Insulin mimetic peptide fusion protein, mutant and applications thereof
A fusion protein and mutant technology, applied in the direction of peptide/protein components, peptides, hybrid peptides, etc., can solve the problems of low efficacy and achieve high biological activity, avoid insulin resistance, and low ability to stimulate cell division.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0069] The pharmaceutical compositions of the present invention can be prepared by known methods for preparing pharmaceutically acceptable carriers that can be administered to patients, so that an effective amount of nucleotide or polypeptide molecule is combined with a pharmaceutically acceptable carrier to form a mixture. Such suitable carriers are described, for example, in Remington's Pharmaceutical Sciences (Mack Publishing Company, Easton, Pa., USA). On this basis, the pharmaceutical composition may comprise the active compound in association with one or more pharmaceutically acceptable carriers or diluents in a buffer solution having an appropriate pH value and isotonic with physiological fluids or Substances such as complex nucleic acid molecules, polypeptide molecules or fusion eggs. Methods of combining active molecules and carriers or both with diluents are well known to those skilled in the art. The composition may include a targeting agent for delivering the acti...
Embodiment 1
[0089] Embodiment 1: the construction of carrier
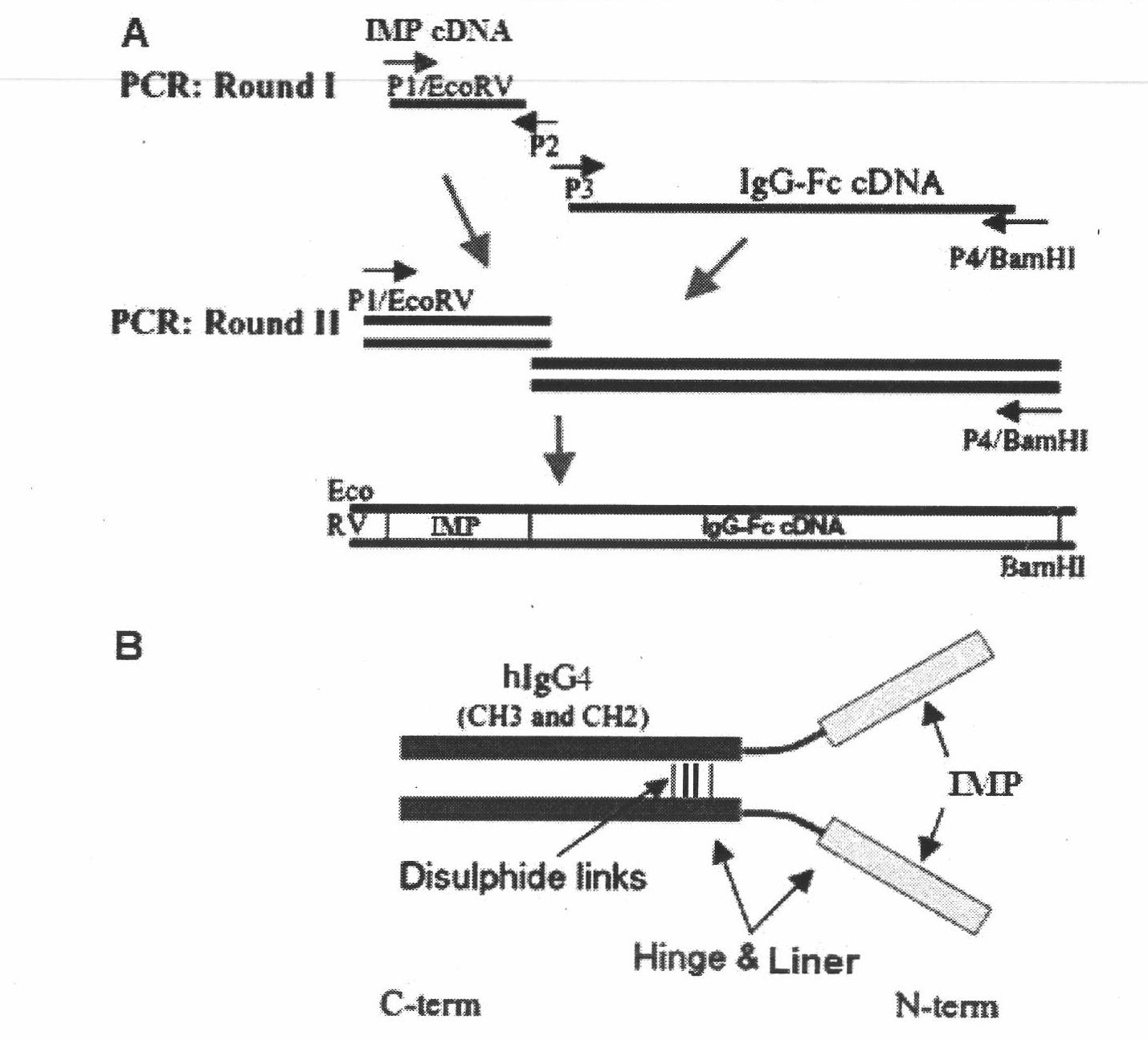
