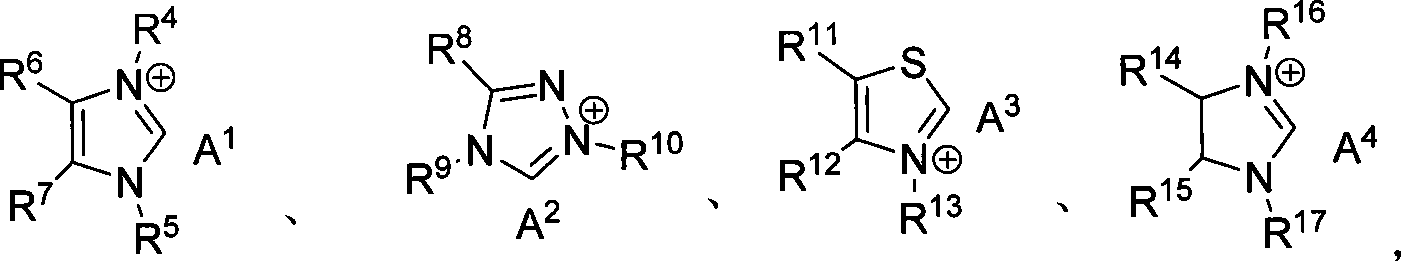
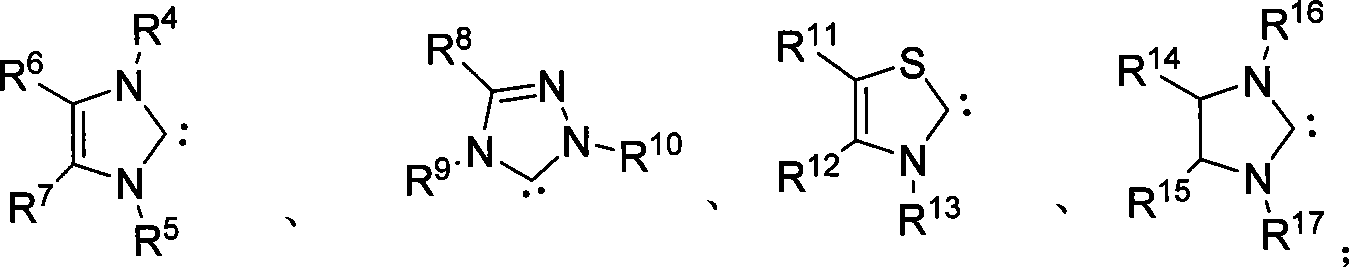
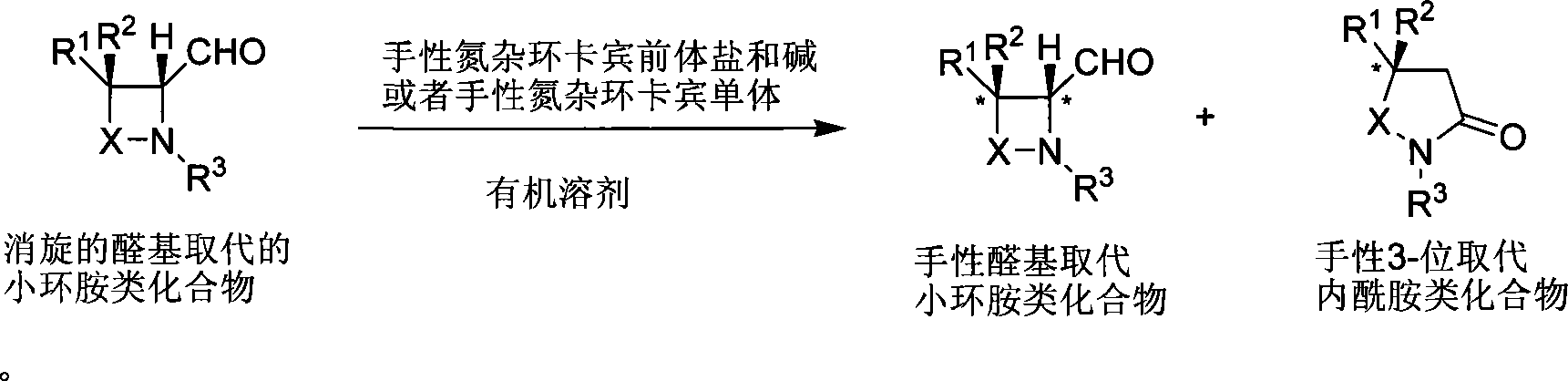
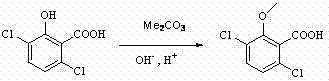Patents
Literature
6279results about How to "High reaction yield" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Process for converting triglycerides to hydrocarbons
ActiveUS20070175795A1High reaction yieldIncrease cetane numberBiofuelsLiquid carbonaceous fuelsBoiling pointHydrocotyle bowlesioides
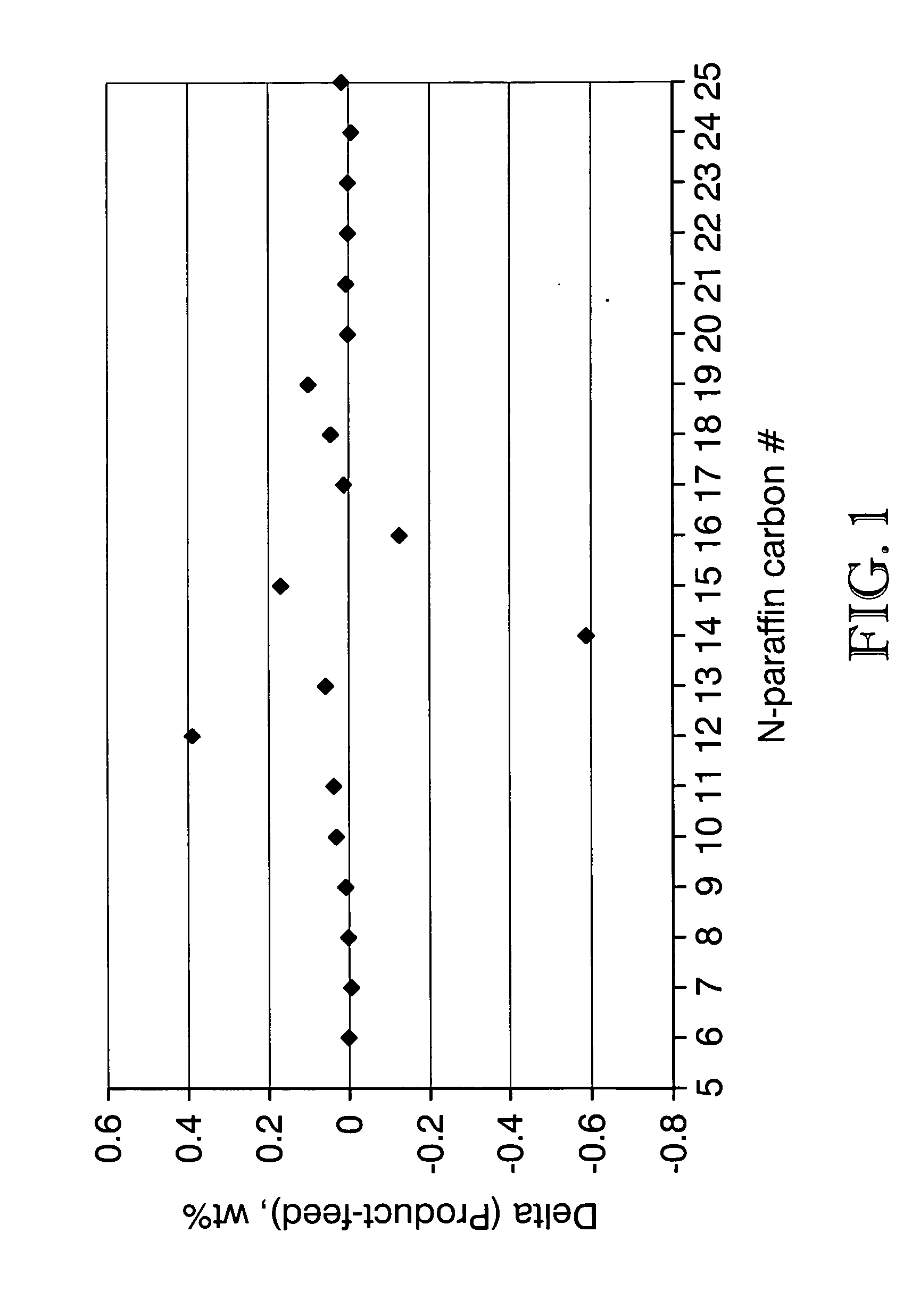
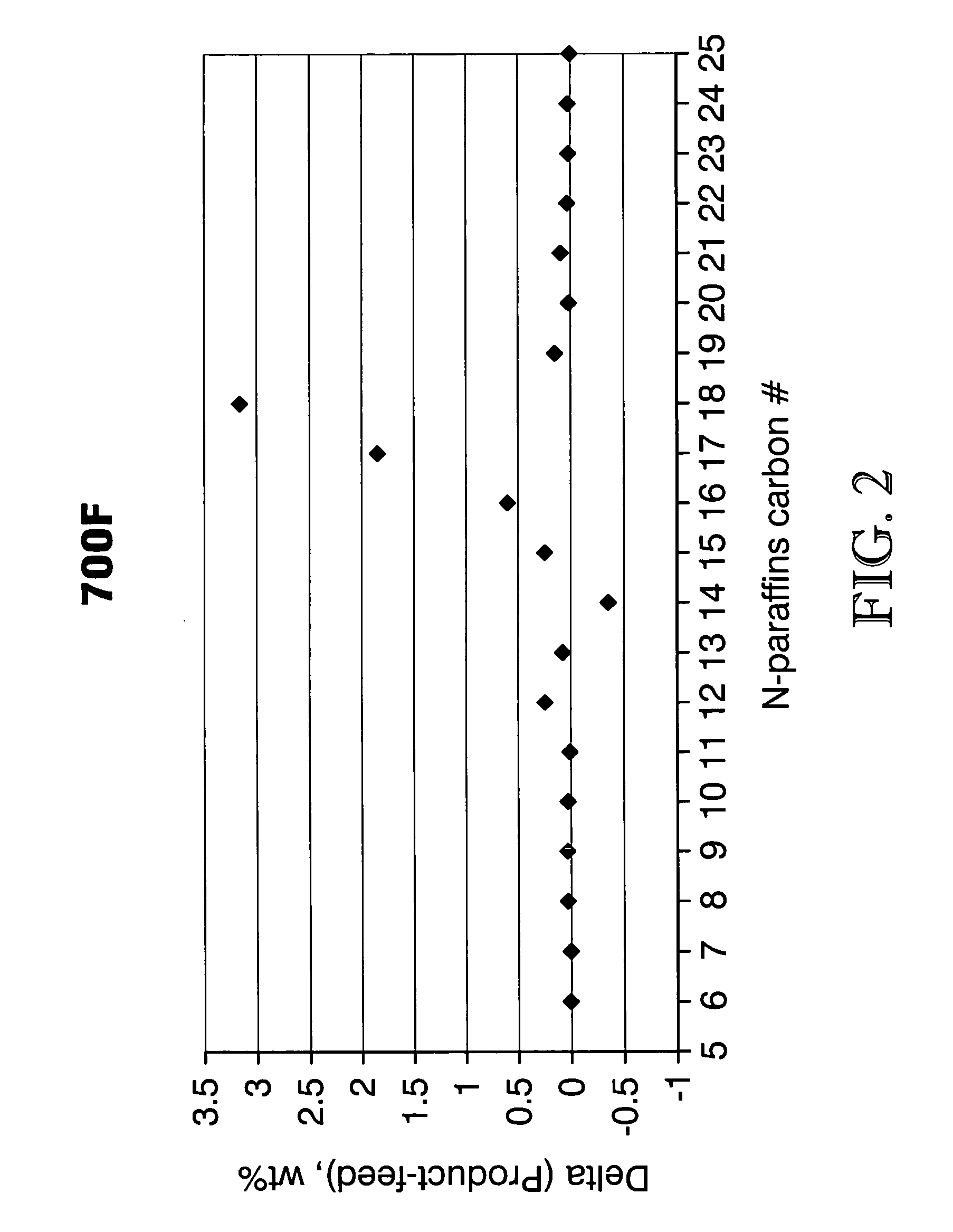
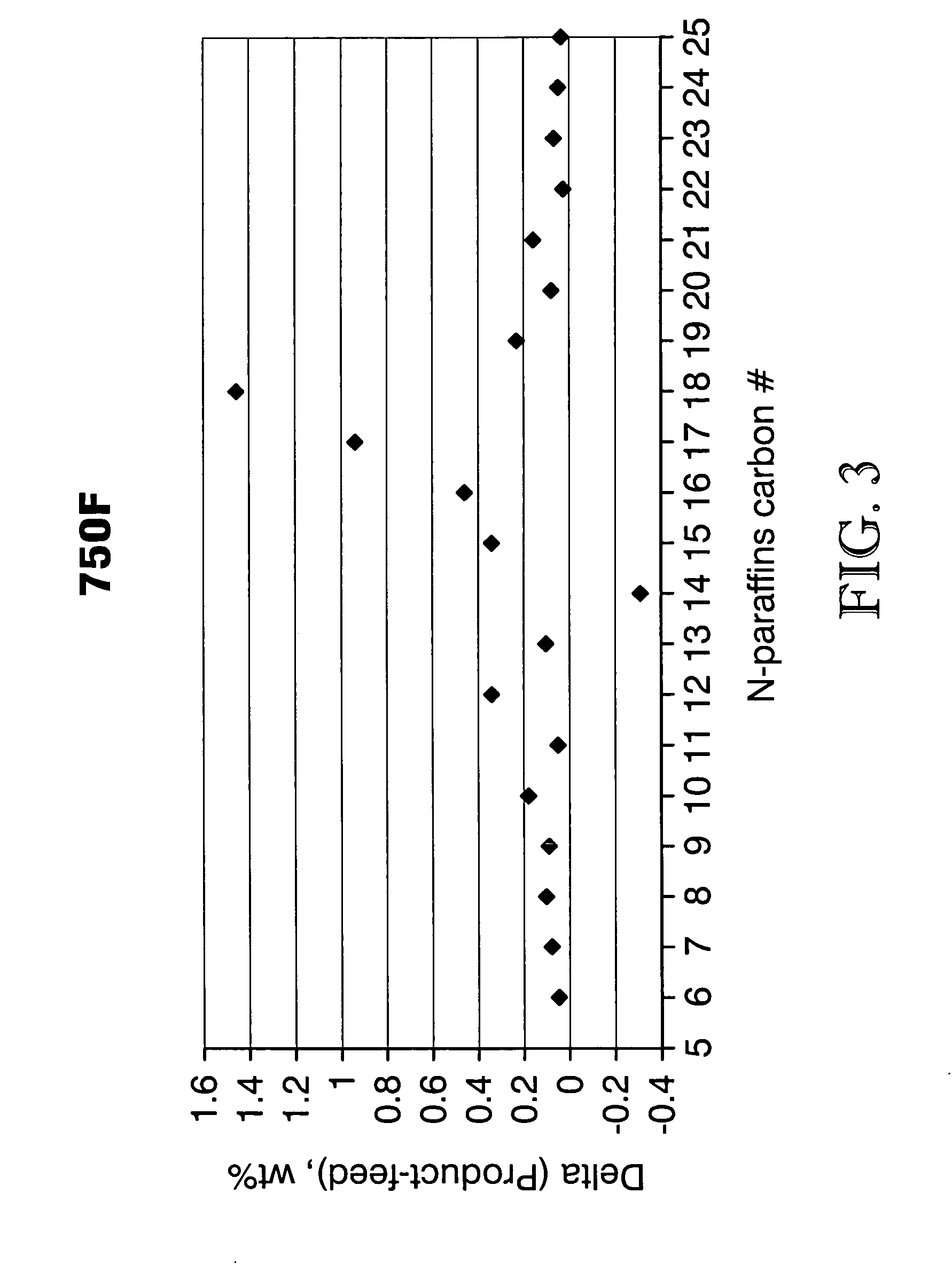
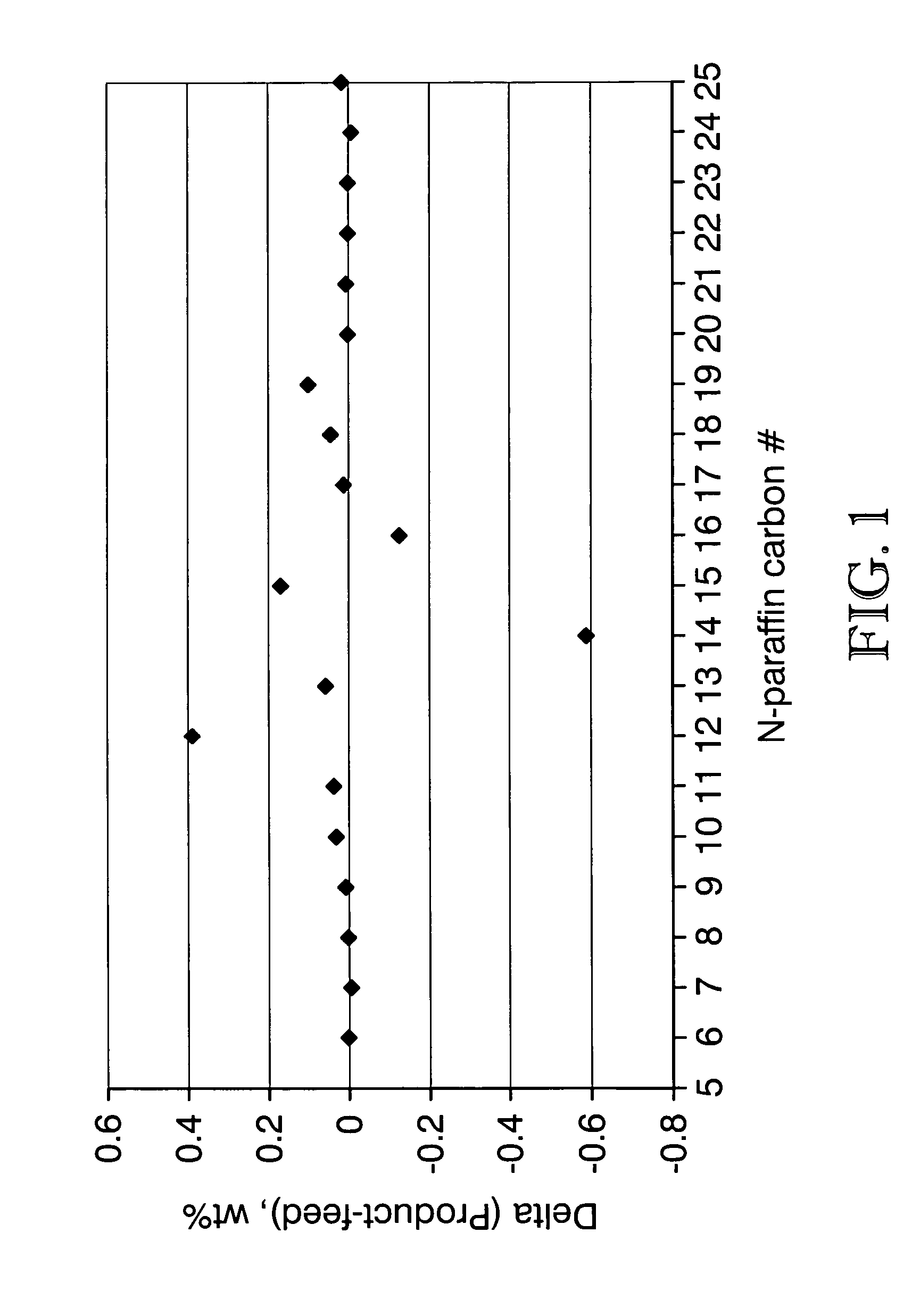
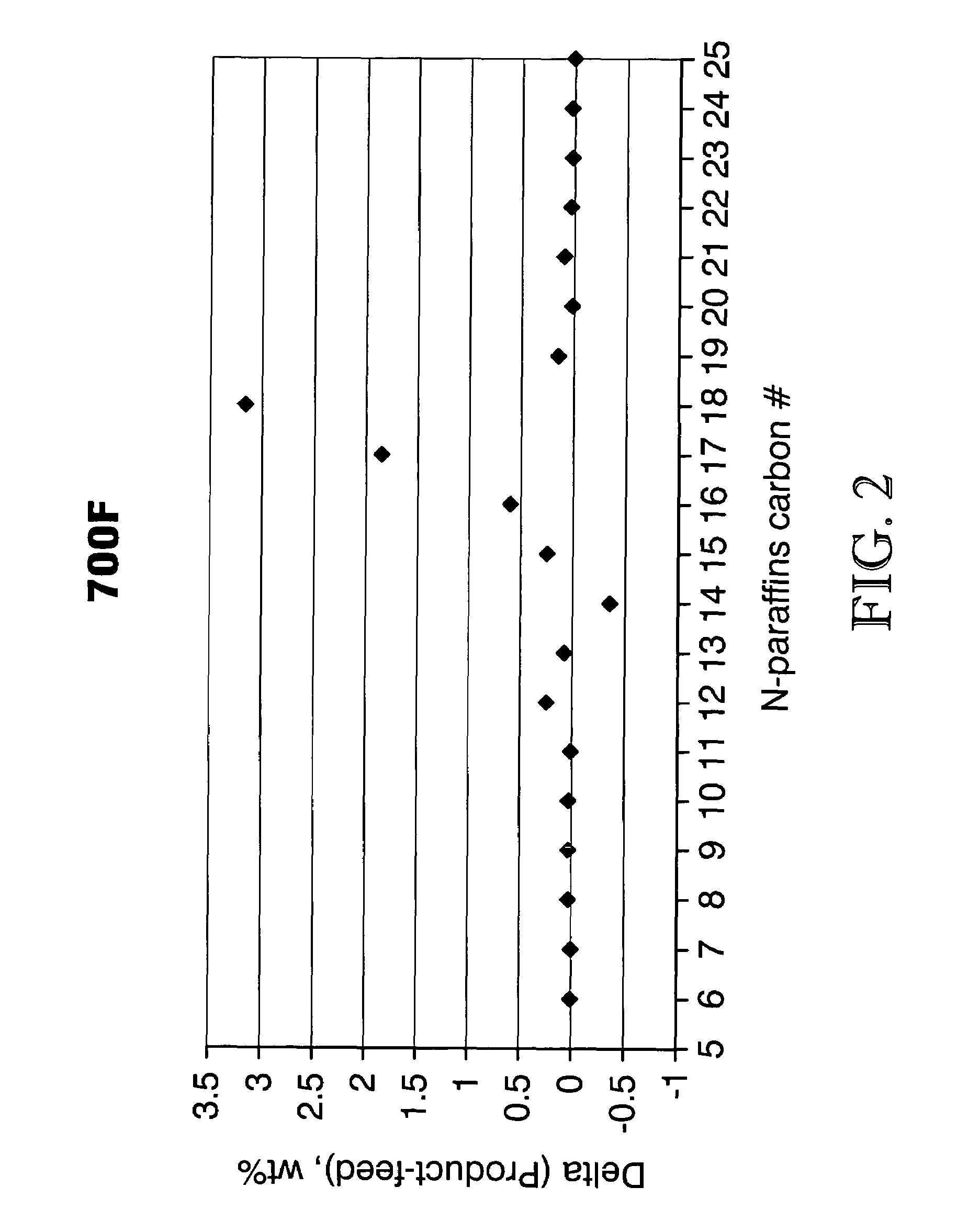
