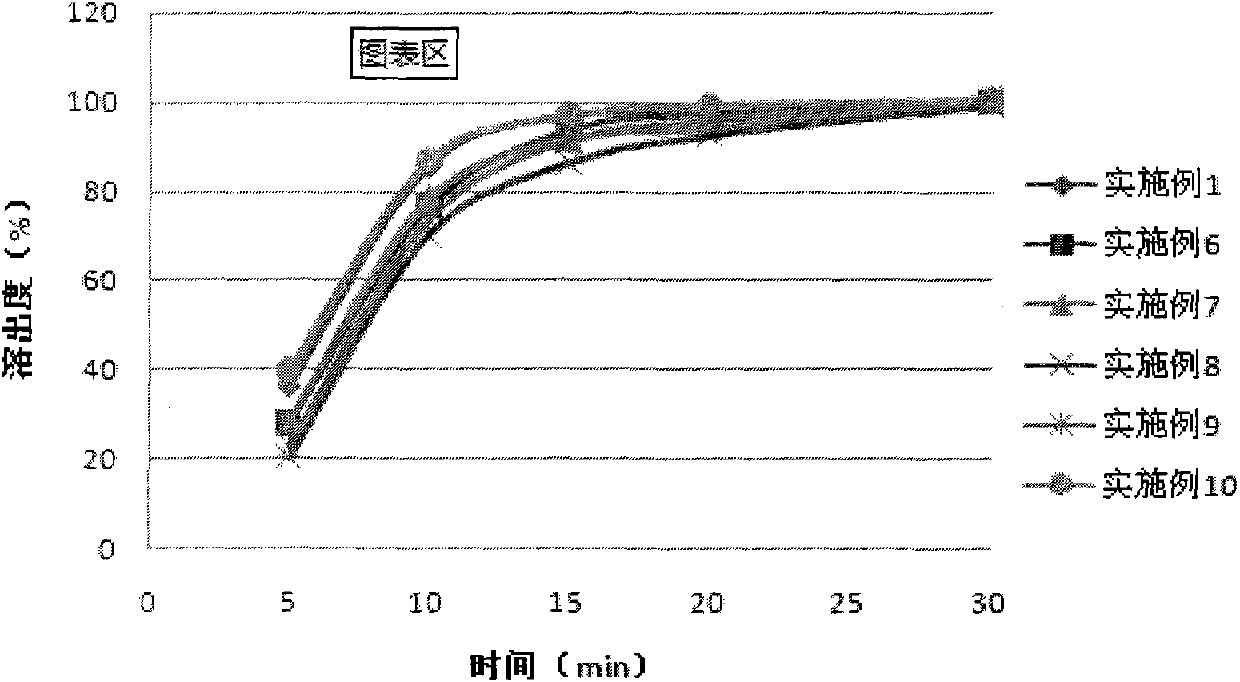
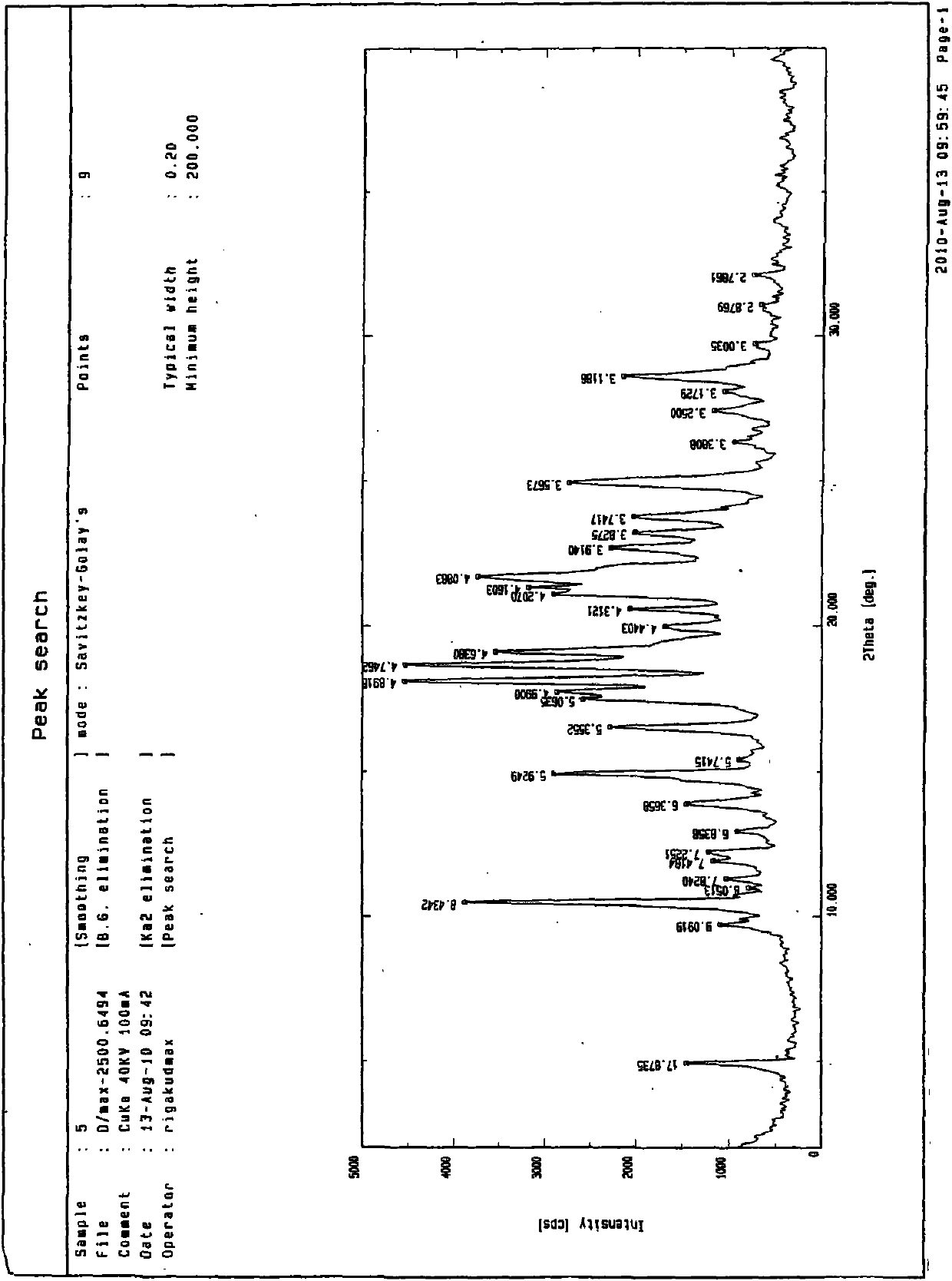
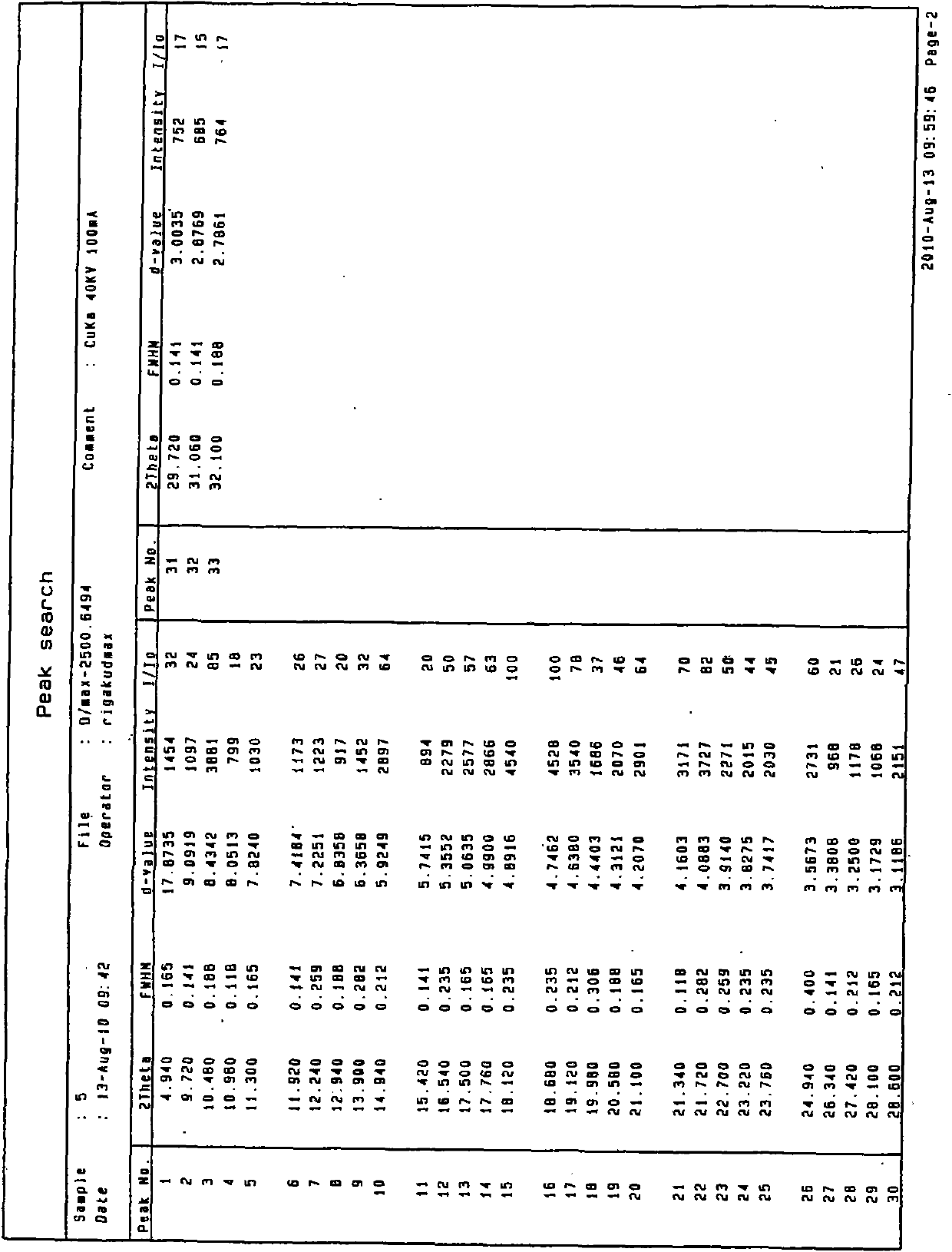
Medicinal preparation containing crystal form a imatinib mesylate
A technology for imatinib mesylate and pharmaceutical preparations, which is applied in the field of pharmaceutical preparations containing α-crystalline imatinib mesylate, and can solve the problems of unsuitable physical properties for preparations, large proportions of main ingredients, and changes in Properties of pharmaceutical preparations, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] 100mg Imatinib Mesylate Tablets
[0045] prescription:
[0046]
