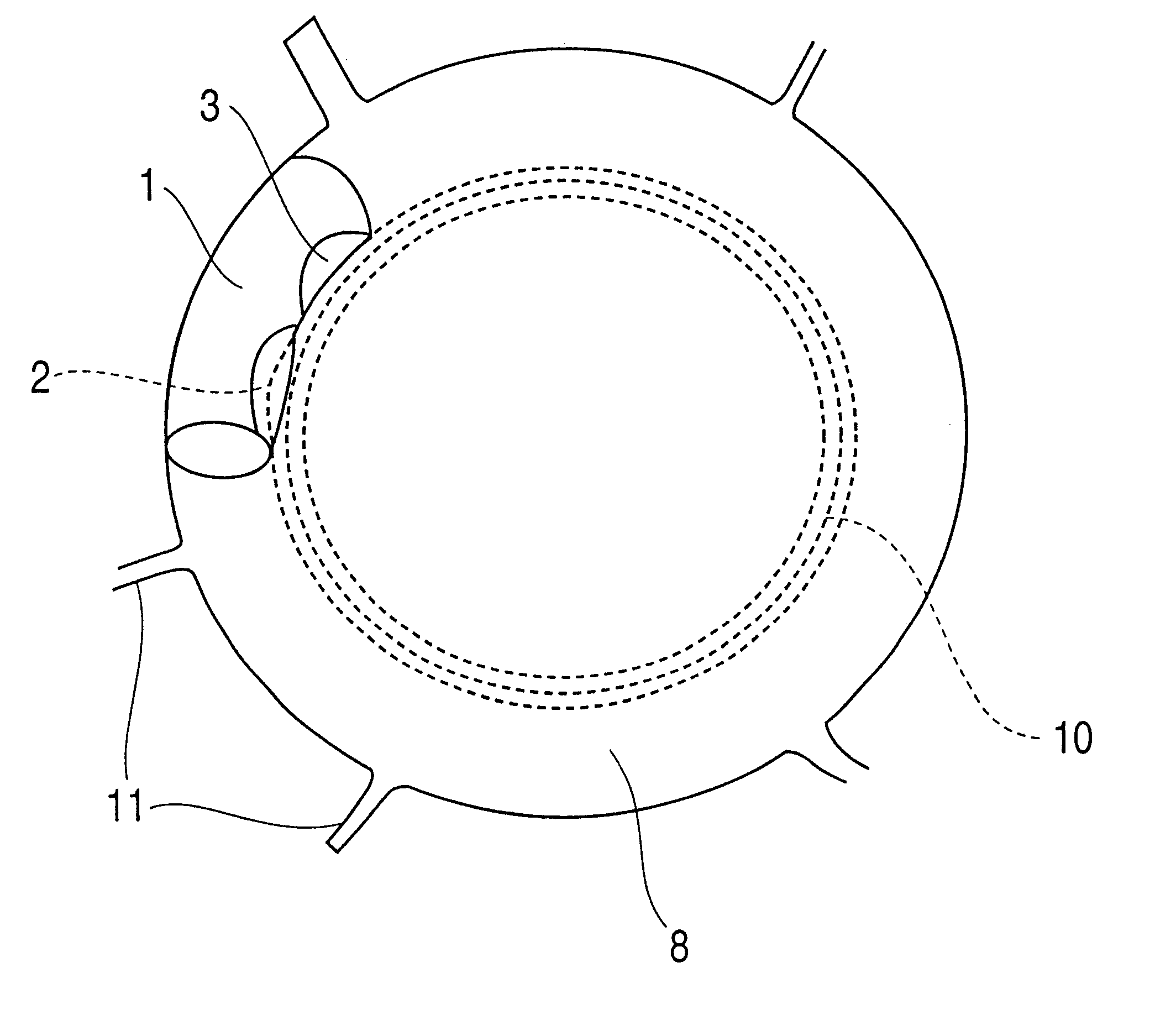
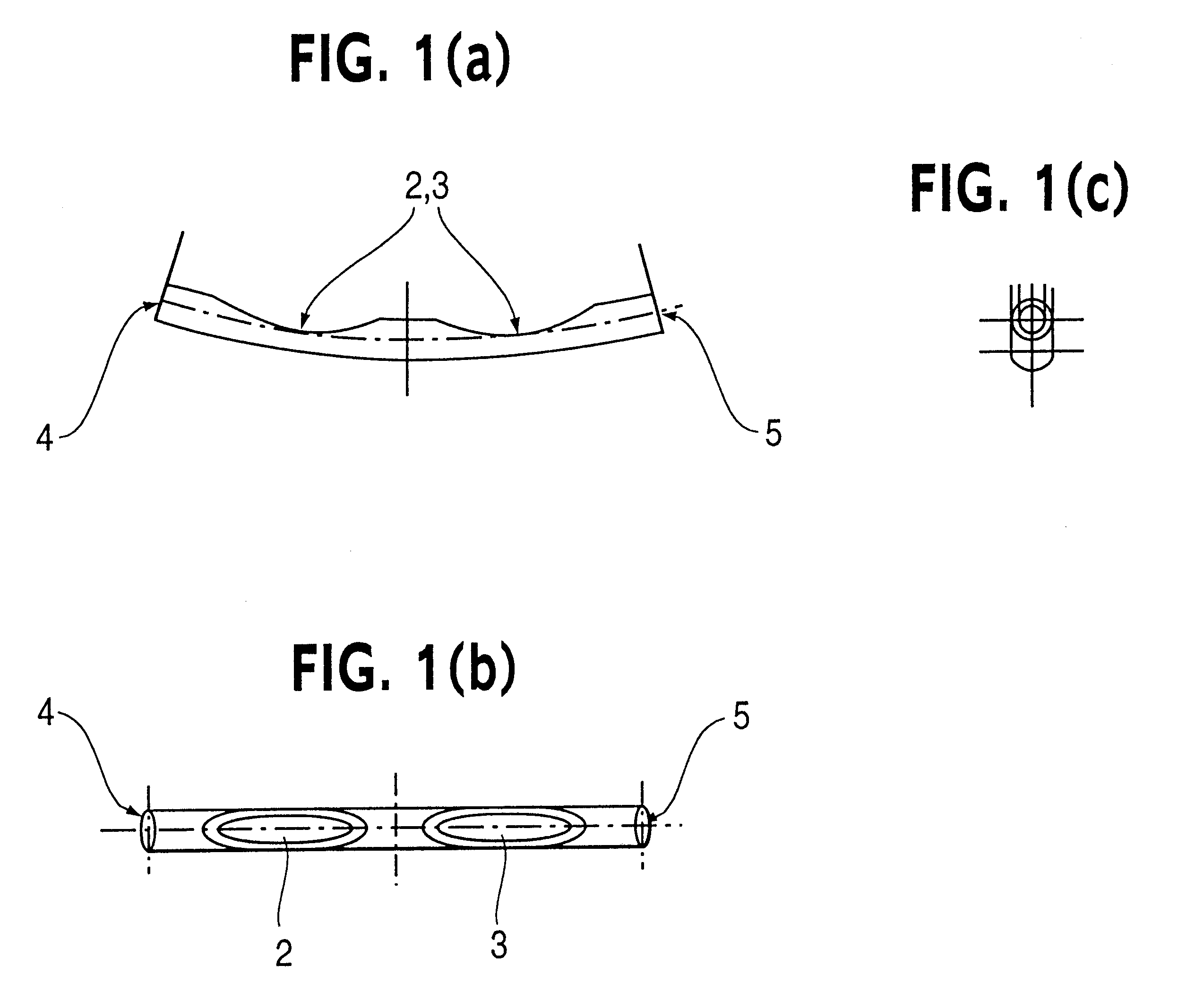
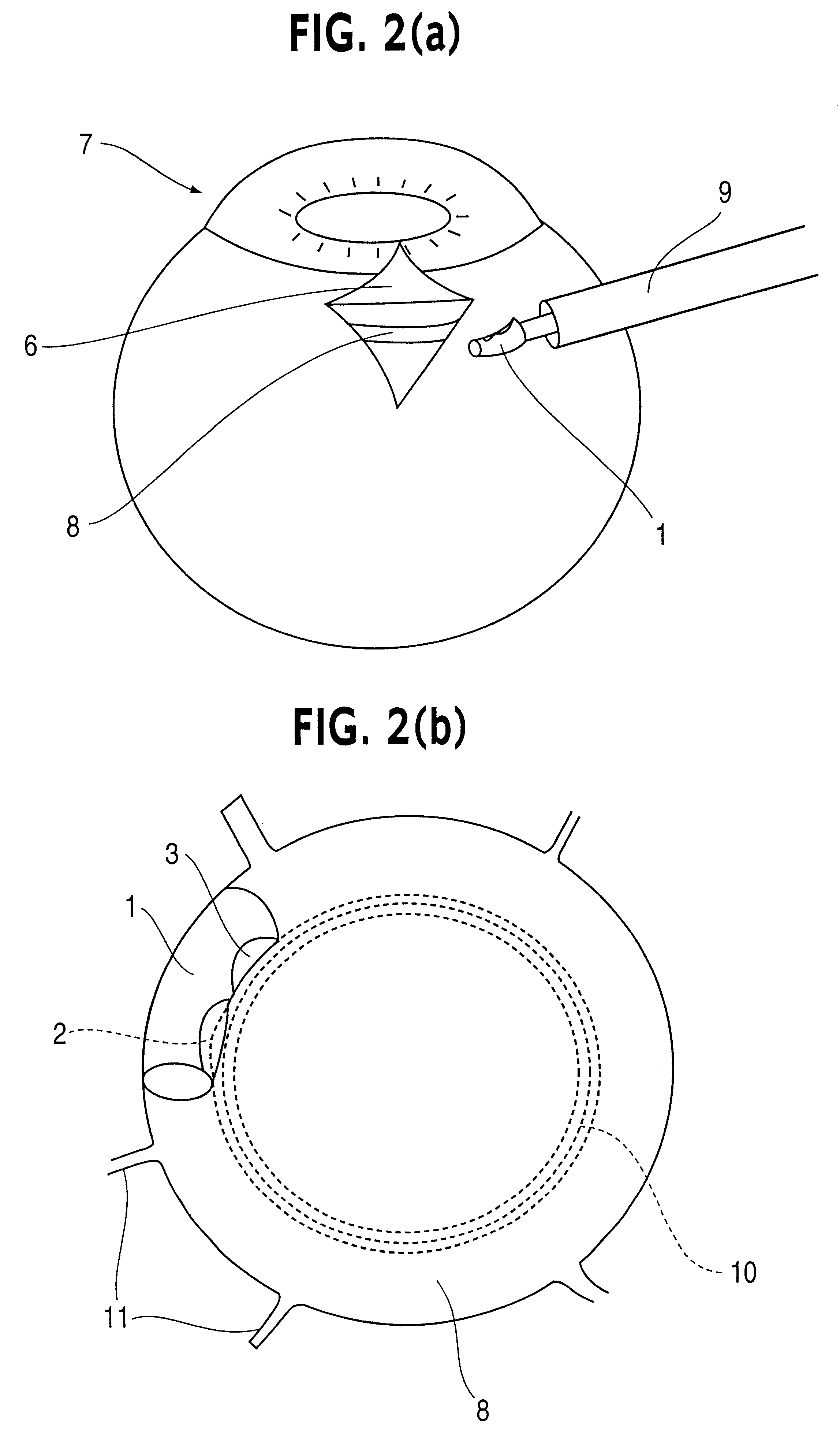
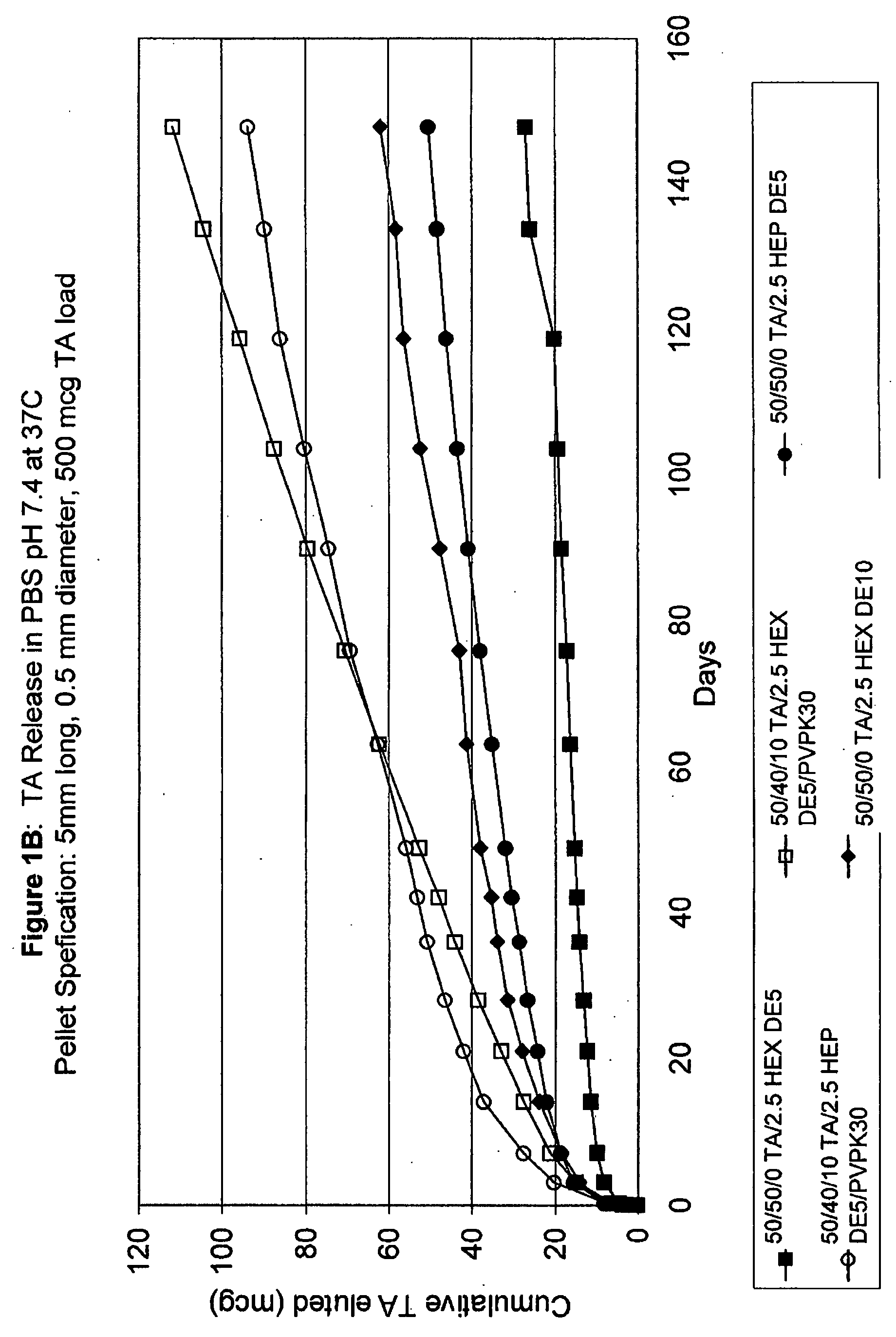
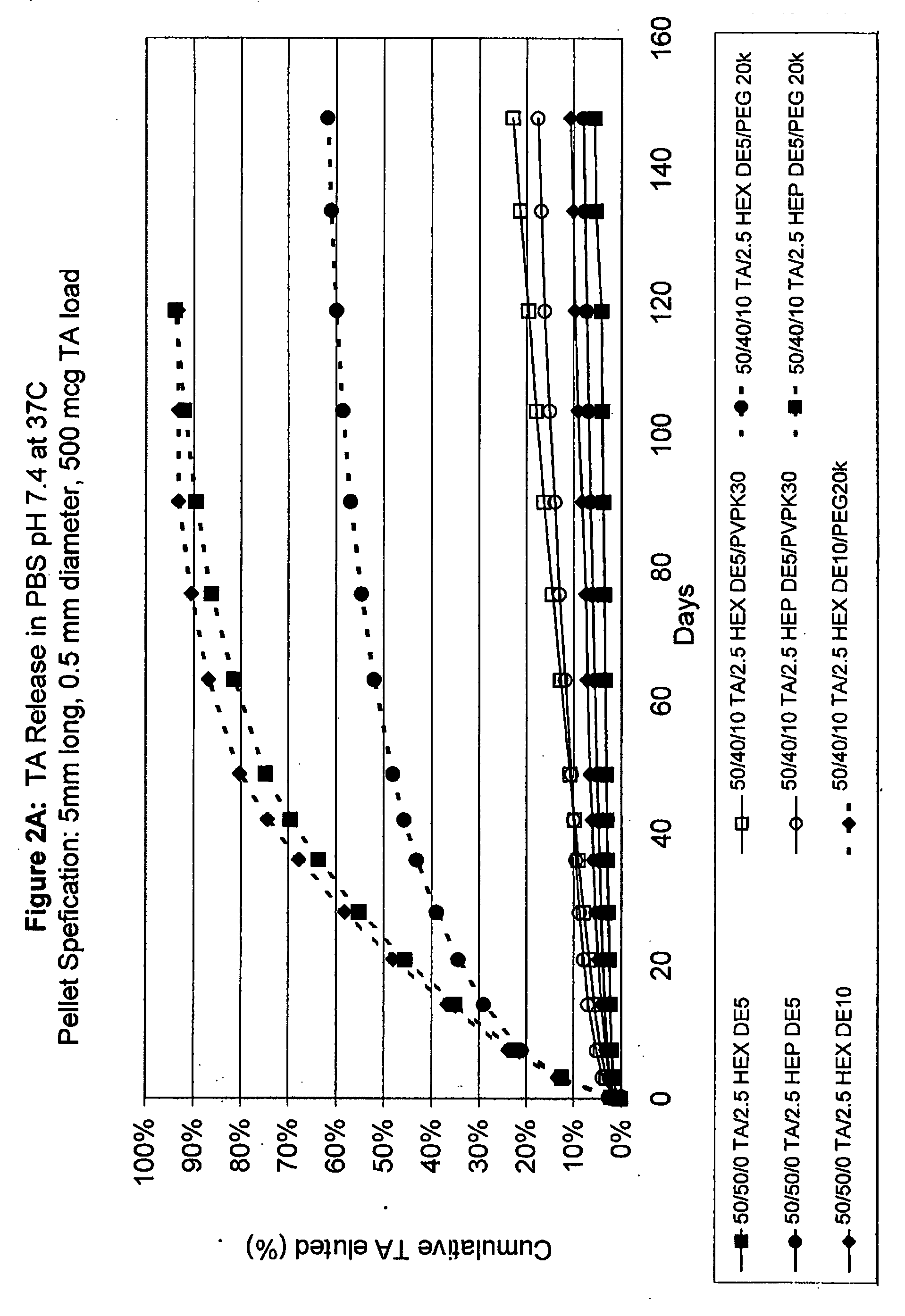
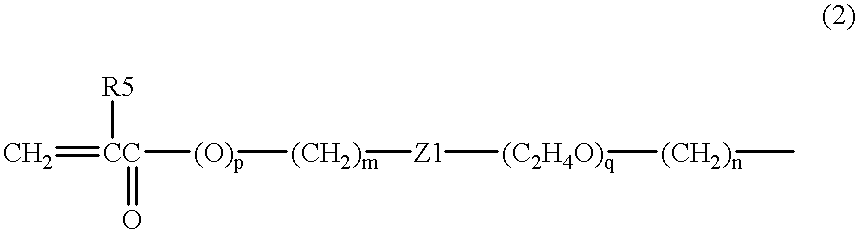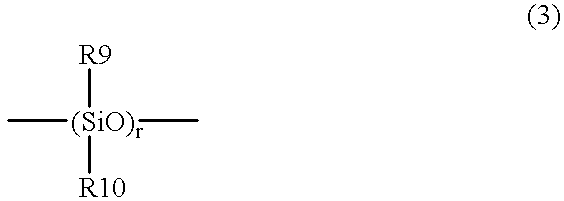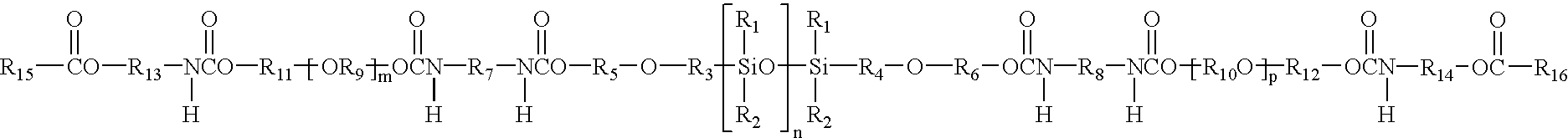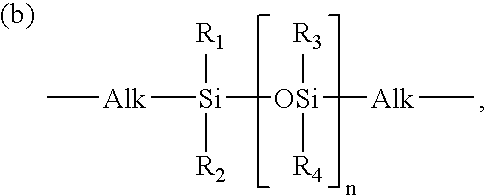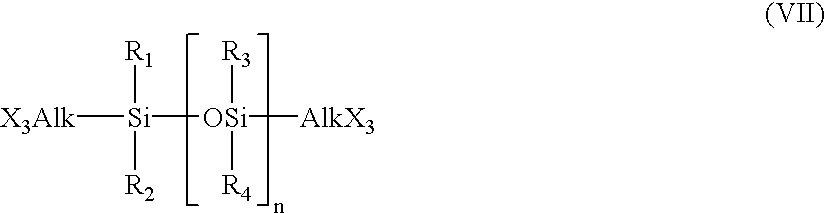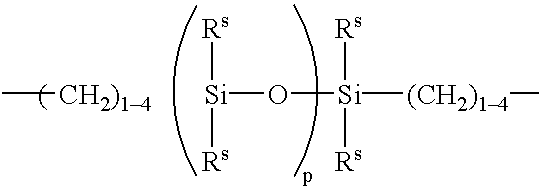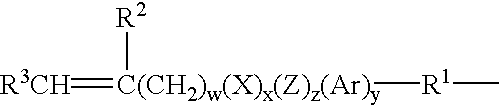Patents
Literature
1018results about "Eye implants" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
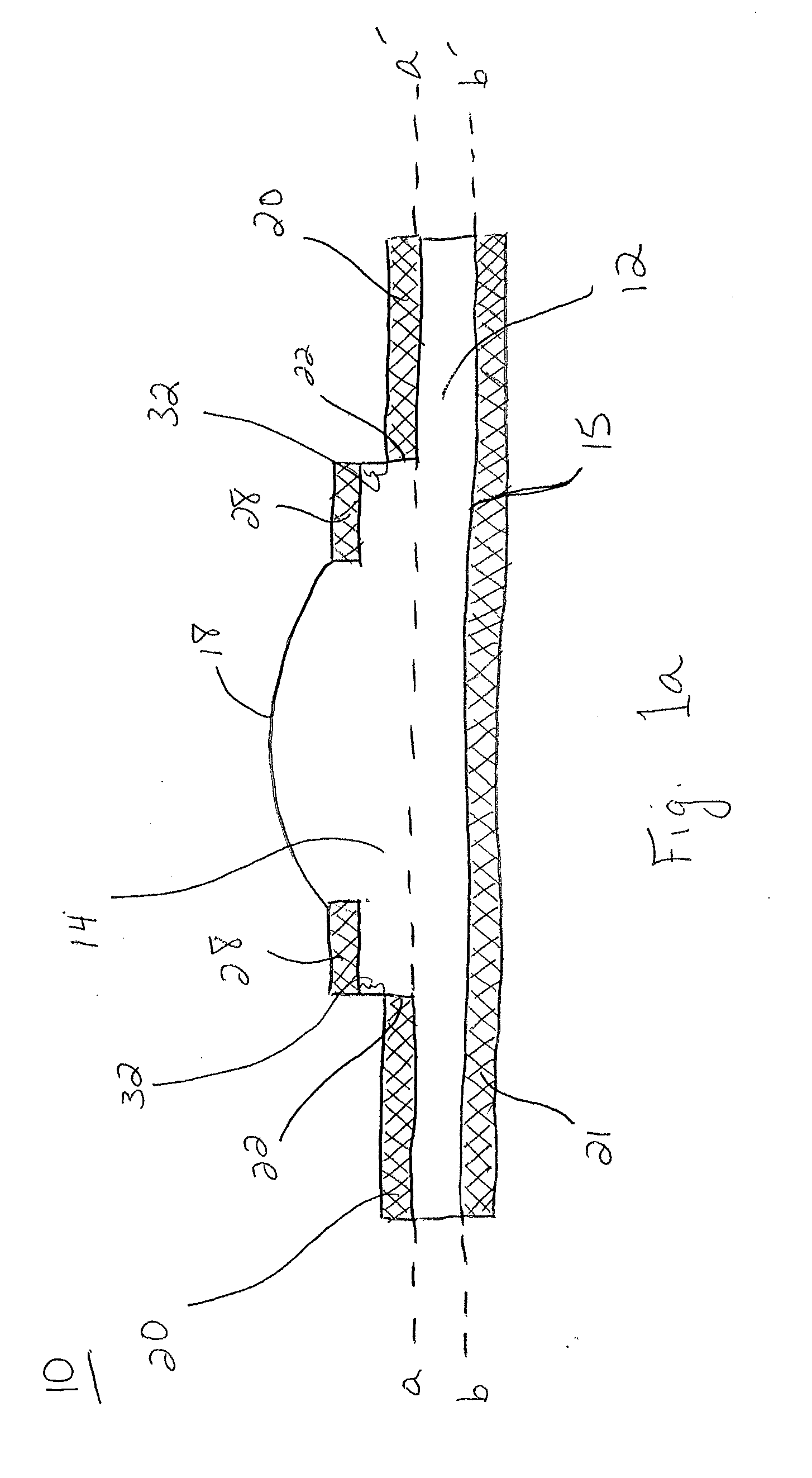
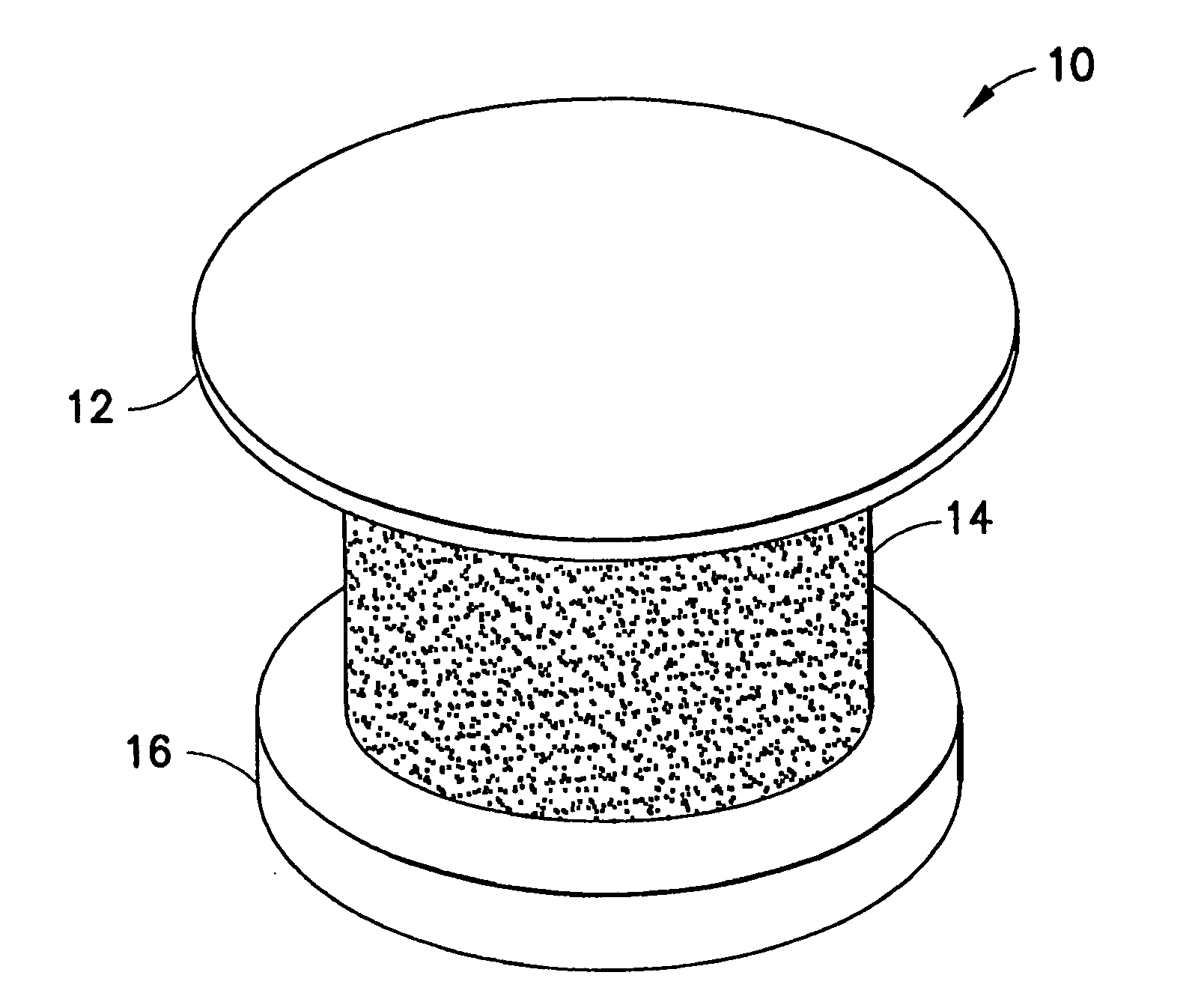
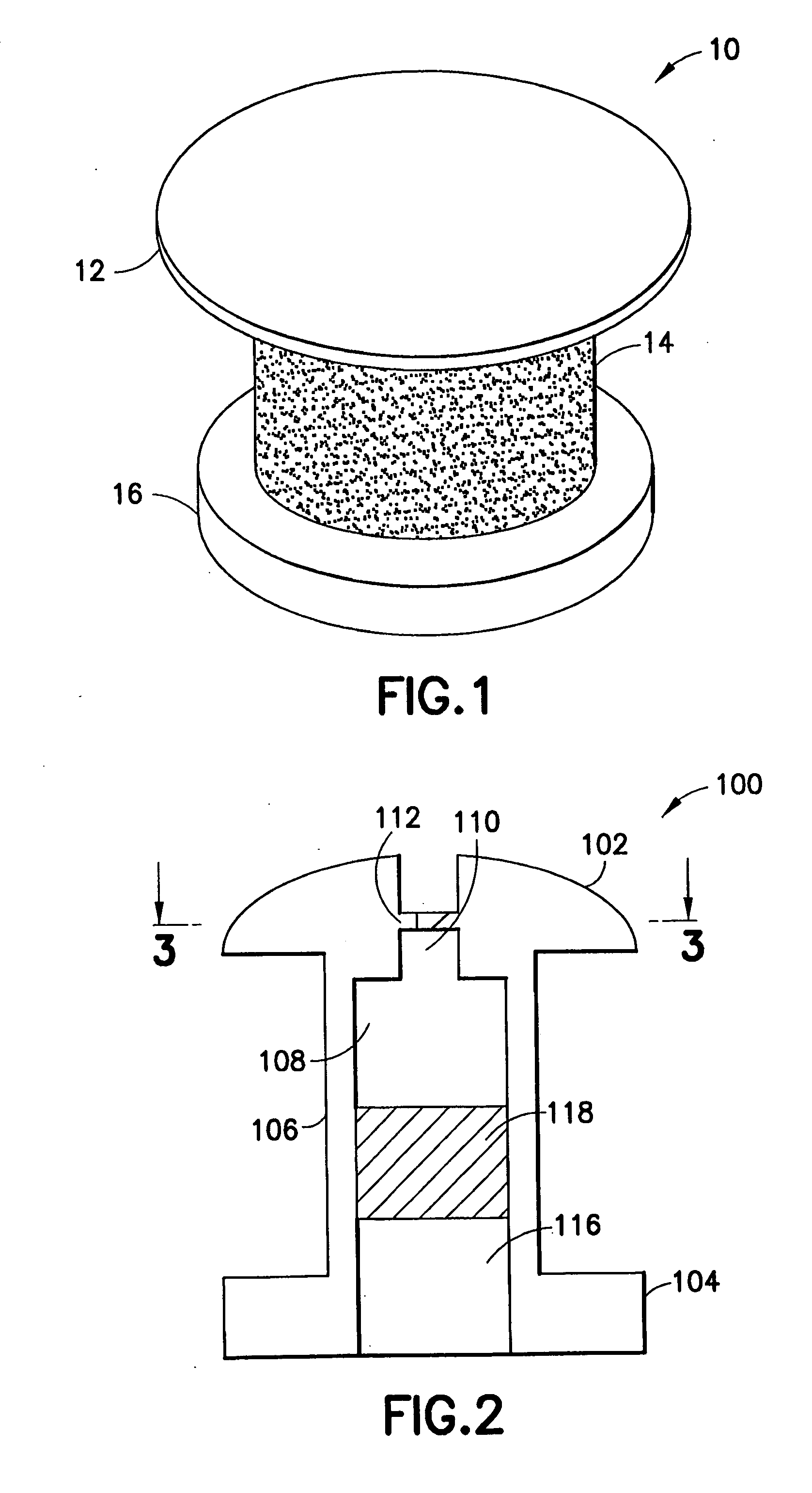
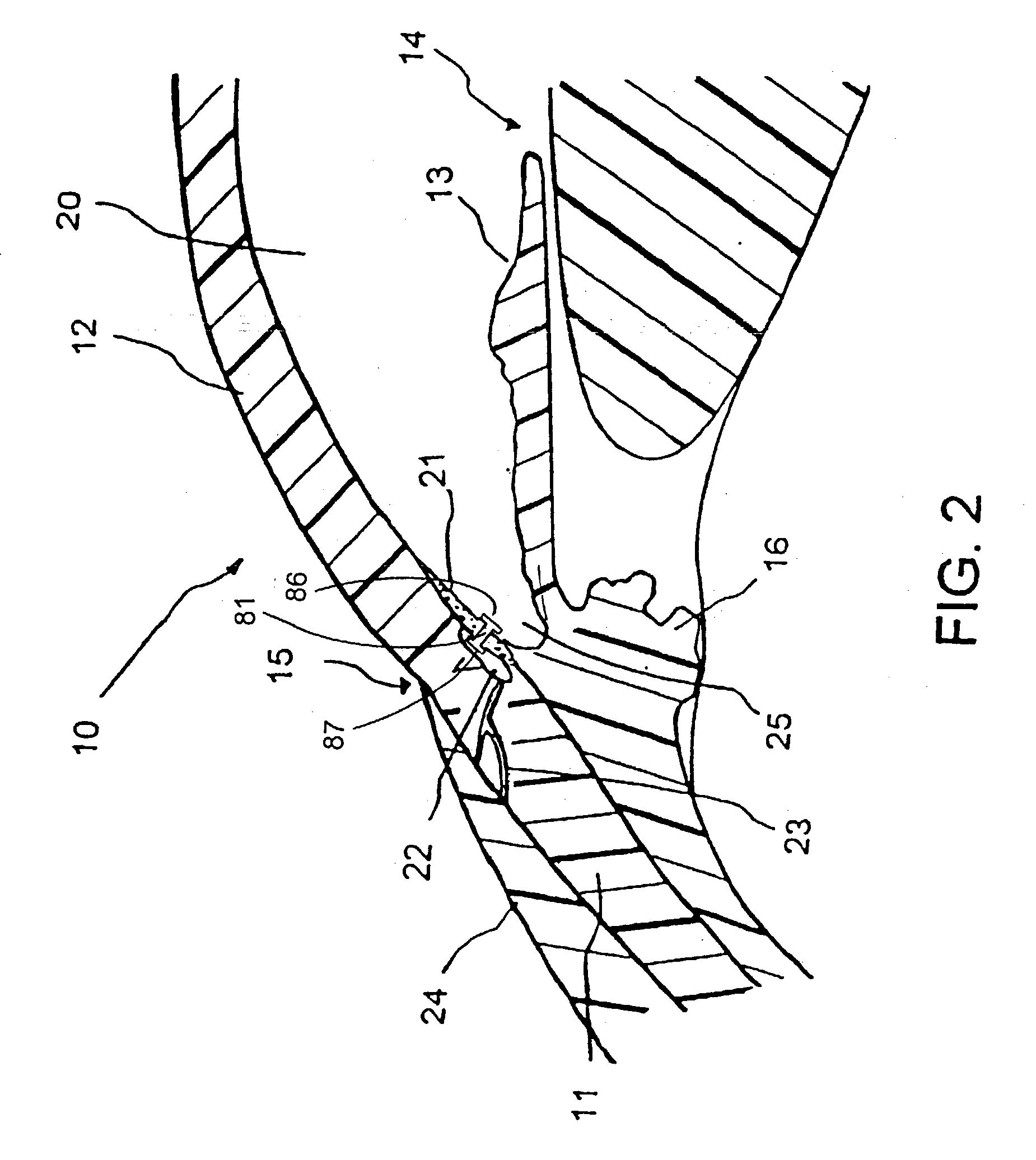
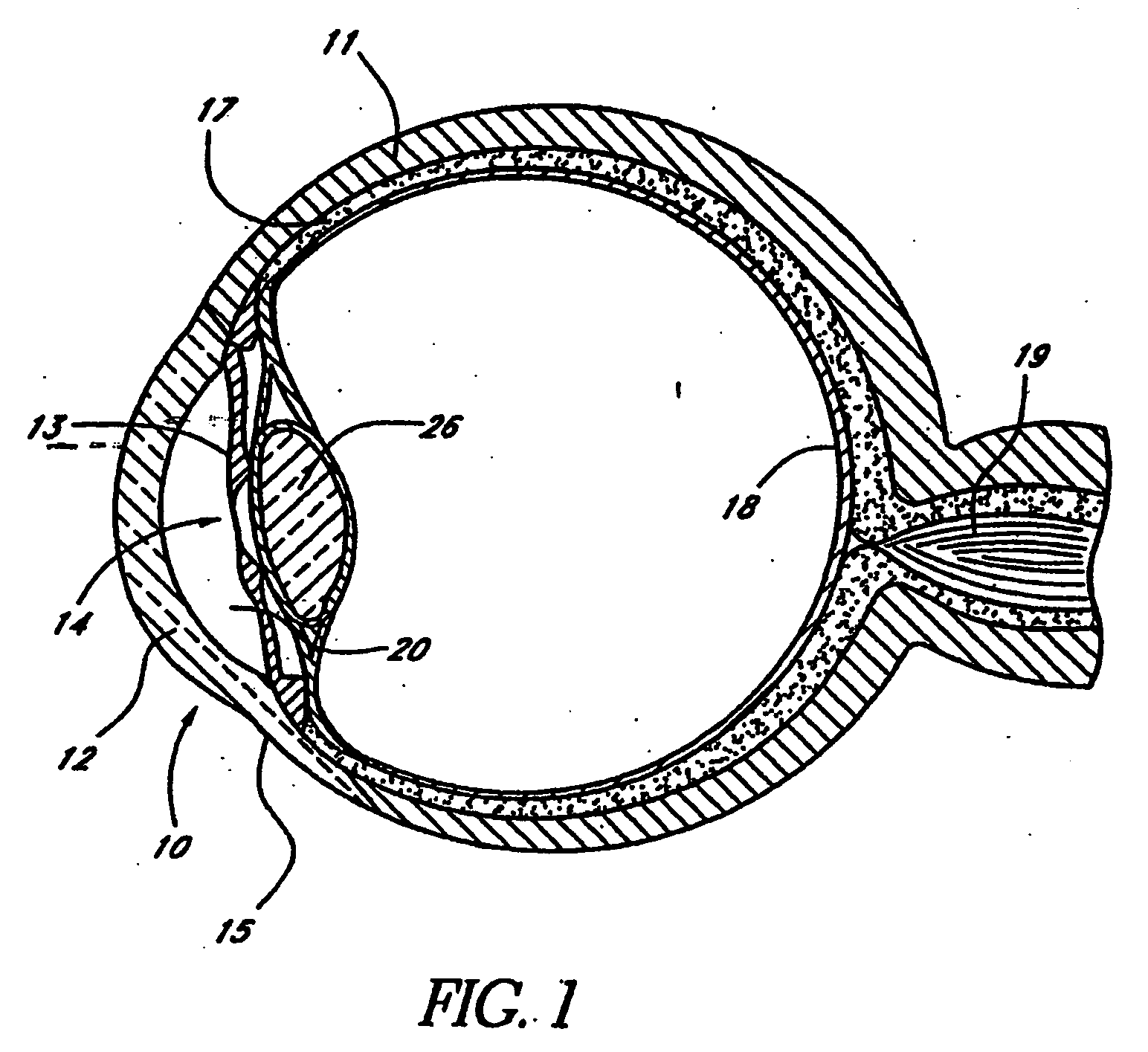
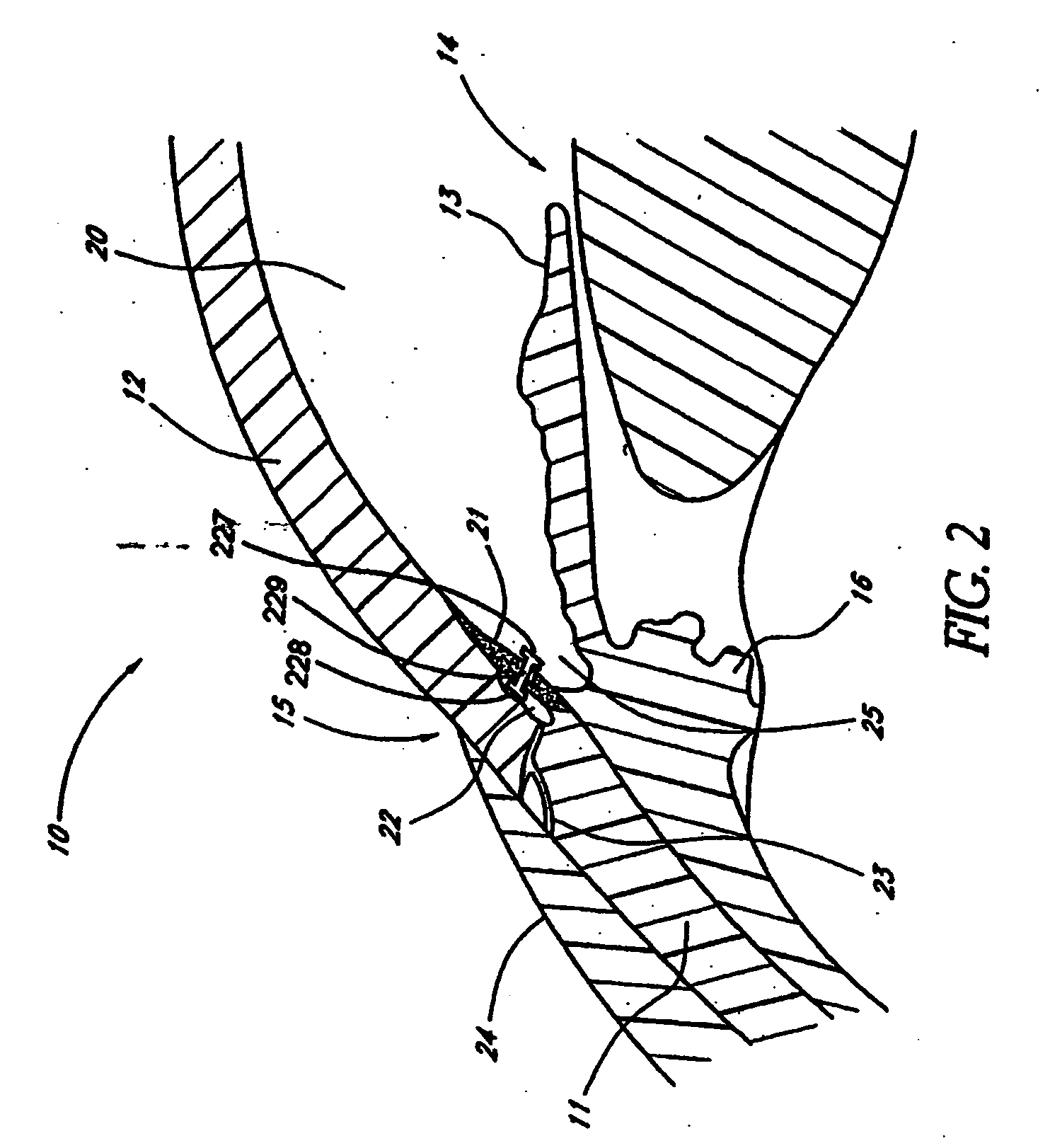
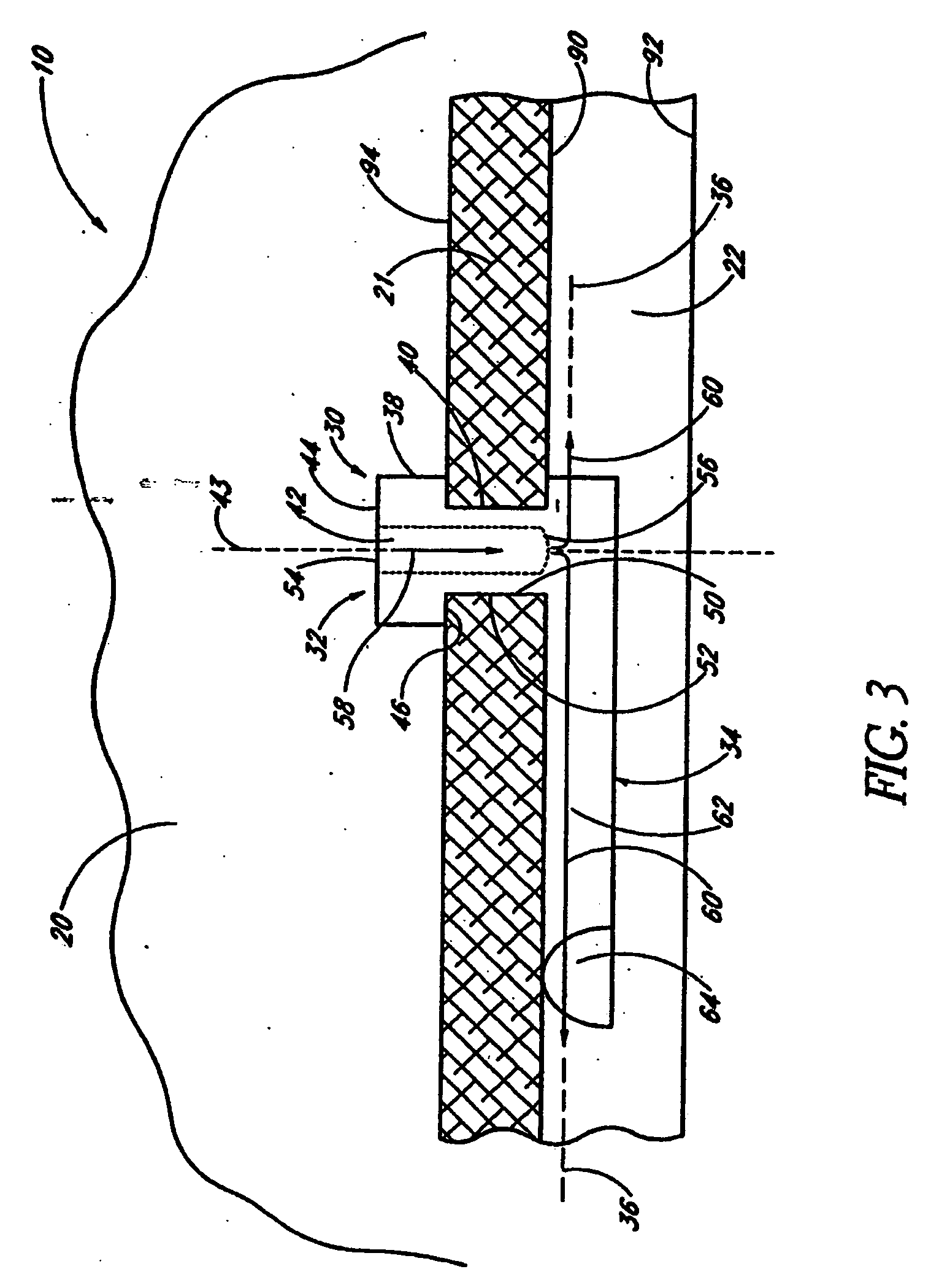
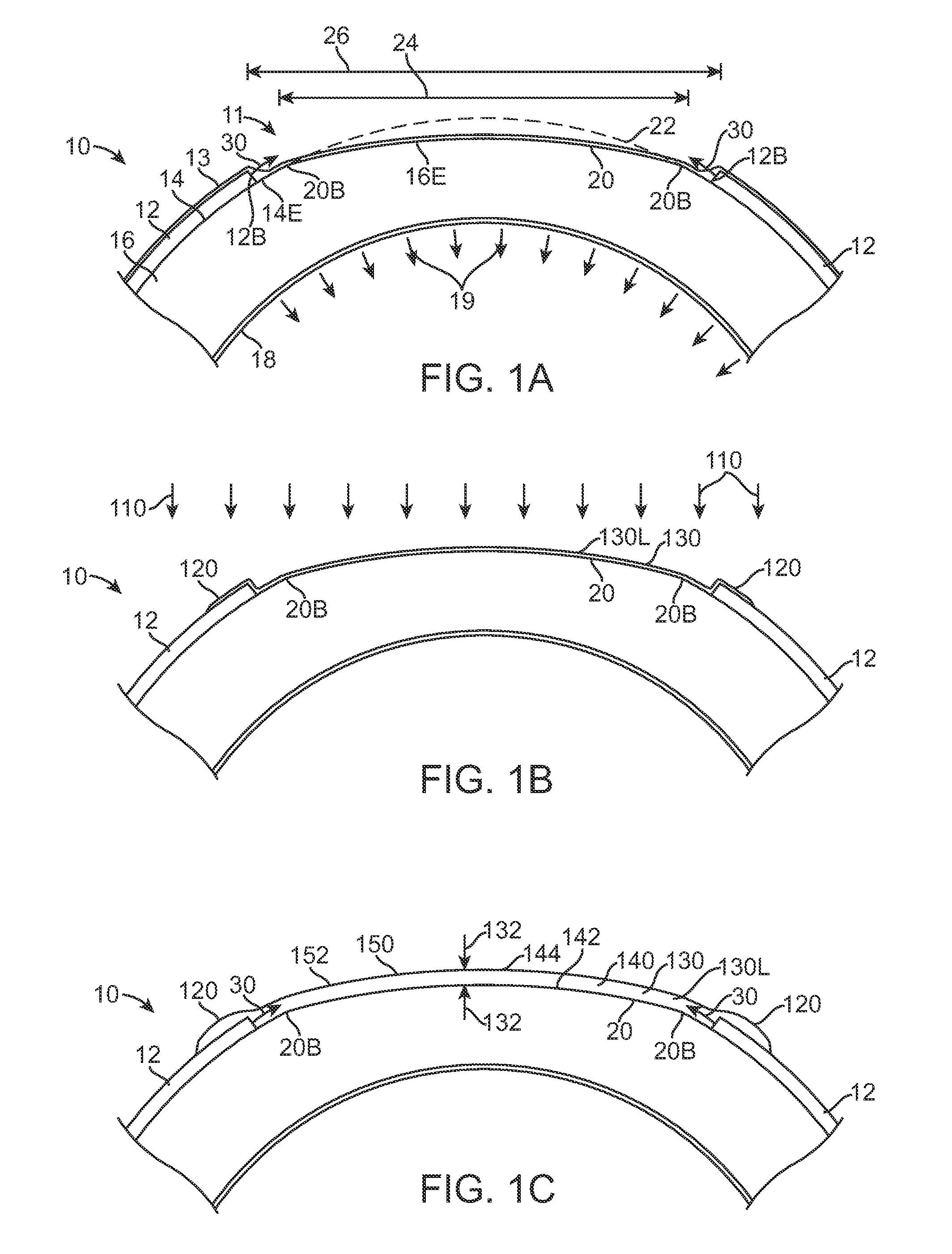
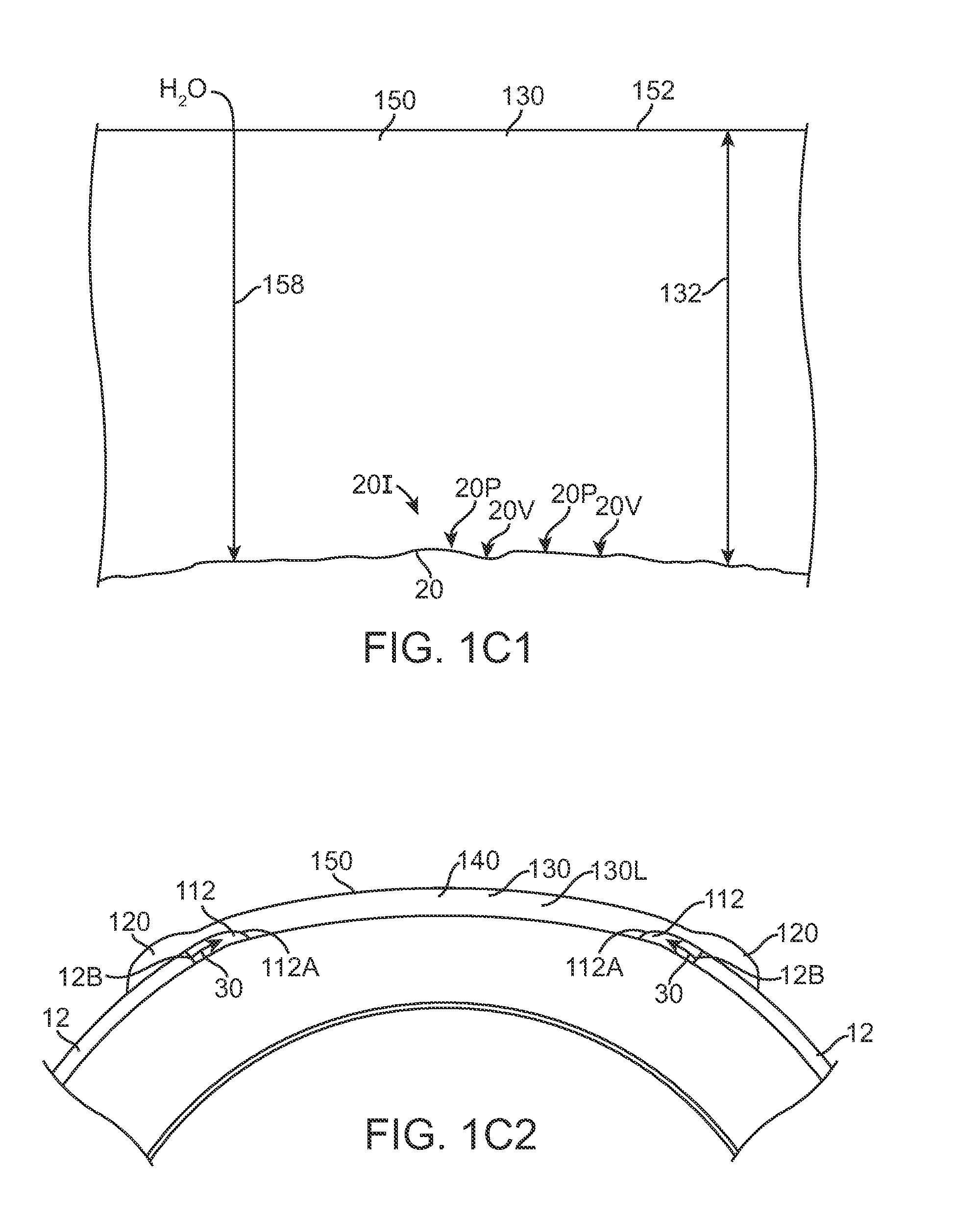
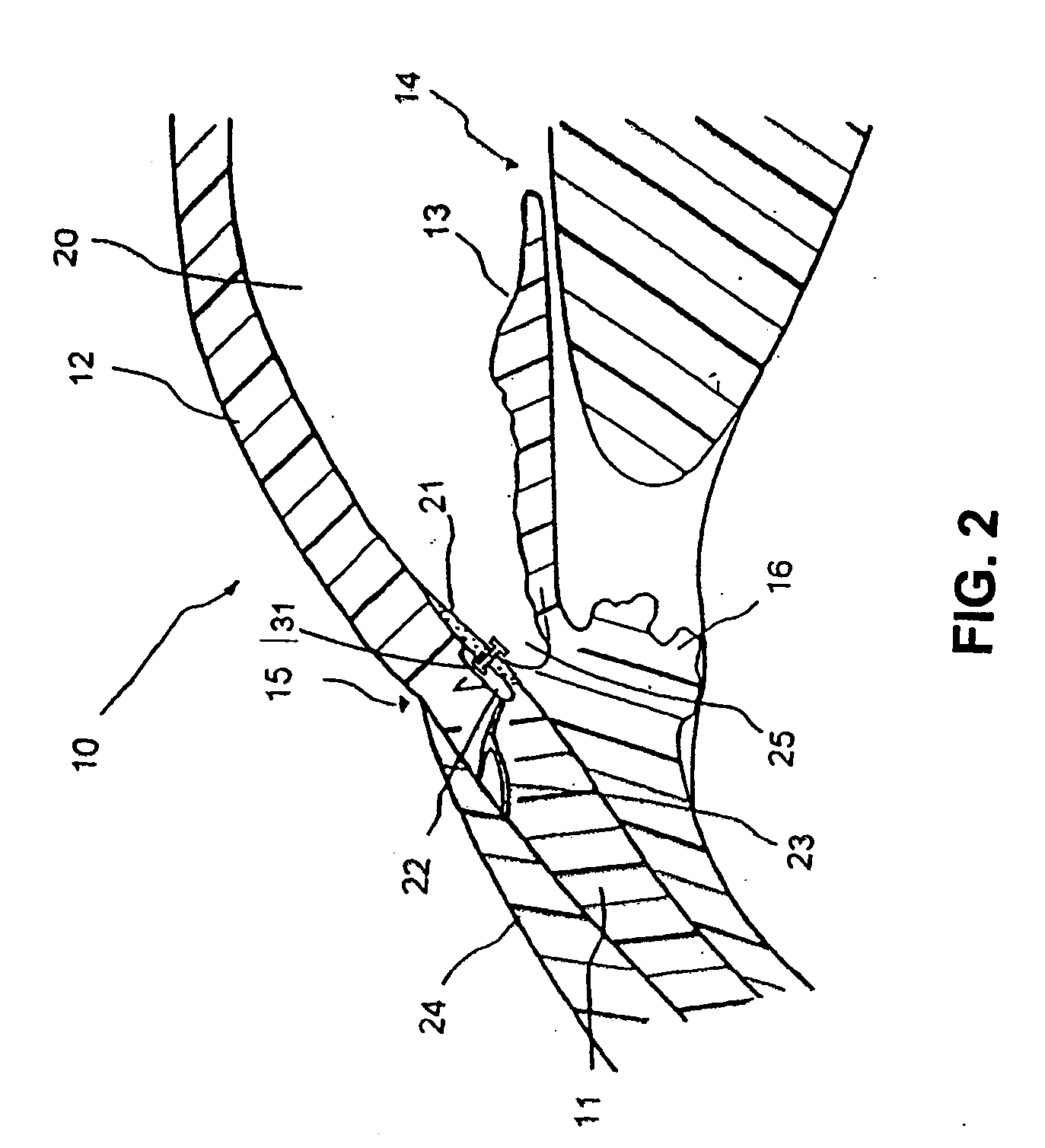
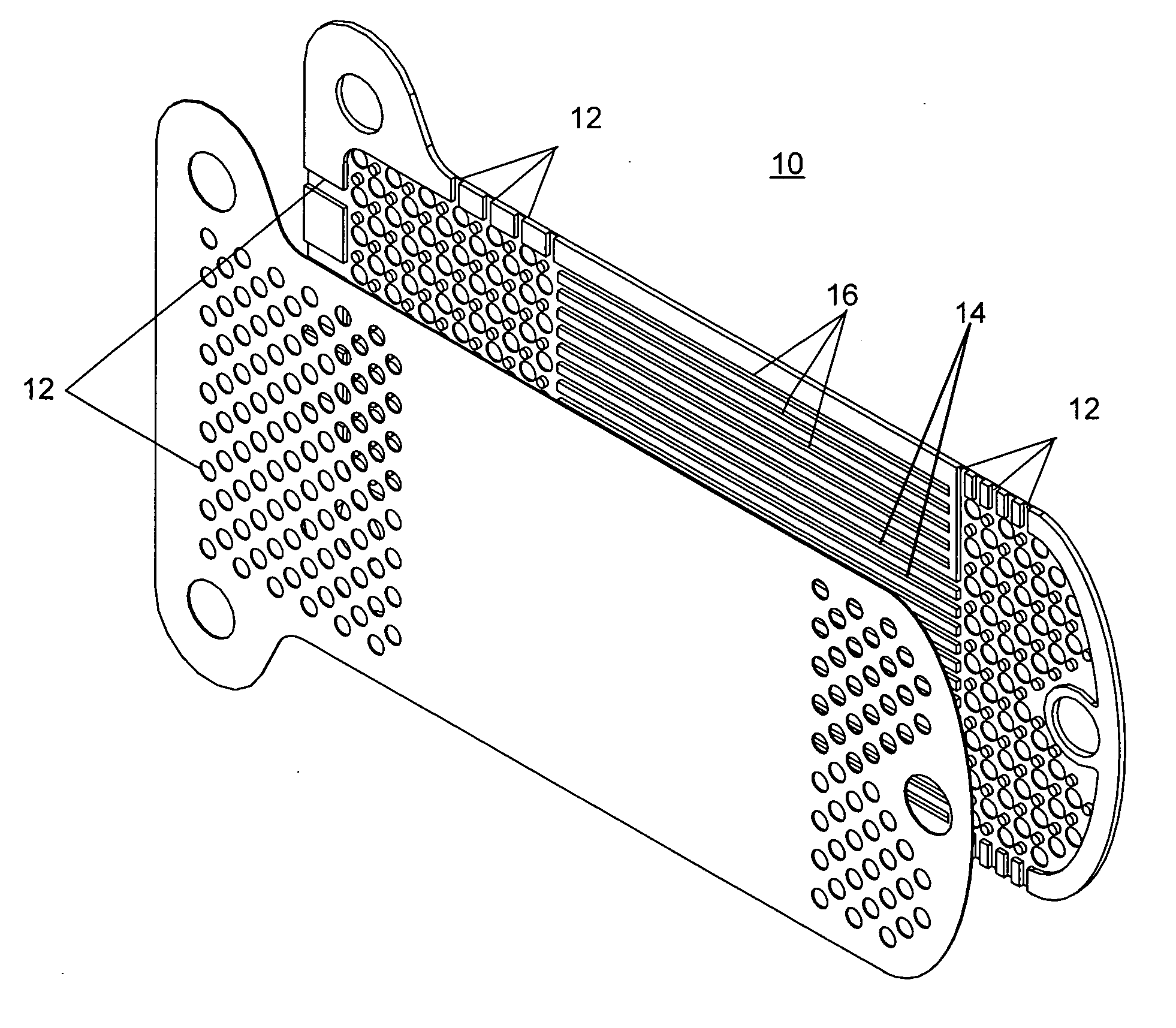
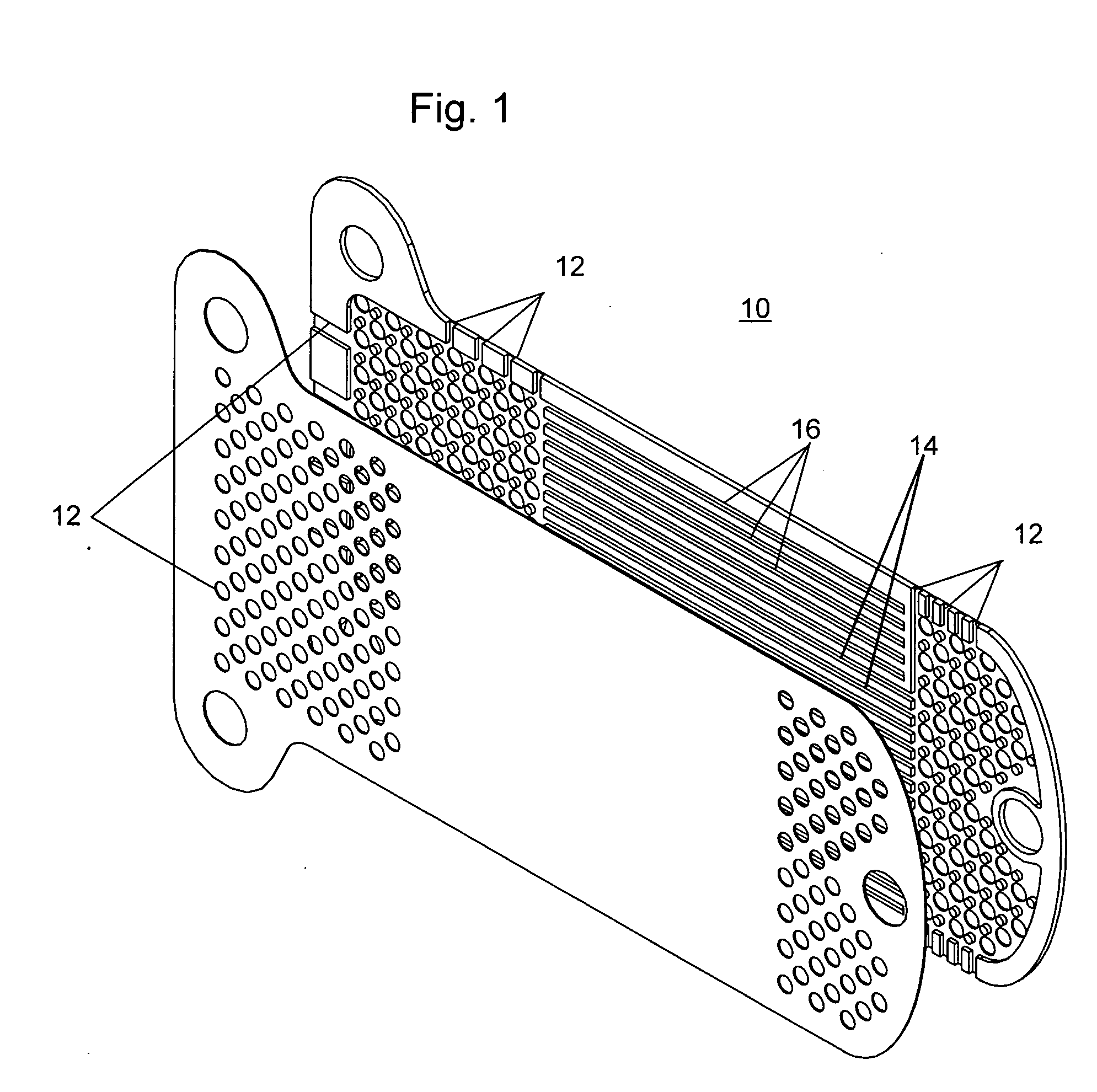
