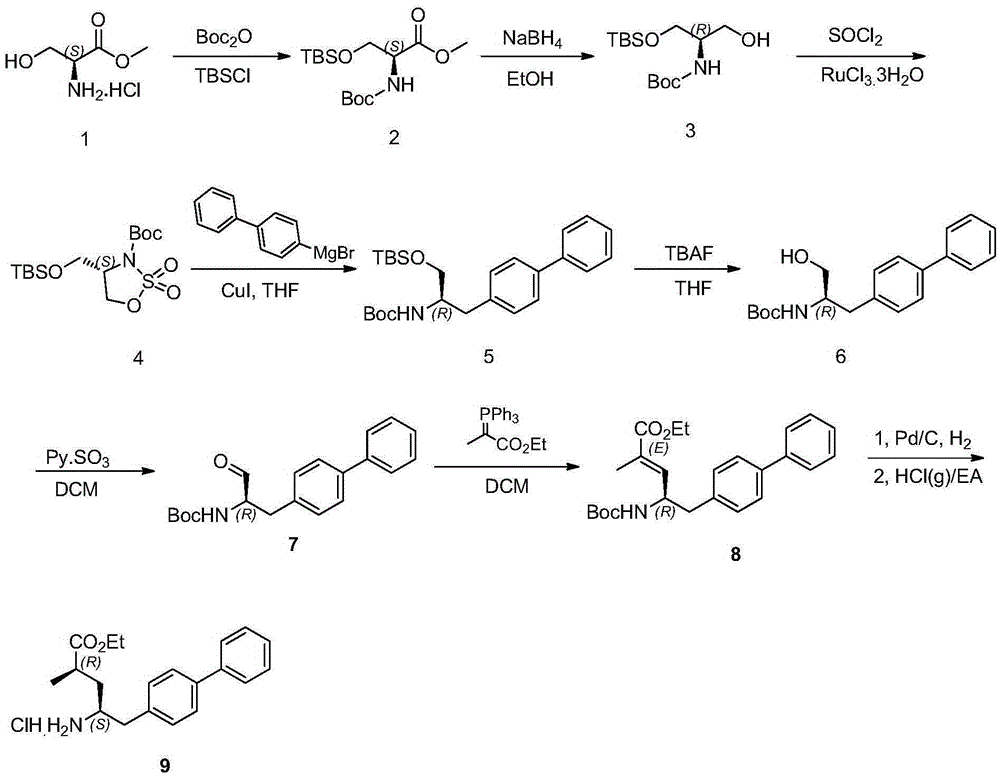
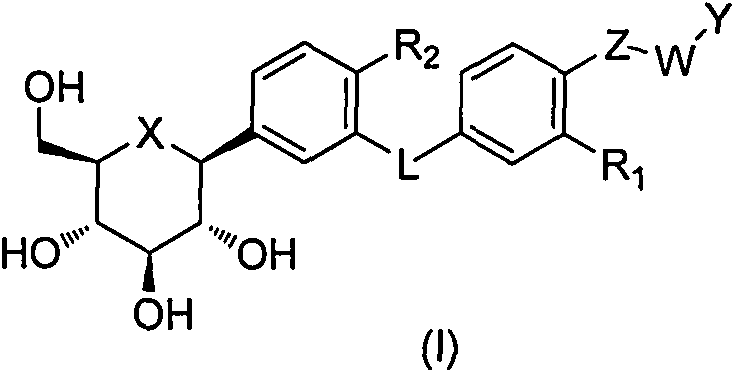
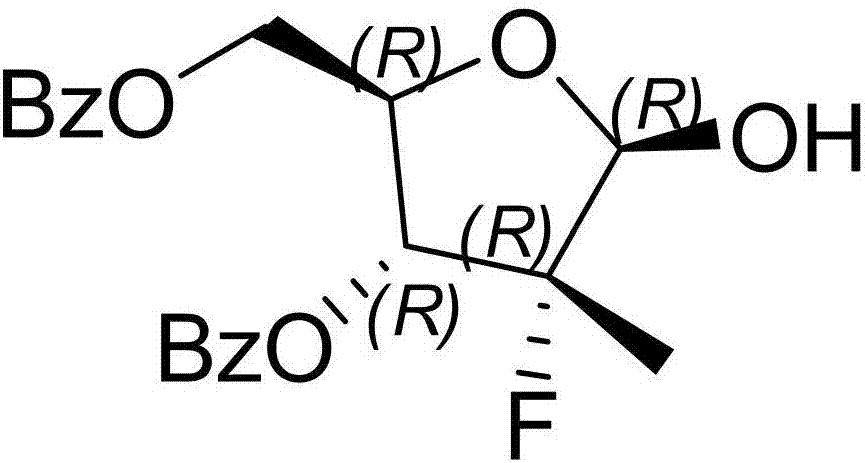
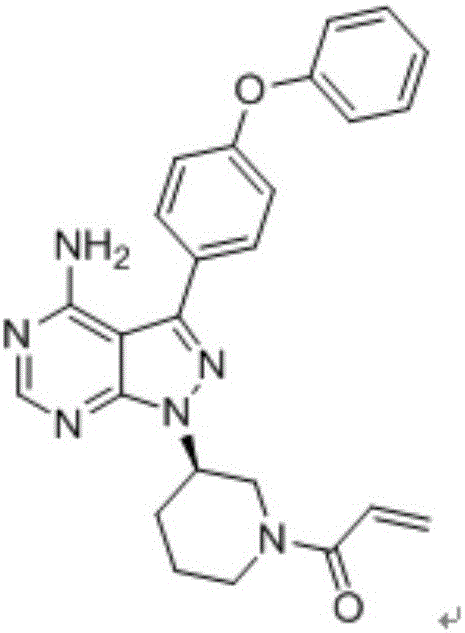
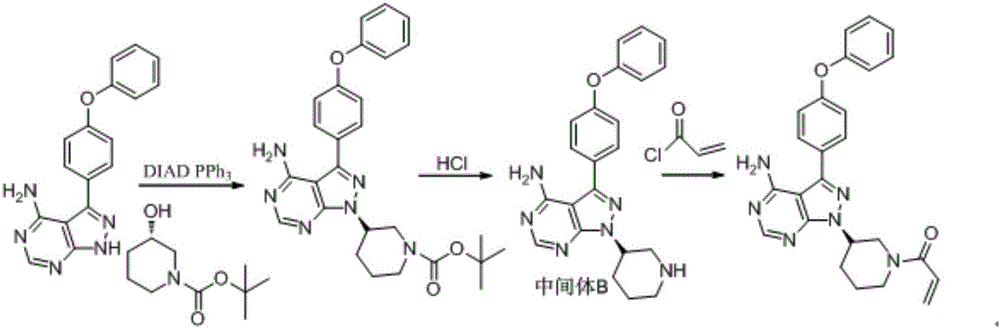
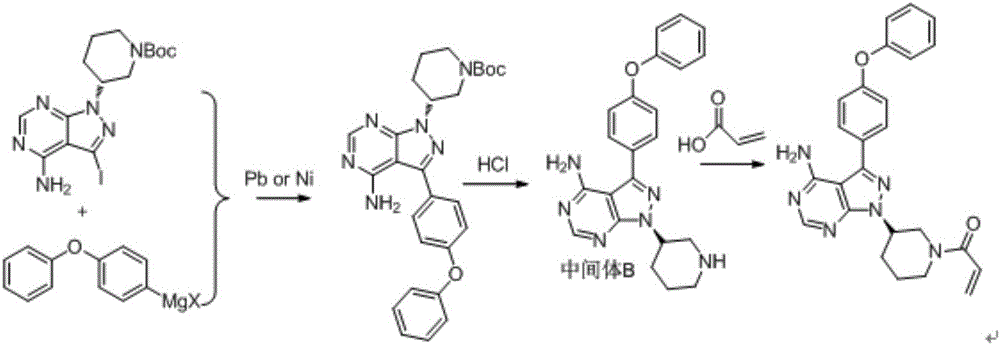
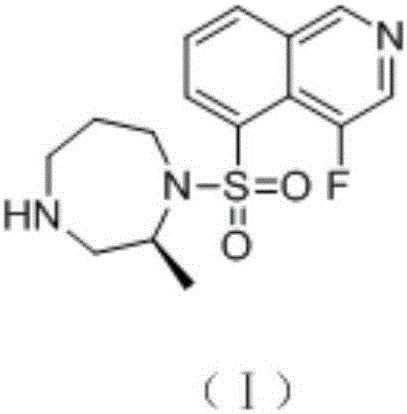
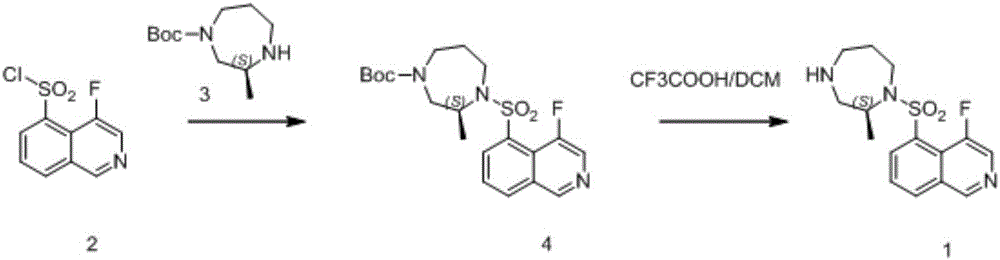
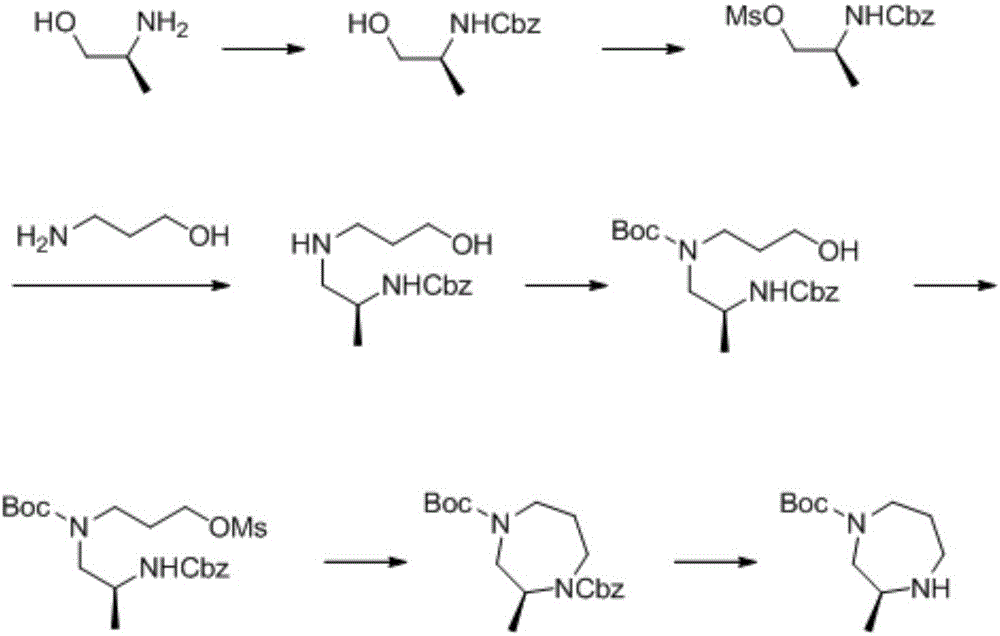
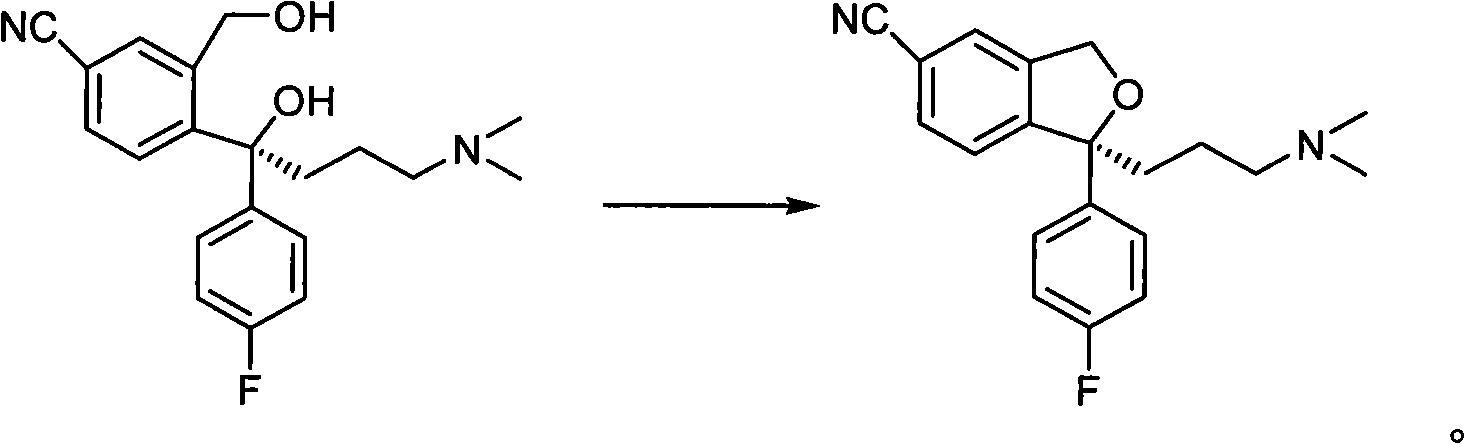
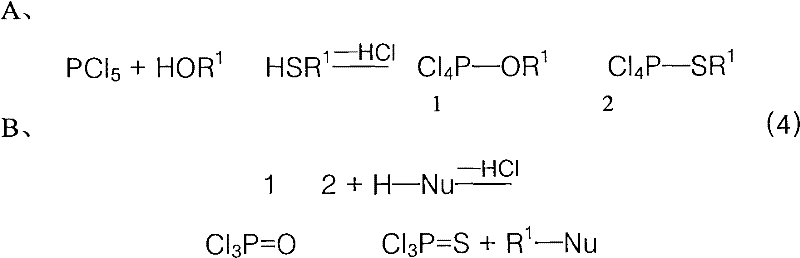Patents
Literature
166 results about "Mitsunobu reaction" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
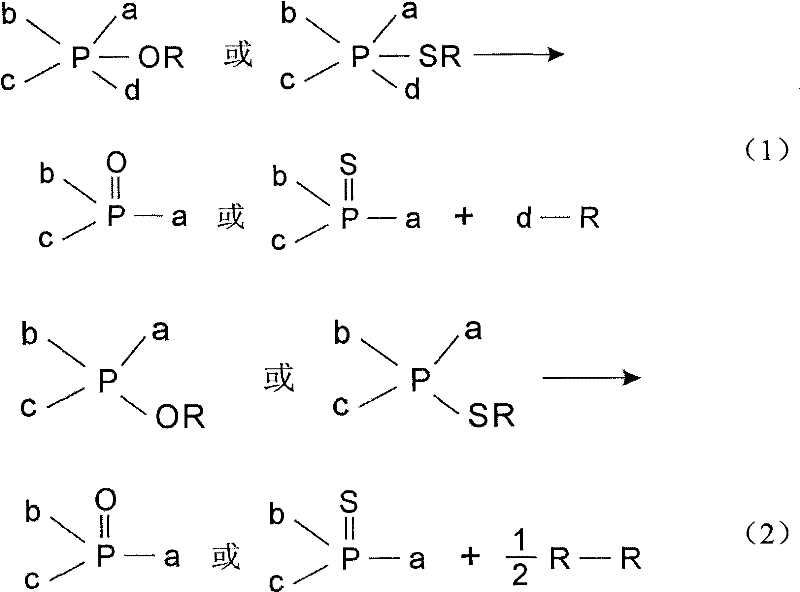
The Mitsunobu reaction is an organic reaction that converts an alcohol into a variety of functional groups, such as an ester, using triphenylphosphine and an azodicarboxylate such as diethyl azodicarboxylate (DEAD) or diisopropyl azodicarboxylate (DIAD). The alcohol undergoes an inversion of stereochemistry. It was discovered by Oyo Mitsunobu (1934–2003).
