Patents
Literature
79results about How to "Reduce difficulty of reaction" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
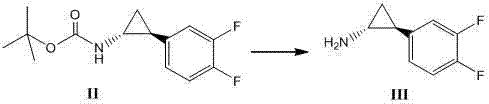
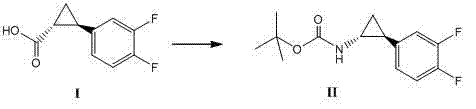
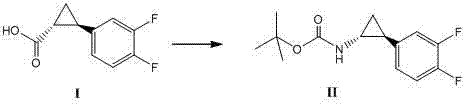
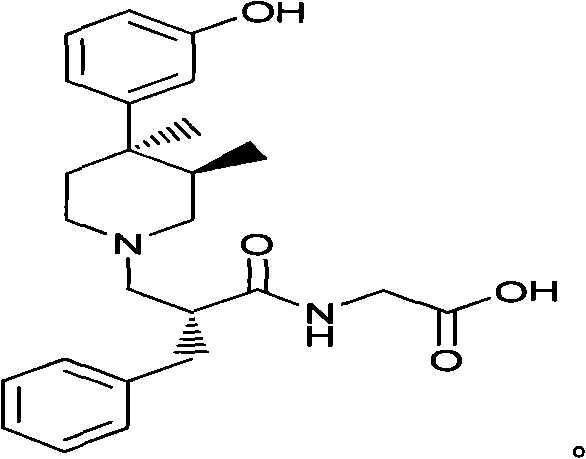
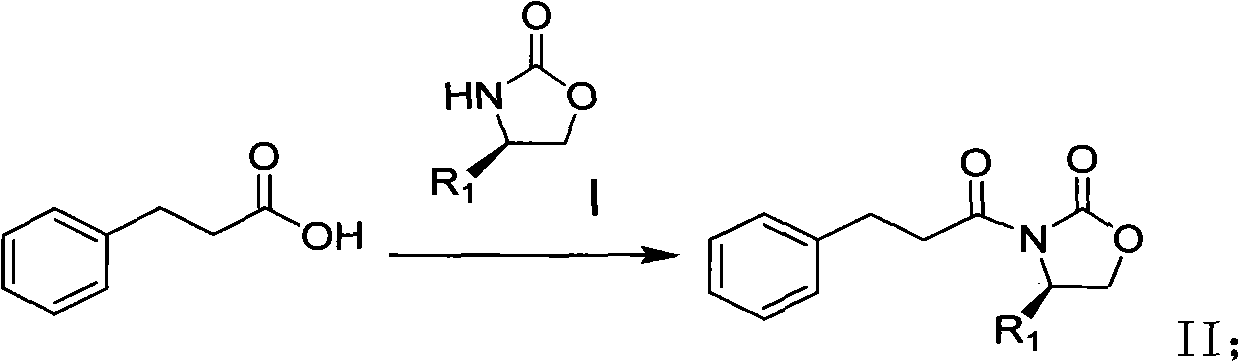
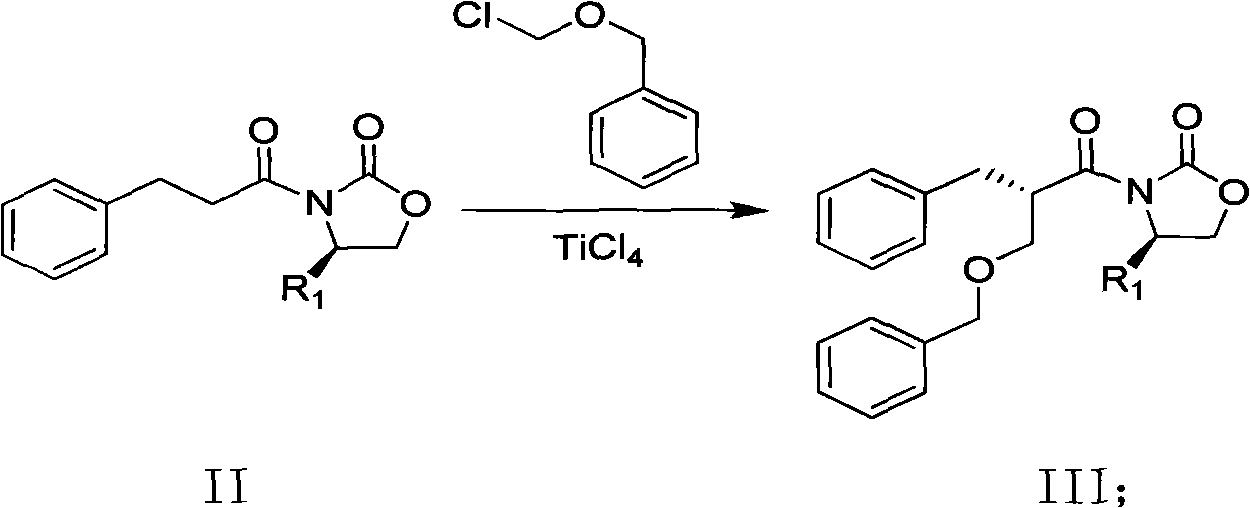
Method for synthesizing trans-(1R,2S)-2-(3,4-difluorophenyl)cyclopropylamine
InactiveCN102249929AReduce difficulty of reactionHigh reaction yieldOrganic compound preparationAmino compound preparationBiochemical engineeringTert-Butyloxycarbonyl protecting group
The invention relates to a method for synthesizing trans-(1R,2S)-2-(3,4-difluorophenyl)cyclopropylamine which is an intermediate for preparing an anticoagulation medicine Ticagrelor. The method provided by the invention mainly comprises the following steps of: synthesizing the trans-(1R,2S)-2-(3,4-difluorophenyl)cyclopropylamine protected by tertiarybutoxy carbonyl through carrying out a rearrangement reaction of DPPA (Diphenylphosphoryl Azide); then removing the protective group of the trans-(1R,2S)-2-(3,4-difluorophenyl)cyclopropylamine protected by the tertiarybutoxy carbonyl and then alkalifying to obtain the product. The whole reaction can be finished through a one-pot boiling synthetic method so that synthesizing steps and synthesizing time are greatly saved, the cost is effectively reduced and the yield is improved; and the method for synthesizing the trans-(1R,2S)-2-(3,4-difluorophenyl)cyclopropylamine has the very active meaning in the industrial production.
Owner:BRIGHTGENE BIO MEDICAL TECH (SUZHOU) CO LTD
A kind of solid chlorine dioxide effervescent tablet and preparation method thereof
ActiveCN102283246AFast dissolutionImprove responsivenessBiocideDisinfectantsDrying AgentsCorrosion inhibitor
The invention belongs to the technical field of chemical engineering and relates to a solid chlorine dioxide effervescent tablet and a preparation method thereof. The solid chlorine dioxide effervescent tablet is prepared from the following raw materials in parts by weight: 8-30 parts of chlorine dioxide matrix, 5-20 parts of halogen source, 0.5-2 parts of cladding passivant, 0-5 parts of corrosion inhibitor, 25-60 parts of acidifier, 0-2 parts of surfactant, 15-24 parts of drying agent and an effervescing agent. By adopting the solid chlorine dioxide effervescent tablet and the preparation method thereof, the safety and the stability of a product and the yield of chlorine dioxide are obviously improved. In the solid chlorine dioxide effervescent tablet and the preparation method thereof,fine chloride is adopted to serve as halogen source, and malic acid is adopted to serve as the the acidifier, thereby, the sediment of calcium salt is avoided, and the corrosivity of the solution of the calcium salt is reduced. Moreover, the safe reliability of the product applied to disinfecting foods and drinking water is ensured.
Owner:石家庄卫科生物科技有限公司
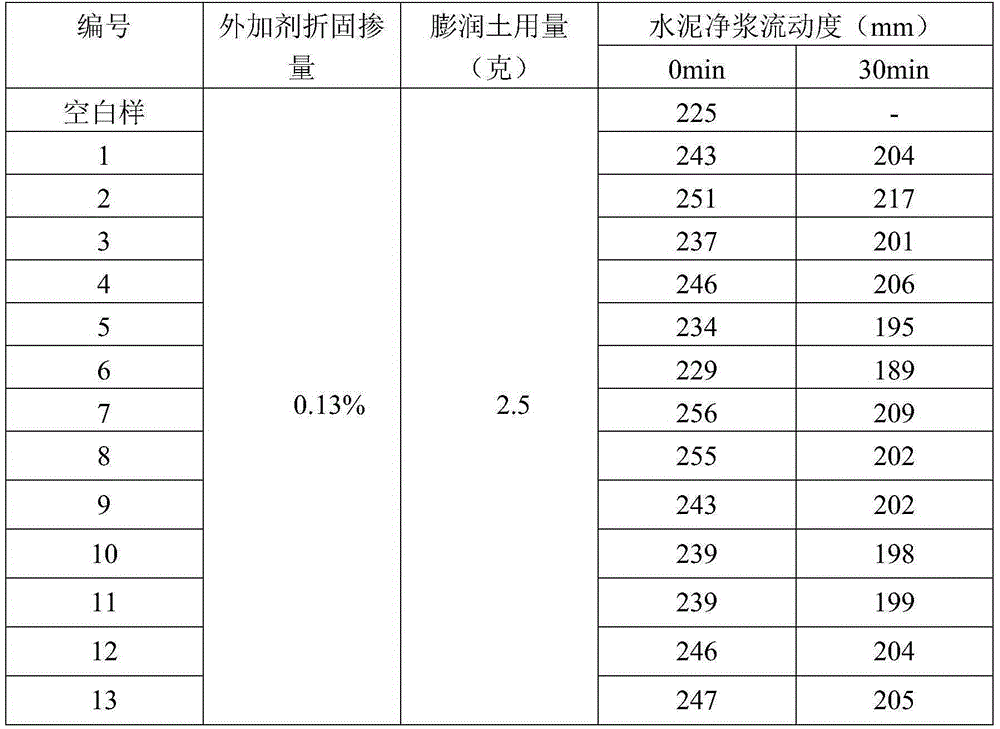
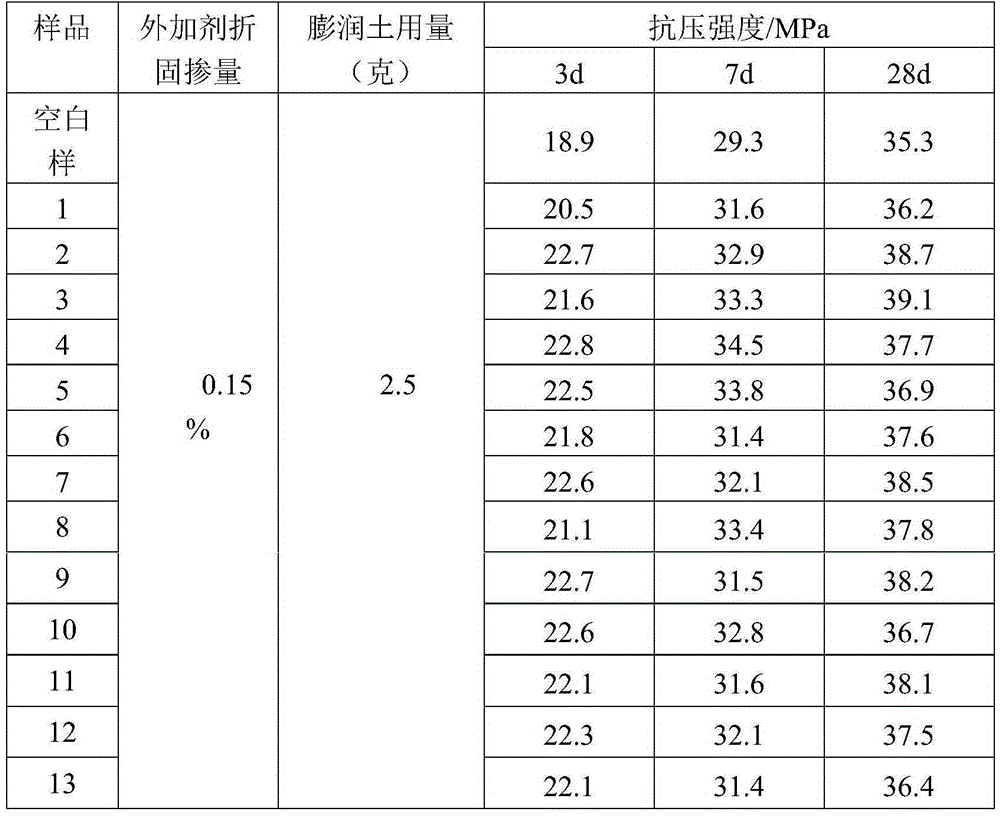
Preparing method for polycarboxylate-type anti-mud water reducer with modified side chain terminal group
ActiveCN105330834AReduce difficulty of reactionImprove reaction efficiencyWork performanceChemical reaction
The invention discloses a preparing method for polycarboxylate-type anti-mud water reducer with a modified side chain terminal group. The preparing method includes the steps that azido-terminated polycarboxylate-type water reducer is prepared first, and then the azido-terminated polycarboxylate-type water reducer and an alkyne-terminated benzene-pyridine derivative are subjected to a click chemical reaction to prepare the polycarboxylate-type anti-mud water reducer with the side chain terminal group being the alkyne-terminated benzene-pyridine derivative. As the terminal hydroxyl group of the polycarboxylate-type water reducer is modified into a five-membered ring and a benzene / pyridine ring, the physical size of the side chain terminal of the polycarboxylate-type water reducer is greatly increased, and the polycarboxylate-type water reducer is not prone to be inserted into a layered structure of clay to achieve the anti-mud effect without affecting the inherent functions. The technical problems that as a side chain of existing polycarboxylate-type water reducer is prone to be inserted into the layered structure of the clay, the polycarboxylate-type water reducer is lost fast, and then the work performance of concrete is lowered are solved.
