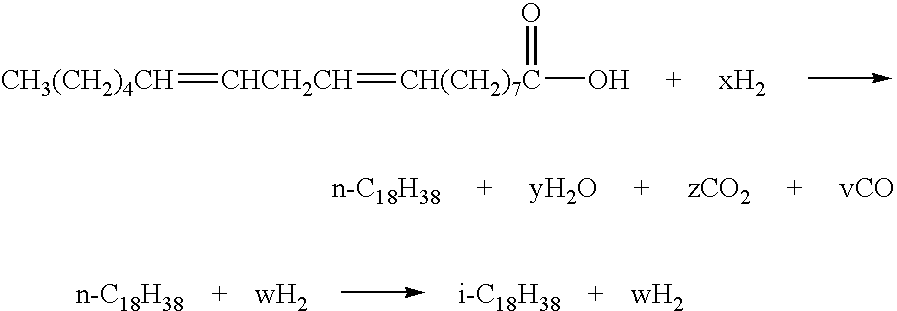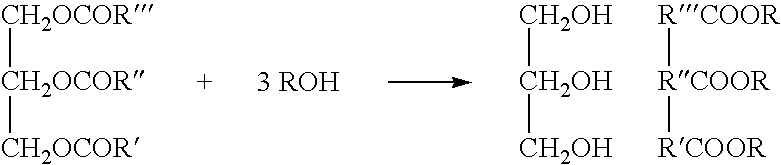Patents
Literature
1716 results about "TG - Triglyceride" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A triglyceride (TG, triacylglycerol, TAG, or triacylglyceride) is an ester derived from glycerol and three fatty acids (from tri- and glyceride).
Solid carriers for improved delivery of active ingredients in pharmaceutical compositions
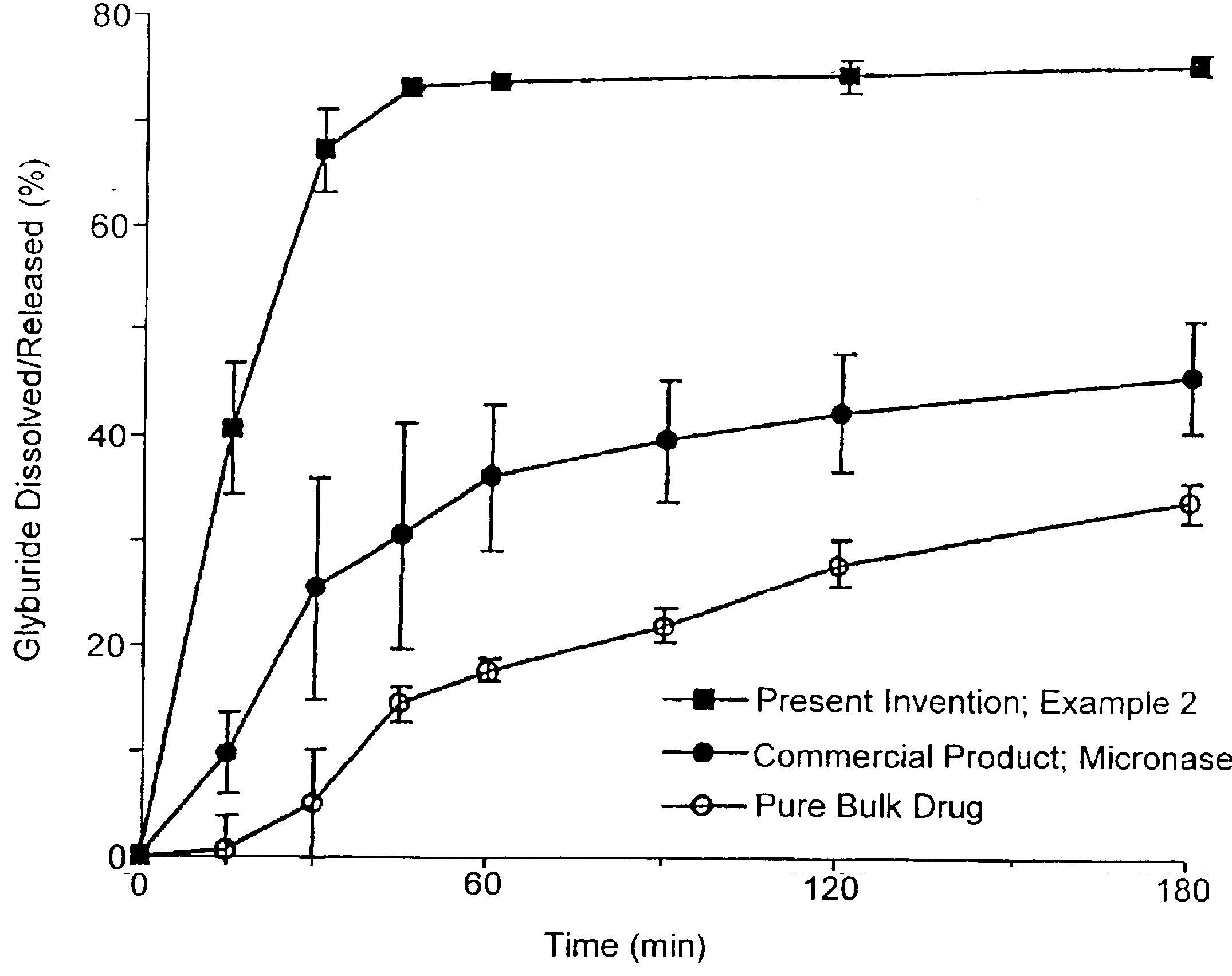
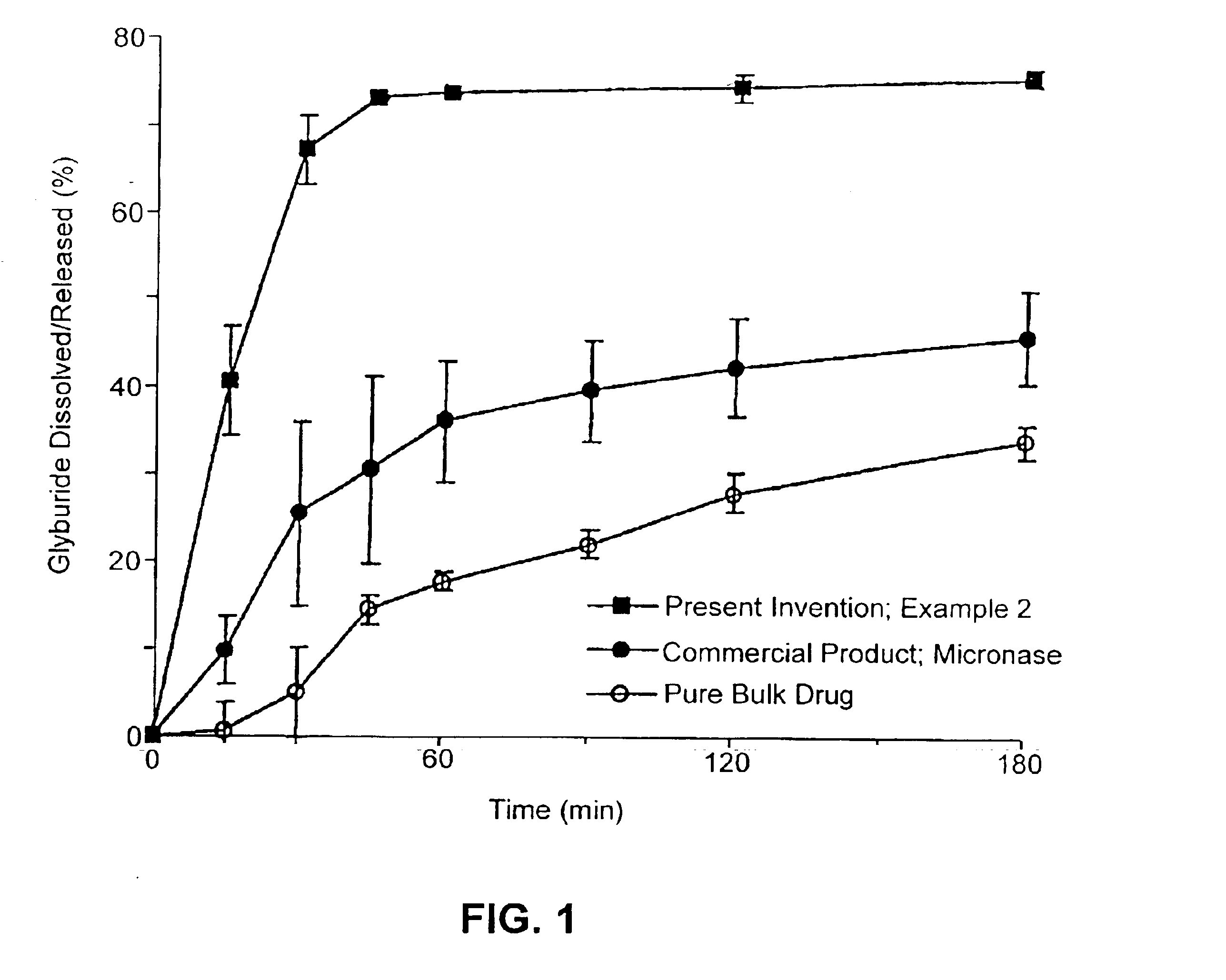
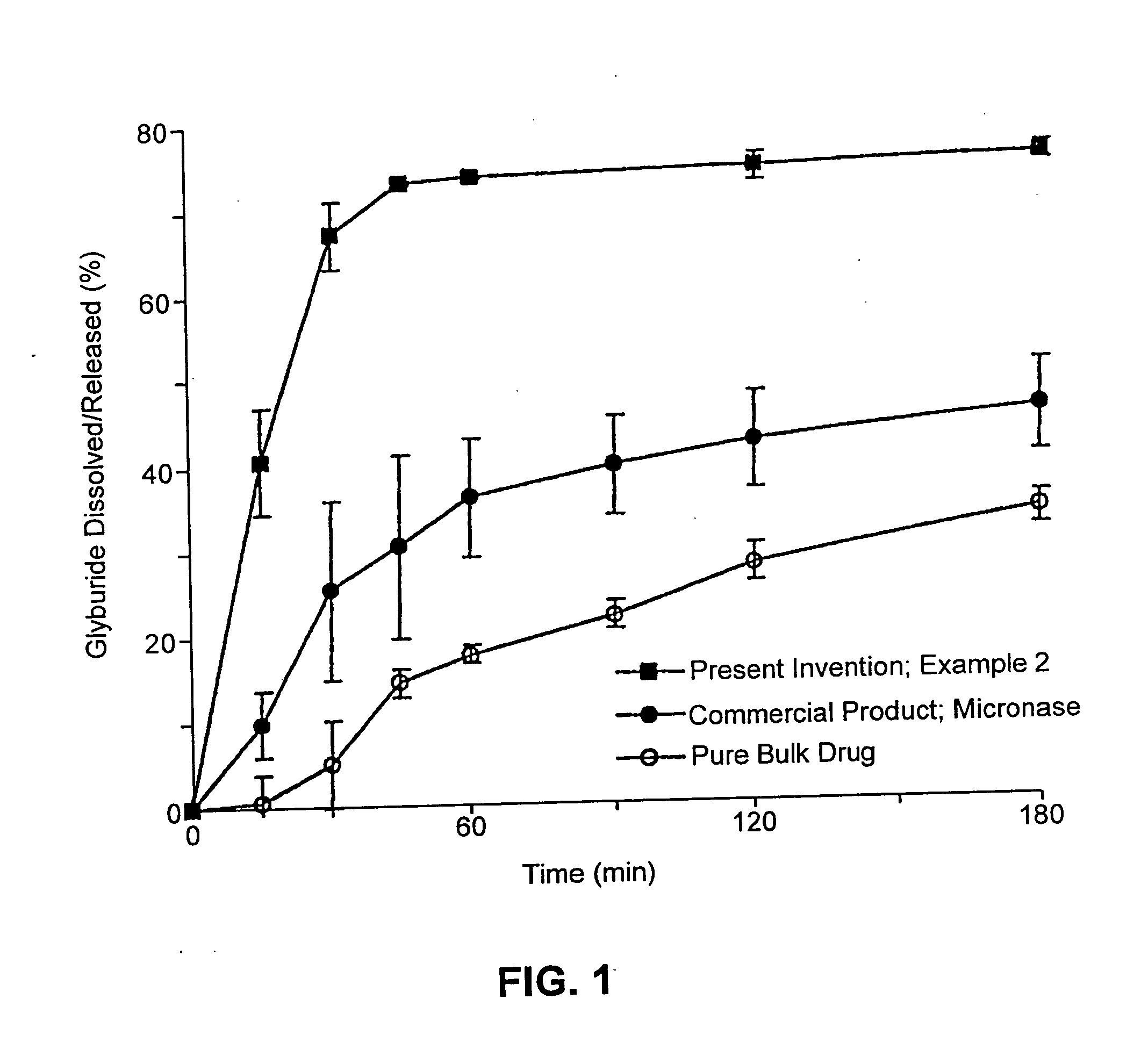
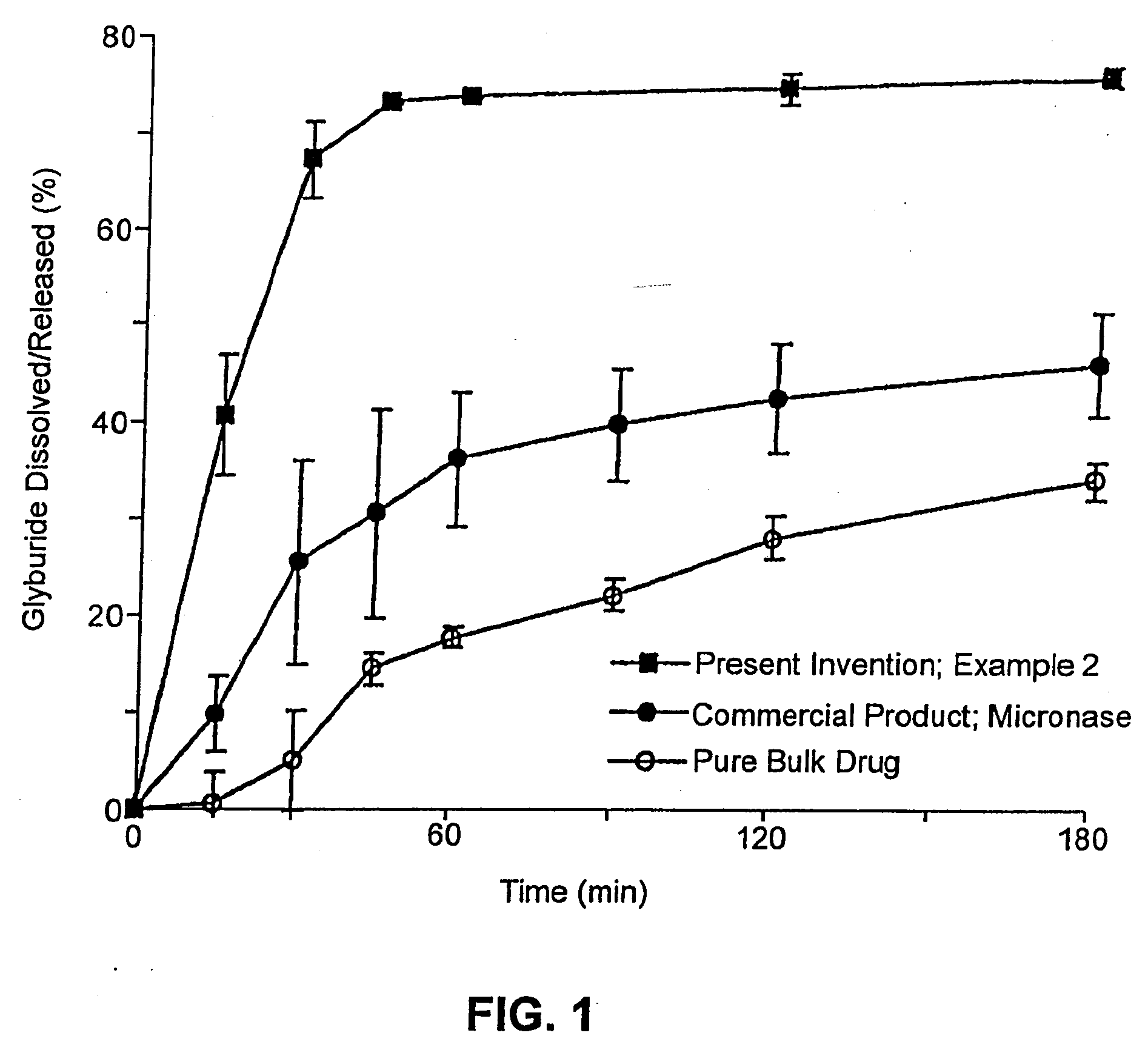
InactiveUS6923988B2Rapid dissolvableMore solubilizedAntibacterial agentsOrganic active ingredientsDiagnostic agentTG - Triglyceride
The present invention provides solid pharmaceutical compositions for improved delivery of a wide variety of pharmaceutical active ingredients contained therein or separately administered. In one embodiment, the solid pharmaceutical composition includes a solid carrier, the solid carrier including a substrate and an encapsulation coat on the substrate. The encapsulation coat can include different combinations of pharmaceutical active ingredients, hydrophilic surfactant, lipophilic surfactants and triglycerides. In another embodiment, the solid pharmaceutical composition includes a solid carrier, the solid carrier being formed of different combinations of pharmaceutical active ingredients, hydrophilic surfactants, lipophilic surfactants and triglycerides. The compositions of the present invention can be used for improved delivery of hydrophilic or hydrophobic pharmaceutical active ingredients, such as drugs, nutritional agents, cosmeceuticals and diagnostic agents.
Owner:LIPOCINE
Solid carriers for improved delivery of active ingredients in pharmaceutical compositions
InactiveUS20060034937A1Good chemical stabilityPromote absorptionGranular deliveryMicrocapsulesDiagnostic agentMedicine
The present invention provides solid pharmaceutical compositions for improved delivery of a wide variety of pharmaceutical active ingredients contained therein or separately administered. In one embodiment, the solid pharmaceutical composition includes a solid carrier, the solid carrier including a substrate and an encapsulation coat on the substrate. The encapsulation coat can include different combinations of pharmaceutical active ingredients, hydrophilic surfactant, lipophilic surfactants and triglycerides. In another embodiment, the solid pharmaceutical composition includes a solid carrier, the solid carrier being formed of different combinations of pharmaceutical active ingredients, hydrophilic surfactants, lipophilic surfactants and triglycerides. The compositions of the present invention can be used for improved delivery of hydrophilic or hydrophobic pharmaceutical active ingredients, such as drugs, nutritional agents, cosmeceuticals and diagnostic agents.
