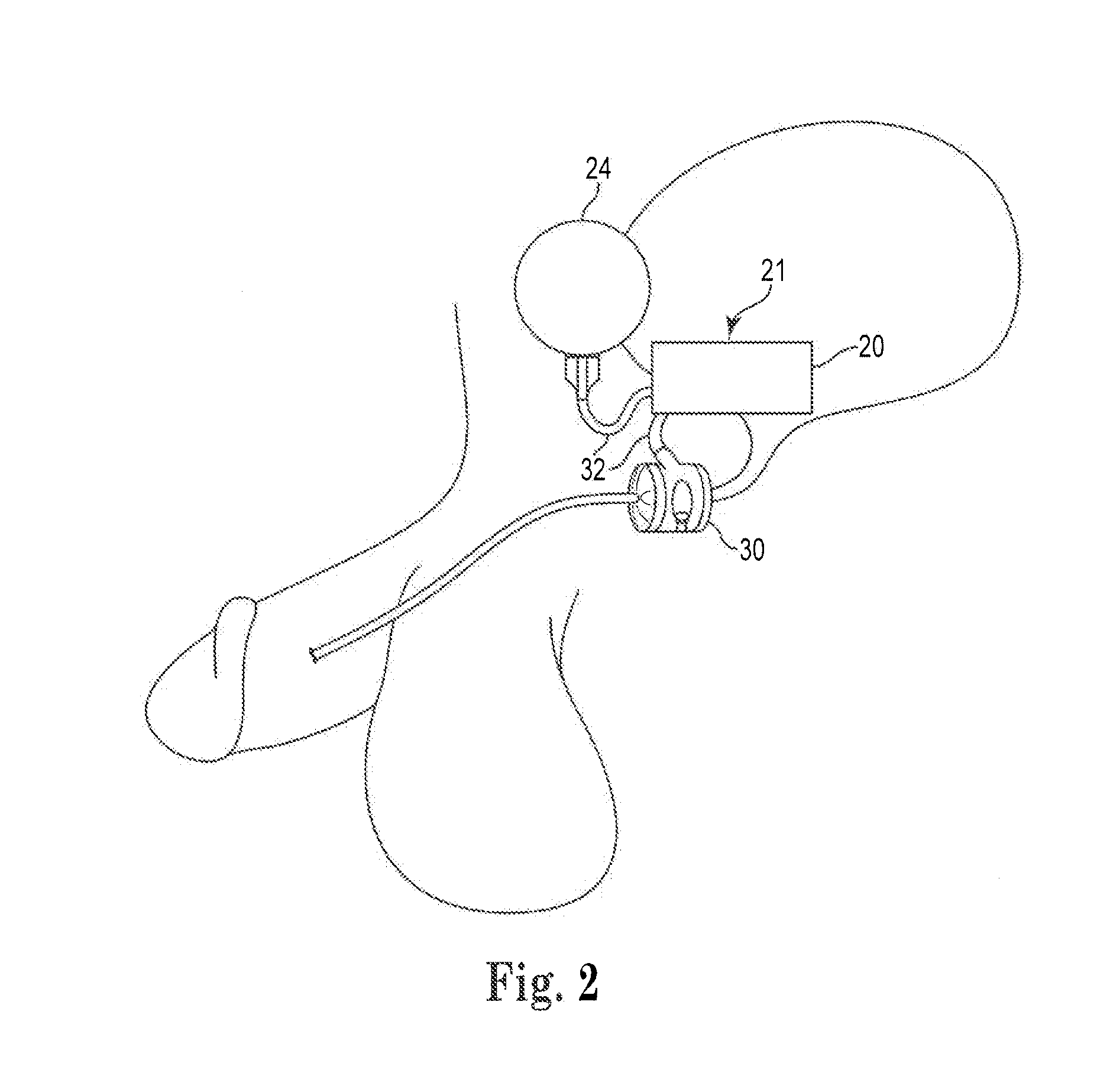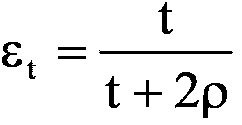Patents
Literature
56 results about "Artificial sphincters" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
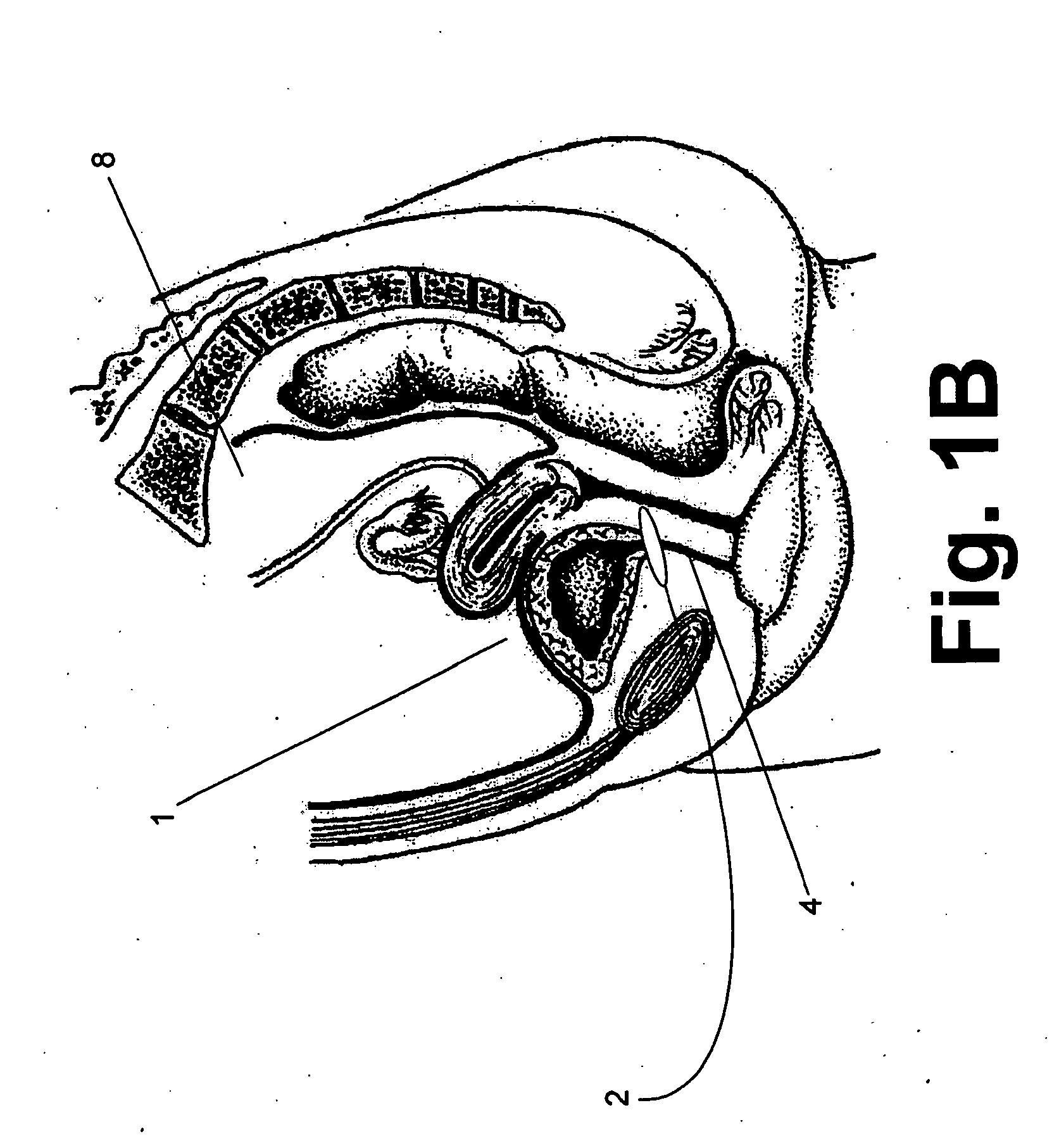
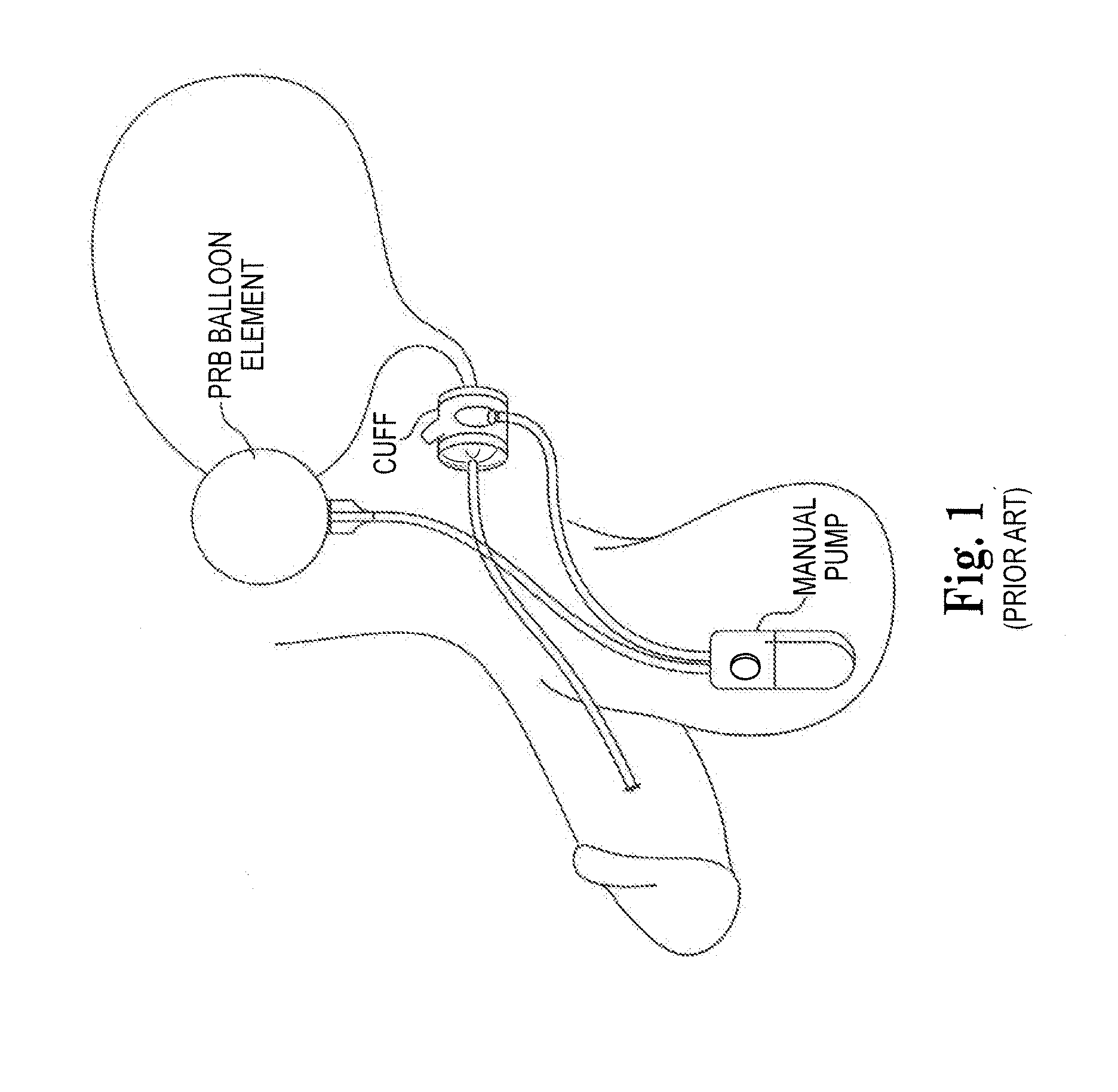
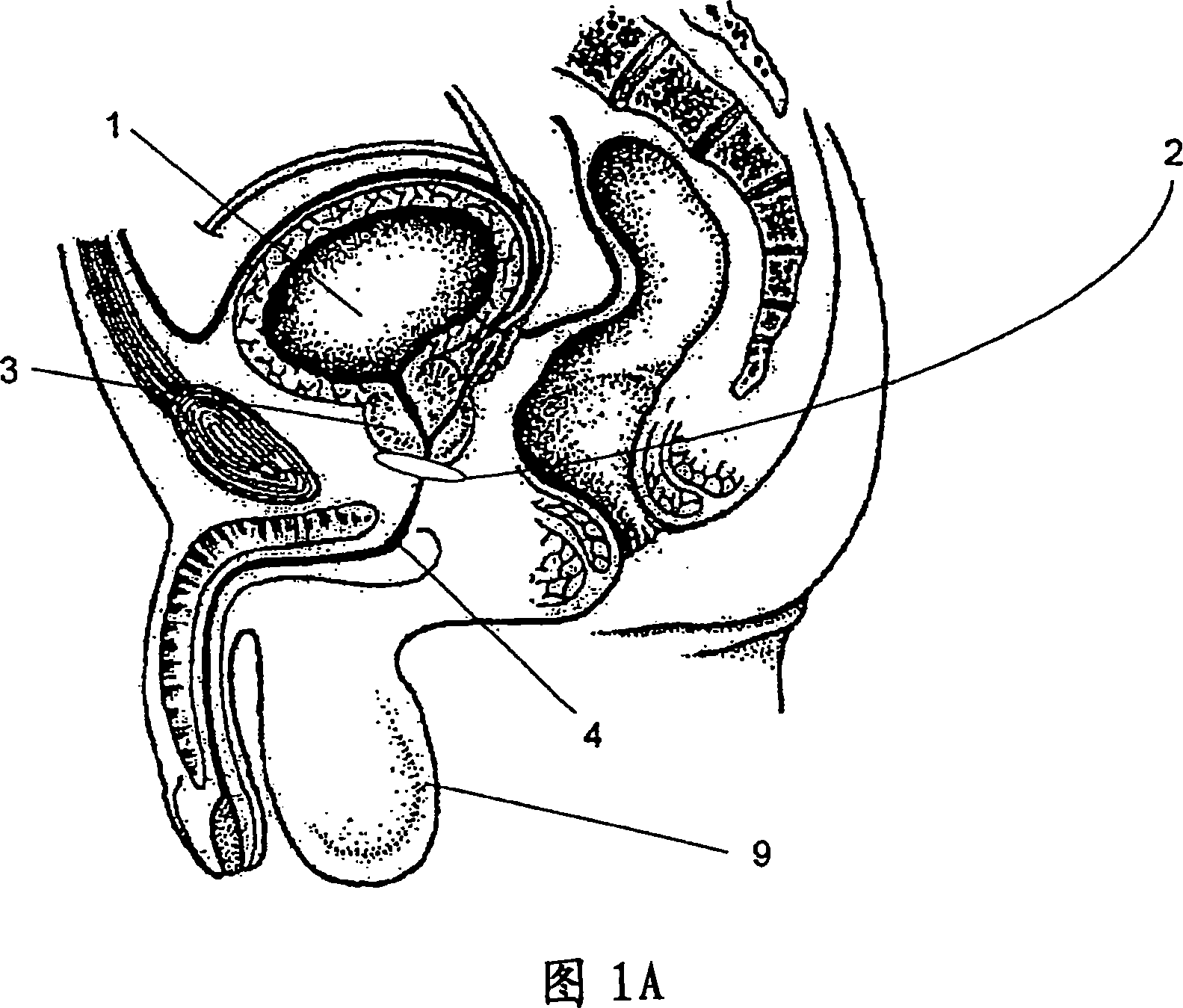
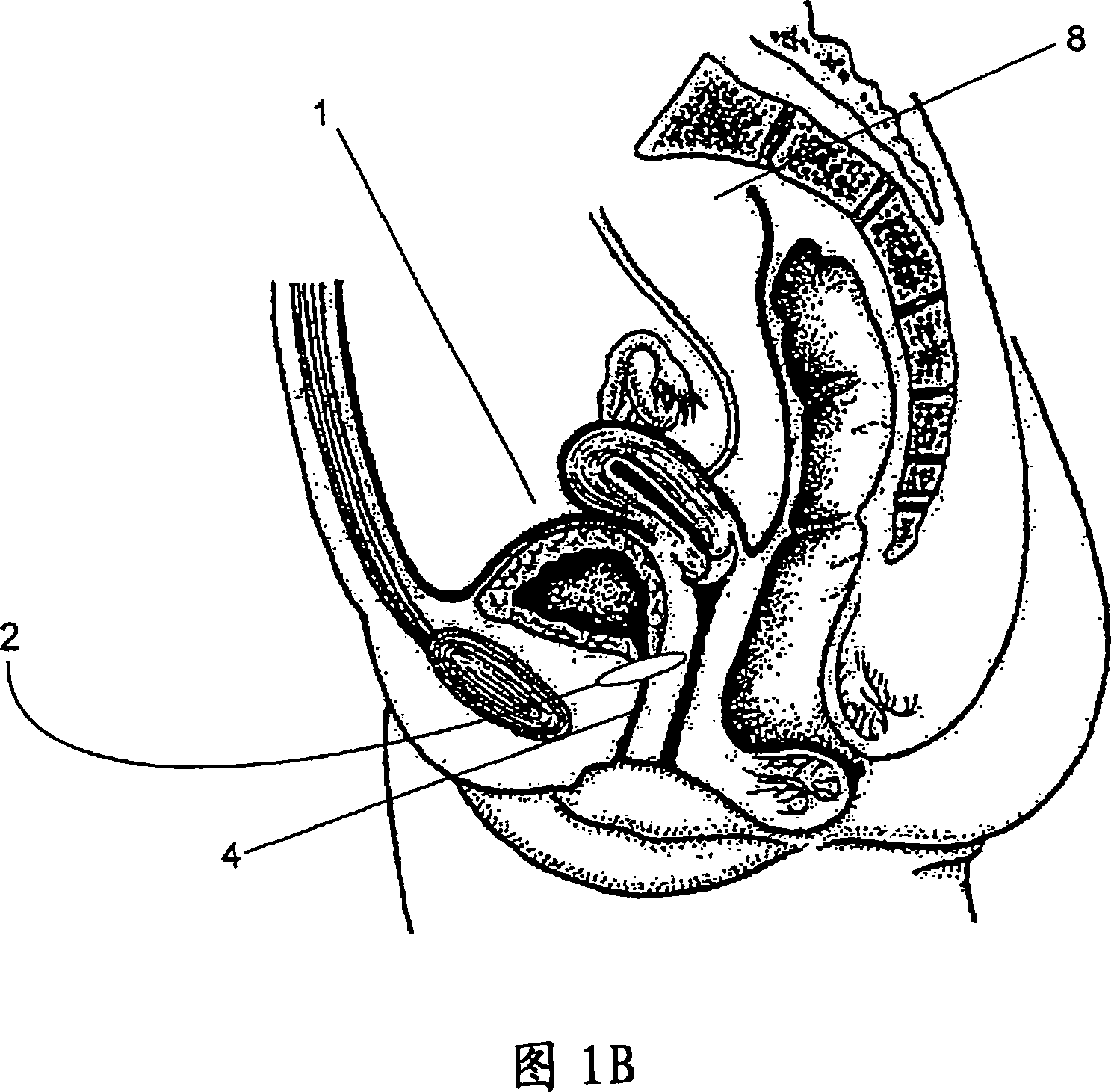
Sphincters are muscles that allow your body to hold in urine. An inflatable artificial (man-made) sphincter is a medical device. This device keeps urine from leaking. It is used when your urinary sphincter no longer works well.
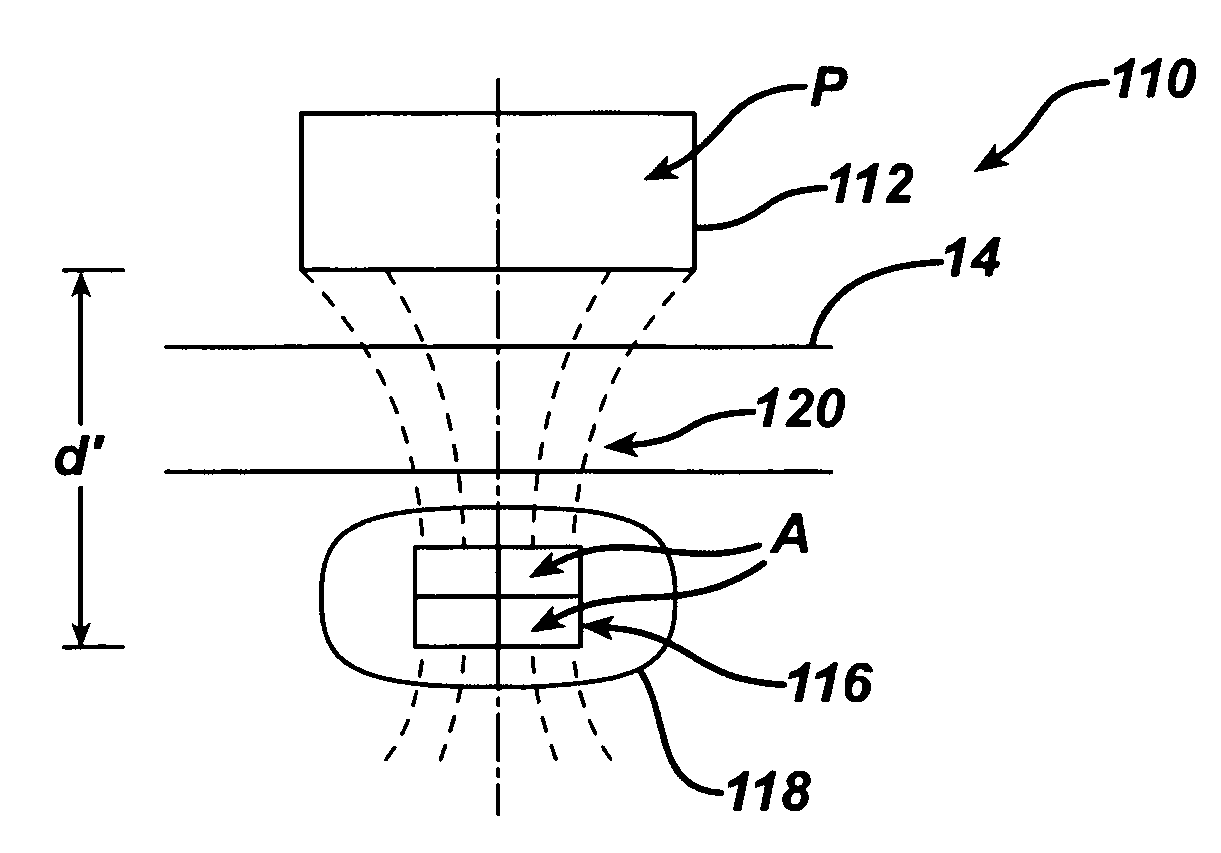
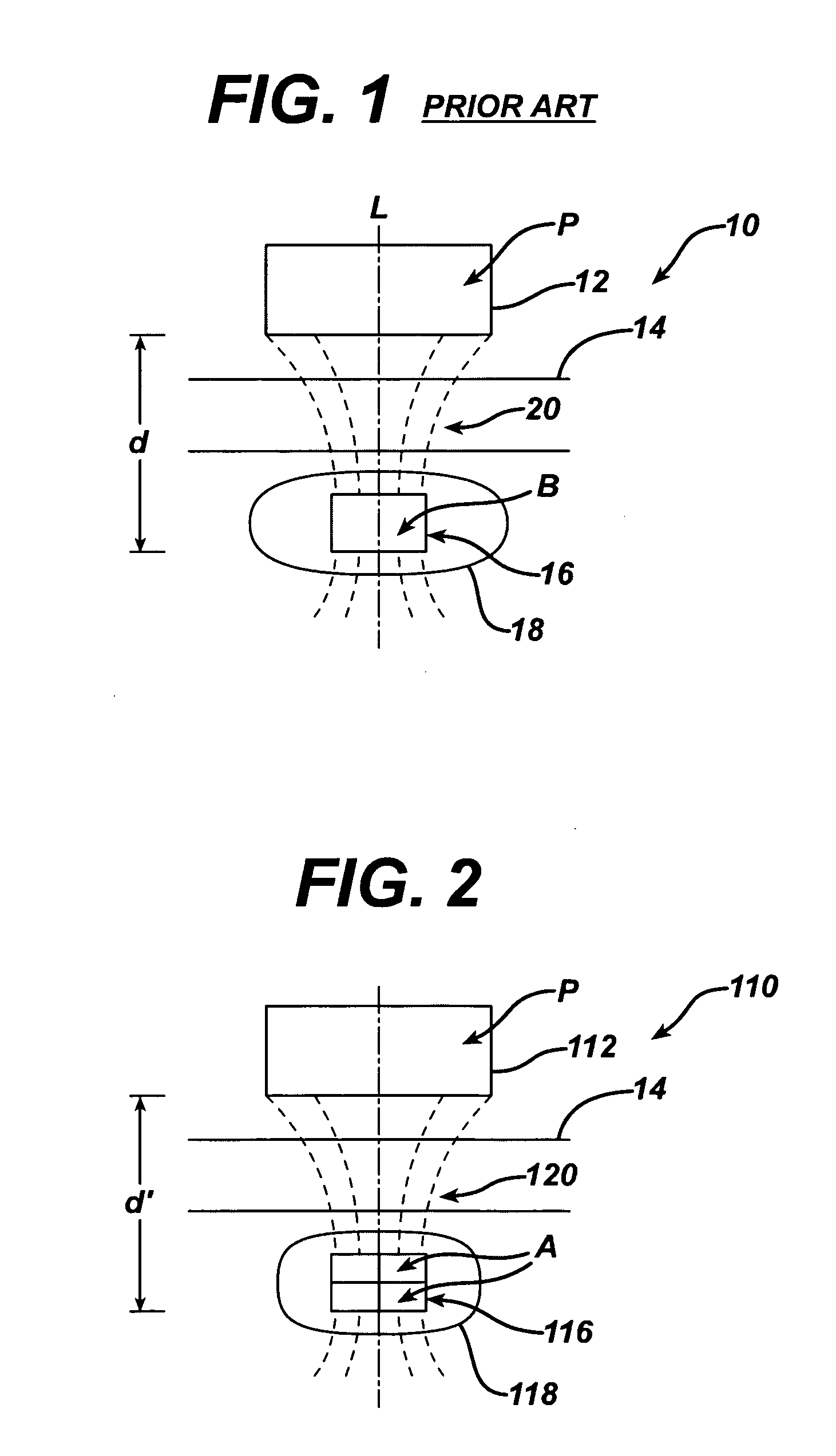
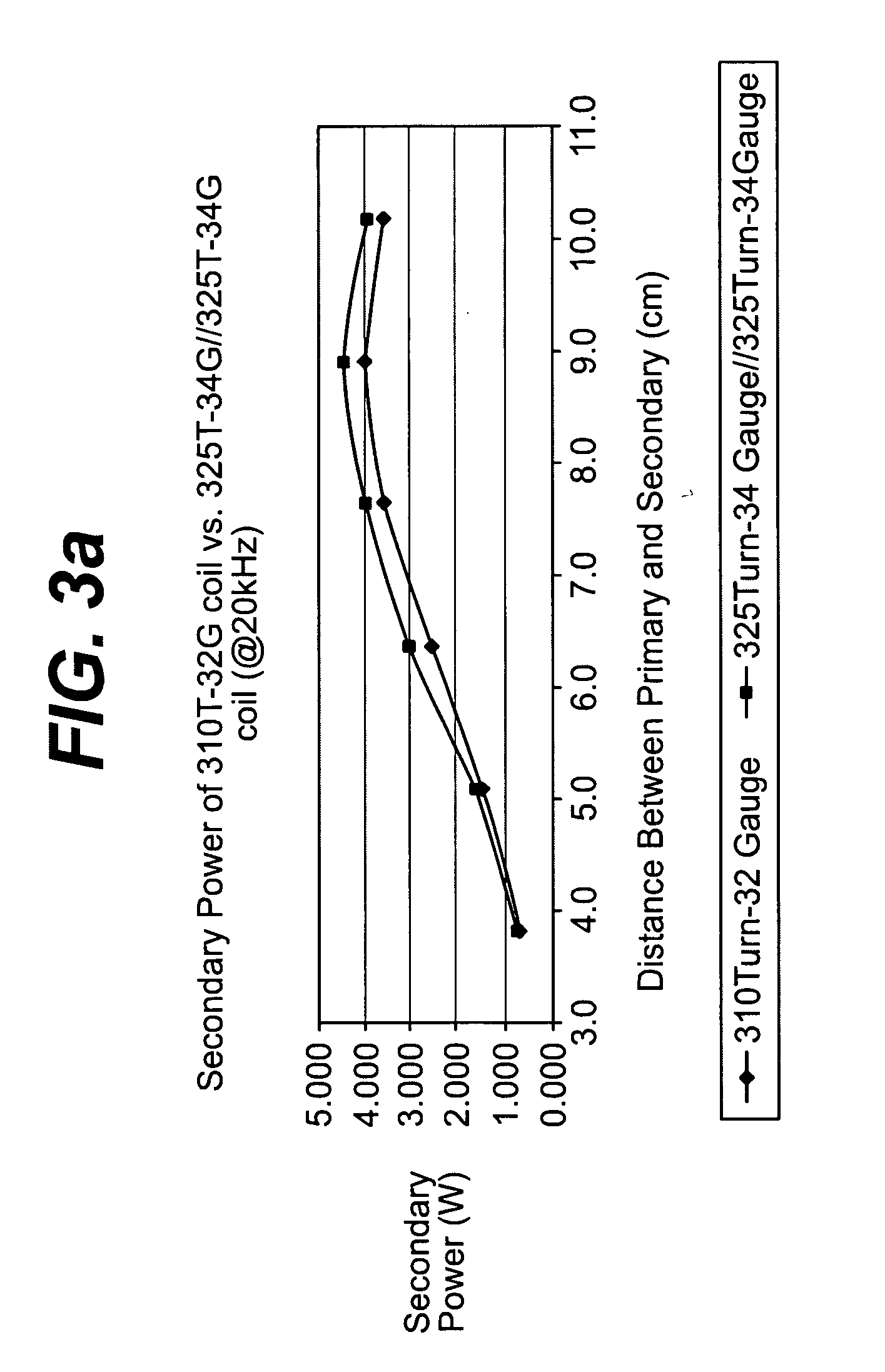
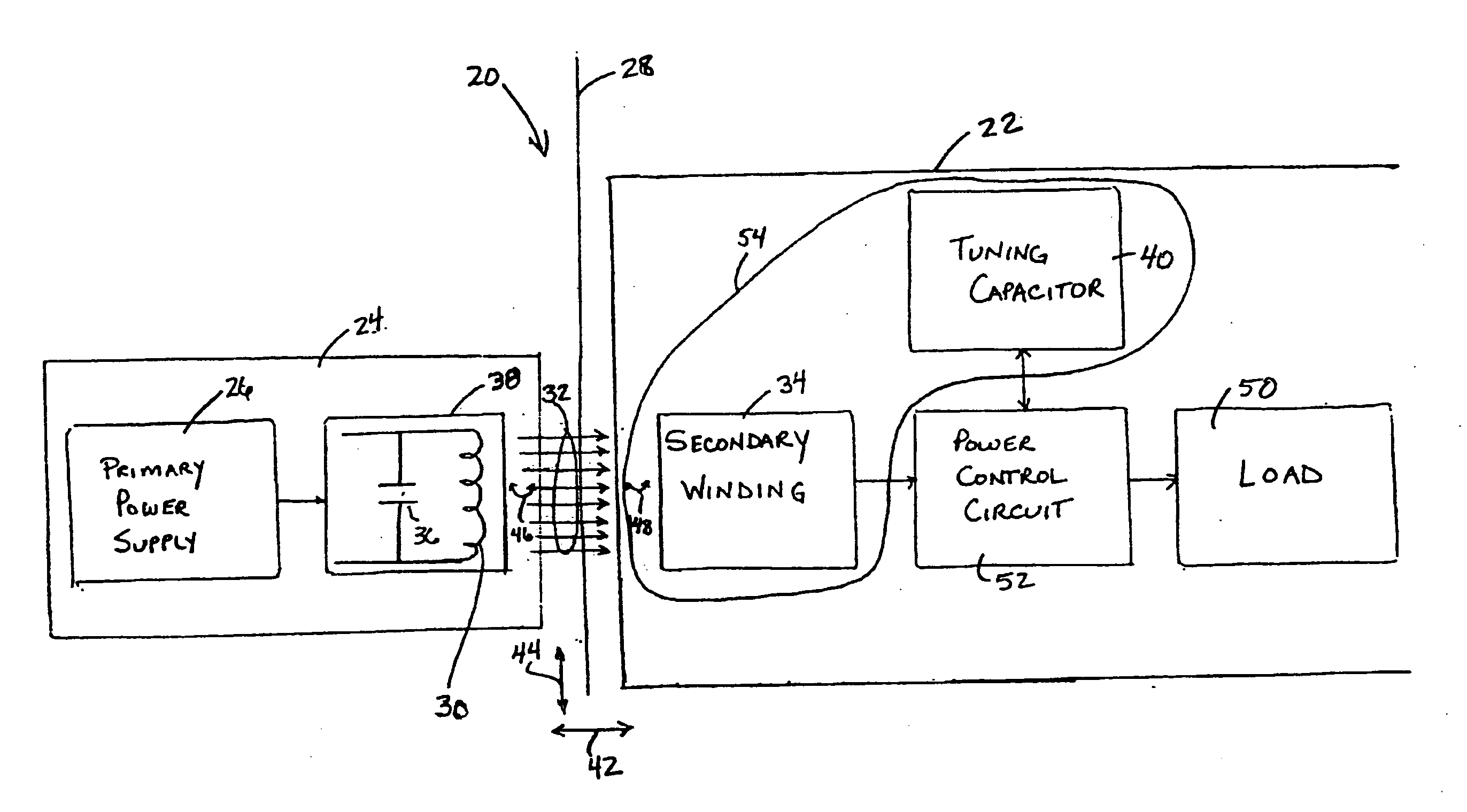
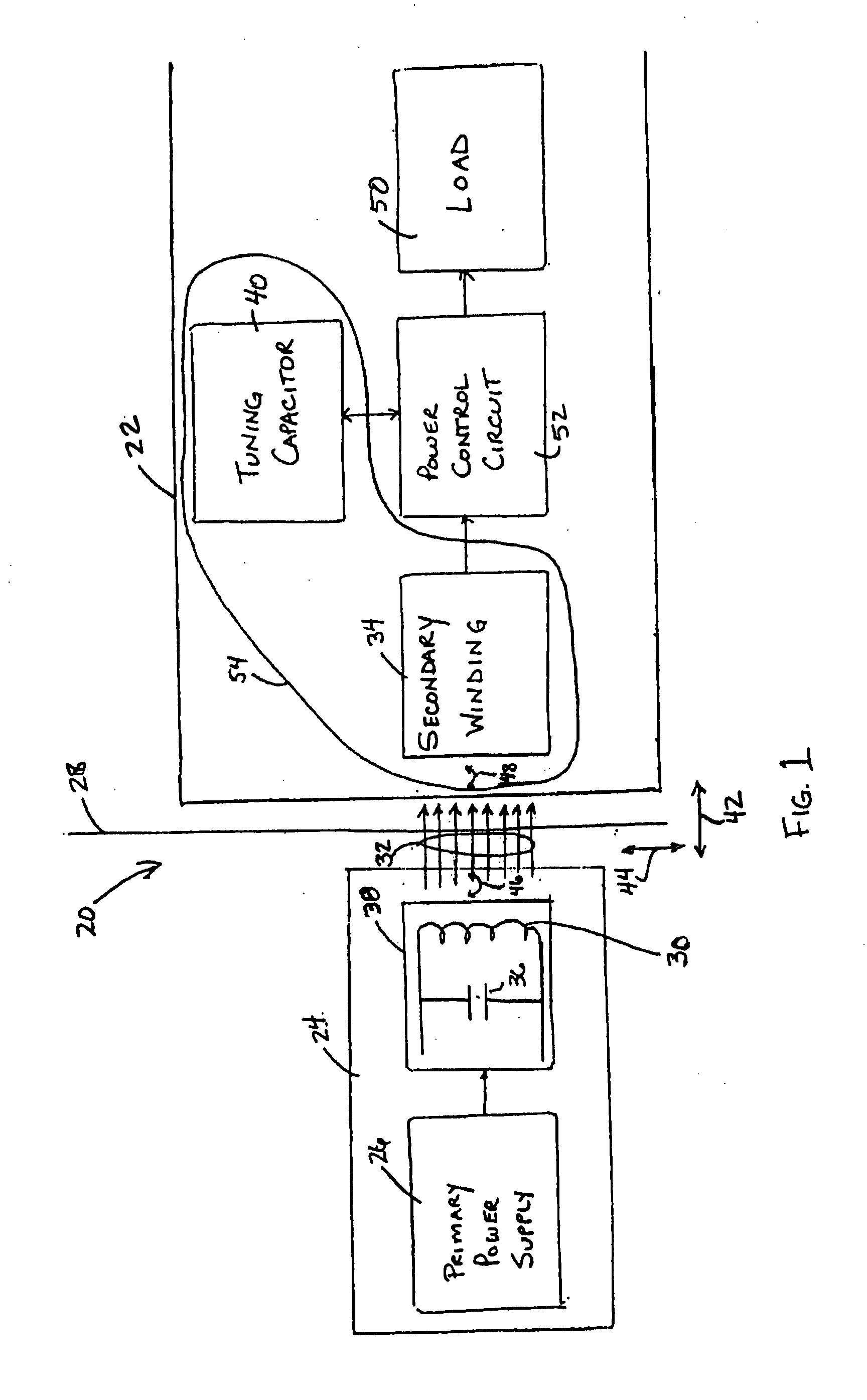
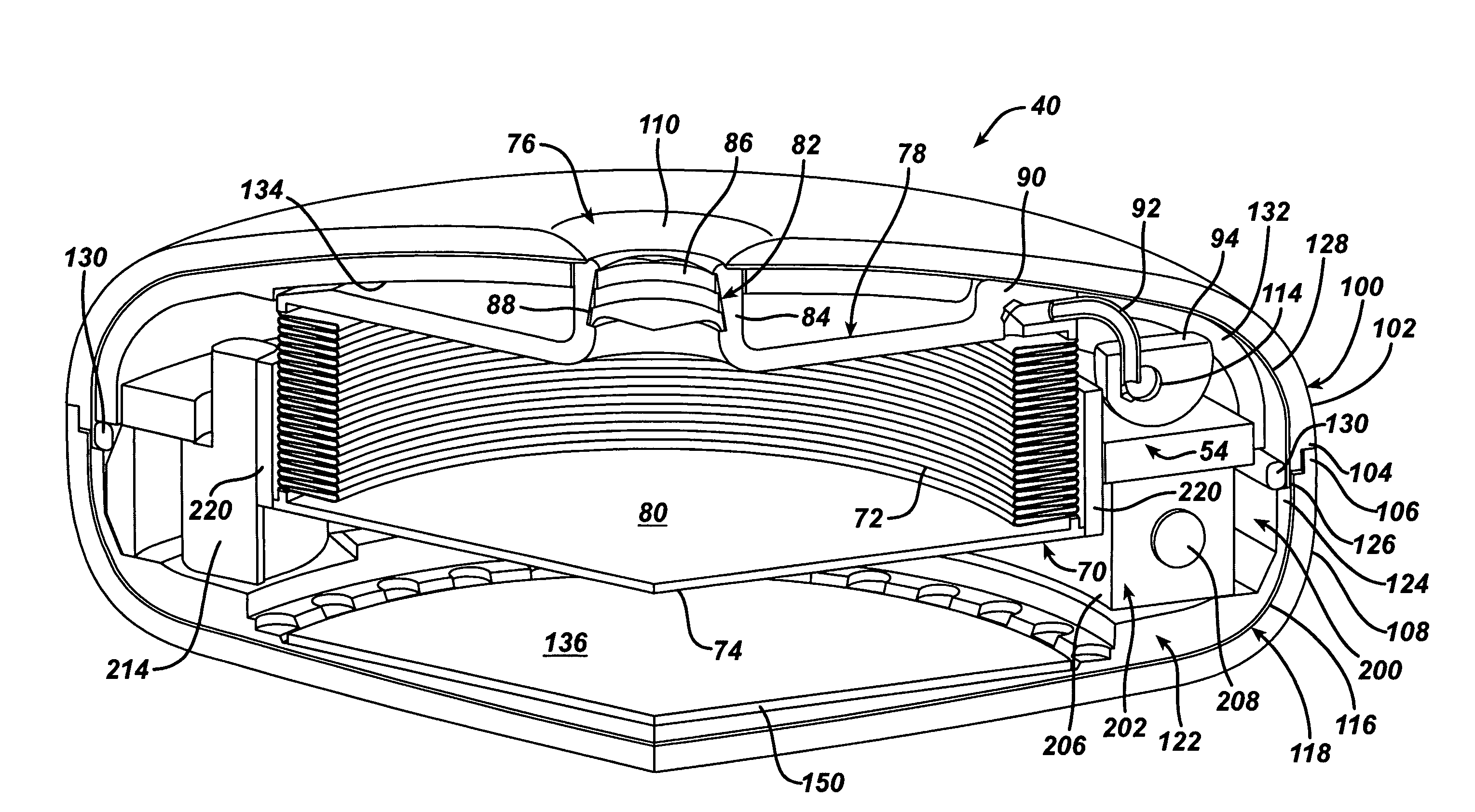
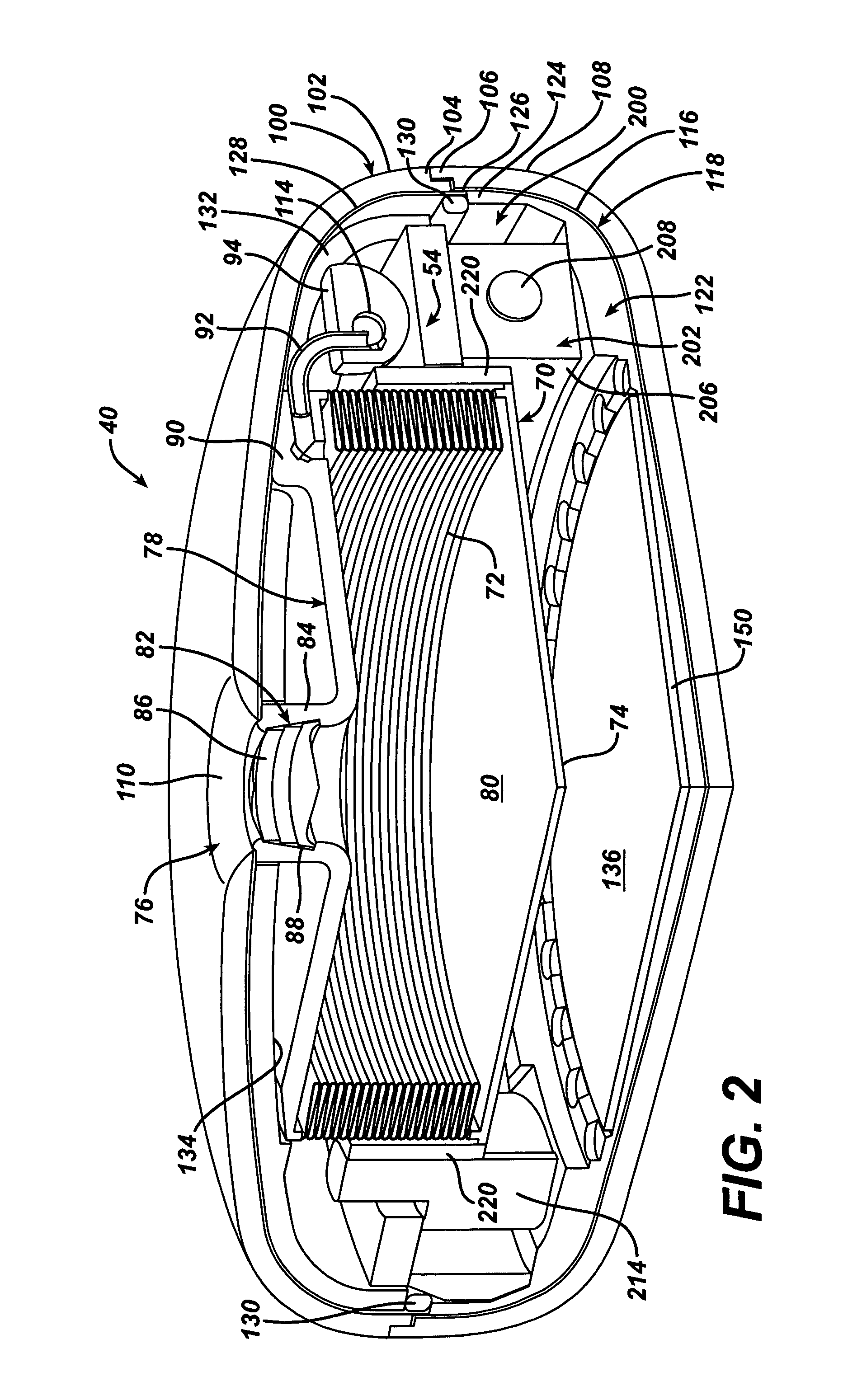
