High-efficiency high-stereoselectivity semisynthesis method of harringtonine and allied alkaloids
A technology of triceps fir and alkaloids, which is applied in chemical instruments and methods, active ingredients of silicon compounds, active ingredients of heterocyclic compounds, etc., can solve the problems of long synthesis lines, is not easy to be applied to industrial production, etc., and achieves short synthesis steps. , easy operation, high chemical yield and high diastereoselectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
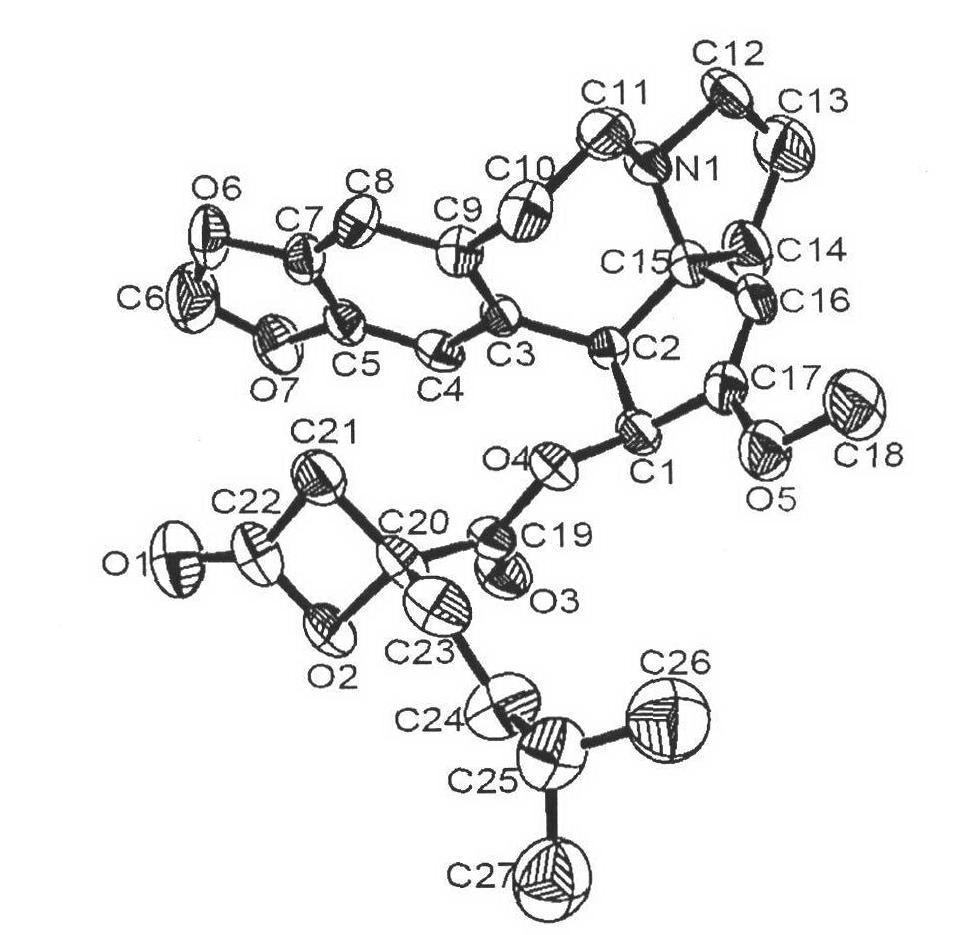
[0048] The preparation of embodiment 1α-ketoacyl harringtonine (16)
[0049]
[0050] To 205mg keto acid (21) (0.75mmol, 1.5mmol) in 3mL CH 2 Cl 2 2,4,6-trichlorobenzoyl chloride (0.26mL, 2mmol) was added dropwise into the solution, then harringtonine (158mg, 0.5mmol), pyridine (0.16mL, 2mmol) in CH 2 Cl 2 (5 mL) solution. After the reaction, extract with 10 mL ether and 10 mL pH=5 buffer solution, and separate the layers. The organic phase was washed again with buffer solution. Combine the aqueous phases and add NaHCO 3 (s) to pH=7, extract with ether, combine the organic phases, wash with saturated NaCl, anhydrous NaCl 2 SO 4 dry. After filtration, it was spin-dried, and the residual solvent and pyridine were removed in vacuum. 171mg of yellow solid was obtained, the yield was 60%.
[0051] Recrystallization gave pale yellow crystals, mp=180-181°C, [α] D =-22° (c 0.5, CHCl 3 , 20°C); 1 H NMR (400MHz, CDCl 3 )δ6.58(s, 1H), 6.56(s, 1H), 5.88(d, J=9.2Hz, 1H), 5...
