Patents
Literature
415 results about "Diabetes treatment" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
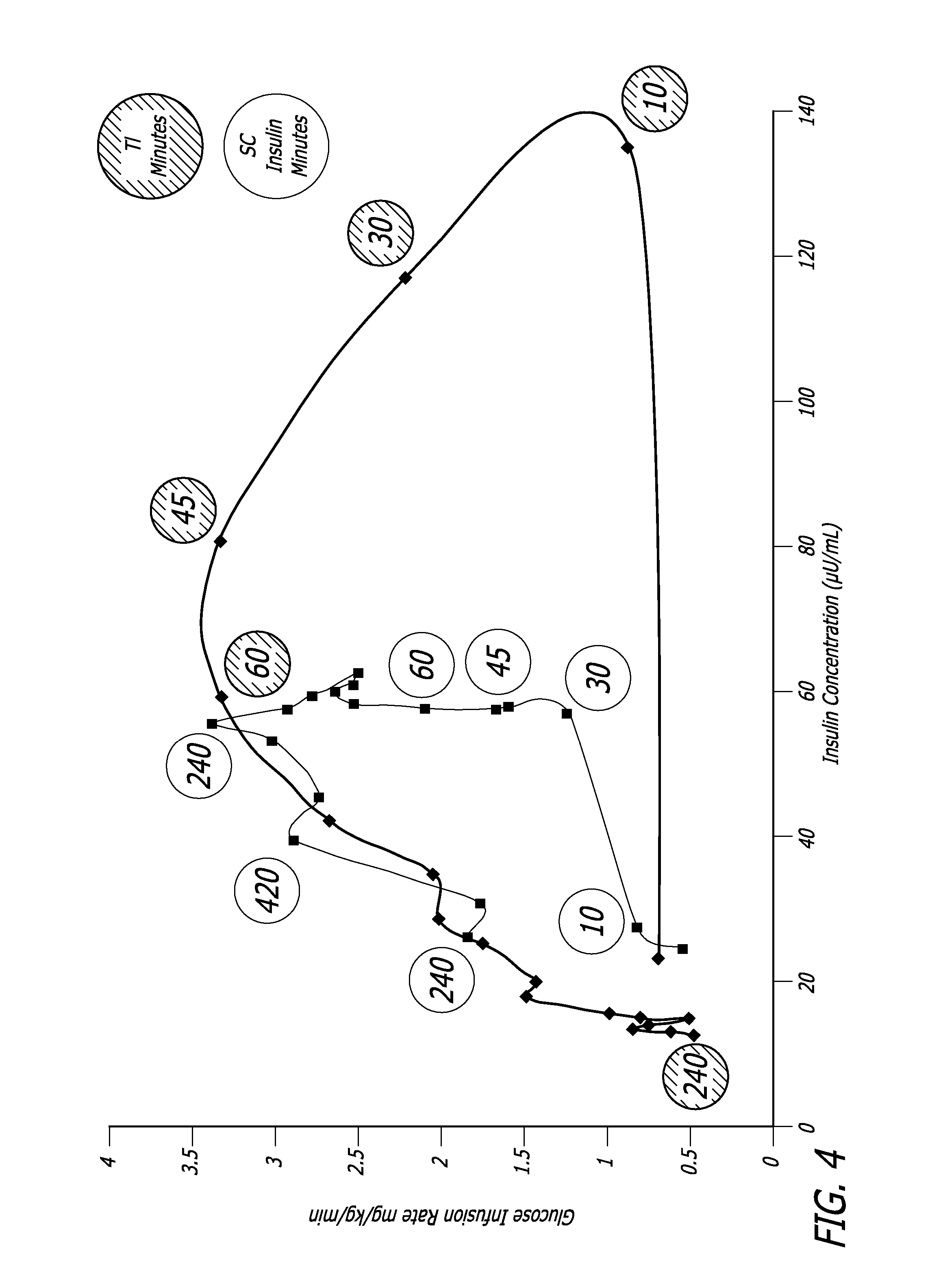
Treatment of Type 2 diabetes. Treatment typically includes diet control, exercise, home blood glucose testing, and in some cases, oral medication and/or insulin. Approximately 40% of people with type 2 diabetes require insulin injections.
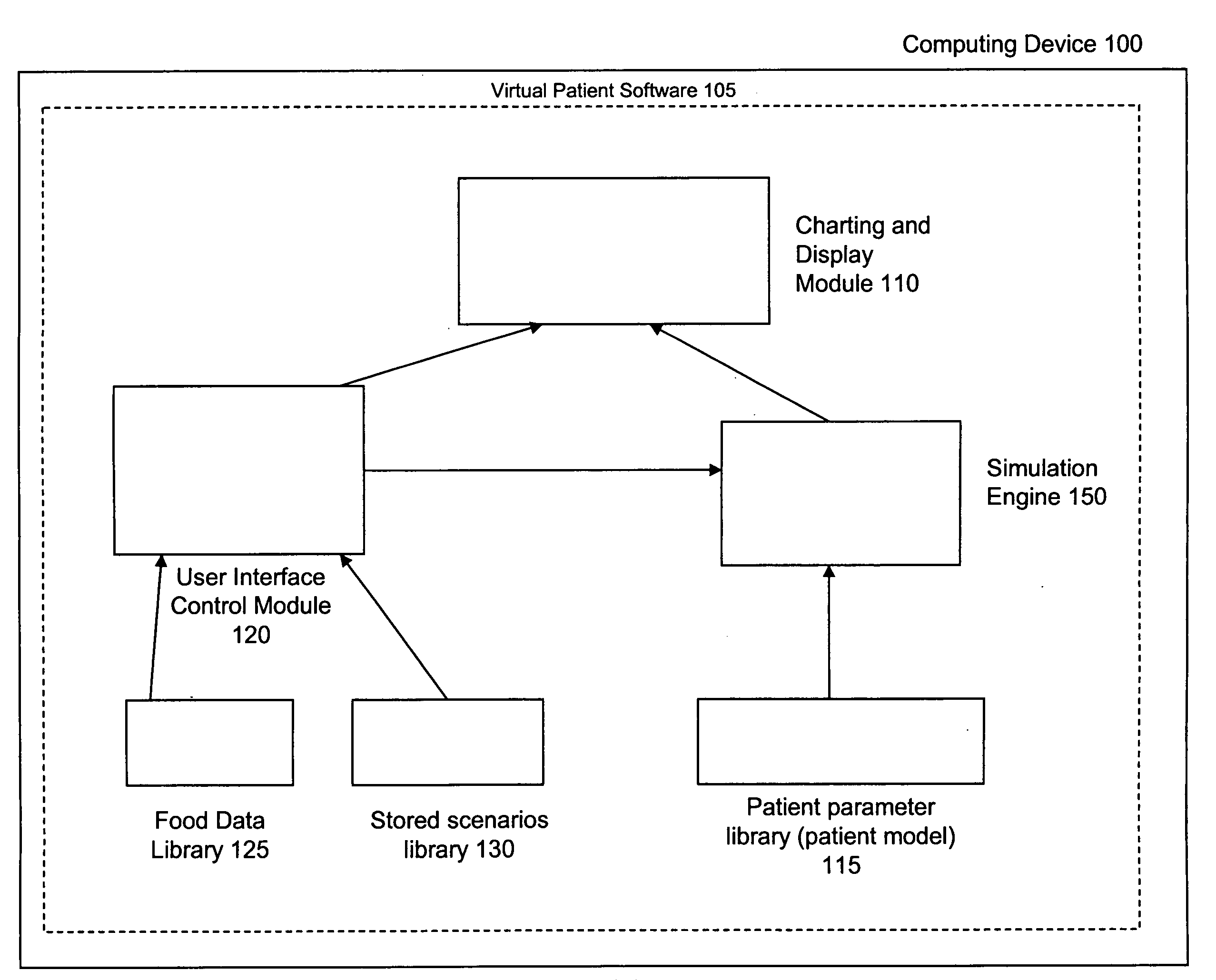
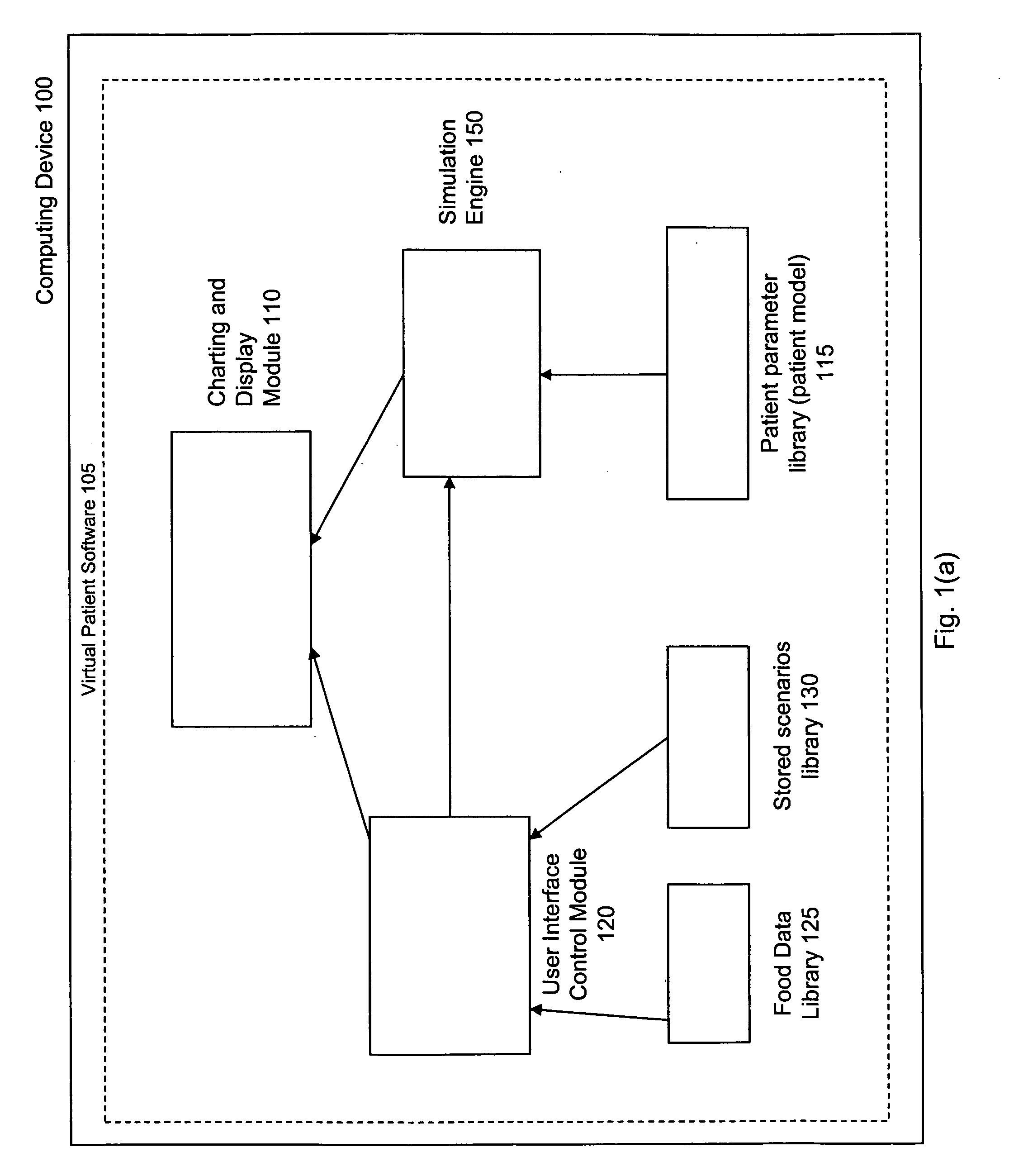
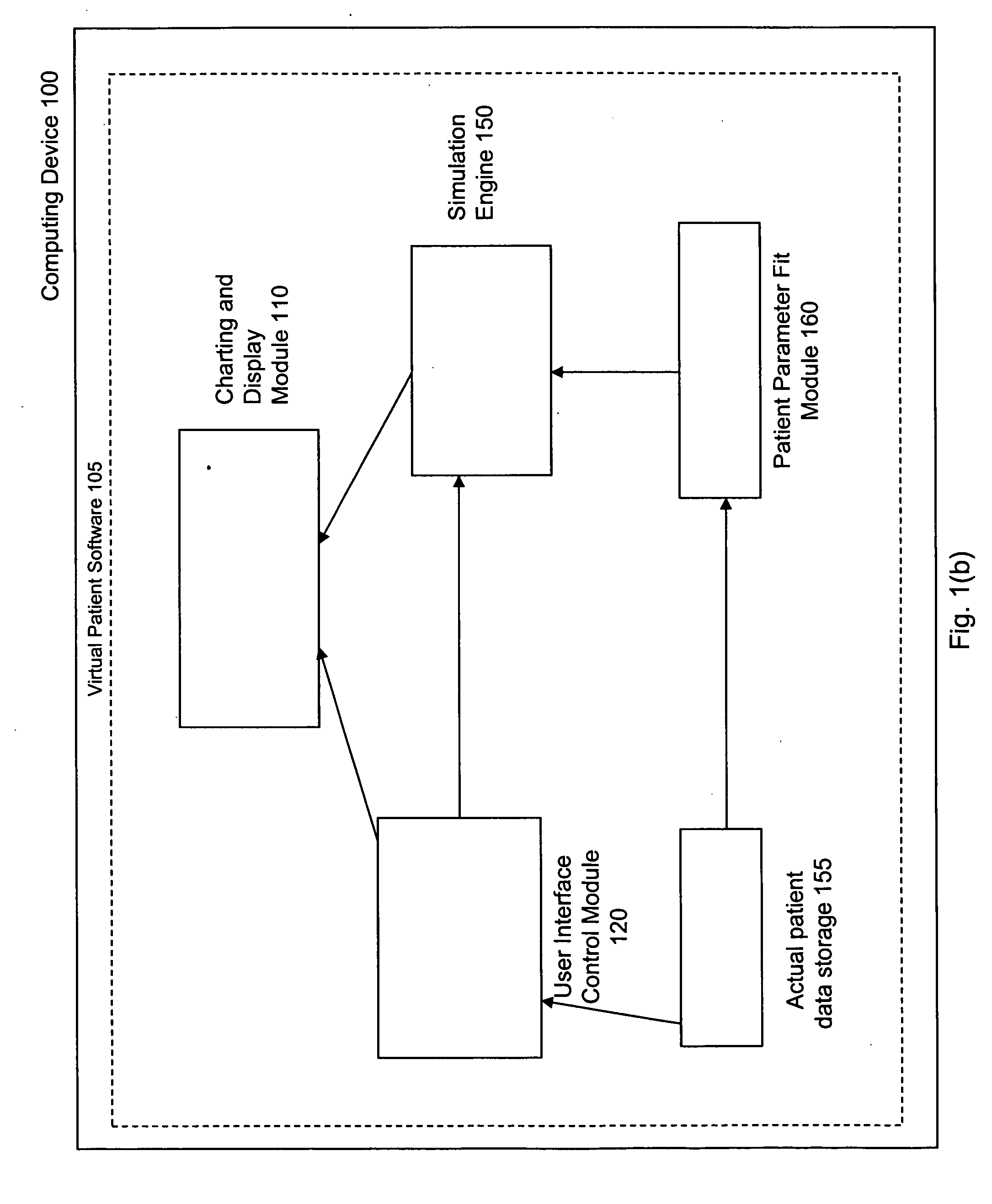
Virtual patient software system for educating and treating individuals with diabetes
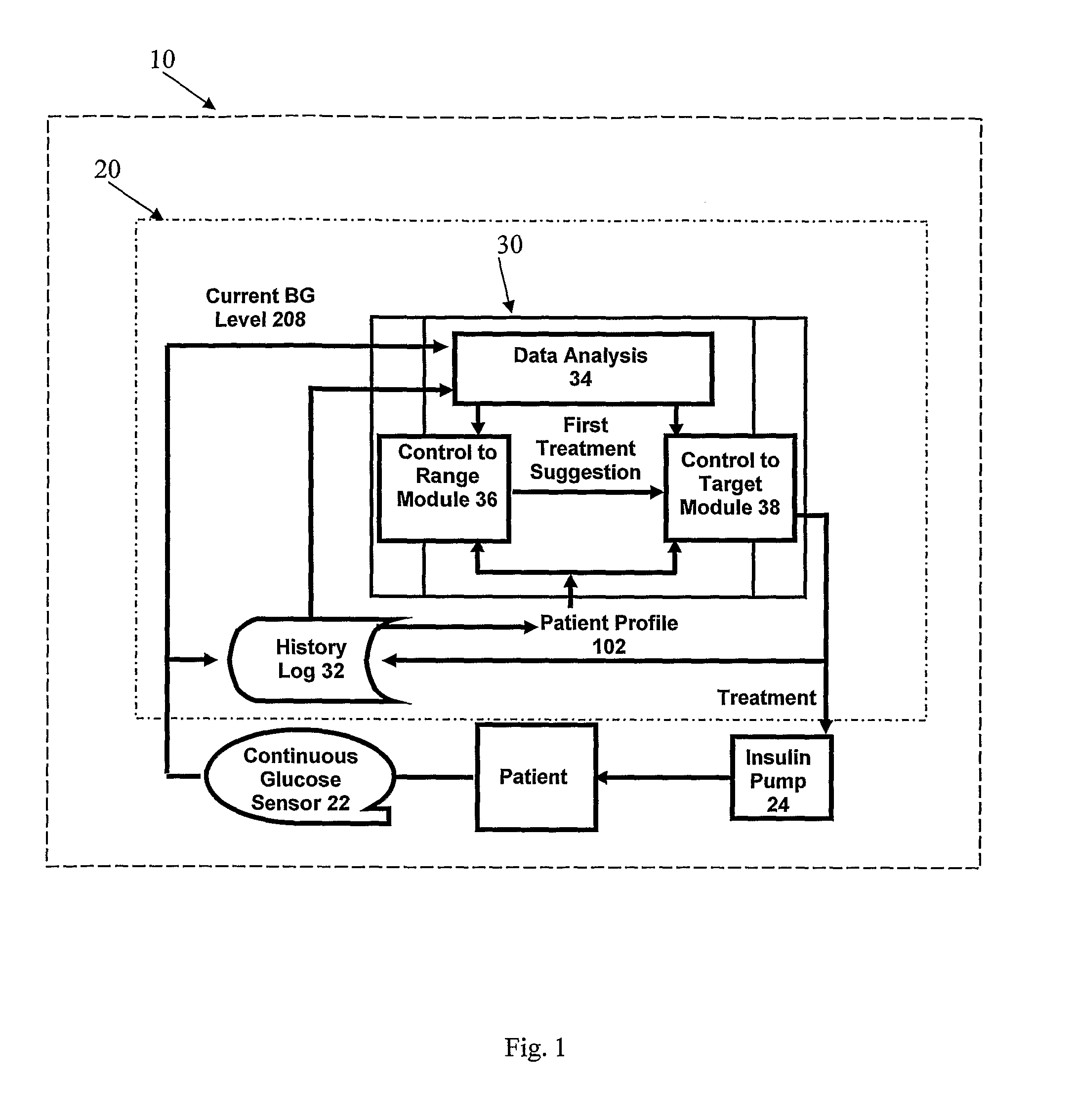
A system to assist an individual in developing a therapy in diabetes treatment of a patient includes a user interface control module, a simulation engine, a charting and display module. The user interface control module receives an input related to the patient and captures a current time of the simulation. The simulation engine receives the input, generates a plurality of blood glucose readings for the patient up to the current time of the simulation based on the input, and to transfers the plurality of blood glucose readings. The charting and display module receives the plurality of blood glucose readings and display the plurality of blood glucose readings. The simulation engine receives patient parameters from a patient parameter library based on a selected patient model.
Owner:MEDTRONIC MIMIMED INC
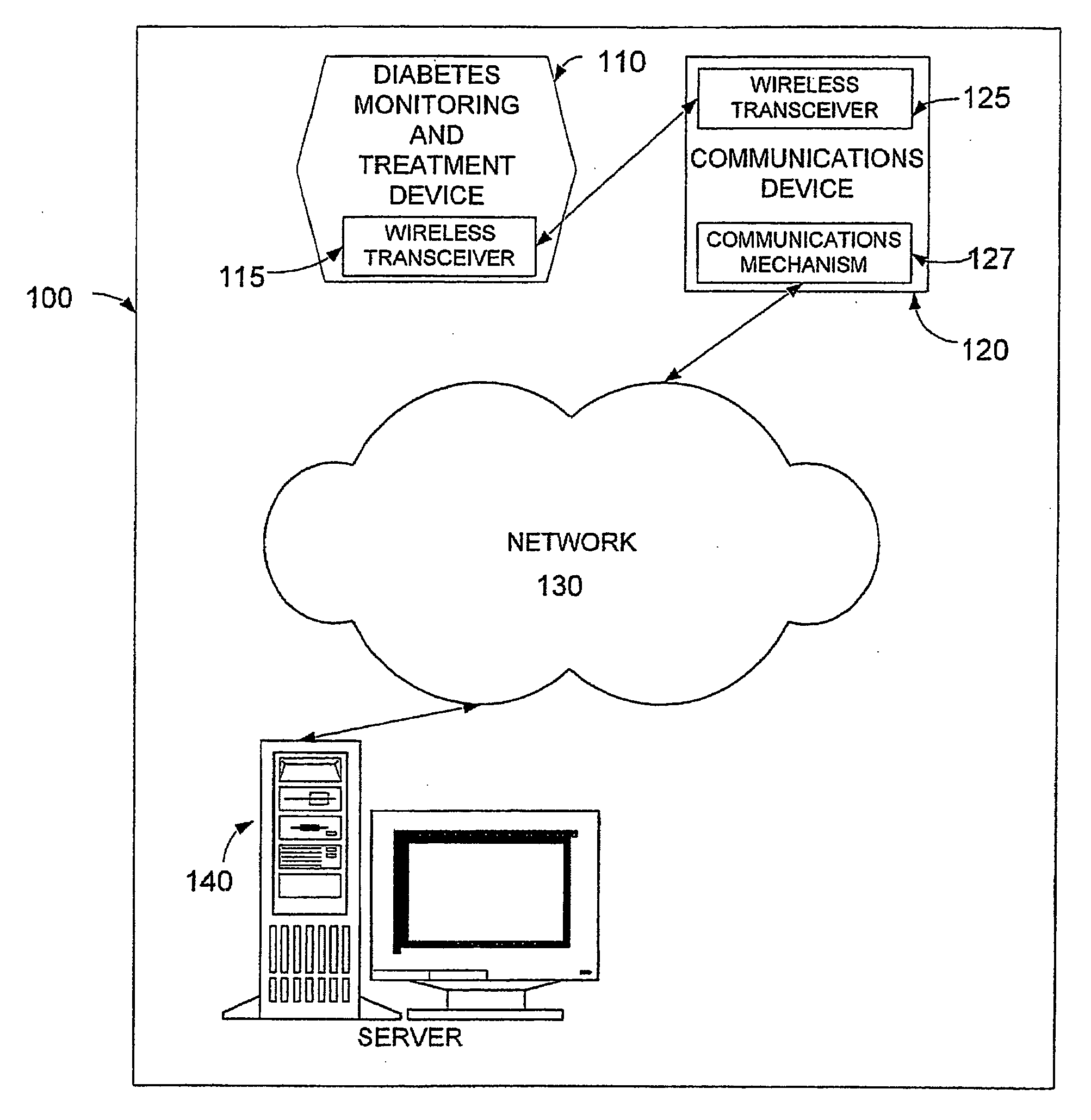
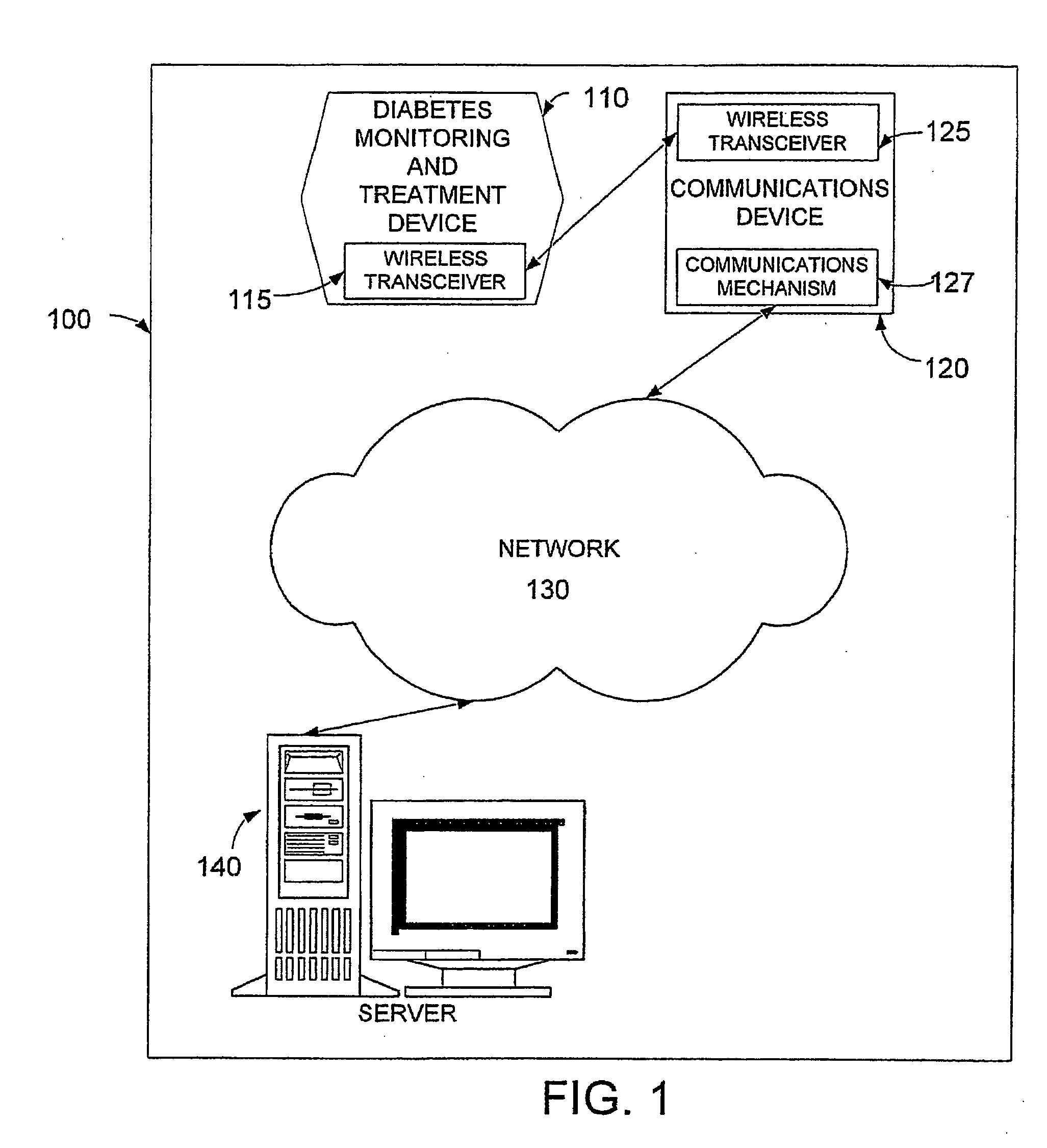
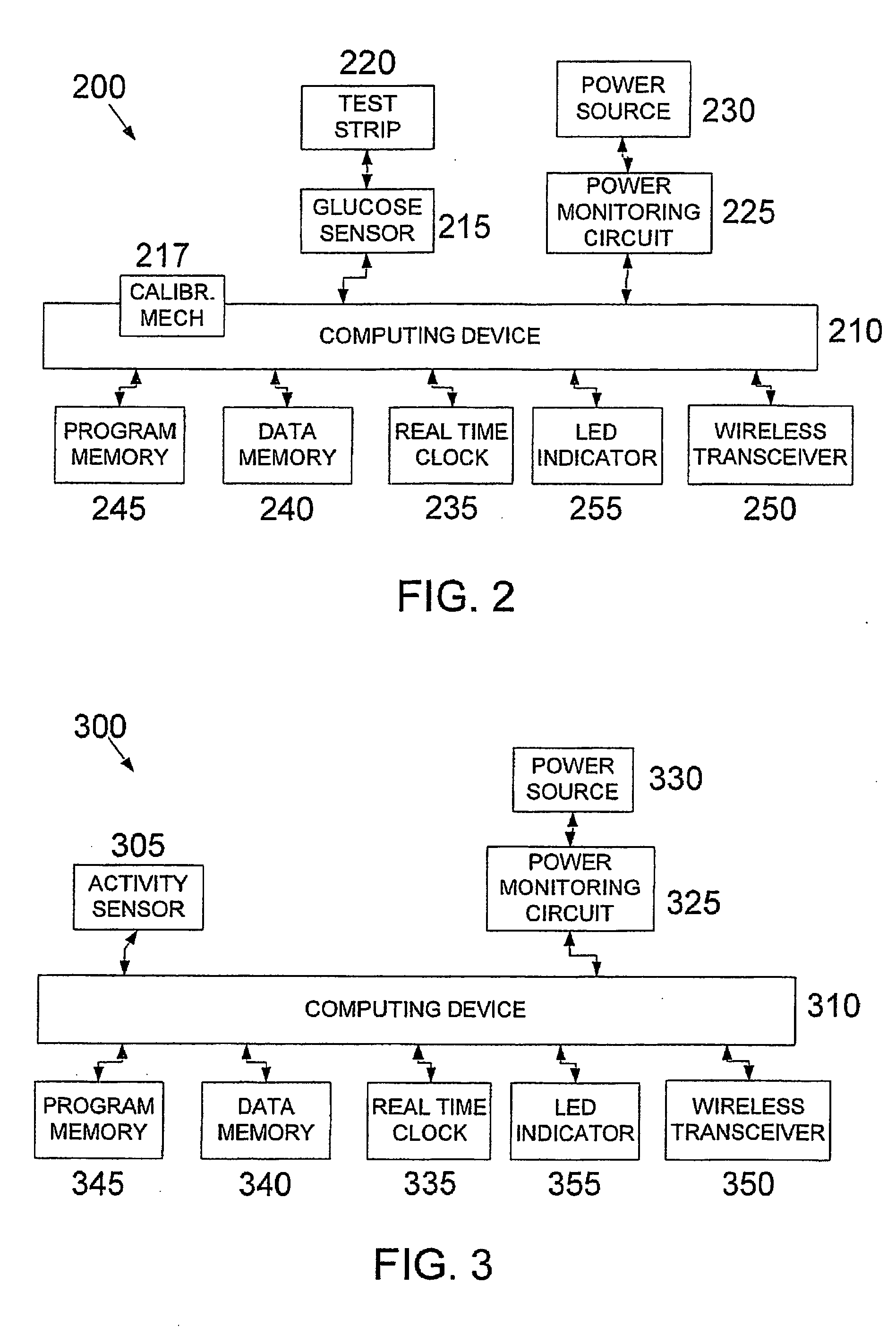
System, device and method for diabetes treatment and monitoring
InactiveUS20060173260A1Improve data accuracyPhysical therapies and activitiesSensorsWireless transceiverGlucose polymers
An apparatus and method may provide a system, apparatus and method for monitoring diabetes, including a server to serve data to a cellular communications network; a communications device including a wireless transceiver; a cellular network to enable data to be communicated between the communication device and the server; and a diabetes monitoring device including a wireless transceiver, to enable data to be communicated between the monitoring device and the communication device. The diabetes monitoring device may include sensors for monitoring one or more of for example blood glucose level, physical activity, energy intake and insulin dosage.