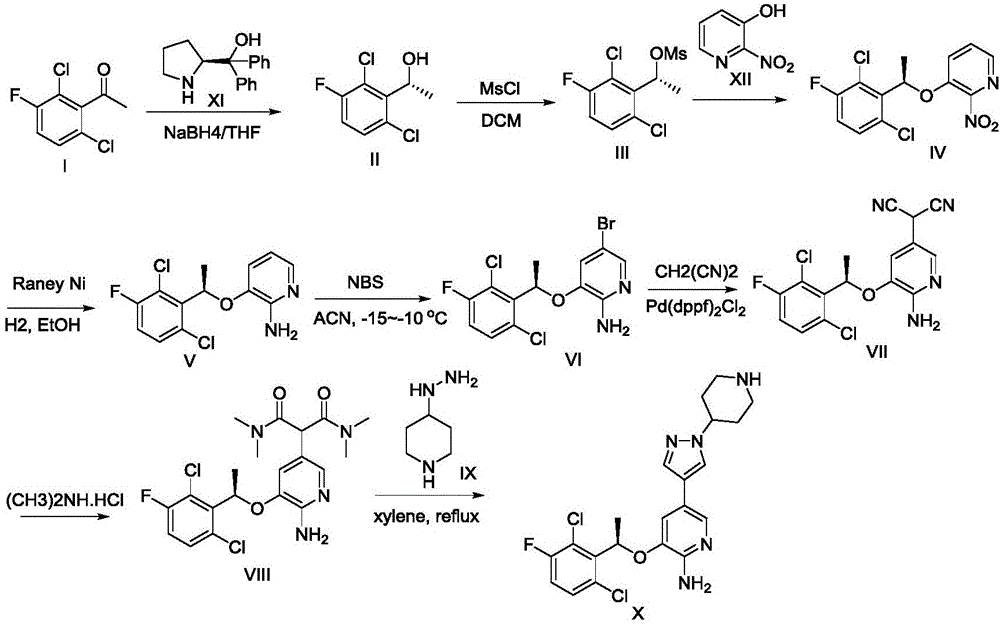
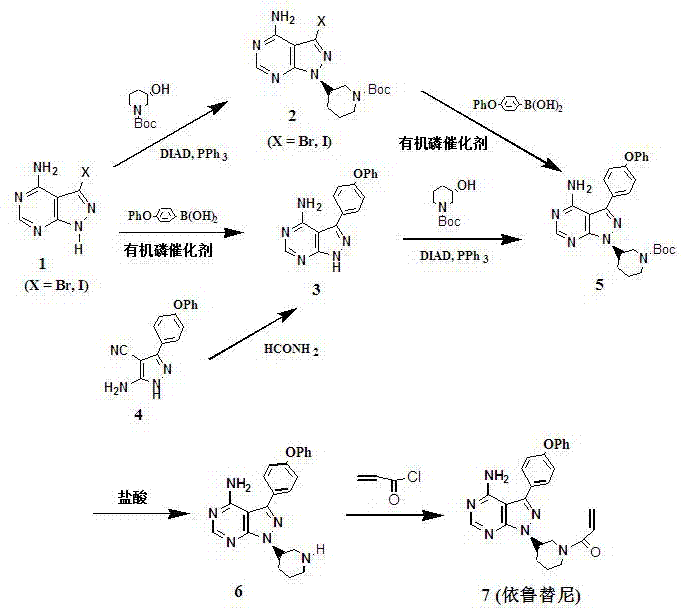
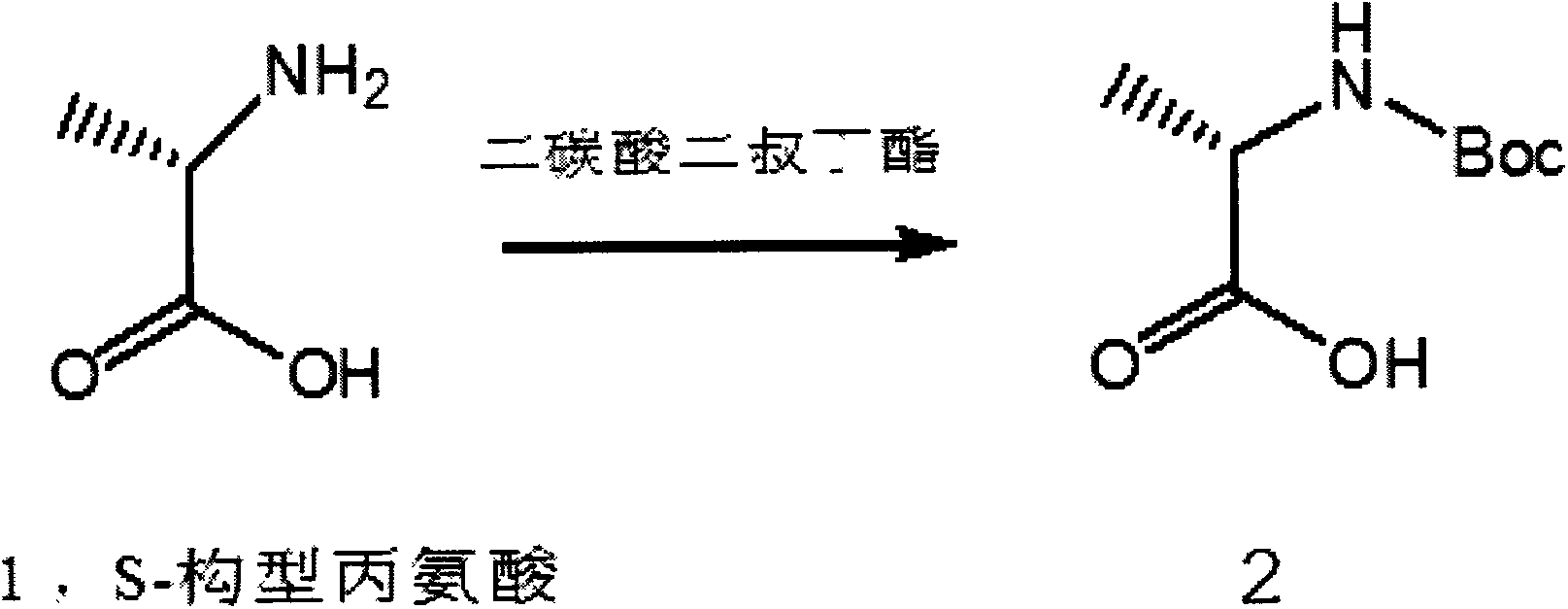
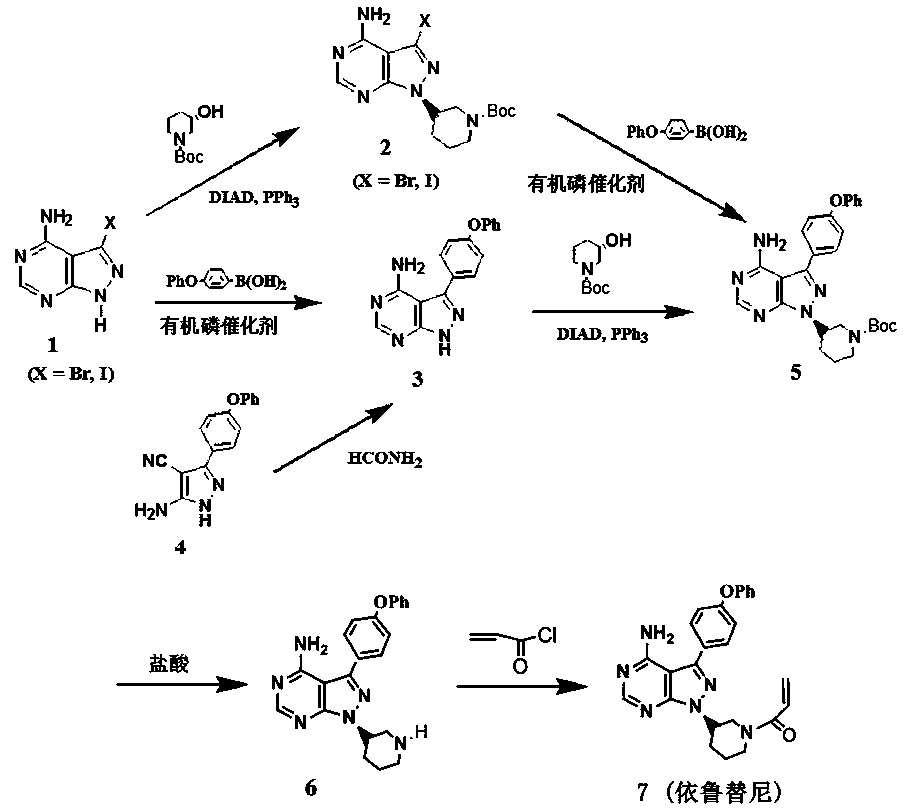
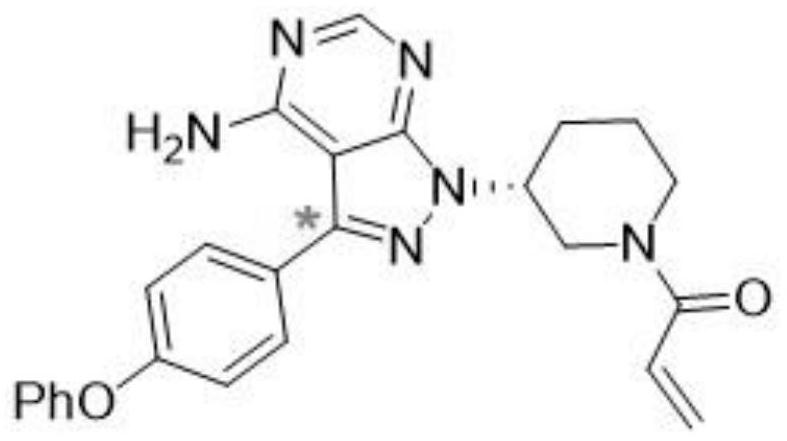
Preparation method for ibrutinib
ActiveCN105859728AHigh purityHigh yieldGroup 4/14 element organic compoundsFormamidine acetateDrugs synthesis
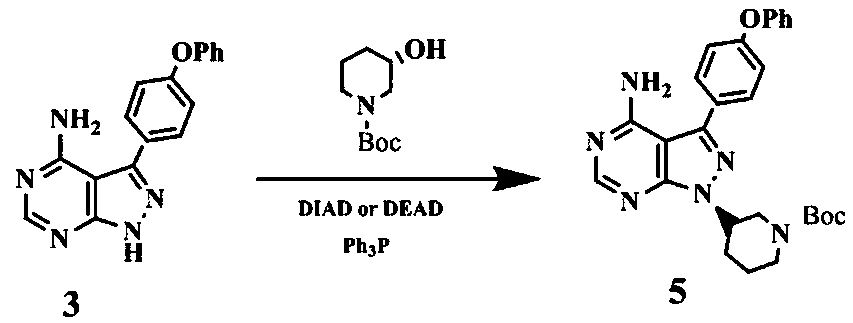
The invention discloses a preparation method for ibrutinib and belongs to the technical field of drug synthesis. The preparation method specifically includes the steps that 3-amino-4-cyano pyrazol and formamidine acetate serve as initial raw materials, and ibrutinib is obtained through a cyclization reaction, a halogenating reaction, a nucleophilic substitution reaction, a Mitsunobu reaction and an amidation reaction. According to the method, the raw materials are easy to obtain, conditions are mild, the process operability and controllability are high, cost is low, the yield is high, fewer side products are generated, purification is easy, and the high-quality product is obtained.