Owner:LIPOCINE
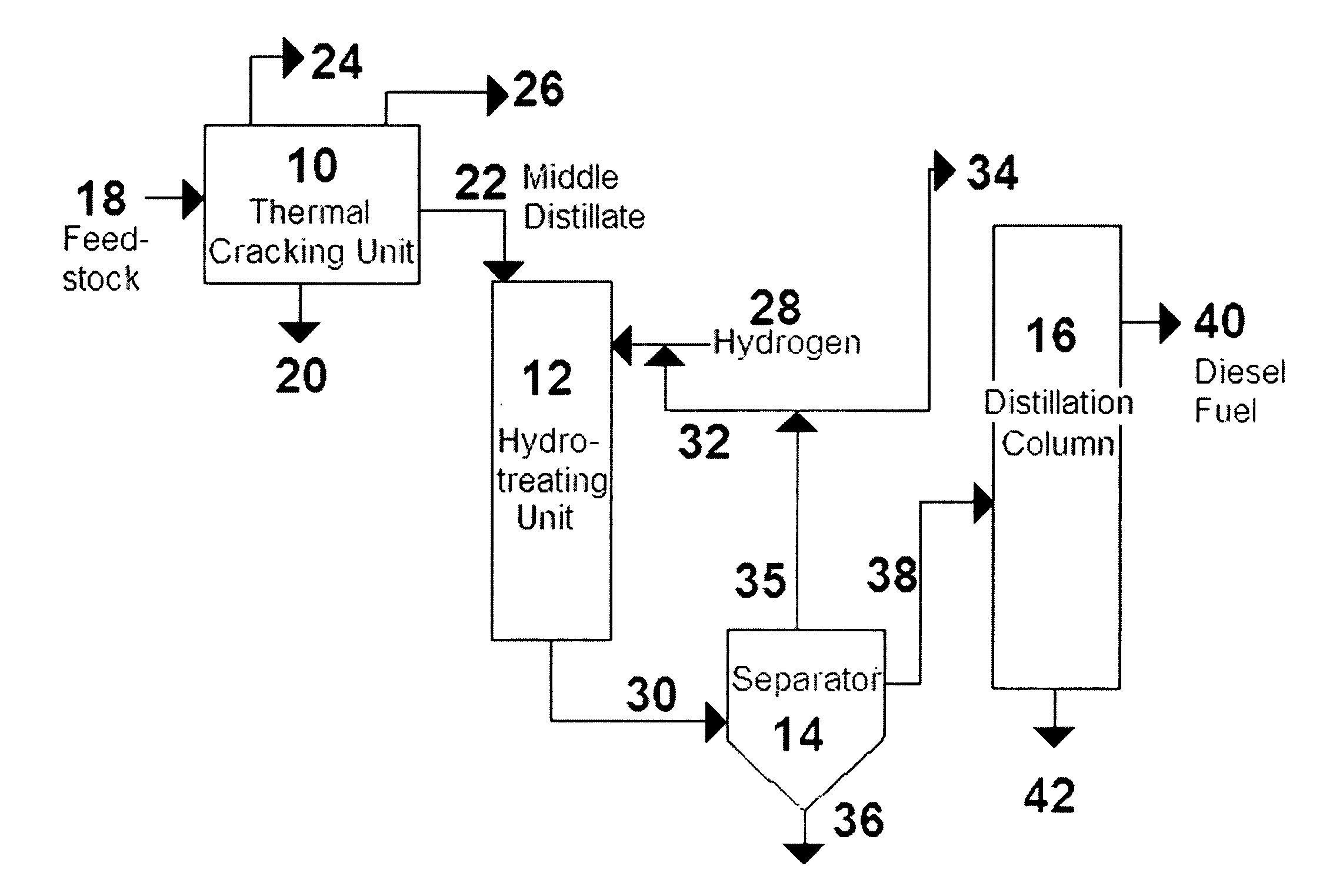
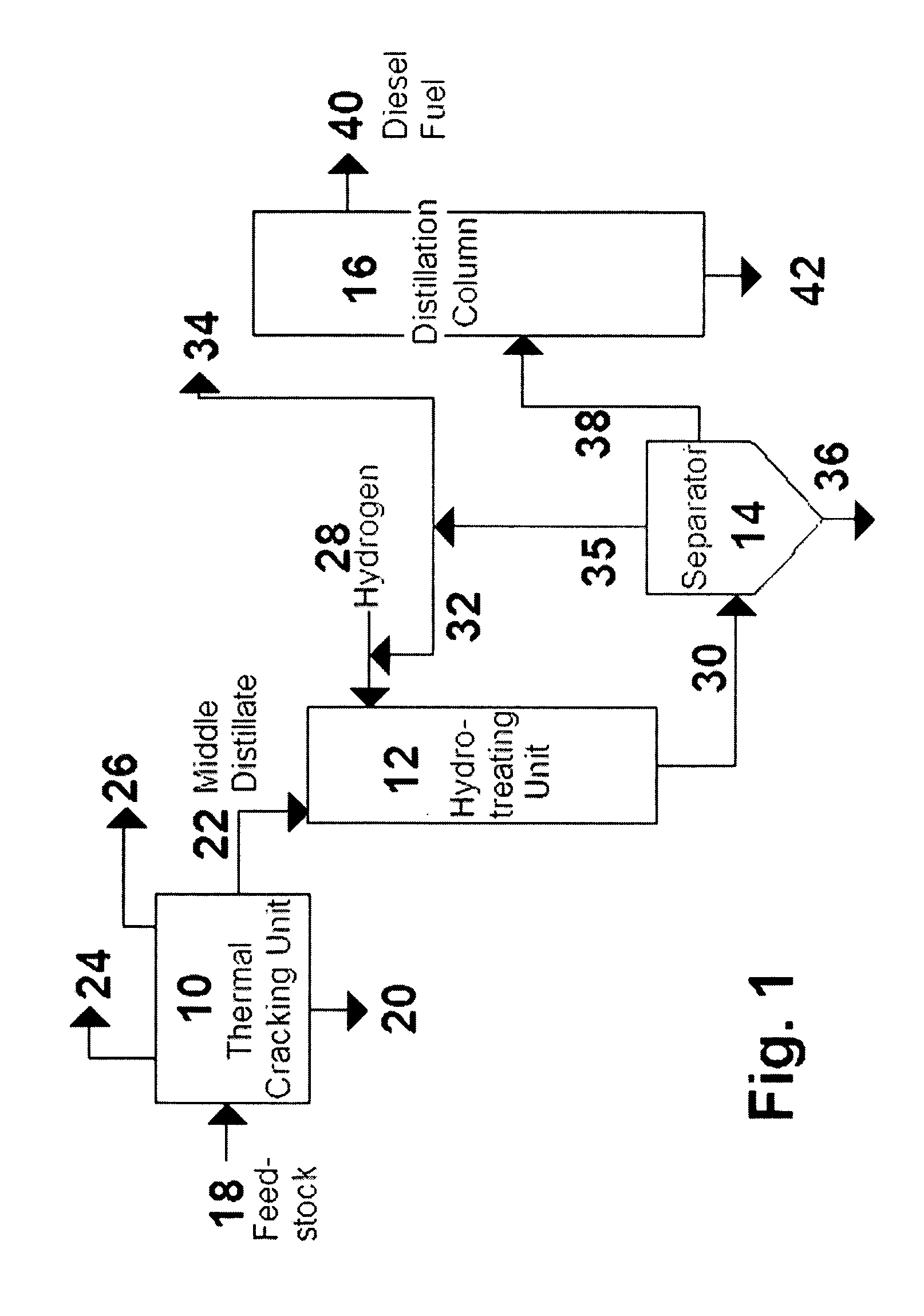
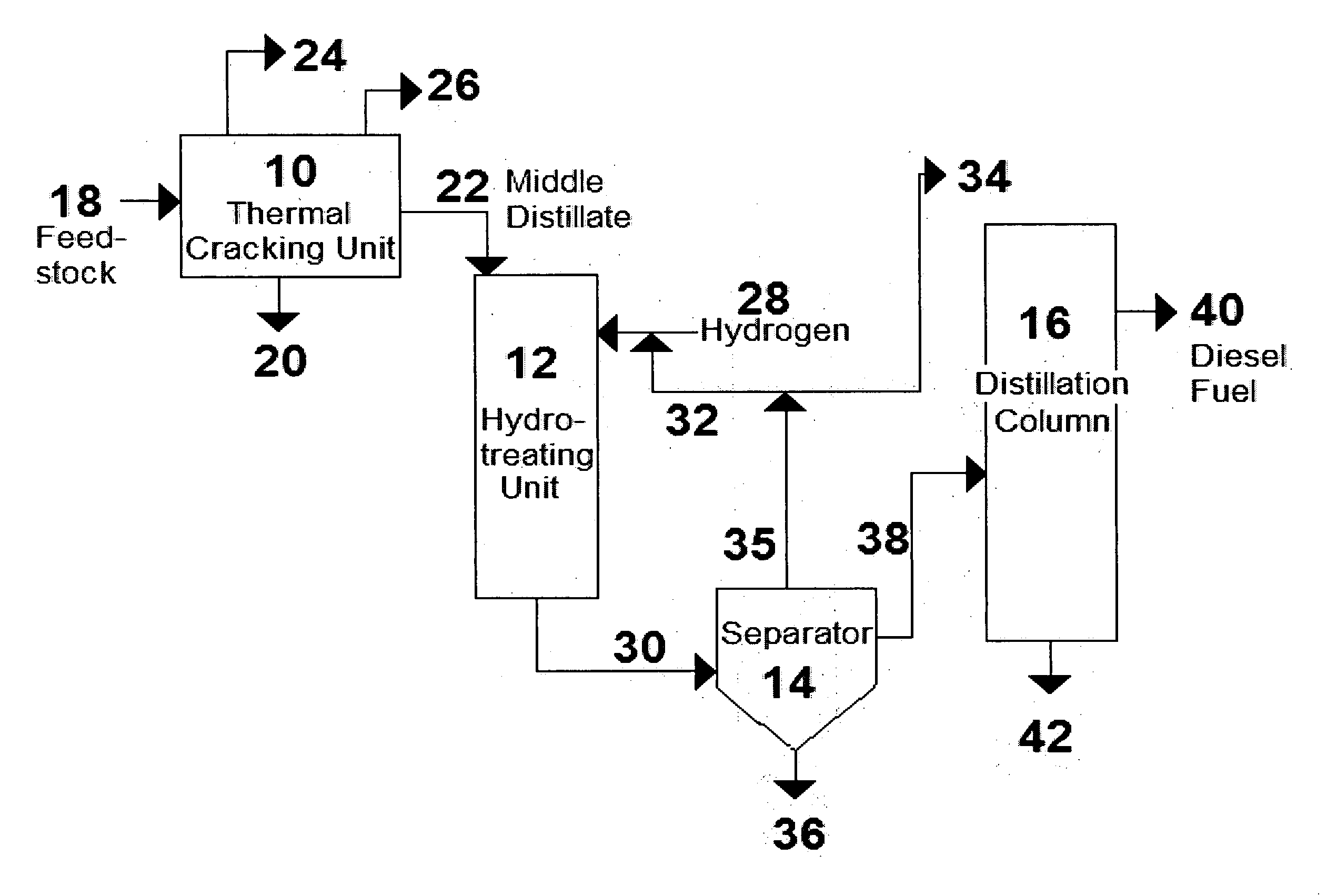
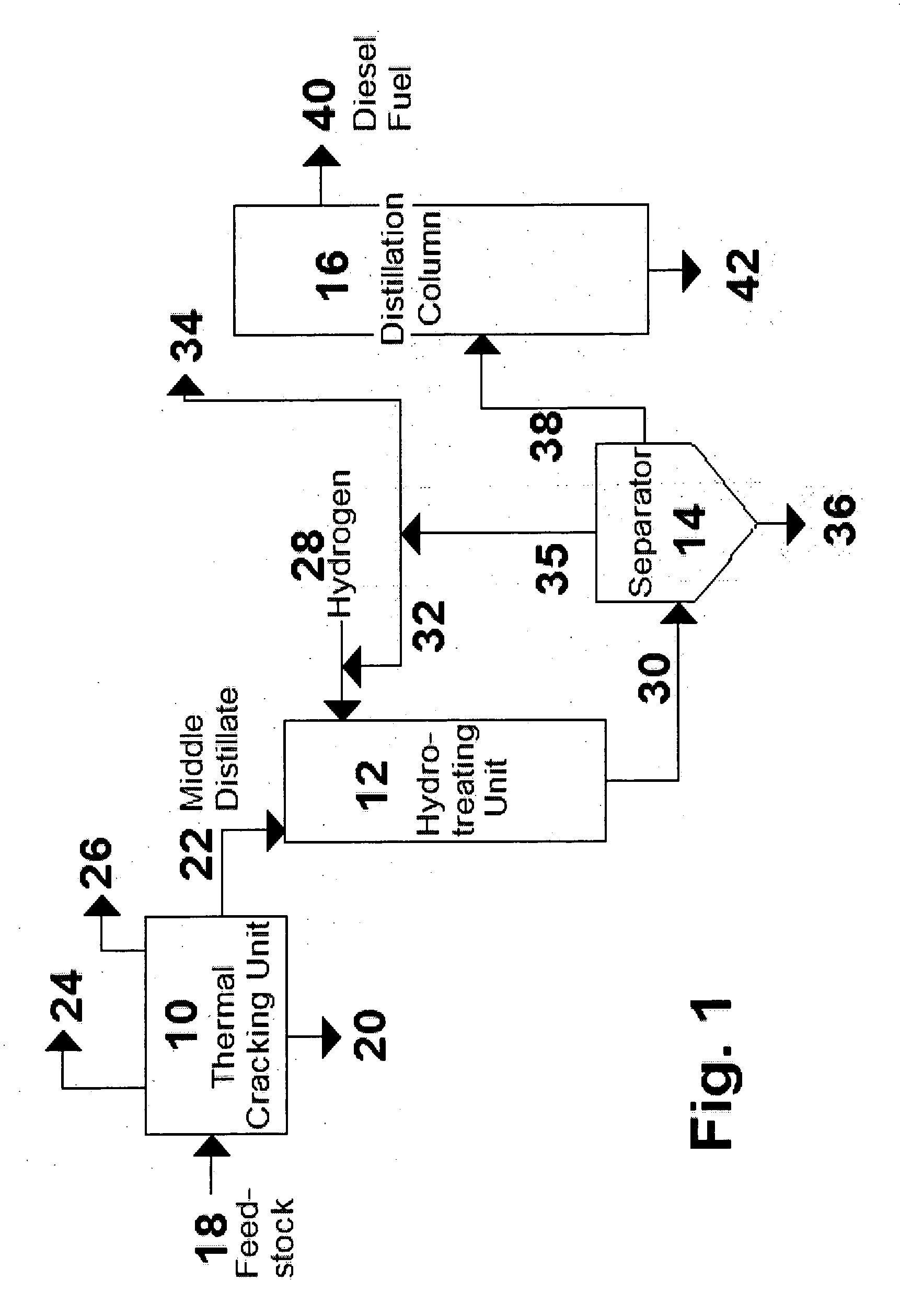
Process for the manufacture of diesel range hydro-carbons
ActiveUS20070006523A1Improve low temperature performanceBig ratioBiofuelsLiquid carbonaceous fuelsChemical industryAlkane
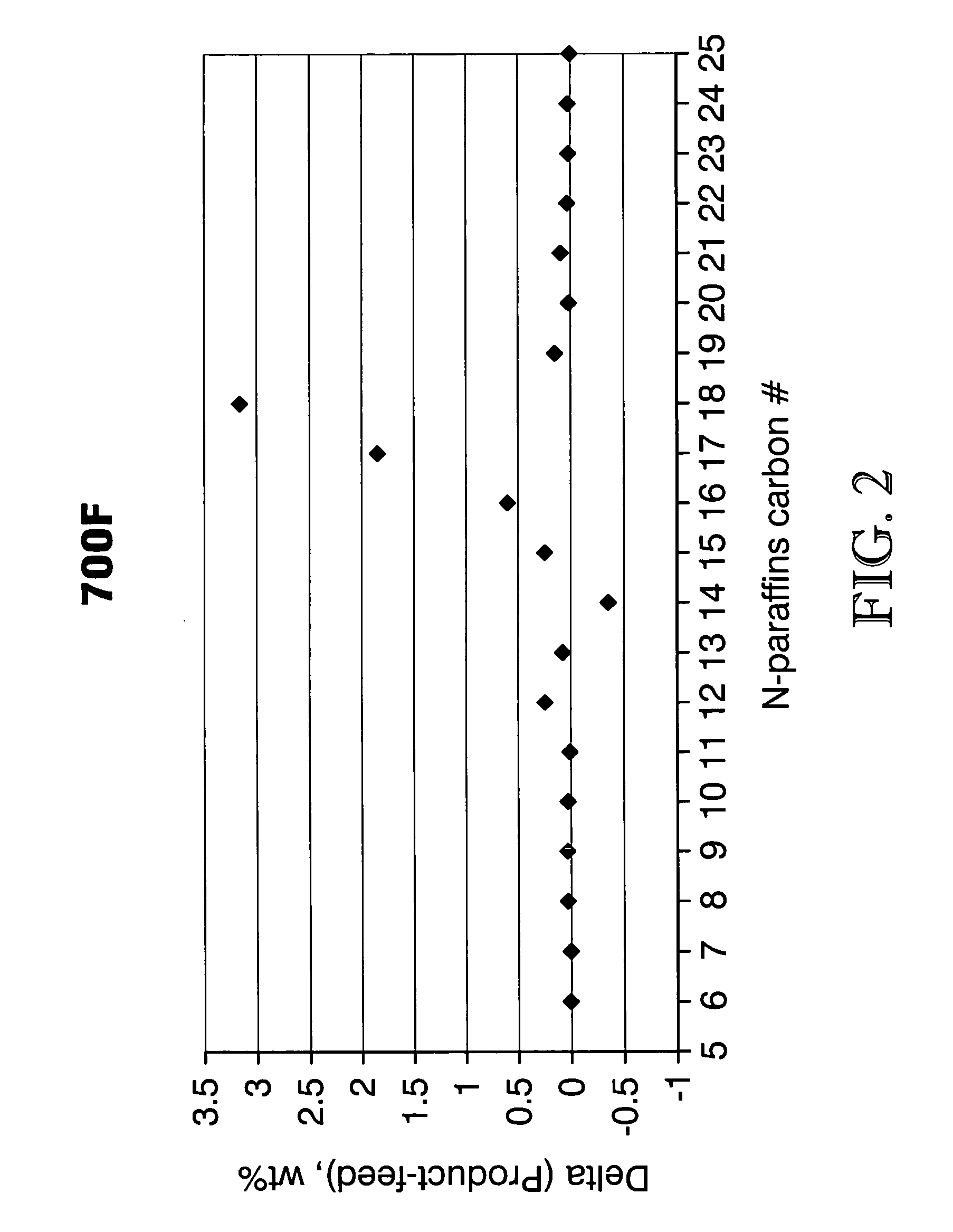
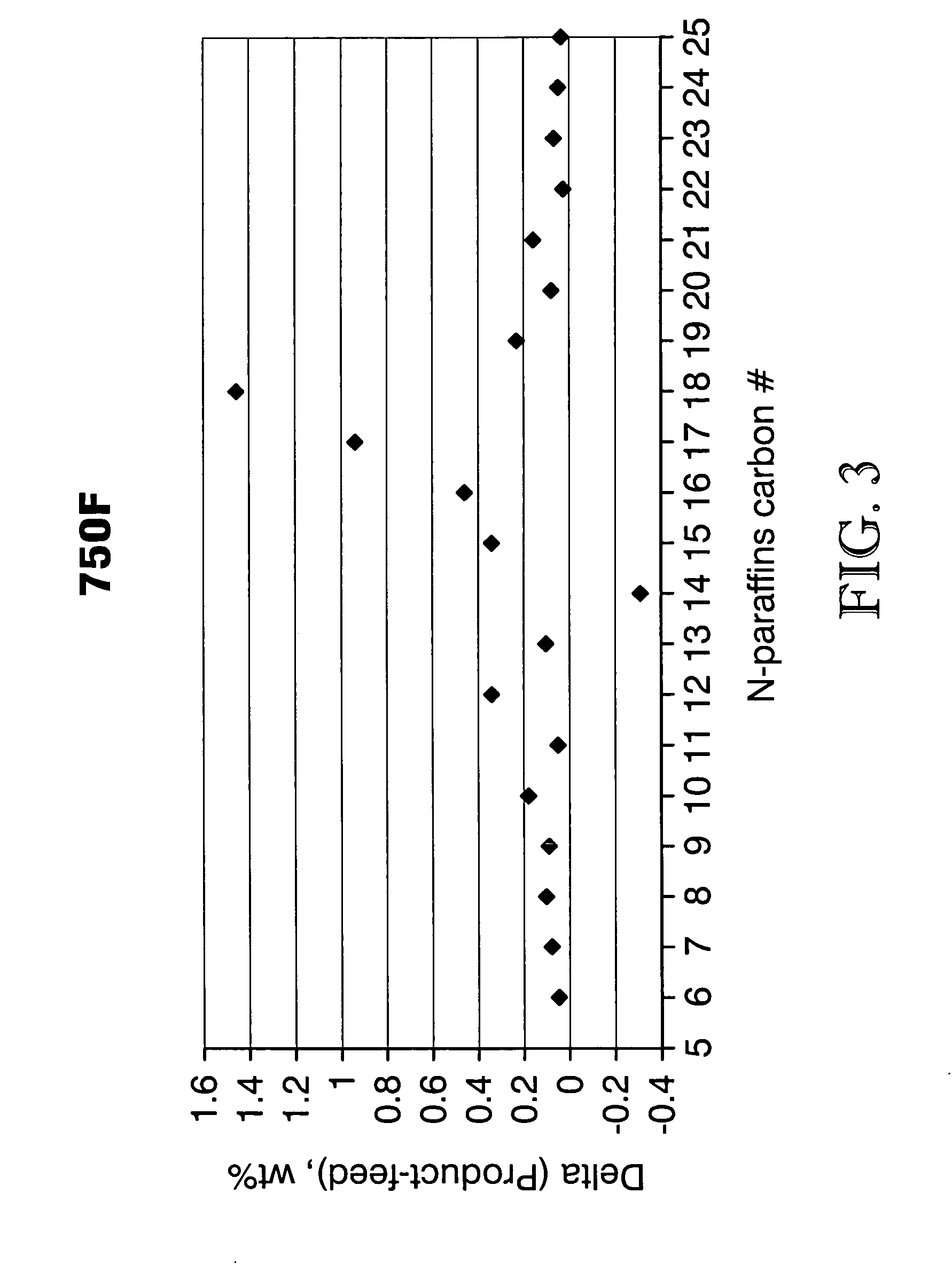
The invention relates to chemical industry and is directed to the production of middle distillate from vegetable oils. In the first step of the production method, the fatty acids or triglycerides of said vegetable oils are hydrogenated to give n-paraffins, and in the second step, the n-paraffins are catalytically converted to paraffins with branched chains. Using this process having two steps, a high-quality middle distillate useful as a component of diesel fuels without any particular specifications may be produced.
Owner:NESTE OIL OY
Pharmaceutical Composition and Method for Treating Hypertriglyceridemia and Hypercholesterolemia in Humans
InactiveUS20090182049A1Lower triglyceridesIncreasing HDL (good) cholesterolBiocideMetabolism disorderDocosahexaenoic acidSerum cholesterol
A method for the treatment or prophylaxis of hypertriglyceridemia and hypercholesterolemia without concomitantly increasing LDL-serum cholesterol, in a human subject requiring such treatment, which method comprises orally administering to the patient an effective amount of a pharmaceutical composition in which the active ingredients comprise a mixture of fatty acids, wherein said mixture comprises at least about 60% by weight a combination of eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) in a weight ratio of EPA:DHA of from about 1.4:1 to about 5:1, wherein said combination is at least about 60% in the triglyceride form of the fatty acids and the balance is at least about 80% of mono and di-glycerides.
Owner:OPHEIM JOAR ARILD
Production of high-cetane diesel fuel from low-quality biomass-derived feedstocks
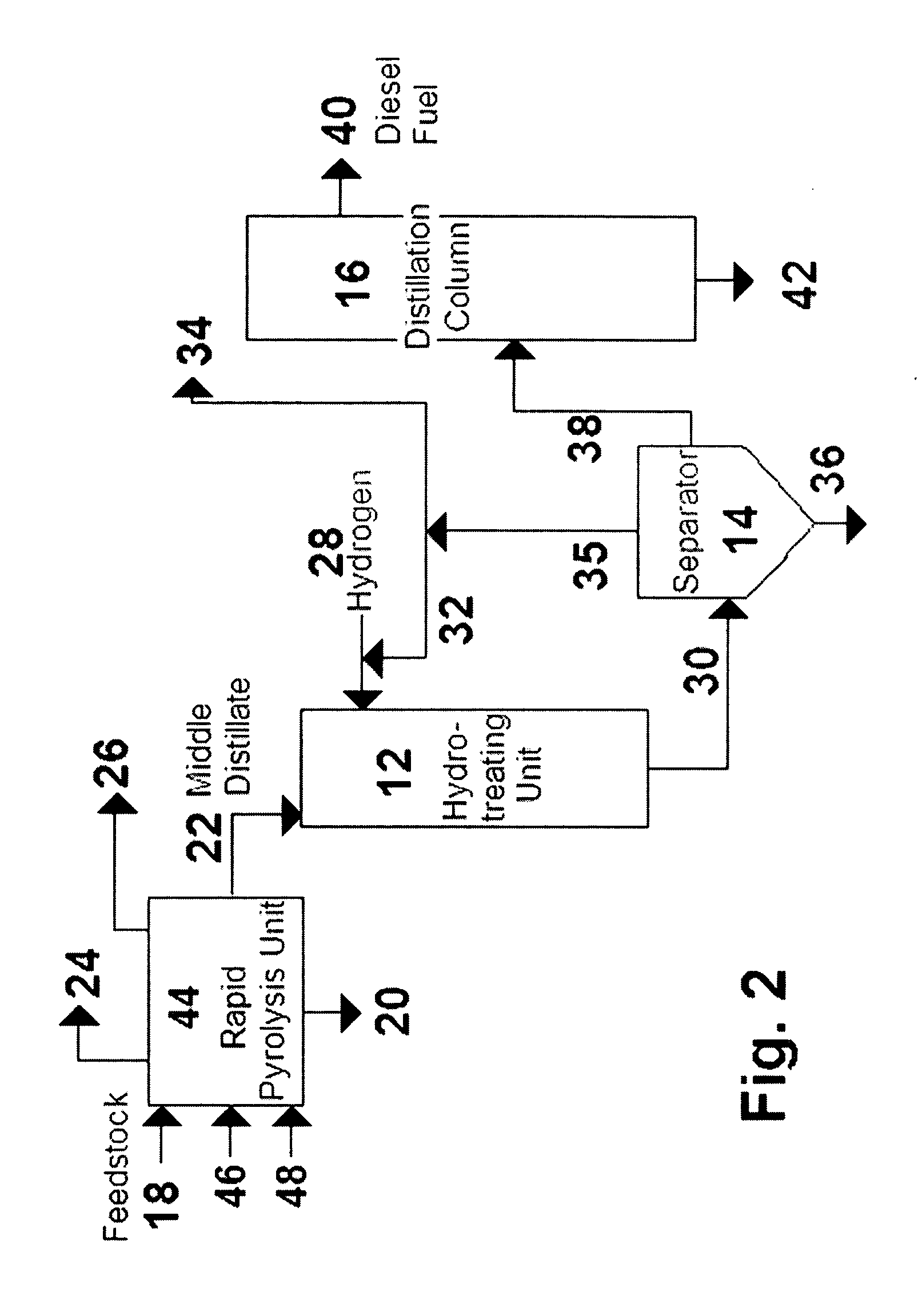
A method is taught for producing diesel fuels of high cetane value from a triglyceride feedstock, comprising pretreating the triglyceride feedstock by thermal cracking or rapid pyrolysis to partially convert the triglycerides and produce a middle distillates stream, and catalytically hydrotreating the middle distillate fraction to produce high cetane value diesel fuels. A biomass-derived diesel fuel is also taught having sulphur content below 10 ppm, a cetane-value of at least 70, a cloud point below 0° C. and a pour point of less than −4° C. A blended diesel fuel is also taught comprising 5 to 20 vol. % of the biomass-derived diesel fuel of the present invention and 80 to 95 vol. % of a petroleum diesel, based on total volume of the blended diesel fuel.
Owner:HER MAJESTY THE QUEEN & RIGHT OF CANADA REPRESENTED BY THE MIN OF NATURAL RESOURCES
Delivery of tetrahydrocannabinol
InactiveUS20070104741A1Avoiding hepatic first-pass metabolismGood choiceBiocideNervous disorderChylomicronTG - Triglyceride
A self-emulsifying drug delivery system to improve dissolution, stability, and bioavailability of drug compounds of dronabinol or other cannabinoids. The drug compound(s) are dissolved in an oily medium (e.g. triglycerides and / or mixed glycerides and / or free fatty acids containing medium and / or long chain saturated, mono-unsaturated, and / or poly-unsaturated free fatty acids) together with at least one surfactant. The surfactant promotes self-emulsification, thereby promoting targeted chylomicron delivery and optimal bioavailability to a mammalian intestinal lumen. A dosage form can optionally include co-solvents, anti-oxidants, viscosity modifying agents, cytochrome P450 metabolic inhibitors, P-GP efflux inhibitors, and amphiphilic / non-amphiphilic solutes to induce semi-solid formation for targeted release rates.
