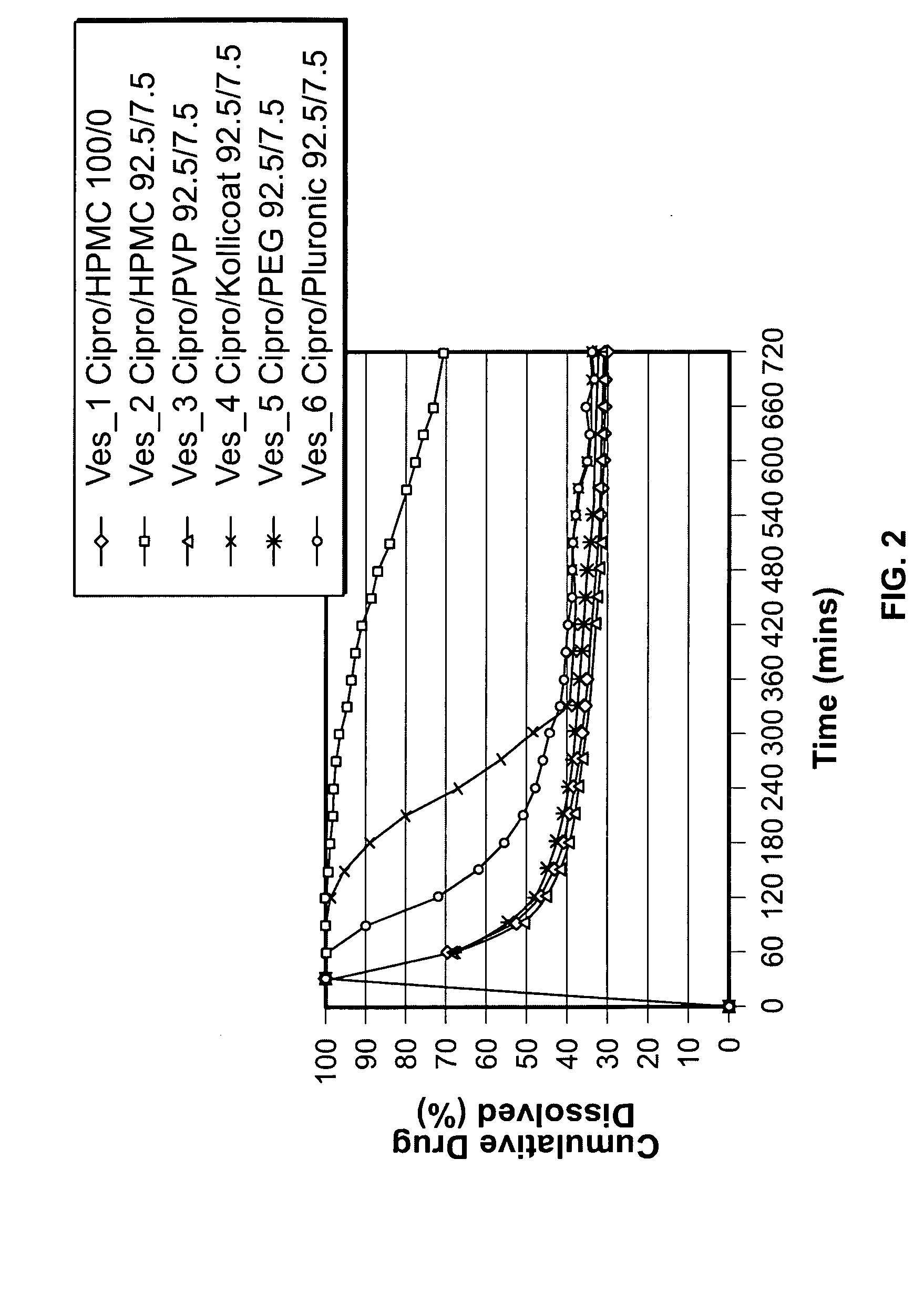
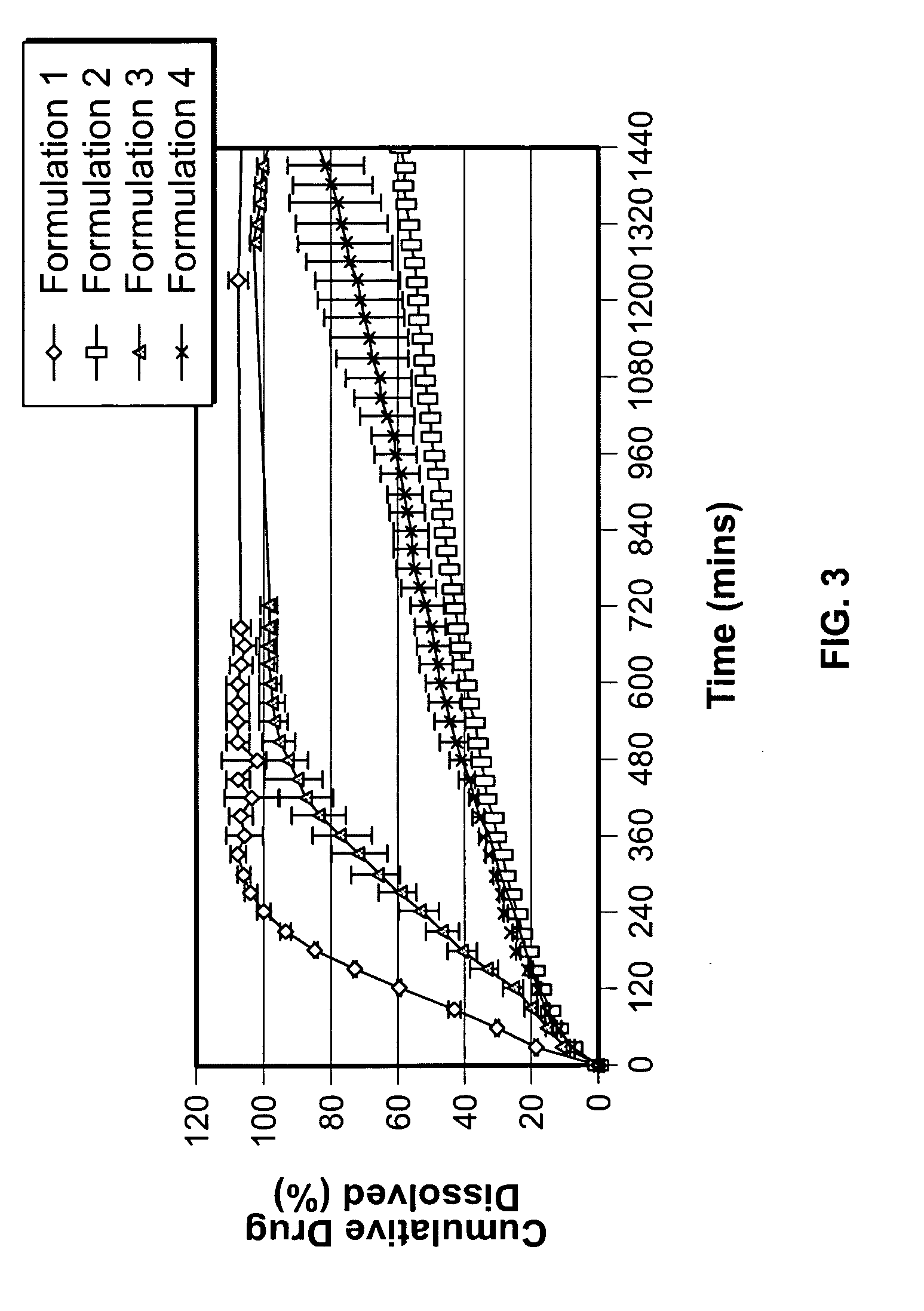
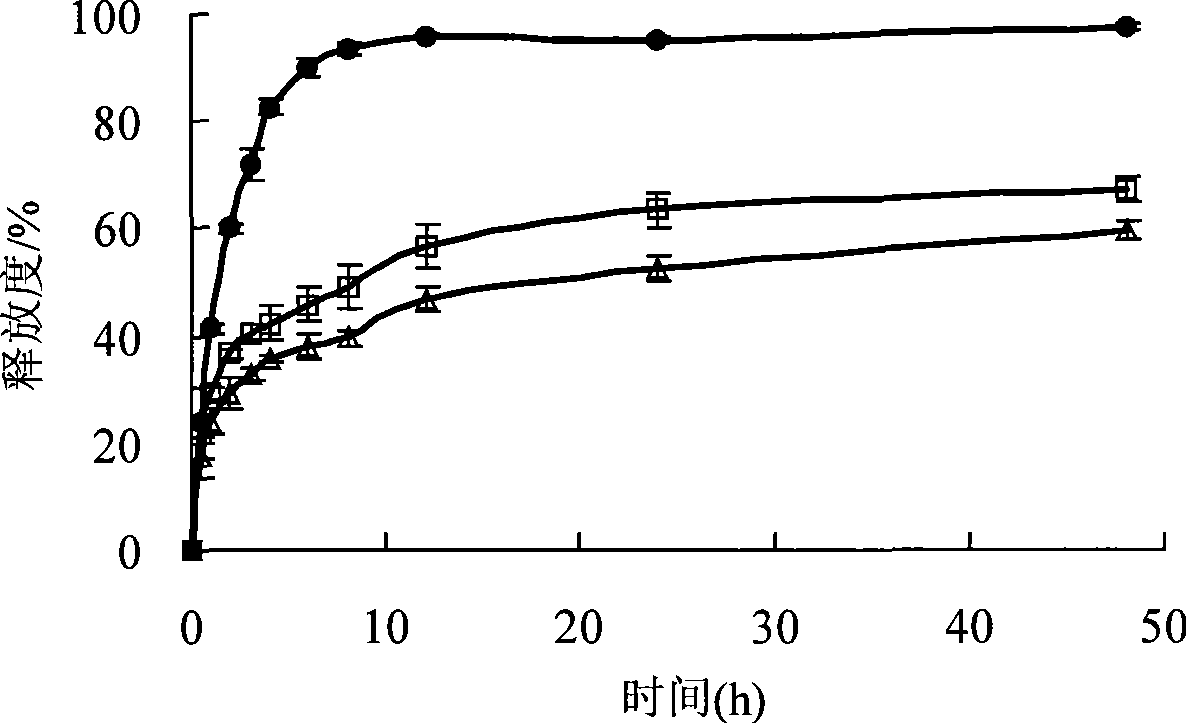
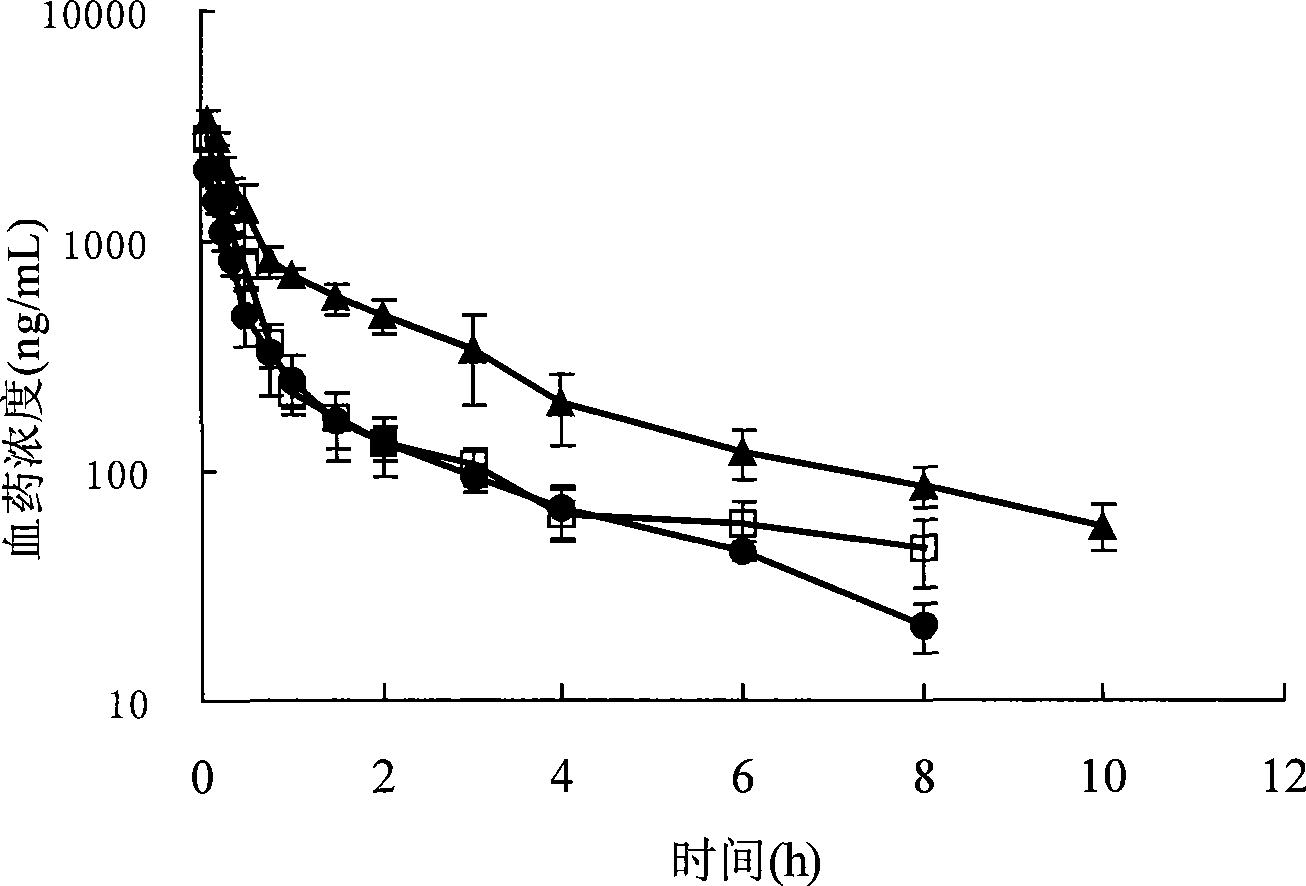
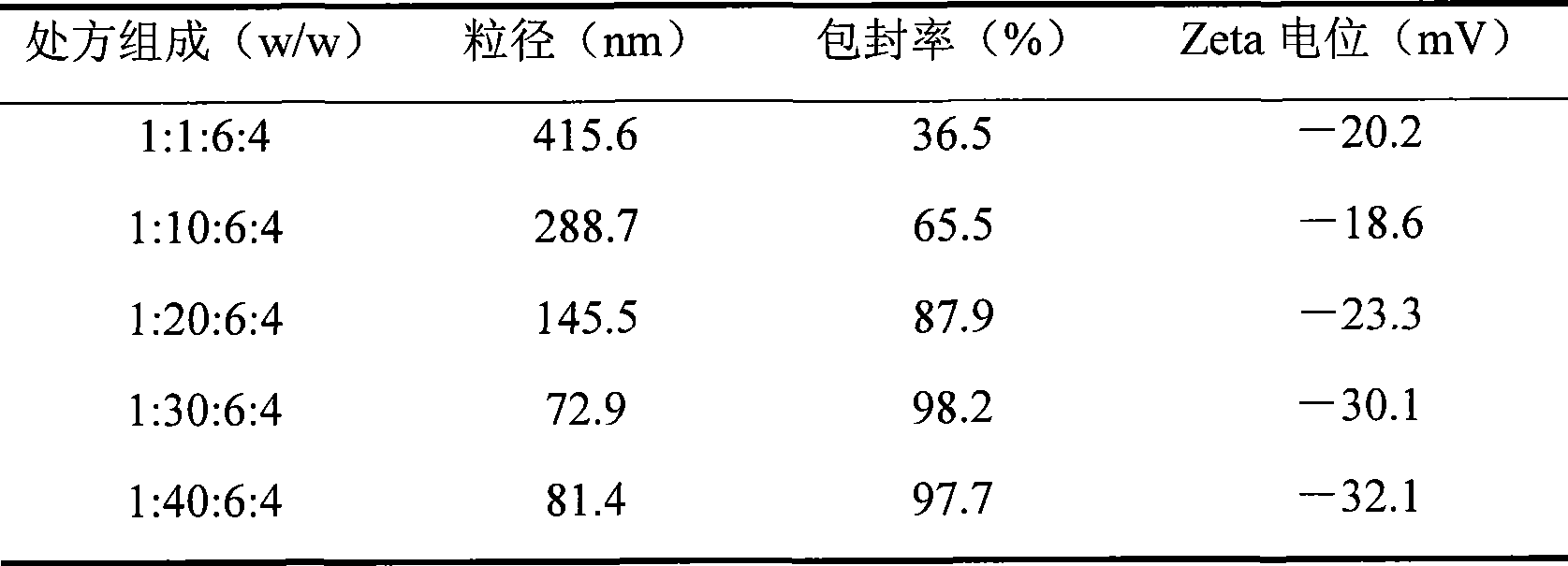
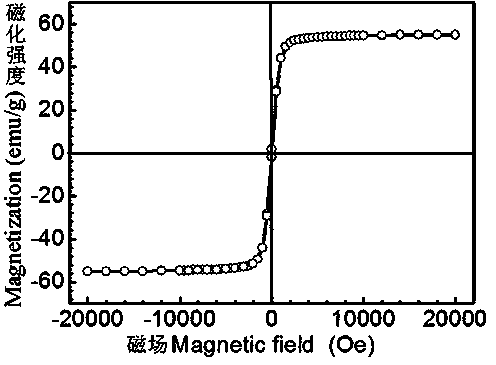
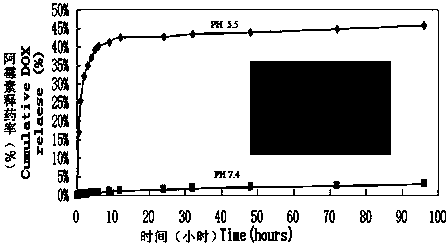
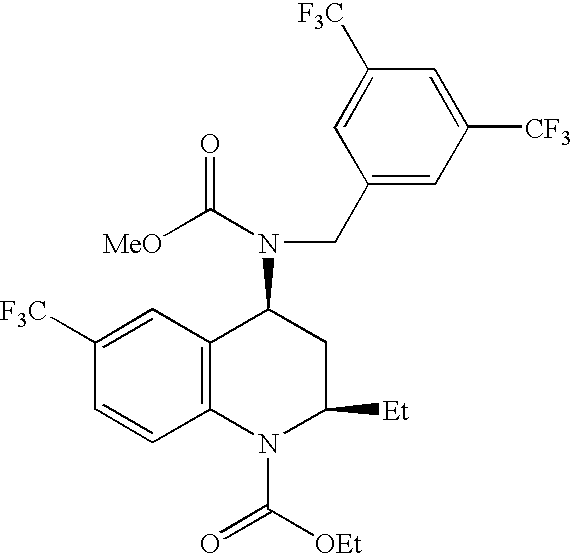
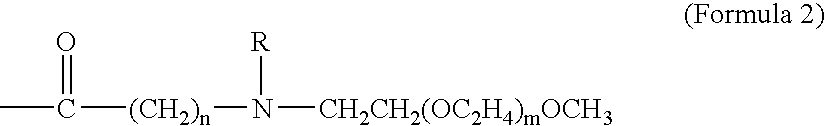Patents
Literature
44results about How to "Low drug load" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
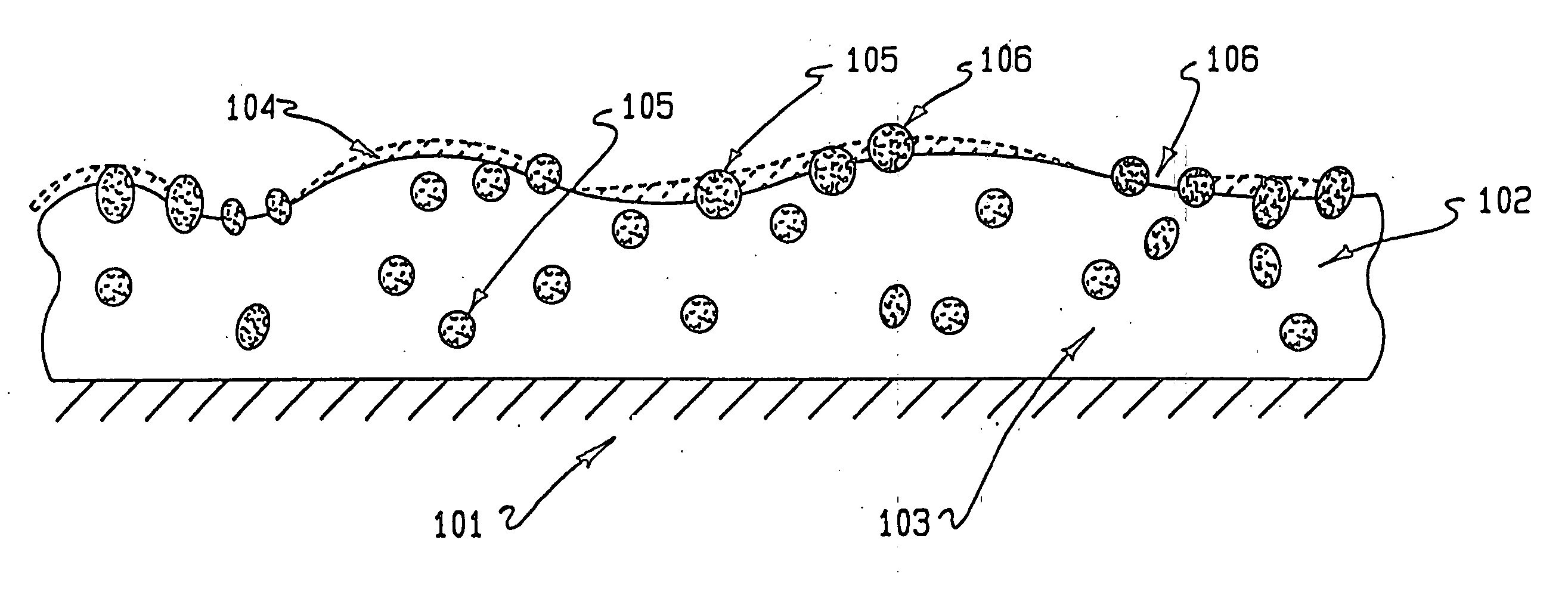
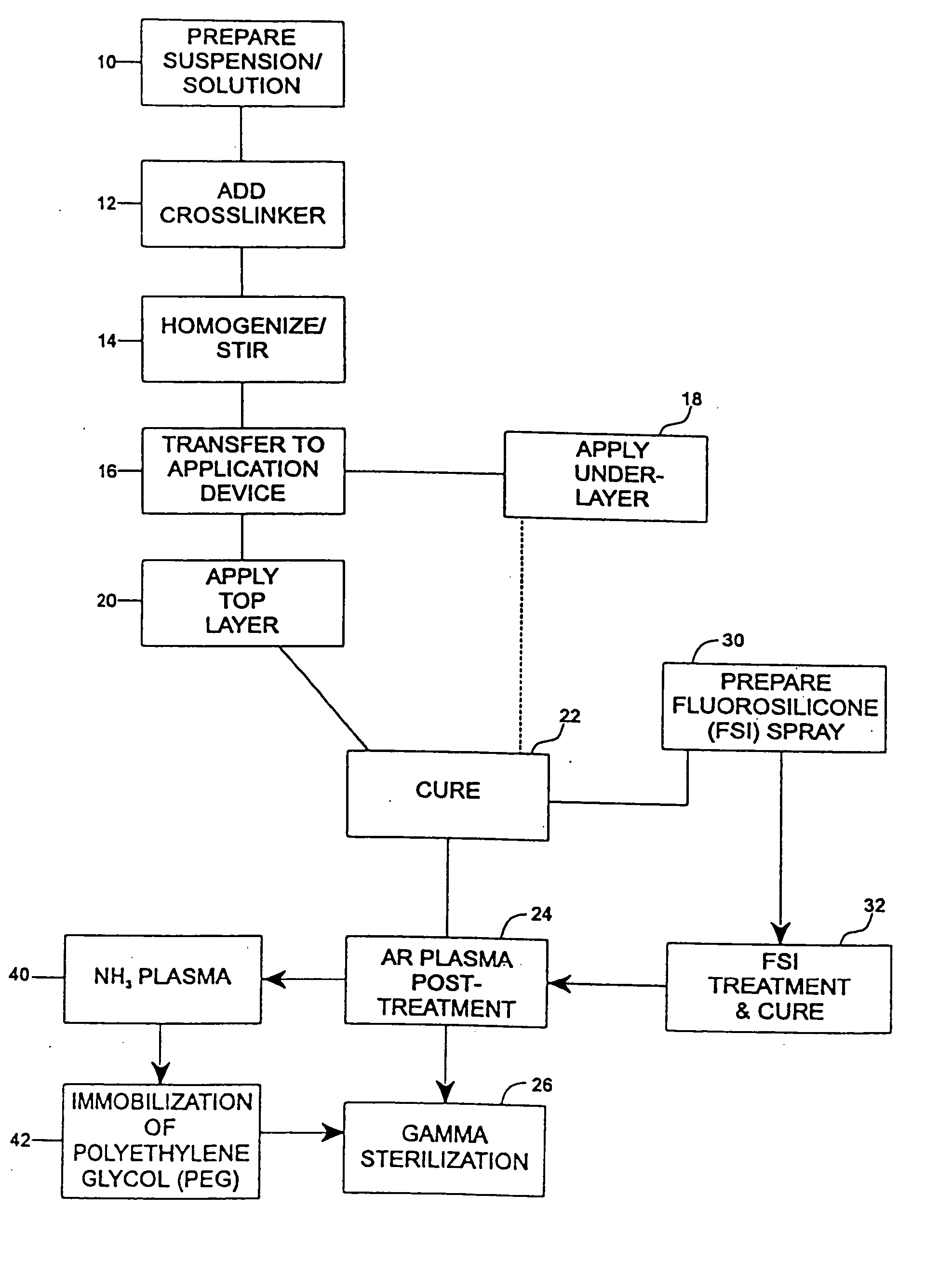
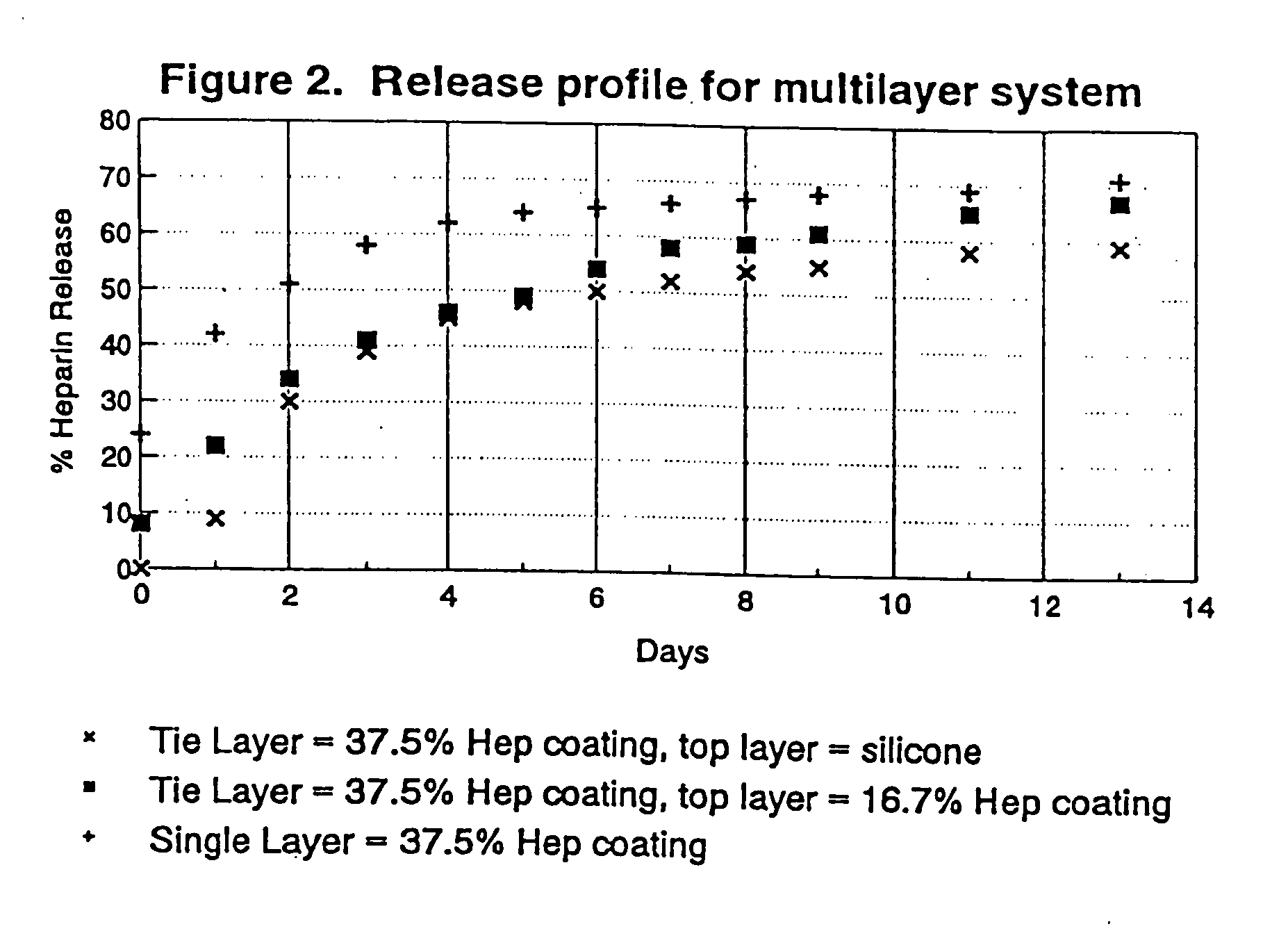
Drug coating with topcoat
InactiveUS20050187611A1Long release timeReduced burst effectStentsSurgeryThrombogenicityDrug coating
A coating and method for a coating an implantable device or prostheses are disclosed. The coating includes an undercoat of polymeric material containing an amount of biologically active material, particularly heparin, dispersed herein. The coating further includes a topcoat which covers less than the entire surface of the undercoat and wherein the topcoat comprises a polymeric material substantially free of pores and porosigens. The polymeric material of the topcoat can be a biostable, biocompatible material which provides long term non-thrombogenicity to the device portion during and after release of the biologically active material.
