Polysulfone side chain grafted polymerized tertiary amine micro-filtration membrane
A polytertiary amine and microfiltration membrane technology, applied in the field of membrane separation, can solve the problems of high operating cost, low retention rate, and inability to remove toxic substances in nanofiltration technology, and achieve the effect of improving separation selectivity and broadening applications.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
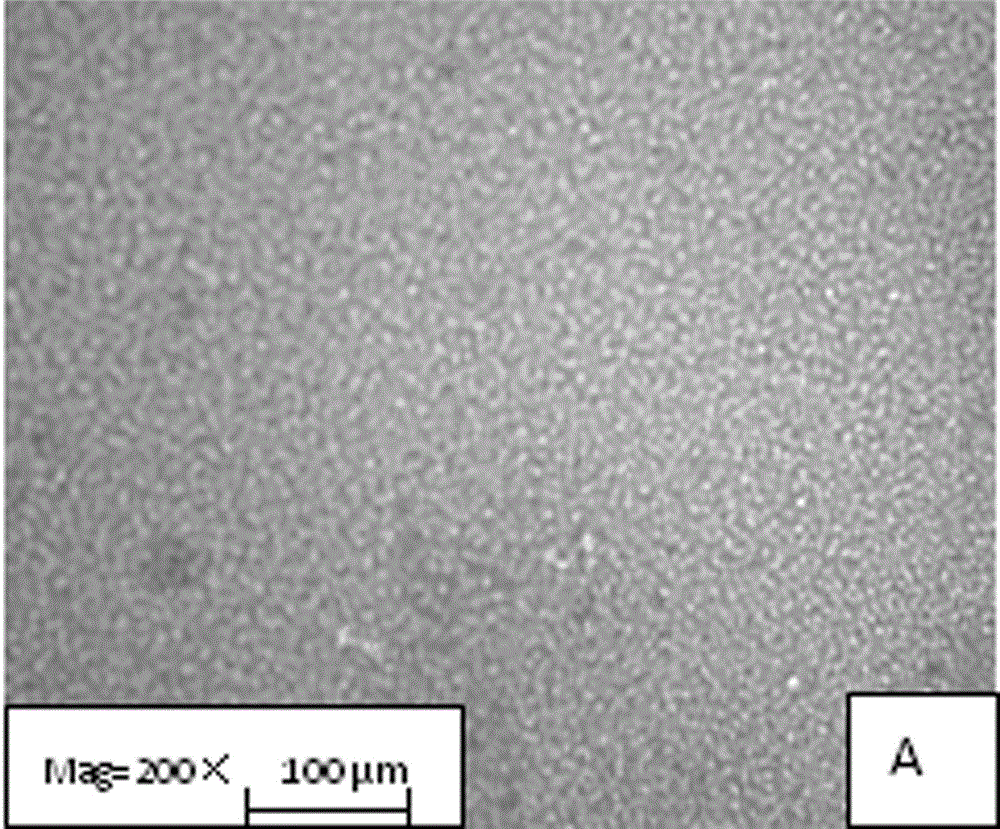
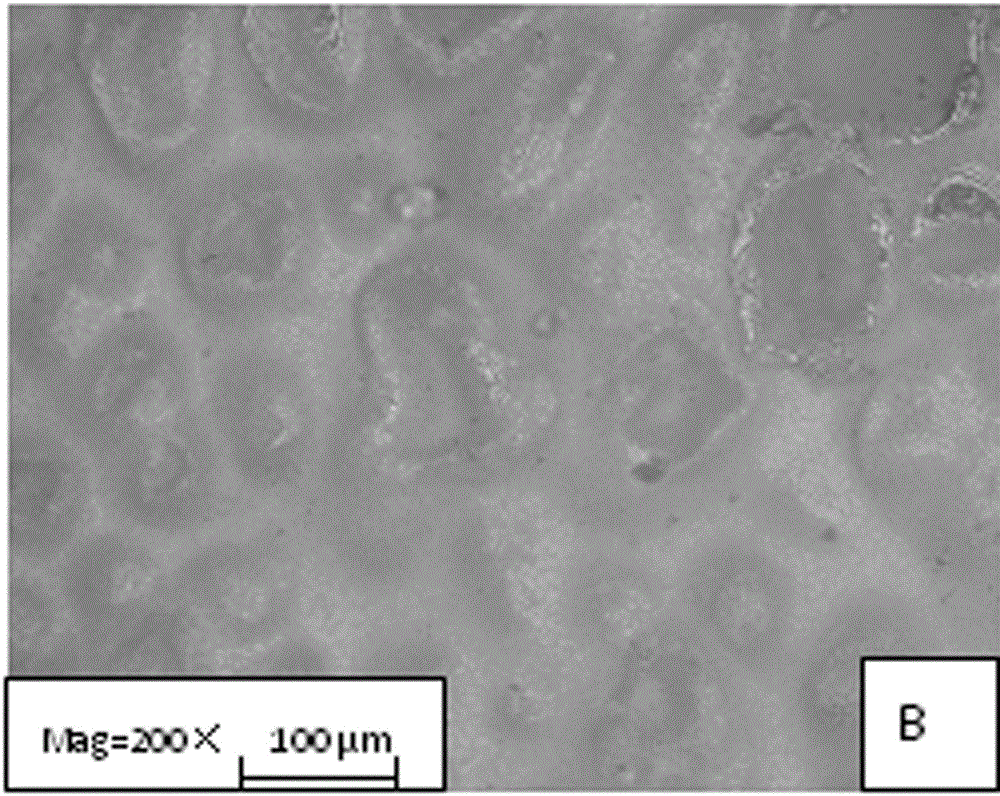
Image
Examples
Embodiment 1
[0043] (1) Preparation of polysulfone side chain grafted polytertiary amine microfiltration membrane
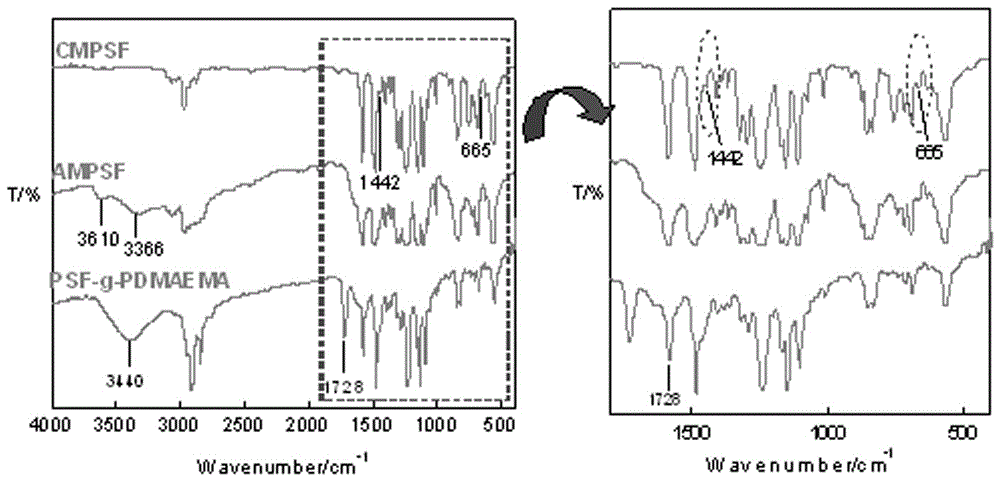
[0044] In the first step, add 1.0g CMPSF and 6mL dimethylacetamide to a four-neck flask, and add 0.22g PEG-400 under stirring conditions, and let it stand for defoaming; pour the solution on a horizontal glass plate, scrape it evenly, and form Uniform and thin layer, quickly put in a water bath, phase inversion into a film; place the film in distilled water for several times of water soaking, after vacuum drying, the CMPSF asymmetric porous base film is obtained;
[0045] In the second step, soak 1g of CMPSF asymmetric porous membrane in ethylenediamine to cause nucleophilic substitution reaction between ethylenediamine and the chloromethyl group on the surface of the CMCPS membrane. Repeated soaking and washing, and vacuum drying, the aminated polysulfone membrane AMPSF was obtained;
[0046] In the third step, in the four-neck flask, add AMPSF membrane and 70 mL of a mixtu...
Embodiment 2
[0051] (1) Preparation of polysulfone side chain grafted polytertiary amine microfiltration membrane
