Patents
Literature
137 results about "Subcutaneous implant" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
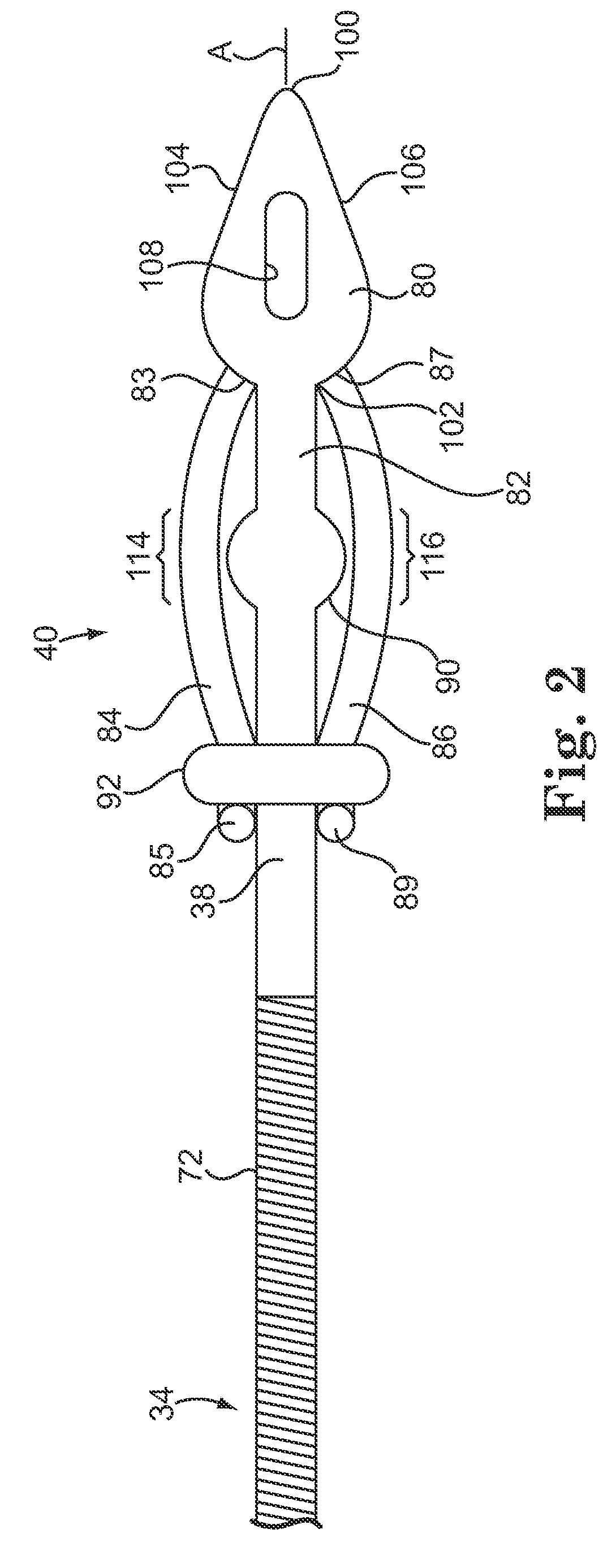
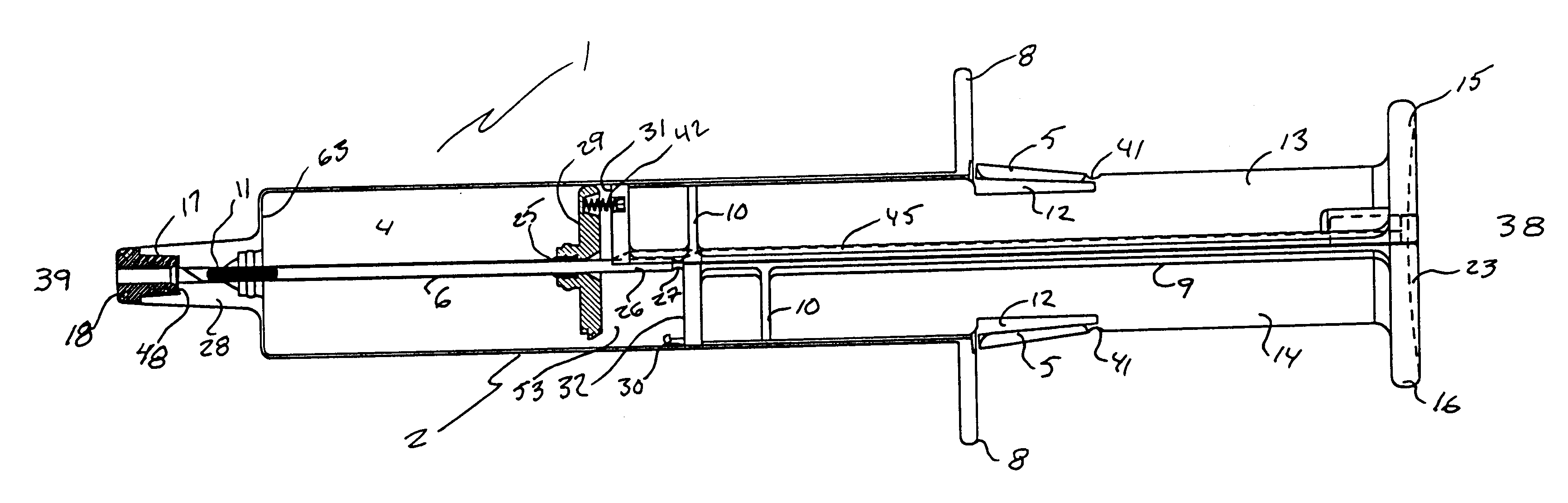
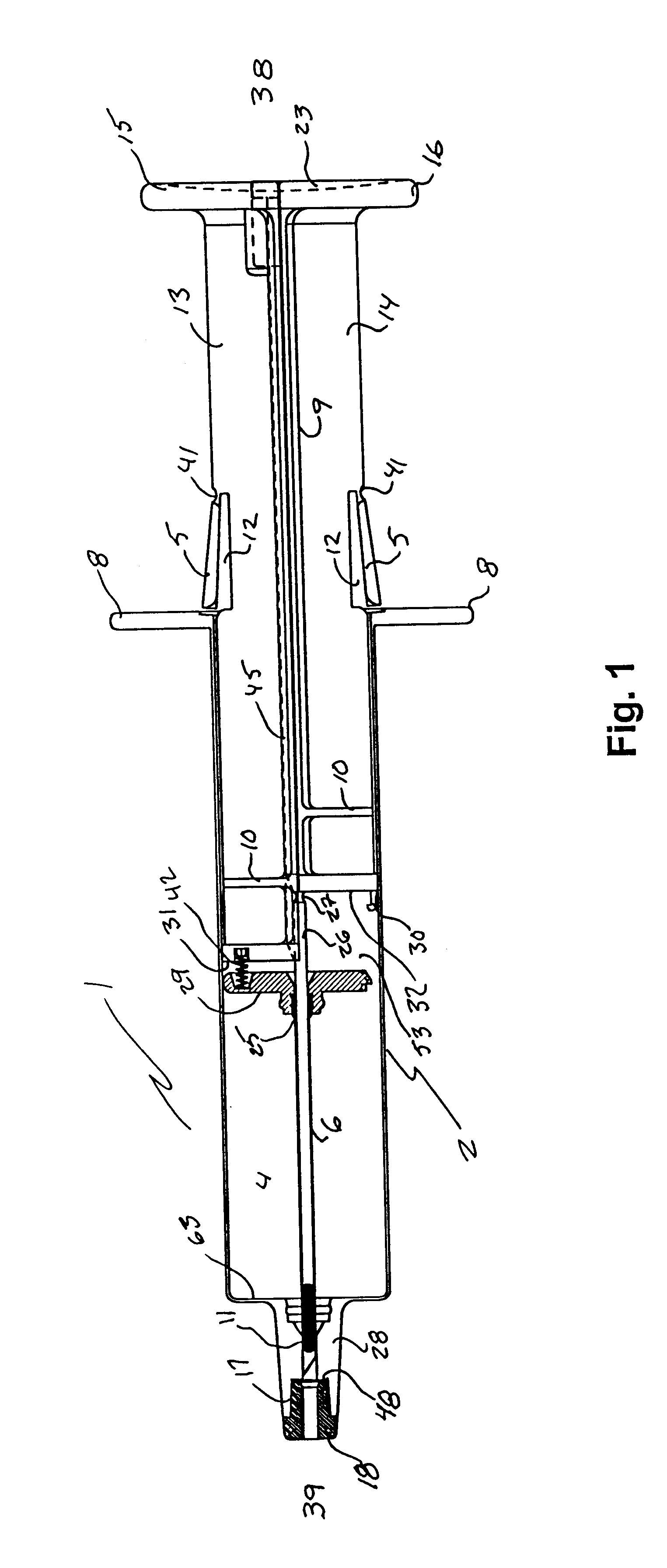
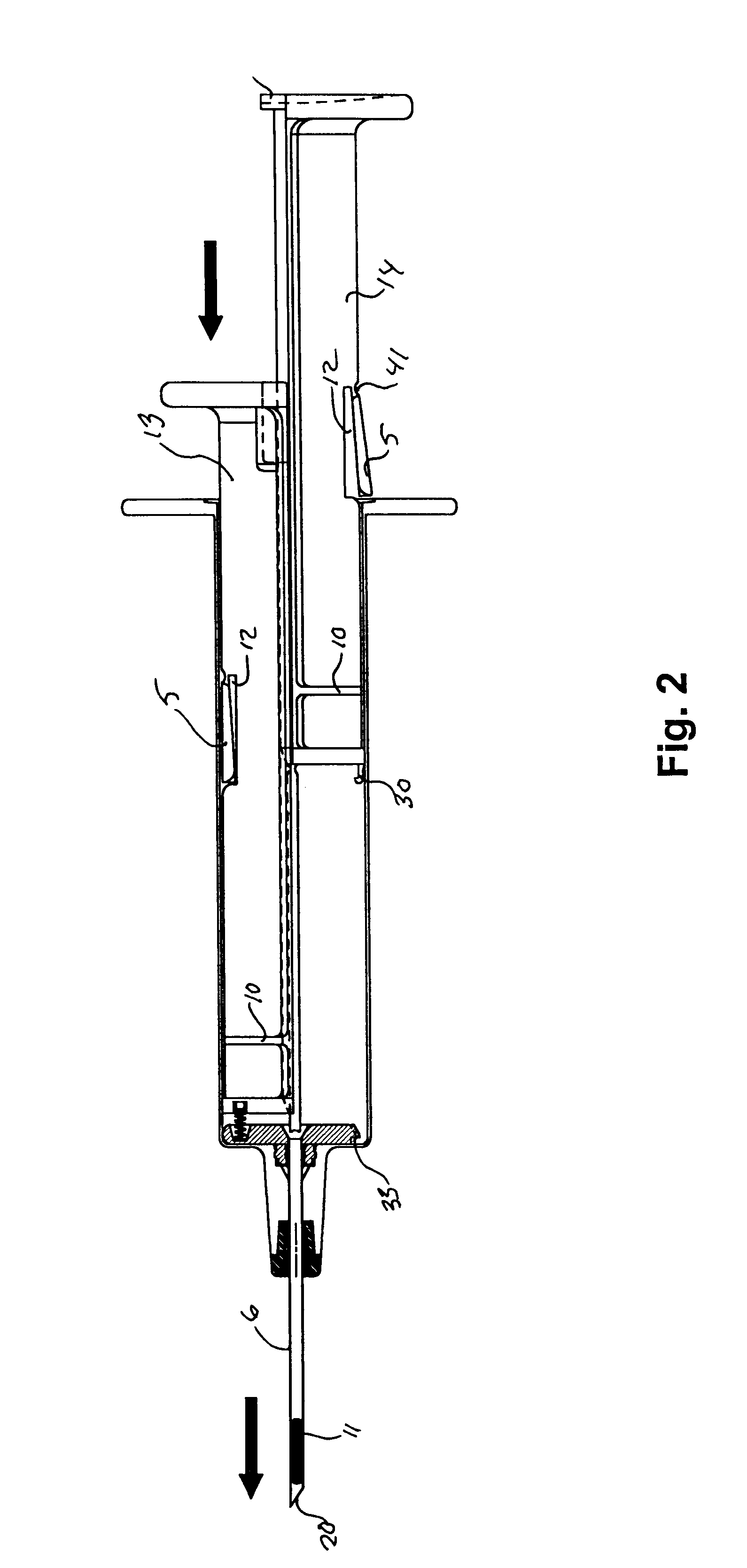
Hypodermic implant device
InactiveUS20030004457A1Avoid spreadingLess-costly disposalMedical devicesInfusion needlesSubcutaneous implantationImplanted device
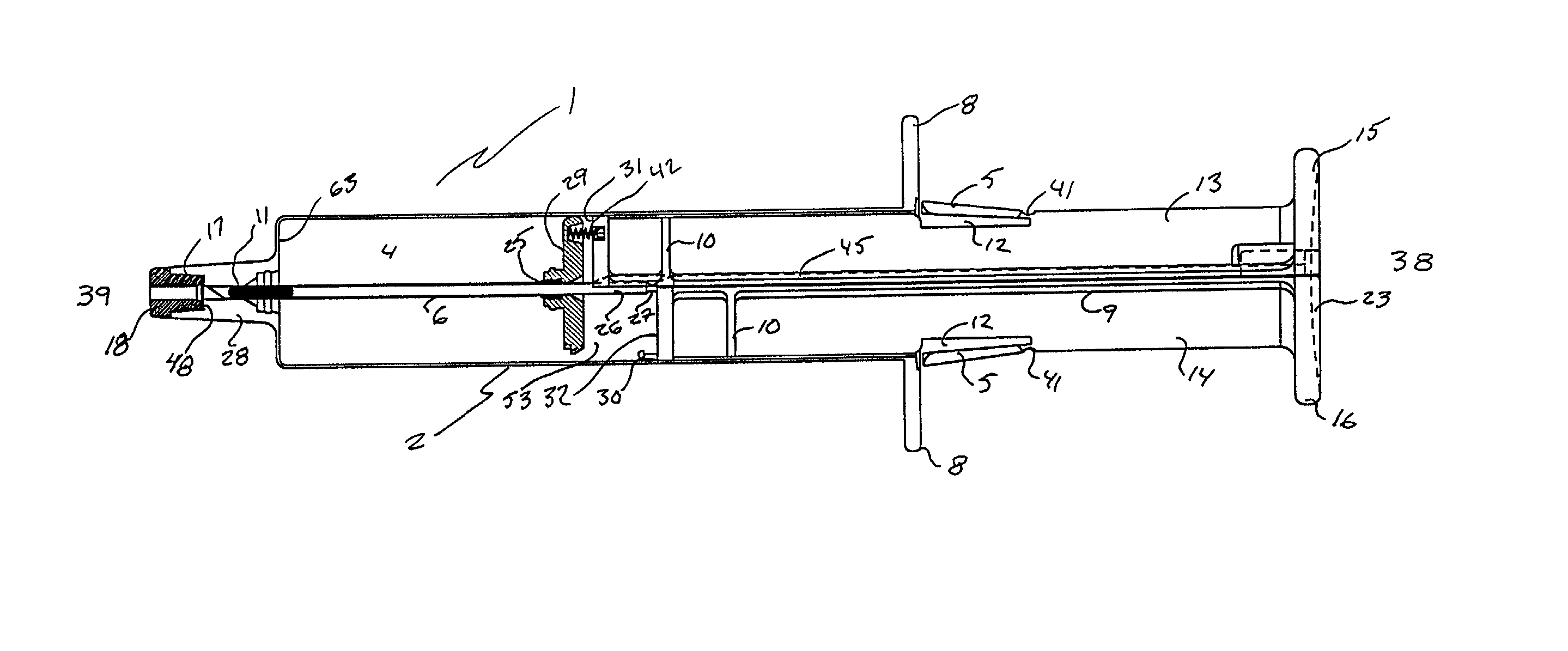
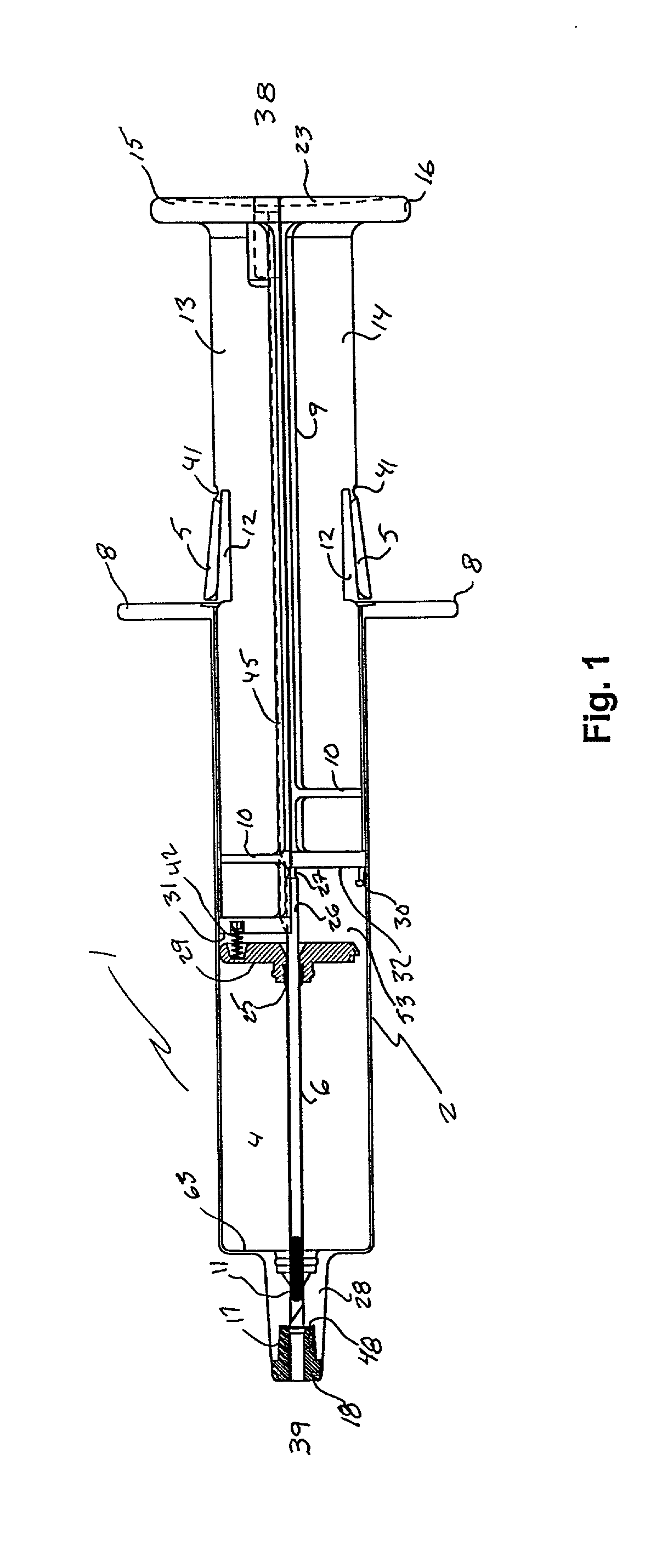
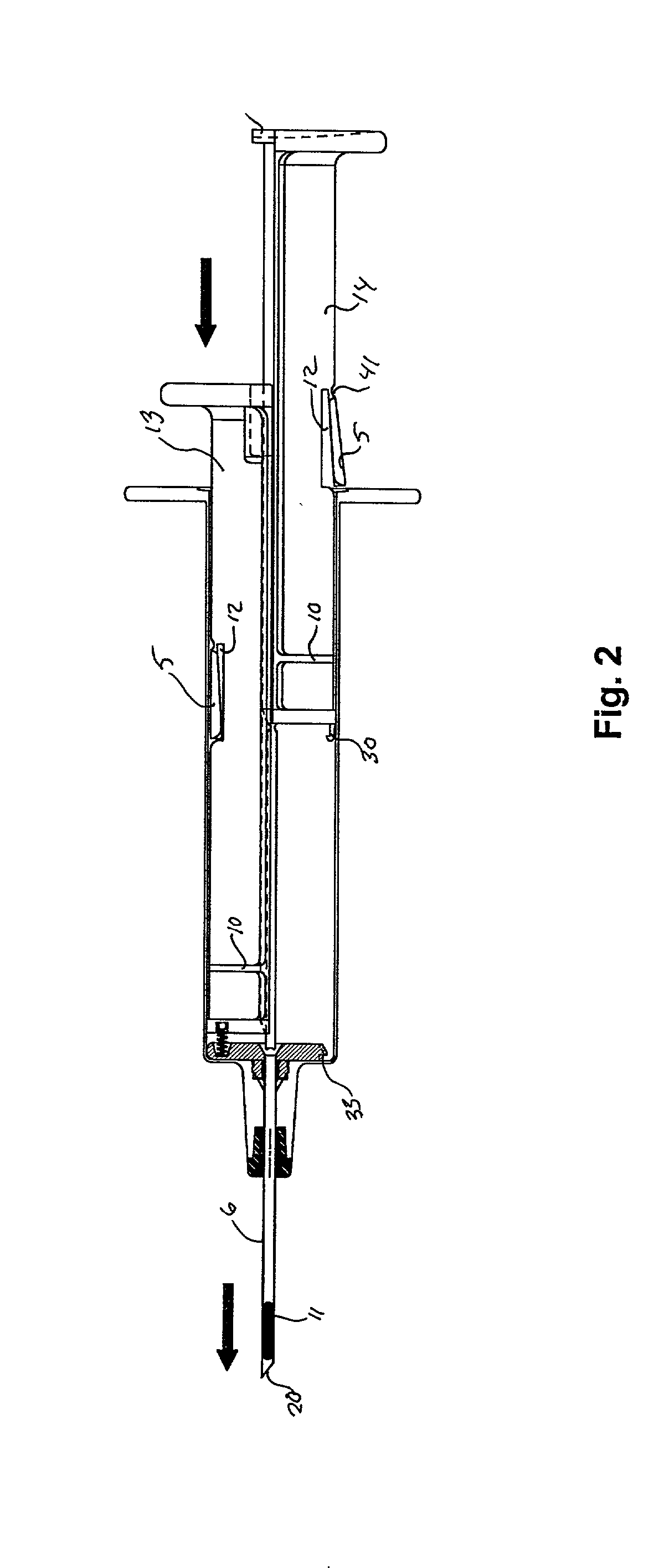
A hypodermic implant device, comprising a barrel; an upper piston segment engaged with an inner wall of the barrel; an axially aligned needle extendably distally out of the barrel for insertion into an object when the upper piston segment is moved distally within the body; a lower piston segment push rod in alignment with the needle, so that upon distal movement of the lower piston segment, the push rod moves distally through the needle expelling a releasable implant housed in the needle; and a means for retraction of the needle into a needle receptacle in the barrel.
