Patents
Literature
128 results about "Endothelial cell growth" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
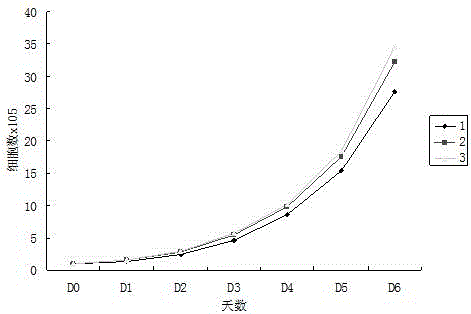
Endothelial Cell Growth Medium is a low-serum (2% V/V) medium optimized for the cultivation of endothelial cells from large blood vessels.
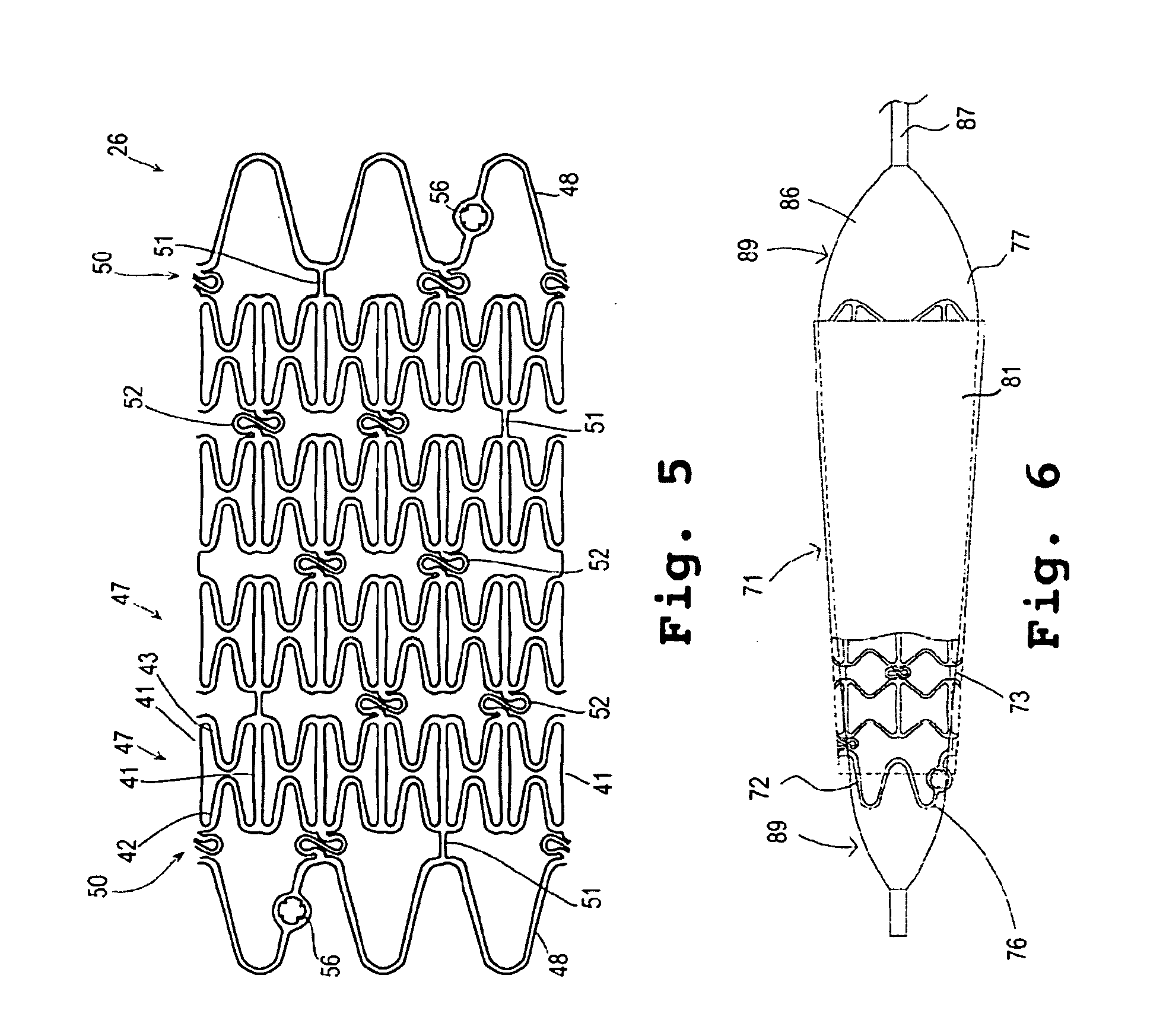
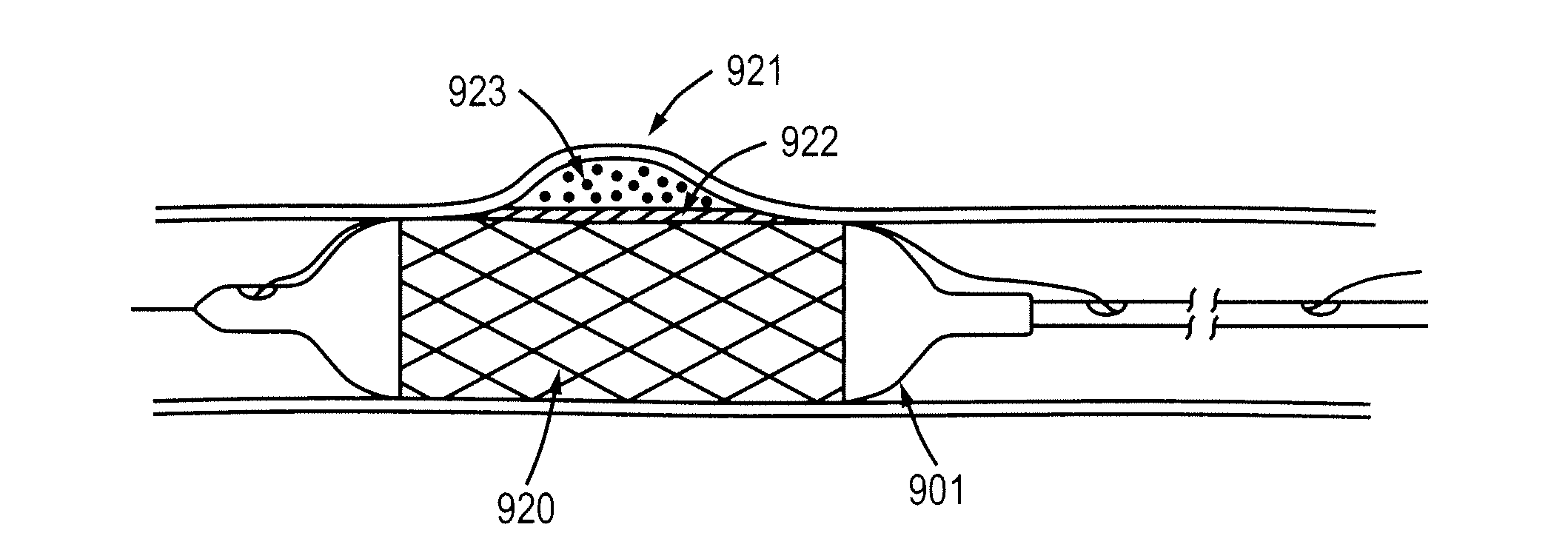
Encased stent
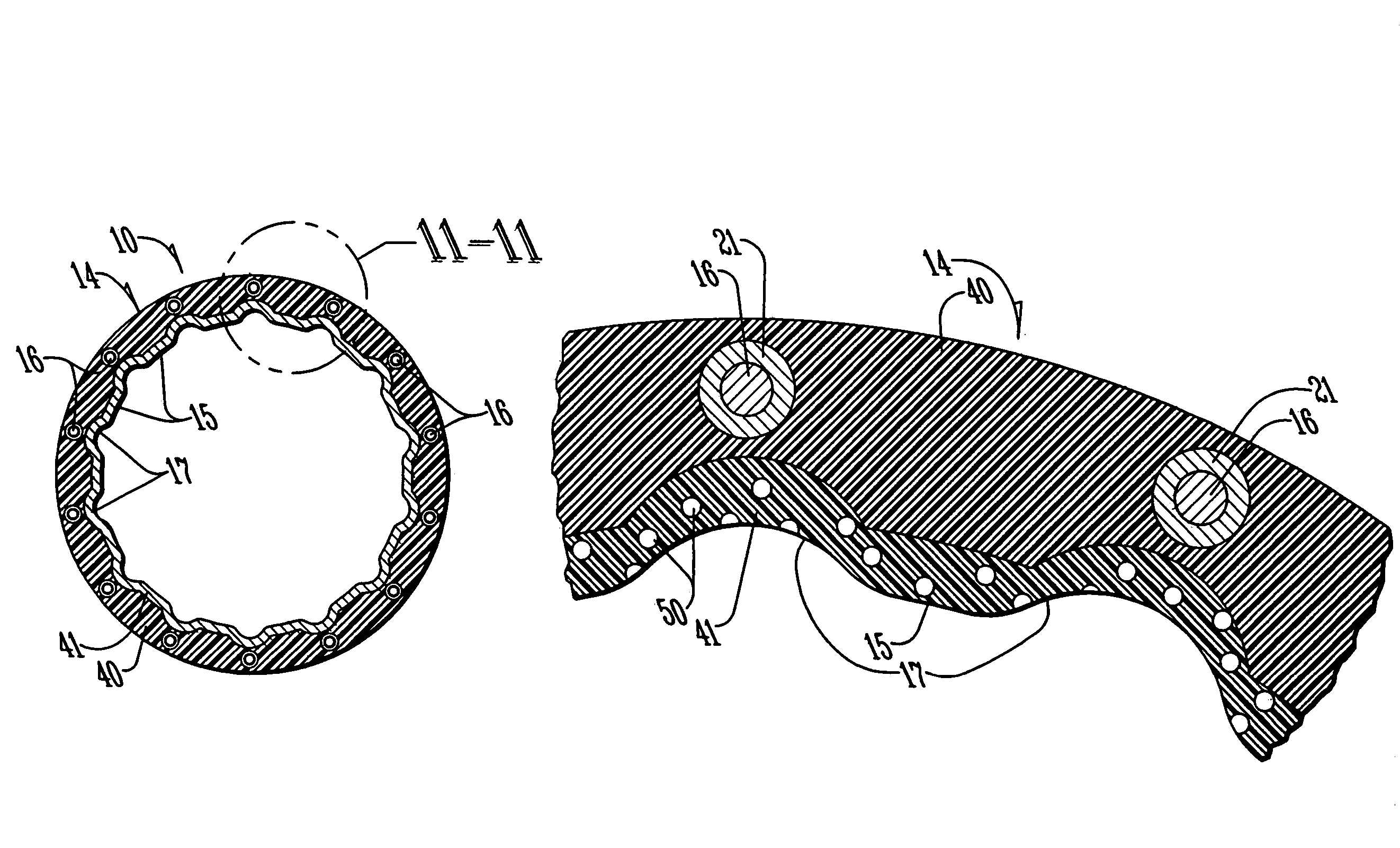
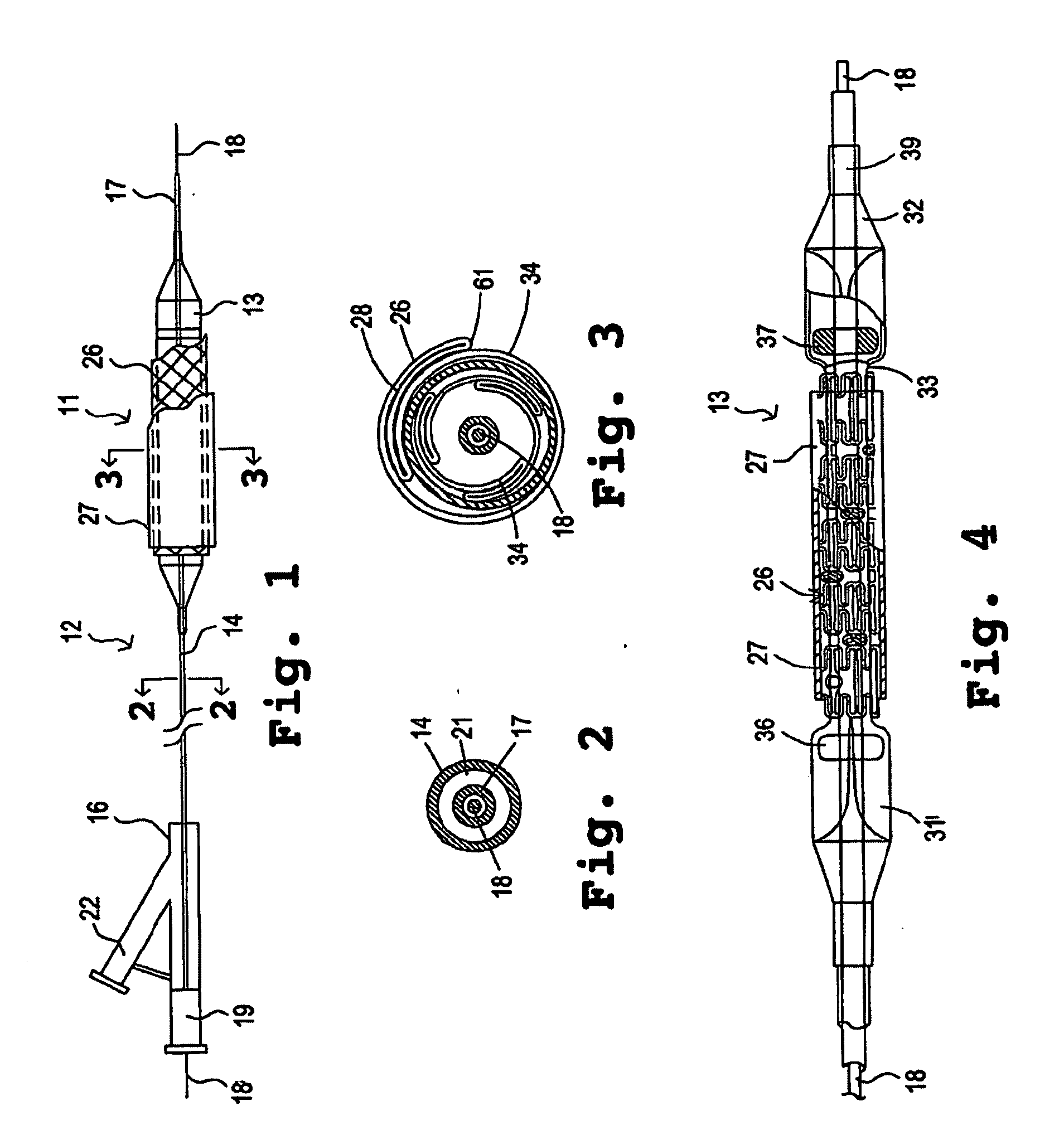
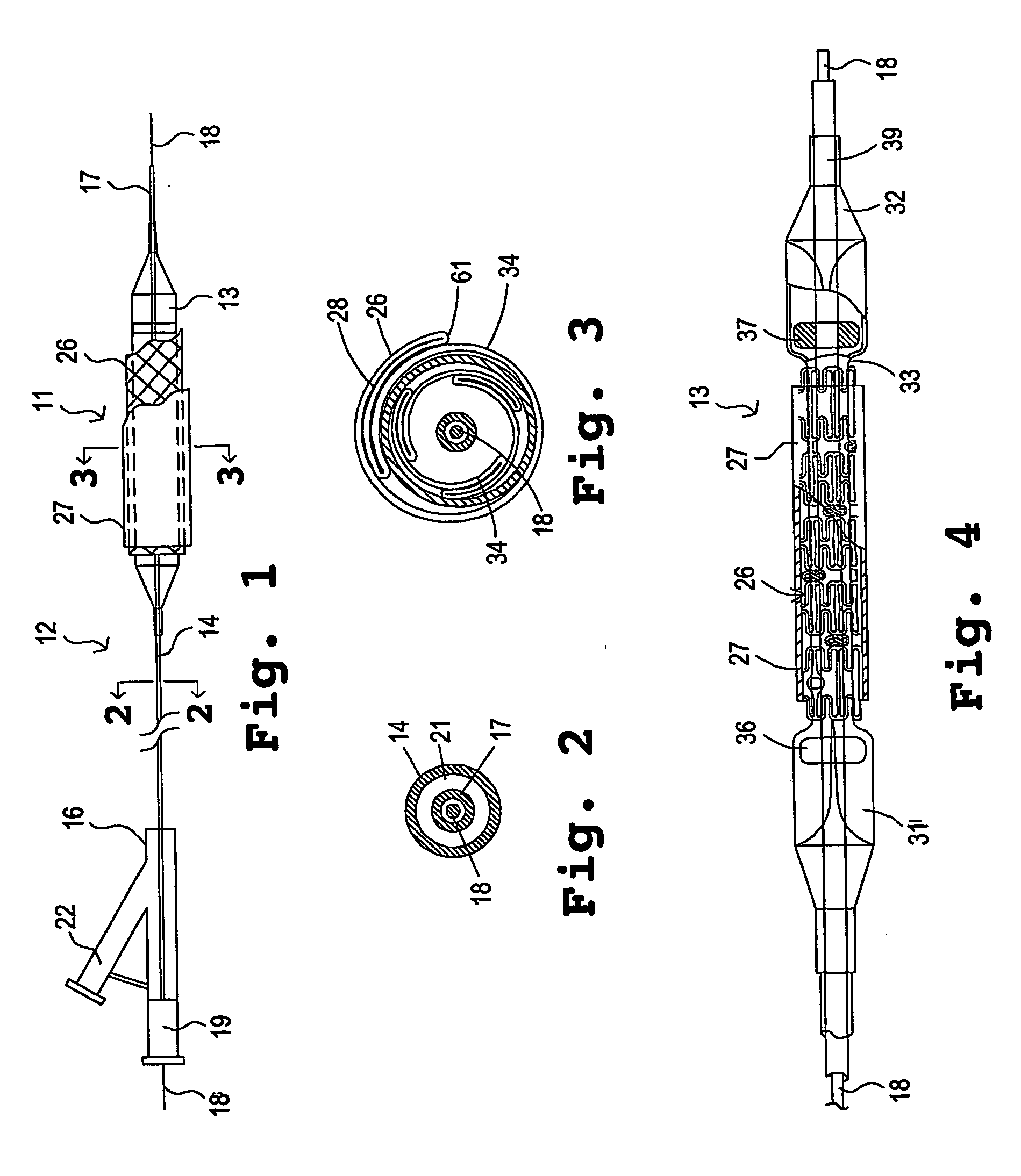
InactiveUS7311727B2Prevent restenosisImprove scalabilityStentsSurgeryCell Cycle InhibitionPolyethylene glycol
An encased stent that discourages restenosis by having a homogenous endothelial cell lining along the inner wall of the stent. The endothelial cell lining may be coated on the stent before the stent is placed in the artery, or the endothelial cell lining may be grown after placement by several factors that encourage such growth and discourage restenosis. The endothelial cells to coat the stent may be genetically modified to enhance the growth of the endothelial cells into a homogeneous lining. The stent has a continuous lining in the form of a multi-layer polymer coating, including a conducting biocorrosion inhibiting layer and a continuous film of polyurethane coupled by a coupling agent to polyethylene glycol. Various drugs and cell factors may be incorporated into the lining, such as anti-thrombin, anti-inflammatory and anti-coagulant drugs, cell cycle inhibitors, and vascular endothelial growth factors.