Owner:MURTY PHARMA
Process for converting triglycerides to hydrocarbons
ActiveUS20070175795A1High reaction yieldIncrease cetane numberBiofuelsLiquid carbonaceous fuelsBoiling pointHydrocotyle bowlesioides
Processes for the conversion of hydrocarbons boiling in the temperature range of from about 80° F. to about 1000° F. to diesel boiling range hydrocarbons, and processes for increasing the cetane number and amount of n-C17 hydrocarbon products in such processes. Diesel boiling range hydrocarbons may be produced by contacting a hydrocarbon boiling in the above-mentioned boiling range with a triglyceride-containing compound to form a mixture, and then contacting the mixture with a hydrotreating catalyst under suitable reaction conditions.
Owner:PHILLIPS 66 CO
Composition comprising beta -hydroxy- beta -methylbutyric acid and at least one amino acid and methods of use
InactiveUS6031000ADecrease in fat massQuality improvementBiocidePeptide/protein ingredientsSerum igeDisease
The present invention provides a composition comprising HMB and at least one amino acid. The present invention also provides a method for the treatment of disease-associated wasting of an animal, a method for decreasing the serum-level of triglycerides of an animal, a method for decreasing the serum viral load of an animal, and a method for redistributing fat in an animal having a visceral region and a subcutaneous region. All methods comprise administering to the animal a composition comprising HMB and at least one amino acid.
Owner:IOWA STATE UNIV RES FOUND
Antidiabetic bicyclic compounds
ActiveUS20070265332A1Useful in treatmentBiocideSenses disorderHypertriglyceridemiaG protein-coupled receptor
Tricyclic compounds containing a cyclopropyl carboxylic acid or carboxylic acid derivative (e.g. amide) fused to a bicyclic ring, including pharmaceutically acceptable salts and prodrugs thereof, are agonists of G-protein coupled receptor 40 (GPR40) and are useful as therapeutic compounds, particularly in the treatment of Type 2 diabetes mellitus, and of conditions that are often associated with this disease, including obesity and lipid disorders, such as mixed or diabetic dyslipidemia, hyperlipidemia, hypercholesterolemia, and hypertriglyceridemia.
Owner:MERCK SHARP & DOHME LLC
Solid carriers for improved delivery of active ingredients in pharmaceutical compositions
InactiveUS20090074859A1Dissolve fastReduce deliveryAntibacterial agentsPowder deliveryDiagnostic agentTG - Triglyceride
The present invention provides solid pharmaceutical compositions for improved delivery of a wide variety of pharmaceutical active ingredients contained therein or separately administered. In one embodiment, the solid pharmaceutical composition includes a solid carrier, the solid carrier including a substrate and an encapsulation coat on the substrate. The encapsulation coat can include different combinations of pharmaceutical active ingredients, hydrophilic surfactant, lipophilic surfactants and triglycerides. In another embodiment, the solid pharmaceutical composition includes a solid carrier, the solid carrier being formed of different combinations of pharmaceutical active ingredients, hydrophilic surfactants, lipophilic surfactants and triglycerides. The compositions of the present invention can be used for improved delivery of hydrophilic or hydrophobic pharmaceutical active ingredients, such as drugs, nutritional agents, cosmeceuticals and diagnostic agents.
Owner:LIPOCINE
Process for converting triglycerides to hydrocarbons
ActiveUS7550634B2High reaction yieldIncrease cetane numberBiofuelsSolid fuelsBoiling pointTG - Triglyceride
Processes for the conversion of hydrocarbons boiling in the temperature range of from about 80° F. to about 1000° F. to diesel boiling range hydrocarbons, and processes for increasing the cetane number and amount of n-C17 hydrocarbon products in such processes. Diesel boiling range hydrocarbons may be produced by contacting a hydrocarbon boiling in the above-mentioned boiling range with a triglyceride-containing compound to form a mixture, and then contacting the mixture with a hydrotreating catalyst under suitable reaction conditions.
