Patents
Literature
110 results about "Imiquimod" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Imiquimod is used to treat actinic keratoses (AK) which are precancerous growths on the skin. AK are caused by too much sun exposure.
Imiquimod cream formulation
InactiveUS20070264317A1Composition is stablePowder deliveryBiocideExcipientPharmaceutical preservatives
Owner:AGIS INDUSTRIES (1983) LTD
Method of stabilizing imiquimod
InactiveUS7902242B2Short lifeImpaired stabilityBiocideOrganic chemistryPharmaceutical formulationOleic Acid Triglyceride
Pharmaceutical formulations and methods including an immune response modifier (IRM) compound and an oleic acid component are provided where stability is improved by using oleic acid have low polar impurities such as peroxides.
Owner:MEDICIS PHARMA CORP
Method of cancer screening; method of cancer treatment; and method of diabetes treatment
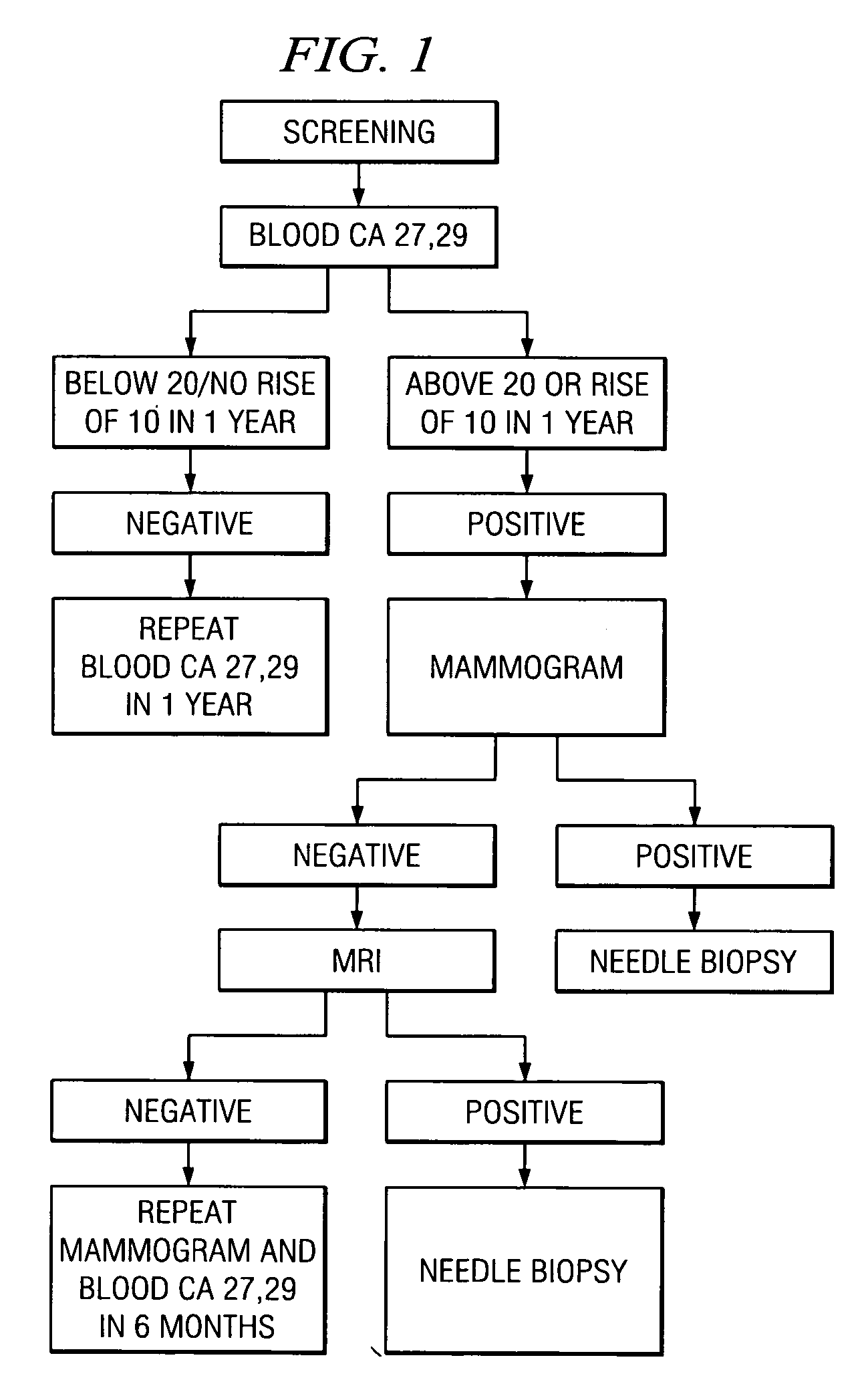
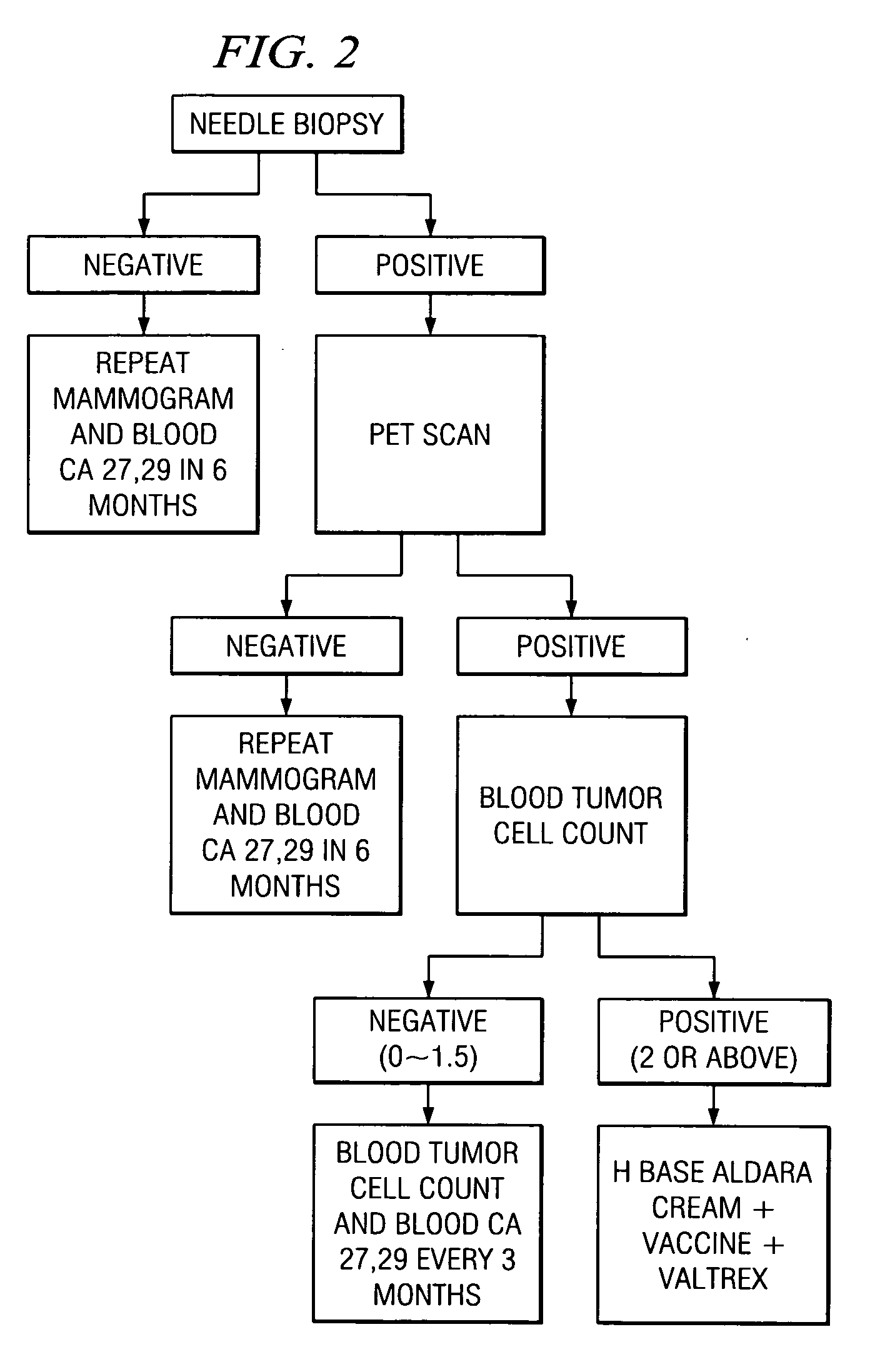
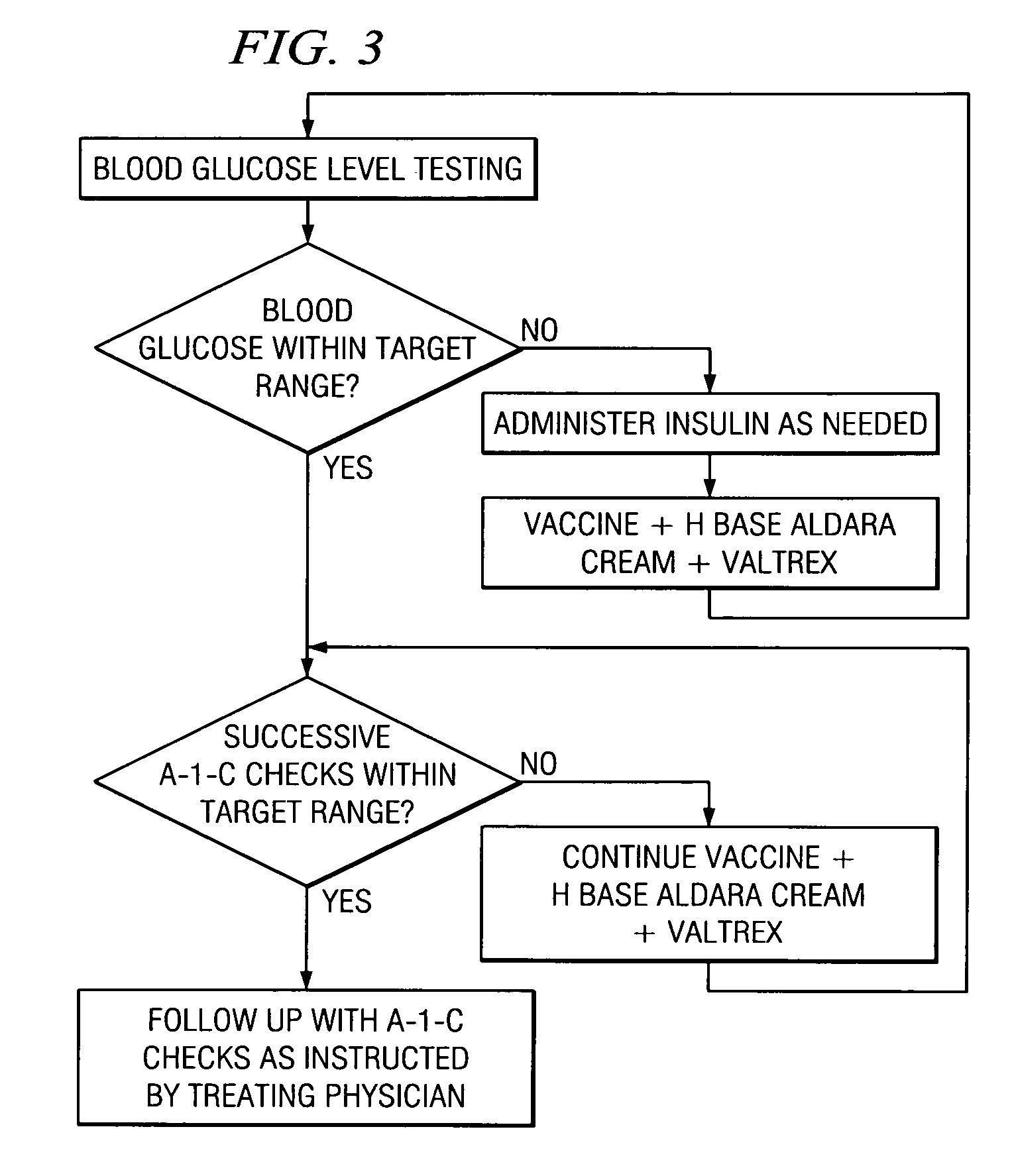
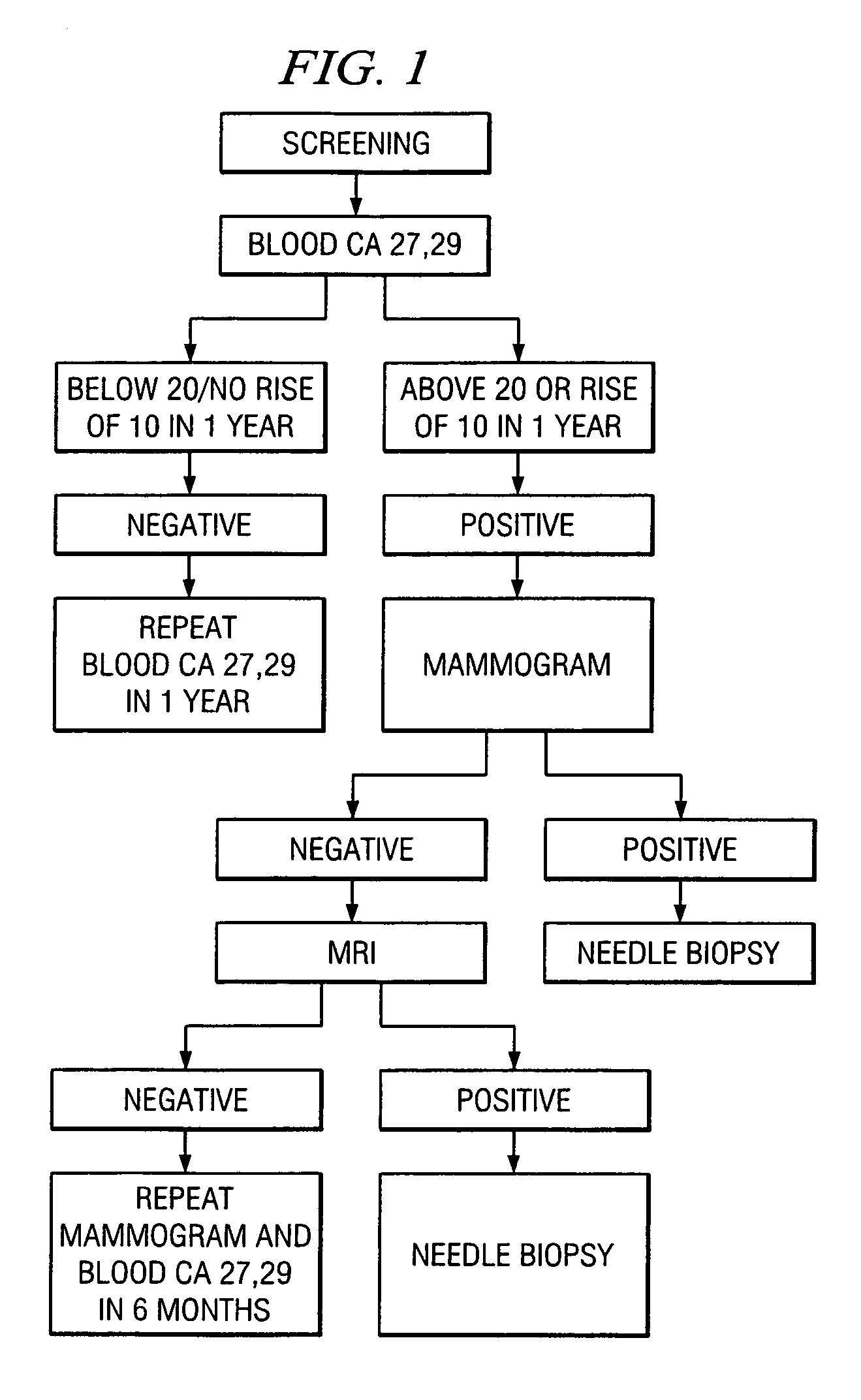
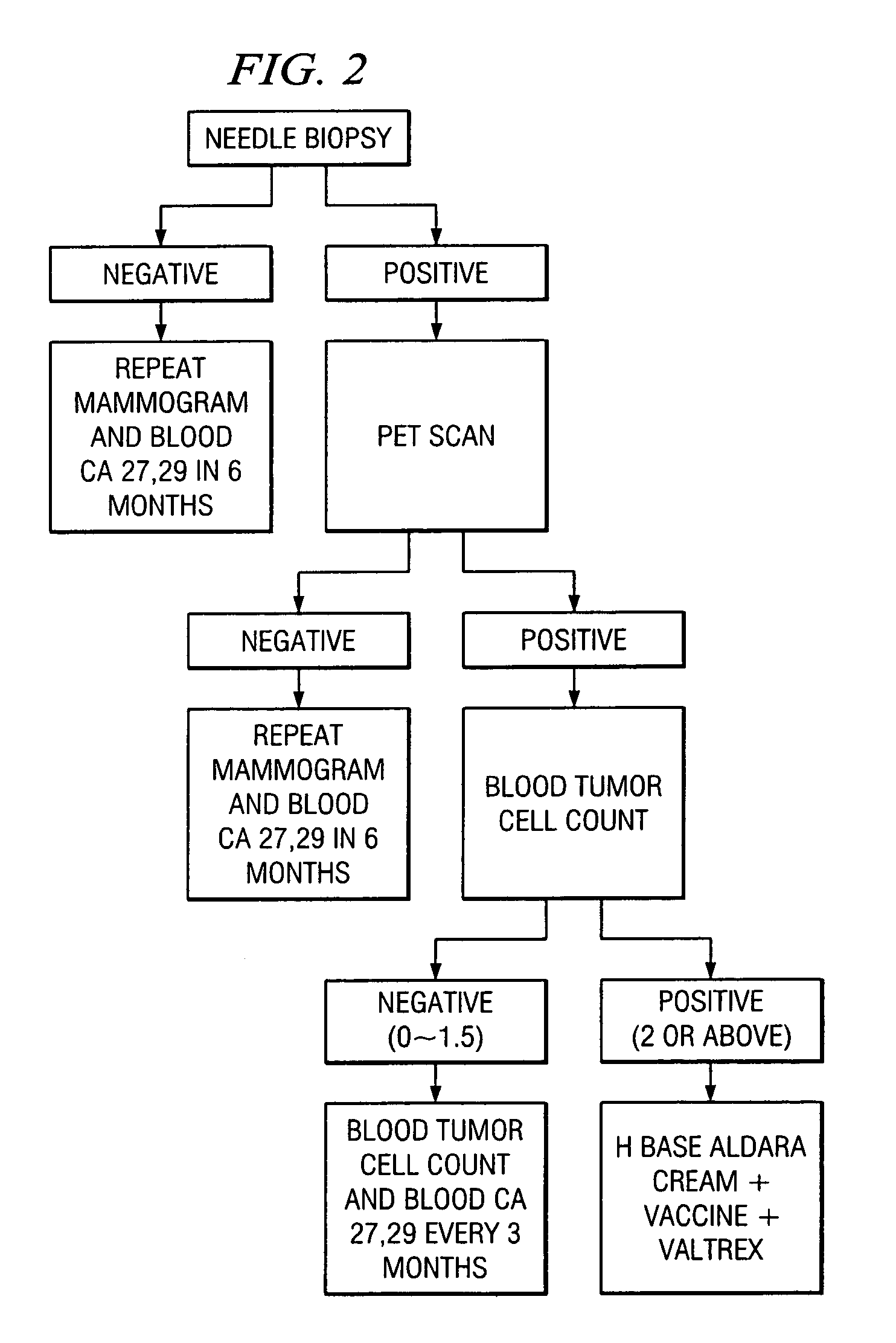
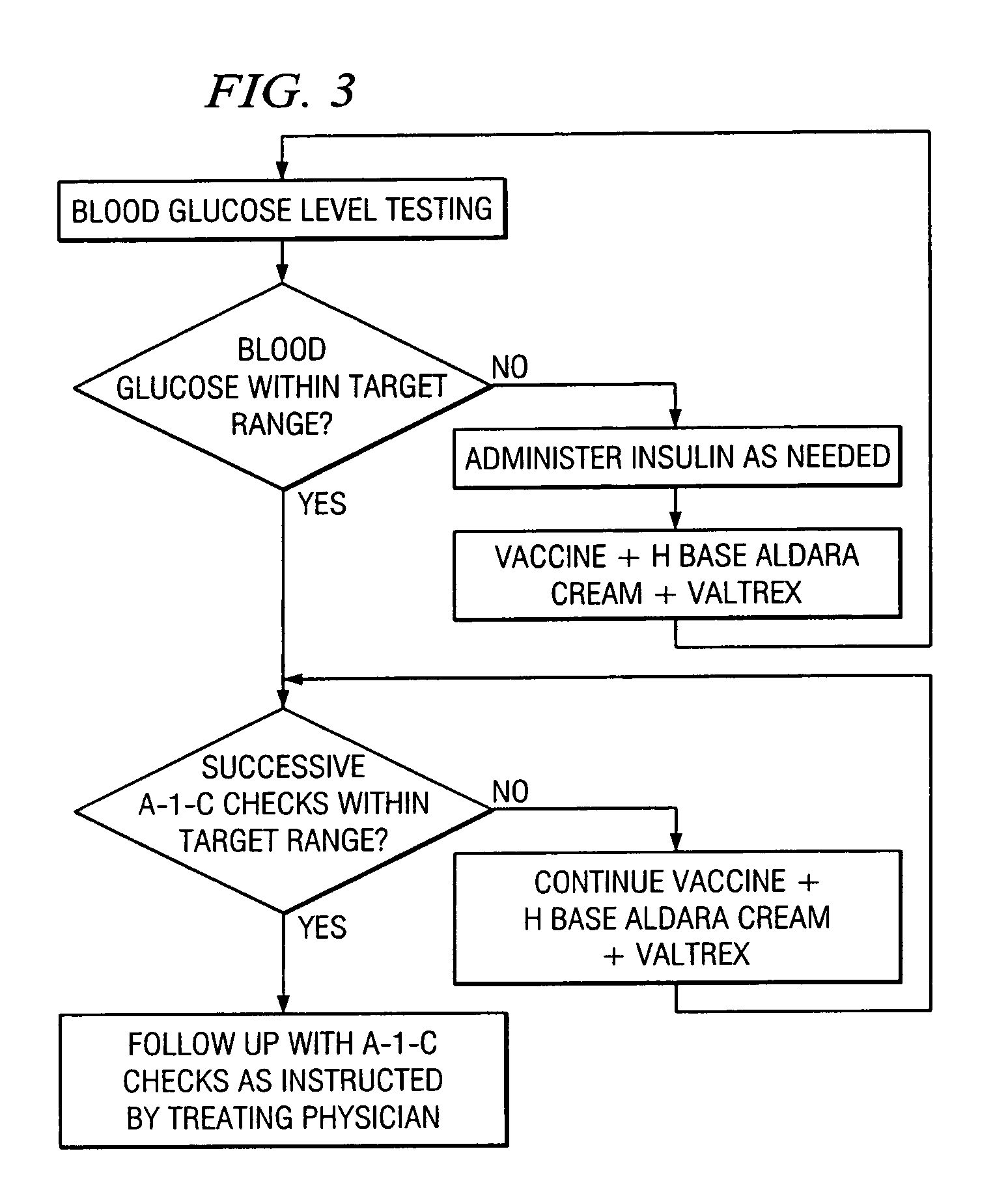
A method of cancer screening comprising the steps of administering the Blood CA 27,29 testing procedure; if the result is positive administering a mammogram; if the result is positive administering an needle biopsy; if the result is positive administering a PET scan; if the result is positive administering a blood tumor cell count. If all of the foregoing steps are positive, the cancer is treated by applying imiquimod transdermally to rotating sites, preferably by mixing ALDARA® (imiquimod) 5% cream with an equal amount of H base cream; administering a vaccine containing tumor necrosis factor, preferably the BCG vaccine; and orally administering VALTREX® (valacyclovir) twice daily. The foregoing treatment method is also effective in treating Type I diabetes, MS, and other epidermal cancers.
