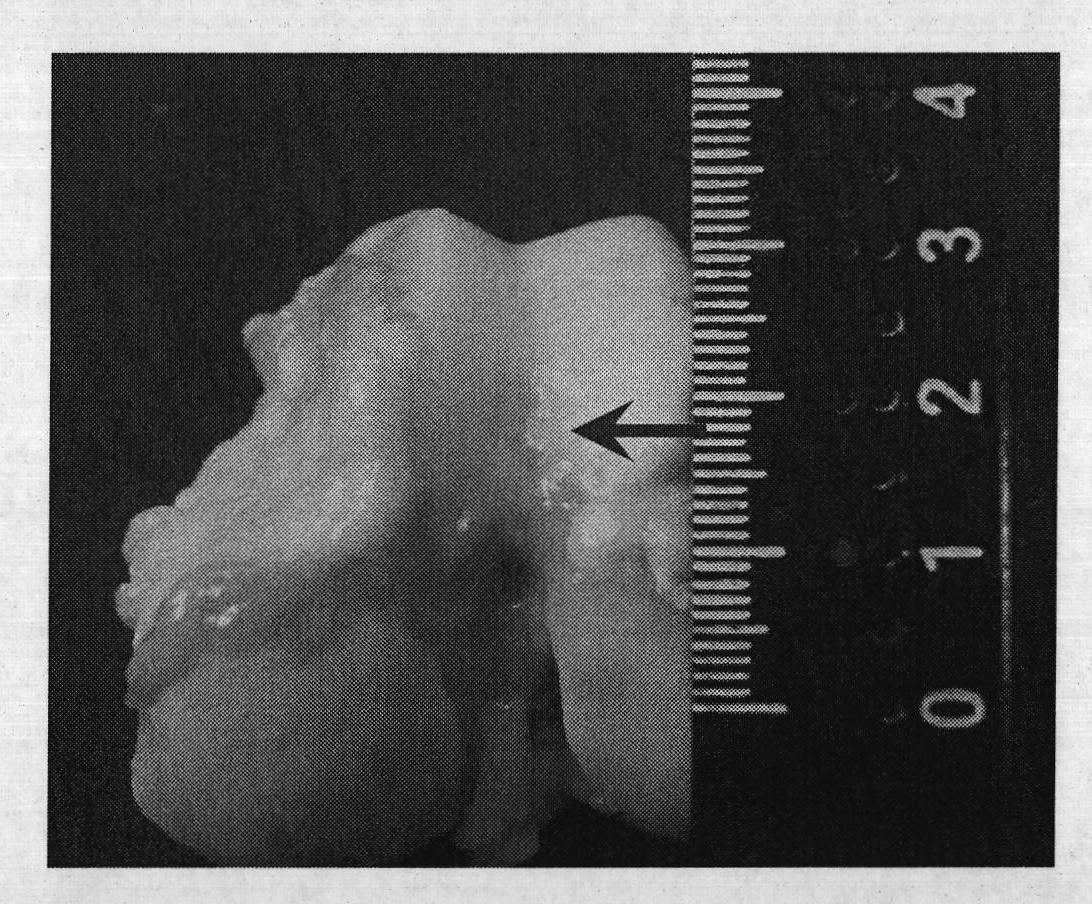
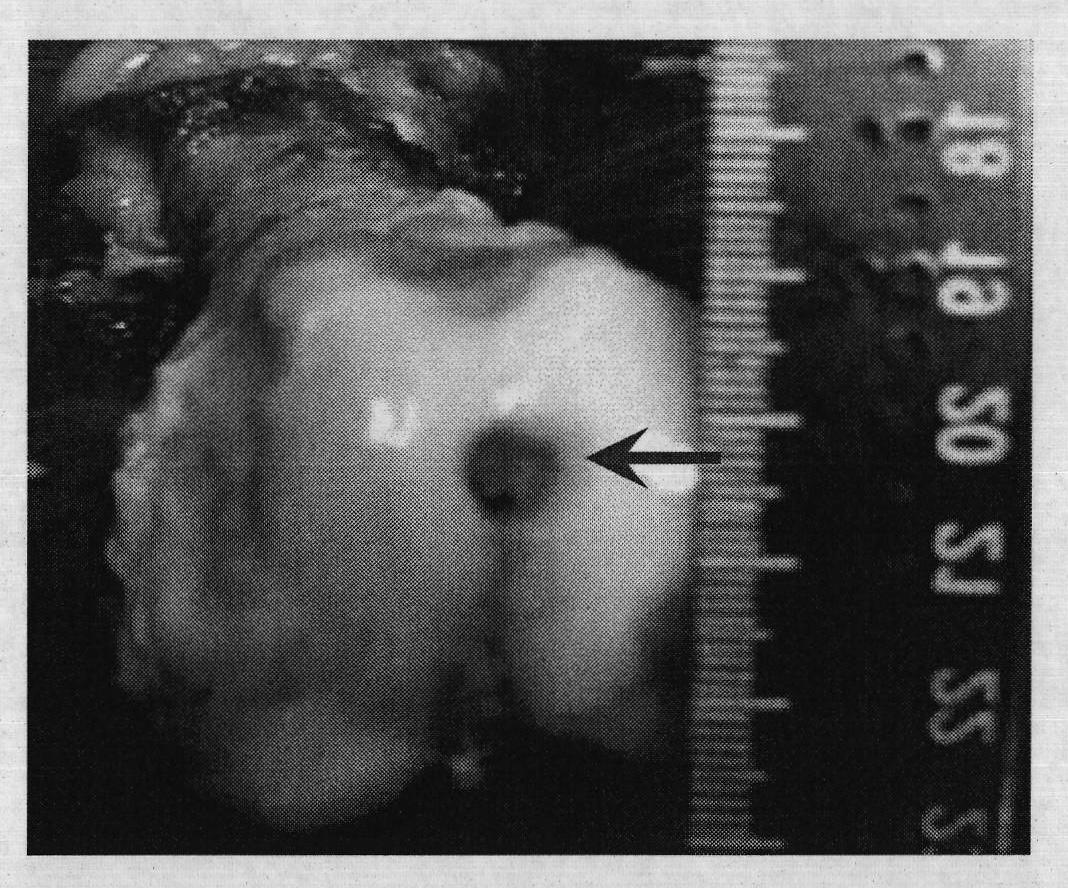
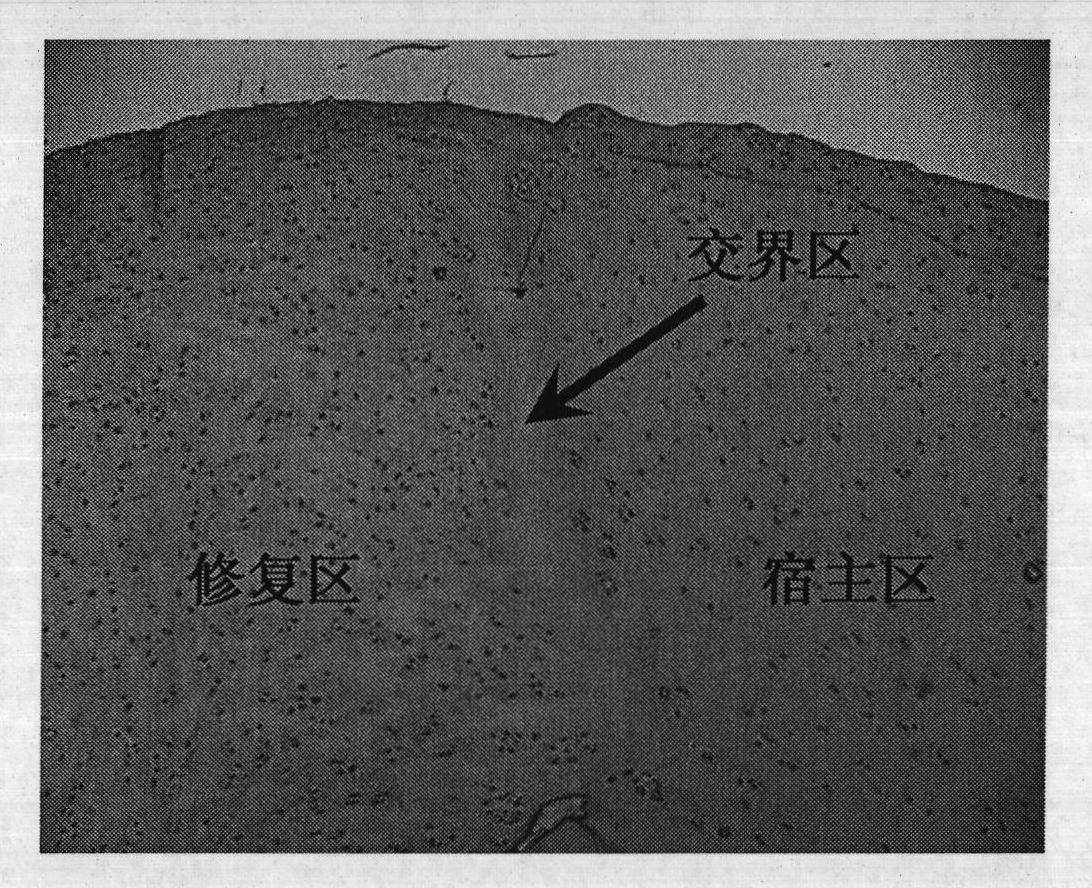
Injection-type cartilage bionic matrix for regenerative repair of cartilage and method for using same
A matrix material, injection-type technology, applied in the field of regenerative medicine, can solve the problems of inconvenience, difference, poor biocompatibility and safety, etc., and achieve the effect of improving safety, convenient use and good repair effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] The injection-type cartilage biomimetic matrix material used for cartilage regeneration and repair in this embodiment is prepared through the following steps:
[0030] a. Take 70% type II collagen, 28% chondroitin sulfate and 2% hyaluronic acid as raw materials by mass percentage;
[0031]b. Dissolve the raw material taken in step a with concentration of 0.05mol / L glacial acetic acid on a clean bench at 0°C to obtain a 10% acidic solution with a mass fraction of the raw material; of course, the raw material mass of the acidic solution in this step The score can be within the range of 10% to 30%;
