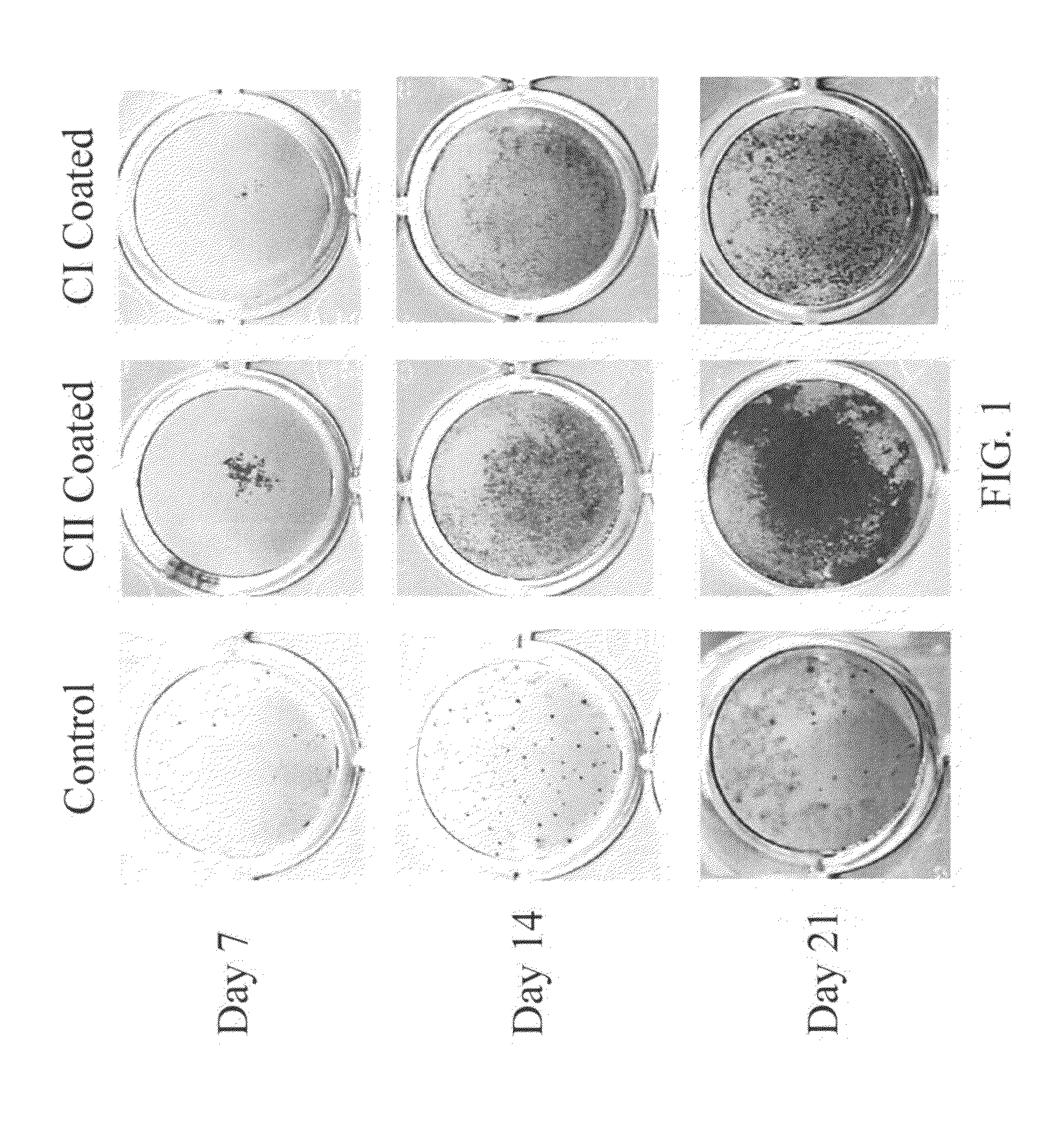
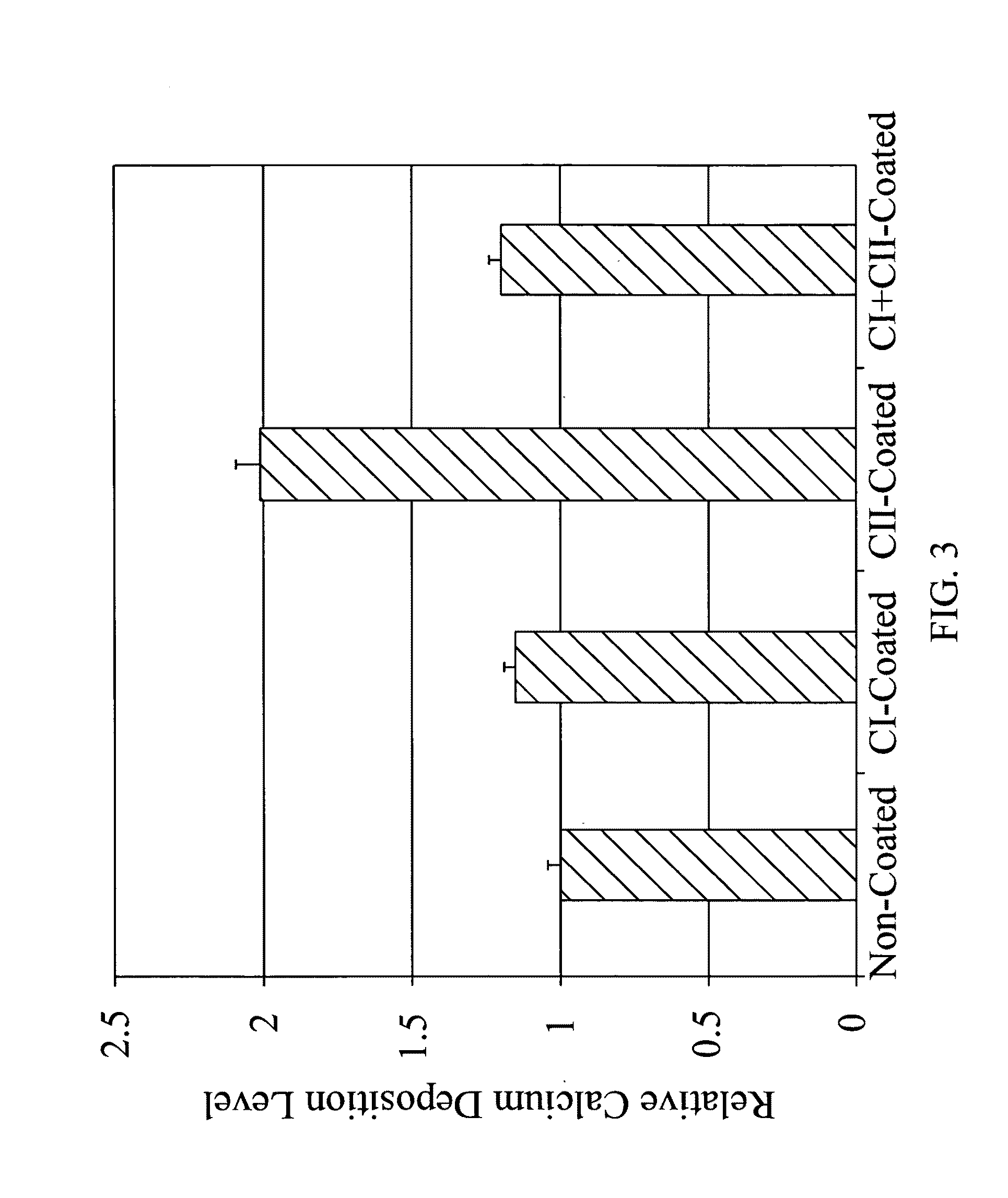
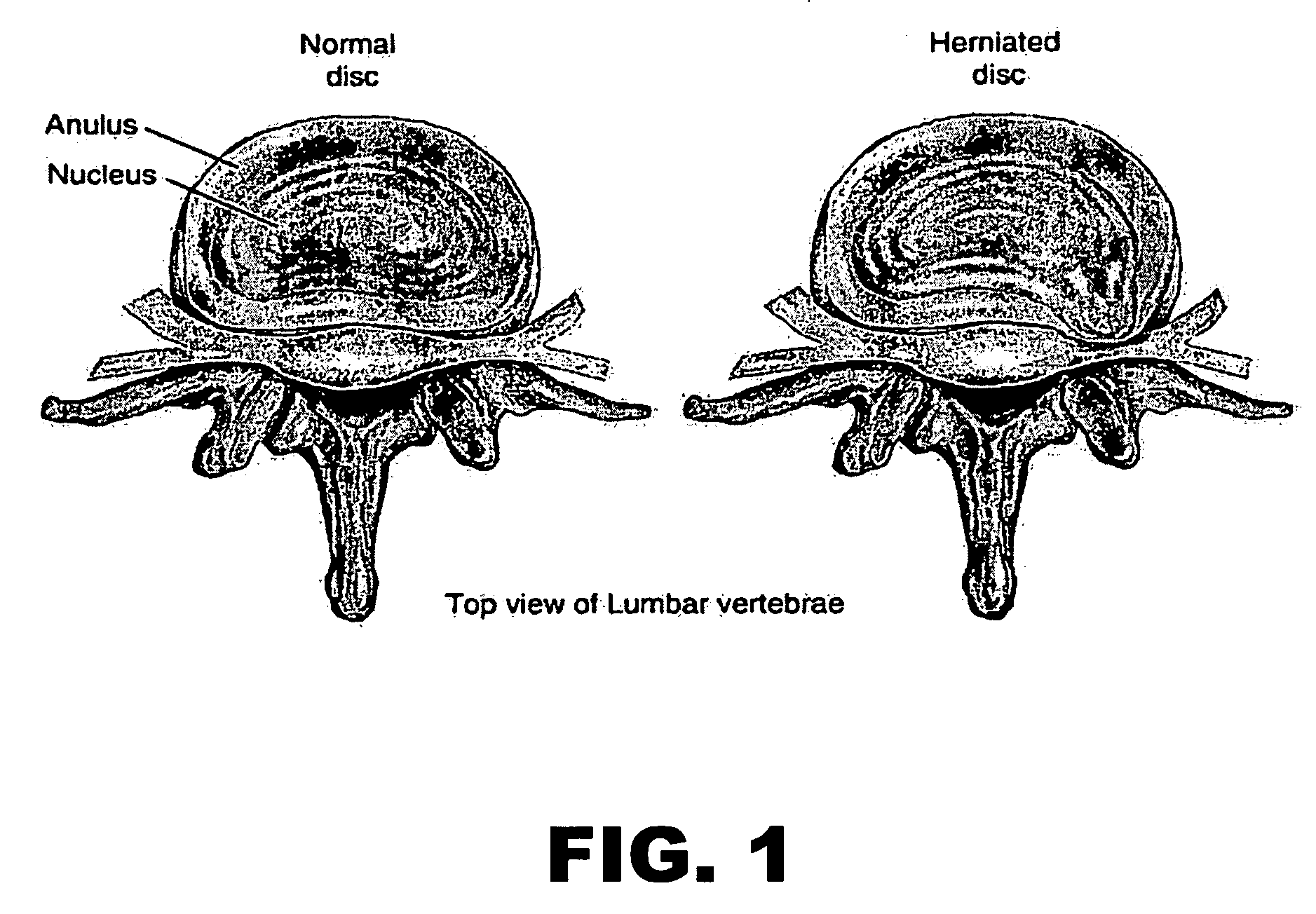
Patents
Literature
171 results about "Type II collagen" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
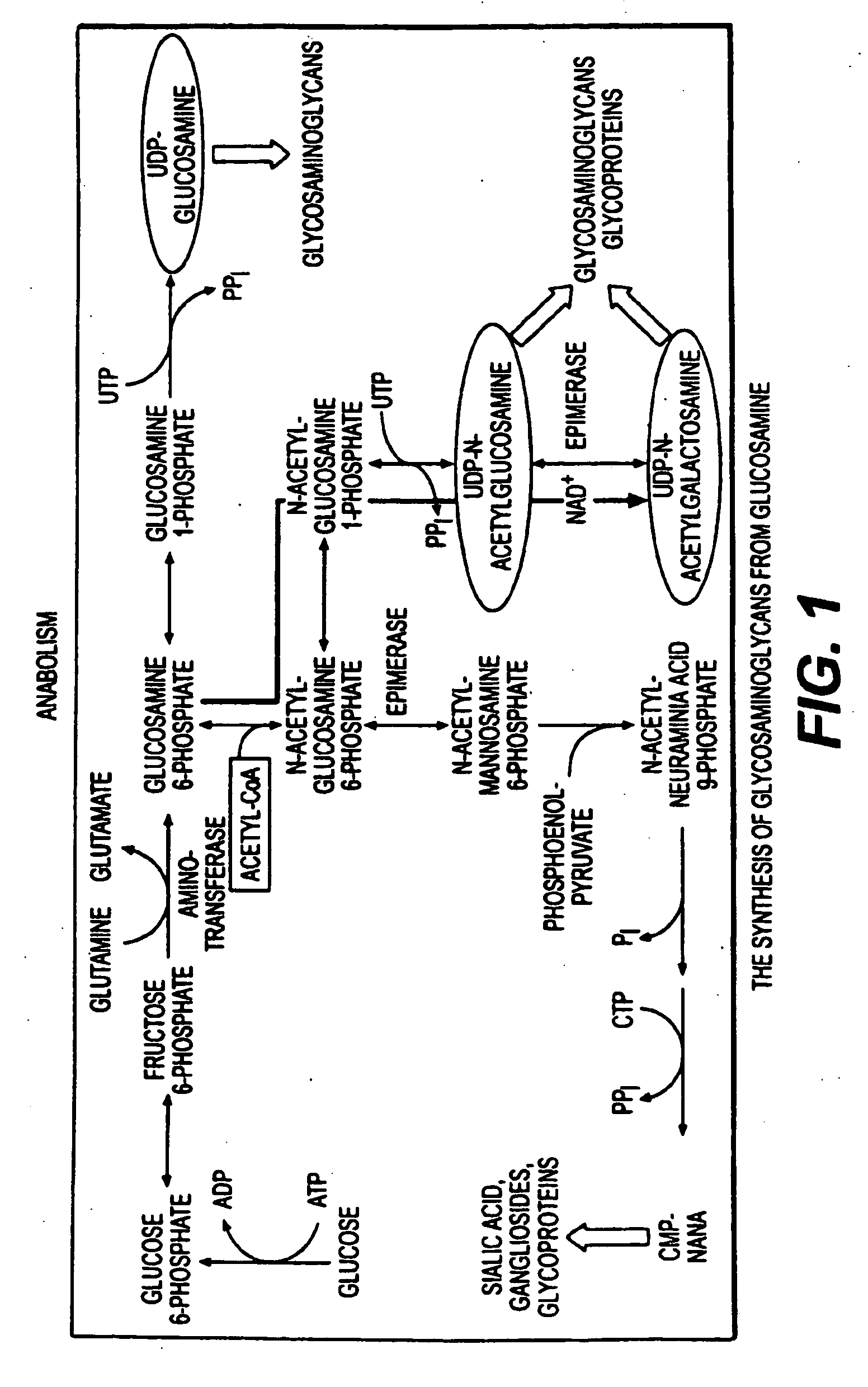
Type II collagen is the basis for articular cartilage and hyaline cartilage, formed by homotrimers of collagen, type II, alpha 1 chains. It makes up 50% of all protein in cartilage and 85–90% of collagen of articular cartilage.