Owner:BOSTON SCI SCIMED INC
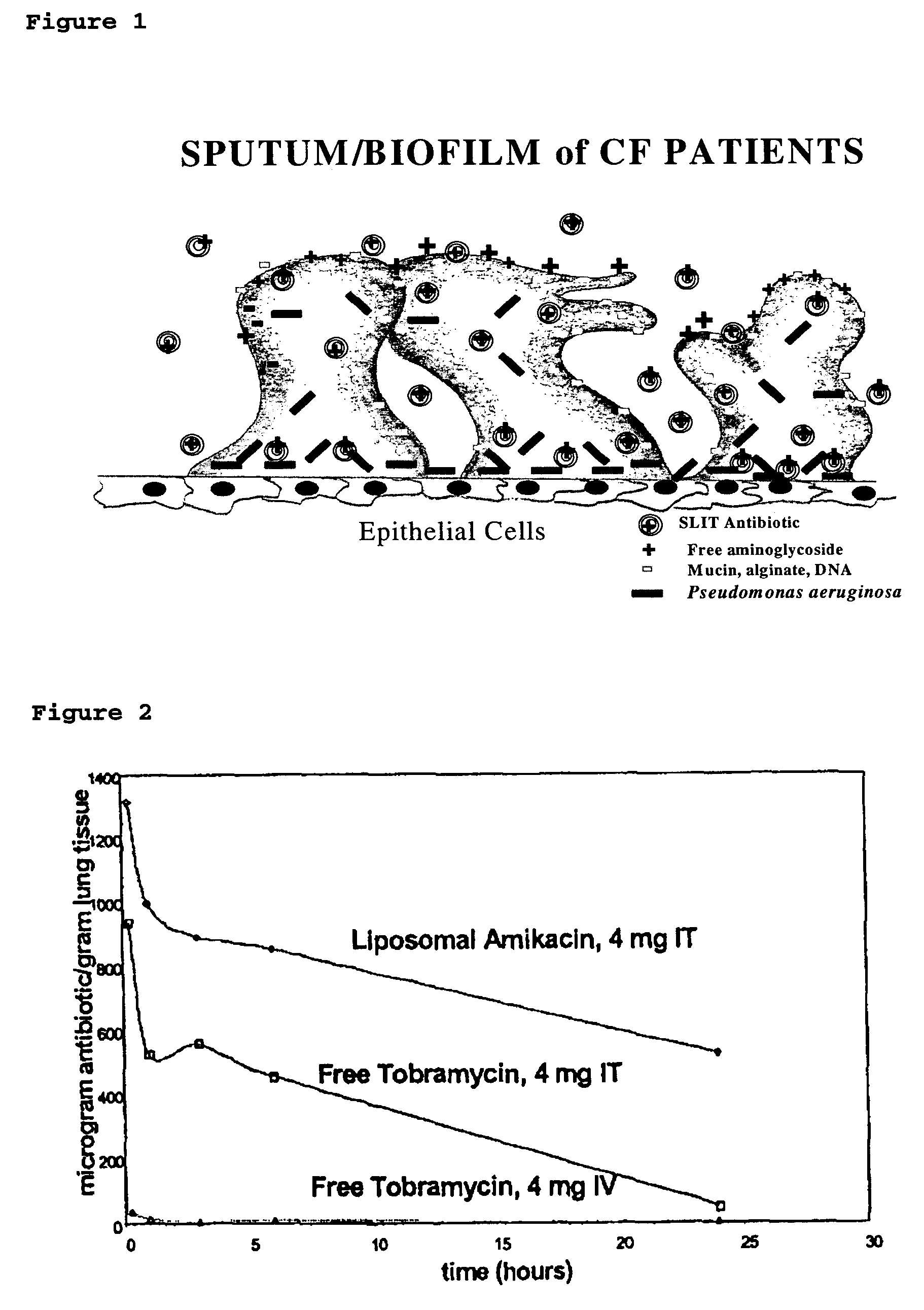
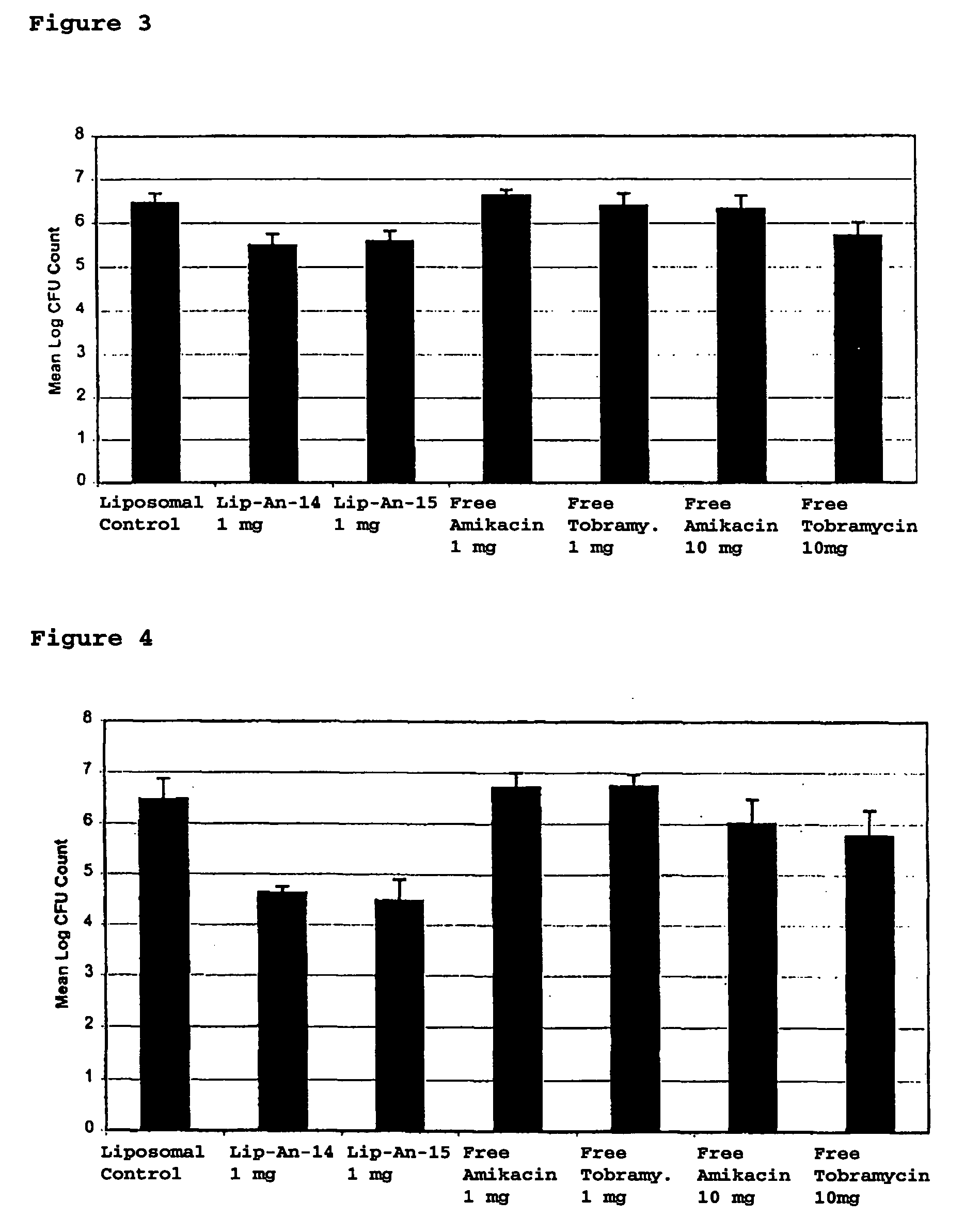
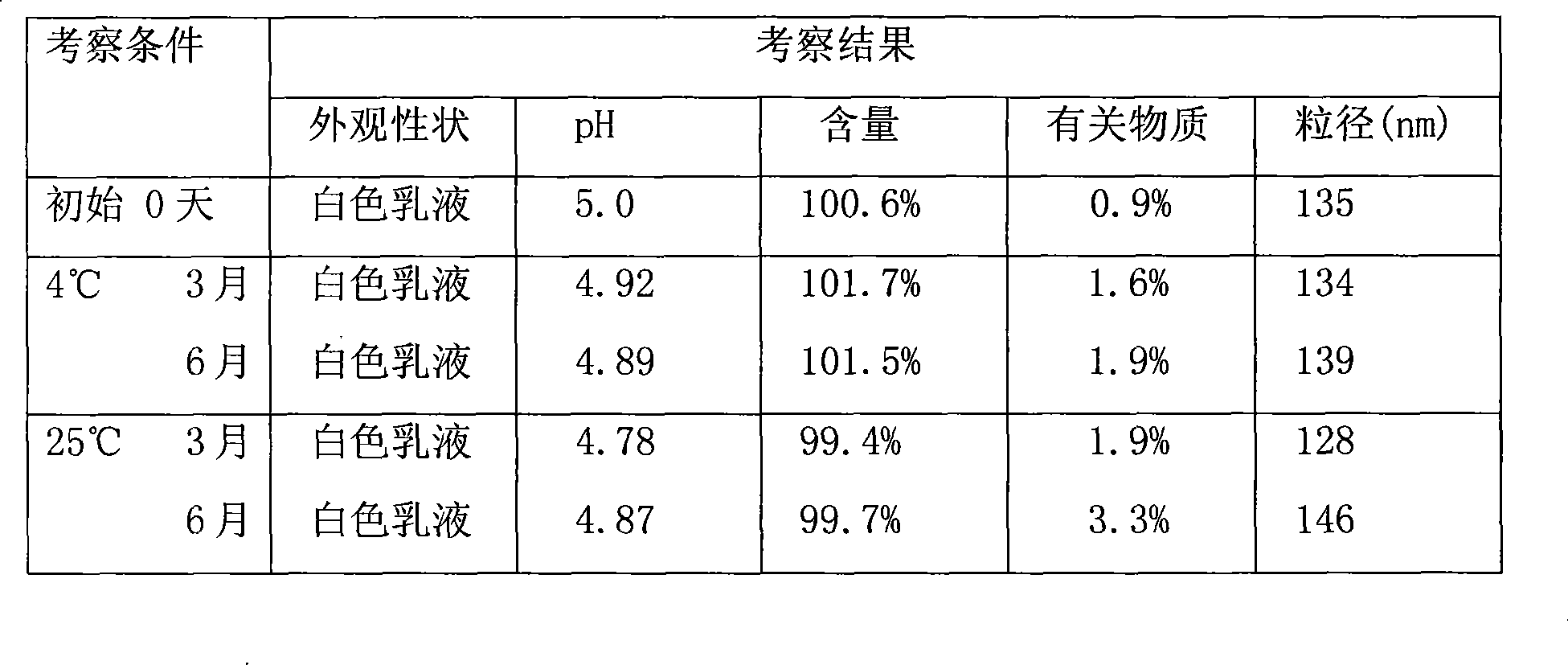
Sustained release of antiinfectives
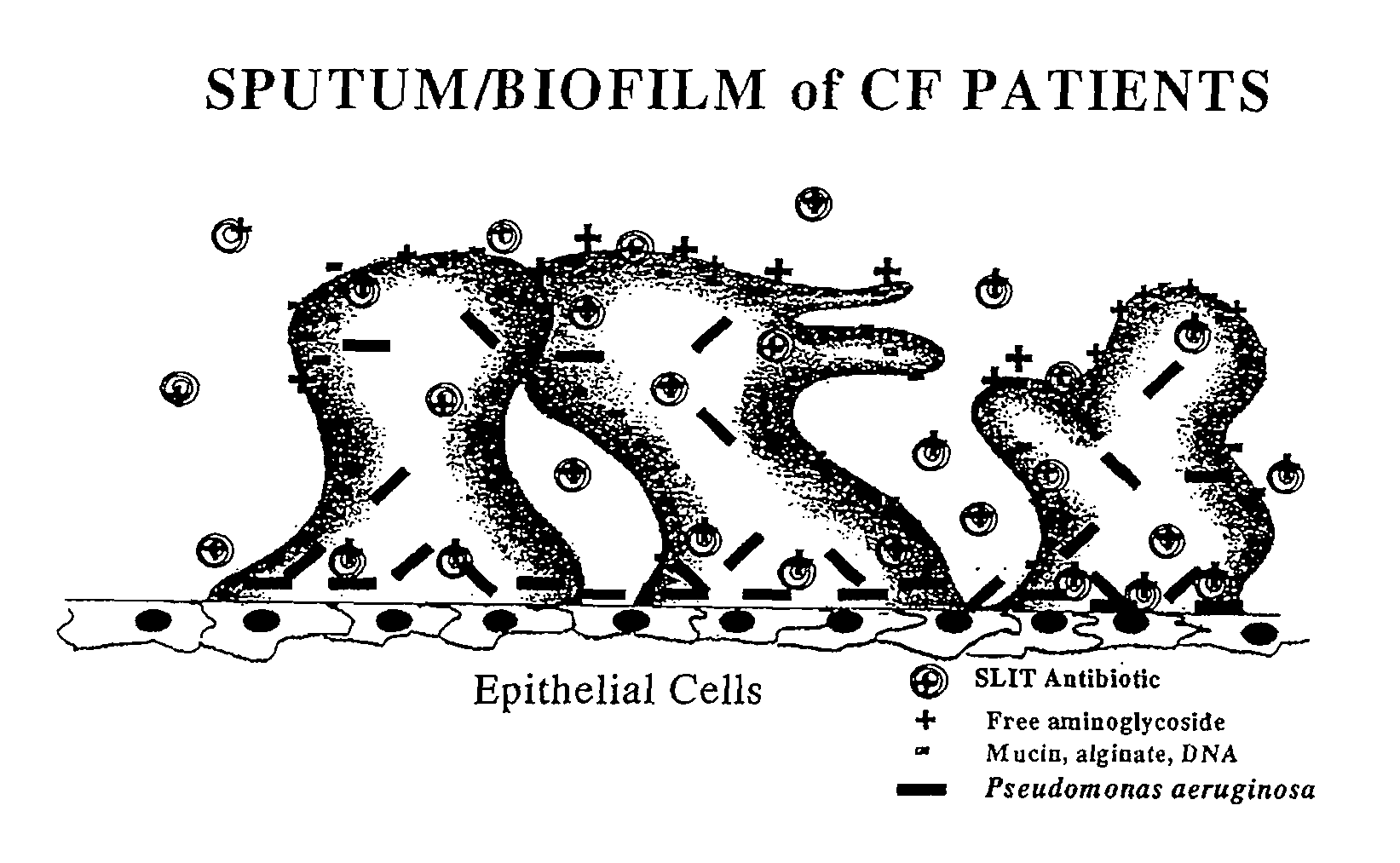
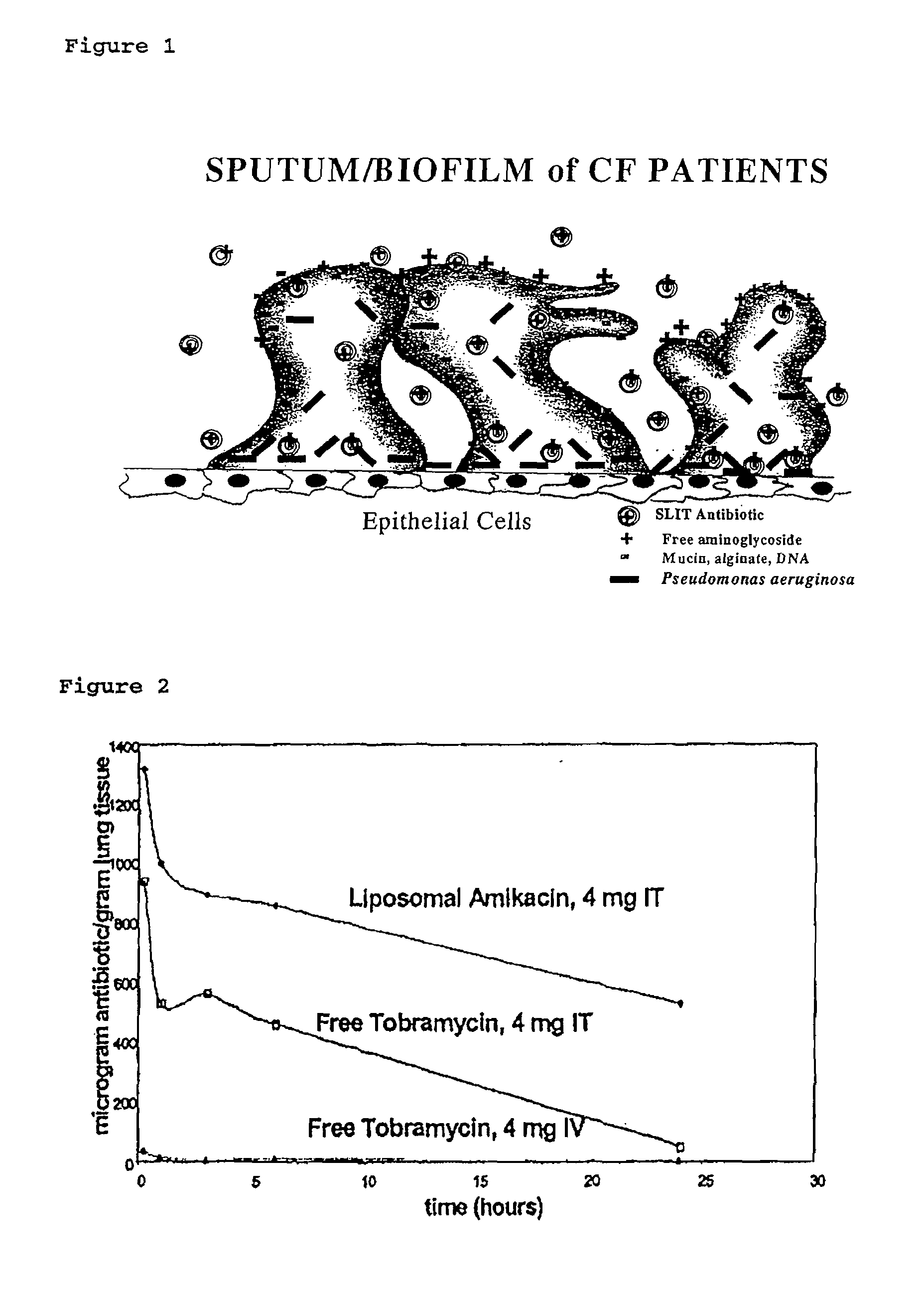
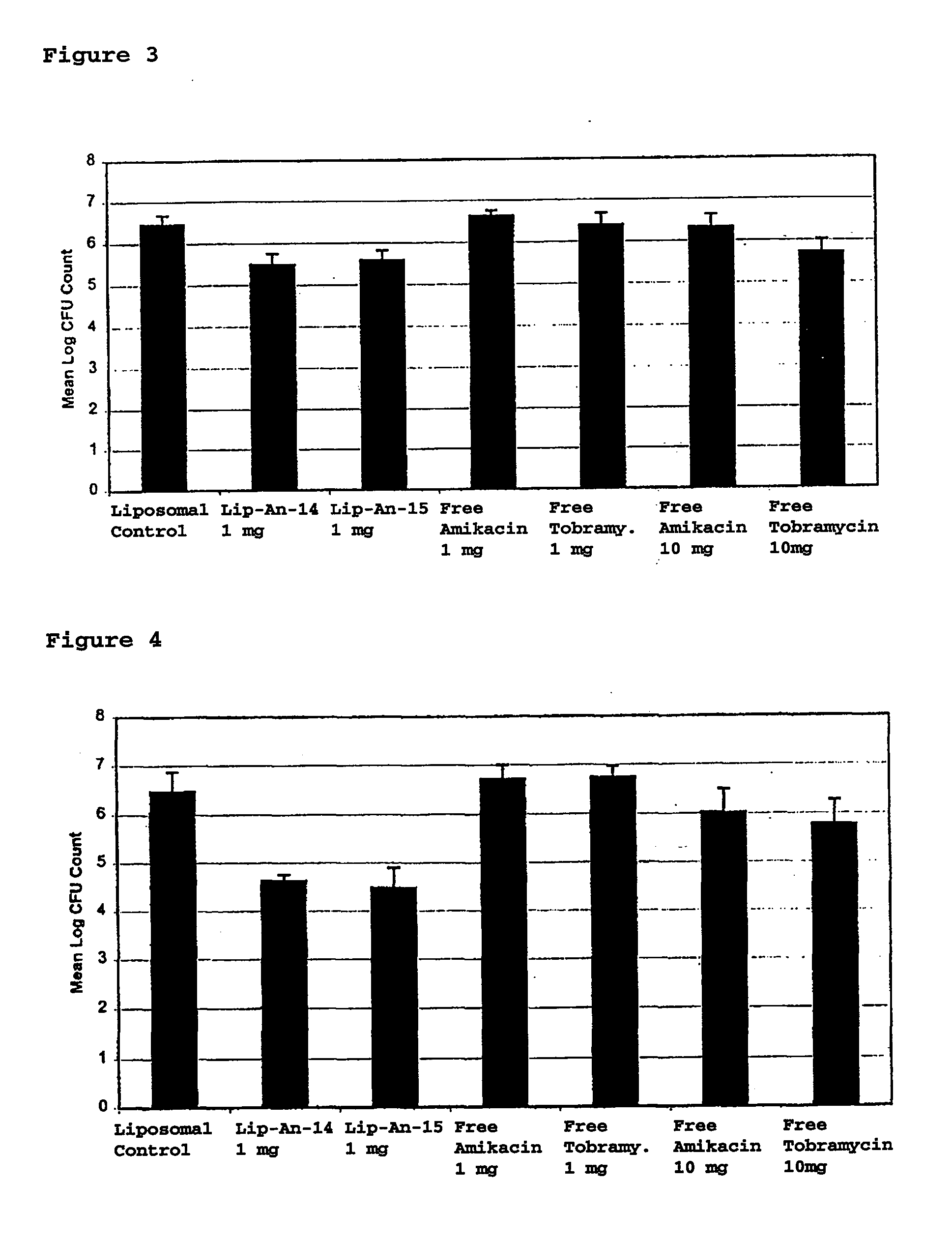
ActiveUS7544369B2Reduce maintenanceEffective targetingAntibacterial agentsPowder deliveryPulmonary infectionCystic fibrosis lungs
Provided, among other things, is a method of treating or ameliorating pulmonary infection in a cystic fibrosis patient comprising pulmonary administration of an effective amount of a liposomal / complexed antiinfective to the patient, wherein the (i) administrated amount is 50% or less of the comparative free drug amount, or (ii) the dosing is once a day or less, or (iii) both.
