Patents
Literature
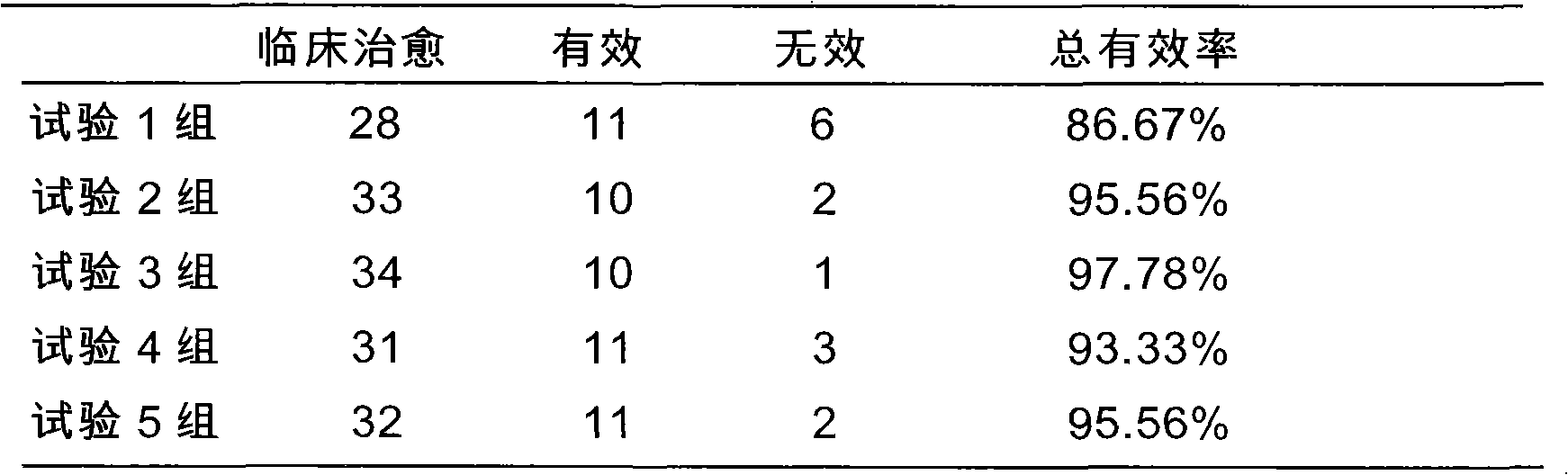
1041 results about "Alcoholic fatty liver" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
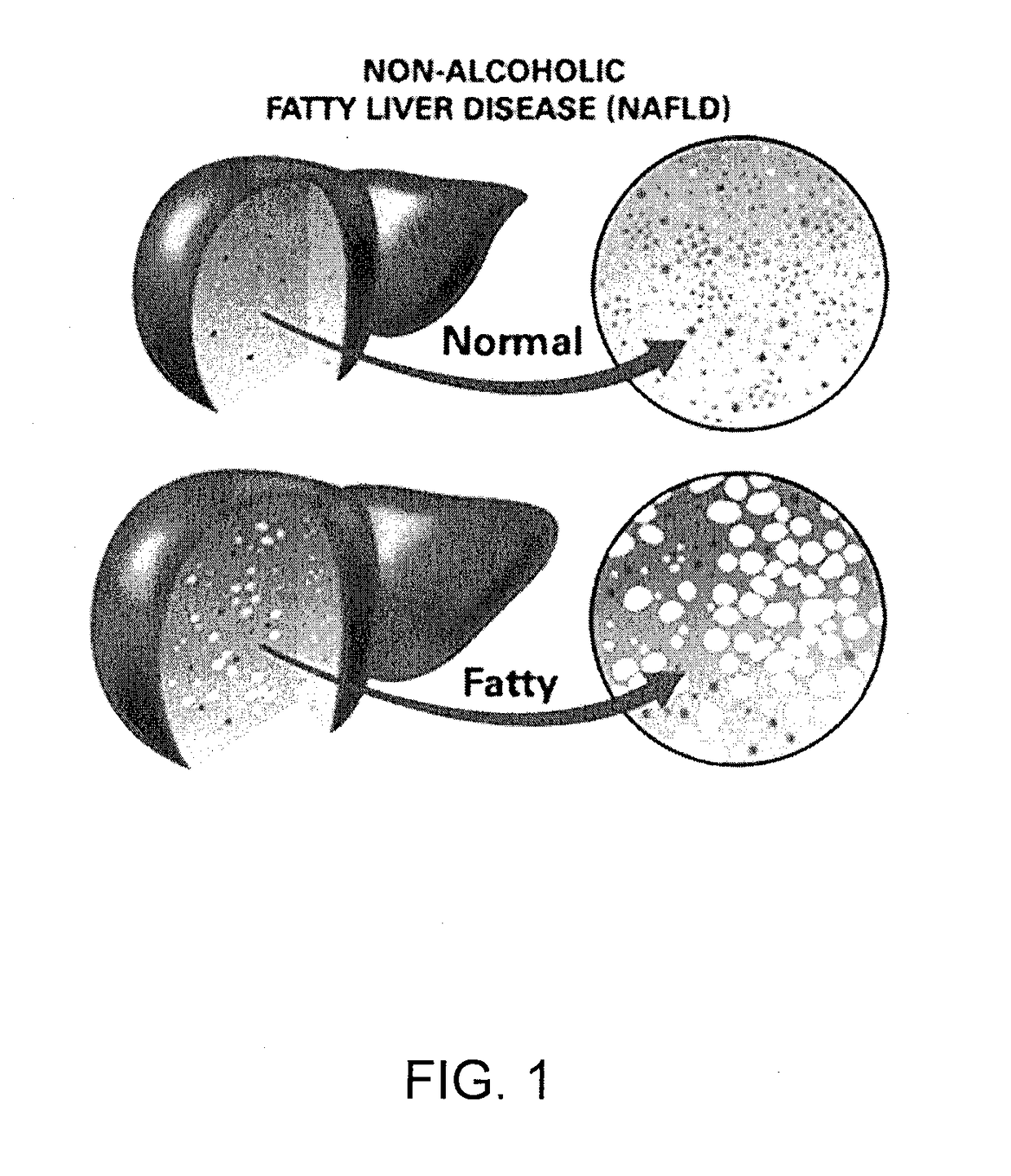
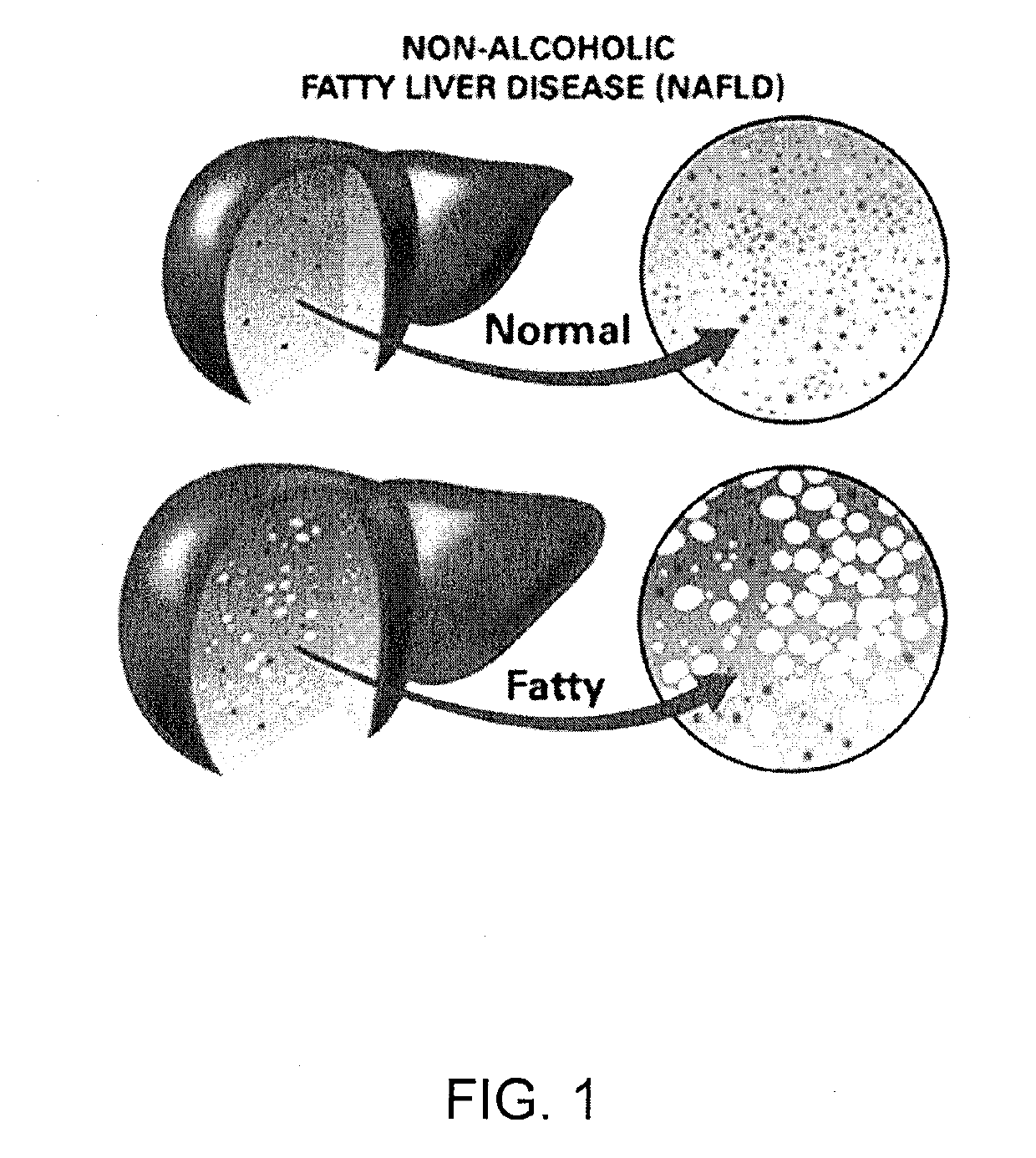
The most common cause of fatty liver in the United States is alcoholism. In alcoholic fatty liver, over consumption of alcohol changes the way that the liver breaks down and stores fats. Often, people with chronic alcoholism also suffer from malnutrition by eating irregularly and not consuming a balanced diet.
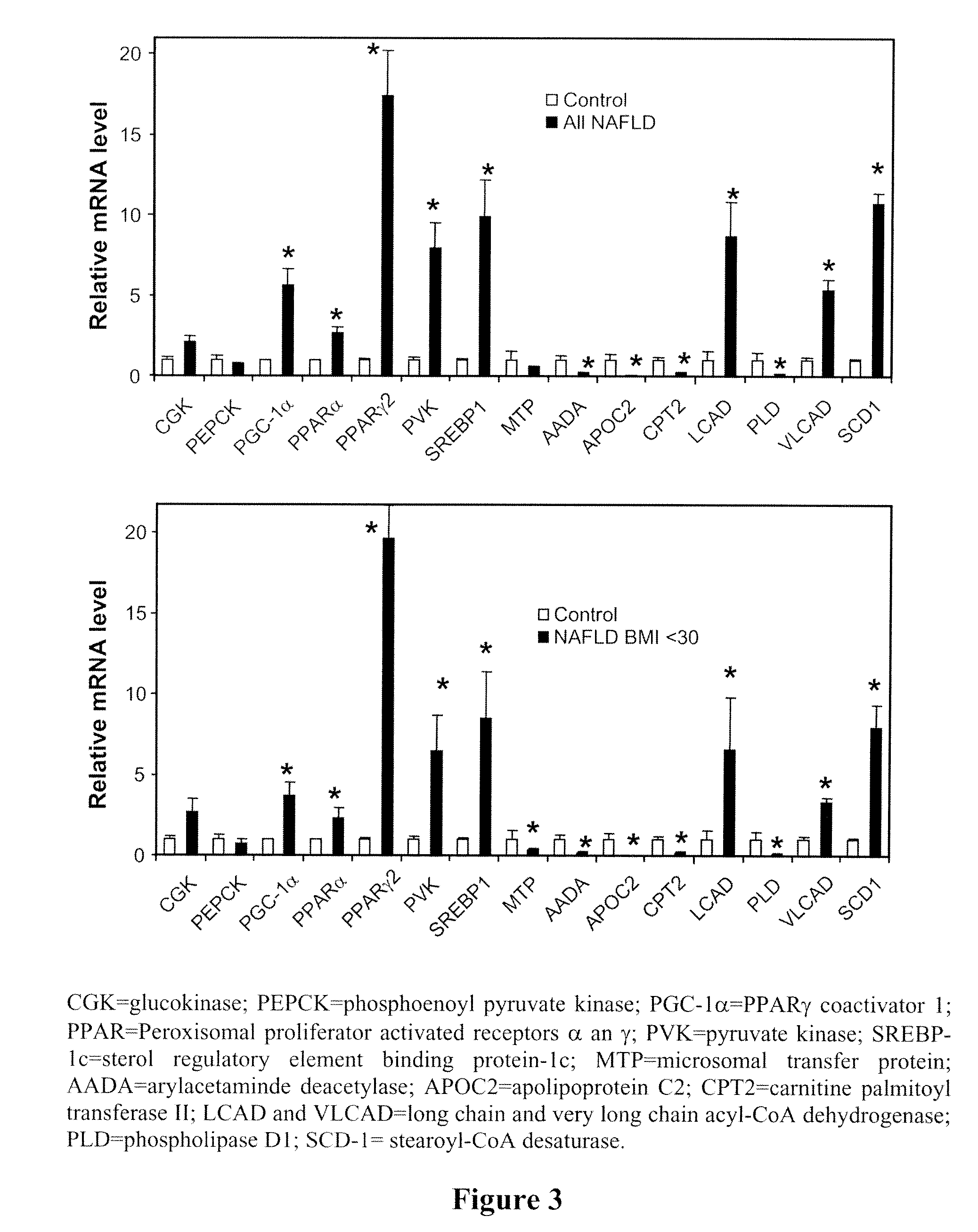
Lysosomal Acid Lipase Therapy for NAFLD and Related Diseases
InactiveUS20090297496A1Improve intracellular localizationIncrease functionHydrolasesPeptide/protein ingredientsPharmaceutical formulationEndocrinology
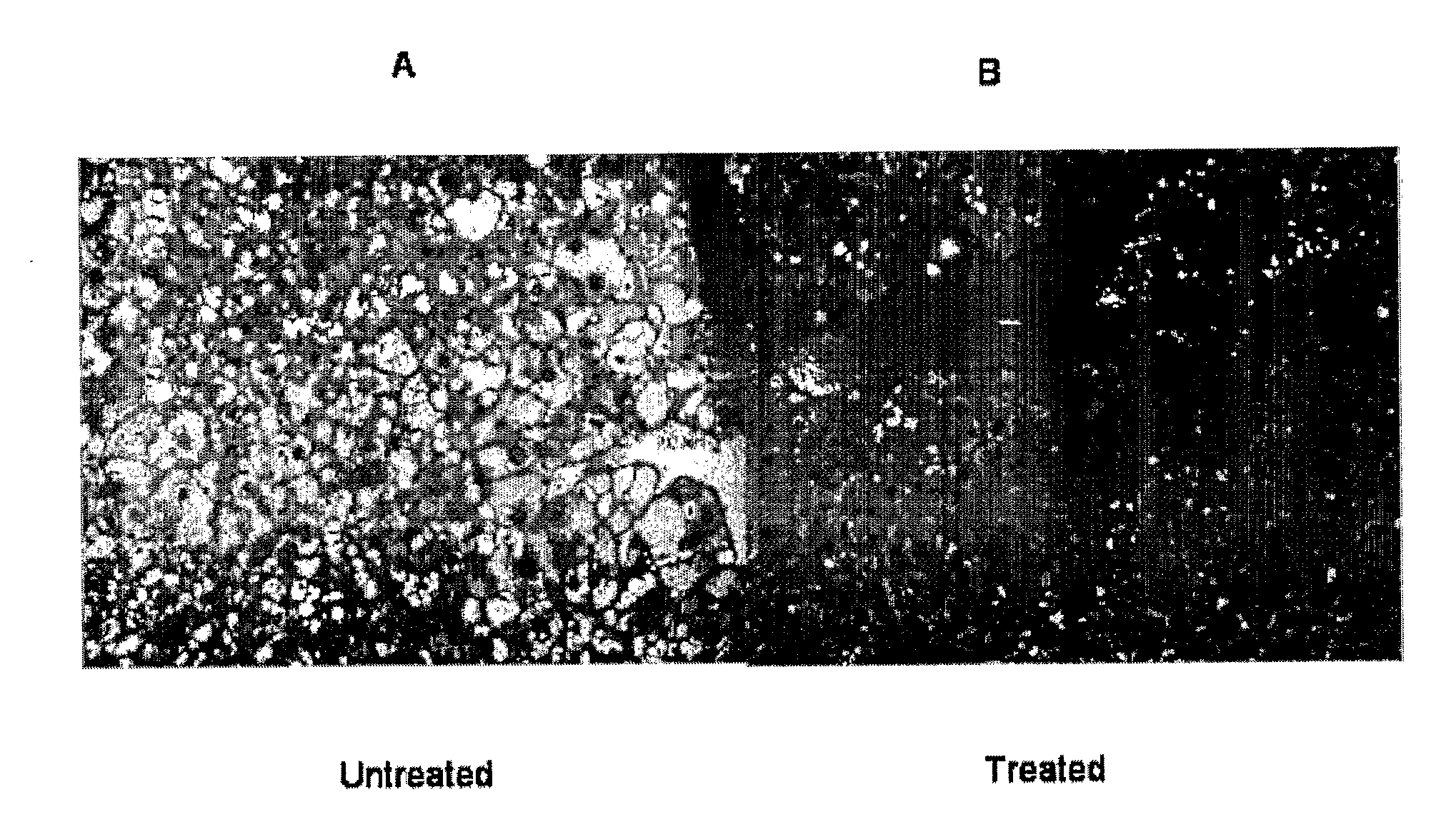
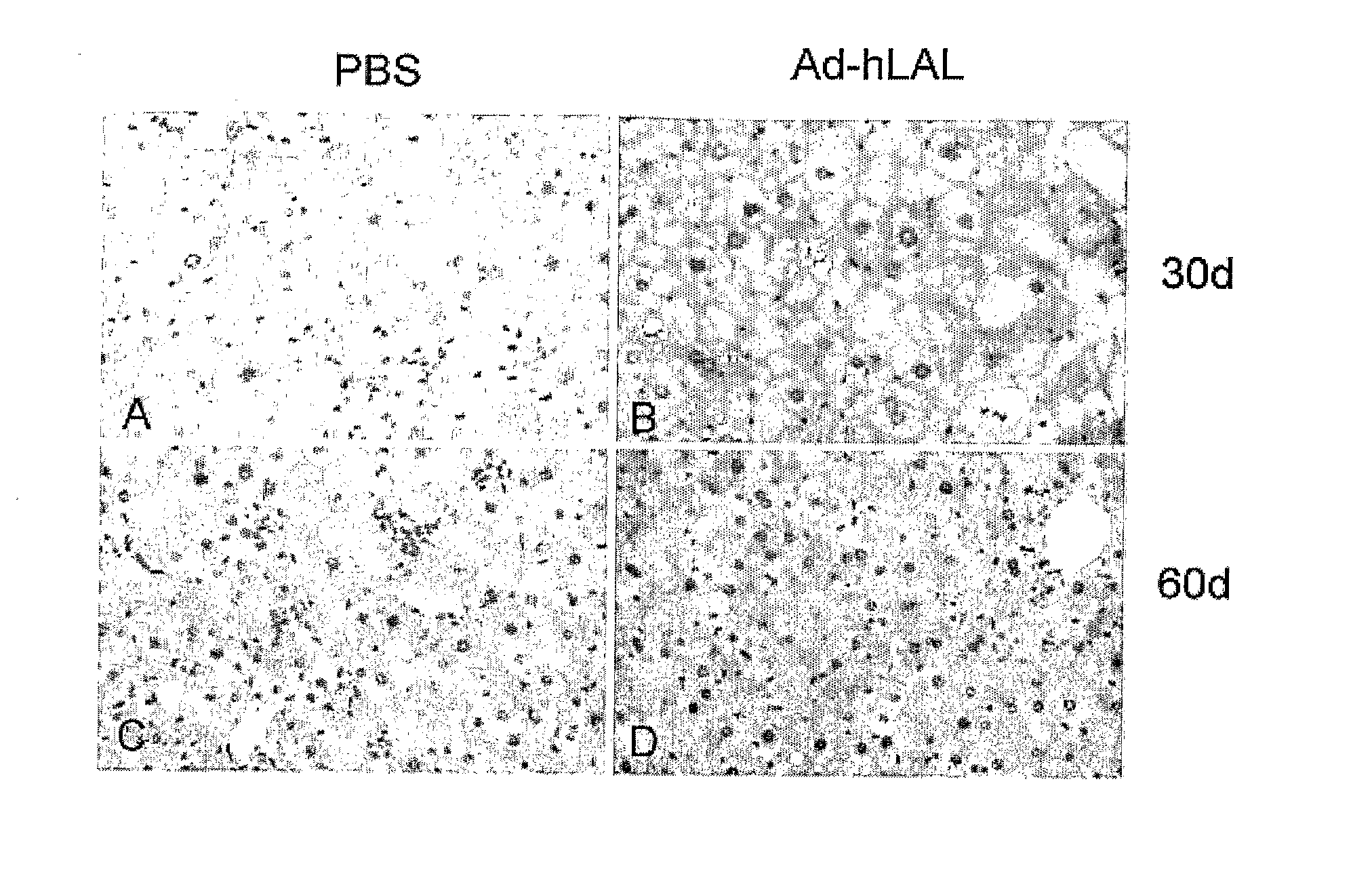
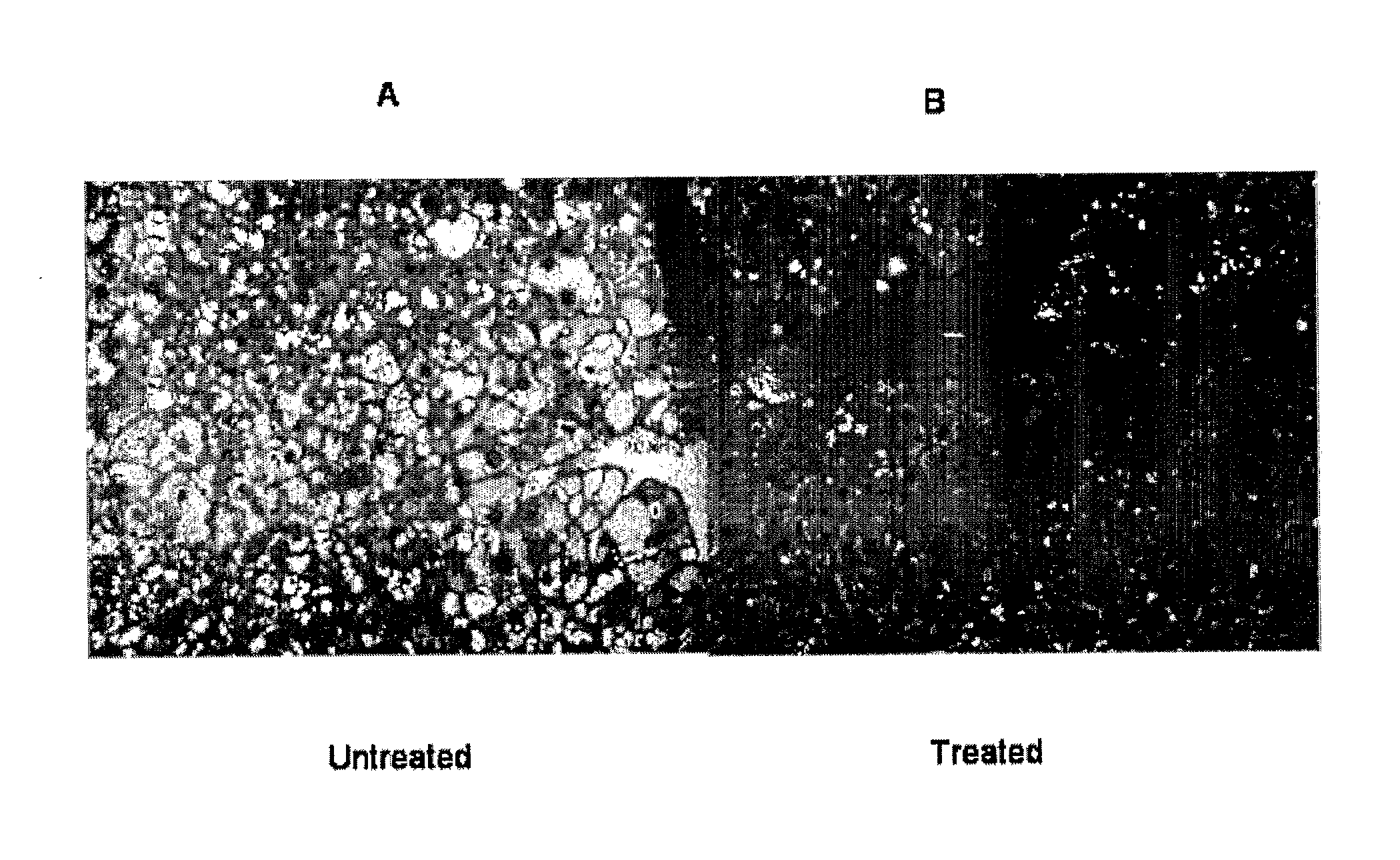
The present invention comprises methods and compositions for the treatment or alleviation of NAFLD (non-alcoholic fatty liver disease) and those conditions associated with NAFLD, including fatty liver disease, nonalcoholic steatohepatitis (NASH) and cirrhosis through the use of pharmaceutical formulations of lysosomal acid lipase or related proteins and / or polypeptides. This invention is also directed to a combination therapy treatment for treating The Metabolic Syndrome. As part of a combination therapy regime for the treatment of The Metabolic Syndrome, pharmaceutical formulations of lysosomal acid lipase or related proteins and / or polypeptides are used as part of the combination therapy regime for treating NAFLD (and NASH), which comprises one of the conditions constituting The Metabolic Syndrome,
Owner:CHILDRENS HOSPITAL MEDICAL CENT CINCINNATI
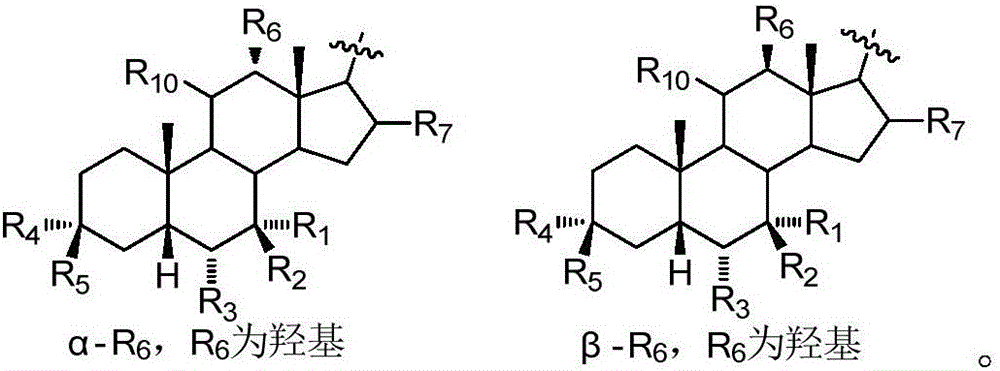
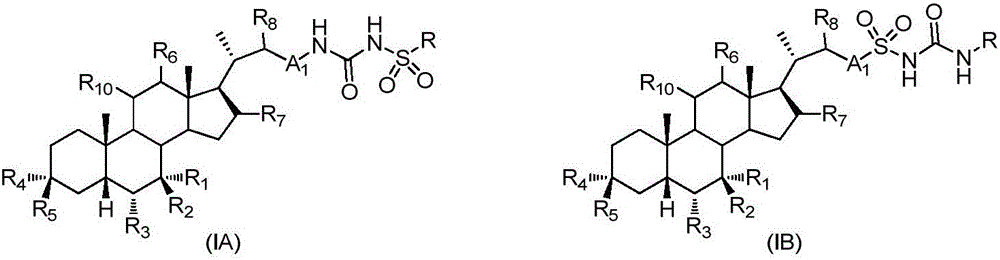
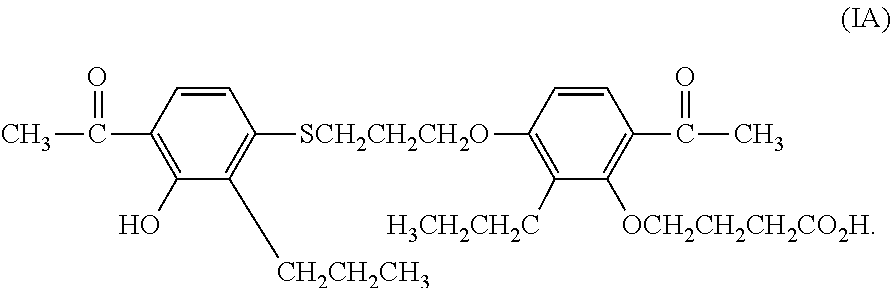
Sulfonylurea derivative and pharmaceutical composition and application thereof
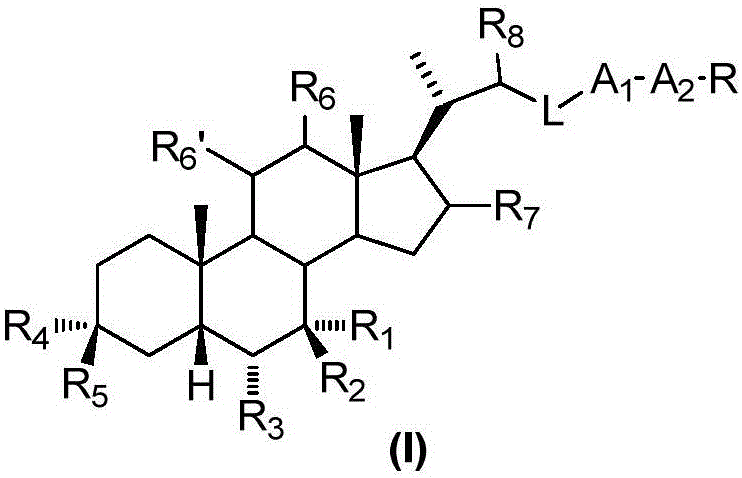
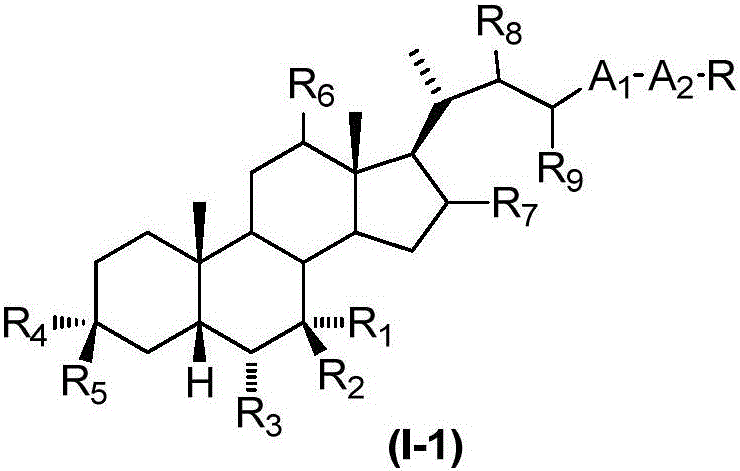
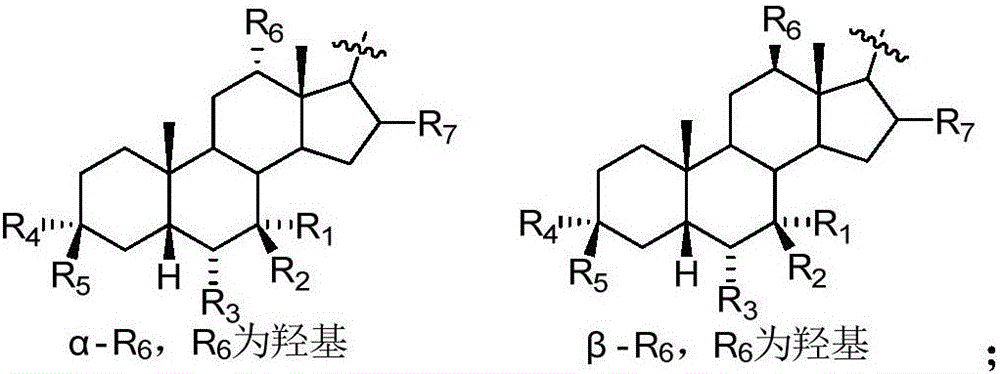
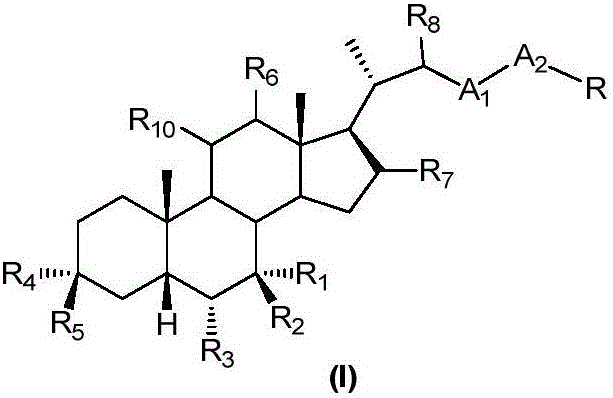