Owner:GMMS
Methods of determining pre or post meal time slots or intervals in diabetes management
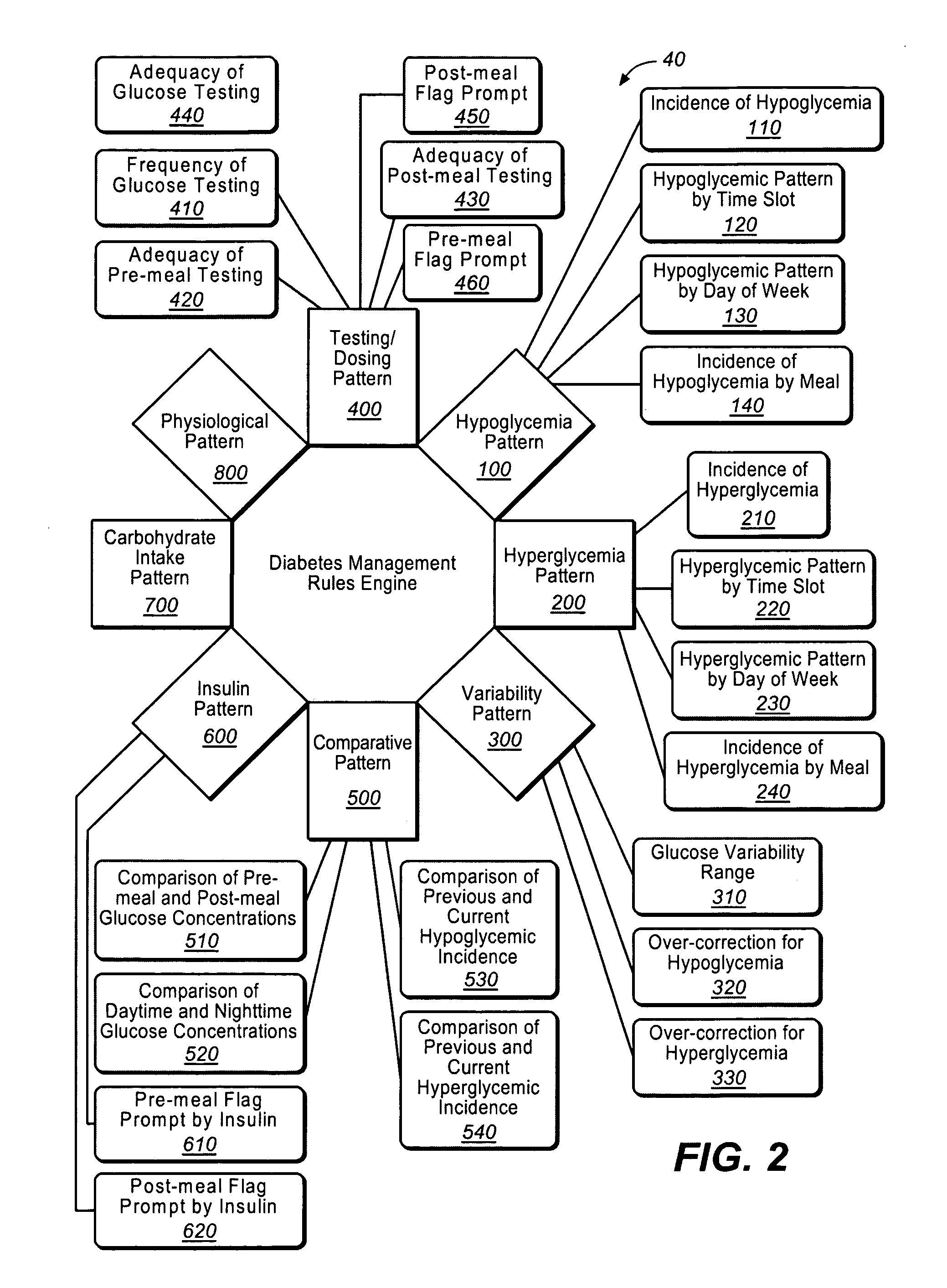
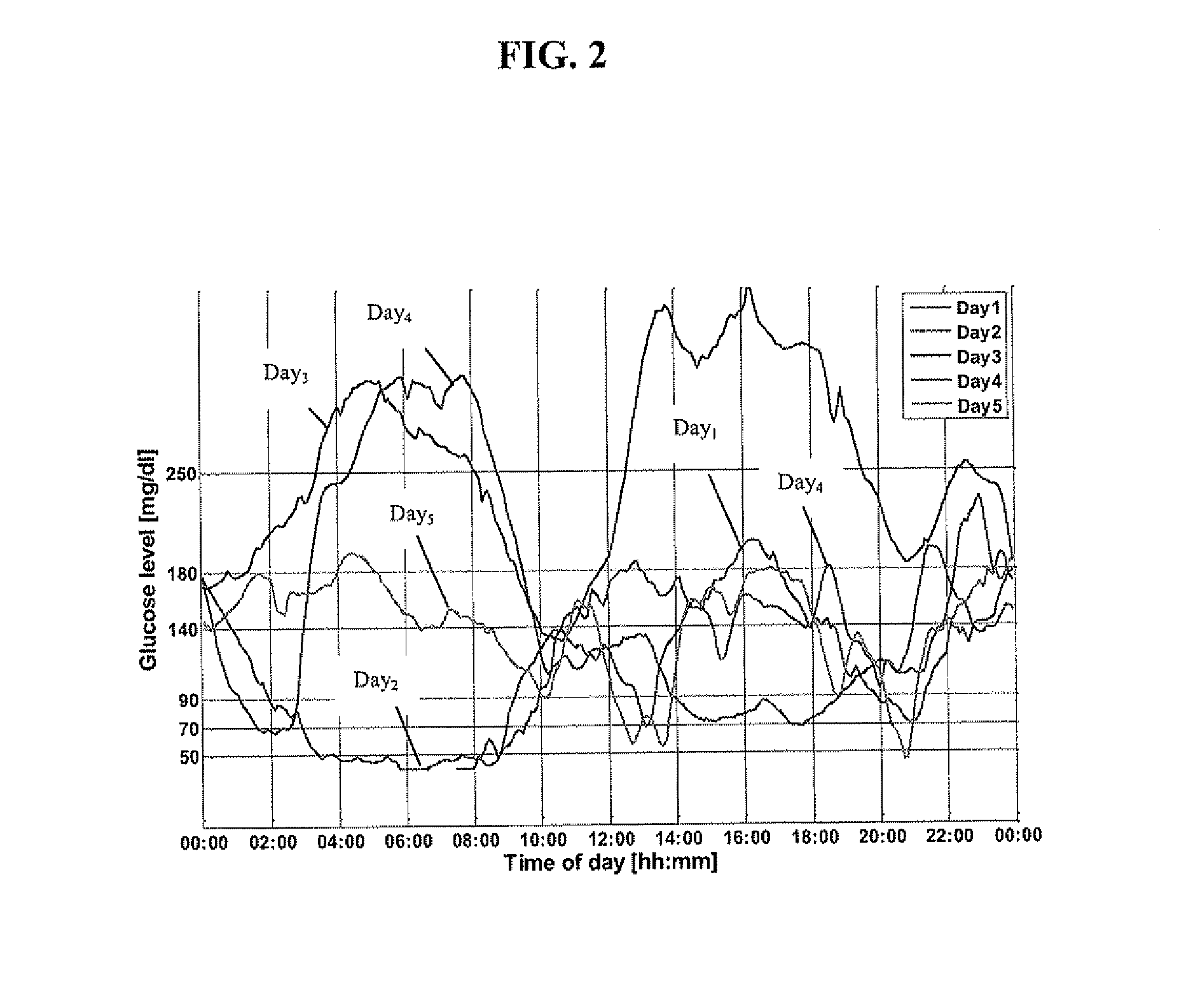
A diabetes management system or process is provided herein that may be used to analyze and recognize patterns for a large number of blood glucose concentration measurements and other physiological parameters related to the glycemia of a patient. In particular, a method of monitoring glycemia in a patient may include storing a patient's data on a suitable device, such as, for example, a blood glucose meter. The patient's data may include blood glucose concentration measurements. The diabetes management system or process may be installed on, but is not limited to, a personal computer, an insulin pen, an insulin pump, or a glucose meter. The diabetes management system or process may identify a plurality of pattern types from the data including a testing / dosing pattern, a hypoglycemic pattern, a hyperglycemic pattern, a blood glucose variability pattern, and a comparative pattern. After identifying a particular pattern with the data management system or process, a warning message may be displayed on a screen of a personal computer or a glucose meter. Other messages can also be provided to ensure compliance of any prescribed diabetes regiments or to guide the patient in managing the patient's diabetes.
Owner:LIFESCAN INC
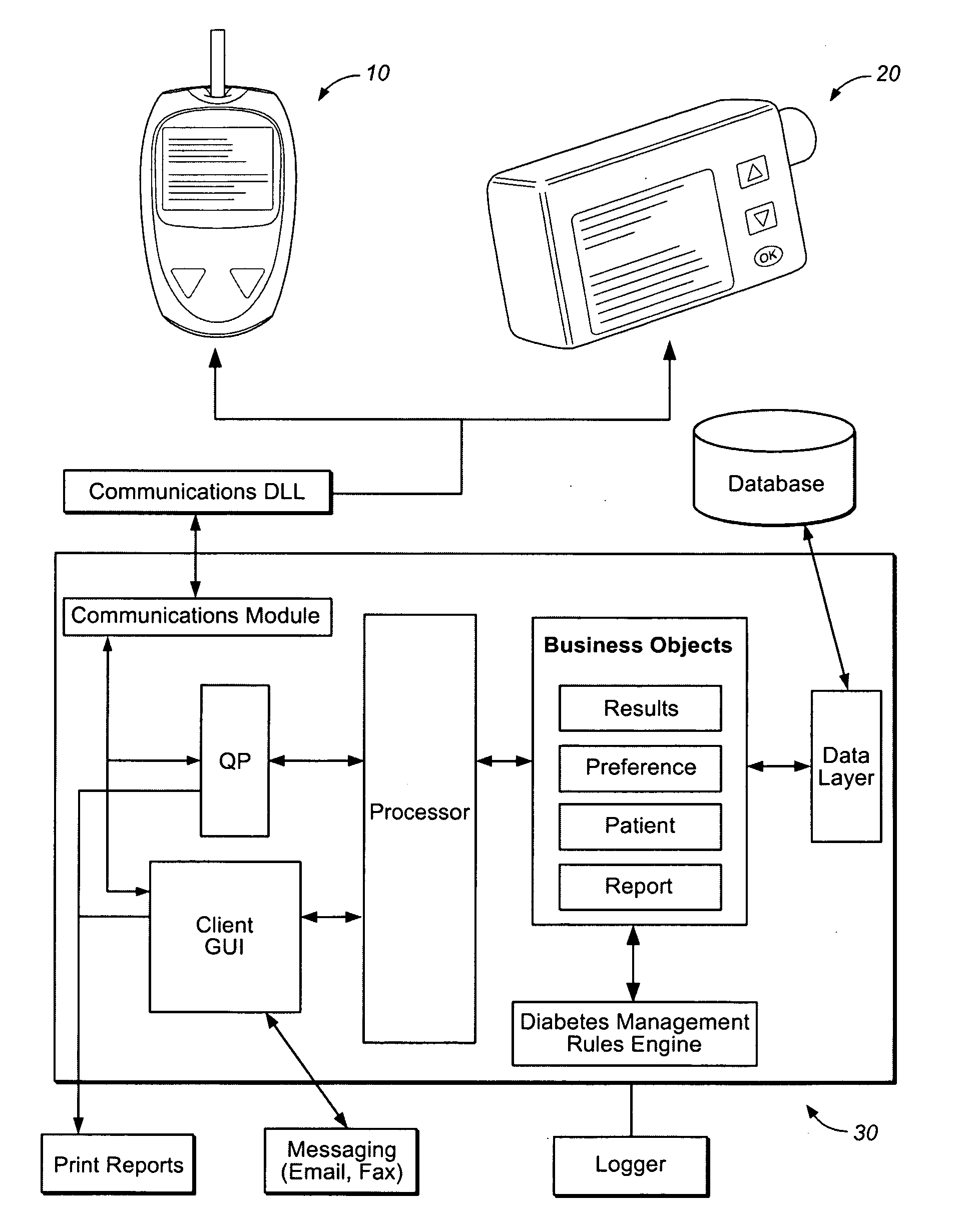
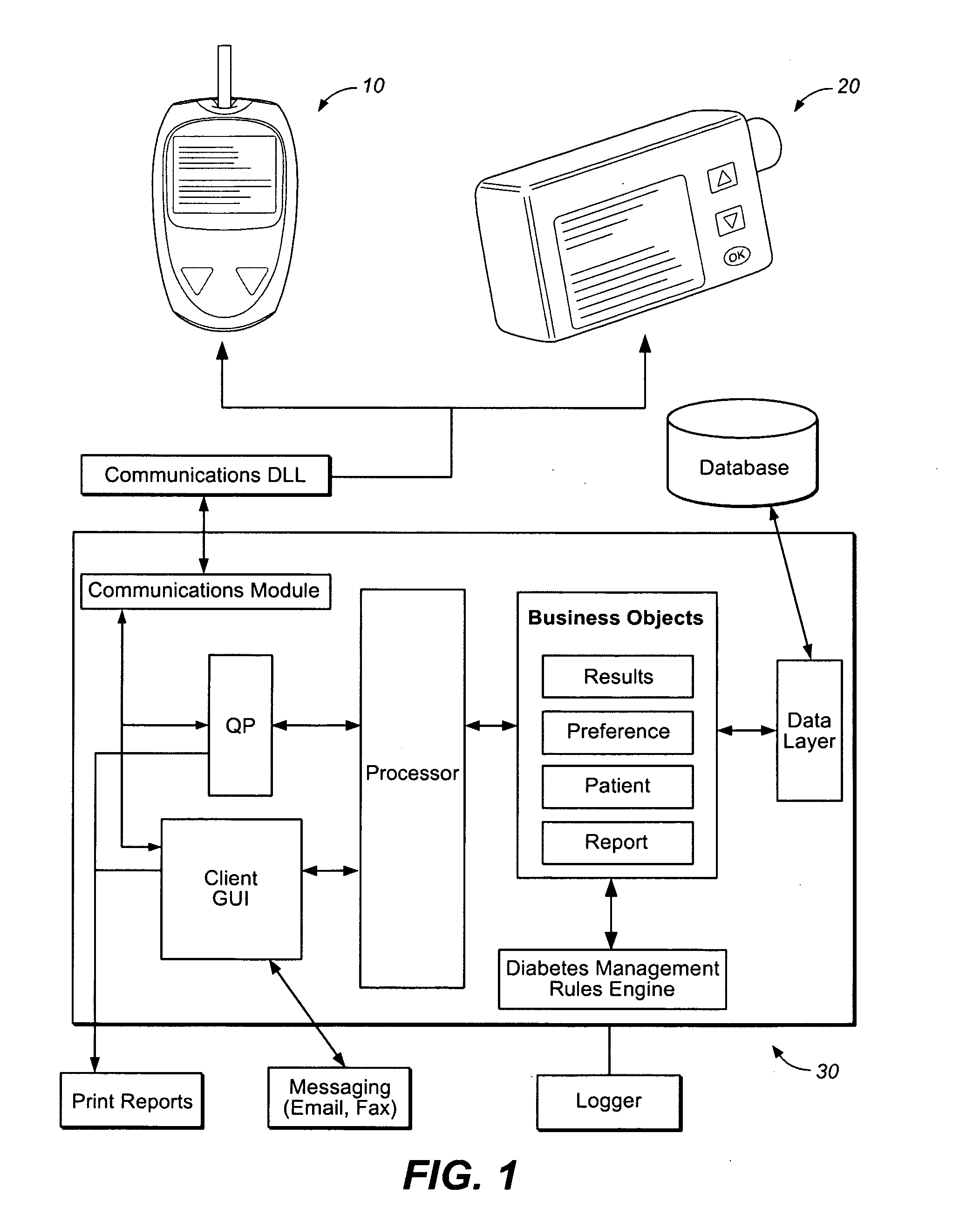
Method and system for automatic monitoring of diabetes related treatments
InactiveUS20120123234A1Easy to controlAvoiding severe hypoglycemia eventMedical simulationInfusion syringesMedicineProcess Measures
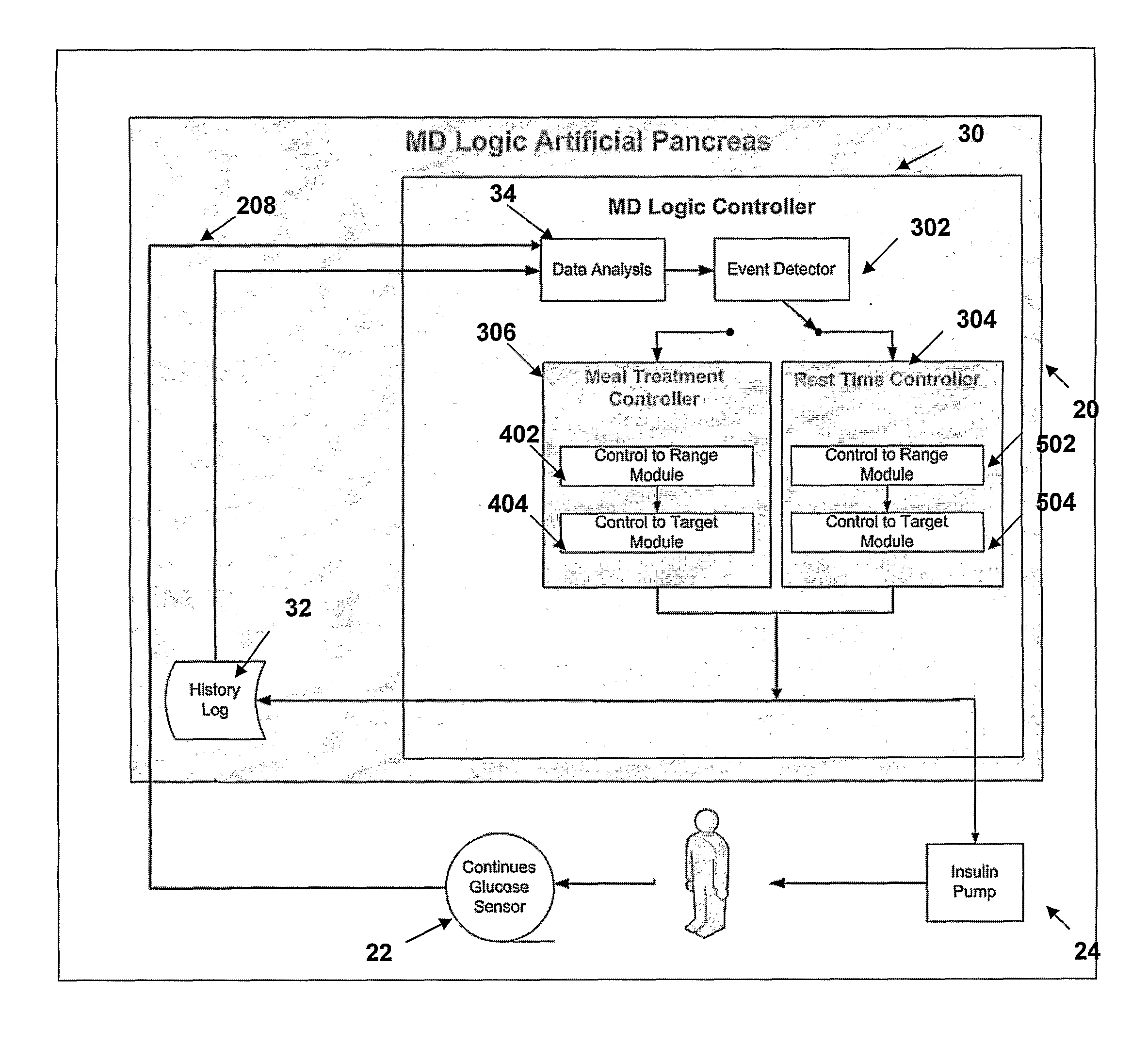
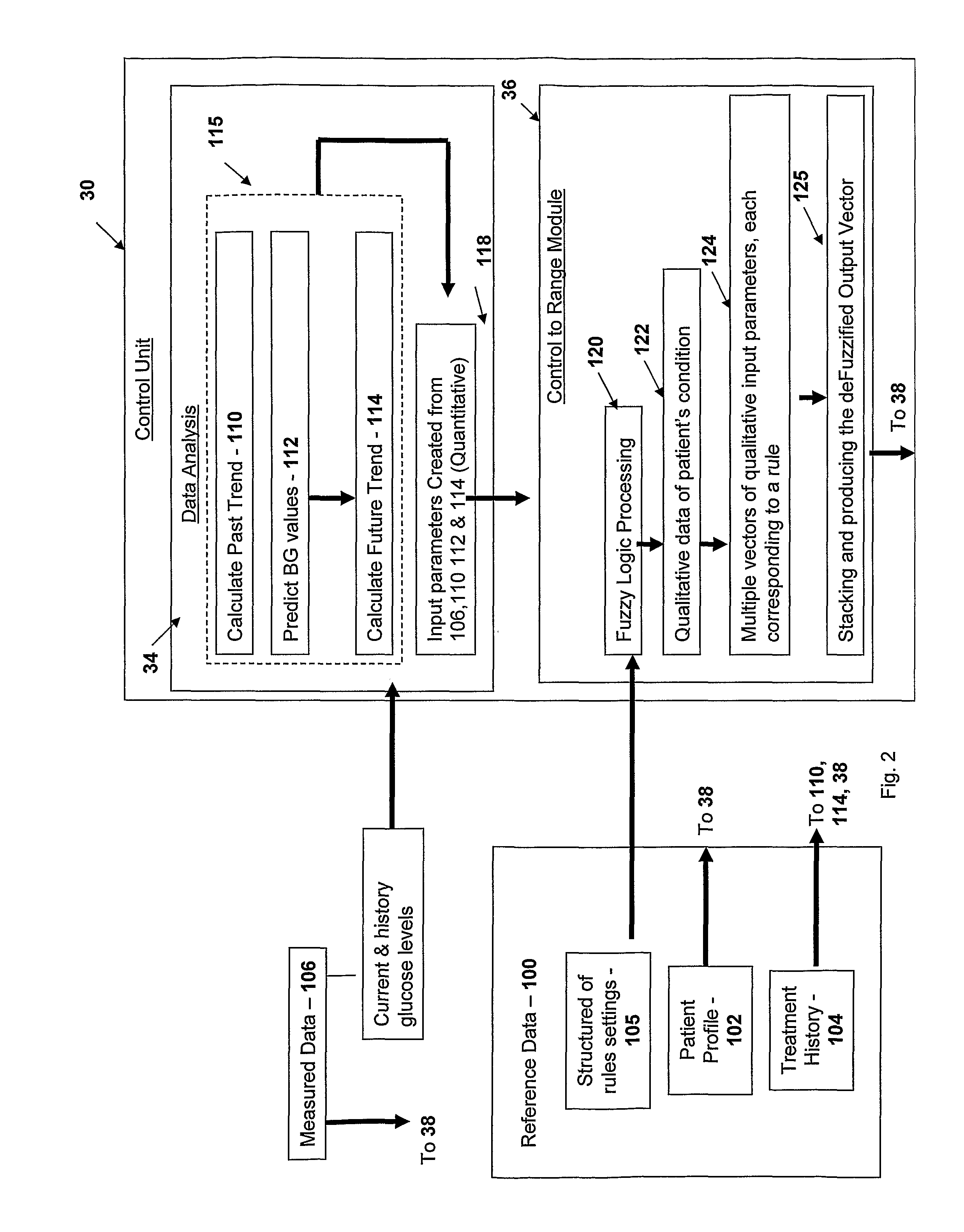
The present invention discloses a monitoring system and method for use in monitoring diabetes treatment of a patient. The system comprises a control unit comprising a first processor module for processing measured data indicative of blood glucose level and generating first processed data indicative thereof, a second processor module comprising at least one fuzzy logic module; the second processor module receives input parameters corresponding to the measured data, the first processed data and a reference data including individualized patient's profile related data, to individualized patient's treatment history related data and processes the received data to produce at least one qualitative output parameter indicative of patient's treatment parameters, such that the second processor module determines whether any of the treatment parameters is to be modified.
