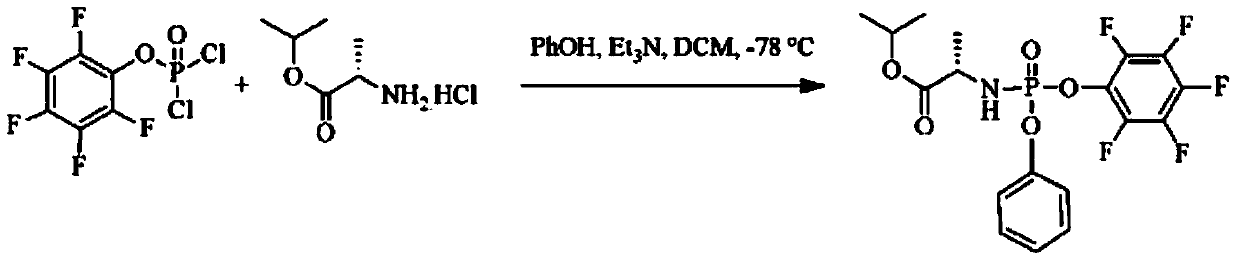
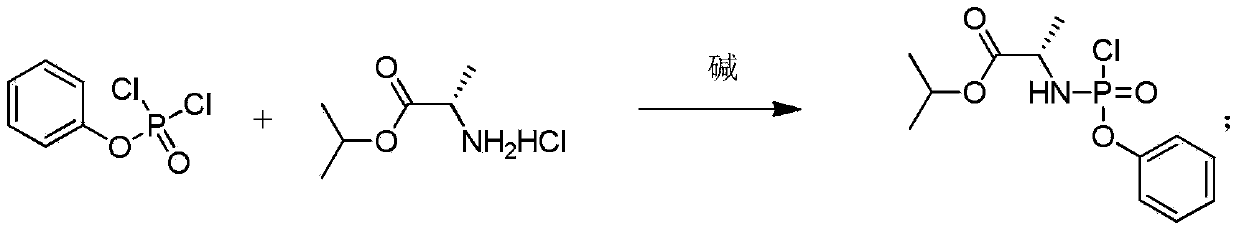
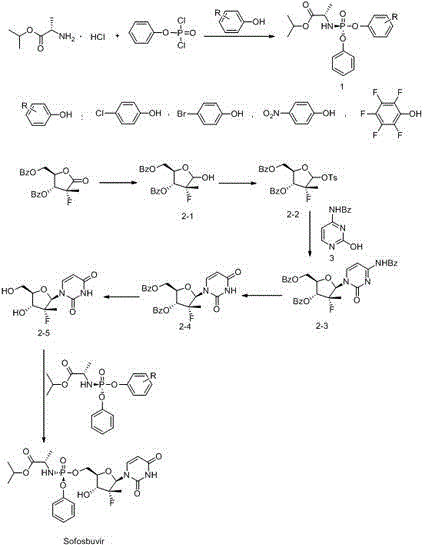
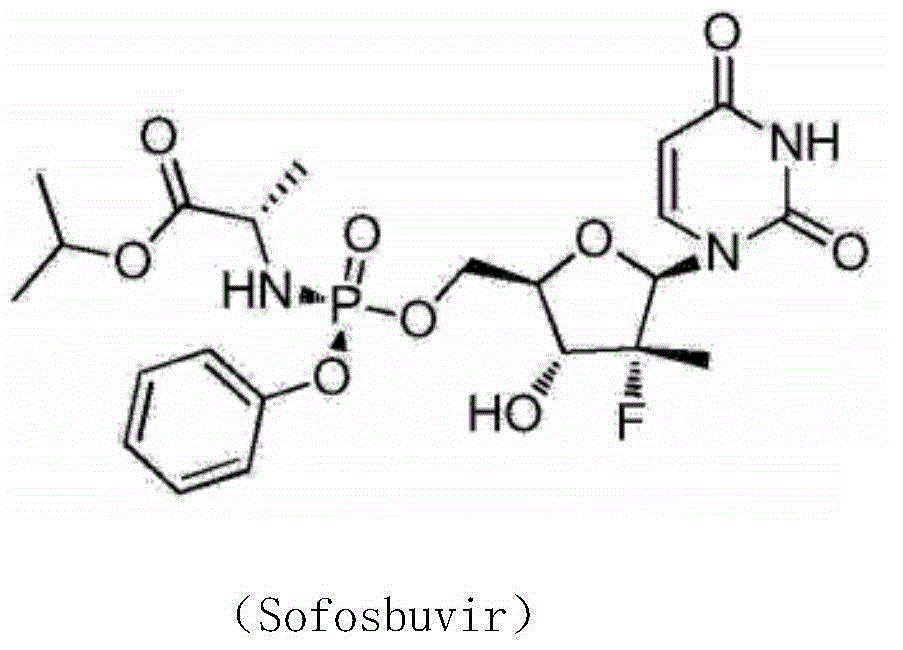
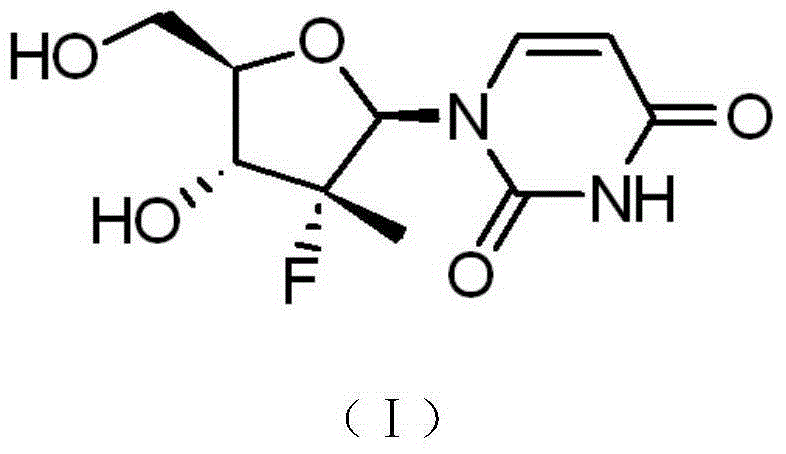
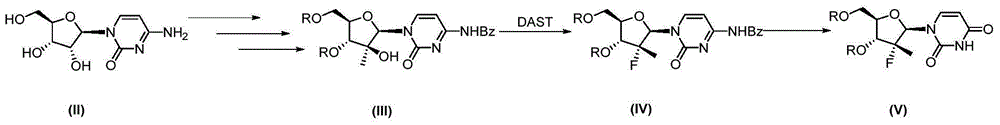
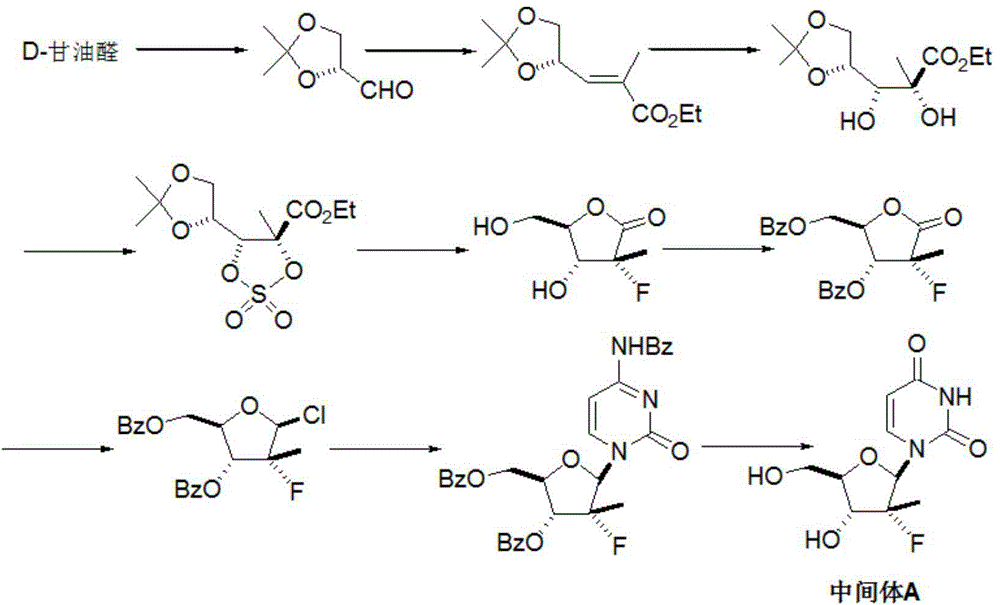
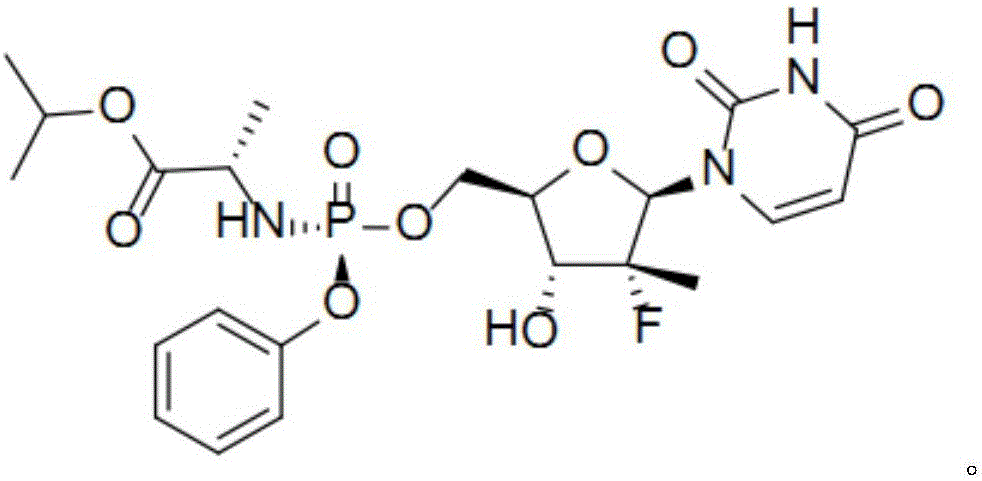
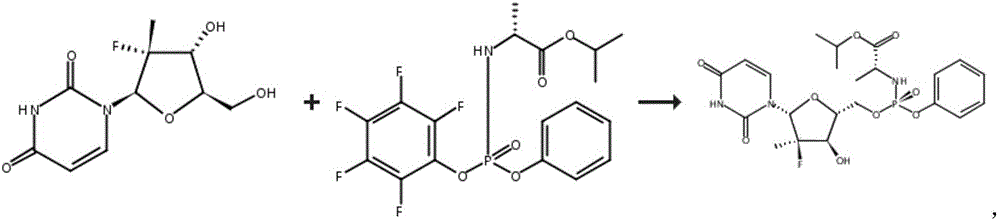
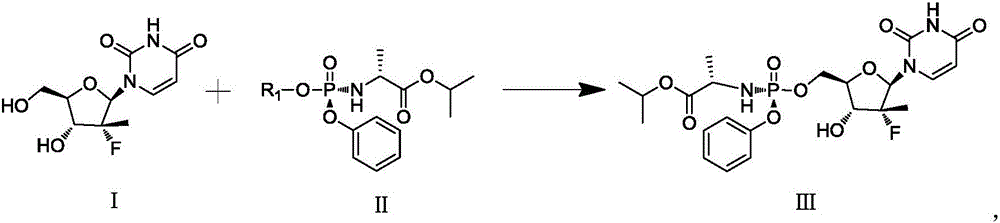
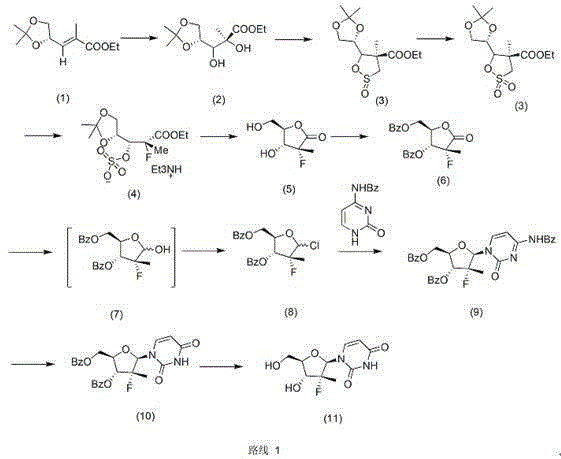
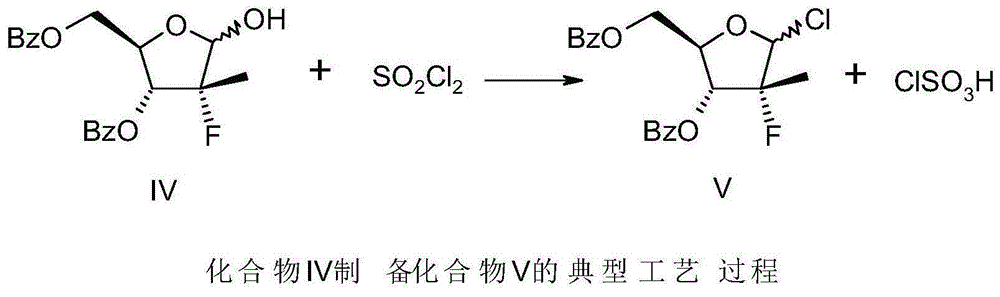
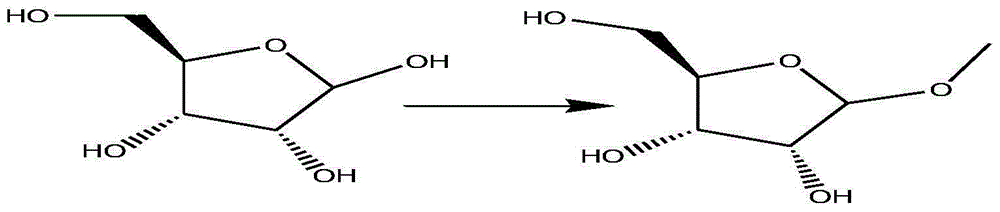
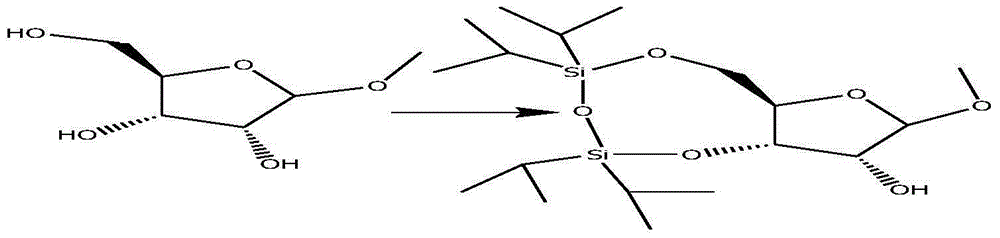
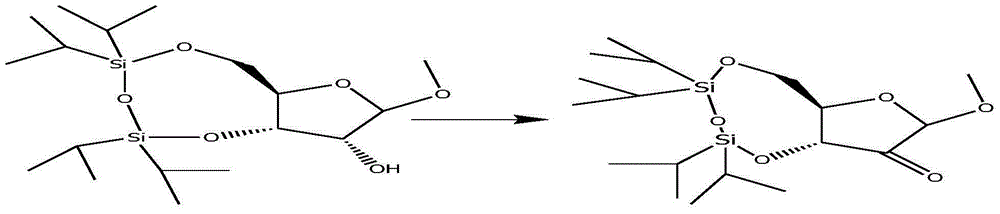