Owner:WOODWARD FAMILY LTD A PARTNERSHIP OF THE STATE OF TEXAS JOHN R WOODWARD GENERAL PARTNER +1
Method of cancer screening; method of cancer treatment; and method of diabetes treatment
A method of cancer screening comprising the steps of administering the Blood CA 27,29 testing procedure; if the result is positive administering a mammogram; if the result is positive administering a needle biopsy; if the result is positive administering a PET scan; if the result is positive administering a blood tumor cell count. If all of the foregoing steps are positive, the cancer is treated by applying imiquimod transdermally to rotating sites, preferably by mixing ALDARA (TM) (imiquimod) 5% cream with an equal amount of H base cream (TM); administering a vaccine that induces production of tumor necrosis factor, preferably the BCG vaccine; and orally administering Valtrex (TM) (valacyclovir) twice daily. The foregoing treatment method is also effective in treating Type I diabetes, MS, and other epidermal cancers.
Owner:LES MEDECINS
Cationic phospholipid-polymer hybridized nanoparticle vaccine adjuvant of common-carrier antigen, MPLA (Monophosphoryl Lipid A) and IMQ (Imiquimod) as well as preparation method and application thereof
ActiveCN108743939AGood biocompatibilityPromote degradationPharmaceutical non-active ingredientsAntibody medical ingredientsDendritic cellPhospholipid
The invention relates to a cationic phospholipid-polymer hybridized nanoparticle vaccine adjuvant of a common-carrier antigen, MPLA (Monophosphoryl Lipid A) and IMQ (Imiquimod) as well as a preparation method and application thereof. The vaccine adjuvant is characterized in that the IMQ as a TLR7 agonist is loaded on a hydrophobic core; the MPLA as a TLR4 agonist is loaded in a phopholipid layer;cationic phospholipid DOTAP (1,2-dioleoy-3-trimethylammonium-propane) in the phopholipid layer is used for adsorbing an antigen; the antigen is protected through hybridized nanoparticles, and the ingestion of the antigen by dendritic cells is improved; immune response after antigen stimulation is improved remarkably through the TLR agonist, and cross-presentation of the antigen is improved remarkably. The hybridized nanoparticles as the vaccine adjuvant can load the antigen and different types of TLR agonists simultaneously, can deliver the antigen through a plurality of immune paths, and promotes the DC activation and maturation. The cross-presentation level is raised, a strong and powerful T-cell killing effect is achieved, cell factor secretion is induced, a long-term memory T-cell reaction is generated, and higher prevention capability for tumors is achieved.
Owner:INST OF BIOMEDICAL ENG CHINESE ACAD OF MEDICAL SCI
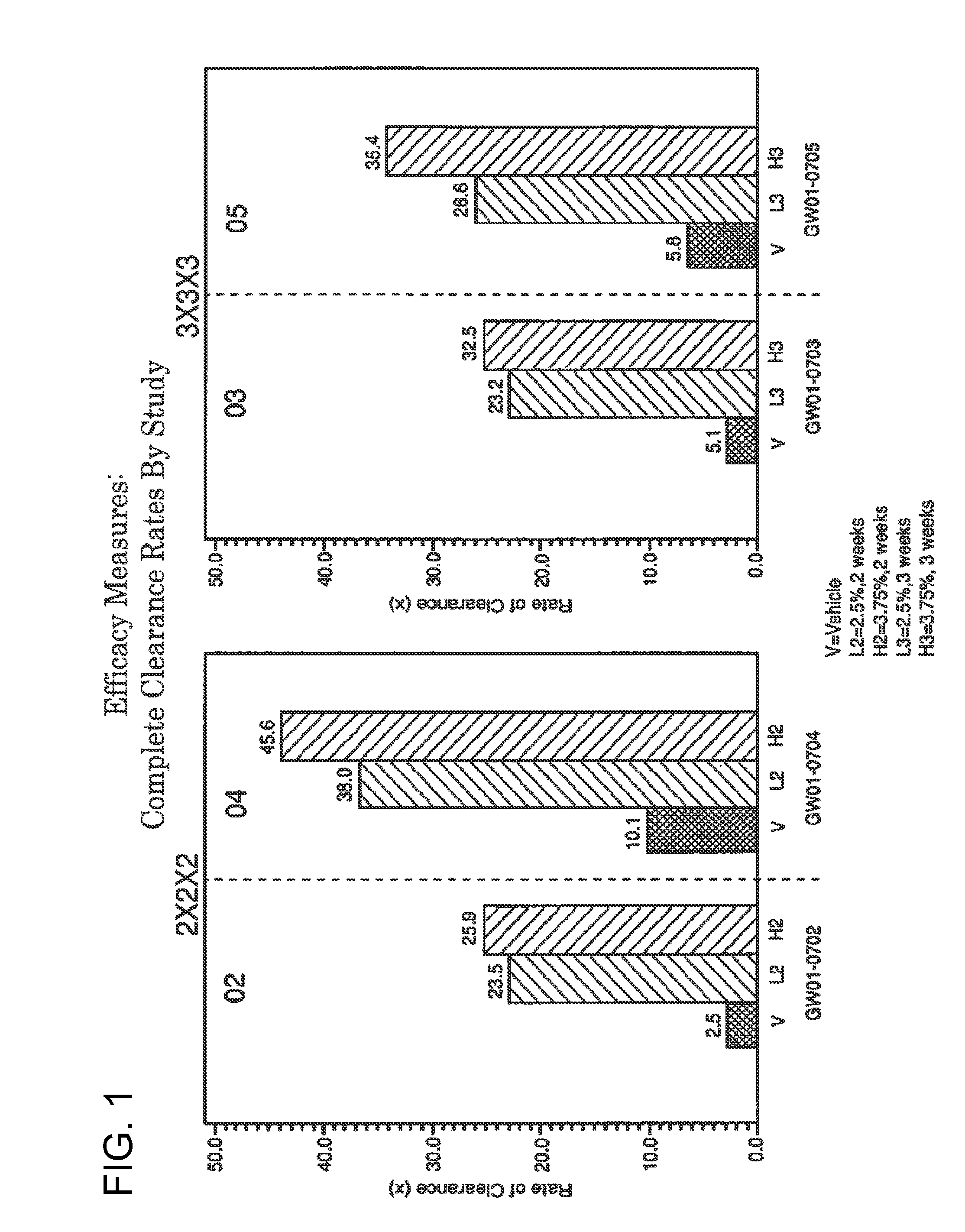
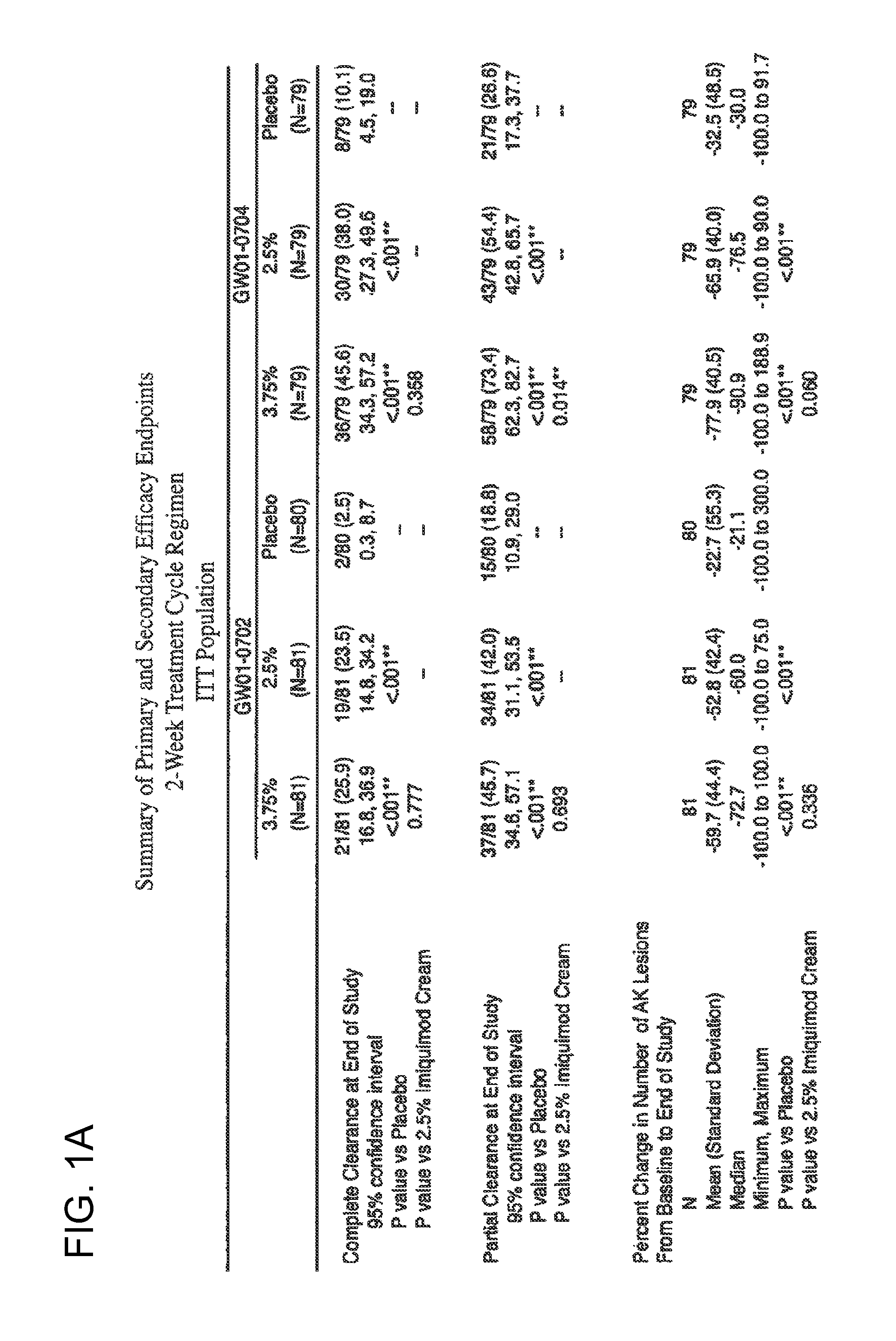
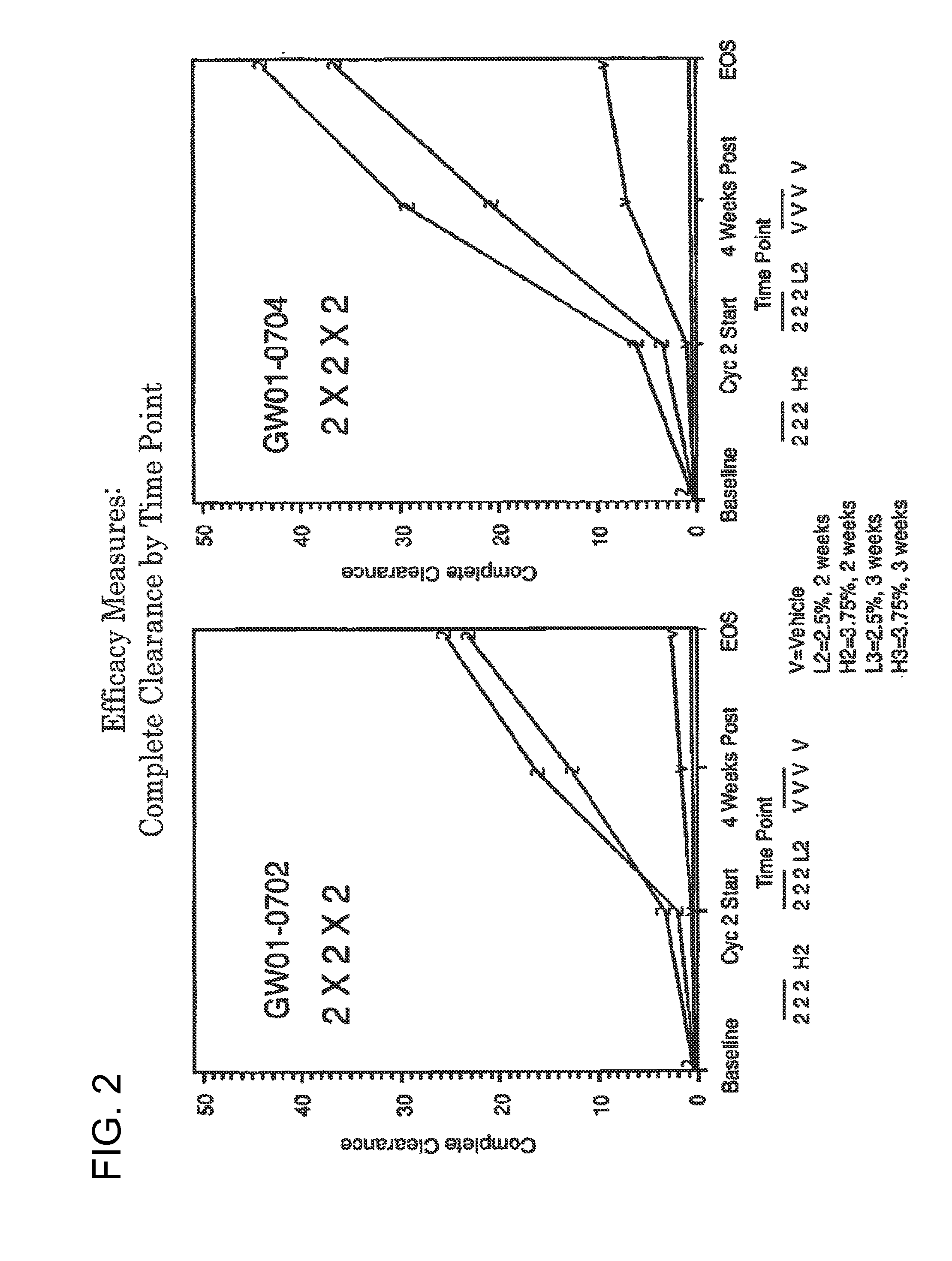
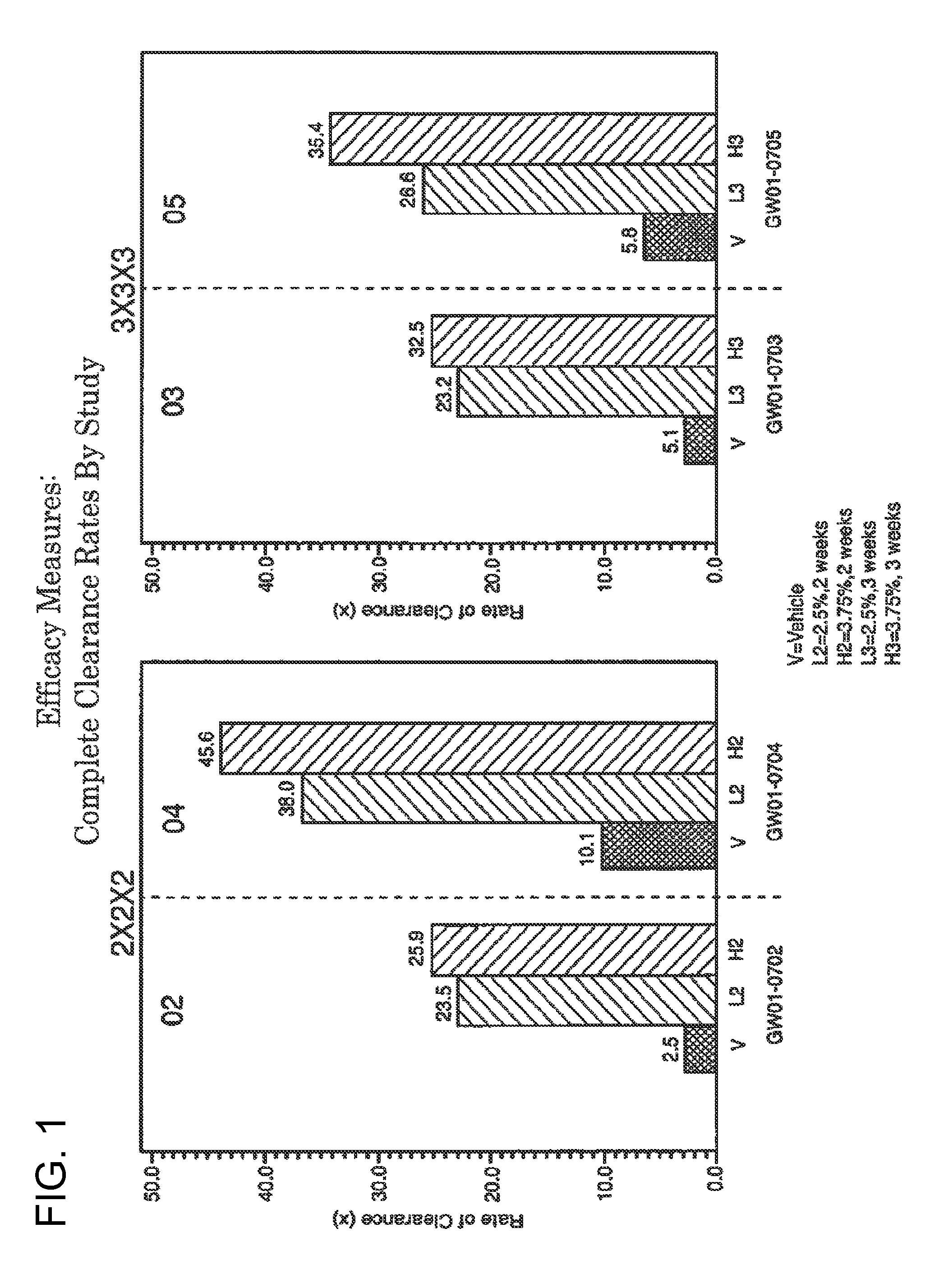
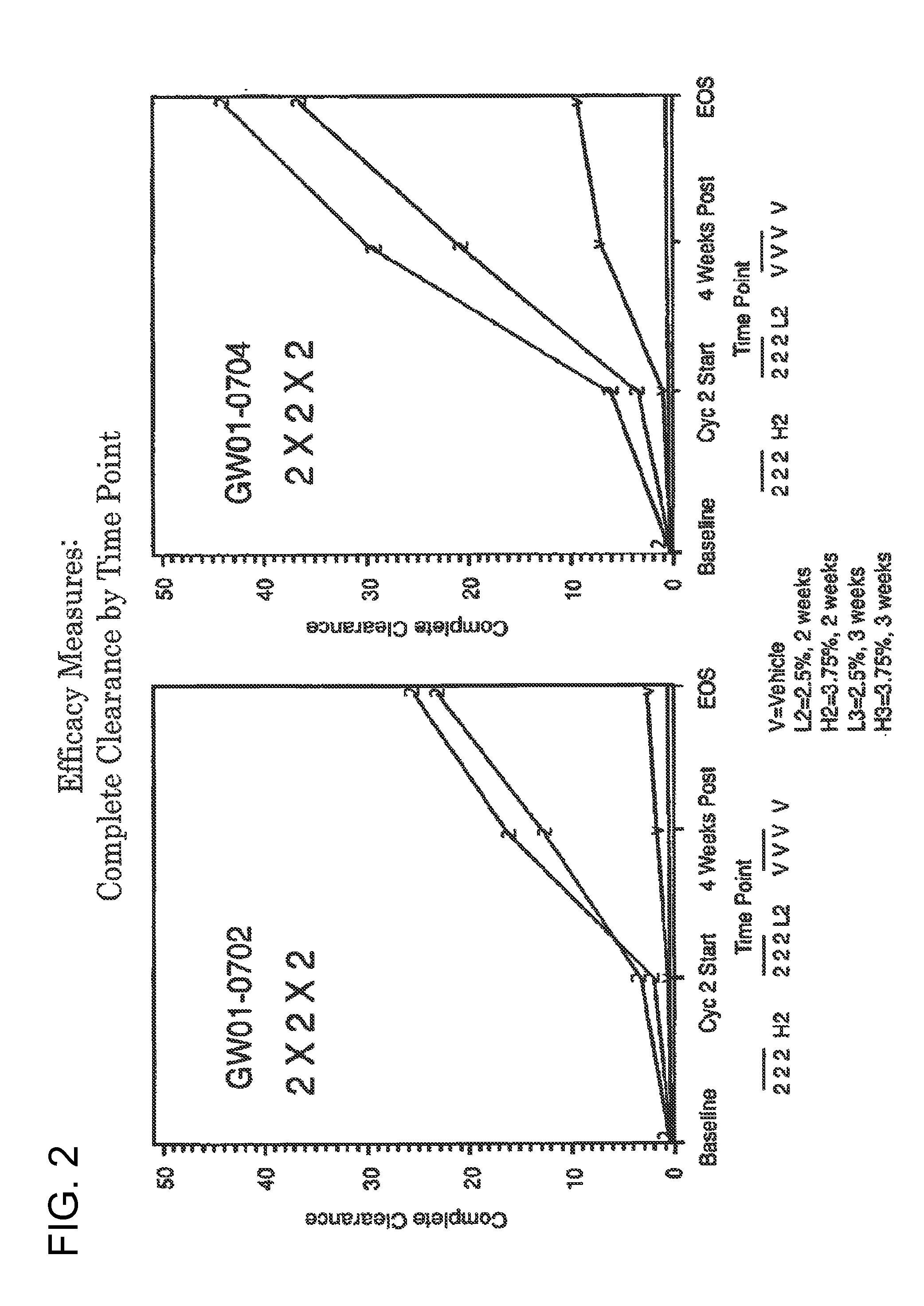
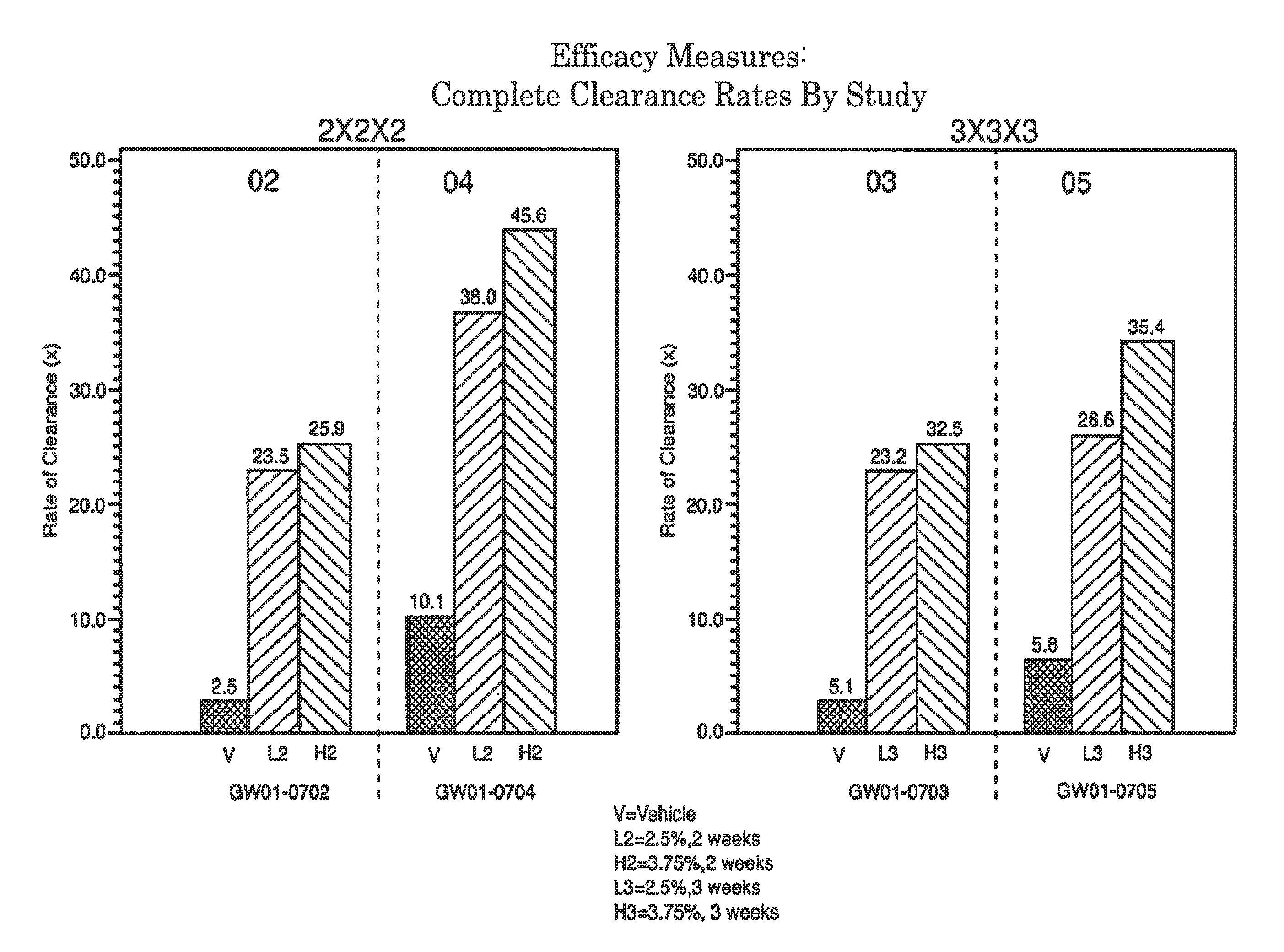
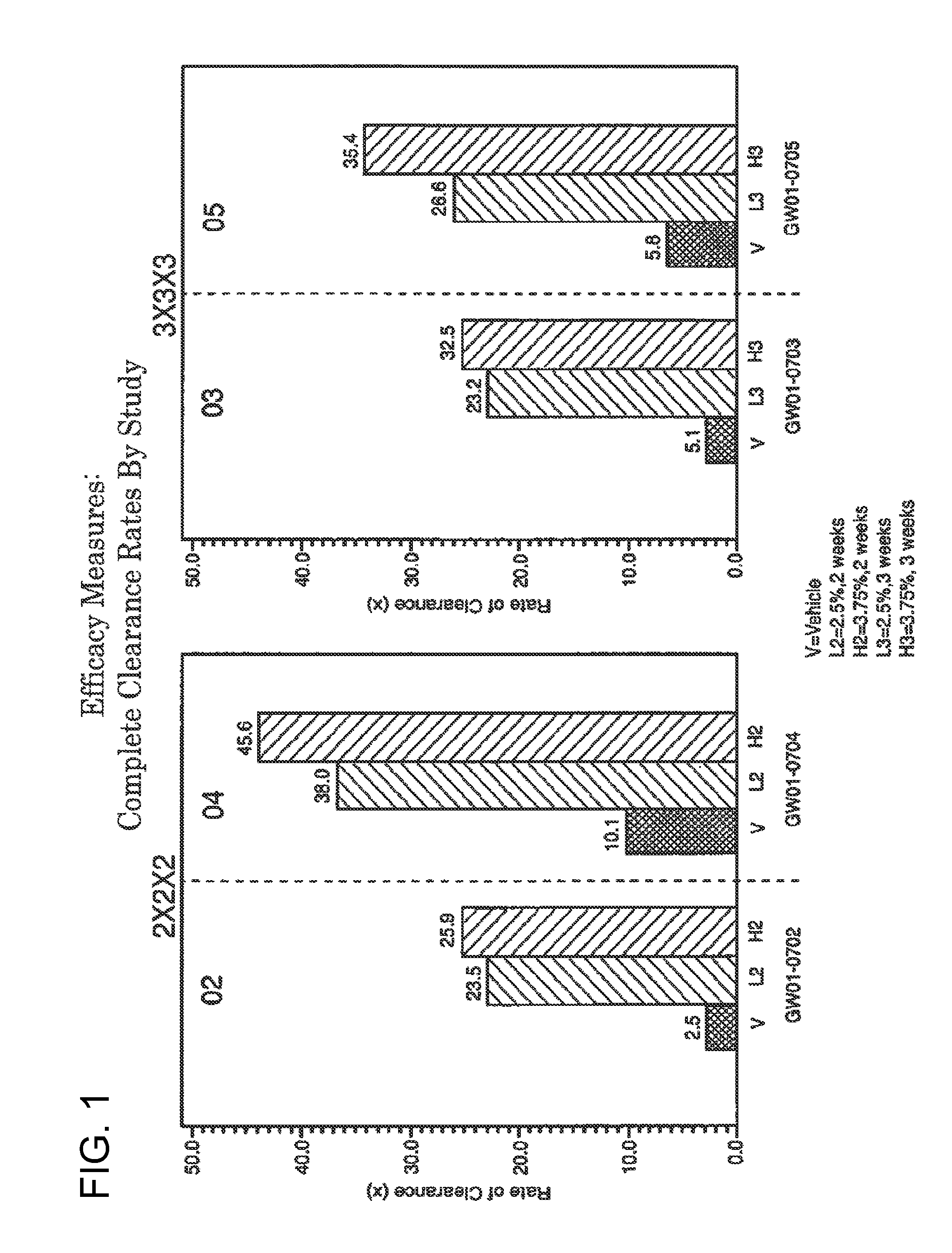
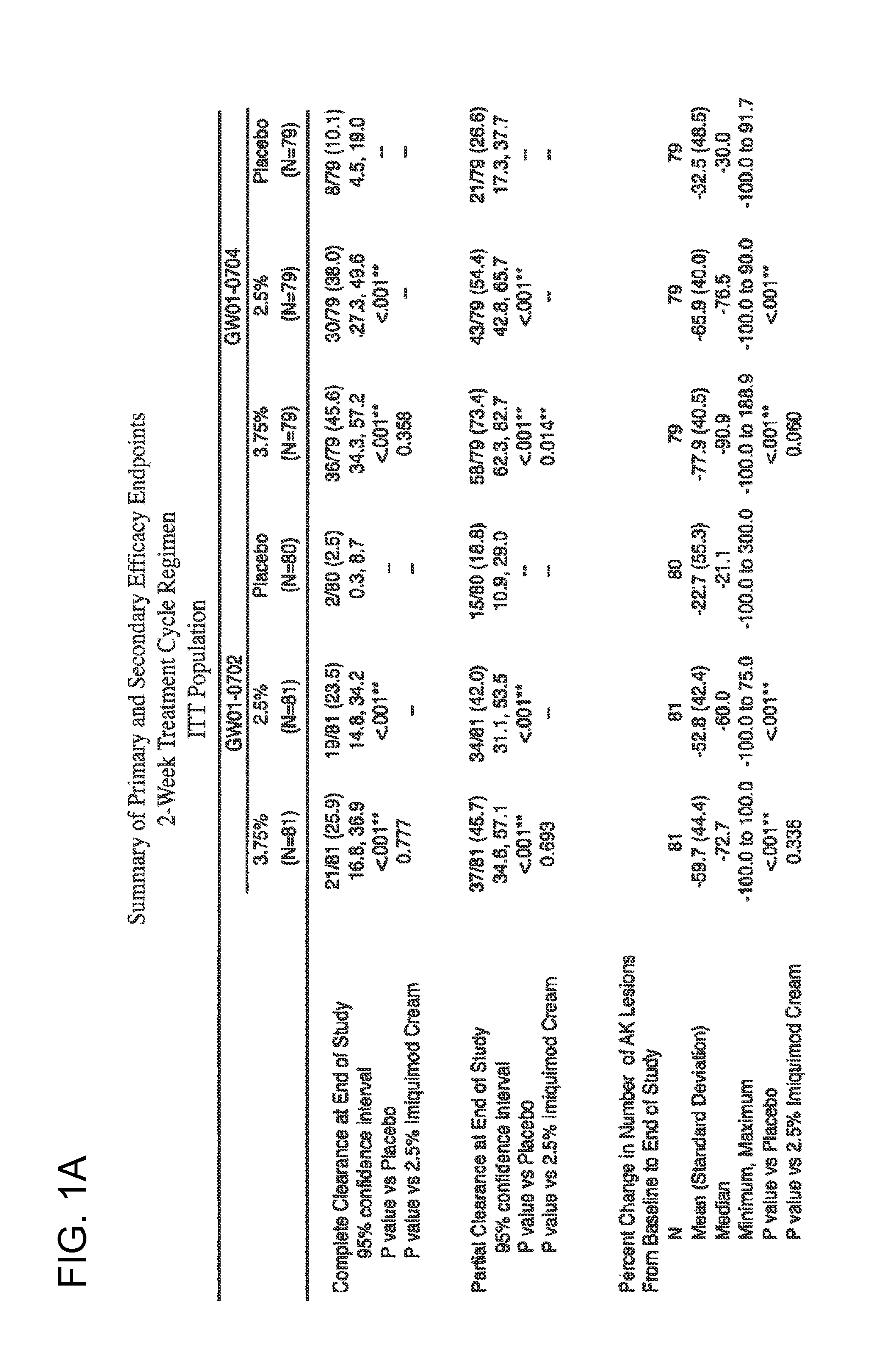
