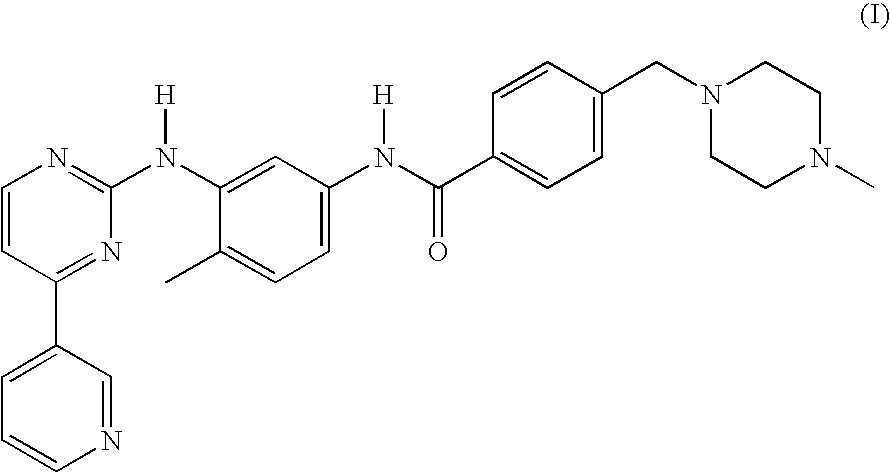
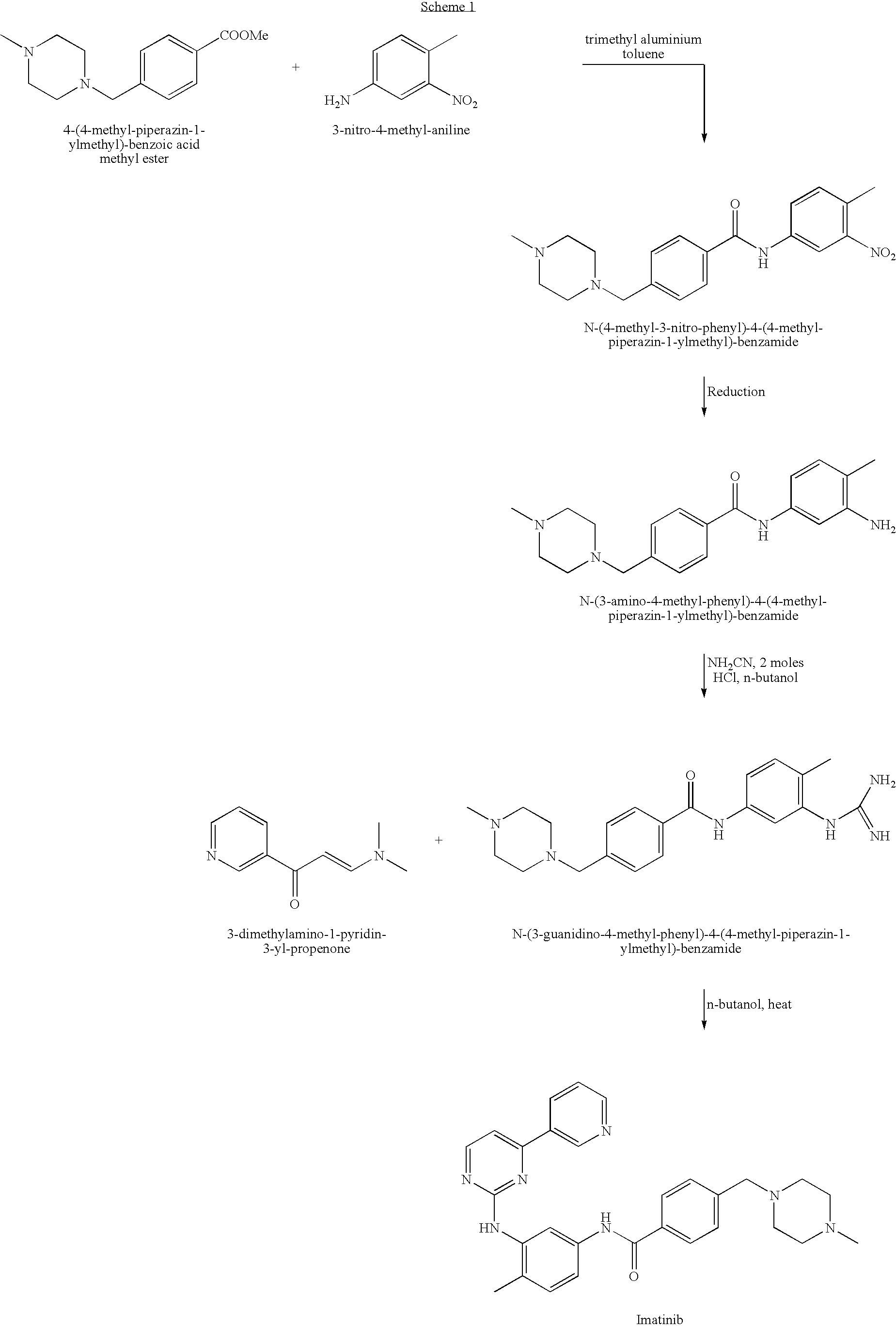
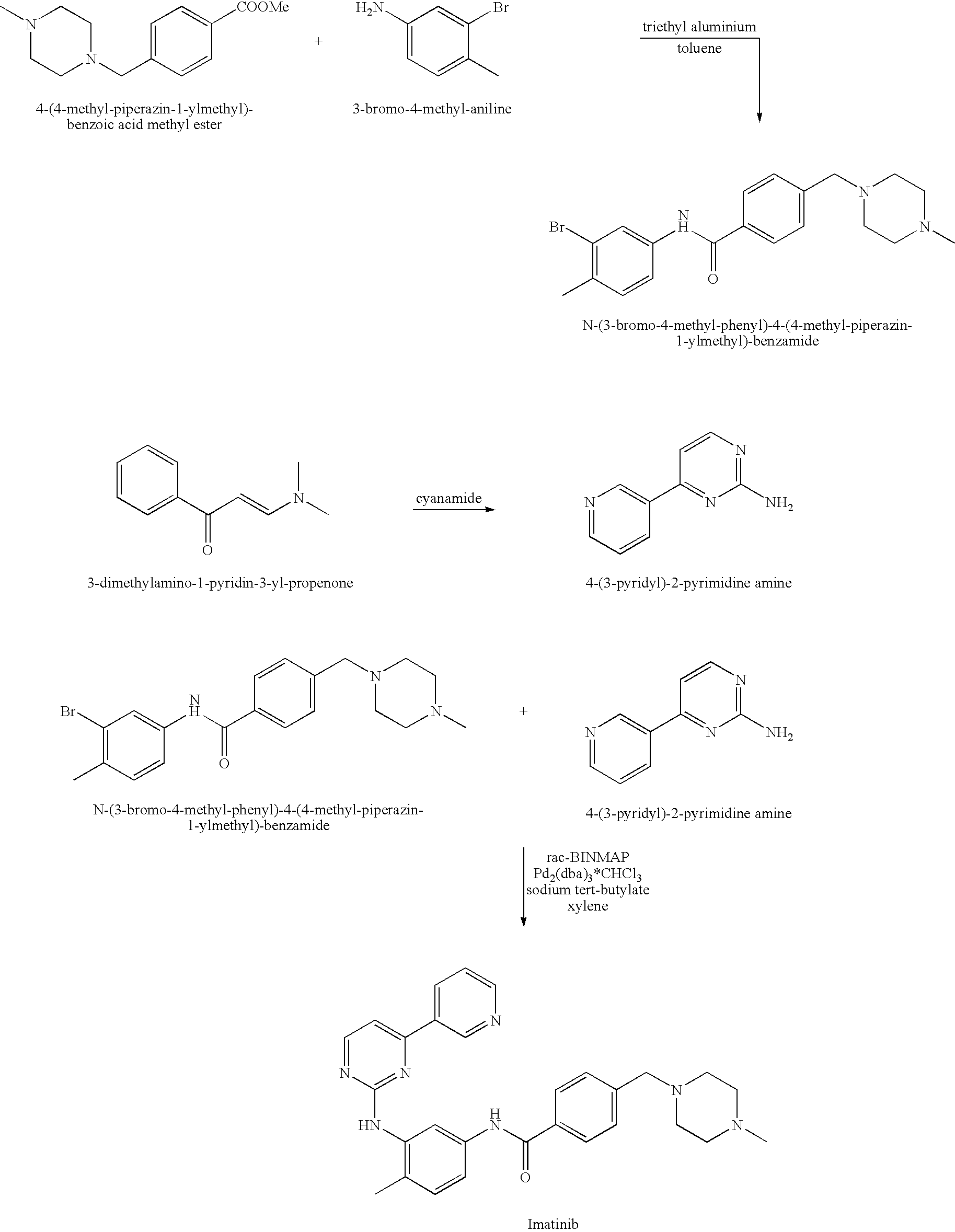
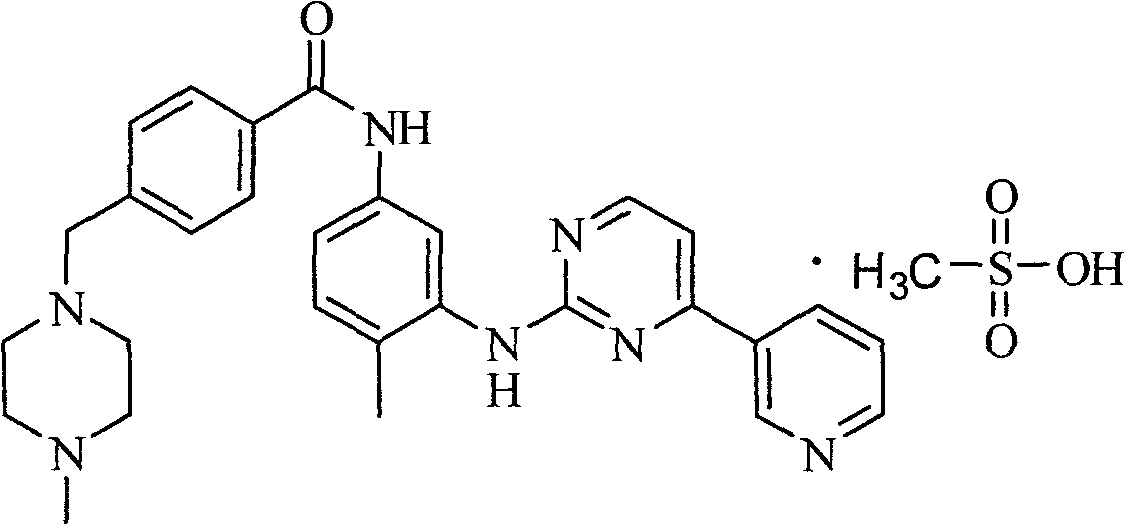
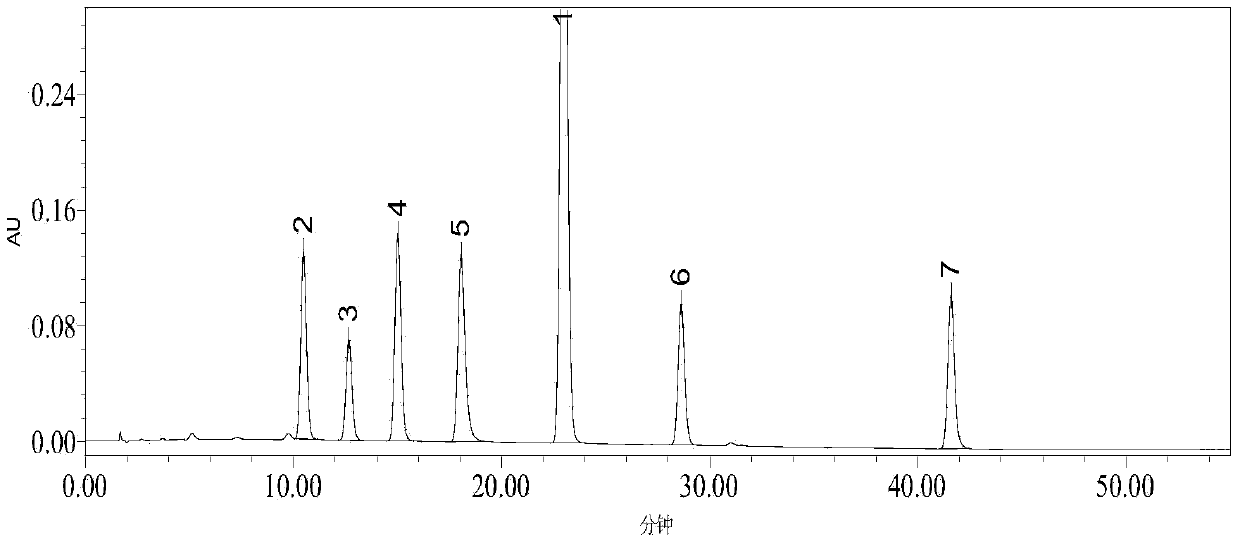
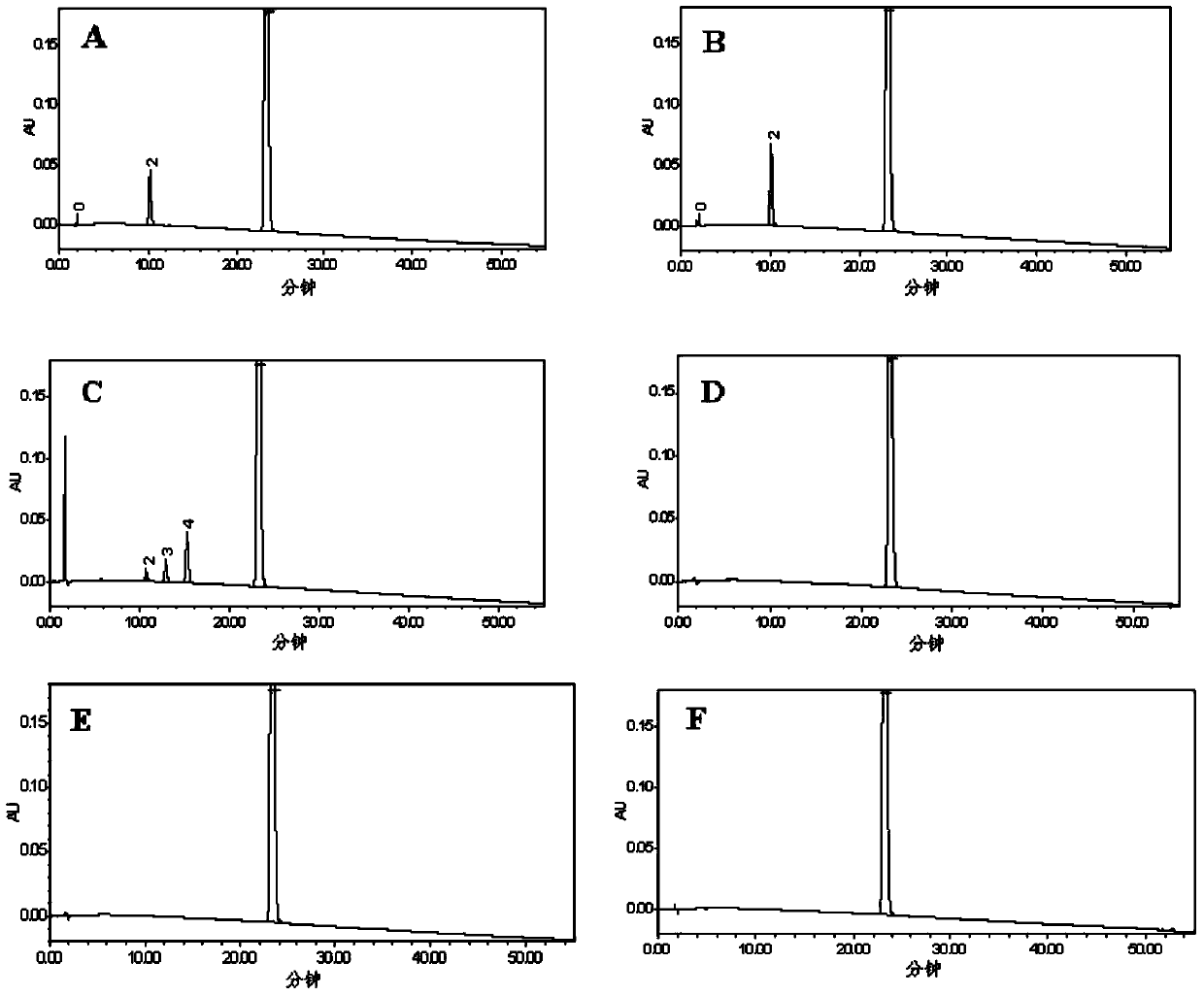
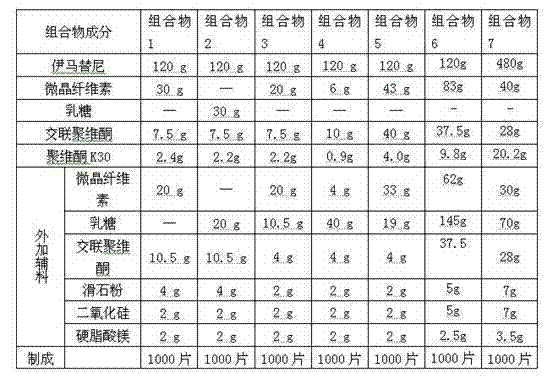
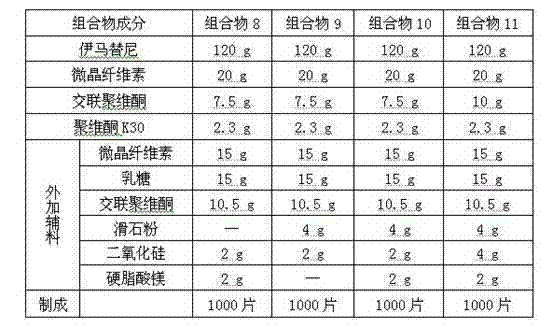
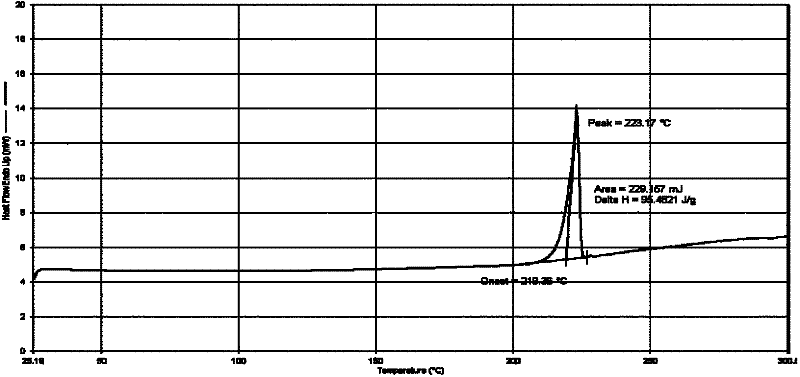
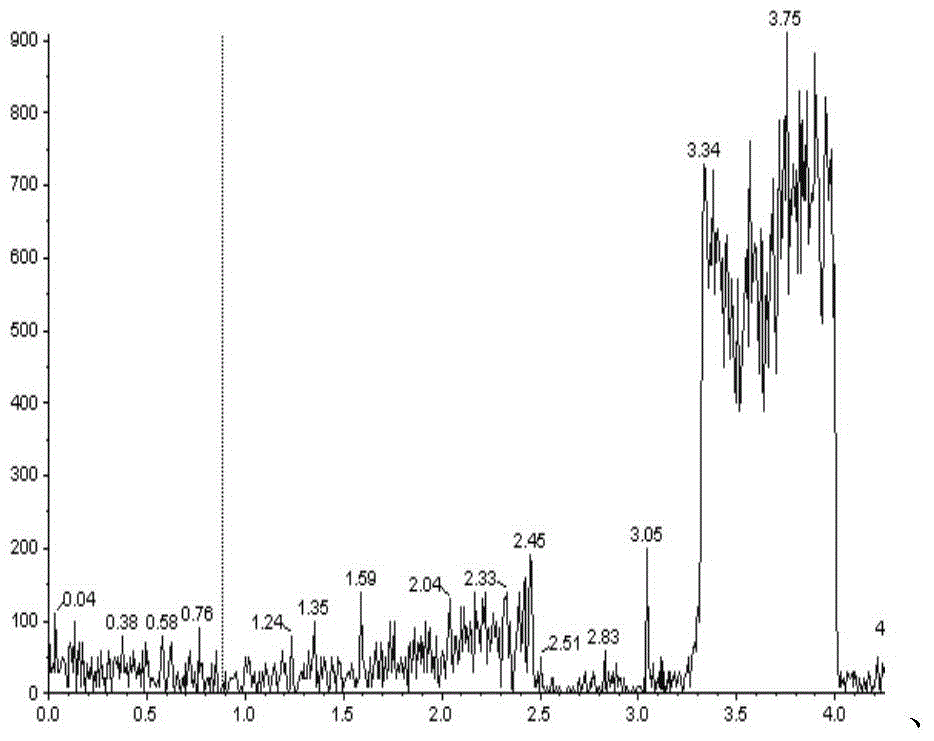
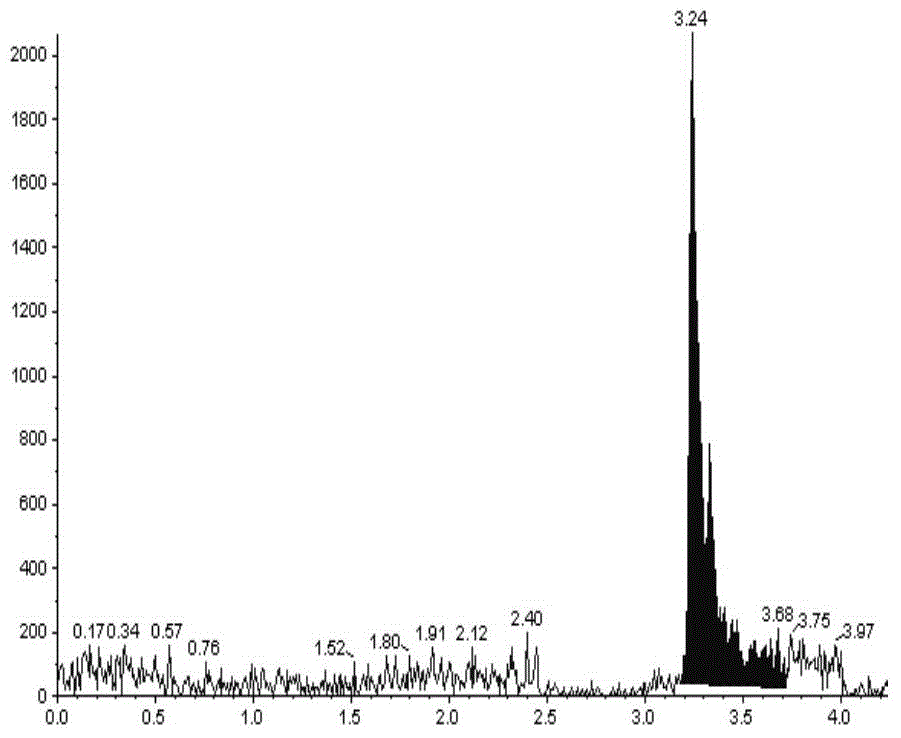
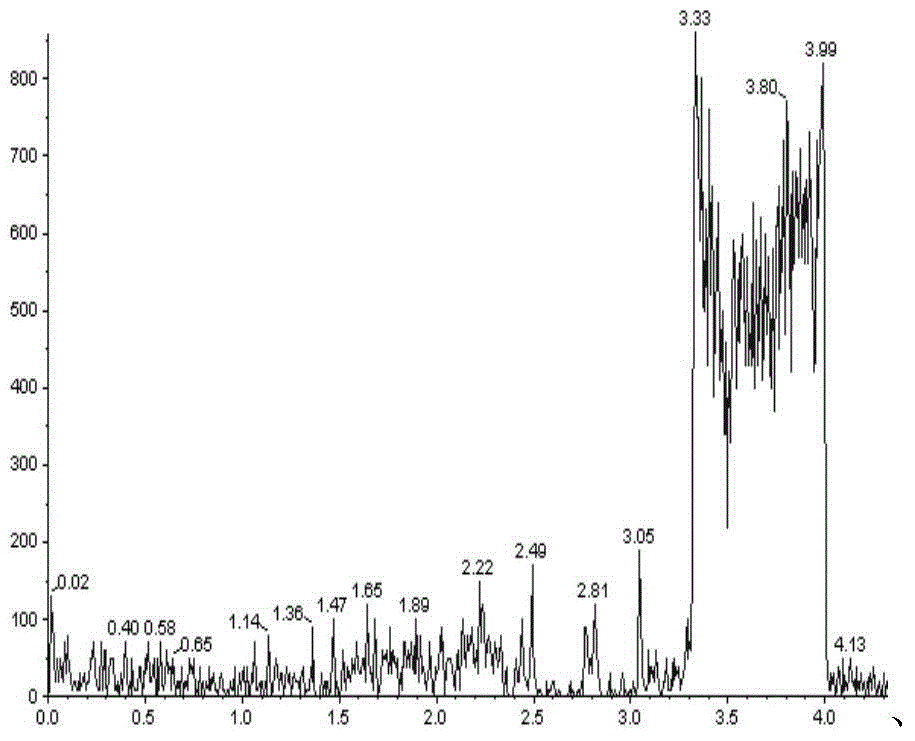
