Patents
Literature
32 results about "Primaquine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Primaquine is used with other medications to prevent and treat malaria caused by mosquito bites in countries where malaria is common.
Rubber additive as well as preparation and application thereof
ActiveCN106749373AEasy to stretchImprove tear resistanceGroup 3/13 element organic compoundsCoatingsPolymer scienceNitrogenous heterocyclic compound
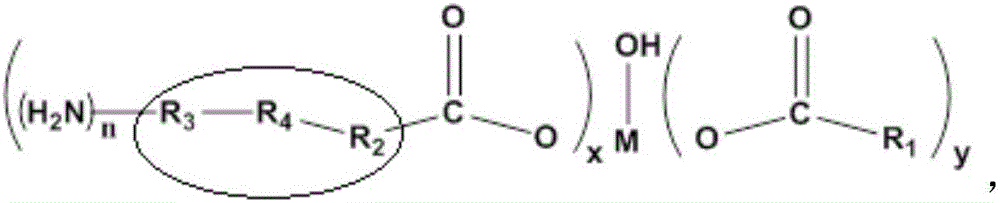
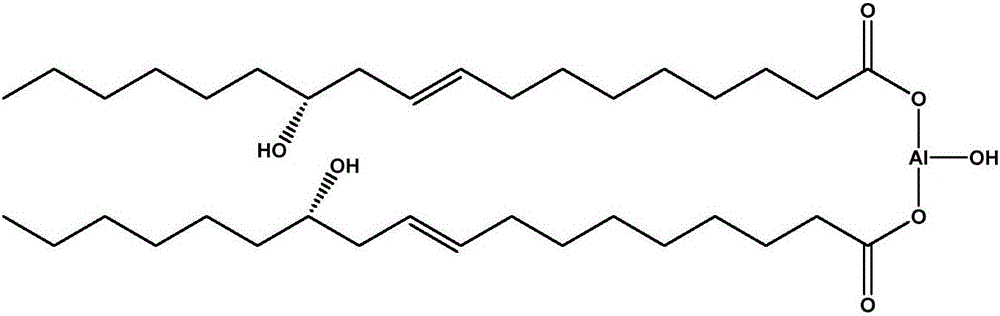
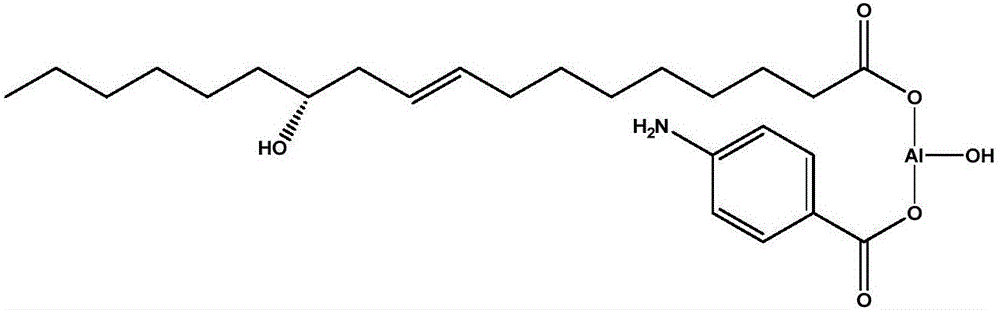
The invention relates to a rubber additive as well as preparation and application thereof. The chemical structural formula of the rubber additive is as shown in the specification, in the formula, M is a metal of which the oxidation state is +3 or +4; R1 is an aliphatic chain of C5-C50 of which a plurality of functional groups are substituted; the substituted functional groups on the chain of R1 include hydroxyl, double bonds or an epoxy group; R2 and R3 are aliphatic chains with 0-50 carbon atoms; R4 is a aromatic ring compound or a nitrogen heterocyclic ring compound; the amino can be connected with R2, R3 and R4 in modes of primaquine, parahelium and tertamino; n is a positive integer not smaller than 1; x is 0 or 1; y is 1 or 2; x+y=2. When the rubber additive is adopted to prepare rubber, the tension strength, the anti-sliding property and the like of the rubber can be remarkably improved.
Owner:TONGJI UNIV +1
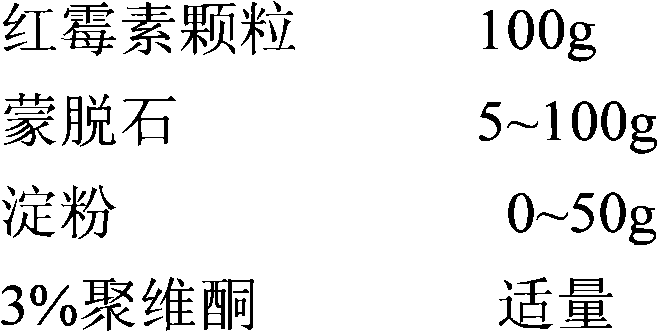
New preparation of erythrocin and relevant drug thereof and preparation method of new preparation
The invention relates to a preparation method of new preparation of erythrocin, which is characterized in that an endothelin core of erythrocin is prepared, and then an isolating layer, a protective layer, a second isolating layer and an improved enteric-coating material layer are applied one by one. In this way, new preparation of the erythrocin which has certain feature of releasing (dissolving) in acid solution (hydrochloric acid solution 9 to 1000) can be formed. The technology of the new preparation can also be widely applied to drugs which, like erythrocin, when being taken orally by a patient, cause the patient to suffer the side effects of stimulation, sickness and the like after degradation in the stomach of the patient or contact with the stomach of the patient, and drugs which the patient needs to take orally to let the blood concentration to reach the peak value in a short time. Such drugs include macrolides of azithromycin, metronidazole of nitroimidazoles, tinidazole, acyclovir as an antiviral drug, ammonium chloride as a phlegm eliminating drug, bromhexine, chloroquine as an antimalarial, nitroquine, artemisinin, dihydroartemisinin, artesunate, primaquine, pyrimethamine, carbarsone and emetine amebicides and so on.
Owner:胡昌勤 +1
Polysulfone side chain grafted polymerized tertiary amine micro-filtration membrane
InactiveCN106179002AHigh separation selectivityGood reference valueMembranesSemi-permeable membranesMethacrylatePhase conversion
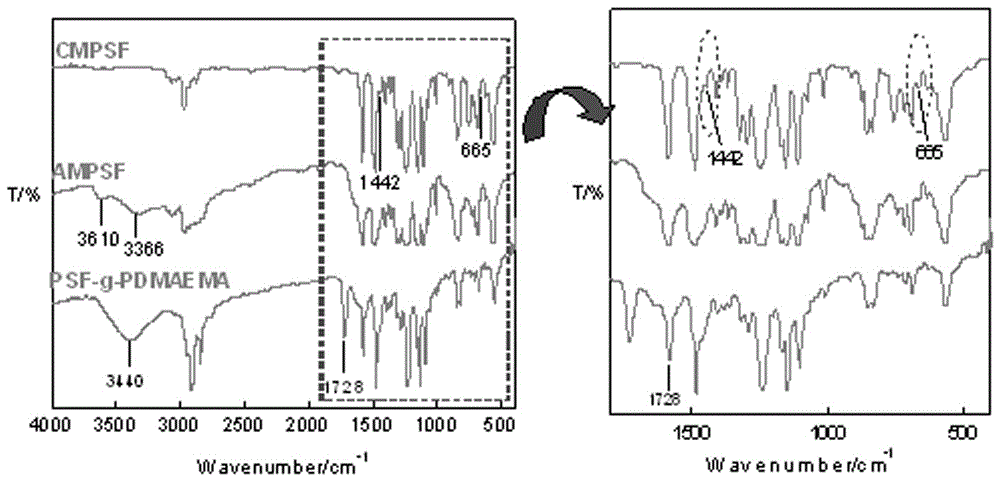
The invention belongs to the field of membrane separation and particularly relates to a polysulfone side chain grafted polymerized tertiary amine micro-filtration membrane. The problems that in the prior art, the reject rate on many micromolecular substances is low, consequently, removal of poisonous substances cannot reach the standard, and the operation cost of the nanofiltration technology is high can be solved. According to a preparation method, a phase conversion film forming method is firstly adopted for preparing a chloromethyl polysulfone (CMPSF) asymmetric micro-filtration membrane; then primaquine groups are introduced to the surface of the micro-filtration membrane through surface chemical modification; finally, on the basis of constructing a -NH2 / S2O82-surface initiating system, tertiary amine monomer dimethyl amino ethyl methacrylate (DMAEMA) is grafted and polymerized on the surface of the membrane, and the porous grafted membrane PSF-g-PDMAEMA grafted with functional macromolecular PDMAEMA is prepared. The prepared polysulfone side chain grafted polymerized tertiary amine micro-filtration membrane can efficiently remove two kinds of poisonous anions such as CrO42- and MoO42-.
Owner:ZHONGBEI UNIV
Enhanced artemisinin-based combination therapy for treating parasitic mediated disease
PendingUS20140256761A1Facilitates early administrationIncidence of recurrenceBiocideAnimal repellantsBerberineCombined Modality Therapy
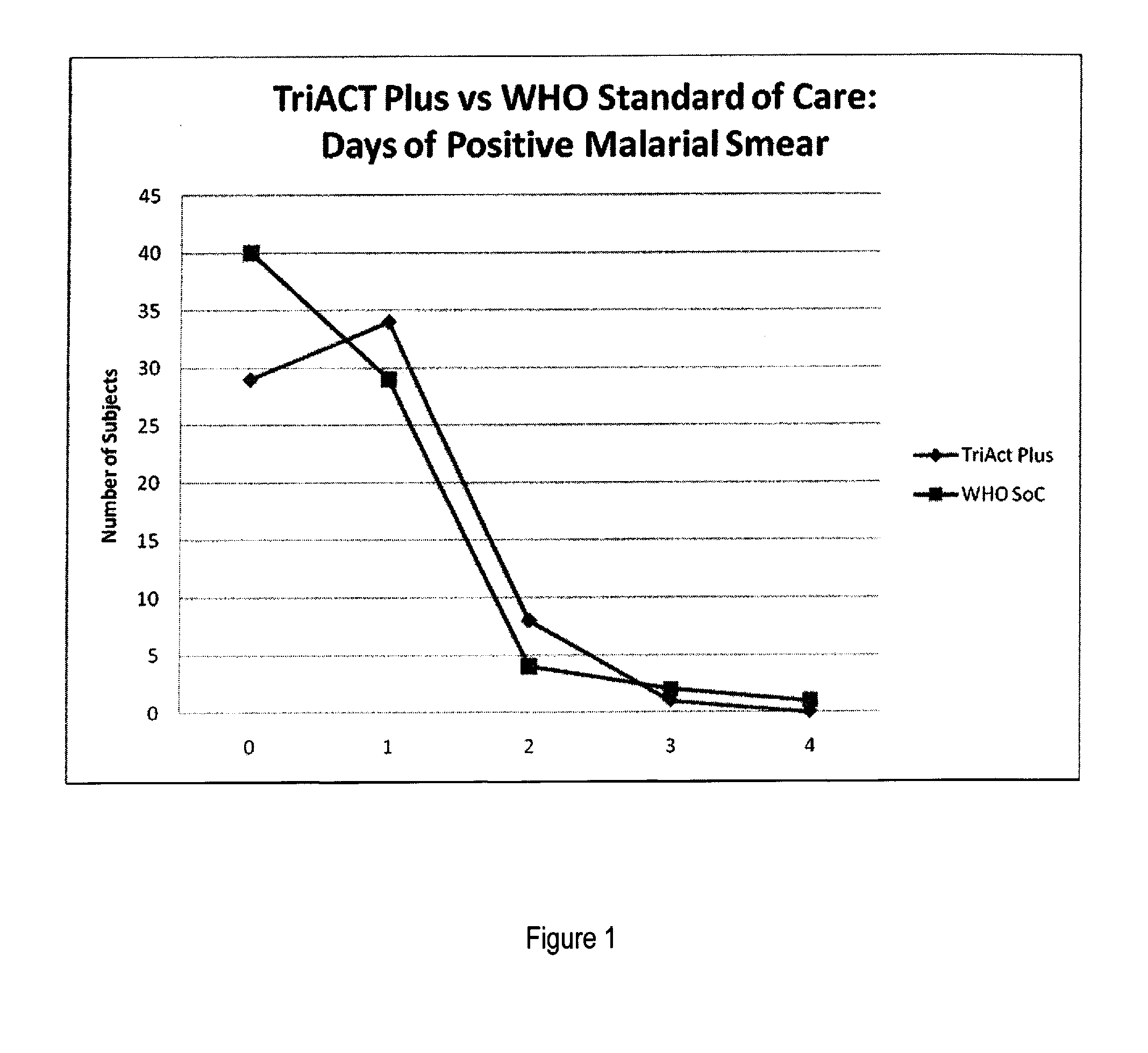
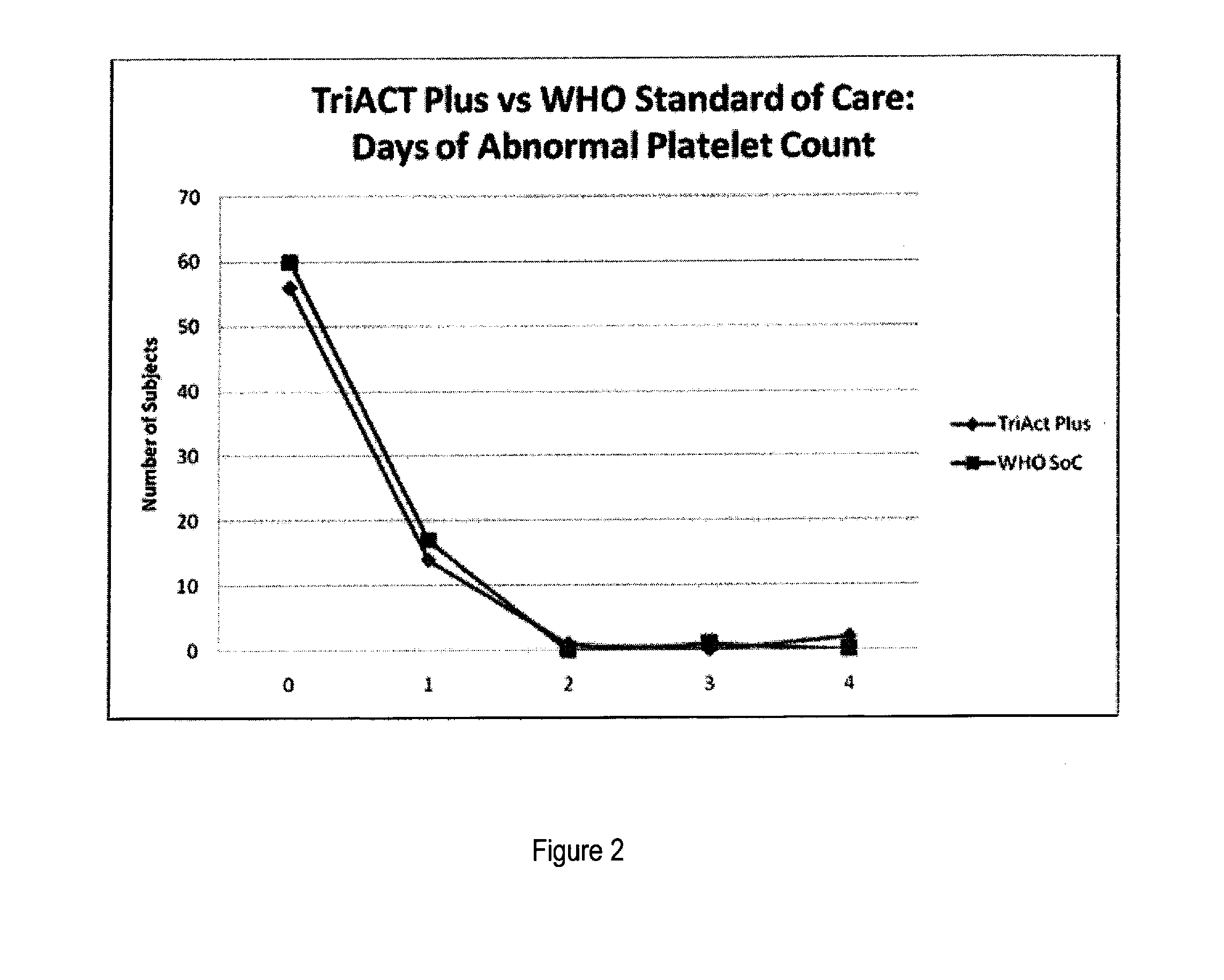
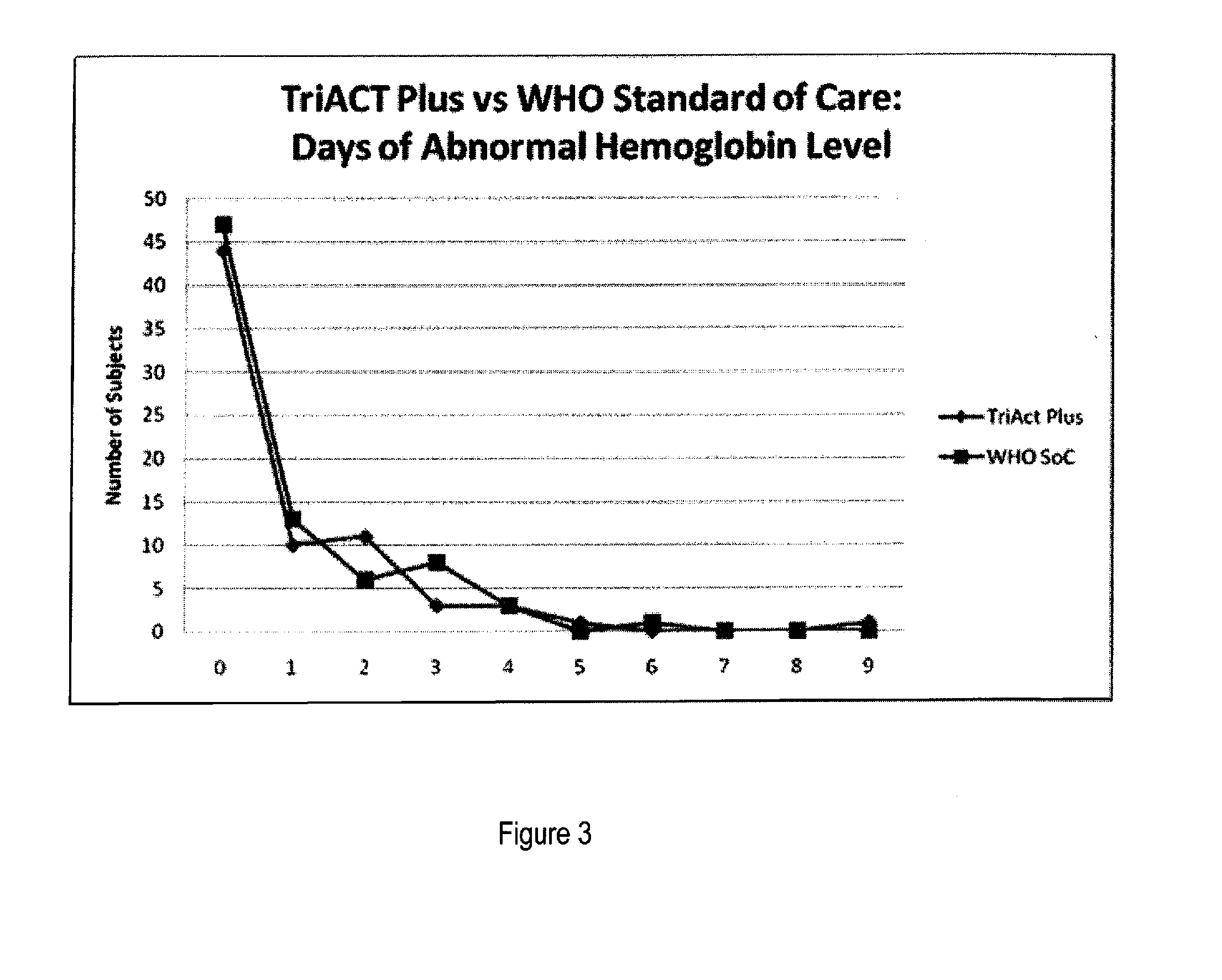
The present invention describes a method of treating individuals suffering from microbial infections, including a parasitic disease such as malaria, by using an improved Artemisinin Combination Therapy (ACT), known as ActRx Tri-ACT Plus. The improved ACT therapy includes administering a combination of four drugs. In one embodiment of the present invention, the method includes administering to an individual a first composition comprising a therapeutically effective amount of an artemether spray sublingually. The individual is then administered a second composition, a therapeutically effective amount of artesunate. A third composition, an effective amount of berberine, or its pharmaceutically acceptable derivatives or salts is administered to the individual. A fourth composition, an effective amount of primaquine, or its pharmaceutically acceptable derivatives or salts is administered to the individual.