Owner:PHILLIPS 66 CO
Method of converting triglycerides to biofuels
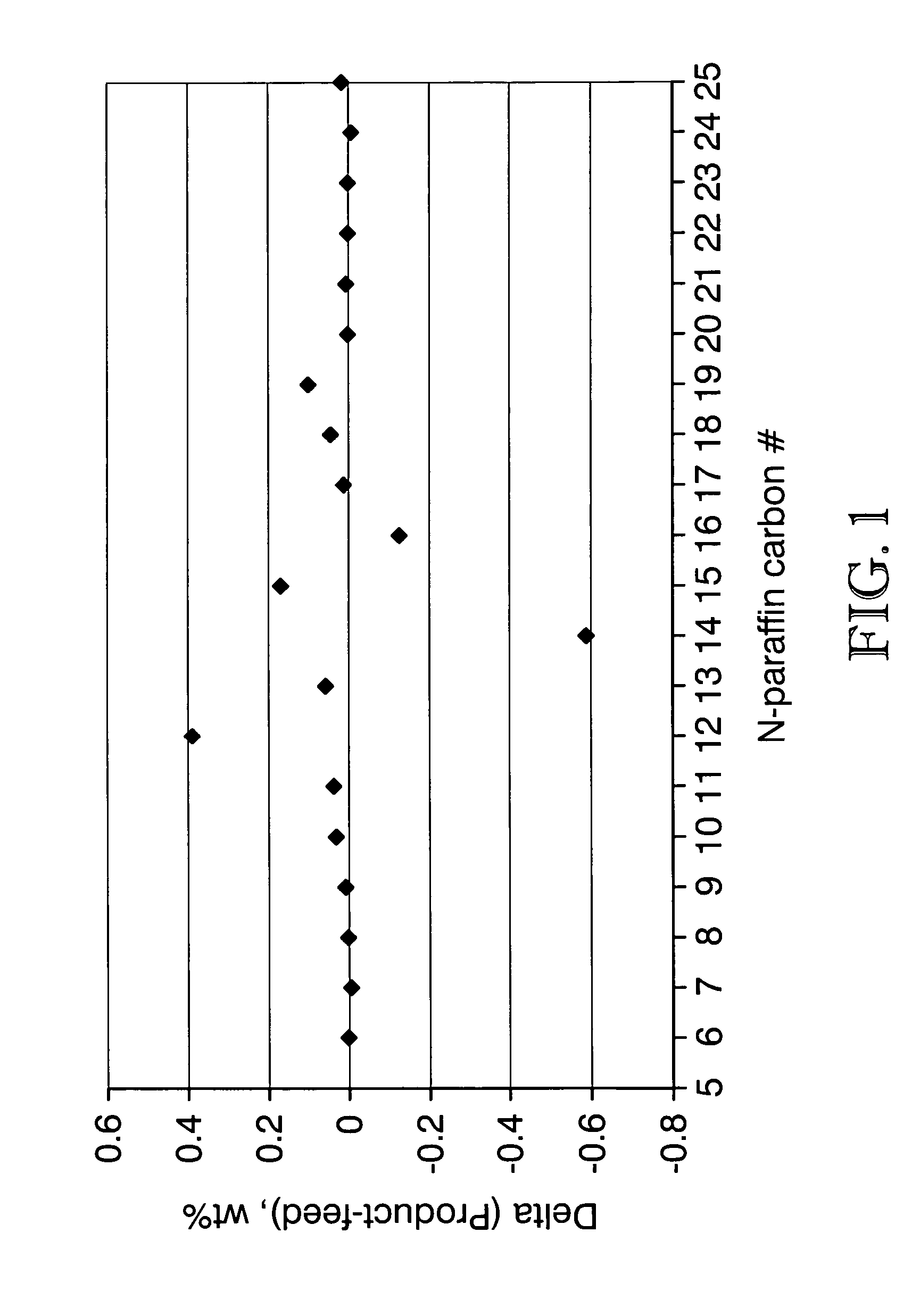
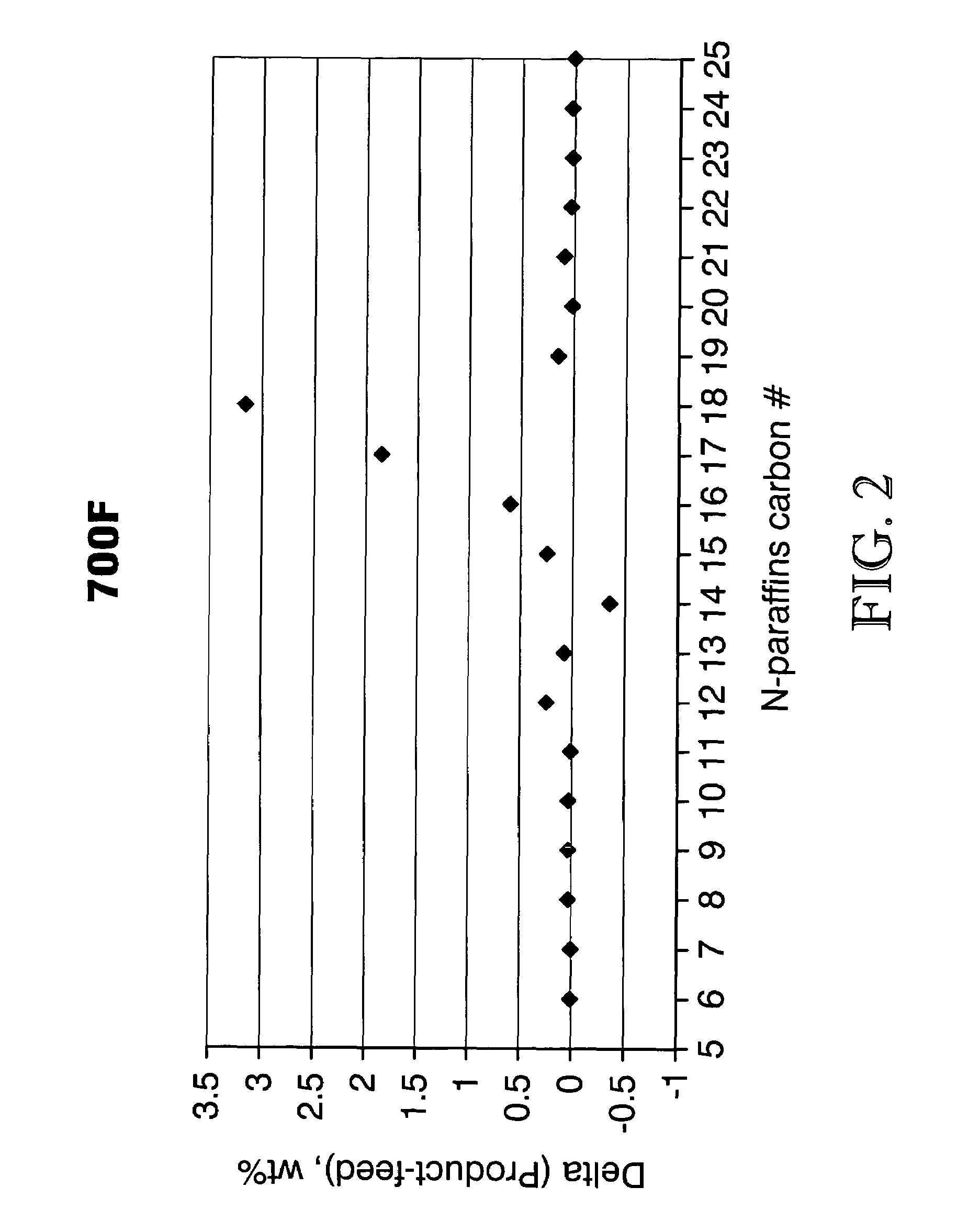
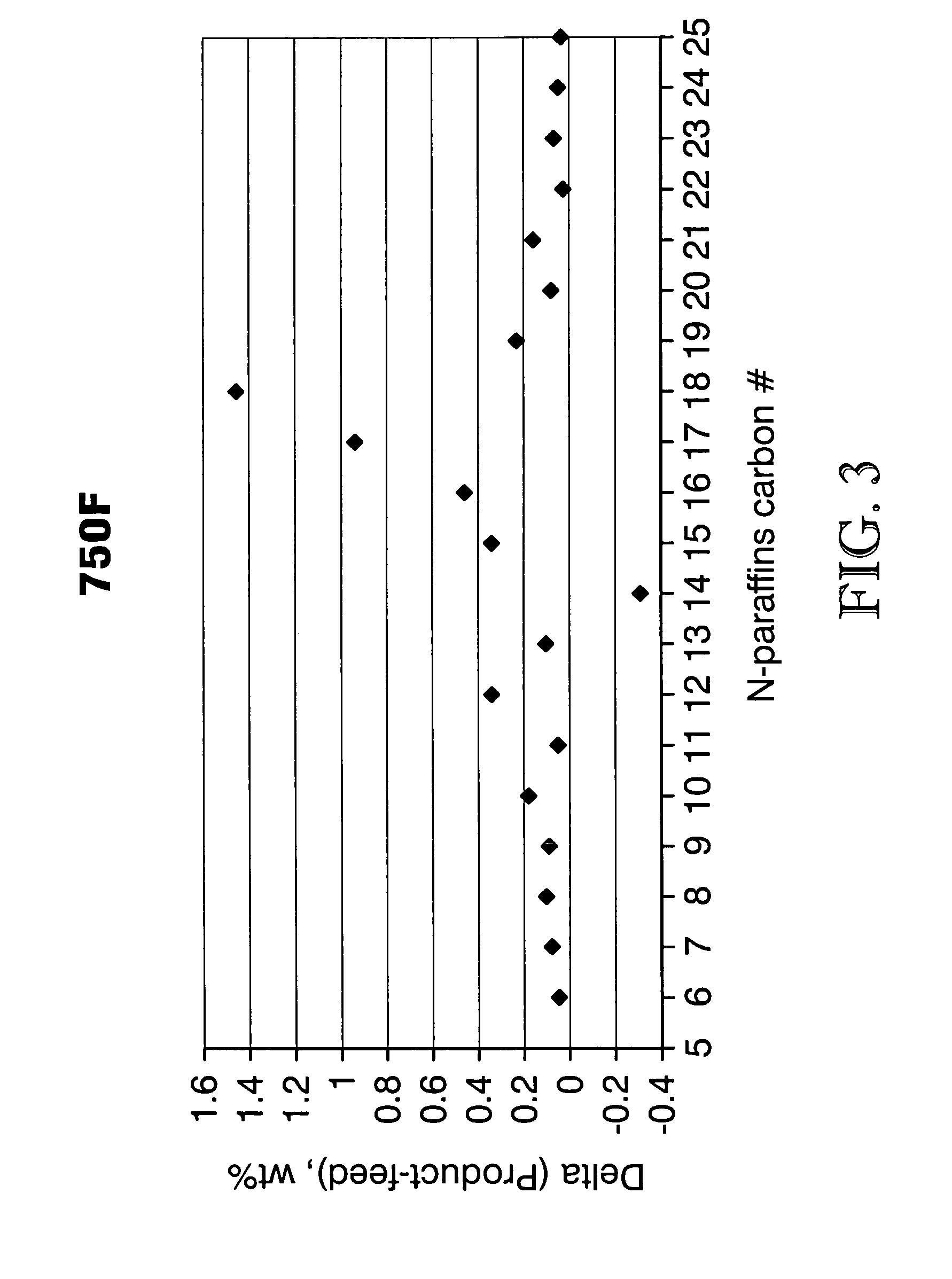
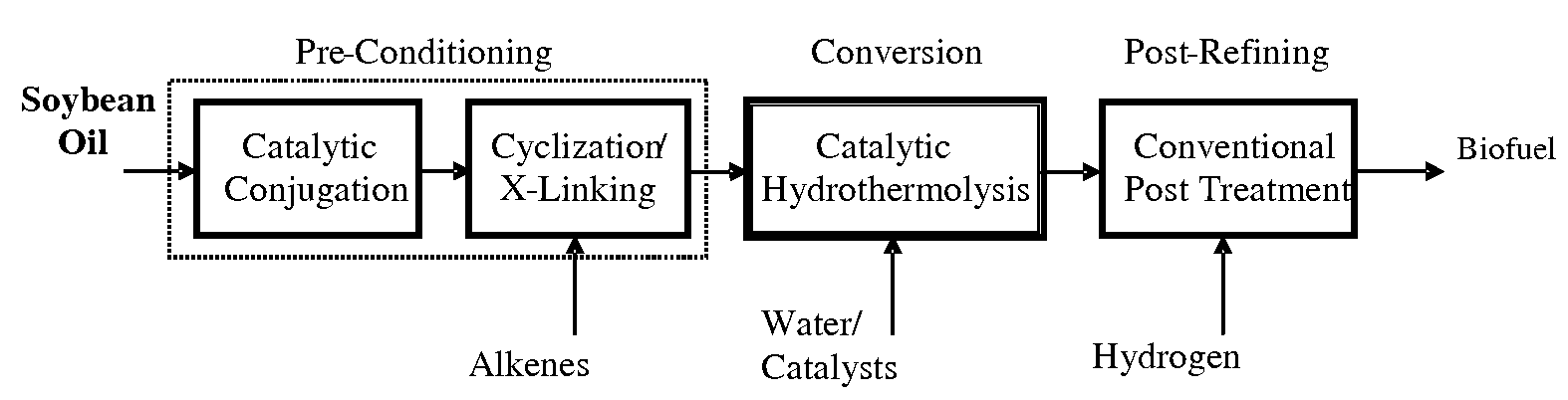
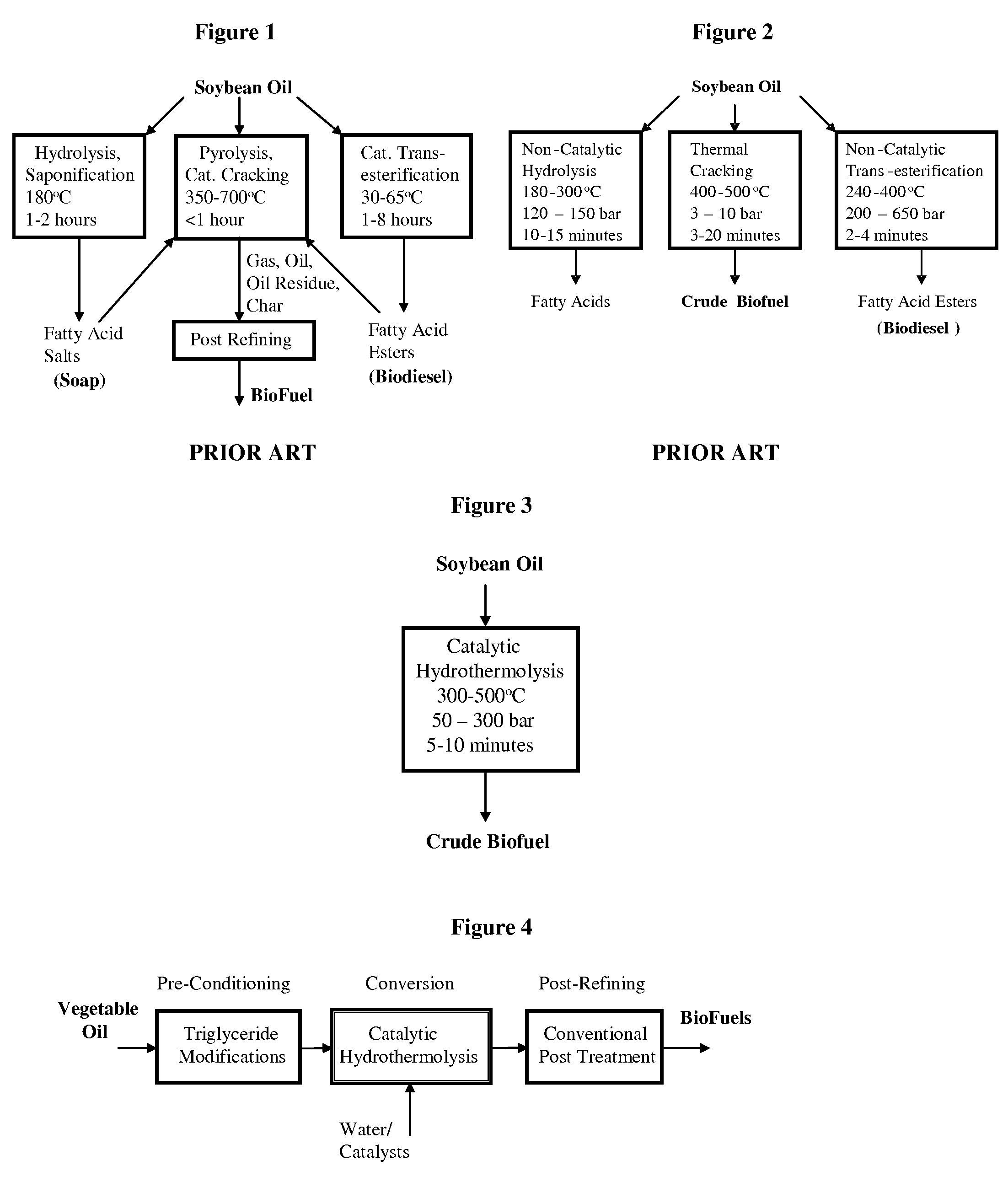
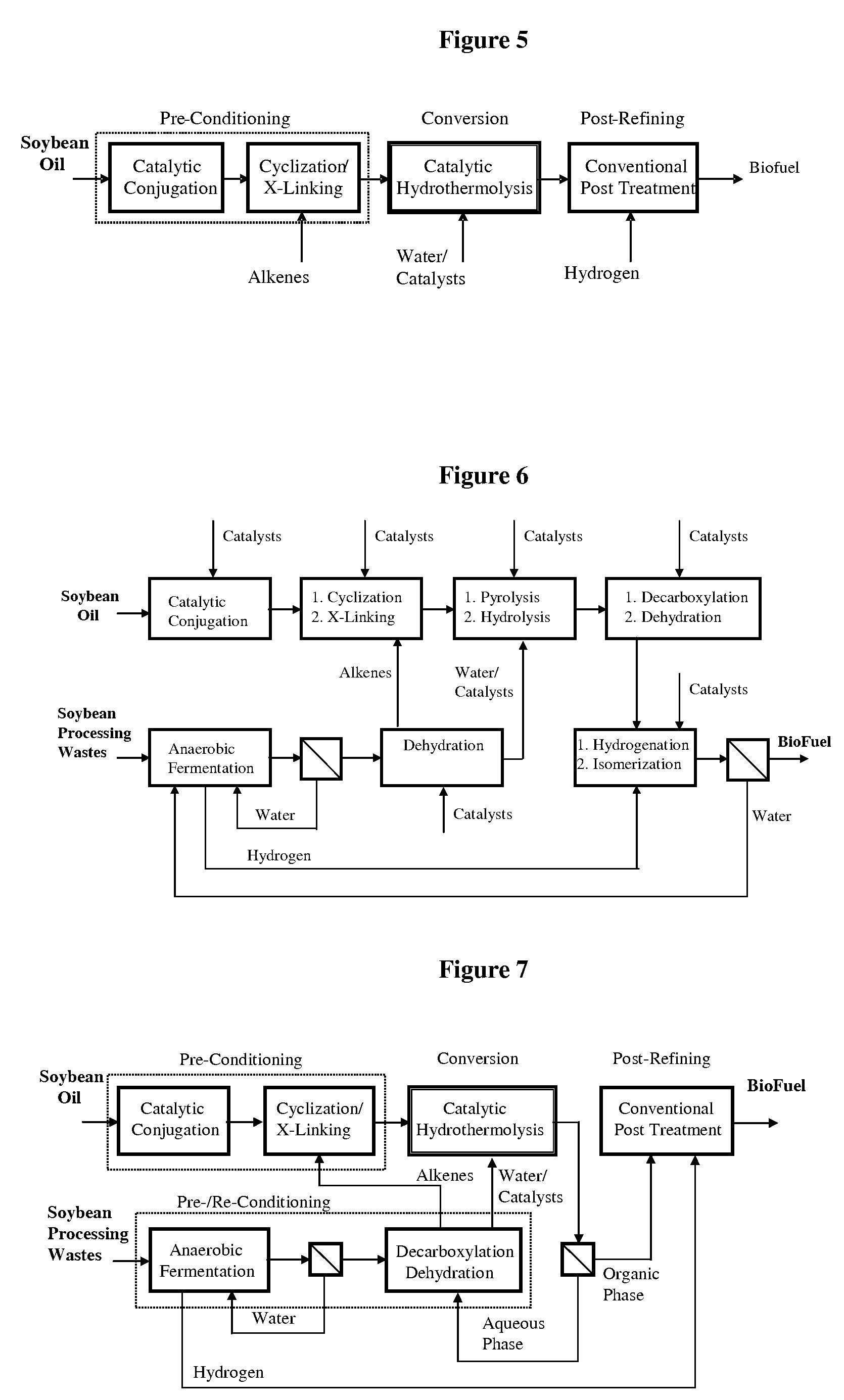
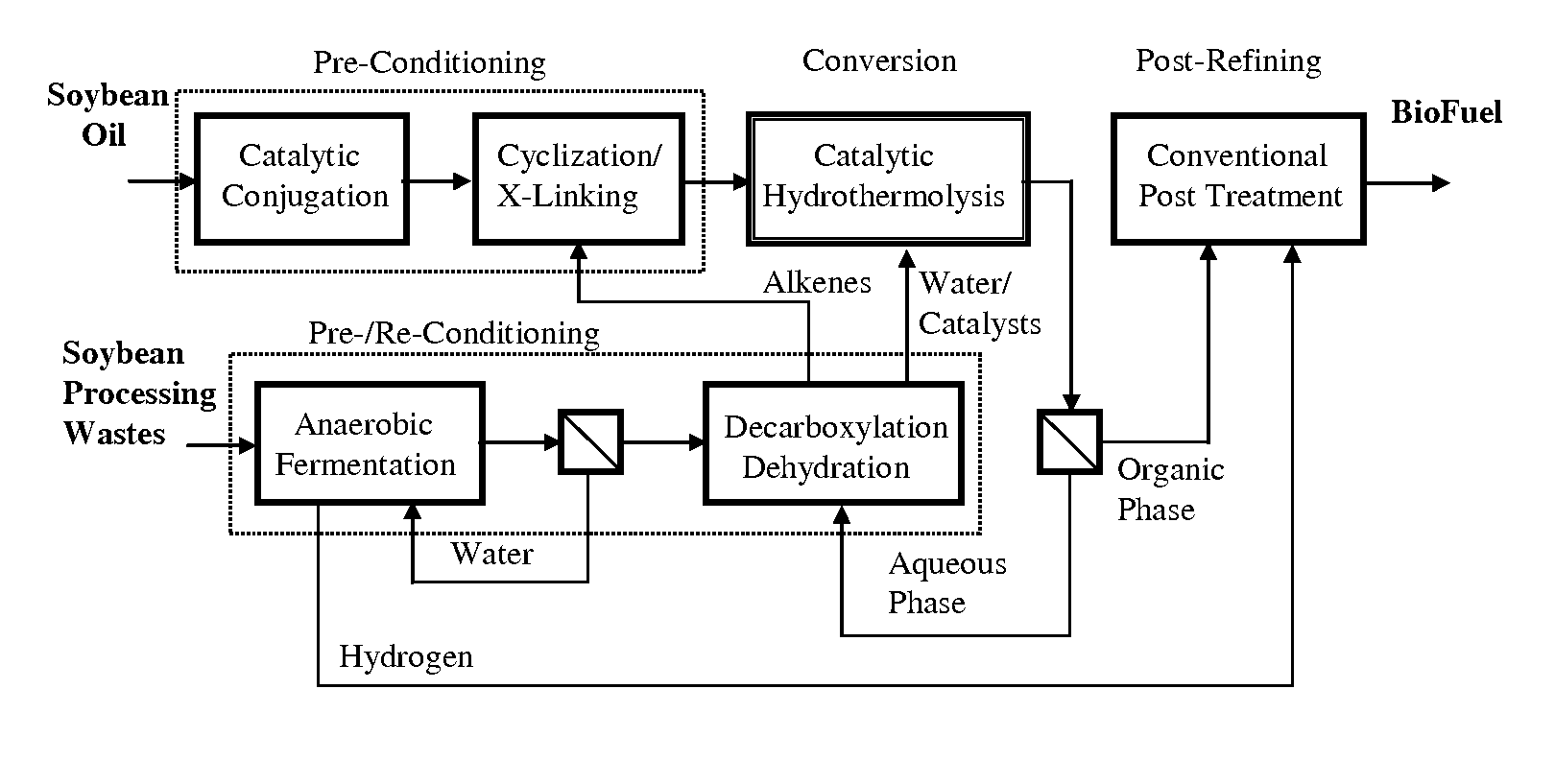
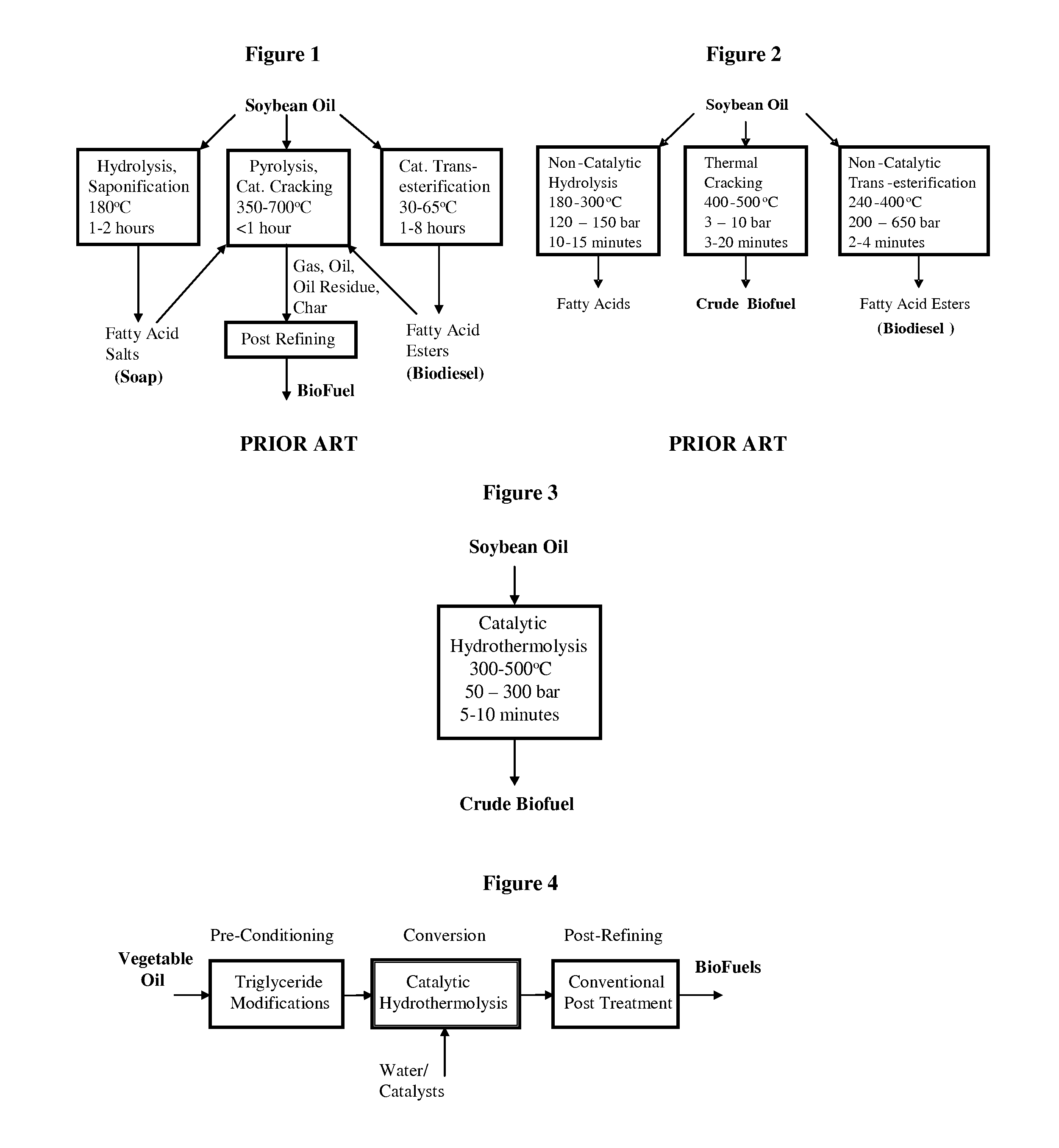
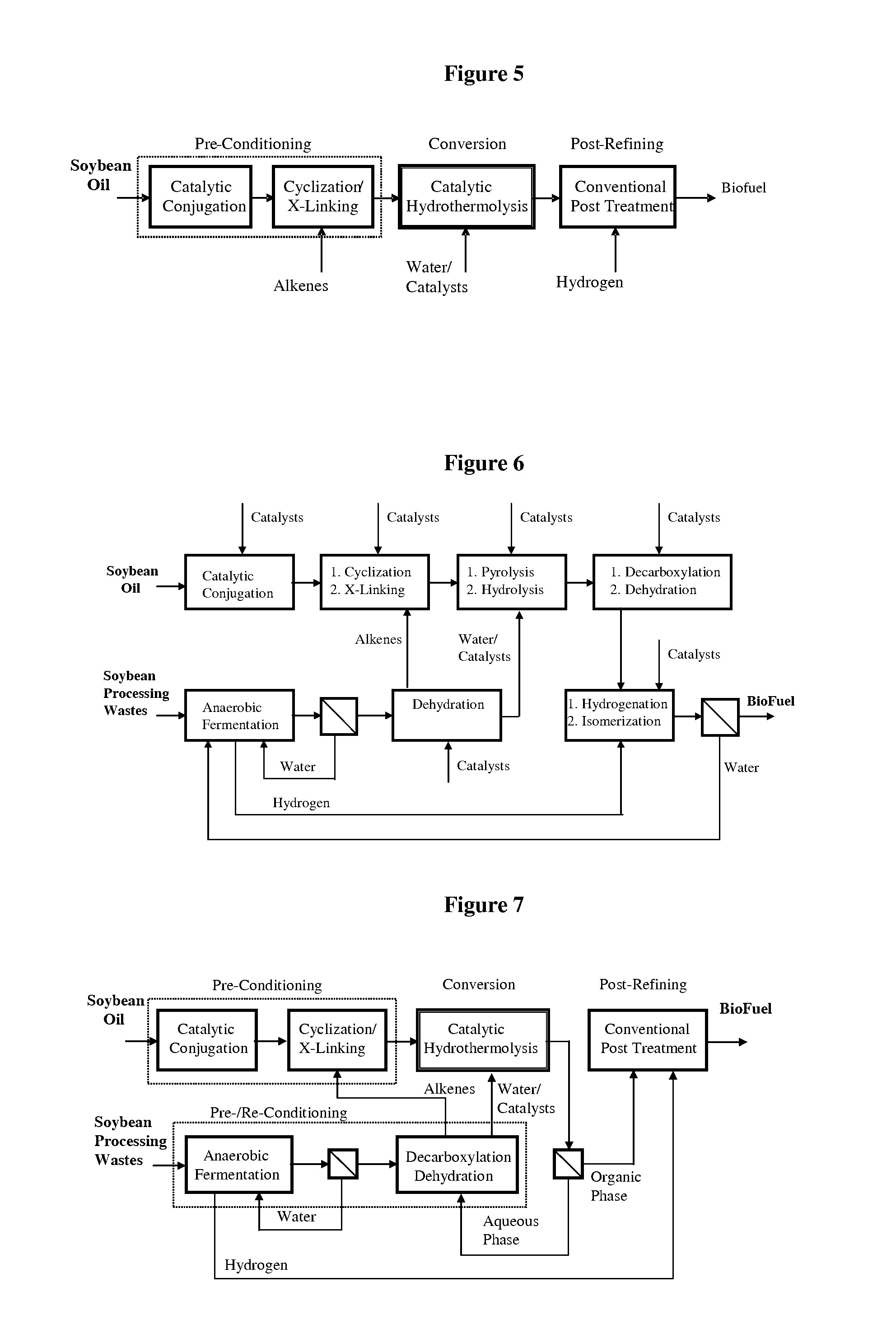
ActiveUS7691159B2Improve chemical and physical and combustion qualityImprove thermal stabilityFatty acid chemical modificationOrganic compound preparationCross-linkIsomerization
A triglyceride-to-fuel conversion process including the steps of (a) preconditioning unsaturated triglycerides by catalytic conjugation, cyclization, and cross-link steps; (b) contacting the modified triglycerides with hot-compressed water containing a catalyst, wherein cracking, hydrolysis, decarboxylation, dehydration, aromatization, or isomerization, or any combination thereof, of the modified triglycerides produce a crude hydrocarbon oil and an aqueous phase containing glycerol and lower molecular weight molecules, and (c) refining the crude hydrocarbon oil to produce various grades of biofuels. A triglyceride-to-fuel conversion process further including the steps of (a) carrying out anaerobic fermentation and decarboxylation / dehydration, wherein the anaerobic fermentation produces hydrogen, volatile acids, and alcohols from fermentable feedstocks, and the decarboxylation / dehydration produces alkenes from the volatile acids and alcohols, respectively; (b) feeding the alkenes to the cyclization process; (c) feeding the hydrogen to the post refining process; and (d) recycling the aqueous phase containing glycerol to the decarboxylation / dehydration process. A biofuel composition including straight-chain, branched and cyclo paraffins, and aromatics. The paraffins are derived from conversion of triglycerides. The aromatics are derived from conversion of either triglycerides, petroleum, or coal.
Owner:APPLIED RES ASSOCS INC
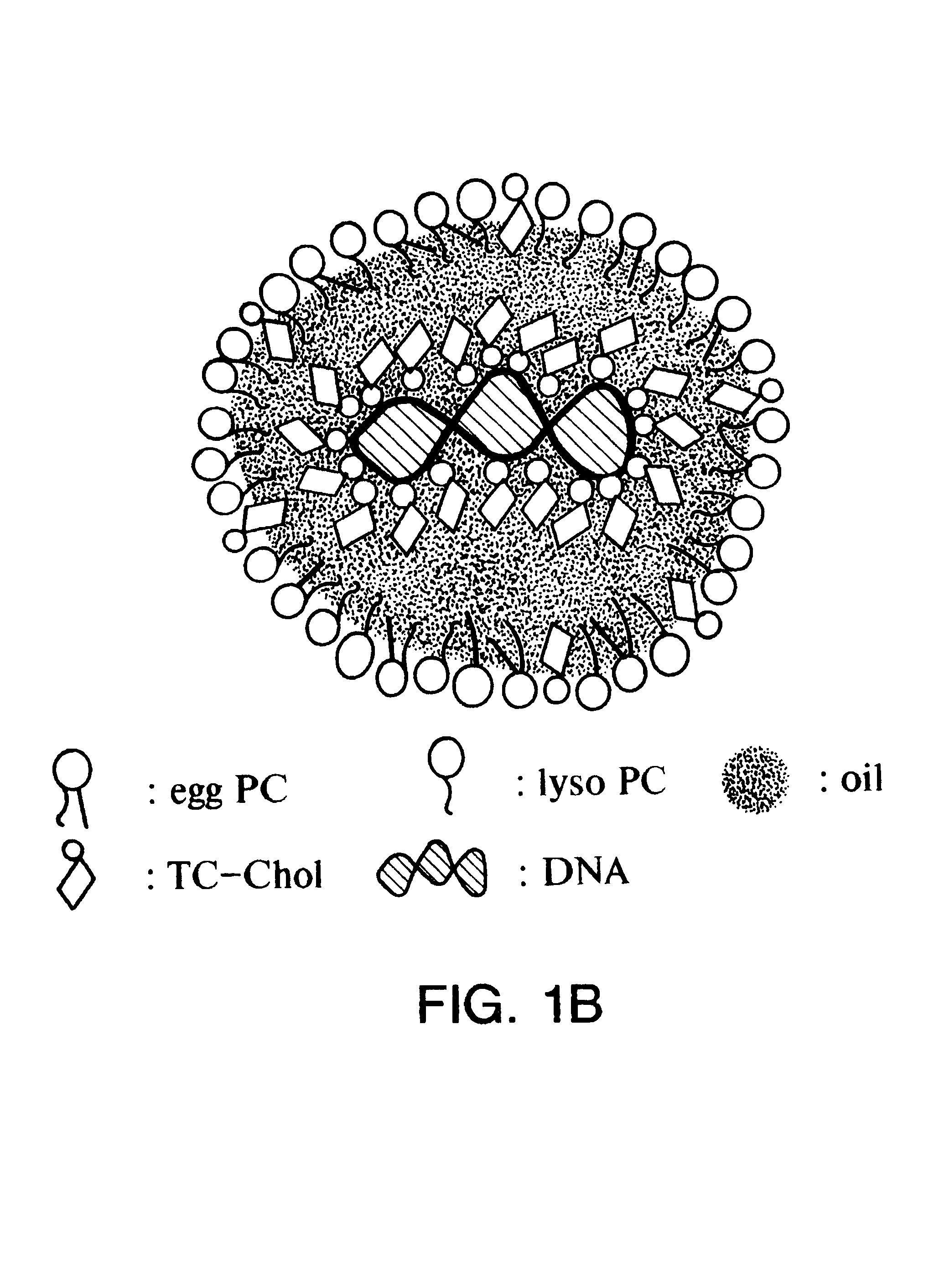
Emulsion formulations for hydrophilic active agents
This invention includes emulsion formulations comprising an aqueous carrier and the following components: triglyceride, cholesterol, phospholipid, at least one charged lipid, at least one hydrophilic biologically active molecule and, optionally, cholesteryl ester, and / or apoprotein; methods of preparing these emulsions; and the use of these emulsions for the delivery of hydrophilic biologically active molecules to cells.
Owner:PITTSBURGH UNIV OF
Production of high-cetane diesel fuel from low-quality biomass-derived feedstocks
ActiveUS20070068848A1Increase cetane numberBiofuelsLiquid hydrocarbon mixture productionTG - TriglyceridePre treatment
A method is taught for producing diesel fuels of high cetane value from a triglyceride feedstock, comprising pretreating the triglyceride feedstock by thermal cracking to partially convert the triglycerides and produce a middle distillates stream, and catalytically hydrotreating the middle distillate fraction to produce high cetane value diesel fuels. A biomass-derived diesel fuel is also taught having sulphur content below 10 ppm, a cetane-value of at least 70, a cloud point below 0° C. and a pour point of less than −4° C. A blended diesel fuel is also taught comprising 5 to 20 vol. % of the biomass-derived diesel fuel of the present invention and 80 to 95 vol. % of a petroleum diesel, based on total volume of the blended diesel fuel.
Owner:HER MAJESTY THE QUEEN & RIGHT OF CANADA REPRESENTED BY THE MIN OF NATURAL RESOURCES
Environmentally benign anti-icing or deicing fluids employing triglyceride processing by-products
InactiveUS6890451B2Improve ice resistanceGreat tractionOther chemical processesTG - TriglycerideGlycerol
Deicing compositions comprised of glycerol-containing by-products of triglyceride processing processes are disclosed.
