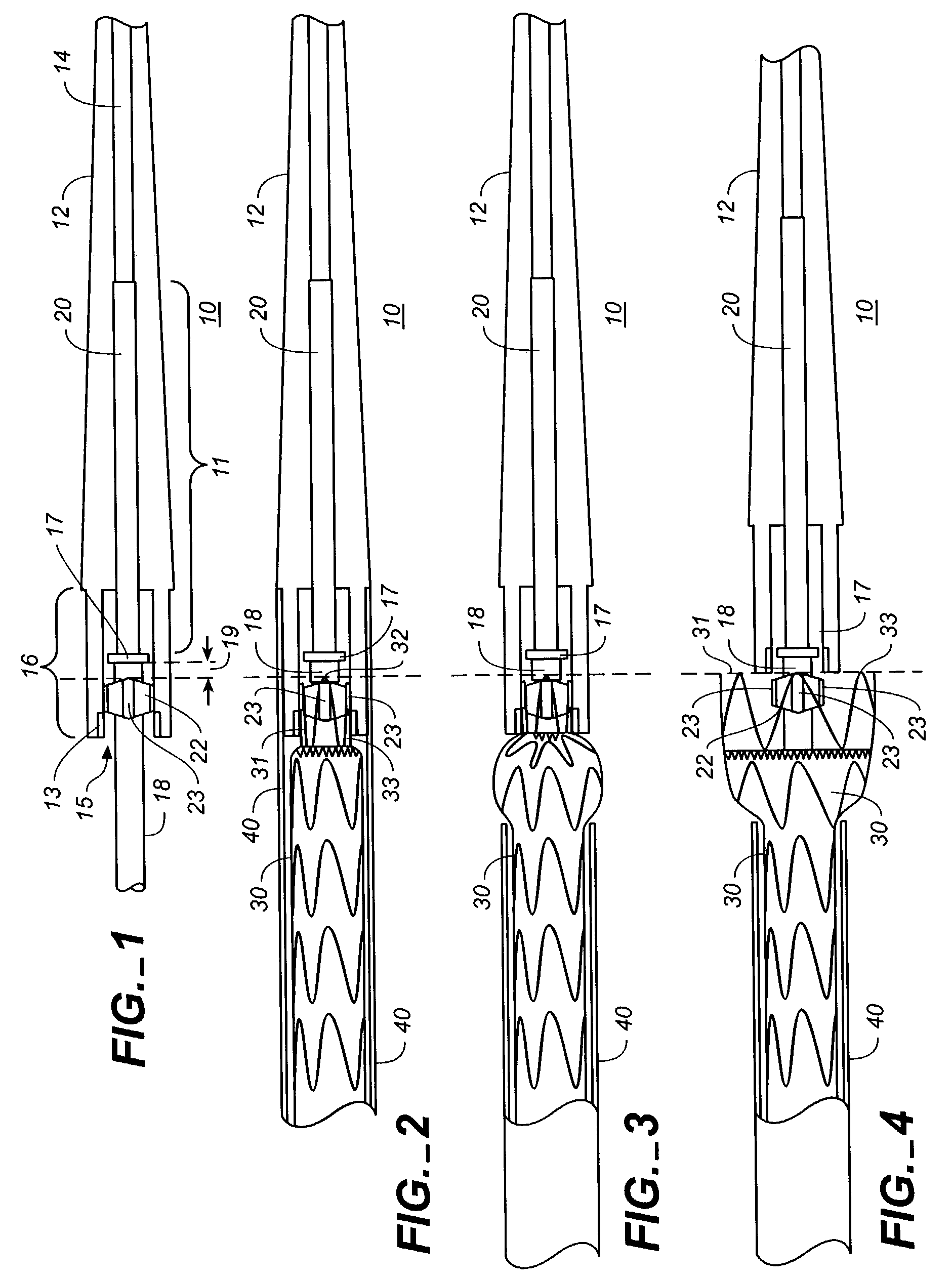
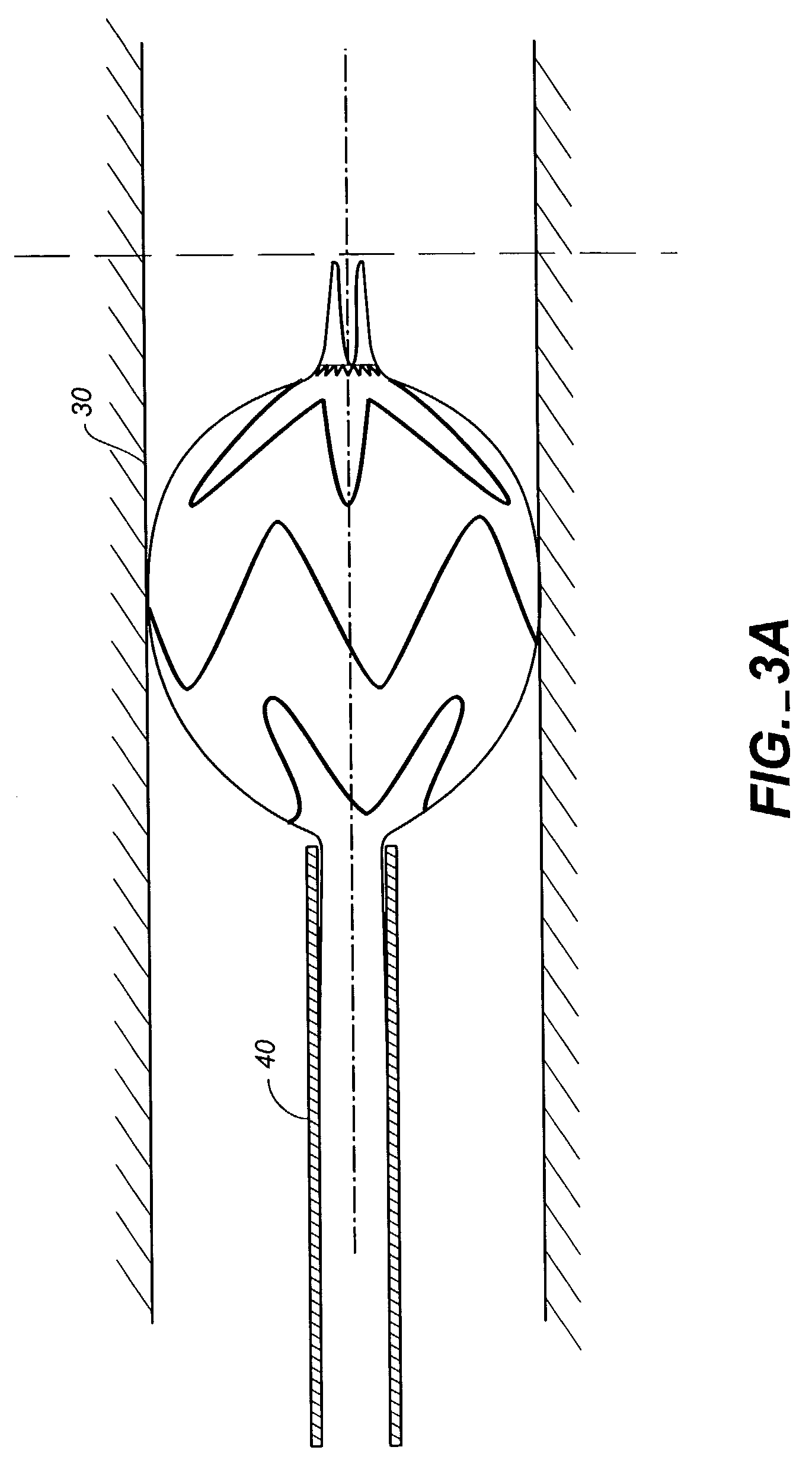
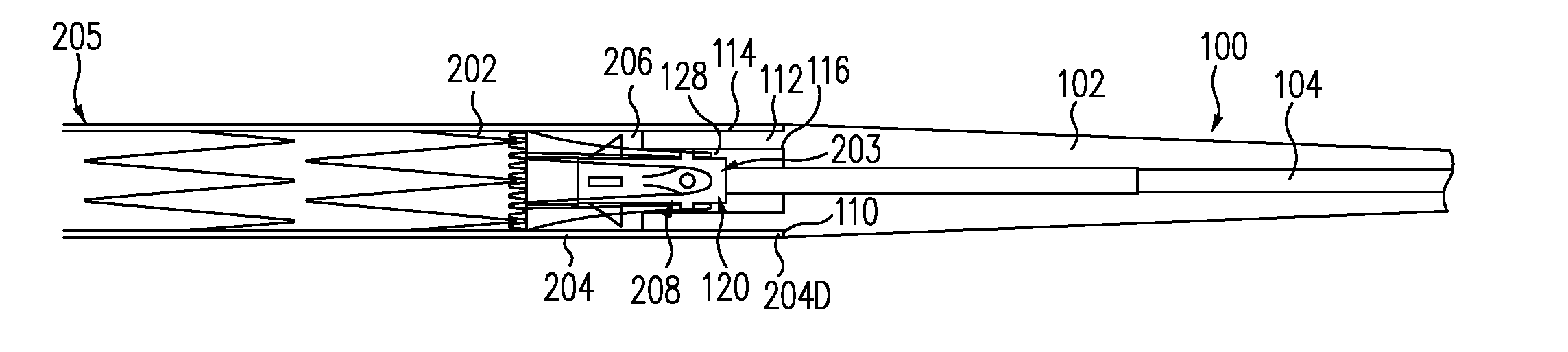
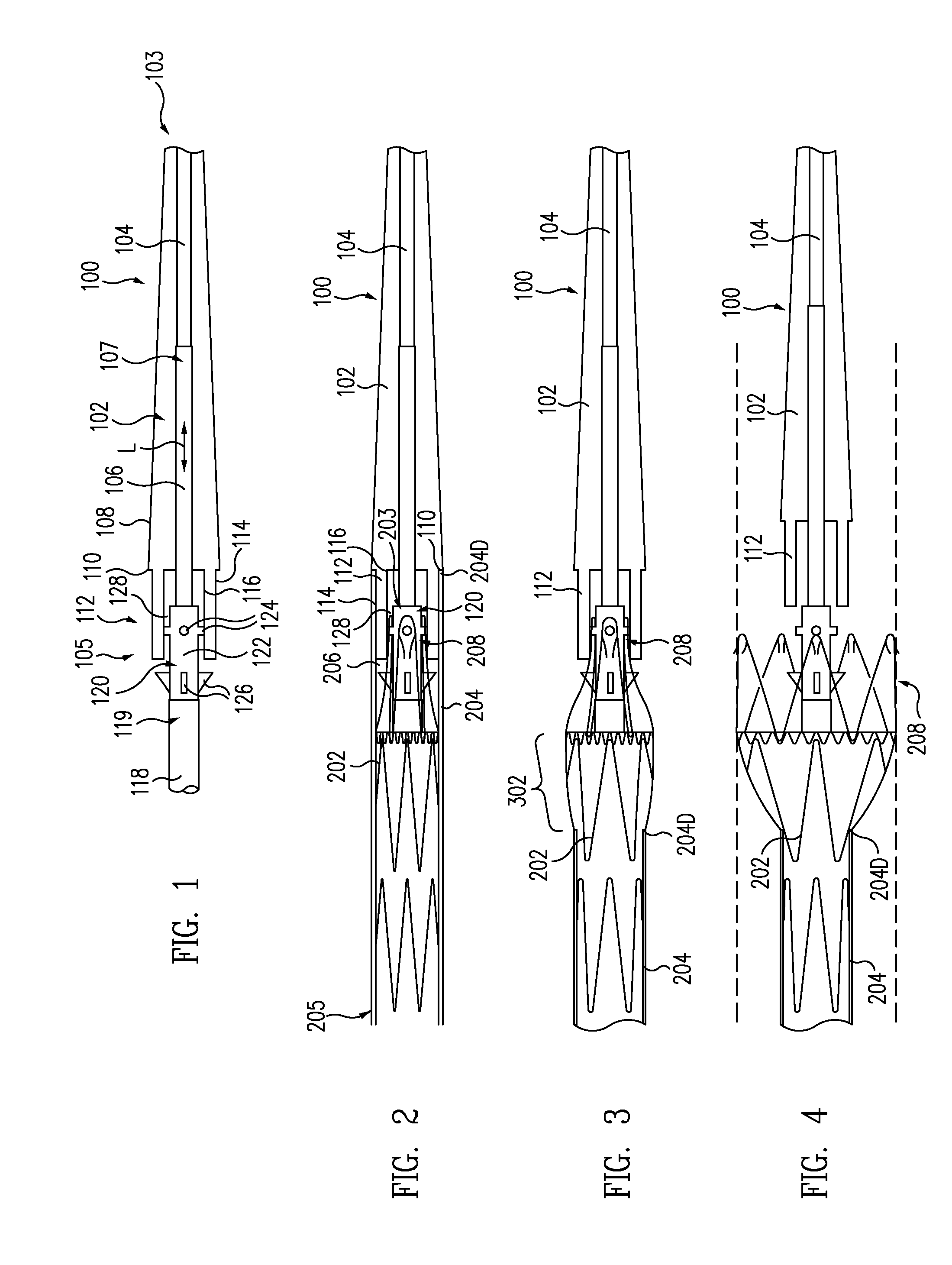
Patents
Literature
1053 results about "Stent grafting" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
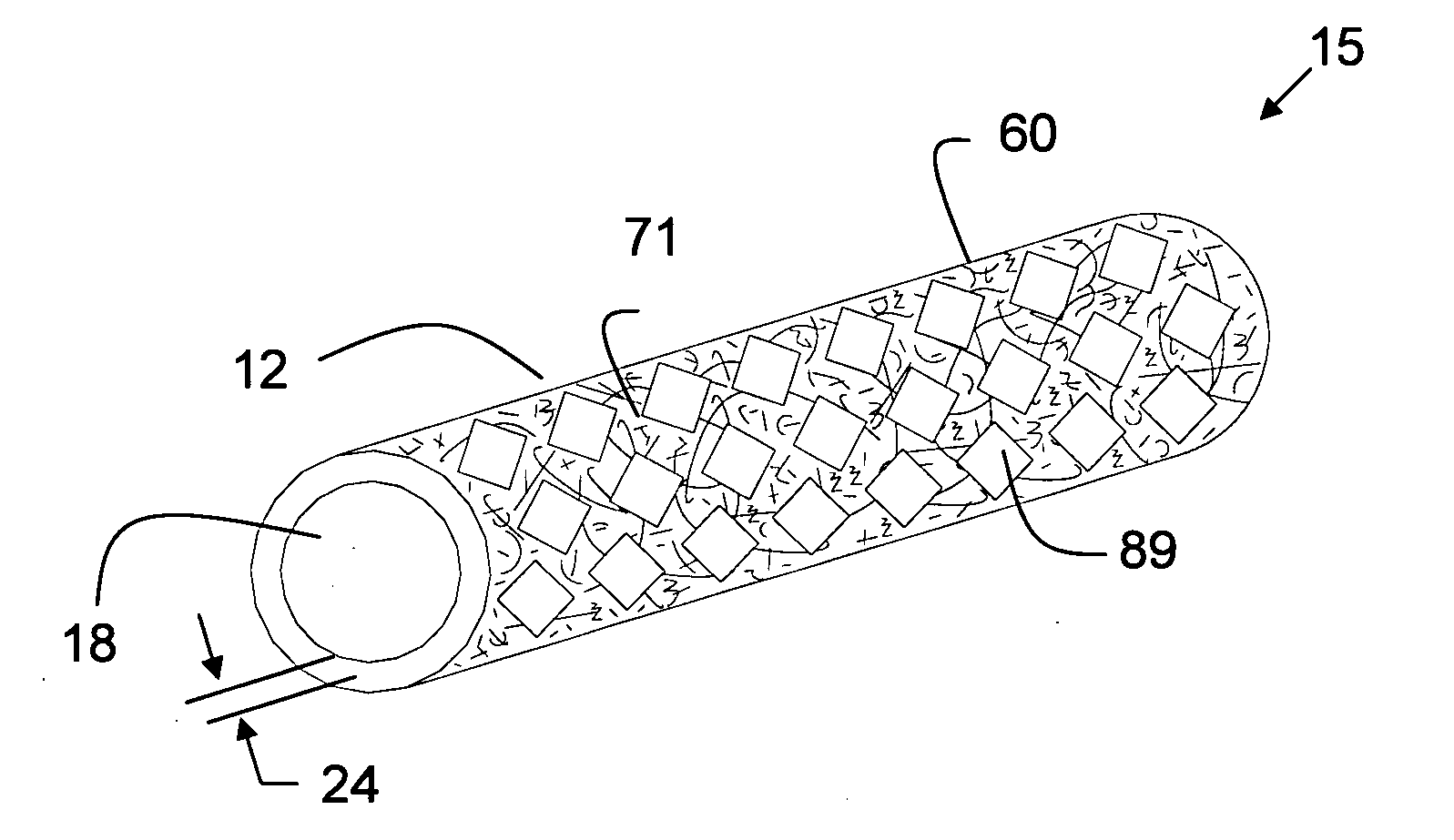
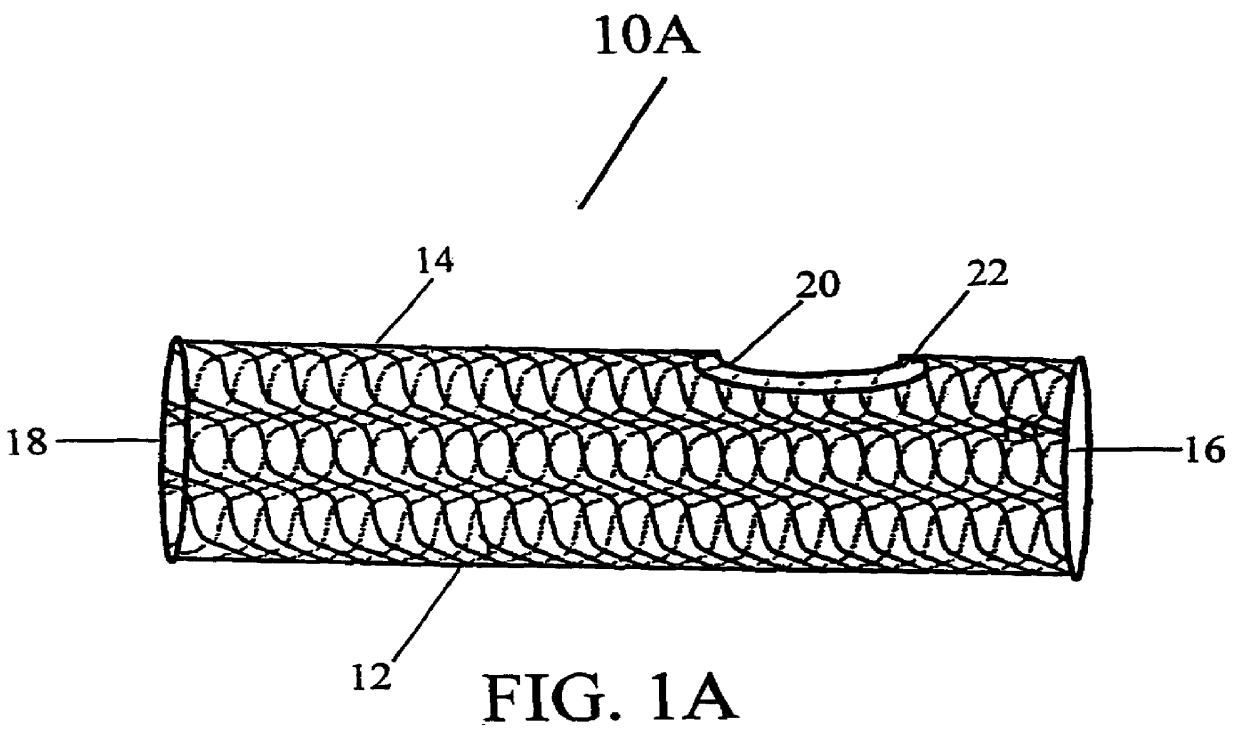
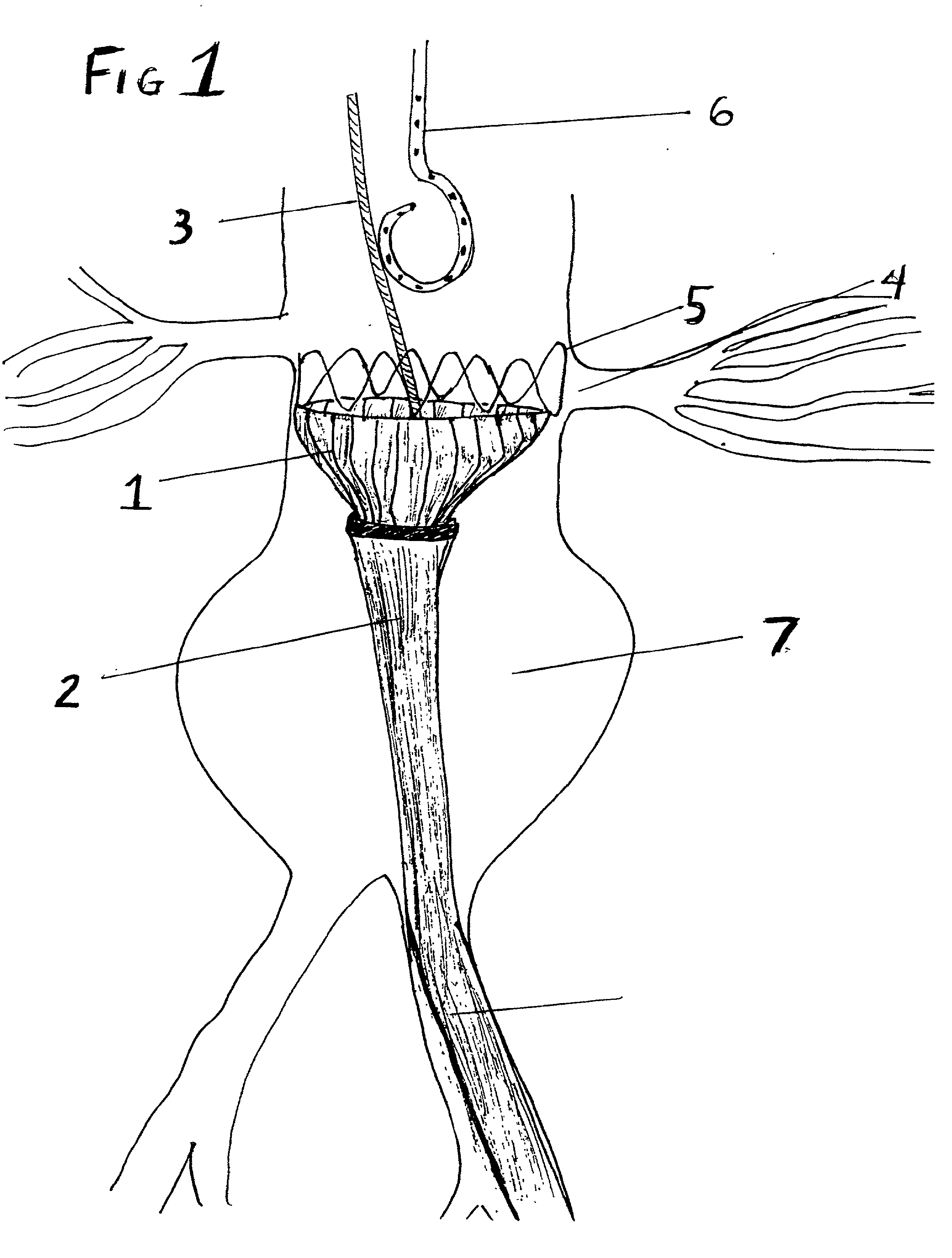
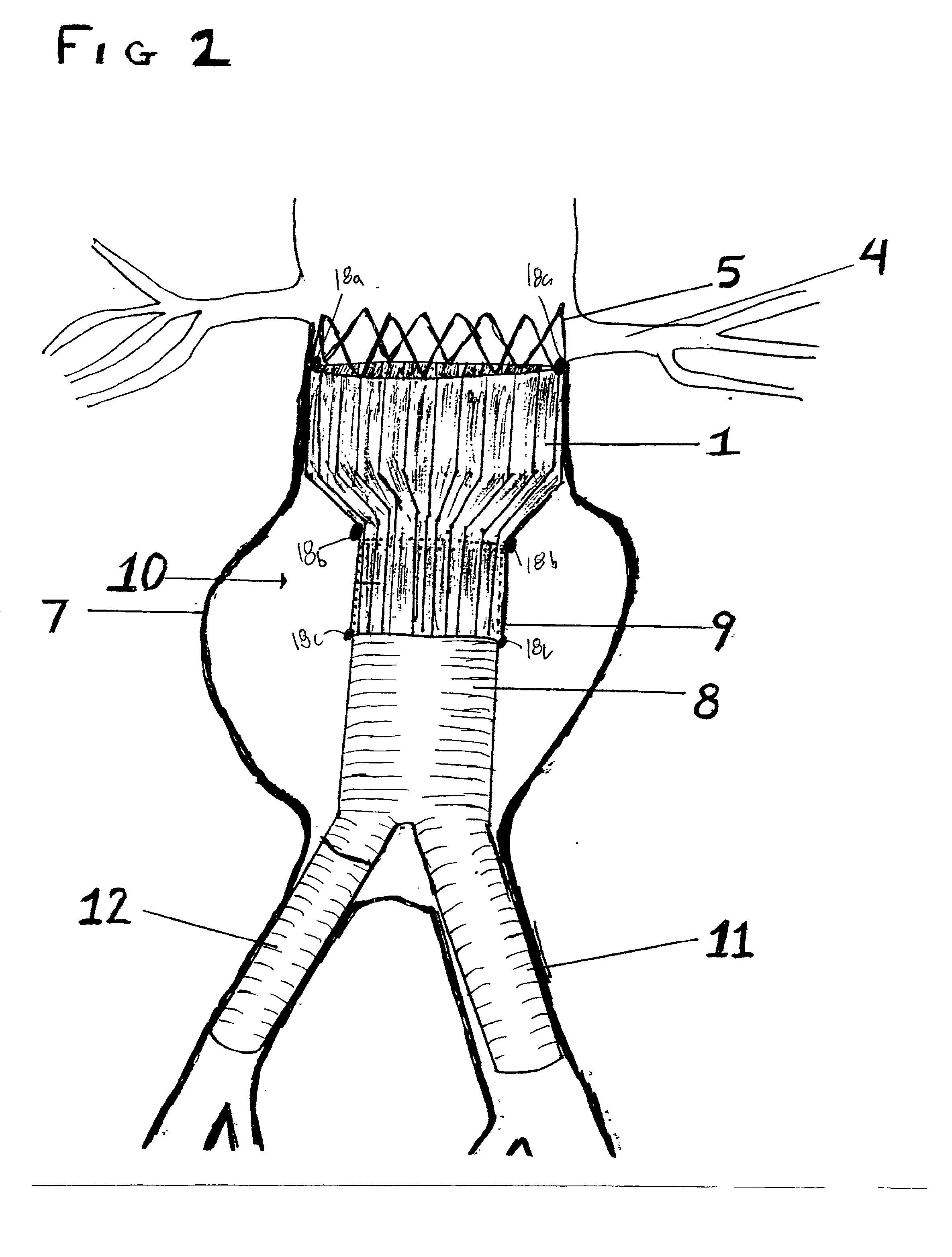
Stent Grafts. Stent grafts are used in transcatheter endovascular aortic repair (EVAR) procedures to seal aortic aneurysms. The grafts usually consist of a self-expanding stent frame that is covered with material to seal the vessel walls and prevent blood leaks feeding the aneurysm.
