Composition for treating angiogenic diseases using extracellular matrix membrane of cartilage-derived cell, and transplant material for cornea or conjunctiva
A technology of chondrocytes and extracellular matrix, applied in corneal or conjunctival grafts, and in the field of compositions for treating corneal angiogenesis-related diseases, can solve problems such as high production costs and production limitations, and achieve prevention and inhibition of angiogenesis , Inhibition of vascularization and vascular invasion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] Example 1: Preparation of CD-ECM and amniotic membrane
[0065] Chondrocyte-derived extracellular matrix membrane (hereinafter referred to as "CD-ECM") used as a composition for treating corneal angiogenesis-related diseases and as a base material for corneal or conjunctival grafts according to exemplary embodiments of the present invention is a membranous scaffold prepared as follows.
[0066]CD-ECM is a membranous extracellular matrix membrane prepared by collecting and culturing chondrocytes derived from knee cartilage in sterile pigs. The CD-ECM used here is a material currently clinically used in orthopedics for the purpose of cartilage regeneration only. The extracellular matrix secreted during monolayer culture of chondrocytes isolated from patella cartilage in sterile pigs is dried in the form of membranes or sheets, decellularized using enzymes such as DNase I, and then washed with PBS buffer rinsing. The extracellular matrix thus obtained is a biofilm with ...
Embodiment 2
[0070] Example 2: Cell Culture
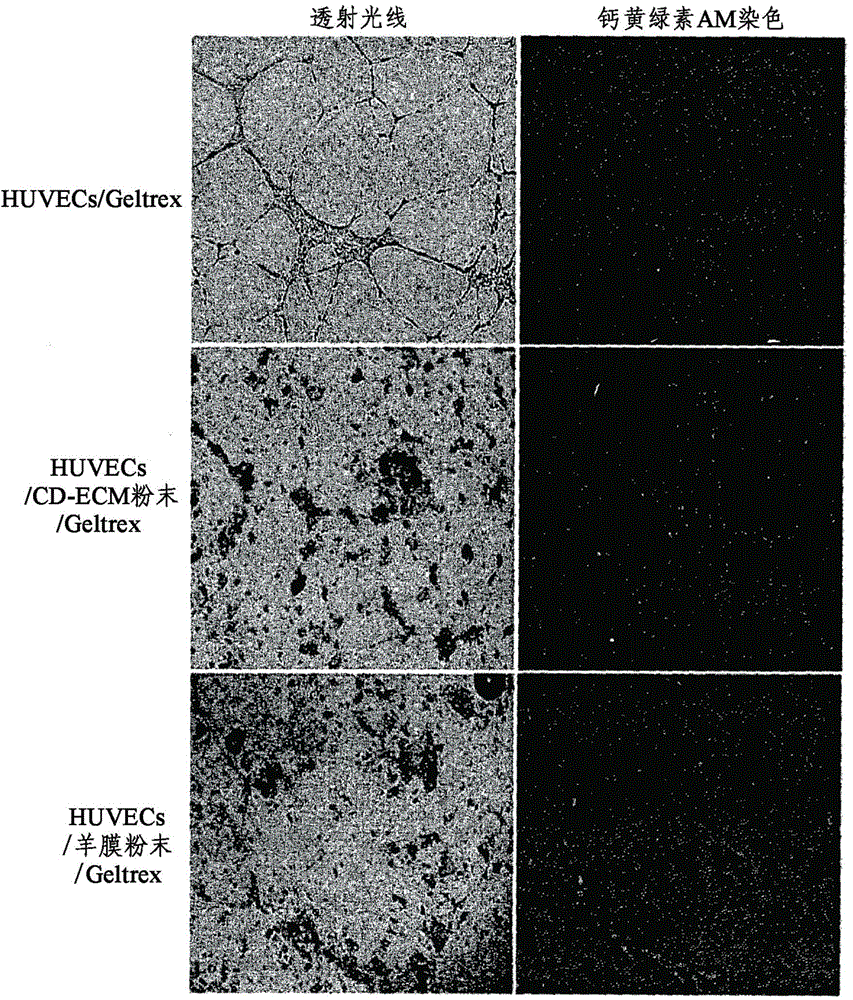
[0071] Vascular endothelial cells and chondrocytes each attached to the CD-ECM of Example 1 and the control group (ie, amniotic membrane) to be used in the in vitro test and the in vivo test were cultured in advance as follows.
[0072] First, human umbilical vein endothelial cells (HUVECs) as vascular endothelial cells were cultured in endothelial cell-containing medium supplemented with growth factors (Promega, Wisconsin, USA), 10 μg / ml bFGF and 2% FBS, and placed in Inoculate 1×10 per plate on the culture plate 6 cells, then, at a temperature of 37 °C in 5% CO 2 cultivated in an incubator. Two days after seeding HUVECs, cells not attached to the culture plate were removed, while cells attached to the culture plate were continued in culture. 14 days after inoculation of human umbilical vein vascular endothelial cells (HUVEC), the human umbilical vein vascular endothelial cells attached to the culture plate were removed by using trypsin, an...