Owner:THE BOARD OF TRUSTEES OF THE UNIV OF ARKANSAS
Composite stent with polymeric covering and bioactive coating
InactiveUS20090062899A1Therapeutic solutionEnhancing endothelial cell growthStentsSurgeryMedicineEndothelial cell growth
A composite expandable stent for delivery into a vessel carrying blood comprising an expandable support frame having first and second end portions. A porous imprevious polymer sleeve having inner and outer surfaces extending over the support frame. A coating is disposed on at least one of the inner and outer surfaces of the polymer sleeve for enhancing endothelial cell growth on the device and polymer sleeve. The stent can be cylindrical or tapered.
Owner:NFOCUS NEUROMEDICAL
Peptide for high performance inhibition of angiogenesis and method for preparing same and use thereof
ActiveCN1699408AImprove and enhance growthImprove and enhance the anti-tumor effectPeptide/protein ingredientsSkeletal disorderEscherichia coliInclusion bodies
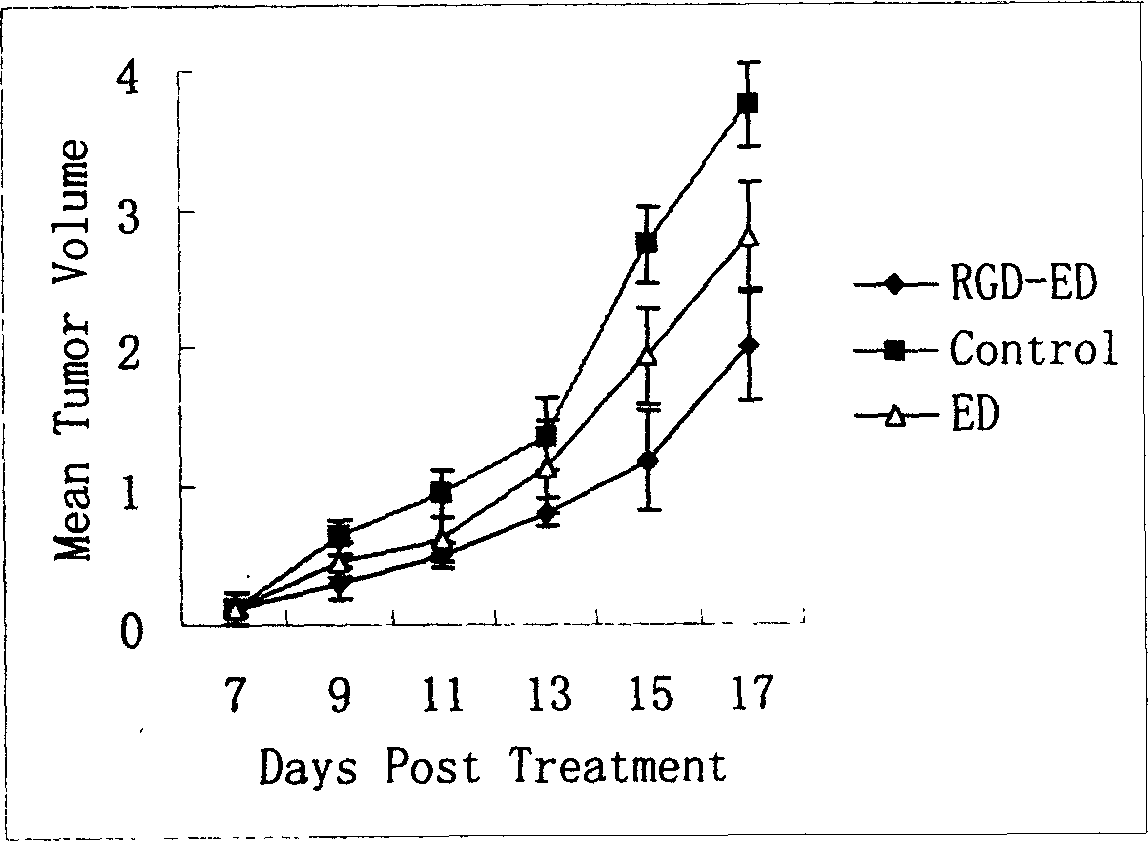
The invention relates to a peptide for high performance inhibition of angiogenesis and method for preparing same and use, wherein high performance blood vessel production inhibiting agent RGD-ED with integration compatibility is designed, the inhibiting agent comprises polypeptide polypeptide-valine-arginine-arginine-alanine-aspartate-arginine-alanine-alanine-valine-praline, its one or two ends are connected with polypeptides containing arginine-glycine-aspartic acid sequence. The RGD-ED provided by the invention can be synthesized. The invention also discloses the expression of one RGD-ED in bacillus coli through gene engineering method, wherein the RGD-ED is prepared through the steps of inclusion body protein segregation, dissolution and renaturation, and ion-exchange chromatography segregation and purification.
Owner:CHINA PHARM UNIV
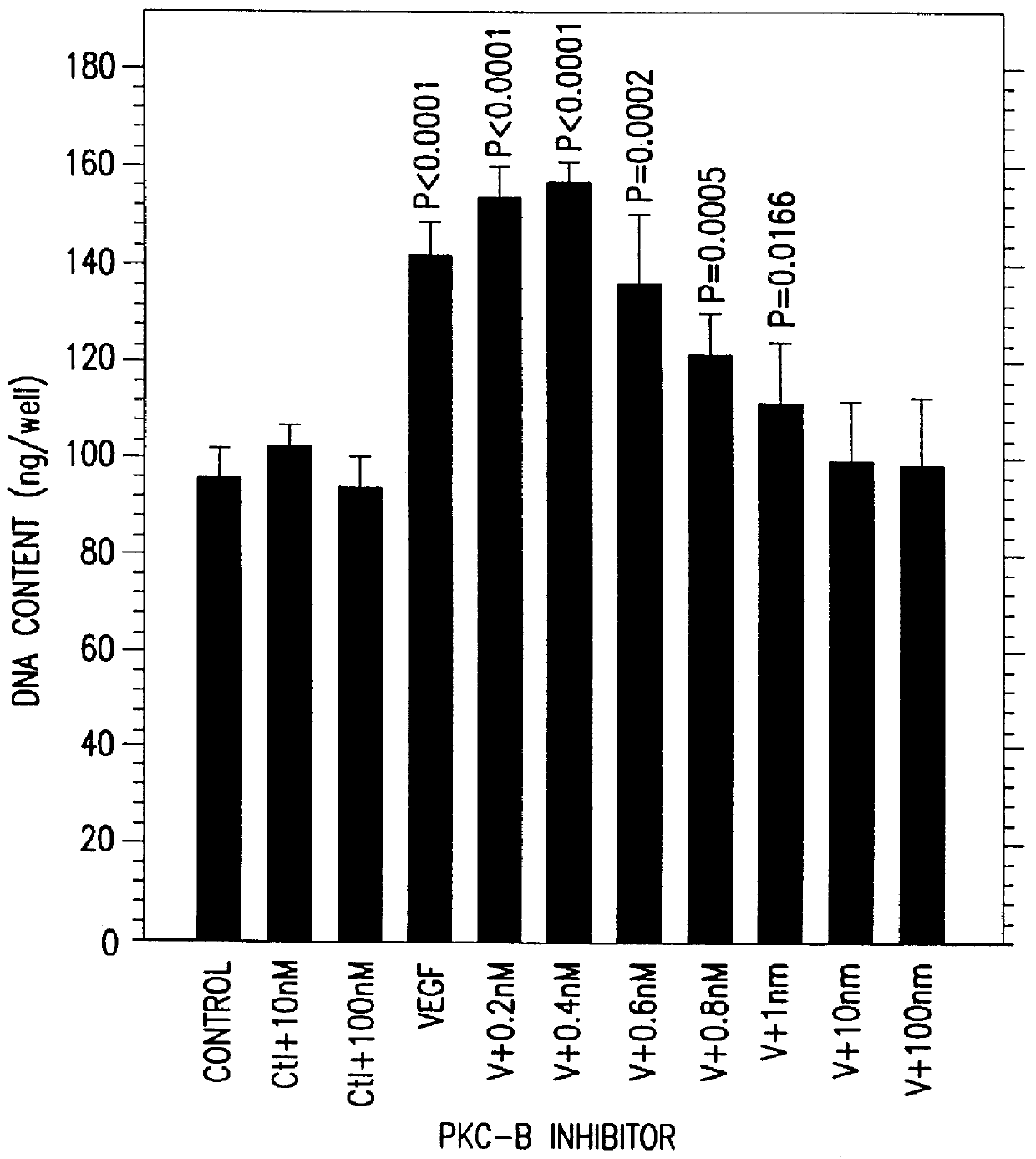
Therapeutic treatment for VEGF related diseases
InactiveUS6284751B1Promote growthPromote absorptionHeavy metal active ingredientsBiocideDiseaseCapillary hyperpermeability
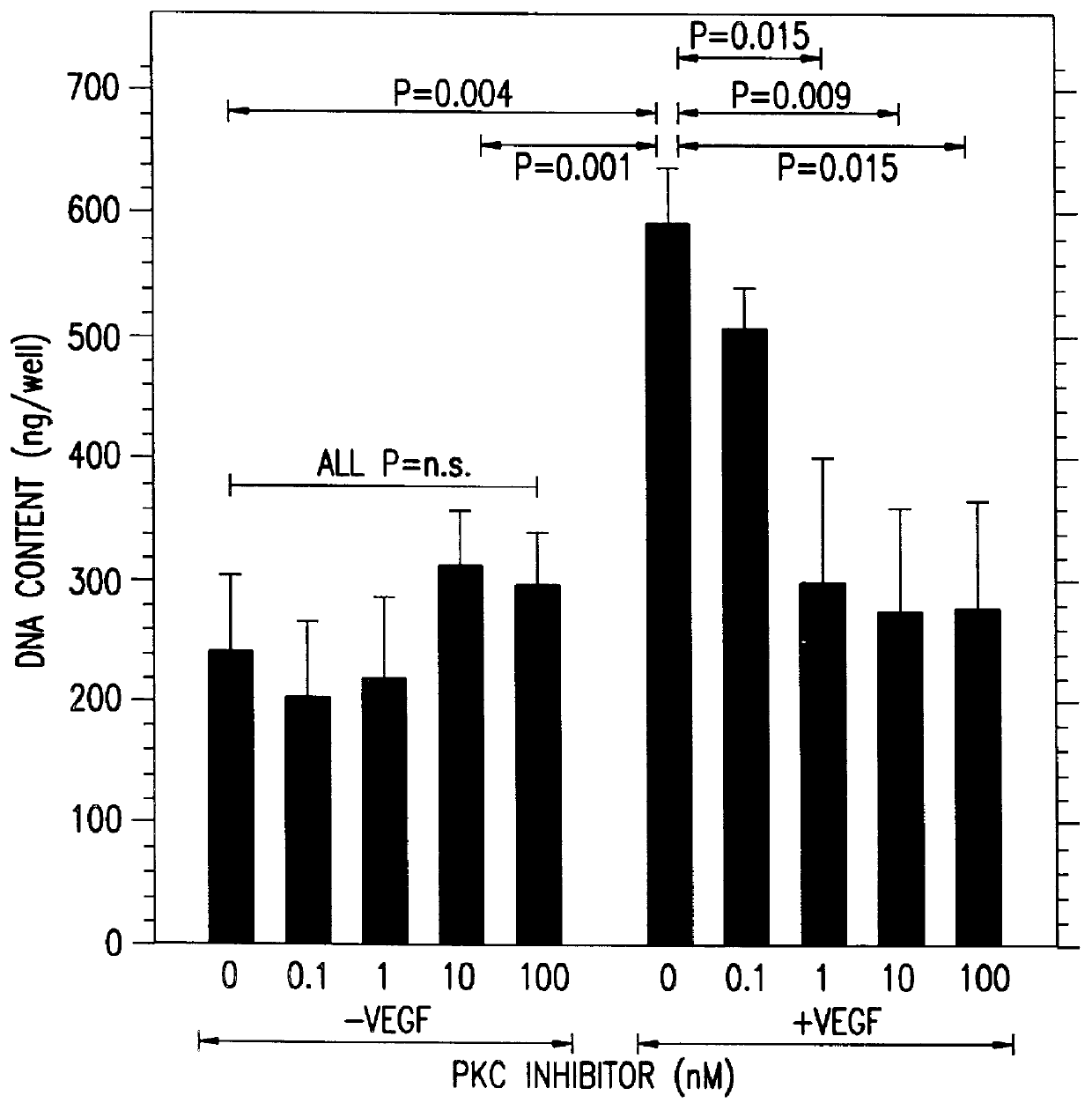
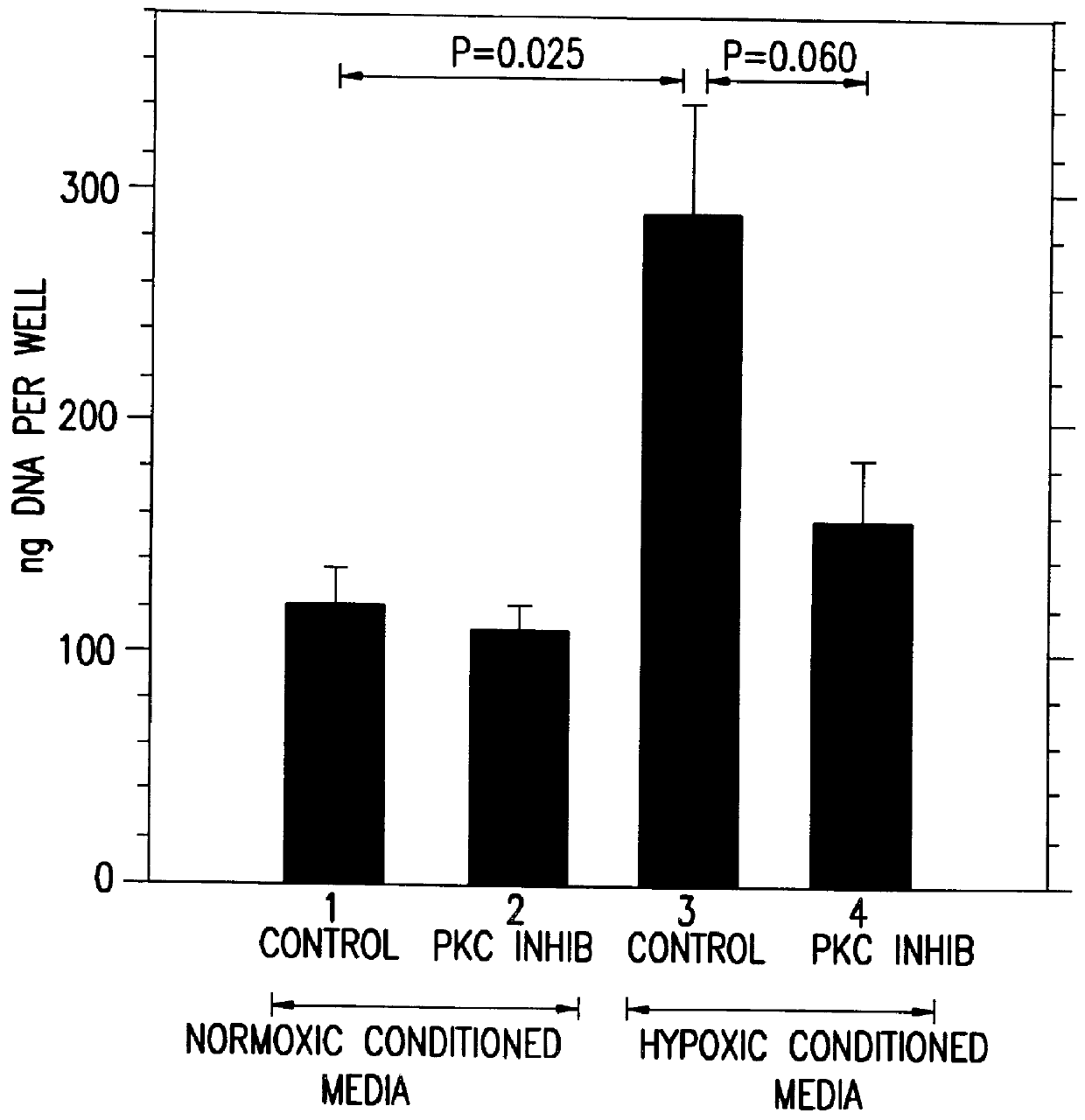
A method for inhibiting VEGF stimulated endothelial cell growth, such as associated with neoplasia, and VEGF stimulated capillary permeability, such as associated with pulmonary edema are disclosed, particularly using the beta-isozyme selective PKC inhibitor, (S)-3,4-[N,N'-1,1'-((2''-ethoxy)-3'''(O)-4'''-(N,N-dimethylamino)-butane)-bis-(3,3'-indolyl)]-1(H)-pyrrole-2,5-dionehydrochloridesalt.
