Electroprocessed Collagen and Tissue Engineering
a technology of electroprocessed collagen and tissue engineering, which is applied in the direction of cell encapsulation, powder delivery, peptide/protein ingredients, etc., can solve the problems of fibrotic encapsulation, lack of cellular infiltration, and rejection of many such polymers
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Fibroblast Growth Factor (FGF) Release from an Implant Comprised of Type I Collagen, PGA and PLA
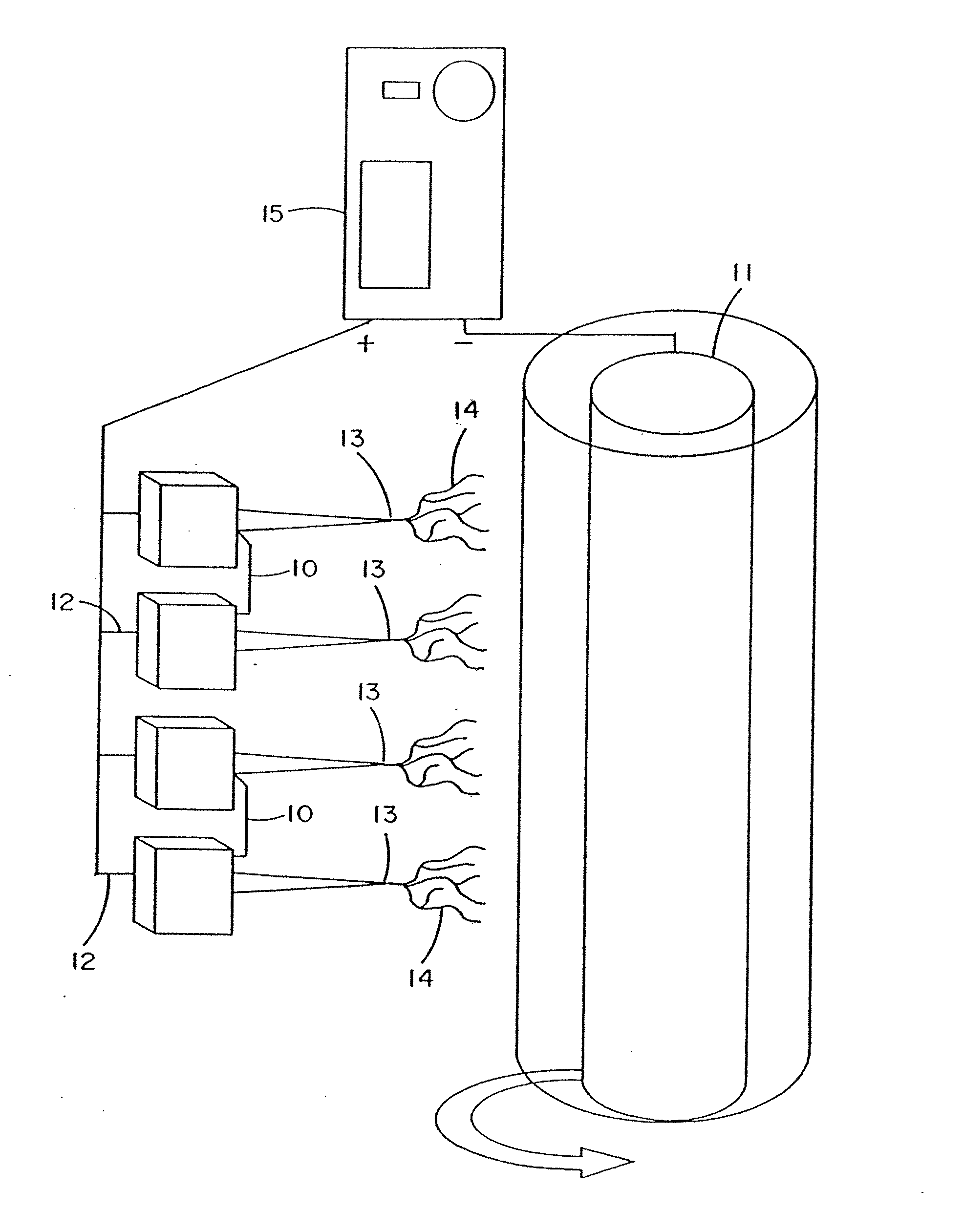
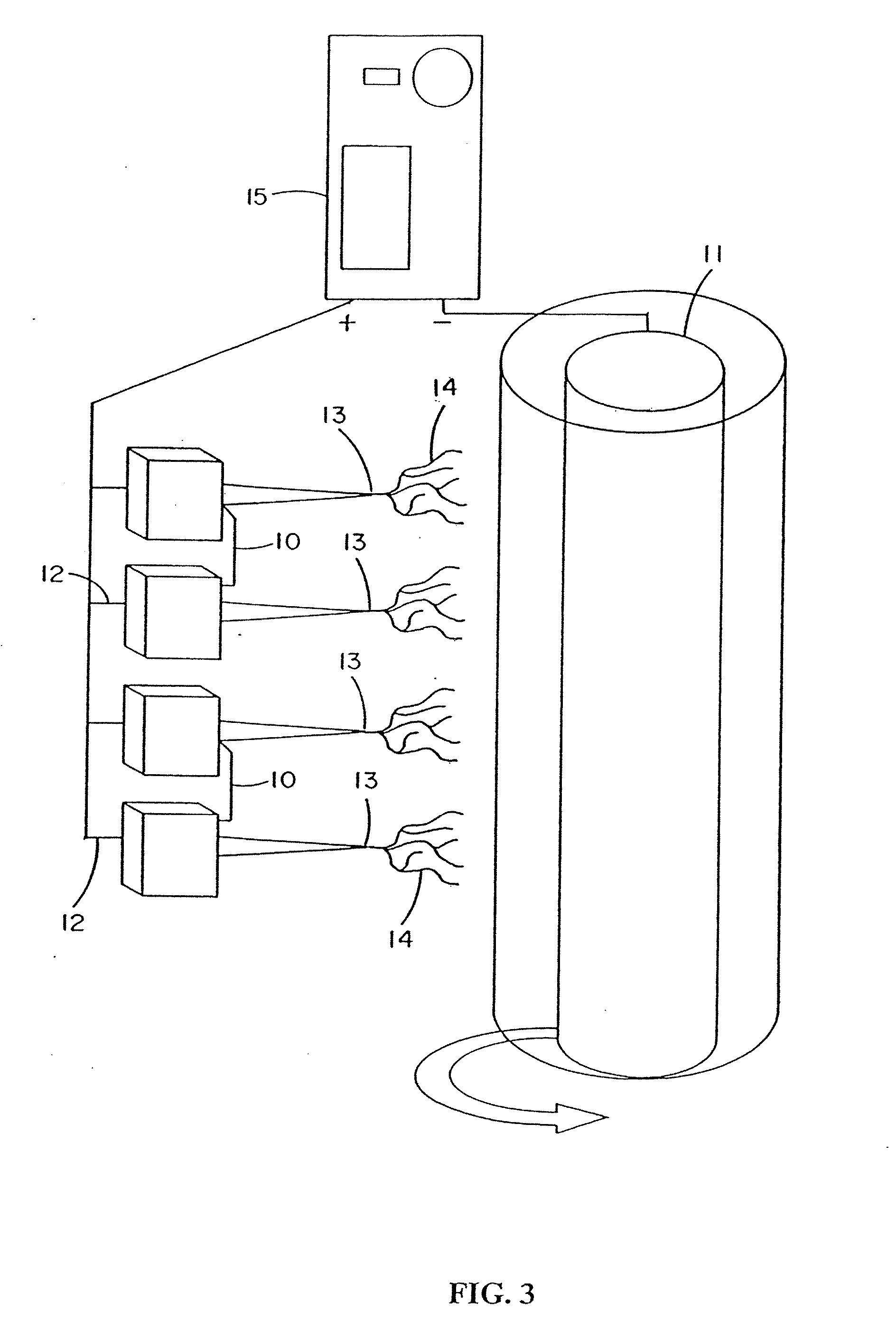
[0272] Fibroblast growth factor (FGF, obtained from Chemicon, Temecula, Calif.) was dissolved in a solution of matrix material comprised of type I collagen (80%), PGA (10%) and PLA (10%). The percentages refer to the weight of the materials with respect to one another. These materials were dissolved in HFIP at a final concentration of 0.08 gm per ml. Sufficient FGF was added to 1 ml of solution to provide an FGF concentration of 50 ng / ml of the collagen / PGA / PLA electrospinning solution. The material was electrospun into the shape of a cylinder onto the outer surface of a grounded and spinning 16 gauge needle about 25-35 mm in length. After completion of electrospinning, the material was removed from the needle and the electrospun cylinder was sutured shut looping a suture around the outside of the construct and pulling tight to seal the ends. A similar result may be obtained by using a ...
example 2
Vascular Endothelial Growth Factor (VEGF) Release from an Implant Material Comprised of Type I Collagen, PGA and PLA
[0273] Vascular endothelial growth factor (VEGF, obtained from Chemicon, Temecula, Calif.) was dissolved in a solution of matrix material comprised of type I collagen (80%), PGA (10%) and PLA (10%) as described in example 1. These materials were dissolved in HFIP at a final concentration of 0.08 gm per ml. Sufficient VEGF was added to 1 ml of solution to provide a VEGF concentration of 50 ng / ml of the collagen / PGA / PLA electrospinning solution. The material was electrospun to form a cylindrical construct and implanted into the rat vastus lateralis muscle using the same procedures set forth in Example 1. VEGF increased the density of functional capillaries that were present throughout the construct. This was evidenced by the presence of capillaries containing red blood cells (RBCs).
