Patents
Literature
327 results about "Stent implantation" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
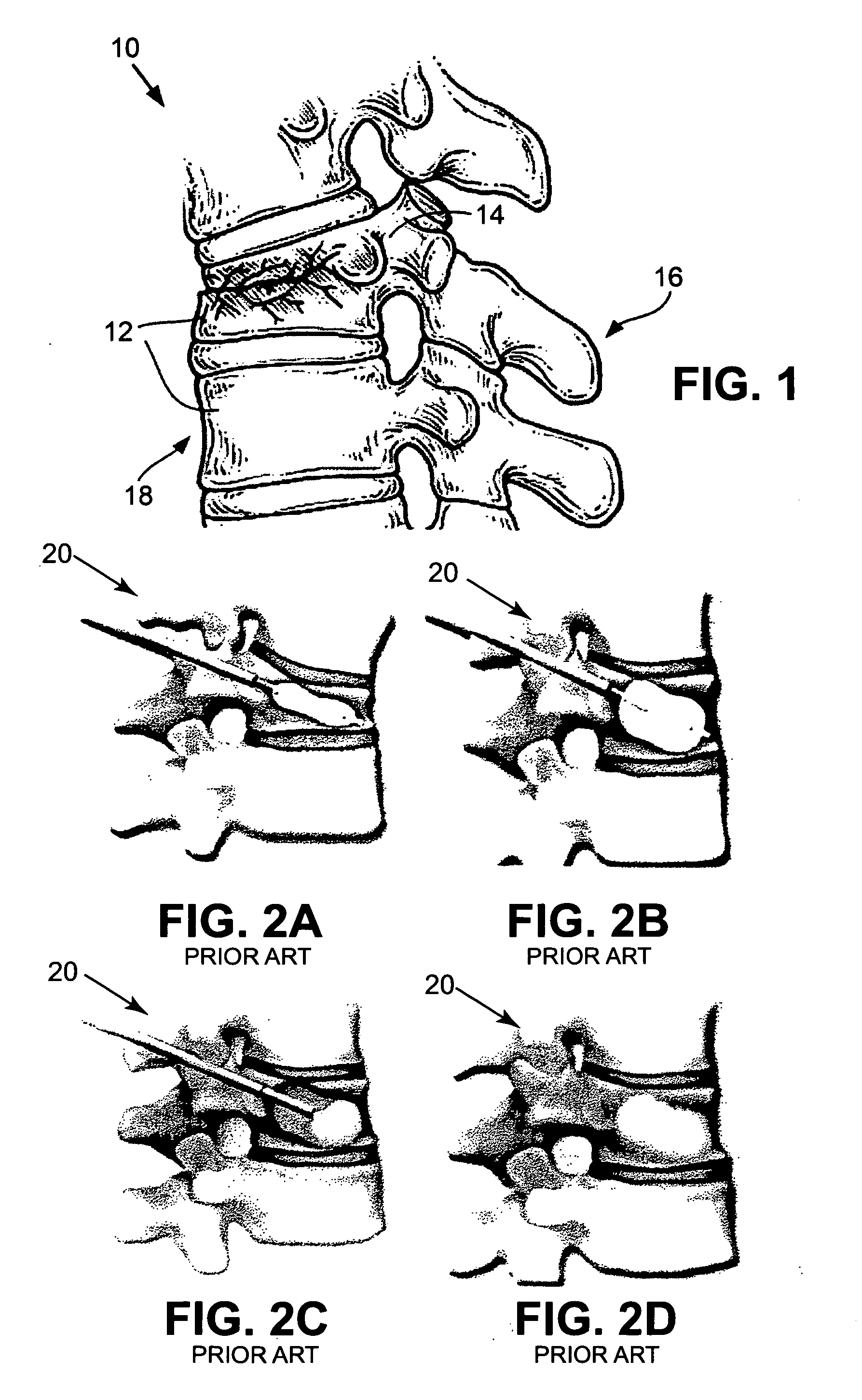
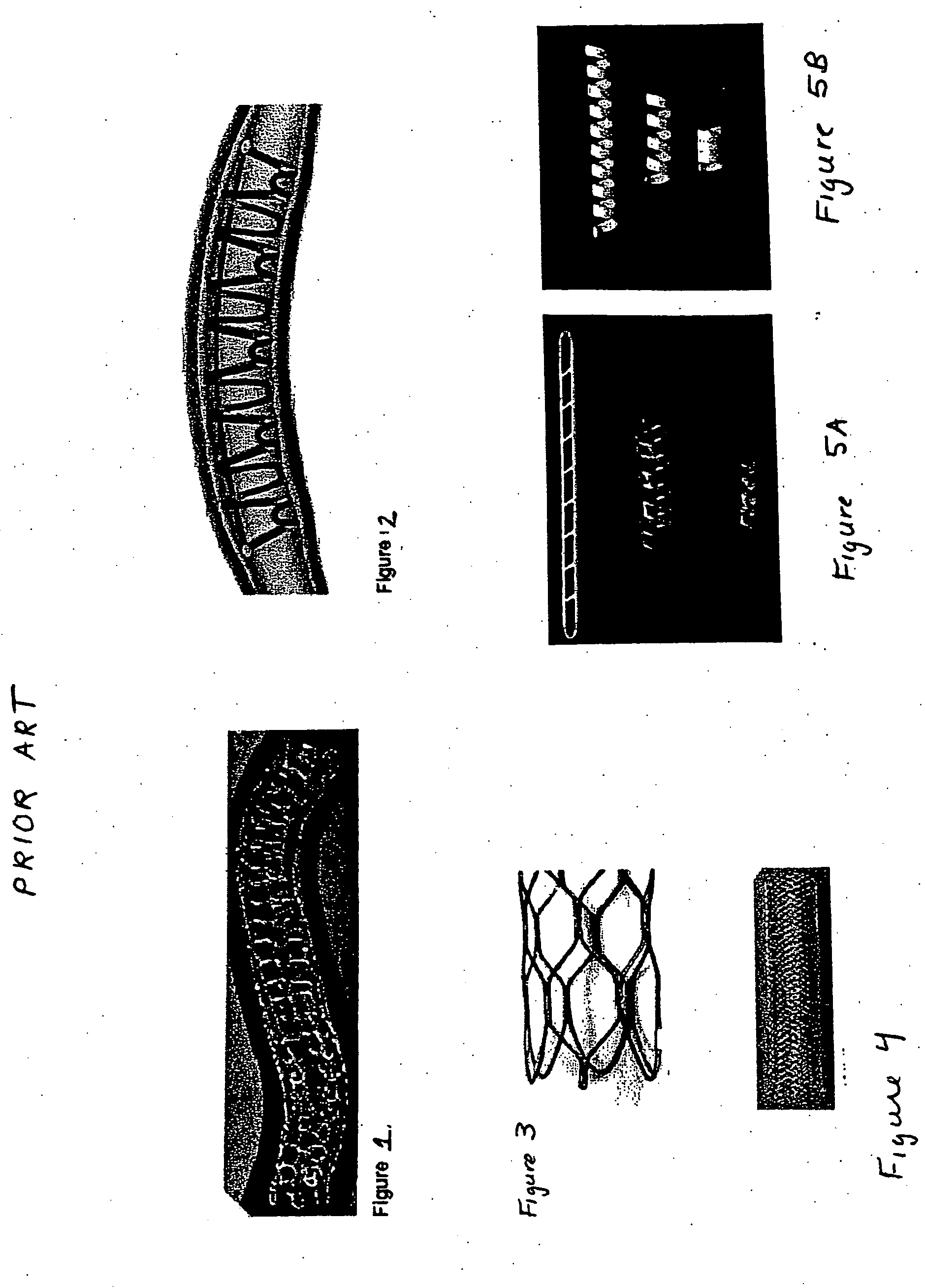
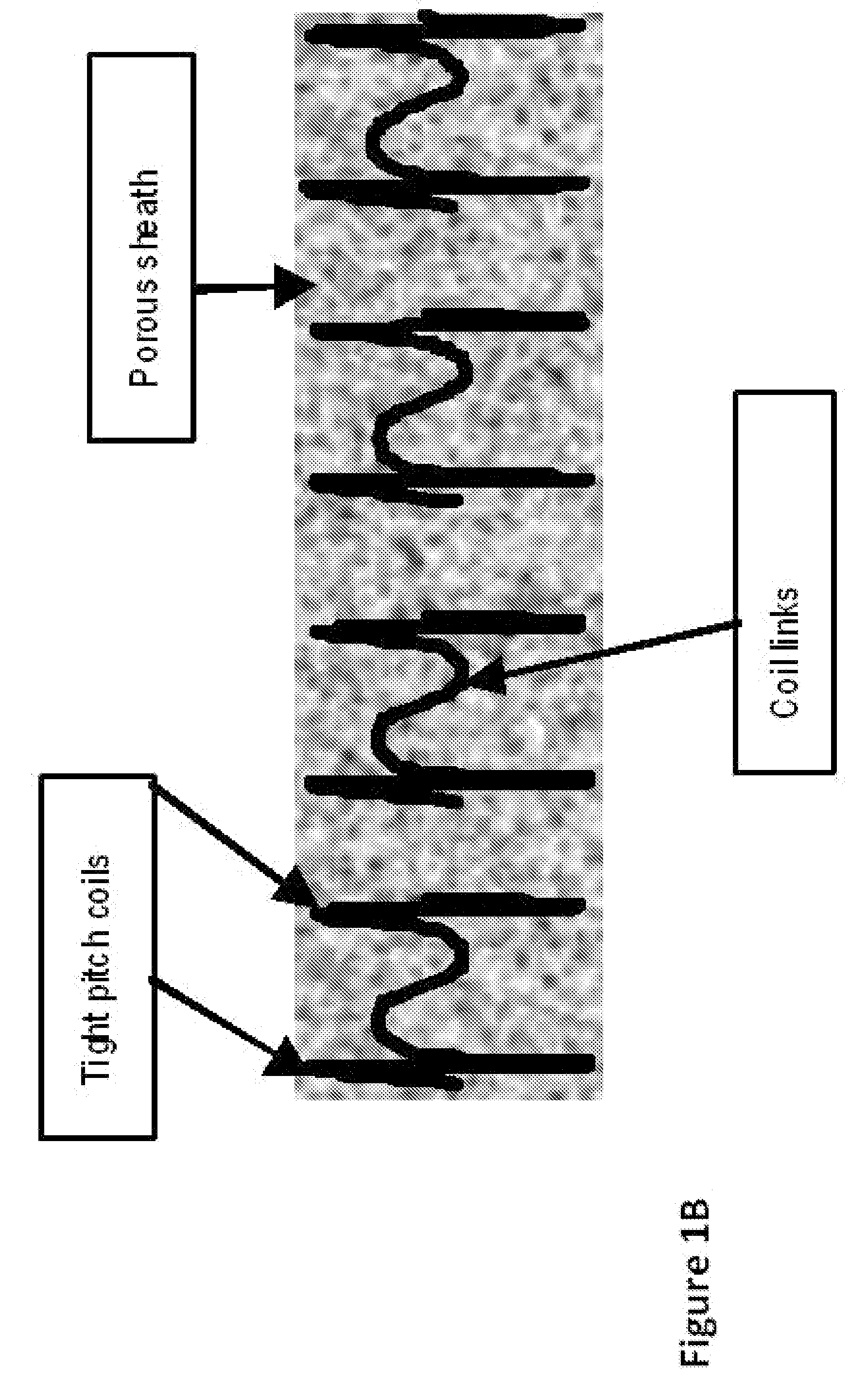
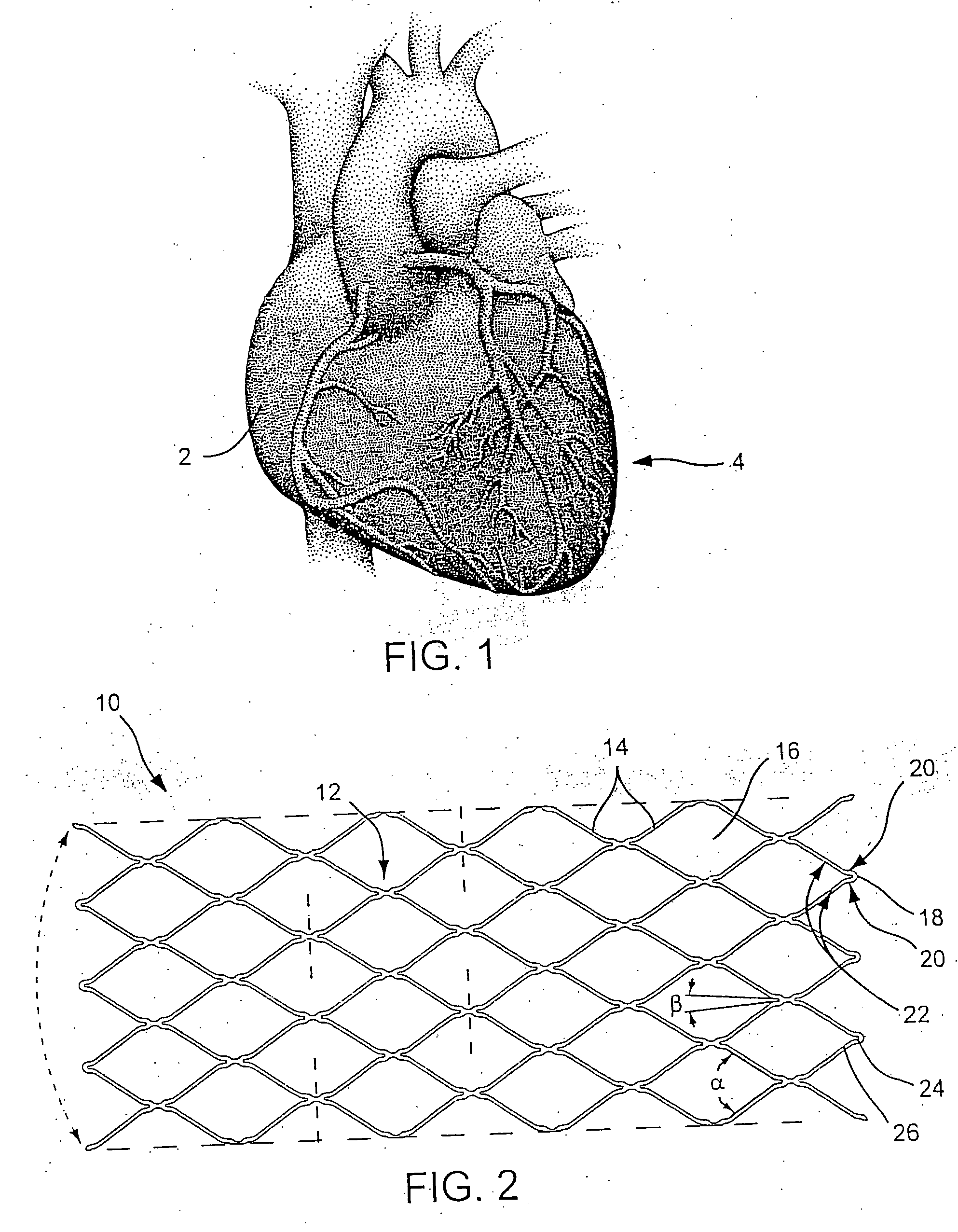
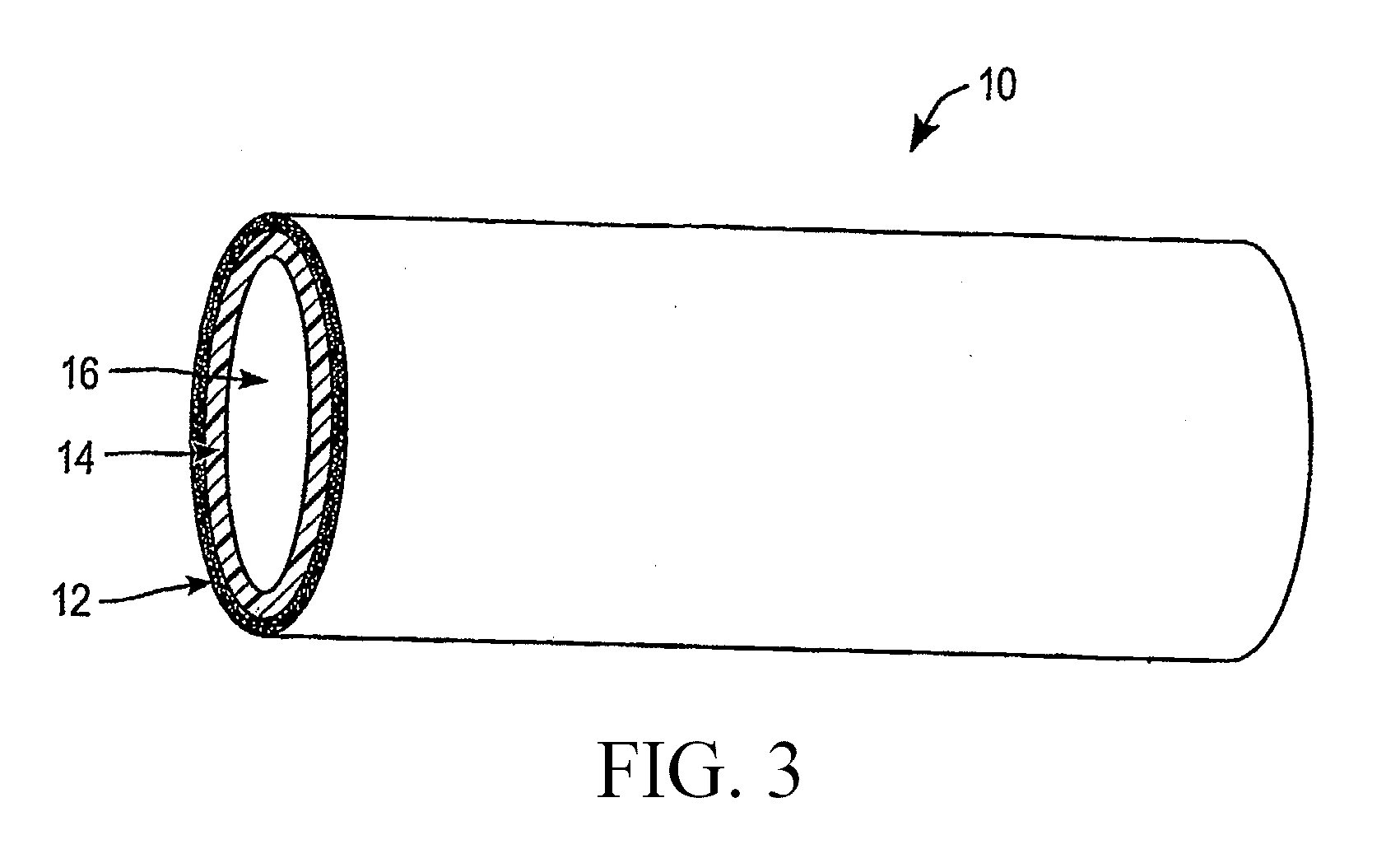
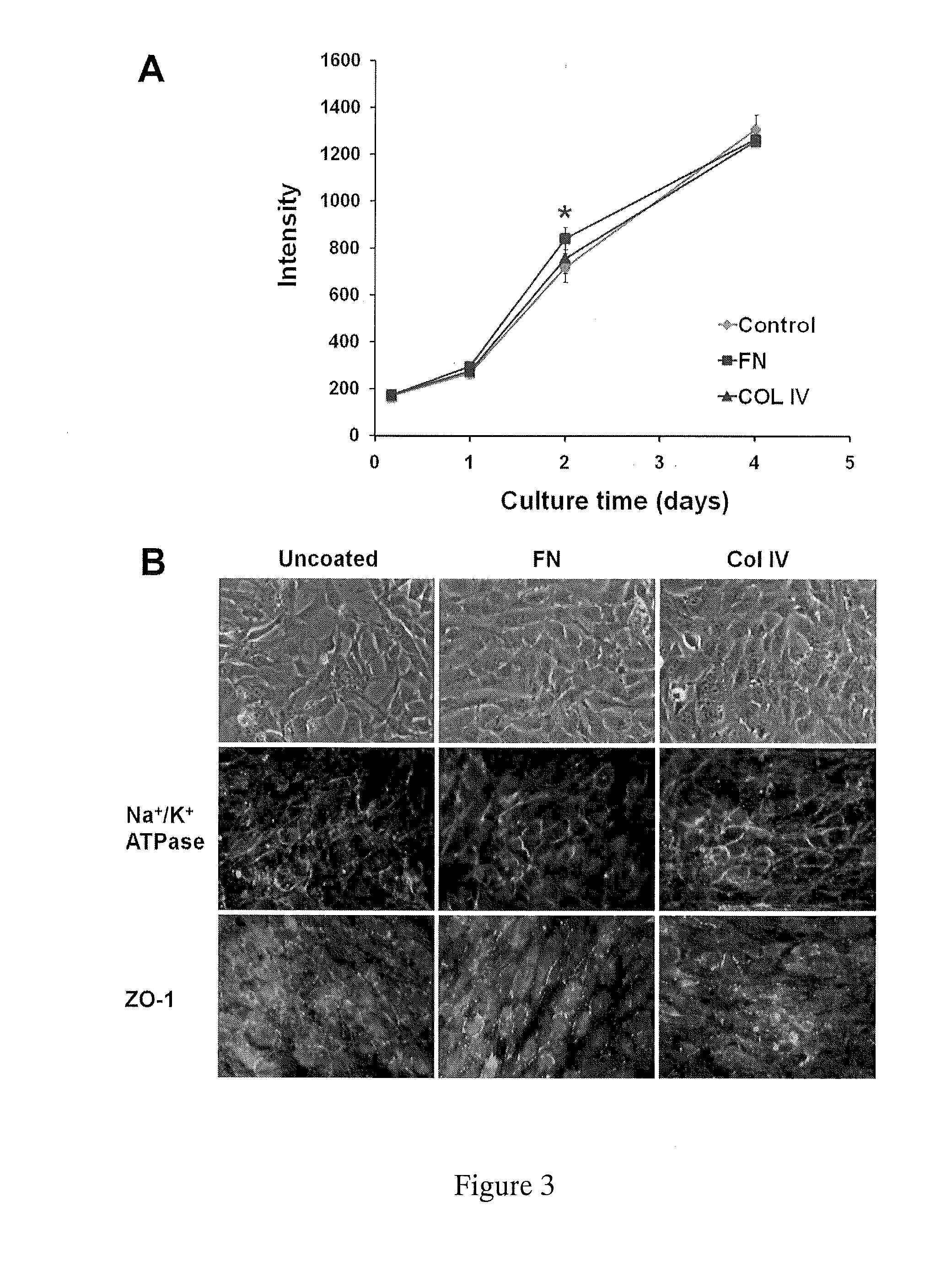
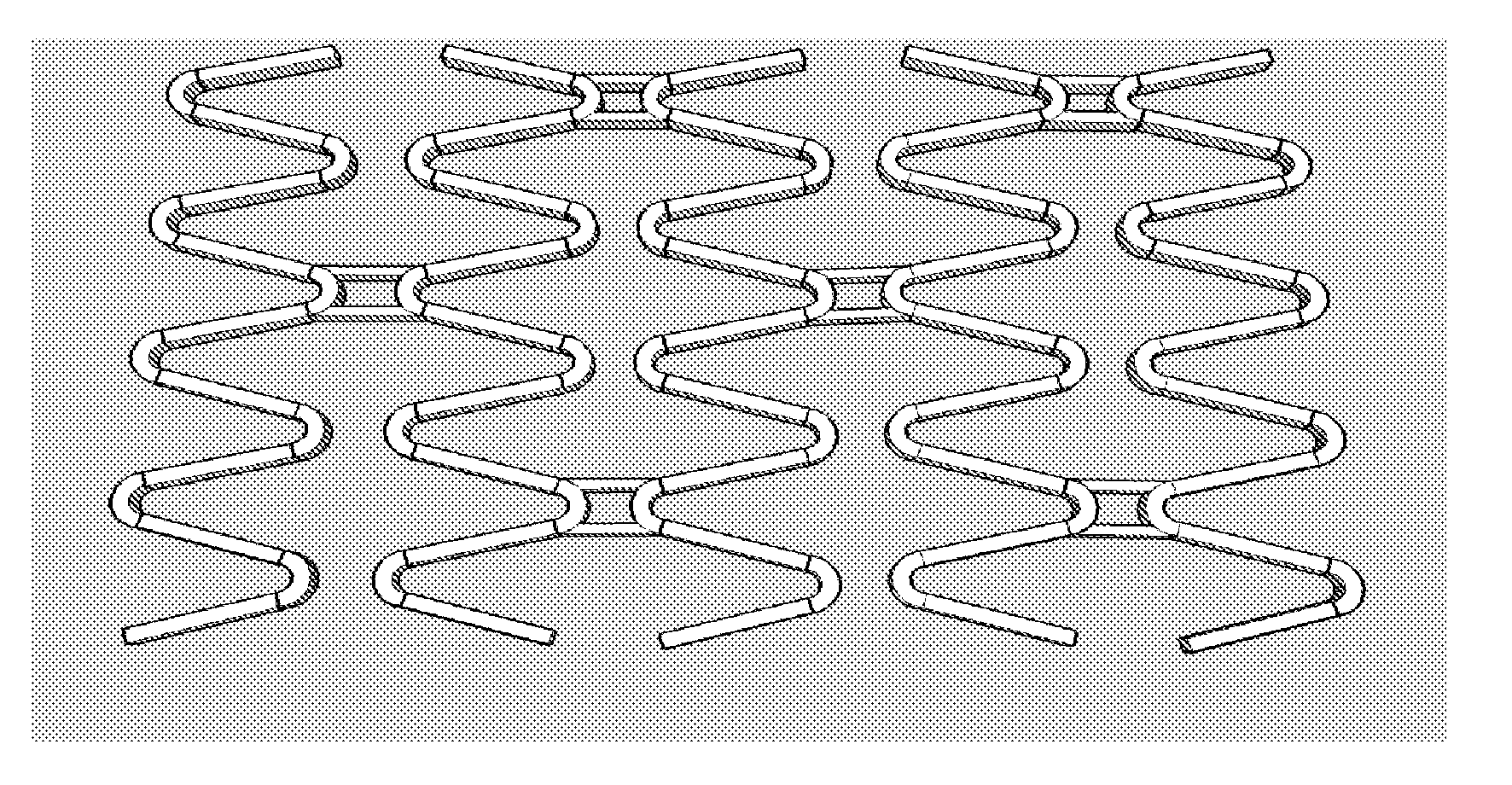
Stent Implantation. Stents are small, metal scaffolds similar in size and shape to the spring found in a ballpoint pen (Figure 3). Stent implantation is not appropriate for every artery. Before stent implantation, the blocked artery usually is treated and dilated with one or more angioplasty balloons.
