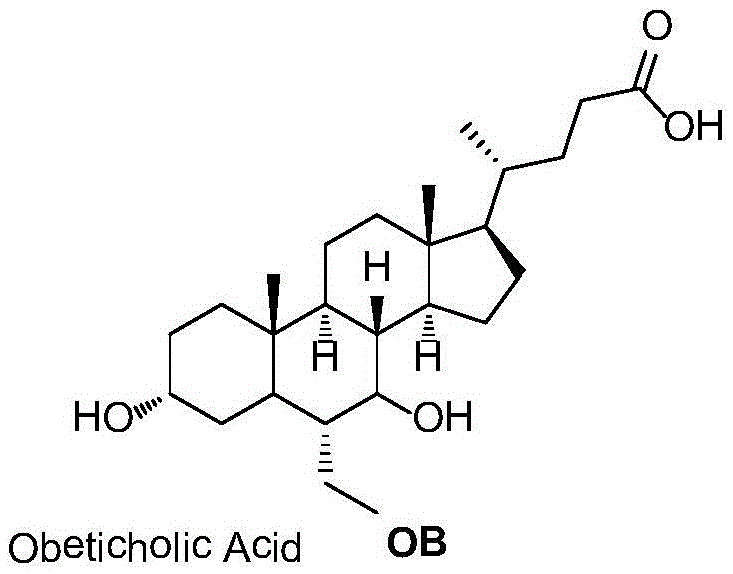
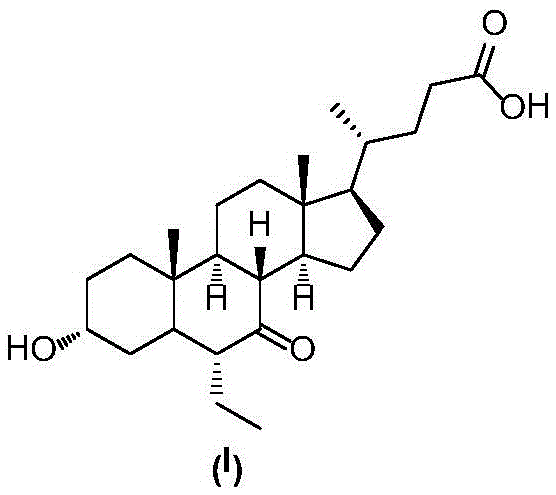
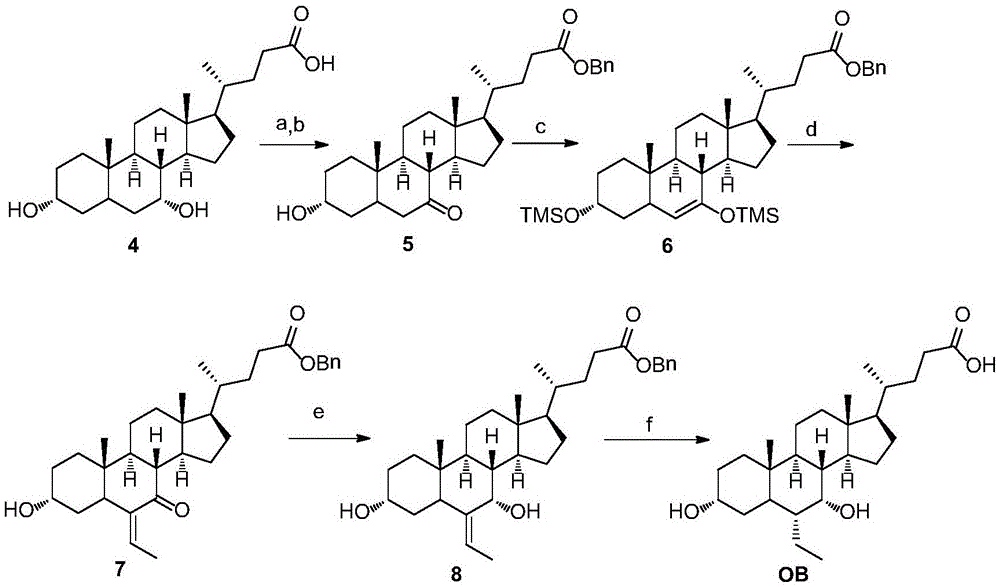
Preparation method for obeticholic acid and intermediate thereof
A technology of obeticholic acid and intermediates, applied in the field of drug synthesis, which can solve problems such as difficult catalytic hydrogenation and incomplete ester hydrolysis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1a3
[0037] Embodiment 1a Preparation of 3α-hydroxyl-7-keto-5β-cholanic acid (III)
[0038]
[0039] Add chenodeoxycholic acid (II) (113g, 0.288mol), sodium bromide (1.78g, 0.0173mol), acetic acid (30mL) and methanol (600mL) successively to the reaction flask, stir at room temperature until all dissolve, and cool to -10°C±2°C, slowly add 13% sodium hypochlorite solution (225mL, 0.39mol) dropwise to the reaction system, control the internal temperature at -10~0°C and stir the reaction until the content of chenodeoxycholic acid (II) is detected by HPLC less than 1%. After the reaction was completed, the ice bath was removed, and the reaction solution naturally rose to room temperature, and 5% sodium bisulfite solution (25 mL) was added dropwise to the reaction system, stirred for 30 minutes, suction filtered, and dried to obtain 3α-hydroxy-7-keto - Crude 5β-cholanic acid (III) (115.83 g). Add the crude product and methanol (1L) into the reaction flask, heat to 65°C, reflux for h...
Embodiment 1b3
[0040] The preparation of embodiment 1b3α-hydroxyl-7-keto-5β-cholanic acid (III)
[0041]
[0042] Add chenodeoxycholic acid (II) (100g, 0.255mol), anhydrous magnesium sulfate (200g), chloroform (300mL) to the reaction flask in turn, stir at room temperature, and add pyridinium chlorochromate dichloride dropwise to the reaction system Methane solution (61 g of pyridinium chlorochromate dissolved in 2.5 L of dichloromethane), and the reaction solution was stirred at room temperature for 30 min. The solid insoluble matter was filtered, the filtrate was washed with water and saturated brine successively, the organic phase was dried over anhydrous sodium sulfate, and concentrated under reduced pressure to obtain 3α-hydroxy-7-keto-5β-cholanic acid (III) (73g, yield 73.3 %, HPLC detection: 97.7%).
Embodiment 2a3
[0043]Example 2a Synthesis of 3α-hydroxyl-7-keto-5β-cholane-24 acid benzyl ester (IVa)
[0044] In the reaction flask, add compound III (20g, 51mmol), anhydrous tetrahydrofuran (450ml), stir, add potassium carbonate (10.5g, 76mmol), heat up to reflux, add benzyl bromide (30ml), reflux reaction, 12h monitoring Reaction (TLC conditions: acetone: dichloromethane: acetic acid = 1:15:1). Reaction finishes, adds triethylamine (30ml), continues reaction to form quaternary ammonium salt and removes excess benzyl bromide, is cooled to room temperature, diatomaceous earth assists and filters solid insoluble matter, filtrate is concentrated under reduced pressure, adds water (200ml), with acetic acid Ethyl ester (200ml*3) was extracted, the organic phases were combined, washed with purified water and saturated sodium chloride, dried over anhydrous sodium sulfate, and concentrated under reduced pressure to obtain 20.3g of compound (IVa), with a yield of 82.8%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com