Patents
Literature
384 results about "Injected material" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
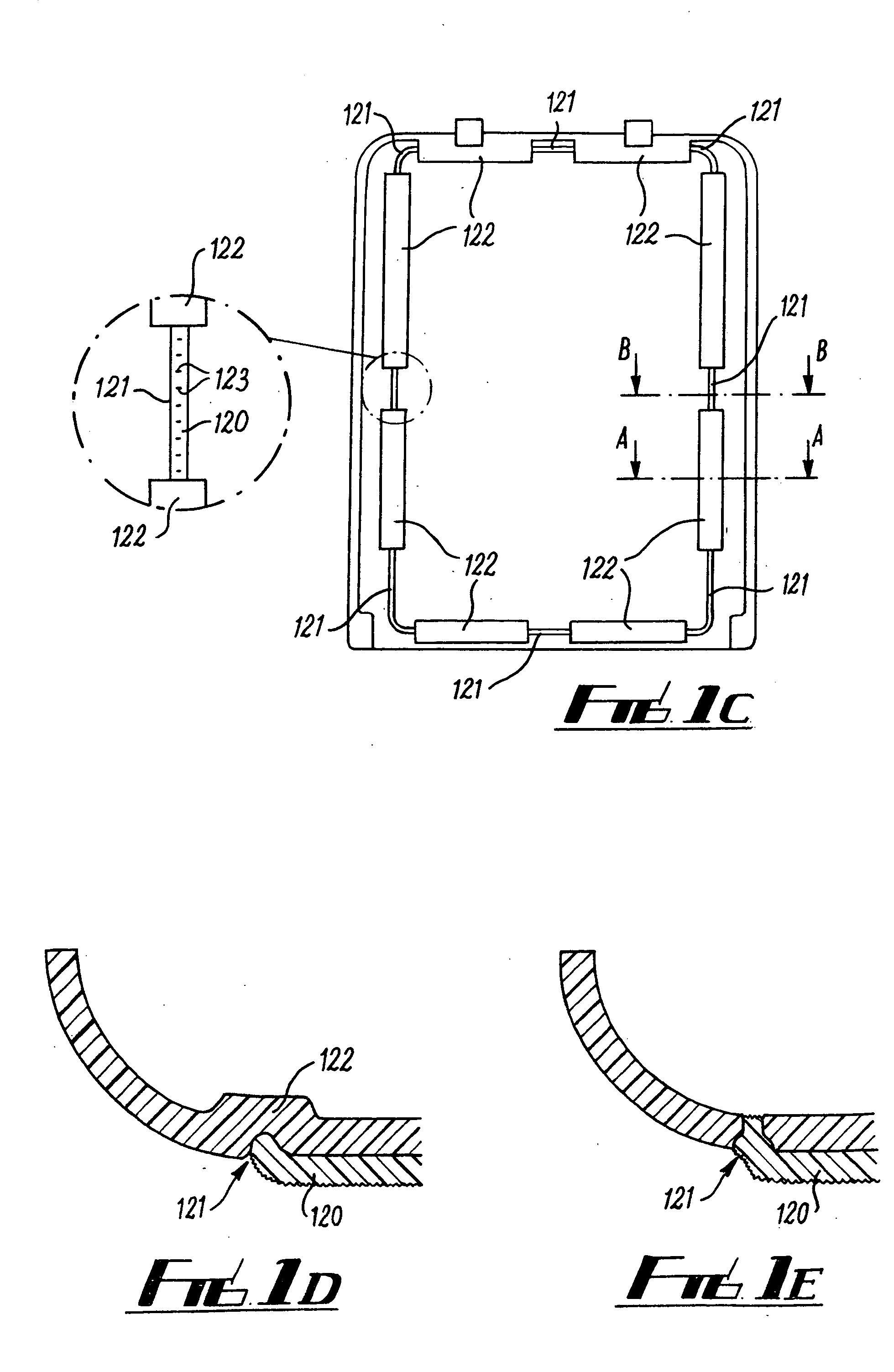
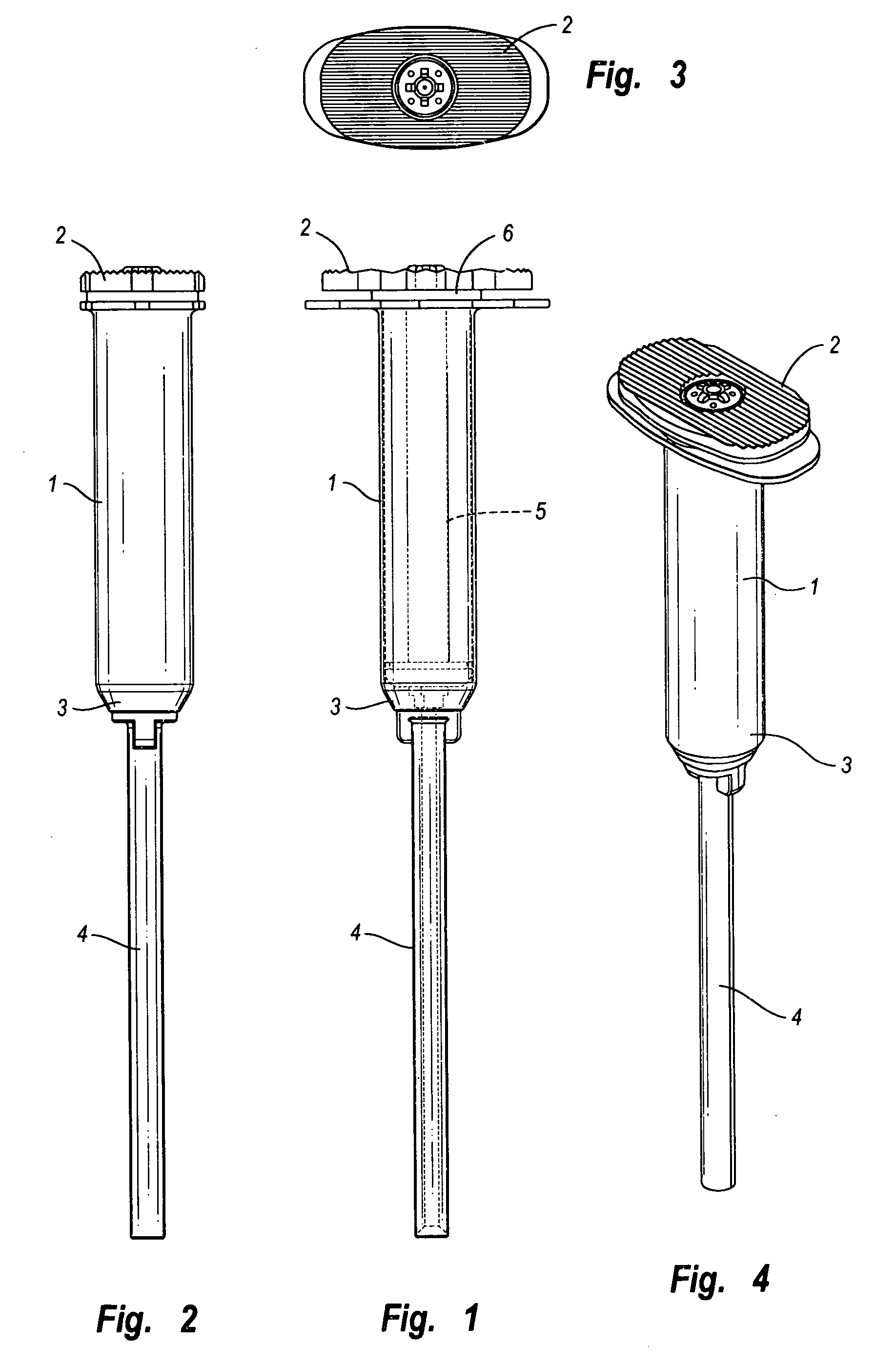
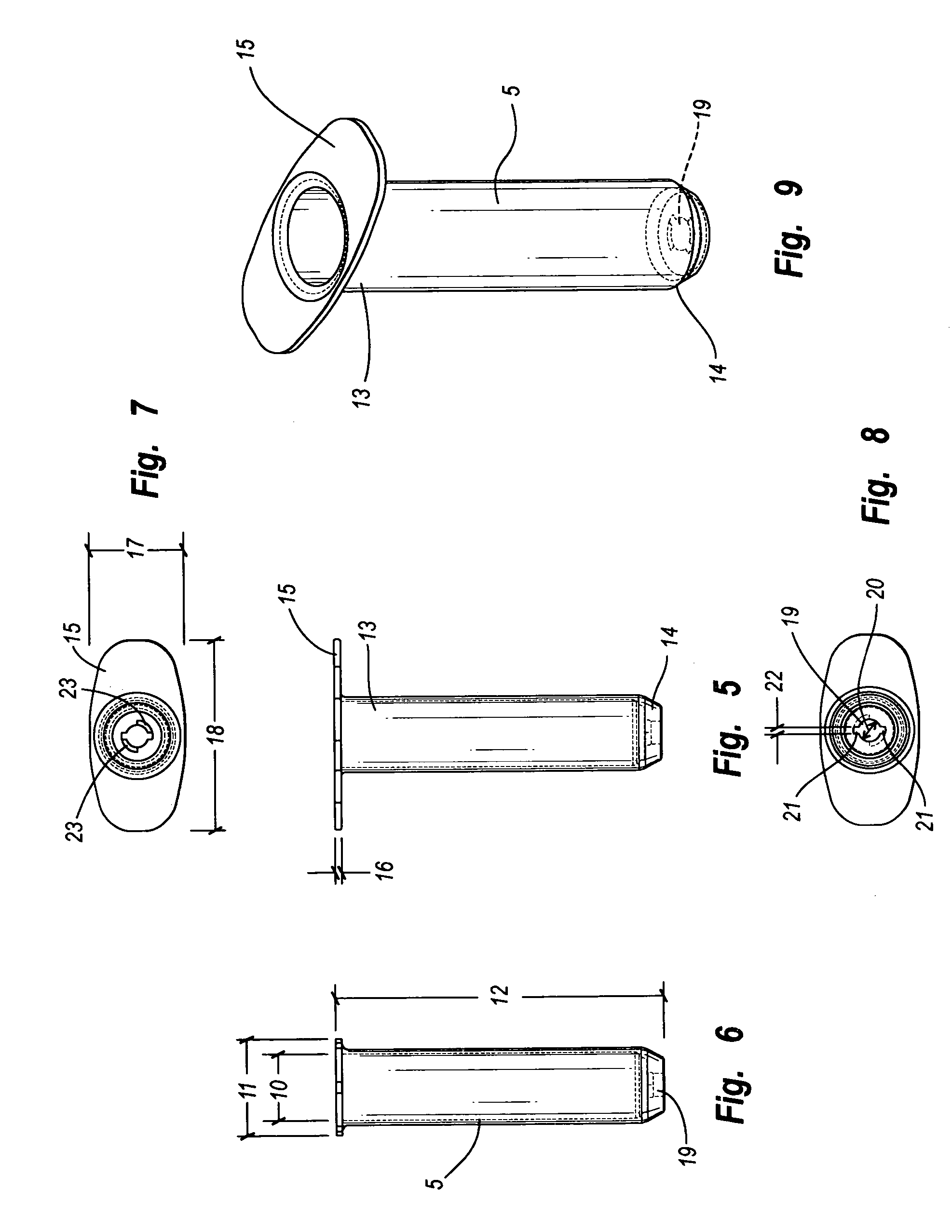
Stapler powered auxiliary device for injecting material between stapler jaws
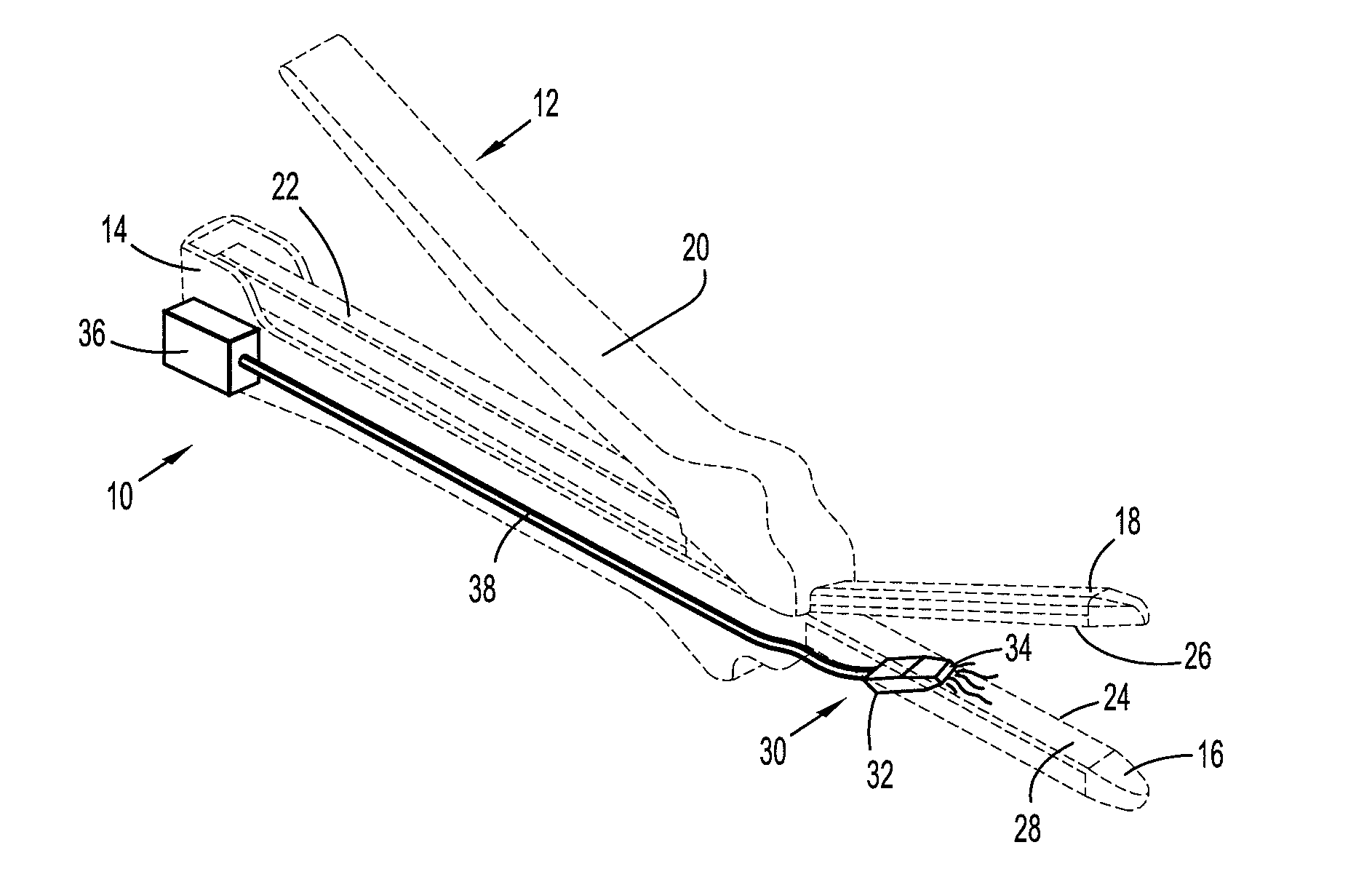
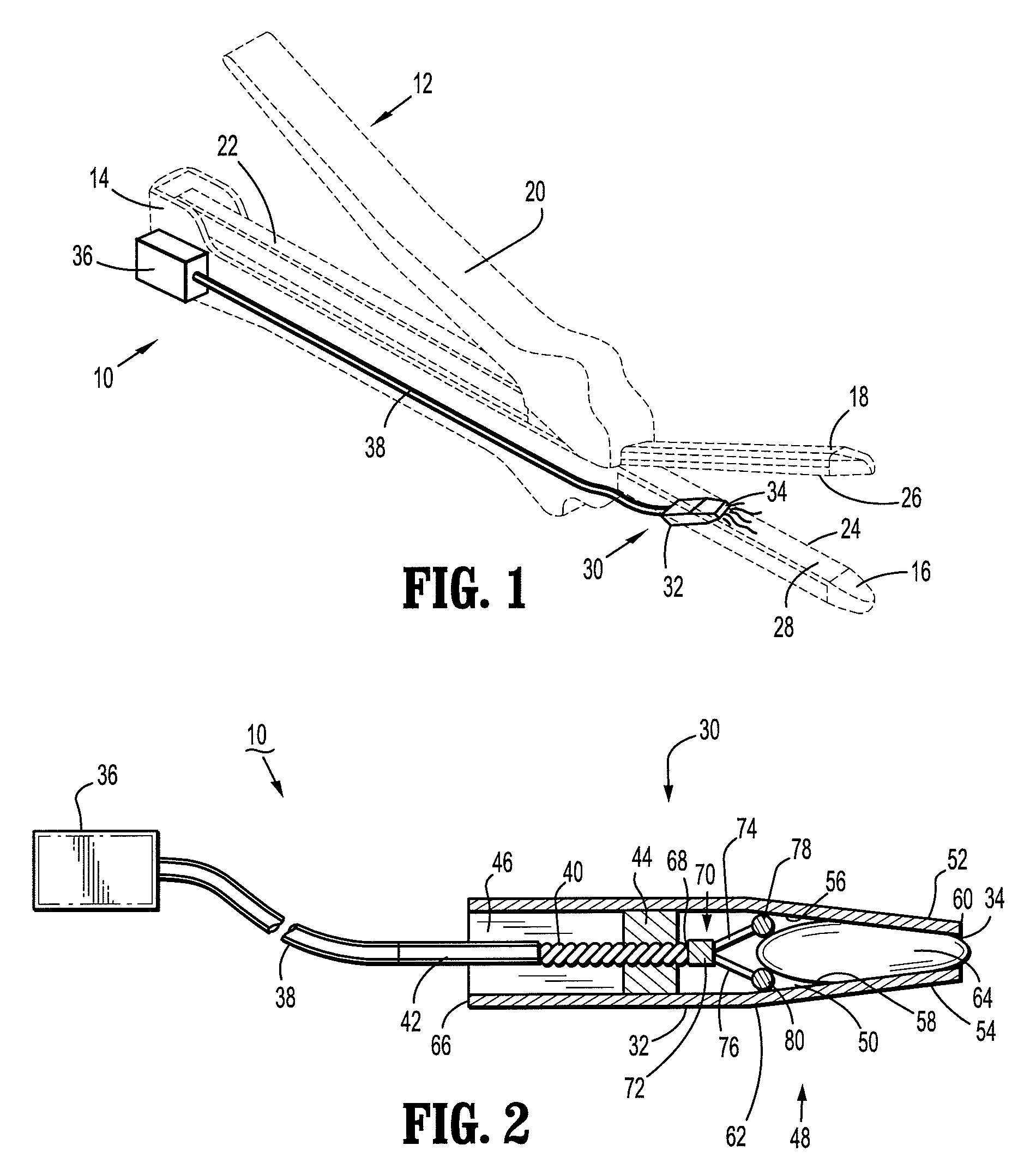
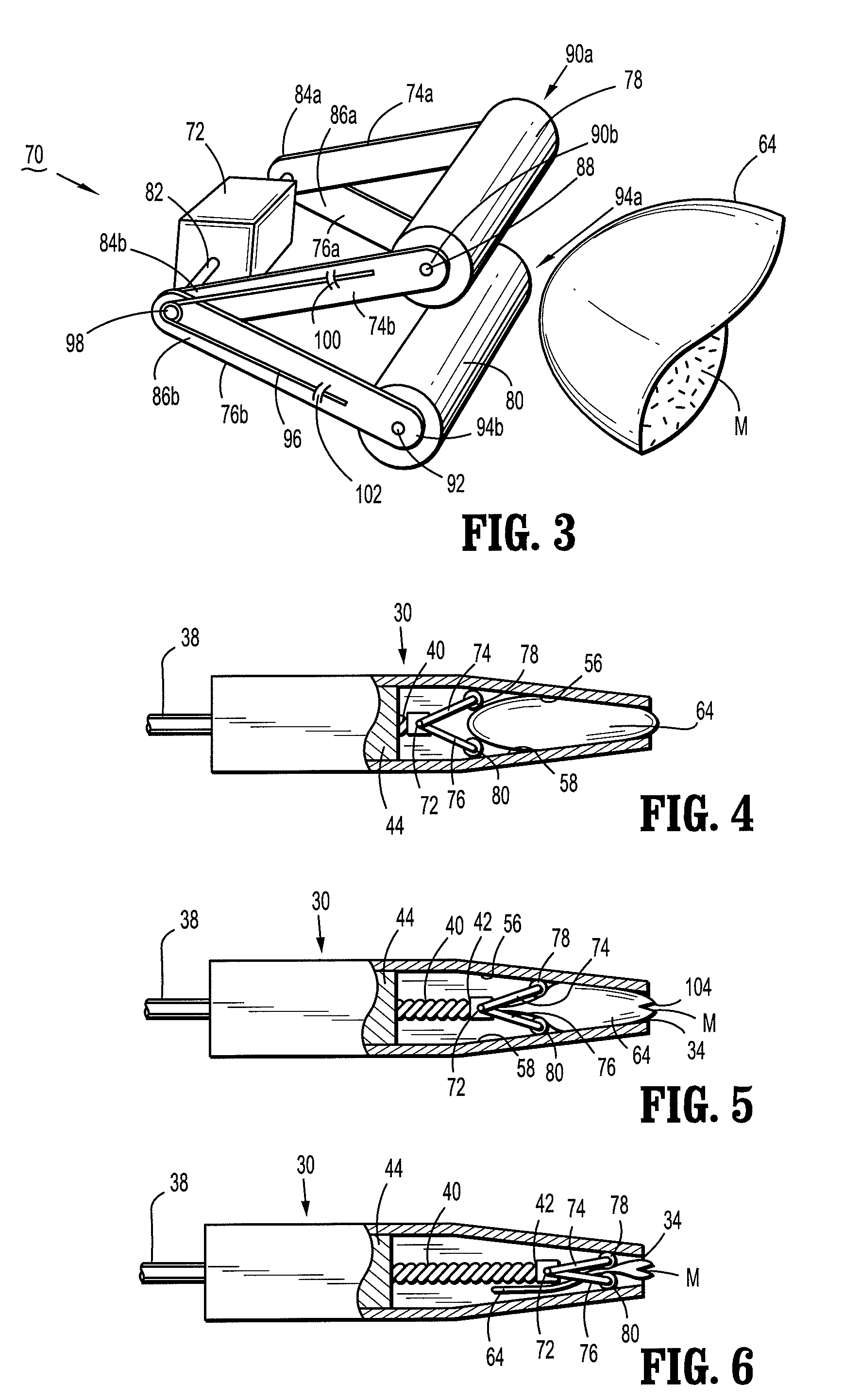
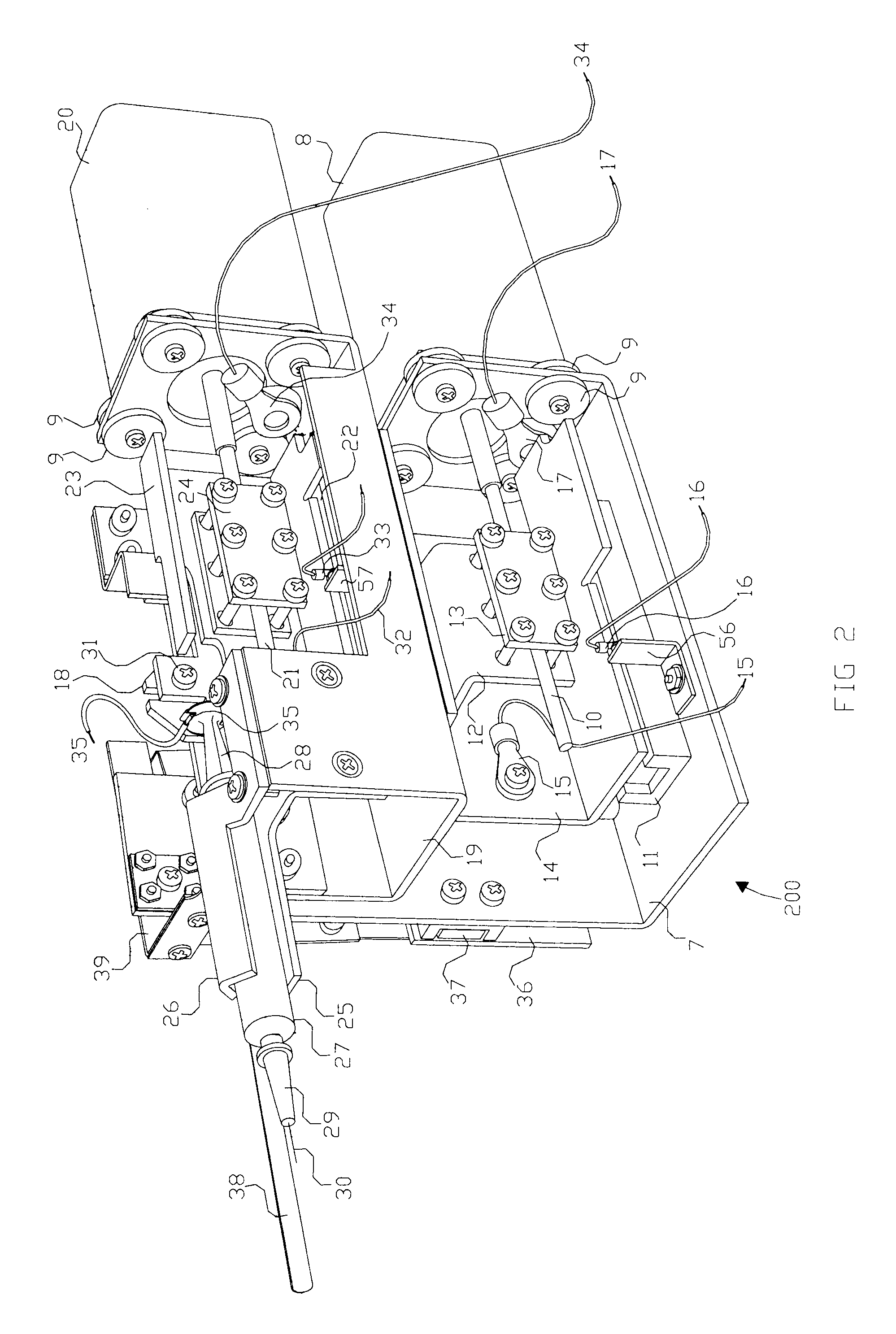
There is disclosed an auxiliary device for injecting material between the jaws of a surgical stapler. The auxiliary device includes a nozzle assembly having a discharge port and a compression assembly having at least one compression roller for forcing material out of the discharge port. The disclosed compression rollers may be formed of an incompressible or compressible material. The auxiliary device also includes a drive mechanism for moving the compression assembly within a nozzle body of the nozzle assembly. There is also disclosed a rupturable capsule for retaining material to be dispensed.
