Proline-4-hydroxylase and application of recombinant expressing host cells of proline-4-hydroxylase
A technology of proline and hydroxylase, applied in the fields of genetic engineering and microorganisms
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
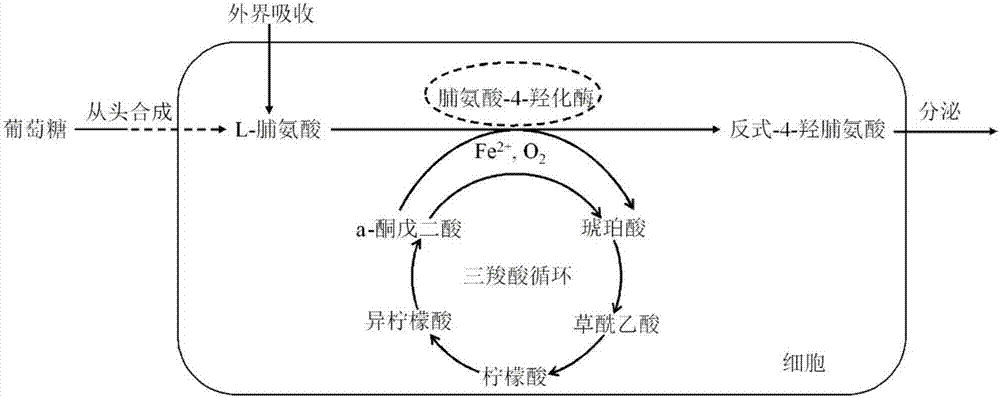
[0030] Codon optimization of proline-4-hydroxylase gene: the original sequence of proline-4-hydroxylase gene sequence is derived from GenBank: KU517670.1, and the gene codon elimination is optimized according to the codon usage frequency of Bacillus Codons with low usage rate, while using the synonymous transformation method to eliminate restriction sites such as BamH I (GGATCC), EcoR I (GAATTC) in the sequence, and remove 3 small hairpins and 2 medium hairpin structures in the sequence, The optimized proline-4-hydroxylase gene sequence is shown in SEQID No.1, and the optimized proline-4-hydroxylase gene sequence was submitted to Suzhou Hongxun Biotechnology Co., Ltd. for artificial synthesis. Considering the secondary structure of mRNA, first of all, it is necessary to ensure that the codon translation pocket consisting of several bases after the ATG start codon is open, so as to reduce the energy potential of ribosomes binding to mRNA, so that ribosomes can Smoothly translat...
Embodiment 2
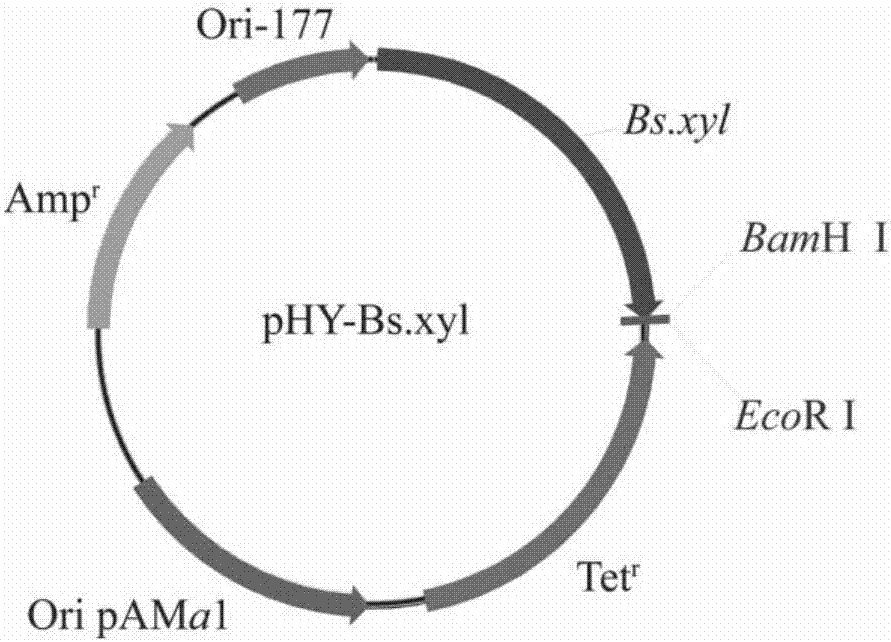
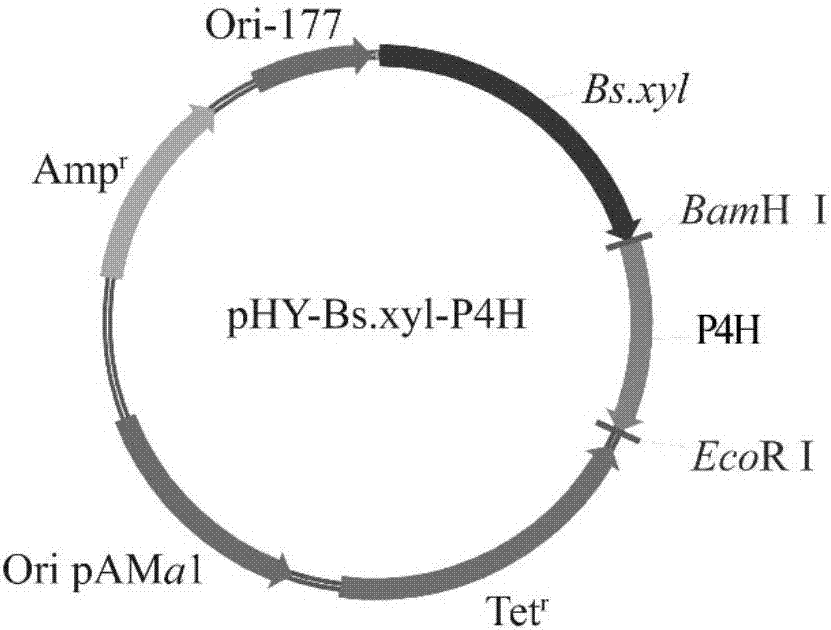
[0032] Construction of the recombinant expression plasmid: the upstream sequence of the codon-optimized proline-4-hydroxylase gene was added with a BamH I (GGATCC) restriction site, and the downstream sequence was added with an EcoR I (GAATTC) restriction site, Linked to pHY-Bs.xyl ( figure 2 Shown), construct carrier pHY-Bs.xyl-P4H, concrete steps are:
[0033] (1) 6.5 μL of proline-4-hydroxylase gene fragment, 2 μL of pHY-Bs.xyl vector, 0.5 μL of T4 ligase, 1 μL of T4 ligase buffer, made into a ligation system, ligated overnight at 16°C, and prepared ligation liquid;
[0034] (2) Add 10 μL of the connection solution prepared in step (1) to 100 μL of JM109 competent cells, then add 10 μL of 5×KCM buffer, mix well, place on ice for 30 minutes, and heat shock at 42°C After 90s, place on ice for 30min, add 500μL of LB medium, incubate at 37°C, 200rpm for 1h, spread on Amp (ampicillin) resistant plate, culture for 12h, extract plasmid for verification, and then further sequenc...
Embodiment 3
[0036] The construction of recombinant Bacillus subtilis WB600 / pHY-Bs.xyl-P4H, the specific method is:
[0037] (1) Inoculate a single colony of Bacillus subtilis WB600 in 20 mL of LB liquid medium, culture overnight at 37°C and 200 rpm;
[0038] (2) Transfer 1mL of the overnight culture to 50mL growth medium GM, and cultivate to OD at 37°C and 200rpm 600 It is about 0.8~0.9;
[0039] (3) Cells were ice-bathed for 10 minutes, and then centrifuged at 6000 rpm for 10 minutes at 4°C to collect the cells;
[0040] (4) Suspend and wash the cells with ice-bathed EP buffer for 4 times, and finally resuspend the cells in 1 mL EP Buffer to make the cell concentration reach about 1-1.3×10 10 CFU / mL, divide competent cells into 1.5mL EP tubes, 60μL per tube;
[0041](5) Add the ligation product to the competent cells, transfer it to the pre-cooled electroporation cup, and transform with 2.0kv pulse electric shock, quickly add 1mL resuscitation medium RM to the electroporation cup, res...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com