[0090] The vectors encoding the fusion protein containing IMP and IgG1-Fc, IMP / IgG-Fc (QL) and IMP / IgG-Fc (QA) vectors were constructed by overlapping PCR (overlap PCR). The IgG4-Fc region contains the IgG4 constant heavy chain (hinge-CH2-CH3 part). The murine IgK secretion leader sequence was fused to the IMP sequence to direct the secretion of the synthetic fusion protein into the culture medium. The cDNA encoding the IMP / hIgG-Fc fusion protein was chemically synthesized and ligated to the PCR-amplified product encoding human IgG4-Fc (hinge, CH2, and CH3), then inserted into the EcoRV and BamHI of the pcDNA3.1 vector The IMP / hIgG-Fc / pcDNA3.1 vector was constructed between the sites. Secretable IMP / hIgG-Fc and its mutant fusion proteins contain an IgG4 constant heavy chain (hinge-CH2-CH3). The mouse IgK secretion leader peptide sequence fused to the IMP sequence directs the secretion of the synthesized protein into the cel...
Embodiment 2
[0096] Example 2: CHO cell expression of IMP / IgG-Fc, IMP / IgG-Fc(QL) and IMP / IgG-Fc(QA) fusion proteins
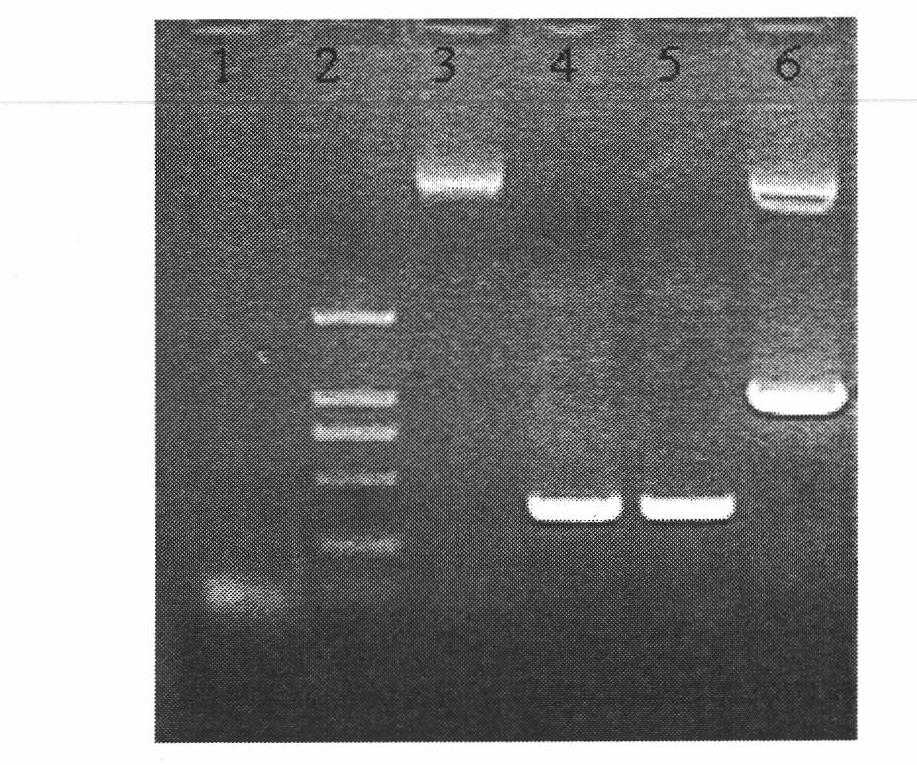
[0097] In order to evaluate the ability of the vector to express and secrete IMP / IgG-Fc fusion protein, the constructed fusion protein vector was transfected into CHO-S cells for transient expression. 48h after transfection, total RNA was extracted from the transfected cells and the expression effect was evaluated by RT-PCR. Application of gene-specific primers detected the expression of the IMP / IgG-Fc fusion protein gene at the transcriptional level and the expression of the IgG-Fc control gene ( figure 2 A). No transcript level expression was detected in the control samples.
