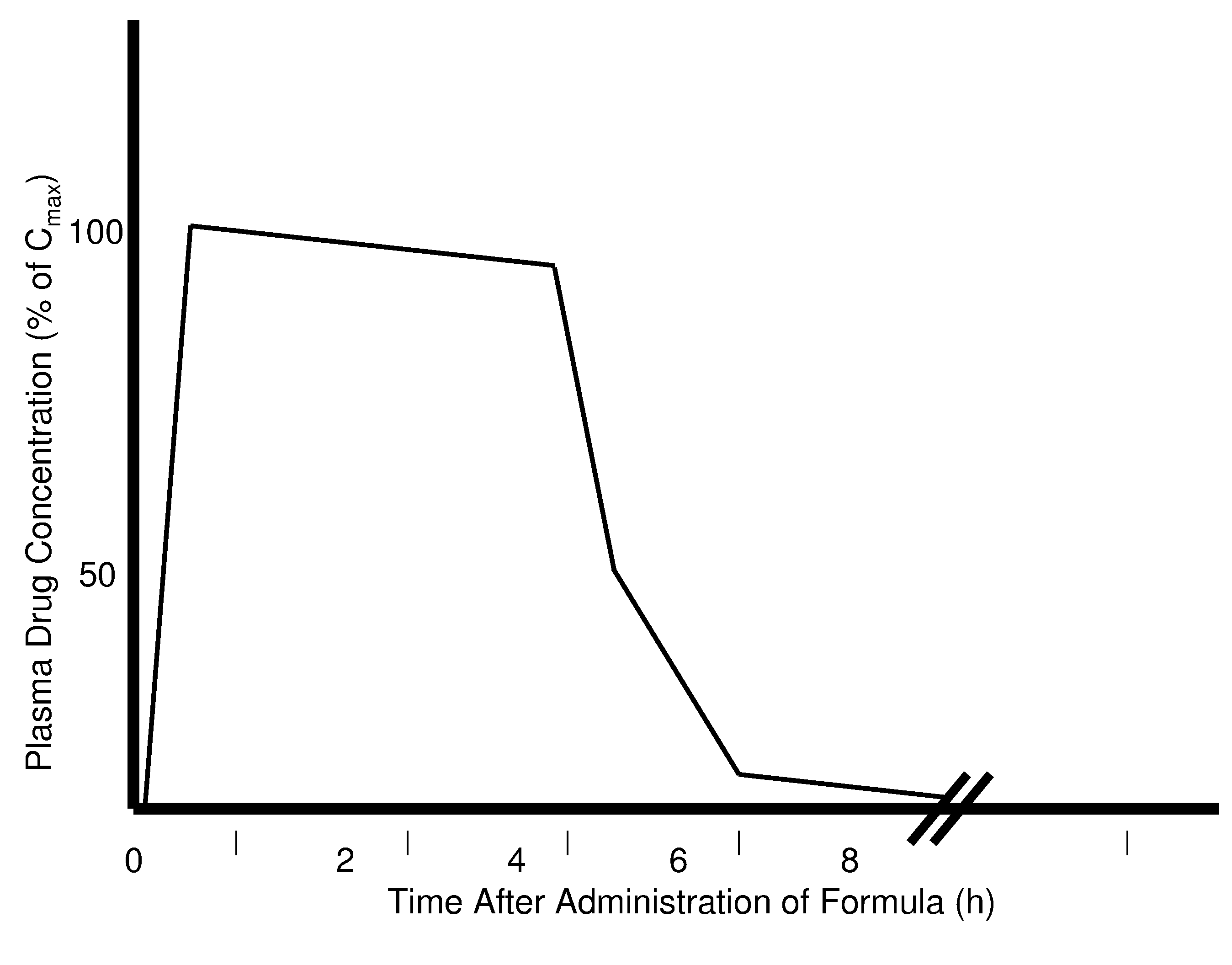
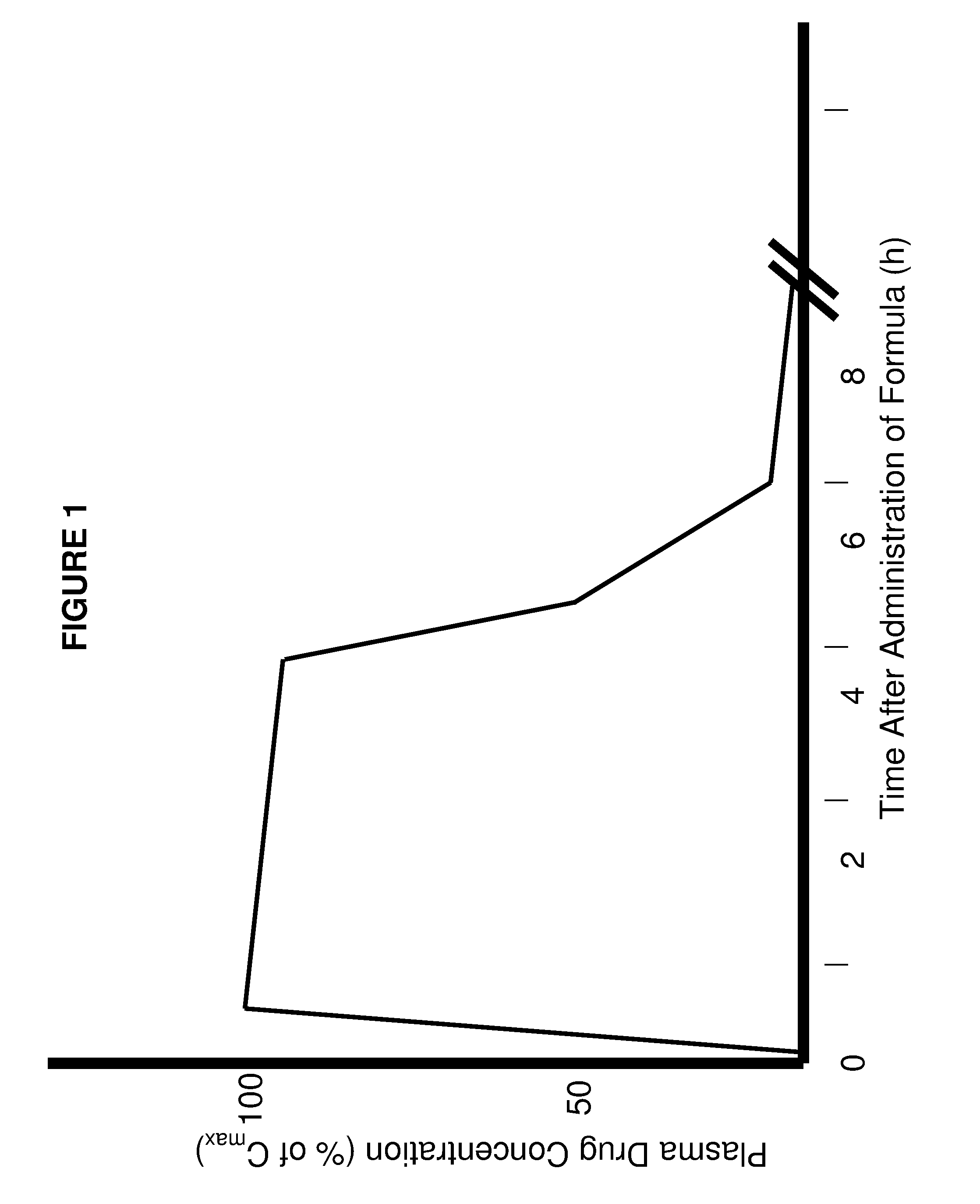
Controlled Release Hydrocodone Formulations
InactiveUS20110262532A1Improve efficiency and qualityGood effectPowder deliveryBiocideControlled releaseHuman patient
A solid oral controlled-release oral dosage form of hydrocodone is disclosed. The dosage form comprising an analgesically effective amount of hydrocodone or a pharmaceutically acceptable salt thereof, and a sufficient amount of a controlled release material to render the dosage form suitable for twice-a-day administration to a human patient, the dosage form providing a C12 / Cmax ratio of 0.55 to 0.85, said dosage form providing a therapeutic effect for at least about 12 hours.
Owner:PURDUE PHARMA LP
Tetracycline compositions for topical administration
Pharmaceutical formulations containing tetracycline for topical administration, as well as methods of making and administering the same, are disclosed.
Owner:WARNER CHILCOTT CO LLC
Tetracycline compositions for topical administration
Multi-part pharmaceutical formulations containing tetracycline for topical administration, as well as methods of making and administering the same, are disclosed.
