DOX-P micelle with anti-tumor drug resistance and preparation method thereof
A DOX-P, drug-resistant technology, applied in the field of DOX-P micelles and their preparation, can solve the problems of non-degradation and accumulation of polyethylene glycol, avoid protein adsorption, high encapsulation efficiency, and drug loading capacity. high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Synthesis of poloxamer 188-doxorubicin conjugate: take 10mmol poloxamer 188, 25mmol succinic anhydride, 20mmol DMAP, 20mmol ethylenediamine, and use 30ml dioxane as an organic solvent, and stir at room temperature for 24h. After the reaction, the reaction solution was transferred to a dialysis bag with a molecular weight cut-off of 7kD, dialyzed for 48 hours using dioxane as the dialysis medium, and freeze-dried to obtain a poloxamer polymer with a carboxyl group at the end; take 10 mmol of carboxyl group at the end Poloxamer polymer, 20mmol doxorubicin, 15mmol EDC (or DCC or EDC·HCL or DIC or DPPA or p-toluenesulfonyl azide or 2,4,6-triisopropylbenzenesulfonyl azide), 15mmol NHS (or sulfo-NHS or DEPBT or HOBT), dissolved in 30ml N,N-dimethylformamide (DMF), DMF can also be replaced with dichloromethane, methyl chloride furan, dimethyl sulfoxide or di Other organic solvents such as oxyhexane, preferably DMF. Then, under the protection of light and nitrogen, stir and re...
Embodiment 2
[0030] Example 2 Synthesis of Poloxamer 188-Adriamycin Conjugate
[0031]Synthesis of poloxamer 188-doxorubicin conjugate: take 10mmol poloxamer 188, 25mmol succinic anhydride, 20mmol DMAP, 20mmol ethylenediamine, and use 30ml dioxane as an organic solvent, and stir at room temperature for 24h. After the reaction, the reaction solution was transferred to a dialysis bag with a molecular weight cut-off of 7kD, dialyzed for 48 hours using dioxane as the dialysis medium, and freeze-dried to obtain a poloxamer polymer with a carboxyl group at the end; take 10 mmol of carboxyl group at the end Poloxamer polymer, 30mmol doxorubicin, 15mmol EDC, 15mmol NHS, dissolved in 30ml N,N-dimethylformamide (DMF), protected from light, under nitrogen protection, stirred at room temperature for 24h; after the reaction Transfer the reaction solution to a dialysis bag, use 1000ml DMF as the dialysis medium, and dialyze for 24 hours, and change the dialysis medium every 6 hours during the dialysis p...