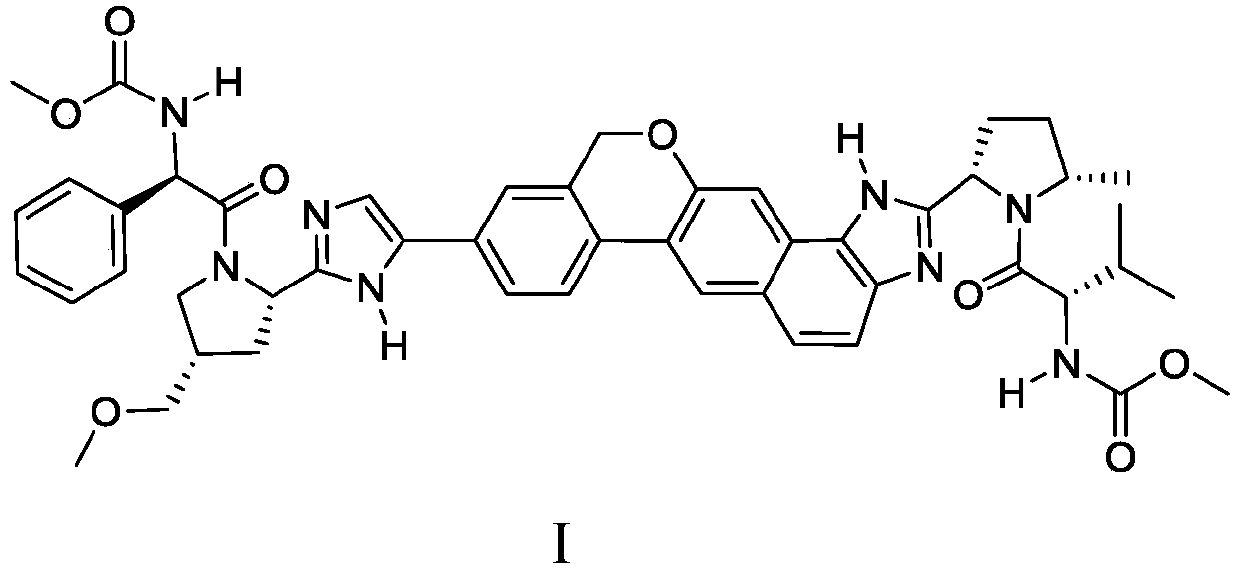
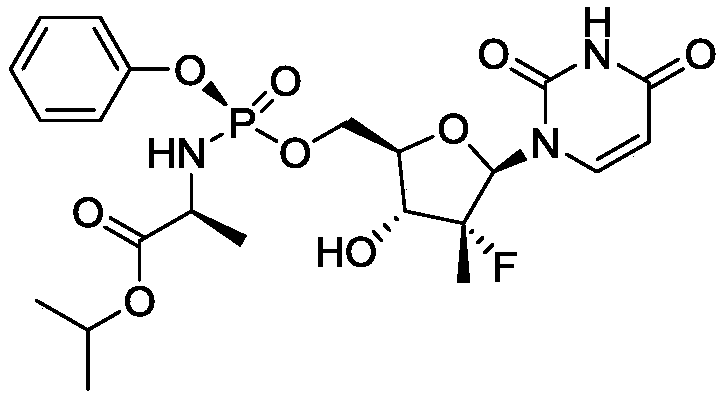
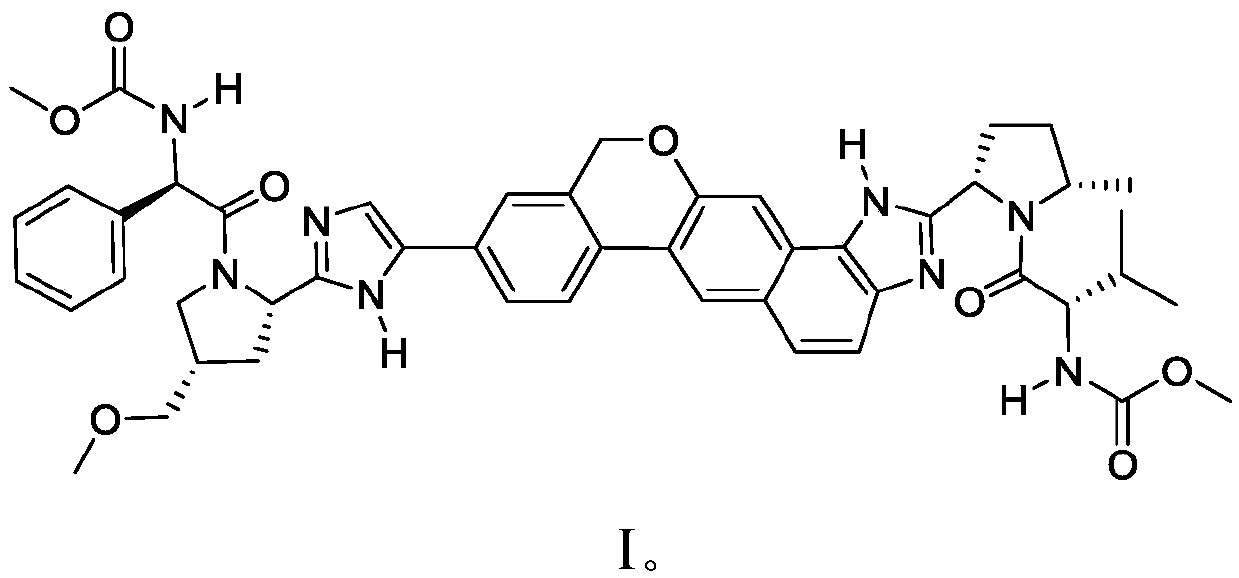
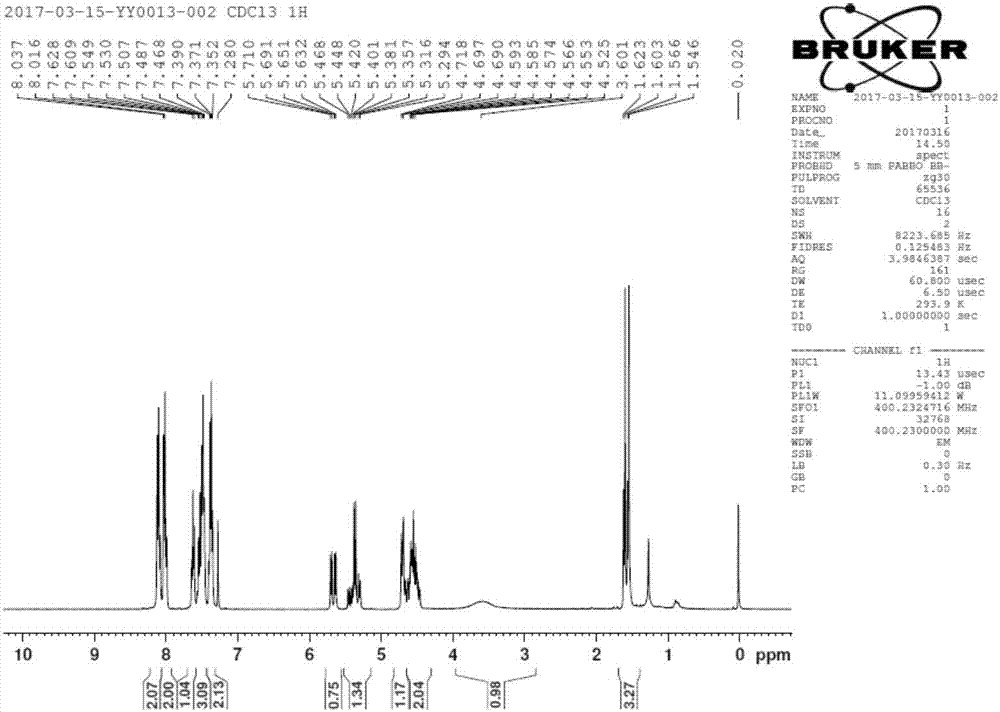
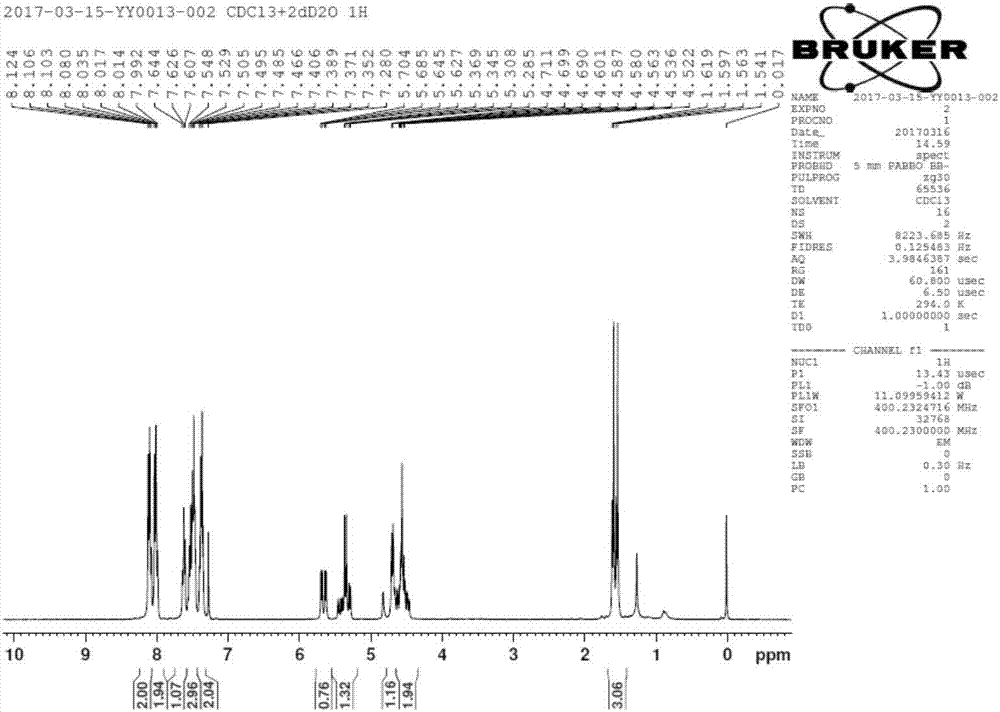
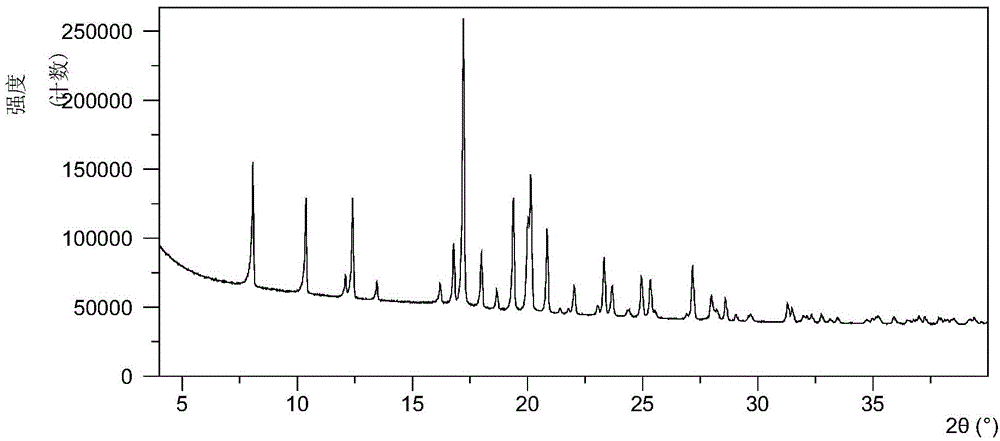
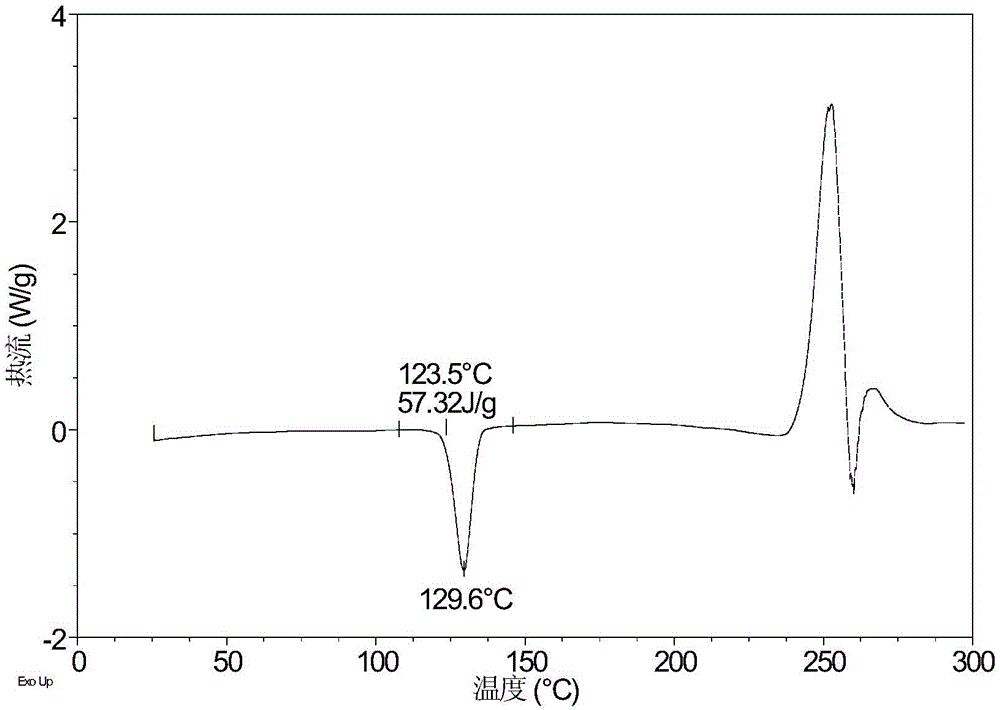
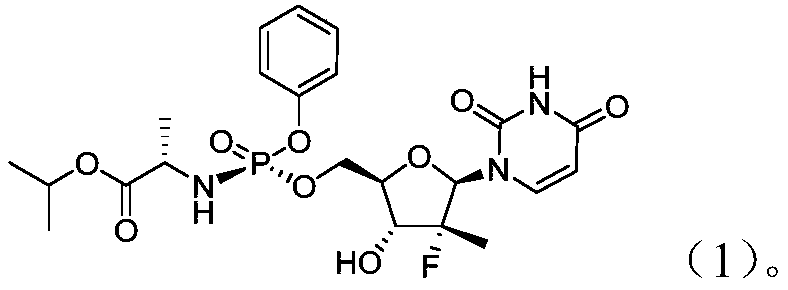
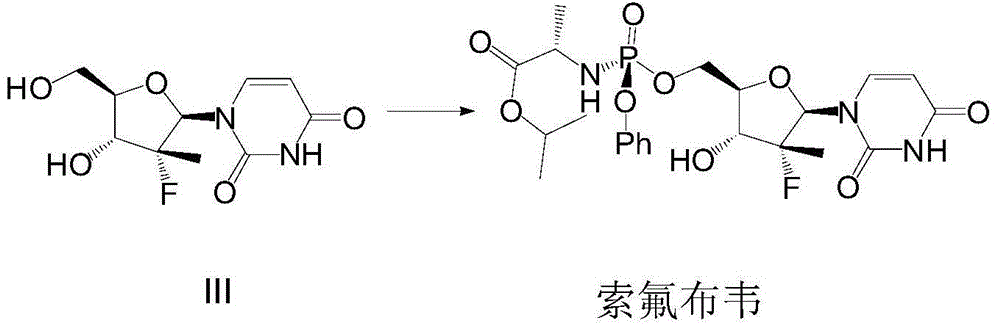
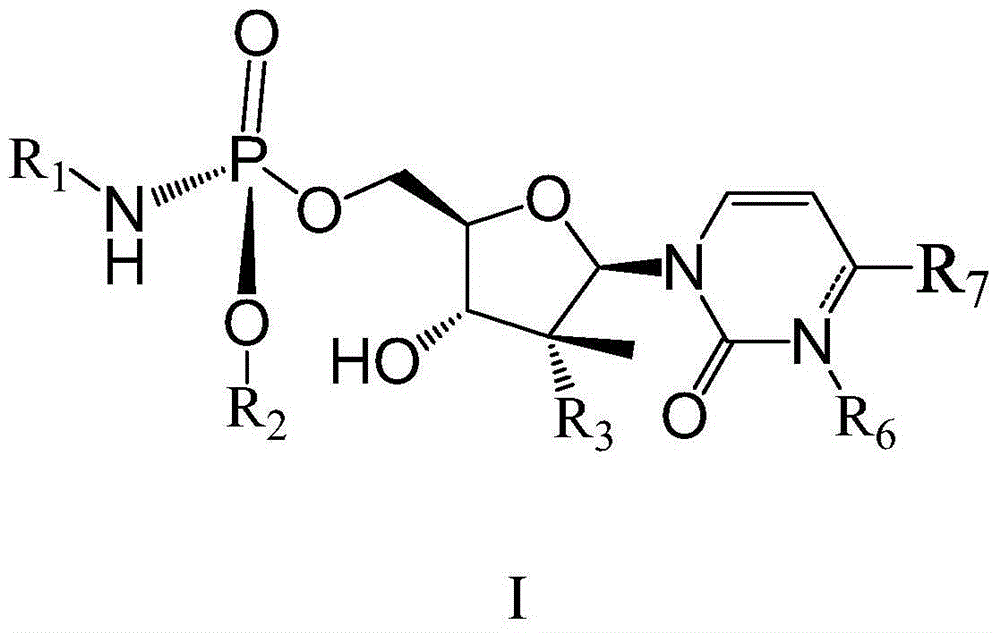
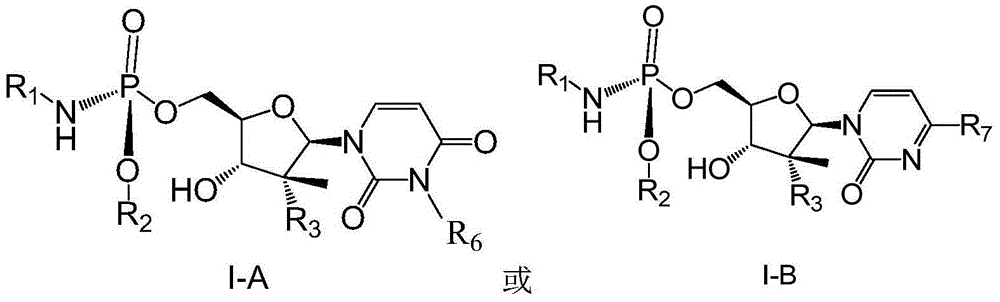
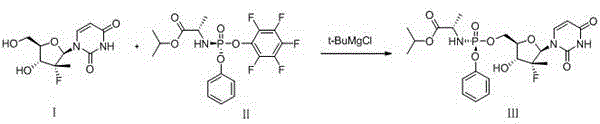
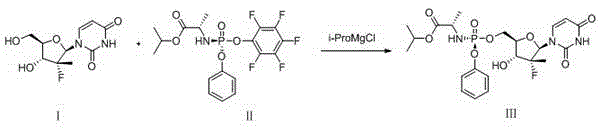
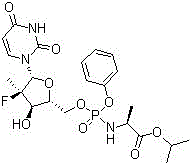
