Patents
Literature
111 results about "Human type" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A human is a member of the species Homo sapiens, which means 'wise man' in Latin. Carolus Linnaeus put humans in the mammalian order of primates. Humans are a type of hominid, and chimpanzees, gorillas, and orangutans are their closest living relatives.
Surgical and examination gloves
InactiveUS6684405B2Avoid stimulationEasy to donPeptide/protein ingredientsSynthetic resin layered productsHuman typeEnzyme inhibitor
An examination or surgical glove having an inner coating of a compound which prevents the degranulation of mast cells which releases medicators of inflammation. The coating includes anti-mast cell and anti-inflammatory agents such cromolyn compounds and human type protease inhibitors.
Owner:LEZDEY JOHN
Animation creation program
InactiveUS7106334B2Simpler and easer operationEasy to operateAnimation3D-image renderingHumanoid robot naoAnimation
The present invention provides an animation creation program whereby the posture of a structure comprising a plurality of links can be determined by means of a simpler and easer operation. In accordance with the movement of one link in the structure comprising a plurality of links, the animation creation program of the present invention automatically determines the spatial positions of the other links of the structure so that the structure retains a posture that is as natural as possible. The animation creation program of the present invention uses, for example, inverse kinematics computation that is a computation method for controlling a human-type robot (humanoid) and is based on a matrix known as a Jacobian and the inverse matrix thereof which is known as the Singularity-Robust Inverse (SR-Inverse). By using this computation method in an animation creation program, in the creation of an animation of a structure comprising a plurality of links, it is possible, when moving one link of a structure displayed on a screen, to automatically determine the positions of the other links of the structure so that the posture of the structure as a whole does not become unnatural, and to create an animation of the structure comprising a plurality of links by means of a simpler and easer operation.
Owner:SEGA CORP
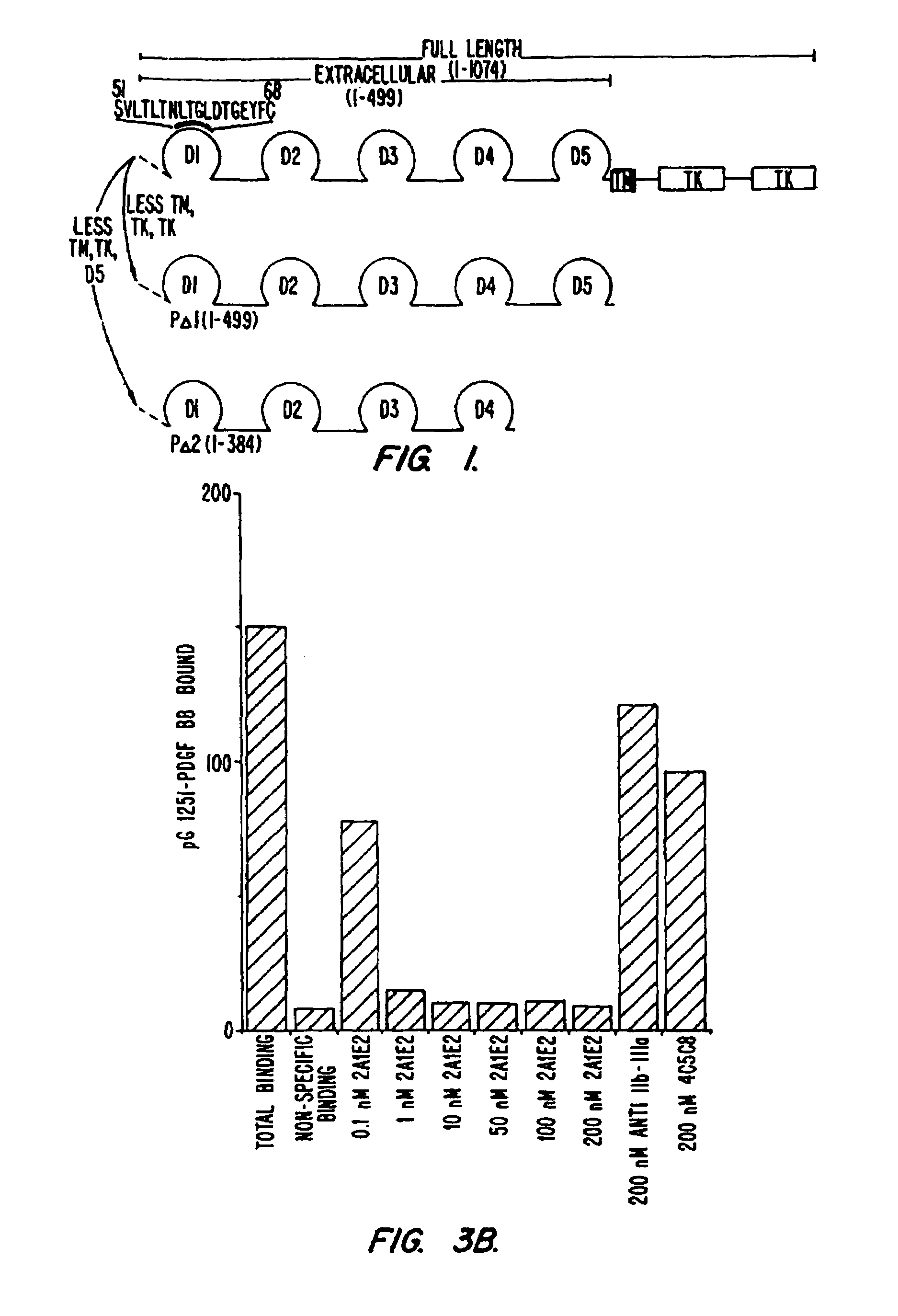
Inhibitory immunoglobulin polypeptides to human PDGF beta receptor
InactiveUS7060271B2Peptide/protein ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsHuman typeReceptor activation
The present invention is directed towards immunoglobulin polypeptides that specifically bind to the extracellular domain of the human type beta PDGF receptor. The binding of the immunoglobulin polypeptides to the receptor inhibits PDGF-induced (or stimulated) receptor activation as indicated by inhibition of receptor phosphorylation and dimerization, and by inhibition of PDGF-mediated mitogenesis, chemotaxis and migration of cells displaying the human PDGF type beta receptor on the cell surface. Nucleic acids encoding the immunoglobulin polypeptides are also included in the invention. The immunoglobulin polypeptides have diagnostic and therapeutic uses.