Owner:KRYPTONITE GRP
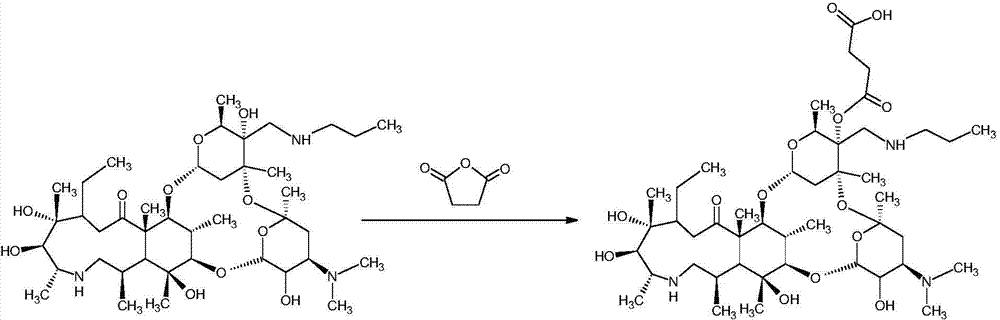
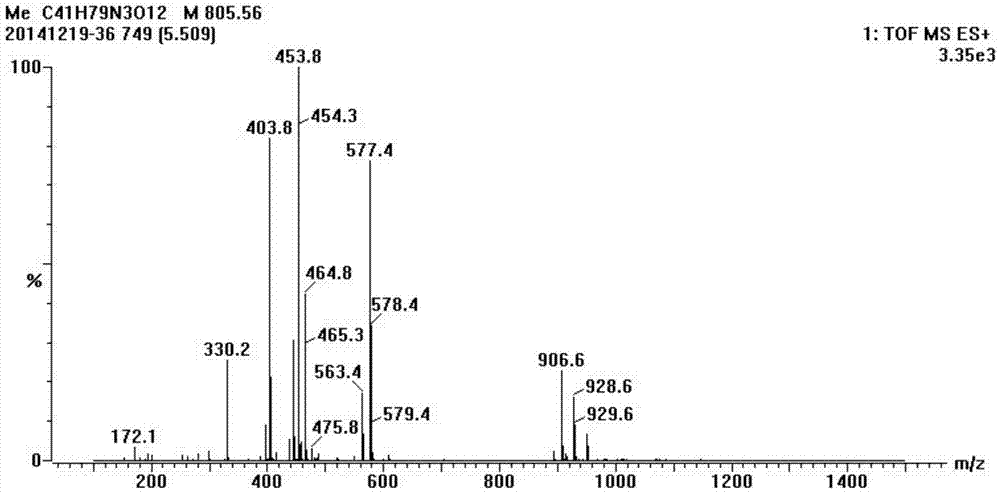
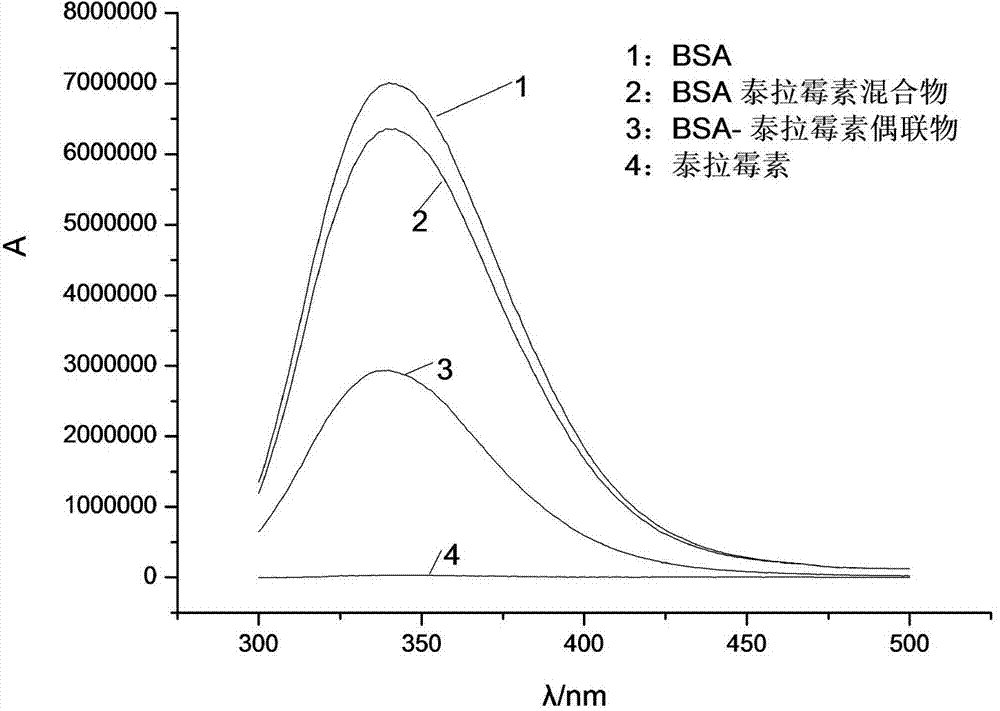
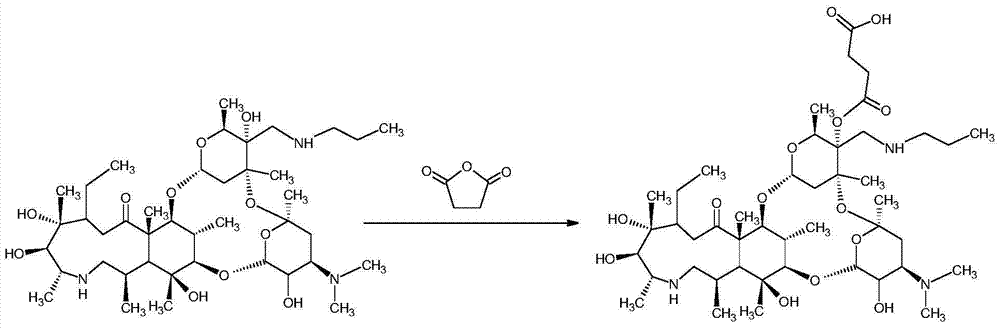
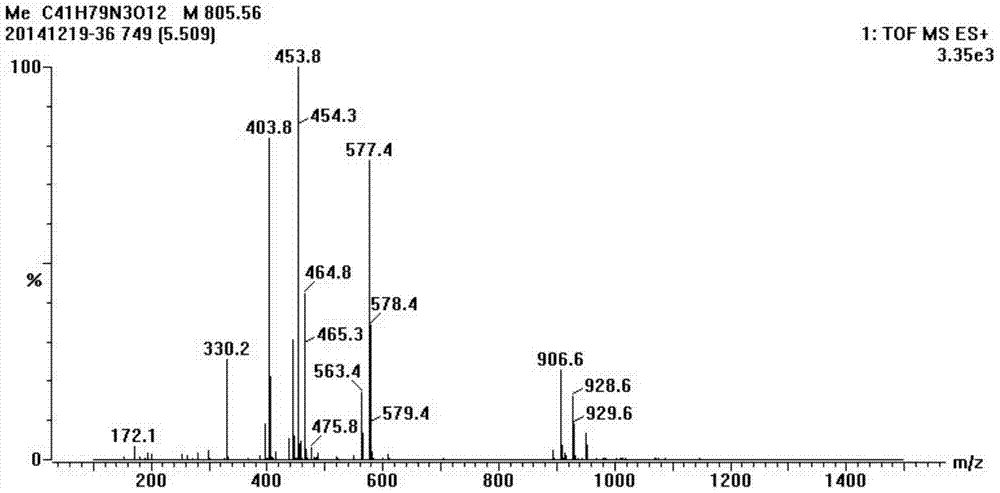
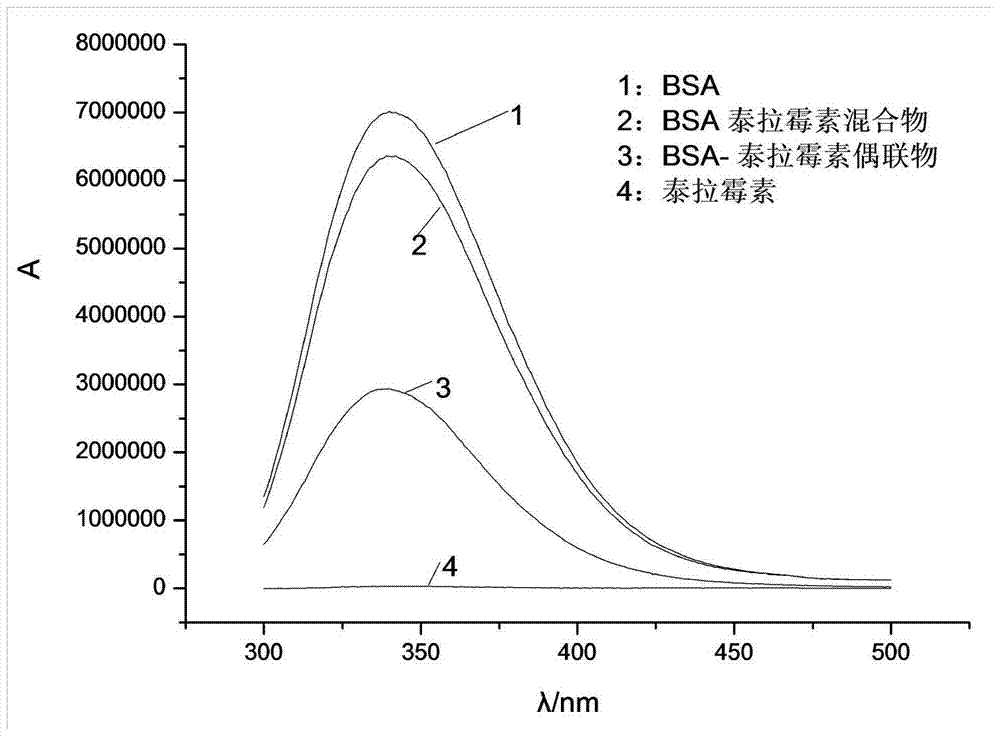
Synthesis method of half-antigen and complete antigen of tulathromycin
The invention discloses a synthesis method of a half-antigen and a complete antigen of tulathromycin and belongs to the technical field of immunology. The method synthesizes the half-antigen of tulathromycin by carrying out hydroxyl on tulathromycin by a succinic anhydride method and introducing free carboxyl. Derivated free carboxyl reacts with primaquine of bovine serum albumin to form an amido bond for coupling by an active ester method to form the complete antigen of tulathromycin. After the antigens are used for immunizing a Balb / c mouse for three times, the valence can reach 1:8000. The antigens have wide practical application prospects.
Owner:JIANGNAN UNIV
Immunosuppressive Cell-Capturing Material and Immunosuppressive Cell-Capturing Column
ActiveUS20140017667A1Reduce concentrationPotential in preventionDead animal preservationMammal material medical ingredientsNystatin GPolymer coatings
This invention discloses an immunosuppressive cell-capturing material comprising a molded body that includes: a readily hydrolyzable condensation polymer having an amino group; a poorly hydrolyzable polymer coating the readily hydrolyzable condensation polymer; and a ligand-conjugated poorly hydrolyzable polymer coating the poorly hydrolyzable polymer, wherein the ligand is at least one selected from the group consisting of a NH2 group, a secondary amino group, a tertiary amino group, a polyamine residue, a basic cyclic polypeptide residue, an aminoglycosidic compound residue, chloroquine, primaquine, mefloquine, imiquimod, and nystatin, and wherein the content of the amino group in the molded body is 150 μmol / g or less. The invention also discloses an immunosuppressive cell-capturing column filled with the capturing material.
