Gene modification method of eukaryotes and corresponding genetically engineered cell, and application thereof
A genetic engineering and genetic modification technology, applied in the field of enhancing the protein synthesis ability of eukaryotic cell-free protein reaction system, to achieve the effect of improving protein synthesis ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0064] In the present invention, the preparation method of the cell extract is not limited, and a preferred preparation method includes the following steps:
[0065] (i) providing cells;
[0066] (ii) washing the cells to obtain washed cells;
[0067] (iii) performing cell disruption treatment on the washed cells to obtain a crude cell extract;
[0068] (iv) performing solid-liquid separation on the crude cell extract to obtain the liquid part, which is the cell extract.
[0069] In the present invention, the solid-liquid separation method is not particularly limited, and a preferred method is centrifugation.
[0070] In the present invention, the centrifugation conditions are not particularly limited, and a preferred centrifugation condition is 5000-100000×g, preferably 8000-30000×g.
[0071] In the present invention, the centrifugation time is not particularly limited, and a preferred centrifugation time is 0.5min-2h, preferably 20min-50min.
[0072] In the present inven...
Embodiment 1
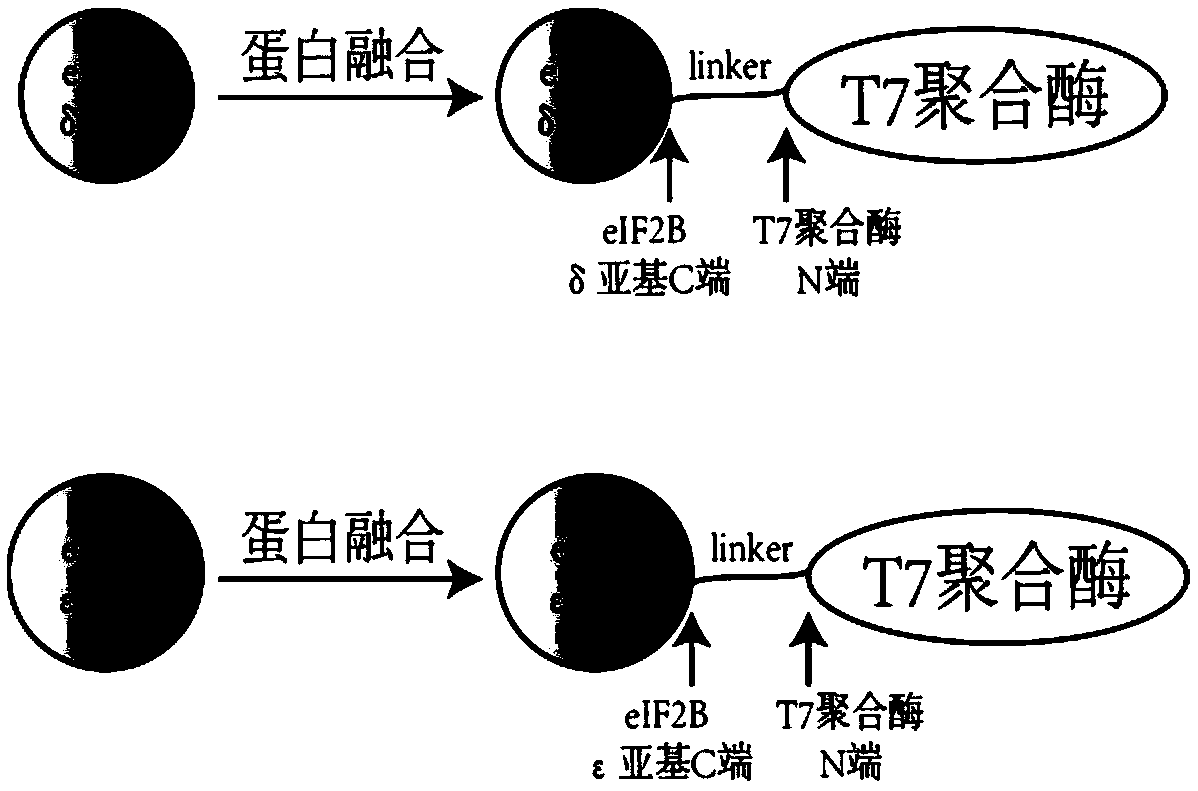
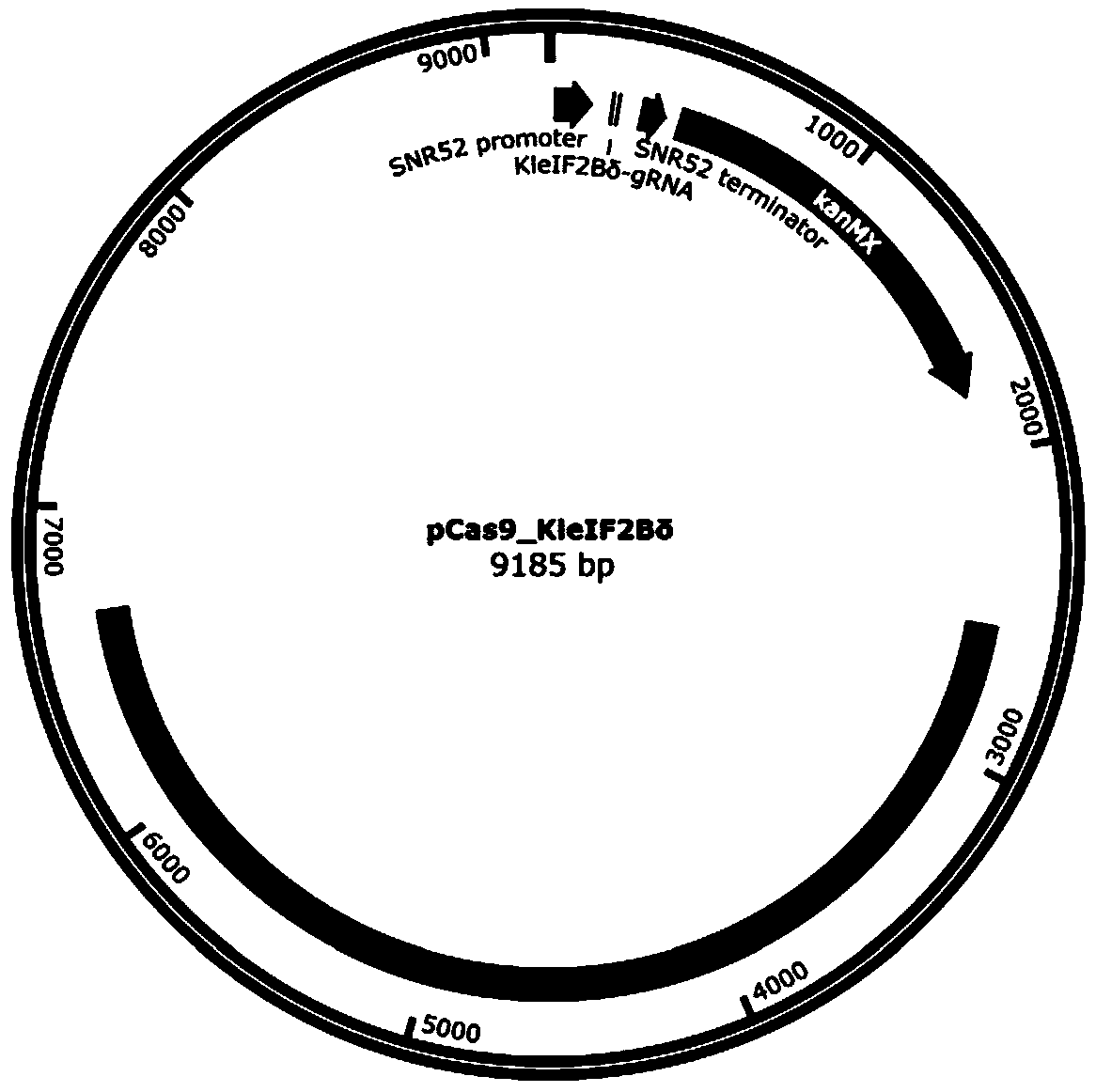
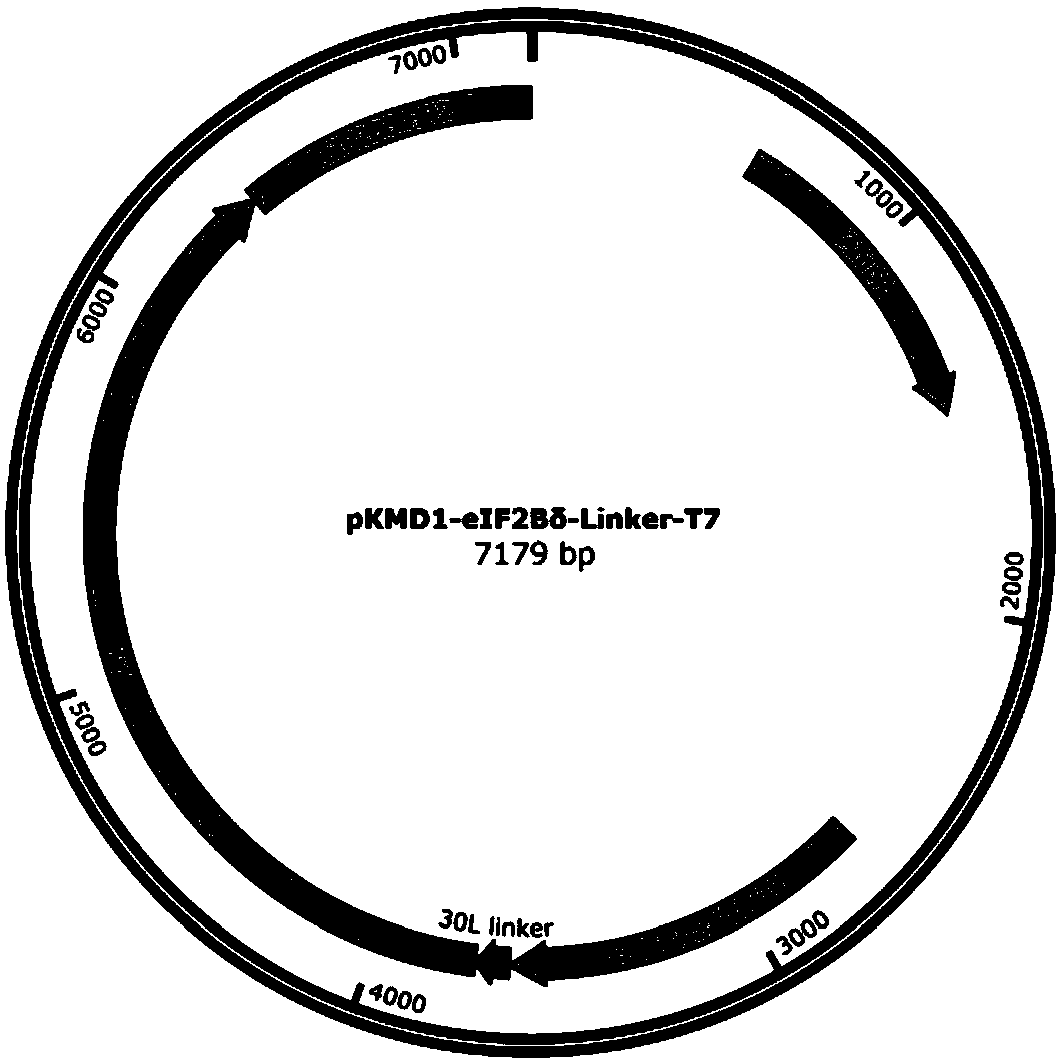
[0098] Example 1 Targeted insertion of T7 RNA polymerase at the C-terminus of eIF2Bδ gene by CRISPR / Cas9
[0099] 1.1 Kl eIF2Bδ CRISPR gRNA sequence determination
[0100] According to Kl To design the C-terminal insertion of eIF2Bδ gene (SEQ ID NO. 1) into T7 RNA polymerase, select the PAM sequence (NGG), and determine the corresponding gRNA sequence. The principle of gRNA selection in this example is: moderate GC content (40%-60%), and avoid the existence of poly T structure. In this example, Kl The eIF2Bδ gRNA sequence is CGACGAAGGTAAGAATGTCA.
[0101] Plasmid construction and transformation methods are as follows: use primer pCas9- Kl eIF2Bδ-gRNA-PF: CGACGAAGGTAAGAATGTCAGTTTTTAGAGCTAGAAATAGC and pCas9- Kl eIF2Bδ-gRNA-PR: TGACATTCTTACCTTCGTCGAAAGTCCCATTCGCCACCCG, using the pCAS plasmid as a template for PCR amplification. Take 17 µL of the amplification product, add 1 µL of Dpn I, 2 µL of 10 × digestion buffer, mix well for 37 o C water bath for 3 h. Take 10 µL of ...
Embodiment 2
[0114] Example 2 Targeted insertion of T7 RNA polymerase at the C-terminus of the eIF2Bε gene by CRISPR / Cas9
[0115] 2.1 Kl eIF2Bε CRISPR gRNA sequence determination
[0116] According to Kl To design the C-terminal insertion of eIF2Bε gene (SEQ ID NO. 3) into T7 transcriptase, select the PAM sequence (NGG), and determine the corresponding gRNA sequence. The principle of gRNA selection in this example is: moderate GC content (40%-60%), and avoid the existence of poly T structure. In this example, Kl The eIF2Bε gRNA sequence is GTATGATTTGGATATCTTAG.
[0117] Plasmid construction and transformation methods are as follows: use primer pCas9- Kl eIF2Bε-gRNA-PF: GTATGATTTGGATATCTTAGGTTTTAGAGCTAGAAATAGC and pCas9- Kl eIF2Bε-gRNA-PR: CTAAGATATCCAAATCATACAAAGTCCCATTCGCCACCCG, pCAS plasmid was used as template for PCR amplification. Take 17 µL of the amplification product, add 1 µL of Dpn I, 2 µL of 10 × digestion buffer, mix well for 37 o C water bath for 3 h. Take 10 µL of D...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com