Application of imatinib mesylate in preparation of drugs for resisting Parkinson's disease (PD)
A technology for imatinib mesylate and Parkinson's disease, which is applied in the field of application of imatinib mesylate in the preparation of anti-Parkinson's disease drugs, and can solve other functions of imatinib mesylate. There is no literature Reporting and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
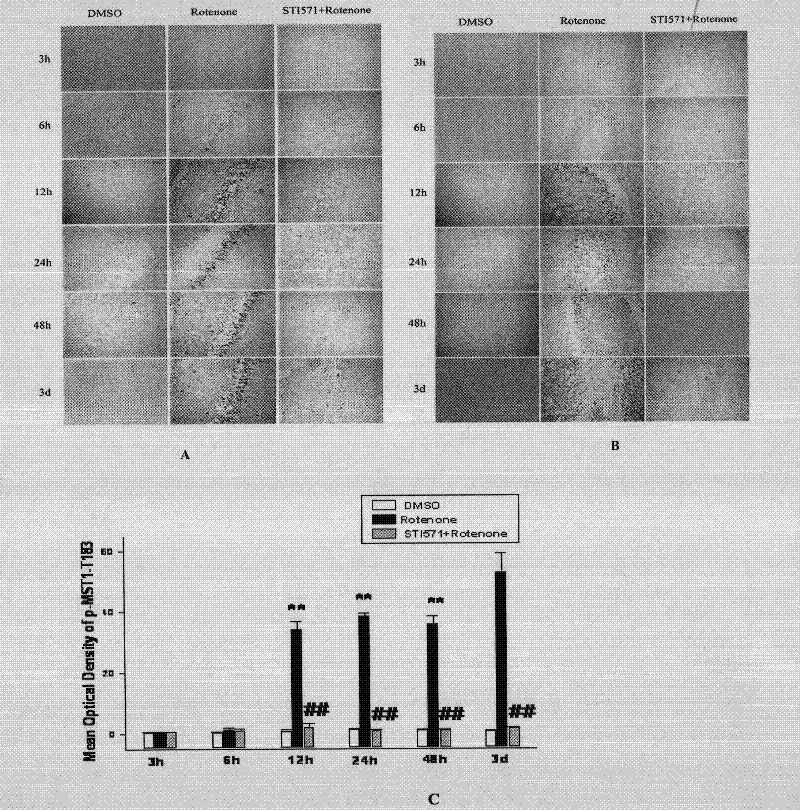
[0030] Embodiment 1, imatinib mesylate (STI571) treats Parkinson's disease
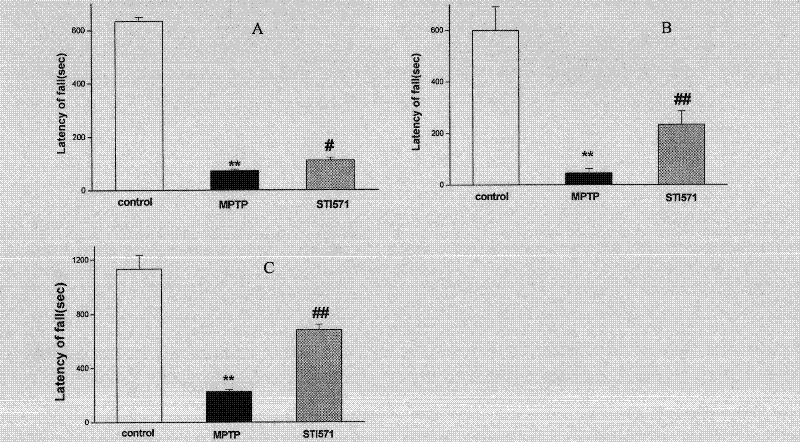
[0031] 1. STI571 can significantly improve MPTP-induced motor dysfunction in mice with chronic Parkinson's disease
[0032] Parkinson's disease is mainly due to pathological changes in the cells located in the substantia nigra of the midbrain, the synthesis of dopamine is reduced, and the excitatory effect of acetylcholine is relatively enhanced. Its incidence rate ranks first among neurodegenerative diseases in the elderly. The main behavioral manifestations are slowness and lack of movement, muscle rigidity, resting tremor, and postural instability.
[0033] 1. Modeling
[0034] Eight-week-old c57BL / 6J mice were randomly divided into 3 groups, 10 mice in each group, administered intraperitoneally in the following manner, once a day, for two consecutive weeks. [1] control group: normal saline; [2] MPTP group: MPTP (20mg / kg, dissolved in normal saline); [3] STI571+MPTP group: intraperitoneal inject...
Embodiment 2
[0064] Embodiment 2, mechanism research
[0065] 1. STI571 can significantly improve MPTP-induced loss of dopamine neurons
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com