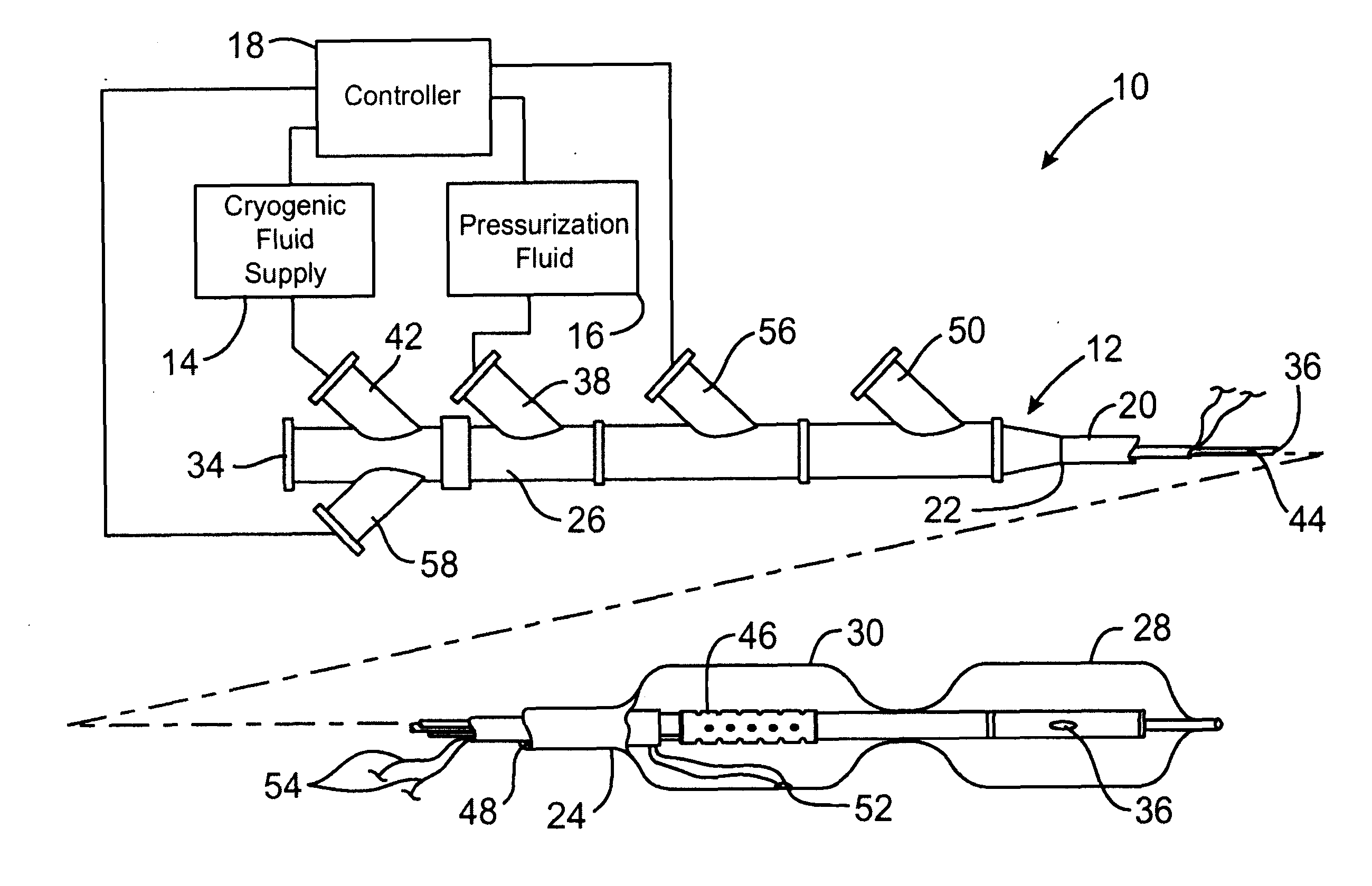
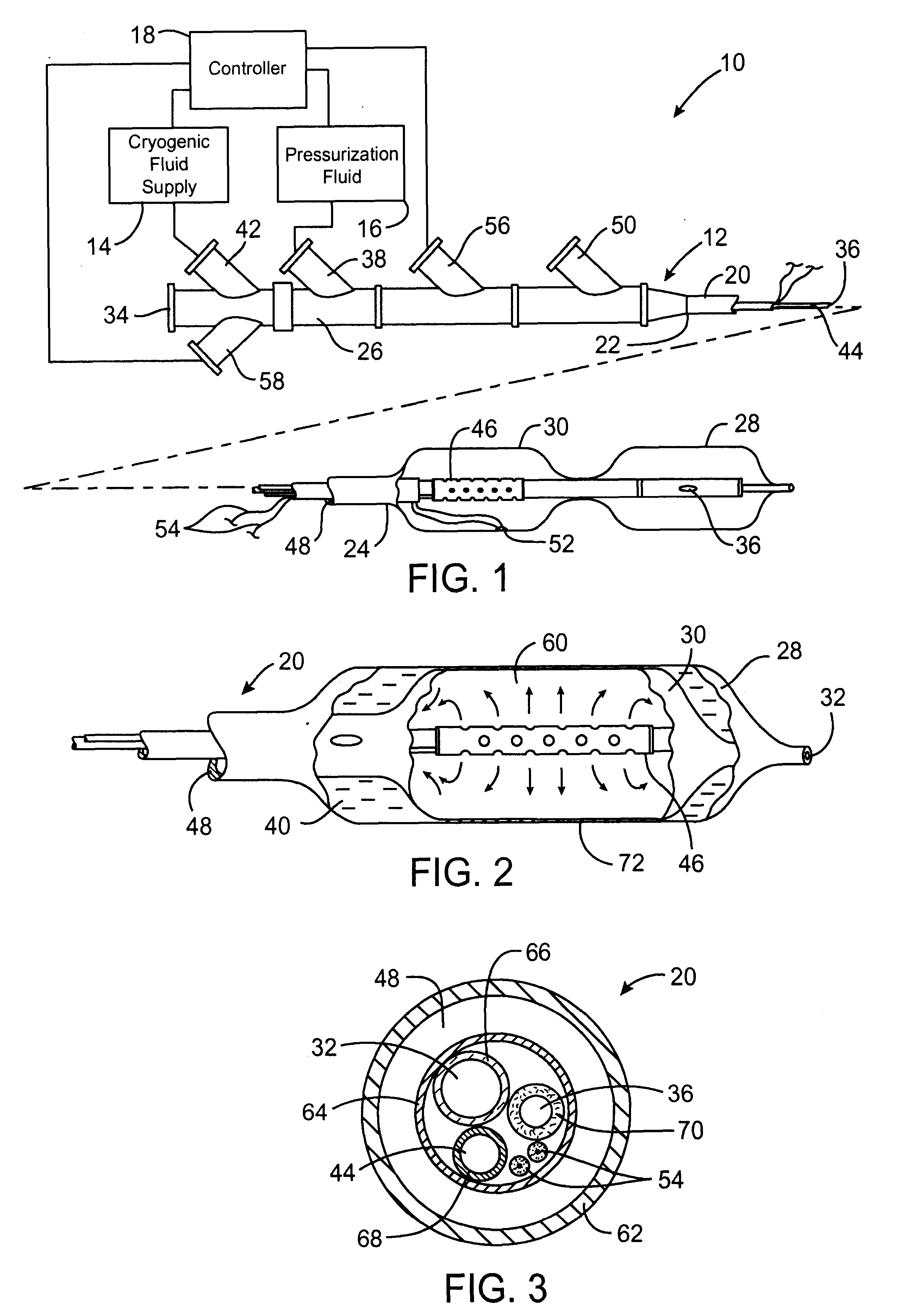
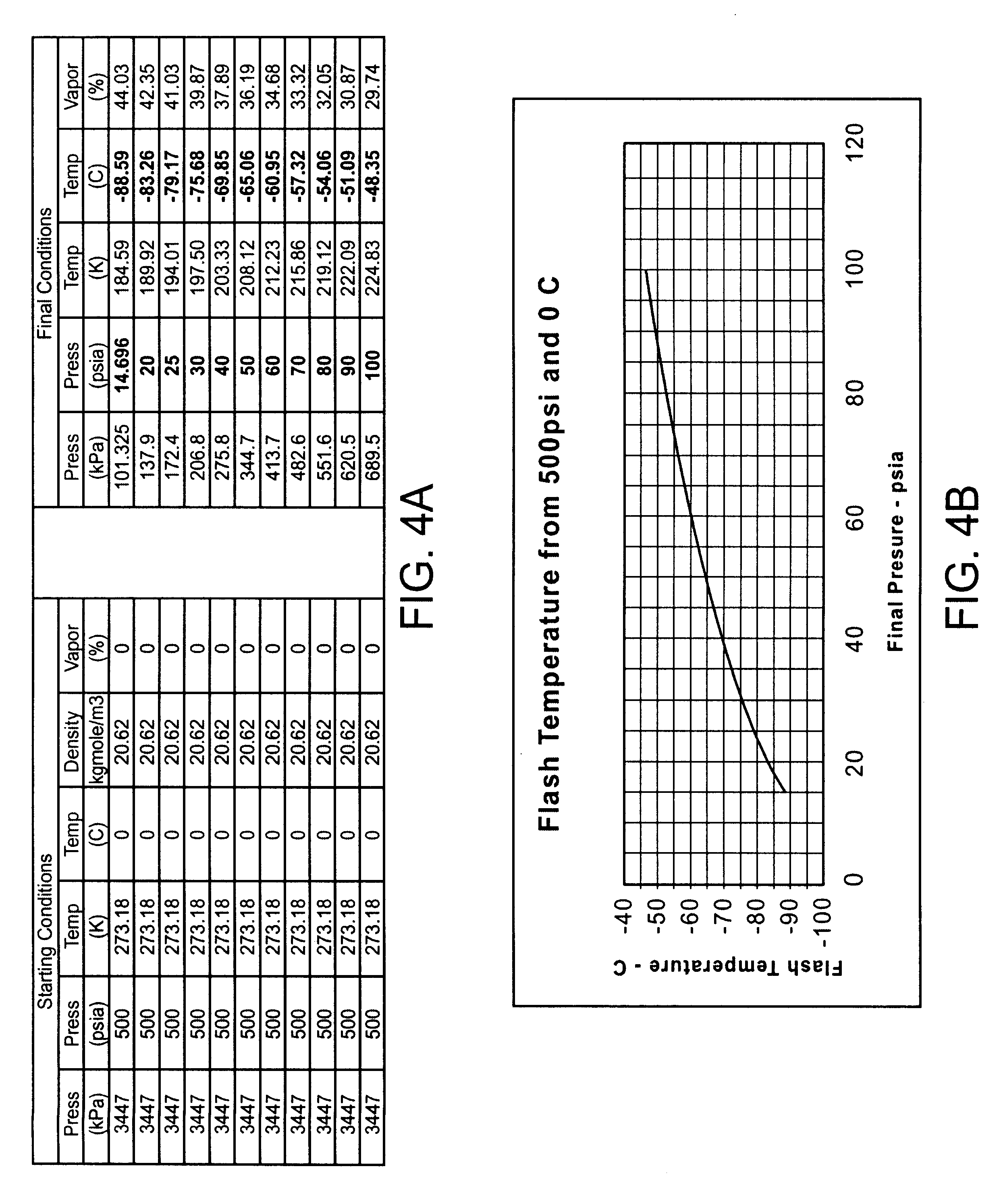
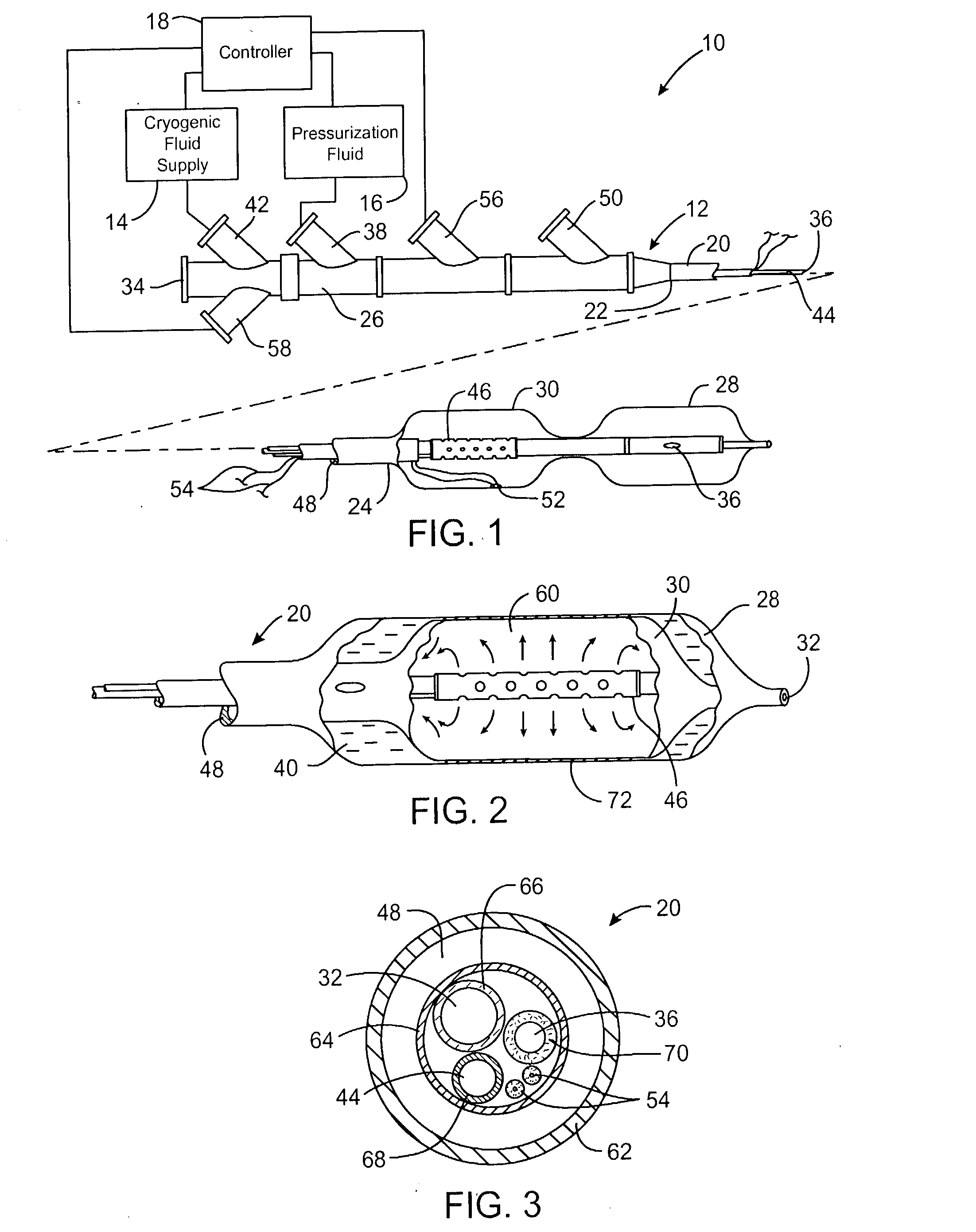
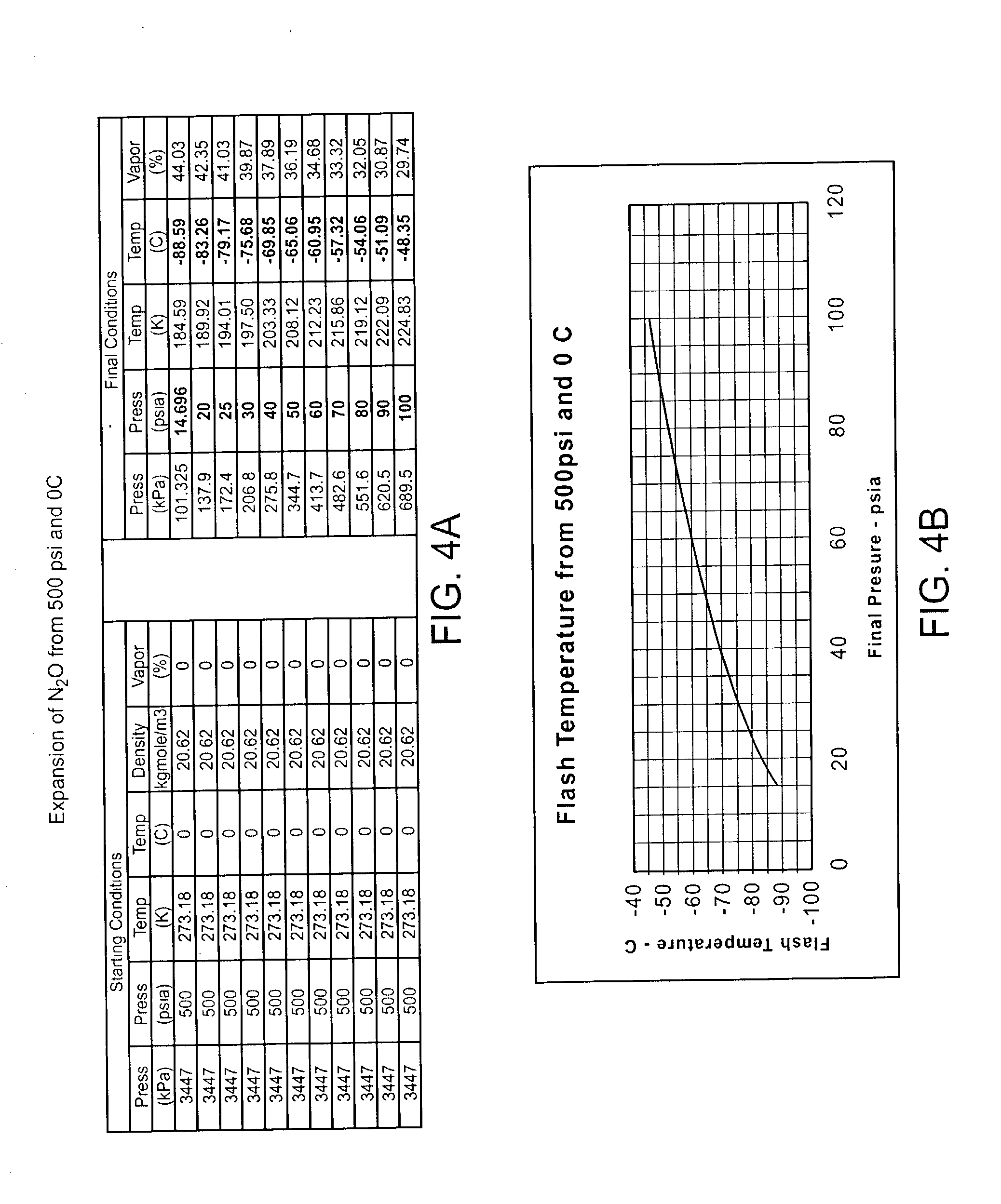
Cryogenically enhanced intravascular interventions
InactiveUS6468297B1Less stenosisLowering indexCatheterSurgical instruments for coolingPercent Diameter StenosisPercutaneous angioplasty
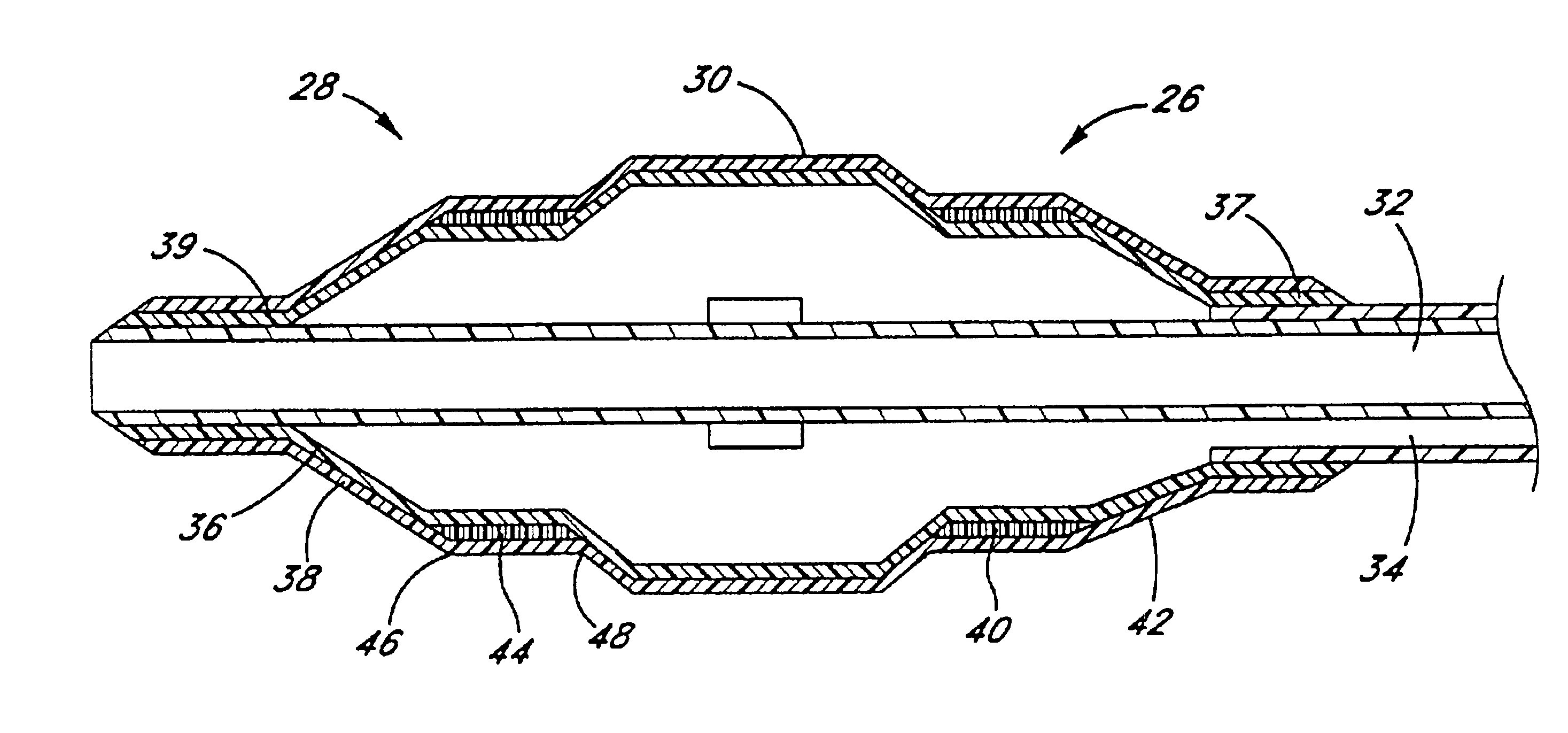
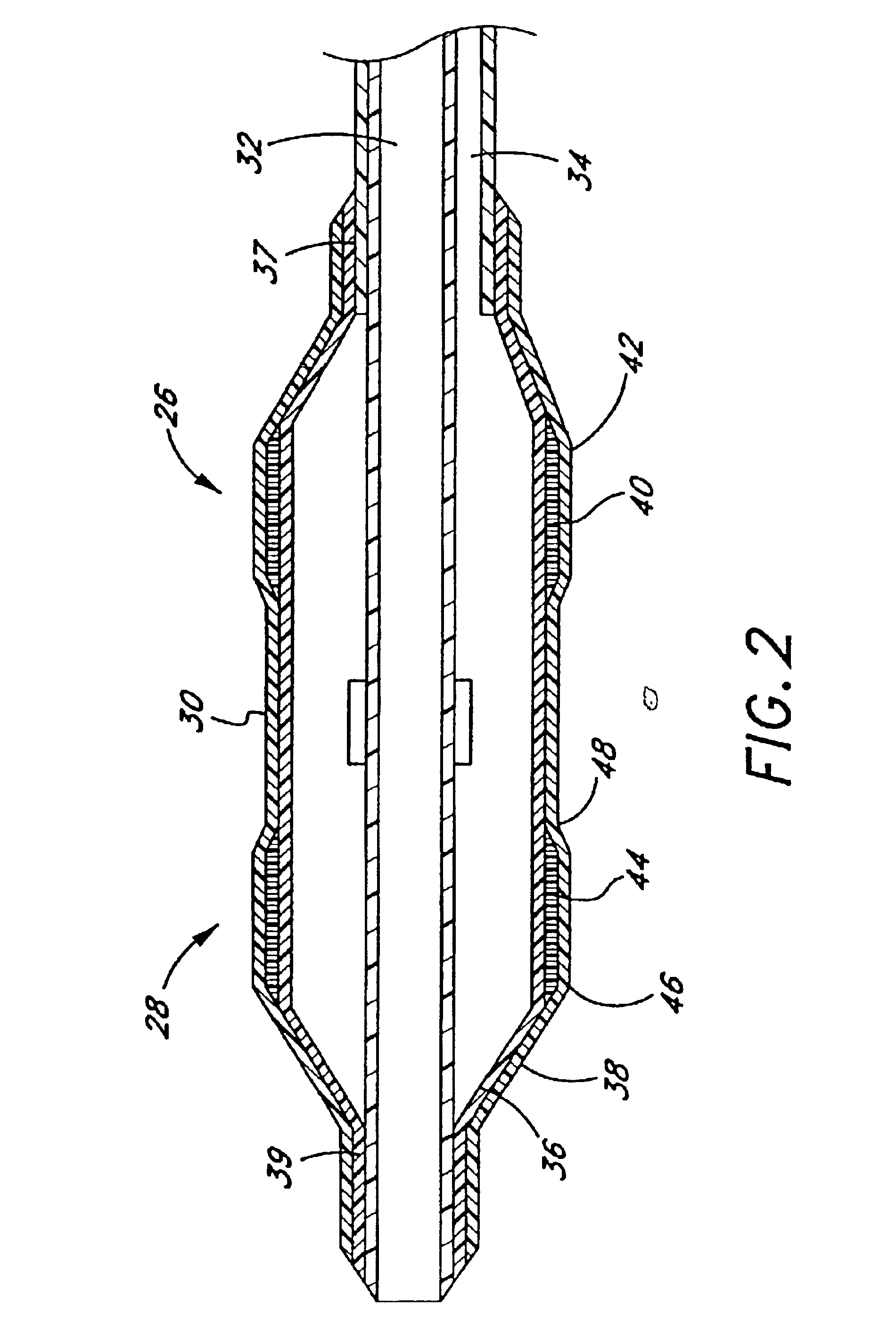
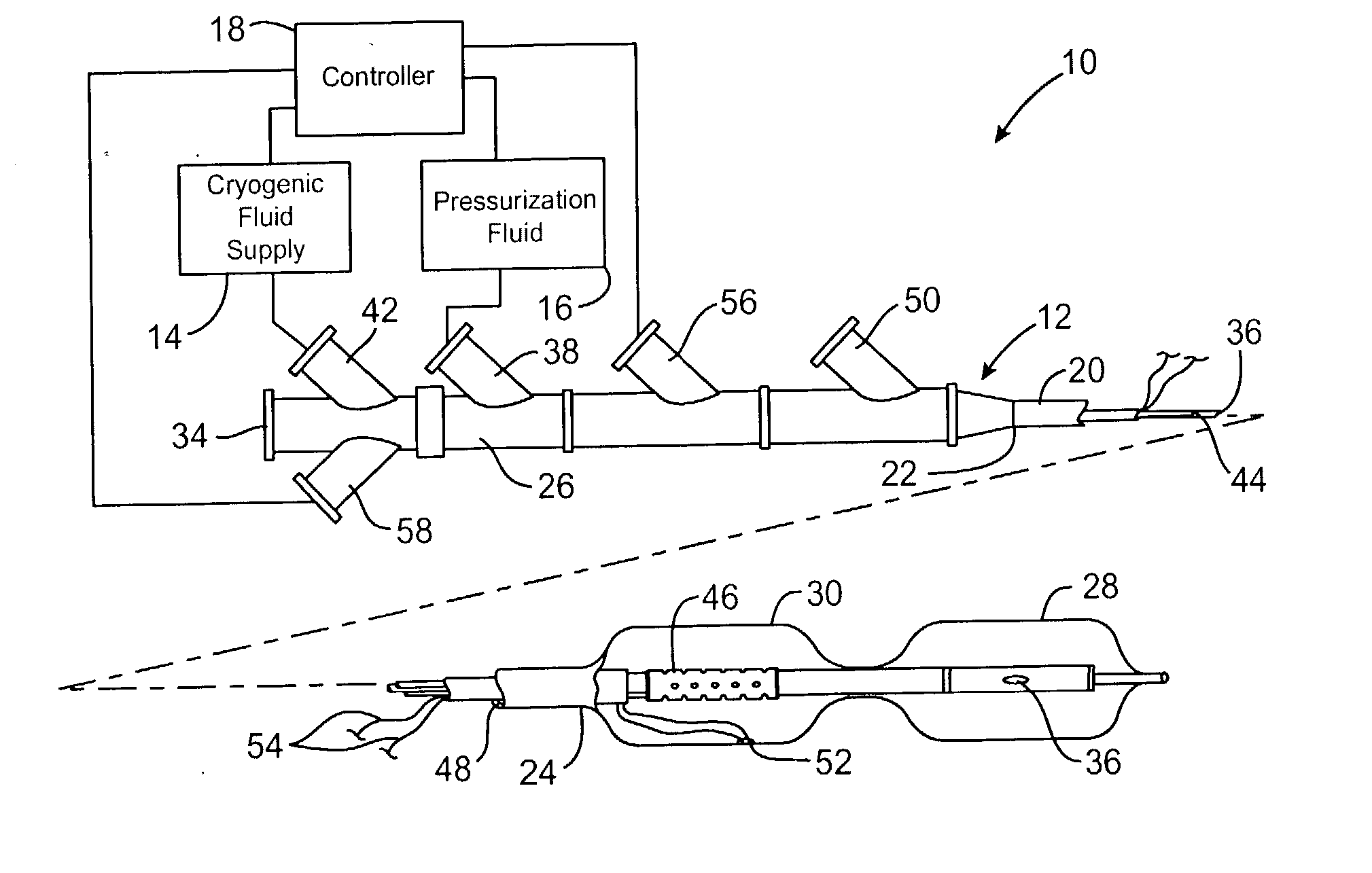
Techniques and devices for treating atherosclerotic disease use controlled cryogenic cooling, often in combination with angioplasty and / or stenting. A combination cryogenic / angioplasty catheter may cool the diseased blood vessel before, during, and / or after dilation. Controlled cooling of the vessel wall reduces actual / observed hyperplasia as compared to conventional uncooled angioplasty. Similar reductions in restenosis may be provided for other primary treatments of the blood vessel, including directional arthrectomy, rotational arthrectomy, laser angioplasty, stenting, and the like. Cooling of vessel wall tissues will often be performed through plaque, and the cooling process will preferably take the thermodynamic effects of the plaque into account.
Owner:BOSTON SCI SCIMED INC
Apparatus and methods for treating bone
InactiveUS20070093899A1Minimally augmentationRecovery heightInternal osteosythesisCannulasInsertion stentBone implant