Owner:COVIDIEN LP
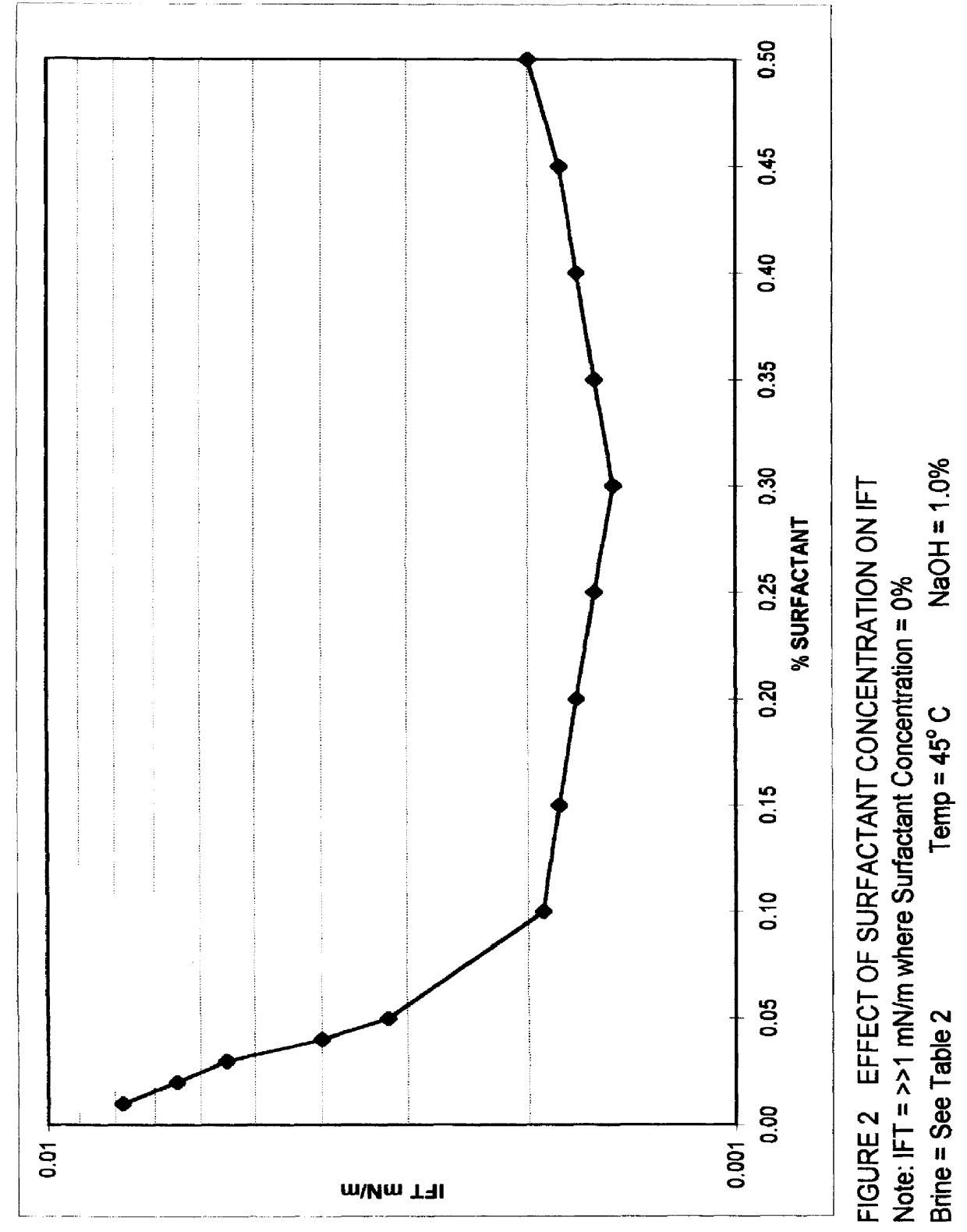
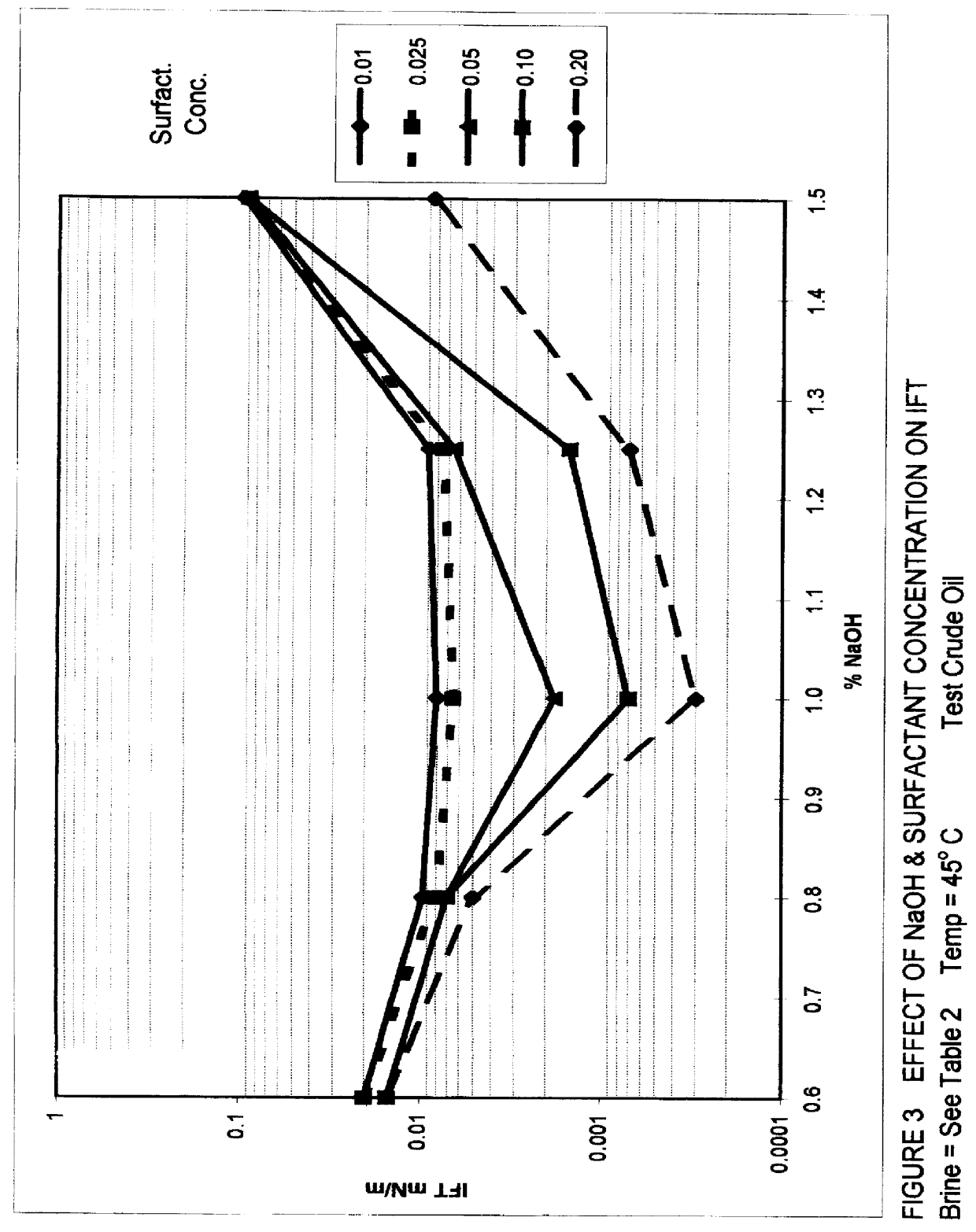
Alkaline surfactant polymer flooding composition and process
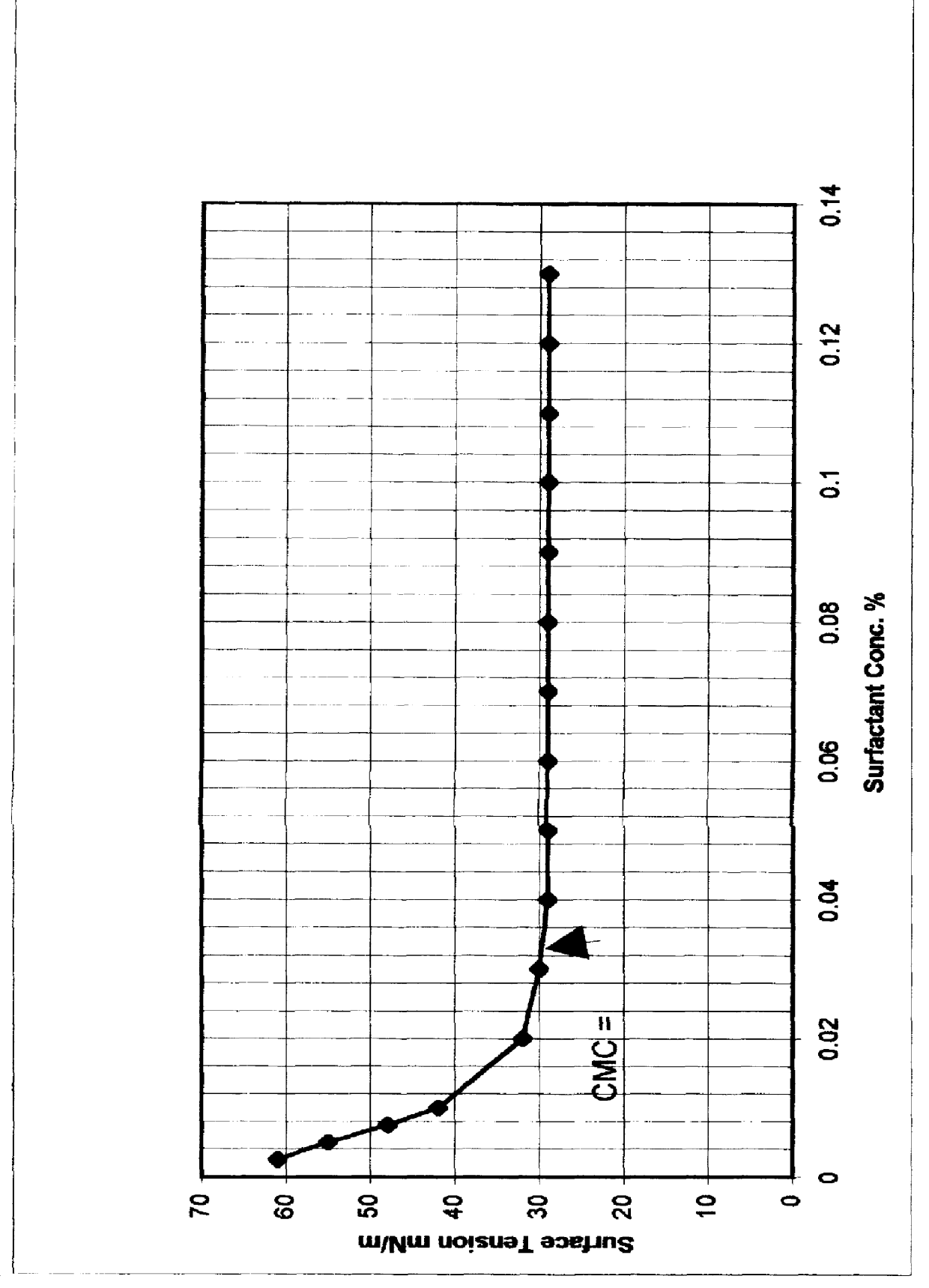
An improved concentrated surfactant formulation and process for the recovery of residual oil from subterranean petroleum reservoirs, and more particularly an improved alkali surfactant flooding process which results in ultra-low interfacial tensions between the injected material and the residual oil, wherein the concentrated surfactant formulation is supplied at a concentration above, at, or, below its CMC, also providing in situ formation of surface active material formed from the reaction of naturally occurring organic acidic components with the injected alkali material which serves to increase the efficiency of oil recovery.
Owner:OIL CHEM TECH
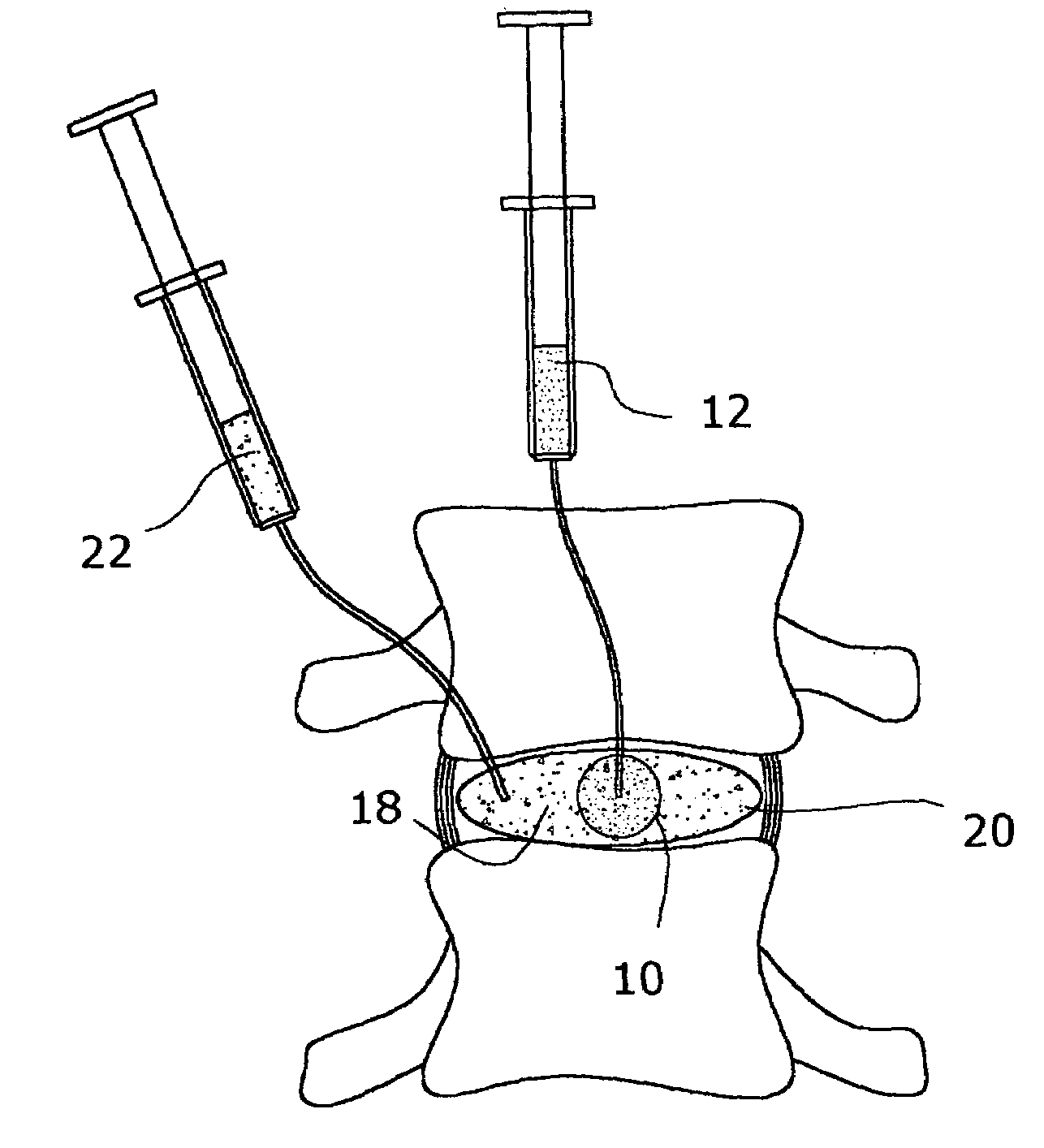
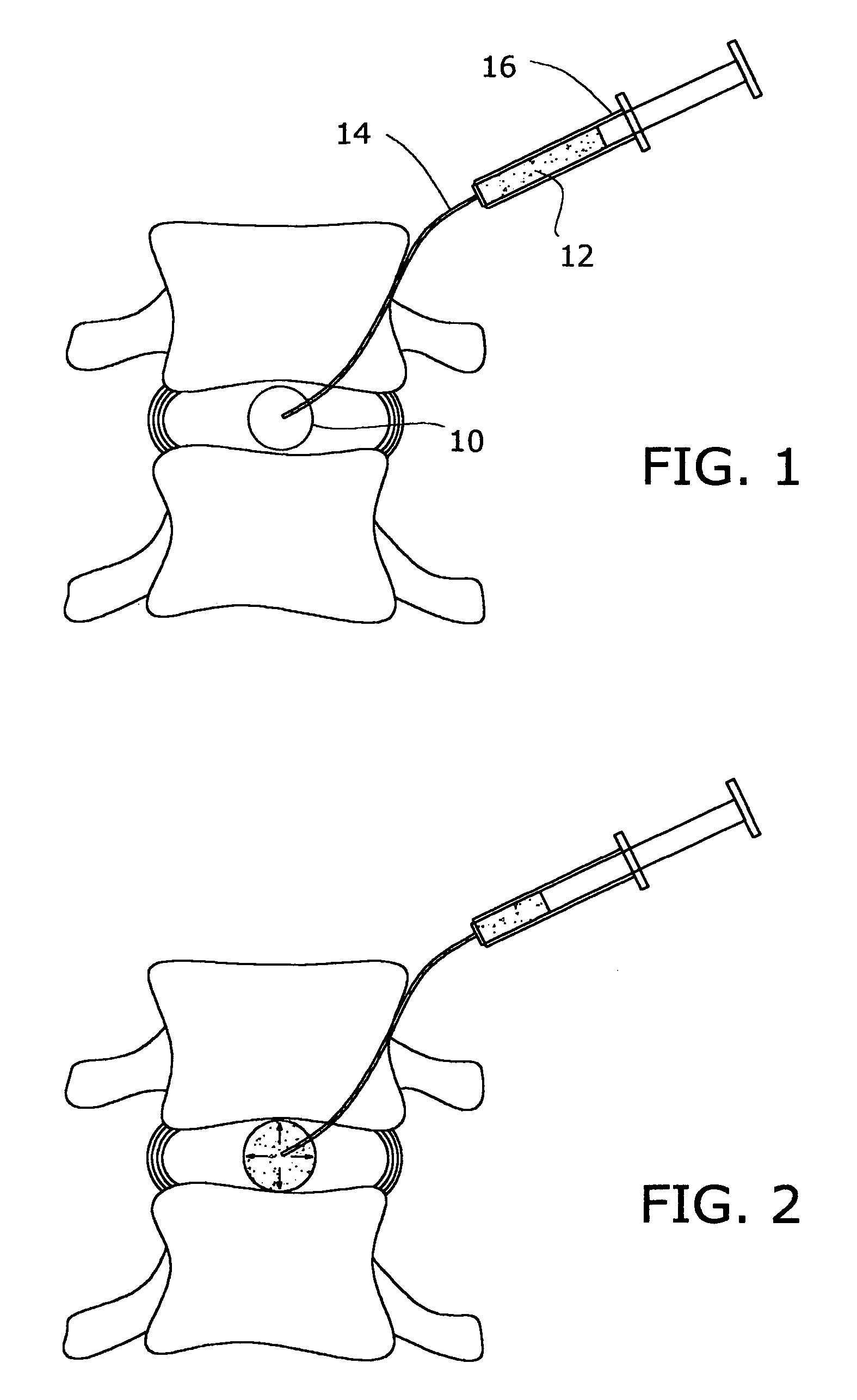
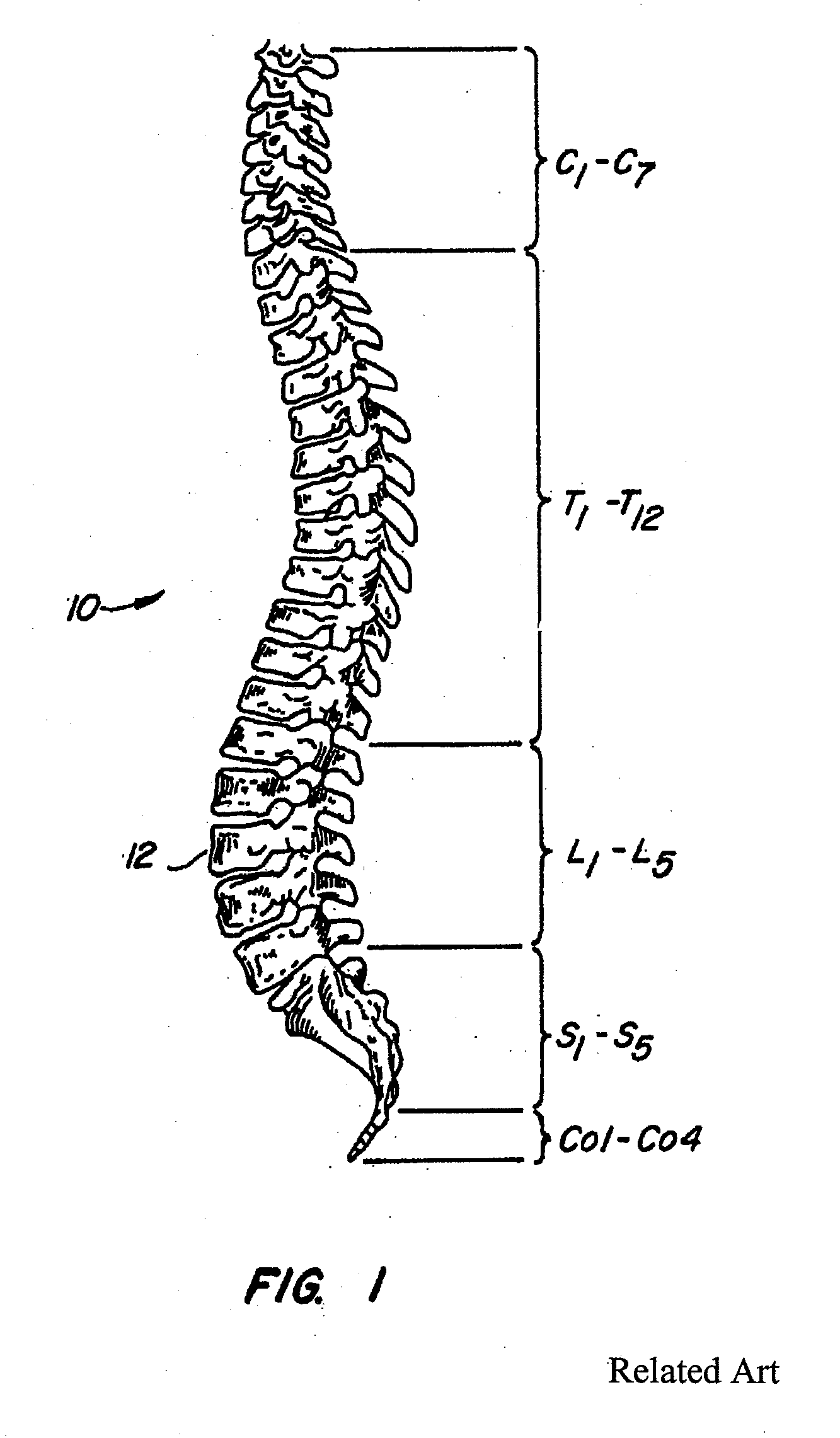
Inflatable nuclear prosthesis
InactiveUS6958077B2Reduce and eliminate abnormal stressSolve the lack of heightJoint implantsSpinal implantsMedicineProsthesis
The nucleus of an intervertebral disc is replaced with a construct including a distendable sack or balloon which is inflated with a hardenable material and is detached in situ when the injected material has hardened. Alternatively, two nested balloons may be inserted, and then filled with materials which have different hardnesses when cured, to simulate a natural disc.