Owner:ELI LILLY & CO
Biological compatibility surface coating of implantation type medical instruments and coating method thereof
The invention discloses a biological compatibility surface coater and self-assembling coating method of planted medical apparatus, which is characterized by the following: the coater is prepared by self-assembling coating method layer by layer, which accelerates esoderma and resists thrombus constituted by two or more macromolecular materials with certain positive and negative load; the positive load material is chitose, chitin, polylysine and so on; the negative load material is disebrin, hyaluronic acid, alginic acid, polyamino acid and so on; the crosslinker and biological active factor or drug can be added to accelerate the growth of the cell; the coater satisfies the request of biologically medical transplantation.
Owner:FUDAN UNIV +1
Matrix Coated Stent
InactiveUS20110172763A1Promote endothelializationSimplifying device manufacture and loadingStentsSurgeryBiocompatibility TestingDisease progression
The present invention relates generally to a drug eluting stent containing metallic surfaces modified in microsphere metallic matrix structure and methods for making same. More specifically, the invention relates to an expandable and implantable vascular stent having at least one matrix layer that promotes improved cellular adhesion properties for healing promotion healing and long term biocompatibility. In the case of coronary stents, the metallic matrix layer promotes re-endothelialization at sites of stent implantation, improves overall healing, and reduces inflammation and intimal disease progression. The microsphere metallic matrix layer may be optionally loaded with one or more therapeutic agent to further improve the function of the implanted stent and further augment clinical efficacy and safety. The active compounds are selected primarily for their anti-proliferative, immunosuppressive, and anti-inflammatory activities, among other properties, which prevent, in part, smooth muscle cell proliferation and promote endothelial cell growth.
Owner:STERLING VASCULAR SYST
Composite stent with polymeric covering and bioactive coating
InactiveUS20060034883A1Therapeutic solutionEnhancing endothelial cell growthStentsSurgeryInsertion stentBiomedical engineering
A composite expandable stent for delivery into a vessel carrying blood comprising an expandable support frame having first and second end portions. A porous imprevious polymer sleeve having inner and outer surfaces extending over the support frame. A coating is disposed on at least one of the inner and outer surfaces of the polymer sleeve for enhancing endothelial cell growth on the device and polymer sleeve. The stent can be cylindrical or tapered.
Owner:NFOCUS NEUROMEDICAL
Composite expandable device with impervious polymeric covering and bioactive coating thereon, delivery apparatus and method
InactiveUS20090043375A1Therapeutic solutionStentsBlood vesselsBiomedical engineeringBioactive coating
A composite expandable device for delivery into a vessel carrying blood comprising an expandable support frame having first and second end portions. An impervious polymer sleeve having inner and outer surfaces extending over the support frame. A coating is disposed on at least one of the inner and outer surfaces of the polymer sleeve for enhancing endothelial cell growth on the device and polymer sleeve.
Owner:NFOCUS NEUROMEDICAL
Preparation method of essence containing human mesenchymal stem cell factors
InactiveCN106344493APromote growthPromote proliferationCosmetic preparationsToilet preparationsFreeze-dryingVascular endothelium
The invention discloses a preparation method of an essence containing human mesenchymal stem cell factors. The preparation method of the essence containing the human mesenchymal stem cell factors comprises the steps: preparation of freeze-dried powder of the human mesenchymal stem cell factors, preparation of a solvent and mixing. The preparation method obtains a high-purity and high-activity stem cell active factor concentrated solution by a cryoconcentration technique and the concentrated solution is freeze-dried to obtainfreeze-dried powder at low temperature in vacuum, so that the factor activity can be kept for a long time; and the solvent contains multiple skin nourishing and moisturizing components and a stem cell lysis solution, can well dissolve the cell factor freeze-dried powder and keeps the cell factor activity. The stem cell essence disclosed by the invention contains vascular endothelial cell growth factors, platelet derived growth factors, epidermal growth factors and other components, can promote skin regeneration, can play roles in moisturizing, tendering, whitening and repairing the skin, and has good application prospects in fields of medical beauty treatment, health protection and the like.
Owner:GENESIS STEMCELL REGENERATIVE MEDICINE ENG CO LTD
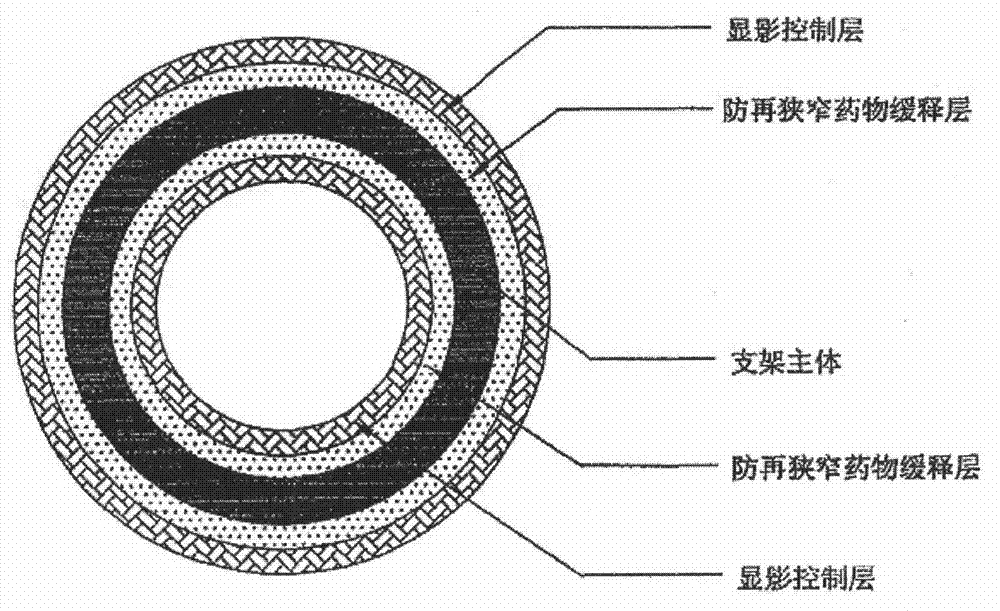
Biodegradable fabric body capable of being developed and conveying device
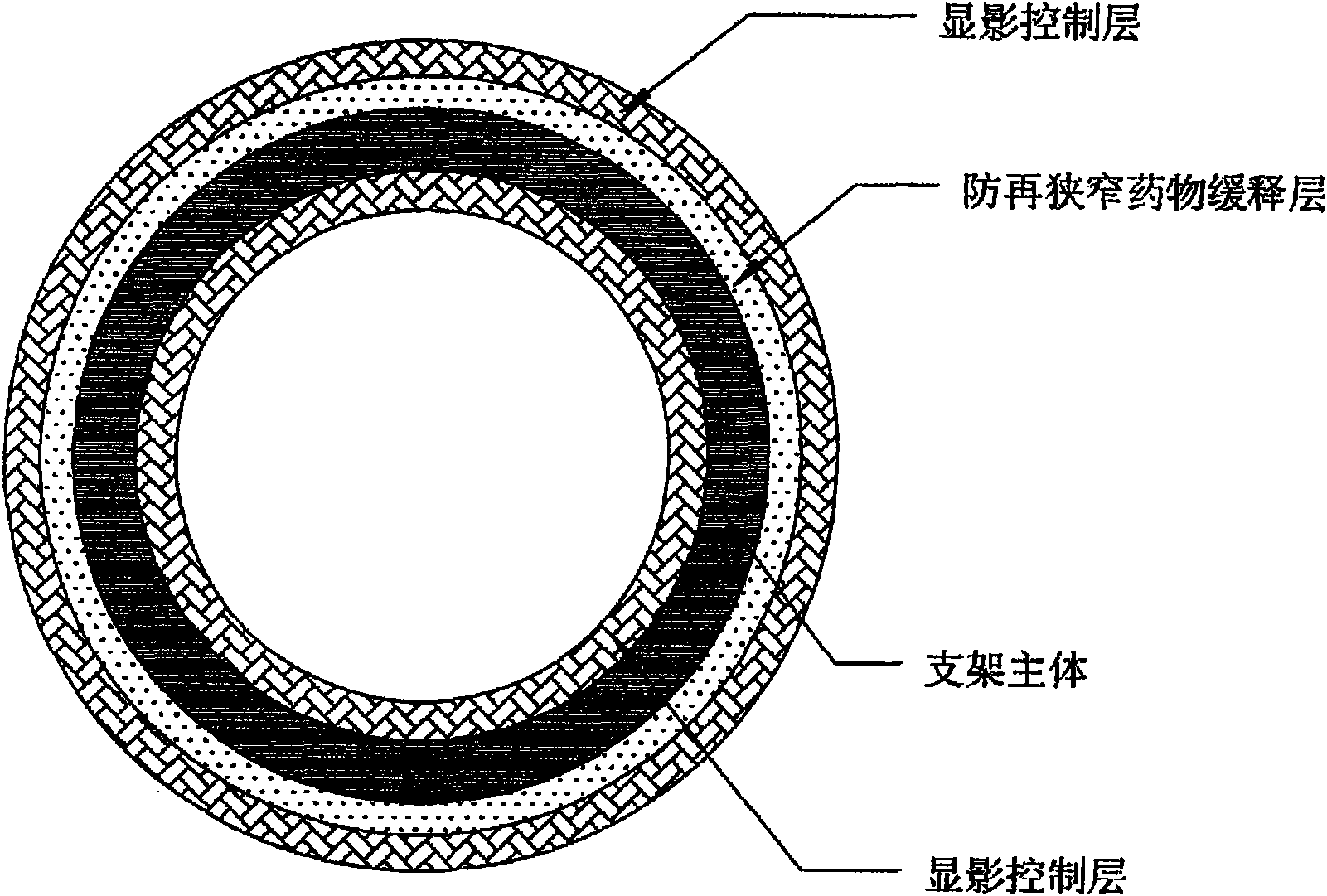
InactiveCN103110444AImprove sealingAvoid the risk of entering the parent vesselSurgeryCoatingsMolecular materialsHemangioma
The invention discloses a biodegradable fabric body capable of being developed and a conveying device. A biodegradable mesh grid serves as a main body. The biodegradable fabric body capable of being developed and the conveying device are characterized in that at least two layers of a sealing layer, a development control layer and a promotion endothelial cell growth layer are arranged on the surface of the mesh grid; the biodegradable mesh grid is woven by composite materials which are obtained by one or various biodegradable high molecular materials in a blending or a copolymerization mode; a form of the biodegradable fabric body is geometry composed of one or a plurality of quadric surfaces and comprises a mushroom shape composed of a spherical shape, a double-elliptical external form and double spherical caps and variants thereof; the mesh grid is a single-layer mesh or a double-layer mesh woven by biodegradable silk. After being implanted into hemangioma, the biodegradable fabric body capable of being developed and the conveying device have a shape memory function and enough mechanical strength, can play a role of embolism blocking hemangioma in a short term and are good in sealing performance. Degraded fragments cannot come off to blood to cause an embolism. The biodegradable fabric body capable of being developed and the conveying device have good biological safety, and the possibility that the degraded fragments cause the embolism is lowered.
Owner:陈平根 +1
Therapeutic treatment for VEGF related ocular diseases
InactiveUS6114320APromote growthPromote cell growthBiocideSenses disorderIsozymeTherapeutic treatment
A method for inhibiting VEGF stimulated endothelial cell growth, such as associated with macular degeneration, and VEGF stimulated capillary permeability, such as associated with macular edema are disclosed, particularly using the isozyme selective PKC inhibitor, (S)-3,4-[N,N'-1,1'-((2''-ethoxy)-3'''(O)-4'''-(N,N-dimethylamino)-butane)-bis-(3,3'-indoly 1)]-1(H)-pyrrole-2,5-dionehydrochloridesalt.
Owner:ELI LILLY & CO +1
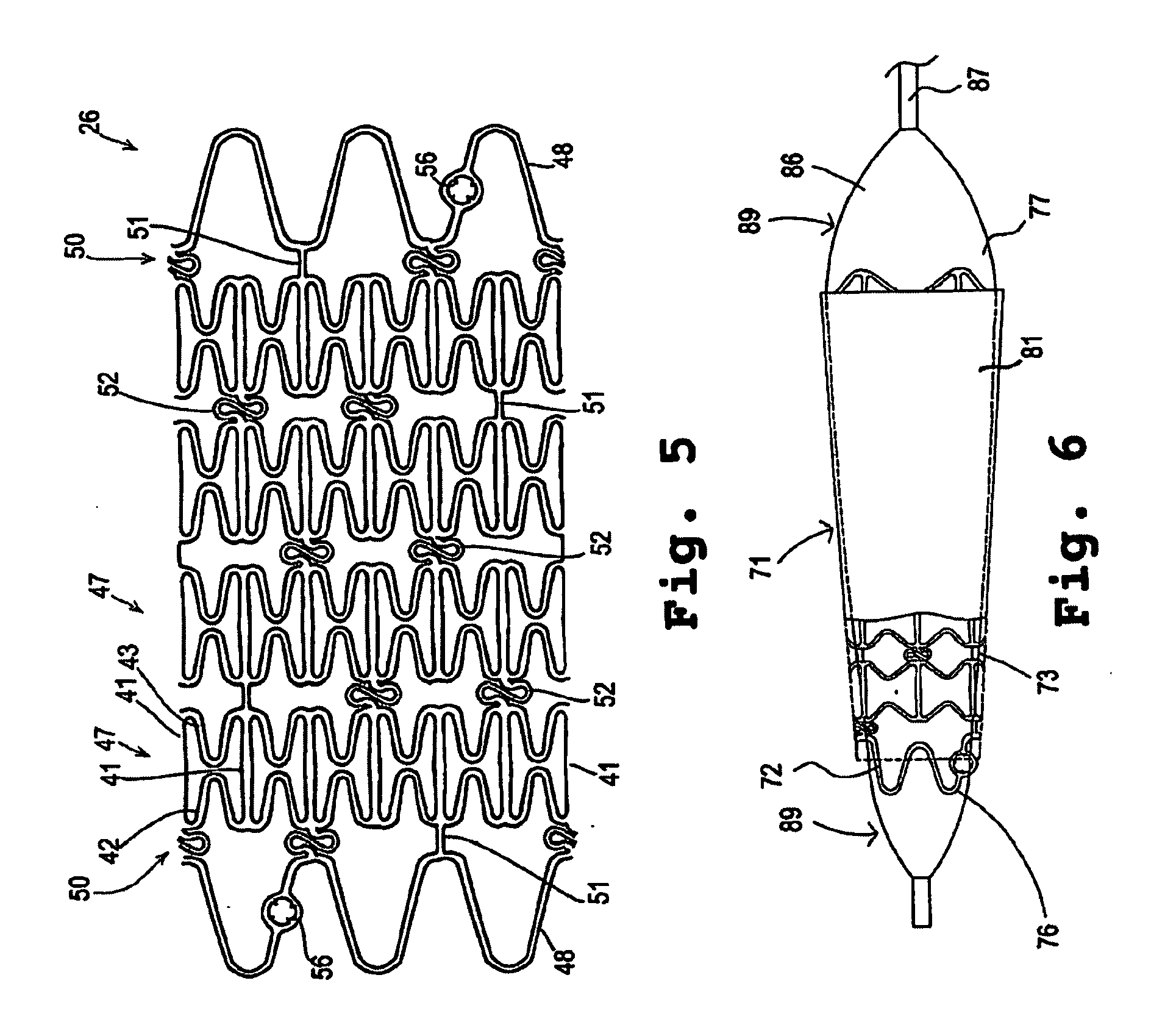
Biodegradable stent with laminated coatings
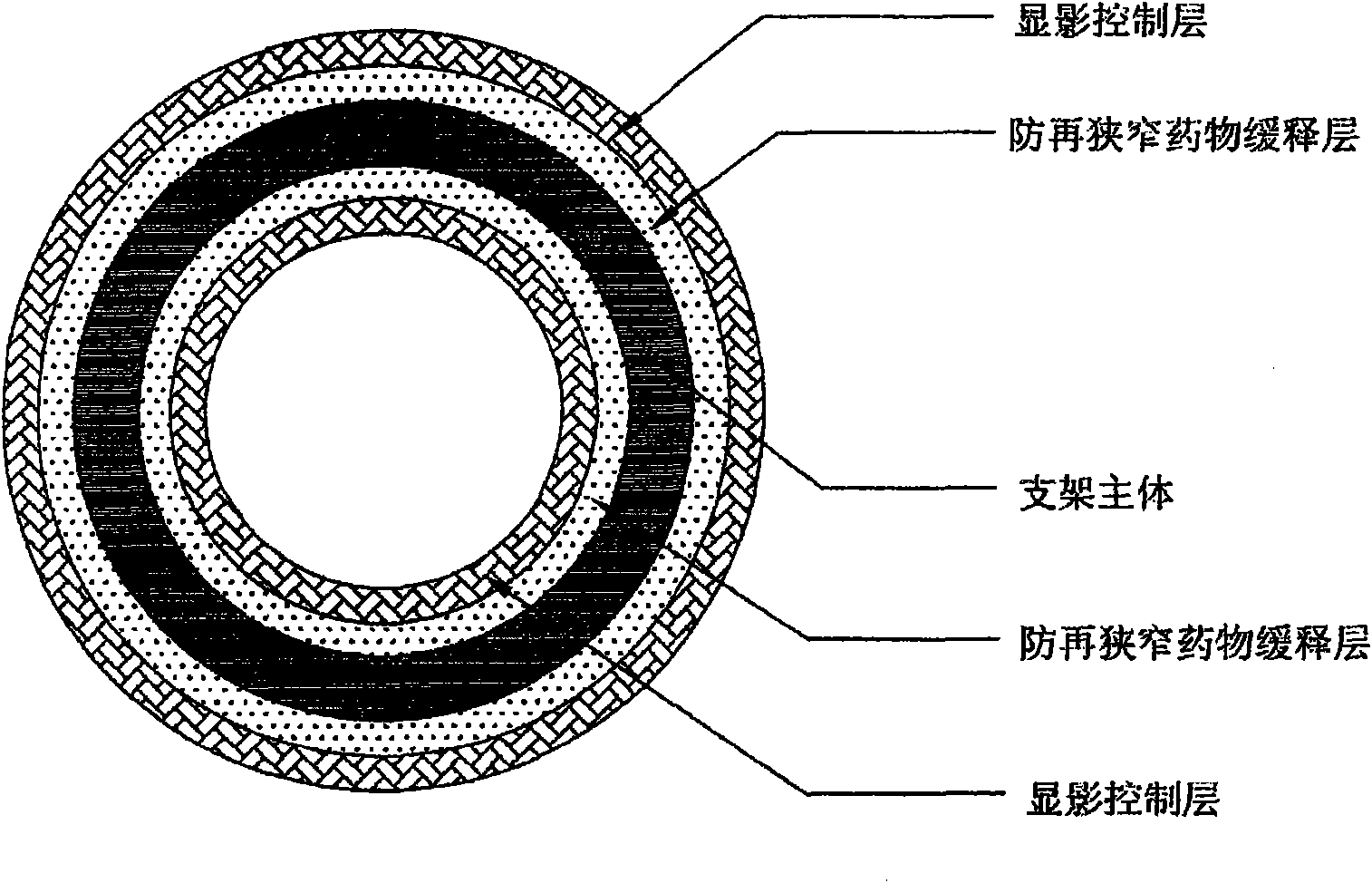
InactiveCN102151185AStable supportImprove the development effectStentsSurgeryControl layerVascular endothelium
The invention belongs to the field of medical apparatus and relates to a biodegradable stent with laminated coatings. The stent comprises a stent main body with a supporting role and at least two layers selected from a medicine coating, an endothelial cell growth promotion layer and a developing control layer; the main body material of the stent is a biodegradable polymer with higher mechanical strength; after implanted into a pathological position, the stent has strong supporting force and can play a role of supporting a pathological blood vessel in a short time; the developing control layer can help a doctor accurately observe the position and the expansion consistency of the stent in the operation so as to prevent adverse events, such as displacement, instant collapse, and the like from happening; medicines for restricting neointimal hyperplasia in the medicine coating can prevent restenosis of the blood vessel after the stent is implanted for a short time; and the endothelial cell growth promotion layer can promote endothelial cells to grow on the surface of the stent to wrap the stent in the vascular endothelium so as to prevent degradable fragments of the stent from falling into the blood to cause embolism.