Implants and methods for minimally invasive augmentation and repositioning of vertebrae may comprise one or more expandable members, e.g., stents, implants, surrounding a balloon-tipped catheter or other expansion device, inserted into a vertebral body or other bone. Expansion of the expandable member within the vertebral body or other bone may reposition the fractured bone to a desired height and augment the bone to maintain the desired height. A bone cement or other filler can be added to further augment and stabilize the vertebral body or other bone.
Owner:SYNTHES USA
Stent with outer slough coating
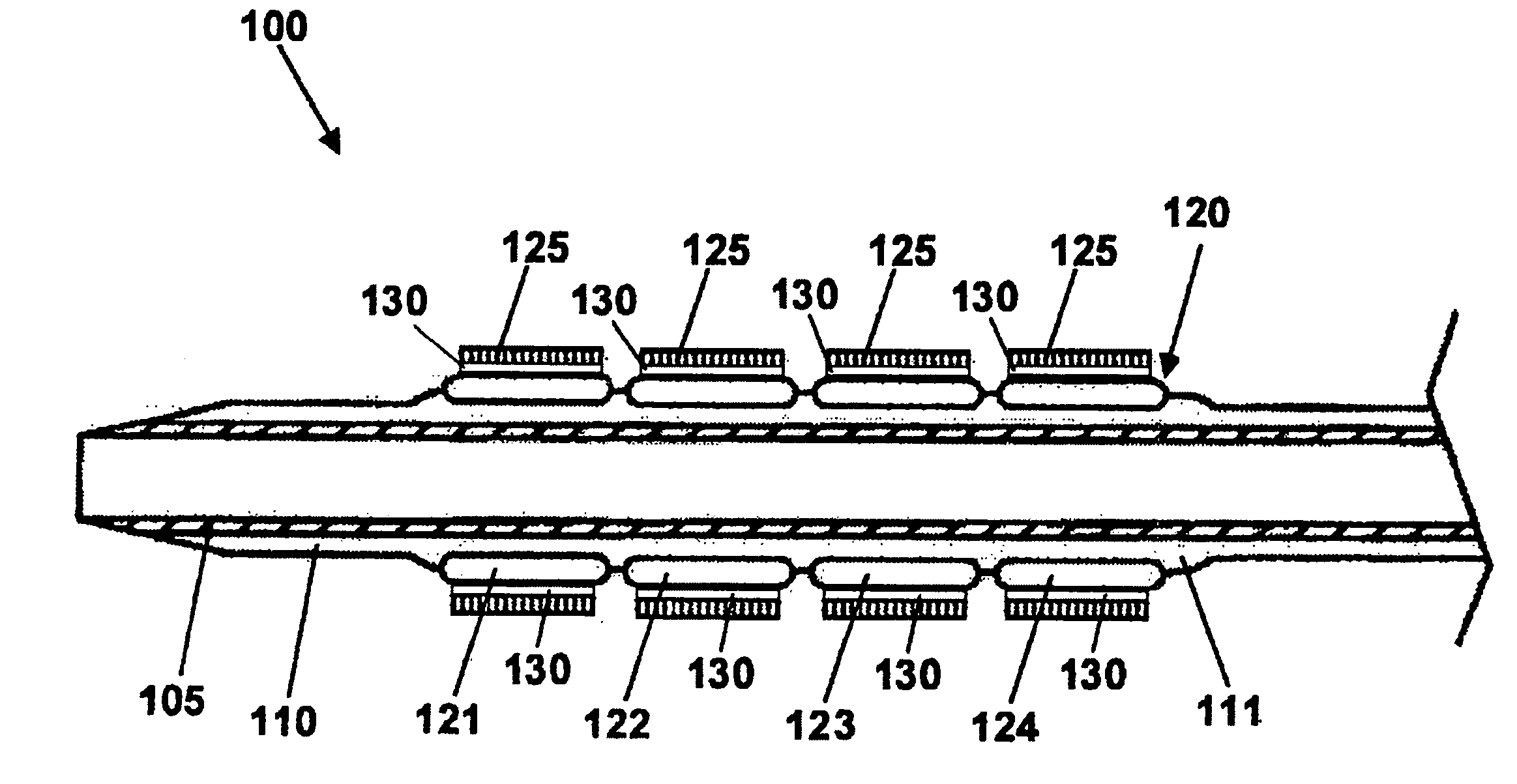
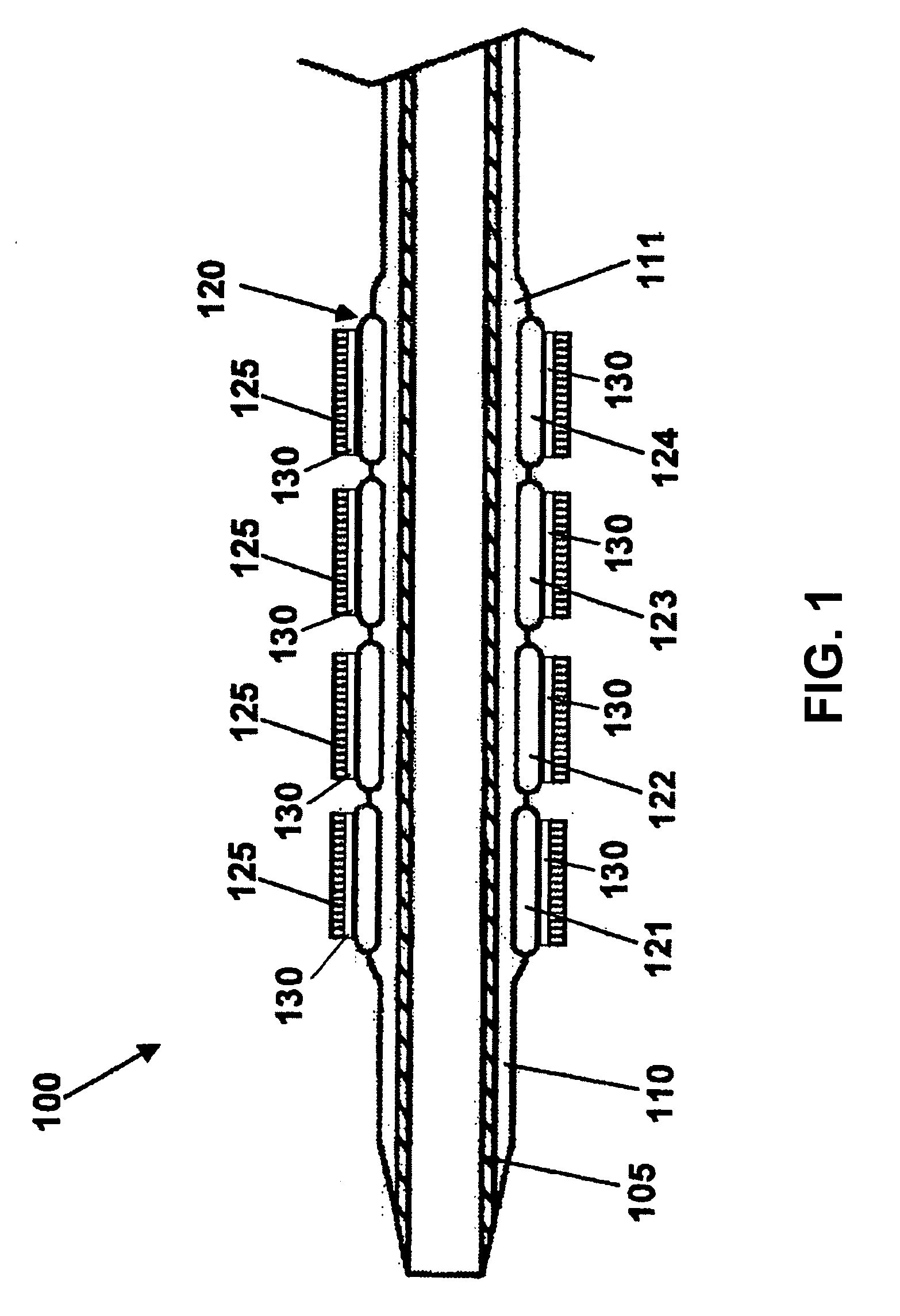
The stent with an outer slough coating 125 of the present invention provides a coated stent having a permanent coating 130 disposed on the stent and a slough coating 125 disposed on the permanent coating 130. The permanent coating 130 includes an anti-proliferative agent and the slough coating 125 includes an anti-inflammatory agent. The slough coating 125 erodes shortly after stent implantation to deliver the anti-inflammatory agent, which treats tissue trauma from the angioplasty and the presence of the stent. Once the slough coating 125 has substantially eroded, the permanent coating 130 delivers the anti-proliferative agent long-term to prevent tissue growth on the stent or within the body lumen, and prevent restenosis. The permanent coating 130 can also include an anti-inflammatory agent.