Owner:WARNER CHILCOTT CO LLC
Rapid acting drug delivery compositions
ActiveUS20050214251A1Improve stabilityQuick effectPowder deliveryPeptide/protein ingredientsNasal cavityBuccal use
Drug formulations for systemic drug delivery with improved stability and rapid onset of action are described herein. The formulations may be administered via buccal administration, sublingual administration, pulmonary delivery, nasal administration, subcutaneous administration, rectal administration, vaginal administration, or ocular administration. In the preferred embodiments, the formulations are administered sublingually or via subcutaneous injection. The formulations contain an active agent and one or more excipients, selected to increase the rate of dissolution. In the preferred embodiment, the drug is insulin, and the excipients include a metal chelator such as EDTA and an acid such as citric acid. Following administration, these formulations are rapidly absorbed by the oral mucosa when administered sublingually and are rapidly absorbed into the blood stream when administered by subcutaneous injection. In one embodiment, the composition is in the form of a dry powder. In another embodiment, the composition is in the form of a film, wafer, lozenge, capsule, or tablet. In a third embodiment, a dry powdered insulin is mixed with a diluent containing a pharmaceutically acceptable carrier, such as water or saline, a metal chelator such as EDTA and an acid such as citric acid. Devices for storing and mixing these formulations are also described.
Owner:ELI LILLY & CO
Apparatus and method for administration of IV liquid medication and IV flush solutions
InactiveUS6953450B2Reduce the number of stepsShorten the timeDiaphragm valvesYielding couplingInterconnectionBuccal administration
The present invention provides a medical liquid administration apparatus and administration method that are particularly apt for intravenous (IV) applications. More particularly, the administration apparatus and method may be employed in conjunction with the administration of liquid medication and one or more flush solutions from multi-dose sources, wherein fluid interconnections between at least one flush solution source and the administration apparatus may be established a single time at the outset of a given procedure. The administration apparatus may include a valve having a control member selectively positionable to provide any selected one of a plurality of closed flow paths through the valve, and a syringe interconnected to the control member for clockwise / counterclockwise co-rotation therewith (e.g. to establish the selected flow path).
Owner:BAXTER ENGLEWOOD
Controlled release pharmaceutical compositions comprising a fumaric acid ester
The present invention relates to controlled release pharmaceutical compositions comprising fumaric acid ester(s) as active substance(s). The compositions are suitable for use in the treatment of e.g. psoriasis or other hyperproliferative, inflammatory or autoimmune disorders and are designated to release the fumaric acid ester in a controlled manner so that local high concentrations of the active substance within the gastrointestinal tract upon oral administration can be avoided and, thereby, enabling a reduction in gastro-intestinal related side-effects.
Owner:BIOGEN SWISS MFG GMBH
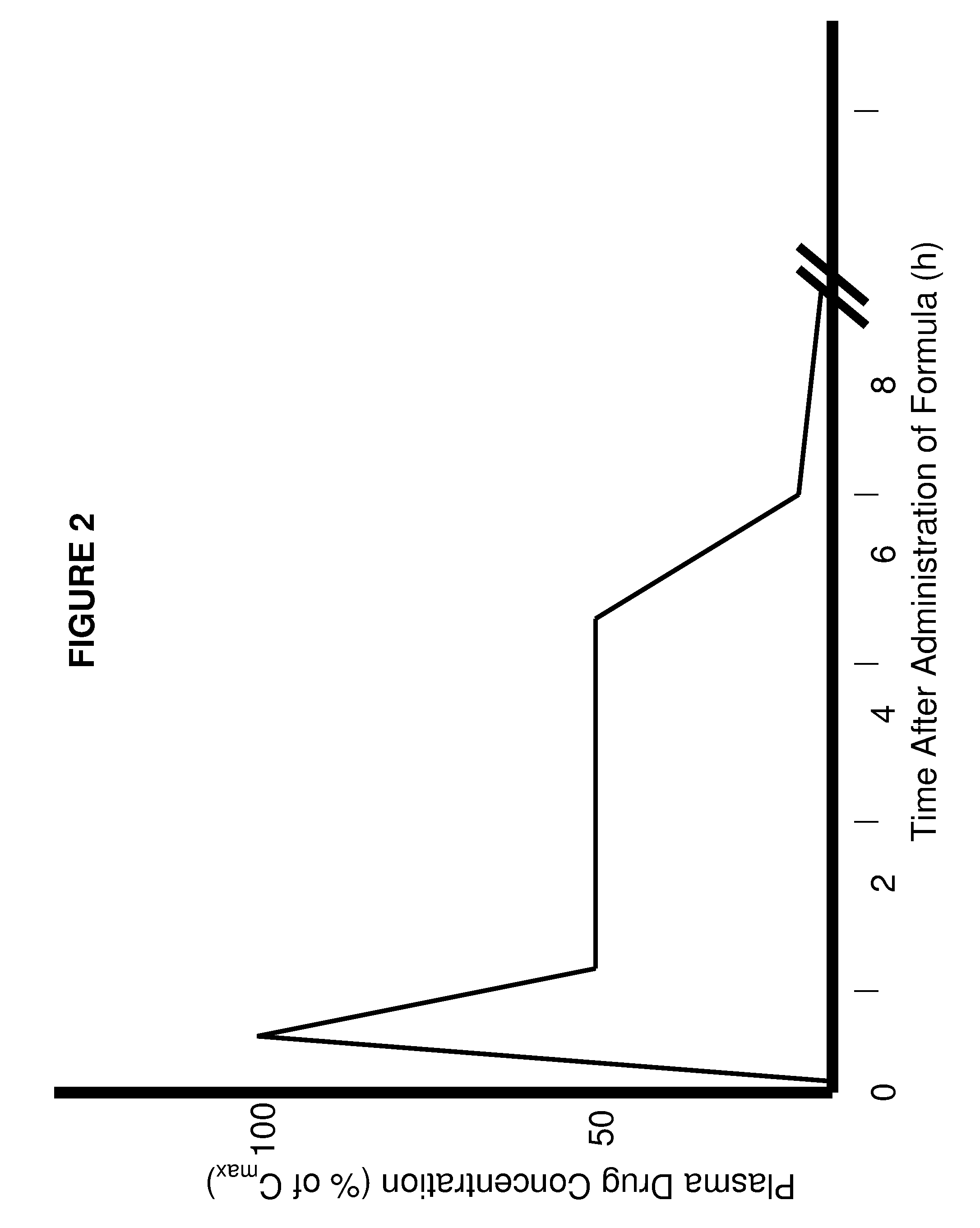
Formulation for intranasal administration
InactiveUS6017963AImprovement in flow property and dispensing accuracyPowder deliveryBiocideInhalationAnalgesic agents
A dosage form for intranasal and / or inhalation administration of an analgesic having low opioid receptor binding activity to a warm blooded animal, that includes a pharmaceutically effective amount of the analgesic. A preferred analgesic is tramadol, a tramadol and / or a pharmaceutically acceptable salt, metabolite or derivative thereof. Methods of making and using the formulation according to the invention are also provided.