Owner:JIANGSU CHINA RAILWAY ARIT NEW MATEIRALS CO LTD
Intermediate of alvimopan and synthesis method thereof
ActiveCN102127005AReduce dosageEasy to buyOrganic compound preparationSulfonic acid esters preparationPropanoic acidPtru catalyst
Owner:BRIGHTGENE BIO MEDICAL TECH (SUZHOU) CO LTD
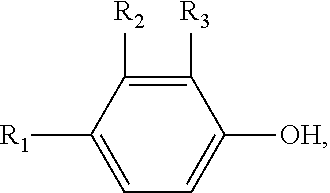
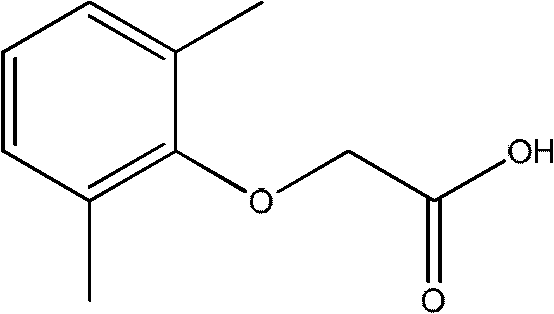
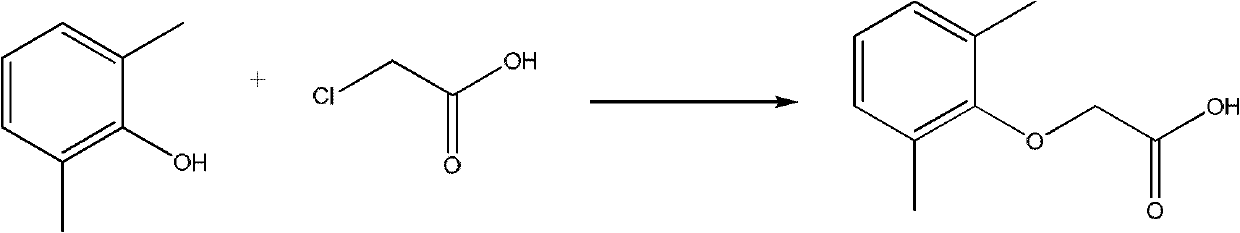
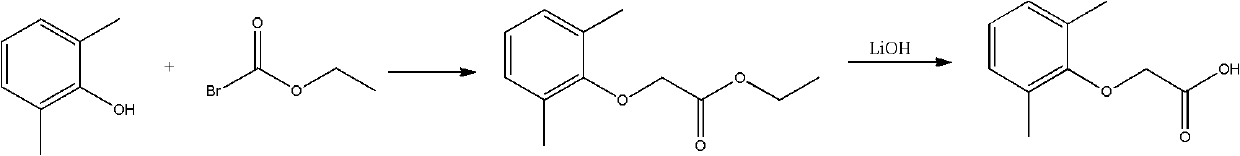
Method for synthesizing 2,6-dimethyl phenoxyacetic acid
InactiveCN101967092AReduce difficulty of reactionQuick responseOrganic compound preparationCarboxylic compound preparationSide productChemistry
The invention discloses a method for synthesizing 2,6-dimethyl phenoxyacetic acid, comprising the following steps of: (1) adding 2,6-dimethyl phenol to an alkali aqueous solution, wherein the 2,6-dimethyl phenol and alkali are added in the mole ratio of 0.5-1.0:1.0; (2) stirring to dissolve the mixture, then filtering after sufficient reaction, increasing the temperature of a filtering material and dewatering to obtain 2,6-dimethyl phenolate; (3) mixing the 2,6-dimethyl phenolate and chloroacetic acid in the mole ratio of 0.5-1.0:1.0, heating till the 2,6-dimethyl phenolate and the chloroacetic acid are completely melted, and reacting in an inert gas atmosphere; (4) after the reaction is finished, cooling to room temperature, adding water or alkali liquor for washing, and filtering to obtain a crude product; and (5) recrystallizing the crude product to obtain a 2,6-dimethyl phenoxyacetic acid pure product. The synthesizing method has the advantages of simple and convenient operation, short reaction time, easy postprocessing, few side product, high productivity, and the like.
Owner:厦门市亨瑞生化有限公司
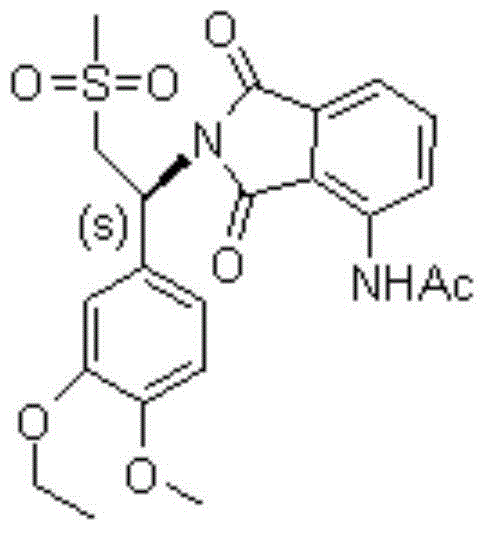
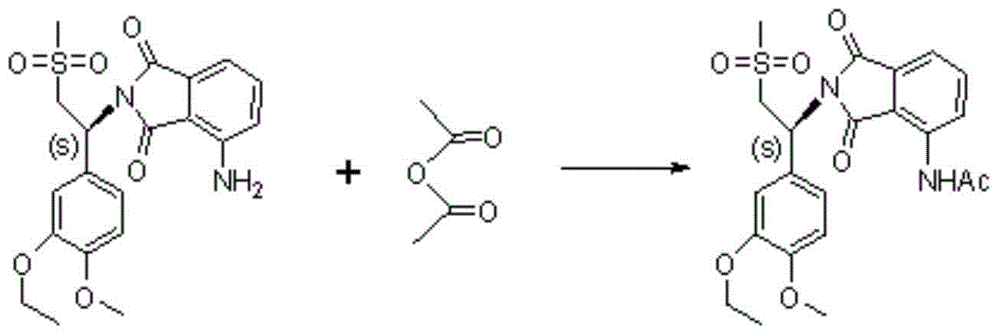
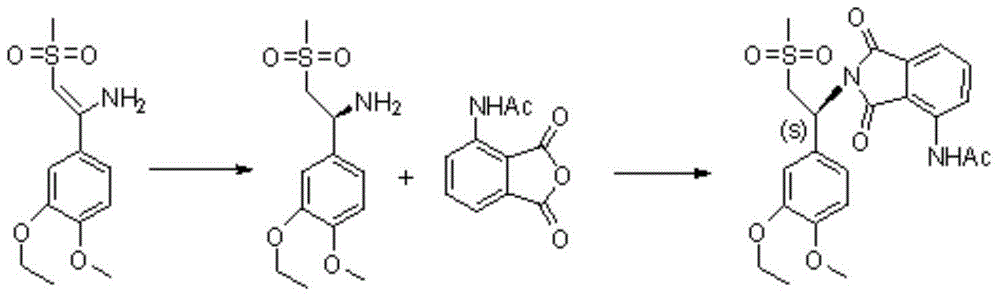
Preparation method of S-type apremilast
The invention discloses a preparation method of apremilast (+)-2-[1-(3-ethoxy-4-methoxyphenyl)-2-mesylethyl]-4-acetamidoisoindolinyl-1,3-dione. The method comprises the following steps: (1) converting a compound 1-(3-ethoxy-4-methoxyphenyl)-2-(methanesulfonyl)ethylamine racemate disclosed as Formula (II) into a salification substance disclosed as Formula (III) by using a resolving agent; and (2) connecting the compound disclosed as Formula (III) with 3-acetamidophthalic anhydride to obtain the compound disclosed as Formula (I). The method can be utilized to obtain the stable apremilast acceptable finished product, has the advantages of mild technological conditions, simple after-treatment, high purity and low reaction cost, and can easily implement industrial production.
Owner:NANJING CORE TECH CO LTD
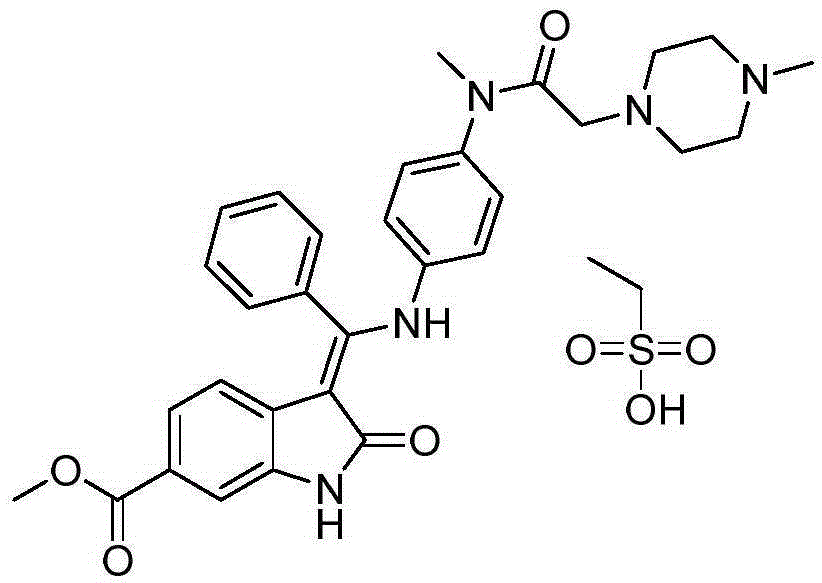
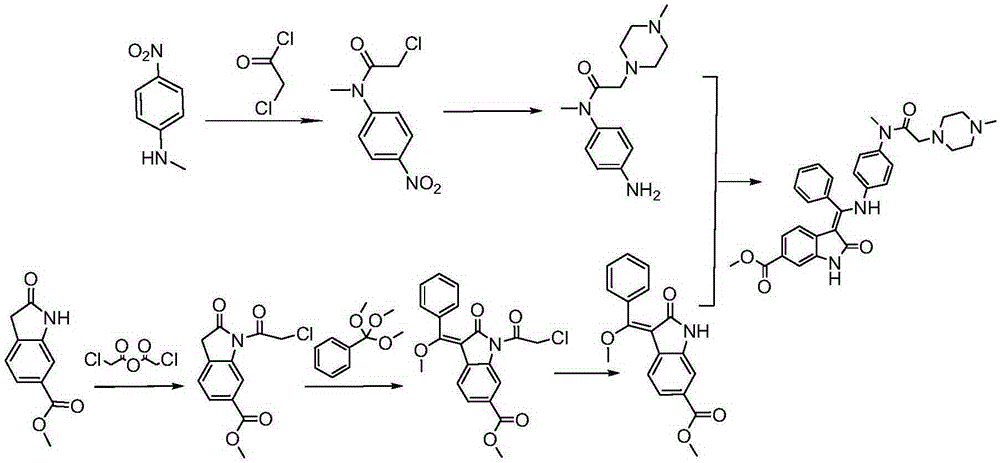
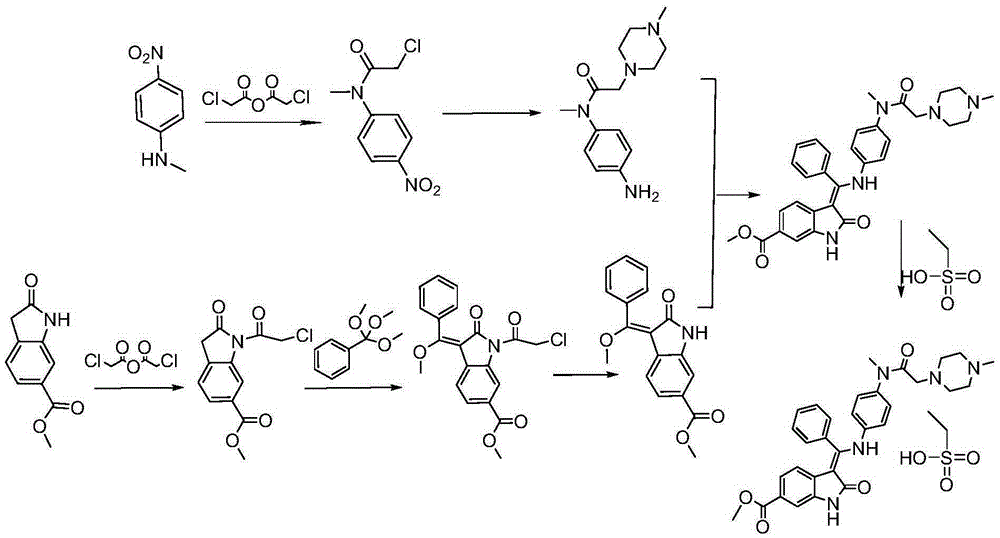
Preparation method of crystalline nintedanib esylate
InactiveCN105418483AReduce usageShort synthetic stepsSulfonic acids salts preparationEthanesulfonic acidSulfonate
The invention discloses a preparation method of crystalline nintedanib esylate (3-Z-[1-(4-(N-((4-methyl-piperazin-1-yl)-methylcarbonyl)-N-methyl-amino)-phenylamino)-1-phenyl-methylene]-6-methoxycarbonyl-2-dihydroindolone monoethyl sulfonate). The method comprises steps as follows: (1) a compound represented in the formula (B) and acylating chlorination reagent chloroacetic anhydride react, and acyl chloride (C) is obtained; (2) the compound represented in the formula (C) and trimethyl orthobenzoate have a condensation reaction, and a compound represented in the formula (D) is obtained; (3) the compound represented in the formula (D) is deprotected, and a compound represented in the formula (E) is obtained; (4) the compound represented in the formula (E) and N-(4-aminophenyl)-N-methyl-2-(4-methylpiperazin-1-yl) acetamide have a condensation reaction, and a compound represented in the formula (F) is obtained; (5) the compound represented in the formula (F) and ethanesulfonic acid have a salification reaction, and a nintedanib esylate compound represented in the formula (A) is obtained. The stable crystalline nintedanib esylate can be obtained with the method, technological conditions are mild, aftertreatment is simple, the purity is high, the reaction cost is low, and industrial production is easy to realize.
Owner:NANJING CORE TECH CO LTD
Preparation method of high-flowability positive ion modified copolyester
The invention relates to a preparation method of high-flowability positive ion modified copolyester. Binary acid I, dihydric alcohol I and high-flowability branched structure modifiers are uniformly mixed and then subjected to esterification reaction, and isophthalic acid-5-sodium sulfonate and dihydric alcohol II esterification products are led in after esterification reaction and subjected to pre-condensation reaction and final polycondensation reaction to prepare the high-flowability positive ion modified copolyester. The high-flowability branched structure modifiers are more than one of pyromellitic anhydride, cyclopentane tetracarboxylic anhydride, benzophenone tetracarboxylic dianhydride, trimellitic anhydride, mellophanic dianhydride, pyromellitic acid, cyclopentane tetracid, benzophenone tetracid, trimellitic acid and mellophanic acid, and the dihydric alcohol II is more than one of butanediol, pentanediol, hexanediol, heptanediol, octylene glycol, nonanediol and decanediol. Two modified components are introduced, so that flow-activation energy is remarkably reduced, acting force of the copolyester and tube walls in the flowing process is reduced, and melt viscosity loss iseffectively controlled.
Owner:DONGHUA UNIV
Preparation method of rosuvastatin intermediate and intermediate compound
InactiveCN104059024AAvoid it happening againReduce difficulty of reactionOrganic chemistryThioureaBenzaldehyde
The invention discloses a preparation method of a rosuvastatin intermediate: 4-(4-fluorobenyl)-6-isopropyl-2-(N-methyl-N-methylsulfonyl amino) pyrimidine-5-formonitrile. The method comprises the following steps: carrying out a reaction on ethyl isobutyrate and acetonitrile to generate isobutyryl acetonitrile; and then carrying out cyclization, methylation, reduction, oxidization and substitution on isobutyryl acetonitrile and p-fluoro benzaldehyde as well as thiourea to prepare the intermediate. The 4-(4-fluorobenyl)-6-isopropyl-2-(N-methyl-N-methylsulfonyl amino) pyrimidine-5-formonitrile prepared by the method is low in cost, simple to react, high in yield and easy to realize industrialized production.