The invention relates to a preparation method and application of a sulfonylurea compound and a composition containing the same component as FXR and / or TGR5 agonist, the FXR and / or TGR5 agonist is a compound shown as a formula (I), or a pharmaceutically acceptable salt, a solvate, a prodrug, an isomer and a stable isotope derivative thereof. The compounds can be used for treatment of FXR and / or TGR5 mediated diseases including primary biliary cirrhosis, nonalcoholic fatty liver, portal hypertension, bile acid diarrhea and cholestasis, type II diabetes and obesity and other field.
Owner:SHANGHAI DE NOVO PHARMA
Application of GLP-1R/GCGR double-target agonist polypeptide to treatment of fatty liver diseases, hyperlipidemia and arteriosclerosis
ActiveCN106046145AImprove biological activityExtended half-lifePeptide/protein ingredientsMetabolism disorderFibrosisDrug biological activity
The invention relates to application of a polypeptide compound having double agonist effects on a glucagon-like peptide-1 receptor and a glucagon receptor. The polypeptide compound has the characteristics of high enzymolysis stability, high bioactivity, no adverse reaction, etc., can alleviate abnormal rise in the levels of total cholesterol and triglyceride in blood induced by diabetes and high-meal diet, lowers the level of liver enzyme, improves liver damage and fibrosis stage and is applicable to prevention or treatment of diseases like non-alcoholic fatty liver diseases, hyperlipidemia and arteriosclerosis.
Owner:SHENZHEN TURIER BIOTECH CO LTD
Thyromimetics for the Treatment of Fatty Liver Diseases
InactiveUS20090232879A1Low in fatPreventing and treating and ameliorating fatty liver diseaseBiocideMetabolism disorderSteatosisReceptor
The present invention is directed toward the use of thyromimetic compounds that are thyroid receptor ligands, pharmaceutically acceptable salts thereof, and to prodrugs of these compounds for preventing, treating, or ameliorating fatty liver diseases such as steatosis, non-alcoholic fatty liver disease, and non-alcoholic steatohepatitis.
Owner:METABASIS THERAPEUTICS INC
Method and System for Treating Non-Alcoholic Fatty Liver Disease
ActiveUS20170106026A1Inhibit progressEarly detectionUnknown materialsObesity treatmentIntestinal microorganismsInsulin resistance
A method and system for treating non-alcoholic fatty liver disease (NAFLD) involves the modulation of the gut microbial of a person suffering from NAFLD, and in particular the provision of a probiotic therapy configured to reduce liver aminotransferases, total-cholesterol, TNF-α and to improve insulin resistance in NAFLD patients.