Owner:GRAY PLANT MOOTY MOOTY & BENNETT PA
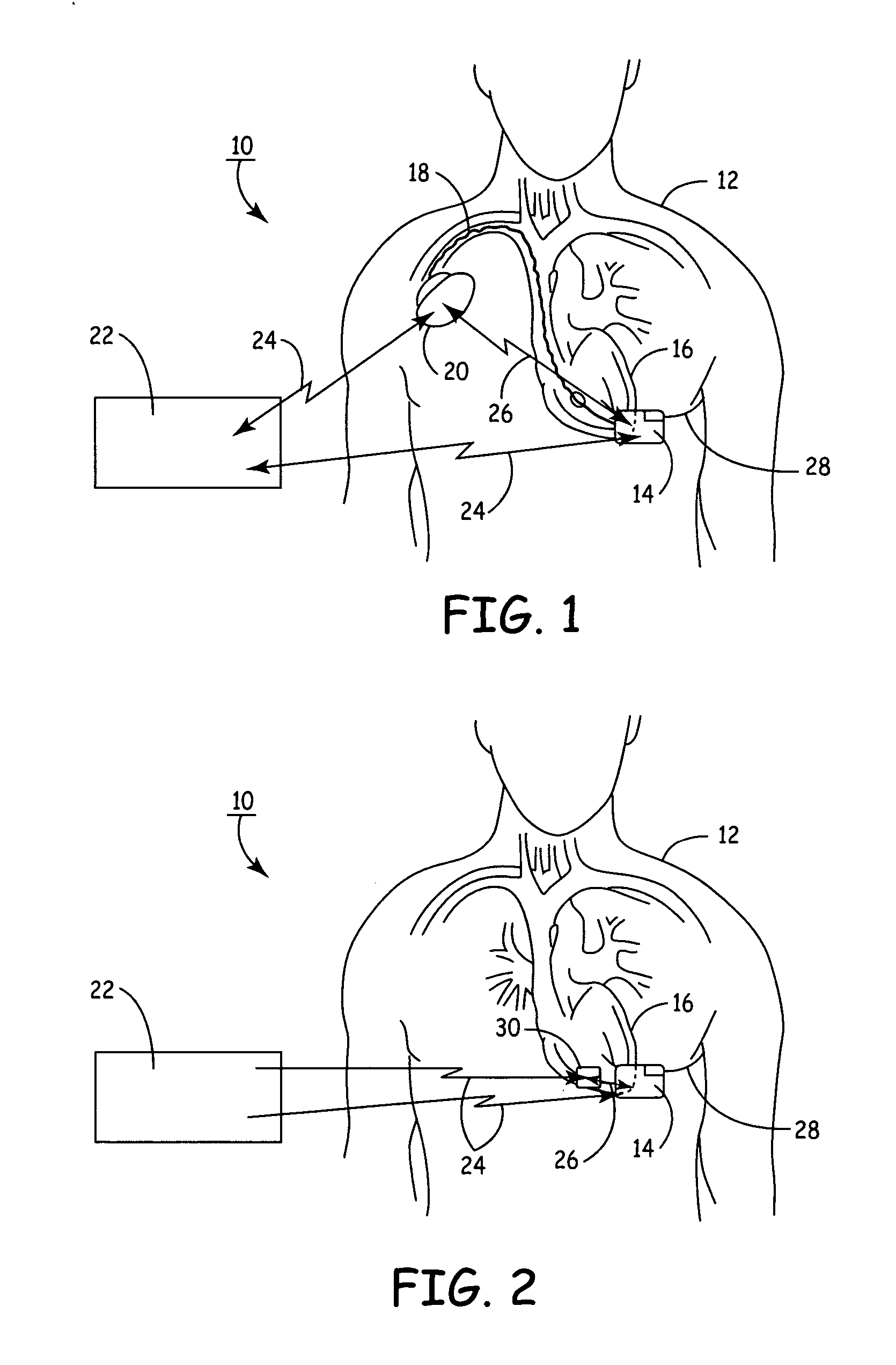
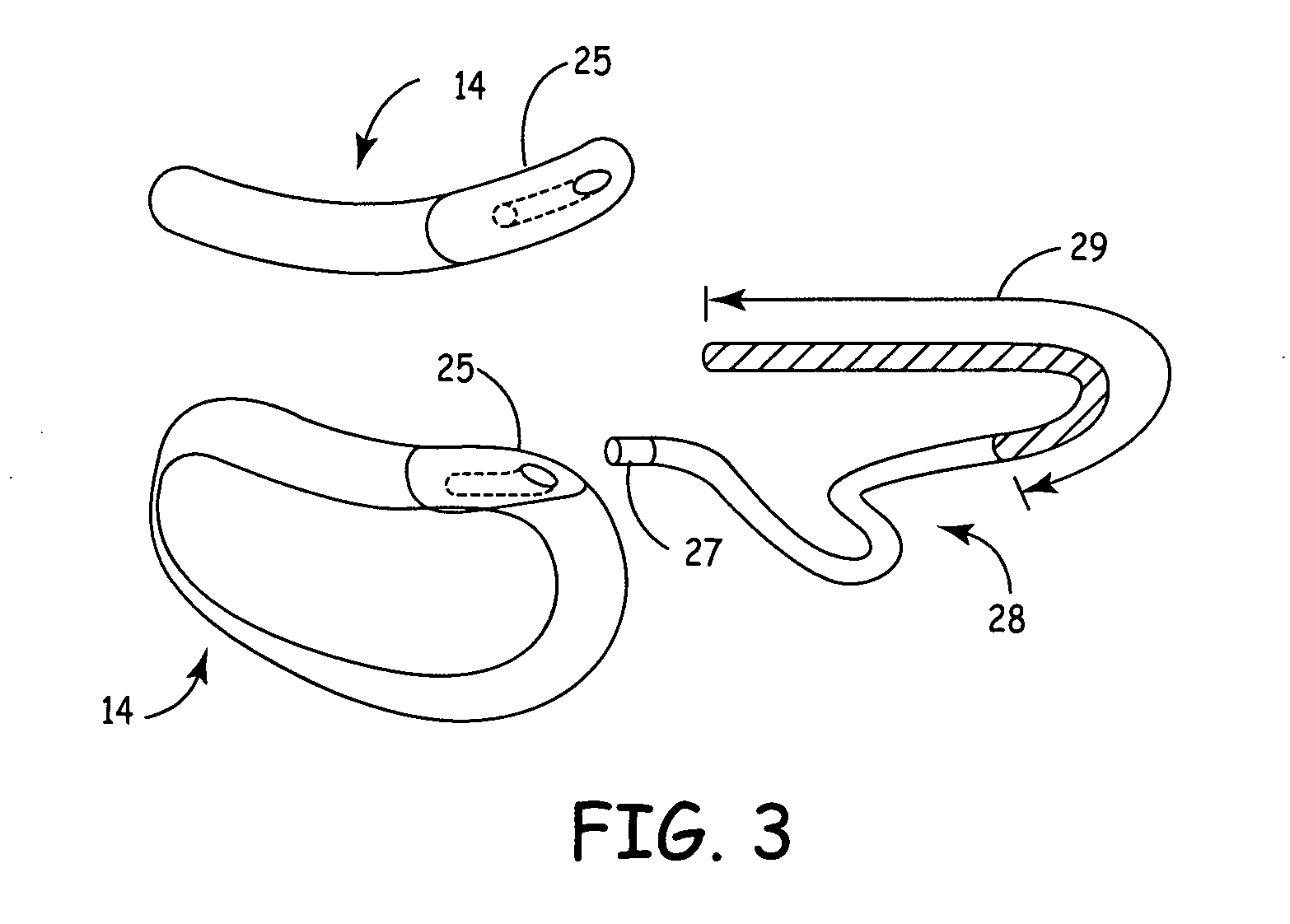
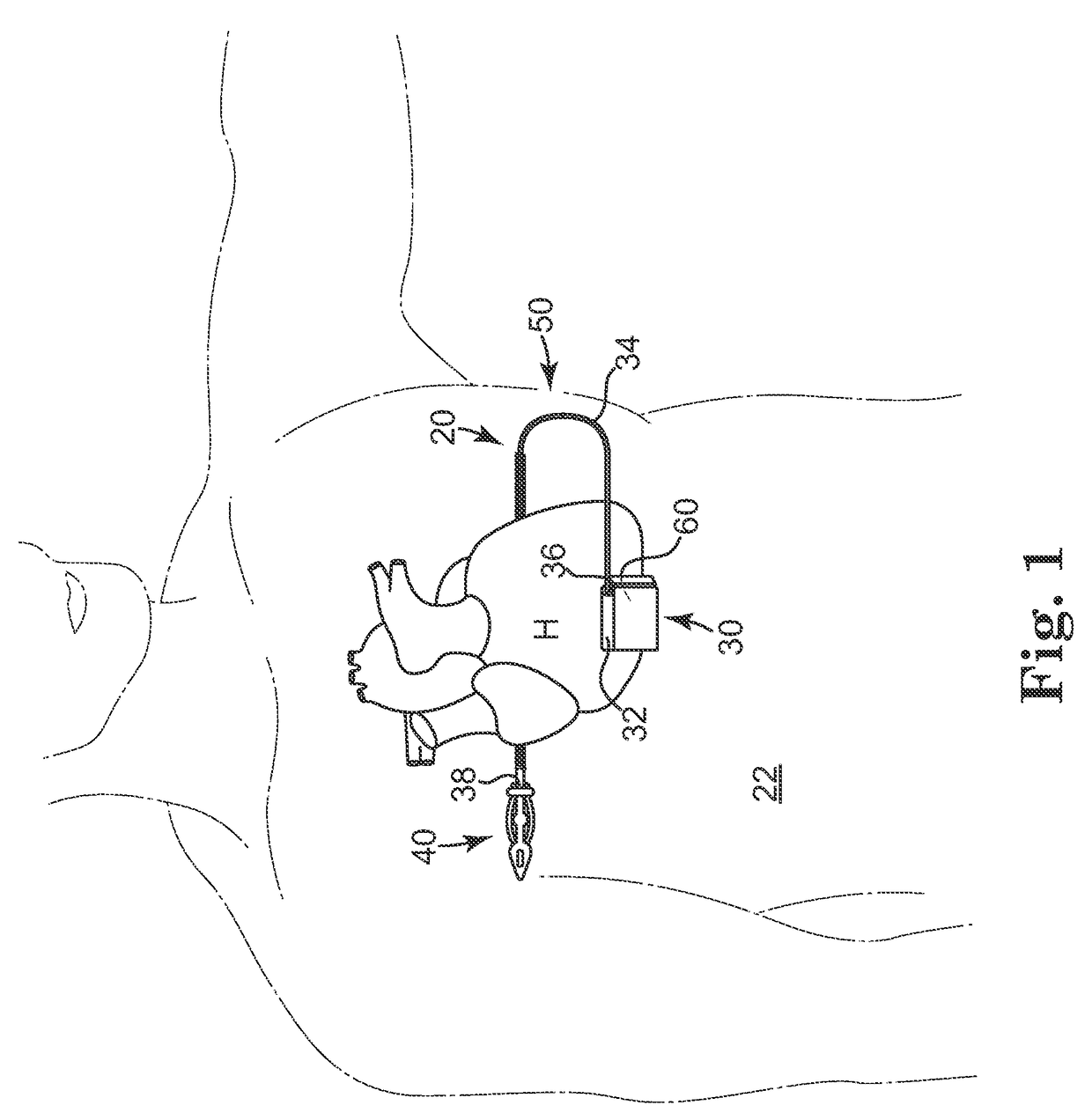
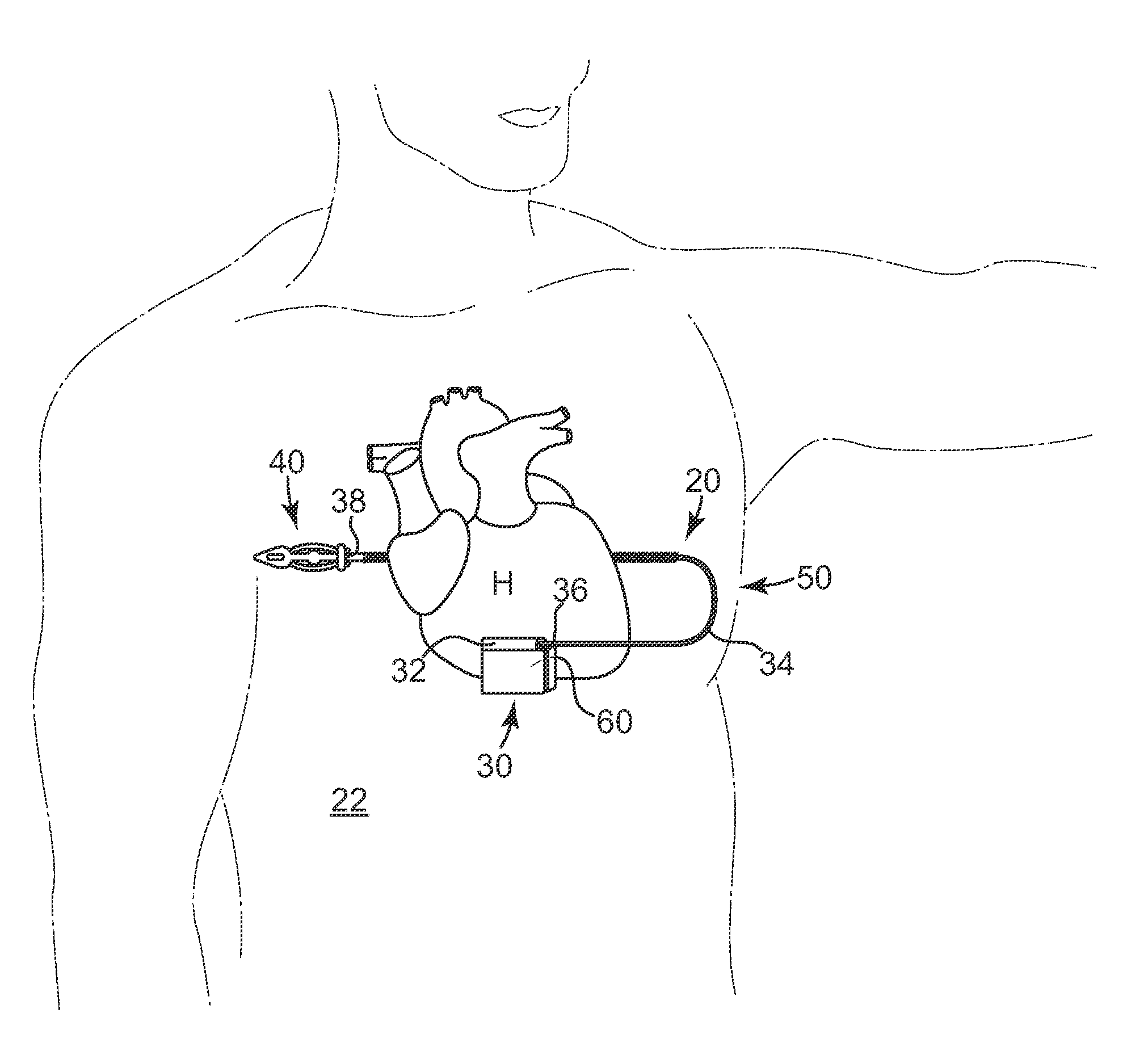
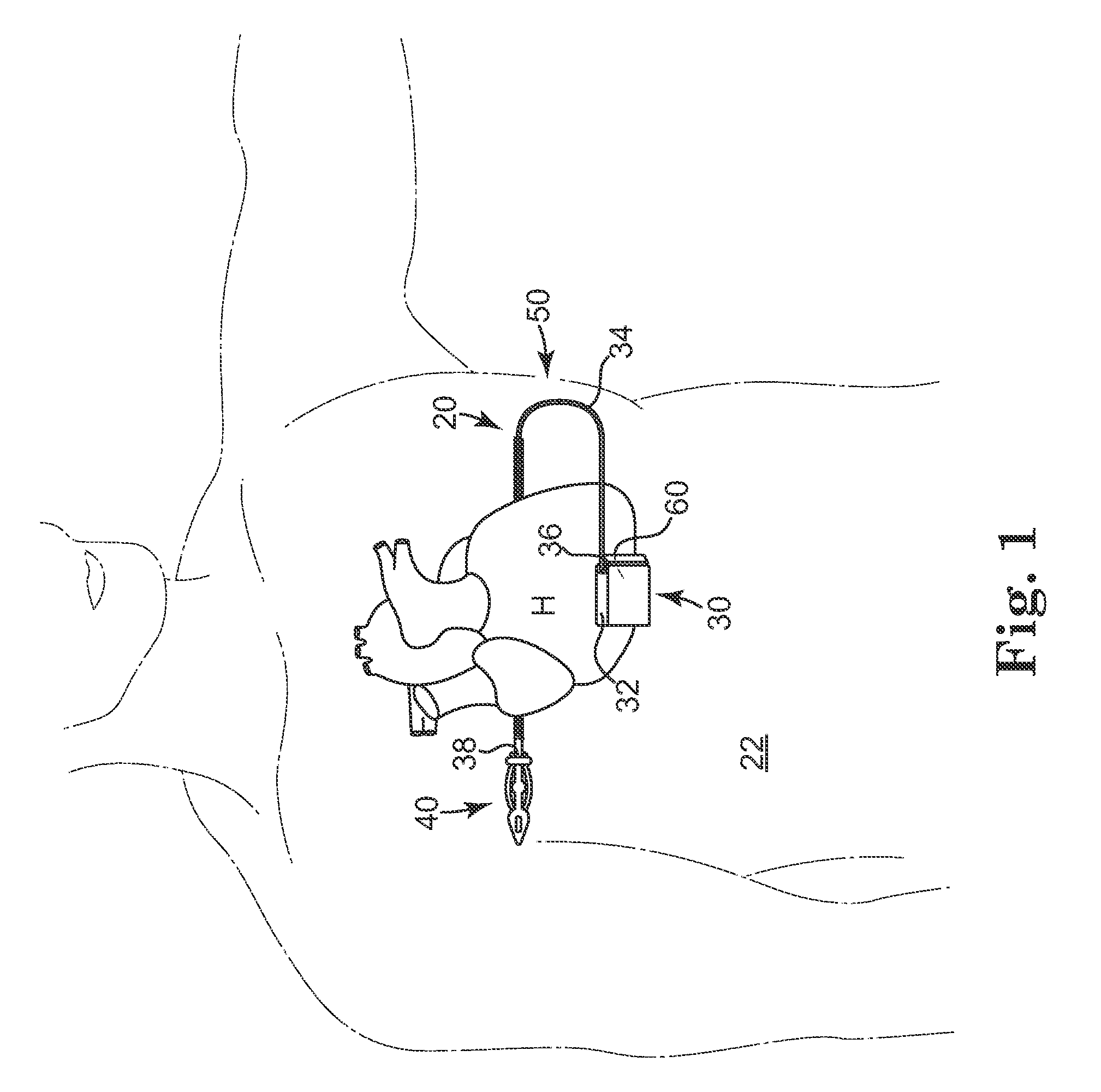
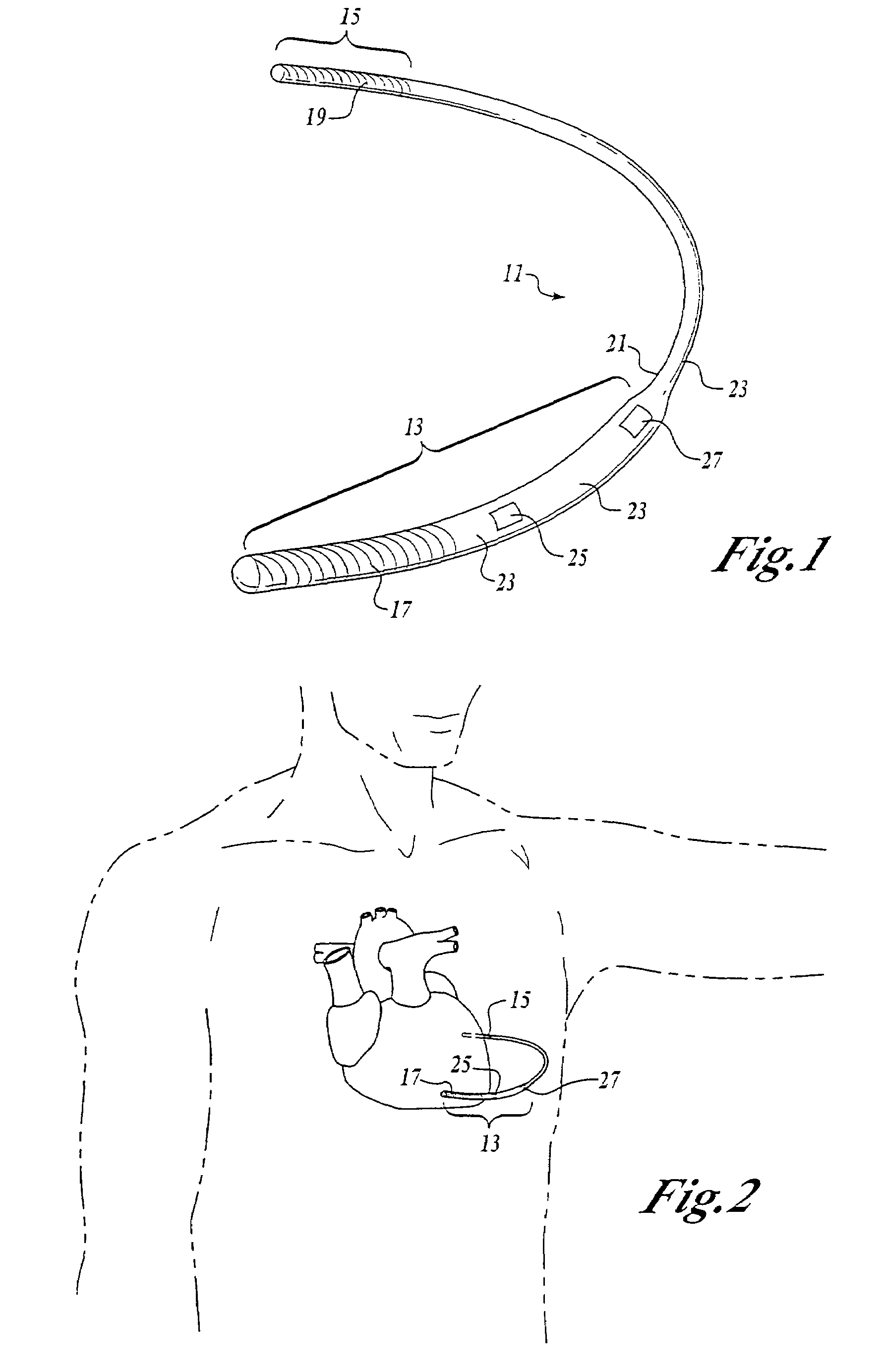
Remotely enabled pacemaker and implantable subcutaneous cardioverter/defibrillator system
ActiveUS20060241701A1Relieve painSafe and effective operationHeart defibrillatorsSubcutaneous implantationCardiac pacemaker electrode
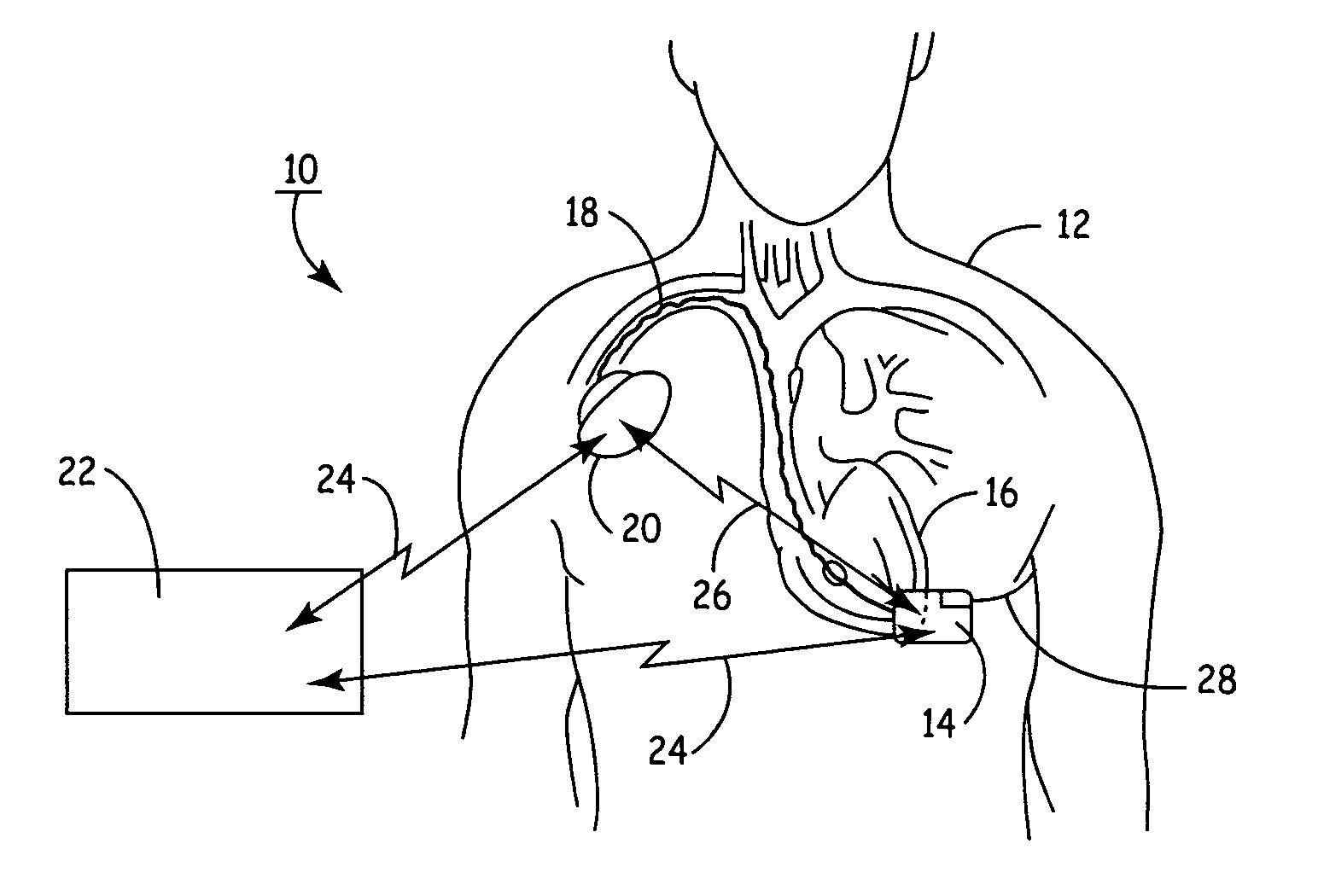
Subcutaneous Implantable cardioverter-defibrillators (SubQ ICDS) are disclosed that are entirely implantable subcutaneously with minimal surgical intrusion into the body of the patient and provide distributed cardioversion-defibrillation sense and stimulation electrodes for delivery of cardioversion-defibrillation shock and pacing therapies across the heart when necessary. The SubQ ICD is implemented with other implantable and external medical devices and communicates to provide drugs and therapy in a coordinated and synergistic manner.
Owner:MEDTRONIC INC
Implantable medical device
InactiveUS20070078391A1Accurate accessEase of operationMedical devicesIntravenous devicesEngineeringFluid injection
A medical device suitable for subcutaneous implantation, generally including a housing having a reservoir, a septum positioned within and supported by the housing, at least one light emitting element placed in position defining relation to the septum, and a pressure actuated, light activating circuitry associated with the at least one light emitting element. The light element(s) may be positioned, for instance, in at least partially surrounding relation around the septum, embedded within or below a translucent housing that supports the septum, positioned within the reservoir and adapted to emit its light through a translucent septum, or positioned on the exterior of the supporting housing. The medical device can be adapted to receive high pressure fluid injections and if so adapted, will include a light emitting element that will provide a visual indication this capacity.
