Synthesis method of sofosbuvir
A synthesis method and compound technology, applied in chemical instruments and methods, preparation of sugar derivatives, sugar derivatives, etc., can solve the problems of difficult industrial production, incomplete reaction, slow reaction, etc., to reduce the generation of solid waste and ensure safety Improved performance and less solid waste
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
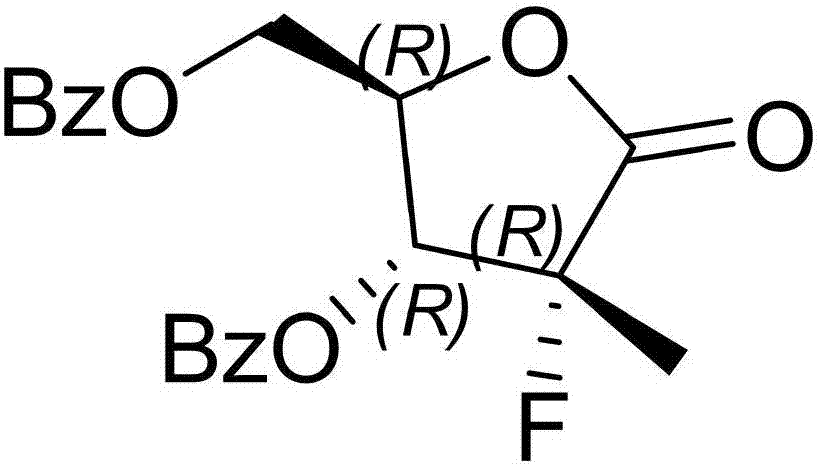
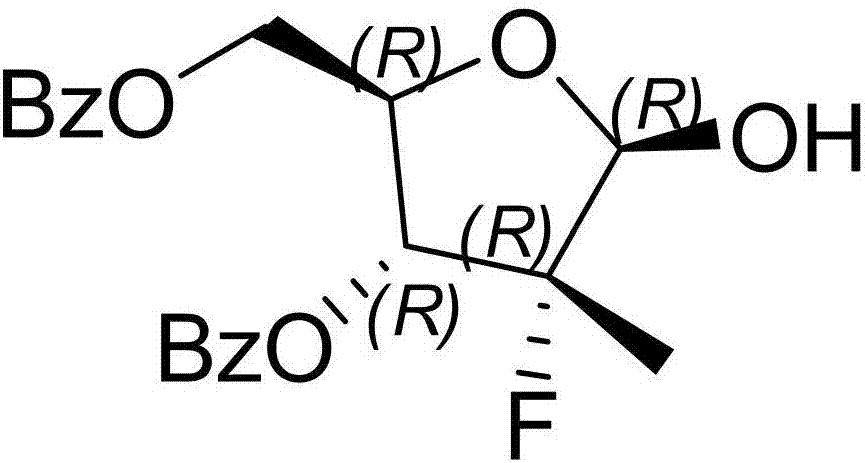
[0033] Embodiment 1, with reference to attached Figure 1-9 As shown, a synthetic method of sofosbuvir of the present invention, ((2R,3R,4R)-3-(benzoyloxy)-4-fluoro-5-hydroxyl-4-methyltetrahydrofuran-2 - Base) Methyl benzoate generates compound 1 through Mitsunobu reaction to generate sulfonate; compound 1 is docked with N-benzoylcytosine to generate compound 2, and compound 2 is used to obtain sofosbuvir. The method adopts Mitsunobu reaction to avoid the generation of isomers, and the isomers can be reduced to below 5%; the method uses sulfonate esters to dock with N-benzoylcytosine, avoiding the use of tin tetrachloride raw materials, and the yield Higher, the post-processing produces less solid waste, and is suitable for large-scale industrial production.
[0034] A kind of synthetic method of sofosbuvir, compound 2 generates compound 3 under the heating of acetic acid aqueous solution.
[0035] A method for synthesizing sofosbuvir, wherein compound 3 is deprotected by ad...
Embodiment 2
[0059] Embodiment 2, with reference to attached Figure 1-9As indicated, 18.6 g (50 mmol) of (2R)-2-deoxy-2-fluoro-2-methyl-D-erythropentanoic acid GAMMA-lactone 3,5-diphenyl was added to a 250 mL reaction bottle Formate and 120 mL of dichloromethane, stir and cool down to 0~10 °C, add the prepared trifluoroethanol-red aluminum toluene solution dropwise into the reaction, keep the temperature below 10 °C, keep stirring after the addition After 2~3 h, there is no remaining raw material for spotting, and ((2R,3R,4R)-3-(benzoyloxy)-4-fluoro-5-hydroxy-4-methyltetrahydrofuran-2-yl)benzene Methyl formate.
[0060] A kind of synthetic method of sofosbuvir, trifluoroethanol-red aluminum toluene solution is to take by weighing 23.3 g (80.74 mmol) red aluminum (70% toluene solution) and 50 mL toluene and add in 100 mL reaction bottle, argon protection Lower the temperature to 0-10 °C, slowly add 8.0 g (80 mmol) of trifluoroethanol dropwise, keep the temperature below 10 °C during the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com