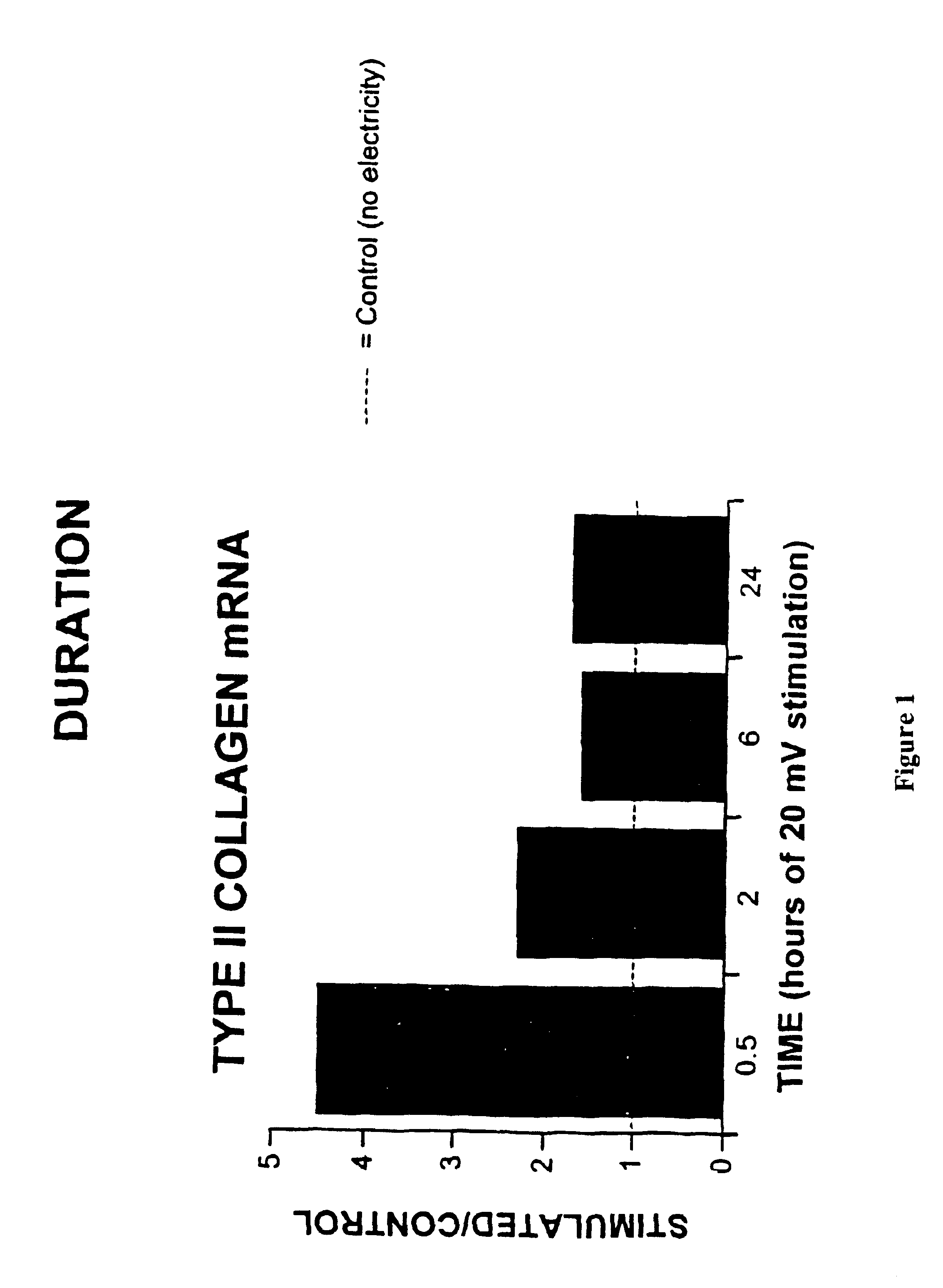
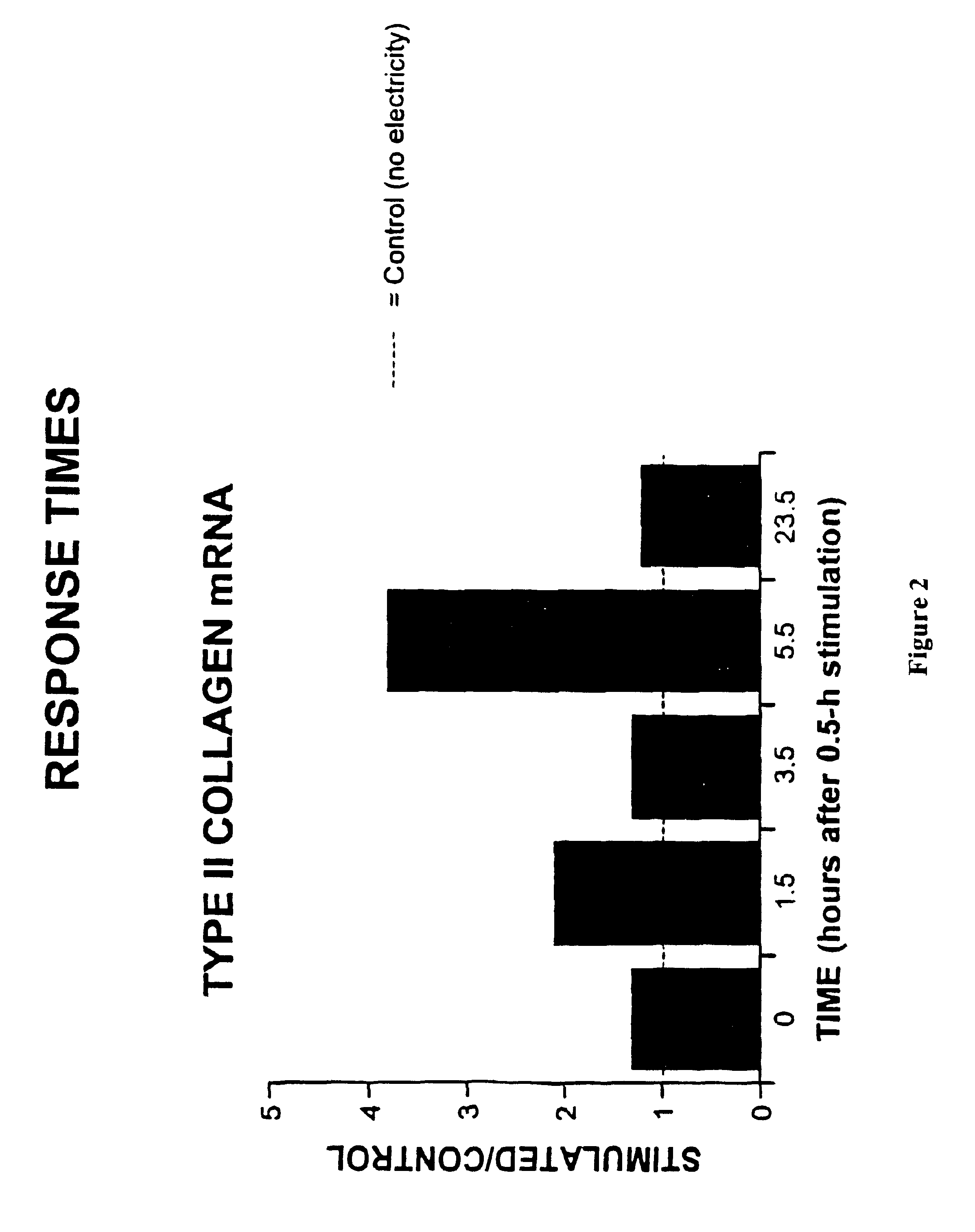
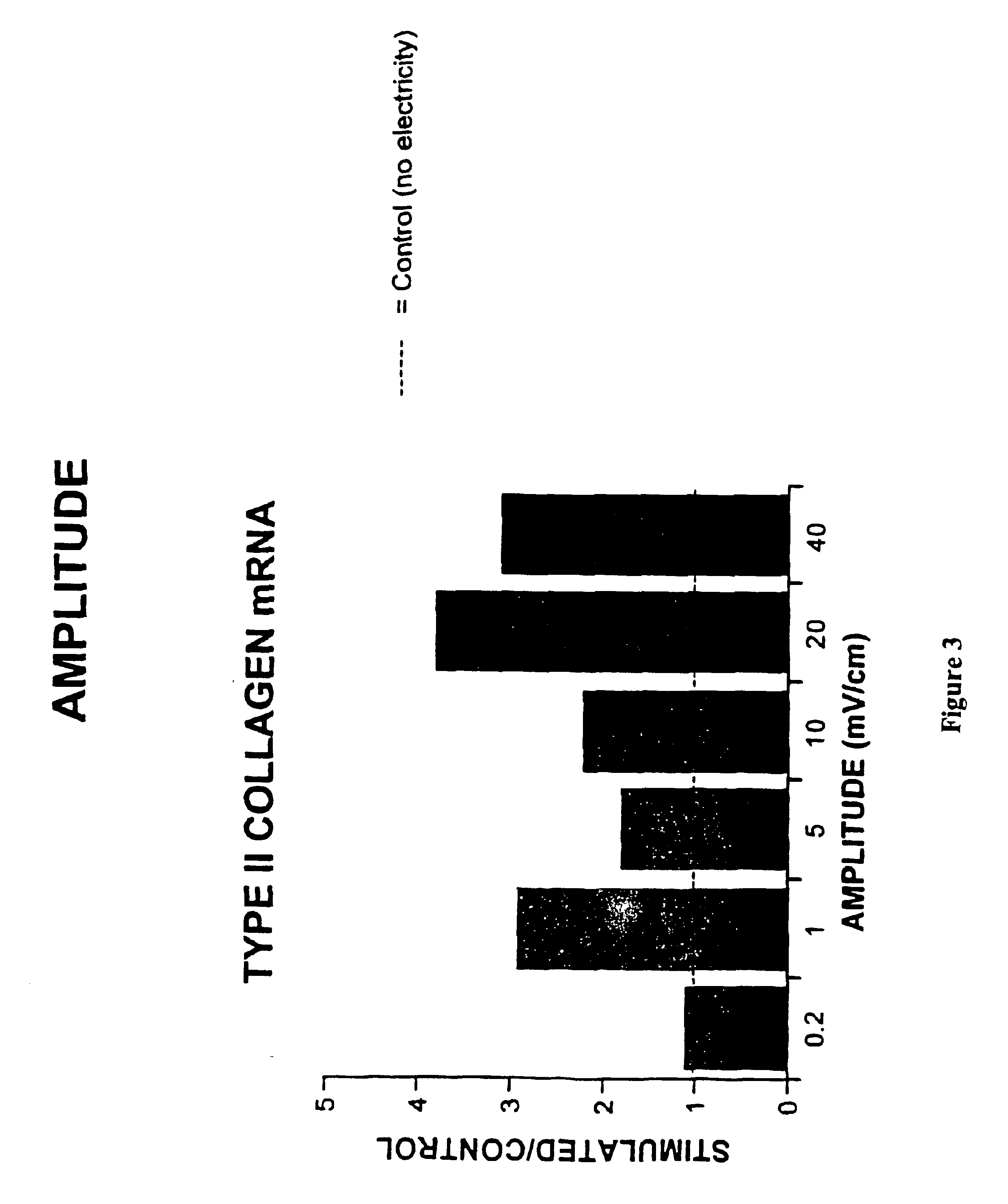
Regulation of type II collagen gene expression using specific and selective electrical and electromagnetic signals
InactiveUS6919205B2Increase typeElectrotherapySkeletal/connective tissue cellsCartilage cellsHuman DNA sequencing
Methods and devices for the regulation of type II collagen gene expression in cartilage cells via the application of specific and selective fields generated by specific and selective electric and electromagnetic signals in the treatment of diseased or injured articular cartilage. By gene expression is meant the up regulation or down regulation of the process whereby specific portions (genes) of the human genome (DNA) are transcribed into mRNA and subsequently translated into protein. Methods and devices are provided for the targeted treatment of injured or diseased cartilage tissue that include generating specific and selective electric and electromagnetic signals that generate specific and selective fields optimized for type II collagen gene expression and exposing cartilage tissue to the specific and selective fields generated by specific and selective signals so as to regulate type II collagen gene expression in such cartilage tissue. The resulting methods and devices are useful for the targeted treatment of osteoarthritis, rheumatoid arthritis, cartilage injury, and cartilage defects.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
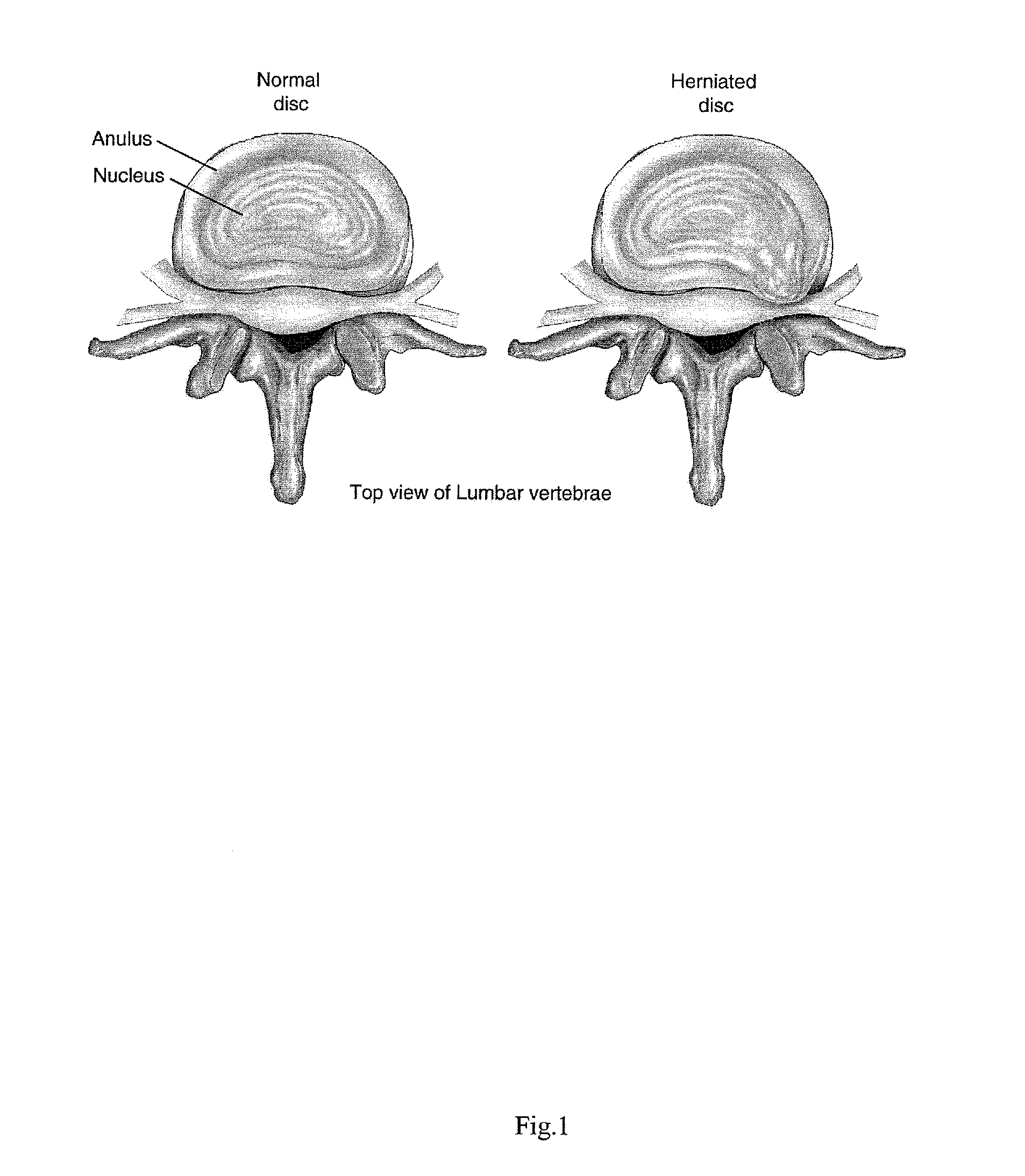
Intervertebral Disc Repair, Methods and Devices Therefor
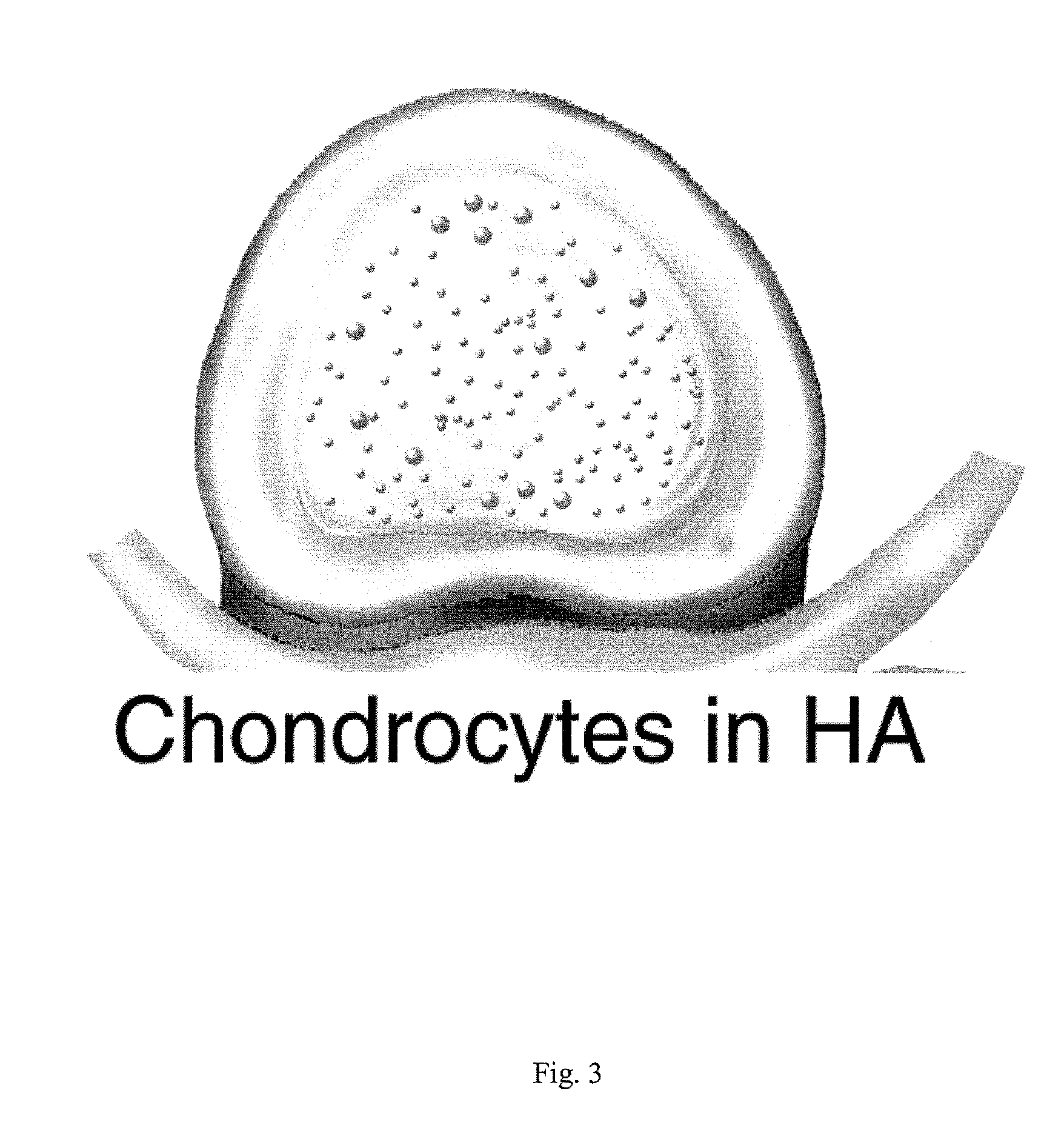
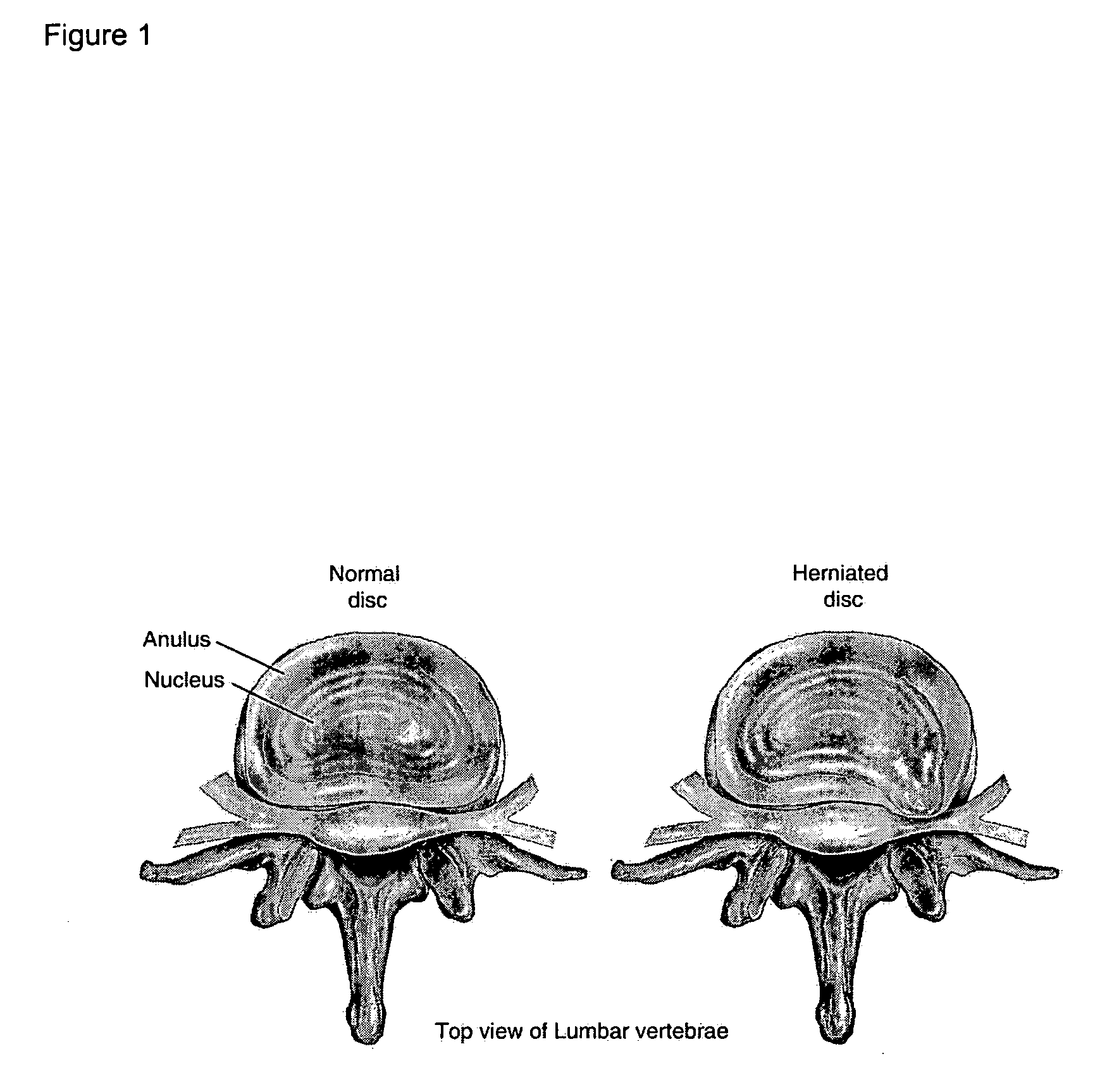
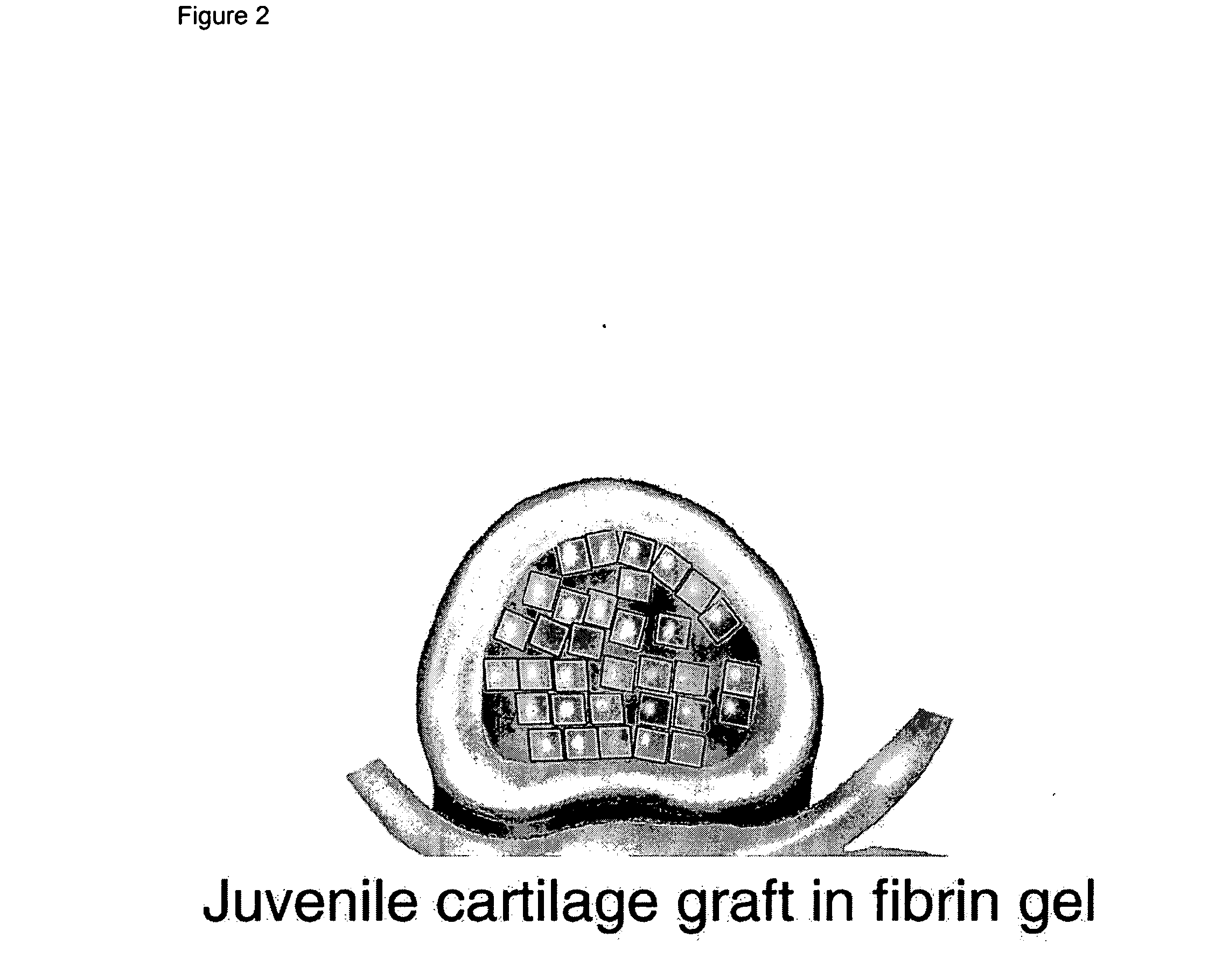
The present application discloses compositions, methods and devices for treatment of a degenerative intervertebral disc. A composition can comprise chondrocytes expressing type II collagen. These chondrocytes can be obtained from human cadavers up to about two weeks following death, and can be grown in vitro. The compositions can further comprise one or more biocompatible molecules. Treatment of a degenerative disc can comprise injecting or implanting a composition comprising the chondrocytes into a degenerative disc through an aperture or incision. If the aperture or incision is closed with a suture or a glue after introduction of the chondrocytes, the closure can withstand over 400 N of compression force.
Owner:VELOCITY FINANCIAL GRP INC ITS SUCCESSORS & ASSIGNS
Integral engineering rack of interface osteochondro tissue with bionic function
InactiveCN101020083AIncrease connection areaImprove connection strengthJoint implantsSubchondral boneBiocompatibility Testing
Owner:THE FIRST AFFILIATED HOSPITAL OF THIRD MILITARY MEDICAL UNIVERSITY OF PLA
Intervertebral disc repair, methods and devices therefor
ActiveUS20050196387A1High molecular weightBiocidePeptide/protein ingredientsHuman cadaverType II collagen