Owner:DREAMED DIABETES
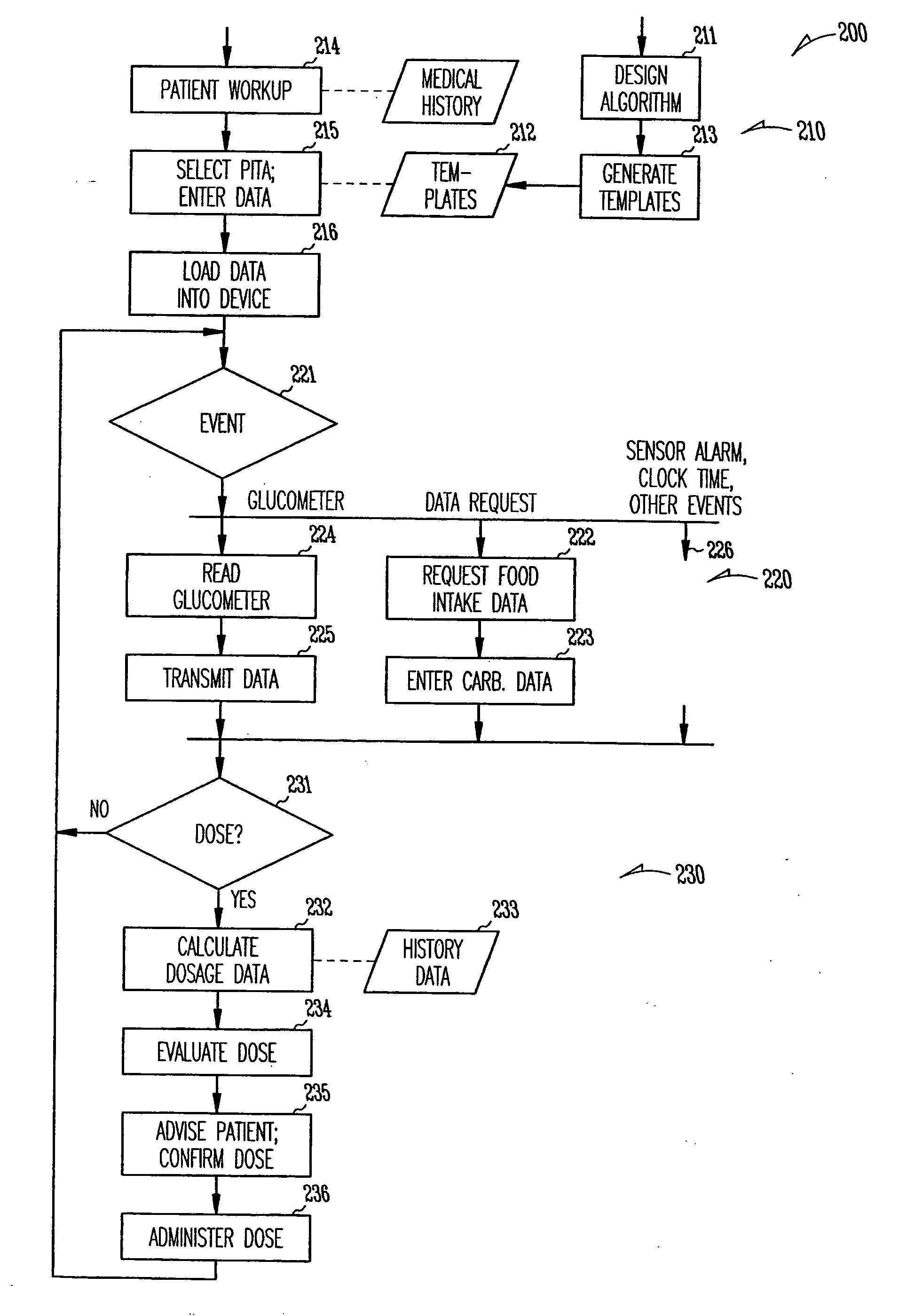
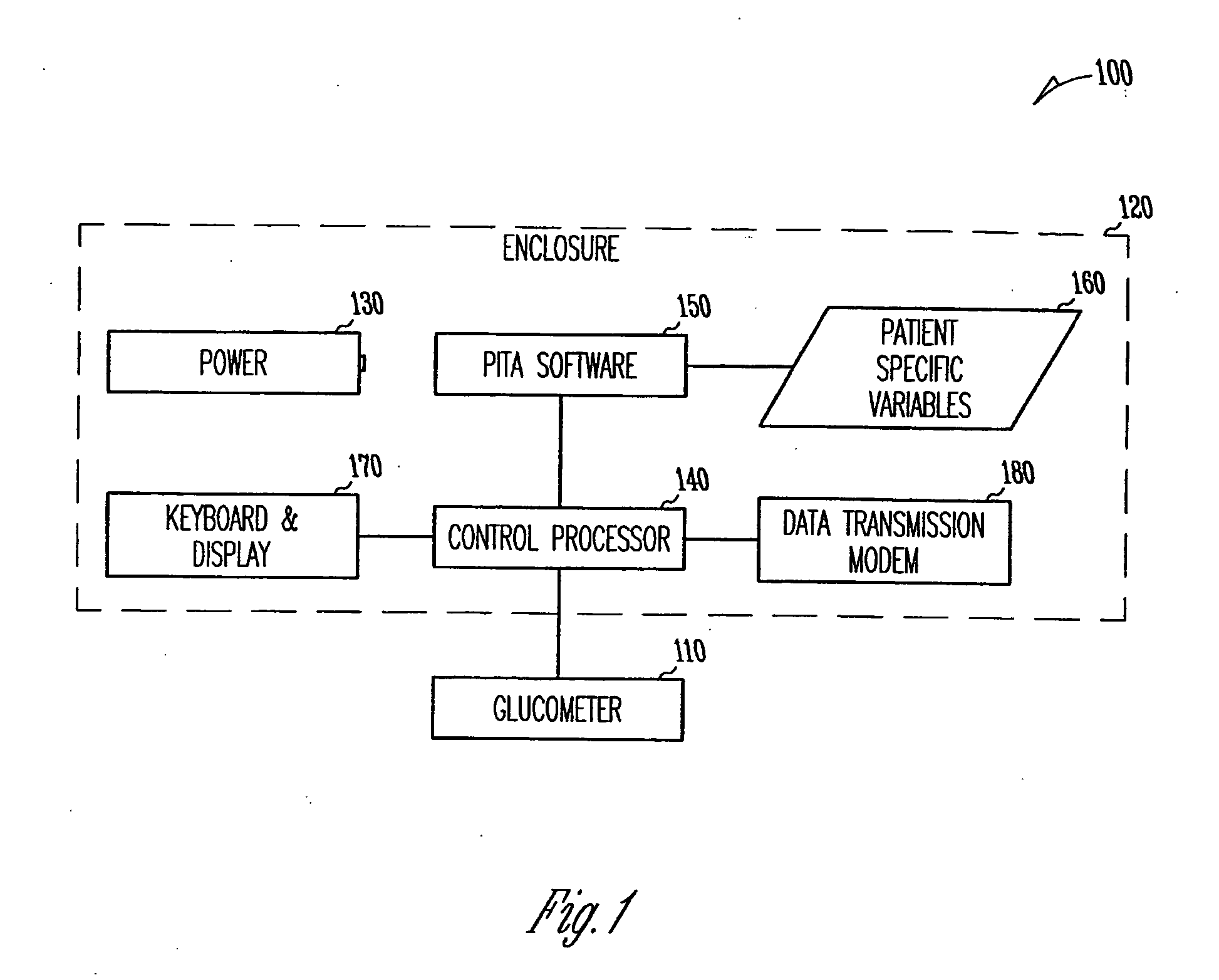
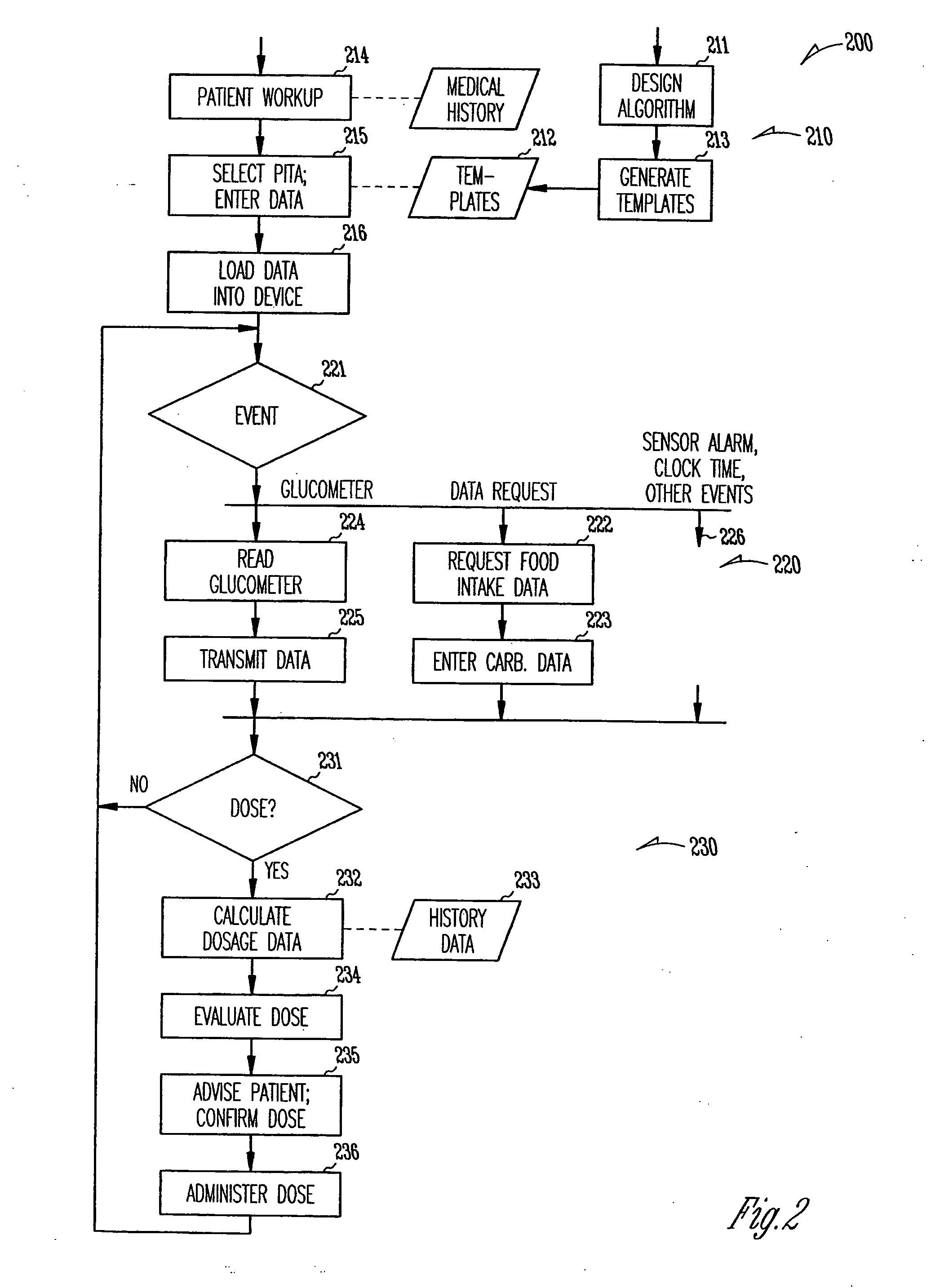
Patient management of diabetes treatment
InactiveUS20050197553A1Local control/monitoringDrug and medicationsPersonalizationPatient management
A portable diabetes management device calculates medication dosages from glucometer readings and data such as food intake entered by the patient, according to a management plan that can be personalized by a health-care professional using a template. The device may also store and communicate past data and plan revisions.
Owner:COOPER COLLEEN
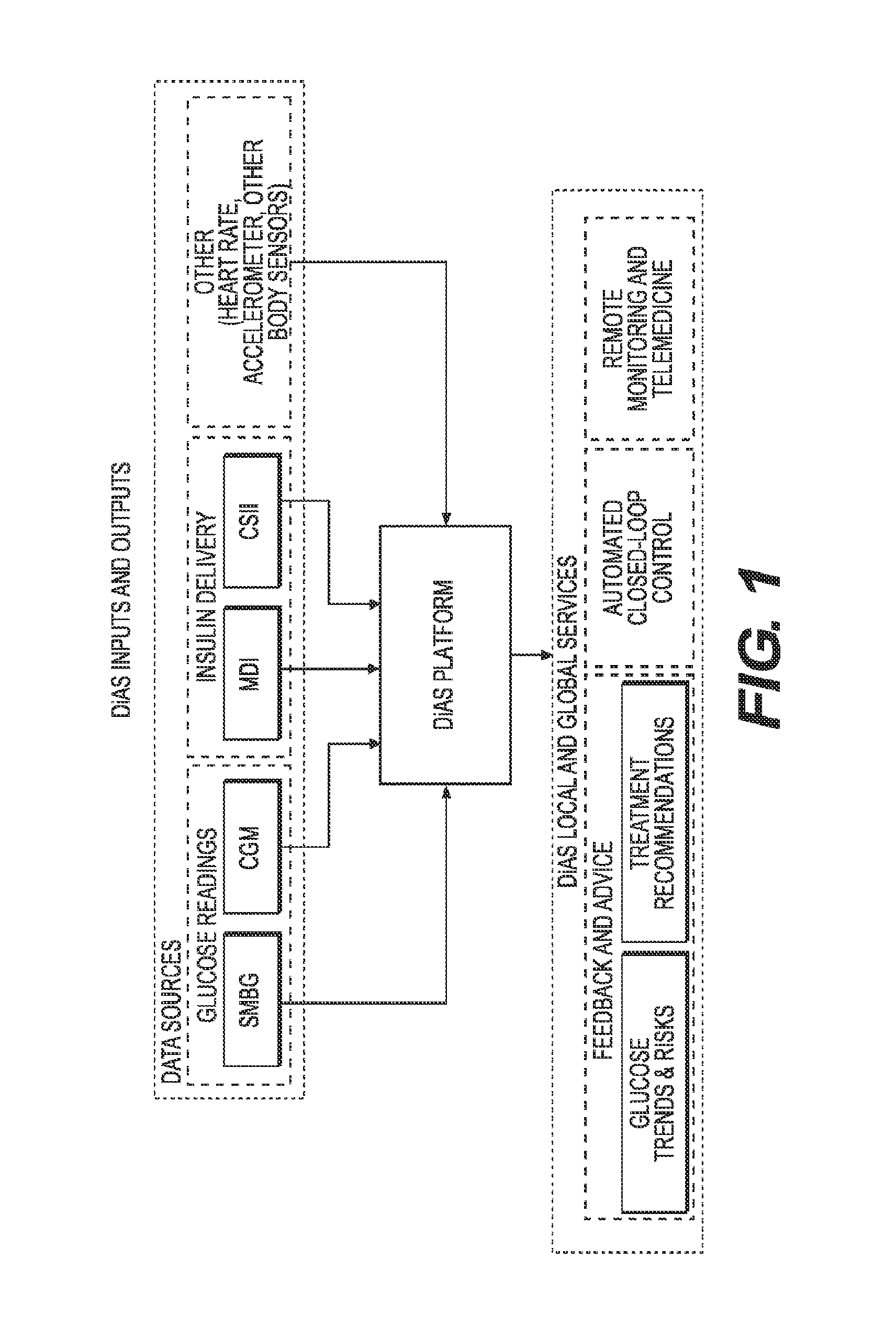
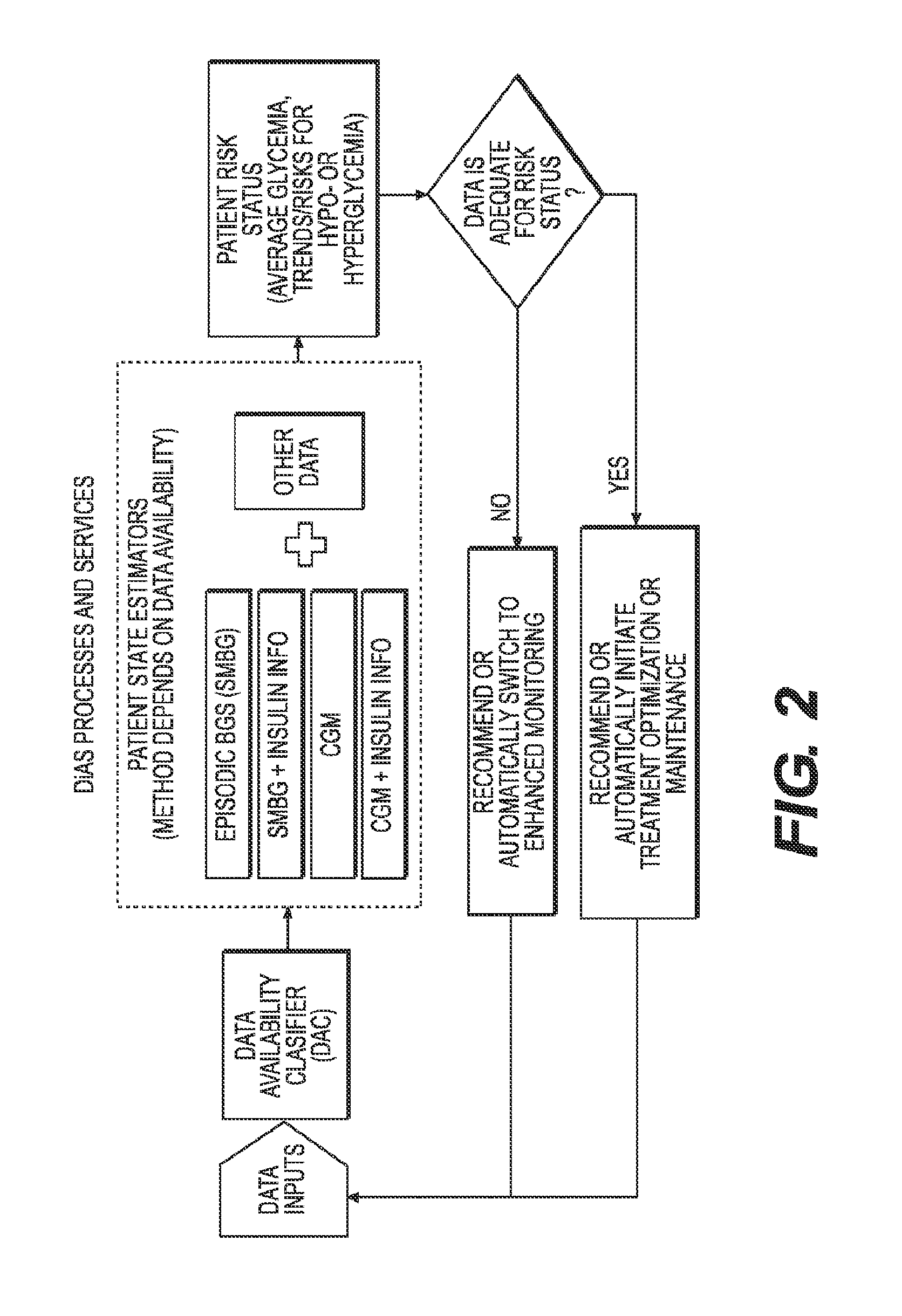
Unified Platform for Monitoring and Control of Blood Glucose Levels in Diabetic Patients
ActiveUS20150018633A1Easy to replaceGuaranteed uptimePeptide/protein ingredientsDrug and medicationsClosed loopSystem call
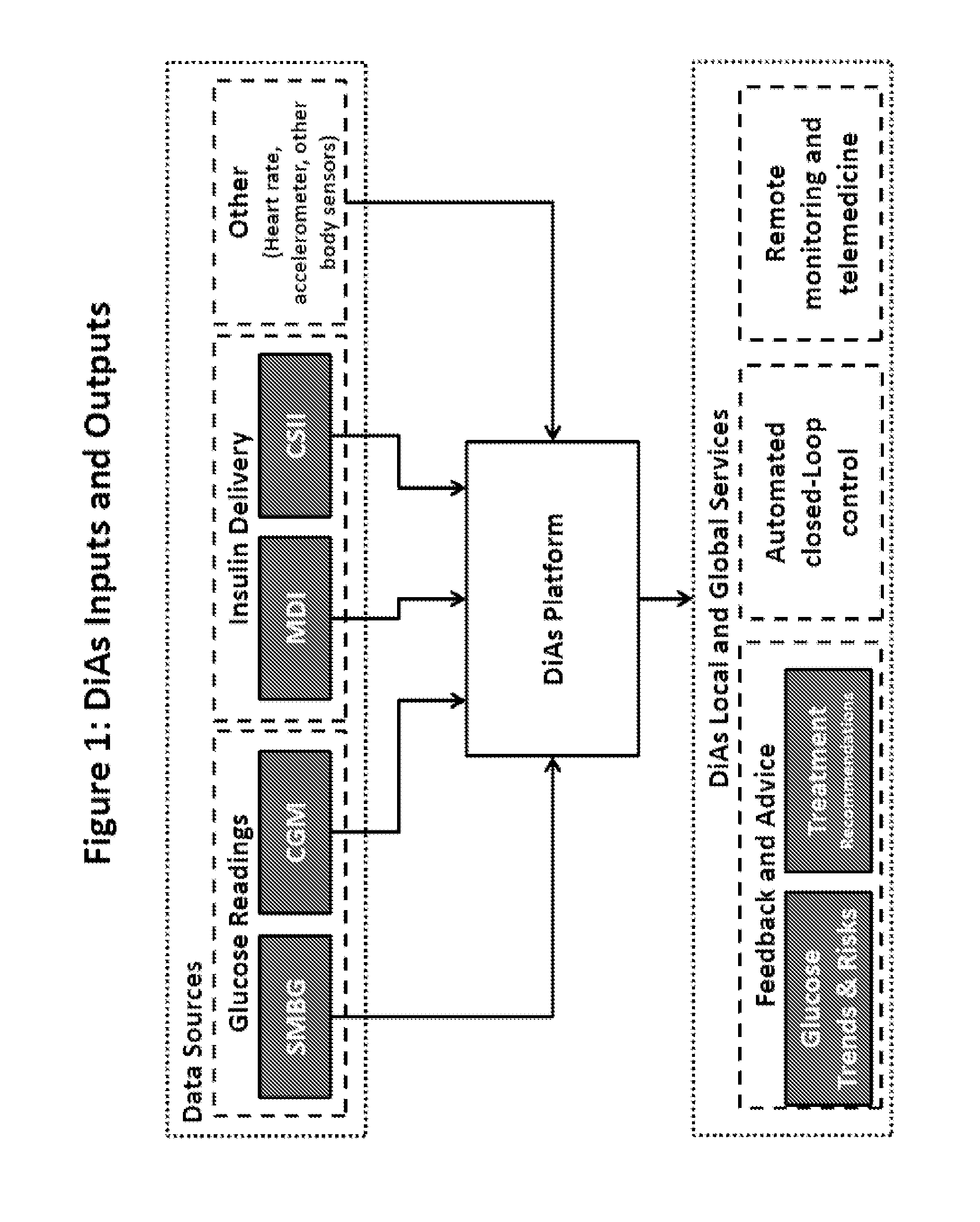
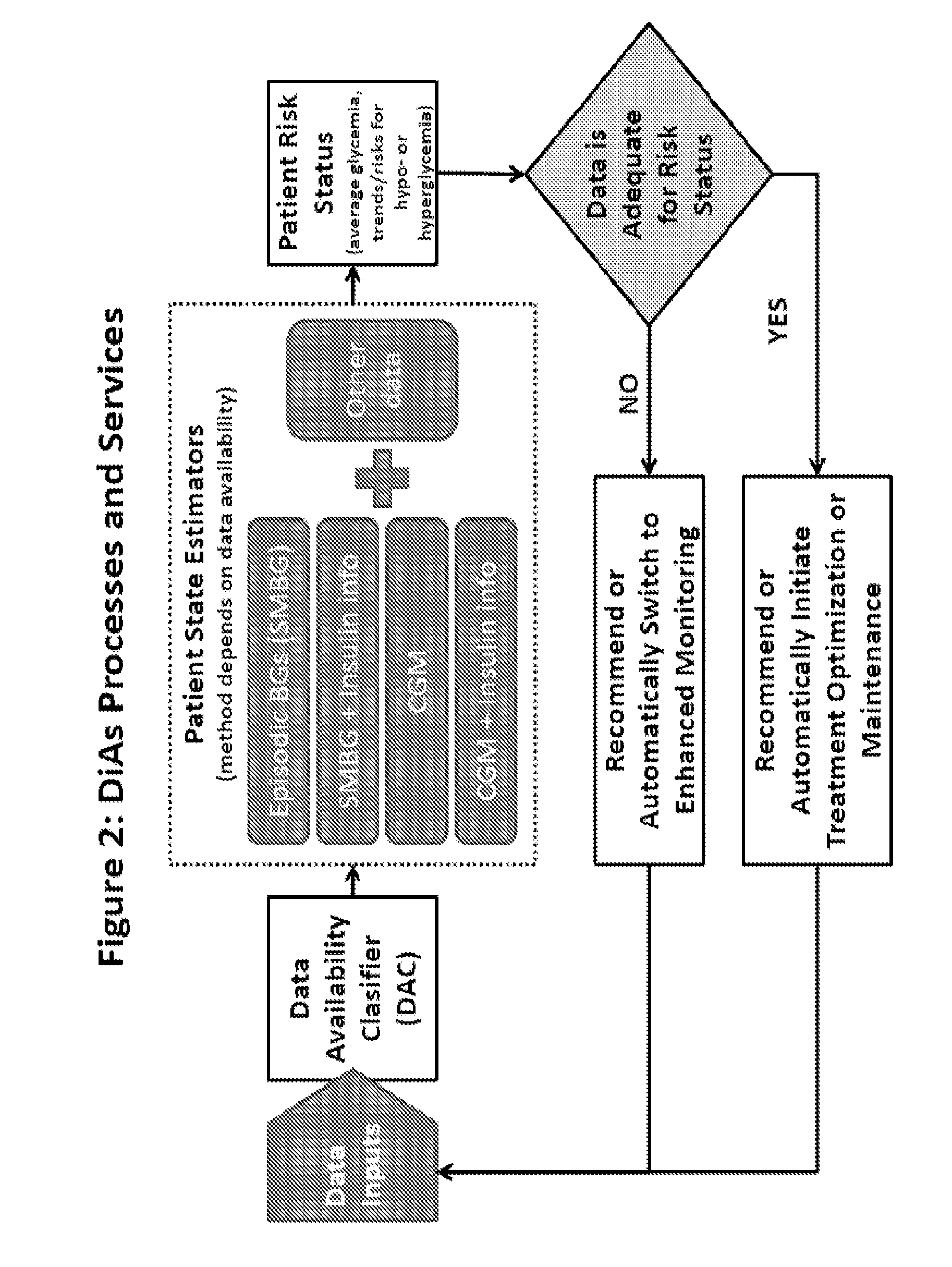
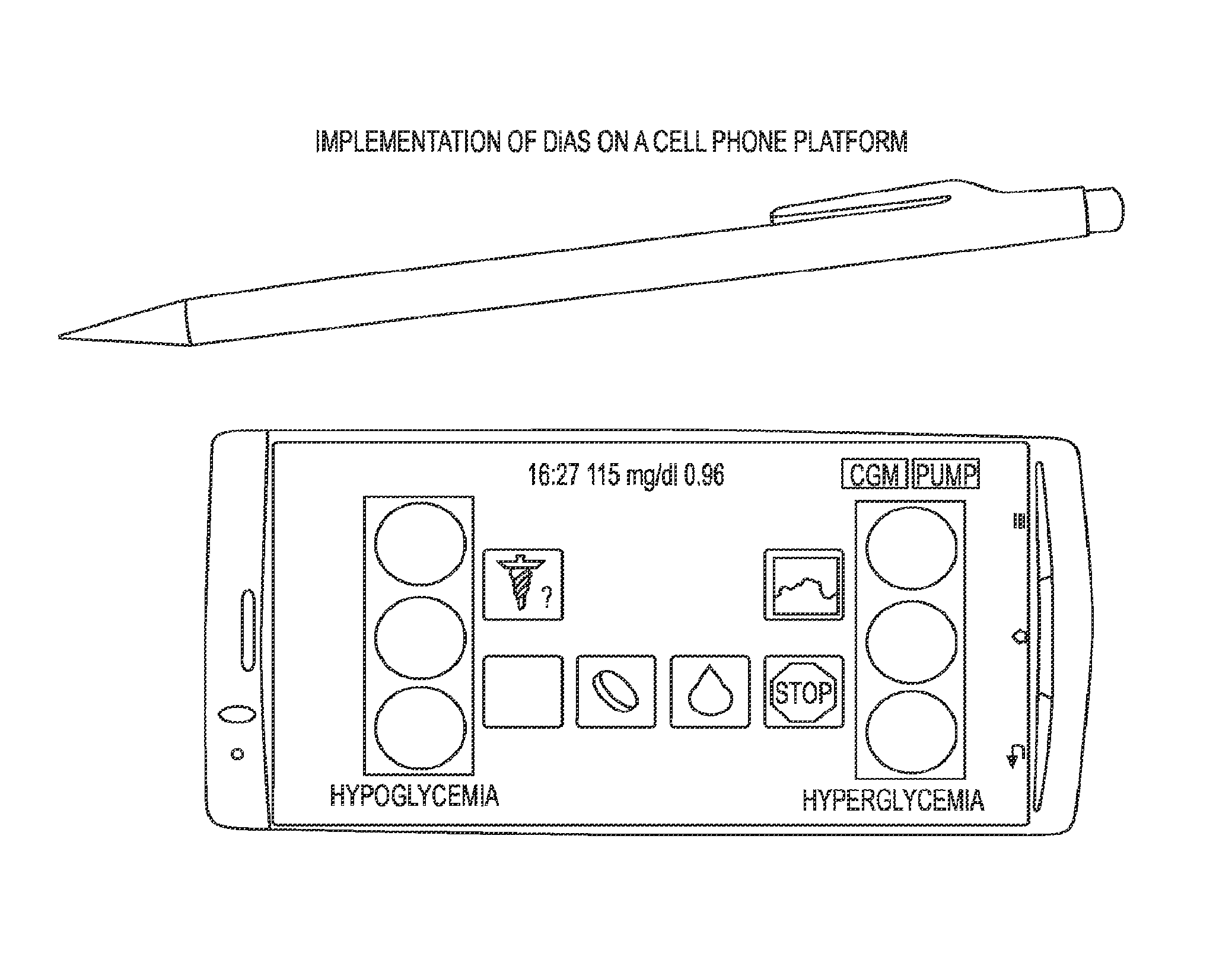
A flexible system capable of utilizing data from different monitoring techniques and capable of providing assistance to patients with diabetes at several scalable levels, ranging from advice about long-term trends and prognosis to real-time automated closed-loop control (artificial pancreas). These scalable monitoring and treatment strategies are delivered by a unified system called the Diabetes Assistant (DiAs) platform. The system provides a foundation for implementation of various monitoring, advisory, and automated diabetes treatment algorithms or methods. The DiAs recommendations are tailored to the specifics of an individual patient, and to the patient risk assessment at any given moment.
Owner:UNIV OF VIRGINIA ALUMNI PATENTS FOUND
Superior control of blood glucose in diabetes treatment
InactiveUS20060239934A1Easy to controlReduce riskOrganic active ingredientsPowder deliveryPostprandial HypoglycemiaGlucose fluctuations