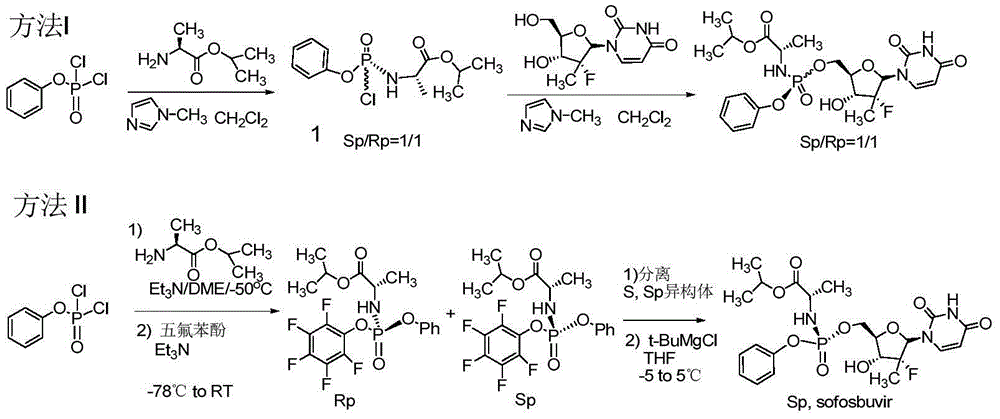
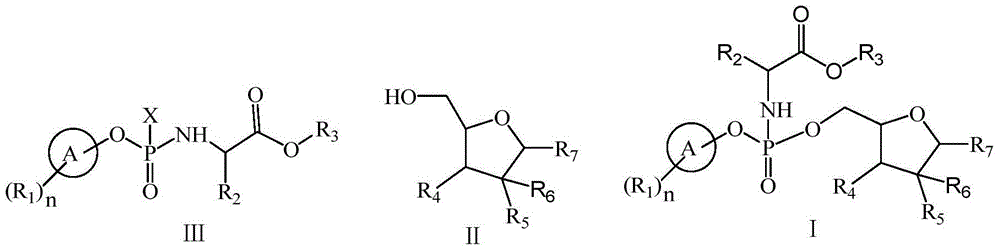
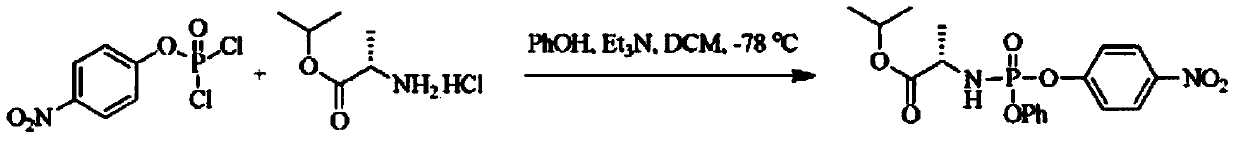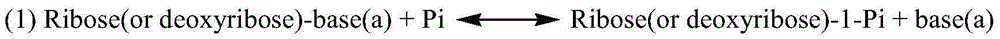Patents
Literature
208 results about "Sofosbuvir" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
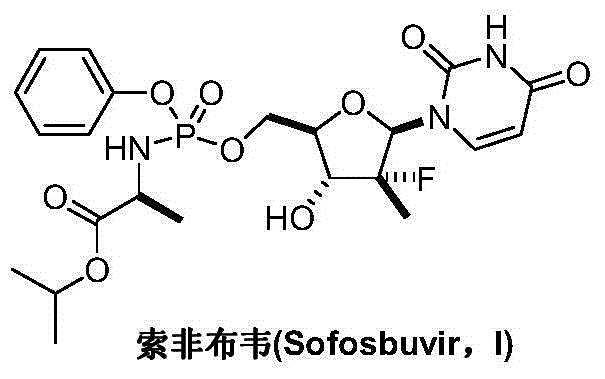
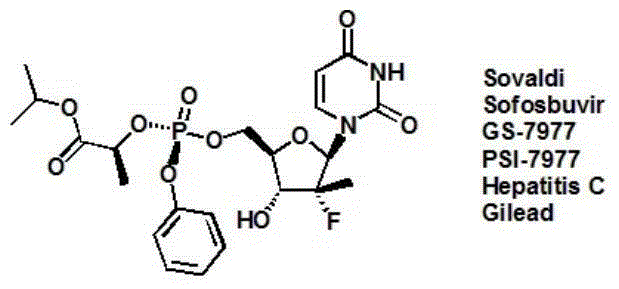
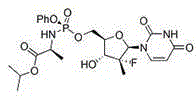
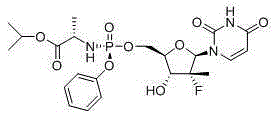
Sofosbuvir is used with other antiviral medications (such as ribavirin, peginterferon, daclatasvir) to treat chronic (long-lasting) hepatitis C, a viral infection of the liver.