Owner:NAT UNIV CORP SHIGA UNIV OF MEDICAL SCI
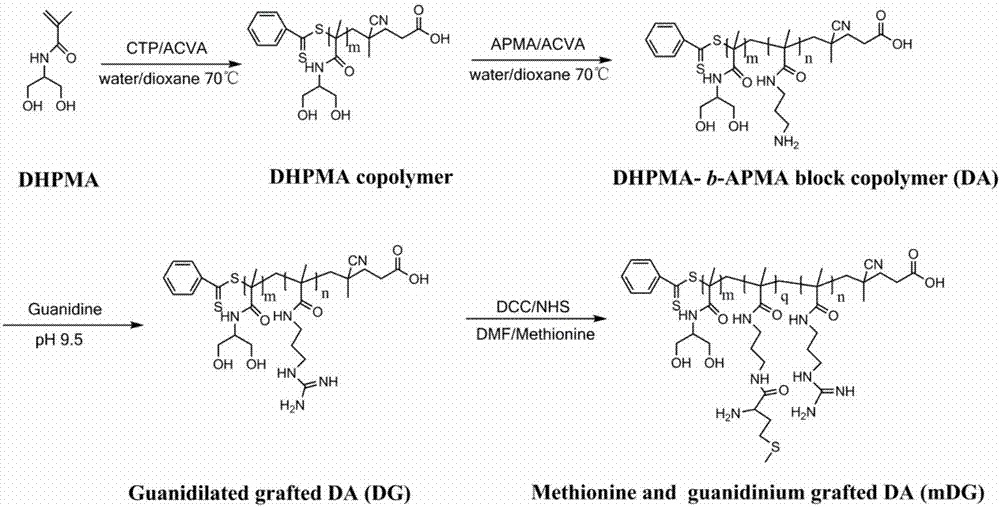
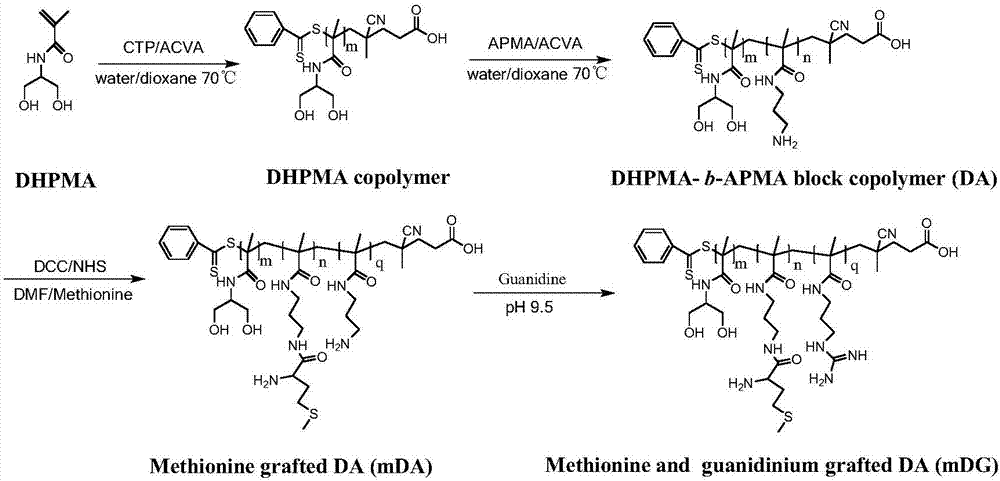
Cationic polymer gene carrier and preparation method and application thereof
ActiveCN106893054AEasy to carryImprove bindingGenetic material ingredientsOther foreign material introduction processesTumor targetPolymer science
Provided is a cationic polymer gene carrier. N-3-aminopropyl methacrylamide and N-(1,3-dihydroxyl-2-propyl) methacrylamide serve as monomers to synthesizea block polymer N-(1,3-dihydroxyl-2-propyl) methacrylamide-b-N-3-aminopropyl methacrylamide polymer, then a guanidine group is coupled to a chain segment of the N-3-aminopropyl methacrylamide to obtain the N-(1,3-dihydroxyl-2-propyl) methacrylamide-b-N-3-guanidinomethacrylamide polymer; or methionine having tumor targeting property is grafted and a primaquine group is guanidinated to obtain N-(1,3-dihydroxyl-2-propyl) methacrylamide-b(N-3-aminopropyl methacrylamide-g-methionine)-b-guanidinomethacrylamide polymer. The invention further provides a preparation method of the gene carrier. The gene carrier can carry a gene drug, has good biocompatibility and tumor targeting property and is large in application prospect.
Owner:JIANGSU CANCER HOSPITAL
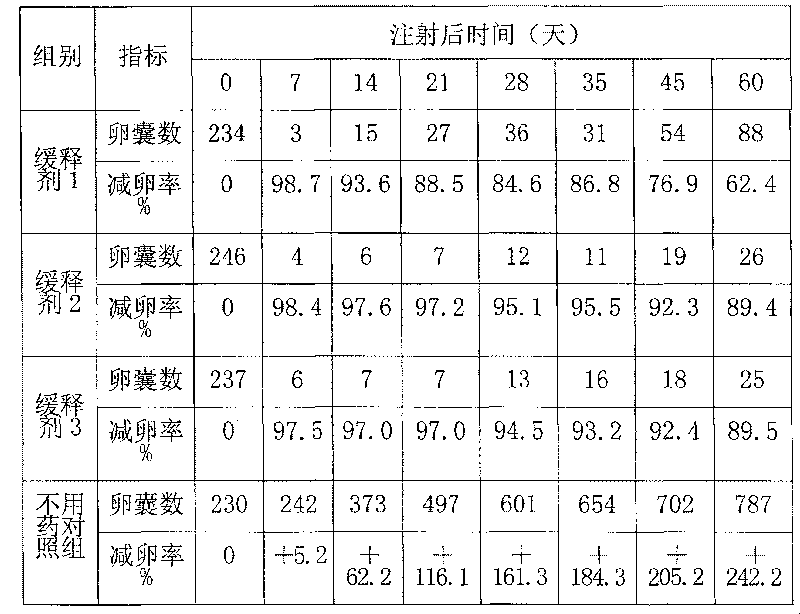
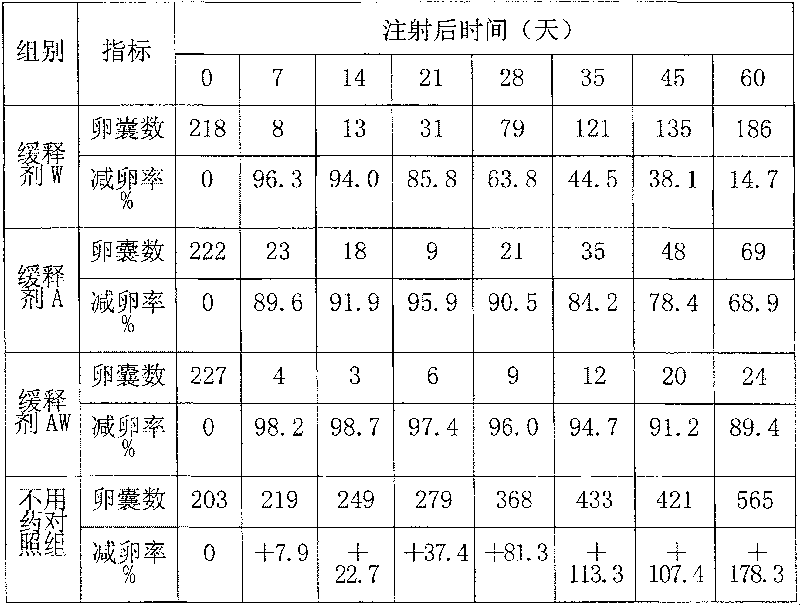
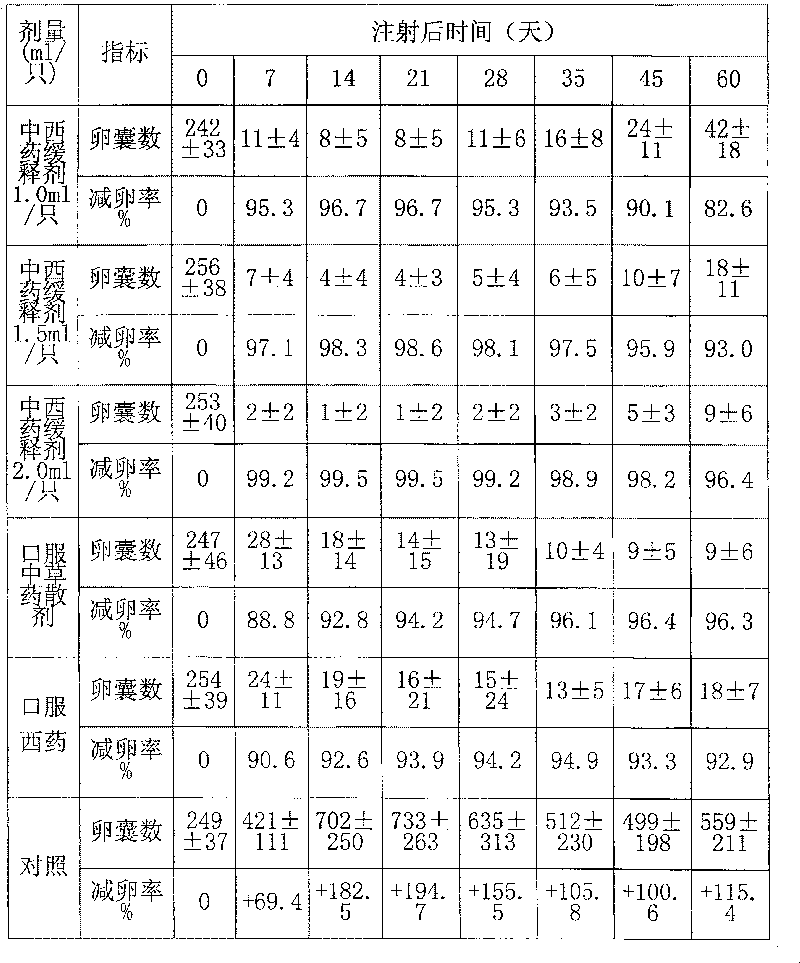
Chinese and Western medicine united sustained-release injection for preventing and controlling coccidiosis of rabbit and its preparation method
InactiveCN101085076ALower resistanceDelay drug resistanceAntiparasitic agentsHeterocyclic compound active ingredientsCoccidiosisBeta-Cyclodextrins
The invention relates to a combination Western-Chinese pharmaceutical slow release injection for treating rabbit coccidiosis and process for preparation, wherein the constituents of the injection include (by weight percent): sweet wormwood 3-6 parts, flavescent sophora root 3-6 parts, Chinese pulsatilla root 2-5 parts, hairy vein agrimony 2-5 parts, bupleurum root 1-3 weight parts, garden burnet root 1-3 parts, astragalus root 1-3 parts, primaquine 0.06-0.35 part, beta-cyclodextrin 2-4 parts. The preparing process consists of drying the raw material herbs, disintegrating, mixing, ethanol extracting, water extracting, removing foreign substance, mixing the extract with primaquine and beta-cyclodextrin, grinding, dressing and adjusting soup volume.
Owner:ZHEJIANG ACADEMY OF AGRICULTURE SCIENCES
Compound artemisinin
The present invention provides a novel combination comprising artemisinin in the form of tablets and related dosage forms for pediatric use, such as granules, suppository, suspension syrup and dry powder, for the treatment of human malarias including multiple-resistant subtertian malaria, tertian malaria and quartan malaria. Said combination is comprised of artemisinin, piperaquine and primaquine. Clinical tests in Southeast Asia countries where malaria is epidemic demonstrate that, apart from having high and rapid therapeutic effect possessed by the most excellent domestic and foreign artemisinin-type anti-malarial drugs, the present combination is also featured with shorter course of treatment, less side effect, lower material cost, and more convenience for administration, and its ability of rapidly killing gametophyte and cutting off infection source thereby blocking spreading of malaria is a further improvement.
Owner:ARTEPHARM CO LTD CHINA
Composition containing artemisinin for treatment of malaria
The present invention provides a novel combination comprising artemisinin in the form of tablets and related dosage forms for pediatric use, such as granules, suppository, suspension syrup and dry powder, for the treatment of human malarias including multiple-resistant subtertian malaria, tertian malaria and quartan malaria. The combination is comprised of artemisinin, piperaquine and primaquine. Clinical tests in Southeast Asia countries where malaria is epidemic demonstrate that, apart from having high and rapid therapeutic effect possessed by the most excellent domestic and foreign artemisinin-type anti-malarial drugs, the present combination is also featured with shorter course of treatment, less side effect, lower material cost, and more convenience for administration, and its ability of rapidly killing gametophyte and cutting off infection source thereby blocking spreading of malaria is a further improvement.
Owner:ARTEPHARM CO LTD CHINA
Immunosuppressive cell-capturing material and immunosuppressive cell-capturing column
InactiveUS9127250B2Reduce concentrationPotential in preventionOther blood circulation devicesOther chemical processesPolymer coatingsNystatin
Owner:NAT UNIV CORP SHIGA UNIV OF MEDICAL SCI
Malaria-resisting arteannuin naphthoquine compound composition
ActiveCN101116665AQuick clearStop transmissionSuppositories deliverySolution deliverySide effectCurative effect
The invention discloses a medicine combination used to cure or prevent malaria, which basically consists of arteannuin or derivatives thereof, naphthoquine and primaquine. The invention can be used in curing malignant malaria, quartan malaria and tertian malaria and is particularly suitable to cure malignant malaria with multi-drug resistance through adopting one dose therapeutics. The invention has good curative effect, less toxic and side effect and low cost, thereby being particularly suitable for fast control and elimination of malaria in various regions.
Owner:李国桥 +1
A kind of synthetic method of hapten and complete antigen of telamycin
The invention discloses a synthesis method of a half-antigen and a complete antigen of tulathromycin and belongs to the technical field of immunology. The method synthesizes the half-antigen of tulathromycin by carrying out hydroxyl on tulathromycin by a succinic anhydride method and introducing free carboxyl. Derivated free carboxyl reacts with primaquine of bovine serum albumin to form an amido bond for coupling by an active ester method to form the complete antigen of tulathromycin. After the antigens are used for immunizing a Balb / c mouse for three times, the valence can reach 1:8000. The antigens have wide practical application prospects.
