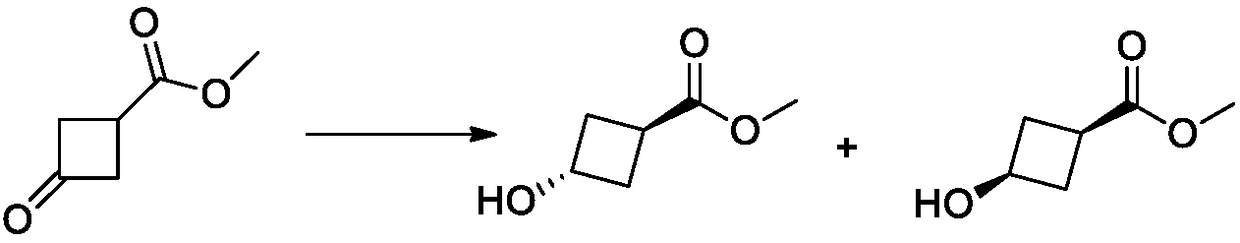
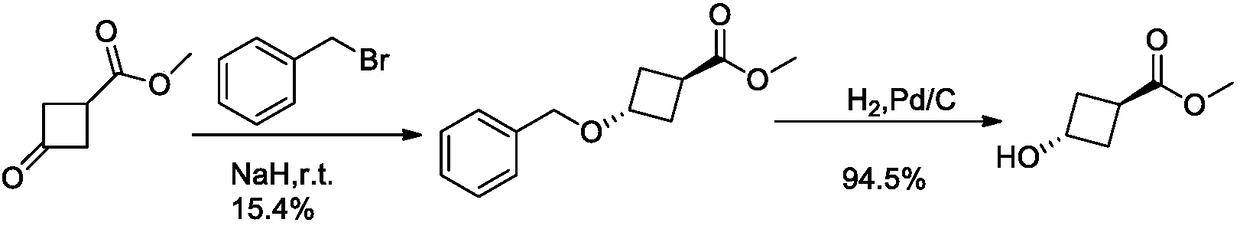
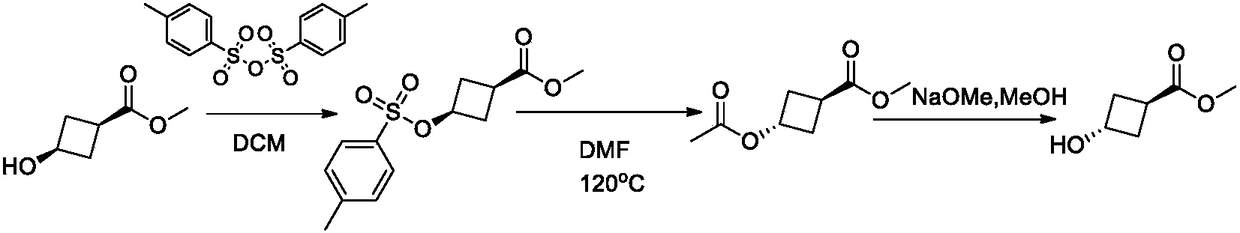
Method for synthesizing trans-3-hydroxy cyclobutyl formic acid
A technology of hydroxycyclobutyl carboxylate and hydroxycyclobutyl, which is applied in the field of synthesis of trans-3-hydroxycyclobutyl carboxylic acid, can solve problems such as poor stereoselectivity, low yield, and difficulty in scaling up industrial production, and achieve a reaction Mild, high yield, cost-saving effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] The preparation of embodiment 1 cis-3-hydroxycyclobutyl carboxylate methyl ester
[0048] Dissolve methyl 3-carbonyl-cyclobutanecarboxylate (3075g, 24.0mol, 1.0eq.) in 30L tetrahydrofuran, cool down to -78~-60°C, add dropwise 20L lithium tri-tert-butoxy aluminum hydride (9154g , 36.0mol, 1.5eq.) tetrahydrofuran solution, after 4 hours of heat preservation reaction, TLC test after the completion of the reaction, dropwise added 6mol. L -1 The reaction was quenched with hydrochloric acid, the pH of the reaction solution was adjusted between 5 and 6, and the temperature was controlled at about 0°C. 20L of ethyl acetate was added to dilute and stirred for 30min. Pad Celite to filter, the filter cake was washed 3 times with ethyl acetate, and the filtrate was separated. The aqueous phase was extracted and washed with ethyl acetate, all organic phases were combined, dried, and concentrated to obtain light yellow liquid methyl cis-3-hydroxycyclobutylcarboxylate (2733 g, 21.0 ...
Embodiment 2
[0051] The preparation of embodiment 2 cis-3-hydroxycyclobutyl carboxylate methyl ester
[0052] Dissolve methyl 3-carbonyl-cyclobutanecarboxylate (3075g, 24.0mol, 1.0eq.) in tetrahydrofuran (30L), cool down to -78~-60°C, add dropwise tetrahydrofuran solution of lithium triethylborohydride (43L, 36.0mol, 1.5eq.), heat preservation reaction 6h, TLC test after the completion of the reaction, drop 6mol. L -1 The reaction was quenched with hydrochloric acid, the pH of the reaction solution was adjusted between 5 and 6, and the temperature was controlled at about 0°C. 20L of ethyl acetate was added to dilute and stirred for 30min. Pad diatomaceous earth to filter, the filter cake is washed 3 times with ethyl acetate, the filtrate liquid separation, the organic phase and the water phase are separated, wherein the water phase is extracted with ethyl acetate again, all organic phases are combined, dried and concentrated to obtain a light yellow liquid Formula - methyl 3-hydroxycyclo...
Embodiment 3
[0054] The preparation of embodiment 3 trans-p-nitrobenzoic acid (3-methoxycarbonyl cyclobutyl) ester
[0055] Dissolve methyl cis-3-hydroxycyclobutylcarboxylate (2730g, 21.0mol, 1.0eq) prepared in Example 1 in tetrahydrofuran (30L), cool to -10°C, add p-nitrobenzoic acid (4178g , 25.0mol, 1.2eq.), diethyl azodicarboxylate (4354g, 25.0mol, 1.2eq.), triphenylphosphine (6557g, 25.0mol, 1.2eq.). Then, under the protection of nitrogen, the mixture was stirred overnight at room temperature for 16 h. TLC detected that the reaction was complete, the tetrahydrofuran was rotated off, methyl tert-butyl ether (20 L) was added, and stirred for 0.5-1 h. Filter and wash the filter cake twice with methyl tert-butyl ether. All filtrates were collected and the filtrate was washed with saturated aqueous sodium bicarbonate solution. Liquid separation, drying and concentration. The crude product was then slurried with a mixed solvent of ethyl acetate / petroleum ether, filtered, and concentrate...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com