Unitary surgical device and method
a surgical device and unitary technology, applied in the field of surgical devices, can solve the problems of debilitating pain in the joint, frequent damage to the meniscus in twisting injuries, and adverse effects on cartilage health
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
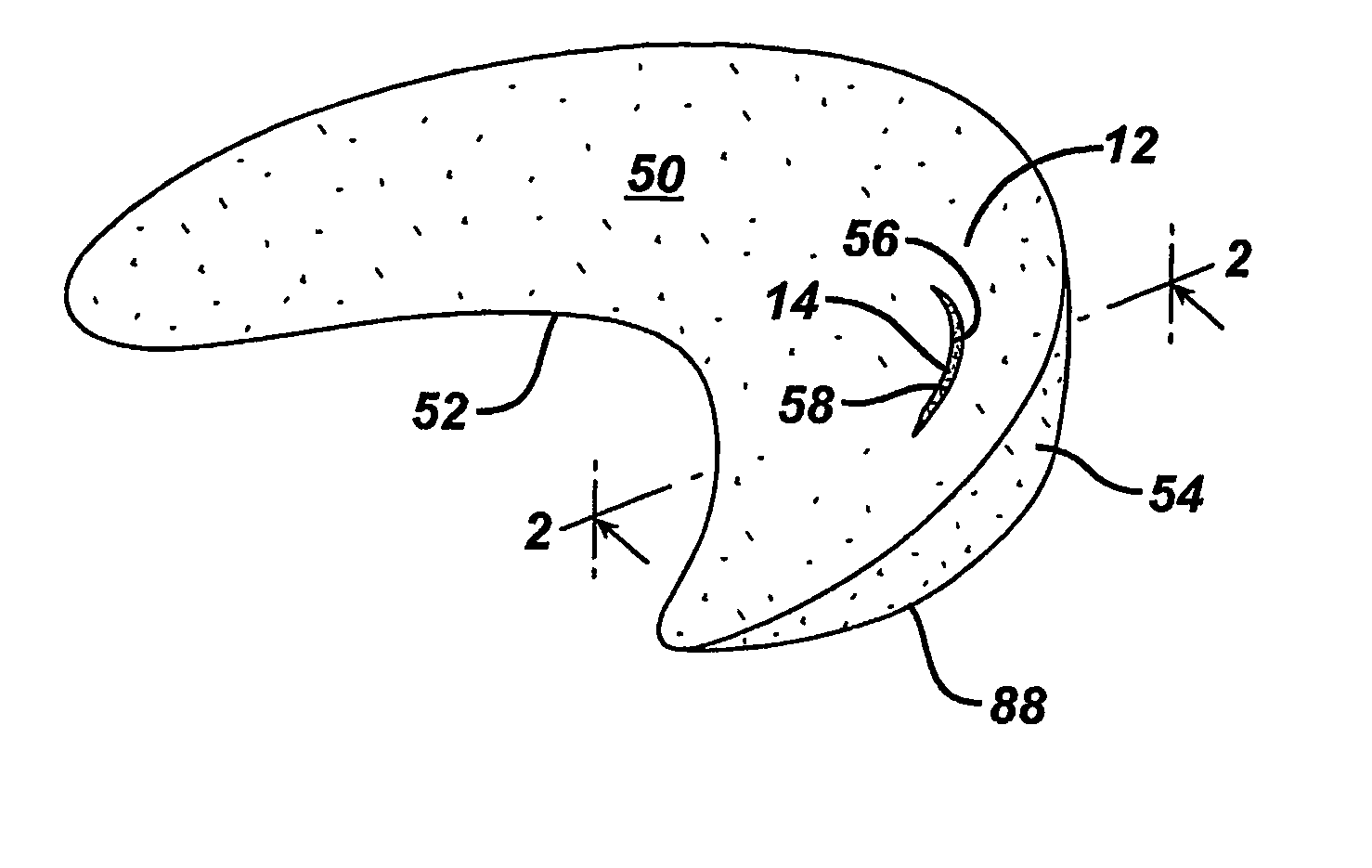
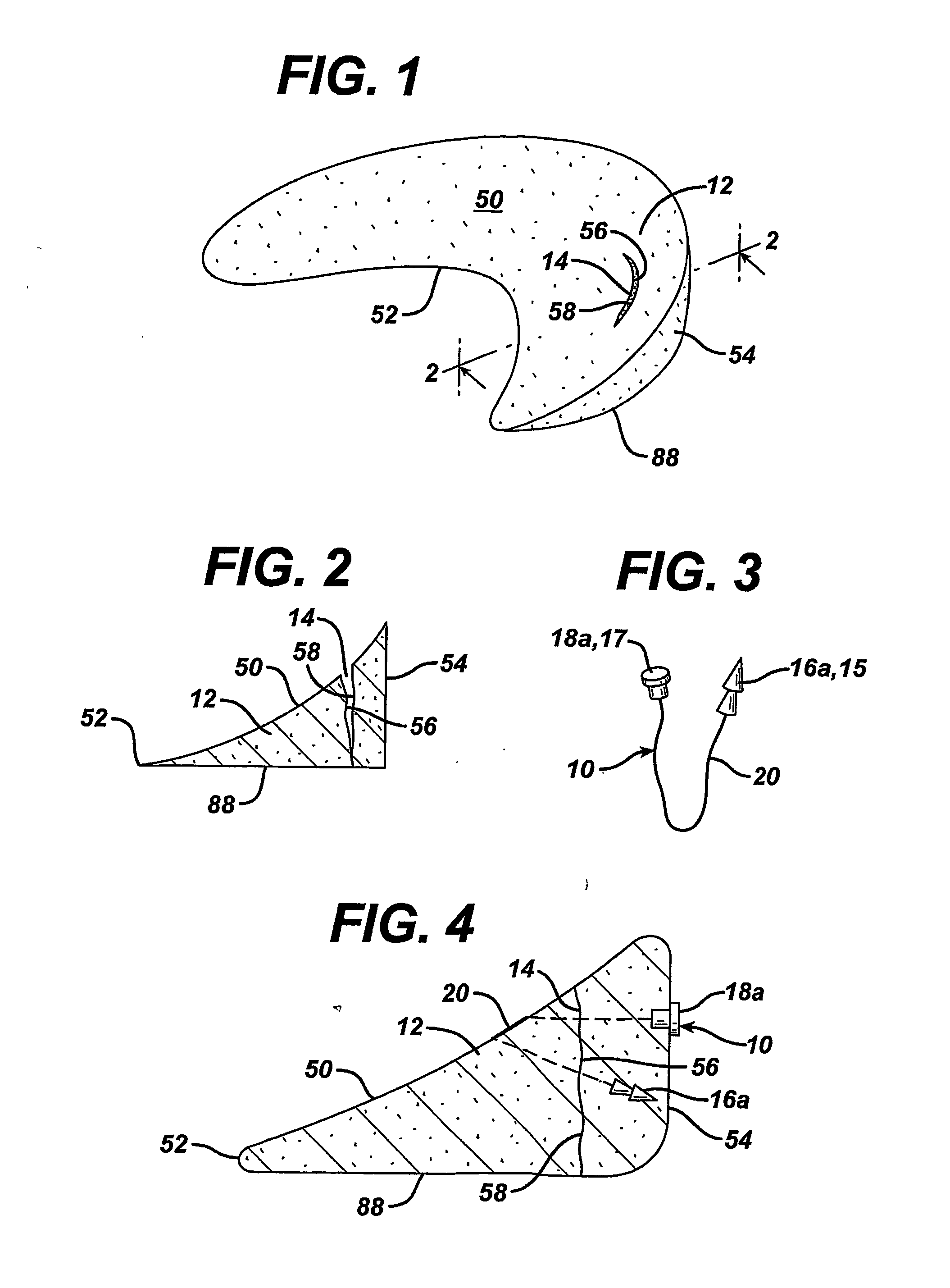
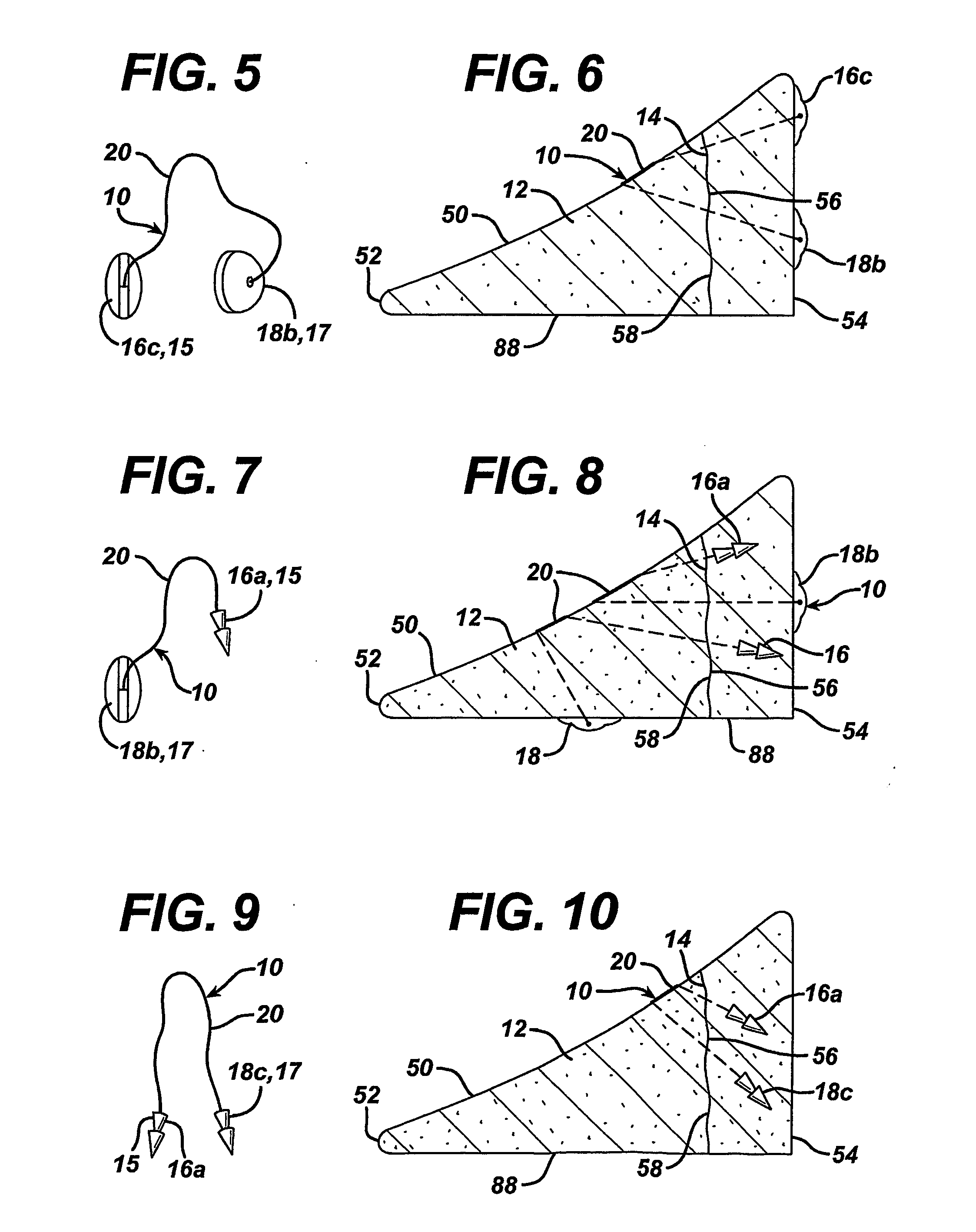
[0080] A variety of unitary surgical devices 10 utilizing the principles of the present invention are illustrated in the accompanying drawings. The illustrated surgical devices 10 are for implantation in a patient for repairing a body tissue in the patient. The illustrated embodiments would most commonly be used in repairing intra-articular fibrocartilage, such as the meniscus of the knee, although the invention is not so limited unless expressly called for in the claims. A meniscus, or part of a meniscus, is diagrammatically illustrated at 12 in the accompanying drawings (FIGS. 1-2, 4, 6, 8, 10, 12, 15-17, 19, 21, 24-26, 29, 31, 33, 36, 38, 40, 46-49, 51 and 52). An example of a meniscal tear is shown at 14 in FIGS. 1-2, 4, 6, 8, 10, 46, 47 and 51. The invention is also expected to be useful in the treatment of damaged and diseased intra-articular fibrocartilage in other body parts as well.
[0081] As used herein “unitary” refers to the fact that the surgical devices 10 include at l...
PUM
| Property | Measurement | Unit |
|---|---|---|
| density | aaaaa | aaaaa |
| density | aaaaa | aaaaa |
| density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com