Owner:EURO-CELTIQUE SA
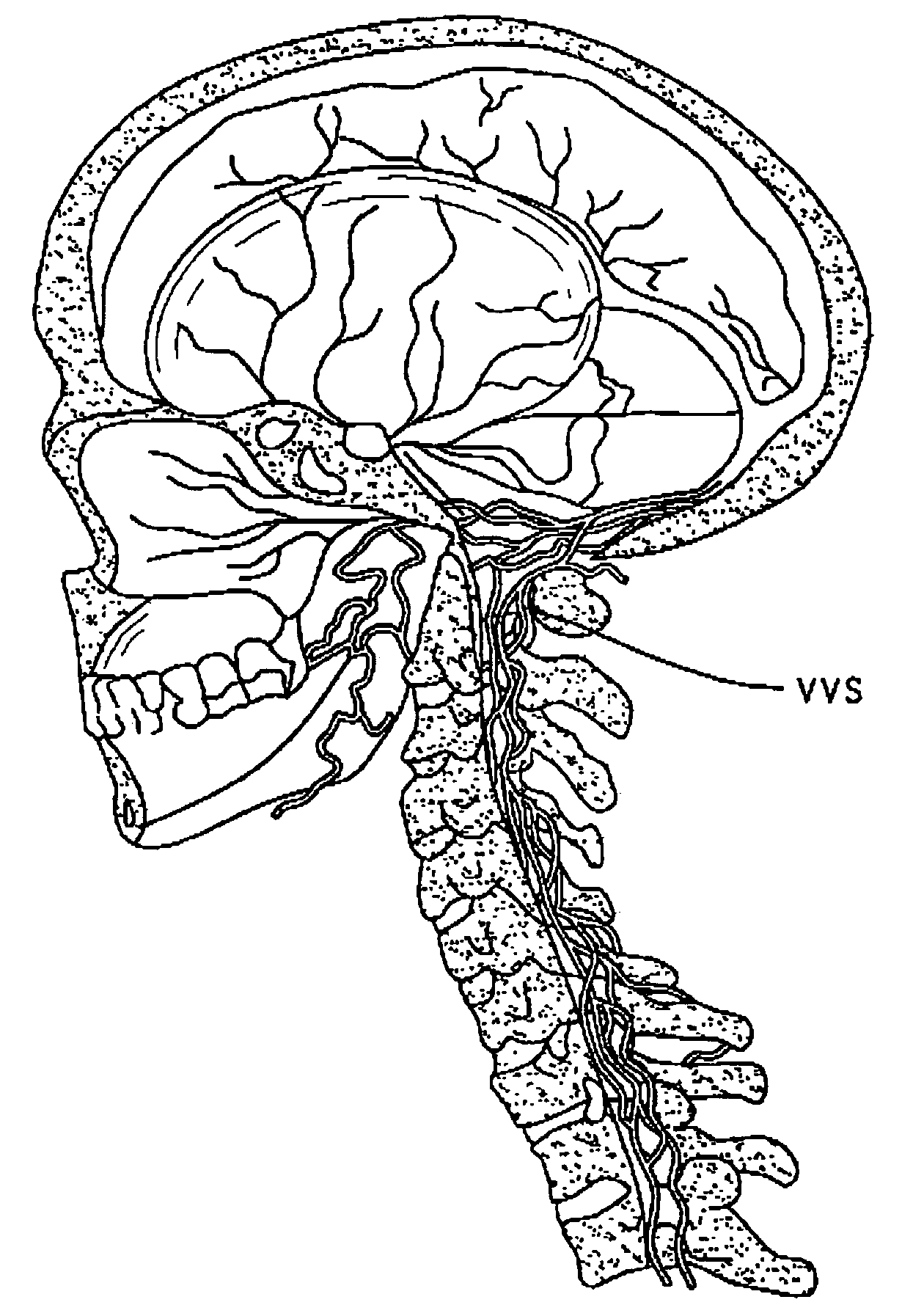
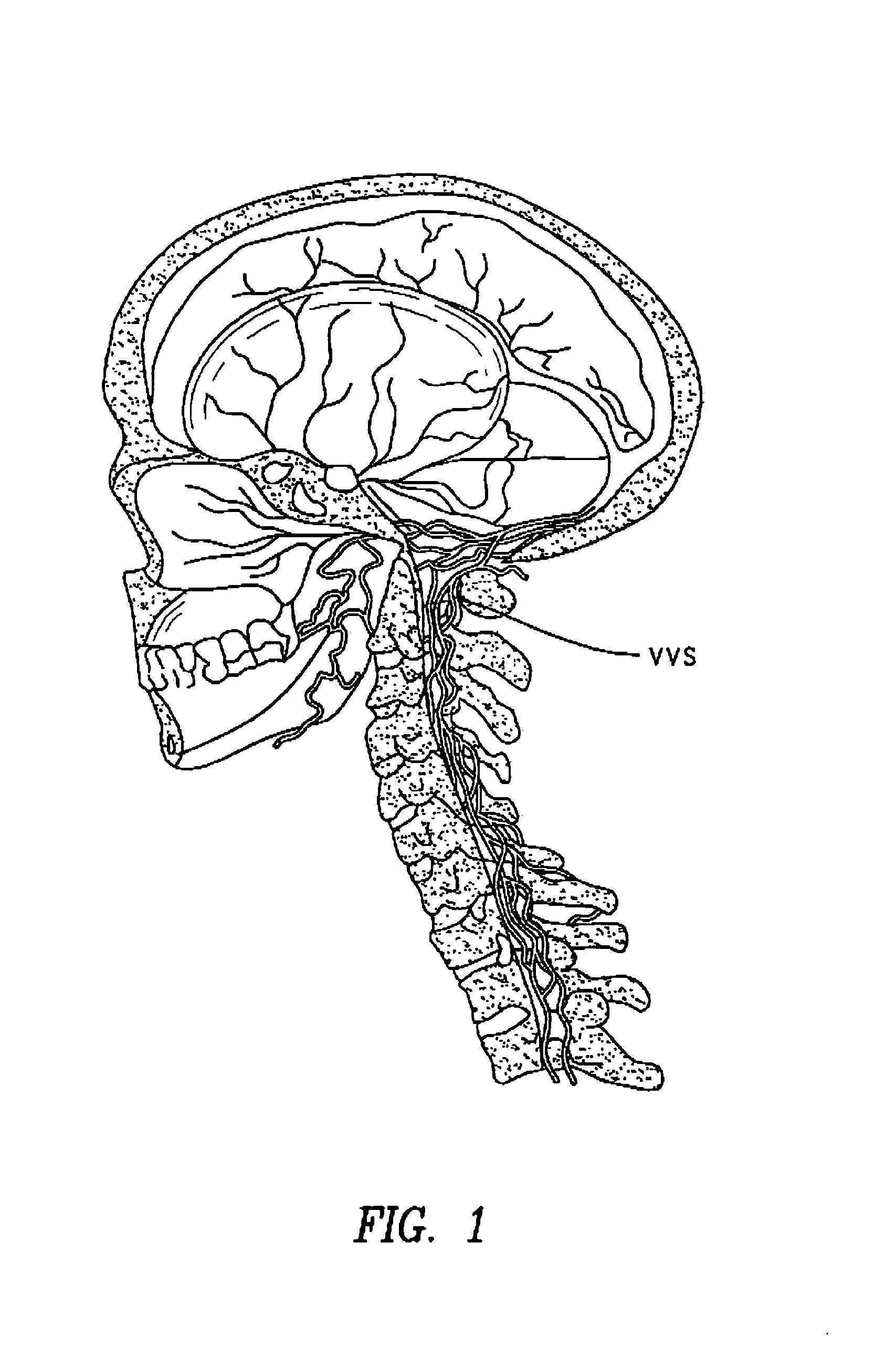
Method of delivering a TNF antagonist to the brain of a human by perispinal administration without direct intrathecal injection
InactiveUS7214658B2Improve cognitive functionReduce deliveryAnimal cellsOrganic active ingredientsEtanerceptTnf antagonists
The present invention provides specific methods of using and administering etanercept to improve cognitive function in a human, for both the treatment and prevention of cognitive impairment, or, alternatively, to enhance cognitive function including Alzheimer's Disease, Idiopathic Dementia, and Traumatic Brain Injury. The methods of the present invention include the perispinal administration of etanercept. For the purposes of this patent “perispinal” is to be considered as referring to “perispinal extrathecal;” therefore direct intrathecal administration is excluded. Perispinal administration leads to enhanced delivery of etanercept to the brain in a therapeutically effective amount, via the vertebral venous system and / or the cerebrospinal fluid. Delivery of etanercept to the brain utilizing the methods of the present invention includes the use of the vertebral venous system to deliver etanercept to the brain via retrograde venous flow. Physical maneuvers are used to enhance delivery of etanercept to the brain via this route.