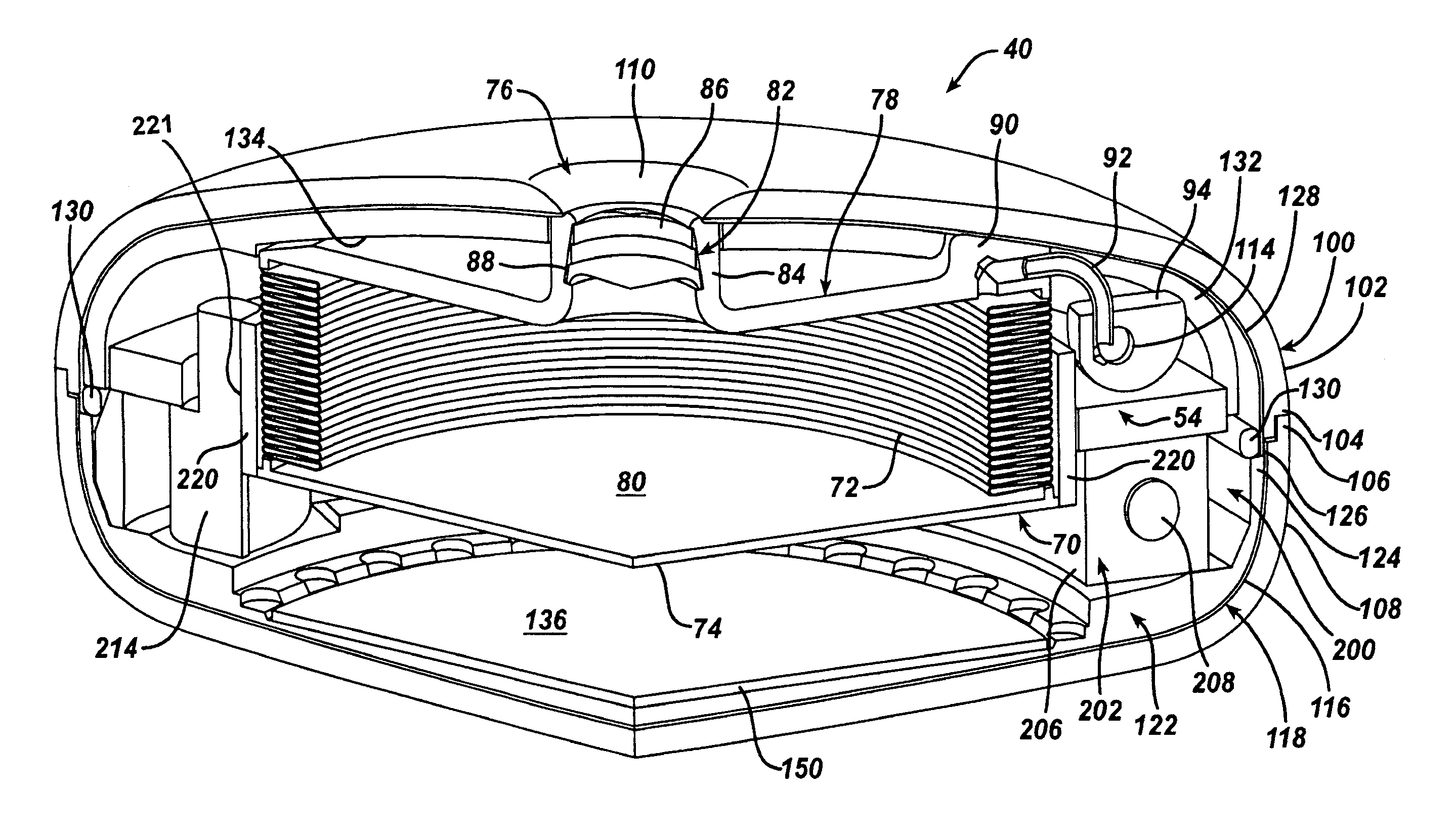
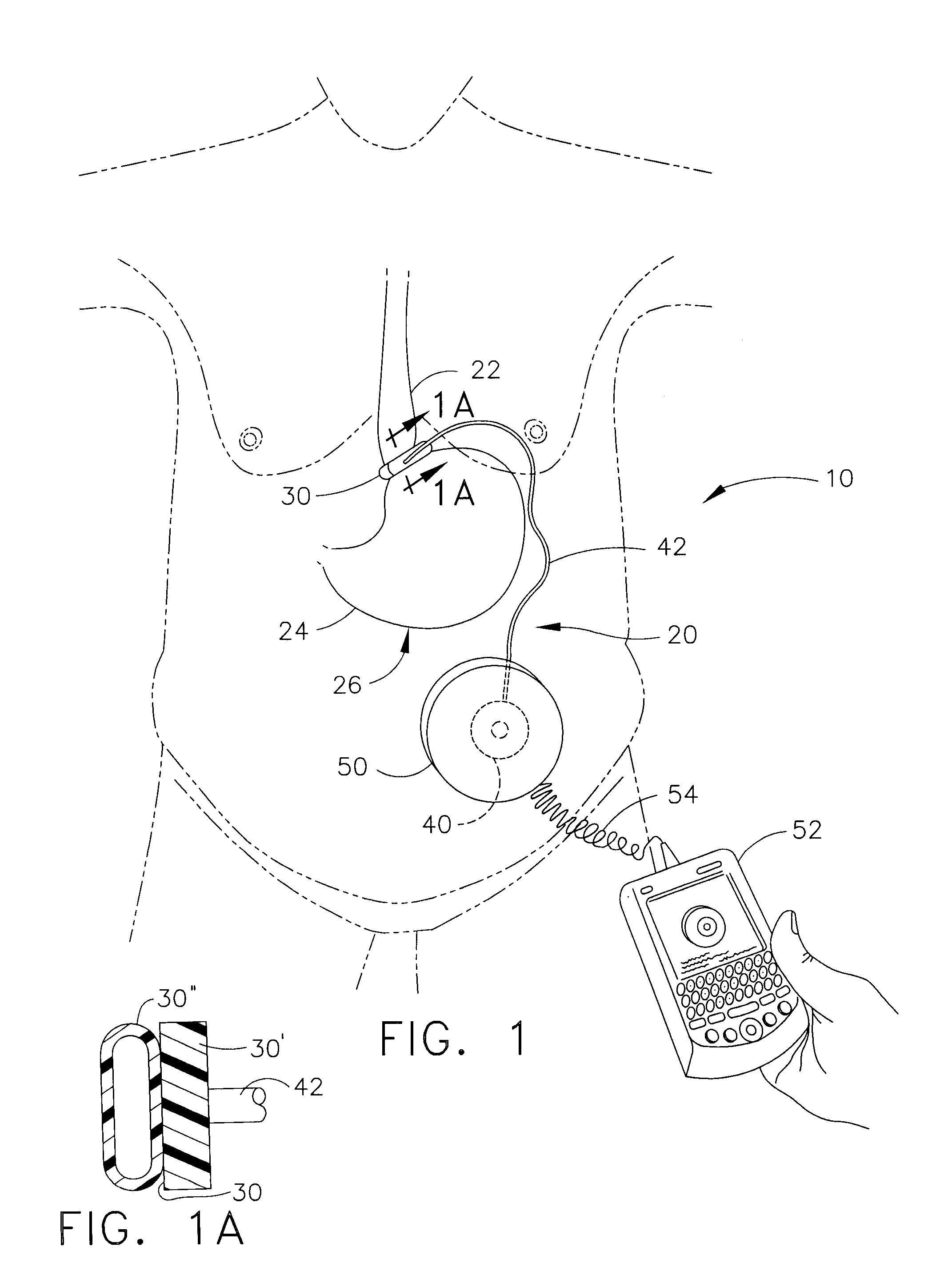
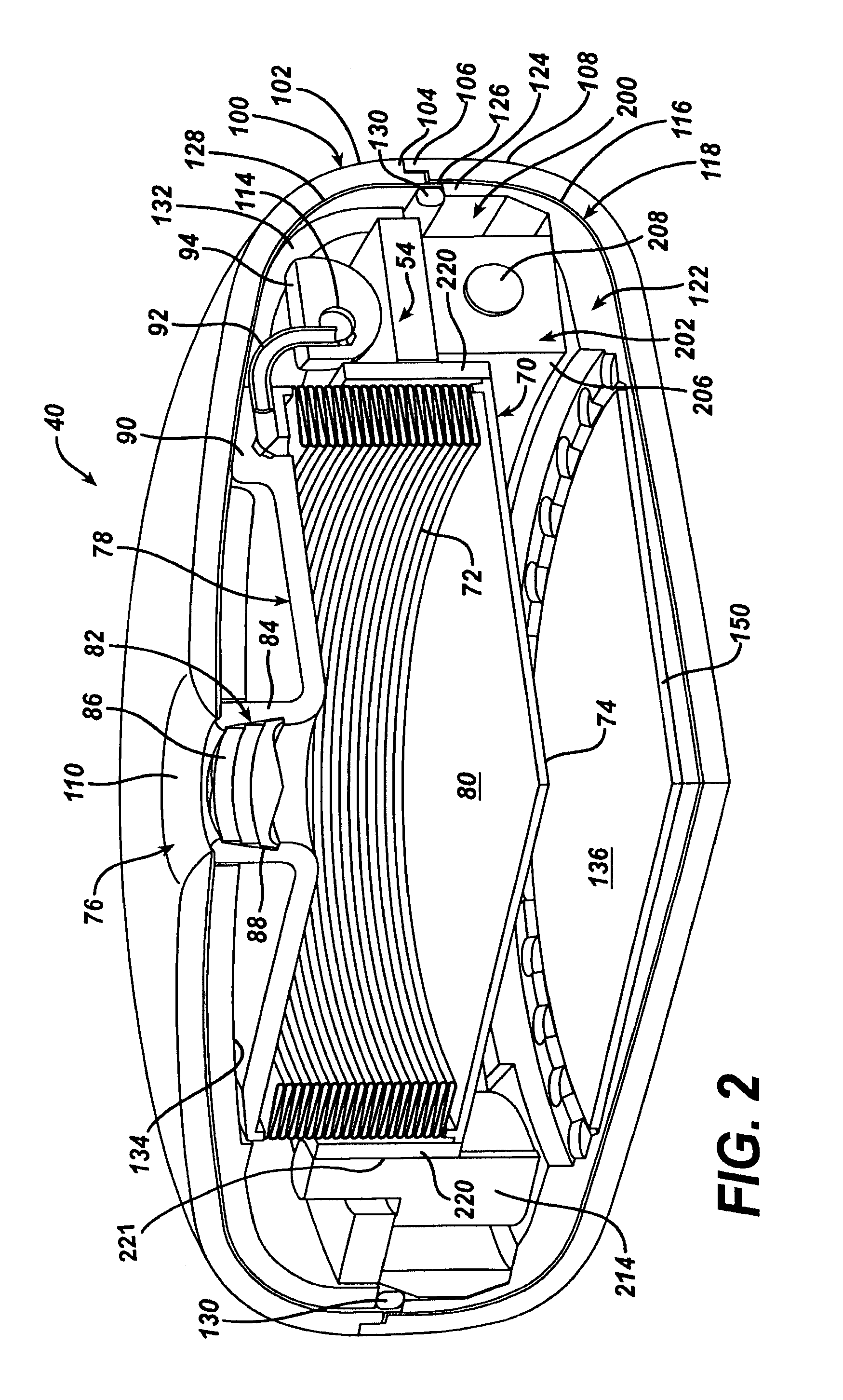
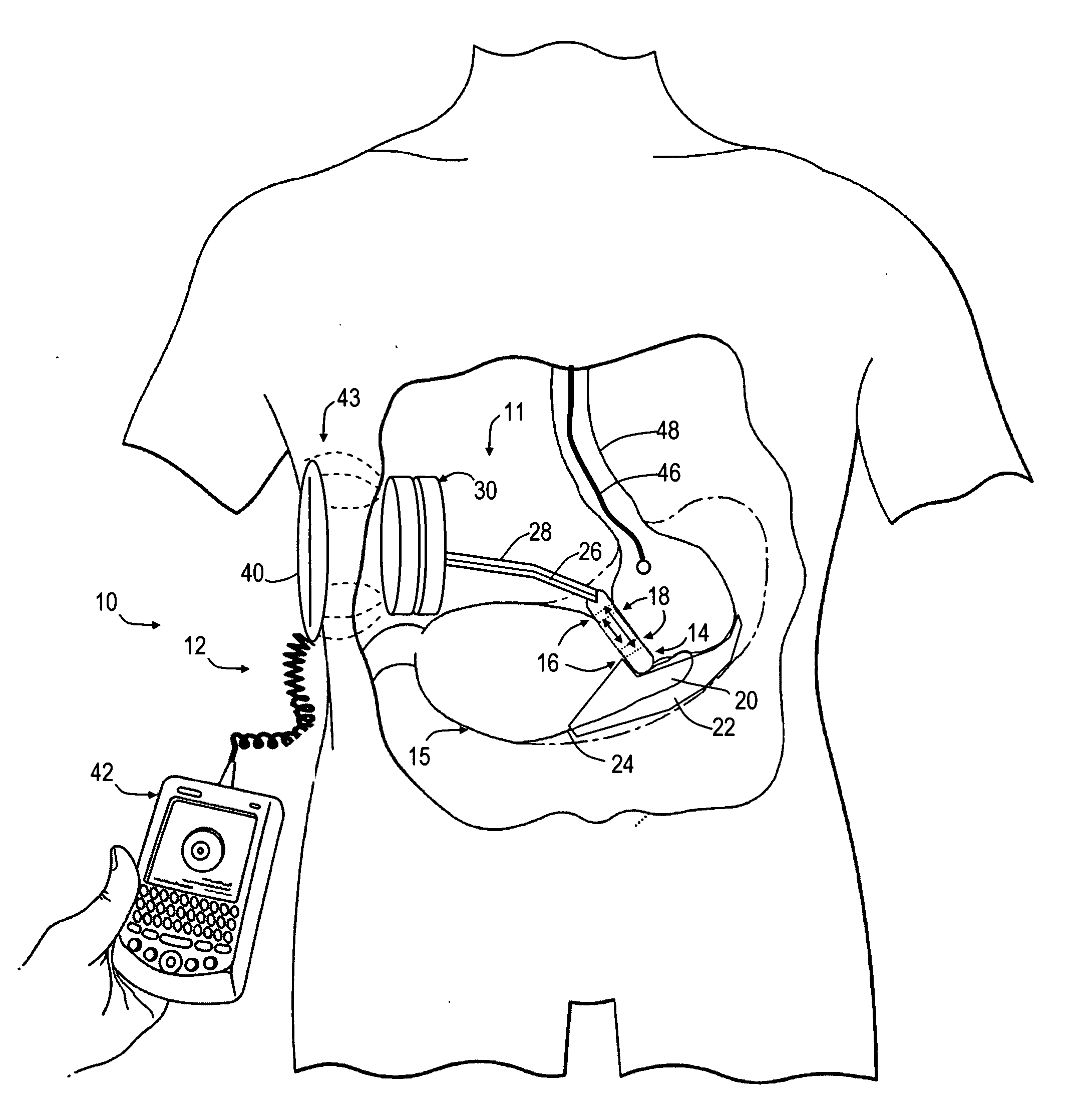
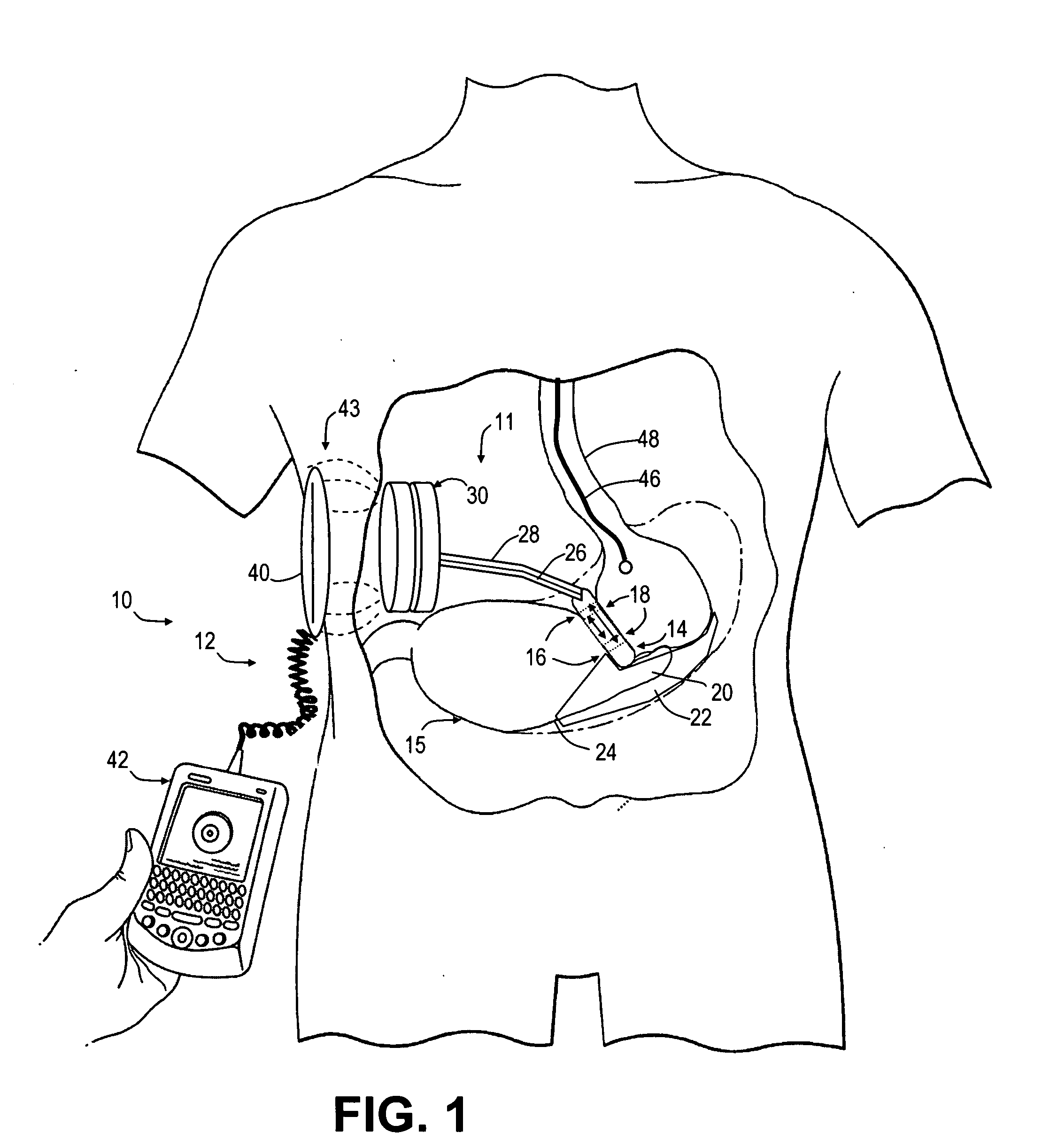
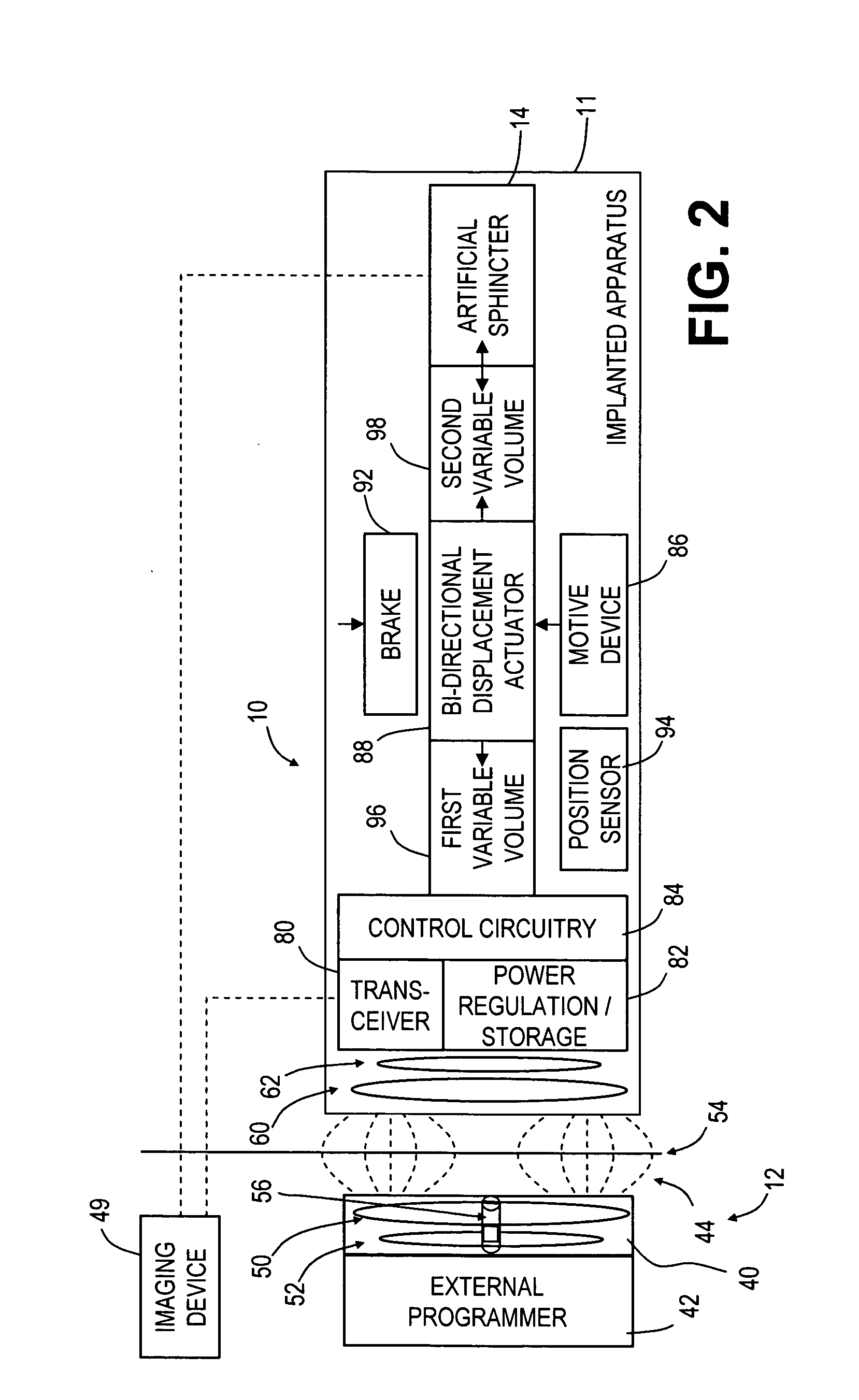
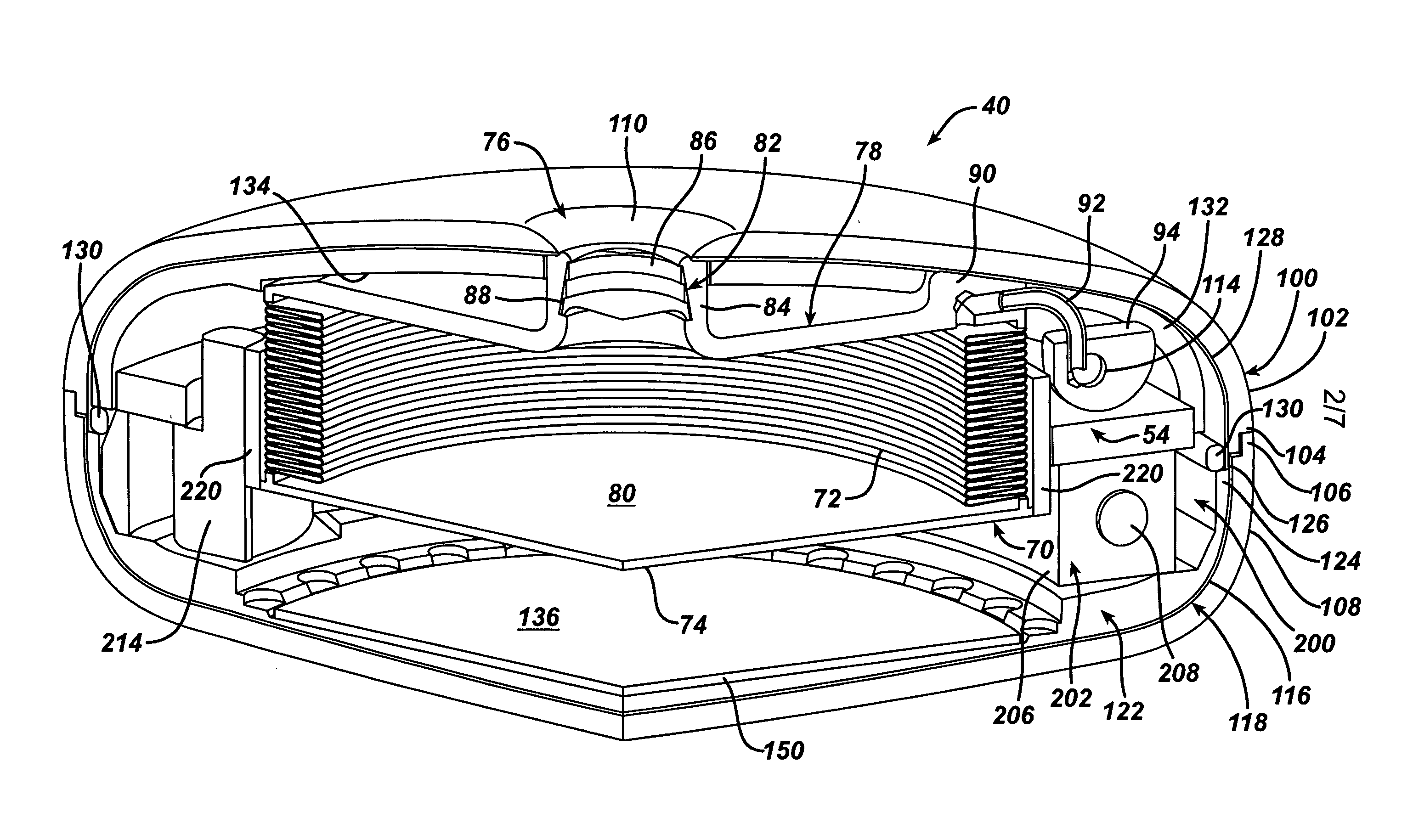
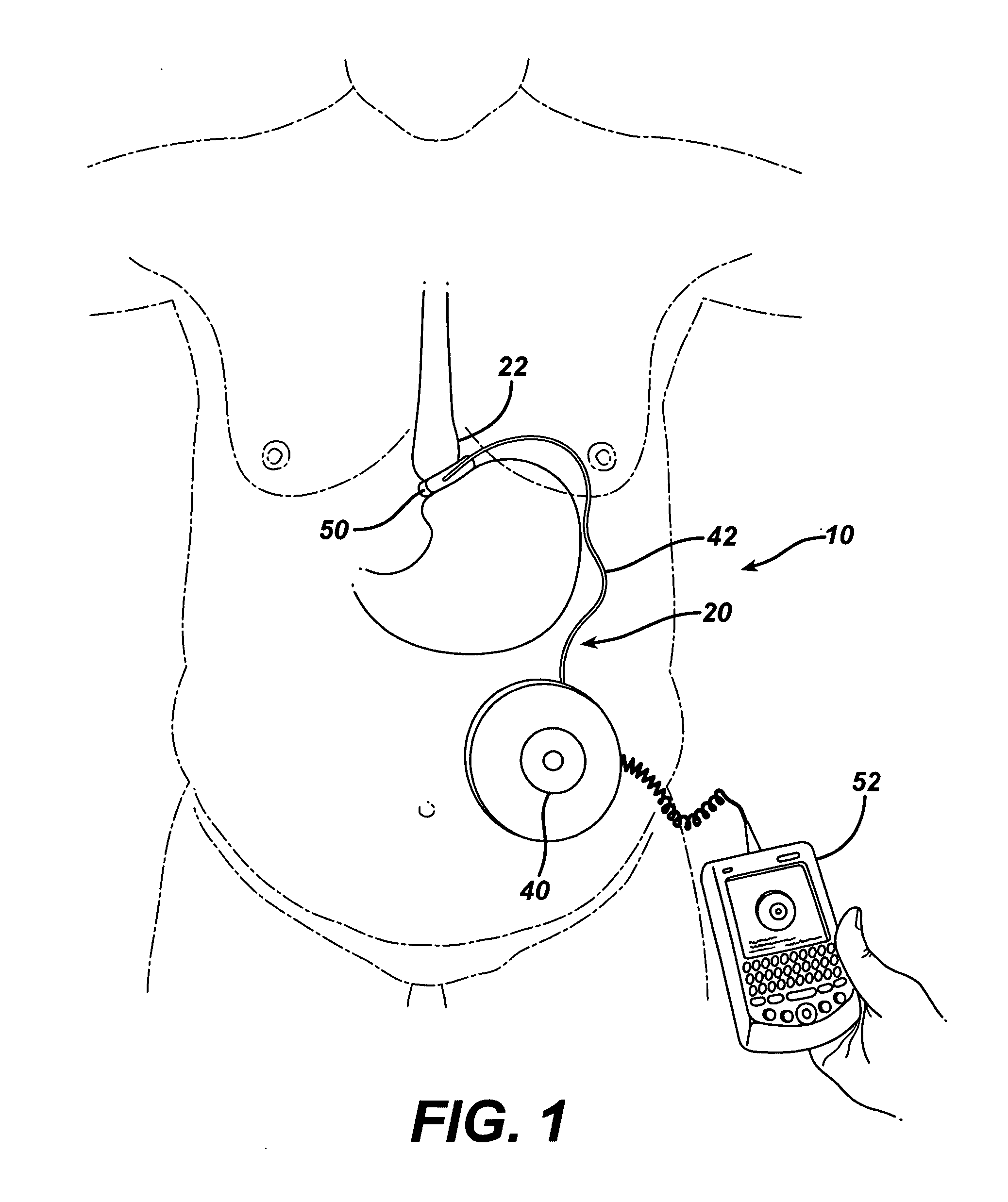
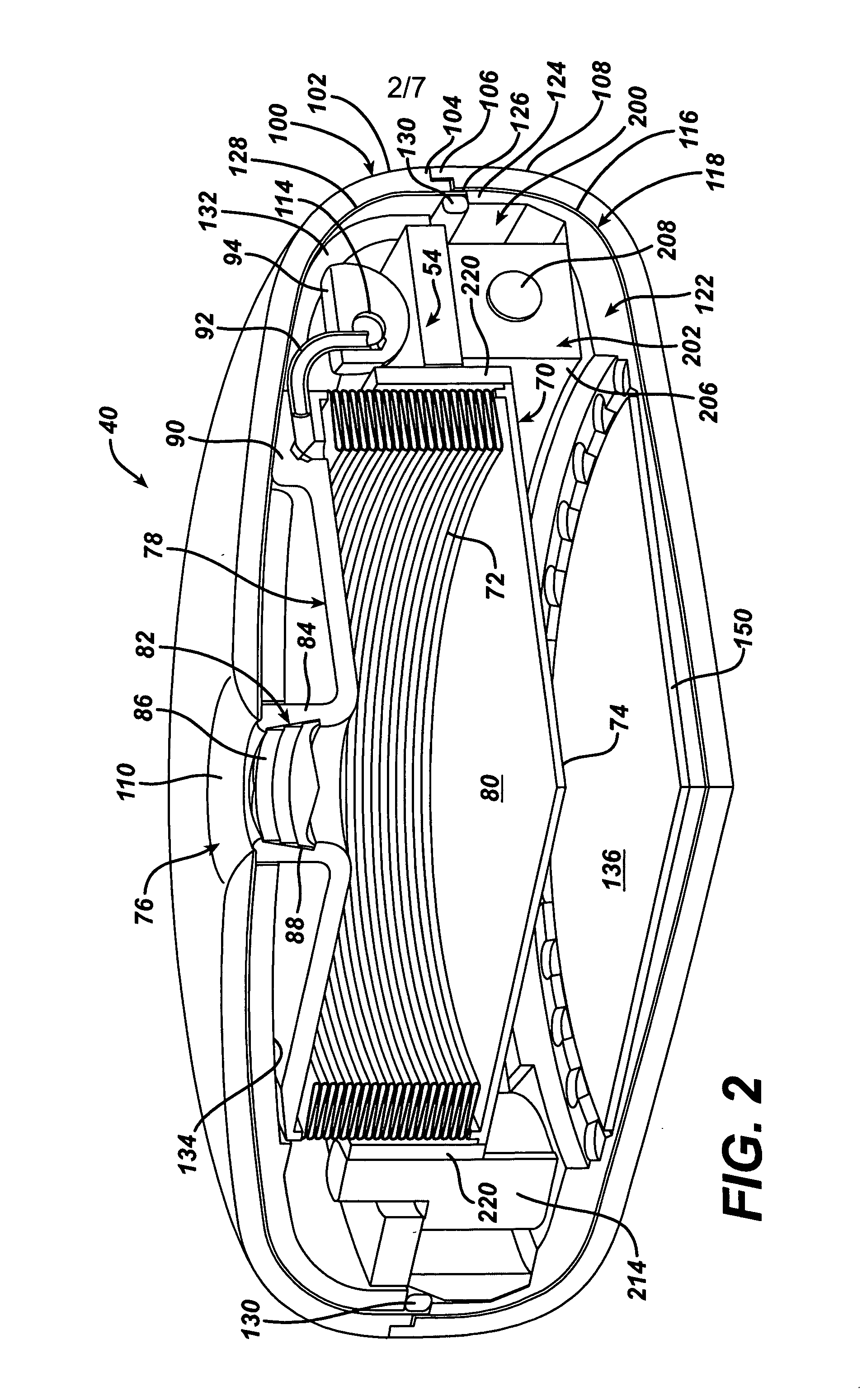
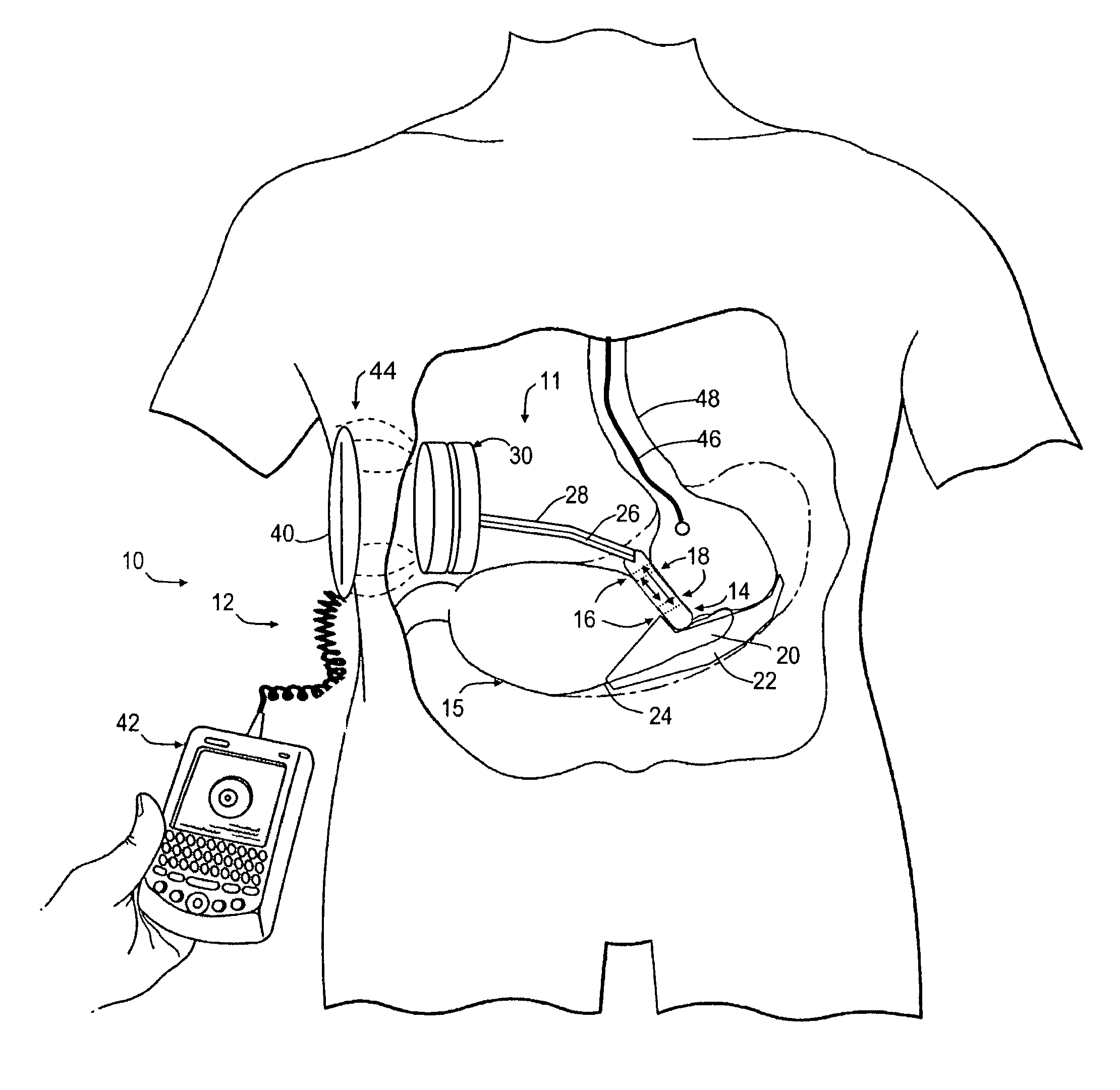
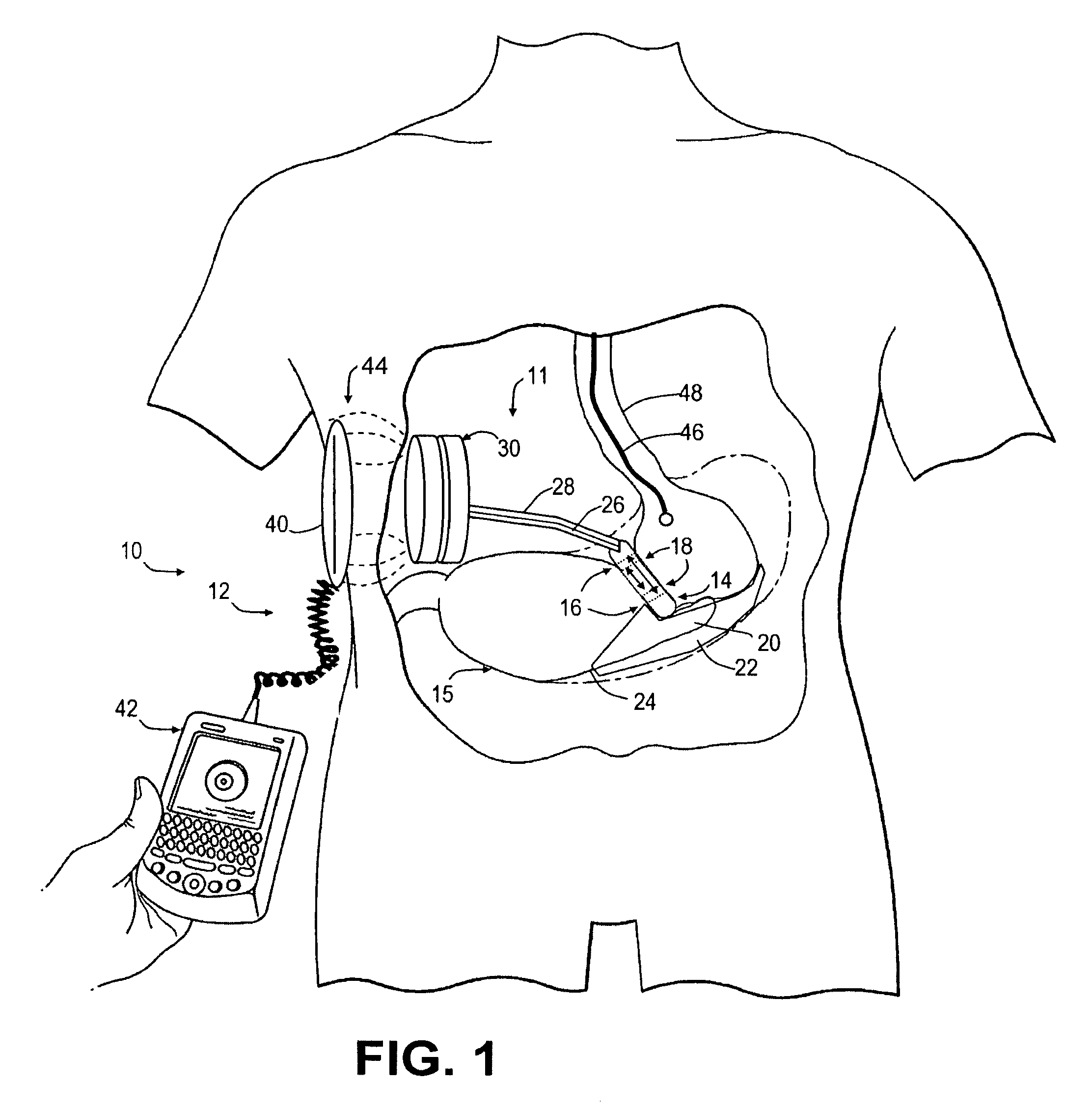
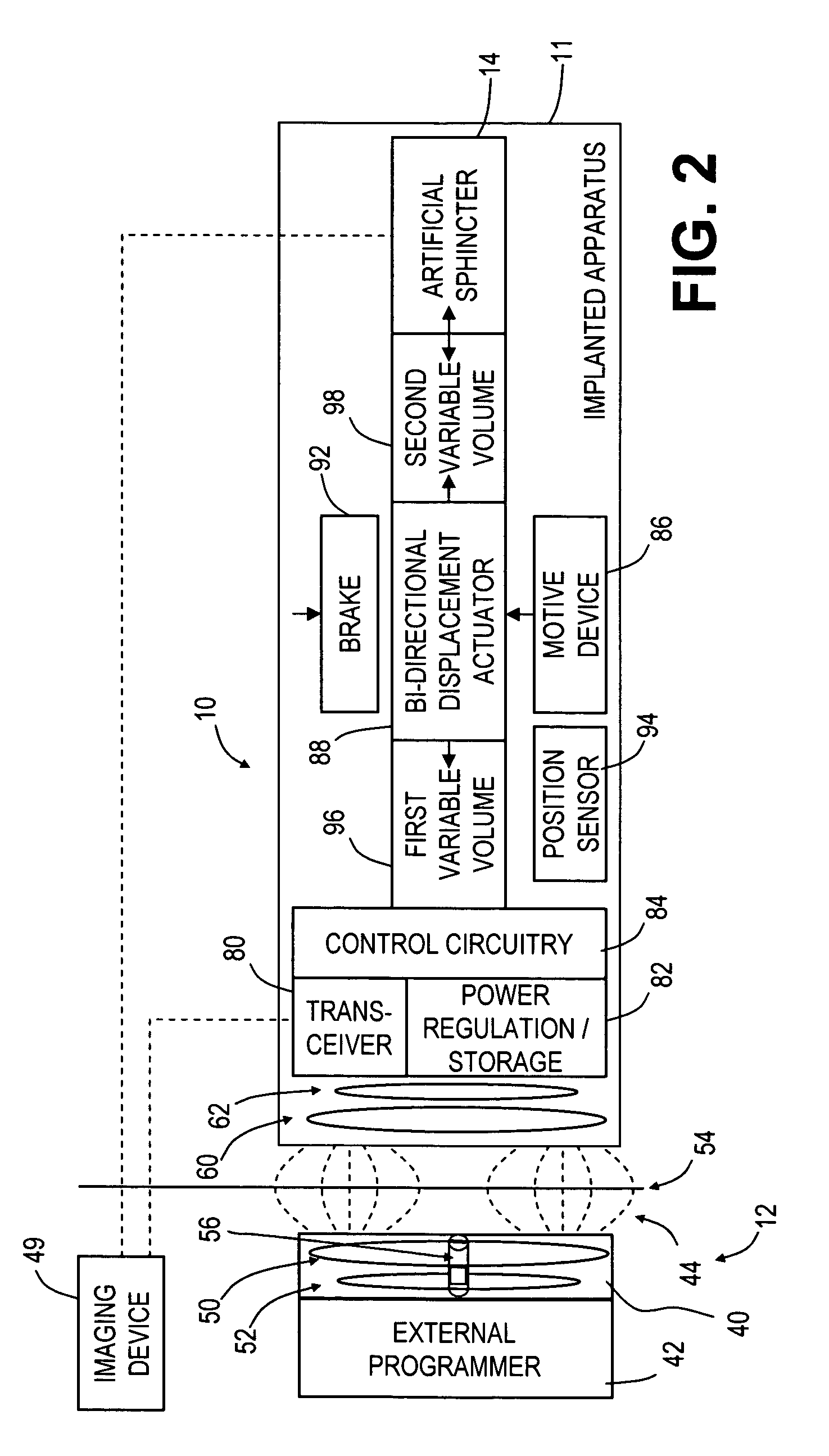
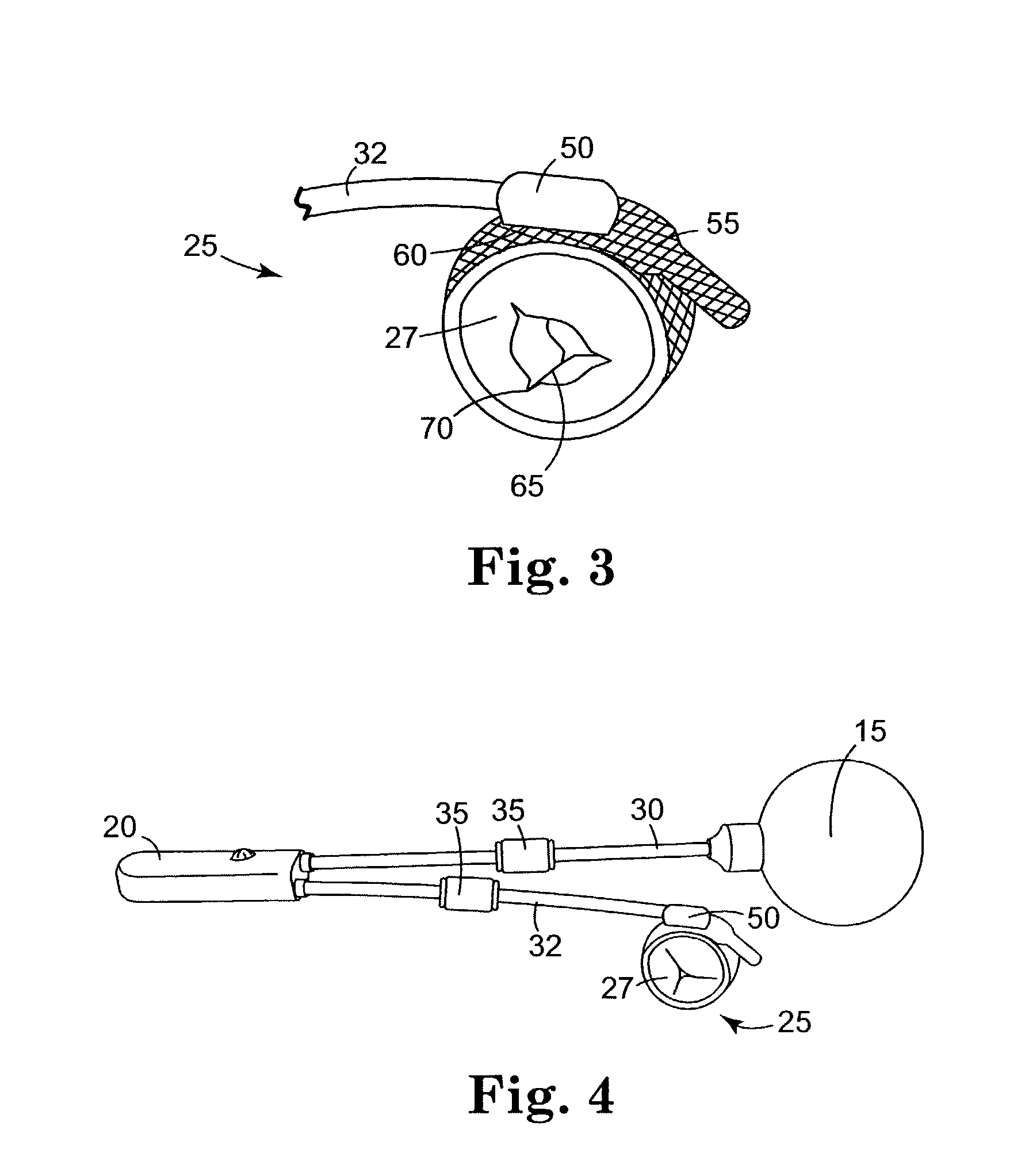
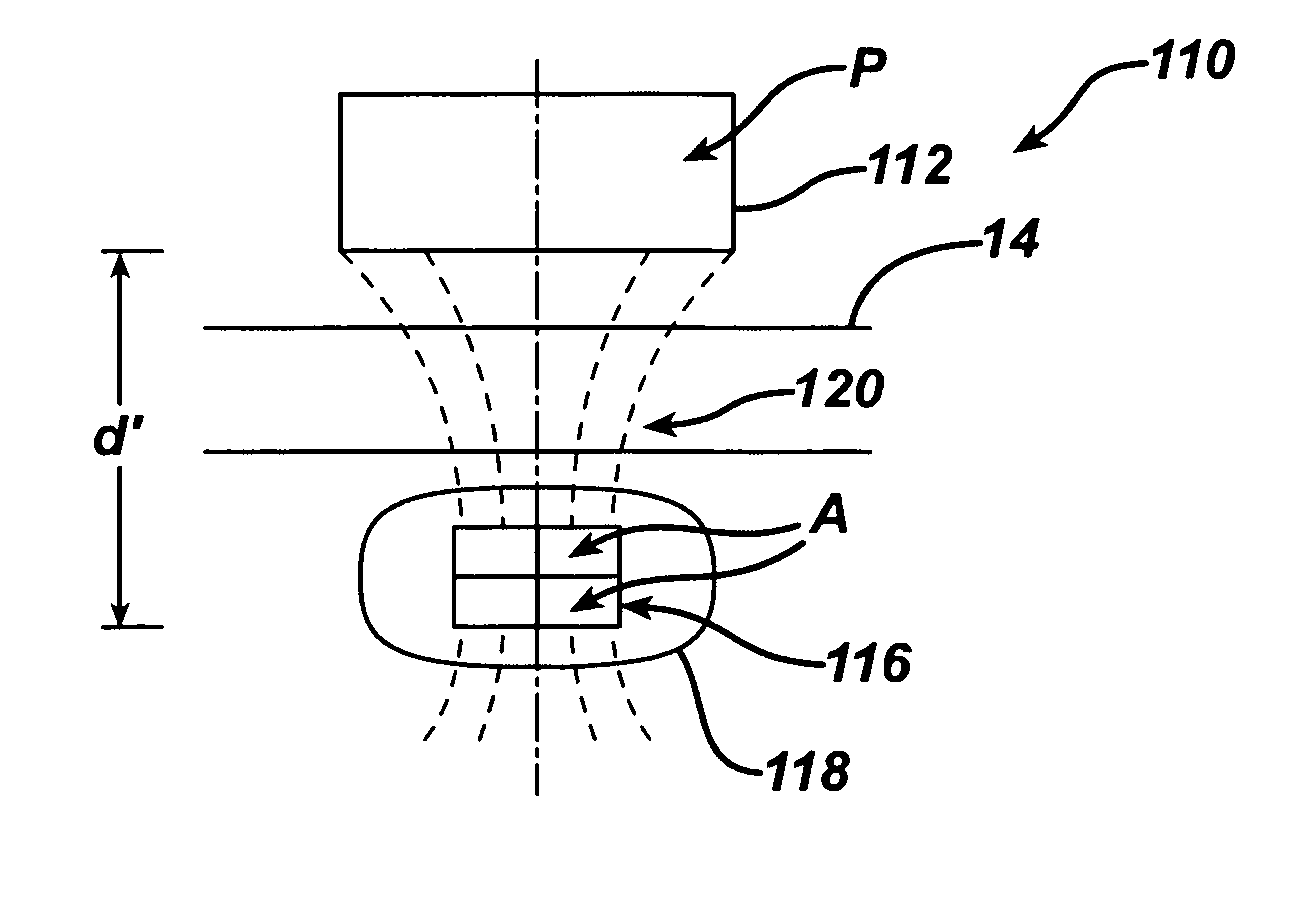
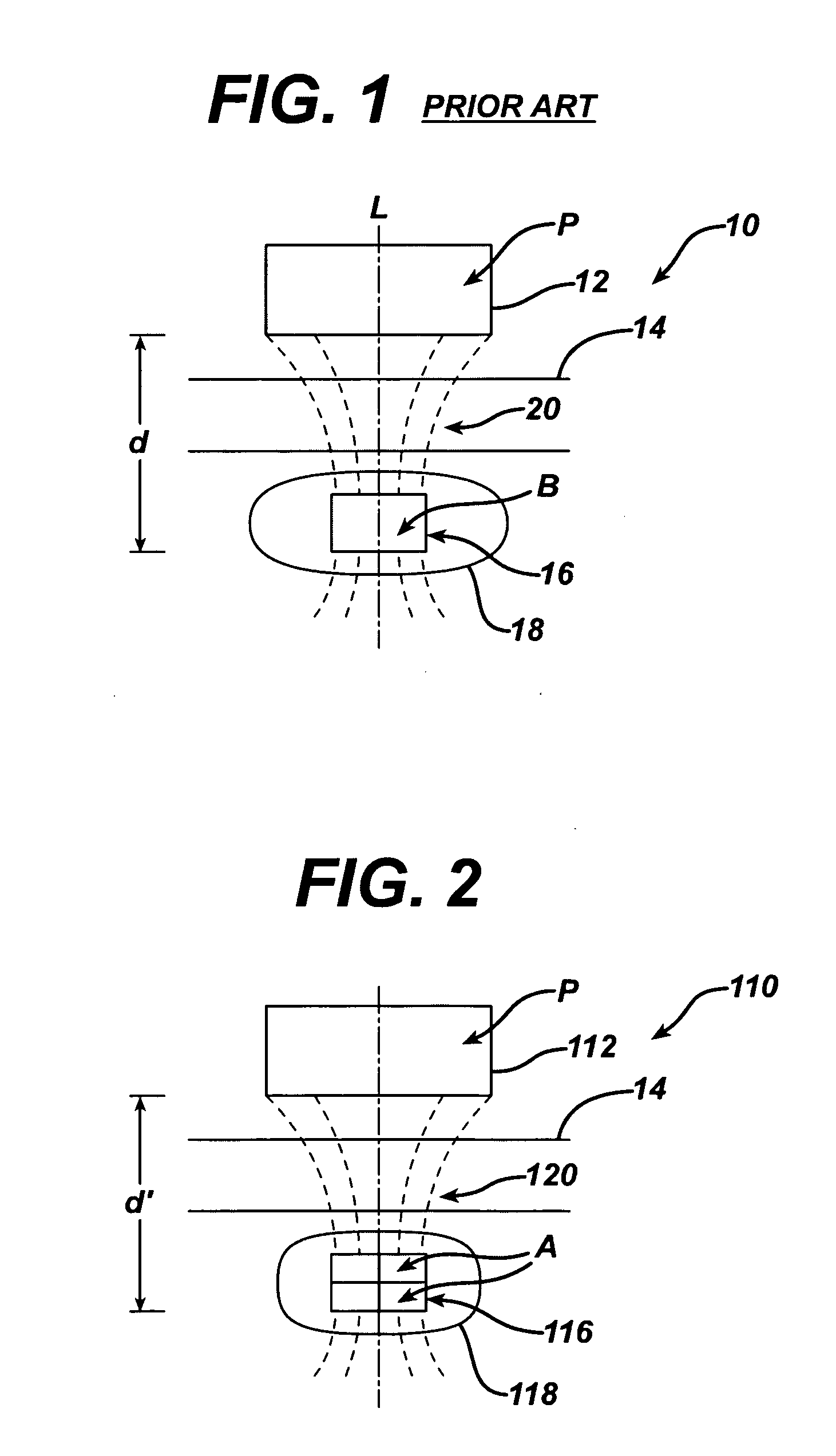
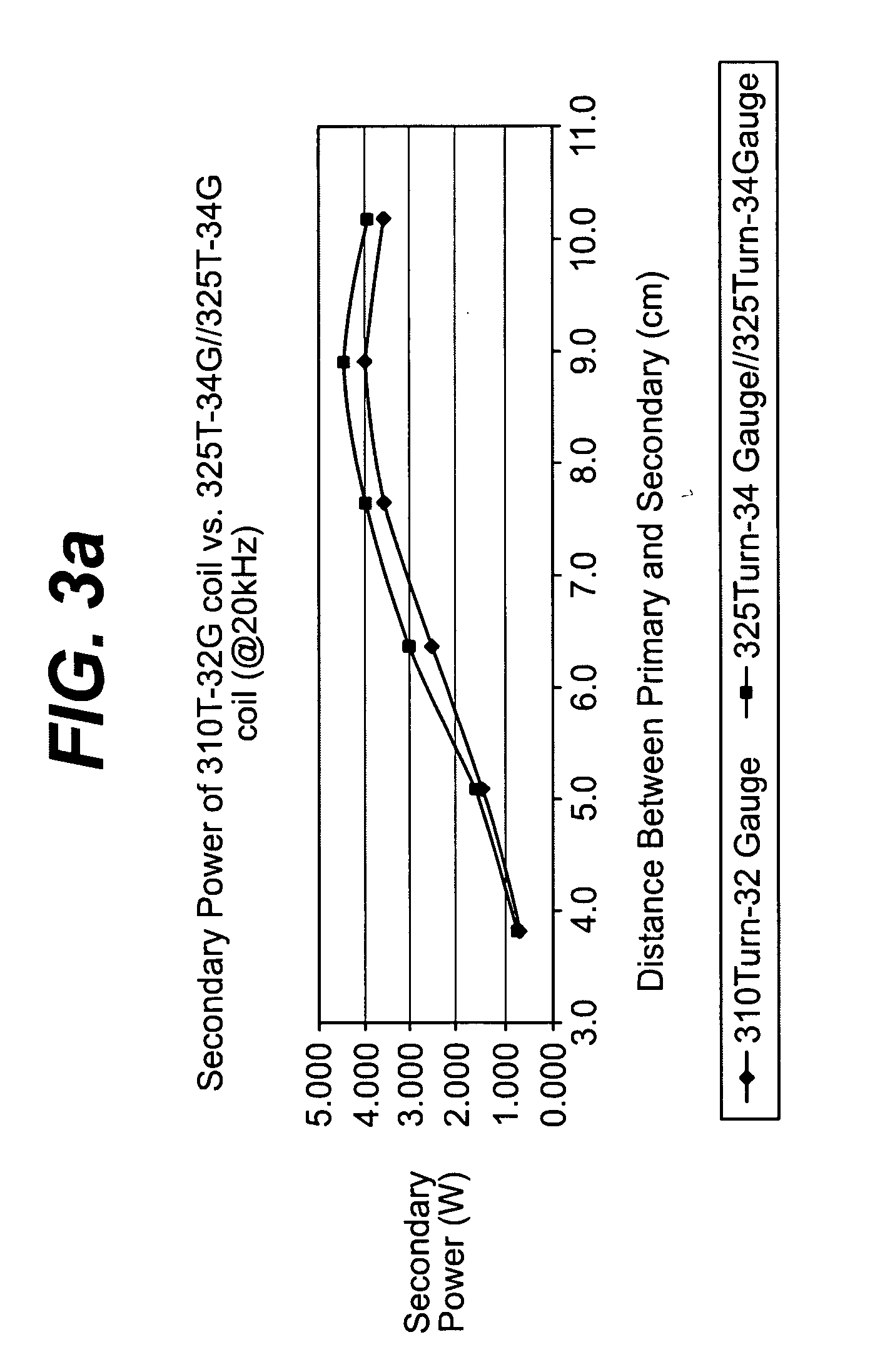
Spatially decoupled twin secondary coils for optimizing transcutaneous energy transfer (TET) power transfer characteristics
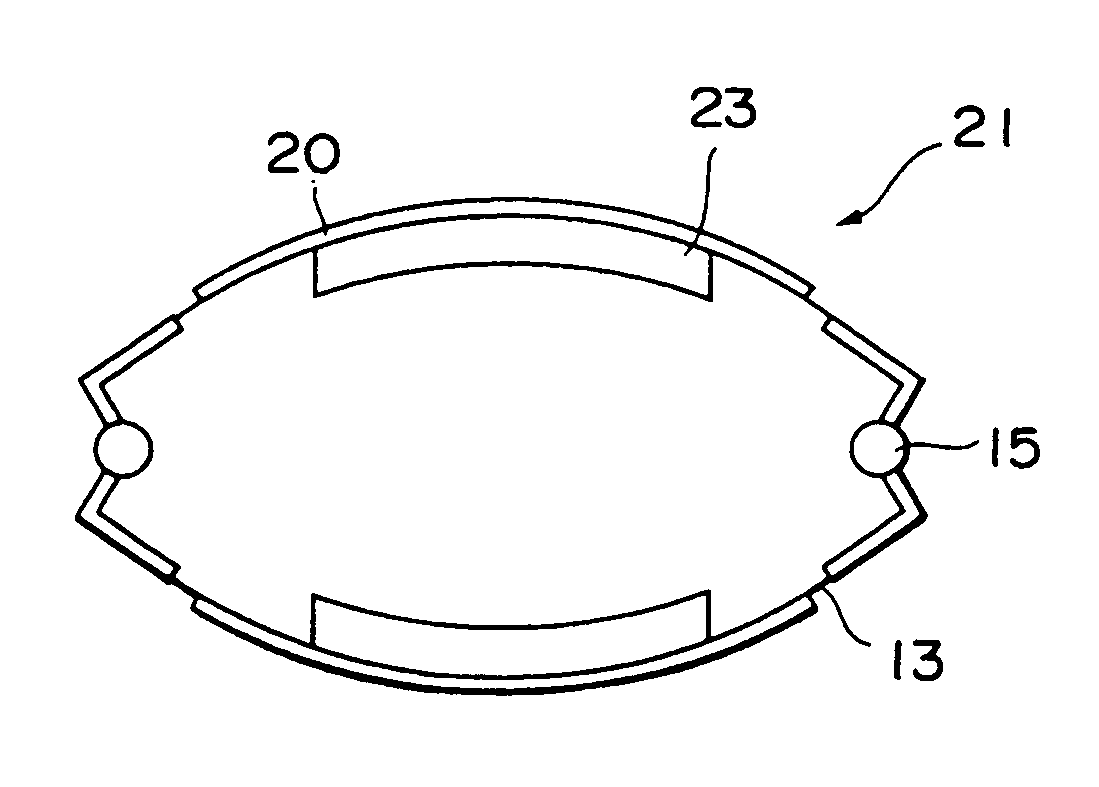
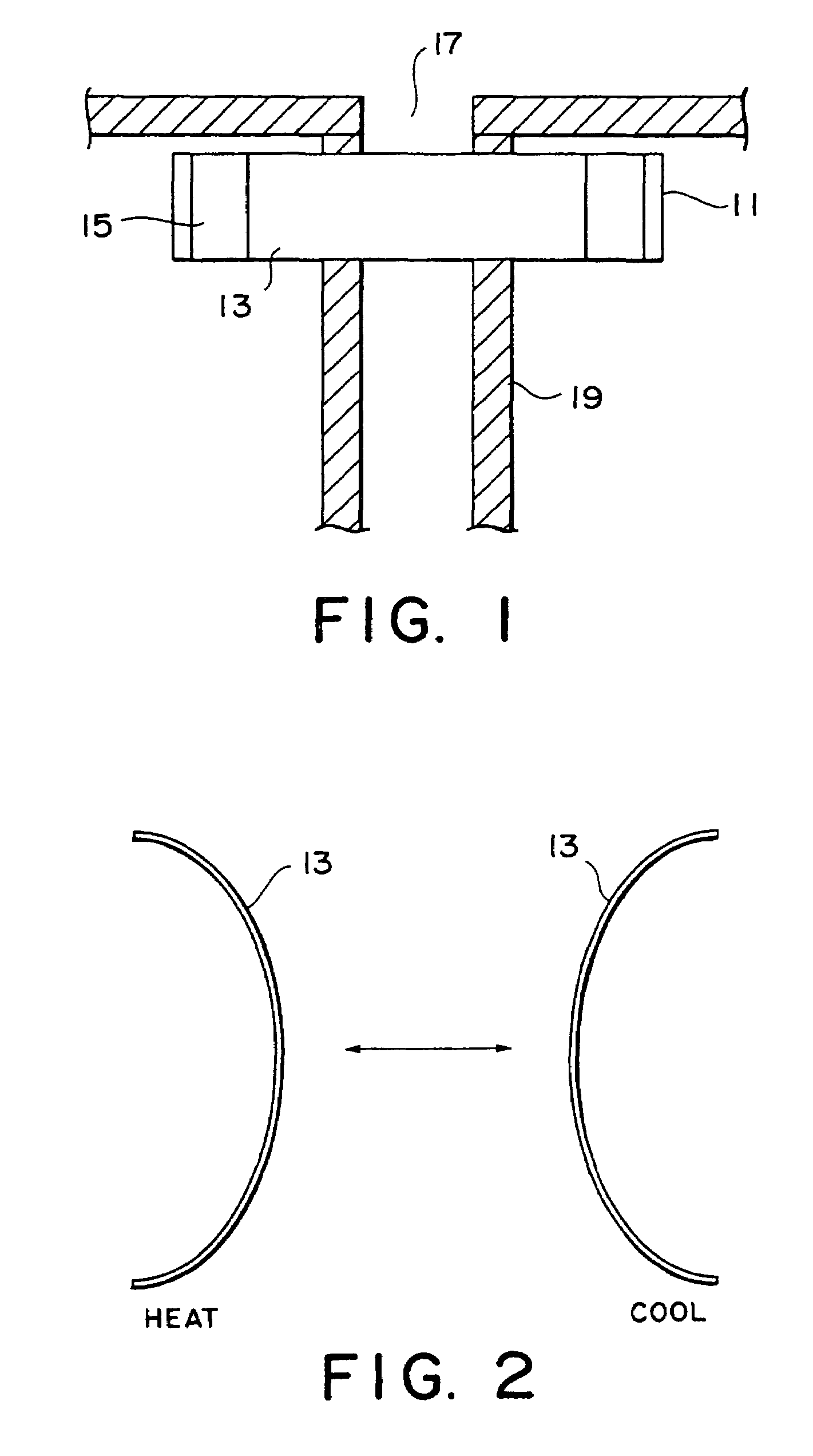