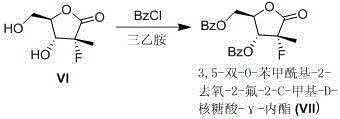
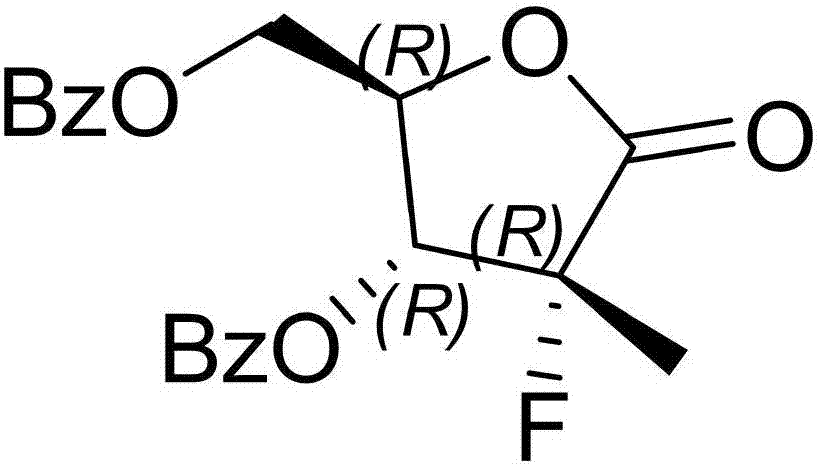
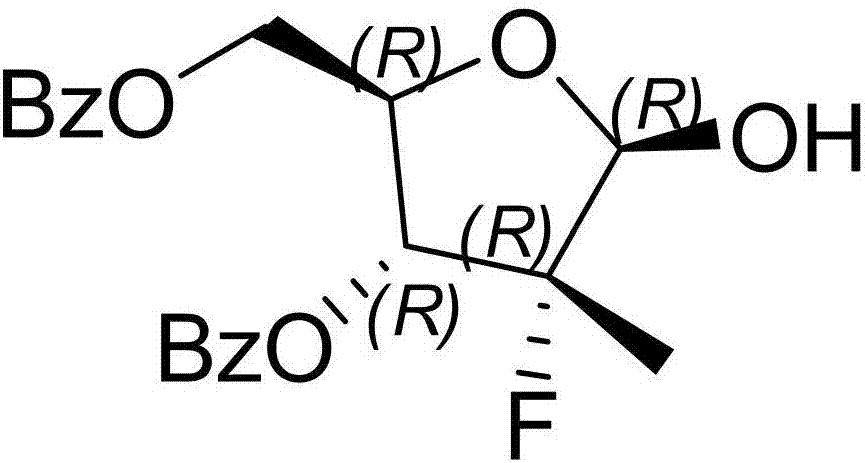
Preparation method of 3,5-dibenzoyl-2-deoxy-2-fluoro-2-methyl-D-ribo-gamma-lactone
InactiveCN103804446AMild reaction conditionsEasy to operateSugar derivativesSugar derivatives preparationAlkanePhenacyl
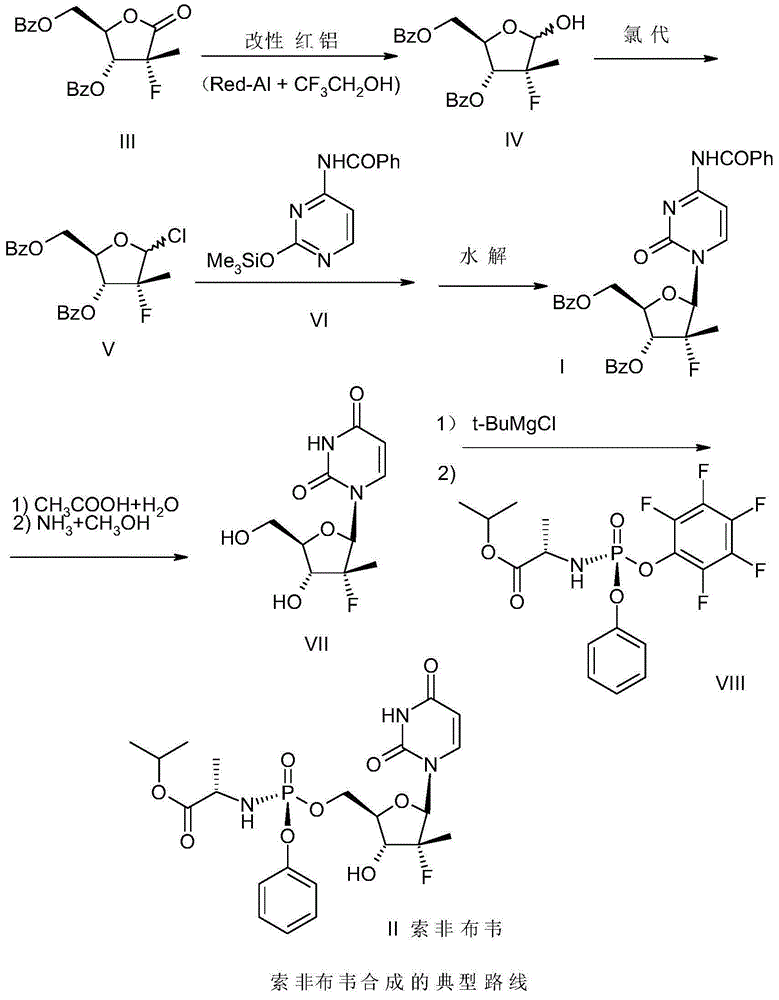
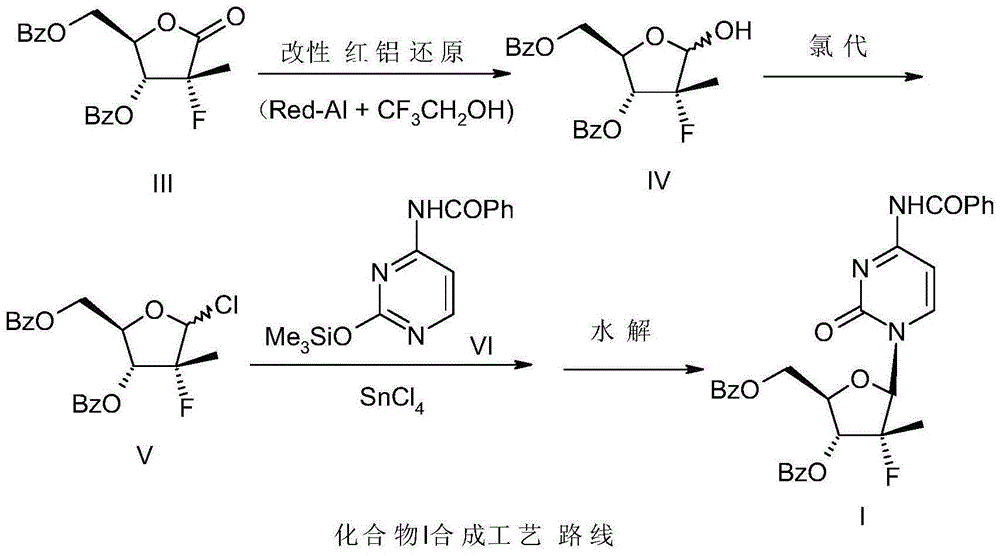
The invention provides a preparation method of 3,5-dibenzoyl-2-deoxy-2-fluoro-2-methyl-D-ribo-gamma-lactone (sofosbuvir). The method comprises the following steps: adding alkali into a mixed solution of L-alanine isopropyl ester or an acid salt thereof and phenyl dichlorophosphate for reacting to obtain (S)-2-phenoxy-chloro-phosphoryl amino isopropyl propionate, reacting the (S)-2-phenoxy-chloro-phosphoryl amino isopropyl propionate with 4-trifluoromethylphenol in the presence of alkali at the temperature of 0-10 DEG C to obtain racemic (S)-2-[(4-trifluoromethyl-phenoxyl)-phenoxy-phosphoryl amino)]alanine isopropyl ester, dissolving the racemic (S)-2-[(4-trifluoromethyl-phenoxyl)-phenoxy-phosphoryl amino)]alanine isopropyl ester into an ether or alkane solvent at the normal temperature, cooling to 50-10 DEG C below zero, and separating (S)-2-[(S)-(4-trifluoromethyl-phenoxy)]-phenoxy-phosphoryl amino)]alanine isopropyl ester out; and reacting the (S)-2-[(S)-(4-trifluoromethyl-phenoxy)]-phenoxy-phosphoryl amino)]alanine isopropyl ester with (2'R)-2'-deoxy-2'-fluoro-2'-methyluridine in the presence of organic alkali with high steric hindrance to obtain the 3,5-dibenzoyl-2-deoxy-2-fluoro-2-methyl-D-ribo-gamma-lactone. The method has the advantages of simple steps, mild reaction and suitability for industrial production.
Owner:苏州东南药业股份有限公司
Preparation method of sofosbuvir intermediate
ActiveCN104151352AEnvironmental protection and green patentsEnvironmental Protection and Green LiteratureGroup 5/15 element organic compoundsKetone solventsKinetic resolution
The invention discloses a preparation method of a sofosbuvir intermediate, which comprises the following step: in an anhydrous non-protonic solvent, carrying out dynamic kinetic resolution on a compound disclosed as Formula (II) under the action of an organic alkali and / or inorganic alkali to prepare a compound disclosed as Formula (I), wherein the non-protonic solvent is one or more of ester solvent, ketone solvent and ether solvent, the organic alkali and / or inorganic alkali account / accounts for 0.1-10 wt% of the compound disclosed as Formula (II), the temperature of the dynamic kinetic resolution is 10-30 DEG C, and the time of the dynamic kinetic resolution is 5-10 hours. The preparation method has the advantages of high conversion rate, high product purity and low cost, and is environment-friendly.
Owner:CHEMVON BIOTECH CO LTD
Preparation method of ribofuranose phosphate derivative
ActiveCN104610404APurity is easy to controlHigh yield of docking reactionSugar derivativesSugar derivatives preparationPhosphateGrignard reagent
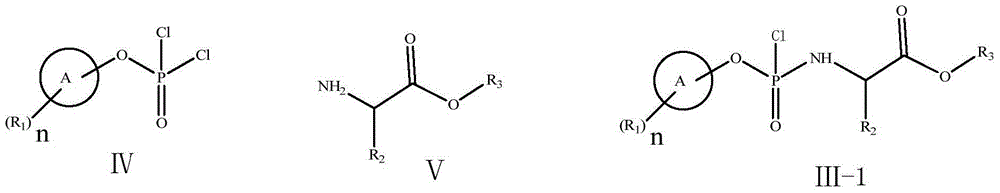