Owner:TACT IP
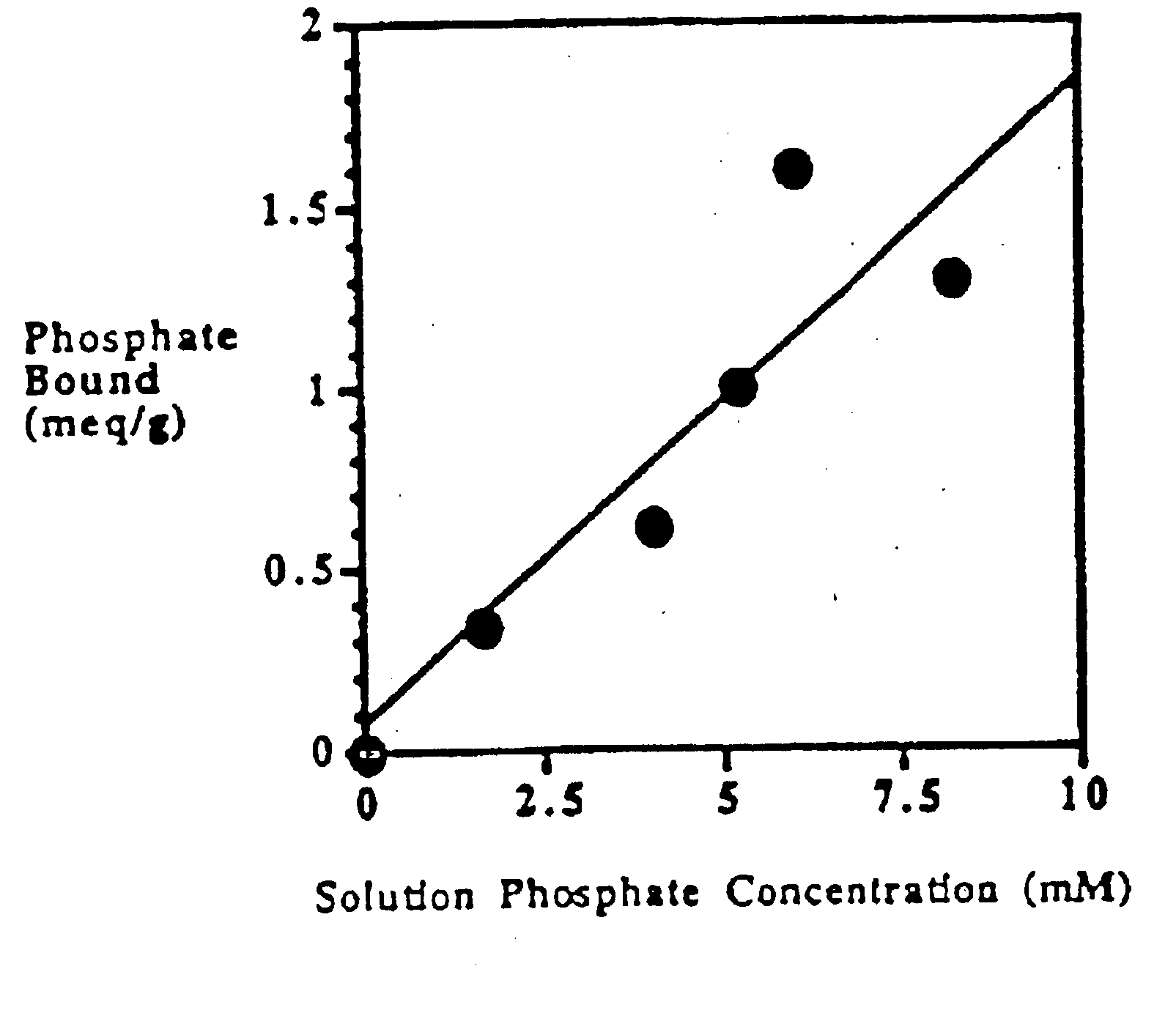
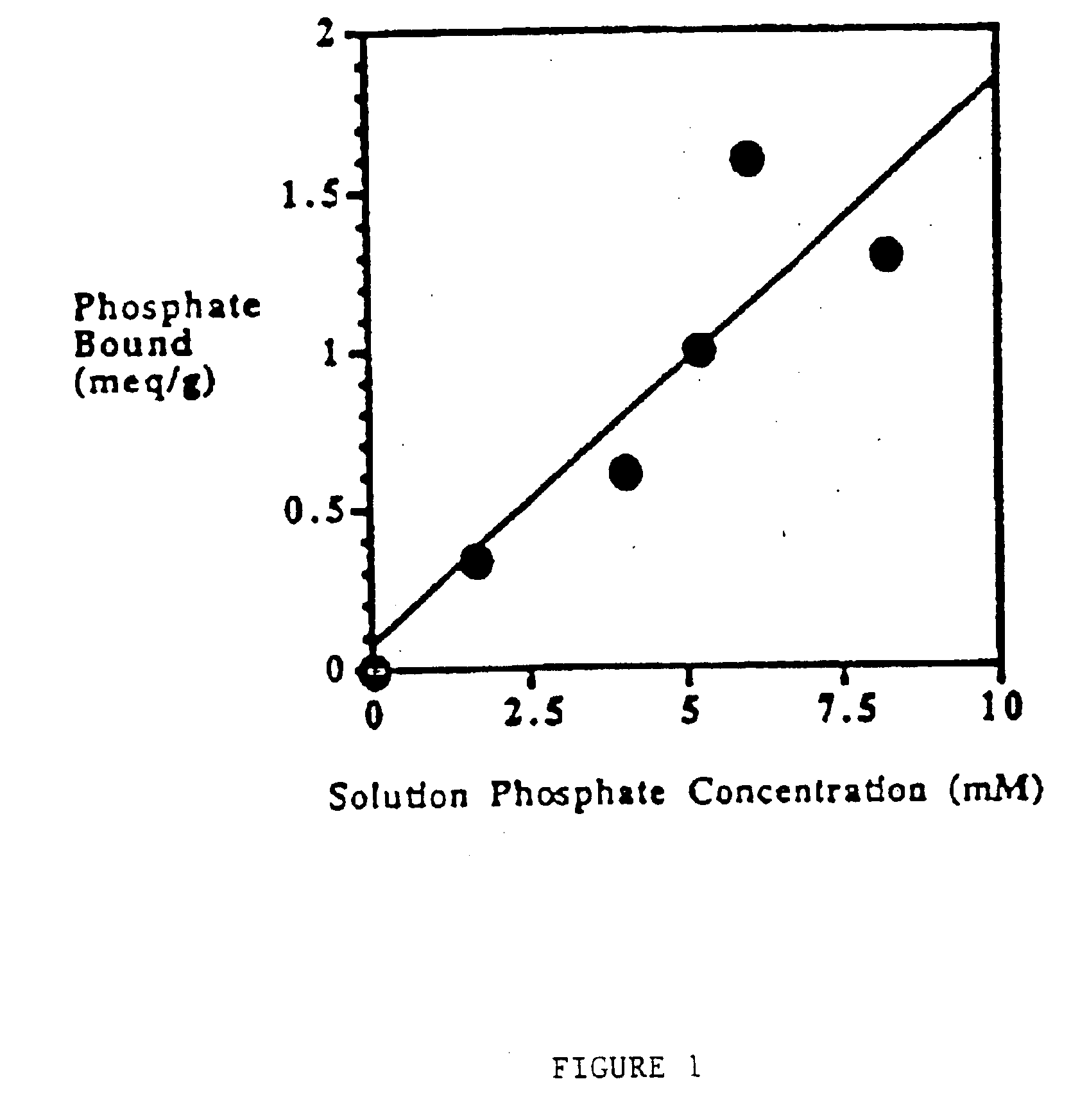
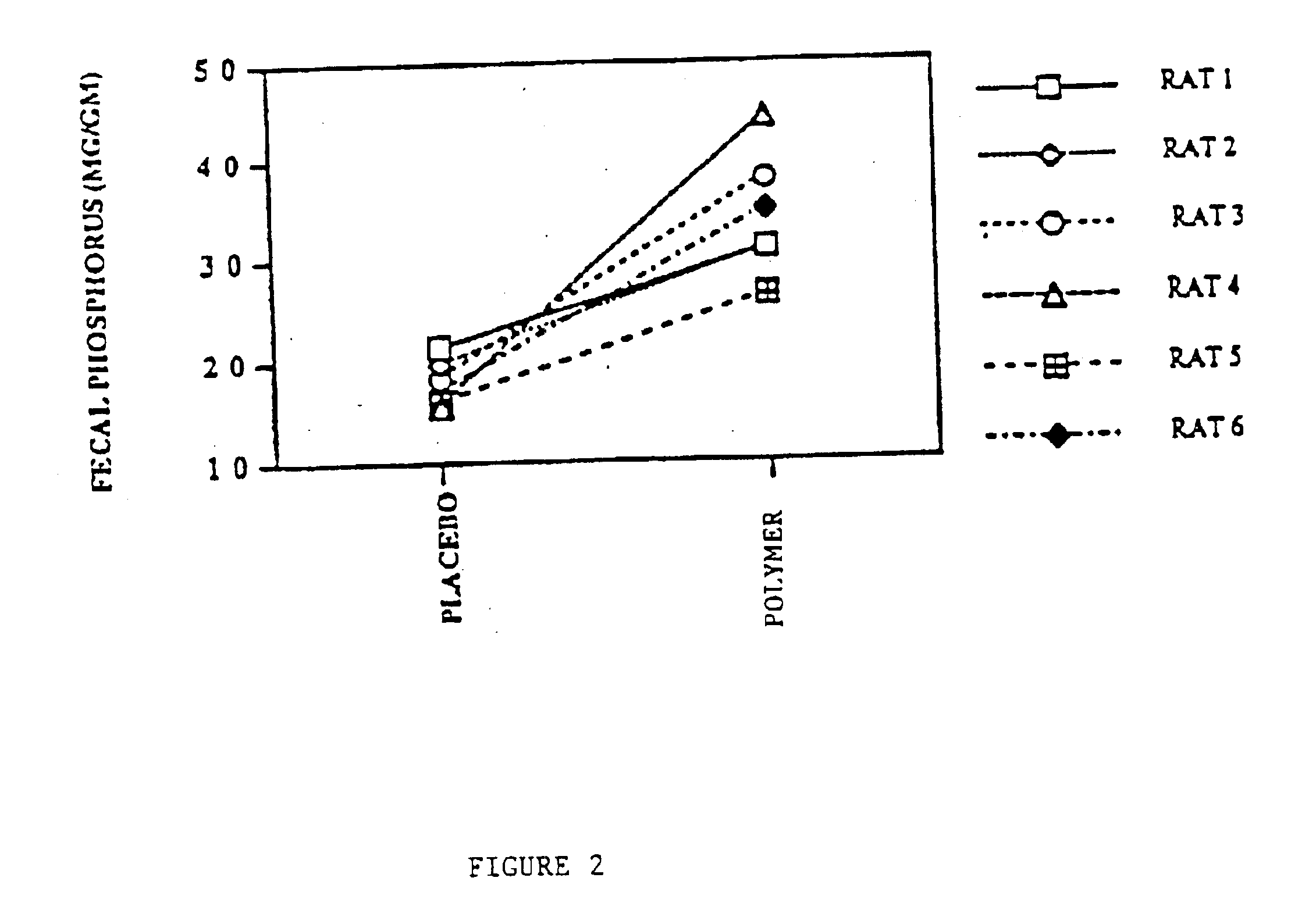
Method of making phosphate-binding polymers for oral administration
InactiveUS6858203B2Low serum levelsPromote absorptionPowder deliveryMetabolism disorderOral medicationBuccal administration
Phosphate-binding polymers are provided for removing phosphate from the gastrointestinal tract. The polymers are orally administered, and are useful for the treatment of hyperphosphatemia.