Tear and abrasion resistant expanded material and reinforcement
InactiveUS20070207186A1Increase flexibilityLess complex manufacturing processStentsSurgeryDiseaseEngineering
The present invention is a more durable expanded material that enables thinner wall thicknesses and a more flexible reinforcement suitable for stenting. The present invention is especially useful in the construction of grafts, stents, and stent-grafts which are used, for example, in repairing or replacing blood vessels that are narrowed or occluded by disease, aneurismal blood vessels, or other medical treatments. The inventive material and configurations allow expansion or contraction in size or adjustment in size in an incremental manner so that the optimum size, shape, and fit with other objects can be obtained. The present invention is also optionally capable of more accurately delivering one or more active ingredients such as drugs over longer periods of time. The present invention optionally includes surface modifications and additives that increase the surface adhesion of active ingredients, coatings, or combinations thereof. Finally, the present invention optionally includes growing cells on the inventive material so that the expanded material, reinforcement, or combinations thereof are useful, for example, in producing lab-grown blood vessels or organs.
Owner:SCANLON JOHN JAMES +1
Multiple-sided intraluminal medical device
A multiple-sided medical device comprises a closed frame of a single piece of wire or other resilient material and having a series of bends and interconnecting sides. The device has both a flat configuration and a second, folded configuration that comprises a self-expanding stent. The stent is pushed from a delivery catheter into the lumen of a duct or vessel. One or more barbs are attached to the frame of the device for anchoring or to connect additional frames. A covering of fabric or other flexible material such as DACRON, PTFE, or collagen, is sutured or attached to the frame to form an occlusion device, a stent graft, or an artificial valve such as for correcting incompetent veins in the lower legs and feet. A partial, triangular-shaped covering over the lumen of the device allows the valve to open with normal blood flow and close to retrograde flow.
Owner:COOK MEDICAL TECH LLC
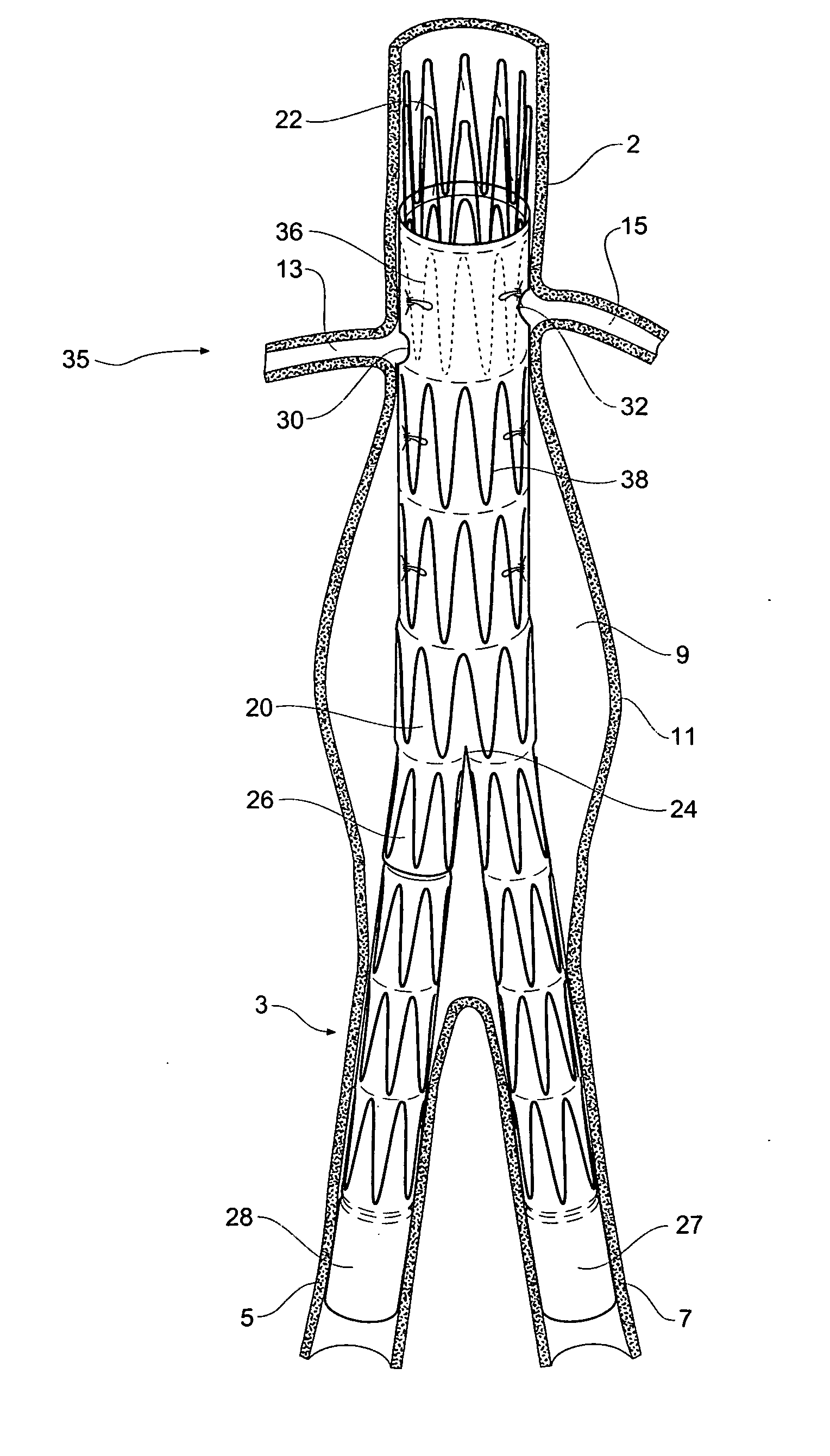
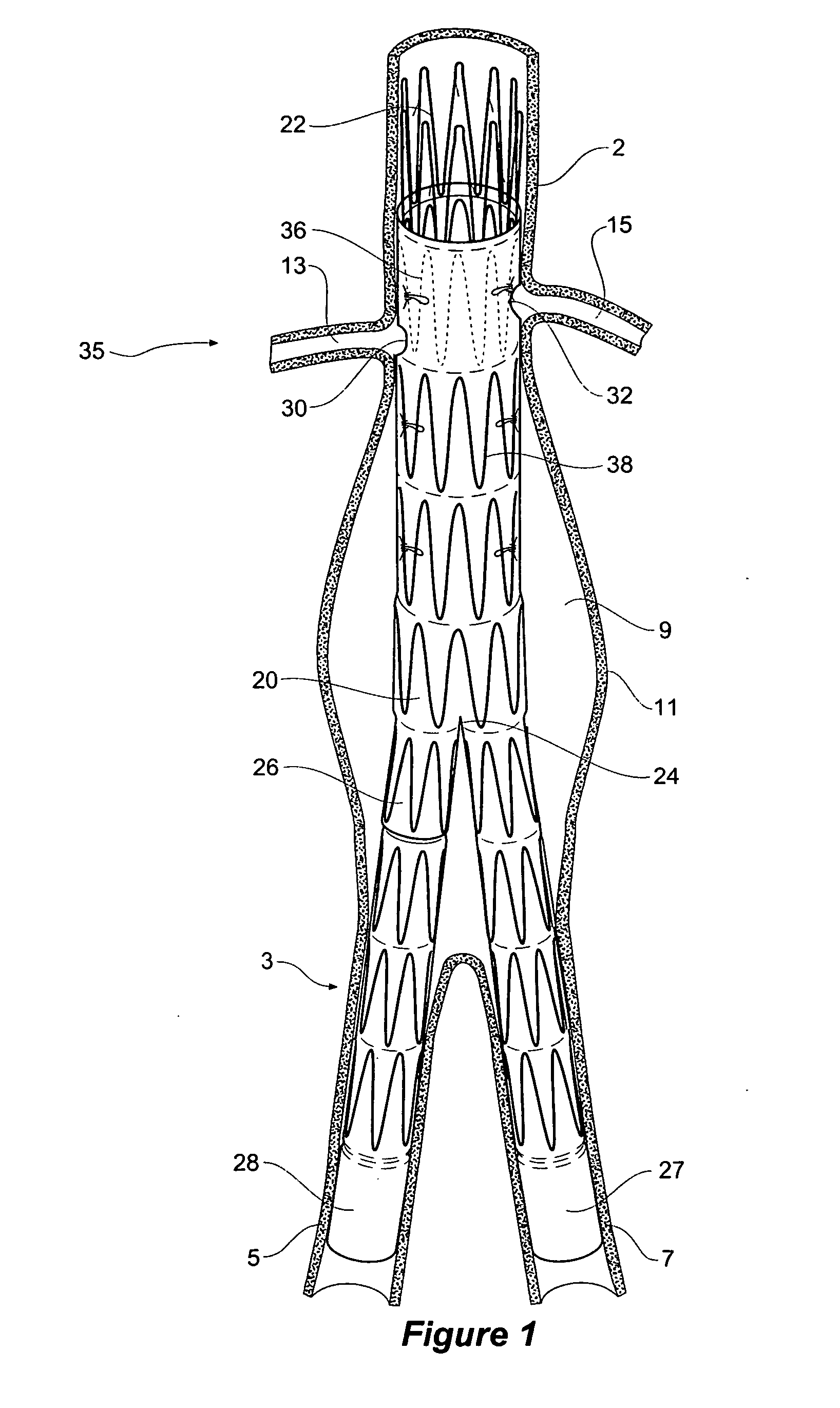
Stent or graft support structure for treating bifurcated vessels having different diameter portions and methods of use and implantation
A self-expanding stent structure is provided having a main portion that expands to a first diameter and a branch portion that expands to a second diameter, different the first diameter, the main portion having a link portion that forms a flexible linkage to, and forms part of, the branch portion. The self-expanding structure may be compressed to a reduced diameter for delivery, and resumes an expanded diameter during deployment. The self-expanding stent structure also may be advantageously incorporated in an asymmetric stent-graft system. Methods of use are also provided, wherein the main portion of the self-expanding structure, when deployed in a trunk vessel, may be used to anchor the branch portion in a branch vessel.
Owner:UFLACKER RENAN
Modular intraluminal prosteheses construction and methods
InactiveUS6193745B1Unnecessary expansive forcePrevent radial movementStentsBlood vesselsDiseaseExtensibility
The present invention provides modular intraluminal tubular prostheses, particularly stents and stent-grafts, for the treatment of disease conditions, particularly aneurysms. Modular sections of the prostheses, or "prosthetic modules," may be selectively combined to form a composite prosthesis having characteristics which are tailored to the specific requirements of the patient. Each prosthetic module preferably includes one or more standard interface ends for engaging another module, the module / module interface typically comprising ends which overlap and / or lock within a predetermined axial range. Advantageously, the axial length, cross-section, perimeter, resilient expansive force, axial flexibility, liner permeability, liner extensibility, radial conformability, liner / tubal wall sealing and anchoring, and other prosthetic characteristics may be varied along the axis of the composite prosthesis, and also along the axis of each prosthetic module. The modules are preferably individually introduced into a lumen system of a patient body so that the composite prosthesis is assembled in situ. Ideally, selection of appropriate prosthetic modules and the flexibility of the interface overlap range provides a custom fit intraluminal prosthesis which provides a therapy tailored to the individual patient's needs.
Owner:MEDTRONIC AVE
Actively Controllable Stent, Stent Graft, Heart Valve and Method of Controlling Same
Sealable and repositionable implant devices are provided to increase the ability of endovascular grafts and valves to be precisely deployed or re-deployed, with better in situ accommodation to the local anatomy of the targeted recipient anatomic site, and with the ability for post-deployment adjustment to accommodate anatomic changes that might compromise the efficacy of the implant. A surgical implant includes a self-expanding stent of a shape-memory material set to a given shape. The stent has a wall with a portion having a first thickness and a second portion having a thickness greater than the first. The second portion defines a key-hole shaped longitudinal drive orifice. The implant includes a selectively adjustable assembly having adjustable elements and being operable to force a configuration change in at least a portion of the self-expanding stent. The adjustable elements have a part rotatably disposed within the longitudinal drive orifice.
Owner:EDWARDS LIFESCI CARDIAQ
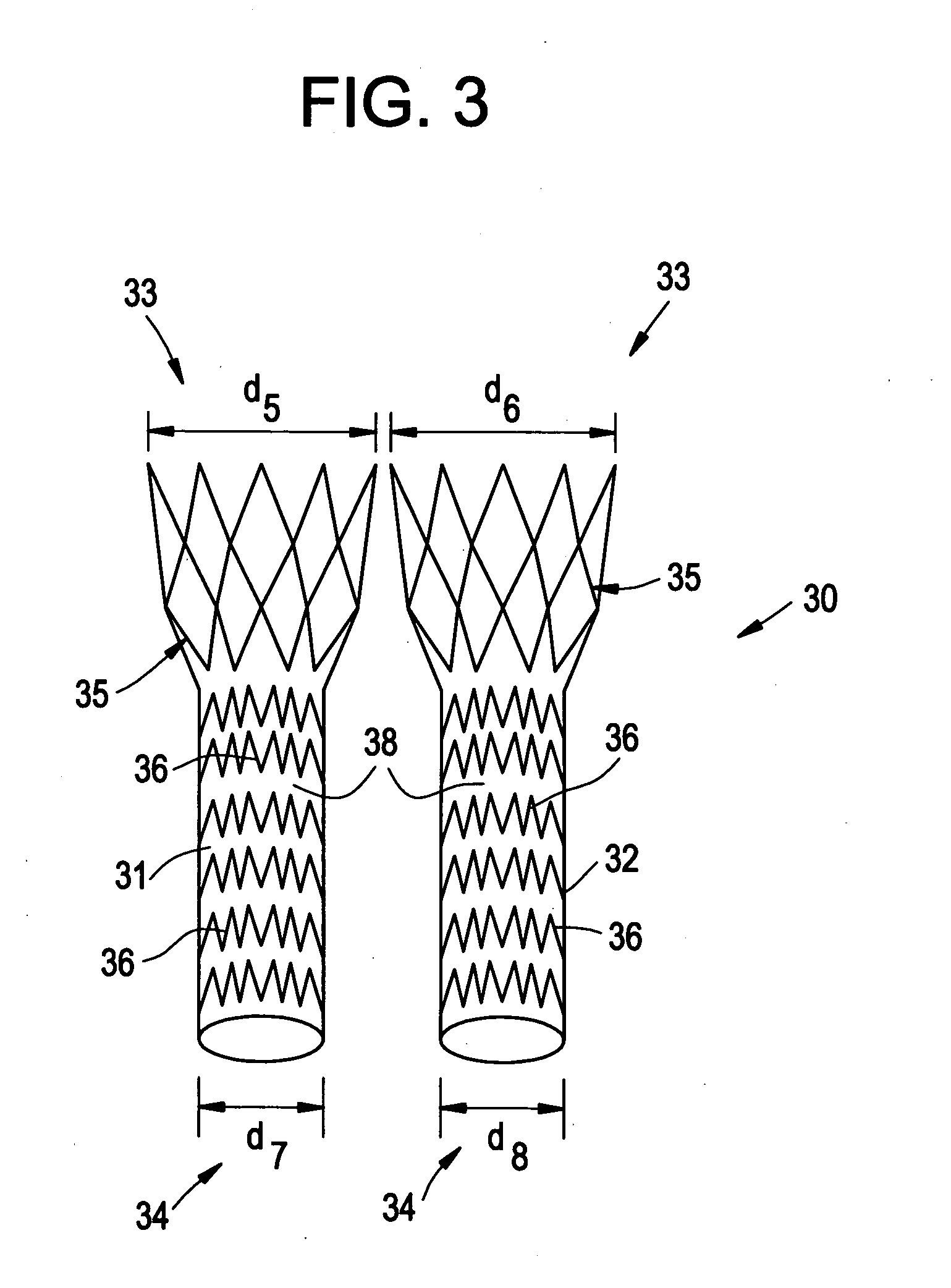
Stent-graft-membrane and method of making the same
A braided self-expandable stent-graft-membrane made of elongated members forming a generally tubular body. A membrane layer and graft layer are disposed on a endoprosthesis such as a stent. The membrane layer is substantially impermeable to fluids. The outermost layer is biocompatible with the body tissue. The innermost layer is biocompatible with the fluid in the passage. An embodiment includes a graft layer disposed on the inside of a stent and a membrane layer disposed on the outside of the stent. The innermost layer is biocompatible with the fluid in the passage. The stent-graft-membrane is used at a treatment site in a body vessel or organ where it is desirous to exclude a first fluid located outside the endoprosthesis from reaching a second fluid located in the lumen. The membrane may be made of silicone or polycarbonate urethane. The graft may be braided, woven, spun or spray-cast PET, PCU, or PU fibers. The layers may include ePTFE or PTFE.
Owner:LIFEPORT SCI
Actively Controllable Stent, Stent Graft, Heart Valve and Method of Controlling Same
Sealable and repositionable implant devices are provided with features that increase the ability of implants such as endovascular grafts and valves to be precisely deployed or re-deployed, with better in situ accommodation to the local anatomy of the targeted recipient anatomic site, and / or with the ability for post-deployment adjustment to accommodate anatomic changes that might compromise the efficacy of the implant. A surgical implant includes an implant body and a selectively adjustable assembly attached to the implant body, the assembly having adjustable elements and being operable to cause a configuration change in a portion of the implant body and, thereby, permit implantation of the implant body within an anatomic orifice to effect a seal therein under normal physiological conditions.
Owner:EDWARDS LIFESCI CARDIAQ
Degradable implantable medical devices
InactiveUS20060229711A1Reduce probabilityLower resistanceStentsBlood vesselsVascular implantBlood vessel
Devices and methods are provided for an implantable medical device which is degradable over a clinically relevant period of time. The medical devices may have the form of implants, graft implants, vascular implants, non vascular implants, wound closure implants, sutures, drug delivery implants, biologic delivery implants, urinary tract implants, inter-uterine implants, organ implants, bone implants including bone plates, bone screws, dental implants, spinal disks, or the like. In preferred embodiments, the implantable medical device comprises an implantable luminal prosthesis, such as vascular and non-vascular stents and stents grafts.
Owner:ELIXIR MEDICAL CORP
Methods and devices for forming vascular anastomoses
Methods and devices for forming an anastomosis utilize a graft vessel secured to a vessel coupling that is fixed to a target vessel without using suture. The vessel coupling may be collapsed for introduction into the target vessel and then expanded to engage the vessel wall. The vessel coupling may be a stent attached to a graft vessel to form a stent-graft assembly. The anastomosis may be carried out to place the graft and target vessels in fluid communication while preserving native proximal flow through the target vessel, which may be a coronary artery. As a result, blood flowing through the coronary artery from the aorta is not blocked by the vessel coupling and thus is free to move past the site of the anastomosis.
