Patents
Literature
1794 results about "ECM Protein" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
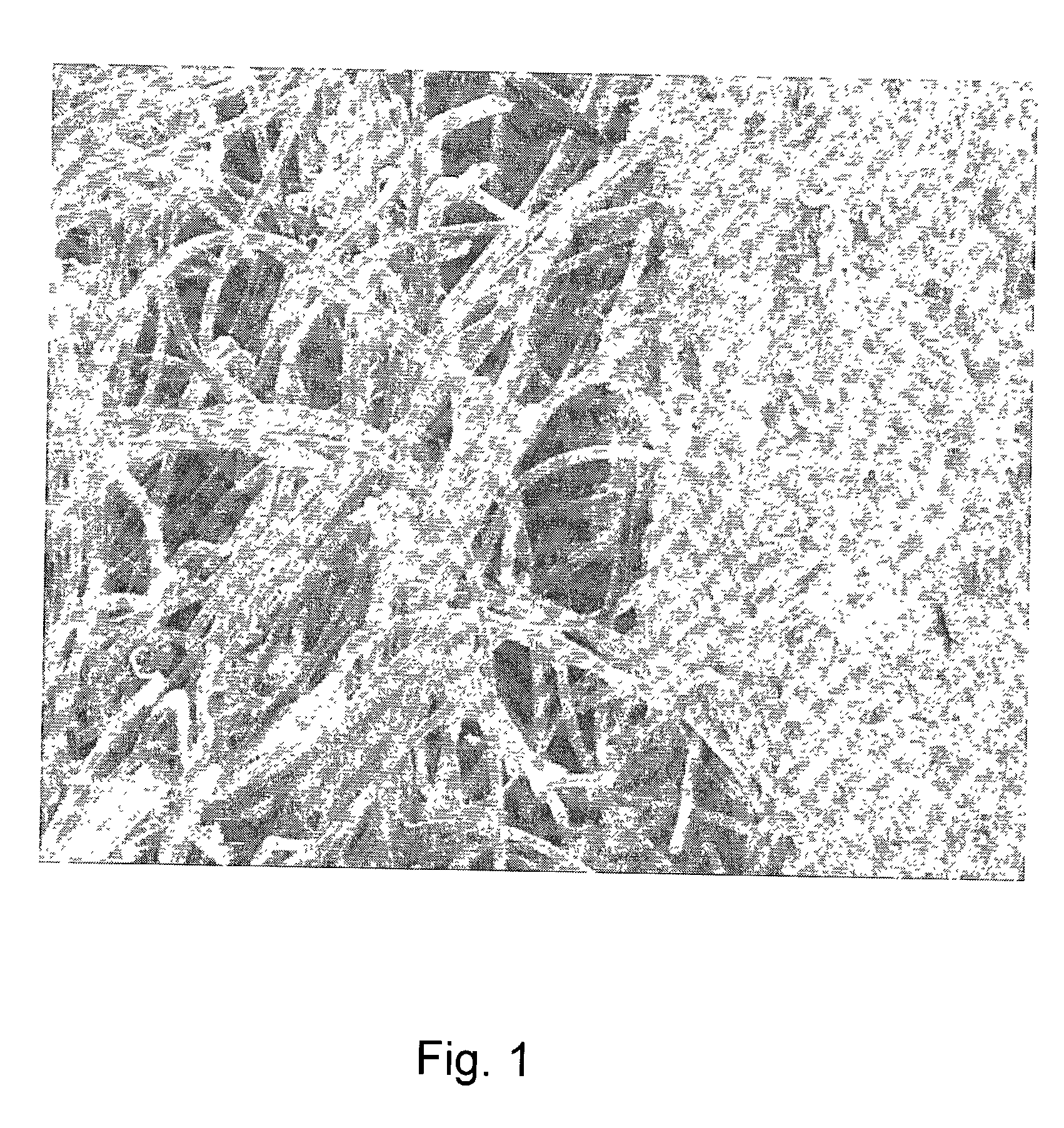
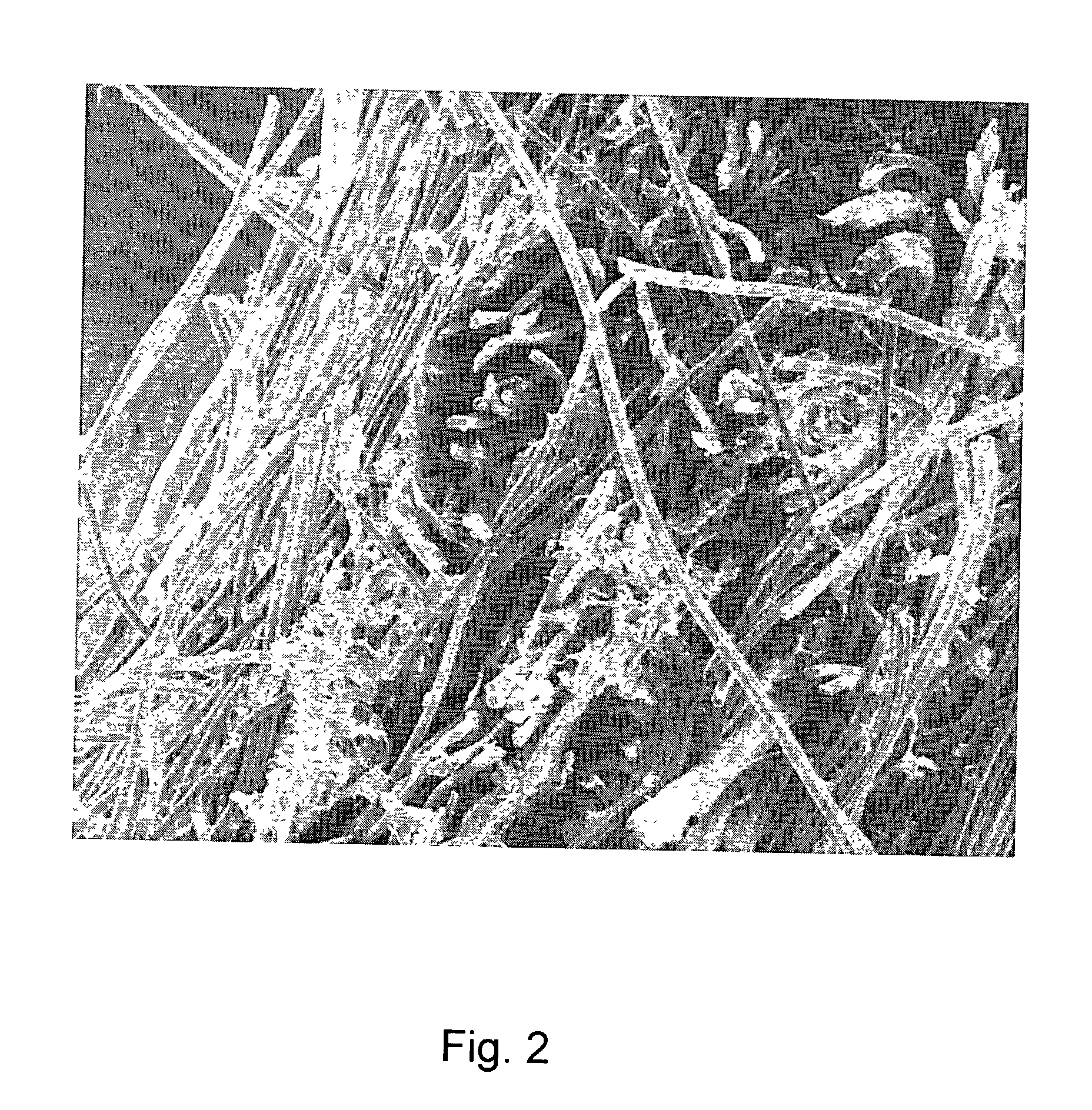
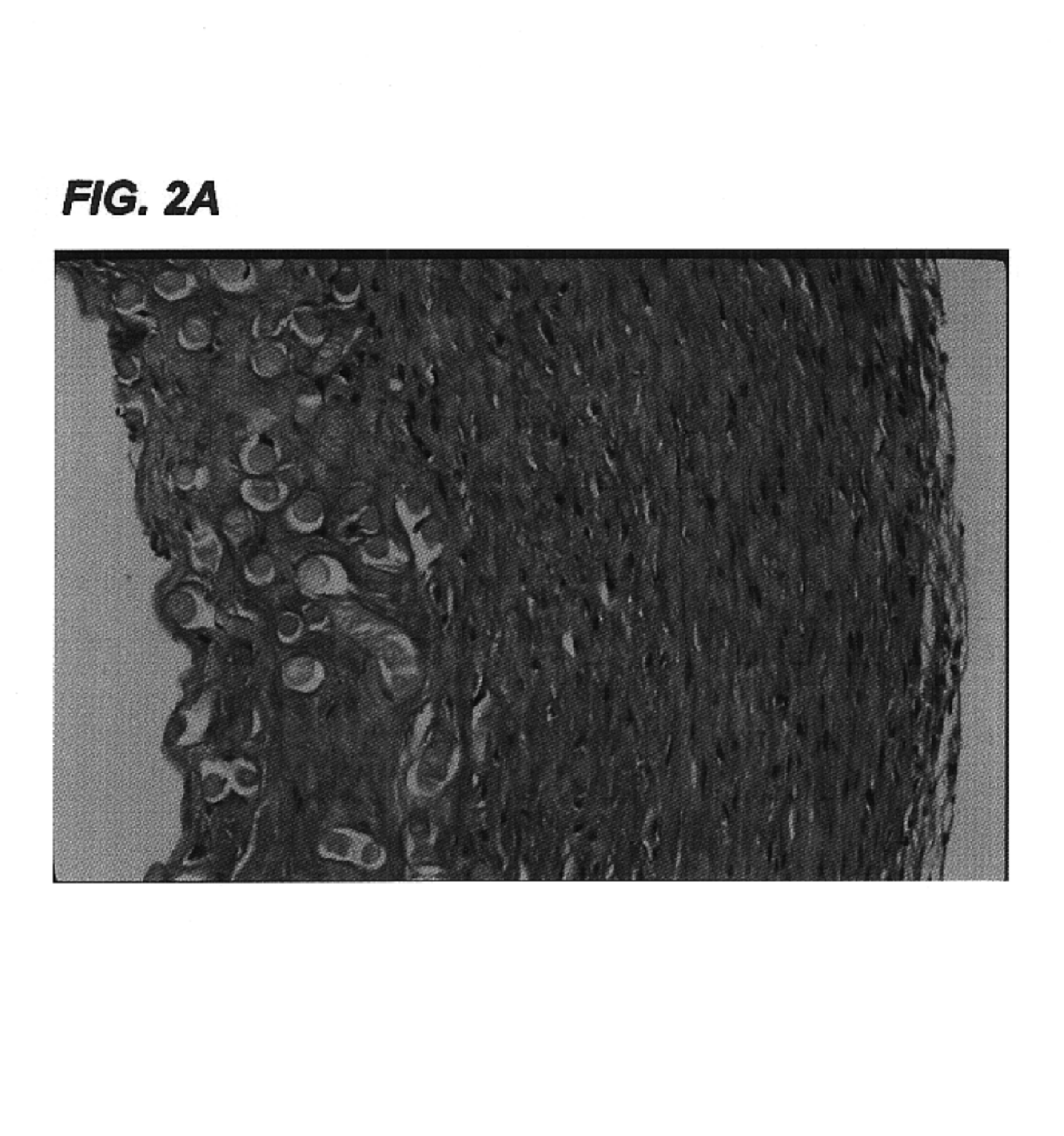
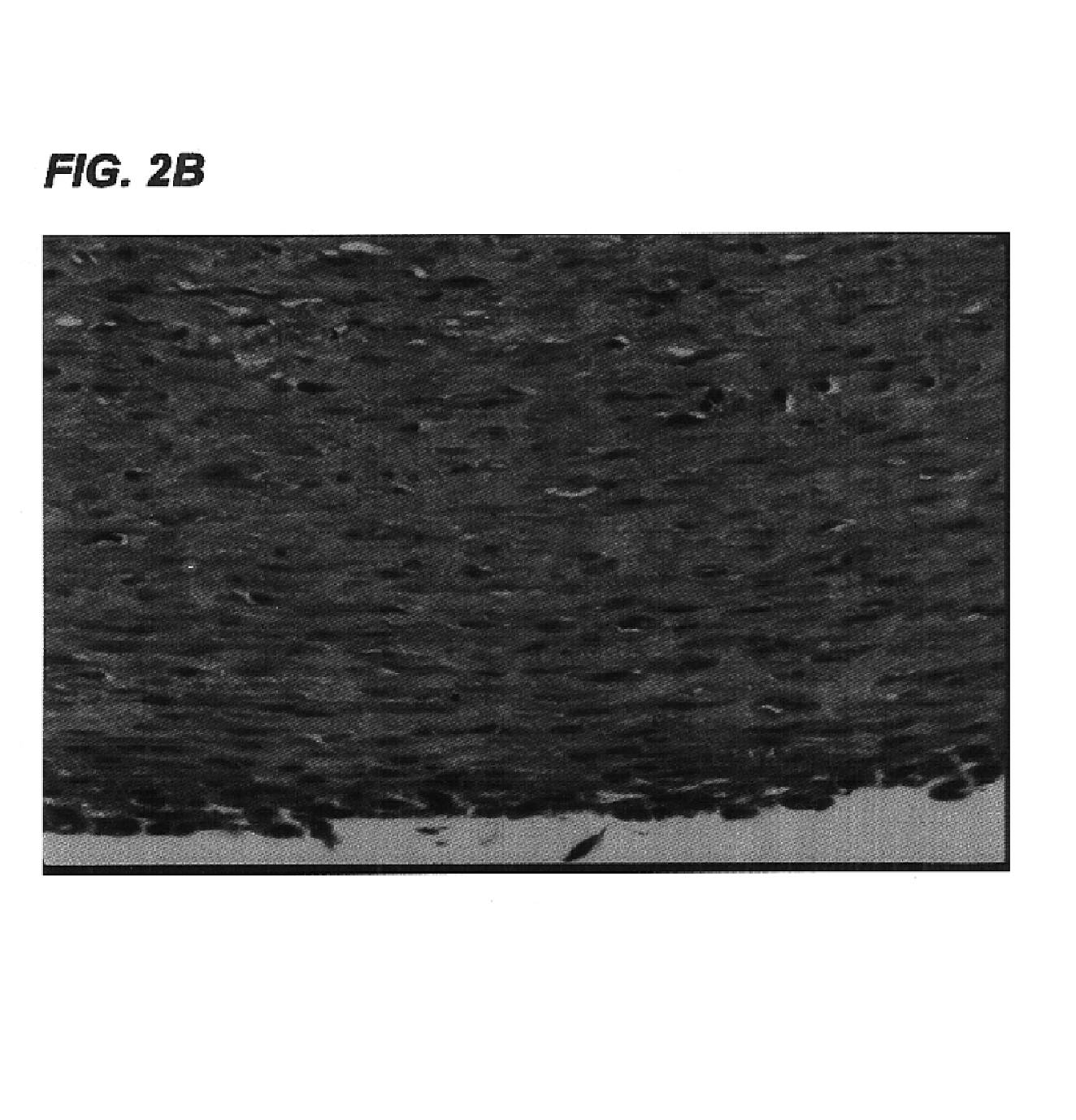
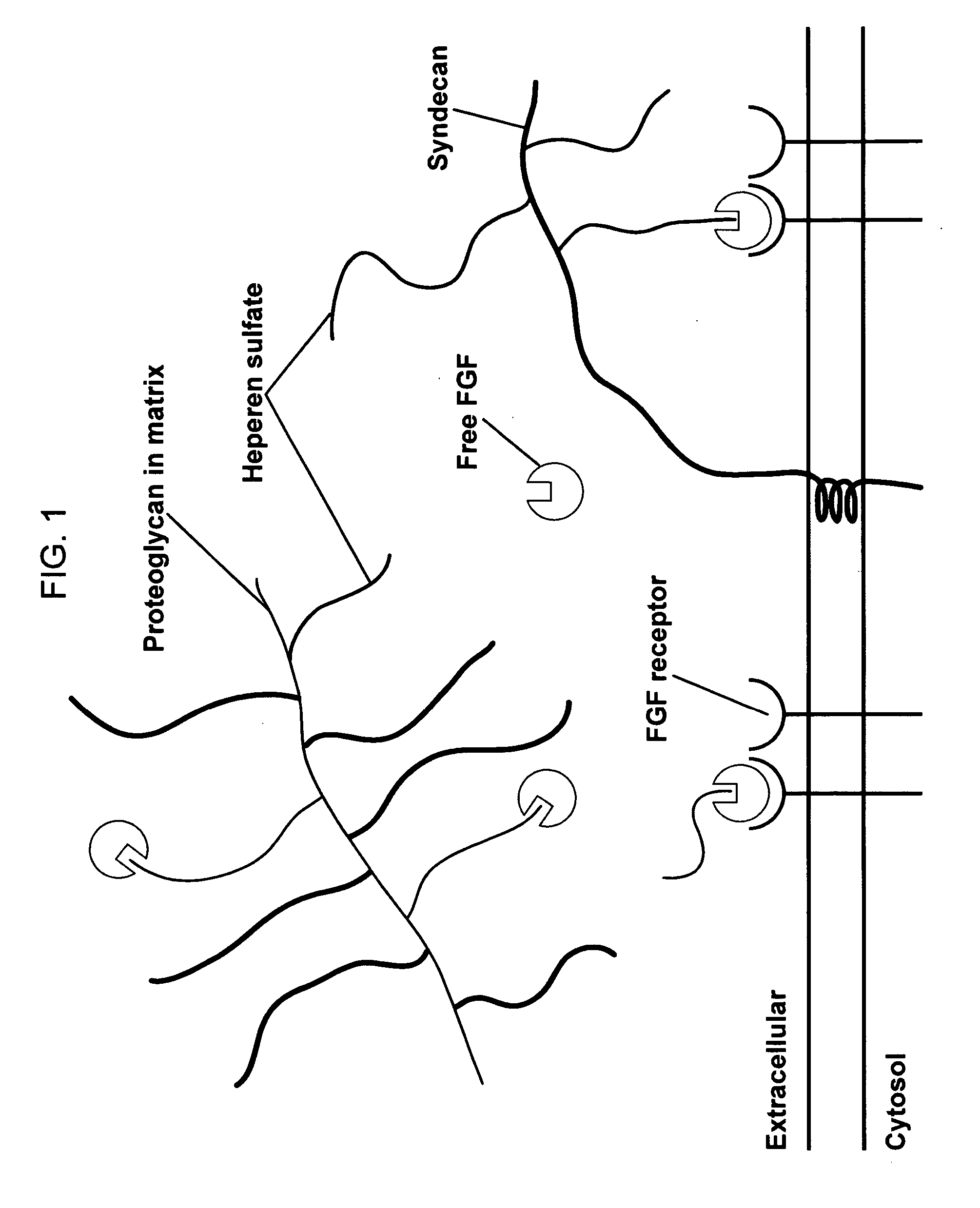
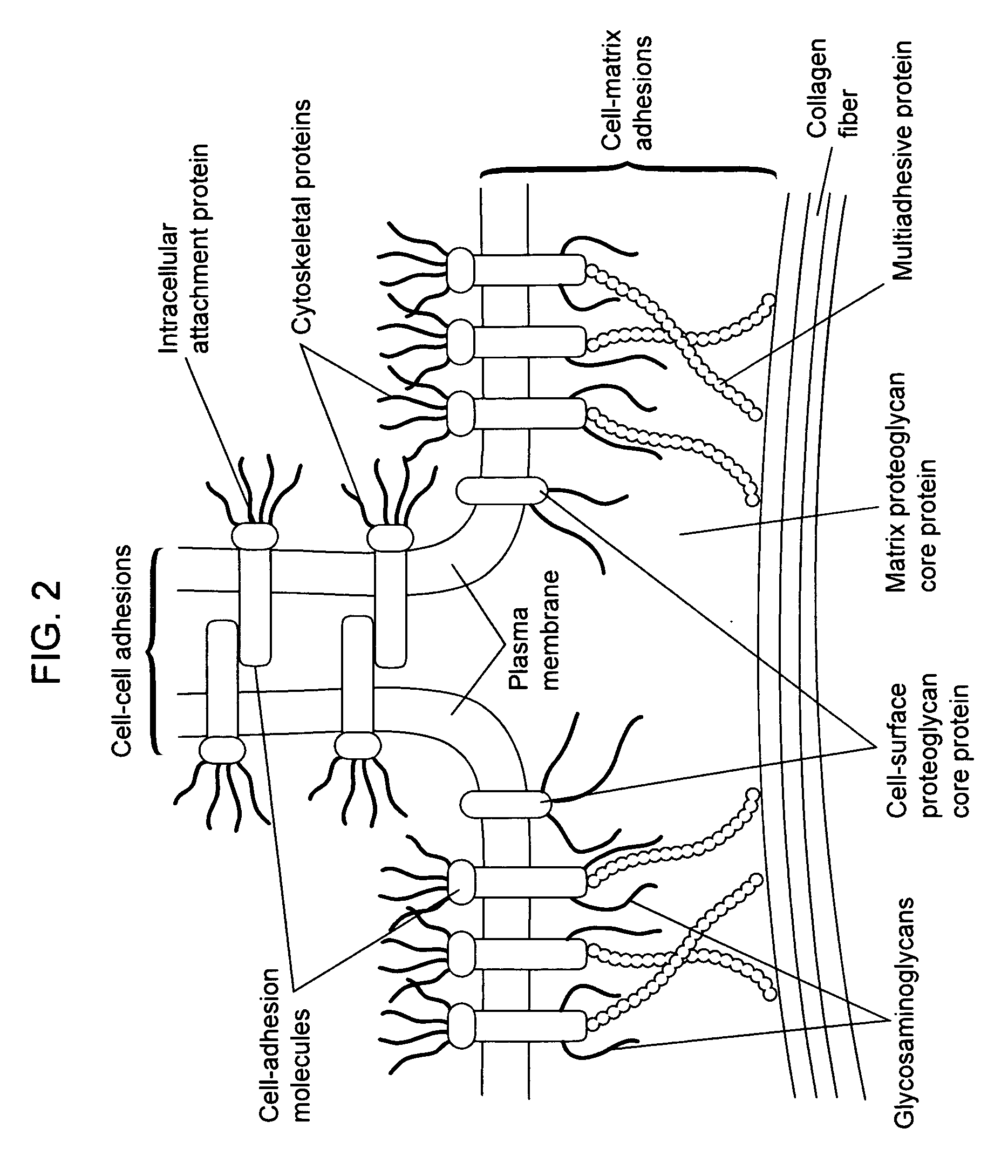
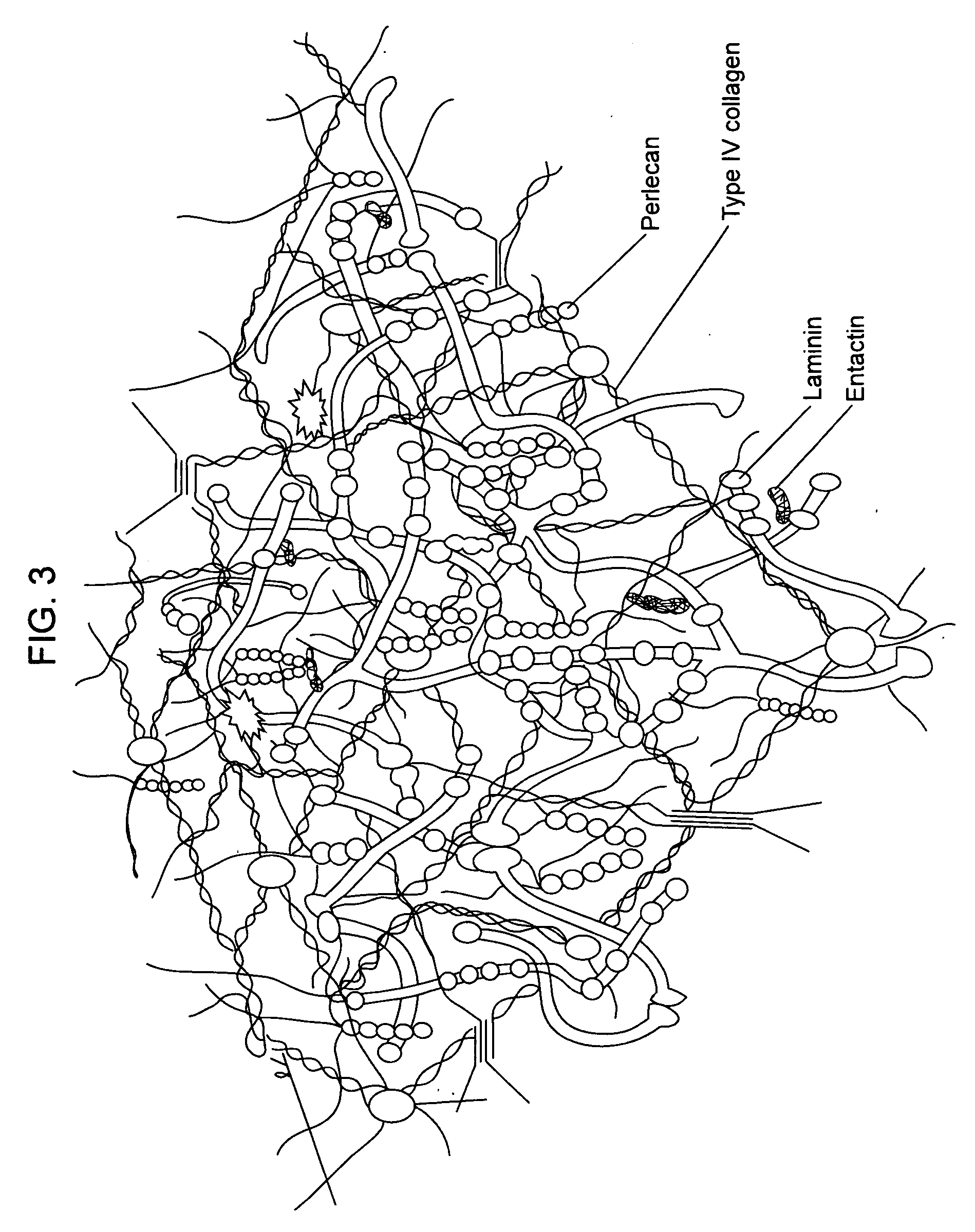
Collagens are the most abundant protein in the ECM. In fact, collagen is the most abundant protein in the human body and accounts for 90% of bone matrix protein content. Collagens are present in the ECM as fibrillar proteins and give structural support to resident cells.