Owner:INSMED INC
Paclitaxel lipid composite
ActiveCN101396346AImprove solubilityGood dispersionOrganic active ingredientsPharmaceutical non-active ingredientsEmulsionDrug loading dose
The invention discloses a paclitaxel lipid complex. The paclitaxel lipid complex consists of paclitaxel and lipid material. The weight proportion of the paclitaxel and the lipid material is 1 to 1 - 19, the preferential proportion is 1 to 2 - 10, and the more preferential proportion is 1 to 3 - 6. The lipid material is selected from natural lipid and synthetic lipid or the mixture thereof. The paclitaxel lipid complex can also contain antioxidation stabilizer. The invention also discloses a preparation method of the paclitaxel lipid complex and the application in the preparation of injection submicron emulsion and dry emulsion. The paclitaxel lipid complex has high solubility in oil, and the prepared submicron emulsion has the advantages of high drug loading quantity, high stability, high safety and low irritability.
Owner:BEIJING WEHAND BIO PHARMA CO LTD
Sustained Release of Antiinfectives
InactiveUS20100068257A1Reduce maintenanceEffective targetingPowder deliveryBiocidePulmonary infectionCystic fibrosis lungs
Provided, among other things, is a method of treating or ameliorating pulmonary infection in a cystic fibrosis patient comprising pulmonary administration of an effective amount of a liposomal / complexed antiinfective to the patient, wherein the (i) administrated amount is 50% or less of the comparative free drug amount, or (ii) the dosing is once a day or less, or (iii) both.
Owner:INSMED INC
Oral delivery system comprising a drug/polymer complex
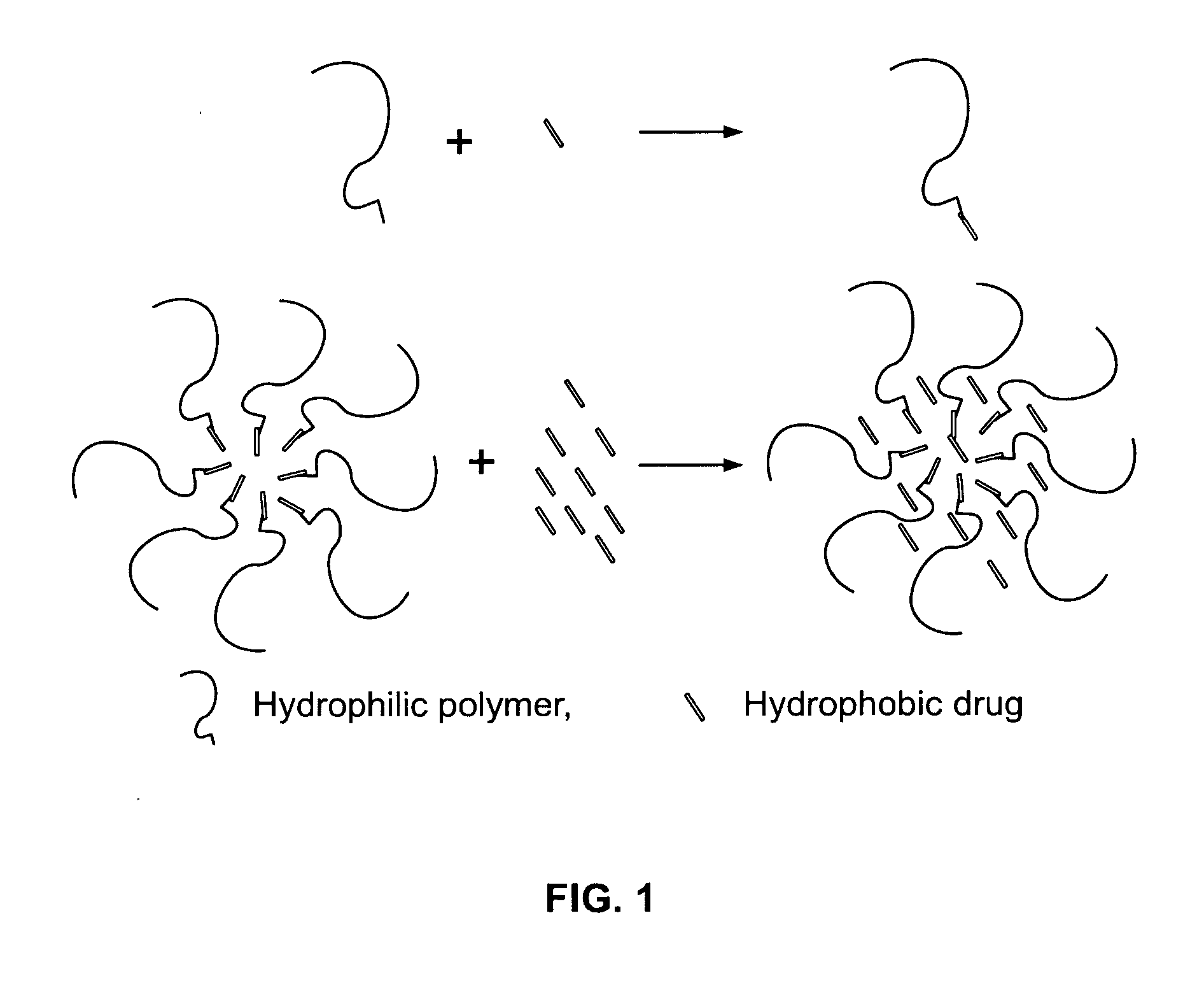
InactiveUS20050287212A1High low solubility drug loadingIncrease volumePowder deliveryPill deliverySolubilityHydrophilic polymers
This invention pertains to the enhanced delivery of orally administered pharmaceutical agents and methods, dosage forms and devices thereof. In particular, the invention is directed to methods including providing a low solubility drug having a pKa between about 6 and about 9; dissolving the low solubility drug in an aqueous solution, wherein a pH of the aqueous solution is less than about 6.0; dissolving a hydrophilic polymer in the aqueous solution, wherein the weight ratio of the hydrophilic polymer to the low solubility drug is less than or equal to about 0.15; lyophilizing the aqueous solution to obtain a lyophilized powder. Also disclosed are drug formulations made according to the method, and dosage forms that include the drug formulations.
Owner:ALZA CORP
Medicine carrying fibroin microsphere and preparation thereof
InactiveCN101244277AGood denaturation effectHigh drug loadingOrganic non-active ingredientsGranular deliveryEmbedding rateOrganic solvent
The invention discloses a drug-loaded microsphere and the preparation method, belonging to the technical field of biomedicine, which comprises following steps: adopting water / oil / water multiple emulsion technology and self-assembly technology of protein to fully mix the water soluble medicine and regenerated silk fibroin solution; adding the mixture in oil phase with emulsifier with certain amount during stirring for emulsification; adding organic solvent and stirring, thereby the silk fibroin is denaturalized and Beta structured to form milky crystalline silk fibroin fine particle, and the medicine is embedded; removing supernatant liquid through centrifugal effect; adding organic solvent; further crystallizing the silk fibroin and embedding the medicine; removing solvent and residual oil phase through centrifugal effect and collecting the microsphere. The average particle size of the silk fibroin drug-loaded microsphere is between 5.84 to 86.27Mum, the embedding rate is 42 to 99%, the drug loading dosage is 2.5 to 8.5%. The drug-loaded microsphere has the advantages of applicability to requirements for different purposes and wide application prospect.
Owner:SUZHOU UNIV
Biogastrone acid prosome liposome with long circulation function and preparation method thereof
InactiveCN101366698AAchieve long cycleSmall particle sizeOrganic active ingredientsMetabolism disorderFreeze-dryingCholesterol
The invention belongs to the new technical field of a drug preparation and in particular relates to a precursor liposome containing a glycyrrhetinic acid and a method for preparing the same. The precursor liposome containing the glycyrrhetinic acid consists of glycyrrhetinic acid, phospholipids, cholesterol, a surfactant and a water-soluble material; a suspension solution of the glycyrrhetinic acid liposome is prepared by an ethanol injection method or a thin-film dispersion method; and solid liposome powder is prepared by a freeze drying method or a spray drying method. The precursor liposome has simple process and good repeatability, is suitable for industrialized production; through drug administration by intravenous injection, the precursor liposome has long-circulating function, can remarkably reduce the toxicity of drug and achieve the function of prolonging the drug effect; and through oral administration, a solid preparation of the liposome can increase absorption and improve bioavailability by three to five times.
Owner:CHINA PHARM UNIV
Asymmetric magnetic mesoporous silica rod supporting chemotherapeutic and gene drugs and application thereof to tumor diagnosis and treatment
InactiveCN104225599AGood treatment effectReal-time monitoring of treatment effectGenetic material ingredientsInorganic non-active ingredientsBiocompatibility TestingMesoporous silica
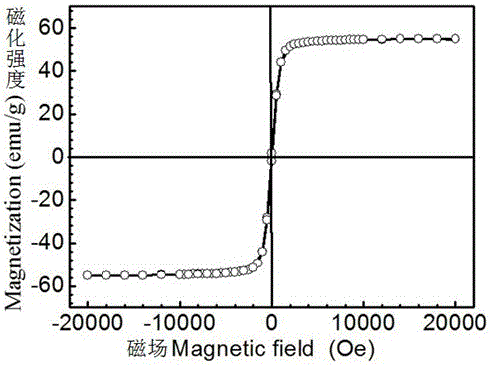
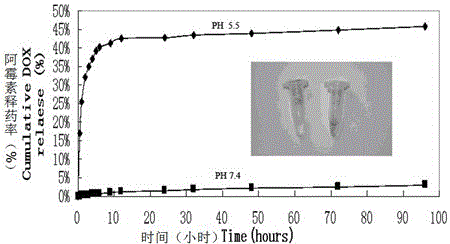
The invention relates to the field of nanometer drug carriers, and concretely relates to an asymmetric magnetic mesoporous silica rod supporting chemotherapeutic and gene drugs and application thereof to tumor diagnosis and treatment. The asymmetric magnetic mesoporous silica rod is prepared by employing spherical magnetic ferrite nanoparticles and ethyl orthosilicate through a sol-gel method, and the asymmetric magnetic mesoporous silica rod is subjected to surface functionalization modification, and is successively loaded with a chemotherapeutic drug, coated by a positive high-molecular polymer and loaded with a gene drug, so that a target product is obtained. The chemotherapeutic drug is connected with the silica rod through functionalization of the mesoporous surface, and the silica rod is endowed with the pH-responsive drug release characteristic, also the biocompatibility of the composite material is increased and the in-vivo cycling time is prolonged, and gene is supported in an electrostatic adsorption mode. The composite material is injected into a living body via an intravenous route, the characteristics of nanoparticle in-vivo passive targeting, gene guiding and pH-responsive drug release of the composite material are utilized, also the cooperativity of the multidrug resistant gene and the chemotherapeutic drug is utilized, and in-vitro magnetic targeting, NMR imaging and other technologies are applied to diagnosis and treatment of malignant tumors.
Owner:JILIN UNIV
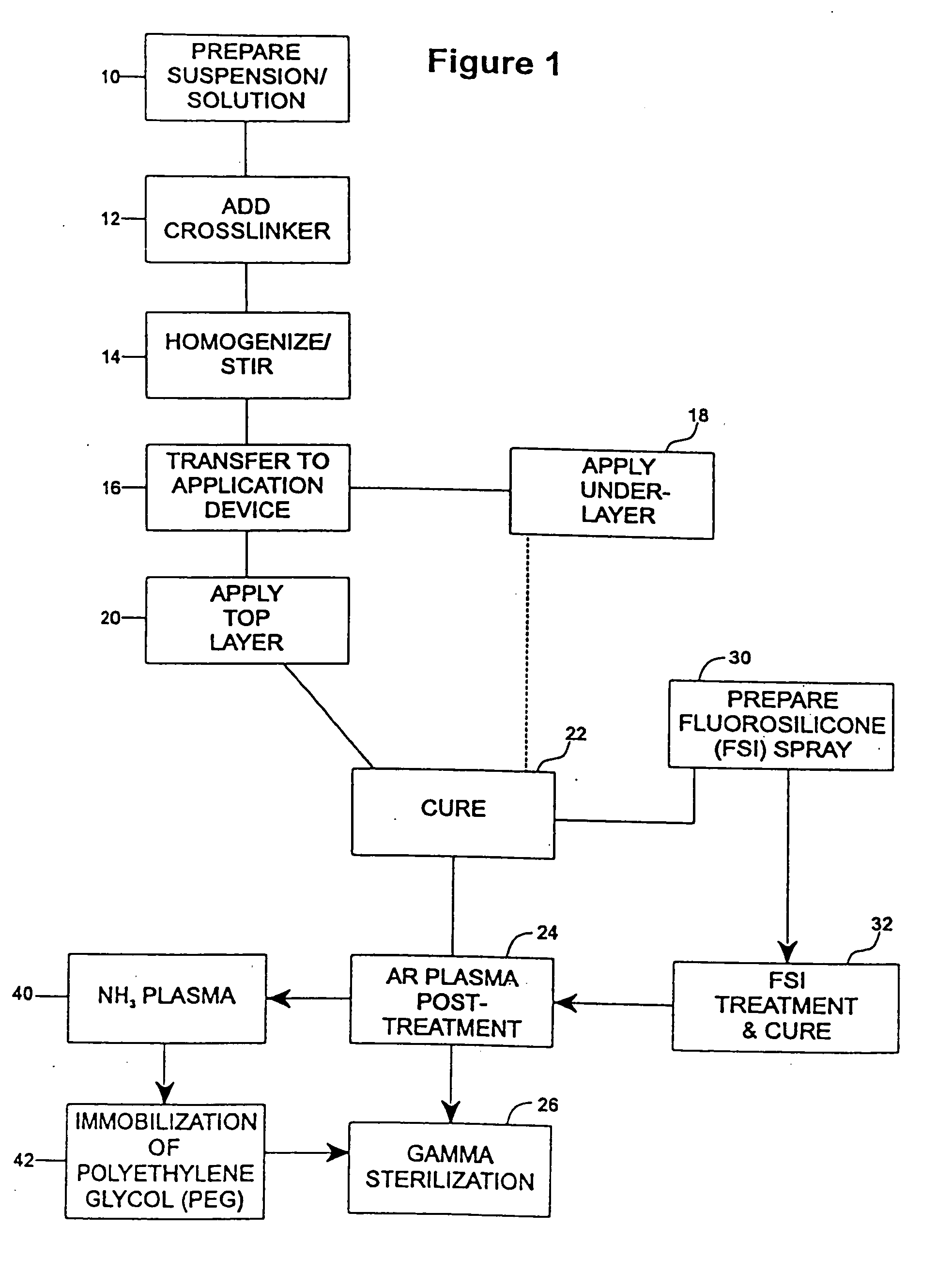
Drug coating with topcoat
InactiveUS20050208200A1Increase coating thicknessLow drug loadStentsSurgeryThrombogenicityDrug coating
A coating and method for a coating an implantable device or prostheses are disclosed. The coating includes an undercoat of polymeric material containing an amount of biologically active material, particularly heparin, dispersed therein. The coating further includes a topcoat which covers less than the entire surface of the undercoat and wherein the topcoat comprises a polymeric material substantially free of pores and porosigens. The polymeric material of the topcoat can be a biostable, biocompatible material which provides long term non-thrombogenicity to the device portion during and after release of the biologically active material.