The invention discloses a preparation method of a ribofuranose phosphate derivative. The preparation method comprises preparation steps as follows: L-alanine isopropyl ester hydrochloride, phenol dichlorophosphate and substituted phenol are taken as starting materials and have a docking reaction under the action of alkali; (2R)-2-deoxy-2-difluoro-2-methyl-D-erythropentonic acid GAMMA-lactone and 3,5-dibenzoate reduce carbonyl in a dichloromethane or ether solvent into an alcoholic hydroxyl group under the action of a strong reducing agent; an intermediate with a formula 2-1 has a reaction with paratoluensulfonyl chloride under the action of alkali to obtain p-toluenesulfonates; an intermediate with a formula 2-2 and a benzoyl cytosine derivative have a docking reaction under the action of a condensing agent; an intermediate with a formula 2-3 converts cytosine into uracil under the action of organic acid; benzoyl protection for an intermediate with a formula 2-4 is released under the action of an alkaline agent; an intermediate with a formula 2-5 and an intermediate with a formula 1 are docked under the action of a Grignard reagent to obtain Sofosbuvir.
Owner:NANTONG CHANGYOO PHARMATECH CO LTD
Crystal forms of sofosbuvir and preparation method of crystal forms
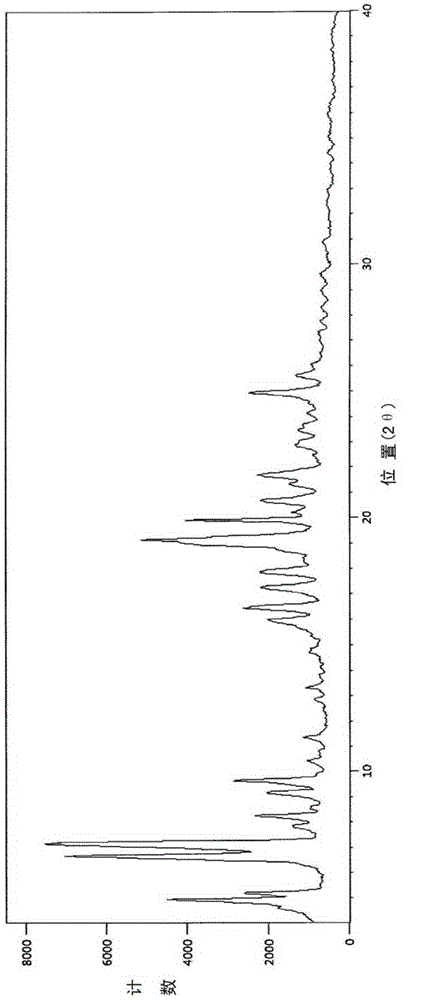
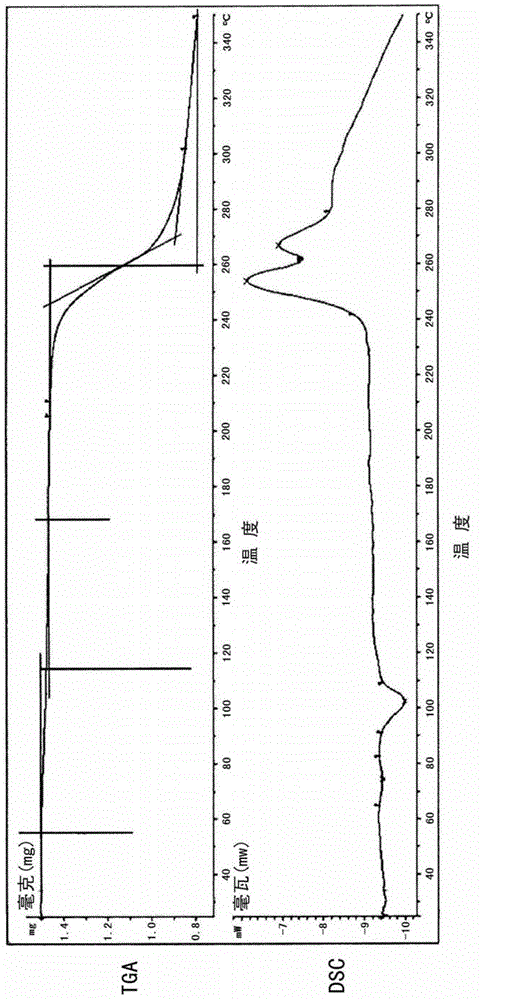
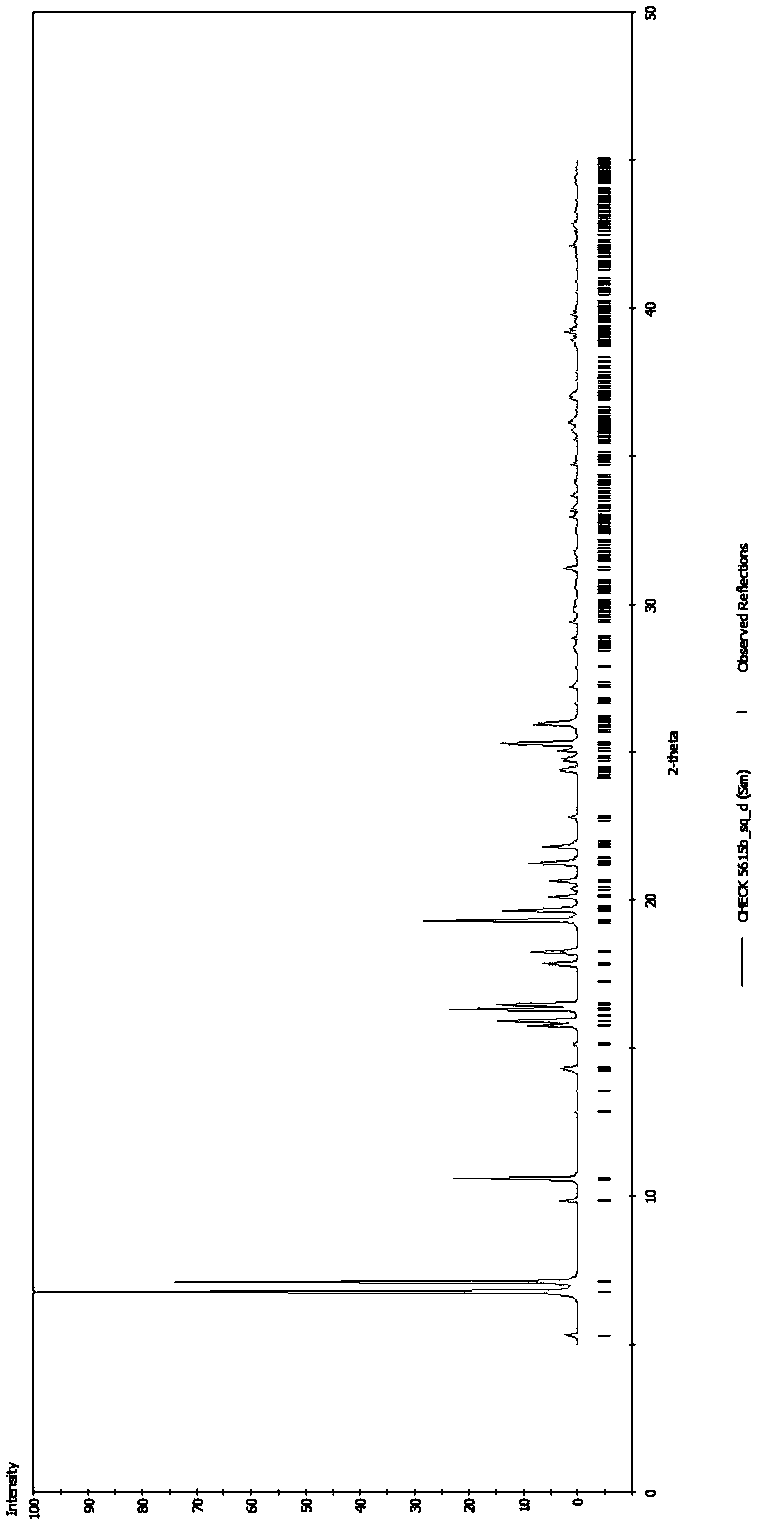
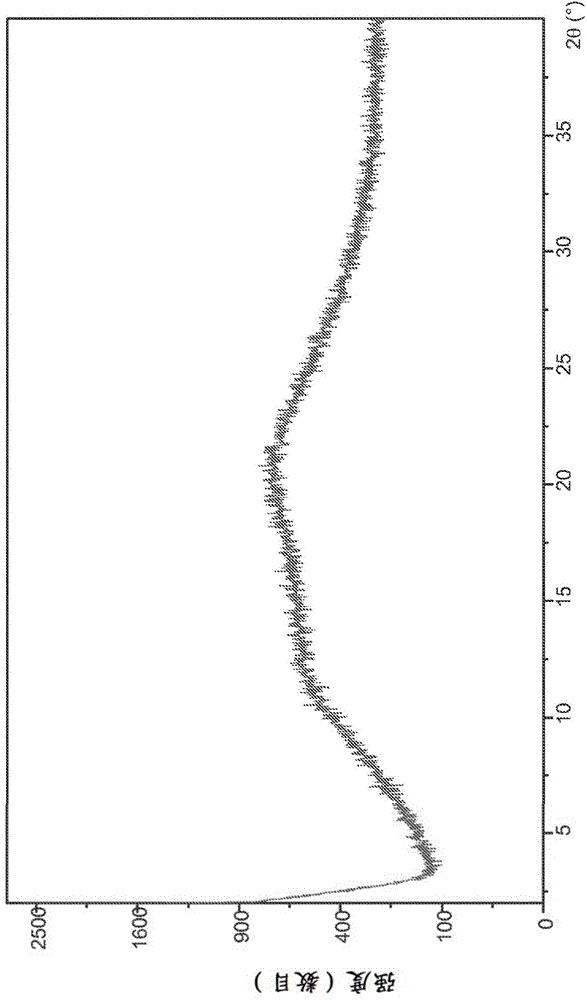
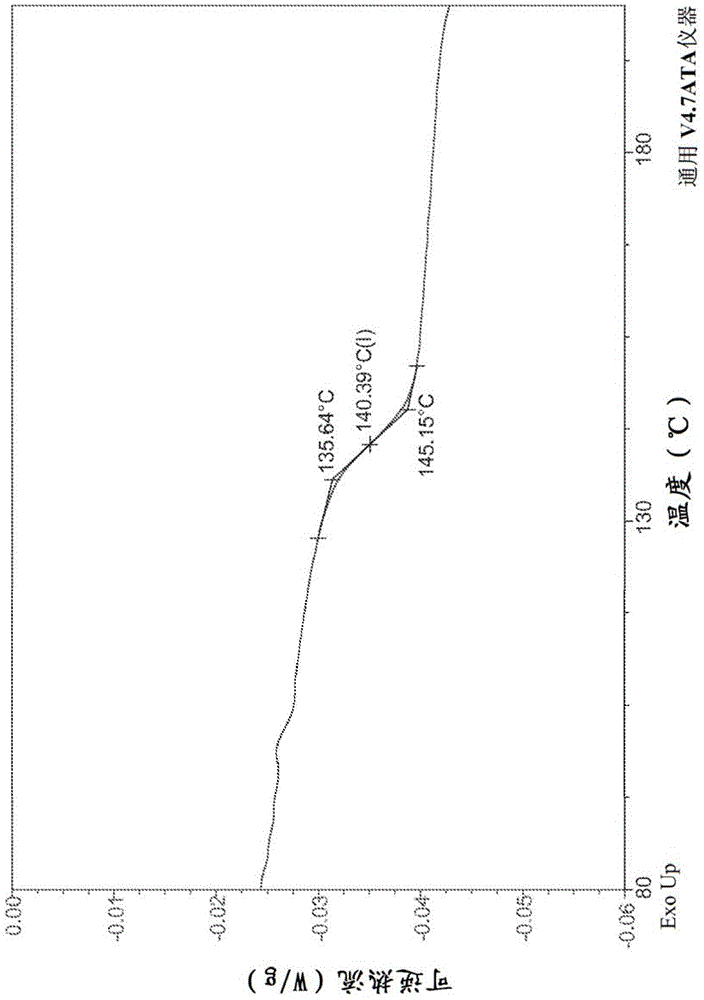
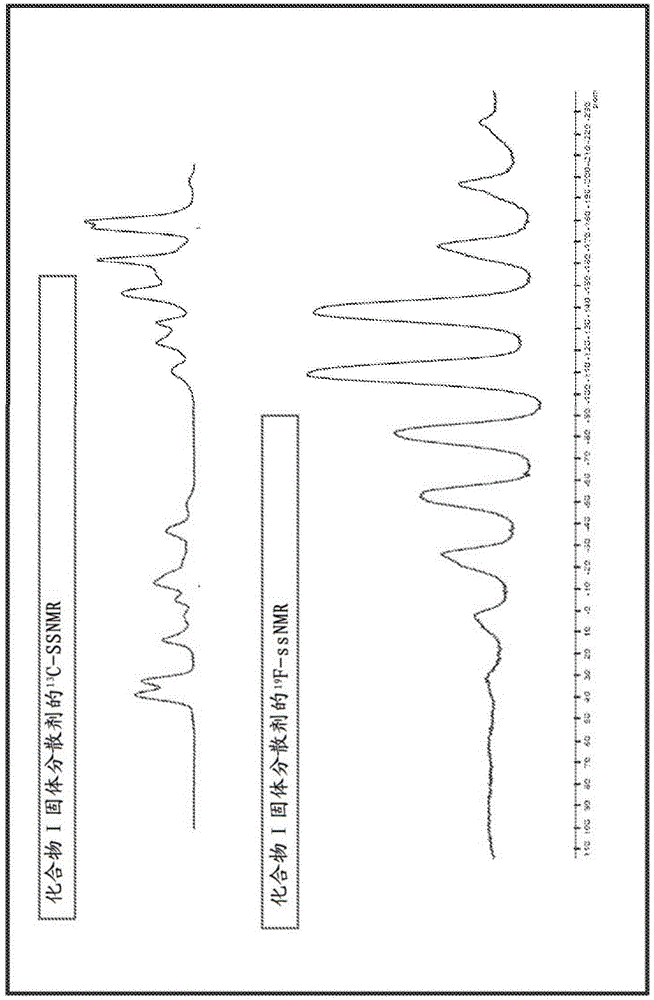
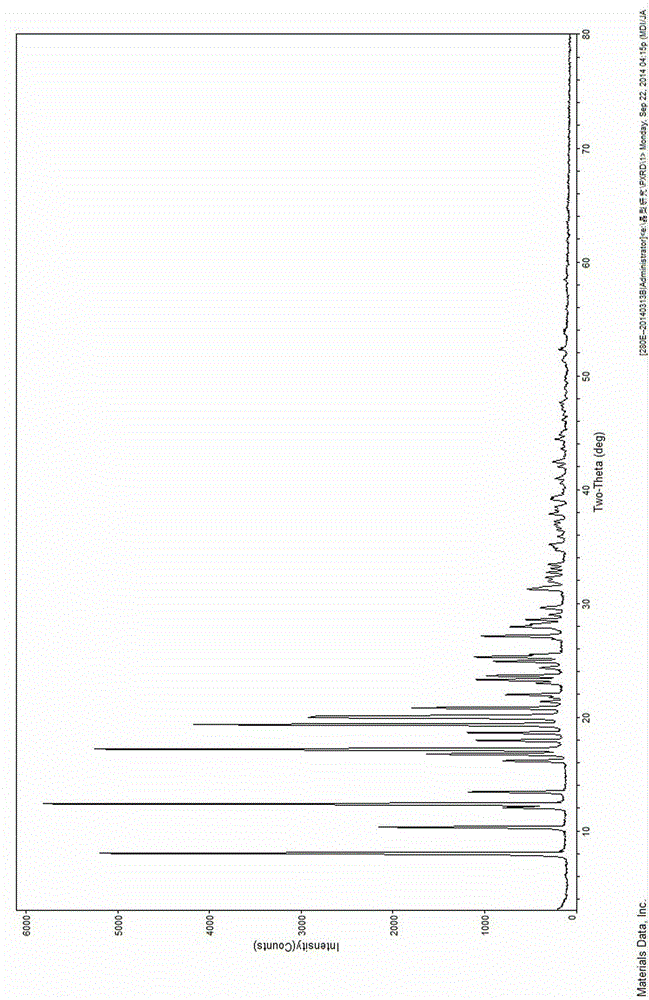
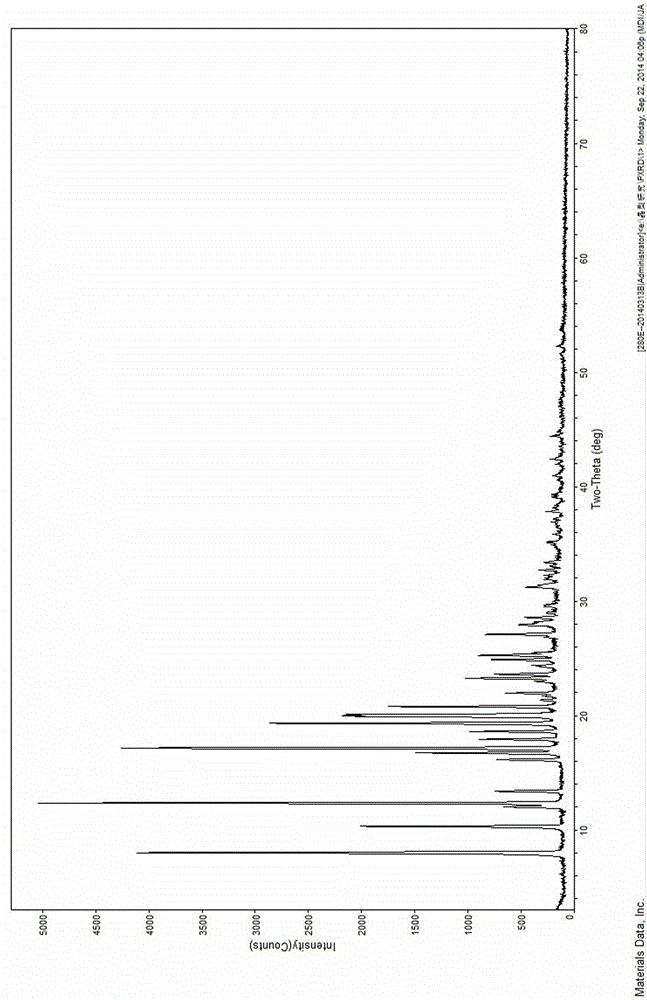
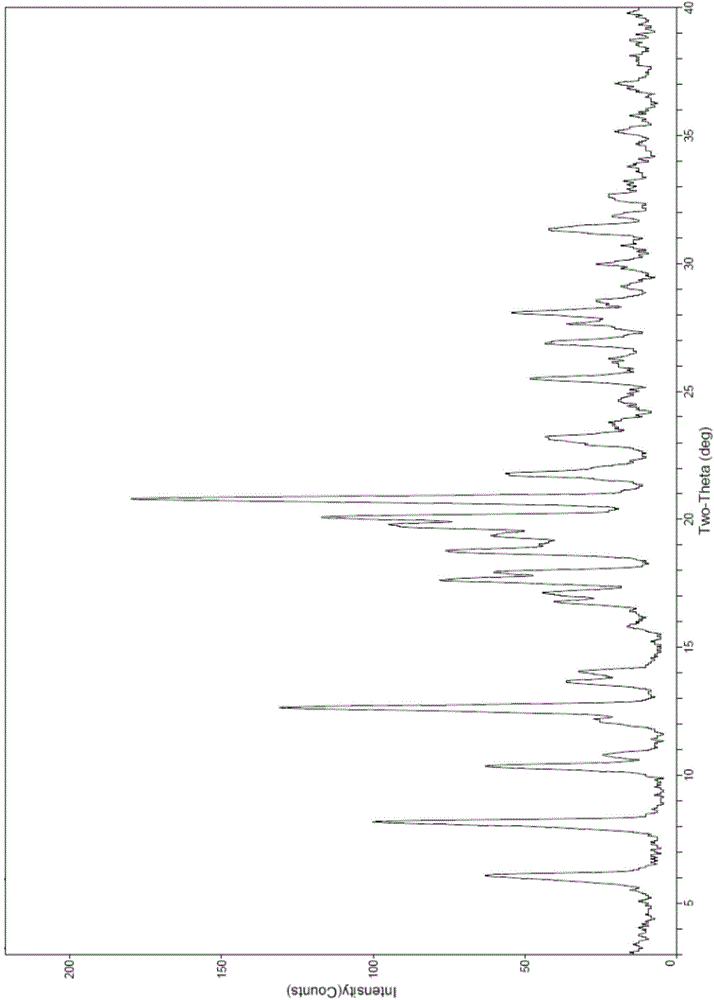
The invention discloses four novel crystal forms H1, H2, H3 and H4 of sofosbuvir, and a method for preparing the four crystal forms. The structures and the lattice parameters of the four novel crystal forms of the sofosbuvir are determined by monocrystal X-ray diffraction analysis; characteristic peaks of the crystal form H1 are formed at the diffraction angles 2theta of 4.9 degrees, 6.6 degrees, 7.1 degrees, 8.2 degrees, 9.6 degrees, 16.5 degrees, 19.0 degrees, 19.2 degrees, 19.9 degrees, 24.9 degrees and the like; the characteristic peaks of the crystal form H2 are formed at the diffraction angles 2theta of 6.7 degrees, 7.0 degrees, 9.7 degrees, 19.1 degrees, 19.3 degrees, 19.8 degrees, 20.3 degrees, 24.9 degrees, 25.1 degrees, 25.6 degrees and the like; the characteristic peaks of the crystal form H3 are formed at the diffraction angles 2theta of 5.1 degrees, 6.7 degrees, 7.1 degrees, 9.6 degrees, 15.8 degrees, 17.2 degrees, 19.3 degrees, 19.9 degrees, 20.7 degrees, 24.9 degrees and the like; and the characteristic peaks of the crystal form H4 are formed at the diffraction angles 2theta of 5.5 degrees, 5.6 degrees, 10.0 degrees, 10.9 degrees, 10.9 degrees, 16.6 degrees, 19.8 degrees, 20.0 degrees, 20.4 degrees, 25.0 degrees and the like. The four novel crystal forms of the sofosbuvir respectively have the advantages of excellent physical and chemical properties, good stability, good solubleness, simpleness in preparation and operation and the like.