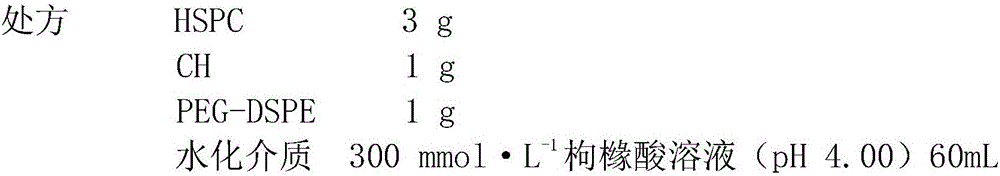Patents
Literature
136 results about "Imatinib mesylate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
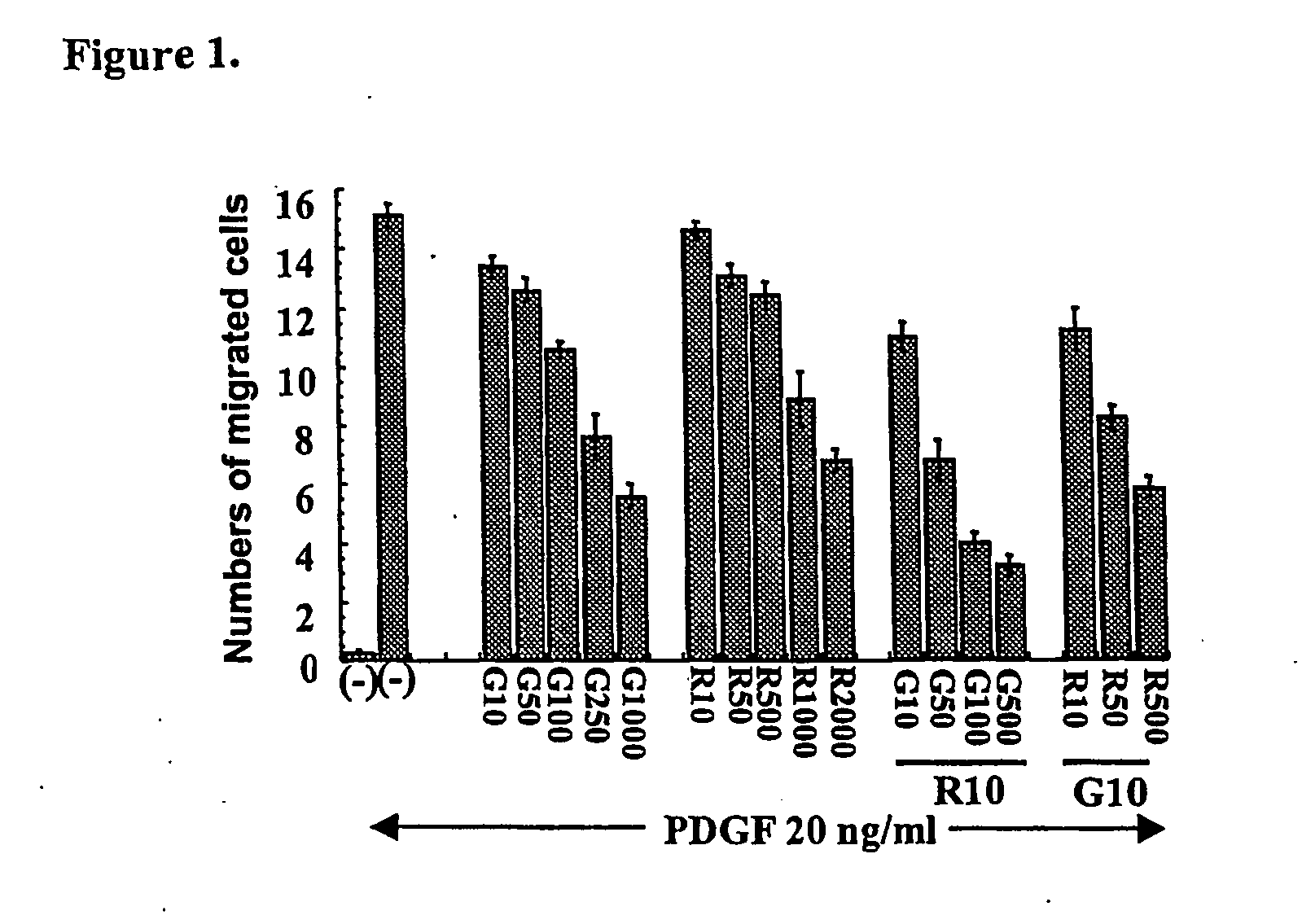
Methods and compounds for the treatment of vascular stenosis
This invention features a method of treatment for vascular stenosis or restenosis using a combination of N-phenyl-2-pyrimidine derivatives such as imatinib mesylate and PI3K inhibitors, such as rapamycin.