Owner:SUDDABY LOUBERT
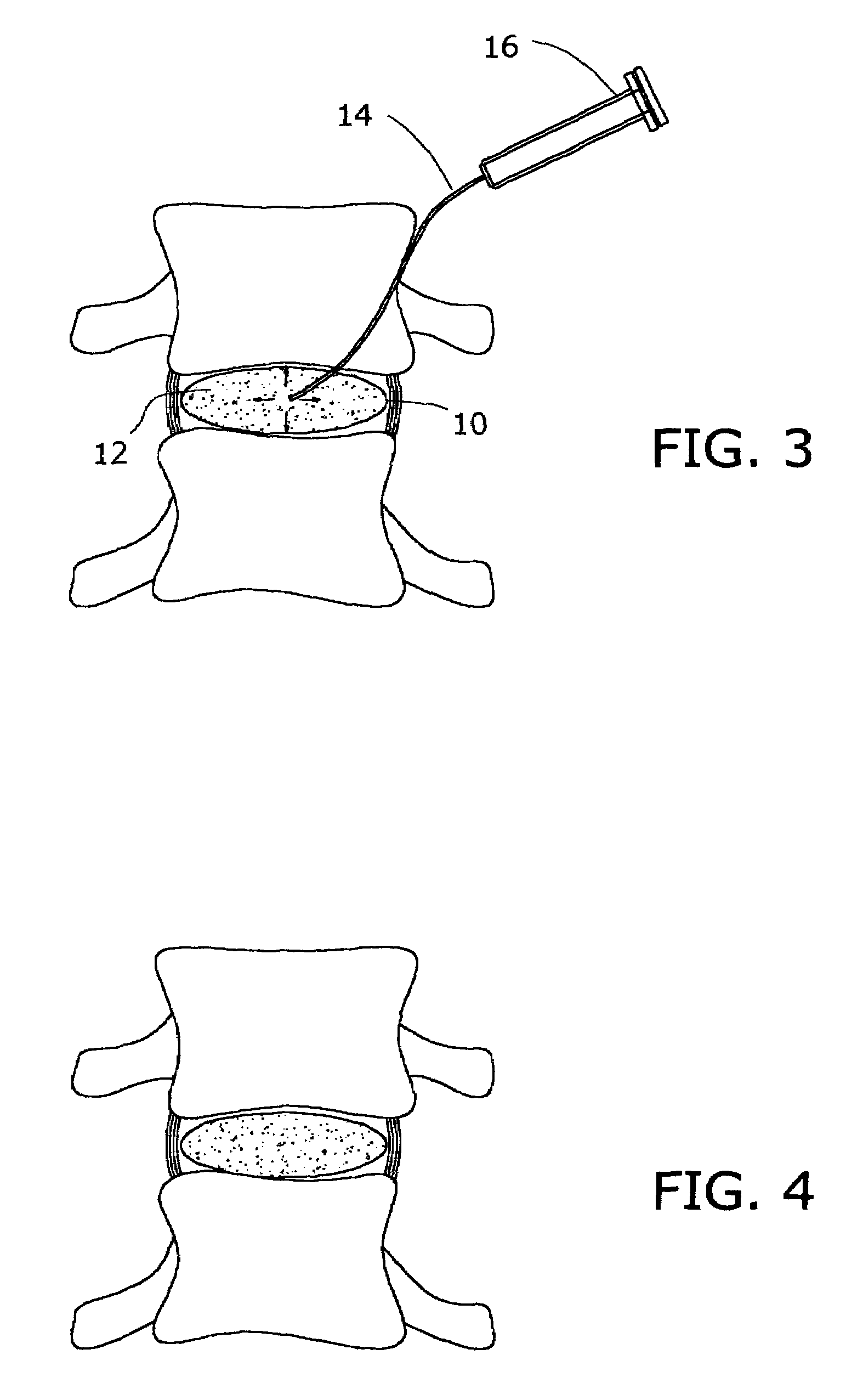
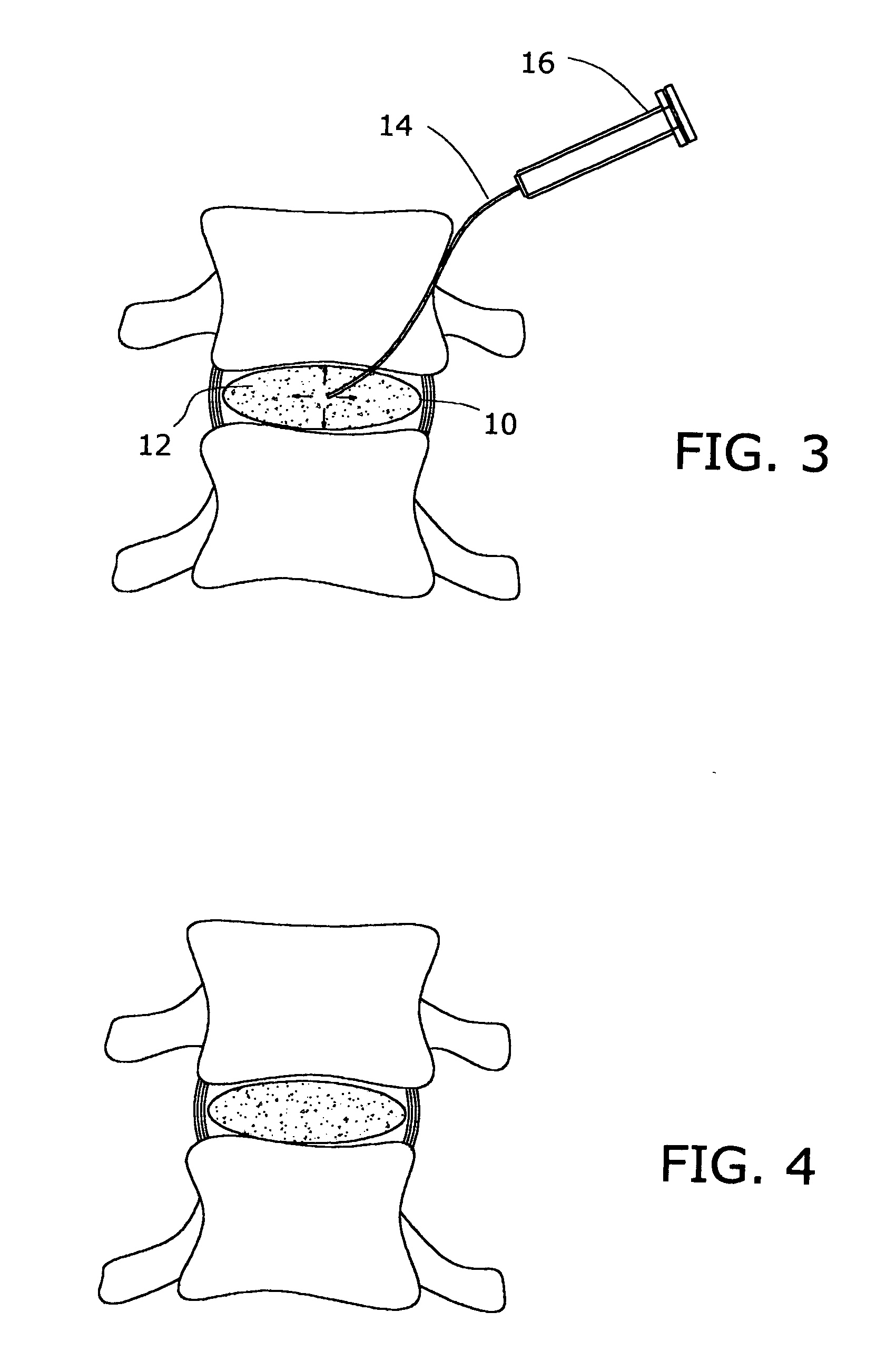
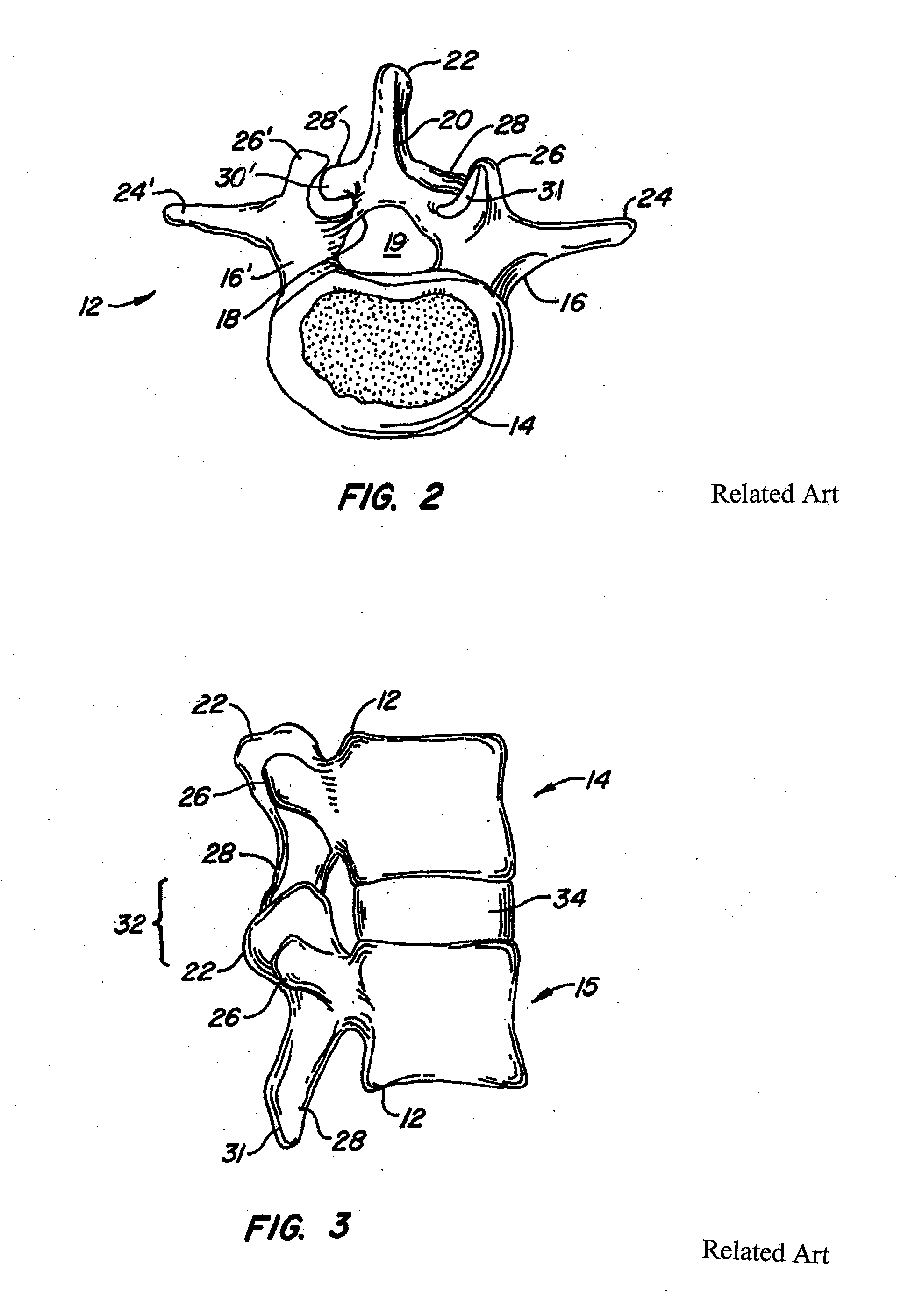
Apparatus and method for fixation of osteoporotic bone
InactiveUS6887246B2Safely introducedSatisfy safety performance requirementsSurgical needlesJoint implantsSurgical treatmentOsteoporotic bone
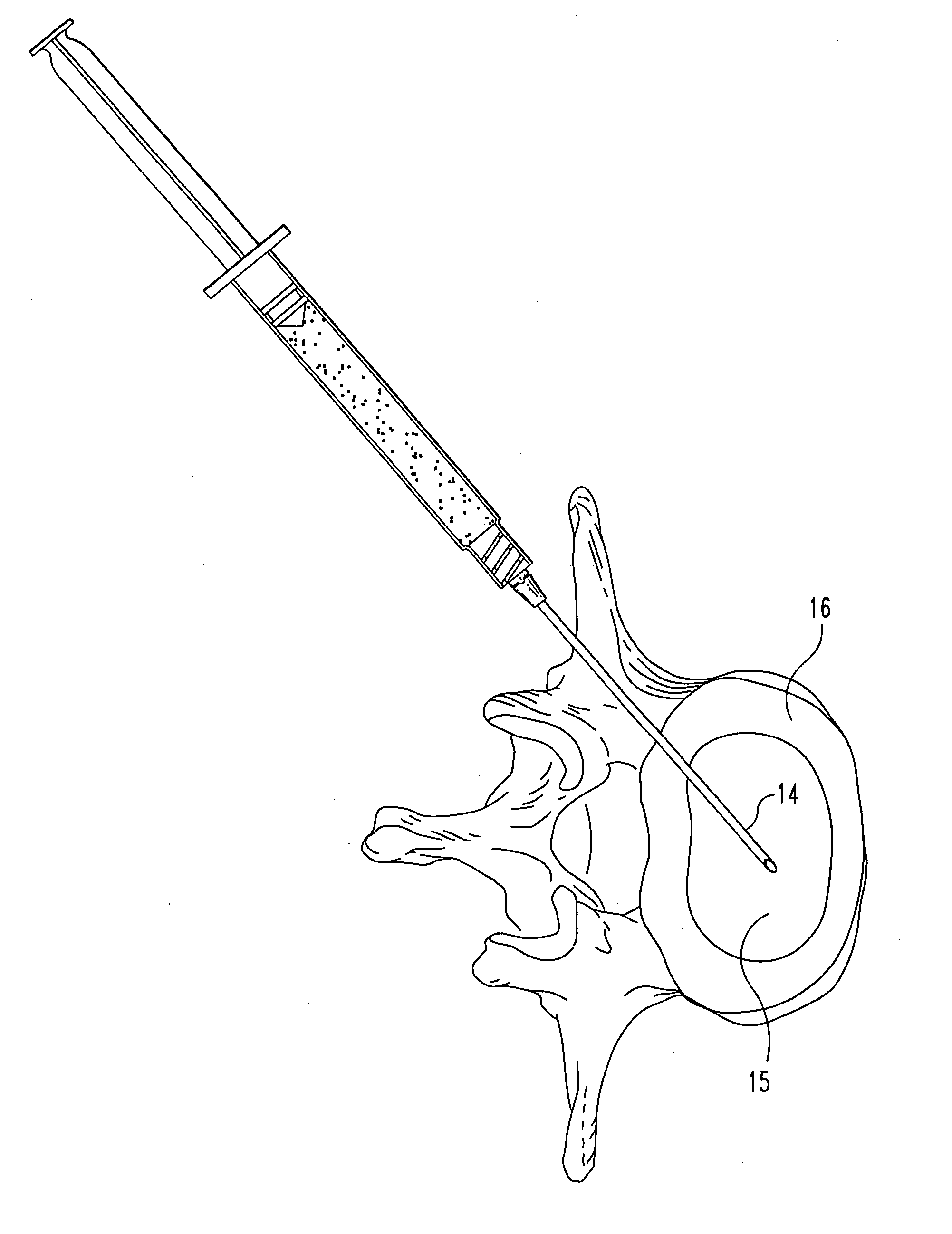
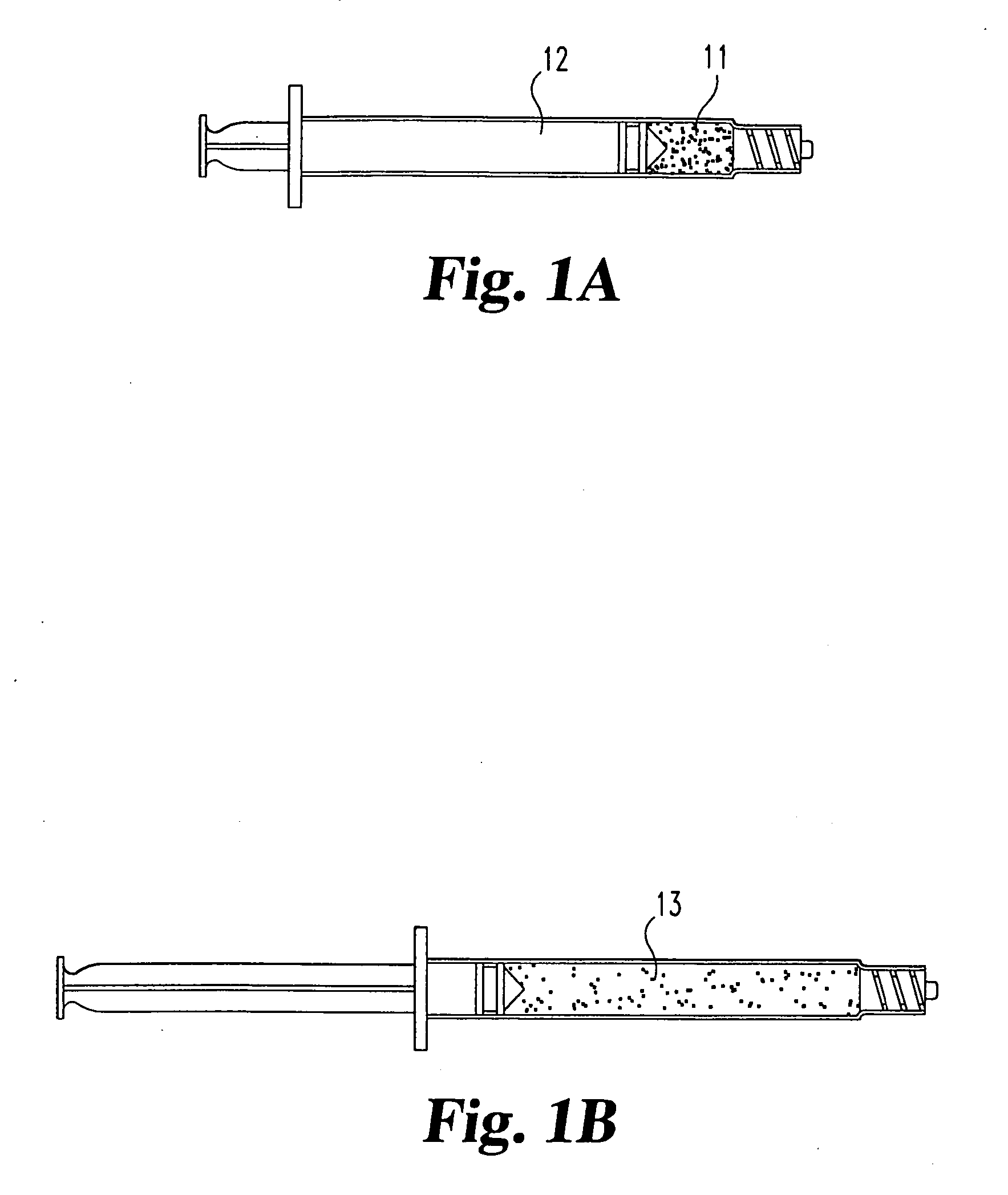
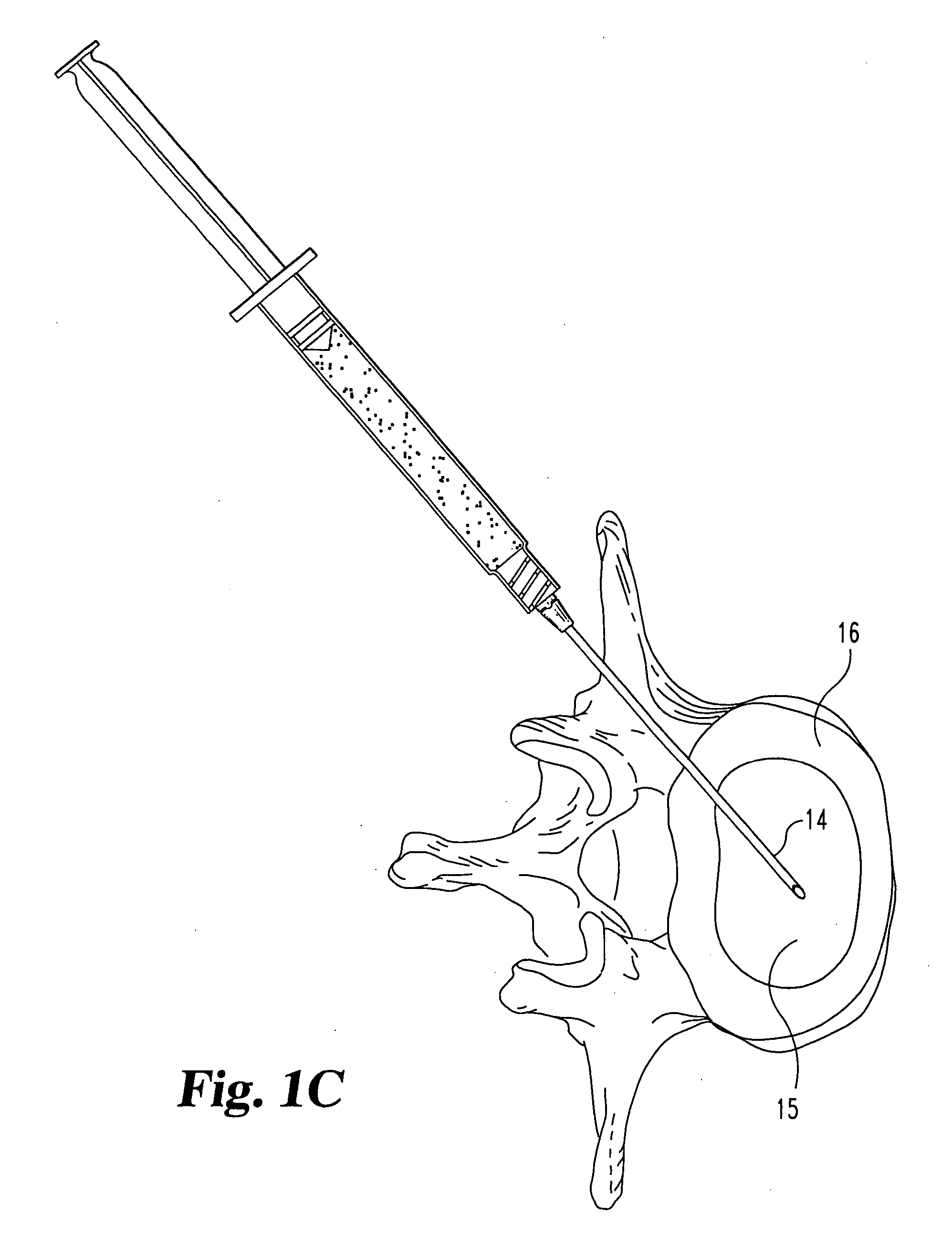
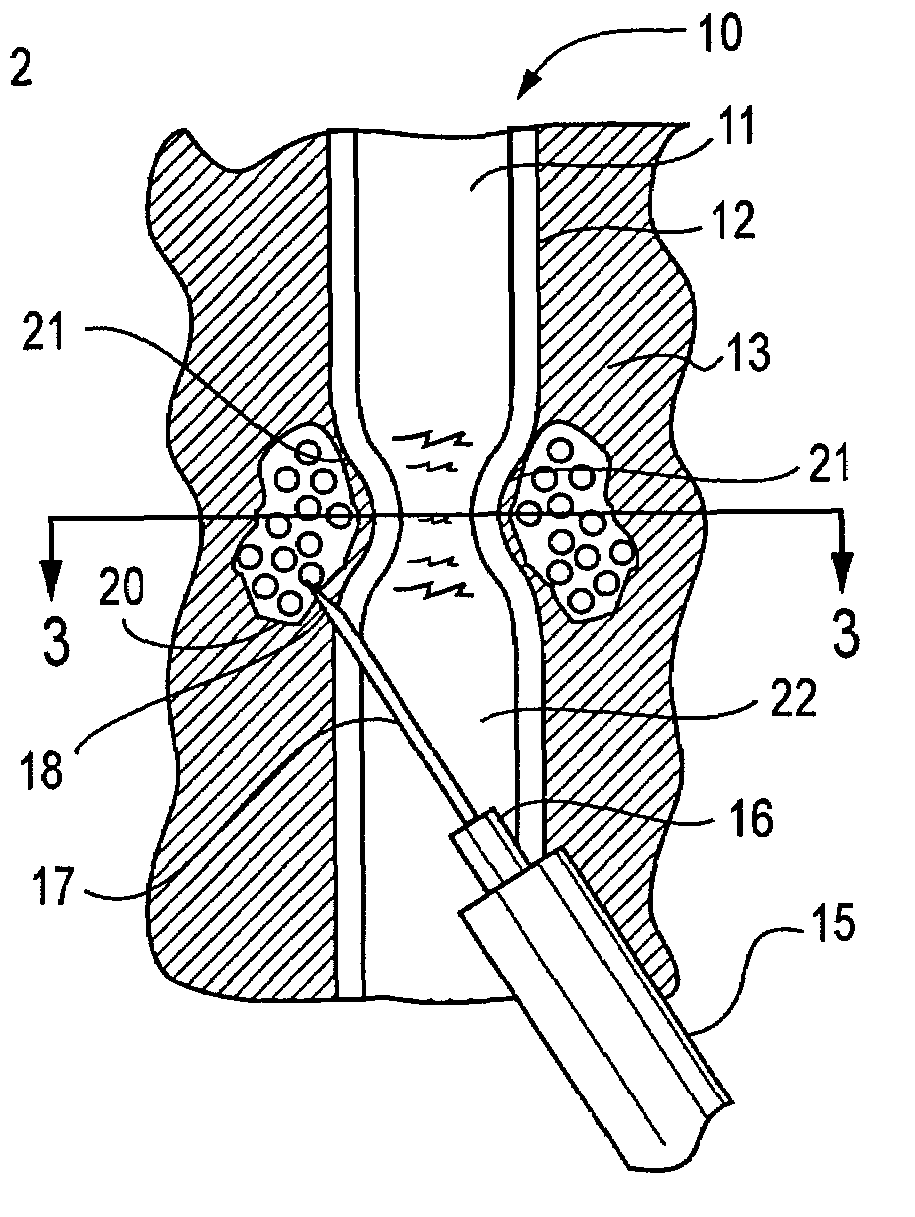
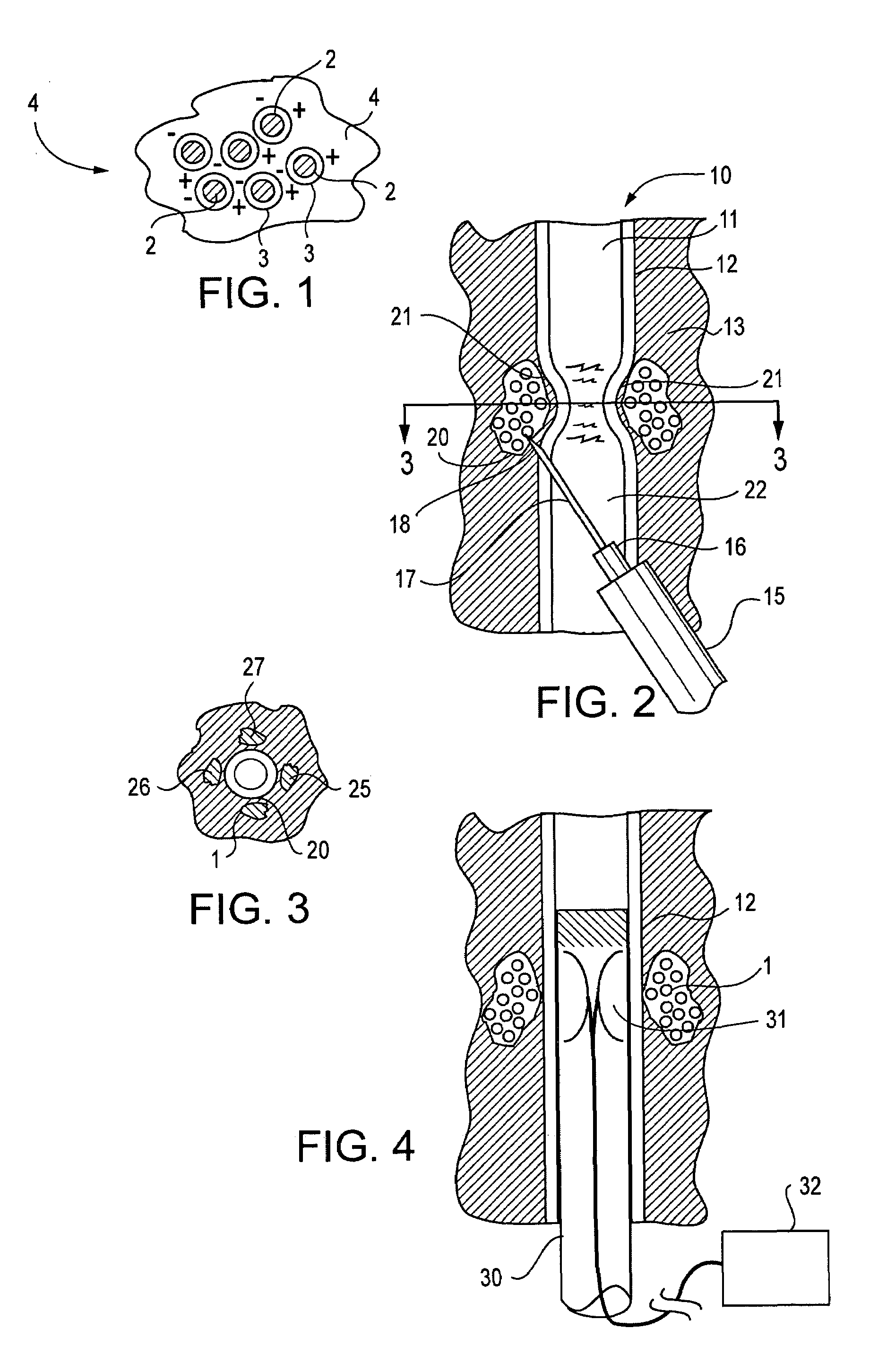
A Novel surgical apparatus and method of use in osteoplasty and other methods of injecting materials into a subject for medical purposes. The present invention particularly relates to the surgical treatment of traumatic, pathogenic, or osteoporotic bone conditions of the human and other animal body systems and more particularly, to a novel apparatus and method for injection of a material into a lesion of a vertebral body or other bony structure.