Owner:CHARM PHARMATECH NANJING
Sofosbuvir film coating tablet preparation and preparation method thereof
InactiveCN104546783AWell mixedImprove liquidityOrganic active ingredientsDigestive systemPolythylene glycolStearic acid
The invention discloses a sofosbuvir film coating tablet preparation. The preparation is prepared from the following active ingredients in percentage by mass: 25.0-40.0 percent of sofosbuvir, 2.0-8.0 percent of a disintegrating agent, 50.0-80.0 percent of a diluting agent and 0.5-1.5 percent of a lubricating agent, wherein the disintegrating agent is one or more of croscarmellose sodium, hydroxypropyl methylcellulose and sodium carboxymethyl starch, one or two of sodium carboxymethylcellulose and hydroxypropyl methylcellulose as first choice, and preferably sodium carboxymethylcellulose; the diluting agent is one or more of microcrystalline cellulose, mannitol, lactose and polyethylene glycol, one or two of microcrystalline cellulose and mannitol as first choice, and preferably microcrystalline cellulose; and the lubricating agent is one or more of magnesium stearate, talcum powder, aerosil and calcium stearate, one or two of magnesium stearate and aerosil, and preferably magnesium stearate. The preparation has the advantages of simple process, high yield, good stability and the like, and is easy for large-scale industrial production.
Owner:ANHUI YELLEN PHARMA
Sofosbuvir monocrystal M and preparation method and applications of sofosbuvir monocrystal M
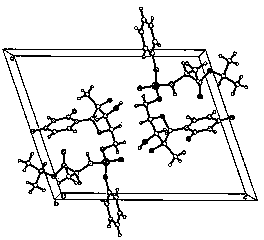
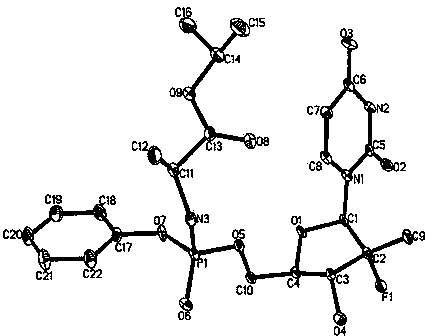
The invention discloses a sofosbuvir monocrystal M and a preparation method and applications of the sofosbuvir monocrystal M. The sofosbuvir monocrystal prepared according to the preparation method disclosed by the invention belongs to a monoclinic system, and the space group is P21; the unit cell parameters are as follows: a=13.816 (3) A, b=5.973 (2) A, c=17.666 (4) A, and Beta=108.96 (3); the unit cell volume is 1378.7 (3) A3, and the number Z of molecules in a unit cell is 2. Solvents selected in the sofosbuvir monocrystal M disclosed by the invention are methylene chloride, chloroform, ethanol, isopropanol, acetonitrile, and C3-C7 alkyl cyanoacetate or C3-C7 dialkyl carbonate or C3-C7 dialkyl oxalate, and the like, and the sofosbuvir monocrystal is prepared by using a solvent evaporation method. The sofosbuvir monocrystal prepared according to the preparation method disclosed by the invention has good stability.
Owner:汤律进 +3
Synthesis method of intermediate compound of sofosbuvir
InactiveCN104987355AHigh yieldMild reaction conditionsSugar derivativesSugar derivatives preparationSodium methoxideSynthesis methods
The invention provides a synthesis method of an intermediate compound of sofosbuvir as shown in formula (I) (see specification). The synthesis method of the intermediate compound of sofosbuvir, a synthetic route of which is as follows: (see specification) the synthesis method comprises the following steps: taking (3R, 4R, 5R)-3-fluoro-dihydro-4-hydroxl3-methyl furan-2(3H)-ketone as an initial raw material to react with pivaloyl chloride to generate an intermediate product (XII); reducing the intermediate product to obtain an intermediate product (XIII); enabling the intermediate product (XIII) to react with pivaloyl chloride to generate an intermediate product (XIV); generating an intermediate product (XV) by virtue of the reaction of the intermediate product (XIV) and a hydrogen bromide; performing silyl-hilbert-johnson reaction for the intermediate product (XV) and uracil to generate an intermediate product (XVI); enabling the intermediate product (XVI) to react with sodium methoxide to obtain a target product. The synthesis method of the intermediate compound (I) is moderate in reaction condition, simple in procedure, low in cost, environment-friendly and favorable for the industrialized production.
Owner:SHANGHAI TWISUN BIO PHARM
Preparation method for sofosbuvir
InactiveCN104478976AEase of industrial productionRaw materials are easy to getSugar derivativesSugar derivatives preparationUridine NucleotidesSofosbuvir
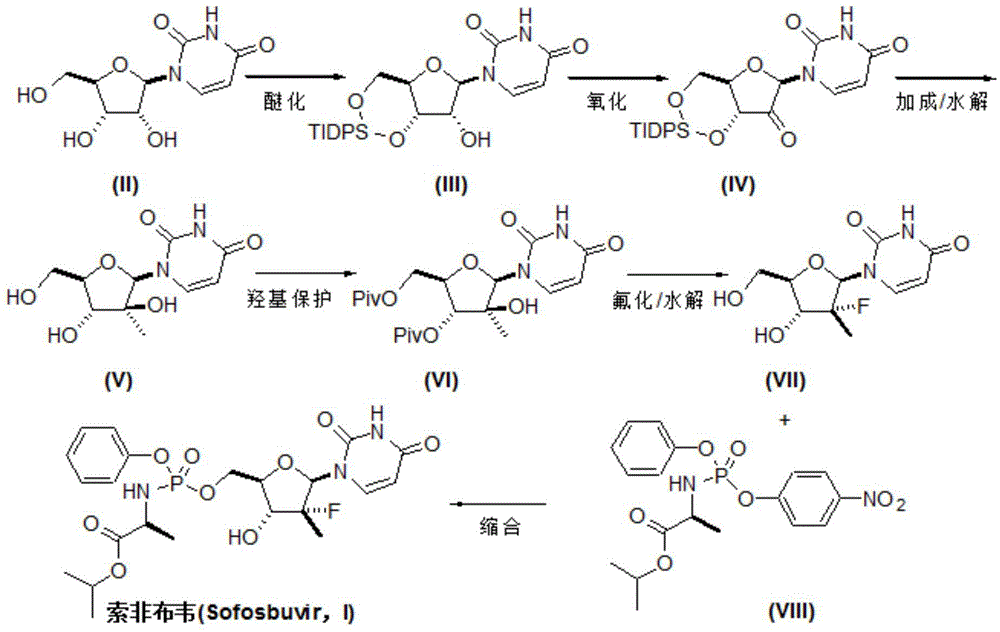
The invention discloses a preparation method for preparing sofosbuvir (Sofosbuvir,I) with uridine as a raw material and through etherification, oxidation, addition, condensation and other steps; the preparation method has the advantages of easily obtained raw materials, concise process, economy, and environmental protection, and is suitable for industrialized production.
Owner:SUZHOU MIRACPHARMA TECH
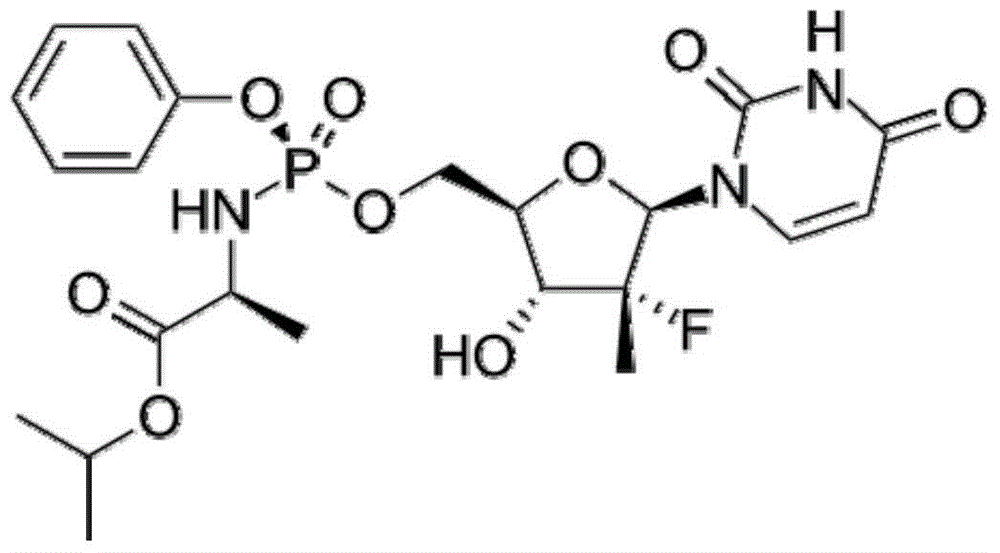
Antiviral nucleoside phosphoramidate and pharmaceutical composition and applications thereof
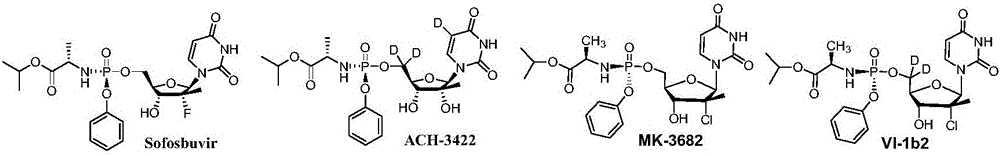
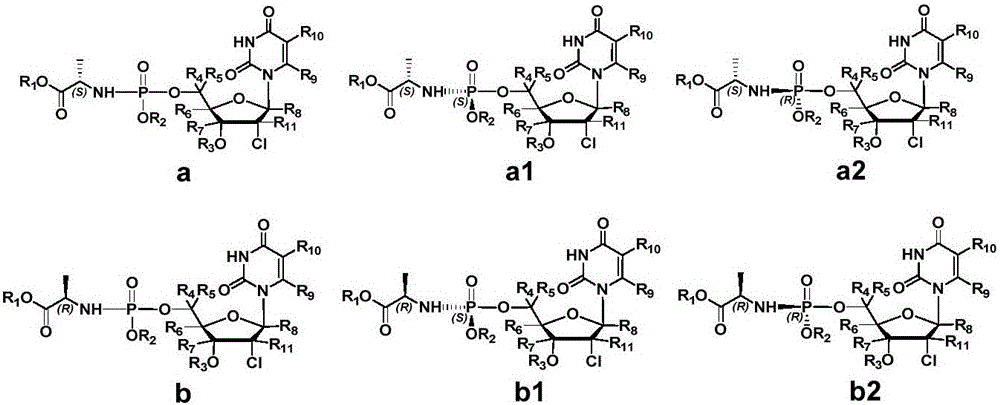
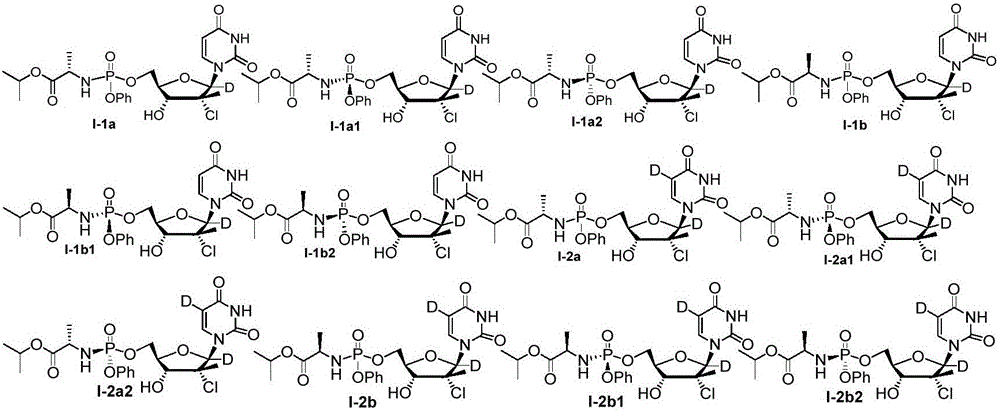
The invention provides an antiviral nucleoside phosphoramidate and a pharmaceutical composition and applications thereof. The nucleoside phosphoramidate compound is prepared by connecting nucleoside with phosphate through phosphorus-oxygen bonds. The structural formulas of the nucleoside phosphoramidate compound are represented by a, a1, a2, b, b1, and b2. The invention also discloses stereoisomers, pharmaceutically acceptable salts, hydrates, solvates, or crystals of the nucleoside phosphoramidate compound. The anti-hepatitis C activity of the provided novel nucleoside phosphoramidate is obviously better than that of sofosbuvir used in clinic. On the saccharide ring, the fluorine atoms are replaced by chlorine atoms, and the cytotoxicity of measure cell lines is prominently reduced. By systematically modifying and optimizing the basic groups, saccharide rings, and prodrugs, the anti-hepatitis C activity of partial synthesized compounds is 2 to 10 times higher than that of sofosbuvir. At the same time, the key parts of metabolism are optimized, the metabolism stability and chemical stability of synthesized compounds in plasma are better, compared with those of sofosbuvir.