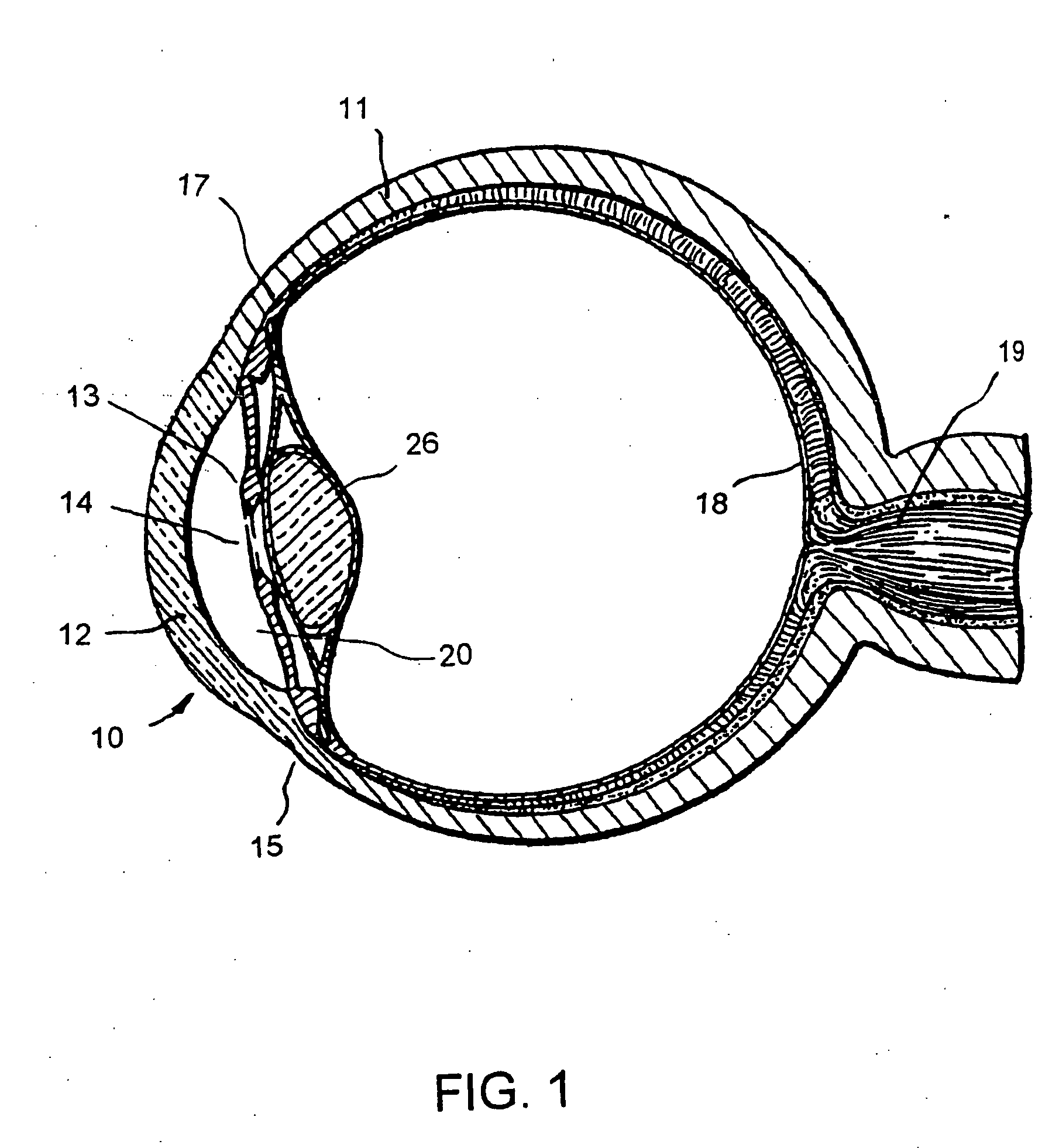
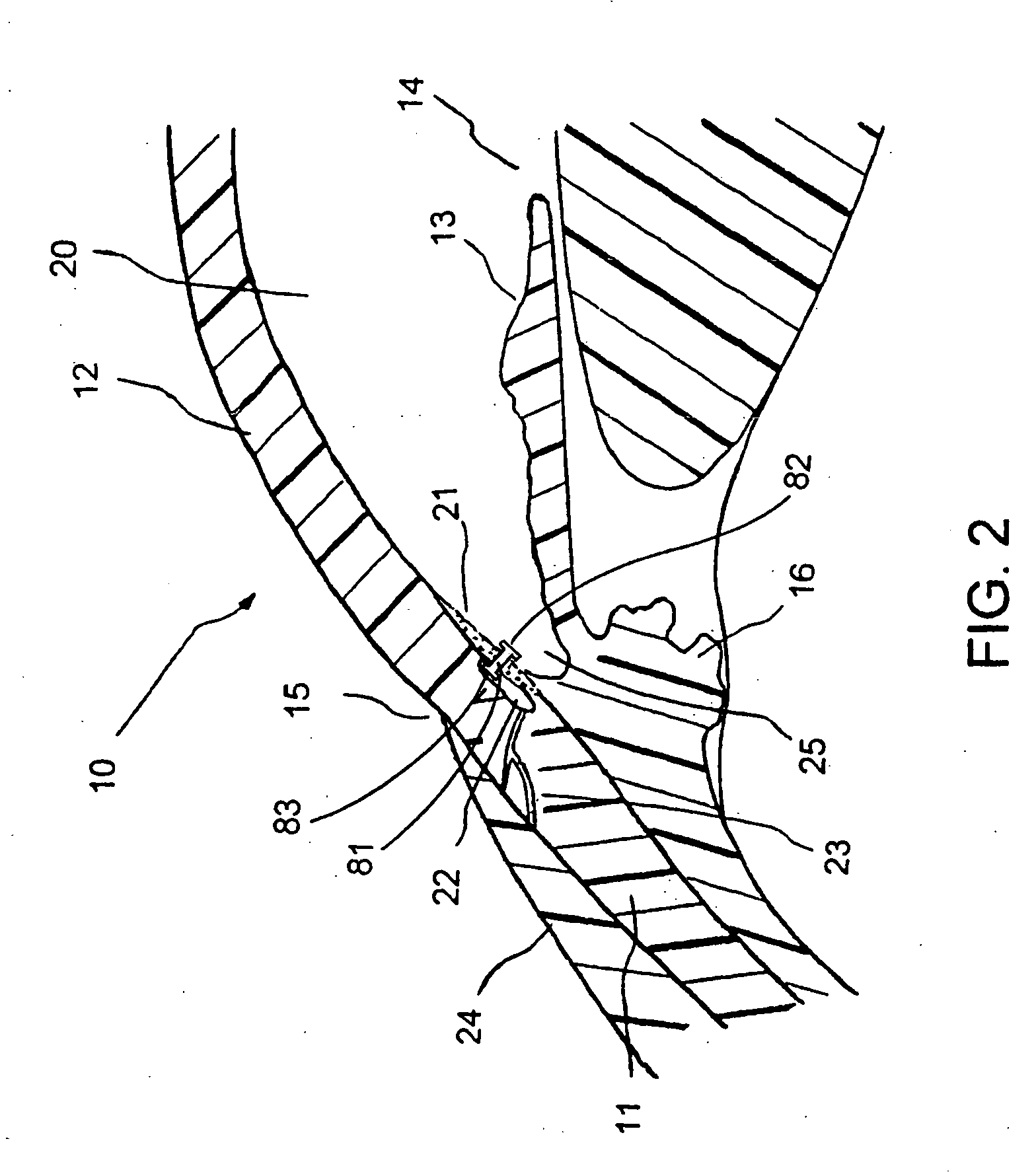
Extended wear ophthalmic lens
InactiveUS5760100AExcellent ion permeabilityGood water permeabilityLiquid surface applicatorsEye implantsExtended wear contact lensesIon permeation
Owner:NOVARTIS AG
Long wearable soft contact lens
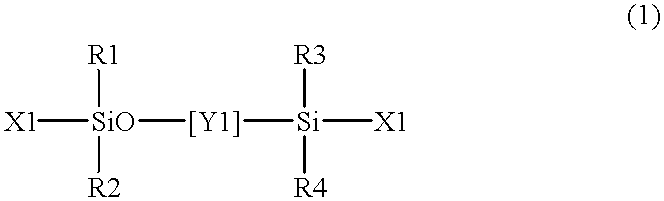
The present invention relates to a soft contact lens, and provides a contact lens which shows small and stable contact angle to water at its surface in water as well as in air, little deposition in wearing, high oxygen permeability, no adhesion of lens to a cornea and superior extended-wearing characteristics. The present invention provides a hydrogel soft contact lens which has contact angle at a lens surface in a range of 10-50° by the captive bubble method in water and 30-90° by the sessile drop method in air, oxygen permeability of not less than 30 and water content of not less than 5%, and also a hydrogel soft contact lens consisting of a polymer comprising a hydrophilic siloxanyl monomer shown by a specified general formula.