Owner:MILLENNIUM PHARMA INC
Therapeutic products with enhance ability to immunomodulate cell functions
InactiveUS20070135621A1Effective therapyImprove abilitiesImmunoglobulins against blood group antigensAntipyreticFc(alpha) receptorFc receptor
The present invention relates to a method for the production and the selection of human or chimaeric or humanized antibodies or molecules that comprise the Fc region of human IgG, capable of modulating the activity of one or several particular Fc receptors, such as the triggering of inhibitory functions through the human type IIB receptors of IgG (FcgammaRIIB / CD32).
Owner:INST NAT DE LA SANTE & DE LA RECHERCHE MEDICALE (INSERM) +1
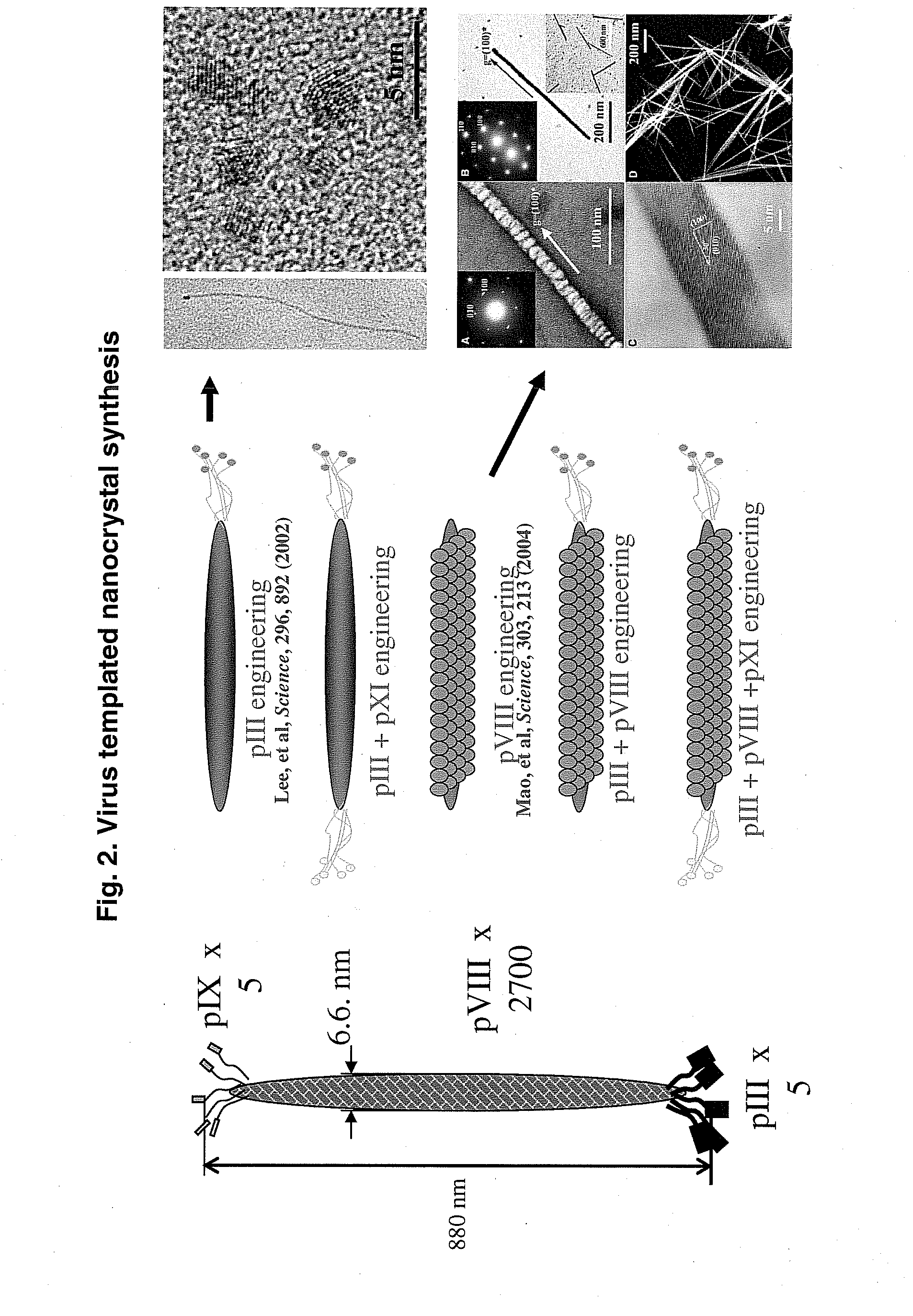
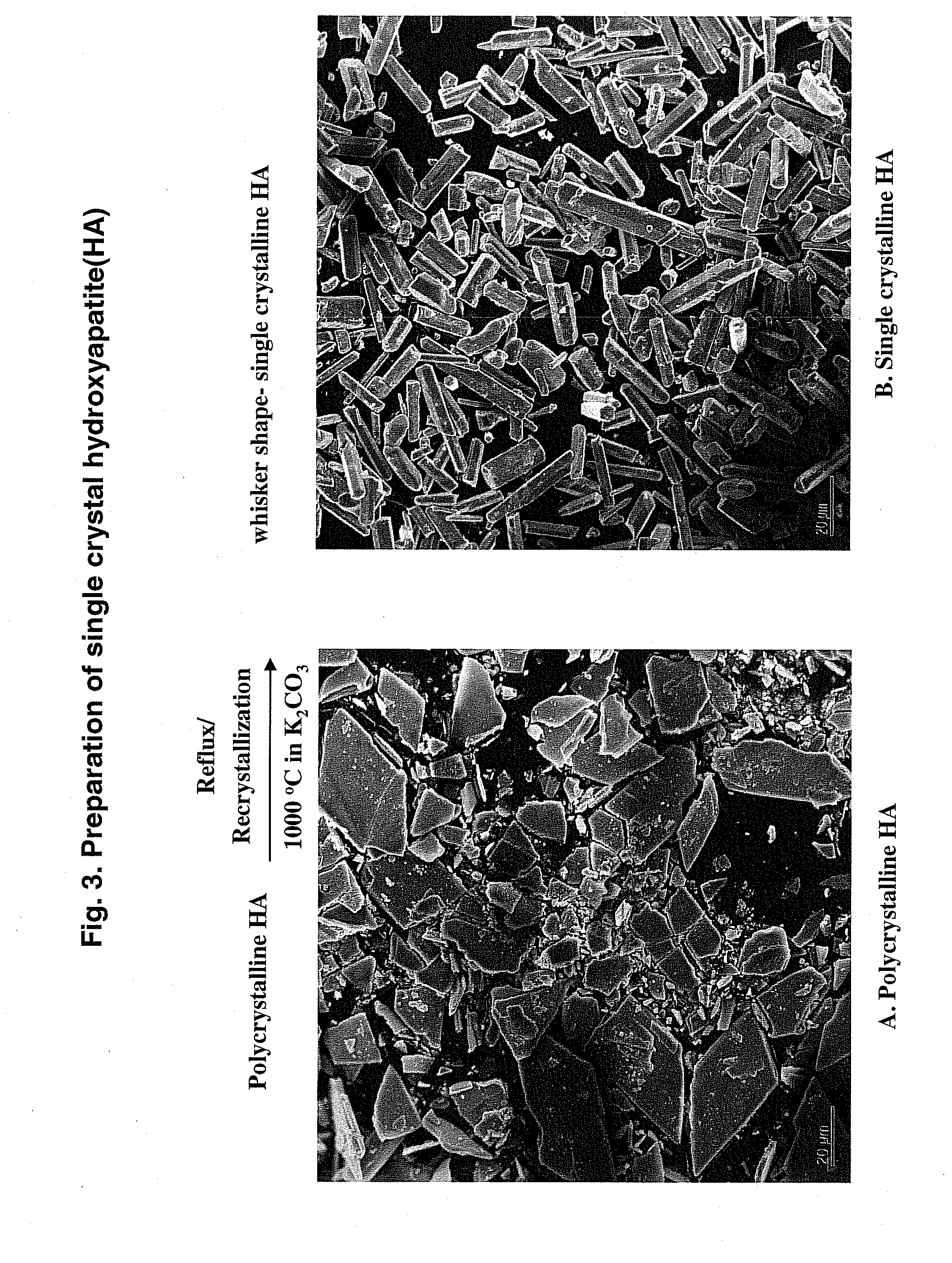
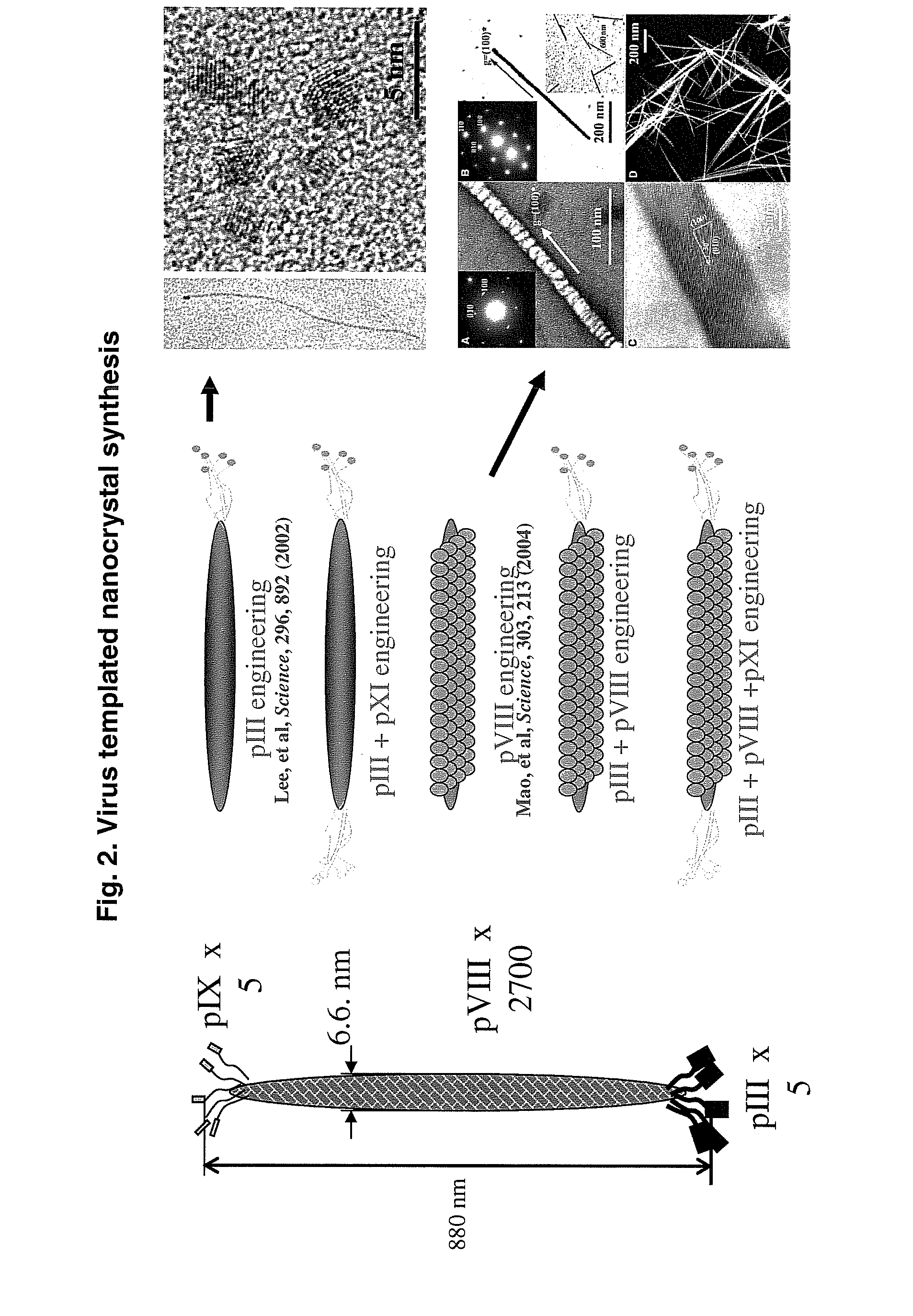
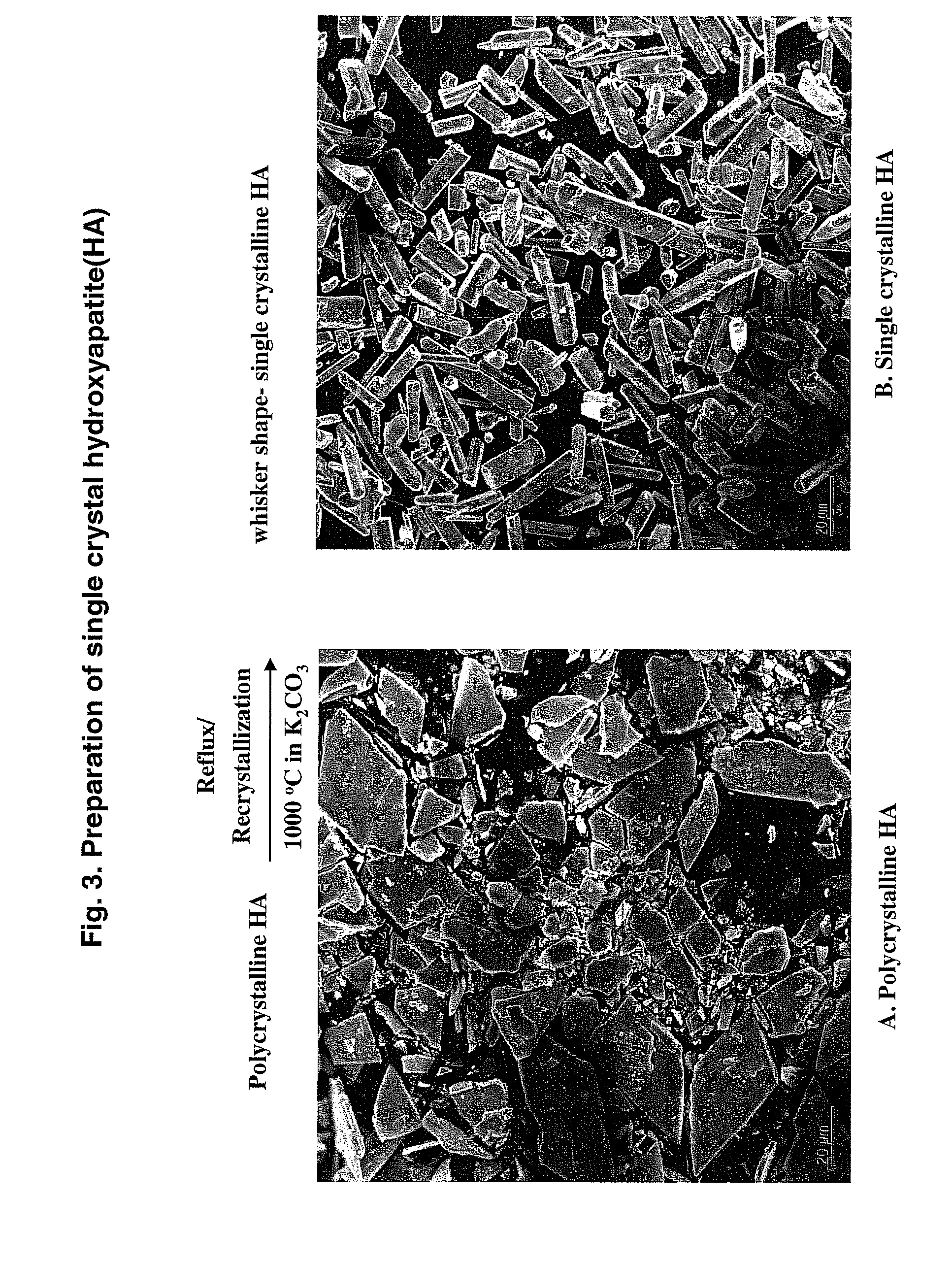
Hydroxyapatite-Binding Peptides for Bone Growth and Inhibition
ActiveUS20080279908A1Inhibiting mineral growthPhosphatesPeptide/protein ingredientsCell-Extracellular MatrixApatite
Hydroxyapatite (HA)-binding peptides are selected using combinatorial phage library display. Pseudo-repetitive consensus amino acid sequences possessing periodic hydroxyl side chains in every two or three amino acid sequences are obtained. These sequences resemble the (Gly-Pro-Hyp)x repeat of human type I collagen, a major component of extracellular matrices of natural bone. A consistent presence of basic amino acid residues is also observed. The peptides are synthesized by the solid-phase synthetic method and then used for template-driven HA-mineralization. Microscopy reveal that the peptides template the growth of polycrystalline HA crystals ˜40 nm in size.
Owner:RGT UNIV OF CALIFORNIA
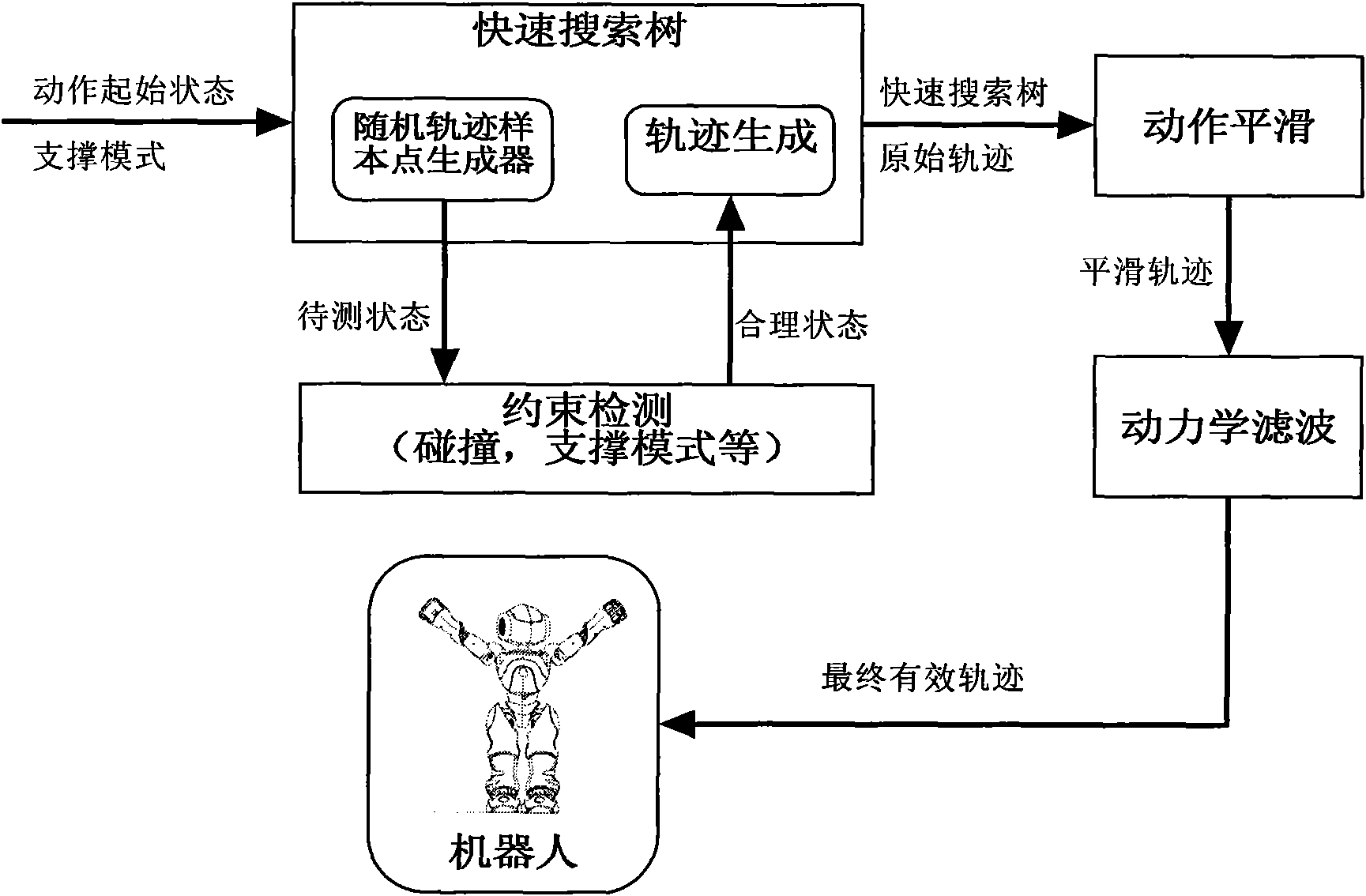
Human type robot kicking action information processing method based on rapid search tree
ActiveCN102375416AReliable executionImprove robustnessSelf-moving toy figuresProgramme control in sequence/logic controllersInformation processingGyroscope
The invention relates to a human type robot kicking action information processing method based on a rapid search tree. The method comprises the following steps: 1) according to information acquired by a joint position sensor, an acceleration sensor and a gyroscope, obtaining current state of the robot through a forward movement model under an uncertain environment, and obtaining the current robotstate through the forward movement model; 2) according to the current state and a termination state of kicking of the robot, computing each required joint movement trail of the robot through the rapid random search tree; 3) performing the smooth processing on the joint movement trail in the step 2) through a movement smooth filter, and modifying through a kinetics filter to obtain a stable final kicking movement trail. Compared with the prior art, the method provided by the invention has the advantages of guaranteeing that the robot can reliably kick the ball under the uncertain environment.