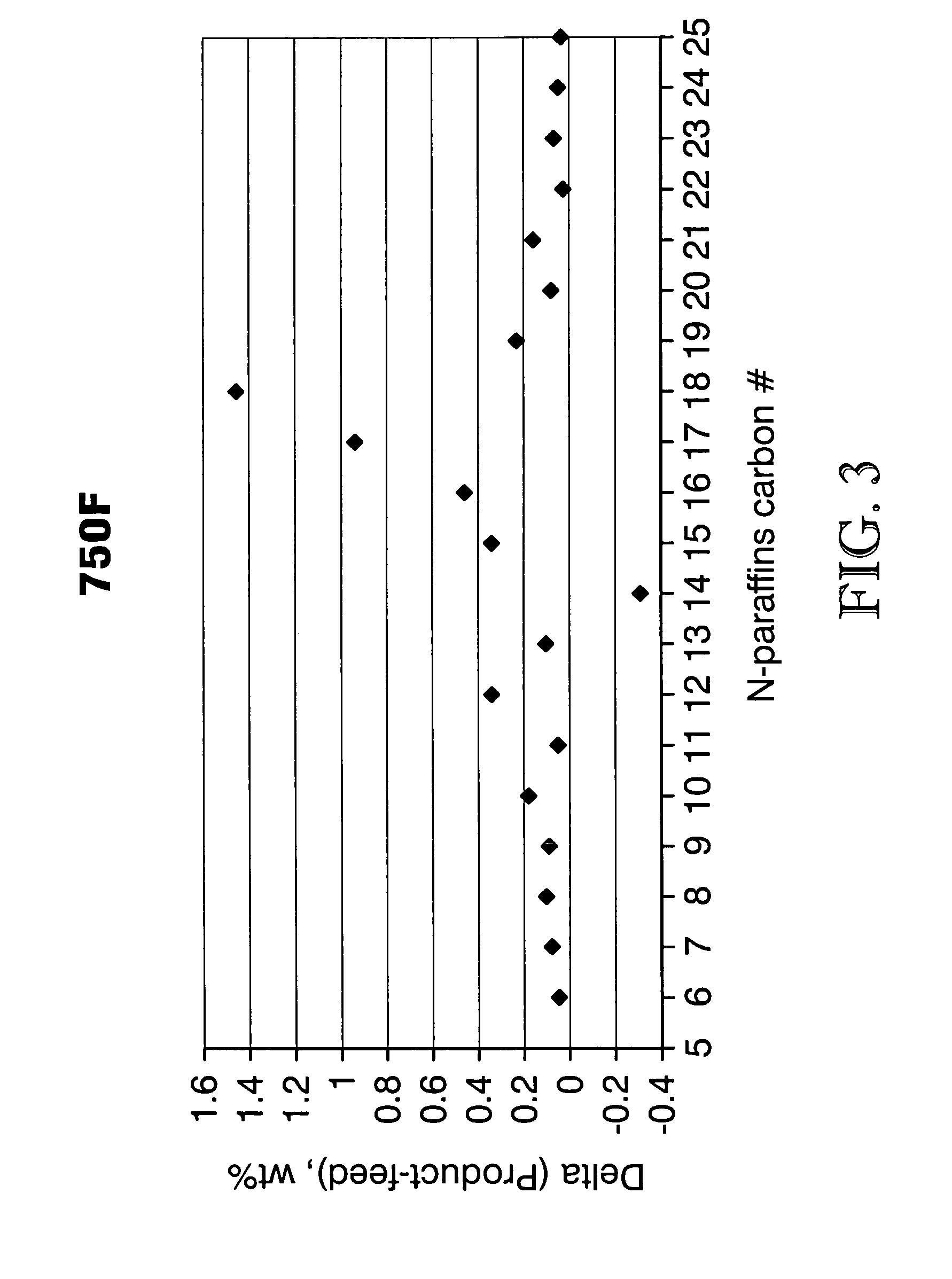
Processes for the conversion of hydrocarbons boiling in the temperature range of from about 80° F. to about 1000° F. to diesel boiling range hydrocarbons, and processes for increasing the cetane number and amount of n-C17 hydrocarbon products in such processes. Diesel boiling range hydrocarbons may be produced by contacting a hydrocarbon boiling in the above-mentioned boiling range with a triglyceride-containing compound to form a mixture, and then contacting the mixture with a hydrotreating catalyst under suitable reaction conditions.
Owner:PHILLIPS 66 CO
Process for converting triglycerides to hydrocarbons
ActiveUS7550634B2High reaction yieldIncrease cetane numberBiofuelsSolid fuelsBoiling pointTG - Triglyceride
Processes for the conversion of hydrocarbons boiling in the temperature range of from about 80° F. to about 1000° F. to diesel boiling range hydrocarbons, and processes for increasing the cetane number and amount of n-C17 hydrocarbon products in such processes. Diesel boiling range hydrocarbons may be produced by contacting a hydrocarbon boiling in the above-mentioned boiling range with a triglyceride-containing compound to form a mixture, and then contacting the mixture with a hydrotreating catalyst under suitable reaction conditions.