Hybrid biologic-synthetic bioabsorbable scaffolds
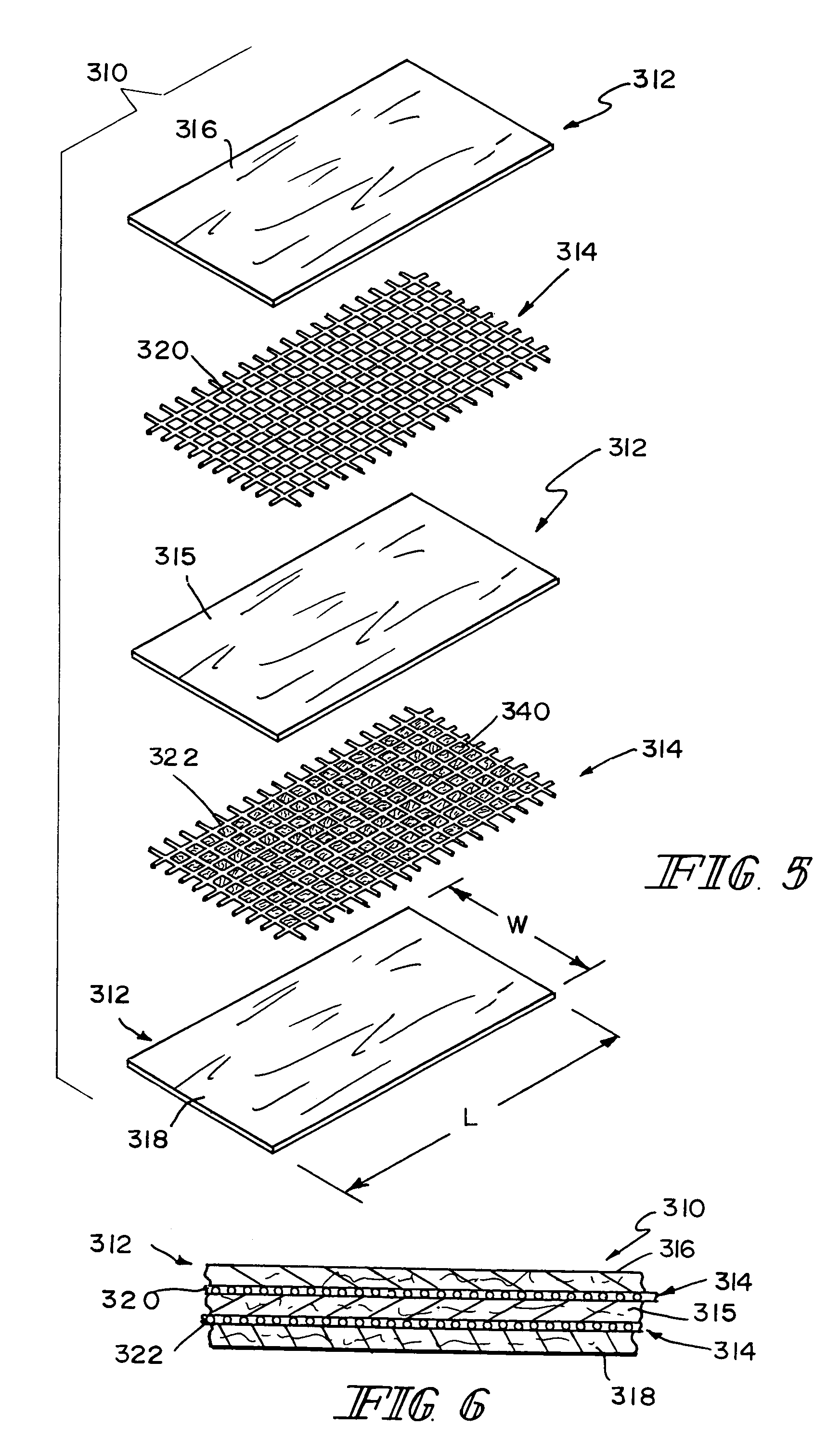
ActiveUS8366787B2Increase surface areaGood mechanical integritySuture equipmentsBone implantBioabsorbable scaffoldCell-Extracellular Matrix
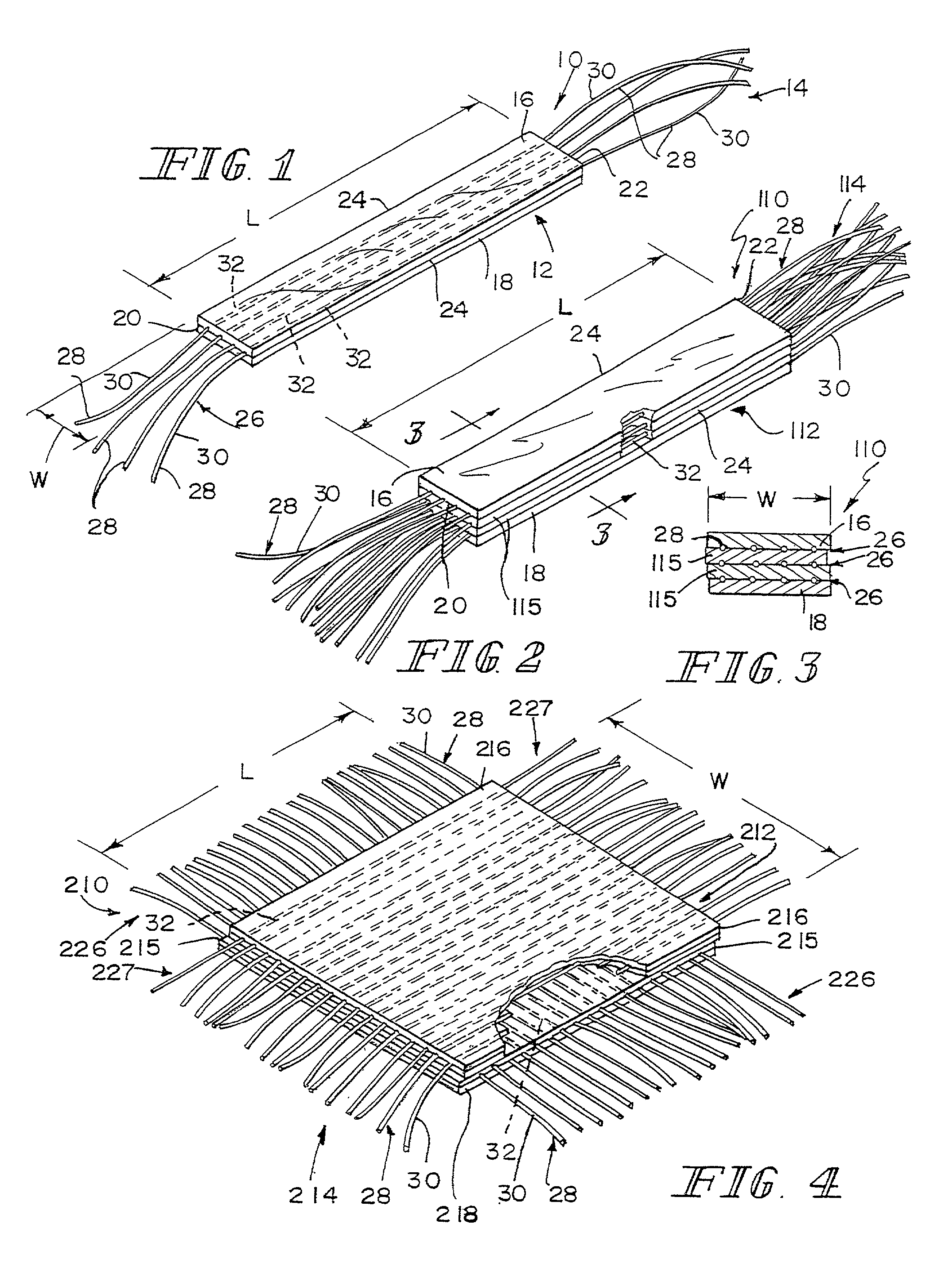
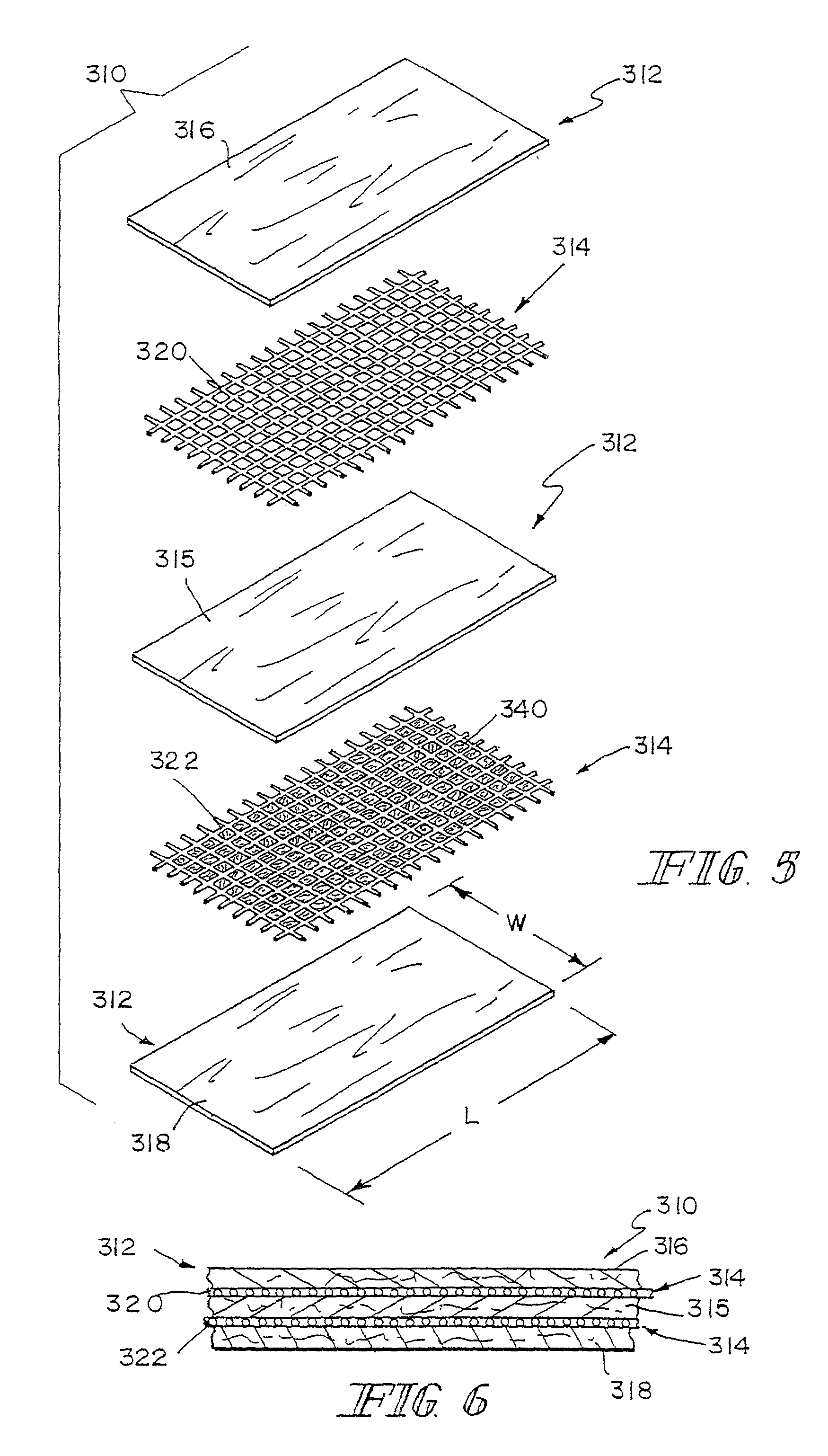
A bioprosthetic device is provided for soft tissue attachment, reinforcement, and or reconstruction. The device comprises a naturally occurring extracellular matrix portion and a three-dimensional synthetic portion. In illustrated embodiments, the naturally occurring extracellular matrix portion comprises layers of small intestine submucosa, and the three-dimensional synthetic portion comprises a foam or a three-dimensional mesh, textile, or felt.