Owner:MEDTRONIC VASCULAR INC
Method and apparatus for transmyocardial direct coronary revascularization
InactiveUS6929009B2Facilitate valvingShortening and thickeningEar treatmentCannulasVeinHeart chamber
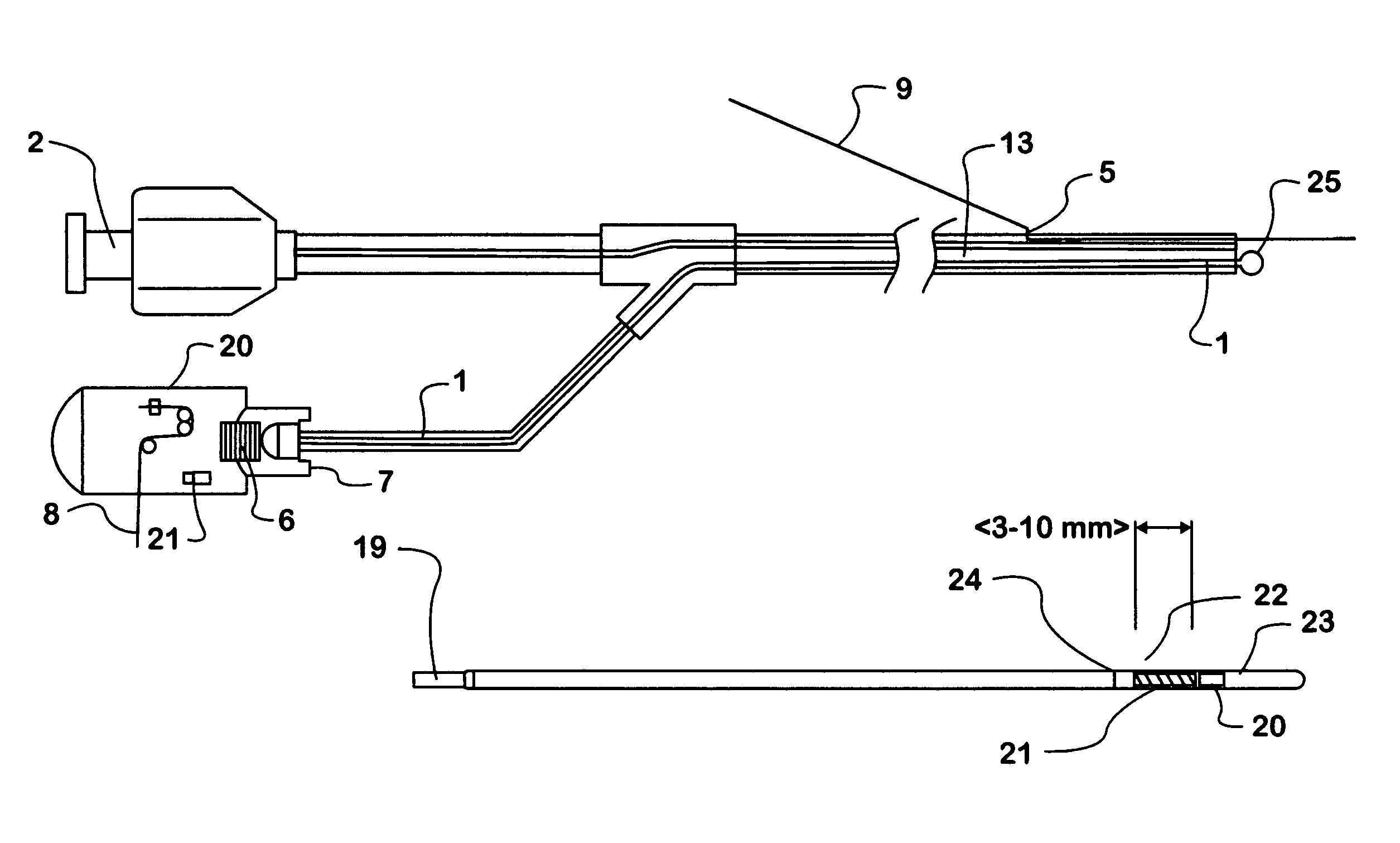
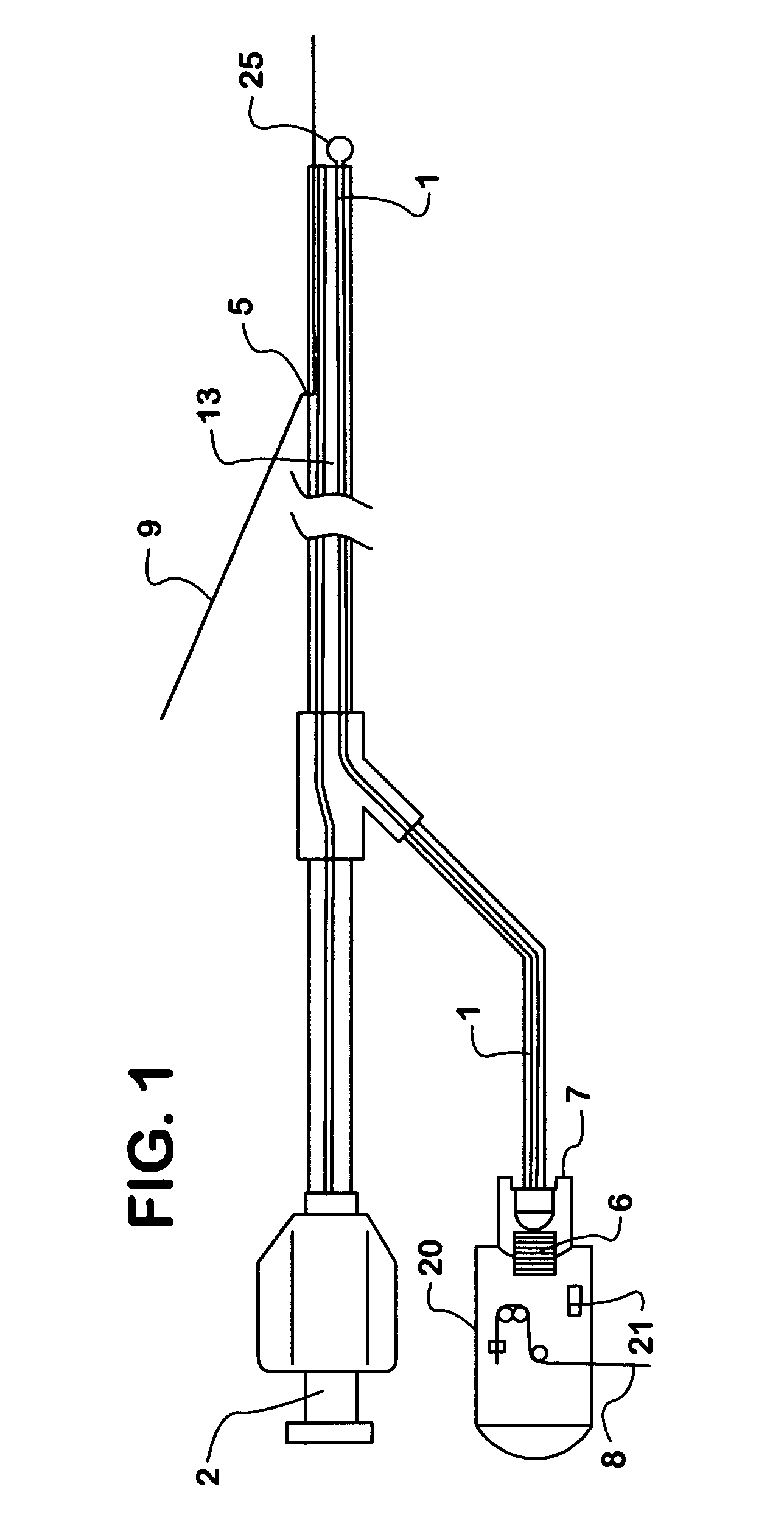
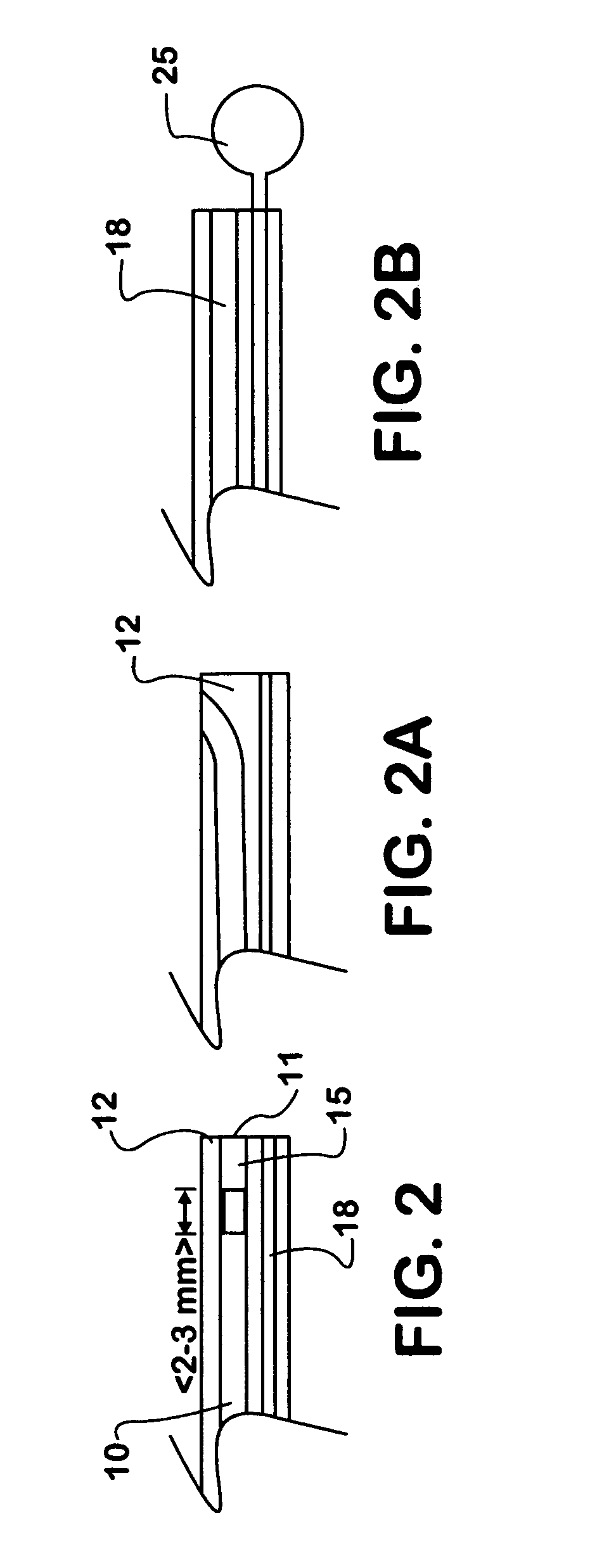
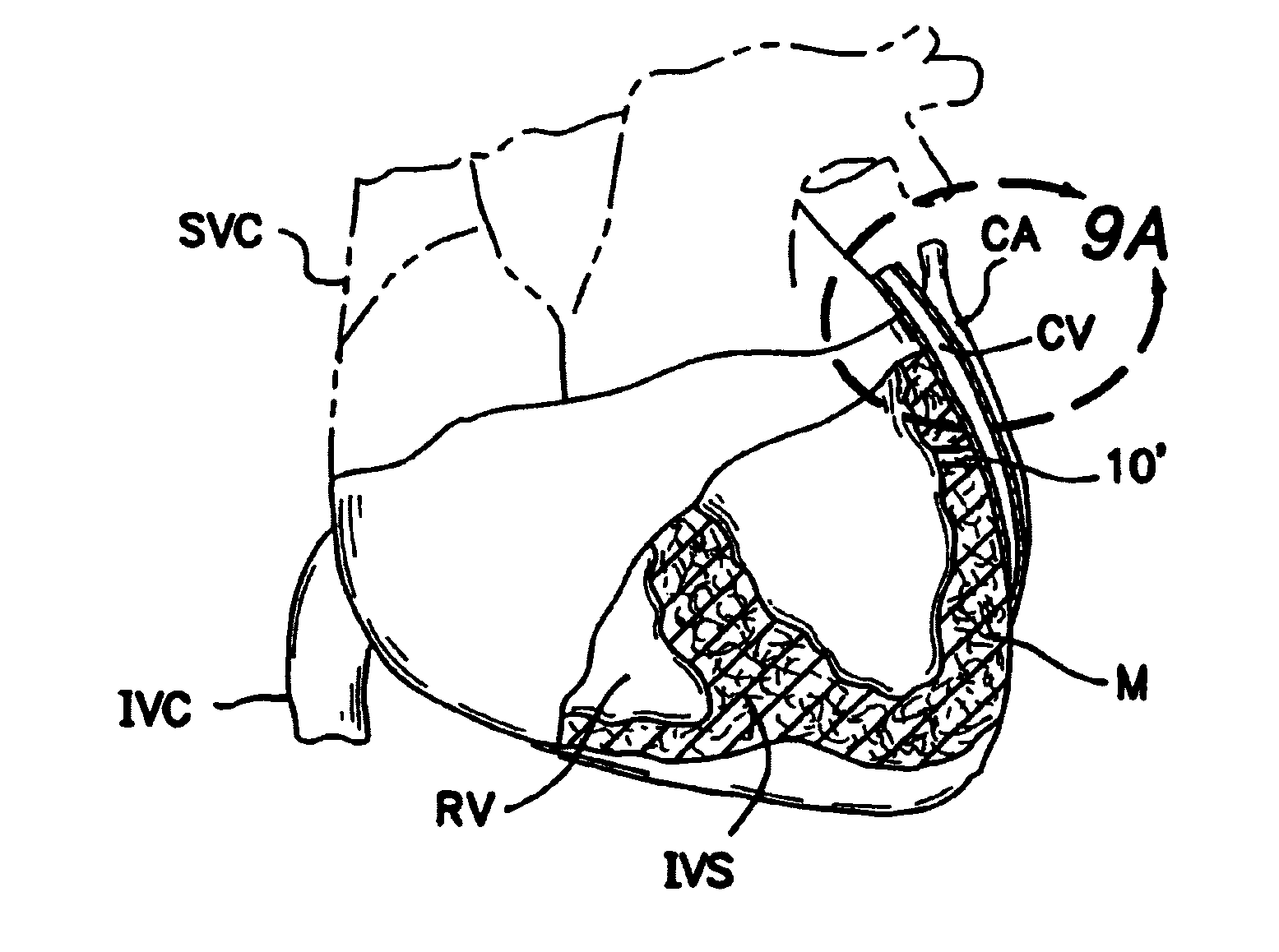
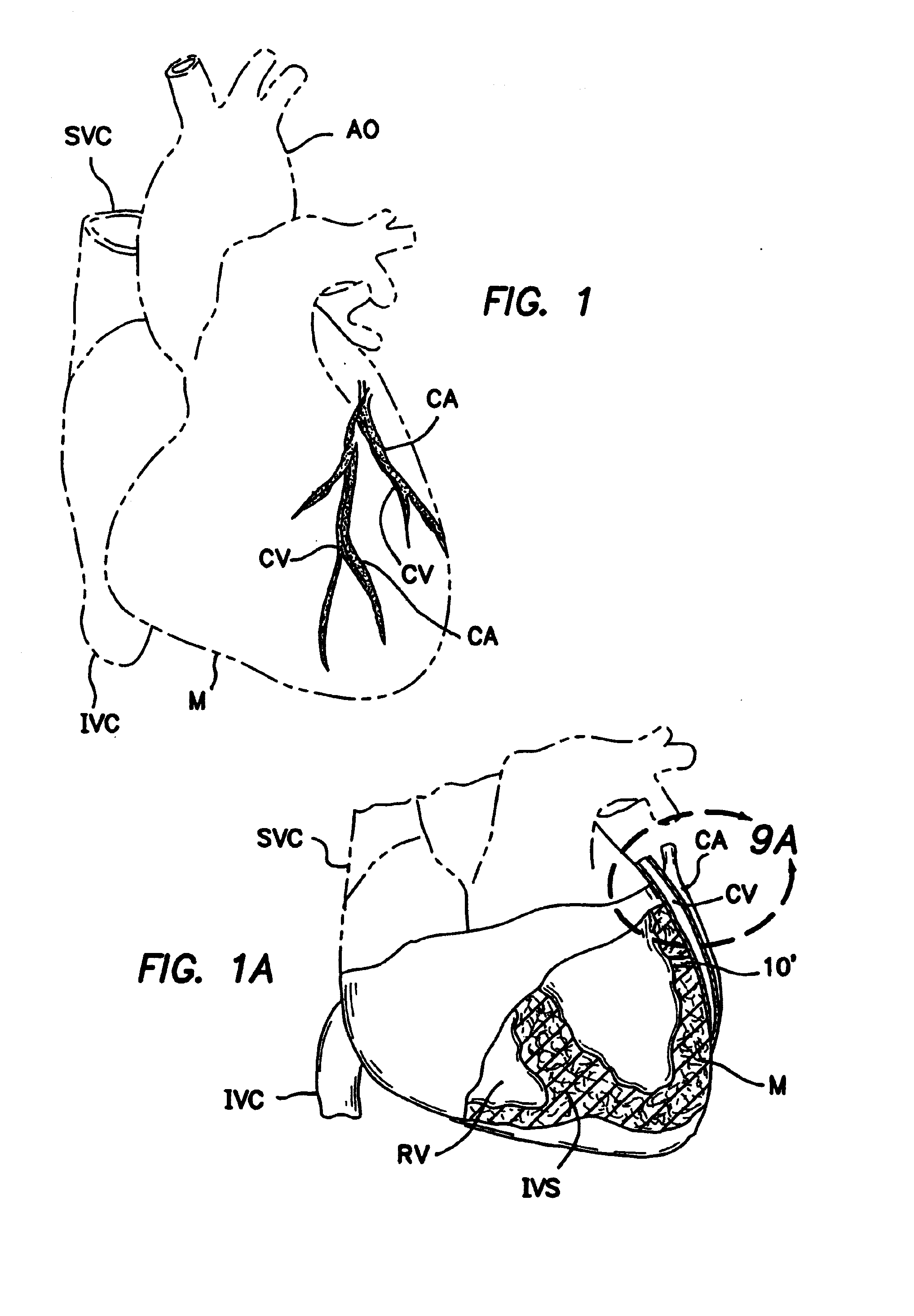
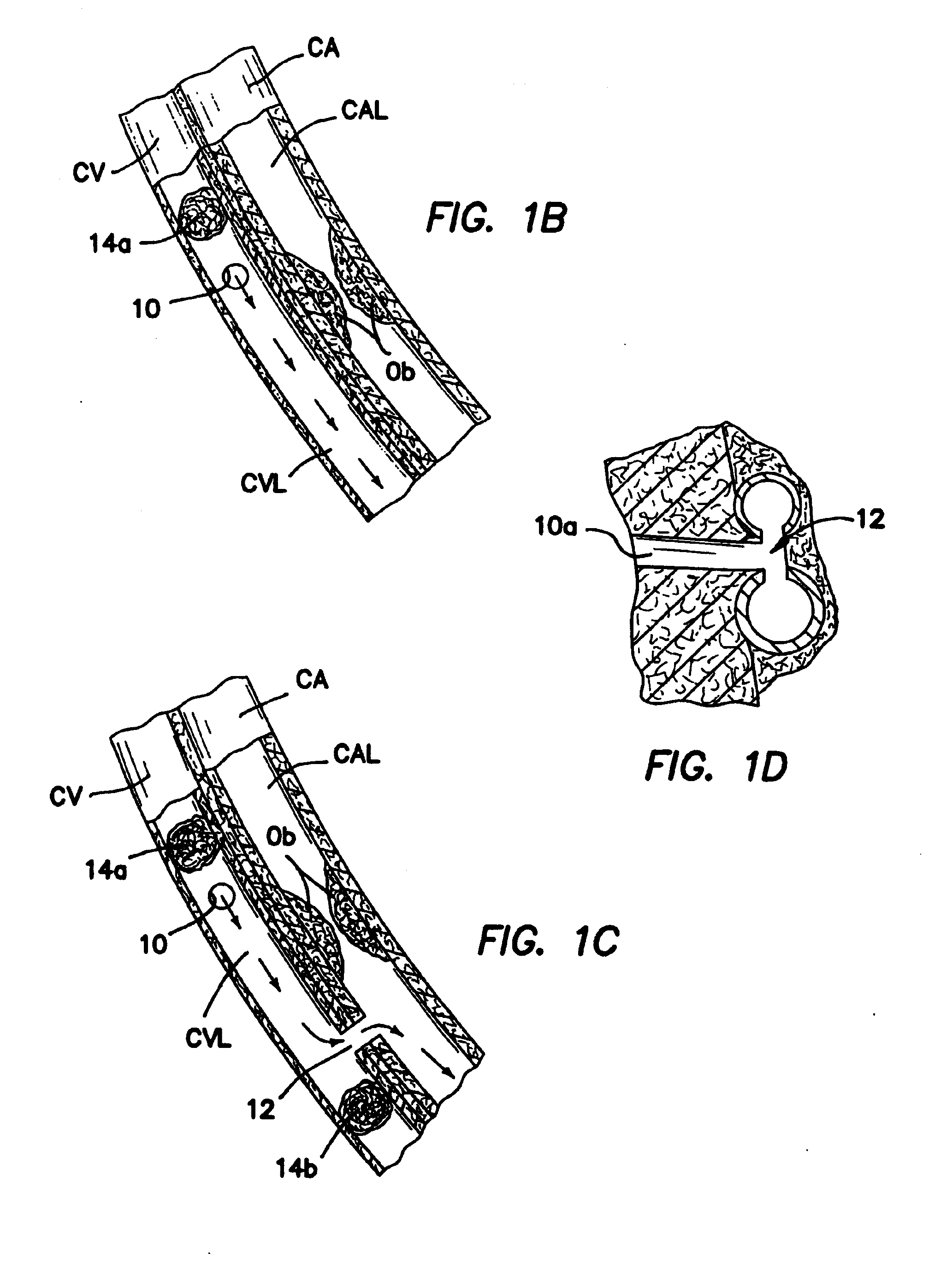
Methods and apparatus for direct coronary revascularization wherein a transmyocardial passageway is formed between a chamber of the heart and a coronary blood vessel to permit blood to flow therebetween. In some embodiments, the transmyocardial passageway is formed between a chamber of the heart and a coronary vein. The invention includes unstented transmyocardial passageways, as well as transmyocardial passageways wherein protrusive stent devices extend from the transmyocardial passageway into an adjacent coronary vessel or chamber of the heart. The apparatus of the present invention include protrusive stent devices for stenting of transmyocardial passageways, intraluminal valving devices for valving of transmyocardial passageways, intracardiac valving devices for valving of transmyocardial passageways, endogenous tissue valves for valving of transmyocardial passageways, and ancillary apparatus for use in conjunction therewith.
Owner:MEDTRONIC VASCULAR INC
Electromagnetic photonic catheter for reducing restenosis
InactiveUS6962584B1Reduce spreadImprove scalabilitySurgical instrument detailsCatheterPhotonicsPercent Diameter Stenosis
The method of vascular treatment for restenosis or vulnerable plaque after an invasive procedure, such as for example angioplasty, stenting with or without drug coating, or drug delivery, comprises: inserting a catheter or hollow guide wire to the treatment location; delivering light through the catheter in the wavelength range of about 700–2500 nm; and moving the light to treat the affected region.
Owner:STONE GREGG W +5
Methods and apparatus for transmyocardial direct coronary revascularization
InactiveUS7159592B1Easy to movePromote formationEar treatmentCannulasCoronary revascularizationCoronary revascularisation
Methods and apparatus for direct coronary revascularization wherein a transmyocardial passageway is formed between a chamber of the heart and a coronary blood vessel to permit blood to flow therebetween. In some embodiments, the transmyocardial passageway is formed between a chamber of the heart and a coronary vein. The invention includes unstented transmyocardial passageways, as well as transmyocardial passageways wherein protrusive stent devices extend from the transmyocardial passageway into an adjacent coronary vessel or chamber of the heart. The apparatus of the present invention include protrusive stent devices for stenting of transmyocardial passageways, intraluminal valving devices for valving of transmyocardial passageways, intracardiac valving devices for valving of transmyocardial passageways, endogenous tissue valves for valving of transmyocardial passageways, and ancillary apparatus for use in conjunction therewith.
Owner:MEDTRONIC VASCULAR INC
Glaucoma stent system
Owner:GLAUKOS CORP
Sliding restraint stent delivery systems
InactiveUS20050209672A1Conveniently “ lock-down ”Good locking functionStentsBlood vesselsBody organsInsertion stent
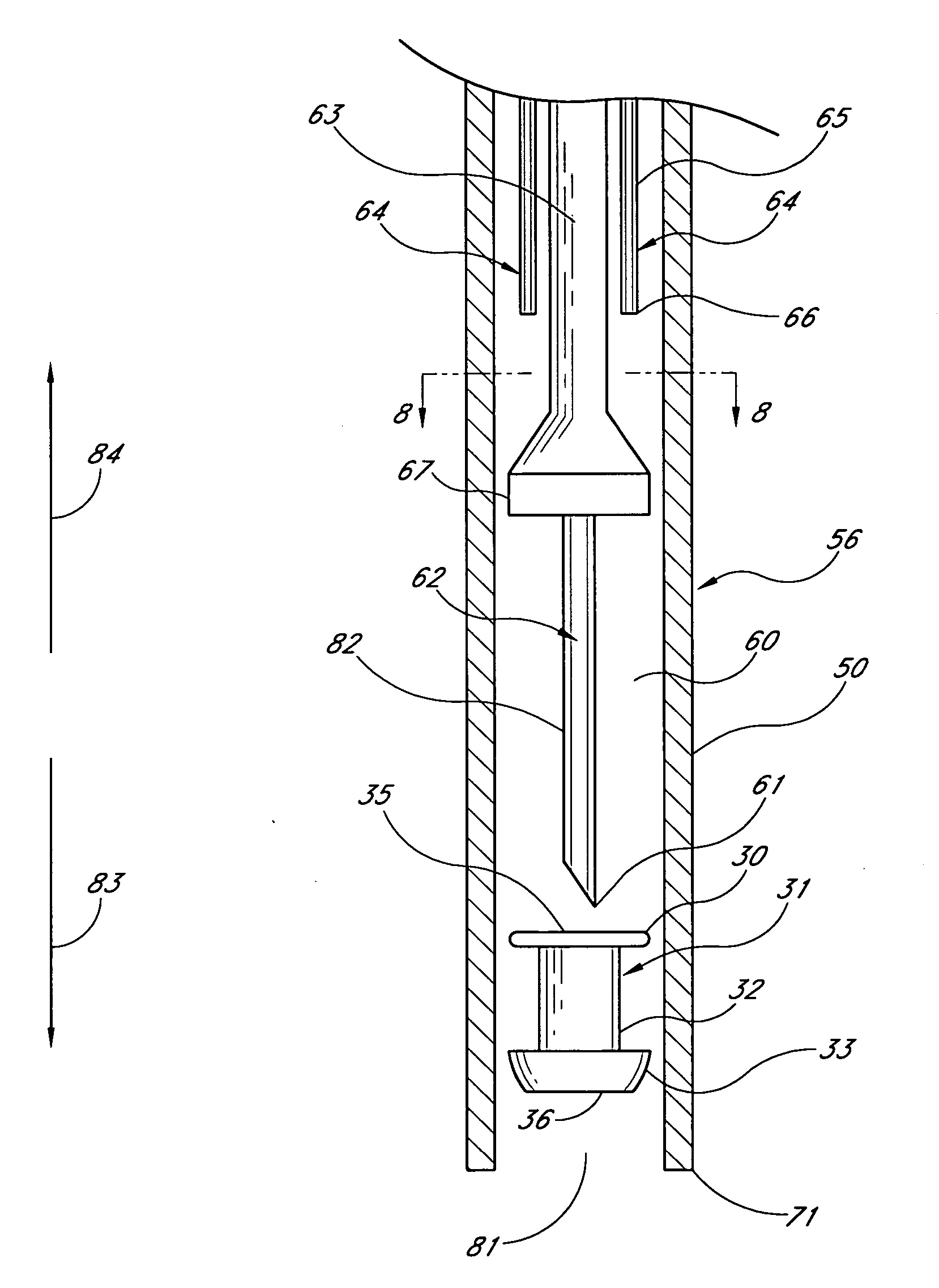
Medical device and methods for delivery or implantation of prostheses within hollow body organs and vessels or other luminal anatomy are disclosed. The subject technologies may be used in the treatment of atherosclerosis in stenting procedures. For such purposes, a self-expanding stent may be deployed in connection with an angioplasty procedure with a sliding restraint based delivery system adapted for simplified use. In the system, the sliding restraint is sized, in coordination with a fixed sleeve accepting a core wire to actuate the restraint to effect an anchoring function with the sleeve so that the stent is not inadvertently advanced during deployment.
Owner:BIOSENSORS INT GROUP
Optimized stent jacket
ActiveUS20100241214A1Reducing platelet aggregationReduce aggregationStentsSurgeryFiberAdditive ingredient
A method of stenting, comprises: implanting a stent assembly in a vessel of a subject, the stent assembly, including: a stent jacket, comprising an expansible mesh structure, formed of fibers of a diameter between about 7 micrometers and about 18 micrometers, the diameter having a property of forming a substantially stable layer of endothelial cells, covering the fibers, thus reducing platelet aggregation, and an expansible stent, operatively associated with the stent jacket. The method further comprises administering to the subject an active pharmaceutical ingredient (API) comprising a platelet aggregation reducer for a shortened time period, not exceeding six months, the shortened time period being a consequence of the property. In accordance with some embodiments, the administration of a platelet aggregation.