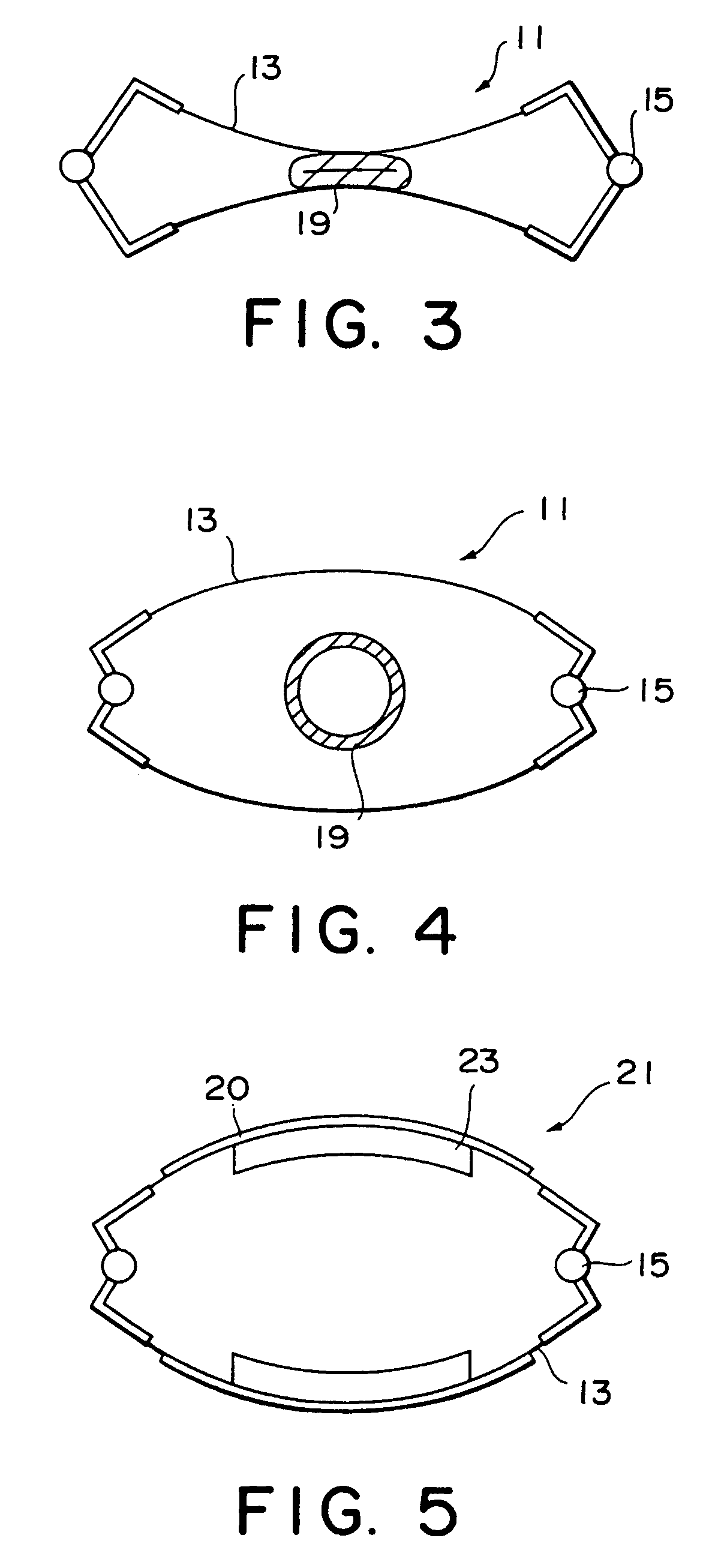
ActiveUS7191007B2Raise transfer toImplantation is simpleElectrotherapyConversion without intermediate conversion to dcEnergy transferImplanted device
An implantable device, such as an infuser device for bidirectional hydraulically controlling a medical artificial sphincter, enhances power transfer characteristics to a secondary coil thereby allowing implantation to greater physical depths and / or enclosing the secondary coil within a housing of the infuser device. The enhanced power transfer is achieved with multiple coils that are longitudinally aligned and physical and electrical parallel to form the secondary loop of a transcutaneous energy transfer system (TET) instead of a single coil. It better optimizes the power transfer from a parallel tuned tank circuit primary coil to an implanted secondary series tuned tank circuit coil.