Owner:COOPERVISION INT LTD
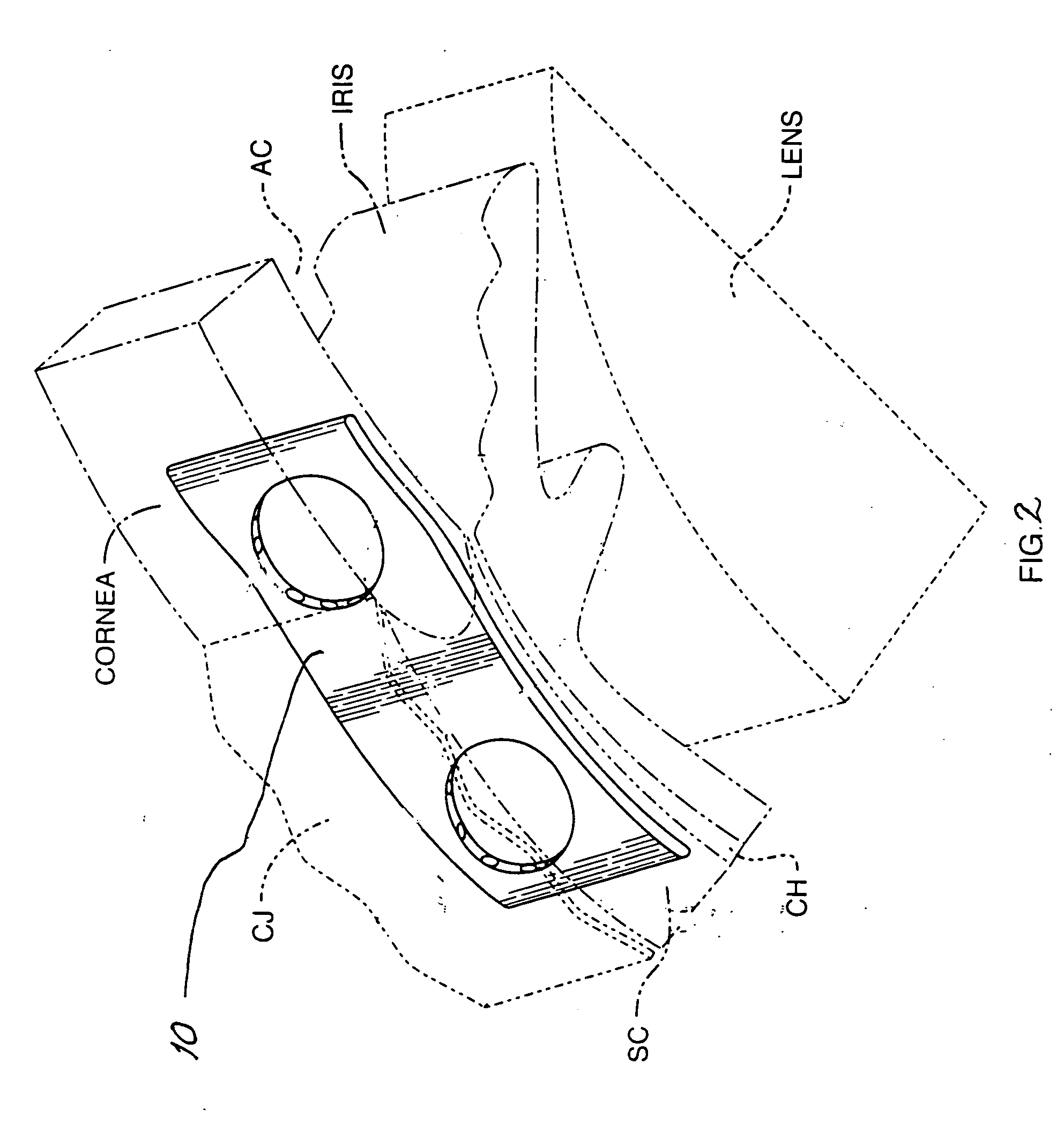
Artificial Cornea and Method of Making Same
Owner:WL GORE & ASSOC INC
Biocompatible implant and use of the same
InactiveUS20060252981A1Good biological affinityStrong enoughImpression capsEye implantsRough surfaceBiological body
The present invention provides an implant capable of being cellularized in treatment of an injured organ or tissue in organisms. The present inventors found that a biocompatible implant comprising a biological molecule and a support is capable of being cellularized. The implant can be used instead of conventional implants which essentially comprise cells. The present invention provides a biocompatible implant comprising A) a biological molecule and B) a support. The present invention also provides A) a first layer having a rough surface, B) a rough surface; B) a second layer having a strength which allows the support to resist in vivo shock. The first layer is attached to the second layer via at least one point.
Owner:CARDIO