Owner:MLI ASSOC
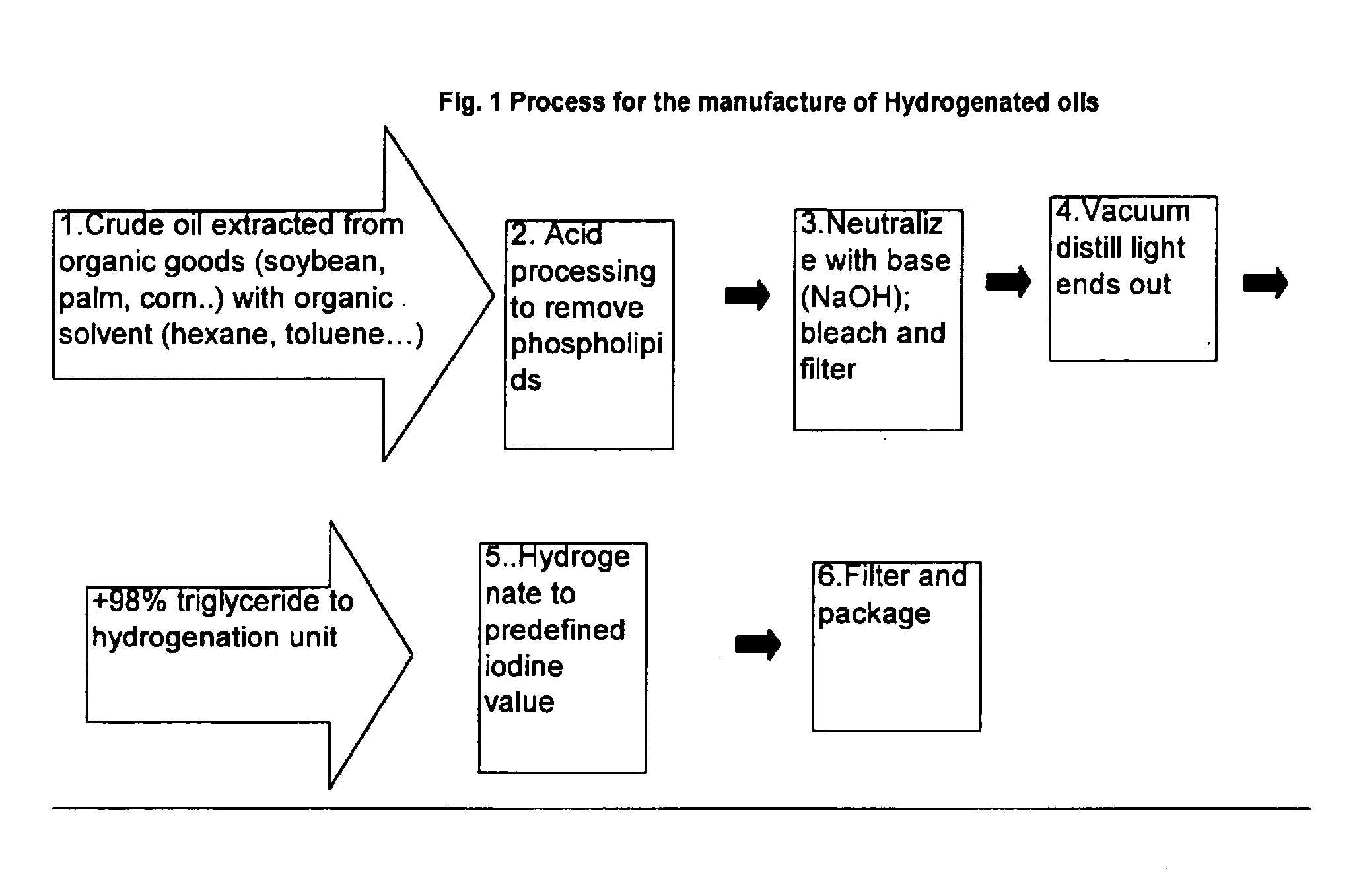
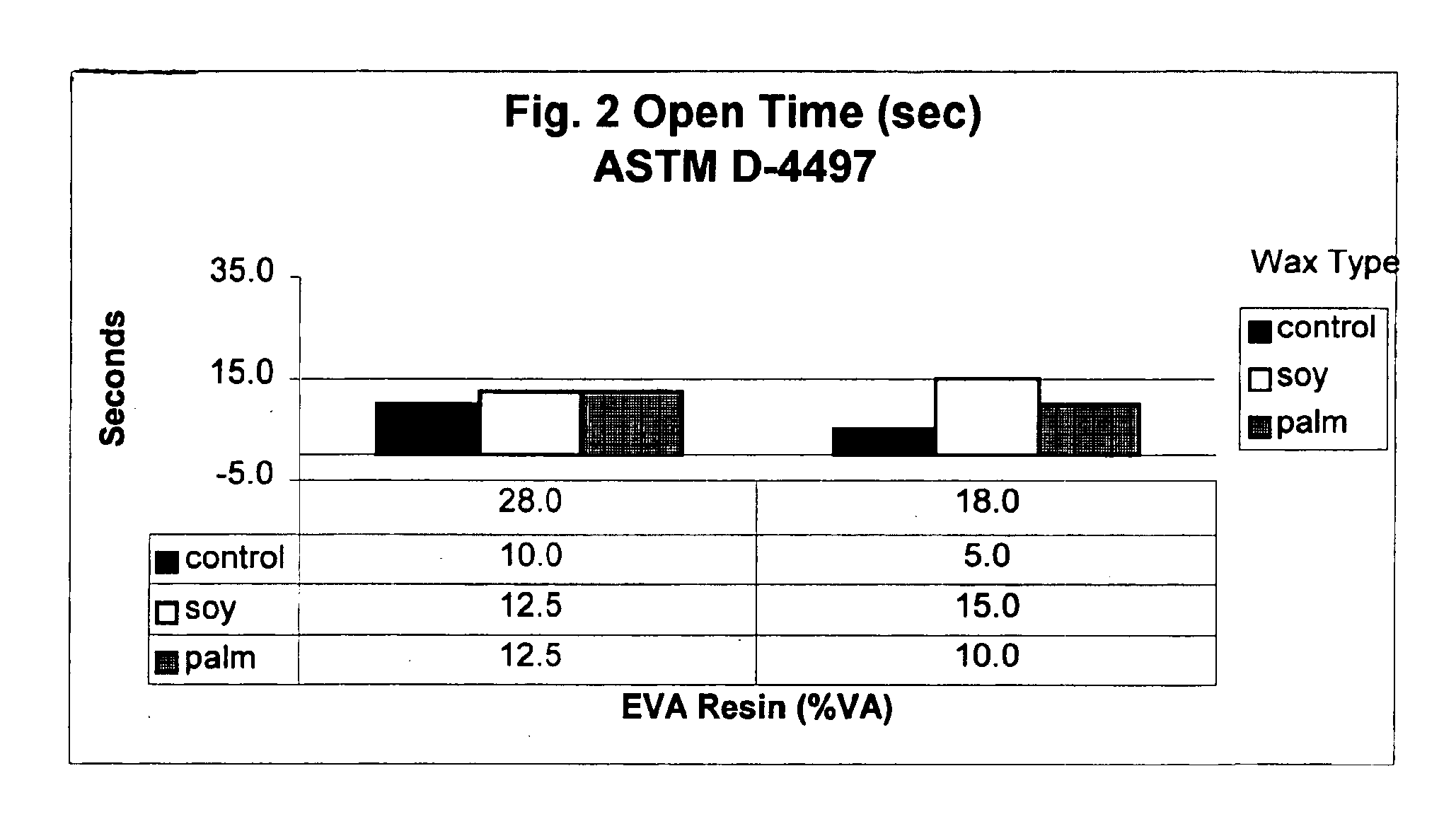
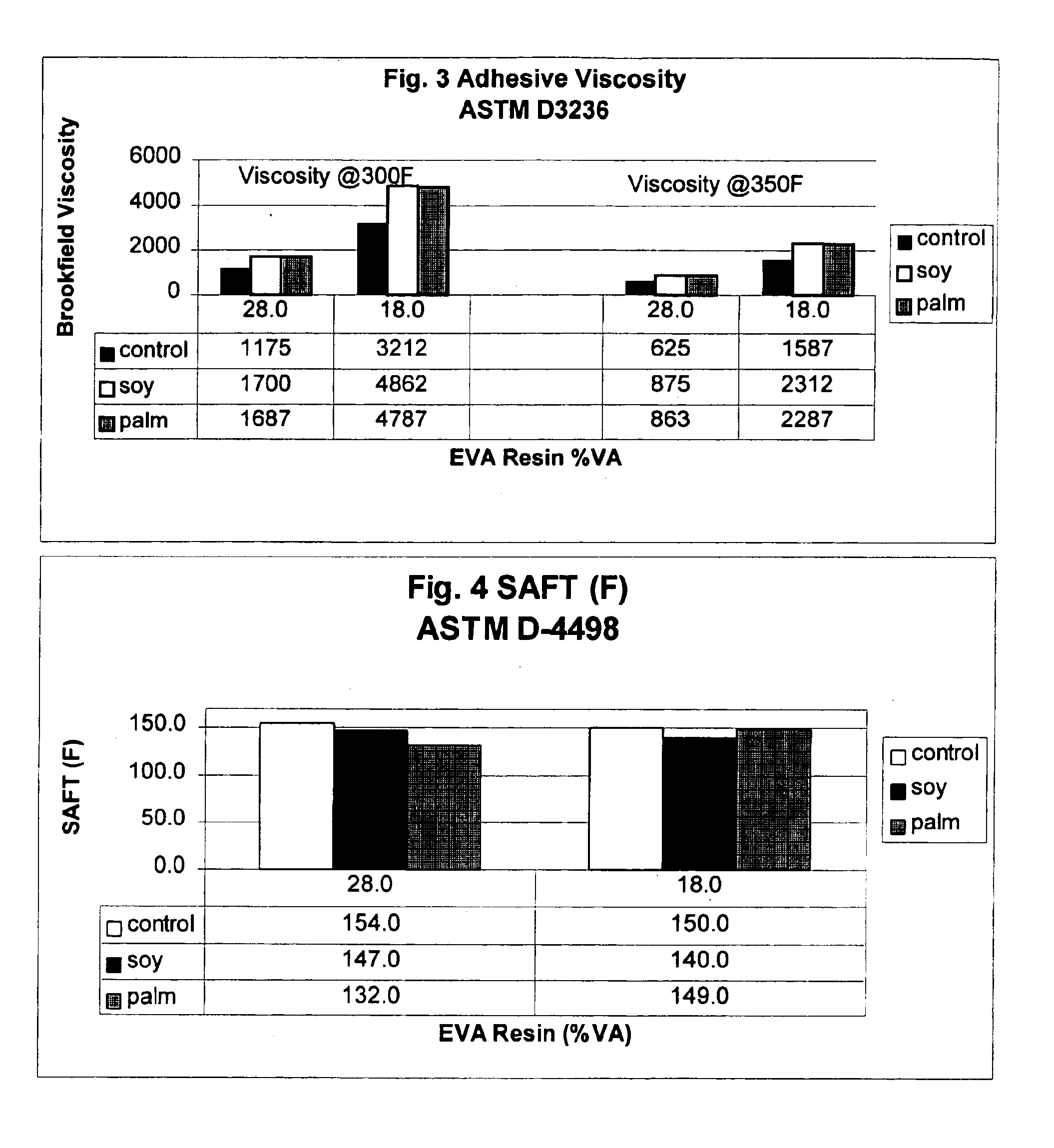
Wax for hot melt adhesive applications
InactiveUS6890982B2Produced economicallyProperty securityNon-macromolecular adhesive additivesFilm/foil adhesivesTG - TriglycerideStearic acid
Waxes prepared from hydrogenated plant oils, such as palm and soybean, are used as substitutes for petroleum derived waxes in hot-melt adhesive compositions. Unlike petroleum-derived or synthetic waxes, adhesive compositions comprising these waxes, which are obtained from naturally derived, renewable resources, achieve adhesion performance similar to conventional adhesives containing petroleum-derived waxes. The inventive waxes have a low iodine value (between 2-5), and melting points between approximately 120-165 degrees F. (Mettler Drop Point). These waxes comprise a triglyceride whose fatty acids are predominantly stearic acid (C18). The naturally derived waxes are used as an alternative to petroleum and synthetically derived waxes in the manufacture of adhesives used to bond paper, wood, glass, plastic and metal in a variety of manufacturing operations.
Owner:MARCUS OIL & CHEM
Water stable fibers and articles comprising starch, and methods of making the same
Water stable fibers and articles made therefrom are formed from a thermoplastic composition comprising destructured starch, polyhydric alcohol, triglyceride, and optionally acid. Processes for making water stable compositions may comprise melt extruding a mixture of destructured starch, polyhydric alcohol, triglyceride, and optionally acid, to form an extrudate, and heating the mixture, extrudate, or both to provide a water stable article.
Owner:THE PROCTER & GAMBLE COMPANY
Absorbable implants and methods for their use in hemostasis and in the treatment of osseous defects
ActiveUS20050065214A1Stimulate bone healing processLower potentialBiocidePowder deliveryBarium saltTG - Triglyceride
Two (or more), -component, body-implantable, absorbable, biocompatible, putty, and non-putty hemostatic tamponades for use in surgery. Component 1 is a finely powdered bulking material, preferably less than 50 microns, e.g. the calcium, magnesium, aluminum, or barium salts of saturated or unsaturated carboxylic acids containing about 6 to 22 carbon atoms, hydroxyapatite, DBM, polyglycolide, polylactide, poldioxinones, polycaprolactones, absorbable glasses, gelatin, collagens, mono, and polysaccharides starches. Component 2, a dispersing vehicle, may be esters of C8-C18 monohydric alcohols with C2-C6 aliphatic monocarboxylic acids; C2-C18 monohydric alcohols with polycarboxylic acids; C8-C30 monohydric alcohols; tocopherol and esters thereof with C2-C10 aliphatic monocarboxylic acids or polycarboxylic acids; absorbable 10-14C hydrocarbons; free carboxylic acids such as oleic, capric, and lauric; dialkyl ethers and ketones; alkyl aryl ethers and ketones, polyhydroxy compounds and esters and ethers thereof; (ethylene oxide / propylene oxide copolymers), oils e.g. olive oil, castor oil and triglycerides.
Owner:ABYRX
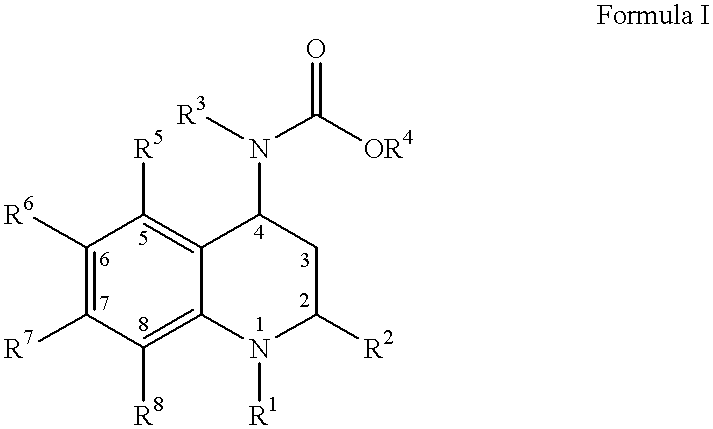
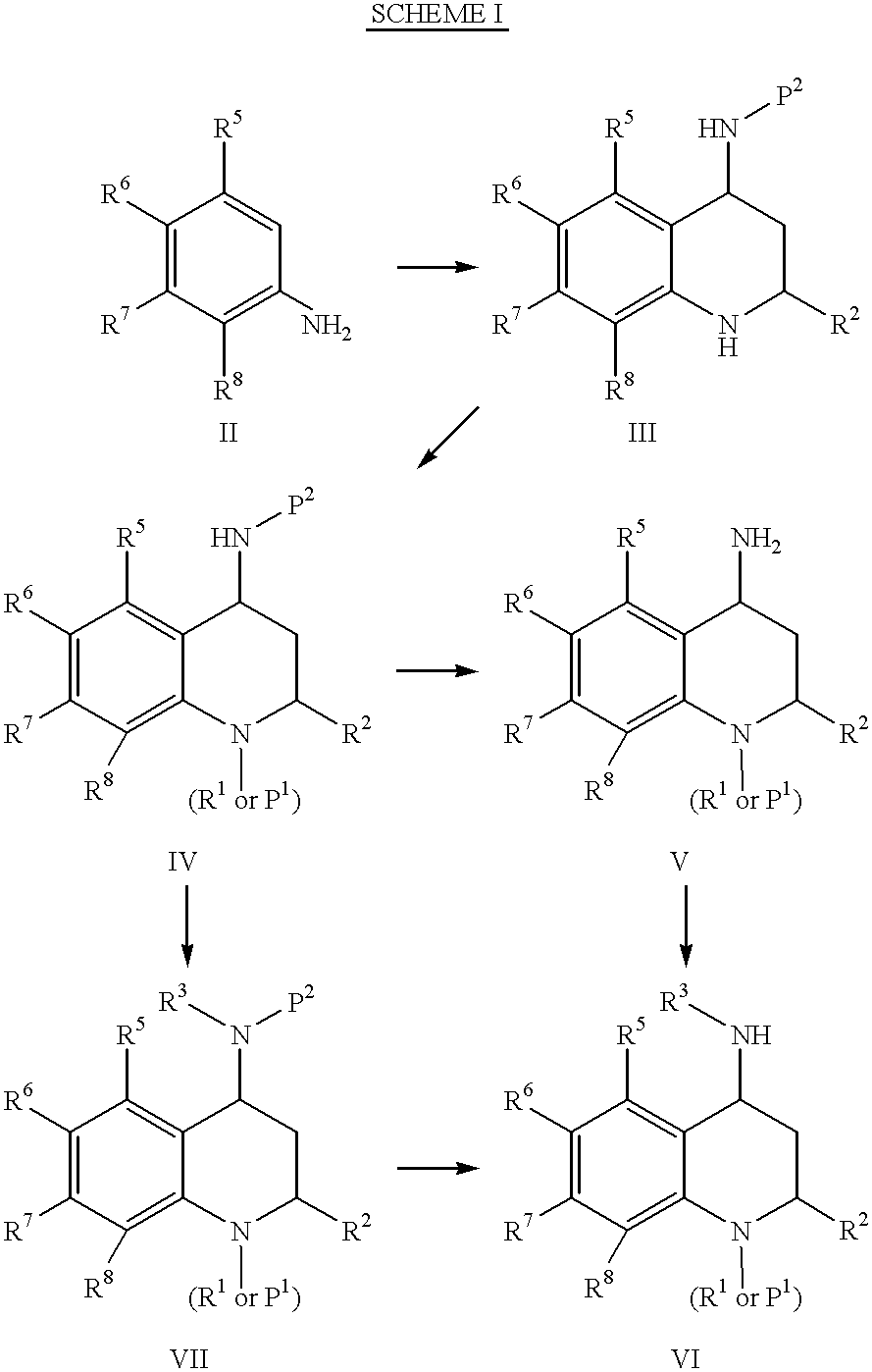
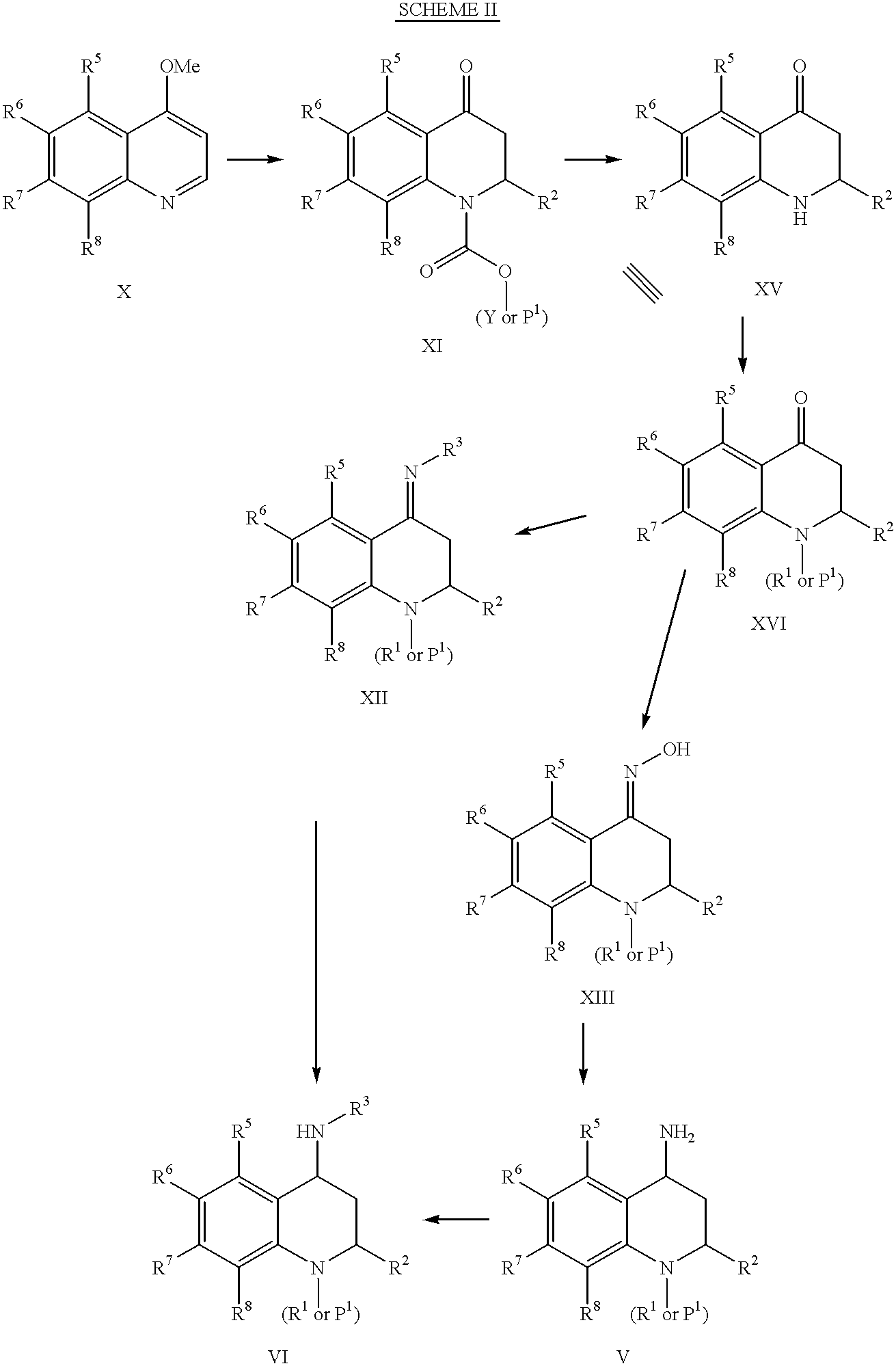
4-carboxyamino-2-substituted-1,2,3,4-tetrahydroquinolines
Owner:PFIZER INC
Absorbable implants and methods for their use in hemostasis and in the treatment of osseous defects
ActiveUS20060013857A1Lower potentialMinimally inhibit osteogenesis and subsequent bone healingSurgical adhesivesSkeletal disorderBarium saltApatite
Two (or more), -component, body-implantable, absorbable, biocompatible, putty, and non-putty hemostatic tamponades for use in surgery. Component 1 is a finely powdered bulking material, preferably less than 50 microns, e.g. the calcium, magnesium, aluminum, or barium salts of saturated or unsaturated carboxylic acids containing about 6 to 22 carbon atoms, hydroxyapatite, DBM, polyglycolide, polylactide, poldioxinones, polycaprolactones, absorbable glasses, gelatin, collagens, mono, and polysaccharides starches. Component 2, a dispersing vehicle, may be esters unsubstituted and N-substituted pyrrolidones of C8-C18 monohydric alcohols with C2-C6 aliphatic monocarboxylic acids; C2-C18 monohydric alcohols with polycarboxylic acids; C8-C30 monohydric alcohols; tocopherol and esters thereof with C2-C10 aliphatic monocarboxylic acids or polycarboxylic acids; absorbable 10-14C hydrocarbons; free carboxylic acids such as oleic, capric, and lauric; dialkyl ethers and ketones; alkyl aryl ethers and ketones, polyhydroxy compounds and esters and ethers thereof; (ethylene oxide / propylene oxide copolymers), oils e.g. olive oil, castor oil and triglycerides.
Owner:ABYRX
Oral capsule formulation with increased physical stability
InactiveUS7011846B2Improve capsule stabilityImprove stabilityPharmaceutical non-active ingredientsCapsule deliveryMonoglycerideOral medication
A formulation for a stabilized capsule for oral administration of a hydrophobic pharmaceutically active agent; comprising a non-aqueous solubilizer selected from 2-pyrrolidone, N-alkylpyrrolidones and combinations thereof; and a capsule stabilizing agent selected from mono-, di- and triglycerides, mono- and di-fatty esters of polyethylene glycol, fatty acids and combinations thereof wherein capsule integrity is maintained for at least 24 hours is disclosed.