Owner:南京红太阳医药研究院有限公司
Dihydropyrimidine compound as well as preparation method and application thereof
ActiveCN113801097AImprove securityGood inhibitory effectOrganic chemistryAntipyreticDiseaseMetabolite
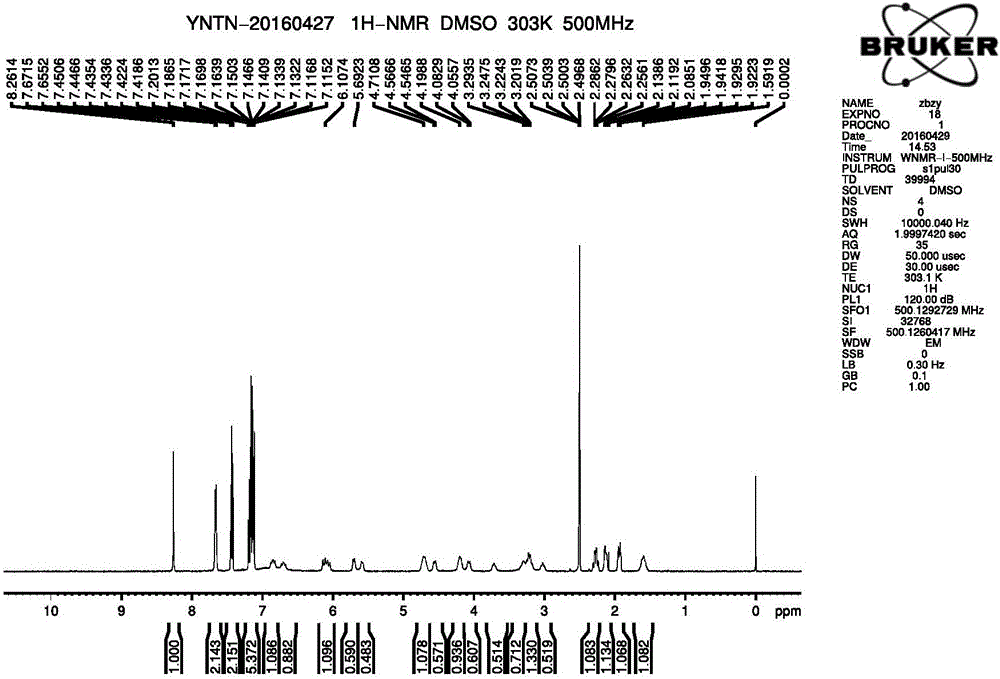
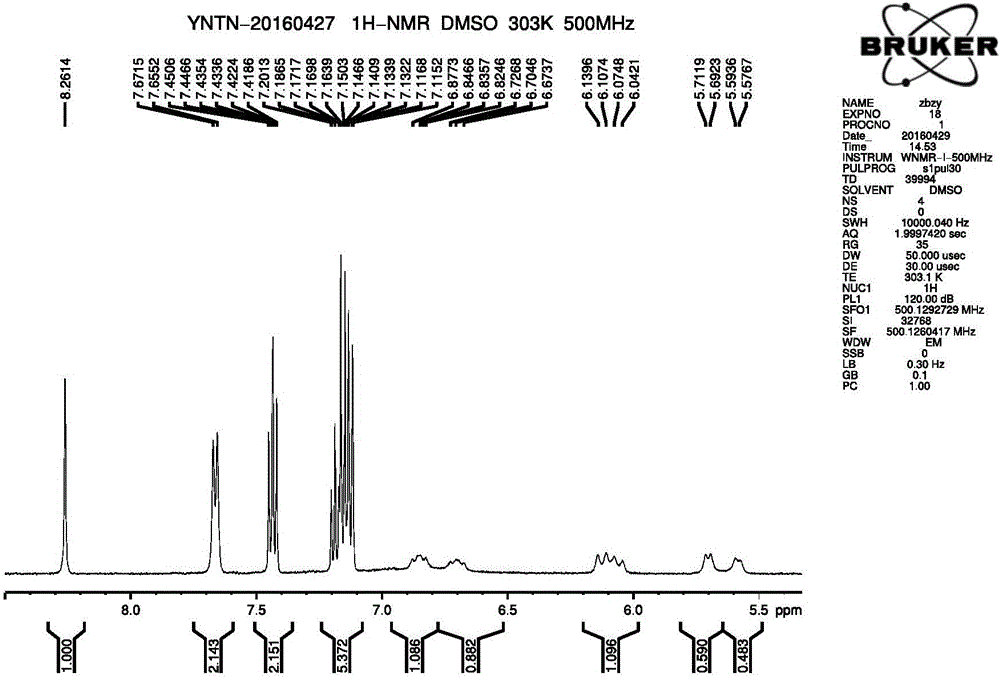
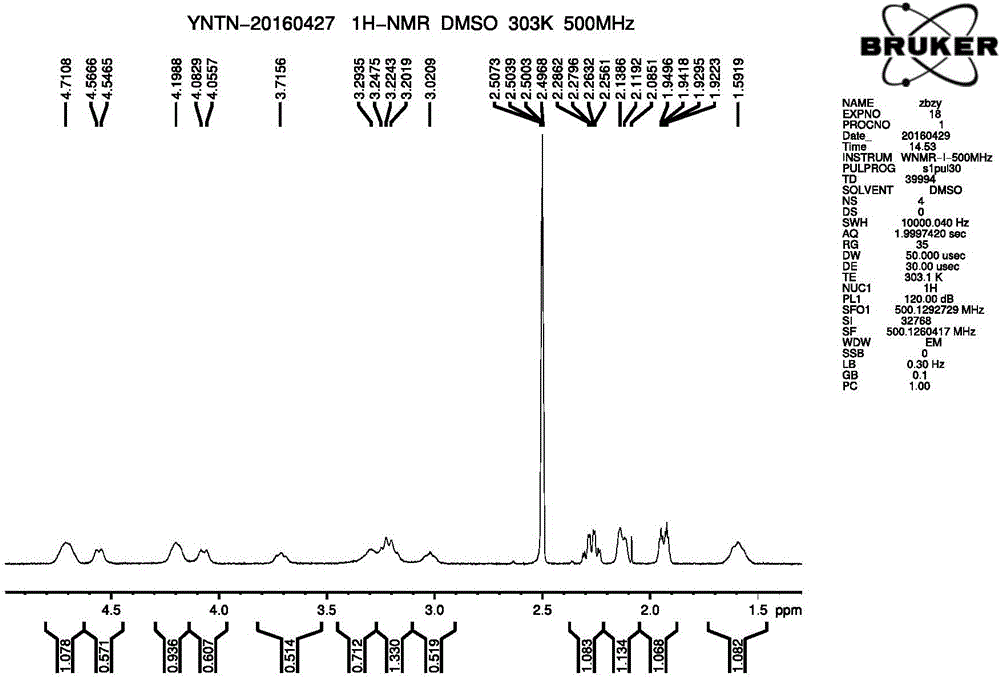
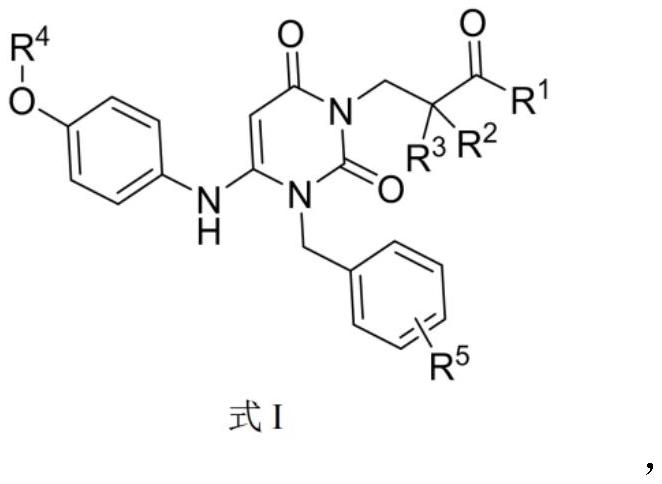
The invention discloses a dihydropyrimidine compound as well as a preparation method and an application thereof, and belongs to the technical field of medicinal chemistry. The structure of the dihydropyrimidine compound provided by the invention is as shown in a formula I in the specification. The preparation method provided by the invention comprises the following steps: carrying out substitution reaction on a compound a and a compound k under the catalysis of alkali to generate a compound b; performing substitution reaction on the compound c and the compound d under an alkaline condition to obtain a compound e; carrying out Mitsunobu reaction on the compound e and f to obtain an intermediate g, and carrying out coupling reaction on the compound g and b to generate a compound h. The invention provides the application of the compound shown in the formula I or salt, solvate, allomer, metabolite, nitric oxide and prodrug thereof in preparation of drugs for treating or preventing P2X3 and / or / P2X2 / 3 receptor related diseases. The dihydropyrimidine compound disclosed by the invention has a good P2X3 receptor antagonism effect and better safety.
Owner:CHENGDU SHIBEIKANG BIOLOGICAL MEDICINE TECH CO LTD
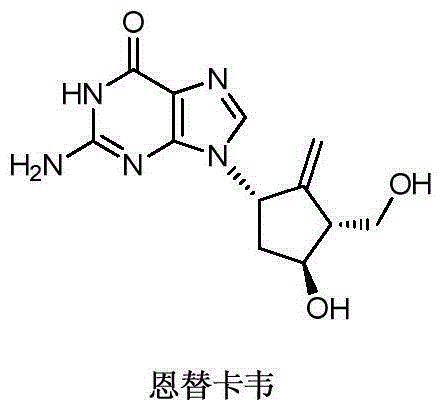
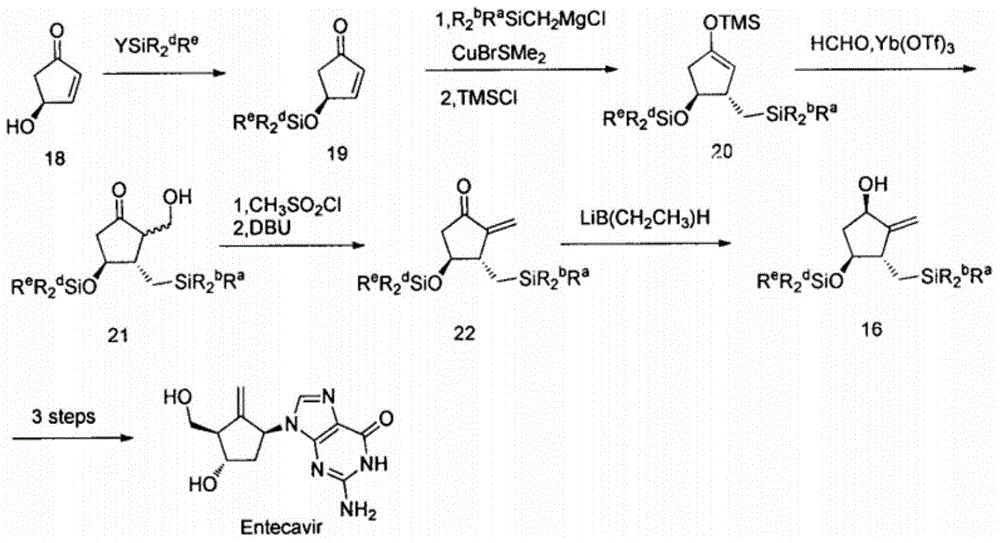
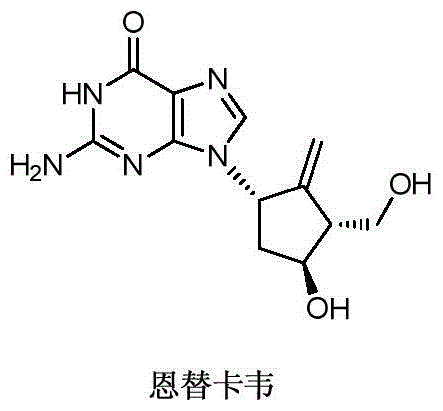
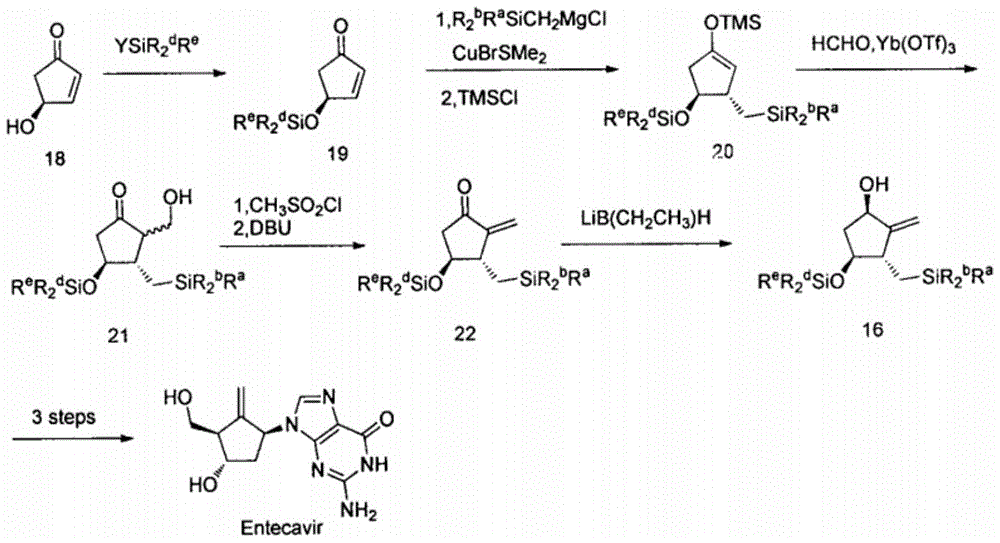
Novel synthetic method for entecavir compound
ActiveCN105037363ASolve rare problemsDosage controllableGroup 4/14 element organic compoundsKetoneAdipate
The invention belongs to the field of drug synthesis and relates to an entecavir compound and a synthetic method of an intermediate of the entecavir compound. The novel synthetic method comprises: by taking (S)-3-hydroxyl dimethyl adipate as an initial raw material, preparing an intermediate 9 through hydroxyl TBS protection, Dieckmann condensation reaction, ketone protection to ketal, ester group reduction to hydroxyl, hydroxyl protection, deprotection, ketone to silyl enol ether and Rubottom oxidizing reaction; and preparing entecavir from the intermediate 9 through wittig reaction, Mitsunobu reaction, silicon preventing radical group removal and basic hydrolysis. The novel synthetic method provided by the invention is mild and easily controllable in reaction condition, simple to operate, high in product yield, high in purity and suitable for industrialized mass production.
Owner:SHANDONG LUOXIN PARMACEUTICAL GROUP STOCK CO LTD +1
Preparation method of drug intermediate (S)-3-hydroxytetrahydrofuran
InactiveCN106957287ASource less restrictiveHigh catalytic activityOrganic chemistry methodsFermentationCarboxylic salt3-Hydroxytetrahydrofuran
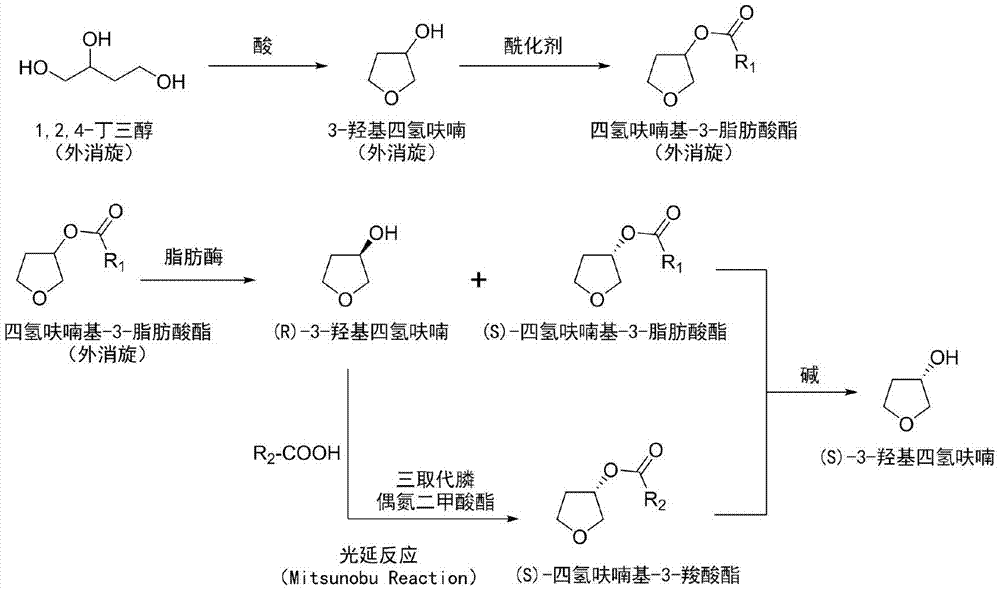
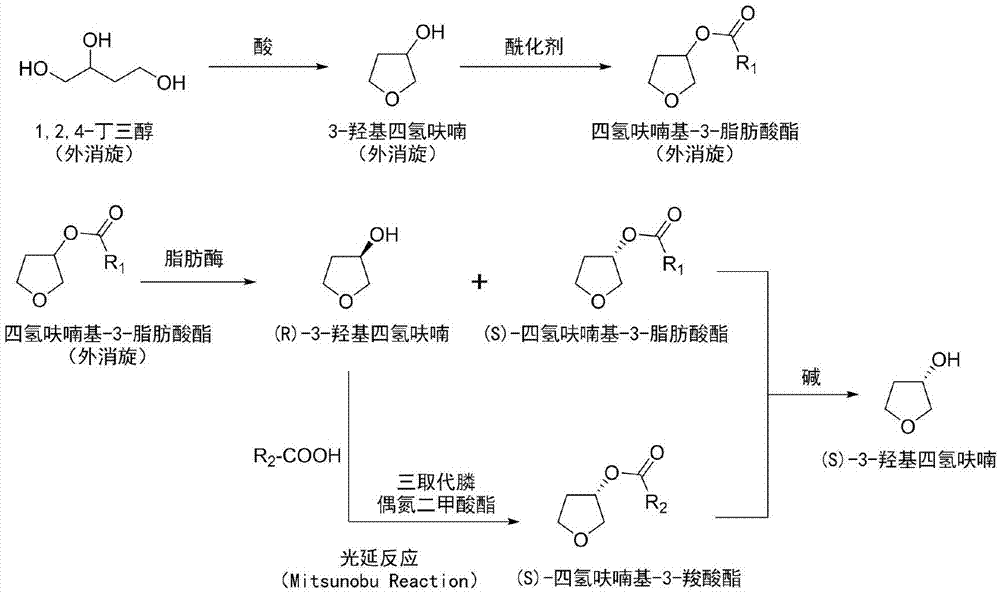
The invention provides a preparation method of a drug intermediate (S)-3-hydroxytetrahydrofuran. The method comprises the following steps: by taking racemic 1,2,4-butantriol as a raw material, synthesizing racemic 3-hydroxytetrahydrofuran; and carrying out esterification to obtain racemic tetrahydrofuryl-3-fatty acid ester. After the (R)-tetrahydrofuryl-3-fatty acid ester in a racemic mixture is hydrolyzed through lipase, the hydrolyzed (R)-tetrahydrofuryl-3-fatty acid ester is converted into (S)-tetrahydrofuryl-3-carboxylate by utilizing a Mitsunobu reaction under the condition that a hydrolyzed product is not separated; and finally, all tetrahydrofuryl ester is hydrolyzed under an alkaline condition to obtain a final product (S)-3-hydroxytetrahydrofuran.