Owner:DEPUY SYNTHES PROD INC
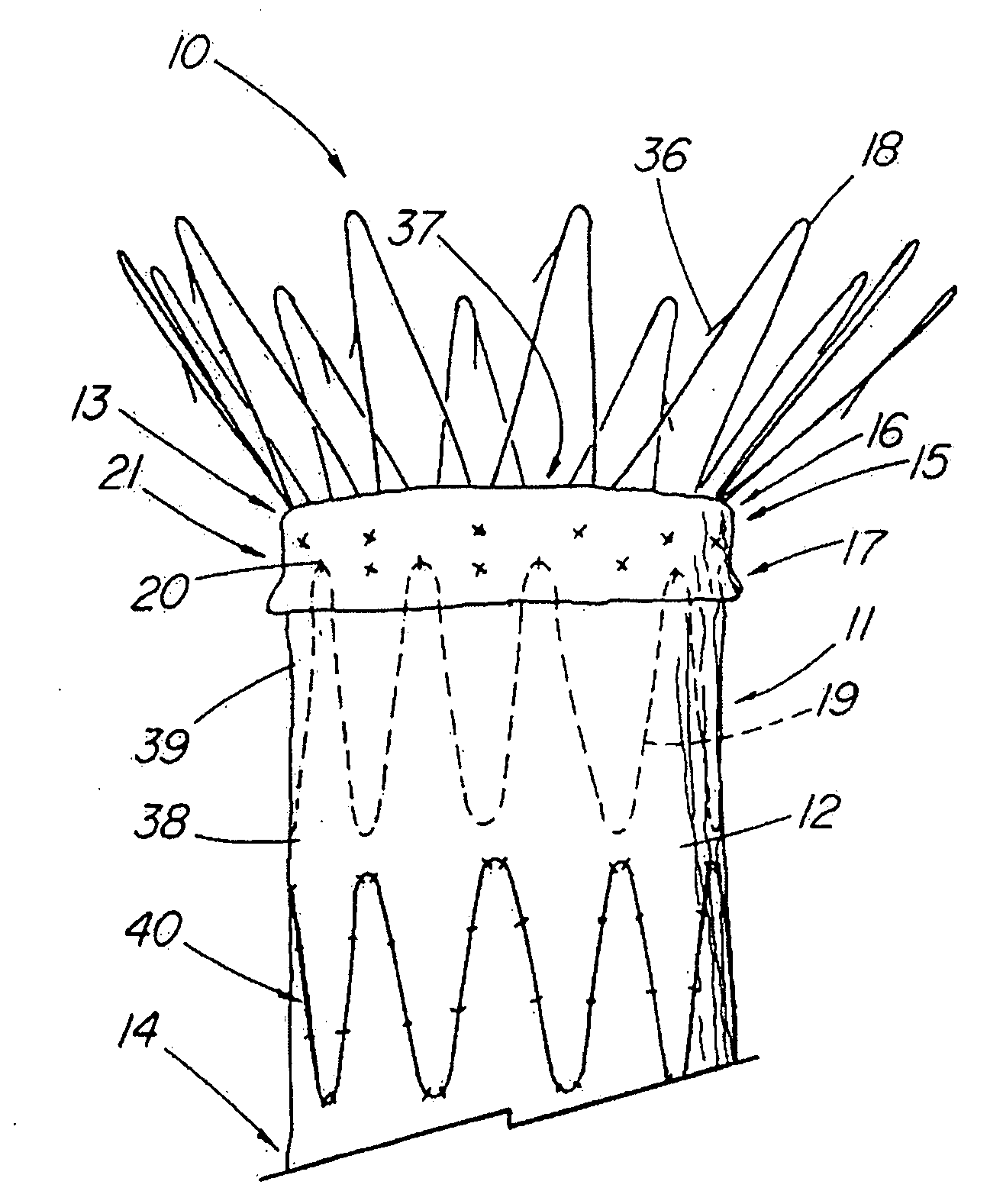
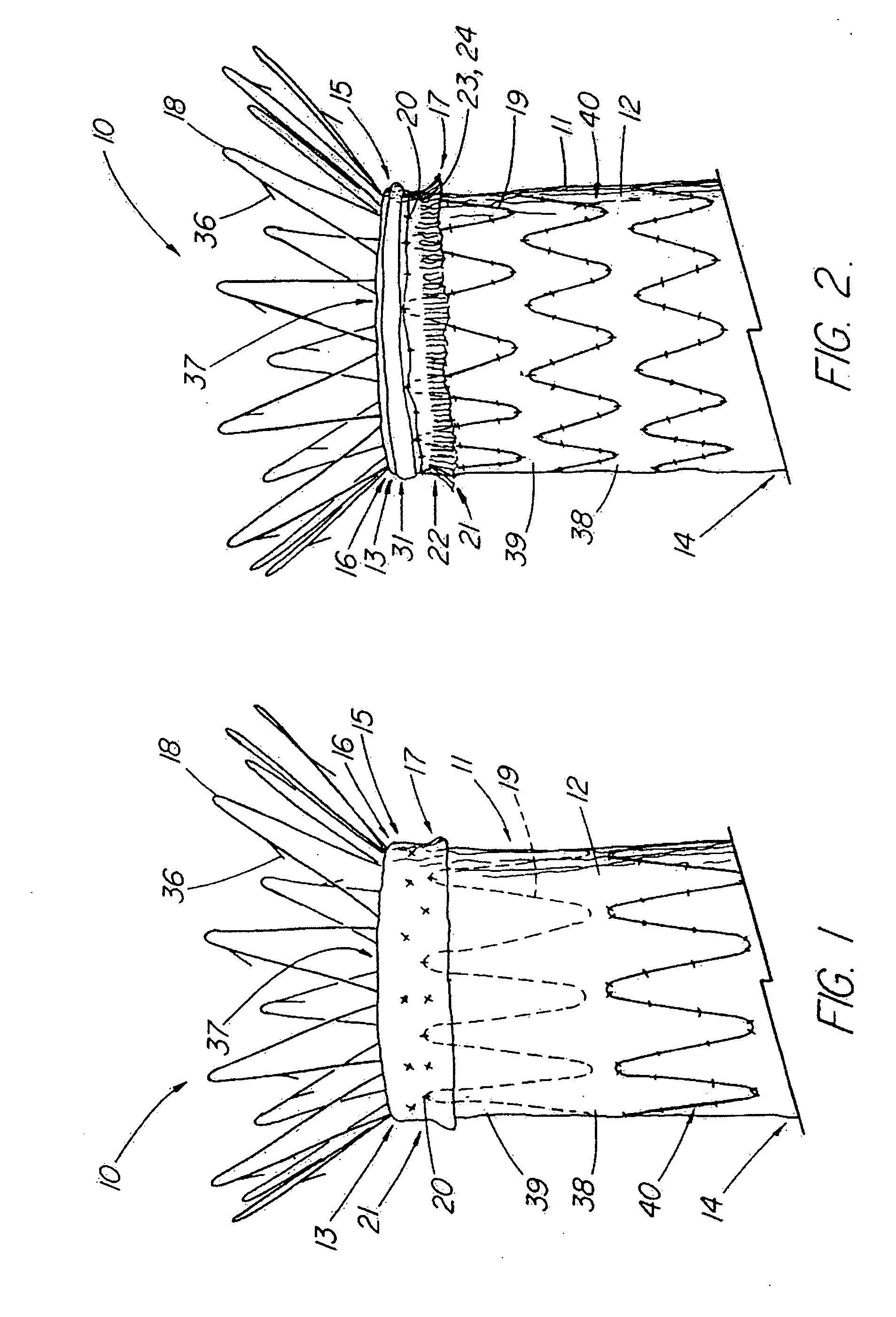
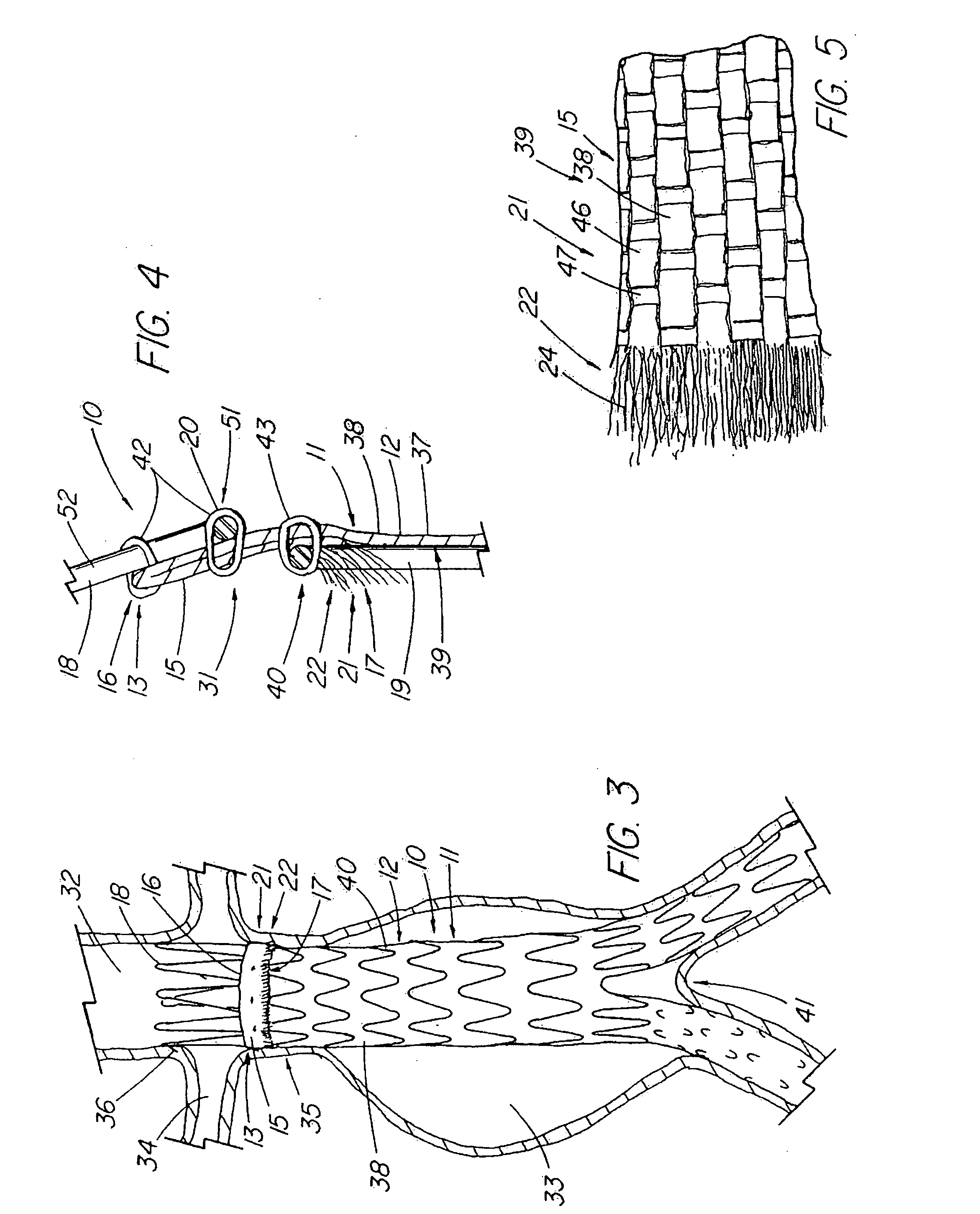
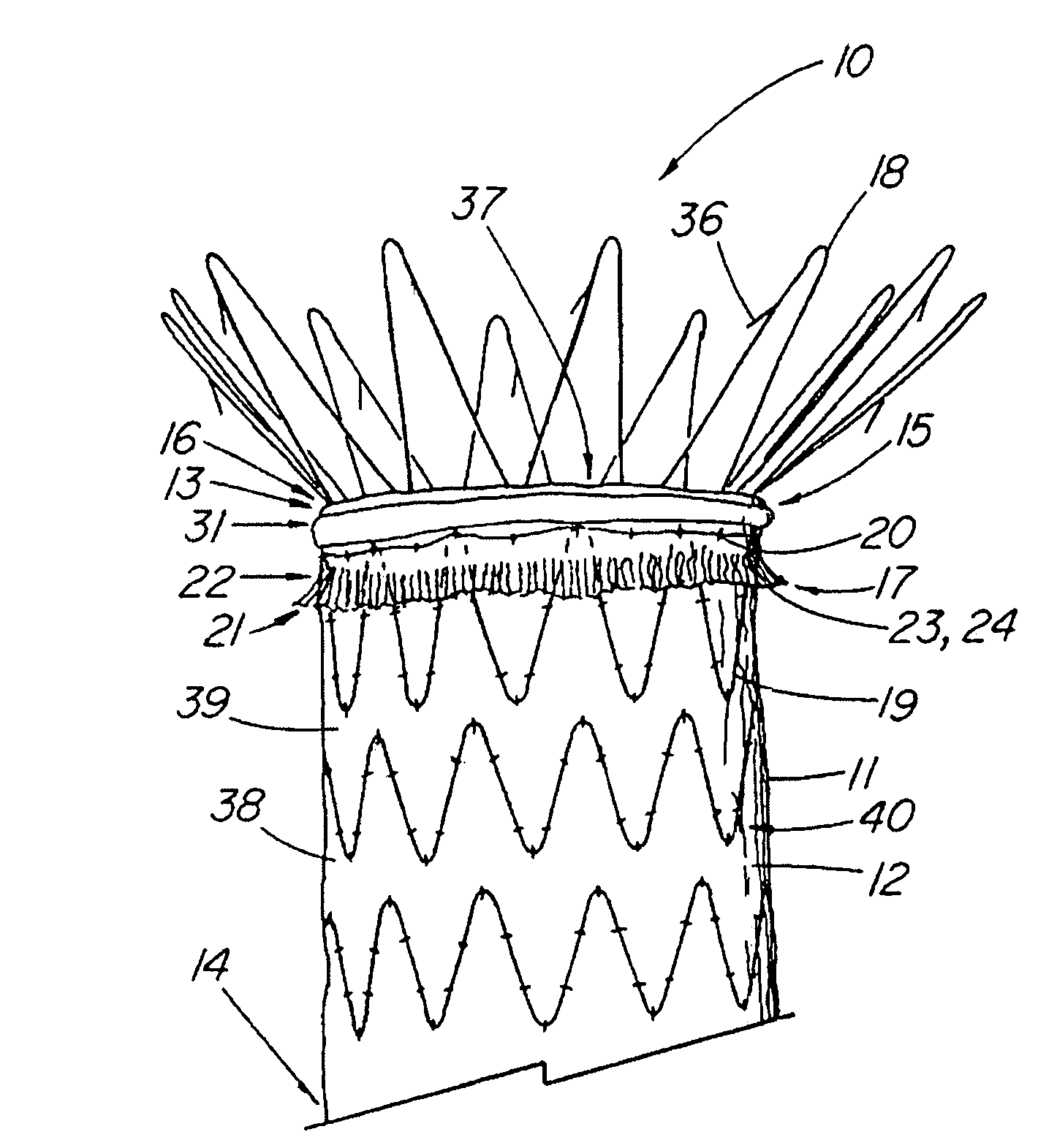
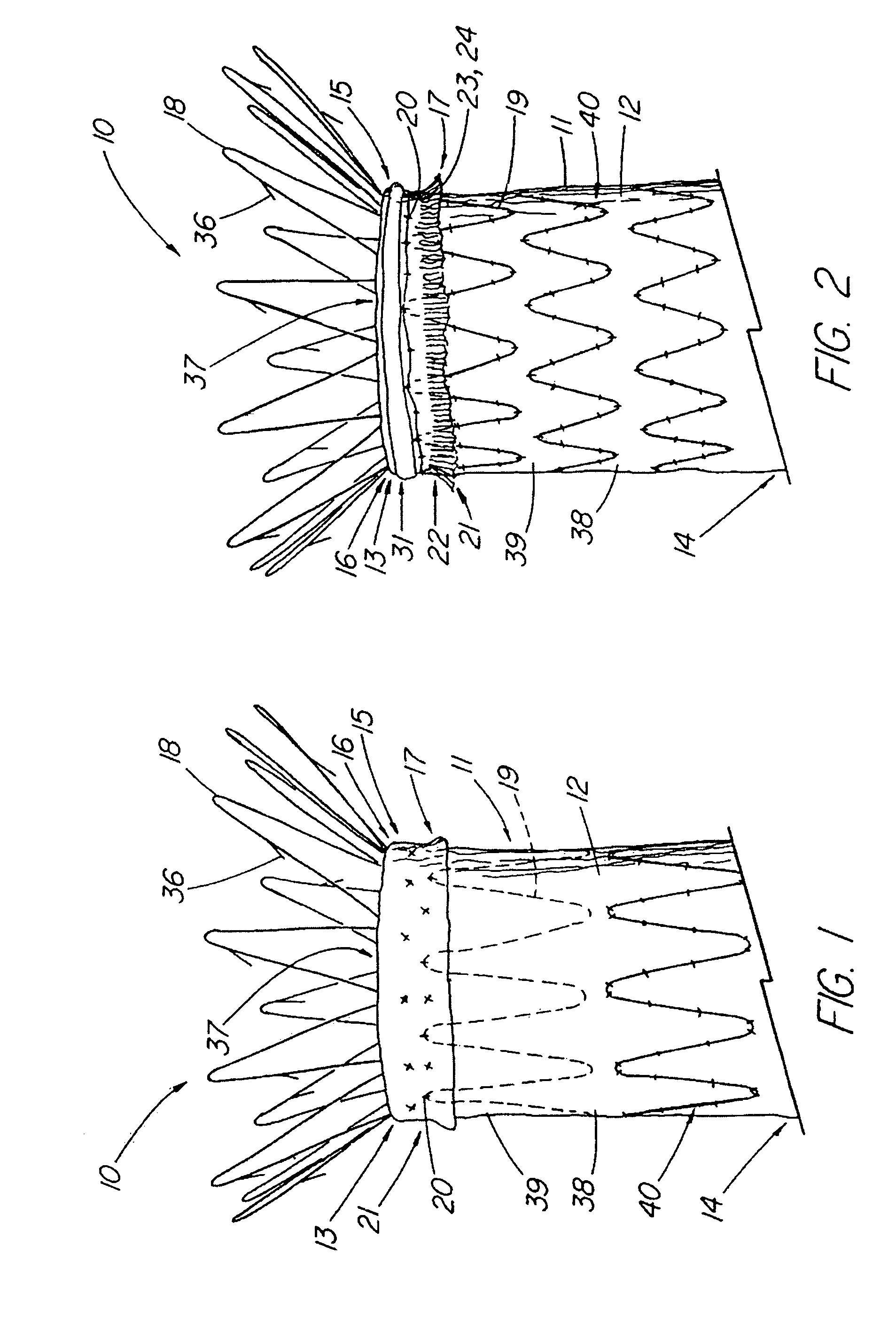
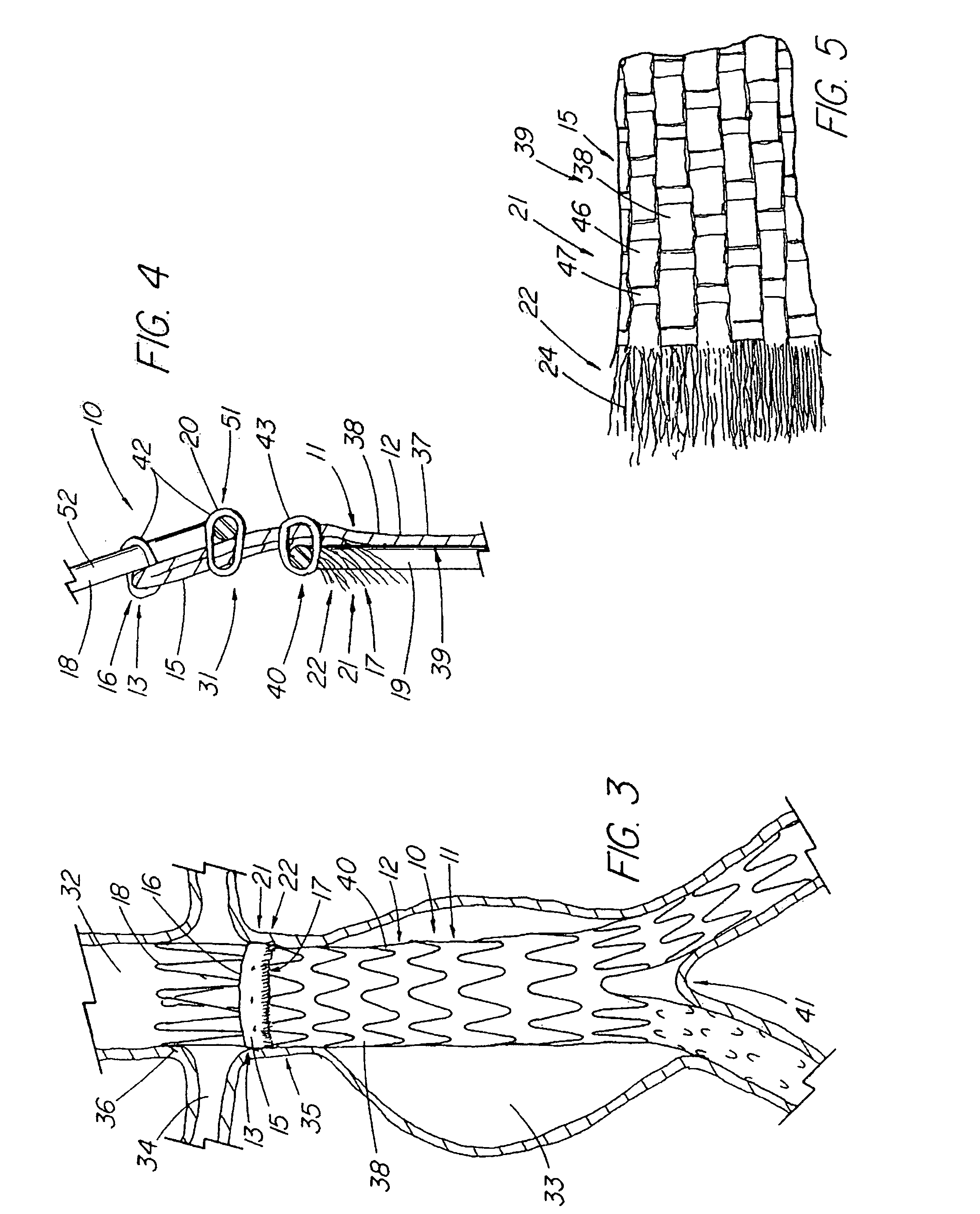
Unitary surgical device and method
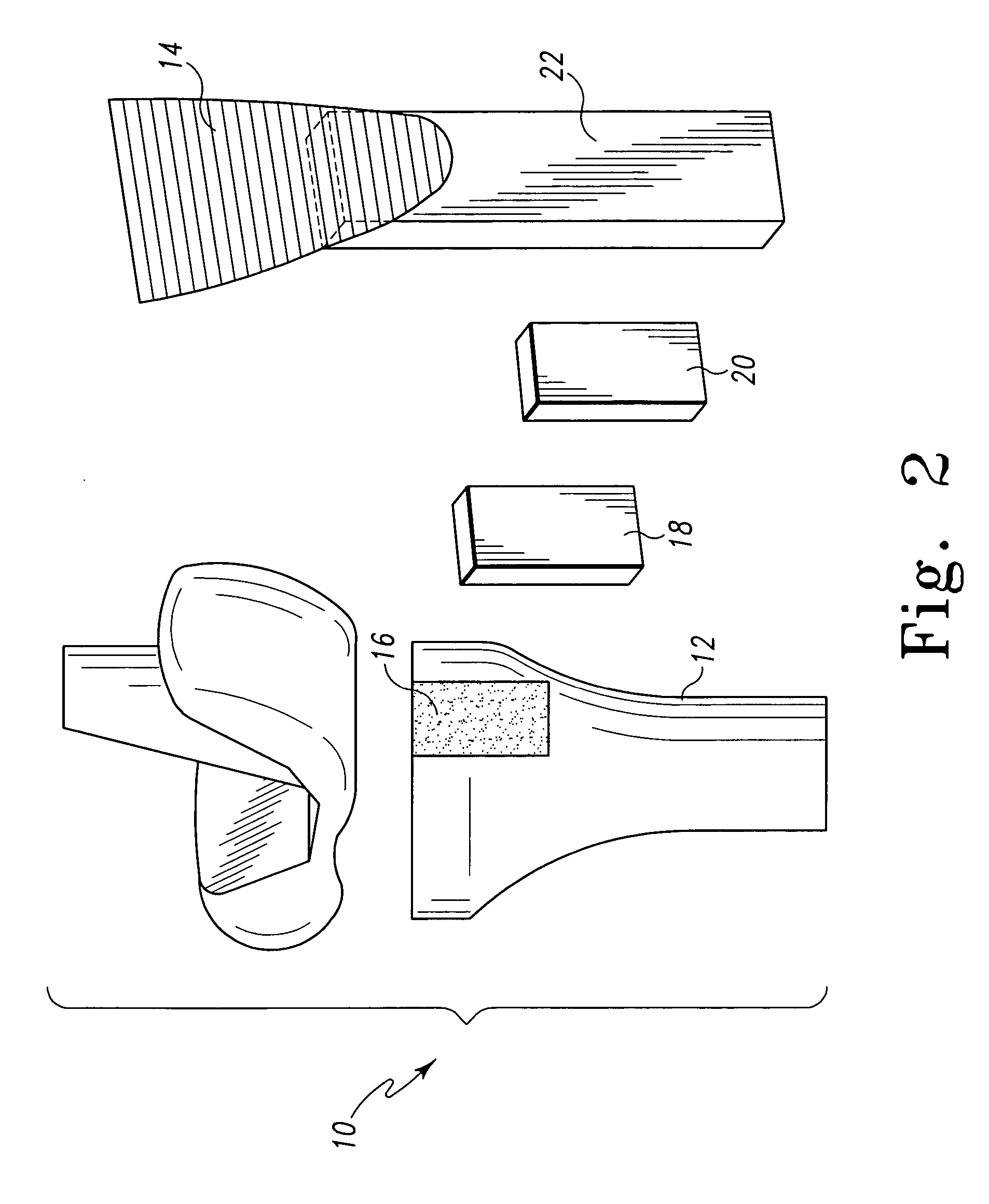
Unitary surgical devices (10) are disclosed. One group of the illustrated devices has a pair of biocompatible, bioresorbable anchors (16, 18) connected to fixed lengths suture. The anchors (16, 18) and fixed length of suture are connected to each other prior to surgery. Another group of unitary surgical devices has a pair of fixating mechanisms (15, 17) connected to a base (21) prior to surgery. The second group of illustrated devices generally includes extracellular matrix material either as part of the base (21) or supported on the base (21). The extracellular matrix material serves as tissue regenerating material. In the second group of unitary surgical devices, the fixating mechanisms illustrated generally comprise suture, anchors or pre-formed holes in the base. All of the illustrated unitary surgical devices are useful in repairing a damaged meniscus. The first group of unitary surgical devices can be used to approximate inner surfaces of a tear in the meniscus. The second group of devices can be used either as an insert to be placed between and approximated to the inner surfaces of the tear or as an insert to replace a void in the meniscus left after a menisectomy.
Owner:DEPUY PROD INC
Reinforced small intestinal submucosa
InactiveUS7160333B2Improve mechanical propertiesEnhanced handling propertyBone implantSurgeryCell-Extracellular MatrixSmall intestine
Bioprosthetic devices for soft tissue attachment, reinforcement, or construction are provided. The devices comprise a sheet of naturally occurring extracellular matrix and a sheet of synthetic mesh coupled to the naturally occurring extracellular matrix portion.