Owner:JIANGNAN UNIV
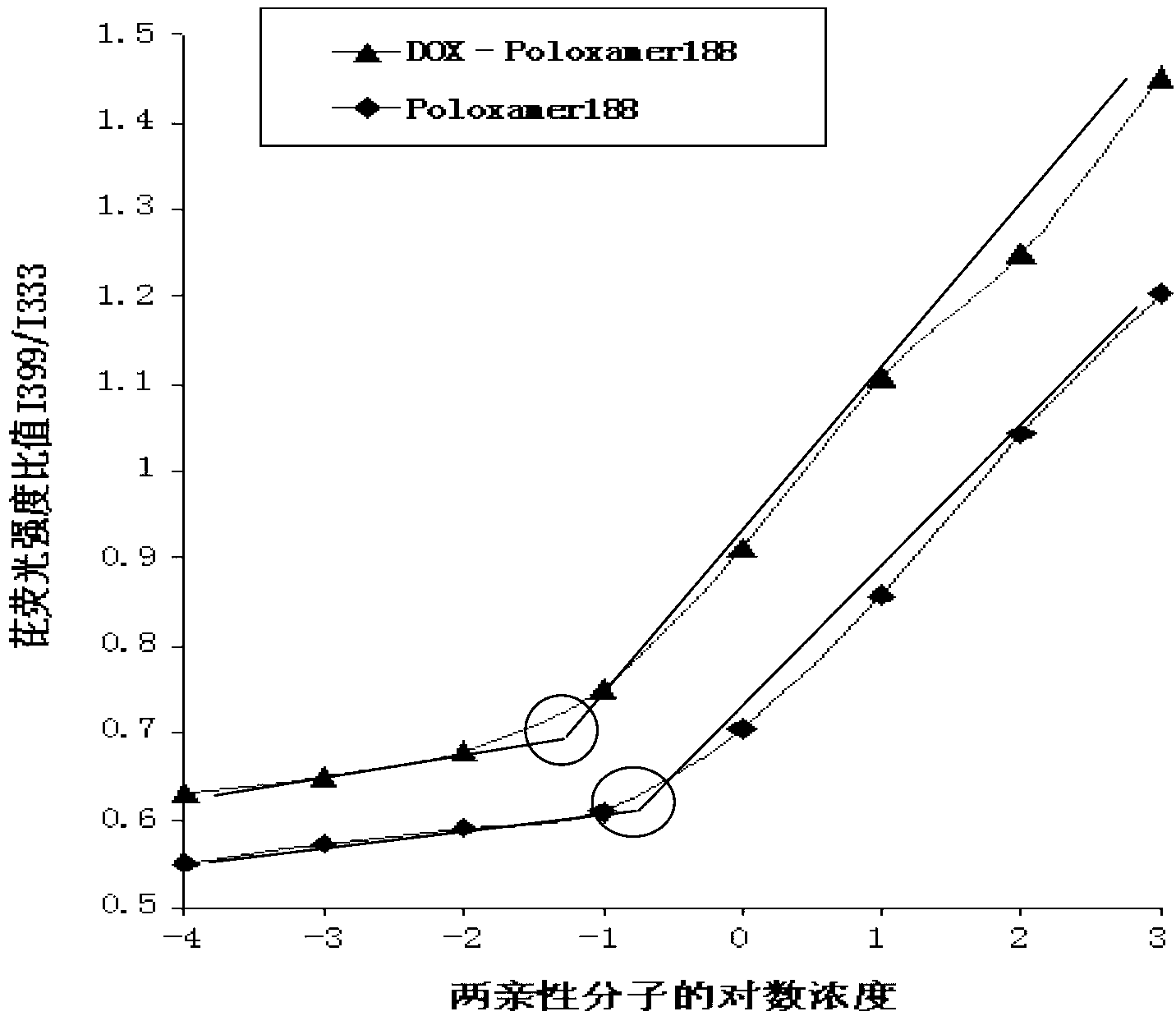
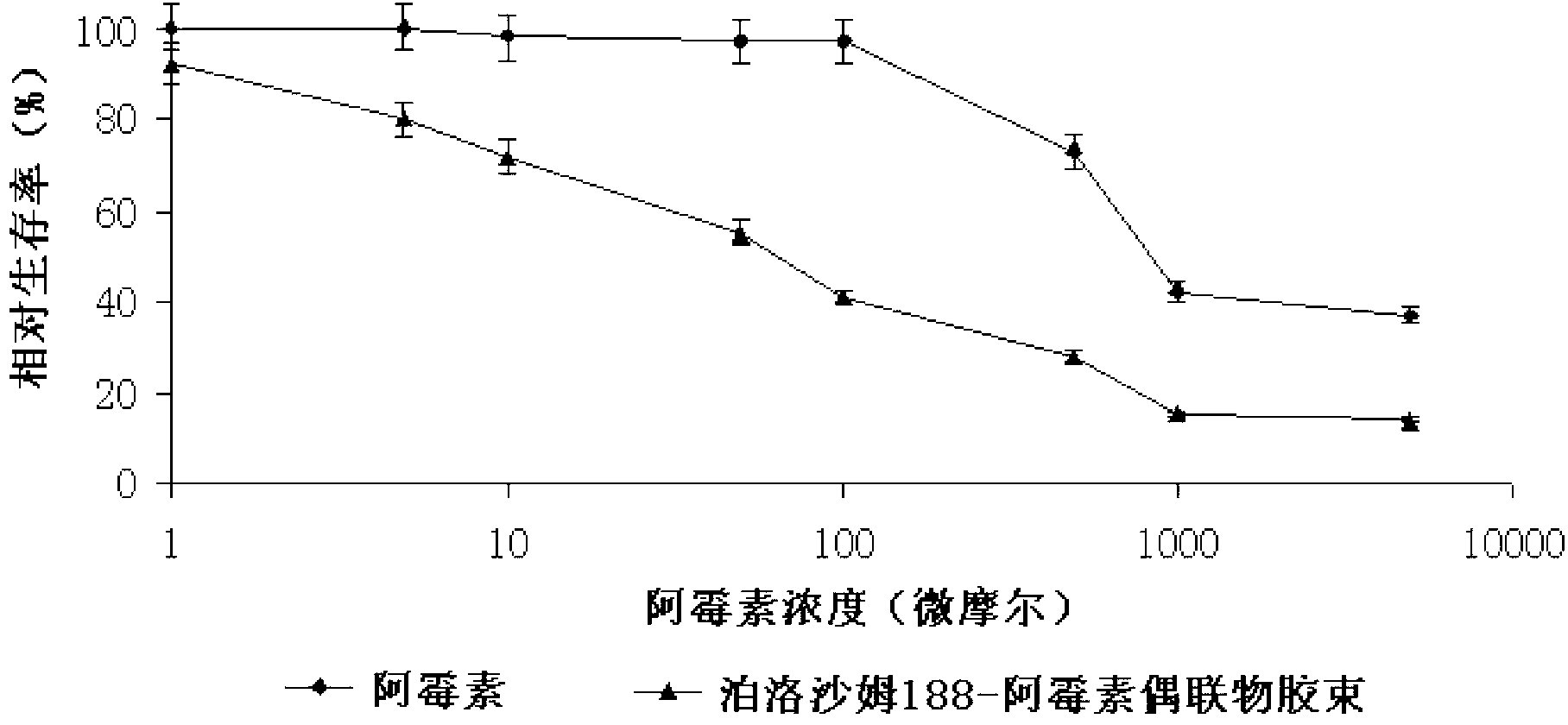
DOX-P micelle with anti-tumor drug resistance and preparation method thereof
InactiveCN102989002ALow critical micelle concentrationParticle size controllableOrganic active ingredientsSolution deliveryWilms' tumorPolymer
The invention discloses poloxamer-adriamycin conjugate (DOX-P) micelle with anti-tumor drug resistance and a preparation method of the DOX-P micelle. The synthetic process of the DOX-P comprises the steps of firstly, reacting poloxamer with succinic anhydride to obtain high-molecular polymer of which the tail end contains carboxyl; and then, directly bonding the carboxyl at the tail end of the polymer with primaquine group in the adriamycin in an amido bond mode. The DOX-P has the capability of forming the micelle; and compared with the adriamycin bulk drug, the adriamycin subjected to covalent binding in the DOX-P micelle has the advantages that the anti-tumor activity of the adriamycin is maintained, the drug resistance of the tumor is overcome, and the stability of the adriamycin is better than that of poloxamer micelle.
Owner:吴燕
Malaria-resisting arteannuin naphthoquine compound composition
ActiveCN101116665BQuick clearStop transmissionSuppositories deliverySolution deliverySide effectCurative effect
The invention discloses a medicine combination used to cure or prevent malaria, which basically consists of arteannuin or derivatives thereof, naphthoquine and primaquine. The invention can be used in curing malignant malaria, quartan malaria and tertian malaria and is particularly suitable to cure malignant malaria with multi-drug resistance through adopting one dose therapeutics. The invention has good curative effect, less toxic and side effect and low cost, thereby being particularly suitable for fast control and elimination of malaria in various regions.
Owner:李国桥 +1
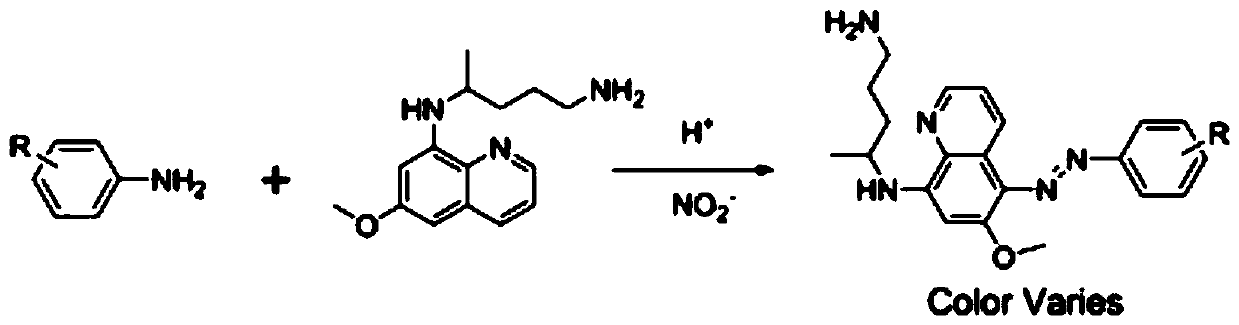
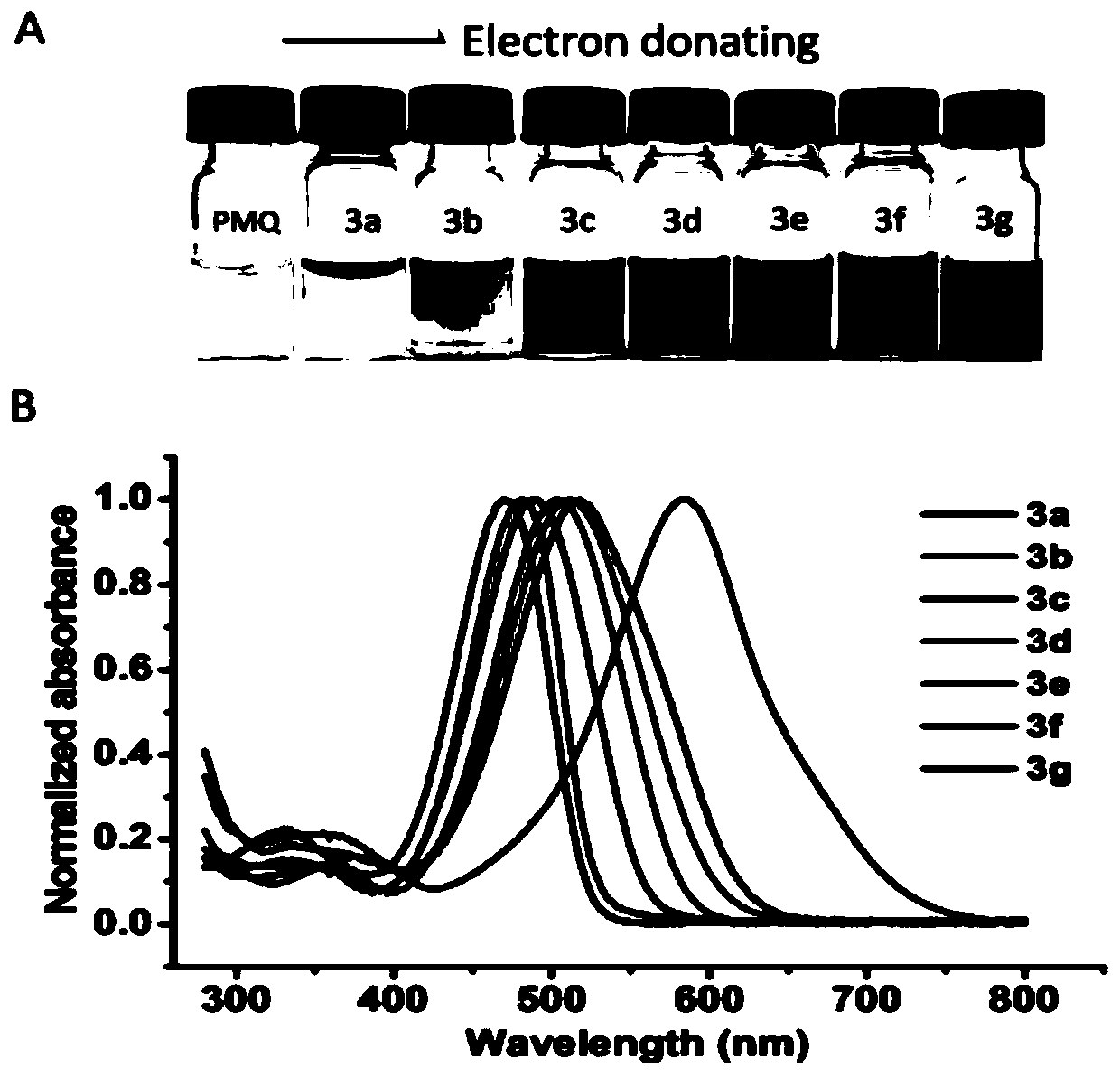
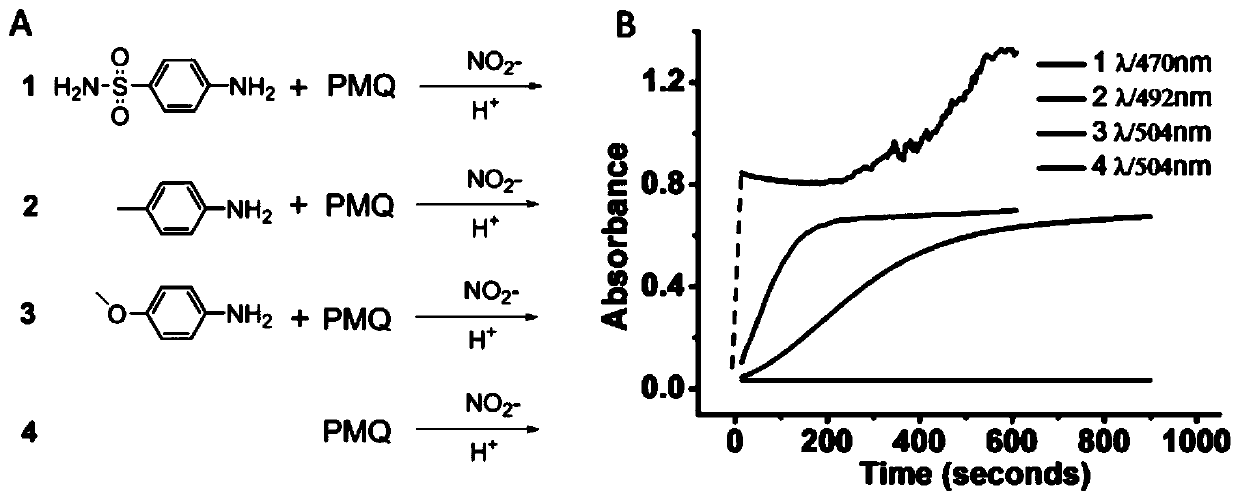
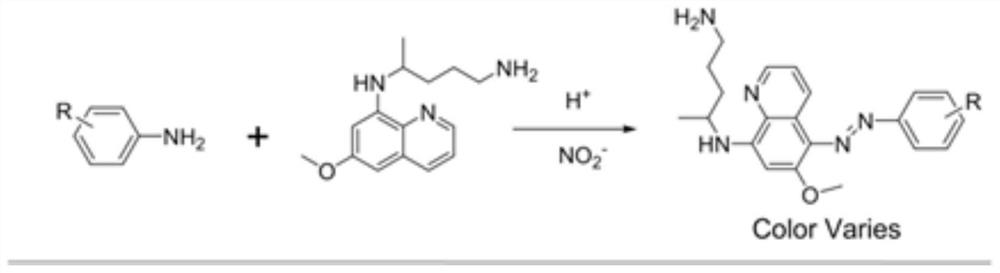
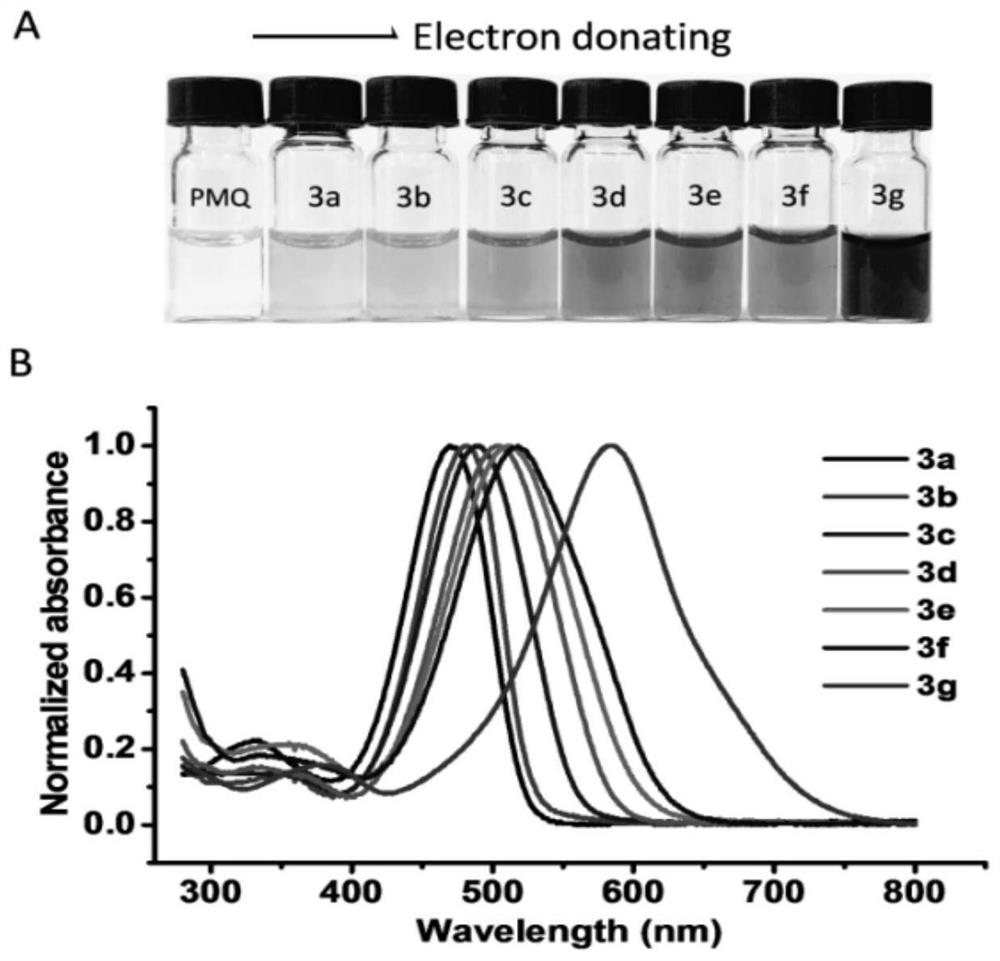
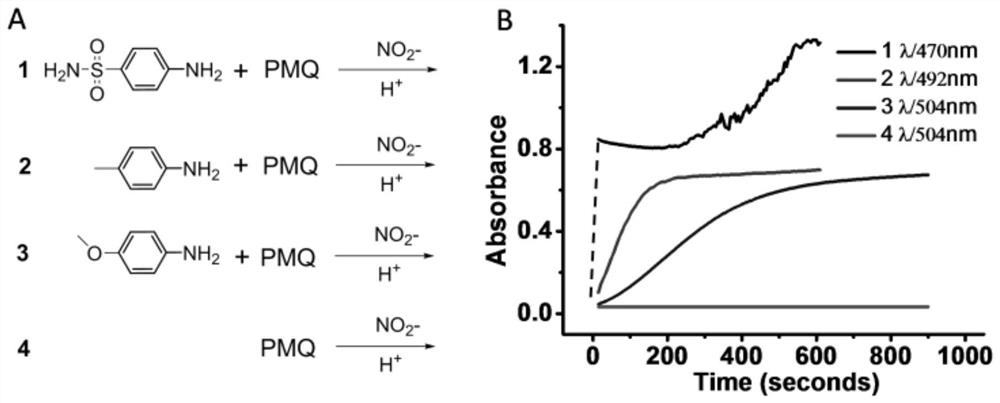
Colorimetric detection method for primaquine drugs
ActiveCN109916887ADark colorLow detection limitMaterial analysis by observing effect on chemical indicatorColor/spectral properties measurementsNitriteSerum samples
The invention relates to a colorimetric detection method for primaquine drugs. In an acidic environment and in the presence of nitrite, para substituent-containing aniline can produce a Griess reaction with primaquine to form a colored azo product; a UV-Vis absorption spectrum and a maximum light absorption value of the colored product are determined; formation of the colored azo product and a change of a light absorption value thereof are detected in the presence of the primaquine with different concentrations by a microplate reader; a standard curve of the concentration of the primaquine andthe light absorption value of the azo product is established; and the concentration of the primaquine drugs in a to-be-tested sample is determined by a corresponding relation between the concentration of the primaquine and the light absorption value of the azo product on the standard curve. The method is successfully used for quantitatively analyzing and synthetizing the concentration of the primaquine in urine, and a detection limit is as low as 0.63 [mu]M. The method can also detect the primaquine from a serum sample within a clinically relevant concentration range.