Owner:ZIMMER BIOMET SPINE INC
Compositions and methods for treating intervertebral discs with collagen-based materials
ActiveUS20050119754A1Enhance the imagePromote healingInternal osteosythesisLigamentsIntervertebral discInjected material
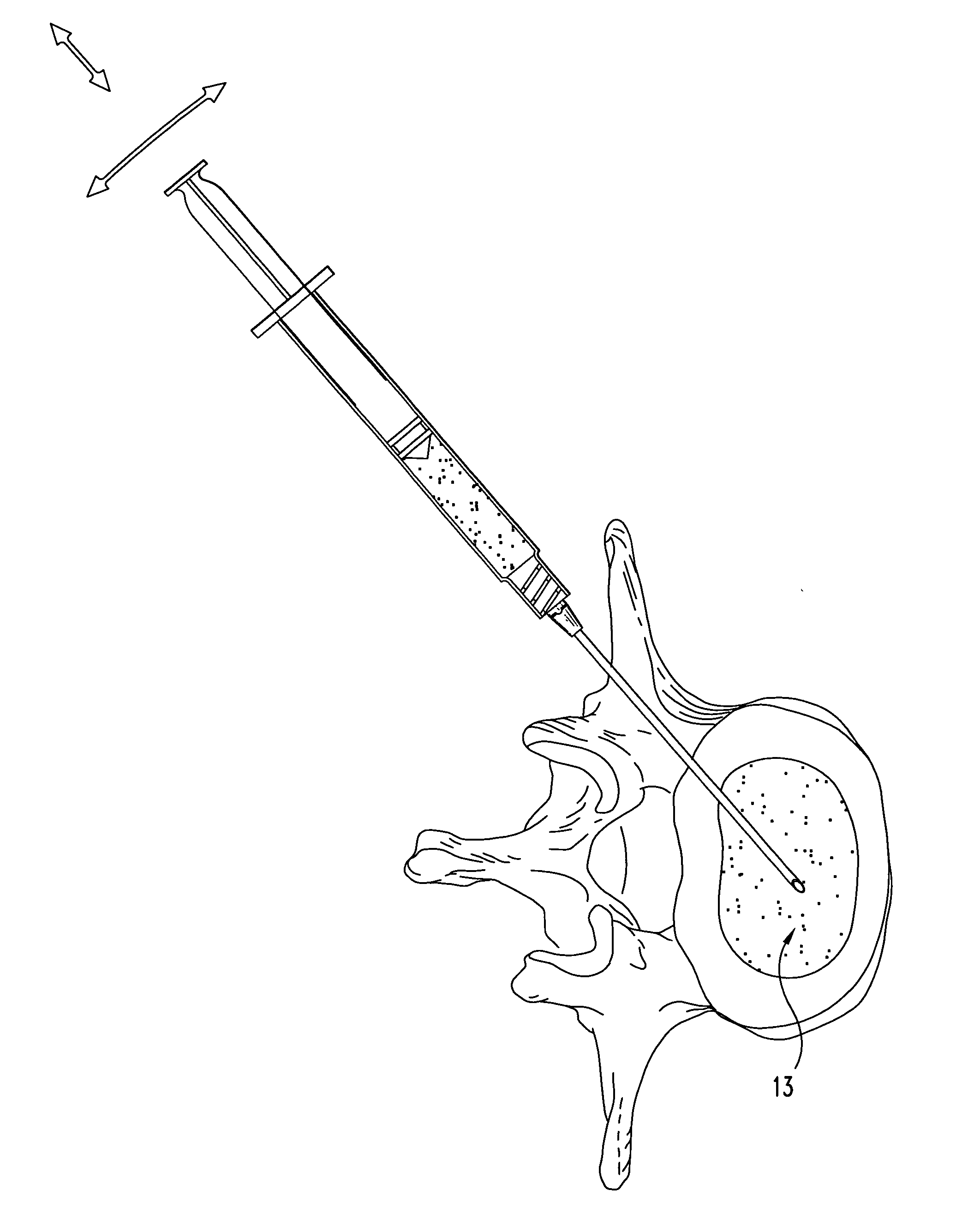
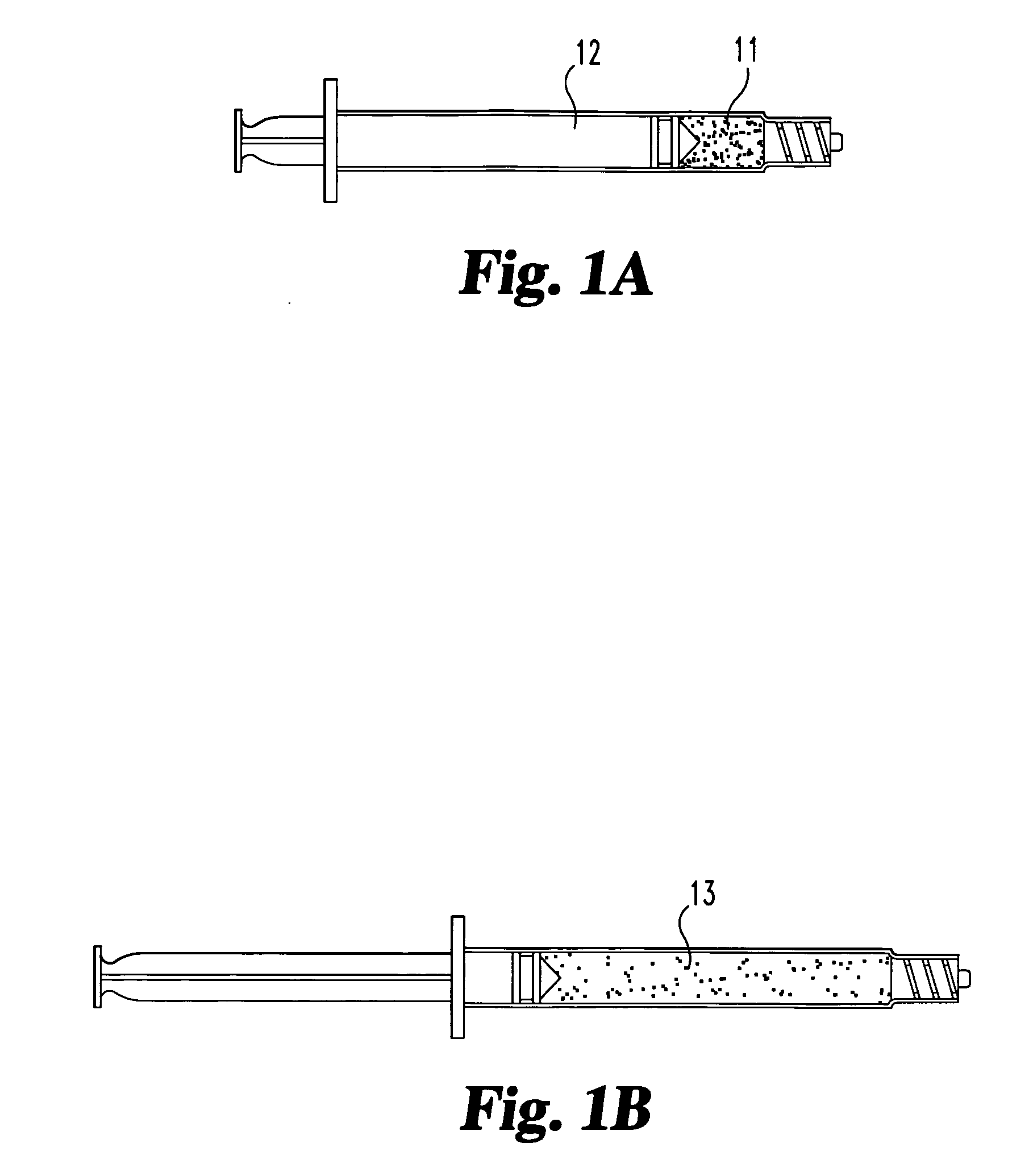
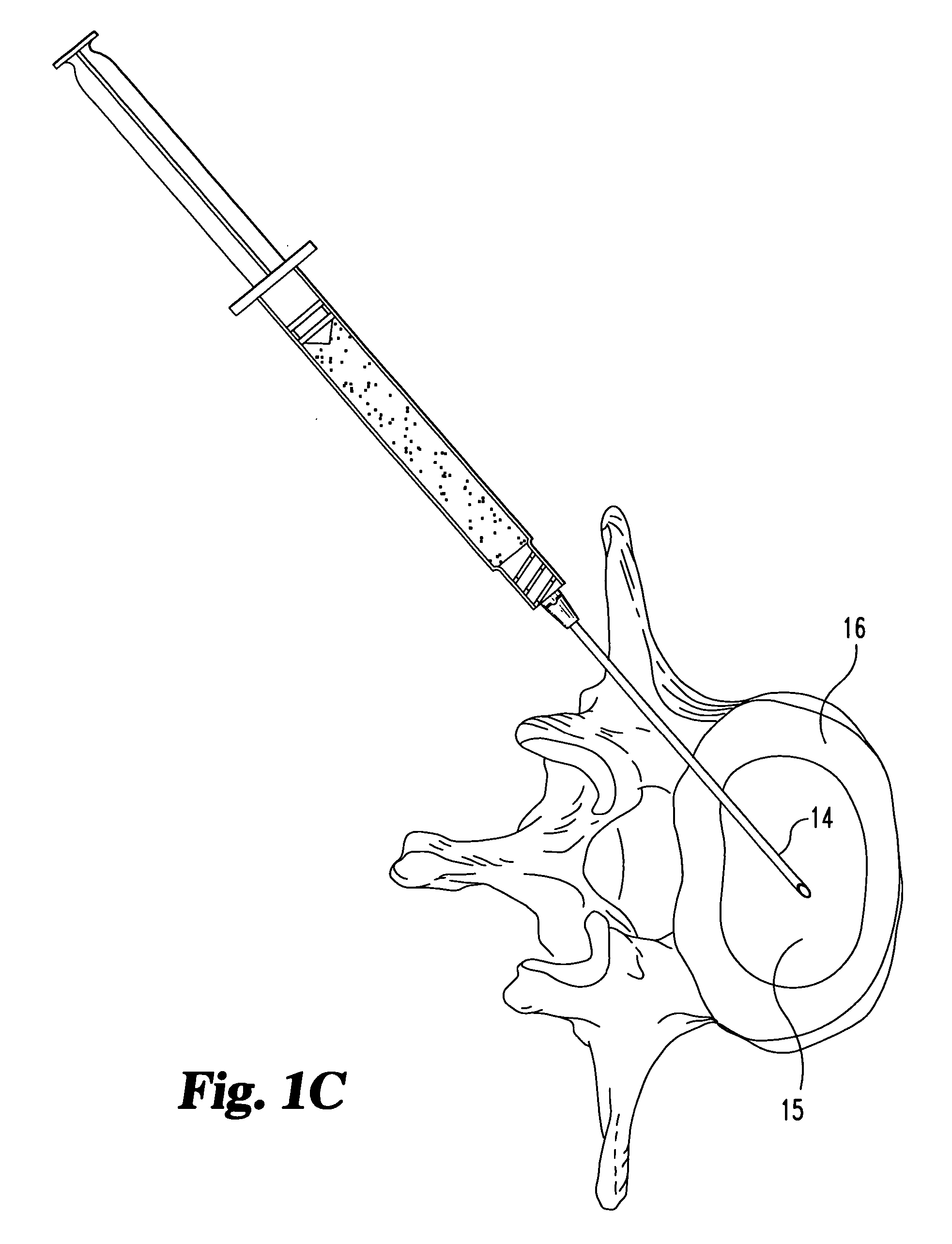
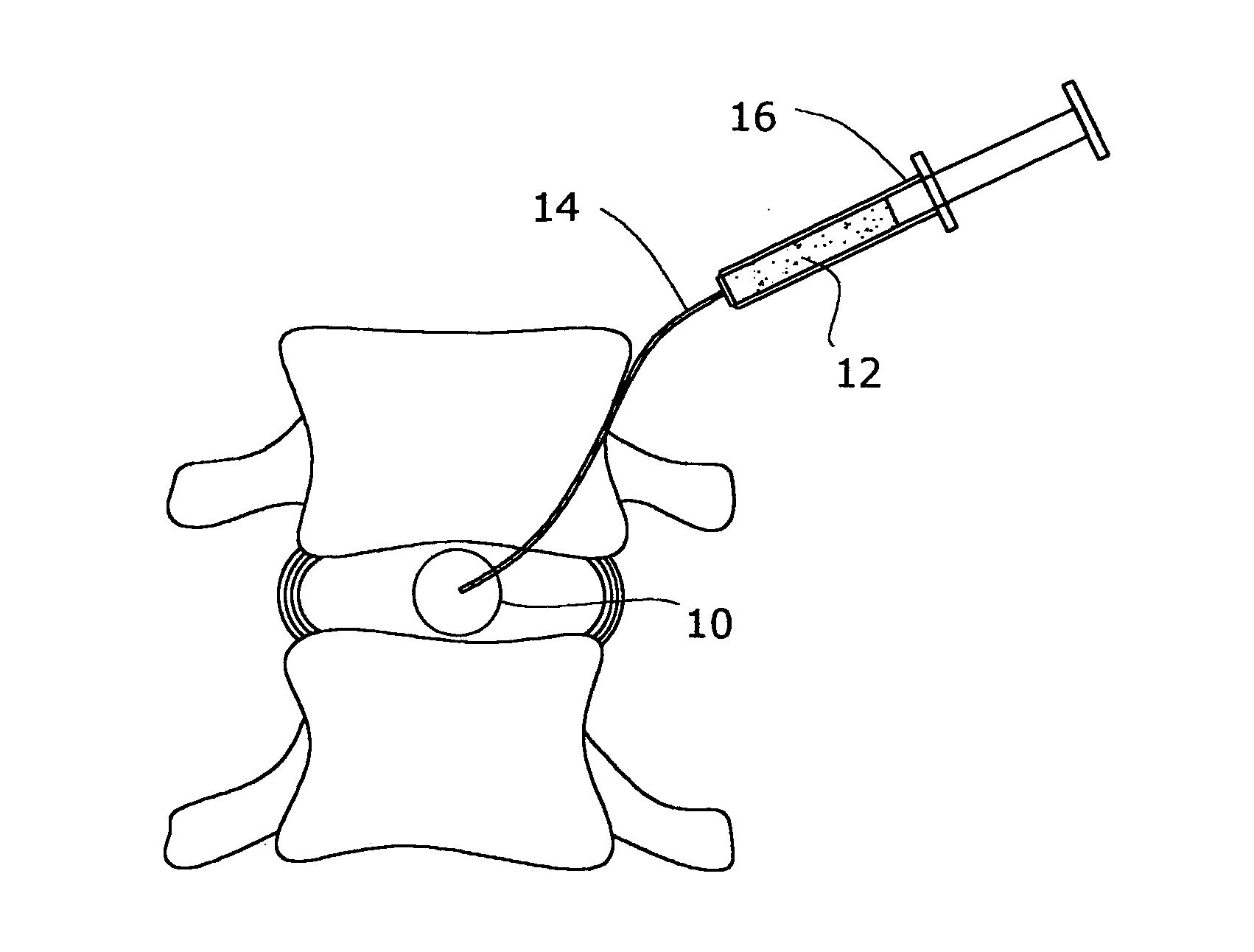
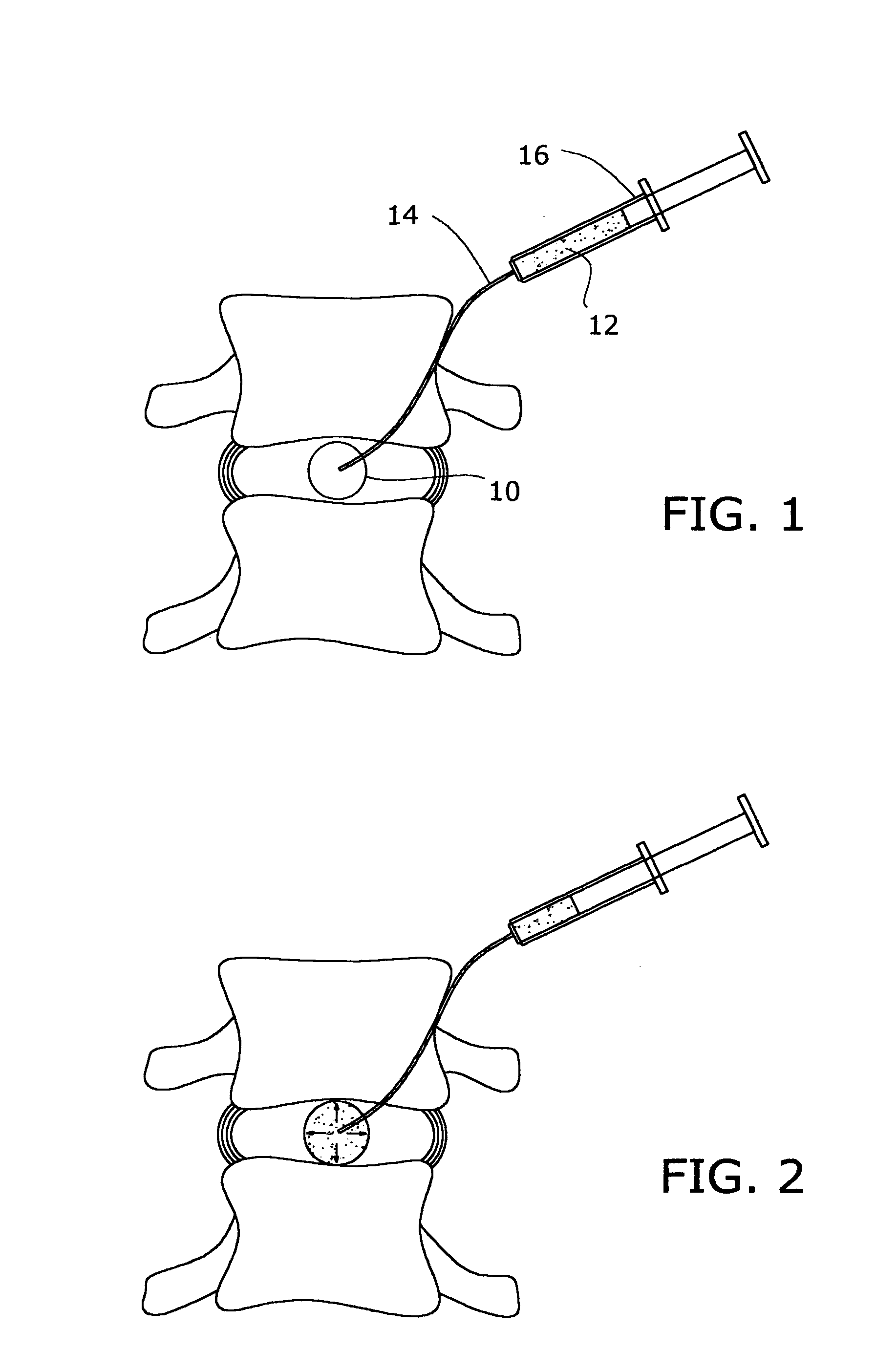
A method of augmenting an intervertebral disc nucleus by injecting or otherwise adding to a disc nucleus a plurality of particles of natural, collagen-rich tissue. The mean particle size of the pieces of natural, collagen-rich tissue may be between 0.25 mm and 1.0 mm. The particles may be dehydrated before implantation, and rehydrated after implantation, or they may be implanted in a “wet” state—such as a slurry or gel. Radiocontrast materials may be included to enhance imaging of the injected material. Other additives may include analgesics, antibiotics, proteoglycans, growth factors, stem cells, and / or other cells effective to promote healing and / or proper disc function.
Owner:WARSAW ORTHOPEDIC INC
Polymeric joint complex and methods of use
InactiveUS20060085075A1Increase and decrease constraintEasy for flexibilityFinger jointsWrist jointsHuman bodyArtificial joints
The invention describes a variety of implantable artificial joint complexes adapted for implantation within a target joint space within a human body. The joint complexes comprise: an expandable joint segment adapted to fit within the target joint space; and at least one of a first cannulated anchor adapted to engage the expandable joint segment and adapted to engage a bony structure adjacent the target joint space; and a second anchor adapted to engage the expandable joint segment and adapted to engage a bony structure adjacent a target joint space. The invention also discloses methods of implanting a patient specific artificial joint complex. The methods include the steps of: accessing a target joint space by creating an access hole through an adjacent bony structure; inserting a joint complex device having a cannulated anchor and an expandable joint segment through the access hole with the expendable joint segment being positioned between the surfaces forming the joint; injecting material into the expandable joint segment; and sealing access to the target joint space.
Owner:GMEDELAWARE 2
Inflatable nuclear prosthesis
ActiveUS20050027358A1Restores sufficient disc space heightRestore mobilityJoint implantsSpinal implantsMedicineProsthesis
The nucleus of an intervertebral disc is replaced with a construct including a distendable sack or balloon which is inflated with a hardenable material and is detached in situ when the injected material has hardened. Alternatively, two nested balloons may be inserted, and then filled with materials which have different hardnesses when cured, to simulate a natural disc.
Owner:SUDDABY LOUBERT
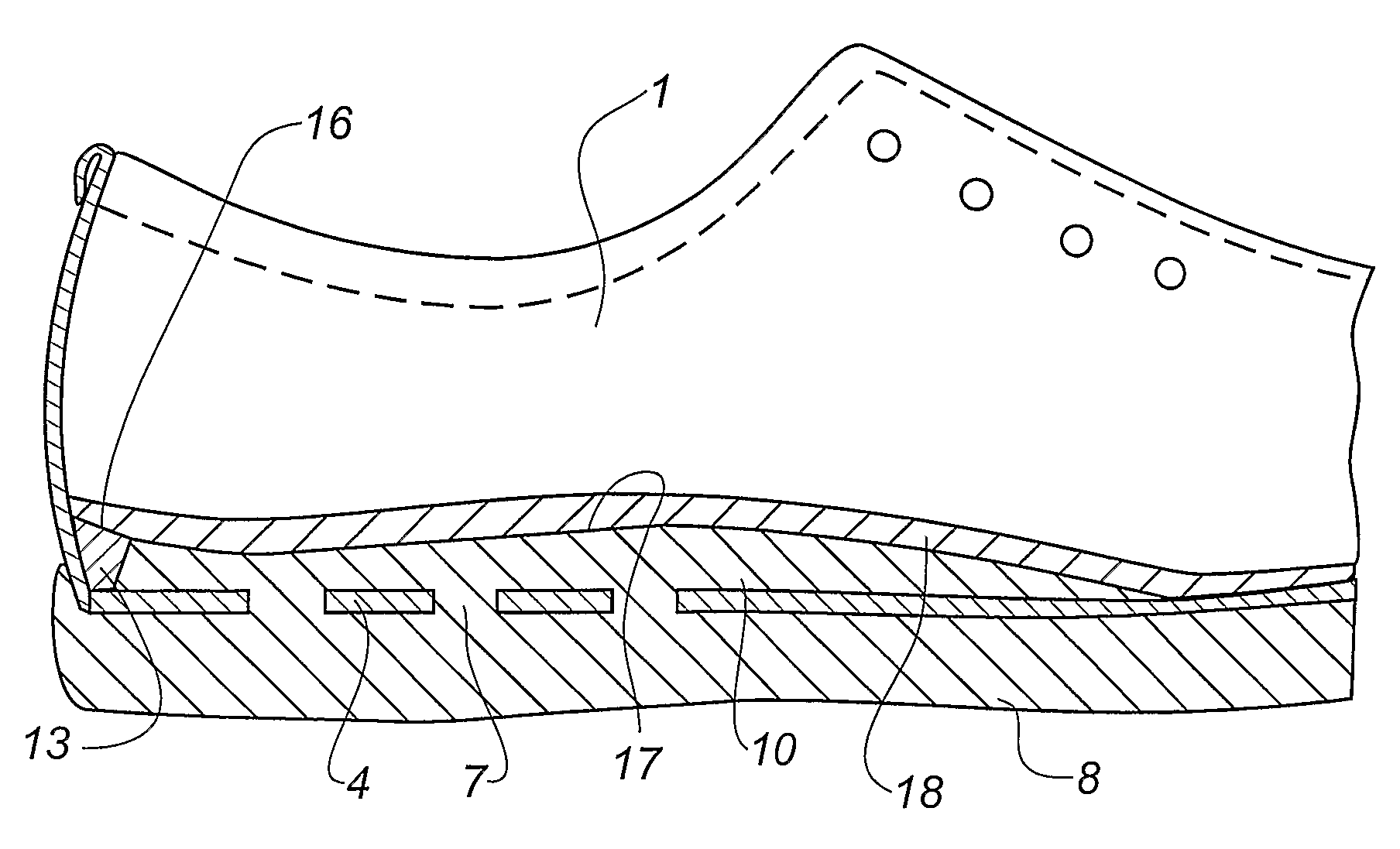
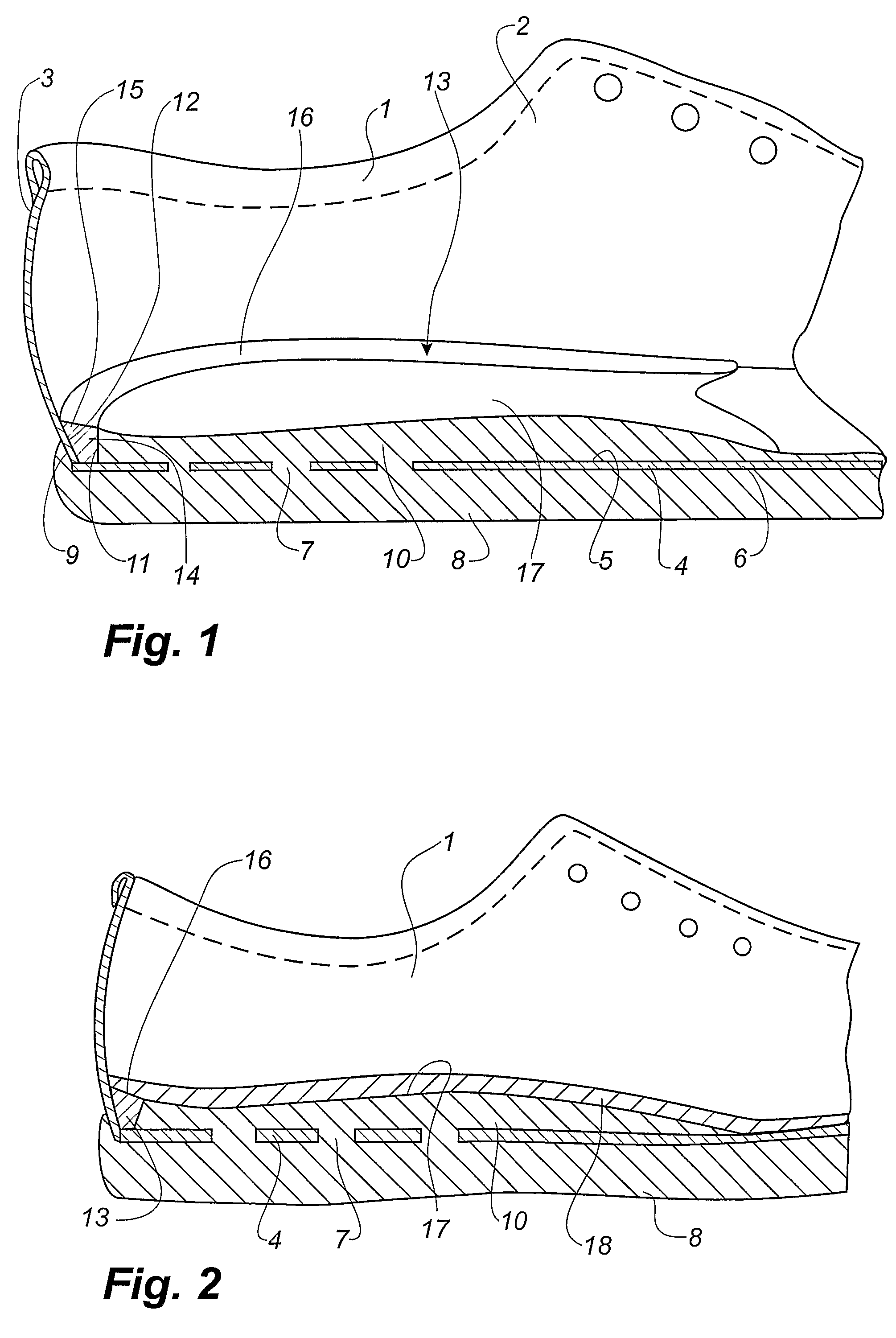
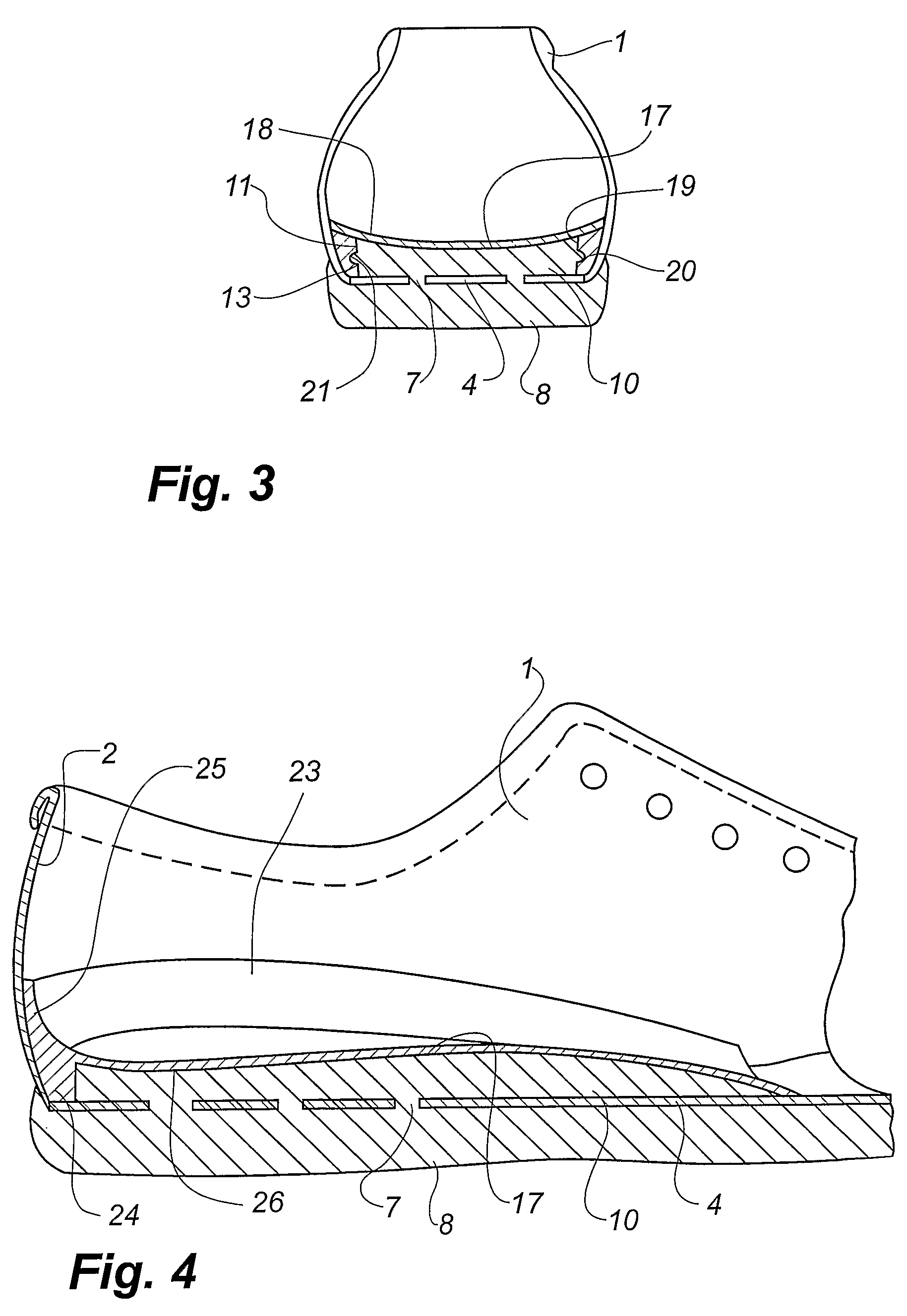
Shoe and a method of making shoes
InactiveUS7743530B2Reduce manufacturing costHigh densityLastingSolesPlastic materialsInjected material
A method of making a shoe, said shoe having an interior sole (10) and an outsole (8) being integrally moulded onto an upper (1) with an insole (4), comprising the following steps: providing of a last (30) having a wedge-shaped recess (32) is its heel area, said recess being defined by a projecting rib (33), providing an upper (1) with an insole (4) on the last (30), said insole (4) being provided with a hole (7) in the heel area, arranging the lower part of the last provided with the upper (1) and the insole (4) in a mould (34), injecting a plastic material into a closed mould cavity formed by the last and the mould, allowing the injected material to harden, whereby the rib (32) of the last (30) forms a corresponding groove (12) between the interior sole (10) and the inner face of the upper (1), removing the upper (1) with the moulded-on soles (8, 10) from the last (30), providing a separately formed insert (13) of a shape substantially corresponding to the shape of the groove (12) and placing the insert in the groove (12).