Owner:DEPUY SYNTHES PROD INC
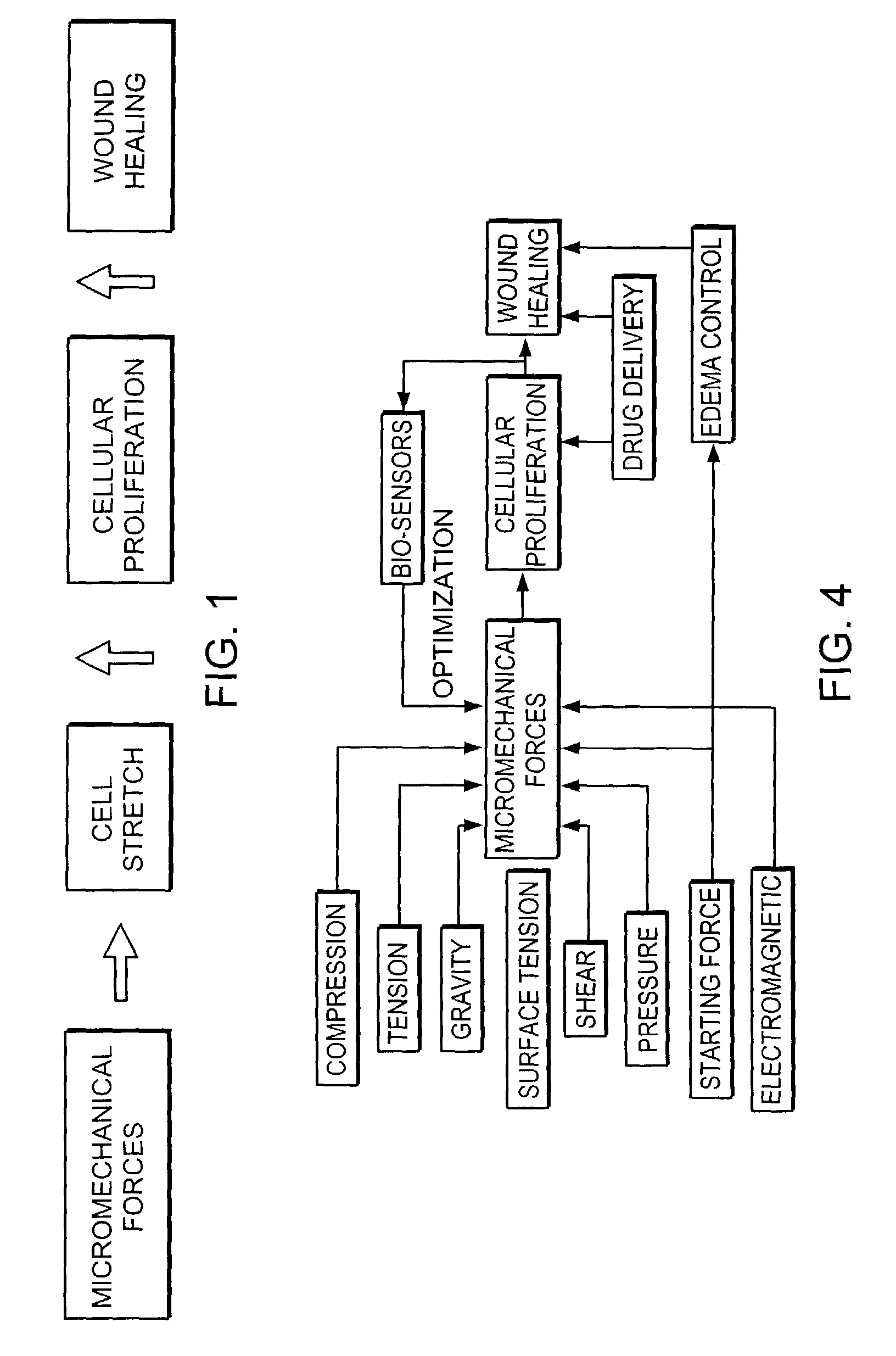
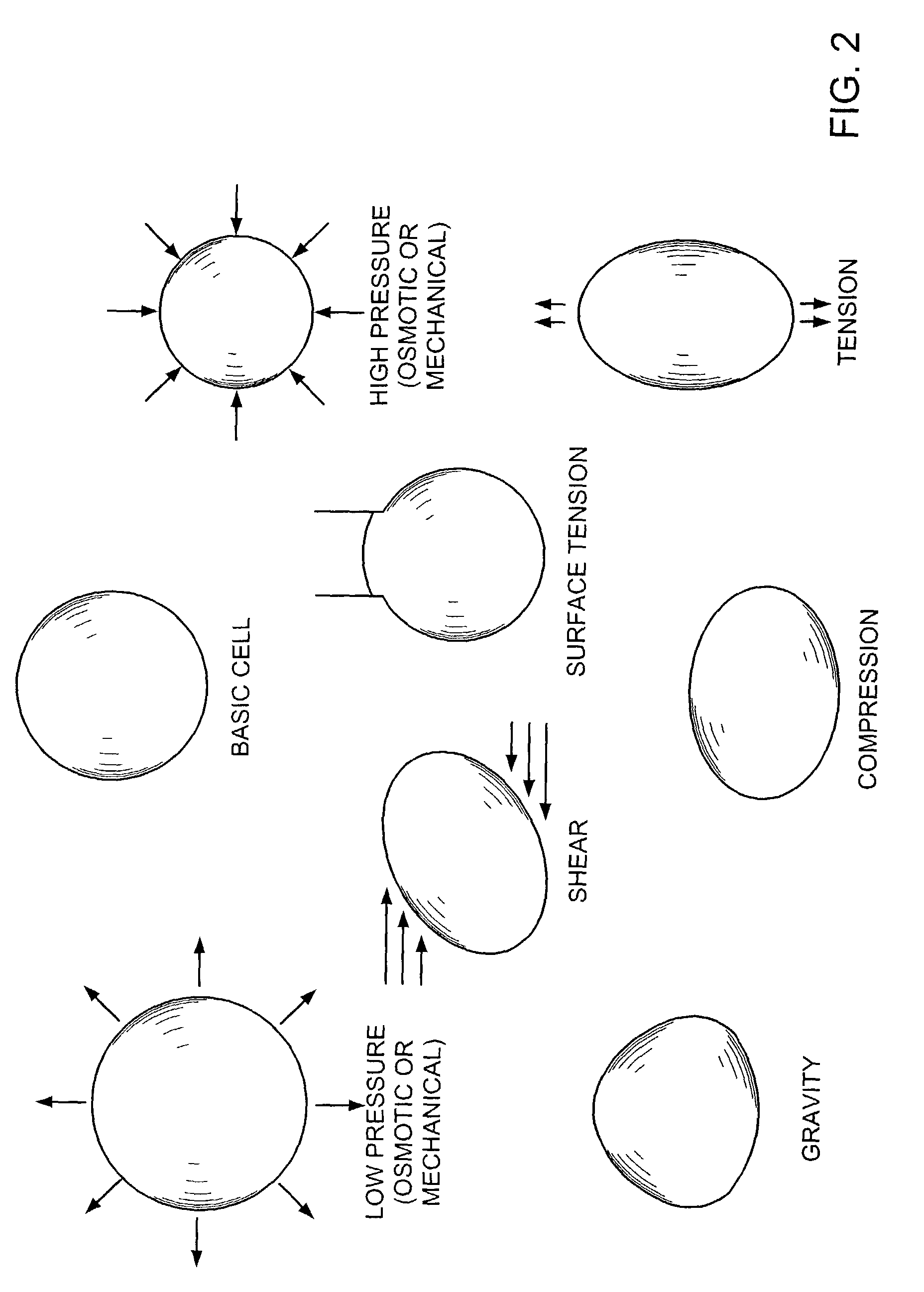
Methods and apparatus for application of micro-mechanical forces to tissues
InactiveUS7494482B2Accelerate tissue ingrowthEnhancing tissue repairNon-adhesive dressingsBone implantMicron scaleCell-Extracellular Matrix
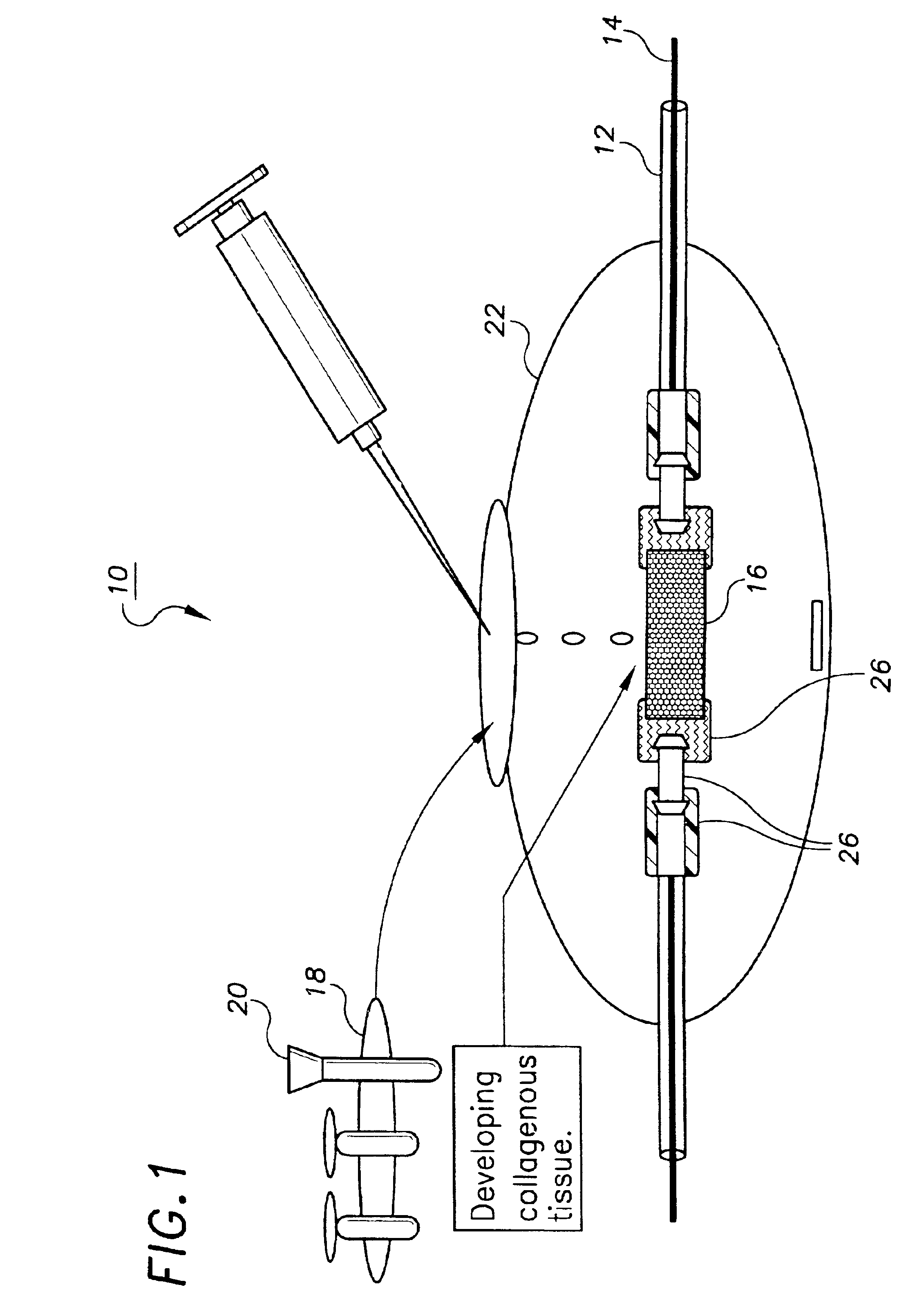
Methods and devices for transmitting micromechanical forces locally to induce surface convolutions into tissues on the millimeter to micron scale for promoting wound healing are presented. These convolutions induce a moderate stretching of individual cells, stimulating cellular proliferation and elaboration of natural growth factors without increasing the size of the wound. Micromechanical forces can be applied directly to tissue, through biomolecules or the extracellular matrix. This invention can be used with biosensors, biodegradable materials and drug delivery systems. This invention will also be useful in pre-conditioned tissue-engineering constructs in vitro. Application of this invention will shorten healing times for wounds and reduce the need for invasive surgery.
Owner:MASSACHUSETTS INST OF TECH +2
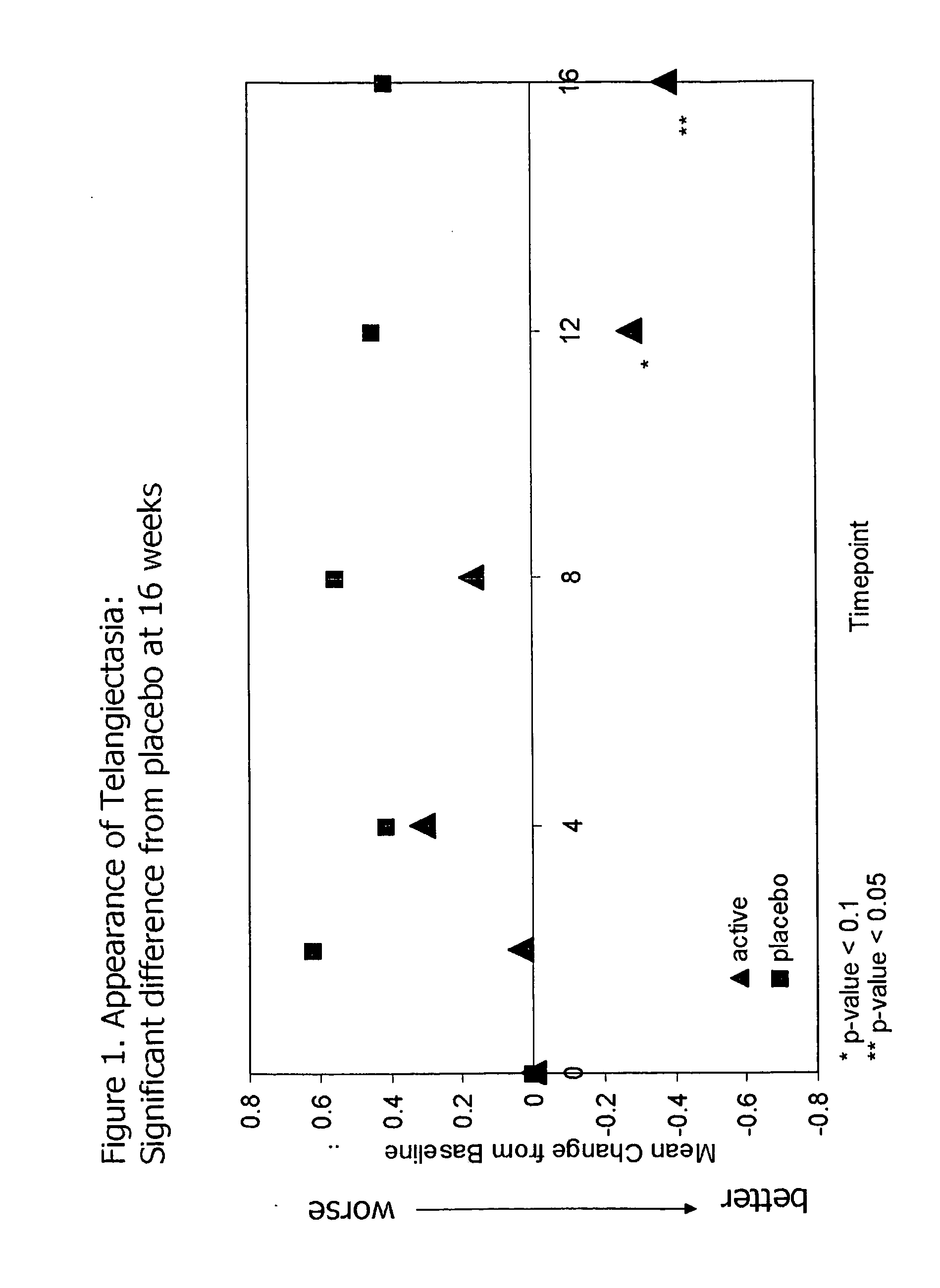
Use of matrix metalloproteinase inhibitors in skin care
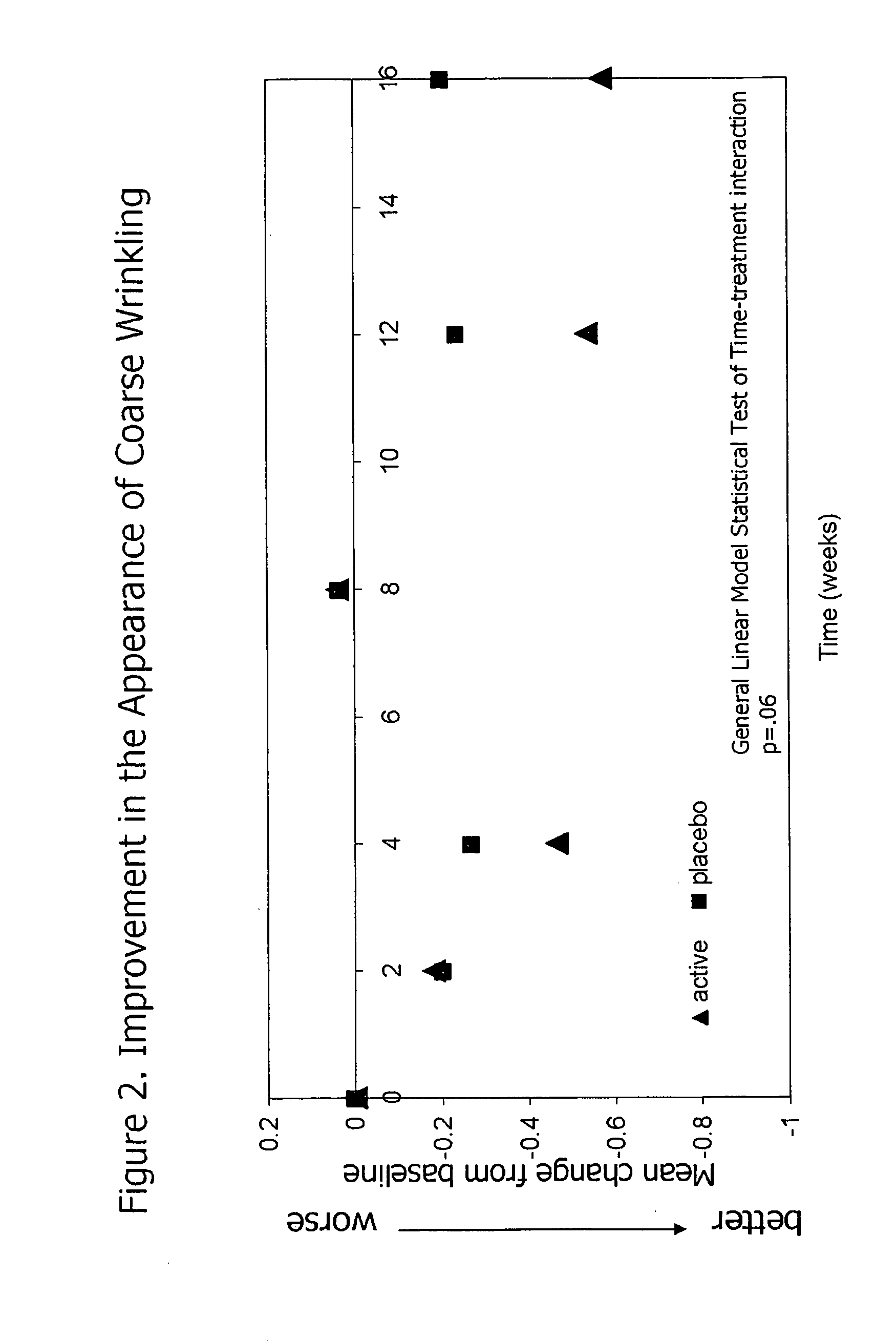
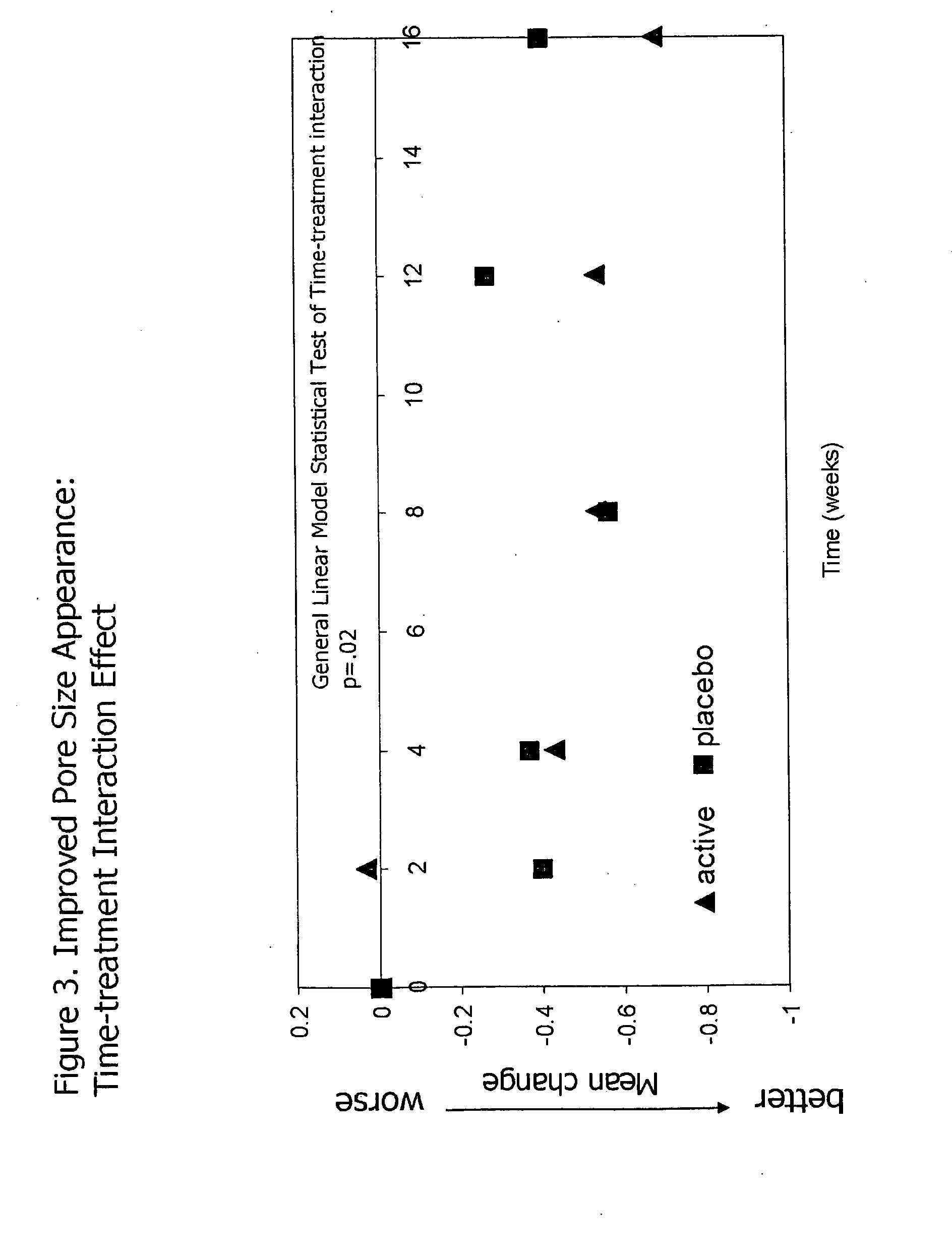
InactiveUS20090068255A1Preventing and reducing of and sun damageImprove skin appearanceBiocideCosmetic preparationsWrinkle skinDisease
The application of matrix metalloproteinase (MMP) inhibitors to the skin inhibits the degradation of proteins found in the skin including collagen, elastin, and other basement membrane and extracellular matrix protein. MMP inhibitors may be used in both cosmetic compositions and pharmaceutical compositions for application to skin. MMP inhibitors are formulated with a cosmetically suitable vehicle or pharmaceutically acceptable excipient for application to the skin as creams, lotions, ointments, solutions, face masks, etc. As cosmetics, the inventive MMP inhibitor compositions are applied to the skin to prevent or reduce the appearance of wrinkles, pigmentation changes, loss of elasticity, or other effects associated with aging or sun damage. As pharmaceuticals, the inventive MMP inhibitor compositions may also be applied to the skin to treat or prevent a skin disease (e.g., proliferative disease, inflammatory disease).