Owner:南京甘宁生物科技有限公司
Combination formulation of two antiviral compounds
Disclosed are pharmaceutical compositions having an effective amount of substantially amorphous ledipasvir and an effective amount of substantially crystalline sofosbuvir.
Owner:GILEAD PHARMASSET LLC
Novel sofosbuvir crystal
The invention relates to a novel sofosbuvir crystal. The novel sofosbuvir crystal has good stability and dissolvability. The invention also provides a method for preparing the novel sofosbuvir crystal. The method realizes crystallization under action of a solvent and an anti-solvent. The method has good reappearance, easily realizes condition control, produces crystals with a high yield and high purity and is suitable for industrial large-scale production.
Owner:BEIJING WINSUNNY PHARMA CO LTD
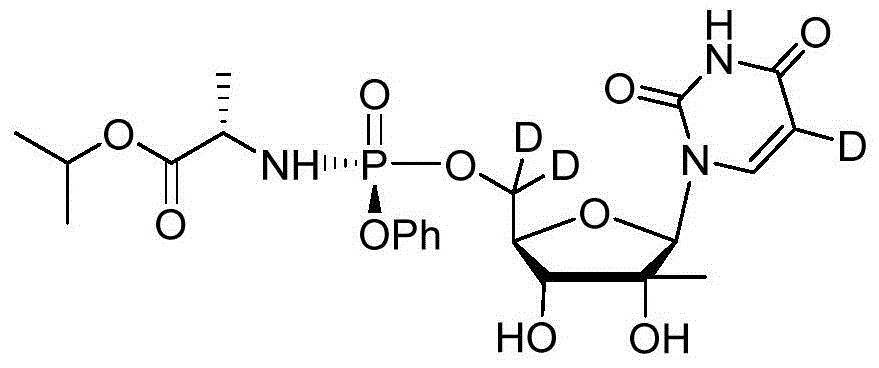
Deuterated Sofosbuvir and use thereof
InactiveCN104672288AIsotope introduction to sugar derivativesOrganic active ingredientsStereoisomerismPhosphoramidate
The invention discloses deuterated Sofosbuvir and application thereof. Deuterated Sofosbuvir is a nucleoside phosphoramidate compound shown in the formula I or II, or stereisomer, salt, hydrate, solvate or crystal of the nucleoside phosphoramidate compound. The compound and the composition are used for treating flaviviridae viruses, and particularly used for treating infection of hepatitis c virus (HCV); and the deuterated Sofosbuvir has good HCV resistance.
Owner:中科云和(北京)生物医药科技有限公司
Method for preparing crystalline form 6 of Sofosbuvir
ActiveCN105801645ASolve the blockageEasy to operateOrganic active ingredientsSugar derivativesProcess conditionsSofosbuvir
The invention relates to a method for preparing crystalline form 6 of Sofosbuvir. The method has the advantages of simple operation, stable process conditions, good reproducibility, high yield, and good purity; viscous jelly does not generate in the middle process, and the blocking problem of a discharge port is solved; the method is suitable for industrial production with higher economic value.
Owner:SHANGHAI ARYL PHARMTECH CO LTD +1
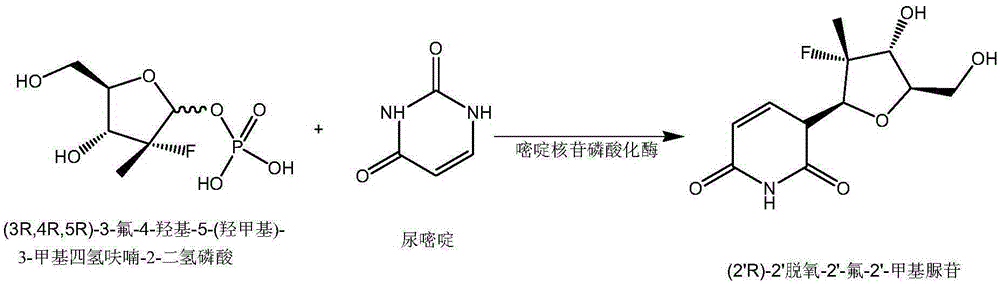
Pyrimidine nucleoside phosphorylase gene and application thereof
The invention relates to the fields of biotechnology and biological medicine, in particular to a pyrimidine nucleoside phosphorylase gene and the application thereof. The nucleotide sequence of the pyrimidine nucleoside phosphorylase gene is shown in SEQ ID NO.1, and the amino acid sequence of pyrimidine nucleoside phosphorylase coded by the gene is shown in SEQ ID NO.2. A genetically engineered bacterium containing the gene is obtained by transferring the gene into escherichia coli. By culturing the genetically engineered bacterium and optimizing the fermentation technology, large-scale production of recombined pyrimidine nucleoside phosphorylase is achieved, and the recombined pyrimidine nucleoside phosphorylase can be used for catalyzed synthesis of Sofosbuvir midbody (2'R)-2'-deoxygenation-2'-fluorine-2'-methyl uridine and production of other nucleoside bulk pharmaceutical chemicals and medical intermediates.
Owner:JIANGSU ALPHA PHARM CO LTD
An improved sofosbuvir preparation method
ActiveCN106397515AMild reaction conditionsEasy to implement factory scale-upSugar derivativesSugar derivatives preparationAfter treatmentOrganic solvent
An improved sofosbuvir preparation method is provided. The method adopts (2'R)-2'-deoxy-2'-fluoro-2'-methyluridine, and other raw materials and prepares the sofosbuvir through reacting in an organic solvent under existence of a Lewis acid and an alkali. The method has advantages of mild reaction conditions, safe agent using, simple and convenient operation, convenient after-treatment, and the like, and is prone to large-scale production in a factory.
Owner:SUNSHINE LAKE PHARM CO LTD
Preparation method for hepatitis C virus resisting drug sofosbuvir intermediates
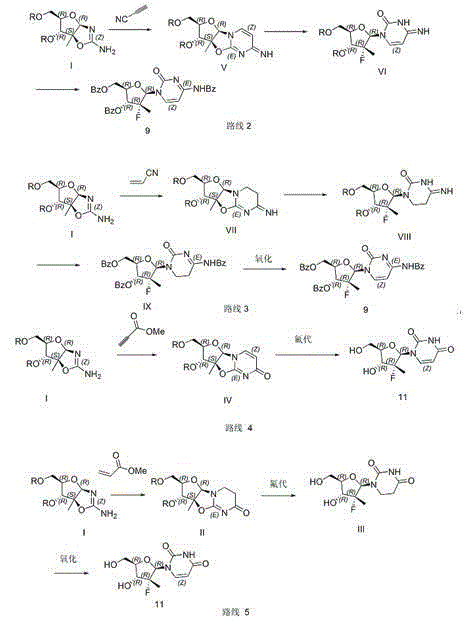
The invention relates to a preparation method for sofosbuvir intermediates 9, 11. The preparation method comprises the following steps: by taking a compound I as an initial material, carrying out an additive reaction with electrophilic reagents acrylonitrile, cyanoacetylene, propargyl ester and acrylate and carrying out ring-closing reaction to prepare corresponding intermediates (VII, V, IV and II); carrying out a ring-opening reaction on the intermediates (VII, V, IV and II) and a fluorination reagent to obtain corresponding intermediates (VIII, VI, II and III), carrying out a reaction on the intermediate VIII and benzoyl chloride in an alkaline condition to prepare an intermediate IX, and further carrying out dehydrogenation reaction on the intermediate IX in the presence of an oxidizing agent to prepare an intermediate 9; carrying out a reaction on the intermediate VI and benzoyl chloride in the alkaline reaction to prepare the intermediate 9; and further carrying out dehydrogenation reaction on the intermediate III in the presence of an oxidizing agent to prepare the intermediate 11.
Owner:CONSCI PHARMA
Synthetic method of sofosbuvir intermediate
InactiveCN105061535AHigh yieldReduce usageSugar derivativesSugar derivatives preparationCytosineN dimethylformamide
The invention relates to a synthetic method of sofosbuvir intermediate-(2'R)-N- benzoyl-2'-deoxy-2'-fluoro-2'-methylcytidine-3',5'-dibenzoate represented by a formula (I) (shown in the specification). The synthetic method comprises the steps of generating a reduction product compound IV of a compound III from the compound III in the presence of modified red aluminum, generating a chlorinated compound V from the compound IV through sulfoxide chloride chlorination under the catalysis of N,N-dimethylformamide, after separating organic materials and aluminum salt from the compound V, directly condensing the compound V with a compound VI N-benzoyl-O-(trimethylsilyl)cytosine, and carrying out hydrolysis by virtue of an acetic acid solution, so as to obtain (2'R)-N- benzoyl-2'-deoxy-2'-fluoro-2'-methylcytidine-3',5'-dibenzoate represented by the formula (I). The synthetic method has the advantages of high yield, convenience in operation and suitability in industrial production.
Owner:JIANGSU COBEN PHARMA CO LTD
Preparation method of sofosbuvir
ActiveCN106565805ARaw materials are easy to getLow costSugar derivativesSugar derivatives preparationPhosphateSide chain
The invention relates to the technical field of medicine, in particular to a preparation method of sofosbuvir. The method adopts D-ribose as the starting material, and carries out esterification reaction, hydroxyl protection reaction, hydroxyl oxidation reaction, methylation reaction, fluorination reaction, docking reaction, hydrolysis, phosphate ester side chain connection and a series of reactions, thus finally obtaining the product sofosbuvir. The preparation method of sofosbuvir provided by the invention adopts D-ribose as the raw material, the raw materials are easily available and low in cost, and the one-step hydrolysis reaction adopted by the invention has few step, therefore the method is easy for industrialization.
Owner:常州市勇毅生物药业有限公司
Combination formulation of two antiviral compounds
ActiveCN105517540AEliminate or mitigate the impactImprove bioavailabilityOrganic active ingredientsDigestive systemChemical compoundSofosbuvir
Disclosed are pharmaceutical compositions comprising Compound I, having the formula (I): and an effective amount of sofosbuvir wherein the sofosbuvir is substantially crystalline. Also disclosed are methods of use for the pharmaceutical composition.
Owner:GILEAD SCI INC
Method for preparing sofosbuvir intermediate and recovering byproduct
ActiveCN107245064AReduce security risksReduce energy consumptionEther preparationRefluxMethylbenzoprim
The invention relates to a method for preparing a sofosbuvir intermediate [(2R,3R,4R)-3-(benzoyloxy)-4-fluoro-5-hydroxy-4-methyltetrahydrofuran-2-yl] methylbenzoate represented by formula (II) and recovering a byproduct (2R,3R,4S)-4-fluoro-2,5-dihydroxy-4-methylpentane-1,3-diyl]dibenzoate represented by formula (III). The method comprises the following steps: (1) reacting a compound (I) 3,5-dibenzoyl-2-deoxy-2-fluoro-2-methyl-D-ribosinic acid-gamma-lactone in a first solvent under the action of a reducing agent at a temperature of from 0 DEG C to the reflux temperature of the first solvent for 1-20 hours to produce the sofosbuvir intermediate (II) and the byproduct (III); and (2) filtering the above product to obtain a filter cake which is the byproduct (III), dissolving the filter cake in a second solvent, and reacting the obtained solution in an acidic environment with the pH value of 1-6 under the action of an oxidant and a catalyst at a temperature from 0 DEG C to the reflux temperature of the second solvent for 0.5-5 hours in order to generate the compound (I).
