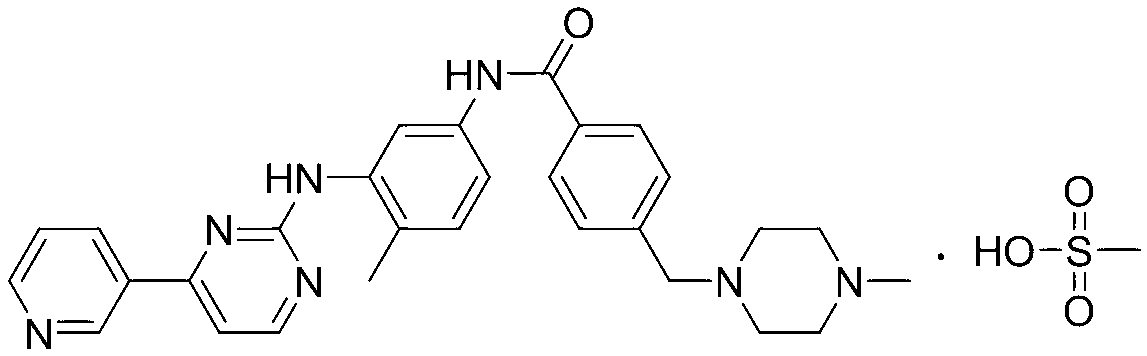
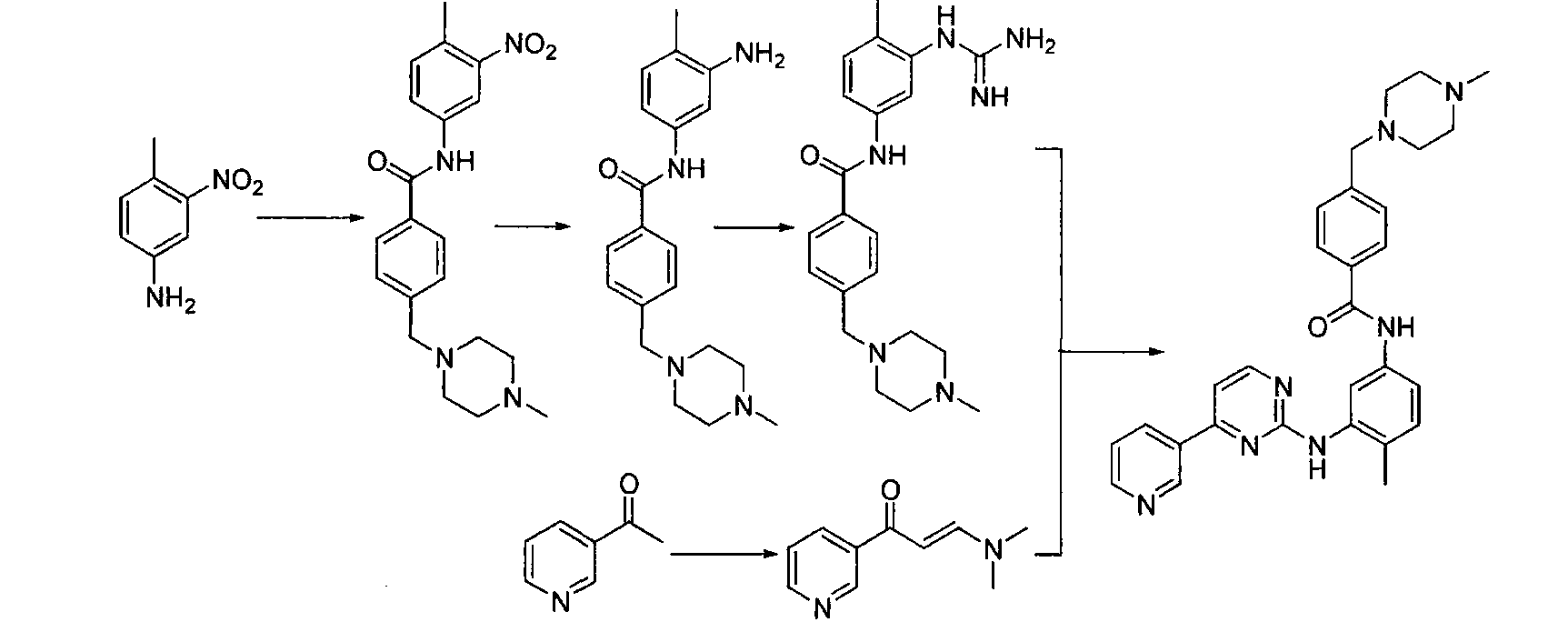
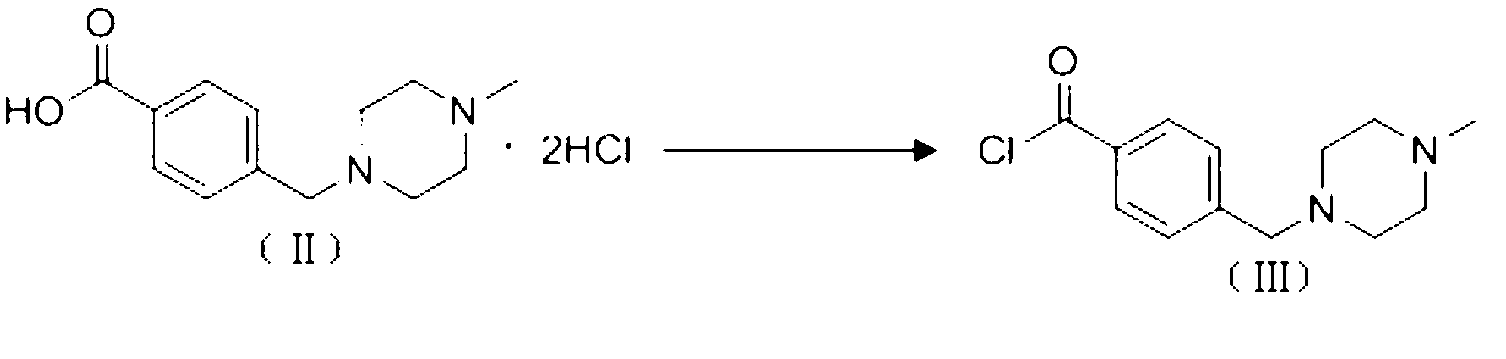
Preparation method of alpha-crystal form imatinib mesylate
A technology of imatinib mesylate and crystal form, which is applied in the field of chemical drug synthesis, and can solve the problems of incomplete reaction, unsuitability for industrial production, and high production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0031] Example Preparation of α crystal form imatinib mesylate
[0032] (1) Synthesis of 4-[(4-methylpiperazin-1-yl)methyl]benzoyl chloride (formula (Ⅲ))
[0033] In a 1000mL four-necked round-bottomed flask, install a mechanical stirrer, a reflux condenser, and a thermometer, add 200mL of toluene to the round-bottomed flask, start stirring, add 50g of imatic acid (4-[(4-methylpiperazine-1 -yl)methyl]benzoic acid dihydrochloride), slowly add 25 mL of thionyl chloride under stirring, heat up to 80-90°C, keep stirring for 4 hours, turn off the heating and naturally cool down to room temperature, concentrate in vacuo to obtain oily thing. used directly in the next step.
[0034] (2) 4-(4-methyl-1-piperazine)methyl-N-4-methyl-3-4-(3-pyridine)-2-pyrimidineaminophenyl-aniline (formula (IV)) Synthesis
[0035] In a 1000mL three-necked flask, add 37g of imaamine (N-(5-amino-2-methylphenyl)-4-(3-pyridyl)-2-aminopyrimidine), add 150mL of pyridine and 35ml of triethylamine, Add drop...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com