Owner:MEDTRONIC INC
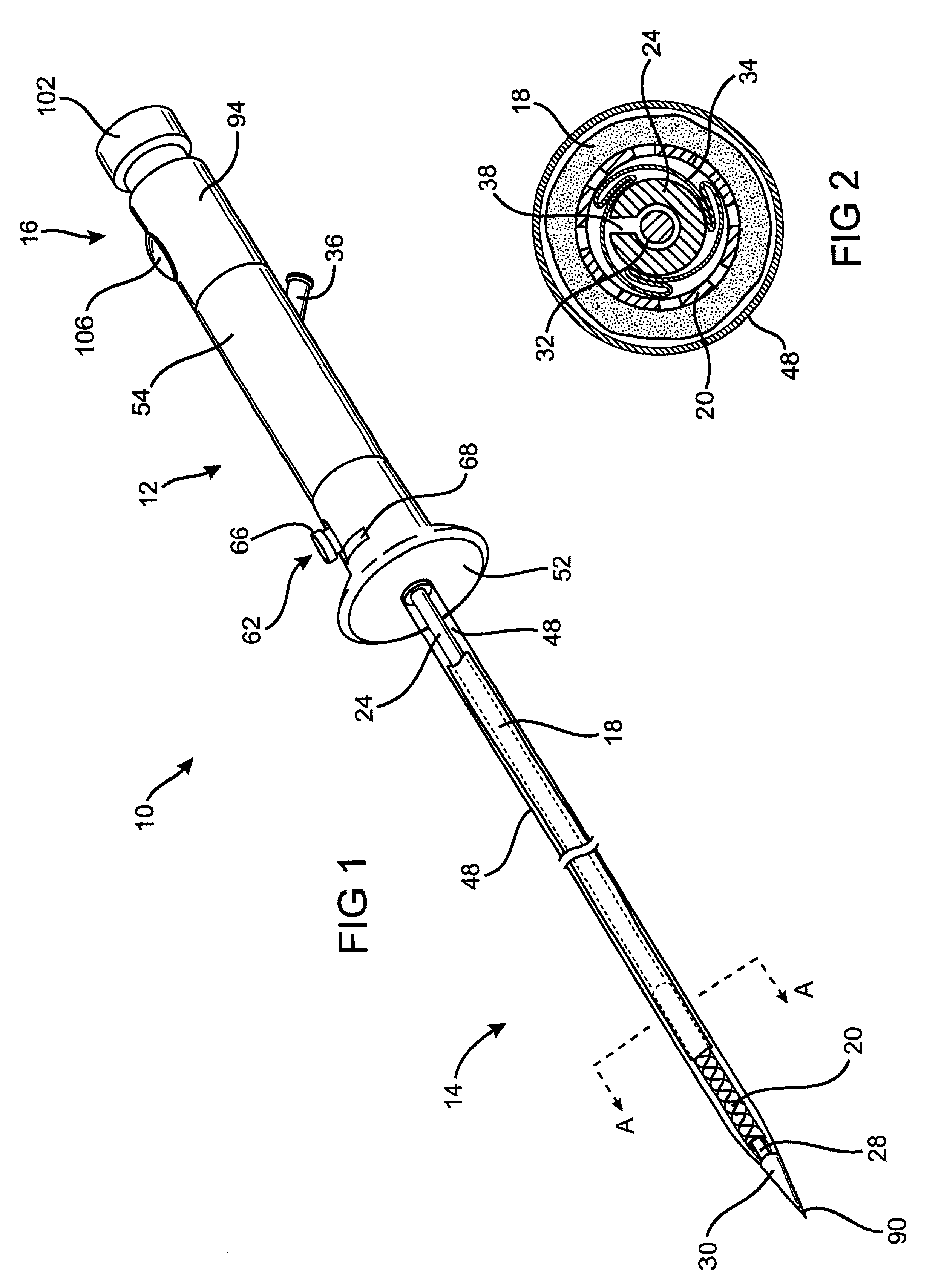
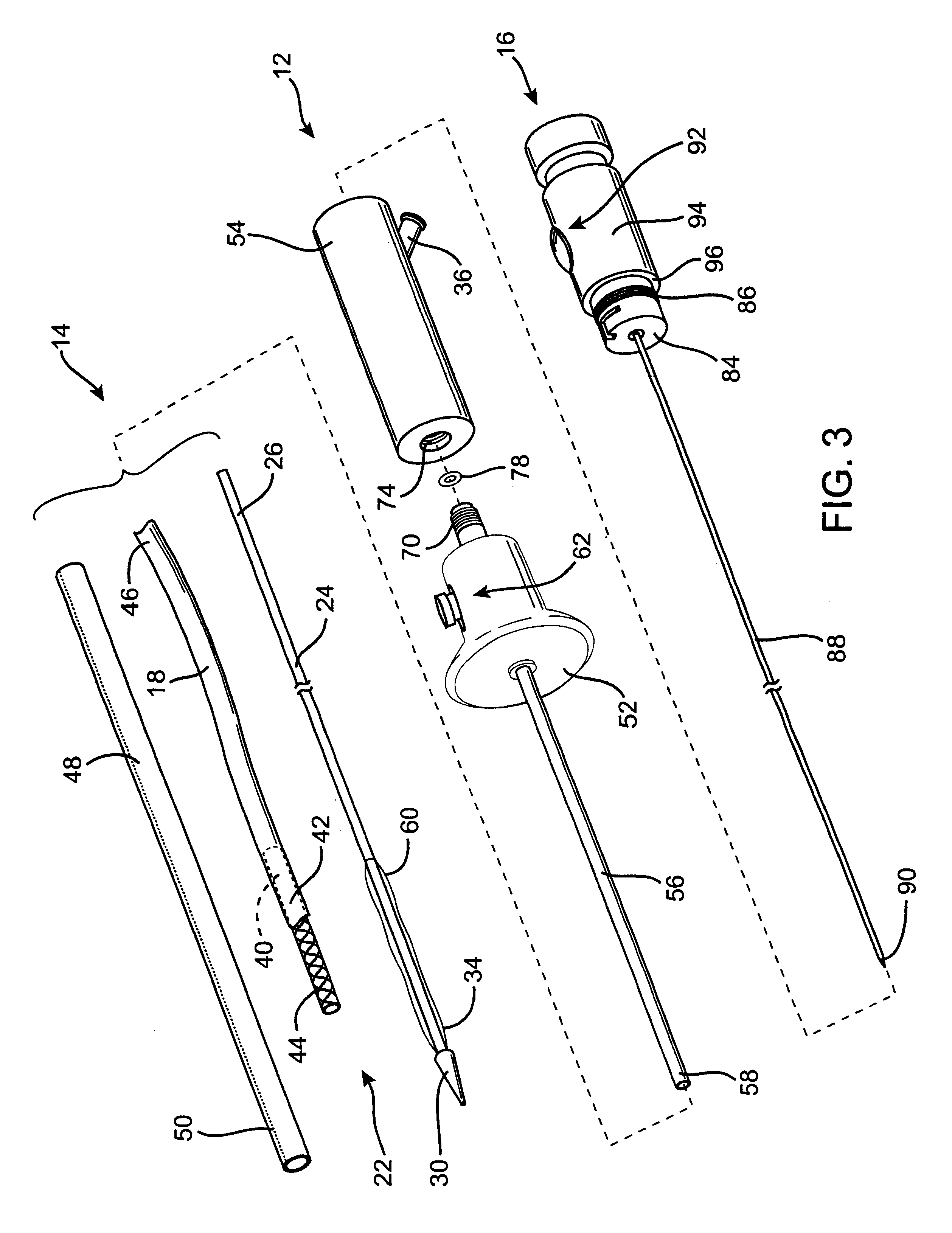
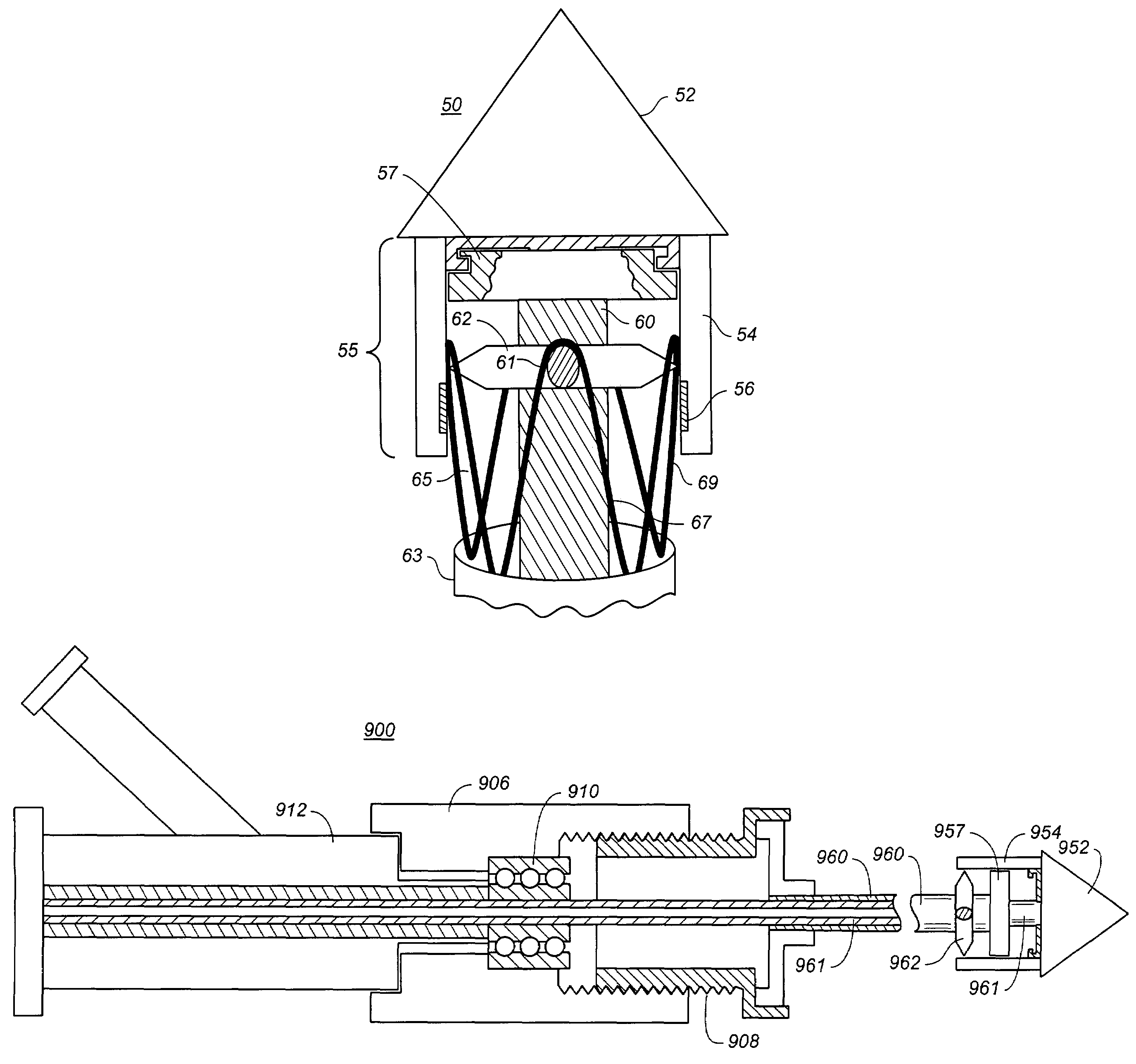
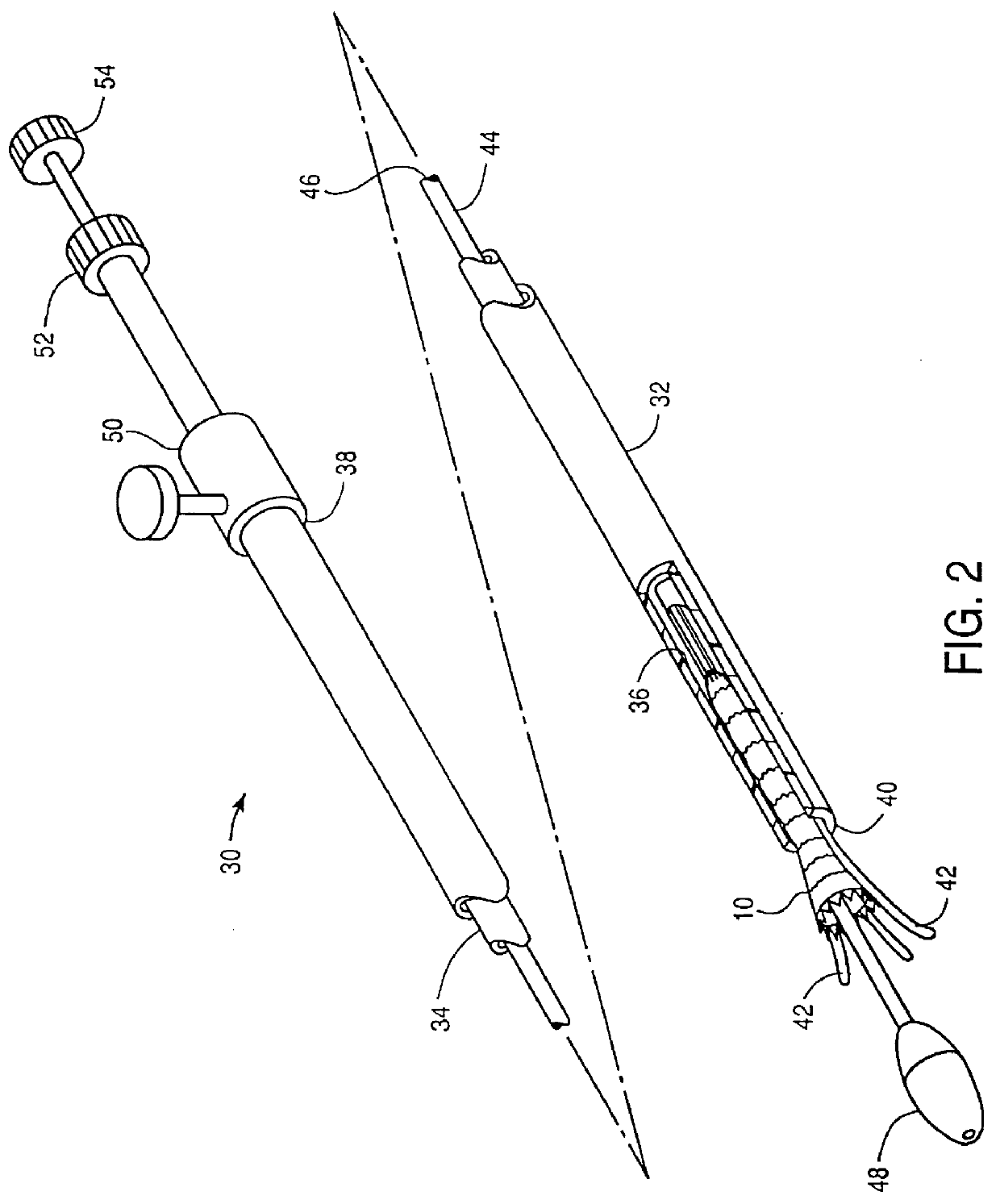
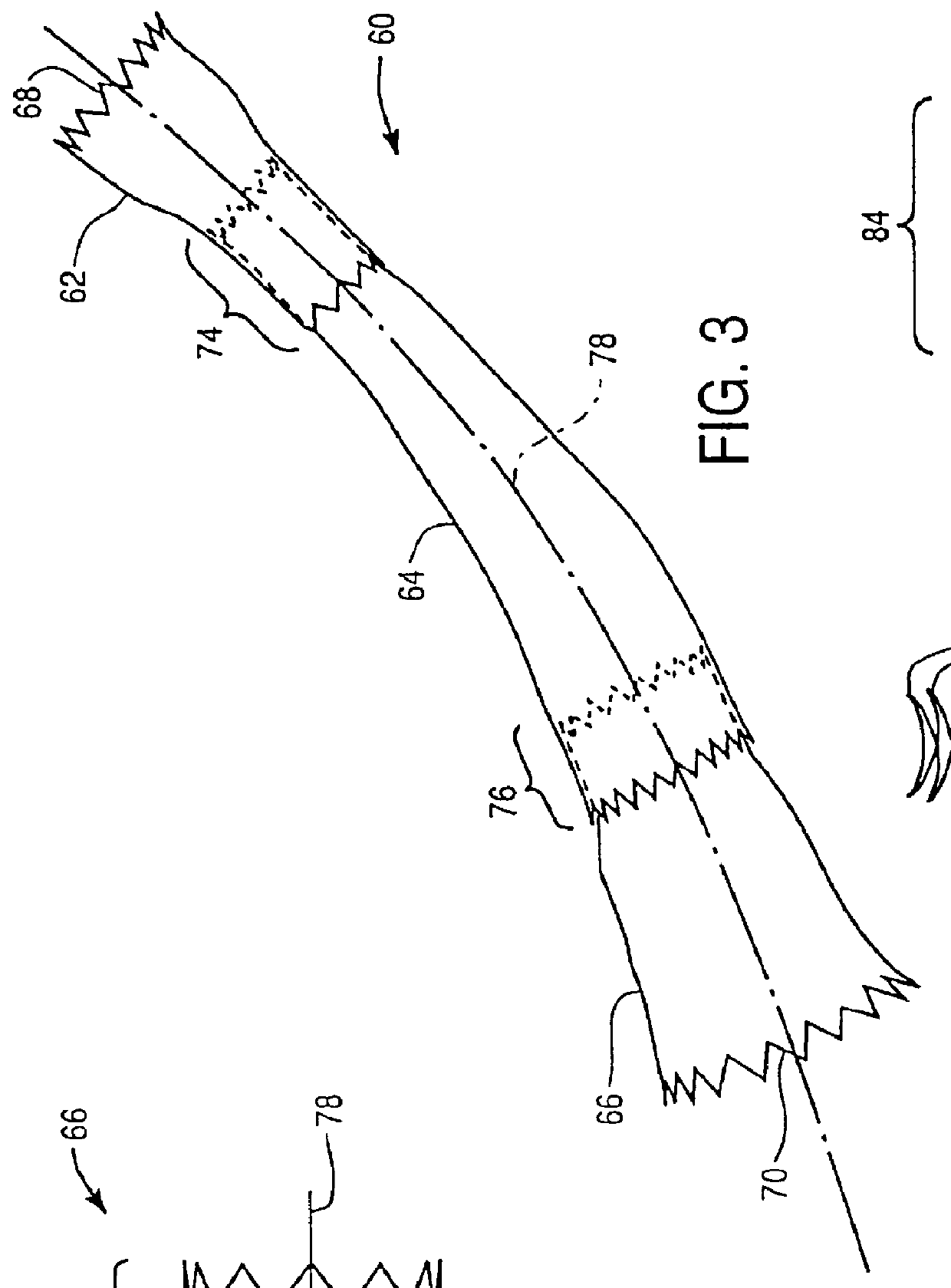
Controlled deployment delivery system
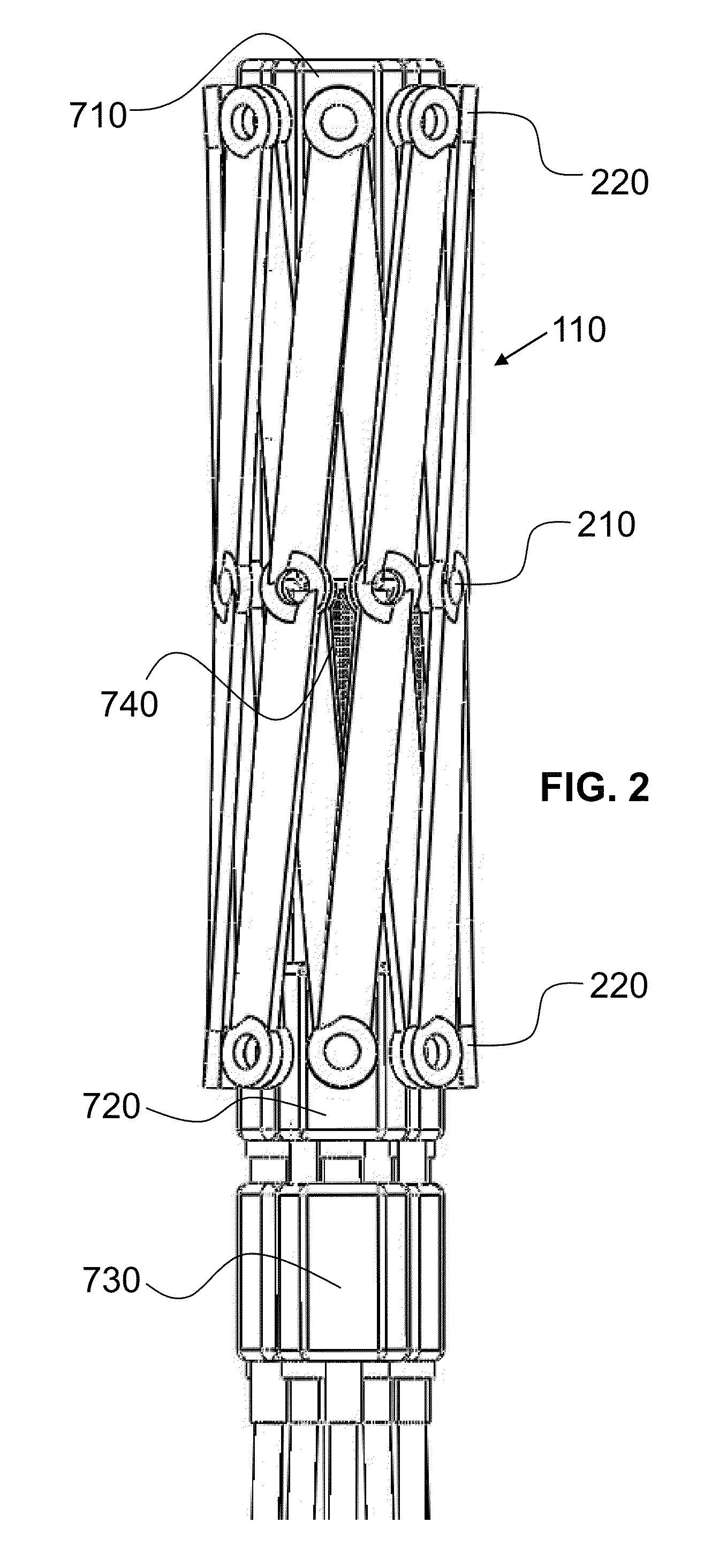
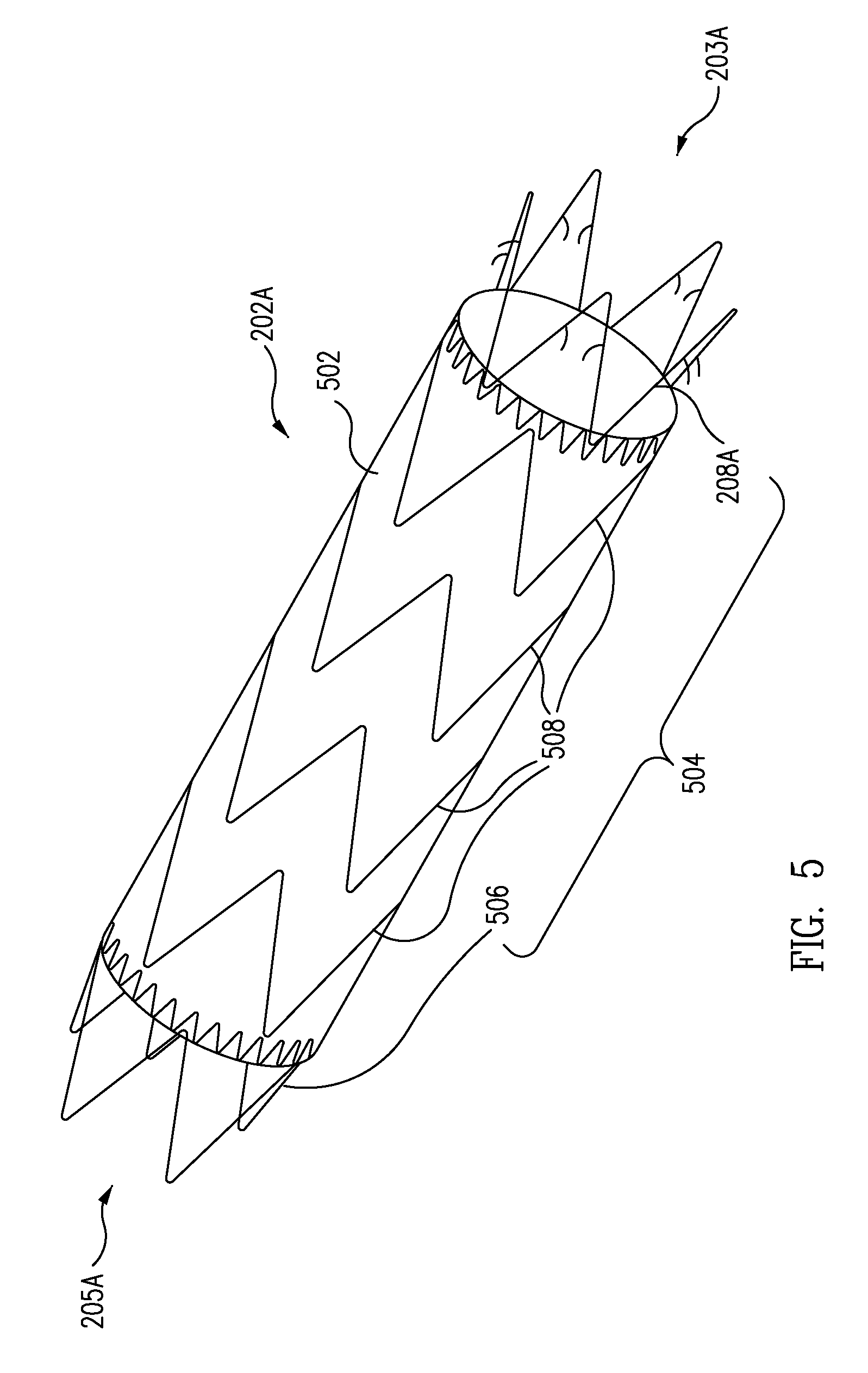
A controlled stent-graft deployment delivery system (10 50 or 900) includes a stent-graft (30 or 63), a retractable primary sheath (40) containing the stent-graft in a first constrained diameter configuration, an outer tube (18) within the retractable primary sheath and within the stent-graft, and an inner tube (20) within the outer tube, where the inner tube and the outer tube both axially move relative to the retractable primary sheath and to each other. The system further includes a cap (15) coupled to a distal end of the inner tube and configured to retain at least a portion of a proximal area of the stent-graft in a radially compressed configuration. A distal assembly (100) provides controlled relative axial movement between the outer tube and the inner tube enabling the release of the proximal end (65, 67, 68, and 69) of the stent-graft from the cap and from the radially compressed configuration.