Owner:LIVING PROOF INC
Decellularized extracellular matrix of conditioned body tissues and uses thereof
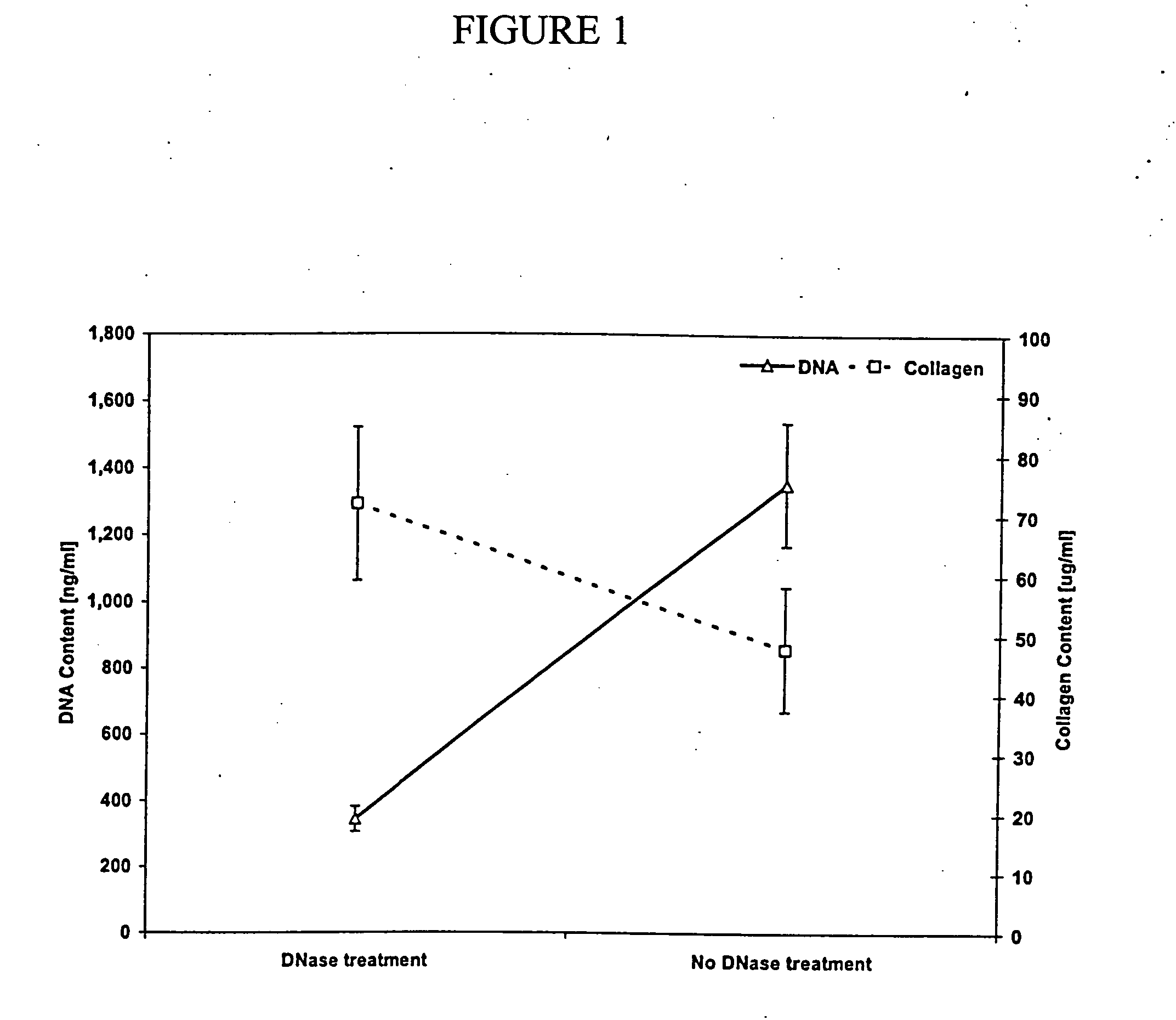
InactiveUS20050013870A1Increase in sizeHigh strengthGastrointestinal cellsGenetically modified cellsCell-Extracellular MatrixECM Protein
The present invention relates generally to decellularized extracellular matrix of conditioned body tissues. The decellularized extracellular matrix contains a biological material, preferably vascular endothelial growth factor (VEGF), produced by the conditioned body tissue that is in an amount different than the amount of the biological material that the body tissue would produce absent the conditioning. The invention also relates to methods of making and methods of using said decellularized extracellular matrix. Specifically, the invention relates to treating defective, diseased, damaged or ischemic cells, tissues or organs in a subject by administering, injecting or implanting the decellularized extracellular matrix of the invention into a subject in need thereof. The invention is further directed to a tissue regeneration scaffold for implantation into a subject inflicted with a disease or condition that requires tissue or organ repair, regeneration and / or strengthening. Additionally, the invention is directed to a medical device, preferably a stent or an artificial heart, having a surface coated or covered with the decellularized extracellular matrix of the invention or having a component comprising the decellularized extracellular matrix of the invention for implantation into a subject, preferably a human. Methods for making the tissue regeneration scaffold and methods for manufacturing a coated or covered medical device having a component comprising decellularized extracellular matrix of conditioned body tissues are also provided.
Owner:BOSTON SCI SCIMED INC
Methods, compositions and systems for local delivery of drugs
ActiveUS20110195123A1Reduce degradationImprove permeabilityBiocidePowder deliveryCell-Extracellular MatrixBrachytherapy
Implantable medical device eluting drug locally and in prolonged period is provided, including several types of such a device, the treatment modes of implementation and methods of implantation. The device comprising of polymeric substrate, such as a matrix for example, that is used as the device body, and drugs, and in some cases additional scaffolding materials, such as metals or additional polymers, and materials to enhance visibility and imaging. The selection of drug is based on the advantageous of releasing drug locally and in prolonged period, where drug is released directly to the extracellular matrix (ECM) of the diseased area such as tumor, inflammation, degeneration or for symptomatic objectives, or to injured smooth muscle cells, or for prevention. One kind of drug is the gene silencing drugs based on RNA interference (RNAi), including but not limited to si RNA, sh RNA, or antisense RNA / DNA, ribozyme and nucleoside analogs. The modes of implantation in some embodiments are existing implantation procedures that are developed and used today for other treatments, including brachytherapy and needle biopsy. In such cases the dimensions of the new implant described in this invention are similar to the original implant. Typically a few devices are implanted during the same treatment procedure.
Owner:SILENSEED LTD
Mastopexy and Breast Reconstruction Prostheses and Method
InactiveUS20080097601A1Easy to handleResistant to biodegradationMammary implantsBandagesMastopexyCell-Extracellular Matrix
Mastopexy and breast reconstruction prostheses and implantation method that allow for radiographic imaging of the breast tissue. The prostheses are arcuate and elongate optionally meshed to conform with breast tissue when implanted. Prostheses are made from naturally occurring extracellular matrix, primarily collagen, that, allows for mammographic imaging without interference as is expected from synthetic materials.
Owner:ORGANOGENESIS
Endoluminal device with extracellular matrix material and methods
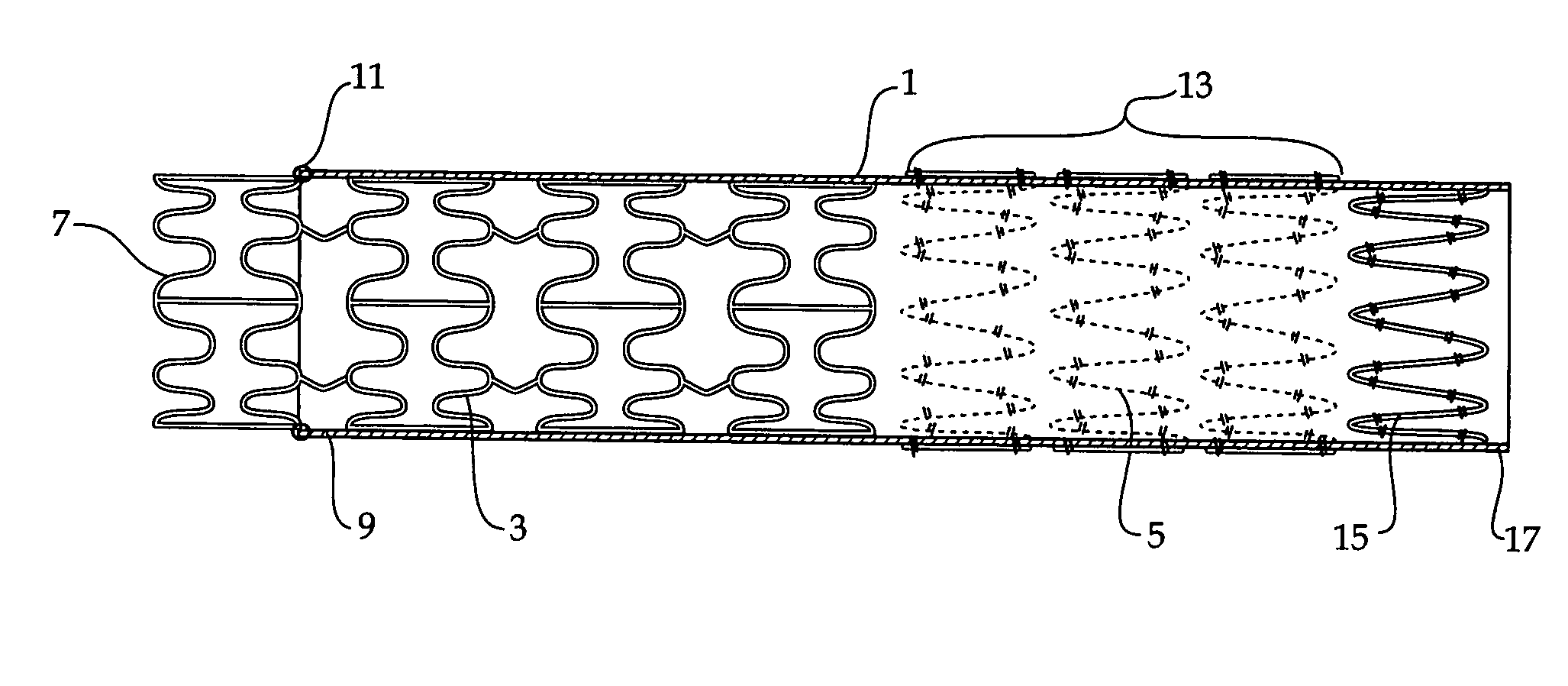
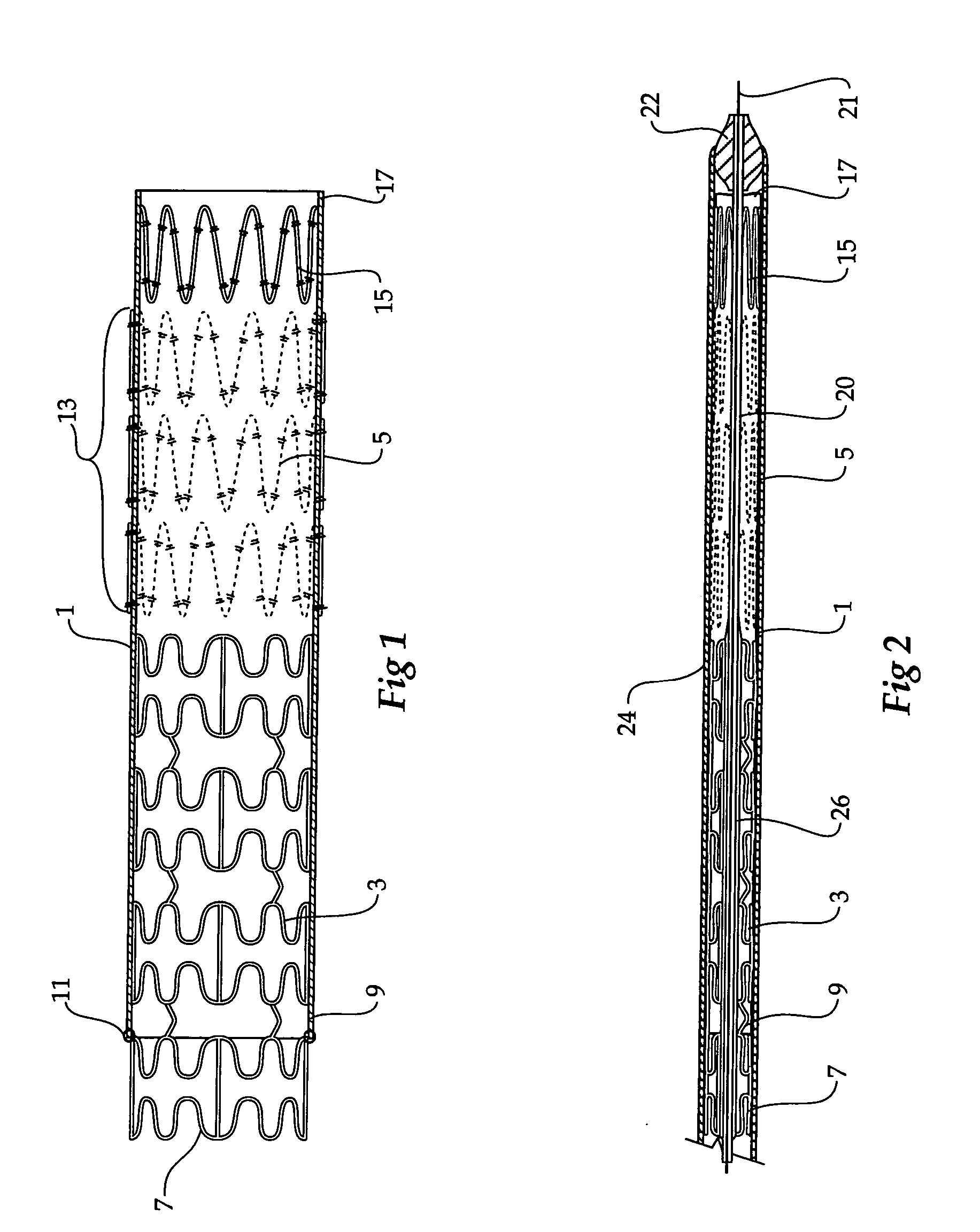
ActiveUS20050273155A1Reducing endoluminal device endoleaksStentsBlood vesselsCell-Extracellular MatrixSynthetic materials
An endoluminal device comprises a stent and a tubular graft supported by the stent. The graft has a proximal and a distal opening and comprises a synthetic material and a bioremodelable material. The bioremodelable material is disposed on an exterior surface in at least one band adjacent at least one of the proximal and distal openings.
Owner:COOK MEDICAL TECH LLC +1
Hybrid biologic/synthetic porous extracellular matrix scaffolds
ActiveUS20030021827A1Wide of injuriesWide supportSuture equipmentsSurgical needlesCell-Extracellular MatrixECM Protein
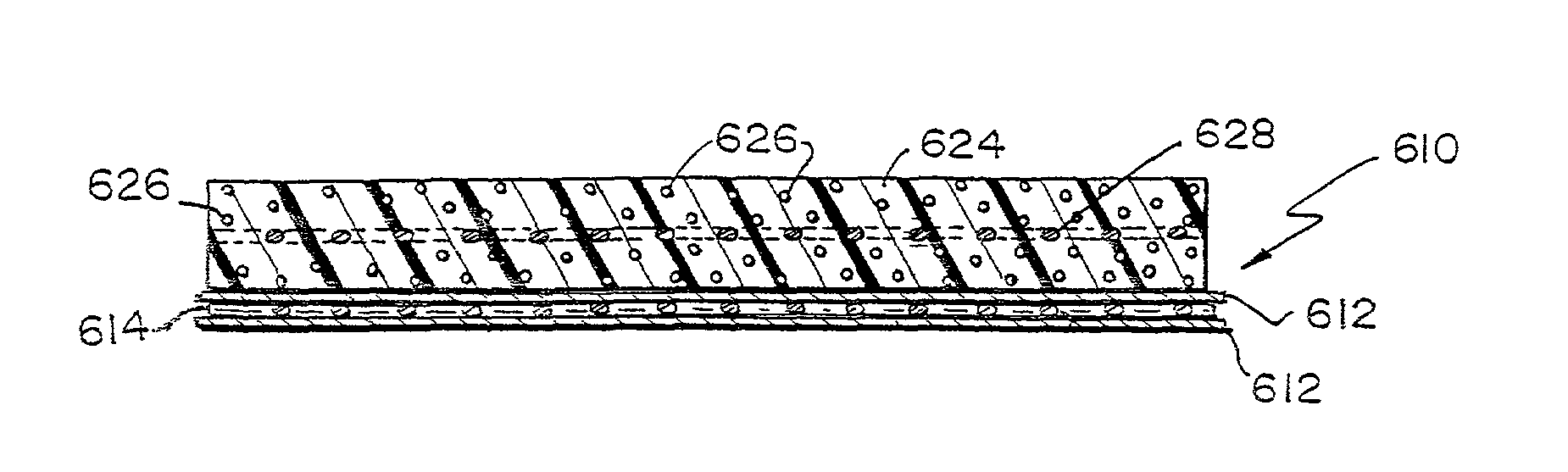
Methods of making a hybrid biologic / synthetic scaffold for repairing damaged or diseased tissue are provided. The methods include the step of suspending pieces of an extracellular matrix material in a liquid to form a slurry, and coating a synthetic mat with the slurry, or mixing or layering the slurry with a synthetic polymer solution. The liquid is subsequently driven off so as to form a foam. Porous implantable scaffolds fabricated by such a method are also disclosed.
Owner:DEPUY SYNTHES PROD INC
Decellularized tissue engineered constructs and tissues
InactiveUS6962814B2Low immunogenicityReduction in immuneBiocidePeptide/protein ingredientsGrowth phaseCell-Extracellular Matrix
New methods for producing tissue engineered constructs and engineered native tissues are disclosed. The methods include producing a tissue engineered construct by growing cells in vitro on a substrate and then decellularizing the construct to produce a decellularized construct consisting largely of extracellular matrix components. The construct can be used immediately or stored until needed. The decellularized construct can be used for further tissue engineering, which may include seeding the construct with cells obtained from the intended recipient of the construct. During any of the growth phases required for production of the construct, the developing construct may be subjected to various tissue engineering steps such as application of mechanical stimuli including pulsatile forces. The methods also include producing an engineered native tissue by harvesting tissue from an animal or human, performing one or more tissue engineering steps on the tissue, and subjecting the tissue to decellularization. The decellularized, engineered native tissue may then be subjected to further tissue engineering steps.
Owner:DUKE UNIV
Decullularized extracellular matrix of conditioned body tissues and uses thereof
InactiveUS20050181016A1Good curative effectMinimize delayGastrointestinal cellsGenetically modified cellsAbnormal tissue growthCell-Extracellular Matrix
The invention is directed to an apparatus, such as a medical device, having a surface coated or covered with a decellularized extracellular matrix or having a component comprising the decellularized extracellular matrix for implantation into a subject, preferably a human. In one embodiment of the invention, a decellularized extracellular matrix is used to form a bodily implant such as a vein, an artery, an esophagus, or a ventricular restraining device. In some embodiments of the invention, the decellularized extracellular matrix is configured to be a time released therapeutic. In another embodiment of the invention, a decellularized extracellular matrix forms an aneurysm treatment device, such as an aneurysm coil, a seal, a pouch, or a filler. In a further embodiment of the invention, decellularized extracellular matrix is used to embolize lesions, tumors, or vessels. Methods for making the tissue regeneration scaffold and methods for manufacturing a coated or covered medical device having a component comprising decellularized extracellular matrix of body tissues are also provided.