Owner:ECCO SKO
Collagen-based materials and methods for augmenting intervertebral discs
ActiveUS20050197707A1Enhance the imagePromote collagen crosslinkingInternal osteosythesisPeptide/protein ingredientsIntervertebral discInjected material
A method of augmenting an intervertebral disc by injecting particles of collagen-based material into the disc. The particles may be dehydrated before implantation, and rehydrated after implantation, or they may be implanted in a “wet” state—such as a slurry or gel. Radiocontrast materials may be included to enhance imaging of the injected material. Other additives may include analgesics, antibiotics, proteoglycans, growth factors, and / or other cells effective to promote healing and / or proper disc function.
Owner:WARSAW ORTHOPEDIC INC
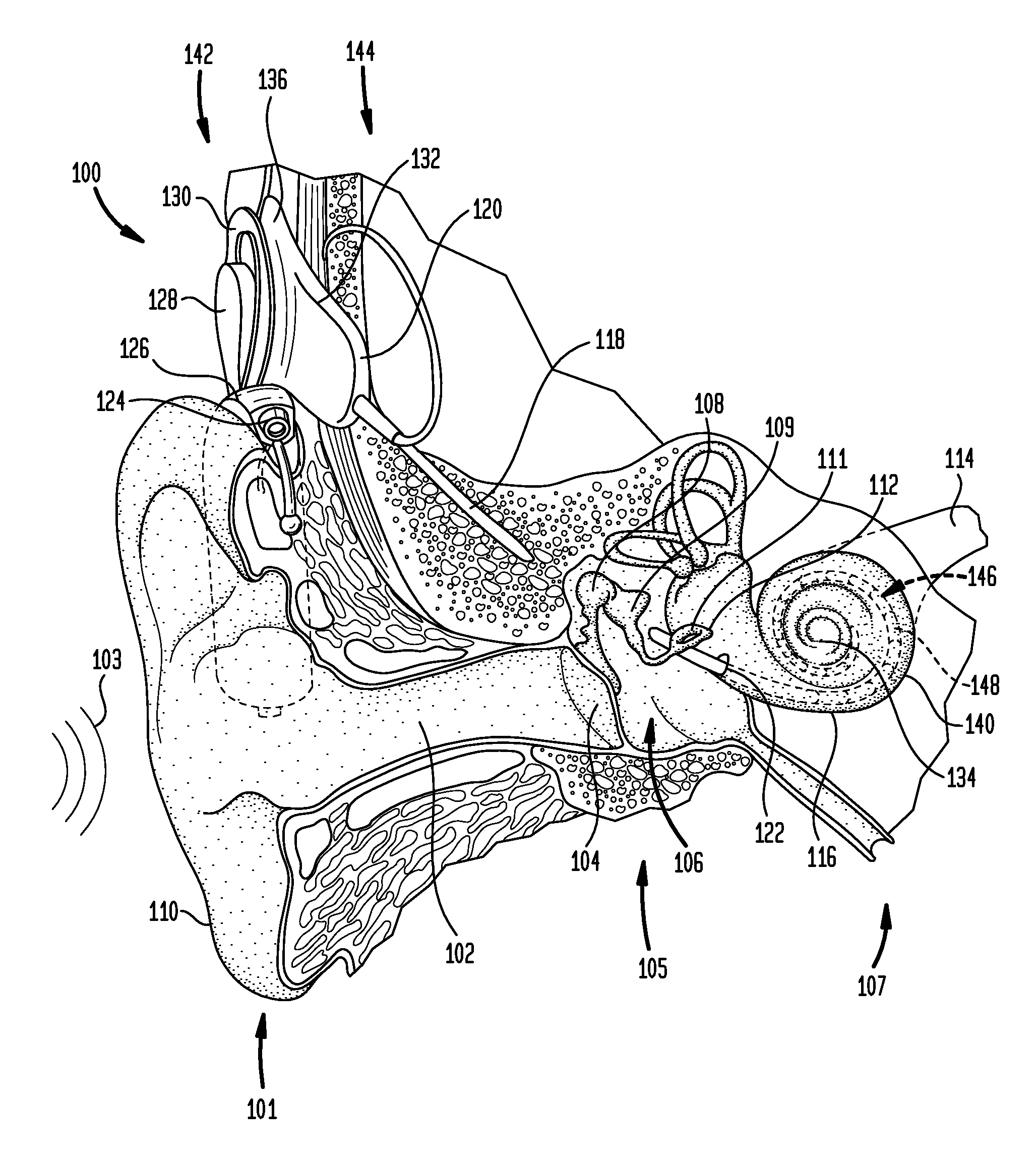
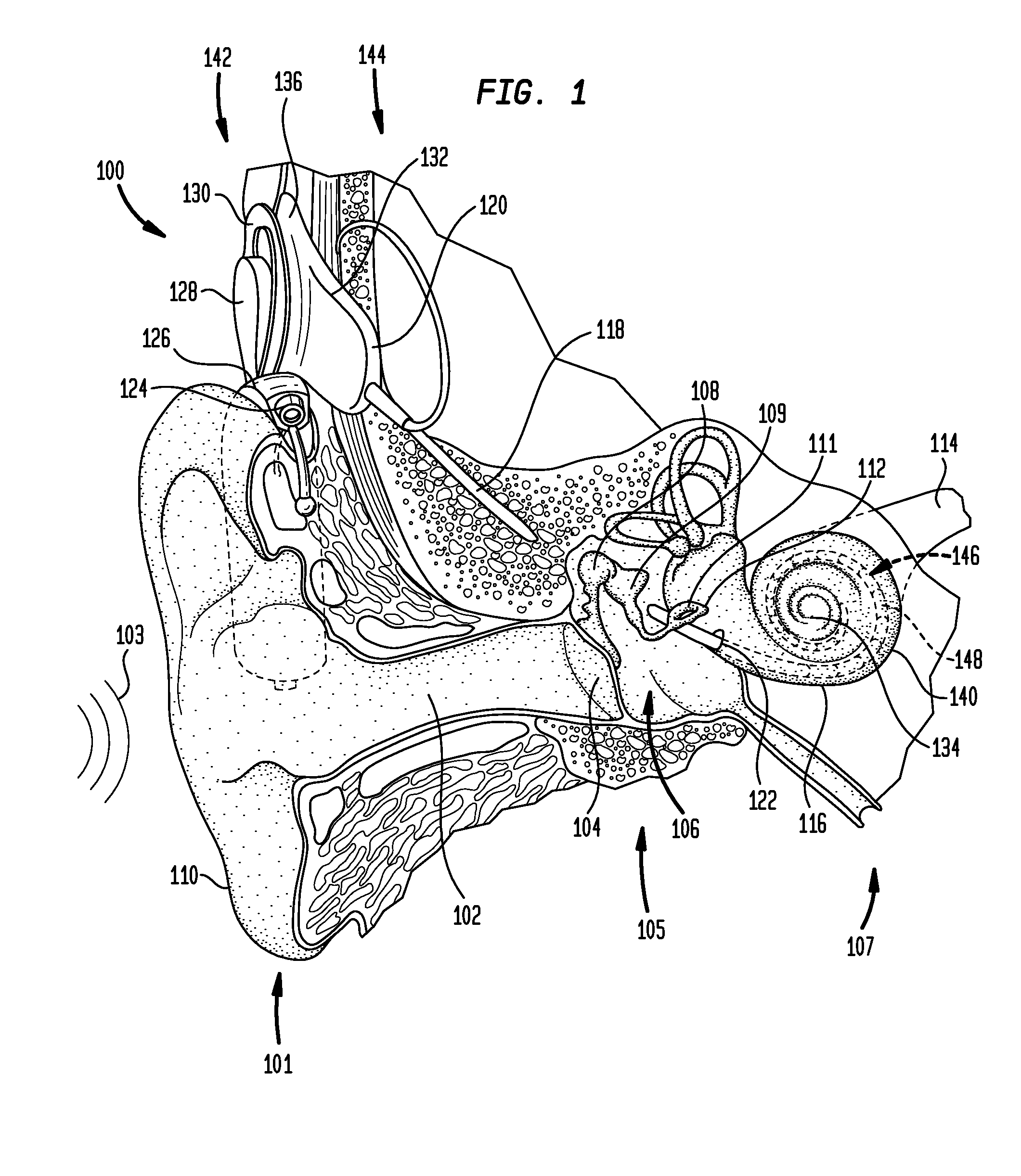
Manufacturing an electrode carrier for an implantable medical device
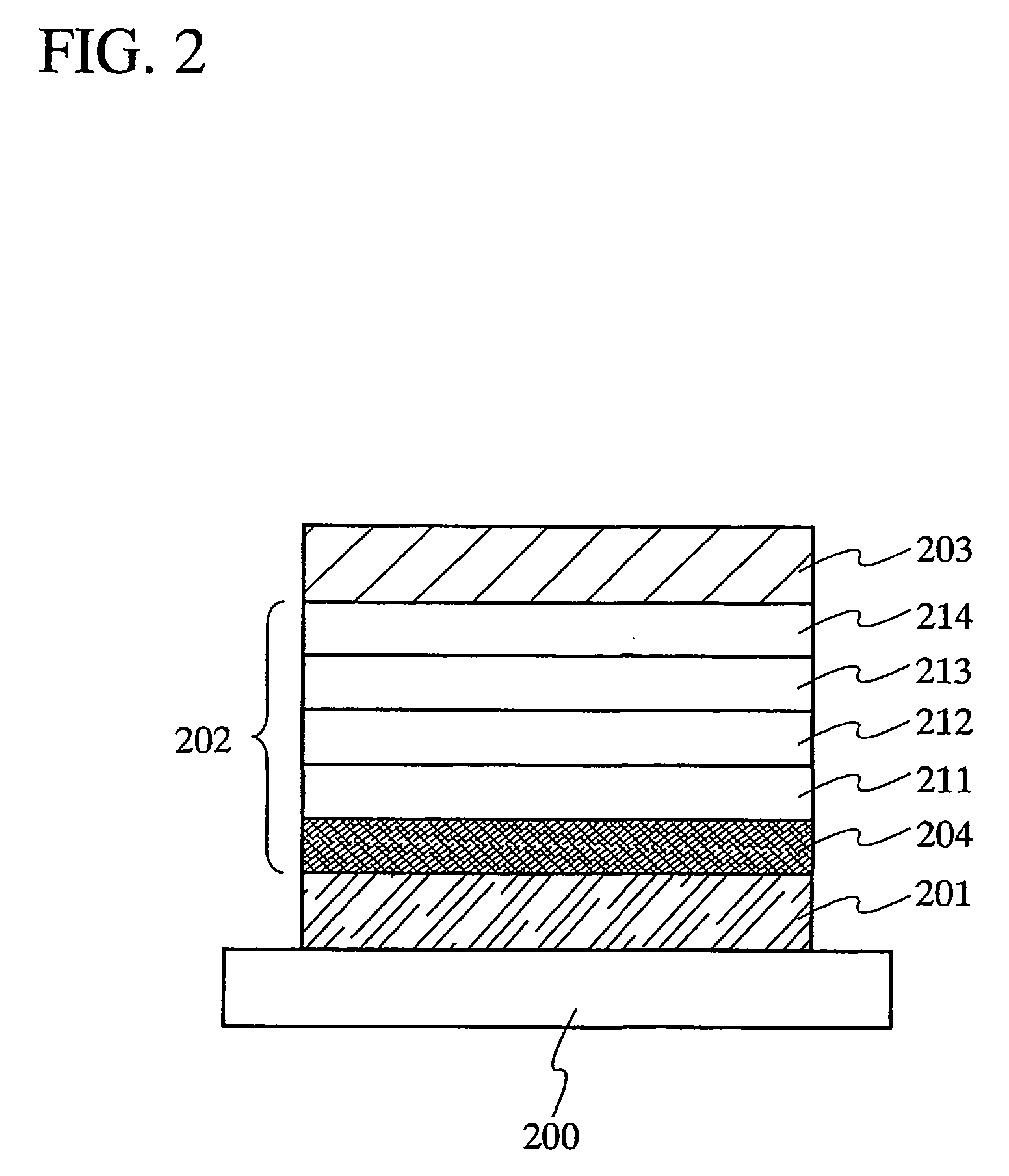
According to one aspect of the present invention, there is provided a method for manufacturing an electrode carrier comprising one or more electrode contacts of an implantable medical device, comprising: coupling at least one electrode to a base plate, securing the base plate in a mold, injecting the mold with injection material at least partially around the at least one electrode coupled to the base plate, curing the injected material, and decoupling the at least one electrode from the base plate such that the cured injection material is separated from the base plate.
Owner:COCHLEAR LIMITED
Device and Method for Injecting Fluids or Gels
ActiveUS20110230833A1Avoiding blood vessel injuryUniform layersAutomatic syringesMedical devicesSkin surfaceInjected material
A device for successively injecting material at predetermined distances between successive injections to body tissue along a desired path of skin surface includes an injector sub-assembly having a needle configured for injecting the material into body tissue, and a displacement sub-assembly configured to facilitate precise displacement of the needle or of the device along the path between successive injections.
Owner:LANDMAN MANIA +2
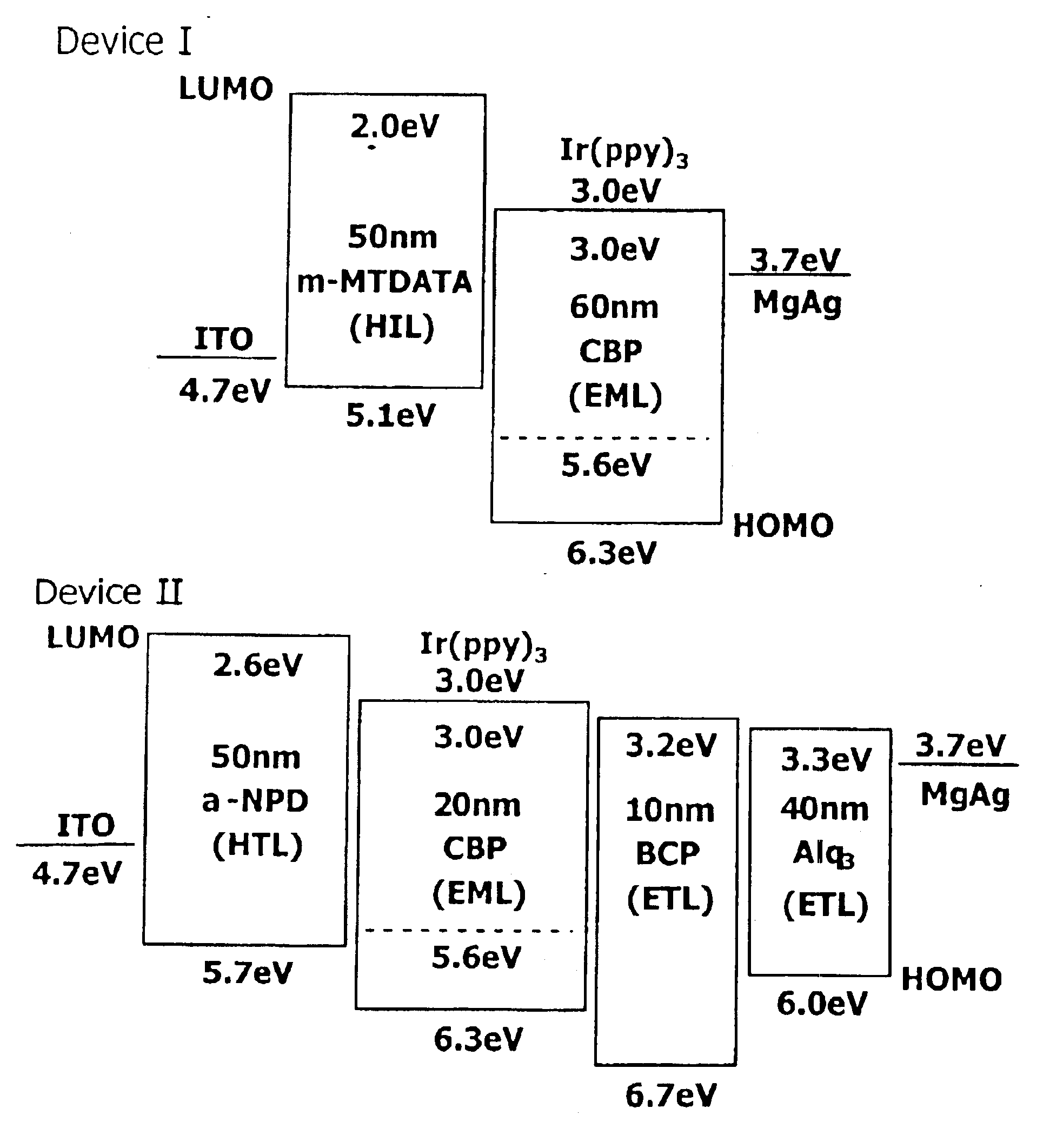
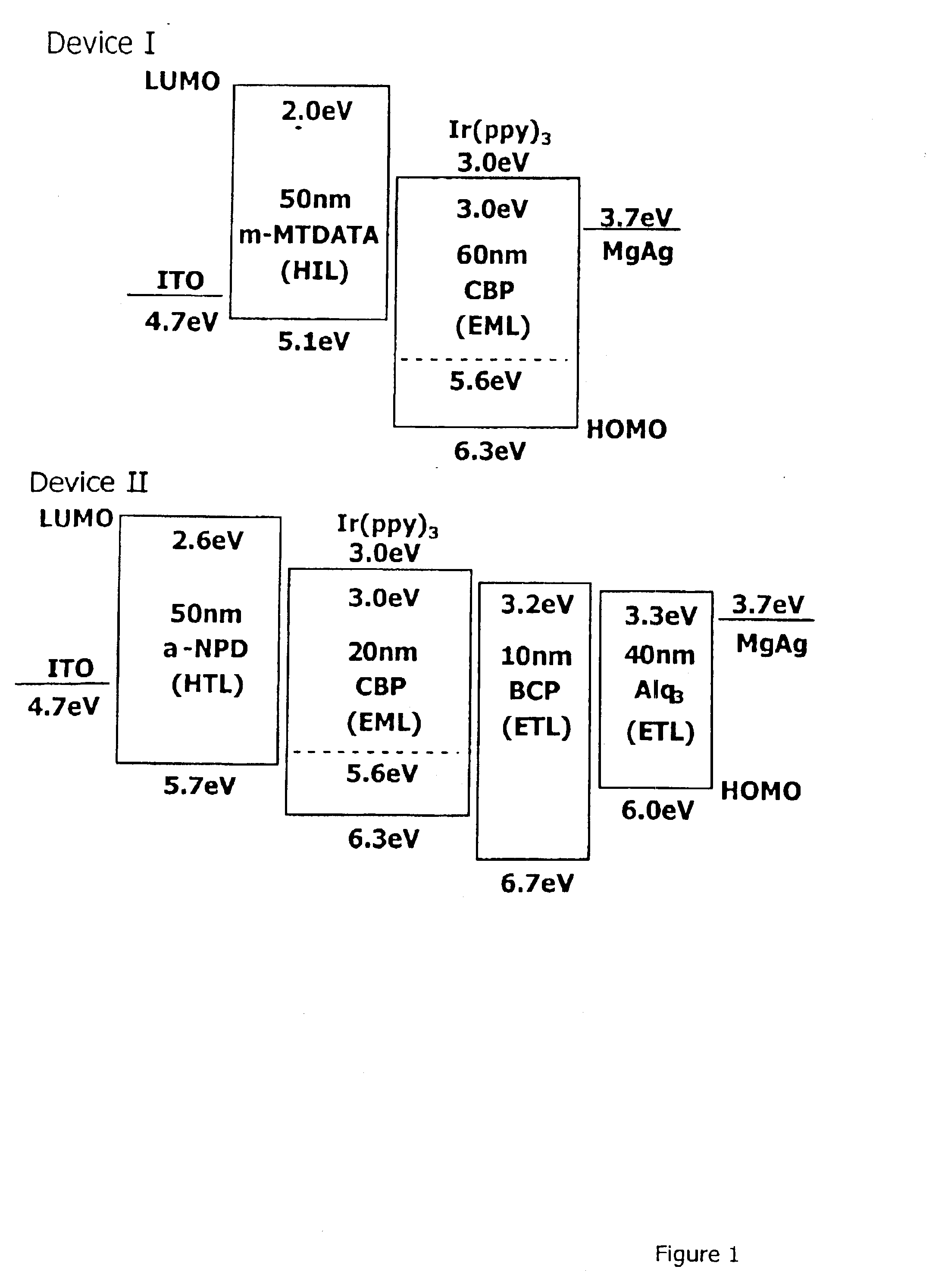
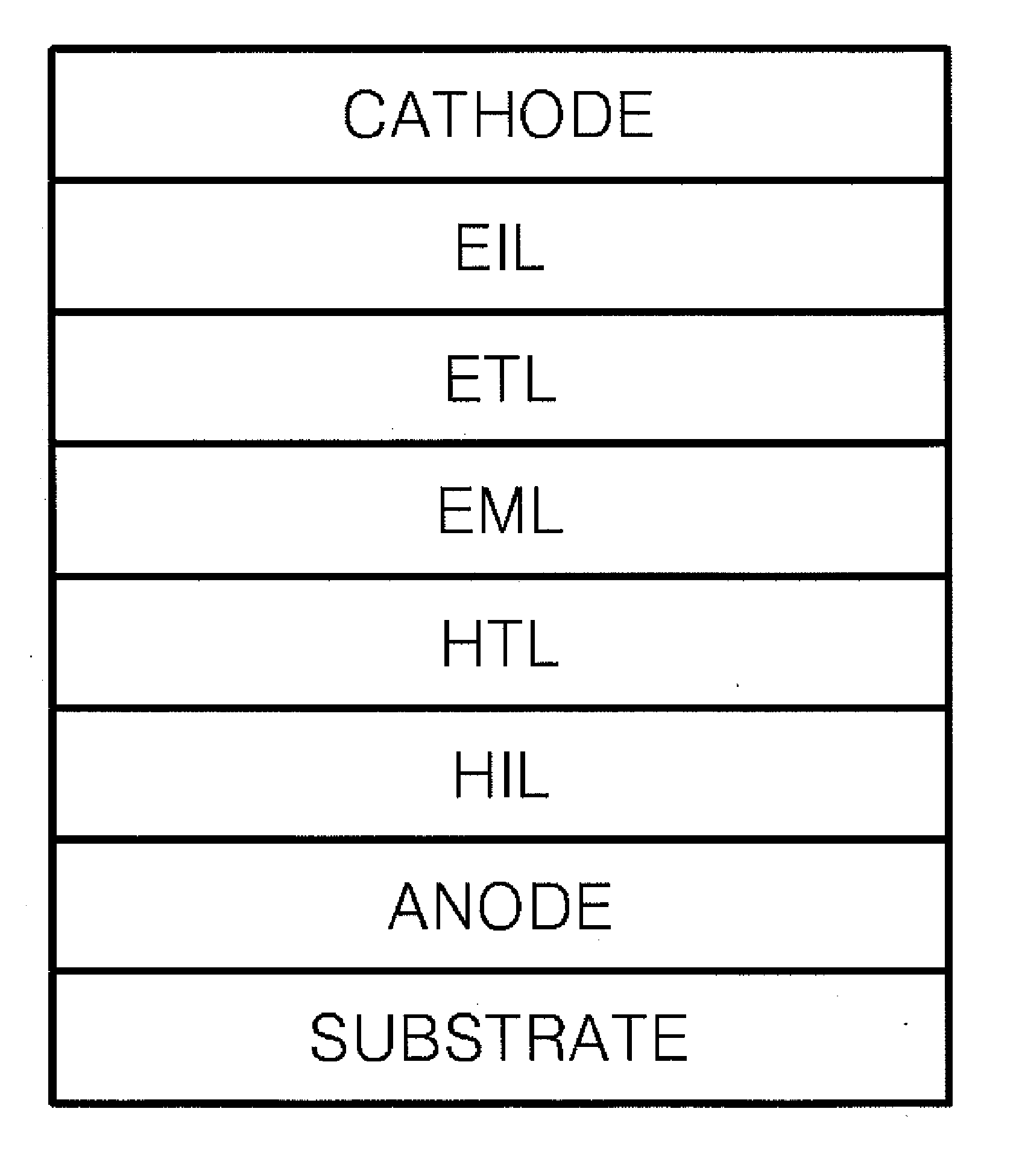
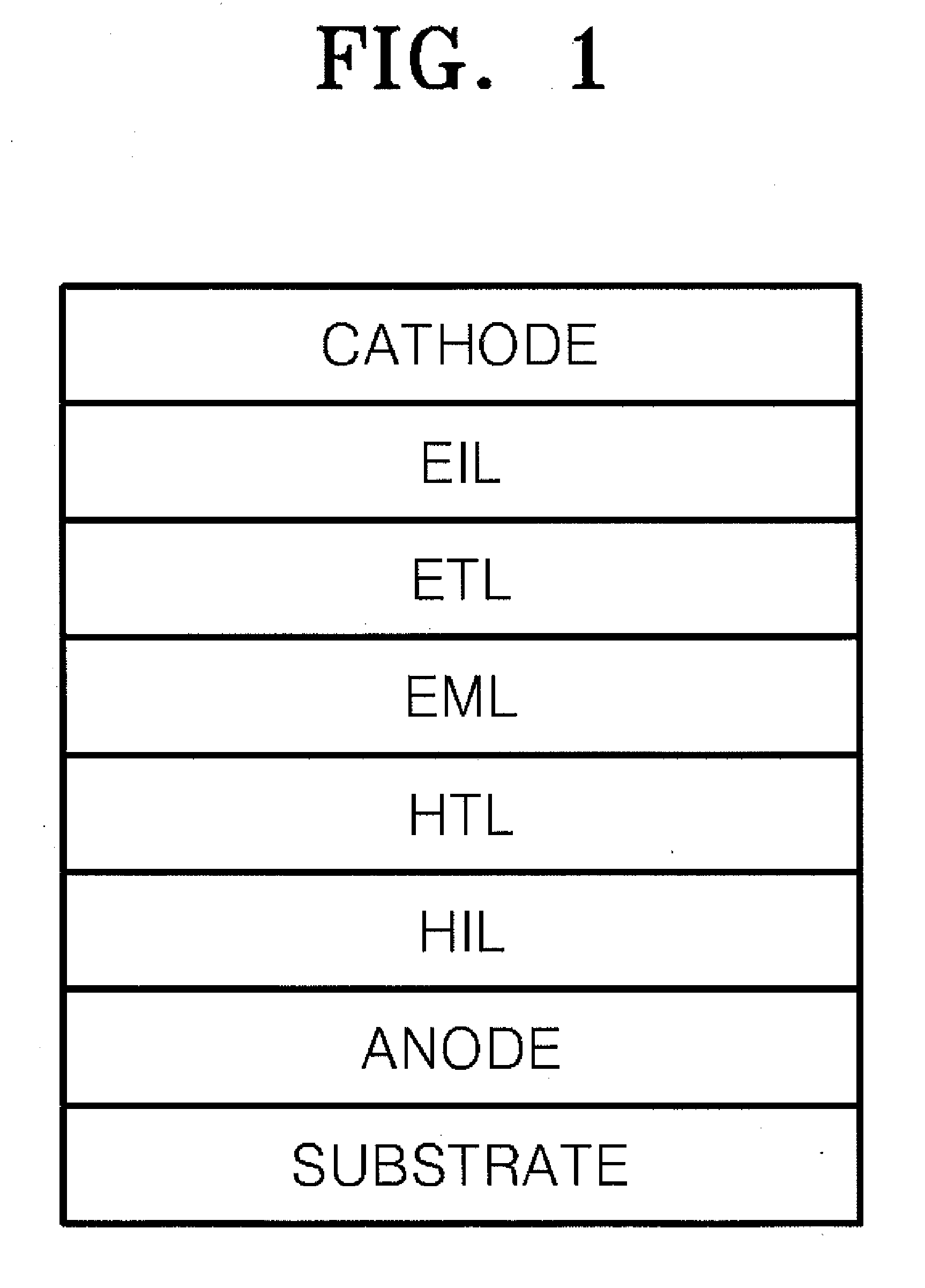
Material for organoelectro-luminescence device and use thereof
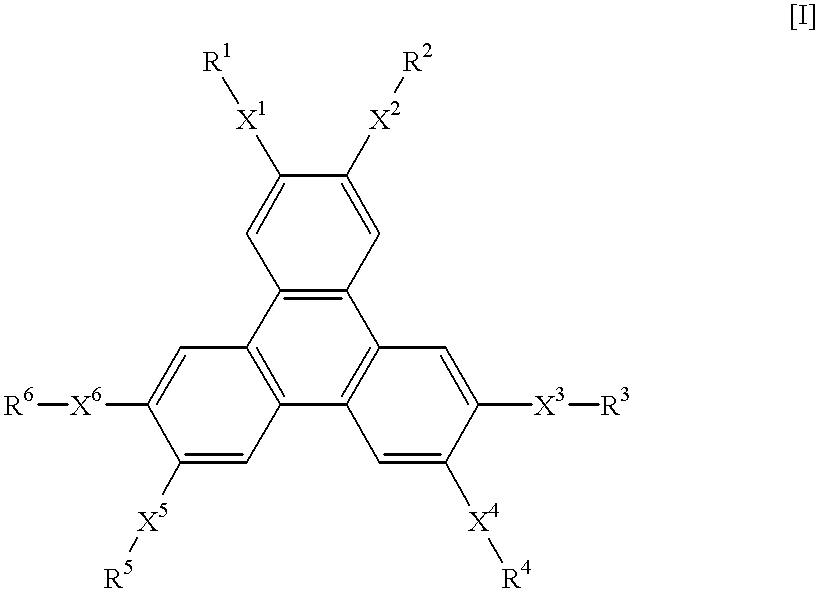
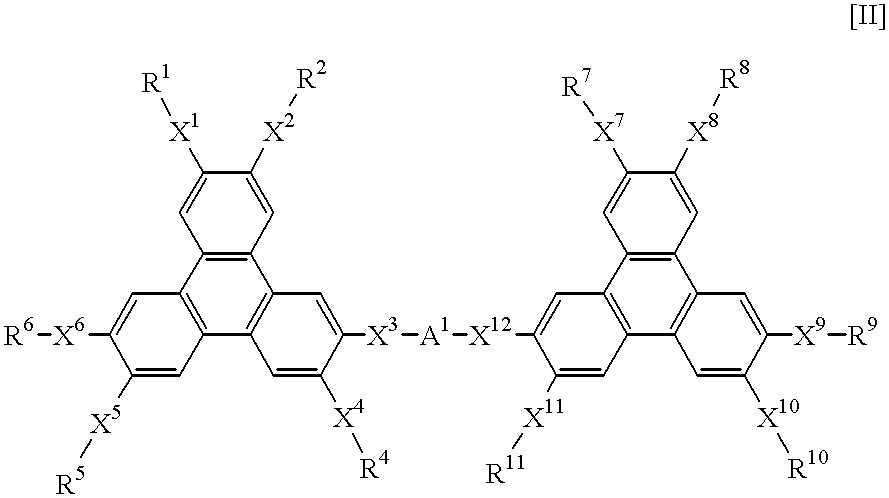
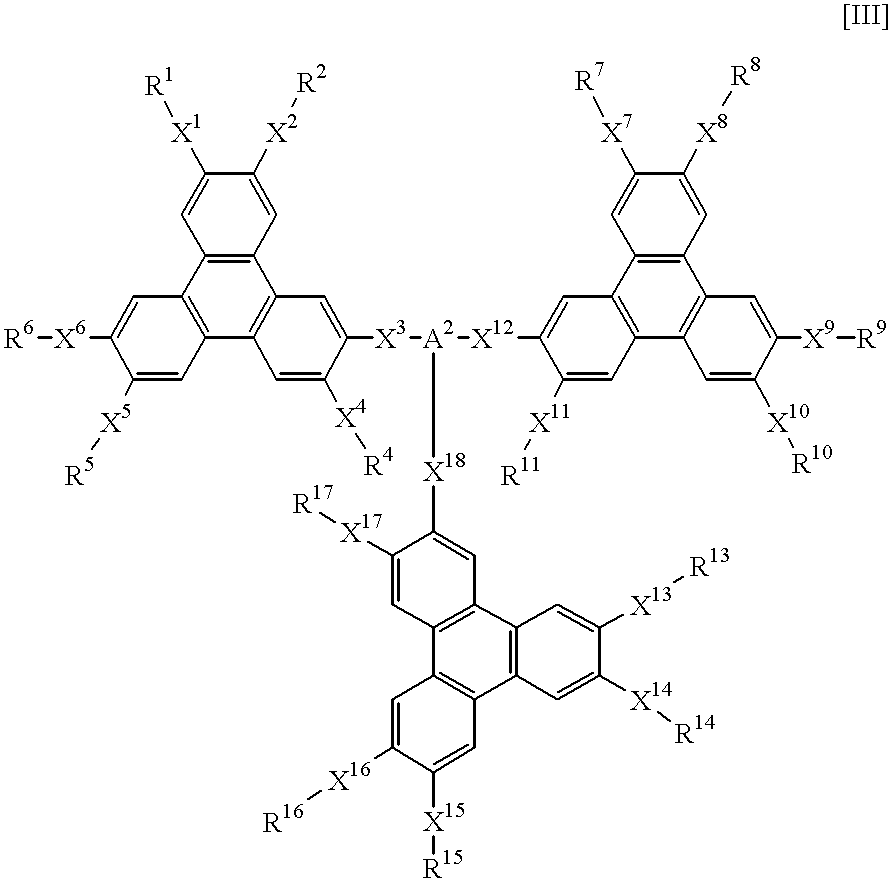
A hole-injecting material for an organic EL device, having excellent hole-injecting capability and durability and having the formula [I], [II] or [III] specified in claim 1, and an organic electroluminescence device obtained by forming either a light-emitting layer or a plurality of organic compound thin layers including the light-emitting layer between a pair of electrodes composed of a cathode and an anode, wherein at least one layer contains the above material.
Owner:TOYO INK SC HOLD CO LTD
Material for organoelectroluminescence device and use thereof
A hole-injecting material for an organic EL device, having excellent hole-injecting capability and durability and having the formula [I], [II] or [III] specified in claim 1, and an organic electroluminescence device obtained by forming either a light-emitting layer or a plurality of organic compound thin layers including the light-emitting layer between a pair of electrodes composed of a cathode and an anode, wherein at least one layer contains the above material.