Owner:杭州述康生物技术有限公司
Synthesis process for compound crizotinib
The invention provides a new synthesis method for crizotinib. An atomic economic reaction is adopted to reduce environmental pollution. A high-optical purity raw material is obtained by chiral prolinol induced chiral reduction; a chiral centre is constructed through an SN2 substitution reaction; post-processing and purification difficulties caused by Mitsunobu reaction are overcome. Malononitrile derivative is constructed by adopting a coupling reaction of malononitrile and bromo-pyridinium derivative; N,N-dicarboamide derivatives are obtained by performing aminolysis on N,N-dimethylamine hydrochloride; in the N,N-dicarboamide derivatives, N,N-dimethylamine serving as an easy-to-leave group and hydrazine perform a ring closing reaction to construct a pyrazolone ring, so that an expected final product, namely crizotinib, is obtained. According to the method, though continuous steps are used, the reaction of each step is high, the optical purity is high, and the total yield is also high. In addition, raw materials used in the synthesis method are low in cost and easily obtained; the using amount of a catalyst is small; total cost is easy to control. An operating process is simple and convenient and easy to control, and is suitable for industrial production.
Owner:甘肃皓骏药业有限公司
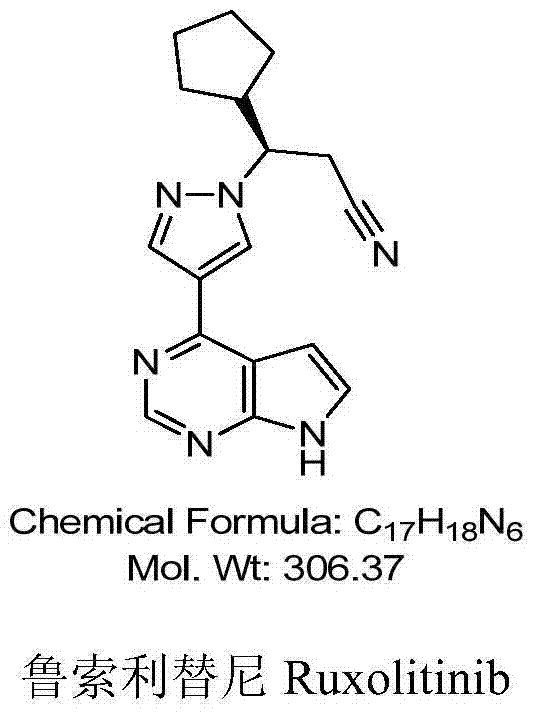
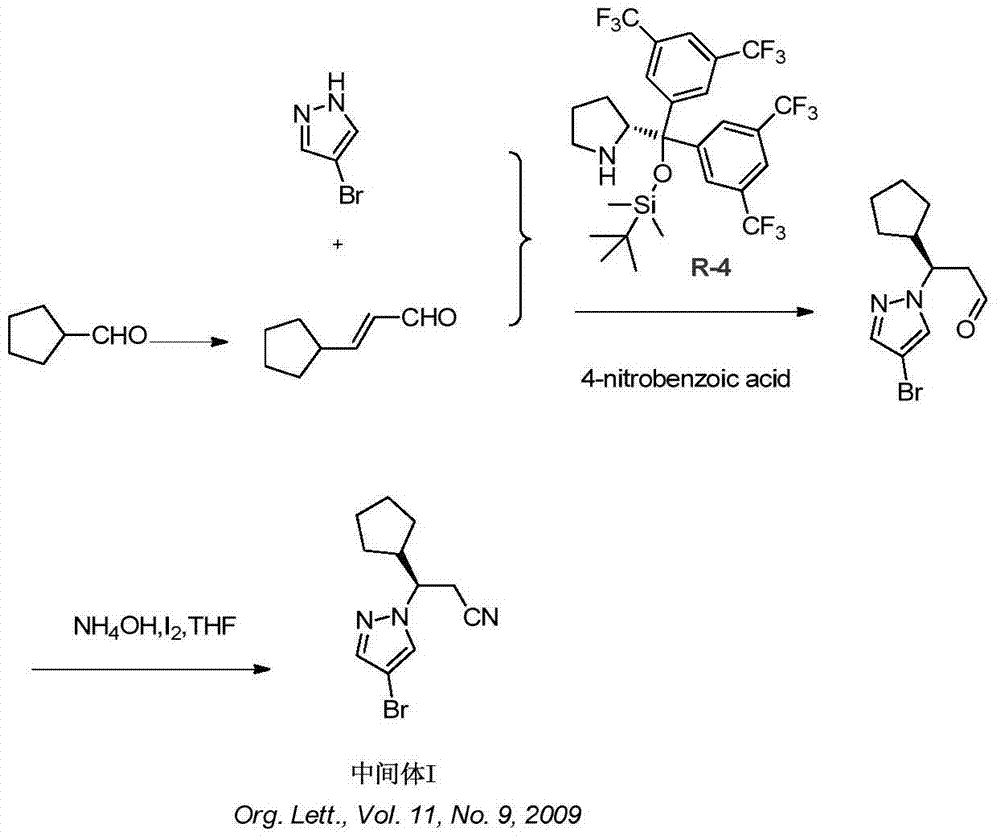
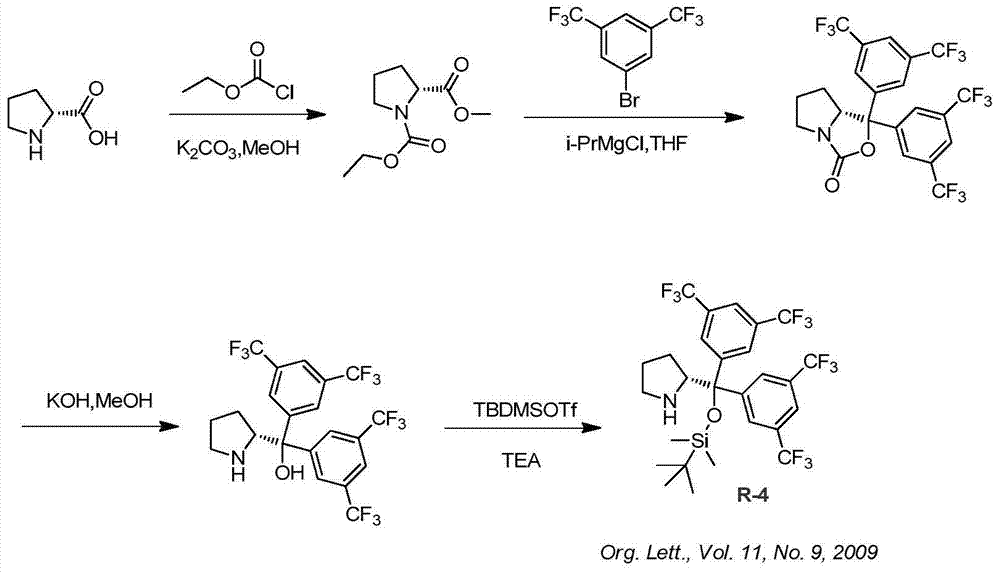
Synthesis method of ruxolitinib intermediate
The invention relates to a synthesis method of a ruxolitinib intermediate. The method comprises the following steps: firstly, carrying out catalytic reaction on cyclopentane methyl formate and acetonitrile to prepare 3-cyclopentyl-3-oxypropionitrile; carrying out enzymatic asymmetric reduction on 3-cyclopentyl-3-oxypropionitrile to generate chiral alcohol (S)-3-cyclopentyl-3-oxypropionitrile; and carrying out Mitsunobu reaction and 4-bromopyrazole coupling on (S)-3-cyclopentyl-3-oxypropionitrile to obtain ruxolitinib intermediate (3R)-3-(4-bromo-1H-pyrazole-1-yl)-3-cyclopentane propionitrile. The synthesis method has the advantages of short route, low cost, mild condition and good stereoselectivity, and is suitable for industrialized mass production.
Owner:BIOCOMPOUNDS PHARMACEUTICAL INC +1
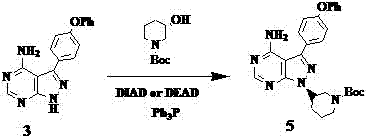
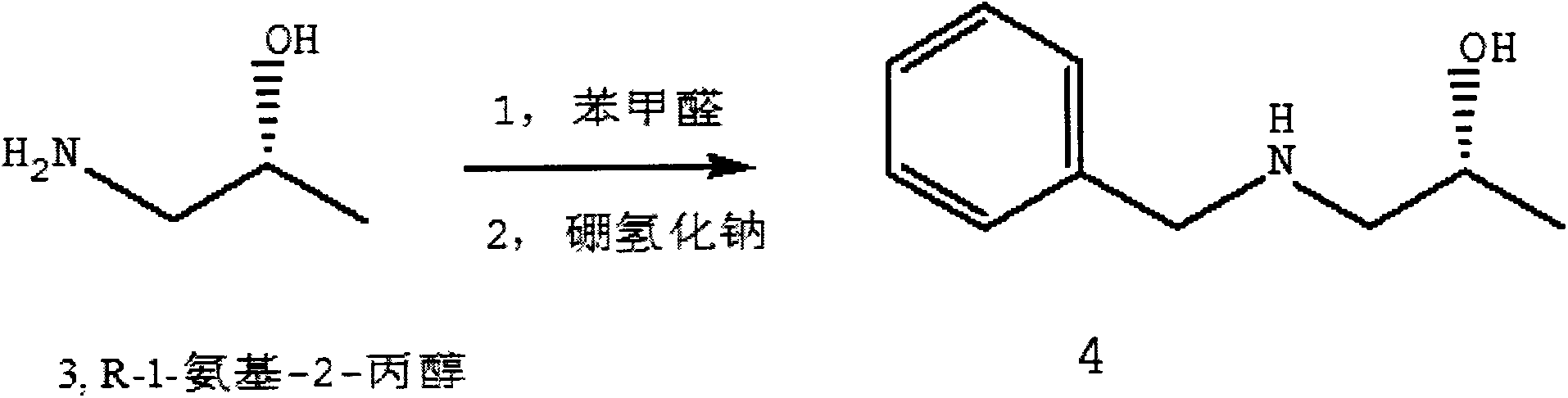
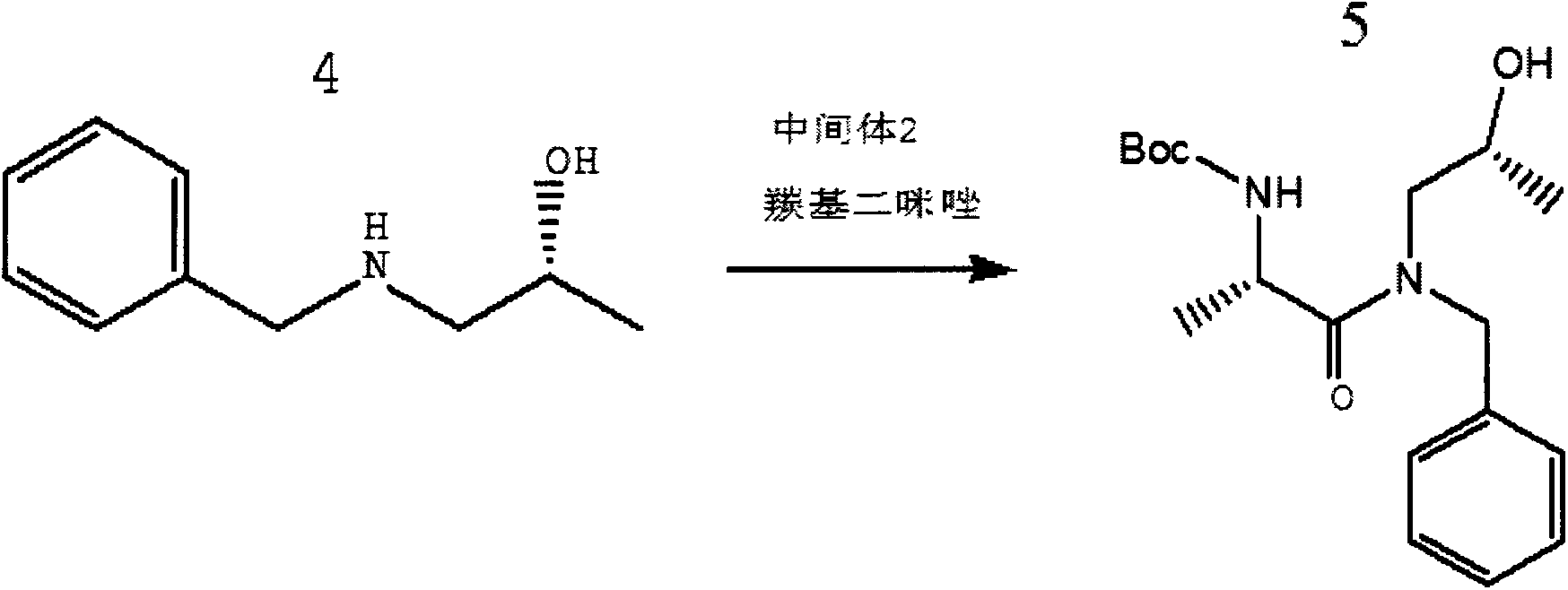
Preparation method of ibrutinib
ActiveCN106008526AHigh yieldQuality improvementOrganic chemistryBulk chemical productionTert-Butyloxycarbonyl protecting groupMitsunobu reaction
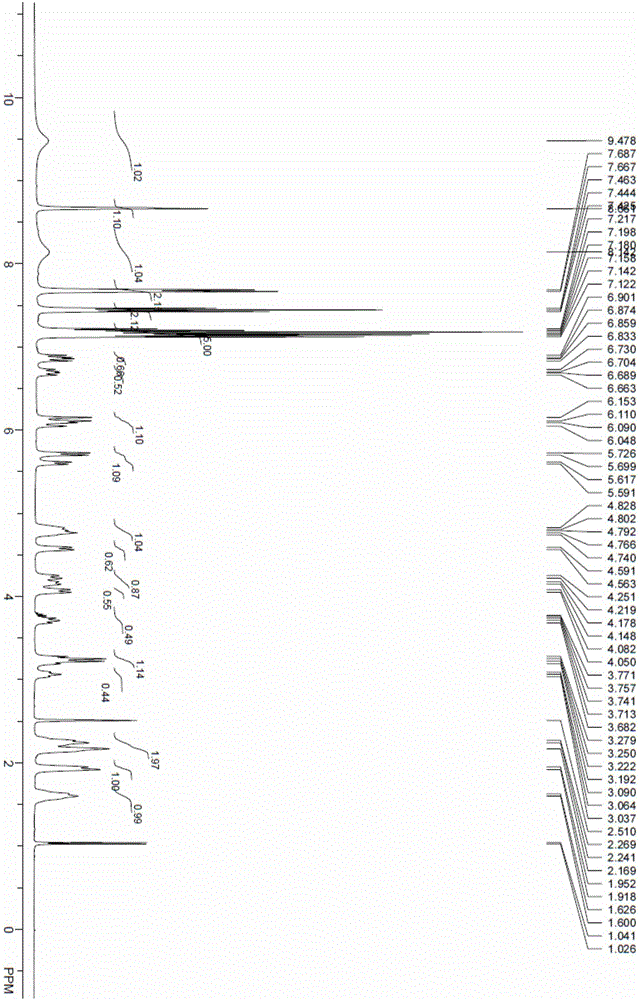
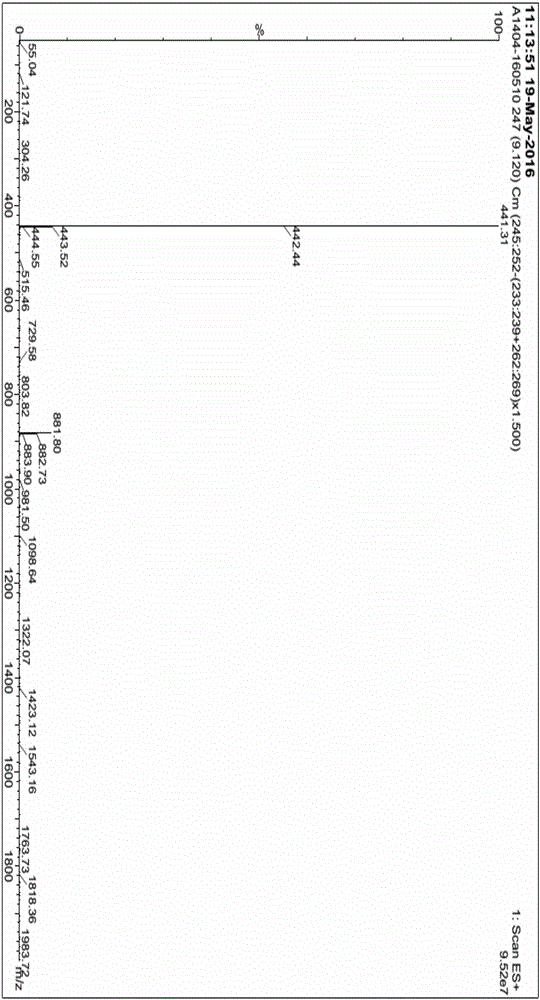
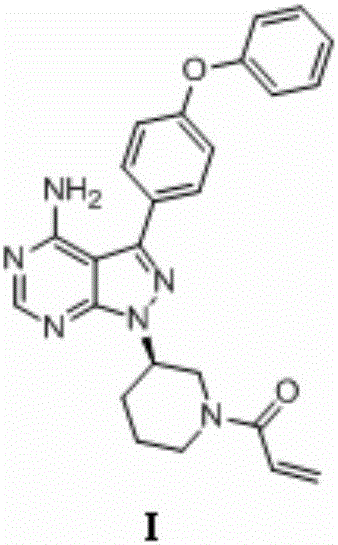
The invention relates to the technical field of medicine, in particular to a preparation method of ibrutinib. The preparation method of ibrutinib comprises the following steps that 4-amino-3-(4-phenoxy phenyl)-1H-pyrazol[3,4-d]pyrimidine and S-1-tert-butyloxycarbonyl-3-hydroxyl piperidine are prepared into a compound shown in the formula IV (please see the formula in the description) through a Mitsunobu reaction, and a Boc protecting group of the compound shown in the formula IV is removed to prepare a compound shown in the formula V (please see the formula in the description); the compound shown in the formula V and acrylic ester are prepared into a compound shown in the formula I (please see the formula in the description) in the presence of a catalyst and an activating agent. According to the preparation method, the reaction process is mild in condition, few reaction steps are needed, high temperature and copious cooling are not needed, no high-toxicity reagent is adopted, and the whole synthesizing process is stable and controllable; ibrutinib prepared through the method is high in yield and quality and has the advantages of being good in stability, high in purity, convenient to store and the like.
Owner:BIOCOMPOUNDS PHARMACEUTICAL INC +1
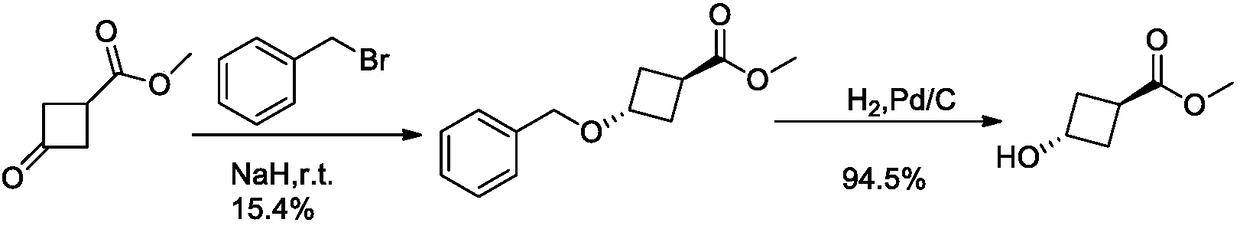
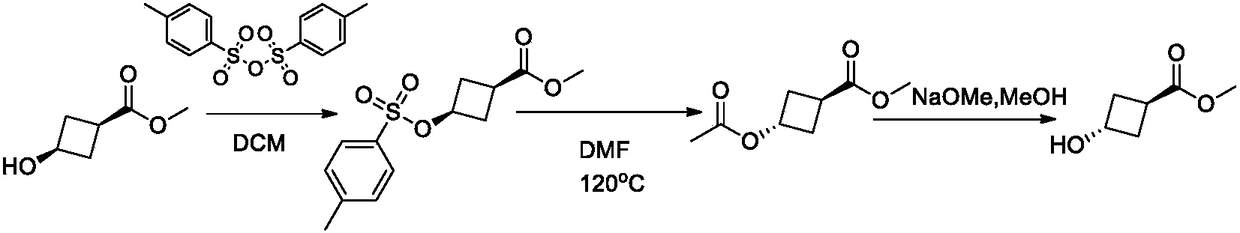
Method for synthesizing trans-3-hydroxy cyclobutyl formic acid
ActiveCN108129288AHigh stereoselectivityMild responseOrganic compound preparationCarboxylic acid esters preparationFormateSynthesis methods
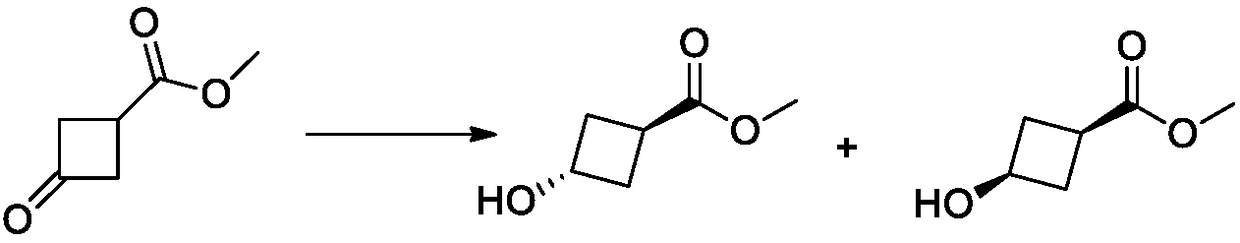
The invention belongs to the field of organic synthesis and discloses a method for synthesizing trans-3-hydroxy cyclobutyl formic acid. The method comprises the following steps: by using an appropriate reducing agent, reducing 3-carbonyl-cyclobutane formate (C1-C6 alkyl ester) into single trans-3-hydroxy cyclobutyl formic ether in an efficient stereoselectivity manner, and further performing a Mitsunobu reaction and hydrolysis, thereby obtaining single trans-3-hydroxy cyclobutyl formic acid. The method provided by the invention is high in synthesis method raw material availability, mild in reaction condition, good in stereoselectivity and relatively high in yield; in addition, aftertreatment and purification are both easy to operate, and the method is applicable to industrial amplified production.
