Thyromimetics for the Treatment of Fatty Liver Diseases
a technology of fatty liver disease and thyromimetics, which is applied in the field of thyromimetics, can solve the problems of limiting the rate of fatty acid oxidation, affecting the effect of beneficial or detrimental effects, etc., and achieve the effects of reducing the fat content in the liver, preventing, treating or ameliorating fatty liver disease, and modulating gene expression
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0959]Examples of the method of the invention includes the following. It will be understood that these examples are exemplary and that the method of the invention is not limited solely to these examples.
[0960]For the purposes of clarity and brevity compounds are referred to by compound numbers (from the Table below) in the biological examples below.
CompoundStructureNumber17 7 6cis-13-1TRIAC18
example a
Chronic Exposure to Thyroid Receptor Agonists in Normal Rats
[0961]The purpose of these studies was to compare the difference in efficacy to clear liver triglyceride content between T3 and various T3 mimetics that are carboxylic acids and T3 mimetics that are phosphonic acids. In one example, T3 and Compounds 7 and 17, which differ only in that for Compound 7, the X moiety of Formula II is —P(O)OH2 and for Compound 17, X is —C(O)OH, were compared. In the same example TRIAC and Compound 6, which differ only in that for Compound 6, X is —P(O)OH2 and for TRIAC, X is —C(O)OH, were compared. Efficacy was measured by analyzing total liver triglycerides.
[0962]Methods: Normal rats (Sprague-Dawley) were maintained on a standard diet. Compounds 7, 17, 6, TRIAC or T3 were administered by continuous infusion using an osmotic pump (Alzet; subcutaneous implant) at a dose of 1 mg / kg / day. The compounds were dissolved in 0.1N NaOH solution and the pH adjusted to 7.4-8.0. The compounds were brought up...
example b
Chronic Exposure to Thyroid Receptor Agonists in ob / ob Mice
[0966]The purpose of these studies was to compare the difference in efficacy to clear liver triglyceride content between Compound cis-13-1 and T3 in ob / ob mice.