Owner:INSPIRE M D LTD
Permanent thrombus filtering stent
InactiveUS20070093744A1Stroke preventionGood blood pressureStentsSurgeryInsertion stentStent implantation
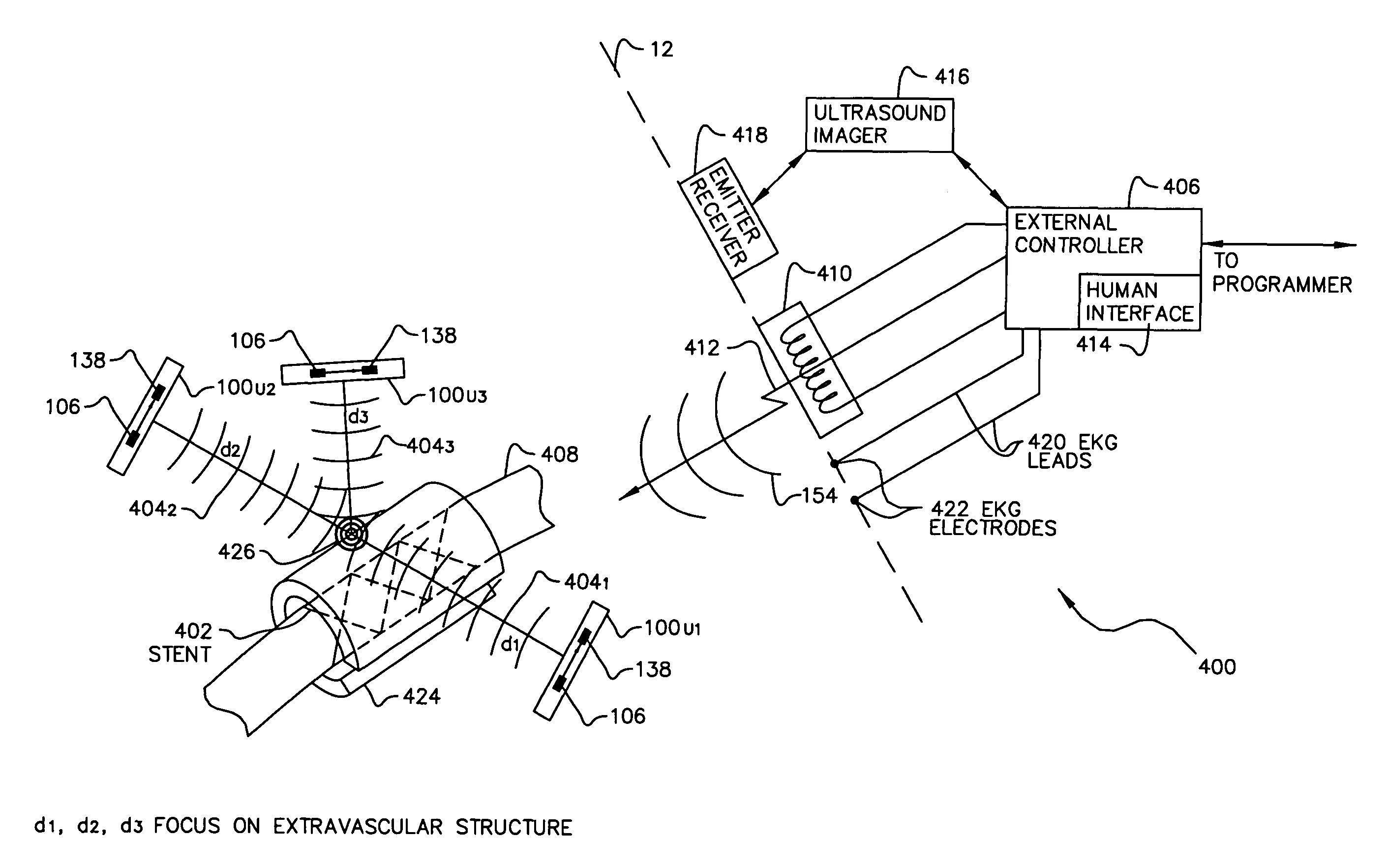
A permanent thrombus and plaque filtering stent blocks and / or filters potential emboli in patients undergoing intravascular treatment and / or stent implantation. The stent has a plurality of movable magnetic or ultrasonic agitating elements attached thereto, which when remotely activated move, vibrate or rotate to break up the thrombus, plaque or tissue debris.
Owner:THE GENERAL HOSPITAL CORP
Embolic protection devices and methods
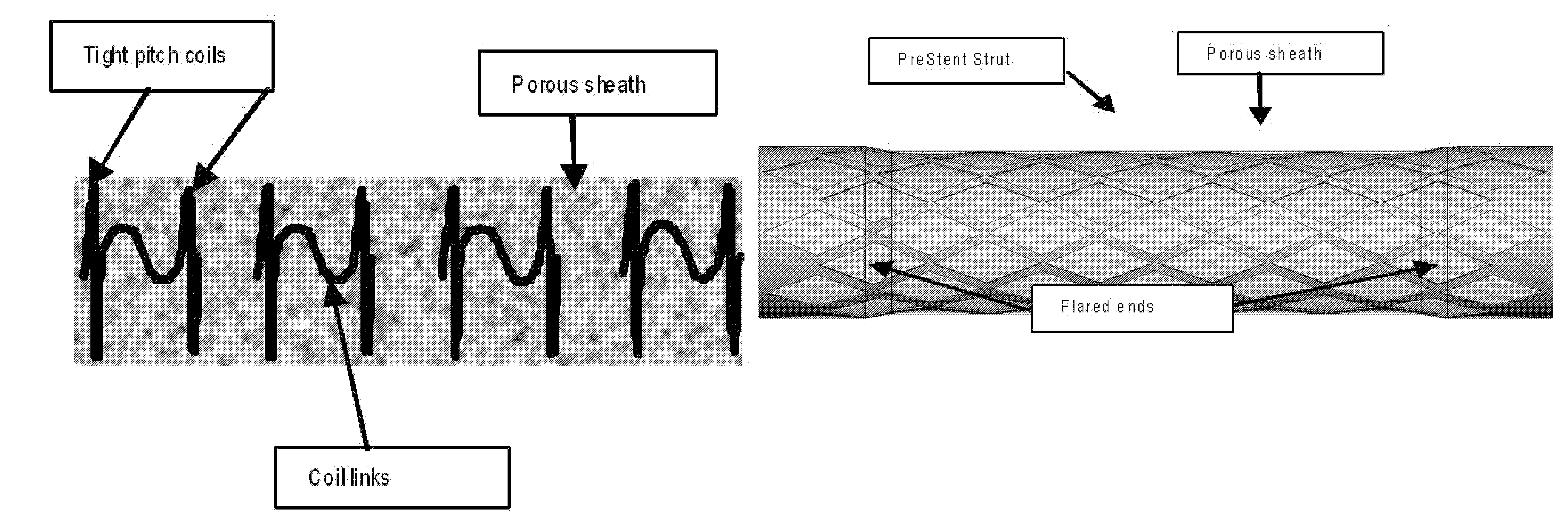
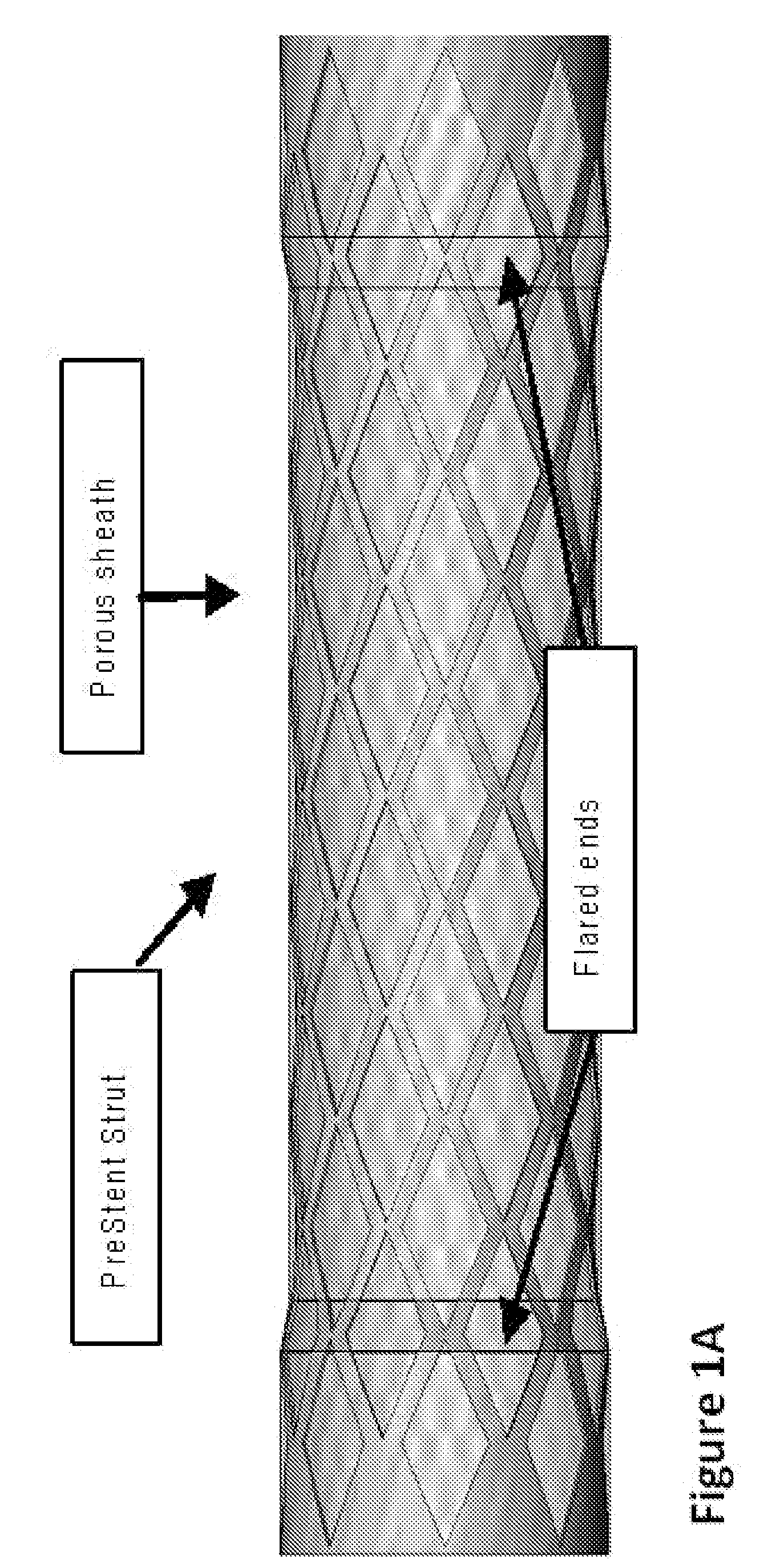
InactiveUS20090043330A1Facilitate safe insertionImprove securityStentsDiagnosticsSupporting systemEmbolic Protection Devices
The present invention provides a PreStent comprising a sheath and frame designed for preparing a vessel passageway for the subsequent delivery of a stent. The PreStent can be self-expanding or balloon-expandable. Also provided is a supporting system for delivering the PreStent safely. The supporting system includes a delivery catheter, one or more occlusion balloon, optionally one or more dilation balloon, and a retention sheath for the self-expanding type of PreStent. The PreStent of the present invention is flexible for use with a variety of stent and guidewire models such that it can easily be incorporated with existing devices to improve stenting procedures.
Owner:SPECIALIZED VASCULAR TECH
Composite stent with inner and outer stent elements and method of using the same
An endoprosthesis comprising a stent, a cover fully covering the stent wherein the cover has variable porosity in the radial direction; and an adhesion layer connecting the stent to the cover. Another aspect of the invention is a method of implanting an endoprosthesis which includes a stent, providing a cover with variable porosity in the radial direction, connecting the stent to the cover with an adhesion layer to form a covered stent, and implanting the covered stent within a body lumen of a patient.
Owner:BOSTON SCI SCIMED INC
Computerized workflow method for stent planning and stenting procedure
ActiveUS7650179B2Optimize locationStentsBlood flow measurement devicesStent implantationComputer-aided
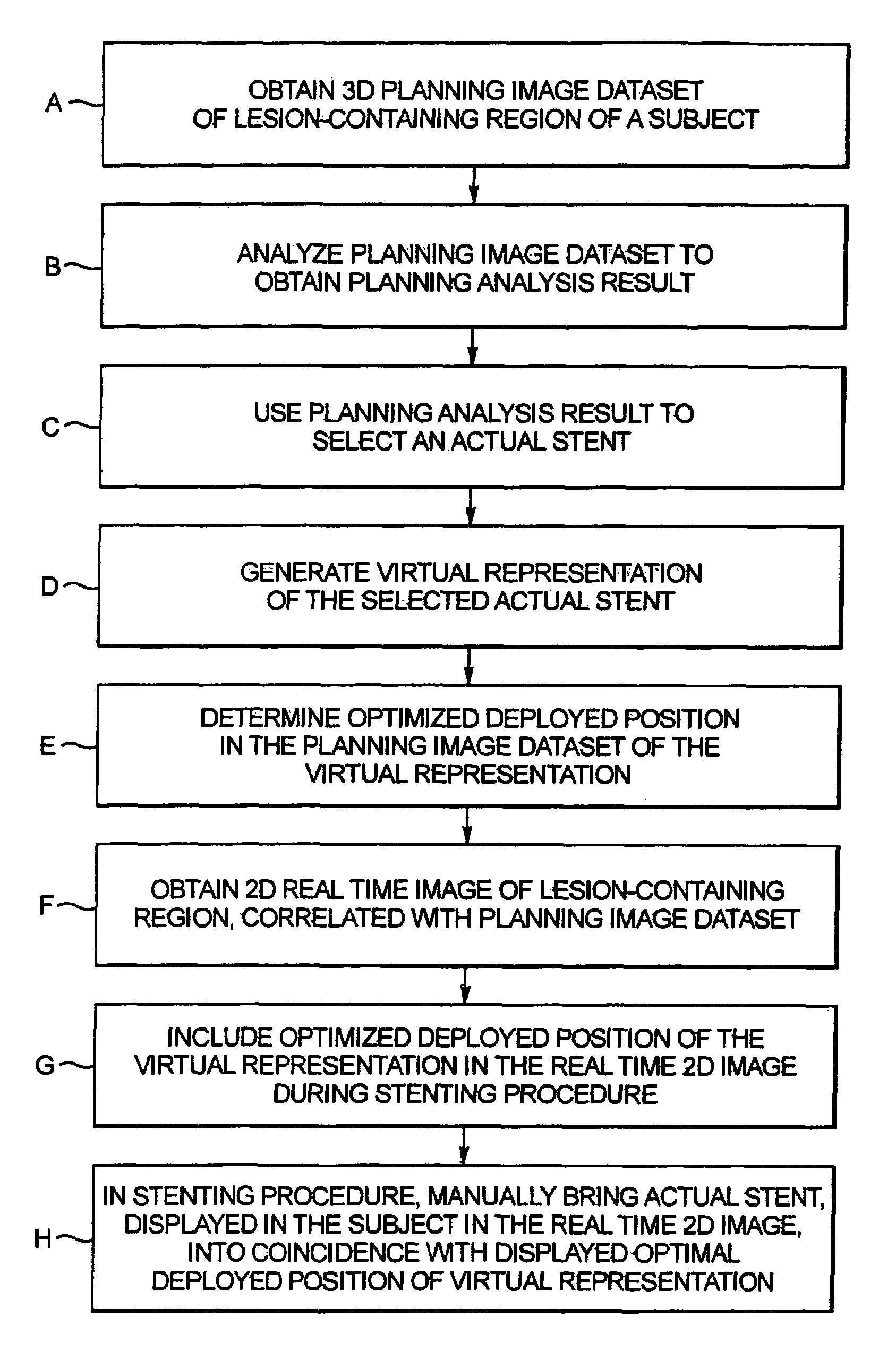
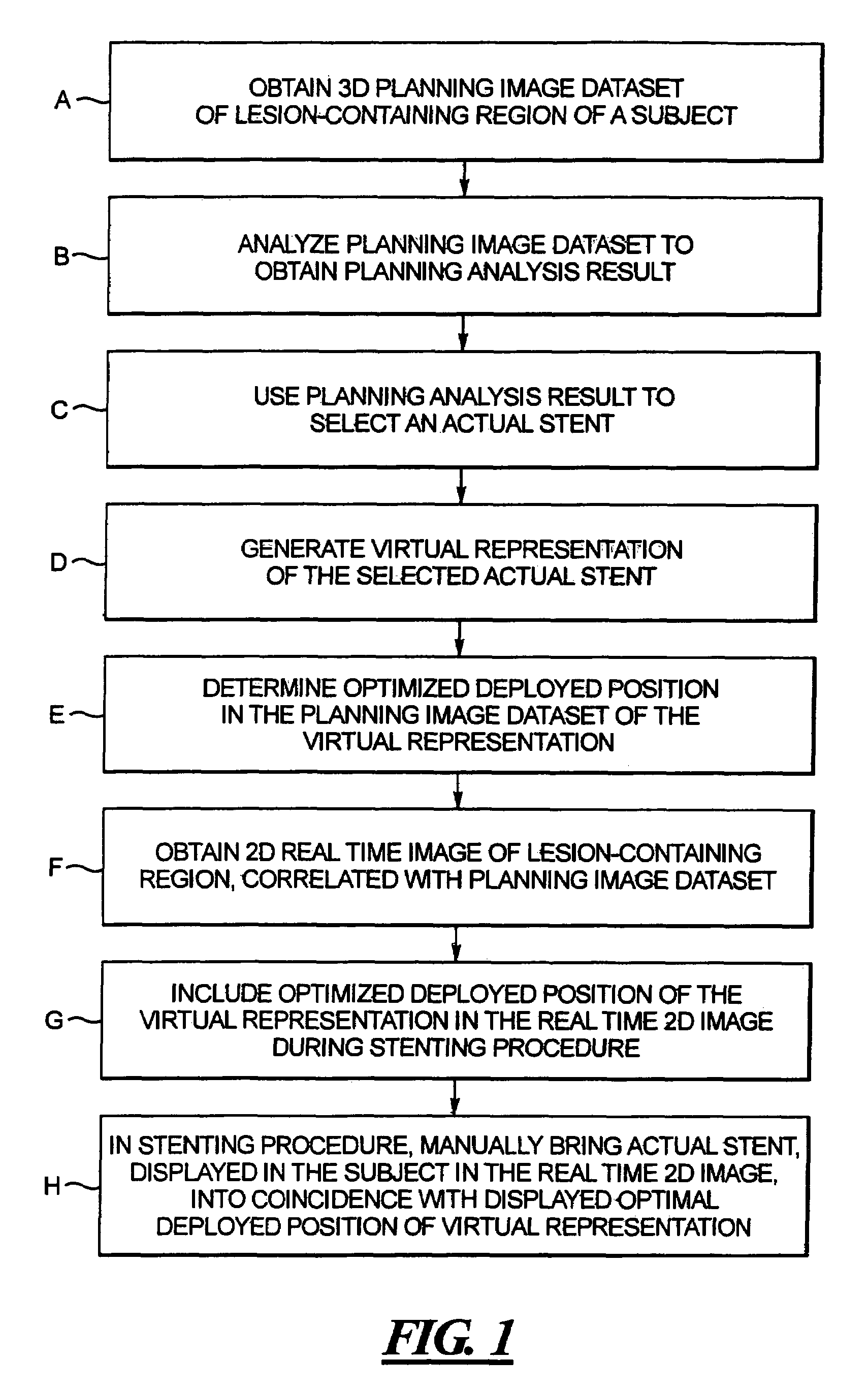
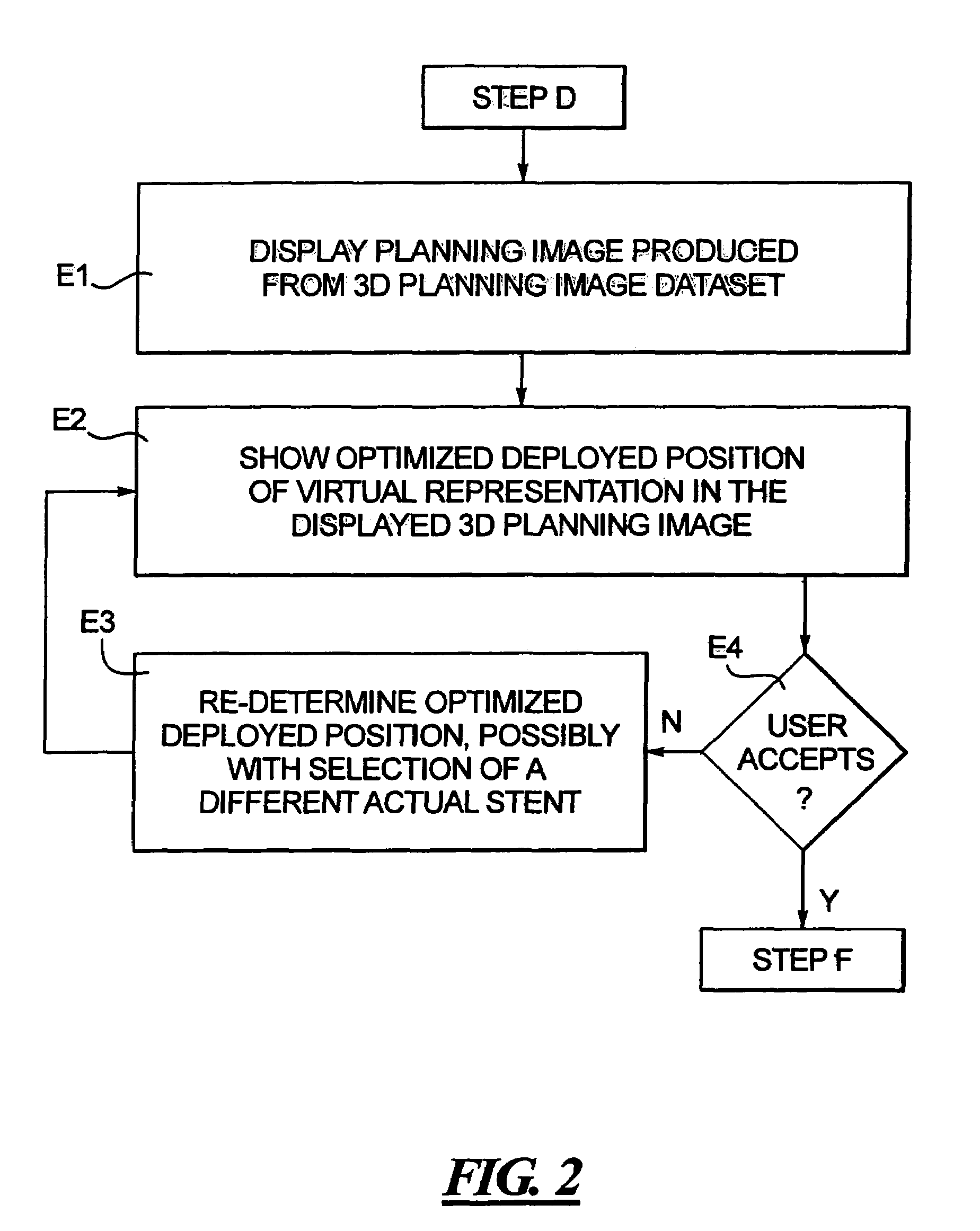
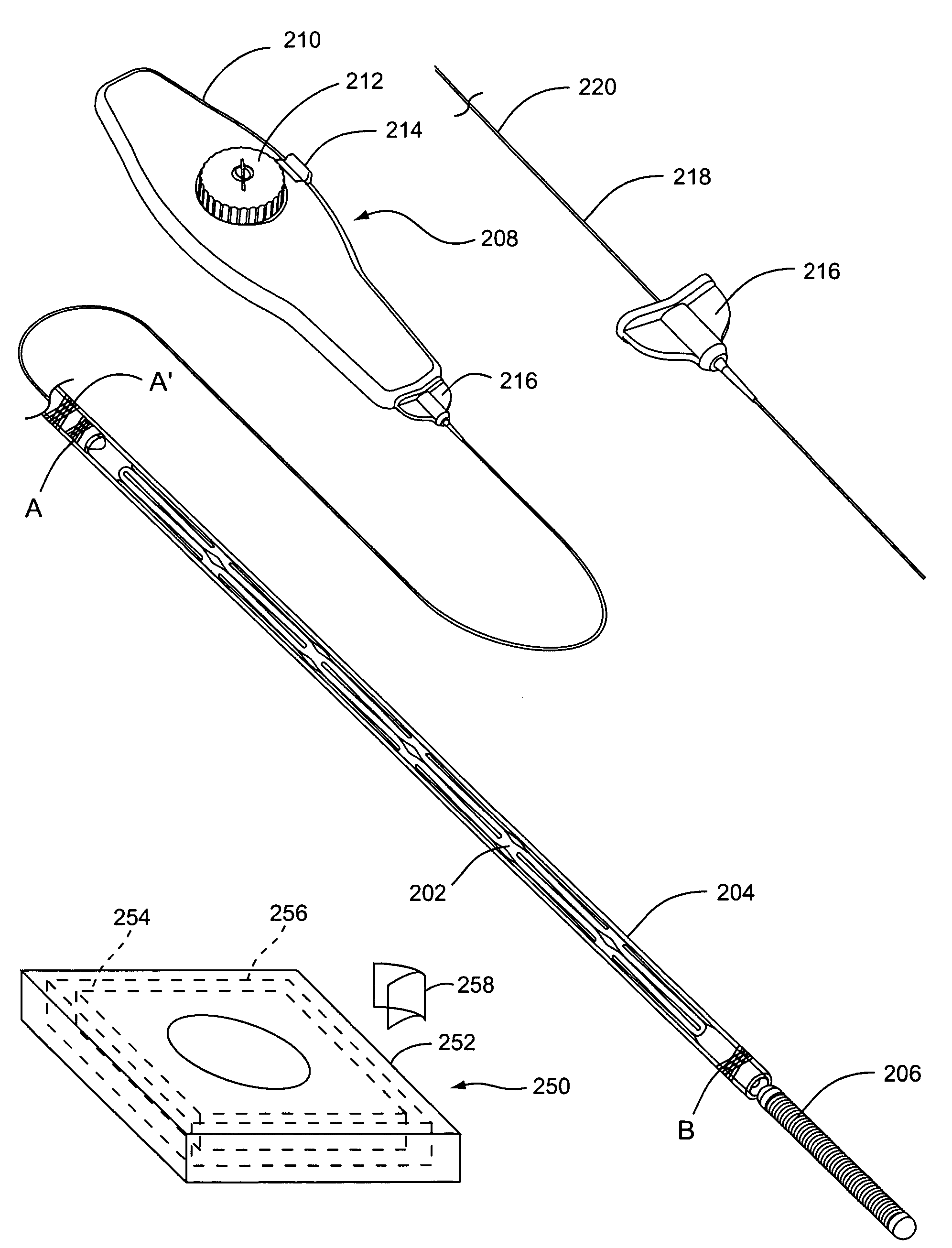
In a computerized workflow method for stent planning and conducting a stenting procedure, characteristics of a lesion to be stented are determined from a 3D planning image of the region and selection of an actual stent for stenting the lesion is made with computer-assisted analysis of the lesion based on the characteristics. A virtual stent is electronically generated based on the actual stent, and, using the virtual stent, a best position for the actual stent, for effectively stenting the lesion, is determined. A real time 2D image of the lesion-containing region is displayed during the stenting procedure, with the virtual stent included therein at the aforementioned best position. A physician manually guides the actual stent relative to the lesion during the stenting procedure until the position of the actual stent, as seen in the displayed real time 2D image, coincides with the virtual stent in that image.