Owner:ETHICON ENDO SURGERY INC
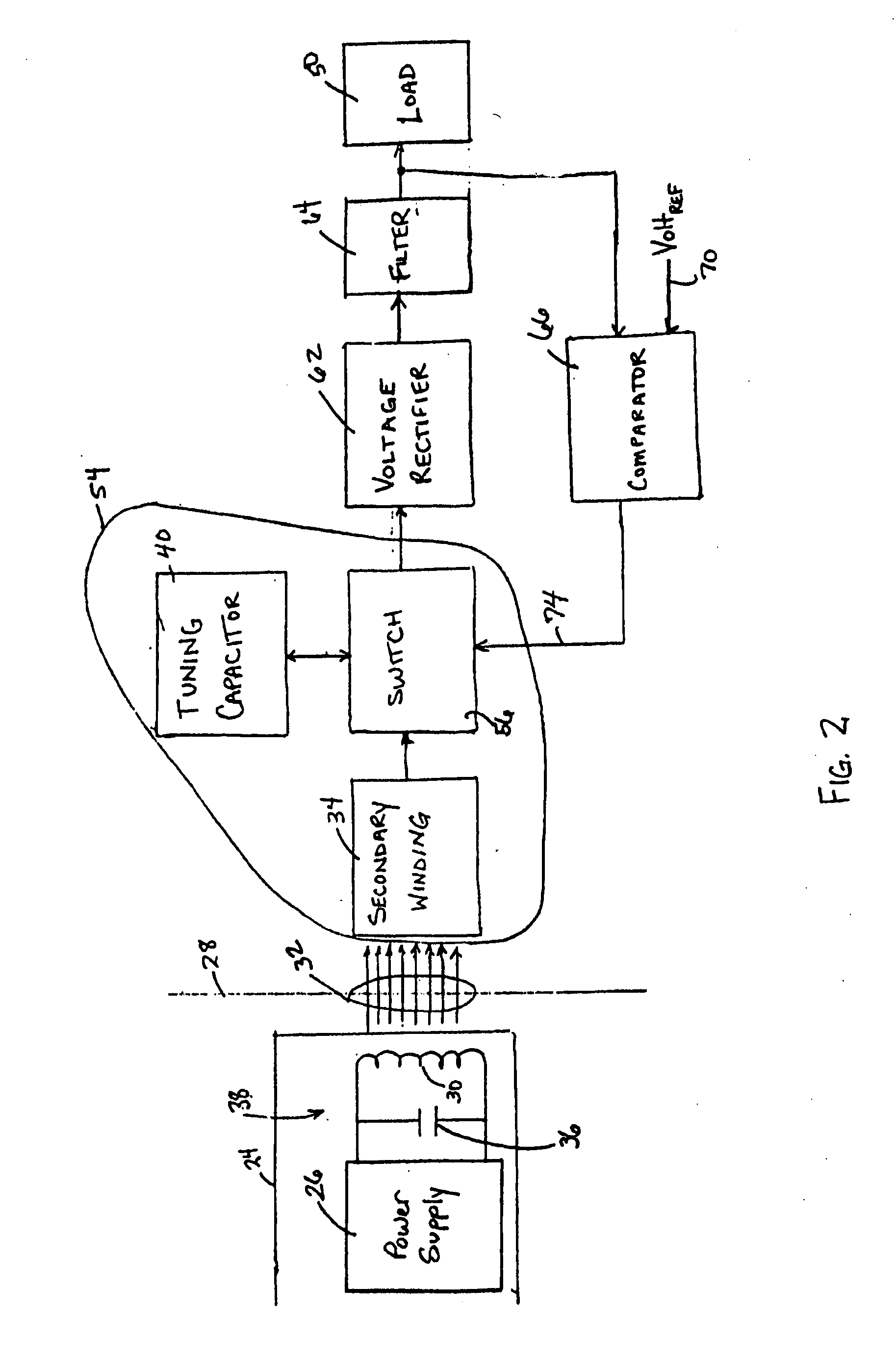
Medical implant having closed loop transcutaneous energy transfer (TET) power transfer regulation circuitry
InactiveUS20050288739A1Not easy to damageLess susceptible to inoperabilityElectrotherapyAnti-incontinence devicesElectrical batteryClosed loop
An implantable medical device, such as a bi-directional infuser device for hydraulically controlling an artificial sphincter (e.g., adjustable gastric band) benefits from being remotely powered by transcutaneous energy transfer (TET), obviating the need for batteries. In order for active components in the medical device to operate, a sinusoidal power signal received by a secondary coil is rectified and filtered. An amount of power transferred is modulated. In one version, a voltage comparison is made of a resulting power supply voltage as referenced to a threshold to control pulse width modulation (PWM) of the received sinusoidal power signal, achieving voltage regulation. Versions incorporate detuning or uncoupling of the secondary coil to achieve PWM control without causing excessive heating of the medical device.
Owner:ETHICON ENDO SURGERY INC
Complaint implantable medical devices and methods of making same
InactiveUS6936066B2Give flexibilityFacilitating transmural endothelializationStentsHeart valvesSurgical GraftMetallic materials
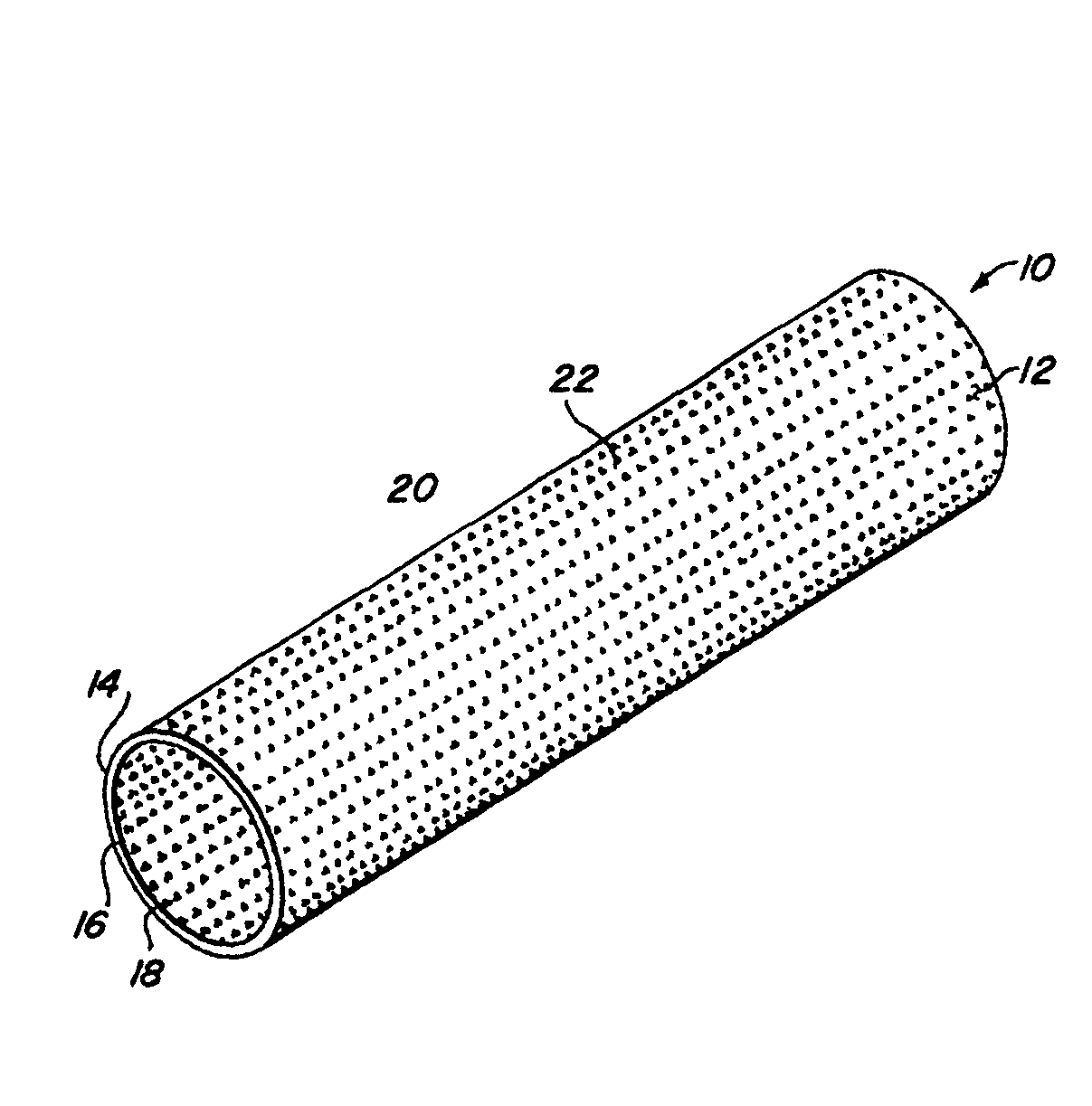
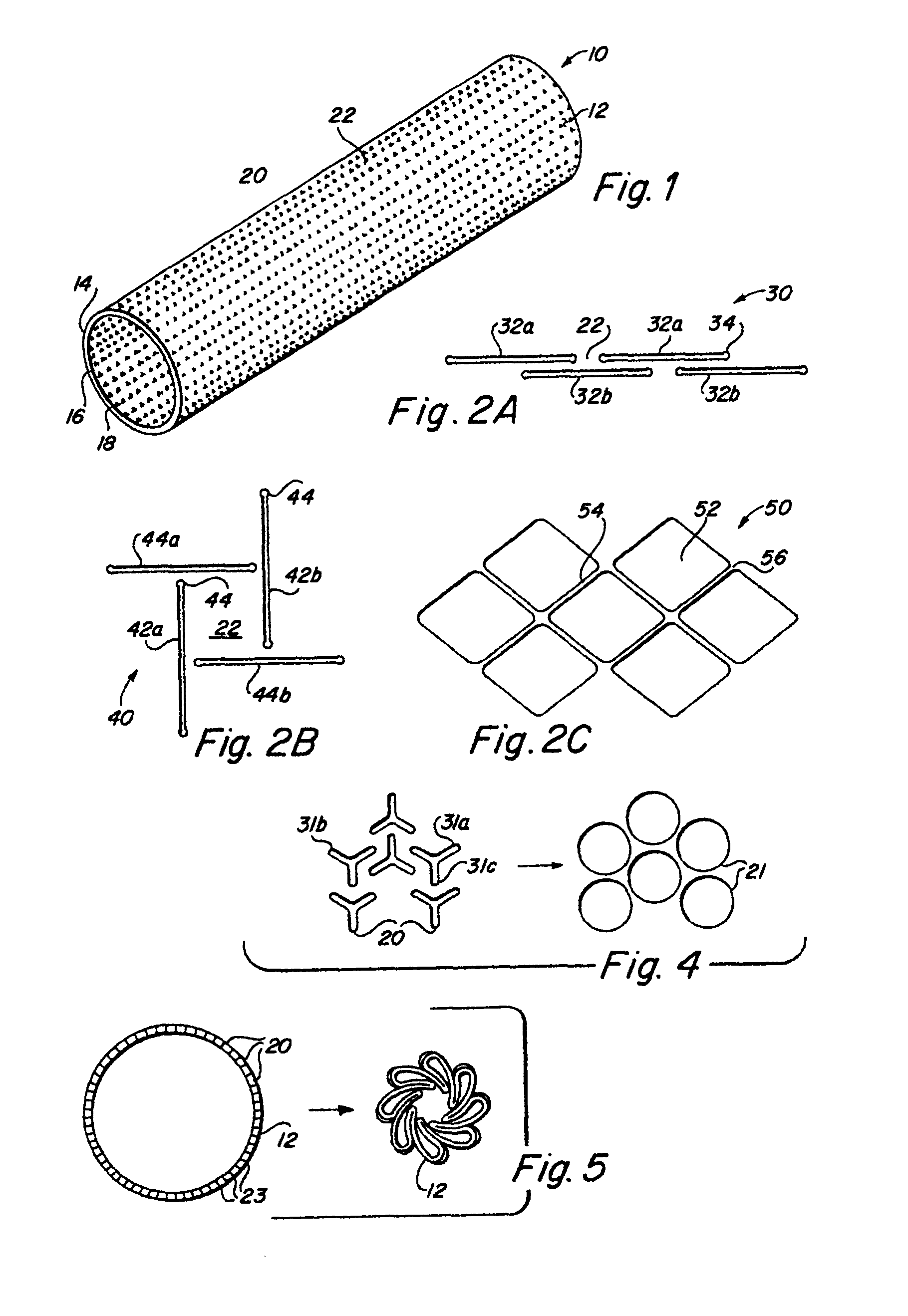
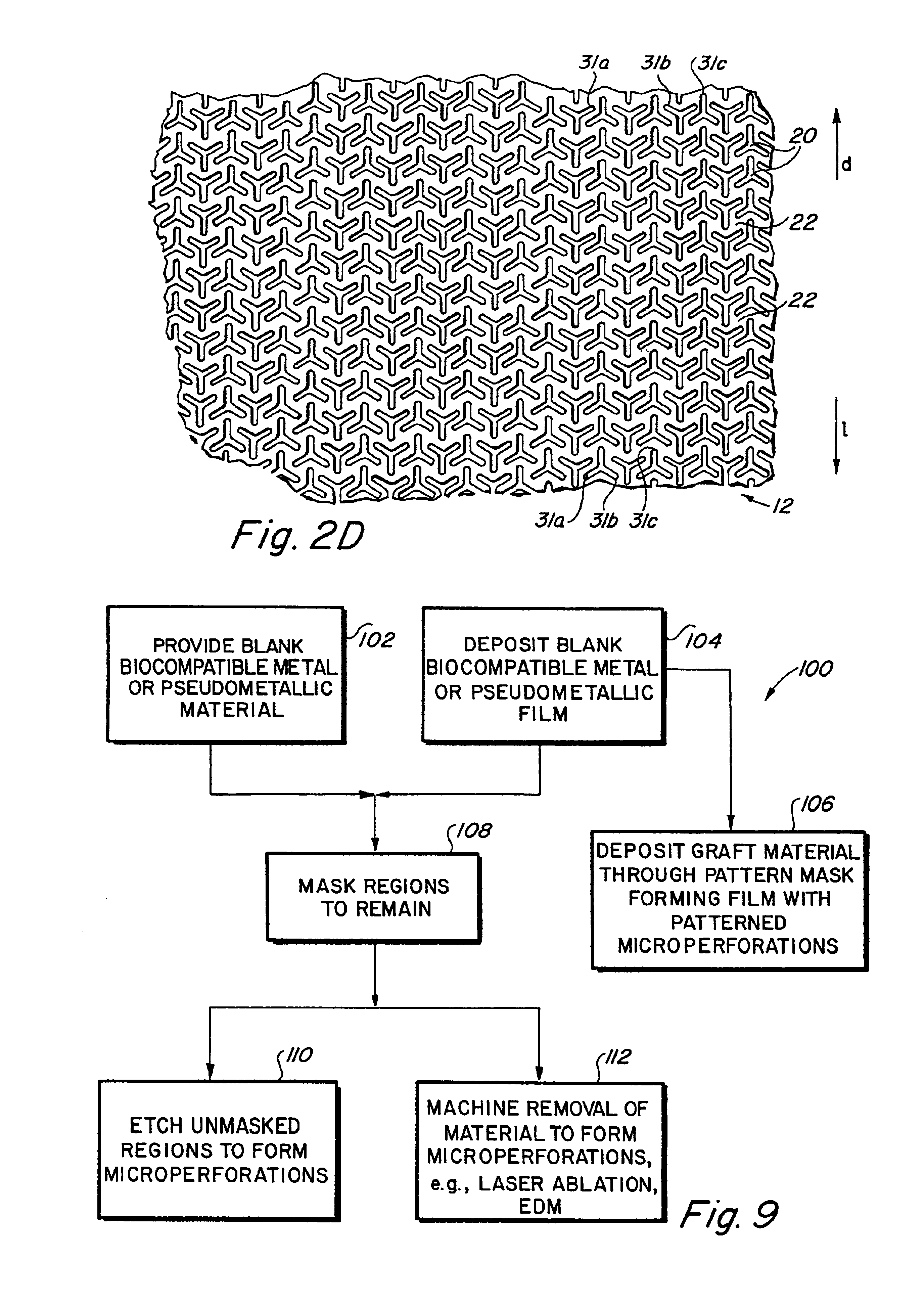
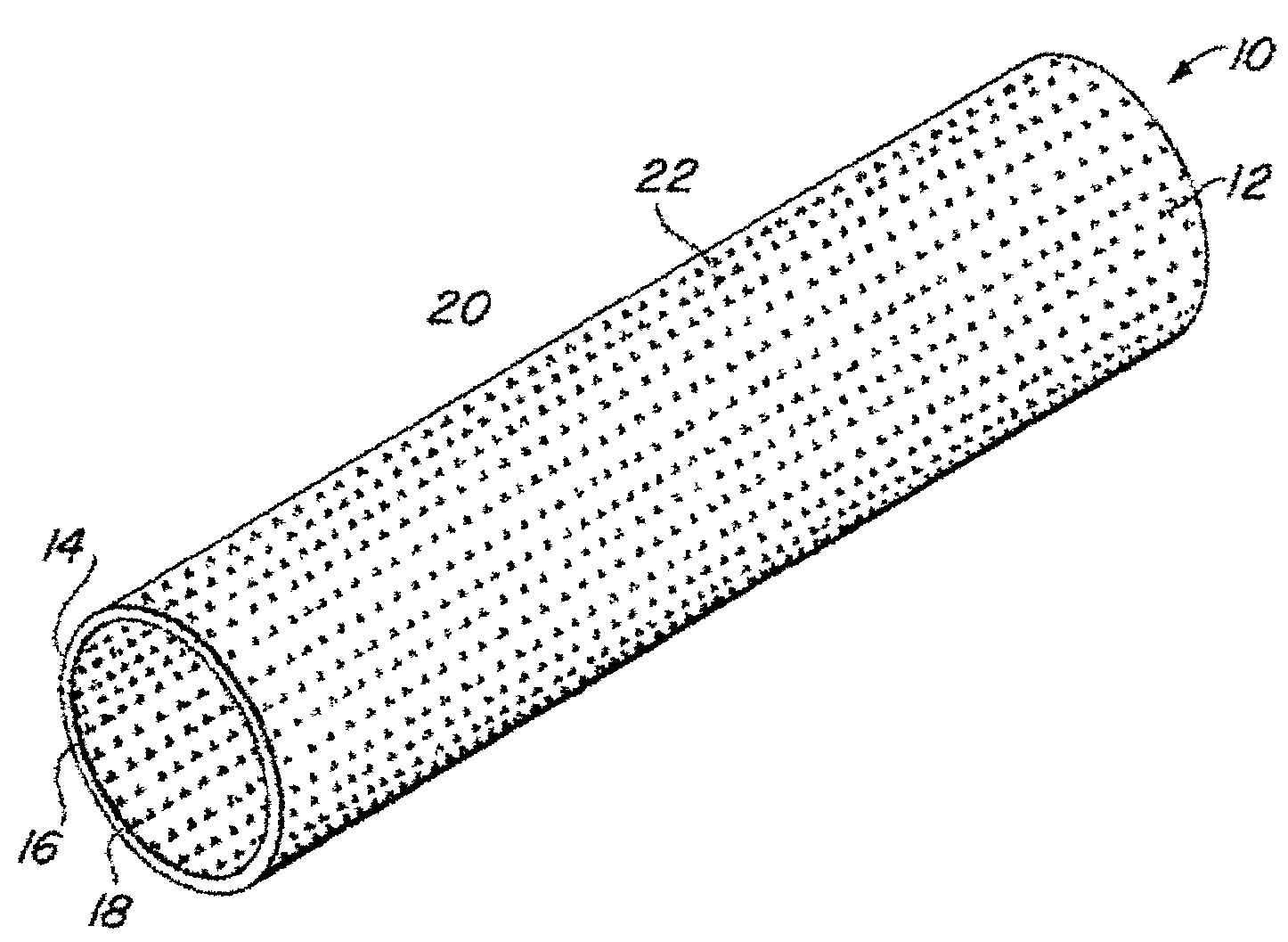
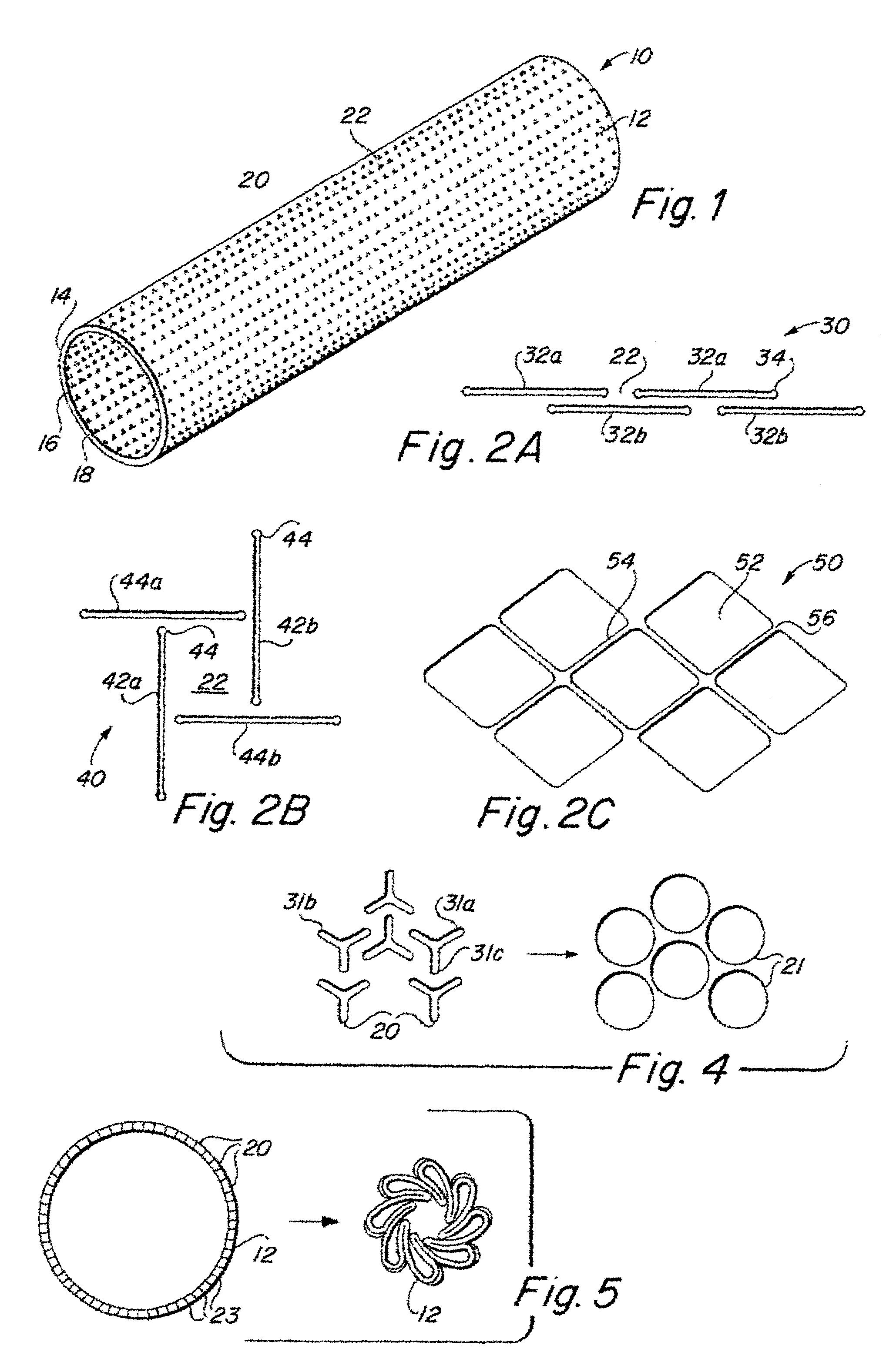
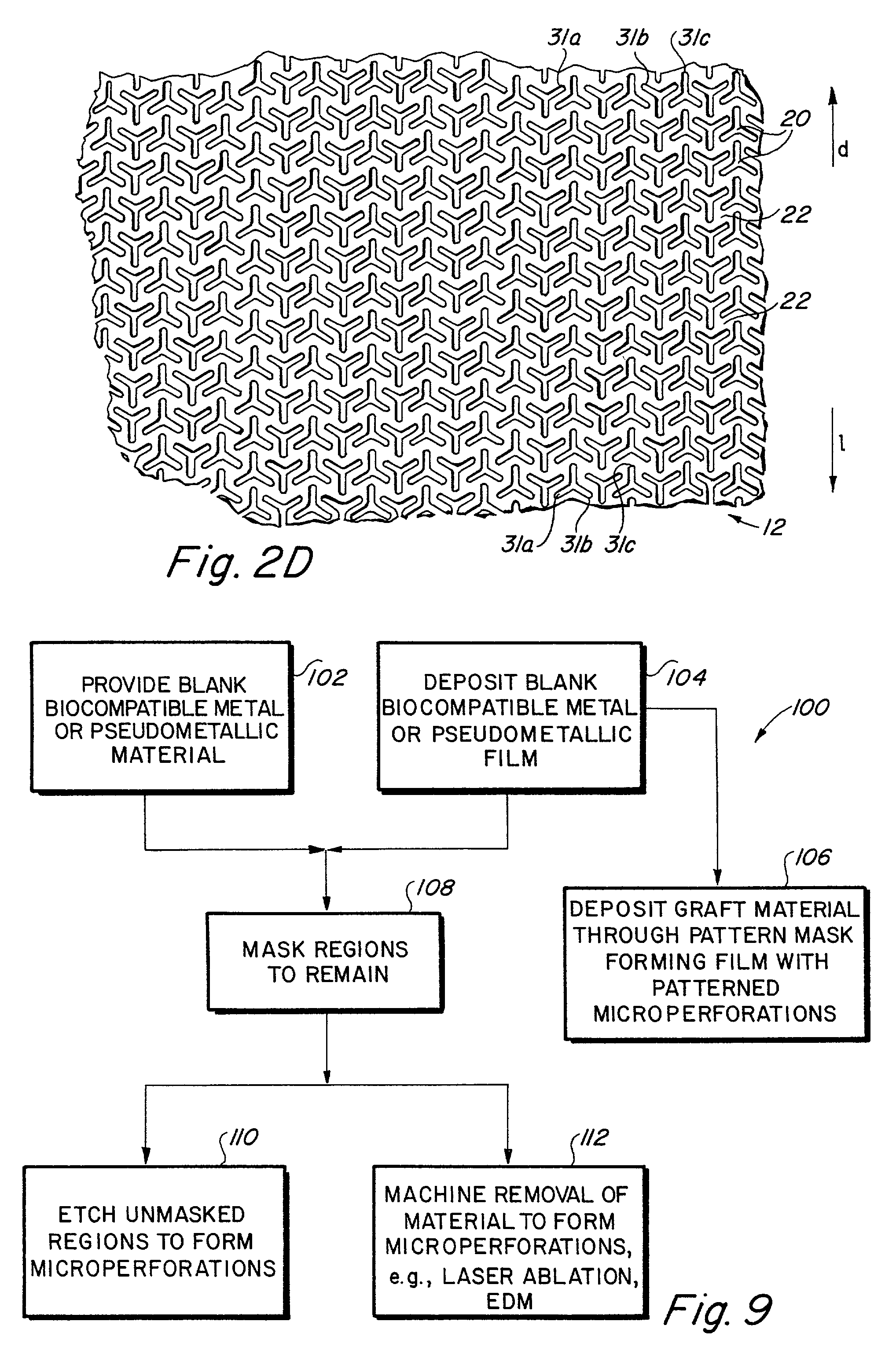
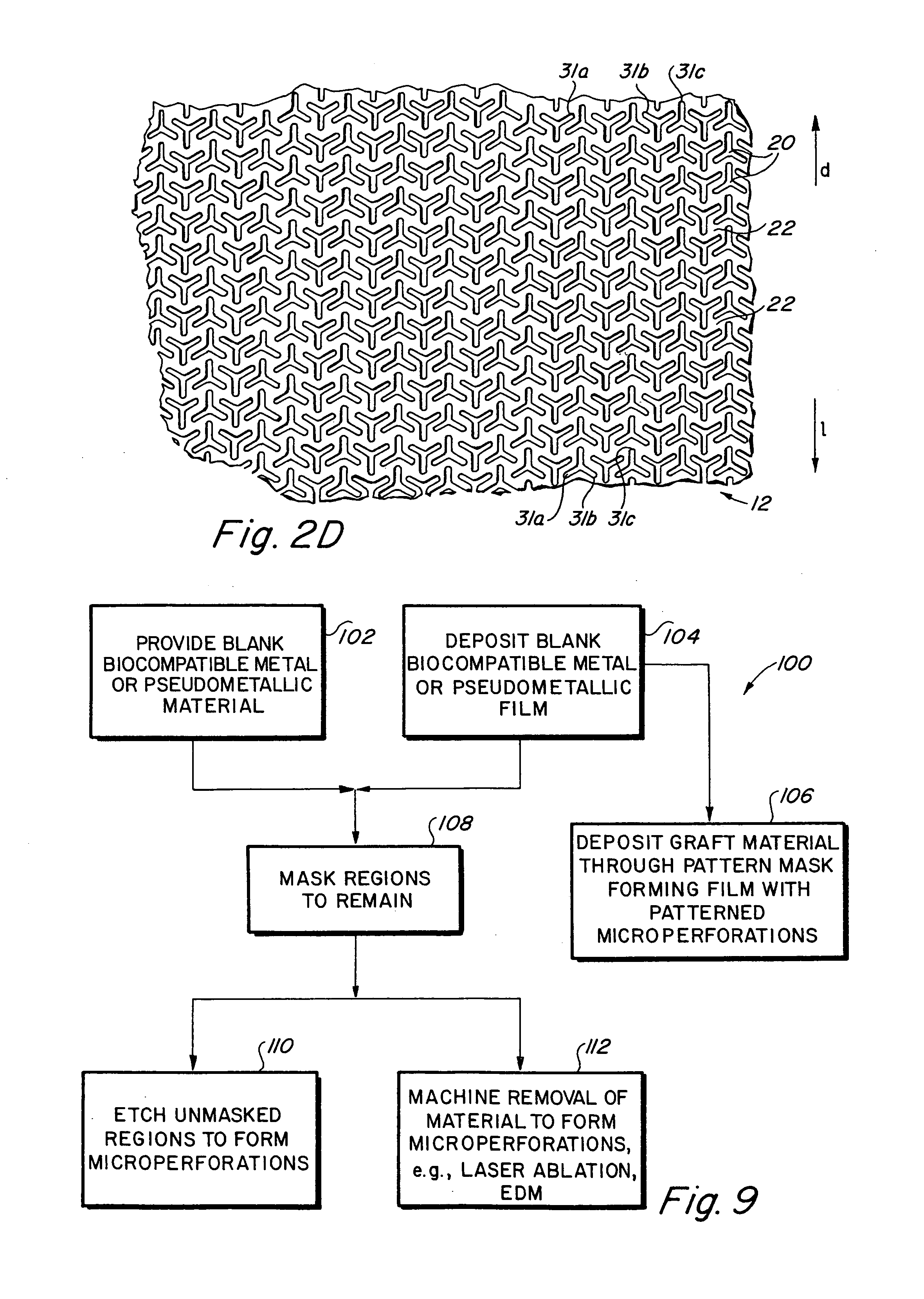
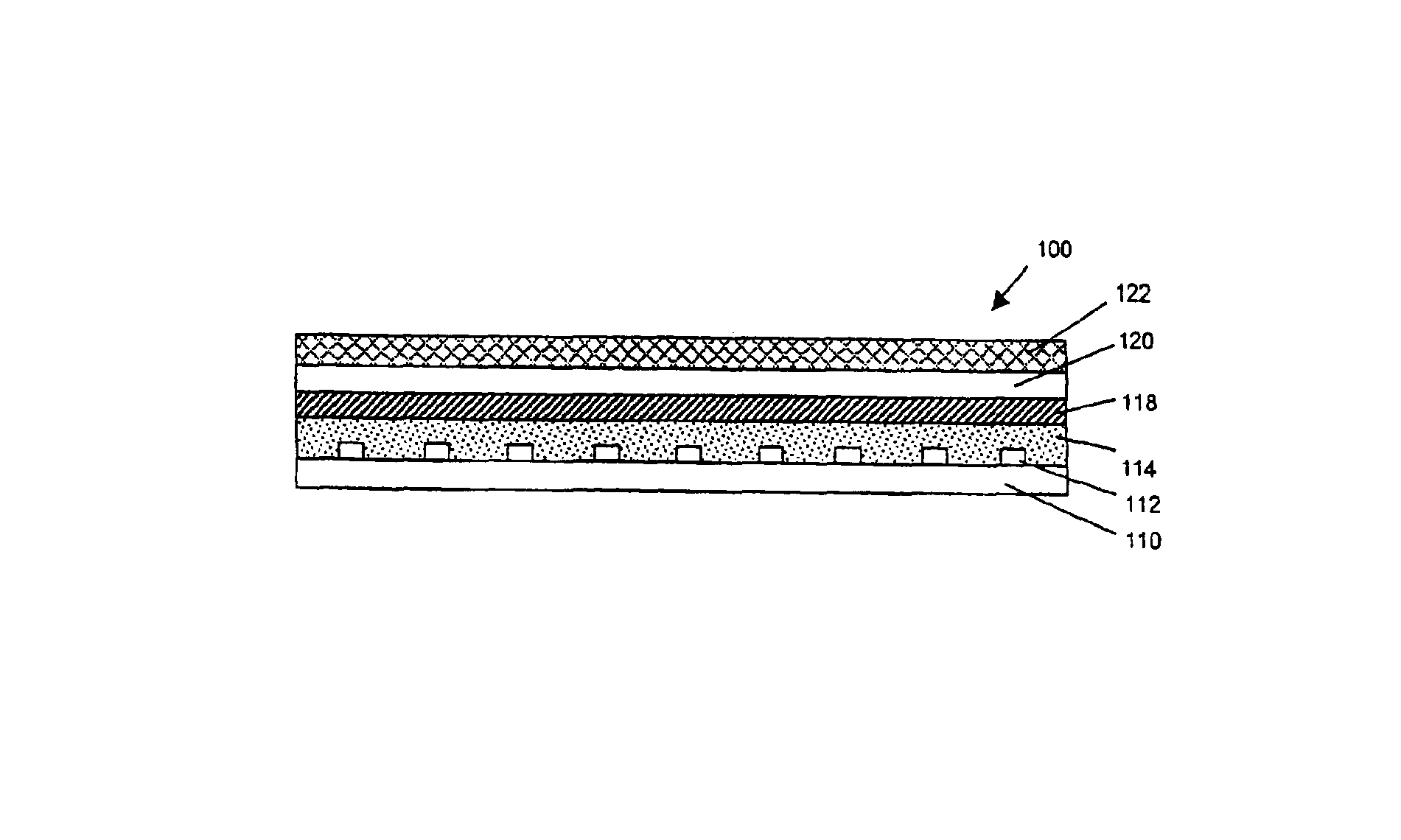
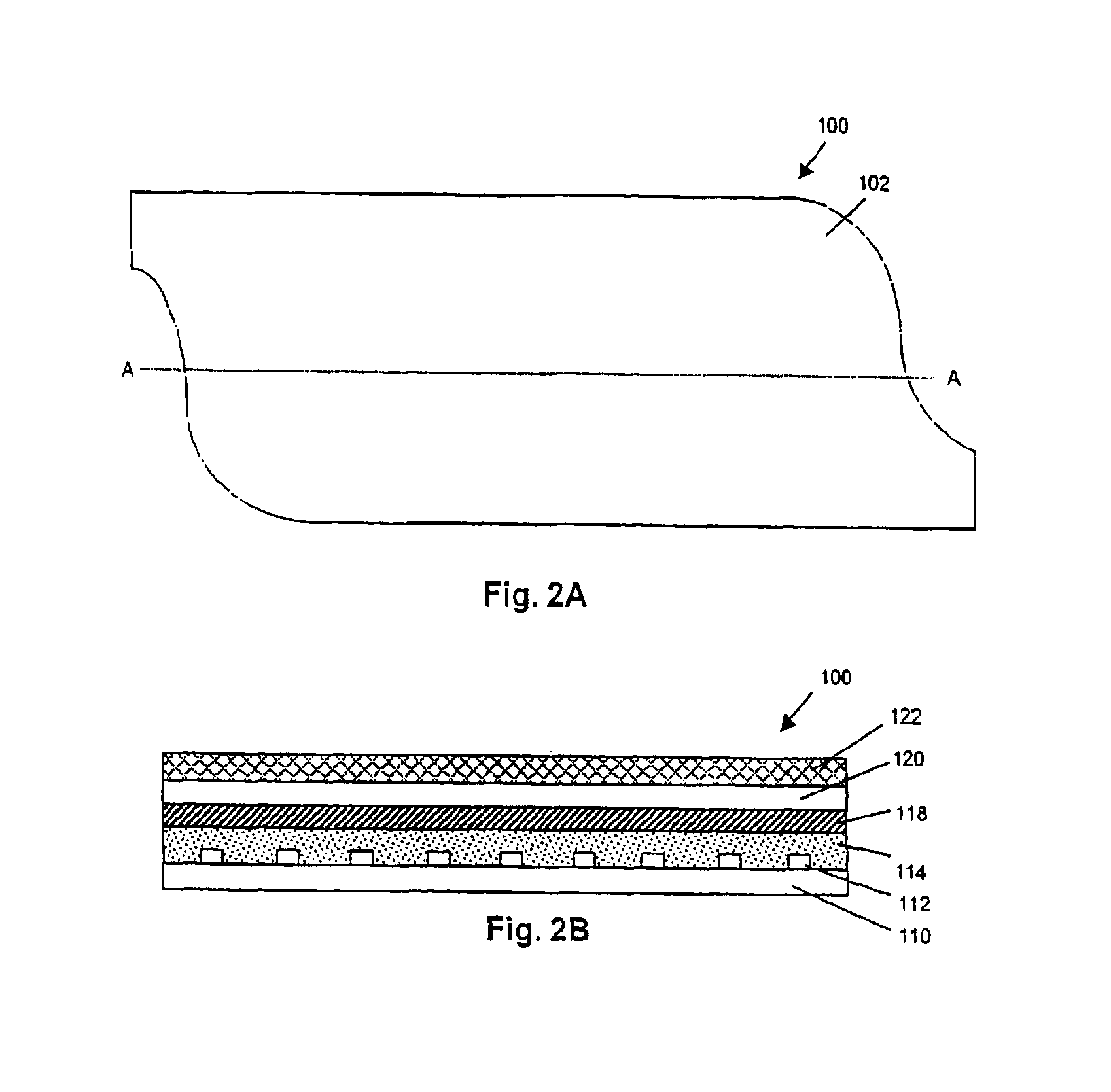
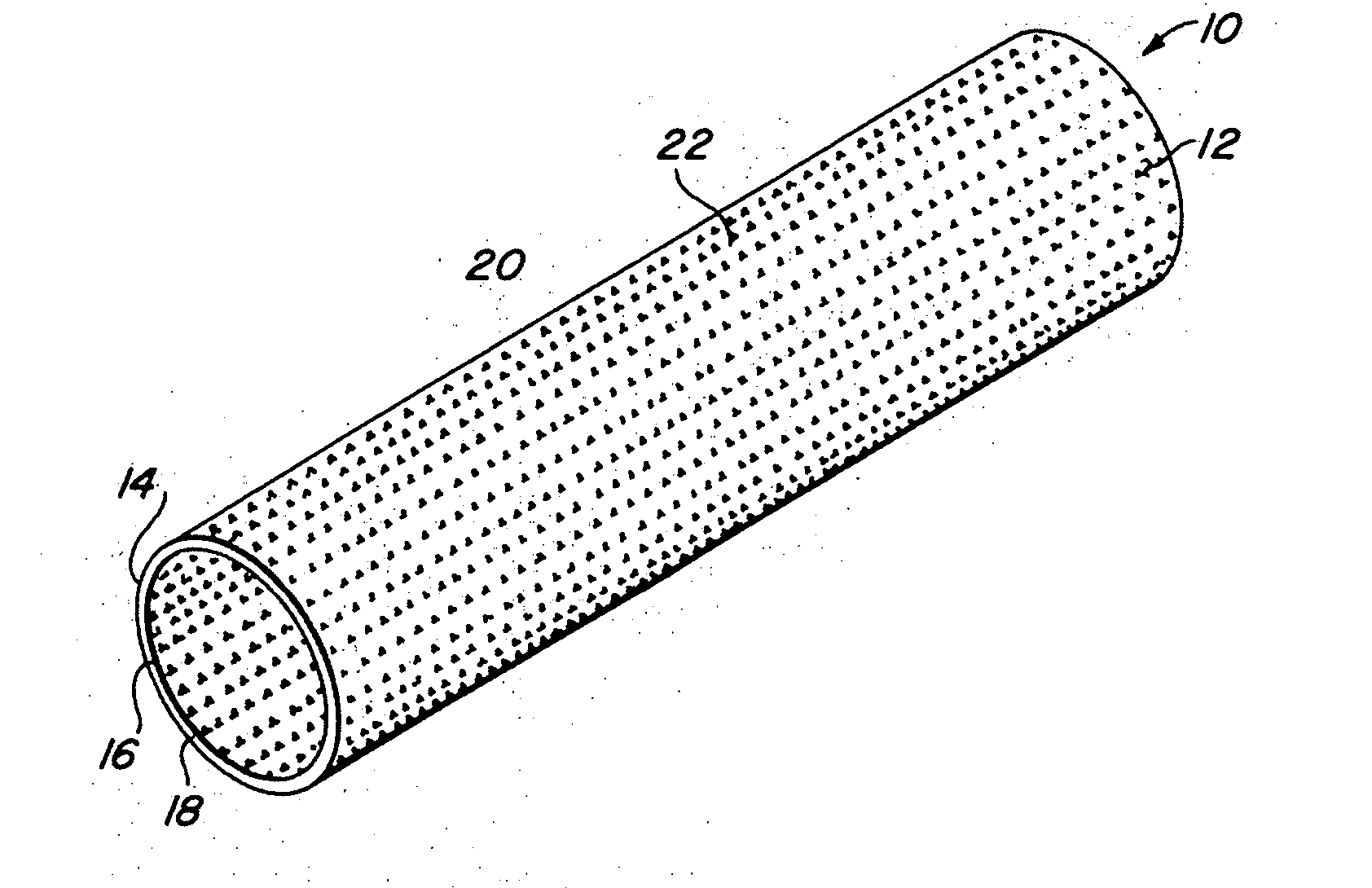
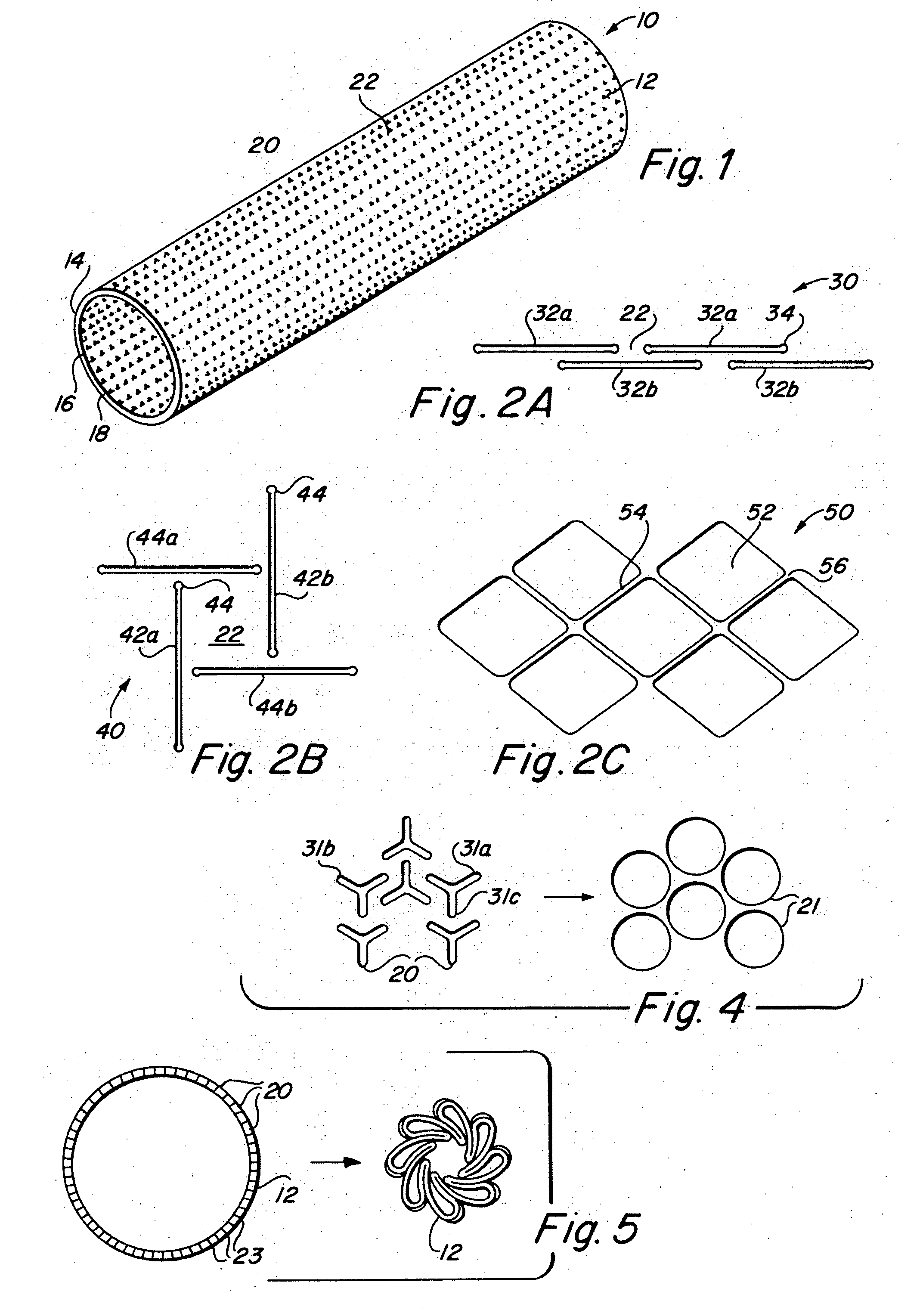
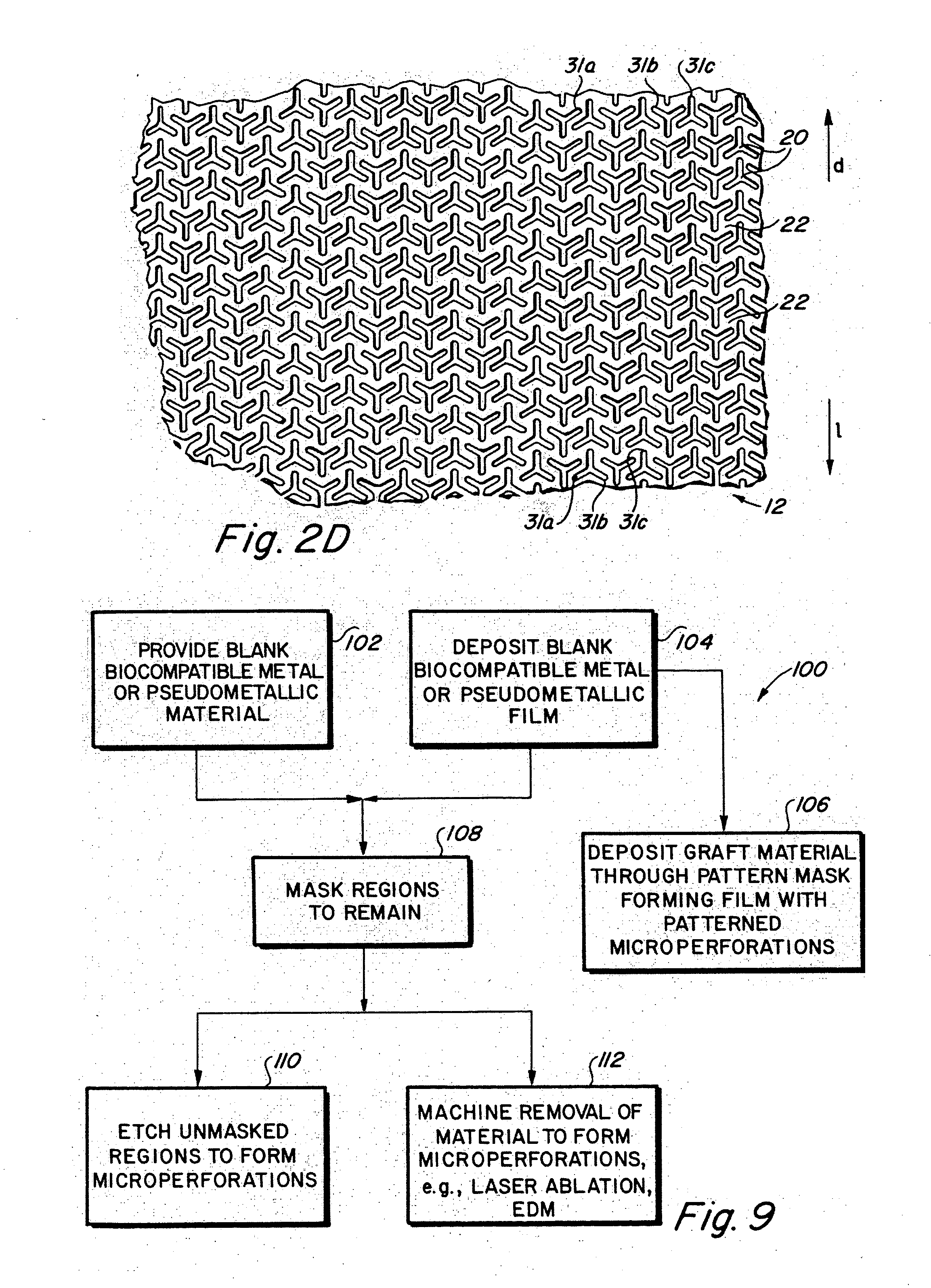
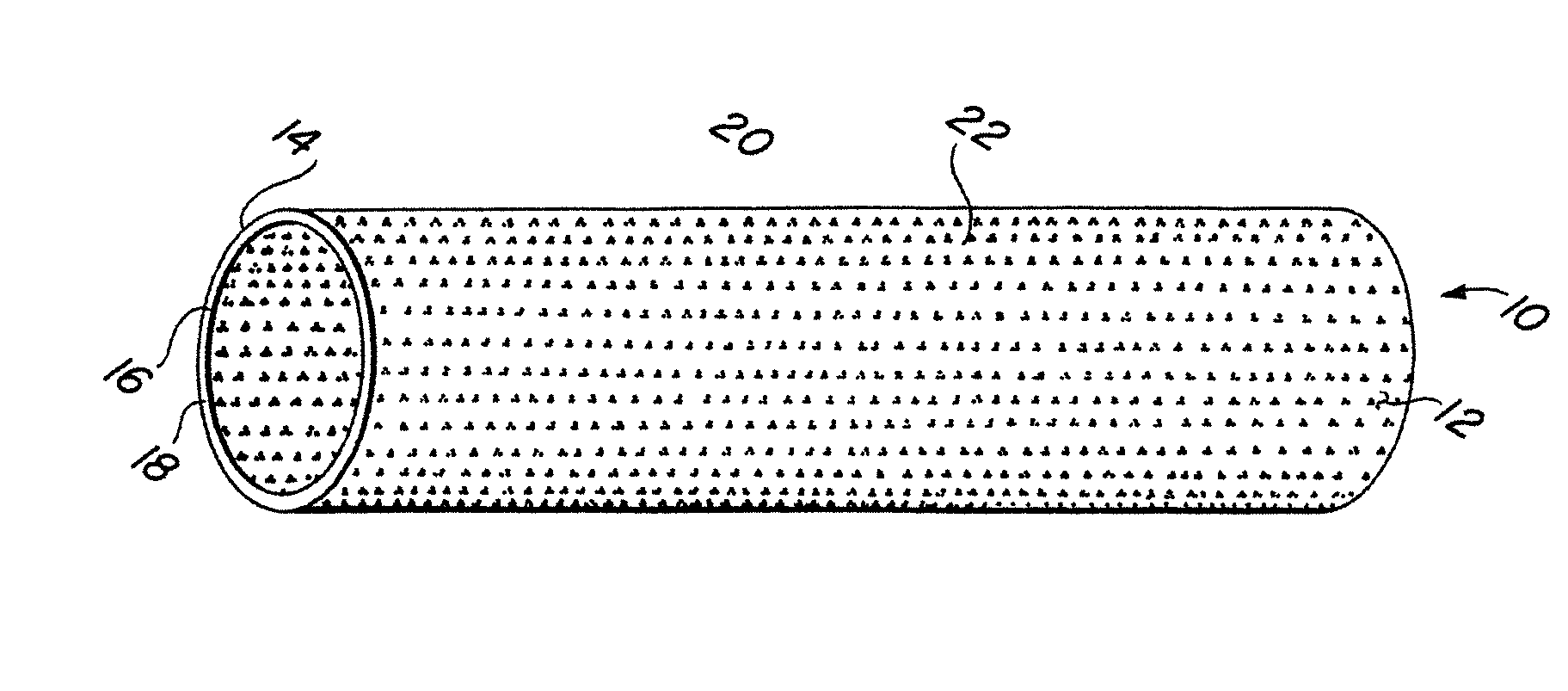
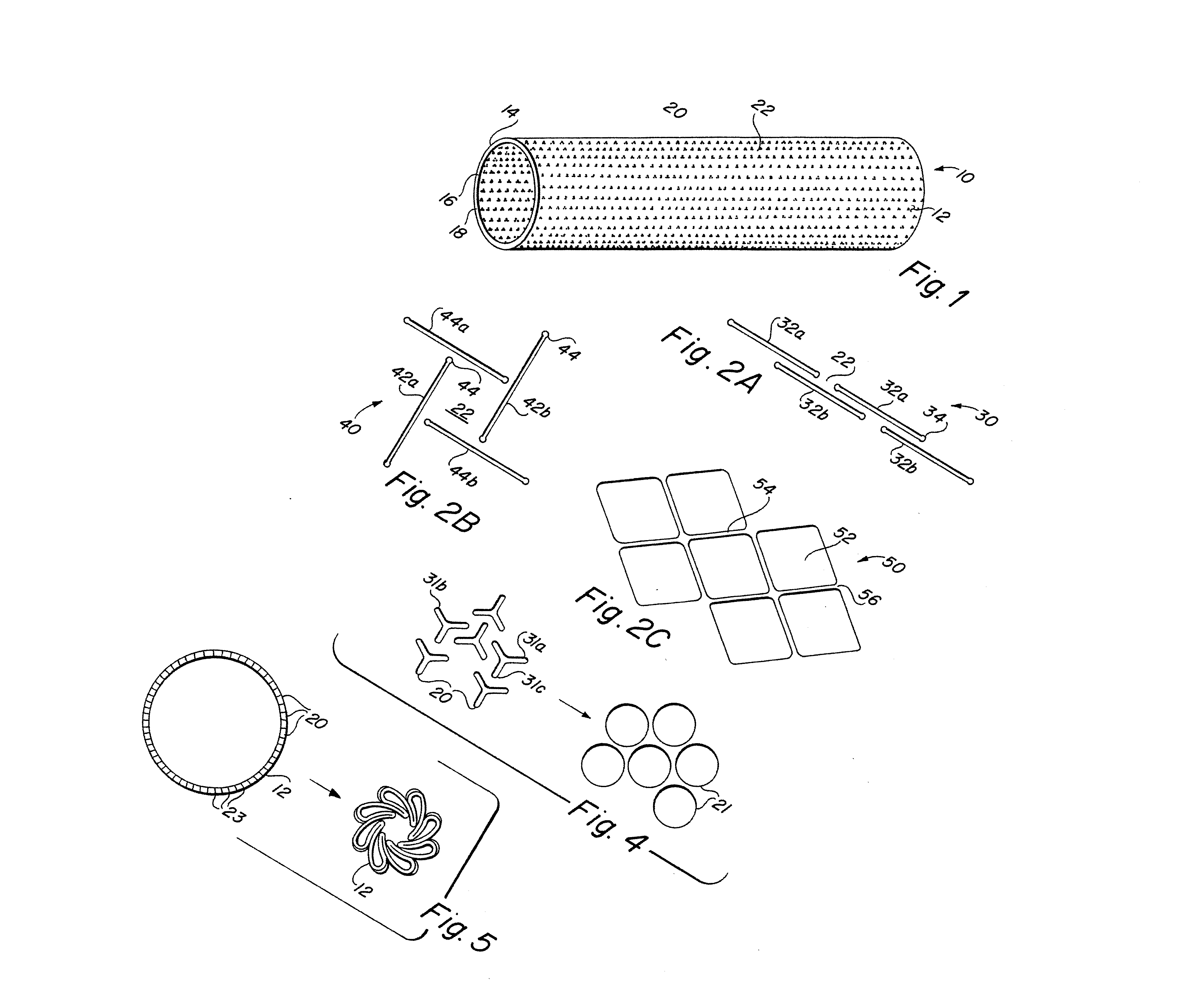
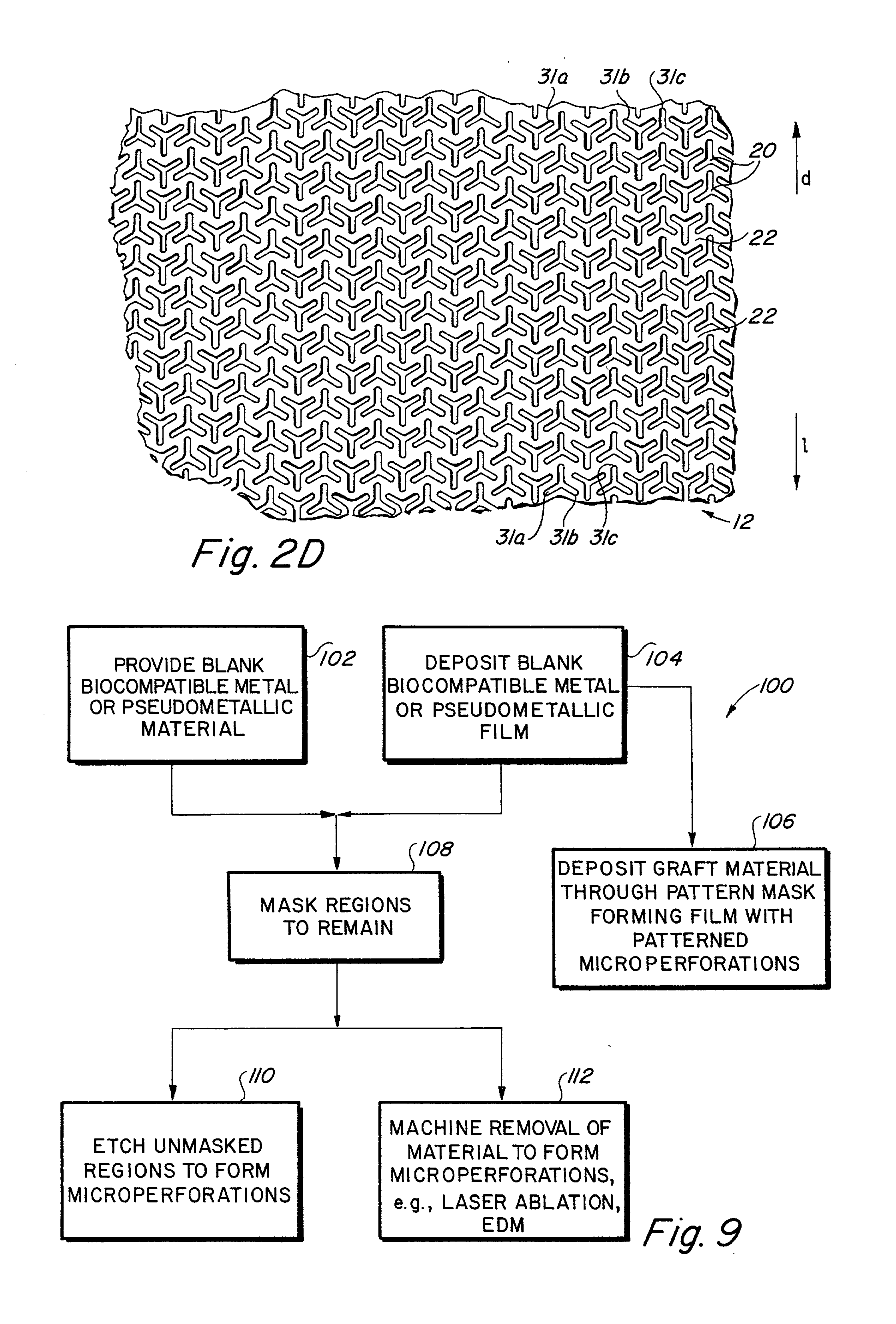
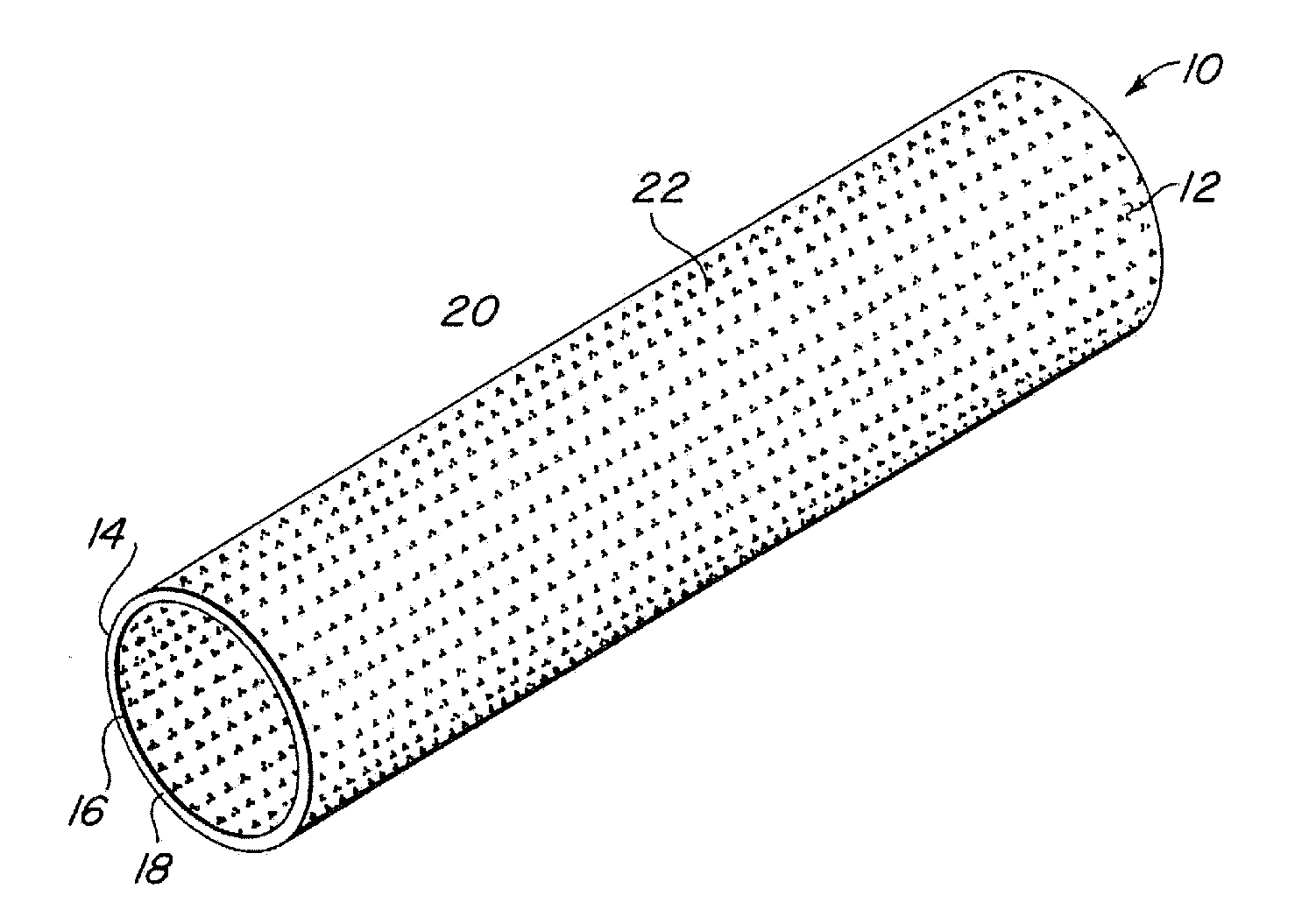
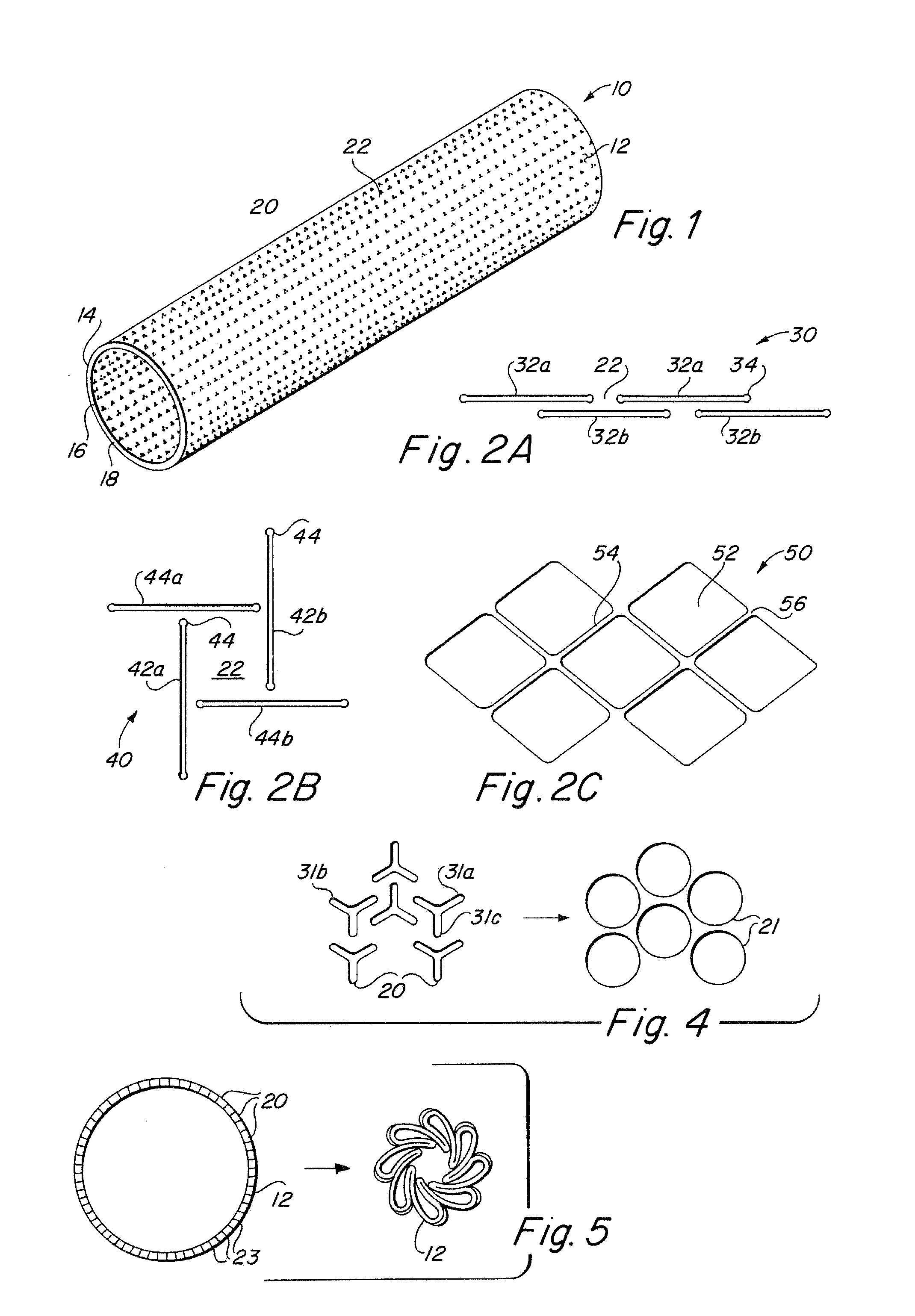
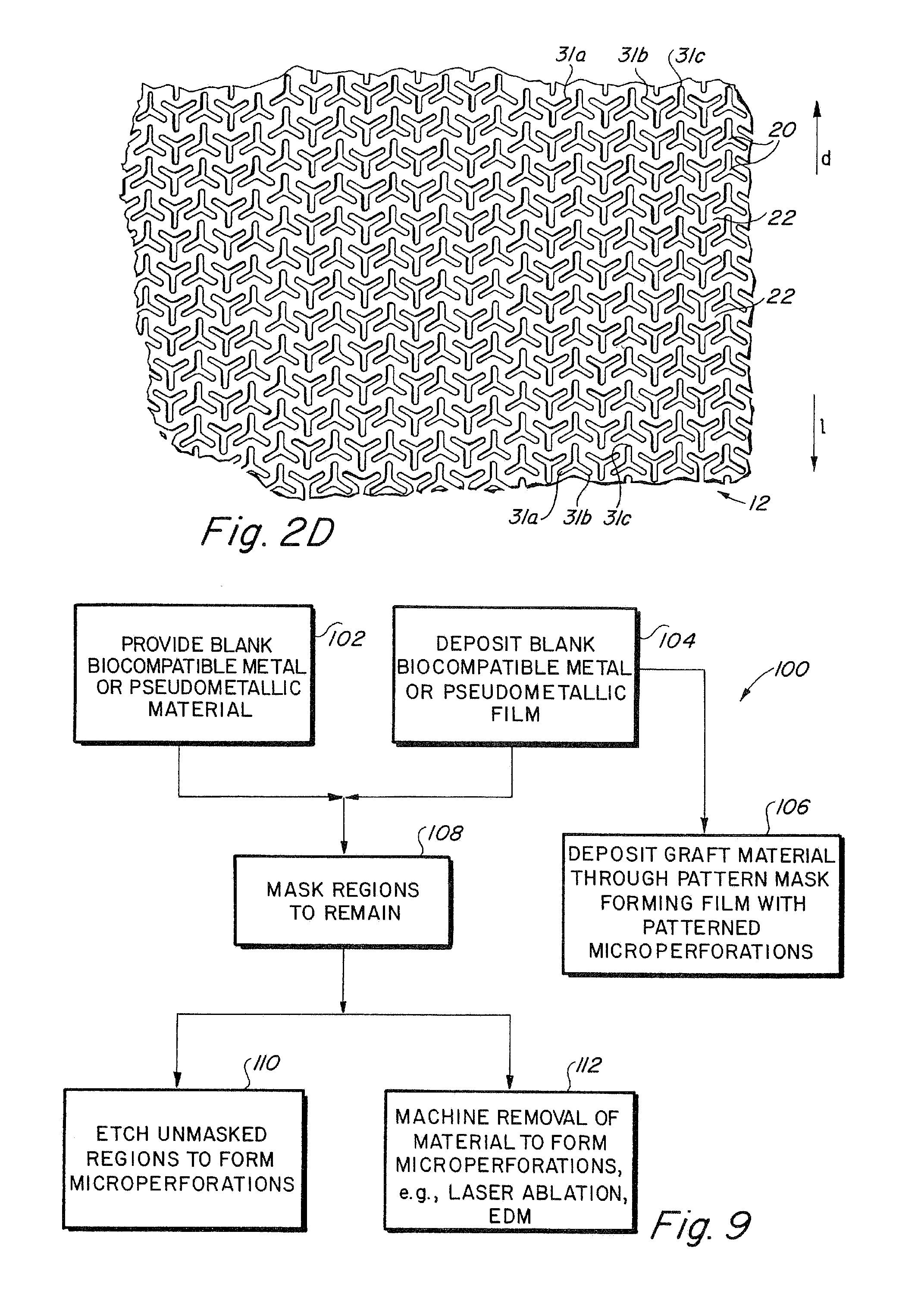
Implantable medical grafts fabricated of metallic or pseudometallic films of biocompatible materials having a plurality of microperforations passing through the film in a pattern that imparts fabric-like qualities to the graft or permits the geometric deformation of the graft. The implantable graft is preferably fabricated by vacuum deposition of metallic and / or pseudometallic materials into either single or multi-layered structures with the plurality of microperforations either being formed during deposition or after deposition by selective removal of sections of the deposited film. The implantable medical grafts are suitable for use as endoluminal or surgical grafts and may be used as vascular grafts, stent-grafts, skin grafts, shunts, bone grafts, surgical patches, non-vascular conduits, valvular leaflets, filters, occlusion membranes, artificial sphincters, tendons and ligaments.
