Cannulated injection system
a cannulated injection and cannula technology, applied in the field of cannulated injection systems, can solve the problems of difficult material placement, affecting the choice of injection device and the manner, and difficulty in removing trapped air
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0086] Referring Now to the Drawings
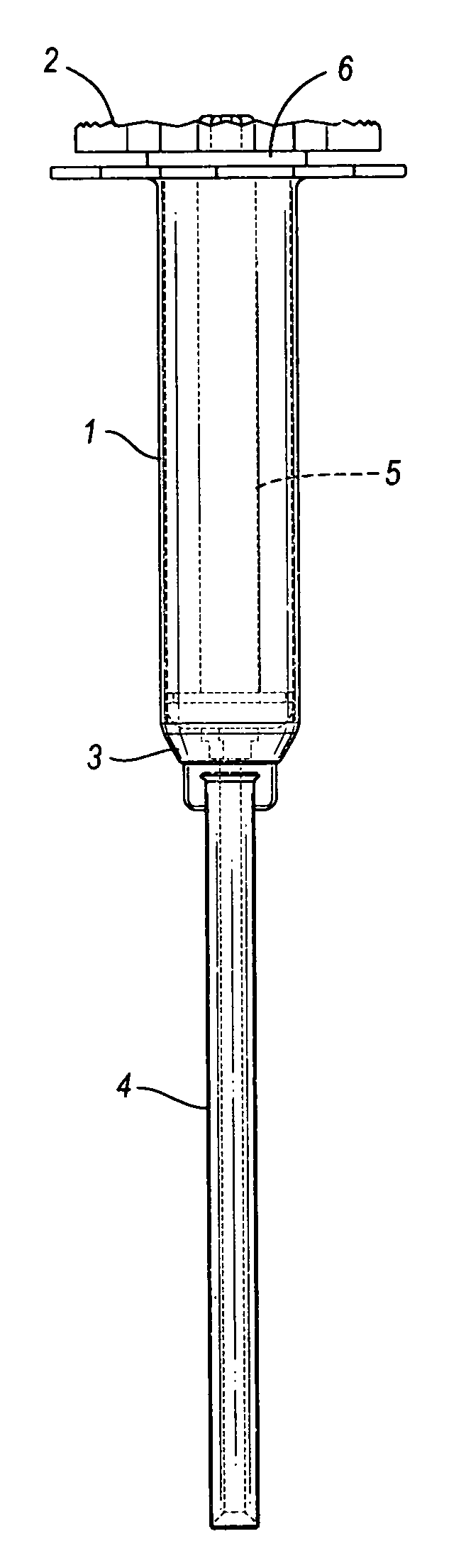
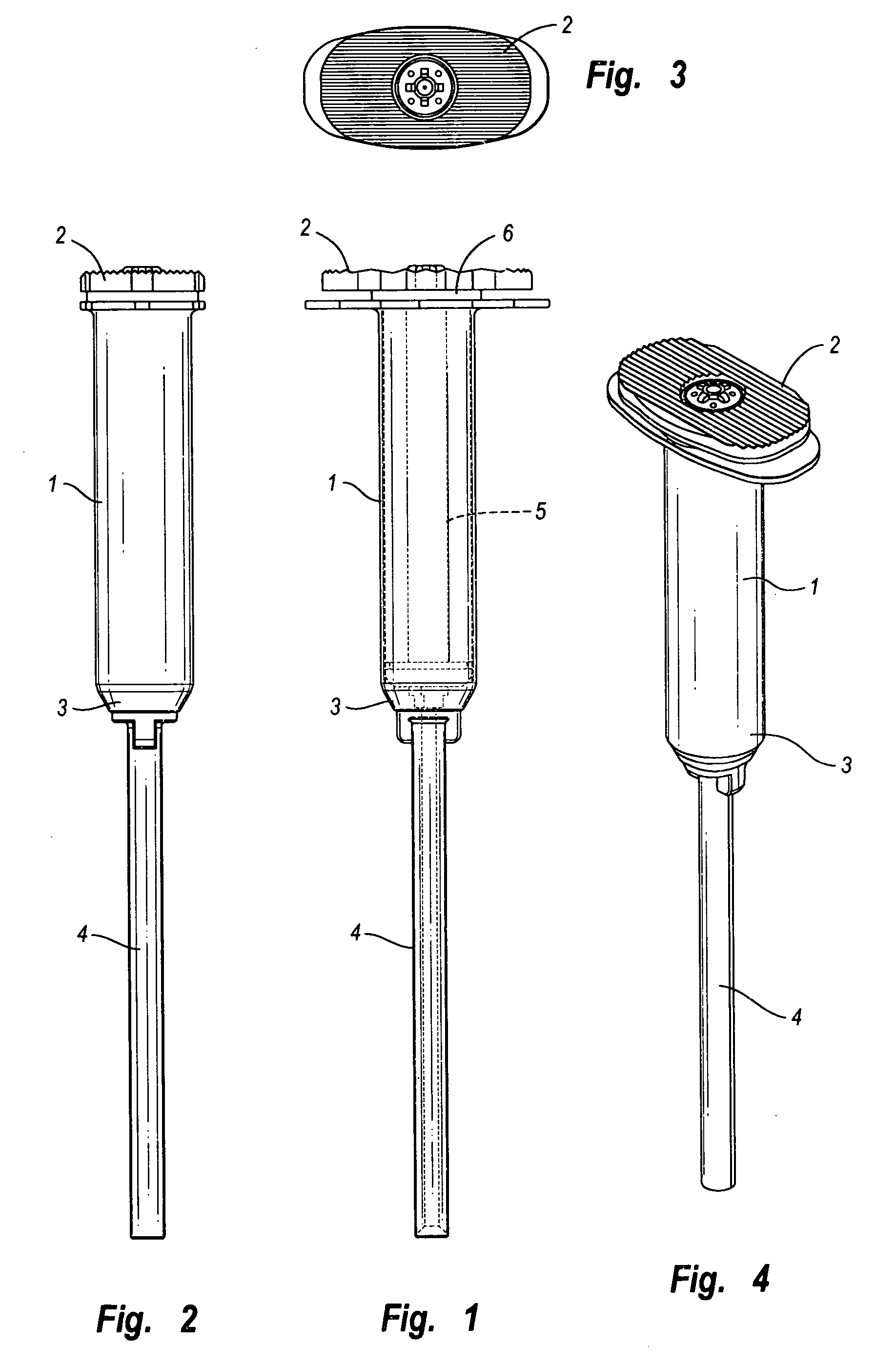
[0087] In the portion of the invention shown in FIGS. 1 through 4, cannulated injection system 1 has a proximal end 2 and a distal end 3 to which is mounted nozzle 4. System 1 has a hollow body 5 and a piston assembly 6 in the body.