Embodiment 2
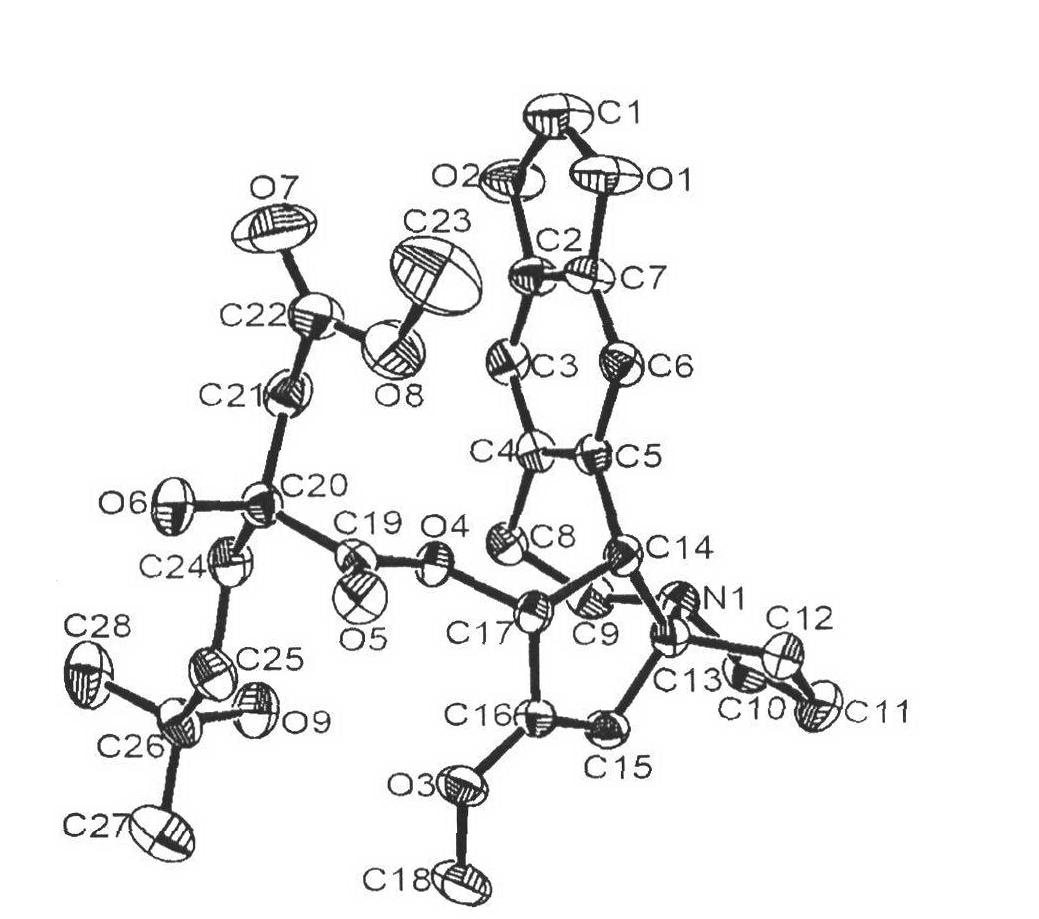
[0052] Preparation of Example 2α-ketoacyl harringtonine (17)
[0053]
[0054] The harringtonine (1.6g, 4mmol), Et 3 A solution of N (triethylamine, 1.4 mL, 10 mmol) and 4-dimethylaminopyridine (DMAP 97 mg, 0.8 mmol) in 20 ml of dichloromethane was slowly added dropwise to ketoacid (22) (1.728 g, 6 mmol) and 2, 4,6-trichlorobenzoyl chloride in 25 mL of dichloromethane solution. After the reaction is complete, add 50ml of saturated sodium bicarbonate solution, separate the layers, extract the aqueous phase with 30mL ether three times, combine the organic phases, wash twice with 50mL of pH=5 buffer solution, and wash with 10% Na 2 CO 3 Wash twice with solution, once with saturated saline, and with anhydrous Na 2 SO 4 Dry, spin dry, dissolve in n-hexane, filter, and place in the refrigerator to precipitate a white solid to obtain a total of 1.75 g of white solid, with a yield of 75%. mp=114-115°C; [α] D =-113° (c 0.5, CHCl 3 , 20°C); 1 HNMR (400MHz, CDCl 3 )δ6.58(s, 1H...