Owner:GUANGZHOU UNIVERSITY OF CHINESE MEDICINE
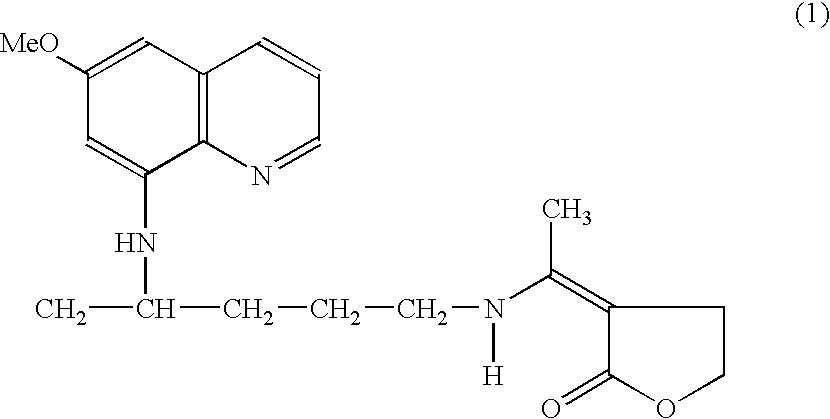
Method for the treatment of malaria by the use of primaquine derivative N1-(3-ethylidinotetrahydrofuran-2-one)-N4- (6-methoxy-8-quinolinyl)-1,4-pentanediamine as gametocytocidal agent
InactiveUS7183291B2Low toxicityFacilitating controlled deliveryBiocideOrganic chemistryPhysiologyMalaria
The present invention a novel use of primaquine derivative N1-(3-ethylidinotetrahydrofuran-2-one)-N4-(6-methoxy-8-quinolinyl)-1,4-pentanediamine in the treatment and controlling the spread of malaria. In particular, the present invention discloses a method of treatment of malaria by the use of primaquine derivative N1-(3-ethylidinotetrahydrofuran-2-one)-N4-(6-methoxy-8-quinolinyl)-1,4-pentanediamine as a gametocytocidal agent.
Owner:COUNCIL OF SCI & IND RES
A kind of colorimetric detection method of primaquine drugs
ActiveCN109916887BDark colorLow detection limitMaterial analysis by observing effect on chemical indicatorColor/spectral properties measurementsSerum samplesAniline
The invention relates to a colorimetric detection method for primaquine drugs. Under the conditions of an acidic environment and the presence of nitrite, aniline with substituents at the para-position can undergo a Griess reaction with primaquine to form a colored azo product; Determine the UV-Vis absorption spectrum and the maximum absorbance value of the above-mentioned colored product; under the presence of different concentrations of primaquine detected by a microplate reader, the formation of the above-mentioned colored azo product and the change of its absorbance value; establish the primaquine concentration and azo The standard curve of the product absorbance value; through the corresponding relationship between the concentration of primaquine on the standard curve and the absorbance value of the azo product, the concentration of the primaquine drug in the sample to be tested is determined. This method has been successfully used to quantify the concentration of primaquine in synthetic urine with a detection limit as low as 0.63 μM. The method also detects primaquine from serum samples in a clinically relevant concentration range.
Owner:GUANGZHOU UNIVERSITY OF CHINESE MEDICINE