Owner:TONGJI UNIV
Insulin mimetic peptide fusion protein, mutant and applications thereof
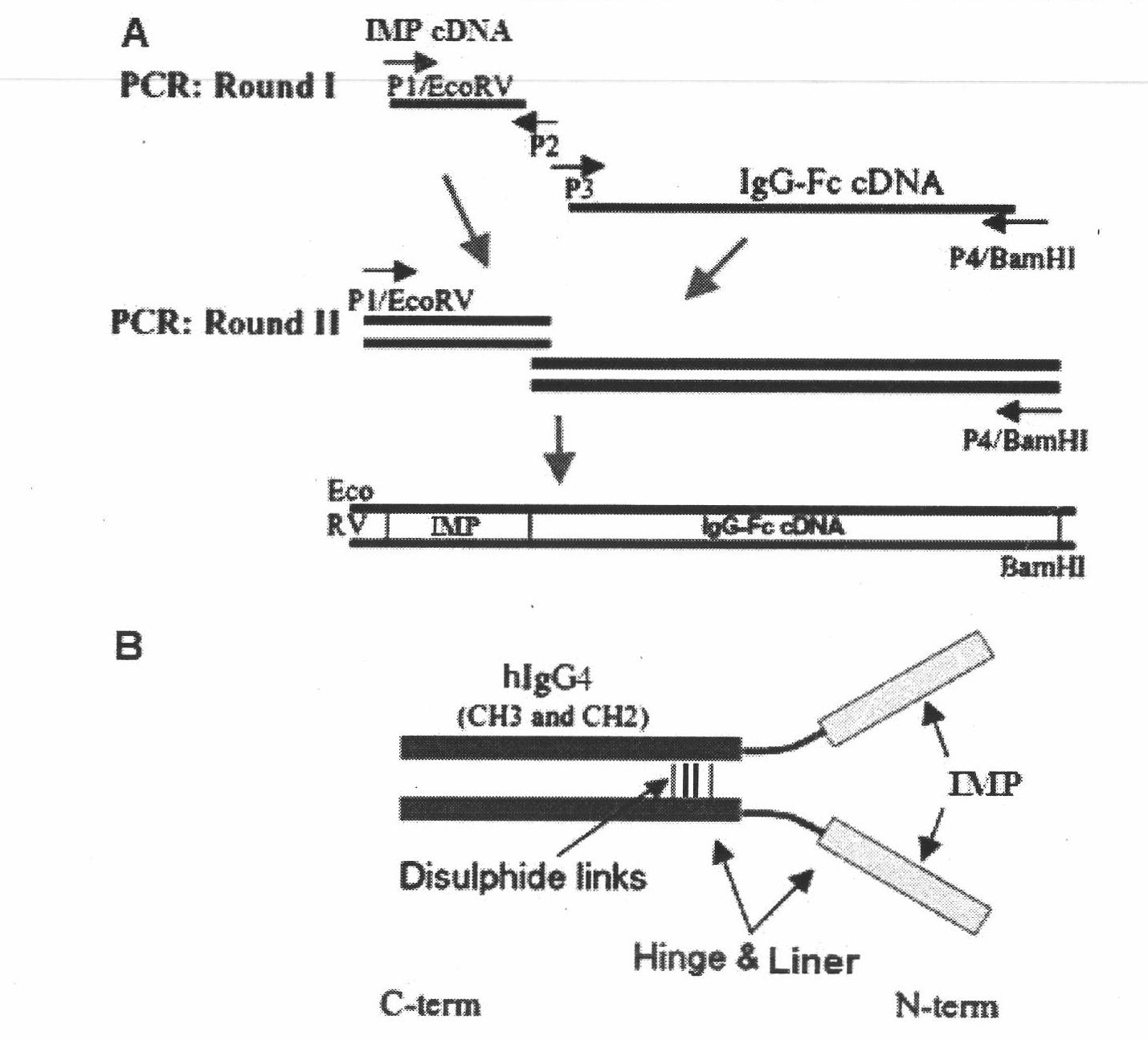
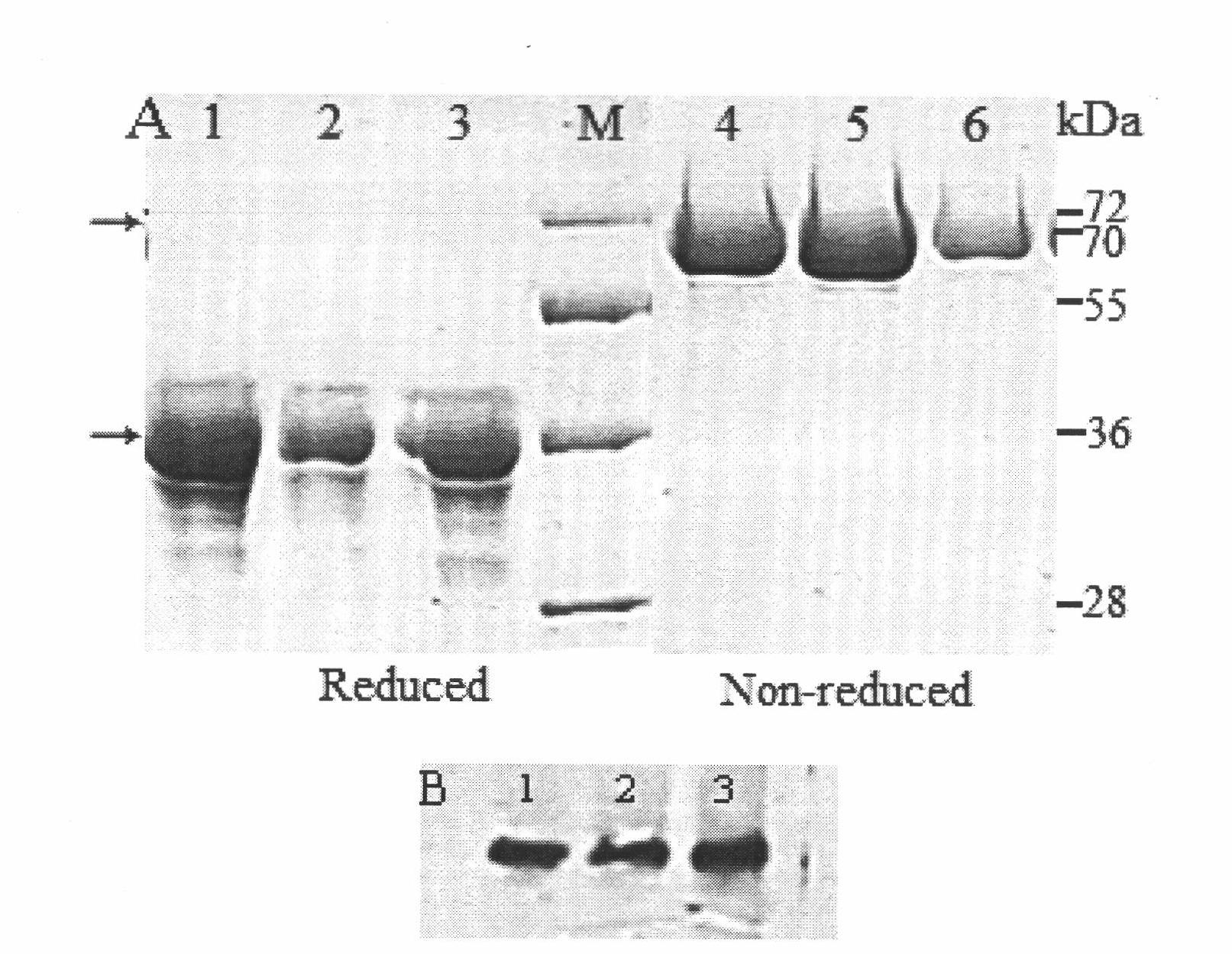
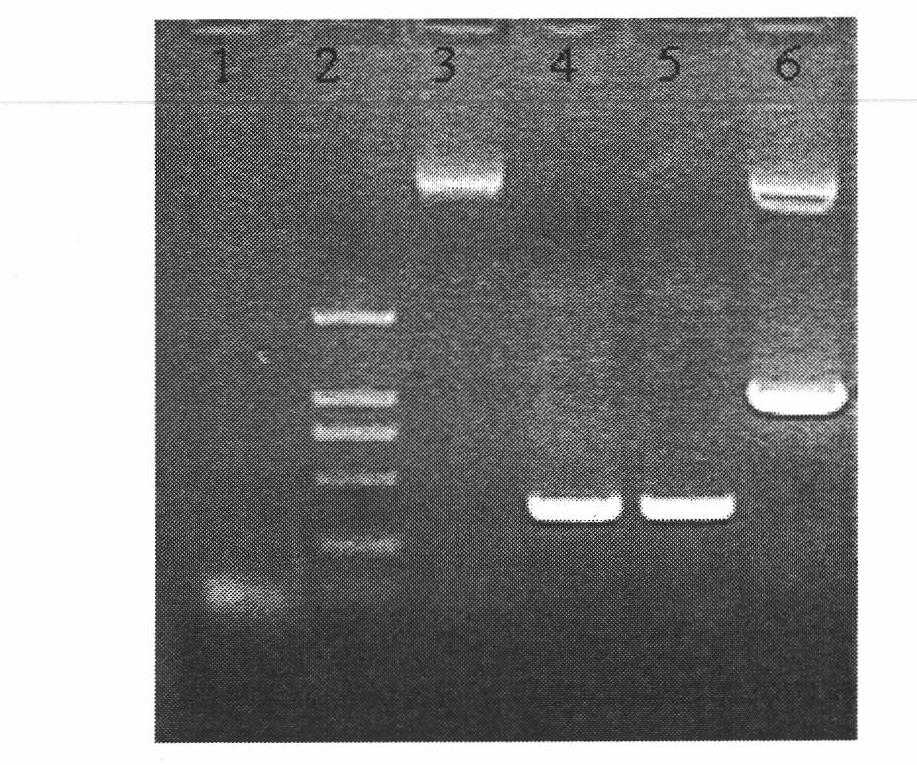
ActiveCN102516393AAvoid resistanceLow splitting powerBacteriaPeptide/protein ingredientsHalf-lifeHuman type
The invention relates to an amino acid sequence of a fusion protein for treating human type I and type II diabetes, and a production method and an application thereof. The invention relates to a noninsulin diabetes treatment method which can be used for avoiding insulin resistance. The fusion protein related to the invention is formed by fusing an insulin mimetic peptide and an IgG-Fc or IgG-Fc mutant through a connecting peptide, so that the in-vivo half-life of the insulin mimetic peptide can be prolonged remarkably while the blood sugar lowering activity of the insulin mimetic peptide is kept. The mutant of IgG-Fc can be used for further prolonging the in-vivo half-life of the fusion protein.
Owner:XUANZHU BIOPHARMACEUTICAL CO LTD +1
Method for establishing type 2 diabetes animal model and application of type 2 diabetes animal model in screening of blood sugar reducing medicaments
The invention provides a method for establishing a type 2 diabetes animal model. An animal is fed with a high-fat and high-sugar feed for a period of time, and the animal is drenched with alcohol with certain concentration at the same time. The attack process of human type 2 diabetes is simulated in the method; and the prepared animal model has typical characteristics of the type 2 diabetes such as high fasting blood glucose, high insulinemia, hyperlipidemia and impaired glucose tolerance, and is suitable for mechanism research of the type 2 diabetes and screening of a large quantity of medicaments.
Owner:NORTHEAST NORMAL UNIVERSITY
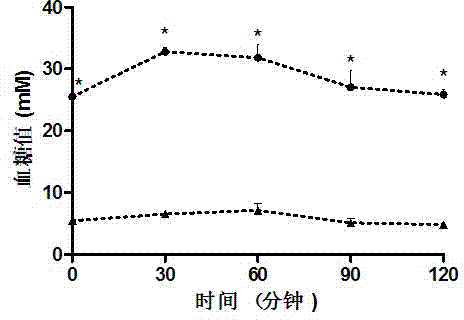
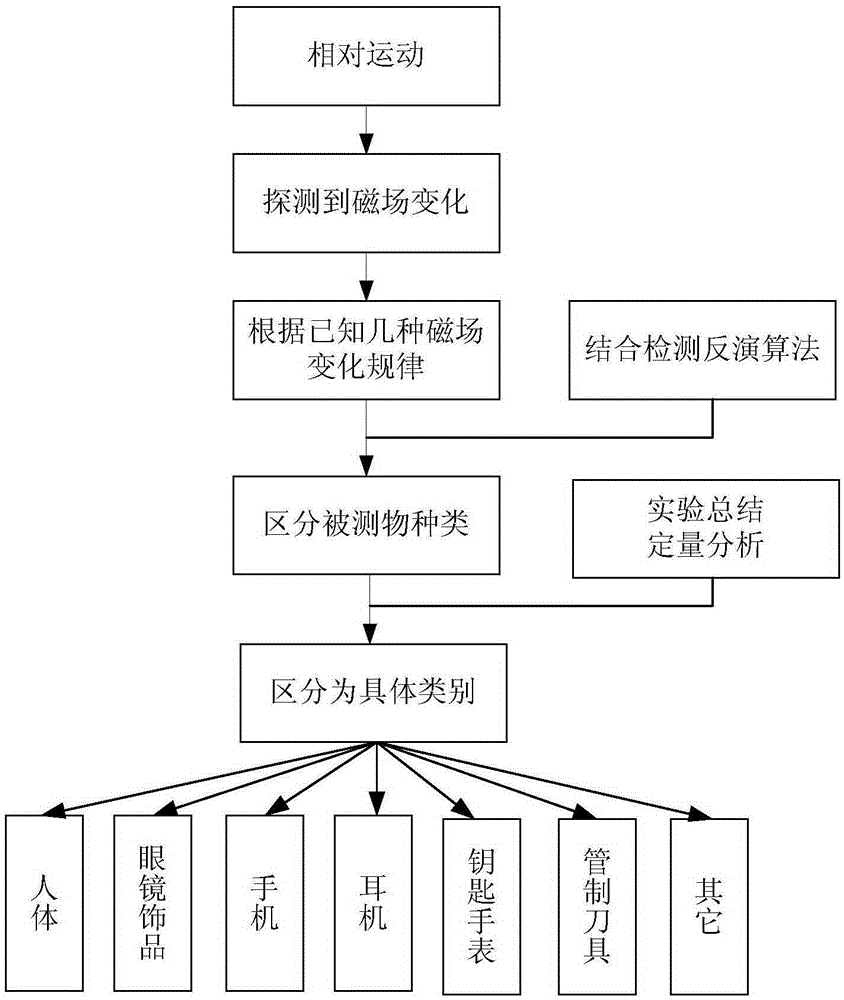
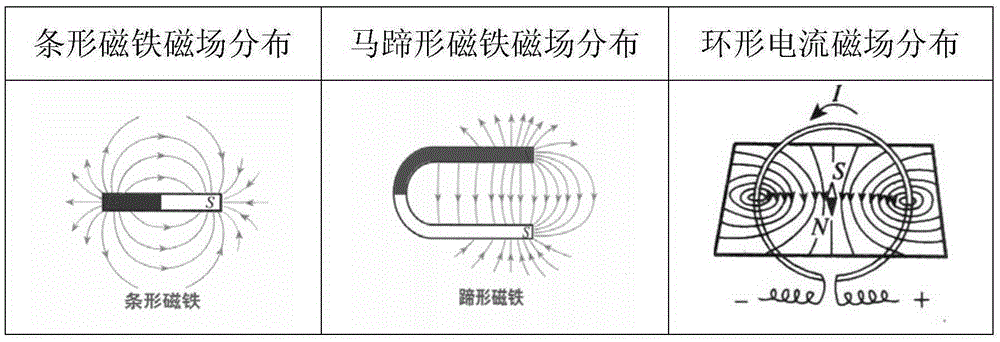
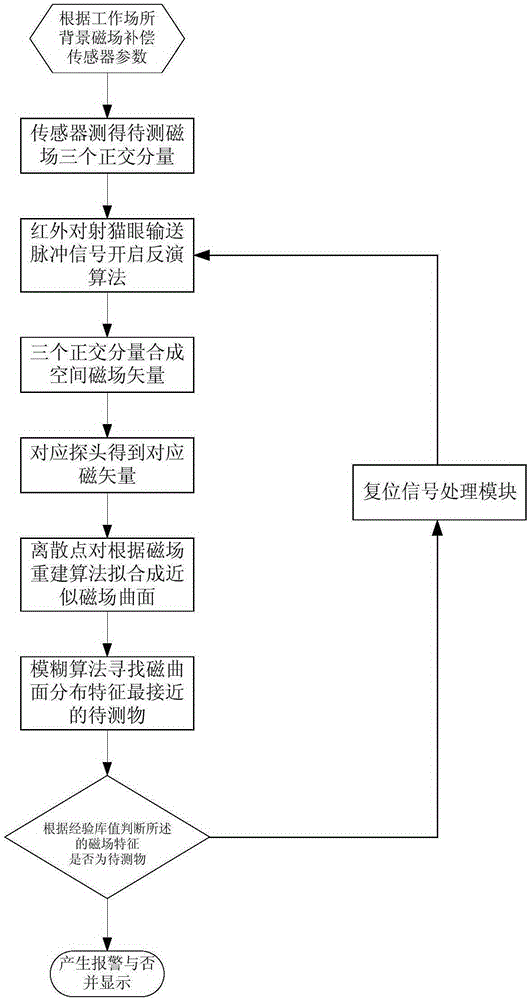
Weak magnetic detection method
ActiveCN105403922AReduce false alarm rateElectric/magnetic detectionAcoustic wave reradiationHuman typeFalse alarm
The invention belongs to the technical field of article detection and specifically relates to a weak magnetic detection method which carries out detection by taking a weak magnetic field feature of a detected object as a basis. According to the technical scheme provided by the invention, the detected object can be distinguished according to concrete types comprising a human type, an eyeglass ornament type, a communication recording equipment type such as mobile phone earphones and the like, a key watch type, a controlled knife type and other types. Products further developed based on a basic principle of the method include yet are not confined to mobile phone detection doors, radiation-free metal safety check doors, package detectors, metal lossless flow detection devices and the like. On the basis that the type of the detected object is distinguished by use of the magnetic field feature of the detected object, the purpose of relevant detection is realized, and the rate of false alarm is substantially reduced.