Owner:ZHEJIANG UNIV
Preparation method of alpha-crystal form imatinib mesylate
InactiveCN103058991AShort synthetic stepsEasy to operateOrganic chemistryBenzoic acidOrganic solvent
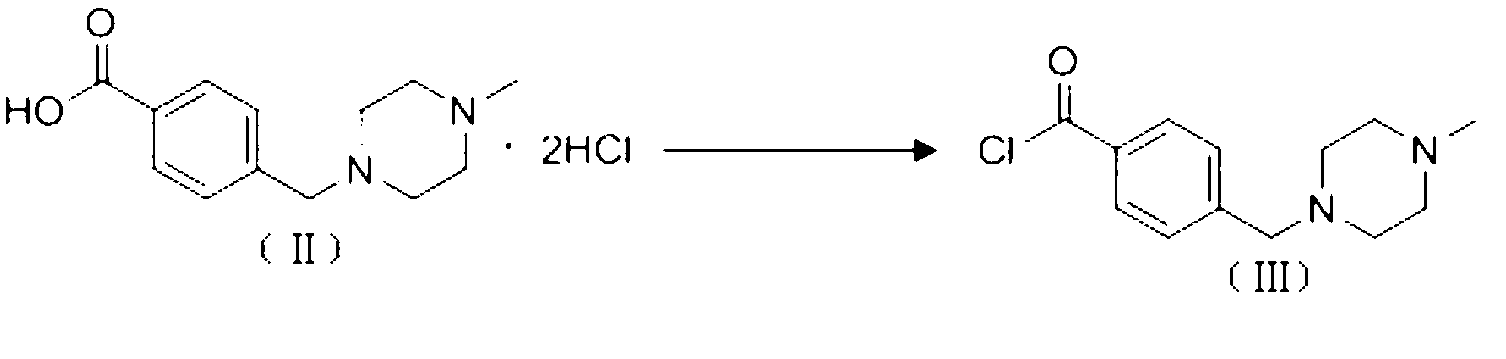
The invention discloses a preparation method of alpha-crystal form imatinib mesylate. The preparation method comprises the following steps that 1, 4-[(4-methyl piperazine-1-yl)methyl]benzoic acid dihydrochloride shown in the formula II is converted into an acyl chloride shown in the formula III by an acylating chlorination reagent; 2, the acyl chloride shown in the formula III and N-(5-amino-2-methylphenyl)-4-(3-pyridyl)-2-aminopyrimidin undergo a condensation reaction in the presence of an alkali to produce a compound shown in the formula IV; and 3, the compound shown in the formula IV and methylsulfonic acid undergo a salt forming reaction in the presence of an organic solvent to produce a compound shown in the formula I. The preparation method can realize preparation of stable alpha-crystal form imatinib mesylate and allows mild process conditions. The stable alpha-crystal form imatinib mesylate can be after-treated simply and has high purity. The preparation method has a low reaction cost and is suitable for industrial production.
Owner:NANJING CORE TECH CO LTD
Low-surface-alkalinity lithium nickel cobalt aluminate positive electrode material and preparation method thereof
InactiveCN108417796ALow structural firmnessSmall latticeCell electrodesSecondary cellsPhosphatePyrophosphate
The invention discloses a low-surface-alkalinity lithium nickel cobalt aluminate positive electrode material and a preparation method thereof. The lithium nickel cobalt aluminate positive electrode material is obtained by performing calcining on metal hydrogen phosphate and a lithium nickel cobalt aluminate positive electrode active material in oxygen flow. By adoption of metal hydrogen phosphatewhich is decomposed into pyrophosphate in calcining, reactant activity is improved, and a reaction with the residual alkali in the lithium nickel cobalt aluminate positive electrode active material ispromoted to generate a Li<3>PO<4> coating layer; compared with phosphate, hydrogen phosphate is lower in structural firmness, lower in lattice energy and easier in reaction, so that the calcining temperature can be obviously lowered; dry method coating of the lithium nickel cobalt aluminate positive electrode active material is converted from high temperature solid phase reaction (700 DEG C) in the prior art into a medium temperature solid phase reaction (400-600 DEG C), so that reaction difficulty is greatly lowered; and in addition, simple process, low cost and high production efficiency are achieved, so that realization of large-scale industrial production can be facilitated.
Owner:CHENGDU ORGANIC CHEM CO LTD CHINESE ACAD OF SCI +1
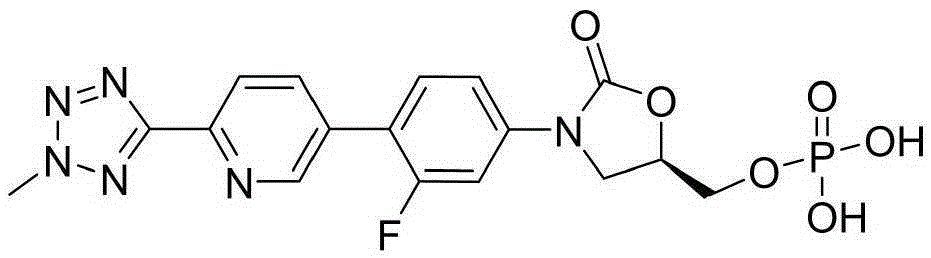
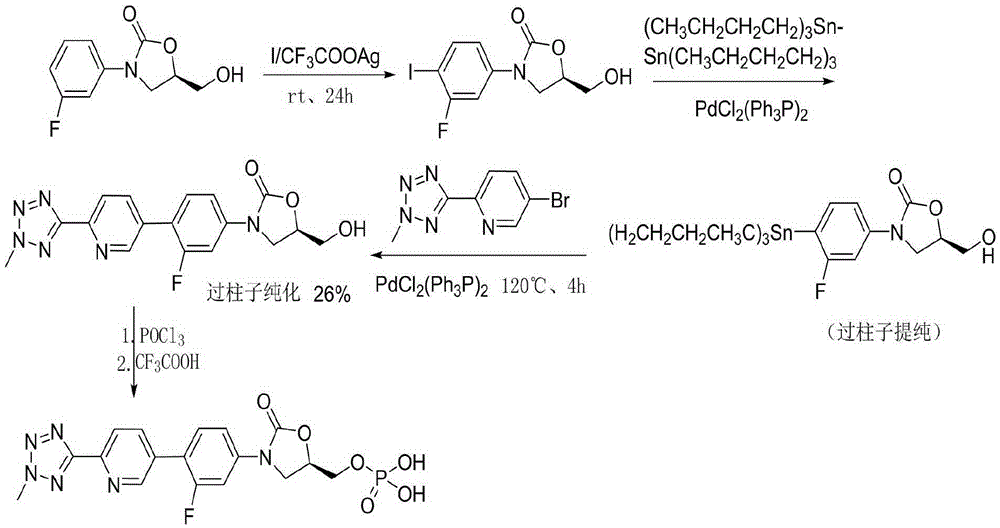
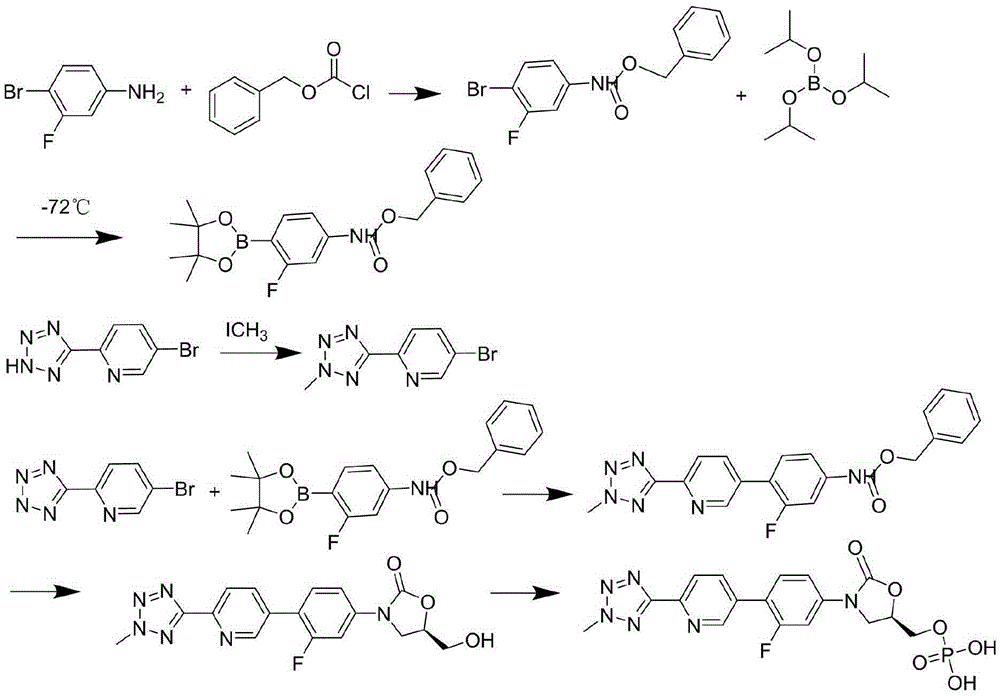
Preparation method of tedizolid phosphate
InactiveCN105418681AShort synthetic stepsEasy to operateGroup 5/15 element organic compoundsPhosphateBromine
The invention belongs to the technical field of synthesis of chemical medicines and particularly relates to a preparation method of tedizolid phosphate. (5R)-3-(4-bromo-3-fluorophenyl)-5-(hydroxymethyl)oxazolidin-2-one and 2-(2-methyl-2H-tetrazolyl-5-yl) pyridine-5-boronic acid pinacol ester are taken as raw materials, and (R)-3-(4-(2-(2-methyl tetrazole-5-yl) pyridine-5-yl)-3-fluorophenyl)-5-(hydroxymethyl)oxazolidin-2-ketone phosphate is prepared with a four-step method. The method has a mild process condition, aftertreatment is simple, the purity is high, the reaction cost is low, and industrial production is easy to realize.
Owner:NANJING CORE TECH CO LTD
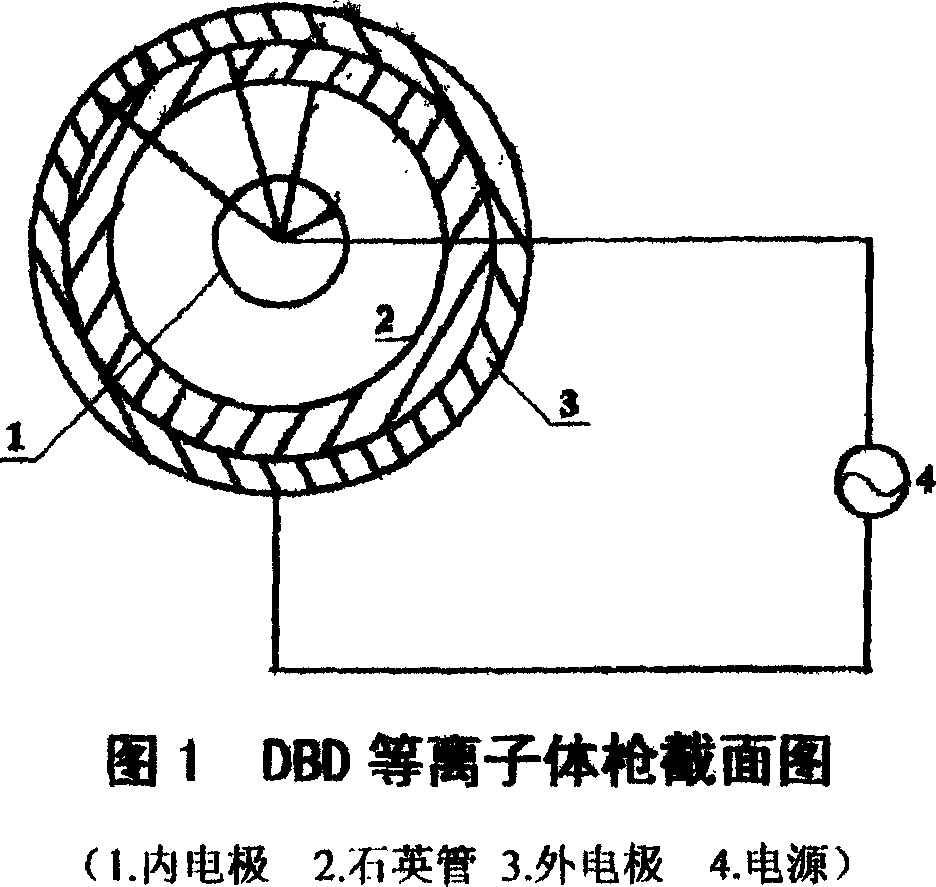
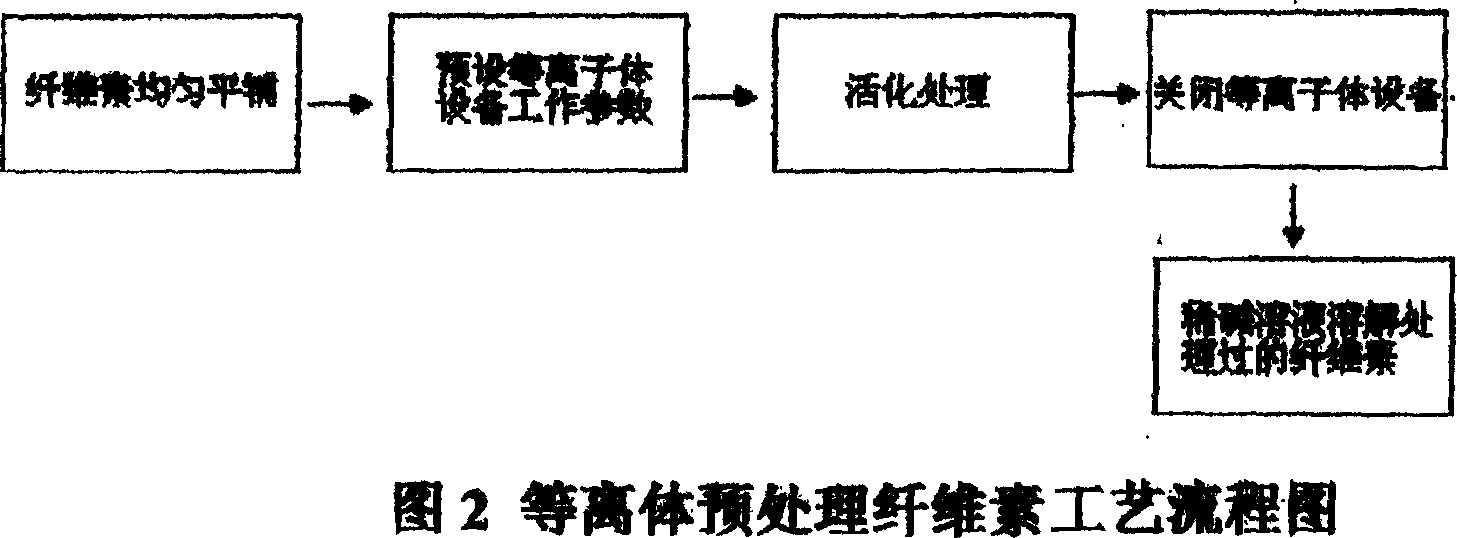
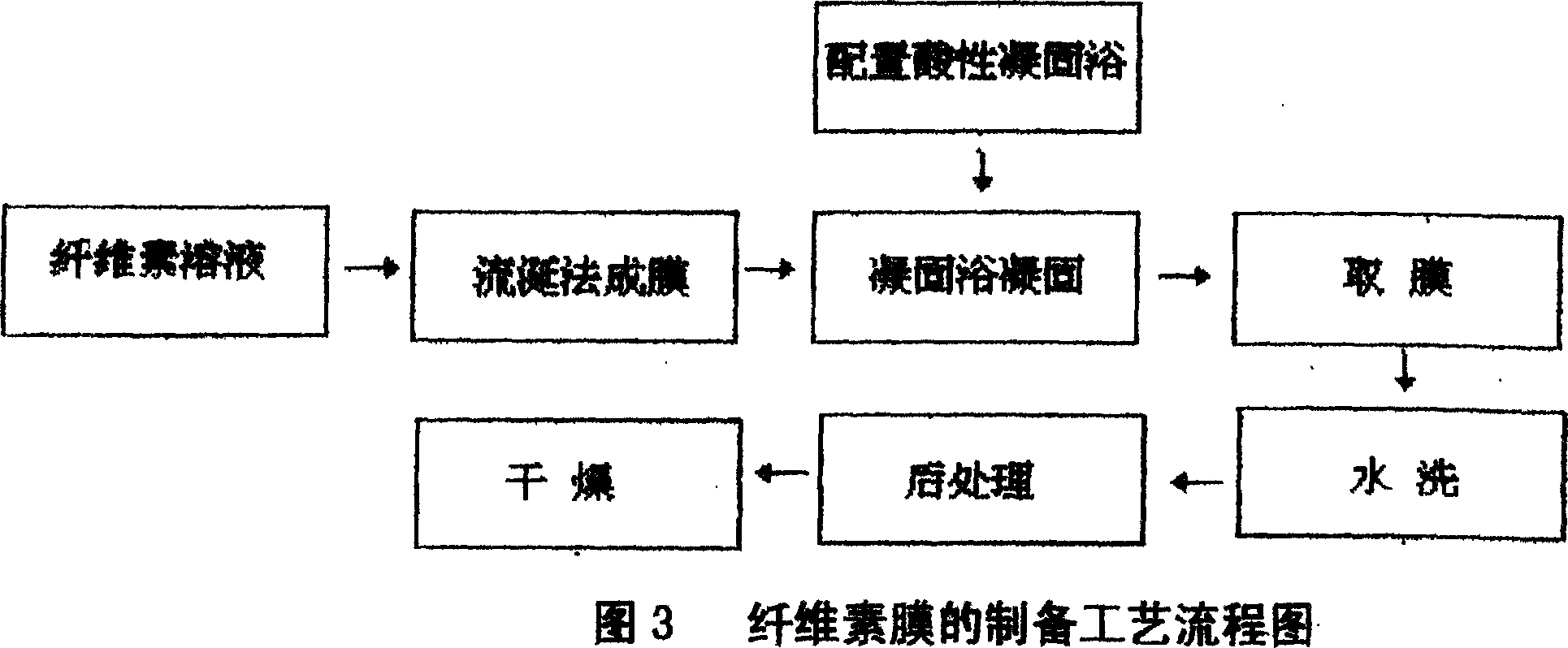
Preparation method of cellulose film
InactiveCN1948369ANo pollution in the processPotential to increase accessibilityCelluloseBiological activation
This invention relates to a preparation of cellulose membrane which is no pollution,low cost and naturally degradable,by the method of surface activation about cellulose by using hypothermic plasmas body to administer radiation and dissolving cellulose by using dilute alkali solution. The prepared cellulose membrane can be used in the area of packaging and printing.