Owner:SIEMENS HEALTHCARE GMBH
Portal design for stent for treating bifurcated vessels
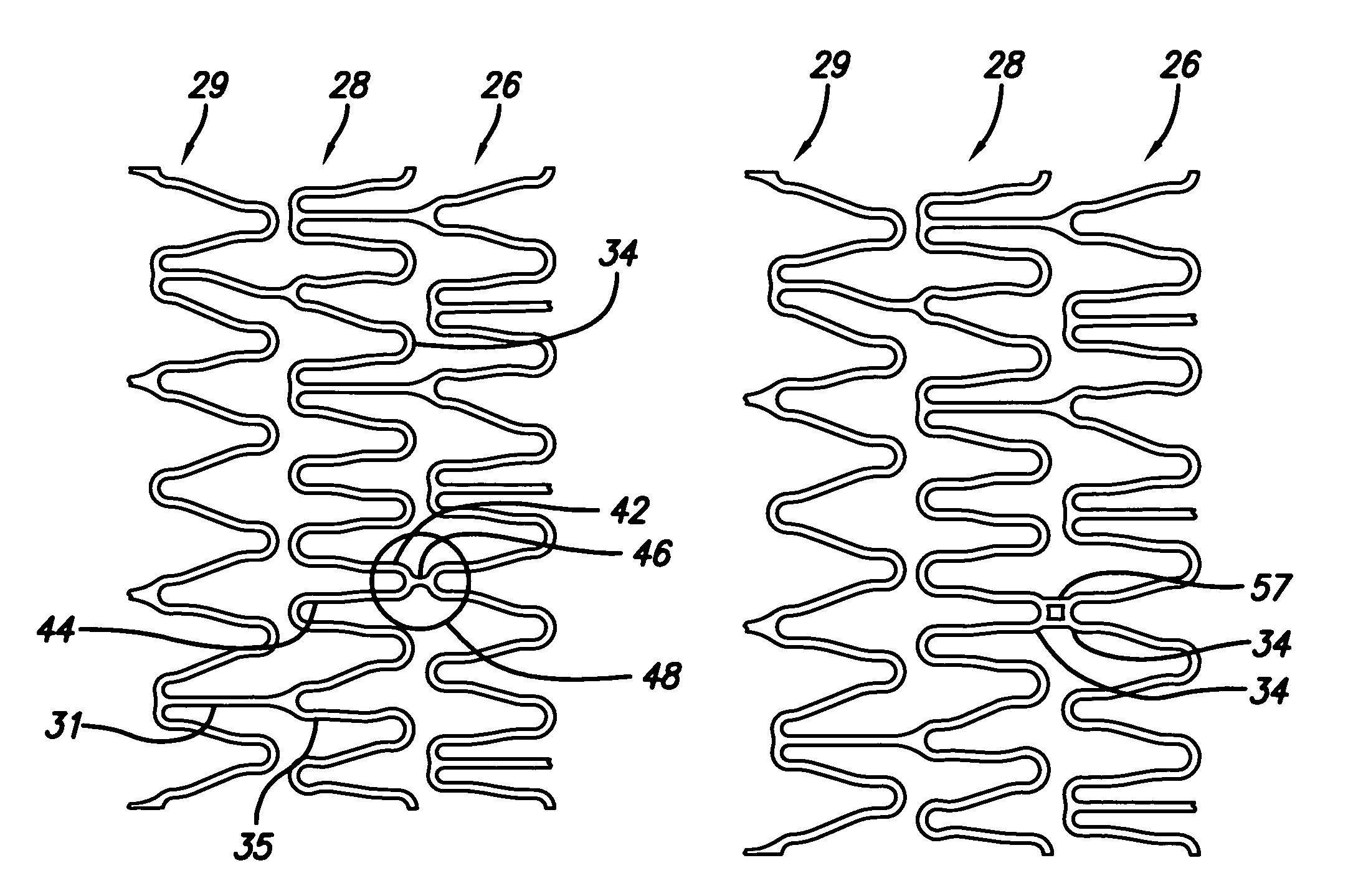
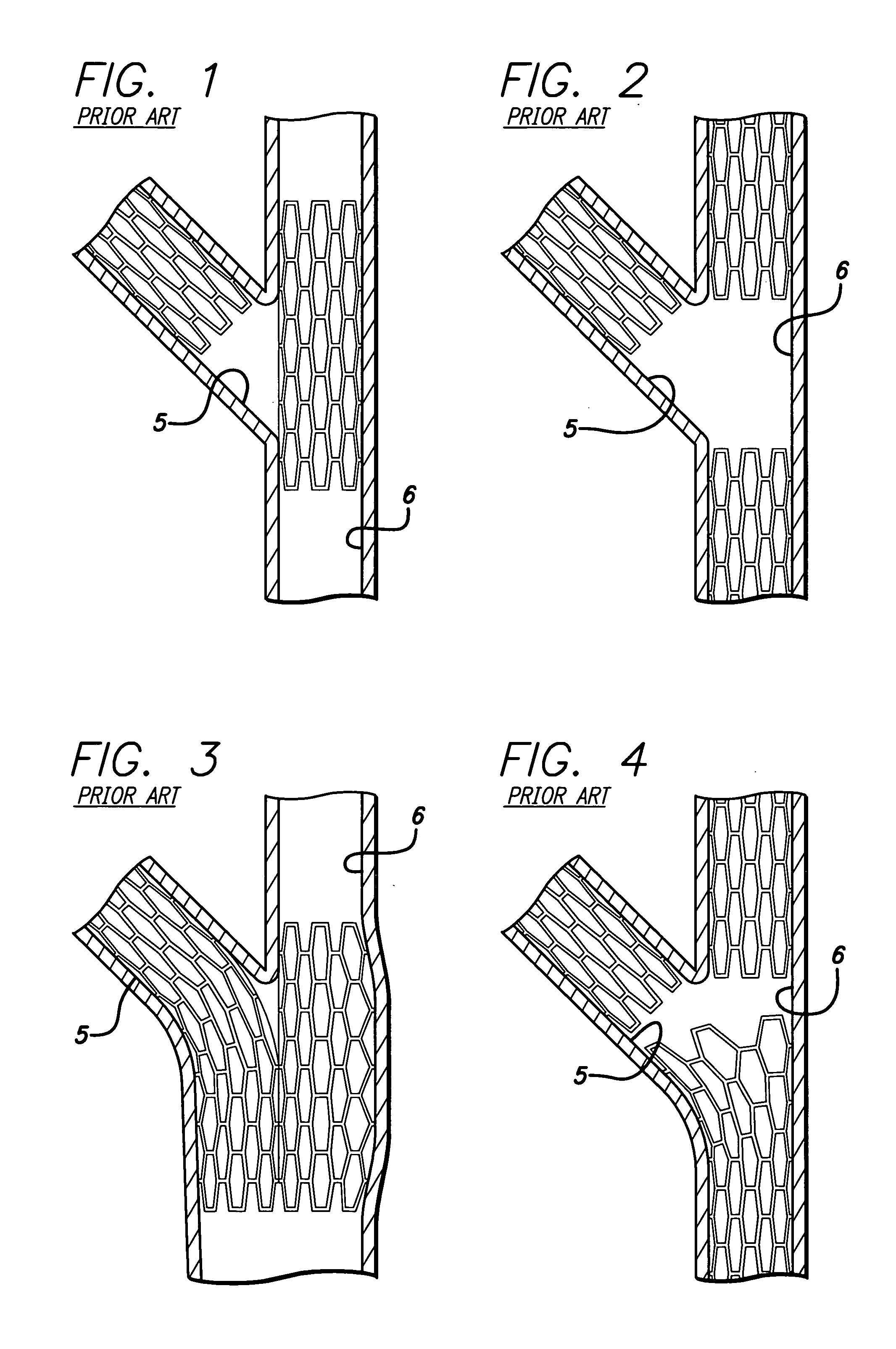
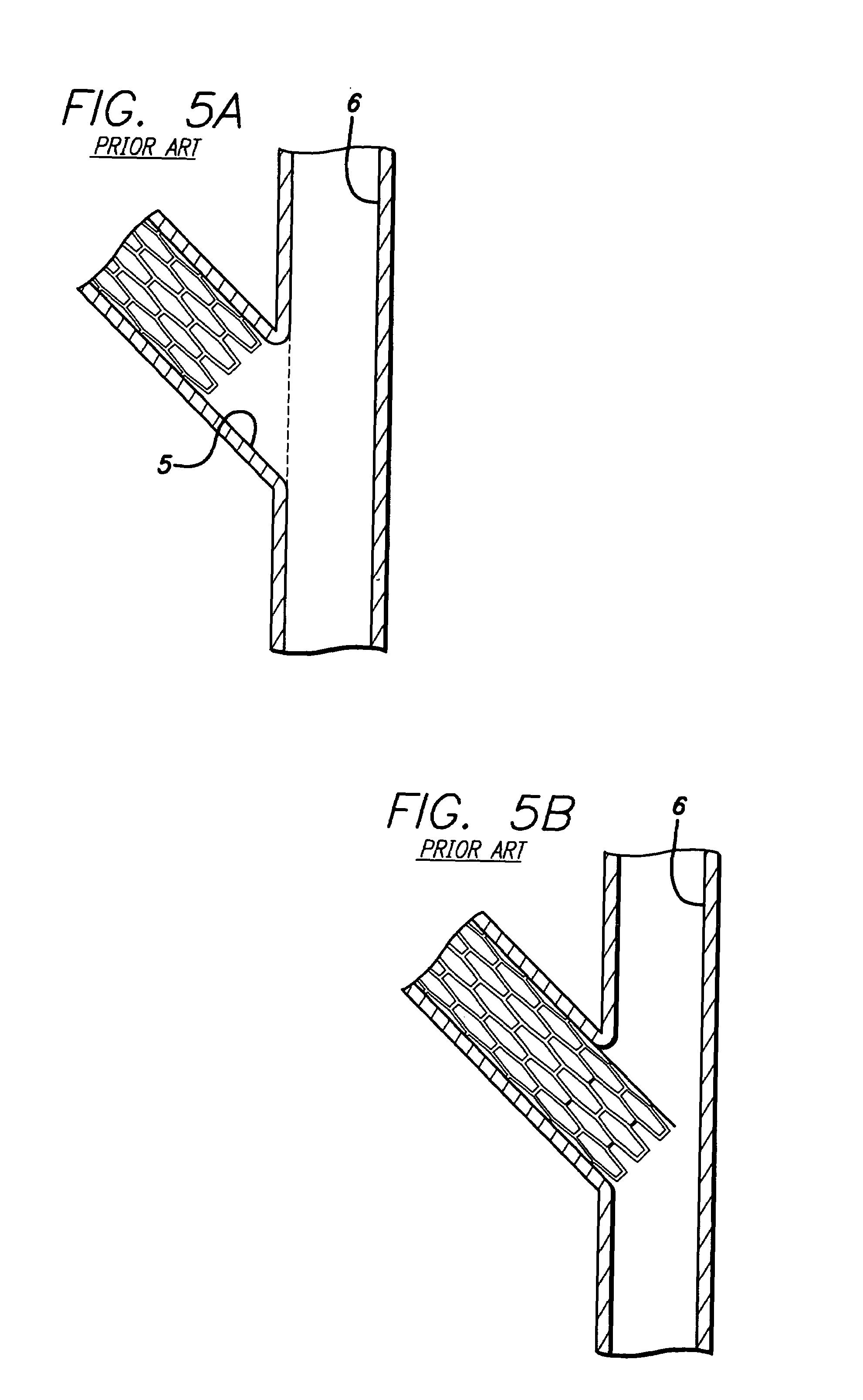
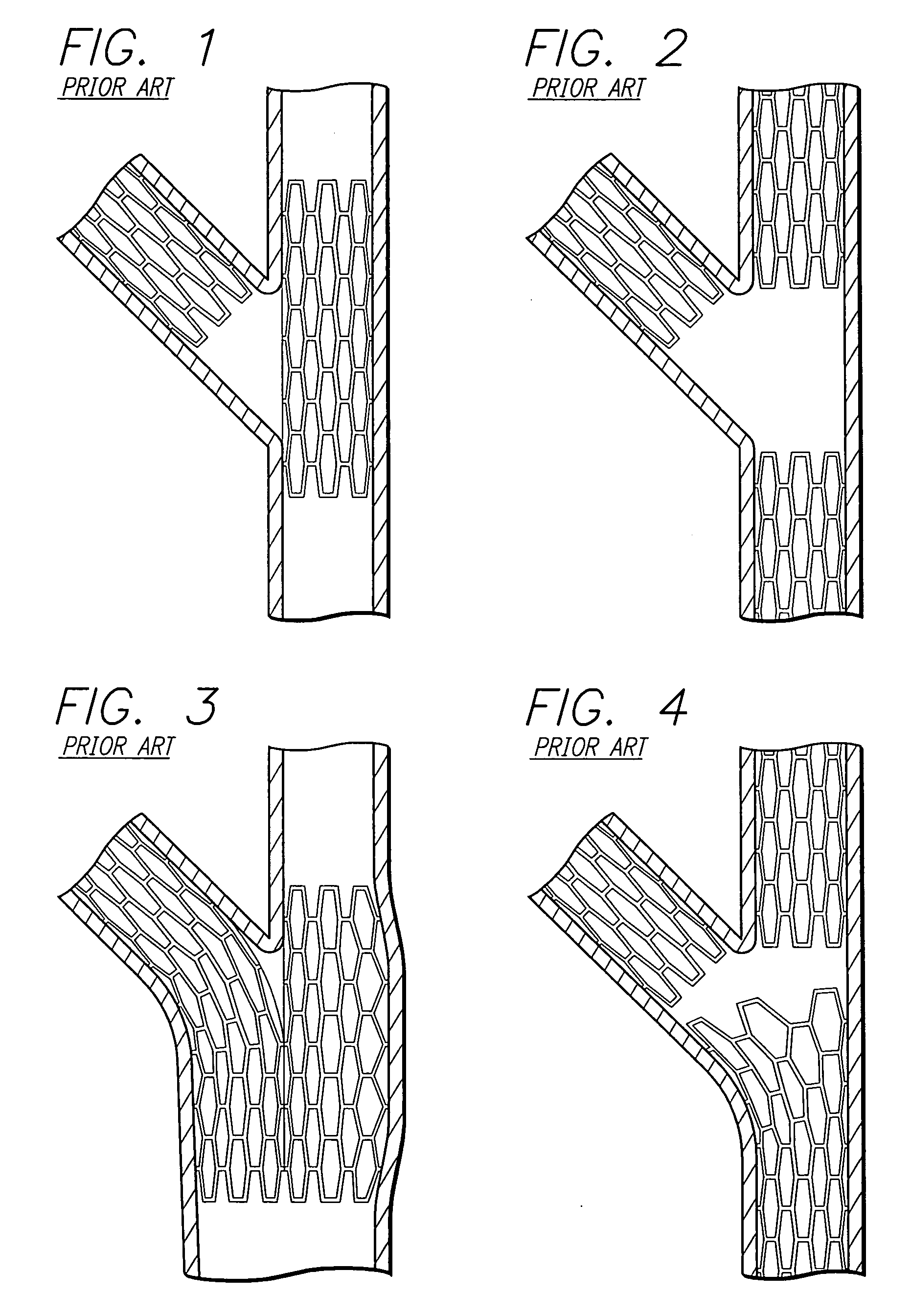
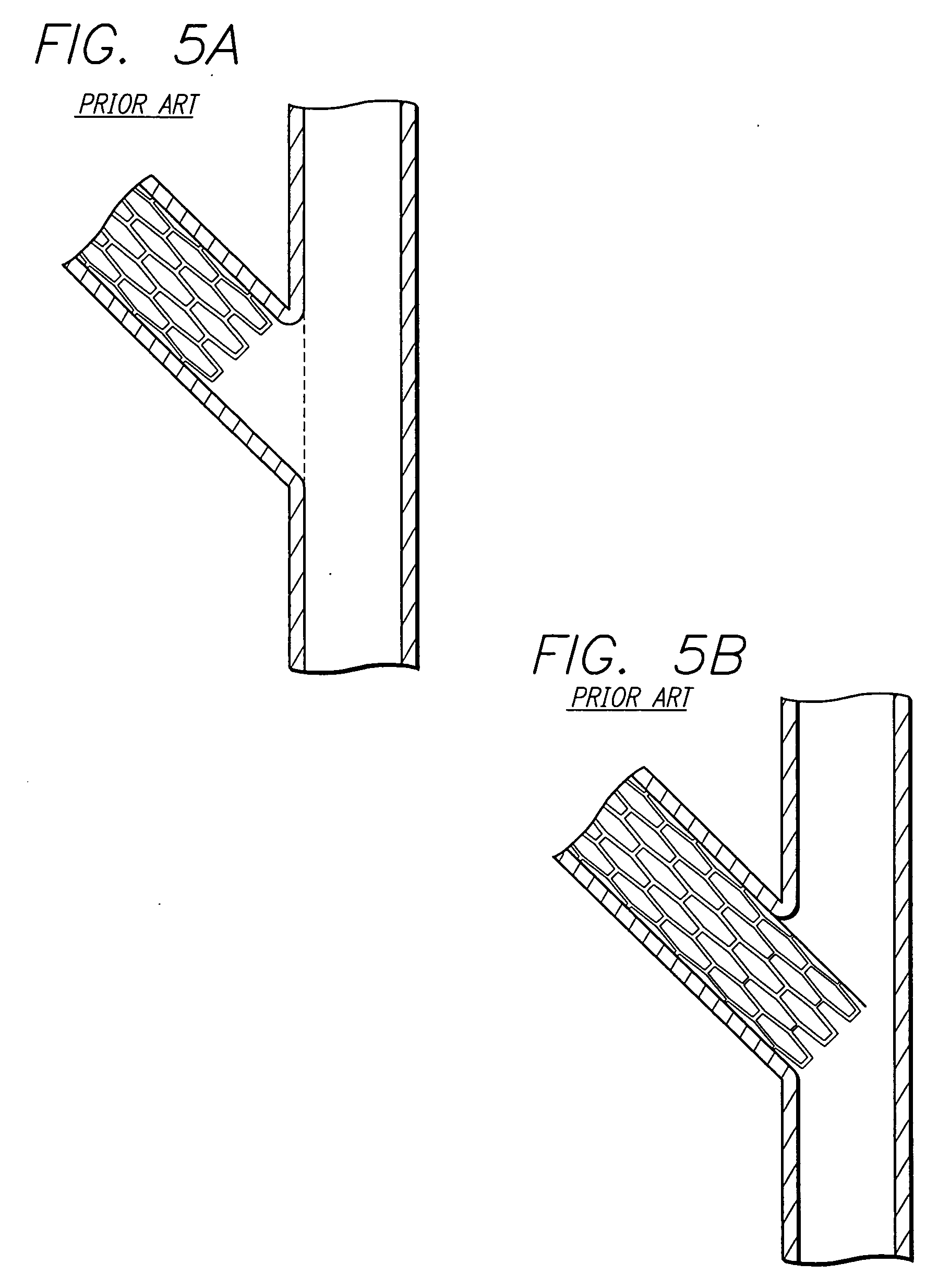
A stent pattern includes an improved portal region for repairing a main vessel and a side branch vessel forming a bifurcation. More particularly, the stent has rings aligned along a common longitudinal axis that are connected by links, where the stent has a proximal section, a distal section, and a central section (portal region). The number of rings and the expanded diameter of the sections are varied to create a “trap door” capable of expanding to a slightly larger diameter than the proximal section and the distal section of the stent. The configuration of the stent pattern of the portal region prevents the occurrence of portal overlap of immediately adjacent rings into the portal region during stent deployment. The stent is implanted at a bifurcation so that the proximal section and the distal section are in the main vessel, and the central section contacts at least a portion of the opening to the side branch vessel. A second stent can be implanted in the side branch vessel and abut the expanded central section to provide full coverage of the bifurcated area in the main vessel and the side branch vessel.
Owner:ABBOTT CARDIOVASCULAR
Stent delivery system with diameter adaptive restraint
InactiveUS20050209670A1Improve space efficiencyWide applicabilityStentsBlood vesselsBody organsInsertion stent
Medical device and methods for delivery or implantation of prostheses within hollow body organs and vessels or other luminal anatomy are disclosed. The subject technologies may be used in the treatment of atherosclerosis in stenting procedures. For such purposes, a self-expanding stent may be deployed in connection with an angioplasty procedure with a stent delivery system having a diameter adaptive restraint. Upon withdrawal of the restraint, the stent is freed, while the restraint or connections thereto assumes a reduced diameter within a tubular body of the delivery guide.