Owner:BEIJING HANGXING MACHINERY MFG CO LTD
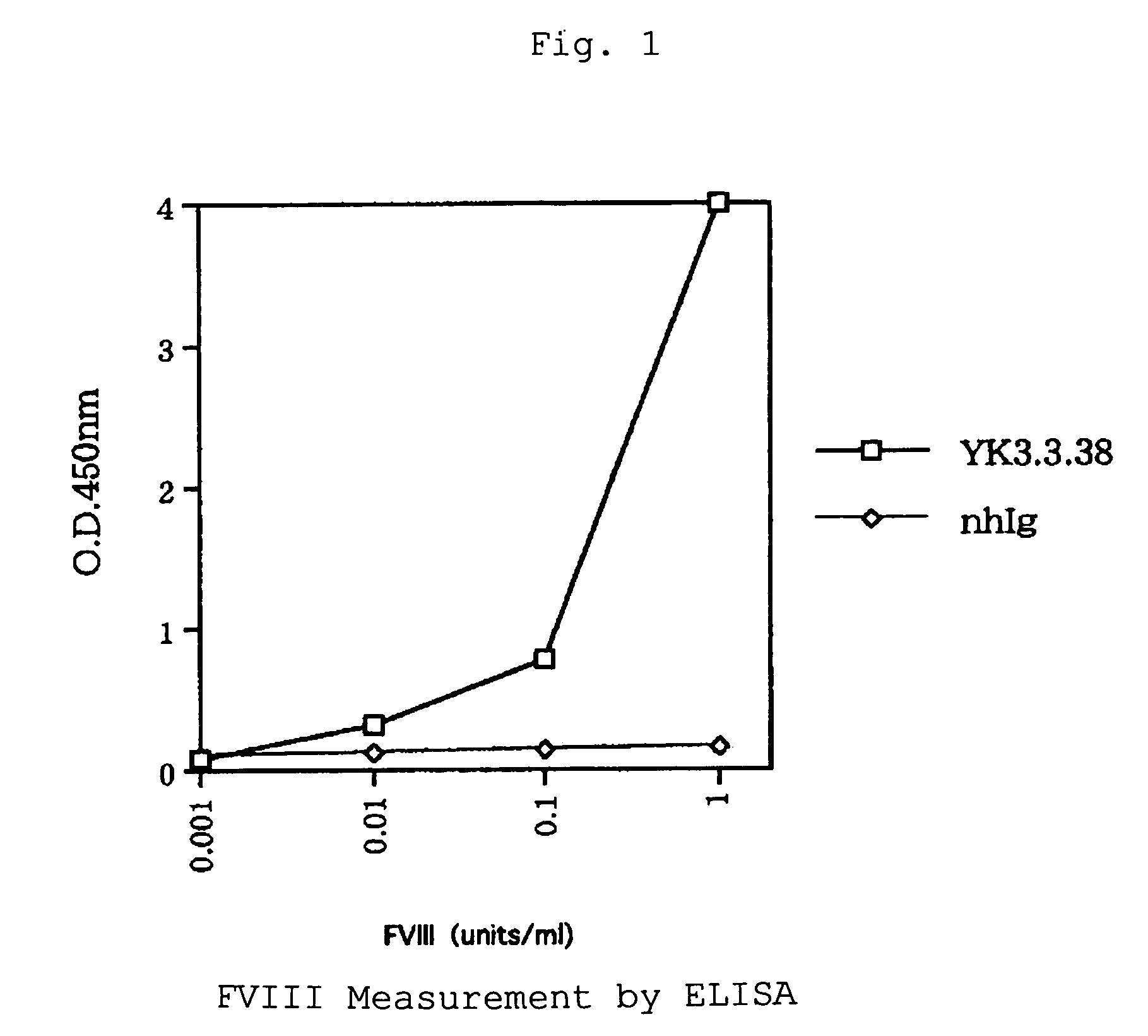
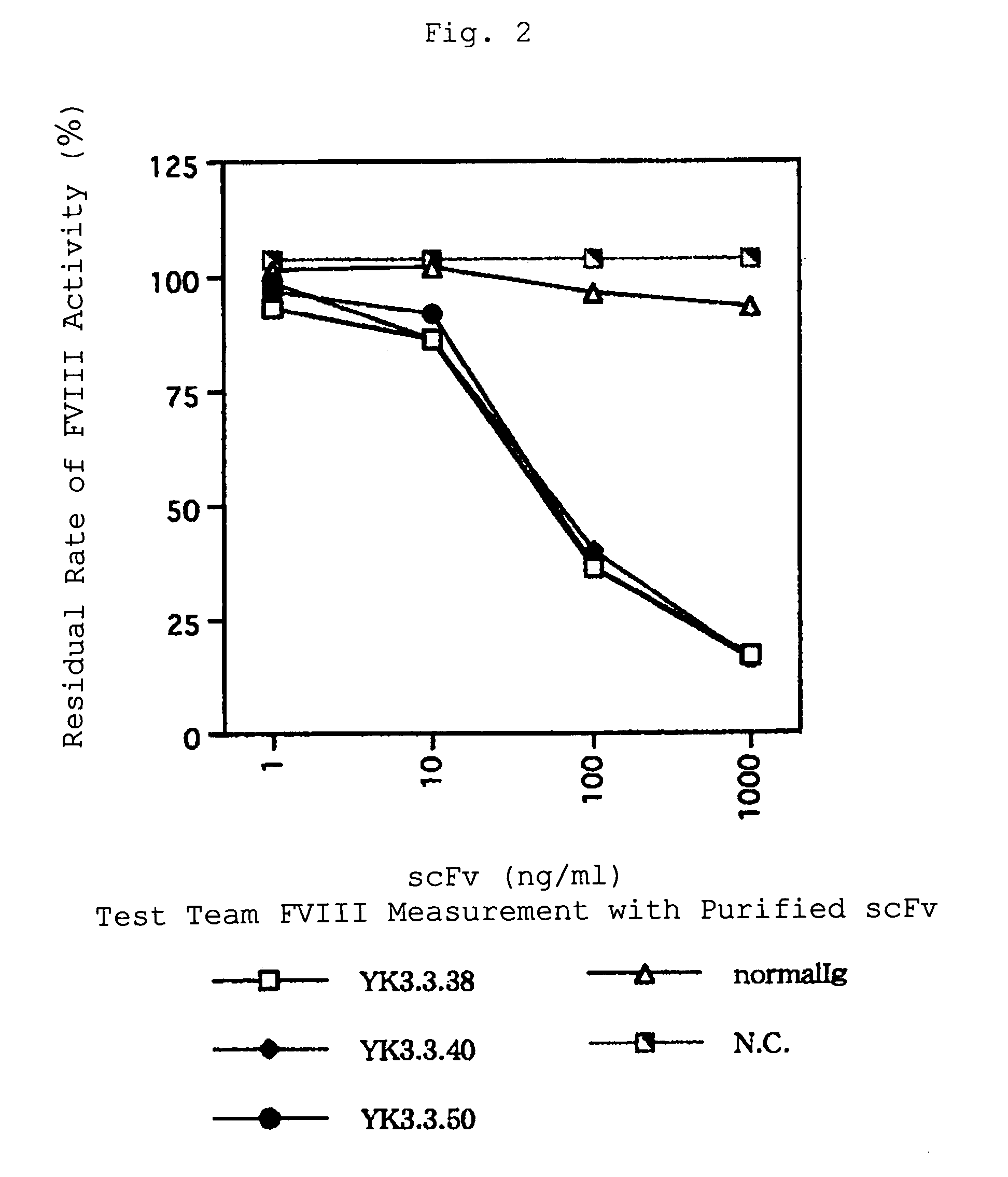
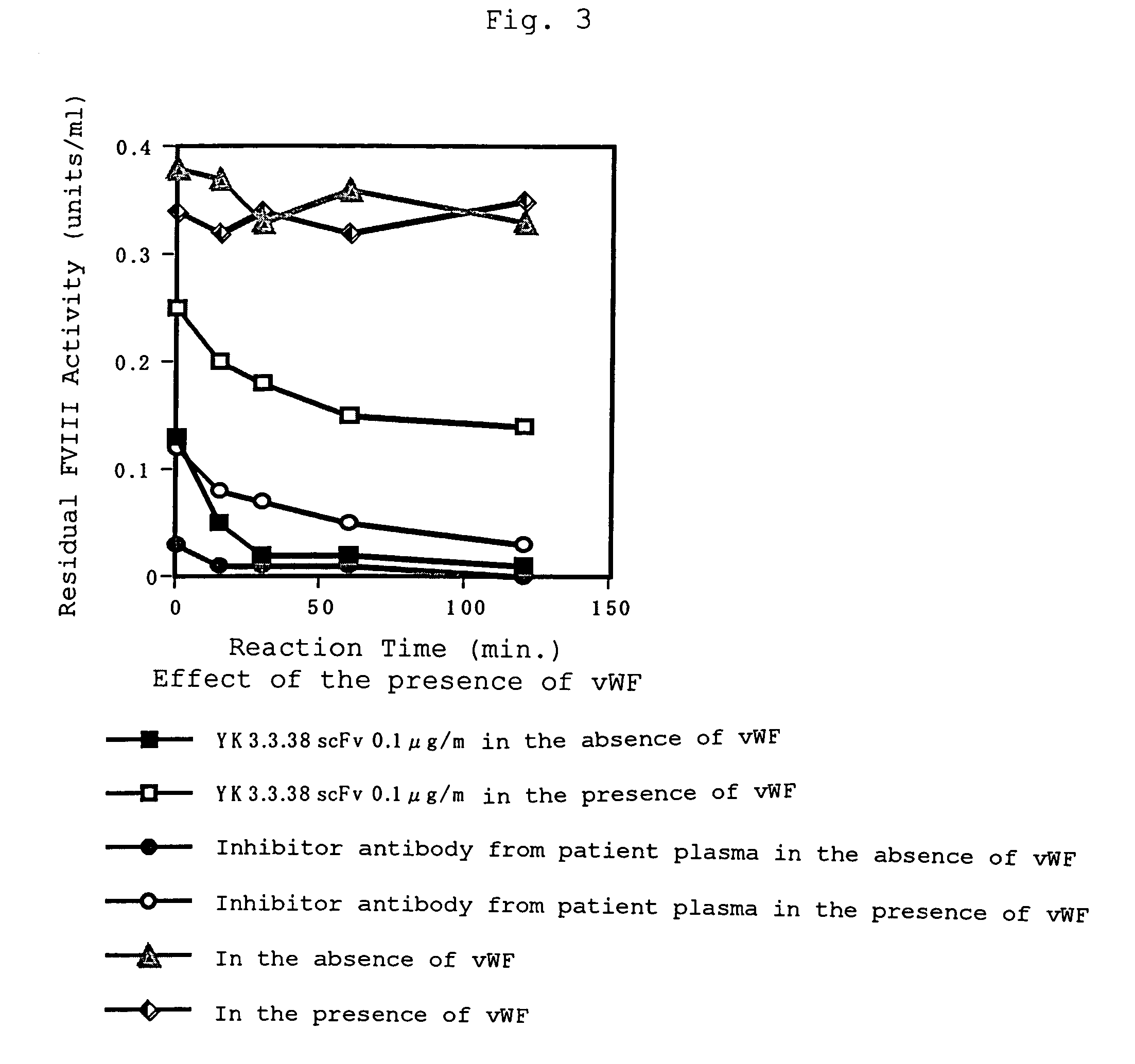
Human-type anti-blood coagulation factor VIII antibody
InactiveUS7214785B2Immunoglobulins against blood coagulation factorsFactor VIIBlood coagulation factor VIIIRandom combination
The present invention provides a human antibody against blood coagulation factor VIII (hereinafter also referred to as “FVIII”) and an antibody fragment that binds to human FVIII and specifically inhibits the coagulation activity of human FVIII. ScFv display phage libraries, prepared by using scFv DNAs constructed by random combinations of immunoglobulin VH chain genes and VL chain genes from lymphocytes from hemophilia A patients, is reacted with FVIII immobilized to a solid phase via anti-FVIII monoclonal antibody, and scFv clones capable of binding to FVIII are cloned to reveal VH and VL chains of FVIII-specific antibody.