Methods related to the treatment of diabetes and improving the control of blood glucose levels are provided. In particular, methods are provided for effectively reducing postprandial glucose excursions while reducing the incidence of clinically significant late postprandial hypoglycemia by administered an insulin composition in a form suitable for pulmonary administration. Additionally, methods for effectively reducing post-prandial glucose excursions while reducing the incidence of clinically significant late postprandial hypoglycemia by administered an insulin composition in a form suitable for pulmonary administration along with a long-acting basal insulin.
Owner:MANNKIND CORP
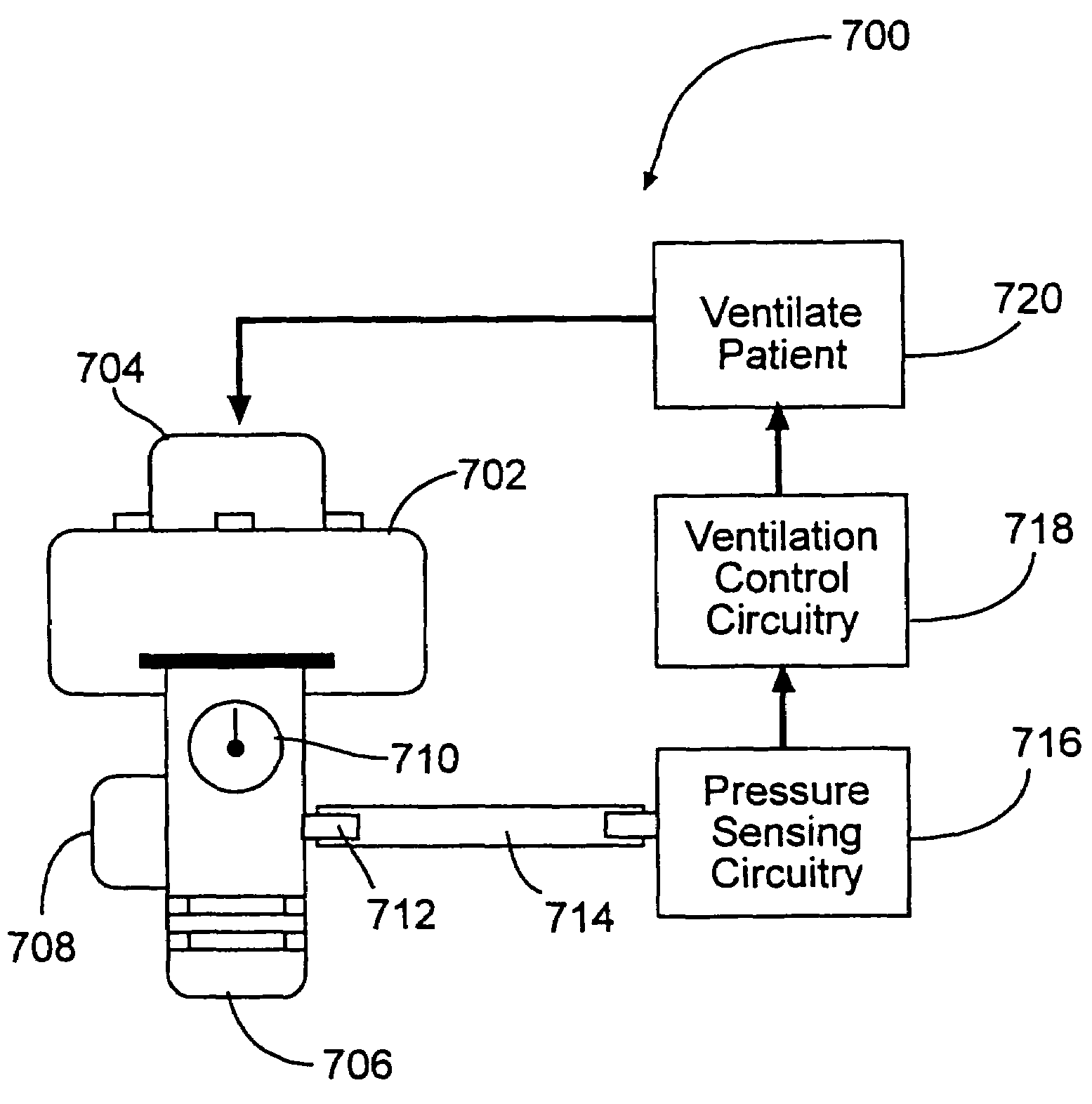
Diabetes treatment systems and methods
InactiveUS7204251B2Increase loopDecrease and prevent flowCompressorElectrotherapyHeart rightInhalation
Owner:ZOLL MEDICAL CORPORATION
Monitoring device for management of insulin delivery
ActiveUS20120246106A1Minimize changesDrug and medicationsMedical devicesInsulin activityTreatment management
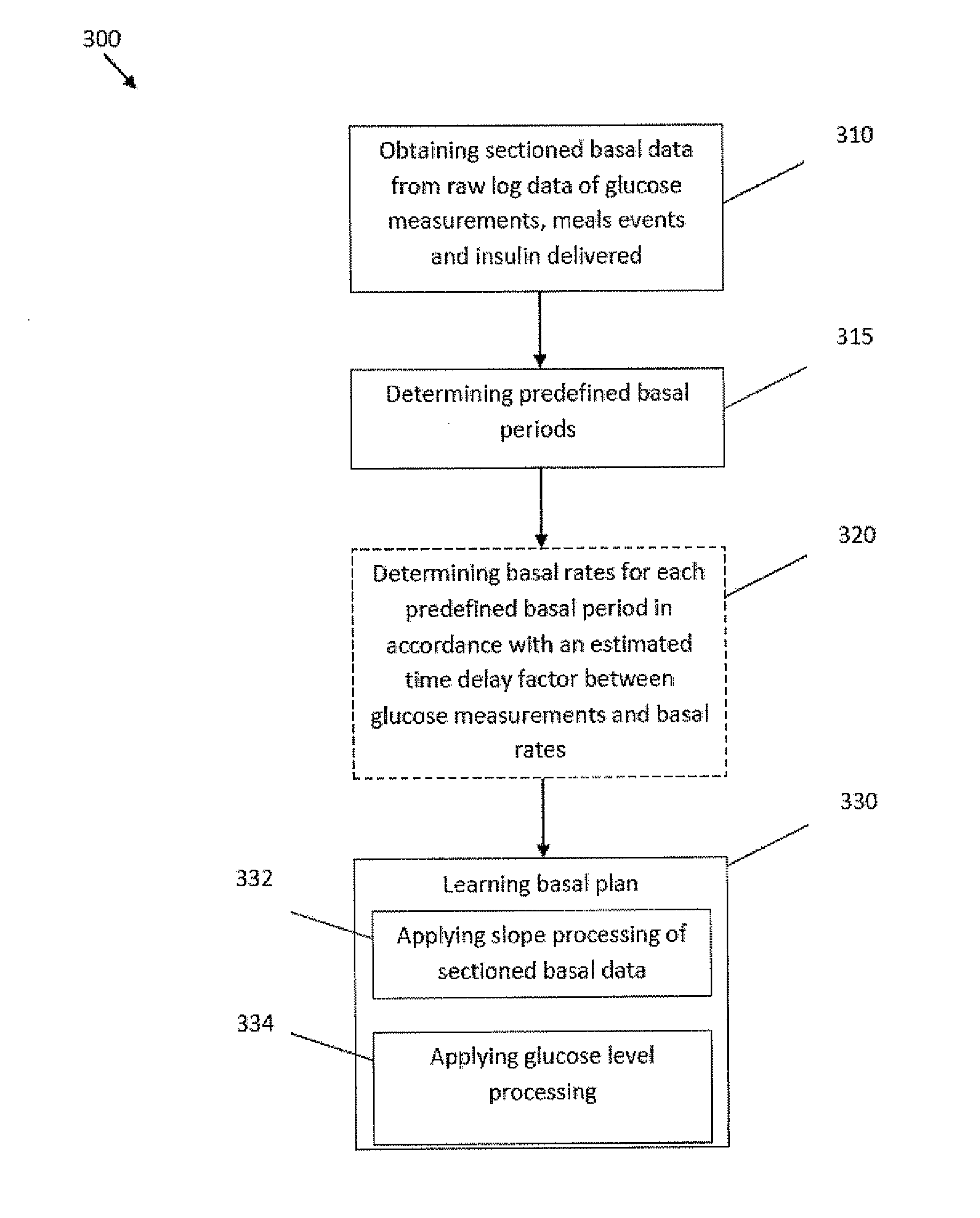
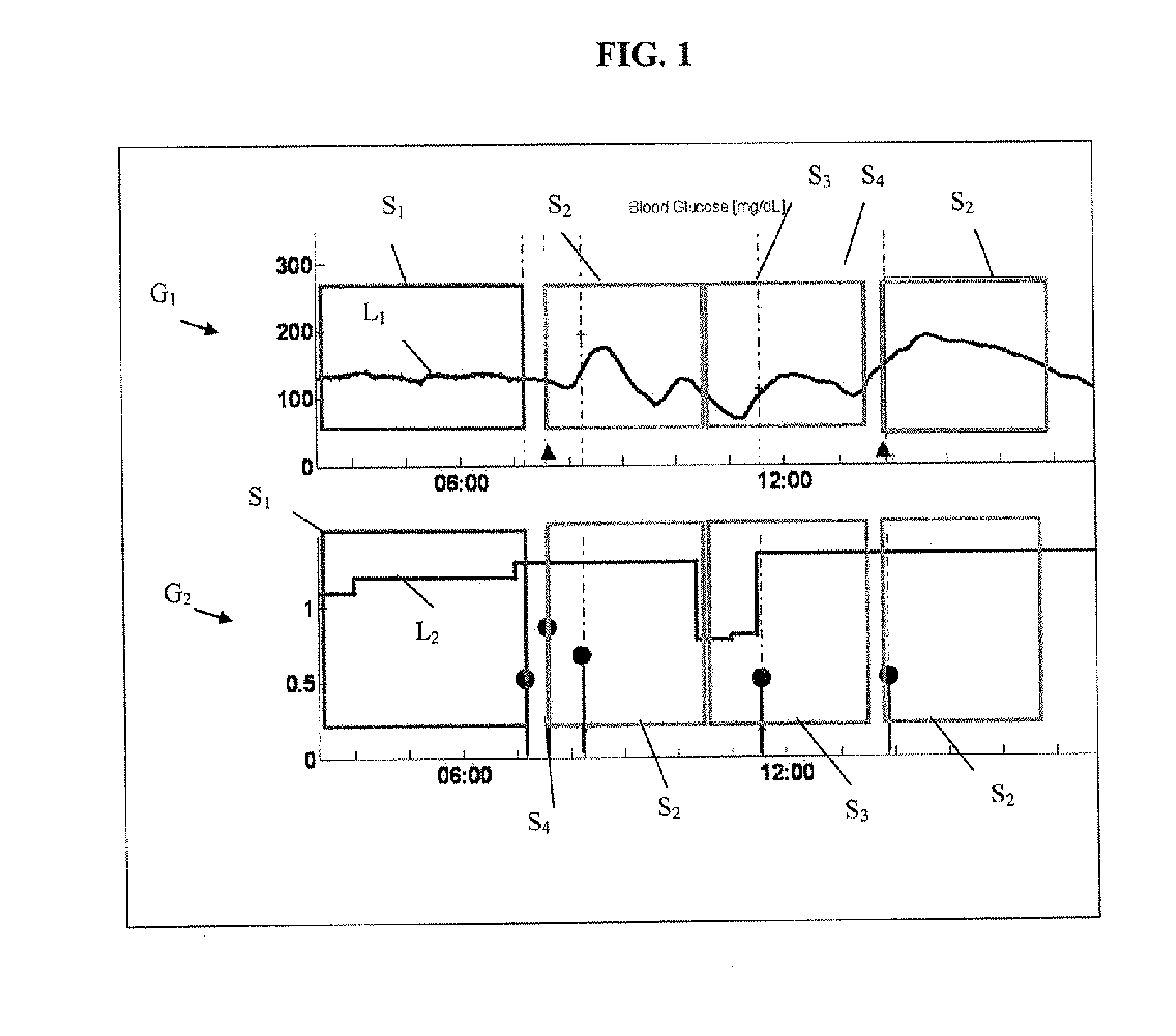
Monitoring system and method for use with diabetic treatment management. The system includes: a communication interface configured to permit access to stored raw log data, obtained over a certain time, being indicative of glucose measurements, meals consumed and insulin delivery; and a control unit including an unsupervised learning controller configured to receive and process said raw log data and determine at least one global insulin pump setting of basal rate, correction factor, carbohydrate ratio and insulin activity curve parameters. The system may include a processing unit including a first processor for processing measured data indicative of blood glucose level and generating first processed data, a second processor including at least one fuzzy logic module which receives input parameters corresponding to the measured data, the first processed data and a reference data, and processes the data to produce a qualitative output parameter to determine whether any treatment parameter should be modified.
Owner:DREAMED DIABETES
Diabetes therapy
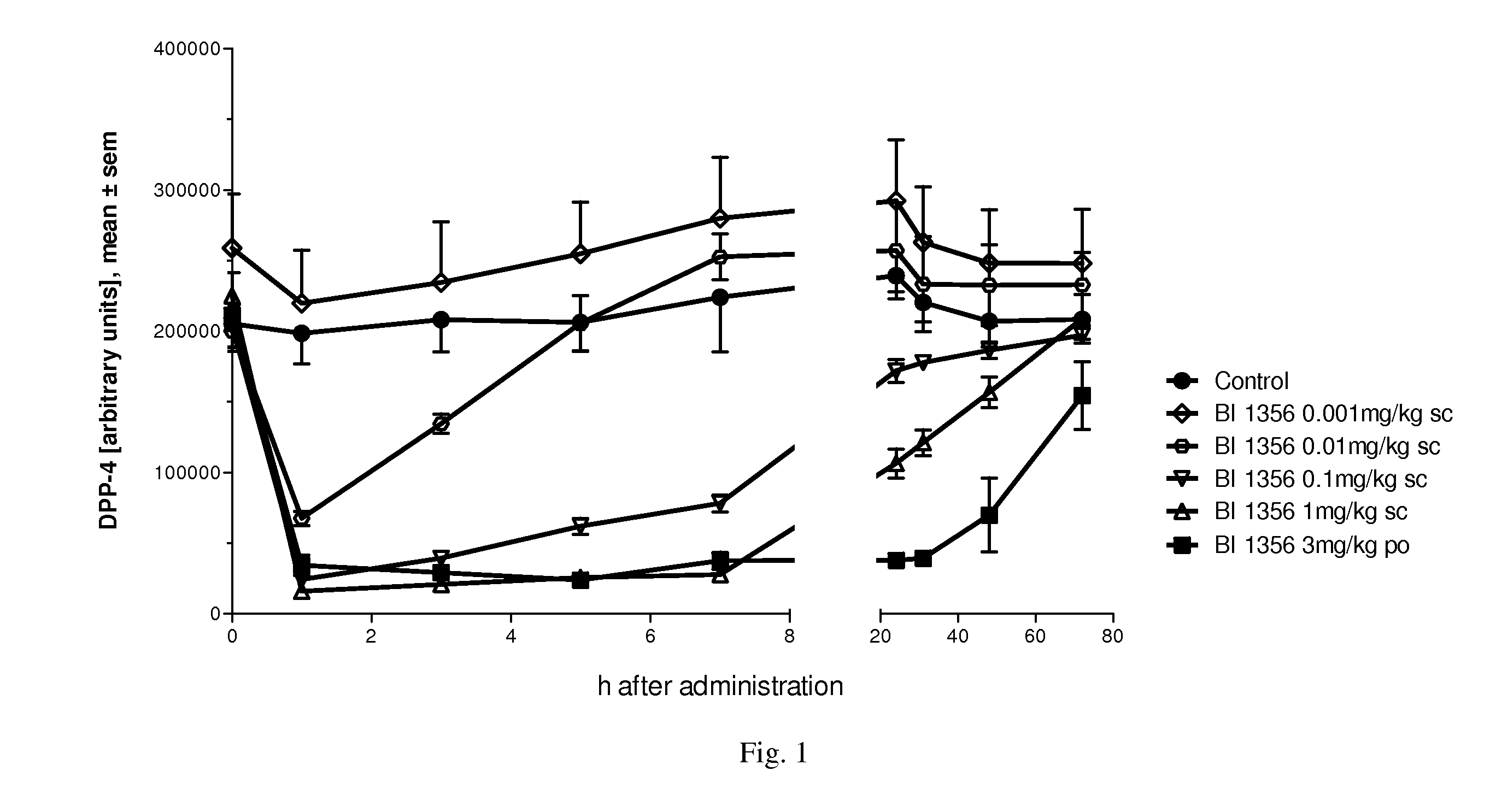
The present invention relates to methods for treating and / or preventing metabolic diseases comprising the combined administration of a DPP-4 inhibitor and a long-acting insulin. The invention further relates to a DPP-4 inhibitor for subcutaneous or transdermal use.