Owner:MEDTRONIC VASCULAR INC
Delivery System for Stent-Graft With Anchoring Pins
ActiveUS20080114442A1Proximal anchor stent ring is facilitatedSmooth and easy retractionStentsBlood vesselsStent graftingInsertion stent
A delivery system for an endoprosthesis includes a spindle having a spindle body and spindle pins extending radially outward from the spindle body. The delivery system further comprises a tip comprising a sleeve, the spindle pins extending from the spindle body toward the sleeve. The endoprosthesis includes a proximal anchor stent ring having spindle pin catches and anchor pins. The spindle pins of the spindle extend into the spindle pin catches and the sleeve radially constrains the anchor pins.
Owner:MEDTRONIC VASCULAR INC
Mated main and collateral stent and method for treatment of arterial disease
The present invention is directed to the use of a stented graft having predetermined and sized lateral openings for the treatment of arterial disease at or around the intersection of multiple arteries, thereby ensuring blood flow through such arteries to collateral organs. In particular, the lateral opening of a main stent supporting a main artery has a collar with either at least two detents or inlets spaced about the annular extent thereof. The main collar mates with a collateral collar provided at the proximal end of the collateral stent having the other of at least two detents or inlets spaced about the annular extent thereof at intervals coincident with the inlets or detents on the main collar to mate and lock the main stent to the collateral stent supporting a collateral artery.
Owner:TAHERI SYDE A
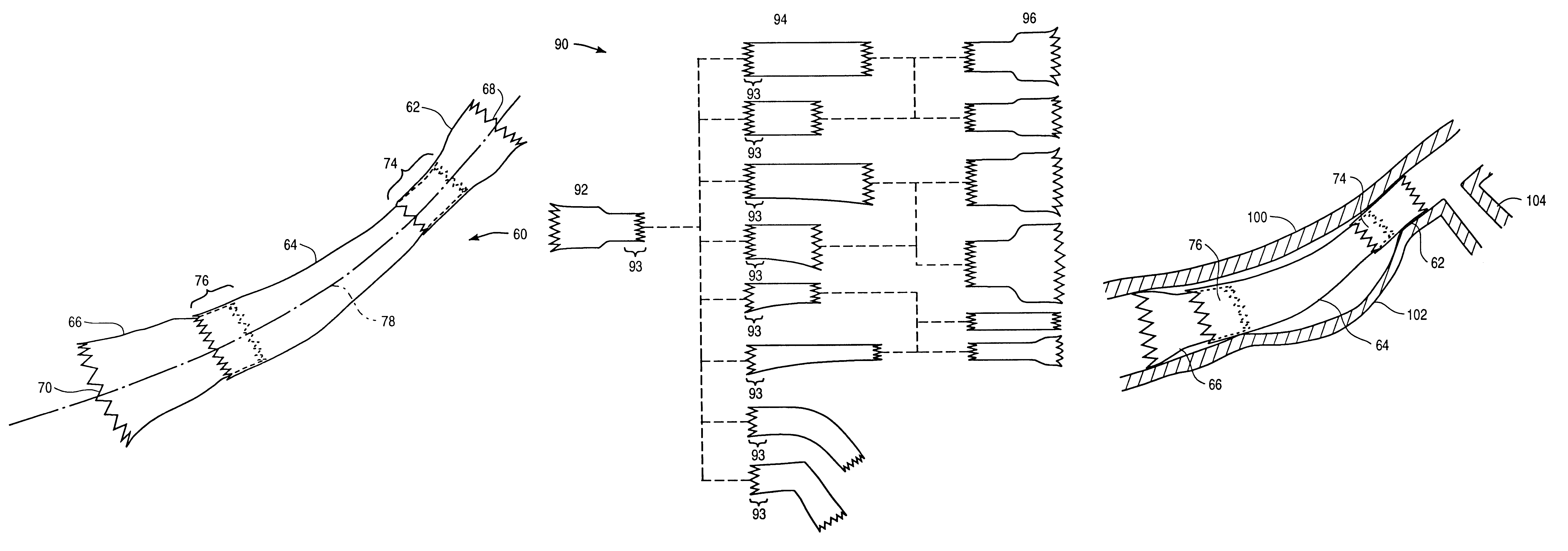
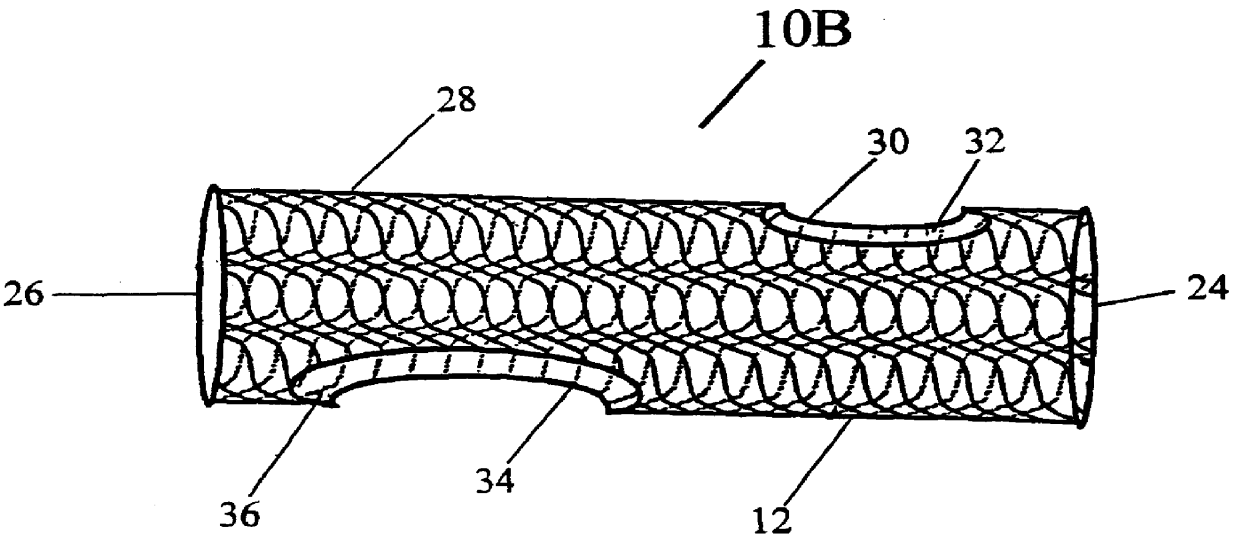
Stitched stent grafts and methods for their fabrication
InactiveUS6123722AUnnecessary expansive forcePrevent radial movementStentsBlood vesselsExtensibilityStent grafting
The present invention provides modular intraluminal tubular prostheses, particularly stents and stent-grafts, for the treatment of disease conditions, particularly aneurysms. Modular sections of the prostheses, or "prosthetic modules," may be selectively combined to form a composite prosthesis having characteristics which are tailored to the specific requirements of the patient. Each prosthetic module preferably includes one or more standard interface ends for engaging another module, the module / module interface typically comprising ends which overlap and / or lock within a predetermined axial range. Advantageously, the axial length, cross-section, perimeter, resilient expansive force, axial flexibility, liner permeability, liner extensibility, radial conformability, liner / tubal wall sealing and anchoring, and other prosthetic characteristics may be varied along the axis of the composite prosthesis, and also along the axis of each prosthetic module. The modules are preferably individually introduced into a lumen system of a patient body so that the composite prosthesis is assembled in situ. Ideally, selection of appropriate prosthetic modules and the flexibility of the interface overlap range provides a custom fit intraluminal prosthesis which provides a therapy tailored to the individual patient's needs.
Owner:MEDTRONIC AVE
Tapered endovascular stent graft and method of treating abdominal aortic aneurysms and distal iliac aneurysms
An endovascular stent graft is provided for use in treating abdominal aortic aneurysms. The endovascular stent has a tapered section which allows it to accommodate markedly large aortas such as the abdominal aorta and still connects to standard modular aortic stent grafts. Methods of utilizing the stent to treat abdominal aortic aneurysms and distal iliac aneurysms are also provided.
Owner:COOK MEDICAL TECH LLC
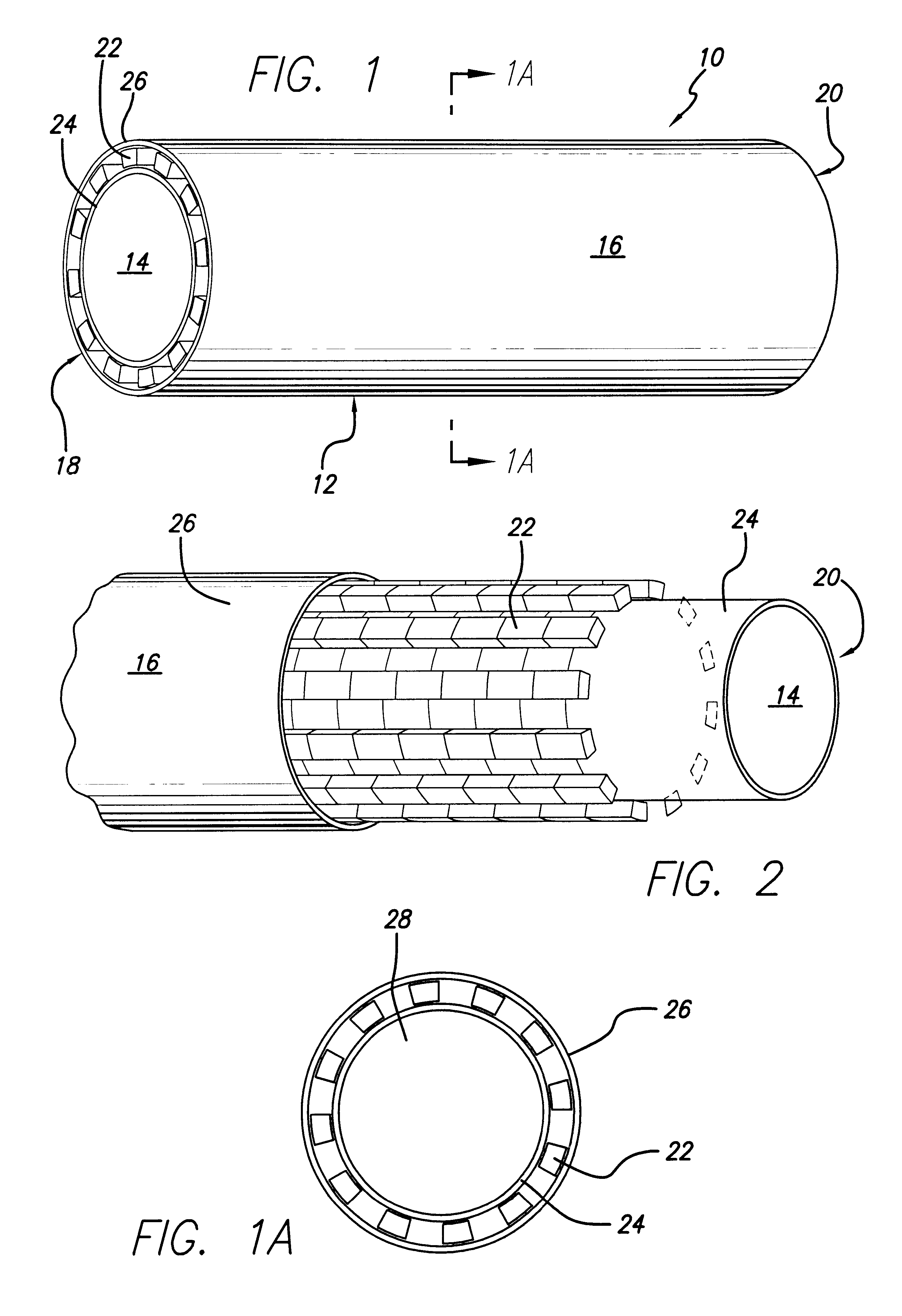
Encapsulated stent
InactiveUS6383214B1Reduce thrombosisEasy to separateStentsBlood vesselsStent graftingPolytetrafluoroethylene
An encapsulated stent having a stent or structural support layer sandwiched between two biocompatible flexible layers. One preferred embodiment has a stent cover which includes a tubular shaped stent that is concentrically retained between two tubular shaped grafts comprised of expanded polytetrafluoroethylene. Another preferred embodiment has a stent graft which includes at least one stent sandwiched between the ends of two tubular shaped grafts wherein at least a portion of the grafts are unsupported by the stent. Still another embodiment includes an articulating stented graft which includes a plurality of stents spaced apart from one another at a predetermined distance wherein each stent is contained between two elongated biocompatible tubular members. The graft / stent / graft assemblies all have inseparable layers.
Owner:BARD PERIPHERAL VASCULAR
Self-aligning stent graft delivery system, kit, and method
ActiveUS20050049667A1Reduce the possibilityIncreases blood-tight vascular connectionStentsBlood vesselsDistal portionStent grafting
A delivery system and kit for endovascularly delivering a prosthesis along a guidewire to an curved implantation site includes a control handle having a handle body with a prosthesis movement control assembly movably disposed thereto. A proximal end of a catheter is connected to the control handle. A prosthesis delivery assembly is movably disposed in the catheter. The delivery assembly has a guidewire lumen slidably receiving therein the guidewire and having a curved distal end orienting the delivery assembly when passing through a curved vessel portion. A method for automatic endovascular alignment of the prosthesis includes loading it in the distal delivery assembly, positioning the guidewire into the curved implantation site, and threading the guidewire lumen over the guidewire with the delivery assembly and into the implantation site to at least approximately align the curved distal portion to a curve of the curved implantation site and, thereby, orient the prosthesis.
Owner:BOLTON MEDICAL INC
Intravascular deliverable stent for reinforcement of vascular abnormalities
InactiveUS20070168019A1Avoid interactionReduce overall outer diameterStentsCatheterVascular Skin TumorSaphenous veins
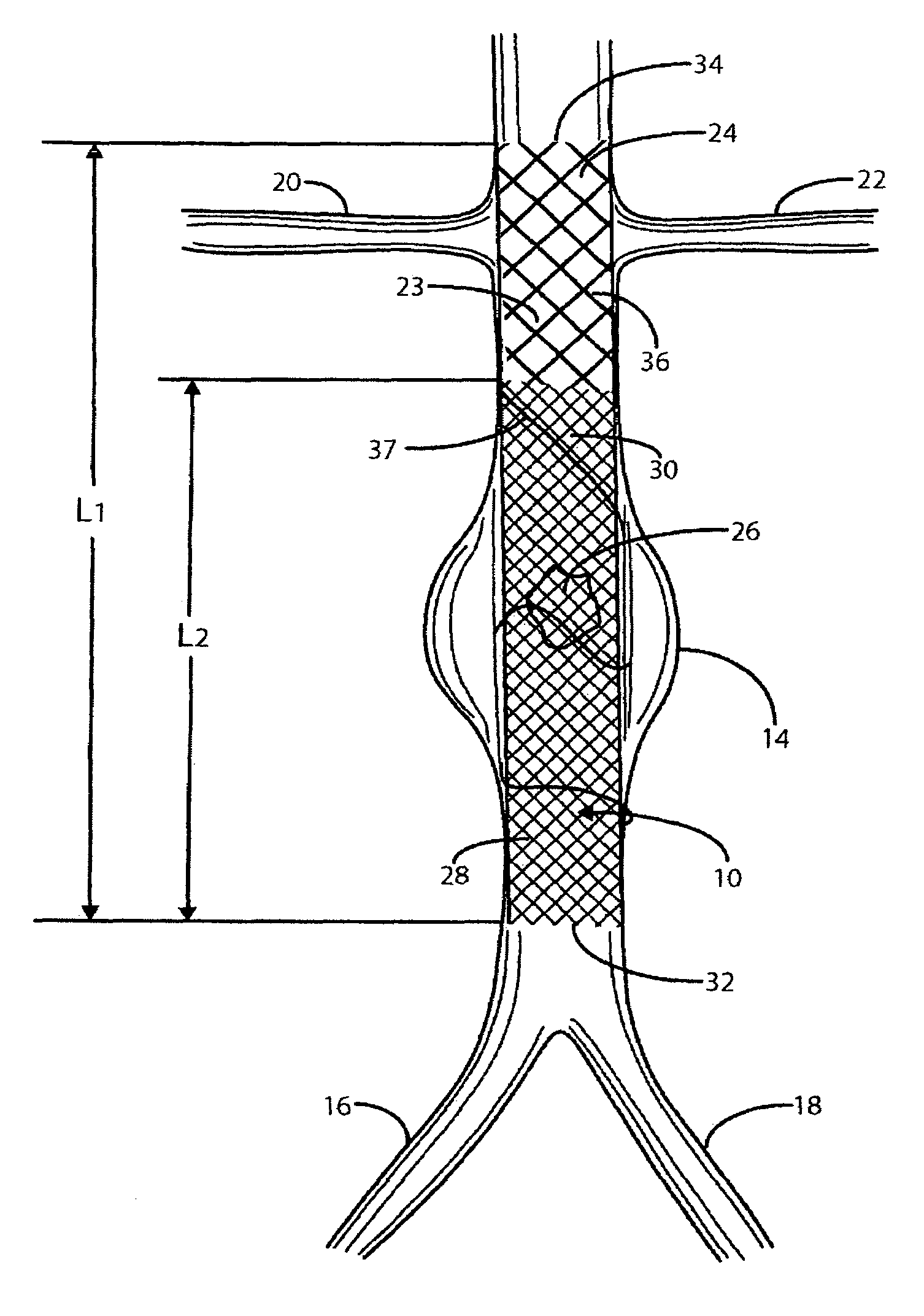
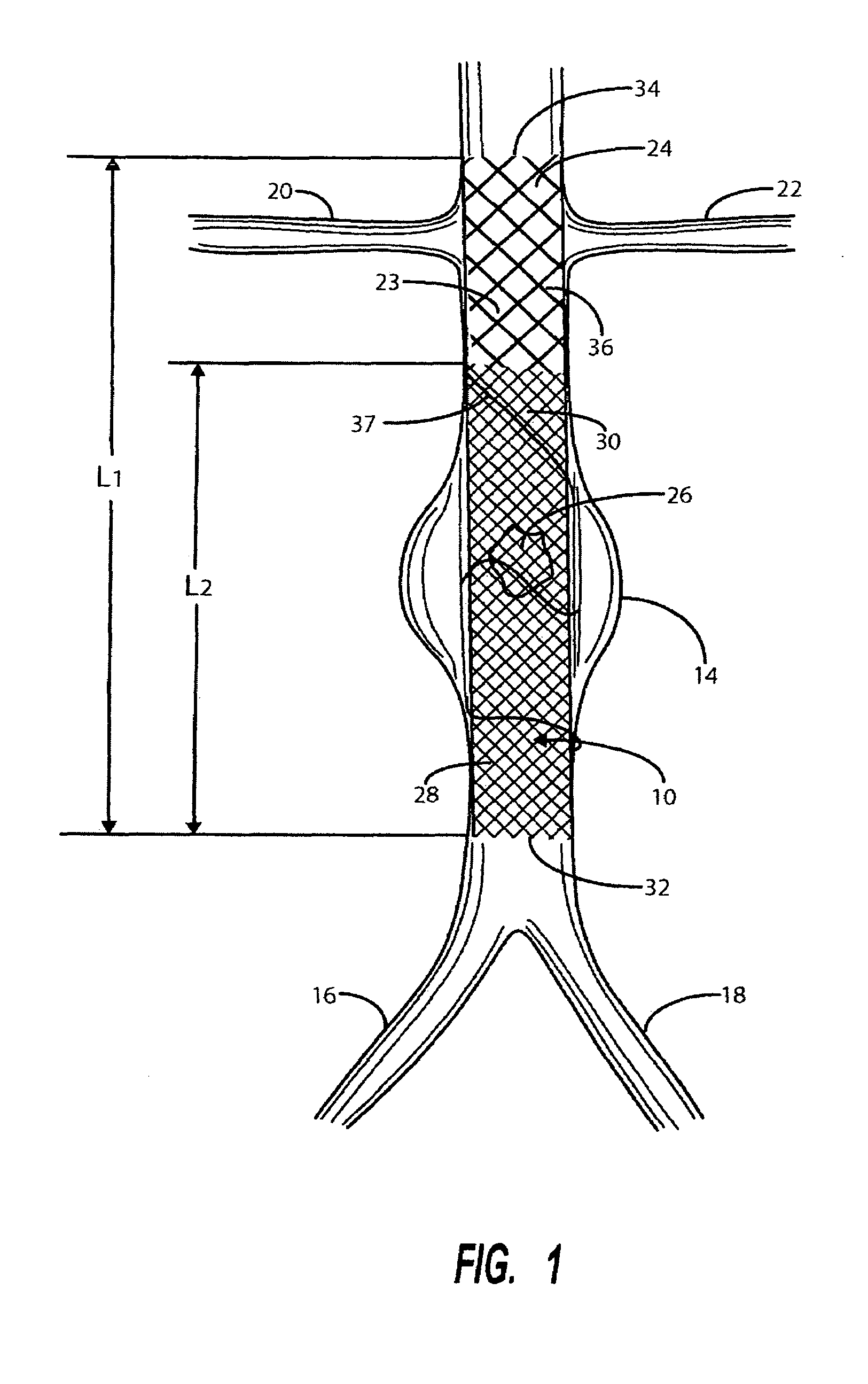
A catheter deliverable stent / graft especially designed to be used in a minimally invasive surgical procedure for treating a variety of vascular conditions such as aneurysms, stenotic lesions and saphenous vein grafts, comprises an innermost tubular structure and at least one further tubular member in coaxial arrangement. In one embodiment, the innermost tubular structure is of a length (L1) and is formed by braiding a relatively few strands of highly elastic metallic alloy. The pick and pitch of the braid are such as to provide relative large fenestrations in the tubular wall that permit blood flow through the wall and provide the primary radial support structure. A portion of the innermost tubular structure of a length L1 is surrounded by a further braided tubular structure having relatively many strands that substantially inhibit blood flow through the fenestrations of the innermost tubular structure. The composite structure can be stretched to reduce the outer diameter of the stent / graft, allowing it to be drawn into a lumen of a delivery catheter. The catheter can then be advanced through the vascular system to the site of treatment and then released, allowing it to self-expand against the vessel wall. Various optional embodiments are disclosed that allow one skilled in the art to tailor the design to the specific application.