Owner:JURIDICAL FOUND THE CHEMO SERO THERAPEUTIC RES INST
Zika virus disease vaccine taking human Ad5 replication-defective adenovirus as vector
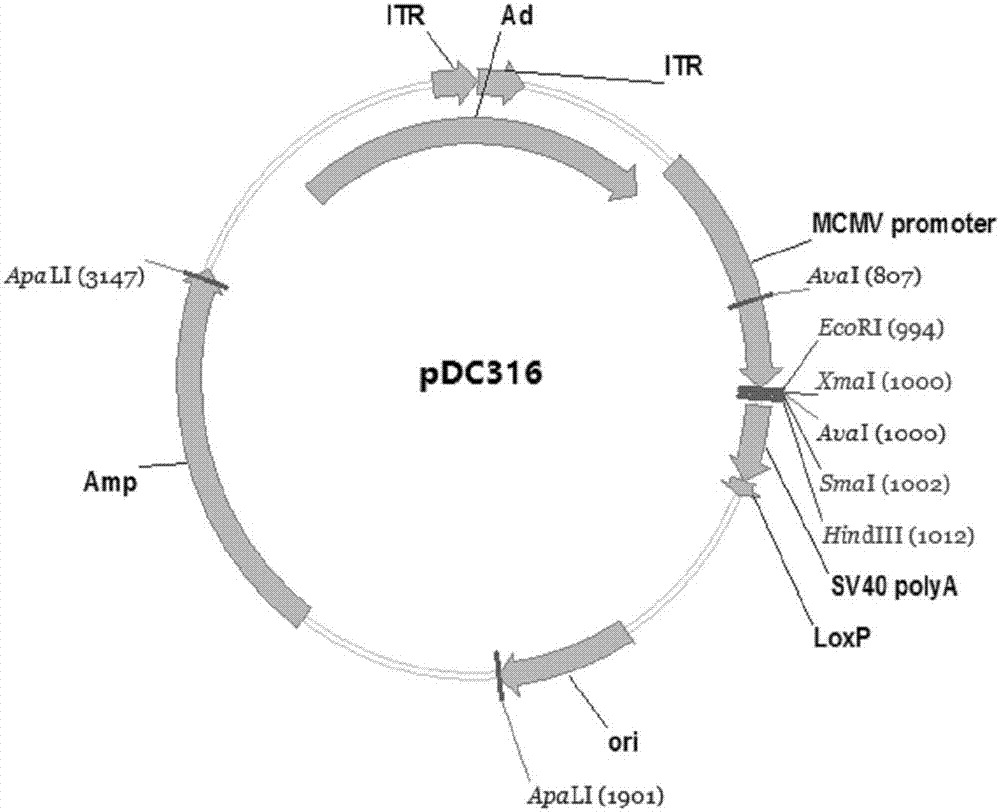
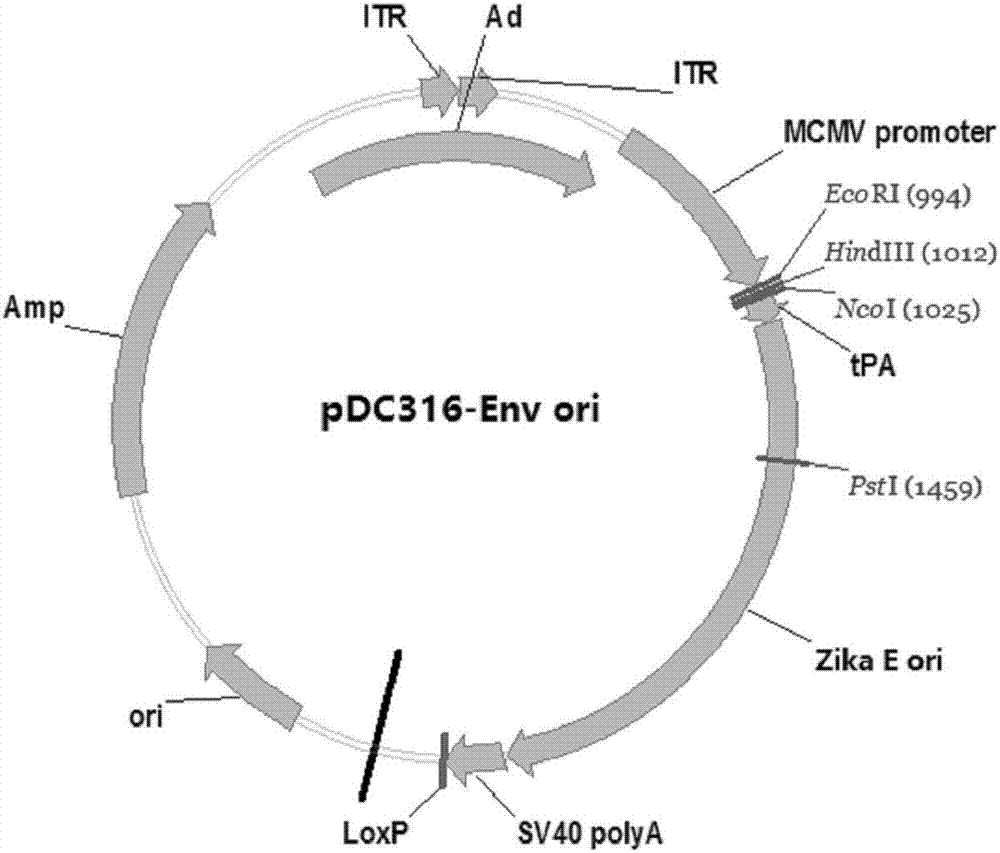
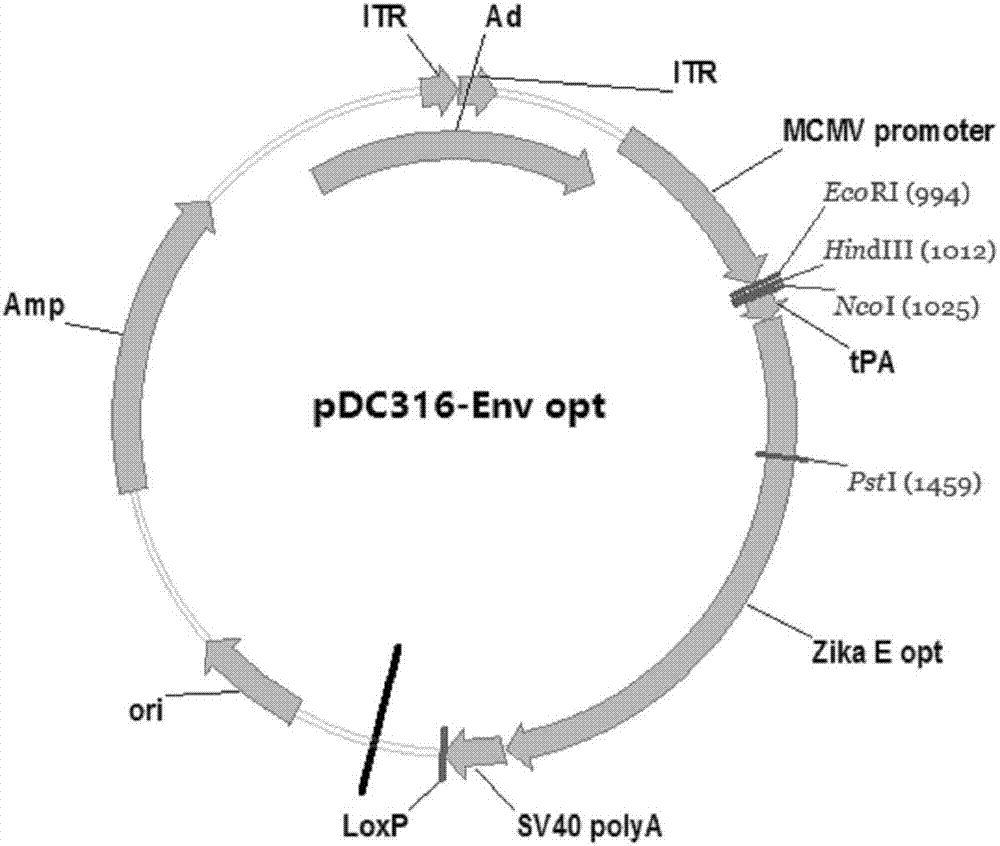
ActiveCN107190013ARapid responseRapid immune responseSsRNA viruses positive-senseViral antigen ingredientsInfected cellShuttle vector
The invention discloses a codon-optimized nucleotide sequence capable of expressing an Env protein of a zika virus. The sequence can be fused with a protein prM and a protein prM endogenous signal peptide of a codon-optimized zika virus; after the sequence is inserted into a shuttle vector pDC316, the sequence and an auxiliary vector pBHGlox_E1, 3Cre realize cotransfection of a cell HEK293, so as to package an E1 and E3 combined missing-recombinant adenovirus taking a replication-defective human type-5 adenovirus as a vector; the recombinant adenovirus vector can efficiently express an envelope protein of the zika virus in an infected cell. After the nucleotide sequence-inserted recombinant adenovirus serving as a vaccine is immune to an animal for single time, strong humoral immune and cellular immune responses can be quickly induced. A zika vaccine taking the recombinant adenovirus as the vector is suitable for large-scale and rapid preparation and can be used for emergent vaccination for a large scale of people in a zika outbreak and prophylactic immunization for people at ordinary times.
Owner:INST OF BIOENG ACAD OF MILITARY MEDICAL SCI OF THE CHINESE
Method for secretory production of glycoprotein having human-type sugar chain using plant cell
A method for the secretory production of a glycoprotein having a human-type sugar chain, comprising a step of introducing a gene of an enzyme capable of performing a transfer reaction of a galactose residue to a non-reducing terminal acetylglucosamine residue, and a gene of heterologous glycoprotein, to obtain a transformed plant cell, a step of culturing the plant cell, and a step of recovering the culture medium of the plant cell.
Owner:PHYTON HLDG
Method for producing recombinant human type II collagen single chain by Pichia pastoris
InactiveCN110029111AOptimize secondary structureEliminate useConnective tissue peptidesPeptide preparation methodsPichia pastorisNucleotide
Owner:JIANGSU TRAUTEC MEDICAL TECH CO LTD
Method For Secretory Production of Glycoprotein Having Human-Type Sugar Chain Using Plant Cell
InactiveUS20120060239A1TransferasesOther foreign material introduction processesBiotechnologyHuman type
A method for the secretory production of a glycoprotein having a human-type sugar chain, comprising a step of introducing a gene of an enzyme capable of performing a transfer reaction of a galactose residue to a non-reducing terminal acetylglucosamine residue, and a gene of heterologous glycoprotein, to obtain a transformed plant cell, a step of culturing the plant cell, and a step of recovering the culture medium of the plant cell.
Owner:PHYTON HLDG
Method for establishing SD mouse fat and diabetes research model through diet and STZ induction
InactiveCN109221011AExplain stabilityEasy to get materialsOrganic active ingredientsAnimal husbandryAnimal scienceHuman type
The invention provides a method for establishing an SD mouse fat and diabetes research model through diet and STZ induction. Through high-fat feed and STZ induction, the attacking characteristics of fat and diabetes of humans are induced, and an animal model for establishing pathogenesis of fat and type-2-diabetes caused by insulin resistance and partial defects of pancreatic beta cells is provided. Mice are divided into a normal control group, a fat group and a diabetes group, and each group has six SD male mice. The high-fat feed is adopted for feeding mice for eight weeks, a pure-fat insulin resistance model is induced, after the mice are fed with the high-fat feed for eight weeks, injection of STZ small doze in batch is conducted, and establishment of the diabetes animal model is induced. The method can better simulate the attacking characteristics of human type-2-diabetes caused by improper diet and enjoinment factors currently, and compared with other modeling, the method has better scientific significance of clinical and fundamental research combination.
Owner:任慧雯
Method and kit for simultaneously detecting human type A and type B respiratory syncytial viruses and human metapneumoviruses
ActiveCN104017901AMicrobiological testing/measurementMicroorganism based processesBiotechnologyConserved sequence
The invention belongs to the field of biotech applications and relates to a multiplex fluorescence RT-PCR (reverse transcription-polymerase chain reaction) method including internal quality control and a kit for simultaneously detecting human type A and type B respiratory syncytial viruses and human metapneumoviruses. Specific primers and probes are designed for N genes, L genes and conserved sequences of the L genes of representive strains of the human type A and type B respiratory syncytial viruses and the human metapneumoviruses, the one-step multiplex fluorescence RT-PCR quick detection method including the internal quality control is simple and quick to operate, the tediousness of a conventional single fluorescence RT-PCR single-hole single-detection method is avoided, the operation program is simplified, the test cost is reduced, and strong technical support is provided for aspects of field detection, hygienic evaluation, clinical diagnosis and the like of the viruses by virtue of relatively high specificity, sensitivity, high efficiency and stability of the method.