Owner:BEIJING INSTITUTE OF GRAPHIC COMMUNICATION
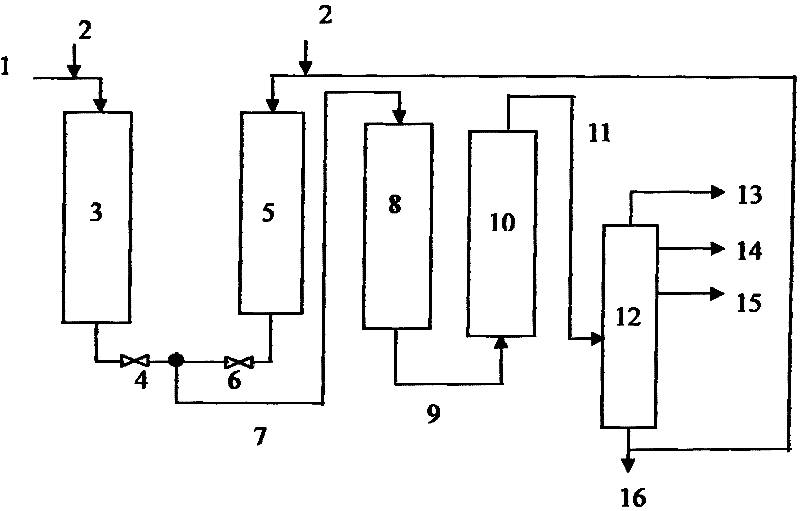
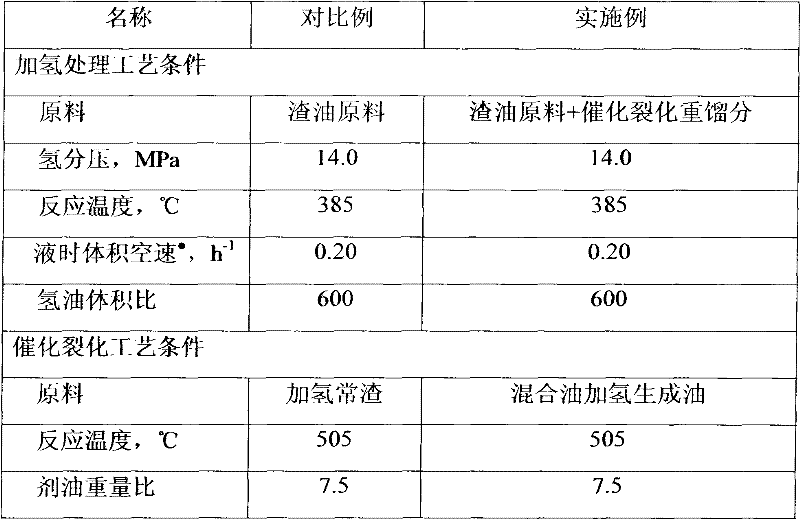
Residual oil hydrogenation treatment and catalytic cracking combination method
ActiveCN102453544AHigh aromatic contentLow cetane numberTreatment with hydrotreatment processesGas phaseFractionation
The present invention discloses a residual oil hydrogenation treatment and catalytic cracking combination method. According to the method, the residual oil hydrogenation treatment process comprises two hydrogenation protection reactors, wherein the two hydrogenation protection reactors are arranged in parallel connection, the feed material of the first hydrogenation protection reactor is the residual oil raw material, and the feed material of the second hydrogenation protection reactor is the catalytic cracking heavy fraction; the effluents of the two reactors are mixed, and the resulting mixture enters a hydrogenation treatment reaction zone to carry out a hydrogenation reaction; the effluent of the hydrogenation reaction is subjected to gas-liquid separation, wherein the resulting gas phase is circularly adopted for the hydrogenation reaction, and the liquid phase directly enters a catalytic cracking apparatus without fractionation; the effluent of the catalytic cracking reaction is separated to obtain dry gas, liquefied gas, catalytic cracking gasoline, and catalytic cracking heavy fraction after catalytic cracking of the gasoline, wherein the catalytic cracking heavy fraction is adopted as the feed material of the second hydrogenation protection reactor. With the method, the operating period of the residual oil hydrogenation apparatus can be prolonged, the maximum amount of the catalytic cracking gasoline can be produced, and the equipment investment can be saved.
Owner:CHINA PETROLEUM & CHEM CORP +1
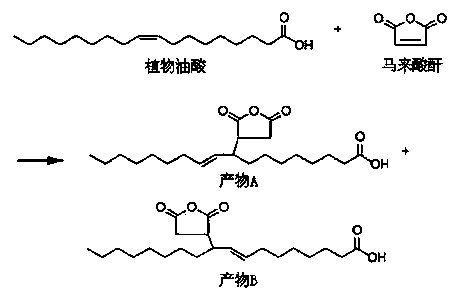
Preparation method of maleic anhydride grafted vegetable oil acid
ActiveCN103664841AHigh yieldReduce difficulty of reactionOrganic chemistryPolymer scienceVegetable oil
The invention discloses a preparation method of maleic anhydride grafted vegetable oil acid. The preparation method is characterized by comprising steps as follows: vegetable oil acid, maleic anhydride, a catalyst and a solvent are added into a reaction kettle and heated for an Alder-Ene reaction, unreacted raw materials and solvent are boiled off through pressure reduction after the reaction, and the maleic anhydride grafted vegetable oil acid is obtained. The preparation method is simple in process, raw materials are safe, non-toxic, easy to obtain and low in cost, the conditions are mild, and the obtained product is light in color and can be used for the next step, so that the industrialized implementation is facilitated.
Owner:山东艾孚特科技有限公司 +2
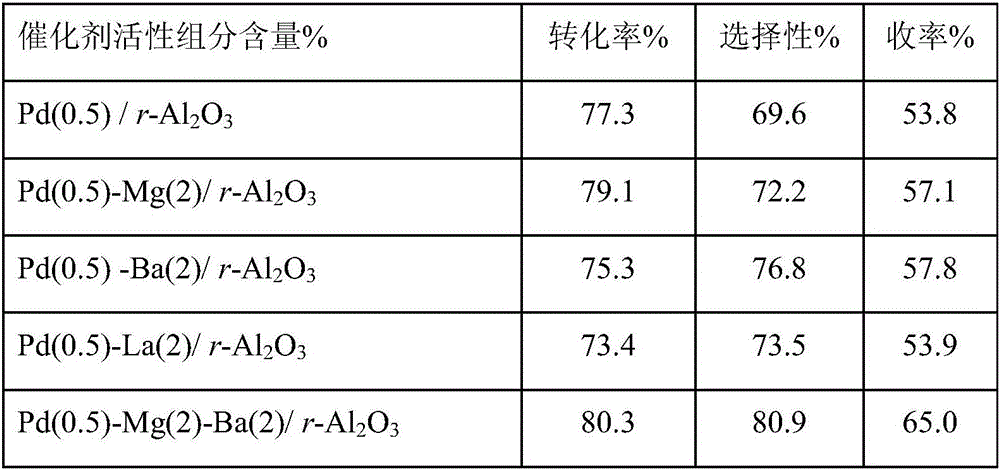
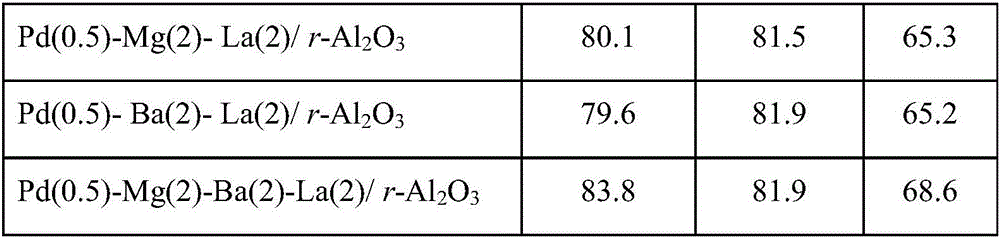
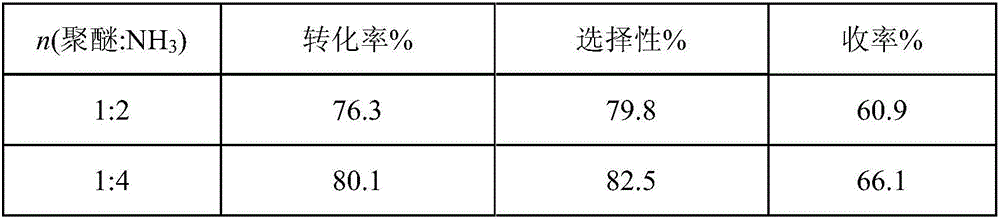
Method for preparing polyether amine through normal pressure catalysis
ActiveCN106832251AReduce difficulty of reactionSimple reaction conditionsMetal/metal-oxides/metal-hydroxide catalystsHydrogenFixed bed
The invention relates to a method for preparing polyether amine through normal pressure catalysis, and belongs to the technical field of organic high-molecular compound preparation or chemical processing. Polyoxyethylene ether, liquid ammonia and hydrogen gas are used as raw materials; the raw materials are introduced into a tubular fixed bed reactor filled with catalysts; the reaction temperature is controlled at 160 to 210 DEG C; the polyether air speed is 0.1 to 4 g / h / g / cat; the ratio of the feeding speed rate of the polyether to the feeding speed rate of liquid ammonia to the feeding speed rate of the hydrogen gas is 1:(3 to 12):(3 to 12); after the reaction is completed, filtering is performed to obtain the polyether amine product. When the method is applied to the polyether amine production, the advantages of mild reaction condition, high controllability and the like are realized.