[0052] In the first step, add 0.8g CMPSF and 7mL dimethylacetamide to a four-neck flask, and add 0.2g PEG-400 under stirring conditions, and let it stand for defoaming; pour the solution on a horizontal glass plate, scrape it evenly, and form Uniform and thin layer, quickly put in a water bath, phase inversion into a film; place the film in distilled water for several times of water soaking, after vacuum drying, the CMPSF asymmetric porous base film is obtained;
[0053] In the second step, 1 g of CMPSF asymmetric porous membrane is soaked in ethylenediamine, so that ethylenediamine and the chloromethyl group on the surface of the CMCPS membrane undergo a nucleophilic substitution reaction. After soaking for 15 minutes at 20 ° C, the membrane is taken out and washed with methanol and Distilled water was soaked and washed repeatedly, and vacuum-dried to obtain the aminated pol...
Embodiment 3
[0059] (1) Preparation of polysulfone side chain grafted polytertiary amine microfiltration membrane
[0060] In the first step, add 0.9gCMPSF and 6mL dimethylacetamide to a four-necked flask, and add 0.2gPEG-400 under stirring conditions, and let it stand for degassing; pour the solution on a horizontal glass plate, scrape it evenly, Form a uniform thin layer, quickly put it into a water bath, and phase transform into a film; place the film in distilled water for several times of water soaking, and after vacuum drying, the CMPSF asymmetric porous base film is obtained;
[0061] In the second step, 1 g of CMPSF asymmetric porous membrane is soaked in ethylenediamine, so that ethylenediamine and the chloromethyl group on the surface of the CMCPS membrane undergo a nucleophilic substitution reaction. After soaking for 18 minutes at 22 ° C, the membrane is taken out and washed with methanol and Distilled water was soaked and washed repeatedly, and vacuum-dried to obtain the amina...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Degree of grafting | aaaaa | aaaaa |
| Degree of grafting | aaaaa | aaaaa |
| Degree of grafting | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com