Owner:VACTRONIX SCI LLC
Self-supporting metallic implantable grafts, compliant implantable medical devices and methods of making same
Implantable medical grafts fabricated of metallic or pseudometallic films of biocompatible materials having a plurality of microperforations passing through the film in a pattern that imparts fabric-like qualities to the graft or permits the geometric deformation of the graft. The implantable graft is preferably fabricated by vacuum deposition of metallic and / or pseudometallic materials into either single or multi-layered structures with the plurality of microperforations either being formed during deposition or after deposition by selective removal of sections of the deposited film. The implantable medical grafts are suitable for use as endoluminal or surgical grafts and may be used as vascular grafts, stent-grafts, skin grafts, shunts, bone grafts, surgical patches, non-vascular conduits, valvular leaflets, filters, occlusion membranes, artificial sphincters, tendons and ligaments.
Owner:VACTRONIX SCI LLC
Thermodynamically driven reversible infuser pump for use as a remotely controlled gastric band
InactiveUS7351240B2Small volumeAvoid changeAnti-incontinence devicesFlexible member pumpsEngineeringPiezo electric
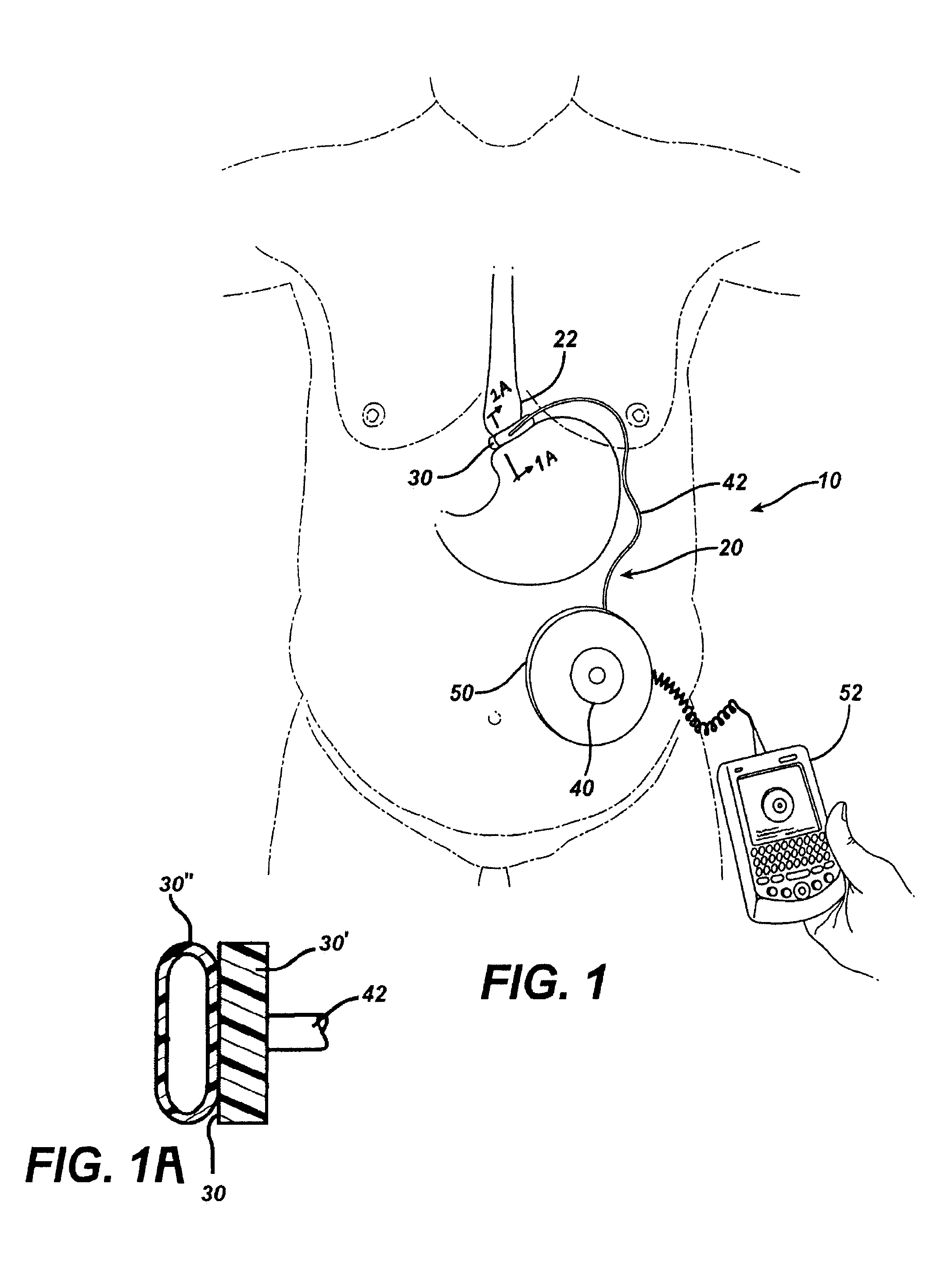
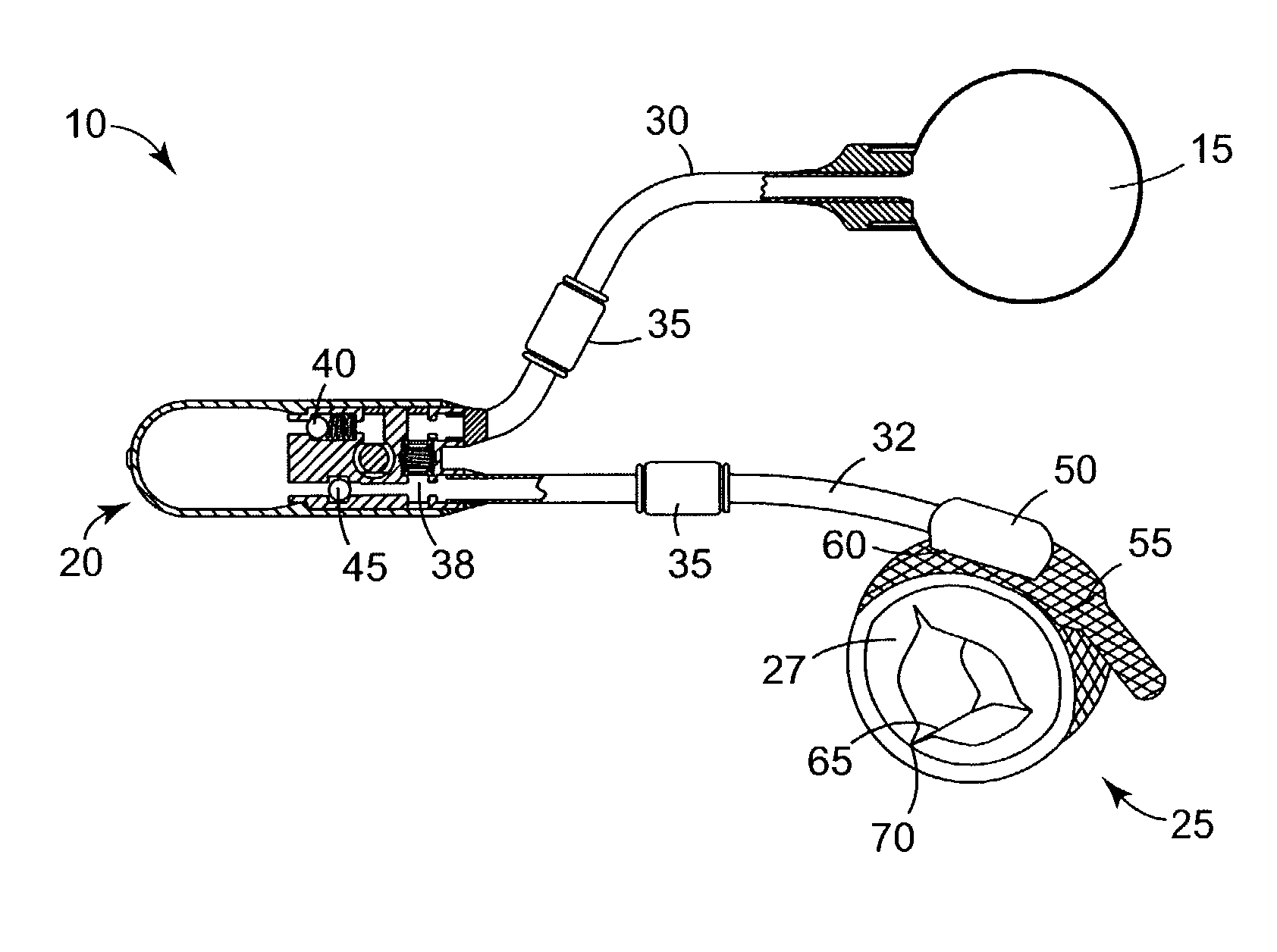
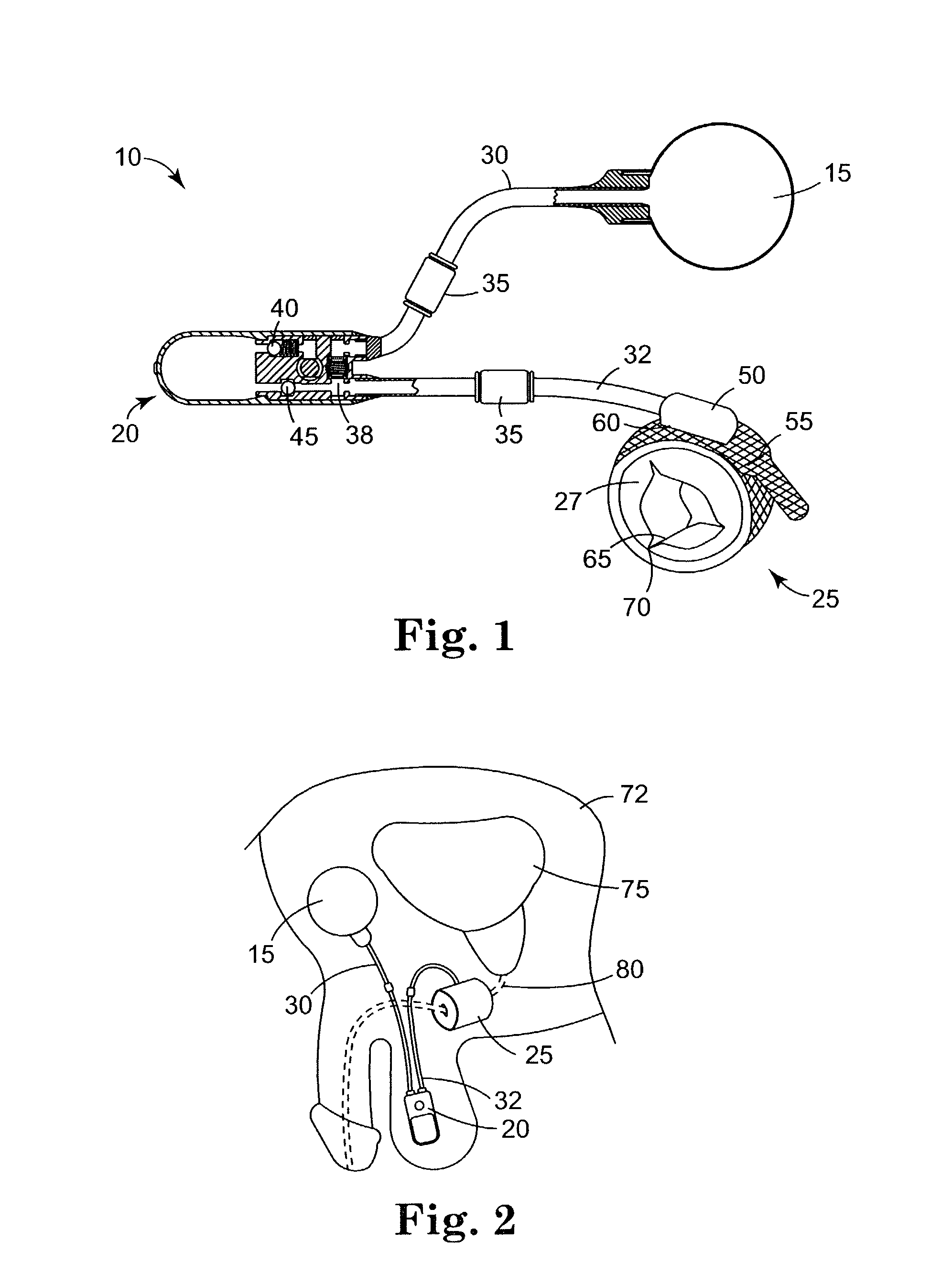
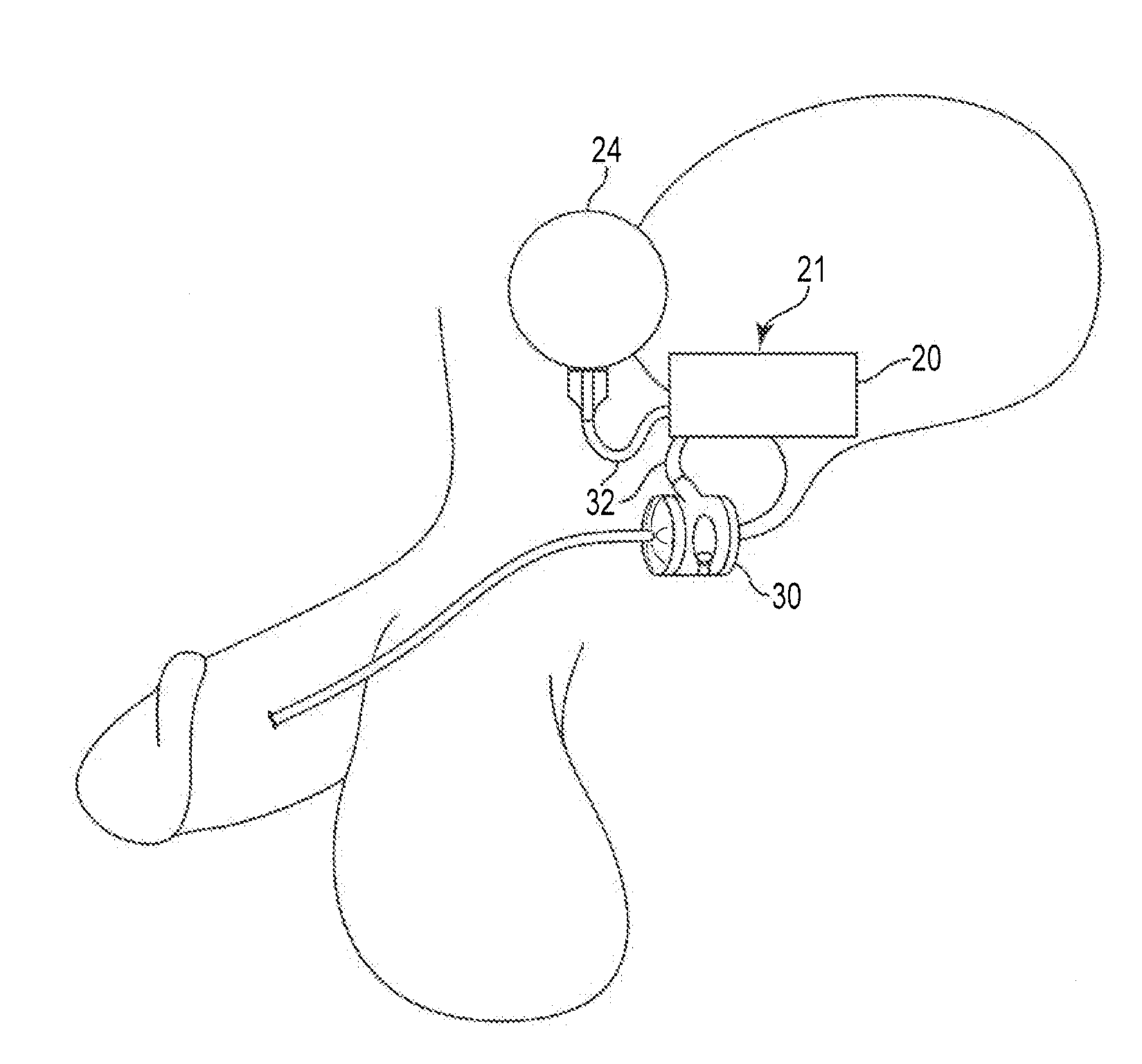
An implantable artificial sphincter system provides long-term adjustment via transcutaneous energy transfer (TET), minimizing invasive adjustment through adding or removing fluid via a syringe. An infuser device provides bi-directional fluid transfer via a flexible conduit to a sphincter band, such as a gastric band, by a combination of thermodynamic actuation and a piezo-electrically disengaged drum brake assembly that thereby achieves a desirable small volume device. A propellant within a propellant cavity surrounds a metal bellows accumulator biased at body temperature to either expand or collapse the bellows accumulator with the opposite direction of movement effected by a thermal element that heats in combination with a negatively-biased propellant or cools in combination with a positively-biased propellant. A drum brake assembly locks the metal bellows accumulator in place between adjustments by thermodynamic actuation by activating piezo-electric stack actuators that disengage calipers from a brake drum attached to the bellows accumulator.
Owner:ETHICON ENDO SURGERY INC
Bi-directional infuser pump with volume braking for hydraulically controlling an adjustable gastric band