Owner:BOEHRINGER INGELHEIM INT GMBH
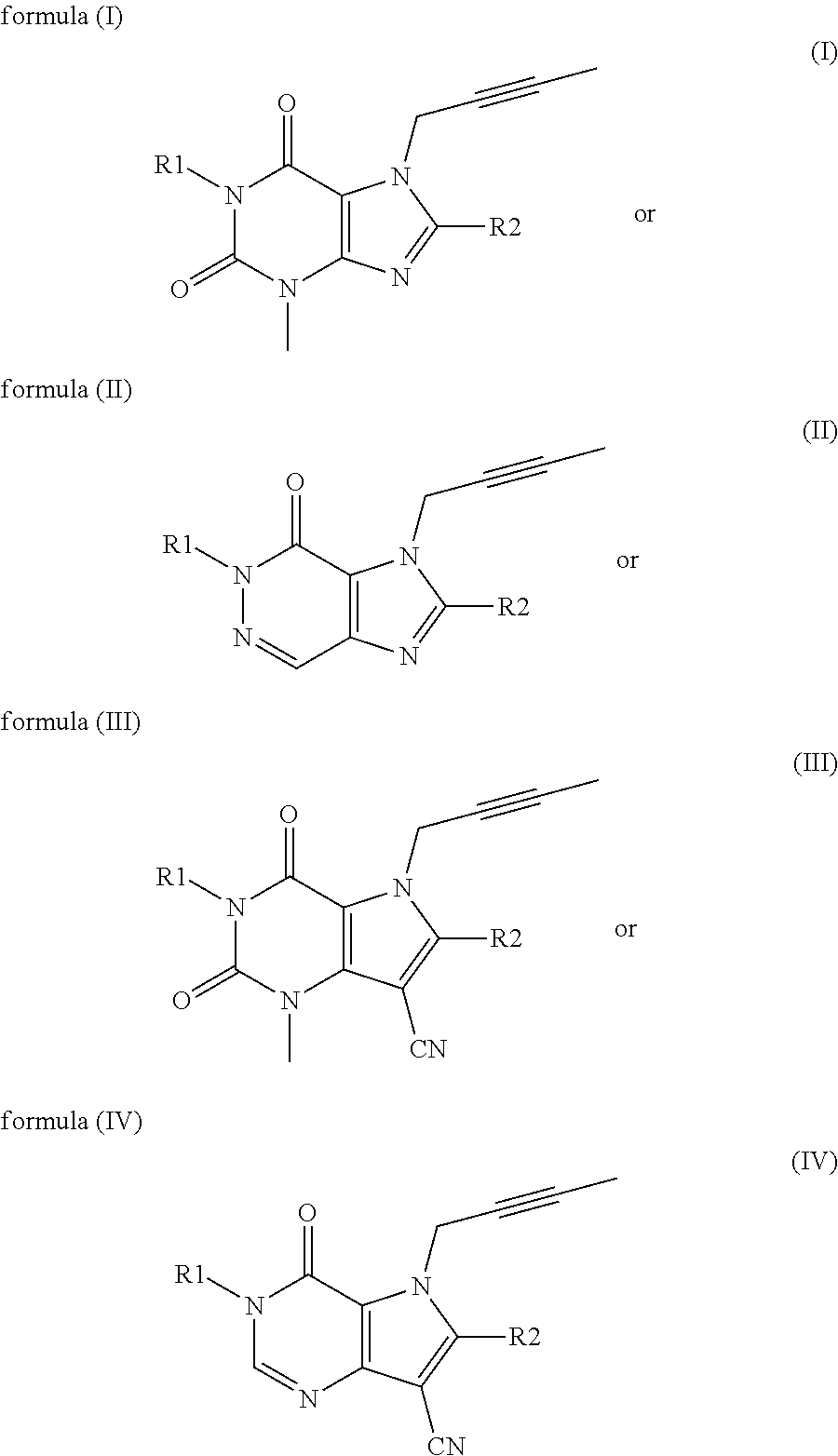
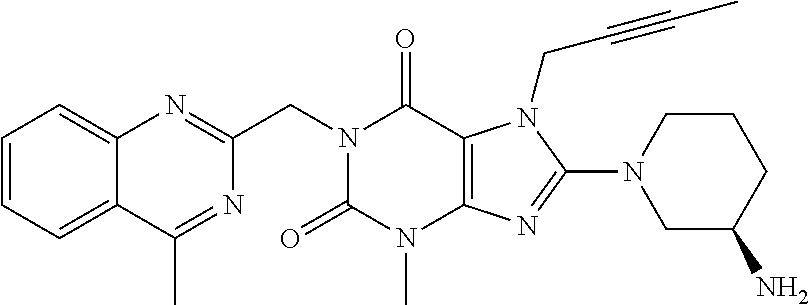
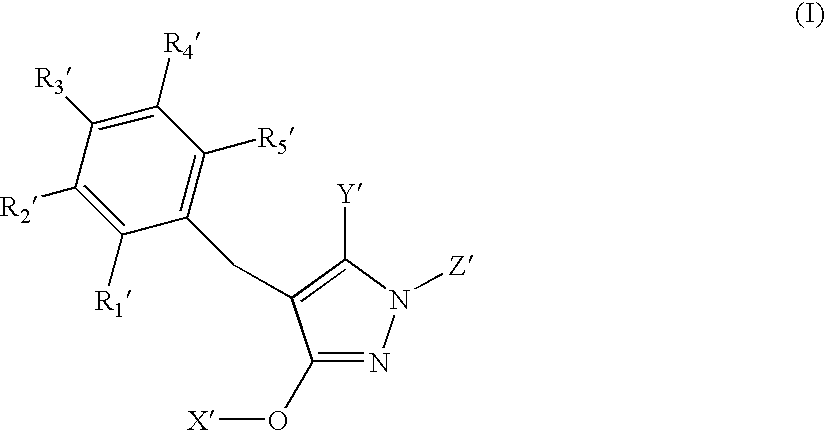
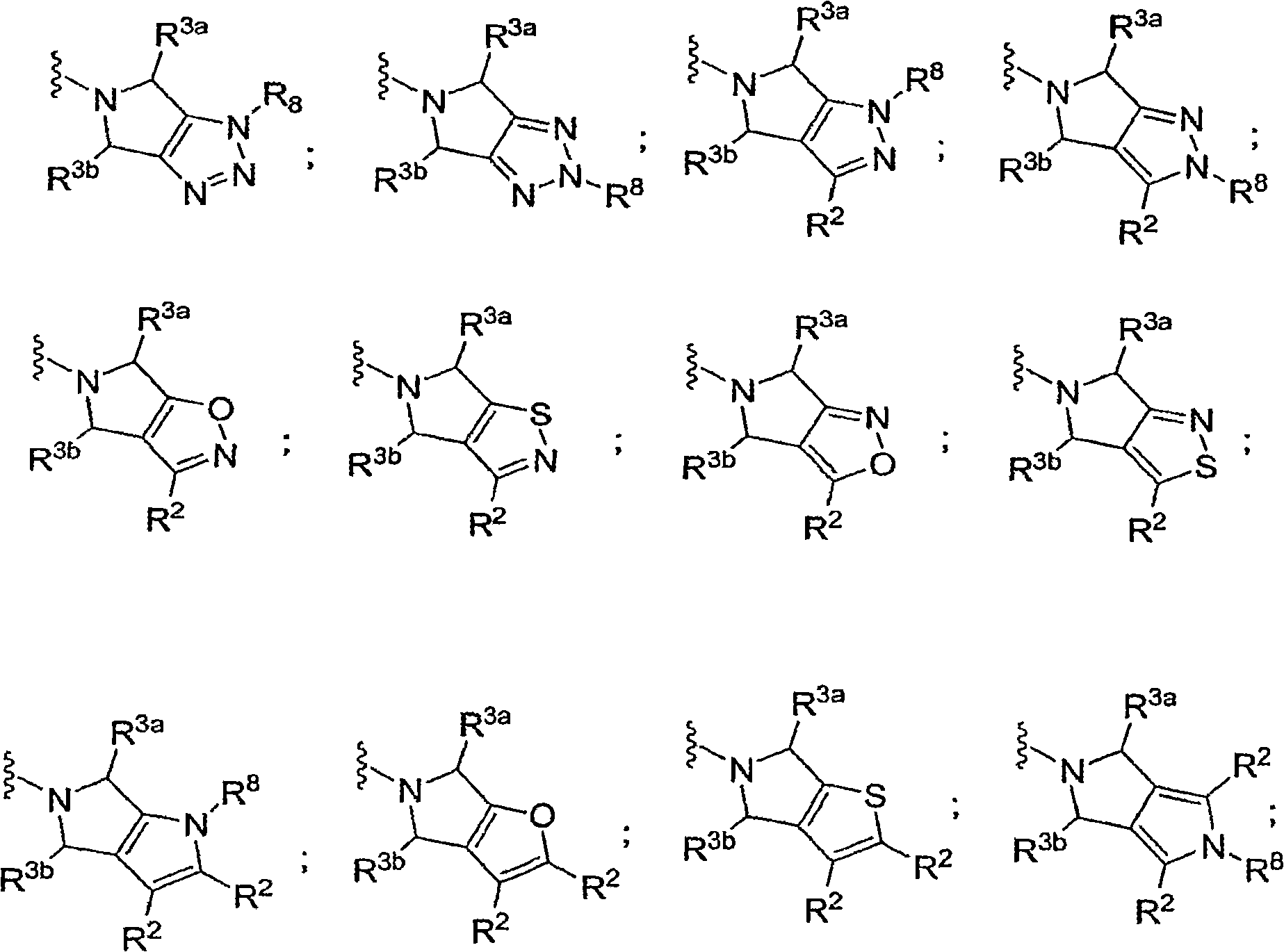
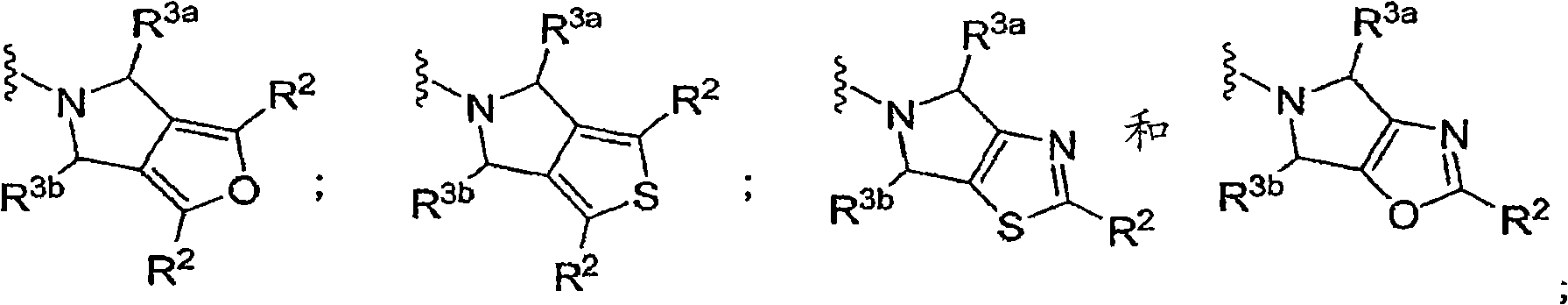
N-substituted pyrazole-O-glycoside derivatives and therapeutic agent for diabetes containing the same
The present invention relates to pyrazole derivatives represented by the following formulas and analogues thereof, which can be used for a therapeutic agent for diabetes.
Owner:AJINOMOTO CO INC
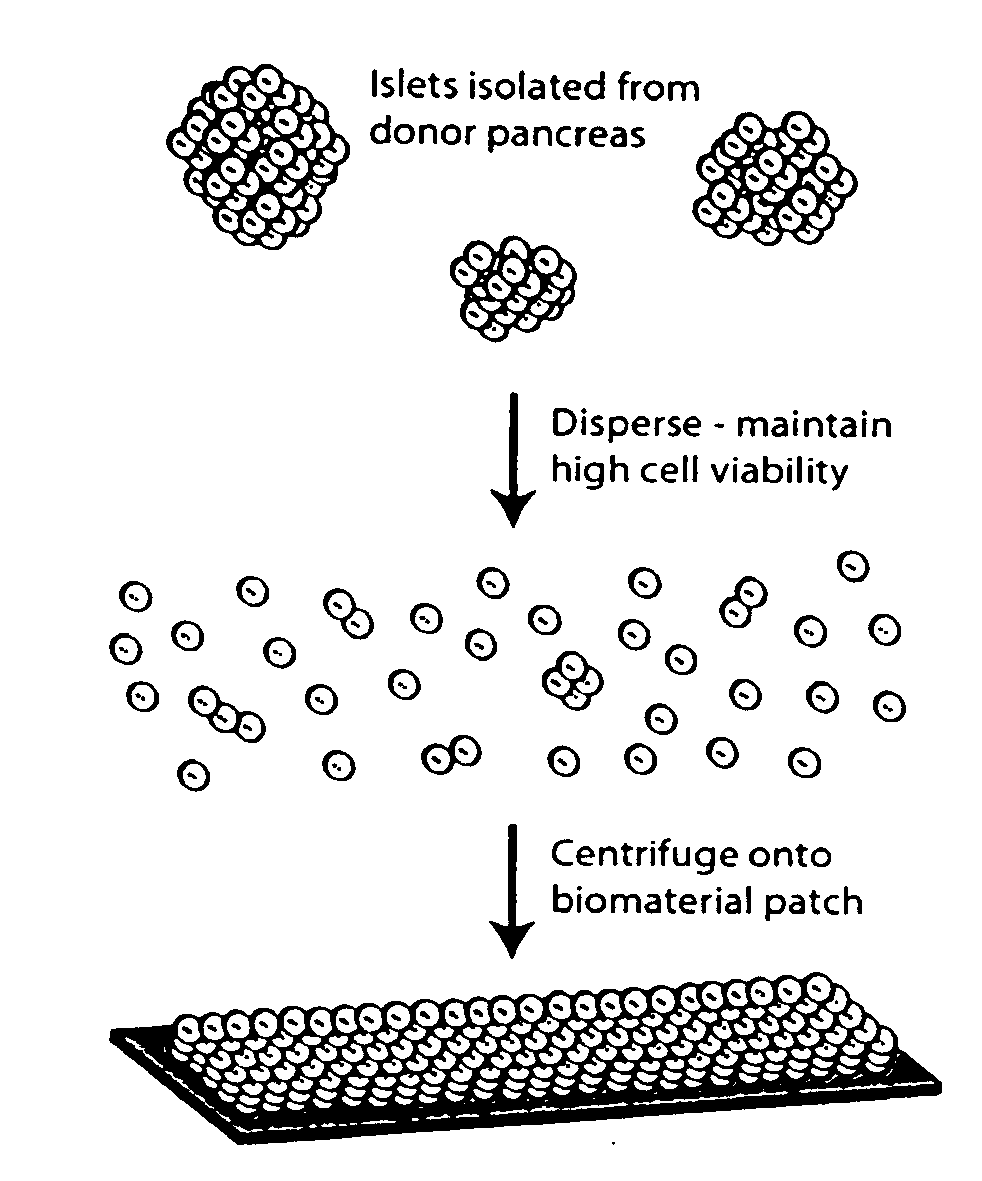
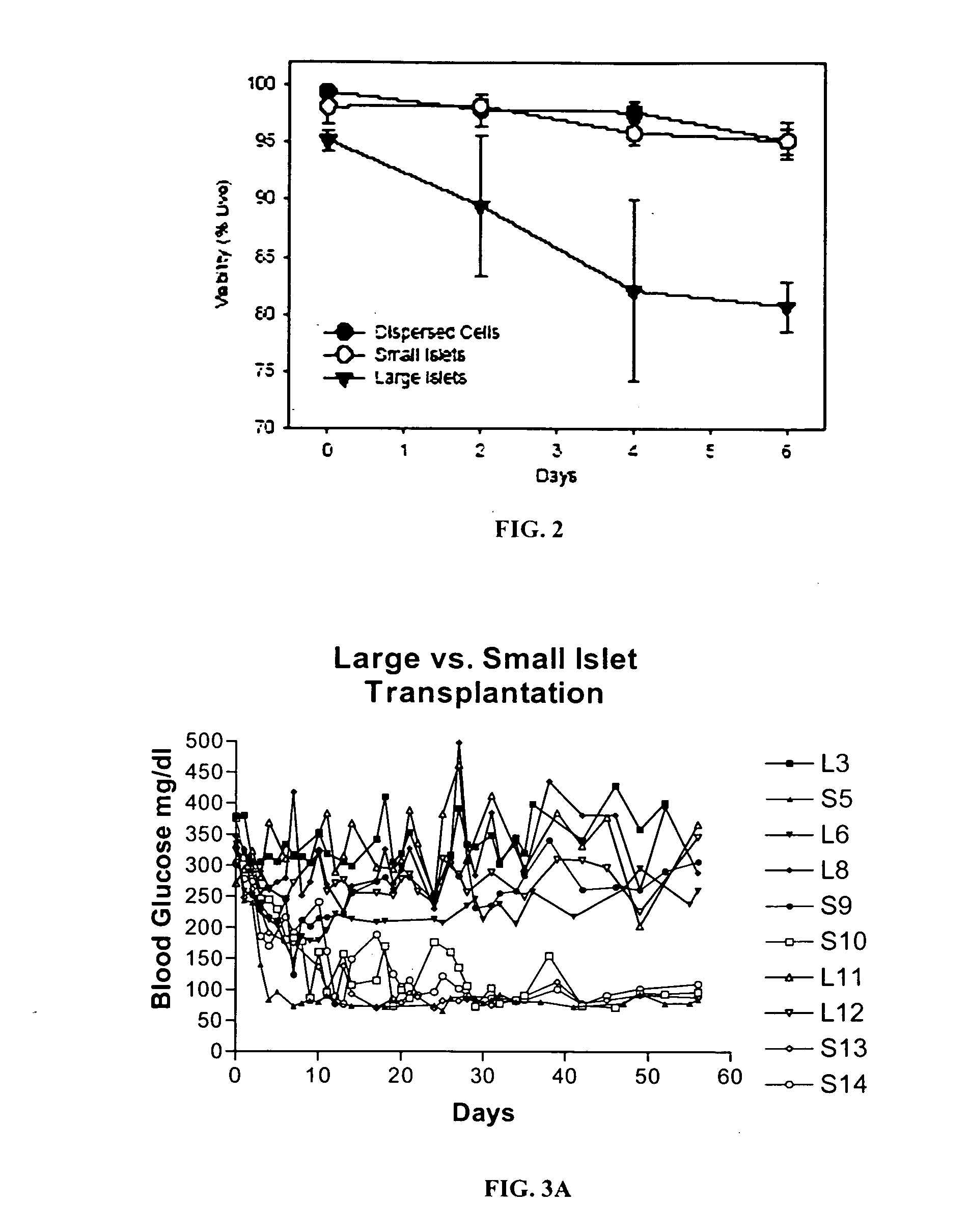
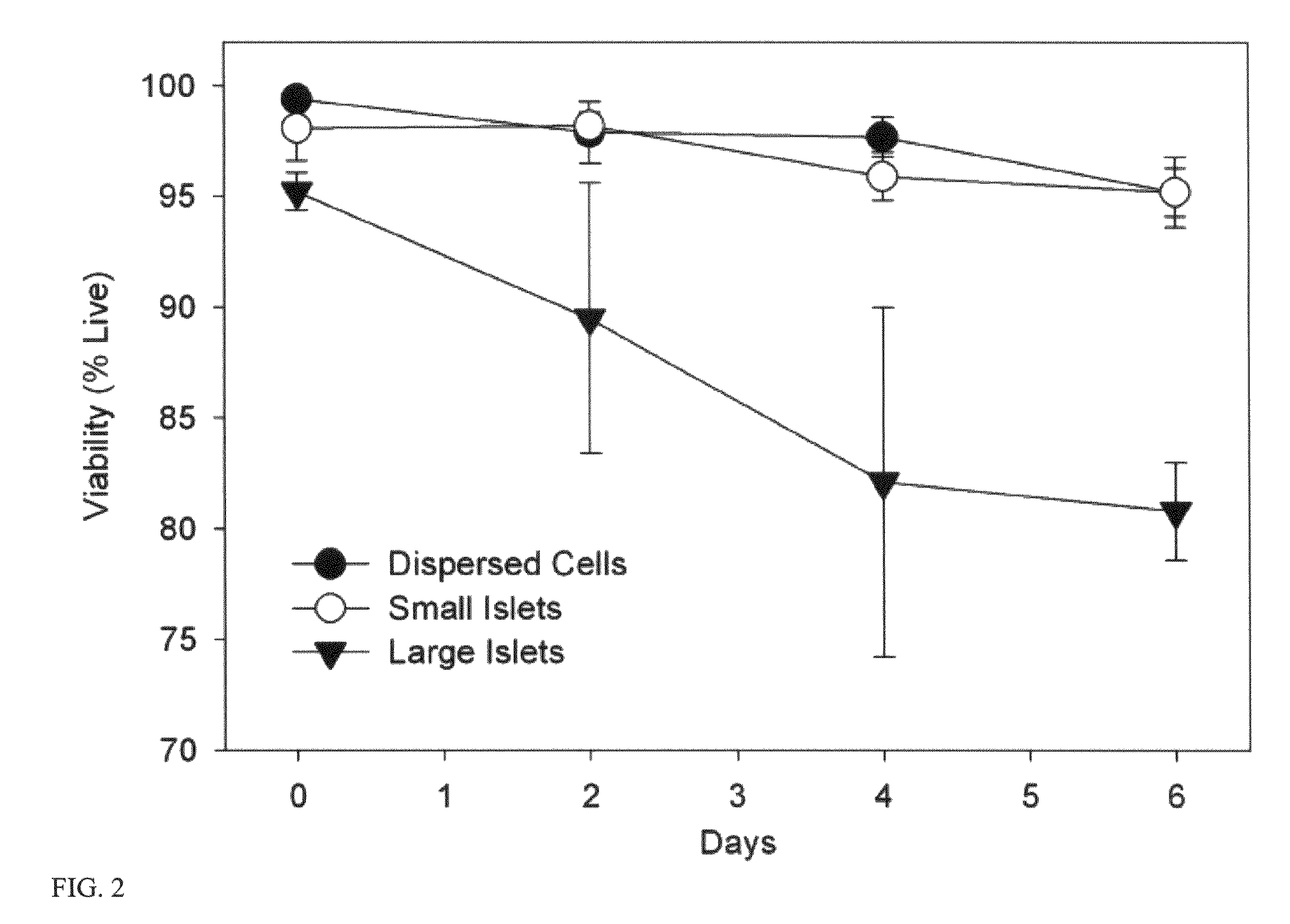
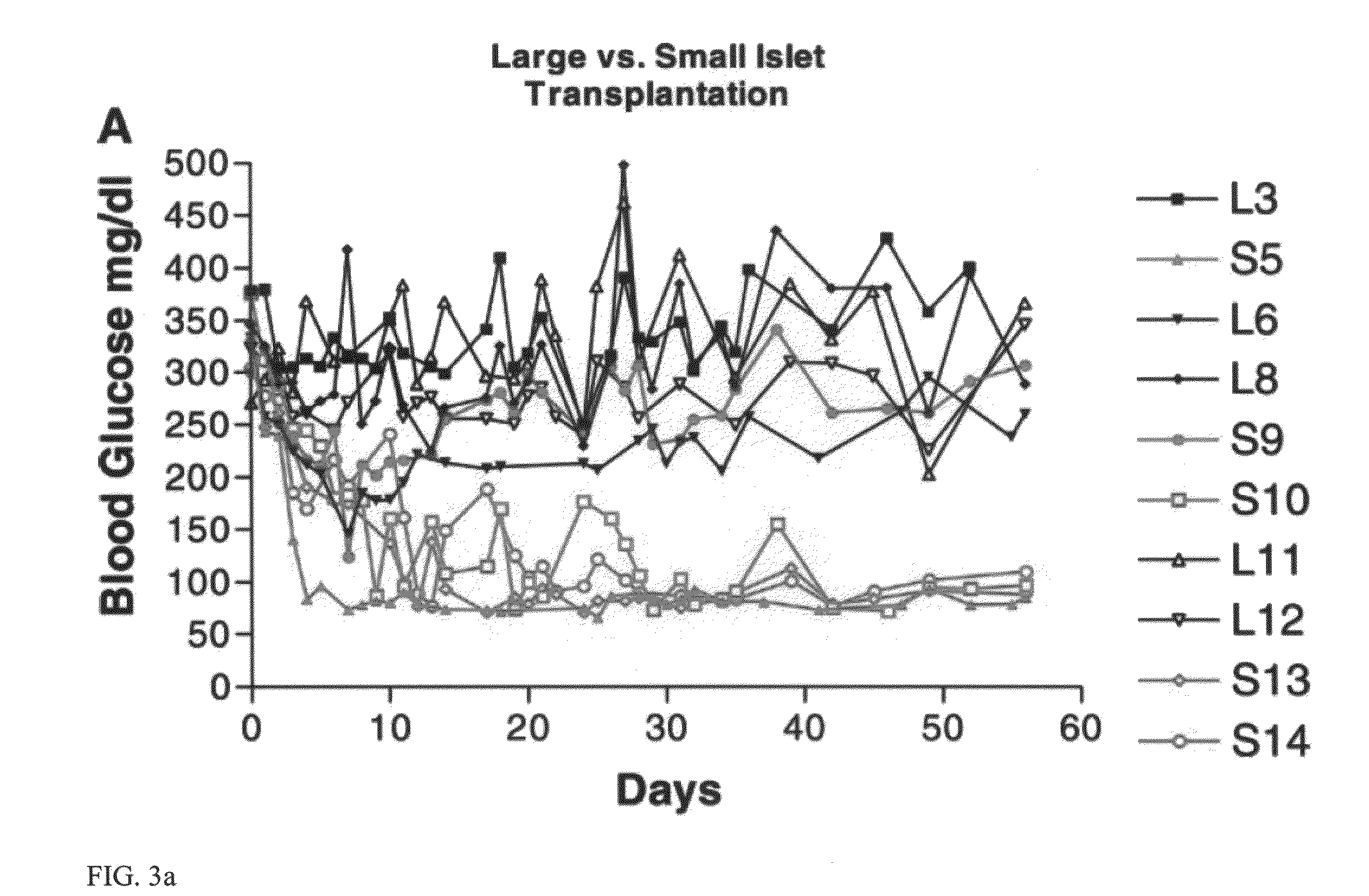
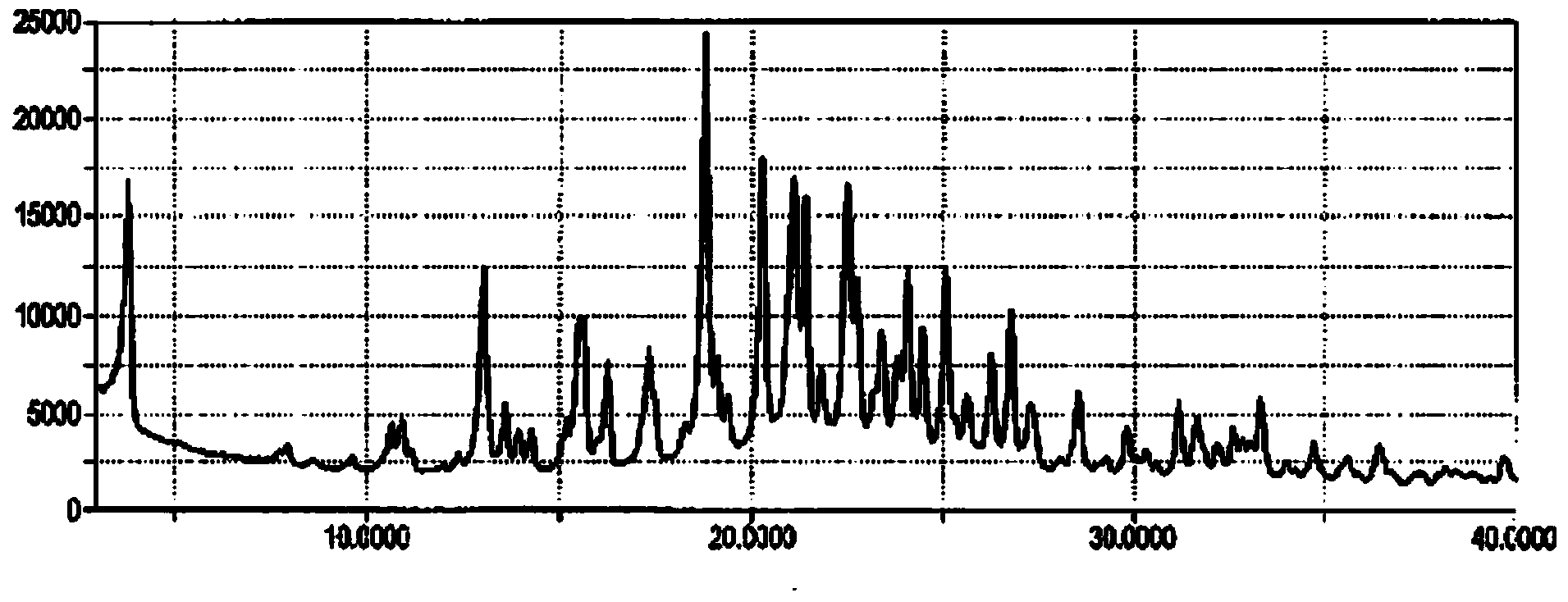
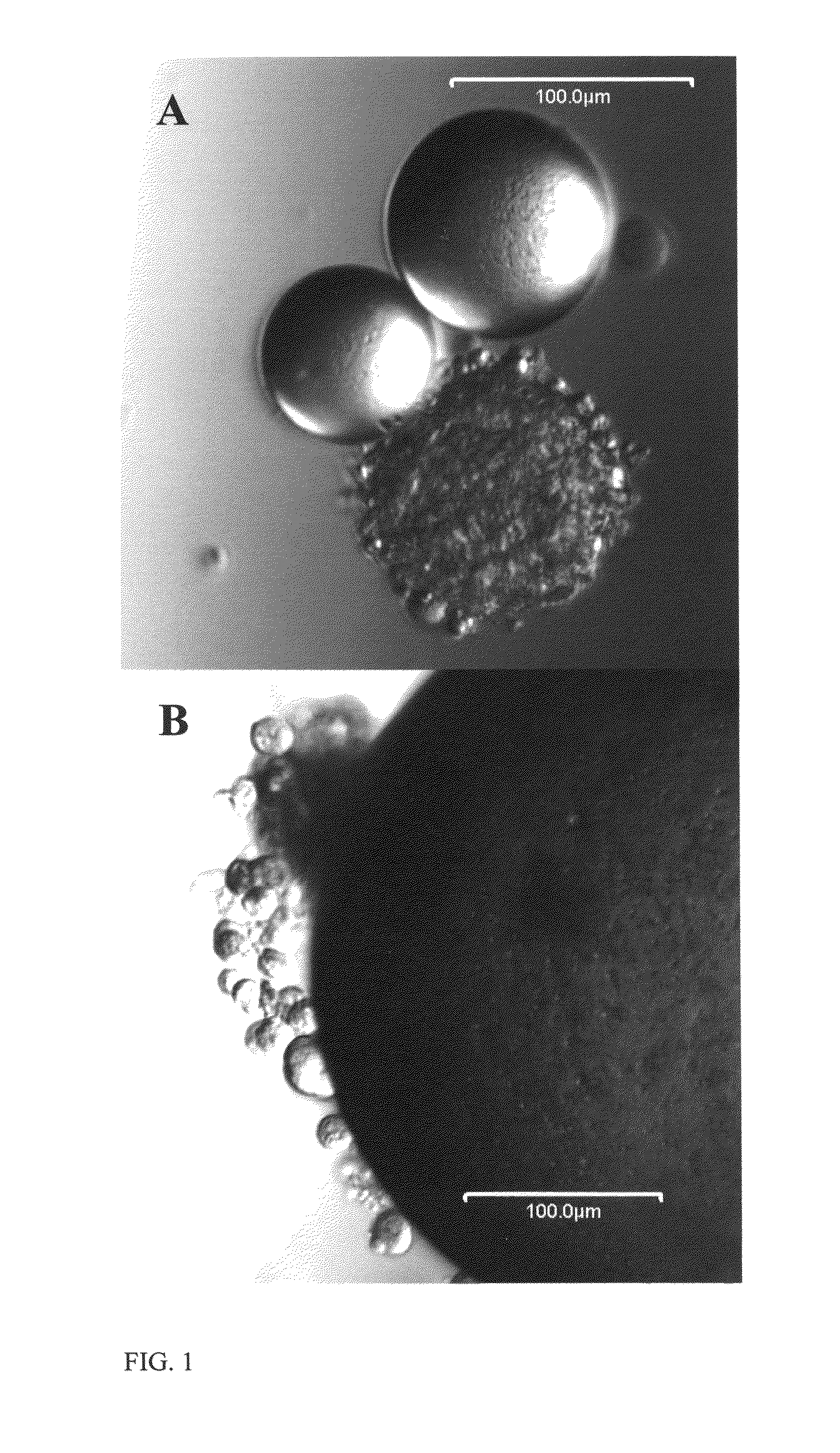
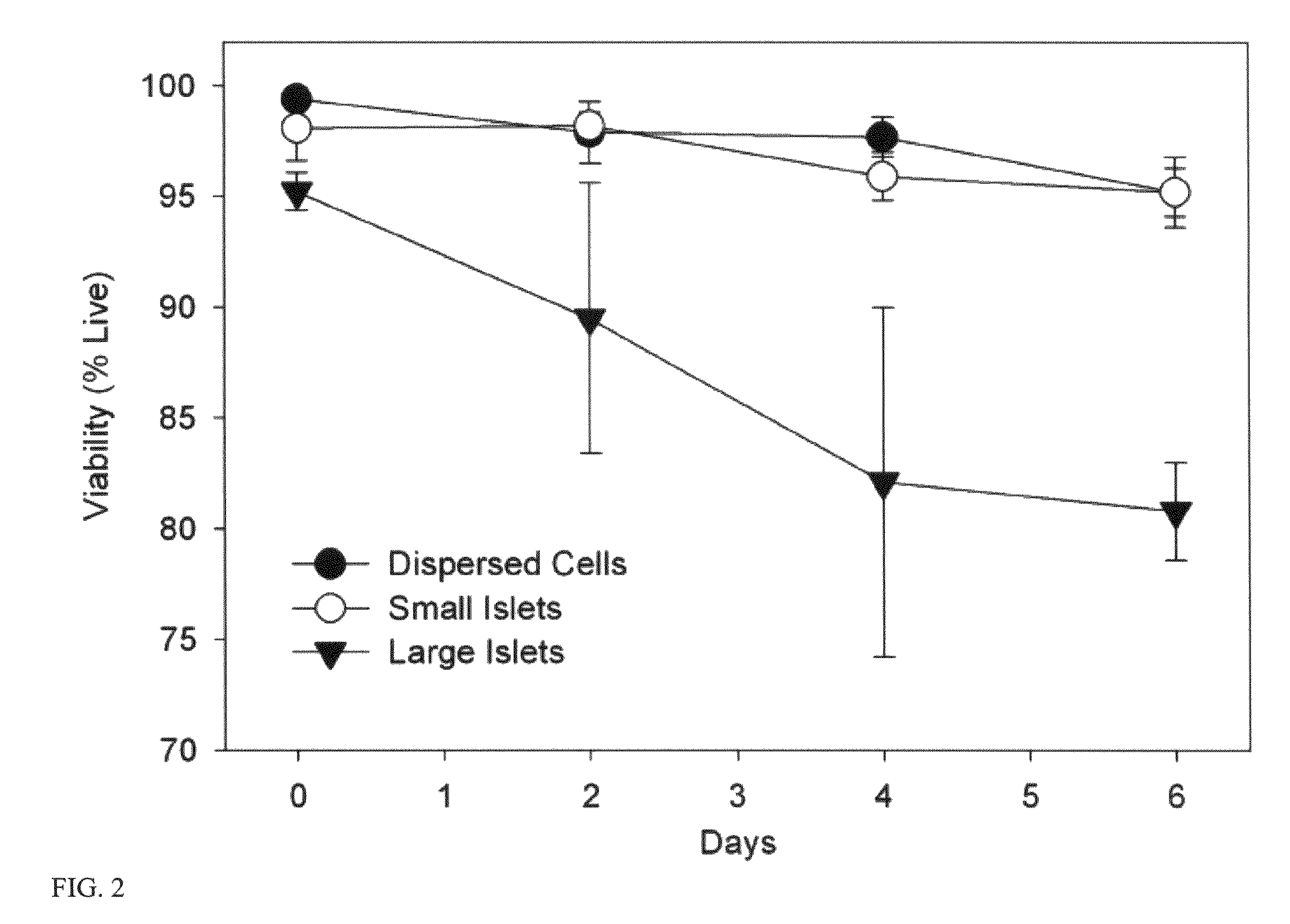
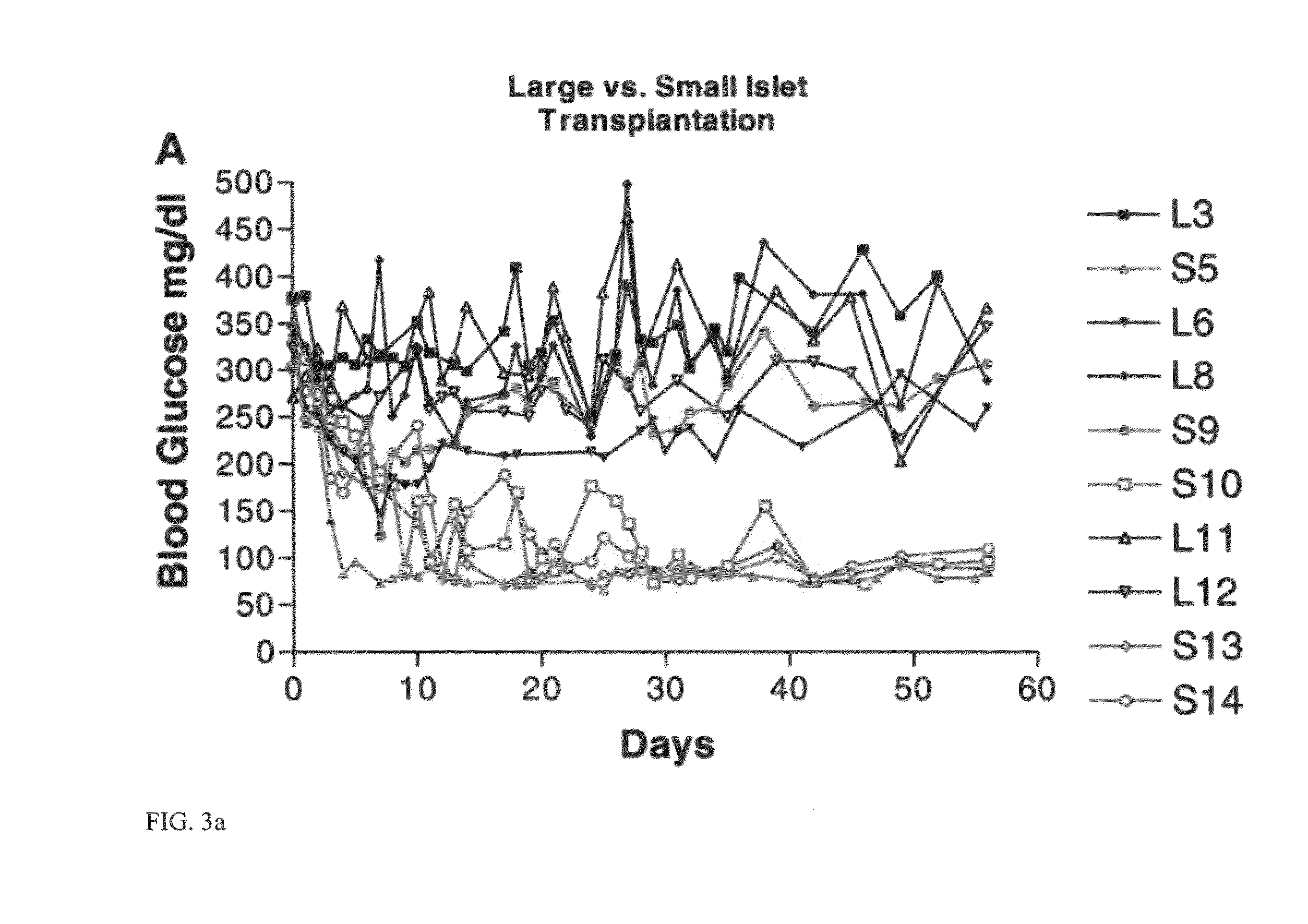
Templated islet cells and small islet cell clusters for diabetes treatment
InactiveUS20080103606A1Improve survivabilityGood adhesionCell dissociation methodsPancreatic cellsIslet cellsBiomaterial scaffold
Owner:KANSAS UNIV OF +1
Method of treating diabetes informed by social determinants of health
PendingUS20210319887A1Easy diagnosisEasy to solvePhysical therapies and activitiesMarket predictionsDiseaseTherapy adherence
Disclosed herein is an invention that is a medical treatment method for diabetes, its comorbidities and its complications where their treatment requires lifestyle modification. The invention changes or alters lifestyle by identifying what is valuable or harmful to the health-related determinants of the pattern-of-life of a person, modifying the such determinants of health of the person as the person navigates their pattern of life, applying the resulting insights to modify the lifestyle of the person, promoting and improving therapy adherence and compliance and thereby improving the health of the person. The method is intended to prevent diabetes, to increase the early detection of diabetes, to diagnose diabetes, to delay the progression of diabetes, to reduce the severity of diabetes and to operationalize the insights from lifestyle modification through disease risk assessment, medical decision-making, comprehensive care plan management and patient outreach, engagement and retention in the care plan.
Owner:AMUSENEERING TECH LLC
Central data exchange node for system monitoring and control of blood glucose levels in diabetic patients
ActiveUS20160331310A1Easy to replaceGuaranteed uptimeInertial sensorsMedical devicesNODALClosed loop
A flexible system capable of utilizing data from different monitoring techniques and capable of providing assistance to patients with diabetes at several scalable levels, ranging from advice about long-term trends and prognosis to real-time automated closed-loop control (artificial pancreas). These scalable monitoring and treatment strategies are delivered by a unified system called the Diabetes Assistant (DiAs) platform. The system provides a foundation for implementation of various monitoring, advisory, and automated diabetes treatment algorithms or methods. The DiAs recommendations are tailored to the specifics of an individual patient, and to the patient risk assessment at any given moment. A central data exchange node or server collects patient data from individual DiAs devices and provides safety assurance, monitoring, telemedicine and database building for the DiAs system.
Owner:UNIV OF VIRGINIA ALUMNI PATENTS FOUND
Multi-Transgenic Pigs for Diabetes Treatment
ActiveUS20110038841A1Reduce needInhibit the inflammatory responseBiocideVectorsTransgenePancreatic islets
The present invention provides certain animals, and in particular porcine animals, tissue and cells derived from these, which lack any expression of functional alpha 1,3 galactosyltransferase (αGT) and express one or more additional transgenes which make them suitable donors for pancreatic islet xenotransplantation. Methods of treatment and prevention of diabetes using cells derived from such animals are also provided.
Owner:REVIVICOR INC
Monitoring device for management of insulin delivery
ActiveUS8954373B2Minimize changesDrug and medicationsMedical devicesInsulin activityLearning controller