Antimalarial green tea chewing gum
InactiveCN101623296AFull of nutritionNo side effectsChewing gumPharmaceutical delivery mechanismDiseaseSide effect
The invention relates to an antimalarial green tea chewing gum which is characterized by being made from the following raw materials by weight portion: 0.1-5 portions of artemisinin, 0.3-5 portions of tea polysaccharide, 0.6-8 portions of naphthoquine, 0.01-0.15 portion of primaquine, 10-70 portions of sugar, 1-30 portions of starch and 5-40 portions of gum base. The antimalarial green tea chewing gum is made according to the steps of fully mixing the gum base, the sugar and the like, heating the mixture into the paste, cooling the paste below 40 DEG C, adding slowly and stirring the crystalloid artemisinin, the tea polysaccharide, the naphthoquine and the primaquine to the paste, forming a block and cutting the block. Besides, the artemisinin or the derivative thereof, the tea polysaccharide, the naphthoquine and the primaquine can be added to the raw material of the maltose, or the crunchy candy or the granular candy to made into the antimalarial green tea maltose, the antimalarial green tea crunchy candy or the antimalarial green tea granular candy. The antimalarial green tea chewing gum tastes fragrant, sweet and nice, is rich in nutrition, has no toxic side effect and can cool the throat and prevent malaria after being taken for a long time.
Owner:乔新光
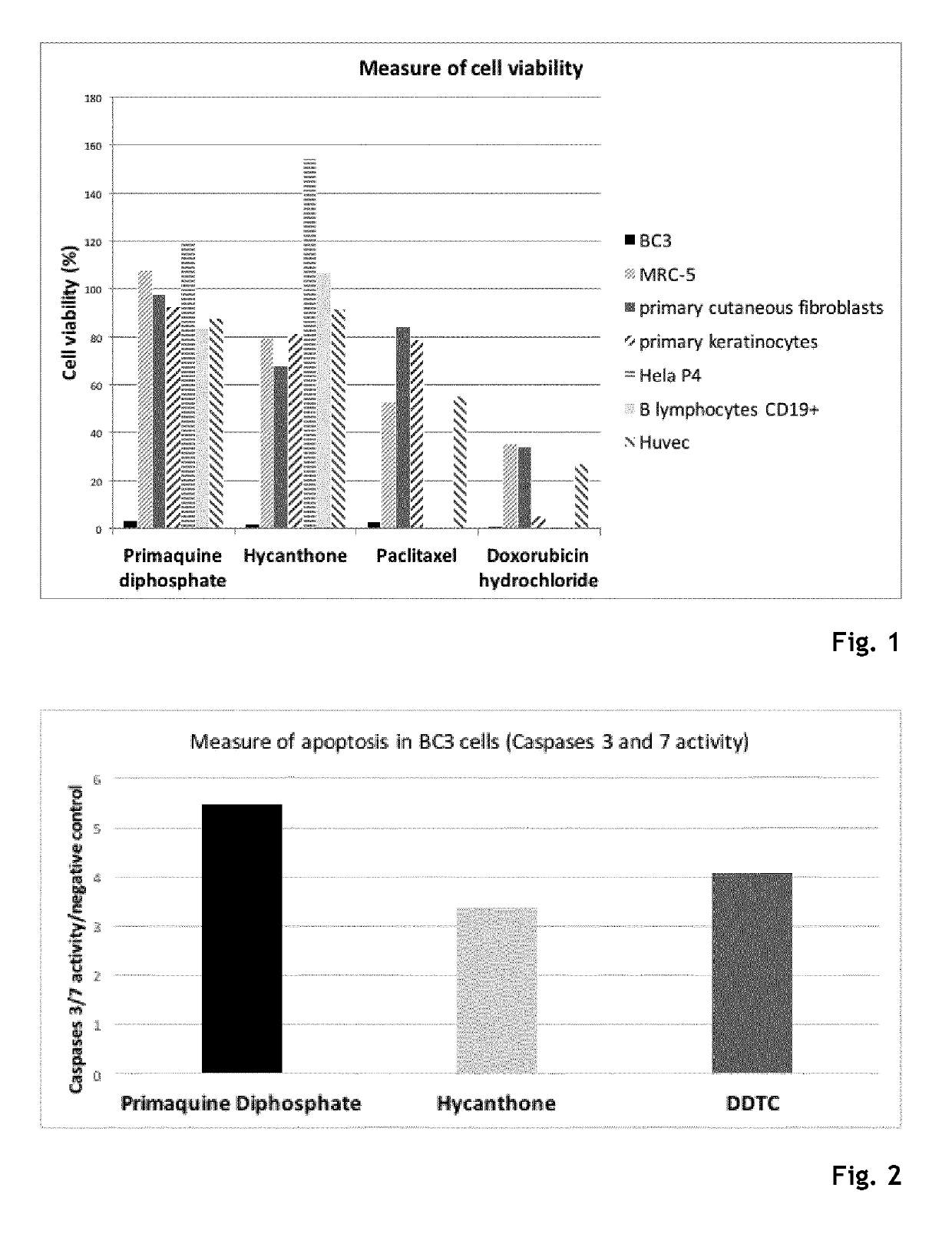
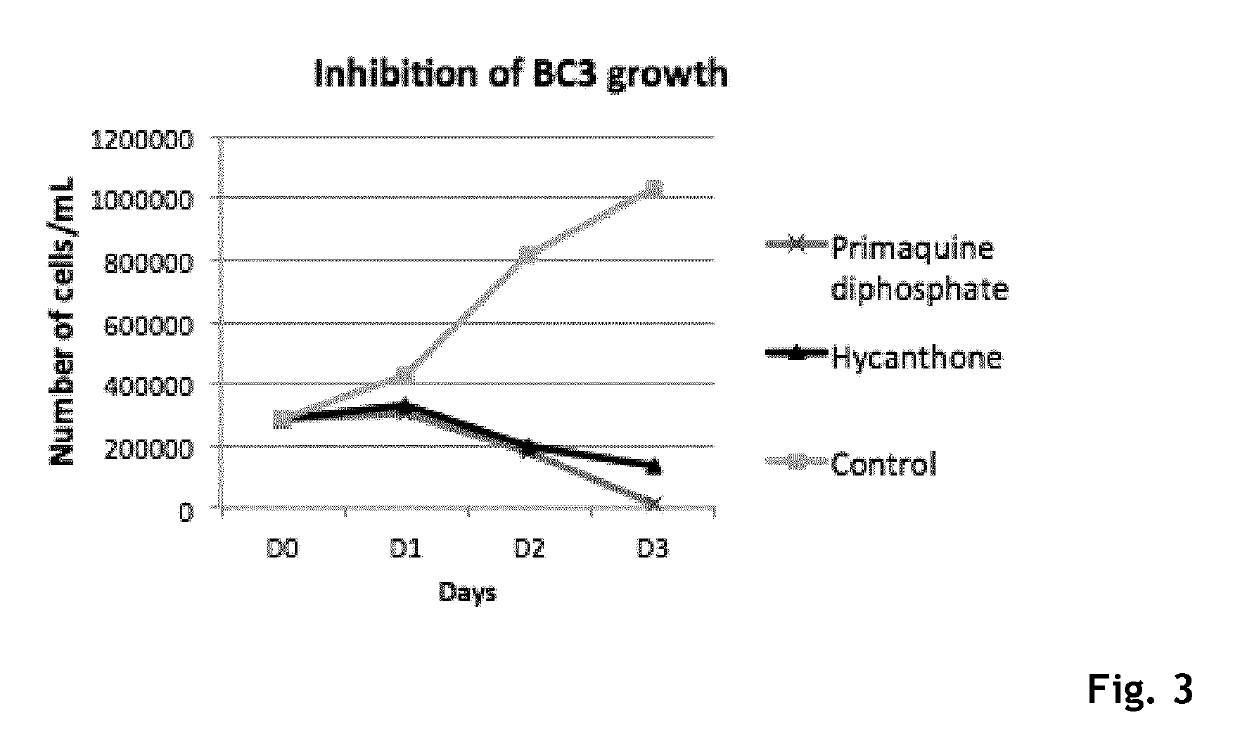
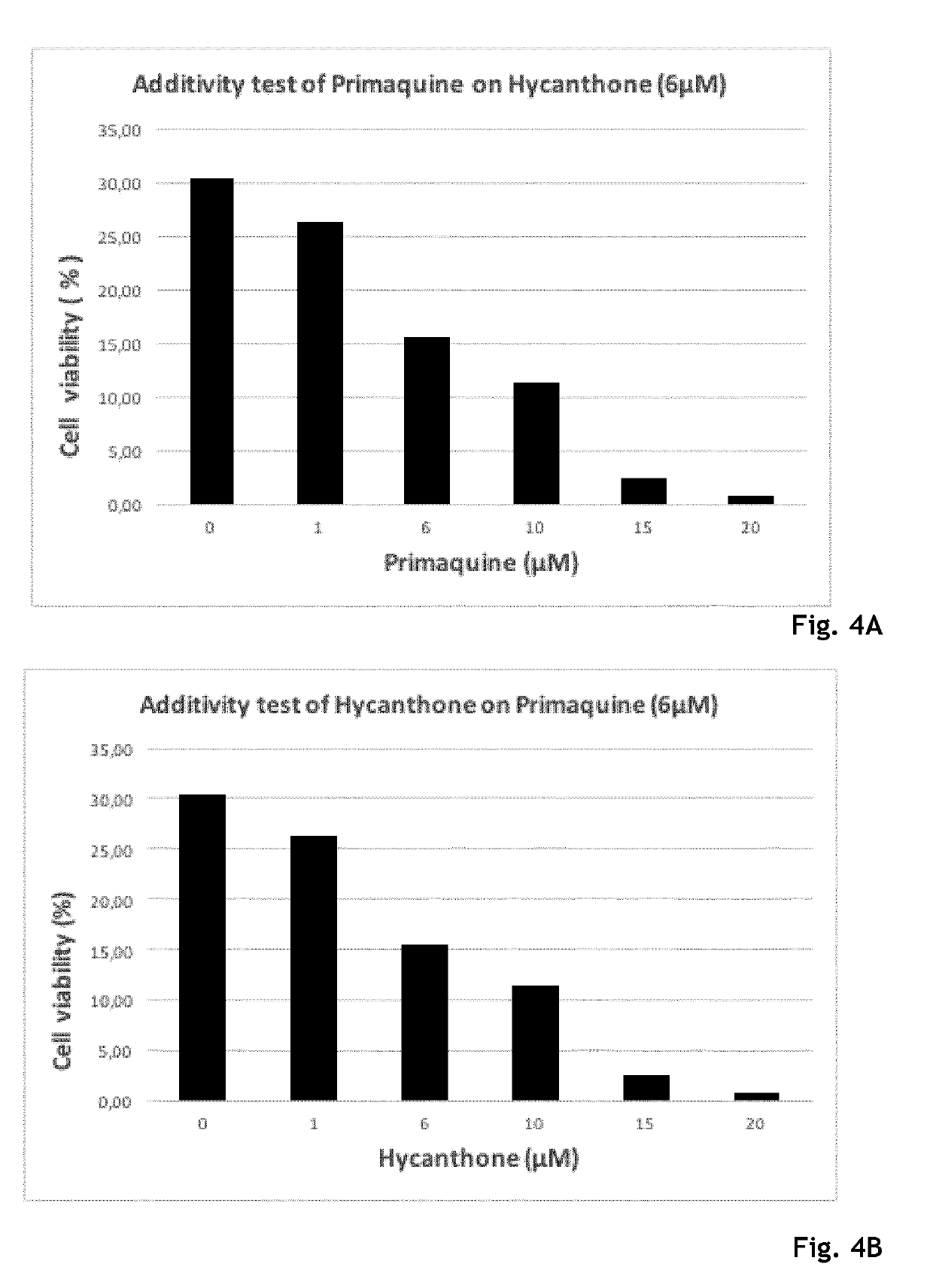
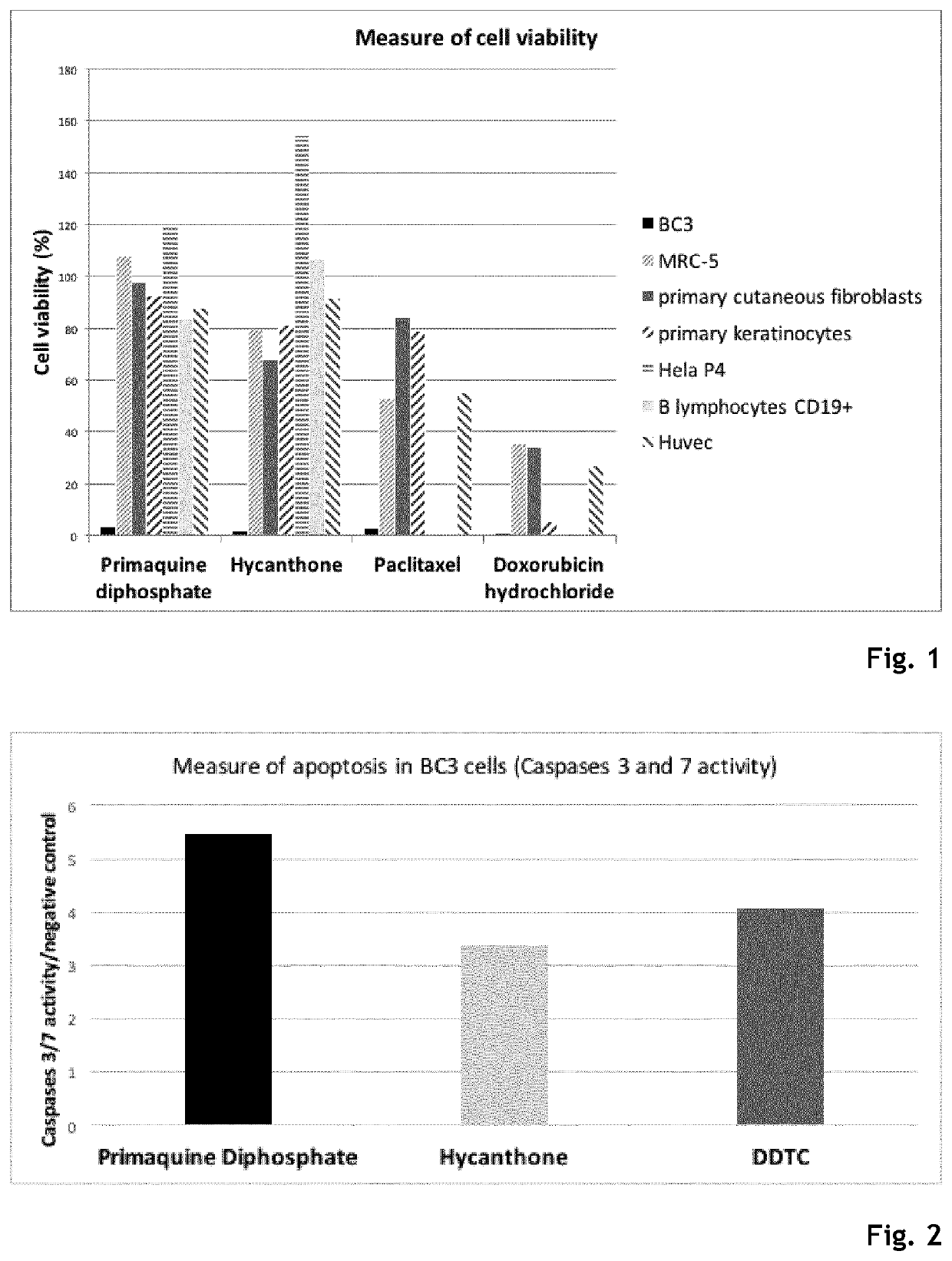
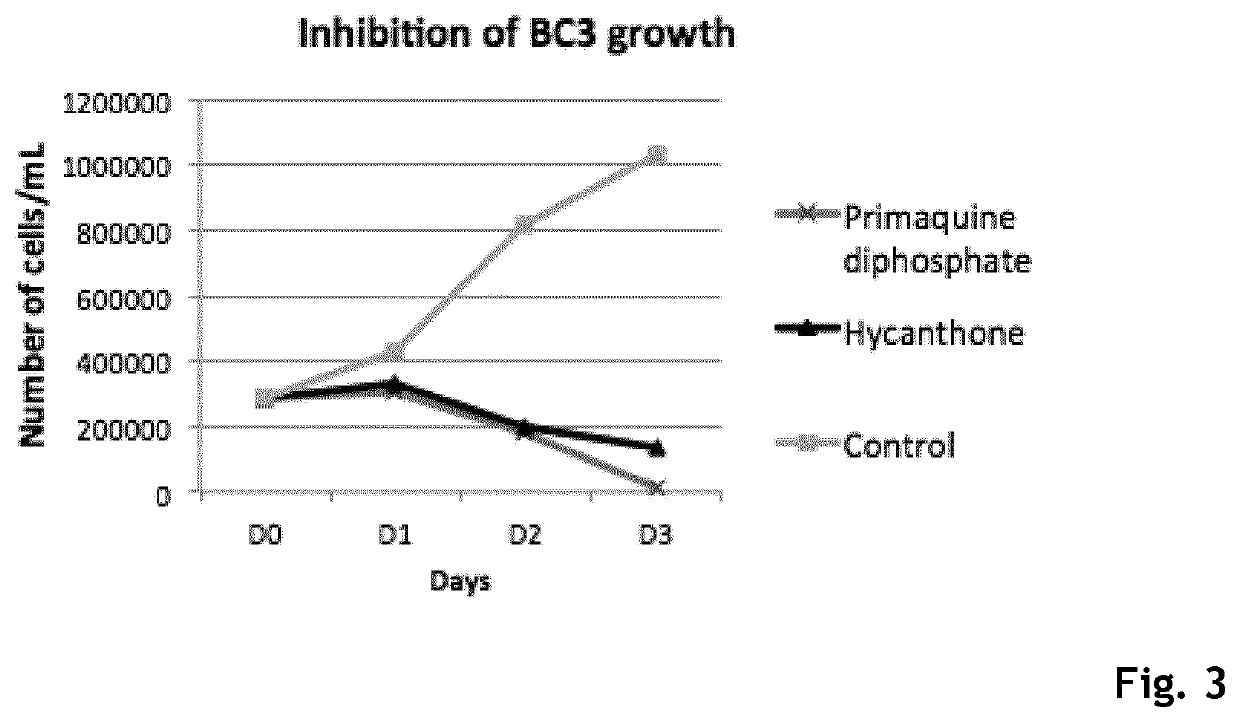
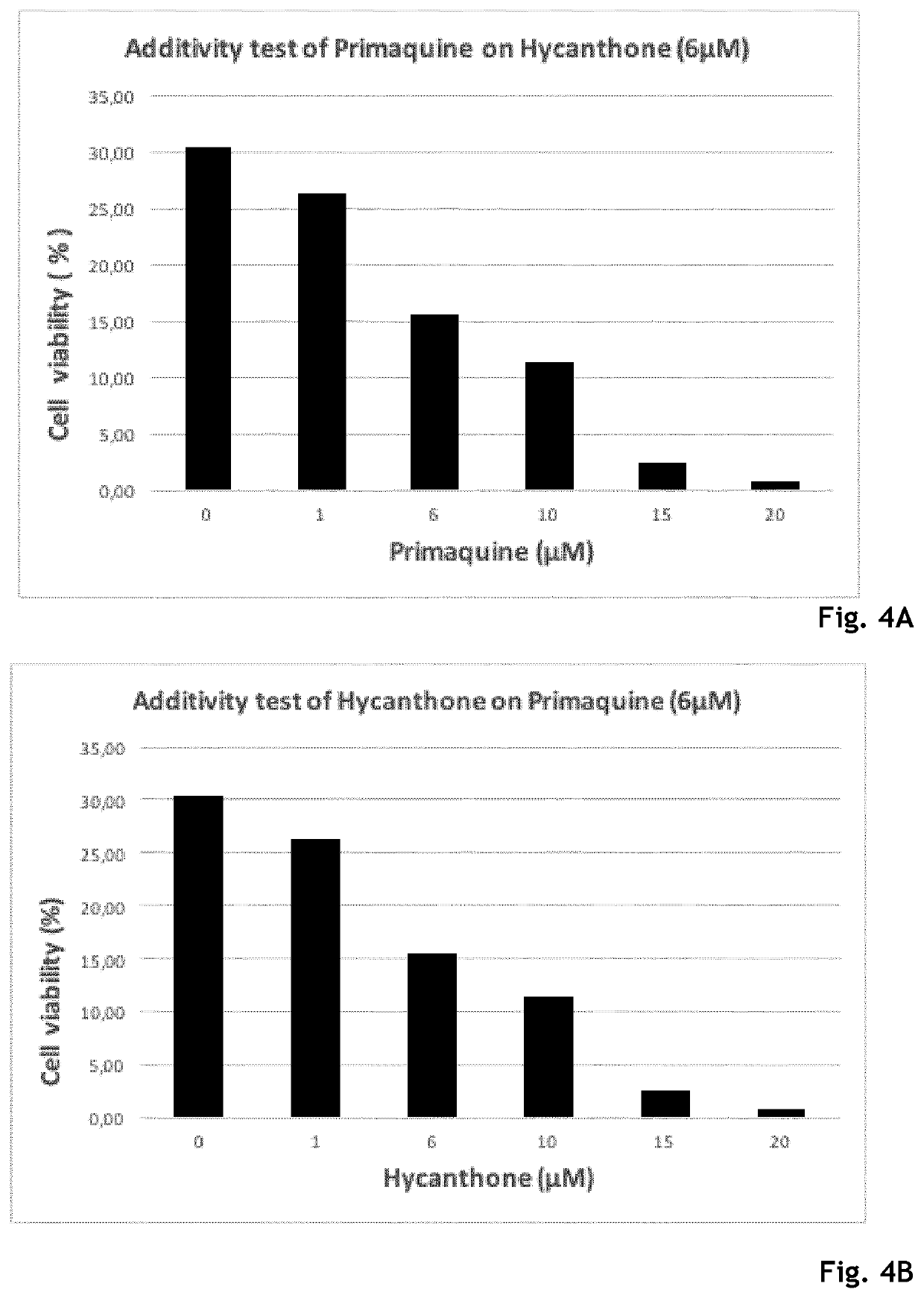
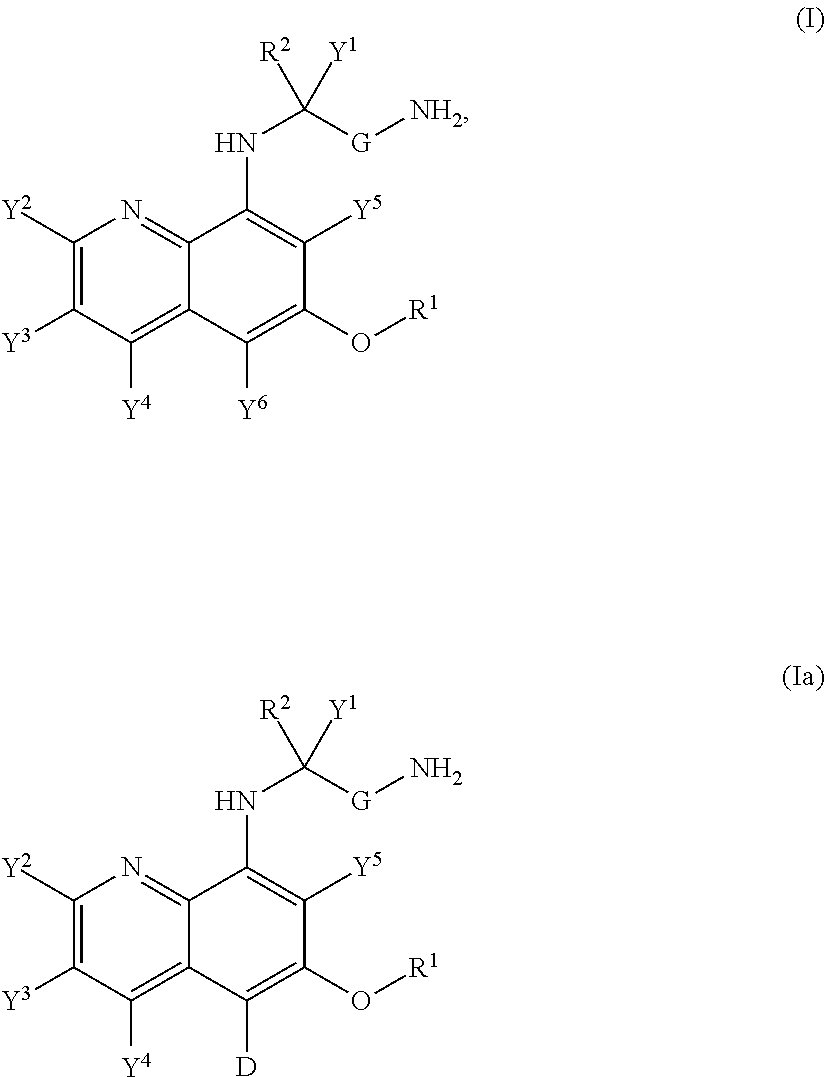
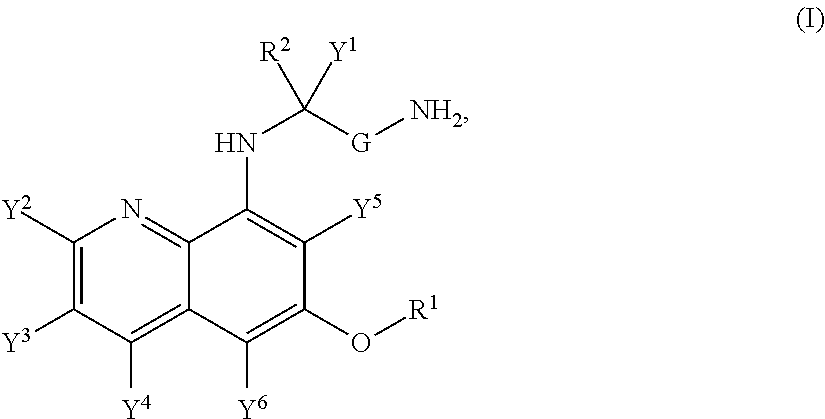
Hycanthone derivatives and primaquine derivatives for use in the prevention and/or the treatment of disorders associated to gammaherpesvirus
The present invention relates to compounds of the following general formula (I) or (II) or a pharmaceutically acceptable salt and / or solvate thereof, for use in the prevention and / or the treatment of disorders associated to gammaherpesvirinae, in particular to the human herpesvirus 8 (HHV8) or the human herpes virus 4 (HHV4), and pharmaceutical compositions containing such compounds.