Owner:ST JUDE MEDICAL CARDILOGY DIV INC
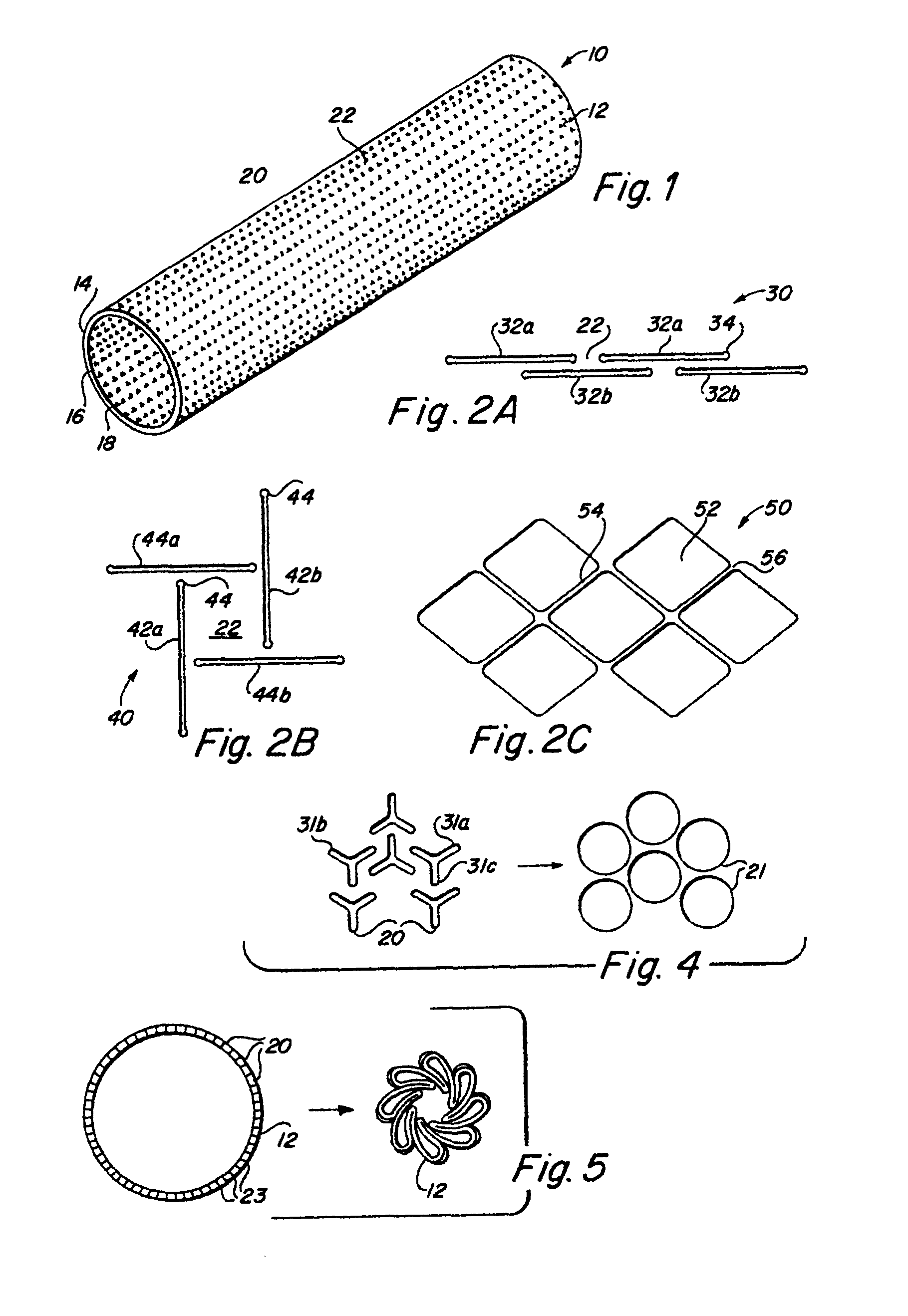
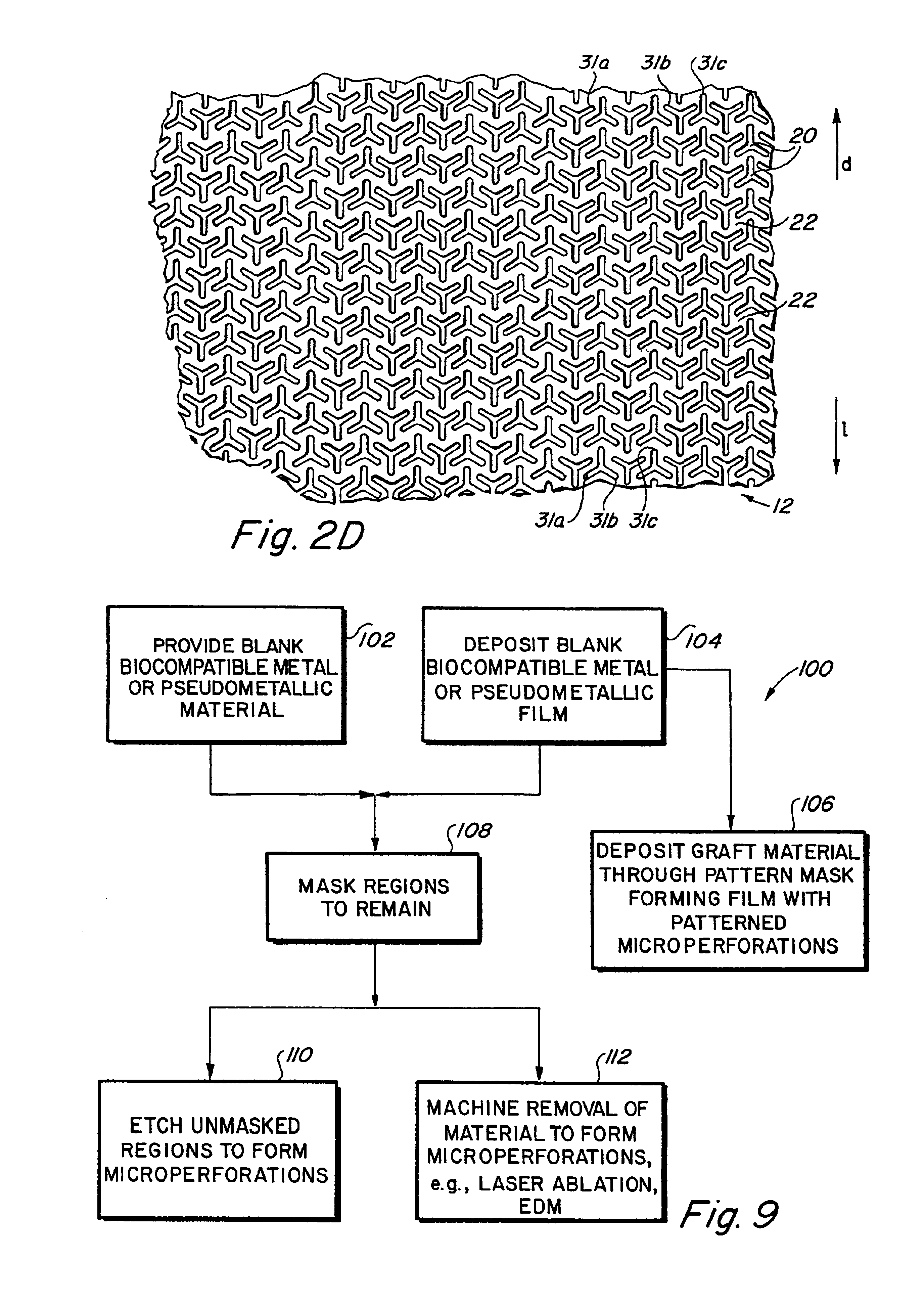
Complaint implantable medical devices and methods of making same
InactiveUS6936066B2Give flexibilityFacilitating transmural endothelializationStentsHeart valvesSurgical GraftMetallic materials
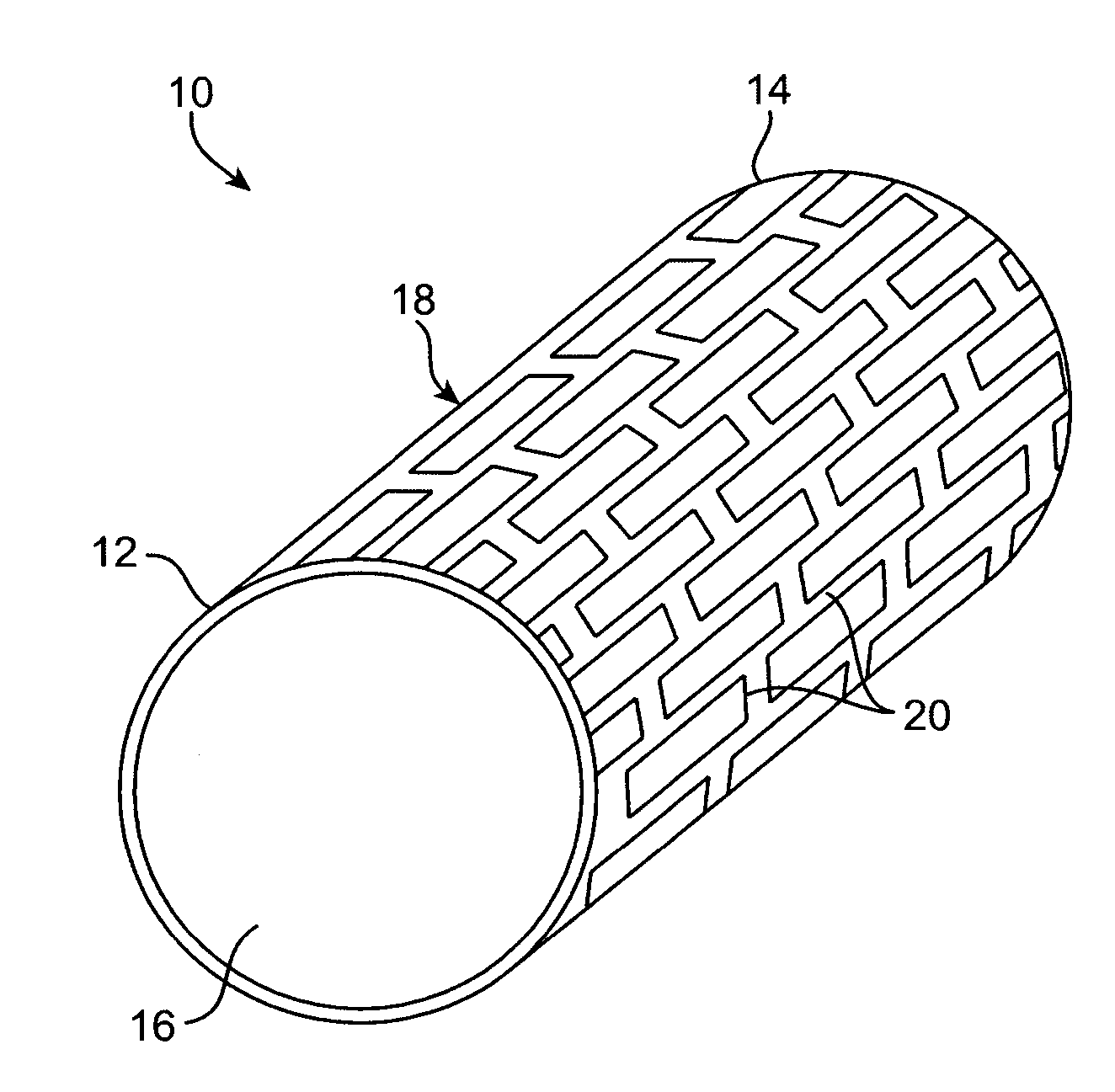
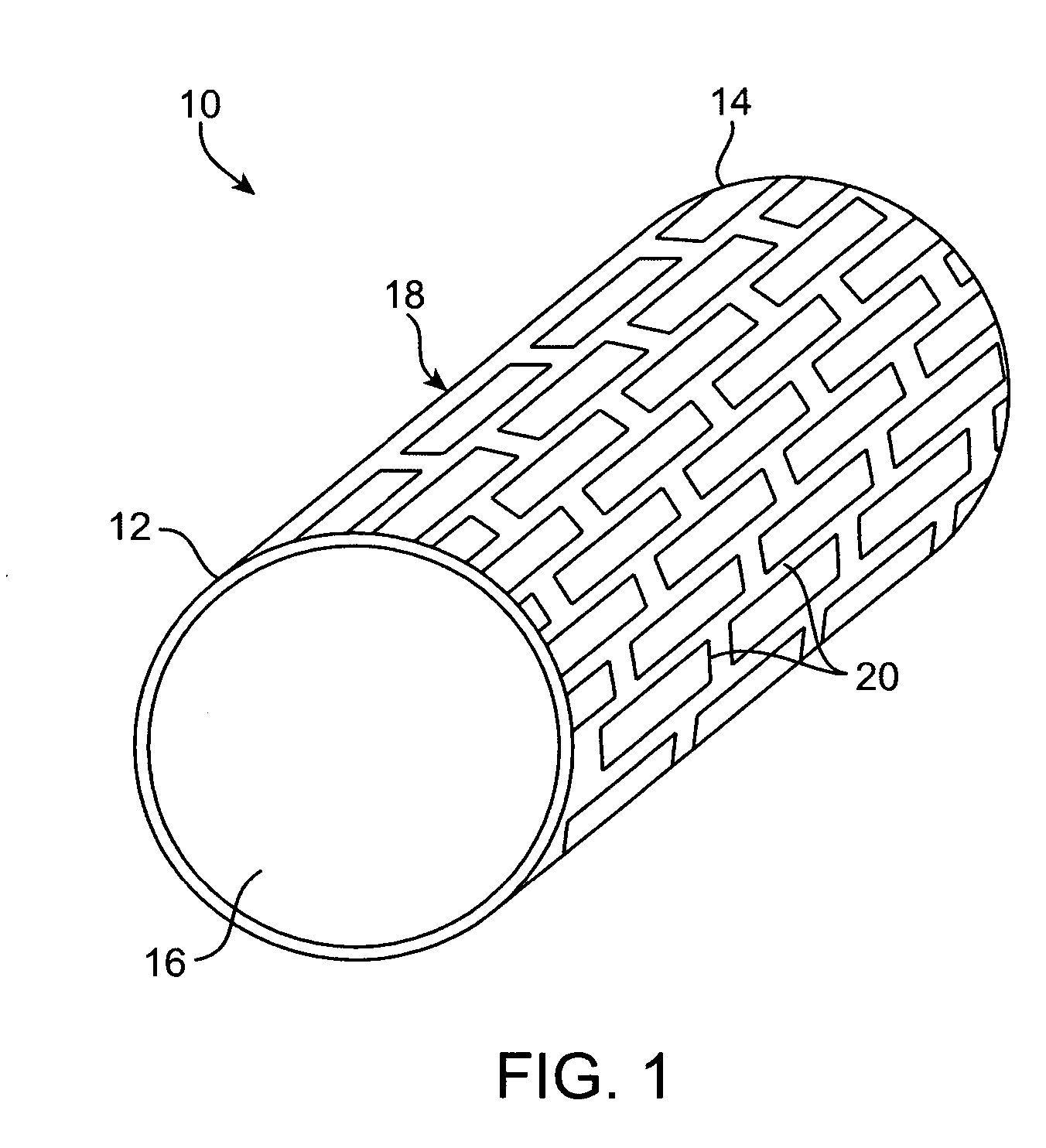
Implantable medical grafts fabricated of metallic or pseudometallic films of biocompatible materials having a plurality of microperforations passing through the film in a pattern that imparts fabric-like qualities to the graft or permits the geometric deformation of the graft. The implantable graft is preferably fabricated by vacuum deposition of metallic and / or pseudometallic materials into either single or multi-layered structures with the plurality of microperforations either being formed during deposition or after deposition by selective removal of sections of the deposited film. The implantable medical grafts are suitable for use as endoluminal or surgical grafts and may be used as vascular grafts, stent-grafts, skin grafts, shunts, bone grafts, surgical patches, non-vascular conduits, valvular leaflets, filters, occlusion membranes, artificial sphincters, tendons and ligaments.
Owner:VACTRONIX SCI LLC
Stent-graft with anchoring pins
ActiveUS7655034B2Proximal anchor stent ring is facilitatedSmooth and easy retractionStentsBlood vesselsStent graftingProsthesis
Owner:MEDTRONIC VASCULAR INC
Intravascular devices and fibrosis-inducing agents
InactiveUS20050149173A1Reducing perigraft leakageFacilitate “anchoring”StentsPeptide/protein ingredientsFibrosisCoil embolization
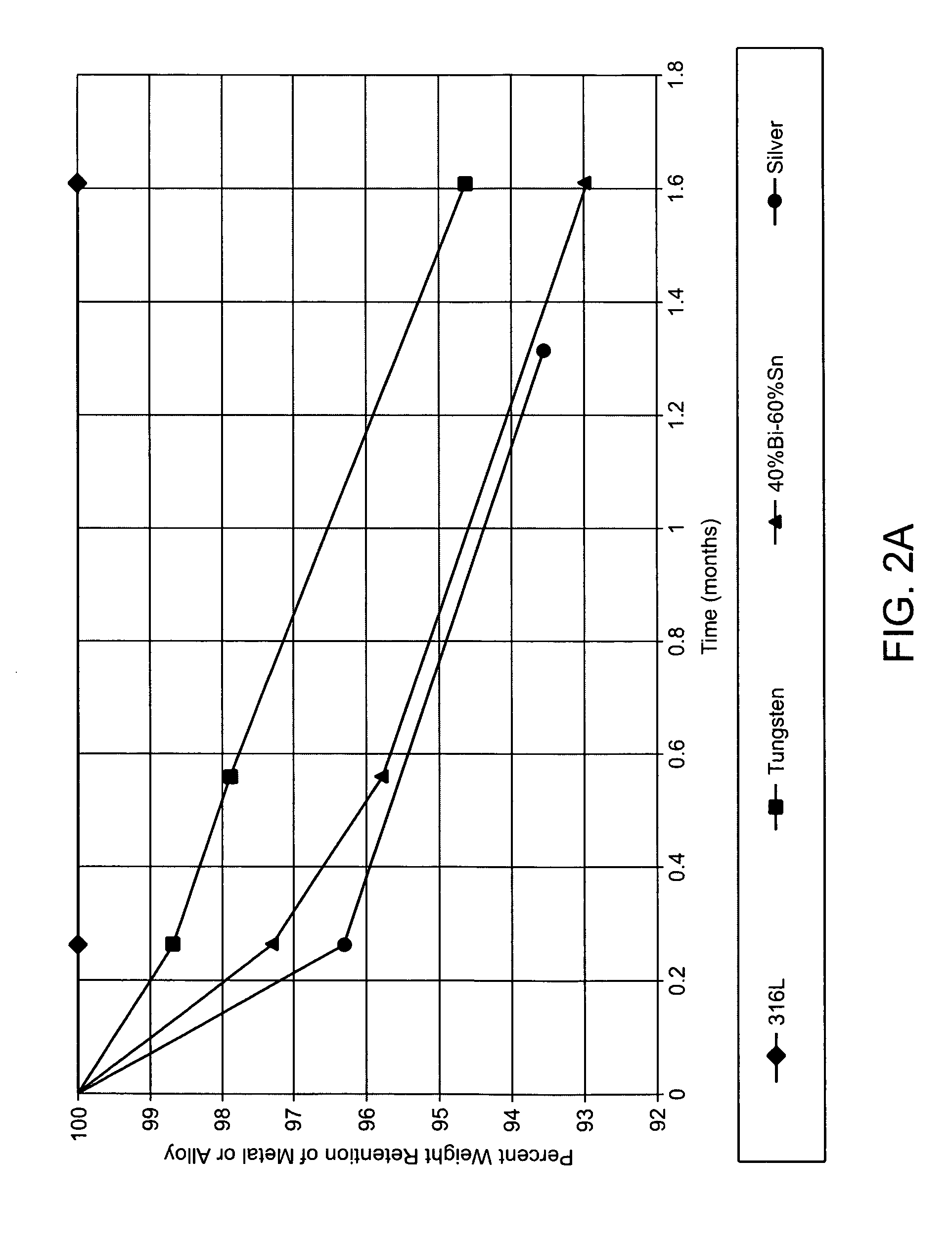
Intravascular devices (e.g., stents, stent grafts, covered stents, aneurysm coils, embolic agents and drug delivery catheters and balloons) are used in combination with fibrosing agents in order to induce fibrosis that may otherwise not occur when the implant is placed within an animal or to promote fibrosis betweent the devices and the host tissues. Compositions and methods are described for use in the treatment of aneurysms and unstable arterial (vulnerable) plaque.
Owner:ANGIOTECH INT AG (CH)
Stiffened balloon catheter for dilatation and stenting
The present invention pertains to a stiffened balloon which can be used in angioplasty, endovascular, and valvuloplasty procedures or as a delivery balloon to deliver a stent or a stent-graft. Longitudinally discontinuous stiffening members connected to the expandable balloon stiffen the balloon but allow it to be navigated through curved passages. Projections on the stiffening members may engage, incise, crush, fracture, or pierce occlusions or retain a stent or stent-graft. Radio-opaque portions may be referenced to determine orientation. Longitudinally continuous stiffening members bearing projections and connected to the balloon perform in a similar manner.