example 3
Release of VEGF from Constructs of Electroprocessed Collagen, PGA, and PLA
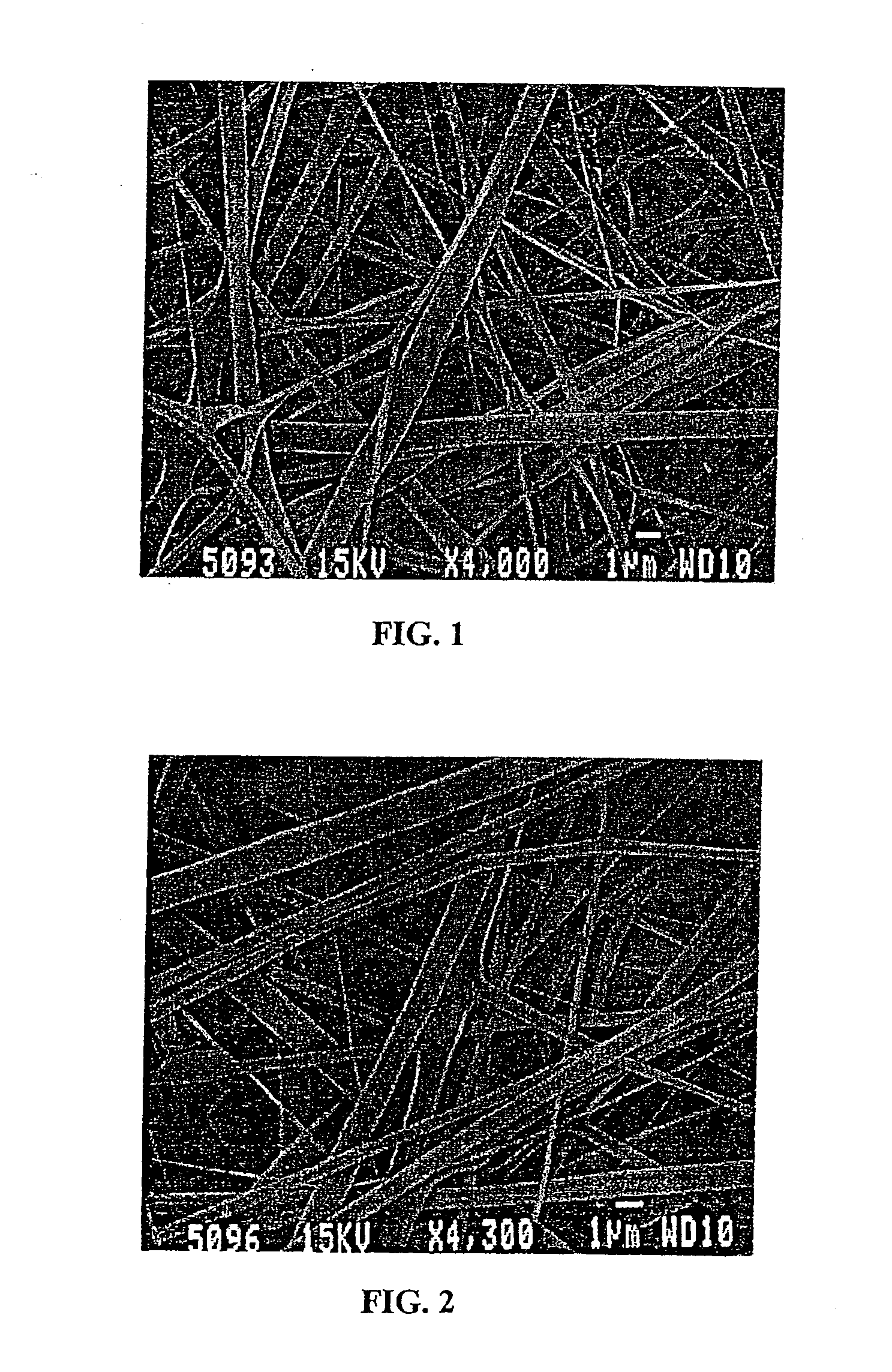
[0274] Constructs of electroprocessed collagen and PGA:PLA copolymer, with VEGF spun into the matrix were prepared using 80% collagen and 20% PGA:PLA. The collagen and PGA:PLA were dissolved in HFIP at a final combined concentration of 0.08 gm per ml. Solutions were prepared in which different amounts of VEGF were added to 1 ml of the solution of collagen and PGA:PLA copolymer. Separate solutions for electrospinning were prepared containing 0 ng, 25 ng, 50 ng, and 100 ng each of VEGF in 1 ml of electrospinning solution. Constructs were prepared for each solution by electrospinning 1 ml of material onto a cylindrical construct (2 mm in diameter). The constructs were removed from the target needle and cut in half. One half of each electrospun sample was then exposed to glutaraldehyde vapor fixation to cross link the fibers of collagen. Cross-linking was accomplished by exposing the constructs to glutaraldehyde...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com