The present application discloses compositions, methods and devices for treatment of a degenerative intervertebral disc. A composition can comprise chondrocytes expressing type II collagen. These chondrocytes can be obtained from human cadavers up to about two weeks following death, and can be grown in vitro. The compositions can further comprise one or more biocompatible molecules. Treatment of a degenerative disc can comprise injecting or implanting a composition comprising the chondrocytes into a degenerative disc.
Owner:VELOCITY FINANCIAL GRP INC ITS SUCCESSORS & ASSIGNS
Novel chondrocyte epimatrix membrane and preparation method thereof
InactiveCN102188748ANo biological immunogenicitySuitable for growthProsthesisAdditive ingredientBlood vessel
The invention discloses a novel chondrocyte epimatrix membrane and a preparation method thereof. The membrane contains cell epimatrix ingredients which have bioactivity and cell activity, wherein the cell epimatrix comprises the following main ingredients in percentage by weight: 80 to 95 percent of bionic type II collagen in an acellular cartilage matrix and 10 to 20 percent of hyaluronic acid, 10 to 20 percent of aminopolysaccharide and the like which serve as a chondrocyte epimatrix; the chondrocyte epimatrix biological membrane prepared from the acellular cartilage matrix has the bionic chondrocyte epimatrix ingredients, is excellent in cell consistency, does not arouse immunological rejection of host cartilage tissue, can be used for repairing the damage of hyaline cartilage tissue of joints and reconstructing the hyaline cartilage tissue, and also can be used for a seed cell inoculating vector and a growth factor sustained-release vector; and the chondrocyte epimatrix contains blood vessel inhibiting factor ingredients, so the membrane also can be used for preventing the conglutination of tissue such as muscle tendons.
Owner:成都军区昆明总医院
Use of anabolic agents, anti-catabolic agents, antioxidant agents, and analgesics for protection, treatment and repair of connective tissues in humans and animals
InactiveUS20070141181A1Repair and treat and prevent damageBiocideAntipyreticSuper oxide dismutaseS-Adenosyl-l-methionine
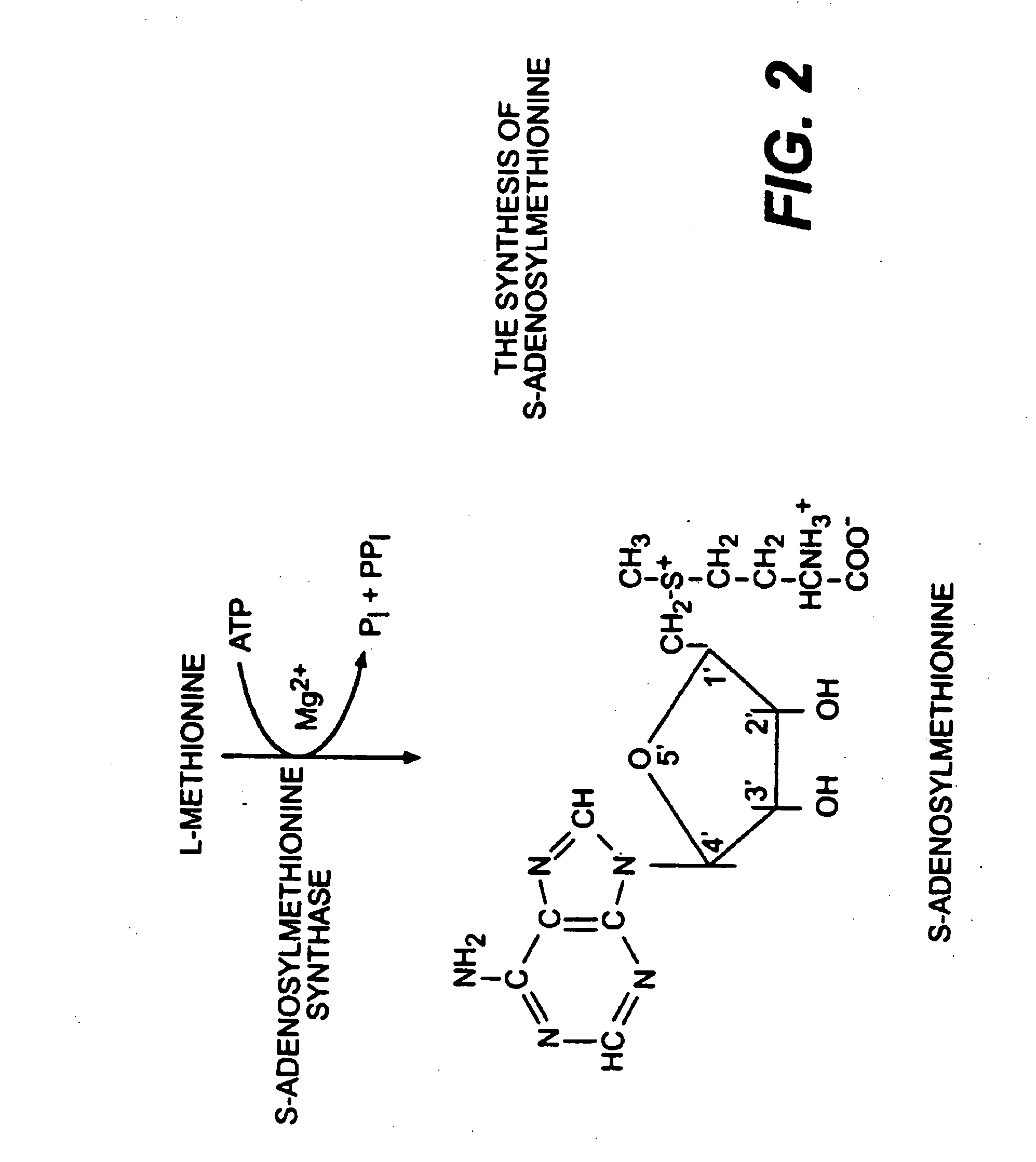
The present invention relates to compositions for the modulation of inflammation in connective tissues in humans and animals and the modulation of markers of such inflammation, including COX-2, TNF-α, IL-1β, iNOS, p38, and chemokines, comprising any or all of anabolic, anti-catabolic, anti-oxidant and analgesic agents, including aminosugars, S-adenosylmethionine, arachadonic acid, GAGs, including pentosan, collagen type II, tetracyclines or tetracycline-like compounds, diacerin, super oxide dismutase, L-ergothioneine, methylsulfanylmethane, one or more avocado / soybean unsaponifiables, and an analgesic, e.g., acetaminophen, and to methods of treating humans and animals by administration of these novel compositions to humans and animals in need thereof.