Owner:ANGIODYNAMICS INC
Subcutaneously implantable power supply
InactiveUS20030004546A1Reduction of repeat traumaSave thousandHeart defibrillatorsPhotovoltaicsSubcutaneous implantMechanical engineering
Owner:CASEY DON E
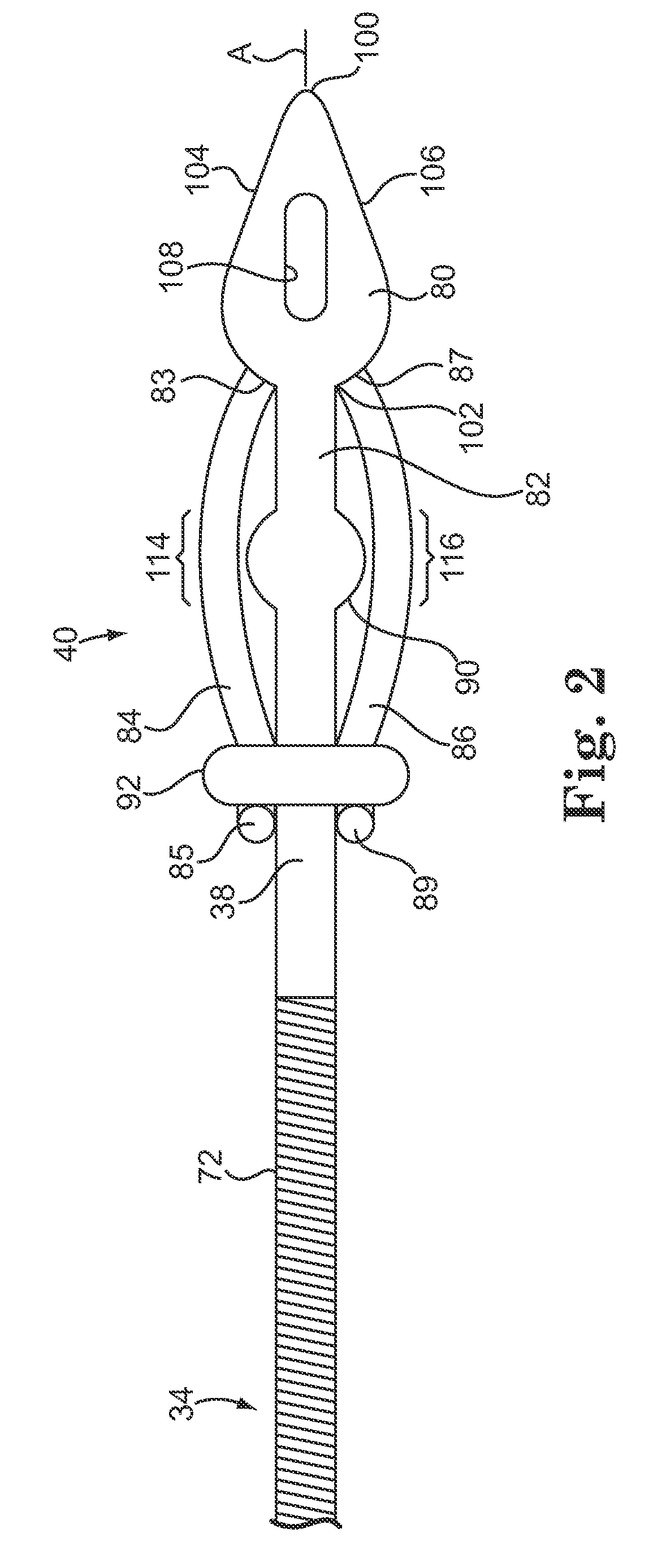
Subcutaneous electrode with improved contact shape for transthorasic conduction
One embodiment of the present invention provides a lead electrode assembly for use with an implantable cardioverter-defibrillator subcutaneously implanted outside the ribcage between the third and twelfth ribs comprising the electrode.
Owner:CAMERON HEALTH
Implantable pulse generator for providing functional and/or therapeutic stimulation of muscles and/or nerves and/or central nervous system tissue
ActiveUS20050278000A1Efficient chargingImprove the quality of lifeElectrotherapyElectromyographyMicrocontrollerPrimary cell
Improved assemblies, systems, and methods provide an implantable pulse generator for prosthetic or therapeutic stimulation of muscles, nerves, or central nervous system tissue, or any combination. The implantable pulse generator is sized and configured to be implanted subcutaneously in a tissue region. The implantable pulse generator includes an electrically conductive laser welded titanium case. Control circuitry is located within the case, and includes a primary cell or rechargeable power source, a receive coil for receiving an RF magnetic field to recharge the rechargeable power