Method for producing recombinant human type II collagen single chain by Pichia pastoris
A technology of collagen and Pichia pastoris, applied in the field of genetic engineering, can solve problems such as low translation and expression efficiency, failure to meet purity requirements, and insufficient protein purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
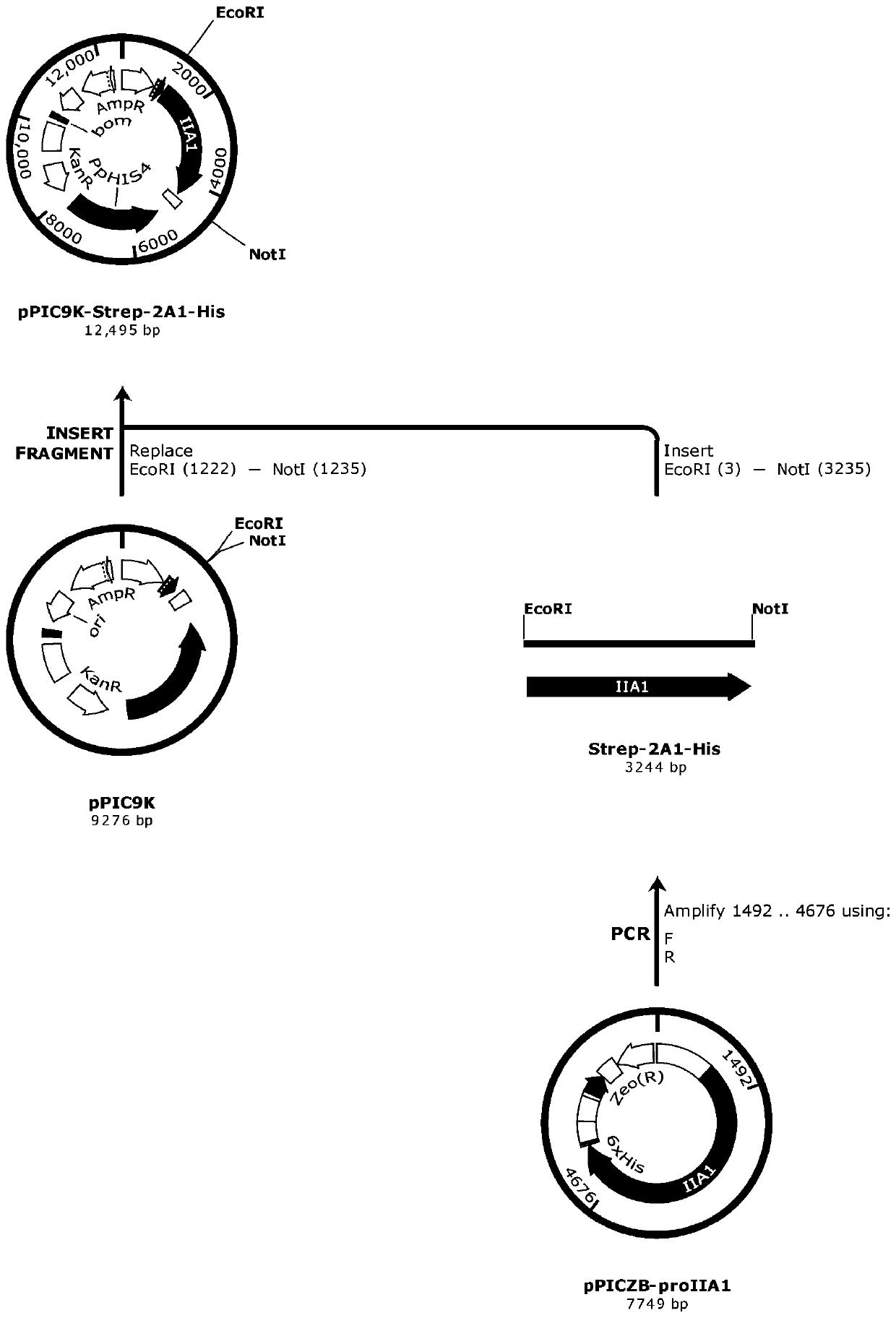
[0131] The construction of embodiment 1 recombinant expression vector
[0132] The full length of the recombinant human type Ⅱ collagen single chain to be expressed is 1078 amino acids, the N-terminal is a Srtep-tagⅡ tag, and the C-terminal is a 6×His tag. This gene recombines the amino acids of the human type Ⅱ collagen single chain The sequence is shown in SEQ ID NO.2. 1.1 Optimization and synthesis of gene sequence
[0133] Based on Genebank gene sequence NM_001844.4 and recombinant human type II collagen single-chain amino acid sequence SEQID NO.2, aiming at Pichia pastoris codon preference, GC content of DNA sequence, mRNA secondary structure, CpG island, repeat The sequence, PolyA site, RNA unstable region and other parameters were systematically calculated and optimized, and the synonymous conversion method was used to eliminate the restriction sites such as EcoRI (GAATTC) and NotI (GCGGCCGC) in the sequence. Compared with the coding sequence of the natural type II co...