Owner:上海毕得医药科技股份有限公司
A kind of new synthetic method of entecavir compound
ActiveCN105037363BSolve rare problemsDosage controllableGroup 4/14 element organic compoundsWittig reactionDrugs synthesis
The invention belongs to the field of drug synthesis and relates to an entecavir compound and a synthetic method of an intermediate of the entecavir compound. The novel synthetic method comprises: by taking (S)-3-hydroxyl dimethyl adipate as an initial raw material, preparing an intermediate 9 through hydroxyl TBS protection, Dieckmann condensation reaction, ketone protection to ketal, ester group reduction to hydroxyl, hydroxyl protection, deprotection, ketone to silyl enol ether and Rubottom oxidizing reaction; and preparing entecavir from the intermediate 9 through wittig reaction, Mitsunobu reaction, silicon preventing radical group removal and basic hydrolysis. The novel synthetic method provided by the invention is mild and easily controllable in reaction condition, simple to operate, high in product yield, high in purity and suitable for industrialized mass production.
Owner:SHANDONG LUOXIN PHARMA GRP CO LTD +1
Method for preparing midbody of heart failure medicine
ActiveCN105254589AComplianceFew reaction stepsGroup 4/14 element organic compoundsCarbamic acid derivatives preparationThiazoleMitsunobu reaction
The invention discloses a compound which is tert-butyl (S)-4-(((tert-butyl dimethyl silicane) oxy) methyl)-1,2,3-oxy thiazole-3-carboxylic acid 2,2-dioxide, a preparation method thereof, and a method for synthesizing a midbody of a heart failure medicine by using the compound. By using the method disclosed by the invention, DEAD which has exposure danger is not used, the reaction steps can be shortened, mitsunobu reaction can be avoided, the synthesis process is relatively low in cost, relatively environment-friendly and applicable to industrial capacity, and the method is simple to operate, and as API, can relatively well meet the requirements of the State Food and Drug Administration.
Owner:SHANGHAI BOC CHEM CO LTD
C-aryl glucoside derivative, as well as medical composition, preparation method and application thereof
InactiveCN105017236AEnhanced inhibitory effectEffective treatmentOrganic active ingredientsOrganic chemistryArylChemical composition
The invention relates to a C-aryl glucoside derivative, as well as a medical composition, a preparation method and application thereof. The preparation method comprises the following steps of: method I, in a solvent, under the action of alkali, performing a deacetylation protecting group reaction on compounds 1-f; method II: 1, performing a Mitsunobu reaction on components 2-g and (as shown in the description); and 2, performing a deacetylation protecting group reaction on the compounds 2-f obtained in the step 1; and method III: 1, mixing compounds 3-g and (as shown in the description), and performing a nucleophilic substitution reaction; and 2, performing a deacetylation protecting group reaction on the compounds 3-f obtained in the step 1. The medical composition comprises the C-aryl glucoside derivative, salt and / or prodrug of the C-aryl glucoside derivative which are / is acceptable in pharmacy, and auxiliary materials. The invention further relates to the C- aryl glucoside derivative, and application of salt or medical compositions of the C- aryl glucoside derivative, which is acceptable in pharmacy, for preparing SGLT inhibitors. The C- aryl glucoside derivative disclosed by the invention provides a new direction for research of the SGLT inhibitors. (As shown in the description)
Owner:SHANGHAI DE NOVO PHARMA
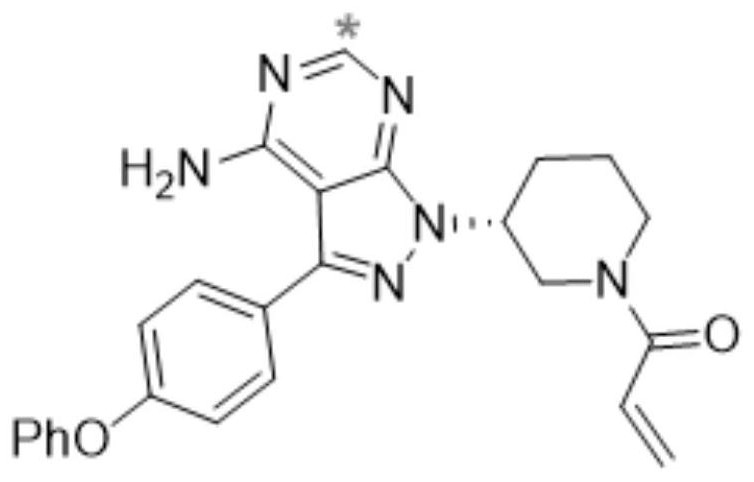
Separation and purification method of ibrutinib intermediate
InactiveCN106967071AImprove separation and purification efficiencyOrganic chemistryPurification methodsChloride
The invention discloses a separation and purification method of an ibrutinib intermediate-(3R)-4-amino-3-(4-phenoxyphenyl)-1-(1-tert-butoxycarbonylpiperidine-3-group)-1-1H-pyrazole [3, 4-d] pyrimidine. The separation and purification method includes: after Mitsunobu reaction for preparing the intermediate stops, adding magnesium chloride into a mixture; cooling after back-flowing; filtering to remove compound precipitate of magnesium chloride and triphenyl phosphine oxide to obtain the intermediate high in purity. Column chromatography is omitted in the process, so that the separation and purification method is high in efficiency and low in cost.
Owner:FUJIAN INST OF MICROBIOLOGY
Application of LJ reaction in mitsunobu reaction
InactiveCN102442957APhosphorus halides/oxyhalidesGroup 5/15 element organic compoundsMitsunobu reactionCleaner production
The invention relates to application of a novel heterogeneous reaction in an LJ molecule in a mitsunobu reaction. Phosphorus pentahalide substitutes for a classic mitsunobu reaction coupling reagent, so that the yield in most of the mitsunobu reactions is improved, the cost is reduced, and the standard of clean production process is reached.