Owner:ZHEJIANG HUANGMA TECH +3
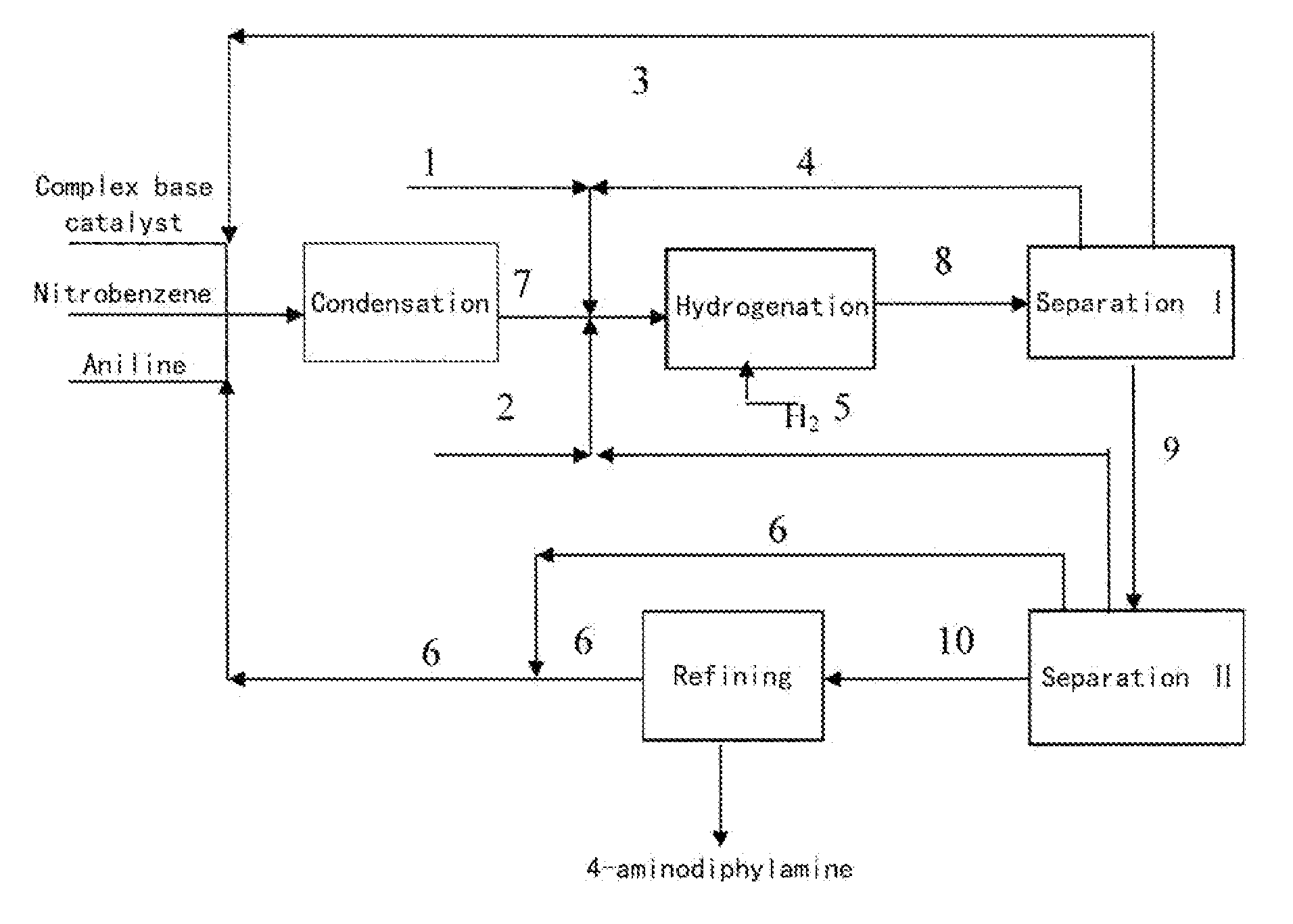
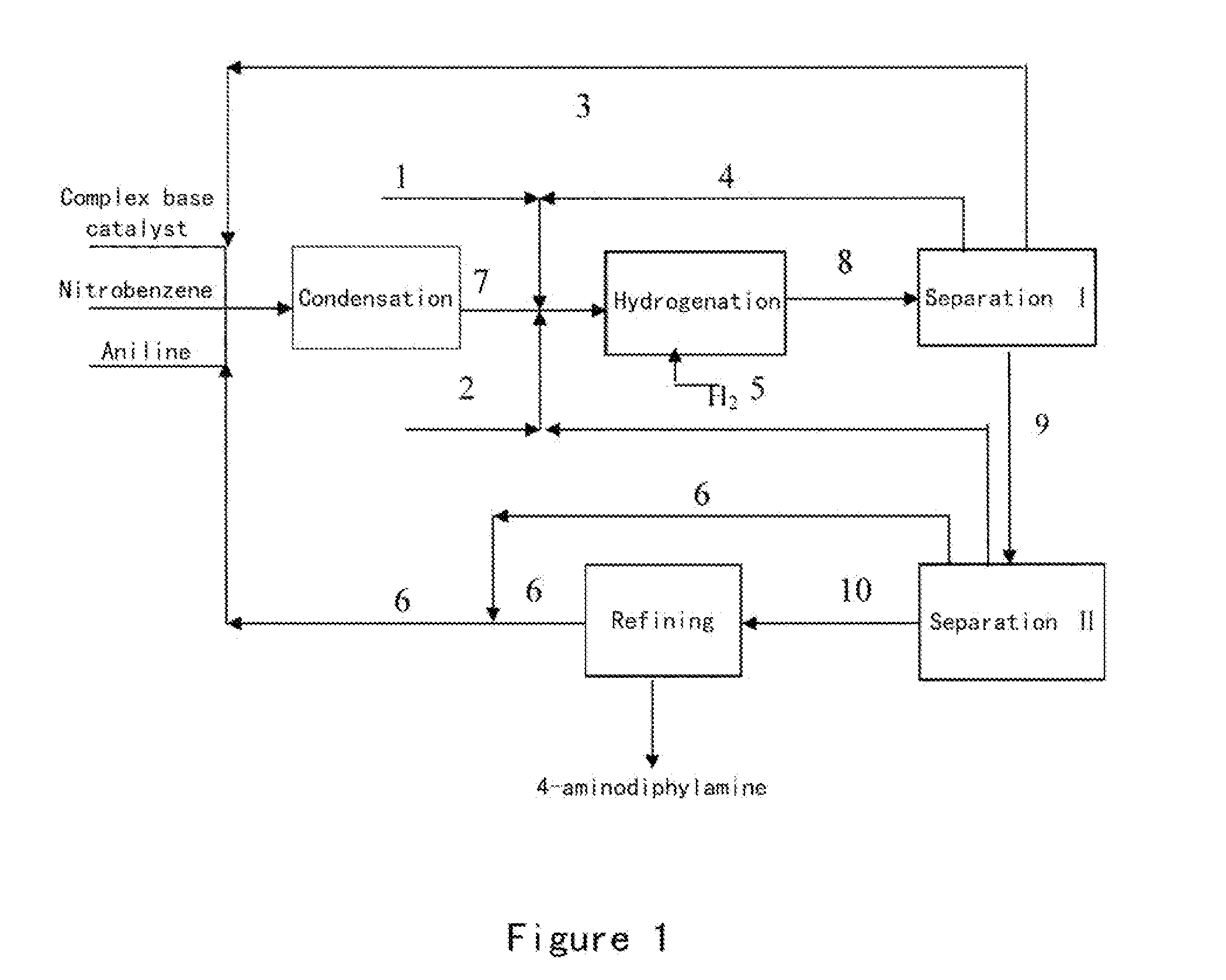
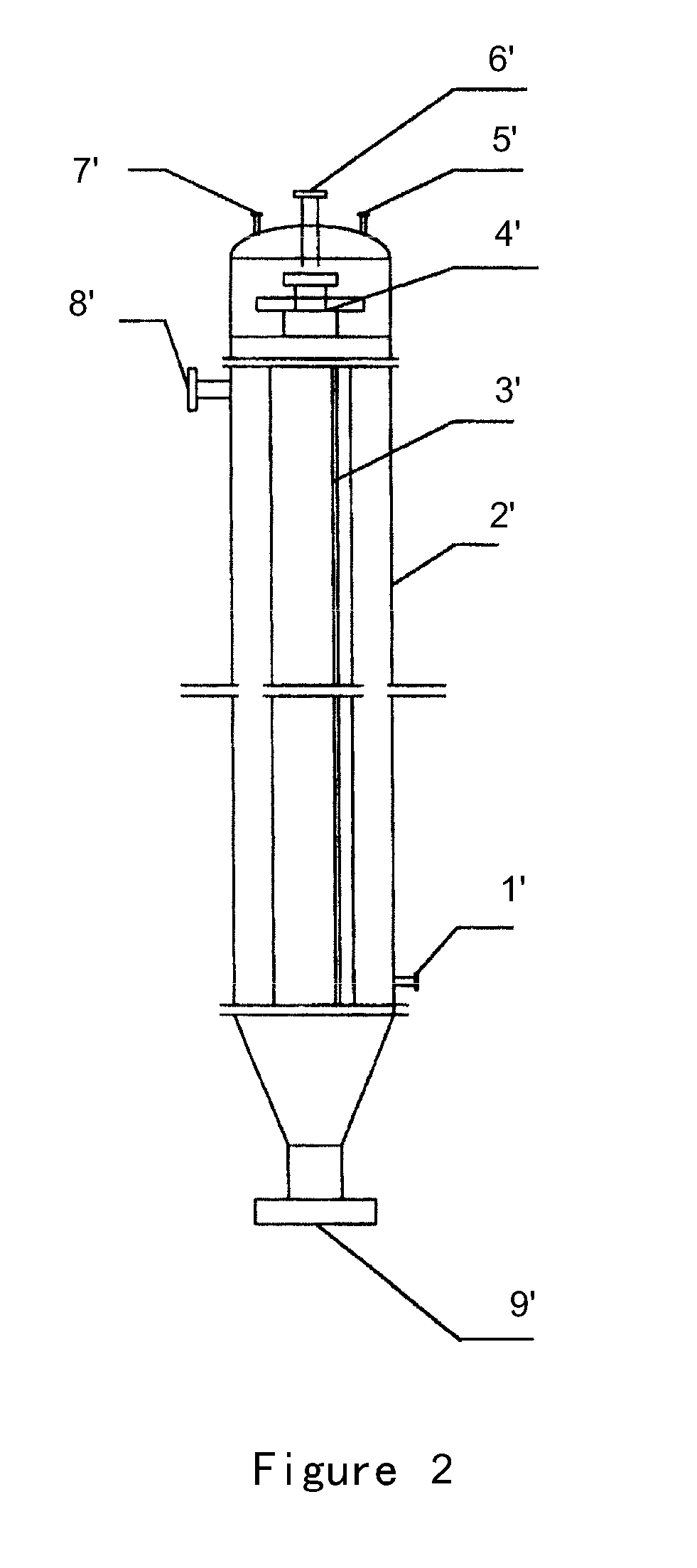
Process for preparing 4-aminodiphenylamine
ActiveUS20120053367A1Increase rangeHigh yieldOrganic compound preparationAmino compound preparation by condensation/addition reactionsChemical compositionPhenazine
A method for refining an organic composition containing 4-aminodiphenylamine, aniline, azobenzene, and phenazine, having the steps of feeding the organic composition to a first rectification column, producing a first effluent composition containing aniline, azobenzene, phenazine, and a small amount of 4-aminodiphenylamine at the top of the first rectification column and a second effluent composition containing crude 4-aminodiphenylamine at the bottom of the first rectification column, feeding the second effluent composition to a second rectification column, and producing a 4-aminodiphenylamine composition at the top of the second rectification column and a residual composition at the bottom of the second rectification column.
Owner:SENNICS CO LTD
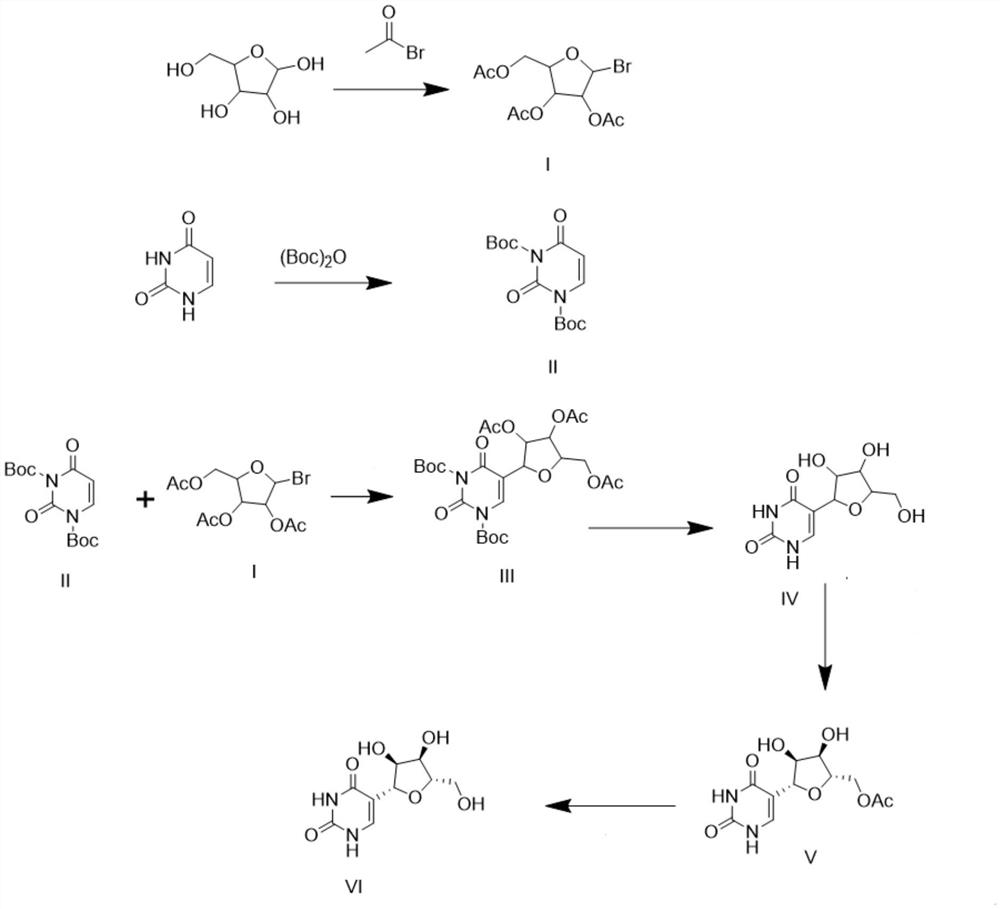
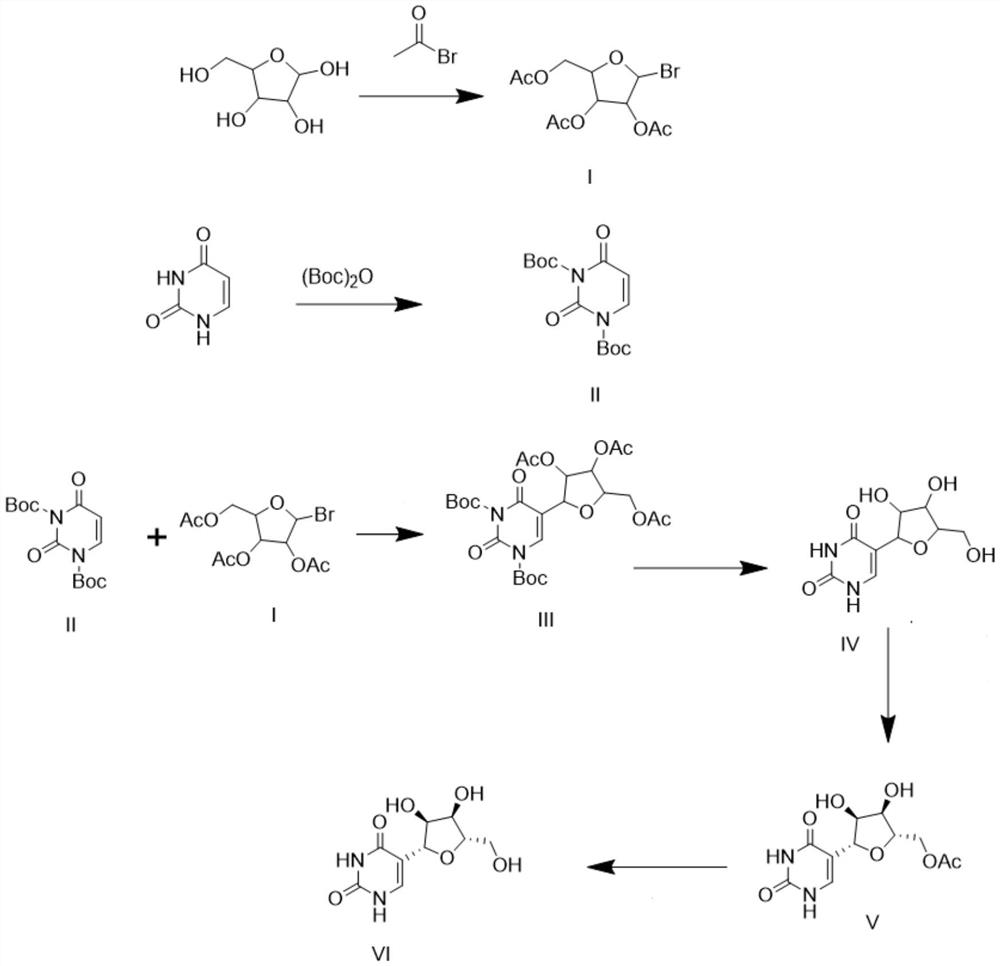
New synthesis method of pseudouridine
PendingCN114702481AReduce usageFew reaction stepsOrganic chemistry methodsFermentationAcetic anhydrideReaction step
The invention discloses a novel synthesis method of pseudouridine, which comprises the following steps: 1) taking D-ribose as a reaction initiator, and dropwise adding acetyl bromide into an organic solvent to obtain an intermediate I; 2) using uracil as a reaction initiator, eliminating reactive hydrogen in an organic solvent by using strong Lewis base, and adding (Boc) 2O in batches to obtain a corresponding intermediate II; and (3) carrying out condensation reaction on the compound I and the compound II in an organic solvent under the condition of strong Lewis base to obtain an intermediate III, removing a protecting group by using trifluoroacetic acid to obtain an intermediate IV, adding acetic anhydride, carrying out chiral resolution by using lipase to obtain a compound V, and finally removing a protecting group under an alkaline condition to obtain a final product VI. The product is a pseudouridine product. According to the method, mild and safe chemical reagents and enzyme chiral resolution are adopted, the reaction steps are shortened, the reaction difficulty is reduced, and therefore the technical bottleneck of green synthesis is achieved.