Owner:GRAYZEL JEFFREY +1
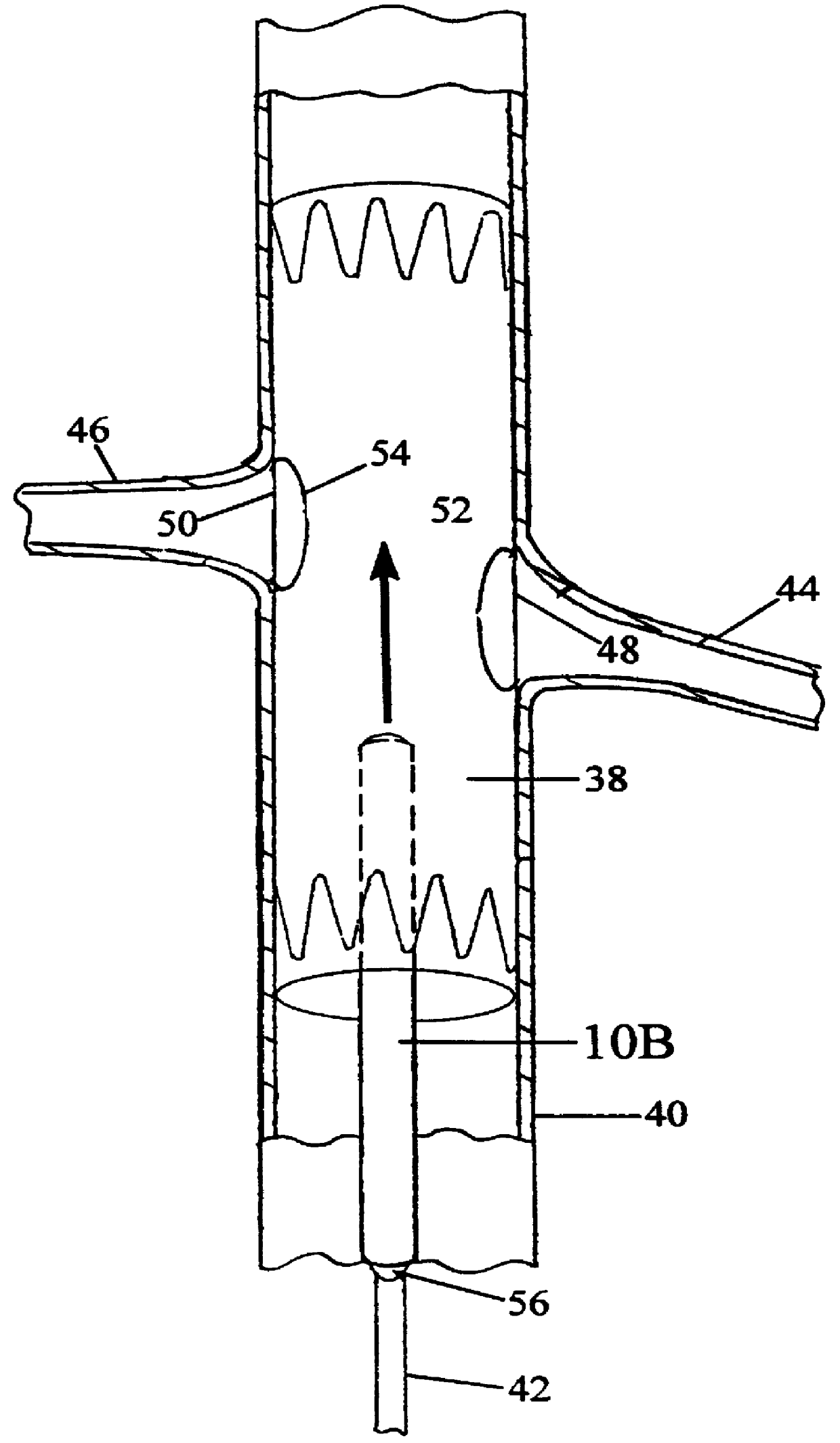
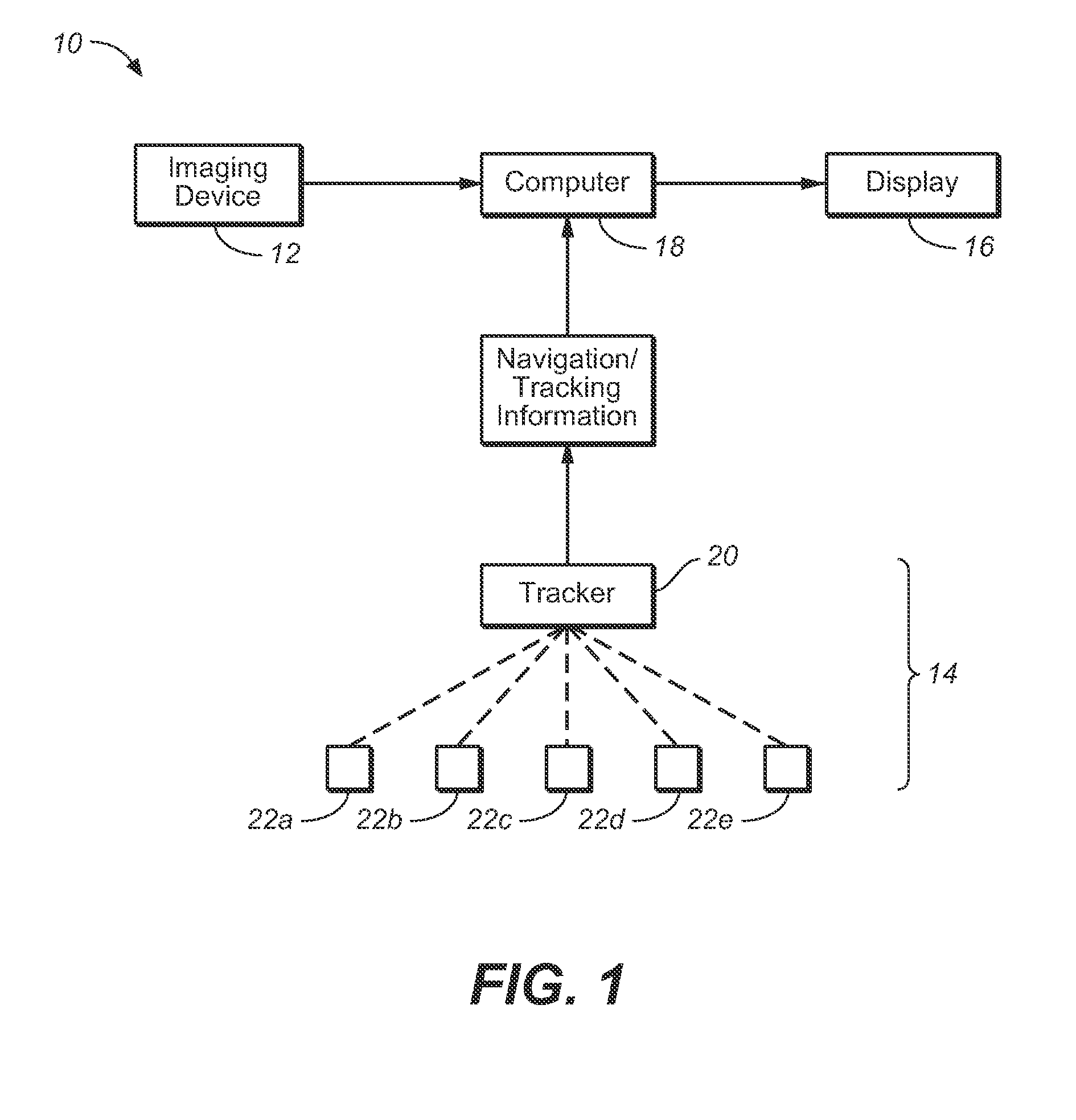
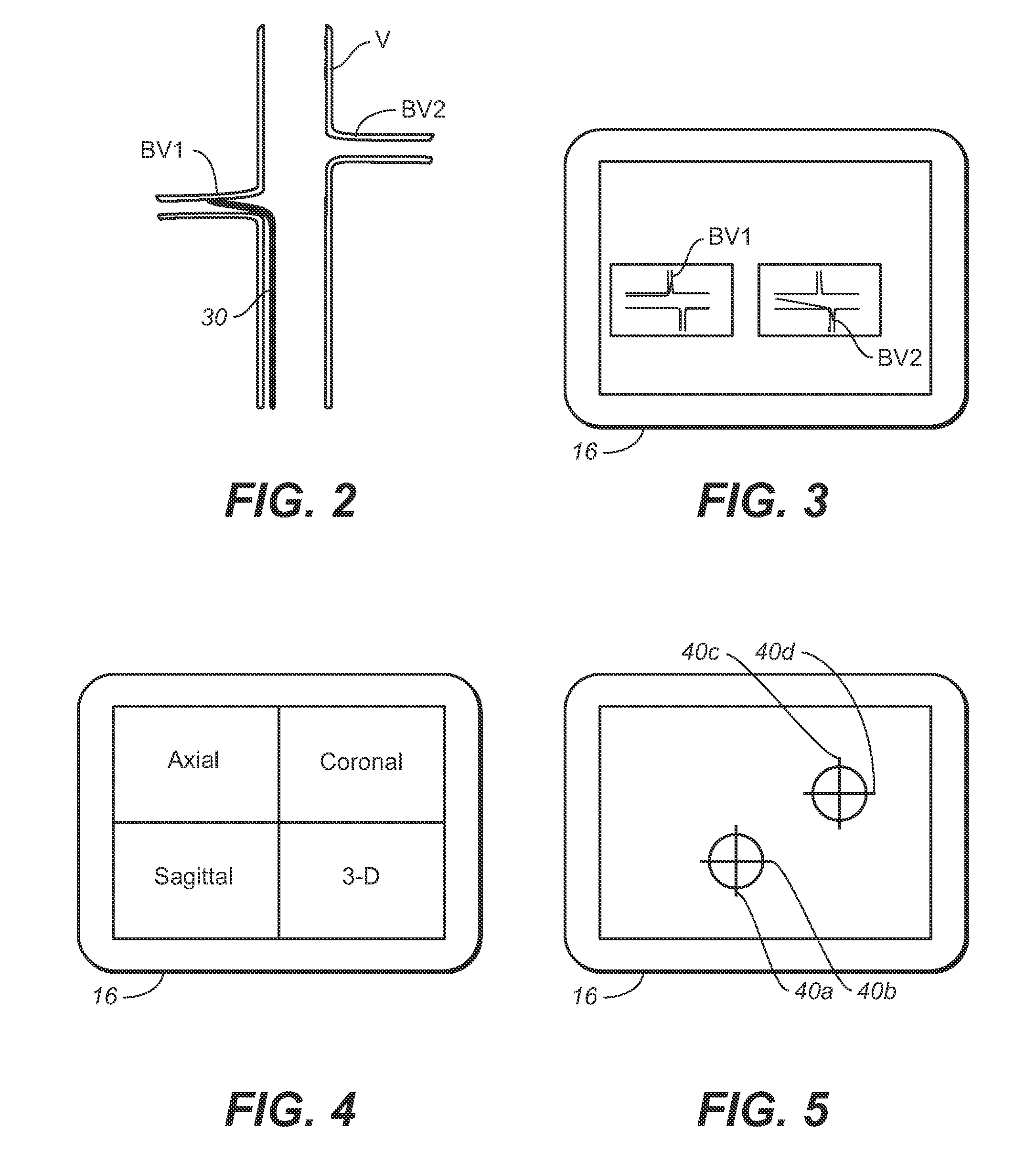
Vessel Position and Configuration Imaging Apparatus and Methods
One or more markers or sensors are positioned in the vasculature of a patient to facilitate determining the location, configuration, and / or orientation of a vessel or certain aspects thereof (e.g., a branch vessel), determining the location, configuration and / or orientation of a endovascular devices prior to and during prosthesis deployment as well as the relative position of portions of the vasculature and devices, generating an image of a virtual model of a portion of one or more vessels (e.g., branch vessels) or devices, and / or formation of one or more openings in a tubular prosthesis in situ to allow branch vessel perfusion when the prosthesis is placed over one or more branch vessels in a patient (e.g., when an aortic abdominal artery stent-graft is fixed to the aorta superior to the renal artery ostia).
Owner:MEDTRONIC VASCULAR INC
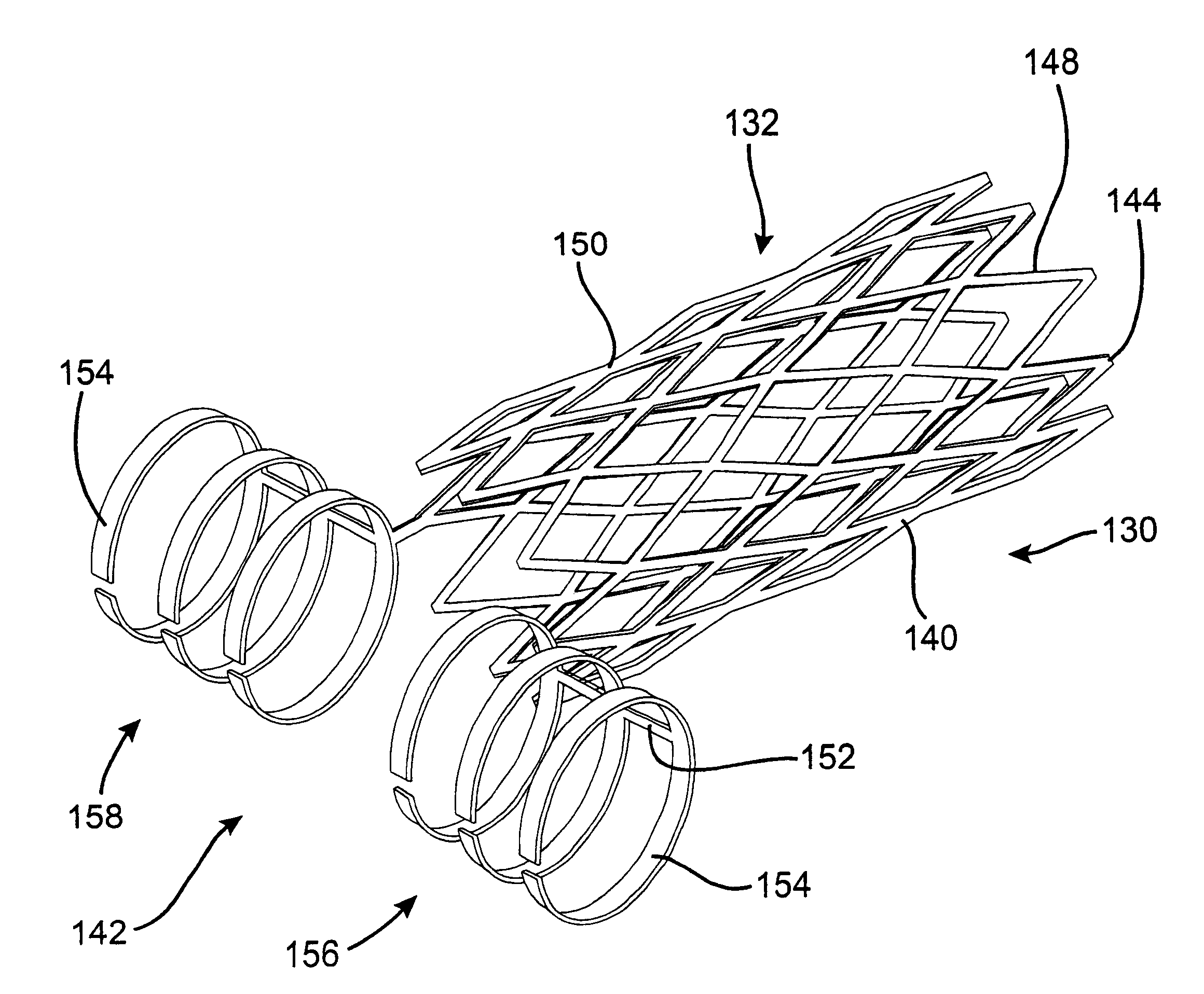
Assembly of stent grafts
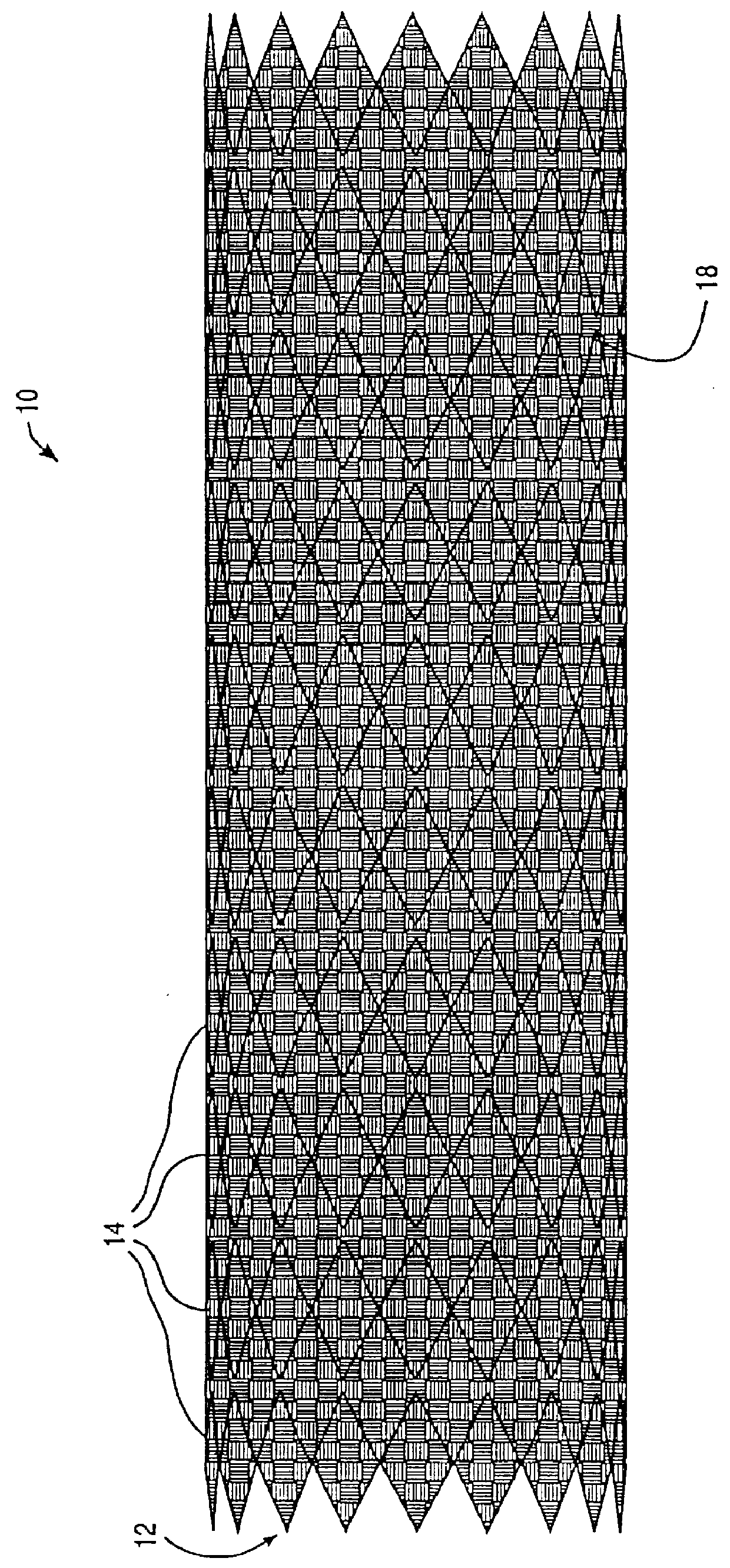
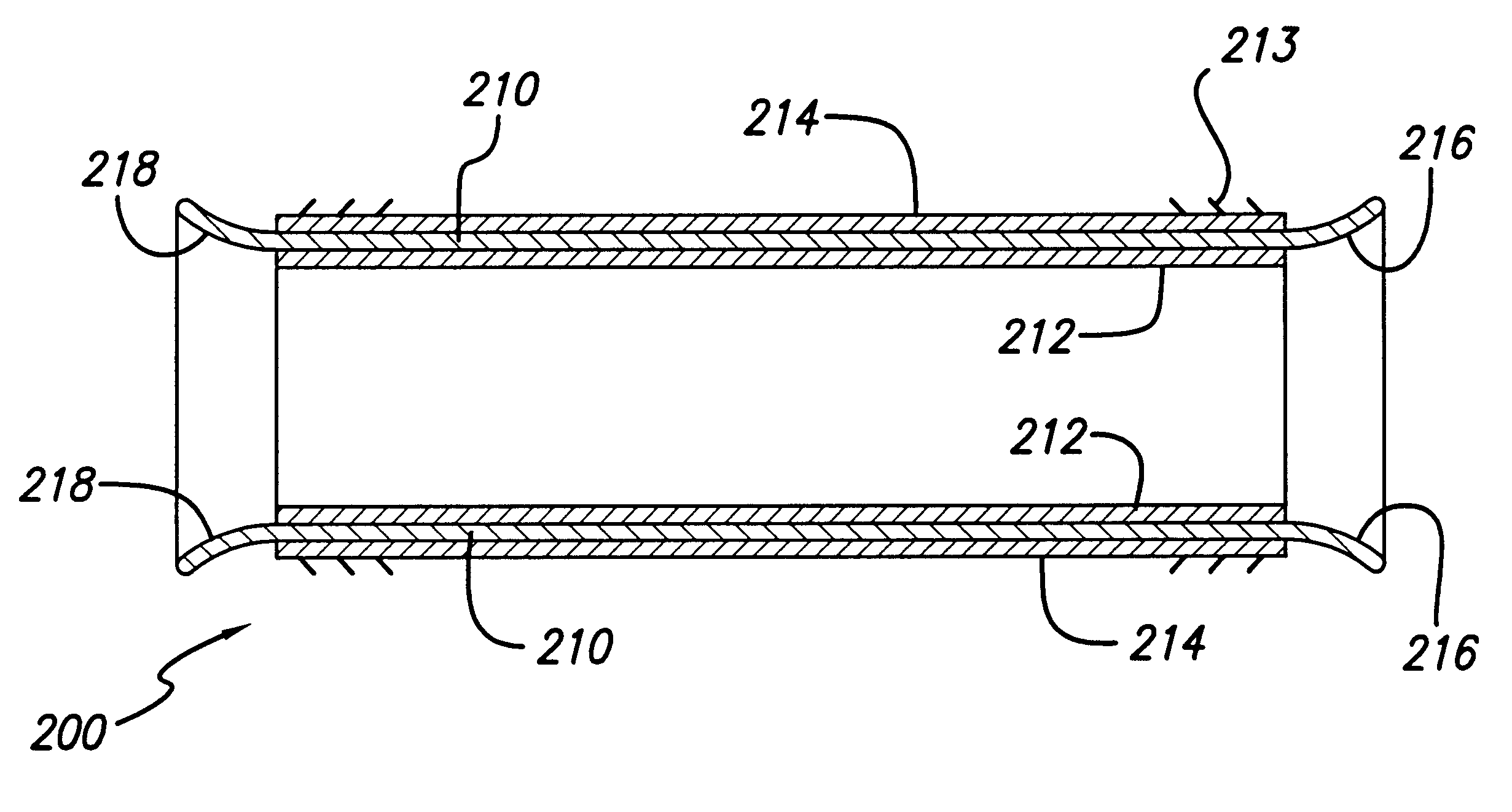
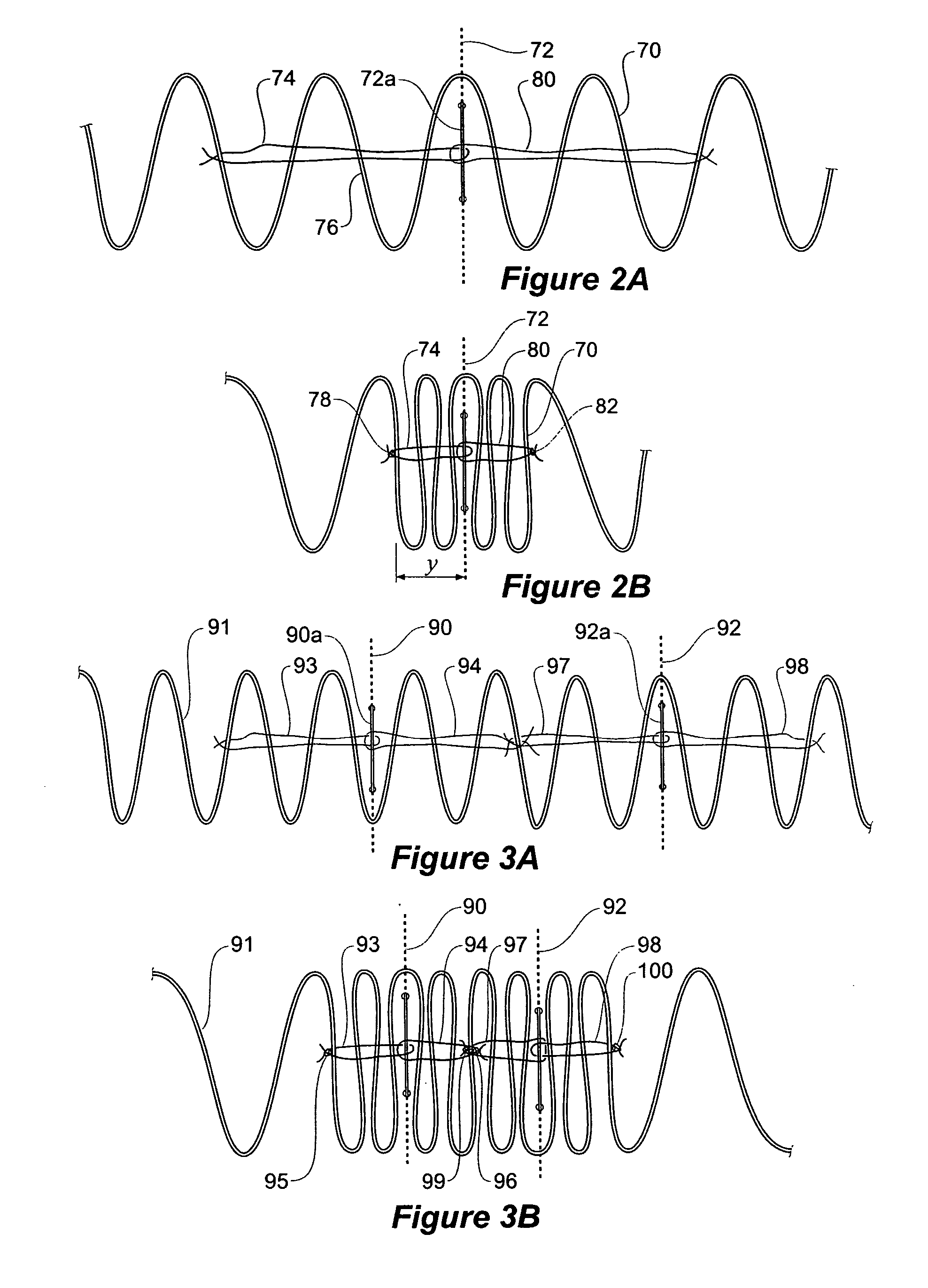
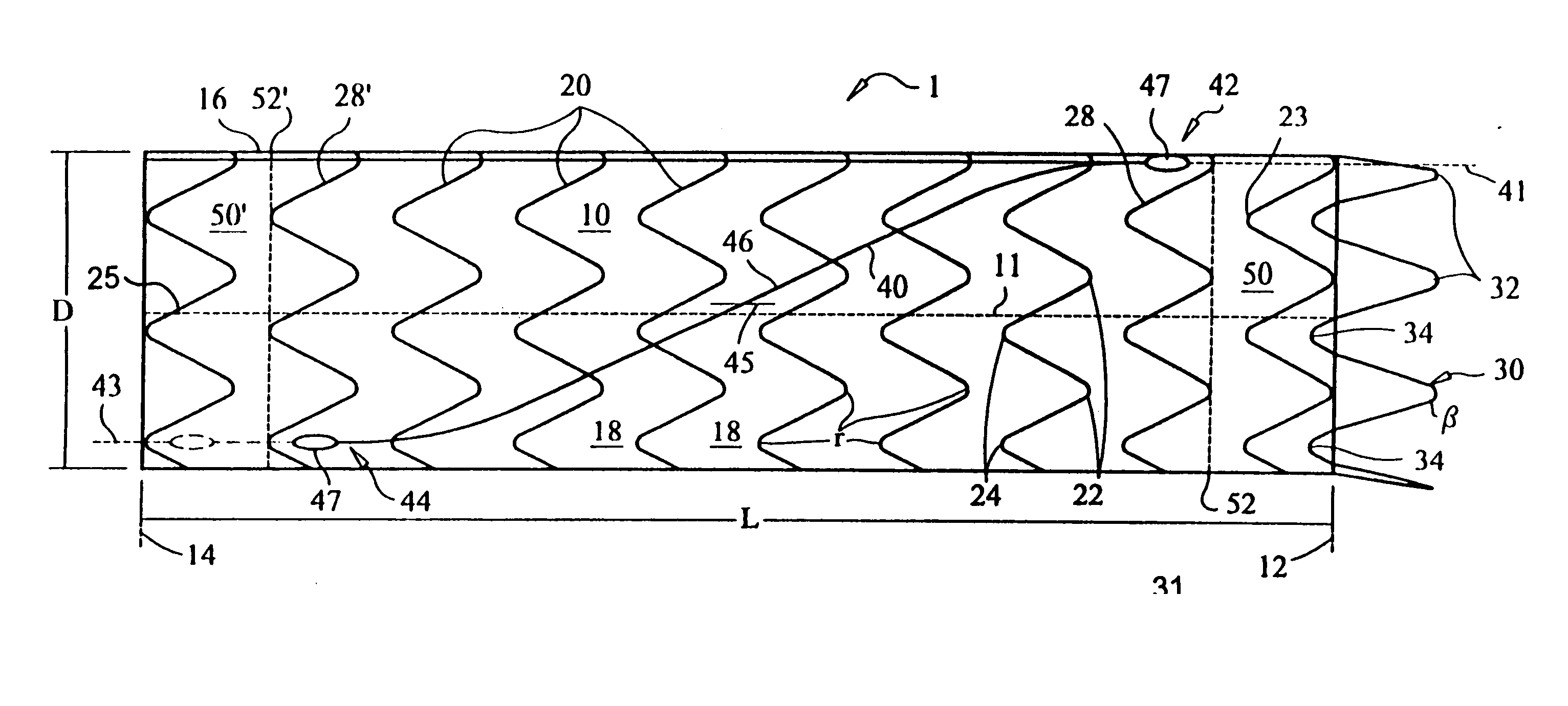
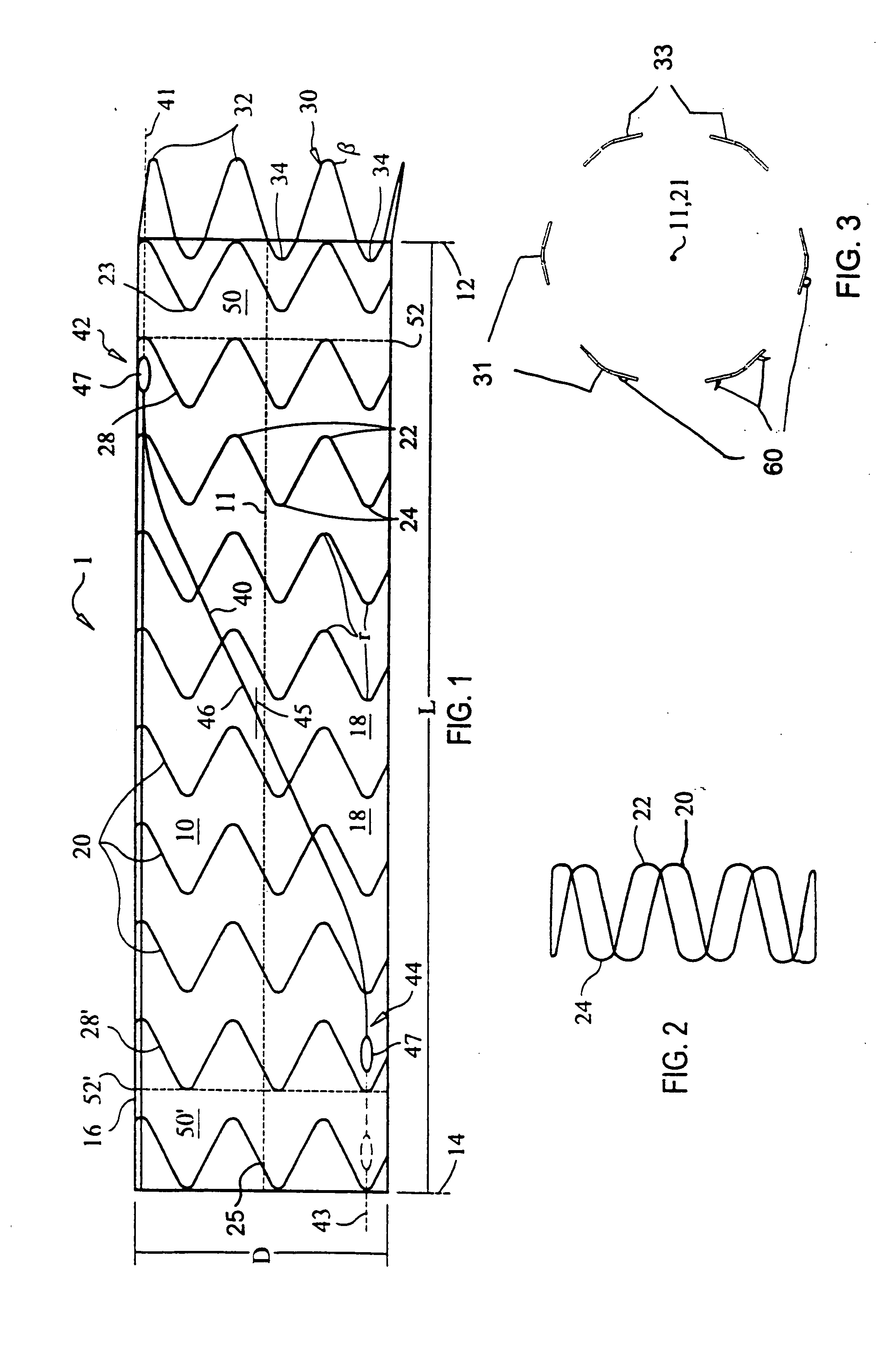
ActiveUS20070043425A1Reduce the overall diameterReduce distanceStentsBlood vesselsStent grafting% diameter reduction
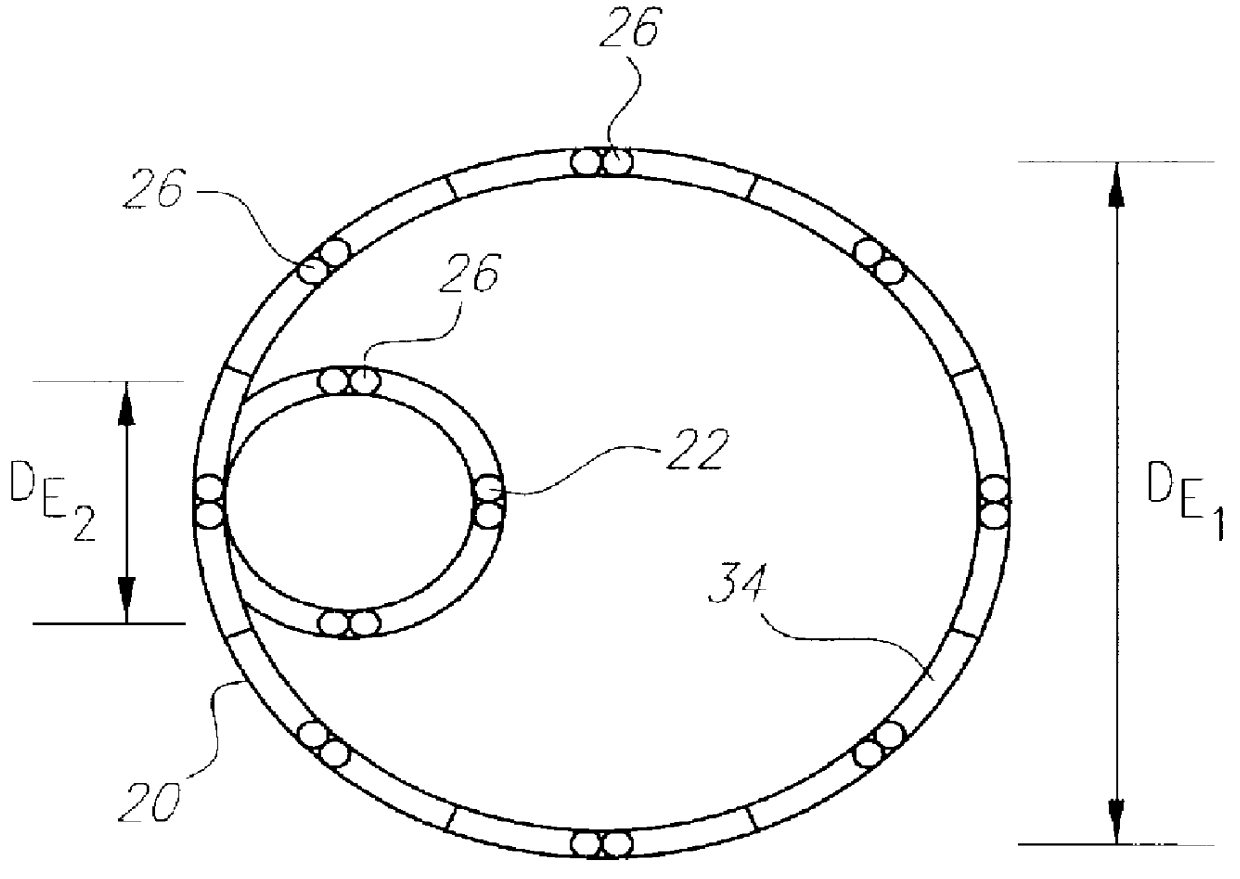
A method of assembling of a stent graft (20) including temporarily diameter reduction arrangements to enable partial release of a stent graft to assist with positioning before complete release. The diameter reduction arrangement includes a release wire (72) and flexible threads (74, 80) extending to struts (76) of a self expanding stent (70) either side of the release wire and being pulled tight. Removal of the release wire enables full expansion of the self expanding stent.
Owner:COOK MEDICAL TECH LLC
Stent graft
ActiveUS20050049674A1Reduce the possibilitySmooth connectionStentsMechanical apparatusStent graftingInsertion stent
A vascular repair device includes a tubular graft body and a structural framework having at least two stents connected to the graft body. Stents of the structural framework can each be respectively connected to graft body adjacent the proximal and ends and the support member is shorter than the separation distance therebetween to form a gimbal at least at one end. A first stent is connected along an entirety thereof and a second stent is connected at distal apices thereof. Distal apices of the second stent have radii of curvature smaller than proximal apices. A curved longitudinal support member can be connected to the graft body, the support member being substantially symmetrical with respect to the centerline. The support member can be connected to the graft body independent of the structural framework. At least one of the ends of the support member can have a curved longitudinal extremity.
Owner:BOLTON MEDICAL INC
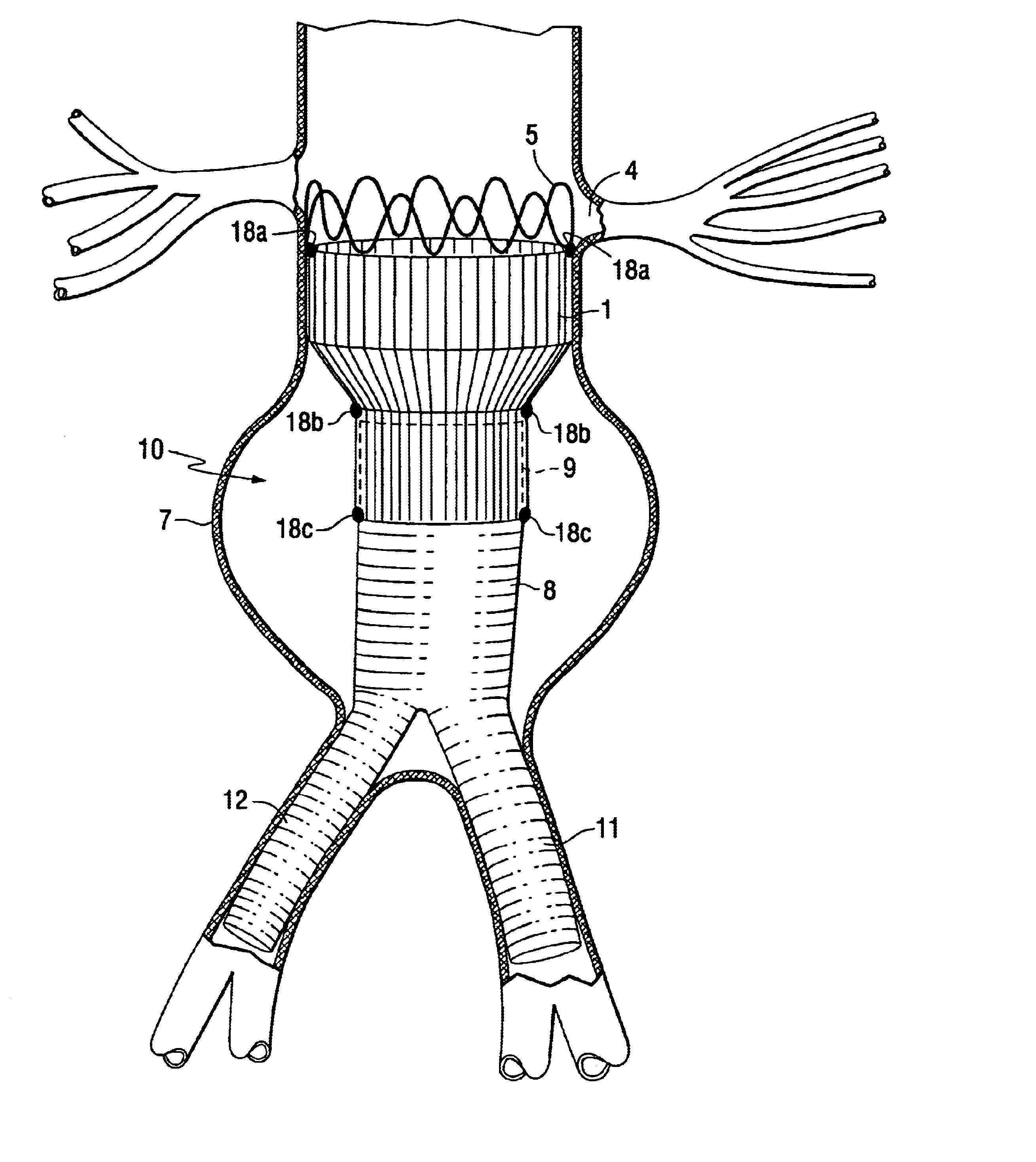
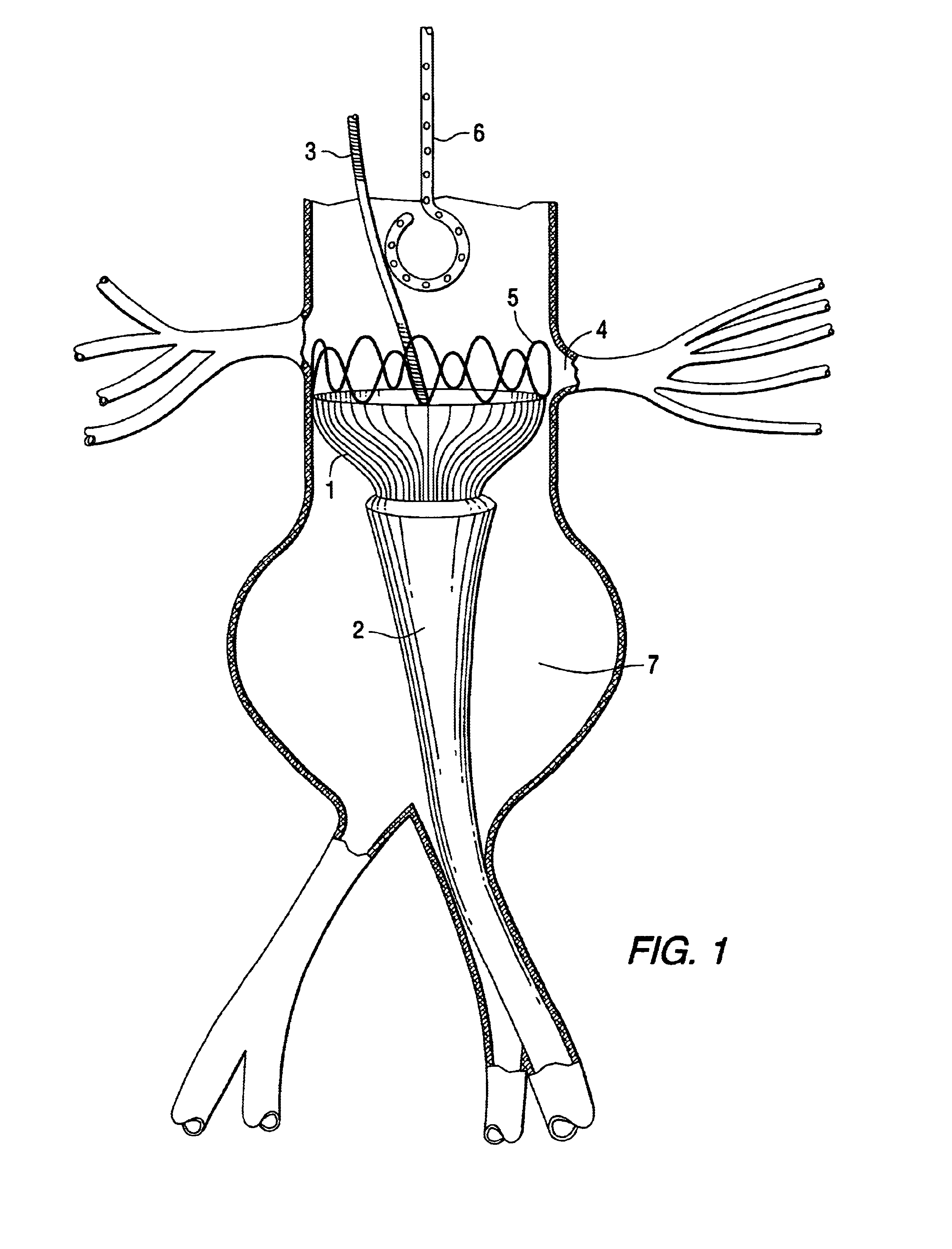
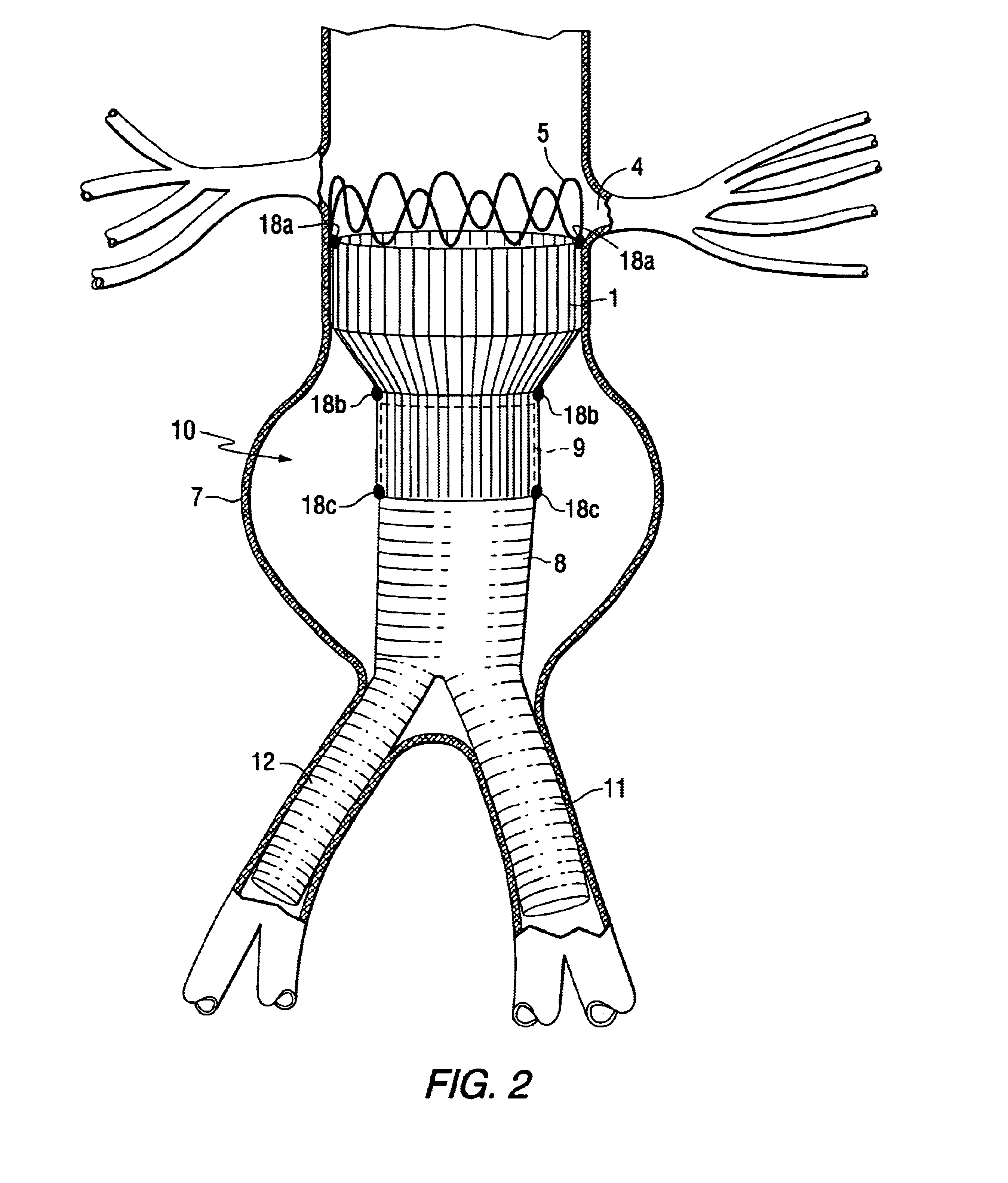
Modular stent graft assembly and use thereof