[0047] In the table *: 119.5 mg of imatinib mesylate, calculated as 100 mg of imatinib. In the table, 1 represents internal auxiliary materials, and 2 represents external auxiliary materials.
[0048] Preparation Process:
[0049] Pulverize imatinib mesylate in α crystal form, mix with the auxiliary materials added in and pass through a 60-mesh sieve; add absolute ethanol, mix in a high-speed shear mixer, use a 40-mesh sieve (0.425mm) to make granules, and use The oven dries the wet granules. Thoroughly mix dry granules with additional excipients. The blend was directly compressed (see Figure 2).
Embodiment 2
[0051] This embodiment uses isopropanol as a binder, and the purpose is to compare with Embodiment 1.
[0052] Prescription: with embodiment 1
[0053] Process: Grind the α-crystalline imatinib mesylate, mix it with the added PVPP, and pass through a 60-mesh sieve; add isopropanol, mix in a high-speed shear mixer, use a 40-mesh sieve to make granules, and use an oven to sieve Wet granules dry.
Embodiment 3
[0055] In this example, 98% ethanol is used as a binder, and the purpose is to compare with Example 1.
[0056] Prescription: with embodiment 1
[0057] Process: Pulverize imatinib mesylate in α crystal form, mix it with the added PVPP and pass through a 60-mesh sieve; add 98% ethanol, mix in a high-speed shear mixer, use a 40-mesh sieve to make granules, and use an oven to sieve Wet granules dry.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Angle of repose | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com