Owner:南京艾斯特医药科技有限公司
Preparation method of nano-TiO2
ActiveCN108609650ASmall particle sizeReduce difficulty of reactionNanotechnologyTitanium dioxideAlcoholUltraviolet lights
The invention belongs to the technical field of nano materials and particularly relates to a preparation method of nano-TiO2. The preparation method comprises steps as follows: step 1, N-butyl titanate is added to absolute ethanol and ultrasonically dispersed for 20-40 min, and a Ti alcohol solution is obtained; step 2, polyvinylpyrrolidone is added to the Ti alcohol solution and stirred until polyvinylpyrrolidone is completely dissolved, then sealed ultrasonic reaction is performed for 1-3 h, and a Ti alcohol dispersion is obtained; step 3, methylcellulose is added to water and stirred at constant temperature until methylcellulose is completely dissolved, and a dispersed aqueous solution is obtained; step 4, the dispersed aqueous solution is slowly dropwise added to the Ti alcohol dispersion and subjected to the ultrasonic reaction for 2-4 h, and a mixed slightly-turbid solution is obtained; step 5, the mixed slightly-turbid solution is added to a reaction kettle and stirred at the constant temperature to be gelatinized, a product is filtered while still hot and washed by absolute ethanol at the constant temperature, and gel is obtained; step 6, the gel is placed in aqueous ethanol, subjected to the ultrasonic reaction for 1-3 h and filtered, then ultraviolet light activation is performed, and nano-TiO2 is obtained.
Owner:绍兴市秀臻新能源科技有限公司
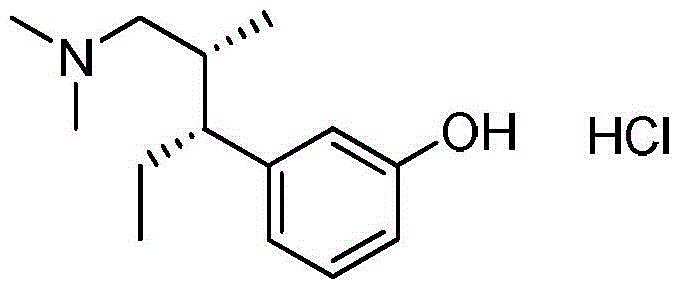
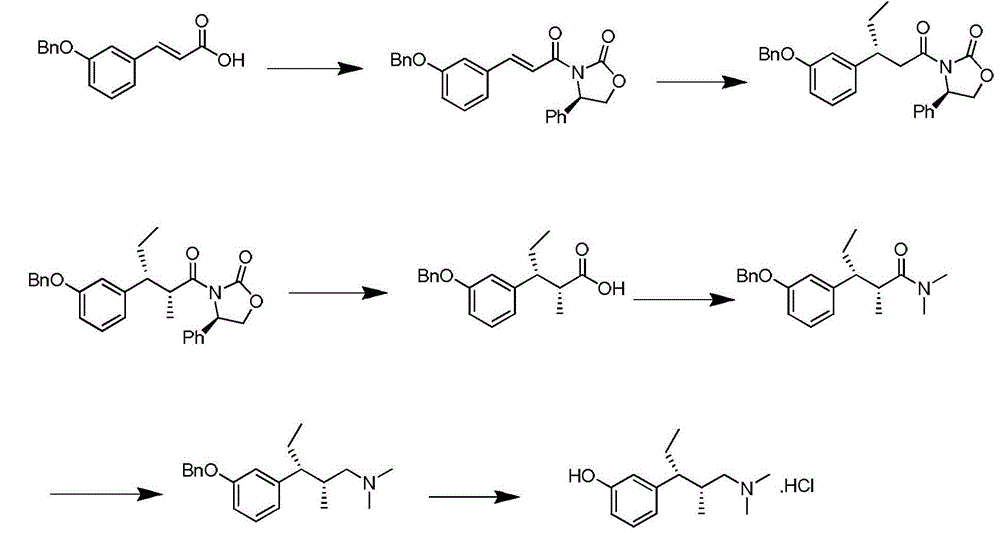
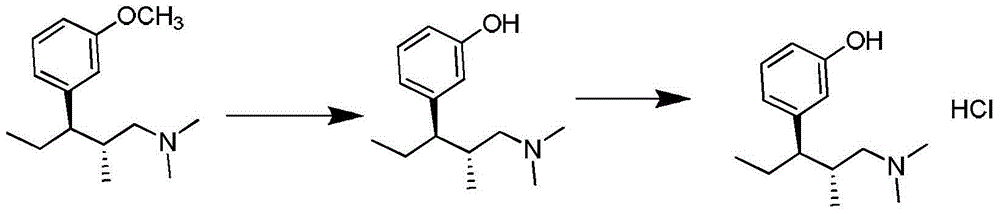
Preparation method of new crystal form tapentadol hydrochloride
InactiveCN103553940AReduce usageShort synthetic stepsOrganic compound preparationAmino-hyroxy compound preparationTapentadol HydrochlorideSolvent
The invention discloses a preparation method of new crystal form tapentadol hydrochloride, which comprises the following steps of (1) converting (2R, 3R)-3-(3-methoxyphenyl)-N,N,2-trimethyl-amylamine hydrochloride by use of a demethylation reagent into the formula (III): (-)-(1R, 2R)-3-(3-dimethylamino-1-ethyl-2-methylphenyl)phenol; (2) salifying the compound of formula (III) with hydrochloric acid in a mixed solvent of acetonitrile and isopropyl alcohol to obtain a compound of formula (I): (-)-(1R, 2R)-3-(3-dimethylamino-1-ethyl-2-methylphenyl)phenol hydrochloride. By adopting the method disclosed by the invention, stable new crystal form tapentadol hydrochloride can be obtained; moreover, the process conditions are mild, the after-treatment is simple, the purity is high, the reaction cost is low, and industrial production is easy to realize.
Owner:NANJING CORE TECH CO LTD
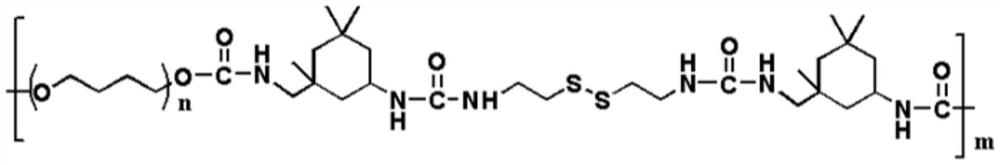
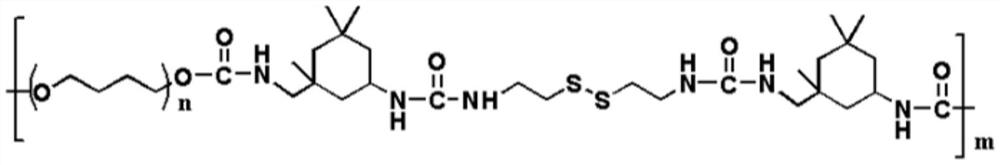
High-strength self-repairing polyurethane material and preparation method thereof
The invention relates to a high-strength self-repairing polyurethane material and a preparation method thereof. The high-strength self-repairing polyurethane material is a tough and recyclable polyurethane elastomer with rapid and efficient self-repairing capability. According to the invention, compared with the existing self-repairing elastomer and hydrogel with low strength (generally less than 10MPa) and poor toughness, the high-strength self-repairing polyurethane material has higher practical application value due to high strength and high toughness; the elastomer is composed of a soft segment, a hard segment and a chain extender, wherein hydrogen bonds, disulfide bonds and soft / hard segment microphase separation in the elastomer can achieve a synergistic effect, so that the elastomer has high strength and excellent self-repairing characteristics at the same time; the preparation method of the self-repairing polyurethane elastomer is simple and easy to implement; and due to the fact that the self-repairing polyurethane elastomer is high in strength, good in toughness, high in transparency, high in self-repairing efficiency and capable of being recycled, the self-repairing polyurethane elastomer has potential application prospects in the aspects of synthetic rubber, coating, flexible electronics and the like.
Owner:NORTHWESTERN POLYTECHNICAL UNIV +1
Preparation method for enrofloxacin-d5
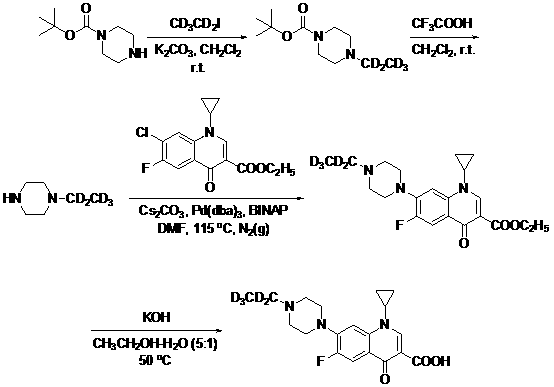
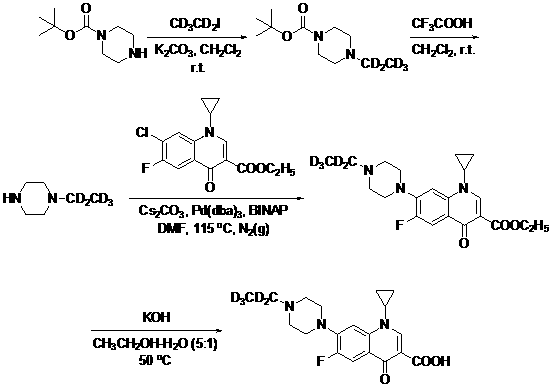
ActiveCN108675959AGuaranteed abundanceSynthetic reaction is simpleIsotope introduction to heterocyclic compoundsQuinolineIsotope
The invention discloses a preparation method for enrofloxacin-d5. The preparation method comprises the following steps: performing a Buchwald-Hartwig coupling reaction on 1-ethyl-piperazine-d5 and 1-cyclopropyl-7-chloro-6-fluoro-1,4-dihydro-4-oxoquinoline-3-carboxylic ethyl ester, and preparing 1-cyclopropyl-6-fluoro-1,4-dihydro-4-oxo-7-(4-ethyl-1-piperazinyl)-3-quinoline carboxylic ethyl ester-d5; and after alkaline hydrolysis, to obtain the enrofloxacin-d5. The preparation method is capable of, through performing the coupling reaction on an esterified carboxylic group and ethyl piperazine, enabling a synthetic reaction to be more easily performed, the difficulty of the reaction is reduced, and a yield of products is very high. In addition, the isotope abundance can be guaranteed in the high-temperature reaction conditions, a reaction process is more easily controlled, the repeatability and the stability are higher, and the conditions of large-scale production are satisfied.
Owner:雅安职业技术学院
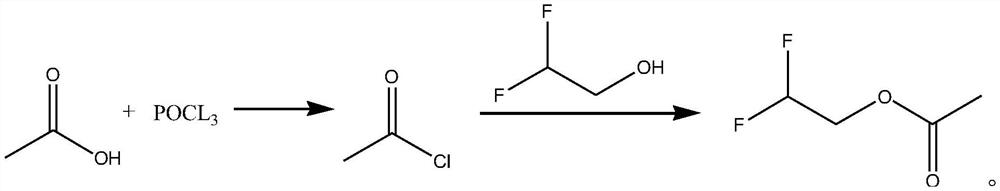
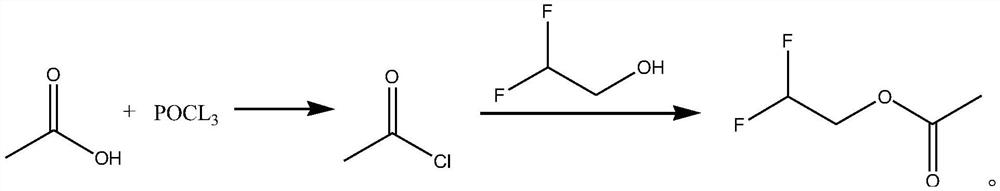
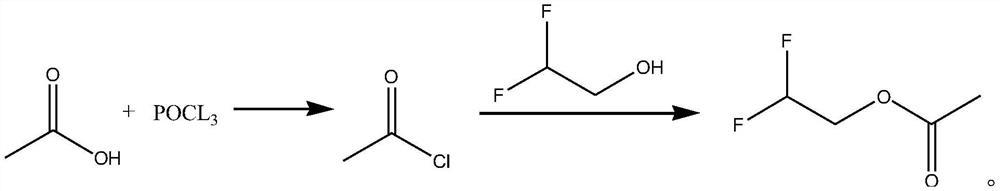
Synthetic method of difluoroethanol acetate
PendingCN114507134AReduce difficulty of reactionSimple ingredientsPreparation from carboxylic acid halidesOrganic compound preparationElectrolytic agentAcetic acid
The invention discloses a synthesis method of difluoroethanol acetate, and relates to the technical field of battery electrolyte additives, the synthesis method comprises the following steps: taking glacial acetic acid, cooling to below 5 DEG C, slowly dropwise adding phosphorus oxychloride, carrying out acylating chlorination reaction, standing for layering, directly dissolving an obtained acetyl chloride crude product in dichloromethane, cooling to below 5 DEG C again, slowly dropwise adding difluoroethanol, and carrying out acylating chlorination reaction to obtain the difluoroethanol acetate. And after dropwise adding, carrying out acylation reaction under the catalytic action of an acid capturing agent to obtain the difluoroethanol acetate. According to the method, acetic acid is taken as a raw material, difluoroethanol acetate is synthesized through a one-pot method, post-treatment is not needed in the process, and the reaction difficulty is reduced; the adopted raw materials are simple and easy to obtain, and the production cost is reduced.