Owner:BIOSENSORS INT GROUP
Stent with drug-delivery system
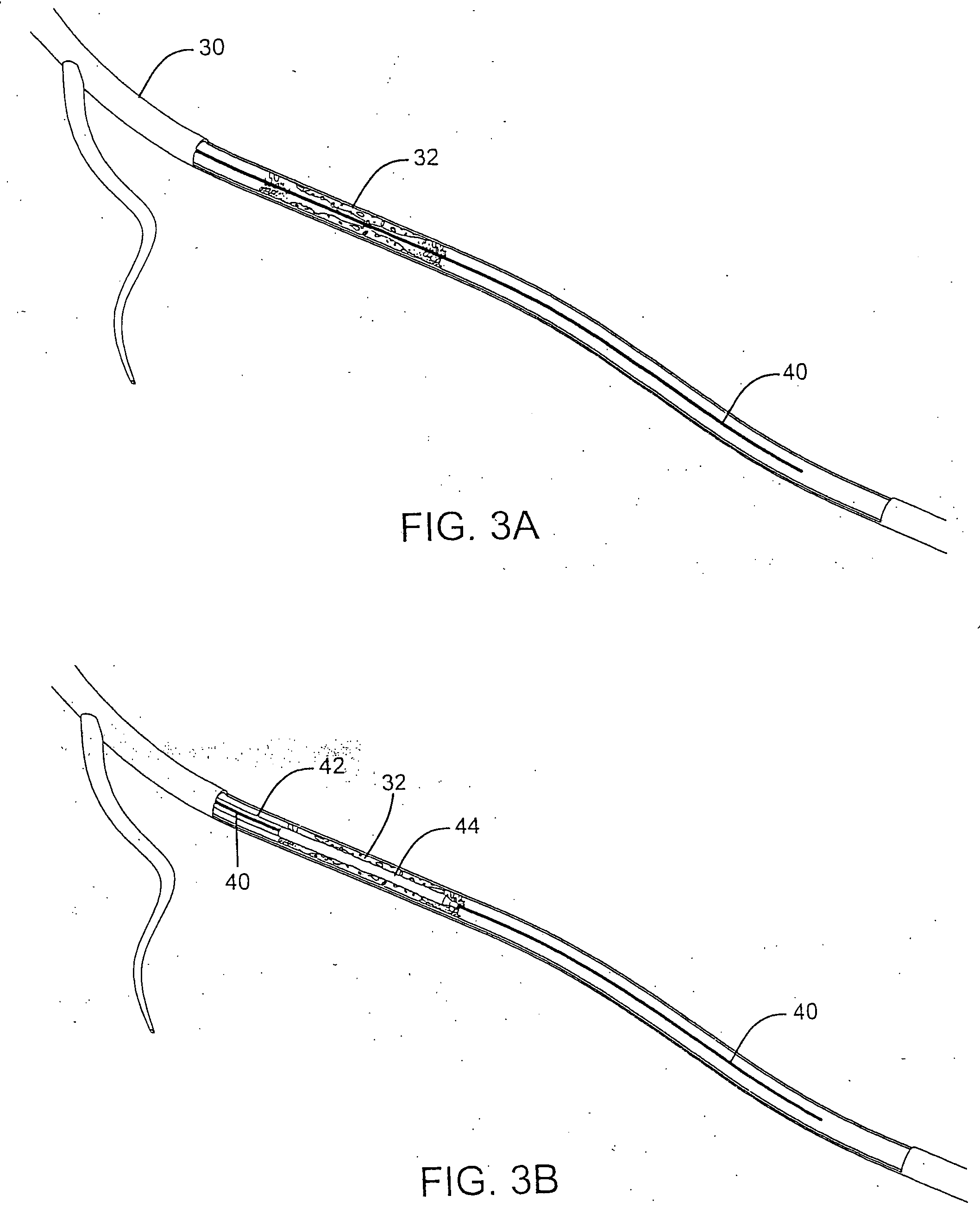
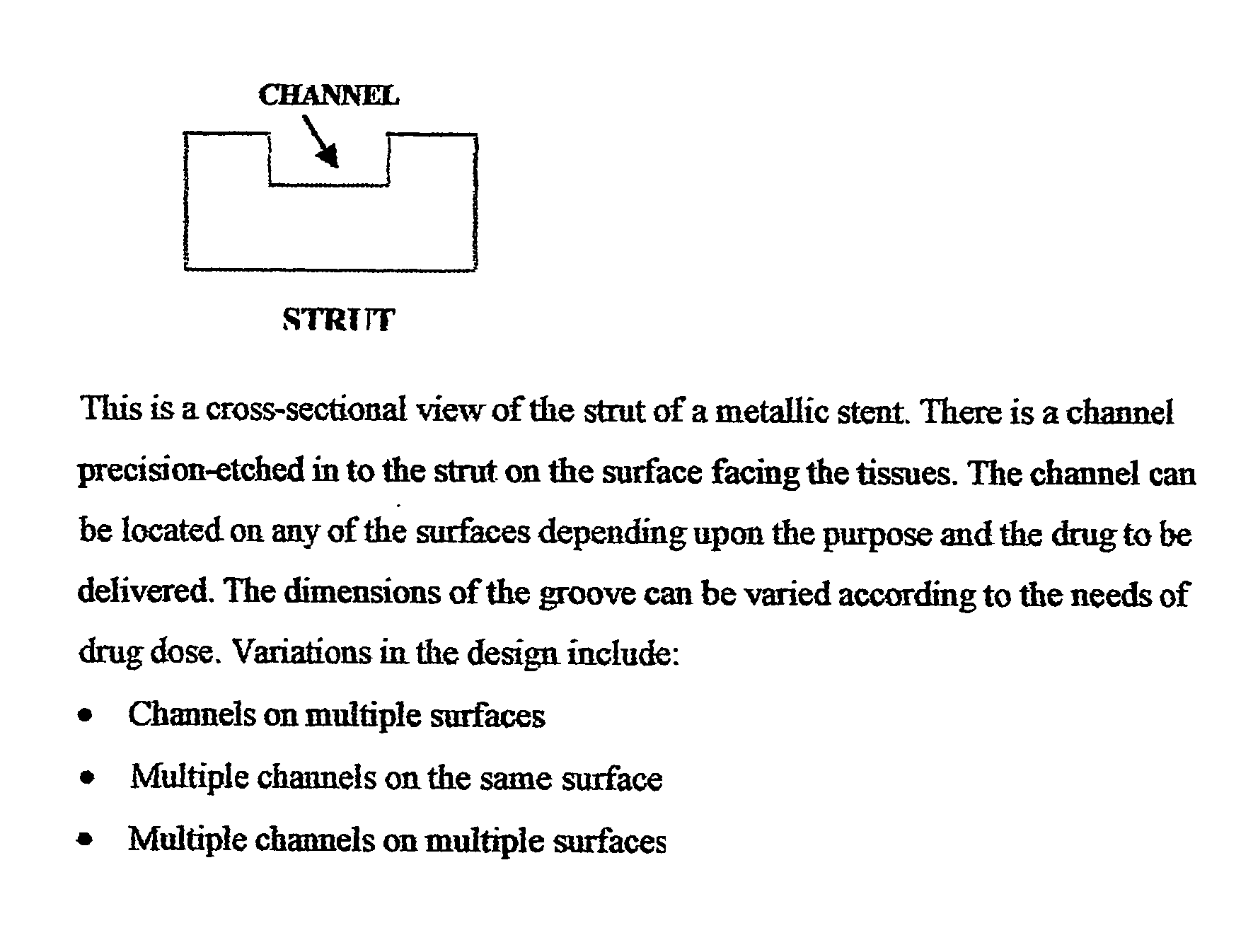
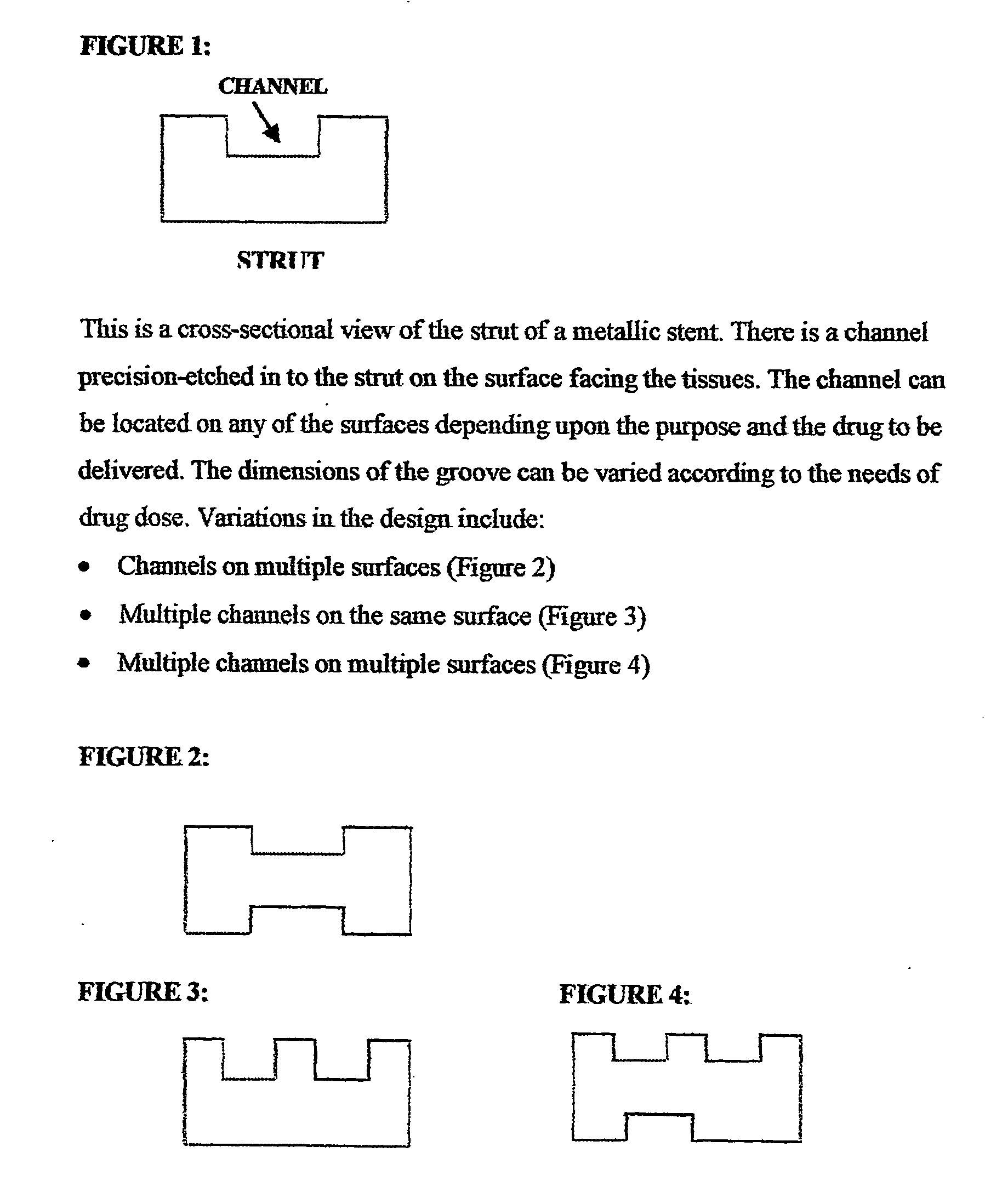
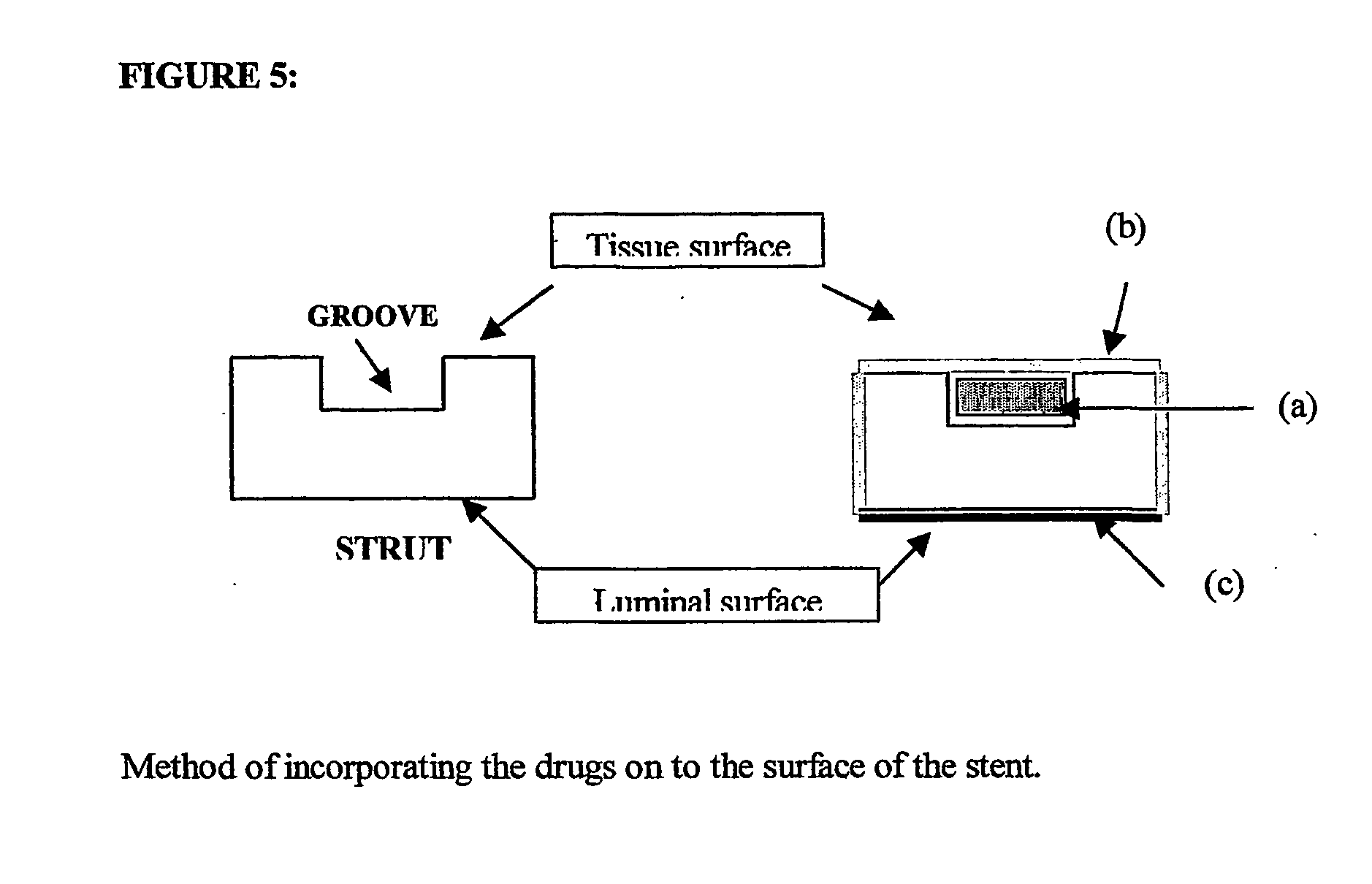
A unique design of the stent that incorporates a drug-reservoir running along the stent struts. One or more drugs can be incorporated within the reservoir. Additionally other drugs can be coated on the surface of the strut enclosing the reservoir drug to facilitate sequential release of drugs. Thus, the design incorporating sequential release of multiple drugs facilitates to tackle the sequential complex biologic processes involved in renarrowing following stent implantation. The design also ensures that the drug present in the reservoir is released exclusively into the adjacent tissue without getting washed away into the blood flowing within the lumen.
Owner:BADARI NAYANAN NAGARADA GADDE +1
Focalized stent implantation
Disclosed is a focal balloon having at least one reference zone and a focal zone. In one embodiment, the reference zone and focal zone are inflatable to a first generally cylindrical profile at a first pressure. At a second, greater pressure, the focal section expands to a second, greater diameter, while the reference zone remains substantially at the first diameter. In an alternate embodiment, the focal zone and the reference zone are inflatable to their respective predetermined diameters at the inflation pressure, in the absence of constricting lesions or anatomical structures. Multiple lobed and drug delivery embodiments are also disclosed.
Owner:RADIANCE MEDICAL SYST
Cryogenically enhanced intravascular interventions
InactiveUS20030109912A1Reduce stenosisPrevent restenosisCatheterSurgical instruments for coolingPercent Diameter StenosisPercutaneous angioplasty
Techniques and devices for treating atherosclerotic disease use controlled cryogenic cooling, often in combination with angioplasty and / or stenting. A combination cryogenic / angioplasty catheter may cool the diseased blood vessel before, during, and / or after dilation. Controlled cooling of the vessel wall reduces actual / observed hyperplasia as compared to conventional uncooled angioplasty. Similar reductions in restenosis may be provided for other primary treatments of the blood vessel, including directional arthrectomy, rotational arthrectomy, laser angioplasty, stenting, and the like. Cooling of vessel wall tissues will often be performed through plaque, and the cooling process will preferably take the thermodynamic effects of the plaque into account.
Owner:BOSTON SCI SCIMED INC
Nanoporous stents with enhanced cellular adhesion and reduced neointimal formation
The present invention relates generally to medical devices containing nanoporous surfaces and methods for making same. More specifically, the invention relates to implantable vascular stents or other biomedical devices having at least one nanoporous layer that promotes improved cellular adhesion properties that promote healing and long term biocompatibility. In the case of stents, the nanoporous layer promotes re-endothelialization at sites of stent implantation vasculature, improves overall healing, and reduces inflammation and intimal disease progression. The nanoporous layer may be optionally loaded with one or more therapeutic agent to further improve the function of the implanted stent and further augment clinical efficacy.
Owner:UNIV OF VIRGINIA ALUMNI PATENTS FOUND
Catheter assembly and method for treating bifurcations
InactiveUS20080009933A1Improve delivery capabilitiesSave effortStentsBlood vesselsInsertion stentBlood vessel
An improved stent design and stent delivery catheter assembly for repairing a main vessel and a side branch vessel forming a bifurcation. The stent includes rings aligned along a common longitudinal axis and connected by links, where the stent has one or more portals for aligning with and partially expanding into the opening to the side branch vessel. The stent is implanted at a bifurcation so that the main stent section is in the main vessel, and the portal section covers at least a portion of the opening to the side branch vessel. A second stent can be implanted in the side branch vessel and abut the expanded central section to provide full coverage of the bifurcated area in the main vessel and the side branch vessel. Radiopaque markers on the stent and on the tip of the delivery catheter assist in aligning the portal section with the opening to the side branch vessel.
Owner:ABBOTT CARDIOVASCULAR
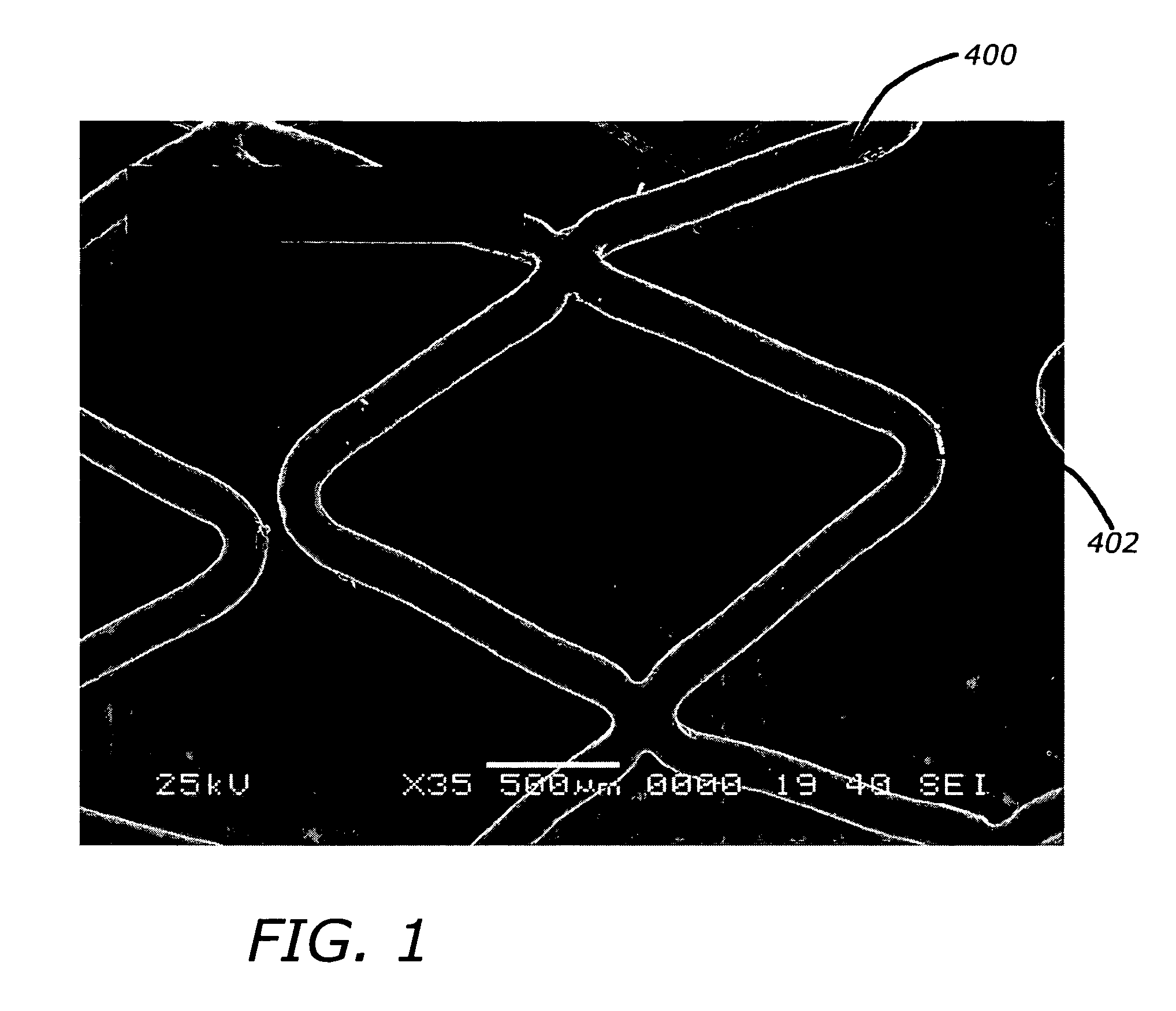
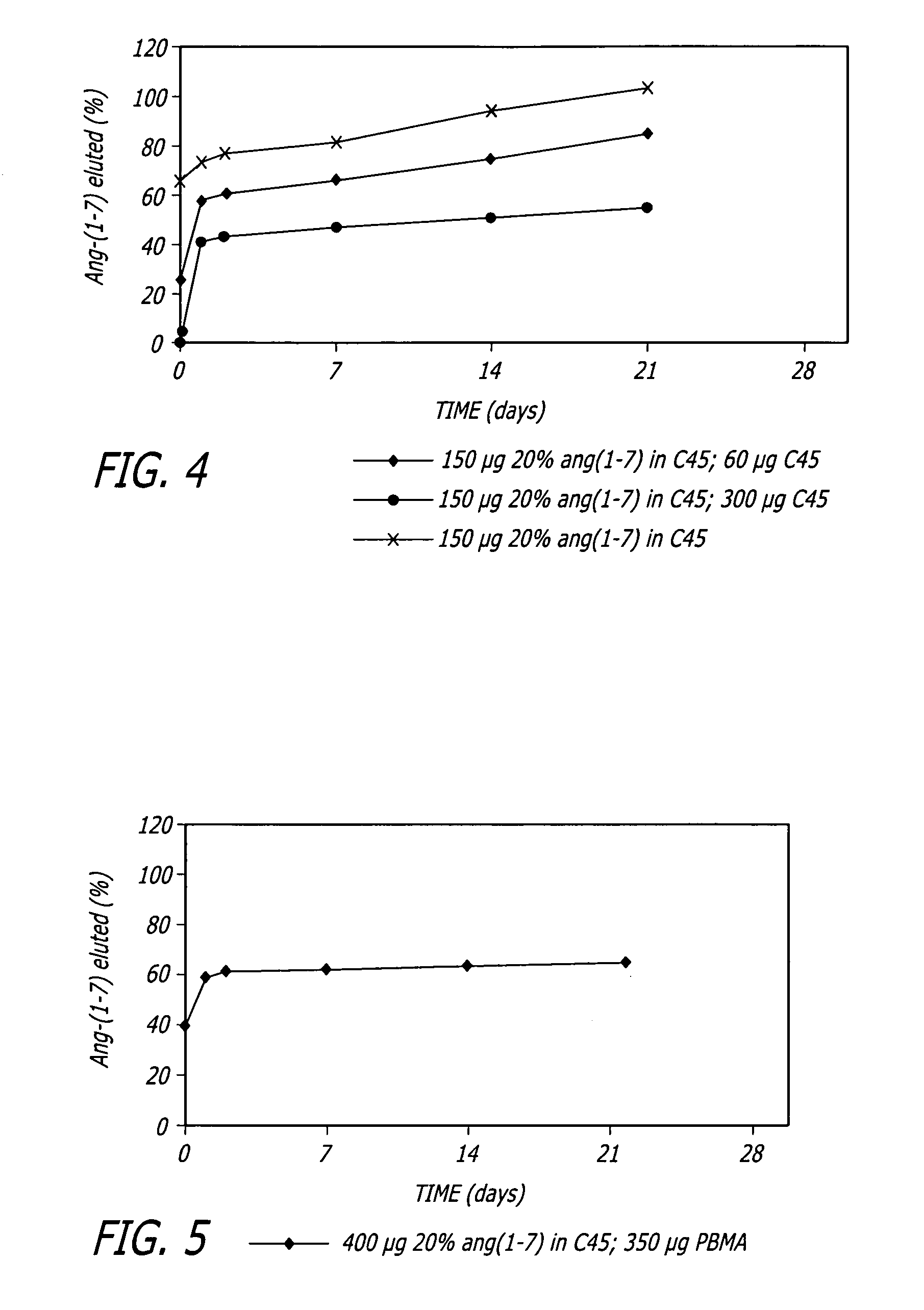
Angiotensin-(1-7) eluting polymer-coated medical device to reduce restenosis and improve endothelial cell function
InactiveUS7176261B2Function increasePrevent restenosisAngiotensinsPeptide/protein ingredientsVascular endotheliumPercent Diameter Stenosis
Medical devices with polymer coatings designed to control the release of angiotensin-(1-7) receptor agonists from medical devices are disclosed. The present application also discloses providing vascular stents with angiotensin-(1-7) receptor agonist-containing controlled-release coatings. Methods for treating or inhibiting post-stent implantation restenosis as well as improving vascular endothelial function in patients are also provided.