Owner:NUTRAMAX LABORATORIES INC
Bone implant and manufacturing method thereof
InactiveUS20110262486A1Readily availableHigh activityBiocideHeavy metal active ingredientsBiopolymerBone implant
The invention discloses a bone implant and a manufacturing method thereof. The manufacturing method of the bone implant comprises a step of coating or mixing type II collagen with at least one porous bone material comprising metals, bio-ceramics, natural biopolymers and synthetic polymers. Another manufacturing method of the bone implant comprises the steps of loading type II collagen with or without at least one porous bone material in a container, and lyophilizing the type II collagen to generate a type II collagen sponge construct with or without the porous bone material as the bone material. The manufactured bone implants are effective, with or without loading cells having differentiation tendency towards osteogenesis, to facilitate bone repair upon introduction of the bone implant into various osseous defects.
Owner:TAIPEI MEDICAL UNIV
Intervertebral Disc Repair, Methods and Devices Therefor
The present application discloses compositions, methods and devices for treatment of a degenerative intervertebral disc. A composition can comprise chondrocytes expressing type II collagen. These chondrocytes can be obtained from human cadavers up to about two weeks following death, and can be grown in vitro. The compositions can further comprise one or more biocompatible molecules. Treatment of a degenerative disc can comprise injecting or implanting a composition comprising the chondrocytes into a degenerative disc.
Owner:VELOCITY FINANCIAL GRP INC ITS SUCCESSORS & ASSIGNS
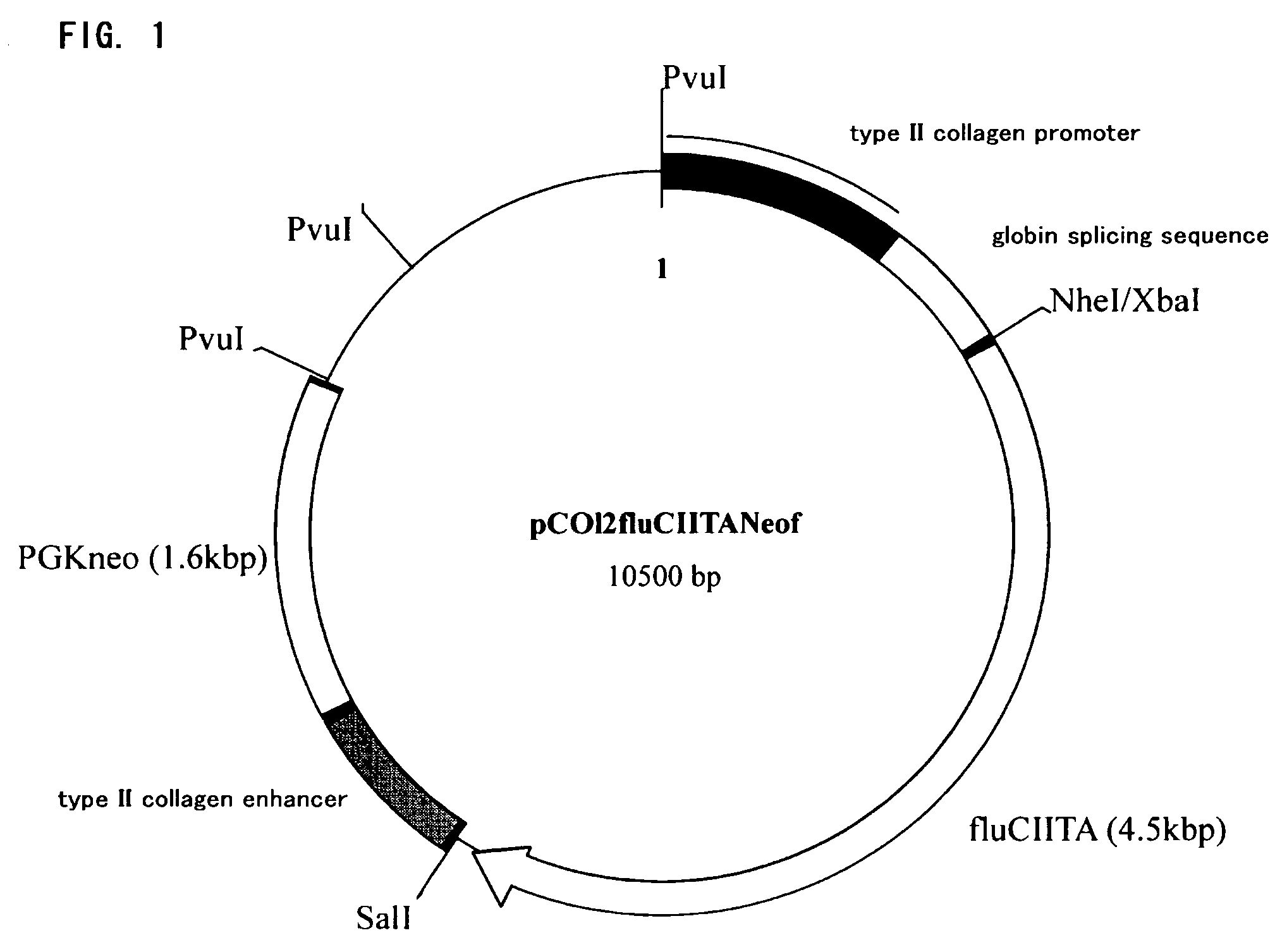
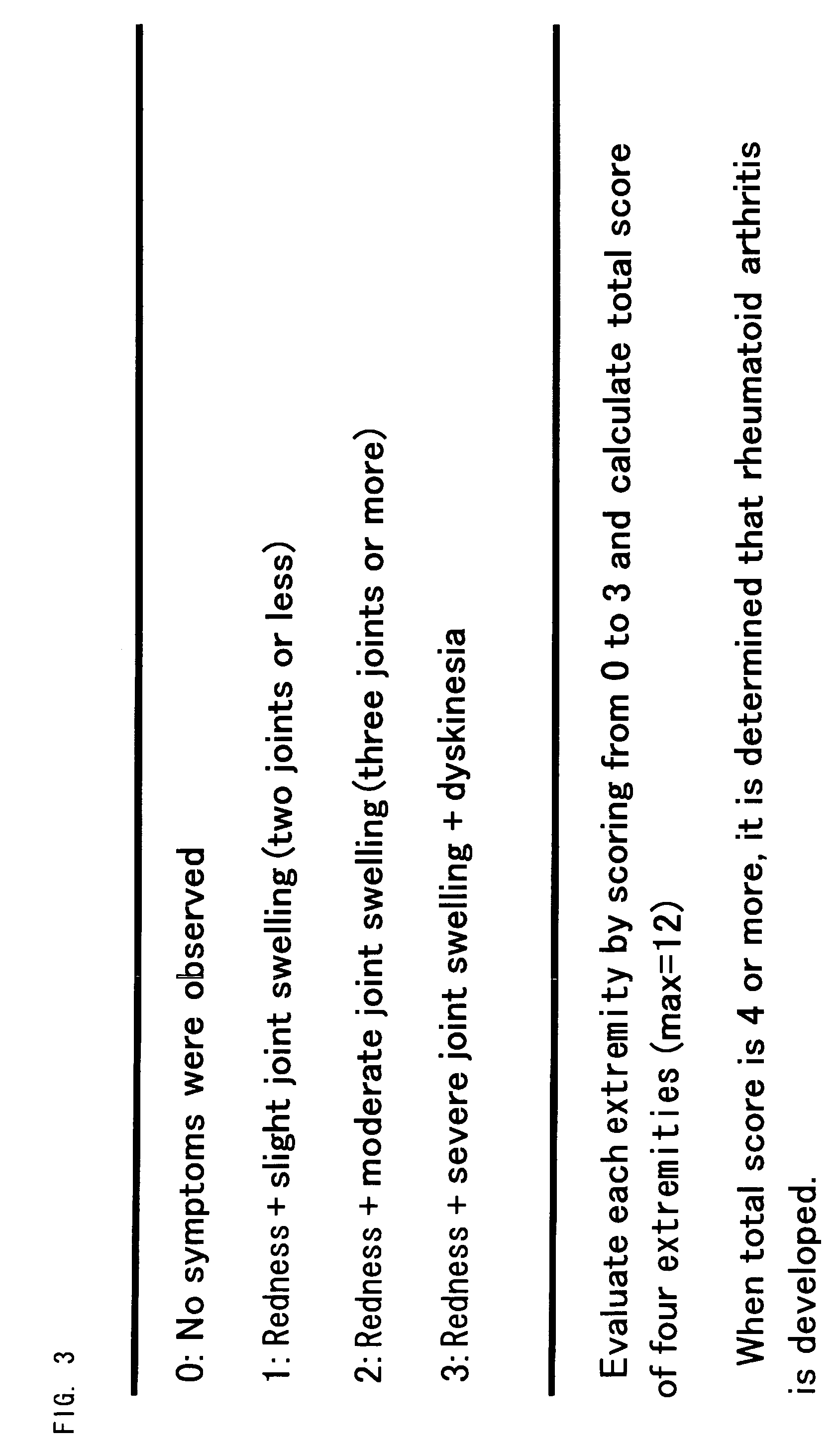
Transgenic nonhuman mammal representing the pathologic conditions of human rheumatoid arthritis
InactiveUS7745690B2High sensitivityAccurate observationAnimal cellsBiological testingMammalMHC class II
It is intended to provide a transgenic nonhuman mammal well representing the pathologic conditions of human rheumatoid arthritis. A transgenic nonhuman mammal is obtained by transferring a foreign DNA, wherein a DNA selected from the group consisting of an MHC class II transcriptional activator gene, the activity domain of an MHC class II transcriptional activator gene and a variant of an MHC class II transcriptional activator gene is provided under the control of a type II collagen promoter, into a cell at the early stage of development.
Owner:NAGOYA INDUSTRIAL SCIENCE RESEARCH INST
Preparation method of cartilage extract containing non-denatured type II collagen
InactiveCN106916870AHigh purityHigh extraction rateConnective tissue peptidesFermentationSubtilisinAlkaline protease
The invention provides a preparation method of cartilage extract containing non-denatured type II collagen. The method comprises the following steps: degreasing, disinfecting, homogenizing, enzymatic hydrolysis, filtration and drying. In the enzymatic hydrolysis step, the pH of a slurry obtained in the homogenizing step is adjusted to 2.5-8.5, an enzyme accounting for 0.001-2%of the weight of cartilages is added, a clear liquid accounting for 1 / 20 to 1 / 500 of the weight of the cartilages and obtained through juicing pawpaw and / or pineapples and filtering the obtained juice is added, the enzymatic hydrolysis is carried out at 25-50 DEG C for 12-48 h, and the enzyme is preferably selected from one or more of pepsin, subtilisin, alkaline proteases and metalloproteases. The cartilage extract obtained through the preparation method has the advantages of high purity, high extraction rate, and easiness in large-scale production, and richness in non-denatured type II collagen and chondroitin sulfate.
Owner:BEIJING SEMNL BIOTECHNOLOGY CO LTD +1
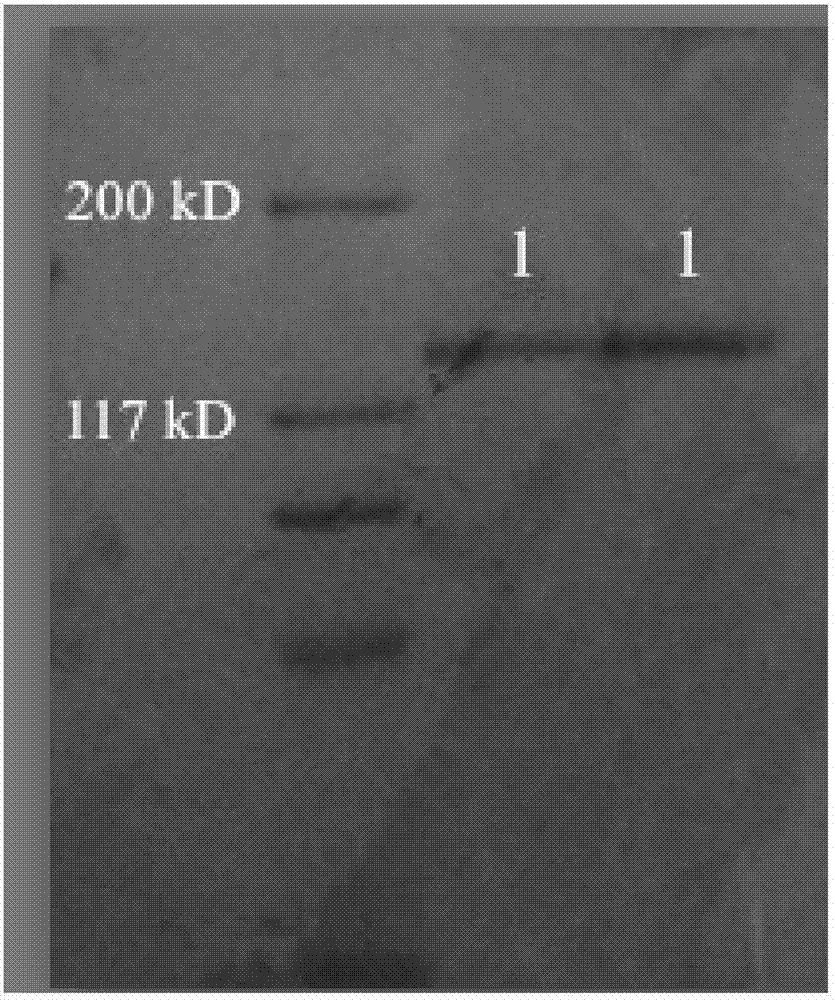
Method of Separating Collagen From the Various Animal Tissues for Producing Collagen Solution and Product Using the Same
ActiveUS20080118947A1Improve customer satisfactionQuality improvementConnective tissue peptidesPeptide/protein ingredientsPhosphateFractionation
A method for separation the collagen from the various animal tissues is disclosed for preparing collagen solution and product using the same. The porcine tissues are processed to have proper form and size for acid-treatment. The acid-treatment is repeated with pepsin to separate type I or II collagens. The separated collagen is salt-treated for fractionation and ethanol-treated for obtaining 5˜10% of collagen from the initial tissue weight. The prepared tissues are processed for separating collagen through the collagen separating process. The separated collagen is processed for preparing product. The method for preparing product is comprised: treating a collagen solution having a predetermined concentration under a neutral condition at a low temperature, followed by overnight treatment at a temperature of 30 to 35° C.; concentrating collagen by centrifugation; and dissolving the thus-concentrated collagen in refrigerated weakly-acidic solvent or phosphate buffered saline (PBS), thereby preparing collagen having a concentration of 1 to 5 mg / mL.