Owner:PHILLIPS 66 CO
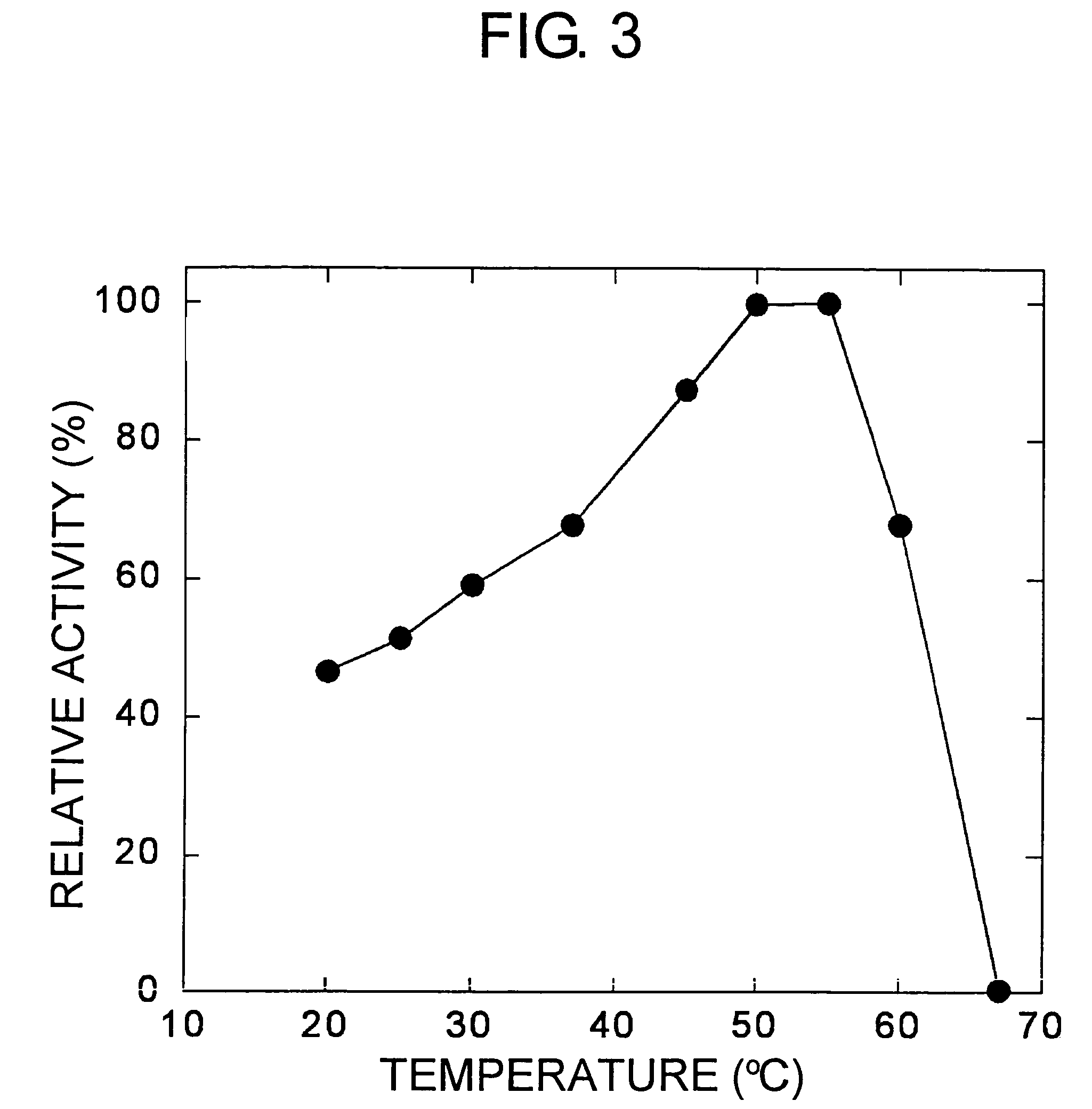
Carbonyl reductases, polynucleotides comprising DNA encoding the same, methods for producing the same, and methods for producing optically active alcohol utilizing the same
Owner:DAICEL CHEM IND LTD
Method of producing reactive silicon group-containing polyether oligomers
The invention provides a method of producing a reactive silicon group-containing polyether oligomer which comprises reacting (a) a polyether oligomer having main chain of a polyether and, in each molecule, at least one unsaturated group represented by the general formula (1):(in the formula, R1 is a hydrocarbon group containing not more than 10 carbon atoms and R2 is a divalent organic group containing 1 to 20 carbon atoms and one or more species selected from the group consisting of hydrogen, oxygen and nitrogen atoms as a constituent atom) or the general formula (2):on a side chain or at a terminus with (b) a reactive silicon group-containing compound in the presence of (c) a group VIII transition metal catalyst to introduce the reactive silicon group into said polyether oligomer (a),wherein the reaction is carried out in the presence of (d) a sulfur compound.
Owner:KANEKA CORP
Oxyether triazole compound, and preparation method and application thereof
InactiveCN103772305AHigh reaction yieldHigh purityOrganic chemistryLuminescent compositionsSimple Organic CompoundsDiphenyl ether
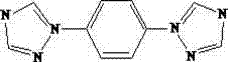
The invention discloses a preparation method of 1-(4-(4-(1H-1,2,4-triazolyl-1-yl)phenoxy)phenyl)-1H-1,2,4-triazole. The organic compound is prepared by heating 4,4'-dibromodiphenyl ether, triazole, potassium carbonate and copper oxide by a one-pot process. The preparation method has the characteristics of simple technological operation, low production cost and low environmental pollution, and is suitable for large-scale industrial production. The invention also discloses application of the 1-(4-(4-(1H-1,2,4-triazolyl-1-yl)phenoxy)phenyl)-1H-1,2,4-triazole in photoelectric materials.
Owner:TIANJIN NORMAL UNIVERSITY
Process for producing aromatic hydrocarbon
InactiveUS20100185034A1Simple and economical procedureHigh reaction yieldMolecular sieve catalystsMolecular sieve catalystHydrogenReaction temperature
Provided is a process for producing an aromatic hydrocarbon using a molybdenum-containing solid catalyst, more specifically a process for producing an aromatic hydrocarbon efficiently from a lower hydrocarbon gas essentially containing methane by activating the molybdenum-containing solid catalyst with maintaining a high yield for a long period of time.The process comprises a pre-contacting step of allowing a molybdenum-containing solid catalyst to contact with a pre-contacting gas comprising at least one selected from a lower hydrocarbon and a hydrogen gas; and a reaction step of allowing the pre-contacted catalyst to contact with a raw material gas essentially containing methane, to generate an aromatic hydrocarbon, wherein the starting temperature in the pre-contacting step is lower than the reaction temperature, and the temperature during the pre-contacting step from the beginning to the end is not over the reaction temperature.
Owner:MITSUI CHEM INC +1
Method and composition for chemical synthesis using high boiling point organic solvents to control evaporation
InactiveUS6177558B1Reduce evaporationHigh reaction yieldMaterial nanotechnologySequential/parallel process reactionsChemical synthesisChemical Moiety
A method for reducing evaporation of a liquid reagent solution during solid phase, micro-scale chemical synthesis of a molecule comprising sub-units on an open environment solid support surface. The method includes the steps of providing an open solid support surface including at least one binding site which is functionalized with a reactive chemical moiety; and depositing a substantially controlled and minute volume of liquid reagent solution onto the support surface, and in contact with the binding site. The reagent solution includes reactants contained in at least one relatively high boiling point solvent, in contrast to standard organic solvents for such reagents. Application of a high boiling point solvent substantially reduces evaporation of the reagent solution in the open environment during synthesis on the solid support while enabling the maintenance of a substantially high reaction yield.
Owner:METRIGEN
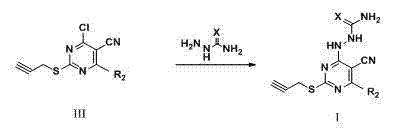
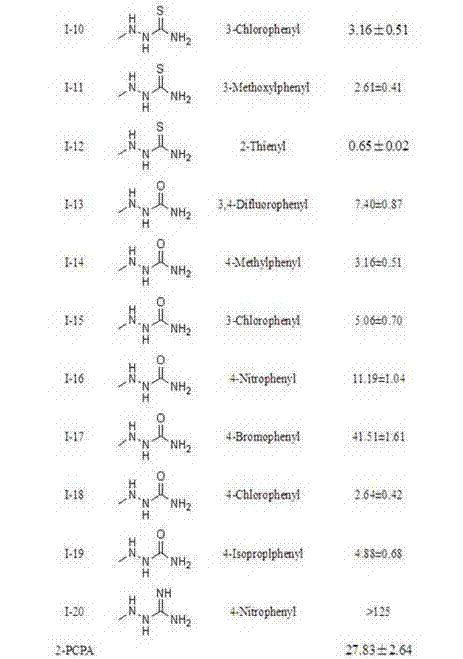
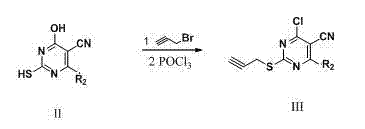
Pyrimidine derivatives containing semicarbazide and terminal alkyne structural units, and preparation methods and applications of pyrimidine derivatives
ActiveCN104119280AHigh activityMild reaction conditionsOrganic active ingredientsOrganic chemistryAlkynePharmaceutical Substances
The invention belongs to the field of medicinal chemistry, and discloses pyrimidine compounds containing semicarbazide and terminal alkyne structural units, and preparation methods and applications of the pyrimidine compounds in preparation of antitumor drugs by taking lysine specific demethylase 1 (hereafter referred to as LSD1) as a target. A pyrimidine active fragment is built by adopting a three-component one-pot method, and then the target compounds are prepared by substitution, chlorination and ammonification reaction. The general formulas of the compounds are as shown in the formula I in the specification. An in vitro anti-tumor activity experiment and an LSD1 inhibition activity experiment prove that the compounds have obvious inhibiting and killing action on a plurality of tumor cells by inhibiting the activity of the LSD1, can be used as lead compounds for further development, and are applied to preparation of the antitumor drugs.