Owner:BETH ISRAEL DEACONESS MEDICAL CENT INC
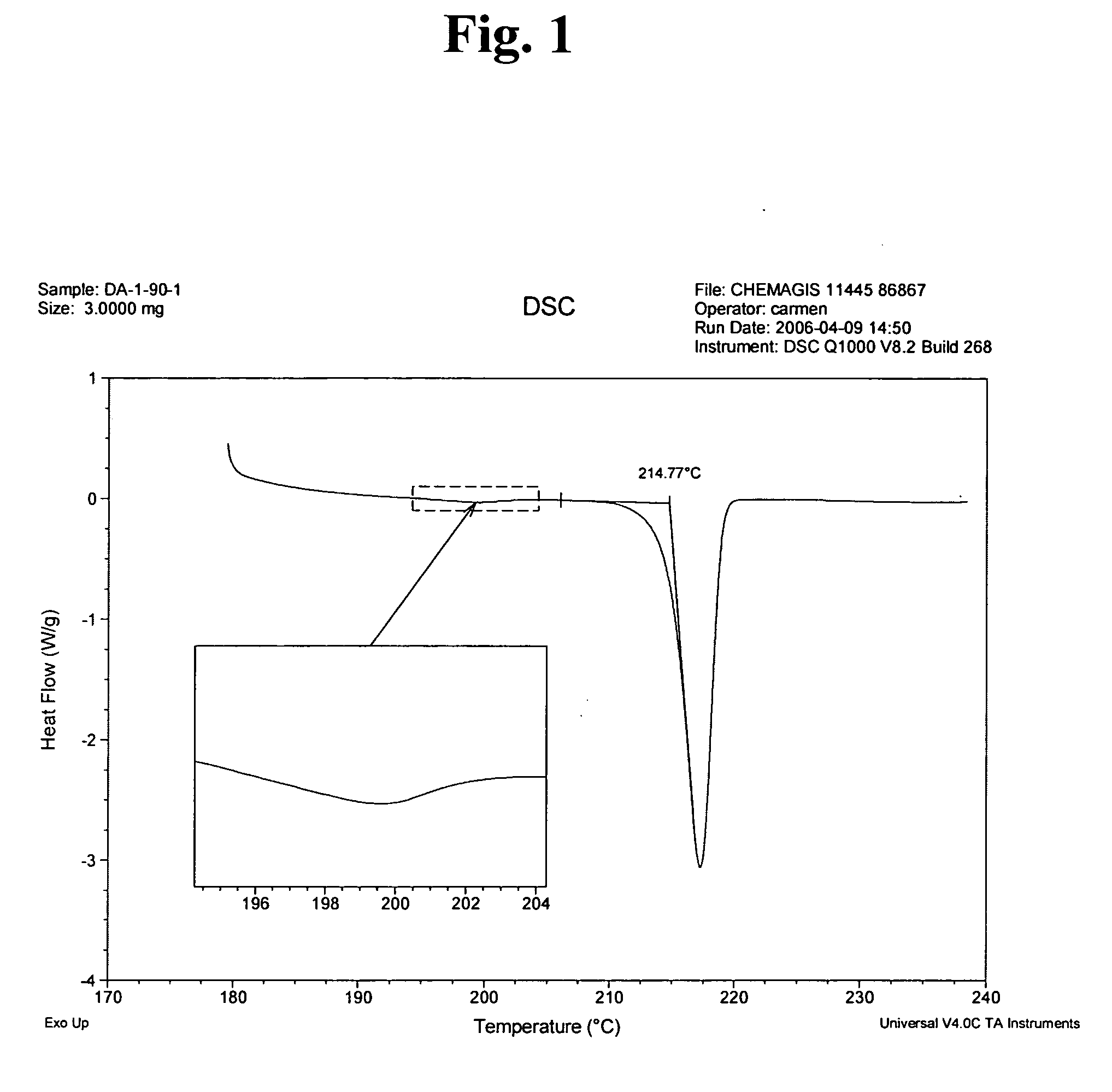
Imatinib mesylate alpha form and production process therefor
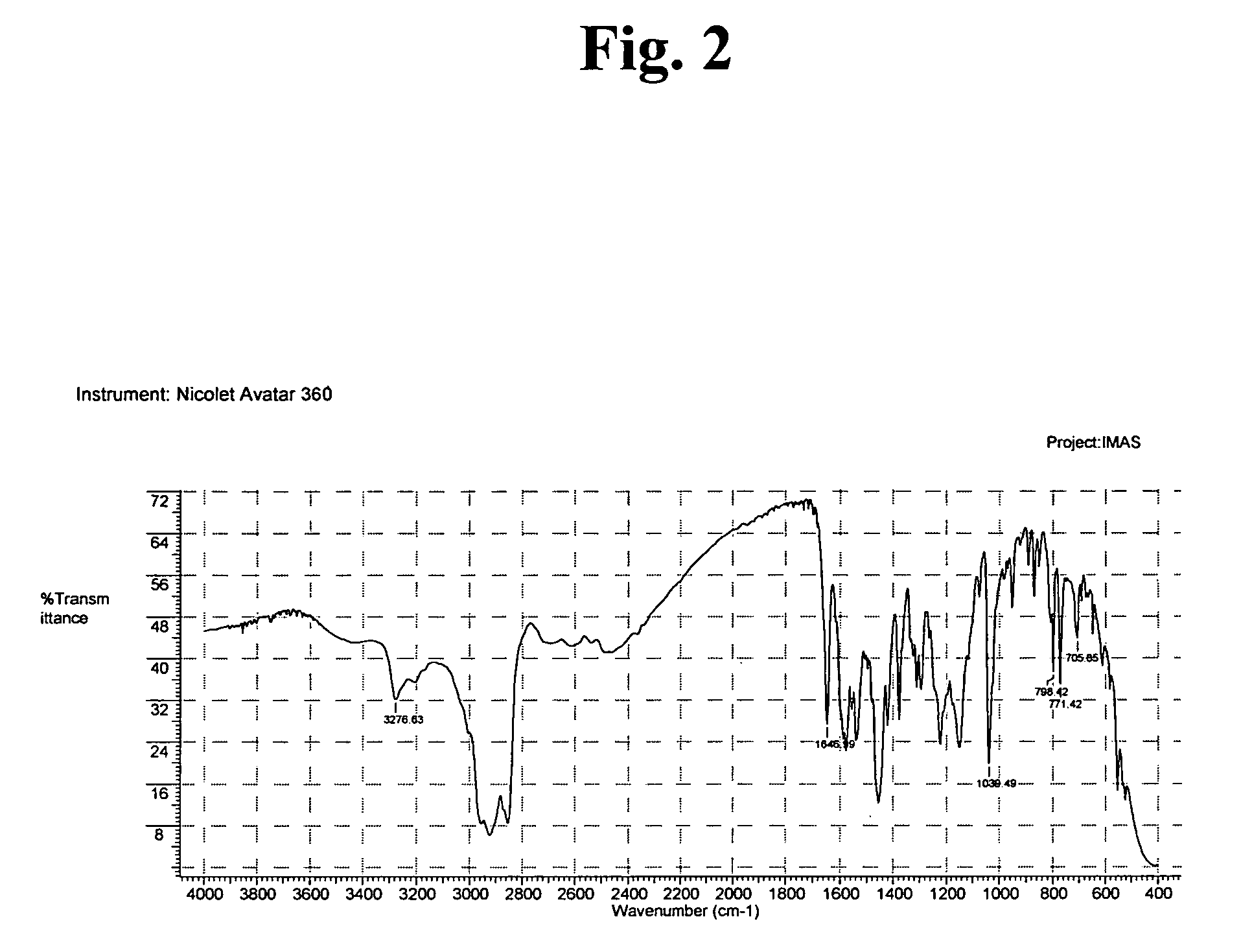
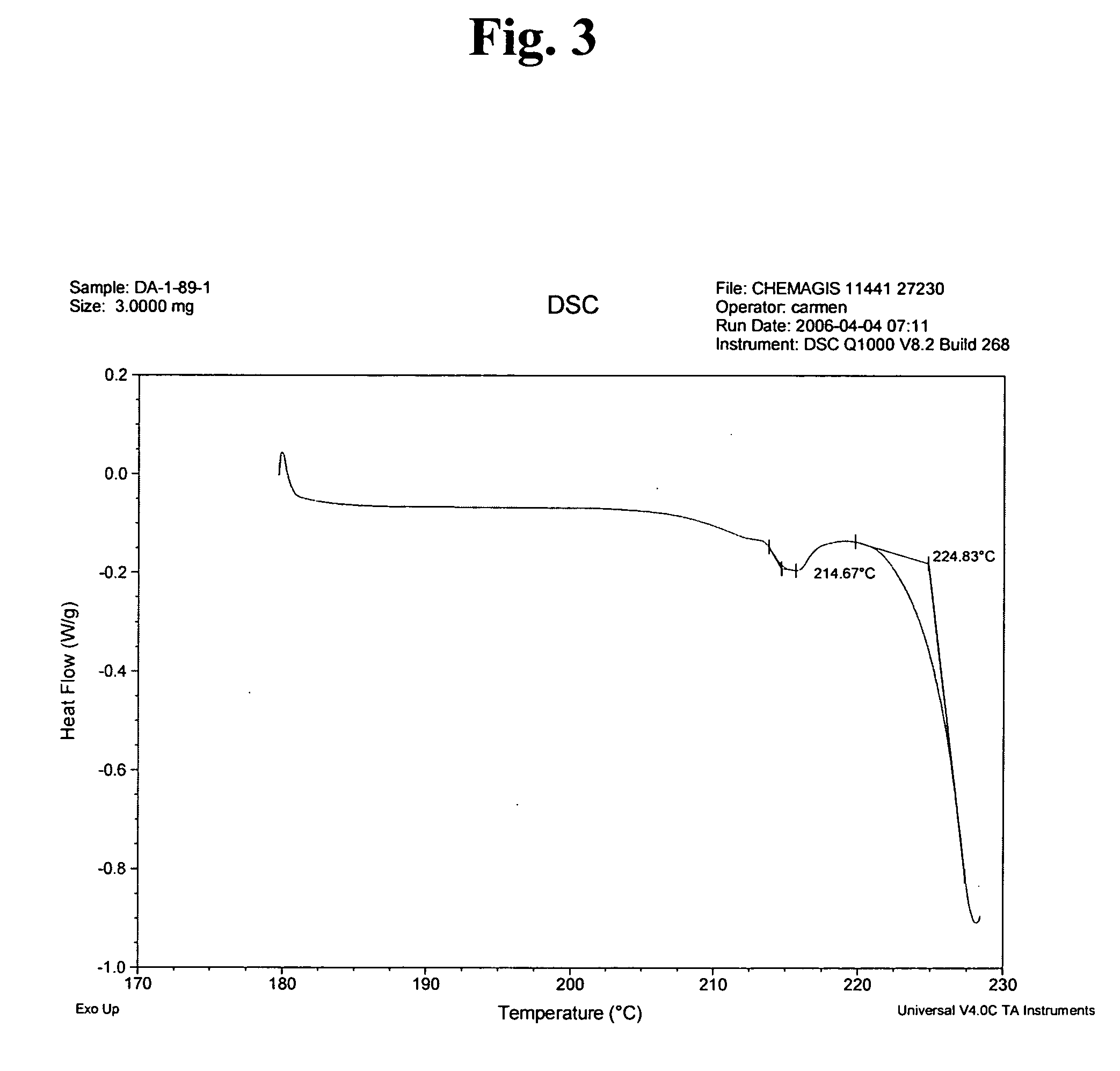
InactiveUS20060223816A1Appropriate compositionOrganic active ingredientsOrganic chemistryOrganic solventSeed crystal
Provided is a process for preparing crystalline imatinib mesylate in substantially pure α-form, which preferably includes crystallizing imatinib mesylate from an organic solvent containing imatinib and methanesulfonic acid, and seed crystals of imatinib mesylate α-form, wherein the seed crystals are added before imatinib mesylate begins to precipitate from the mixture. Also provided are stable, free-flowing imatinib mesylate crystals in substantially pure α-form, and a pharmaceutical composition containing the stable, free-flowing imatinib mesylate crystals.
Owner:CHEMAGIS
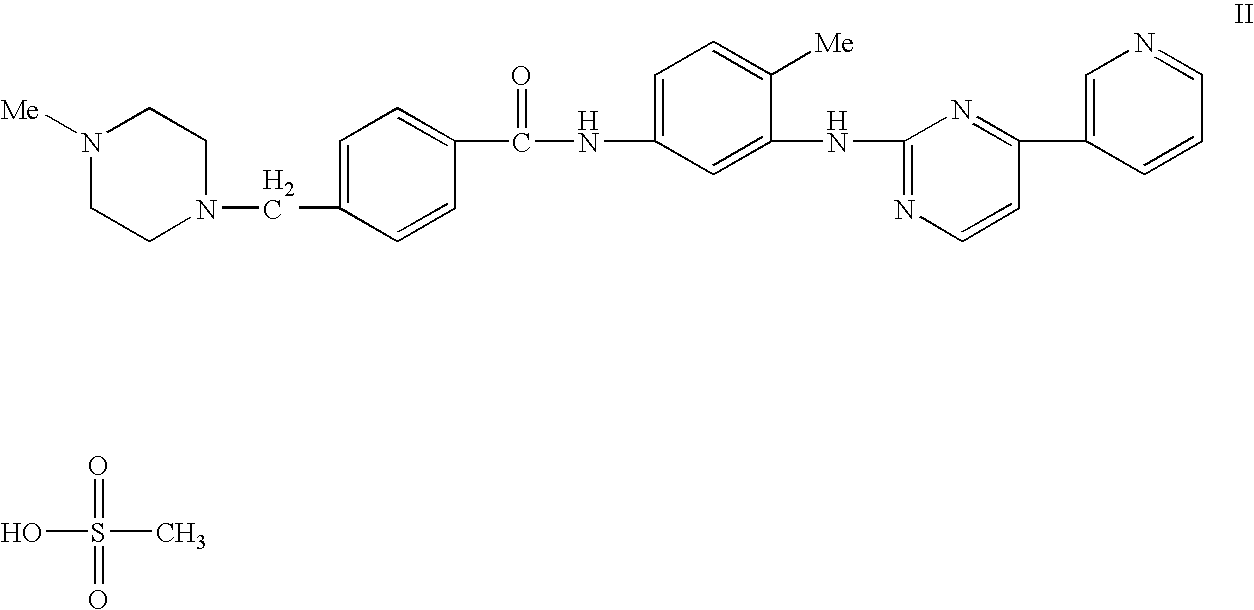
Medical devices comprising a protein-tyrosine kinase inhibitor to inhibit restonosis
Implantable medical devices having an anti-restenotic coatings are disclosed. Specifically, implantable medical devices having coatings of proteintyrosine kinase inhibitors are disclosed. The anti-restenotic protein-tyrosine kinase inhibitor is 4+4-Methyl-1-piperazinyl)methyl]-N-[4-methyl-3-[[4-(3-pyridinyl)-2pyrimidinyl]amino]-phenyl]benzamide methanesulfonate and pharmaceutically acceptable derivatives thereof (imatinib mesylate). The anti-restenotic medial devices include stents, catheters, micro-particles, probes and vascular grafts. The medical devices can be coated using any method known in the art including compounding the protein-tyrosine kinase inhibitor with a biocompatible polymer prior to applying the coating. Moreover, medical devices composed entirely of biocompatible polymer-protein-tyrosine kinase inhibitor blends are disclosed. Additionally, medical devices having a coating comprising at least one proteintyrosine kinase inhibitor in combination with at least one additional therapeutic agent are also disclosed. Furthermore, related methods of using and making the antirestenotic implantable devices are also disclosed.
Owner:MEDTRONIC VASCULAR INC
Stable Crystal Form of Imatinib Mesylate and Process for the Preparation Thereof
InactiveUS20070265288A1Stable and non-hygroscopicUseful in therapyOrganic active ingredientsBiocideMethane sulfonic acidPyridine
The invention relates to a stable, non hygroscopic alpha crystalline form of methane sulfonic acid addition salt of 4-(4-methyl piperazin-1yl methyl)-N-[4-methyl-3-(4-pyridin-3-yl) pyrimidin-2-yl amino)phenyl]-benzamide (imatinib mesylate). A process for the preparation of the crystalline form is also described.
Owner:CIPLA LTD
Imatinib production process
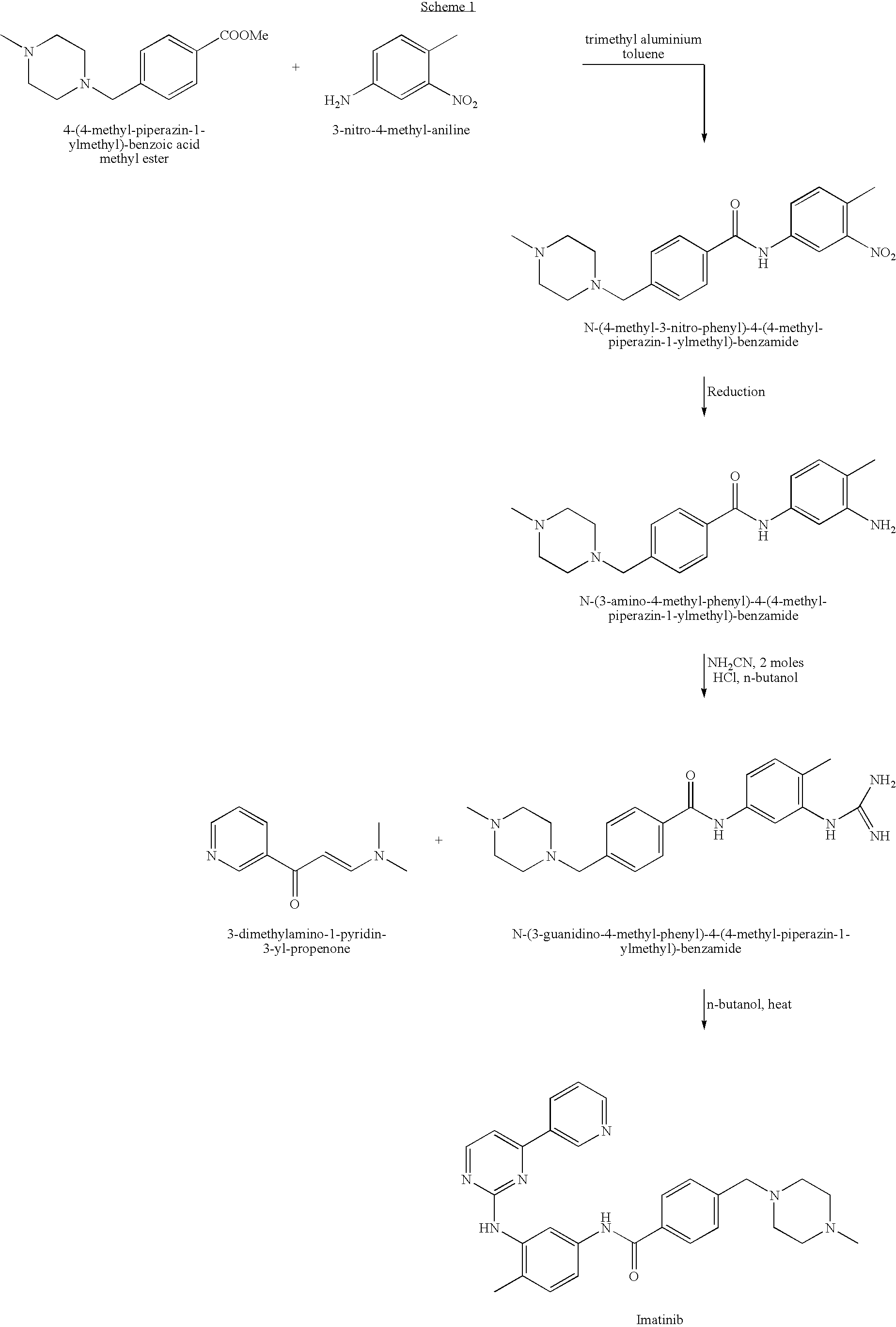
Provided is a process for producing imatinib and salts thereof, e.g., imatinib mesylate. The process includes reacting4-(4-methyl-piperazin-1-ylmethyl)-benzoic acid with N-(5-amino-2-methylphenyl)-4-(3-pyridyl)-2-pyrimidine-amine in the presence of a carboxylic acid coupling reagent, to produce imatinib, and optionally converting the imatinib into a salt.
Owner:WAVELENGTH ENTERPRISES LTD
Imatinib mesylate composition and preparation method thereof
ActiveCN102349874AReduce usagePromote dissolutionOrganic active ingredientsPill deliveryBULK ACTIVE INGREDIENTOncology
The invention provides an imatinib mesylate composition and a preparation method thereof. The imatinib mesylate composition is prepared from imatinib mesylate used as an active ingredient through a dry granulation process, and the dissolution rate of imatinib mesylate is effectively improved.
Owner:CSPC ZHONGQI PHARM TECH (SHIJIAZHUANG) CO LTD +1
Effervescent tablet containing imatinib mesylate and preparation method thereof
InactiveCN101401797AEasy to storeEasy to carryOrganic active ingredientsPharmaceutical delivery mechanismEffervescent tabletPharmacy
The invention relates to the technical field of pharmaceutical preparation, in particular to an effervescent tablet containing imatinib mesylate and a method for preparing the same. The effervescent tablet comprises 25 to 500 milligrams of the imatinib mesylate and an acid base pair which is acceptable in pharmacy; furthermore, a filling agent, an adhesive, a disintegrating agent, a lubricating agent, a sweetening agent and a flavour modifying agent which are acceptable in pharmacy can be added in the effervescent tablet. The effervescent tablet containing the imatinib mesylate has the advantages of faster action speed compared with the tablets and capsulated drugs in the market, has convenient use and good taste in taking, and is more suitable for children, the elderly, and patients who can not swallow solid medicines.