Owner:CELLONTECH
Tissue engineered bone-cartilage complex tissue graft and preparation method thereof
The invention provides a tissue engineered bone-cartilage composite tissue transplant and a preparation method thereof, and belongs to the tissue engineering field. The method comprises the following steps: seed cells are implanted on a scaffold material and cultured in vitro to form the tissue engineered bone-cartilage composite tissue transplant; the seed cells are osteogenic stem cells; the scaffold material comprises an osteogenic part and a chondroblast part, and the osteogenic part is added with an osteoblast induction factor and modified by a type-I collagen in a preparation process; and the chondroblast part is added with a chondroblast induction factor and modified by a type-II collagen. Bone marrow mesenchymal stem cells are successfully induced into osteocytes and chondrocytes, and bone tissues and cartilage tissues are formed after the obtained transplant is transplanted in vivo. Satisfactory effect is obtained from the tissue engineered bone-cartilage composite tissue which is constructed by the transplant.
Owner:JINAN UNIVERSITY
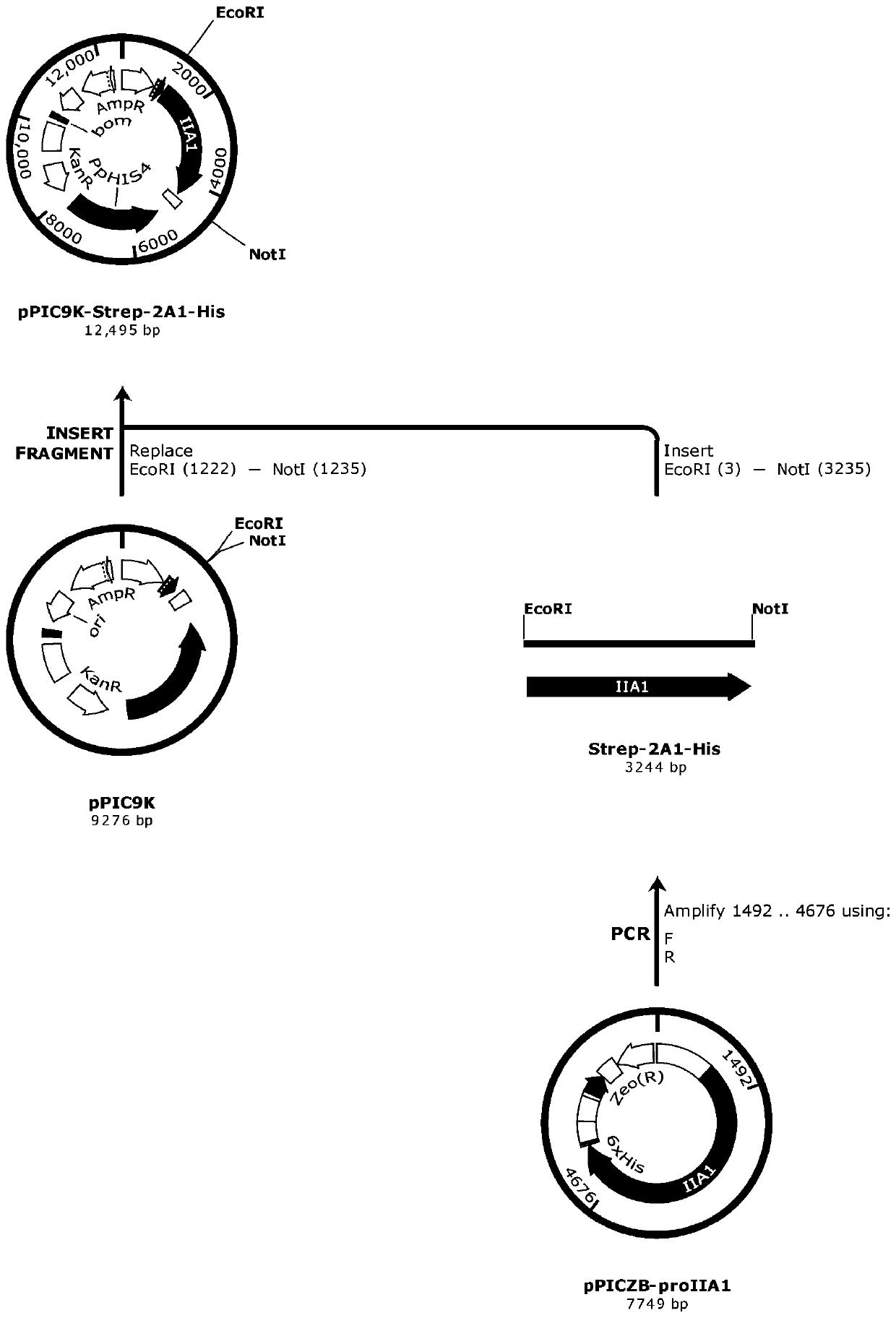
Method for producing recombinant human type II collagen single chain by Pichia pastoris
InactiveCN110029111AOptimize secondary structureEliminate useConnective tissue peptidesPeptide preparation methodsPichia pastorisNucleotide
Owner:JIANGSU TRAUTEC MEDICAL TECH CO LTD
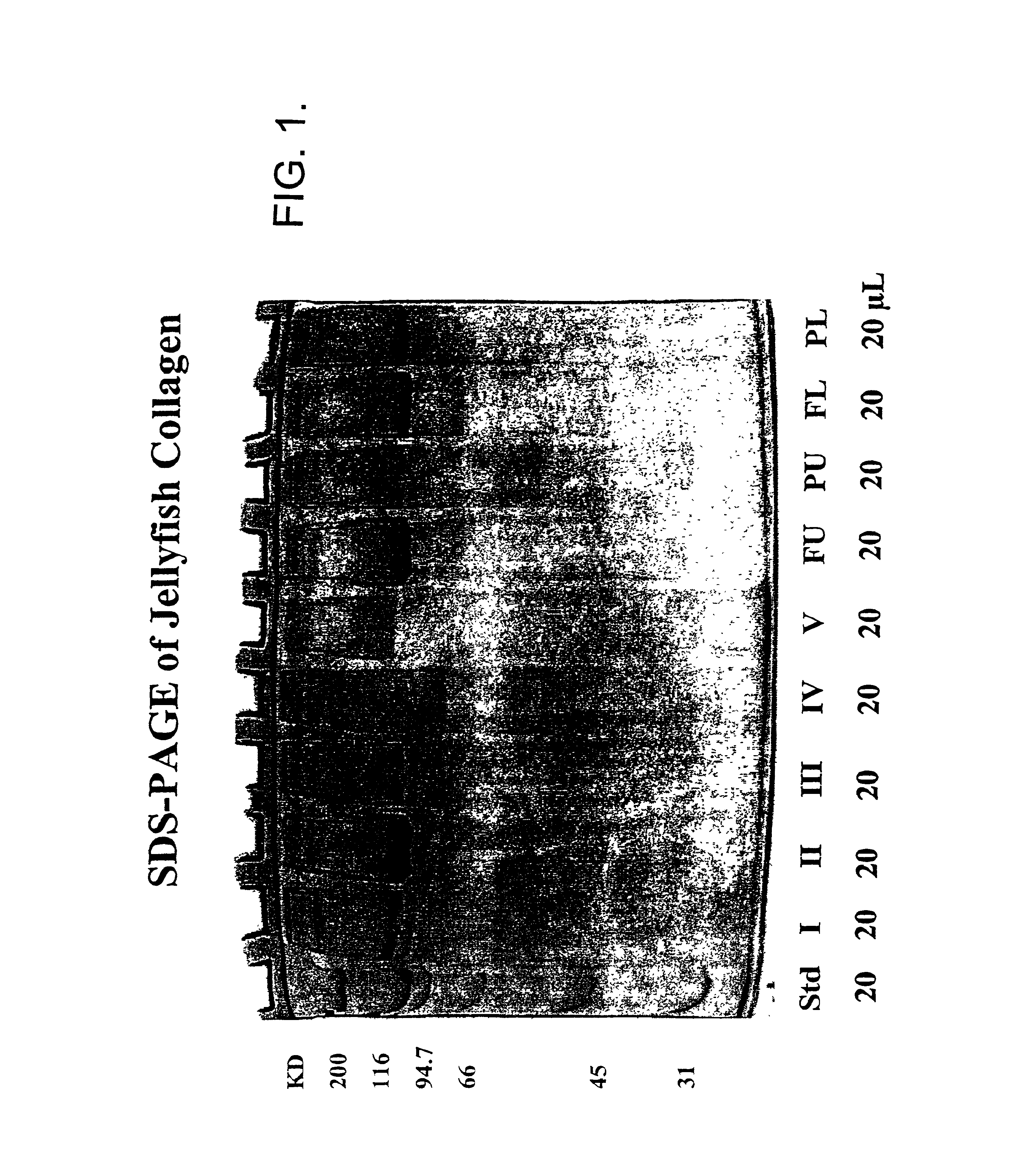
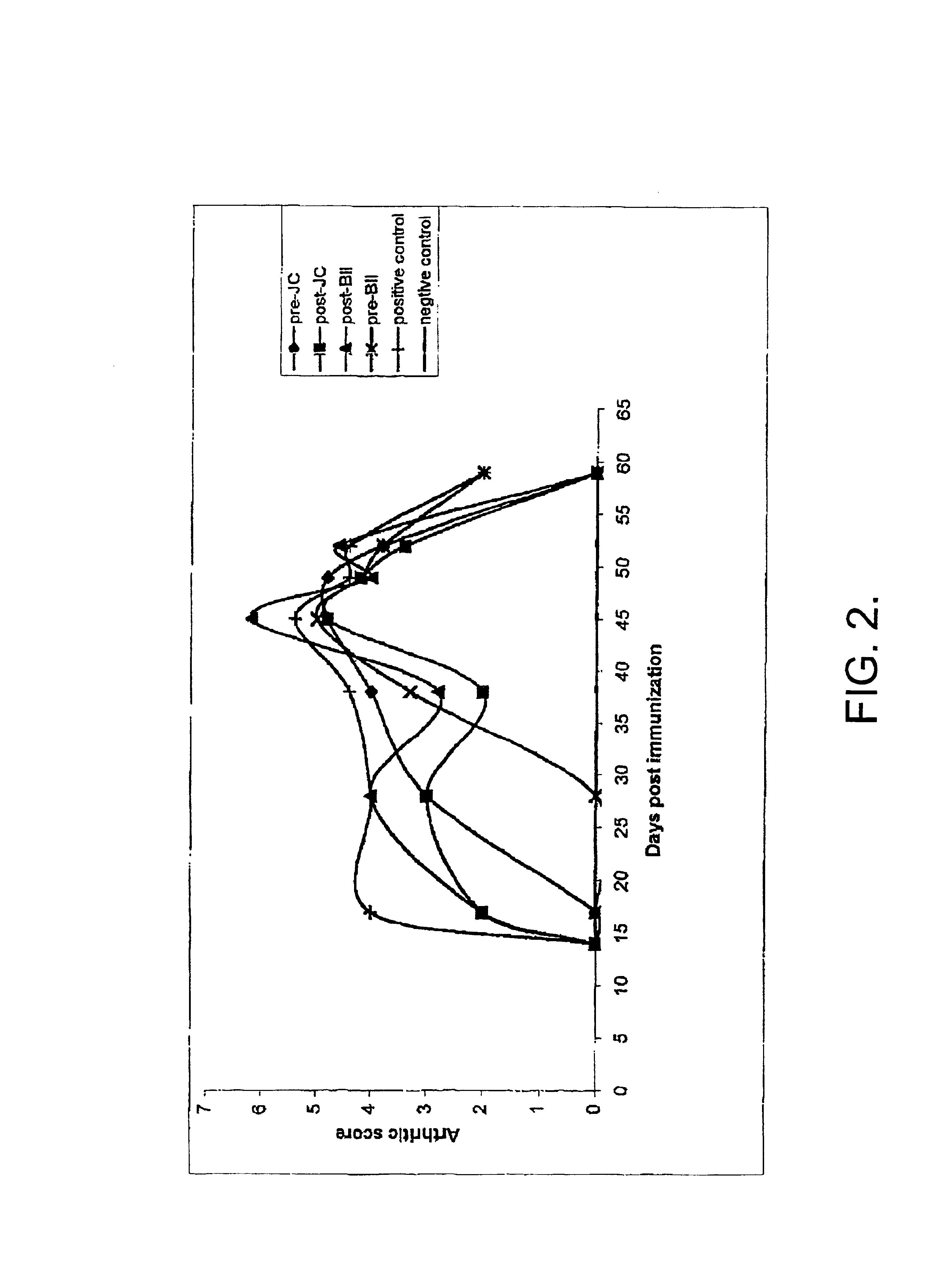
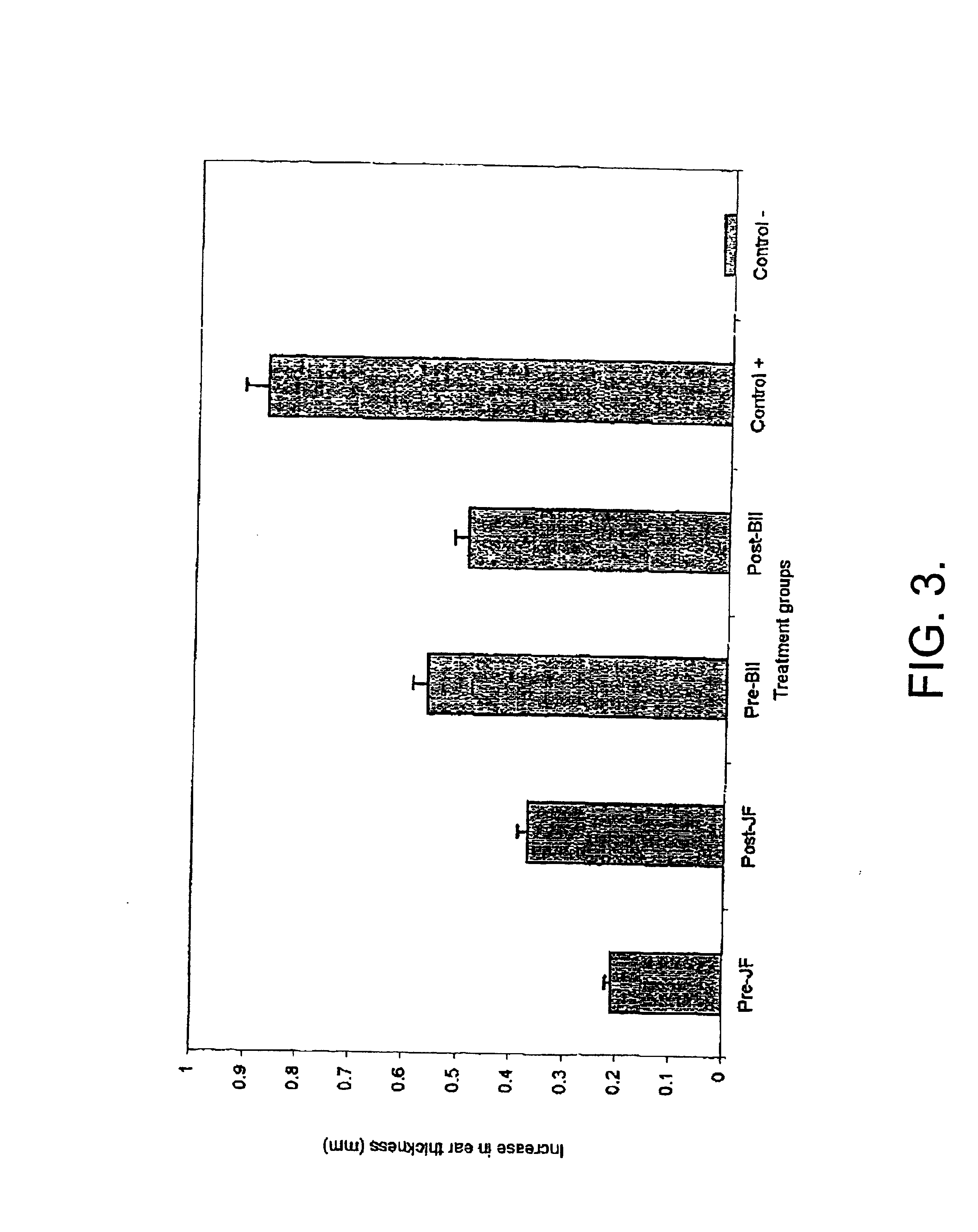
Use of jellyfish collagen (type II) in the treatment of rheumatoid arthritis
Compositions of substantially homogeneous type II-like collagen from invertebrates belonging to the class Scyphozoa, phylum Coelenterata, i.e., jellyfish, particularly Stomolophus meleagris, and methods for its extraction are provided. Methods for the treatment of arthritis, in particular rheumatoid arthritis, by administering an effective amount of the collagen-containing compositions of the invention so as to induce immune tolerance, are also provided.