Owner:TOYO INK SC HOLD CO LTD
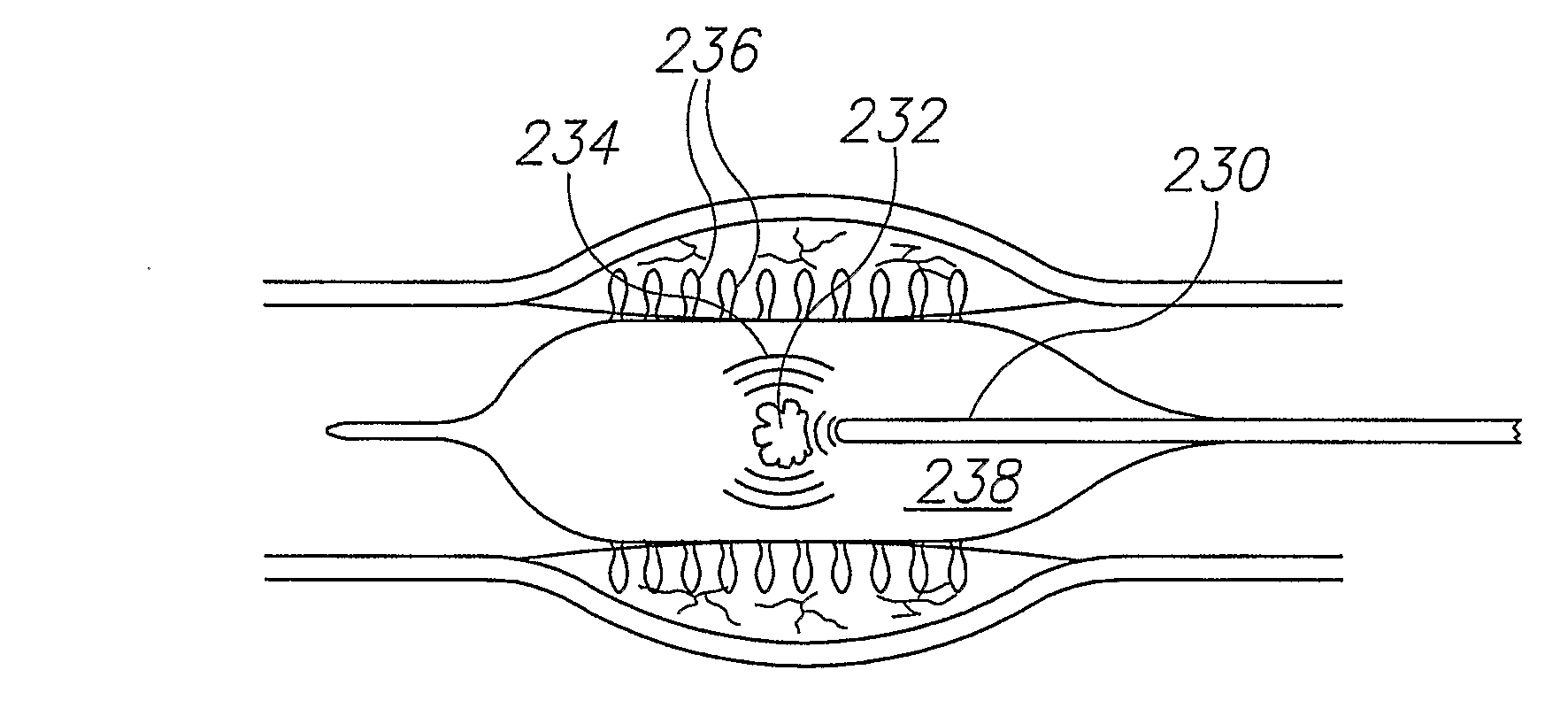
Material Delivery System
InactiveUS20070299392A1Improve uniformityAvoid tearingStentsBalloon catheterInjected materialDelivery system
A balloon for injecting material into a wall of a hollow organ of a human, comprising: an expandable balloon body having a surface and having an axis; at least one predefined ejection port on said body adapted for ejection of fluid therefrom, in a transaxial direction; and an impulse source configured for and adapted to eject material out of said point at a velocity and shape suitable for mechanically penetrating tissue adjacent said port.
Owner:BY PASS
Rotationally Oscillating Injector
InactiveUS20080213899A1Bioreactor/fermenter combinationsBiological substance pretreatmentsInjected materialPenetrated oocyte
A microinjection device is provided that includes an injection element defining a longitudinal axis, and that further includes a motor. The injection element is rotatable about the longitudinal axis by the rotational motor. The injection element is for penetrating a target, such as a cell. A microinjection system is provided that includes the microinjection device and a control unit. The control unit is for controlling a rotational amplitude and a frequency of oscillation of the injection element. A method for penetrating a target to facilitate injecting material therein is provided that includes providing the material to an injection element, contacting the target with a distal end of the injection element, rotating the injection element about a longitudinal axis to form a hole in the target, and penetrating the target with the injection element via the hole formed in the target. A method for performing intra-cytoplasmic sperm injection is provided that includes providing a solution comprising sperm to an injection element, contacting an oocyte with a distal end of the injection element, rotating the injection element alternately clockwise and counterclockwise about a longitudinal axis to form a hole in the oocyte, penetrating the oocyte with the distal end of the injection element via the hole formed in the oocyte, and expelling the solution comprising sperm into the penetrated oocyte.
Owner:UNIV OF CONNECTICUT
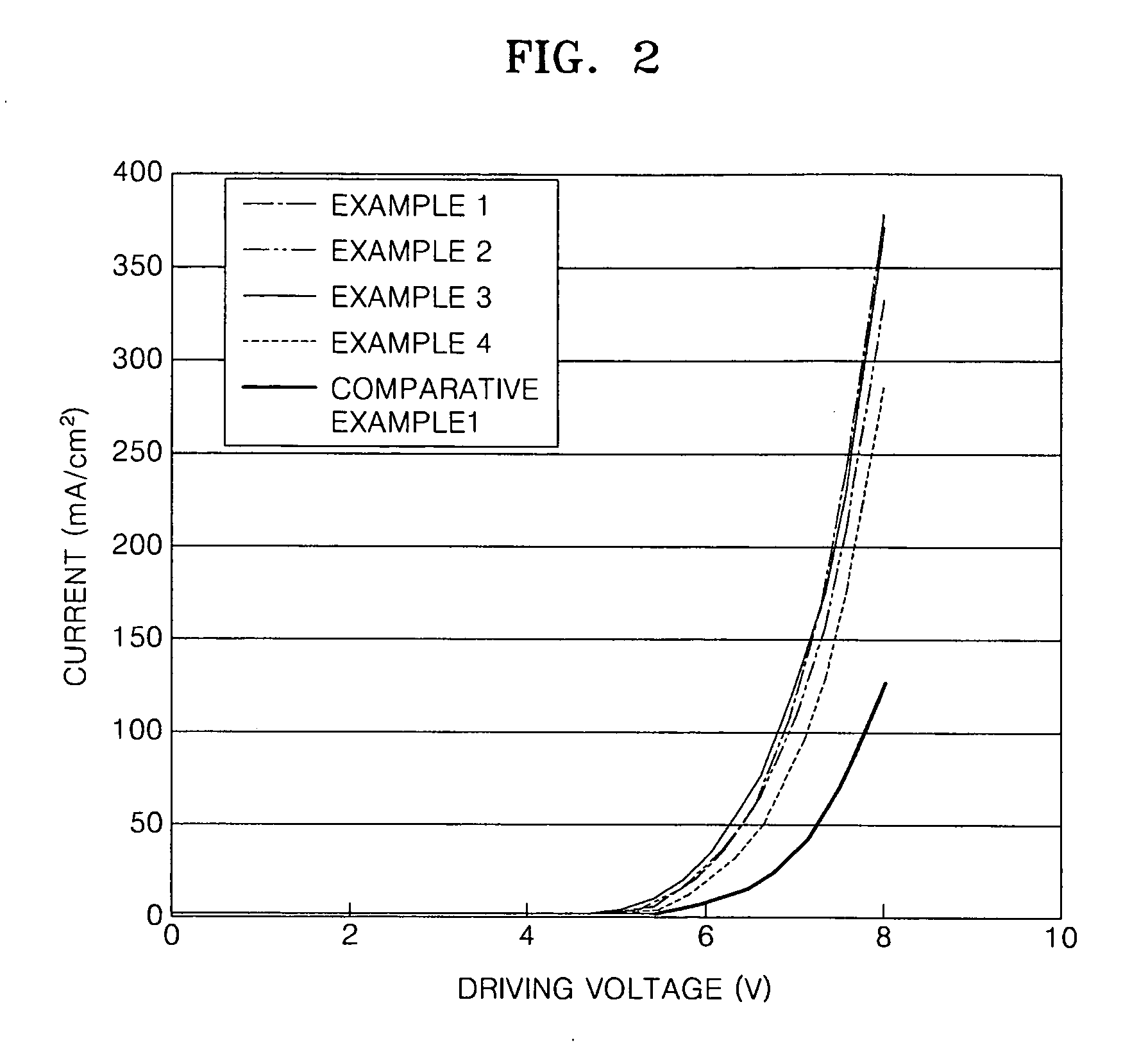
Silicon-containing compound and organic electroluminescent device employing the same
ActiveUS20100051914A1Improve efficiencyReduce the driving voltageSilicon organic compoundsSolid-state devicesCarbazoleOrganic light emitting device
Provided are a silicon-containing compound having carbazole and fluorene in its molecule and an organic electroluminescent device including an organic layer employing the same. The silicon-containing compound is represented by the following formula:The silicon-containing compound has excellent electrical characteristics and a charge transporting capability, the silicon-containing compound can be used as a hole injecting material, a hole transporting material, and / or a light emitting material that are suitable for all-color fluorescent and phosphorescent organic light emitting devices such as red, green, blue, and white fluorescent and phosphorescent organic light emitting devices. When the silicon-containing compound is used to manufacture an organic electroluminescent device, the organic electroluminescent device has a high efficiency, a low driving voltage, high luminosity, and a long lifetime.