Owner:BEIJING TRADE STAR MEDICAL TECH
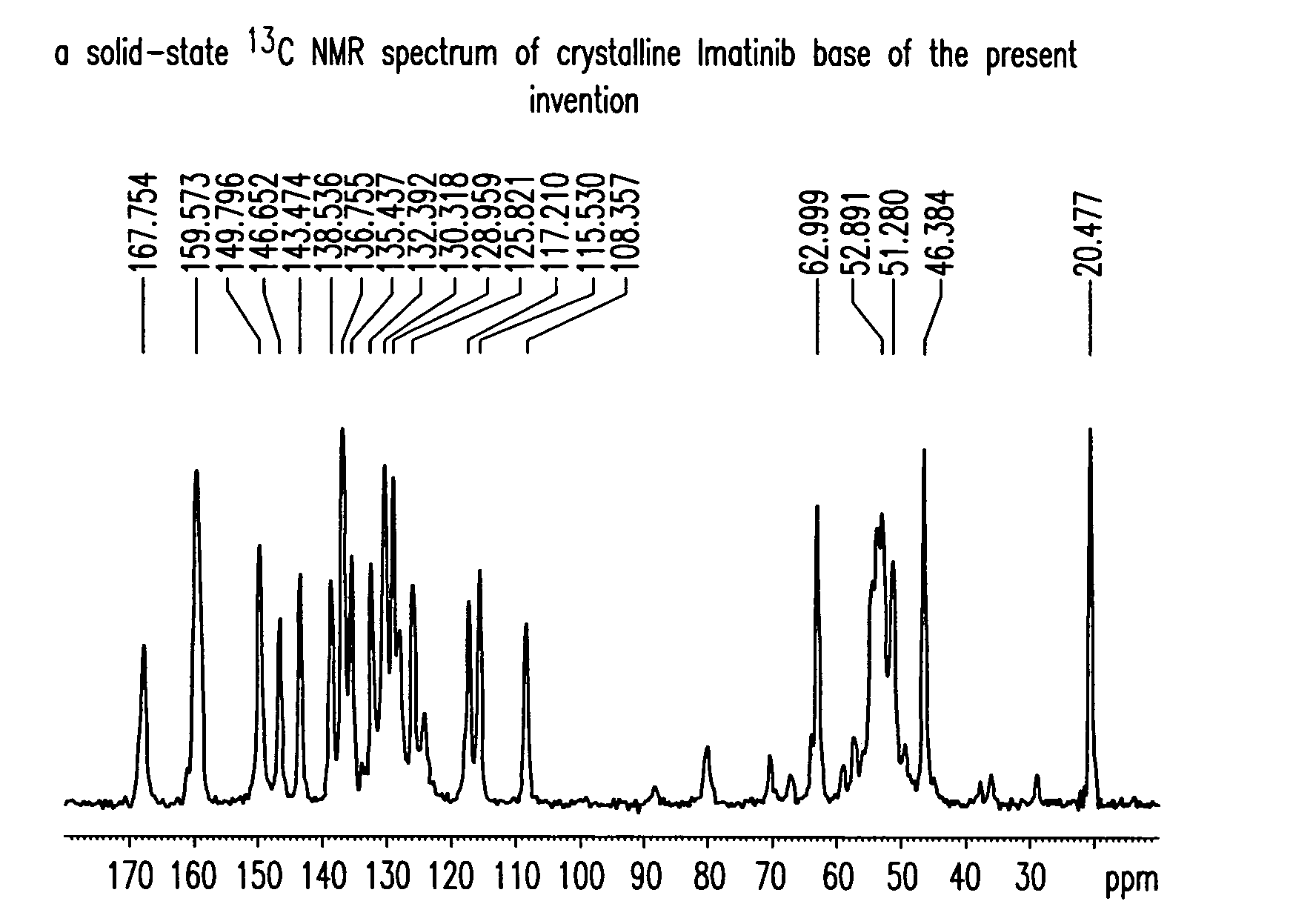
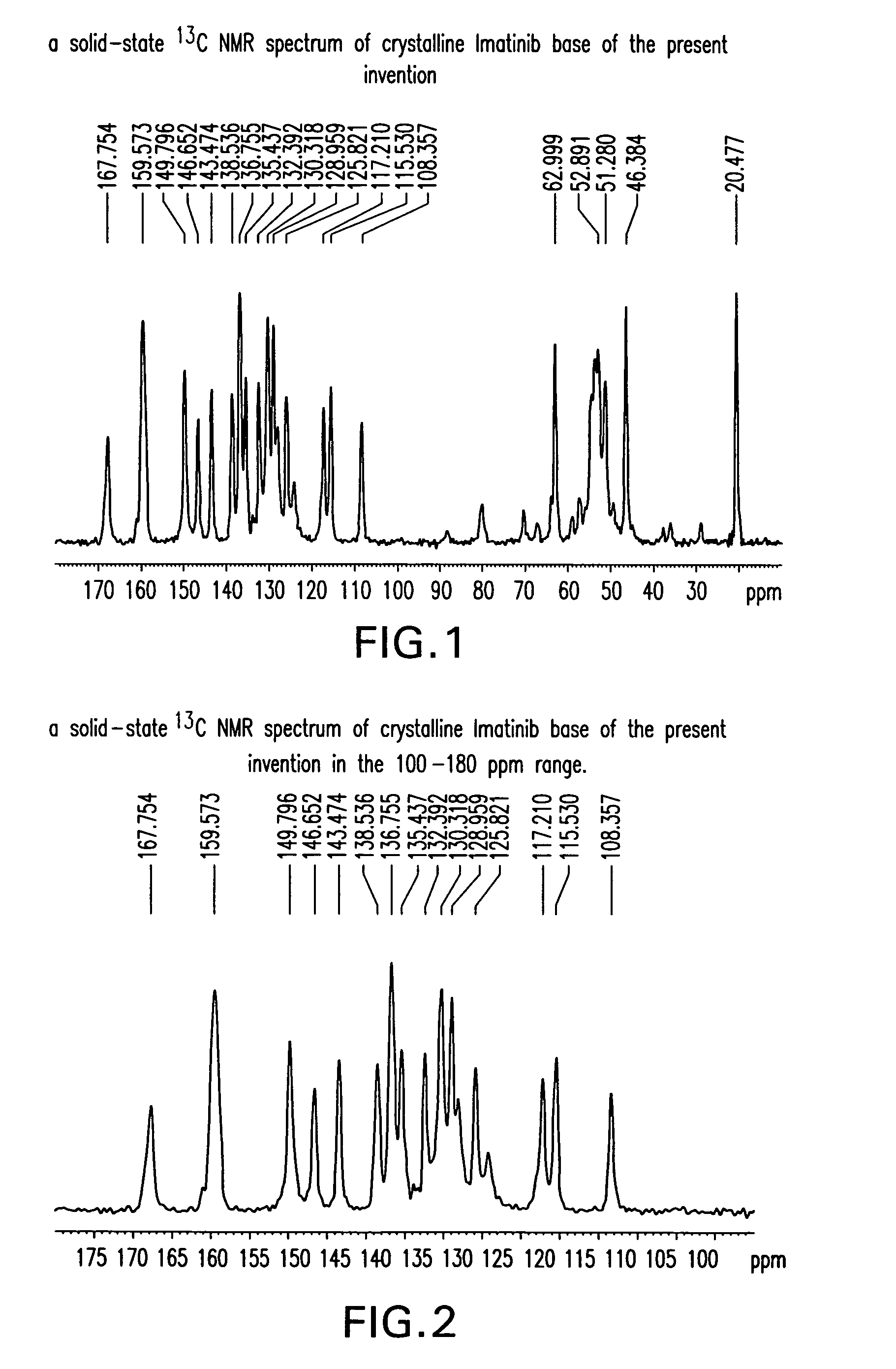
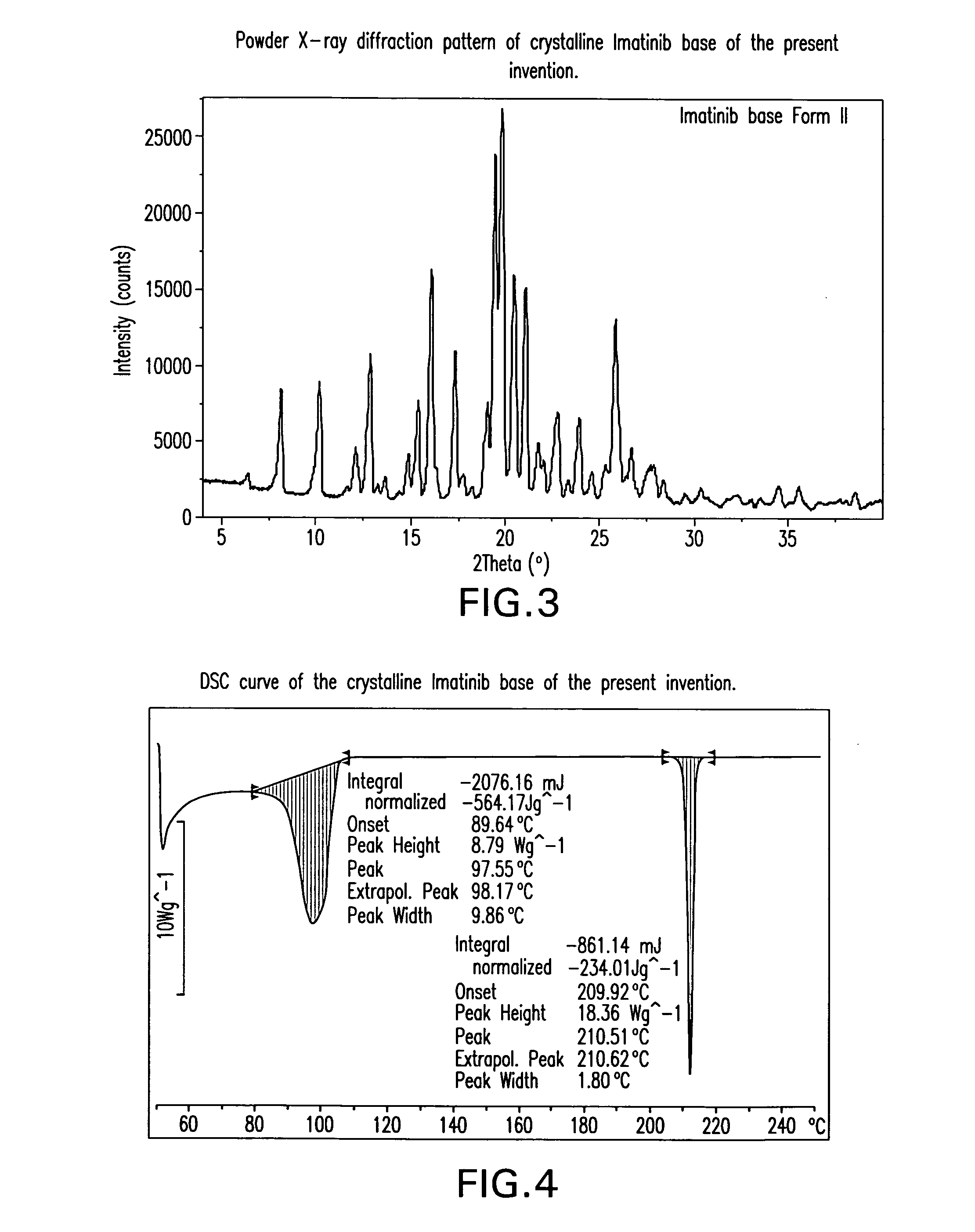
Imatinib base, and imatinib mesylate and processes for preparation thereof
The present invention provides crystalline forms of imatinib base, imatinib base free of desmethyl imatinib, and imatinib mesylate free of desmethyl imatinib mesylate, processes of their preparation and pharmaceutical compositions of imatinib mesylate.
Owner:SICOR INC
Methods and compositions for the treatment of graft failure
InactiveUS20050261283A1Reduce inhibitionReduce productionBiocideOrganic chemistryPlateletTunica intima
The present invention provides methods and compositions for treating graft failure resulting from neointimal hyperplasia. These methods and compositions feature the use of platelet derived growth factor receptor (PDGFR) inhibitor compounds, such as N-phenyl-2-pyrimidine compounds (e.g., imatinib mesylate) to inhibit the biological activity of the PDGFR.
Owner:BETH ISRAEL DEACONESS MEDICAL CENT INC
Imatinib production process
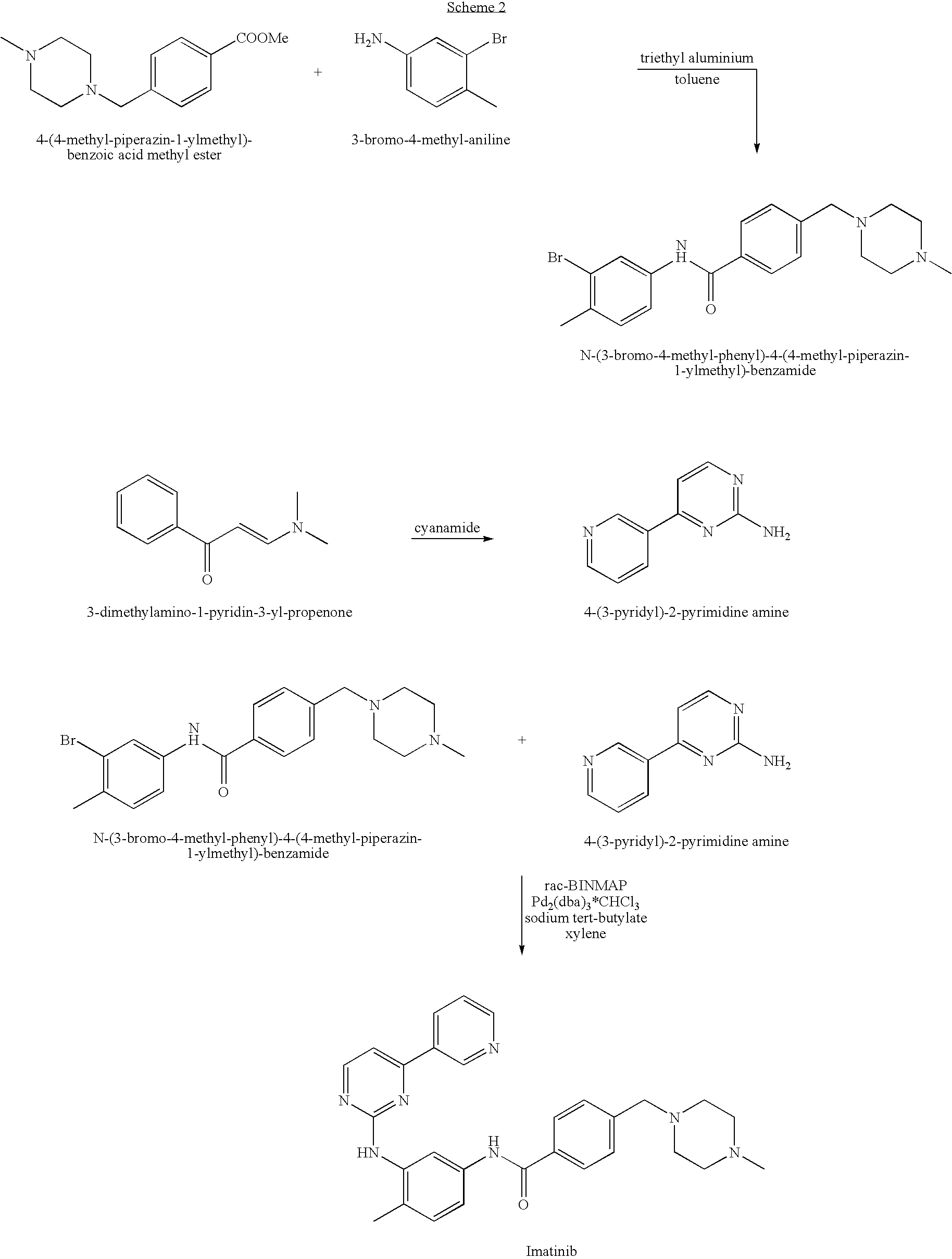
Provided is a process for producing imatinib and salts thereof, e.g., imatinib mesylate. The process includes reacting 4-(4-methyl-piperazin-1-ylmethyl)-benzoic acid with N-(5-amino-2-methylphenyl)-4-(3-pyridyl)-2-pyrimidine-amine in the presence of a carboxylic acid coupling reagent, to produce imatinib, and optionally converting the imatinib into a salt.
Owner:WAVELENGTH ENTERPRISES LTD
Imatinib mesylate orally disintegrating tablets and preparation method thereof
InactiveCN101401795APromote dissolutionQuickly exert the therapeutic effect of the whole bodyOrganic active ingredientsPill deliveryOrally disintegrating tabletPharmaceutical formulation
The invention relates to the technical field of pharmaceutical preparation, in particular to an orally disintegrating tablet containing imatinib mesylate and a method for preparing the same, wherein the orally disintegrating tablet comprises effective amount of the imatinib mesylate and a pharmaceutic adjuvant which is acceptable in pharmacy and can rapidly collapse and release drugs in an oral cavity. The orally disintegrating tablet containing the imatinib mesylate has the advantages of faster action speed compared with the prior tablets and capsulated drugs, has convenient use and good taste in taking, and is more suitable for children, the elderly, and patients who can not swallow solid medicines.