Owner:GENZYME CORP
Topical administration of basic antifungal compositions to treat fungal infections of the nails
Methods and topical pharmaceutical formulations are provided for the treatment of nail fungus (onychomycosis). The invention involves a pharmacologically active antifungal agent, plus a pharmaceutically acceptable base in a formulation having a pH of 7.5 to about 13.0, preferably about 8.0 to 11.5, and most preferably about 8.5 to 10.5. These basic formulations permeate the nail and are effective in treating fungal infections of the nail and surrounding tissues.
Owner:DERMATRENDS INC
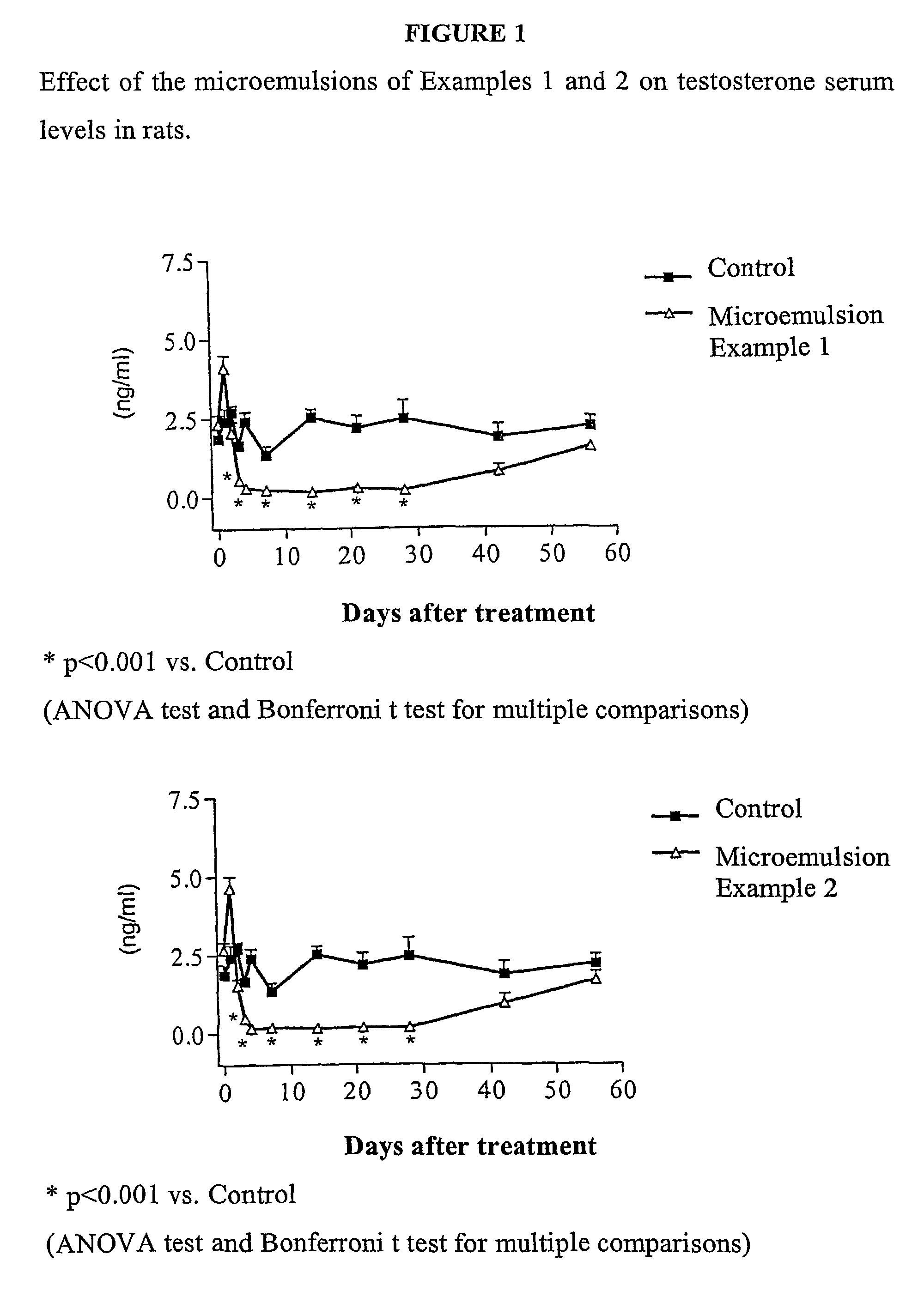
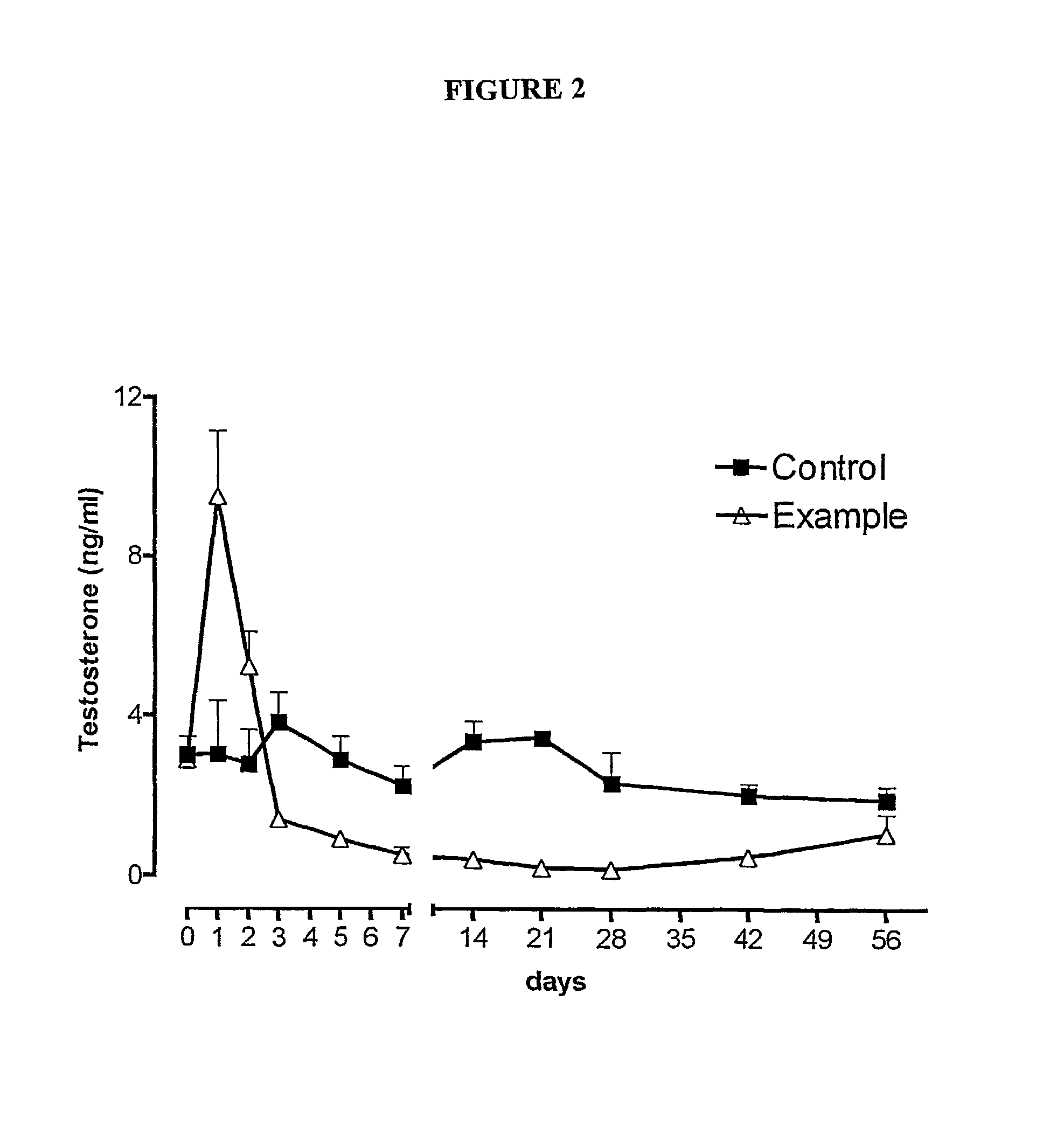
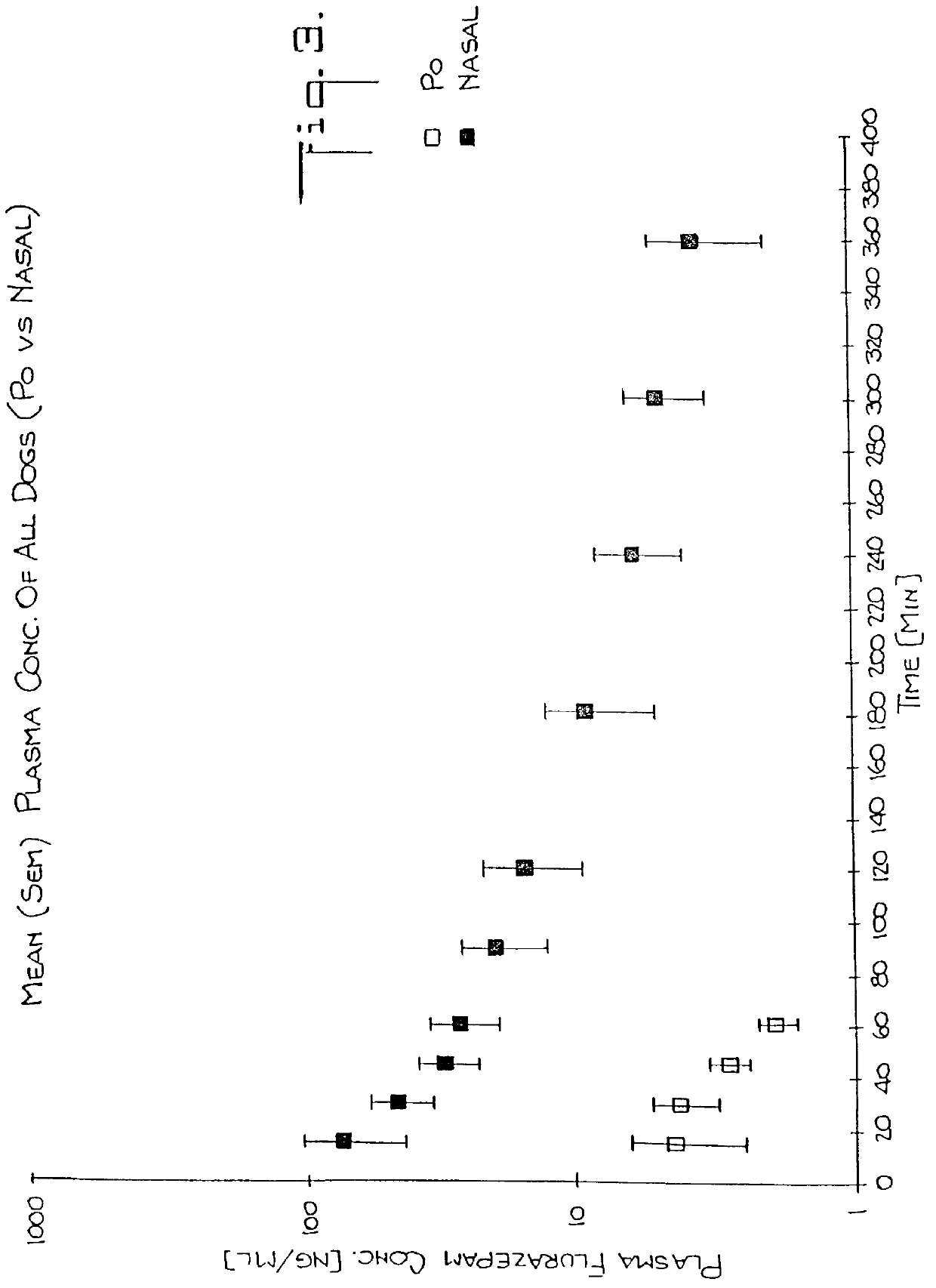
Nasal administration of benzodiazepine hypnotics
Nasal administration of benzodiazepines is described as providing improved therapeutic effects as compared to conventional delivery techniques. The compositions comprise a benzodiazepine hypnotic in a pharmaceutically acceptable nasal carrier.
Owner:QUESTCOR PHARMA
Parenteral formulations of dopamine agonists