Owner:SHANGHAI MICROPORT MEDICAL (GROUP) CO LTD
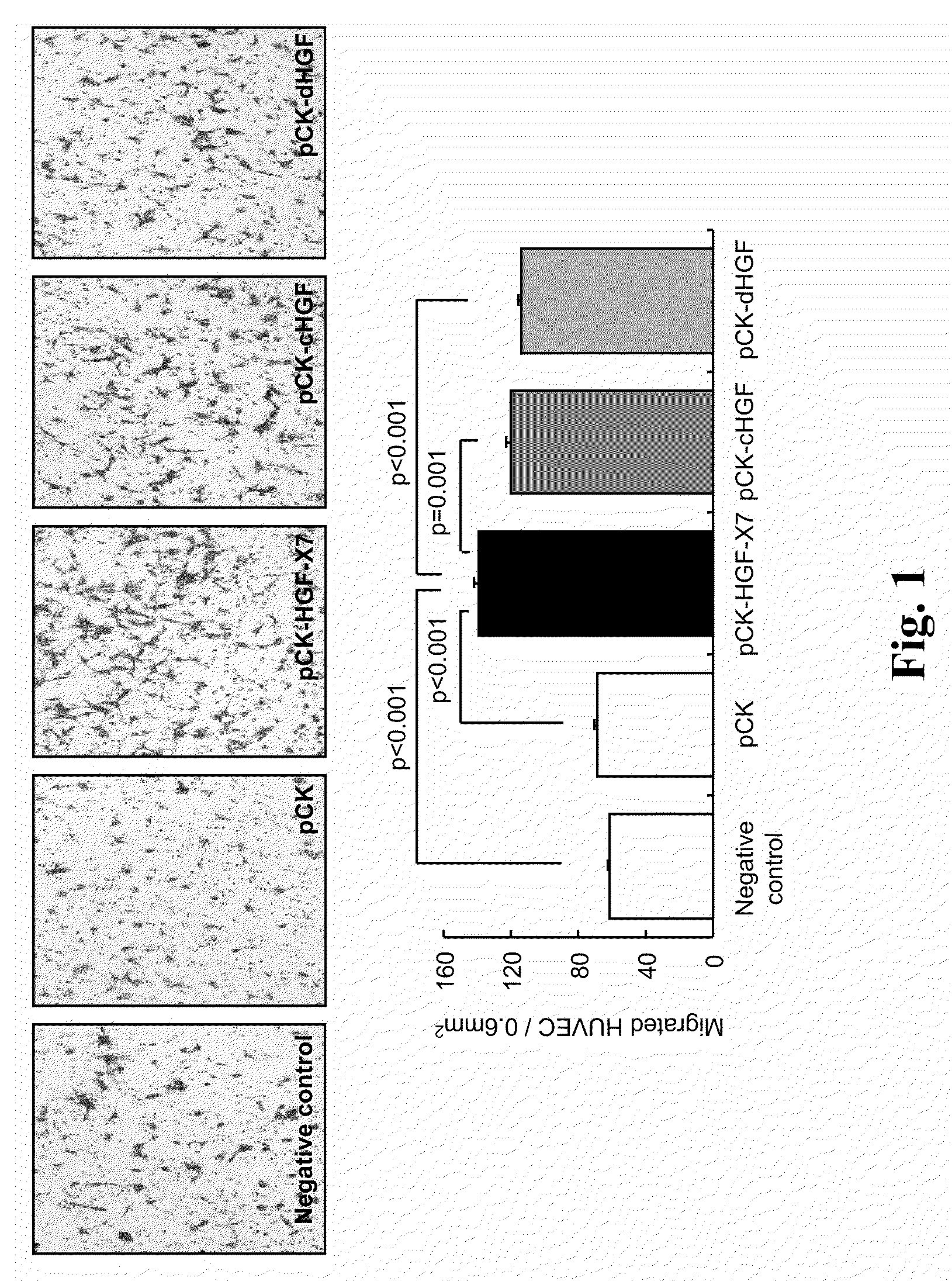
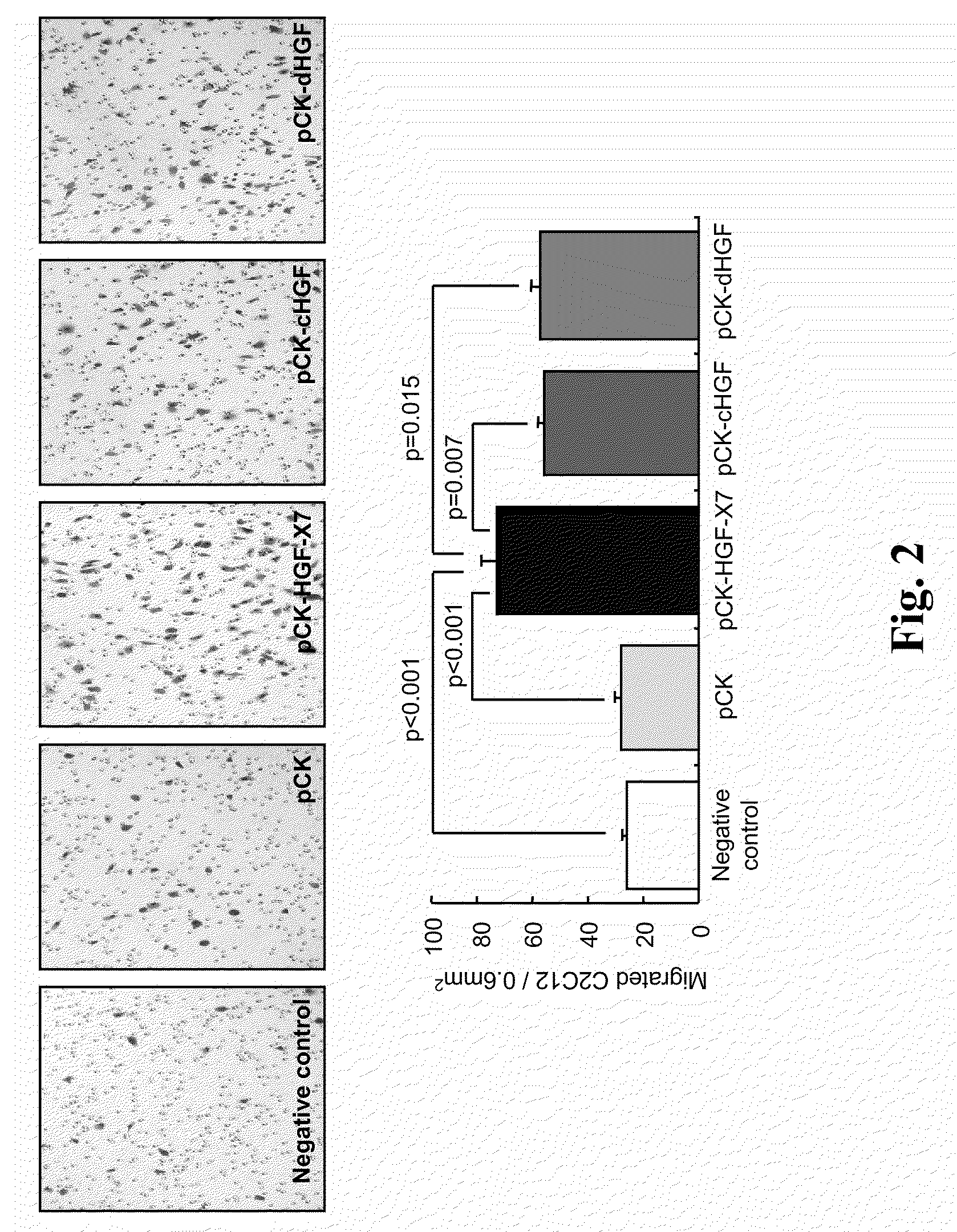
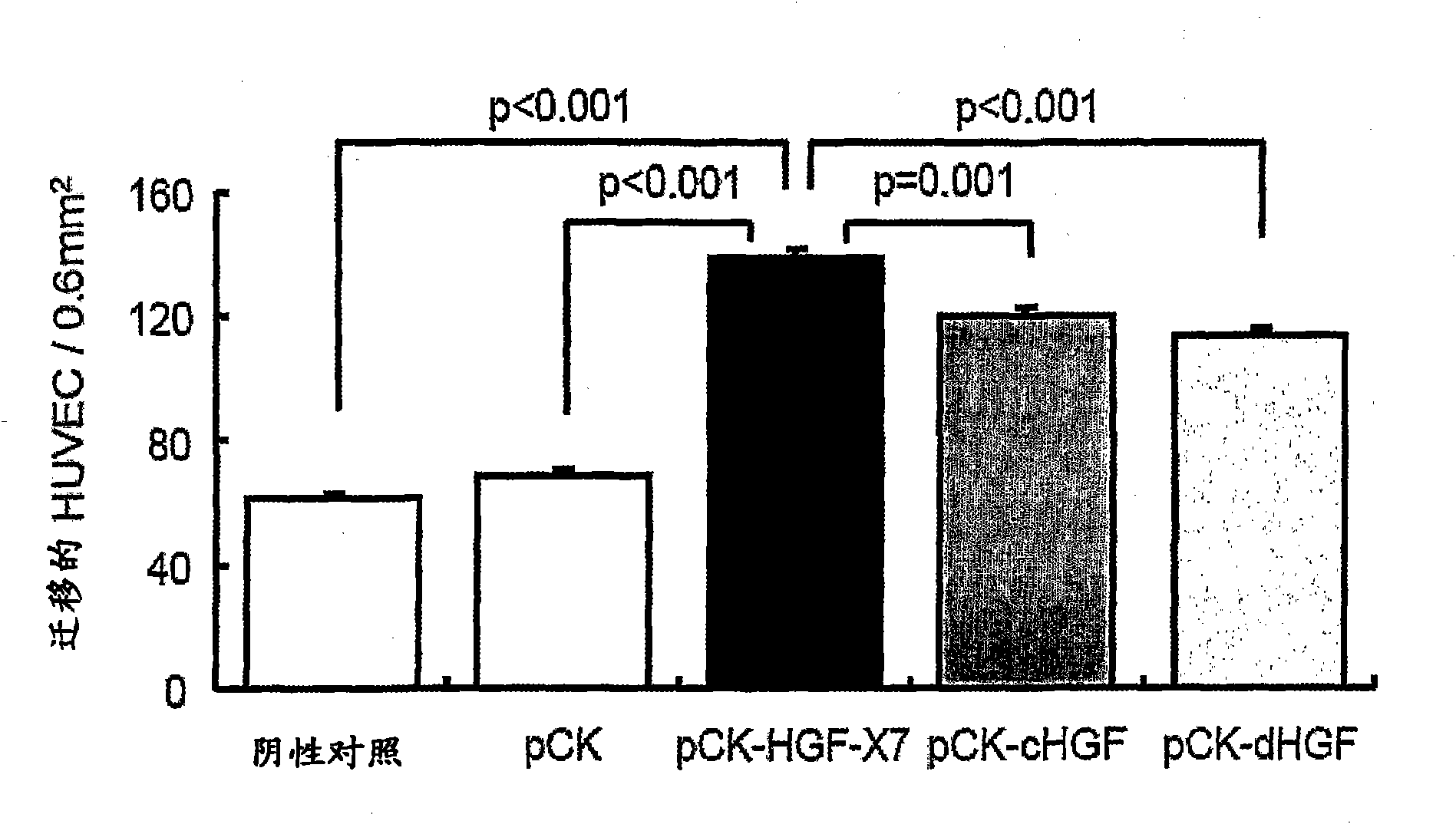
Treatment and Prevention of Cardiac Conditions Using Two or More Isoforms of Hepatocyte Growth Factor
InactiveUS20090202606A1Promote endothelializationPromotes and accelerates re-endothelializationPeptide/protein ingredientsCarbohydrate active ingredientsEndothelial NOSHepatocyte growth factor
The present invention relates to methods for treating or preventing cardiac conditions in a subject comprising administering to the subject two or more isoforms of hepatocyte growth factor (HGF). The present invention further relates to methods for promoting endothelial cell growth in a blood vessel comprising administering to the blood vessel two or more isoforms of hepatocyte growth factor (HGF). In one embodiment the two or more isoforms of HGF are administered as one or more polynucleotides encoding the isoforms.
Owner:HELIXMITH CO LTD
Preparation method of coating having endothelium bionic function
InactiveCN103330960AImprove bindingStrong chemical stabilitySurgeryCoatingsSmooth muscleNitric oxide
The invention discloses a preparation method of a coating having an endothelium bionic function, which comprises the following step: fixing 3,3-diselenodipropionic acid (SeDPA) having nitrogen monoxide catalytic activity and heparin on the surface of an amino-enriched coating. The functional coating shows an endothelium-like bionic function, reflects excellent anticoagulant property, and has excellent functions of inhibiting proliferation of smooth muscle cells and promoting growth of endothelial cells.
Owner:SOUTHWEST JIAOTONG UNIV
Preparation method of a mechanically enhanced cell-loaded microchannel hydrogel
InactiveCN102266589AImprove mechanical propertiesGuaranteed stabilityProsthesisFiberEndothelial cell culture
The invention relates to a preparation method of a mechanically-enhanced cell-loaded microchannel hydrogel, which comprises the following steps: preparing a mixed solution of hydrogel sol and cell culture fluid, and pouring the sol-cell mixed liquid into a polymethyl methacrylate die into which capillary tubes or fibers are inserted, thereby enabling primary crosslinking; drawing out the capillary tubes or fibers, introducing the hydrogel sol into microchannels of the cell-loaded hydrogel, and reinserting the capillary tubes or fibers, or sucking excess hydrogel sol out of the microchannels and carrying out ultraviolet irradiation, thereby enabling secondary crosslinking, so that the cell-loaded hydrogel with thick hydrogel layer is formed in the wall regions of the microchannels; and drawing out the capillary tubes or fibers, introducing endothelial cell culture fluid into the microchannels of the cell-loaded hydrogel, and culturing so that the endothelial cells grow and spread to form endothelial microchannels. The introduction of the thick hydrogel layer can maintain the stability of the microchannels in the hydrogel, and prevent shedding and intrusion of the endothelial cells;and the culture fluid in the microchannels can be filtered and screened.