[0032] c. Titrate and neutralize the acidic solution obtained in step b with an aqueous solution of sodium hydroxide with a concentration of 1 mol / L on a clean bench at 0°C until the pH is 7.0 to obtain the required injection-type cartilage for cartilage regeneration and repair Biomimetic matrix material; the sodium hydroxide used here can also be replaced by potassium hydrox...
Embodiment 2
[0036] The injection-type cartilage biomimetic matrix material used for cartilage regeneration and repair in this embodiment is prepared through the following steps:
[0037] a. Take 75% type II collagen, 24% chondroitin sulfate and 1% hyaluronic acid as raw materials by mass percentage;
[0038] b. Dissolving the raw material obtained in step a with hydrochloric acid with a concentration of 0.1mol / L on a clean bench at 4°C to obtain an acidic solution with a mass fraction of the raw material of 15%;
[0039] c. Titrate and neutralize the acidic solution obtained in step b with an aqueous solution of potassium hydroxide with a concentration of 1 mol / L on a clean bench at 4°C until the pH is 7.5 to obtain the required injection-type cartilage for cartilage regeneration and repair Biomimetic matrix materials.
[0040] The obtained injection-type cartilage biomimetic matrix material for cartilage regeneration and repair is sealed and stored at low temperature (0-4° C.) for futur...
Embodiment 3
[0042] The injection-type cartilage biomimetic matrix material used for cartilage regeneration and repair in this example was prepared through the following steps:
[0043] a. Take 65% type II collagen, 30% chondroitin sulfate and 5% hyaluronic acid as raw materials by mass percentage;
[0044] b. Dissolving the raw material obtained in step a with hydrochloric acid with a concentration of 0.01mol / L on a clean bench at 0°C to obtain an acidic solution with a mass fraction of the raw material of 20%;
[0045] c. Titrate and neutralize the acidic solution obtained in step b with an aqueous potassium hydroxide solution with a concentration of 2 mol / L on a clean bench at 4°C until the pH is 7.3 to obtain the required injection-type cartilage for cartilage regeneration and repair Biomimetic matrix materials.
[0046] The obtained injection-type cartilage biomimetic matrix material for cartilage regeneration and repair is sealed and stored at 0-4°C for future use.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com