Owner:SEED HEALTH INC
Compositions and methods for treating nonalcoholic fatty liver disease-associated disorders
The invention relates to compositions containing cholesterol absorption inhibitors alone or in combination with other therapeutic agents for treating non-alcoholic fatty liver disease-associated disorders by administering a therapeutically effective amount of the compositions to a subject in need thereof.
Owner:IRONWOOD PHARMA
Method and system for reducing the likelihood of developing liver cancer in an individual diagnosed with non-alcoholic fatty liver disease
ActiveUS20190117709A1Inhibit progressEarly detectionOrganic active ingredientsDigestive systemFiberMonoacylglycerol acyltransferase
A method for reducing the likelihood of developing non-alcoholic steatohepatitis (NASH) in an individual diagnosed with non-alcoholic fatty liver disease involves providing in the gut of an individual a population of beneficial bacteria selected from the group consisting of Lactobacillus species, and at least 6 grams per day of fiber to the individual to maintain a therapeutically effective amount of the beneficial bacteria in the gut of the individual. In certain embodiments, monoacylglycerolacyltransferase-3 (MGAT3) synthesis is inhibited to lower triacylglycerol (TAG) production, while in others, expression of diacylglycerolacyltransferase-2 (DGAT-2) is inhibited. The beneficial bacteria are preferably modified to produce increased amounts of butyrate and are also encapsulated in a frangible enclosure. Levels of Roseburia are preferably increased while the levels of Akkermansia spp. in the individual's gut microbiome are reduced.
Owner:SEED HEALTH INC
Pharmaceutical compositions for the treatment/prophylaxis of non-alcoholic fatty liver disease
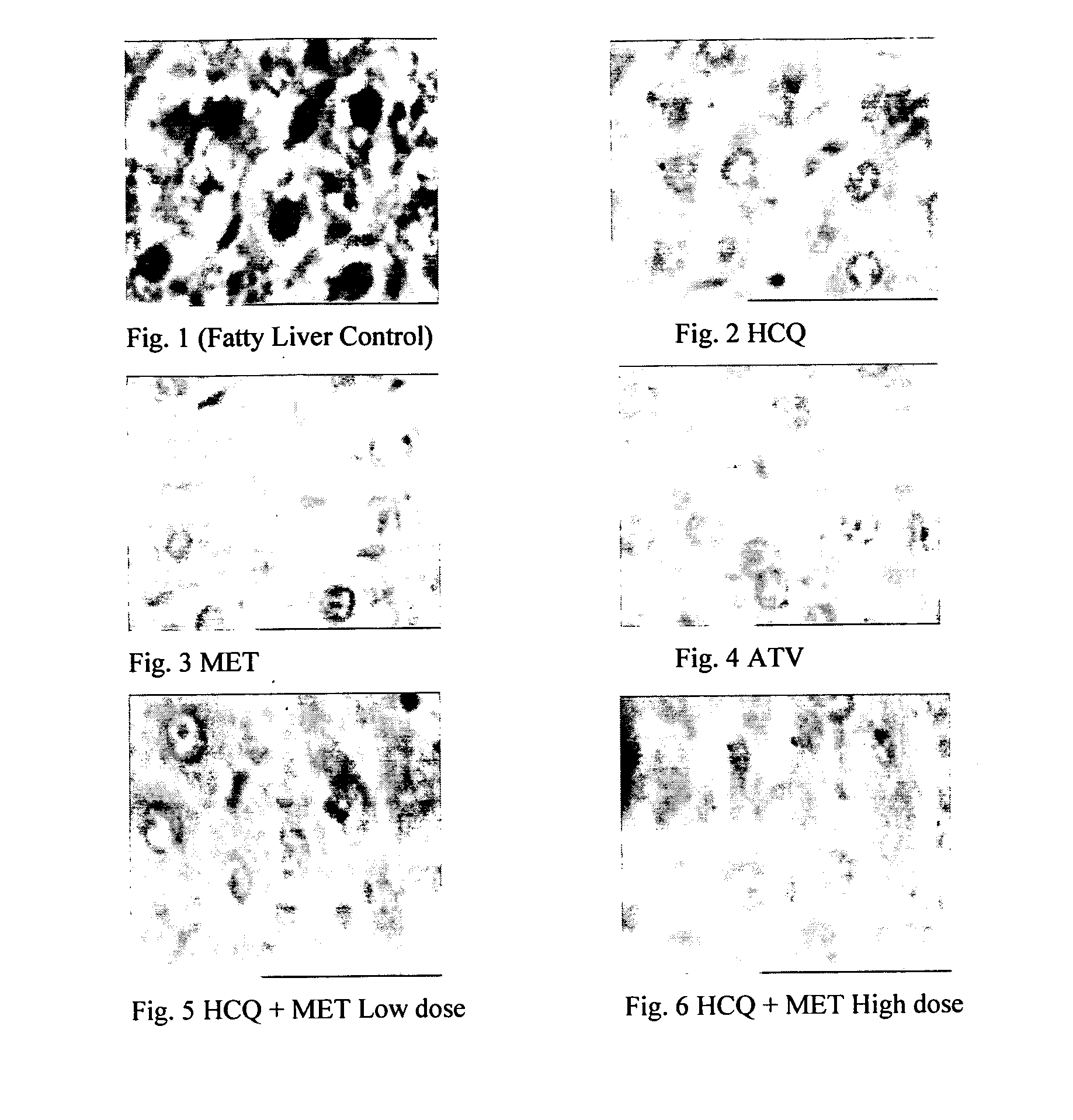
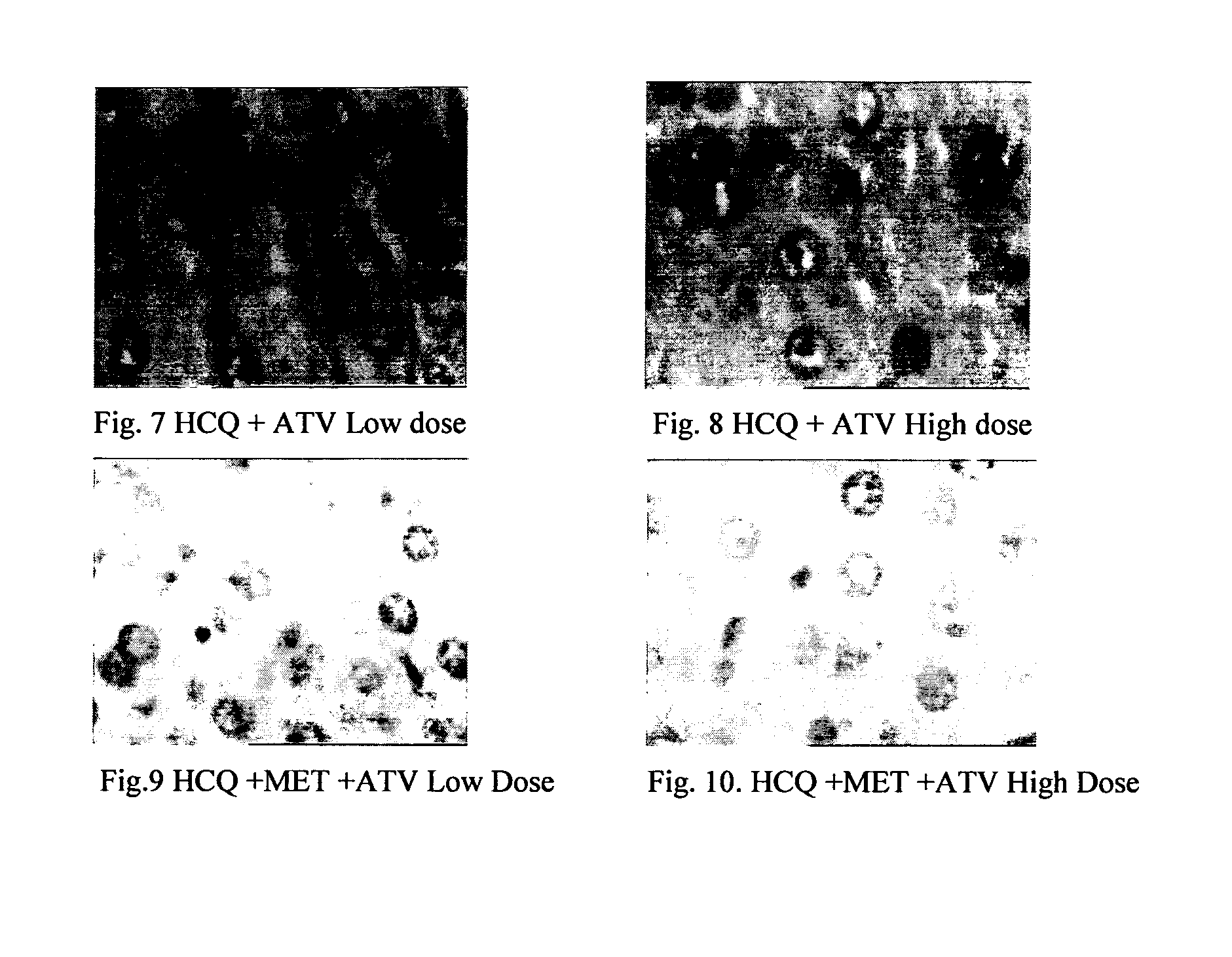
InactiveUS20120202849A1Lowering/preventing accumulation of fatBiocideOrganic chemistryDiseaseHydroxychloroquine
Disclosed herein is a novel synergistic pharmaceutical composition comprising hydroxychloroquine with insulin sensitizing agents and lipid lowering agents such as statins along with pharmaceutical excipients / carriers useful in treating Non-Alcoholic Fatty Liver Disease.
Owner:IPCA LAB LTD
Acc inhibitor combination therapy for the treatment of non-alcoholic fatty liver disease
The present invention provides methods of treating, stabilizing or lessening the severity or progression of a non-alcoholic fatty liver disease using an ACC inhibitor alone or with one or more additional therapeutic agents.
Owner:GILEAD APOLLO LLC +1
Pharmaceutical composition for the prevention or the treatment of non-alcoholic fatty liver disease and the method for prevention or treatment of non-alcoholic fatty liver disease using the same