Embodiment 2
[0310] Embodiment 2 Construction of recombinant Pichia pastoris engineering bacteria
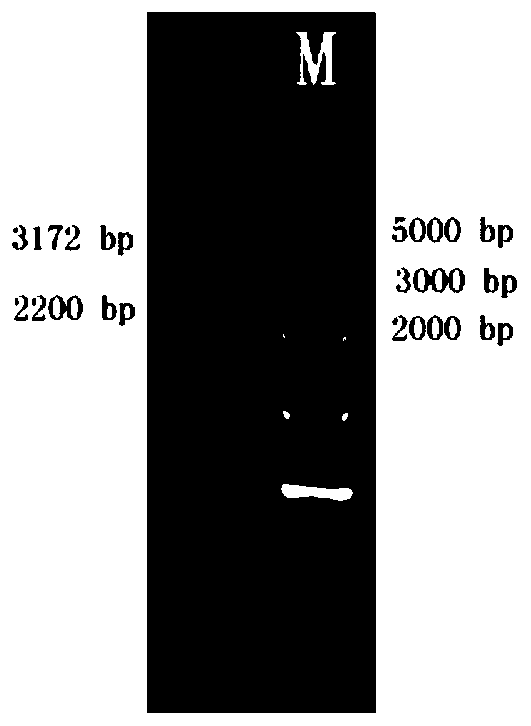
[0311] 2.1 Linearization of expression vector pPIC9K-Strep-2A1-His
[0312] Restriction endonuclease SalI was used for digestion and digestion at 37°C overnight, and the reaction system was as follows:
[0313]
[0314] Then 0.7% agarose gel electrophoresis was used to detect whether the cutting was complete. After the cutting was complete, the enzyme cutting solution was treated with a plasmid extraction kit, and the linearized plasmid was recovered, so that the volume was controlled at about 10 μL.
[0315] 2.2 Preparation of Pichia pastoris SMD1168 competent cells
[0316] 1) Pick a single colony of yeast SMD1168, inoculate it into a test tube containing 5mL of YPD liquid medium, and culture it overnight at 30°C and 220rpm with shaking;
[0317] 2) Inoculate 50 μL of the overnight culture into a 500 mL Erlenmeyer flask containing 50 mL of fresh YPD liquid medium, culture at 30°C and ...
Embodiment 3
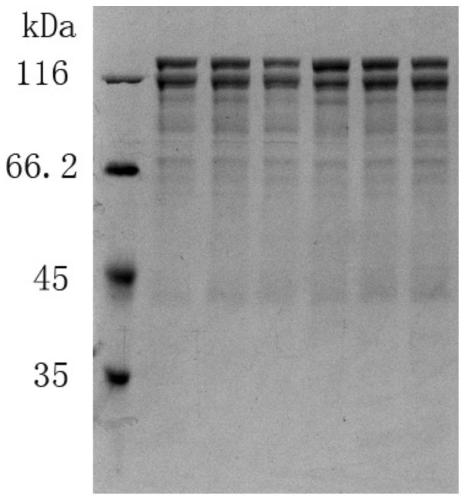
[0353] Example 3 Yeast Recombinant Collagen Expression, Purification and Preparation
[0354] Strep-Tactin affinity chromatography resin was purchased from IBA Life Science Company, and Ni-NTA His Bands affinity chromatography resin was purchased from Merck Company.
[0355] 3.1 Fermentation-induced expression of yeast engineered bacteria
[0356] 1) Use YPD culture to cultivate Pichia engineered bacteria based on overnight activation at 30°C and 220rpm;
[0357] 2) Take the above-mentioned bacterial solution and transfer it to BMGY medium, and cultivate the engineering bacteria at 30°C and 220rpm for 16-18h, until OD600=2.0-6.0;
[0358] 3) Centrifuge at 1500g for 5min at room temperature to collect the bacteria, resuspend the bacteria with 10mL of BMMY medium to make OD600=1.0, put the obtained bacteria solution in a 100mL sterile Erlenmeyer flask, and continue shaking at 28°C and 220rpm to cultivate;
[0359] 4) adding methanol to the medium every 24 hours to a final con...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com