InactiveUS20100035886A1Reducing elevated cardiovascular-related inflammatory factorReducing elevated cardiovascular-related inflammatory factorsBiocideMetabolism disorderParenteral nutritionParenteral Dosage Form
This invention relates to stable pharmaceutical compositions for parenteral administration comprising dopamine agonists and peripheral acting agents useful for treatment of metabolic disorders or key elements thereof. The parenteral dosage forms exhibit long stable shelf life and distinct pharmacokinetics.
Owner:VEROSCI
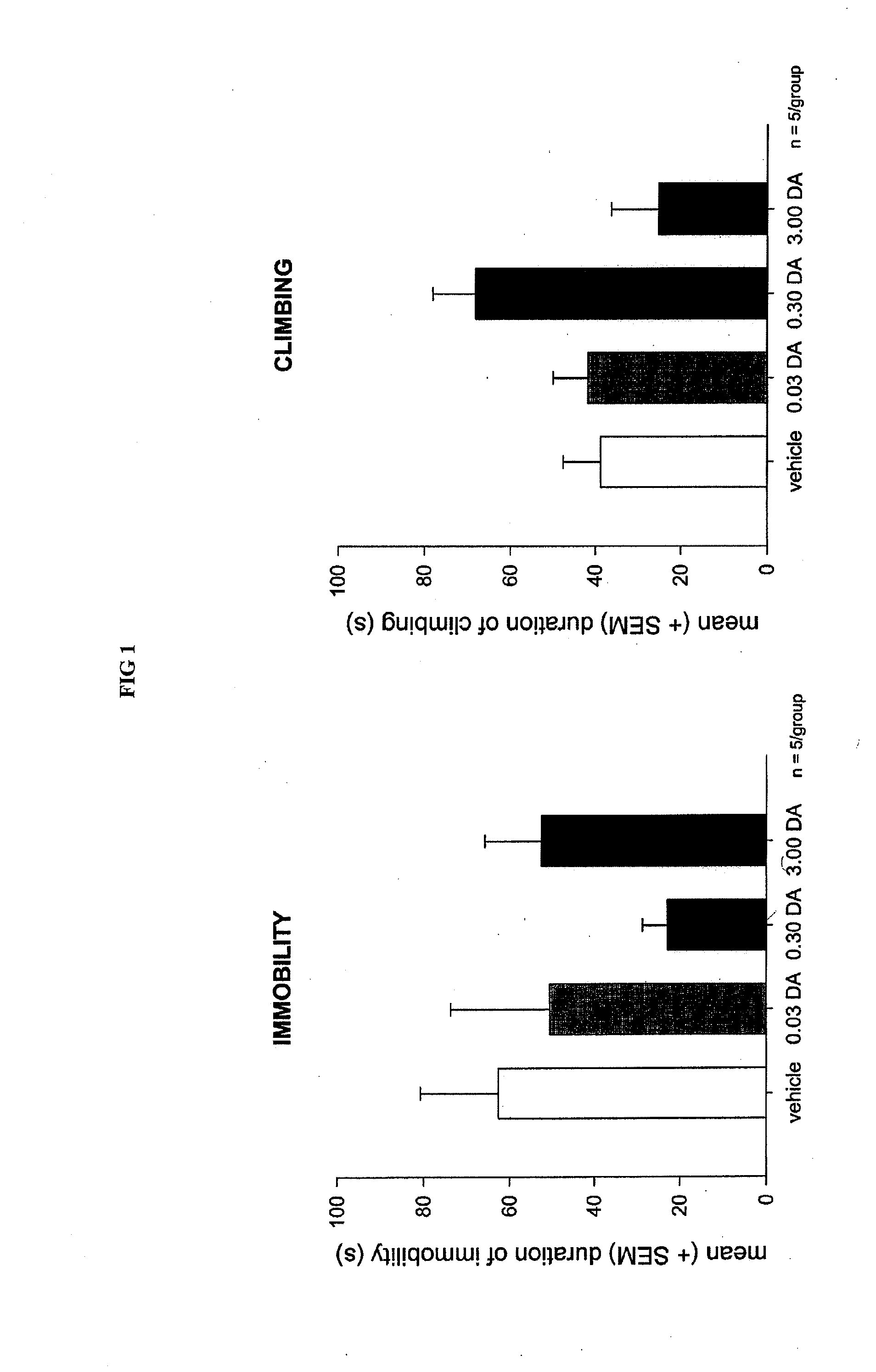
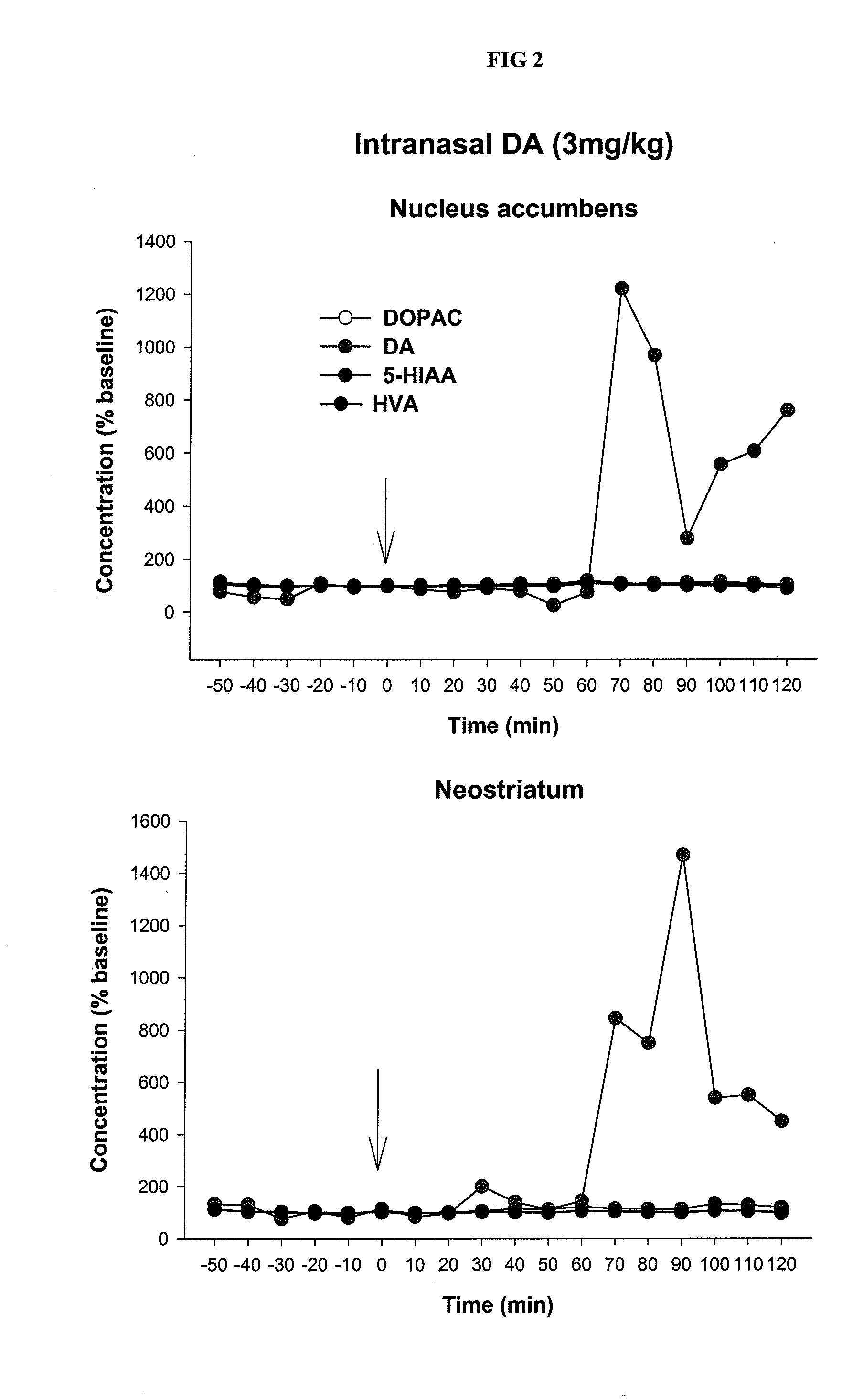
Controlled release delivery system for nasal application of neurotransmitters
InactiveUS20090227550A1Improve bioavailabilityEffective serum levelOrganic active ingredientsBiocideNoseProgesterones
This invention relates to a galenical gel formulation for nasal administration of neurotransmitters / neuromodulators such as dopamine, serotonin or pregnenolone and progesterone. The special lipophilic or partly lipophilic system of the