Owner:AUBURN UNIV
Method of inducing or enhancing chondrogenesis with extracellular matrix containing BMP-4
InactiveUS6932977B2Induce and enhance chondrogenesisEnhancing chondrogenesisOrganic active ingredientsBiocideCell-Extracellular MatrixECM Protein
A method and composition are provided for inducing or enhancing chondrogenesis in vivo or in vitro. The method is performed by exposing the cells in vitro or in vivo to an extracellular matrix comprising of type I collagen, type II collagen or a mixture of type I collagen or type II collagen and hyaluronate and further containing BMP-4 or a combination of BMP-4 and GDF-5.
Owner:DEPUY SPINE INC (US)
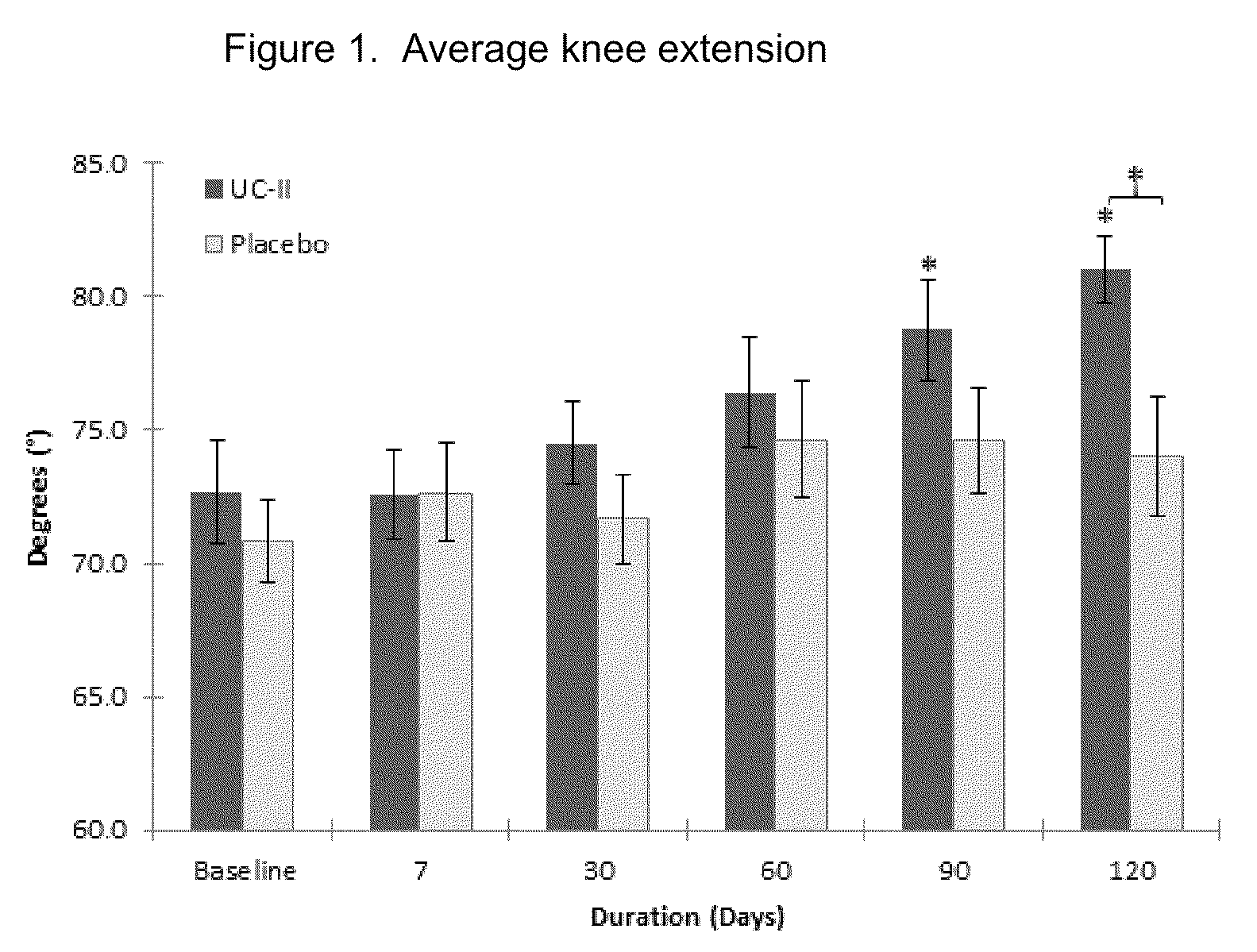
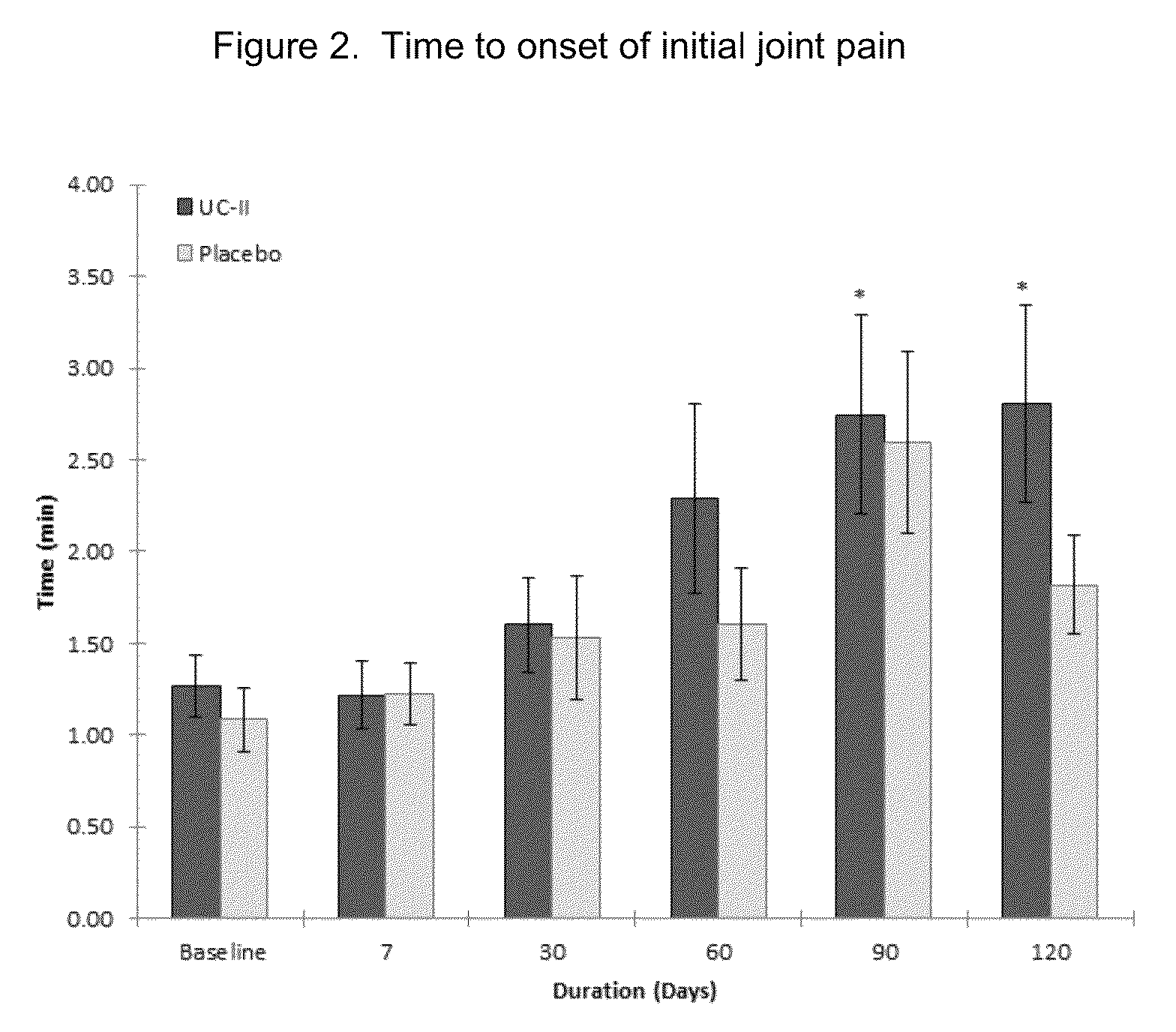
Method of reducing exercise-induced joint pain in non-arthritic mammals
ActiveUS9066926B2Reduce joint painExtended maintenance periodPeptide/protein ingredientsAntipyreticJoint painAnesthesia
The present invention relates to a method of treating exercise-induced joint pain in arthritis-free mammals by the administration of undenatured Type II collagen.
Owner:LONZA GREENWOOD LLC
Method for extracting undenatured type II collagen from squid cartilage
The invention provides a method for extracting undenatured type II collagen from squid cartilage. The method includes: freeze-drying squid cartilage; crushing the freeze-dried squid cartilage, and sieving to obtain cartilage powder; suspending the cartilage powder in HCL solution, EDTA solution and NaOH solution to obtain precipitate; adding acetic acid solution and protease into the precipitate, stirring, centrifuging and collecting supernate; adding saturated NaCl solution into the supernate until NaCl concentration is 1-1.5M, allowing standing for 16-24h, centrifuging, and removing supernate to obtain first precipitate; dissolving the first precipitate by using acid, performing dialysis, and performing freeze-drying to obtain the undenatured type II collagen. The purity of the undenatured type II collagen acquired by extracting is not lower than 95%.
Owner:SHANGHAI FISHERIES RES INST
Elastic protein peptide powder and preparation method thereof
InactiveCN108968077AImprove aging phenomenonIncrease elasticityFood ingredient functionsProtein food ingredientsCRANBERRY JUICEElastin peptides
The invention discloses elastic protein peptide powder. The key points of the technical scheme are characterized in that the elastic protein peptide powder is prepared from the following components inparts by weight: 2,300-2,700 parts of fish collagen hydrolysate, 600-800 parts of blueberry juice powder, 600-800 parts of grape powder, 230-270 parts of cranberry juice powder, 50-350 parts of xylitol, 90-130 parts of green plum powder, 80-120 parts of acerola cherry fruit powder, 40-80 parts of type II collagen peptide hydrolysate, 80-120 parts of L-calcium lactate, 30-70 parts of soybean peptide, 8-14 parts of skipjack elastic protein peptide, 2-5 parts of blood orange powder, 10-14 parts of cubilose peptide, 80-120 parts of beet juice powder, 60-80 parts of citric acid and 1-5 parts of sucralose. The invention aims at providing the elastic protein peptide powder which can remarkably improve skin ageing.
Owner:杭州相生相成科技有限公司
Oral remedy for rheumatoid arthritis and functional food
InactiveUS6010722AHighly effective oral immune toleranceAvoid immune responsePeptide/protein ingredientsAntipyreticDiseaseOral medication
PCT No. PCT / JP96 / 01623 Sec. 371 Date May 18, 1998 Sec. 102(e) Date May 18, 1998 PCT Filed Jun. 13, 1996 PCT Pub. No. WO96 / 41644 PCT Pub. Date Dec. 27, 1996Oral drugs and functional foods of the present invention contain type-II collagen denatured (fragmented) under specific conditions. Rheumatoid arthritis (RA) has been considered to be an autoimmune disease against type-II collagen as an antigen. Since denatured type-II collagen of the invention induces high oral immune tolerance and inhibits immune responses against type-II collagen, it can prevent and treat RA. Particularly, RA can effectively be prevented and treated by simple oral administration of type-II collagen.
Owner:NIPPON HAM
Method of Reducing Exercise-induced Joint Pain in Non-arthritic Mammals
ActiveUS20150119335A1Relieve knee painRelieve painPeptide/protein ingredientsAntipyreticJoint painAnesthesia
The present invention relates to a method of treating exercise-induced joint pain in arthritis-free mammals by the administration of undenatured Type II collagen.
Owner:LONZA GREENWOOD LLC
Composition for preventing and treating bone arthritis and application thereof
ActiveCN109106944AHas a repairing effectInhibit inflammationOrganic active ingredientsHydrolysed protein ingredientsCartilage collagenMedicine
The invention provides a composition for preventing and treating bone arthritis. The composition is characterized by comprising non-denaturing type II collagen and cartilage collagen peptide accordingto a weight ratio of 1:(0.5 to 30), preferably 1:(1 to 20), more preferably 1:(1.5 to 10), and most preferably 1:(1.5 to 6). The invention also provides application of the composition in preparationof a product for preventing and treating the bone arthritis, wherein the product is a medicine or health-care food. The composition for preventing and treating bone arthritis has the advantages that the cartilage collagen peptide can be used as a raw material needed for synthesizing cartilage tissues, the degradation of joints is prevented, and the repair function is realized on the injured joints; the non-denaturing type II collagen can inhibit the generation of inflammation, so as to relieve and treat the arthritis; proved by experiment results, the combination of the cartilage collagen peptide and the non-denaturing type II collagen can achieve a synergistic effect on the preventing and treatment of arthritis.