Owner:BEIJING TRADE STAR MEDICAL TECH
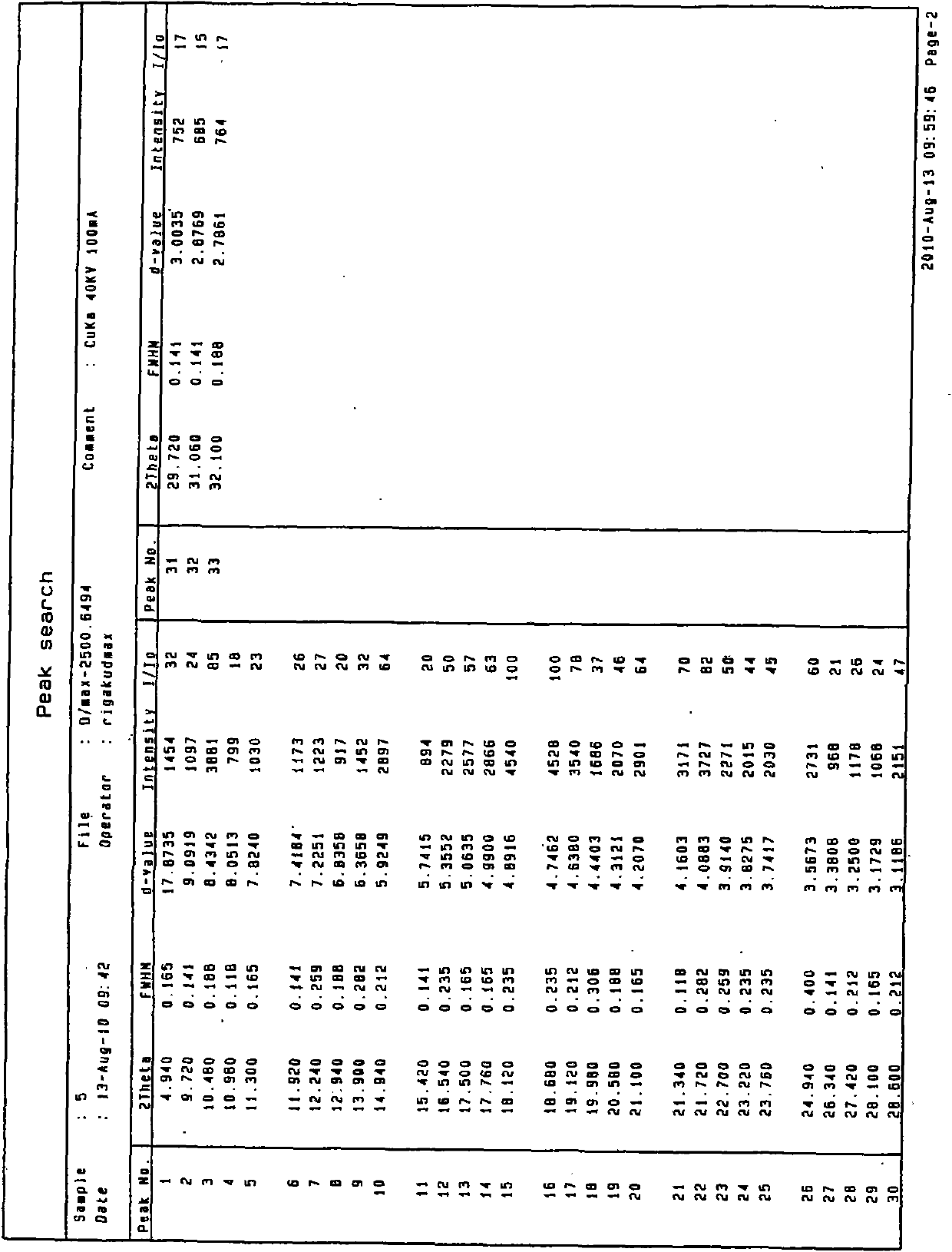
Method for determining content and impurity limit of imatinib mesylate based on HPLC-DAD (High performance liquid chromatography-diode array detection) method
ActiveCN103852544AAvoid damageExtended service lifeComponent separationGradient elutionChromatography column
The invention discloses a method for determining the content and the impurity limit of imatinib mesylate based on a HPLC-DAD (High performance liquid chromatography-diode array detection) method. A C18 chromatographic column is used; a methanol-triethylamine water solution is used as a flowing phase; the volume percentage concentration of the triethylamine water solution is 0.5-1 percent; the pH value of the triethylamine water solution is 7.5-11; the flowing speed is 0.8-1.2 ml / min and the detection wavelength is 200-400 nm during a gradient elution process; the process of the gradient elution lasts for 0-55 minutes and the volume percentage concentration of the triethylamine water solution is 65-15 percent. Under the chromatographic condition, the separation degrees among all components are greater than 2.0; the linear range ranges from 9.09 to 90.92 microgram.ml<-1> (r=0.9998); the detection limit is 0.3ng. The method is simple, accurate, high in sensitivity and good in repeatability, and avoids the influence on agents and the service life of the chromatographic column due to octane sulfonic acid sodium ions.
Owner:JIANGSU KANION PHARMA CO LTD
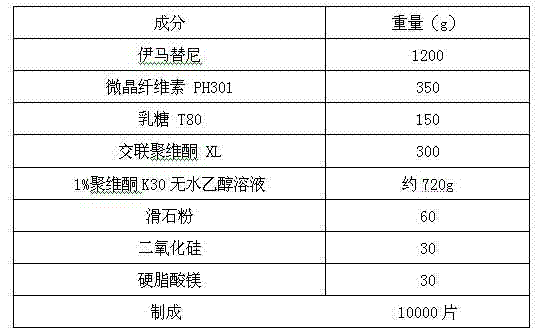
Imatinib-containing composition and preparation method thereof
InactiveCN102240291ALow costImprove stabilityOrganic active ingredientsAntineoplastic agentsMagnesium stearateLactose
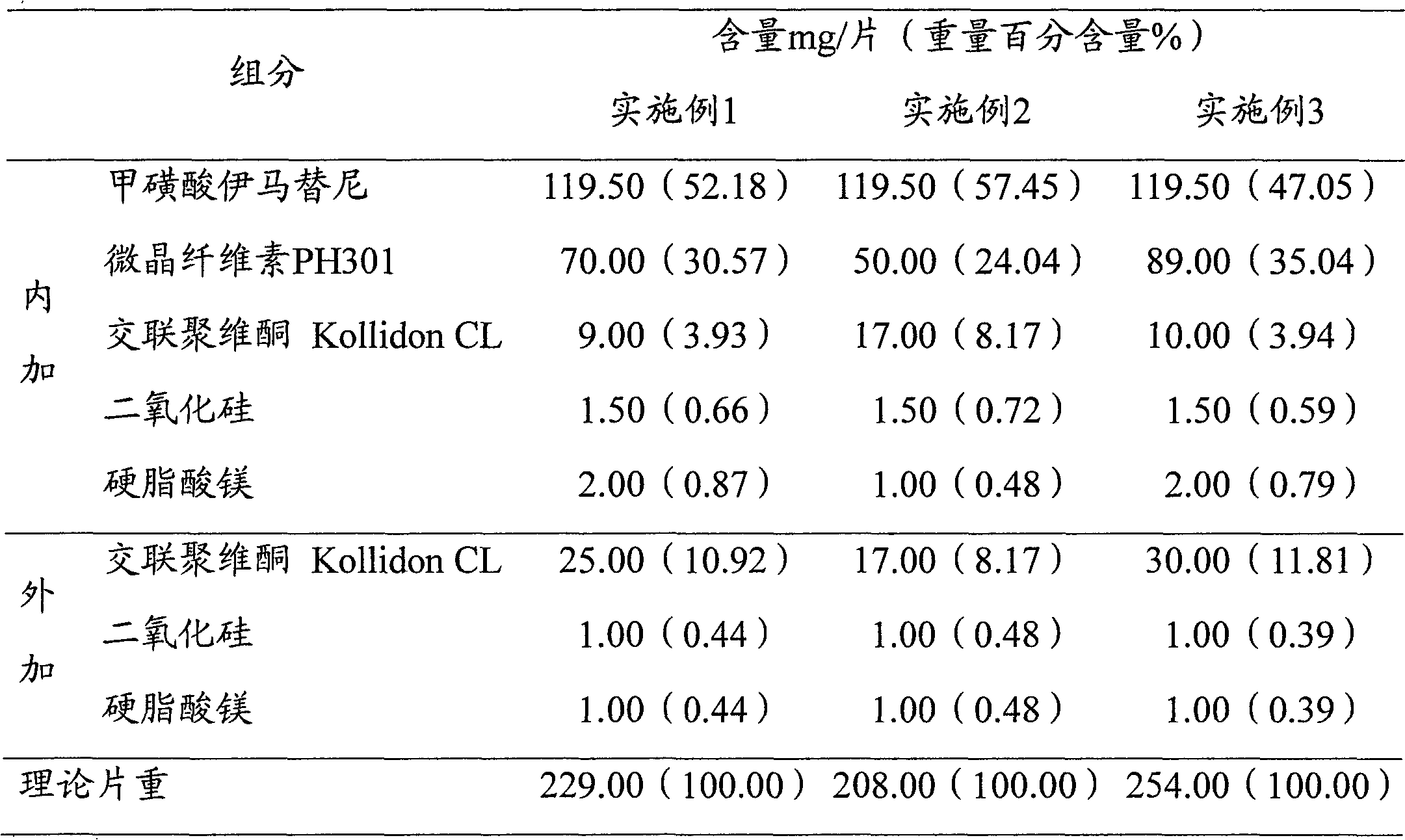
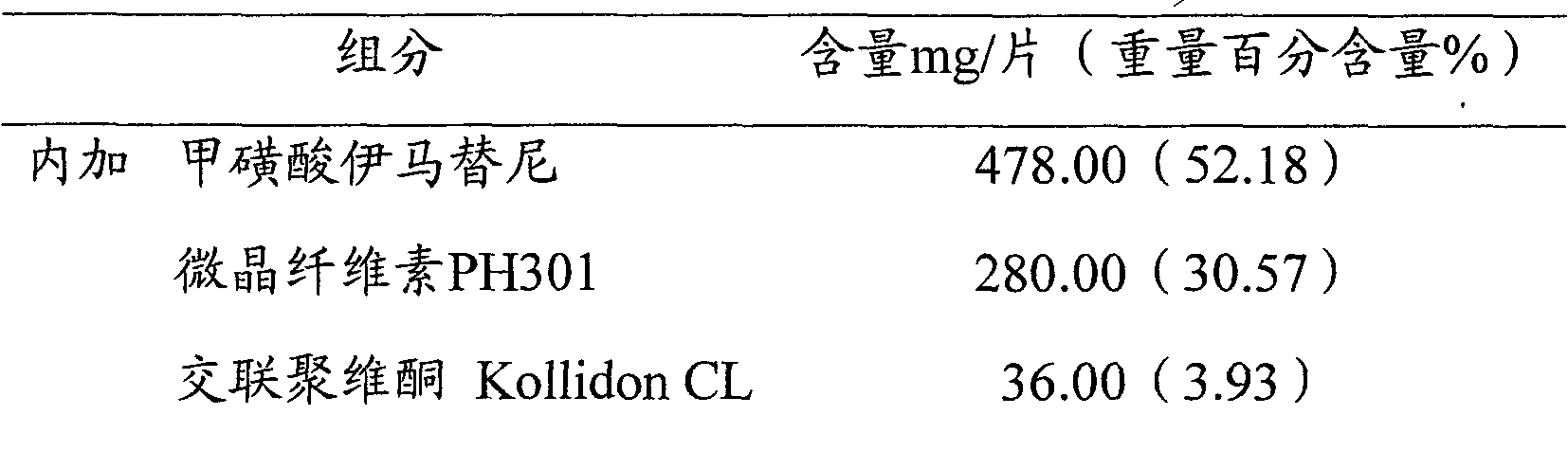
The invention relates to an imatinib-containing composition and a preparation method thereof, belonging to the technical field of pharmaceutical preparations. The imatinib-containing composition disclosed by the invention is composed of imatinib, a diluent, a disintegrating agent, an adhesive and a lubricant, wherein the imatinib is imatinib mesylate alpha crystal; the diluent is a mixture of microcrystalline cellulose and lactose; the disintegrating agent is cross-linked polyvidone; the adhesive is polyvidone K30; and the lubricant is a mixture of magnesium stearate, talc powder and silica micropowder. The preparation method is simple and efficient in process, and can be used for industrial mass production, and the problem that the imatinib mesylate alpha crystal has strong hydroscopicityis and is not easy to form preparation is completely solved.
Owner:HANGZHOU HUADONG MEDICINE GRP PHARMA RES INST
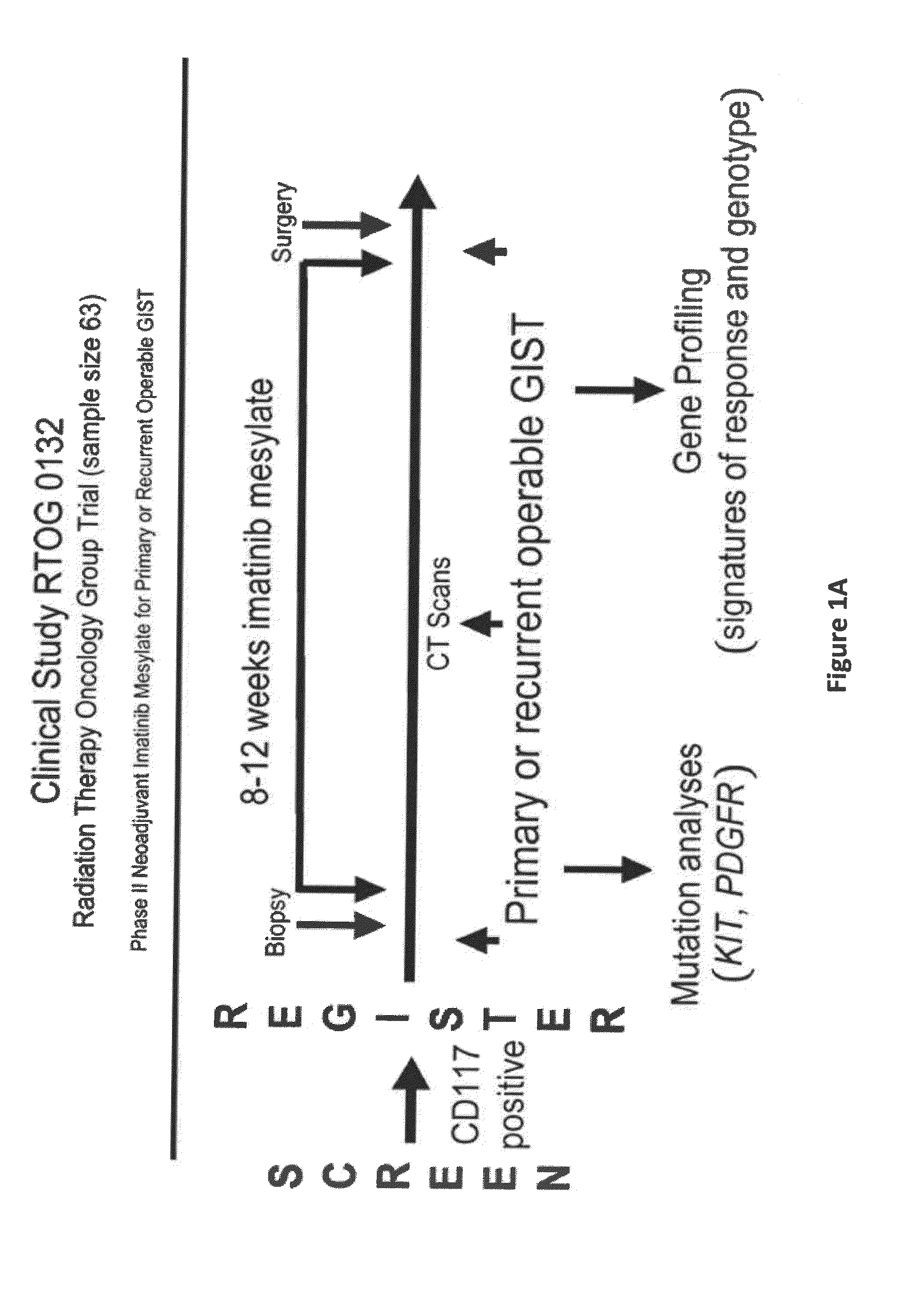
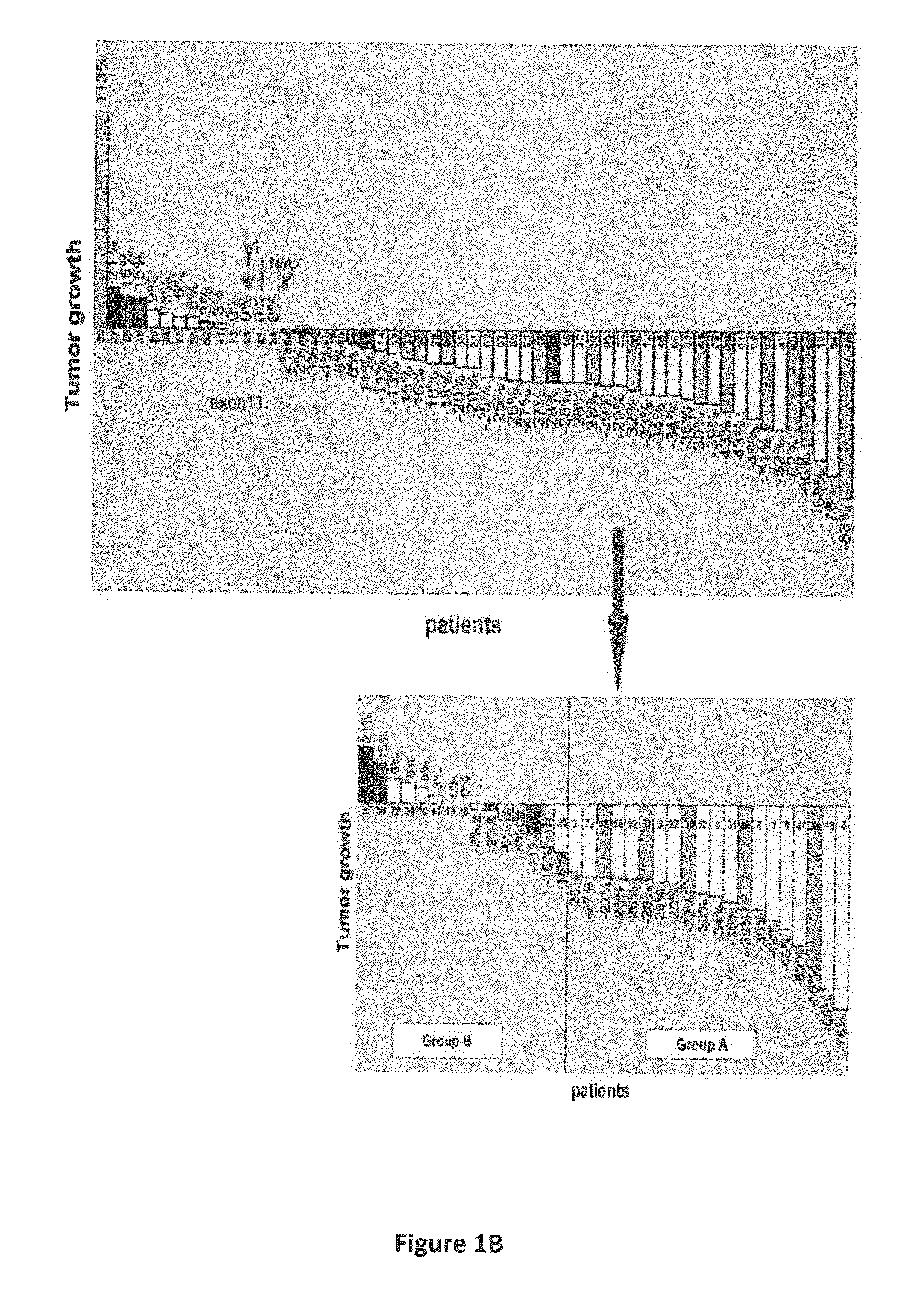
Gene expression signatures associated with response to imatinib mesylate in gastrointestinal stromal tumors and use thereof for predicting patient response to therapy and identification of agents which have efficacy for the treatment of cancer
Compositions and methods are disclosed for identifying agents useful for the treatment of malignancy, particularly GISTs which are resistant to imatinib mesylate (IM). In a preferred embodiment, agents which sensitize cancer cells to IM are provided.