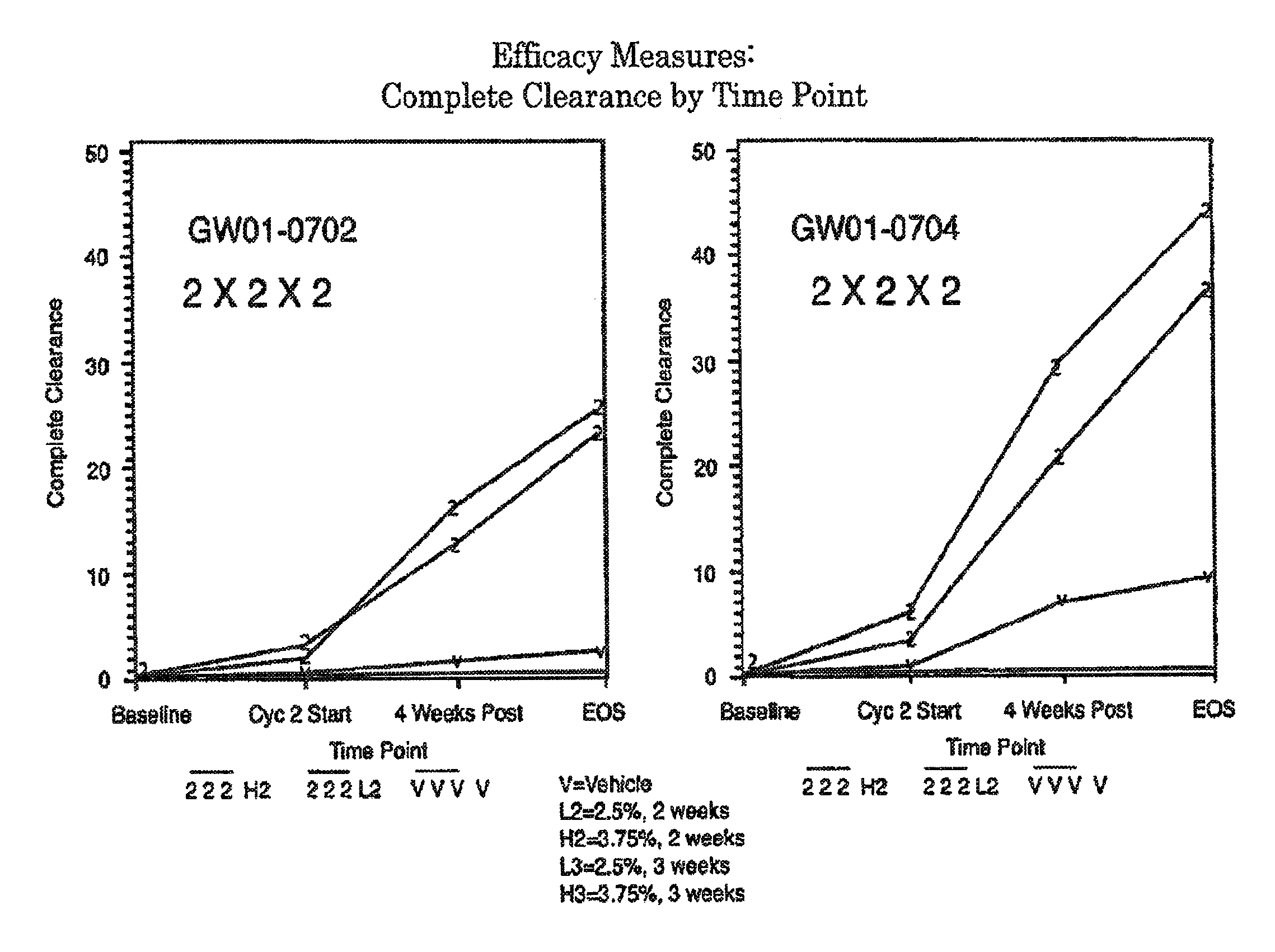
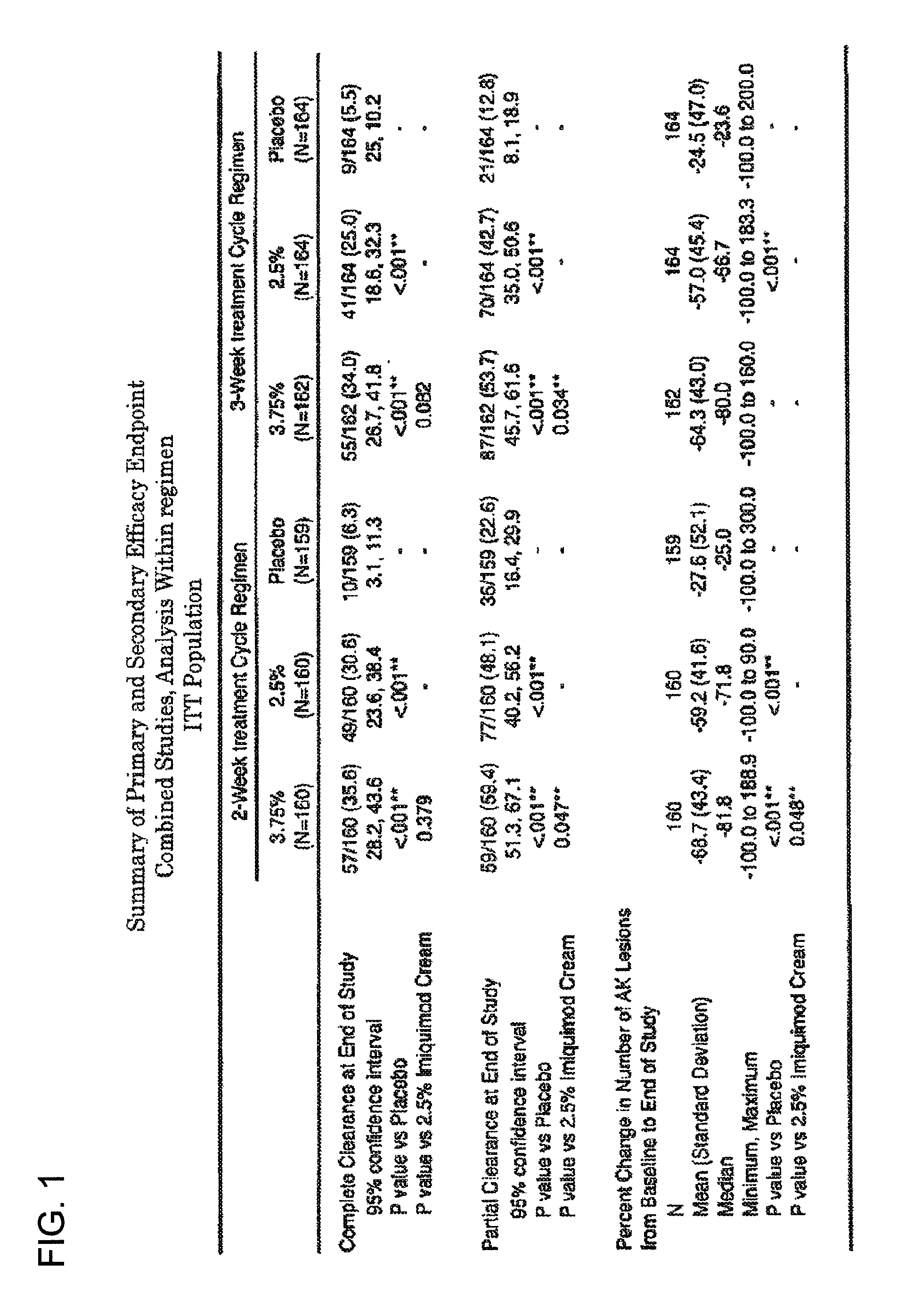
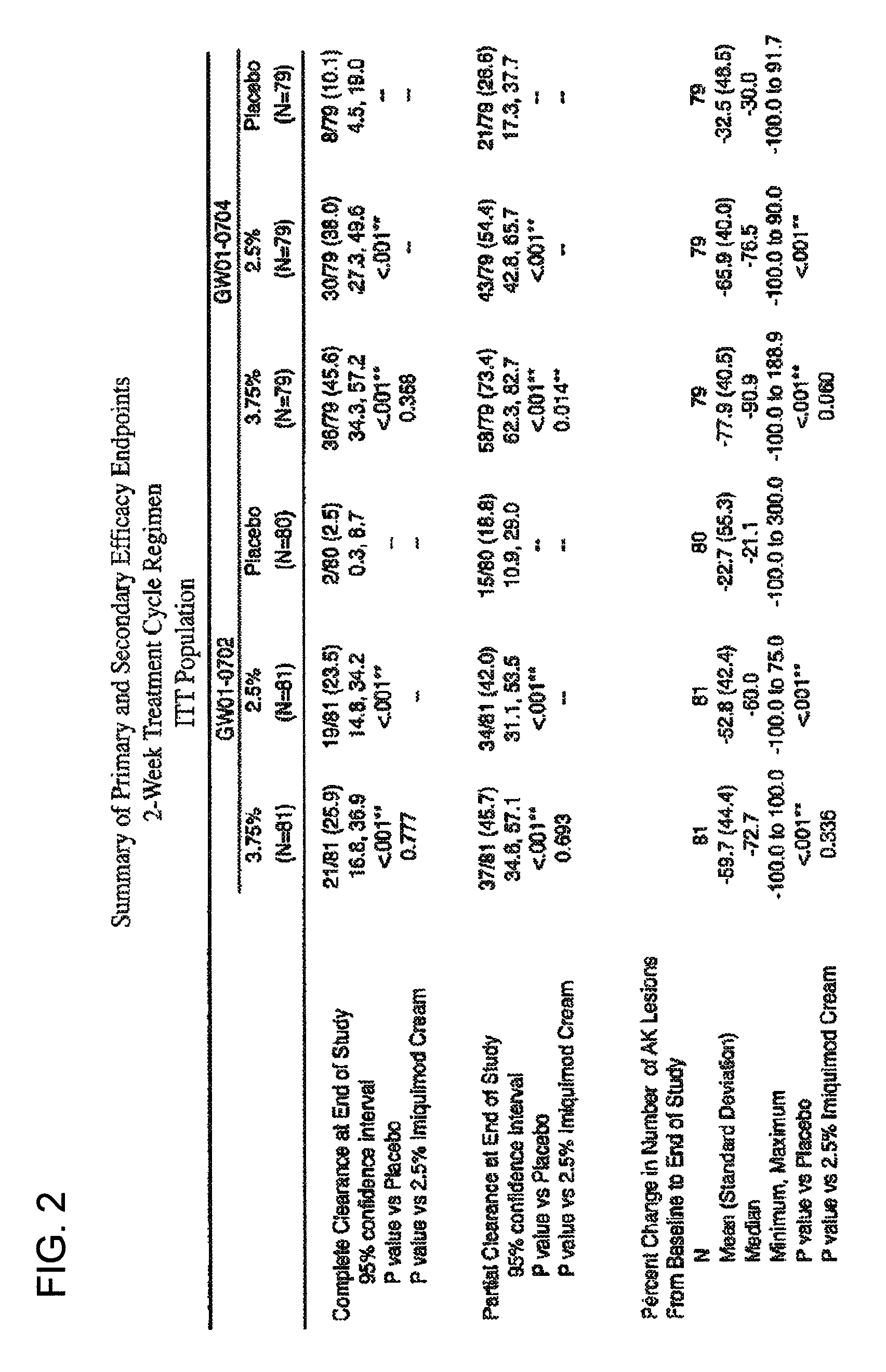
2 x 2 x 2 WEEK DOSING REGIMEN FOR TREATING ACTINIC KERATOSIS WITH PHARMACEUTICAL COMPOSITIONS FORMULATED WITH 3.75 % IMIQUIMOD
ActiveUS20110257218A1Improved imiquimodReduced strengthBiocideOintment deliveryDosing regimenRegimen
Owner:MEDICIS PHARMA CORP
3 x 3 x 3 WEEK TREATMENT REGIMEN FOR TREATING ACTINIC KERATOSIS WITH PHARMACEUTICAL COMPOSITIONS FORMULATED WITH 2.5% IMIQUIMOD
InactiveUS20110257217A1Improved imiquimodReduced strengthBiocideOintment deliveryDosing regimenRegimen
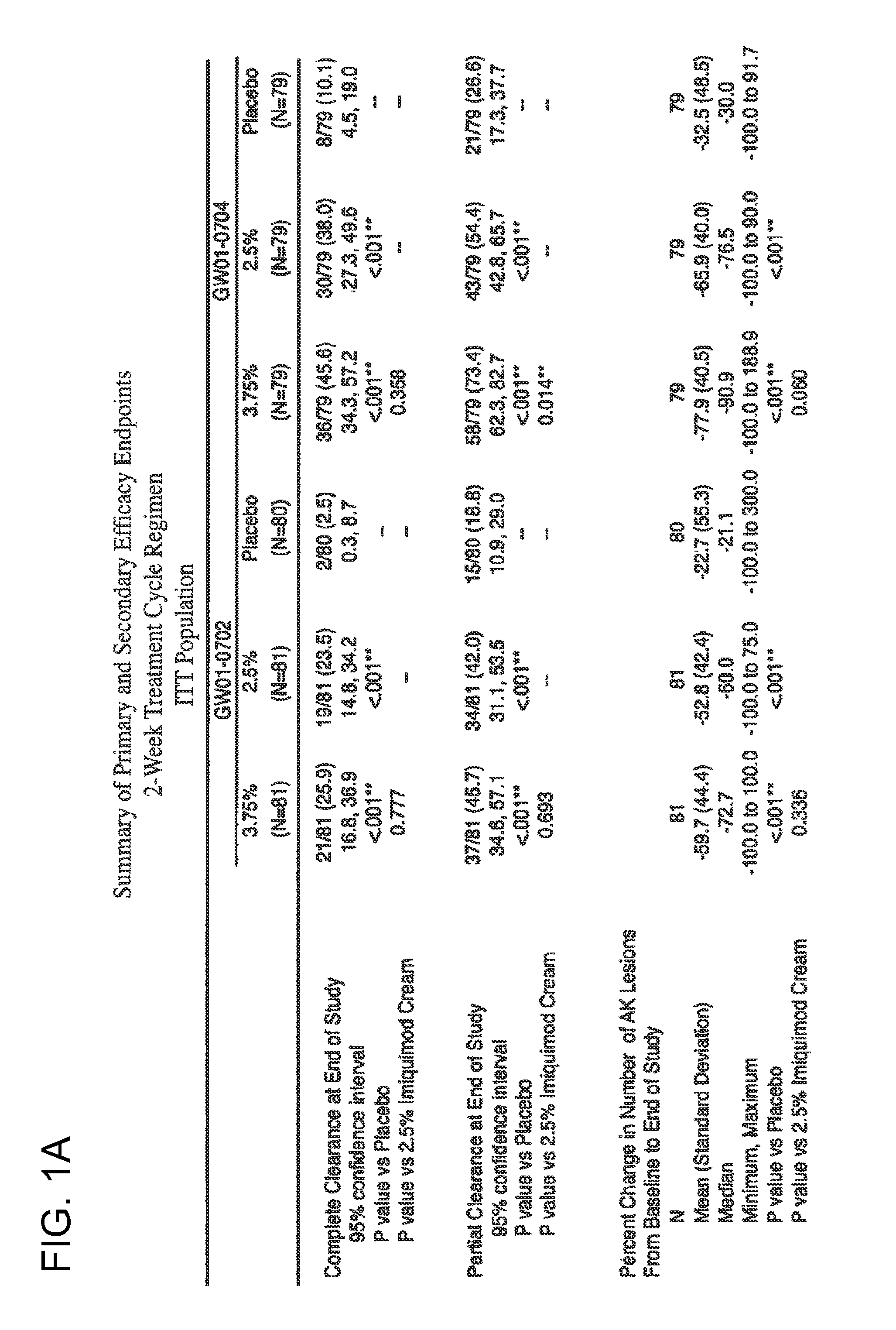
Pharmaceutical formulations and methods for the topical or transdermal delivery of 1-isobutyl-1H-imidazo[4,5-c]-quinolin-4-amine or 1-(2-methylpropyl)-1H-imidazo[4,5-c]quinolin-4-amine, i.e., imiquimod, to treat actinic keratosis with short durations of therapy, than currently prescribed for the commercially available Aldara® 5% imiquimod cream, as now approved by the U.S. Food & Drug Administration (“FDA”), are disclosed and described. More specifically, lower dosage strength imiquimod formulations to deliver an efficacious dose of imiquimod for treating actinic keratosis with an acceptable safety profile and dosing regimens that are short and more convenient for patient use than the dosing regimen currently approved by the U.S. Food & Drug Administration (“FDA”) for Aldara° 5% imiquimod cream to treat actinic keratosis are also disclosed and described.
Owner:MEDICIS PHARMA CORP
Preparation method of ointment for treating infantile hemangioma
InactiveCN106729614AAvoid bleedingPromote circulationOrganic active ingredientsAerosol deliveryVitamin CVitamin B12
The invention relates to a preparation method of an ointment for treating infantile hemangioma. The preparation method comprises the following steps: adding timolol hydrogenmaleate, propranolole hydrochloride, tacrolimus, pingyangmycin, vitamin C, vitamin E and vitamin B12 to water according to certain mass ratio, heating to 30-85 DEG C of the temperature, adding a thickener, imiquimod and a wetting agent according to certain mass ratio after stirring and dissolving, and uniformly stirring, and preparing the ointment for treating the infantile hemangioma after cooling. The ointment for treating the infantile hemangioma is an external used medicine, particularly suitable for the infants inconvenient for taking medicines and injection.
Owner:刘腾
Lower dosage strength imiquimod formulations and short dosing regimens for treating genital and perianal warts
InactiveUS20110207766A1Low dosage strengthSimplified dosing regimenBiocideOintment deliveryDosing regimenRegimen