Owner:BOSTON SCI SCIMED INC
Decellularized bone marrow extracellular matrix
ActiveUS20050013872A1Minimizes and avoids immune responsePeptide/protein ingredientsSkeletal disorderCell-Extracellular MatrixInsertion stent
The invention is directed to compositions comprising decellularized bone marrow extracellular matrix and uses thereof. Methods for repairing or regenerating defective, diseased, damaged or ischemic tissues or organs in a subject, preferably a human, using the decellularized bone marrow extracellular matrix of the invention are also provided. The invention is further directed to a medical device, preferably a stent or an artificial heart, and biocompatible materials, preferably a tissue regeneration scaffold, comprising decellularized bone marrow extracellular matrix for implantation into a subject.
Owner:BOSTON SCI SCIMED INC
System and method for attaching soft tissue to an implant
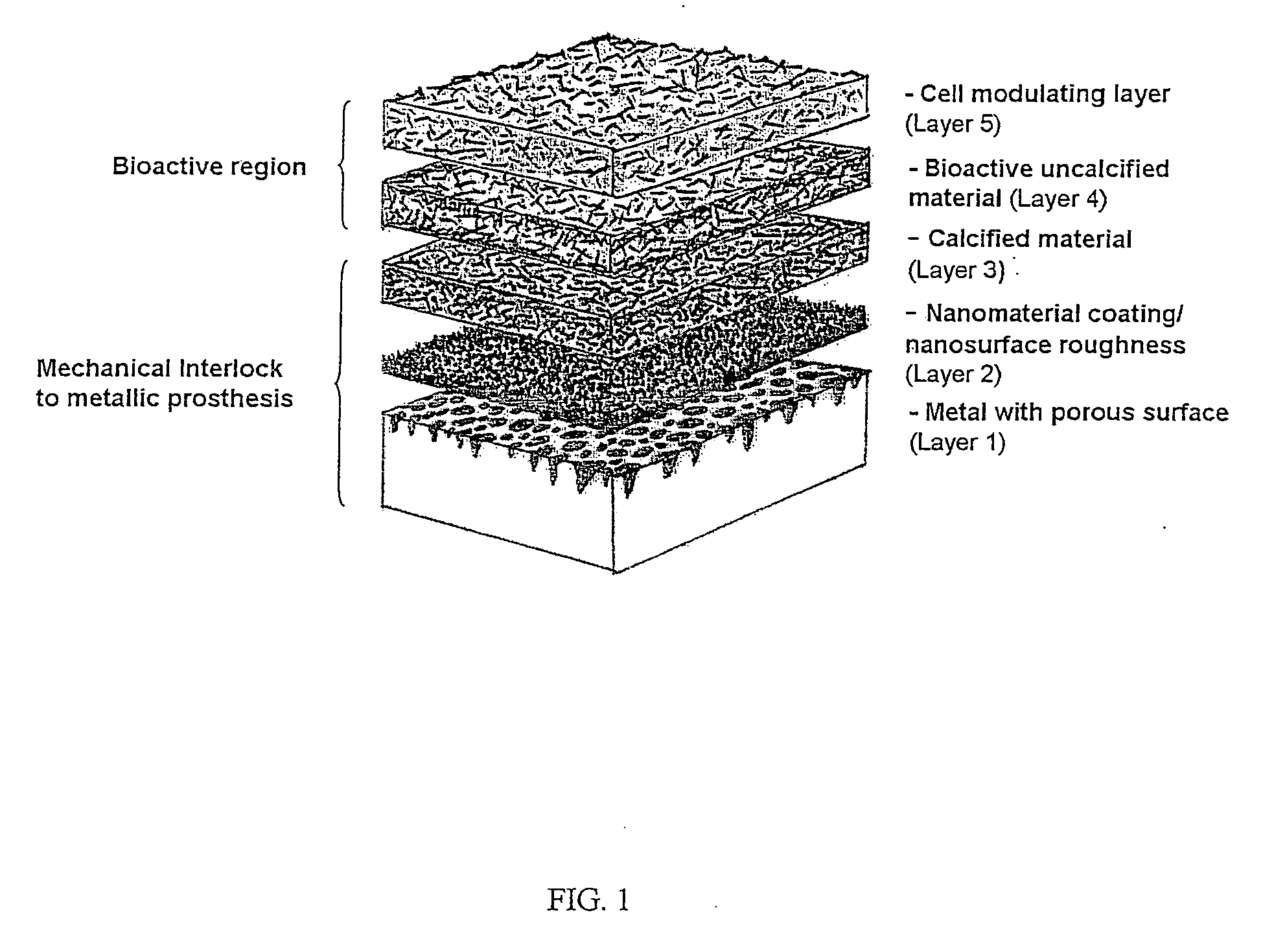
ActiveUS20060105015A1Firmly connectedIncreased pull-out strengthAnodisationBiocideCell-Extracellular MatrixProsthesis
One embodiment of the present invention is directed to compositions and methods for enhancing attachment of soft tissues to a metal prosthetic device. In one embodiment a construct is provided comprising a metal implant having a porous metal region, wherein said porous region exhibits a nano-textured surface, and a biocompatible polymer matrix coating the nano-textured surface. The polymer matrix coating comprises a naturally occurring extracellular matrix with biocompatible inorganic materials distributed within the matrix, or a biocompatible polymer and an osteo-inductive agent.
Owner:DEPUY SYNTHES PROD INC +1
Compositions for regenerating defective or absent myocardium
ActiveUS20070014773A1Restoring cardiac functionEffective amountBiocideOrganic active ingredientsCell-Extracellular MatrixRegenerative process
Compositions of the invention for regenerating defective or absent myocardium comprise an emulsified or injectable extracellular matrix composition. The composition may also include an extracellular matrix scaffold component of any formulation, and further include added cells, proteins, or other components to optimize the regenerative process and restore cardiac function. Methods for regenerating defective or absent myocardium apply a composition to a site of myocardium in need of regeneration using a delivery mode appropriate for the particular formulation.
Owner:CORMATRIX CARDIOVASCULAR INC
Self-assembling peptides incorporating modifications and methods of use thereof
ActiveUS20050181973A1Peptide/protein ingredientsAntibody mimetics/scaffoldsCell-Extracellular MatrixTissue repair
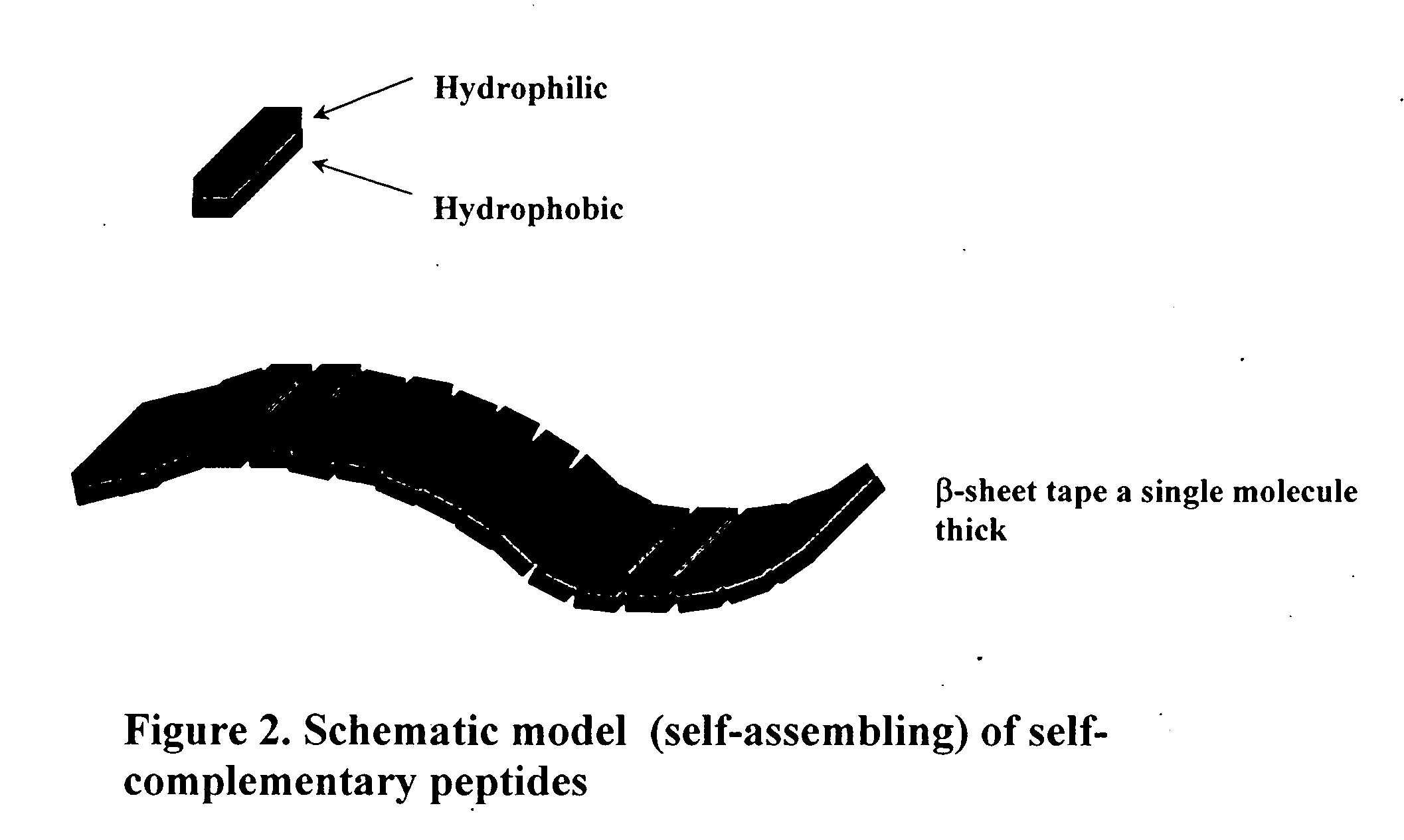
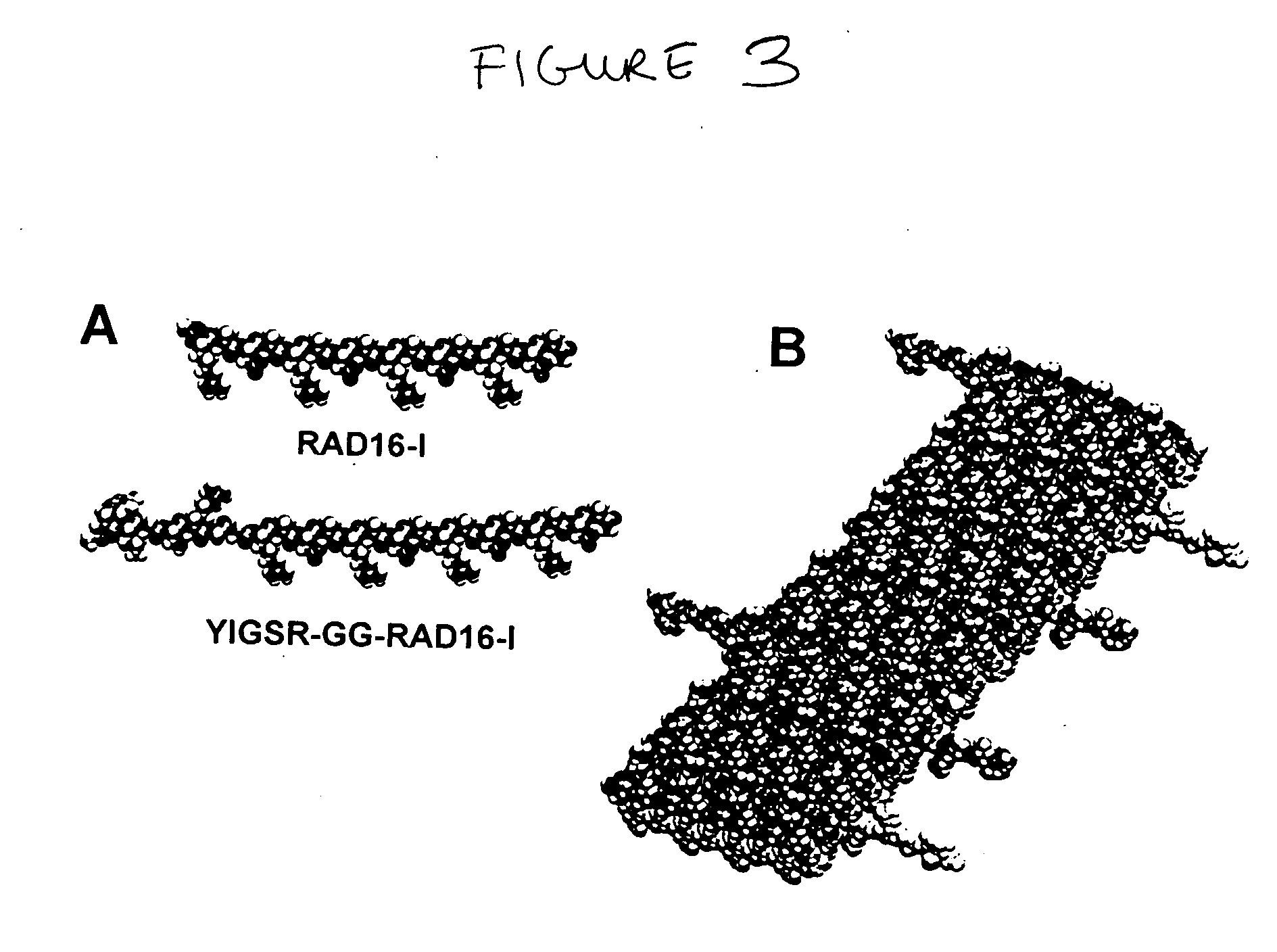
The invention provides a self-assembling peptide comprising (a) a first amino acid domain that mediates self-assembly, wherein the domain comprises alternating hydrophobic and hydrophilic amino acids that are complementary and structurally compatible and self-assemble into a macroscopic structure when present in unmodified form; and (b) a second amino acid domain that does not self-assemble in isolated form. In certain embodiments of the invention the second amino acid domain comprises a biologically active peptide motif, e.g., a peptide motif found in a naturally occurring protein, or a target site for an interaction with a biomolecule. In certain embodiments of the invention the naturally occurring protein is a component of the extracellular matrix, e.g., a component of the basement membrane. The invention further provides scaffolds comprising the self-assembling peptides and methods of using the scaffolds including for cell culture, tissue engineering, and tissue repair.
Owner:MASSACHUSETTS INST OF TECH
Adipose-derived stem cells and lattices
InactiveUS20050282275A1Promote growthFacilitate differentiationGenetic material ingredientsMammal material medical ingredientsCell-Extracellular MatrixConnective tissue fiber
The present invention provides adipose-derived stem cells and lattices. In one aspect, the present invention provides a lipo-derived stem cell substantially free of adipocytes and red blood cells and clonal populations of connective tissue stem cells. The invention also provides a method of isolating stem cells from adipose tissues. The cells can be employed, alone or within biologically-compatible compositions, to generate differentiated tissues and structures, both in vivo and in vitro. Additionally, the cells can be expanded and cultured to produce hormones and to provide conditioned culture media for supporting the growth and expansion of other cell populations. In another aspect, the present invention provides a lipo-derived lattice substantially devoid of cells, which includes extracellular matrix material from adipose tissue. The lattice can be used as a substrate to facilitate the growth and differentiation of cells, whether in vivo or in vitro, into anlagen or even mature tissues or structures.
Owner:UNIVERSITY OF PITTSBURGH +1
Multi-layer cell encapsulation for tissue engineering
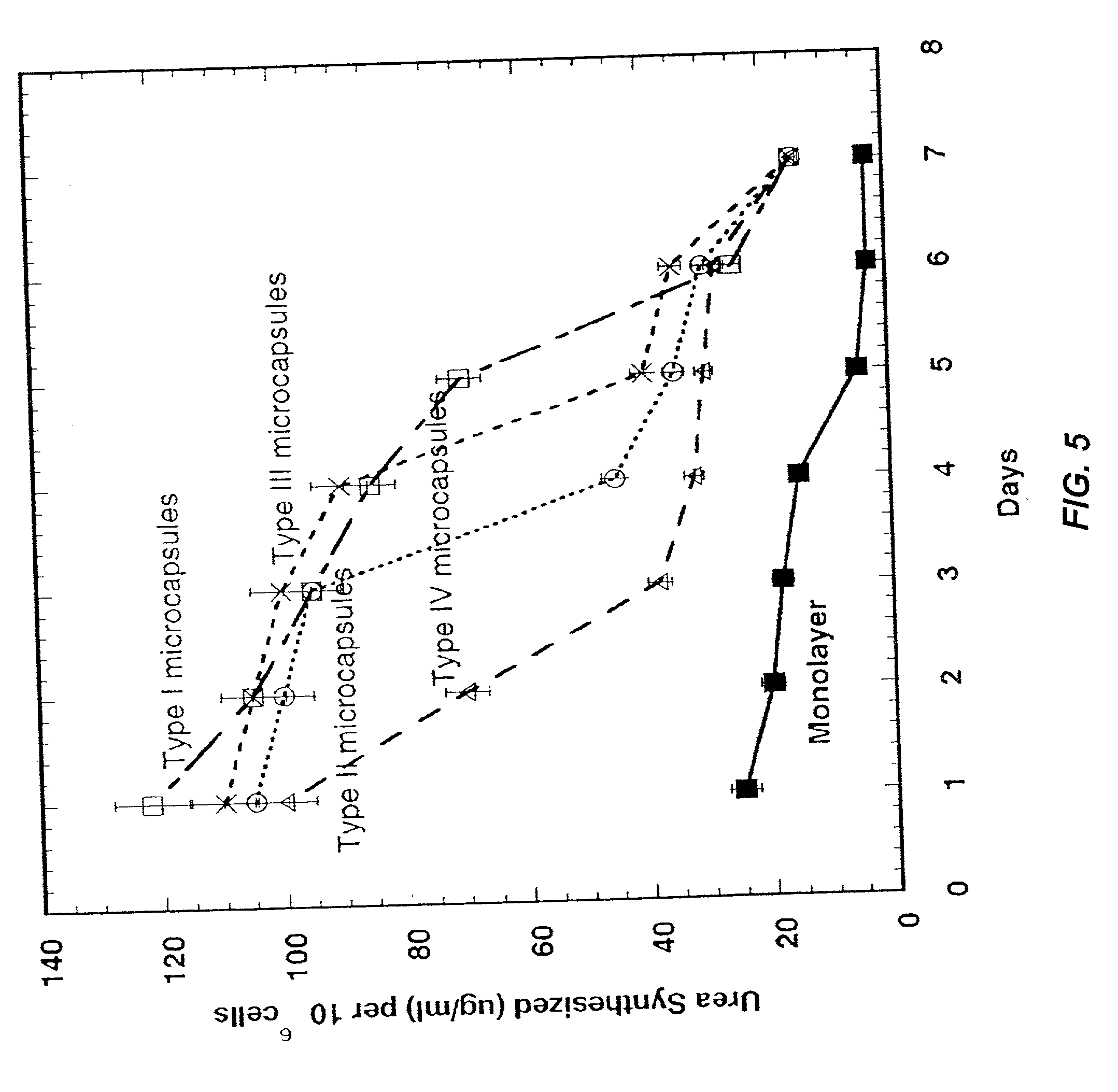
A multi-layered microcapsule has an inner extracellular matrix and an outer shell. The inner extracellular matrix includes a first inner layer of biopolymer and a second intermediate layer of polymer that provides partial immune-protection and holds the first layer in place. The outer shell can form an exoskeleton to provide mechanical stability. Each of the individual layers can be varied to optimize mechanical stability, cell function, and immuno-protection.
Owner:AGENCY FOR SCI TECH & RES +1
Acellular matrix repairing gel and new method for preparing the same
ActiveCN104971380ARetain biological activityImprove securityProsthesisCell-Extracellular MatrixClinical value
The invention relates to the field of bio-materials, and especially relates to an acellular matrix repairing gel and a new method for preparing the same. The invention discloses the acellular matrix repairing gel and the method for preparing the same, wherein the method includes steps of acellular treatment and gelatinization treatment on tissue and organs from mammal animals to prepare the acellular matrix repairing gel. The acellular matrix repairing gel is eliminated in immunogenicity of heterologous and foreign tissue, so that activity of extracellular matrix components of the tissue is maintained as more as possible. The gel can specially repair damaged tissue and organs of human body, is strong in applicability, is suitable for requirements of various irregular-shaped repair zones and different position environments in body and has a huge clinical value.