invention leads to high bioavailability of the active ingredient in plasma and brain caused by sustained serum levels and / or direct or partly direct transport from nose to the brain.
Owner:MATTERN PHARMA
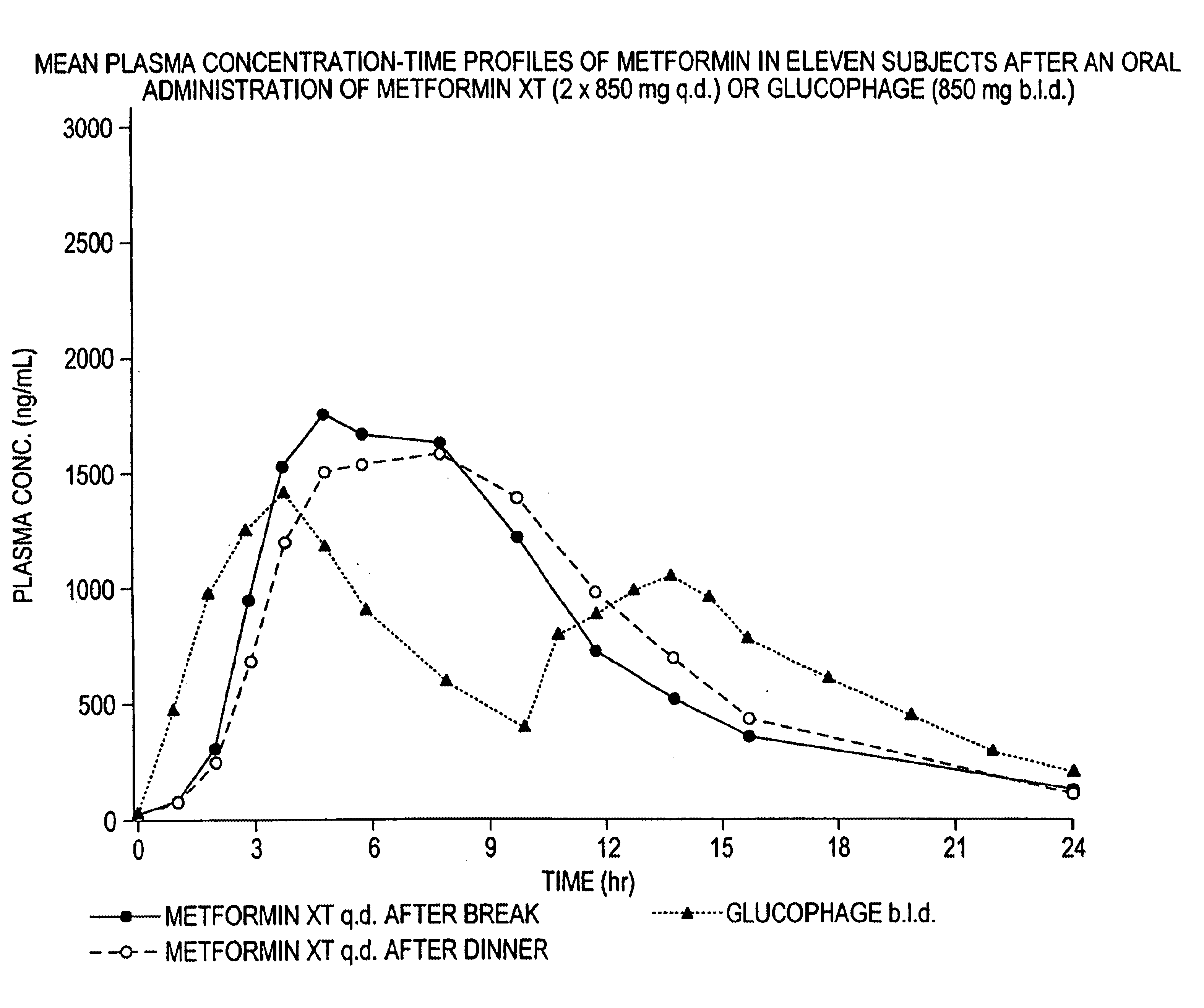
Controlled release metformin compositions
InactiveUS6866866B1Effective controlImprove bioavailabilityOrganic active ingredientsCoatingsCo administrationBlood plasma
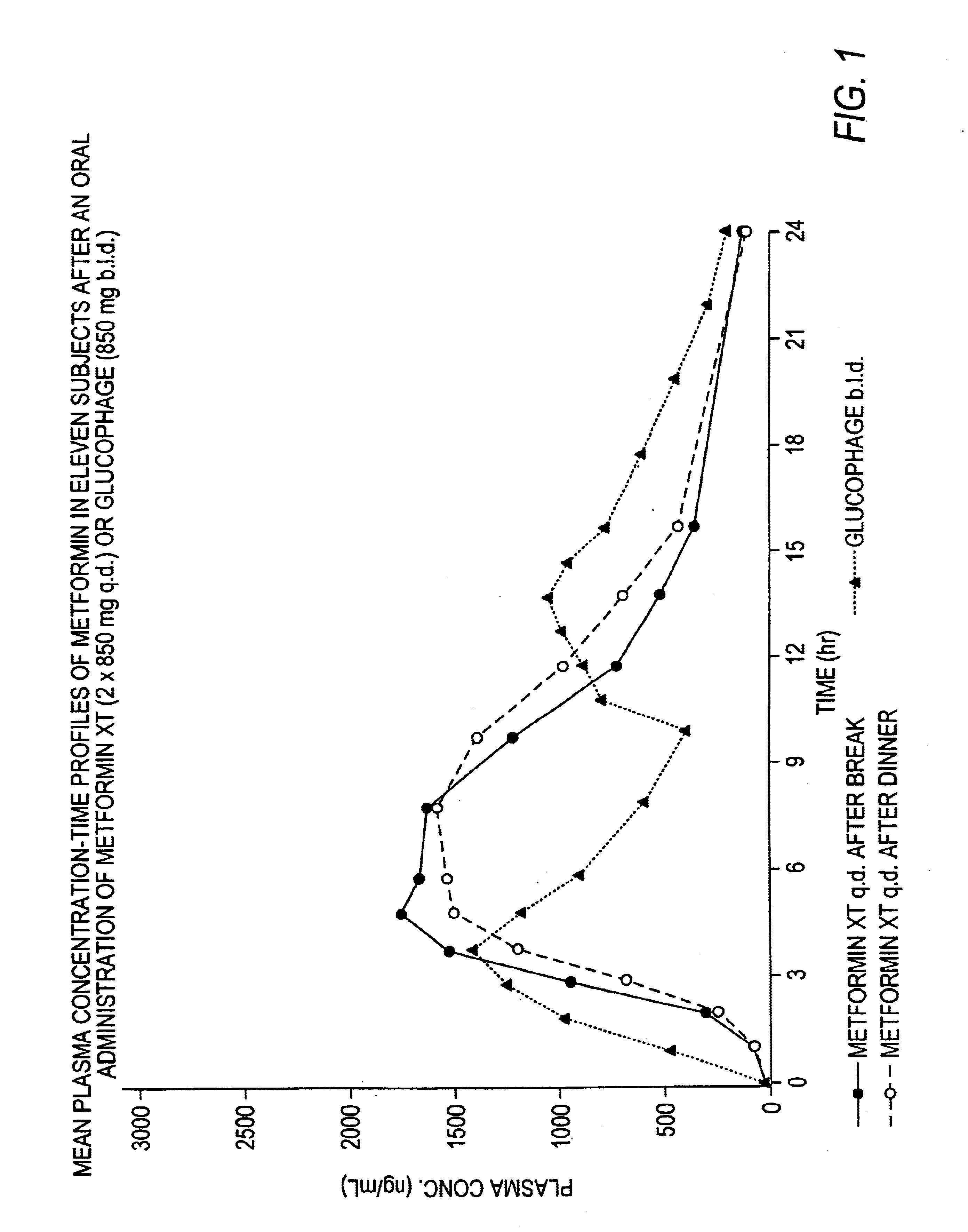
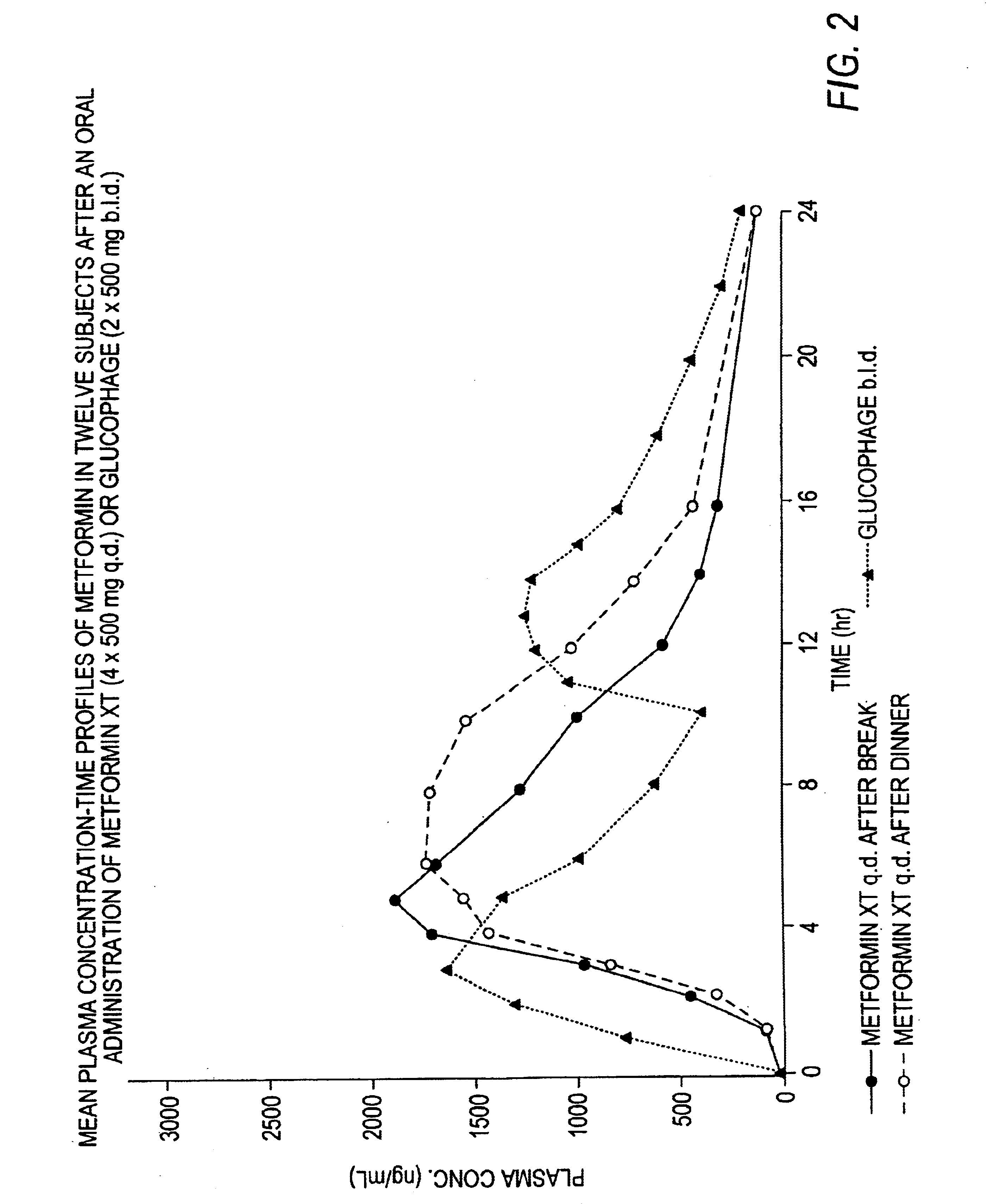
A composition for treating patients having non-insulin-dependent diabetes mellitus (NIDDM) by administering a controlled release oral solid dosage form containing preferably a biguanide drug such as metformin, on a once-a-day basis. The dosage form provides a mean time to maximum plasma concentration (Tmax) of the drug which occurs at 5.5 to 7.5 hours after oral administration on a once-a-day basis to human patients. Preferably, the dose of drug is administered at dinnertime to a patient in the fed state.
Owner:ANDRX LABS
Oral administration form for an acid liable active proton pump inhibitor
Novel administration form for acid-labile active compounds are described. The novel administration forms have no enteric layers and are suitable for oral administration.
Owner:TAKEDA GMBH
Controlled Release Pharmaceutical Compositions Comprising a Fumaric Acid Ester
InactiveUS20080299196A1Decrease glass transition pointLow film forming temperatureBiocideSenses disorderDiseaseHigh concentration
The present invention relates to controlled release pharmaceutical compositions comprising fumaric acid ester(s) as active substance(s). The compositions are suitable for use in the treatment of e.g. psoriasis or other hyperproliferative, inflammatory or autoimmune disorders and are designed to release the fumaric acid ester in a controlled manner so that local high concentrations of the active substance within the gastrointestinal tract upon oral administration can be avoided and, thereby, enabling a reduction in gastro-intestinal related side-effects.
Owner:BIOGEN SWISS MFG GMBH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com