Ocular implant and methods for making and using same
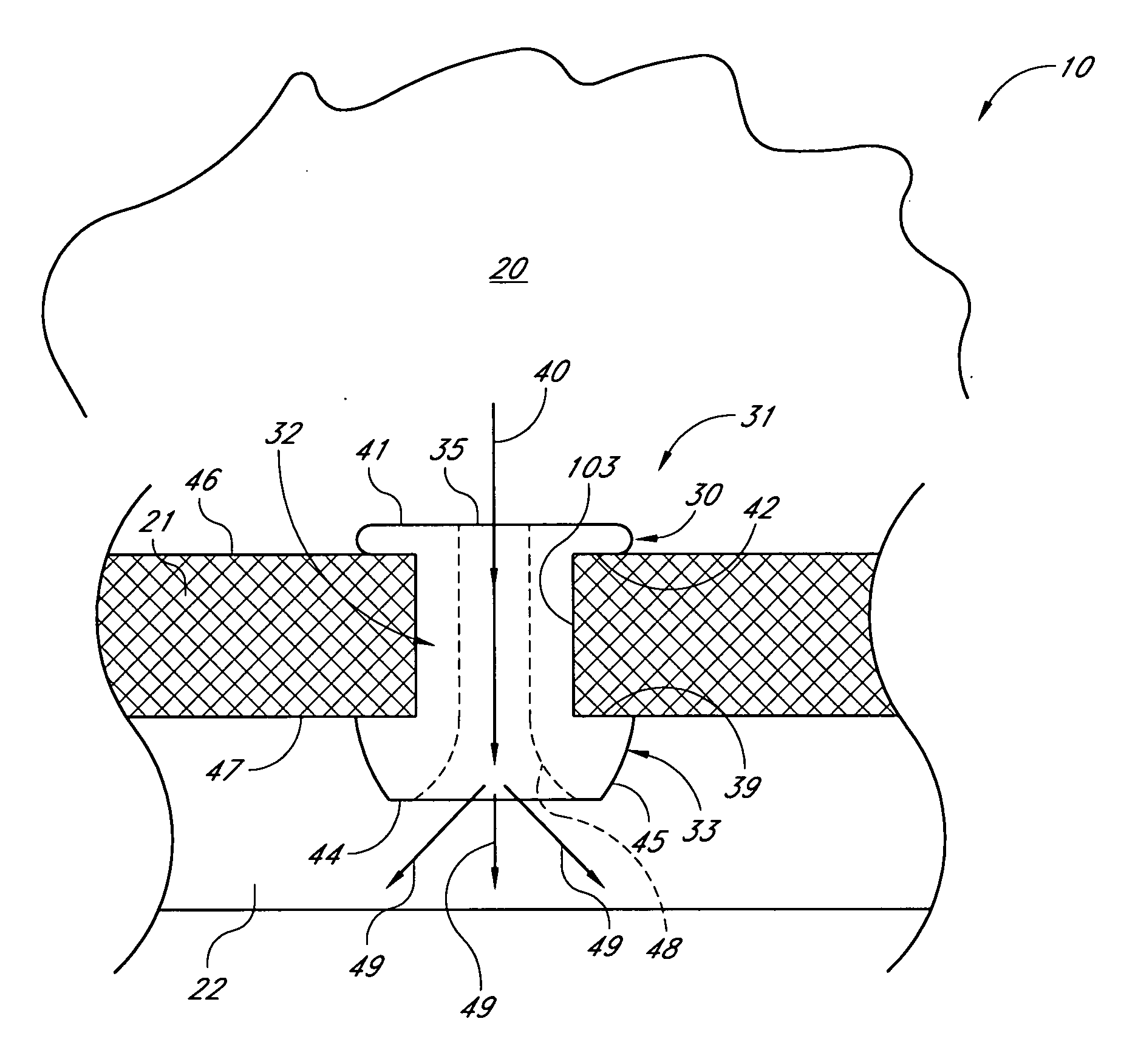
InactiveUS20050119737A1MinimizeLower eye pressureEye implantsEye surgeryAqueous humorImplanted device
An ocular implant device that is insertable into either the anterior or posterior chamber of the eye to drain aqueous humor and / or to introduce medications. The implant can include a substantially cylindrical body with a channel member that regulates the flow rate of aqueous humor from the anterior chamber or introduces medications into the posterior chamber, and simultaneously minimizes the ingress of microorganisms into the eye.
Owner:BECTON DICKINSON & CO
Drug delivery device
Drug delivery devices, and methods of delivering pharmaceutically active agents to a target tissue within a body using such devices, are disclosed. One drug delivery device includes a body having an internal surface for placement proximate a target tissue and a well having an opening to the internal surface. An inner core comprising a pharmaceutically active agent is disposed in the well.