Owner:山东隽秀生物科技股份有限公司
Electroprocessed collagen and tissue engineering
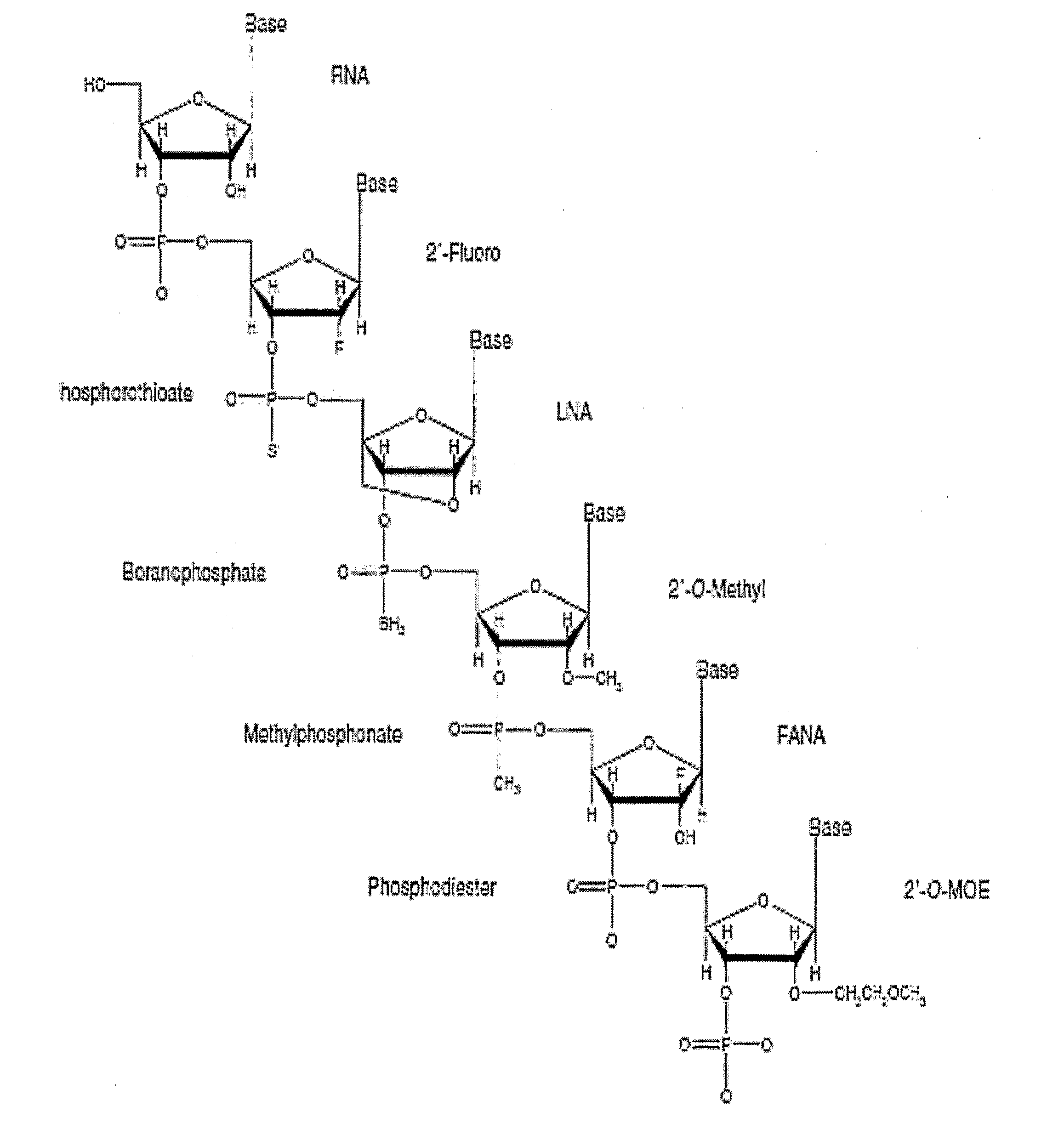
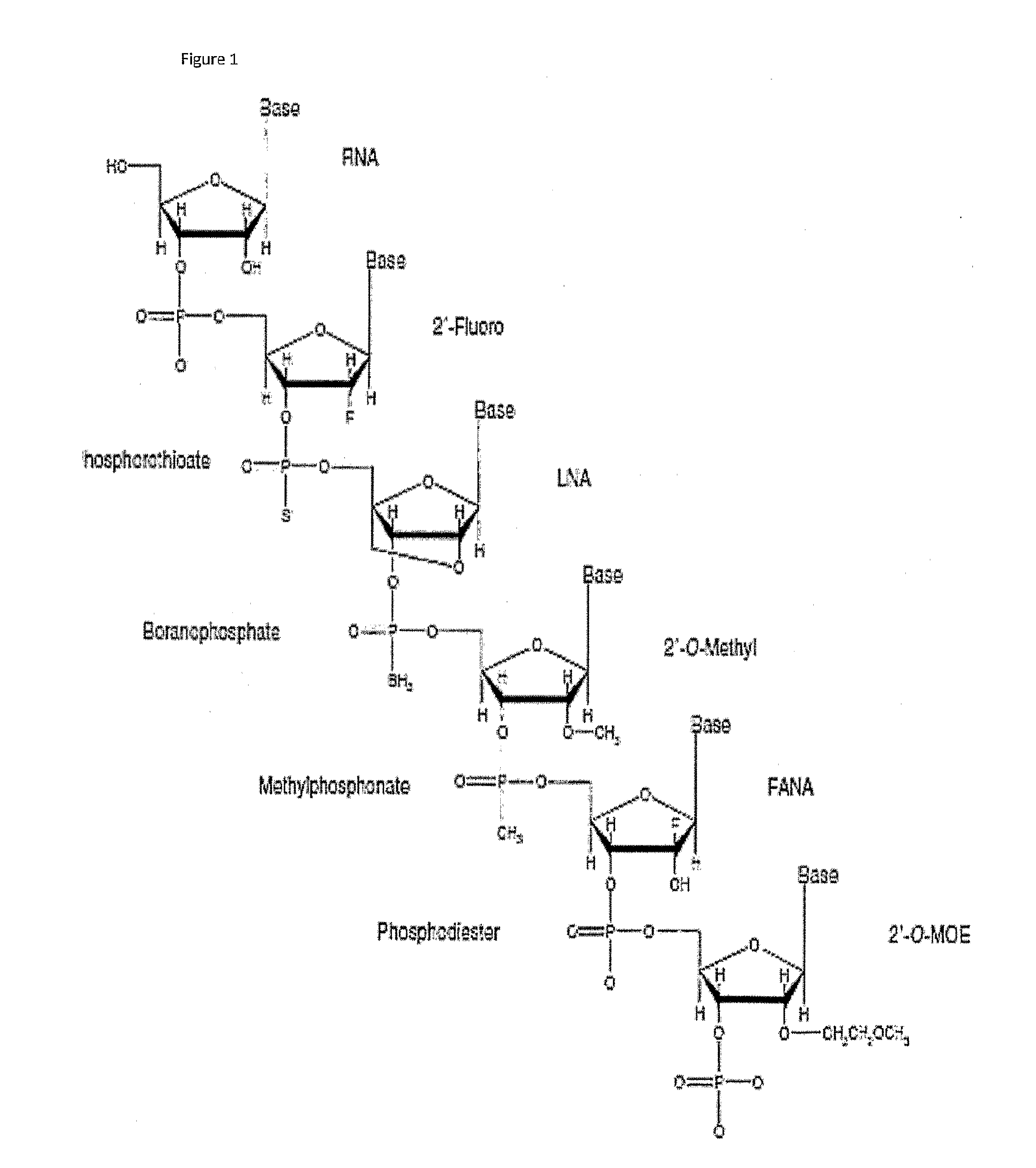
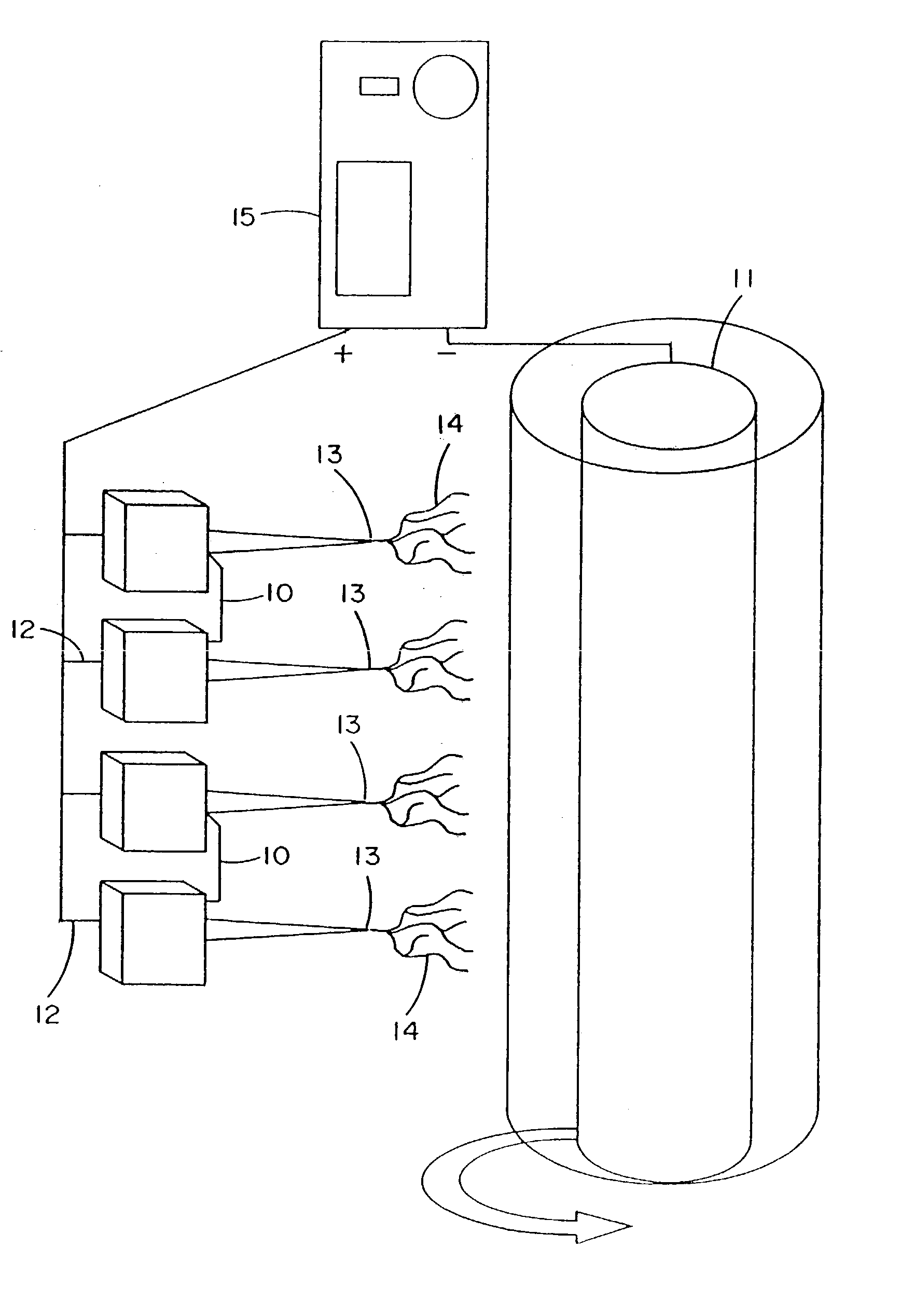
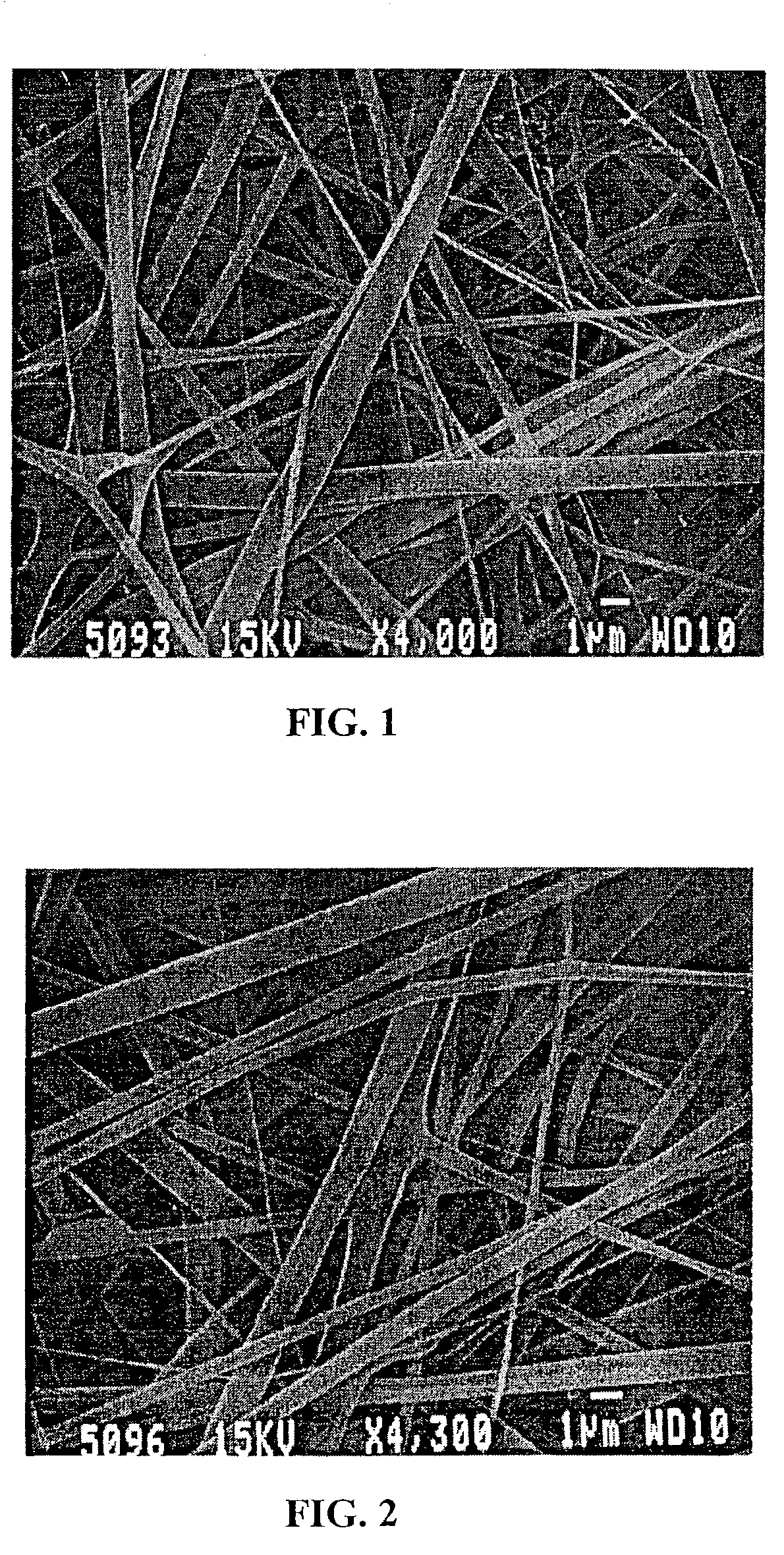
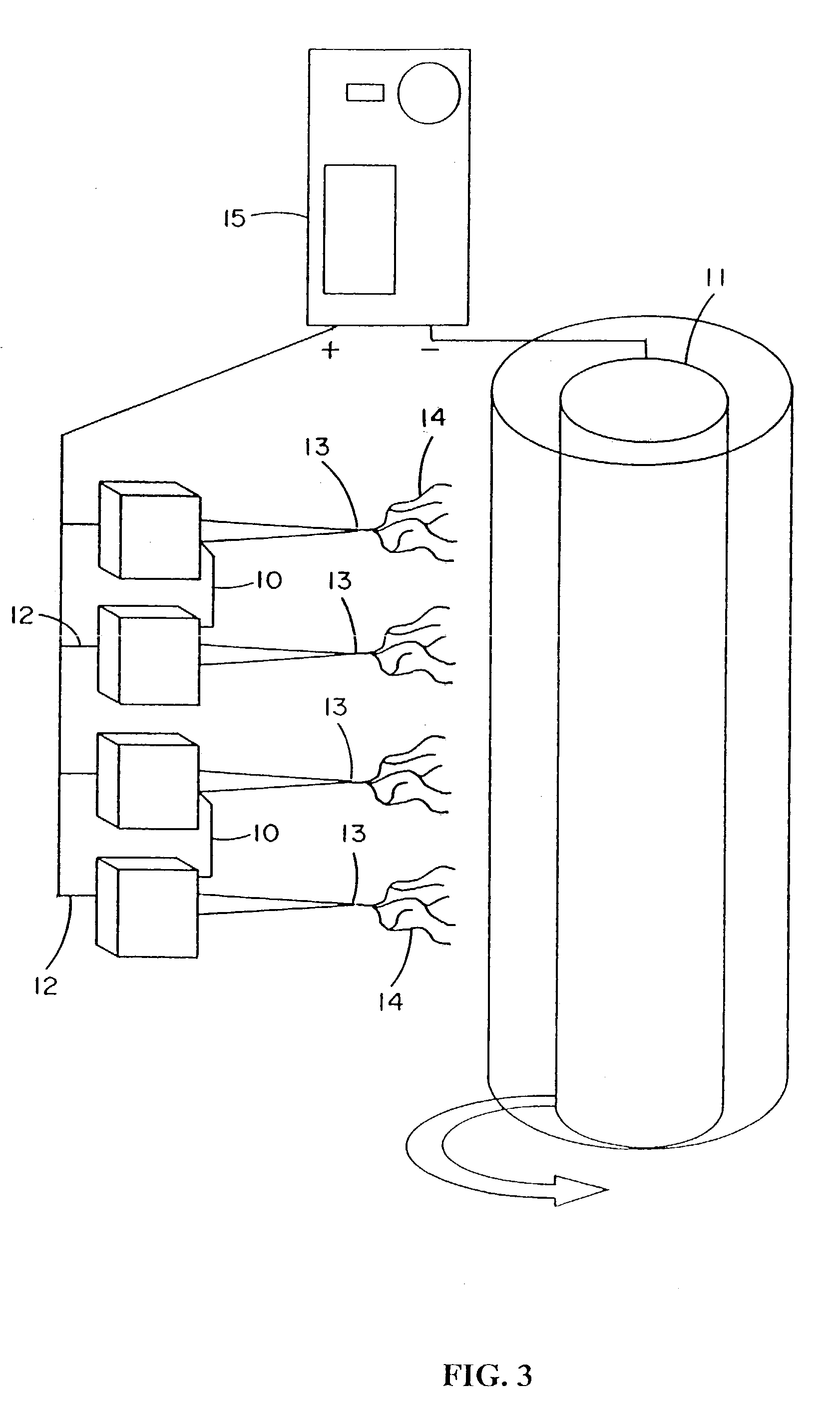
InactiveUS7615373B2Monocomponent protein artificial filamentPeptide/protein ingredientsCell-Extracellular MatrixECM Protein
The invention is directed to formation and use of electroprocessed collagen, including use as an extracellular matrix and, together with cells, its use in forming engineered tissue. The engineered tissue can include the synthetic manufacture of specific organs or tissues which may be implanted into a recipient. The electroprocessed collagen may also be combined with other molecules in order to deliver substances to the site of application or implantation of the electroprocessed collagen. The collagen or collagen / cell suspension is electrodeposited onto a substrate to form tissues and organs.