Owner:ZHENGZHOU UNIV
Microfluidic radiosynthesis system for positron emission tomography biomarkers
InactiveUS20090036668A1Fast and efficient and compact mannerSmall amountGaseous chemical processesSugar derivativesHands freeEngineering
Methods and devices for a fully automated synthesis of radioactive compounds for imaging, such as by positron emission tomography (PET), in a fast, efficient and compact manner are disclosed. In particular, the various embodiments of the present invention provide an automated, stand-alone, hands-free operation of the entire radiosynthesis cycle on a microfluidic device with unrestricted gas flow through the reactor, starting with target water and yielding purified PET radiotracer within a period of time shorter than conventional chemistry systems. Accordingly, one aspect of the present invention is related to a microfluidic chip for radiosynthesis of a radiolabeled compound, comprising a reaction chamber, one or more flow channels connected to the reaction chamber, one or more vents connected to said reaction chamber, and one or more integrated valves to effect flow control in and out of said reaction chamber.
Owner:SIEMENS MEDICAL SOLUTIONS USA INC
Method for synthesis of cobalt nanoparticle and bamboo-like nitrogen doped carbon nanotube composite material
The invention discloses a method for synthesis of a cobalt nanoparticle and bamboo-like nitrogen doped carbon nanotube composite material. The method includes: dissolving a soluble cobalt salt and an amine polymer in a hydrophilic reagent according to a mole ratio of 1:(2-200), performing evaporation at 60DEG C, conducting grinding after cooling, performing calcination at 400-1400DEG C under nitrogen atmosphere, then treating the sample with acid, and carrying out washing, centrifugation and drying so as to obtain the cobalt nanoparticle and bamboo-like nitrogen doped carbon nanotube composite material. The obtained cobalt nanoparticles have small particle size and are employed to coat the head of a carbon nanotube evenly so as to combine tightly with the carbon nanotube. The composite material has application prospects in fuel cell anode materials, lithium ion battery cathode materials and the like. The method designed by the invention has the advantages of easily available raw materials, simple process and no pollution, short preparation period, mild reaction conditions, low cost, and mass synthesis capability, etc.
Owner:BEIJING INSTITUTE OF TECHNOLOGYGY
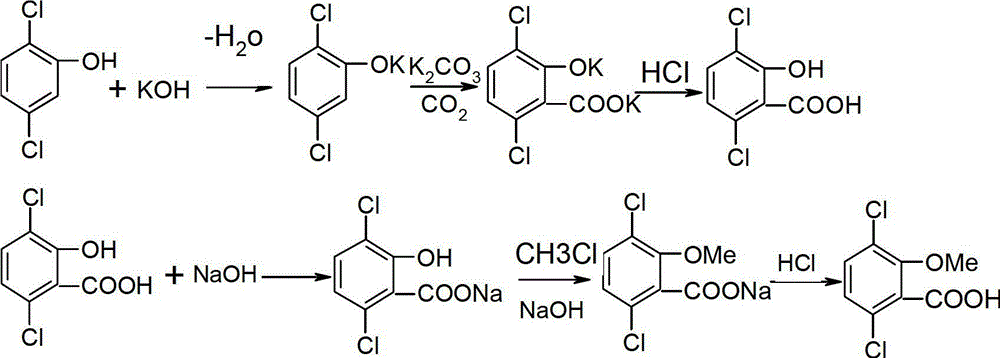
Synthetic process of herbicide dicamba
ActiveCN102942474AImprove one-way yieldImprove quality and efficiencyOrganic compound preparationCarboxylic compound preparationMethoxylaricinolic acidFixed bed
The invention relates to a preparation method of herbicide dicamba. The preparation method includes: (1) liquid potassium hydroxide and 2, 5-dichlorophenol are reacted according to molar ratio of 0.95:1-1:1 to obtain 2, 5-dichlorophenol potassium; (2) at the presence of anhydrous potassium carbonate and catalyst, the 2, 5-dichlorophenol potassium obtained in the step (1) is reacted with CO2 to generate 3, 6-dichlorosalicylic acid, pressure of CO2 is controlled to be 4-6MPa, reaction temperature ranges from 100 DEG C to 160 DEG C, and molar ratio of anhydrous potassium carbonate and 2, 5-dichlorophenol potassium is 1-2:1; and (3) in alkaline condition, at the temperature of 70-100 DEG C, the 3, 6-dichlorosalicylic acid obtained in the step (2) and chloromethane are reacted under the action of the catalyst through a tube fixed-bed reactor according to molar ratio 1:1-3.5, saponification and acidification are performed to obtain 3, 6-dichloro-2-methoxysalicylic acid, namely the dicamba. The process is high in reaction yield, simple in reaction condition, good in product quality, small in three wastes and low in energy consumption.
Owner:JIANGSU YOUJIA CHEM +2
Method for preparing 4-nitrodiphenylamine and 4-nitrosodiphenylamine from carbanilide
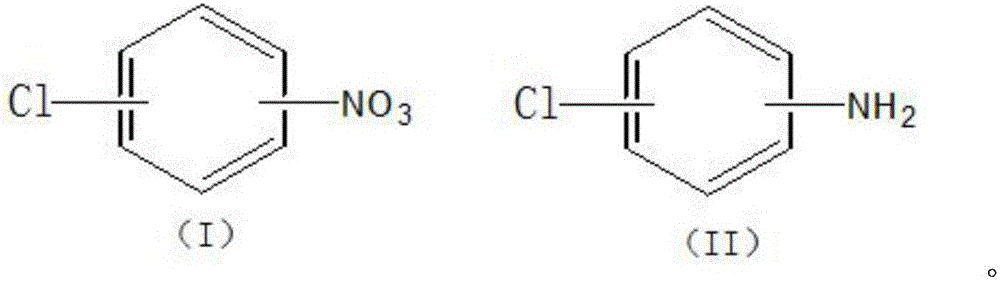
InactiveUS6137010AHigh yieldImprove responseOrganic compound preparationAmino preparation by hydrogen substitutionAniline4-aminodiphenylamine
This invention relates to a process for preparing 4-nitrodiphenylamine and 4-nitrosodiphenylamine to be used for 4-aminodiphenylamine as an intermediate of antiozonant, wherein carbanilide is reacted with nitrobenzene in the presence of an appropriate base, while simultaneously adding aniline to the mixture so as to regenerate some amounts of carbanilide as a starting material. According to this invention, 4-nitrodiphenylamine and 4-nitrosodiphenylamine can be prepared in a higher selectivity and conversion rate via a continuous reaction by recycling carbanilide, a starting material, while adding a certain amount of aniline during the process. Further, the amount of waste water can be significantly reduced compared to the conventional method without any corrosive materials harmful to the environment.
Owner:KOREA KUMHO PETROCHEMICAL CO LTD
Oleanolic acid derivative, and preparation method and purpose thereof
InactiveCN102070697AHigh reaction yieldReduce manufacturing costSteroidsMethylaminesMedicinal chemistry
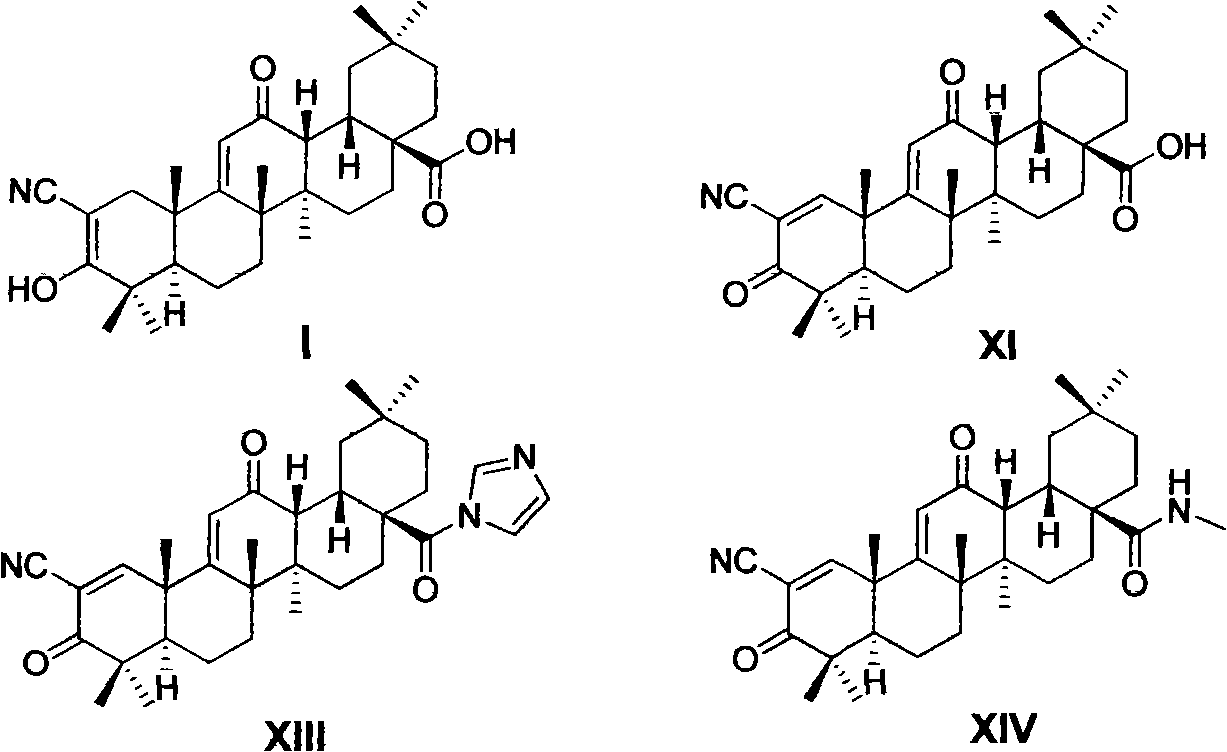
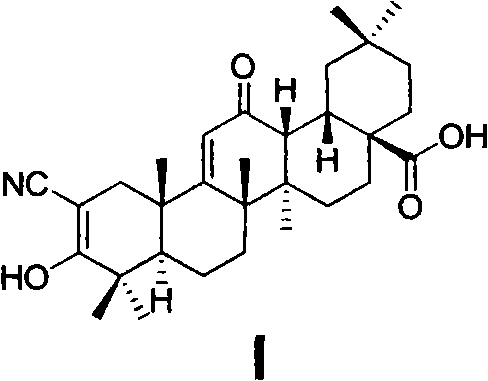
The invention relates to the field of medicine, in particular to an oleanolic acid derivative shown as a formula I, and pharmaceutically acceptable salts, a preparation method and purpose thereof. The invention also discloses application of the compound shown by the formula I to the preparation of anti-tumor medicine of 2-cyano-3, 12-dioxo oleanane-1, 9(11)-diene-28-carboxylic acid (XI, CDDO), 2-cyano-3, 12-dioxo oleanane-1, 9(11)-diene-28-carboxylic imidazole (XIII, CDDO-Im) and 2-cyano-3, 12-dioxo oleanane-1, 9(11)-diene-28-carboxyl methylamine (XIV, CDDO-MA).