Owner:BOSTON SCI SCIMED INC
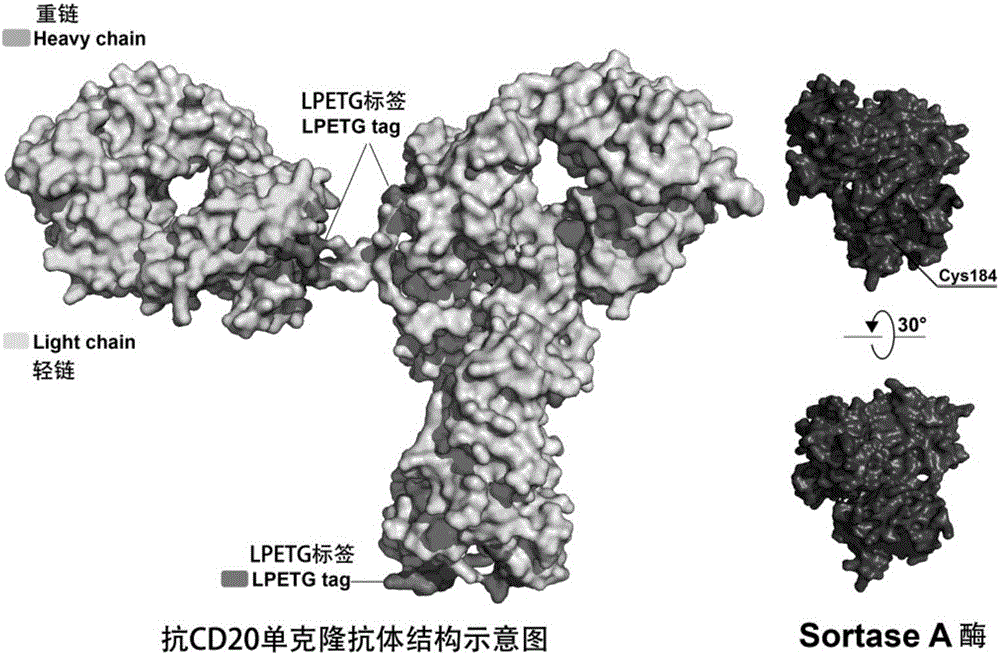
Antibody conjugated medicine and preparation method and application thereof
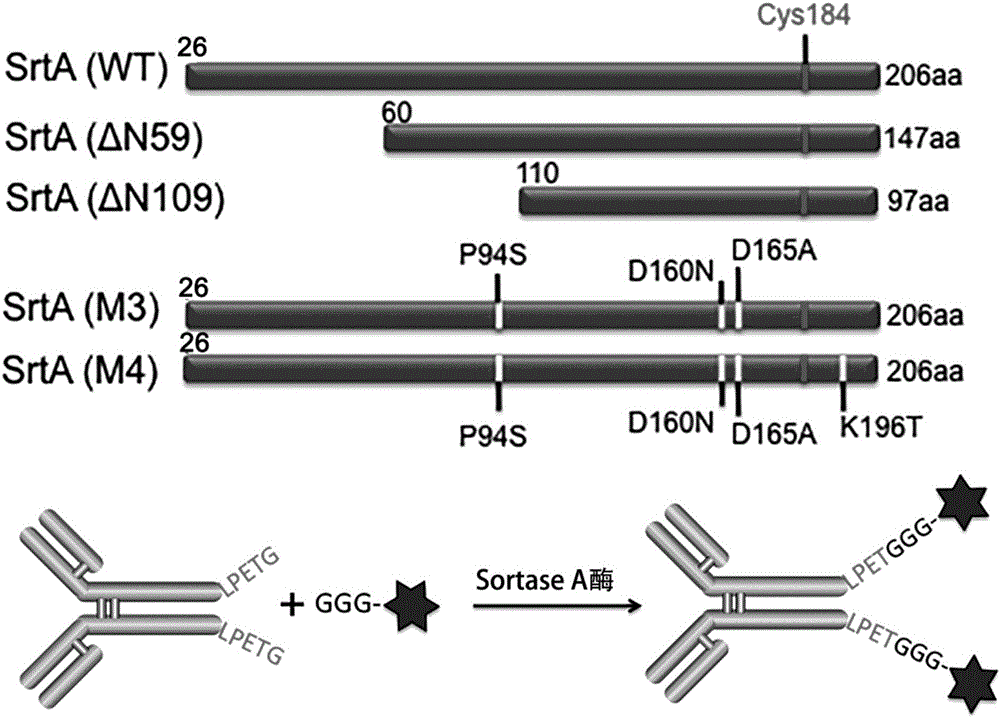
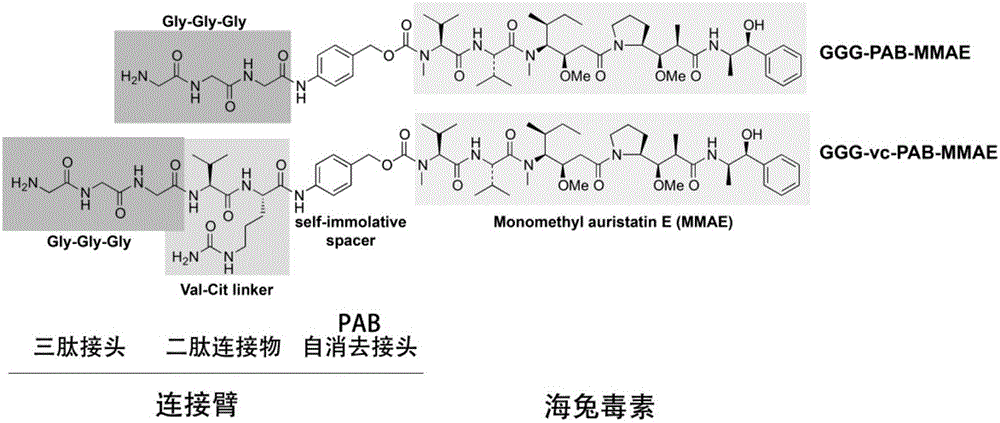
ActiveCN106237341AHigh anti-tumor activity in vitro and in vivoAvoid the use of organic reagentsPharmaceutical non-active ingredientsDepsipeptide ingredientsAnti cd20Drug
The invention discloses an antibody conjugated medicine and a preparation method and application thereof. The antibody conjugated medicine is formed by connecting an antibody and a medicine through a connecting arm. The antibody is an anti-CD20 monoclonal antibody with heavy chain containing LPXTG sequence. The medicine is aplysiatoxin or a derivative thereof. The connecting arm contains a short-peptide linker for connecting the antibody and a self-elimination linker for connecting the medicine. The short-peptide linker contains at least 1-3 continuous glycines. The antibody conjugated medicine has high uniformity and can achieve higher in-vitro and in-vivo antineoplastic activity with less drug loading capacity (DAR) than CD20 targeting ADC prepared by other chemical methods. IC50 (median inhibitory concentration) for Ramos cells can reach 0.005 nanogram / milliliter.
Owner:ZHEJIANG UNIV
Double-balloon drug coating balloon dilatation catheter
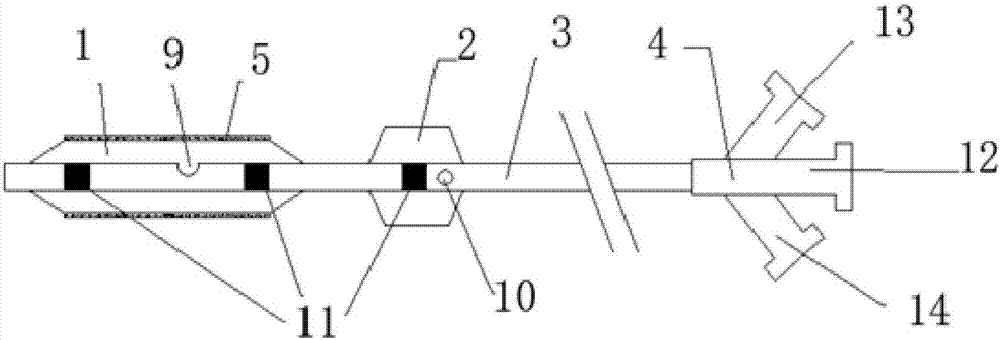
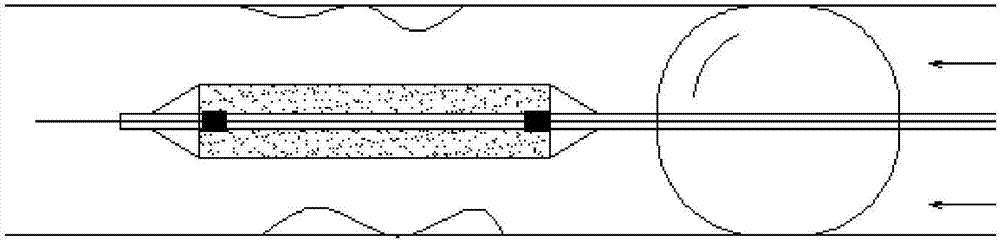
InactiveCN106994204AReduce sheddingMitigate washout effectSurgeryDilatorsBalloon dilatation catheterFirst Fill
The invention discloses a double-balloon drug coating balloon dilatation catheter. The double-balloon drug coating balloon dilatation catheter comprises a push rod, and the far end of the push rod is fixedly provided with a working balloon for attaching drug coating and a blocking balloon for block blood flow; the inside of the push rod is provided with a guide wire cavity for mounting a guide wire in a through mode, a first filling cavity for filling the working balloon, and a second filling cavity for filling the blocking balloon, wherein he first filling cavity is communicated with the inner cavity of the working balloon, and the second filling cavity is communicated with the inner cavity of the blocking balloon. By means of the double balloons, the double-balloon drug coating balloon dilatation catheter can reduce flushing effects of blood flow on drug to reduce drug loss, and meanwhile, can have no increase in the diameter to reduce surgical difficulty and surgical injuries.
Owner:HANGZHOU WEIQIANG MEDICAL TECH CO LTD
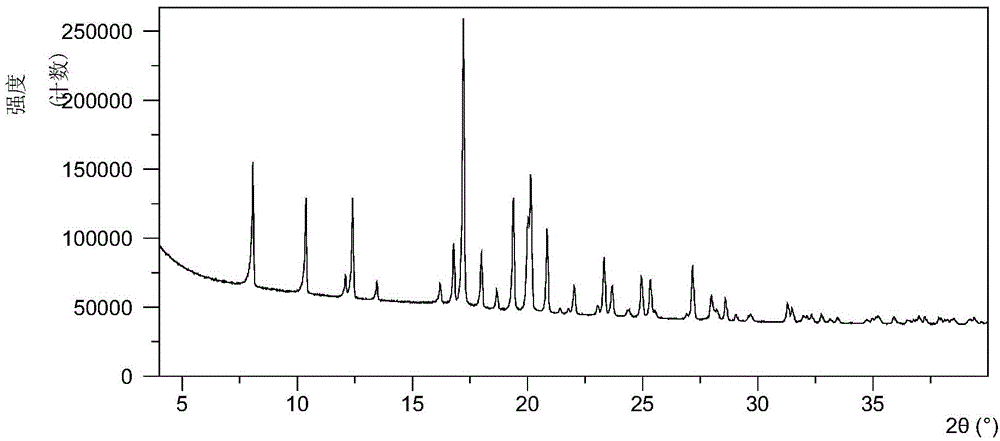
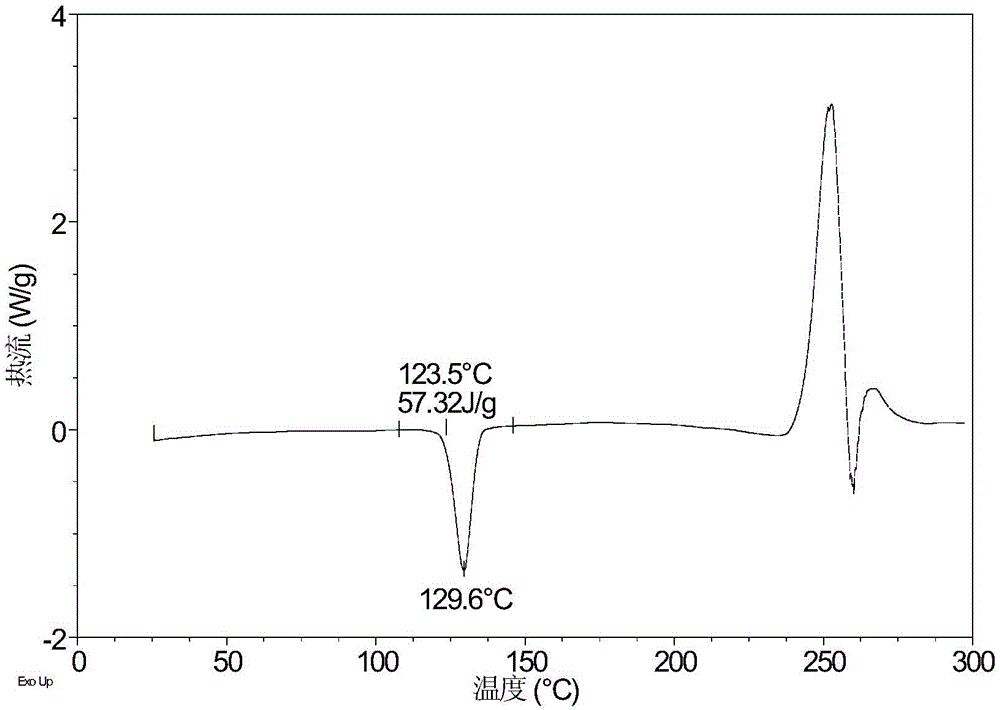
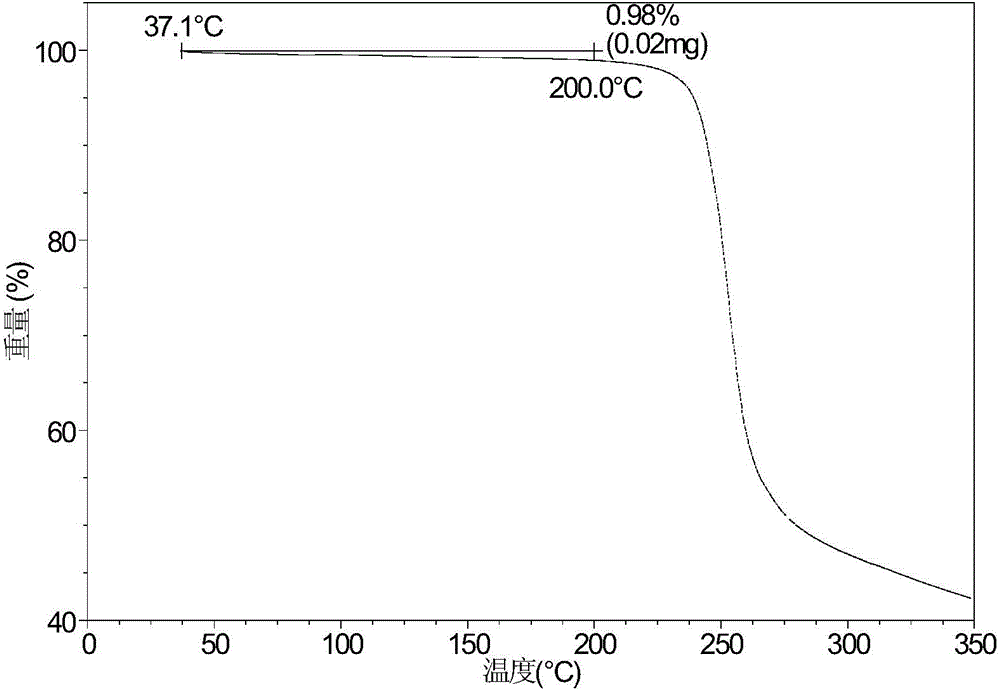
Crystal form A of sofosbuvir and preparation method thereof
InactiveCN104974205AImprove solubilityImprove efficacyOrganic active ingredientsSugar derivativesSolubilityHigh humidity
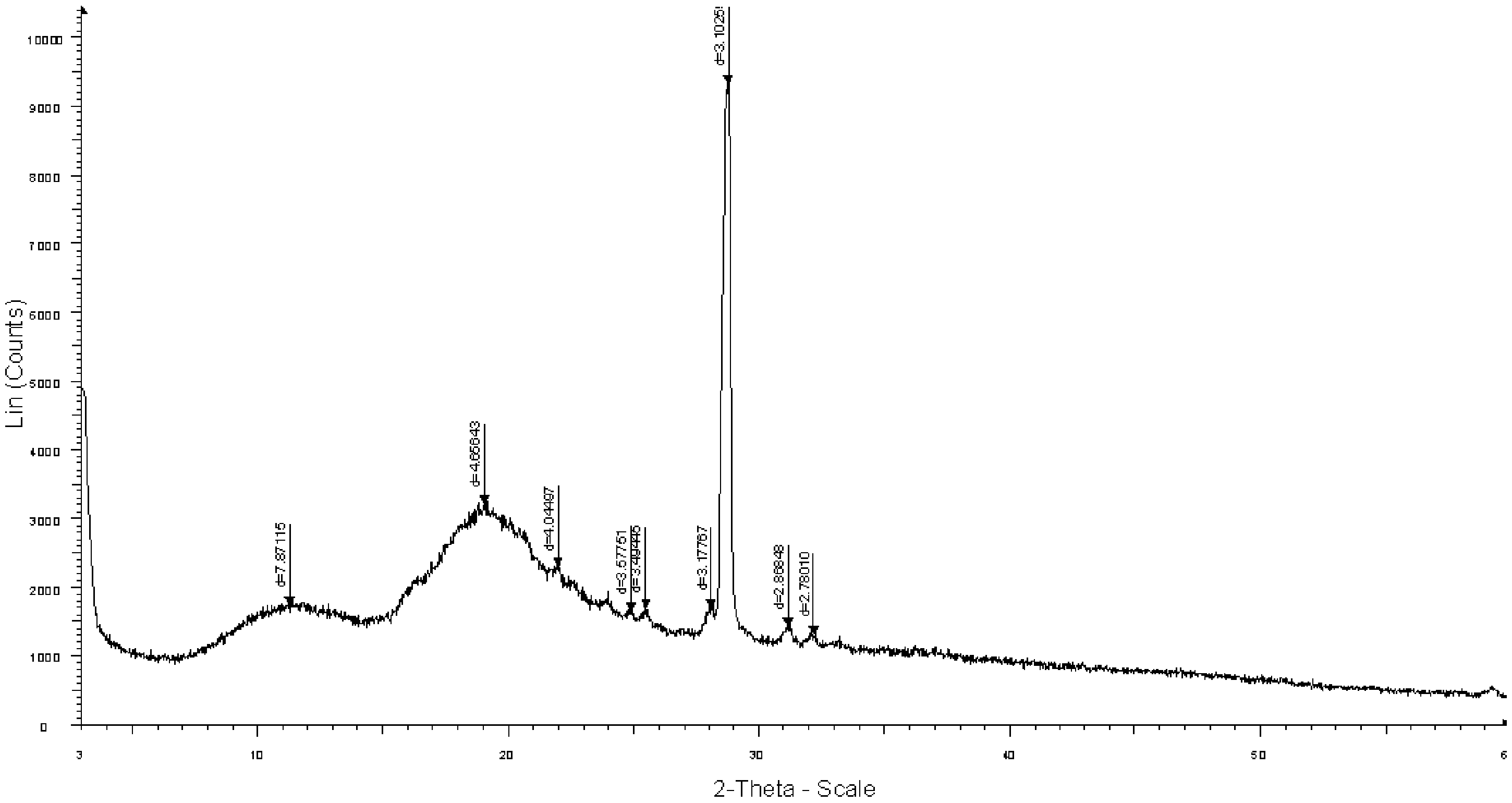
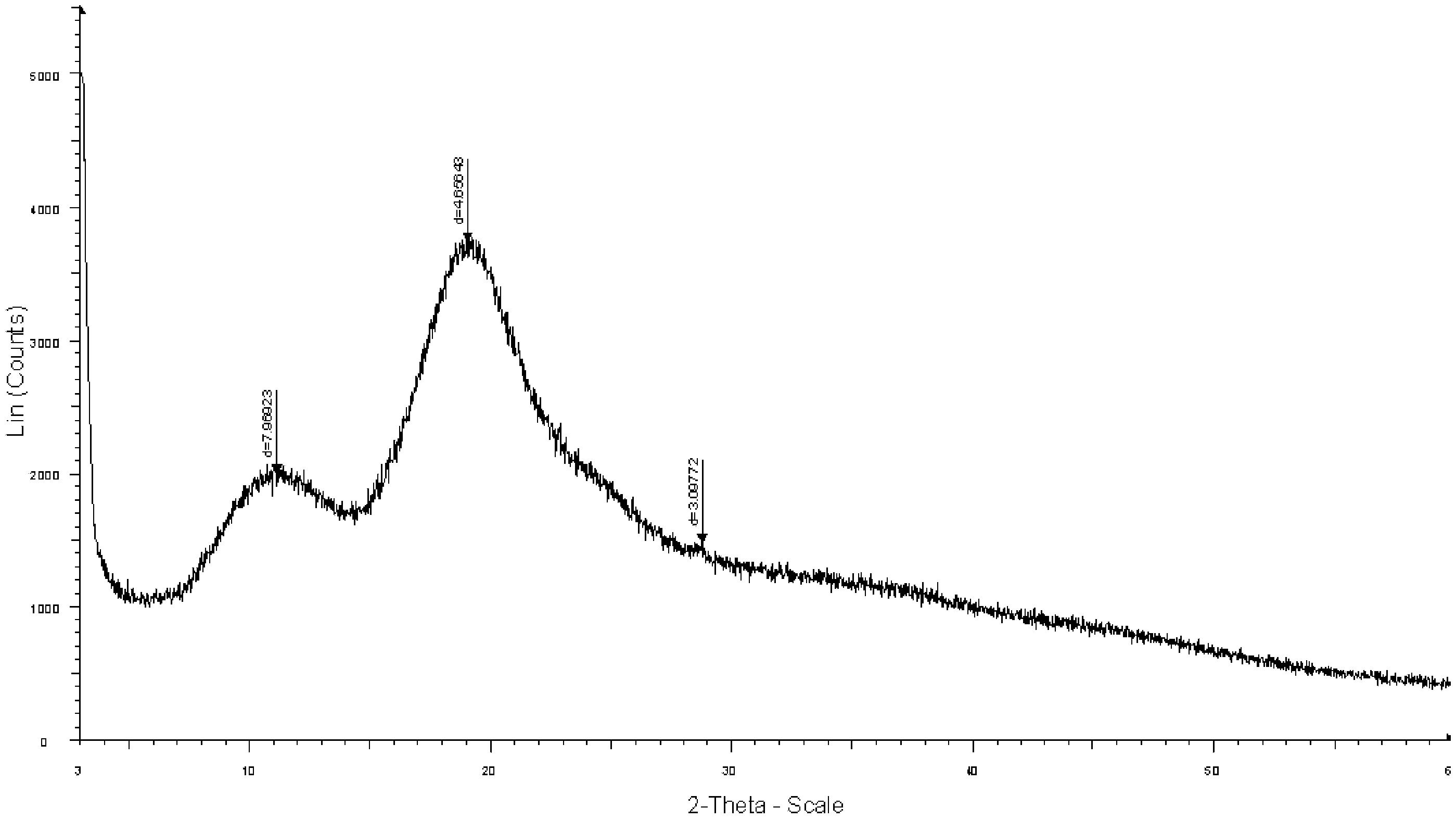
The invention provides a crystal form A of sofosbuvir and a preparation method thereof. The crystal form A is characterized in that an X-ray powder diffraction spectrum has characteristic peaks at the 2[theta] value being 12.4 + / - 0.2 degrees, 19.4 + / - 0.2 degrees, 27.1 + / - 0.2 degrees, 13.5 + / - 0.2 degrees, 25.5 + / - 0.2 degrees and 16.8 + / - 0.2 degrees. The crystal form A is higher in solubility in a bio-medium, is beneficial to drug efficiency increasing, can reduce the carrying amount of the drug, is low in hygroscopicity and is not liable to deliquesce due to high humidity, so that the drug can be stored for a long time conveniently.