Owner:JIANGSU UNINOVO BIOLOGICAL TECH
Vivo assay for anti angiogenic compounds
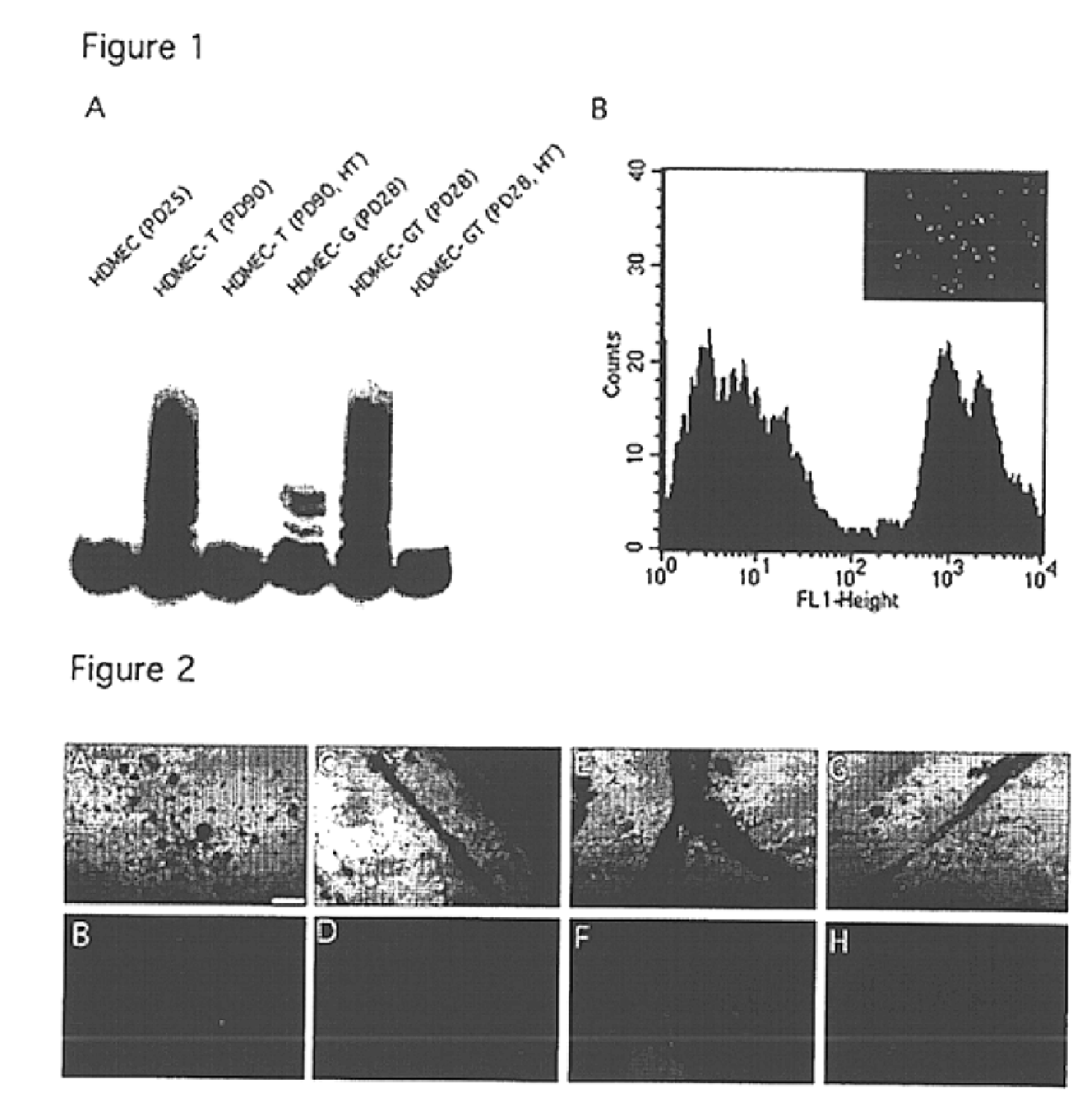
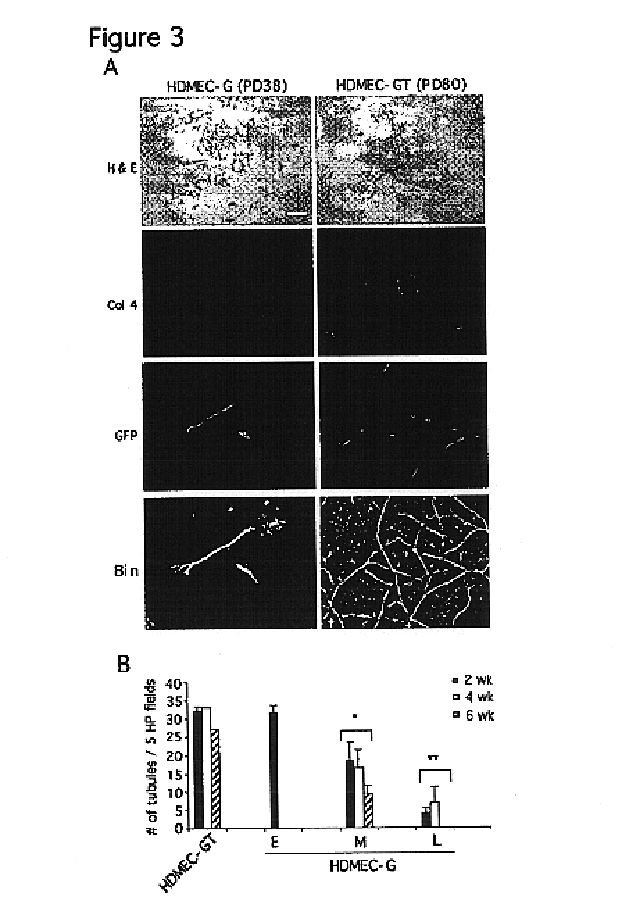
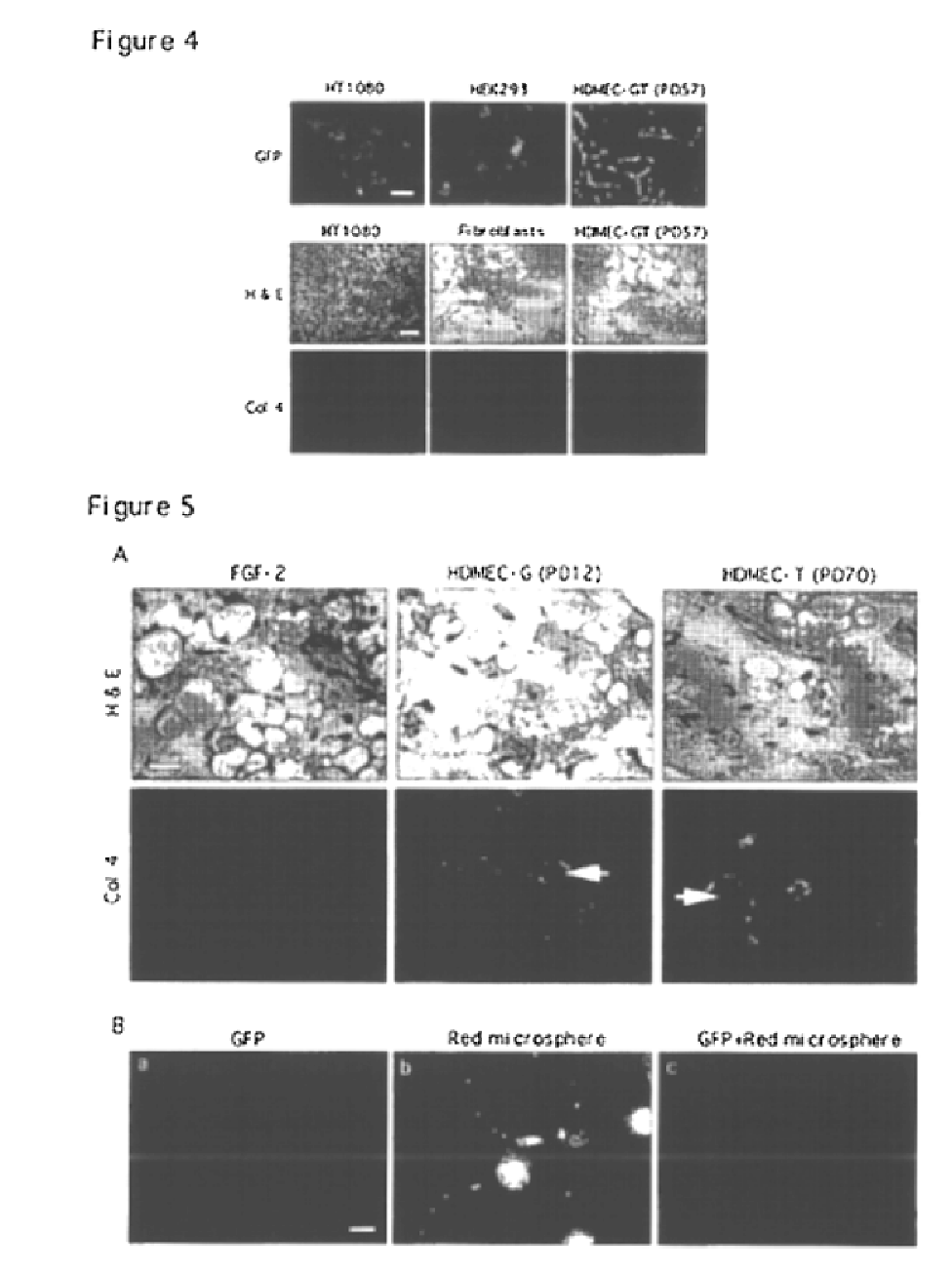
We report the use of telomerase-immortalized human microvascular endothelial cells in the formation of functional capillary blood vessels in vivo. Previously we showed the superior in vitro survival of human telomerase reverse transcriptase (hTERT)-transduced human endothelial cells. Here we show that retroviral-mediated transduction of hTERT in human dermal microvascular endothelial cells (HDMEC) results in cell lines that form microvascular structures when subcutaneously implanted in severe combined immunodeficiency (SCID) mice. The human origin of xenografted microvaculature was confirmed both by basement membrane immunoreactivity with anti-human type IV collagen staining and visualization of fluorescent vessels containing HDMEC that were co-transduced with hTERT and green fluorescent protein (eGFP). The lack of human vascular structures after implantation of HT1080 fibrosarcoma cells, 293 human embryonic kidney cells or human skin fibroblasts demonstrated the specificity of HDMEC at forming capillaries. Intravascular red fluorescent microspheres injected into the host circulation were found within green “telomerized” microvessels indicating functional murine-human vessel anastamoses. Whereas primary HDMEC-derived vessel density decreased steadily with time, telomerized HDMEC maintained durable vessels 6 weeks after xenografting. Modulation of implant vessel density by exposure to different angiogenic and angiostatic factors demonstrated the utility of this system for the study of human microvascular remodeling in vivo.
Owner:HERRON G SCOTT
Hydroxyapatite-binding peptides for bone growth and inhibition
Hydroxyapatite (HA)-binding peptides are selected using combinatorial phage library display. Pseudo-repetitive consensus amino acid sequences possessing periodic hydroxyl side chains in every two or three amino acid sequences are obtained. These sequences resemble the (Gly-Pro-Hyp)x repeat of human type I collagen, a major component of extracellular matrices of natural bone. A consistent presence of basic amino acid residues is also observed. The peptides are synthesized by the solid-phase synthetic method and then used for template-driven HA-mineralization. Microscopy reveal that the peptides template the growth of polycrystalline HA crystals ˜40 nm in size.
Owner:RGT UNIV OF CALIFORNIA
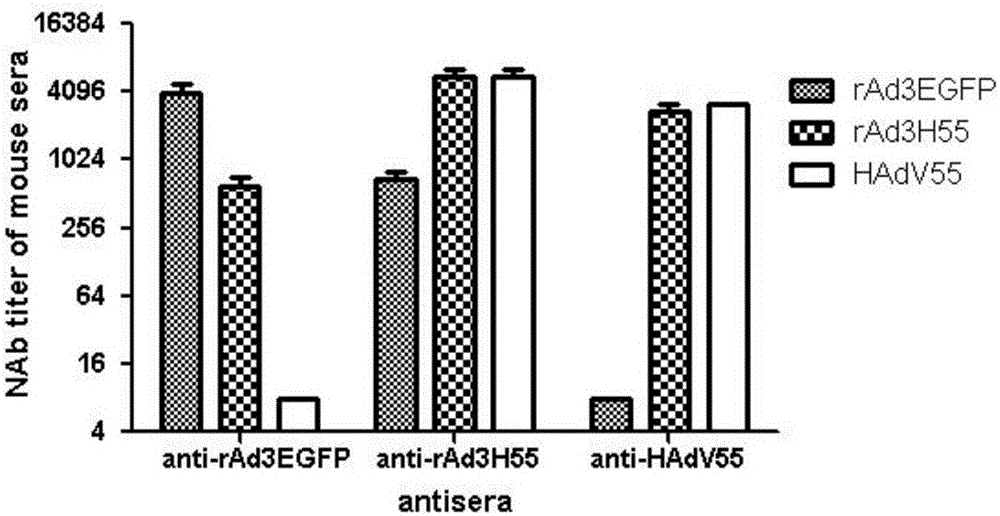
Recombinant adenovirus and tetravalent adenovirus vaccine and preparation method thereof
ActiveCN106318916ANo recombinationHigh neutralization potencyViral antigen ingredientsVirus peptidesHuman typeSerotype
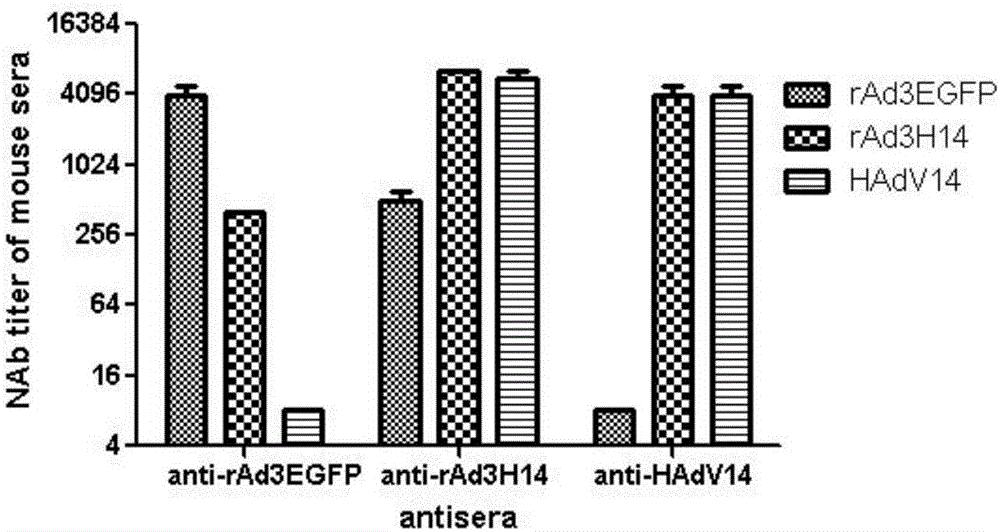
The invention discloses a recombinant adenovirus and tetravalent adenovirus vaccine and a preparation method thereof. The tetravalent recombinant adenovirus vaccine contains a recombinant type 3 adenovirus strain, a recombinant type 7 adenovirus strain, a recombinant type 14 adenovirus strain and a recombinant type 55 adenovirus strain. The preparation method disclosed by the invention comprises the following steps: preparing recombinant shuttle plasmids containing hexon gene segments, and performing in-bacteria homologous recombination with a recombinant human type 3 adenovirus strain, thereby obtaining a recombinant adenoviral genome in which the hexon gene segments are replaced by type 7, type 14 and type 55; transfecting cells, rescuing to obtain recombinant human type 3, 7, 14 and 55 recombinant adenoviruses with different main capsid protein-hexon proteins; purifying, mixing according to the same protein content, and inactivating by using beta-propiolactone, thereby obtaining the tetravalent adenovirus vaccine. The tetravalent adenovirus vaccine is capable of inducing neutralizing antibody responses to four types of serotype adenoviruses, and the neutralizing titer is 500-1000.