Embodiment 3
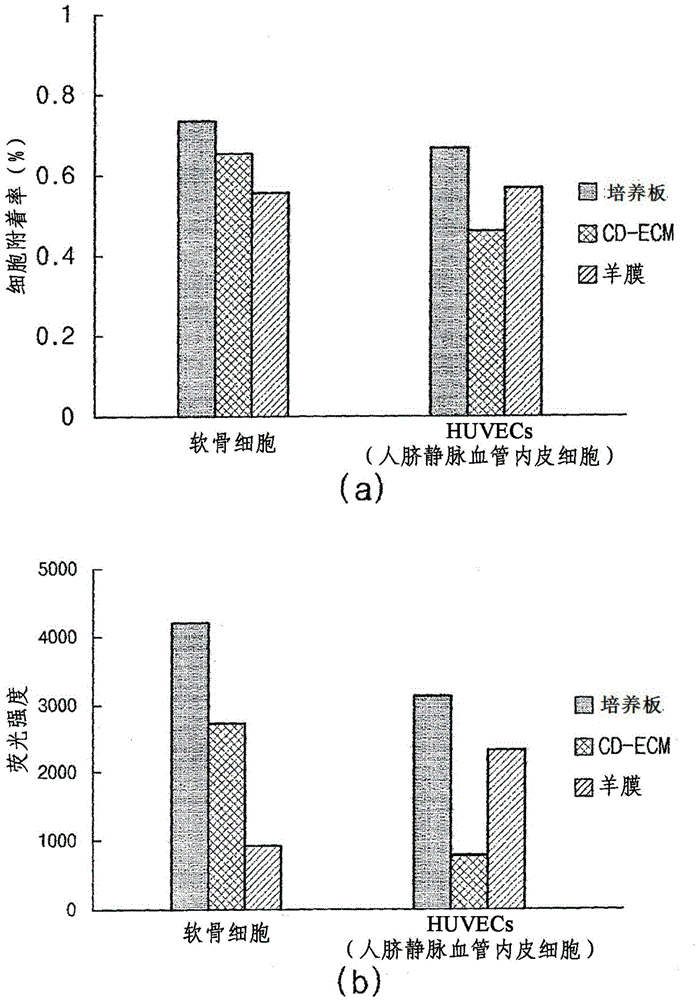
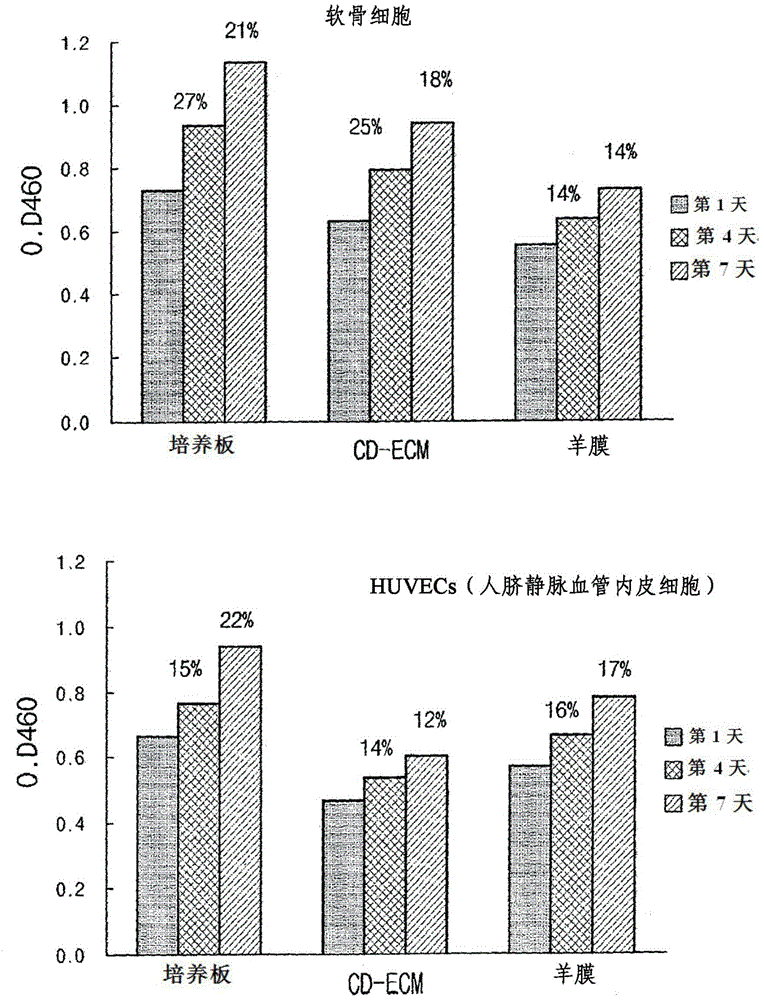
[0074] Example 3: Cell Attachment Analysis of Chondrocytes and Vascular Endothelial Cells (eg, HUVECs)
[0075] Depending on the materials of the CD-ECM of Example 1 and the control group (ie, amnion), the difference in attachment rate between chondrocytes and vascular endothelial cells (eg, HUVEC) was confirmed as follows.
[0076] First, CD-ECM (5 mm in diameter) and amnion were each independently attached to a 24-well dish, dried, and sterilized using ionized gas. Then, the 24-well dishes on which CD-ECM and amnion were independently coated were inoculated with 1×10 3 chondrocytes and 1×10 3 vascular endothelial cells. For fluorescent staining of cells, 40 μl of calcein AM (2 μg / ml) (Invitrogen, Wisconsin, USA) was added thereto, and then, at a temperature of 37° C. in 5% CO 2 cultured in an incubator for 24 hours. Here, a medium supplemented with 10% FBS and DMEM was used for culturing chondrocytes, while a medium supplemented with 10 μg / ml bFGF, 2% FBS, and endothelia...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com