InactiveCN102883721AInhibit progressInhibitory activityOrganic active ingredientsMetabolism disorderSitagliptinBULK ACTIVE INGREDIENT
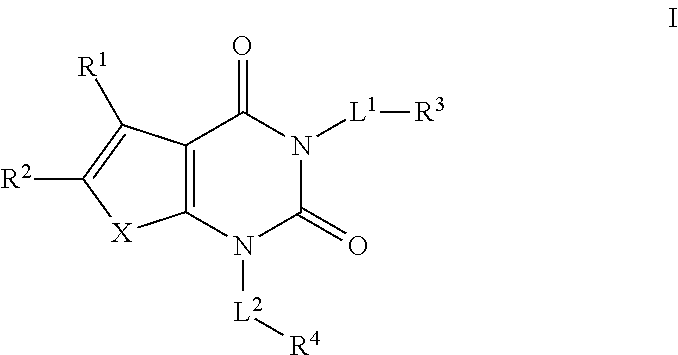
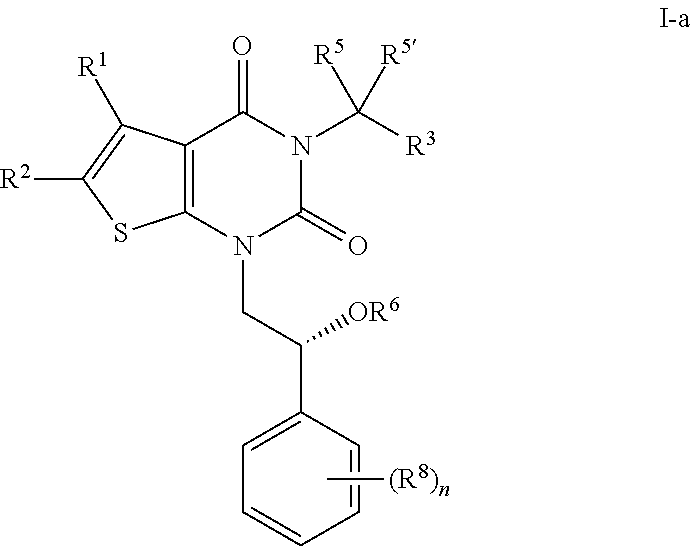
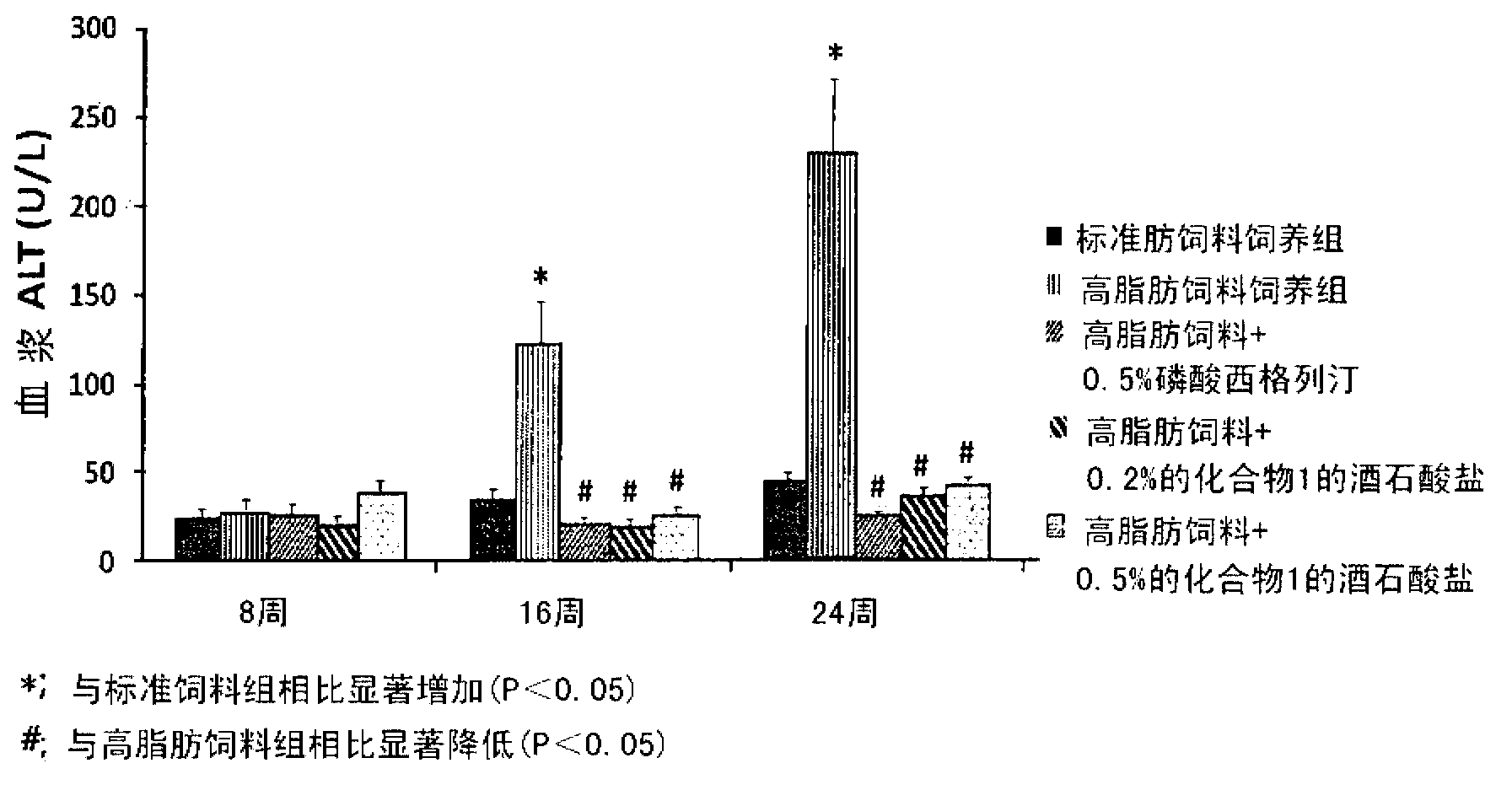
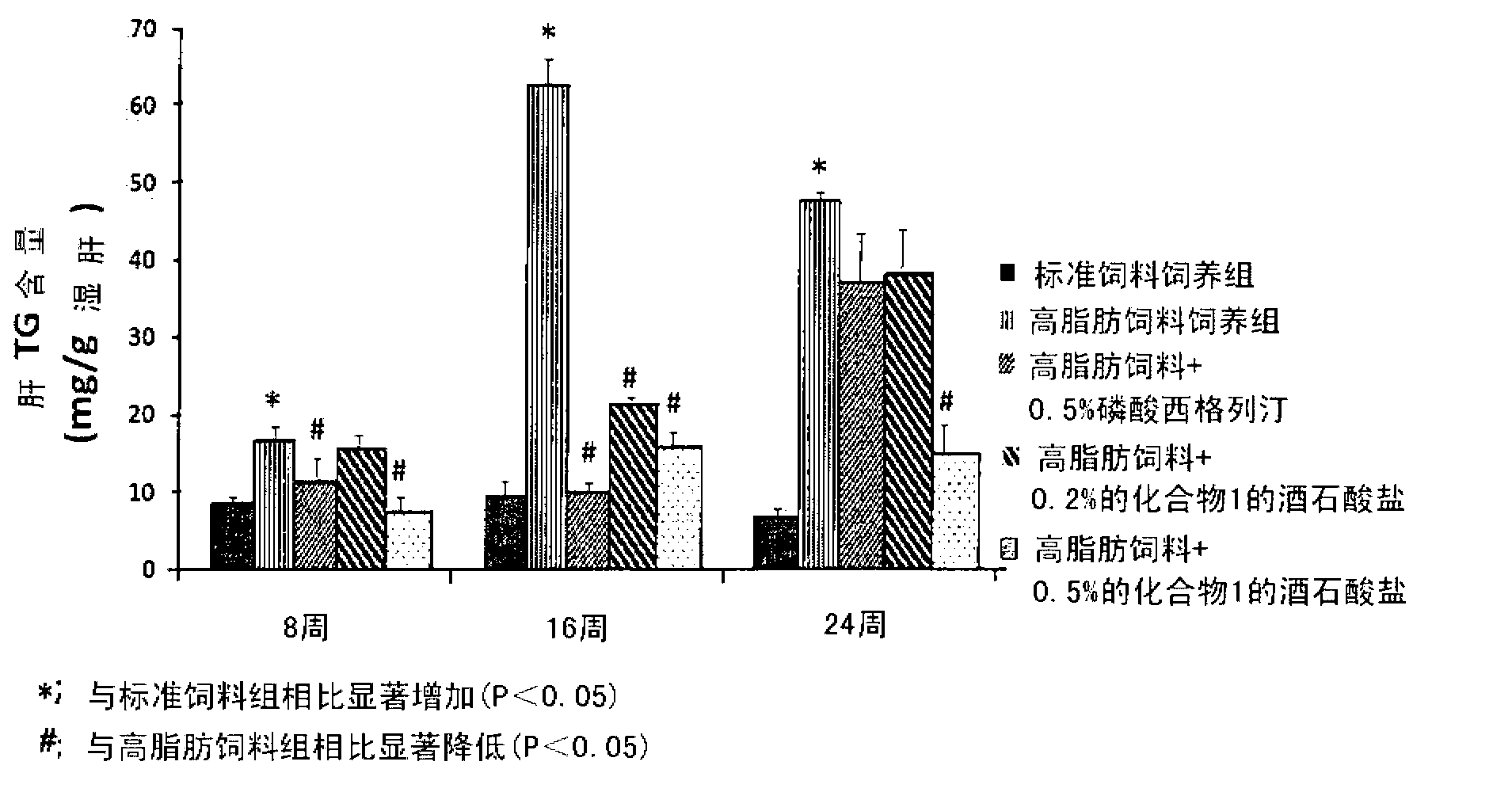
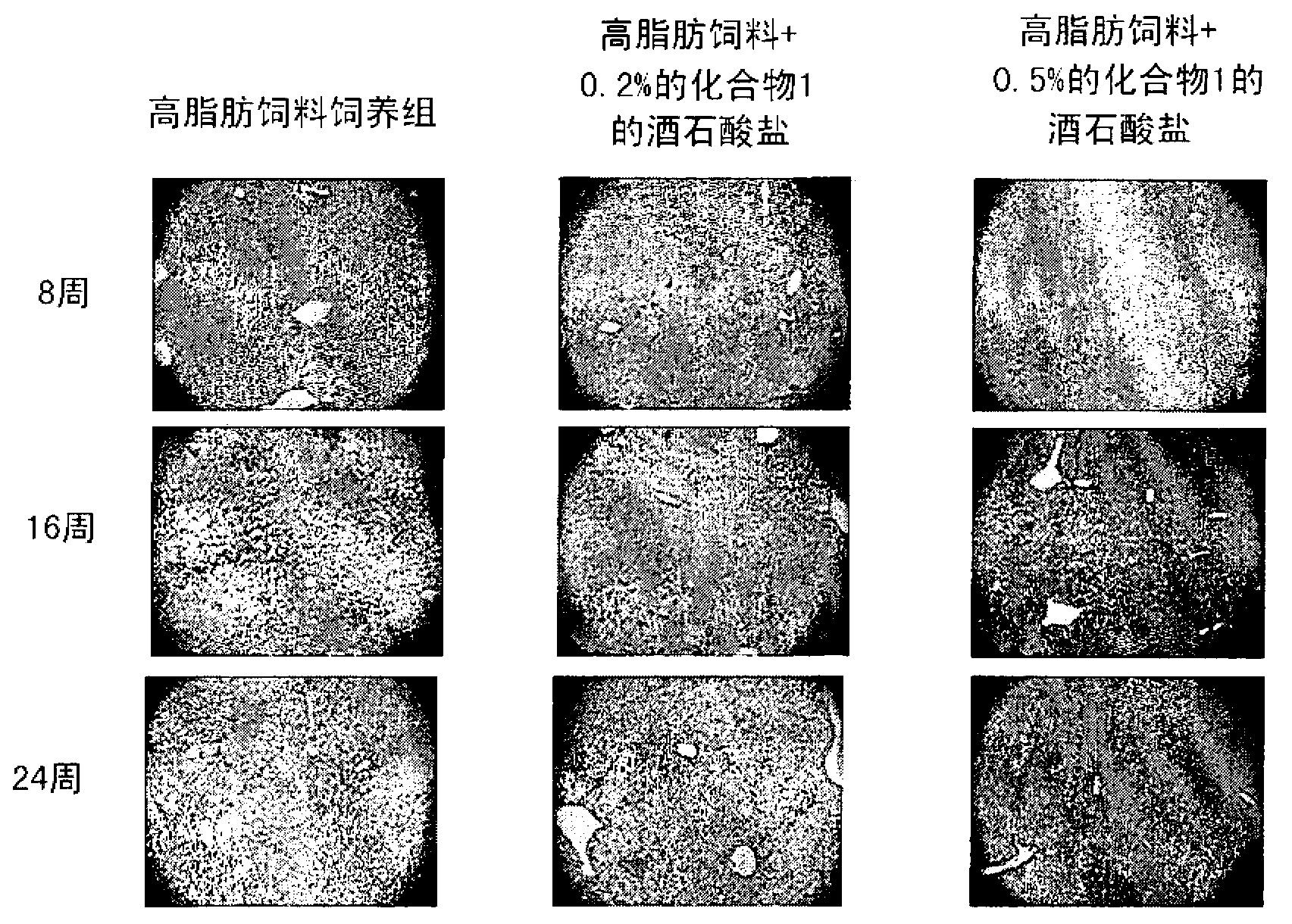
The present invention provides a pharmaceutical composition for the prevention and treatment of a non-alcoholic fatty liver disease (NAFLD), containing an active ingredient selected from the group consisting of Compound 1 represented by formula 1, sitagliptin, vildagliptin, linagliptin or a pharmaceutically acceptable salt thereof. Further, the present invention provides a method for the prevention or treatment of a non-alcoholic fatty liver disease, including administering an effective amount of an active ingredient selected from the group consisting of Compound 1 represented by formula 1, sitagliptin, vildagliptin, linagliptin or a pharmaceutically acceptable salt thereof to a mammal including a human in need thereof.; Further, the present invention provides use of Compound 1 represented by formula 1, sitagliptin, vildagliptin, linagliptin or a pharmaceutically acceptable salt thereof, for manufacturing a pharmaceutical composition for the prevention or treatment of a non-alcoholic fatty liver disease.
Owner:DONG A PHARMA
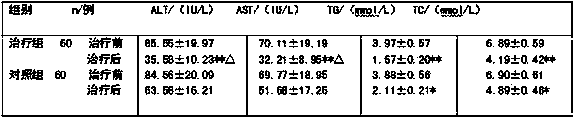
Traditional Chinese medicinal composition for treating alcoholic fatty liver and preparation method thereof
The invention discloses a traditional Chinese medicinal composition for treating alcoholic fatty liver and a preparation method thereof. The traditional medicinal composition contains the following raw materials of: by weight, 15-35 parts of Artemisia capillaries, 15-30 parts of Radix Puerariae, 15-35 parts of Poria cocos, 10-25 parts of couchgrass root, 10-25 parts of Herba Eupatorii, 10-25 parts of cassia seed, 4-10 parts of green tea, 5-12 parts of haw, 5-12 parts of red sage root, 5-12 parts of sweet wormwood, 5-12 parts of cortex lycii, 8-15 parts of burdock, 4-10 parts of Caulis Bambusae In Taeniam, 4-10 parts of polygonatum rhizome, 4-10 parts of ligustrum lucidum, 4-10 parts of Ligusticum wallichii and 2-8 parts of licorice. The traditional Chinese medicinal composition can be made into any clinically acceptable oral medicinal preparation including pill, particulate agent, capsules or tablet and the like. It shows through clinical observation result that the traditional Chinese medicinal composition has substantial curative effects for alcoholic fatty liver. Safety observation indicates that the traditional Chinese medicinal composition is safe in clinic usage and has no toxic and side effect.
Owner:黄文珍
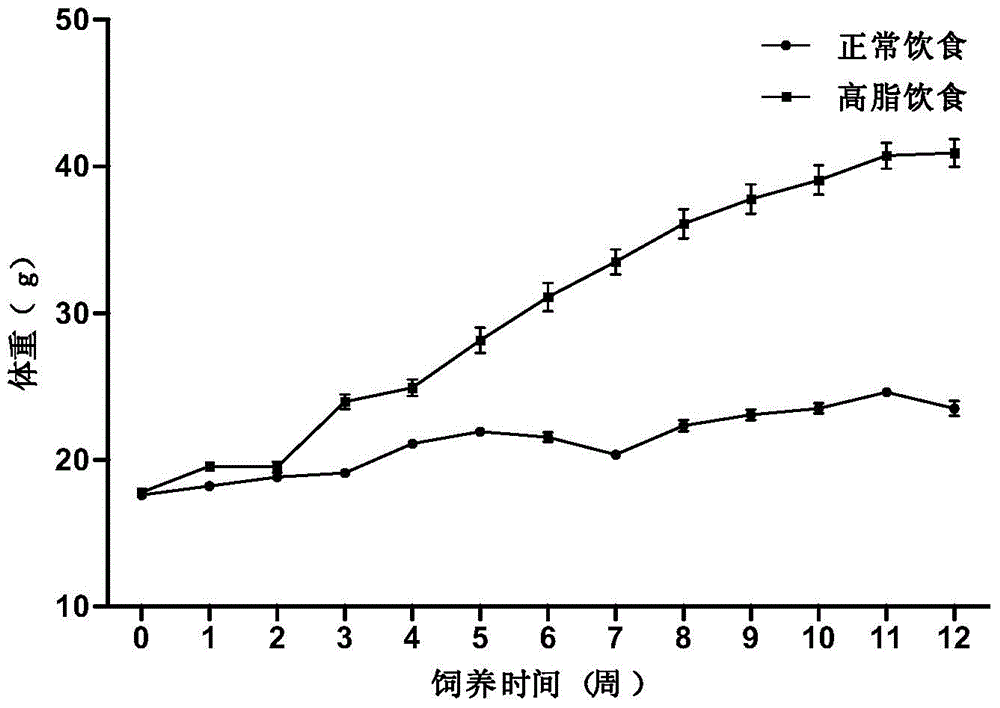
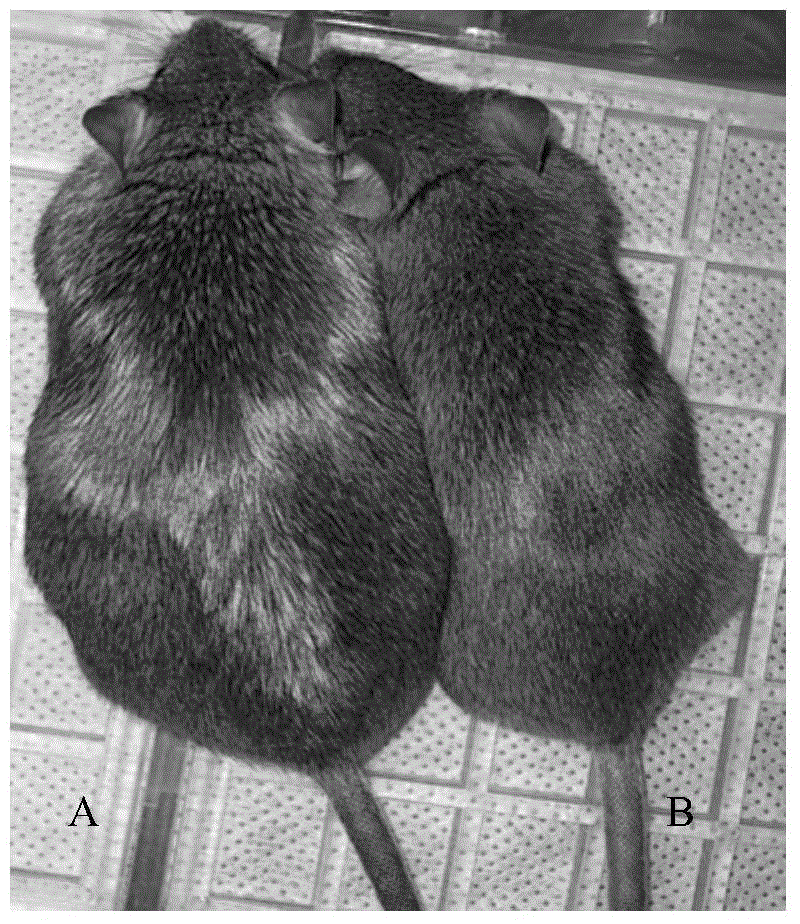
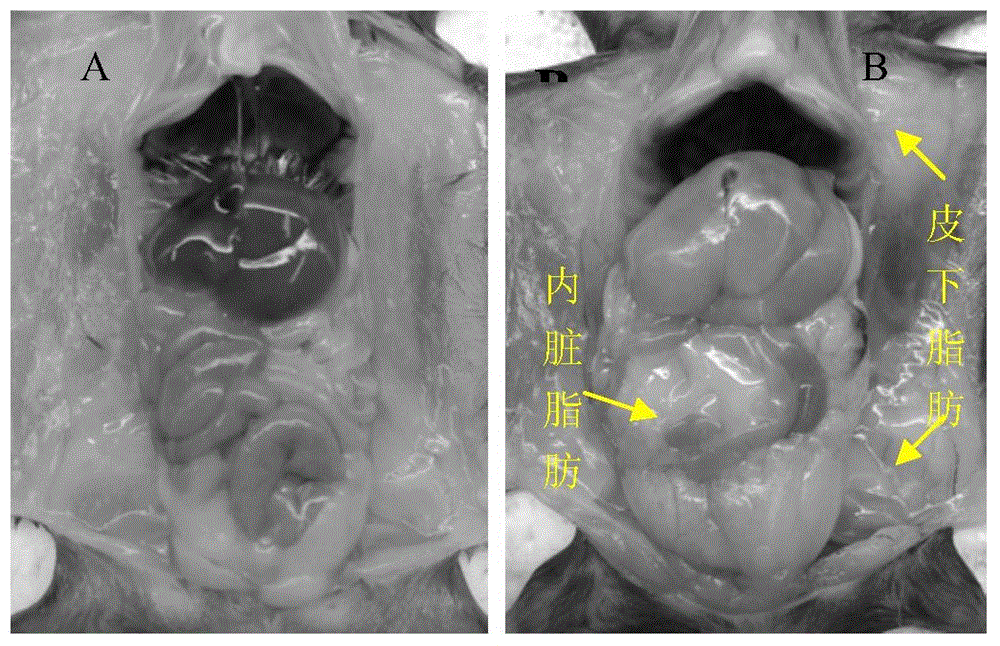