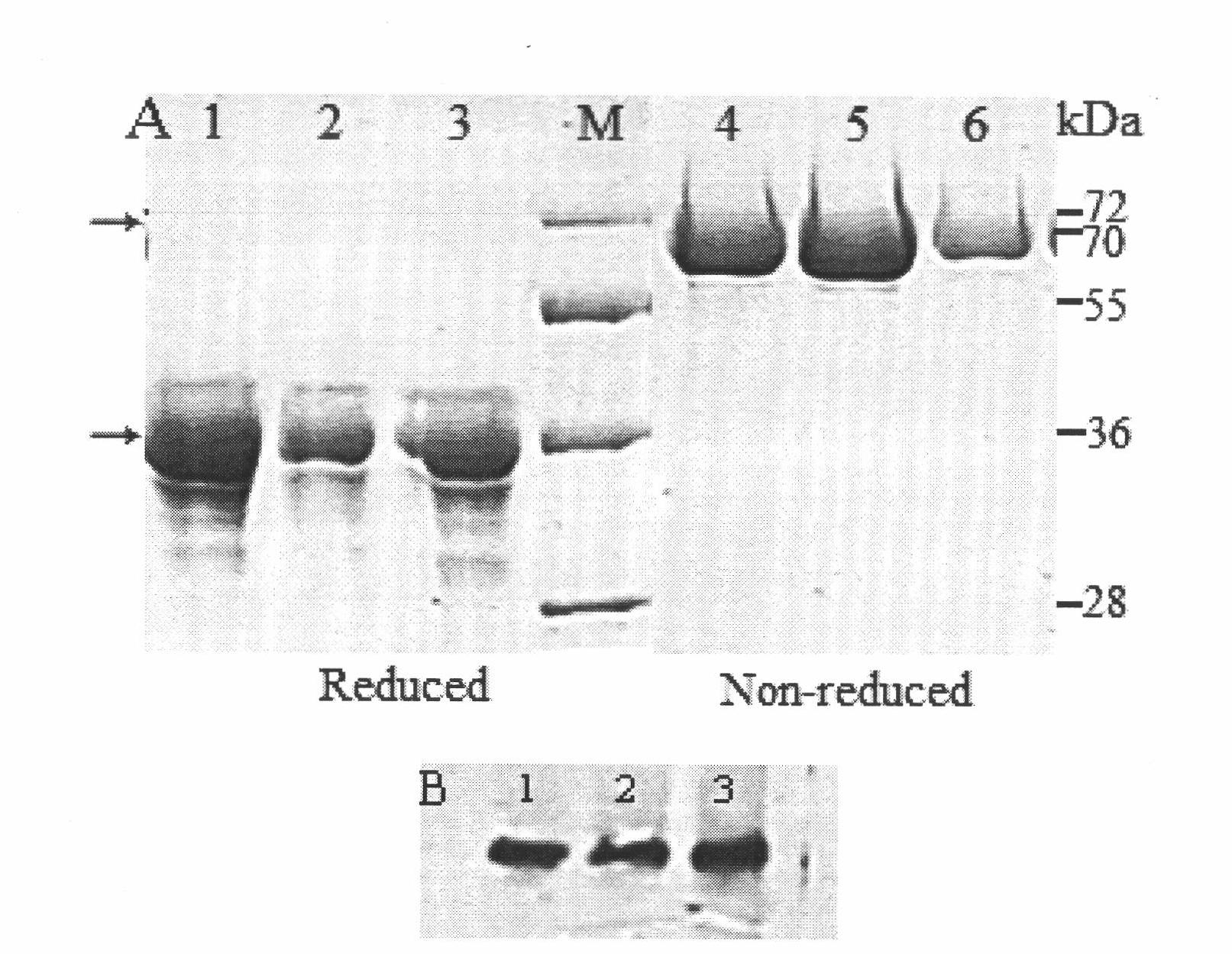
[0098] Transfected CHO-S cell lysates and cell culture fluid were detected by Western blot and anti-mouse antibody to confirm the expression of the fusion protein at the translational level. Such as figure 2 As shown in B, Fc fusion proteins were detected in both culture medium and cell lysate...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com