Owner:CHINA PHARM UNIV
Synthetic method for agomelatine
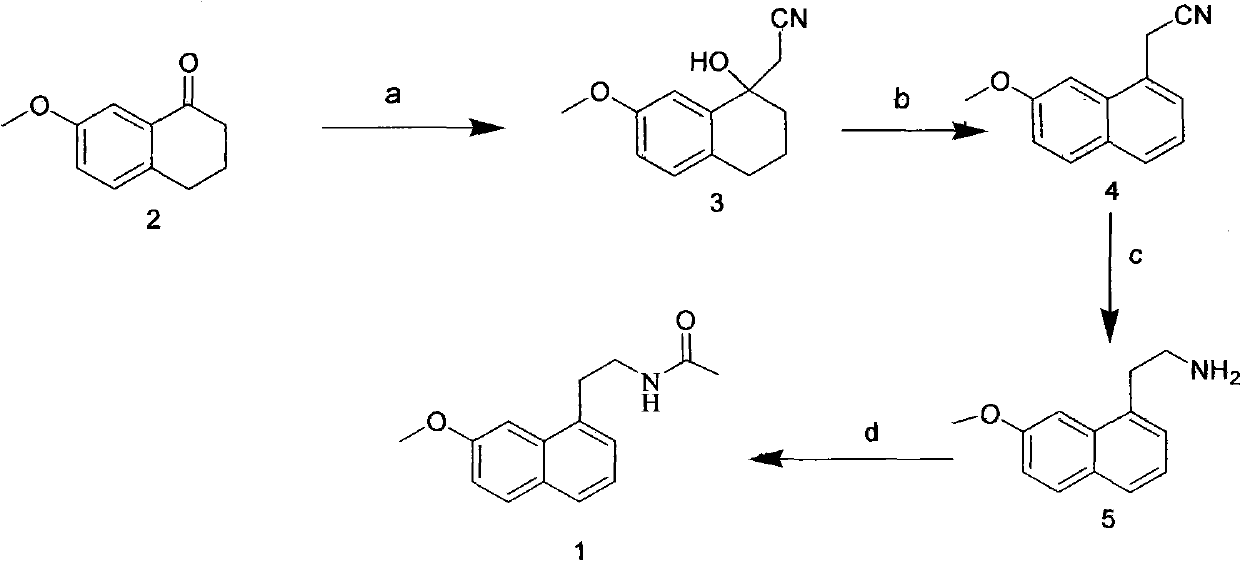
InactiveCN101792400AHigh reaction yieldMild reaction conditionsOrganic compound preparationCarboxylic acid amides preparationButyl lithiumEthylamines
The invention relates to a synthetic method for agomelatine. The method comprises the following steps: reacting 7-methoxy-1-tetralone (2) serving as a raw material with acetonitrile under the action of n-butyl lithium to obtain 1-hydroxy-7-methoxy-1,2,3,4-tetralin-1-naphthyl acetonitrile (3); then uniformly mixing the compound (3) and acetic acid solvent or toluene, adding dichloro dicyano benzoquinone into the mixture for reacting at the temperature of between 50 and 150 DEG C for 4 to 20 hours to obtain a compound (4); adding the compound (4) into uniformly mixed solution of lithium aluminum hydride and tetrahydrofuran for reacting at the temperature of between 0 and 60 DEG C for 5 to 24 hours to obtain (7-methoxy-1-naphthyl) ethylamine (5); and finally uniformly mixing the compound (5)and triethylamine or naphthyridine, and adding acetylchloride into the mixture for reacting at the temperature of between 0 and 25 DEG C for 1 to 5 hours to obtain the agomelatine (1). The synthetic method for the agomelatine of the invention has the advantages of high yield, low cost, high controllability, simple processing after the reaction and environment protection and is suitable for industrial production of the melatonin antidepressant.
Owner:EAST CHINA NORMAL UNIVERSITY
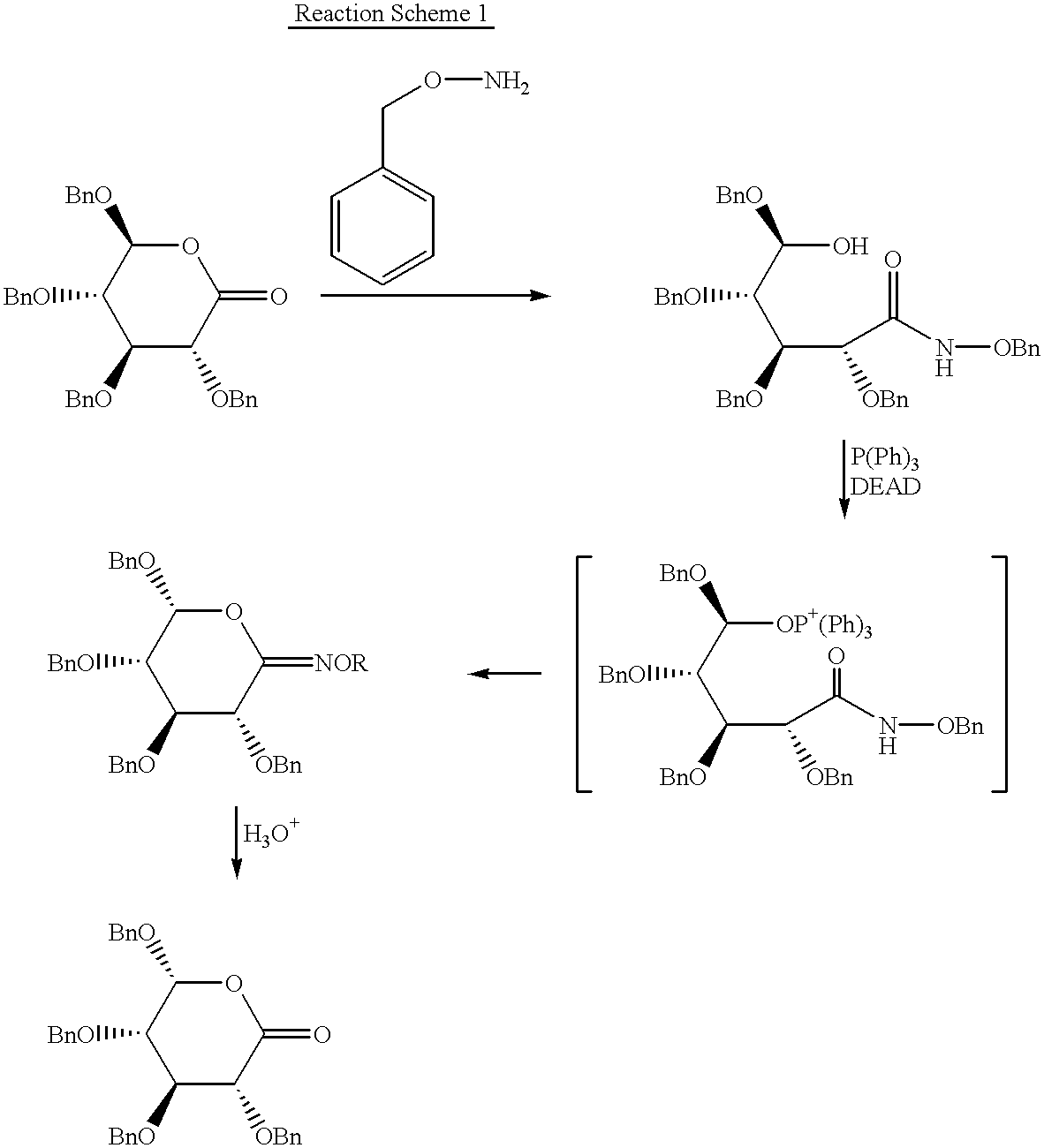
Chirality conversion method in lactone sugar compounds
InactiveUS6448415B1Process stabilityHigh reaction yieldSugar derivativesSugar derivatives preparationRiboseSugar
The present invention relates to a new process for effectively converting the chirality of 4- or 5-position carbon of a 1,4-lactone- or 1,5-lactone sugar compound which comprises reacting the lactone compound with secondary amine and sulfonyl group-containing compound. The compound of which chirality is converted according to the present invention can be advantageously used for preparing such expensive rare sugar compounds as L-ribose, D-talose, etc.
Owner:HANCHEM
Method for synthesizing aldehyde substituted small ring amines compounds with high enantioselectivity and 3-substituted lactams compounds with optical activity
InactiveCN101125817AIncrease production capacityEasy to handleOrganic chemistryEnantio selectivityMetal
The invention provides a method of a racemoid aldehyde group-ubstituted small cyclic amine compound, a high efficiency high enantio-selectivity synthesized chiral aldehyde group-substituted small cyclic amine compound and the synthesis of 3-substituted lactam compound with certain optical activity. Compared with the existing method, the method has wide adaptive substrate, and the catalyst is easily acquired, the reaction condition is mild, the operation is simple and convenient and the reaction efficiency is high. No adding of any metal salt compound is required, thus facilitating the production and the processing of the medicine.