Owner:MEDTRONIC INC
Staged stent delivery systems
InactiveUS20070055339A1Reduce aggregate forceReduce frictional forceStentsBlood vesselsProsthesisBody organs
Medical devices and methods for delivery or implantation of prostheses within hollow body organs and vessels or other luminal anatomy are disclosed. The subject technologies may be used in the treatment of atherosclerosis in stenting procedures or a variety of other procedures. The various systems described employ self expanding stent restrained by tubular restraints. The systems are configured to reduce restraint actuation force relative to simple-sheath based stent delivery systems by actuation in a staged fashion.
Owner:BIOSENSORS INT GROUP
Endothelial scaffolds
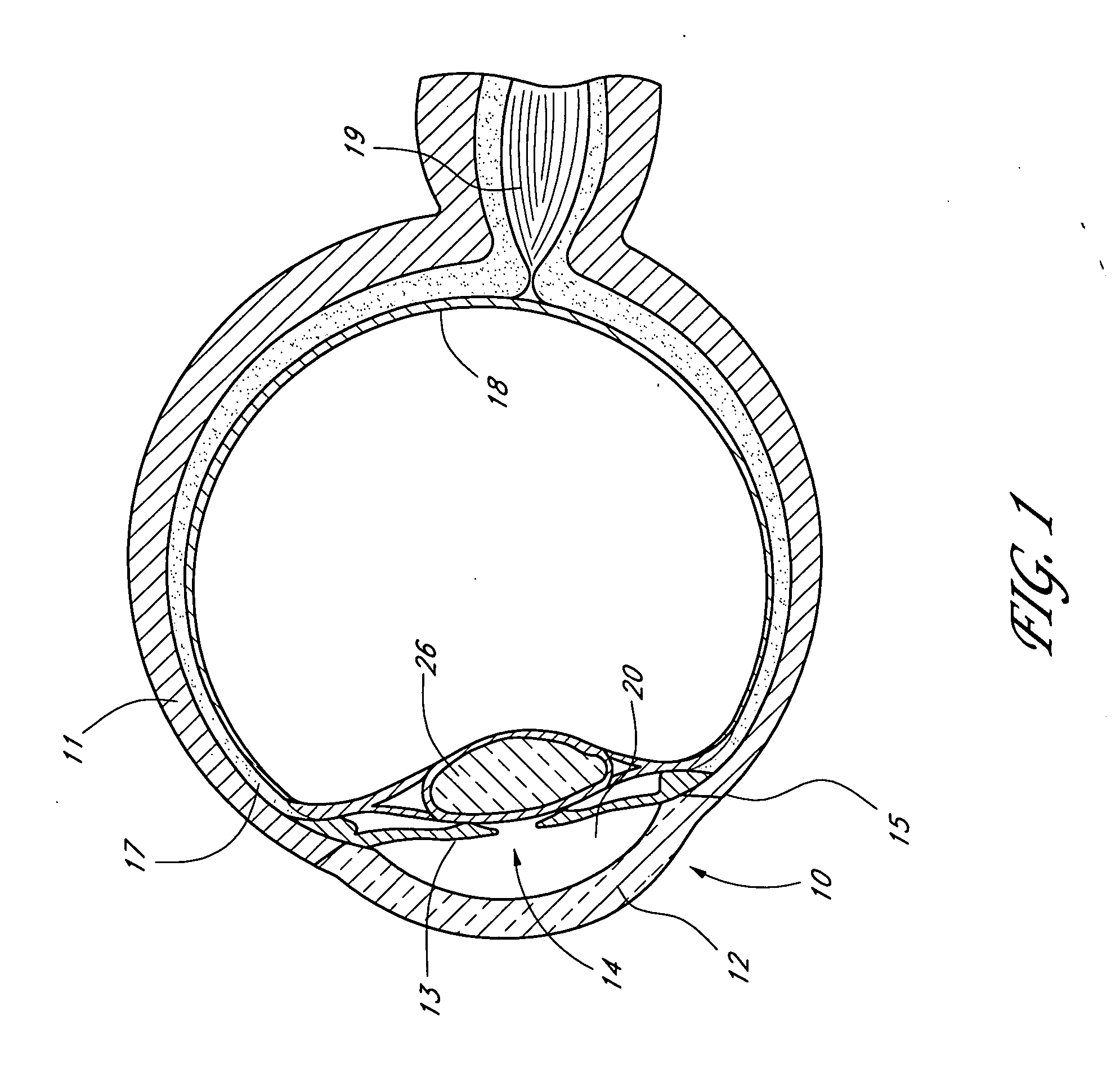
Provided herein is an endothelial scaffold comprising, consisting of, or consisting essentially of decellularized corneal stroma. In some embodiments, the scaffold has cultured endothelial cells seeded thereon. Methods of treating a patient in need of corneal endothelial transplant are also provided, including implanting the scaffold as described herein onto a cornea of the patient (e.g., by deep keratectomy).
Owner:WAKE FOREST UNIV HEALTH SCI INC
Bioresorbable stent
A bioresorbable endoluminal prosthesis for placement in a body lumen having a stent substrate of a first metallic material that has a lower electrical potential than a standard reference electrode. The stent substrate is coated with a biodegradable polymer having a second metallic material dispersed therein, wherein the second metallic material has a higher electrical potential than the standard reference electrode. After implantation of the stent within the body lumen, the second metallic material is present in the polymeric coating in a sufficient concentration to cause galvanic corrosion of the first metallic material such that over time the stent substrate is bioresorbed.
Owner:MEDTRONIC VASCULAR INC
Implantable Stent with Degradable Portions
InactiveUS20070288084A1Increase usable surface areaQuickly degradableStentsBlood vesselsStent implantationBlood vessel
Disclosed herein are stents with degradable part and / or sections of the stent. The degradable portions of the disclosed stents can provide increased surface area for a period of time for bioactive material deposition or enhanced radiopacity at various locations along the length of the stent. The stents of the present invention also can stent portions of a vessel that are damaged by stent implantation but do not necessarily require long term stenting by providing degradable end sections on the stents of the present invention.
Owner:MEDLOGICS DEVICE CORP
Bioresorbable Stent
A bioresorbable endoluminal prosthesis for placement in a body lumen having a stent substrate of a first metallic material that has a lower electrical potential than a standard reference electrode. The stent substrate is coated with a biodegradable polymer having a second metallic material dispersed therein, wherein the second metallic material has a higher electrical potential than the standard reference electrode. After implantation of the stent within the body lumen, the second metallic material is present in the polymeric coating in a sufficient concentration to cause galvanic corrosion of the first metallic material such that over time the stent substrate is bioresorbed.
Owner:MEDTRONIC VASCULAR INC
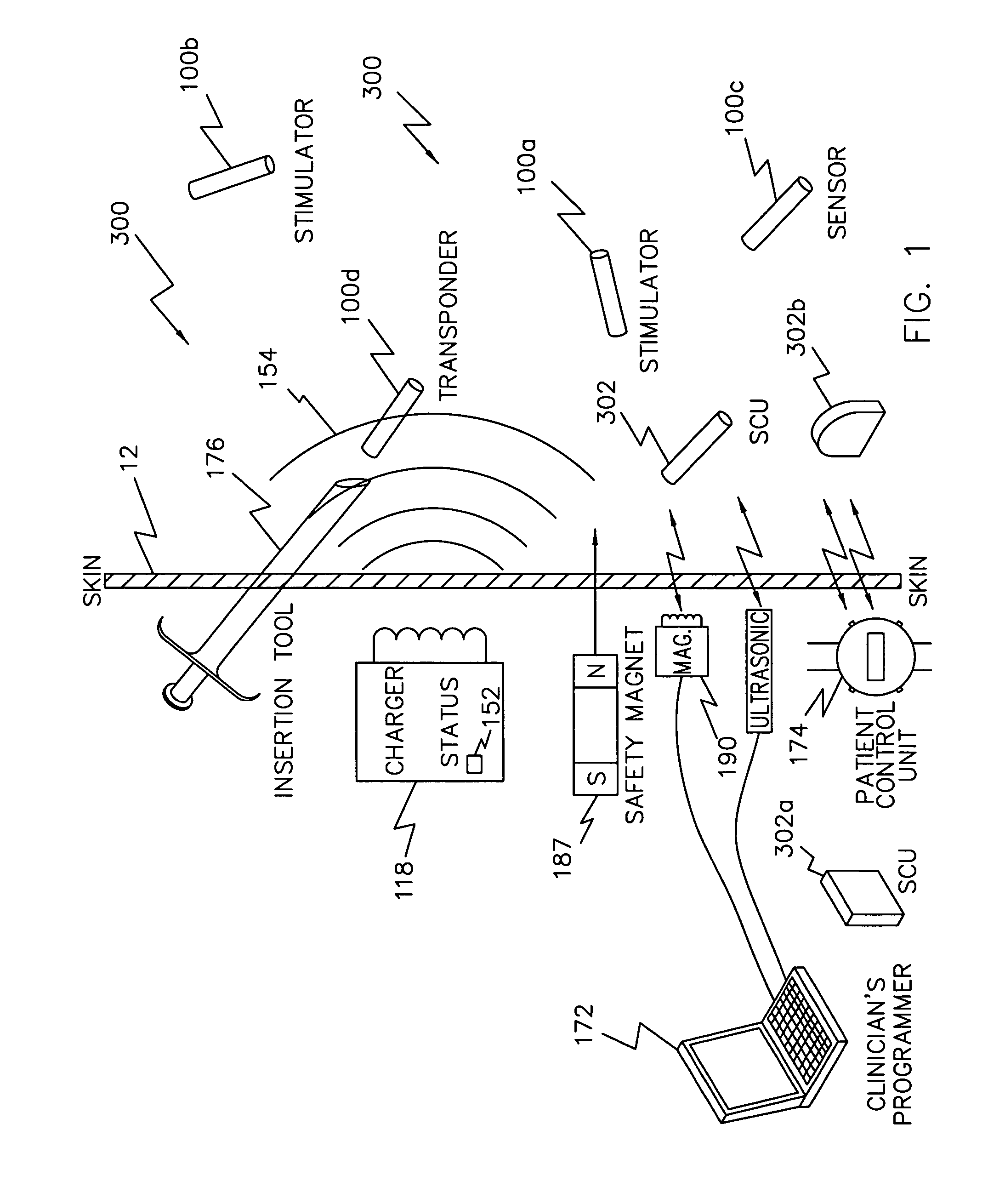
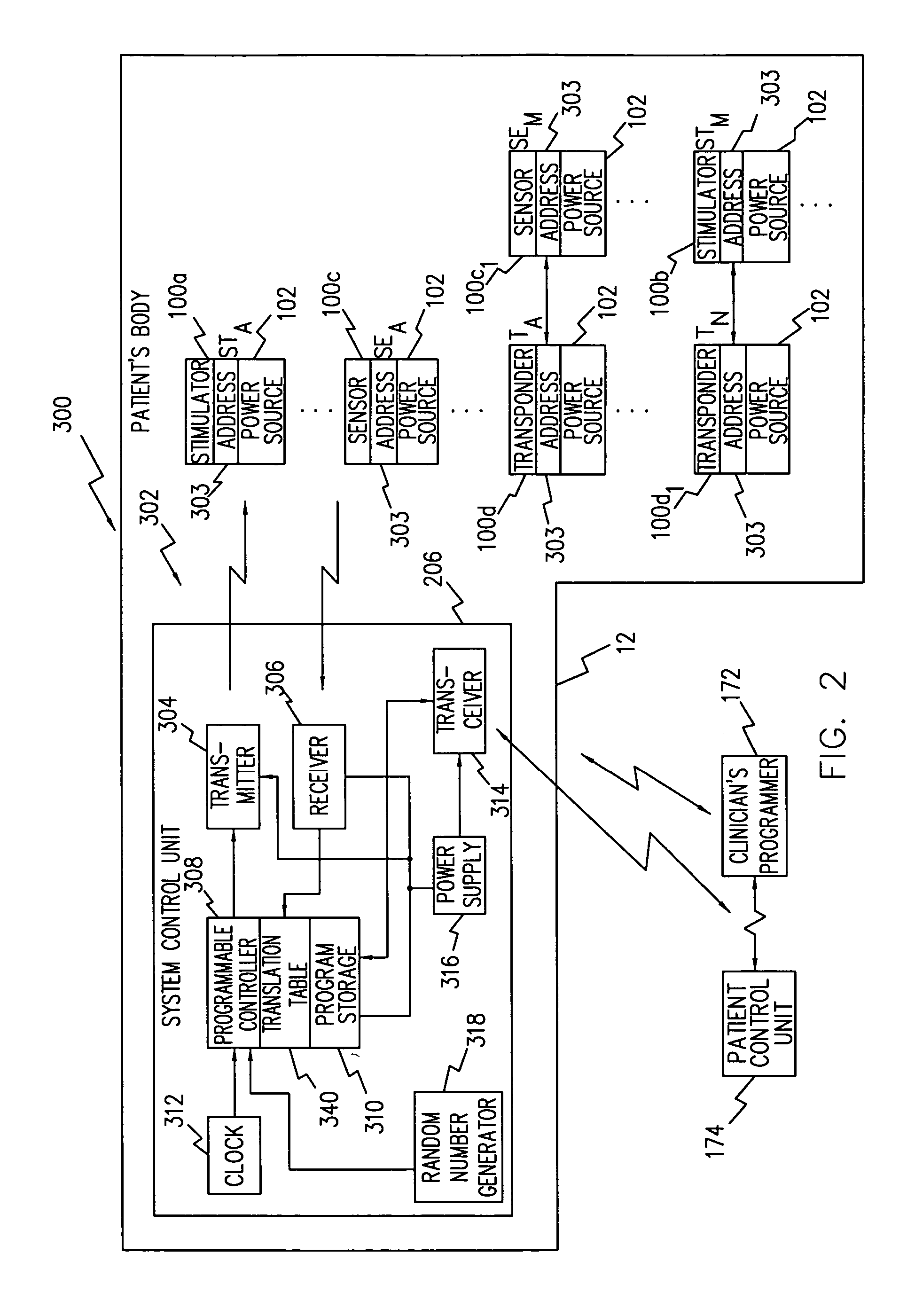
System of implantable ultrasonic emitters for preventing restenosis following a stent procedure
A system and method that minimizes plaque accumulation on a stent and thereby restenosis that could require a subsequent invasive medical procedure following implantation of the stent in a patient. A plurality of electrically-powered, biocompatible devices, implantable via injection, are positioned within the patient proximate to the stent and under control of a externally-placed controller are commanded to emit ultrasonic waves corresponding to the mechanical resonance of the stent. By controlling the frequency and the relative phases of the ultrasonic waves, the accumulation of plaque on the stent can thus be minimized.
Owner:ALFRED E MANN FOUND FOR SCI RES
Matrix Coated Stent
InactiveUS20110172763A1Promote endothelializationSimplifying device manufacture and loadingStentsSurgeryBiocompatibility TestingDisease progression
The present invention relates generally to a drug eluting stent containing metallic surfaces modified in microsphere metallic matrix structure and methods for making same. More specifically, the invention relates to an expandable and implantable vascular stent having at least one matrix layer that promotes improved cellular adhesion properties for healing promotion healing and long term biocompatibility. In the case of coronary stents, the metallic matrix layer promotes re-endothelialization at sites of stent implantation, improves overall healing, and reduces inflammation and intimal disease progression. The microsphere metallic matrix layer may be optionally loaded with one or more therapeutic agent to further improve the function of the implanted stent and further augment clinical efficacy and safety. The active compounds are selected primarily for their anti-proliferative, immunosuppressive, and anti-inflammatory activities, among other properties, which prevent, in part, smooth muscle cell proliferation and promote endothelial cell growth.
Owner:STERLING VASCULAR SYST
Left auricle plugging device and transport system
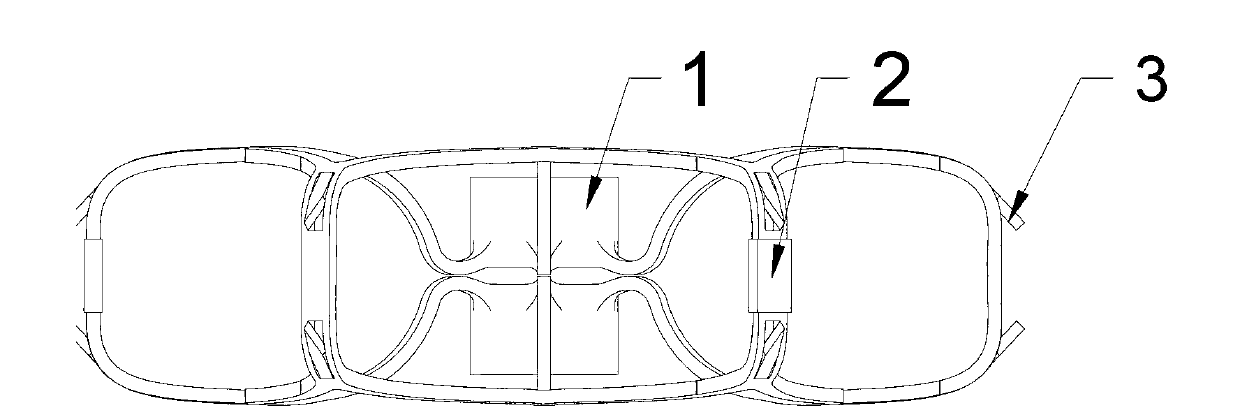
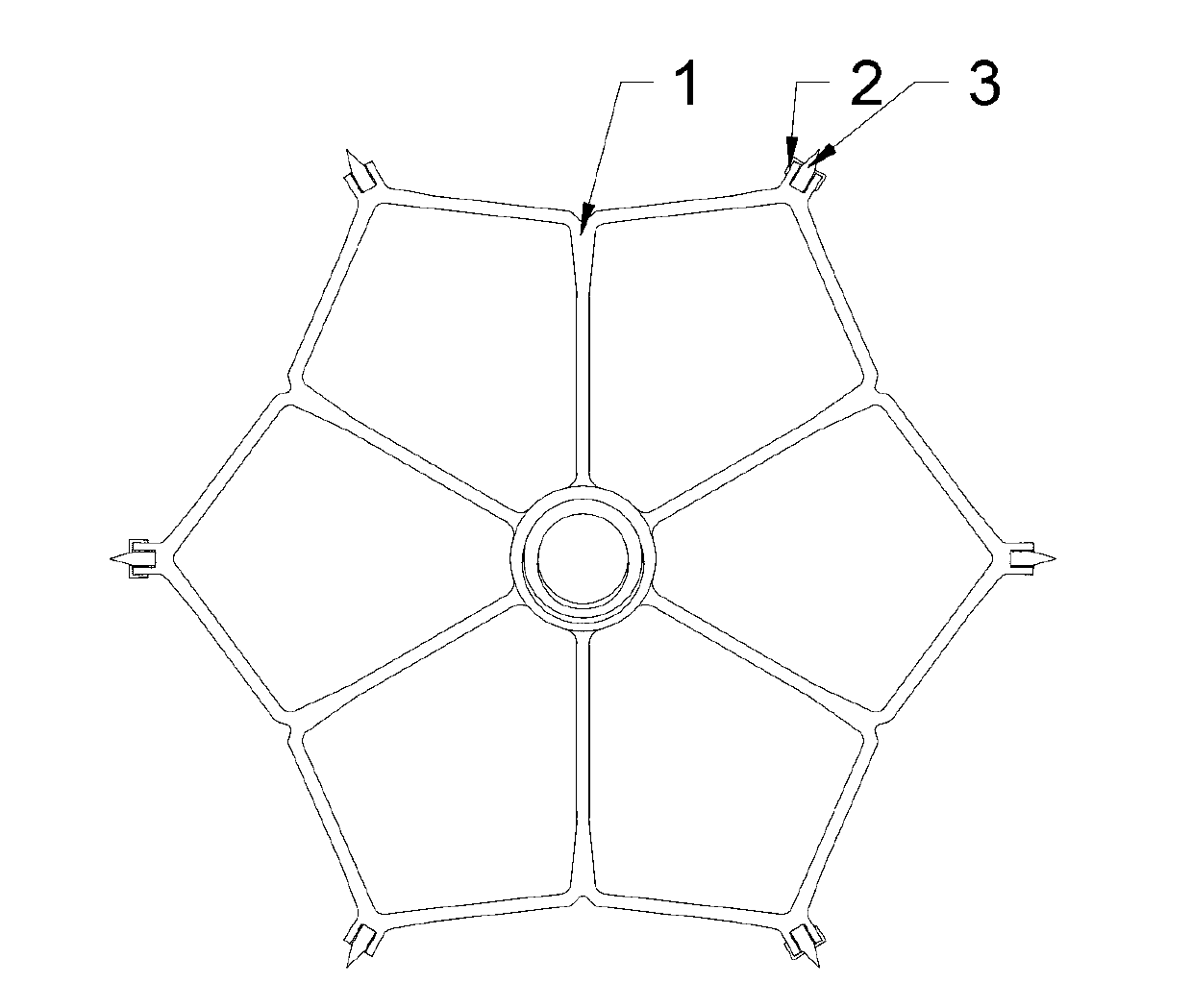
The invention discloses a left auricle plugging device and a transport system. The left auricle plugging device comprises a stent and a plugging device body. Firstly, the stent is implanted and fixed on the left auricle inlet position, the double-tray-shaped plugging device body is released on the stent for plugging a left auricle, and therefore the problem of cerebral apoplexy caused by left auricle thrombus because of atrial fibrillation can be solved. The left auricle plugging device is provided with the self-expandable nitinol stent, the stent is firmly fixed on the left auricle inlet position by depending on anchoring thorns, and then the appropriate plugging device body is released on the stent. The transport system transports the left auricle plugging device to the left auricle position. Compared with the prior art, the stent is firstly fixed on the left auricle inlet position, then the double-tray-shaped plugging device body is released on the stent, and therefore release accuracy and implanting stability of the plugging device body are improved, and the plugging device body has selectivity. After release, the stent and the the plugging device body can be recovered to a catheter for relocation or replacement.
Owner:APT MEDICAL HUNAN INC
Stent systems
InactiveCN101605509ASolve operational problemsSolve assembly problemsStentsBlood vesselsBody organsStent implantation
Medical devices and methods for delivery or implantation of prostheses within hollow body organs and vessels or other luminal anatomy are disclosed. The subject technologies can be used in the treatment of atherosclerosis in stenting procedures or be used in variety of other procedures. The systems can employ a self expanding stent restrained by one or more members released by an electrolytically erodable latch.
Owner:BIOSENSORS INT GROUP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com