Owner:NOVARTIS AG
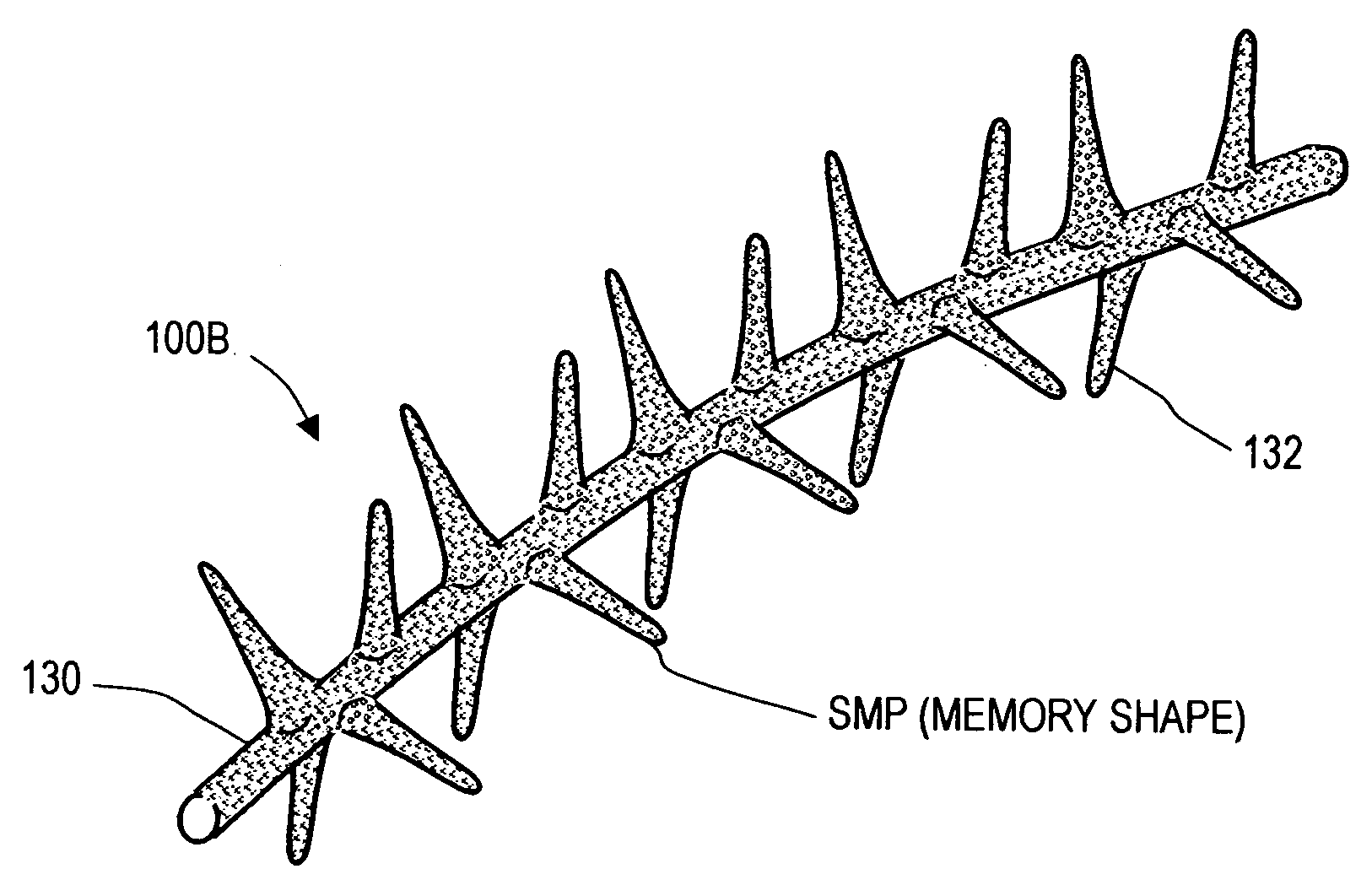
Implants for treating ocular hypertension, methods of use and methods of fabrication
A stent for treating ocular hypertension by providing means for enhancing outflows of aqueous humor from the anterior chamber. An exemplary stent is fabricated of a shape memory polymer (SMP) that can withstand very large reversible inelastic strains for storing energy in a temporary reduced cross-sectional shape. In one embodiment, the stent in a temporary shape is introduced into a targeted tissue volume in and about the eye's aqueous outflow pathways. Following minimally invasive implantation of the stent, body temperature or another stimulus causes the stent to move from its temporary shape to its memory shape thereby releasing stored energy to retract the tissue to open flow pathways or increase tissue permeability. In another embodiment, the SMP stent body has interior flow passageways to provide addition fluid outflow means. In several embodiments, the stent can be of a shape memory alloy material.
Owner:SHADDUCK JOHN H
Expandable glaucoma implant and methods of use
Disclosed is an implant for use in an eye with glaucoma, the implant including an inlet section in fluid communication with an outlet section, the inlet section being sized and shaped to fit at least partially in the anterior chamber of the eye, and the outlet section being sized and shaped to fit at least partially in Schlemm's canal of the eye. The implant also includes an expandable substrate suitable for expansion in the eye to assist in retaining the implant in the eye.
Owner:GLAUKOS CORP
Injectable glaucoma implants with multiple openings
ActiveUS20050271704A1Reduce morbidityAvoiding hypotonyEye implantsEye surgerySchlemm's canalIntraocular pressure
Intraocular stents and applicators are disclosed for treating glaucoma. The stents are configured to extend between the anterior chamber of the eye and Schlemm's canal for enhancing outflow of aqueous from the anterior chamber so as to reduce intraocular pressure. The stents can have features for anchoring the stent into Schlemm's canal as well as preventing the walls of Schlemm's canal from closing the outlet of the stents. The applicators can be steerable so as to make implantation easier. Additionally, the applicators can be configured to hold a plurality of stents so that multiple stents can be implanted through one incision without removing the applicator from the incision between serial implantations.
Owner:GLAUKOS CORP
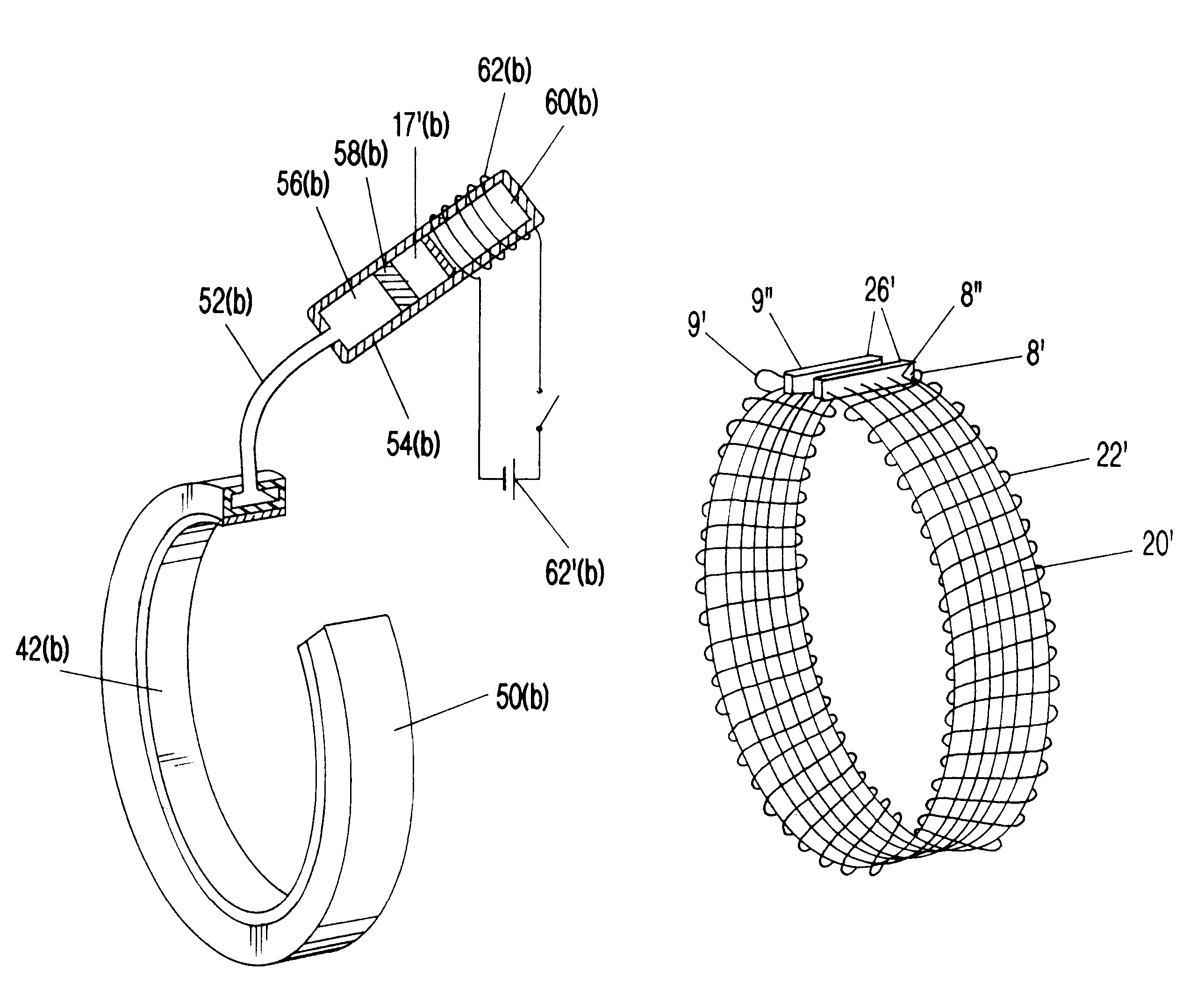
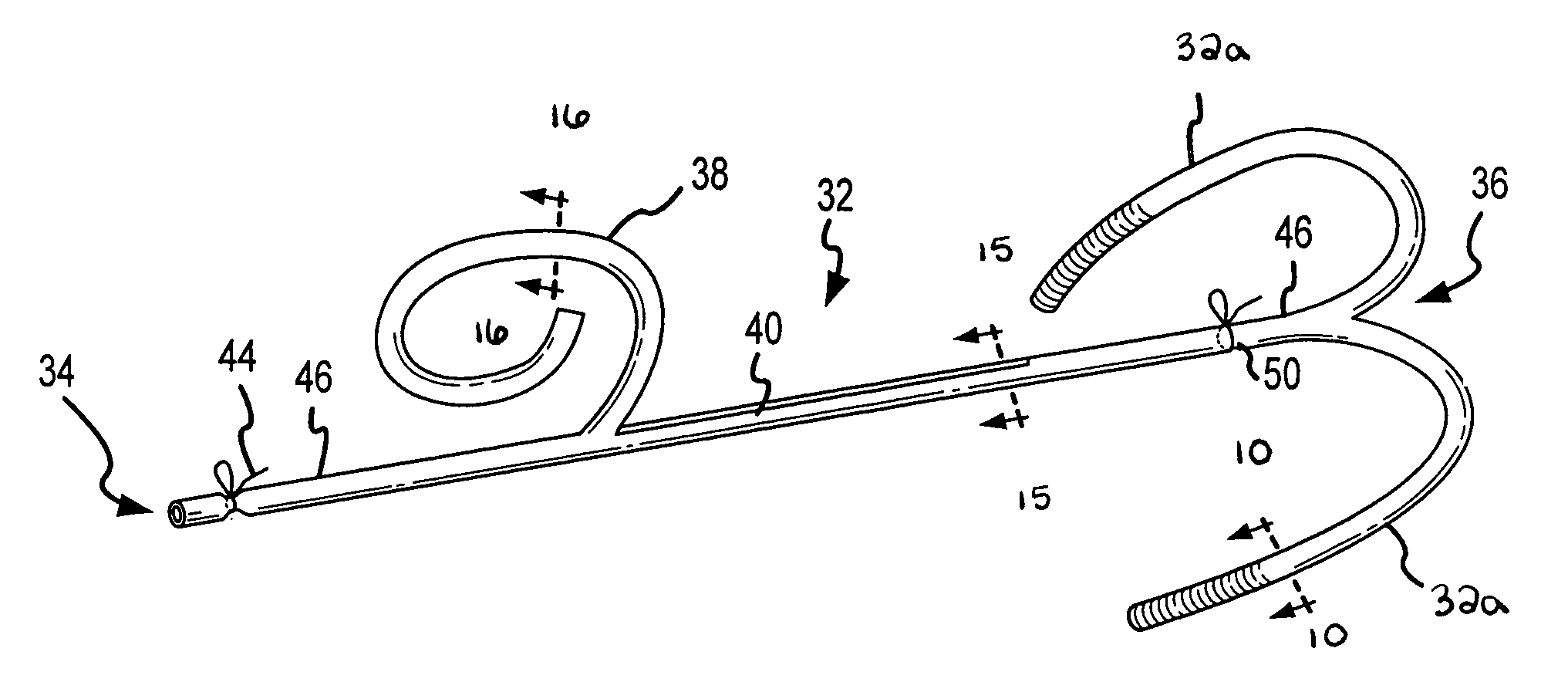
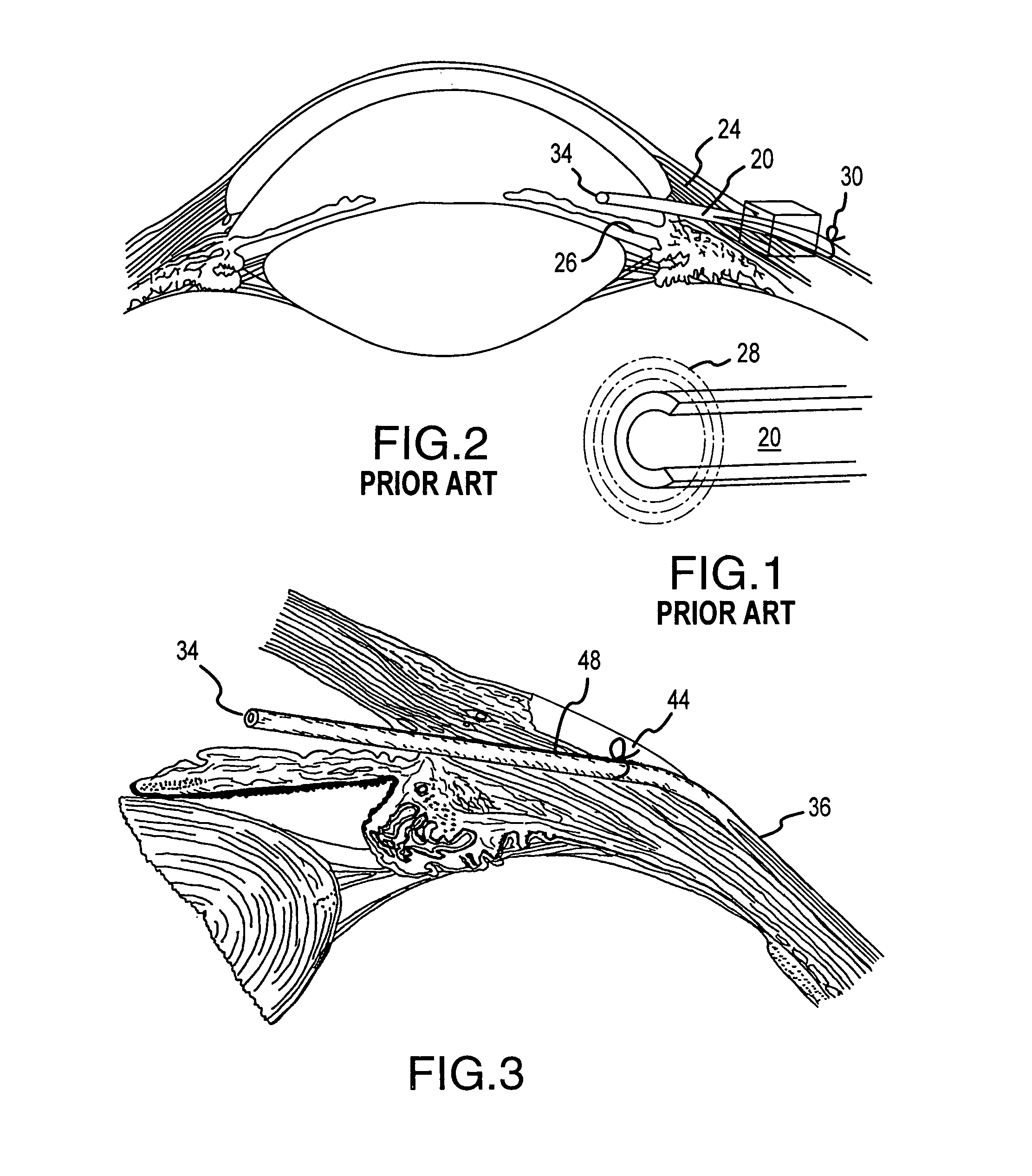
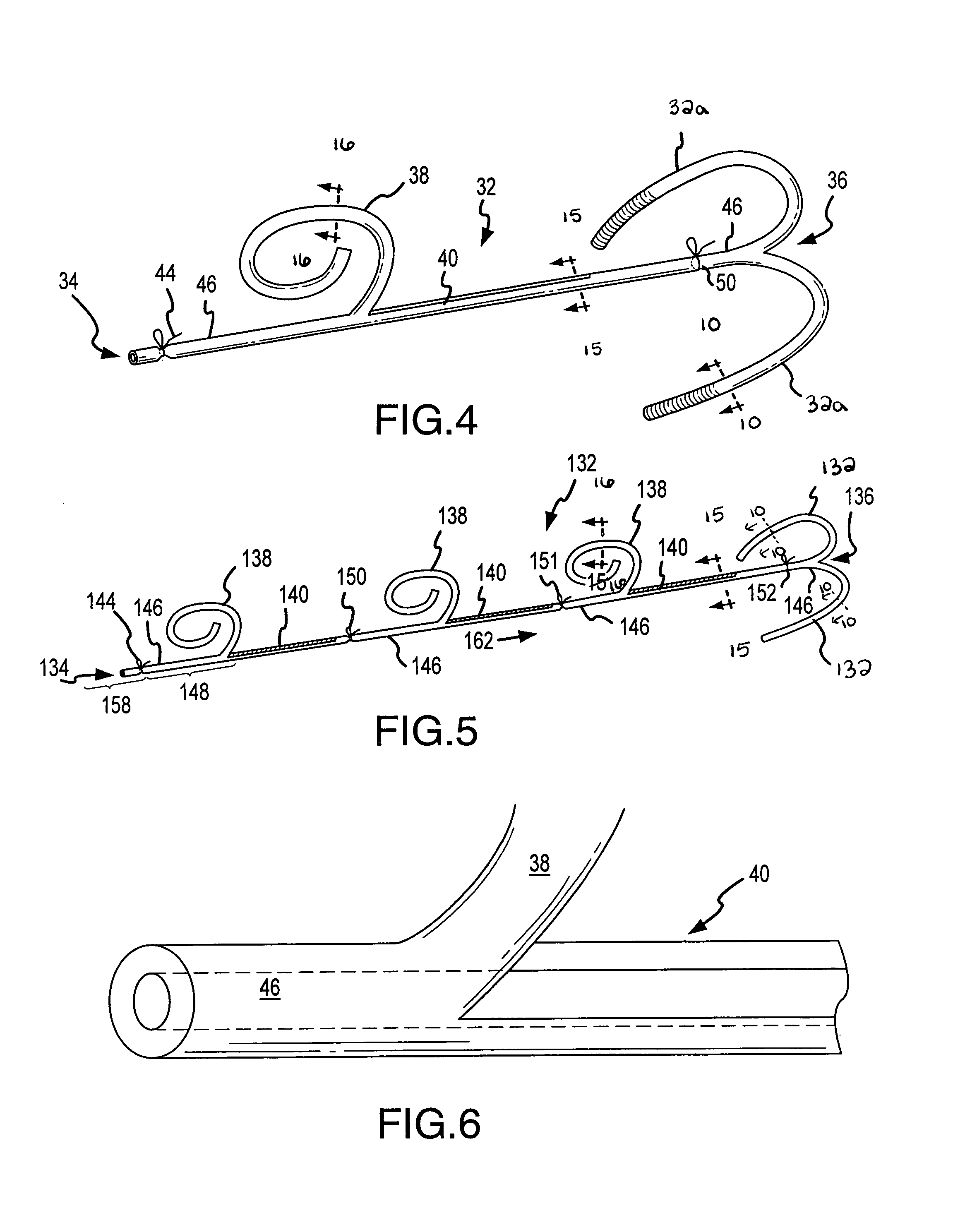
Segmented scleral band for treatment of presbyopia and other eye disorders
InactiveUS6197056B1Increase the effective working distanceLaser surgeryEye implantsDiseaseCiliary body
A segmented scleral expansion band adapted for implantation within or fastening to a segment of the sclera of an eye lying outside of and adjacent to the ciliary body of the eye, is formed from a number of arcuate segments, curved to match the curvature of the globe of the eye, and joined together at each end to form a complete scleral expansion band. The band is implanted in the sclera of the eye by forming circumferential tunnels, inserting the band segments through the tunnels, and joining the ends of the segments to form a complete scleral expansion band. The scleral expansion band is useful in treating presbyopia and other ocular disorders.
Owner:REFOCUS OCULAR INC
Therapeutic device for pain management and vision
ActiveUS20100036488A1Increase moistureRelieve painSenses disorderEye implantsEpitheliumTherapeutic Devices
A therapeutic lens for the treatment of an epithelial defect comprises a layer of therapeutic material disposed over the stroma and / or Bowman's membrane to inhibit water flow from the tear liquid to the stroma and / or Bowman's membrane, such that corneal deturgescence can be restored to decrease corneal swelling and light scattering. The layer may cover and protect nerve fibers to decrease pain. The layer may comprise an index of refraction to inhibit light scatter from an anterior surface of the stroma and / or Bowman's membrane. The lens may comprise a curved anterior surface that provides functional vision for the patient when the epithelium regenerates. The layer of therapeutic material can be positioned on the eye in many ways, for example with a spray that is cured to adhere the layer to the exposed surface of the stroma and / or Bowman's membrane.