source, and a microcontroller for control of the implantable pulse generator. Improved assemblies, systems, and methods also provide a stimulation system for prosthetic or therapeutic stimulation of muscles, nerves, or central nervous system tissue, or any combination. The stimulation system provides at least one electrically conductive surface, a lead connected to the electrically conductive surface, and an implantable pulse generator electrically connected to the lead.
Owner:MEDTRONIC URINARY SOLUTIONS
Subcutaneous electrode for transthoracic conduction with low-profile installation appendage and method of doing same
One embodiment of the present invention provides a lead electrode assembly for subcutaneous implantation including an electrode; a riser coupled to the electrode; and a head coupled to the riser.
Owner:CAMERON HEALTH
Thermally reversible implant and filler
The invention relates to the use of a thermal reversible gel, such as a copolymer composition, as a biological filler or implant. The gel has a semi-solid form at body temperature, but upon cooling to a temperature below a threshold level, the gel is liquefied and can be re-shaped, re-sized, manipulated or removed from the body. The gel may be used as a subcutaneous implant, a biological filler, joint or tissue spacer, for wrinkle filling or other cosmetic implants, as a soft-tissue replacement for reconstructive surgery, or as a barrier within the lumen of a biological structure, such as a blood vessel. The implant may be used to provide reversible birth control by providing, for example, a reversible barrier to the cervix or a reversible blockage of the lumen of the vas deferens.