[0967]Methods: ob / ob mice were maintained on a standard diet. Compound cis-13-1 was administered at doses of 3, 10 and 30 mg / kg / d orally in a CMC suspension. T3, 100 nmole / kg / d, was administered as an aqueous solution subcutaneously. Liver triglycerides were analyzed as described in example A. Epididymal fat pads were removed and weighed. Clinical chemistry analysis was performed by LabCorp (San Diego, Calif.).
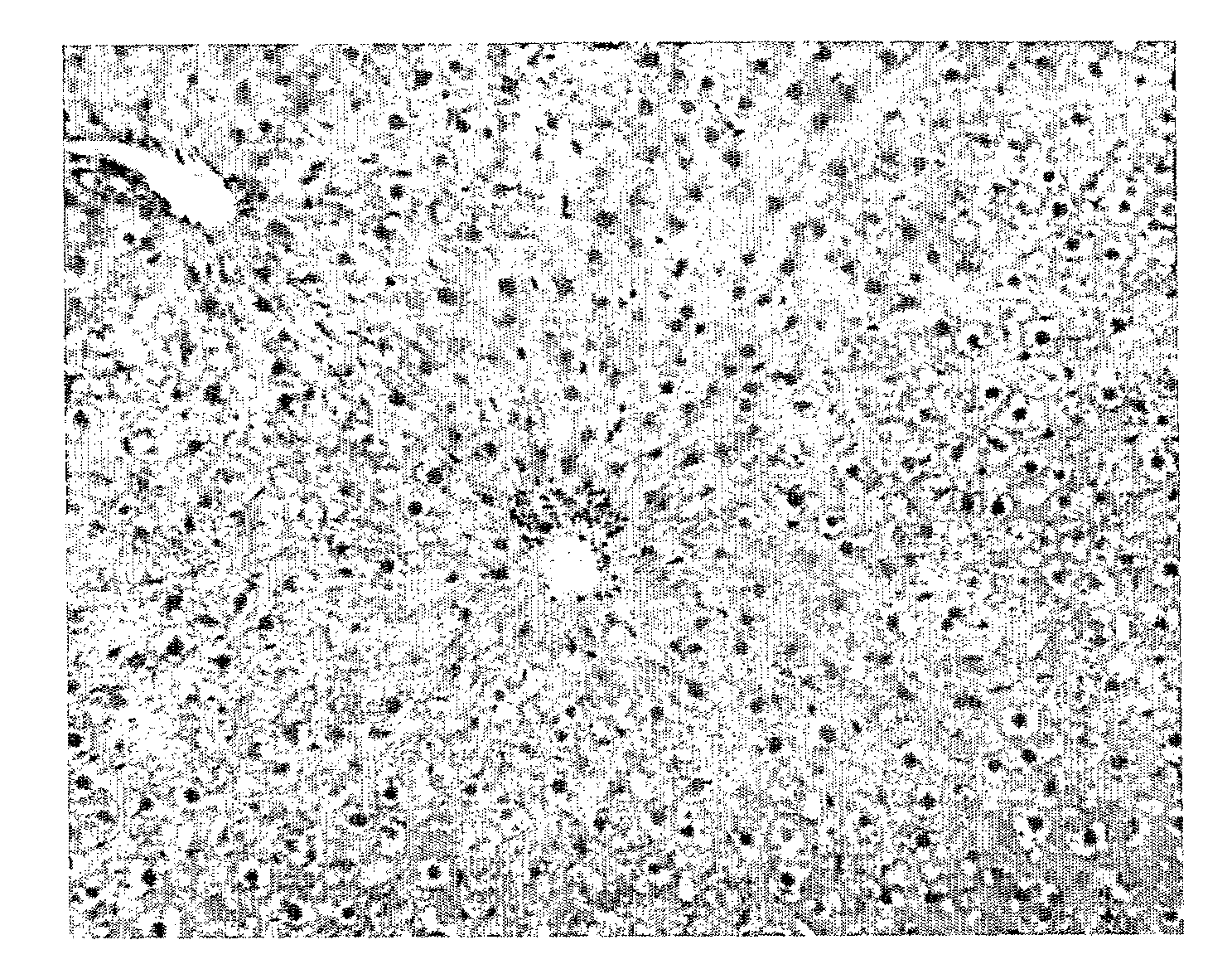
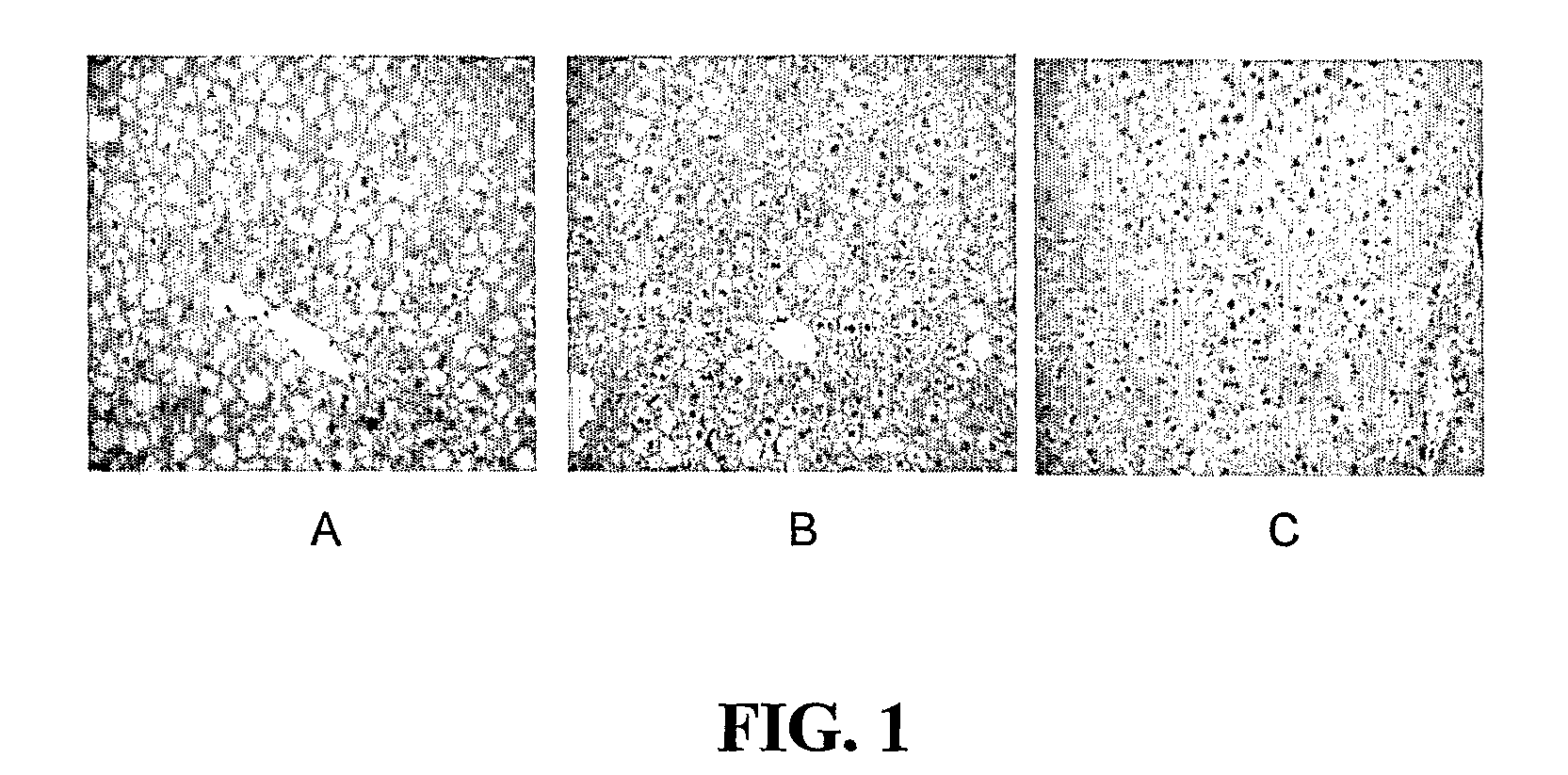
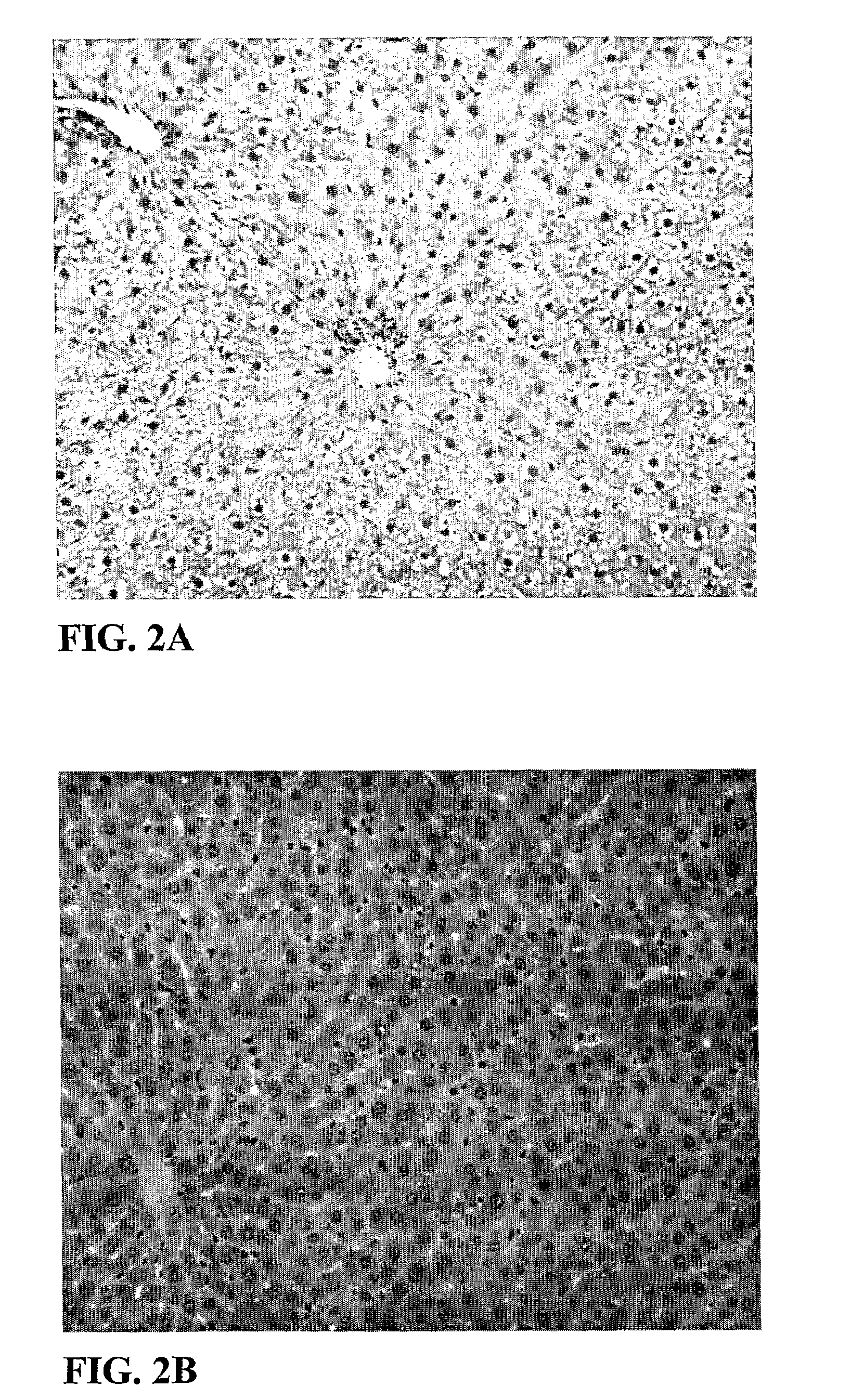
[0968]Results: T3 did not significantly decrease liver triglyceride content (FIG. 1). However, Compound cis-13-1 decreased hepatic triglyceride content at 10 and 30 mg / kg / d (FIG. 1). Compound cis-13-1 did not decrease epididymal fat pad (EFP) weight. T3 significantly decreased EFP weight, consistent with a well described effect of T3 on lipolysis. Treatment of o...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molar density | aaaaa | aaaaa |
| Molar density | aaaaa | aaaaa |
| Dimensionless property | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com