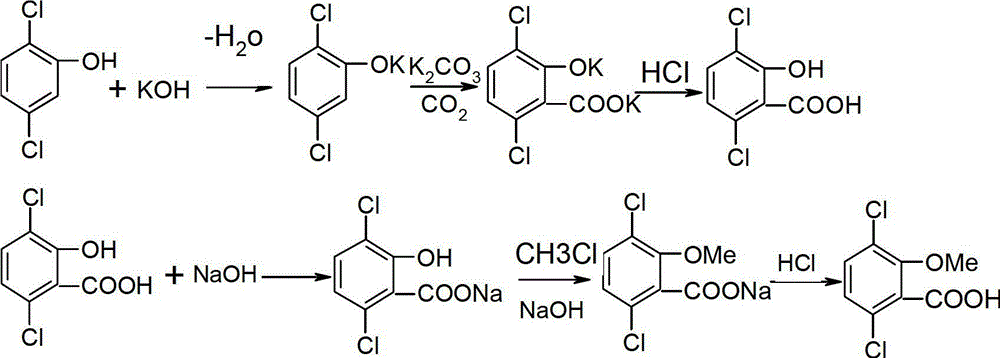
Synthetic process of herbicide dicamba
A technology for dicamba and herbicides, which is applied in the preparation of organic compounds, the preparation of carboxylates, organic chemistry, etc., can solve the problems of long reaction time, high energy consumption and low yield, etc., and achieves long reaction time and single-pass yield. Low rate and good product quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] 1) Synthesis of potassium 2,5-dichlorophenate
[0028] 300g of anhydrous toluene and 65g of 2,5-dichlorophenol were added to the 500ml reaction flask, 21.2g of KOH was added under stirring at room temperature, and the reaction was continued for 1hr-2hr. After removing the water, cool down. A toluene solution of potassium 2,5-dichlorophenolate was obtained almost quantitatively.
[0029] 2) Synthesis of 3,6-dichlorosalicylic acid
[0030] 379g of the toluene solution of potassium 2,5-dichlorophenolate obtained in step 1), 96g of powdered anhydrous potassium carbonate, 3g of butanol and 3g of triethylamine were added to a 500ml autoclave. After introducing an appropriate amount of carbon dioxide, start heating and stirring, control the temperature in the kettle to 130 ° C, and slowly introduce CO 2 to a pressure of 5.0MPa. Add CO slowly as the reaction progresses 2 To ensure that the temperature and pressure in the kettle are maintained at 130°C and 5.0MPa. And in t...
Embodiment example 2
[0034] 1) Synthesis of potassium 2,5-dichlorophenate
[0035] 300g of anhydrous xylene and 65g of 2,5-dichlorophenol were added to the 500ml reaction flask, 21.2g of KOH was added under stirring at room temperature, and the reaction was continued for 1hr-2hr. After removing the water, cool down. An almost quantitative xylene solution of potassium 2,5-dichlorophenolate was obtained.
[0036] 2) Synthesis of sodium 3,6-dichlorosalicylate
[0037] 80.1 g of dried and ground potassium 2,5-dichlorophenate, 96 g of powdered anhydrous potassium carbonate, 2.8 g of octanol and 4.2 g of diisopropylamine were added to a 500 ml autoclave. After introducing an appropriate amount of carbon dioxide, start heating and stirring, control the temperature in the kettle to 135 ° C, and slowly introduce CO 2 to a pressure of 6.0MPa. Add CO slowly as the reaction progresses 2 To ensure that the temperature and pressure in the kettle are maintained at 135°C and 6.0MPa. And keep in this state f...
Embodiment 3
[0041] 1) Synthesis of potassium 2,5-dichlorophenate
[0042] 300g of anhydrous toluene and 65g of 2,5-dichlorophenol were added to the 500ml reaction flask, 22.2g of KOH was added under stirring at room temperature, and the reaction was continued for 1hr-2hr. After removing the water, cool down. A toluene solution of potassium 2,5-dichlorophenolate was obtained almost quantitatively.
[0043] 2) Synthesis of sodium 3,6-dichlorosalicylate
[0044] 379g of the toluene solution of potassium 2,5-dichlorophenolate obtained in step (1), 96g of powdered anhydrous potassium carbonate, 4g of butanol and 2g of triethylamine were added to a 500ml autoclave. After introducing an appropriate amount of carbon dioxide, start heating and stirring, control the temperature in the kettle to 140 ° C, and slowly introduce CO 2 to a pressure of 6.0MPa. Add CO slowly as the reaction progresses 2 To ensure that the temperature and pressure in the kettle are maintained at 140°C and 6.0MPa. And ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com