Monitoring system and method for use with diabetic treatment management. The system includes: a communication interface configured to permit access to stored raw log data, obtained over a certain time, being indicative of glucose measurements, meals consumed and insulin delivery; and a control unit including an unsupervised learning controller configured to receive and process said raw log data and determine at least one global insulin pump setting of basal rate, correction factor, carbohydrate ratio and insulin activity curve parameters. The system may include a processing unit including a first processor for processing measured data indicative of blood glucose level and generating first processed data, a second processor including at least one fuzzy logic module which receives input parameters corresponding to the measured data, the first processed data and a reference data, and processes the data to produce a qualitative output parameter to determine whether any treatment parameter should be modified.
Owner:DREAMED DIABETES
Human glucose concentration continuous monitoring device based on optical fiber surface plasmon resonance
InactiveCN101819136ASimple structureReduce volumePhase-affecting property measurementsHuman bodyConcentrations glucose
The invention discloses a human glucose concentration continuous monitoring device based on optical fiber surface plasmon resonance. An optical fiber SPR (Surface Plasmon Resonance) probe is implanted in a human body; light emitted by a light source reaches the optical fiber SPR probe via an optical fiber coupler; after SPR attenuation, reflected light reaches a spectrograph through the optical fiber coupler; and a computer analyzes and processes data output by the spectrograph and displays monitoring results. A micro-cavity is fixed outside the optical fiber SPR probe, one side of the micro-cavity is a semitransparent film, and only glucose and other micromolecules can enter the micro-cavity. GGBP proteins or borate polymers are fixed on the goldfilm surface of the optical fiber SPR probe and can carry out specificity absorption to the glucose molecules, thereby improving the measurement sensitivity. The invention can realize human glucose concentration continuous monitoring by measuring the glucose concentration of human body tissue fluid, has high measurement precision and good stability and can realize 24-hour human glucose concentration continuous monitoring and provide comprehensive glucose concentration change data for better guiding the diabetes treatment.
Owner:TIANJIN UNIV
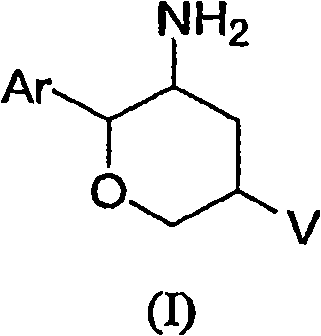
Aminotetrahydropyrans as dipeptidyl peptidase-IV inhibitors for the treatment or prevention of diabetes
The present invention is directed to novel substituted aminotetrahydropyrans of structural formula (I) which are inhibitors of the dipeptidyl peptidase-IV enzyme and which are useful in the treatment or prevention of diseases in which the dipeptidyl peptidase-IV enzyme is involved, such as diabetes and particularly Type 2 diabetes. The invention is also directed to pharmaceutical compositions comprising these compounds and the use of these compounds and compositions in the prevention or treatment of such diseases in which the dipeptidyl peptidase-IV enzyme is involved.
Owner:SCHERING AG
Glucagon like peptide-1 mutant polypeptide and preparation method, medicinal composition and use thereof
InactiveCN102363633AImprove compliancePeptide/protein ingredientsMetabolism disorderHalf-lifeRetention time
The invention discloses a glucagon like peptide(GLP)-1 mutant polypeptide and a preparation method, a medicinal composition and use thereof. The mutant polypeptide is formed by bonding a Cys-containing elongation peptide to the N terminal of the glucagon like peptide-1 mutant, the mutant polypeptide itself folds to form a disulfide bond. The glucagon like peptide-1 mutant polypeptide is used for preparing a medicinal composition for treating diabetes and treating and preventing obesity. For overcoming the drawbacks of short retention time in body and need of daily injection administration of clinic GLP-1 analogue, the invention provides the GLP-1 analogue which has a longer half-life period. With the longer half-life period, the GLP-1 mutant polypeptide is not required to be injected into a patient every day, and can increase the obedience of the patient effectively.
Owner:天津拓飞科技有限公司
Multiple agent diabetes therapy
InactiveUS7323543B2Organic active ingredientsPeptide/protein ingredientsActrapid insulinPancreatic hormone
A pharmaceutical composition includes at least two of agents I)-iii), wherein agent i) is selected from the group consisting of an insulin, an insulin analog, a physiologically active fragment of said insulin and a physiologically active fragment of said insulin analog, agent ii) is selected from the group consisting of an insulin-related peptide, an insulin-related peptide analog, a physiologically active insulin-related peptide fragment and a physiologically active insulin-related peptide analog fragment, and agent iii) is an insulin sensitizer.