Owner:NEXIS VISION LIQUIDATING TRUST +2
Ocular plug formed from placenta derived collagen biofabric
The present invention relates to ocular plugs formed from a biodegradable material. The plugs comprises a shaft and, optionally, a cap. The ocular plugs are intended to occlude, and to repair, discontinuities in the sclera, whether formed deliberately during injection or surgical foray into the eye, or accidentally. The method further provides methods of making the ocular plug. the invention also provides methods of using the ocular plugs to occlude and repair discontinuities in the sclera, or to deliver biologically active compounds to the sclera or the eye. Finally, the invention provides kits comprising one or more ocular plugs in a container.
Owner:LIU QING +1
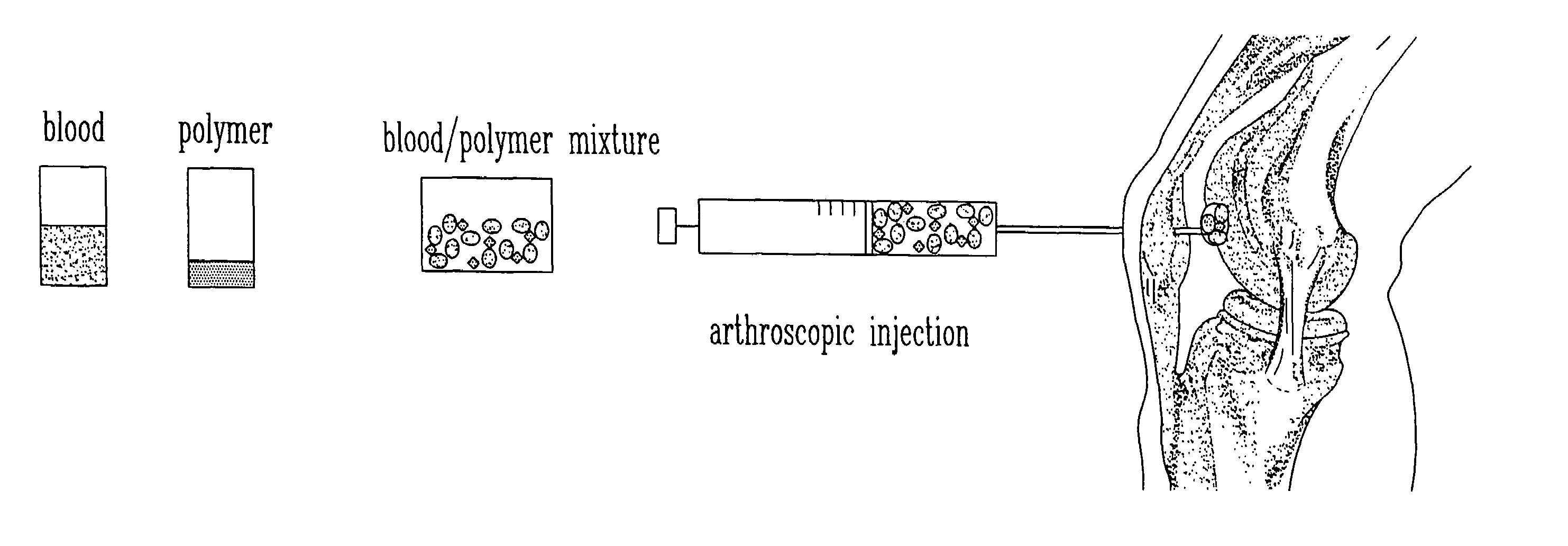
Composition and method for the repair and regeneration of cartilage and other tissues
InactiveUS7148209B2Add supportImprove coagulation/solidificationBiocidePeptide/protein ingredientsAbnormal tissue growthRepair tissue
Owner:SMITH & NEPHEW ORTHOPAEDICS
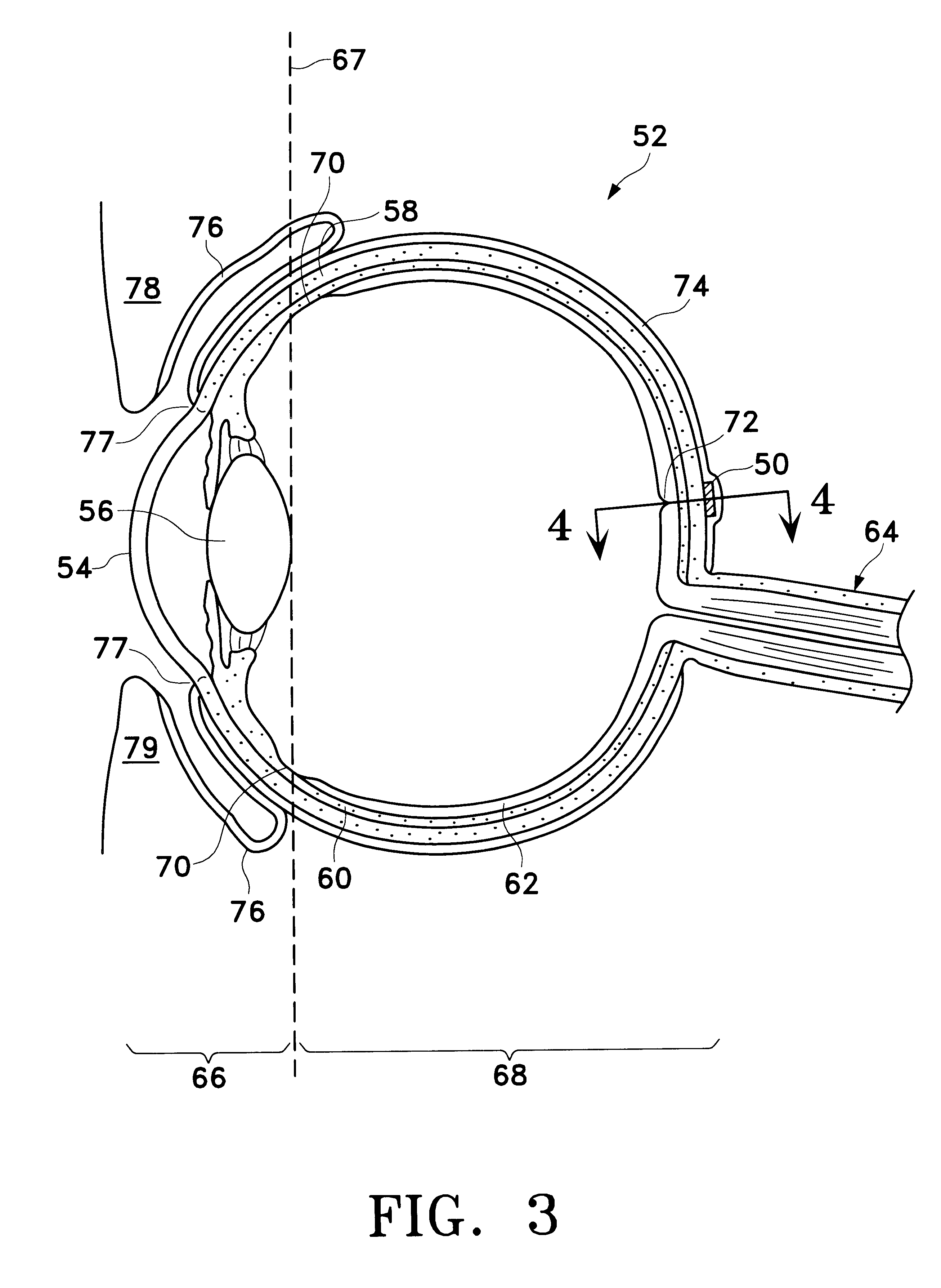
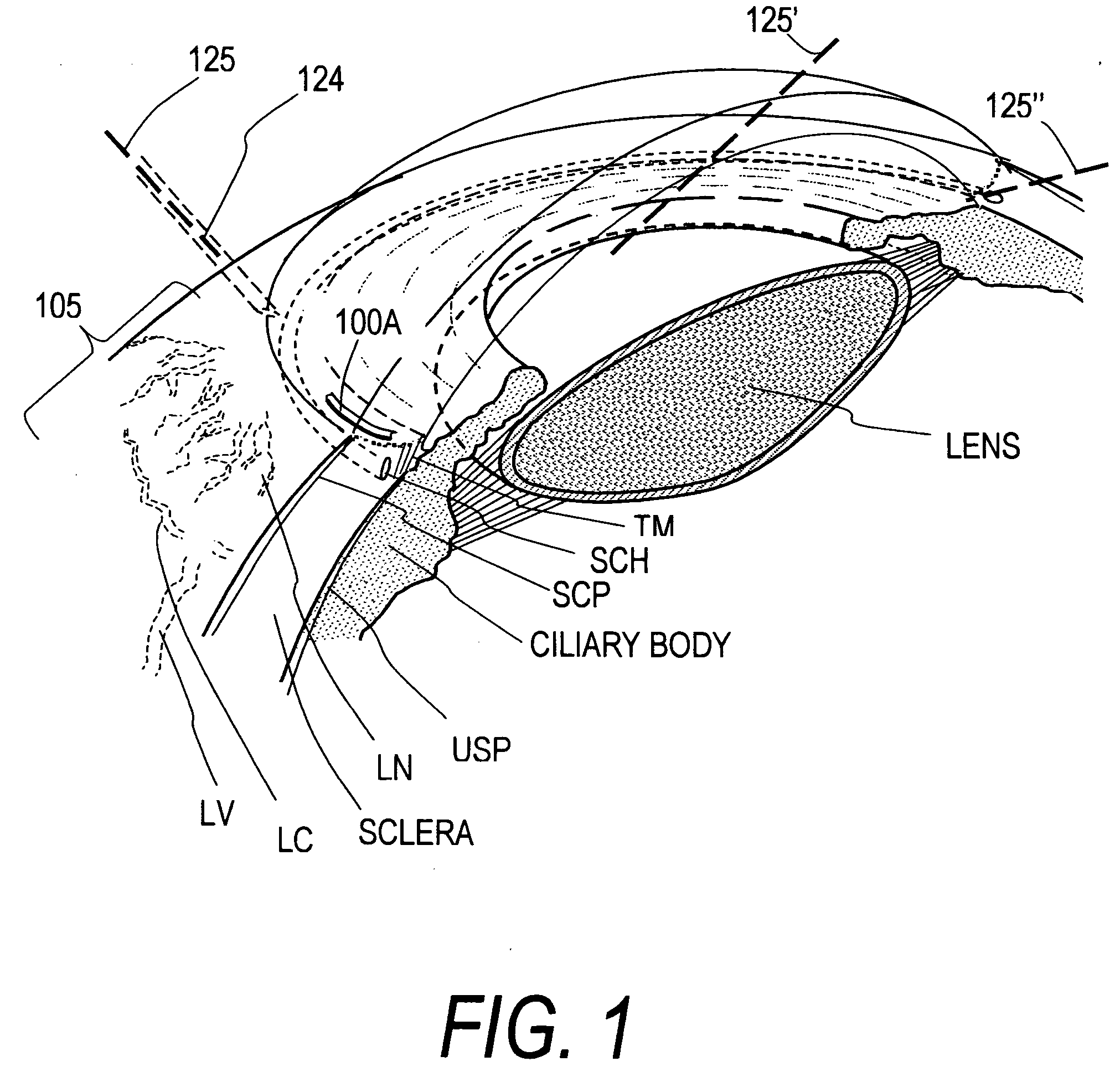
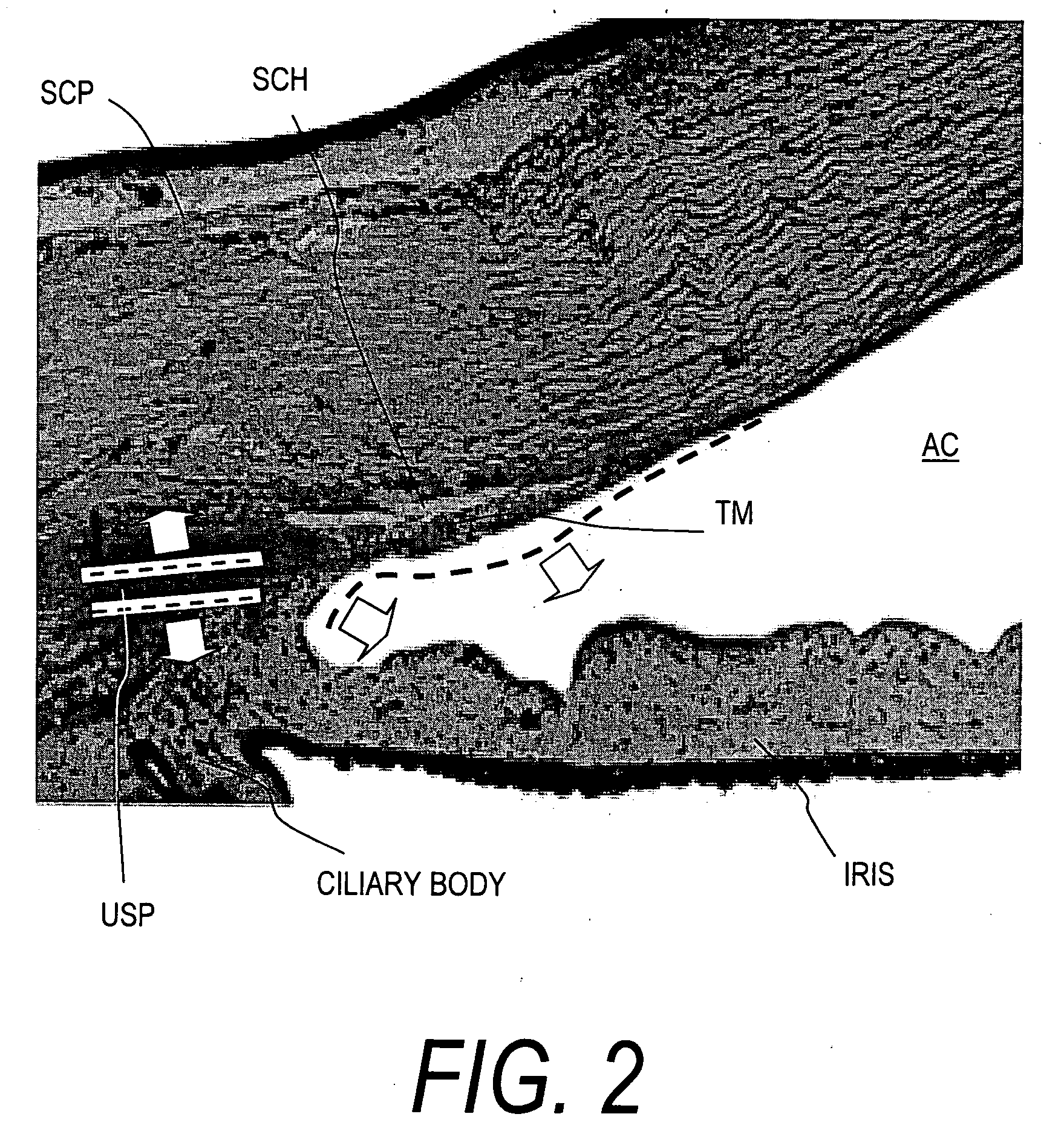
Device for improving in a targeted manner and/or permanently ensuring the ability of the aqueous humor to pass through the trabecular meshwork
A device is disclosed for selectively improving and / or permanently ensuring the permeability for ocular aqueous humour through the trabecular formations into Schlemm's canal. A small tubular element is provided, whose wall material encloses a hollow duct that presents an open configuration on both ends along the longitudinal extension of the hollow duct, so that the size and the shape of the small tubular element correspond approximately to the internal contour of Schlemm's canal, and the wall material as well as the wall thickness are so selected that upon introduction into Schlemm's canal the small tubular element keeps the canal open and dilates adjacent trabecular formations.
Owner:NEUHANN THOMAS
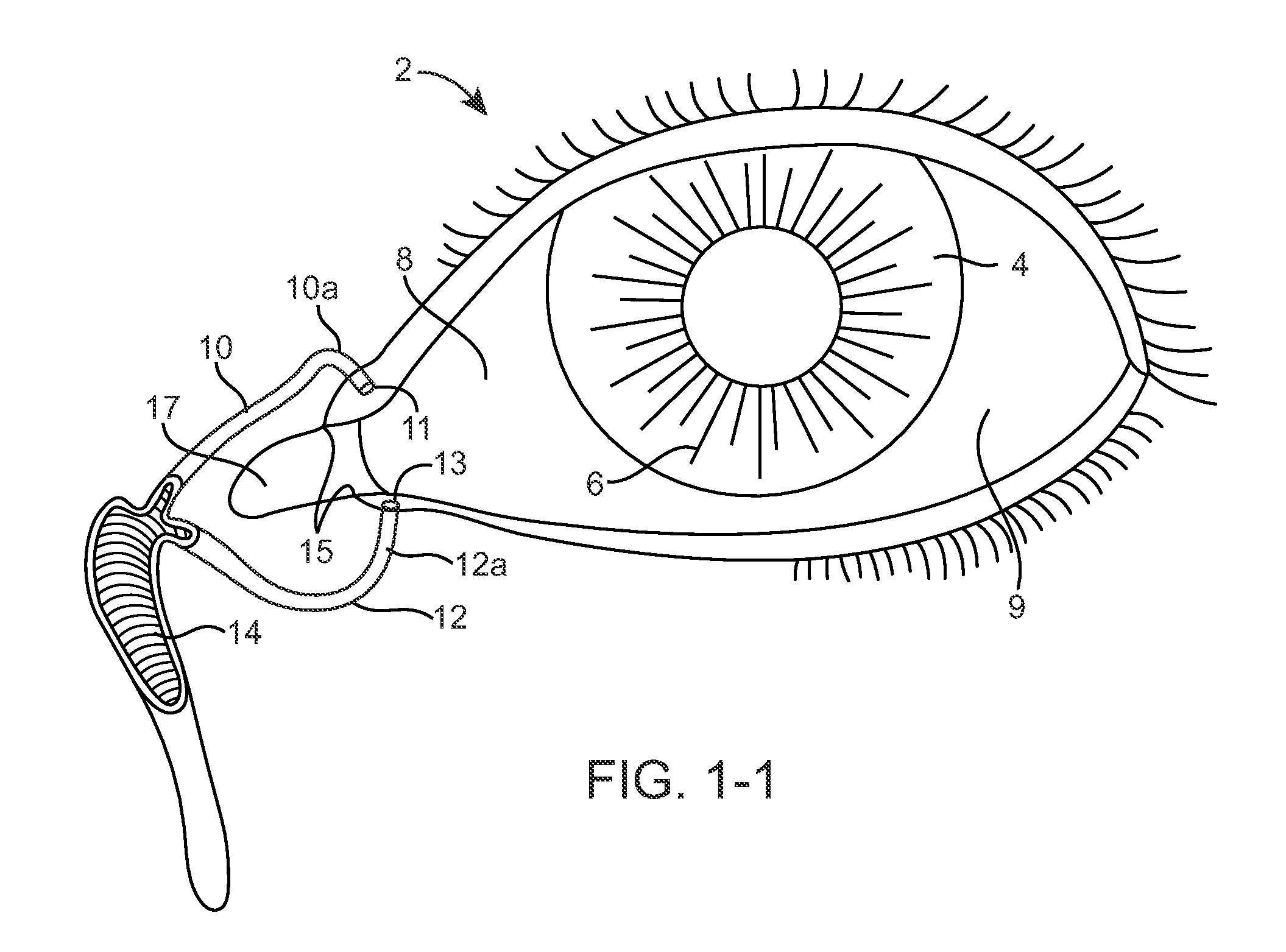
Nasolacrimal Drainage System Implants for Drug Therapy
ActiveUS20070243230A1Reduce deliveryAvoid flowAntibacterial agentsSenses disorderShape-memory alloyImplanted device
Implant devices, systems and methods for insertion into a punctum of a patient optionally comprises a drug core and a sheath body disposed over the drug core. The drug core includes a therapeutic agent deliverable into the eye, and the sheath defines at least one exposed surface of the drug core. The exposed surface(s) of the drug core may contact a tear or tear film fluid and release the therapeutic agent at therapeutic levels over a sustained period when the implant is implanted for use. The implant may include a retention element to retain the drug core and sheath body near the punctum, optionally comprising a shape memory alloy that can resiliently expand. An occlusive element may be attached to the retention element to at least partially occlude tear flow through the canalicular lumen.
Owner:MATI THERAPEUTICS
Contrast-enhanced ocular imaging
The invention relates generally to medical devices and methods for ocular imaging and, more particularly, to devices and methods for increasing contrast in an eye in which an imaging contrast agent is introduced into an aqueous humor outflow channel. For example, in one embodiment, the outflow channel may be Schlemm's Canal, or in another embodiment, the outflow channel may be an episcleral vein. Also disclosed are methods for implanting a trabecular stent via an ab extemo procedure with assistance of enhanced magnetic resonance imaging to restore a part or all of the normal physiological function of directing aqueous outflow for maintaining a normal intraocular pressure in an eye.
Owner:GLAUKOS CORP
Shunt for the treatment of glaucoma
InactiveUS20060069340A1Reducing ocular hypertensionLaser surgeryEye implantsIntraocular pressureFlow diverter
A system is provided for reducing intraocular pressure, the system having: an implantable shunt, the implantable shunt with a planar member, at least one microchannel disposed within that planar member, and a laser whereby at least one fenestration may be introduced into the microchannel.