Owner:CRYSTAL PHARMATECH CO LTD
Solid Adsorbates of Hydrophobic Drugs
InactiveUS20090169583A1Easily incorporateLow bioavailabilityPowder deliveryBiocideHydrophobeMedicine
A solid pharmaceutical composition comprises a solid adsorbate comprising a hydrophobic drug, a lipophilic vehicle, and a porous substrate, wherein the hydrophobic drug and lipophilic vehicle are adsorbed to the porous substrate.
Owner:BEND RES
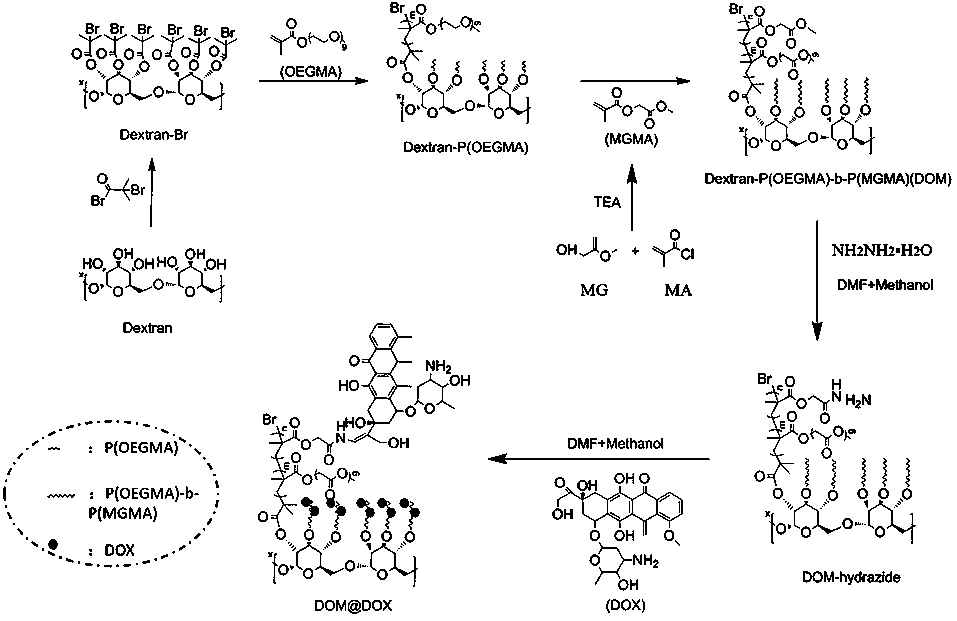
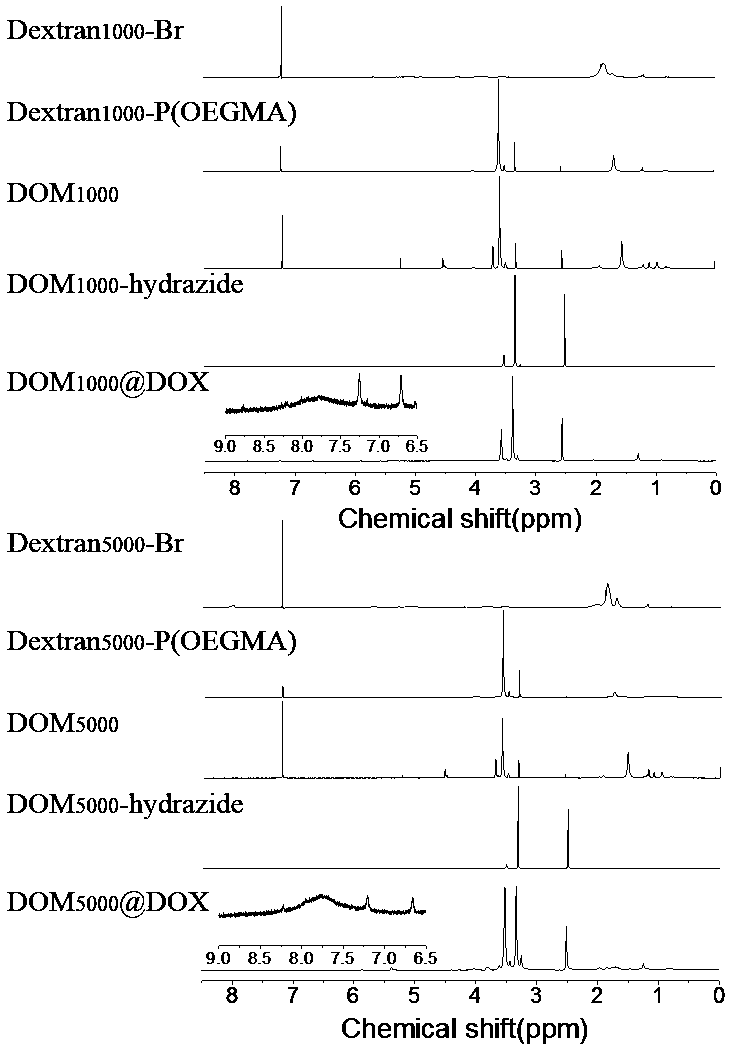
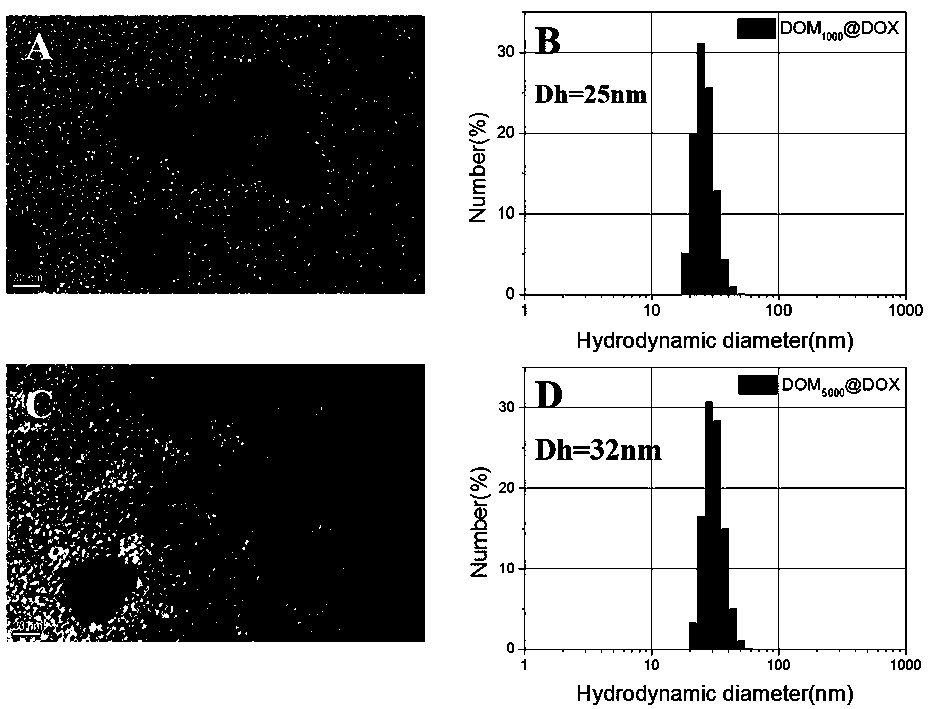
Preparation method and application of acid-responsive polymer drug based on dextran
InactiveCN108774301AEfficiently control the sizeIncrease upload volumeOrganic active ingredientsPharmaceutical non-active ingredientsSolubilityCancer cell
The invention particularly relates to an acid-responsive polymer drug based on dextran and application of the acid-responsive polymer drug. In a molecular formula of the polymer drug, the value rangeof x is 3 to 30, and m and n separately indicate polymerization degrees of a hydrophobic block and a hydrophilic block. A method comprises the following synthetic steps: (1) using dextran to synthesize an ATRP initiator (Dextran-Br); (2) introducing the hydrophilic and hydrophobic blocks by utilizing ATRP polymerization reaction; (3) using N2H4-H2O to substitute an ester group on MGMA, thus obtaining an acid-responsive precursor; and (5) using amino and carbonyl on doxorubicin to react to form hydrazone bonds to synthesize an acid-responsive polymerization prodrug. In a cancer cell weak acid environment, the polymerization prodrug can be selectively released and has the advantages of higher micelle stability, high drug loading amount and the like, and can effectively overcome the defects in a drug delivery system of the nano technology that hydrophobic drug molecules are poor in solubility, high in toxicity and side effects and the like.
Owner:SOUTHWEST UNIVERSITY
In-situ gel film agent with biological adhesion and preparation method thereof
InactiveCN104095804AHigh strengthLarge spreading areaOrganic active ingredientsPharmaceutical delivery mechanismTreatment effectDrug release
The invention provides a water-soluble cyclodextrin derivative clathrate of fluorouracil, and an in-situ gel film agent containing the clathrate and having biological adhesion and a preparation method thereof. According to the invention, cyclodextrin derivative-hydroxypropyl-beta-cyclodextrin is used to include fluorouracil hardly soluble in water so as to allow the solubility of fluorouracil in water to be improved and fluorouracil to have a slow release effect, so the in-situ gel film agent is prepared; the in-situ gel film agent is a novel cavity drug delivery preparation and comprises the hydroxypropyl-beta-cyclodextrin clathrate of fluorouracil, a temperature-sensitive gel skeletal material and a biologically adhesive material. The preparation is a freely flowable solution before usage, rapidly forms a gel film on the surface of a cavity, has a great drug release area, good biological compatibility and good gel strength and biological adhesion, is capable of firmly adhering on the mucous membrane of the cavity, prolongs action time, realizes uniform drug distribution, is beneficial for absorption of the drug and diffusion of the drug to peripheral tissue and improves bioavailability and treatment effects of the drug.
Owner:INST OF MATERIA MEDICA AN INST OF THE CHINESE ACAD OF MEDICAL SCI
Amphiphilic prodrugs
InactiveUS20040180840A1Good water solubilityImprove conveniencePowder deliveryBiocideOligomerPolymer
The present invention provides amphiphilic prodrugs comprising a therapeutic compound conjugated to an PEG-oligomer / polymer and methods for using said prodrugs to enable oral drug delivery and / or delivery of drugs across the blood brain barrier into the central nervous system.
Owner:BIOCON LTD
Flower-shaped lactose loaded curcumin nano dry powder inhalant and preparation method thereof
InactiveCN111956631ALarge specific surface areaImprove liquidityPowder deliveryKetone active ingredientsLACTOSE MONOHYDRATEDrug carrier
The invention relates to a flower-shaped lactose loaded curcumin nano dry powder inhalant and a preparation method thereof, and belongs to the technical field of medical treatment. The flower-shaped lactose loaded curcumin nano dry powder inhalant consists of a drug carrier and curcumin solid lipid nanoparticles; the drug carrier is flower-shaped lactose; the flower-shaped lactose is prepared by performing a reaction on lactose monohydrate and citric acid; the flower-shaped lactose has a pore surface area of 30 plus and minus 7m2 / g, a pore volume of 0.7 plus and minus 0.3cm3 / g, a diameter peakvalue of 3.4, 5.6 and 12.4nm and an aerodynamic particle size of 2 to 4mum, and is adjustable in crude powder size; the curcumin solid lipid nanoparticles have an entrapment rate of 87.73%, a drug-loading rate of 7.72%, a particle size of 156.9 plus and minus 2.2nm, a polydispersity coefficient of 0.480 and an average Zeta potential of minus 24.8mV; and the dry powder inhalant is administrated toa respiratory system by a dry powder inhaler.
Owner:常州道宁医药科技有限公司 +1
Nano Chinese medicine threewingnut/Tripterygium prepn and its prepn process
InactiveCN1398612AImprove bioavailabilityGood dispersionPowder deliveryIndividual molecule manipulationWater bathsEvaporation
The new nano Chinese medicine threwingnut / Tripteerygium preparation consists of threwingnut / Tripterrygium extraction, general lactone or general alkaloid 0.1-0.6 wt%, carrier 0.1-0.8 wt%, surfactant 0.8-3.8 wt% and water. The preparation process includes dissolving threwingnut / Tripteerygium extraction, general lactone or general alkaloid in alcohol, dissolving carrier in cyclohexane, mixing through stirring, depression rotary evaporation to eliminate organic solvent and to form medicine film onto the wall of flask, vacuum drying to eliminate organic solventm, dissolving surfactant in water and adding it to the film, and final supersonic treatment to obtain yellow transparent colloid solution.