Owner:FOX CHASE CANCER CENTER
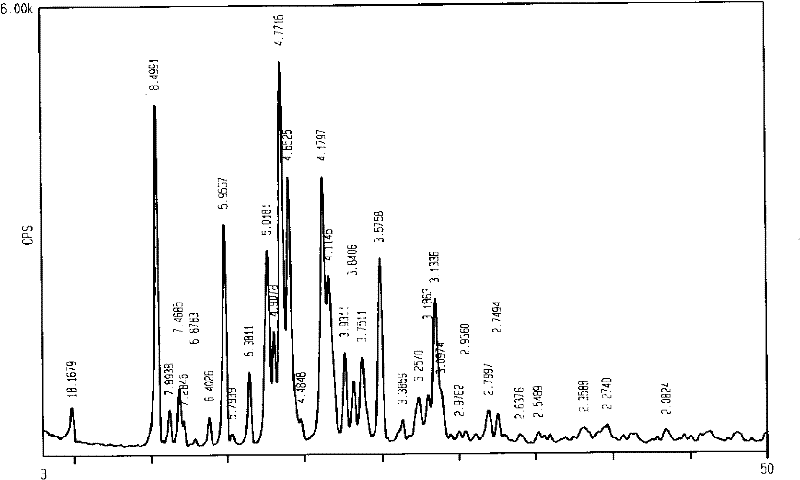
Preparation method of alpha-crystal form imatinib mesylate
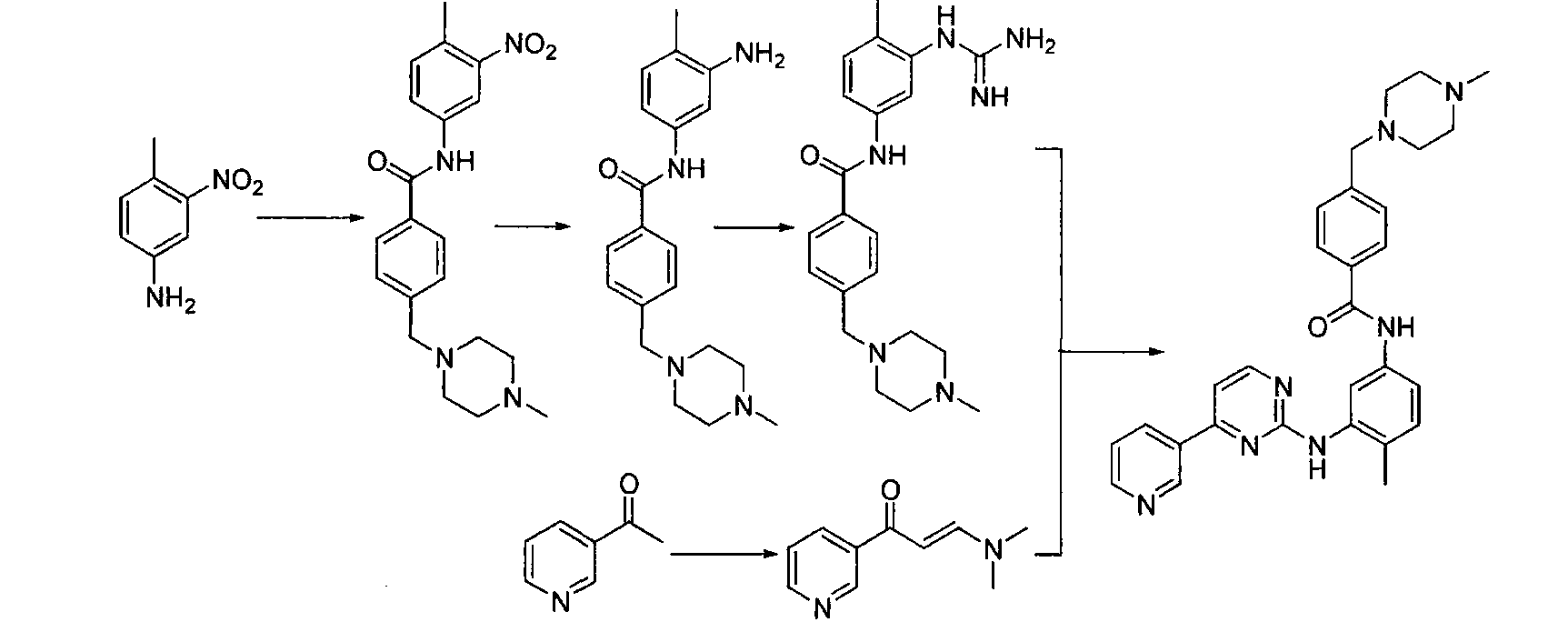
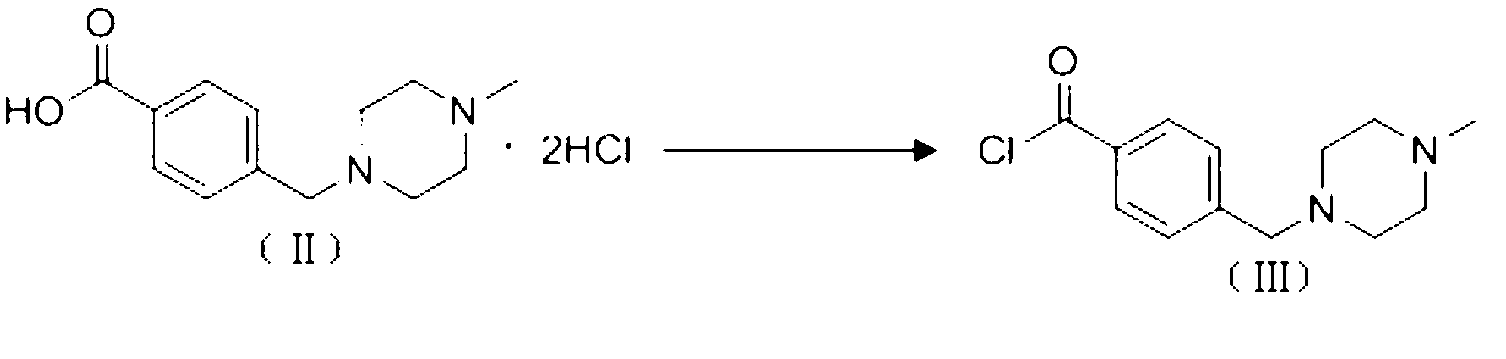
InactiveCN103058991AShort synthetic stepsEasy to operateOrganic chemistryBenzoic acidOrganic solvent
The invention discloses a preparation method of alpha-crystal form imatinib mesylate. The preparation method comprises the following steps that 1, 4-[(4-methyl piperazine-1-yl)methyl]benzoic acid dihydrochloride shown in the formula II is converted into an acyl chloride shown in the formula III by an acylating chlorination reagent; 2, the acyl chloride shown in the formula III and N-(5-amino-2-methylphenyl)-4-(3-pyridyl)-2-aminopyrimidin undergo a condensation reaction in the presence of an alkali to produce a compound shown in the formula IV; and 3, the compound shown in the formula IV and methylsulfonic acid undergo a salt forming reaction in the presence of an organic solvent to produce a compound shown in the formula I. The preparation method can realize preparation of stable alpha-crystal form imatinib mesylate and allows mild process conditions. The stable alpha-crystal form imatinib mesylate can be after-treated simply and has high purity. The preparation method has a low reaction cost and is suitable for industrial production.
Owner:NANJING CORE TECH CO LTD
Imatinib mesylate gastric-soluble pellet tablet and preparation method thereof
ActiveCN105663064AEasy to takeEasy to carryOrganic active ingredientsPill deliveryAdhesiveFluidized bed
The invention discloses an imatinib mesylate pellet tablet and a preparation method thereof. The preparation method comprises the following steps: firstly, preparing a main drug, namely imatinib mesylate, into a gastric-soluble coating pellet by a fluidized bed coating process, and then mixing the coating pellet with a filling agent, a disintegrating agent, an adhesive, a plasticizing agent, a lubricating agent and the like to prepare the gastric-soluble tablet by a compressing dry granulation technology. The tablet can be directly swallowed and can be quickly disintegrated into pellets in water so as to be convenient for patients with dysphagia and especially children to take. The accurate dose of the tablet can be ensured, and the tablet can be taken safely and conveniently.
Owner:HENAN LIFE PHARMA CO LTD
Medicinal preparation containing crystal form a imatinib mesylate
The invention discloses a medicinal preparation containing crystal form a imatinib mesylate. The medicinal preparation comprises crystal form a imatinib mesylate, a disintegrating agent, a diluent, a lubricating agent and a flow aid, wherein the using amount of the imatinib mesylate accounts for 50-80 percent of the total weight of the medicinal preparation; the using amount of the disintegrating agent is 15-30 percent of the total weight of a tablet; the using amount of the diluent is 0-20 percent of the total weight of the tablet; the using amount of the lubricating agent is 0.1-2 percent of the total weight of the tablet; and the using amount of the flow aid is 0.1-2 percent of the total weight of the tablet. Due to the adoption of the medicinal preparation prepared in the invention, the defects of poor flowability of a crystal form a and difficulty in preparing a solid preparation can be well overcome, the prepared medicinal preparation is dissolved quickly, and the crystal form does not change in preparing and storing processes.
Owner:TIANJIN TAIPU PHARMA SCI & TECH DEV
Method for preparing alpha-imatinib mesylate
ActiveCN102190649AHigh crystal purityReduce manufacturing costOrganic chemistryOrganic solventSolvent
The invention discloses a method for preparing alpha-imatinib mesylate, and belongs to the field of medicines. The method comprises the following steps of: suspending imatinib base in a mixed solvent of water and an organic solvent, stirring, and adjusting the temperature to be 20-30DEG C; dissolving methanesulfonic acid, and dripping into reaction liquid; after the dripping is finished, standingfor clarifying the liquid, heating to the temperature of between 50 and 60DEG C, and keeping the temperature to react for 0.5 to 1 hour; and reducing the temperature to 32-40DEG C, adding alpha-imatinib mesylate seed crystals, dripping the organic solvent, continuously reducing the temperature to 20-30DEG C, separating out crystals, filtering, and drying to obtain a product. The preparation method has the advantages of simple operation, short reaction time, high yield, high purity of alpha-imatinib mesylate, low production cost, and suitability for industrial production.
Owner:山东安信制药有限公司
Imatinib mesylate chewable tablet and preparation method thereof
InactiveCN101401794AImprove complianceImprove stabilityOrganic active ingredientsPill deliveryIrritationPharmaceutical formulation
The invention relates to the technical field of pharmaceutical preparation, in particular to a chewable tablet containing imatinib mesylate and a method for preparing the same. The chewable tablet containing the imatinib mesylate consists of the principal imatinib mesylate, a filling agent, a flavour modifying agent, a glidant, an odor modifying agent, and an adhesive. The chewable tablet containing the imatinib mesylate can be applied to patients having difficulty in orally taking the common tablets, has good drug compliance, can be dissolved in saliva after chewing and being absorbed by oral or esophageal mucosa, and has high bioavailability. The chewable tablet not only has sweet taste and good mouthfeel, but also modifies penetrating odors and has flavor; the chewable tablet is convenient to take and carry around; and the chewable tablet is prepared by a direct tabletting method, and has good drug stability, simple production process, and low cost.
Owner:BEIJING TRADE STAR MEDICAL TECH
Imatinib mesylate tablet and preparation method thereof
InactiveCN103222965APromote dissolutionDissolution completeOrganic active ingredientsPharmaceutical non-active ingredientsImatinib mesylateCrospovidones
The invention discloses an imatinib mesylate tablet and a preparation method thereof. The imatinib mesylate tablet comprises 8-30% of crospovidone and 8 to 40% of silica. The preparation method comprises the following steps of carrying out granulation of imatinib mesylate and a waterless organic solvent, drying the granules, uniformly mixing the granules, crospovidone, silica, a filler and a lubricant, and carrying out tabletting. The preparation method solves the problem of a slow dissolution rate of a preparation obtained by the prior art.
Owner:QINGDAO UNIV
Imatinib mesylate tablet
ActiveCN102302464AOrganic active ingredientsPharmaceutical non-active ingredientsPoor mobilityMedicine
The invention discloses an imatinib mesylate tablet. The imatinib mesylate tablet comprises the following components in percentage by weight: 40-60% of alpha-crystal imatinib mesylate, 5-20% of antisticking agent, 20-50% of disintegrating agent and 0.2-1% of lubricating agent, wherein the sum of the weight percent of each component is 100%. According to the invention, through adding the antisticking agent, the defects of poor mobility, poor compressibility, poor stickness, static electricity and the like of alpha-crystal imatinib mesylate are avoided effectively; and the imatinib mesylate tablet prepared by alpha-crystal imatinib mesylate has consistent hardness, disintegration, dissolution and other quality standards with original tablet prepared by beta-crystal imatinib mesylate, and the detect that only beta-crystal imatinib mesylate is required as the raw material for preparing the imatinib mesylate tablet in the existing industry is solved.