Embodiment 3
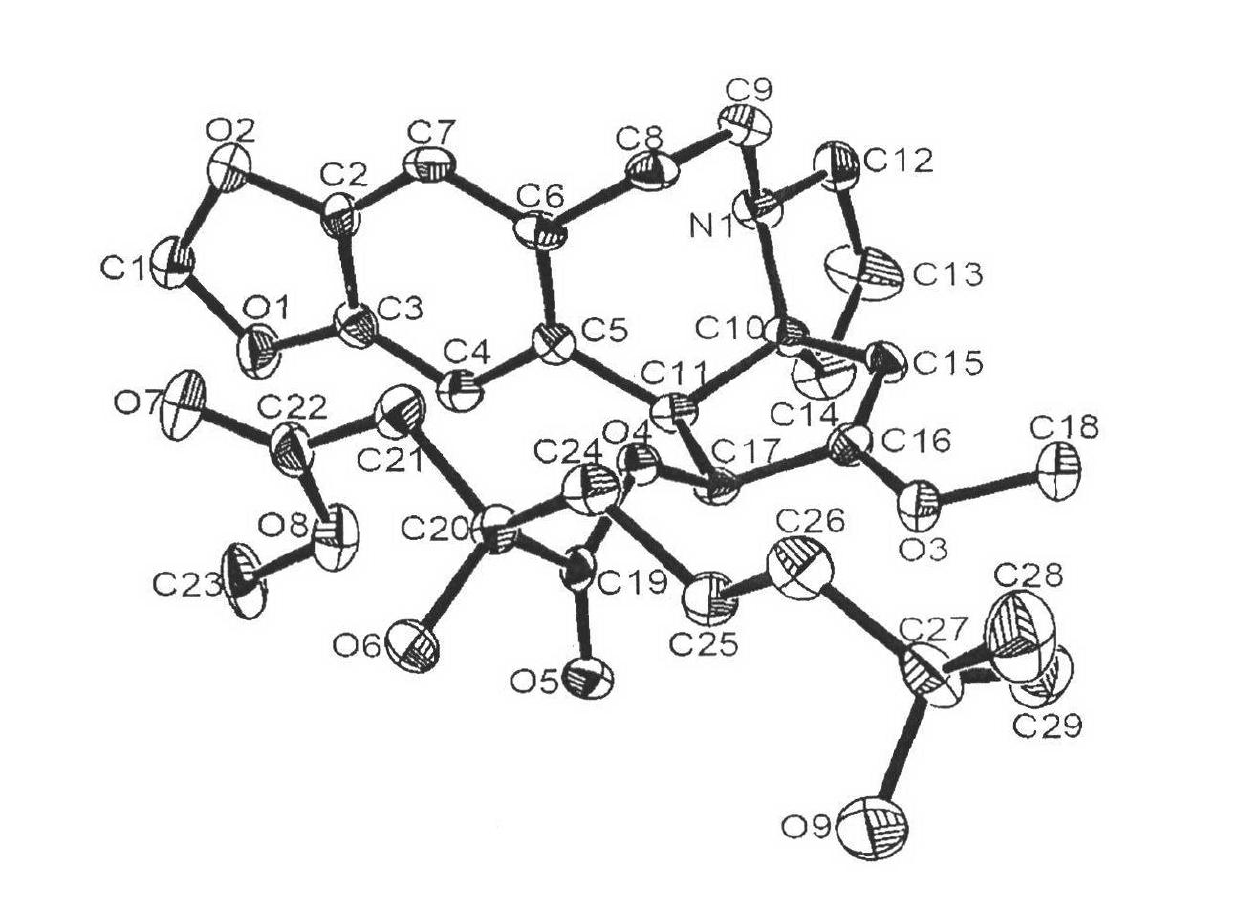
[0055] Preparation of Example 3α-ketoacyl harringtonine (18)
[0056]
[0057] The same method as in Example 1, except that compound (18) was obtained from compound (23), and recrystallized to obtain 179 mg of light yellow solid, with a yield of 81%. mp=98-100°C; [α] D =-125.4° (c 1.0, CHCl 3 , 20°C); 1 H NMR (400MHz, CDCl 3 )δ6.56(s, 1H, ArH), 6.54(s, 1H, ArH), 5.86(d, J=9.2Hz, 1H, ArCHCH), 5.82(s, 2H, OCH 2 O), 5.09(s, 1H, vinyl H), 3.81(d, J=9.2Hz, 1H, ArCHCH), 3.68(s, 3H, OCH 3 ), 3.19 (td, J=11.6, 7.6Hz, 1H, CH 2 ), 3.04 (m, 1H, CH 2 ), 2.91 (m, 1H, CH 2 ), 2.57 (m, 2H, CH 2 ), 2.33 (m, 2H, CH 2 ), 2.22 (m, 1H, CH 2 ), 2.02 (dt, J=12.0, 9.6Hz, 1H, CH 2 ), 1.88 (m, 1H, CH 2), 1.72 (m, 2H, CH 2 ), 1.41 (septet, J=6.8Hz, 1H, (CH 3 ) 2 CH), 1.24(m, 2H, CH 2 ), 0.80 (d, J=6.8Hz, 6H, (CH 3 ) 2 CH)ppm; HRMS(EI)m / z calcd for C 25 h 32 NO 6 (M+H) + 442.2220 found 442.2224.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com