Owner:WUHAN INSTITUTE OF TECHNOLOGY
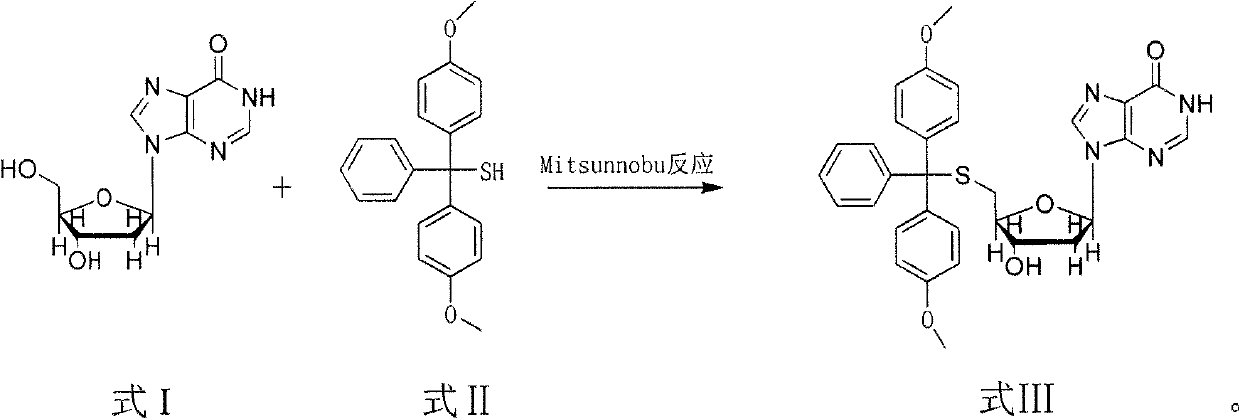
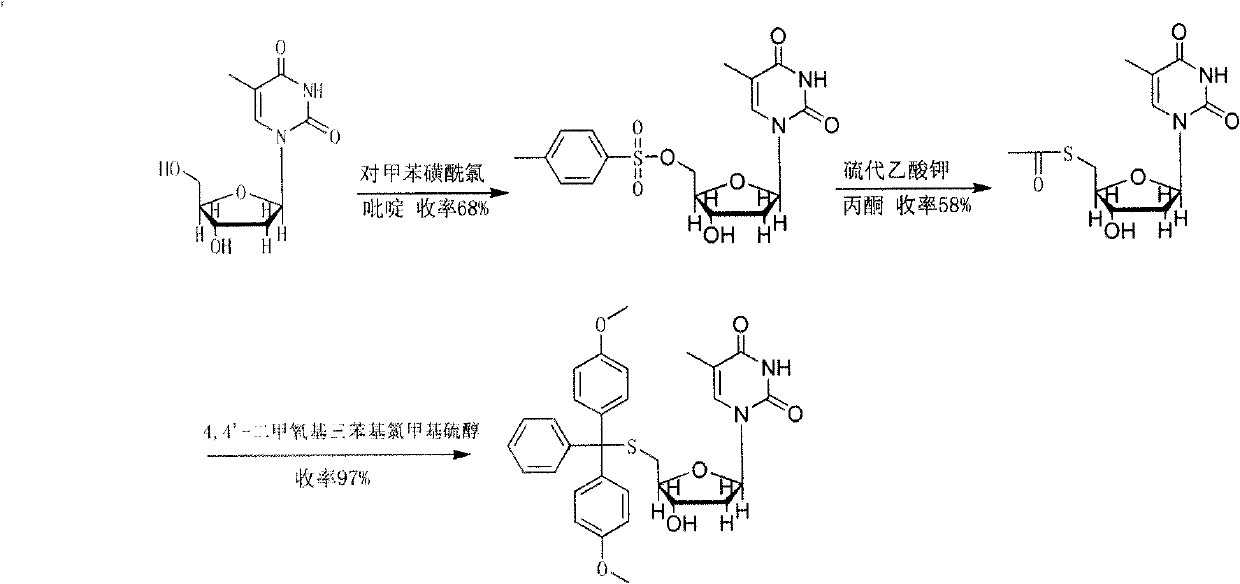
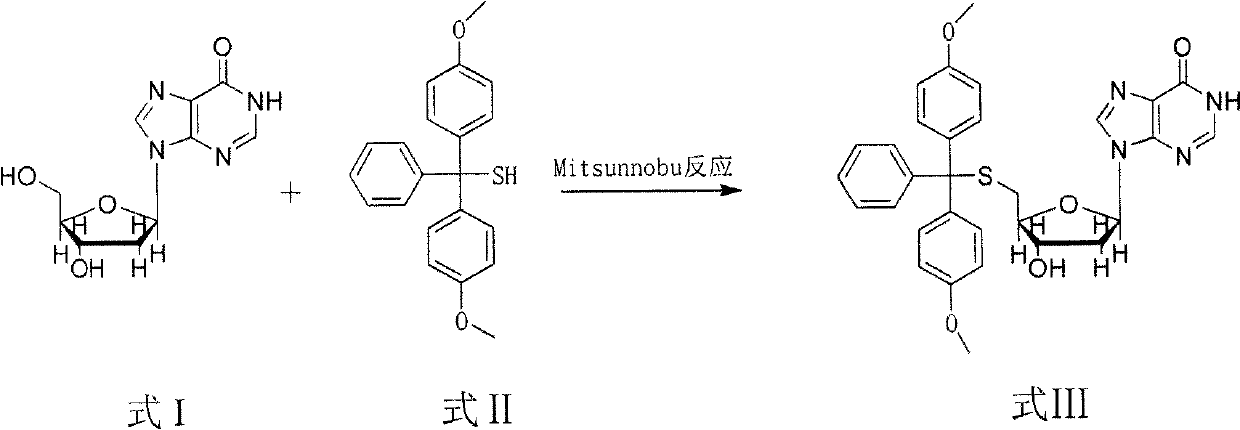
Method for synthesizing and preparing 5'-S-(4, 4'-dimethoxytriphenylmethyl)-2'-deoxyinosine
ActiveCN103073606AMild reaction conditionsHigh selectivitySugar derivativesSugar derivatives preparationOrganic solventRoom temperature
The invention discloses a method for synthesizing and preparing 5'-S-(4, 4'-dimethoxytriphenylmethyl)-2'-deoxyinosine. The method comprises the following steps of: under inert gas protection, carrying out mitsunobu reaction on 2'-deoxyinosine and 4, 4'-dimethoxytriphenylmethyl mercaptan in an organic solvent at room temperature under effects of an azo reagent, a phosphine coordination compound, organic alkali or protonic acid, i.e. carrying out intermolecular dehydration reaction on 5'-hydroxyl of the 2'-deoxyinosine and the 4, 4'-dimethoxytriphenylmethyl mercaptan to obtain a C-S bond, and obtaining the 5'-S-(4, 4'-dimethoxytriphenylmethyl)-2'-deoxyinosine. The method for synthesizing and preparing the 5'-S-(4, 4'-dimethoxytriphenylmethyl)-2'-deoxyinosine has the beneficial effects of being wide in raw material sources, low in price, high in yield, mild in reaction condition, good in selectivity, simple in operation process, convenient in post-treatment, and suitable for large-scale industrial production.
Owner:NAT INST OF PHARMA R & D CO LTD
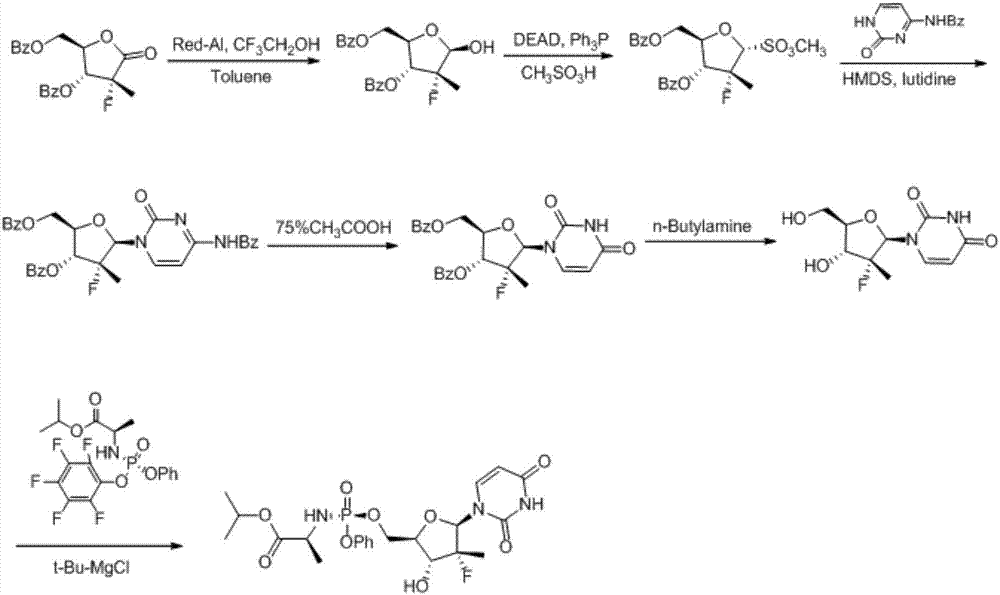
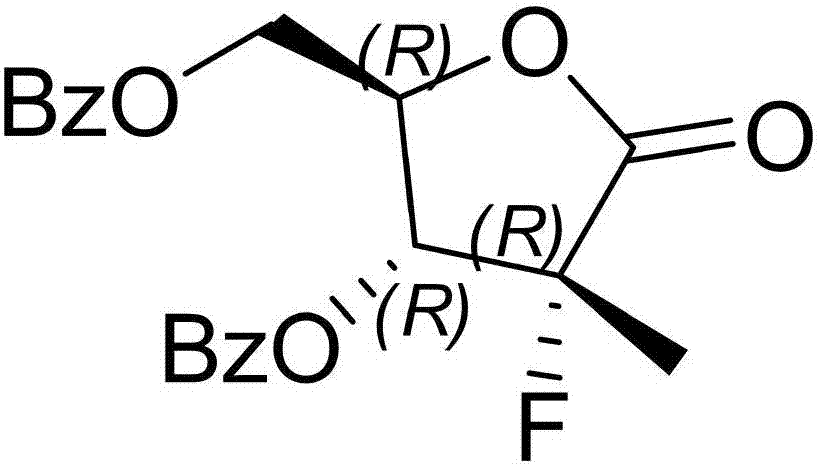
Synthesis method of sofosbuvir
ActiveCN106905398AAvoid generatingReduce usageSugar derivativesSugar derivatives preparationSulfonateSynthesis methods
The invention provides a synthesis method of sofosbuvir. The synthesis method of the sofosbuvir comprises the following steps: performing mitsunobu reaction on ((2R,3R,4R)-3-benzoyloxy)-4-fluorine-5-hydroxyl-4-methyltetrahydrofuran-2-yl)methyl benzoate to produce sulfonate to obtain a compound 1; abutting the compound 1 and N-benzoylcytosine to produce a compound 2. The method adopts mitsunobu reaction to avoid production of an isomer, and the isomer is reduced to 5 percent or below; according to the method, sulfonate and N-benzoylcytosine are abutted, so the use ofa stannic chloride raw material is avoided; furthermore, the yield is high and few solid waste is generated during aftertreatment, so that the method is suitable for large-scale industrialized production.
Owner:ZHEJIANG FORESTRY UNIVERSITY
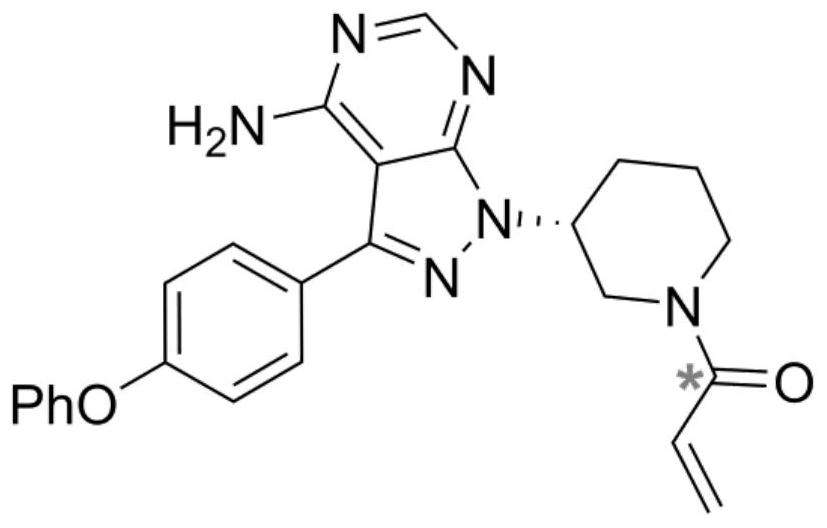
Synthesis and purification process of ibrutinib intermediates
InactiveCN106083860ASave man hoursLow costOrganic chemistryTert-Butyloxycarbonyl protecting groupMitsunobu reaction
The invention relates to a synthesis and purification process of ibrutinib intermediates, in particular to a process to obtain ibrutinib intermediates with the purity being greater than 97 percent by performing Mitsunobu reaction and Boc removal processes on 3-(4-phenoxyl phenyl)-1H-pyrazole[3,4-d]pyridine-4-amine and (s)-1-t-butyloxycarboryl-3-hydroxypiperidine, triphenylphosphine and DIAD to obtain intermediate mixing samples, using solvents for crystallization to obtain intermediate coarse products, and then recrystallizing the coarse products by a mixed solvent method. According to the process, a one-pot method is used for obtaining the ibrutinib intermediates; the reaction process is simple; the yield is high; meanwhile, an efficient refining method is also provided.
Owner:AMICOGEN CHINA BIOPHARM CO LTD
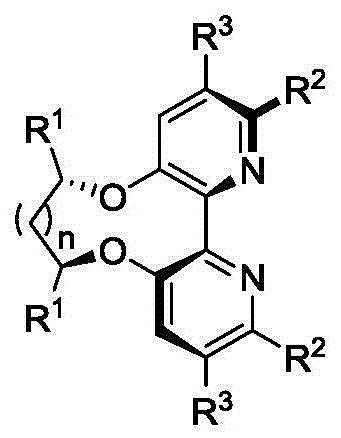
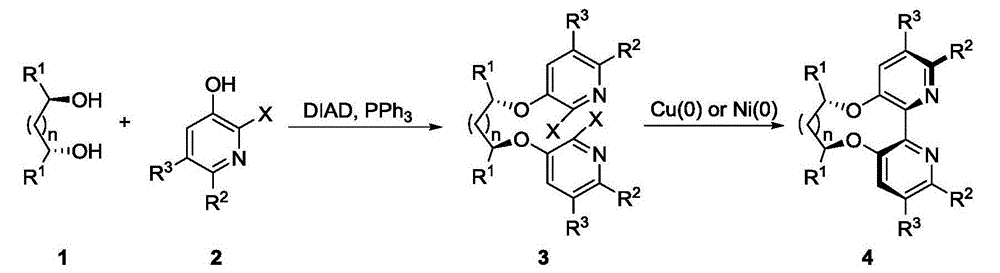
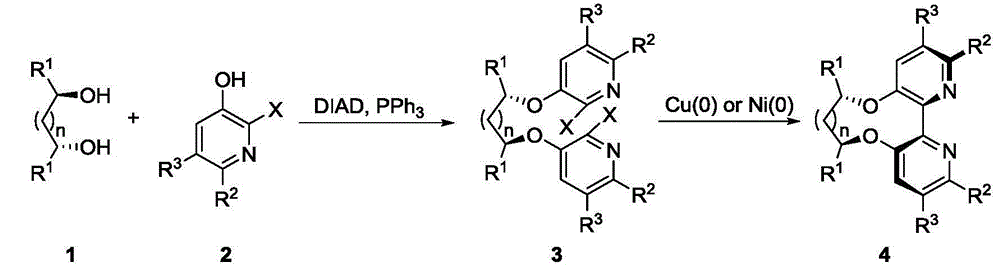
Dipyridine ligands with axial chirality and synthetic method thereof
The invention relates to dipyridine ligands with axial chirality. A synthetic method adopts 3-hydroxy-2-halogen pyridine as an initial raw material, and includes loading a chiral skeleton with the pyridine through a Mitsunobu reaction with a chiral diol, and achieving pyridine coupling through an Ullman reaction catalyzed by nickel (0) or copper (0) to obtain the dipyridine ligands induced by the chiral diol. The method is simple and convenient in operation and high in yield. The synthetic method is more practical when being compared with traditional methods.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
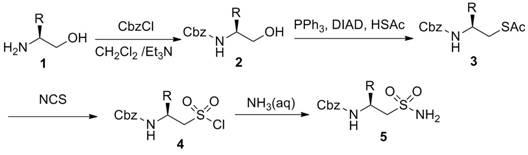
Preparation method of N-carbobenzoxy-2-amino-alkyl sulfonamide
InactiveCN102675165ARaw materials are non-toxic and easy to obtainSimple and fast operationSulfonic acid amide preparationImideCombinatorial chemistry
The invention provides a preparation method of N-carbobenzoxy-2-amino-alkyl sulfonamide. The preparation method of N-carbobenzoxy-2-amino-alkyl sulfonamide includes using vicinal alkamine as material, and sequentially performing carboxybenzyl protection, Mitsunobu reaction, NCS (N-chlorosuccinimide) oxidation and ammonolysis reaction to obtain the N-carbobenzoxy-2-amino-alkyl sulfonamide. The materials used in the method are nontoxic and easy to obtain, simple and convenient to operate, high in reproducibility and high in yield. The preparation method is a current most effective method of synthesizing amino-alkyl sulfonamide. The obtained compound can be used as an enzymatic inhibitor, raw materials to prepare sulfonopeptide and the like.