Owner:GUANGZHOU GIR MEDICINE CO LTD +1
Preparation method of OLETF rat fasting blood-glucose elevation model
The invention discloses a preparation method of an OLETF rat fasting blood-glucose elevation model, belonging to animal zoological medicinal models and feeds specially used for specifically animals. In the invention, an OLETF rat is feed by high-fat feed in 6-8 weeks, and the high-fat feed comprises the following raw materials in percentage by weight: 60-65 percent of standard feed, 10-12 percentof yolk powder, 10-12 percent of lard, 8-12 percent of sugar-free full-cream powder, 2-14 percent of cane sugar and 1-2 percent of water. The OLETF rat fed by the feed can simulate the morbidity process of human type II diabetes more and is good for the research of the type II diabetes; and the invention also shortens the time the OLETF rat develops into the diabetes, accelerates the molding, saves the experimental period and reduces the experimental cost.
Owner:天津市公安医院
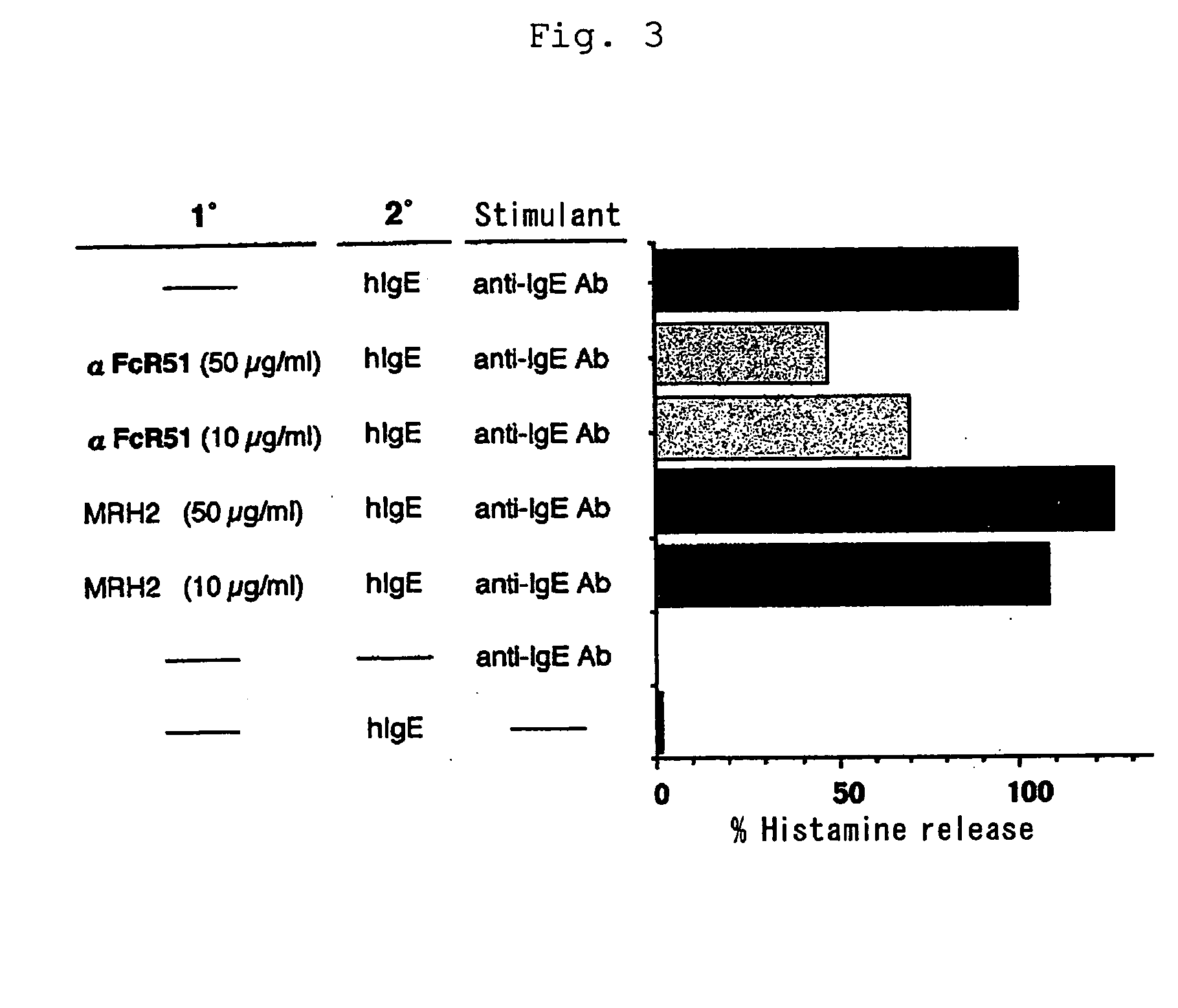
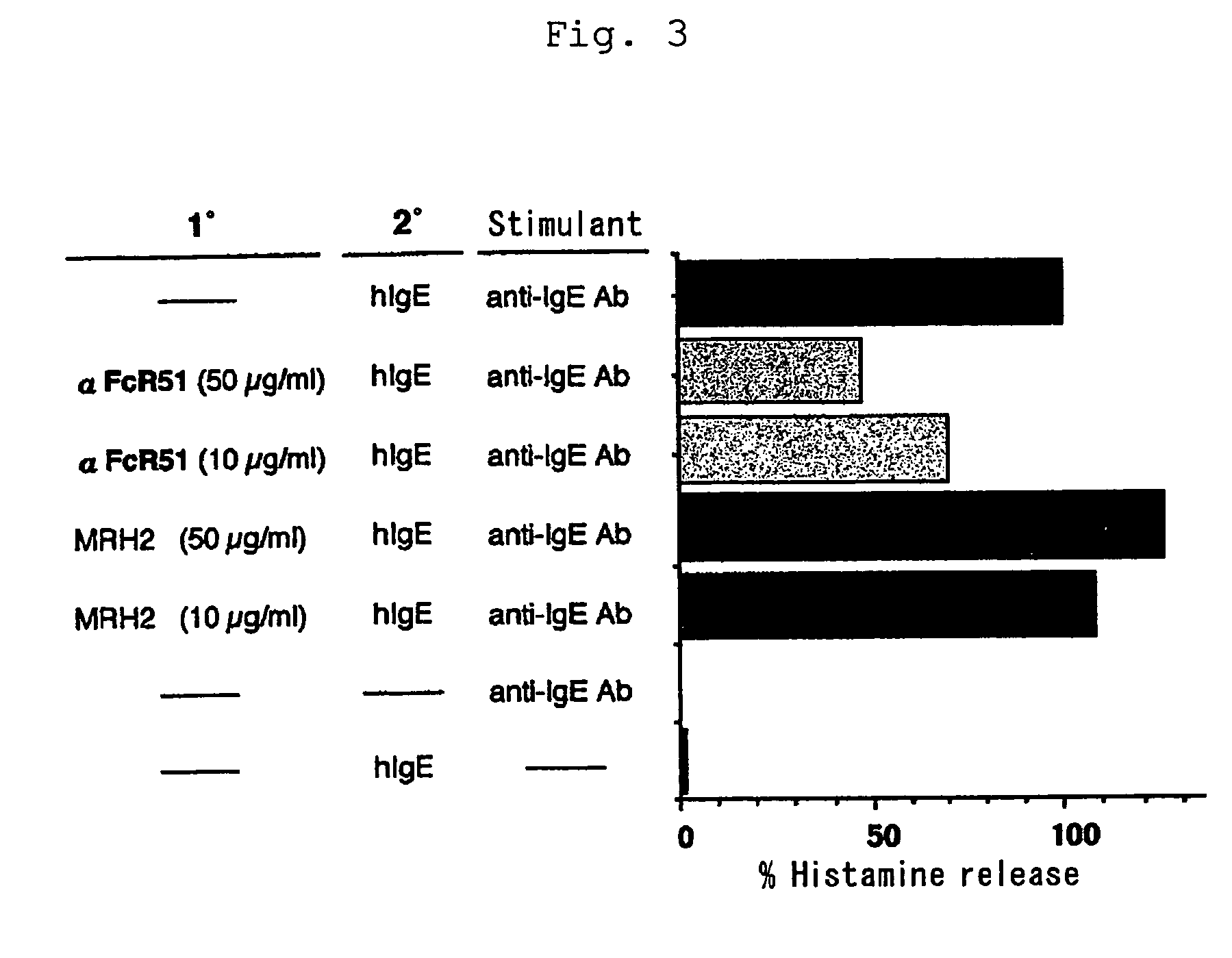
Human type antihuman ige receptor antibody and fragment
InactiveUS20050170451A1Specific and fundamental efficacyLack of efficacyAnimal cellsSugar derivativesPhage antibodiesAntibody fragments
An antibody or an antibody fragment is provided that is efficacious for treating allergic diseases in which IgE is involved. A human antibody and a fragment thereof to a receptor (FcεRI) having high affinity to Fc portion of IgE was obtained using phage antibody display technique. The human anti-IgE receptor antibody and a fragment thereof of the invention has an activity to inhibit the binding between IgE and IgE receptor and hence is expected to be useful as a medicament for treating allergic diseases caused by the binding between IgE and IgE receptor.
Owner:JURIDICAL FOUND THE CHEMO SERO THERAPEUTIC RES INST
Recombinant human collagen and application thereof
InactiveCN110003324AInefficient translationOptimize secondary structureCosmetic preparationsConnective tissue peptidesHuman typeFermentation
The invention provides recombinant human collagen and application thereof. An amino acid sequence encoding the recombinant human collagen is as shown in SEQ ID NO: 1, and the recombinant human collagen comprises an amino terminal affinity purification tag, a human type-I collagen alpha2 chain mature peptide chain and a carboxyl terminal affinity purification tag in sequence from the amino terminal. The recombinant human collagen has the advantages that by designing the specific affinity purification tags at both ends of the human type-I collagen alpha2 chain mature peptide chain, separation and purification and detection of the recombinant human collagen are easy; besides, the recombinant human collagen has a higher molecular weight and special physical and chemical properties, fermentation and expression are easy, the yield is high, and the application range is wide.
Owner:JIANGSU TRAUTEC MEDICAL TECH CO LTD
Dynein mosaic type recombinant human type-B adenovirus and preparation method thereof
ActiveCN107267469AHigh infection efficiencyMicroorganism based processesFermentationGolden hamsterHuman type
The invention discloses a dynein mosaic type recombinant human type-B adenovirus and a preparation method thereof. The skeleton of the dynein mosaic type recombinant human type-B adenovirus is a human type-B adenovirus genome, and a base sequence which encodes a receptor binding domain of dynein is a base sequence which encodes a corresponding domain of a human type-C adenovirus. By a molecular cloning method, Ad5-knob gene fragments are cloned and replaced to recombinant shuttle plasmids, in-vitro recombinant on the Ad5-knob gene fragments and a recombinant human type-3 adenovirus genome is realized, obtained knob gene fragments are replaced into type-5 recombinant human type-3 adenovirus genome, and therefore, dynein mosaic type recombinant human type-3 adenovirus rAd3-FK5 is obtained. The dynein mosaic type recombinant human type-3 adenovirus rAd3-FK5 can be infected with mouse primitive epithelial cells and golden hamster lung and kidney primitive cells in vitro, and the infection efficiency of the dynein mosaic type recombinant human type-3 adenovirus rAd3-FK5 is close to that of Ad5, and is much higher than that of a parent strain rAd3E, in golden hamster cells, significant copying exists, and the dynein mosaic type recombinant human type-B adenovirus can be used for small animal model research of human type-3 adenovirus vaccines and antiviral drug evaluation.
Owner:THE FIRST AFFILIATED HOSPITAL OF GUANGZHOU MEDICAL UNIV (GUANGZHOU RESPIRATORY CENT)
Human type II diabetes gene-slit-3 located on chromosome 5q35
InactiveUS20060141462A1Less-effective and ineffectiveBetter understandingNervous disorderMetabolism disorderHuman typeSusceptibility gene
Association of Type II diabetes and a locus on chromosome 5q35 is disclosed. In particular, the gene SLIT-3 with this locus is shown by linkage analysis to be a susceptibility gene for Type II diabetes. Pathway targeting for drug delivery and diagnosis applications in identifying those have Type II diabetes or at risk of developing Type II diabetes, in particular those that are non-obese are described.
Owner:DECODE GENETICS EHF
Modifiable loca of human type 3 adenovirus hexon and application thereof
The invention provides modifiable amino acid loca in hypervariable regions of human type 3 adenovirus hexon. The modifiable loca are positioned in the hypervariable regions of the hexon and have at least one amino acid locus in a sequence shown as SEQ ID NO:1-4. The amino acid loca are positioned in hypervariable regions 1, 2, 5, and 7 of the human type 3 adenovirus hexon. The invention also provides the application of the modifiable amino acid loca to the show of extrinsic protein and the preparation of immunogen, medicaments and detection reagents. The modifiable amino acid loca lay the foundation for a virus show technology platform which is researched and developed by using a technology platform of modifiable extrinsic polypeptides in the hypervariable regions of the human type 3 adenovirus hexon as vaccines or antigens.
Owner:GUANGZHOU INST OF RESPIRATORY DISEASE
Methods for glyco-engineering plant cells for controlled human o-glycosylation
This invention discloses the development of a novel platform for recombinant production of bioactive glycoproteins and cancer specific vaccines in plants. Plants and plant cell cultures have been humanized with respect to human mucin-type protein O-glycosylation. A panel of plant cell factories for production of recombinant glycoproteins with designed human O-glycosylation, including an improved cancer vaccine candidate, has been developed. The platform provides basis for i) production of an essentially unlimited array of O-glycosylated human glycoprotein therapeutics, such as human interferon α2B and podoplanin, and ii) for further engineering of additional cancer specific O-glycans on glycoproteins of therapeutical value. Currently, mammalian cells are required for human O-glycosylation, but plants offer a unique cell platform for engineering O-glycosylation since they do not perform human type O-glycosylation. Introduction of O-glycosylation into plant cells requires i) that wild-type plant cells do not modify the target peptide substrates and ii) that the appropriate enzymes and substrates are introduced into of plant cells such that O-glycosylation in the secretory pathway proceed and the glycosylated peptide substrates are preferentially exported to the exterior of the cell or accumulated in the cell. In this invention i) the integrity of transiently and stably expressed ‘mucin’ type target peptides in plants cells has been determined and ii) mucin-type O-glycosylation has been established in plants by transient and stable introduction of a Pseudomonas aeruginosa C4-epimerase, the human polypeptide GalNAc-transferases T2 and T4 (GalNAc-T2 and T4) and various human target peptides or proteins. In the present invention GalNAc-T2 and -T4 have been used to produce a Tn cancer glycoform of MUC1.