Owner:NINGBO TEAM PHARMA
Crystal form A of sofosbuvir and preparation method thereof
InactiveCN104974205AImprove solubilityImprove efficacyOrganic active ingredientsSugar derivativesSolubilityHigh humidity
The invention provides a crystal form A of sofosbuvir and a preparation method thereof. The crystal form A is characterized in that an X-ray powder diffraction spectrum has characteristic peaks at the 2[theta] value being 12.4 + / - 0.2 degrees, 19.4 + / - 0.2 degrees, 27.1 + / - 0.2 degrees, 13.5 + / - 0.2 degrees, 25.5 + / - 0.2 degrees and 16.8 + / - 0.2 degrees. The crystal form A is higher in solubility in a bio-medium, is beneficial to drug efficiency increasing, can reduce the carrying amount of the drug, is low in hygroscopicity and is not liable to deliquesce due to high humidity, so that the drug can be stored for a long time conveniently.
Owner:CRYSTAL PHARMATECH CO LTD
Sofosbuvir tablet and preparation method thereof
ActiveCN105287424ASolve the problem of water hardening glueSolve the problem of poor fluidity and incapable of industrial productionOrganic active ingredientsDigestive systemMedicineDissolution
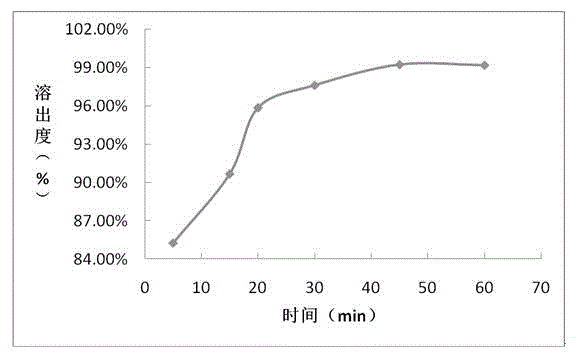
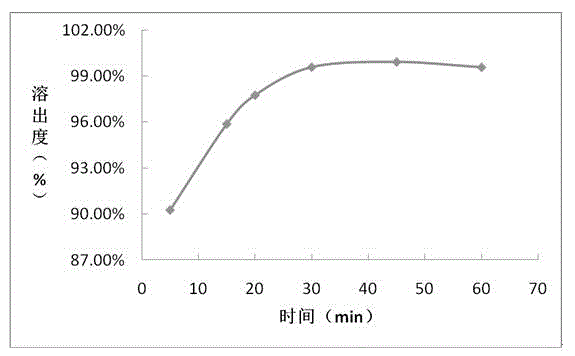
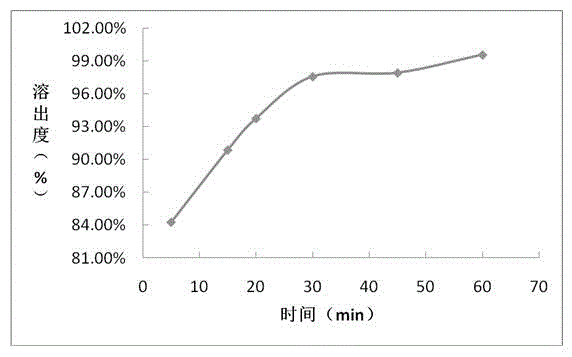
The invention provides a sofosbuvir tablet and a preparation method thereof. The sofosbuvir tablet comprises 10-41%, by total weight of the sofosbuvir tablet, of sofosbuvir, 1-3%, by total weight of the sofosbuvir tablet, of first diluting agent, 53-86%, by total weight of the sofosbuvir tablet, of second diluting agent, 3-10%, by total weight of the sofosbuvir tablet, of disintegrating agent and 0.5-3.0%, by total weight of the sofosbuvir tablet, of lubricating agent. The preparation method is a powder directly-mixing and tabletting method and comprises the following steps: (1) uniformly mixing the sofosbuvir and the first diluting agent to obtain a mixture I; (2) adding and uniformly mixing the second diluting agent and the disintegrating agent in the mixture I to obtain a mixture II; and (3) adding the lubricating agent into the mixture II, totally mixing, tabletting, coating and packaging. Compared with the prior art, the sofosbuvir tablet provided by the invention has the advantages of greatly improving the dissolution rate of the sofosbuvir preparation, enabling the production efficiency to be higher and the preparation cost to be lower, and being capable of adapting to large-scale industrial production.
Owner:SHIJIAZHUANG NO 4 PHARMA
Method for measuring related substances of Sofosbuvir tablet by using HPLC
ActiveCN104569276APrevent hydrogen bondingImproved Chromatographic Retention BehaviorComponent separationPhosphoric acidGradient elution
The invention provides a method for measuring related substances of a Sofosbuvir tablet by using HPLC. The method is characterized in that in an anti-phase C18 column, a phosphoric acid solution or a mixed solution of the phosphoric acid solution and an organic phase is adopted to carry out gradient elution detection on a Sofosbuvir tablet test solution, wherein termination processing is carried out on silica gel particles of the anti-phase C18 column. The method provided by the invention can effectively separate and measure the related substances of the Sofosbuvir tablet, and also has the characteristics of being good in specificity, high in sensitivity, convenient, rapid, economical, and low in cost.
Owner:SUNSHINE LAKE PHARM CO LTD
Application of sofosbuvir in preparation of medicines for preventing and treating coronavirus
InactiveCN111467363ALow half effective concentrationLow effective concentrationOrganic active ingredientsAntiviralsDiseasePharmaceutical drug
The invention discloses an application of sofosbuvir in preparation of medicines for preventing and treating coronavirus, and particularly discloses an application of sofosbuvir or a pharmaceuticallyacceptable salt, isotope, stereoisomer, mixture of stereoisomers, tautomer, ester, amide or prodrug thereof in preparation of medicines for preventing and / or treating diseases caused by coronavirus. The coronaviruses are novel coronaviruses SARS-Cov-2, SARS-CoV, HCoV 229E, NL63, OC43, HKU1 and MERS-CoV. The half effective concentration of sofosbuvir on novel coronavirus of Corona Virus Disease 2019 is 1.12 [mu]M, the toxicity is low, and a good treatment window is provided.
Owner:SHENZHEN INST OF ADVANCED TECH CHINESE ACAD OF SCI
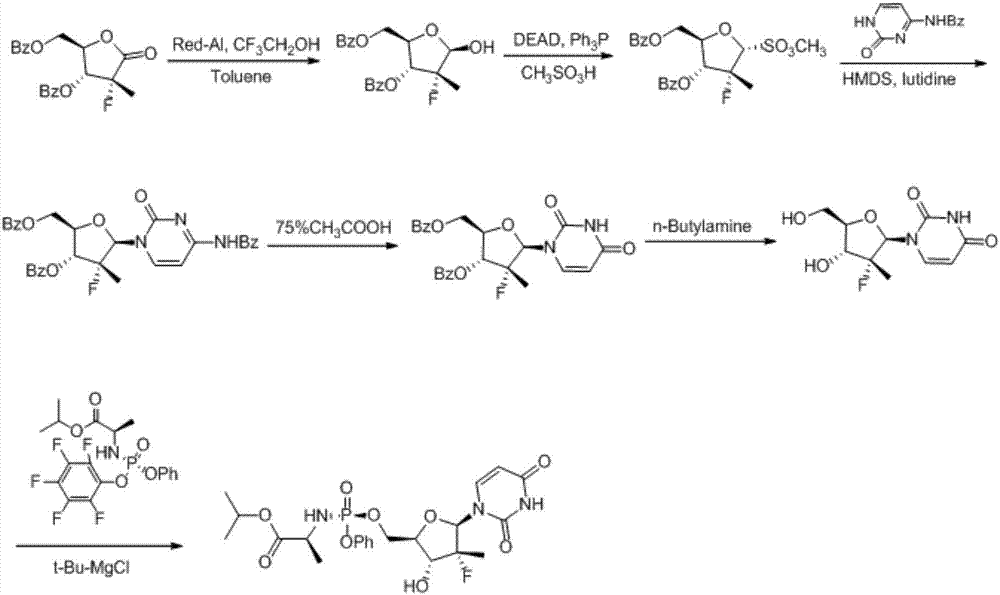
(2'R)-2'-deoxy-2'-halogenated-2'-methyluridine derivative, preparation method and uses thereof
InactiveCN106188193AQuality improvementInhibit side effectsOrganic active ingredientsSugar derivativesDiseasePhosphorylation
The present invention discloses a compound represented by a formula I, a preparation method and an intermediate thereof, uses of the compound represented by the formula I in preparation of drugs for treatment of viral infection diseases, uses of the compound represented by the formula I in preparation of sofosbuvir and analogs thereof, and a method for preparing the sofosbuvir and the analogs thereof by using the compound represented by the formula I. According to the method of the present invention, the new reaction route is designed, such that the side reaction caused by the use of tert-butyl magnesium chloride in the phosphorylation reaction is avoided, the generated impurity is reduced, and the difficulty of the subsequent purification process is reduced. According to the present invention, the test results prove that the method has characteristics of mild reaction conditions, easy operation, high yield, stable product quality and high product purity, and can be used for industrial scale enlargement production. The formula I is defined in the specification.
Owner:SUZHOU VIGONVITA LIFE SCIENCES CO LTD +2
Preparation method of sofosbuvir
ActiveCN105669804AEasy to controlReduce the impactSugar derivativesSugar derivatives preparationGrignard reagentEvaporation
The invention discloses a preparation method of sofosbuvir. The preparation method includes following steps: in the absence of water and oxygen, enabling (2'R)-2'-deoxidized-2'-fluorine-2'-methyluridine, N-[(S)-(2, 3, 4, 5, 6-pentafluorophenoxy) phenoxy phosphoryl]-L-alanine isopropyl ester and Grignard reagent to react in the presence of a reaction solvent; dissolving mother liquid of sofosbuvir in a solvent, adding an adsorbent, depressurizing for concentration to be dry, adding solvent, pulping at room temperature, suction filtering, collecting filtrate, depressurizing for evaporation to dryness to obtain a oily product, adding solvent into the oily product, heating for dissolving to clearness, and cooling to 0-5 DEG C for crystallization to obtain recycled pentafluorophenyl; enabling D-alanine isopropyl ester and phenyl dichlorophosphate to be in reaction at minus 70-minus 80 DEG C, and adding a solvent solution of pentafluorophenyl and alkali for reaction to obtain a compound shown in a formula II. In the synthesis process of sofosbuvir, molecular pentafluorophenyl is removed, and pentafluorophenyl is recycled and used for preparation of an intermediate of sofosbuvir synthesis materials, so that pentafluorophenyl is recycled and influence on environment is reduced.
Owner:NANTONG CHANGYOO PHARMATECH CO LTD
Preparation method of sofosbuvir
ActiveCN105153257AReduce manufacturing costSuitable for industrial productionSugar derivativesGroup 5/15 element organic compoundsSofosbuvirStereoselectivity
The invention relates to a preparation method of sofosbuvir, and specifically provides a quite stereoselective preparation method, which can make the prepared required isomer enrich under the condition of not separating intermediate, so that the preparation method can reduce production cost greatly, and is suitable for commercial process.
Owner:CHIA TAI TIANQING PHARMA GRP CO LTD
Preparation for sofosbuvir key intermediate
The invention relates to preparation for a sofosbuvir key intermediate. With (E)-(S)-3-(2,2-dimethyl-1,3-dioxolane-4-base)-2-ethyl methacrylate being an initial raw material, preparation of 3,5-bis-O-benzoyl-2-deoxidation-2-fluorine-2-C-methyl-D-riboic acid-gamma-lactone is achieved through an epoxidation reaction, fluoridation, a de-acetonylidene cyclization reaction and a benzoyl reaction. According to the method, a synthesis route is short, and in the reaction process, a reagent that oxidability is high, and in the aftertreatment process, potassium permanganate or sodium permanganate and high-corrosivity SOCl2 deep in waste water color will generate a lot of waste water will not be involved.
Owner:WISDOM PHARM CO LTD
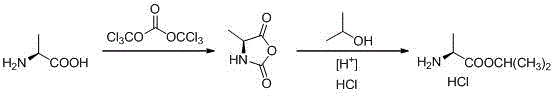
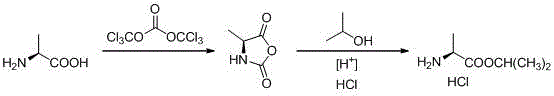
Novel preparation method of L-alanine isopropyl ester hydrochloride
InactiveCN106518694AStrong irritantHighly corrosiveOrganic compound preparationAmino-carboxyl compound preparationMedicinal chemistryPhosgene
The invention provides a novel preparation method of L-alanine isopropyl ester hydrochloride, and relates to a preparation method of an intermediate of a new drug sofosbuvir for treating chronic hepatitis. The method sequentially comprises the following steps that L-alanine is adopted as a raw material to react with triphosgene for ring closure, after ring opening is conducted through isopropanol under the acidic condition, salt formation is conducted, and the product L-alanine isopropyl ester hydrochloride is obtained. According to the novel preparation method, a brand-new synthetic route is provided, the adopted raw material is wide and sufficient in source, the cost is low, the reaction conditions are mild, the processes are simple, all the reactions are conventionally operated, the condition that a large quantity of high-irritation raw materials such as thionyl chloride are used is avoided, and a good industrial prospect is achieved.
Owner:ZHEJIANG GENEBEST PHARMA
Synthesis method of sofosbuvir
ActiveCN106905398AAvoid generatingReduce usageSugar derivativesSugar derivatives preparationSulfonateSynthesis methods
The invention provides a synthesis method of sofosbuvir. The synthesis method of the sofosbuvir comprises the following steps: performing mitsunobu reaction on ((2R,3R,4R)-3-benzoyloxy)-4-fluorine-5-hydroxyl-4-methyltetrahydrofuran-2-yl)methyl benzoate to produce sulfonate to obtain a compound 1; abutting the compound 1 and N-benzoylcytosine to produce a compound 2. The method adopts mitsunobu reaction to avoid production of an isomer, and the isomer is reduced to 5 percent or below; according to the method, sulfonate and N-benzoylcytosine are abutted, so the use ofa stannic chloride raw material is avoided; furthermore, the yield is high and few solid waste is generated during aftertreatment, so that the method is suitable for large-scale industrialized production.
Owner:ZHEJIANG FORESTRY UNIVERSITY
Popular searches
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com