Owner:HUAZHONG UNIV OF SCI & TECH +1
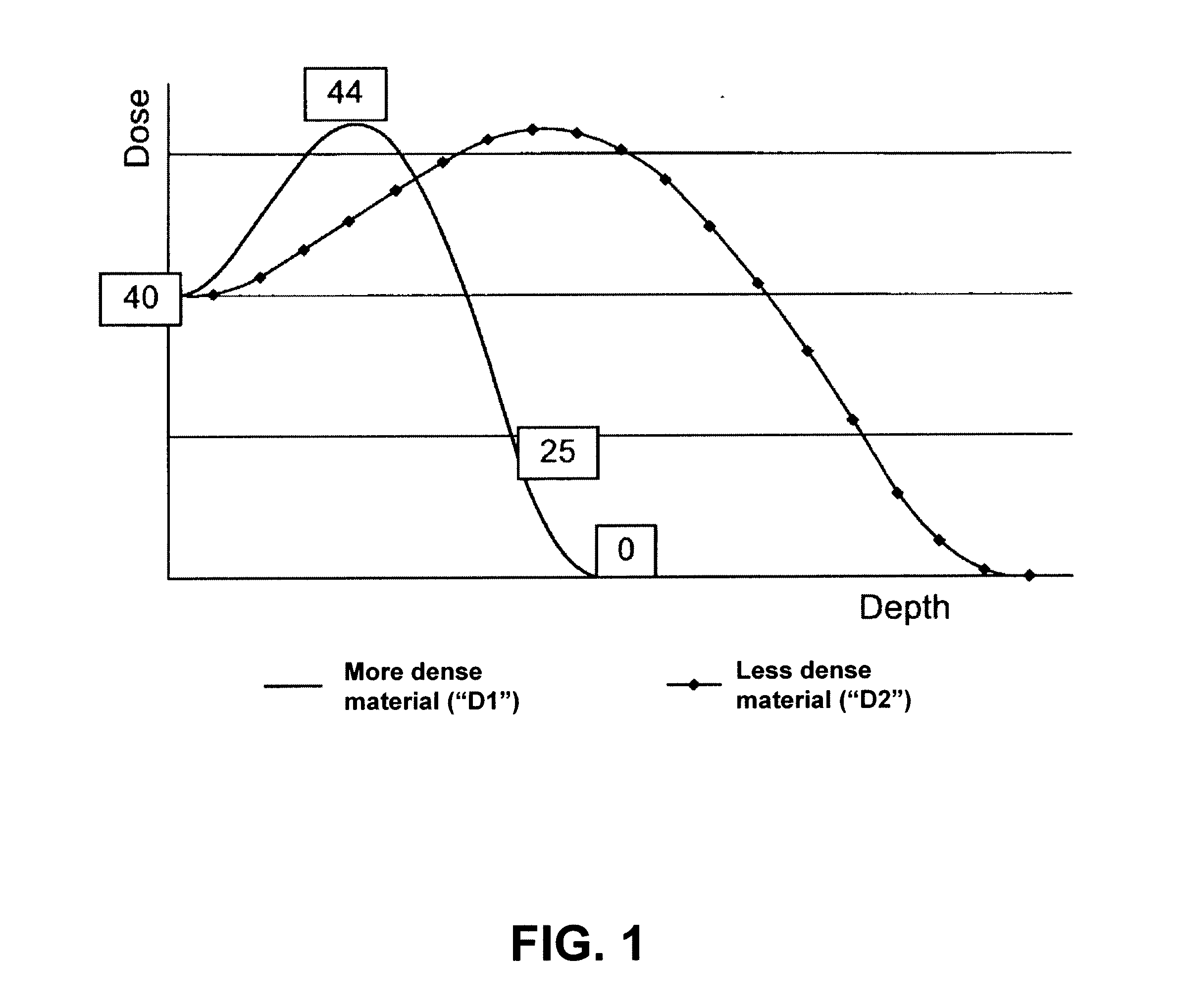
Radiation sterilization of implantable medical devices
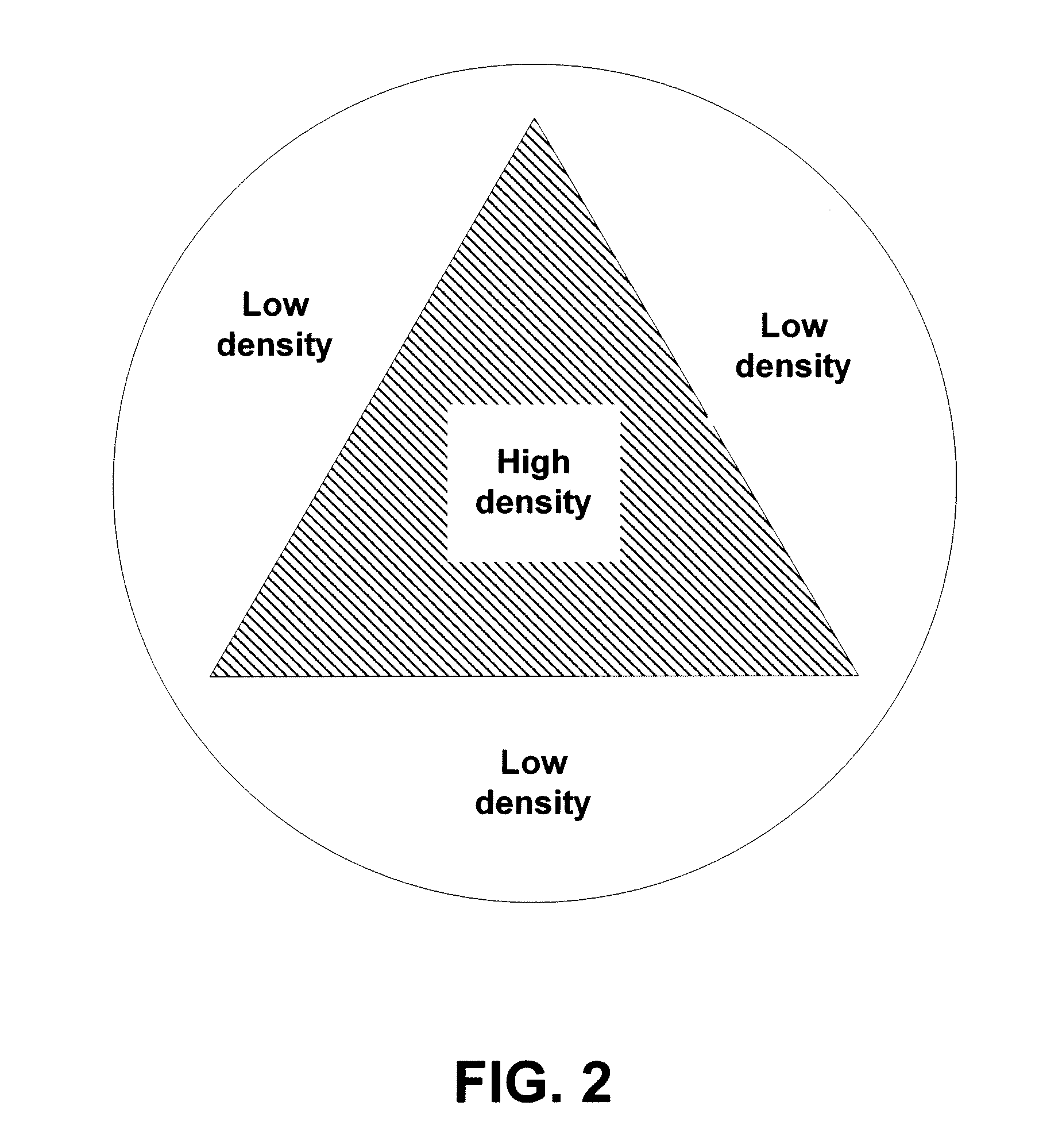
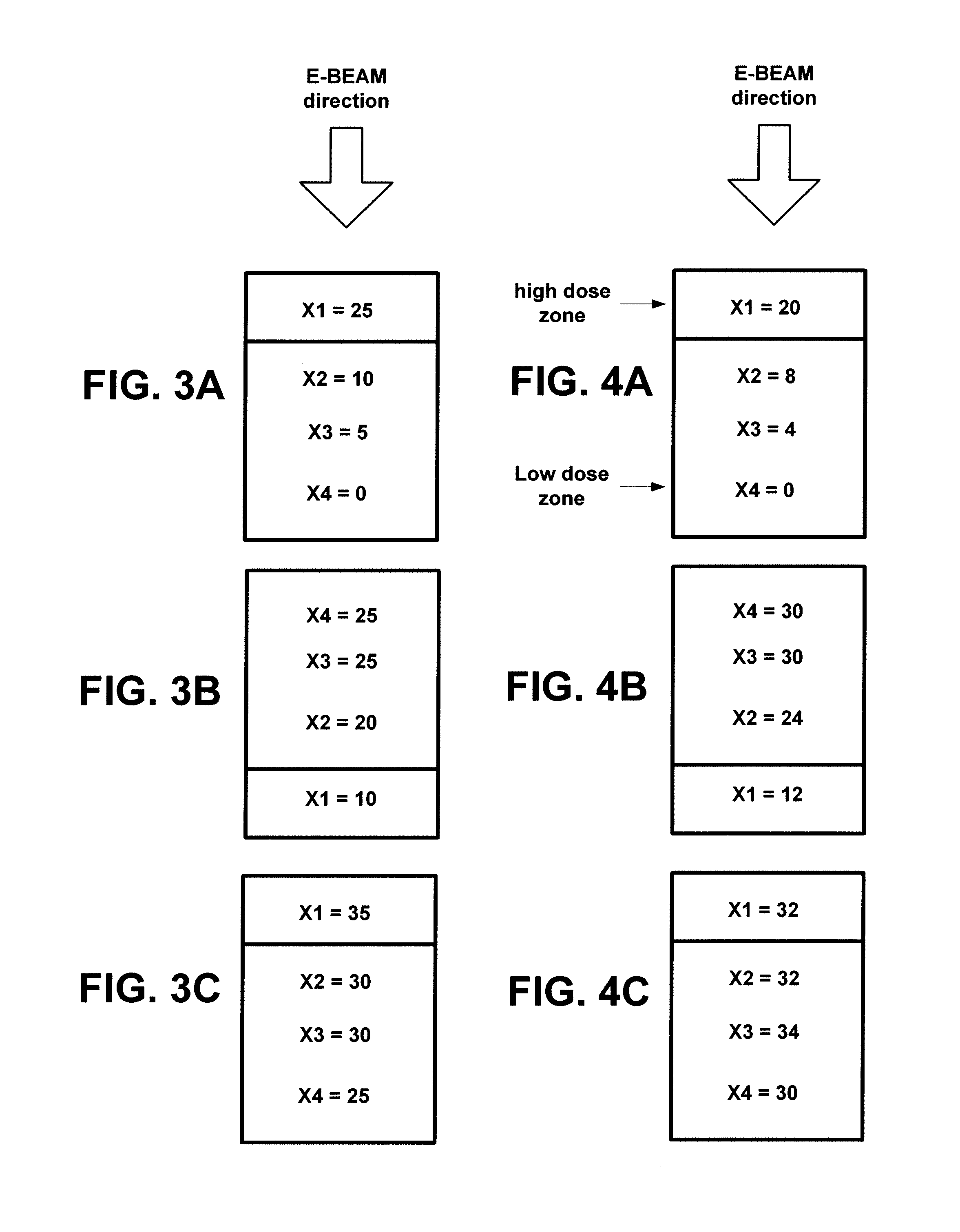
InactiveUS8981316B2Increase temperatureLow drug loadMaterial analysis using wave/particle radiationElectric discharge tubesDose levelRadiation Sterilization
A system and method for sterilization of medical devices includes methods for reducing the variance in dose levels over the medical device by either varying the dose levels for each pass before a radiation source, such as an electron beam, or by increasing the number of passes before a radiation source.
Owner:ABBOTT CARDIOVASCULAR
Radiation Sterilization of Implantable Medical Devices
InactiveUS20110240882A1Increase temperatureLow drug loadMaterial analysis by optical meansRadiation therapyDose levelMedical device
A system and method for sterilization of medical devices includes methods for reducing the variance in dose levels over the medical device by either varying the dose levels for each pass before a radiation source, such as an electron beam, or by increasing the number of passes before a radiation source.
Owner:ABBOTT CARDIOVASCULAR
Asymmetric magnetic mesoporous silica rods co-loading chemotherapy and gene drugs and their application in tumor diagnosis and treatment
InactiveCN104225599BGood treatment effectReal-time monitoring of treatment effectGenetic material ingredientsInorganic non-active ingredientsMesoporous silicaBiocompatibility
The invention relates to the field of nanometer drug carriers, and concretely relates to an asymmetric magnetic mesoporous silica rod supporting chemotherapeutic and gene drugs and application thereof to tumor diagnosis and treatment. The asymmetric magnetic mesoporous silica rod is prepared by employing spherical magnetic ferrite nanoparticles and ethyl orthosilicate through a sol-gel method, and the asymmetric magnetic mesoporous silica rod is subjected to surface functionalization modification, and is successively loaded with a chemotherapeutic drug, coated by a positive high-molecular polymer and loaded with a gene drug, so that a target product is obtained. The chemotherapeutic drug is connected with the silica rod through functionalization of the mesoporous surface, and the silica rod is endowed with the pH-responsive drug release characteristic, also the biocompatibility of the composite material is increased and the in-vivo cycling time is prolonged, and gene is supported in an electrostatic adsorption mode. The composite material is injected into a living body via an intravenous route, the characteristics of nanoparticle in-vivo passive targeting, gene guiding and pH-responsive drug release of the composite material are utilized, also the cooperativity of the multidrug resistant gene and the chemotherapeutic drug is utilized, and in-vitro magnetic targeting, NMR imaging and other technologies are applied to diagnosis and treatment of malignant tumors.
Owner:JILIN UNIV
Interventional or implantable medical instrument drug coating and preparation method thereof
PendingCN111588914AImprove the hydrophilic and hydrophobic balancePromote absorptionCatheterCoatingsPharmaceutical drugHydrophilic matrix
The invention relates to an interventional or implantable medical instrument drug coating and a preparation method thereof. The core of the interventional or implantable medical instrument drug coating is a grafted drug copolymer. The preparation method comprises the following steps: under the action of a catalyst, an active drug is covalently connected with a hydrophilic matrix to prepare the grafted drug copolymer, the grafted drug copolymer is dissolved and dispersed in a solvent, and then covered on the surface of an interventional or implantable medical instrument, and drying is performed. The covalent linkage manner can enhance the homogeneity of the drug and the matrix coating, improve the hydrophilicity and hydrophobicity balance of the drug and the adhesion-release balance of thedrug coating and the surface of the medical instrument, and enhance the efficiency that the drug is absorbed by tissues, penetrates through tissue stroma, and is taken in by cells. The homogeneity, uniformity and stability of the medical instrument drug coating are enhanced, the drug loading capacity is reduced, the drug loss is reduced, the drug entering blood circulation is reduced, and the absorption of the drug in target tissues and cells is increased, so that the toxicity is reduced, and the drug efficacy is improved.
Owner:LIAONING YINYI BIOTECH CO LTD
Lu AE58054 hydrochloride crystal form A, preparation method and uses thereof
InactiveCN105175307AImprove stabilityPrevent crystallizationOrganic active ingredientsNervous disorderSolubilityX-ray
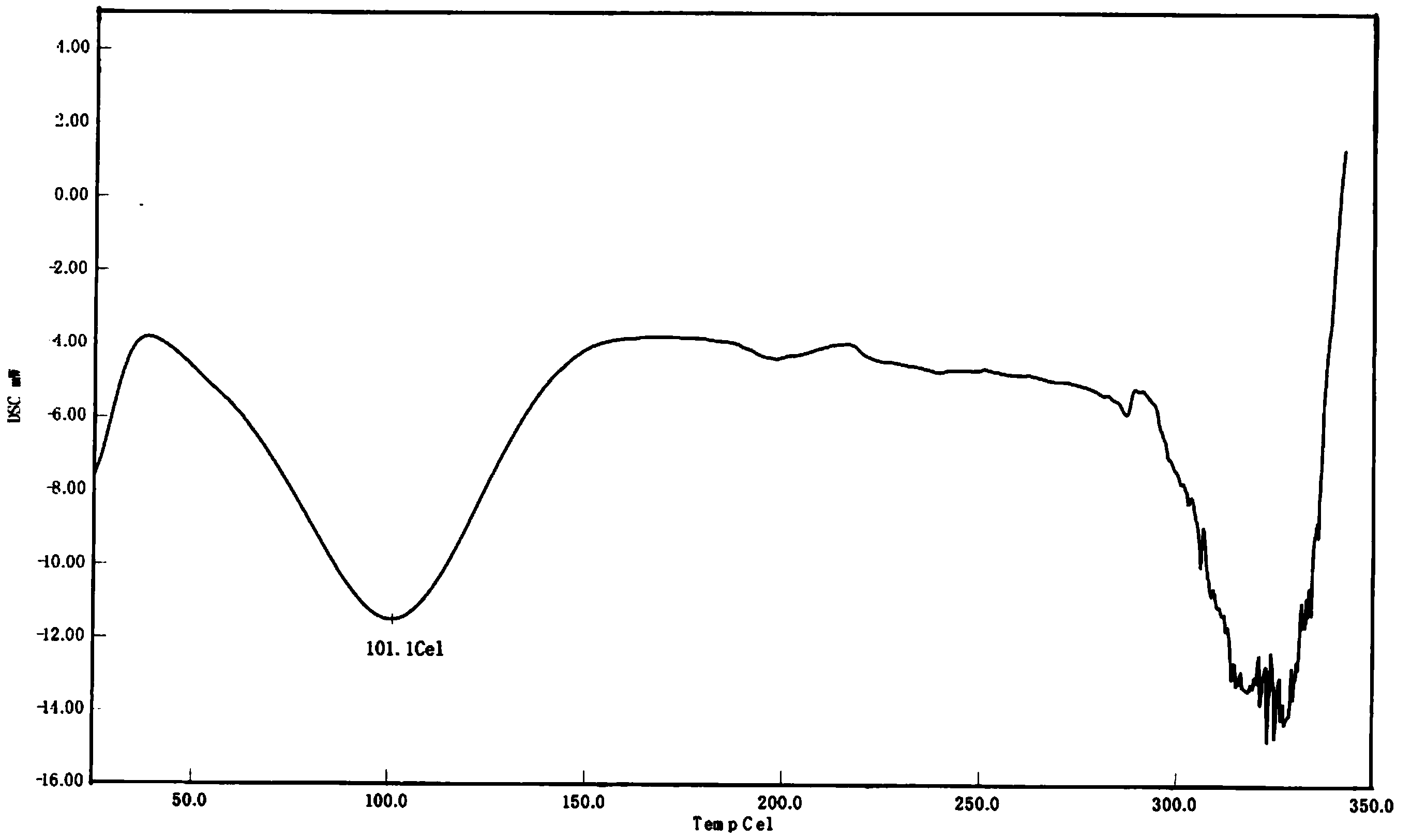
The present invention relates to a Lu AE58054 hydrochloride crystal form A, a preparation method and uses thereof, wherein the hydrochloride crystal form A has the characteristic peaks in the 25 DEG C X-ray powder diffraction spectrum (Cu K[alpha] radiation) when the 2[theta] value is 17.1+ / -0.2 DEG, 17.8+ / -0.2 DEG, and 21.4+ / -0.2 DEG. According to the present invention, the crystal form A preparation process has characteristics of simple operation and low cost, and the crystal form A has characteristics of good stability, low hygroscopicity, high solubility and easy drug absorption, and provides important significance for reduction of drug loading and preparation of Alzheimer's disease treatment preparations.