Embodiment 3
[0032] Example 3 Preparation and Characterization of Poloxamer 188-Adriamycin Conjugate Micelle
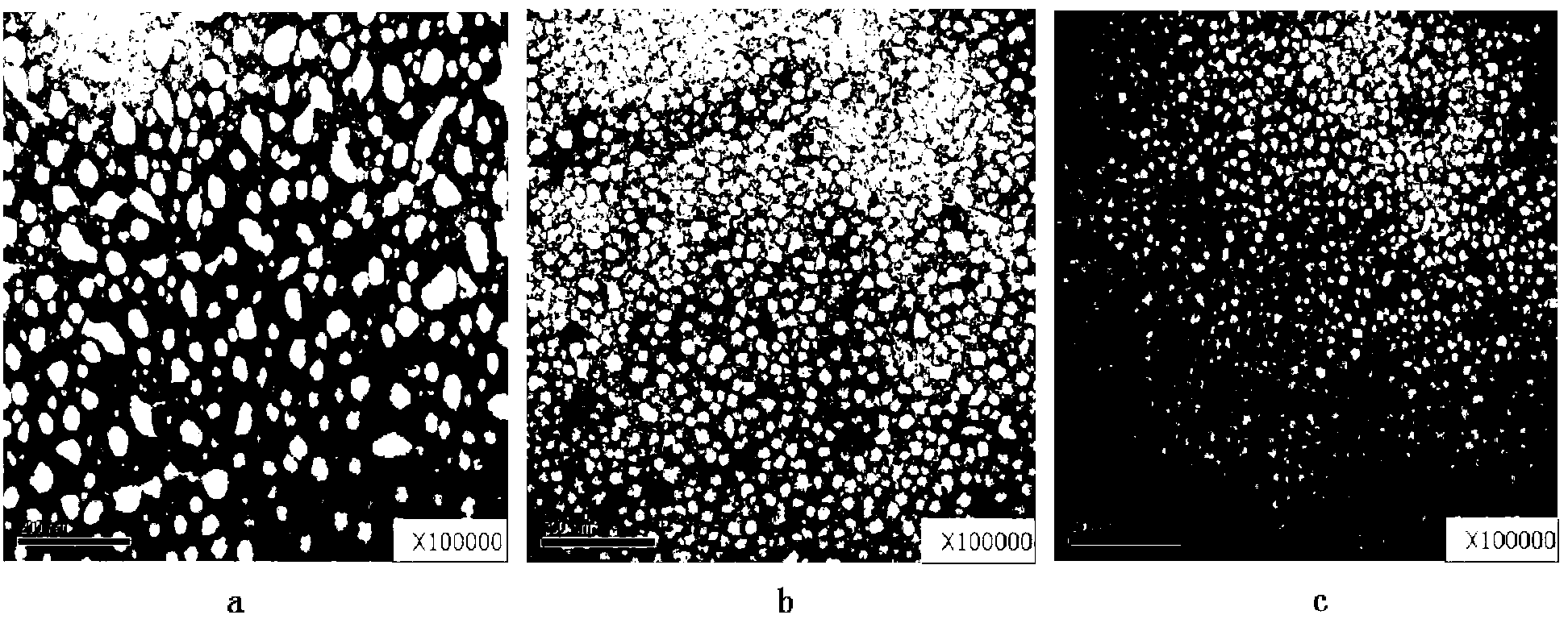
[0033] Preparation of poloxamer 188-doxorubicin conjugate micelles: 100 mg of poloxamer 188-doxorubicin conjugate obtained in Example 1 was dissolved in 10 ml of water, and high-pressure homogeneous circulation was performed twice under ice bath, 0.22 Filter with a μm filter membrane to obtain poloxamer 188-doxorubicin conjugate micelles.
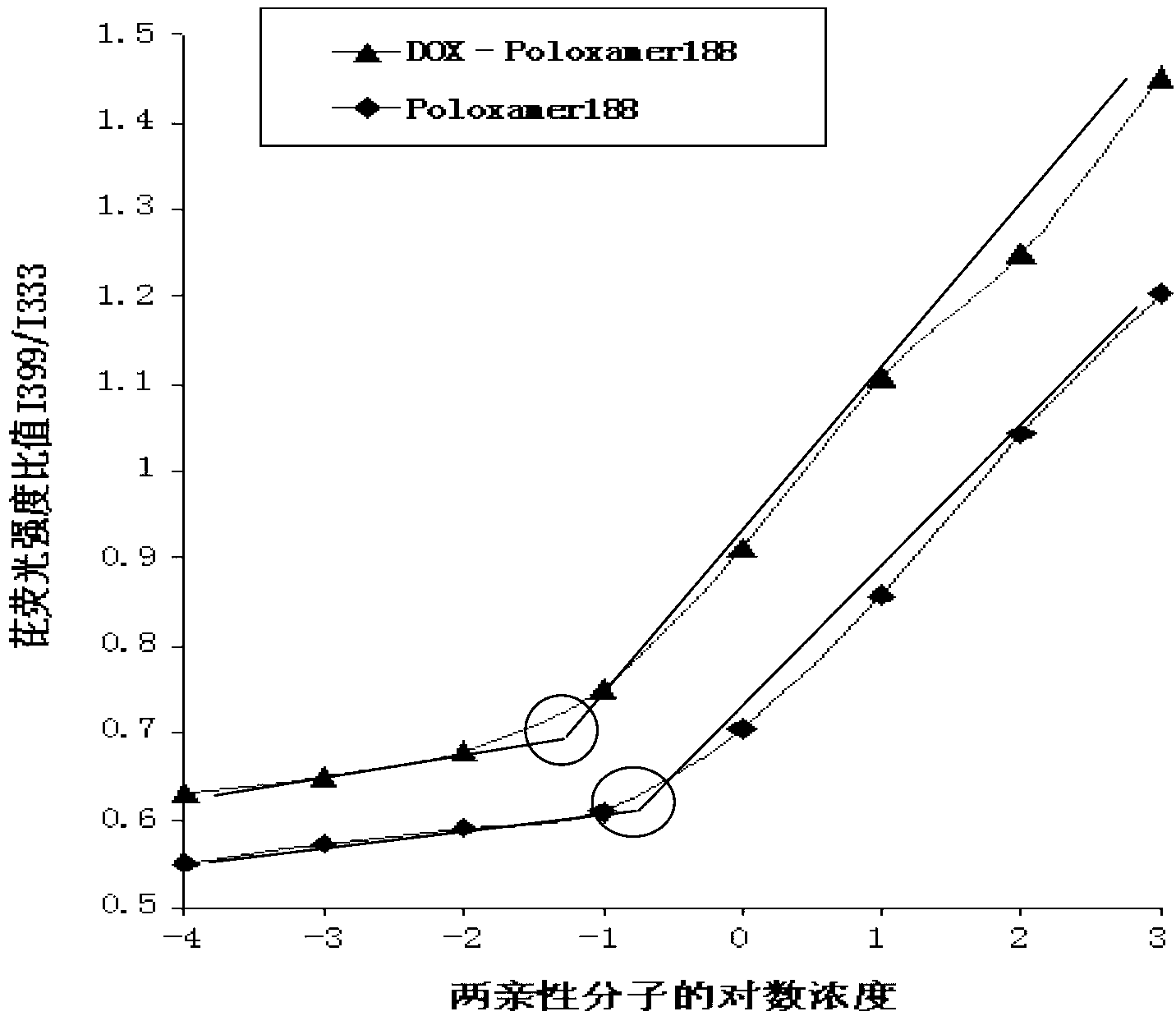
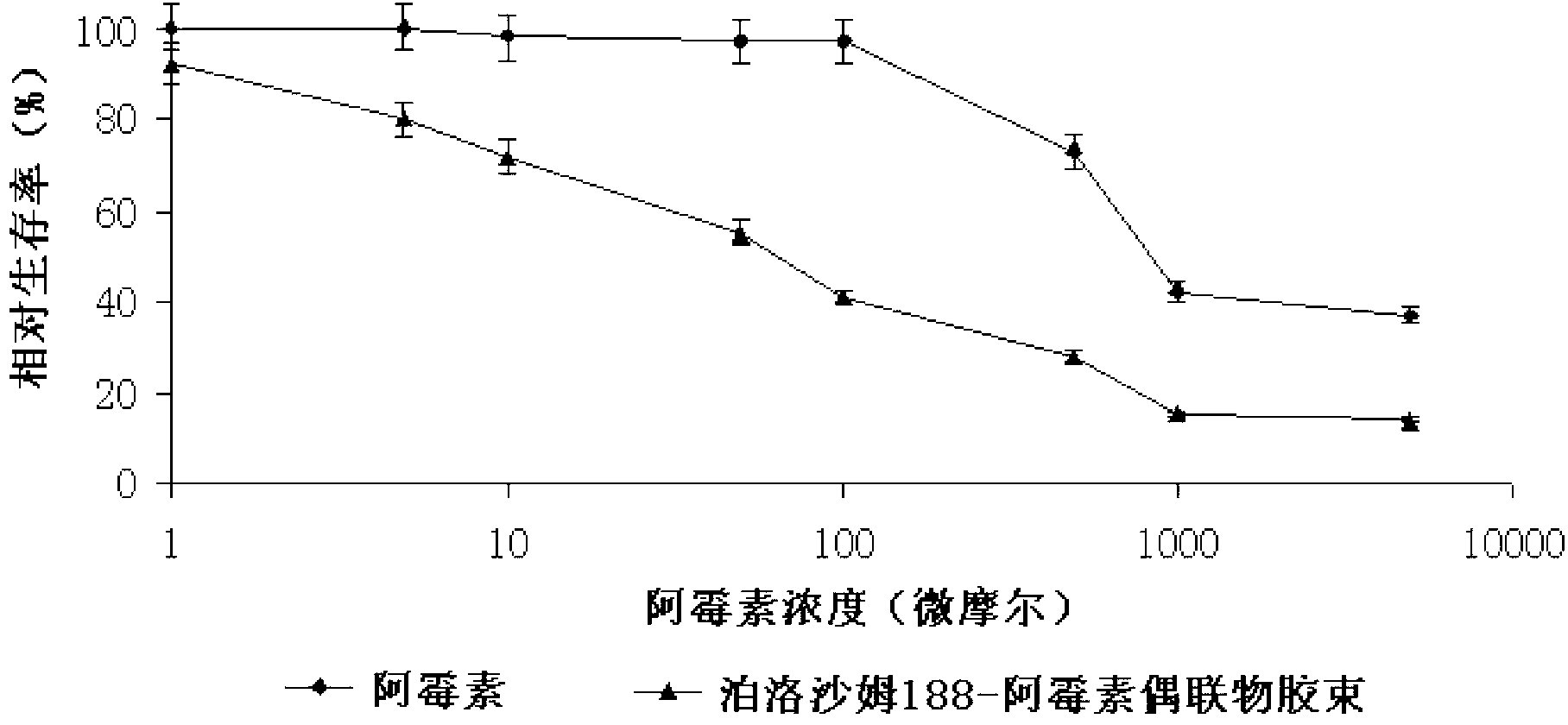
[0034] Characterization observation: observe the poloxamer 188-doxorubicin conjugate micelles with transmission electron microscope (TEM, Hitachi, H-7500, Japan), record the shape and particle size of the micelles, and determine the doxorubicin by HPLC The changes in the microscopic properties and drug content over time were observed, and the changes in micelle particle size and potential were measured by a nanoparticle size and potentiodynamic analyzer (Malvern Nano ZS90).
[0035] Table 1 Characterization observation results of poloxamer 188...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com