Owner:SUPERNUS PHARM INC
Low-oil pharmaceutical emulsion compositions comprising progestogen
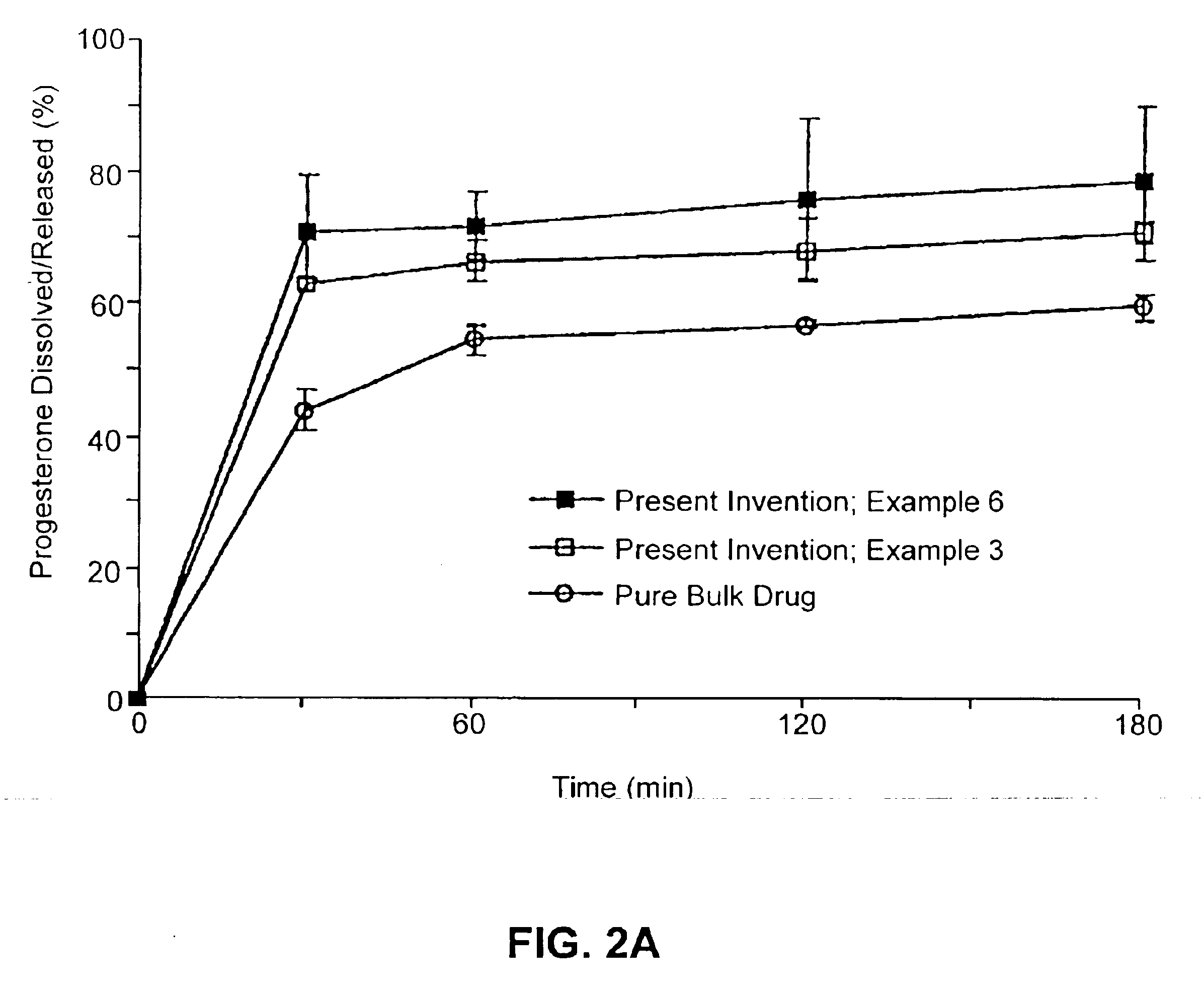
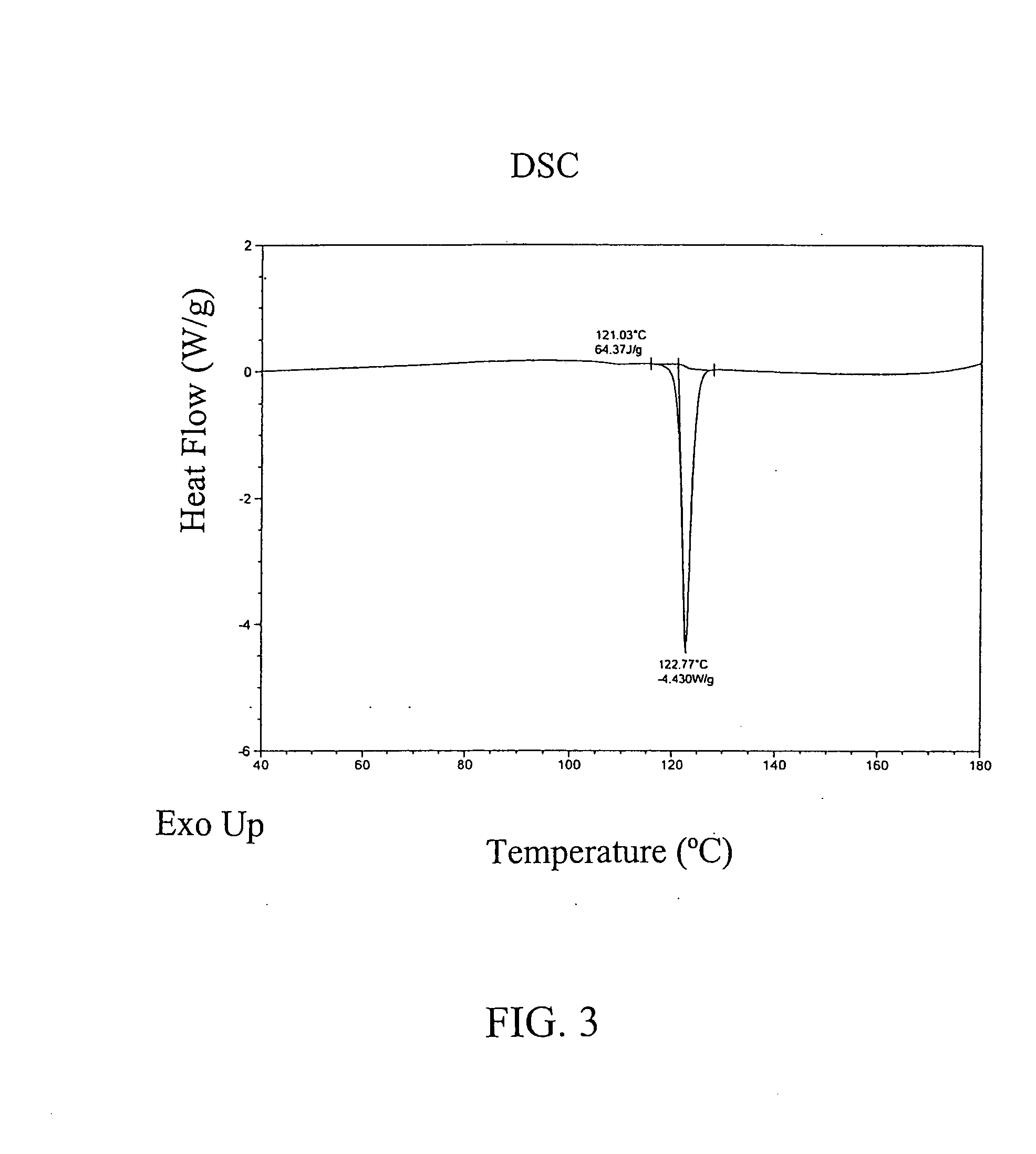
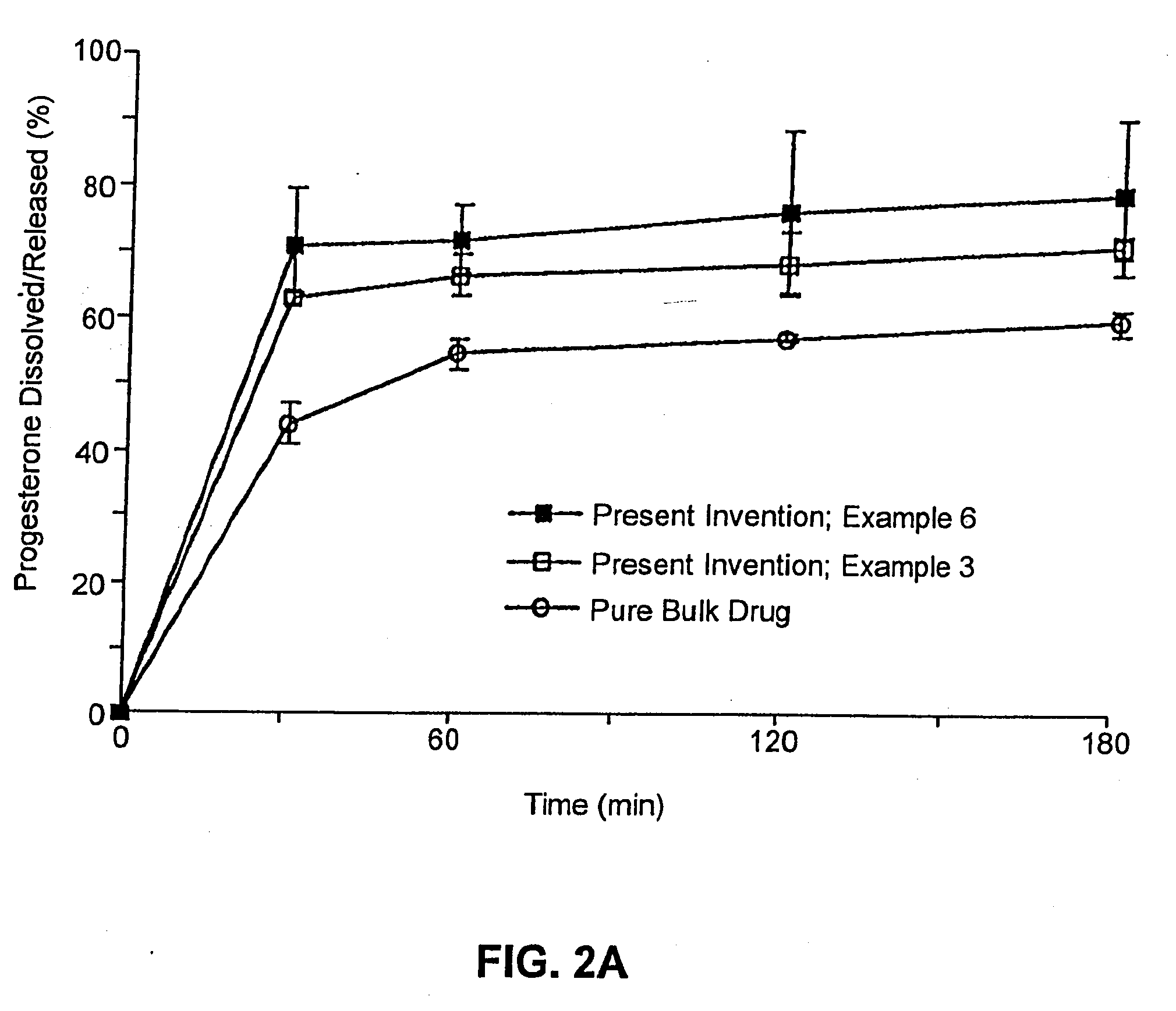
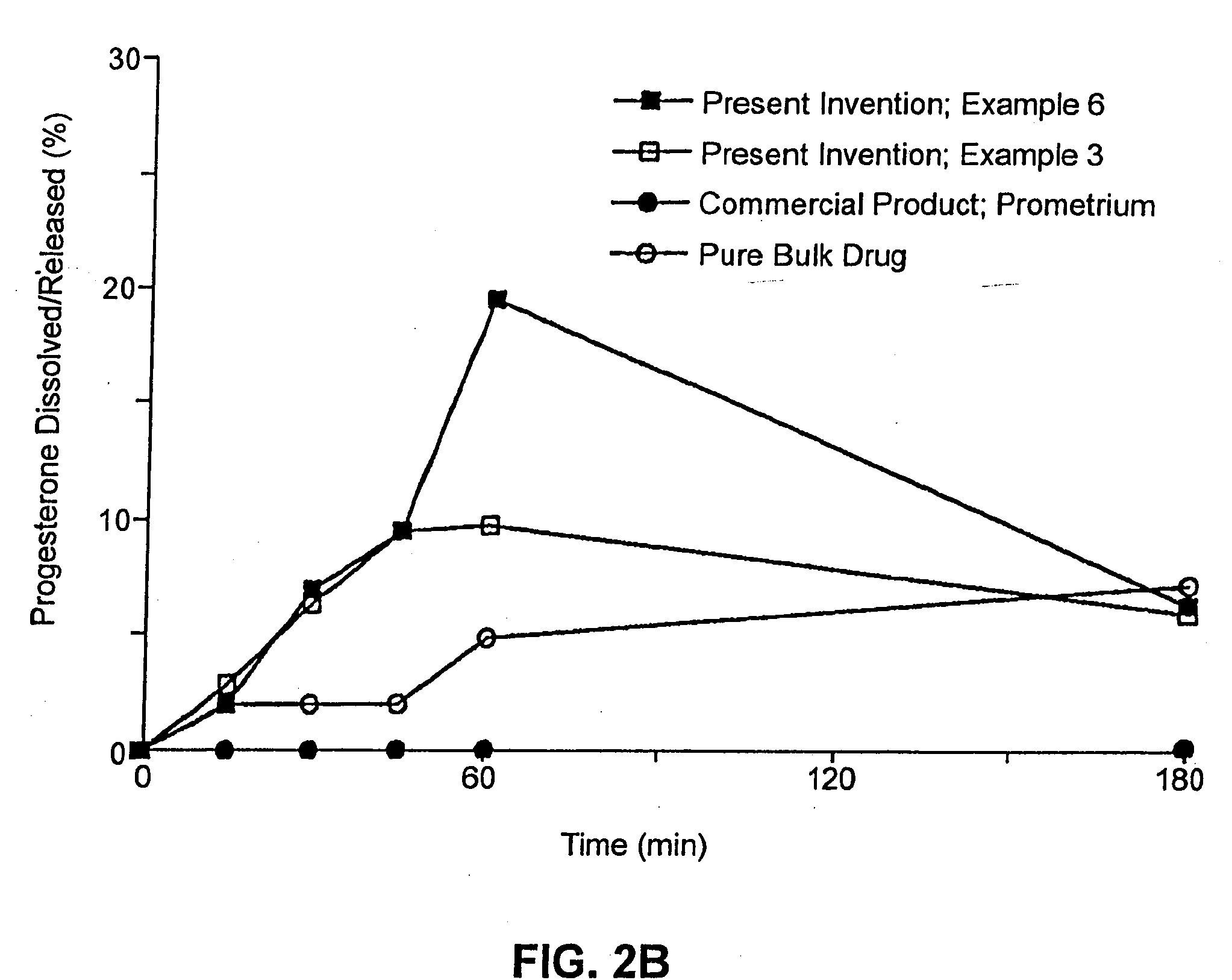
ActiveUS20110262494A1High progesterone solubilityElevation in histamine releaseOrganic active ingredientsNervous disorderIUD with progestogenTG - Triglyceride
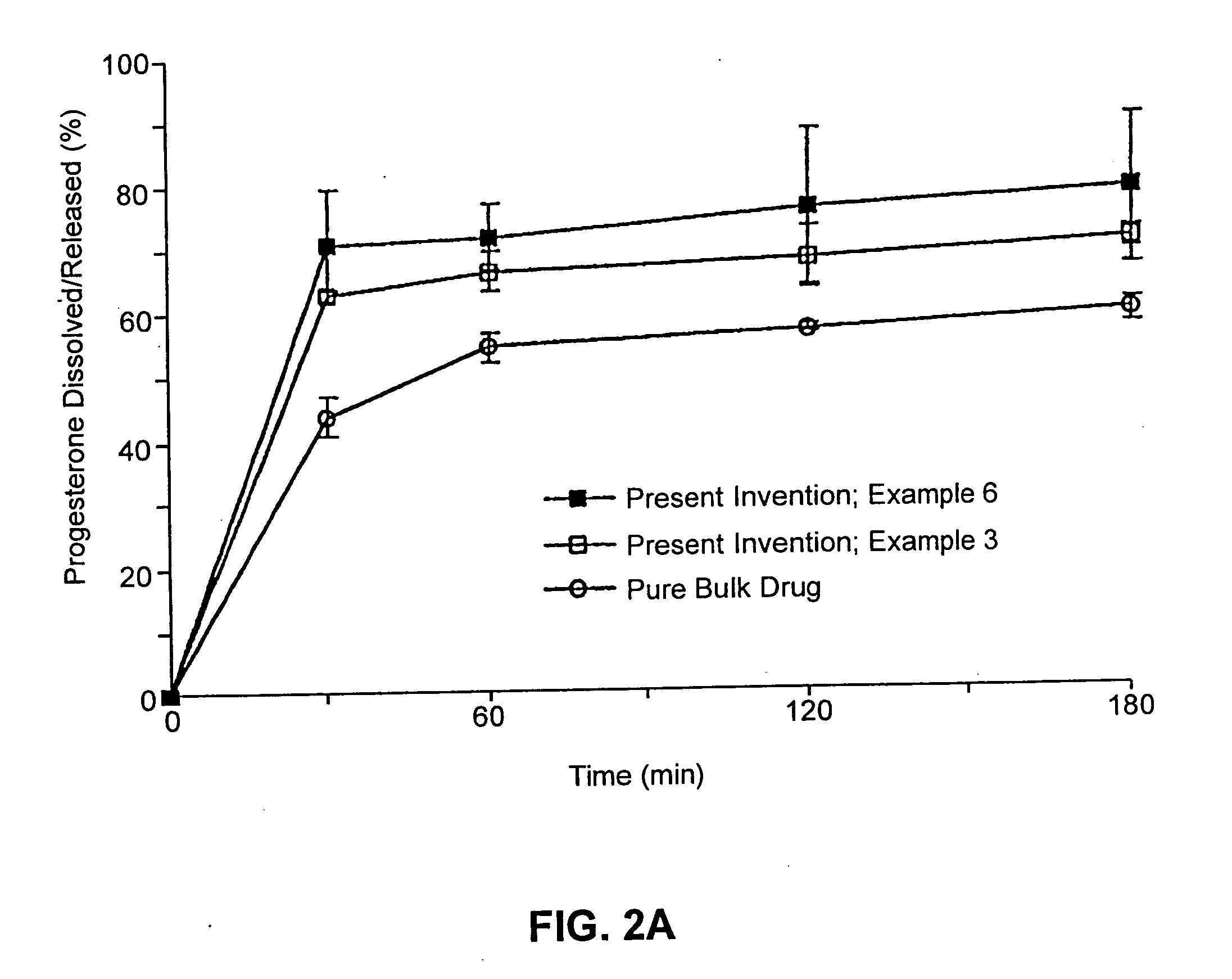
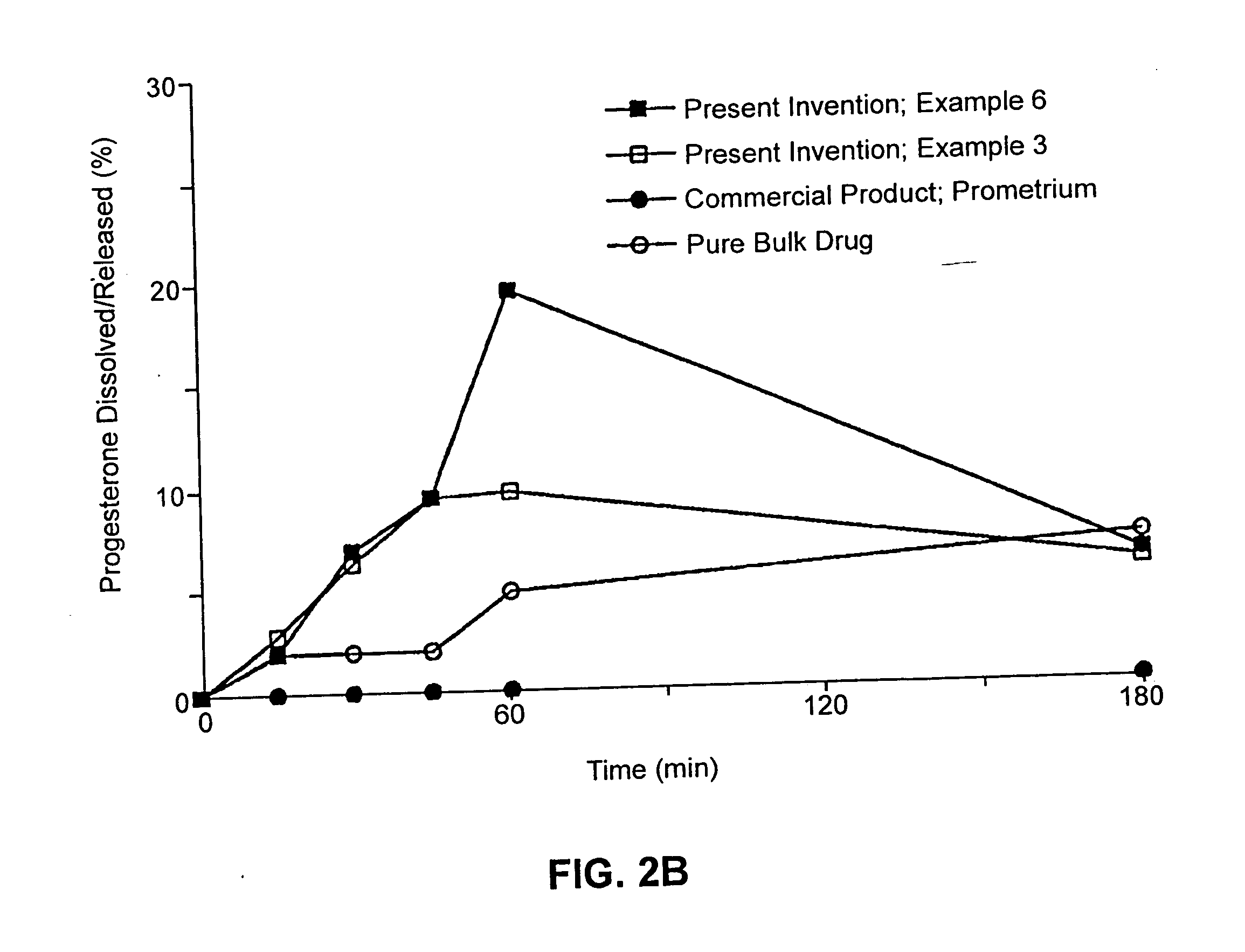
Described are sterile, ready-to-use, pharmaceutical oil-in-water emulsion compositions for parenteral administration comprising:0.015 to 0.5% wt / vol progesterone;0.5 to 10% wt / vol oil, wherein the oil comprises at least 85% wt. / wt. triglyceride;0.0425 to 4.1% wt / vol phospholipid;80-99.4% wt / vol aqueous medium;wherein the composition has an osmolality in the range of 200-1000 mOsm / kg.Also described are methods of making such compositions and method of using such compositions in therapeutic or prophylactic treatment, such as treatments comprising intravenous administration of the pharmaceutical composition.