Pharmaceutical formulations and methods for the topical or transdermal delivery of 1isobutyl-1H-imidazo[4,5-c]-quinolin-4-amine or 1-(2-methylpropyl)-1H-imidazo[4,5 c]quinolin-4-amine, i.e., imiquimod, to treat genital / perianal warts with shorter durations of therapy than currently prescribed for the commercially available Aldara® 5% imiquimod cream, as now approved by the U.S. Food & Drug Administration (“FDA”), are disclosed and described. More specifically, lower dosage strength imiquimod formulations to deliver an efficacious dose of imiquimod for treating genital / perianal warts with an acceptable safety profile and dosing regimens that are shorter and more convenient for patient use than the dosing regimen currently approved by the U.S. Food & Drug Administration (“FDA”) for Aldara® 5% imiquimod cream to treat genital / perianal warts are also disclosed and described.
Owner:MEDICIS PHARMA CORP
Method for preparing dendritic cells with capacity to induce cancer-specific immune response, and pharmaceutical composition and kit incorporating dendritic cells for preventing or treating cancer or inhibiting metastasis
InactiveCN102439137AEnhanced ability to induce immune responsesLow toxicity and very safePeptidesMammal material medical ingredientsDendritic cellCancer metastasis
The present invention relates to a method for preparing mature dendritic cells, having a significantly improved capacity for inducing a cancer-specific immune response; a pharmaceutical composition for preventing or treating a cancer or inhibiting cancer metastasis, containing the mature dendritic cells prepared by using the above method; and a pharmaceutical kit incorporating the mature dendritic cells and an imiquimod prepared by the above method. Accordingly, it is possible to prepare safe mature dendritic cells which significantly improves the induction capacity of the cancer-specific immune response and lowers toxicity. The mature dendritic cells are effective in preventing and treating cancer, and inhibiting metastasis. In parallel with administering the cancer-specific mature dendritic cells, the imiquimod used for preparing the pharmaceutical kit improves cancer immunotherapy and prevents or treats a cancer and inhibits cancer metastasis.
Owner:JW PHARMA CORP
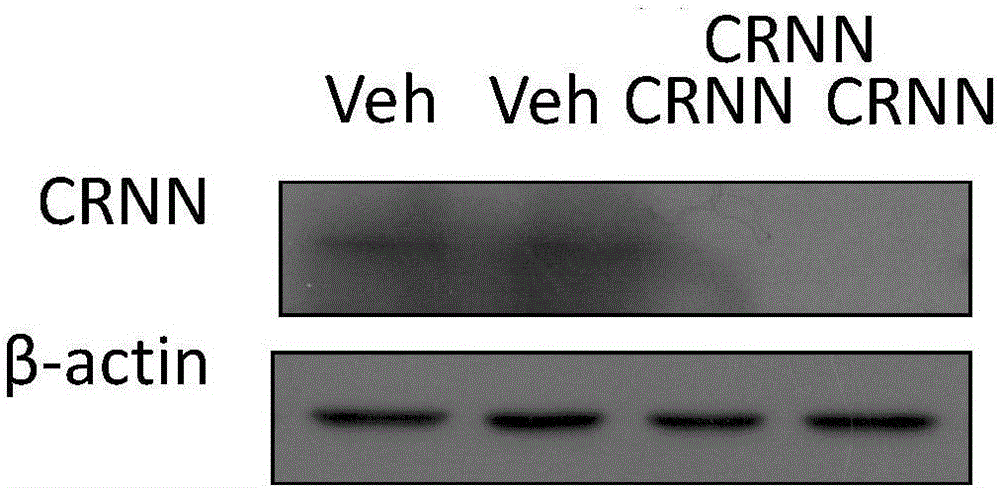
Application of Cornulin as target in preparation of drug for preventing and treating psoriasis
ActiveCN106620694AInhibition returns to normalIncreasing the thicknessOrganic active ingredientsGenetic material ingredientsAkt signallingDrug target
The invention discloses an application of Cornulin as a target in preparation of a drug for preventing and treating psoriasis. Cornulin is taken as the drug target on the gene level and / or the protein level for the drug. Cornulin is positively correlated with development of psoriasis, when a Cornulin antibody with proper concentration is used for treating an imiquimod mouse model of psoriasis, scales and skin lesion thickness can be improved, and AKT signals can be inhibited to inhibit development of psoriasis and inhibit skin lesion for returning to normal; compared with other psoriasis treating drugs, and the Cornulin antibody has the advantages that the intervention target is clearer, that is, AKT signal related molecular pathways in inflammatory pathways for psoriasis incidence are targeted.