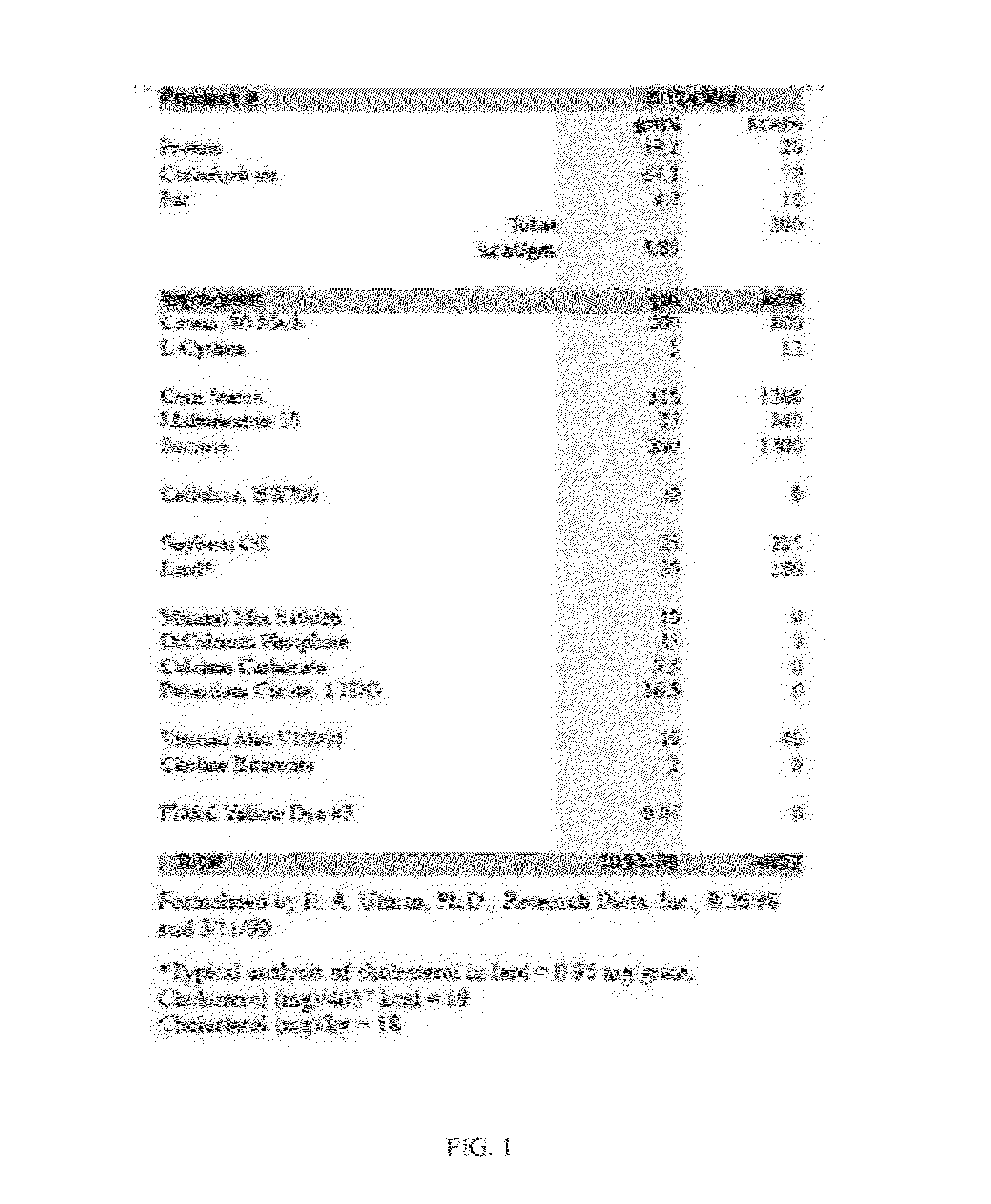
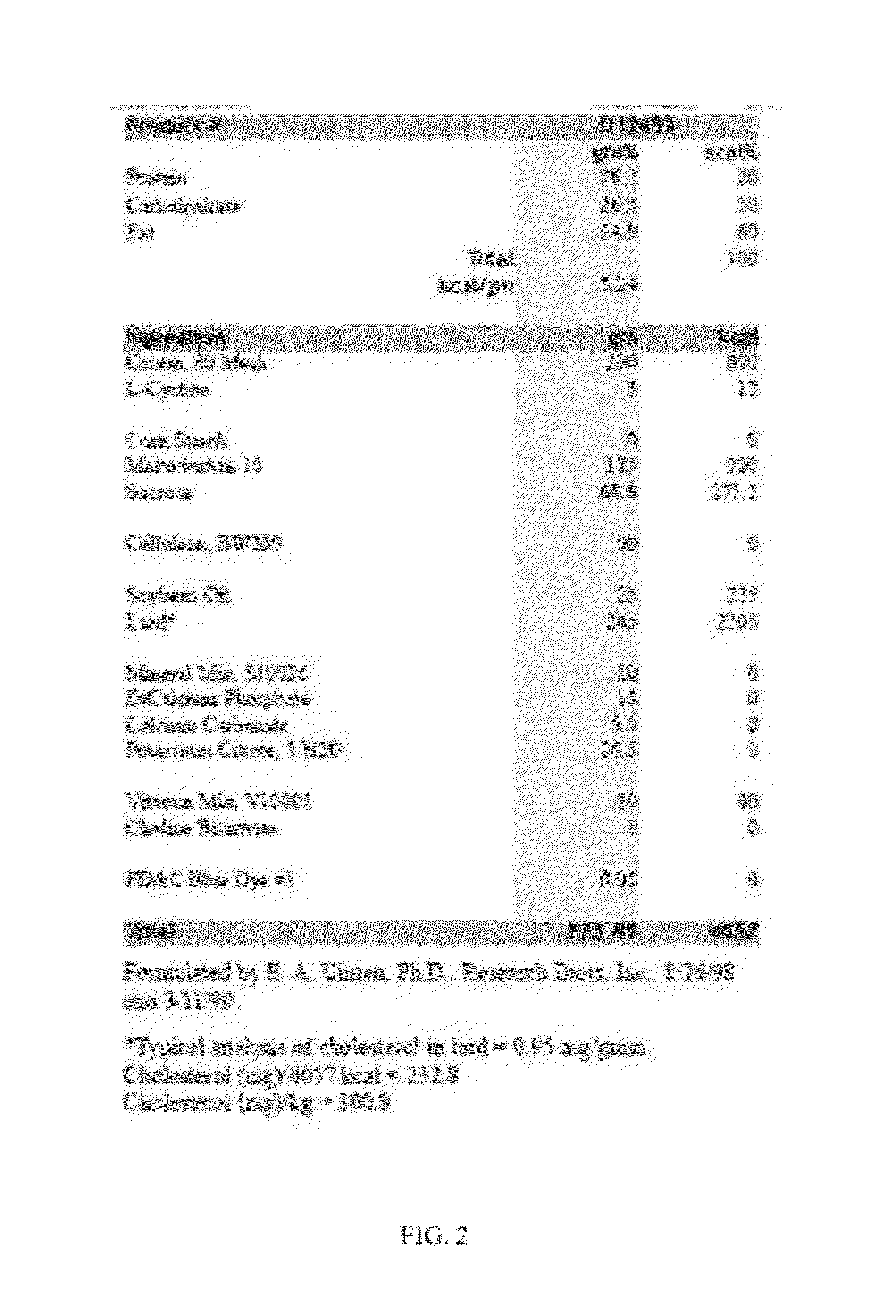
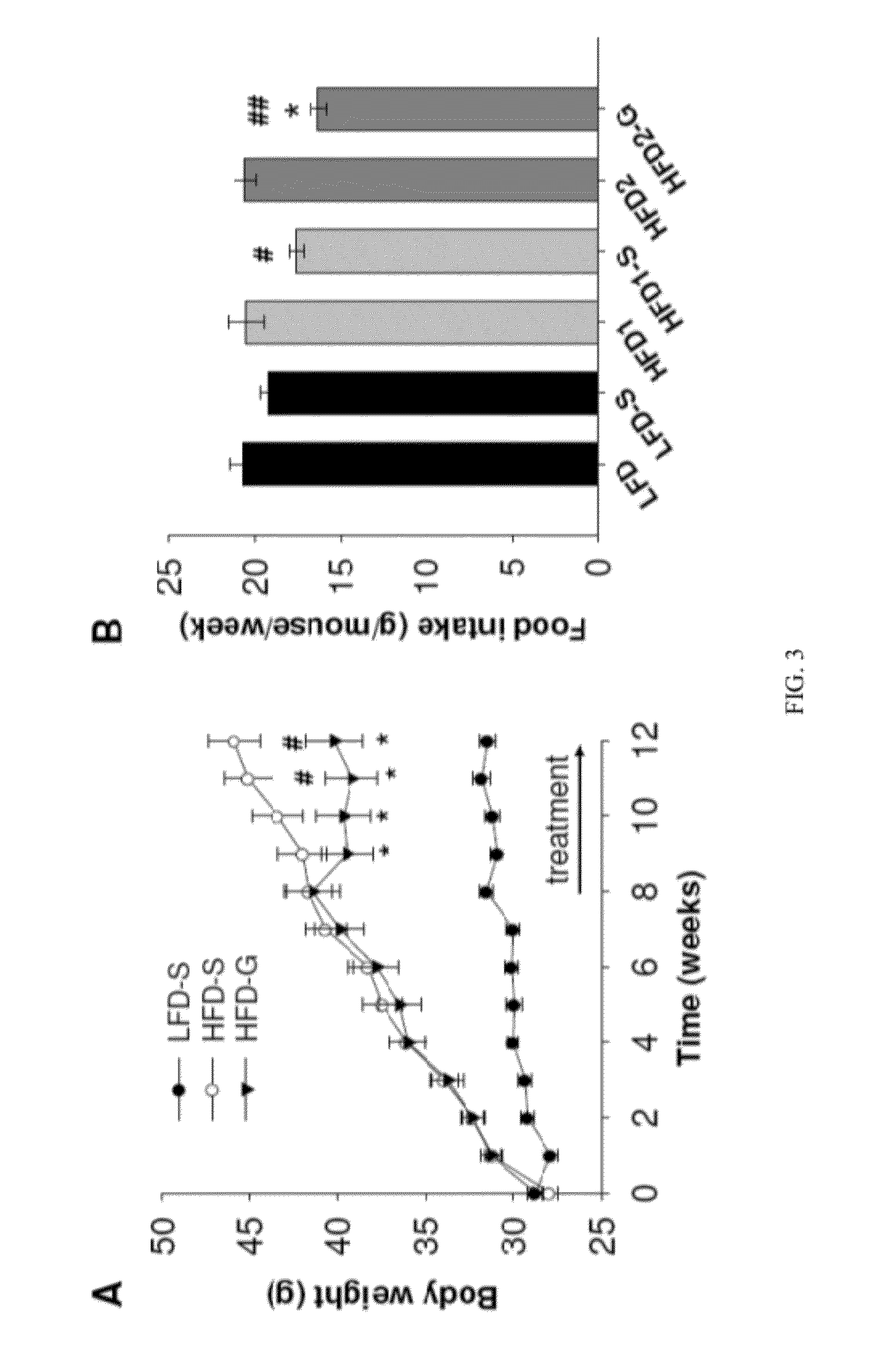
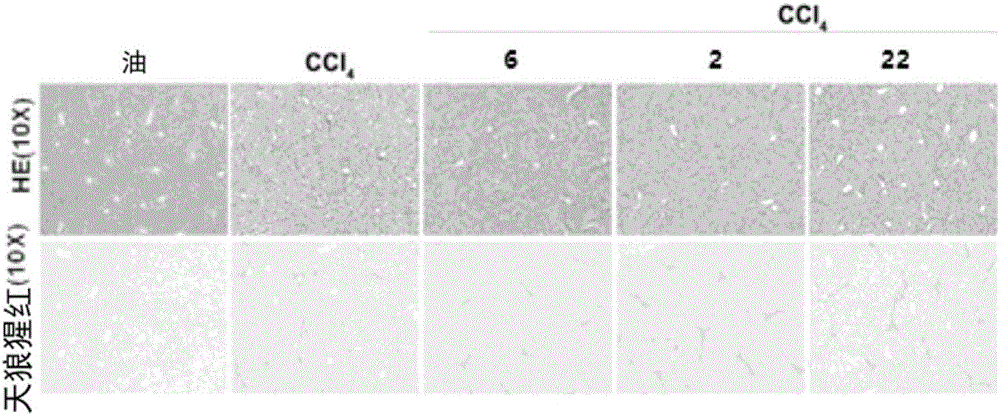
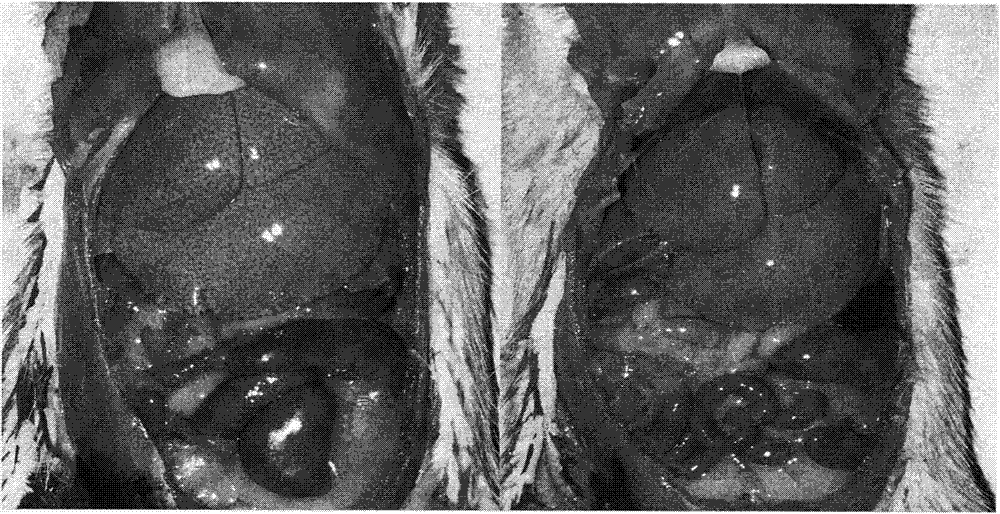
High-fat feed and application thereof in building animal model with non-alcoholic fatty liver
InactiveCN102106476AEasy to operateEasy to controlAnimal feeding stuffAccessory food factorsAnimal scienceCholesterol
The invention discloses high-fat feed and application thereof in building an animal model with non-alcoholic fatty liver. The high-fat feed comprises the following raw materials in mass percent: 80.5% of rat breeding feed, 10% of egg, 7% of lard oil, 2% of cholesterol and 0.5% of No.3 bile salt. The model built in the invention can subsequently experience former three stages in the development ofthe clinical disease course of the non-alcoholic fatty liver: simple fatty liver, steato hepatitis and fibrosis. The model is used for studying the pathogenesis as well as the development mechanism of the non-alcoholic fatty liver, studying the prevention and treatment of the non-alcoholic fatty liver at different stages, and particularly for screening new medicines for preventing and treating fibrosis of the non-alcoholic fatty liver, screening measures for preventing and treating fibrosis of the non-alcoholic fatty liver and objectively evaluating the methods for preventing and treating thenon-alcoholic fatty liver at each stage.
Owner:ZHEJIANG ACAD OF MEDICAL SCI
Markers of non-alcoholic fatty liver disease (nafld) and non-alcoholic steatohepatitis (nash) and methods of use thereof
Novel methods for assessing the level of triglycerides in the liver of a subject are described, comprising determining the amount of a lipid metabolite in a sample from a body fluid of the subject. The methods may be used, for example, in diagnosing and monitoring liver disorders such as steatosis, NAFLD and NASH.
Owner:METABOLON
Use of probes for unbound metabolites
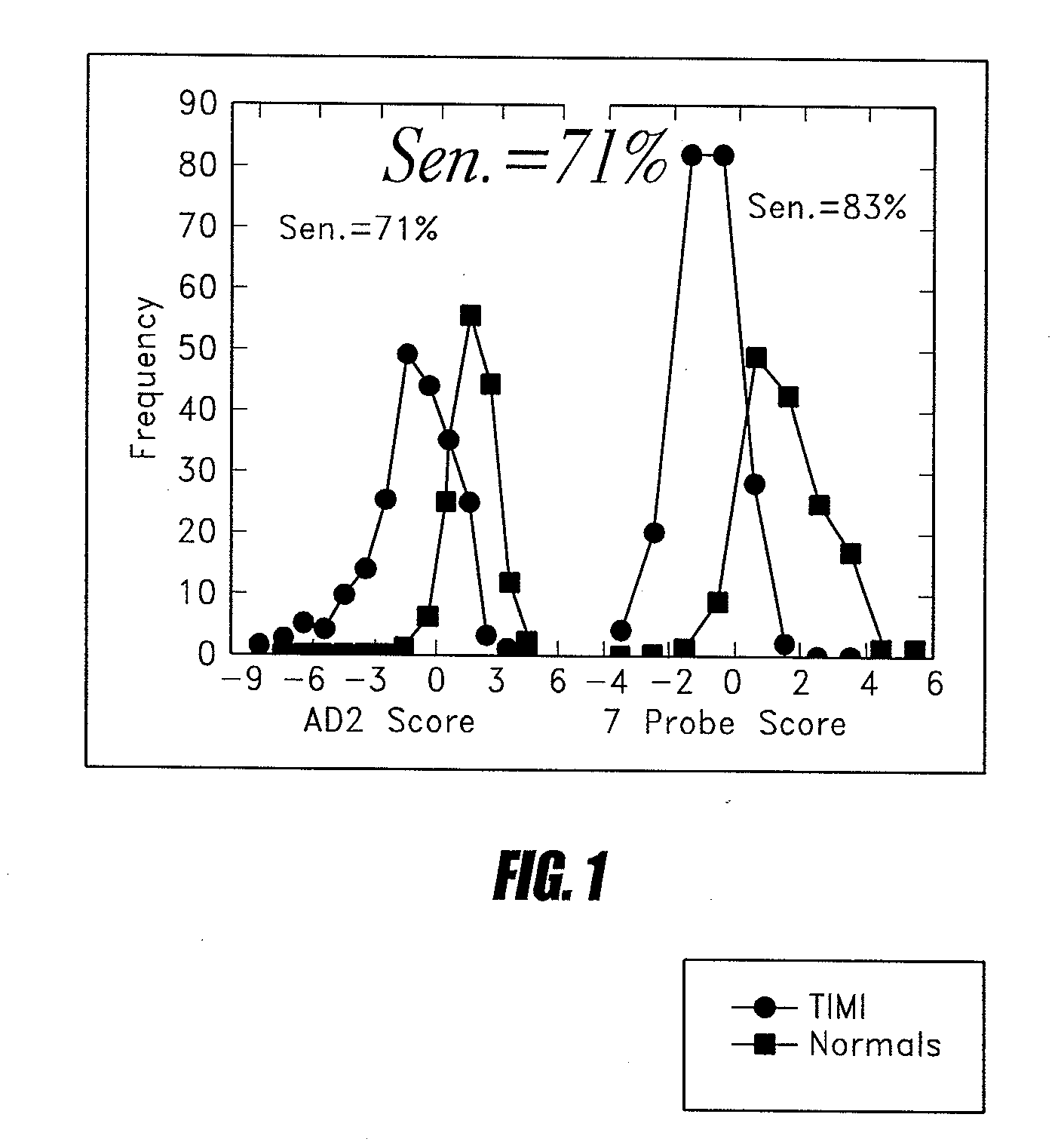
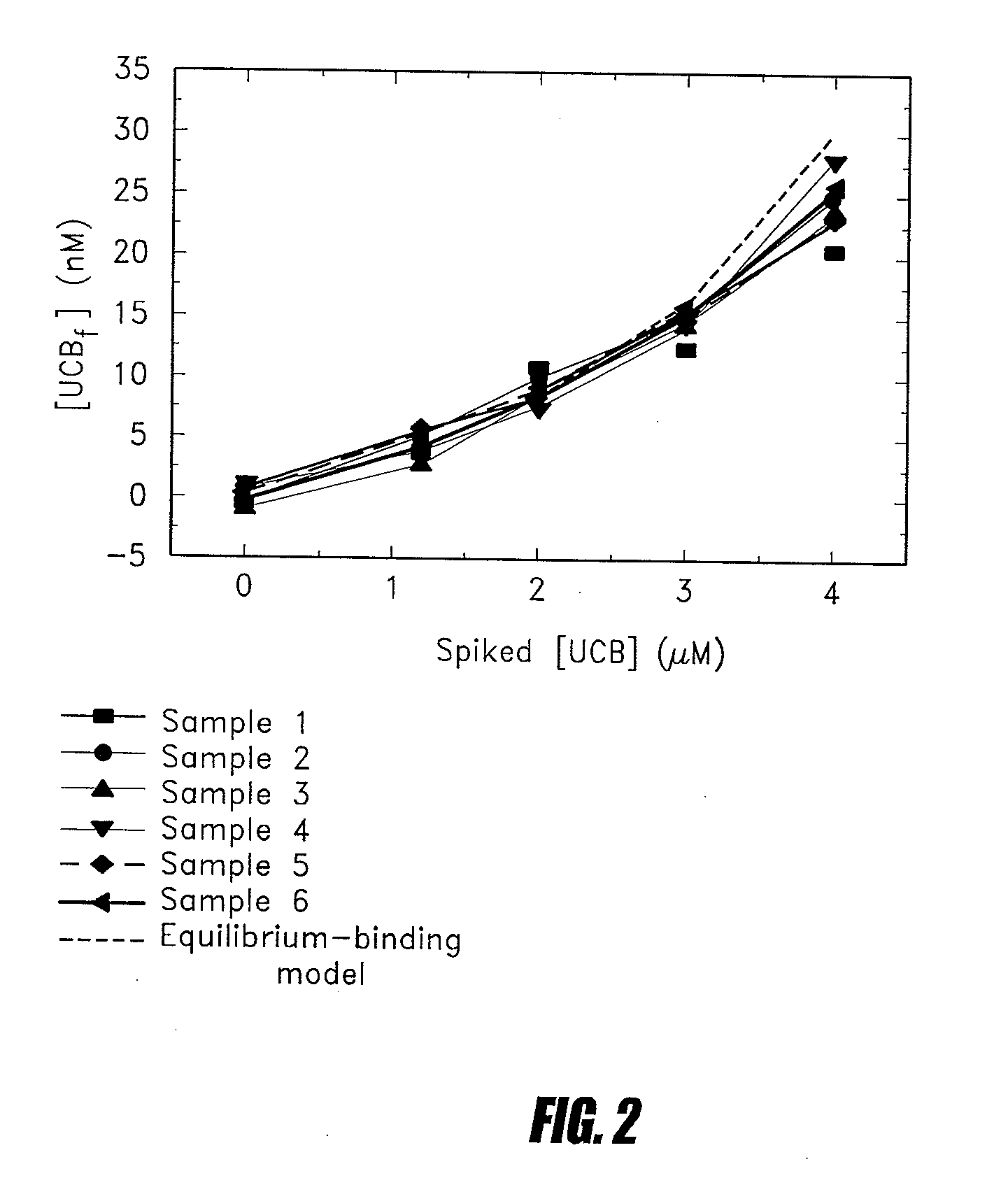
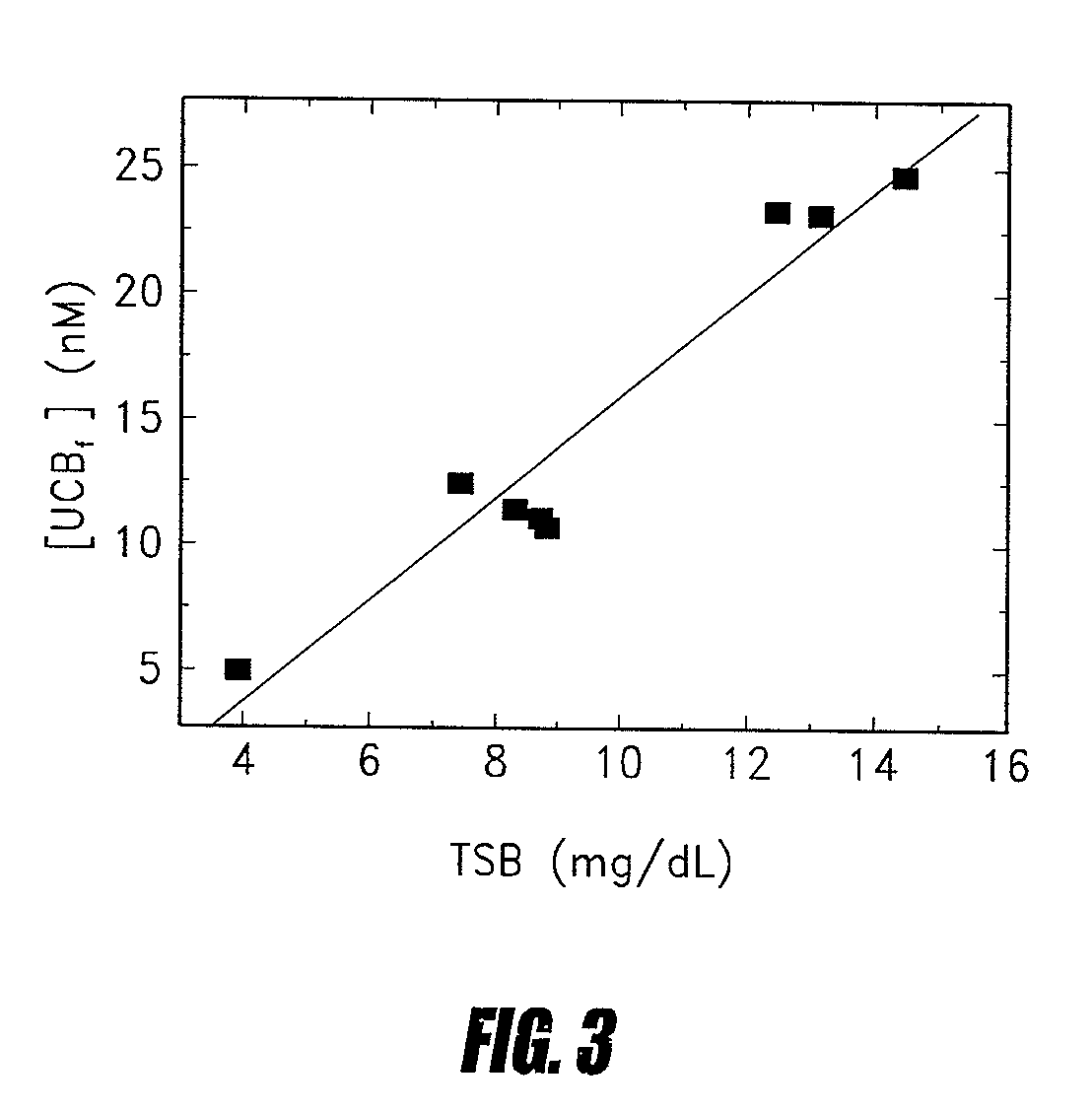
Methods of determining levels of unbound metabolites are disclosed. Probes derived from fatty acid binding protein muteins are described that bind preferentially to a number of unbound metabolites including oleate, stearate, linoleate, palmitate, arachidonate and unconjugated bilirubin. A profile for a patient is determined using one or more of the described probes. The profile is useful in diagnosis of disease, particularly myocardial infarction, non-alcoholic fatty liver disease (NAFLD), diabetes, stroke, sepsis and neonatal jaundice. The responses of multiple probes to a test sample are used to classify the degree of acute coronary syndrome by comparison to multi-probe profiles generated from unstable angina, non ST elevation myocardial infarction, and ST elevation myocardial infarction.
