Pharmaceutical composition for preventing in-stent restenosis
A composition and restenosis technology, which is applied in the direction of drug combination, surgical drug, pharmaceutical formula, etc., can solve the problem of high preoperative complication rate, achieve the effect of improving motor dysfunction, preventing recurrence, and preventing in-stent restenosis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
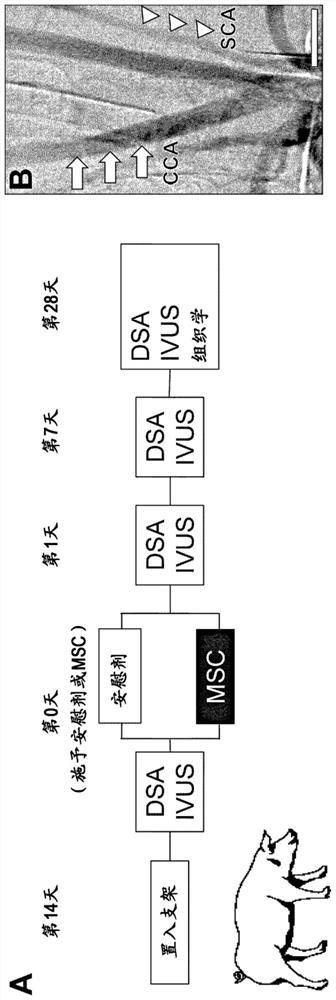
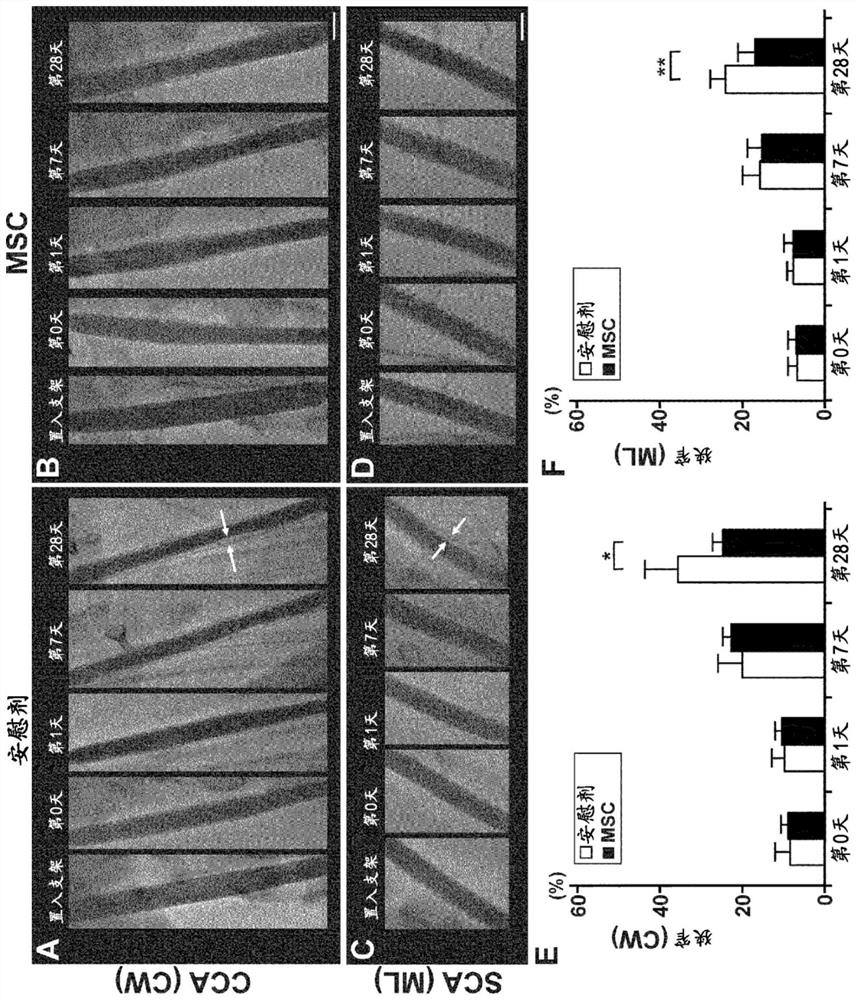
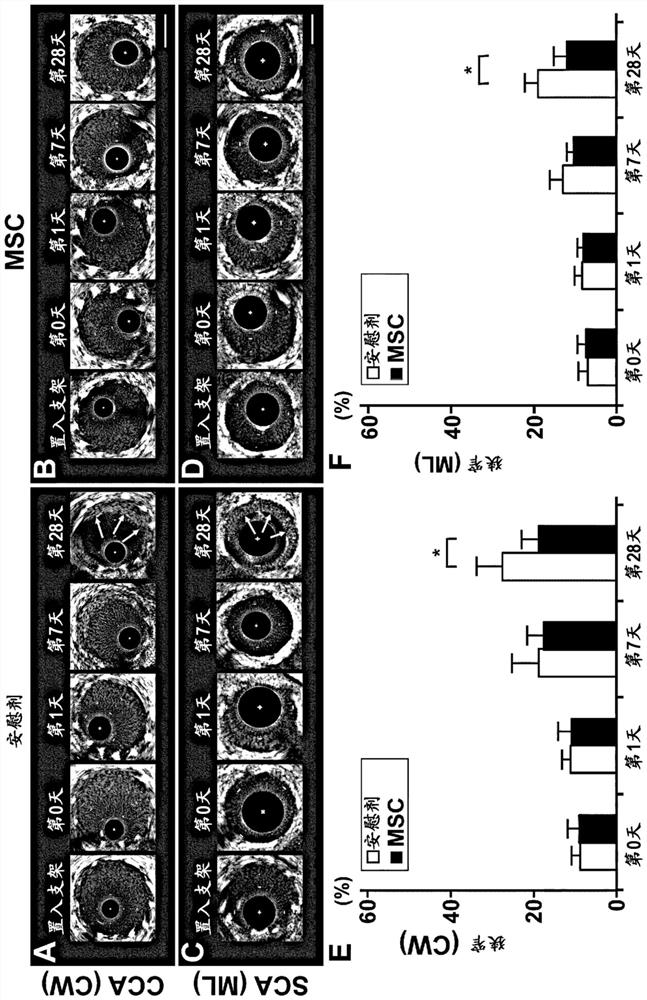
[0090] 1. Materials and methods
[0091] (1) Preparation of human mesenchymal stem cells
[0092] Human MSCs were prepared according to previous reports. That is, MSC (P2) was purchased from Lonza Corporation (Walkersville, MD), diluted with Dulbecco's modified eagle medium (Dulbecco's modified eagle medium), added 10% FBS (Life Technologies company), 2mM L-glutamine ( Sigma-Aldrich Company), 100U / ml penicillin-streptomycin (Sigma-Aldrich Company), inoculated into 150mm tissue culture dish (IWAKI Company), in 5% CO 2 Incubate for several days at 37°C in a humidified atmosphere. When the cells reached confluence, trypsin-EDTA solution (Mediatech Inc.) was used to detach the adherent cells, and 1 × 10 4 cells / ml for subculture. Thus, MSCs were proliferated to 1×10 with a short culture time (passage: 4 times) 8 cell. The proliferated MSCs were isolated, and on the day of stent insertion, the preservation solution [RPMI (Life Technologies Company) 8 ml, autologous serum 8 ml...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com