Owner:BEIJING UNIV OF CHEM TECH
Preparation method of 1,4-dioazo-cycloheptane derivative
The invention discloses a preparation method of a 1,4-dioazo-cycloheptane derivative. The preparation method comprises the steps of carrying out multiple coupling on a compound of a formula (VI) and a compound of a formula (V) at -5-0 DEG C in aromatic hydrocarbon or a halogenated hydrocarbon solvent in the presence of alkali, after the coupling, removing hydroxyl protection from the compound of the formula (VI) by virtue of TBAF, carrying out self-cyclization by virtue of a Mitsunobu reaction to obtain a compound of a formula (II), and removing amino protection from the compound of the formula (II) by virtue of a hydrochloric acid ethyl acetate solution, so as to obtain the target compound 1,4-dioazo-cycloheptane derivative. The preparation method has the advantages that synthetic steps are few, the reaction condition of each step is mild, and the operation is simple convenient, so that the production cost is lowered; and more importantly, the yield of the 1,4-dioazo-cycloheptane derivative synthesized by the method is high.
Owner:WUCHANG UNIV OF TECH +1
Method for preparing escitalopram
InactiveCN101607951AGood shape retentionWill not racemizeNervous disorderOrganic chemistryOrganic solventEscitalopram
The invention discloses a method for preparing escitalopram represented by a formula (I), which comprises the following step: reacting S-diol represented by a formula (II) in an aprotic organic solvent under the action of a phosphine complex compound, an azo reagent and a proton supplying agent in an atmosphere of an inert gas to obtain the escitalopram represented by the formula (I). The method uses a Mitsunobu reaction in the cyclization preparation of escitalopram represented by the structural formula (I) for the first time and has the unexpected advantages of excellent product configuration retention, prevention of racemization in a cyclization process and high optical purity and yield. In addition, the method of the invention is simple in operation, mild in condition, simple and convenient in post processing and suitable for large-scale industrial production.
Owner:SHANGHAI INST OF PHARMA IND +1
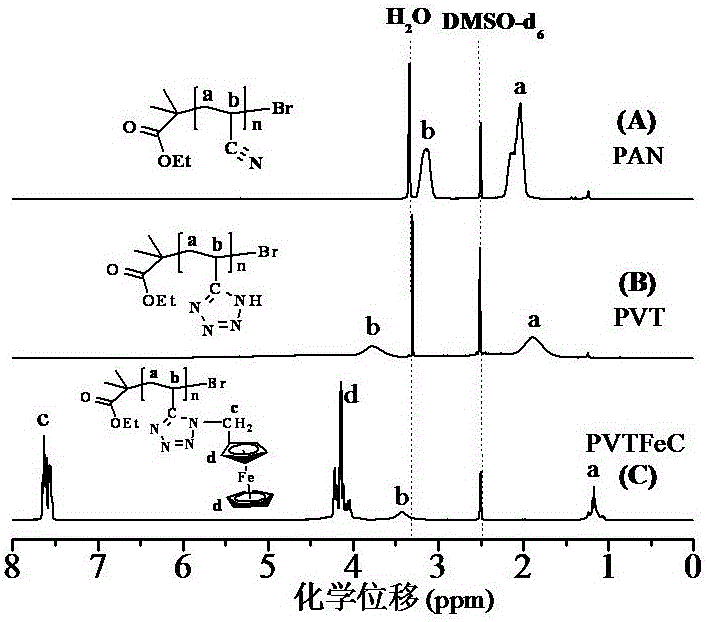
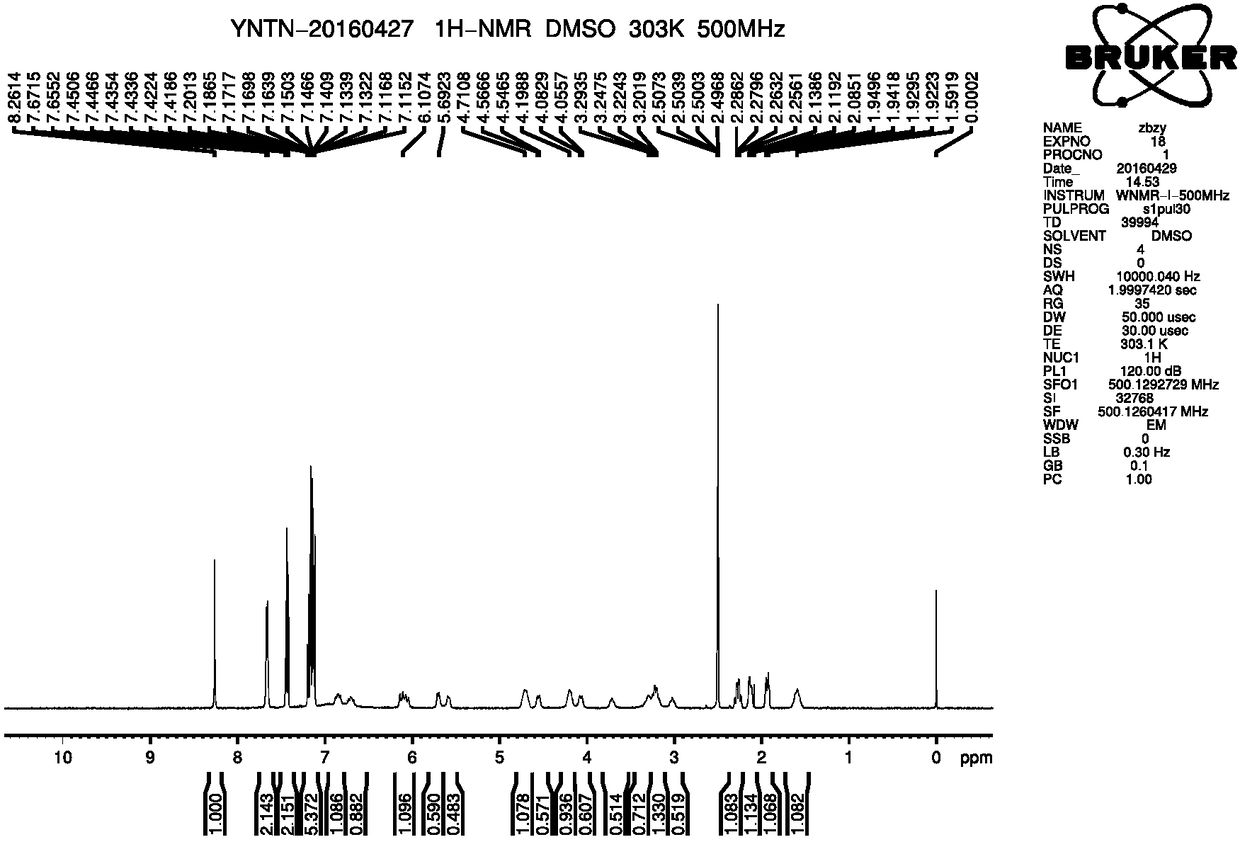
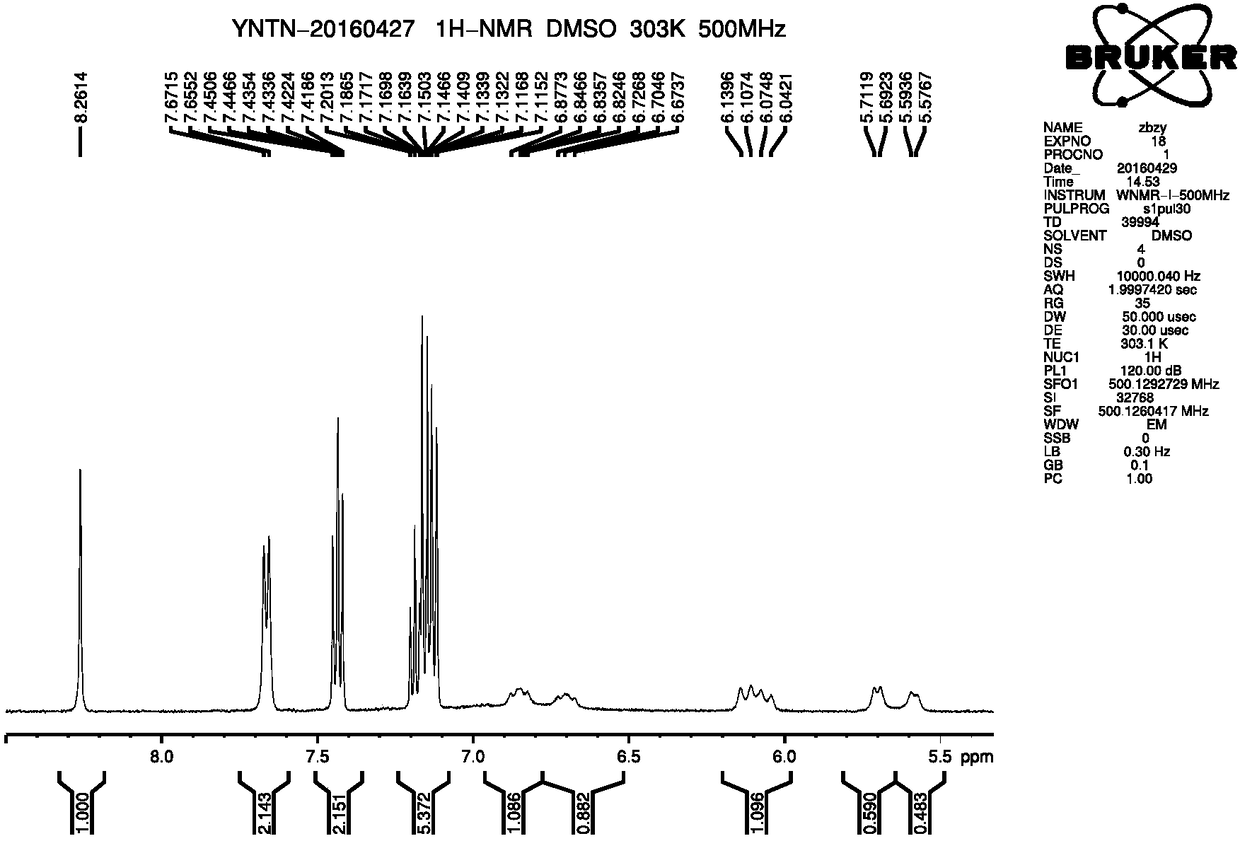
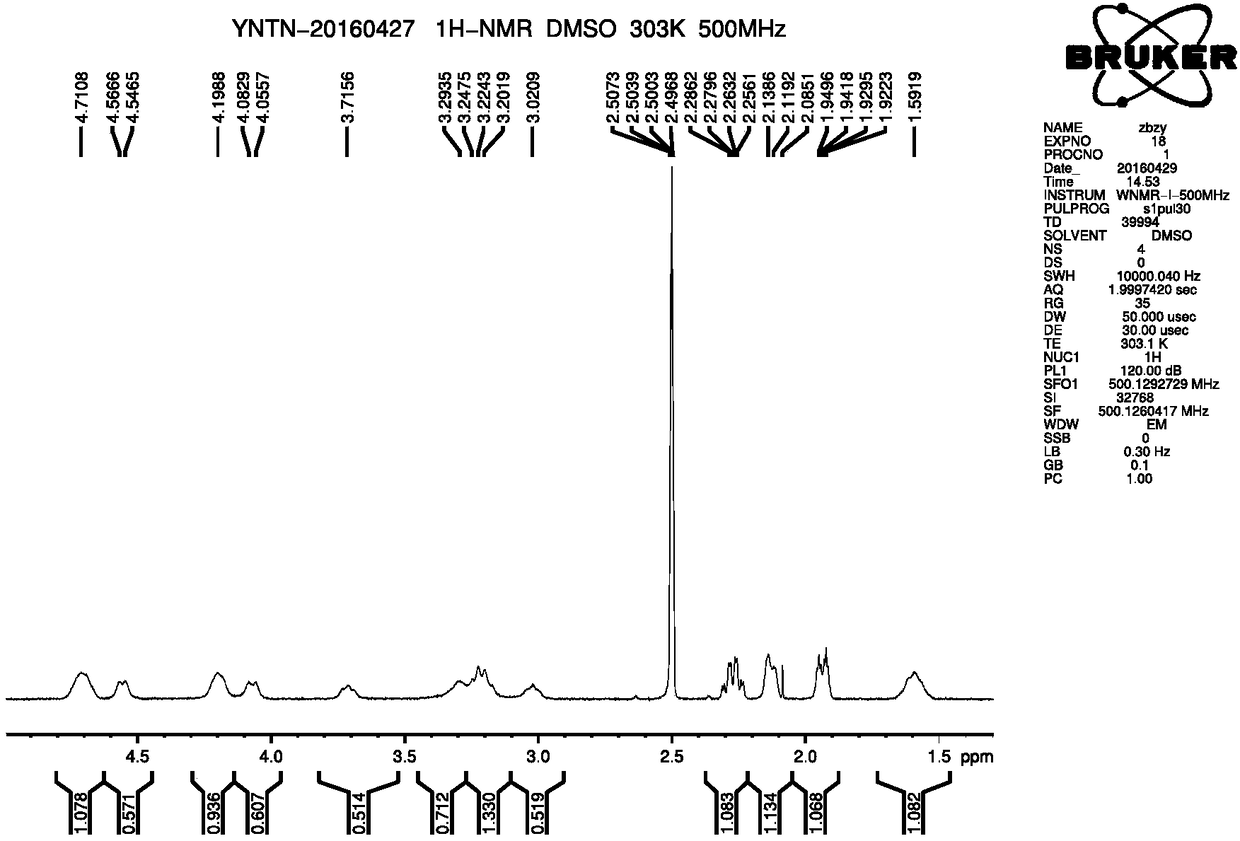
Novel method for preparing ferrocenyl polymer from controllable type polyacrylonitrile resin
The invention discloses a novel method for preparing a ferrocenyl polymer from controllable type polyacrylonitrile resin. The reaction of the novel method comprises the following steps of (1) preparing the polyacrylonitrile resin into a reaction system of polyvinyl tetrazolium (PVT) through a nitrile based click chemistry reaction, and enabling polyacrylonitrile, sodium azide, a catalyst and a solvent to be subjected to a reaction at certain temperature for a certain time so as to obtain an intermediate product PVT; and (2) preparing a reaction system of the ferrocenyl polymer (PVT-FeC) through a Mitsunobu reaction, and enabling the intermediate product PVT prepared in the step (1), ferrocenemethanol, triphenylphosphonium and diethyl azodicarboxylate to be subjected to a reaction at appropriate temperature for a certain time so as to obtain the ferrocenyl polymer. The molecular weight of the obtained polymer can be conveniently designed, besides, the end group of the polymer can still have activity, and the polymer can be used for synthesizing some other functional copolymers with a topological structure.