InactiveUS7374565B2Increase varietyAvoid changeAnti-incontinence devicesPharmaceutical delivery mechanismPiezo electricEngineering
An implantable artificial sphincter system provides long-term adjustment via transcutaneous energy transfer (TET), minimizing invasive adjustment through adding or removing fluid via a syringe. An infuser device provides bi-directional fluid transfer via a flexible conduit to a sphincter band, such as a gastric band, by a combination of thermodynamic actuation and a piezo-electrically disengaged drum brake assembly that thereby achieves a desirable small volume device. A propellant within a propellant cavity surrounds a metal bellows accumulator biased at body temperature to either expand or collapse the bellows accumulator with the opposite direction of movement effected by a thermal element that heats in combination with a negatively-biased propellant or cools in combination with a positively-biased propellant. A drum brake assembly locks the metal bellows accumulator in place between adjustments by thermodynamic actuation by activating piezo-electric stack actuators that disengage calipers from a brake drum attached to the bellows accumulator.
Owner:ETHICON ENDO SURGERY INC
Compliant implantable medical devices and methods of making same
Owner:VACTRONIX SCI LLC
Electroactive polymer based artificial sphincters and artificial muscle patches
InactiveUS6921360B2Lower esophageal sphincter dysfunctionAnti-incontinence devicesSurgeryLower esophagusElectroactive polymer actuators
Provided are artificial muscle patches, which are adapted to be implanted adjacent a patient's heart, and artificial sphincter cuffs, which are adapted to be implanted around a body lumen, such as the urethra, the anal canal, or the lower esophagus. The devices of the present invention comprise: (a) one or more electroactive polymer actuators; and (b) a control unit for electrically controlling the one or more electroactive polymer actuators to expand or contract the devices.
Owner:BOSTON SCI SCIMED INC
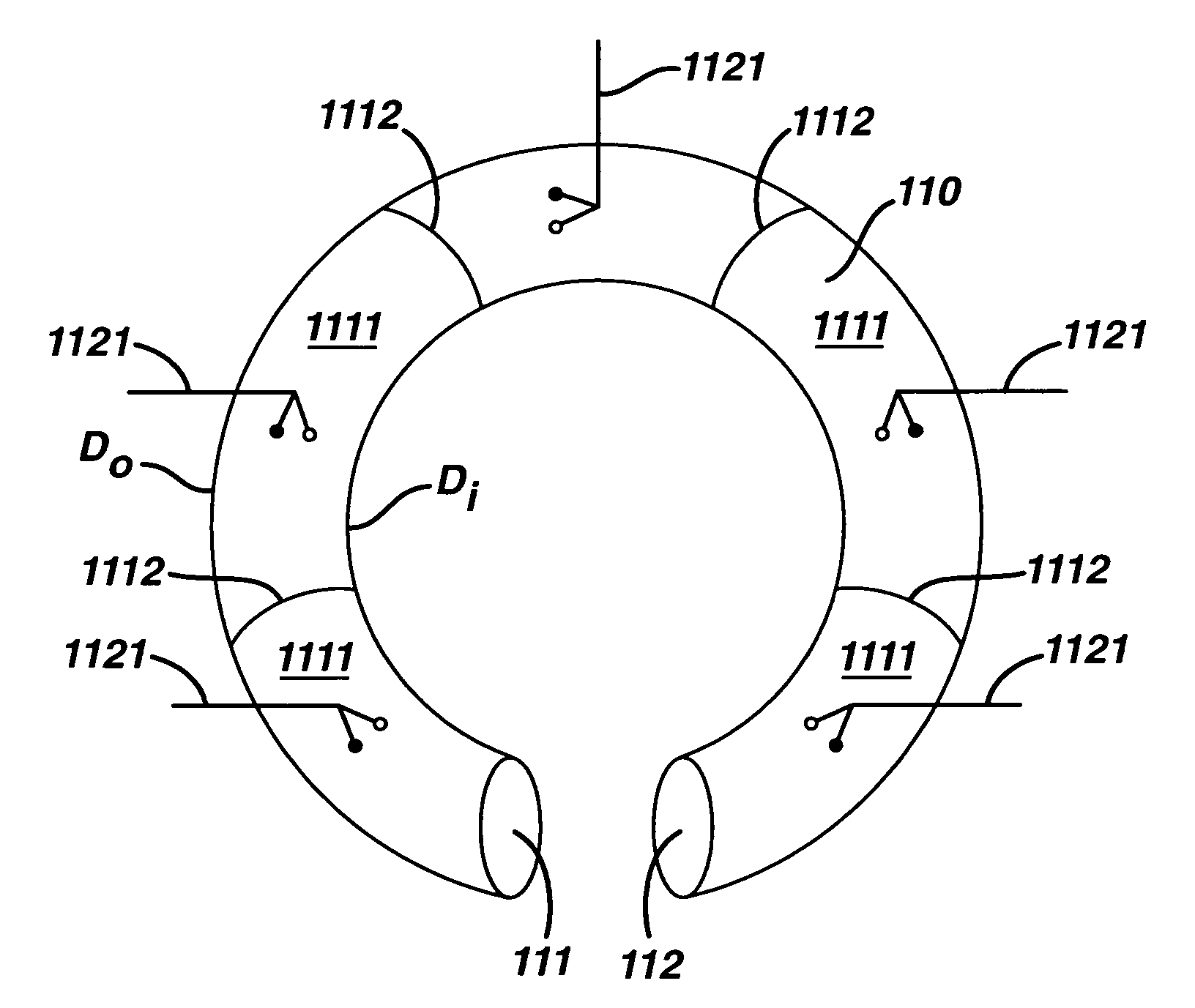
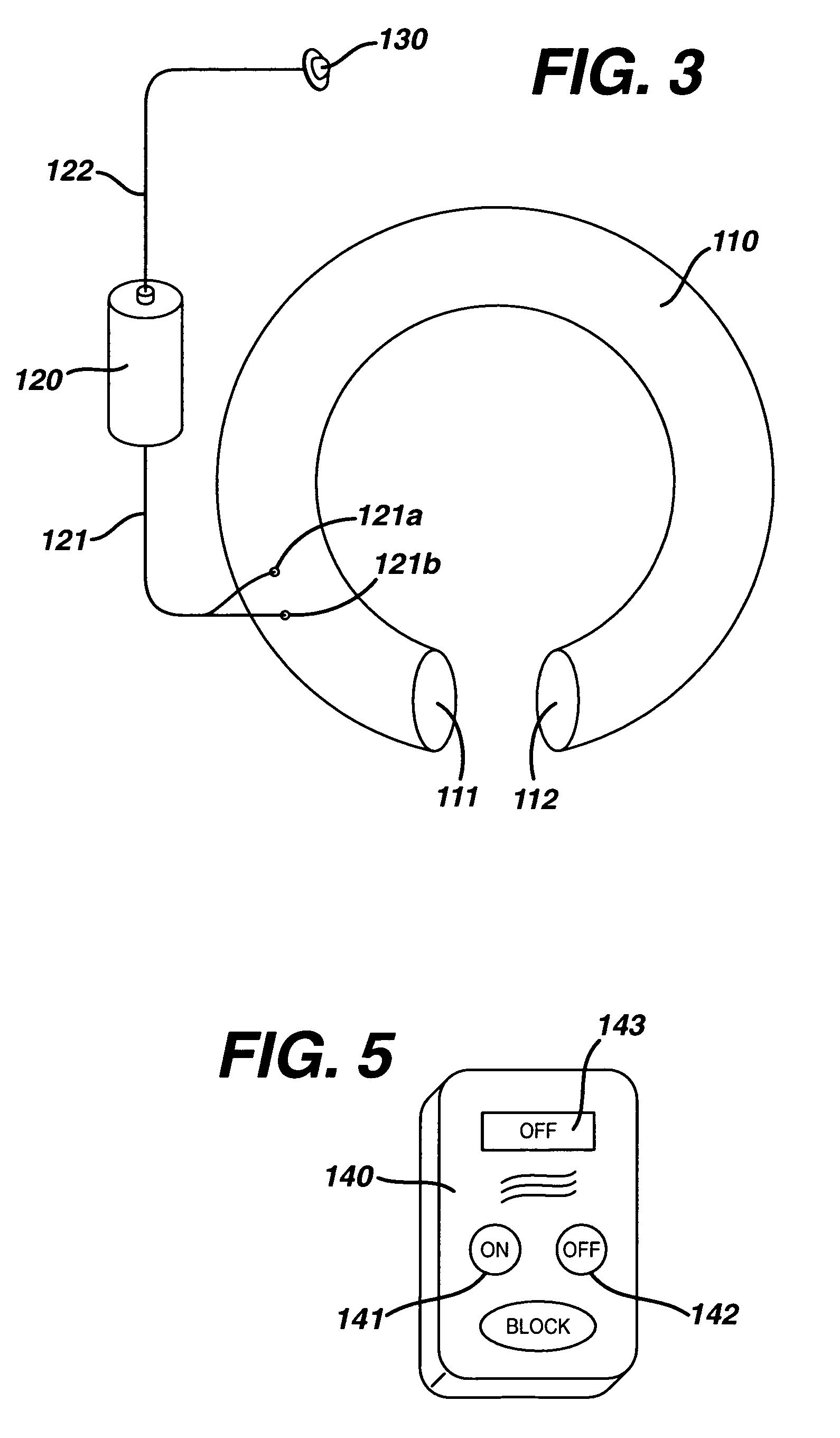
Magnetic resonance imaging (MRI) safe remotely adjustable artificial sphincter
An implantable artificial sphincter system provides long-term adjustment via transcutaneous energy transfer (TET), minimizing invasive adjustment through adding or removing fluid via a syringe. An infuser device provides bi-directional fluid transfer via a flexible conduit to a sphincter band, such as a gastric band. Materials are nonferrous and nonmagnetic so as to be magnetic resonance imaging (MRI) safe, being substantially immune to strong magnetic fields and not introducing an electromagnetic interference / compatibility (EMIC) hazard.