Owner:XI AN JIAOTONG UNIV
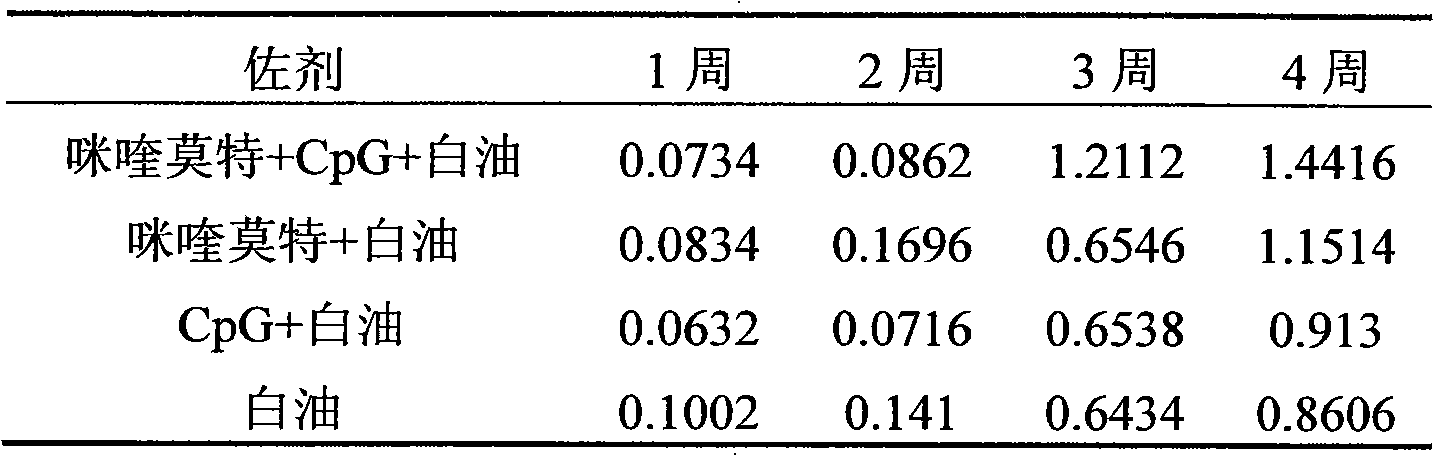
Compound immunological adjuvant and vaccine
ActiveCN101850117AHigh activityImprove immune responseViral antigen ingredientsAntiviralsMicroorganismRecombinant peptide
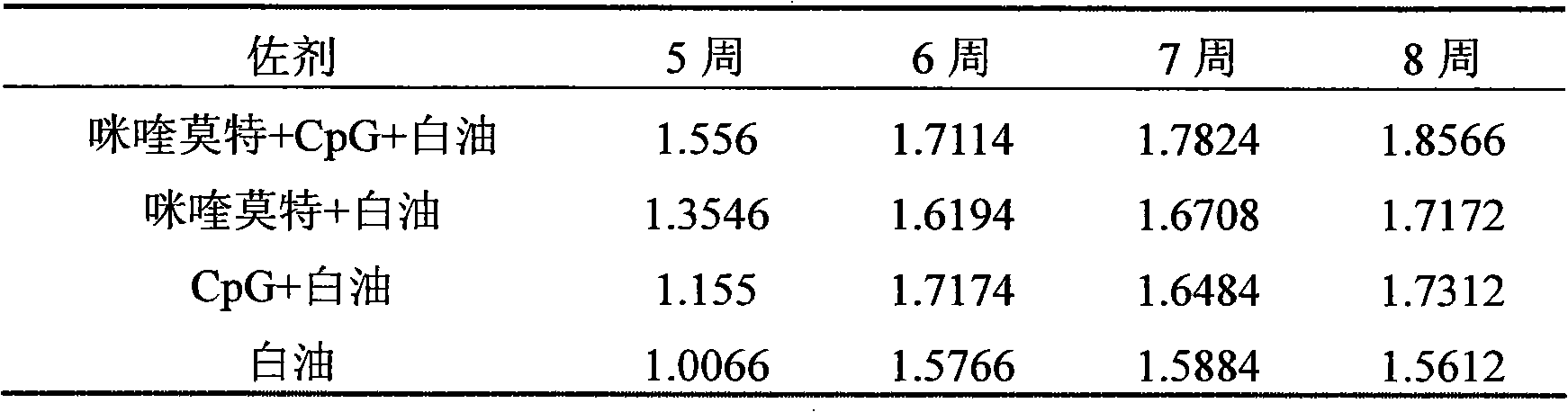
The invention relates to a compound immunological adjuvant comprising a water phase solution and an oil phase solution which are respectively prepared, wherein the water phase solution is a CpG water solution the concentration of which is 0.5 to 2.0mg / mL; the oil phase solution is an imiquimod white oil solution the concentration of which is 0.25 to 1.0mg / mL; and the volume ratio of the water phase solution to the oil phase solution is 1:6. The invention also relates to a vaccine prepared from one or more antigens selected from attenuated live full microorganisms, inactivated microorganisms, recombinant peptide and proteins, synthetic peptide and cracked microorganisms and the water phase solution and the oil phase solution in the compound immunological adjuvant according to the volume ratio of 1:1:6. By the compounding of imiquimod, CpG and a white oil component, the compound immunological adjuvant has an obvious synergistic effect and enhances the immunological activity reactions of Th1 and Th2, so that the activity of stimulated immunological cells is obviously enhanced.
Owner:国家兽用生物制品工程技术研究中心 +2
Imiquimod micro emulsion gels for local skin and preparation method thereof
InactiveCN101756886AImproves transdermal penetrationImprove permeabilityOrganic active ingredientsPharmaceutical delivery mechanismDispersityEmulsion
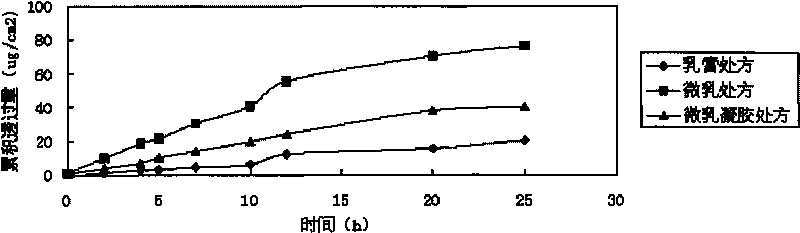
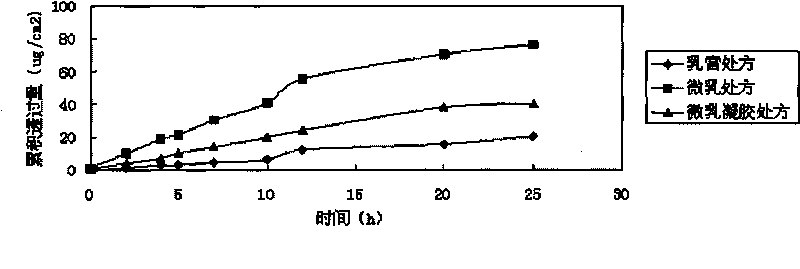
The invention relates to imiquimod micro emulsion gels for local skin, at least comprising the following ingredients by mass percent: 0.1-2.5 percent of imiquimod, 1-24 percent of solvent, 1-16 percent of surface active agent, 1-12 percent of cosurfactant, 1-20 percent of gelatinizing agent and the remaining water; wherein the imiquimod is 1-isobutyl-1H-imidazole(4,5-C) quinoline-4-amine; the preparation method comprises the following steps: the imiquimod is dissolved in the solvent, the surface active agent and the cosurfactant are added in the solvent dissolved with the imiquimod, and the mixture is dispersed uniformly; water with [roper amount is added in the mixture to form micro emulsion; the gelatinizing agent is swelled by water, so as to prepare the solution with a certain concentration, the gelatinizing agent solution is dropped in the imiquimod micro emulsion dropwise, the stirring is carried out while the gelatinizing agent solution is dropped, the pH value is adjusted by adding triethanolamine, so as to prepare the imiquimod micro emulsion gels; the invention has the advantages that: the thorough dispersity of the micro emulsion to medicines, adhesiveness of gels to the skin and deformation flowability thereof under external force are concentrated, so as to lead the medicine to be applied to the affected part and cause the medicine to pass into internal body and reach target spot through the skin; the medicine effect can act on continuously.
Owner:HUAZHONG NORMAL UNIV
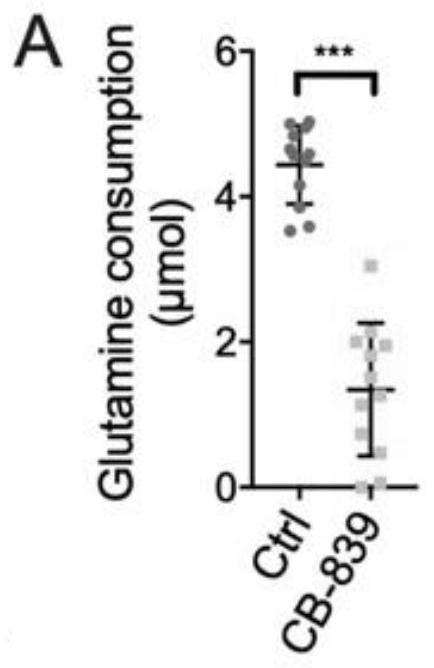
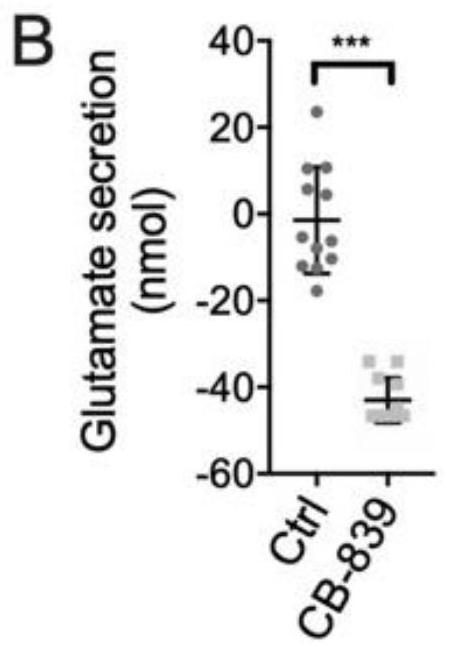
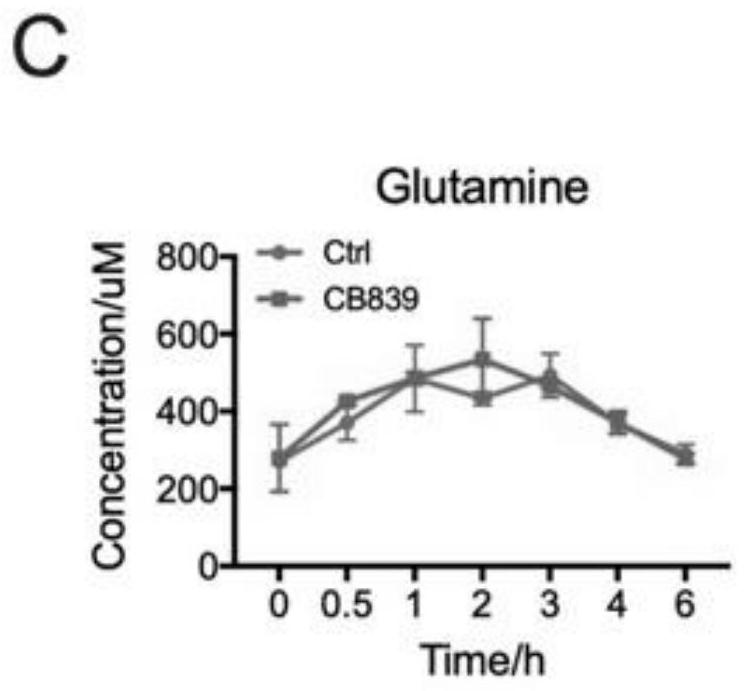
Application of glutaminase inhibitor in preparation of medicine for treating psoriasis
InactiveCN111643669AReduced activityInhibit metabolismDermatological disorderHeterocyclic compound active ingredientsCell activityApoptosis