Owner:KLEINFELD ALAN
Application of composite high-fat forage to construct non-alcoholic fatty liver disease rat model
The invention belongs to the field of animal experiment models, and discloses application of a composite high-fat forage to construct a non-alcoholic fatty liver disease rat model. The high-fat forage is composed of the following raw materials in percent by mass: 77.5% of a rat basic forage, 10% of egg, 10% of coconut oil, 2% of cholesterol, 0.5% of bile salt, and 500mg / kg / d of sodium valproate calculated according to the rat weight. After 8 weeks, rats all have typical non-alcoholic fatty live symptoms, and liver has a lot of fat accumulation along with inflammatory cell infiltration. The model establishing time is short, the success rate is high, and the composite high-fat forage is applicable to pathogenesis research induced by high-fat-diet combined medicines with liver-toxicity side effect, screening of related control measures and efficacy evaluation of treatment medicines.
Owner:NANJING GENERAL HOSPITAL NANJING MILLITARY COMMAND P L A +1
Method of treating non-alcoholic fatty liver disease and steatohepatitis
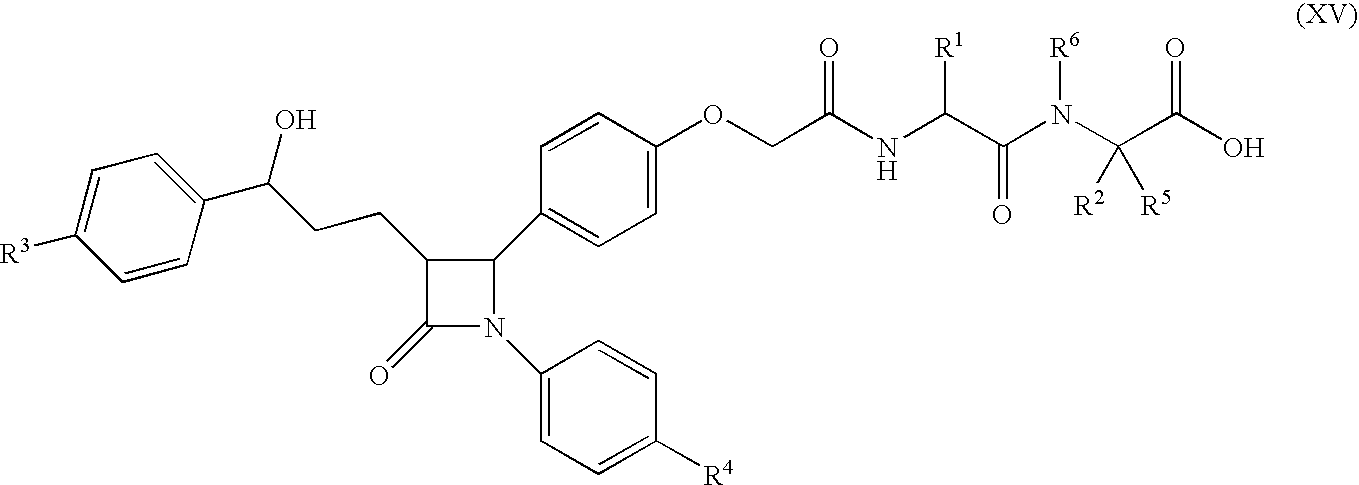
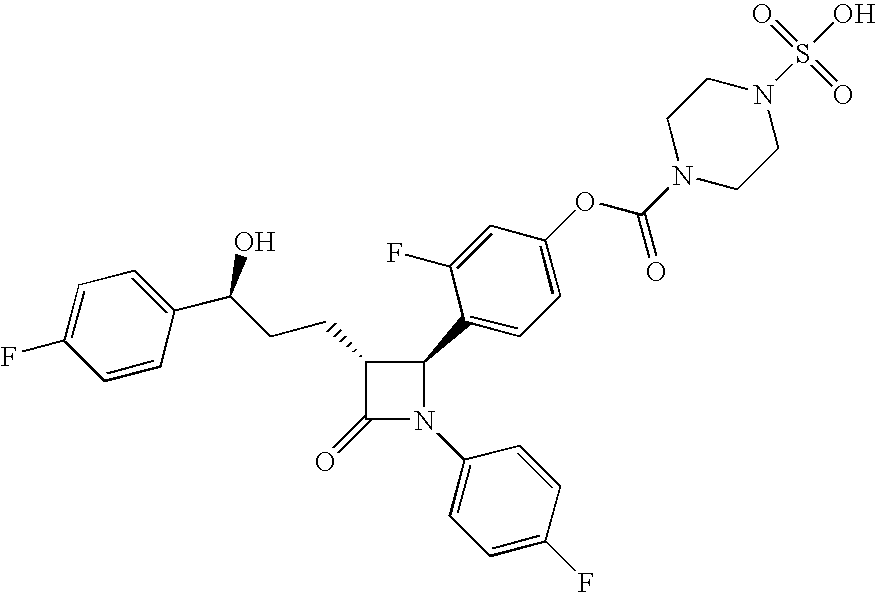
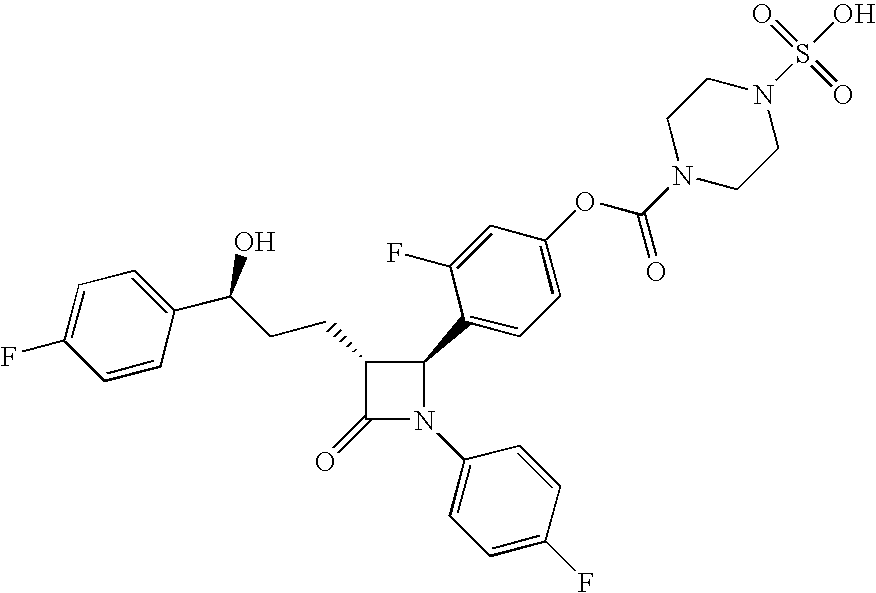
A compound of Formula (I):or a metabolite thereof, or an ester of the compound of Formula (I) or the metabolite thereof, or a pharmaceutically acceptable salt of each thereof, wherein m, n, X1 and X2 are as defined herein, is useful for treating non-alcoholic fatty liver disease (NAFLD) or non-alcoholic steatohepatitis (NASH).
Owner:MEDICINOVA INC
Azabicycloalkane-indole and azabicycloalkane-pyrrolo-pyridine mch-1 antagonists, methods of making, and use thereof
Novel MCH-1 receptor antagonists are disclosed. These compounds are used in the treatment of various disorders, including obesity, anxiety, depression, non-alcoholic fatty liver disease, and psychiatric disorders. Methods of making these compounds are also described in the present invention.
Owner:ALBANY MOLECULAR RESEARCH INC
Progression Inhibitor For Disease Attributed To Abnormal Accumulation Of Liver Fat
The present invention provides pharmaceutical compositions useful as agents for the inhibition of progression of diseases associated with abnormal accumulation of liver lipids. In particular, the pharmaceutical compositions of the present invention which comprise as an active ingredient a sodium / glucose co-transporter 2 inhibitor are highly suitable as an agent for the inhibition of progression of not only common fatty liver but also non-alcholic fatty liver disease (NAFL), non-alcholic steatohepatitis (NASH), hypernutritive fatty liver, diabetic fatty liver, alcholic fatty liver disease toxic fatty liver or the like.