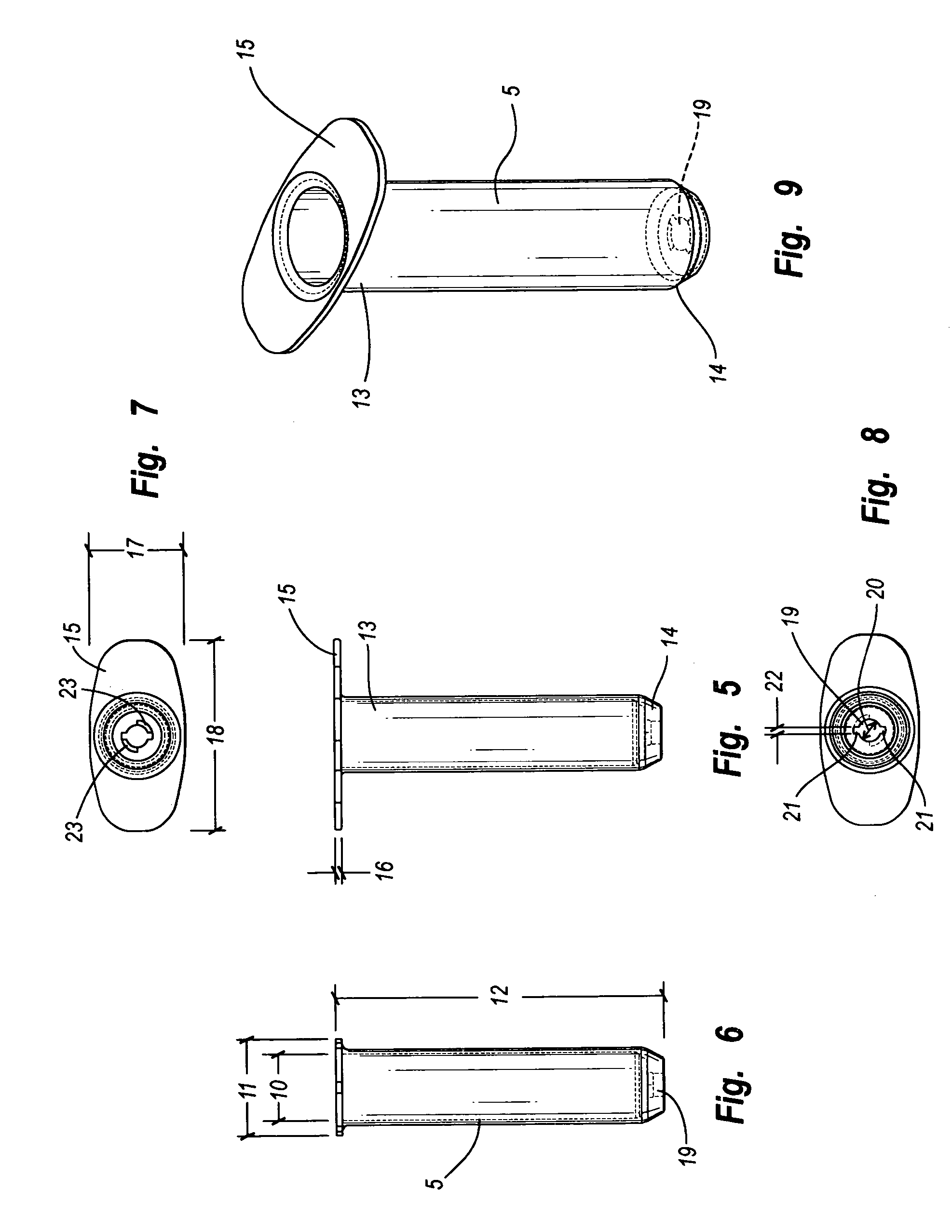
[0088] Referring to FIGS. 5-9, body 5 of inner diameter 10, outer diameter 11 and length 12 has a proximal end 13 and a distal end 14. Proximal end 13 has a flange 15 of thickness 16 and, as viewed in FIG. 7, an approximately rectangular shape of width 17 and length 18. Distal end 14 has a cannulation 19 of diameter 20, grooves 21 of width 22 and shoulders 23.
[0089] Referring to FIGS. 10-14, piston outer member 30 has a proximal end 31 having a flange 32 of thickness 33 and approximate rectangular shape of width 34 and length 35. Protruding from the most proximal surface 36 of flange 32 is cylindrical projection 37 of diameter 38 and height 52, displaced a distance 39 from axis 40 of outer member 30 and at angle 41. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com