Owner:SHANGHAI INST OF ORGANIC CHEM CHINESE ACAD OF SCI
Preparation process of herbicide dicamba
InactiveCN102125035AReduce pollutionHigh reaction yieldBiocideAnimal repellantsMethoxylaricinolic acidSalicylic acid
The invention relates to a preparation process of herbicide dicamba, in particular to a supercritical preparation process. The preparation process of the herbicide dicamba comprises the following steps of: preparing 2,5-dichlorophenol into corresponding sodium phenolate, and then completing carboxylation reaction under a supercritical condition to obtain 3,6-dichlorosalicylic acid; and then completing O-methylation to prepare a product of 3,6-dichloro-2-methoxy salicylic acid, i.e. the dicamba, by taking dimethyl carbonate as a reagent. The invention changes the heterogeneous gas-solid reaction of the traditional process into homogeneous reaction under the supercritical condition, carries out the O-methylation reaction by using the dimethyl carbonate and has the advantages of high reaction yield, good product quality, reduced environmental pollution, lowered energy consumption, and the like.
Owner:上海力智生化科技有限公司
Method for preparing hydroxymethyl-furfural from glucide under low temperature and normal pressure
InactiveCN101475543AReduce equipment costsReduce manufacturing costOrganic chemistryOrganic solventDistillation
The invention provides a method for preparing hydroxymethylfurfural by saccharide at low temperature and normal pressure. The method comprises the following steps: mixing a saccharide raw material and a reaction solvent in a mass ratio of 1:3-50; heating the mixed solvent to a temperature of between 100 and 180 DEG C; adding a catalyst which is 0.01 to 2 percent of the mass of the solvent; reacting in an atmosphere of inert gas protection for 30 minutes to 10 hours; cooling a reaction system; extracting the hydroxymethylfurfural by an organic solvent, and generating the hydroxymethylfurfural by reaction; drying the hydroxymethylfurfural by distillation; and crystallizing the hydroxymethylfurfural to obtain the product. The method has the advantages of high selectivity, high yield, and low cost.
Owner:SHANXI INST OF COAL CHEM CHINESE ACAD OF SCI
Amide-Substituted Silicones and Methods for their Preparation and Use
InactiveUS20080063879A1High reaction yieldLarge particle sizeSilicon organic compoundsFatty acid chemical modificationOrganosiliconMedicine
Owner:DOW CORNING CORP
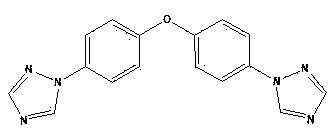
Phenyl bistriazole compound, and preparation method and application thereof
InactiveCN103086988AThe reaction is easy to operateHigh reaction yieldPolycrystalline material growthOrganic chemistrySimple Organic CompoundsBis triazole
The invention discloses a phenyl bistriazole compound, and a preparation method and application thereof, and particularly relates to a 1-[4-(1H-1,2,4-triazole-1-yl)phenyl]-1H-1,2,4-triazole single crystal and a preparation method of 1-[4-(1H-1,2,4-triazole-1-yl)phenyl]-1H-1,2,4-triazole. The organic compound is prepared by heating ethylenediamine, 1H-1,2,4-triazole, potassium carbonate, 1,4-diiodobenzene and cuprous iodide through a one-pot method. The preparation method disclosed by the invention has the characteristics simple process operation, low production cost and less environmental pollution, and is suitable for large-scale industrial production. The 1-[4-(1H-1,2,4-triazole-1-yl)phenyl]-1H-1,2,4-triazole single crystal prepared by the invention can be used in the aspect of photoelectric materials, especially dye and luminescent agents.
Owner:TIANJIN NORMAL UNIVERSITY
Preparation method of methylene diphenyl diisocyanate
ActiveCN106748887AImprove conversion rateInhibition of byproductsIsocyanic acid derivatives preparationOrganic compound preparationReaction rateOrganic phosphorus
The invention provides a preparation method of methylene diphenyl diisocyanate. The method comprises the following steps: performing a salt forming reaction on m-xylylenediamine and hydrogen chloride gas to obtain m-xylylenediamine hydrochloride; mixing the m-xylylenediamine hydrochloride, an organic phosphorus compound and solid phosgene for reaction to obtain methylene diphenyl diisocyanate. In a process of preparing methylene diphenyl diisocyanate, according to the application, an organic phosphorus compound additive is introduced, so that the reaction of m-xylylenediamine hydrochloride is promoted, therefore, the reaction rate and the effective conversion rate are improved.
Owner:SHANDONG EFIRM BIOCHEMISTRY & ENVIRONMENTAL PROTECTION CO LTD
Production of multiple unsaturated fatty acid phytosterin ester
InactiveCN1982326AIncrease contentHigh reaction yieldSteroids preparationVegetable oilOrganic solvent
Production of unsaturated fatty acid plant sterol ester is carried out by alcoholysis reacting for glycerin trilaurate of vegetable oil under action of methanol and catalyst to obtain fatty acid methyl ester, ester exchange reacting for fatty acid methyl ester and plant sterol under action of catalyst, removing un-reacted methanol and fatty acid methyl ester to obtain crude unsaturated fatty acid plant sterol ester, de-coloring and deodorizing to obtain the refined final product. It's cheap, harmless and non-toxic and can be used in food-industry production.
Owner:ZHEJIANG MEDICINE CO LTD XINCHANG PHAMACEUTICAL FACTORY
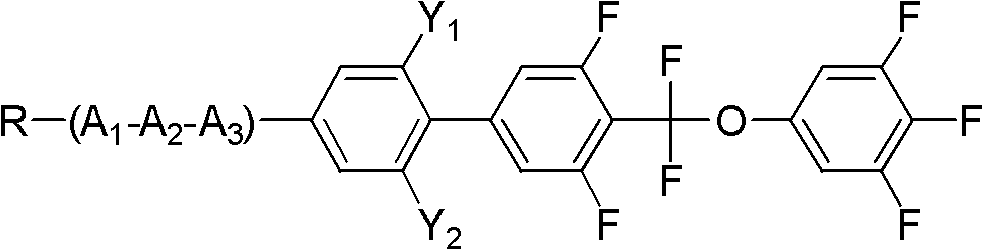
Method for preparing difluoromethoxy bridge type liquid crystal
InactiveCN102675062AFew reaction stepsHigh reaction yieldLiquid crystal compositionsOrganic chemistryReaction intermediateBoric acid
The invention discloses a method for preparing a difluoromethoxy bridge type liquid crystal compound, which comprises the following steps: uniformly mixing a compound shown in a formula II and a compound shown in a formula III with alkali under the condition that a catalyst exists for reaction and obtaining a compound shown in a formula I after reaction. The method has the beneficial effects that a reaction substrate is changed into the compound shown in the formula II and a phenylo boric acid derivative shown in the formula III, the reaction steps are reduced, a reaction intermediate is simplified, the total reaction yield is greatly improved, and different group compounds can use the same intermediate formula I. The method is particularly suitable for preparing the difluoromethoxy bridge type liquid crystal compound. Formula II, formula III and formula I are shown in the instruction.
Owner:SHIJIAZHUANG CHENGZHI YONGHUA DISPLAY MATERIALS CO LTD
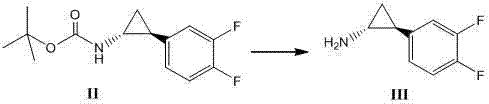
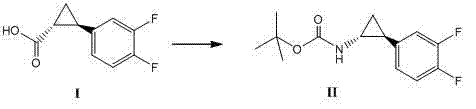
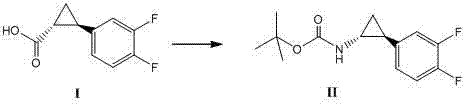
Method for synthesizing trans-(1R,2S)-2-(3,4-difluorophenyl)cyclopropylamine
InactiveCN102249929AReduce difficulty of reactionHigh reaction yieldOrganic compound preparationAmino compound preparationBiochemical engineeringTert-Butyloxycarbonyl protecting group
The invention relates to a method for synthesizing trans-(1R,2S)-2-(3,4-difluorophenyl)cyclopropylamine which is an intermediate for preparing an anticoagulation medicine Ticagrelor. The method provided by the invention mainly comprises the following steps of: synthesizing the trans-(1R,2S)-2-(3,4-difluorophenyl)cyclopropylamine protected by tertiarybutoxy carbonyl through carrying out a rearrangement reaction of DPPA (Diphenylphosphoryl Azide); then removing the protective group of the trans-(1R,2S)-2-(3,4-difluorophenyl)cyclopropylamine protected by the tertiarybutoxy carbonyl and then alkalifying to obtain the product. The whole reaction can be finished through a one-pot boiling synthetic method so that synthesizing steps and synthesizing time are greatly saved, the cost is effectively reduced and the yield is improved; and the method for synthesizing the trans-(1R,2S)-2-(3,4-difluorophenyl)cyclopropylamine has the very active meaning in the industrial production.
Owner:BRIGHTGENE BIO MEDICAL TECH (SUZHOU) CO LTD
Efficient cellulose nanocrystalline preparing method
ActiveCN105777913AHigh yieldImproved particle size distributionPulp properties modificationWashing/displacing pulp-treating liquorsInorganic saltsCellulose
The invention provides an efficient and quick cellulose nanocrystalline preparing method.According to the method, a cellulose raw material is pretreated with aqueous alkali and then subjected to acidolysis, and inorganic salt containing metal ions is added during acidolysis to serve as a promoter to promote acidolysis reaction.Reaction condition is mild and easy to control, raw materials needed by reaction are simple and easy to obtain, and the method can be widely applied to preparation of cellulose nanocrystalline from various cellulose raw materials.