Owner:KISSEI PHARMA
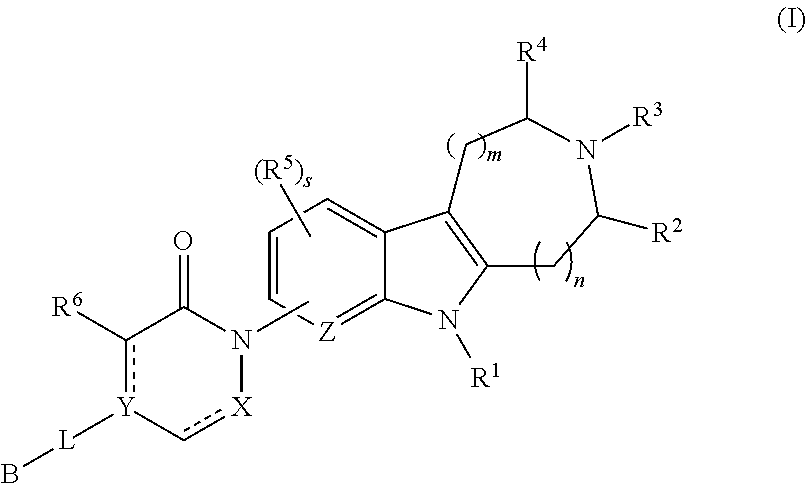
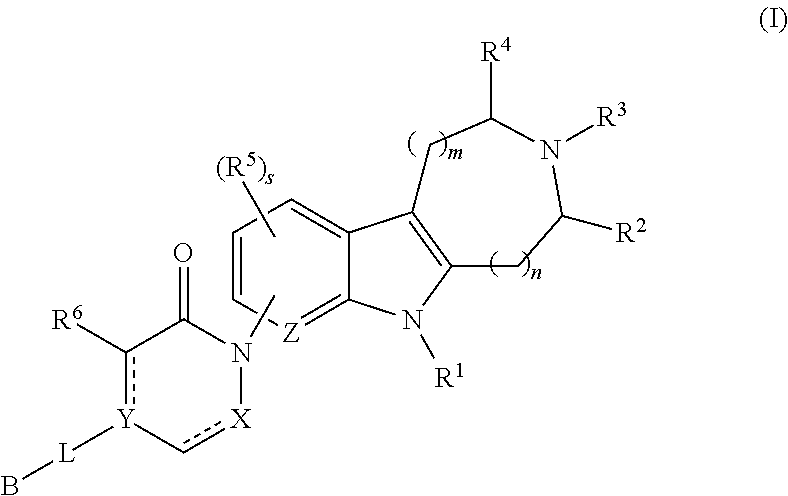
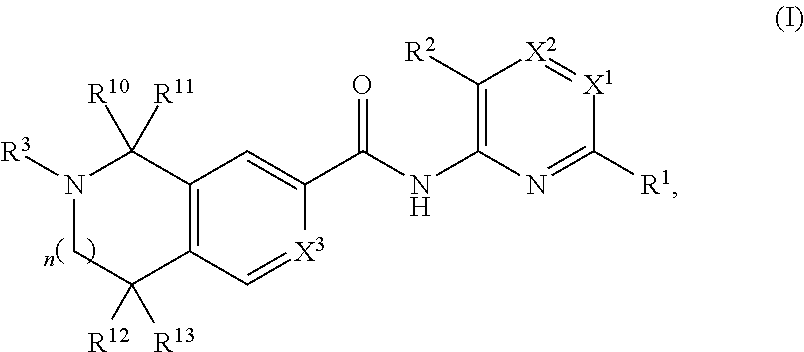
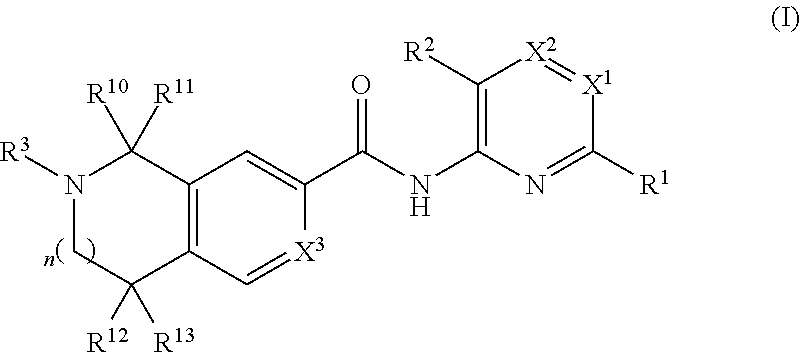
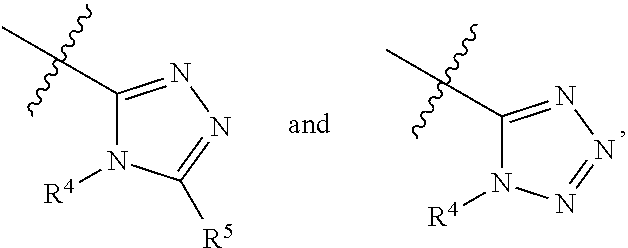
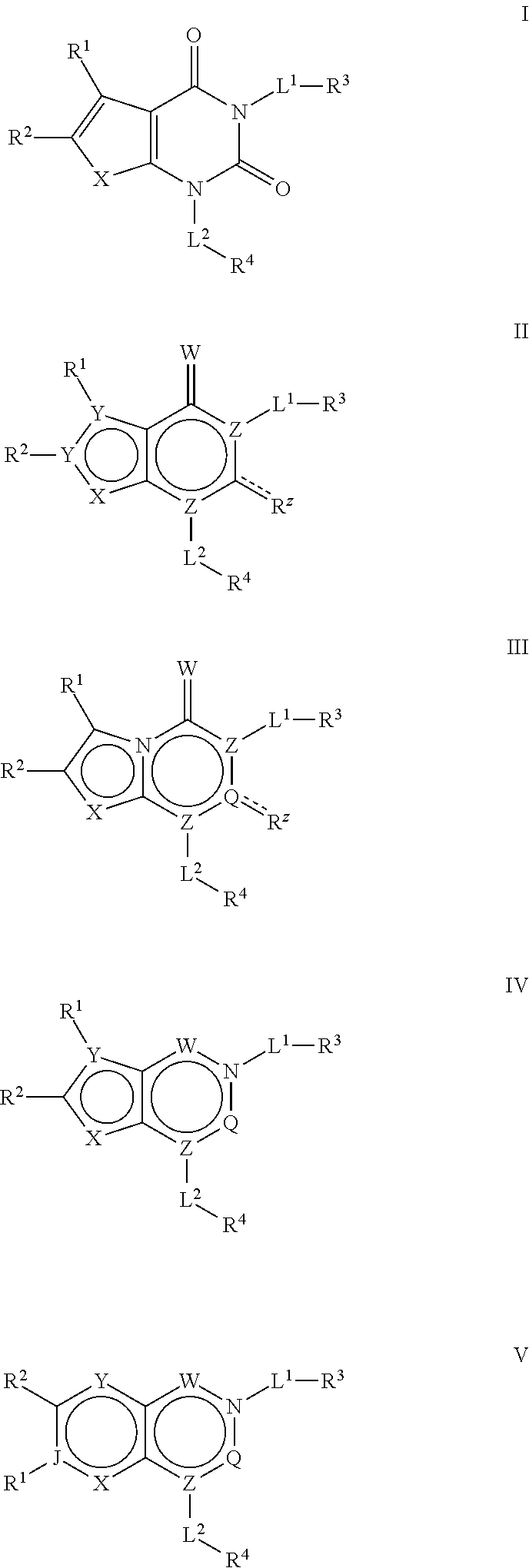
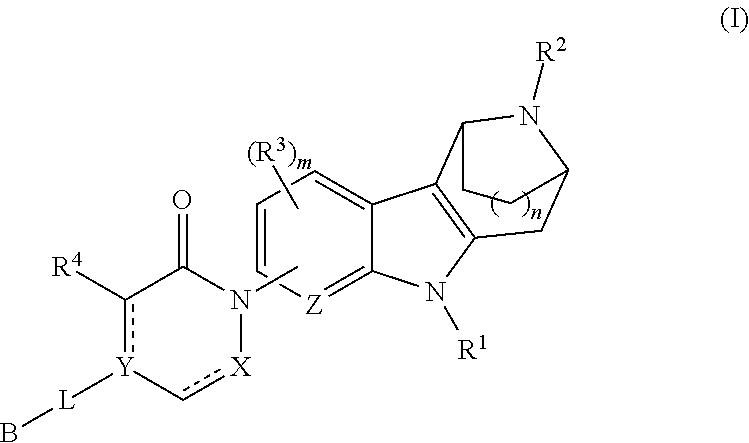
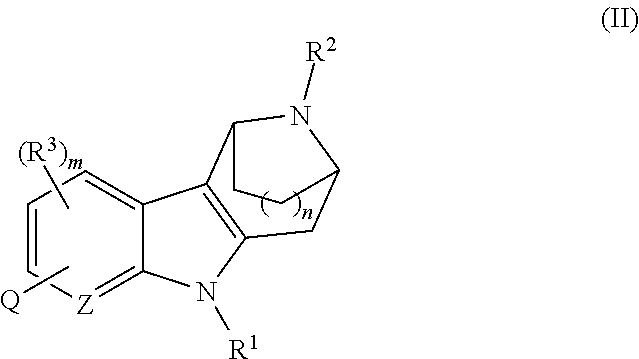
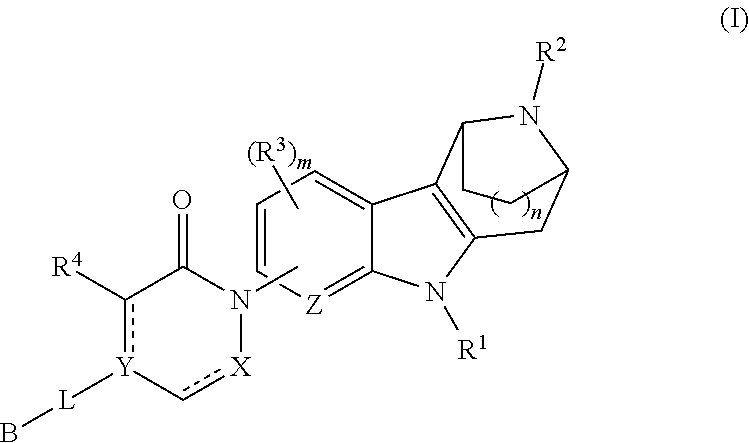
AZINONE-SUBSTITUTED AZEPINO[b]INDOLE AND PYRIDO-PYRROLO-AZEPINE MCH-1 ANTAGONISTS, METHODS OF MAKING, AND USE THEREOF
Novel MCH-1 receptor antagonists are disclosed. These compounds are used in the treatment of various disorders, including obesity, anxiety, depression, non-alcoholic fatty liver disease, and psychiatric disorders. Methods of making these compounds are also described in the present invention.
Owner:HARMONY BIOSCIENCES LLC +1
Application of medicinal composition to preparation of medicament for preventing and treating alcoholic liver damage and fatty liver and lowering blood fat
ActiveCN102058632AEasy to solveImprove development and utilization valueOrganic active ingredientsDigestive systemSecondary hyperlipidemiaAlcoholic liver damage
The invention discloses an ilicis routundae cortex medicinal composition for preventing and treating alcoholic liver damage, alcoholic fatty liver, non-alcoholic fatty liver and hyperlipidemia. The composition is extracted and refined from natural ilicis routundae cortex and is not chemically modified, and is characterized by comprising substances such as pedunculoside, syringin, rotundicacid and the like. The invention also discloses a preparation method of the ilicis routundae cortex composition and application to preparation of a medicament for preventing and treating the alcoholic liver damage, the alcoholic fatty liver and the non-alcoholic fatty liver and lowering blood fat.
Owner:吉林修正药业新药开发有限公司
Compositions and methods for reducing hepatotoxicity associated with drug administration and treating non-alcoholic fatty liver disease, non-alcoholic steatohepatitis and associated cirrhosis
InactiveUS20090239831A1Improve efficiencyEnhancing armamentariumBiocideSalicyclic acid active ingredientsActive agentHigh doses
The present invention relates to the discovery that acetylsalicylic acid (ASA or aspirin), salicylic acid (SA) and related salicylate esters and their pharmaceutically acceptable salts, when coadministered in effective amounts with a drug or other bioactive agent which typically (in the absence of the salicylate compound) produces significant hepatotoxicity as a secondary indication, will substantially reduce or even eliminate such hepatotoxicity. Favorable therapeutic intervention results from the use of the present invention having the effect of reducing hepatotoxicity associated with the administration of certain drugs and other bioactive agents and in certain instances of allowing the administration of higher doses of a compound which, without the coadministration, would produce hepatotoxicity which limits or even negates the therapeutic value of the compound. The invention also relates to methods of reducing the likelihood of a patient at risk for non-alcoholic fatty liver diseases (NAFLD), including non-alcoholic steatohepatitis (NASH), or treating NAFLD or NASH including primary NASH, NASH secondary to liver transplantation (NASH post-liver transplantation) or cirrhosis represent alternative aspects of the present invention.
Owner:YALE UNIV
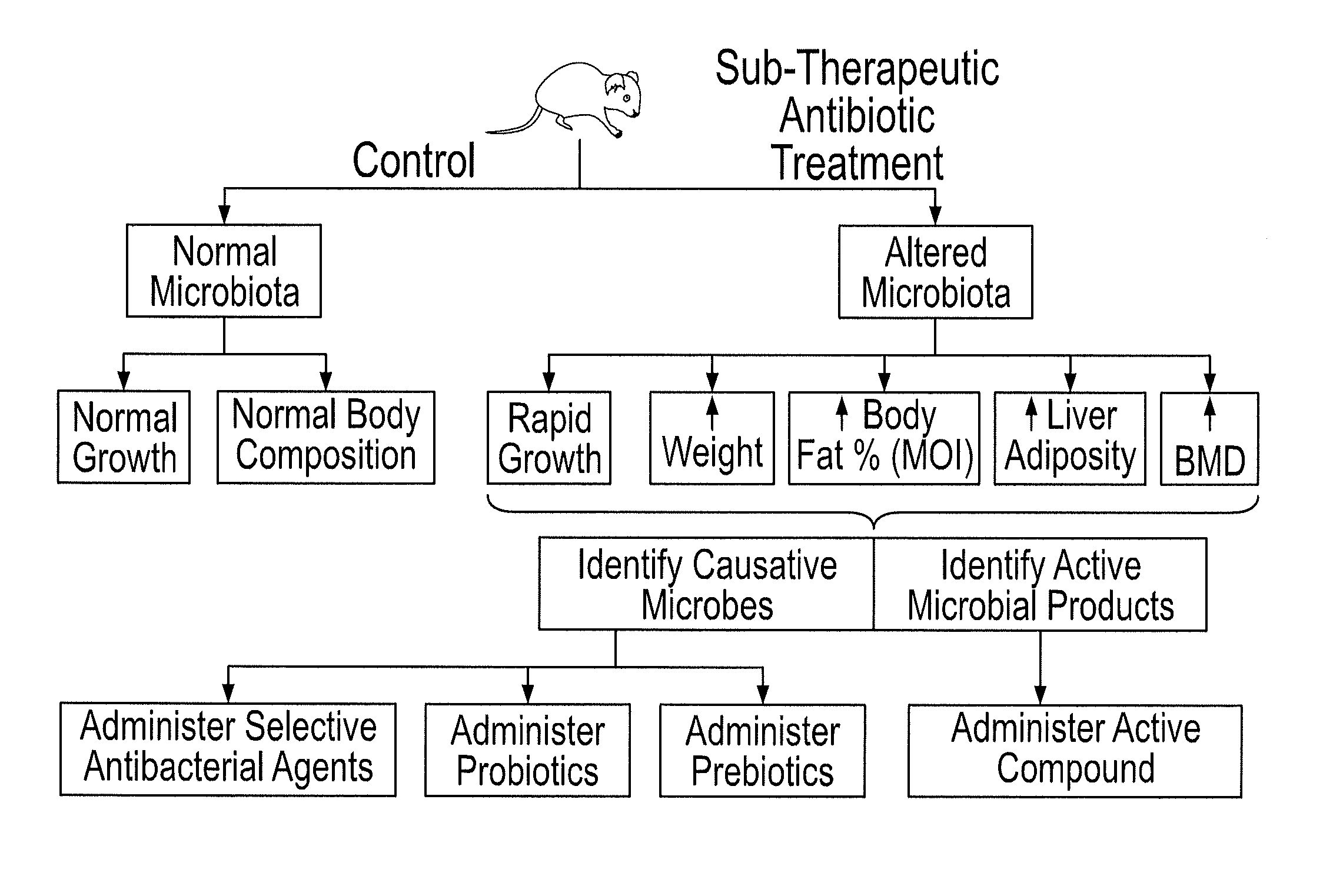
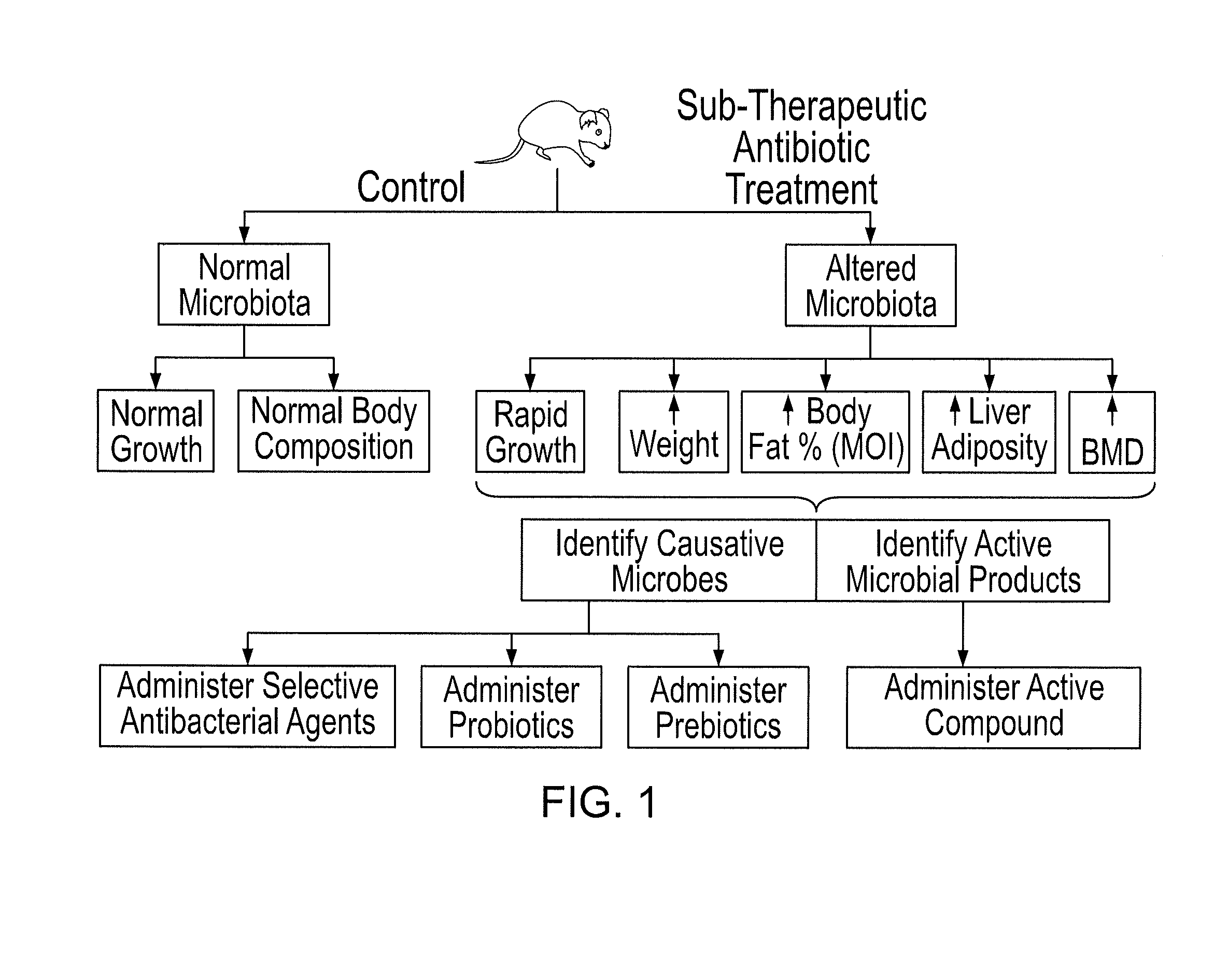
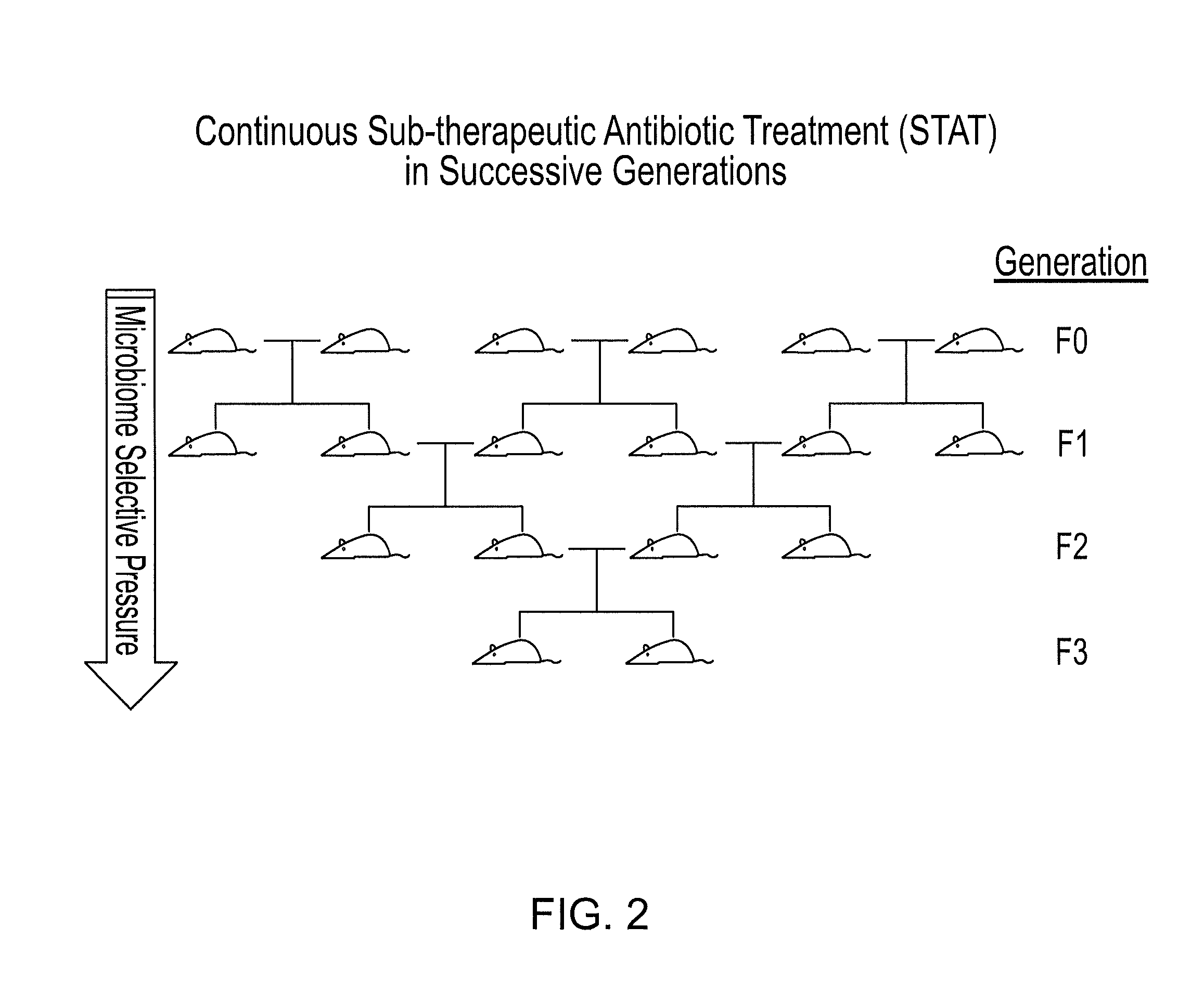
Methods for treating bone disorders by characterizing and restoring mammalian bacterial microbiota
ActiveUS8951512B2Increasing adult height and muscle massBiocideMetabolism disorderIntestinal microorganismsPancreatic hormone
Owner:NEW YORK UNIV
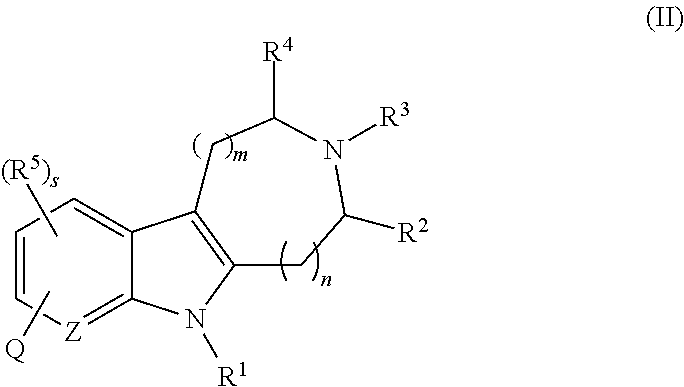
Azinone-substituted azapolycycle mch-1 antagonists, methods of making, and use thereof
Novel MCH-1 receptor antagonists are disclosed. These compounds are used in the treatment of various disorders, including obesity, anxiety, depression, non-alcoholic fatty liver disease, and psychiatric disorders. Methods of making these compounds are also described in the present invention.