Owner:UNIV DE PARIS +4
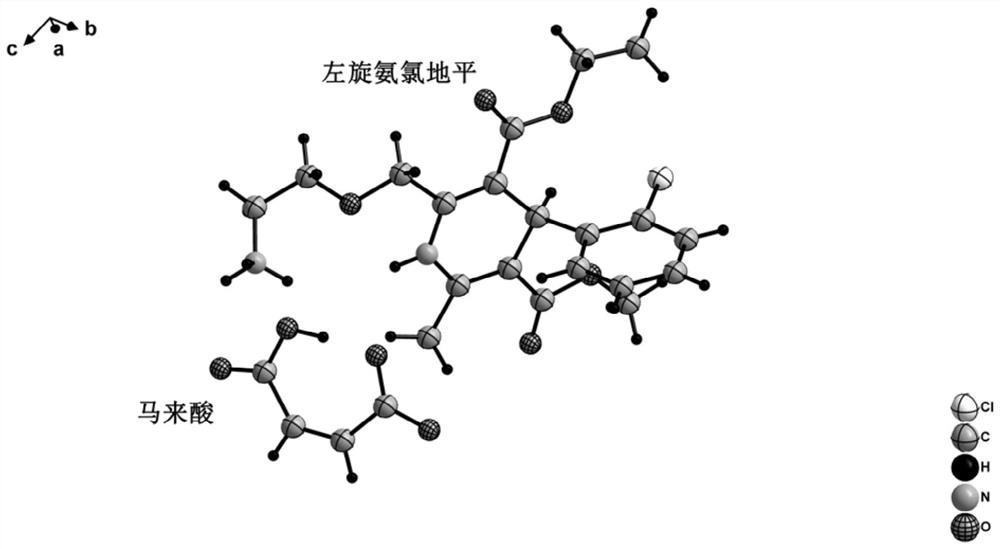
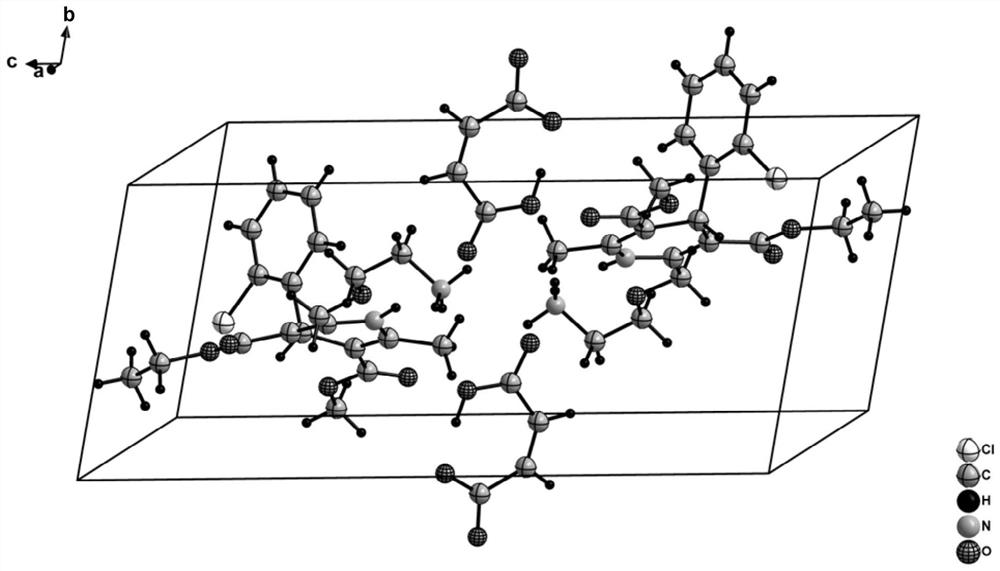
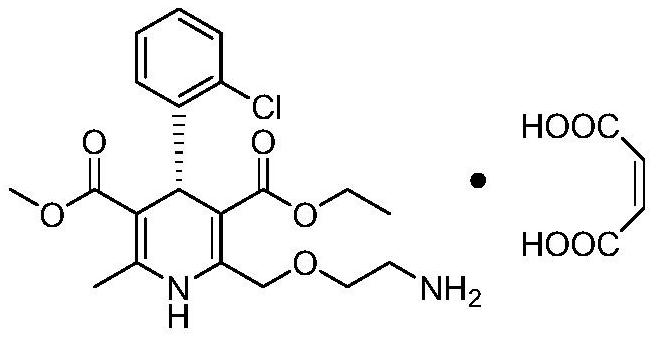
Levoamlodipine maleate eutectic medicine crystallized to triclinic system and preparation method and application of levoamlodipine maleate eutectic medicine
PendingCN111671750AClear crystal formDefine crystallographic parametersOrganic active ingredientsOrganic compound preparationCrystal systemButenedioic acid
Owner:FUDAN UNIV +1
Preparation and preparation method of erythromycin and related drugs
The invention relates to a preparation method of new preparation of erythrocin, which is characterized in that an endothelin core of erythrocin is prepared, and then an isolating layer, a protective layer, a second isolating layer and an improved enteric-coating material layer are applied one by one. In this way, new preparation of the erythrocin which has certain feature of releasing (dissolving) in acid solution (hydrochloric acid solution 9 to 1000) can be formed. The technology of the new preparation can also be widely applied to drugs which, like erythrocin, when being taken orally by a patient, cause the patient to suffer the side effects of stimulation, sickness and the like after degradation in the stomach of the patient or contact with the stomach of the patient, and drugs which the patient needs to take orally to let the blood concentration to reach the peak value in a short time. Such drugs include macrolides of azithromycin, metronidazole of nitroimidazoles, tinidazole, acyclovir as an antiviral drug, ammonium chloride as a phlegm eliminating drug, bromhexine, chloroquine as an antimalarial, nitroquine, artemisinin, dihydroartemisinin, artesunate, primaquine, pyrimethamine, carbarsone and emetine amebicides and so on.
Owner:胡昌勤 +1
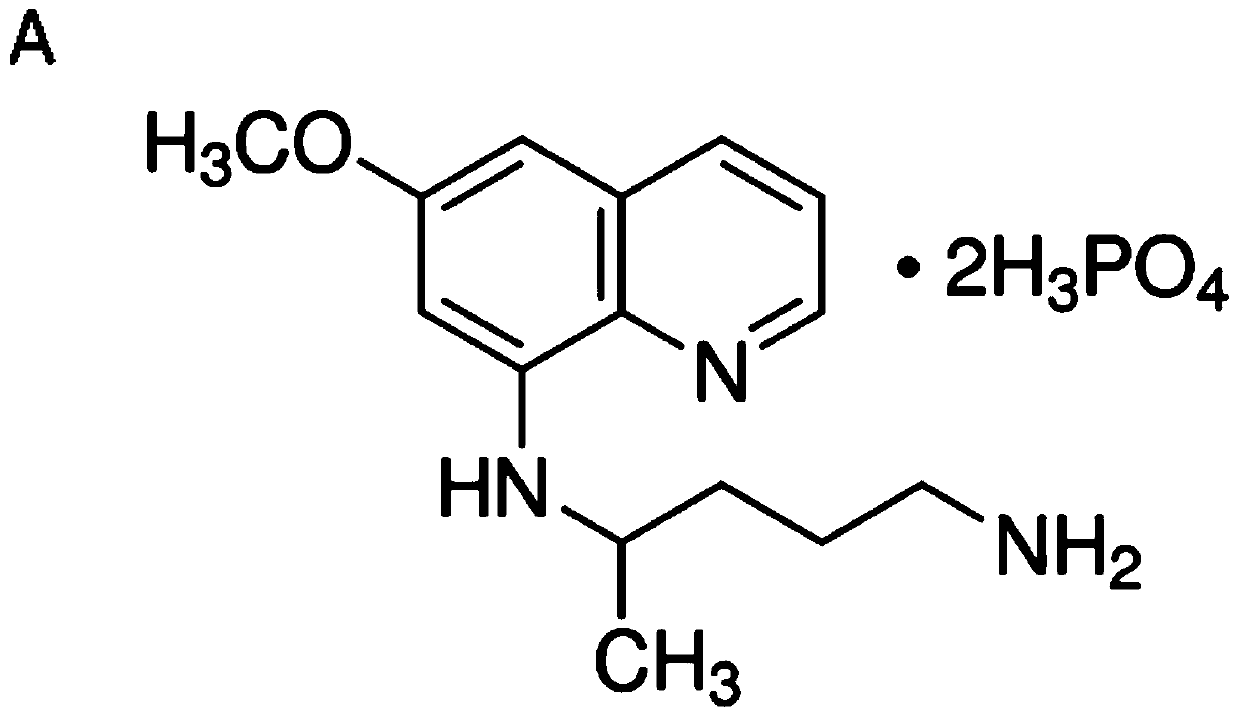
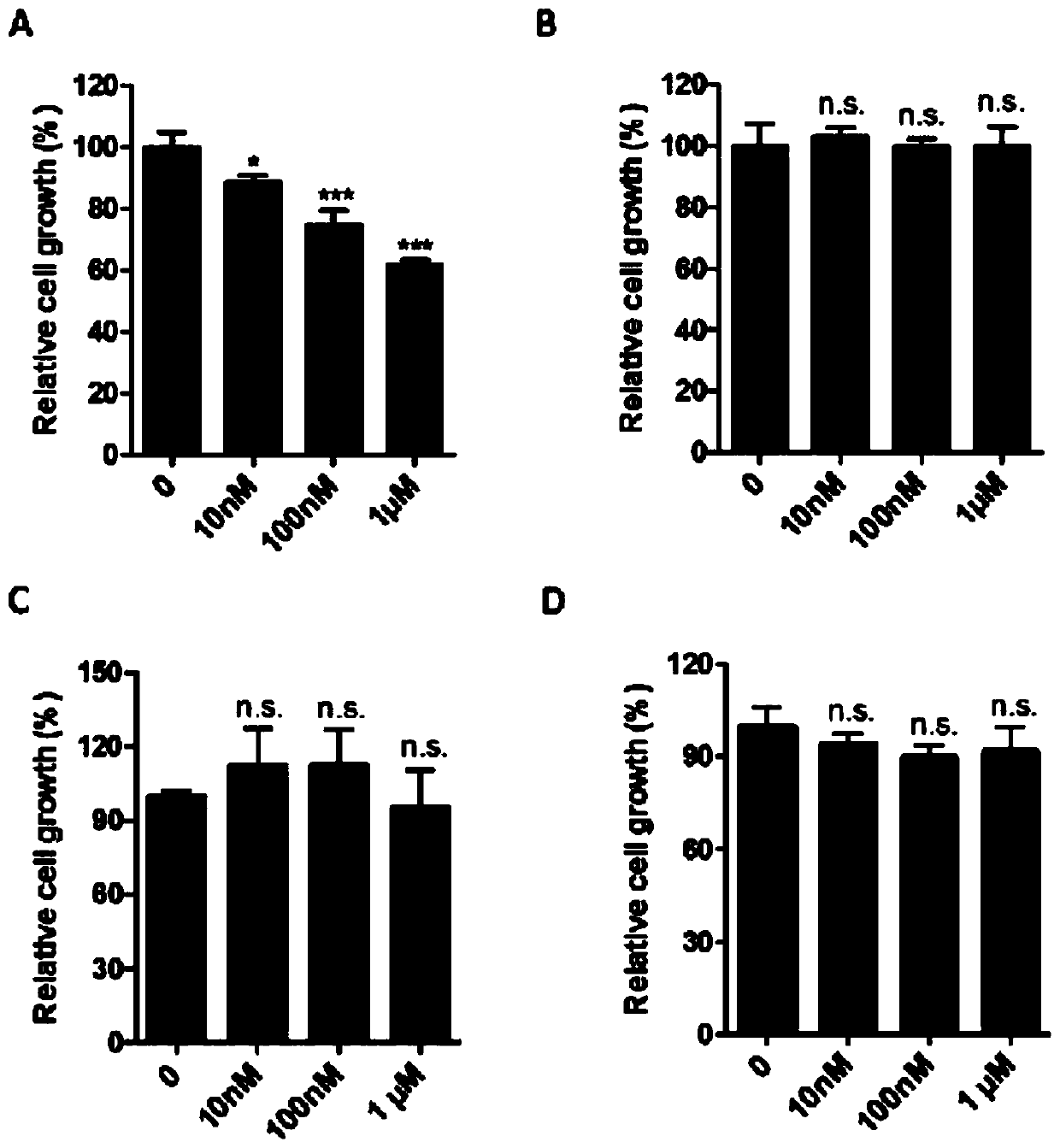
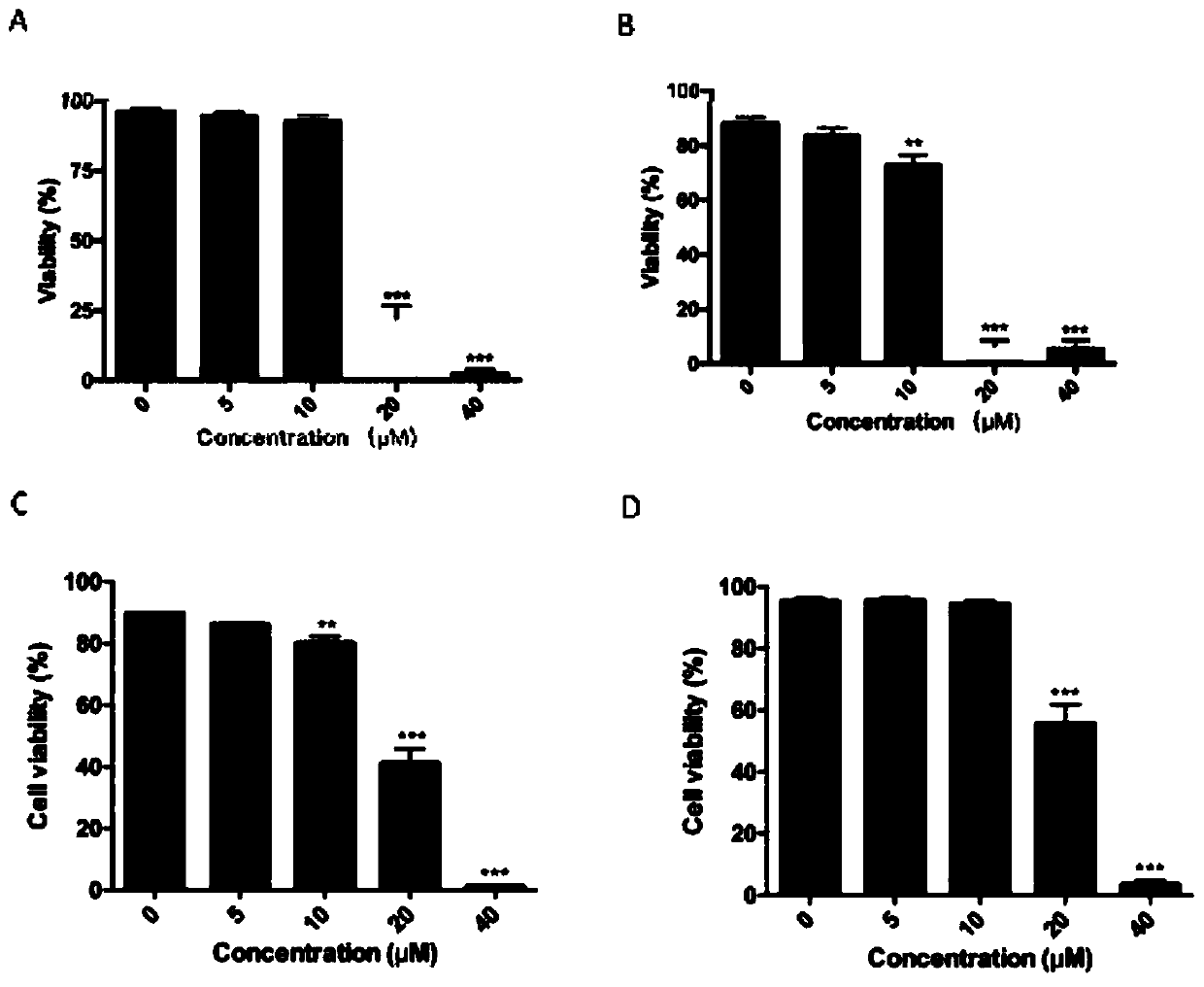
Application of anti-malarial drug-phosphate primaquine in preparation of drugs for treating leukemia
ActiveCN111067899ASignificant anti-leukemia effectGrowth inhibitionOrganic active ingredientsAntineoplastic agentsPeripheral blood mononuclear cellMyeloid leukemia
The invention discloses an application of an anti-malarial drug-phosphate primaquine in preparation of drugs for treating leukemia. Through the system screening on multiple drugs approved by the FDA,an anti-malarial drug-phosphate primaquine is found that the phosphate primaquine has significant anti-leukemia effects. At the cellular level, the phosphate primaquine can significantly inhibit the growth of multiple myeloid leukemia cell lines but has no significant growth inhibiting effects on normal human peripheral blood mononuclear cells and neutrophil, and the phosphate primaquine has moreobvious growth inhibiting effects on acute promyelocytic leukemia cells; at the patient level, the phosphate primaquine can inhibit the primary cell colony forming ability of patients with acute myeloid leukemia; and at the animal level, the phosphate primaquine has obvious inhibition effects on all-trans retinoic acid-resistant acute promyelocytic leukemia. The potential clinical transformation and application of the phosphate primaquine in the treating of the leukemia, especially the acute promyelocytic leukemia, can provide important scientific theoretical bases.
Owner:WENZHOU MEDICAL UNIV
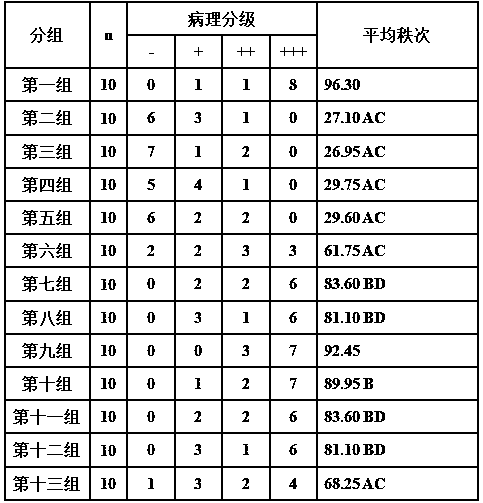
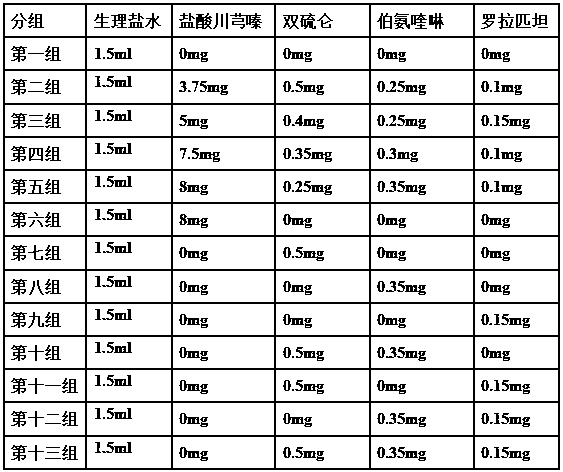
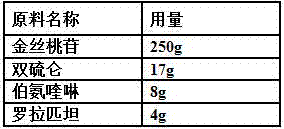
A kind of oral pharmaceutical composition for treating atherosclerosis
ActiveCN107823207BHas anti-atherosclerotic effectWeak anti-atherosclerotic effectOrganic active ingredientsPharmaceutical delivery mechanismRolapitantCardiovascular toxicity
The invention belongs to the field of medicine and especially relates to an oral pharmaceutical composition for treating atherosclerosis. The oral pharmaceutical composition for treating atherosclerosis is prepared from pharmaceutically acceptable pharmaceutic adjuvants and 60-170 parts by weight of ligustrazine hydrochloride, 5-12 parts by weight of disulfiram, 1-7 parts by weight of primaquine and 1-3 parts by weight of Rolapitant. In the composition, Rolapitant can inhibit cardiovascular toxicity of disulfiram and primaquine and eliminate the phenomenon that efficacy is counteracted when disulfiram and primaquine are used in combination. The formula including ligustrazine hydrochloride, disulfiram, primaquine and Rolapitant has good antiatherosclerosis effect and safety.