Owner:SAMSUNG DISPLAY CO LTD
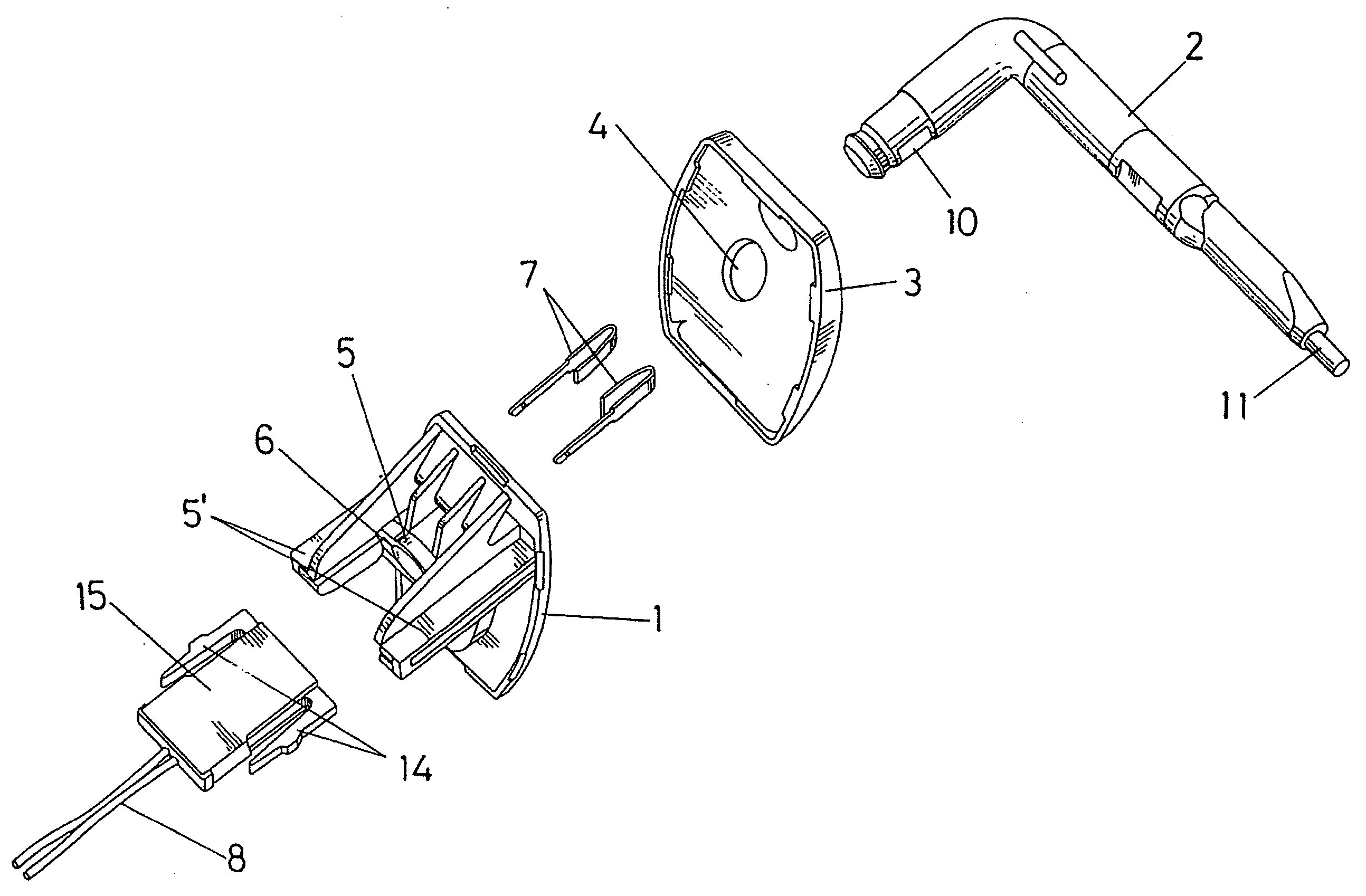
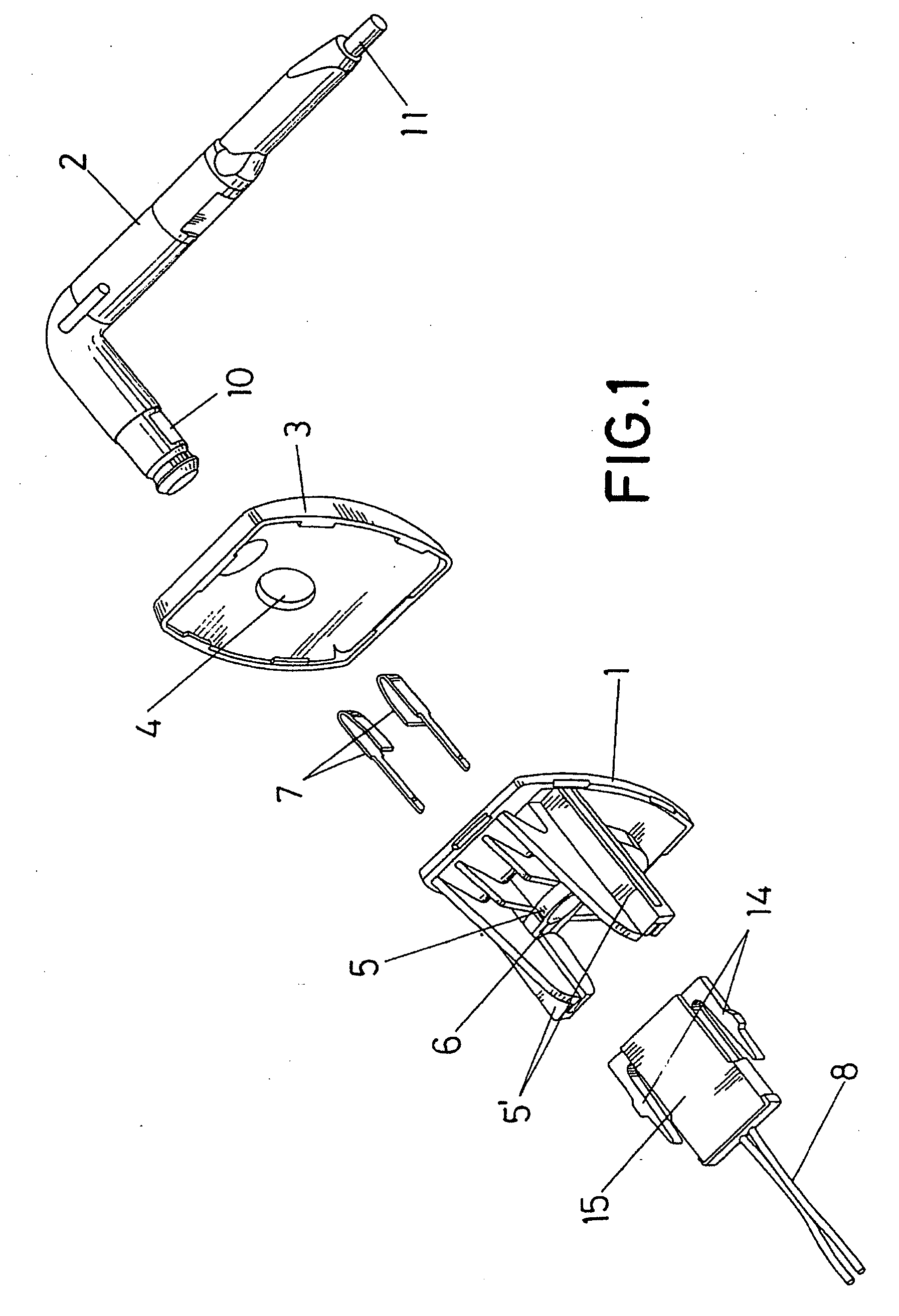
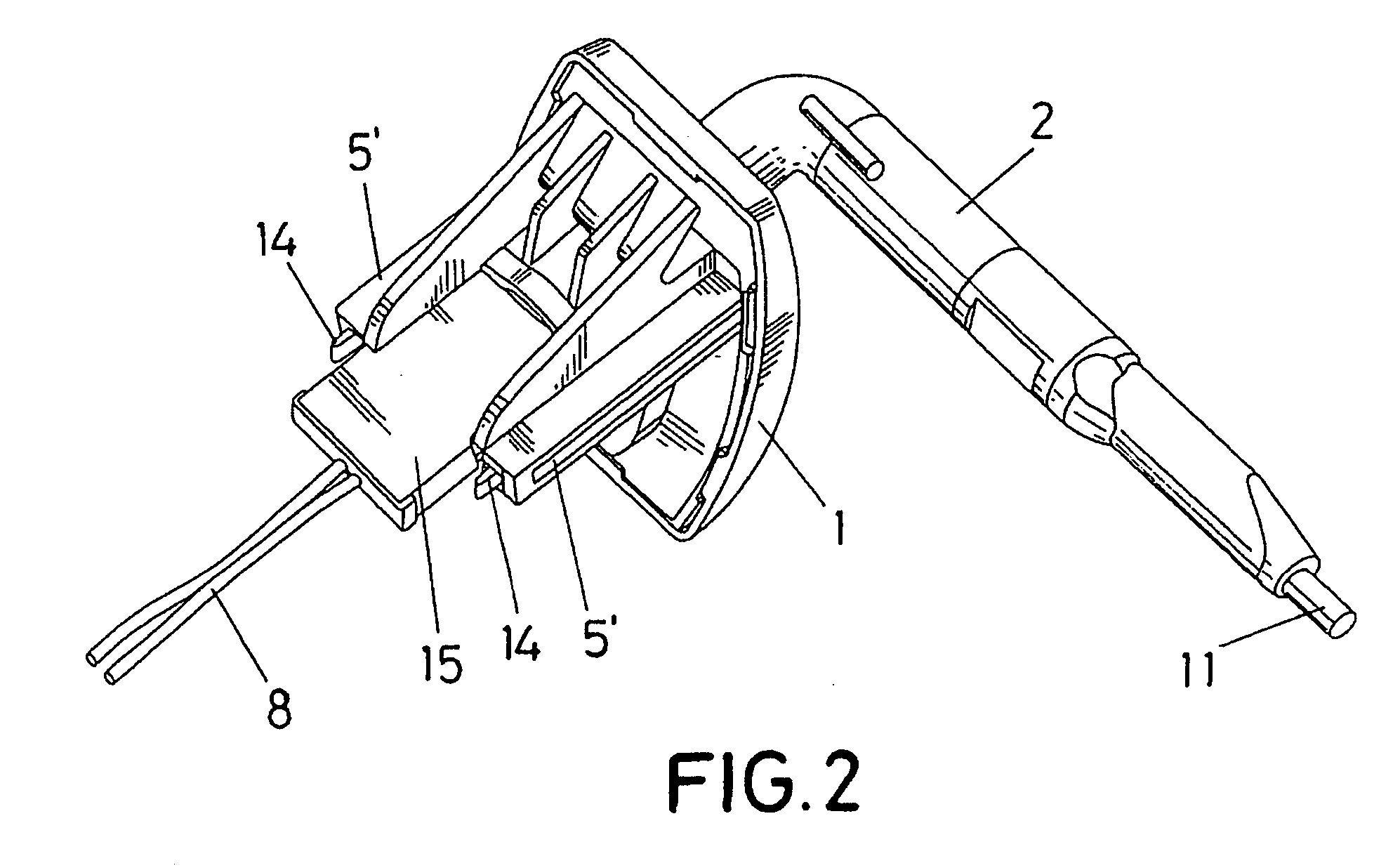
Sun visor with conducting arm for vehicles
The sun visor comprises a plate (1) for fastening to the bodywork of the vehicle and an arm (2) mounted in a hinged manner on the base (1), which in turn constitutes the means of assembly for the eyeshade of the sun visor. The arm (2) has a pair of conductive metallic elements (9) and (9′), located approximately parallel to each other and coated by means of an over-injected material, leaving the end (10) of such metallic elements (9-9′) uncovered to be able to establish contact with strips (7) foreseen in the hole (5) wherein the arm (2) is mounted in a hinged manner, whilst the other end of such elements (9-9′) in one case appears on the exterior in a terminal length (11) and in another of shorter length and defines a terminal end (12) which is uncovered on the arm (2), to be able to establish connection with a contact established on the actual eyeshade of the sun visor, for the enabling or disabling of the electrical device which can interact with said sun visor.
Owner:GRP ANTOLIN ING SA
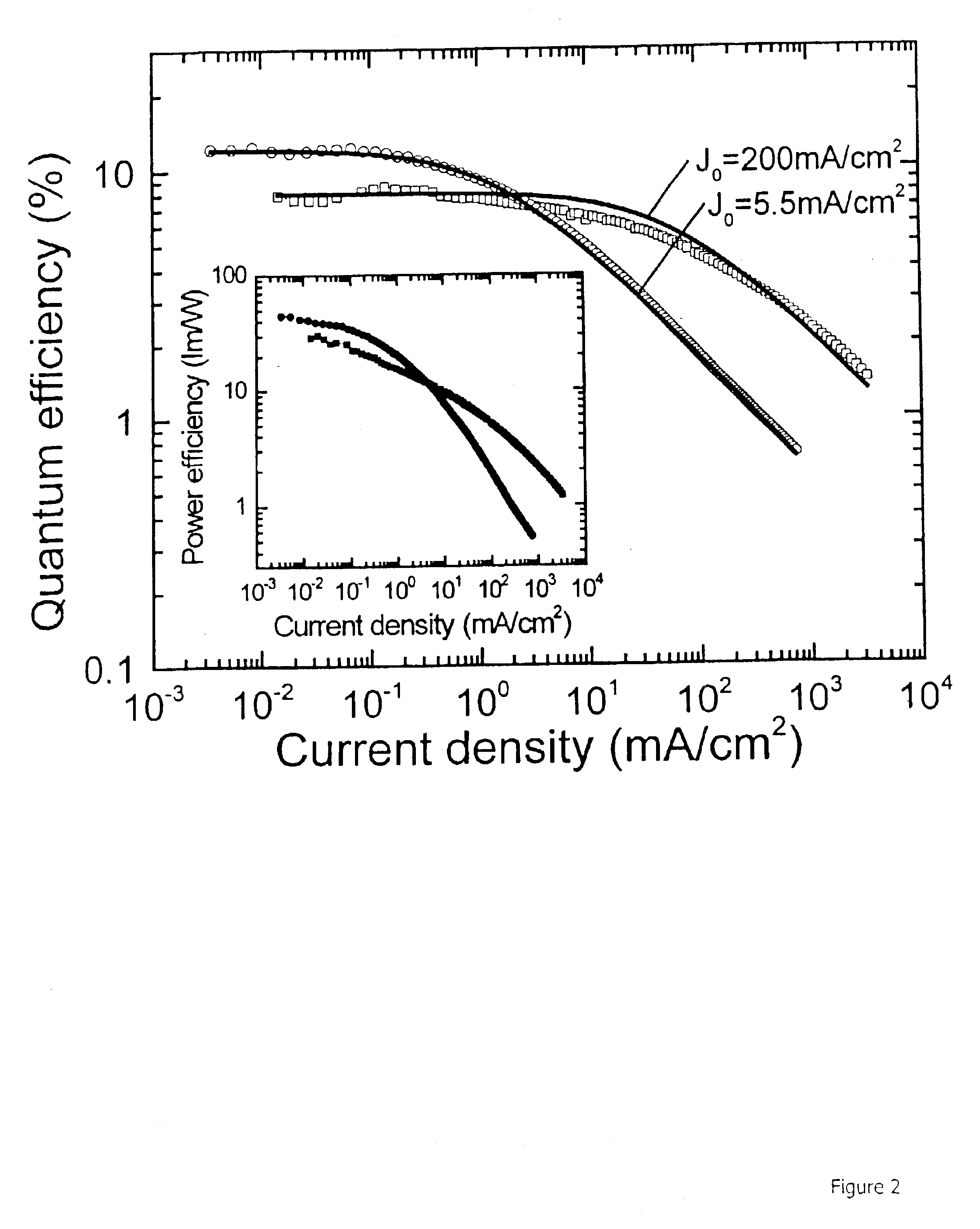
Highly efficient OLEDs using doped ambipolar conductive molecular organic thin films
Owner:THE TRUSTEES FOR PRINCETON UNIV
Polymeric joint complex and methods of use
InactiveUS20080140121A1Increase and decrease constraintEasy to replaceFinger jointsWrist jointsArtificial jointsInjected material
The invention describes a variety of implantable artificial joint complexes adapted for implantation within a target joint space within a human body. The joint complexes comprise: an expandable joint segment adapted to fit within the target joint space; and at least one of a first cannulated anchor adapted to engage the expandable joint segment and adapted to engage a bony structure adjacent the target joint space; and a second anchor adapted to engage the expandable joint segment and adapted to engage a bony structure adjacent a target joint space. The invention also discloses methods of implanting a patient specific artificial joint complex. The methods include the steps of: accessing a target joint space by creating an access hole through an adjacent bony structure; inserting a joint complex device having a cannulated anchor and an expandable joint segment through the access hole with the expendable joint segment being positioned between the surfaces forming the joint; injecting material into the expandable joint segment; and sealing access to the target joint space.
Owner:GMEDELAWARE 2
Low-density thermostable high-alumina lightening casting material
The invention discloses a high-alumina lightweight injecting material with low density, low heat conductivity and stable high temperature volume and preparing method, which comprises the following steps: allocating mass percent with 26-55% lightweight mullite skeletal material, 5-20% floating pearl, 3-10% quartz, 10-25% blue spar, 2-10% silica micronized flour, 2-10% alpha-Al2O3, 10-25% pure alumina cement and 0. 5-5% fiber; getting the product. This invention possesses low volume density, small heat conductivity at 1000 deg. c and good high temperature volume stability, which can be used for long-period under 1400 deg. c.
Owner:上海彭浦特种耐火材料厂有限公司
In-Mould labelling
An In-Mould Labelling process comprising: clamping a label within a mould void of an injection moulding tool; and injecting material into the mould void while the label is clamped. An In-Mould Labelling process comprising: placing a label between a first mould void and a second, separate mould void; and injecting material into the first and second mould voids.
Owner:NOKIA CORP
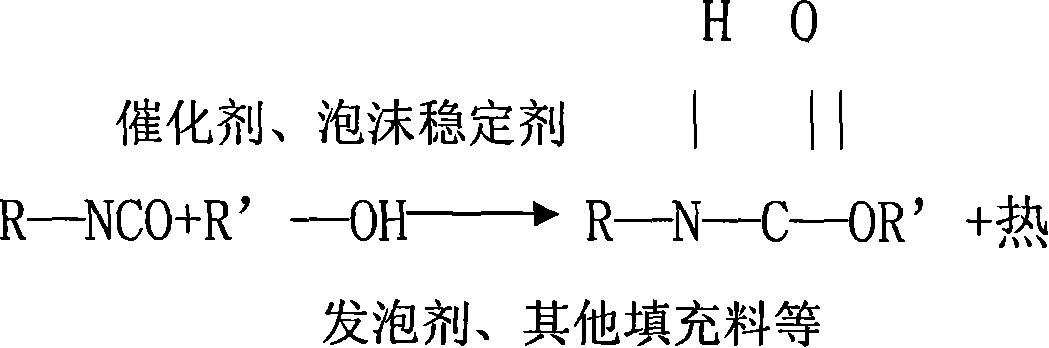
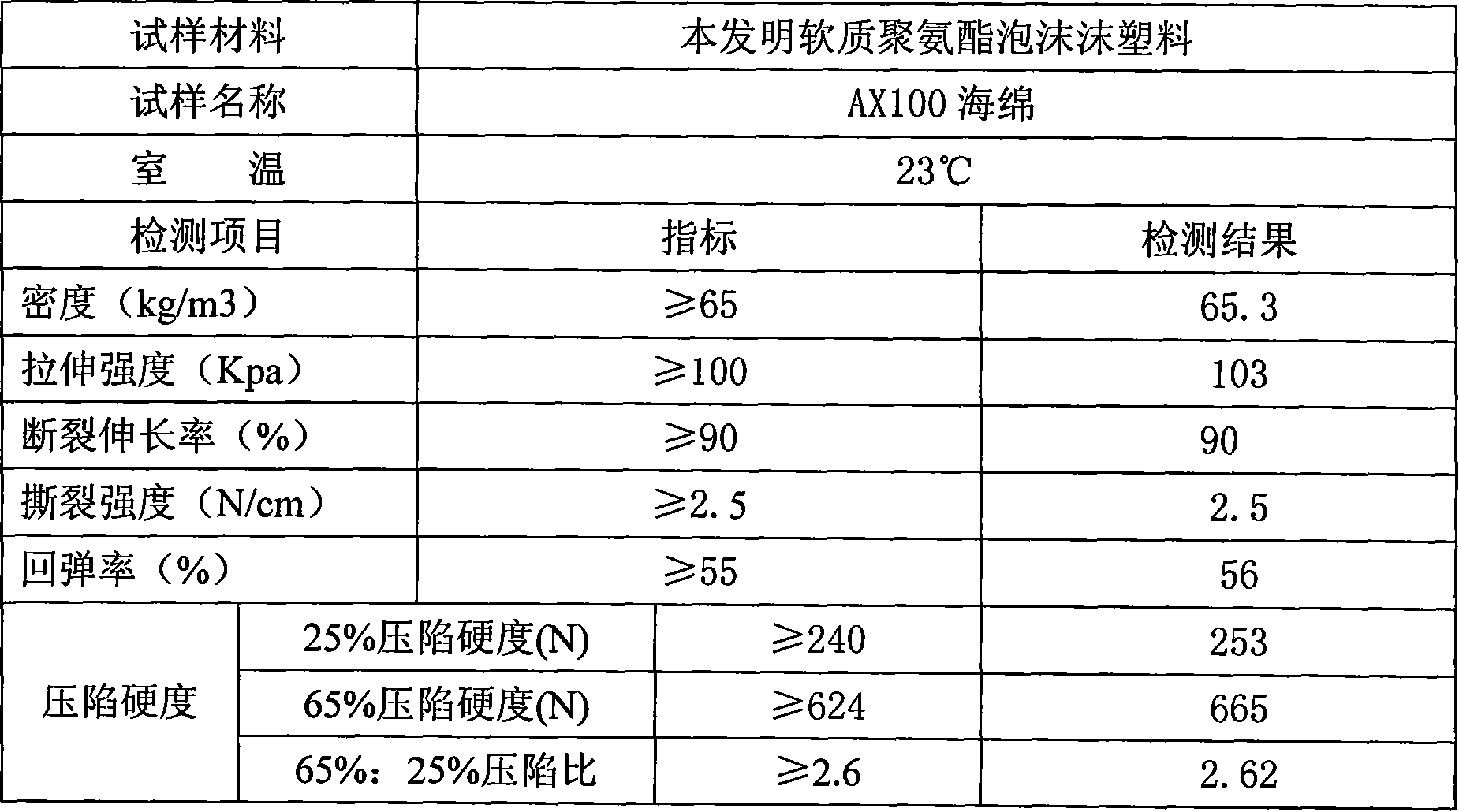
Formula of cold curing foam and forming technique
The invention discloses a formulation of cold curing foam in the polyurethane foaming technical field and a shaping technique, which shapes in molding through injecting material on a high pressure foaming machine after stirring white material and black material which are matched according to weight proportion, wherein white material: black material= 100:42-48, the white material is formed by following raw materials according to weight percentage, 40-68% polyether 3628, 25-53% polyether 330N, 2.8-3.0% water, 0.9-1.0% catalyst 8134, 1.9-2.1% cross linking agent 2186, 0.6-0.8% triethanolamine, 0.3-0.5% silicon oil 8715, 0.1-0.3% silicon oil 10366 and 0.05-0.1% catalyst A-1, and the black material is formed by following raw materials with weight percentages 45-55% TDI and 45-55% MDI. The technique has excellent manufacturability, high rebound property and duty ratio, low technique temperature and short curing time.
Owner:JIANGSU DEXIANG POLYURETHANE SEAT CO LTD
Injection-type cartilage bionic matrix for regenerative repair of cartilage and method for using same
InactiveCN101934092AReduce dwell timeSolve the problem of excessive degradationProsthesisCell-Extracellular MatrixMedicine
The invention discloses an injection-type cartilage bionic matrix for the regenerative repair of cartilage and a method for using the same. The materials, of which the main components are the same as those of the extracellular matrix of the normal articular cartilage, are used as a raw material, and the raw materials undergo dissolution and pH adjustment to form the injection-type cartilage bionic matrix which is in a sol state at the low temperature and at a gel state at the body temperature; and by using the injection-type cartilage bionic matrix as a carrier, the injection-type cartilage bionic matrix is mixed with seed cells at the low temperature and then injected in cartilage deficiency parts, the injected materials undergo phase change by raising the temperature of the deficiency area to turn into the gel, so that the seed cells are fixed in the deficiency parts to conduct the regenerative repair function. The method is simple and easily implemented, the product is conveniently used, the operation injury is small, and the repair effect of the deficiency parts is good; the raw material has high biocompatibility and safety, all components undergo intermolecular bonding under the preparation conditions of the invention, so that the problem that the single bionic material is degraded too fast is solved; and the seed cells are directly mixed with the raw materials, and the culture in vitro is unnecessary so that the detention time in vitro of the seed cells are shortened, and the clinical application is safer.
Owner:THE FIRST AFFILIATED HOSPITAL OF THIRD MILITARY MEDICAL UNIVERSITY OF PLA
Method for fabricating door brick in coke furnace
The prodn. steps are: raw material with fixed size is matched according to fixed matching ratio and is weighed according to fixed matching ratio then is send into puddle mixer to be sturred 2-3 minutes by adding water, the mixed material is send into model with fixed size to be vibrated 2-3 minutes by vibrostand or vibrating stick to make injected material compacted; the material is natural maintained in proper time in the model, remaintained 3 days after form stripping, dried 7 days in drying room, then is used into kln to be fired 3 hours in temp. 1300 deg.C to produce the end product, thickness of the brick is more than 200 mm, evenly reach to 1200-1500 mm.
Owner:山东耐材集团中齐耐火有限公司
Carbazole Derivative, and Light Emitting Element and Light Emitting Device Using the Carbazole Derivative
InactiveUS20080284328A1Excellent in hole injecting propertyReduce the driving voltageOrganic chemistryDischarge tube luminescnet screensHole injection layerInjected material
It is an object of the present invention to provide a material which is excellent in a hole injecting property and a hole transporting property, and to provide a light emitting element and a light emitting device using a material which is excellent in a hole injecting property and a hole transporting property. The present invention provides a carbazol derivative represented by a general formula (1). The carbazol derivative according to the present invention is excellent in the hole injecting property. By using the carbazol derivative according to the present invention as a hole injecting material for a hole injecting layer of a light emitting element, a driving voltage can be reduced. In addition, a lower driving voltage, improvement of the luminous efficiency, a longer life time, and higher reliability can be realized by applying the material to a light emitting element or a light emitting device.
Owner:SEMICON ENERGY LAB CO LTD
Active tissue augmentation materials and method
InactiveUS7326172B2Avoid absorptionPrevent circulatory insufficiencyAnti-incontinence devicesSurgical needlesMedicineInjected material
Active tissue augmenting agents, compositions and methods for use are disclosed. In a typical embodiment, the active augmenting agents of the invention can be used to form an artificial sphincter around a lumen of a human or animal body. In one embodiment, the active augmenting agent comprises magnitizable particles which can provide occlusion of a lumen, such as the urethral lumen, by circumferential attraction of the injected material toward the center of the lumen by the inherent magnetic flux field created from the magnetic dipoles of the magnetic particles.
Owner:TORAX MEDICAL
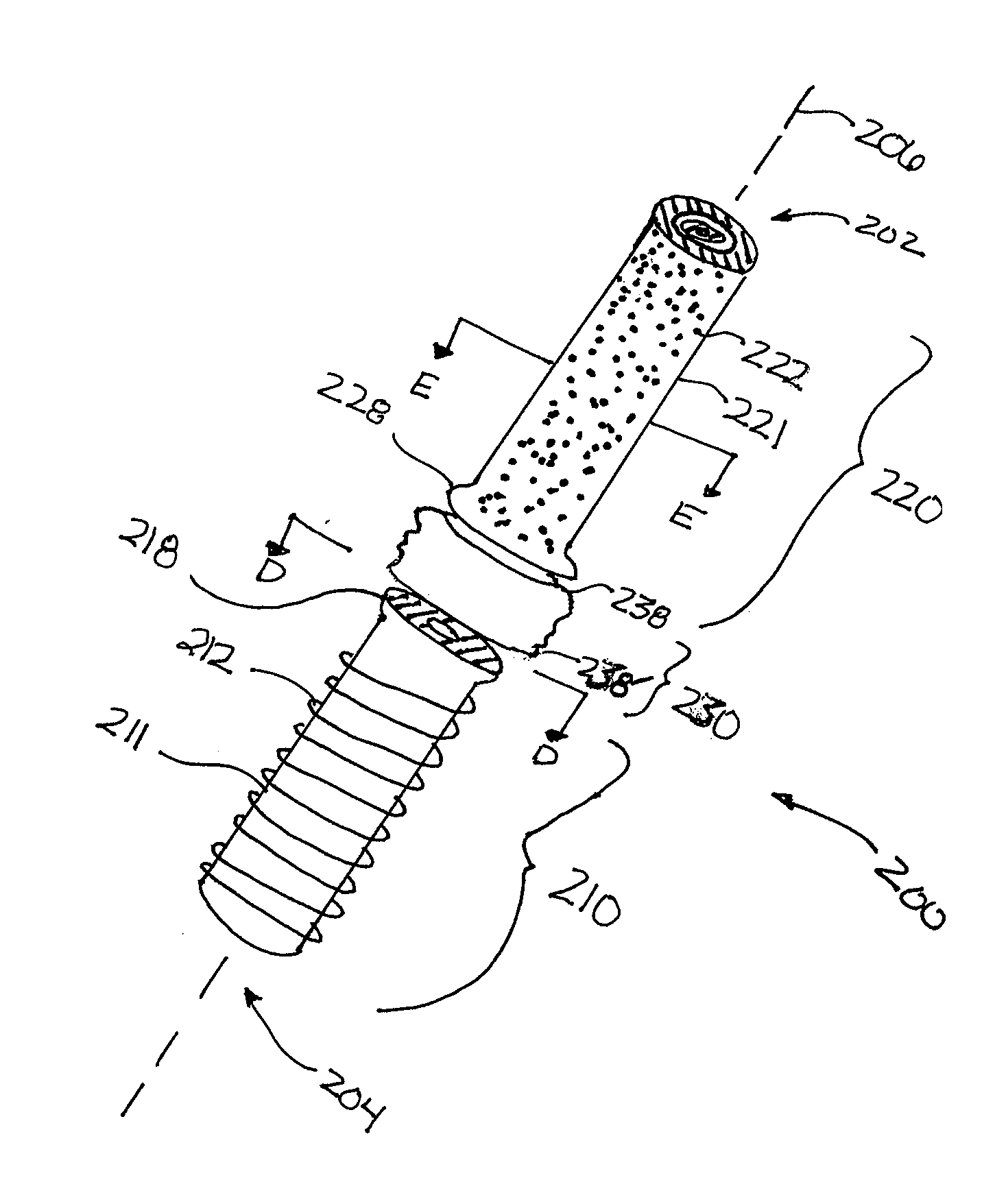
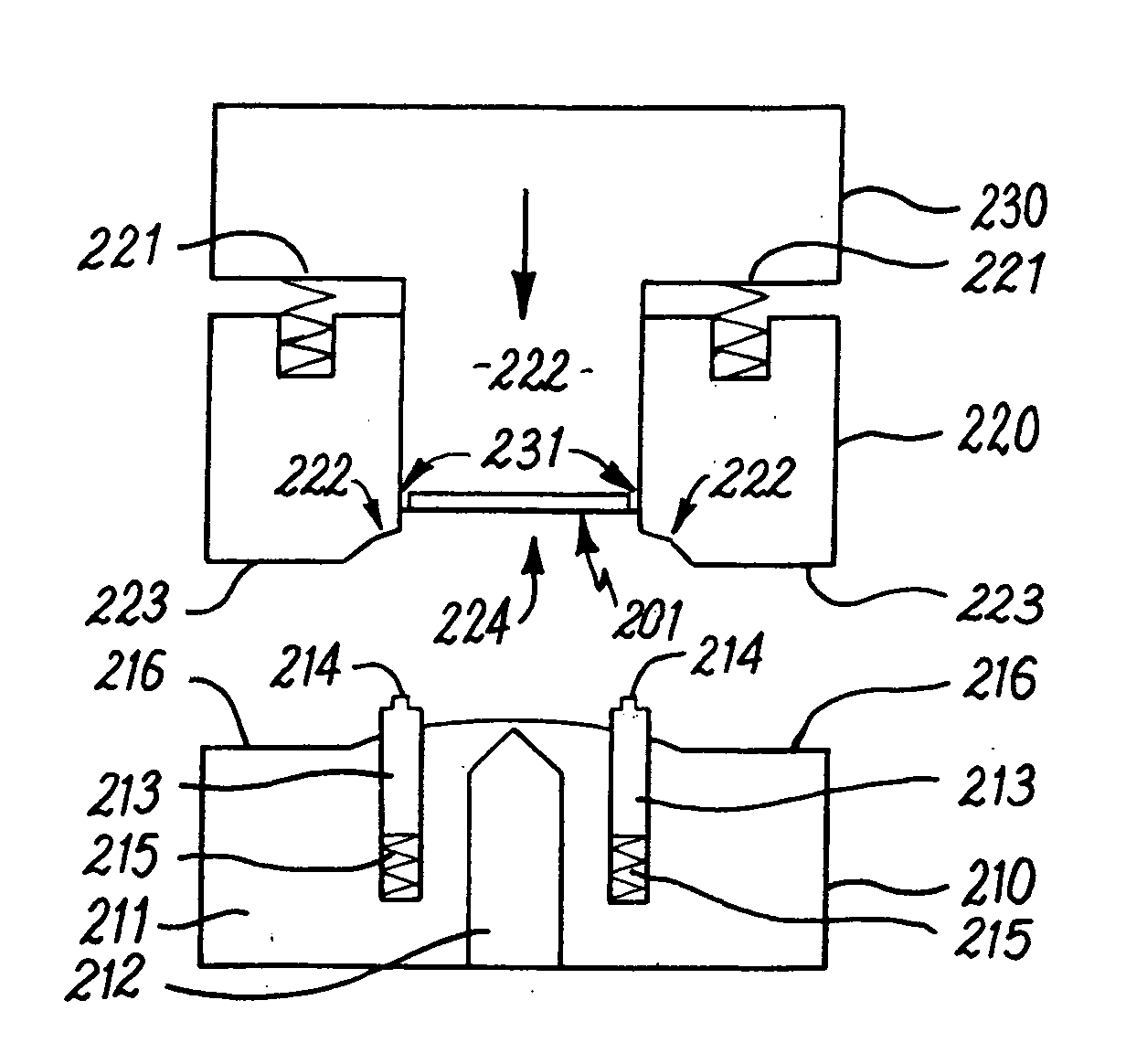
Cannulated injection system
InactiveUS20060253081A1Avoid lostAvoid material lossInfusion syringesSurgical needlesMedicineReciprocating motion
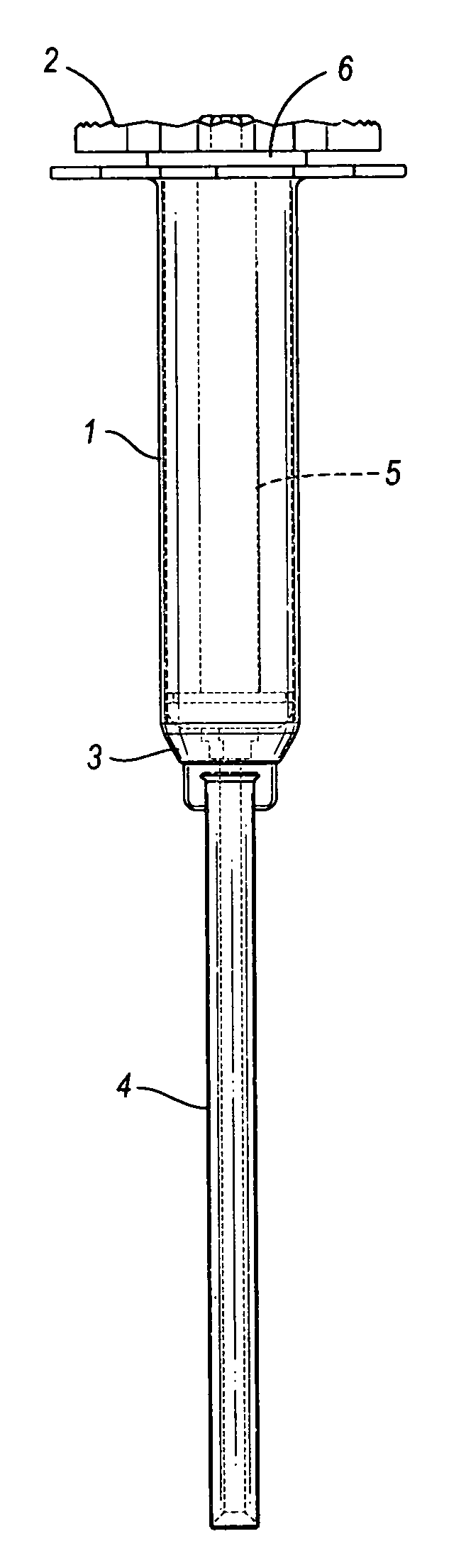
A cannulated injection system having or more hollow bodies to receive material to be injected, a piston in each said hollow body to force material from said body through a nozzle having a passageway sized and selected to accommodate flow of the material therethrough and with the passageway arranged to accommodate a guide wire passed therethrough, the guide wire further being passed through support structure including a piston and hollow body in which said piston reciprocates or a support structure for a plurality of hollow bodies in which pistons reciprocates during use in simultaneously injecting material from said hollow bodies through said injection tip and around a guide wire passing through the tip.