Owner:SHIJIAZHUANG SAN TAI CHEM CO LTD
Preparation method of tofacitinib citrate
InactiveCN104530055AShort synthetic stepsEasy to operateCarboxylic acid salt preparationSolventTOFACITINIB CITRATE
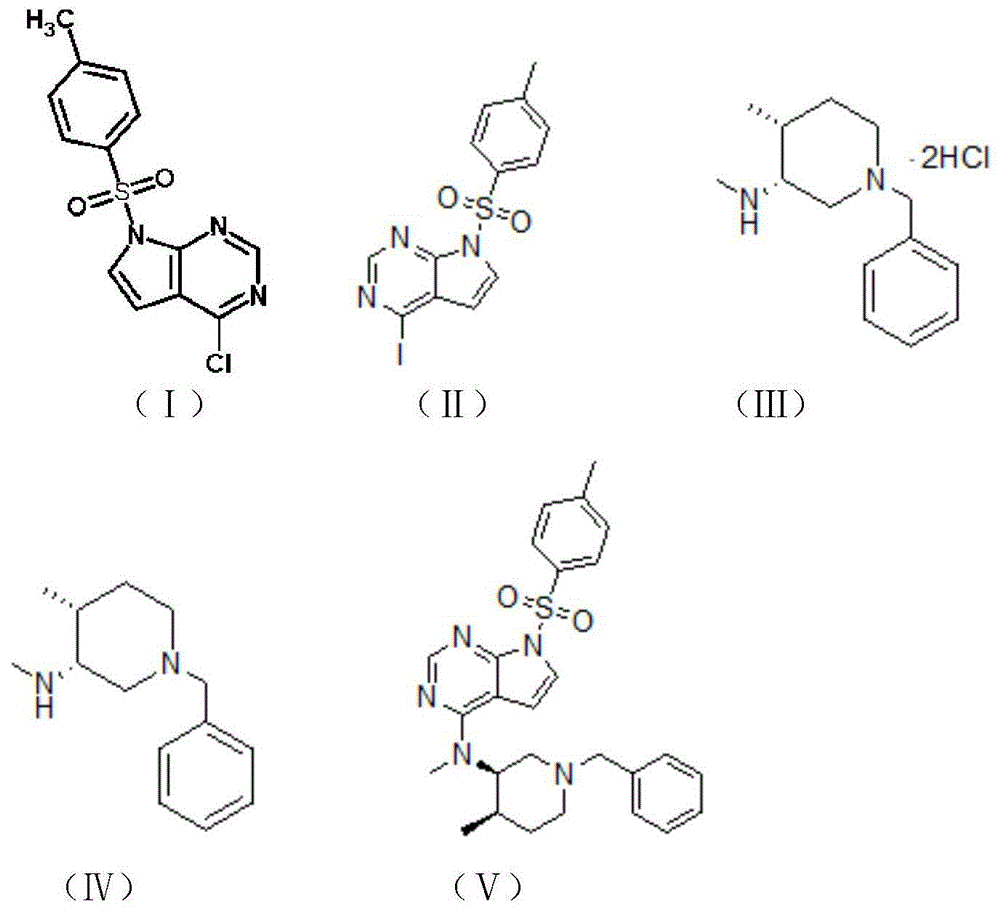
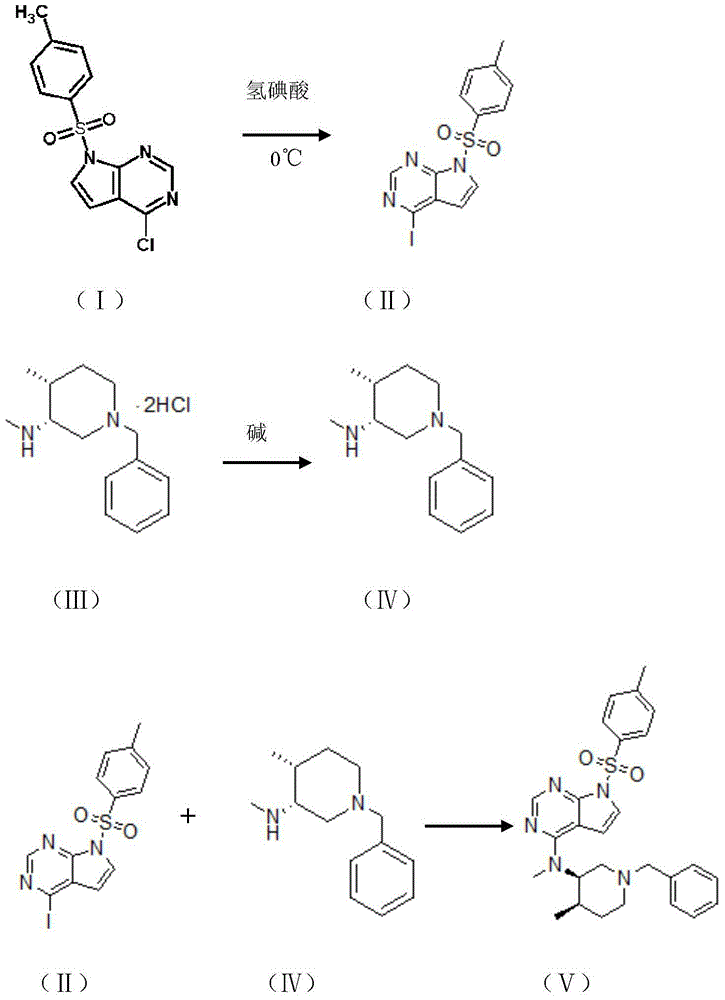
The invention belongs to the field of synthetic drugs and particularly relates to a preparation method of tofacitinib citrate. The preparation method comprises the following steps: (1) adding a compound I into hydroiodic acid to obtain a compound II; (2) dissolving the compound II in a solvent, and adding a base to obtain a compound IV; and (3) reacting the compound IV and the compound II to obtain a compound V, namely, tofacitinib citrate, wherein the compound I, the compound II, the compound III, the compound IV and the compound V have molecular structures as shown in the description. By the preparation method, the reaction time is greatly shortened, the post-treatment is simplified and the cost is reduced.
Owner:NANJING CORE TECH CO LTD
Modified bagasse retarder and preparation method thereof
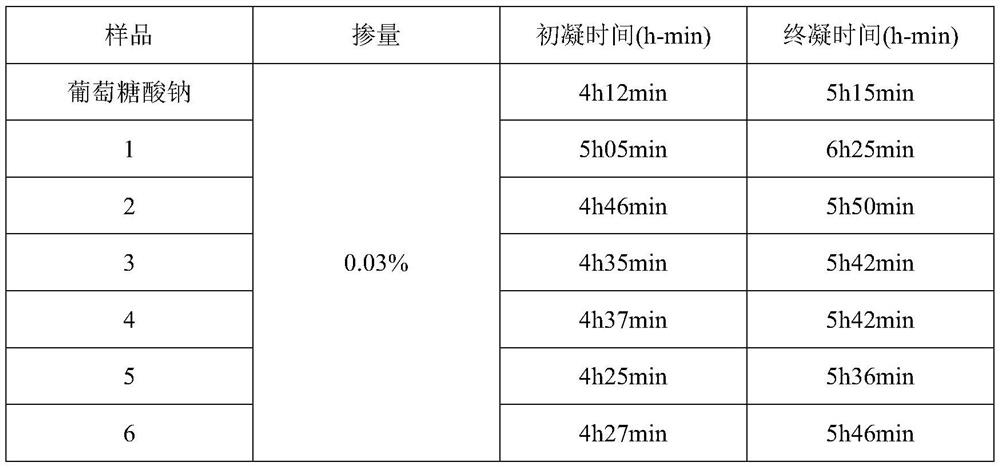
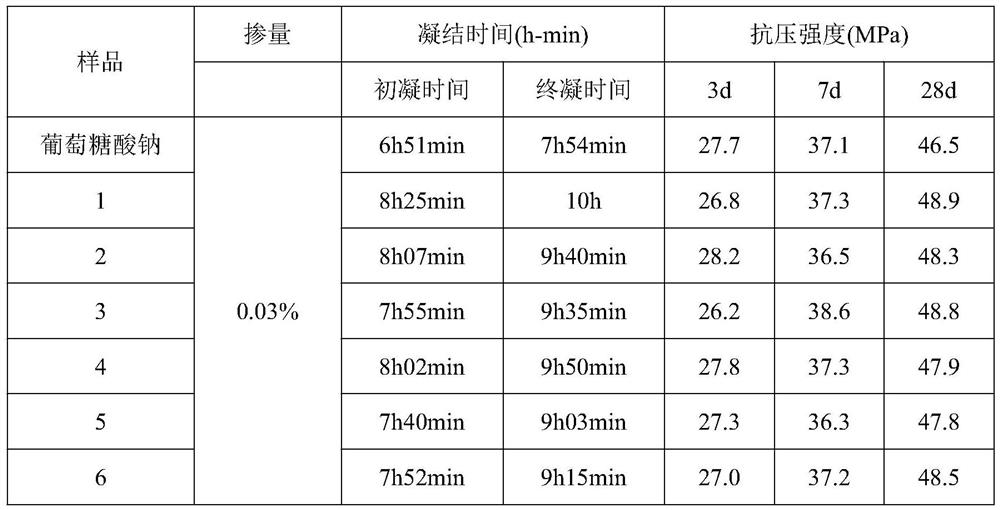
ActiveCN111848978AFollow-up response is simpleReduce difficulty of reactionSulfite saltHemicellulose
The invention discloses a modified bagasse retarder and a preparation method thereof. The preparation method specifically comprises the following steps: pretreating bagasse with sulfite or chlorite, and removing lignin and part of hemicelluloses in the bagasse; and carrying out acidolysis by using composite acid under a catalytic condition. The prepared retarder not only contains hydroxyl and carboxyl, but also contains functional groups such as phosphate groups and the like. The retarding effect of the modified bagasse retarder under the conditions of normal temperature (20 DEG C) and high temperature (50 DEG C) is superior to that of sodium gluconate, and the later strength of concrete is not influenced. Compared with traditional retarders such as sodium gluconate, the modified bagasse retarder has the advantages of being low in cost, environmentally friendly, renewable, simple in production process, wide in application prospect and the like.
Owner:JIANGSU CHINA RAILWAY ARIT NEW MATEIRALS CO LTD
Preparation method of pitavastatin calcium
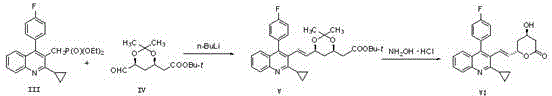
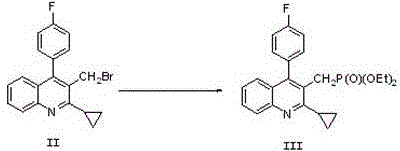
The invention relates to a preparation method of a cholesterol reduction drug, particularly relates to a preparation method of pitavastatin calcium as a crude drug of the cholesterol reduction drug, and aims at the problems that the pitavastatin calcium synthetic technology in the prior art is long in steps and complicated in operation, and uses strongly corrosive reagents which is environmentally unfriendly, causes serious corrosion to equipment, and may not facilitate industrial production. The invention provides the new preparation method of the pitavastatin calcium, the new preparation method is as follows: 3-bromomethyl-2-cyclopropyl-4-(4-fluorophenyl)quinoline is prepared from 2-cyclopropyl-4-(4-fluorophenyl)-3-quinolinecarboxaldehyde by a one step reaction, and then reacts with an organophosphorus reagent to obtain pitavastatin calcium intermediate phosphorus ylide, on the basis of improving of the yield to 80%, the reaction steps are reduced, and the reaction difficulty is reduced, and hydroxylamine hydrochloride is selected as a deprotection reagent, so that the new preparation method is mild in reaction conditions, environmentally friendly, high in yield, and beneficial to industrial production.
Owner:XUZHOU WANBANG JINQIAO PHARMA +1
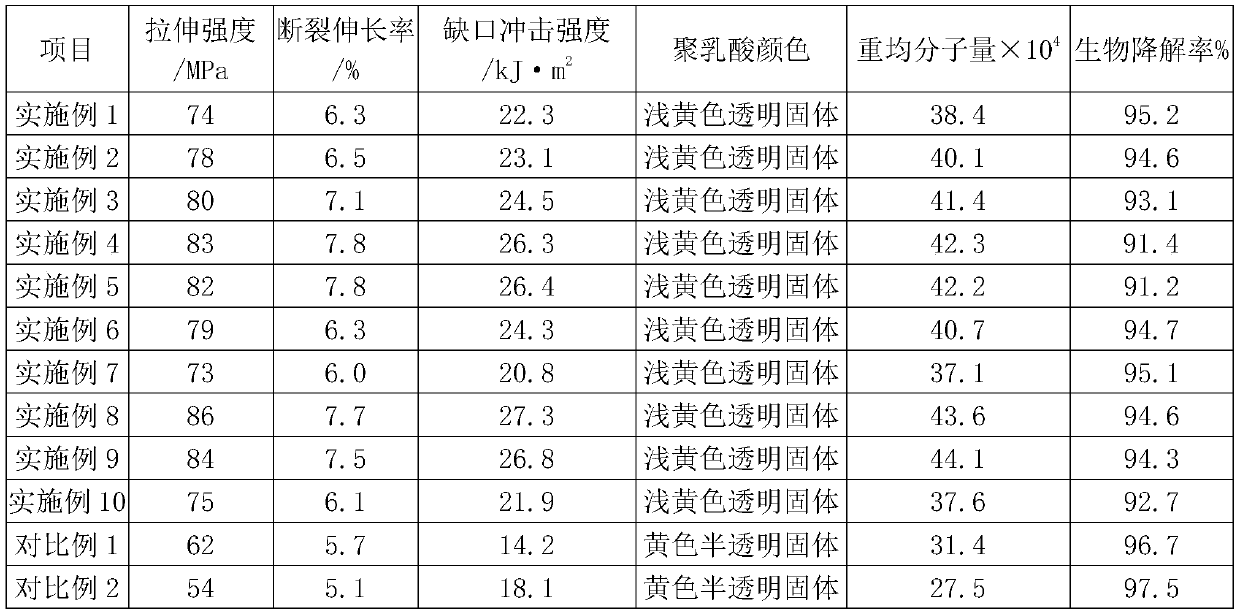
Preparation method of high-molecular-weight polylactic acid
The invention provides a preparation method of high-molecular-weight polylactic acid. The method comprises the following steps: prepolymerization, polymerization, chain extension and crosslinking, wherein a chain extender in the crosslinking step is a dioxazoline chain extender, and a crosslinking agent in the crosslinking step is a polycarboxyl compound. According to the invention, the polycarboxyl compound is used for enabling polylactic acid molecules to react so as to form a net-shaped cross-linked structure, so the molecular weight of polylactic acid is further increased, and the mechanical properties of polylactic acid are improved; meanwhile, it is unexpectedly found that a synergistic effect exists between the used dioxazoline chain extender and the polycarboxyl compound added in the crosslinking step, the molecular weight of the polylactic acid can be increased by adjusting the proportion of the dioxazoline chain extender to the polycarboxyl compound, and the color and lusterof the produced polylactic acid are not affected by chain extension and crosslinking.; moreover, activated clay added in the pre-polymerization step is found to have effect on improving the impact resistance of the polylactic acid, reducing reaction difficulty and further improving the weight-average molecular weight of the polylactic acid.