Owner:XI AN JIAOTONG UNIV
Stem cell culture medium
InactiveCN105200008ADoes not affect potentialHigh speedNervous system cellsSkeletal/connective tissue cellsSodium bicarbonateHuman platelet
The invention discloses a stem cell culture medium. The stem cell culture medium comprises an improved DMEM (Dulbecco's Modified Eagle Medium) / F12 basal culture medium and an additive, wherein the additive comprises sodium bicarbonate, selenium amino acid chelate, recombinant human insulin growth factors, recombinant human basic fibroblast growth factors, recombinant human lactoferrin, ascorbic acid, recombinant human platelet-derived growth factors, recombinant human vascular endothelial cell growth factors, octacosanol, polyvinyl alcohol, polyvinylpyrrolidone and recombinant human epidermal growth factors. According to the stem cell culture medium, the potential of stem cells is not influenced while the stem cells can be proliferated rapidly, the proliferation speed of the stem cells is increased by 3-5 times compared with a common culture medium, and further, the stem cell culture medium can be used for culturing the stem cells of various kinds of tissue and has excellent applicability; the cultured stem cells have high differentiation capability, can be differentiated into multiple functional cells and have very high scientific research and medical application values, culture medium components are exact, the quality is stable, and accordingly, the cultured stem cells are not likely to generate human body rejection reaction after transplanting.
Owner:XINXIANG MEDICAL UNIV
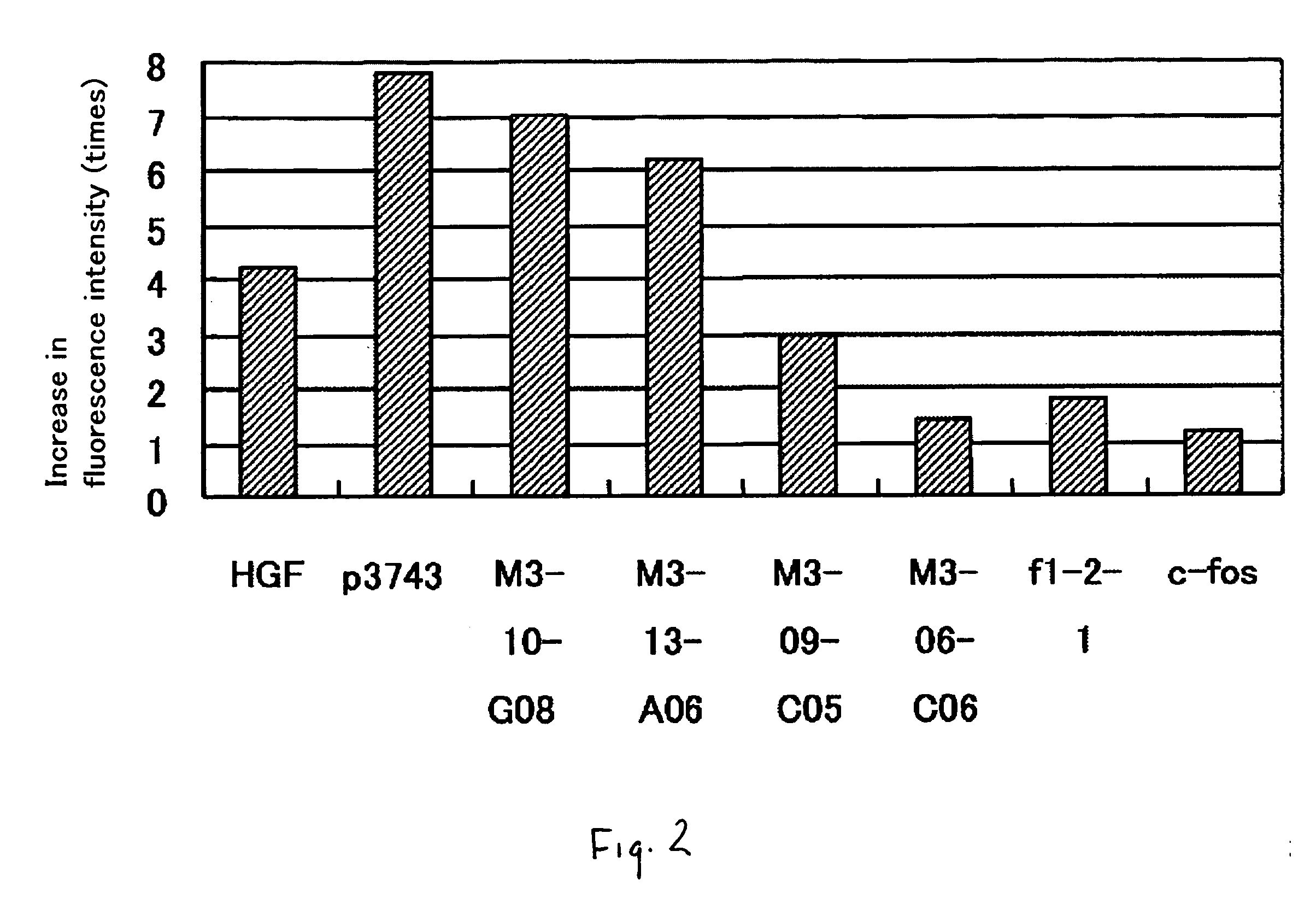
Gene Promoting Vascular Endothelial Cell Growth
ActiveUS20070281888A1Promoting transcriptionAntibacterial agentsOrganic active ingredientsVascular endotheliumAngina
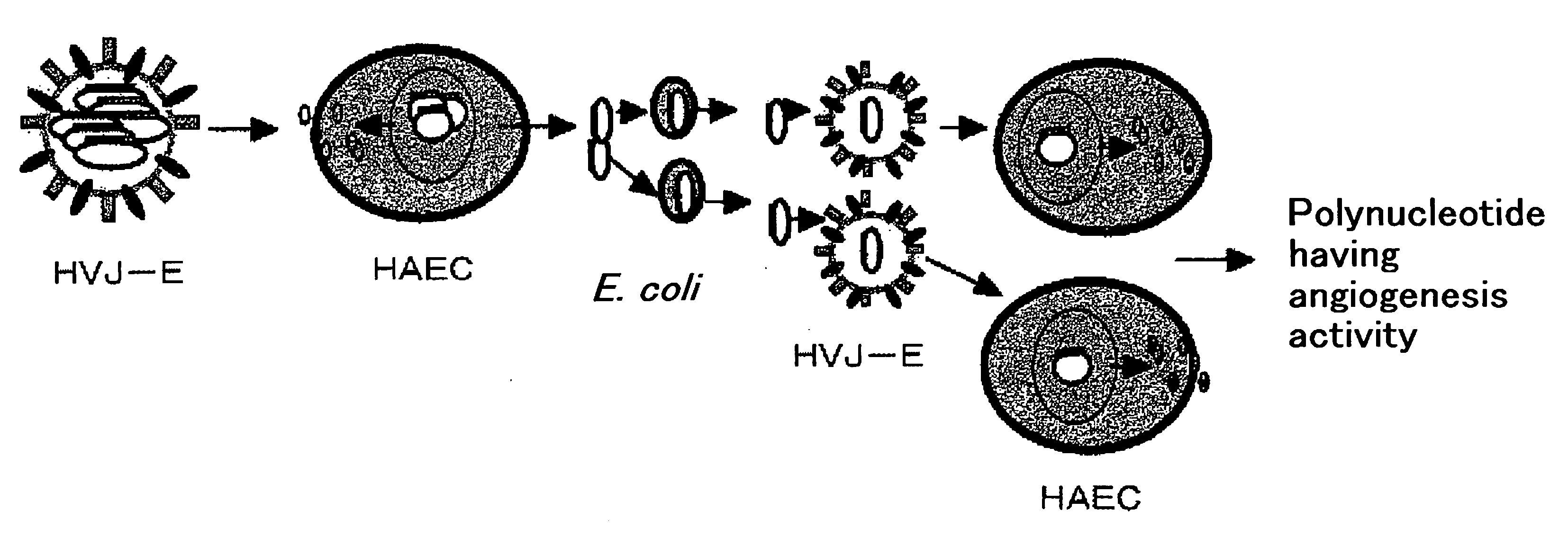
It is intended to provide a novel polypeptide having an activity of growing vascular endothelial cells, an activity of promoting transcription form c-fos promoter, an activity of promoting transciption from VEGF promoter and / or an angiogenic activity; a polynucleotide encoding this polypeptide; the above polypeptide and / or a pharmaceutical composition containing the polypeptide for treating a disease selected from the group consisting of obstructive arteriosclerosis, Buerger's disease, peripheral vascular disorder, angina, myocardial infraction, brain infarction, ischemic heart disease and ischemic brain disease; a method of treating these diseases; and an antibacterial composition. The above problems can be solved by isolating a novel peptide having the above-described activities and a nucleotide encoding this peptide.
Owner:FUNPEP CO LTD
Recombinant proteins containing shiga-like toxin and vascular endothelial growth factor fragments
The present invention is directed to an isolated polypeptide including: (1) the A subunit of Shiga-like bacterial toxin, wherein said subunit has the nucleic acid sequence of SEQ ID NO:9; and (2) human vascular endothelial growth factor wherein the growth factor has the nucleic acid sequence of SEQ ID NO:10; wherein the isolated polypeptide possesses ribosome inactivating activity. The present invention is also directed to compositions for inhibiting endothelial cell growth in a patient.
Owner:SIBTECH
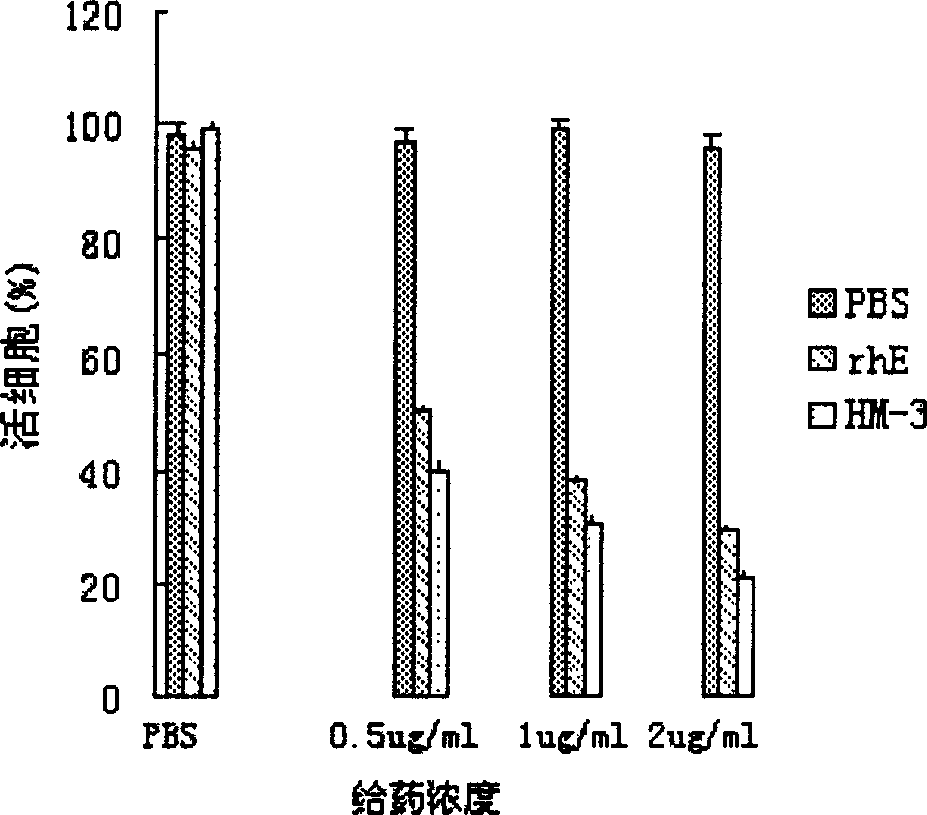
Blood vessel formation inhibitor IIM3 and its preparation method and application
InactiveCN1830487AImprove and enhance growthImprove and enhance the anti-tumor effectPeptide/protein ingredientsAntineoplastic agentsEscherichia coliInclusion bodies
An efficient angiogenesis depressant HM-3 for treating the solid tumors including stomach cancer, lung cancer and liver cancer is prepared through expressing in colibacillus by genetic engineering method, separating the protein of inclusion body, dissolving, re-naturalizing, and separating-purifying by ion change and chromatography.
Owner:徐寒梅
Methods and apparatuses for coating a lesion
InactiveUS20090005849A1Providing elasticityProvide mechanical strengthOrganic active ingredientsBiocideAdjuvant therapyLesion
A method and apparatus to treat regions of a vessel is described. A protein elastin-based polymer is released from the apparatus to coat the vessel lining as a primary therapy or an adjunct therapy with the delivery and deployment of a stent with or without drug coating. The protein elastin-based polymer may include a triblock structure having an elastin pentapeptide as the flanking block and a hydrophilic variant of the pentapeptide as the middle block. Both the flanking and middle blocks can be modified to change the structural and chemical properties of the polymer. In particular, the protein elastin based polymer is adapted to perform at least one of controlling release of a treatment agent, stimulating endothelial cell growth and stabilizing the vulnerable plaque to prevent rupture of the vulnerable plaque.
Owner:ABBOTT CARDIOVASCULAR
Compositions useful for and methods of modulating angiogenesis
InactiveUS20090036373A1Easy to optimizePeptide/protein ingredientsAntipyreticVascular endotheliumAngiogenesis growth factor
Compositions and methods for treating subjects with disorders characterized by aberrant vascular endothelial cell growth are provided. The compositions comprise agents that are combinations of a Wnt pathway stimulator component and a Tie2 pathway repressor component. Particularly useful Wnt pathway stimulators include, but are not limited to, Wnt7b-like molecules. Particularly useful Tie2 pathway repressor components include, but are not limited to, Ang2-like molecules. The methods allow for modulation of vascular endothelial cells, vascular endothelial cell vessels, capillary bed development, and angiogenesis.
Owner:CHILDRENS HOSPITAL MEDICAL CENT CINCINNATI
Non-woven mat and metho of producing same
InactiveUS20100305687A1Promote ingrowthUniform porosityAdhesive processesFilament/thread formingSmooth musclePorosity
A mat having a highly uniform porosity distribution is produced by consolidating 15 or more layers of melt blown webs (or the like) having different orientations. Control over the porosity is provided by using webs that exhibit a narrow, unimodal distribution of fiber diameters over the bulk of its distribution, such as in the top 80%. A compliance of the mats can be chosen by selecting a number and orientation of the webs. It is thus possible to produce mats that are good candidates for vascular grafts, for example. The uniformity of the porosity within the range of 6 μm to 30 μm permits seeding of the vascular graft with endothelial and smooth muscle cells. The mats have the demonstrated ability to retain, and support growth of, smooth muscle cells and endothelial cells.