Owner:YANG ZHANG +7
Human type antihuman IgE receptor antibody and fragment
An antibody or an antibody fragment is provided that is efficacious for treating allergic diseases in which IgE is involved. A human antibody and a fragment thereof to a receptor (FcεRI) having high affinity to Fc portion of IgE was obtained using phage antibody display technique. The human anti-IgE receptor antibody and a fragment thereof of the invention has an activity to inhibit the binding between IgE and IgE receptor and hence is expected to be useful as a medicament for treating allergic diseases caused by the binding between IgE and IgE receptor.
Owner:JURIDICAL FOUND THE CHEMO SERO THERAPEUTIC RES INST
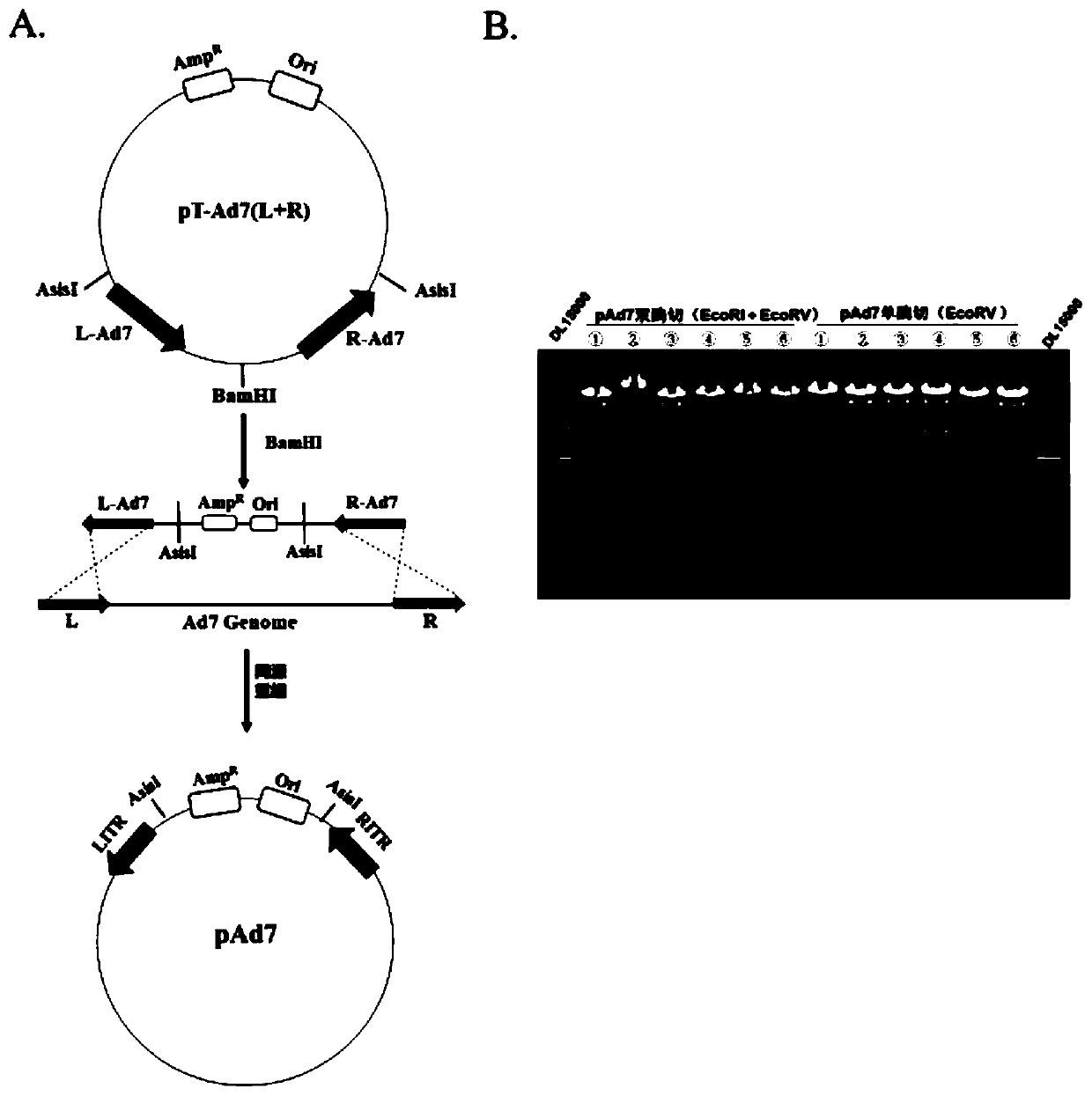
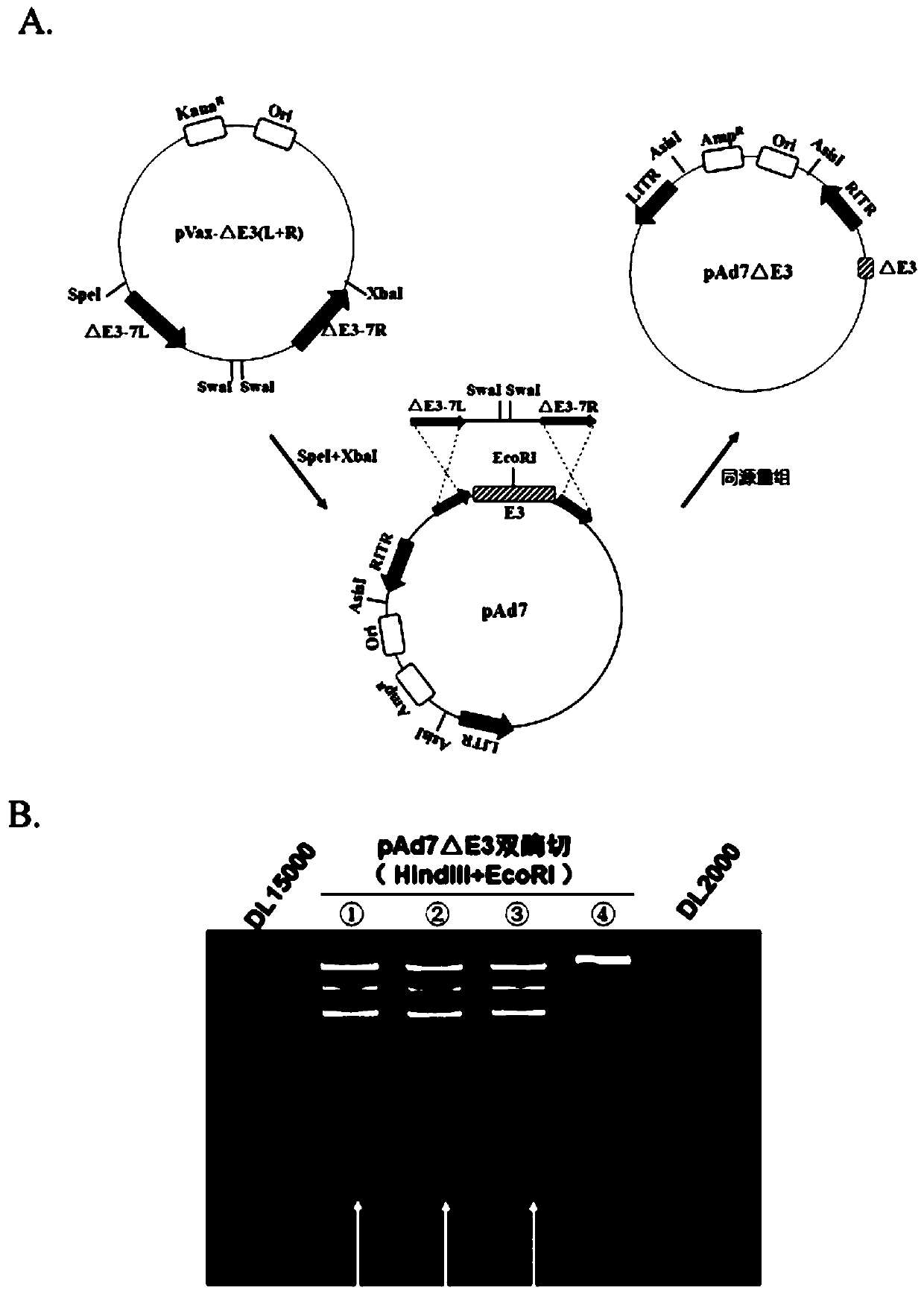
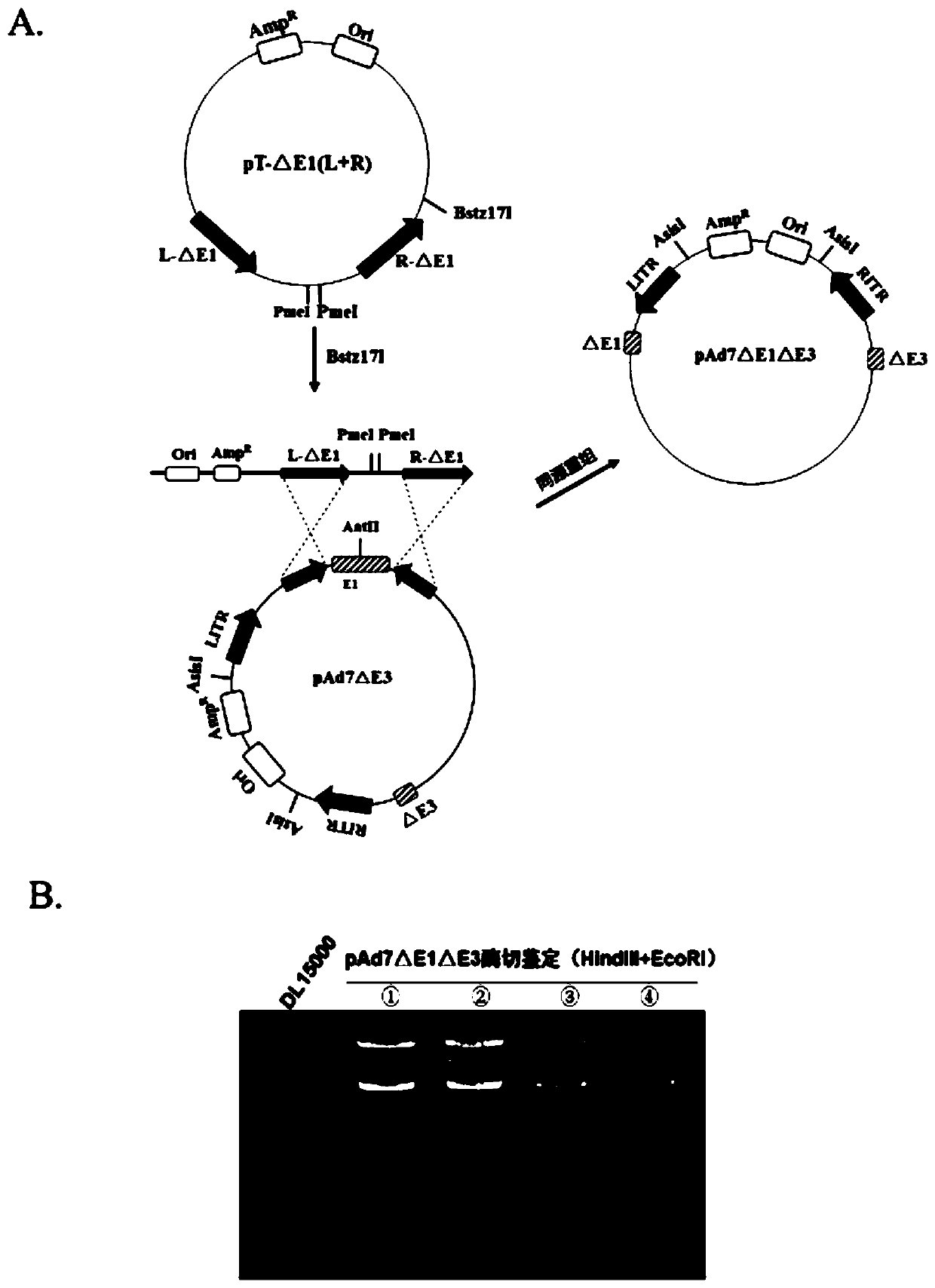
Replication-deficient type recombinant human type 7 adenovirus, preparation method and application thereof
InactiveCN110616199AIncrease production capacityViral antigen ingredientsVirus peptidesHuman typeNeutralizing antibody
The present invention relates to the field of biotechnology and specifically discloses a replication-deficient type human type 7 adenovirus and a preparation method and an application thereof. In thereplication-deficient type human type 7 adenovirus, E1 gene and E3 gene are deleted, E4 gene open reading frames 2, 3, 4, 6 and 6 / 7 are replaced into a corresponding reading frame of Ad5 genome, and aE1 gene region can integrate foreign gene expression box. The replication-deficient type 7 adenovirus can be successfully rescued and mass-produced in HEK293, but does not have replication ability inhuman normal cell lines; and after the replication-deficient type 7 adenovirus carrying foreign genes infects cells, efficient expression of the foreign genes can be realized. The replication-deficient type 7 adenovirus can potentially be applied in development of preventive vaccines against human type 7 adenovirus infection; neutralizing antibodies and drug screening against human type 7 adenovirus infection; application as a gene carrier for development of vaccines for other pathogens; and report tracking systems for biological research, etc.
Owner:GUANGZHOU N BIOMED LTD
Preparation and application of curcumin compound capable of inhibiting activity of human type 1 11beta-hydroxysteroid dehydrogenase
ActiveCN102477013AImprove solubilityInhibition of reducing activityOrganic active ingredientsOrganic chemistry11beta hydroxysteroid dehydrogenaseHuman type
The invention relates to a curcumin compound shown as the formula (1), a preparation method of the compound, and application of the compound as an inhibitor of type 1 11beta-hydroxysteroid dehydrogenase (11beta-HSD1) in a medicine for preventing and treating type 2 diabetes mellitus.
Owner:JINAN UNIVERSITY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com