Owner:ETHICON ENDO SURGERY INC
Thermodynamically driven reversible infuser pump for use as a remotely controlled gastric band
InactiveUS20050277974A1Avoid inconvenienceSmallAnti-incontinence devicesFlexible member pumpsEngineeringPiezo electric
An implantable artificial sphincter system provides long-term adjustment via transcutaneous energy transfer (TET), minimizing invasive adjustment through adding or removing fluid via a syringe. An infuser device provides bi-directional fluid transfer via a flexible conduit to a sphincter band, such as a gastric band, by a combination of thermodynamic actuation and a piezo-electrically disengaged drum brake assembly that thereby achieves a desirable small volume device. A propellant within a propellant cavity surrounds a metal bellows accumulator biased at body temperature to either expand or collapse the bellows accumulator with the opposite direction of movement effected by a thermal element that heats in combination with a negatively-biased propellant or cools in combination with a positively-biased propellant. A drum brake assembly locks the metal bellows accumulator in place between adjustments by thermodynamic actuation by activating piezo-electric stack actuators that disengage calipers from a brake drum attached to the bellows accumulator.
Owner:ETHICON ENDO SURGERY INC
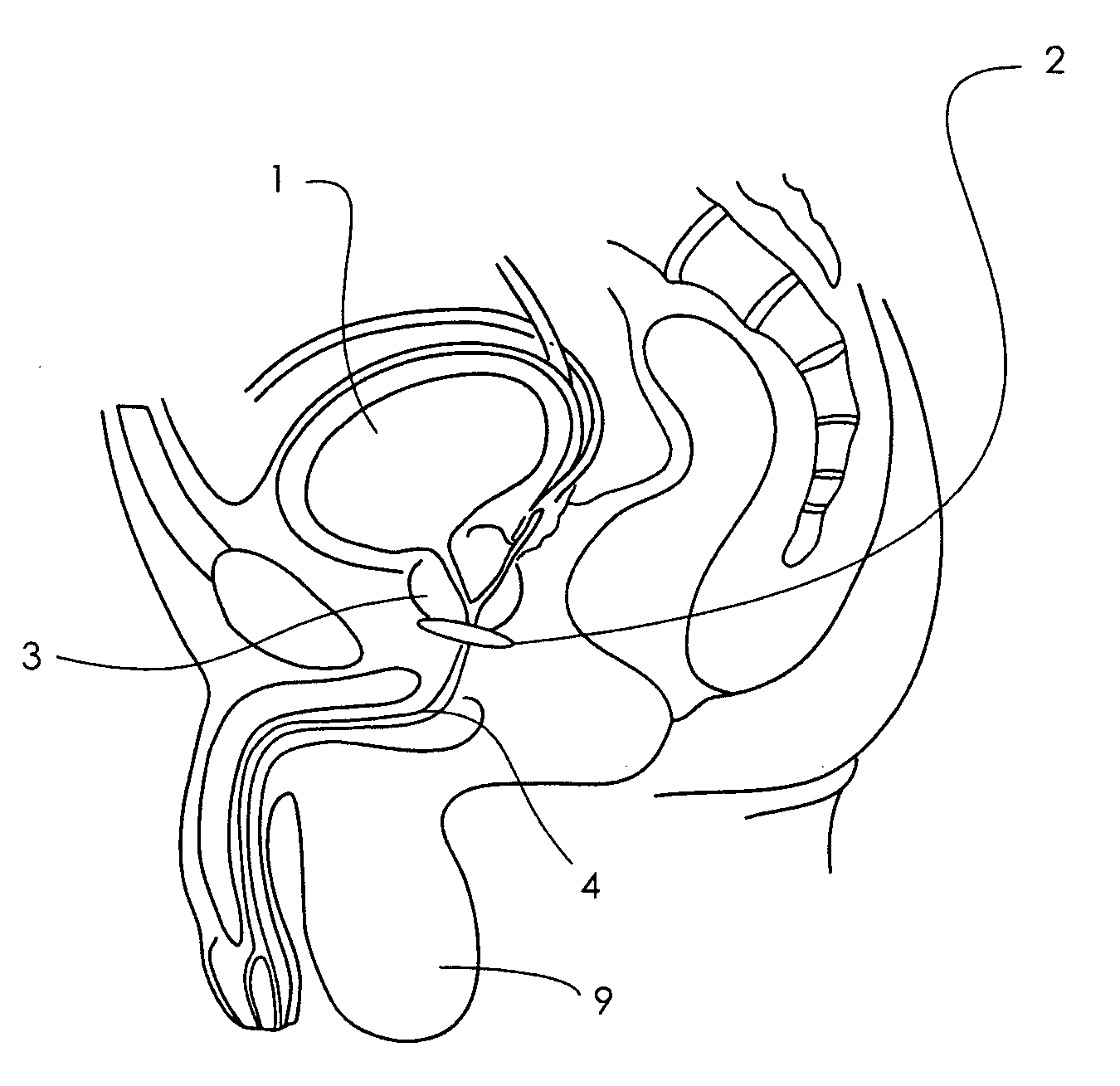
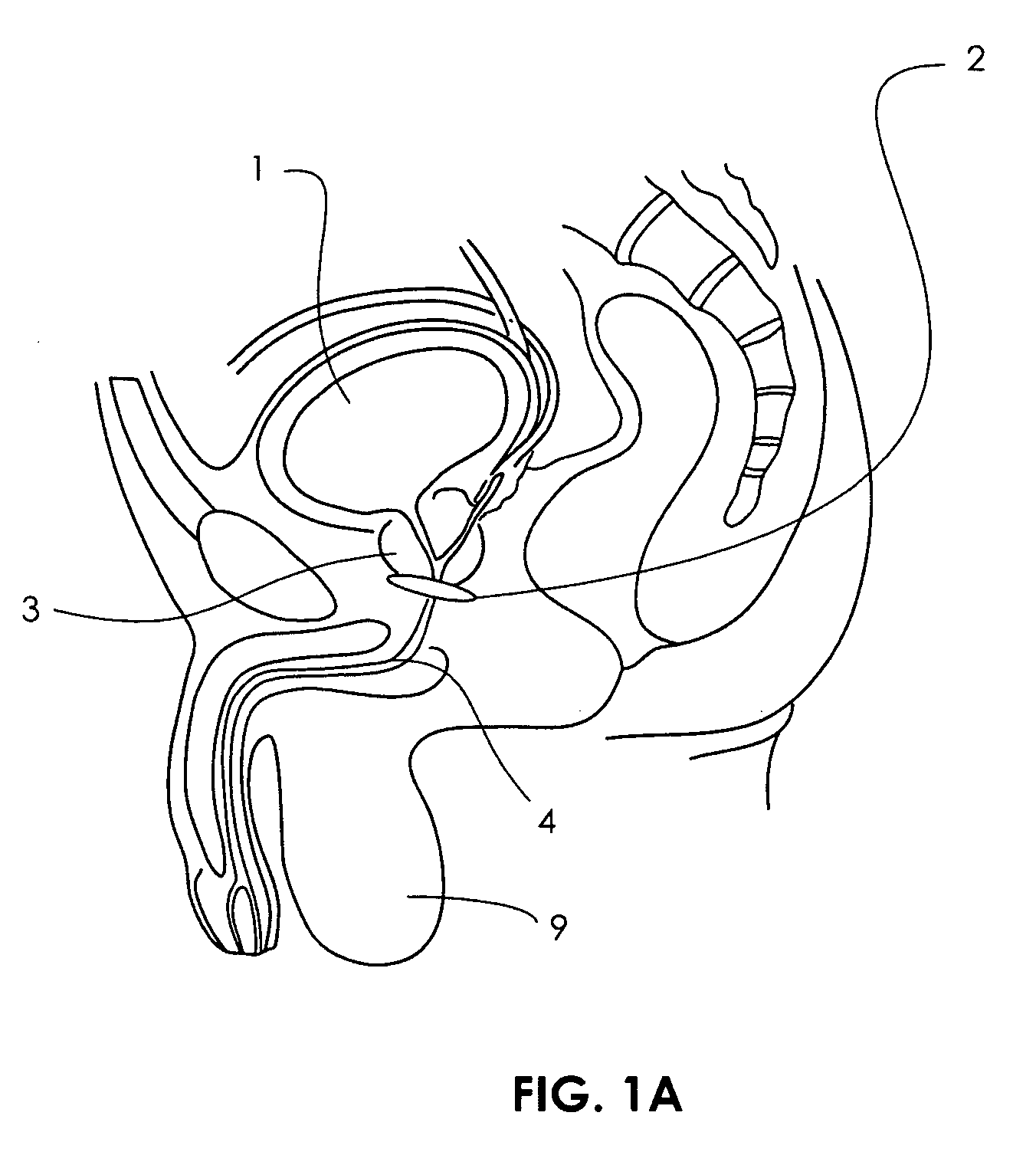
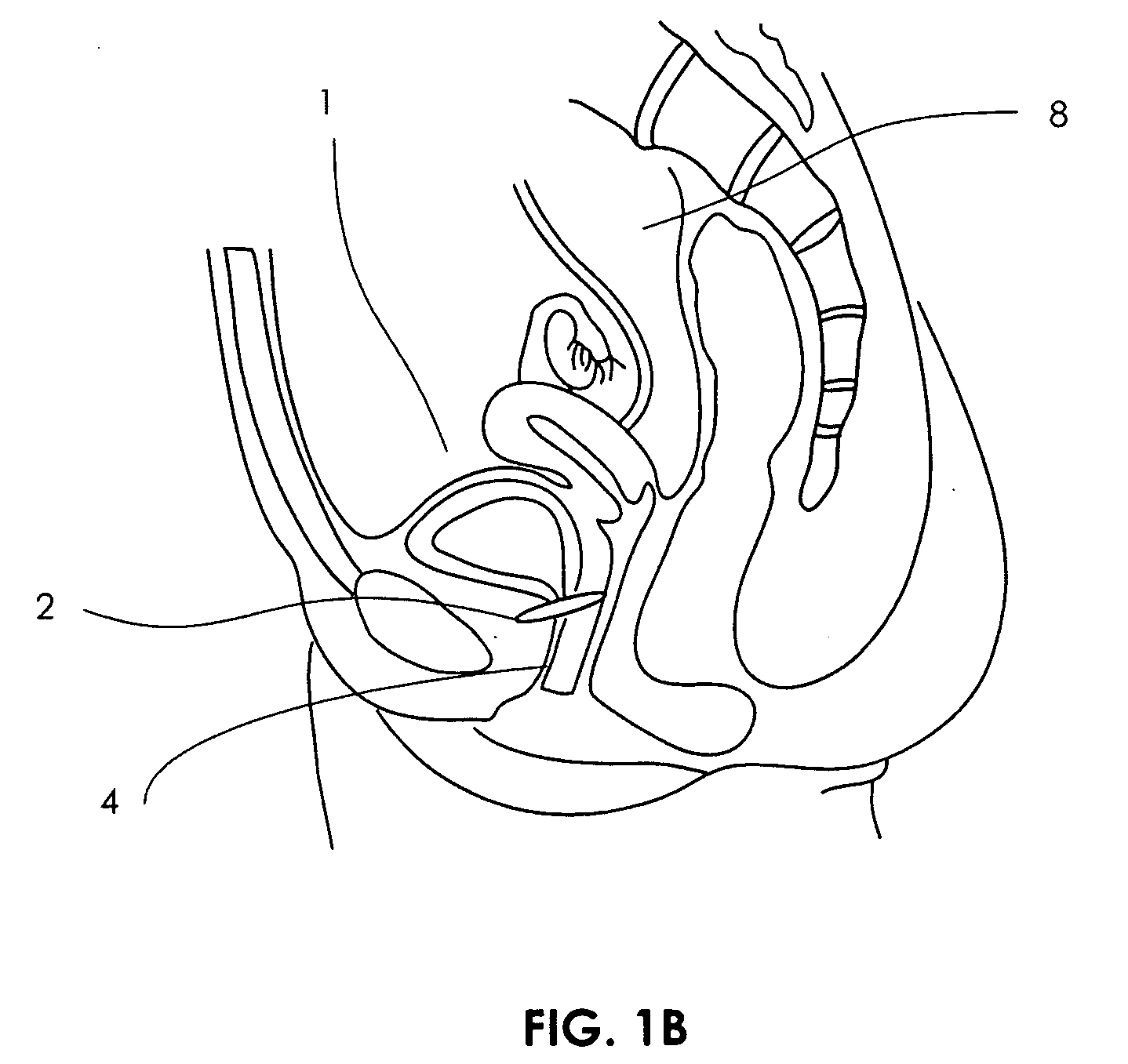
Artificial sphincter
A biologically implantable artificial sphincter system and methods of using the same is disclosed. The artificial sphincter system disclosed herein comprises a support and an electroactive polymer element, both of which are adapted and configured to open and / or close a body cavity. The artificial sphincter systems are useful in the treatment of urinary incontinence, fecal incontinence, and reflux disorders. The implanted artificial sphincter can also provide a signal to the recipient to urinate or defecate.
Owner:PAVAD MEDICAL
Magnetic resonance imaging (MRI) safe remotely adjustable artifical sphincter
Owner:ETHICON ENDO SURGERY INC
Artificial sphincter
InactiveUS6997952B2Sufficient quantityAvoid the risk of infectionStammering correctionAnti-incontinence devicesIntestinal structureShape change
Owner:TOKIN CORP +2
Metallic implantable grafts and method of making same
ActiveUS20050033418A1Promote endothelializationGive flexibilityStentsSurgerySurgical GraftLigament structure
Implantable medical grafts fabricated of metallic or pseudometallic films of biocompatible materials having a plurality of microperforations passing through the film in a pattern that imparts fabric-like qualities to the graft or permits the geometric deformation of the graft. The implantable graft is preferably fabricated by vacuum deposition of metallic and / or pseudometallic materials into either single or multi-layered structures with the plurality of microperforations either being formed during deposition or after deposition by selective removal of sections of the deposited film. The implantable medical grafts are suitable for use as endoluminal or surgical grafts and may be used as vascular grafts, stent-grafts, skin grafts, shunts, bone grafts, surgical patches, non-vascular conduits, valvular leaflets, filters, occlusion membranes, artificial sphincters, tendons and ligaments.
Owner:VACTRONIX SCI LLC
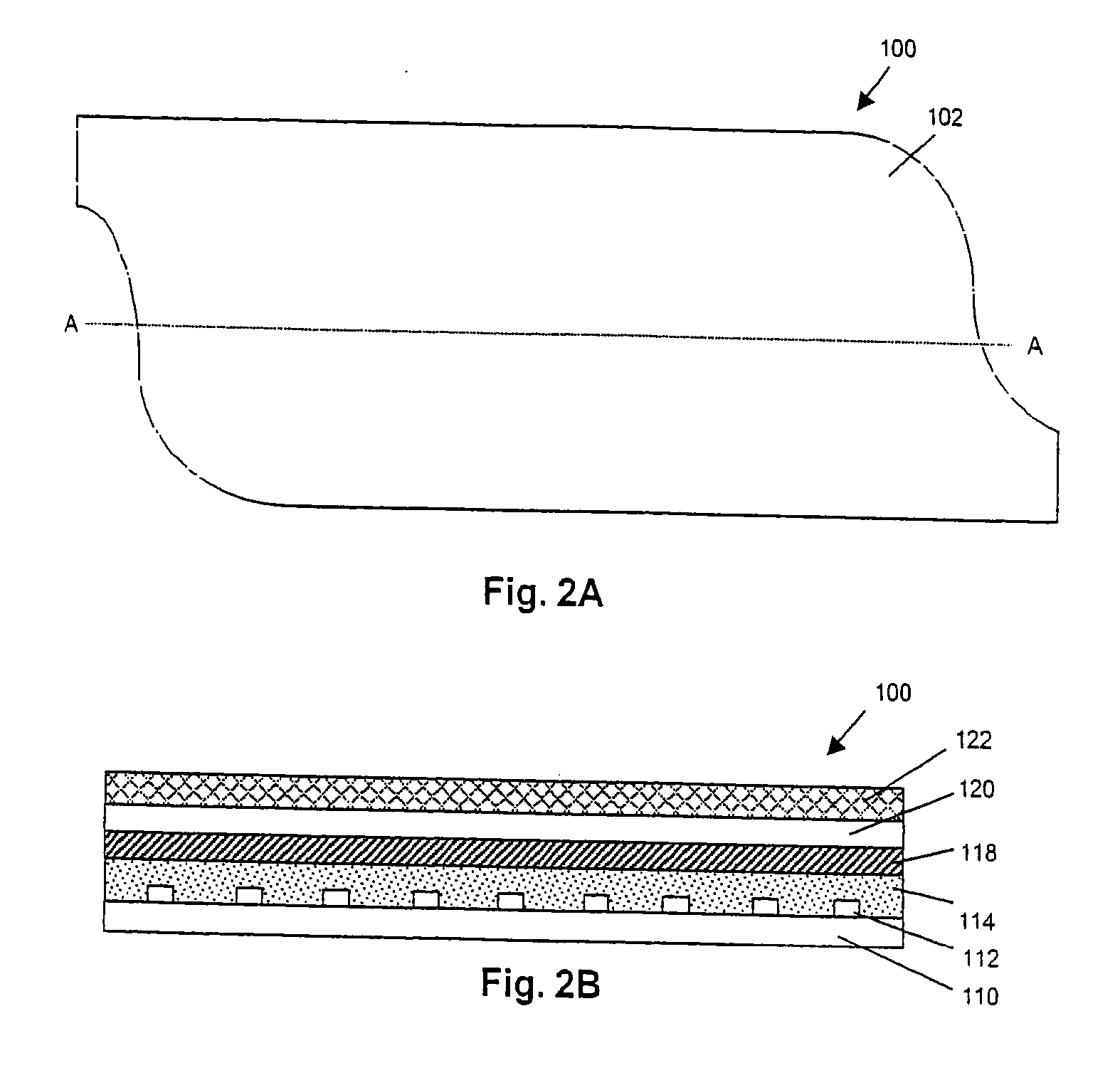
Parylene coated components for artificial sphincters
InactiveUS7011622B2Prevent and inhibit wearPrevent and inhibit and abrasionAnti-incontinence devicesSurgeryParylene coatingVacuum chamber
This invention provides an artificial sphincter including a component coated on at least one contacting surface with a polymeric material, the polymeric coating adapted to expand and return to an original configuration and to prevent or inhibit wear or abrasion of the contacting surface. The polymeric material is parylene in some embodiments. The component adapted for inflation and deflation may be a cuff formed from silicon and adapted to surround a urethra or a rectum. Also provided by the present invention is a method of depositing a coating to a surface of an inflatable component by providing a vacuum chamber system having an inner chamber positioned within an outer chamber. At least one coating material is introduced into at least one of the inner and outer chambers to deposit a coating onto an exposed surface of the component. This invention also provides methods of masking portions of surfaces of an inflatable component to prevent a coating from being deposited on the masked portion.
Owner:BOSTON SCI SCIMED INC
Spatially decoupled twin secondary coils for optimizing transcutaneous energy transfer (TET) power transfer characteristics
ActiveUS20060020305A1Improve power efficiencyImprove energy transferElectrotherapyConversion without intermediate conversion to dcEnergy transferSecondary loop
An implantable device, such as an infuser device for bidirectional hydraulically controlling a medical artificial sphincter, enhances power transfer characteristics to a secondary coil thereby allowing implantation to greater physical depths and / or enclosing the secondary coil within a housing of the infuser device. The enhanced power transfer is achieved with multiple coils that are longitudinally aligned and physical and electrical parallel to form the secondary loop of a transcutaneous energy transfer system (TET) instead of a single coil. It better optimizes the power transfer from a parallel tuned tank circuit primary coil to an implanted secondary series tuned tank circuit coil.
Owner:ETHICON ENDO SURGERY INC
Self-supporting metallic implantable grafts, compliant implantable medical devices and methods of making same
InactiveUS20070250156A1Give flexibilityFacilitating transmural endothelializationStentsBlood vesselsSurgical GraftMetallic materials
Implantable medical grafts fabricated of metallic or pseudometallic films of biocompatible materials having a plurality of microperforations passing through the film in a pattern that imparts fabric-like qualities to the graft or permits the geometric deformation of the graft. The implantable graft is preferably fabricated by vacuum deposition of metallic and / or pseudometallic materials into either single or multi-layered structures with the plurality of microperforations either being formed during deposition or after deposition by selective removal of sections of the deposited film. The implantable medical grafts are suitable for use as endoluminal or surgical grafts and may be used as vascular grafts, stent-grafts, skin grafts, shunts, bone grafts, surgical patches, non-vascular conduits, valvular leaflets, filters, occlusion membranes, artificial sphincters, tendons and ligaments.
Owner:VACTRONIX SCI LLC
Artificial Sphincter System and Method
The present invention provides an artificial sphincter employing an easily controlled electro-mechanical pump system. The artificial sphincter includes an inflatable cuff, a control pump fluidly coupled to the inflatable cuff, and an electro-mechanical pump system. The inflatable cuff is adapted to surround a urethra or rectum of the patient to facilitate continence. An inflation element or balloon can be included to further control pressure to the cuff.
Owner:BOSTON SCI SCIMED INC
Electroactive polymer based artificial sphincters and artificial muscle patches
InactiveUS20050004425A1Improve heart functionLess invasiveAnti-incontinence devicesSurgeryLower esophagusElectroactive polymer actuators
Provided are artificial muscle patches, which are adapted to be implanted adjacent a patient's heart, and artificial sphincter cuffs, which are adapted to be implanted around a body lumen, such as the urethra, the anal canal, or the lower esophagus. The devices of the present invention comprise: (a) one or more electroactive polymer actuators; and (b) a control unit for electrically controlling the one or more electroactive polymer actuators to expand or contract the devices.
Owner:BOSTON SCI SCIMED INC
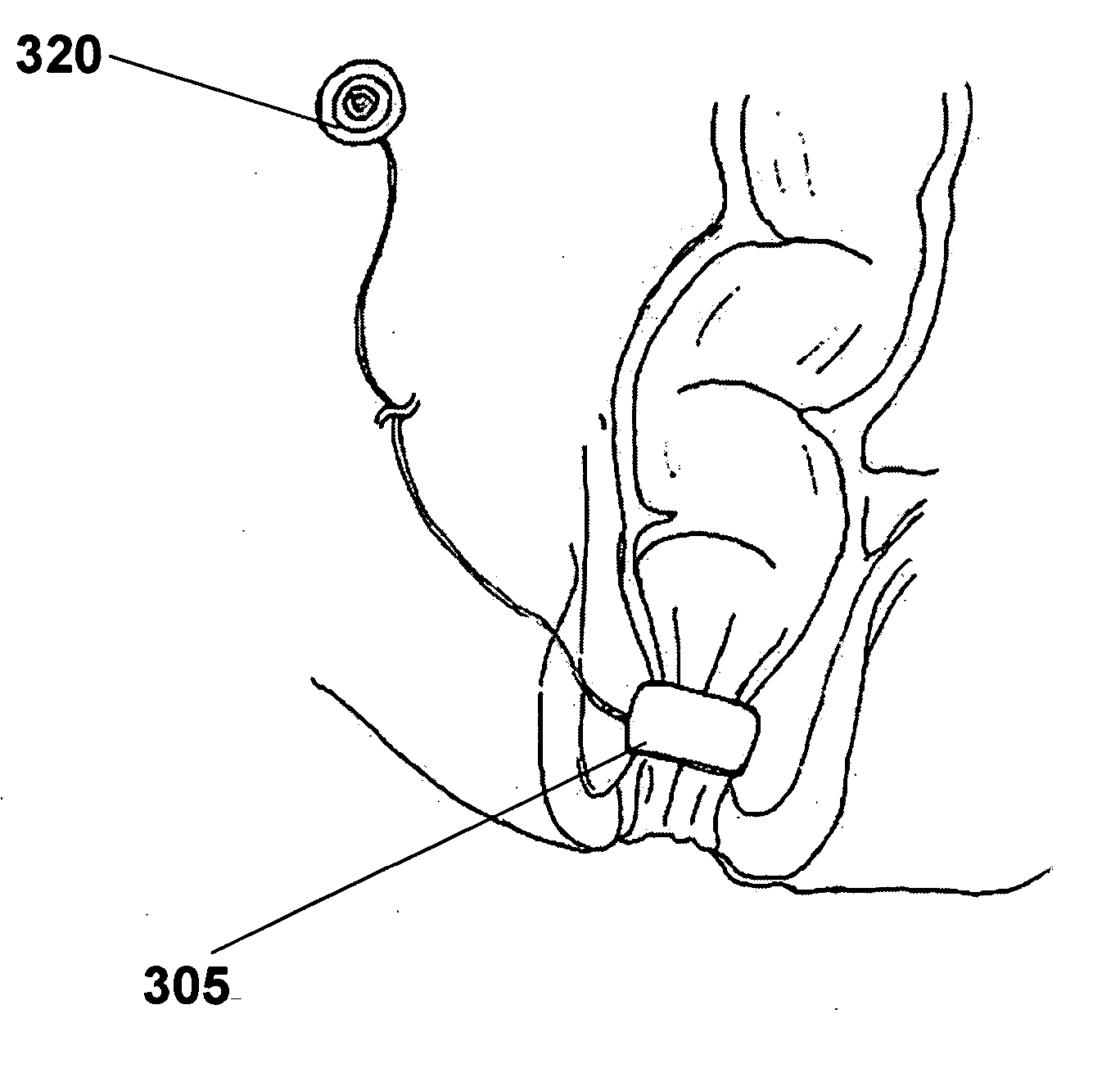
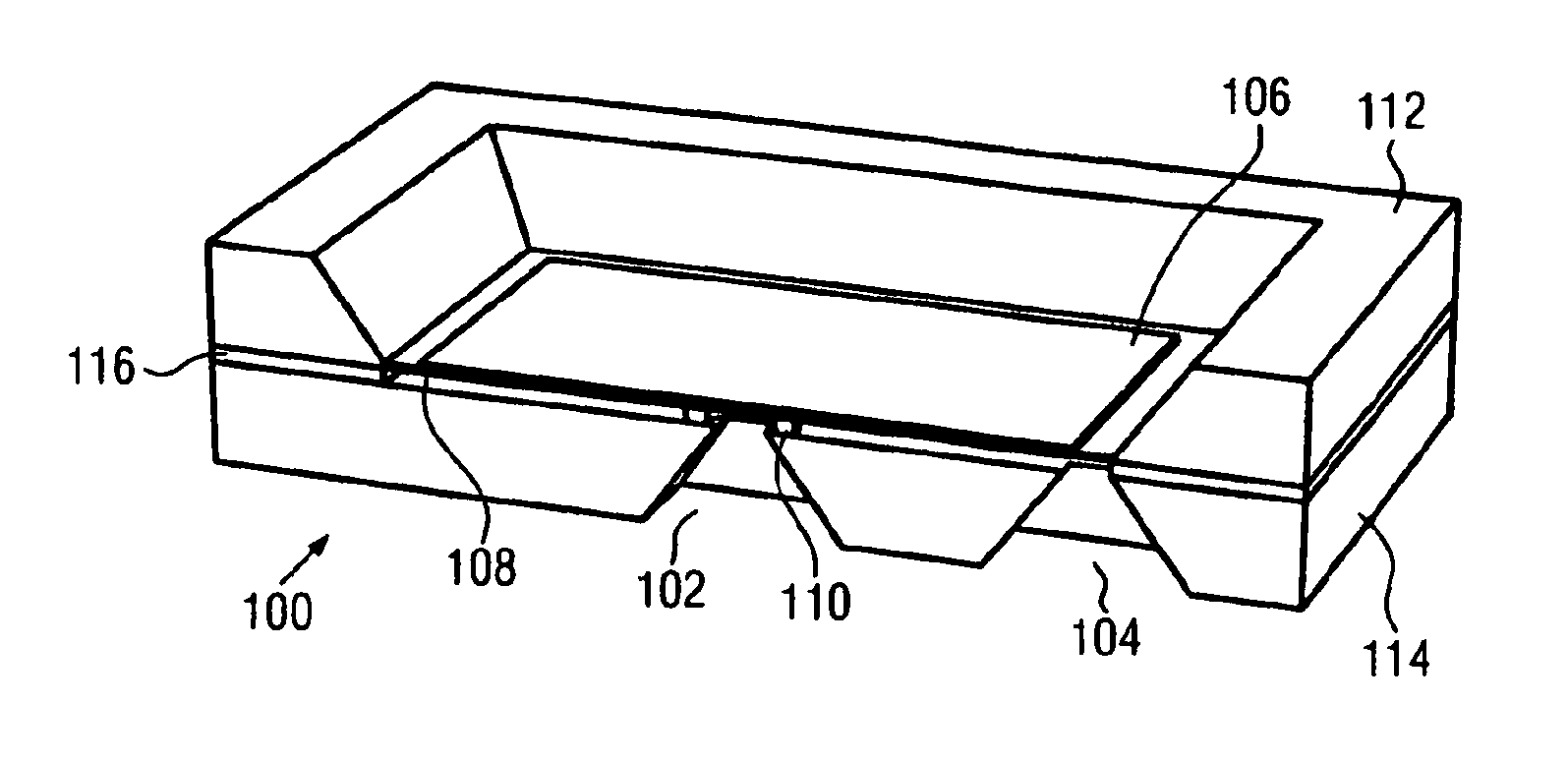
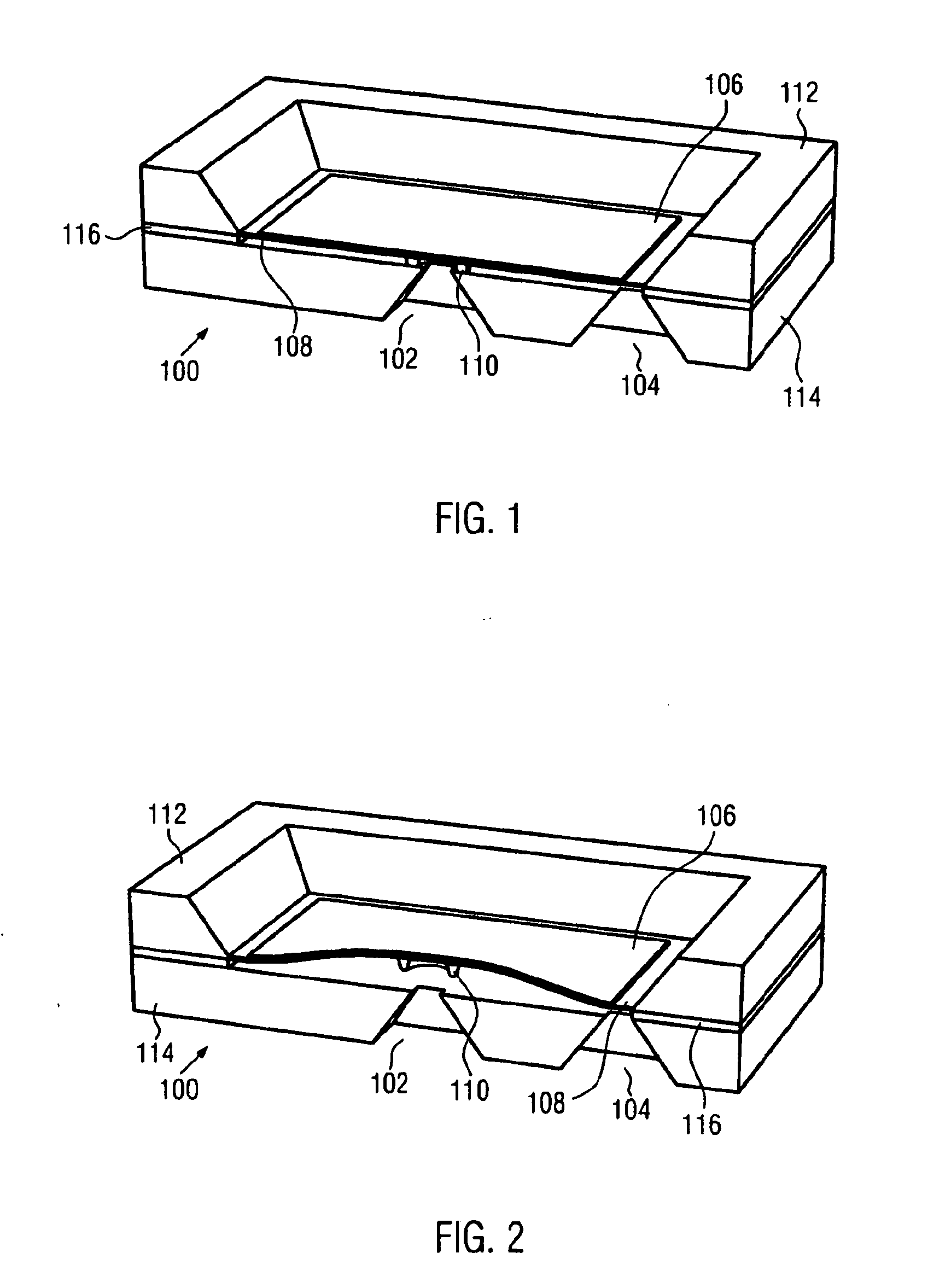
Artificial sphincter with variable viscosity fluid-filled collar
InactiveUS7458930B2Variable viscosityEasy to passAnti-incontinence devicesEndoradiosondesAnatomical conduitMagnetorheological fluid
Systems and methods for supplementing control of an anatomical sphincter. A collar containing variable viscosity fluid surrounds a portion of an anatomical conduit. The flow of bodily fluids through the anatomical conduit occurs according to the viscosity level of the variable viscosity fluid. Electro-rheologic fluid in the collar liquefies in the absence of an electrical potential difference to render the collar pliable, permitting the anatomical conduit to expand and fluid to flow through the anatomical conduit. Electro-rheologic fluid in the collar solidifies in the presence of an electrical potential difference to render the collar firm, restricting the anatomical conduit from expanding and restricting fluid from passing through the anatomical conduit. A control unit, or battery, operable in response to sensed pressure data or according to an external control unit manipulated by a patient, determines when an electrical potential difference is generated to change the state of the electro-rheologic fluid in the collar. Other fluids may be utilized in the collar, such as magneto-rheologic fluids.