Owner:安徽盛美诺生物技术有限公司
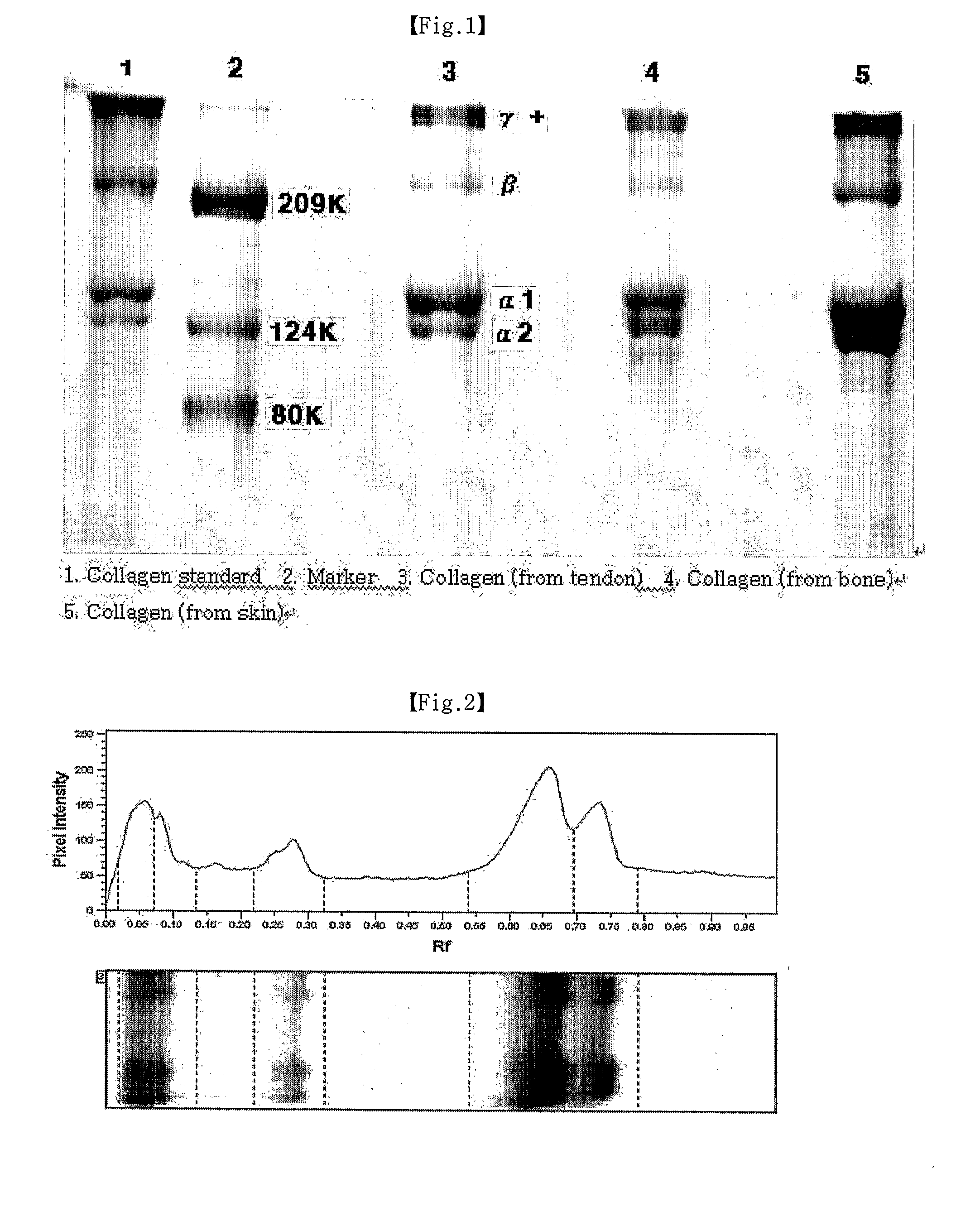
Method for extracting pure type II collagen from chicken cartilage
The invention discloses a method for extracting pure type II collagen from chicken cartilage. The method is characterized by including, using the fresh chicken cartilage as raw material, removing other non-cartilage components, performing degreasing pretreatment, using mixed solution of 4mol / L NaCl and 0.05mol / L Tris-HCl to remove soluble impure protein, using mixed solution (pH 7.5) of 4mol / L guanidine hydrochloride and 0.05mol / L Tris-HCl to remove insoluble impure protein, using acetic acid solution containing 1-2% pepsin for solid matter enzymolysis, then centrifuging to take supernate, adding NaCl until final concentration is 3-4.2mol / L, 1.2-1.8mol / L and 0.8-1.0mol / L, successively salting out to remove other types of collagen, repeatedly adding NaCl until the final concentration is 0.8-1.0mol / L, salting out for 2-5 times, and dialyzing to obtain the pure type II collagen. The type II collagen produced by the method is single in type, high in bioactivity, stable in physical and chemical property. Meanwhile, the method is simple to operate and capable of benefiting large-scale production.
Owner:SICHUAN UNIV
Cartilage targeting double-drug-loaded nano-microspheres with optical thermal response characteristic and preparation method and application thereof
ActiveCN112023060AReduce degenerationImprove abilitiesPowder deliveryOrganic active ingredientsMicrosphereMetal-organic framework
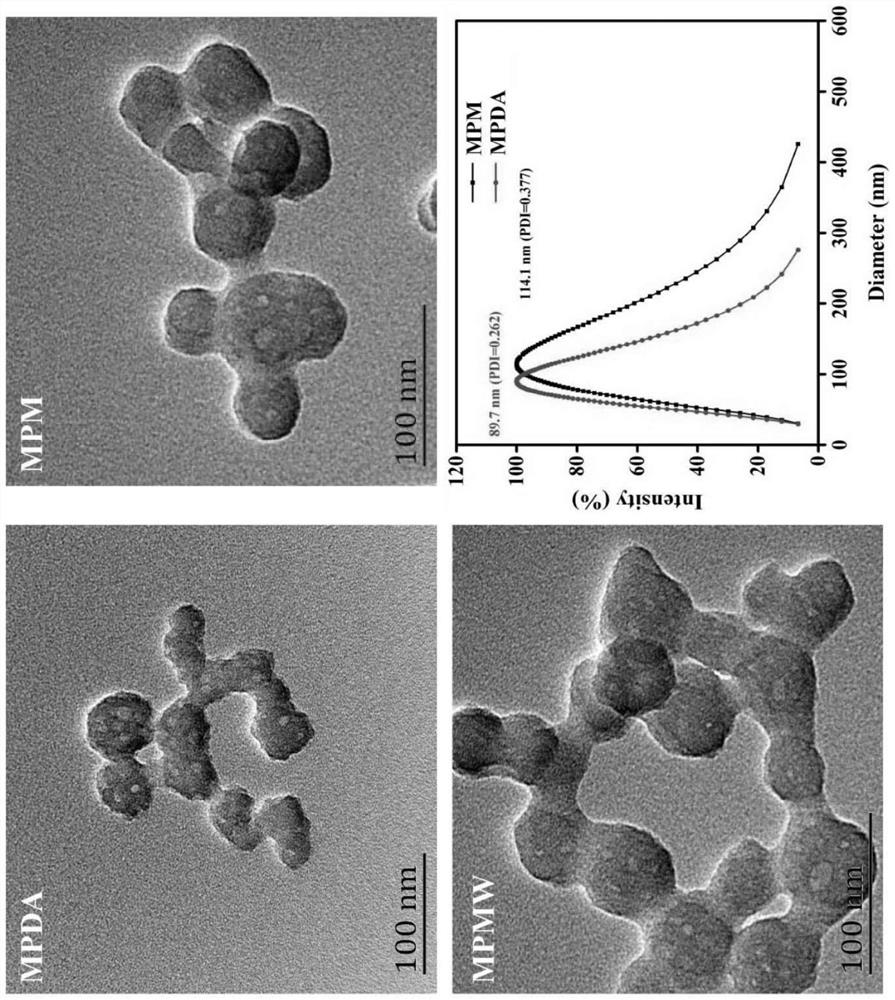
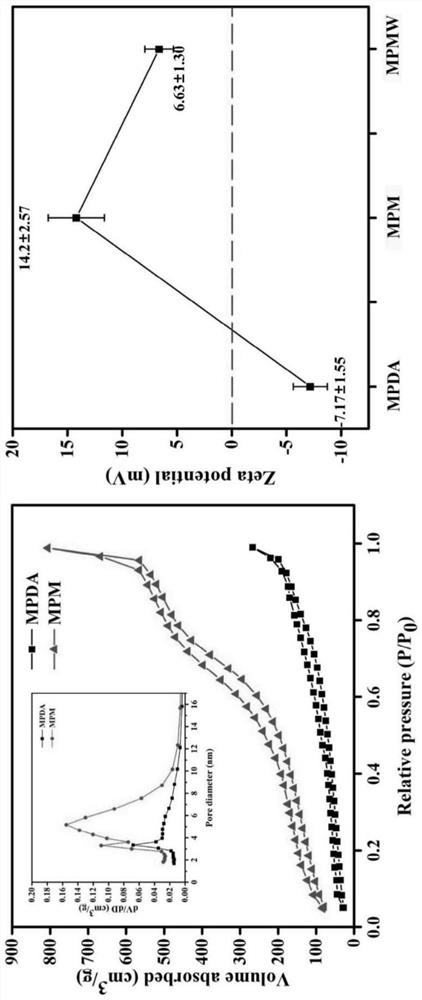
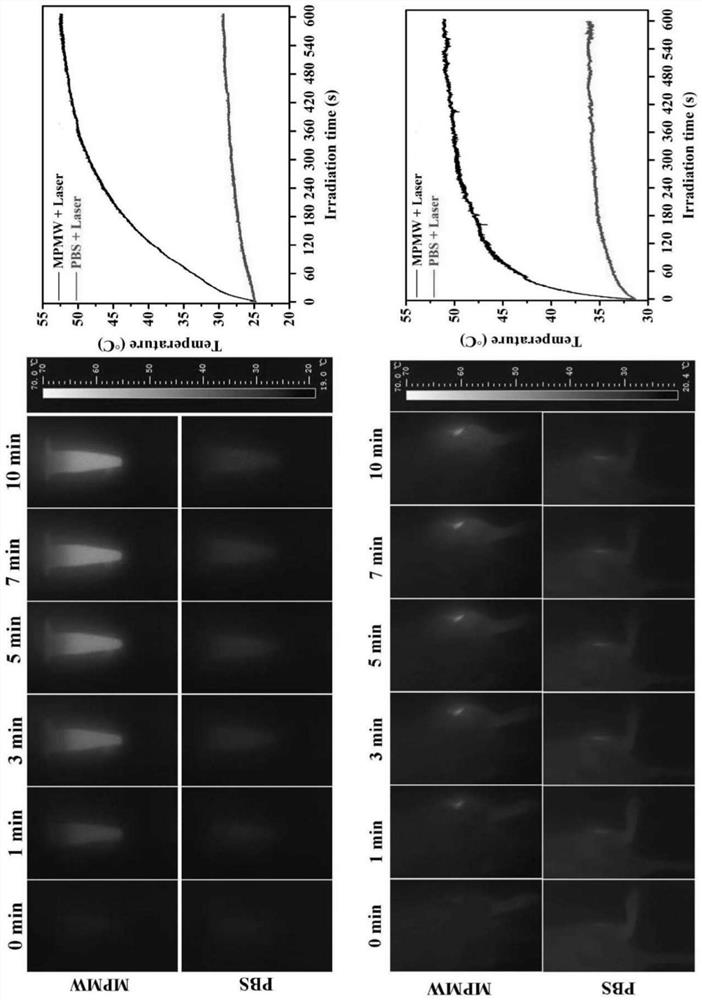
The invention relates to a preparation method and application of cartilage targeting double-drug-loaded nano-microspheres with the optical thermal response characteristic. According to thecartilage targeting double-drug-loaded nano-microspheres with the optical thermal response characteristic, small-particle-size mesoporous polydopamine is used as a matrix, the matrix is sequentially subjected toa cyclic reaction with FeCl3. 6H2O and H3BTC, and the surface of the mesoporous polydopamine is modified to form a metal organic framework. After the synthesized MPDA-MOF (referred to as MPM hereinafter) nano-microspheres react with EDC and NHS solution, carboxyl groups on the surface of MOF are activated, and by connection with amino groups in type II collagen targeting peptide (WYRGRL), composite nano-microspheres (MPMW) of which surfaces are loaded with a cartilage targeting peptide are formed. Double factors of bilirubin (Br) and rapamycin (Rap) are correspondingly loaded in pore passagesof an MOF shell layer and the mesoporous polydopamine, and finally, the RB@MPMW is obtained. The nano-microspheres have the advantages of high optical thermal response characteristic, good biocompatibility, active cartilage targeting, delay on in-vivo cartilage degeneration and the like, and can be used for the targeted therapy of osteoarthritis cartilage degeneration.
Owner:SHANGHAI FIRST PEOPLES HOSPITAL
Method for preparing medical level type II collagen
InactiveCN101301490ARich sourcesLow priceCosmetic preparationsPeptide/protein ingredientsSolid matterArticular cartilage
The invention discloses a method for making medical II collagen, which is characterized in: doing pre-treatment by using fresh articular cartilage as raw material; then using hydrochloric acid carbamidine to extract proteoglycan which comprises preparing a 4M hydrochloric acid carbamidine solution, and regulating the pH value to between 6.5 and 7.5 with alkali, then pouring the Hydrochloric acid carbamidine solution which is 8 to 15 times of the articular cartilage plasma into the cartilage plasma and blend, placing the solution on the shaking bed, shaking for one or two days, centrifuging and obtaining a solid matter, removing residue hydrochloric acid carbamidine solution by ultra-pure water washing for several times; pepsin digestion, which comprises that 2000 to 5000 portions of 0.5 to 0.6M acetic acid solution is added in the solid matter, then 0.1 to 0.3 portions of 2000 to 5000 mass percent solid matter which is pepsin is added in twice , for the first time half of the total amount is added and shaken in the shaking bed for 24 hours ,for the second time the other half is added and shaken for 24 hours, centrifuged, and the supernatant is removed, added in 0.01 to 0.02M aqueous solution of EDTA and 1 to 4 inactivate pepsin; salted out with NaCl; dialyzed with Na2 HPO4 solution, then medical-grade II Collagen is obtained.
Owner:SICHUAN UNIV
Method of separating collagen from the various animal tissues for producing collagen solution and product using the same
ActiveUS7781158B2Efficient separationImprove quality and reliabilityConnective tissue peptidesPeptide/protein ingredientsPhosphateFractionation
A method for separation the collagen from the various animal tissues is disclosed for preparing collagen solution and product using the same. The porcine tissues are processed to have proper form and size for acid-treatment. The acid-treatment is repeated with pepsin to separate type I or II collagens. The separated collagen is salt-treated for fractionation and ethanol-treated for obtaining 5˜10% of collagen from the initial tissue weight. The prepared tissues are processed for separating collagen through the collagen separating process. The separated collagen is processed for preparing product. The method for preparing product is comprised: treating a collagen solution having a predetermined concentration under a neutral condition at a low temperature, followed by overnight treatment at a temperature of 30 to 35° C.; concentrating collagen by centrifugation; and dissolving the thus-concentrated collagen in refrigerated weakly-acidic solvent or phosphate buffered saline (PBS), thereby preparing collagen having a concentration of 1 to 5 mg / mL.