Owner:PAULOS LONNIE +1
Heterocyclic compound and organic electroluminescent device comprising the same
InactiveUS20100033088A1High glass transition temperatureEnhanced charge transport capabilityOrganic chemistryDischarge tube luminescnet screensOrganic light emitting deviceLuminosity
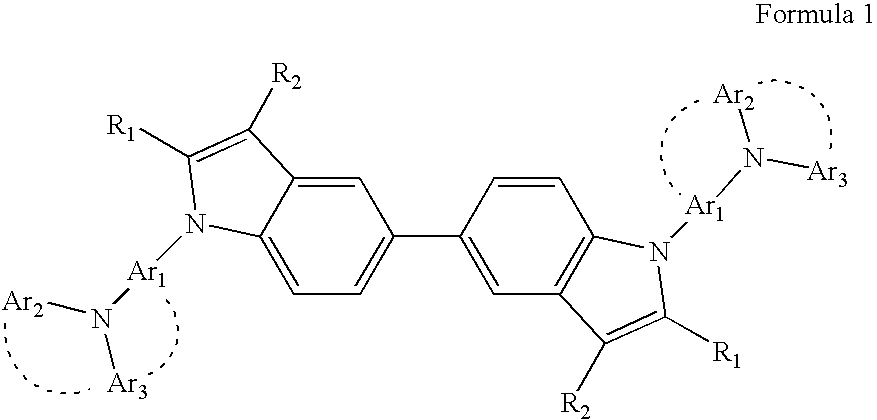
Provided are a heterocyclic compound represented by Formula 1 below and an organic electroluminescent device including an organic layer comprising the heterocyclic compound:wherein Ar1, Ar2, Ar3, R1, and R2 are the same as defined in the detailed description. The heterocyclic compound represented by Formula 1 has excellent electrical characteristics and an excellent charge transporting capability, and thus the heterocyclic compound of Formula 1 can be used as a hole injecting material, a hole transporting material, and / or a light emitting material that are suitable for all-color fluorescent and phosphorescent organic light emitting devices such as red, green, blue, and white fluorescent and phosphorescent organic light emitting devices. In addition, the organic electroluminescent device including an organic layer comprising the heterocyclic compound represented by Formula 1 can have a high efficiency, a low driving voltage, and high luminosity.
Owner:SAMSUNG DISPLAY CO LTD
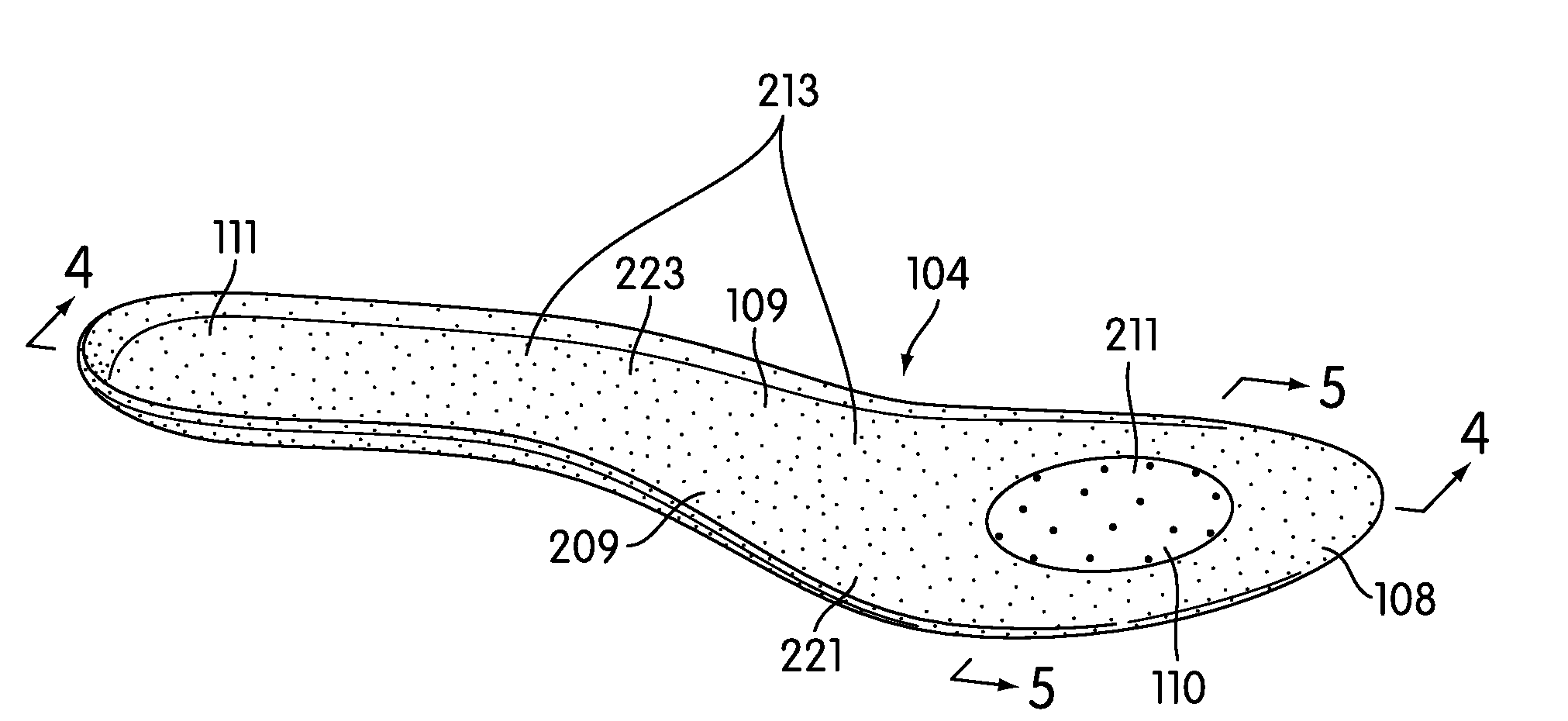
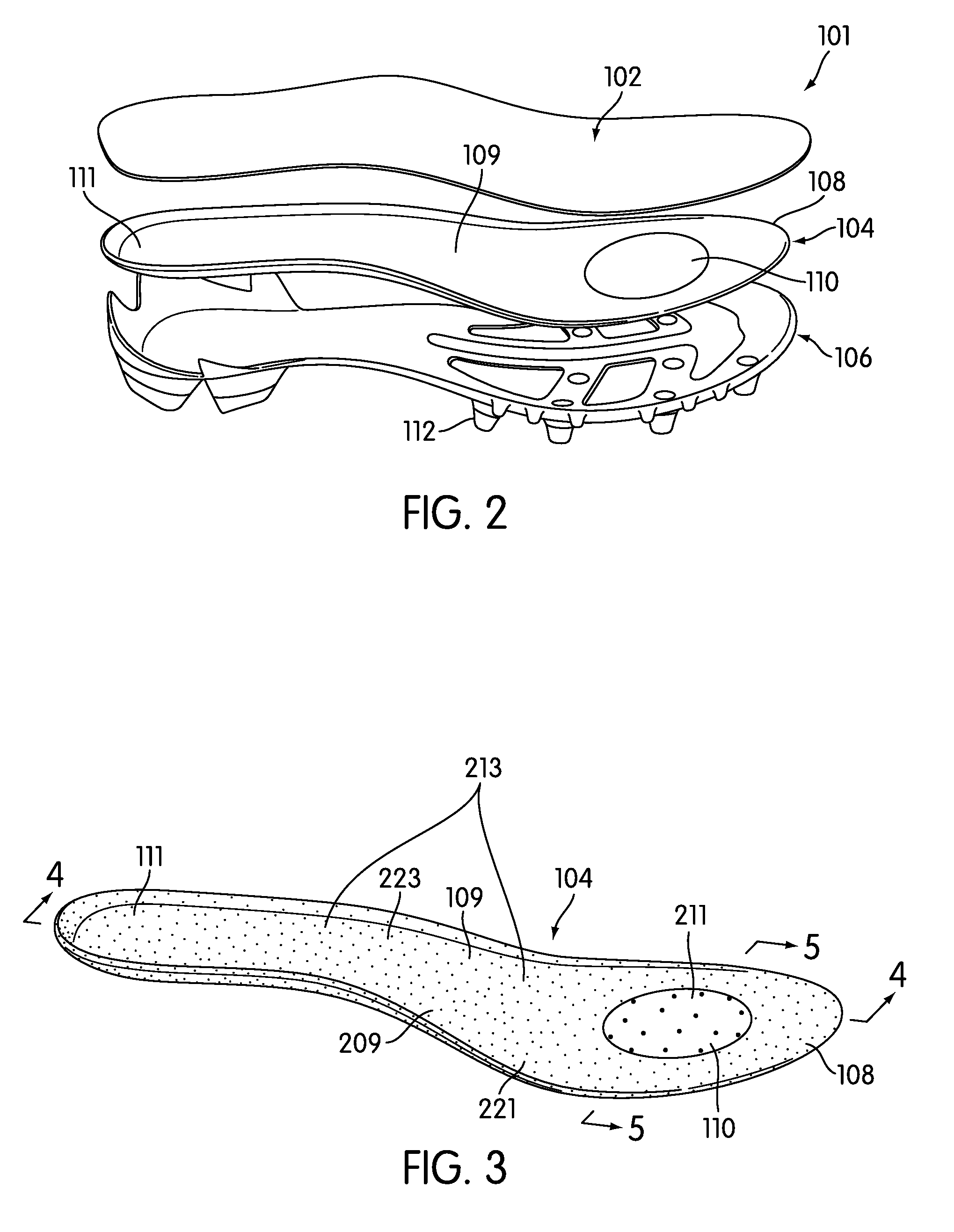
Customization of Inner Sole Board
An inner sole board having varying regions of flexibility is provided for use in an article of footwear. The inner sole board may include different materials along its length at different locations that vary its flexibility. An inner sole board is manufactured in an injection molding process requiring only one mold. The process includes a first step of providing a mold, a second step of providing an injection molding assembly, a third step of preparing an injection molding assembly and mold, a fourth step of injecting material into the mold, and a fifth step of establishing the dimensions of a first portion. During the injection molding process, the flow rate of at least one material may be controlled by a nozzle gate to control the shape and size of the flex zone it creates. In this manner, the inner sole board may be customized for a specific sport or individual.
Owner:NIKE INC
In-situ generation of oxygen-releasing metal peroxides
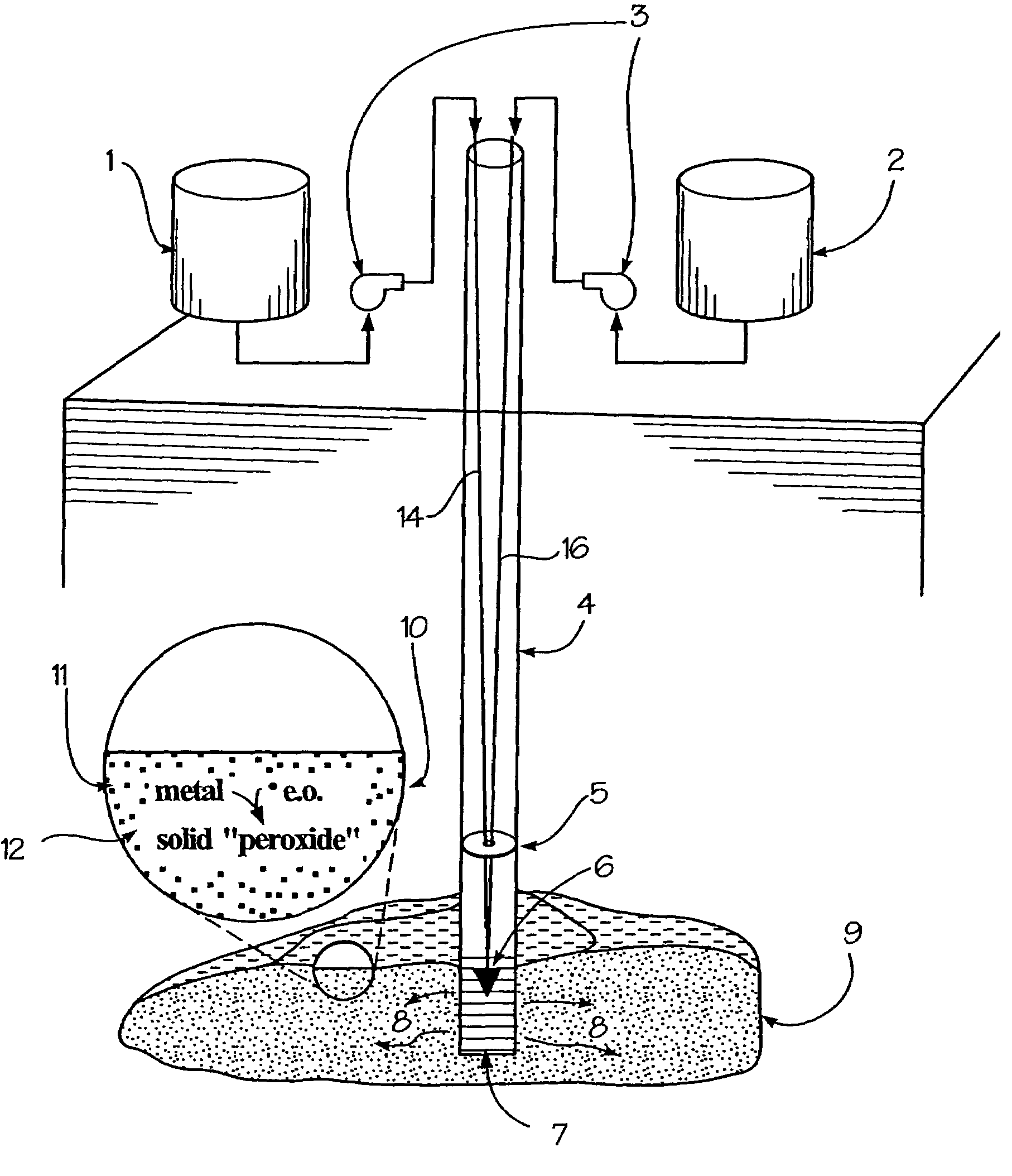
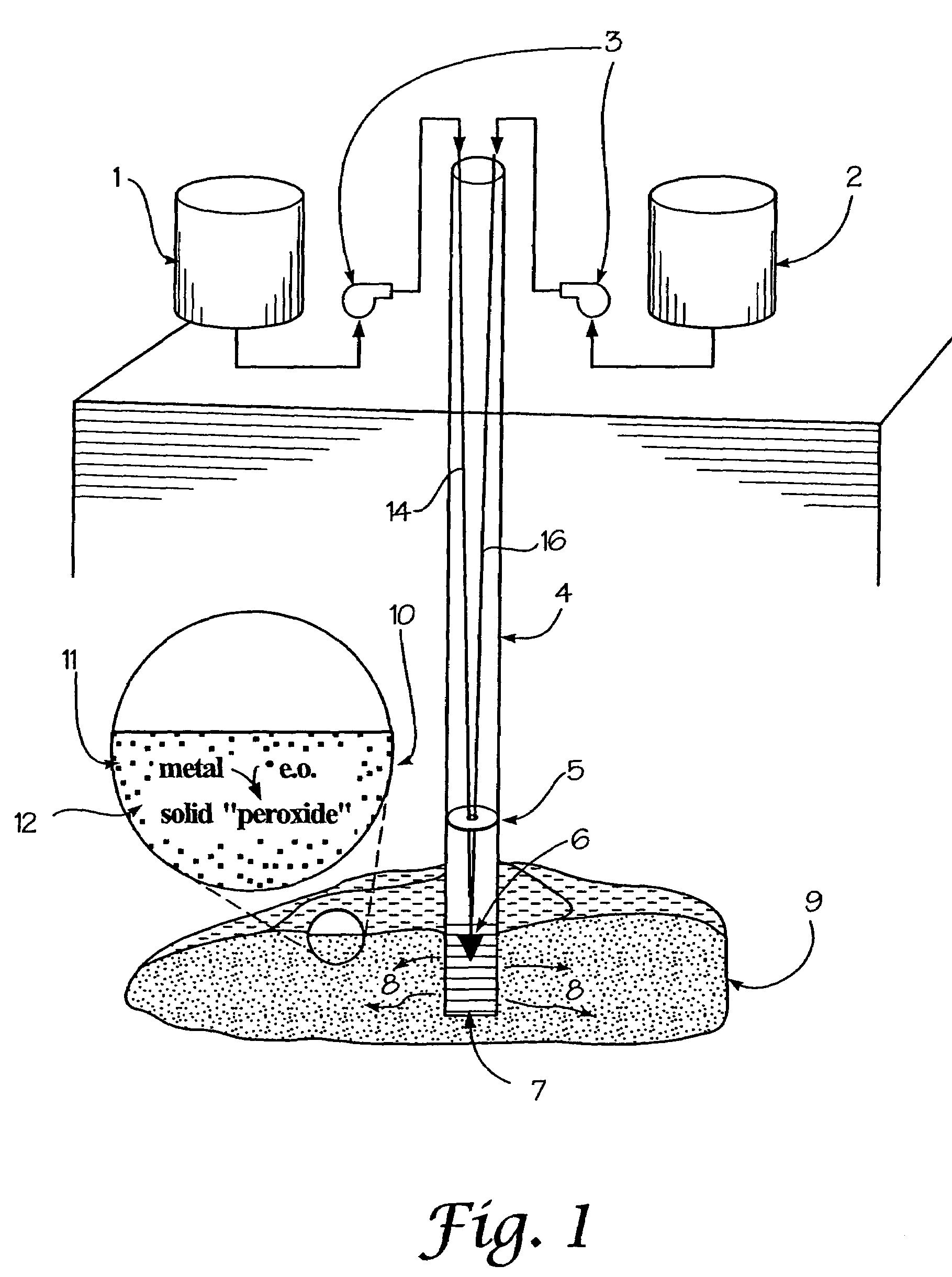
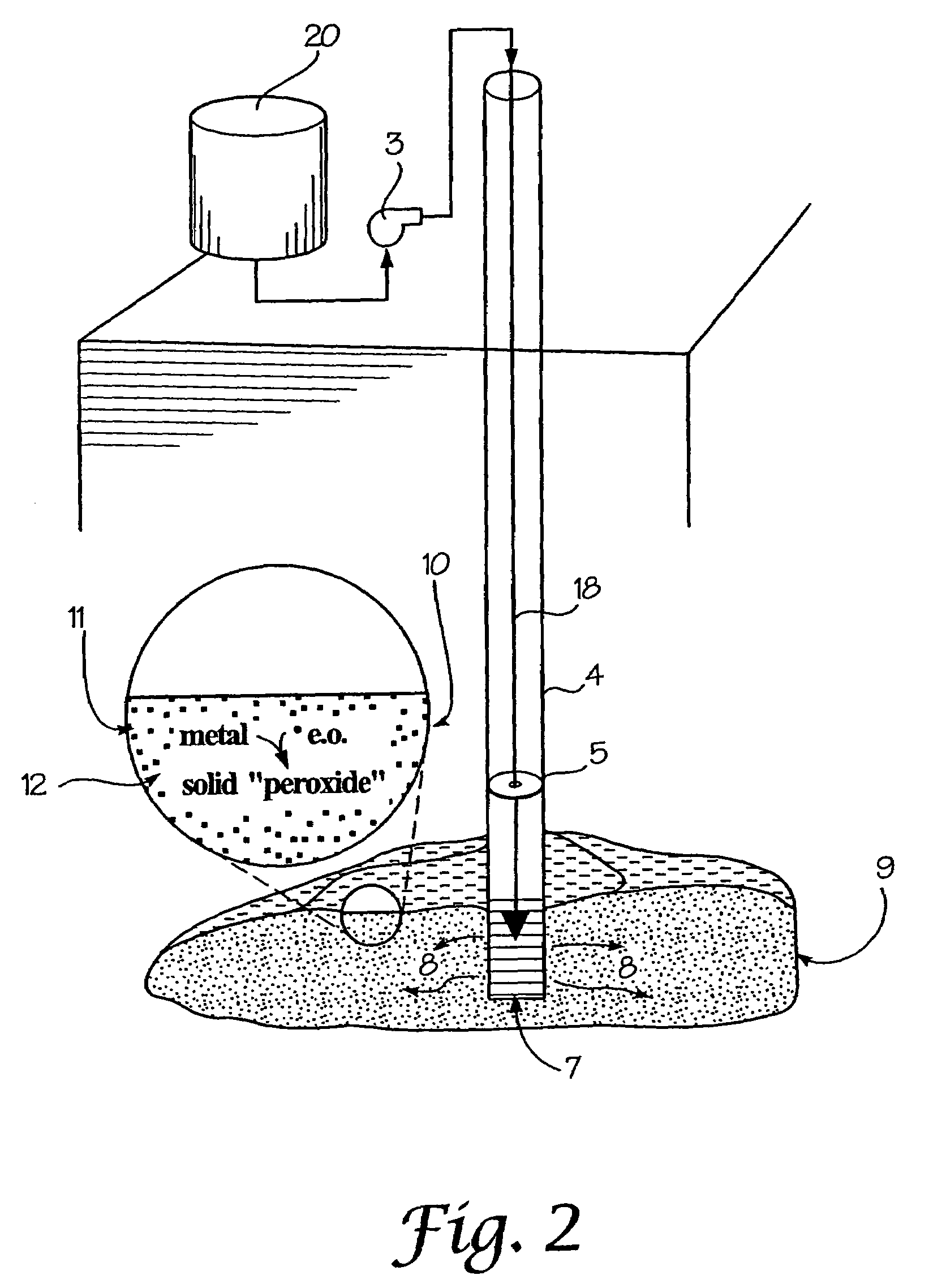
A method for remediation of contaminants in soil and groundwater is disclosed. The method generates oxygen releasing solids in groundwater or soil by injecting an aqueous energetic oxidant solution containing free radicals, oxidative conditions can be created within or ahead of a contaminant plume. Some contaminants may be remediated directly by reaction with the free radicals. Additionally and more importantly, the free radicals create an oxidative condition whereby native or injected materials, especially metals, are converted to peroxides. These peroxides provide a long-term oxygen reservoir, releasing oxygen relatively slowly over time. The oxygen can enhance microbial metabolism to remediate contaminants, can react with contaminant metals either to form immobile precipitants or to mobilize other metals to permit remediation through leaching techniques. Various injection strategies for injecting the energetic oxidant solution are also disclosed.
Owner:SAVANNAH RIVER NUCLEAR SOLUTIONS
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com