Owner:ALBANY MOLECULAR RESEARCH INC
Sulphonylaminocarbonyl derivatives, and pharmaceutical compositions and use thereof
The invention relates to preparation methods of sulphonylaminocarbonyl derivatives and compositions containing the same component and a use of the sulphonylaminocarbonyl derivatives as FXR and / or TGR5 agonists; the agonists are the sulphonylaminocarbonyl derivatives represented by the formula I, or pharmaceutically acceptable salts, prodrugs, solvates, hydrates, polymorphs, isomers, and stable isotope derivatives thereof. The compounds can be used for treatment of diseases and symptoms mediated by FXR and / or TGR5 and other therapeutic fields, wherein the diseases and symptoms include primary biliary cirrhosis, nonalcoholic fatty liver, portal hypertension, bile acid diarrhea and cholestasis, type II diabetes and obesity.
Owner:SHANGHAI DE NOVO PHARMA
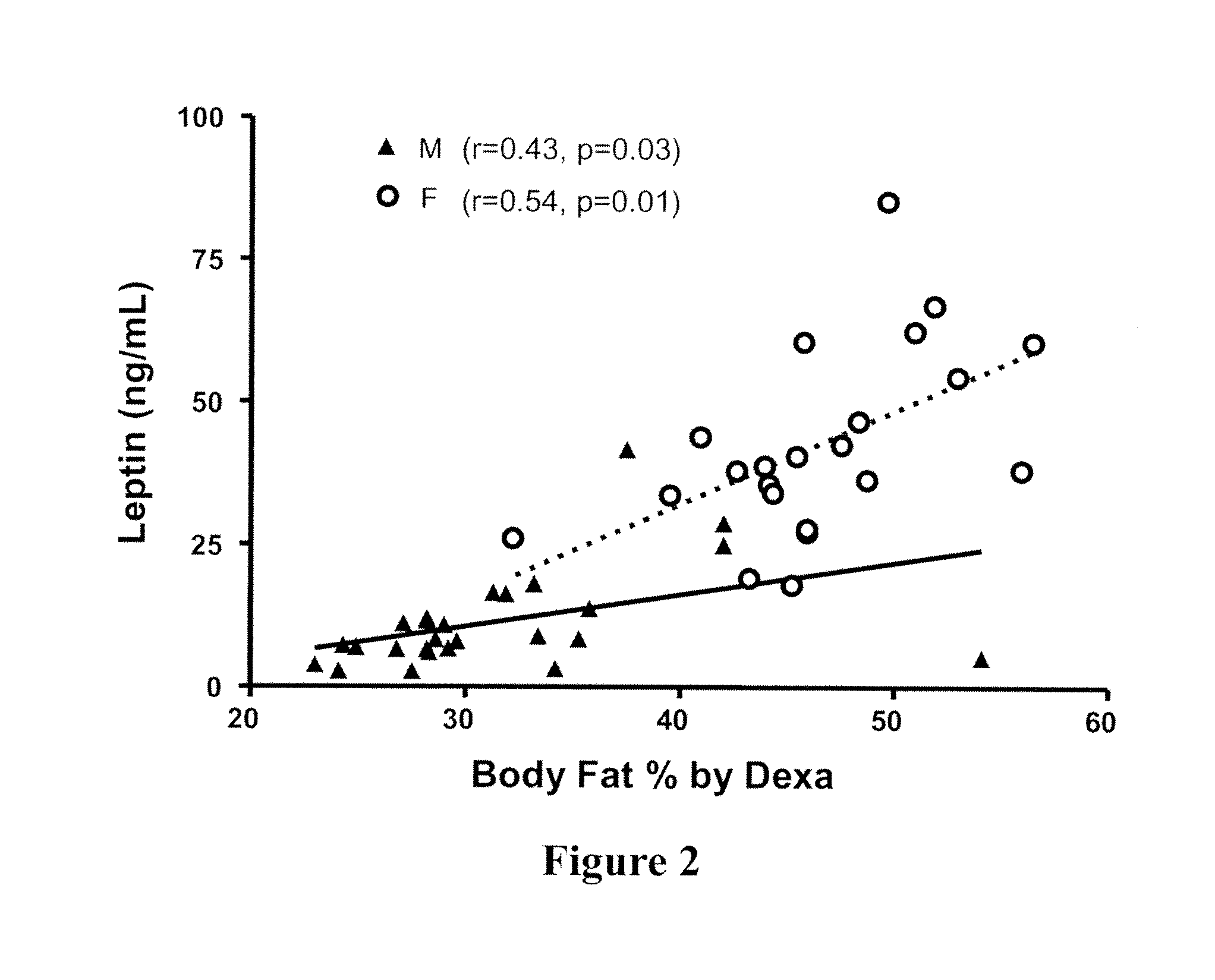
Use of leptin for the treatment of fatty liver diseases and conditions
ActiveUS20110212889A1Liver inflammation is reducedDecrease triglyceride accumulationHormone peptidesPeptide/protein ingredientsSteatosisLeptin Deficiency
The invention generally relates to the use of leptin in the treatment of a leptin-responsive disease or condition in a non-lipodystrophic subject. More particularly, the invention is directed to the use of leptin in the treatment of a fatty liver disease in a non-lipodystrophic subject with a relative leptin deficiency. The invention includes methods for the treatment of nonalcoholic steatohepatitis (NASH), alcoholic fatty liver disease (AFLD), and nonalcoholic fatty liver disease (NAFLD) in a non-lipodystrophic subject. The invention includes the treatment of conditions ranging from ectopic lipid accumulation (steatosis) to cirrhosis.
Owner:RGT UNIV OF MICHIGAN
Apoptosis signal-regulating kinase 1 inhibitors and methods of use thereof
The present invention discloses compounds of Formula (I), and pharmaceutically acceptable salts and esters thereof:which inhibit the Apoptosis signal-regulating kinase 1 (ASK-1), which associated with autoimmune disorders, neurodegenerative disorders, inflammatory diseases, chronic kidney disease, cardiovascular disease. The present invention further relates to pharmaceutical compositions comprising the aforementioned compounds for administration to a subject suffering from ASK-1 related disease. The invention also relates to methods of treating an ASK-1 related disease in a subject by administering a pharmaceutical composition comprising the compounds of the present invention. The present invention specifically relates to methods of treating ASK-1 associated with hepatic steatosis, including non-alcoholic fatty liver disease (NAFLD) and non-alcohol steatohepatitis disease (NASH).
Owner:ENANTA PHARM INC
Method for establishing non-alcoholic fatty liver disease combined with viral hepatitis mouse model
InactiveCN104365543AAffect eatingGood repeatabilityViral/bacteriophage medical ingredientsAnimal feeding stuffLiver histologyHigh fat
The invention discloses a method for establishing a non-alcoholic fatty liver disease combined with viral hepatitis mouse model. The method comprises the steps that high-fat feed is used for feeding a C3H / HeN small mouse for 12 weeks, the body weight, the liver weight, transaminase, the glucolipid metabolism index and liver histological change of the small mouse are observed, and a non-alcoholic fatty liver disease small mouse is cultivated. Then 10 PFU of MHV-3 infected mice are selected, and the survival rate, the liver histology and intrahepatic virus replication of the infected mice are observed, so that the non-alcoholic fatty liver disease combined with viral hepatitis mouse model similar to a human disease process is established. The established model provides a forceful tool for virus dynamics, immunological changes and prevention and control measures systematically studying non-alcoholic fatty liver disease combined with viral hepatitis.
Owner:TONGJI HOSPITAL ATTACHED TO TONGJI MEDICAL COLLEGE HUAZHONG SCI TECH
Methods of treatment of fatty liver disease by pharmacological activation of cholinergic pathways
A method of treating a fatty liver disease in a subject. The method comprises administering to the subject an effective amount of a cholinergic pathway stimulating agent, wherein the fatty liver disease is selected from non-alcoholic fatty liver (NAFL), alcoholic fatty liver (AFL), non-alcoholic steatohepatitis (NASH), alcoholic steatohepatitis (ASH), NASH-associated liver fibrosis, ASH-associated liver fibrosis, non-alcoholic cirrhosis, and alcoholic cirrhosis.
Owner:THE FEINSTEIN INST FOR MEDICAL RES
Semen cassiae, fructus lycii and glossy privet fruit oral solution with functions of tonifying liver and reducing fat
InactiveCN103705854ABlood lipid parameters decreasedImproved liver functionMetabolism disorderDigestive systemMedicinal herbsOral solutions
The invention discloses a semen cassiae, fructus lycii and glossy privet fruit oral solution with functions of tonifying liver and reducing fat. The semen cassiae, fructus lycii and glossy privet fruit oral solution is prepared by adopting the steps of respectively weighing Chinese medicinal herbs: 30 parts of semen cassia, 30 parts of fructus lycii, 30 parts of glossy privet fruit, 20 parts of polygonum multiflorum thumb, 20 parts of gynostemma pentaphylla, 20 parts of raw hawthorn, 20 parts of capillary artemisia, 10 parts of radix curcumae and 10 parts of rhizoma alismatis; and decocting the Chinese medicinal herbs together according to a conventional method, and densely decocting into an oral solution of 2g dried medicinal herbs / ml. A taking method is as follows: the semen cassiae, fructus lycii and glossy privet fruit oral solution is taken twice, 20ml per time. One month is a course of treatment, and the semen cassiae, fructus lycii and glossy privet fruit oral solution is generally taken for three courses of treatment. Fatty, sweet, stodgy and pungent foods are contraindicated in a taking process. The semen cassiae, fructus lycii and glossy privet fruit oral solution has the advantages of low Chinese medicinal herb price, and is convenience to take and process, and has a remarkable treatment effect on non-alcoholic fatty liver disease and hyperlipoidemia. Meanwhile, according to the characteristic of liver deficiency, the semen cassiae, fructus lycii and glossy privet fruit oral solution is suitable for all types of syndrome of the traditional Chinese medicine, is capable of recovering the function of the liver to protect the liver and is capable of removing pathological products to achieve the treatment purpose.
Owner:郭兰春
Serum miRNA maker assemblage for detecting nonalcoholic fatty liver, and its application
InactiveCN104293908AAchieve early detectionRapid non-invasive testingMicrobiological testing/measurementDNA/RNA fragmentationSerum igeMedicine
The invention belongs to the field of biotechnology, and relates to a serum miRNA maker assemblage for detecting nonalcoholic fatty liver, and its application. The serum miRNA maker assemblage for detecting nonalcoholic fatty liver includes the following four has-microRNAs: hsa-miR-122-5p, hsa-miR-1290, hsa-miR-27b-3p and hsa-miR-192- 5p. The serum miRNA maker assemblage can realize early detection and rapid noninvasive detection of the nonalcoholic fatty liver.
Owner:镇江市第三人民医院
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com

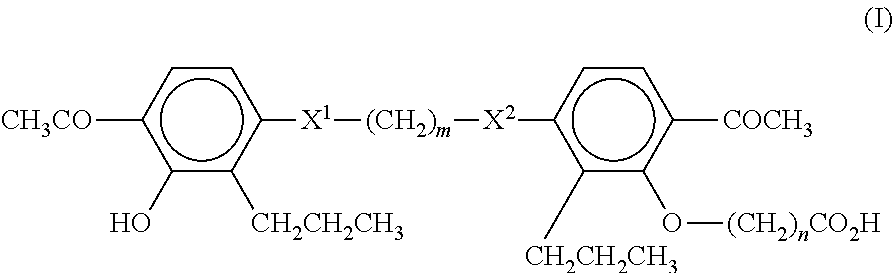
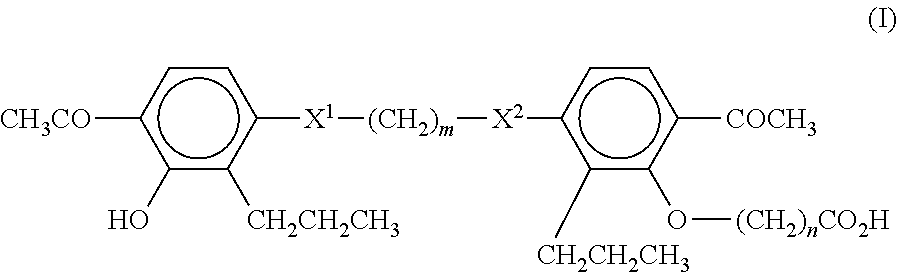
![AZINONE-SUBSTITUTED AZEPINO[b]INDOLE AND PYRIDO-PYRROLO-AZEPINE MCH-1 ANTAGONISTS, METHODS OF MAKING, AND USE THEREOF AZINONE-SUBSTITUTED AZEPINO[b]INDOLE AND PYRIDO-PYRROLO-AZEPINE MCH-1 ANTAGONISTS, METHODS OF MAKING, AND USE THEREOF](https://images-eureka.patsnap.com/patent_img/571c3ea7-937e-44b0-b1a9-b64302e134e3/US20110003793A1-20110106-C00001.png)
![AZINONE-SUBSTITUTED AZEPINO[b]INDOLE AND PYRIDO-PYRROLO-AZEPINE MCH-1 ANTAGONISTS, METHODS OF MAKING, AND USE THEREOF AZINONE-SUBSTITUTED AZEPINO[b]INDOLE AND PYRIDO-PYRROLO-AZEPINE MCH-1 ANTAGONISTS, METHODS OF MAKING, AND USE THEREOF](https://images-eureka.patsnap.com/patent_img/571c3ea7-937e-44b0-b1a9-b64302e134e3/US20110003793A1-20110106-C00002.png)
![AZINONE-SUBSTITUTED AZEPINO[b]INDOLE AND PYRIDO-PYRROLO-AZEPINE MCH-1 ANTAGONISTS, METHODS OF MAKING, AND USE THEREOF AZINONE-SUBSTITUTED AZEPINO[b]INDOLE AND PYRIDO-PYRROLO-AZEPINE MCH-1 ANTAGONISTS, METHODS OF MAKING, AND USE THEREOF](https://images-eureka.patsnap.com/patent_img/571c3ea7-937e-44b0-b1a9-b64302e134e3/US20110003793A1-20110106-C00003.png)