Owner:CELLONTECH
Arthralgia relief composition, arthralgia relief health-care product, and application of arthralgia relief composition
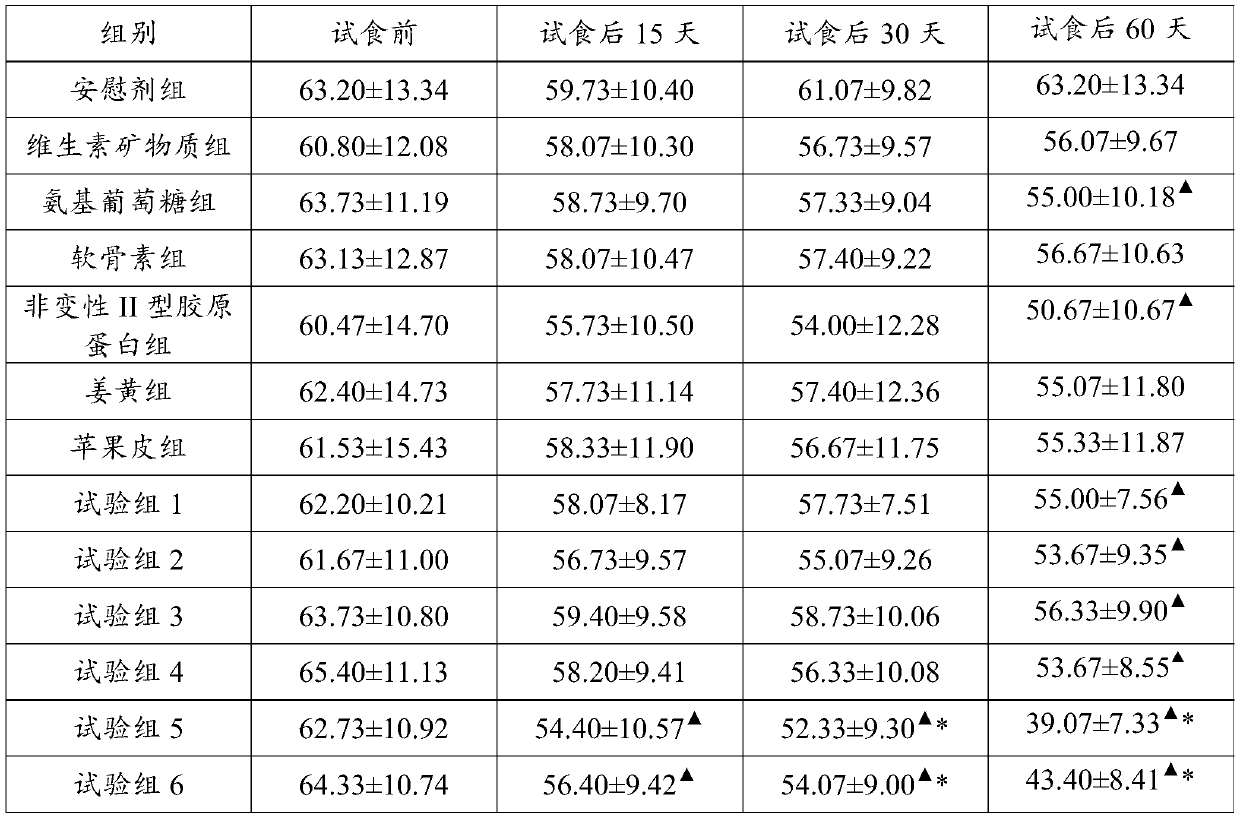
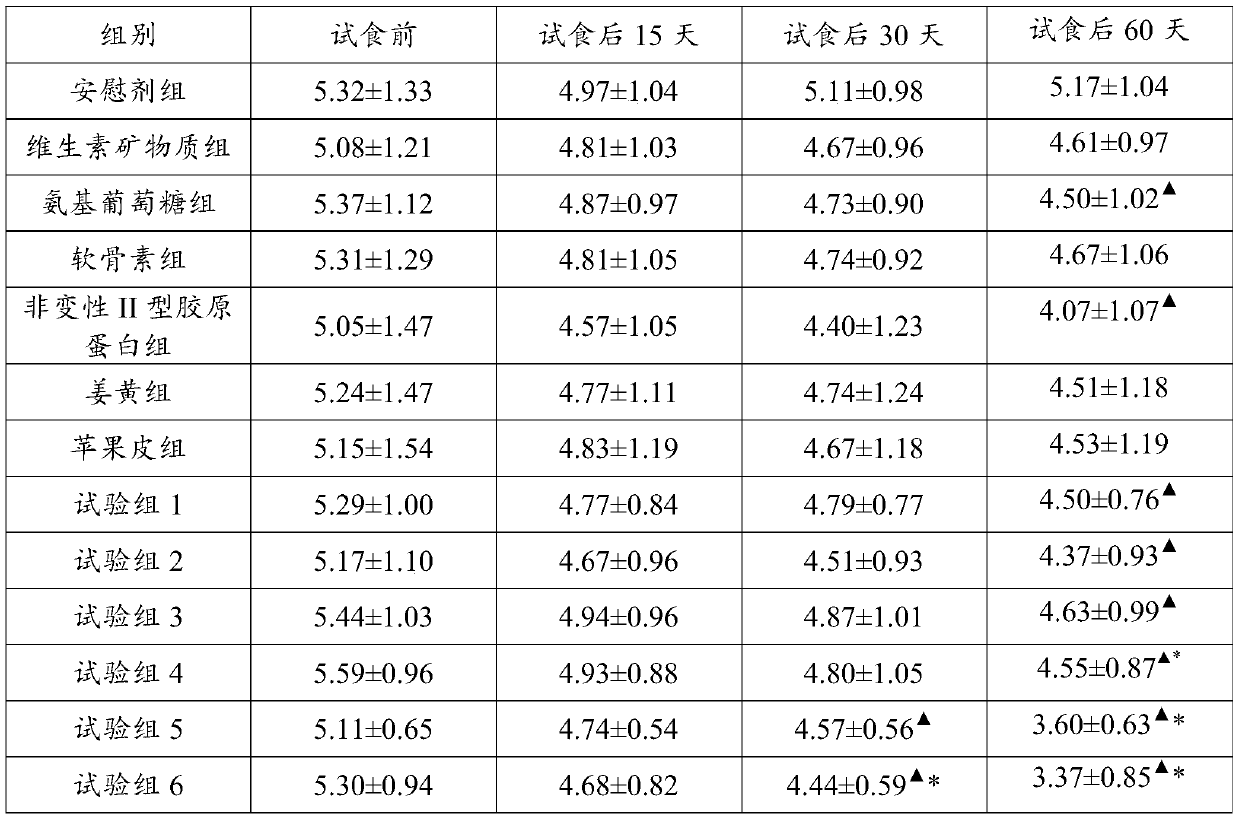
ActiveCN110051822ARelief the painPrevent degenerative changesOrganic active ingredientsHydrolysed protein ingredientsDegenerative changeVitamin K2
The invention discloses an arthralgia relief composition, an arthralgia relief health-care product, and application of the arthralgia relief composition. The arthralgia relief composition comprises D-glucosamine sulfate potassium chloride, calcium carbonate, chondroitin sulfate, type II collagen, non-denatured type II collagen, turmeric extract, vitamin K2 powder, and vitamin D3 powder. Human feeding trials prove that the components are synergistic, allowing arthralgia to be relieved shortly, promoting joint activity improvement, and preventing further degenerative changes in articular cartilages; the arthralgia relief composition has good safety and effect, and a quick, effective scheme is provided for relief of osteoarthritis or rheumatoid arthritis pains.
Owner:BY HEALTH CO LTD
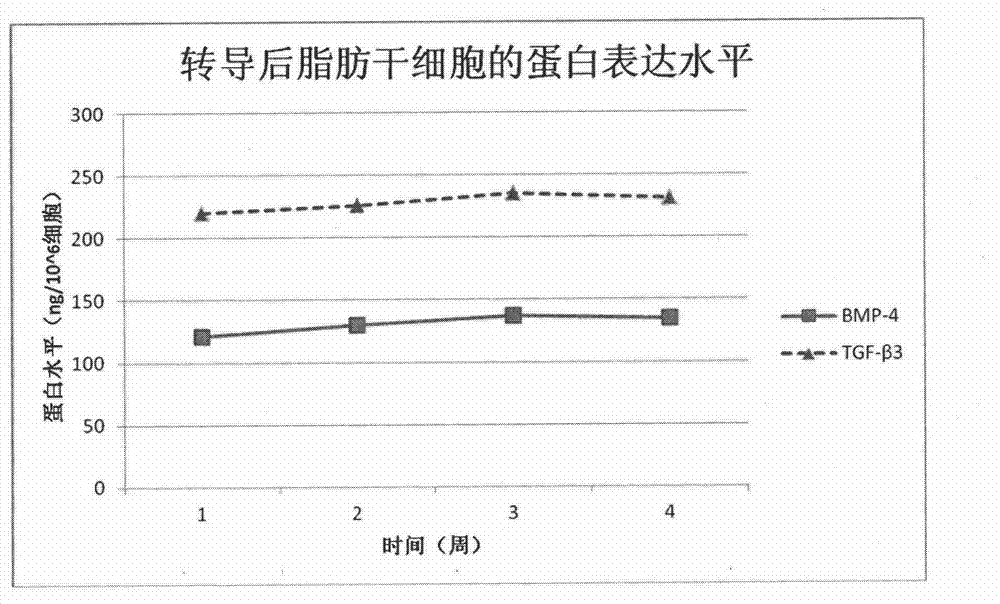
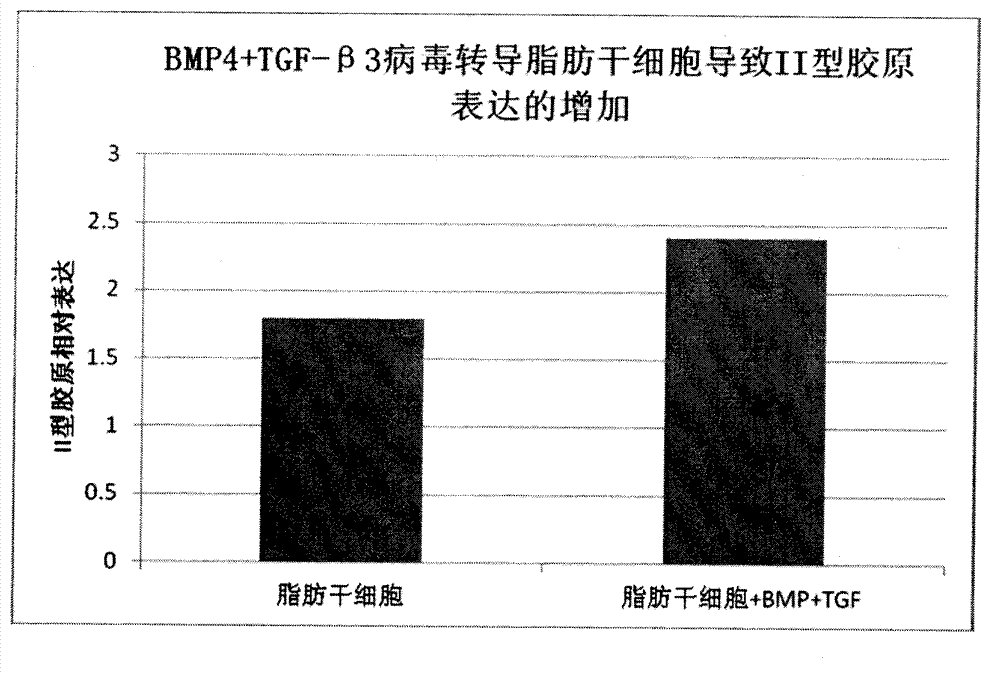
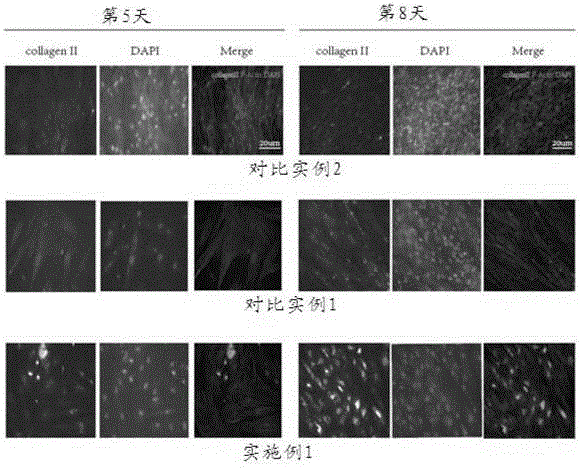
Improved adipose-derived stem cells for cartilage injury repair
InactiveCN103087992AExtend the life cycleImprove repair effectMicroorganism based processesGenetic engineeringCartilage cellsCartilage repair
The adipose-derived stem cells are derived to express bone morphogenetic protein (BMP-4) or transforming growth factor (TCF-beta3) through a gene transduction technology. The performance-enhanced stem cell has high multiplication capacity and cartilage repair capacity and is a good tool for repairing cartilage injury. Considering the functions of the BMP-4 and TCF-beta3 on stem cell regulation and nutrition, the trophic factors are fully expressed by the stem cells through a virus vector transduction method, the capacity of culturing the improved adipose-derived stem cells and cartilage cells in a cartilage induction medium for differentiating into cartilage cells is higher than that of the common adipose-derived stem cells, more type II collagens are generated, and the formed cartilage sphere is large and dense; and meanwhile, the survival cycle of the transplanted stem cells in vivo is prolonged, the repair function of the growth factors and trophic factors on the damaged part is prolonged, and cartilage growth and wound healing are promoted.
Owner:GUANGZHOU LAND BIOLOGY TECH
Preparation method of sturgeon bone II-type collagen peptide
PendingCN107385005AGood joint health functionSuitable for industrial productionConnective tissue peptidesPeptide preparation methodsChain lengthHydrolysis
The invention belongs to the technical field of preparation of functional polypeptide, and particularly relates to a preparation method of sturgeon bone II-type collagen peptide. The preparation method sequentially comprises the following steps: preparing sturgeon bone II-type collagen, preparing sturgeon bone II-type collagen peptide, desalting, concentrating, separating and purifying. The finally prepared II collagen peptide is low-molecular weight sturgeon cartilage II collagen peptide, and has the hydrolysis degree of 9-17% and peptide chain length range of 3-24 amino acids. The collagen peptide prepared by the preparation method is further rich in chondroitin and calcium, and has a good joint health-caring function; and in addition, the preparation method is suitable for industrial production.
Owner:HAILISHENG GROUP +1
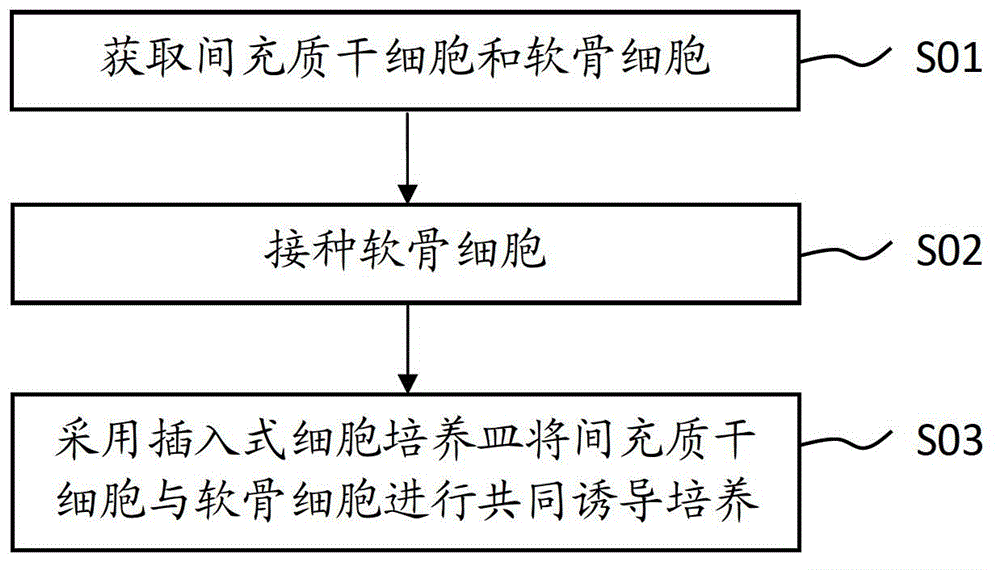
Method for inducing mesenchymal stem cells (MSCs) into chondrocytes
ActiveCN103146645AHigh proliferation rateGuaranteed puritySkeletal/connective tissue cellsCell-Extracellular MatrixECM Protein
The invention provides a method for inducing mesenchymal stem cells (MSCs) into chondrocytes. The method comprises the following steps of: obtaining MSCs and the chondrocytes; inoculating the chondrocytes into a chondrocyte culture solution to be cultured; inoculating MSCs in an inserted cell culture dish, placing the inserted cell culture dish in the chondrocyte culture solution again, and replacing the chondrocyte culture solution into a chondrocyte induced differentiation culture medium for co-culture. By adopting the induction method, MSCs and the chondrocytes can simulate an in-vivo environment to produce a synergy, so that induced MSCs are differentiated into chondrocyte factors to play a role of a synergistic effect for inducing factors with an extracellular matrix secreted by the chondrocytes, so as to obviously shorten the time for inducing MSCs to differentiate into chondrocytes, improve the proliferation rate of the chondrocytes, and simultaneously enhance the expression rate and the expression quantity of type II collagens which induce the differentiated cells and the secretion of glycosaminoglycan (GAG).
Owner:广东省医学医疗有限公司
Preparation method for I/II collagen double-layer composite collagen membrane
ActiveCN103341211AImprove repair effectConform to natural stateCoatingsProsthesisCartilage repairType II collagen
The invention relates to a preparation method for an I / II type double-layer composite collagen membrane and the I / II type double-layer composite collagen membrane prepared by using the preparation method. The preparation method comprises the following steps: 1, extracting type-I collagen and preparing a type-I collagen membrane; 2, extracting type-II collagen and preparing a type-II collagen membrane; and 3, with the type-I collagen membrane as a bottom layer and the type-II collagen membrane as a top layer, carrying out crosslinking so as to form the I / II type double-layer composite collagen membrane. The I / II type double-layer composite collagen membrane provides a good growth environment for chondrocytes and has a good cartilage repair effect.
Owner:张仲文 +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com