Owner:MINIMED
Method of treating a patient
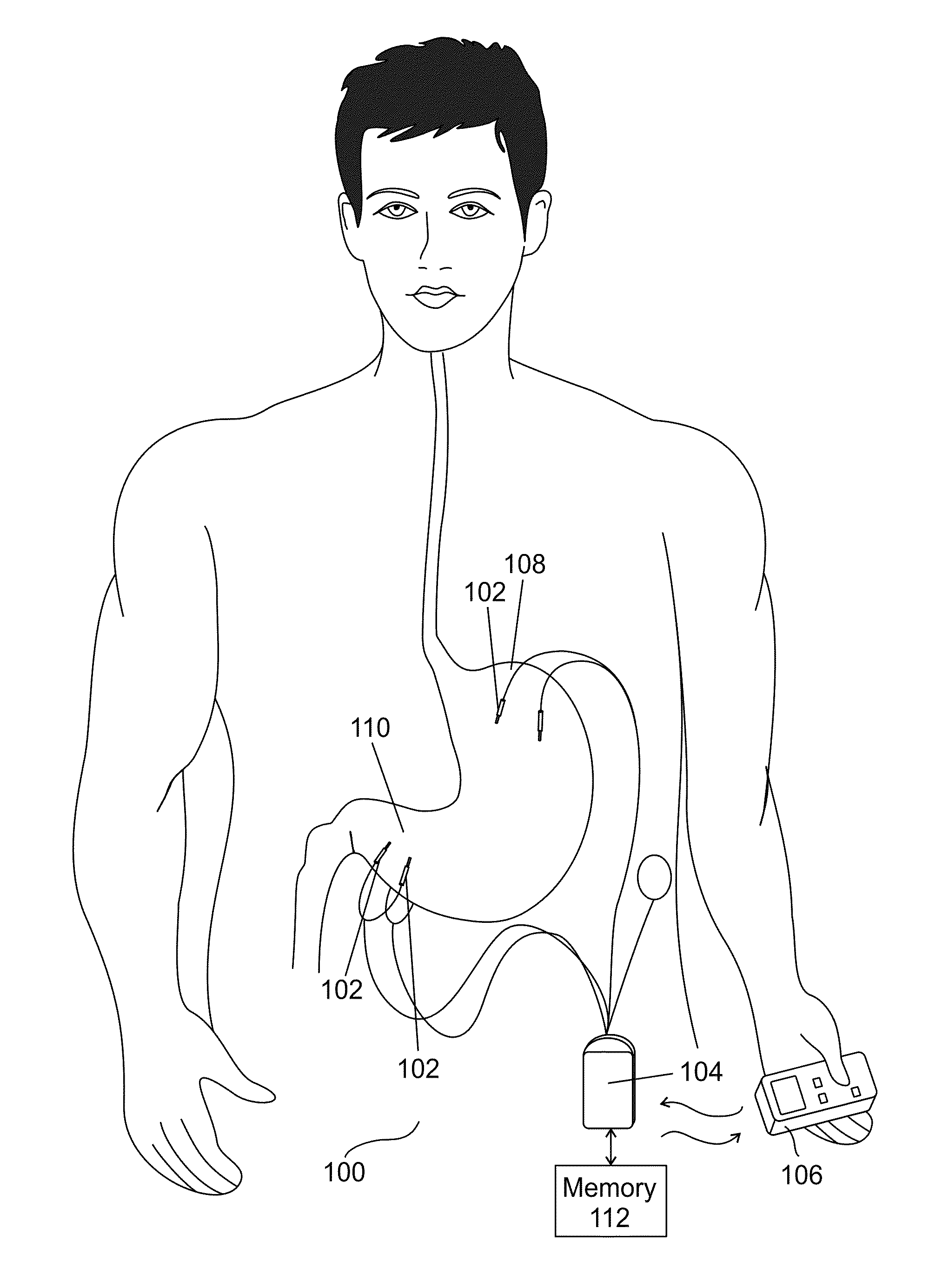
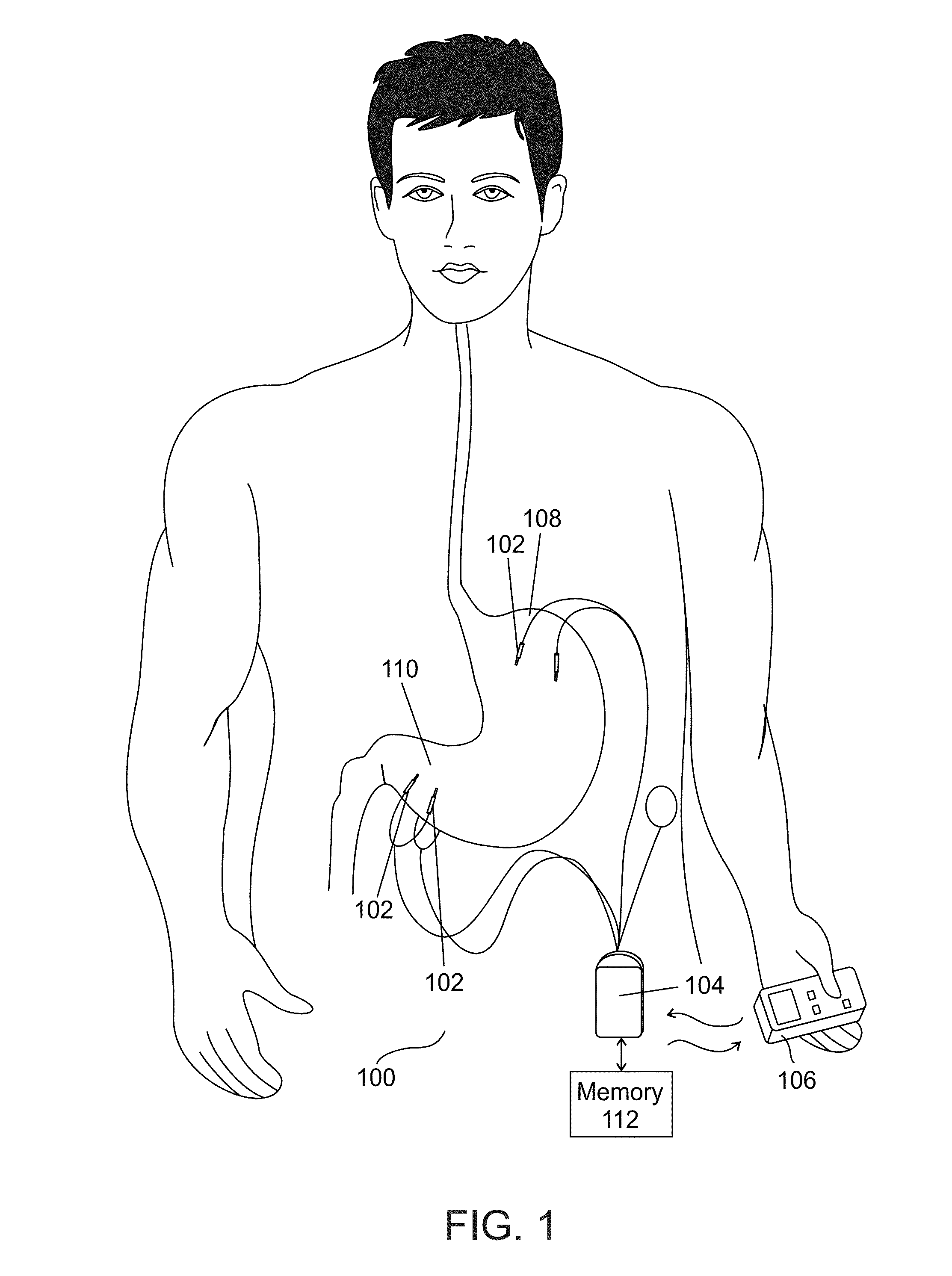
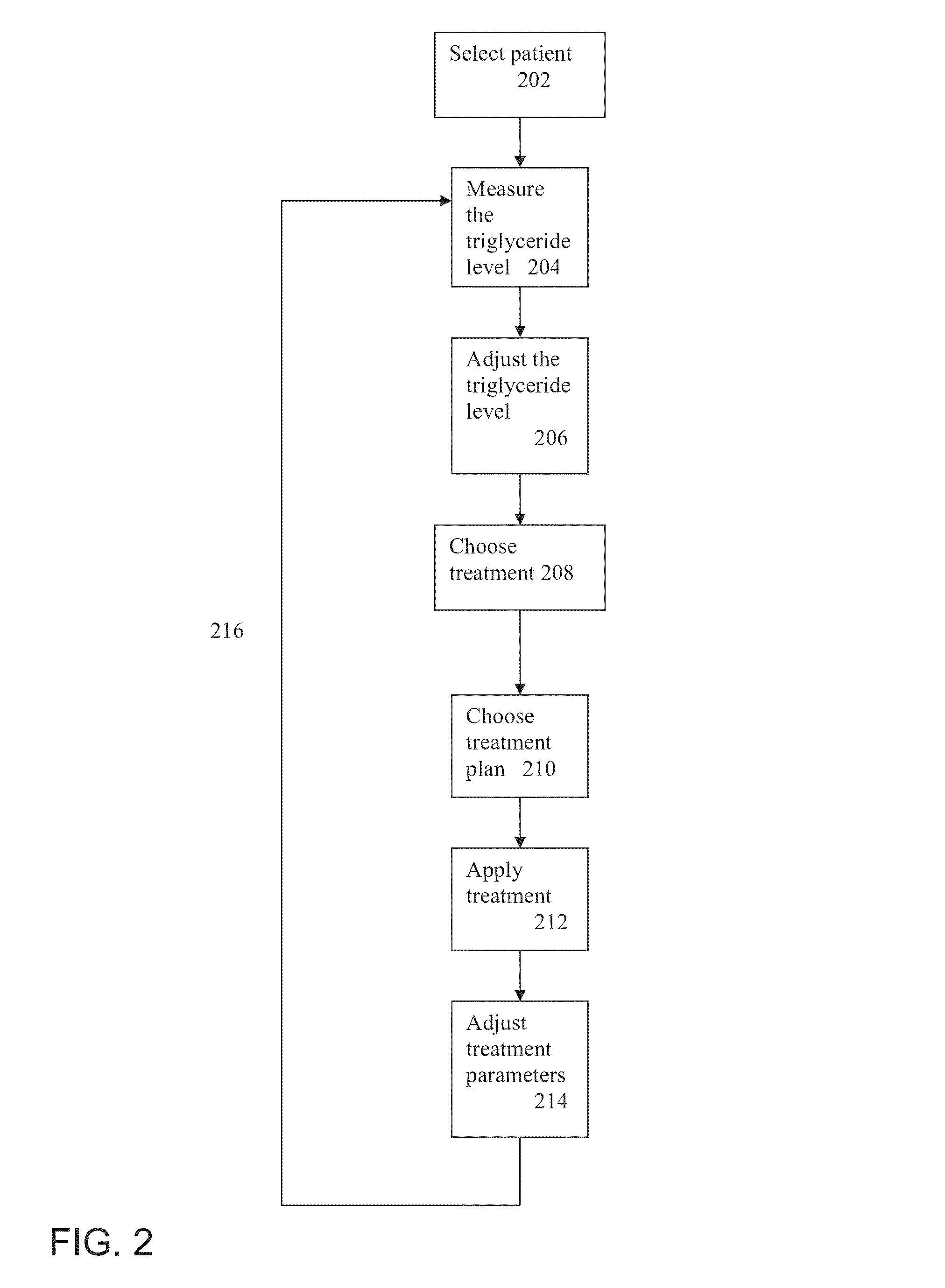
InactiveUS8738127B1Lower blood sugar levelsReduce weightMedical devicesImplantable neurostimulatorsBlood pressureScreening method
There is provided in accordance with an exemplary embodiment of the invention a method of treating diabetic patients comprising measuring a triglyceride level in a diabetic patient and applying a diabetes treatment according to the triglyceride level. Optionally, there is provided a method of screening. Alternatively or additionally, there is provided a method of weight loss. Alternatively or additionally, there is provided a method of relatively reducing HOMA-IR levels. Alternatively or additionally, there is provided a method of relatively reducing blood pressure. An exemplary device to apply the treatment is described.
Owner:TYLERTON INT INC
Templated islet cells and small islet cell clusters for diabetes treatment
ActiveUS20100233239A1Good adhesionImprove survivabilityBiocideBioreactor/fermenter combinationsIslet cellsPancreatic islets
Owner:UNIVERSITY OF KANSAS
Crystal, preparation method and applications of crystal
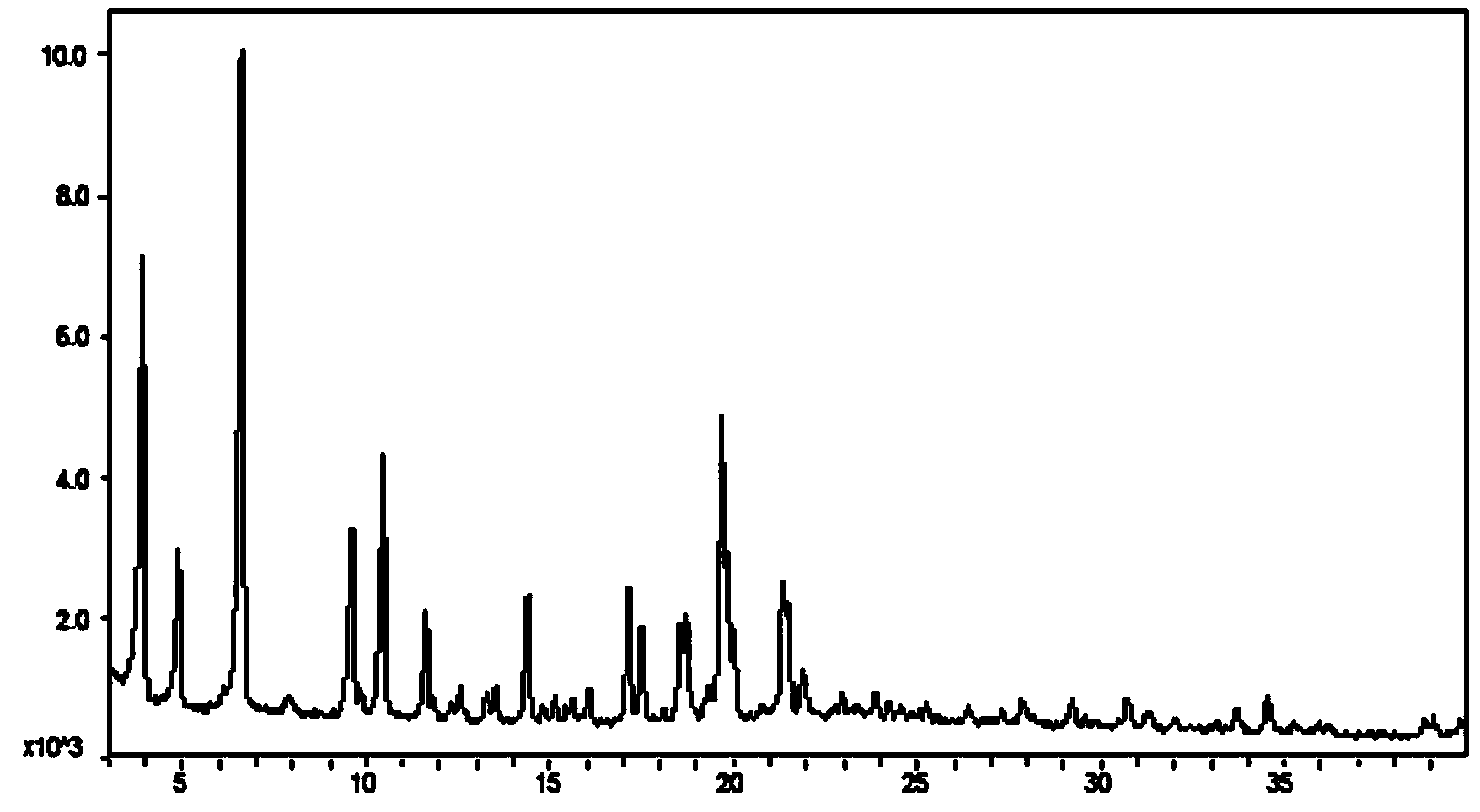
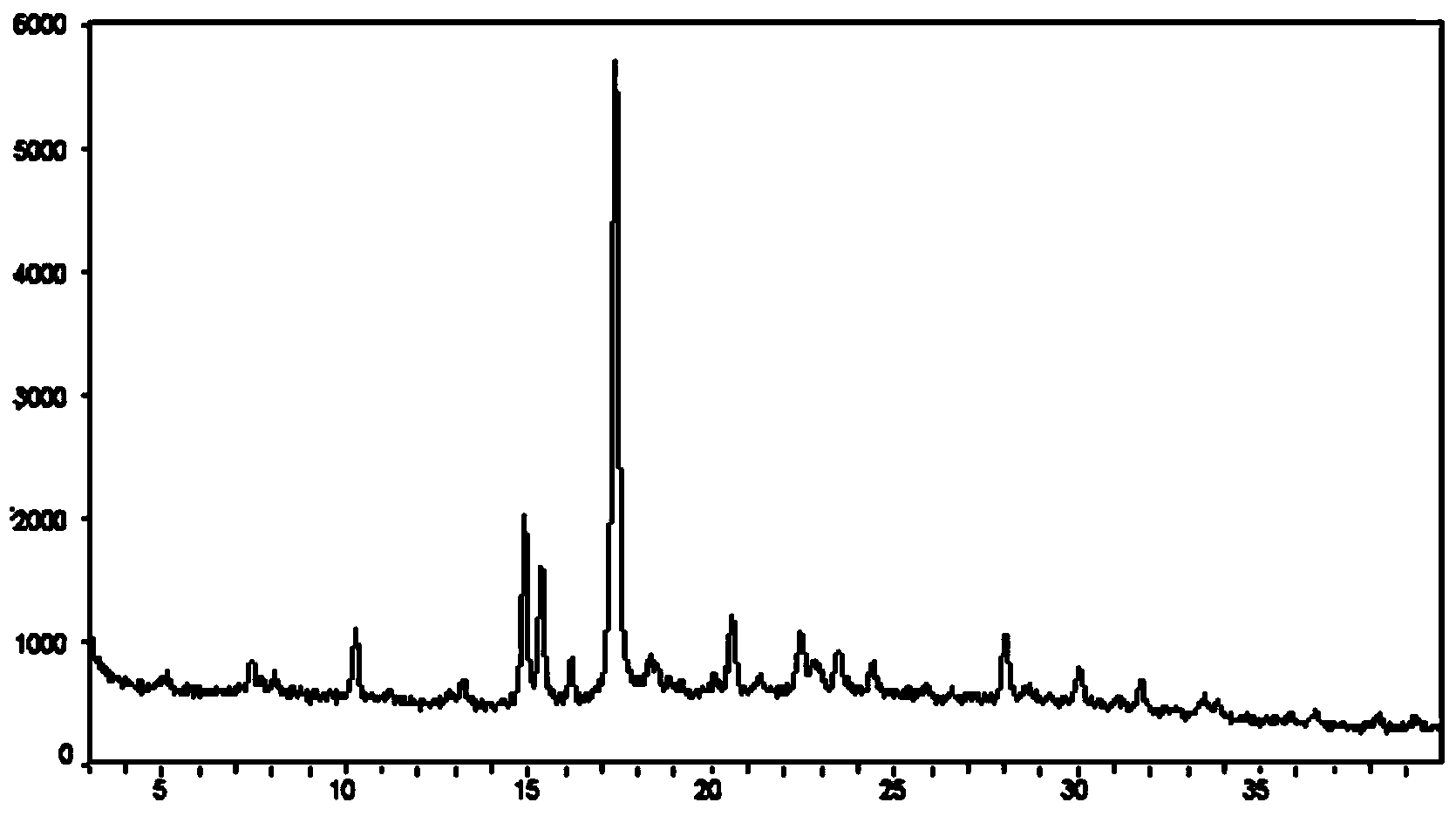
ActiveCN103936726AReduce diabetes complicationsObvious role and effectOrganic active ingredientsNervous disorderBenzeneMethyl group
The invention discloses crystal forms III and IV of 1-(b-D-glucopyranosyl)-4-methyl-3-[5-(4-fluorophenyl)-2-thienylmethyl]benzene. In an X-ray powder diffraction diagram, the crystal forms III and IV at least have characteristic peaks at 2q values being 6.61+ / -0.2, 3.92+ / -0.2 and 19.68+ / -0.2, as well as 17.40+ / -0.2, 15.35+ / -0.2 and 14.91+ / -0.2, respectively. The invention further discloses a preparation method and medical applications of the crystal. The crystal form III is an octanol complex and has more obvious action effect on the diabetes treatment effect. The crystal form IV is an anhydrous complex, and is high in purity; the drying process for preparing the anhydrous complex is simple; on the aspect of preparation technology, the anhydrous complex has obvious advantages compared with the hydrous complex.
Owner:王军
Preparation and applications of mesoporous silica/insulin nanoparticles modified by phenylboronic acid
InactiveCN106236734AStabilize blood sugar levelsAutomatic sensing of glucose concentration changesPeptide/protein ingredientsMetabolism disorderPhenylboronic acidNanoparticle
The invention relates to preparation and applications of mesoporous silica / insulin nanoparticles modified by phenylboronic acid, for effectively solving the problem that the traditional drug carriers release medicines unidirectionally. The technical scheme is as follows: the preparation comprises the following steps: firstly, synthesizing mesoporous silica nanoparticles, modifying amino groups on the surfaces of the mesoporous silica nanoparticles, loading medicine insulin in the mesoporous structure through physical absorption, and further modifying with phenylboronic acid and polysaccharide, thus obtaining the mesoporous silica / insulin nanoparticles modified by phenylboronic acid. The preparation and applications have the advantages that the synthesis process is simple, the prepared nanoparticles have the good biocompatibility, and the releasing valve of medicines can be repeatedly opened and closed, so that the sustained-release effect is achieved on the release of medicines; the mesoporous silica / insulin nanoparticles have a long-time circulation in the body, so that the administration times can be reduced, and thus the mesoporous silica / insulin nanoparticles belong to an innovation in diabetes treatment medicines.
Owner:ZHENGZHOU UNIV
Templated islet cells and small islet cell clusters for diabetes treatment
ActiveUS8735154B2Improve survivabilityGood adhesionBioreactor/fermenter combinationsBiological substance pretreatmentsIslet cellsPancreatic islets
Owner:UNIVERSITY OF KANSAS
Use of Chloroquine to Treat Metabolic Syndrome
InactiveUS20080319010A1Treatment safetyModulate ATM activityBiocideMetabolism disorderHypertension medicationsAntithrombotic Agent
The present invention provides methods and compositions for modulating certain metabolic processes and for treating a variety of disorders associated with metabolic syndrome, including insulin related disorders, ischemia, oxidative stress, atherosclerosis, hypertension, obesity, abnormal lipid metabolism, and stroke by administering an effective dose of a chloroquine compound. The invention also provides methods and compositions relating to administering an effective dose of a chloroquine compound in combination with at least a second pharmaceutically active ingredient or compound including an antihyperglycemic diabetes treatment, an antihypertensive agent, an antithrombotic agent, and / or an inhibitor of cholesterol synthesis or absorption.
Owner:WASHINGTON UNIV IN SAINT LOUIS +1
Diabetes management methods and systems
ActiveCN101088469ATimely feedbackMedical simulationDrug and medicationsAcute hyperglycaemiaDiabetes management
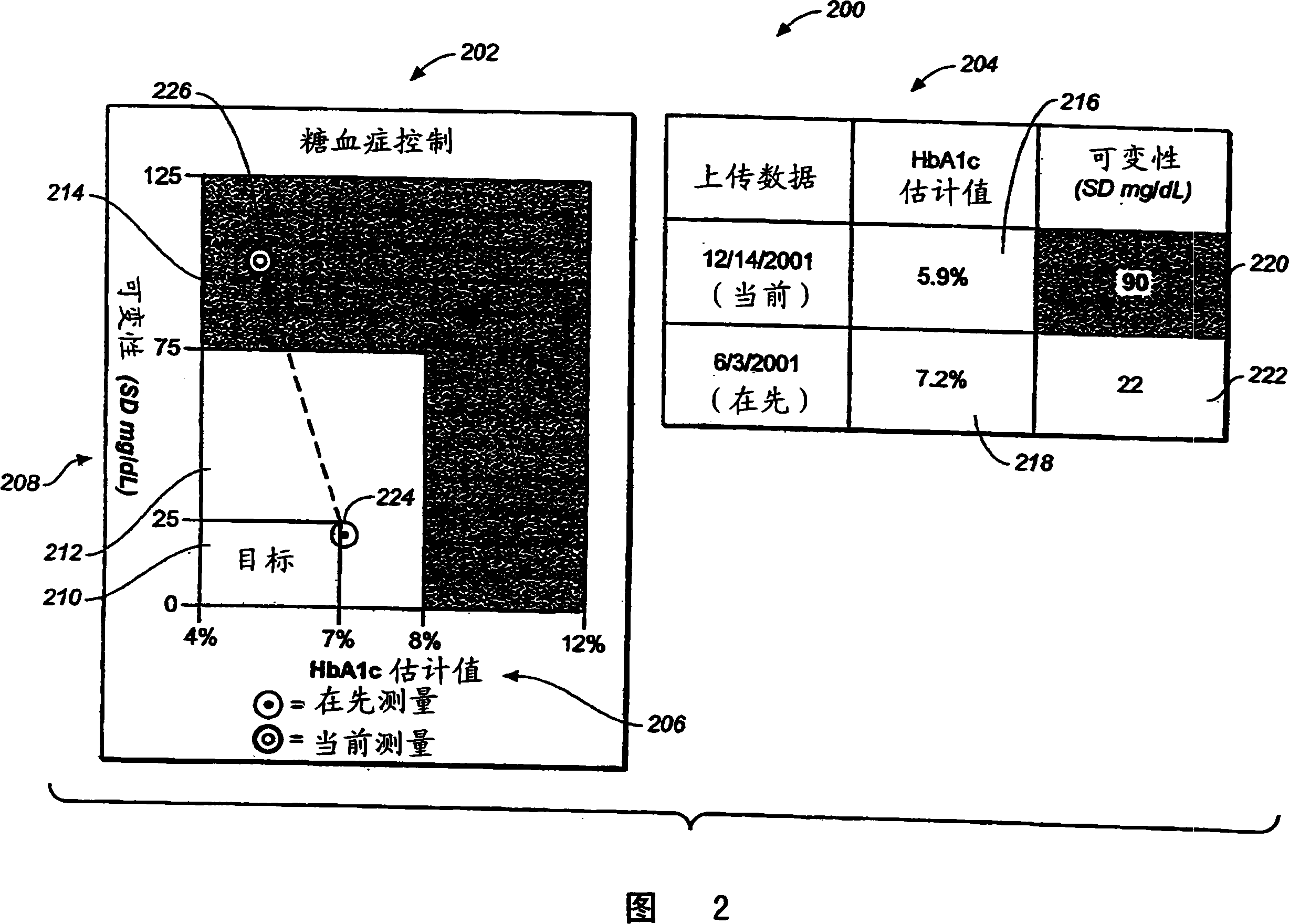
The present invention relates to methods and systems for monitoring the effectiveness of diabetes treatment. Methods and systems in accordance with the present invention provide information relating to variability of glucose levels and hypoglycemia and hyperglycemia. Such information is based on time-stamped blood glucose data obtained from a meter or the like and actual measurements of HbA1c levels are not required.