The invention discloses application of a glutaminase inhibitor in preparation of a medicine for treating psoriasis. In-vitro cell experiments prove that the glutaminase inhibitor can inhibit keratinocyte metabolism glutamine, inhibit miR-31 induced up-regulated keratinocyte mitochondrial respiration, inhibit miR-31 induced mTOR pathway, reduce cell activity and promote cell apoptosis, and has an anti-inflammatory effect; animal experiments prove that the glutaminase inhibitor can be used for effectively treating imiquimod-induced mouse psoriasis-like pathology change, including remarkably reducing the Baker score of skin lesion, effectively reducing the epidermal hypertrophy degree, remarkably reducing the number of subepidermal capillaries, remarkably reducing the number of epidermal Ki67positive cells and reducing the expression of epidermal GLS. Therefore, the glutaminase inhibitor can be used for preparing the medicine for treating psoriasis.
Owner:GUANGDONG HOSPITAL OF TRADITIONAL CHINESE MEDICINE
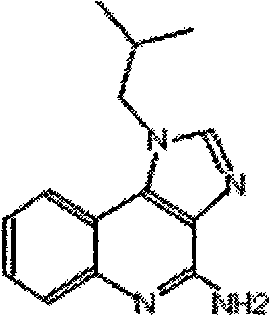
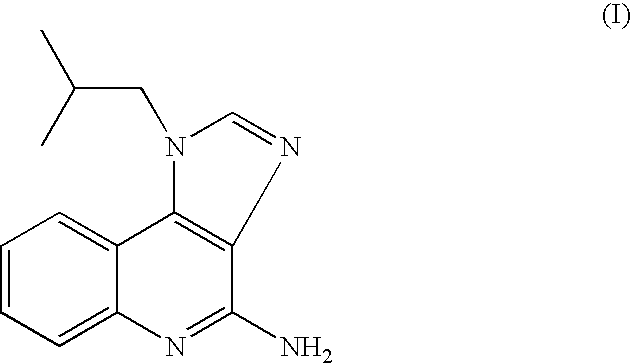
Process for preparing Imiquimod
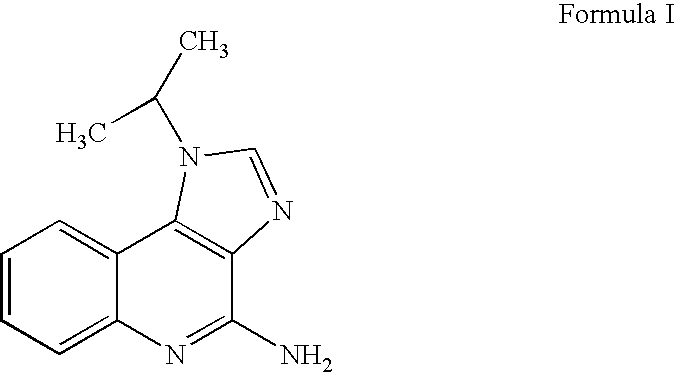
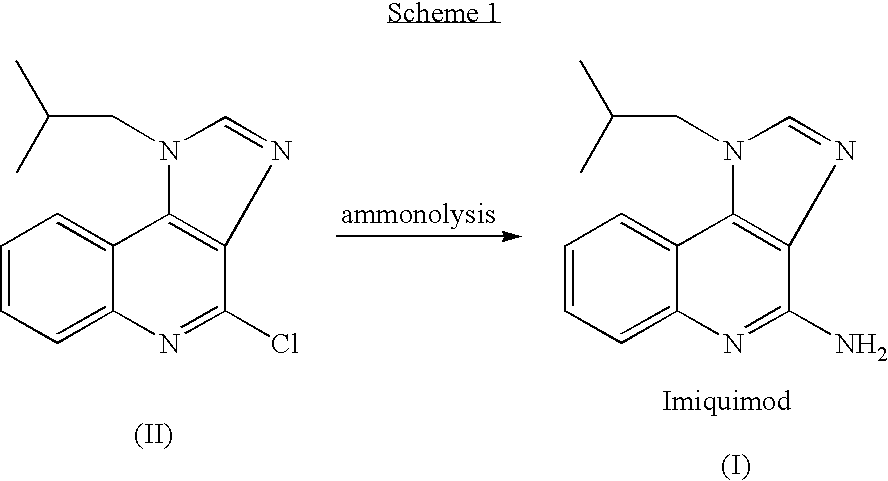
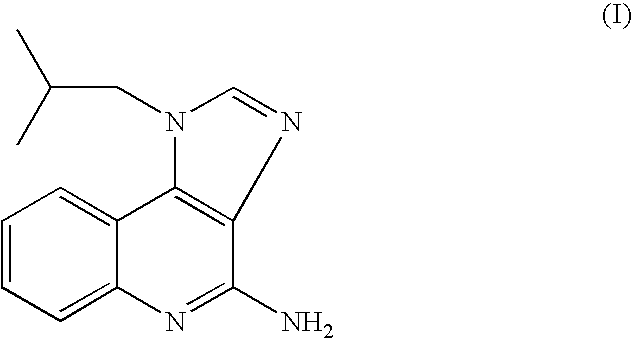
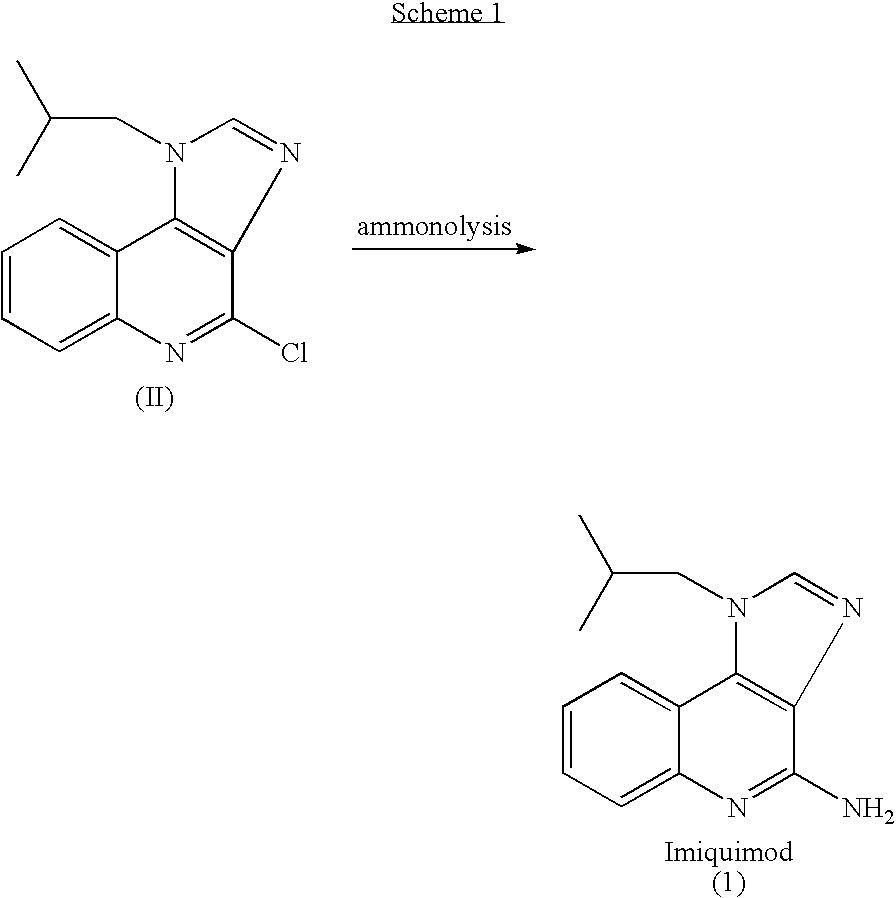
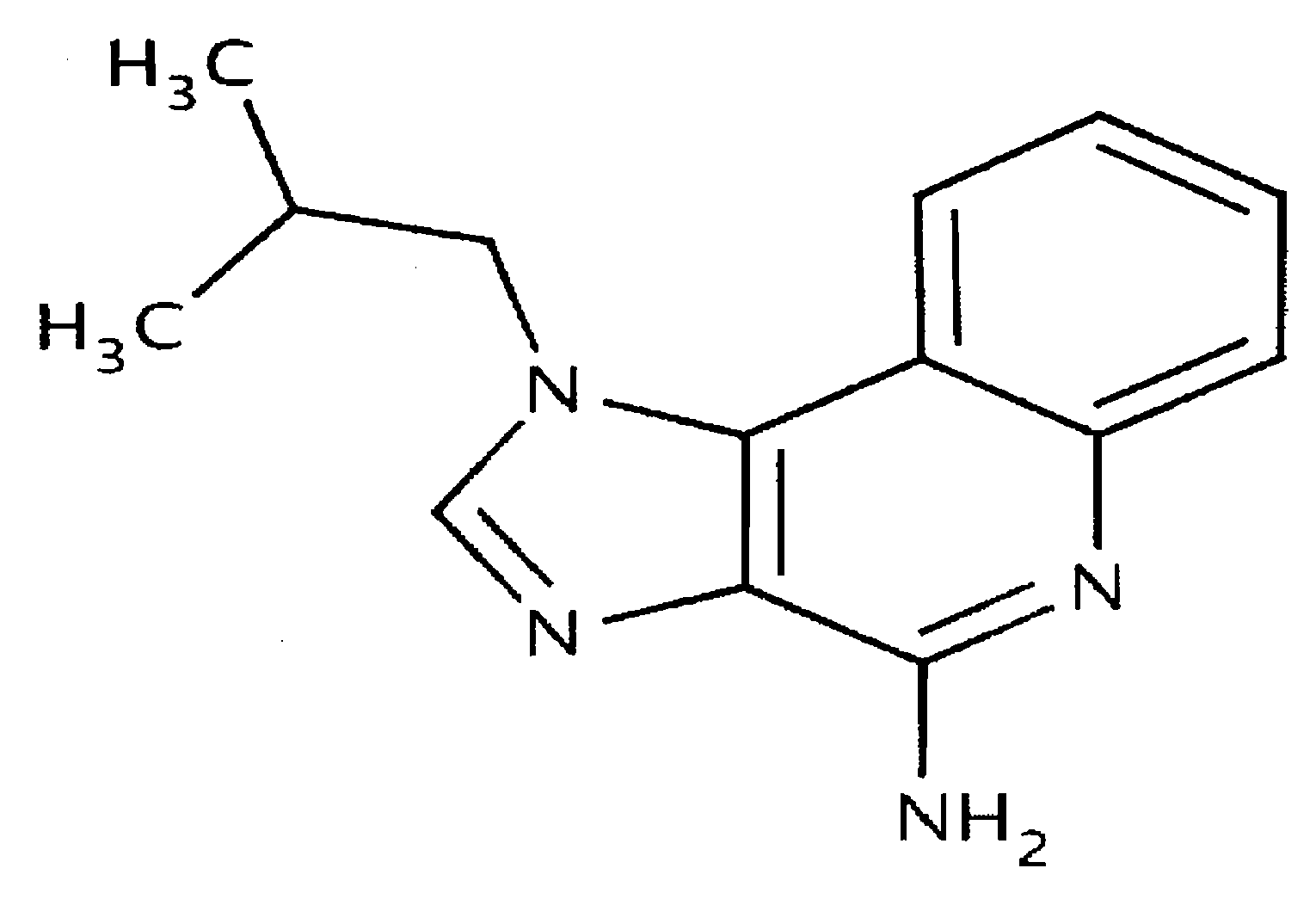
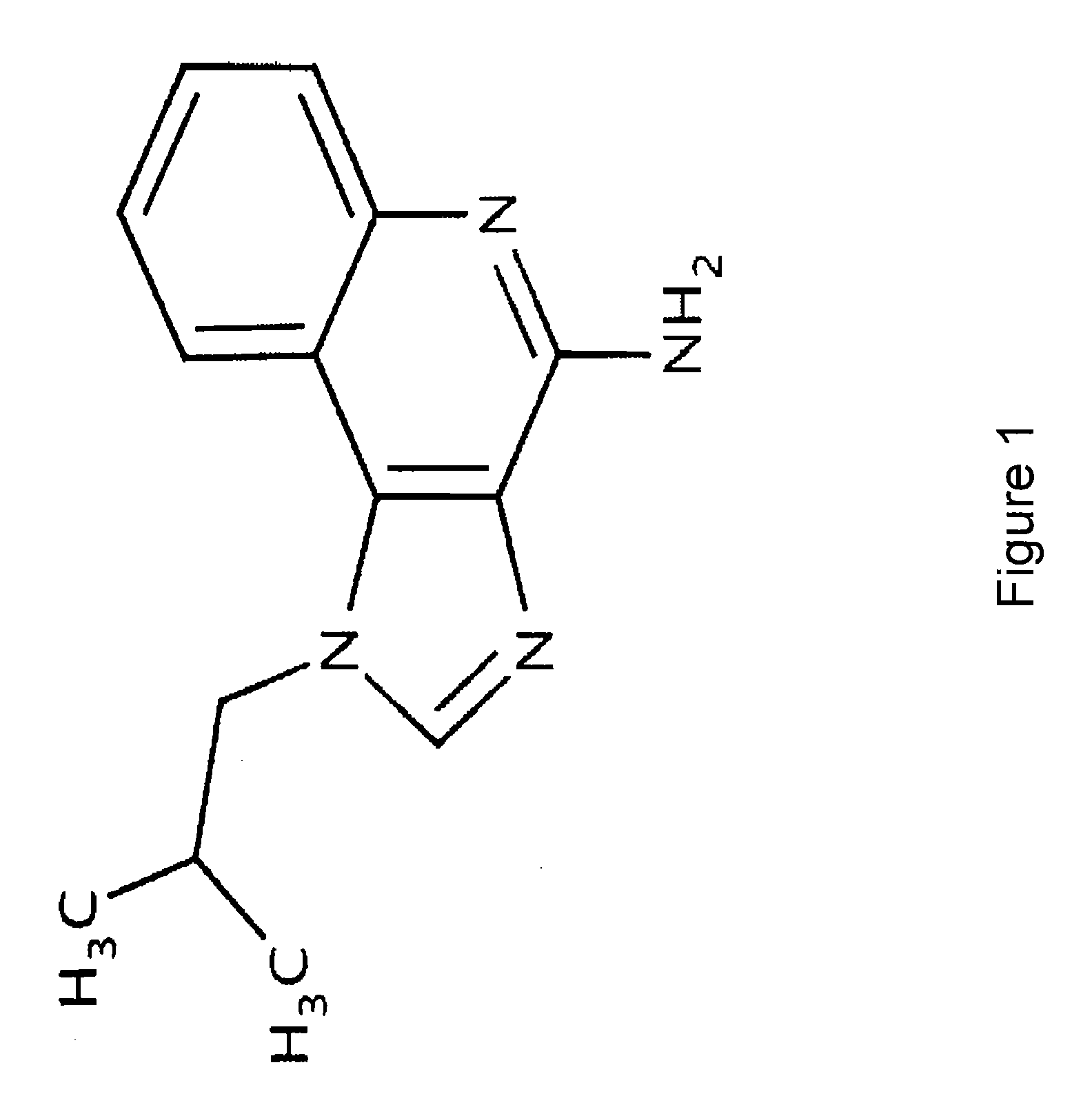
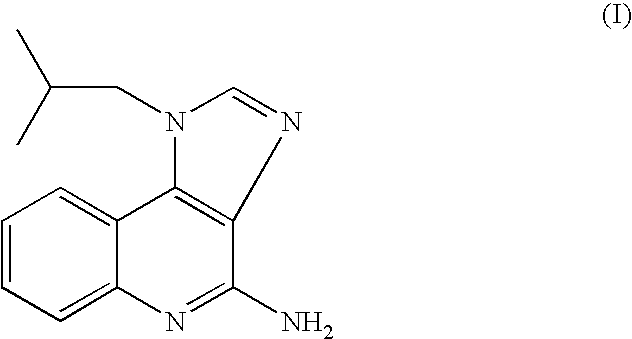
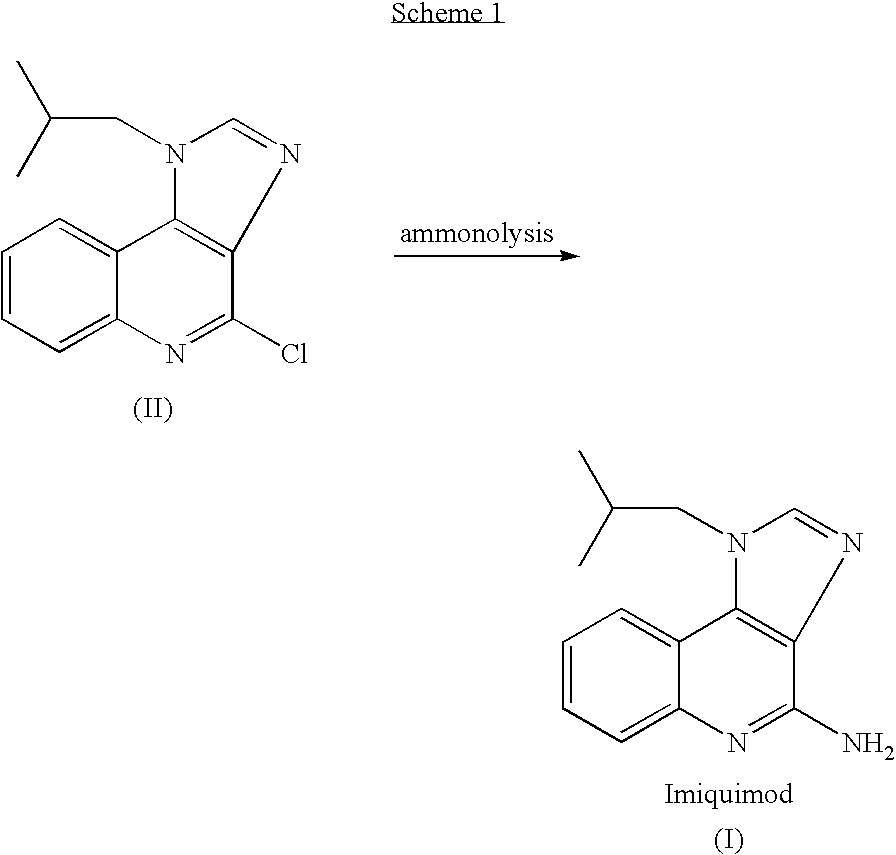
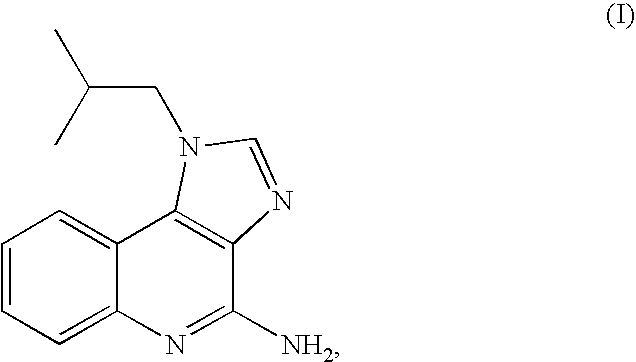
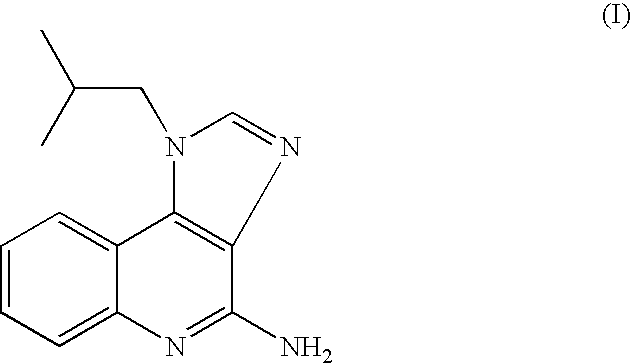
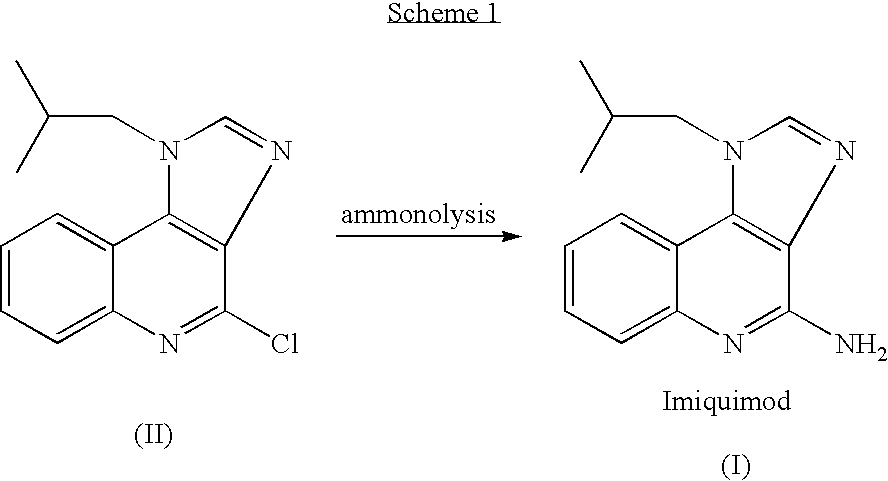
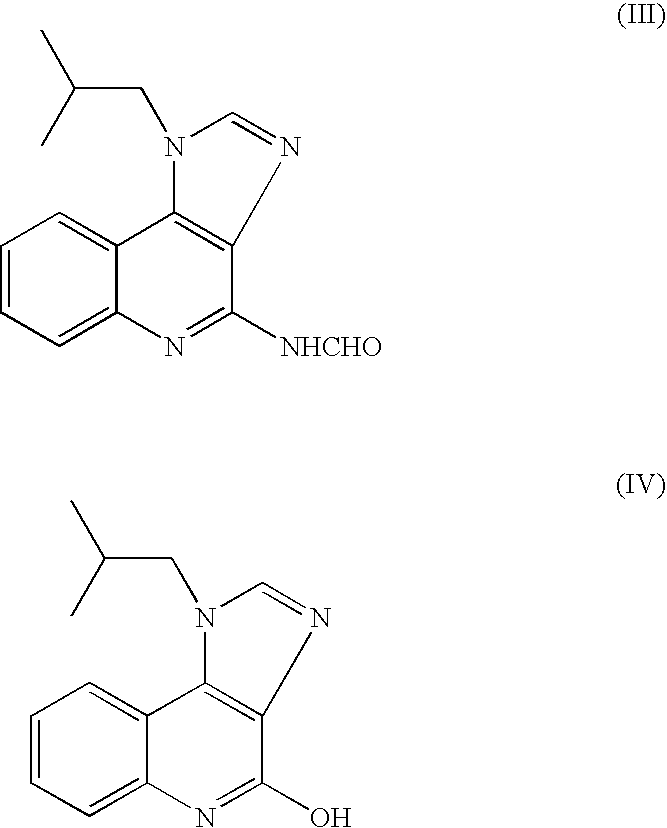
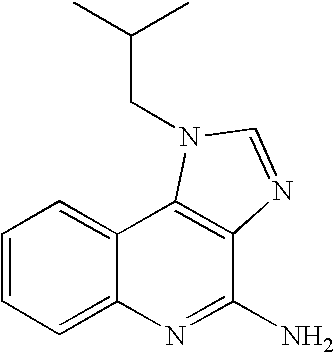
The present invention provides a process for preparing 4-amino-1-isobutyl-1H-imidazo[4,5-c]quinoline (Imiquimod) of formula (I).The process comprises heating 4-chloro-1-isobutyl-1H-imidazo[4,5-c]quinoline of formula (II) with formamide, and optionally with bubbling of gaseous ammonia to afford Imiquimod of formula (I).According to the present invention, by using this process and novel purification methods, essentially as described herein, highly pure Imiquimod is obtained.
Owner:CHEMAGIS
Imiquimod Production Process
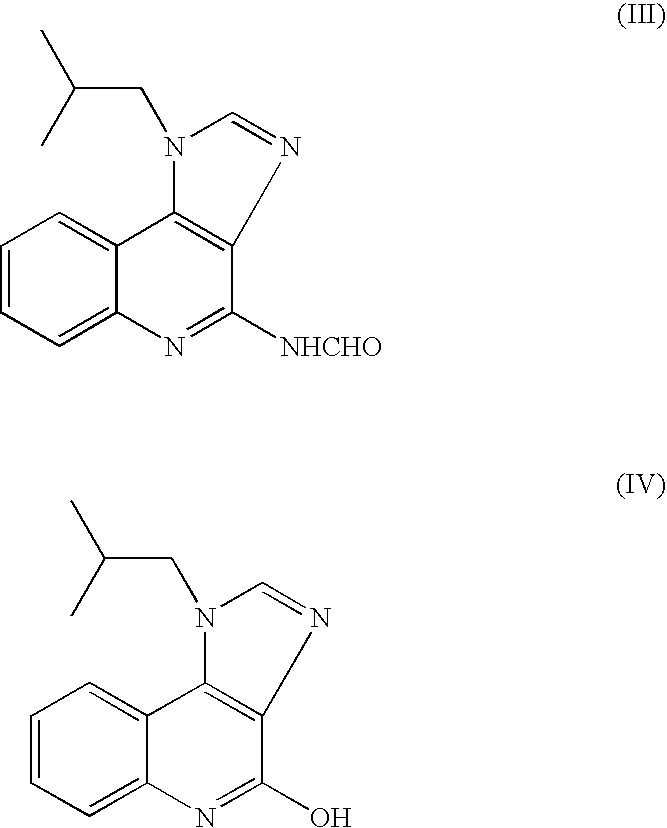
Provided is a process for producing highly pure 4-amino-1-isobutyl-1H-imidazo[4,5-c]quinoline (imiquimod), which includes reacting 4-chloro-1-isobutyl-1H-imidazo[4,5-c]quinoline with a non-gaseous amine precursor. Also provided are methods for isolating highly pure imiquimod. Further provided are intermediates useful in the production of imiquimod, methods for producing such intermediates, and methods for obtaining imiquimod from such intermediates.
Owner:CHEMAGIS
Application of protein tyrosine phosphatase SHP2 inhibitor in preparation of medicine for treating psoriasis
ActiveCN111265529AEasy to prepareReduce the overall heightOrganic active ingredientsDermatological disorderInflammatory factorsDisease
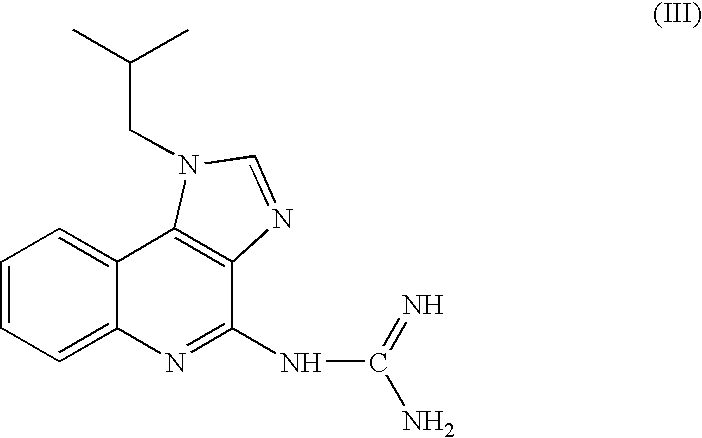
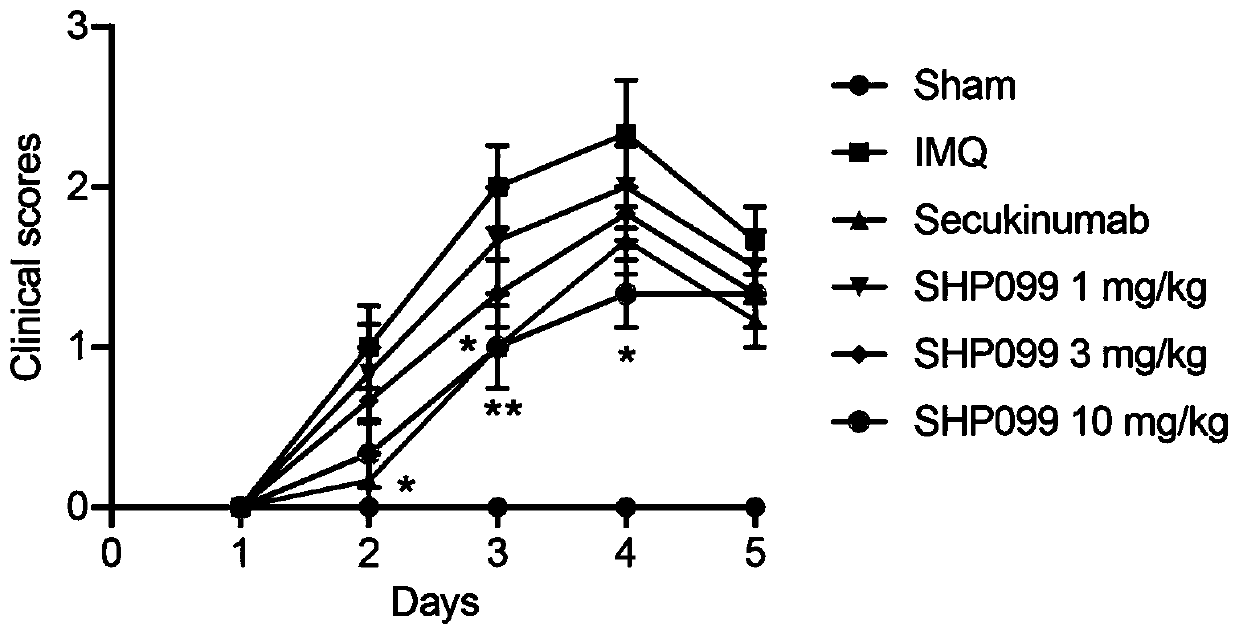
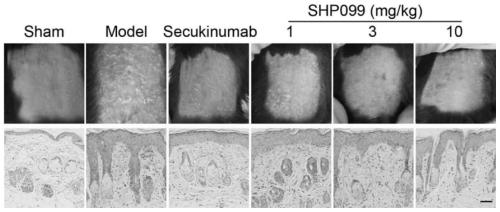
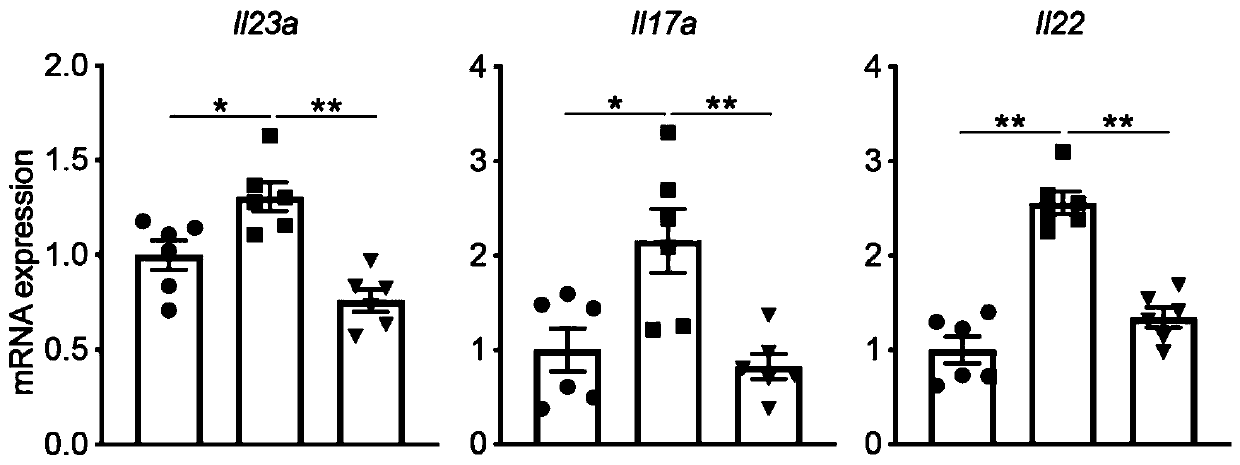
The invention belongs to the technical field of pharmacy. According to the application of a protein tyrosine phosphatase SHP2 inhibitor SHP099 in preparation of the medicine for treating psoriasis, aiming at an imiquimod-induced mouse psoriasis-like disease model, the SHP099 can effectively improve epidermal thickening of mouse skin lesion tissues, inflammatory cell infiltration and the level of inflammatory factors in mouse serum. At the cellular level, the SHP099 can significantly reduce the release of inflammatory factors related to psoriasis.