Owner:SOLX
Ocular pressure regulation
This invention comprises a flexible ocular device for implantation into the eye formed of a biocompatible elastomeric material, foldable to a diameter of 1.5 mm or less, comprising a fluid drainage tube having at one end a foldable plate adapted to locate the device on the inner surface of the sclera in a suprachoroidal space formed by cyclodialysis, said drainage tube opening onto the disc at one end and opening to the anterior chamber when implanted into the eye at its other end, so as to provide aqueous pressure regulation. Also provided are methods for the treatment of glaucoma utilising the flexible ocular device, and an ocular pressure spike shunt.
Owner:ALCON INC
Drug Delivery Methods, Structures, and Compositions for Nasolacrimal System
ActiveUS20070269487A1Avoid expulsionInhibition releaseAntibacterial agentsPowder deliveryEffective treatmentBiomedical engineering
Owner:MATI THERAPEUTICS
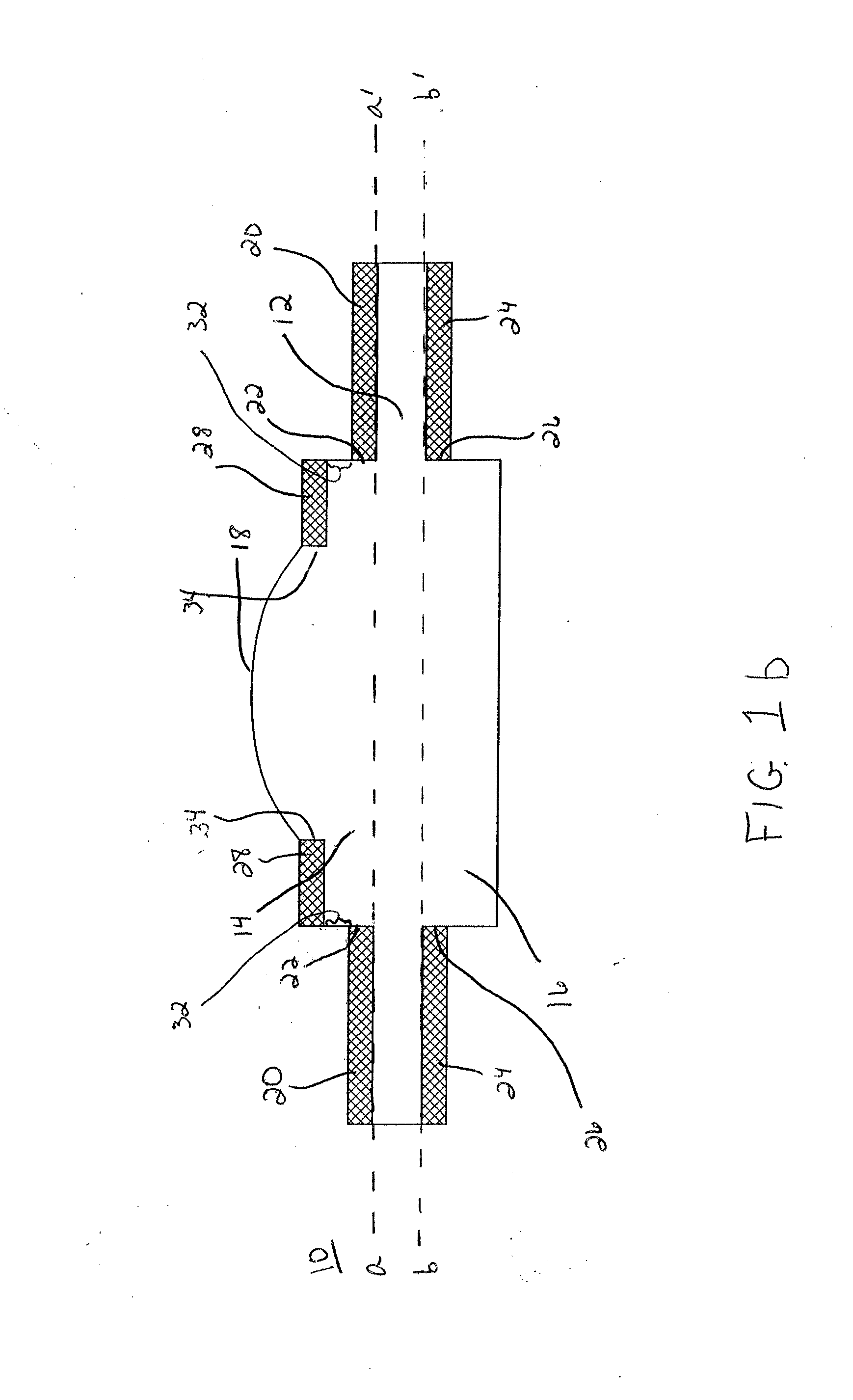
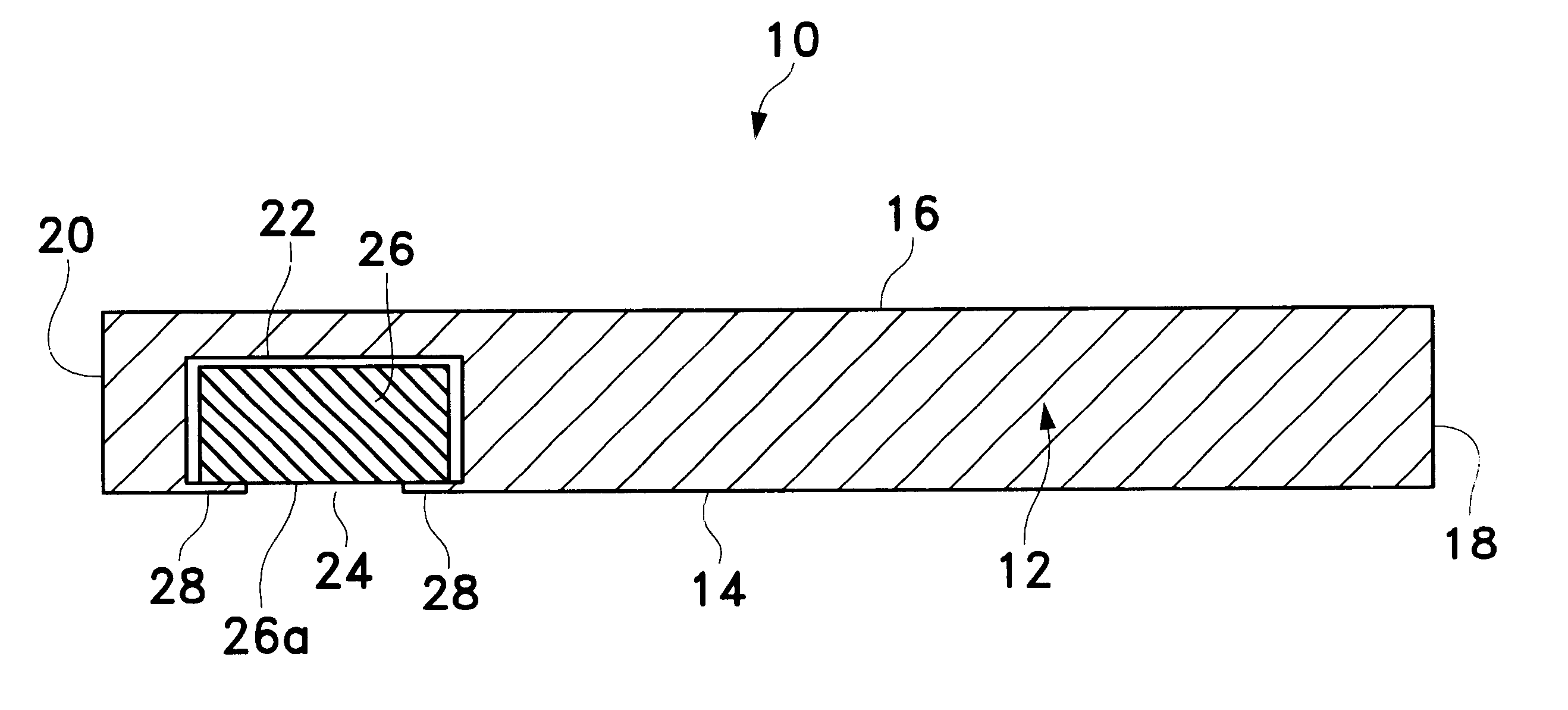
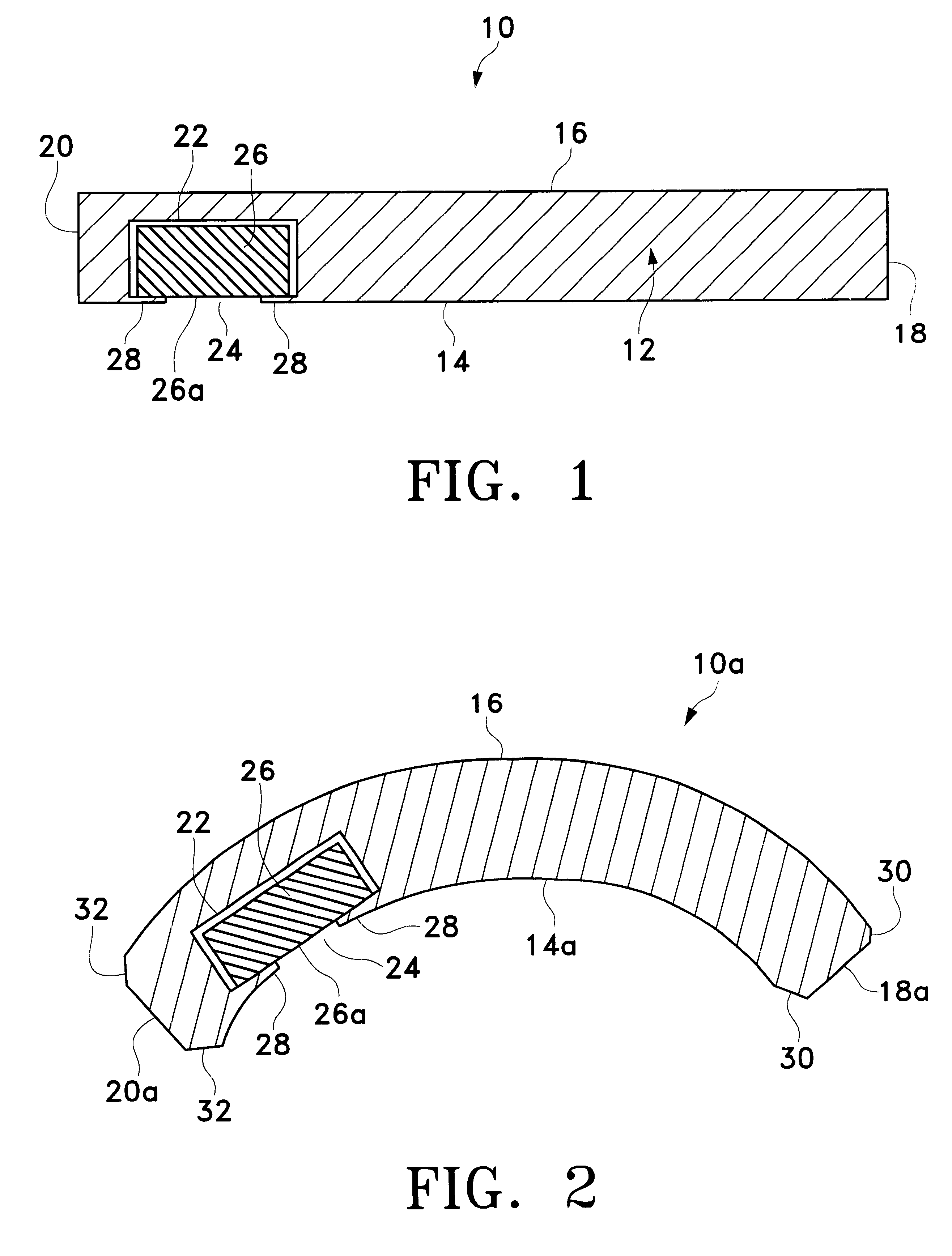
Extended wear ophthalmic lens
InactiveUS6951894B1Sufficient for corneal healthSubstantial adverse impact on ocular health or consumerLiquid surface applicatorsEye implantsExtended wear contact lensesEye movement
An ophthalmic lens suited for extended-wear periods of at least one day on the eye without a clinically significant amount of corneal swelling and without substantial wearer discomfort. The lens has a balance of oxygen permeability and ion or water permeability, with the ion or water permeability being sufficient to provide good on-eye movement, such that a good tear exchange occurs between the lens and the eye. A preferred lens is a copolymerization product of a oxyperm macromer and an ionoperm monomer. The invention encompasses extended wear contact lenses, which include a core having oxygen transmission and ion transmission pathways extending from the inner surface to the outer surface.
Owner:NOVARTIS AG
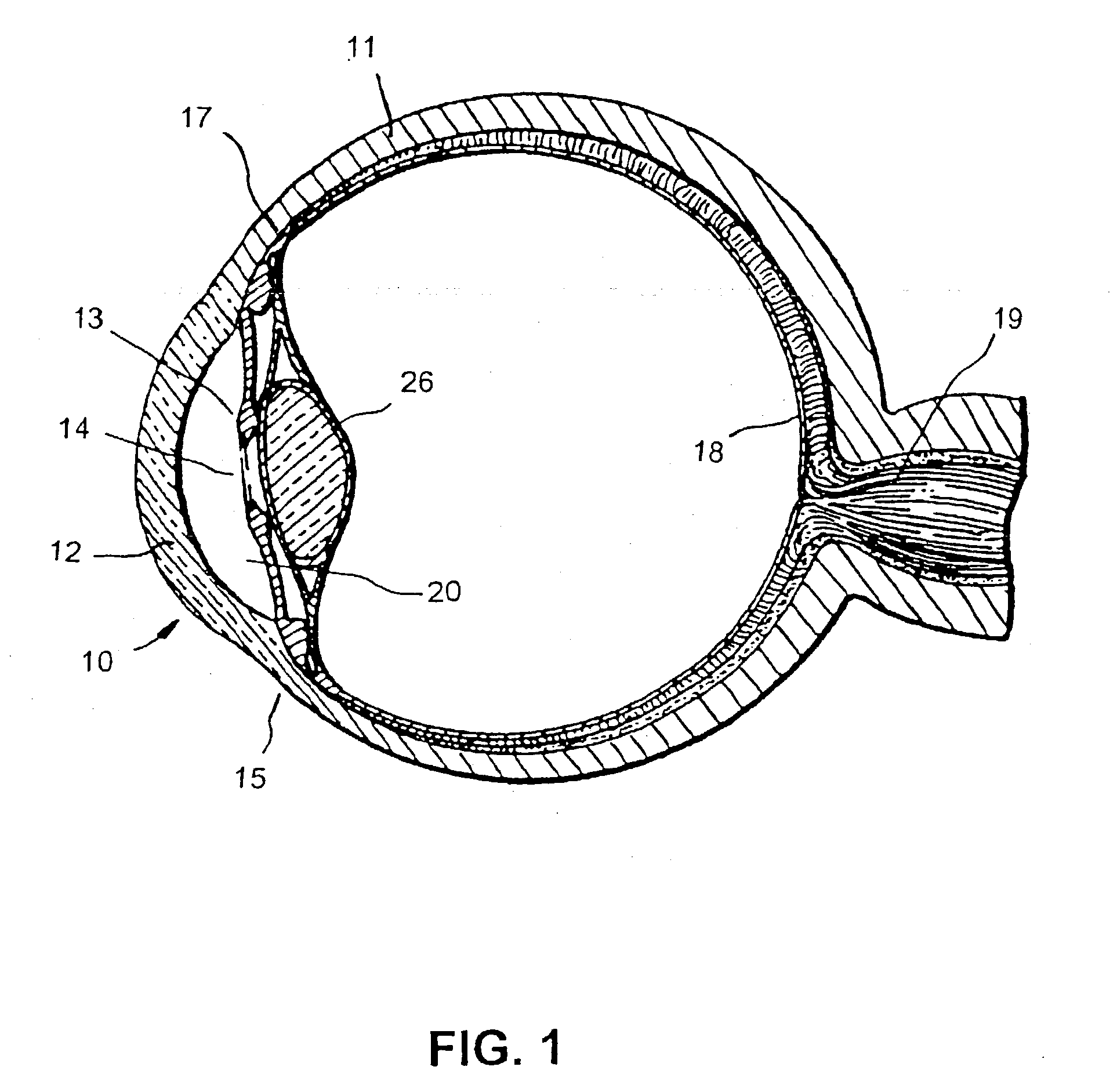
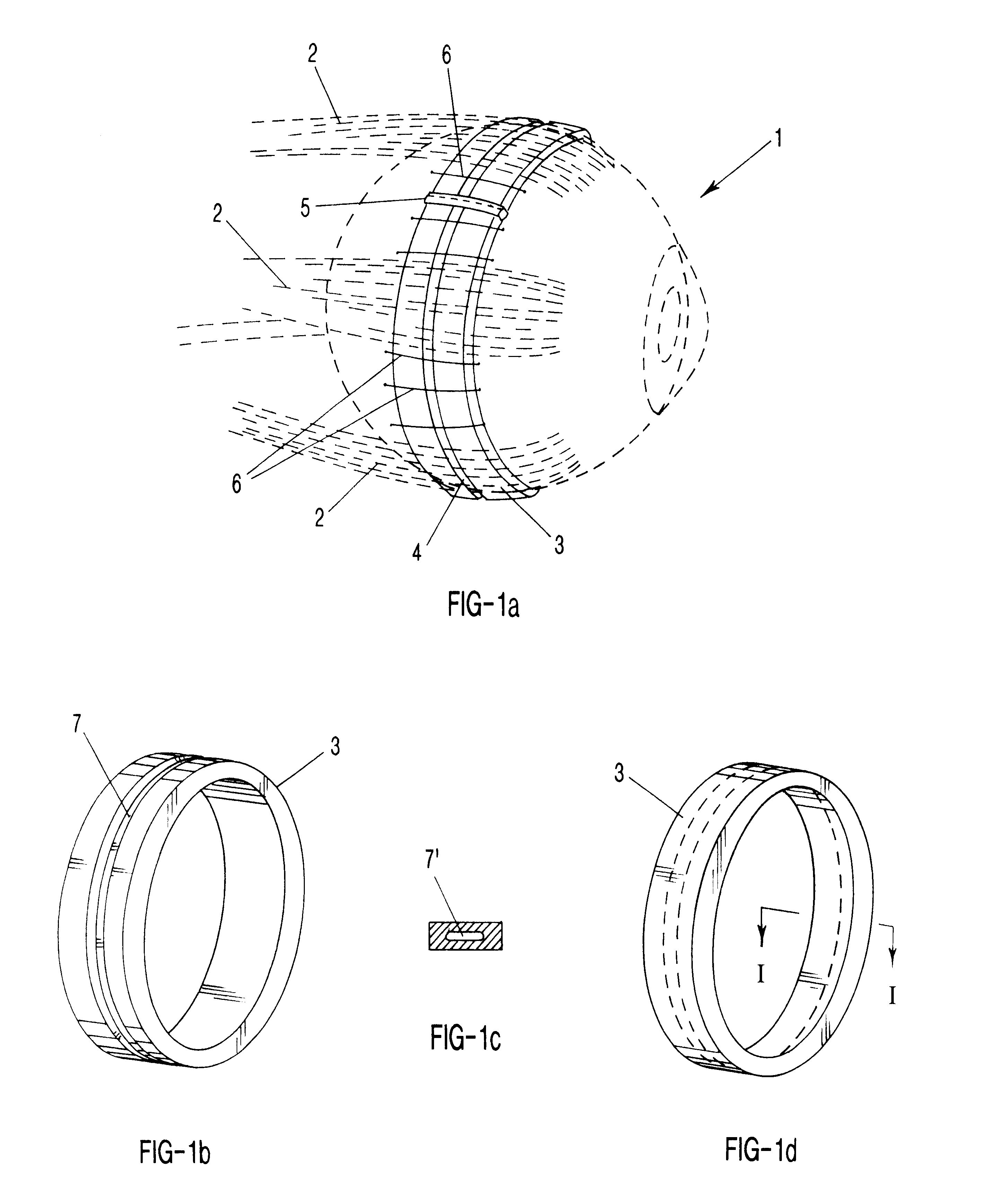
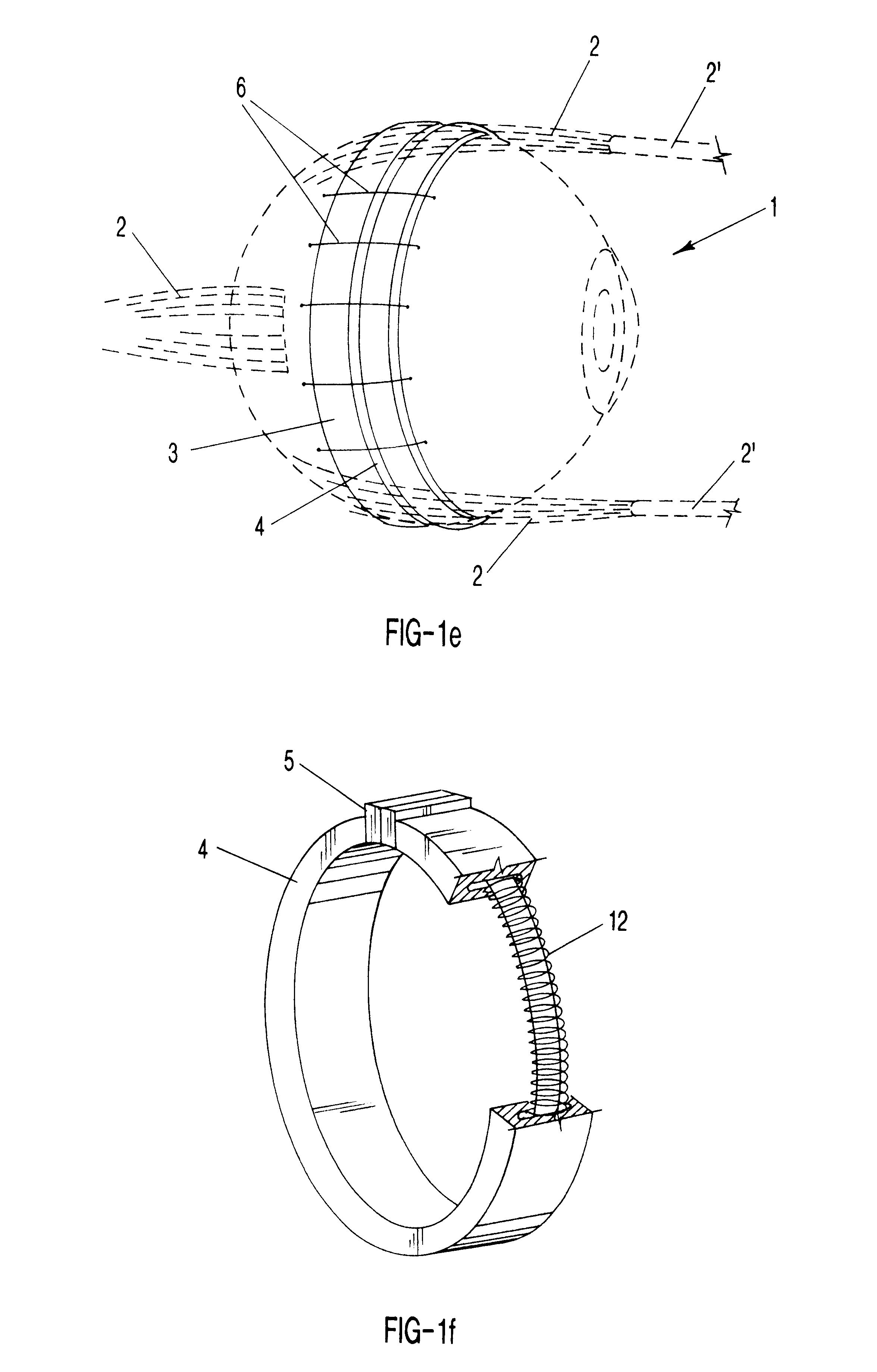
Surgical correction of human eye refractive errors by active composite artificial muscle implants
Surgical correction of human eye refractive errors such as presbyopia, hyperopia, myopia, and stigmatism by using transcutaneously inductively energized artificial muscle implants to either actively change the axial length and the anterior curvatures of the eye globe. This brings the retina / macula region to coincide with the focal point. The implants use transcutaneously inductively energized scleral constrictor bands equipped with composite artificial muscle structures. The implants can induce enough accommodation of a few diopters, to correct presbyopia, hyperopia, and myopia on demand. In the preferred embodiment, the implant comprises an active sphinctering smart band to encircle the sclera, preferably implanted under the conjunctiva and under the extraocular muscles to uniformly constrict the eye globe, similar to a scleral buckle band for surgical correction of retinal detachment, to induce active temporary myopia (hyperopia) by increasing (decreasing) the active length of the globe. In another embodiment, multiple and specially designed constrictor bands can be used to enable surgeons to correct stigmatism. The composite artificial muscles are either resilient composite shaped memory alloy-silicone rubber implants in the form of endless active scleral bands, electroactive ionic polymeric artificial muscle structures, electrochemically contractile endless bands of ionic polymers such as polyacrylonitrile (PAN), thermally contractile liquid crystal elastomer artificial muscle structures, magnetically deployable structures or solenoids or other deployable structures equipped with smart materials such as preferably piezocerams, piezopolymers, electroactive and eletrostrictive polymers, magnetostrictive materials, and electro or magnetorheological materials.