Owner:NAT RES COUNCIL OF CANADA
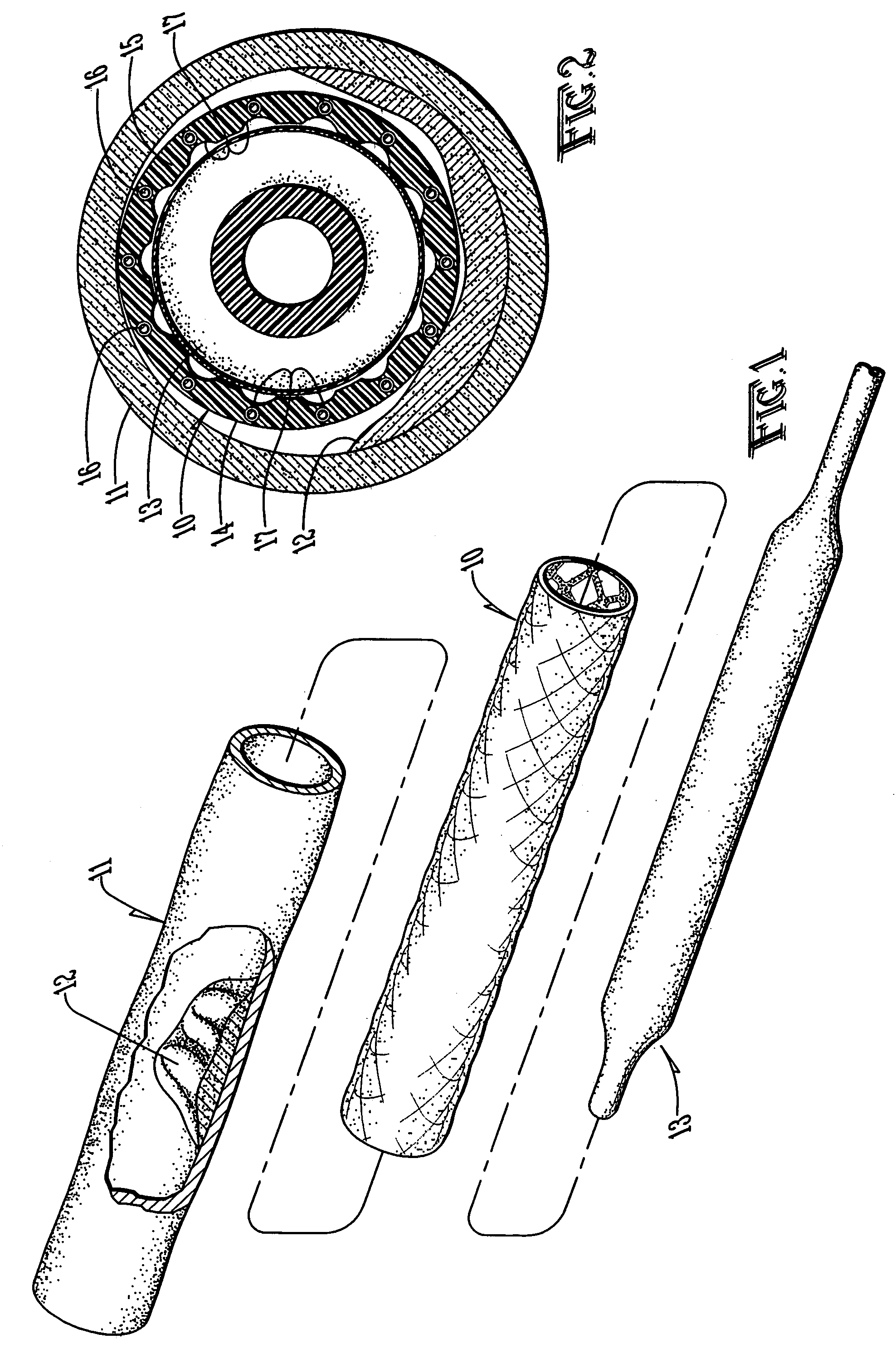
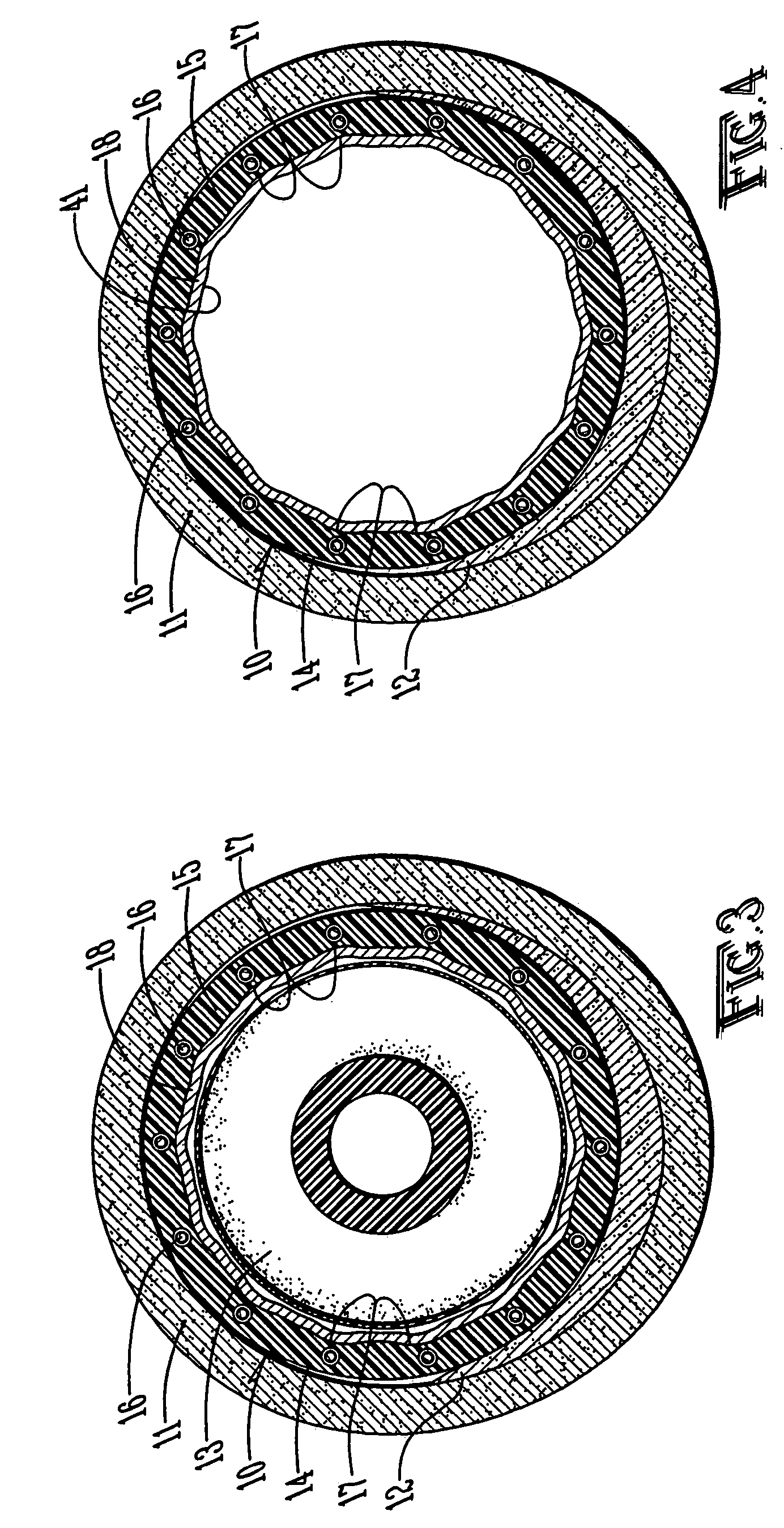
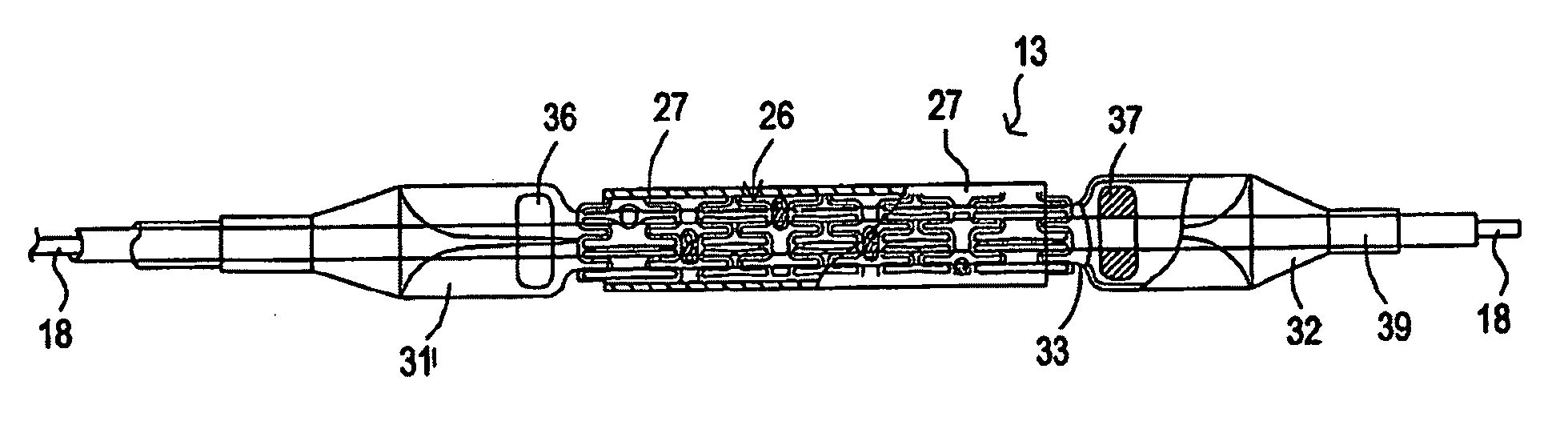
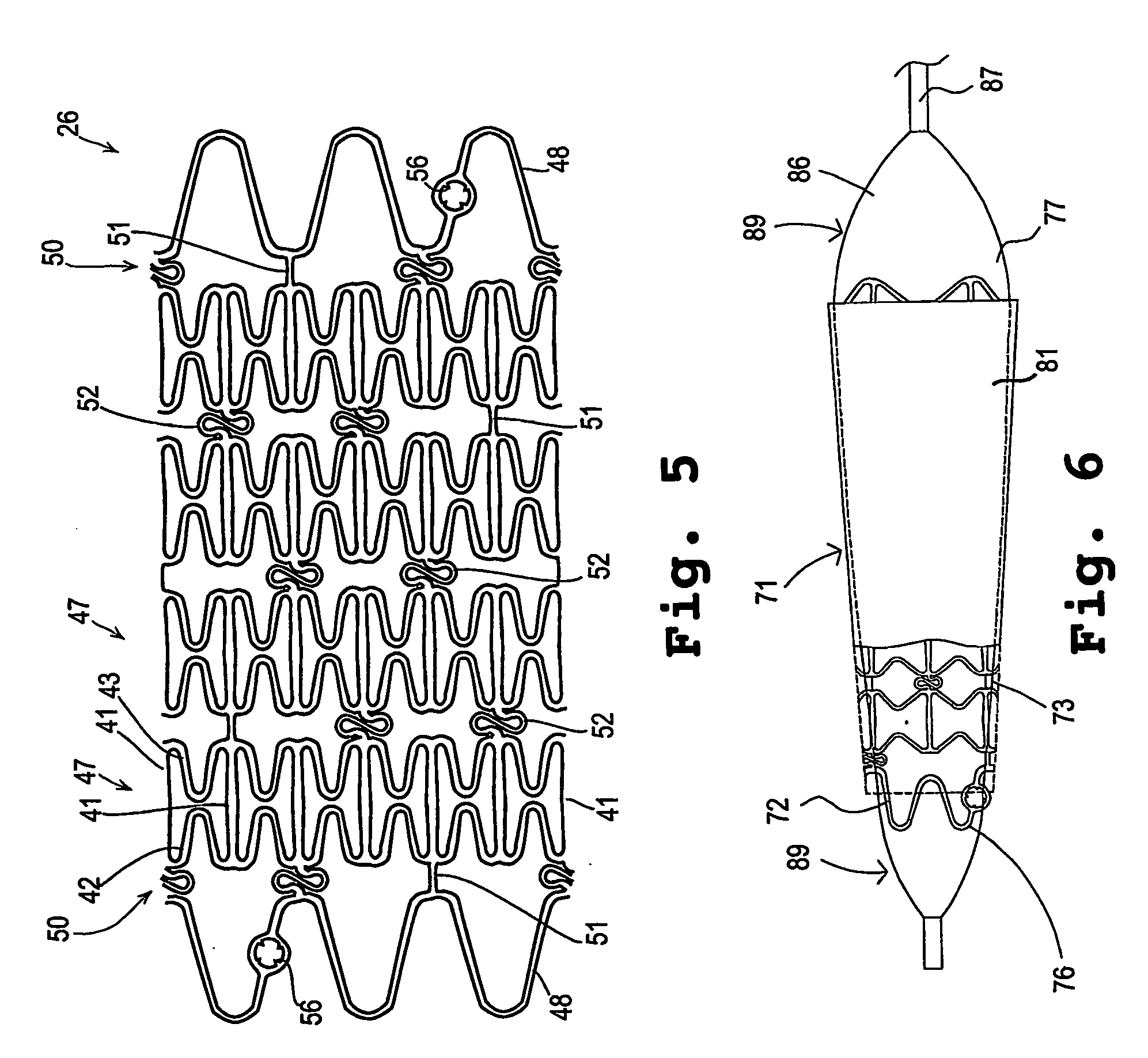
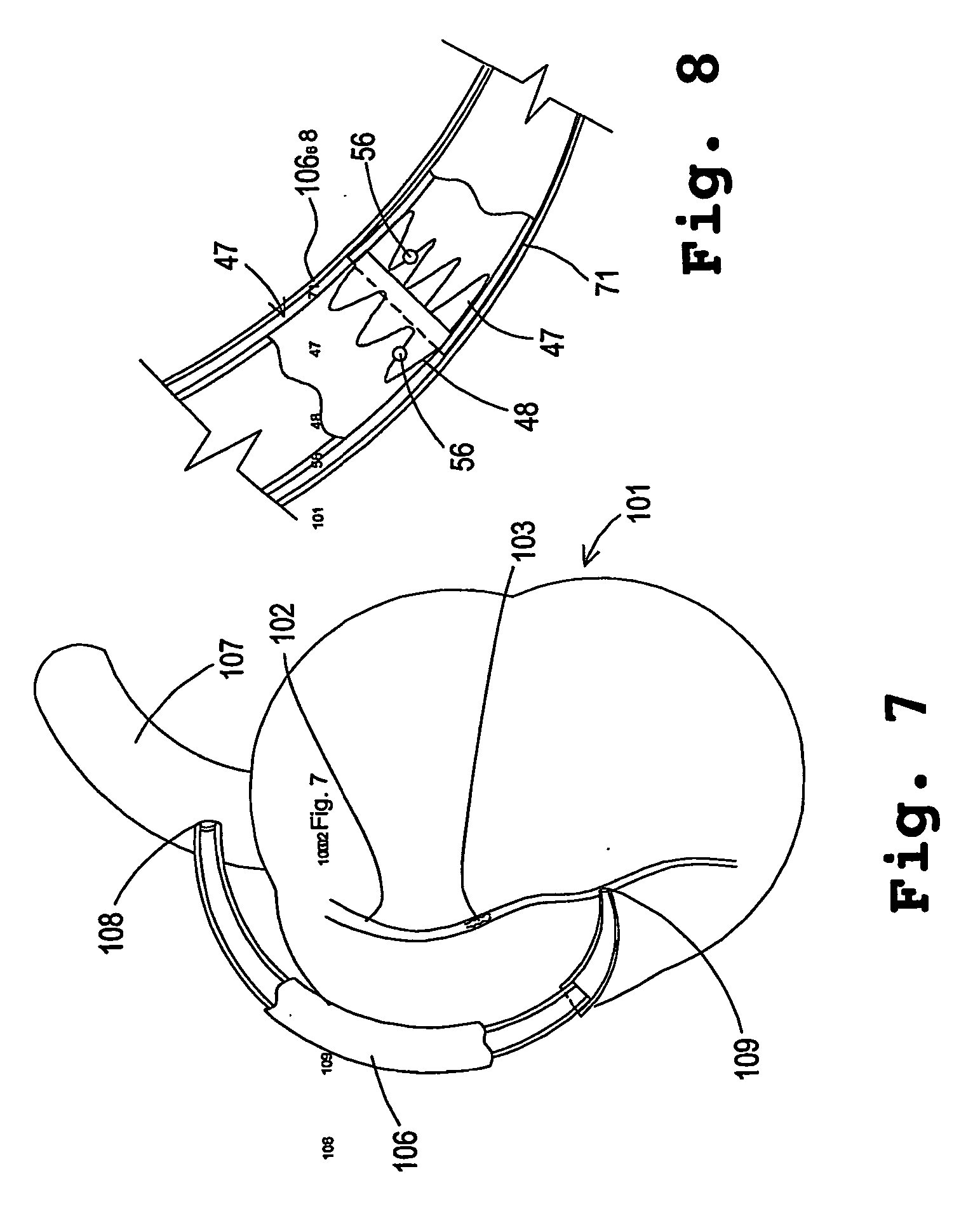
Developable sealed type woven body implantation material and conveying device
The invention discloses a developable sealed type woven body implantation material, which comprises a woven mesh, a sealing film and a developing layer and is characterized in that the woven mesh is a single-layer or double-layer nickel-titanium alloy mesh, the developing layer is one layer of coated developing materials, the sealing film is sewed into any one layer of the single-layer or double-layer nickel-titanium alloy mesh, then, an endothelial cell growth promotion layer is coated at the outer surface of the sealing film, and the developing layer is arranged among or outside any layer of the woven mesh, the sealing film and the endothelial cell growth promotion layer. The conveying device comprises an outer sheath tube (32), an inner sheath tube (31) and a conveying guide wire (30), wherein the inner sheath tube (31) is sheathed in the outer sheath tube (32) and is glidingly connected with the outer sheath tube (32). The woven body implantation material is in a self-expansion type, the integral developing can be realized, the sealing film can seal and isolate the hemangioma, the endothelial cell growth promotion layer promotes the growth of endothelial cells in the surface of the woven body implantation material, the woven body implantation material is coated inside endothelial tissues of blood vessels, and the risk of recent and future thrombus generation is reduced.