Owner:LIFESCAN INC
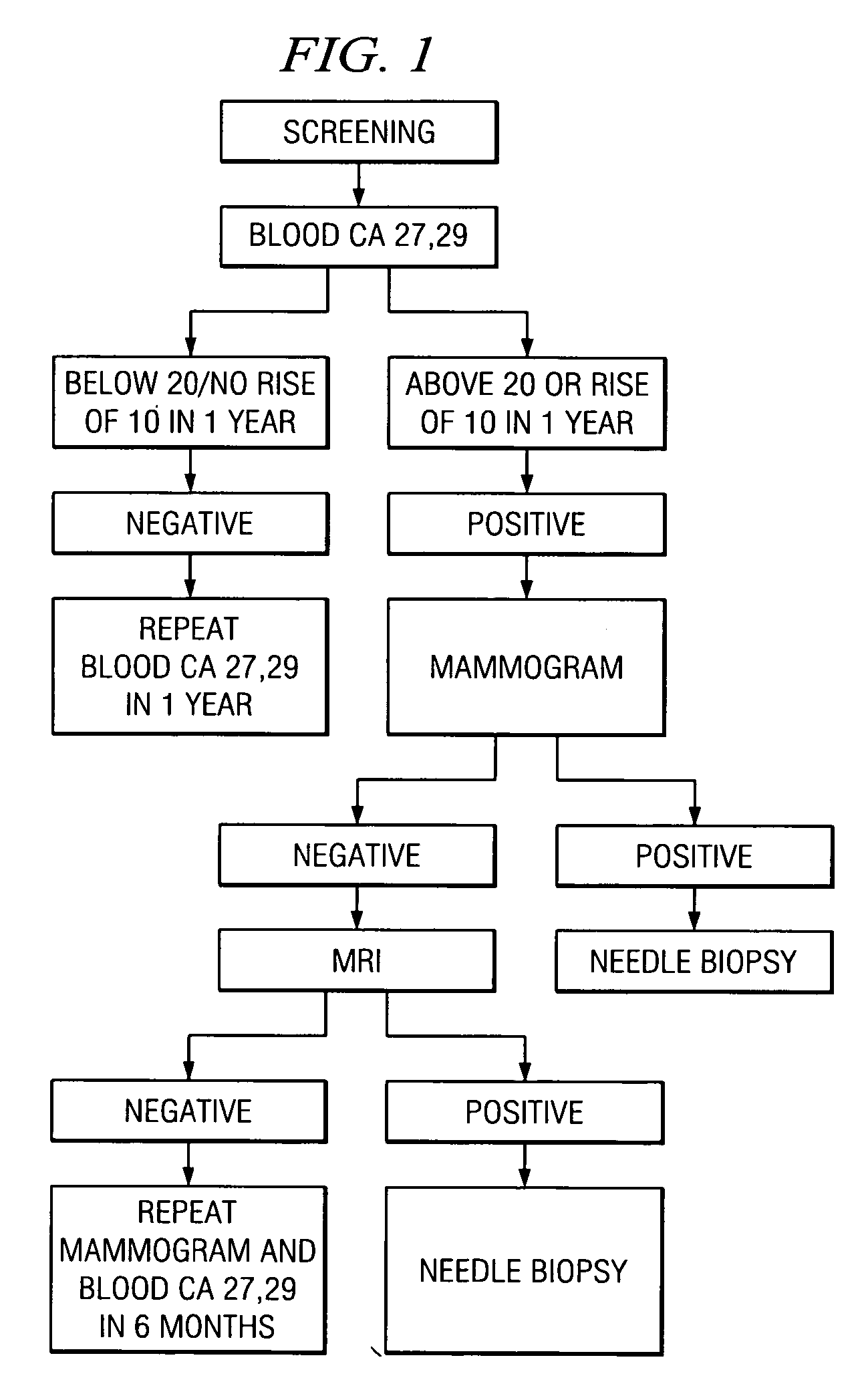
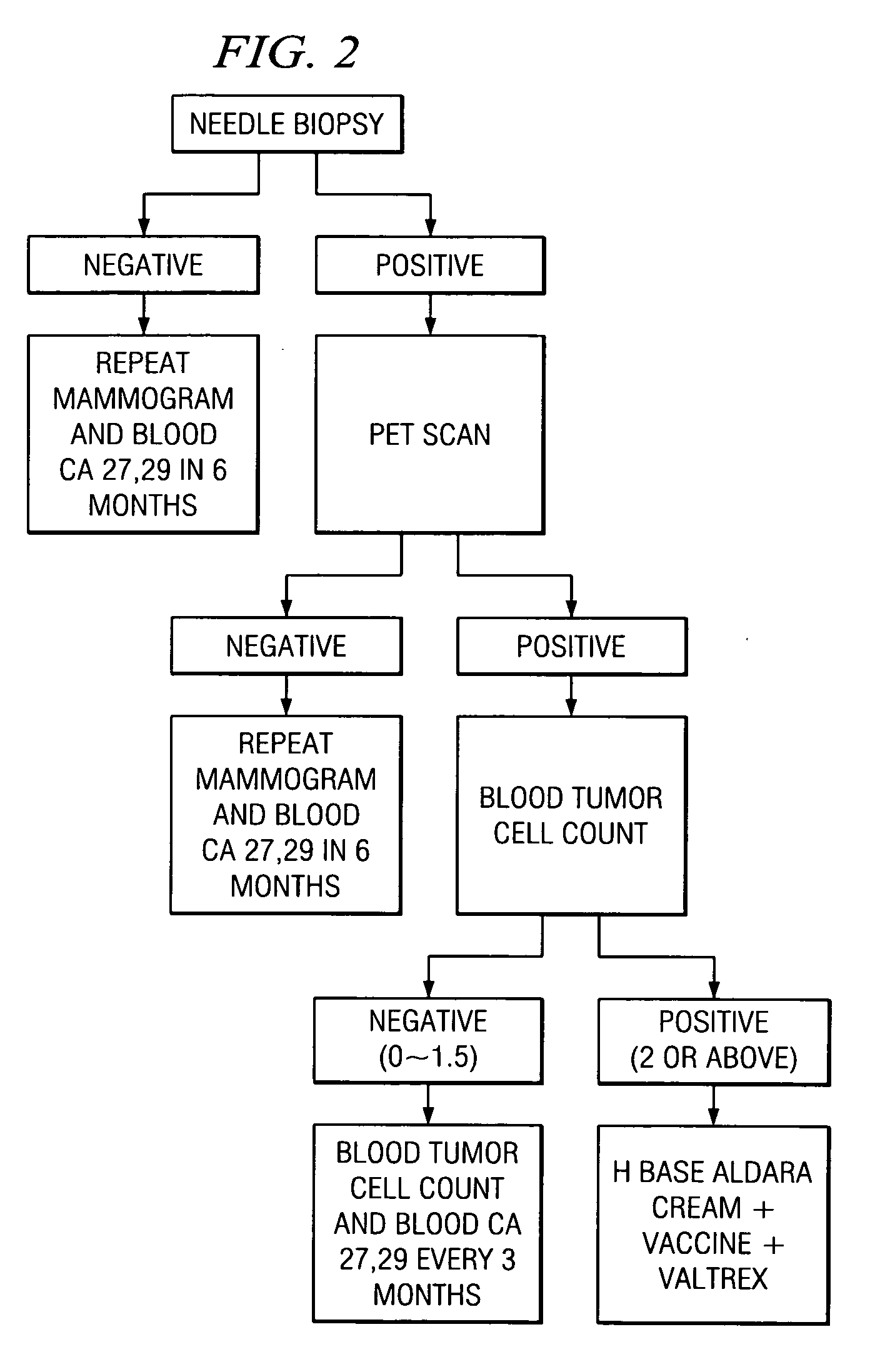
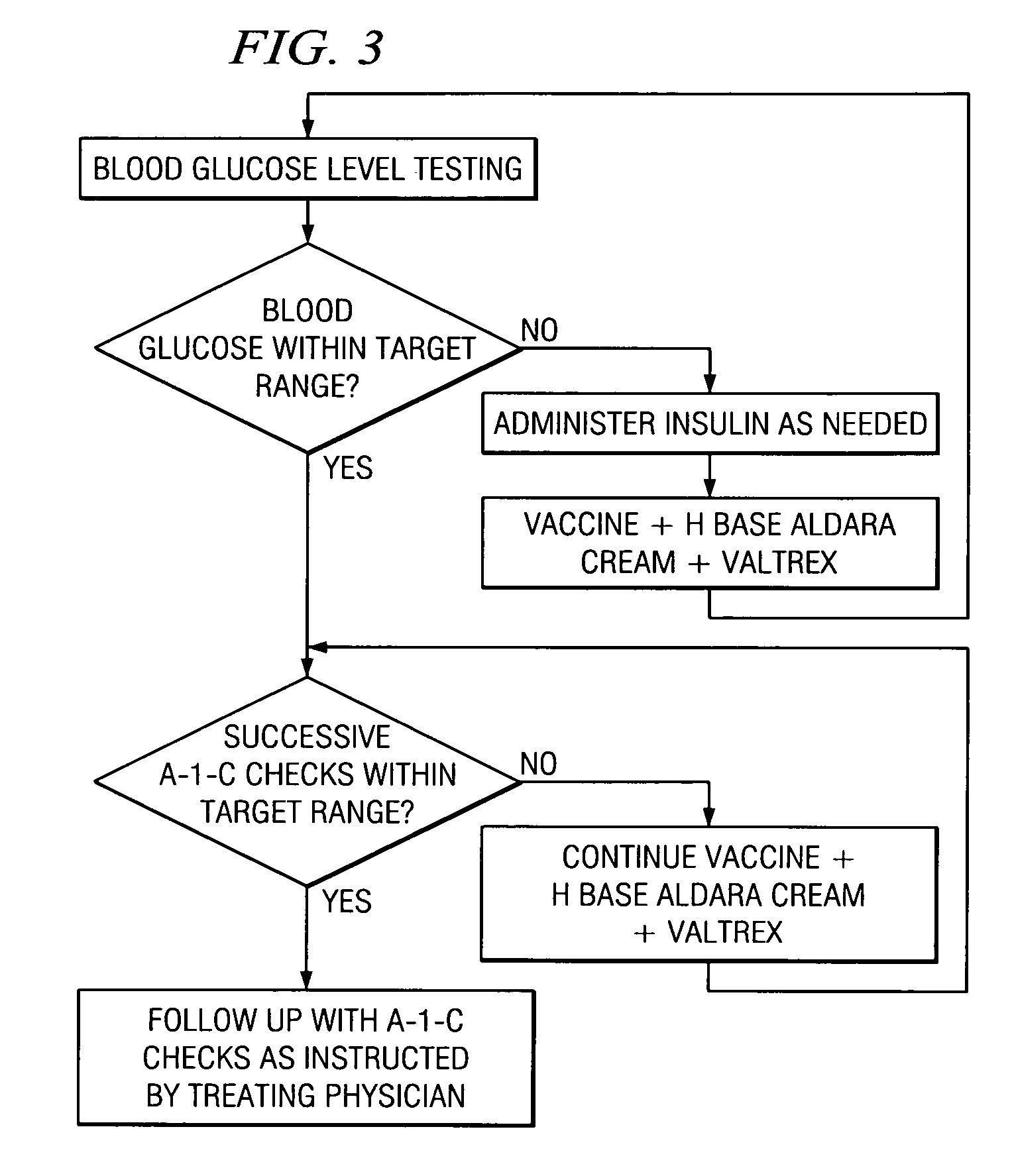
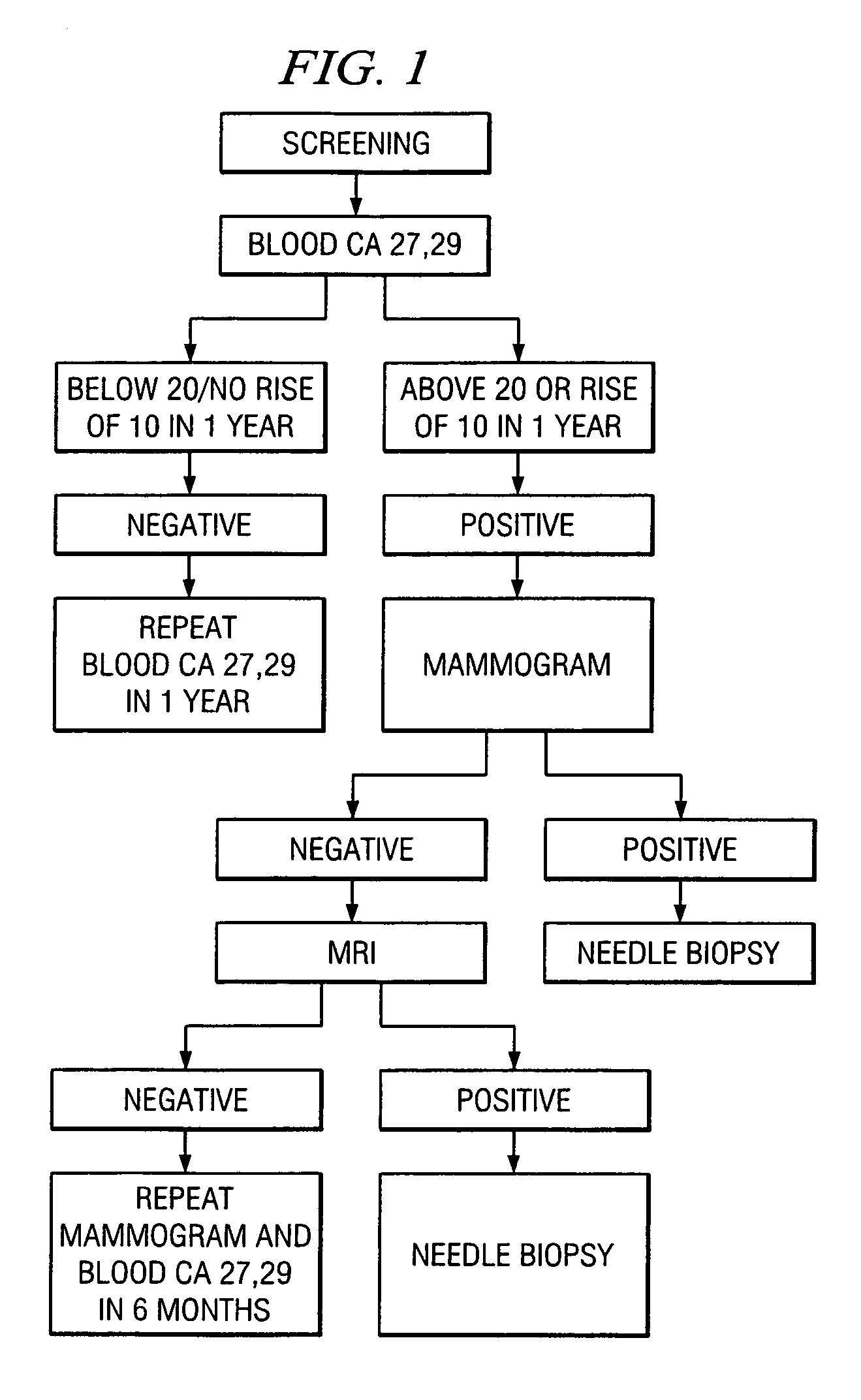
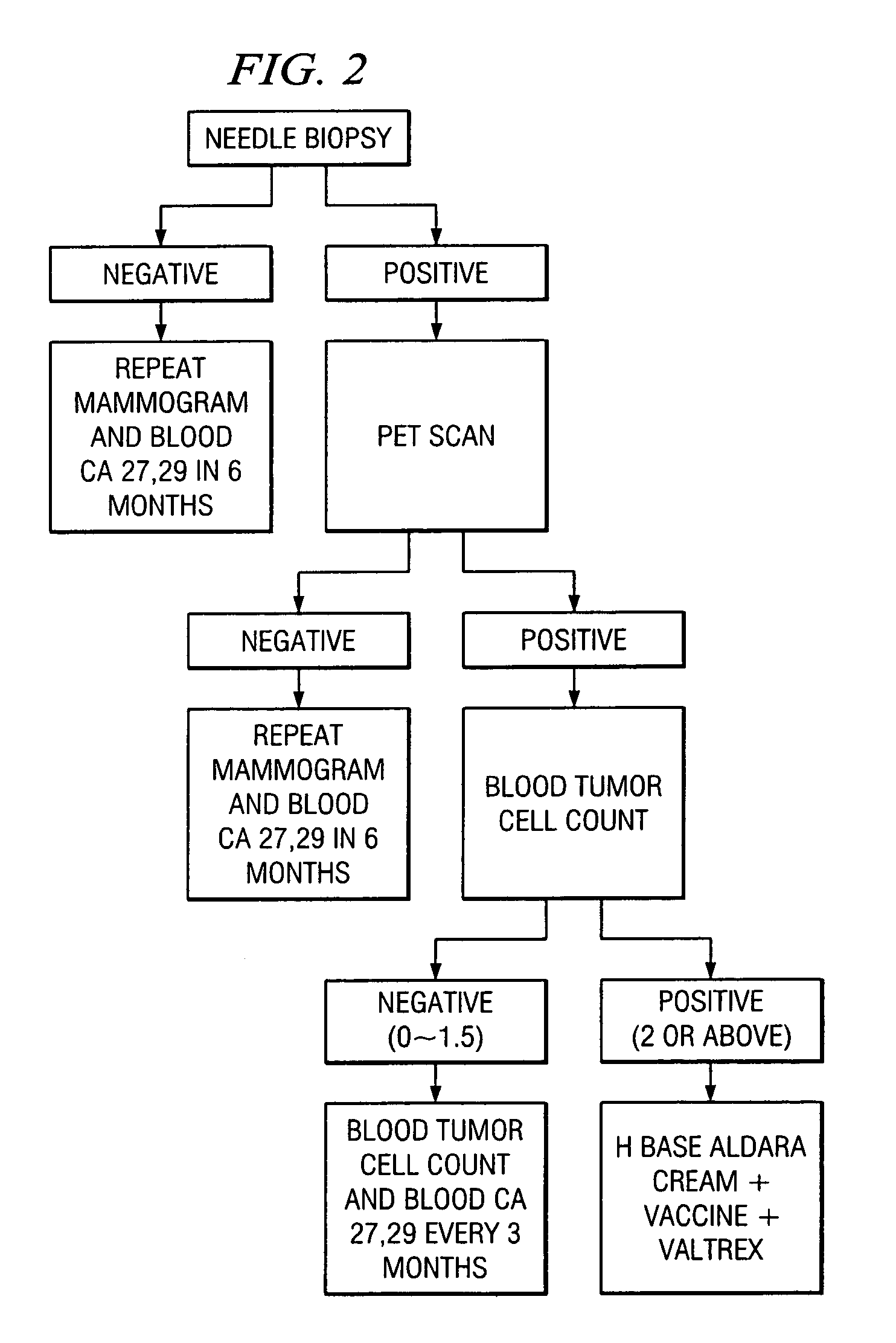
Method of cancer screening; method of cancer treatment; and method of diabetes treatment
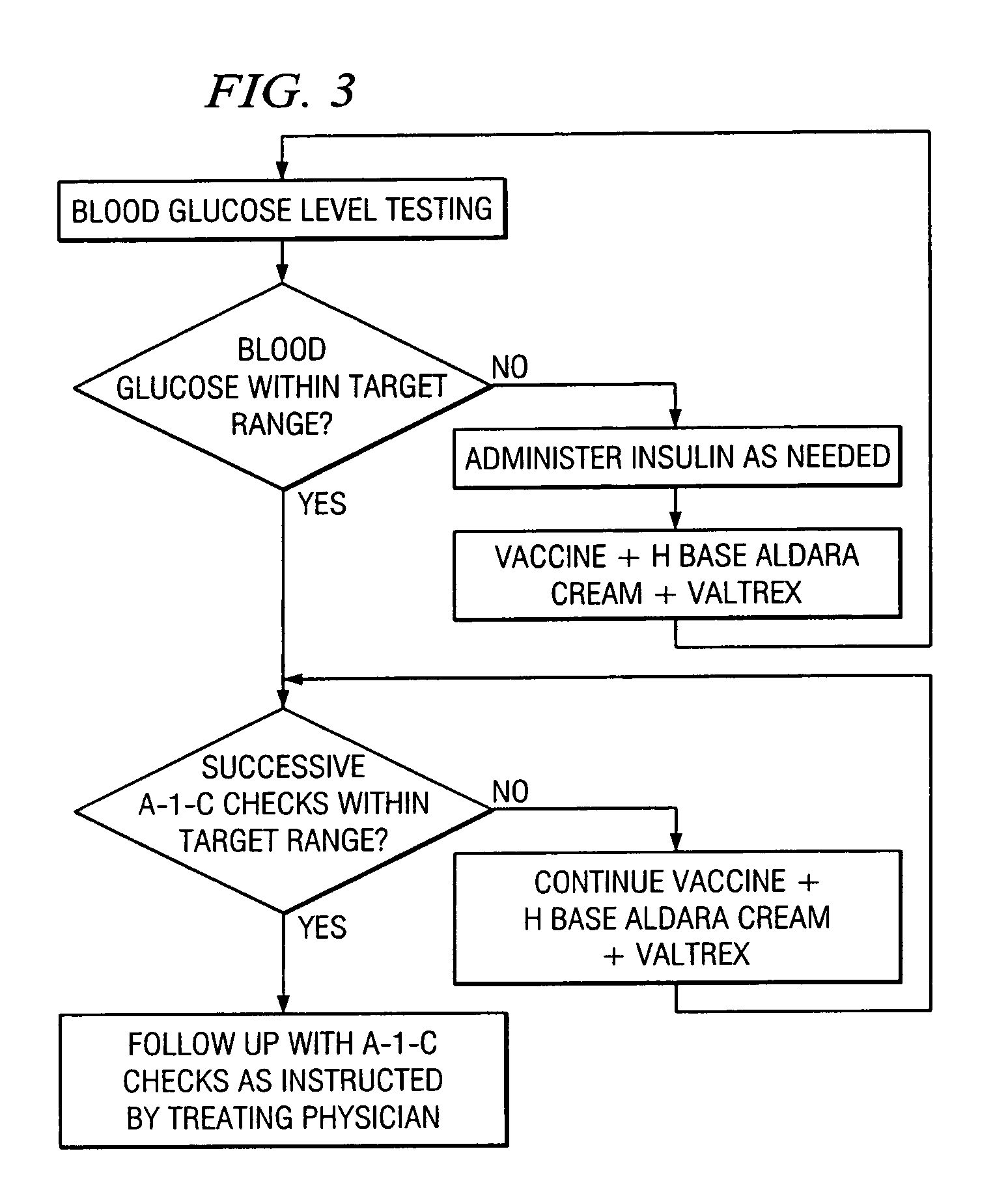
A method of cancer screening comprising the steps of administering the Blood CA 27,29 testing procedure; if the result is positive administering a mammogram; if the result is positive administering an needle biopsy; if the result is positive administering a PET scan; if the result is positive administering a blood tumor cell count. If all of the foregoing steps are positive, the cancer is treated by applying imiquimod transdermally to rotating sites, preferably by mixing ALDARA® (imiquimod) 5% cream with an equal amount of H base cream; administering a vaccine containing tumor necrosis factor, preferably the BCG vaccine; and orally administering VALTREX® (valacyclovir) twice daily. The foregoing treatment method is also effective in treating Type I diabetes, MS, and other epidermal cancers.
Owner:WOODWARD FAMILY LTD A PARTNERSHIP OF THE STATE OF TEXAS JOHN R WOODWARD GENERAL PARTNER +1
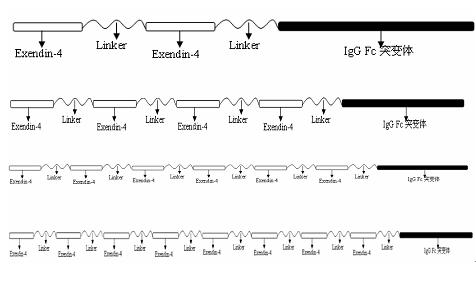
Fusion protein for treating diabetes and preparation method for fusion protein
InactiveCN102558362ACan stimulate secretionReduce physical burdenPeptide/protein ingredientsMetabolism disorderPancreatic hormoneChinese hamster
The invention relates to the technical field of medicines for treating diabetes, in particular to a fusion protein for treating diabetes and a preparation method for the fusion protein. The fusion protein is recombined by 2 to 8 Exendin-4-Linkers and a human IgGFc mutant; Linker is a flexible peptide fragment; and the human IgGFc mutant is an IgG1Fc, IgG2Fc or IgG4Fc mutant. The invention also discloses the preparation method for the fusion protein. The recombined fusion protein obtained by the steps of performing high-level expression in Chinese hamster ovary (CHO) cells, affiliating, and performing ion exchange and molecular sieve chromatography has the bioactivities of stimulating the secretion of insulin and inhibiting glucagon generated after dinner from being released which are possessed by Exendin-4, also has the characteristics of prolonging the half-life period of the Exendin-4 in serum, is used for treating type I and type II diabetes, can effectively reduce the psychological and physiological burden of patients, is safe in use and high in practicability, and can be massively produced and sold.
Owner:DONGGUAN JINLANG BIOTECH
Method of cancer screening; method of cancer treatment; and method of diabetes treatment
A method of cancer screening comprising the steps of administering the Blood CA 27,29 testing procedure; if the result is positive administering a mammogram; if the result is positive administering a needle biopsy; if the result is positive administering a PET scan; if the result is positive administering a blood tumor cell count. If all of the foregoing steps are positive, the cancer is treated by applying imiquimod transdermally to rotating sites, preferably by mixing ALDARA (TM) (imiquimod) 5% cream with an equal amount of H base cream (TM); administering a vaccine that induces production of tumor necrosis factor, preferably the BCG vaccine; and orally administering Valtrex (TM) (valacyclovir) twice daily. The foregoing treatment method is also effective in treating Type I diabetes, MS, and other epidermal cancers.
Owner:LES MEDECINS
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com