Owner:LUDONG UNIVERSITY
Green and environment-friendly preparation method of tenofovir
ActiveCN111205326ALow costThe process is environmentally friendlyGroup 5/15 element organic compoundsOrganic chemistry methodsMethylphosphonic acidPhosphine
The invention discloses a green and environment-friendly tenofovir preparation method which comprises the following steps: dissolving a compound I, S-propylene carbonate and an inorganic weak base inan organic solvent, reacting for 3-6 hours at 85-120 DEG C, cooling to room temperature, and concentrating an obtained system under reduced pressure to obtain an intermediate II; dissolving the intermediate II, hydroxymethylphosphonic acid dialkyl ester and trialkyl (aryl) phosphine in an organic solvent, stirring at room temperature, slowly adding azodicarboxylic acid diester, and reacting for 20minutes to 3 hours to obtain an intermediate III; slowly adding an inorganic strong alkali into the intermediate III, carrying out ice bath, filtering, adjusting the pH value of an obtained filtrate,standing, carrying out suction filtration, washing an obtained filter cake, and carrying out vacuum drying under reduced pressure. According to the method, S-propylene carbonate, adenine and derivatives thereof are taken as initial raw materials; PMPA is generated through configuration inversion of Mitsunobu reaction, the used organic solvents can be recycled, the generated wastewater is mainly aharmless inorganic salt solution, the cost for further treatment and up-to-standard discharge is low, the method is environmentally friendly, the reaction is easy to control, the safety is high, andthe comprehensive economic benefit is high.
Owner:南京道尔医药科技有限公司
A kind of preparation method of ibrutinib
ActiveCN105859728BHigh purityHigh yieldGroup 4/14 element organic compoundsFormamidine acetateMitsunobu reaction
The invention discloses a preparation method for ibrutinib and belongs to the technical field of drug synthesis. The preparation method specifically includes the steps that 3-amino-4-cyano pyrazol and formamidine acetate serve as initial raw materials, and ibrutinib is obtained through a cyclization reaction, a halogenating reaction, a nucleophilic substitution reaction, a Mitsunobu reaction and an amidation reaction. According to the method, the raw materials are easy to obtain, conditions are mild, the process operability and controllability are high, cost is low, the yield is high, fewer side products are generated, purification is easy, and the high-quality product is obtained.
Owner:南京红太阳医药研究院有限公司
Novel technology for trans-2,6-lupetazin
The invention relates to a novel technology for trans-2,6-lupetazin. Chirality substances of S-configuration alanine or R-configuration alanine are adopted, carbonyl imidazole is taken as a condensing agent for preparing dipeptide, walden inversion occurs through a mitsunobu reaction in cyclization so as to obtain a trans-form chirality structure, and then the final product 2S, 6S-lupetazin or 2R, 6R-lupetazin is obtained through reducing by using a reducing agent. Reactions in each step need purification treatment, so that the purity achieves over 98%, and a chemical intermediate of trans 2S, 6S-lupetazin or trans 2R, 6R-lupetazina has pharmaceutical activity and is high in yield.
Owner:PORSE FINE CHEM
Method for using zinc chloride to separate and purify ibrutinib intermediate
InactiveCN109232581AImprove separation and purification efficiencyOrganic chemistryPurification methodsChemistry
The invention discloses a method for using zinc chloride to separate and purify an ibrutinib intermediate, and particularly relates to a separation and purification method of the ibrutinib intermediate (3R)-4-amido-3-(4-phenoxy phenyl)-1-(1-tert-butoxy carbonyl piperidine-3-yl)-1H-pyrazolo[3,4-d]pyrimidine. After Mitsunobu reaction for preparing the intermediate is terminated, zinc chloride is added into a mixture, heated and cooled, composition sediment of zinc chloride and triphenylphosphine oxide is removed by filtering, and the intermediate with good purity is obtained. The process omits column chromatography, and is an efficient and low-cost separation and purification method.
Owner:FUJIAN INST OF MICROBIOLOGY
Synthetic method of entecavir
ActiveCN102924454AEasy to controlRaw materials are cheap and easy to getOrganic chemistryBulk chemical productionEntecavirLithium aluminium hydride
The invention discloses a synthetic method of entecavir. The synthetic method of the entecavir is characterized in that Corey lactone diol serves as initial raw materials, hydroxy group is used for protection, lithium aluminium hydride is used for reduction, then dimethyl tertiary butyl chlorosilane is used to selectively protect the primary hydroxy group, mitsunobu reaction is carried out, 6-substituted guanine is used for condensation, silicon substrate protecting group is removed, alkene is eliminated, then ozone is cut off and reduced, the alkene is eliminated through the hydroxy group, and at last the entecavir is obtained after the protection is removed. The reaction of the synthetic method of the entecavir is easy to control and simple, the raw materials are cheap and easy to obtain, the operation is simple, convenient and environment-friendly, and therefore the synthetic method of the entecavir is suitable for industrialized production.
Owner:苏州市玮琪生物科技有限公司
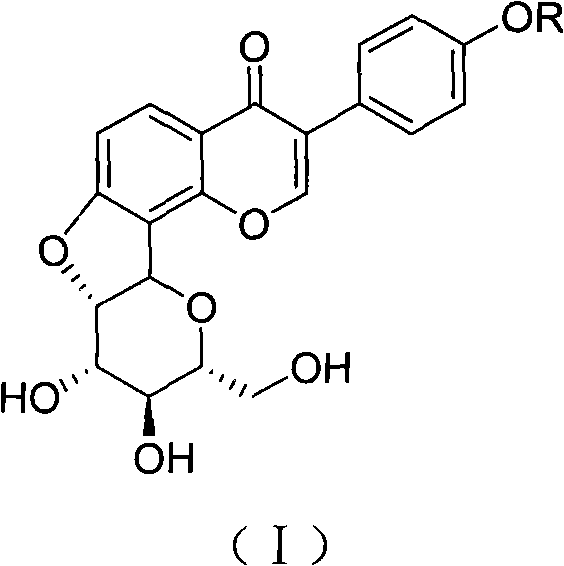
7,2'-dehydration puerarin, salt derivatives as well as preparation method and application thereof
InactiveCN101921282AImprove oral bioavailabilityOvercome the defect of poor oral absorption bioavailabilityOrganic active ingredientsOrganic chemistryDiseaseAngina
The invention relates to a compound 7,2''-dehydration puerarin in a structural formula (I) and salt derivatives thereof. The 7,2''-dehydration puerarin is prepared from puerarin through intramolecular Mitsunobu reaction. The invention can remarkably reduce the arrhythmia duration and prolong the cogulation time. The monomer compound and medical salts thereof can be prepared into orally-taken or injection agents and can be used for treating cardiovascular and cerebrovascular diseases of arrhythmia, coronary disease, angina, myocardial infarction, cerebral infarction, and the like.
Owner:SHANDONG UNIV
Synthesis process of compound D-2- aminoxy-3-methylbutyric acid
InactiveCN102911085ARaw materials are easy to getLower synthesis costOrganic chemistryChemical synthesis3-methylbutyric acid
The invention discloses a synthesis process of compound D-2- aminoxy-3-methylbutyric acid, and belongs to the technical field of chemical synthesis. The synthesis process includes taking natural amino acid L-valine as a raw material, and then synthetizing the non-natural D-2- aminoxy-3-methylbutyric acid by steps of diazotization hydrolysis, acetyl protection, tert-butyl esterification, deacetylation, Mitsunobu reaction, dephthaloyl, tert-butyl ester hydrolysis and the like. The compound is synthetized by raw materials easy to obtain and is low-price; synthesis operations are convenient and simple, reaction conditions are mild and easy to control, reaction selectivity is good, yield is high, and accordingly the compound is suitable for industrial production.
Owner:GANSU RES INSTION OF CHEM IND GRICI
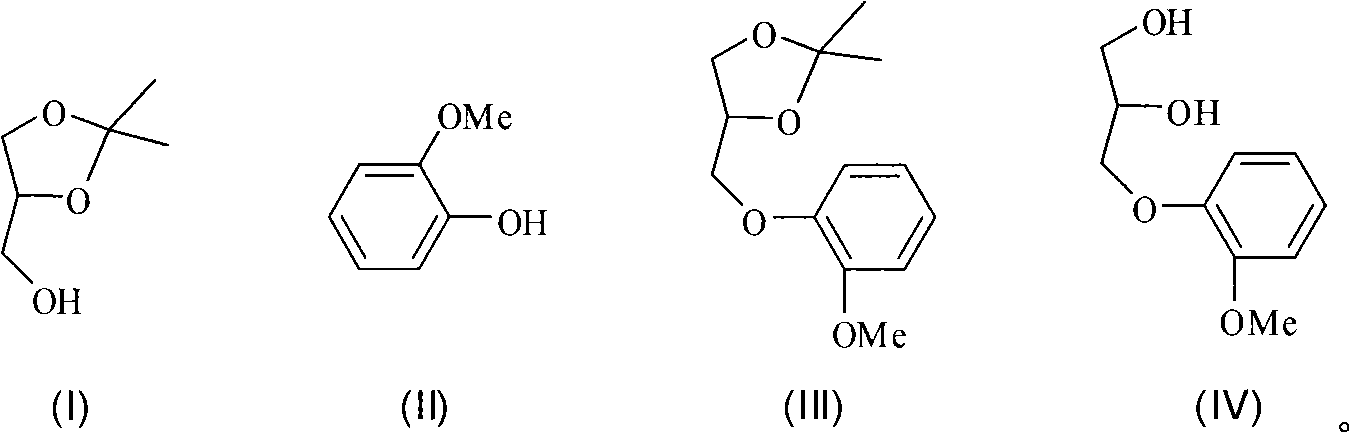
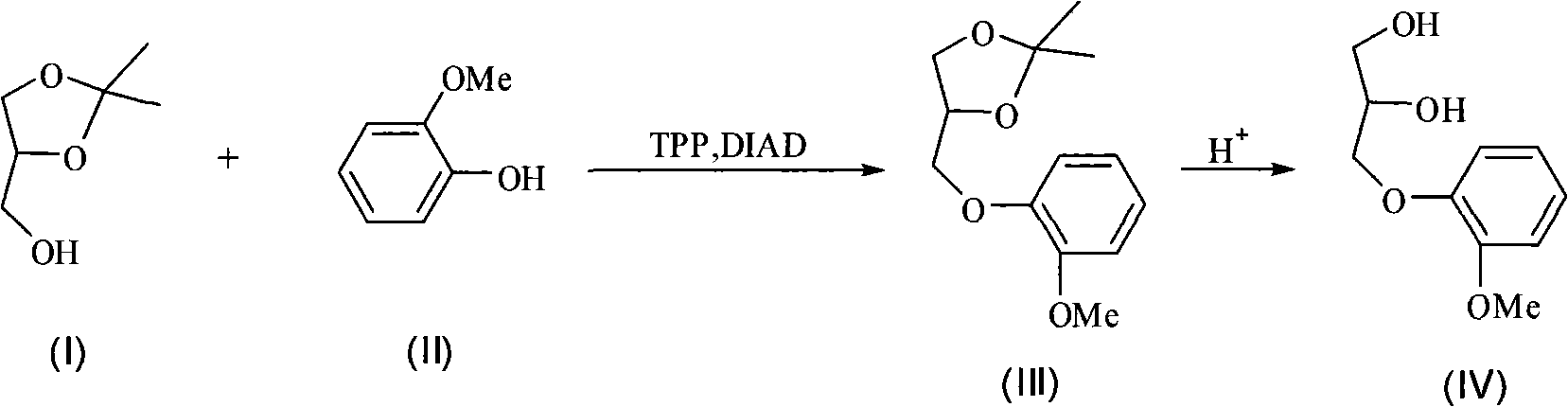
New synthetic method of guaiacol glycerin ether
InactiveCN101525278ASimple methodLow costOrganic chemistryOrganic compound preparationDiisopropyl azodicarboxylateGuaiacol
The invention discloses a new synthetic method of guaiacol glycerin ether with a structure as shown in a formula (IV), isopropylidene glycerol-4-methanol with a structure as shown in formula (I) and guaiacol with a structure as shown in a formula (II) are taken as raw materials, Mitsunobu reaction occurs in organic solvent under the effect of triphenylphosphine and diisopropyl azodicarboxylate to generate intermediate product guaiacol isopropylidene glycerol ether with the structure as shown in formual (III); after full reaction, the obtained intermediate product crude product from separation witnesses hydrolysis reaction directly under acid conditions without purification; after separation and purification of the reaction liquid, the target product guaiacol glycerin with the structure as shown in (IV) ether is obtained. The synthetic method of the invention features application of clean raw material with low cost and easy availability, moderate reaction conditions, simple post treatment processes and adaptability to industrialized production.
Owner:ZHEJIANG UNIV OF TECH
Radioisotope carbon-14 labeled ibrutinib and synthesis method thereof
ActiveCN113024565AInformativeOrganic chemistry methodsBulk chemical productionRadioactive tracerRadio isotopes
The invention provides radioisotope carbon-14 labeled ibrutinib and a preparation method and application thereof. The preparation method comprises the steps that [14C] barium carbonate serves as an initial radioisotope raw material, and the carbon-14 labeled ibrutinib is obtained through [14C] carbon dioxide preparation, carboxylation, halogenation, condensation, methylation, two times of cyclization, Mitsunobu reaction, protecting group removal and the like; a feasible, economical and safe method is provided for the preparation of the 3-site carbon-14 marker in the pyrazolo [3, 4-d] pyrimidine fragment of the ibrutinib molecule for the first time. Furthermore, the related carbon-14 labeled ibrutinib can be used as a radioactive tracer, is mainly used for the fundamental research of the environmental safety problem of the medicine ibrutinib, and can also be used for the fundamental research of the radiopharmacokinetics of the tissue distribution, metabolite structure identification, mass balance and the like of the medicine. Meanwhile, the method provides a reference for the preparation of the carbon-14-containing synthetic building block.
Owner:浙江爱索拓标记医药科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com