Owner:淮北市宇控信息技术有限公司
Hycanthone derivatives and Primaquine derivatives for use in the prevention and/or the treatment of disorders associated to gammaherpesvirus
The present invention relates to compounds of the following general formula (I) or (II) or a pharmaceutically acceptable salt and / or solvate thereof, for use in the prevention and / or the treatment of disorders associated to gammaherpesvirinae, in particular to the human herpesvirus 8 (HHV8) or the human herpes virus 4 (HHV4), and pharmaceutical compositions containing such compounds.
Owner:UNIV DE PARIS +4
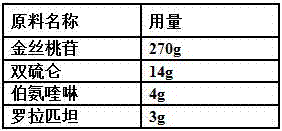
Oral pharmaceutical composition for treating myocardial ischemia-reperfusion injury
ActiveCN107998142AAnti-myocardial ischemia-reperfusion injury is weakEnhanced anti-myocardial ischemia-reperfusion injuryOrganic active ingredientsGranular deliveryCardiac muscleRolapitant
The invention belongs to the field of medicine, and particularly relates to an oral pharmaceutical composition for treating myocardial ischemia-reperfusion injury. The oral pharmaceutical compositionfor treating the myocardial ischemia-reperfusion injury is prepared from pharmaceutically acceptable pharmaceutical adjuvants and hyperin, disulfiram, primaquine and rolapitant, wherein the ratios ofthe hyperoside, the disulfiram, the primaquine and the rolapitant are, by weight, 180-300 parts of the hyperoside, 12-20 parts of the disulfiram, 3-9 parts of the primaquine and 2-5parts of the rolapitant. According to the composition, the rolapitant inhibits cardiovascular toxicity of the disulfiram and the primaquine, and eliminates efficacy counteracting phenomenon when the disulfiram and the primaquine are used in combination; the composition of the hyperin, the disulfiram, the primaquine and the rolapidtant has good resistance on the myocardial ischemia-reperfusion injury and safety.
Owner:德州市洛泰商贸有限公司
Chinese and Western medicine united sustained-release injection for preventing and controlling coccidiosis of rabbit and its preparation method
InactiveCN101085076BLower resistanceDelay drug resistanceAntiparasitic agentsHeterocyclic compound active ingredientsCoccidiosisBeta-Cyclodextrins
The invention relates to a combination Western-Chinese pharmaceutical slow release injection for treating rabbit coccidiosis and process for preparation, wherein the constituents of the injection include (by weight percent): sweet wormwood 3-6 parts, flavescent sophora root 3-6 parts, Chinese pulsatilla root 2-5 parts, hairy vein agrimony 2-5 parts, bupleurum root 1-3 weight parts, garden burnet root 1-3 parts, astragalus root 1-3 parts, primaquine 0.06-0.35 part, beta-cyclodextrin 2-4 parts. The preparing process consists of drying the raw material herbs, disintegrating, mixing, ethanol extracting, water extracting, removing foreign substance, mixing the extract with primaquine and beta-cyclodextrin, grinding, dressing and adjusting soup volume.
Owner:ZHEJIANG ACADEMY OF AGRICULTURE SCIENCES
Green tea xylitol drink additive
InactiveCN101623121AReduce transmissionSignificant effectOrganic active ingredientsAntiparasitic agentsSports drinkMalaria
The invention relates to a green tea xylitol drink additive belonging to the drink additive. The green tea xylitol drink additive is characterized by being made from the following raw materials by weight portion: 100 portions of artemisinin, 5-10 portions of tea polysaccharide, 60-600 portions of naphthoquine, 1-15 portions of primaquine and 10-20 portions of xylitol. When the green tea xylitol drink additive is made, the raw materials can be mixed uniformly for later use. The green tea xylitol drink additive can be added to various common drinks to make the refreshing drink, the soda water, the juice, the soymilk, the coffee, the cocoa, the kvass drink, the healthy medical tea drink, the functional electrolyte sports drink and the like. The invention has the advantages of high efficiency, rapid effect, convenient drinking and the like and can be used for treating falciparum malaria and vivax malaria.
Owner:乔新光
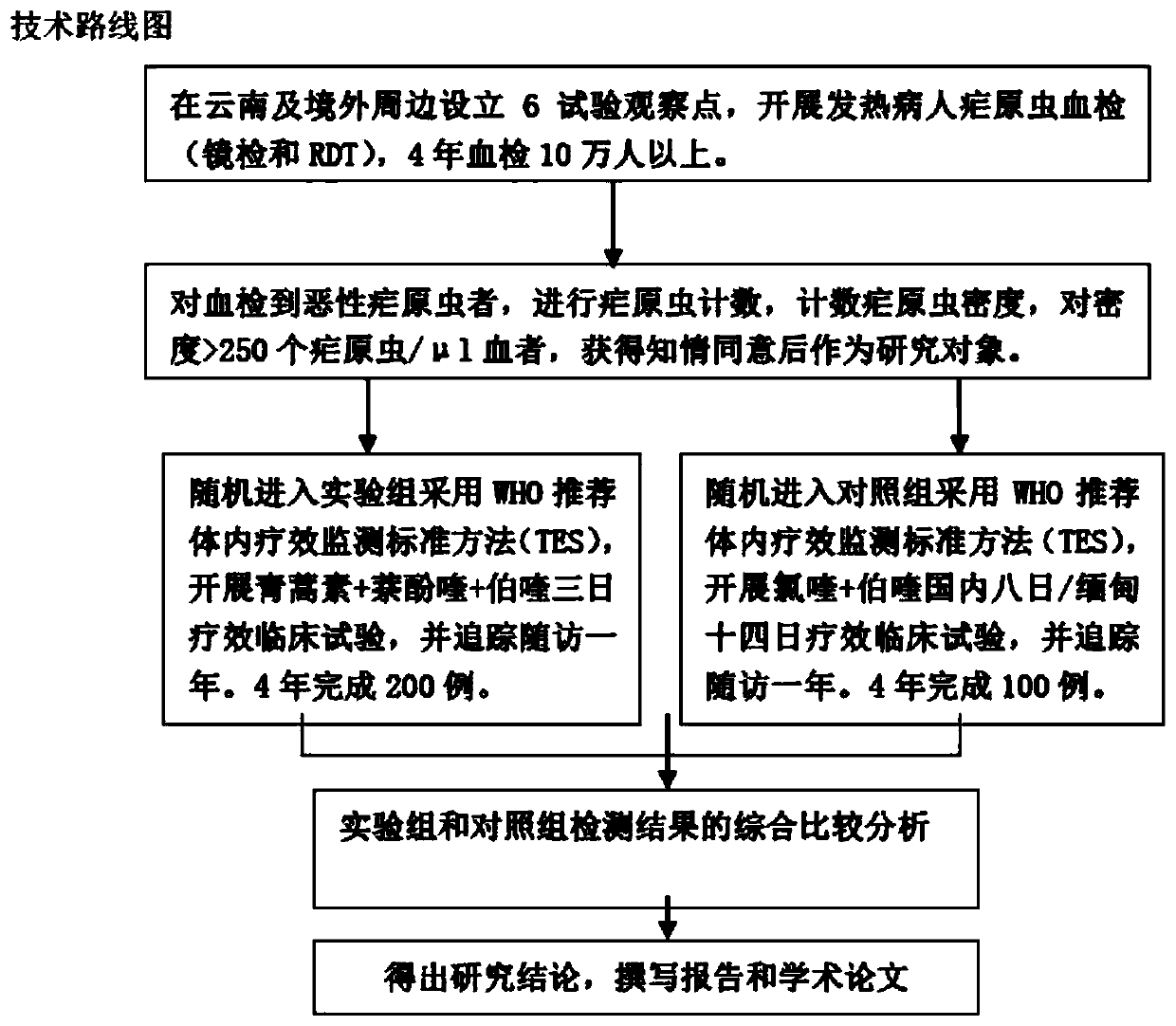
Medicine compatibility scheme for treating vivax malaria and use method of medicine compatibility scheme
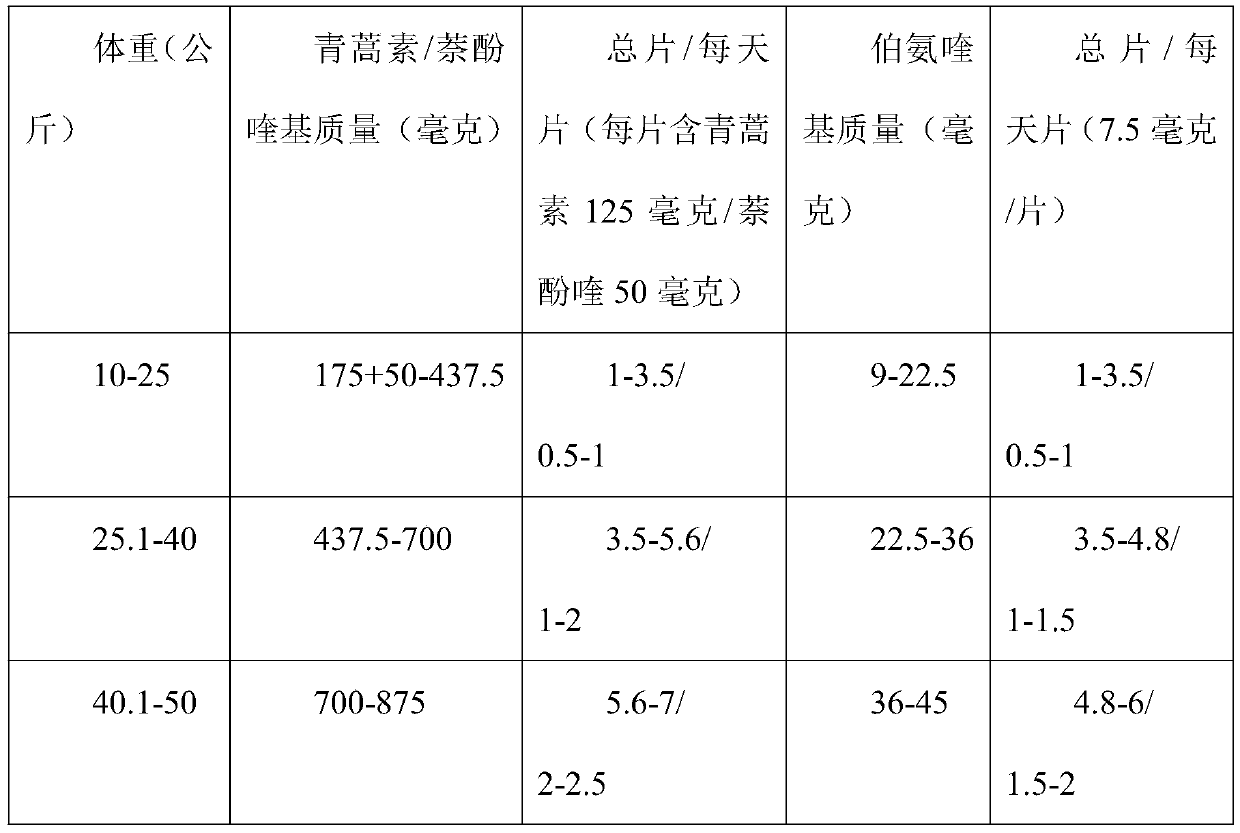
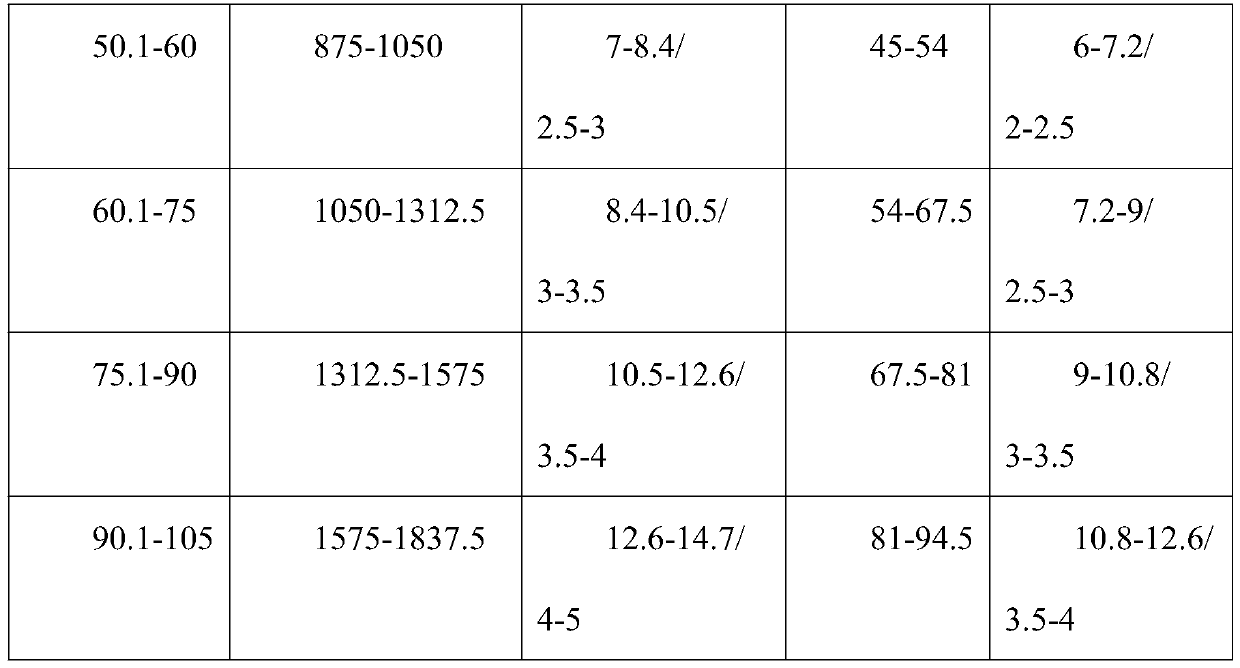
PendingCN111249275ATreatment safetyShort treatment timeOrganic active ingredientsAntiparasitic agentsPharmaceutical drugAlpha-naphthol
The invention discloses a medicine compatibility scheme for treating vivax malaria. The medicine compatibility scheme comprises the following medicine raw materials in a ratio: 17.5mg / kg of artemisinin, 7mg / kg of naphthoquine and 0.9mg / kg of primaquine. The invention further discloses a use method of the medicine compatibility scheme. The use method is that the artemisinin, the naphthoquine and the primaquine of the ratio are taken once per day respectively, and are taken separately for 3 days continuously. The scheme is short in treatment time, that is, can be completed within 3-5 days, and is high in compliance.
Owner:云南省寄生虫病防治所
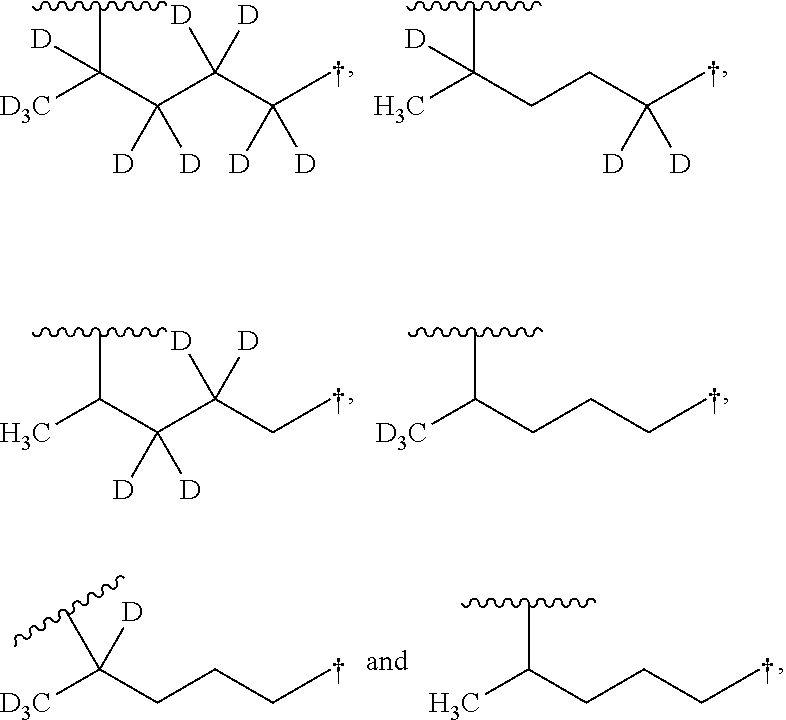
Aminoquinoline Derivatives
Owner:CONCERT PHARMA INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com