Owner:邹旭华
Surface modification method of biomaterial magnesium alloy
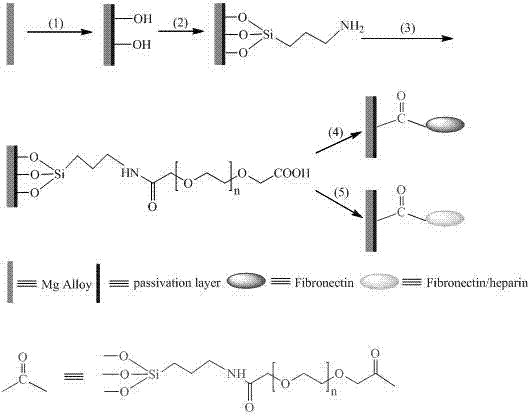
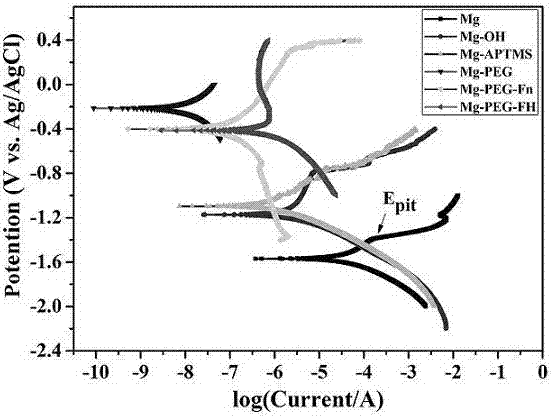
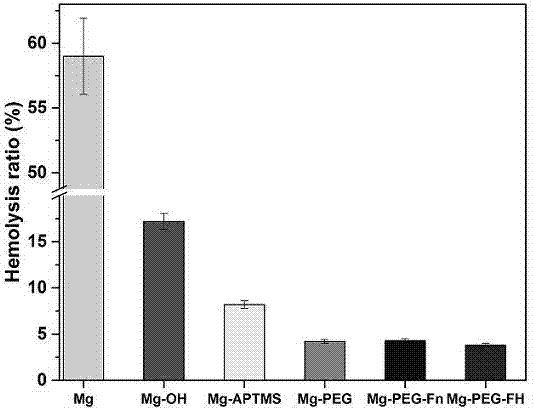
ActiveCN107185055AImprove physiological corrosion resistanceGood blood compatibilitySurgeryPharmaceutical containersSilanesMetallurgy
The invention discloses a surface modification method of a biomaterial magnesium alloy. The method comprises, firstly, performing surface chemical treatment on the surface magnesium alloy; secondly, self-assembling and immobilizing amino silane molecules; lastly, sequentially immobilizing polyethylene glycol, fibronectin and heparin on the surface to structure a multifunctional bioactive surface and meanwhile to improve the physiological corrosion-resistant performance of the magnesium alloy. Through surface modification of the biomaterial magnesium alloy, the method can improve the physiological corrosion-resistant performance of the magnesium alloy and endow the material with good blood compatibility and endothelial cell growth promoting property, thereby laying a good foundation for application of the biomaterial magnesium alloy to the fields of intravascular implantation of materials or devices such as intravascular stents.
Owner:HUAIYIN INSTITUTE OF TECHNOLOGY
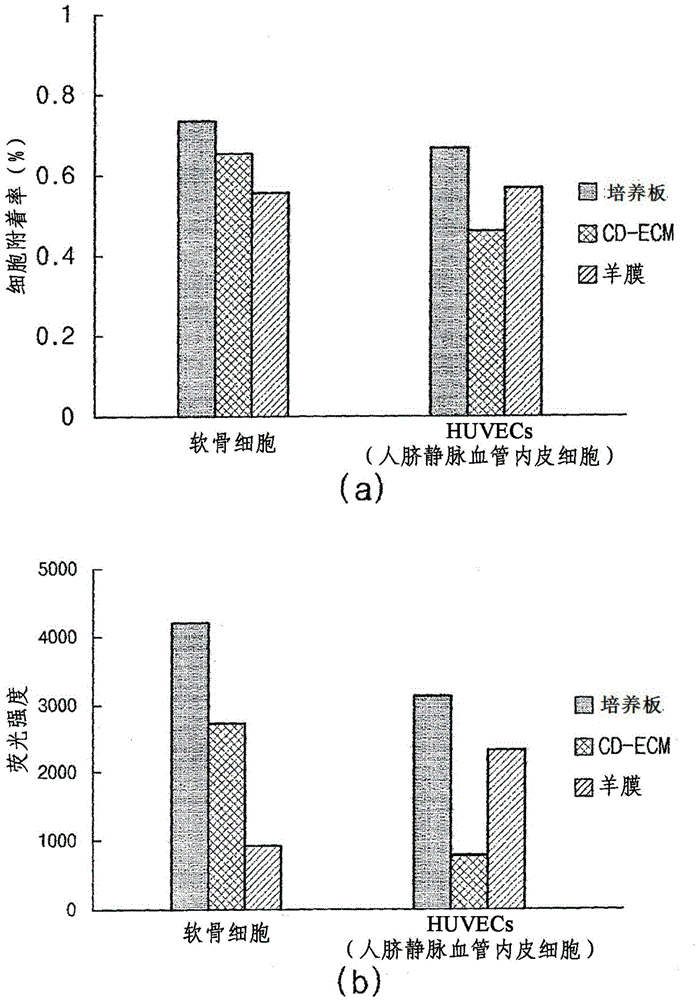
Composition for treating angiogenic diseases using extracellular matrix membrane of cartilage-derived cell, and transplant material for cornea or conjunctiva
InactiveCN104837494ASuppress generationPrevent infiltrationSenses disorderMammal material medical ingredientsConjunctivaCartilage cells
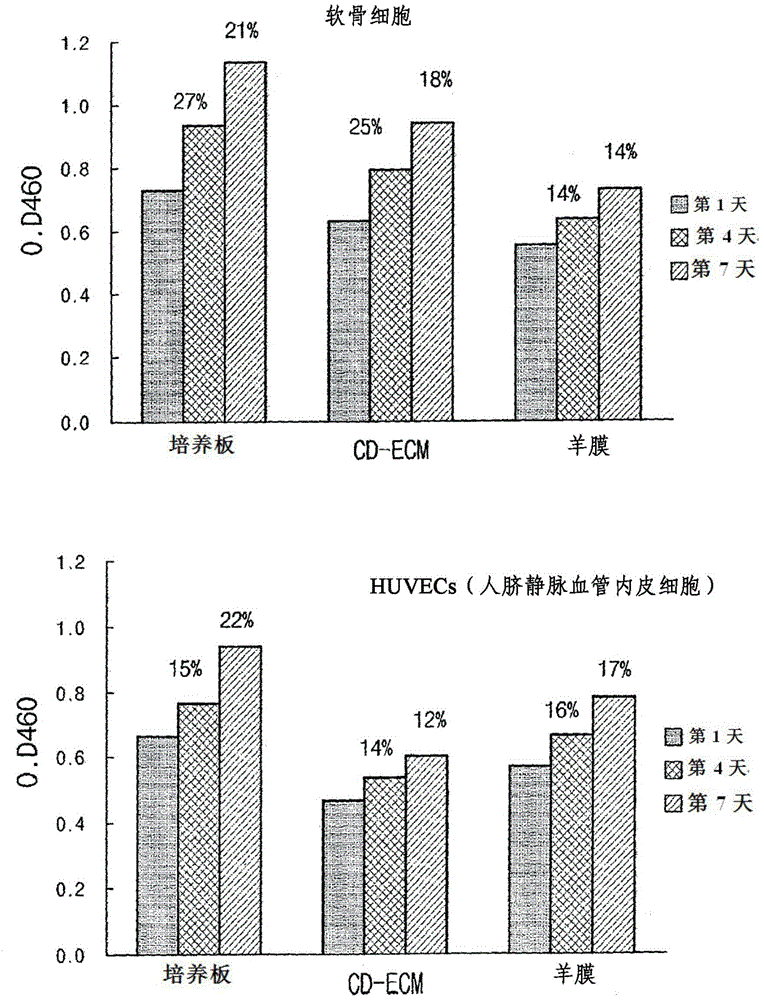
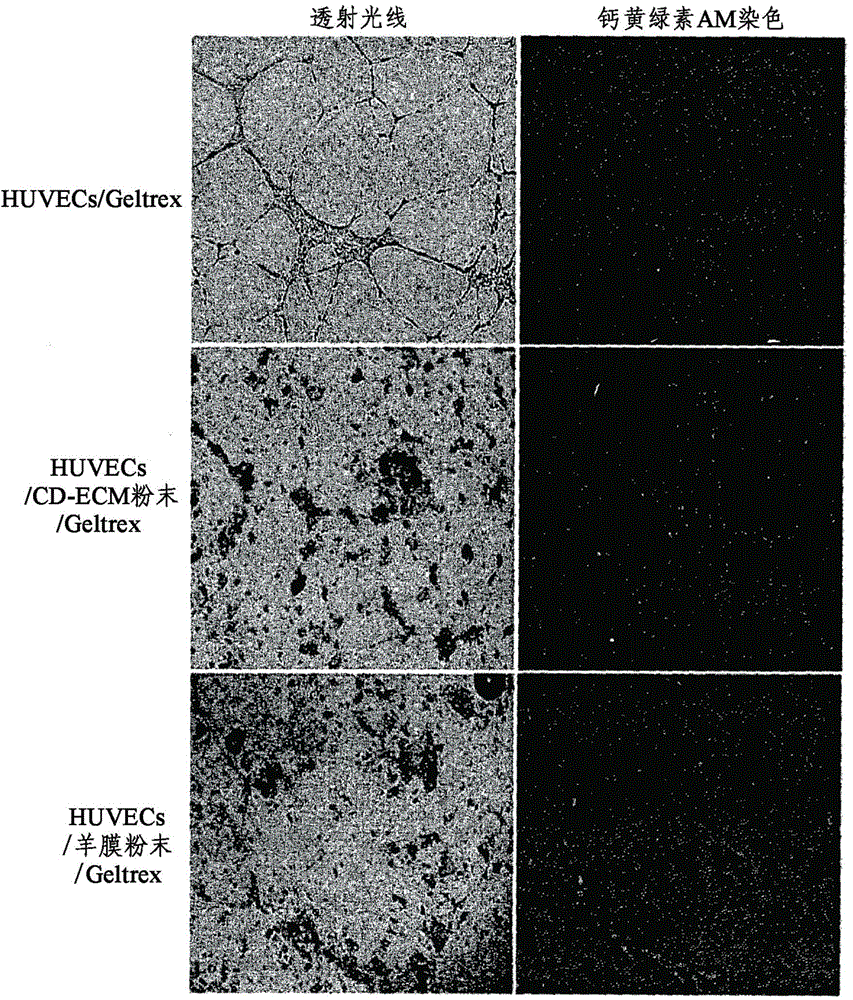
The present invention provides a novel composition for treating angiogenic diseases using an extracellular matrix membrane of a cartilage-derived cell having a membrane or film form, which is excreted from cartilage cells that are cultured when the cartilage cells isolated from the cartilage of an animal is monolayer-cultured, and a transplant material for the cornea or the conjunctiva. The composition for treating the angiogenic diseases and the transplant material for the cornea or the conjunctiva, according to the present invention, suppresses endothelial cells from attaching or proliferating and impedes the movement of endothelial cells, thereby suppressing vascular endothelial growth and vascular penetration and thus ultimately suppressing angiogenesis. [Reference numerals] (AA) Matrigel; (BB) Matrigel / CD-ECM powder; (CC) Matrigel / Amnion powder
Owner:AJOU UNIV IND ACADEMIC COOP FOUND
In-vitro construction method for simulating blood brain barrier through human brain microangiogenesis
ActiveCN108823145AReduce dosageAvoid inaccurate test resultsNervous system cellsArtificial cell constructs3D cell cultureCell culture media
The invention discloses an in-vitro construction method for simulating a blood brain barrier through human brain microangiogenesis. The method comprises the following steps: preparing human brain microvascular endothelial cell suspension and human astrocyte suspension, and preparing fibrinogen mother liquor and thrombin mother liquor; mixing the endothelial cell suspension, the astrocyte suspension, a DMEM (Dulbecco's Modified Eagle Medium) medium, the fibrinogen mother liquor and the thrombin mother liquor, and preparing a mixed cell gel solution; injecting the mixed cell gel solution into amicro-fluidic chip, performing thermostatic incubation to gelation, adding an endothelial growth medium into the micro-fluidic chip, and constructing a 3D cell culture chip; performing continuous culture on the 3D cell culture chip, and enabling the endothelial cells and astrocyte to grow into a brain microvascular network structure, namely correspondingly producing the simulated blood brain barrier. According to the technical scheme provided by the invention, the in-vitro model of the blood brain barrier is successfully constructed, and the characteristics of the blood brain barrier are clearly and accurately reflected.
Owner:WUHAN CHOPPER BIOLOGY
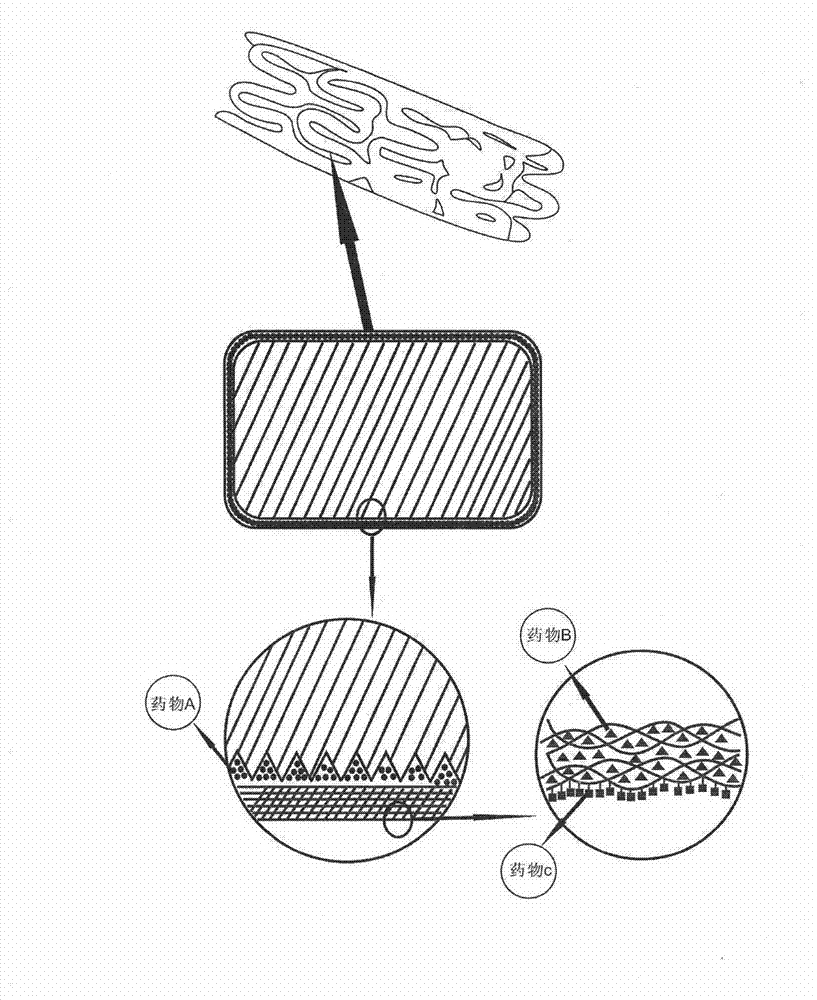
Biodegradable bracket with multiple drugs
The invention provides a biodegradable bracket with multiple drugs, belongs to the field of medical equipment and relates to a biodegradable bracket with a single coating. Besides support function, the bracket further comprises the single coating which contains anti-proliferative drugs, anti-inflammation and anti-immune drugs, drugs for promoting endothelial cells to grow and a developing agent. The bracket main body is made of biodegradable polymers of which mechanical strength is high. After implanted on a pathological position, the bracket has strong support force, so that action of supporting the pathological vessels in short time can be achieved. Because of the developing agent, doctors can accurately obverse the position and the expansion consistency of the bracket during operation, and displacement or other adverse events can be prevented; the anti-proliferative drugs can prevent the vessels from becoming narrow in short time after the bracket is implanted; and the drugs for promoting the endothelial cells to grow can promote the endothelial cells to grow on the surface of the bracket and can cover the bracket in the endothelium of the vessels, so that embolism phenomenon caused by degradation fragments of the bracket dropping in blood can be prevented.
