Controlled release delivery system for nasal application of neurotransmitters
a delivery system and neurotransmitter technology, applied in the field of controlled release delivery system for nasal application of neurotransmitters, can solve the problems of little knowledge on factors controlling the nasal delivery of drugs to the brain, parkinson's disease, and unsuitable administration mode of dopamine for neurological disorders, and achieve the effect of increasing the bioavailability of drugs and effective sustained serum levels
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Nasal Administration of Dopamine to Rats
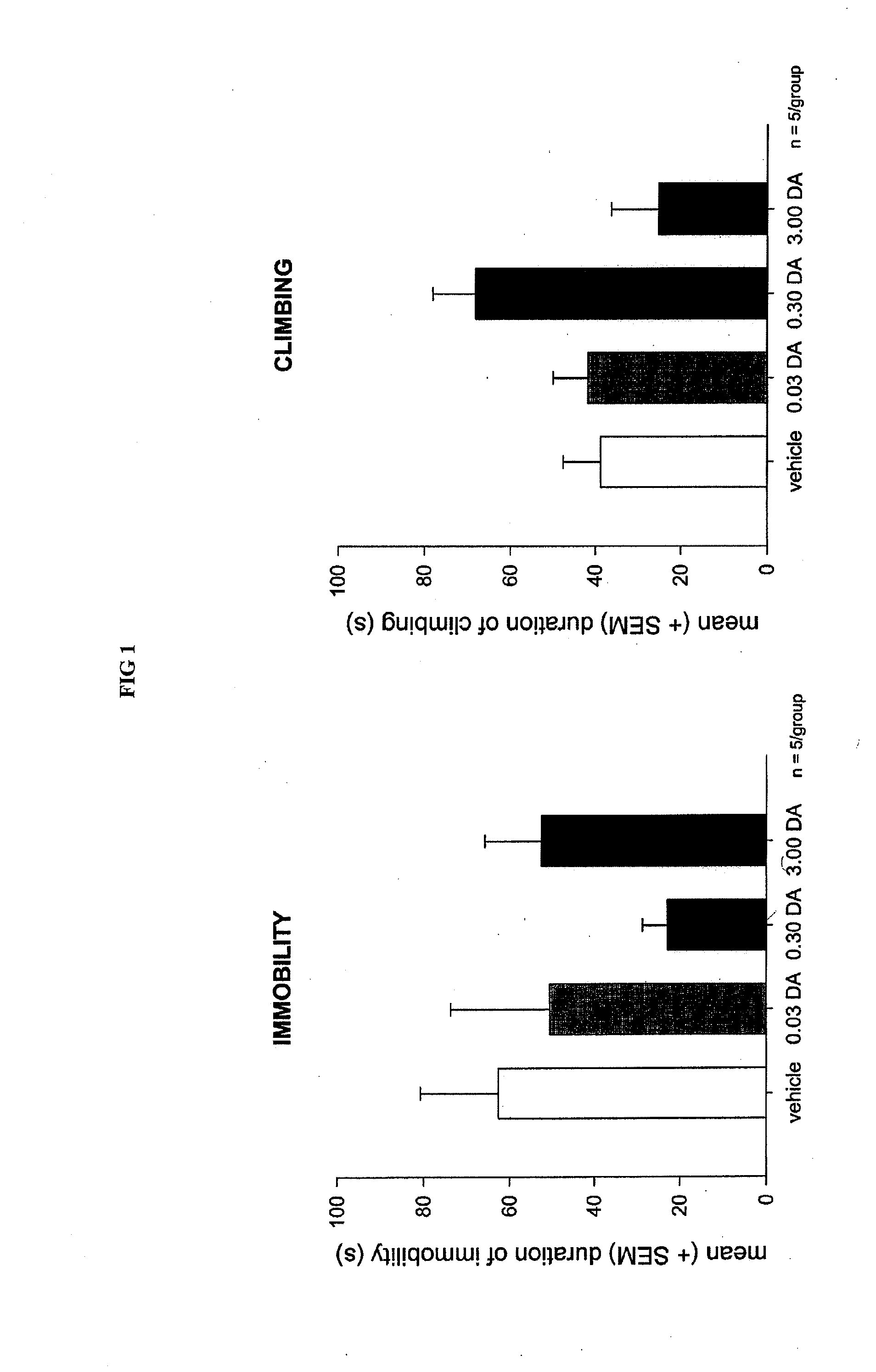
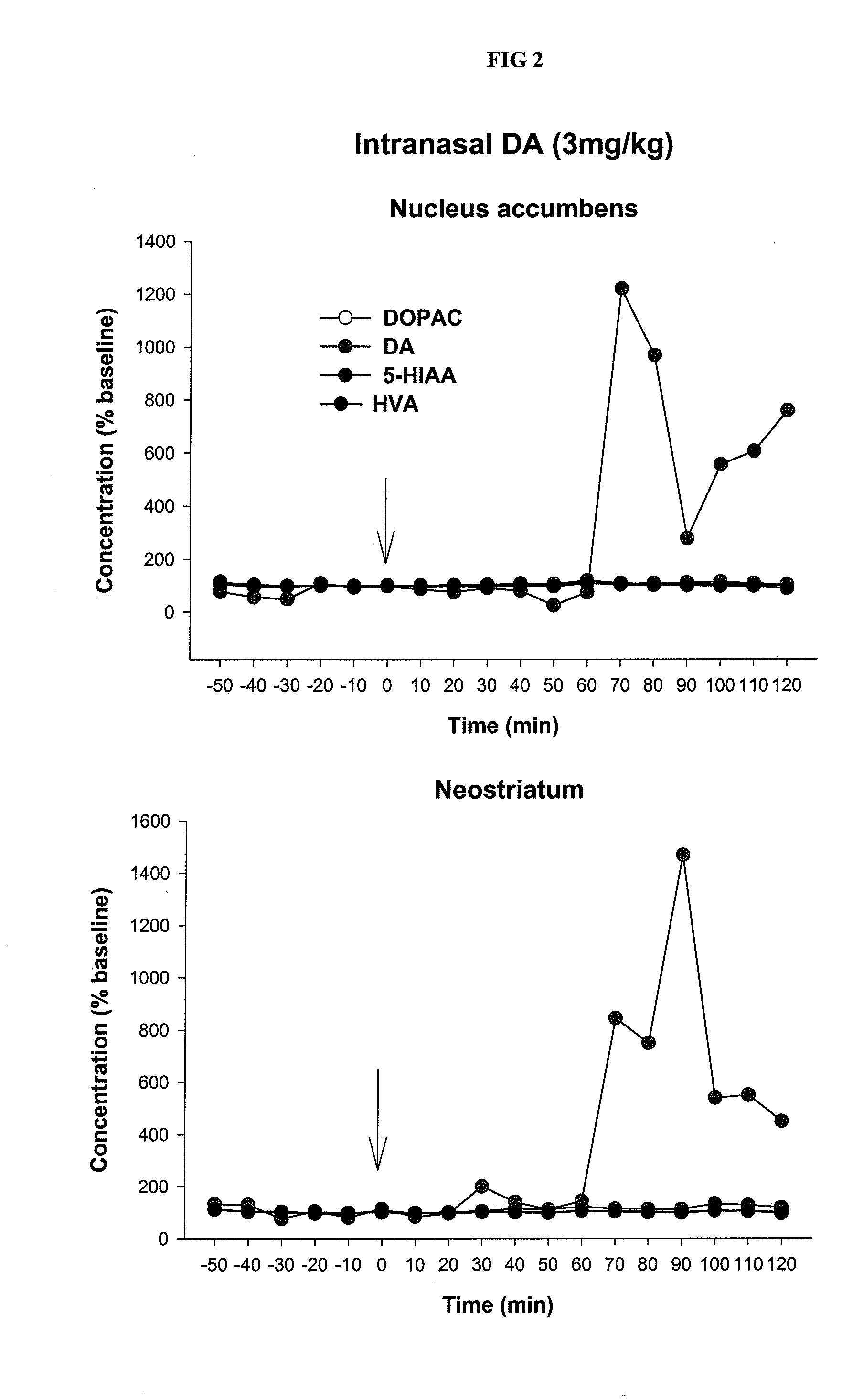
[0078]A dopamine (DA) gel of the inventive formulation was nasally administered to rats used in the validated “forced swimming test.” As shown in FIG. 1, the administration of dopamine results in anti-depressive-like effects. As shown in FIG. 2, strong dopaminergic activity in the neostriatum and ventral striatum (nucleus accumbens) was observed after nasal application of dopamine with the inventive formulation.
[0079]Generally, antidepressants must be administered for an extended length of time before antidepressive effects are observed. Surprisingly, after nasal application to rat of the dopamine gel formulation, antidepressive effects occurred within hours and without any observable side effects, such as those side effects known to occur with desipramine (apathy) or fluoxetine (weight loss).
[0080]After nasal application to rats of dopamine in the inventive gel formulation, the concentration of dopamine in the nucleus accumbens and neostriatu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| lipophilic | aaaaa | aaaaa |
| surface tension | aaaaa | aaaaa |
| hydrophilic | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com