Owner:CRYSTAL PHARMATECH CO LTD
Rheumatic bone-setting transdermal patch and preparation process thereof
InactiveCN101658555AEasy to carry and useStrong medicineAntipyreticAnalgesicsTransdermal patchTreatment effect
The invention relates to a rheumatic bone-setting transdermal patch which is formed by dactylicapnos root, cortex picrasmae, paniculate swallowwort root and rhizoma panacis M which are extracted, andeffective parts are refined, and then transdermal patch excipients are added to prepare the transdermal patch; the transdermal patch obtained by the invention has the characteristics of being convenient for carrying and using, high transdermal adsorption ratio, high effective ingredients and strong treating effect.
Owner:北京化药科创医药科技发展有限公司
Protective agent for nano-emulsion
InactiveCN102018961ALow drug loadHigh drug loadingPharmaceutical non-active ingredientsEmulsion deliveryProtective AgentsDrug product
The present invention provides a protective agent for nano-emulsion, which can improve the drug loading of nano-emulsion and the stability of nano-emulsion. Adding the protective agent into nano-emulsion can improve the drug loading of nano-emulsion. The influence factor of the preparation being investigated, no medicinal crystallization occurs when the nano-emulsion is in the refrigerated condition even in the freezing condition, and therefore the invention can not only improve the quality of medicine but also in favor of the storage and transportation of medicine.
Owner:HEBEI AOXING GROUP PHARMA
DOX-P micelle with anti-tumor drug resistance and preparation method thereof
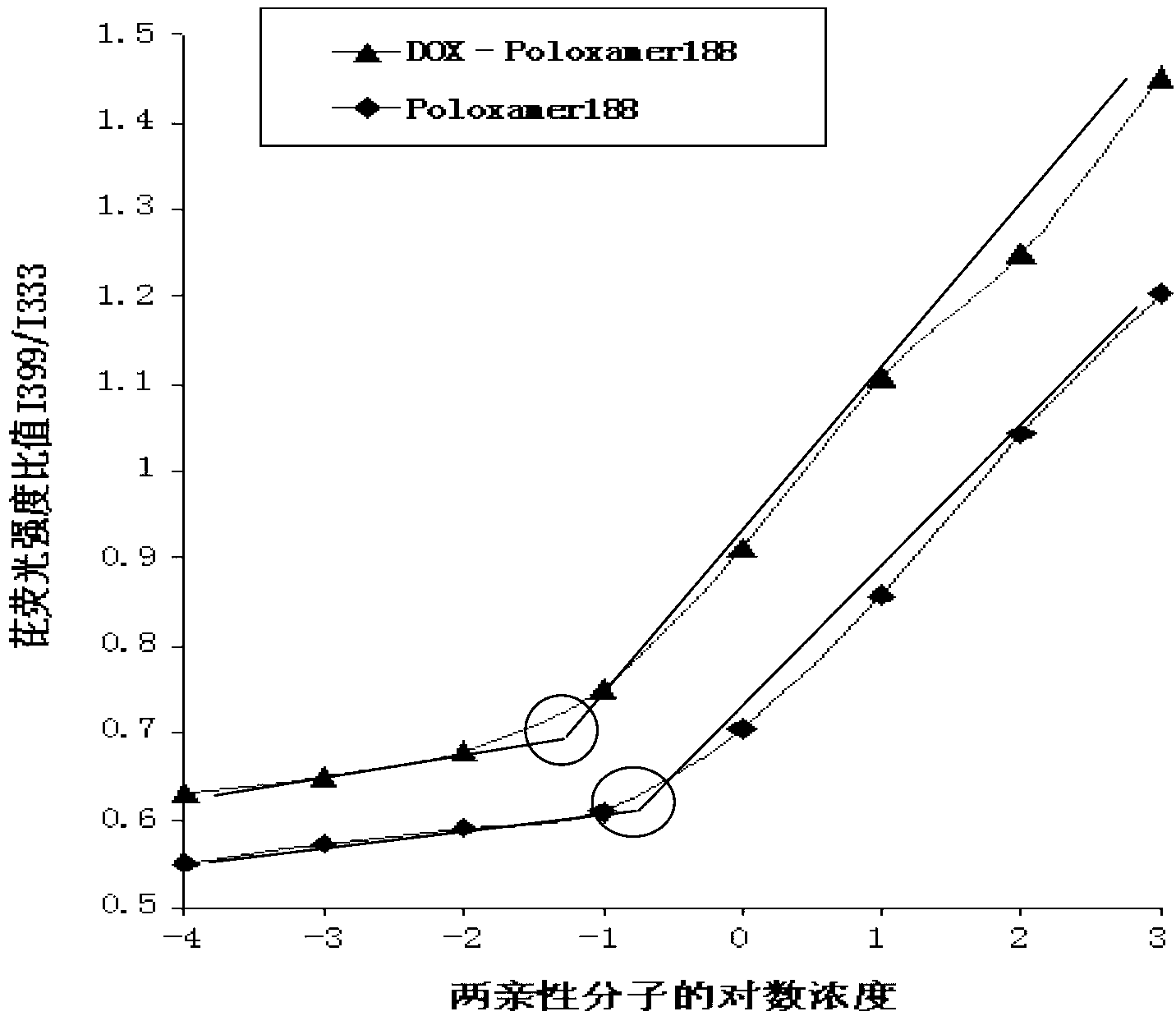
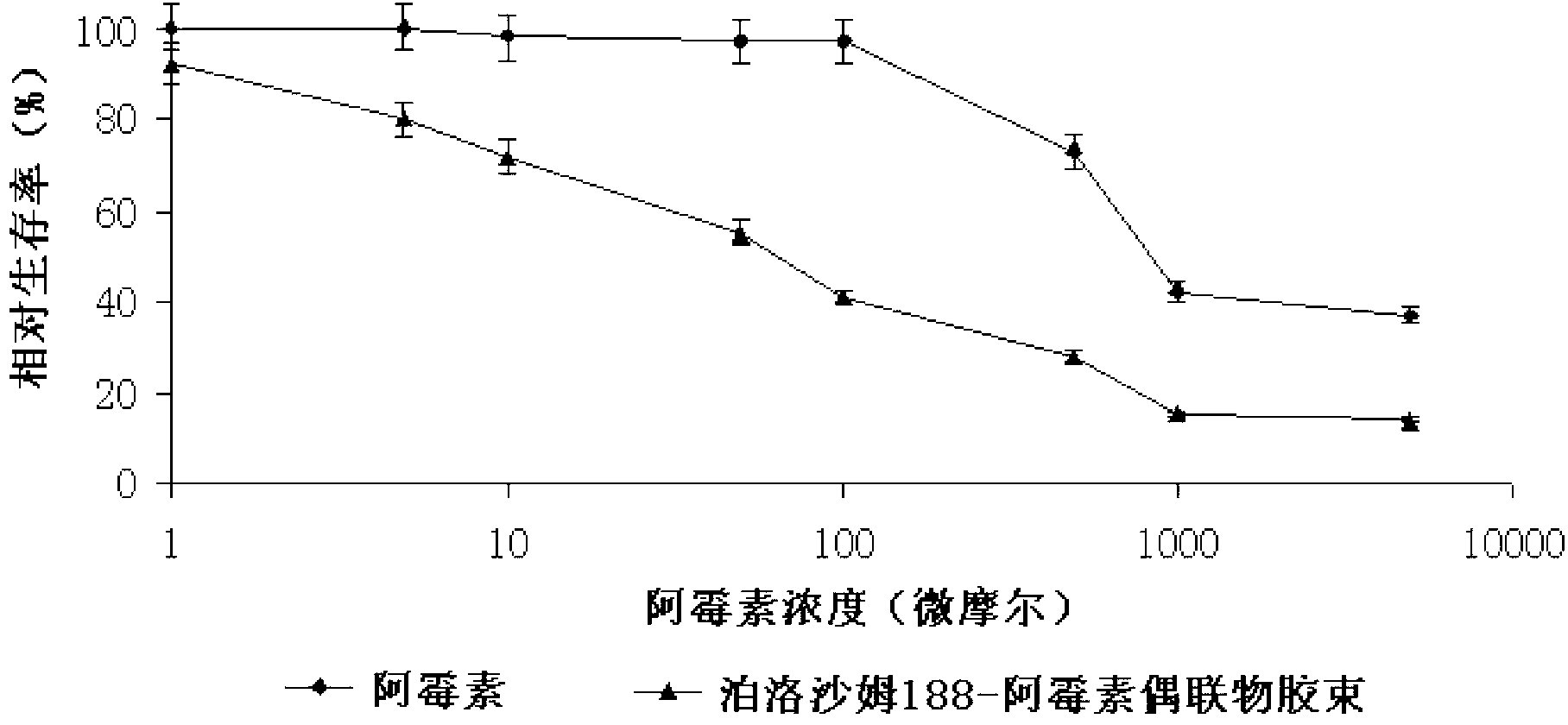
InactiveCN102989002ALow critical micelle concentrationParticle size controllableOrganic active ingredientsSolution deliveryWilms' tumorPolymer
The invention discloses poloxamer-adriamycin conjugate (DOX-P) micelle with anti-tumor drug resistance and a preparation method of the DOX-P micelle. The synthetic process of the DOX-P comprises the steps of firstly, reacting poloxamer with succinic anhydride to obtain high-molecular polymer of which the tail end contains carboxyl; and then, directly bonding the carboxyl at the tail end of the polymer with primaquine group in the adriamycin in an amido bond mode. The DOX-P has the capability of forming the micelle; and compared with the adriamycin bulk drug, the adriamycin subjected to covalent binding in the DOX-P micelle has the advantages that the anti-tumor activity of the adriamycin is maintained, the drug resistance of the tumor is overcome, and the stability of the adriamycin is better than that of poloxamer micelle.
Owner:吴燕
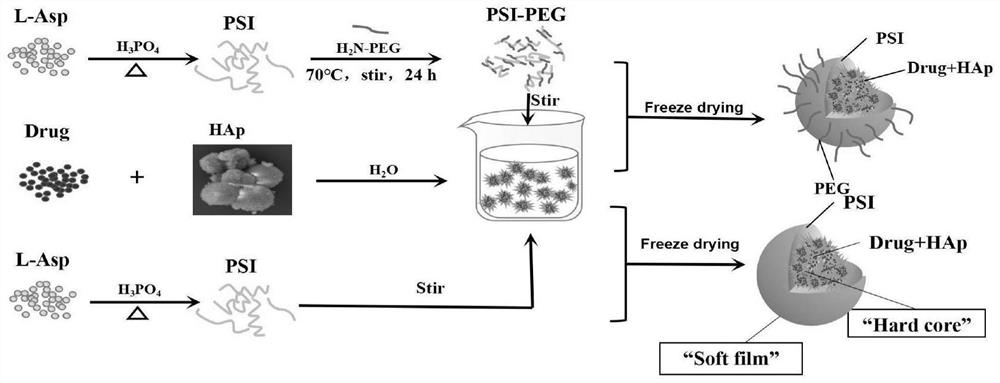
Preparation method of hard-core soft-membrane type nano slow-release drug delivery system for injection
ActiveCN113908137AImprove securitySimple designOrganic active ingredientsInorganic non-active ingredientsFreeze-dryingEngineering
The invention discloses a preparation method of a hard-core soft-membrane type nano slow-release drug delivery system for injection. The preparation method comprises the following steps of: S1, preparing hydroxyapatite; S2, preparing a soft-membrane material PEG-PSI; S3, mixing a drug to be delivered with the prepared hydroxyapatite in a solution, then adding the PEG-PSI dissolved in dimethyl sulfoxide for injection, uniformly mixing, and then centrifuging a preparation at a high speed; and S4, washing, then re-suspending in pure water, and carrying out freeze drying to obtain a final product. According to the preparation method disclosed by the invention, polyethylene glycol modified polysuccinimide with different molecular weights is used as an organic coating material, the hydroxyapatite is used as a drug carrying core, the drug is adsorbed, and a nano preparation form with a hard-core soft-membrane structure is formed through a coating effect of the polysuccinimide on the hydroxyapatite, so that the drug carrying capacity of a nano preparation is increased, the burst release of the nano preparation is avoided, the aggregation of nano particles is reduced, and the physical and chemical stability is improved.
Owner:高州市人民医院
Radiation sterilization of implantable medical devices
InactiveUS20150217009A1Increase temperatureLow drug loadLavatory sanitoryRadiationDose levelMedical device
A system and method for sterilization of medical devices includes methods for reducing the variance in dose levels over the medical device by either varying the dose levels for each pass before a radiation source, such as an electron beam, or by increasing the number of passes before a radiation source.
Owner:ABBOTT CARDIOVASCULAR
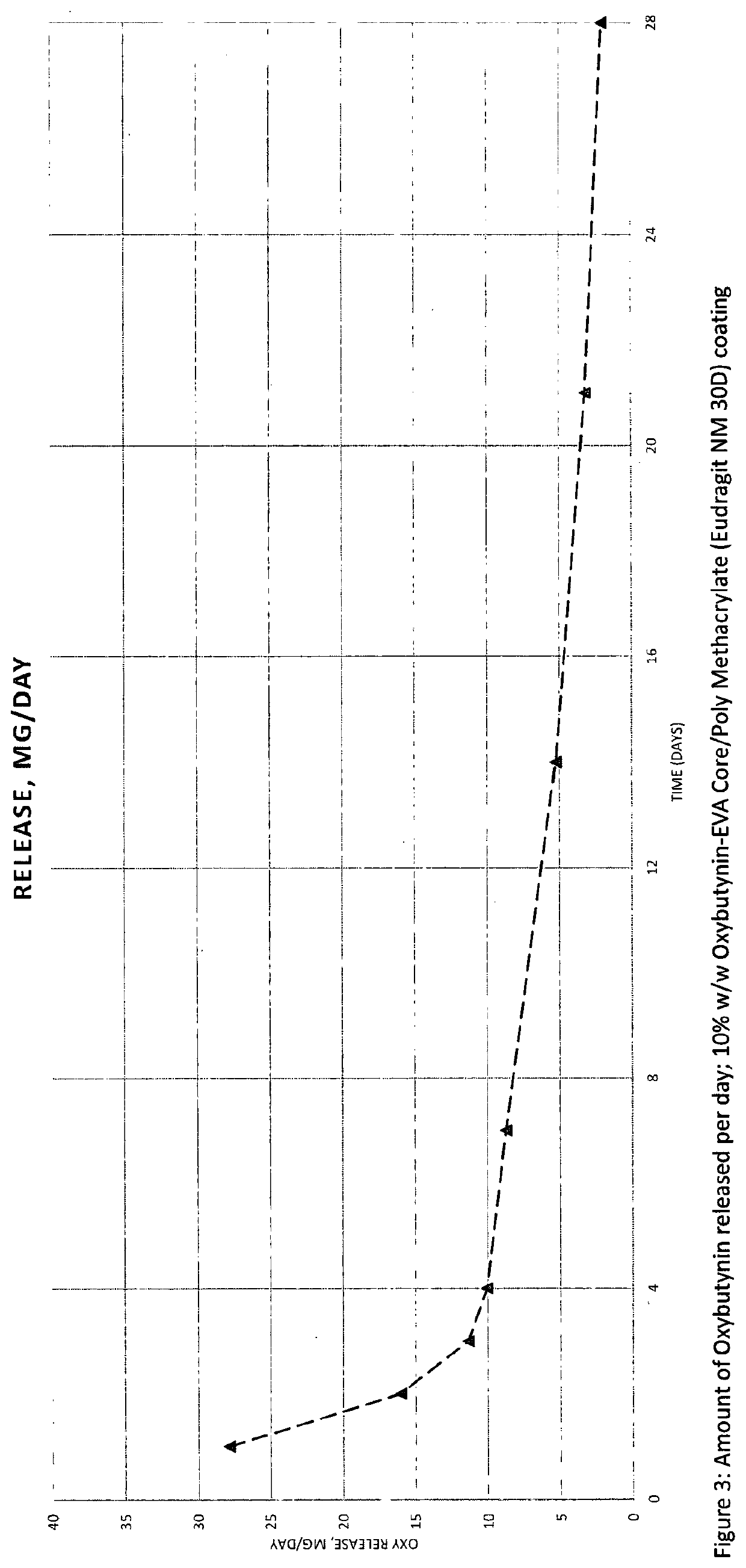
Implantable drug delivery device
PendingUS20200206130A1Improved controlled releasePrevents moisture ingressOrganic active ingredientsPharmaceutical delivery mechanismControl releaseAcrylate ester
Disclosed herein are drug delivery devices and their use as a means of achieving controlled release of one or more active pharmaceutical ingredients. In particular, disclosed herein is an implantable drug delivery device comprising an inner core comprising one or more active pharmaceutical ingredients distributed throughout a first water-insoluble polymer; an intermediate coating positioned around the inner core, said intermediate coating comprising an acrylate polymer; and an outer coating positioned around the intermediate coating, said outer coating comprising a second water-insoluble polymer; wherein the acrylate polymer is formed from one or more monomers of formula (I).
Owner:JUNIPER PHARMA UK LTD
Rheumatic bone-setting transdermal patch and preparation process thereof
InactiveCN101658555BEasy to carry and useStrong medicineAntipyreticAnalgesicsTransdermal patchMedicine
The invention relates to a rheumatic bone-setting transdermal patch which is formed by dactylicapnos root, cortex picrasmae, paniculate swallowwort root and rhizoma panacis M which are extracted, and effective parts are refined, and then transdermal patch excipients are added to prepare the transdermal patch; the transdermal patch obtained by the invention has the characteristics of being convenient for carrying and using, high transdermal adsorption ratio, high effective ingredients and strong treating effect.
Owner:北京化药科创医药科技发展有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com