Owner:BESINS HEALTHCARE LUXEMBOURG (LU)
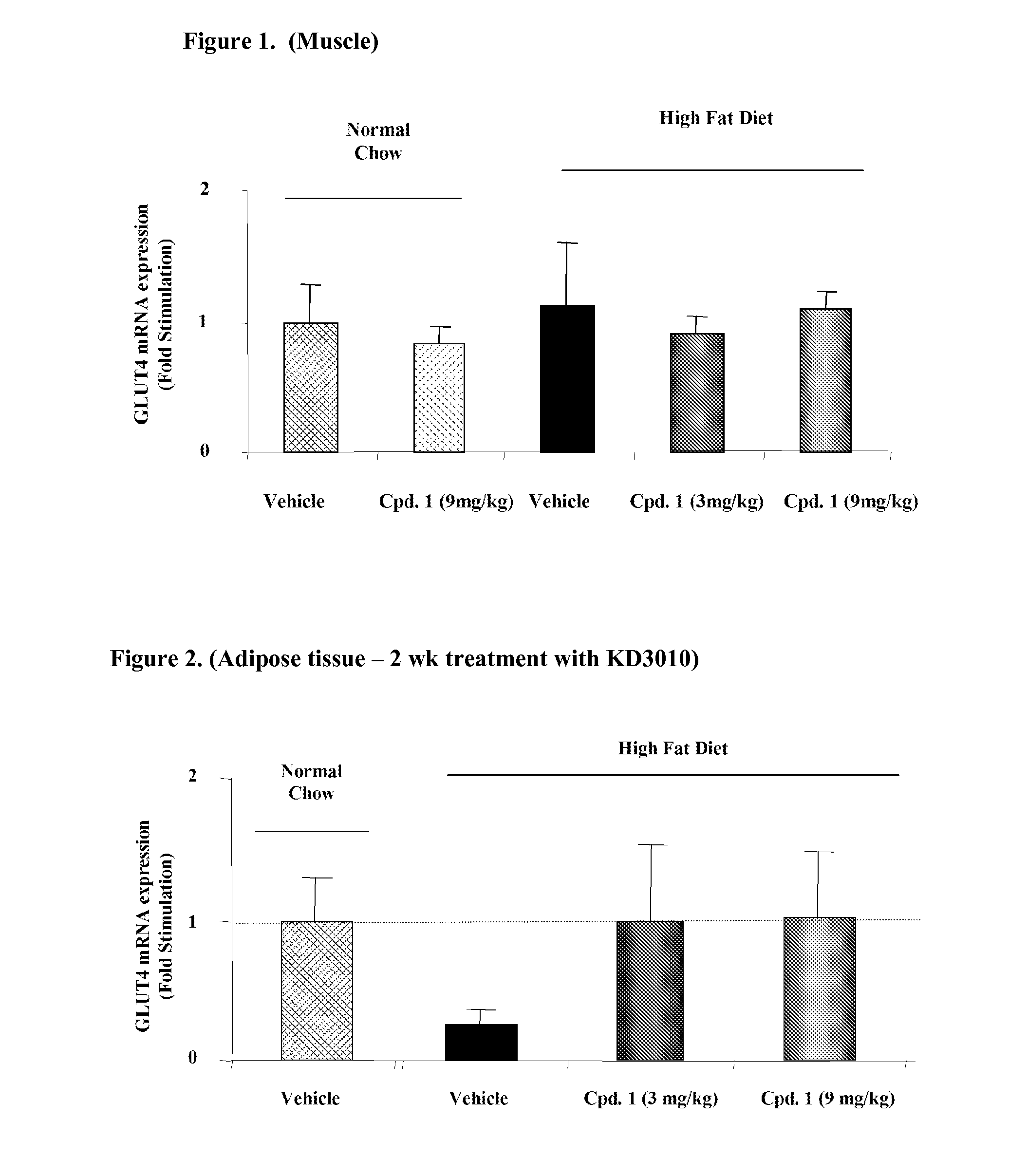
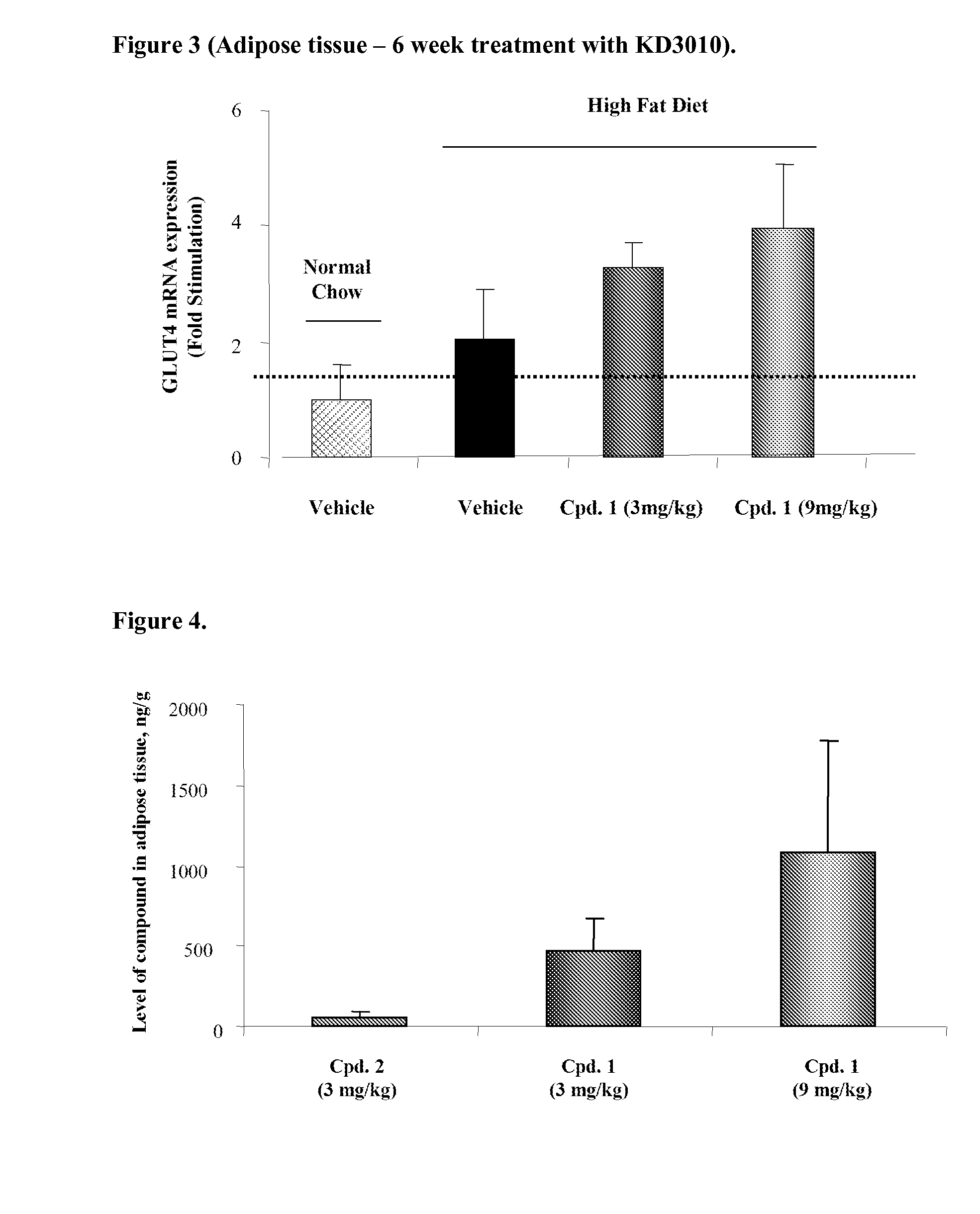
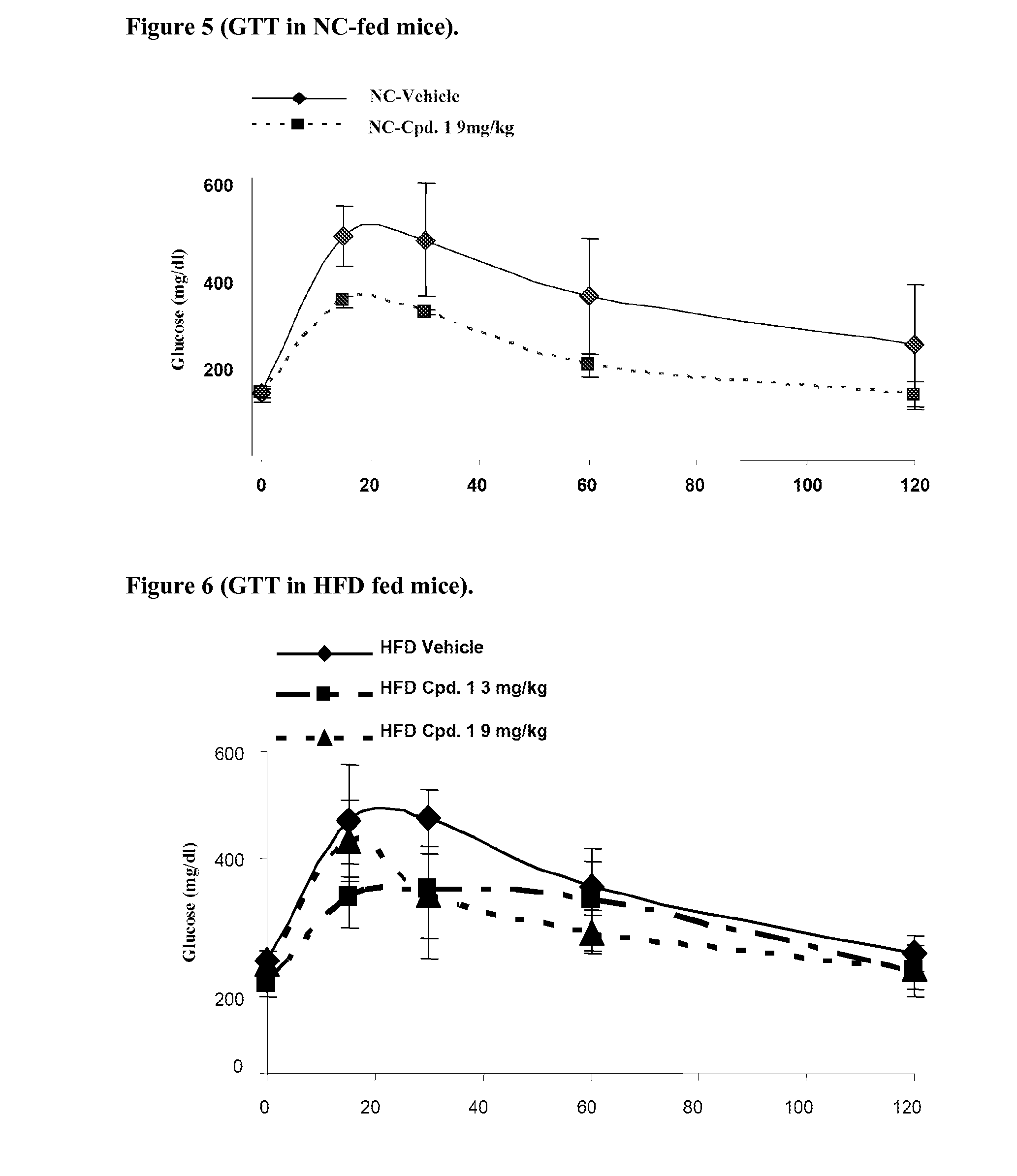
Methods for the upregulation of glut4 via modulation of PPAR delta in adipose tissue and for the treatment of disease
The present invention is directed to novel compositions and their application as pharmaceuticals for the treatment of disease. Methods of upregulation of GLUT4 via activation of peroxisome proliferator activated receptor delta activity in the adipose tissue of a human or animal subject are also provided, for the treatment of conditions such as diabetes, obesity, insulin resistance, metabolic syndrome, and others in which a reduction in insulin resistance, an increase in glucose utilization, a reduction in visceral fat, a reduction in triglyceride (TG) levels, or an increase in levels of high-density lipoprotein (HDL), is beneficial.
Owner:KALYPSYS INC
Method of Converting Triglycerides to Biofuels
ActiveUS20080071125A1Improve chemical and physical and combustion qualityImprove thermal stabilityFatty acid chemical modificationBiofuelsIsomerizationPtru catalyst
A triglyceride-to-fuel conversion process including the steps of (a) preconditioning unsaturated triglycerides by catalytic conjugation, cyclization, and cross-link steps; (b) contacting the modified triglycerides with hot-compressed water containing a catalyst, wherein cracking, hydrolysis, decarboxylation, dehydration, aromatization, or isomerization, or any combination thereof, of the modified triglycerides produce a crude hydrocarbon oil and an aqueous phase containing glycerol and lower molecular weight molecules, and (c) refining the crude hydrocarbon oil to produce various grades of biofuels. A triglyceride-to-fuel conversion process further including the steps of (a) carrying out anaerobic fermentation and decarboxylation / dehydration, wherein the anaerobic fermentation produces hydrogen, volatile acids, and alcohols from fermentable feedstocks, and the decarboxylation / dehydration produces alkenes from the volatile acids and alcohols, respectively; (b) feeding the alkenes to the cyclization process; (c) feeding the hydrogen to the post refining process; and (d) recycling the aqueous phase containing glycerol to the decarboxylation / dehydration process. A biofuel composition including straight-chain, branched and cyclo paraffins, and aromatics. The paraffins are derived from conversion of triglycerides. The aromatics are derived from conversion of either triglycerides, petroleum, or coal.
Owner:APPLIED RES ASSOCS INC
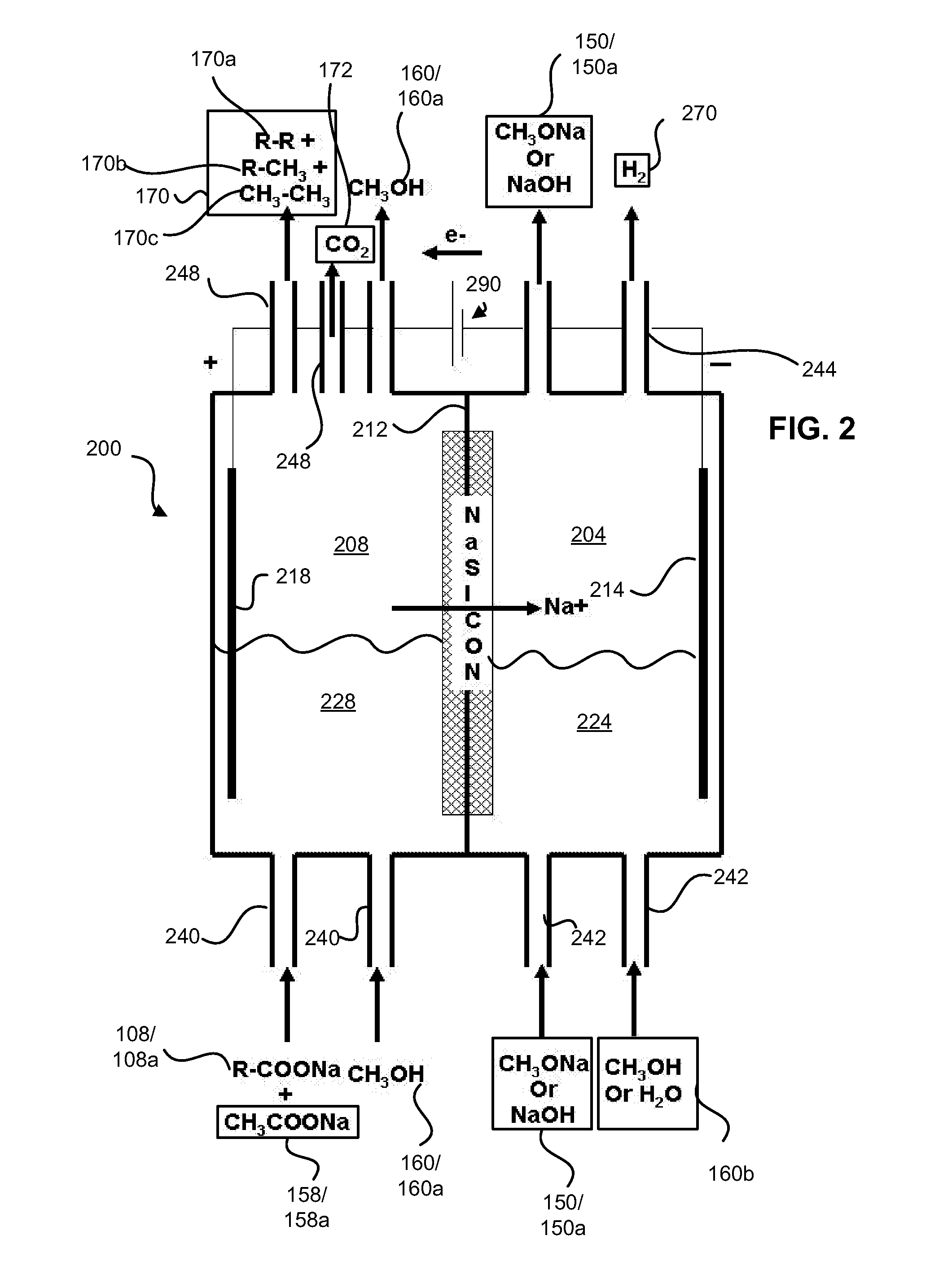
Decarboxylation cell for production of coupled radical products
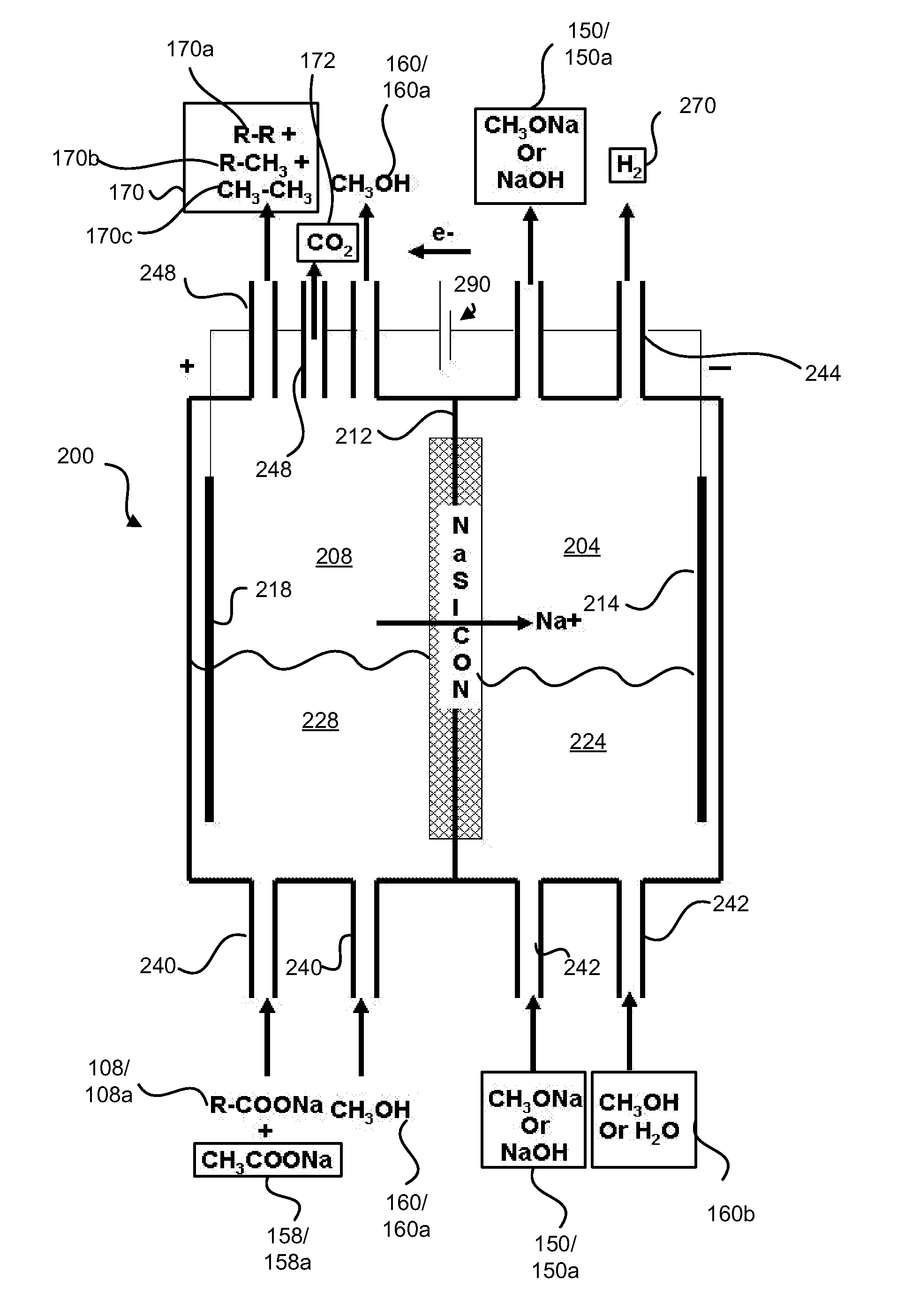
InactiveUS20110024288A1Avoid mixingLow costCellsElectrolytic organic coupling reactionsAlkali ionsTG - Triglyceride
Owner:CERAMTEC
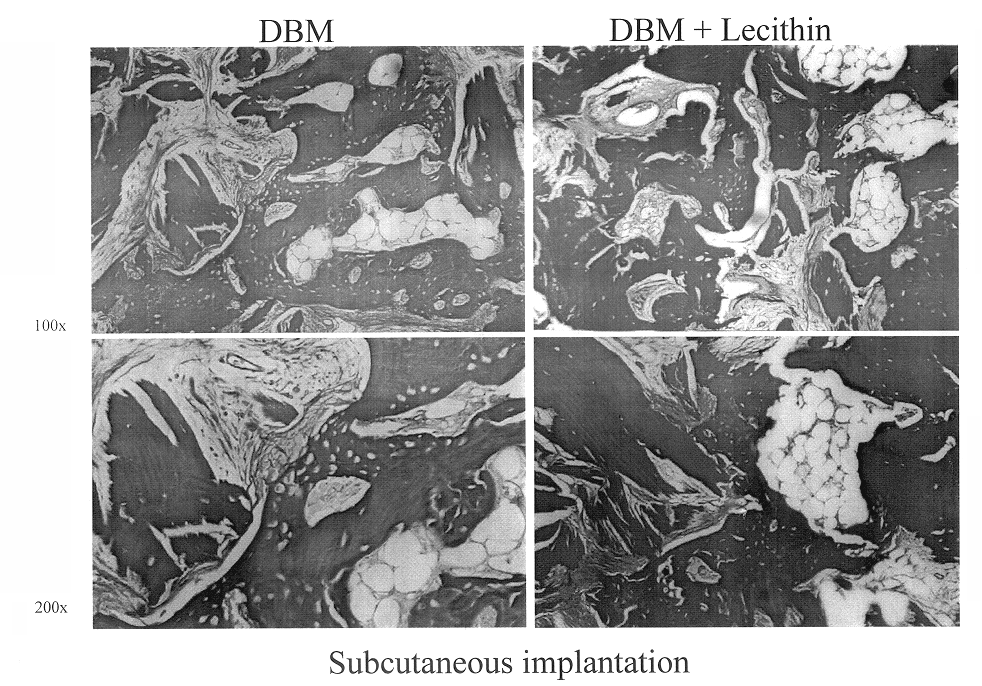
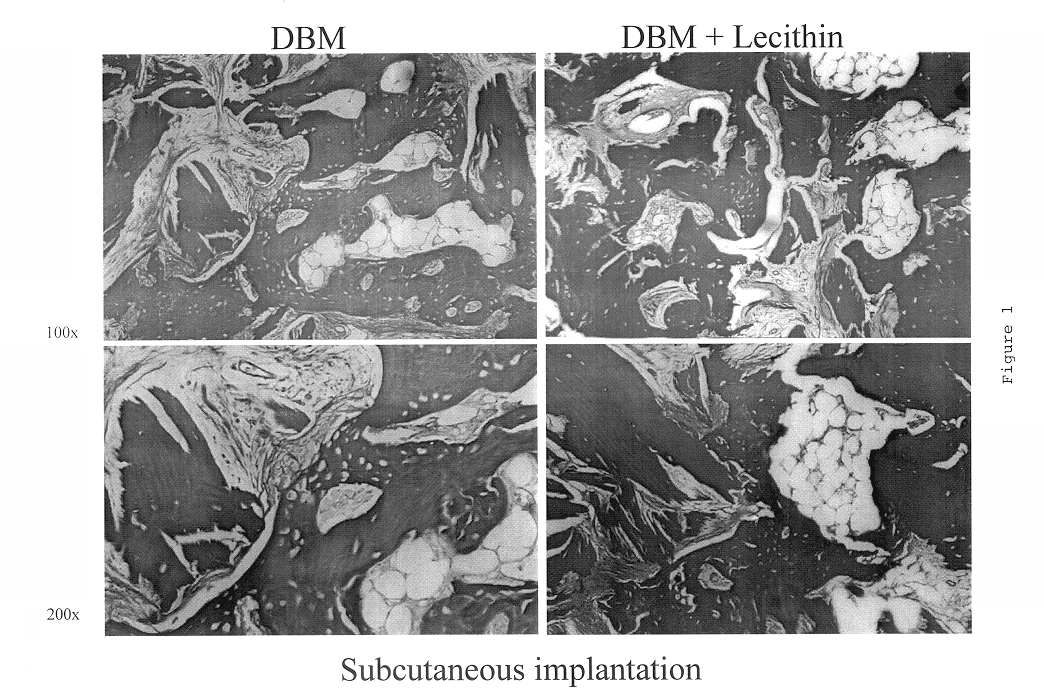
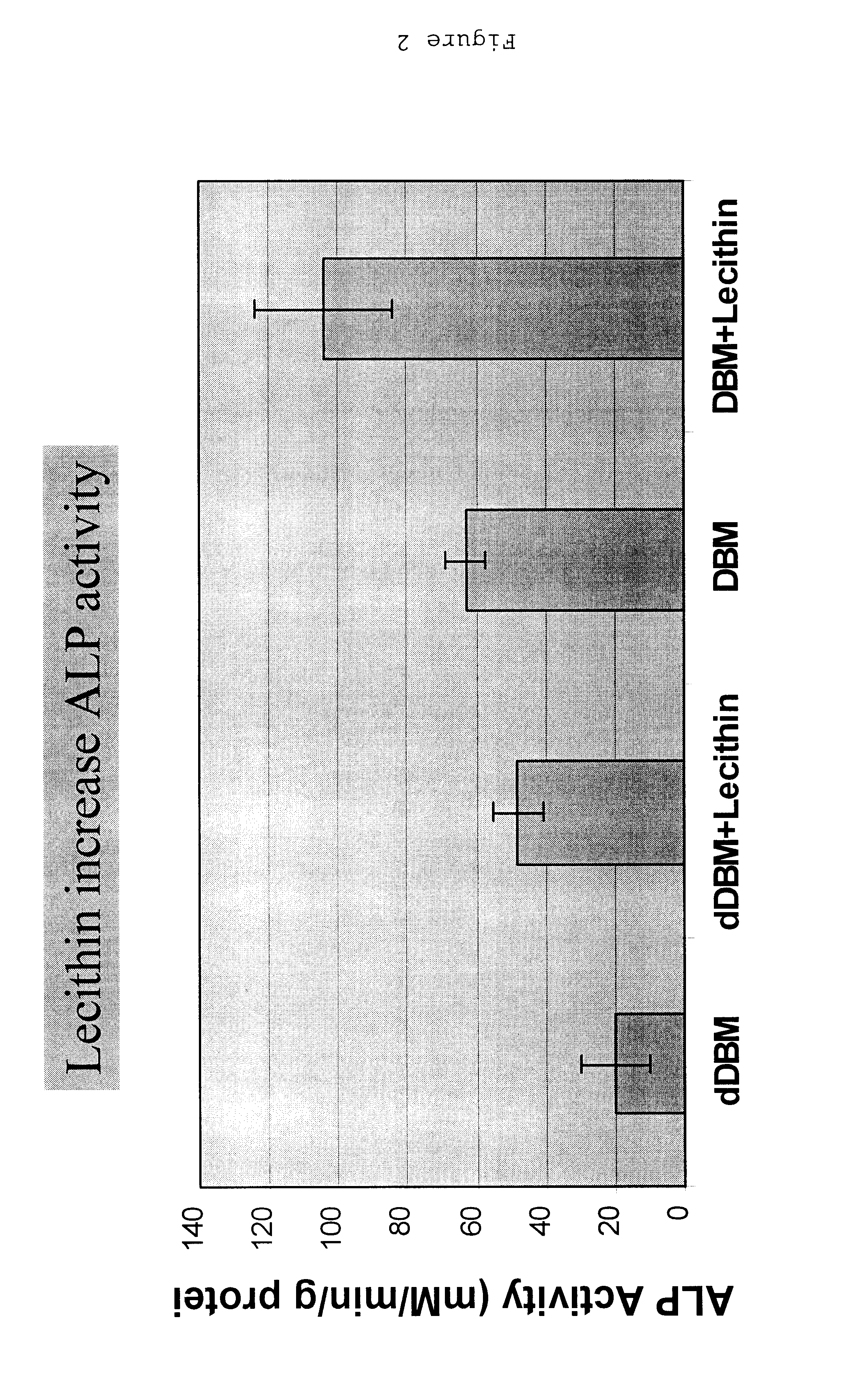
Bone graft material incorporating demineralized bone matrix and lipids
InactiveUS6565884B2Good osteoinductivityEasy to operateBiocidePowder deliveryHydrophilic polymersVitamin C
A demineralized bone putty composition comprises: (1) demineralized bone matrix (DBM); and (2) a lipid fraction selected from the group consisting of lecithin and a mixture of lecithin and triglycerides containing unsaturated fatty acids. The putty composition is moldable, biocompatible, slowly resorbable, and soluble in tissue fluids, and non-extrudable. The composition delivers a biologically active product to animals and humans that will enhance bone formation at sites where bone is lost, deficient, or present in suboptimal amounts. The composition can further comprise calcium, an antioxidant such as Vitamin E or Vitamin C, or a hydrophilic polymer such as methylcellulose or hydroxypropyl methylcellulose.