Owner:CARDINAL HEALTH SWITZERLAND 515 GMBH
Active tissue augmentation materials and method
InactiveUS7326172B2Avoid absorptionPrevent circulatory insufficiencyAnti-incontinence devicesSurgical needlesMedicineInjected material
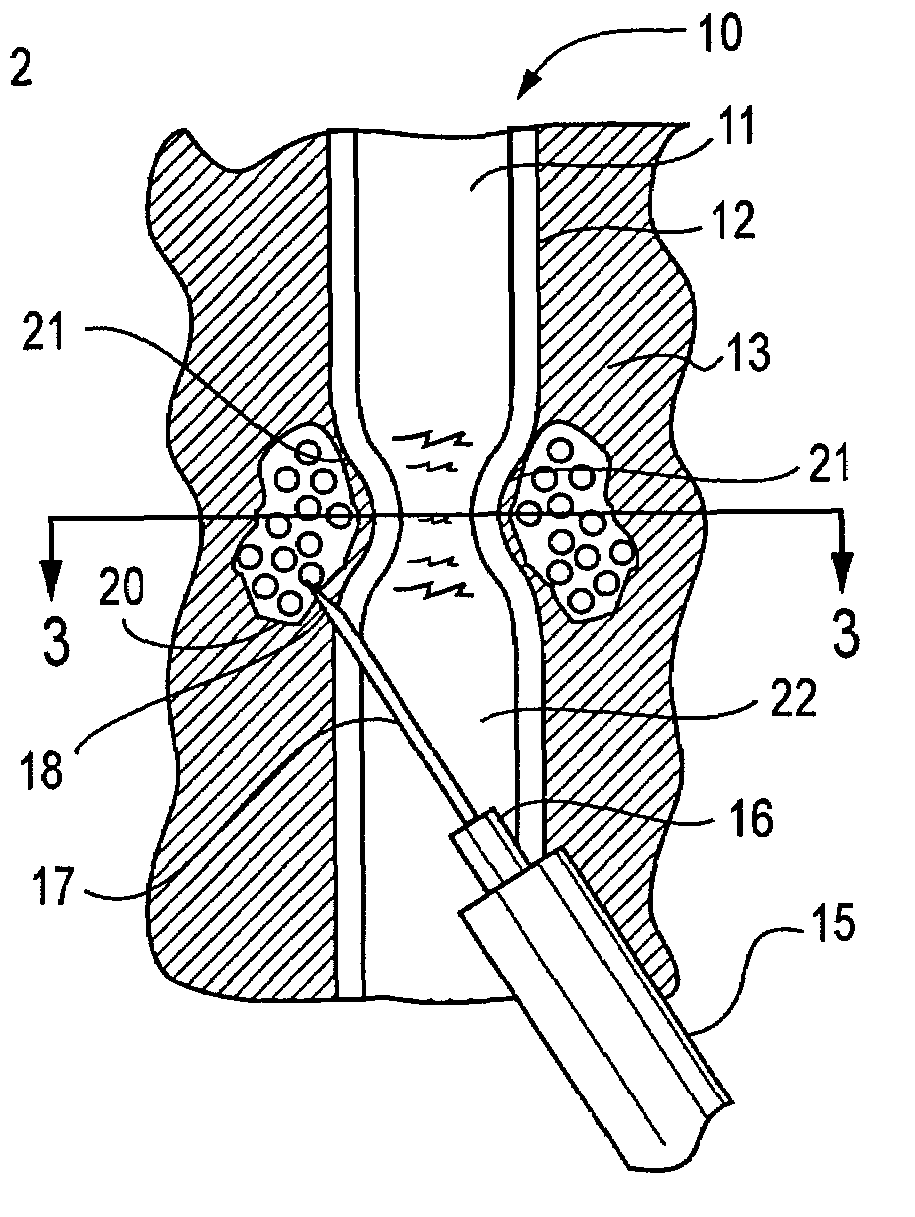
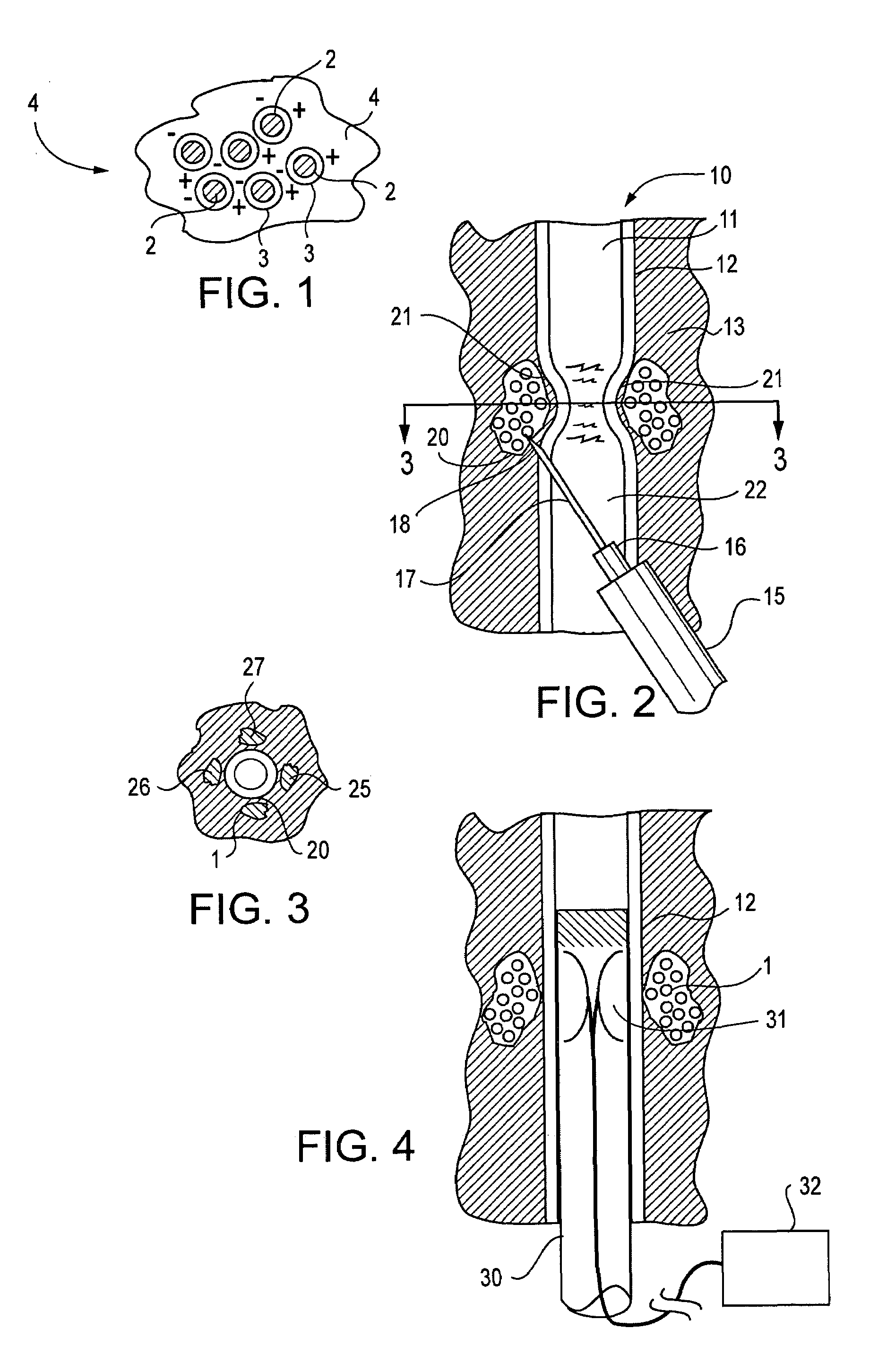
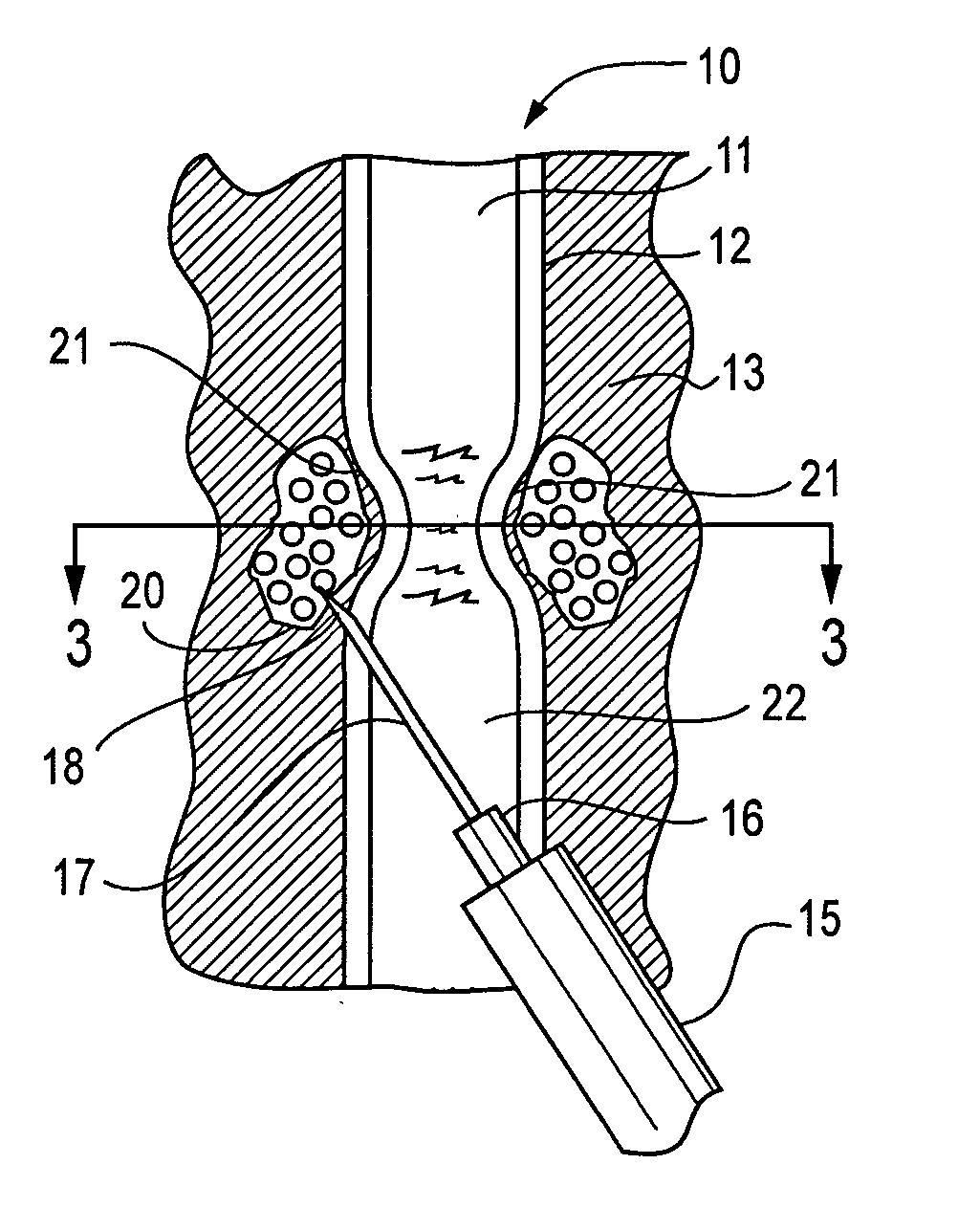
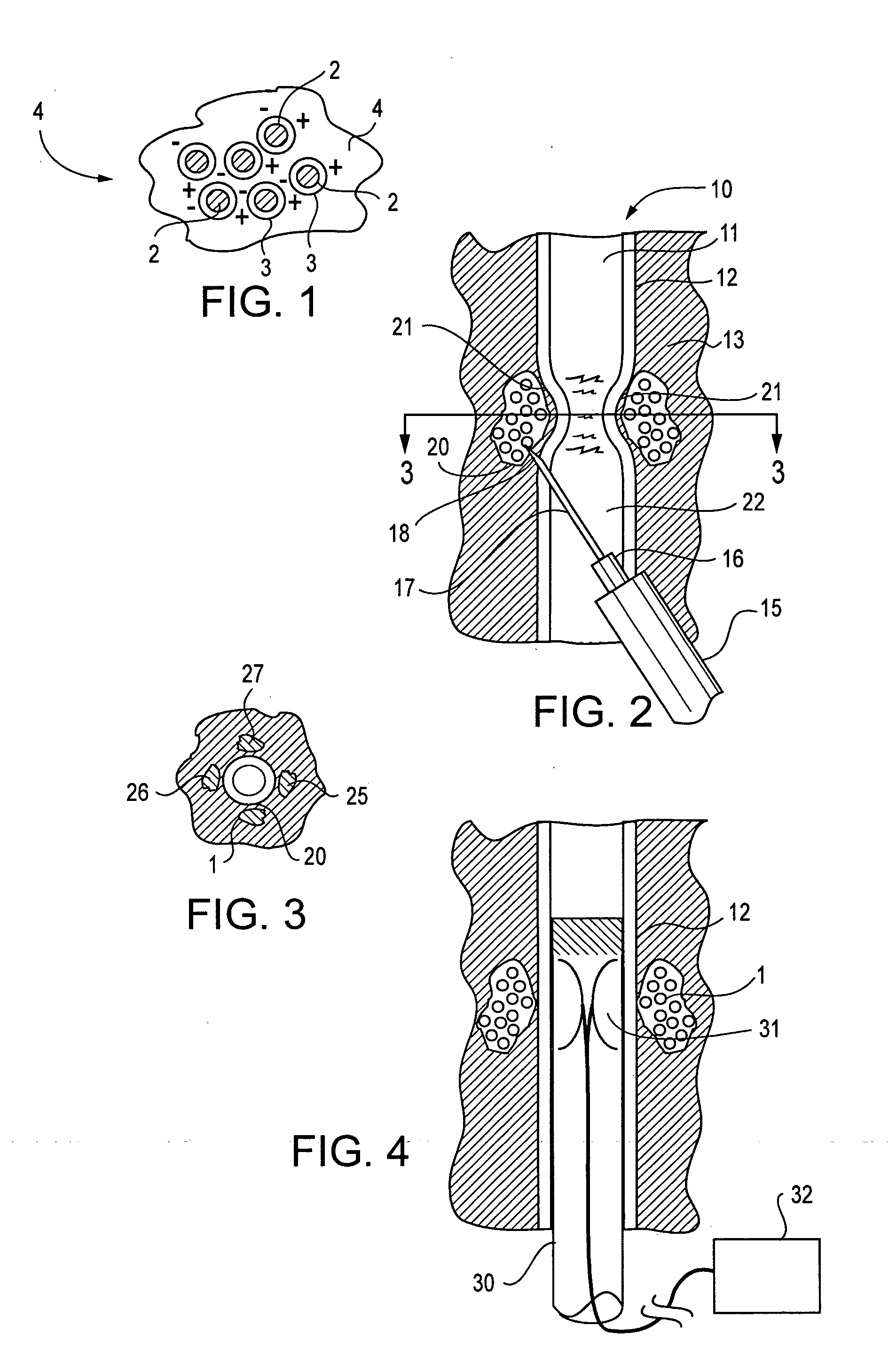
Active tissue augmenting agents, compositions and methods for use are disclosed. In a typical embodiment, the active augmenting agents of the invention can be used to form an artificial sphincter around a lumen of a human or animal body. In one embodiment, the active augmenting agent comprises magnitizable particles which can provide occlusion of a lumen, such as the urethral lumen, by circumferential attraction of the injected material toward the center of the lumen by the inherent magnetic flux field created from the magnetic dipoles of the magnetic particles.
Owner:TORAX MEDICAL
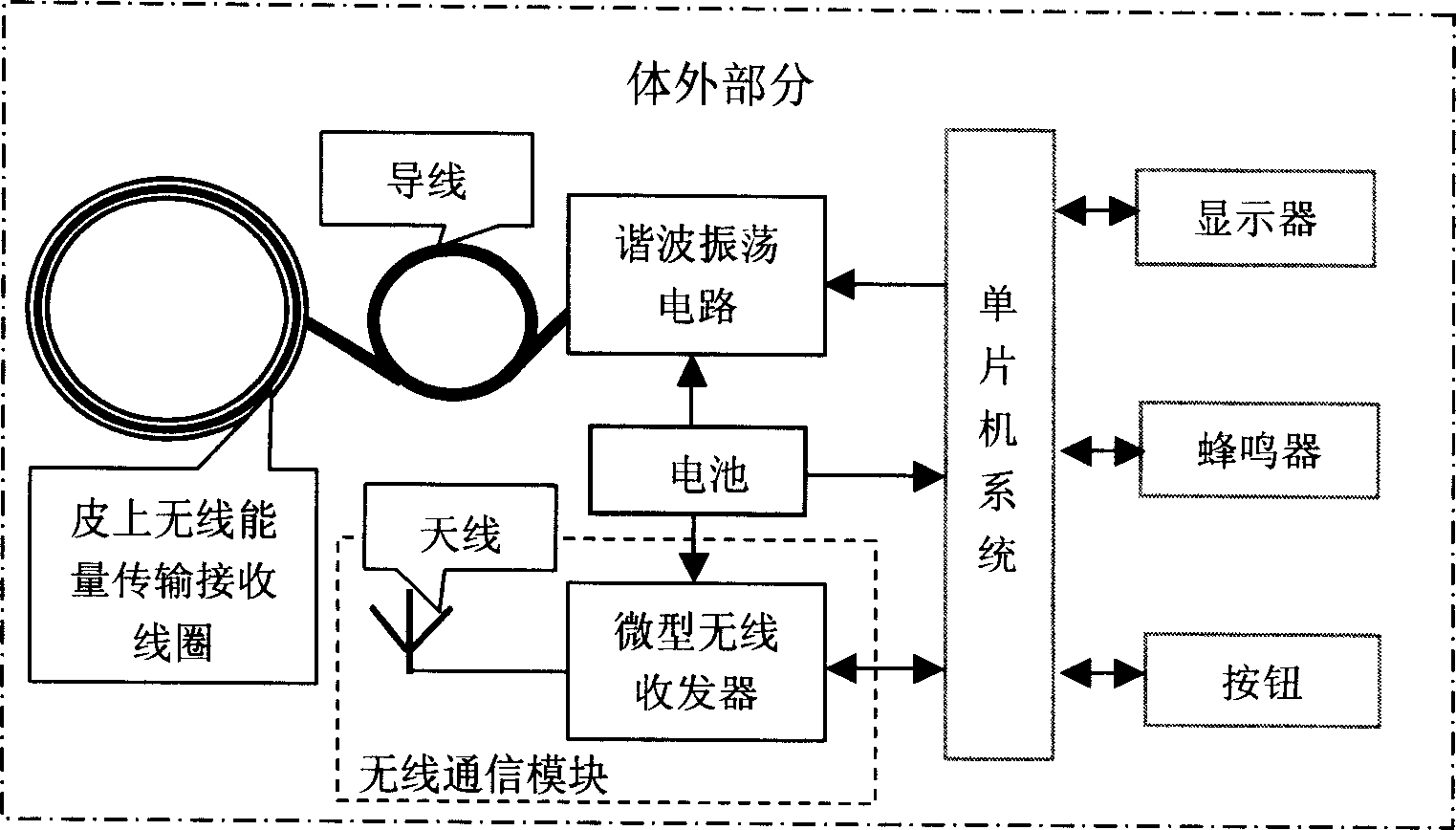
Feed-back control type artificial sphincter ani system
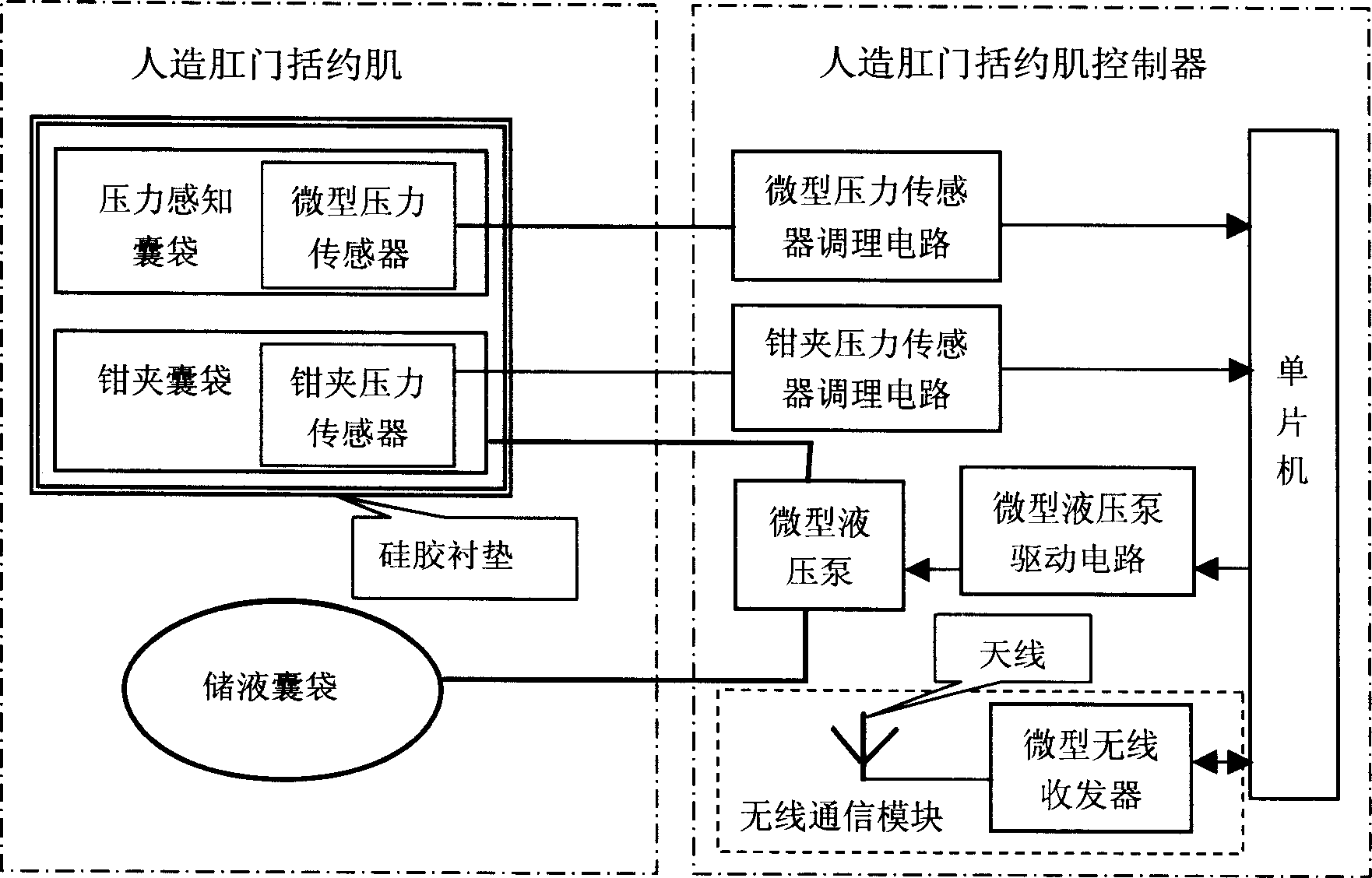
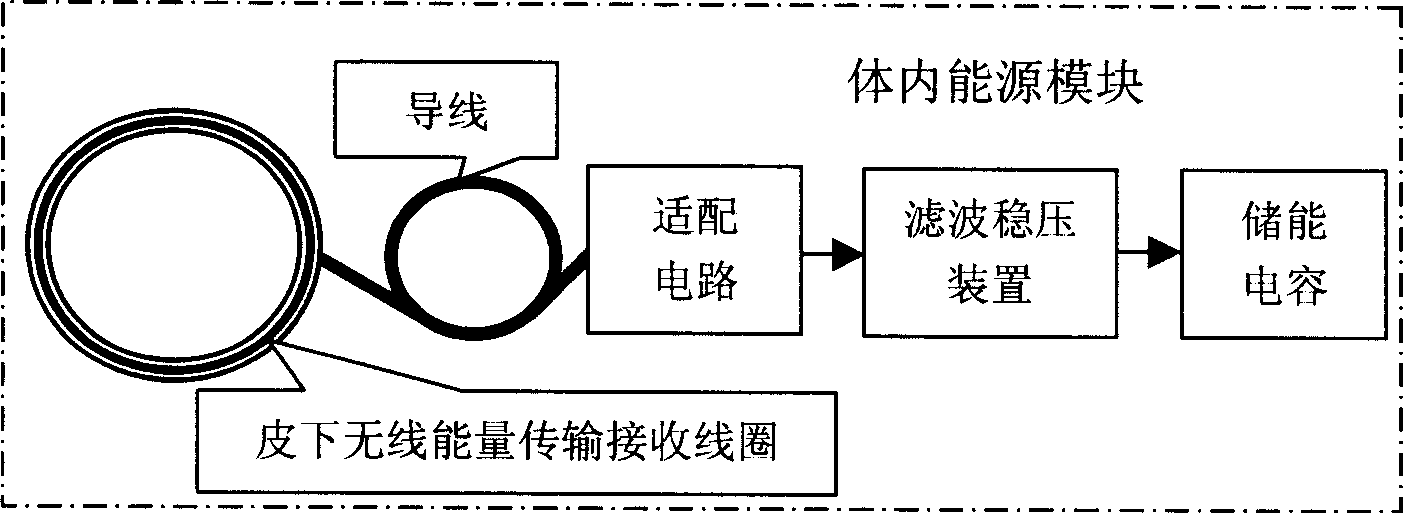
InactiveCN1861020ARealization of wireless power supplySolve the problem of energy supplyColostomyProsthesisMicrocontrollerLife quality
A feedback controlled artificial sphincter system for anus is composed of an internal unit consisting of wireless communication module, the artificial sphincter which comprises pressure sensing bag, miniature pressure sensor, clamping bag, clamping pressure sensor and liquid bag, the artificial sphincter controller which comprises the regulating circuits for said sensors, miniature hydraulic pump and its driver, and single-chip microprocessor, and the power module, and an external unit consisting of wireless transmitting coil on skin, harmonic oscillator, wireless communication module, and single-chip microprocessor system.
Owner:SHANGHAI JIAO TONG UNIV
Artificial sphincter with piezoelectric actuator
InactiveUS20090259093A1Increased durabilityImprove fatigueAnti-incontinence devicesICT adaptationRefluxFecal incontinence
A biologically implantable artificial sphincter system and methods of using the same is disclosed. The artificial sphincter system disclosed herein comprises a sphincter band and a piezoelectric element, both of which are adapted and configured for coordinated operation to open and / or close a body cavity. The artificial sphincter systems are useful in the treatment of urinary incontinence, fecal incontinence, and reflux disorders. The implanted artificial sphincter can also provide a signal to the recipient to urinate or defecate.
Owner:PAVAD MEDICAL
Artificial sphincter
A biologically implantable artificial sphincter system and methods of using the same is disclosed. The artificial sphincter system disclosed herein comprises a support and an electroactive polymer element, both of which are adapted and configured to open and / or close a body cavity. The artificial sphincter systems are useful in the treatment of urinary incontinence, fecal incontinence, and reflux disorders. The implanted artificial sphincter can also provide a signal to the recipient to urinate or defecate.
Owner:PAVAD MEDICAL
Micro-valve having an elastically deformable valve lip, method for producing same and micro-pump
InactiveUS20130186078A1Improve usabilityImprove sealingServomotor componentsRotary clutchesEngineeringActuator
The present invention relates to a micro-valve which is formed from two firmly connected substrates and preferably has an actuator element, for example that is diaphragm-driven, for the controlled opening and closing of a first and / or second passage. The invention further relates to a method for producing such a micro-valve and to a micro-pump which uses at least one such micro-valve. Said micro-pump is intended to be used in particular in conjunction with the development of an artificial sphincter. The micro-valve has a first substrate and a second substrate which are non-detachably joined to each other in order to form a controllable fluid flow section, and at least one first passage and at least one second passage. According to the invention, the micro-valve has at least one elastically deformable seal structure which, for example, can be formed by a photostructurable silicone, to seal off the first and / or second passage.
Owner:ALBERT LUDWIGS UNIV FREIBURG
Biofeedback type artificial sphincter ani system
InactiveCN1718174AAdjust the bowel movement timeAchieve bowel controlLigamentsMusclesAutomatic controlSilica gel
A biologic feedback type artificial sphincter ani system is composed of an artificial sphincter ani consisting of silica gel pad, pressed bag, clamped bag, pressed catheter and clamped catheter, and an artificial sphincter ani controller consisting of pressure sensor and its regulator, miniature liquid pump and its driver, single-chip microprocessor, LCD, buzzer, push button, battery and PCB.
Owner:SHANGHAI JIAO TONG UNIV
Magnetic-ring artificial sphincter and manufacture and application method thereof
The invention relates to a magnetic-ring artificial sphincter, namely an annular structural device based on magnetic bodies. The magnetic-ring artificial sphincter is formed by connecting a plurality of magnetic bodies in series through connection materials. Expansion and shrinkage with reversibility can be achieved through the magnetic force absorption effect of the magnetic bodies and sliding and flexible characteristics of the connection materials. The magnetic-ring artificial sphincter is embedded around cavity pipelines in the bodies, can simulate functions of a normal human sphincter, can enhance or rebuild the sphincter functions of organs reducing in or losing sphincter force, controls normal flow direction of contents of hollow organs, and can substitute or rebuild sphincter tissues at positions of esophagus, cardia, pylorus, ileocecus, anal canals, bladders, positions around urethral canals, alimentary canals and urinary tract stomas and the like. The magnetic-ring artificial sphincter is convenient in operation, few in manufacture process, reliable in function and wide in application prospect in the field of biomedicine.
Owner:创思(绍兴)医疗科技有限公司
Active tissue augmentation materials and method
InactiveUS20060009674A1Avoid absorptionHigh densityAnti-incontinence devicesSurgical needlesMedicineInjected material
Active tissue augmenting agents, compositions and methods for use are disclosed. In a typical embodiment, the active augmenting agents of the invention can be used to form an artificial sphincter around a lumen of a human or animal body. In one embodiment, the active augmenting agent comprises magnitizable particles which can provide occlusion of a lumen, such as the urethral lumen, by circumferential attraction of the injected material toward the center of the lumen by the inherent magnetic flux field created from the magnetic dipoles of the magnetic particles.
Owner:TORAX MEDICAL
Method for preparing two-way memory nickel-titanium alloy for artificial sphincters through rapid solidification
ActiveCN103409663ASimple preparation processImprove performanceLigamentsMusclesTitanium alloyVacuum arc
The invention discloses a method for preparing a two-way memory nickel-titanium alloy for artificial sphincters through rapid solidification. The method comprises the following steps of: weighing high-purity nickel and high-purity titanium which are used as raw materials according to the condition that the atomic ratio of nickel atoms to titanium atoms is 51:49; putting and smelting the raw materials a nonconsumable vacuum arc smelting furnace, absorbing molten metal into a copper die cavity by virtue of a negative-pressure suction casting method carried out by a water-cooled copper die, and carrying out rapid solidification, thereby obtaining a material in the required shape; homogenizing the material; fixing the treated material in a semicircular die with curvature radius, deforming the material under constraint of the die to correspond to the curvature of the die, putting the material together with the die into a resistance furnace to undergo constraint aging at 350-500 DEG C which is maintained for 10-100 hours and quenching the material and the die in water, thus preparing the two-way memory nickel-titanium alloy material for the artificial sphincters. The two-way memory nickel-titanium alloy material has narrow temperature zone response, has a straight shape at normal temperatures of human bodies and has bigger deformation curvature after temperature raising, thus being suitable for serving as the function material of the artificial sphincters.
Owner:SOUTH CHINA UNIV OF TECH
Compliant implantable medical devices and methods of making same
InactiveUS20100154197A1Promote migrationStrict controlVacuum evaporation coatingSputtering coatingSurgical GraftMetallic materials
Implantable medical grafts fabricated of metallic or pseudometallic films of biocompatible materials having a plurality of microperforations passing through the film in a pattern that imparts fabric-like qualities to the graft or permits the geometric deformation of the graft. The implantable graft is preferably fabricated by vacuum deposition of metallic and / or pseudometallic materials into either single or multi-layered structures with the plurality of microperforations either being formed during deposition or after deposition by selective removal of sections of the deposited film. The implantable medical grafts are suitable for use as endoluminal or surgical grafts and may be used as vascular grafts, stent-grafts, skin grafts, shunts, bone grafts, surgical patches, non-vascular conduits, valvular leaflets, filters, occlusion membranes, artificial sphincters, tendons and ligaments.
Owner:VACTRONIX SCI LLC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com