Owner:ORGANOGENESIS +1
Tissue-engineered silk organs
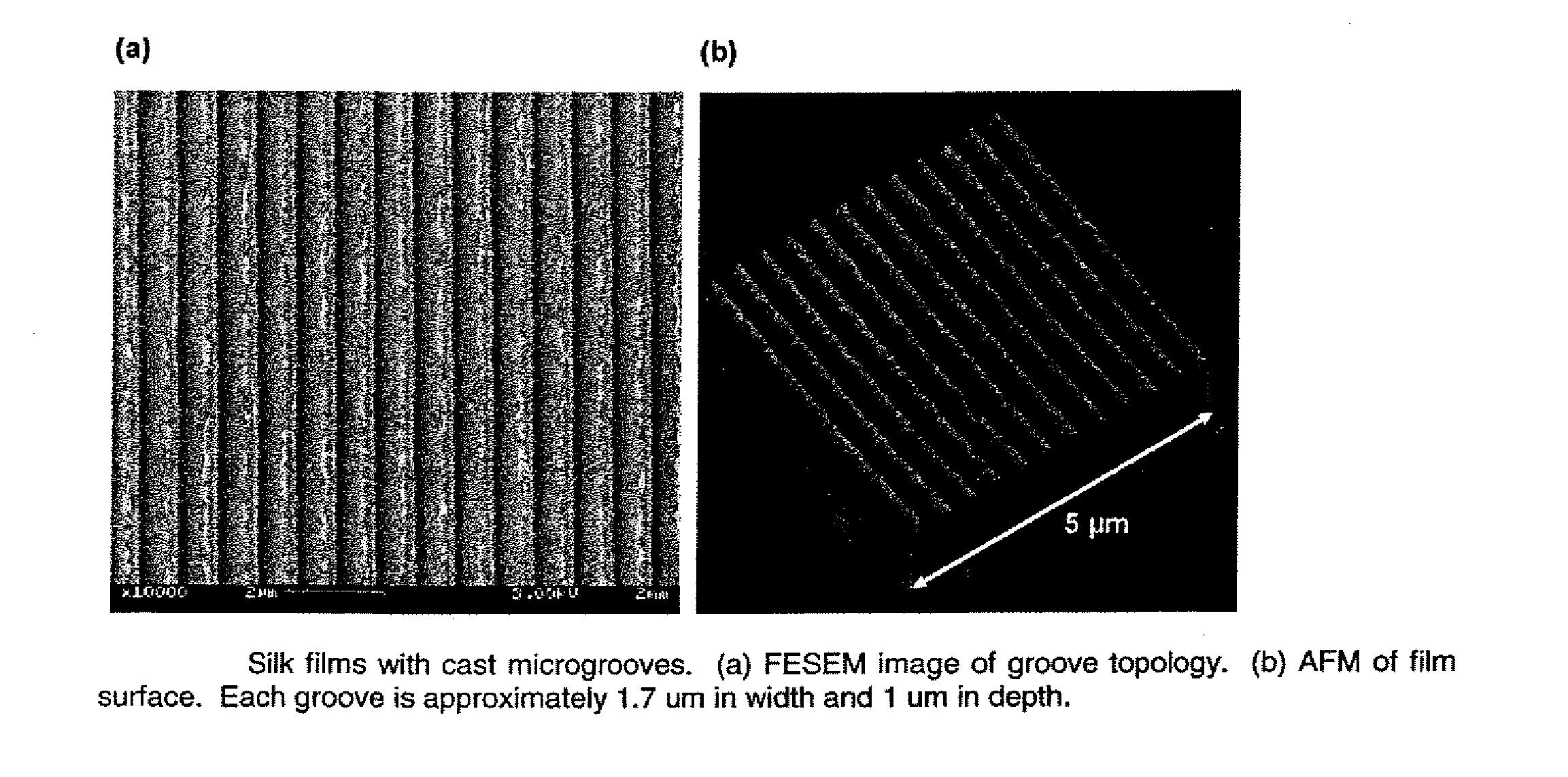
ActiveUS20100191328A1Easy to controlEye implantsNervous system cellsCell-Extracellular MatrixCulture cell
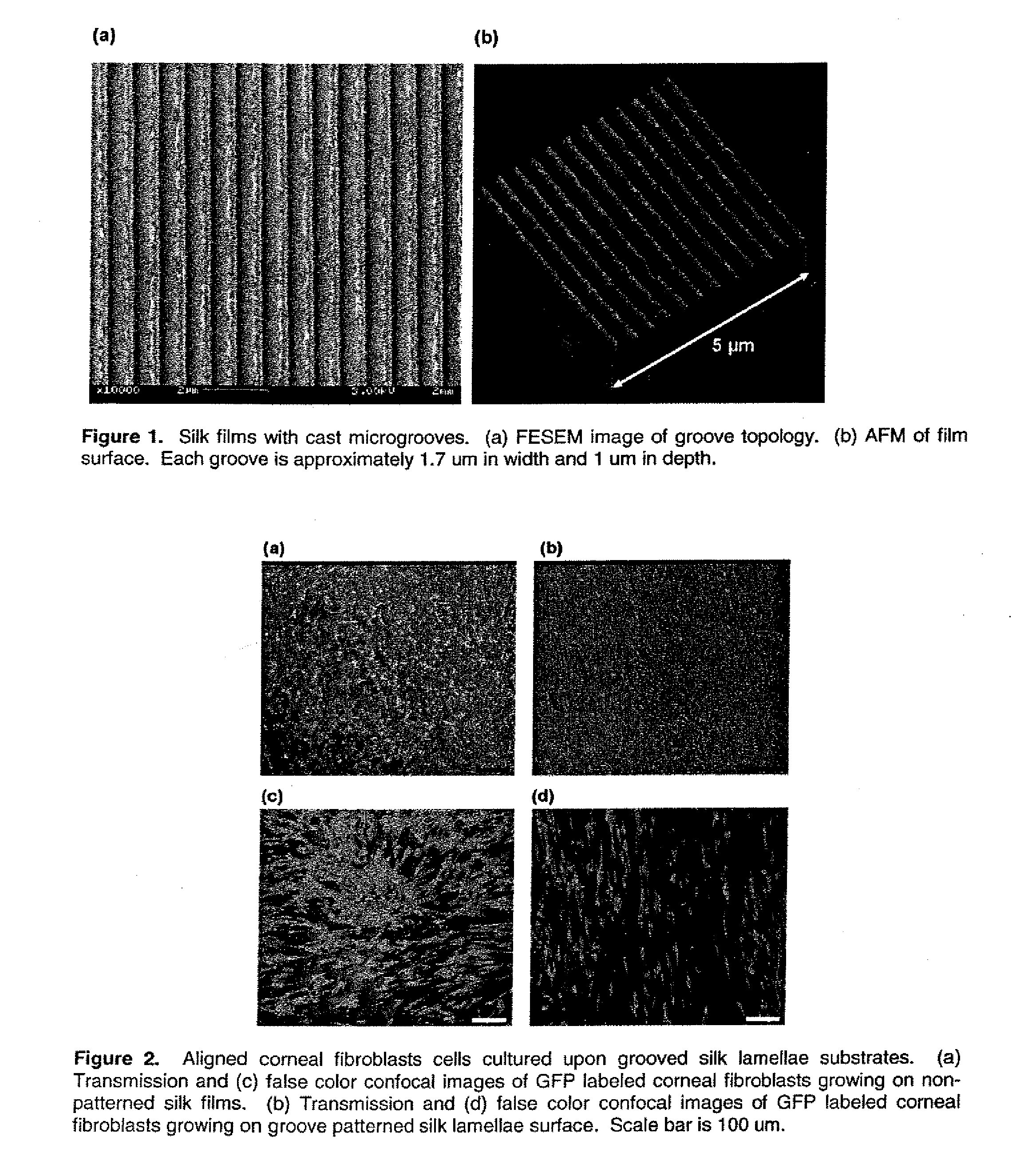
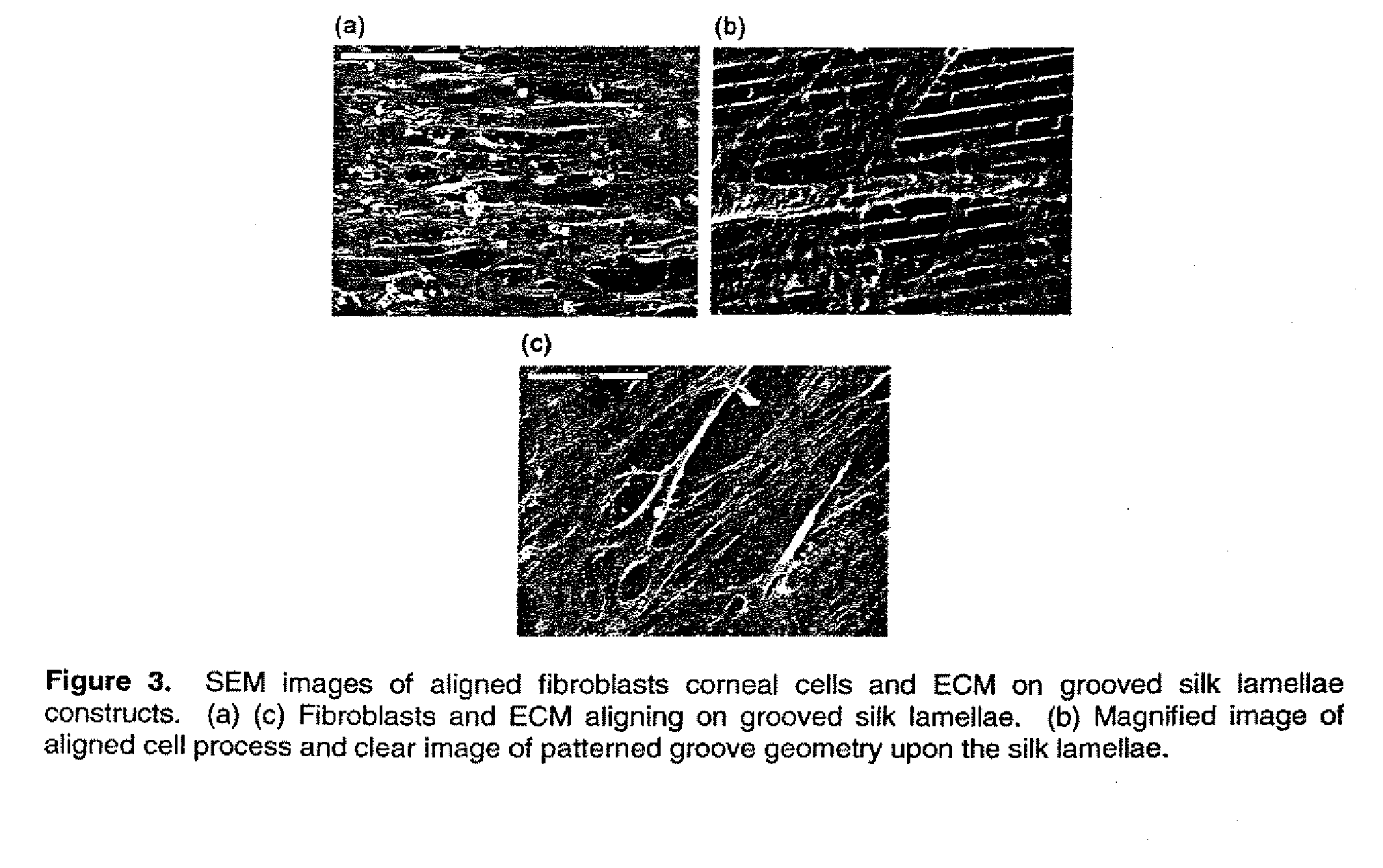
This invention relates to a lamellae tissue layer, comprising a grooved silk fibroin substrate comprising tissue-specific cells. The silk fibroin substrates provides an excellent means of controlling and culturing cell and extracellular matrix development. A multitude of lamellae tissue layers can be used to create a tissue-engineered organ, such as a tissue-engineered cornea. The tissue-engineered organ is non-immunogenic and biocompatible.
Owner:TRUSTEES OF TUFTS COLLEGE TUFTS UNIV
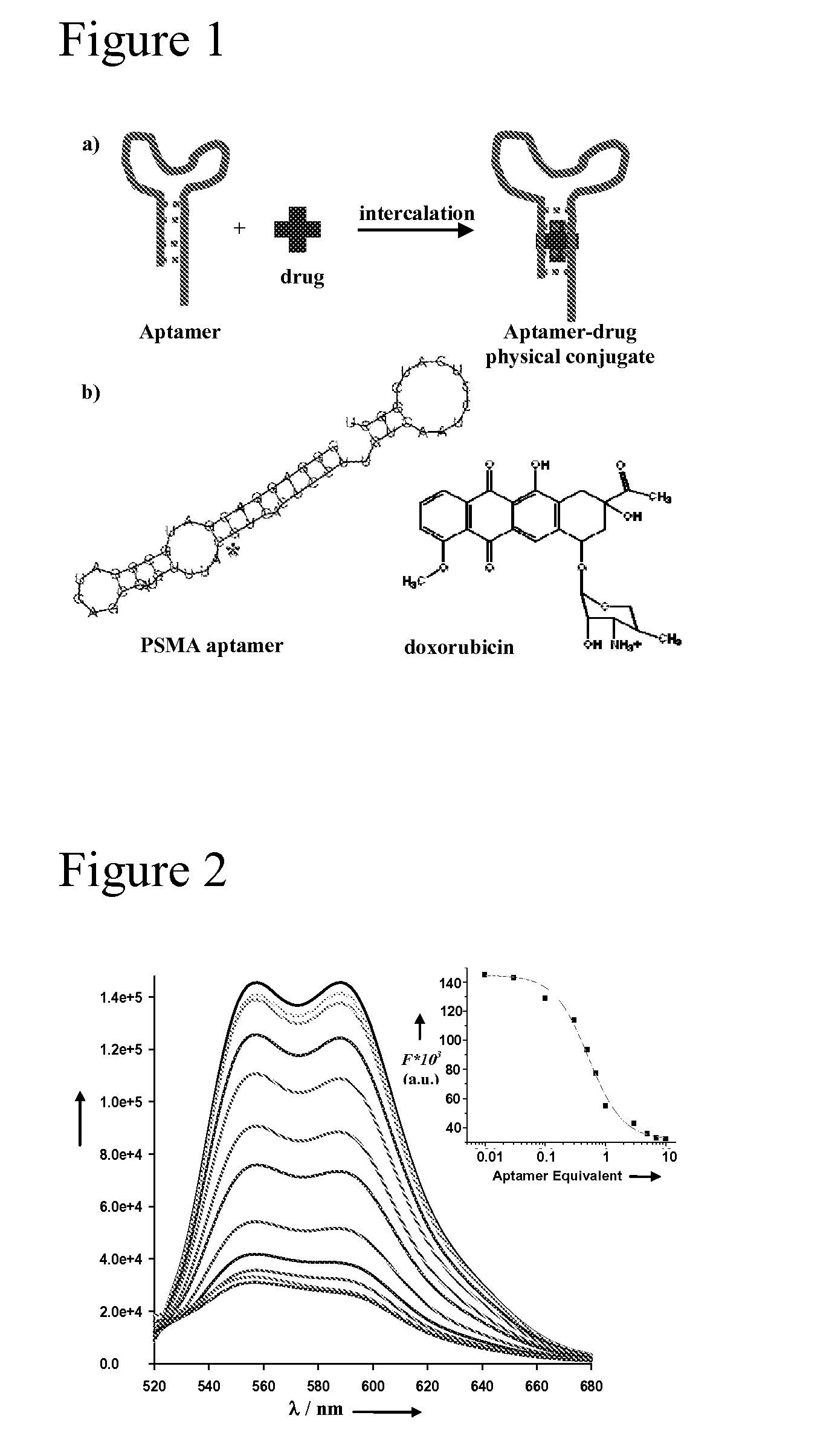
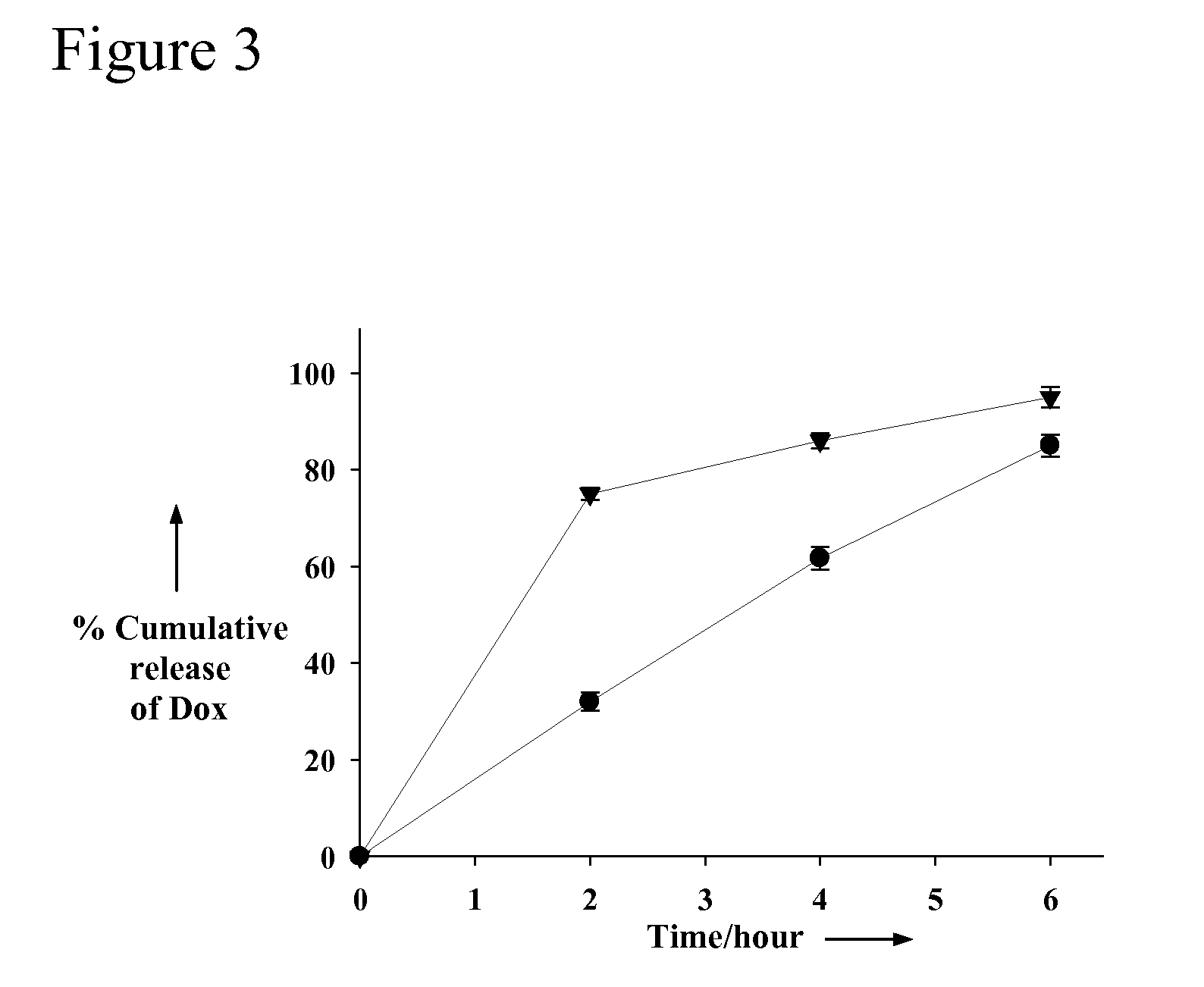
Aptamer-Directed Drug Delivery
InactiveUS20110052697A1Reduce severityReduce morbidityOrganic active ingredientsPowder deliveryCell-Extracellular MatrixDiagnostic agent
The present invention provides systems, methods, and compositions for targeted delivery of a therapeutic agent organs, tissues, cells, extracellular matrix components, and intracellular compartments. The present invention provides a complex comprising a therapeutic or diagnostic agent and a nucleic acid targeting moiety, wherein the agent non-covalently associates with base pairs of the nucleic acid targeting moiety. The invention provides targeted particles comprising a particle and an inventive complex. The present invention provides methods of designing, manufacturing, and using inventive complexes and targeted particles.
Owner:THE BRIGHAM & WOMEN S HOSPITAL INC +2
Electroprocessed Collagen and Tissue Engineering
InactiveUS20080038352A1Powder deliveryMonocomponent protein artificial filamentCell-Extracellular MatrixMedicine
The invention is directed to formation and use of electroprocessed collagen, including use as an extracellular matrix and, together with cells, its use in forming engineered tissue. The engineered tissue can include the synthetic manufacture of specific organs or tissues which may be implanted into a recipient. The electroprocessed collagen may also be combined with other molecules in order to deliver substances to the site of application or implantation of the electroprocessed collagen. The collagen or collagen / cell suspension is electrodeposited onto a substrate to form tissues and organs.
Owner:VIRGINIA COMMONWEALTH UNIV INTPROP FOUND INC +1
Methods and systems for modifying vascular valves
Described are methods and systems for modifying vascular valves in order to reduce retrograde blood flow through the valves. Preferred methods include connecting vascular valve leaflets with at least one remodelable material, such that the valve leaflets become fused by the ingrowth of the patient's native tissue. Preferred remodelable materials include collagenous extracellular matrix material, such as small intestine submucosa.
Owner:COOK MEDICAL TECH LLC
Adipose-derived stem cells and lattices
InactiveUS7470537B2Genetic material ingredientsMammal material medical ingredientsCell-Extracellular MatrixECM Protein
The present invention provides adipose-derived stem cells (ADSCs), adipose-derived stem cell-enriched fractions (ADSC-EF) and adipose-derived lattices, alone and combined with the ADSCs of the invention. In one aspect, the present invention provides an ADSC substantially free of adipocytes and red blood cells and clonal populations of connective tissue stem cells. The ADSCs can be employed, alone or within biologically-compatible compositions, to generate differentiated tissues and structures, both in vivo and in vitro. Additionally, the ADSCs can be expanded and cultured to produce molecules such as hormones, and to provide conditioned culture media for supporting the growth and expansion of other cell populations. In another aspect, the present invention provides an adipose-derived lattice substantially devoid of cells, which includes extracellular matrix material from adipose tissue. The lattice can be used as a substrate to facilitate the growth and differentiation of cells, whether in vivo or in vitro, into anlagen or even mature tissues or structures.
Owner:UNIVERSITY OF PITTSBURGH
Methods for culturing human embryonic stem cells
InactiveUS20050158852A1Improve breeding conditionsImprove survival rateGenetically modified cellsArtificial cell constructsCell-Extracellular MatrixIn vitro growth
The present invention discloses a method for improving growth and survival of single human embryonic stem cells. The method includes the step of obtaining a single undifferentiated HES cell; mixing the single undifferentiated cell with an extracellular matrix (ECM) to encompass the cell; and inoculating the mixture onto feeder cells with a nutrient medium in a growth environment. Therefore the single cells can survive, proliferate and grow in vitro.
Owner:IND TECH RES INST
Endoluminal device with extracellular matrix material and methods
An endoluminal device comprises a stent and a tubular graft supported by the stent. The graft has a proximal and a distal opening and comprises a synthetic material and a bioremodelable material. The bioremodelable material is disposed on an exterior surface in at least one band adjacent at least one of the proximal and distal openings.
Owner:COOK MEDICAL TECH LLC +1
Adipose-derived stem cells and lattices
InactiveUS20050153441A1Promote growthFacilitate differentiationGenetic material ingredientsMammal material medical ingredientsCell-Extracellular MatrixECM Protein
The present invention provides adipose-derived stem cells (ADSCs), adipose-derived stem cell-enriched fractions (ADSC-EF) and adipose-derived lattices, alone and combined with the ADSCs of the invention. In one aspect, the present invention provides an ADSC substantially free of adipocytes and red blood cells and clonal populations of connective tissue stem cells. The ADSCs can be employed, alone or within biologically-compatible compositions, to generate differentiated tissues and structures, both in vivo and in vitro. Additionally, the ADSCs can be expanded and cultured to produce molecules such as hormones, and to provide conditioned culture media for supporting the growth and expansion of other cell populations. In another aspect, the present invention provides a adipose-derived lattice substantially devoid of cells, which includes extracellular matrix material from adipose tissue. The lattice can be used as a substrate to facilitate the growth and differentiation of cells, whether in vivo or in vitro, into anlagen or even mature tissues or structures.
Owner:UNIVERSITY OF PITTSBURGH
Composite stent graft
A composite stent graft has a balloon expandable stent portion ( 3 ), a tubular graft material portion ( 1 ) inside or outside of the balloon expandable stent portion and self expanding stents ( 5 ) associated with the tubular graft material portion. Part ( 7 ) of the balloon expandable stent portion can extend beyond the proximal end ( 9 ) of the tubular graft material portion. The tubular graft material can be polytetrafluoroethylene, Dacron, Thoralon(TM), polyamide, small intestine submucosa, collagenous extracellular matrix material, or any other suitable biocompatible material. A method of deploying which includes flaring a part ( 7 ) of the balloon expandable stent portion is also discussed.
Owner:COOK MEDICAL TECH LLC
Medium for growing human embryonic stem cells
InactiveUS20050037492A1Rapid productionCost-effectiveDrug screeningNervous system cellsGerm layerLipid formation
This disclosure provides an improved system for culturing human pluripotent stem cells. Traditionally, pluripotent stem cells are cultured on a layer of mouse embryonic fibroblast feeder cells to prevent them from differentiating. In the system described here, the role of feeder cells is replaced by defined components added to the culture environment that support rapid proliferation without differentiation. The medium contains an isotonic buffer, a blend of essential nutrients such as protein and lipids, and an effective growth factor or combination of factors that promote proliferation while inhibiting differentiation. Culturing human embryonic stem cells in fresh medium on an extracellular matrix according to this invention causes the cells to expand surprisingly rapidly, while retaining the ability to differentiate into cells representing all three embryonic germ layers. This new culture system allows for bulk proliferation of pPS cells for commercial production of important products for use in drug screening and human therapy.
Owner:ASTERIAS BIOTHERAPEUTICS INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com