Owner:SHANGHAI ACEBRIGHT PHARMA CO LTD
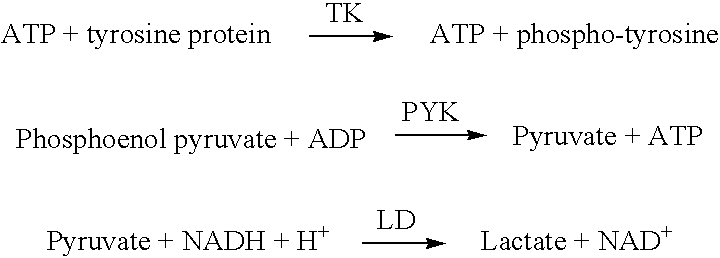
Enzymatic measurement of imatinib mesylate
ActiveUS7300768B2Economical and simpleMicrobiological testing/measurementTransferasesProtein-Tyrosine KinasesKinase
Owner:SALADAX BIOMEDICAL INC
Imatinib mesylate polymorph and pharmaceutical composition thereof
ActiveCN102260242AHigh purityGood physical and chemical propertiesOrganic active ingredientsNervous disorderMedicineImatinib mesylate
Owner:NANJING CAVENDISH BIO ENG TECH +1
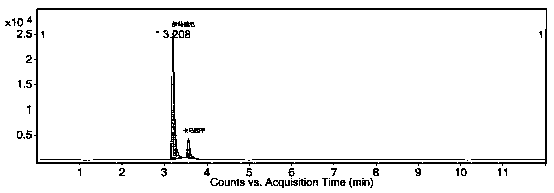
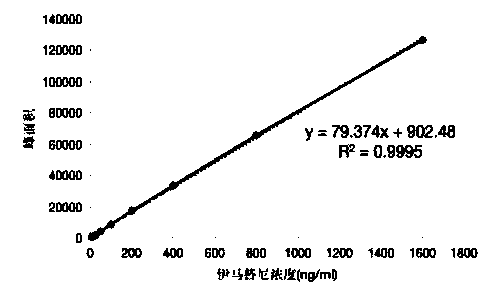
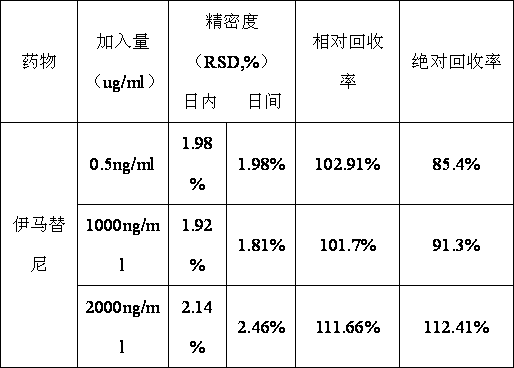
Method for determining trace imatinib in blood sample and application thereof to zero phase clinical trial
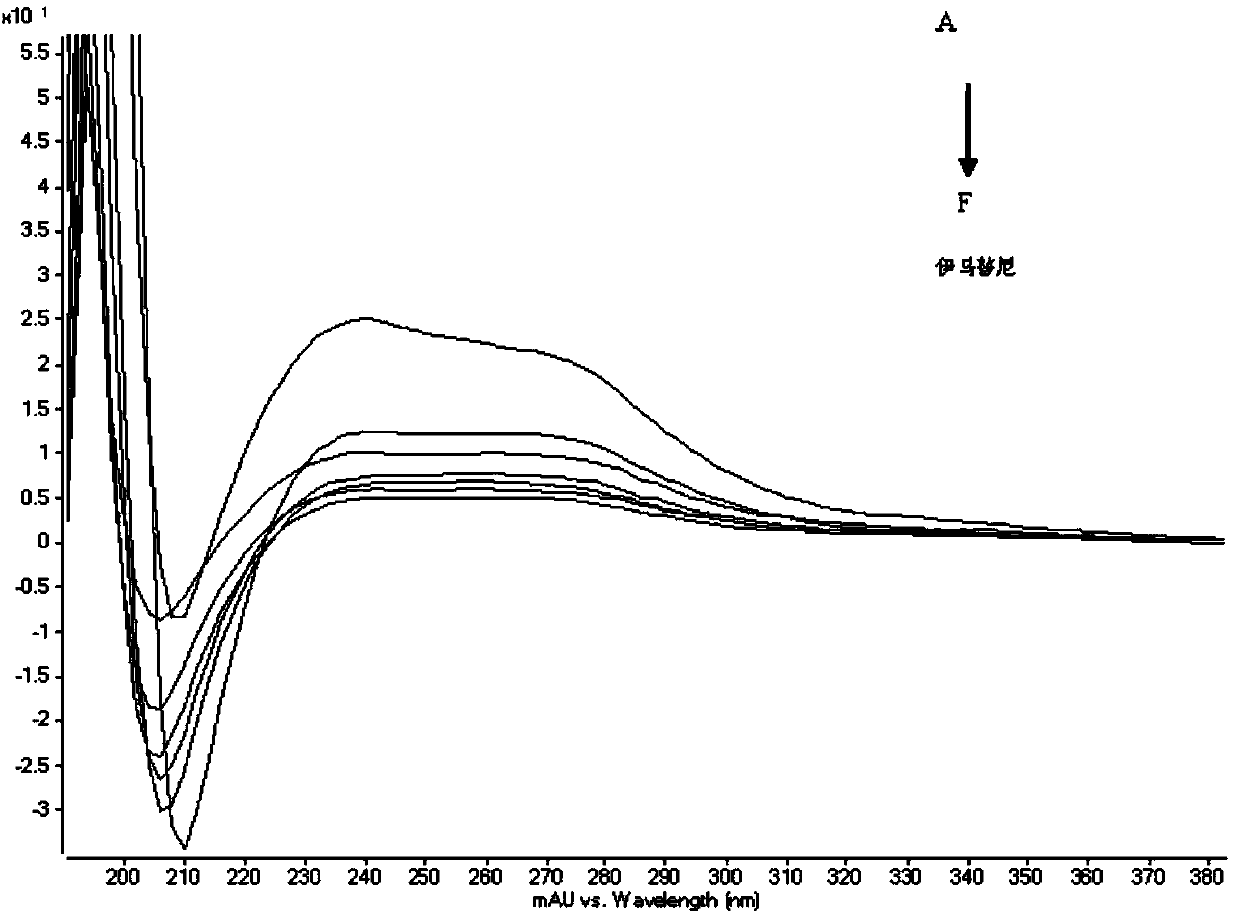
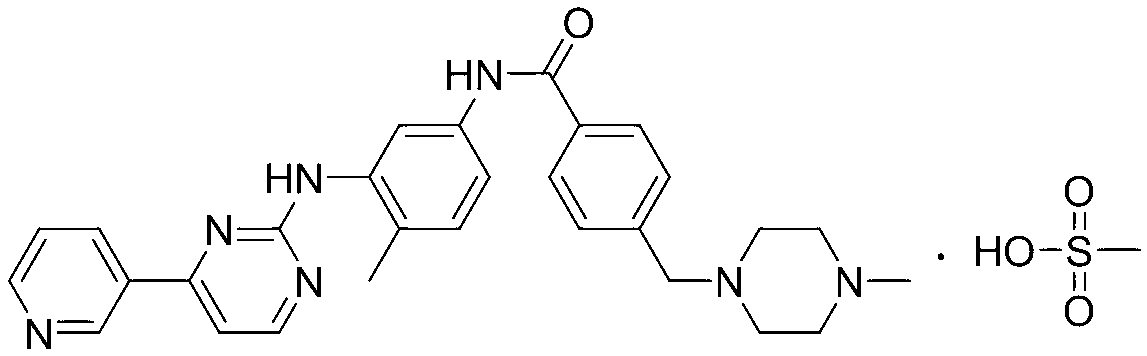
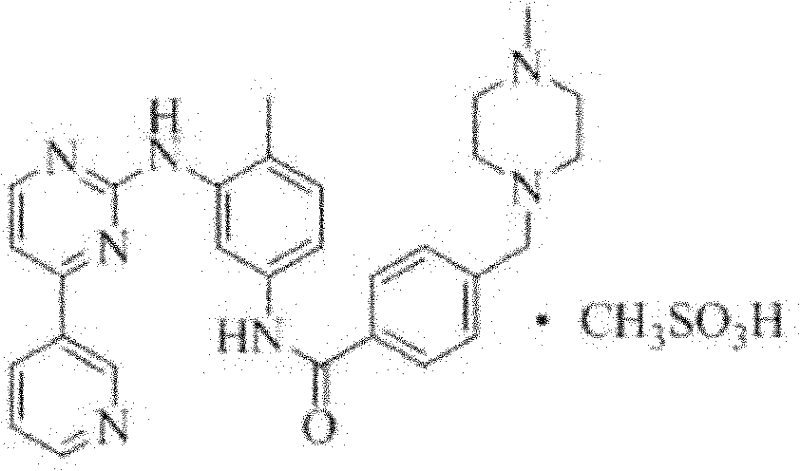
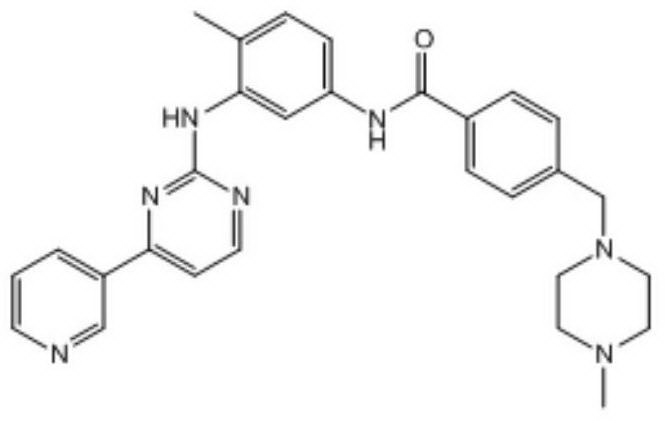
The invention relates to a method for determining trace imatinib in a blood sample and application to zero phase clinical trial, in particular, the invention relates to a method for determining imatinib or pharmaceutical salts of the imatinib in a biological sample. The method adopts the high performance liquid chromatography-tandem mass spectrum for determination and comprises the following steps and the testing conditions: treatment of a tested sample, determination of the sample, and determining a liquid chromatogram condition and a mass spectrum condition and the like. The invention also relates to application of imatinib or pharmaceutical salts of the imatinib to zero phase clinical trial and application of the method to pharmacokinetics experimental study of imatinib or pharmaceutical salts. The result shows that the detection method with the above characteristics can be beneficially used for detecting the concentration of imatinib or pharmaceutical salts of the imatinib such as mesylate in the biological sample such as blood plasma in the medicine zero phase clinical trial. The chemical structure of imatinib mesylate is shown in the description.
Owner:BEIJING SHIJITAN HOSPITAL CAPITAL MEDICAL UNIVERSTY
Nanoparticulate imatinib mesylate formulations
InactiveCN101232870AReduce sizeSmall dosePowder deliveryOrganic active ingredientsStromal tumorDisease
The present invention is directed to a nanoparticulate compositions of imatinib mesylate, or a salt or derivative thereof, having improved pharmacokinetic profiles and reduced fed / fasted variability. The nanoparticulate imatinib mesylate particles of the composition have an effective average particle size of less than about 2000 nm and are useful in the treatment of chronic myeloid leukemia, gastrointestinal stromal tumors and related diseases.
Owner:ELAN PHRMA INT LTD
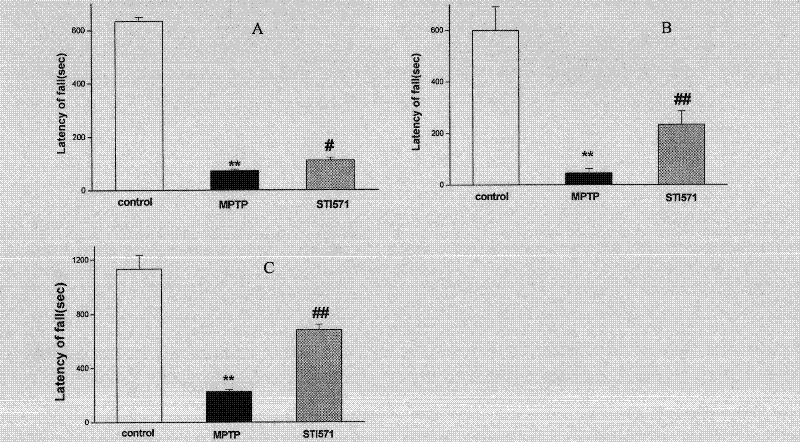
Application of imatinib mesylate in preparation of drugs for resisting Parkinson's disease (PD)
InactiveCN102406648AImprove motor dysfunctionImprove cognitive impairmentOrganic active ingredientsNervous disorderPhosphorylationApoptosis
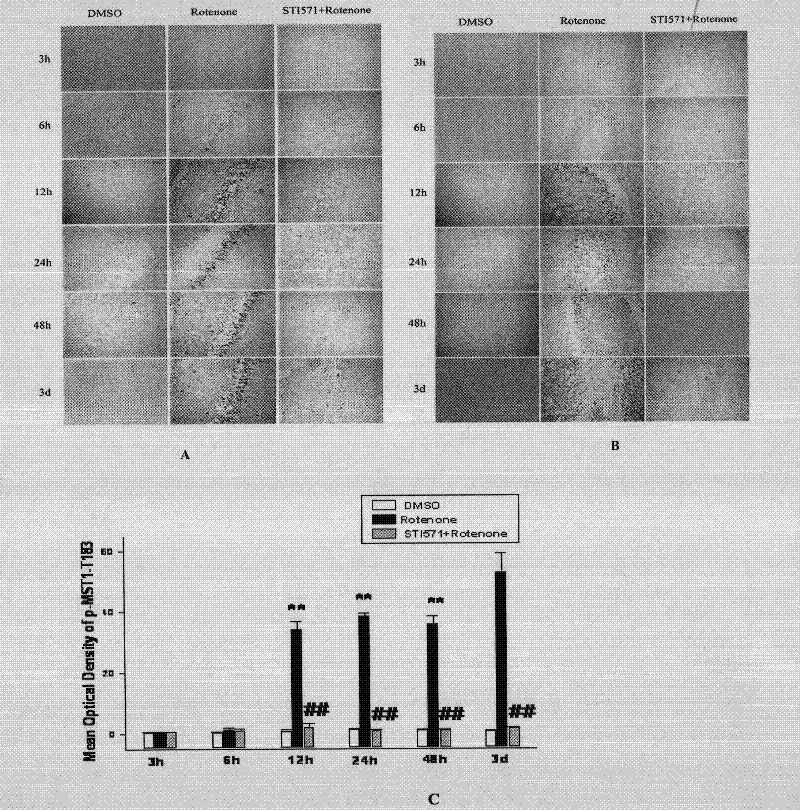
The invention discloses an application of imatinib mesylate in preparation of drugs for resisting Parkinson's disease (PD). The application of imatinib mesylate (STI571) in preparation of drugs for treating neurodegenerative diseases belongs to the protective range of the invention, and the neurodegenerative diseases can be PD. An experiment proves that by continuous administration of the STI571, the dyskinesia induced by MPTP can be obviously improved, and the cognitive dysfunction of mice can be improved. The STI571 can obviously improve the dopaminergic neuron loss induced by the MPTP, can inhibit the rat CA1 and DG (diacylglycerol) region nerve cell apoptosis reaction induced by rotenone, can obviously inhibit the primary substantia nigra neuron apoptosis and Lewy protein expression induced by rotenone, and can inhibit the phosphorylation activation of PKC (Protein Kinase C) and Akt. The STI571 used as a specific inhibitor of c-Ab1 can resist the formation of PD, can improve the behavioral abnormity and cognitive dysfunction of PD, can be used as a new candidate drug for resisting neurodegenerative diseases such as PD and the like, and has favorable application prospects; and the clinical indications of the STI571 are greatly expanded.
Owner:INSITUTE OF BIOPHYSICS CHINESE ACADEMY OF SCIENCES
Imatinib mesylate liposome preparation and preparation method thereof
InactiveCN106727325AGood process reproducibilitySuitable for industrialized mass productionOrganic active ingredientsPharmaceutical non-active ingredientsLipid formationCholesterol
The invention relates to an imatinib mesylate preparation, in particular to an imatinib mesylate liposome preparation. Imatinib mesylate liposome comprises blank liposome and imatinib mesylate liposome original medicines, the blank liposome comprises phospholipid, cholesterol, hydrophilic polymer lipid derivatives, citric acid-sodium citrate solution and alkaline substances, the mass ratio of imatinib mesylate to the phospholipid ranges from 1:8 to 1:30, the mass ratio of the cholesterol to the phospholipid ranges from 1:1 to 1:8, and the mole ratio of the hydrophilic polymer lipid derivatives to the phospholipid ranges from 1:30 to 1:10. A preparation process includes the steps of blank liposome preparation, liposome grading, medicine loading and the like. The imatinib mesylate liposome preparation is high in liposome embedding ratio, uniform in particle size, low in cost and good in process repeatability.