Owner:BIOMET MFG CORP
Statin and Omega-3 Fatty Acids For Lipid Therapy
InactiveUS20090239927A1Lower triglyceride levelsBiocideMetabolism disorderLipid formationTG - Triglyceride
A method of lipid therapy, comprising providing a subject group having a baseline triglyceride level of 200 to 499 mg / dl and being at or near its low-density lipoprotein cholesterol (LDL-C) level goal, and reducing the triglyceride level and the non-high-density lipoprotein cholesterol (non-HDL-C) level of the subject group as compared to treatment with a 3-hydroxy-3-methyl glutaryl coenzyme A (HMG CoA) inhibitor alone, by administering to the subject group an effective amount of an HMG CoA inhibitor and a composition comprising omega-3 fatty acids.
Owner:BOBOTAS GEORGE +3
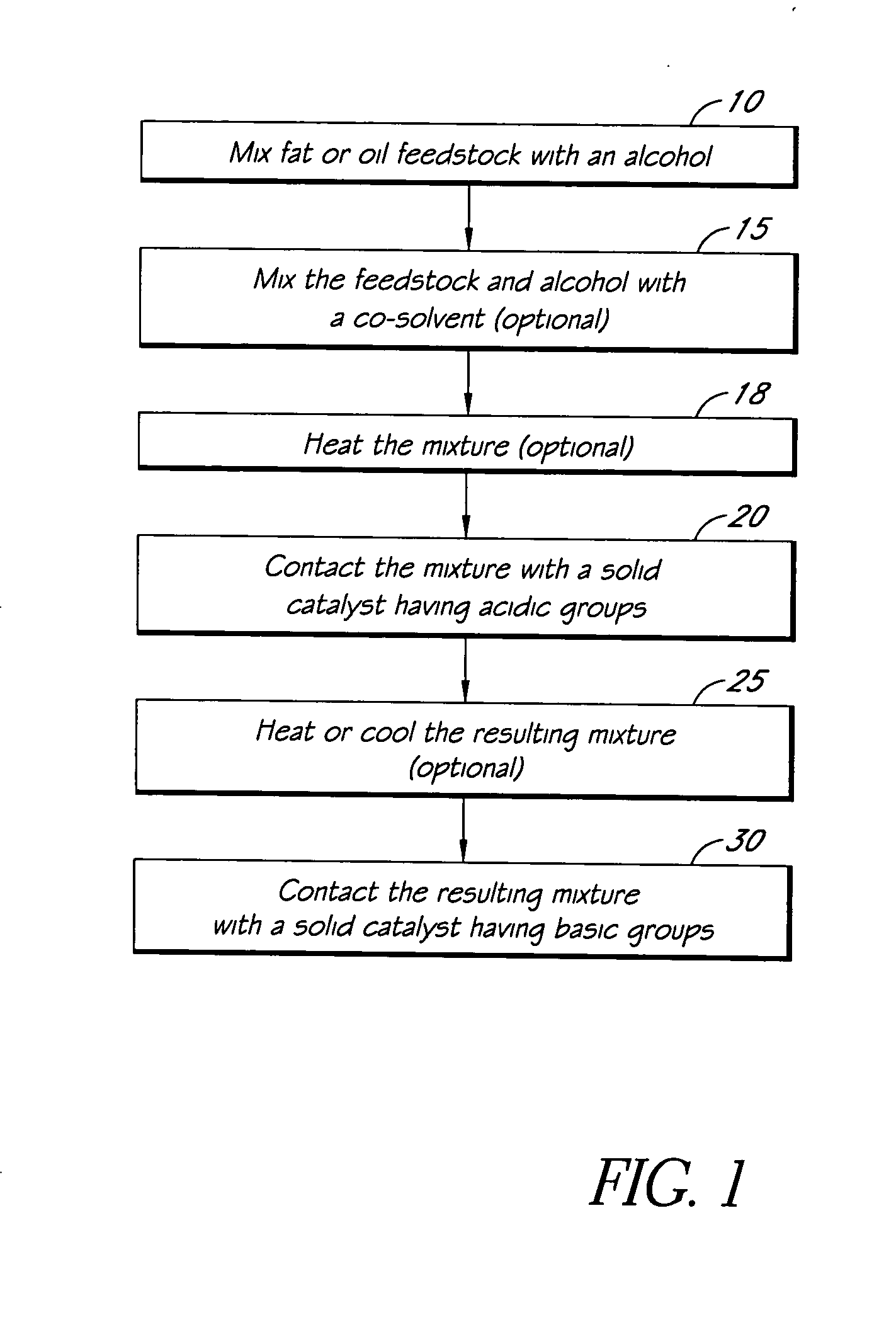
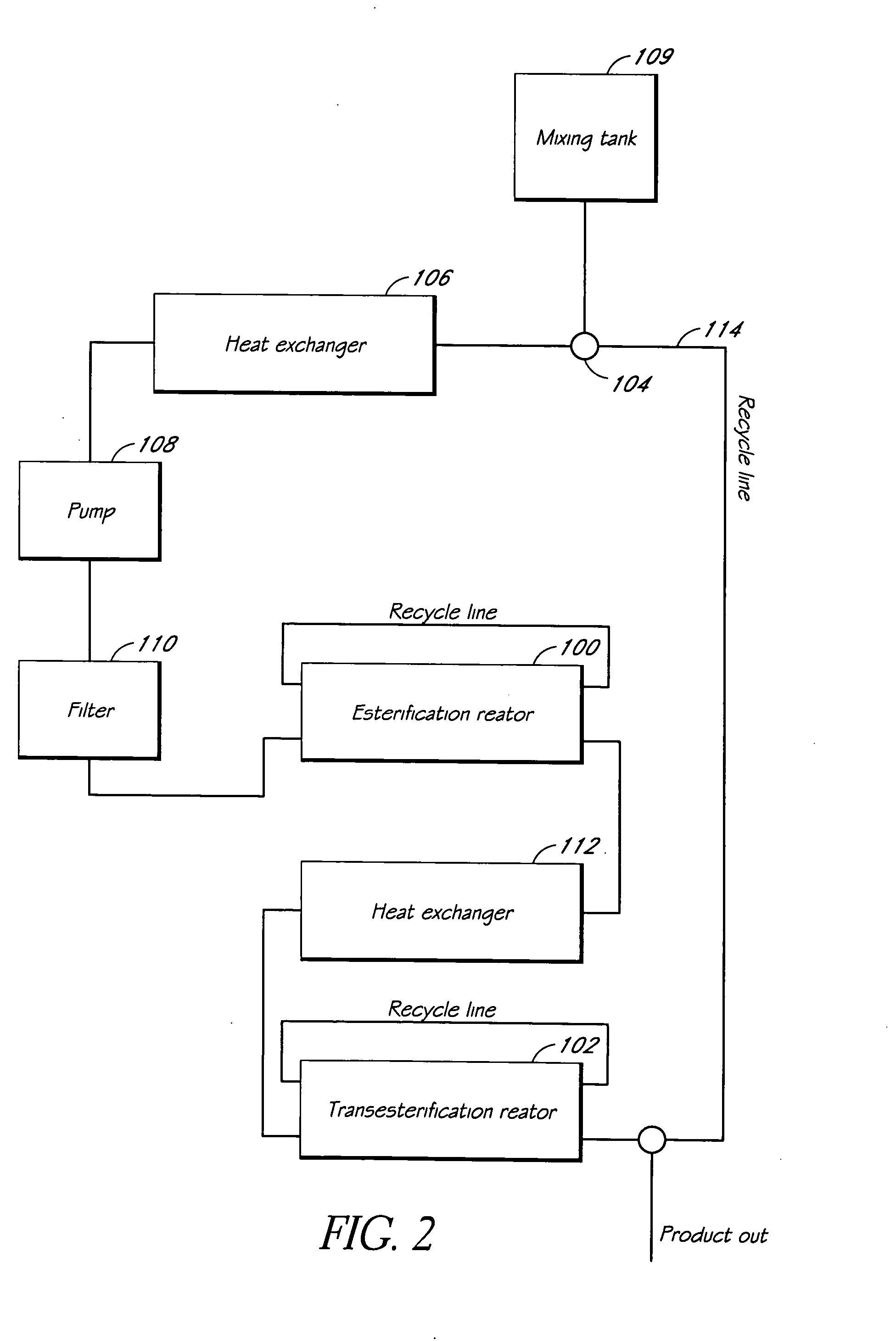
Systems and methods for esterification and transesterification of fats and oils
InactiveUS20060293533A1Fatty oils/acids recovery from wasteFatty acid esterificationAlcoholTransesterification
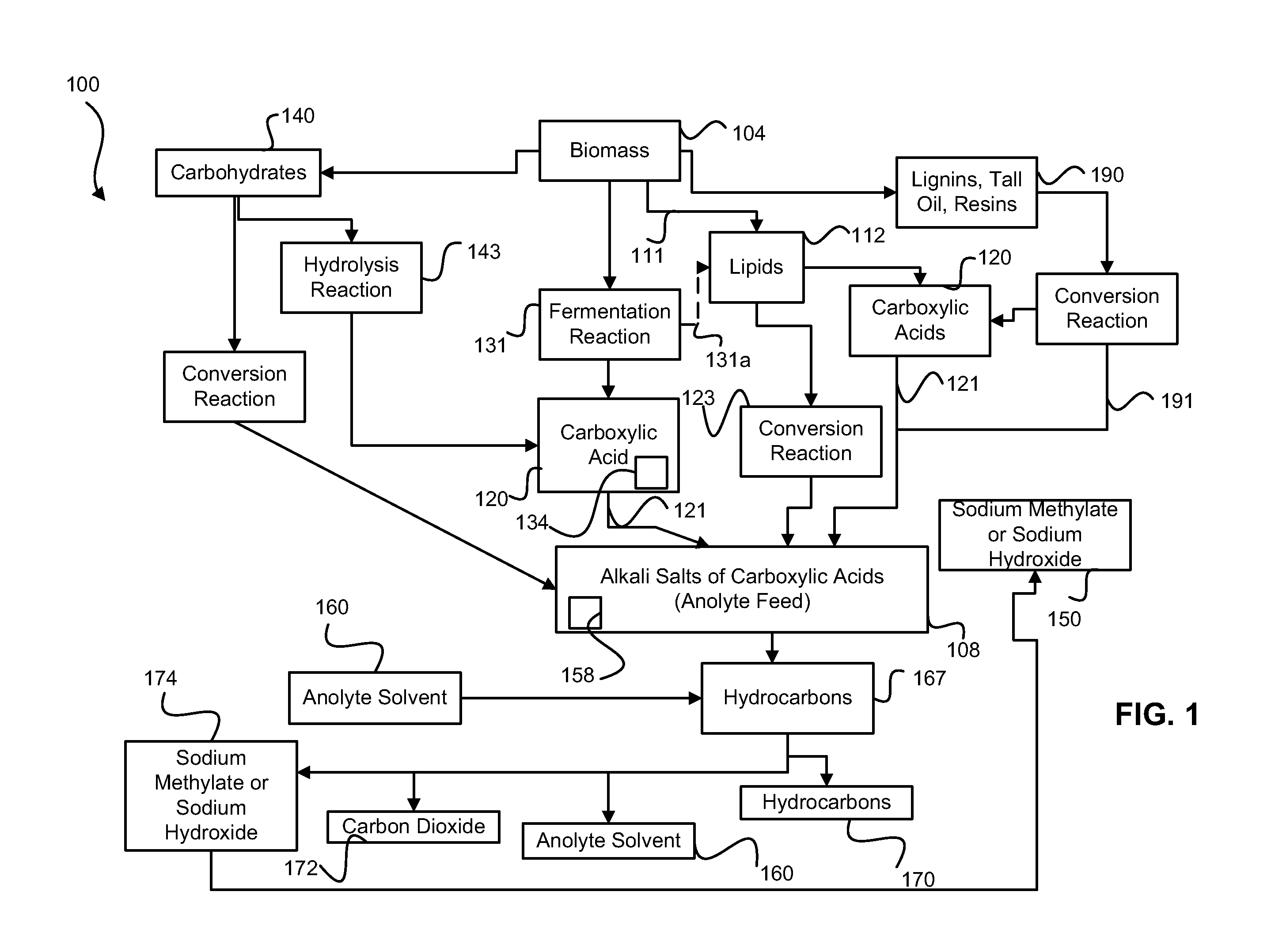
Esterification and transesterification of fats and oils is conducted using one or more heterogeneous solid catalysts in the presence of an alcohol and a cosolvent. In one example, esterification of free fatty acids in fats and oils feedstock is conducted by contacting the feedstock with a solid catalyst having acidic groups. Transesterification of triglycerides in the feedstock is conducted by contacting the feedstock with a solid catalyst having basic groups. Single train continuous plant designs are described for near complete conversion of fats and oils by sequentially contacting the feedstock with heterogeneous acidic solid catalyst and basic solid catalyst.
Owner:BIOSPHERE ENVIRONMENTAL ENERGY
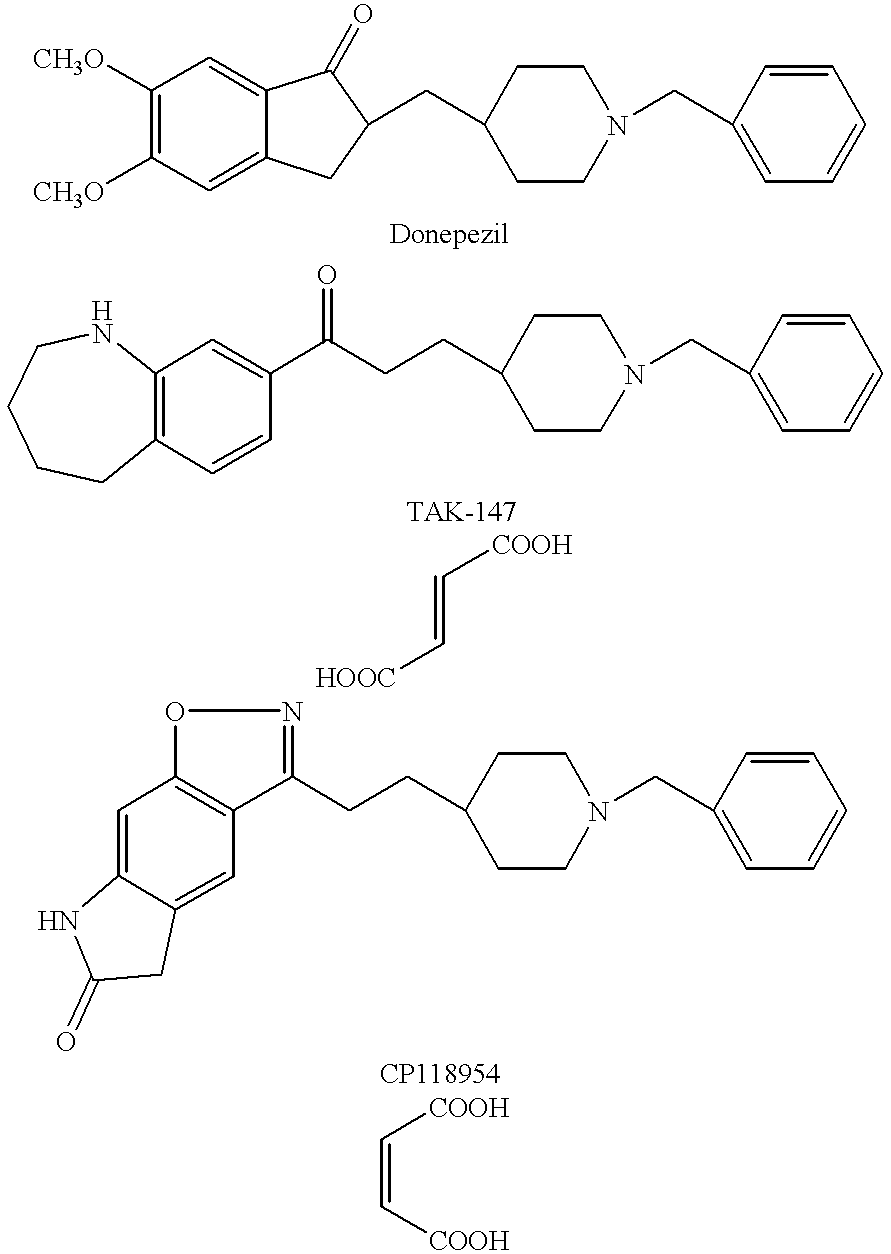
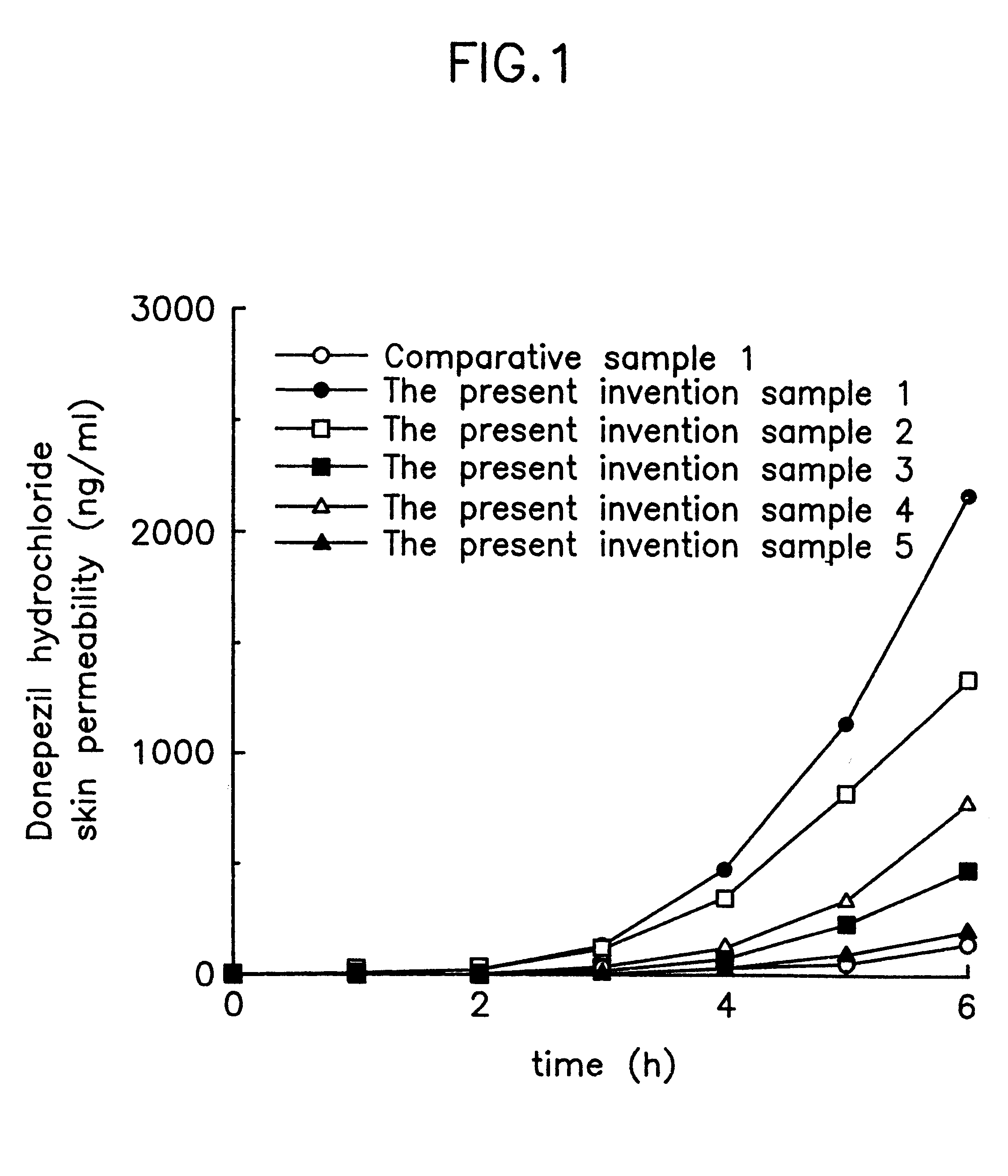
Suppository containing an antidementia medicament
The present invention provides a rectum applicable preparation containing an antidementia medicament, wherein the antidementia medicament is incorporated a triglyceride of a fatty acid and / or a water-soluble macromolecule.
Owner:EISAI CO LTD
Fatty acid compositions and methods of use
InactiveUS20090011012A1Easy to carryConvenient travelBiocideCapsule deliveryCyclosporine toxicityAntioxidant
The invention relates to highly concentrated DHA and EPA formulations in a soft gel capsule. A capsule may contain at least 80% omega-3 fatty acids, salts or derivatives thereof, where EPA and DHA are present in relative amounts of greater than or equal to 3:1 or less than or equal to 1:3, and constitute at least 75% to greater than 95% of the total fatty acids present in the capsule. Capsules of the invention may be provided in a blister package so as to provide clean and protected oils that are easy to travel with. Compliance is improved with one-pill-a-day dosing and the days of the week imprinted on the foil packing. Anitoxidant protection may be provided by rosemary and vitamin C. The invention also provides a methods of treatment, modulation or prophalaxis of coronary disease, altering serum LDL-cholesterol and / or HDL-cholesterol, lowering serum triglycerides, lowering blood pressure, pulse rate, altering the activity of the blood coagulation factor VII complex, mild hypertension, protection from cyclosporine toxicity in kidney transplant, rheumatoid arthritis, development and progression of retinopathy, hypertriglyceridemia, and neurological disorders in a subject.
Owner:BAUM SETH J
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com