Owner:ENVIRONMENTAL ROBOTS
Adjustable inlay with multizone polymerization
The present invention relates to an inlay for correcting refractive error in an eye. The inlay includes a first portion having a first volume that remains substantially constant when exposed to an energy, and a second portion having a second volume that is adapted to change when exposed to the energy. This inlay results in a device that can correct severe ametropic conditions, without ablating any portion of the inlay itself or the cornea.
Owner:MINU
Scleral prosthesis for treatment of presbyopia and other eye disorders
InactiveUS6280468B1Increase the effective working distanceIncrease the working distanceLaser surgeryEye implantsDiseaseOpen angle glaucoma
Presbyopia is treated by implanting within a plurality of elongated pockets formed in the tissue of the sclera of the eye transverse to a meridian of the eye, a prosthesis having an elongated body having a first surface and a second surface opposite the first surface to contact the base and flap of the scleral pocket. The first and second surfaces are spaced apart a distance so that the implanted prosthesis exerts an outward force on the flap of the scleral pocket which results in an outward traction on at least the anterior margin of the scleral pocket. The combined effect of the implanted prostheses is to exert a radially outward traction on the sclera in the region overlying the ciliary body which expands the sclera in the affected region together with the underlying ciliary body. The expansion of the ciliary body restores the effective working distance of the ciliary muscle in the presbyopic eye and thereby increases the amplitude of accommodation. Hyperopia, primary open angle glaucoma and / or ocular hypertension can be treated by increasing the effective working distance of the ciliary muscle according to the invention. A preferred embodiment of the scleral prosthesis has a major surface adapted to contact the base or flap of the pocket and an opposite surface or ridge spaced from the major surface.
Owner:REFOCUS GROUP
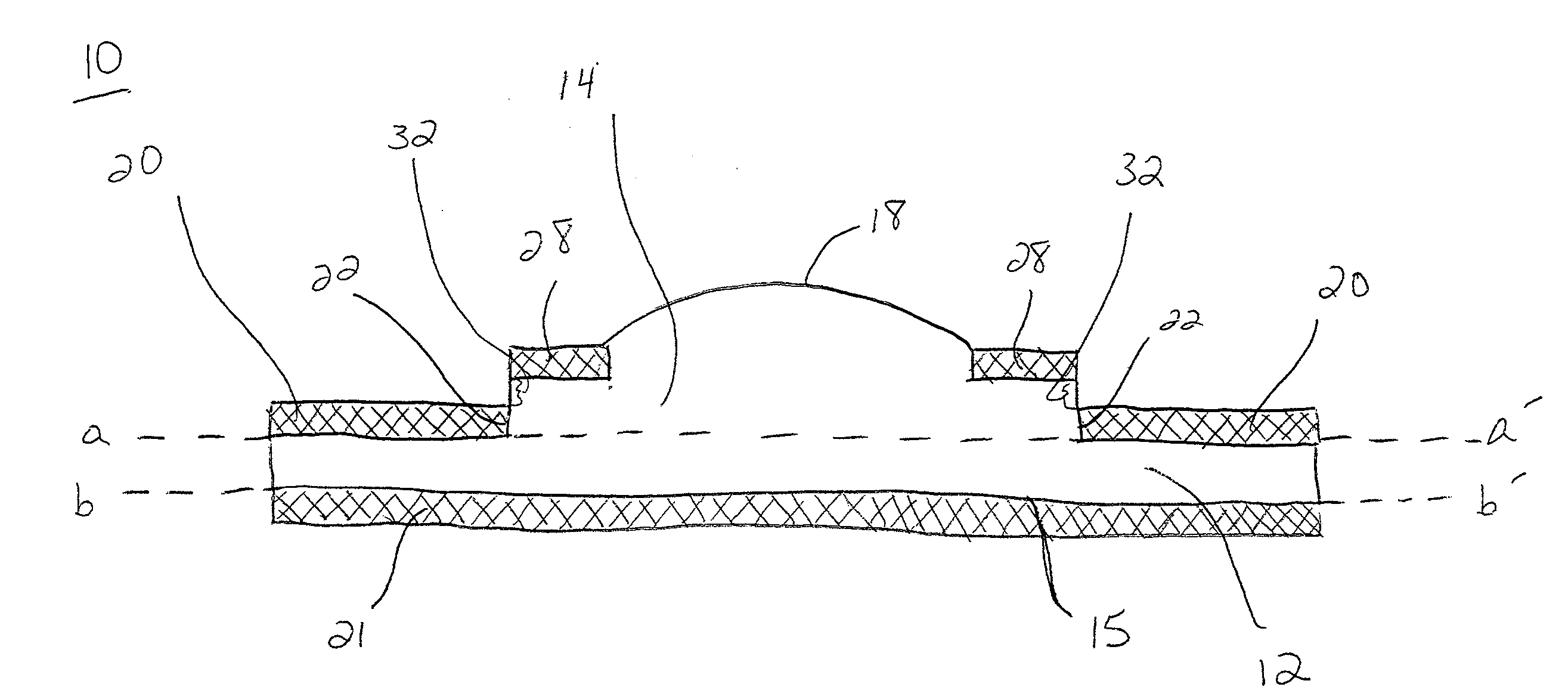
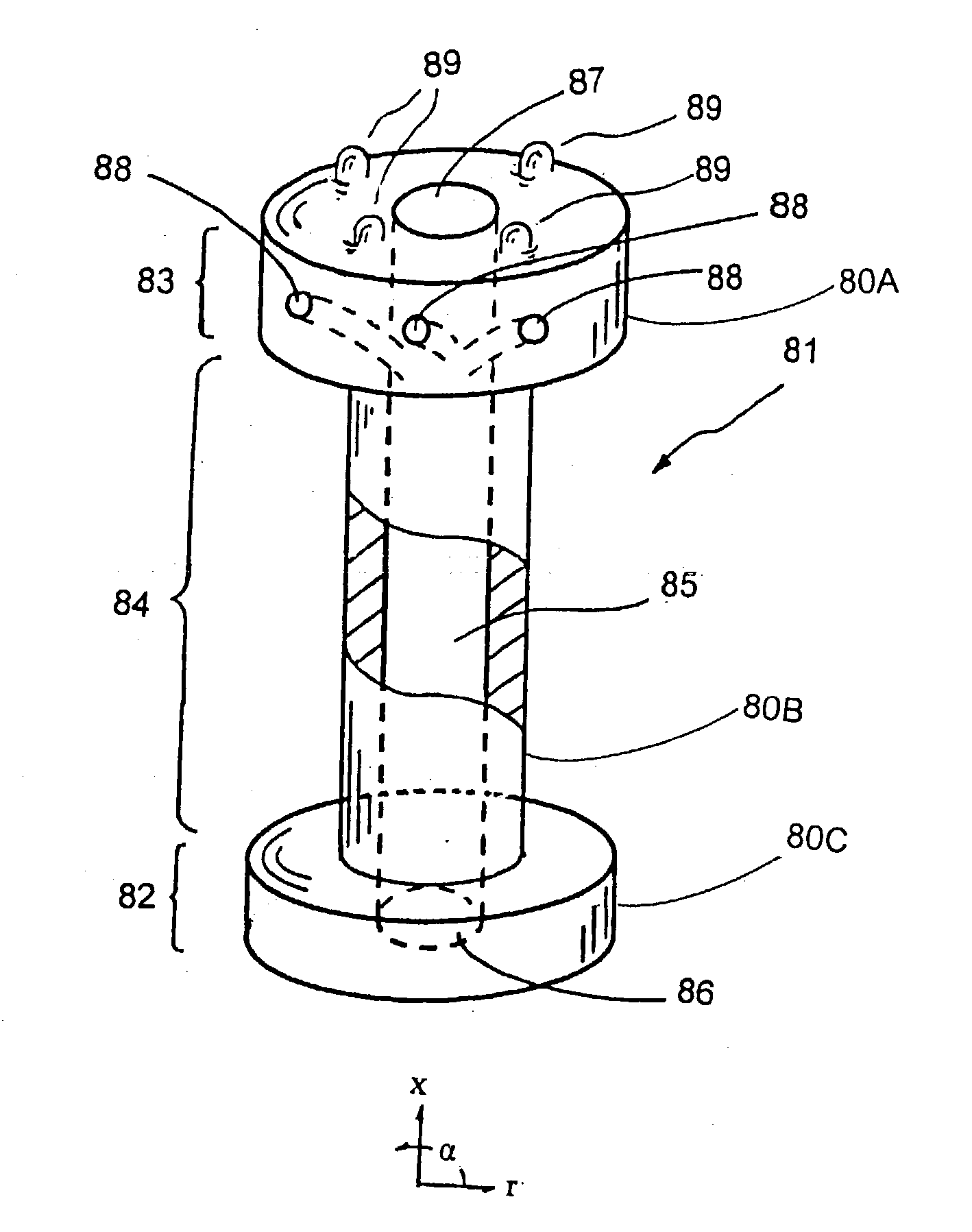
C-shaped cross section tubular ophthalmic implant for reduction of intraocular pressure in glaucomatous eyes and method of use
InactiveUS6962573B1Inhibit migrationReduction in bleb diameterEye implantsEar treatmentOphthalmological implantAqueous humor
A tube for implantation into the eye for replacement conduction of aqueous humor from the chambers of the eyeball to the subconjunctival tissue and ultimately to the venous system is comprised of an elongated fluid conducting conduit having distal and proximate ends, a sidewall and an interior passageway and at least one longitudinally extending opening in the sidewall that exposes the interior passageway and at least one nidi-forming structure carried by the conduit and extending laterally therefrom to implement the formation of at least one aqueous filtration bleb in the tissue of the eyeball. In one embodiment, the tube also contains at least one releasable ligature circumscribing the conduit. In another embodiment, the tube also contains an anchor appended to the conduit to prevent it from migrating from its placement site.
Owner:AQ BIOMED LLC
Fluid infusion methods for glaucoma treatment
InactiveUS20060116626A1Convenient treatmentReduce and inhibit and slow effectLaser surgeryEye implantsFluid infusionInsertion stent
Methods of treating glaucoma are disclosed, such as a method that includes inserting a stent through an incision in an eye; the stent having an inflow portion that is in fluid communication with an outflow portion of the stent; transporting the stent from the incision through the anterior chamber of the eye to an aqueous cavity of the eye, such that the inflow portion of the stent is positioned in the anterior chamber and the outflow portion of the stent is positioned at the aqueous cavity; and infusing fluid from the inflow portion to the outflow portion of the stent.
Owner:GLAUKOS CORP
Posterior Segment Drug Delivery
ActiveUS20100255061A1Reduce frequencyReduce riskOrganic active ingredientsSenses disorderTherapeutic DevicesMedicine
A therapeutic device to release a therapeutic agent comprises a porous structure coupled to a container comprising a reservoir. The reservoir comprises a volume sized to release therapeutic amounts of the therapeutic agent for an extended time when coupled to the porous structure and implanted in the patient. The porous structure may comprise a first side coupled to the reservoir and a second side to couple to the patient to release the therapeutic agent. A plurality of interconnecting channels can extend from the first side to the second side so as to connect a first a plurality of openings on the first side with a second plurality of openings on the second side. Each of the openings on the first side can be connected to each of the openings on the second side with the plurality of interconnecting channels, such that the rate of release of the therapeutic agent can be substantially maintained when one or more of the openings is blocked, for example with particles, cells, bacteria or tissue when the device is implanted for an extended time. The length of the channels extending from the first side to the second side may comprise an effective length greater than a distance across the porous structure from the first side to the second side. The therapeutic device many comprise an expandable retention structure and an expandable reservoir, such that the device can be delivered from a lumen of a delivery device and expand when positioned in the patient. The therapeutic device may comprises a penetrable barrier to inject therapeutic agent into the device when implanted in the patient.
Owner:FORSIGHT VISION5 INC
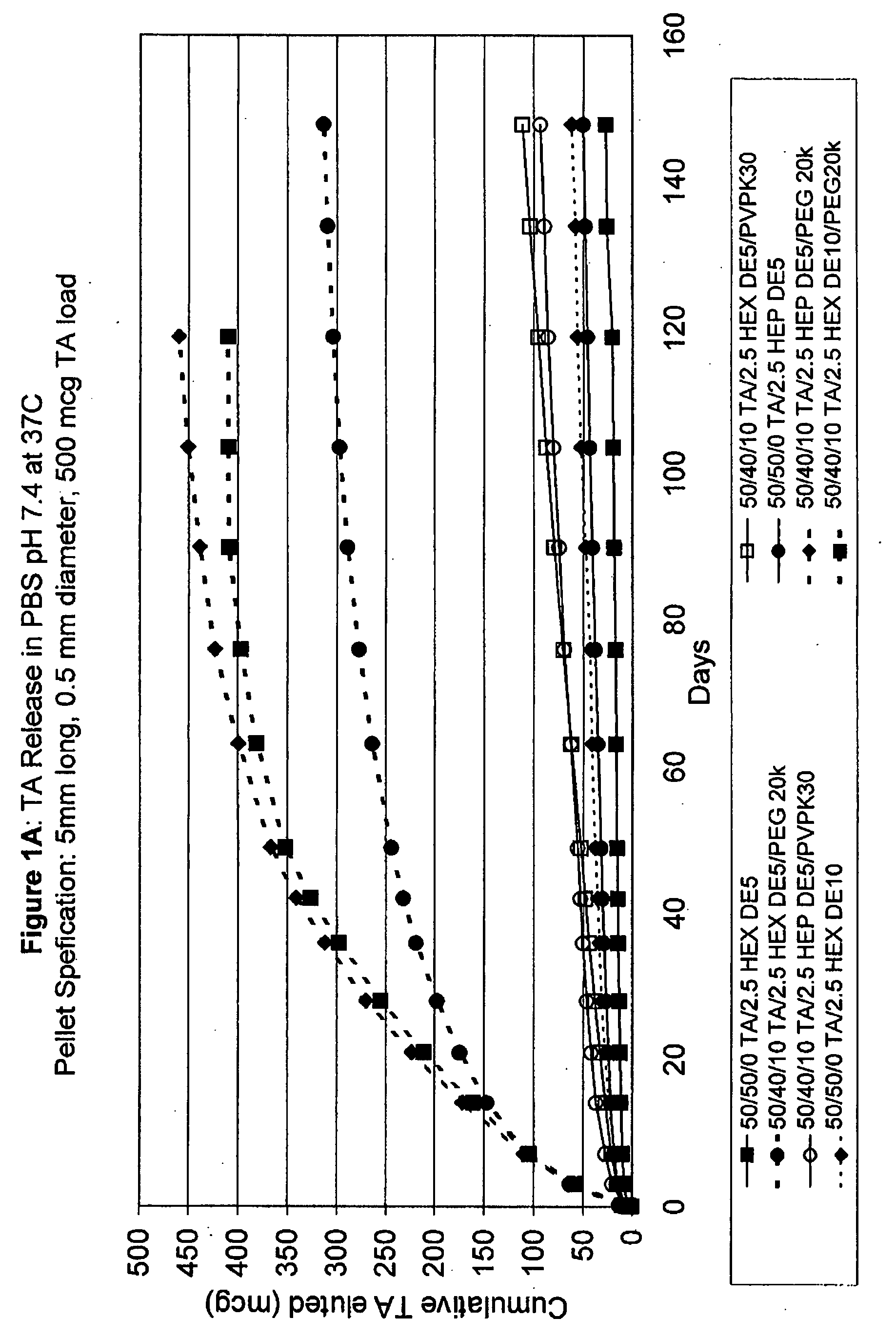
Punctal Plugs and Methods of Delivering Therapeutic Agents
ActiveUS20080181930A1High retention rateIncrease stiffnessBiocideSenses disorderCollagen Punctal PlugsParylene coating
The present invention concerns implantable ocular devices for the sustained release of medication to the eye, and methods for manufacturing and using such devices. In one embodiment, the present invention provides a device comprising: (a) a body comprising a matrix of a prostaglandin and a silicone; (b) a parylene coating on the outer surface of the body; and (c) one or more pores extending from the outer surface of the parylene coating to the outer surface of the body.
Owner:NOVARTIS AG
Glaucoma stent system
Surgical methods and related medical devices for treating glaucoma are disclosed. The method comprises trabecular bypass surgery, which involves bypassing diseased trabecular meshwork with the use of a stent implant. The stent implant is inserted into an opening created in the trabecular meshwork by a piercing member that is slidably advanceable through the lumen of the stent implant for supporting the implant insertion. The stent implant is positioned through the trabecular meshwork so that an inlet end of the stent implant is exposed to the anterior chamber of the eye and an outlet end is positioned into fluid collection channels at about an exterior surface of the trabecular meshwork or up to the level of aqueous veins.
Owner:GLAUKOS CORP
Biodegradable hydrophobic polysaccharide-based drug delivery implants
InactiveUS20070224247A1Sufficient amountLate releaseEye implantsSurgeryPolysaccharideDrug biological activity
Owner:SURMODICS INC
Hydrogel from polysiloxane-containing urethane prepolymer, tris (trimethylsiloxy) silylpropyl methacrylate, and a hydrophilic comonomer
A polyurethane based prepolymer is provided and useful in biomedical devices which provides high oxygen permeability and superior physical properties. A hydrogel is produced from a comonomer mixture containing a polysiloxane-containing urethane prepolymer, tris(trimethylsiloxy)-silylpropyl methacrylate and a hydrophilic comonomer. The hydrogel is especially useful for biomedical materials such as contact lenses and implants.
Owner:BAUSCH & LOMB INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com