Owner:QINGDAO HUANGHAI PHARM CO LTD
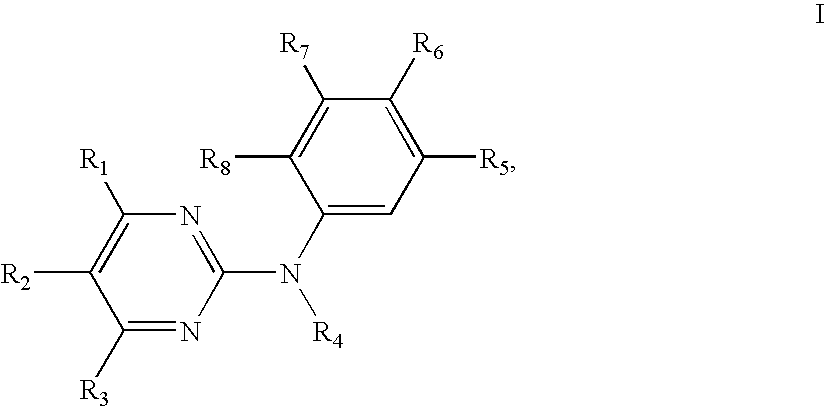
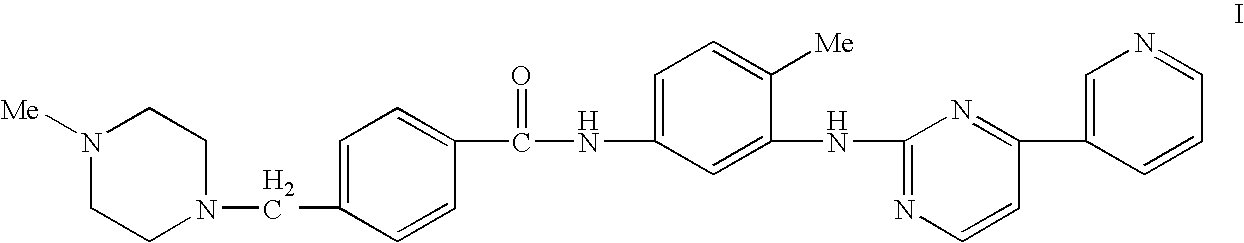
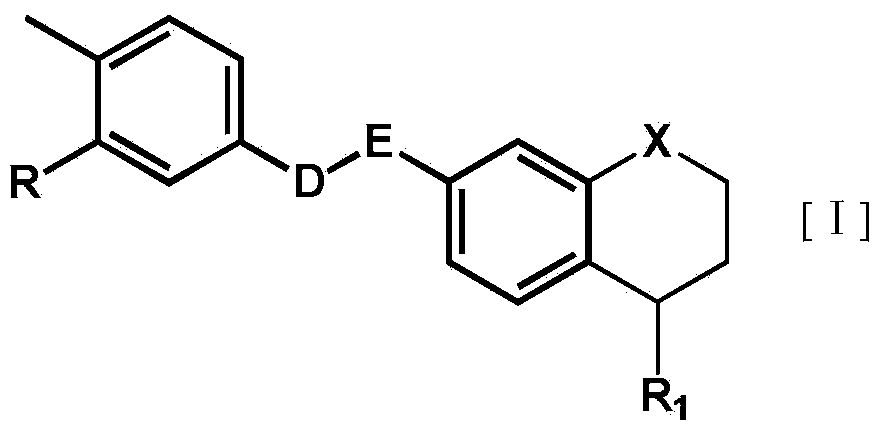
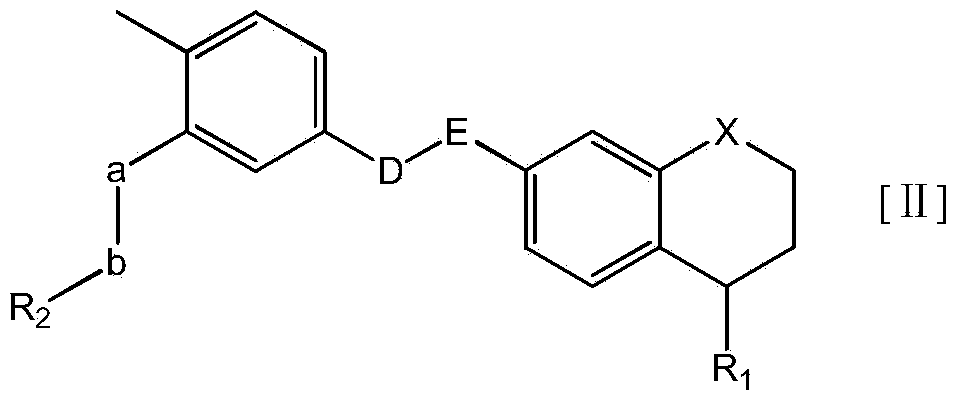
Substituted tetrahydronaphthalene amide compound, pharmaceutically acceptable salt thereof, and preparation method and application
ActiveCN104250253AGood antitumor activityImprove securityOrganic active ingredientsOrganic chemistryTetralinTherapeutic window
The invention relates to substituted tetrahydronaphthalene amide compound as shown in a general formula I (in the Specification), pharmaceutically acceptable salt thereof, and a preparation method and application. The substituted tetrahydronaphthalene amide compound can be used as an antitumor drug, the invention also provides a preparation method of compounds similar to the substituted tetrahydronaphthalene amide compound and pharmaceutical compositions containing the compounds, and in vitro and in vivo anti-tumor effect research results and acute toxicity research results. The substituted benzo-cyclic amide compound as the antitumor drug, has better antitumor activity and safety, particularly resists imatinib mesylate resistance tumors, can be applied to curing tumors, such as leukemia, gastrointestinal stromal tumor, lung cancer, colon cancer, ovarian cancer and kidney cancer, is wide in therapeutic window, and as antitumor agent, the compound has high application value in the field of medicine.
Owner:LIAONING UNIVERSITY
Method and detection reagent for detecting plasma concentration of imatinib mesylate in blood plasma with LC-MS/MS (liquid chromatography-tandem mass spectrometry)
InactiveCN109946404AReduce the impactHigh recovery rateComponent separationBlood plasmaCarbamazepine
The invention discloses a method and a detection reagent for detecting plasma concentration of imatinib mesylate in blood plasma with LC-MS / MS (liquid chromatography-tandem mass spectrometry). The method comprises steps as follows: (i), preparing a standard solution; (ii), preparing an internal standard solution; (iii), pre-processing samples; (iv), drawing a standard curve; (v), measuring the plasma concentration of imatinib mesylate in blood plasma. The detection reagent comprises a moving phase A, a moving phase B, the standard solution and the internal standard solution. The method for detecting the plasma concentration of imatinib mesylate in blood plasma with LC-MS / MS is provided, imatinib and carbamazepine as an internal standard substance can be completely separated with the method, besides, the method is slightly affected by an endogenous substance impurity peak, and recovery rate and precision are high.
Owner:茆勇
Drug composition for treating tumors and application thereof
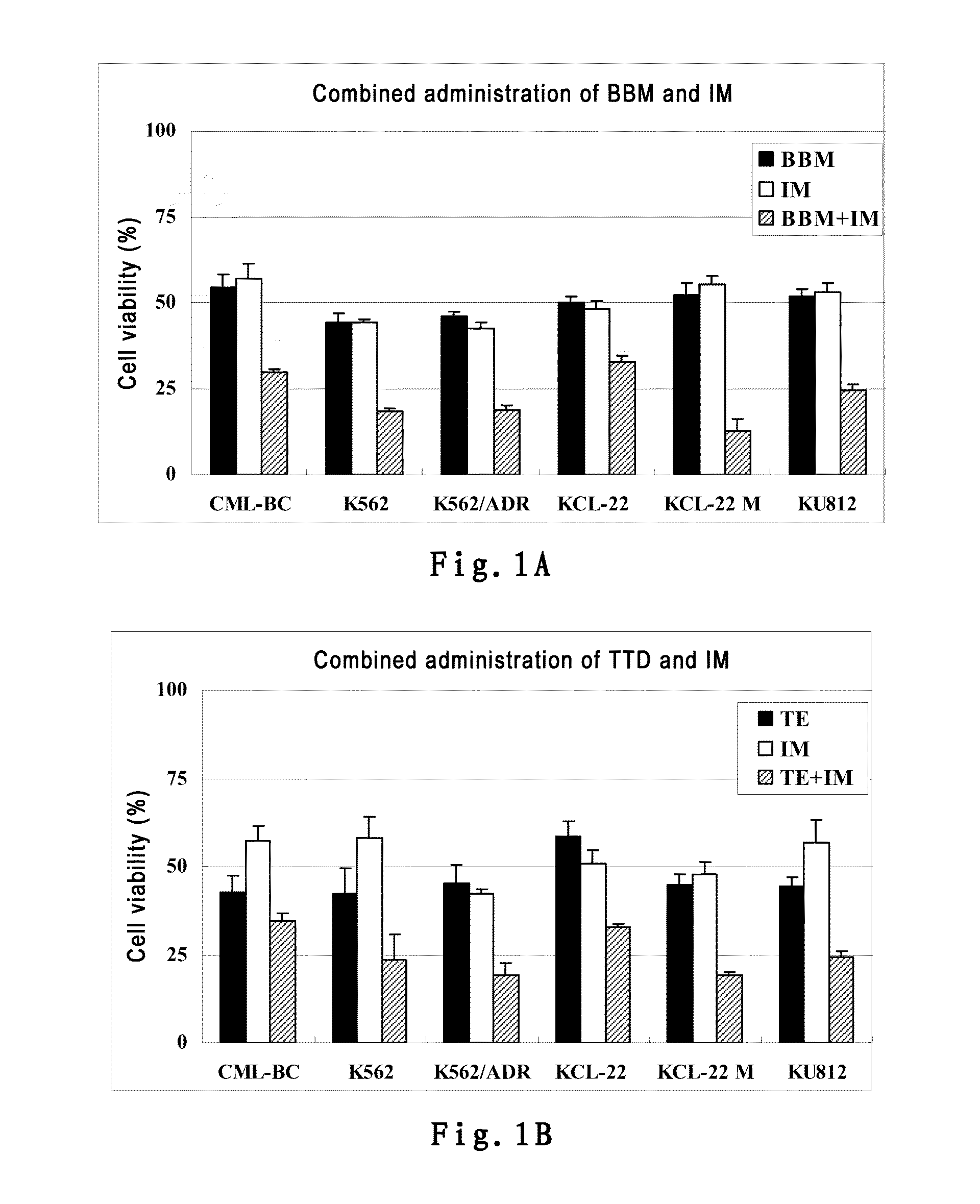
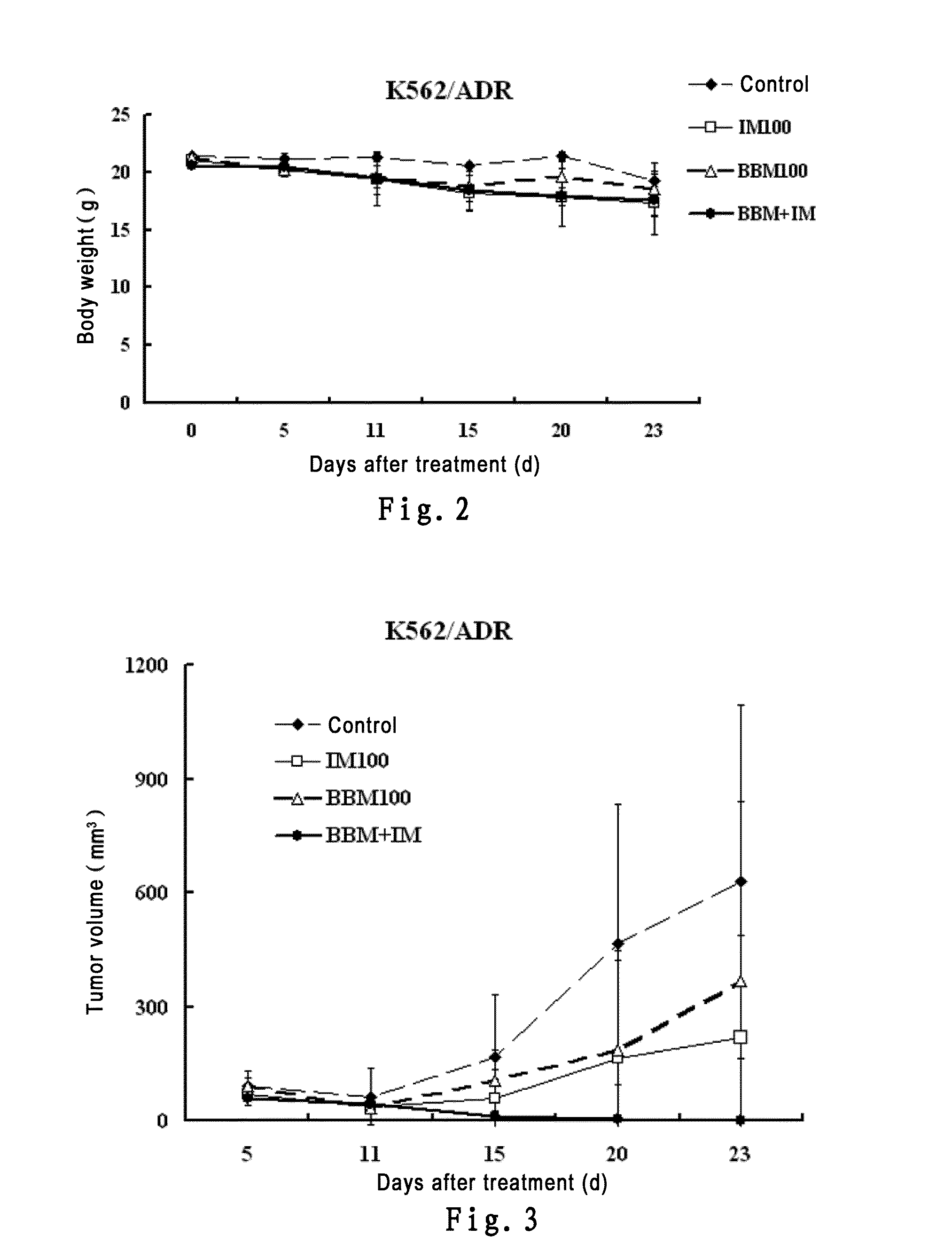
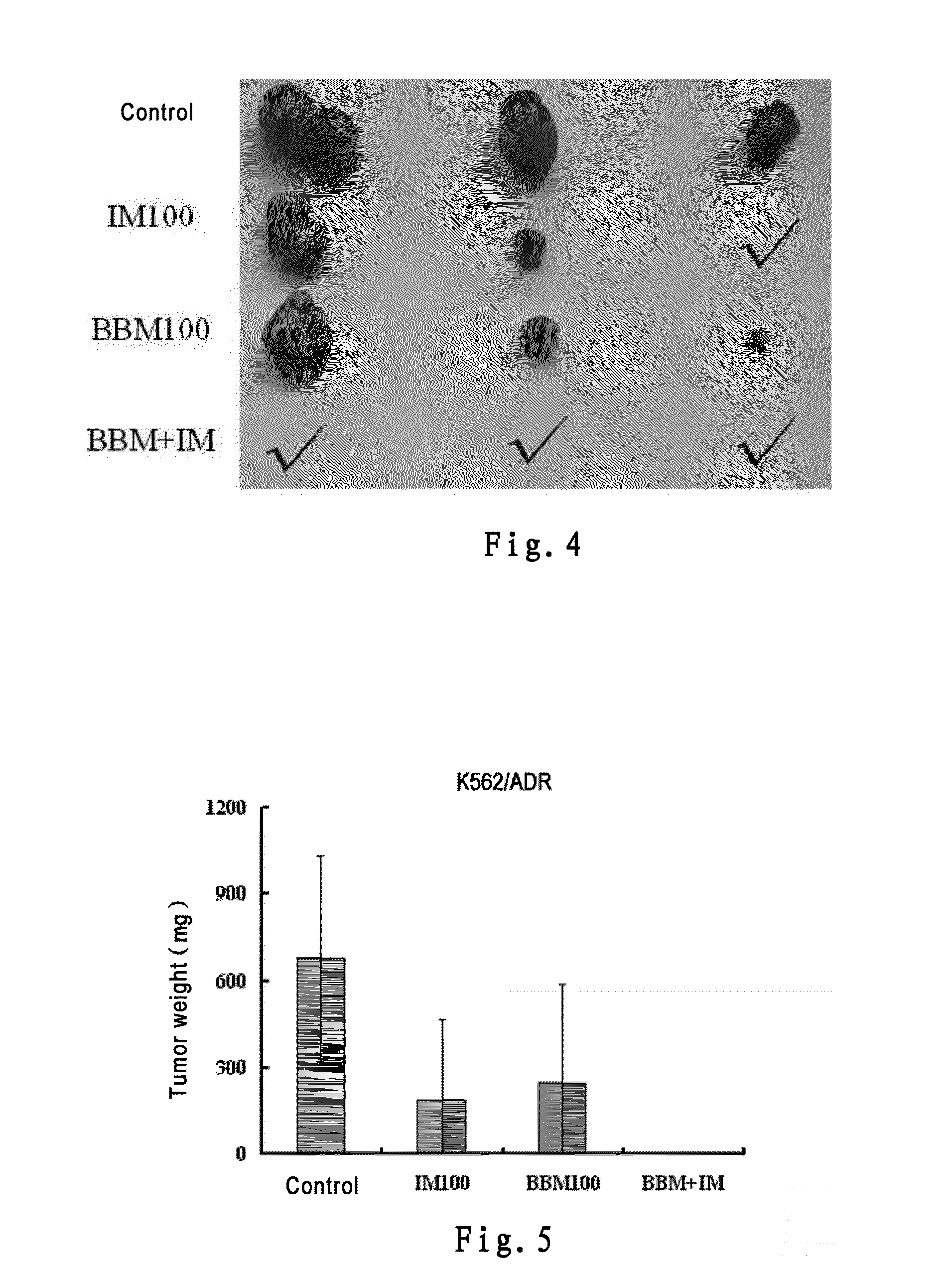
InactiveUS20150238488A1Good treatment effectOrganic active ingredientsAntineoplastic agentsTetrandrineImatinib mesylate
The present invention belongs to the field of medicine and pharmaceutical chemistry, specifically relates to novel antitumor pharmaceutical combinations, and particularly relates to pharmaceutical combinations of bisbenzylisoquinoline alkaloids (e.g. berbamine and tetrandrine) and imatinib mesylates and their use in treating tumors.
Owner:HANGZHOU BENSHENG PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com