Owner:QINGDAO UNIV OF SCI & TECH
Process for producing optically active amino compounds
InactiveUS6221638B1Efficient and inexpensiveHigh yieldTransferasesFermentationCo presenceStereoselectivity
The method for preparing an optically active (R)-amino compound characterized by the method comprising stereoselectively carrying out amino group transfer by action of an (R)-form-specific transaminase in the co-presence of a ketone compound (amino acceptor), and an amino compound (amino donor) of a racemic form or an (R)-form, to give an optically active (R)-amino compound. According to the present invention, it is made possible to easily prepare at a high yield the optically active (R)-amino compounds and the like having an aryl group and the like at their 1-position, which have been conventionally difficult to prepare.
Owner:KANEKA CORP
Porous carbon-loaded composite material catalyst as well as preparation method and application thereof
ActiveCN106732549AImprove transfer abilityModulation distribution characteristicsOrganic compound preparationAmino compound preparationIridiumPorous carbon
The invention discloses a porous carbon-loaded composite material catalyst as well as a preparation method and application thereof. The catalyst consists of a carrier, an active component and a carbon quantum dot, wherein the active component and the carbon quantum dot are loaded on the carrier; the size of the carbon quantum dot is not more than 10 nm; the carrier is porous activated carbon; the active component is one of or a combination of several of platinum, palladium, iridium, ruthenium and rhodium; based on the mass of the carrier, the loading quantity of various metals in the active component is shown as follows: the platinum is 0-10.0 percent by weight, the palladium is 0-10.0 percent by weight, the iridium is 0-10.0 percent by weight, the ruthenium is 0-10.0 percent by weight, and the rhodium is 0-5.0 percent by weight; the loading quantity of the platinum, the rhodium and the palladium is not 0; the total loading quantity of the active component is more than 0.5 percent by weight and is not more than 20 percent by weight; based on the mass of the carrier, the loading quantity of the carbon quantum dot is not more than 15.0 percent by weight. The invention further provides application of the porous carbon-loaded composite material catalyst to the reaction of synthesis of chloroaniline by selective catalytic hydrogenation of chloro-nitrobenzene. The catalyst has the characteristics of being high in conversion rate, high in catalytic activity and high in stability.
Owner:ZHEJIANG UNIV OF TECH
Producing optically active amino compounds
InactiveUS20020192786A1Efficient and inexpensiveSuitable for useBacteriaSugar derivativesMicroorganismNucleotide
To provide a method for preparing optically active compounds, which mainly contain (R)-amino compounds, by microbial enzymes efficiently and inexpensively; a polypeptide having stereoselective transaminase activity which can be suitably used for the above preparation method; and a DNA encoding the polypeptide. A method for preparing an optically active amino compound, characterized in stereoselectively transaminating by acting a transaminase on an amino group acceptor, a ketone compound in the presence of an amino group donor, a primary amine; a DNA comprising a nucleotide sequence encoding a polypeptide having stereoselective transaminase activity; and a polypeptide having stereoselective transaminase activity obtainable from a culture of a microorganism belonging to the genus Arthrobacter.
Owner:KANEKA CORP
Nitrogen-doped activated carbon supported noble metal catalyst and application thereof
ActiveCN107413331AEnhanced electron transfer capabilityRedistribution of electronsOrganic compound preparationAmino compound preparationActivated carbonIridium
The invention discloses a nitrogen-doped activated carbon supported noble metal catalyst and an application thereof. The catalyst is composed of a carrier and an active component; the carrier is nitrogen-doped activated carbon; the active component is one of palladium, platinum, rhodium, ruthenium and iridium; and the load of the active component is not higher than 15wt% based on the mass of the carrier. The invention provides the application of the nitrogen-doped activated carbon supported noble metal catalyst to reaction for preparing an alkyl-containing halogenated aromatic aminocompound shown as a formula (II) by carrying out catalytic hydrogenation reduction on an alkyl-containing halogenated aromatic nitrocompound shown as a formula (I) to show that the nitrogen-doped activated carbon supported noble metal catalyst has the characteristics of high hydrogenation reaction rate, high catalytic activity, high selectivity and high stability.
Owner:ZHEJIANG UNIV OF TECH
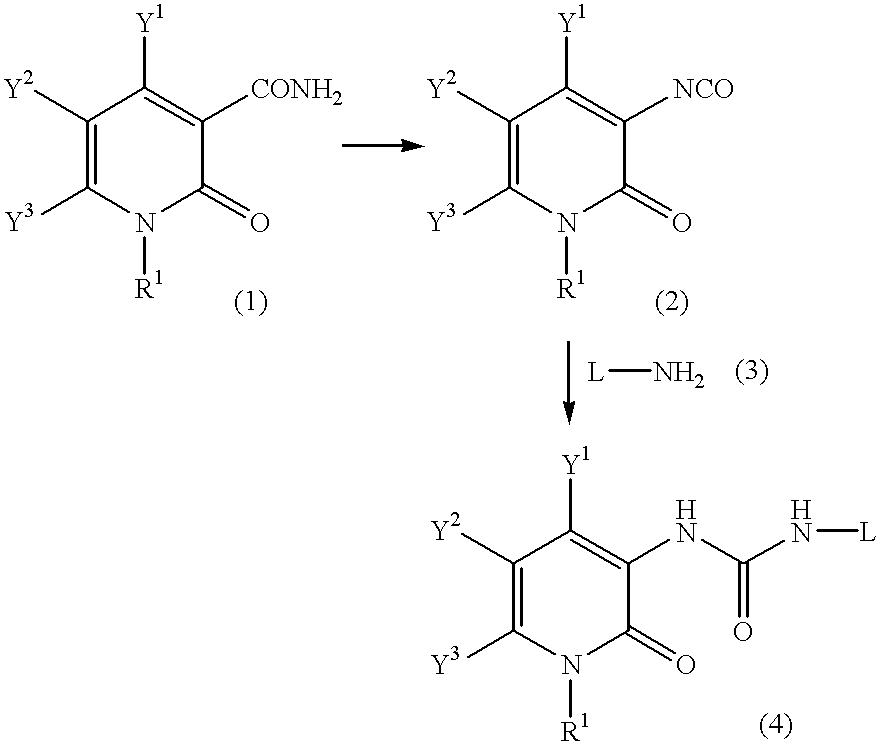
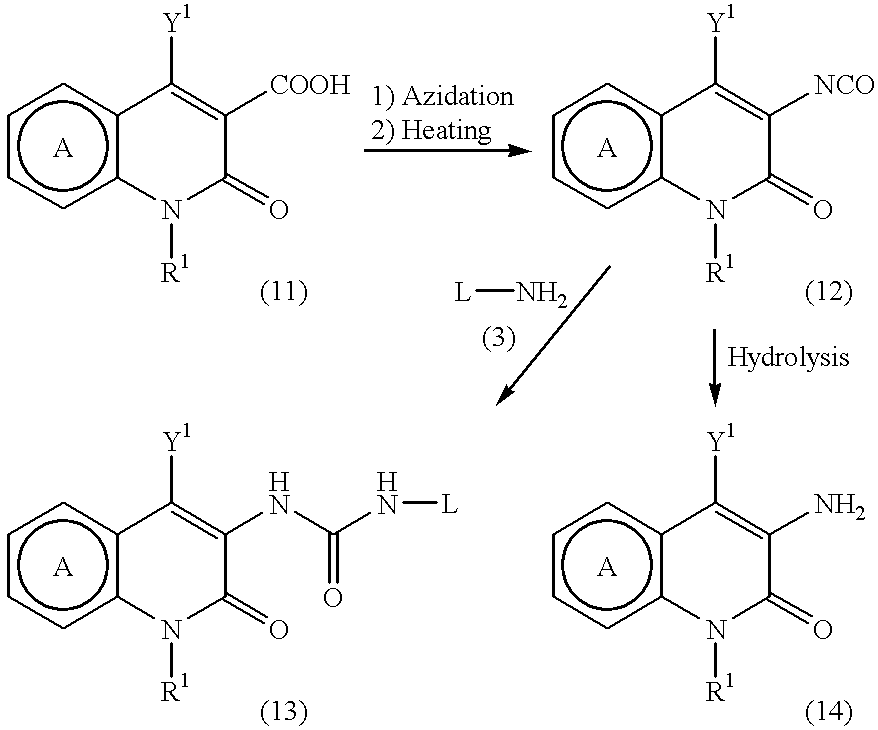
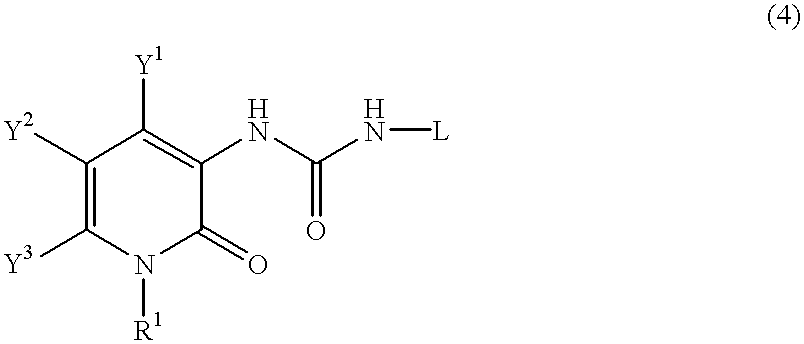
Pyridone derivatives and process for preparing the same
A process for preparing a pyridone derivative (4), which comprises reacting the compound (1) with a hypochlorite or a hypobromite or with lead tetraacetate to give the compound (2), and reacting the compound (2) with the compound (3). Said process is preferably especially from the standpoint of safety. wherein R1 is hydrogen, alkyl, substituted alkyl, etc.; Y1 is hydrogen, alky, substituted alky, etc.; Y2 and Y3 are indenpently hydrogen, halogen, etc.; and L is alkyl, substituted alkyl, etc.
Owner:SUMITOMO DAINIPPON PHARMA CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com