Owner:NANJING UNIV
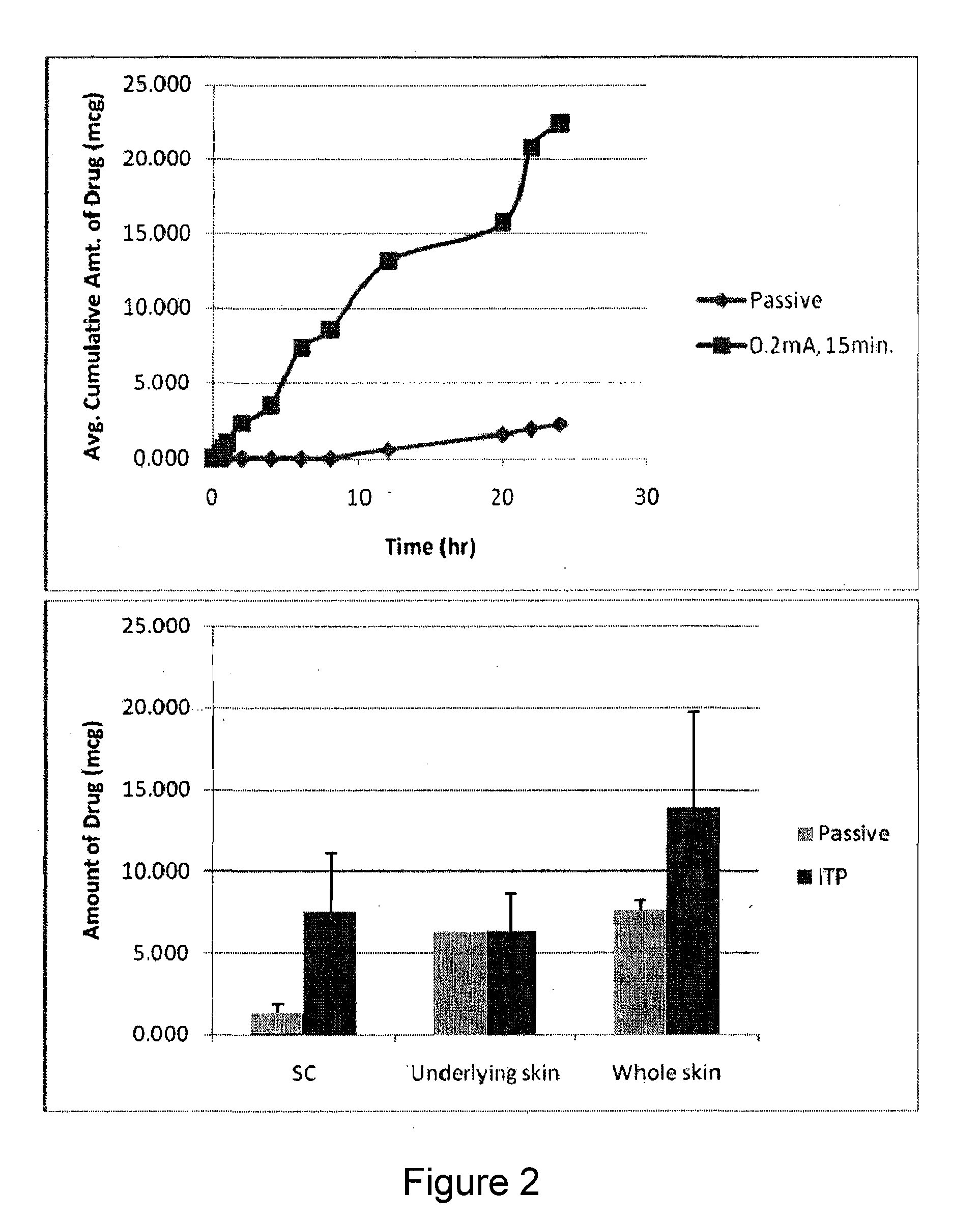
Pharmaceutical Formulations for Iontophoretic Delivery of an Immunomodulator
The present invention describes pharmaceutical formulations and methods suitable for iontophoretic delivery of the formulations to a subject. The formulations comprise an immunomodulator, such as imiquimod, and optionally include various agents and excipients. The formulations can be used as a treatment for skin diseases and conditions such as actinic keratosis, basal cell carcinoma and genital warts. The short term iontophoretic delivery of the formulations results in the creation of a depot effect in the skin of the subject, allowing for a sustained delivery. The shortened delivery time minimizes local side effects at the application site.
Owner:NITRIC BIOTHERAPEUTICS INC
Immunosuppressive Cell-Capturing Material and Immunosuppressive Cell-Capturing Column
ActiveUS20140017667A1Reduce concentrationPotential in preventionDead animal preservationMammal material medical ingredientsNystatin GPolymer coatings
This invention discloses an immunosuppressive cell-capturing material comprising a molded body that includes: a readily hydrolyzable condensation polymer having an amino group; a poorly hydrolyzable polymer coating the readily hydrolyzable condensation polymer; and a ligand-conjugated poorly hydrolyzable polymer coating the poorly hydrolyzable polymer, wherein the ligand is at least one selected from the group consisting of a NH2 group, a secondary amino group, a tertiary amino group, a polyamine residue, a basic cyclic polypeptide residue, an aminoglycosidic compound residue, chloroquine, primaquine, mefloquine, imiquimod, and nystatin, and wherein the content of the amino group in the molded body is 150 μmol / g or less. The invention also discloses an immunosuppressive cell-capturing column filled with the capturing material.
Owner:NAT UNIV CORP SHIGA UNIV OF MEDICAL SCI
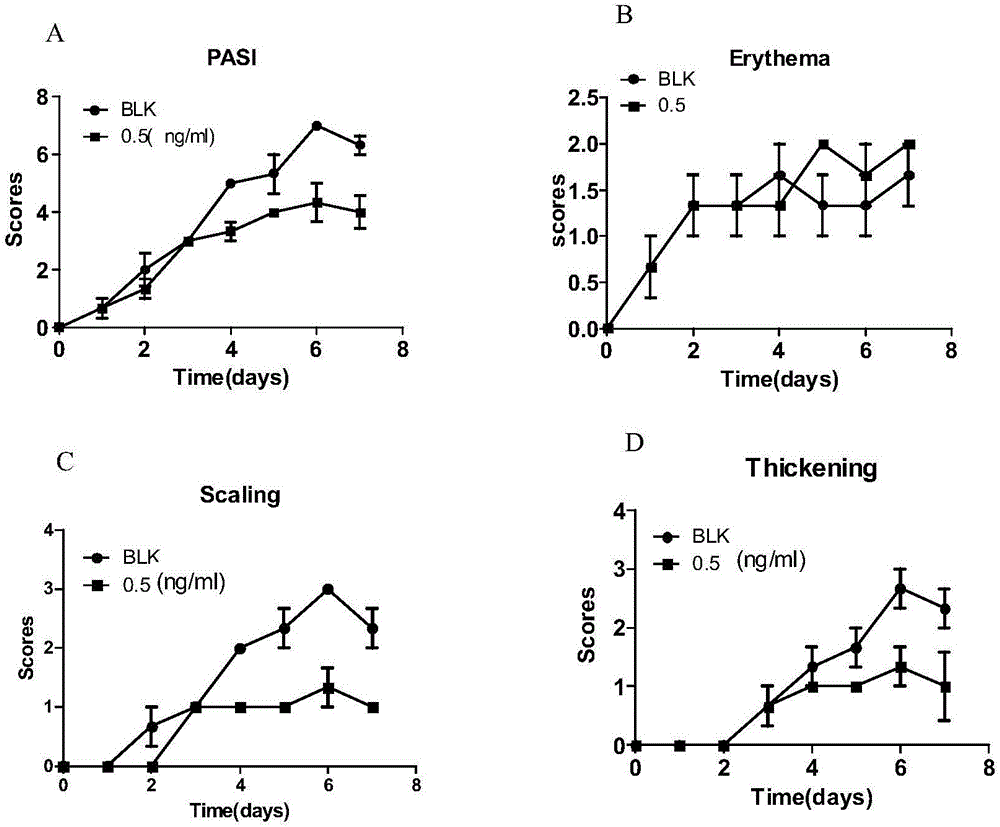
Application of Psoriasin antibody and LL-37 antibody in preparing drug for preventing and treating psoriasis
PendingCN106943594AImprove permeabilityGuaranteed stabilityImmunoglobulins against growth factorsAntibody ingredientsAntiendomysial antibodiesApoptosis
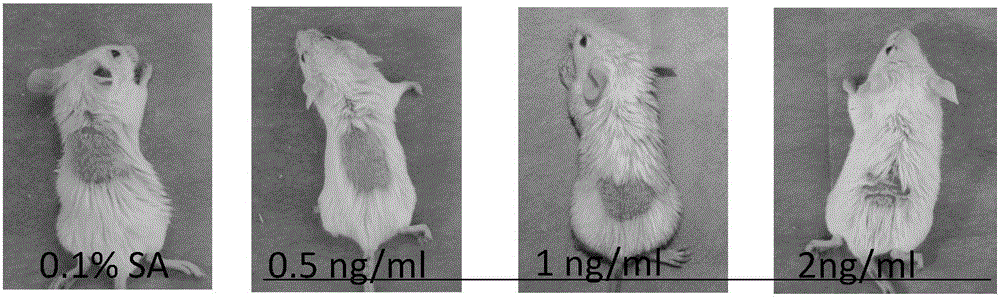
The invention discloses an application of a Psoriasin antibody and an LL-37 antibody in preparing a drug for preventing and treating psoriasis. In comparison to the situation that necessary cell factors and chemotactic factors in a Th cell differentiation inhibiting process are focused on to inhibit inflammation, not only is differentiation inhibition of a Th cell emphasized, but also apoptosis of activated T cells is promoted. Furthermore, by comparing and exploring the optimum rate of the two antibodies by using an external treatment imiquimod mouse model, a result verifies that the treatment effect of 5[mu]g / ml LL37 antibody and 1[mu]g / ml psoriasin antibody is relatively obvious and a relatively high PASI score is acquired.
Owner:XI AN JIAOTONG UNIV
Lower dosage strength pharmaceutical compositions forumlated with 3.75% imiquimod
InactiveUS20110257219A1Improved imiquimodReduced strengthBiocideOintment deliveryDosing regimenRegimen
Pharmaceutical formulations and methods for the topical or transdermal delivery of 1-isobutyl-1H-imidazo[4,5-c]-quinolin-4-amine or 1-(2-methylpropyl)-1H-imidazo[4,5-c]quinolin-4-amine, i.e., imiquimod, to treat actinic keratosis with short durations of therapy, than currently prescribed for the commercially available Aldara® 5% imiquimod cream, as now approved by the U.S. Food & Drug Administration (“FDA”), are disclosed and described. More specifically, lower dosage strength imiquimod formulations to deliver an efficacious dose of imiquimod for treating actinic keratosis with an acceptable safety profile and dosing regimens that are short and more convenient for patient use than the dosing regimen currently approved by the U.S. Food & Drug Administration (“FDA”) for Aldara® 5% imiquimod cream to treat actinic keratosis are also disclosed and described.
Owner:MEDICIS PHARMA CORP
Imiquimod production process
The present invention provides a process for preparing highly pure 4-amino-1-isobutyl-1H-imidazo[4,5-c]quinoline (imiquimod). The process preferably includes heating 4-chloro-1-isobutyl-1H-imidazo[4,5-c]quinoline of formula (II) with ammonia in a polar aprotic solvent at relatively moderate pressure to produce imiquimod, and optionally purifying the imiquimod. The process of the present invention can produce highly pure imiquimod in high yield.
Owner:WAVELENGTH ENTERPRISES LTD
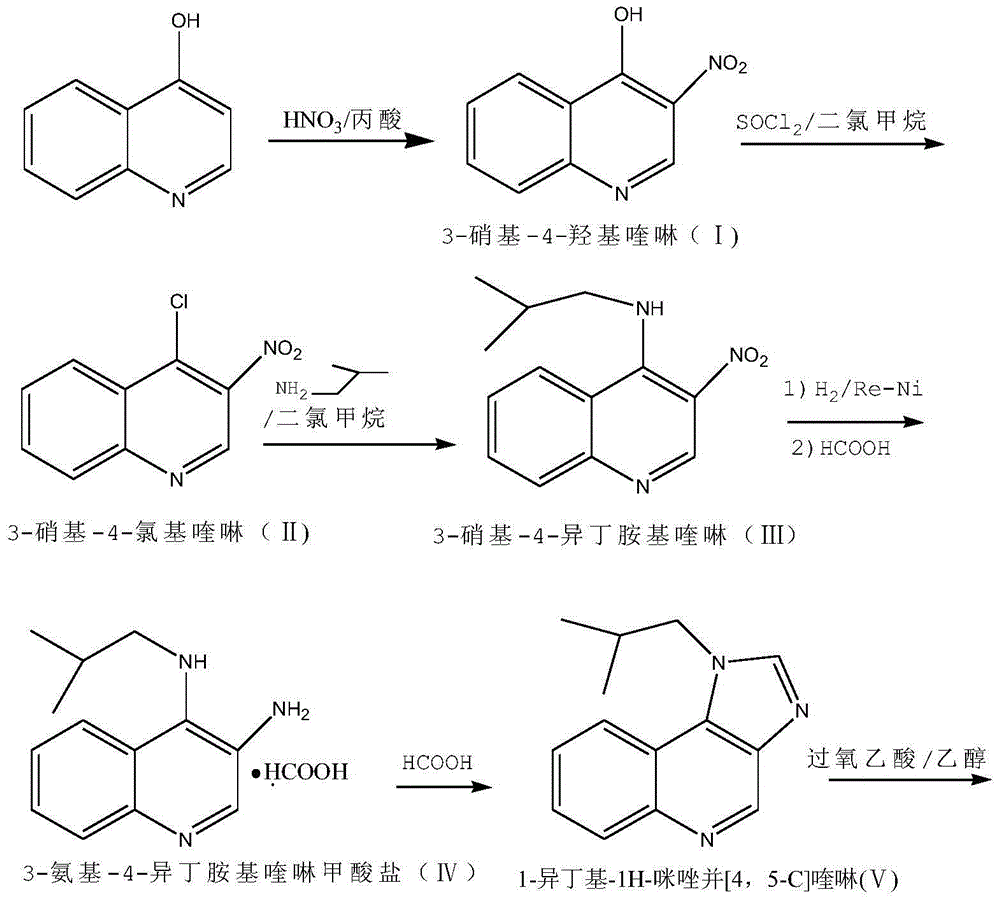
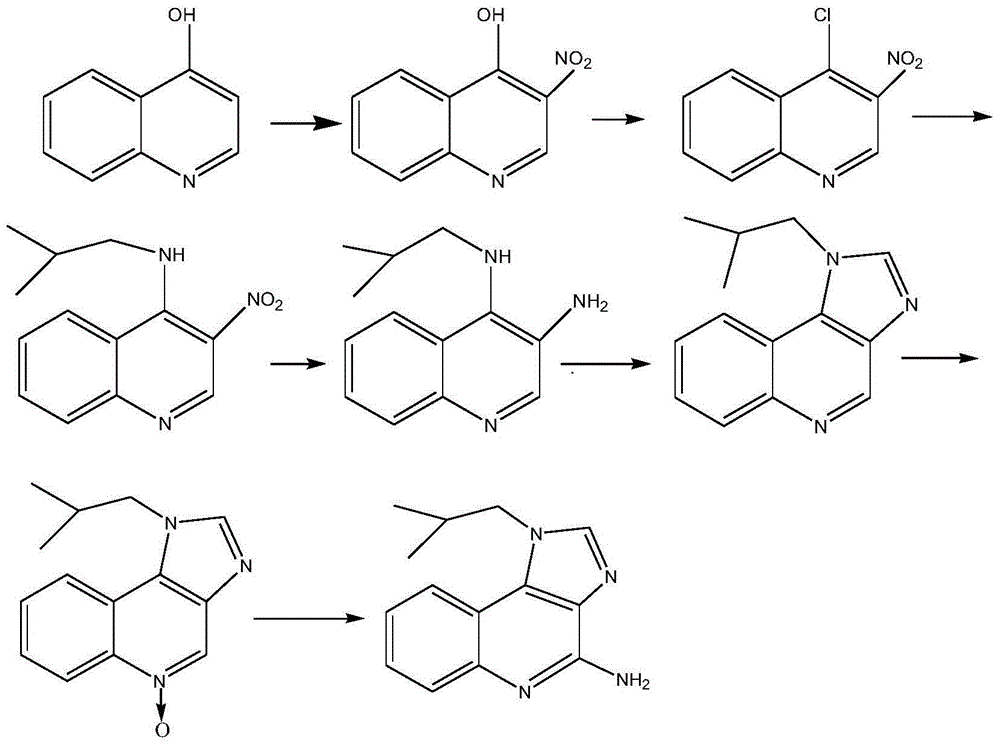
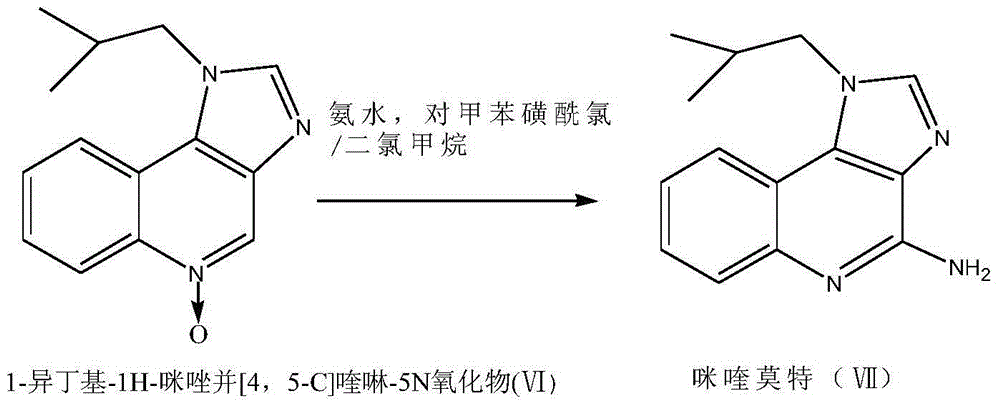
Preparation method of imiquimod
The invention relates to a preparation method of an antiviral drug imiquimod, wherein the preparation method comprises the steps: (1) with 4-hydroxyquinoline as a starting material, nitrifying to obtain 3-nitro-4-hydroxyquinoline; (2) carrying out chlorination and amination on 3-nitro-4-hydroxyquinoline to obtain 3-nitro-4-isobutyl amine quinoline; (3) carrying out hydrogenation on 3-nitro-4-isobutyl amine quinoline to obtain 3-amino-4-isobutyl amine quinoline, and separating in a formate mode; (4) carrying out cyclization, oxidation and ammoniation on 3-amino-4-isobutyl amine quinoline, carrying out a reaction in a same reactor, and thus obtaining an imiquimod crude product; and (5) preparing a hydrochloride of imiquimod from the imiquimod crude product, purifying, and carrying out alkaline hydrolysis to obtain the high-purity imiquimod. In the method, the imiquimod is prepared from 4-hydroxyquinoline as the starting material, and the total yield can reach 55%; the method has the advantages of high yield, less reaction steps, less discharge of three wastes, mild and more complete reaction conditions, and simple and convenient operation, is suitable for industrialized production, and has relatively high practical value. The obtained product is more stable in quality, and the purity can reach more than 99.7%.