Owner:深圳市昕力医疗设备开发有限公司
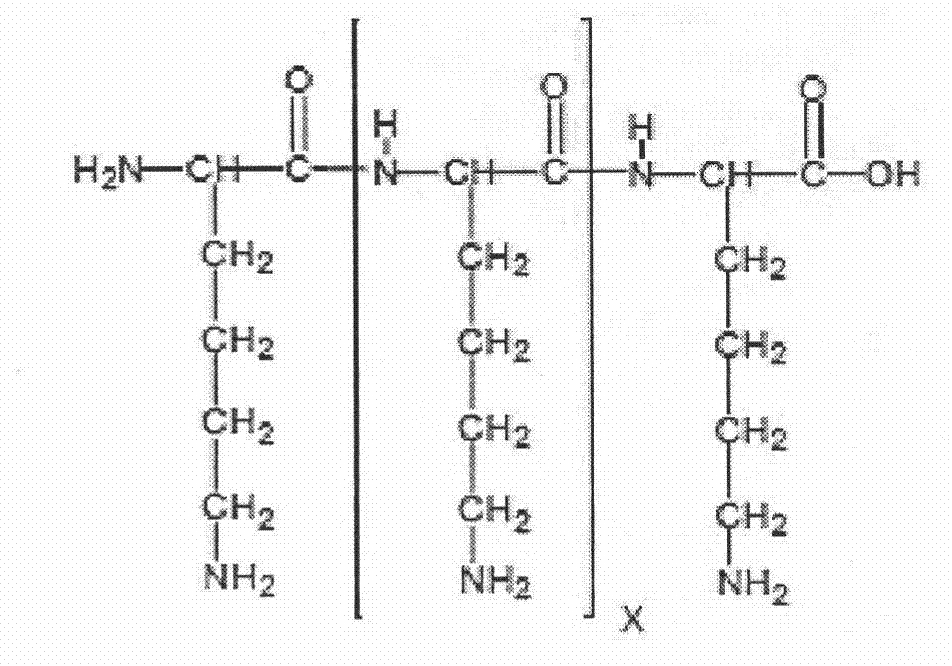
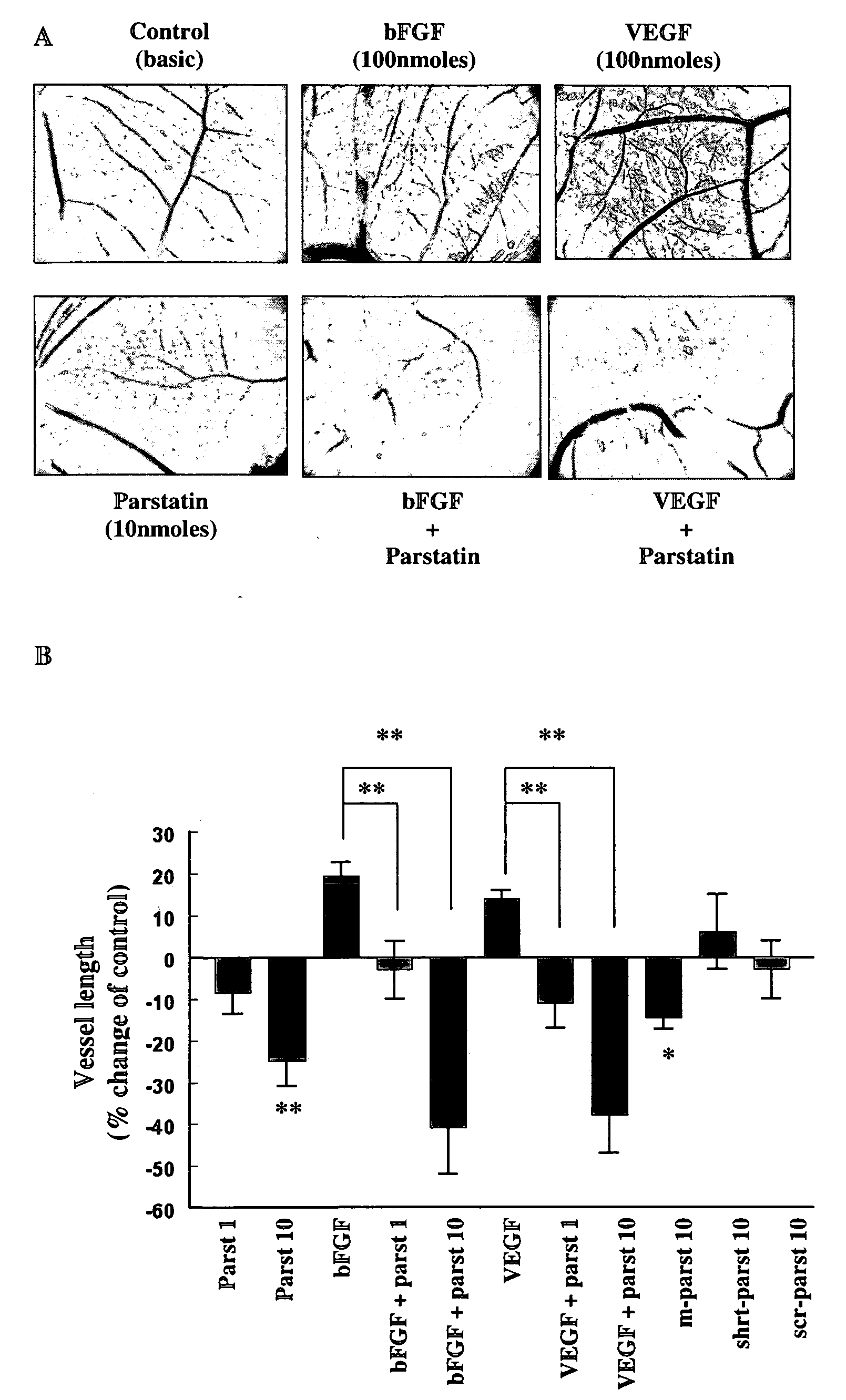
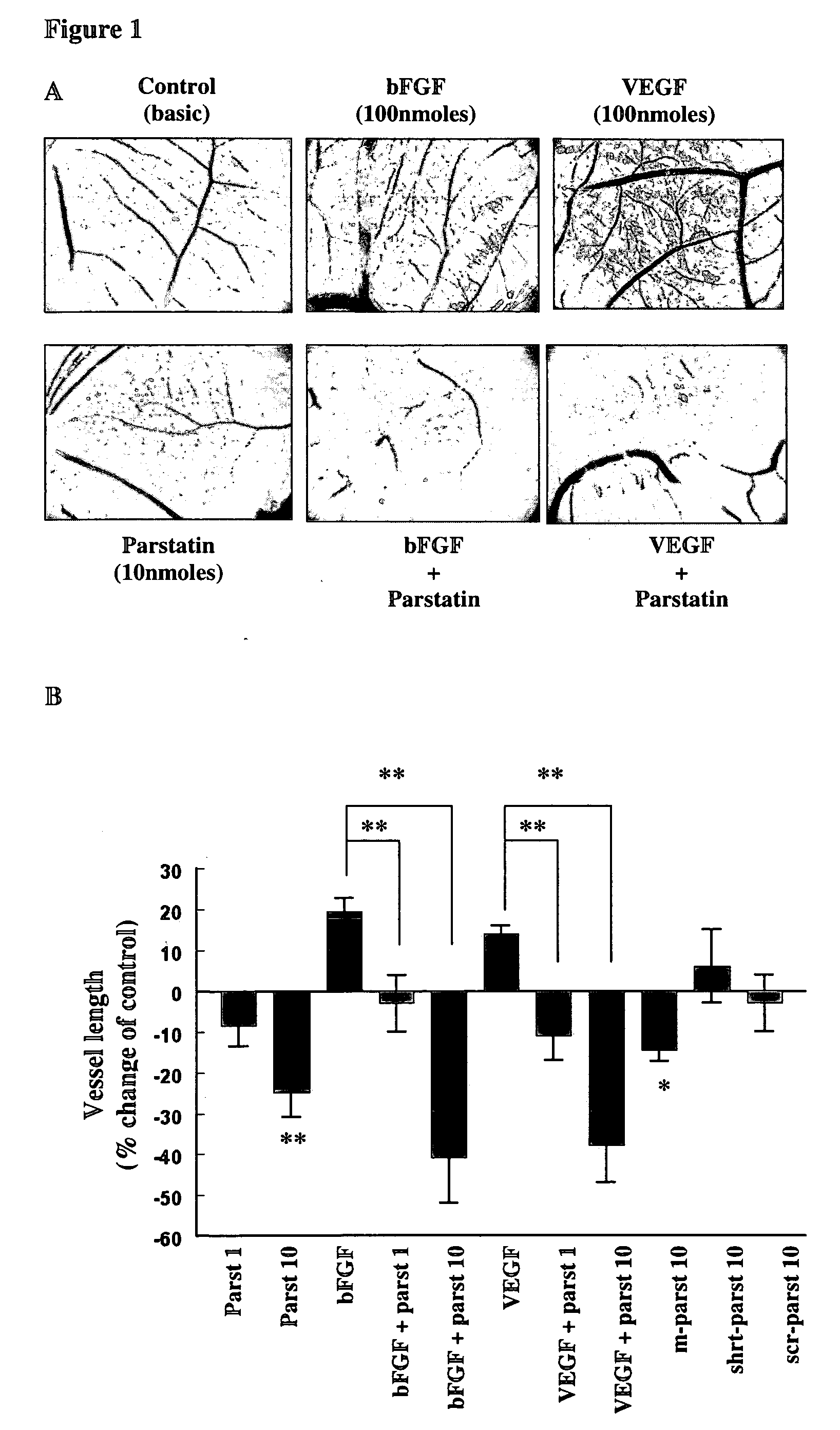
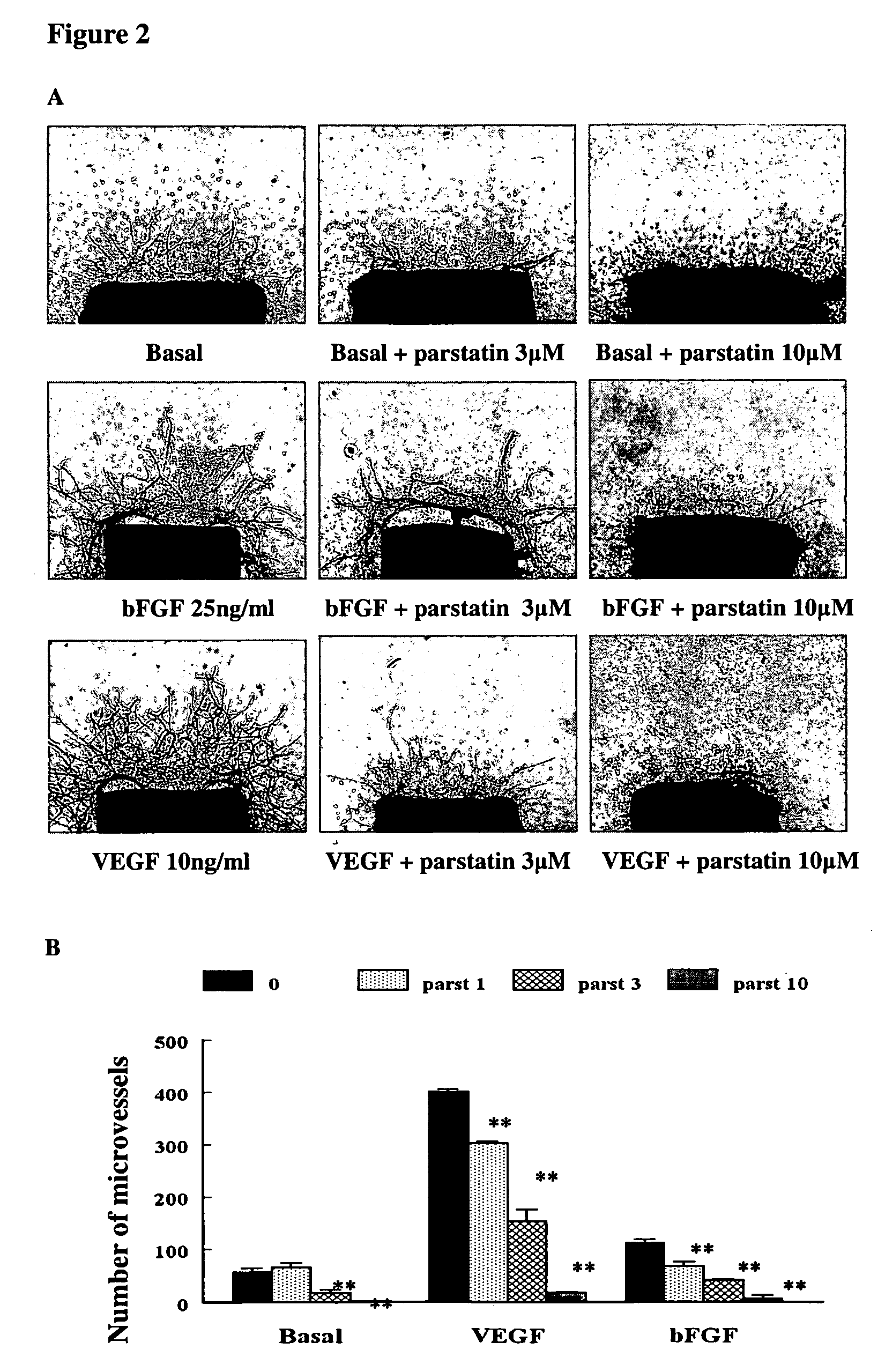
Bioactive parstatin peptides and methods of use
InactiveUS20080242613A1Cell receptors/surface-antigens/surface-determinantsPeptide/protein ingredientsProtein CDisease injury
Bioactive peptides that have a molecular weight of approximately 4.5 kDa and correspond to amino-terminal fragments of protease-activated receptor-1 (PAR-1), which are cleaved and released upon the proteolytic activation of PAR-1 by proteases including, but not limited to, thrombin in humans and animals are disclosed. Such synthetic or recombinantly expressed or endogenously produced or chimeric synthetic peptides that are active in vitro and in vivo and modulate endothelial cell functions and physiological and pathological processes are named herein as parstatin. Parstatin peptides, fragments, analogs, derivatives have the ability to inhibit endothelial cell growth, migration and differentiation, to induce endothelial cell apoptosis, to block angiogenesis and have cardioprotective effects in ischemia / reperfusion injury. Methods for treating angiogenesis-related diseases and endothelium dysfunction-related cardiovascular diseases are disclosed.
Owner:UNIVERSTY OF PATRAS
Artificial Blood Vessel
InactiveUS20080281408A1Not affect activitySimple manufacturing methodStentsCoatingsCollagen VICopolymer
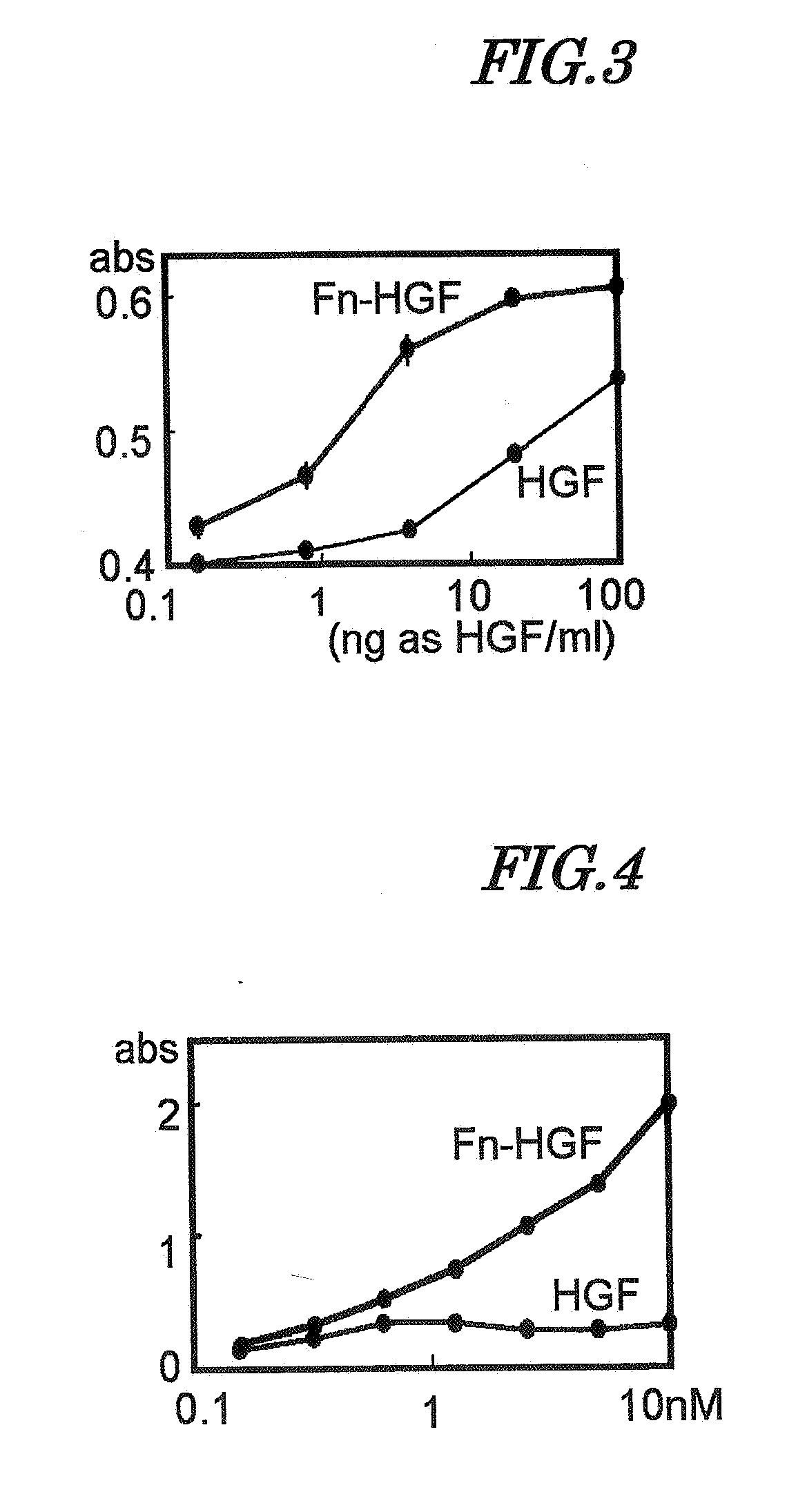
It is an object of the present invention to retain on an artificial blood vessel material an endothelial cell growth-promoting agent, for example the angiogenic factor HGF, without impairing its activity, thereby providing an artificial blood vessel having the function of promoting endothelialization. Such an object can be attained by an artificial blood vessel that includes a porous tubular structure formed from, for example, polytetrafluoroethylene and, layered and immobilized in sequence onto at least the inner surface thereof, (1) a polyamino acid urethane copolymer, (2) collagen or gelatin, and (3) an endothelial cell growth-promoting agent having collagen-binding activity. Preferred examples of the endothelial cell growth-promoting agent include a fusion protein of a polypeptide having collagen-binding activity such as, for example, a fibronectin-derived polypeptide and an angiogenic factor, in particular, HGF.
Owner:NAT UNIV CORP KYUSHU INST OF TECH (JP)
Treatment and prevention of cardiac conditions using two or more isoforms of hepatocyte growth factor
InactiveCN101925362APeptide/protein ingredientsCarbohydrate active ingredientsFactor iiEndothelial NOS
The present invention relates to methods for treating or preventing cardiac conditions in a subject comprising administering to the subject two or more isoforms of hepatocyte growth factor (HGF). The present invention further relates to methods for promoting endothelial cell growth in a blood vessel comprising administering to the blood vessel two or more isoforms of hepatocyte growth factor (HGF). In one embodiment the two or more isoforms of HGF are administered as one or more polynucleotides encoding the isoforms.
Owner:VIROMED CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com