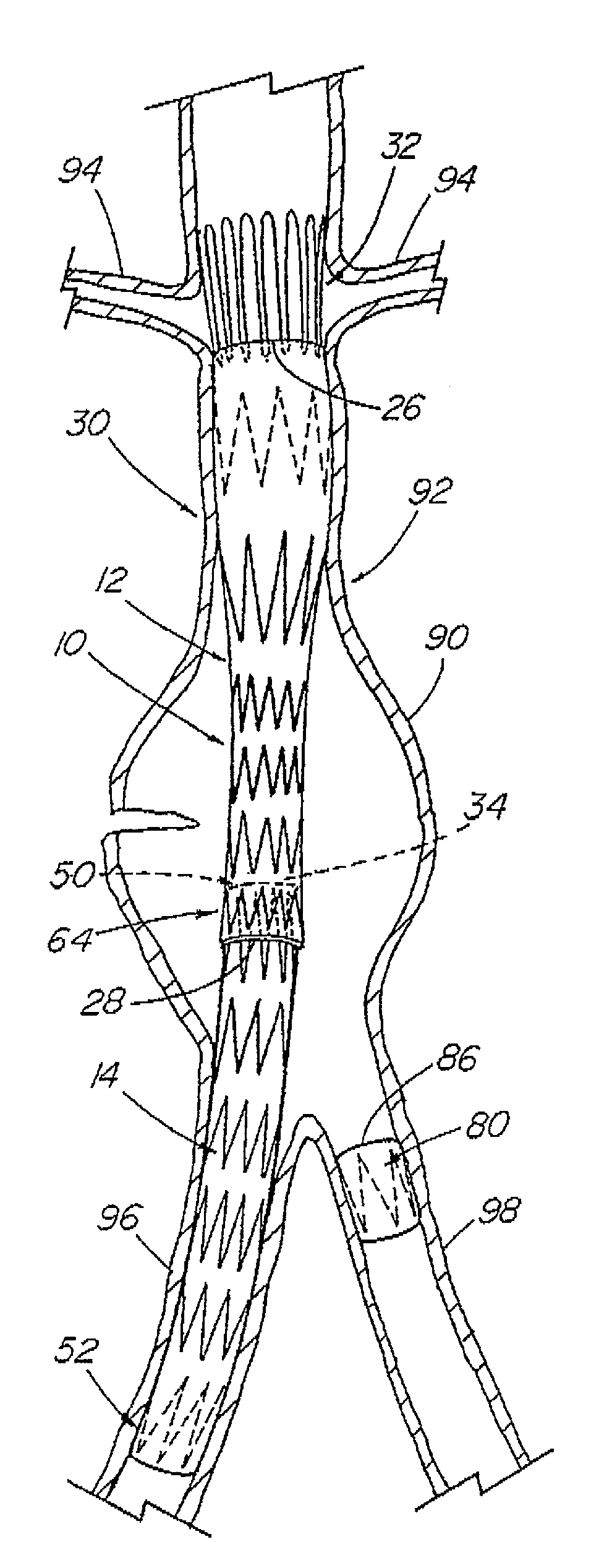
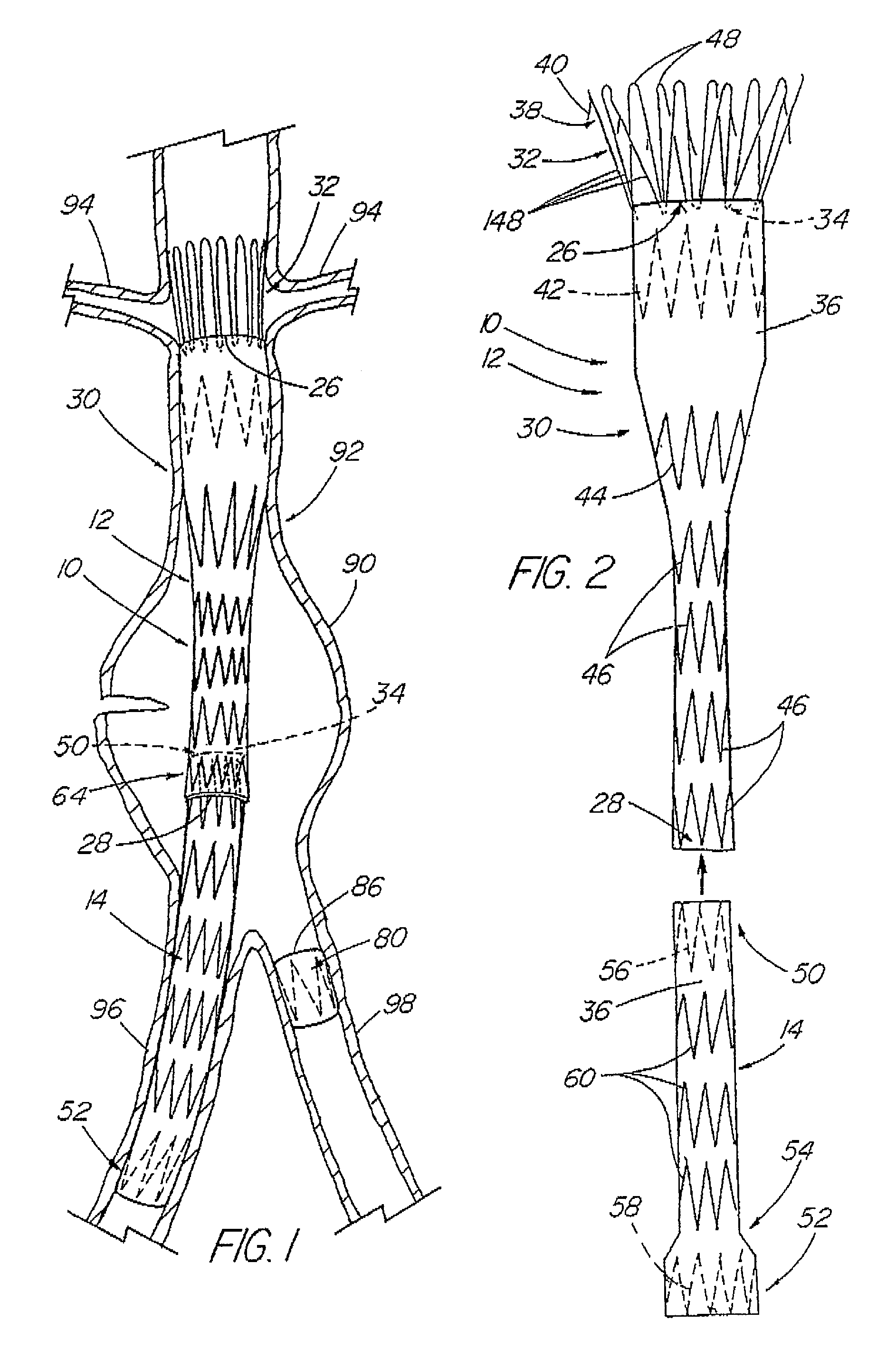
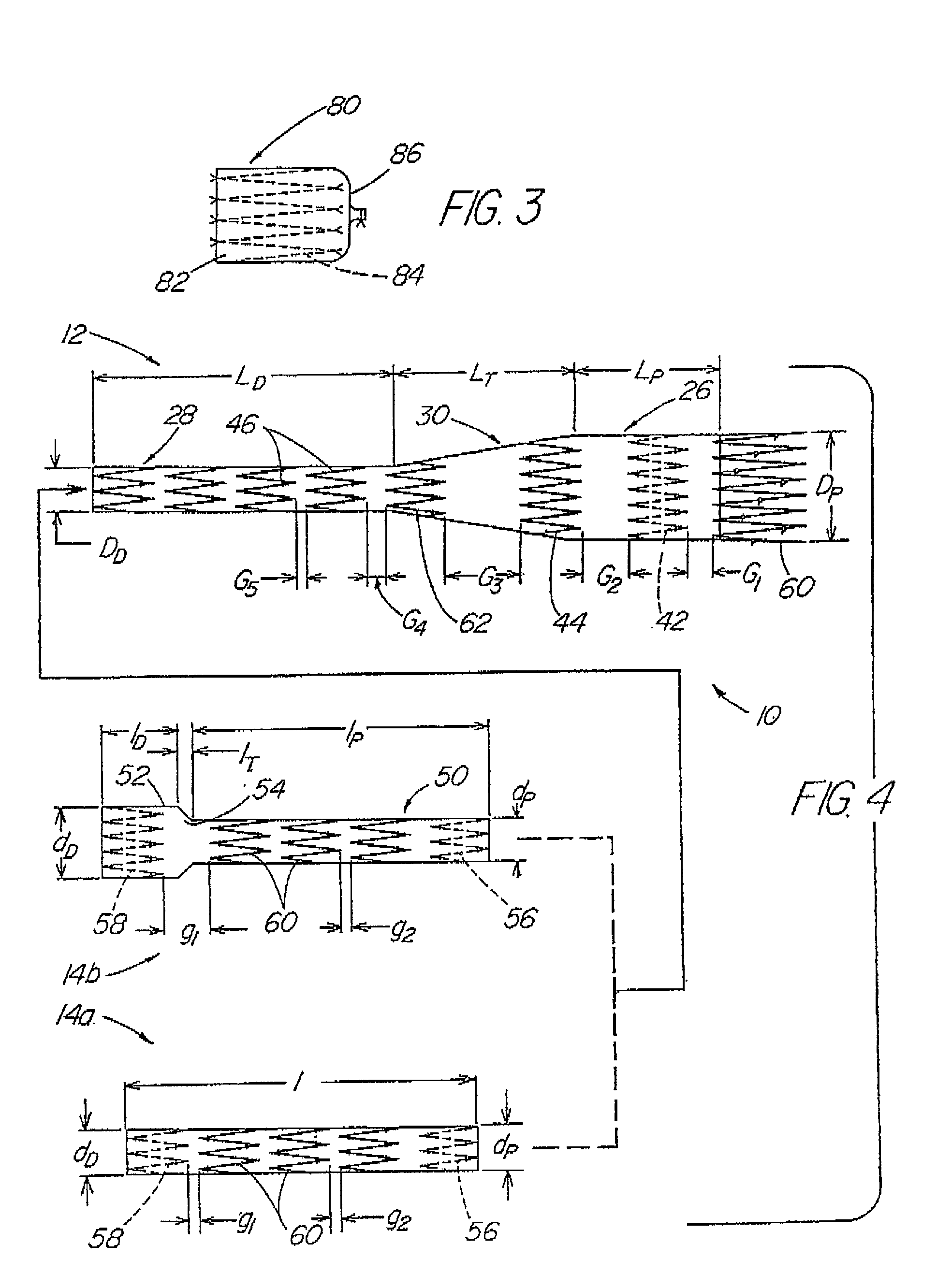
A modular stent graft assembly (10) for repairing a ruptured abdominal aorta aneurysm (90) and having an aortic section tubular graft (12) and an iliac section tubular graft (14). The aortic section graft has a proximal attachment stent (32) thereon for suprarenal attachment of the assembly (10) to the aorta. A proximal end portion (50) of the iliac section graft (14) underlies the distal end portion (28) of the aortic graft (12) and presses outwardly thereagainst forming a a friction fit, at a telescoping region (64). The assembly (10) can be selected from an inventory (300) containing a set of delivery systems (100) of four size aortic section grafts (12) and a set of delivery systems (200) of four size iliac section grafts (14), that together accommodate a large majority of aneurysm sizes, and delivery systems (250) containing four standard sizes of occluders (80).
Owner:COOK MEDICAL TECH LLC
Sheath Capture Device for Stent Graft Delivery System and Method for Operating Same
InactiveUS20080264102A1Reduce the possibilityIncreases blood-tight vascular connectionStentsJewelleryStent graftingExternal catheter
A delivery system for delivering and deploying stent grafts having a proximal stent includes a first lumen and a stent capture device including a capture portion fixedly connected adjacent a first lumen distal end. An outer catheter has a catheter distal end and a catheter inner diameter. A second lumen having a second distal end is slidably disposed about the first lumen and within the outer catheter. A stent graft sheath has a sheath proximal end connected to the second distal end and disposed about the first lumen. The sheath has a sheath distal end and a sheath inner diameter greater than the catheter inner diameter for holding a compressed stent graft. A distal nose cone has a cone proximal end connected to either the capture portion or the first distal end. The nose cone and the capture portion are movably adjustable to selectively capture the sheath distal end therebetween.
Owner:BOLTON MEDICAL INC
Intraluminal stent graft
InactiveUS20070162109A1Minimizes delivery profileEasy to appreciateStentsBlood vesselsStent graftingEndoluminal stent
A modular intraluminal stent graft. The intraluminal stent graft is bifurcated having a primary section and a secondary section extending therefrom. The primary section tapers from a larger diameter at an upstream end to a smaller diameter at a downstream end. The downstream end of the primary section has a pair of independent openings each having an expanded diameter. The secondary section provides a first endoleg having an upstream end that is received through the expanded diameter of one opening of the primary section, and a second endoleg having an upstream end that is received through the second opening of the primary section. The upstream ends of each endoleg, in its expanded state, is larger than the downstream portion of the respective endolegs and expands within the primary section to help assemble the graft in situ. The first and second endolegs also expand within the respective openings each is received within to assemble