Owner:TOPFOND PHARMA CO LTD
Process for preparing Imiquimod
The present invention provides a process for preparing 4-amino-1-isobutyl-1H-imidazo[4,5-c]quinoline (Imiquimod) of formula (I). The process comprises heating 4-chloro-1-isobutyl-1H-imidazo[4,5-c]quinoline of formula (II) with formamide, and optionally with bubbling of gaseous ammonia to afford Imiquimod of formula (I). According to the present invention, by using this process and novel purification methods, essentially as described herein, highly pure Imiquimod is obtained.
Owner:CHEMAGIS
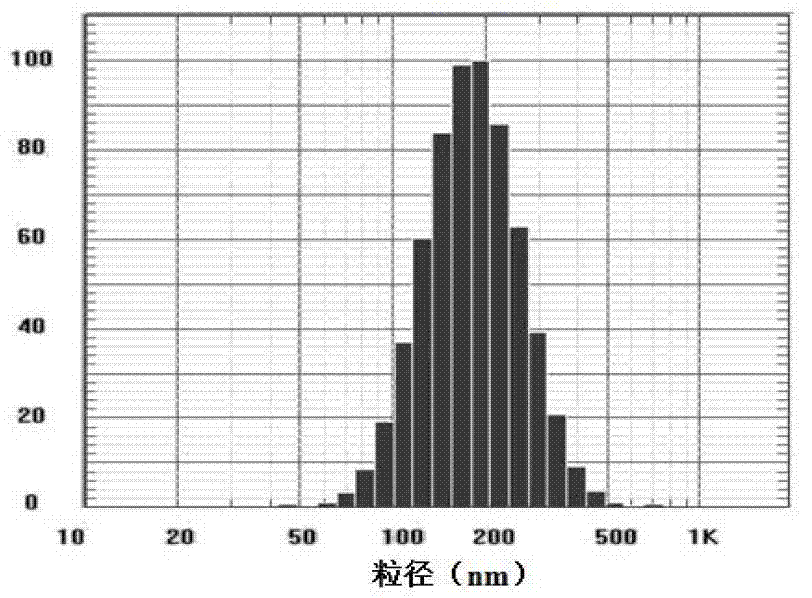
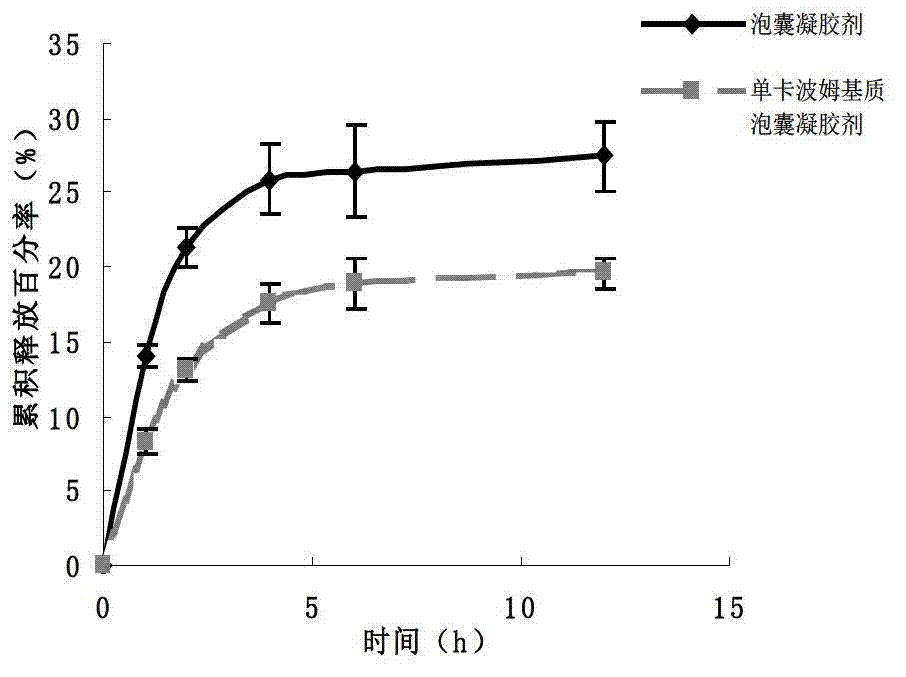
Imiquimod vesicle gel and preparation method for same
ActiveCN103202803AImprove retentionGood curative effectOrganic active ingredientsAerosol deliveryDiseaseMelanoma
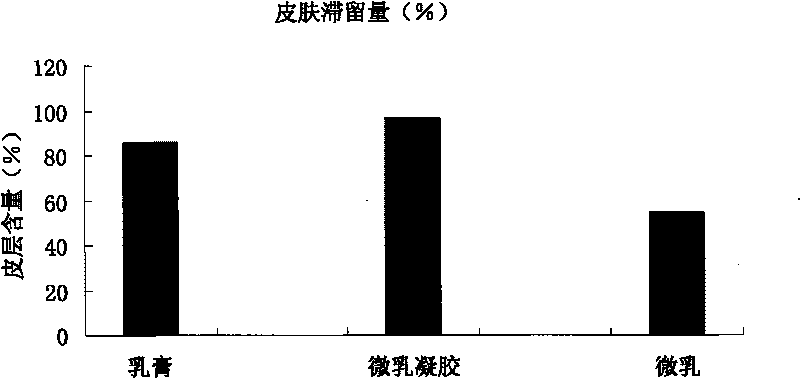
The invention relates to an imiquimod vesicle gel and a preparation method for the same. The vesicle gel is obtained by preparing a vesicle suspension by using imiquimod and the nonionic surfactants of Brij, Span, Poloxamer and the like via self-assembly, then reacting with the mixed gel matrix of carbomer and povidone, and used for treating the diseases of exophytic genital warts, actinic keratosis, skin basal cancer, melanoma and the like via local application on the skin; and by adding povidone in the carbomer gel matrix, the in-vitro medicine release amount can be increased by 42.1%, thus being beneficial to promote the entrance of medicines in the skin to exert the functions. According to the imiquimod vesicle gel and the preparation method for the same disclosed by the invention, the intradermal retention volume of the medicines in 24 hours can be remarkably increased and is 2.1 times that of the commercially available emulsifiable pastes, and the dose of the medicines penetrating through the skin is reduced by 48.7%; and the equivalent intradermal retention volume can be achieved only by a half of the dose of the commercially available emulsifiable pastes, so that the effectiveness of the medicine effect of the medicated parts can be remarkably enhanced, the dose of the medicines entering in the body can be greatly reduced, and the toxic and side effects of the whole body can be reduced.
Owner:SUZHOU UNIV
Combination therapy with low dosage strength imiquimod and photodynamic therapy to treat actinic keratosis
ActiveUS9492682B2Reduced strengthOrganic active ingredientsCosmetic preparationsPhotodynamic therapyTopical treatment
Owner:MEDICIS PHARMA CORP
Pharmaceutical composition for treatment and/or prevention of cancer
PendingCN111936164AEffective treatmentPrevention is effectiveOrganic active ingredientsHybrid immunoglobulinsAntiendomysial antibodiesPharmaceutical drug
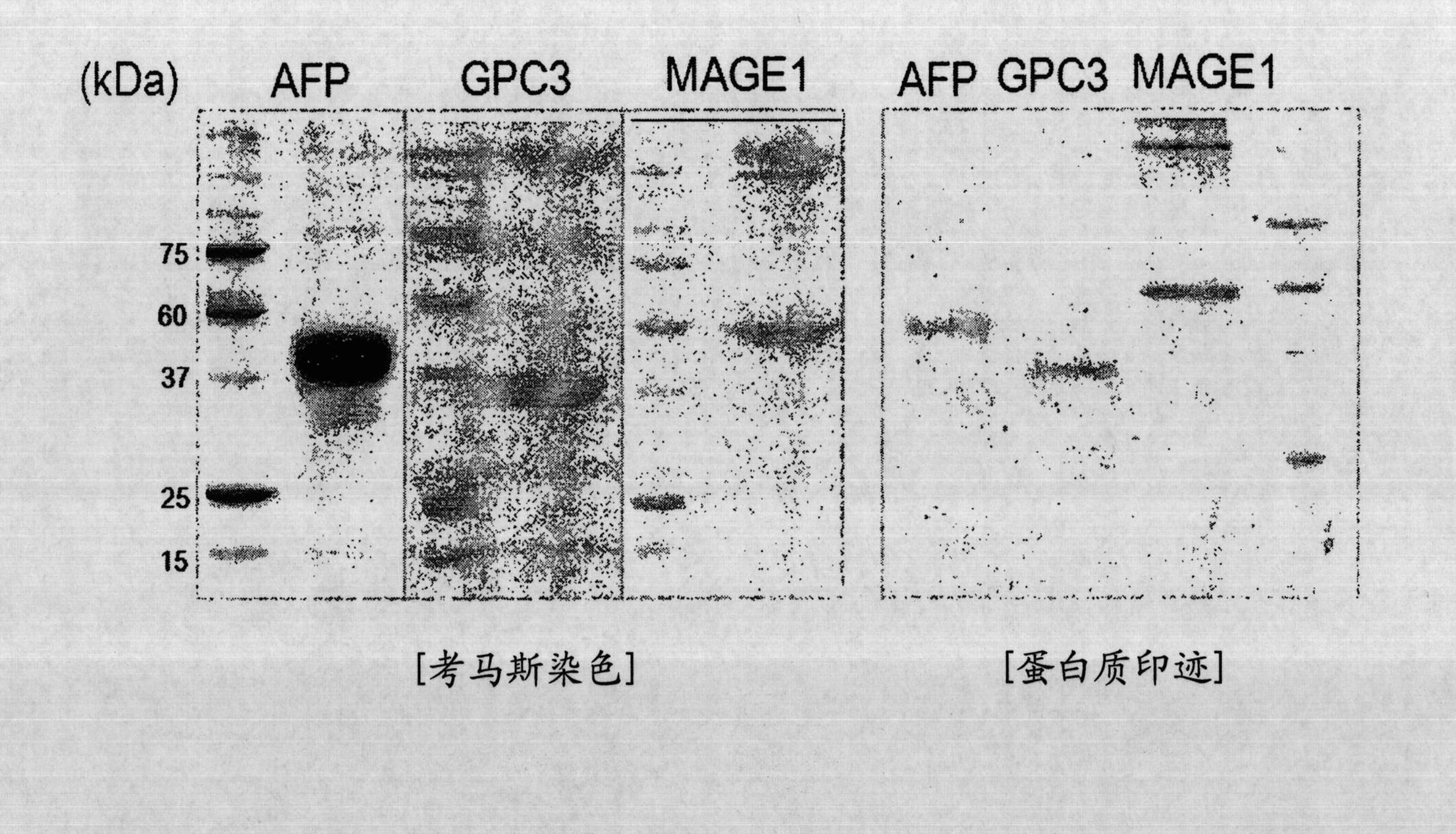
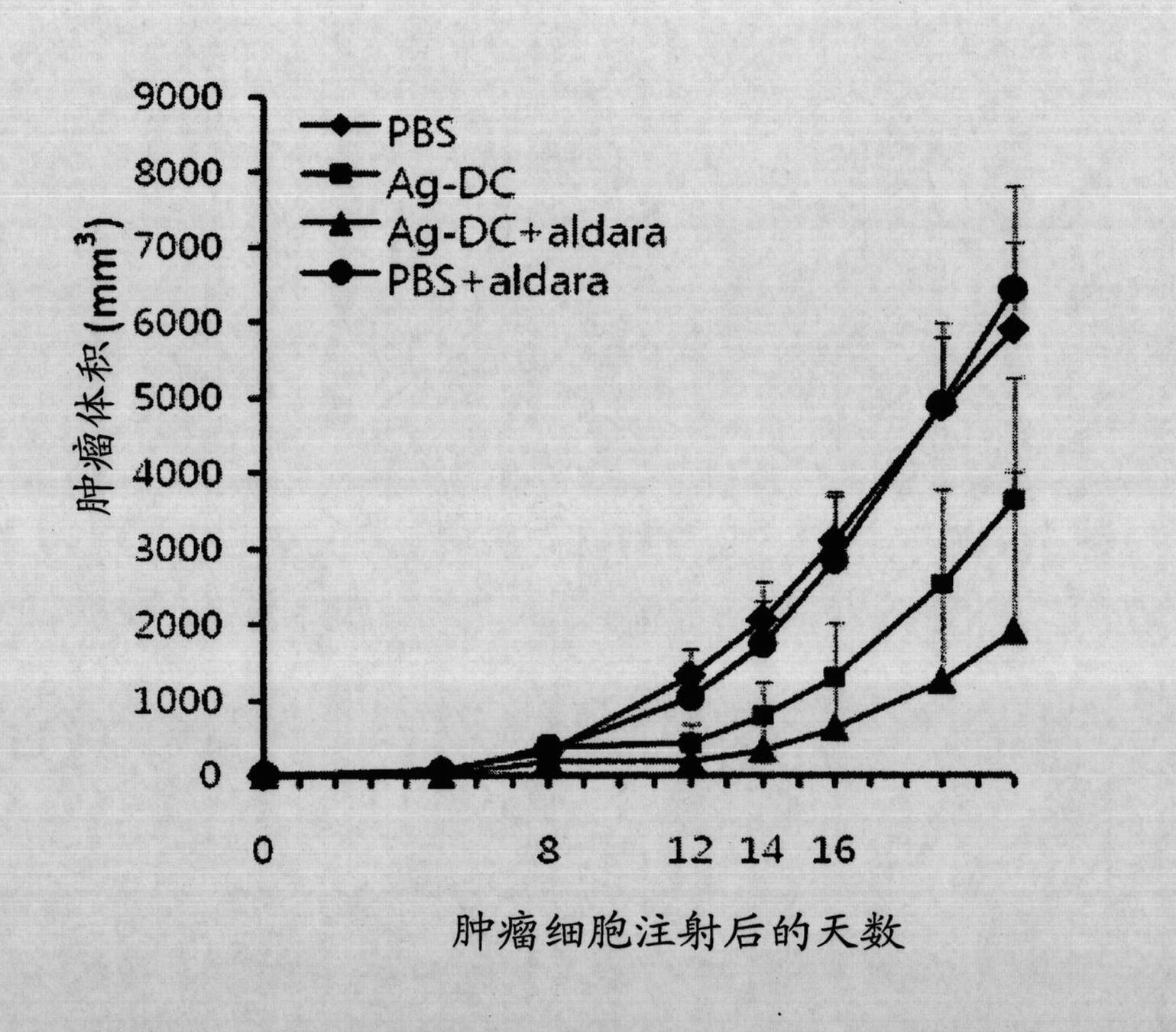
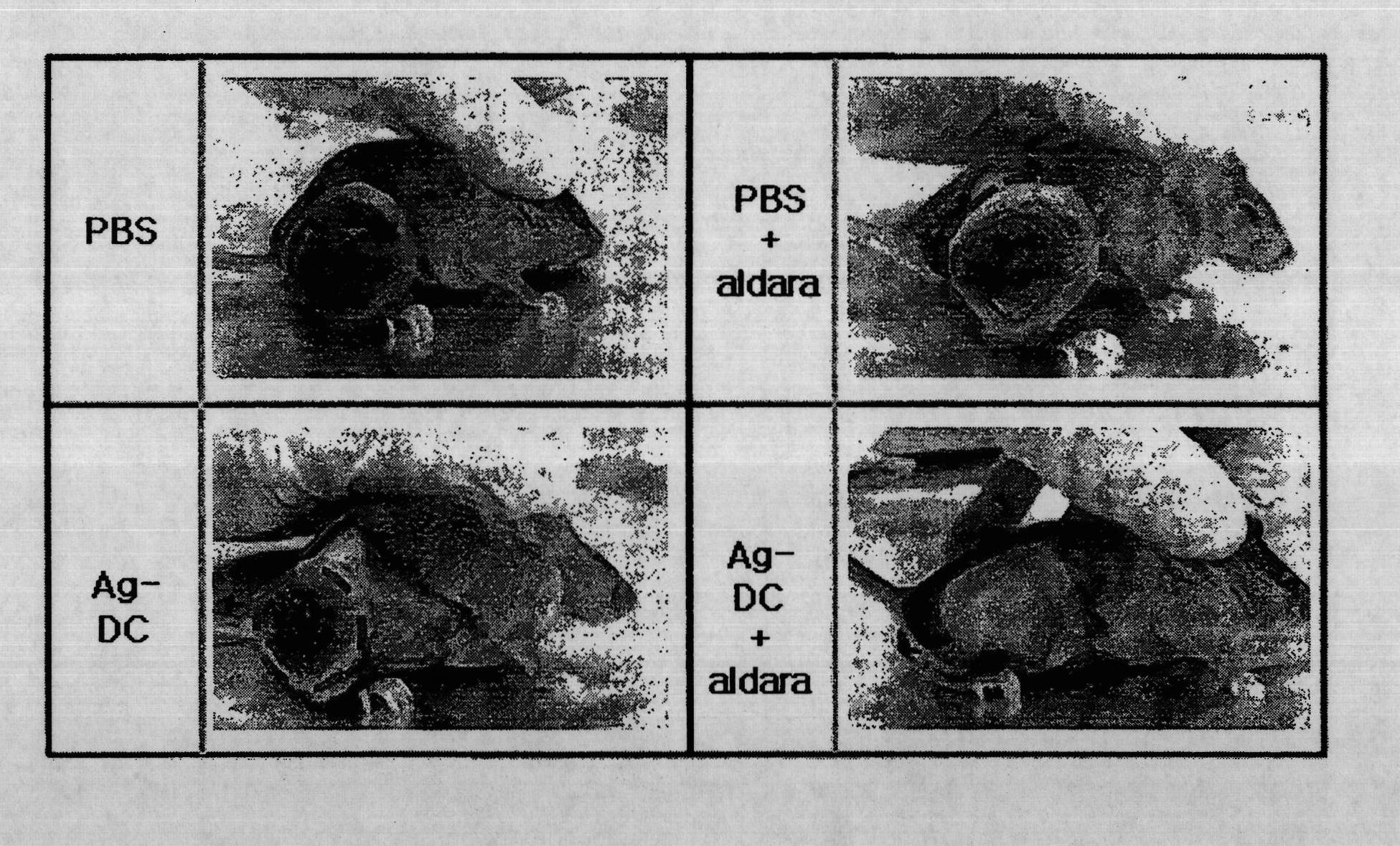
The present invention provides a pharmaceutical product for treatment and / or prevention of cancer, characterized by including imiquimod together or separately in combination with an antibody, or a fragment thereof, having immunological reactivity with a CAPRIN-1 protein. With this pharmaceutical product, it is possible to treat and / or prevent cancer that specifically expresses the CAPRIN-1 proteinon a cell surface.
Owner:TORAY IND INC
Imiquimod turbid liquor and gelling agent thereof
ActiveCN1923170ADoes not interfere with normal functionConvenient treatmentOrganic active ingredientsInorganic non-active ingredientsAlcoholSolvent
Owner:北京科信聚润医药科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com