Owner:上海汉禾生物新材料科技有限公司 +1
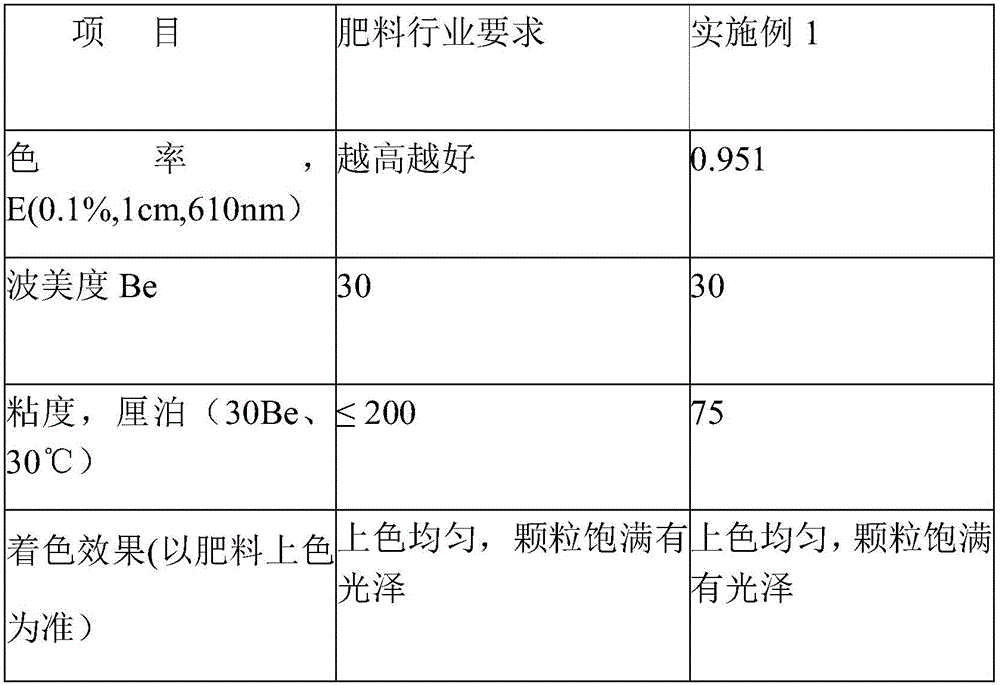
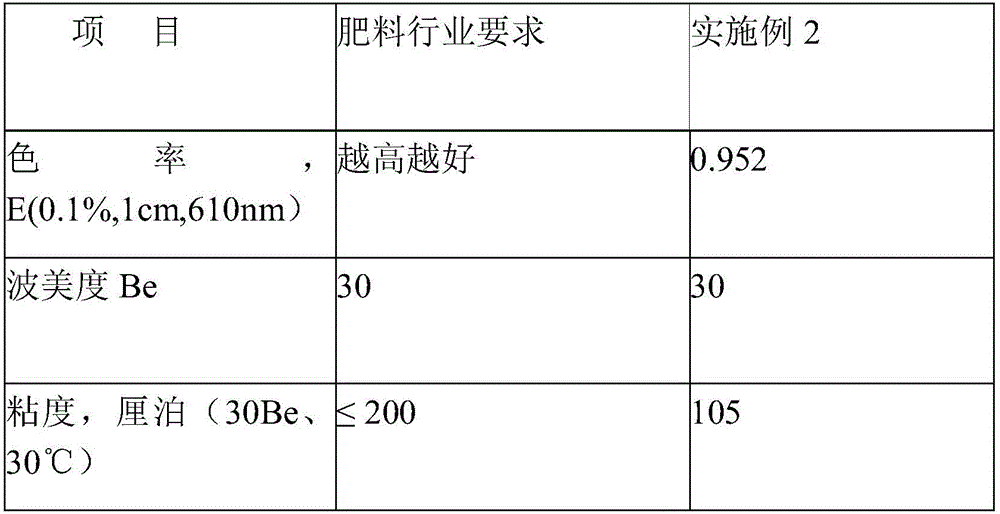
A kind of ammonium sulfite method caramel color process with color rate ≥ 250000ebc
ActiveCN105348852BImprove liquidityAvoid esterificationNatural dyesCoordination polymerizationDouble bond
The invention relates to a process of ammonium sulfite method caramel color with color index being more than or equal to 250000EBC. The process successively comprises the following steps: raw material metering and concentrating, a first time catalyst adding: adding 10 percent to 30 percent sulfite and 5 percent to 15 percent ammonium substances or ammonia substances or amine substances in the concentrated raw material, a first time of pressurized reaction, a second time catalyst adding: adding 0.01 percent to 0.05 percent metallocene catalyst into a material after the ending of the first time of reaction, a second time of pressurized reaction, blending, and metering and filling. According to the process, the ammonium sulfite method caramel color process is first used, after a reaction system generates a lot of heterocyclic rings and olefins, the metallocene catalyst is added to perform the second time of pressurized reaction, transition metal with positive valence in the metallocene catalyst is combined with electron of a monomer molecular to realize stereospecific coordination polymerization, esterification is avoided, reaction difficulty is decreased, carbon chain growth and conjugated double bond increase are performed, so as to obtain the caramel color with the color index being more than or equal to 250000EBC, and the caramel color is good in fluidity.
Owner:QIANHE CONDIMENT & FOOD CO LTD
Synthesis of 3-bromo-5-(2-ethylimidazo[1, 2-a]pyridine-3-carbonyl)-2-hydroxybenzonitrile
ActiveCN111410654APromote conversionHigh yieldOrganic active ingredientsOrganic chemistryOrganic solventEthyl group
The invention discloses a synthetic method of 3-bromo-5-(2-ethylimidazo[1,2-a]pyridine-3-carbonyl)-2-hydroxybenzonitrile, particularly relates to a synthetic method of a compound shown as a formula (III), and particularly relates to a step A or a step B; the preparation method comprises the following steps: A, heating a compound shown as a formula (I) and a compound shown as a formula (II) in an organic solvent for reaction, and heating an obtained reaction product and alkali in the presence of water for continuous reaction to obtain a compound shown as a formula (III); and B, heating the compound shown as the formula (I), a compound shown as a formula (II) and alkali in an organic solvent to react, and heating an obtained reaction product in the presence of water to continuously react toobtain a compound shown as a formula (III).
Owner:JIANGSU ATOM BIOSCI & PHARMA CO LTD
Preparation Method of Polycarboxylate Superplasticizer with Carbon Dioxide
ActiveUS20180155244A1Super cost-effectiveGood competitive advantageNitrogen atmosphereSuperplasticizer
The present invention discloses a preparation method of a polycarboxylate superplasticizer with carbon dioxide, comprising the following steps: (1) preparing a polycarboxylate superplasticizer prepolymer: performing an oxidation-reduction radical polymerization of an unsaturated macromonomer, an unsaturated phenol derivative, a reducing agent, an initiator and a chain transfer agent with different proportions under a nitrogen atmosphere to obtain a novel polycarboxylate superplasticizer prepolymer with different molecular weight; adjusting the pH by adding an alkali; (2) preparing a polycarboxylate superplasticizer: performing a Koble-Schmitt reaction between the polycarboxylate superplasticizer prepolymer and a carbon dioxide for a certain time to obtain the polycarboxylate superplasticizer. The polycarboxylate superplasticizer prepared by the method of the present invention retains the advantages of the existing water-reducing admixture of the polyether monomer compounds, and the production process is simple, safe, controllable, less side effect and has a better cost effective and competitive advantage.
Owner:JIANGSU CHINA RAILWAY ARIT NEW MATEIRALS CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com

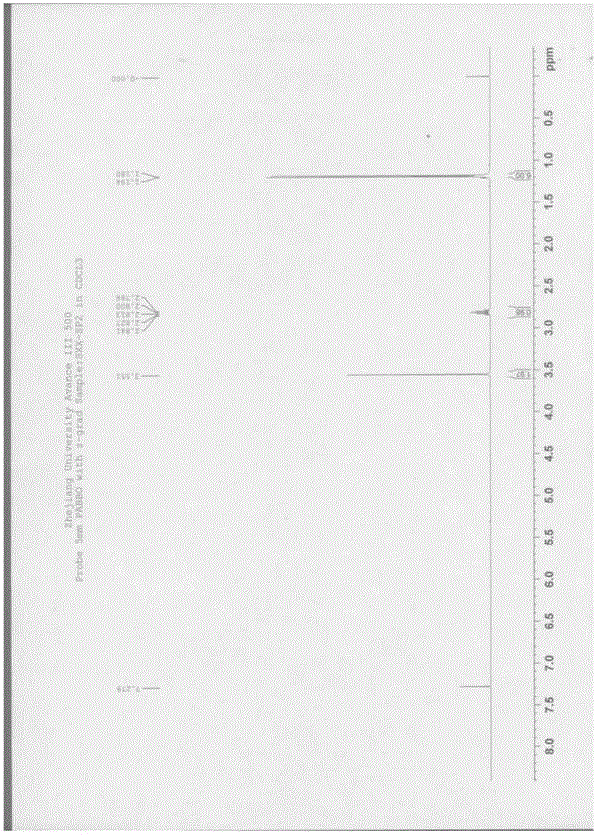


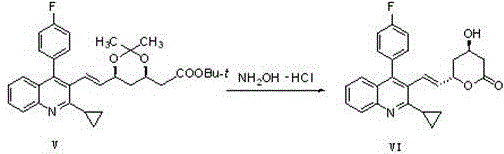

![Synthesis of 3-bromo-5-(2-ethylimidazo[1, 2-a]pyridine-3-carbonyl)-2-hydroxybenzonitrile Synthesis of 3-bromo-5-(2-ethylimidazo[1, 2-a]pyridine-3-carbonyl)-2-hydroxybenzonitrile](https://images-eureka.patsnap.com/patent_img/6abf8bf1-9451-4759-a0f2-1c3be2801829/FDA0002366424220000011.png)
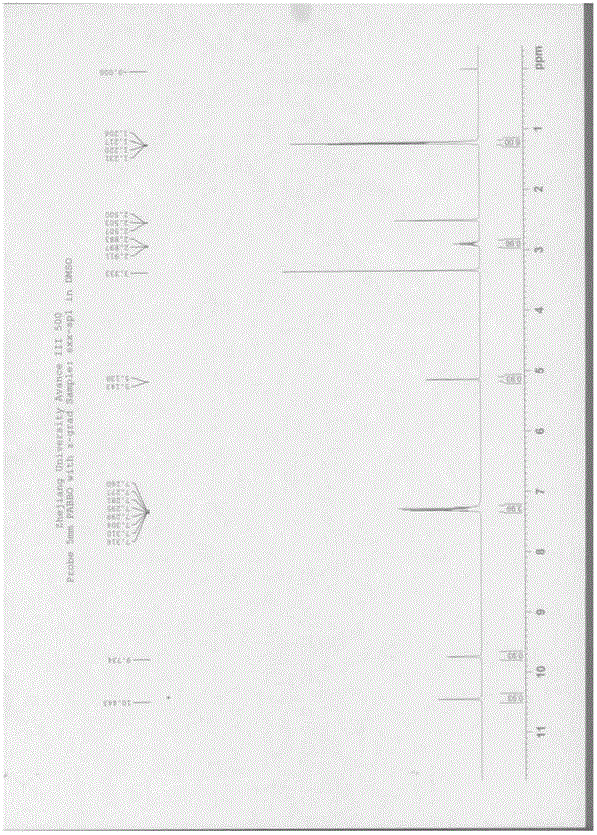
![Synthesis of 3-bromo-5-(2-ethylimidazo[1, 2-a]pyridine-3-carbonyl)-2-hydroxybenzonitrile Synthesis of 3-bromo-5-(2-ethylimidazo[1, 2-a]pyridine-3-carbonyl)-2-hydroxybenzonitrile](https://images-eureka.patsnap.com/patent_img/6abf8bf1-9451-4759-a0f2-1c3be2801829/FDA0002366424220000012.png)
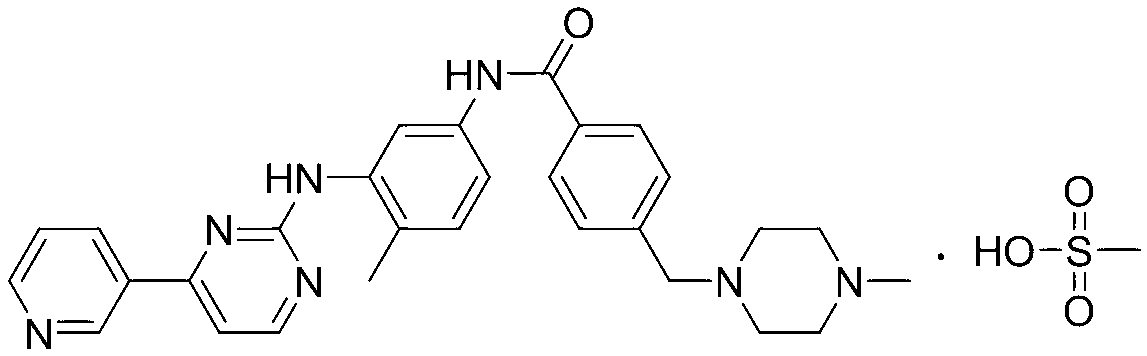
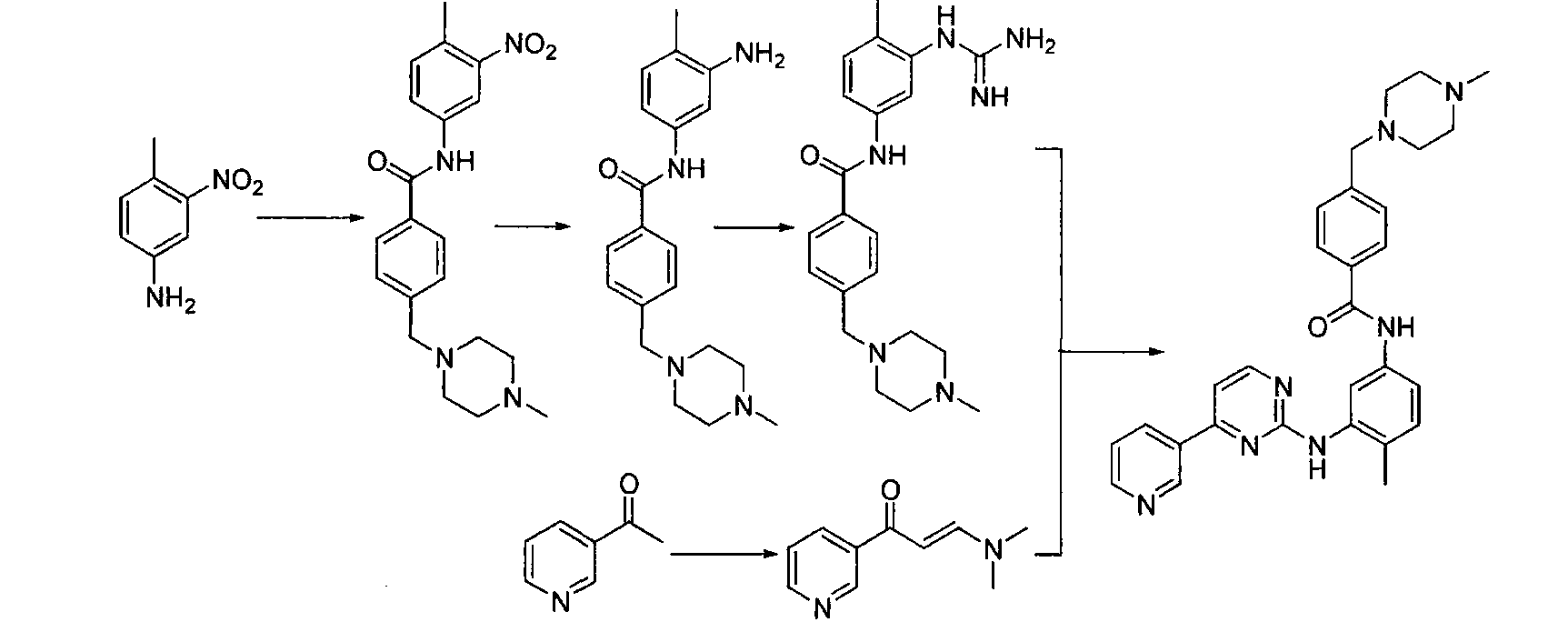
![Synthesis of 3-bromo-5-(2-ethylimidazo[1, 2-a]pyridine-3-carbonyl)-2-hydroxybenzonitrile Synthesis of 3-bromo-5-(2-ethylimidazo[1, 2-a]pyridine-3-carbonyl)-2-hydroxybenzonitrile](https://images-eureka.patsnap.com/patent_img/6abf8bf1-9451-4759-a0f2-1c3be2801829/FDA0002366424220000021.png)