the stent graft in situ as well. The primary section is positioned within a blood vessel trunk, whereas the endolegs of the secondary section are positioned within a blood vessel branched from the blood vessel trunk. A typical application would be to place the primary section within the abdominal aorta infrarenally, with the endolegs positioned in the ipsilateral and contralaterial iliacs, respectively. By minimizing the number of stent segments in the primary section of the stent graft a lower delivery profile is achieved.
Owner:CORDIS CORP
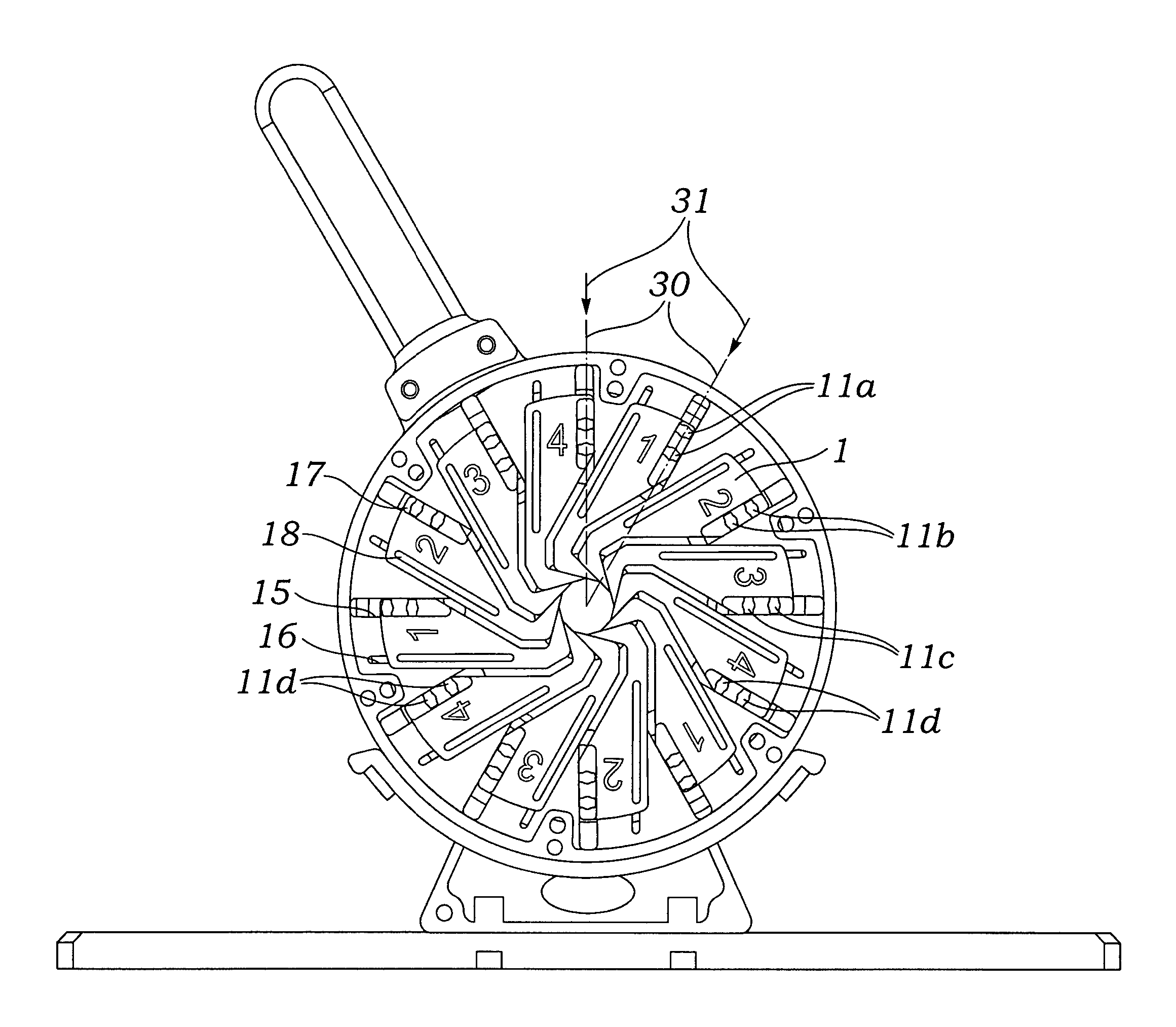
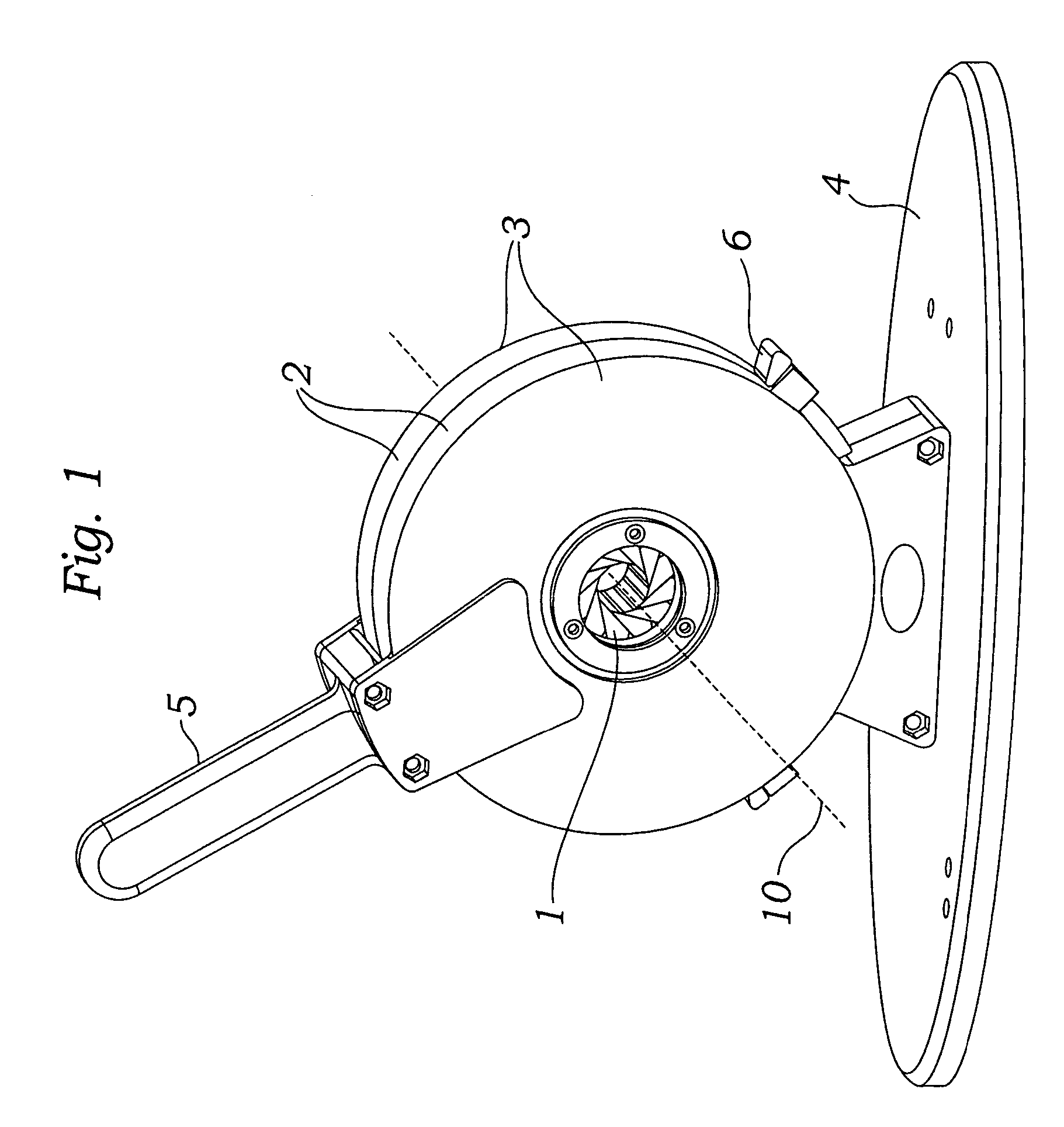
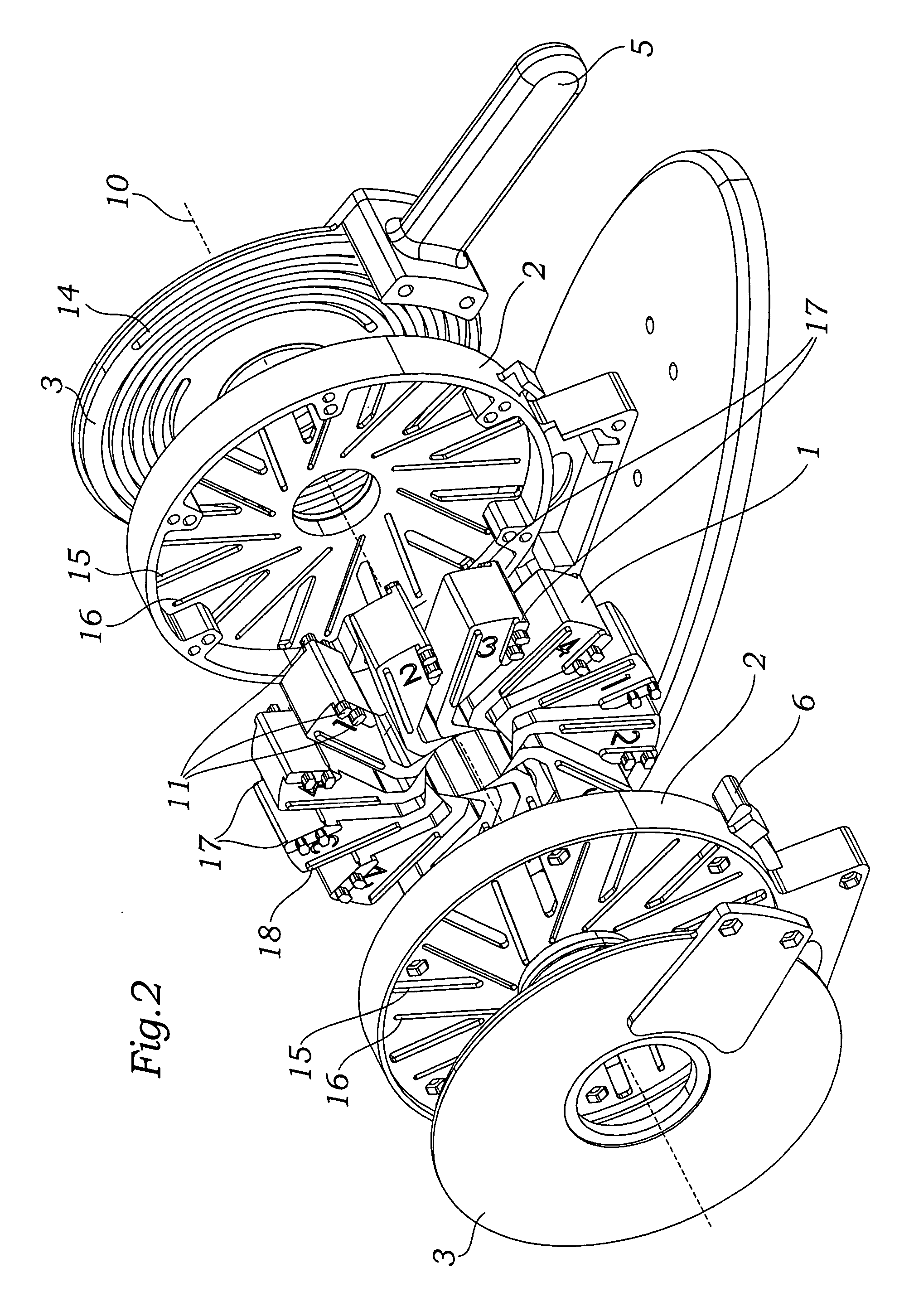
Prosthetic valve crimping device
ActiveUS20070056346A1Prevent rotationMost efficientStentsBalloon catheterProsthetic valveEngineering
An improved crimping mechanism well-suited for use with stented prosthetic heart valves. The crimping mechanism includes a plurality of jaws configured for linear non-rotational movement toward a central axis. A rotational plate is formed with a plurality of spiral grooves or tracks for engaging the jaws. Rotational movement of the spiral tracks produces linear movement of the jaws. Nesting of the inner ends of the jaws permits each to be acted on along different radial lines while their inner faces move together evenly to reduce the crimping aperture in a smooth fashion. The crimping mechanism is particularly well-suited for use with stented prosthetic heart valves, such as a prosthetic aortic valve, though it can also be applied to other stented heart valves, venous valves, and even stent grafts which tend to be fairly large.
Owner:EDWARDS LIFESCIENCES CORP
Stent graft with branch leg
The present invention is directed to a system, apparatus, and method for treating, repairing, and / or replacing an aneurysm, preferably an aortic aneurysm, and most preferably, an abdominal aortic aneurysm. The systems, devices, and methods of the present invention include a first prosthesis or stent gasket, and at least one second prosthesis for bypassing the aneurysm. In preferred embodiments, the second prosthesis includes a branch leg that may be disposed and anchored in an either a cross artery or a downstream artery to facilitate fluid flow. In other preferred embodiments, at least one third prosthesis is provided for establishing a fluid flow channel through a second diseased artery to bypass an aneurysm disposed therein. In accordance with the invention, the first artery may be the abdominal aorta and the second artery may be an iliac artery.
Owner:CARDINAL HEALTH SWITZERLAND 515 GMBH
Tapered endovascular stent graft and method of treating abdominal aortic aneurysms and distal iliac aneurysms
The present invention relates to an improved endovascular stent. The endovascular stent is to be used in treating abdominal aortic aneurysms. The endovascular stent has a tapered section which allows it to accommodate markedly large aortas such as the abdominal aorta and still connect to standard modular aortic stent grafts. Methods of utilizing the stent to treat abdominal aortic aneurysms and distal iliac aneurysms are also provided.
Owner:COOK MEDICAL TECH LLC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com