Crystal form A of sofosbuvir and preparation method thereof
A technology of crystal form and powder diffraction pattern, which is applied to the crystal form A of sofosbuvir and its preparation field, can solve problems such as not being suitable for industrial production, and achieve the effects of reducing drug loading, improving drug efficacy, and high solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0070] The preparation method of sofosbuvir crystal form A
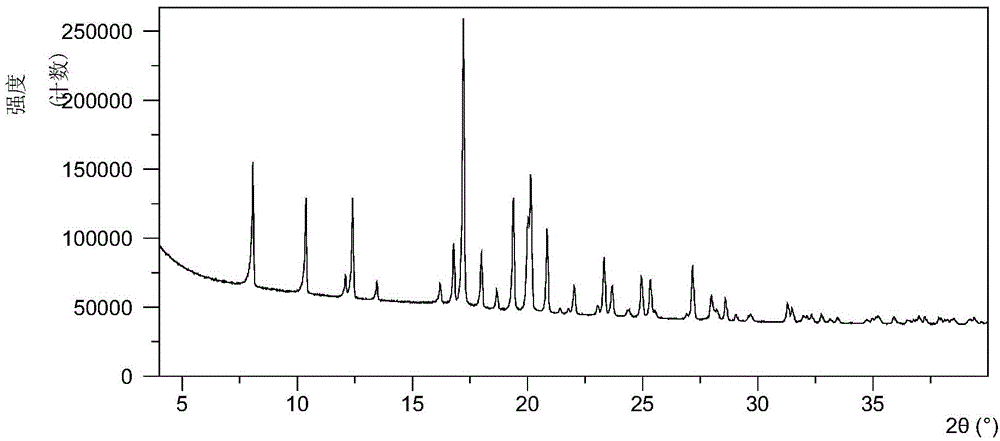
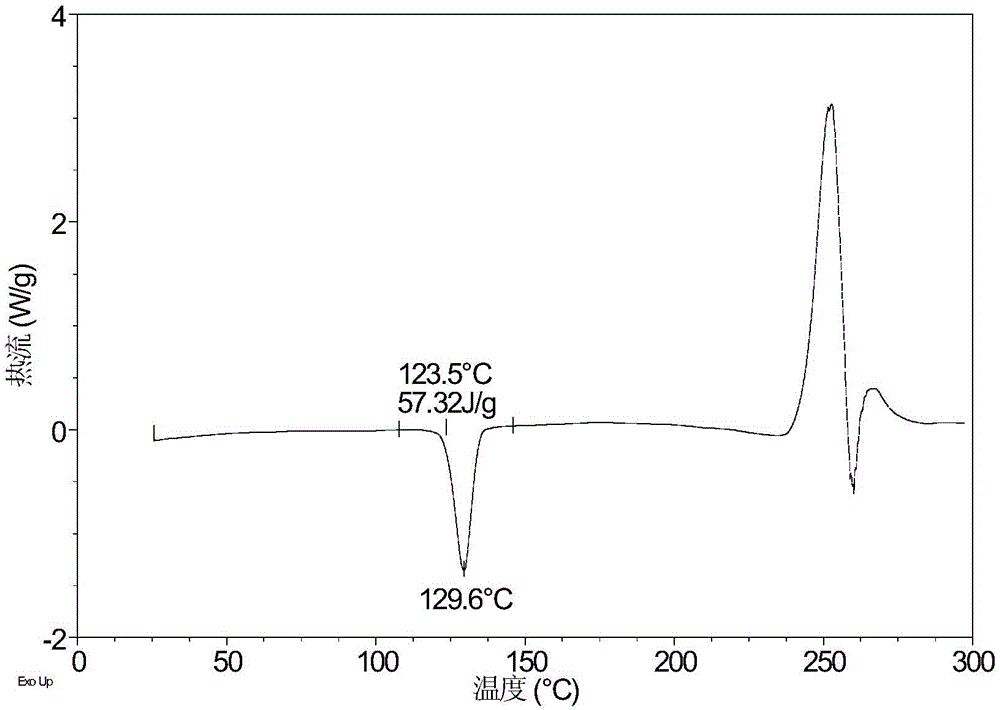
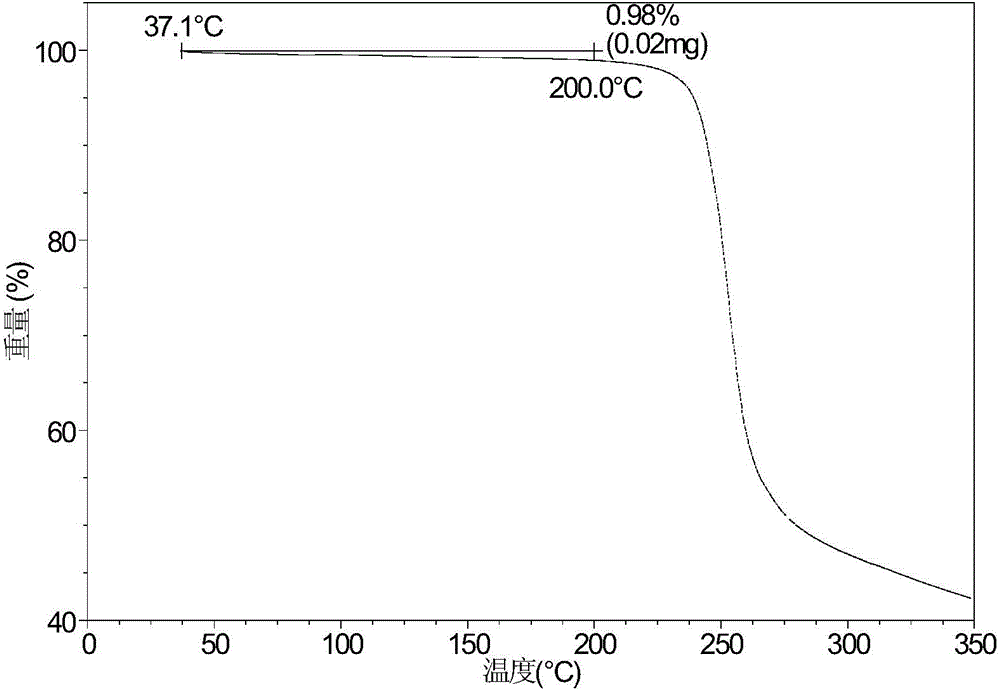
[0071] Take 5.59 mg of sofosbuvir powder and dissolve it in 0.1 mL of ethyl acetate and n-heptane (volume ratio 1:1) mixed solvent, then add 1 mg of high polymer, and volatilize at room temperature to obtain it. Table 2 shows the X-ray powder diffraction data of Form A obtained in this example. Its XRPD pattern is as follows figure 1 , and its DSC graph is shown in figure 2 , and its TGA figure is shown in image 3 .
[0072] Table 2 X-ray powder diffraction data of Form A
[0073] 2theta
[0074] 13.46
Embodiment 2
[0076] The preparation method of sofosbuvir crystal form A
[0077] Dissolve 5.76mg of sofosbuvir powder in 0.1mL of methyl tert-butyl ether and n-heptane (volume ratio 1:1) mixed solvent to obtain a clear solution, then add 1mg of high polymer, and volatilize at room temperature available. Table 3 shows the X-ray powder diffraction data of Form A obtained in this example.
[0078] Table 3 X-ray powder diffraction data of Form A
[0079] 2theta
Embodiment 3
[0081] The preparation method of sofosbuvir crystal form A
[0082] Dissolve 5.58 mg of sofosbuvir in 0.1 mL of methyl isobutyl ketone to obtain a clear solution, then add 1 mg of high polymer, and volatilize at room temperature to obtain. Table 4 shows the X-ray powder diffraction data of Form A obtained in this example.
[0083] Table 4 X-ray powder diffraction data of Form A
[0084] 2theta
[0085] 8.10
[0086] 32.35
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com