Owner:CHENG YU LING +3
Tissue implantable sensor with hermetically sealed housing
ActiveUS20130197332A1Suitable shapeReduce sizeLine/current collector detailsEndoradiosondesConcentrations glucoseDetector array
A tissue-implantable sensor for measurement of solutes in fluids and gases, such as oxygen and glucose, is provided. The sensor includes: i) a detector array including at least one detector; ii) a telemetry transmission portal; iii) an electrical power source; and iv) circuitry electrically connected to the detector array including signal processing means for determining an analyte level, such as glucose level, in a body fluid contacting the detectors. The sensor components are disposed in a hermetically sealed housing having a size and shape suitable for comfortable, safe, and unobtrusive subcutaneous implantation allowing for in vivo detection and long term monitoring of tissue glucose concentrations by wireless telemetry.
Owner:GLYSENS
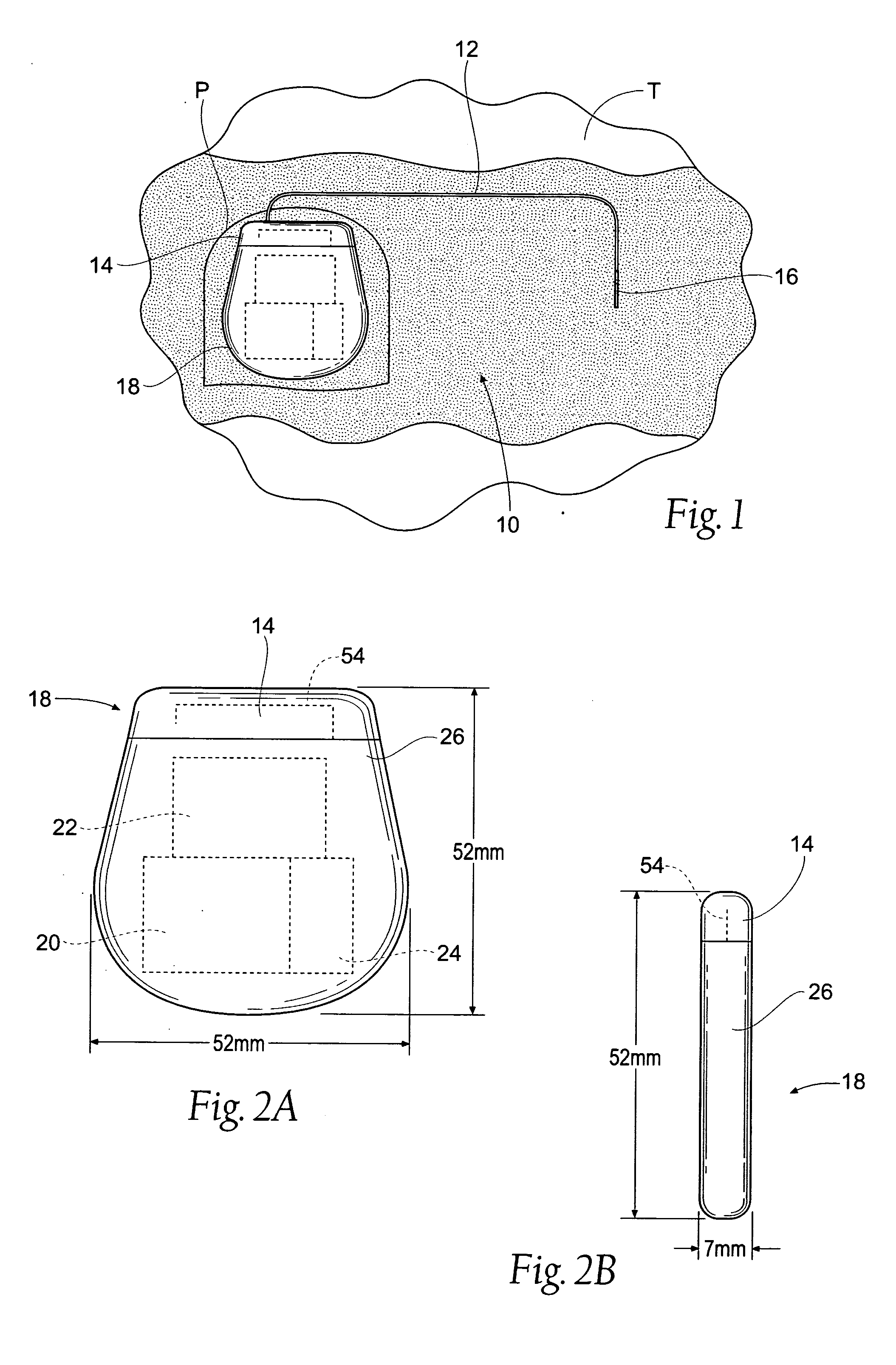
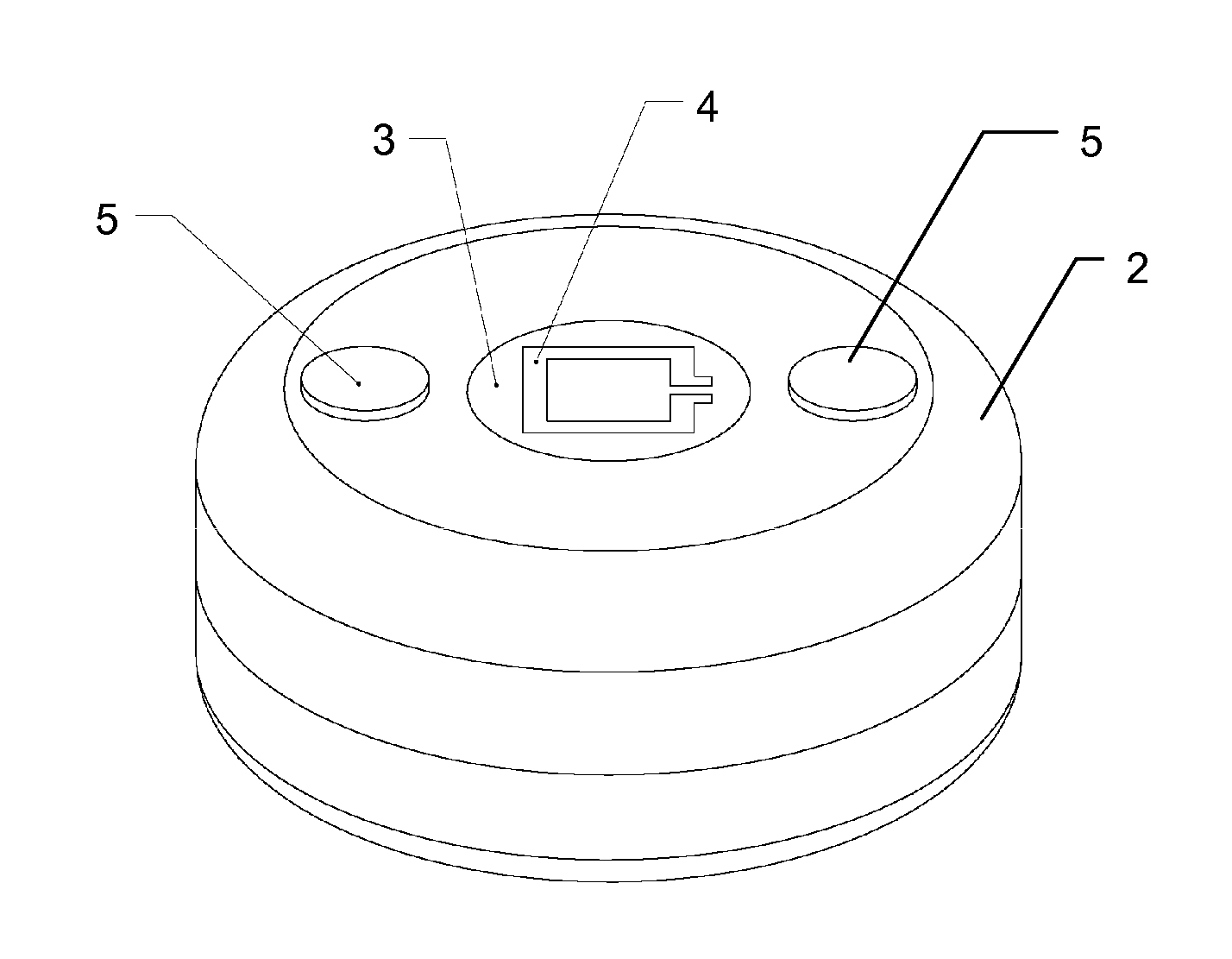
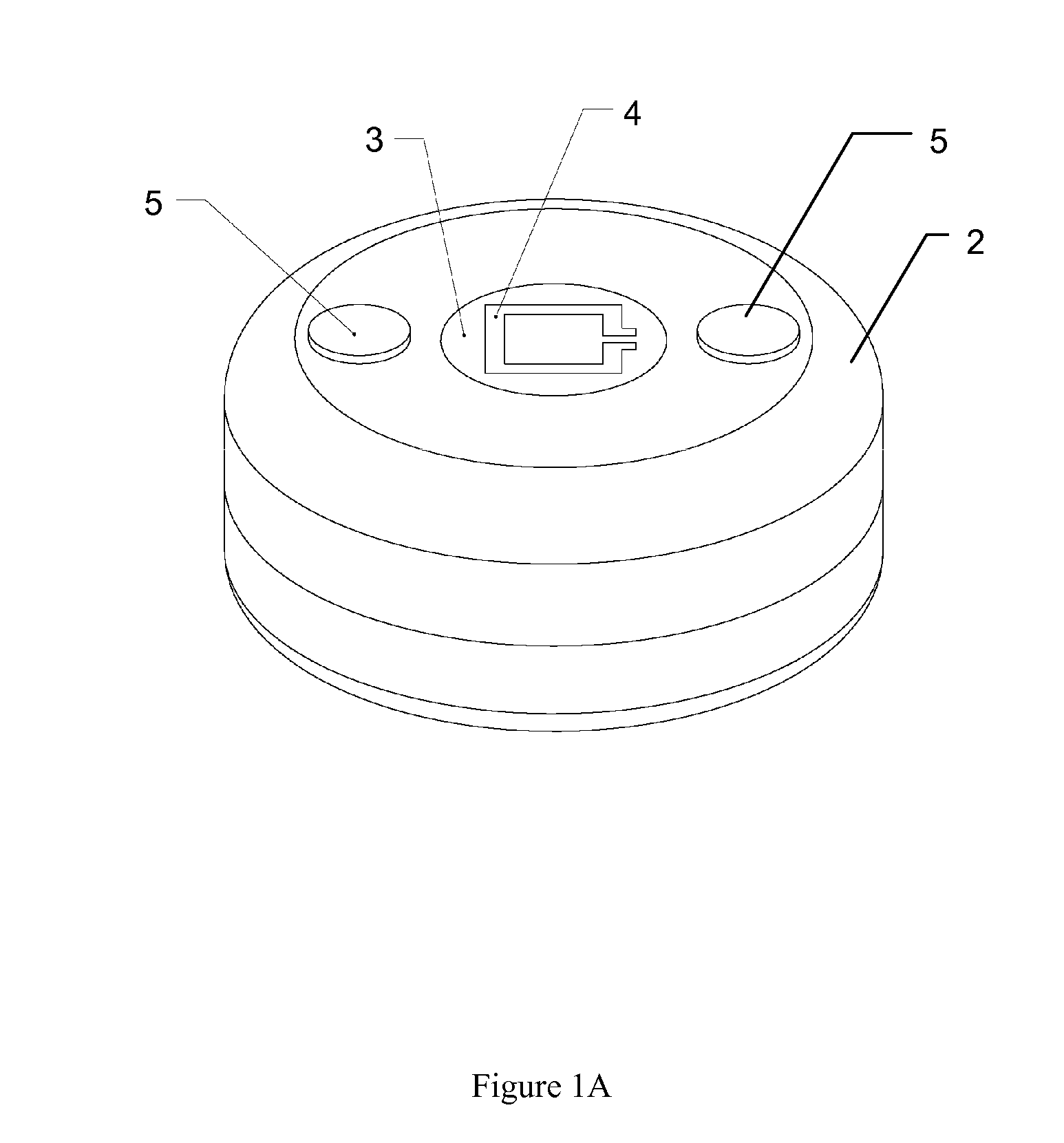
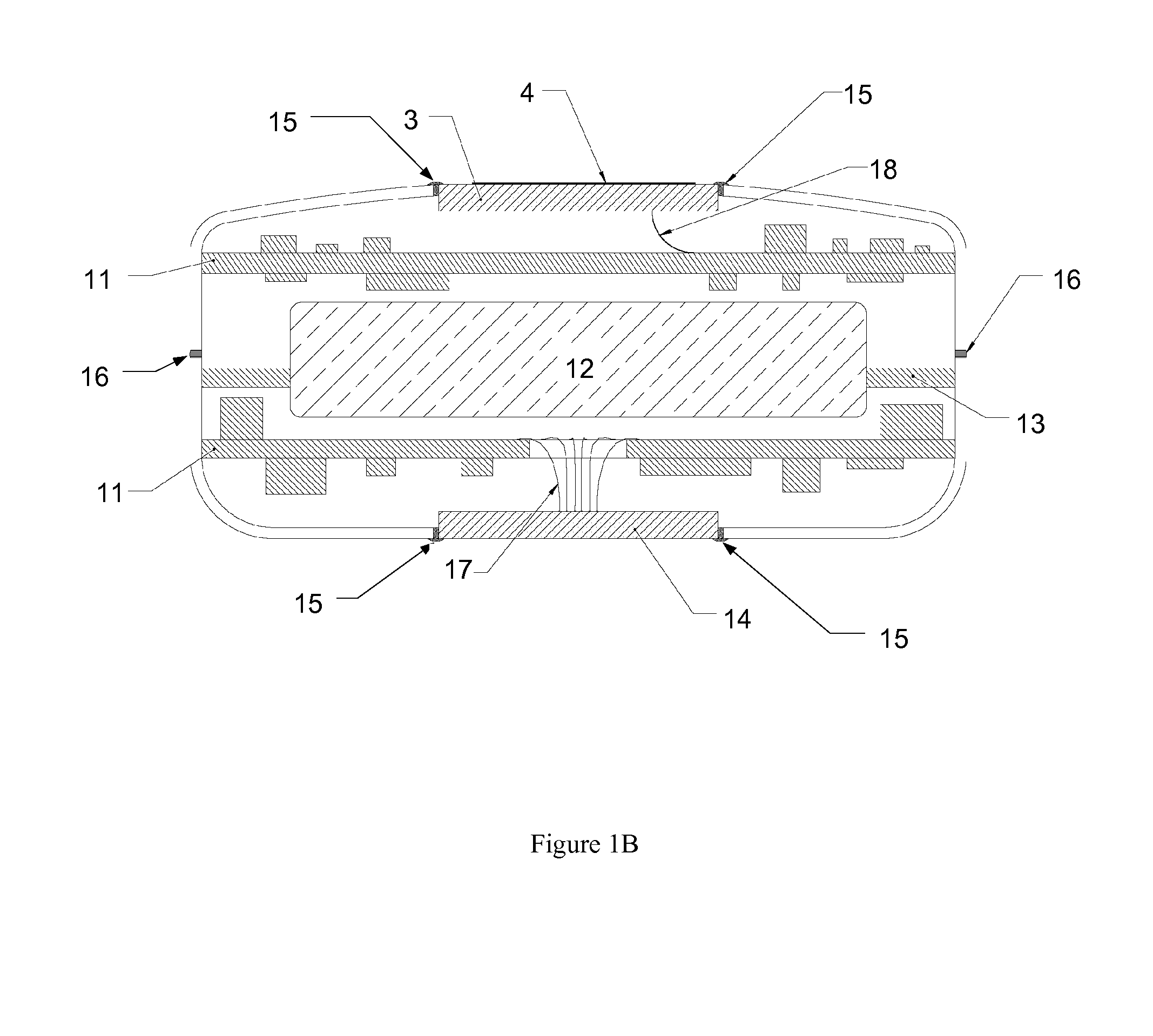
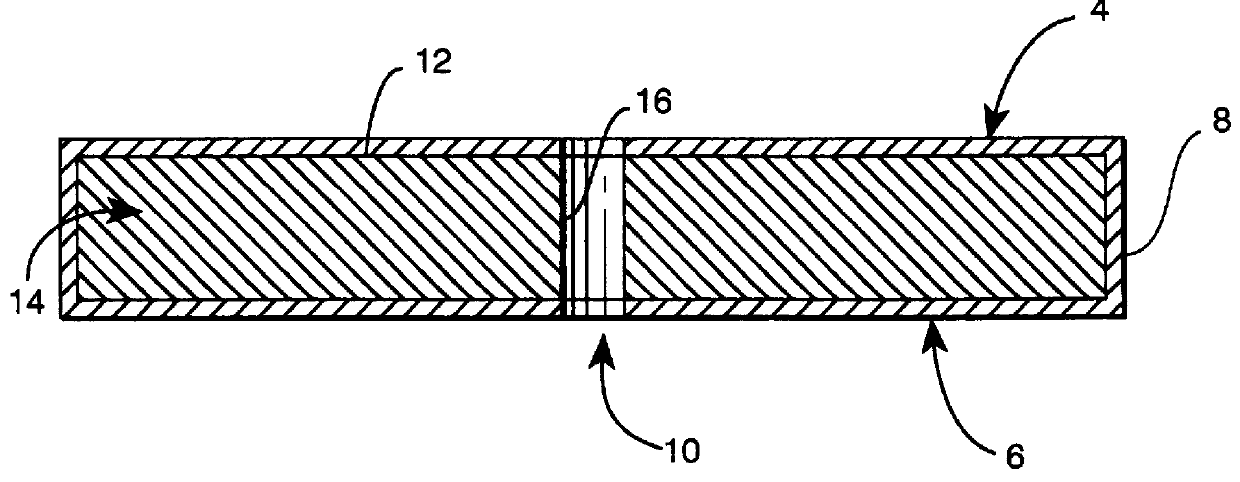
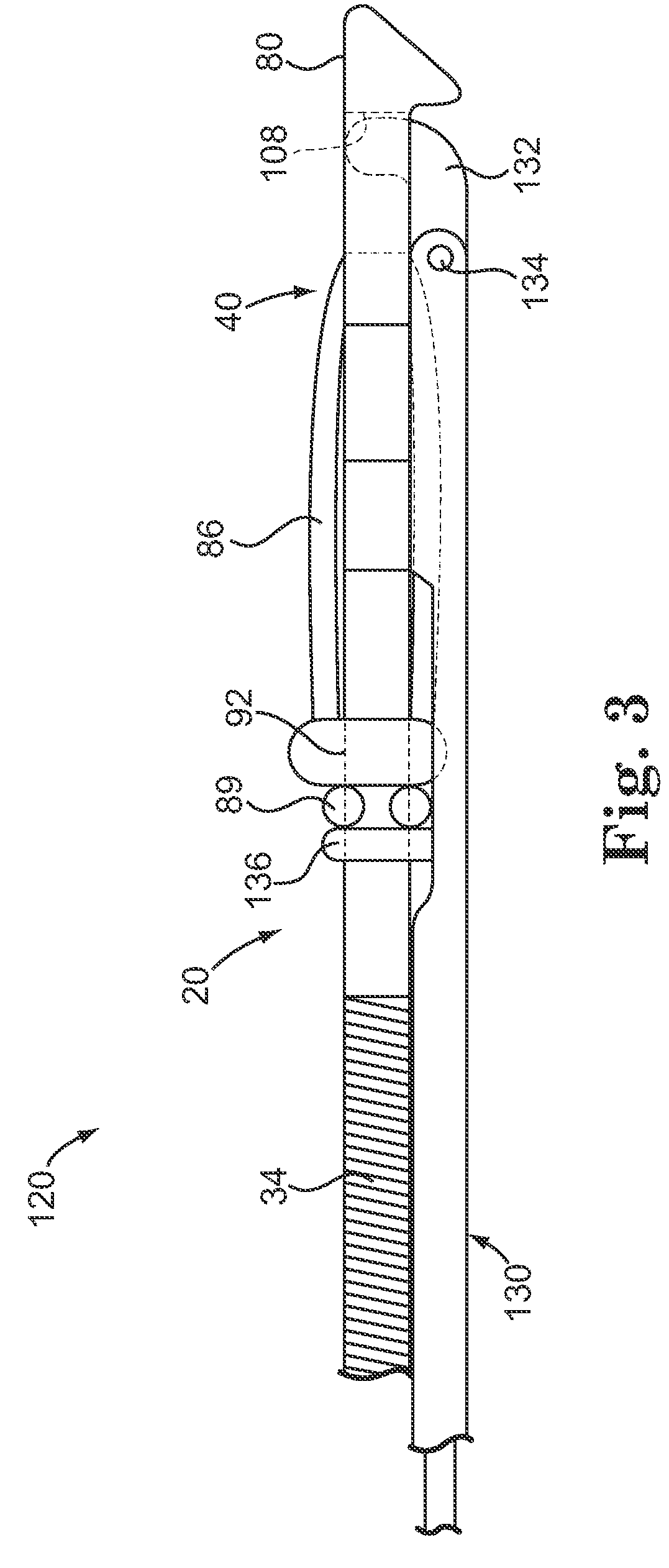
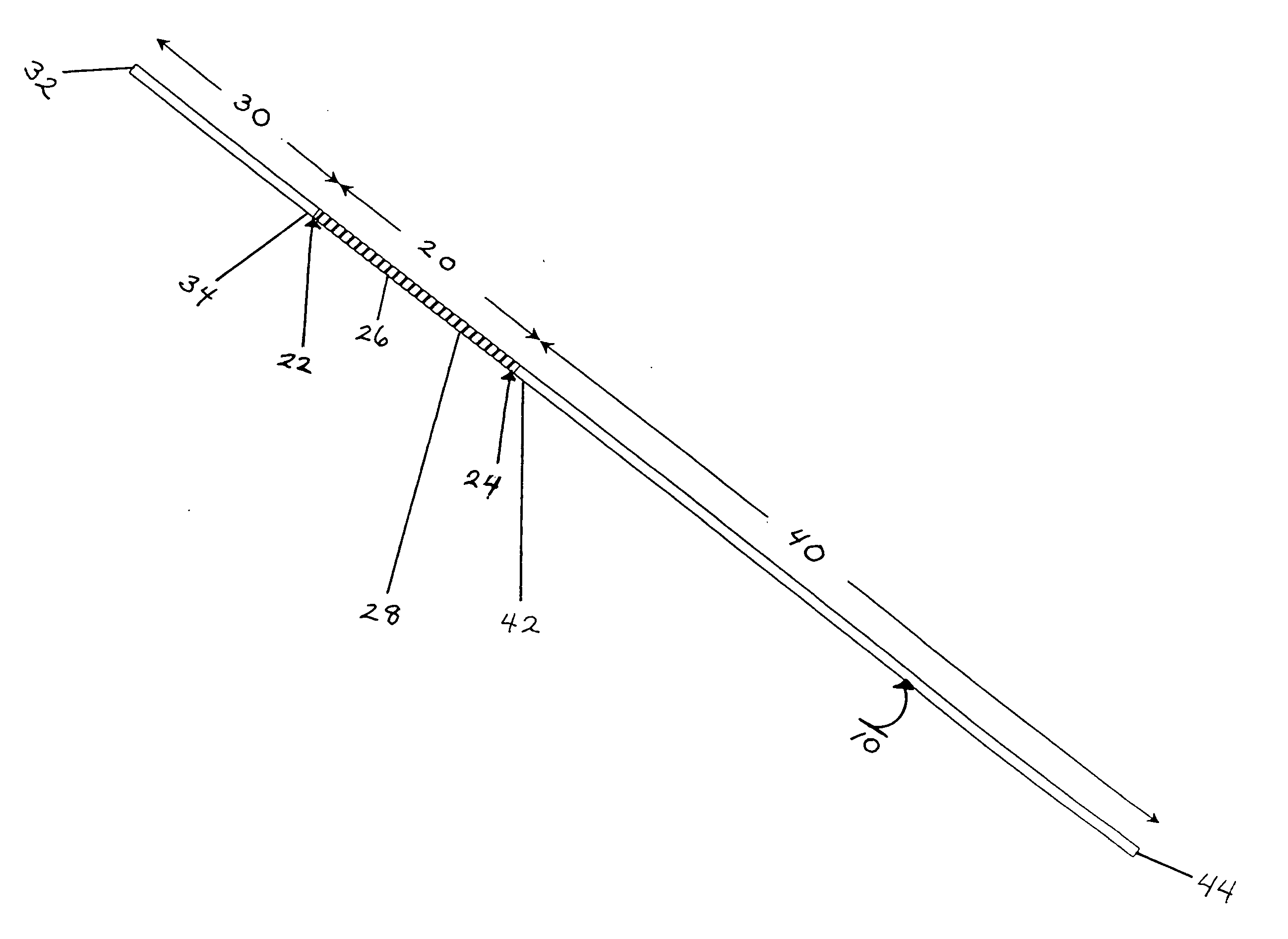
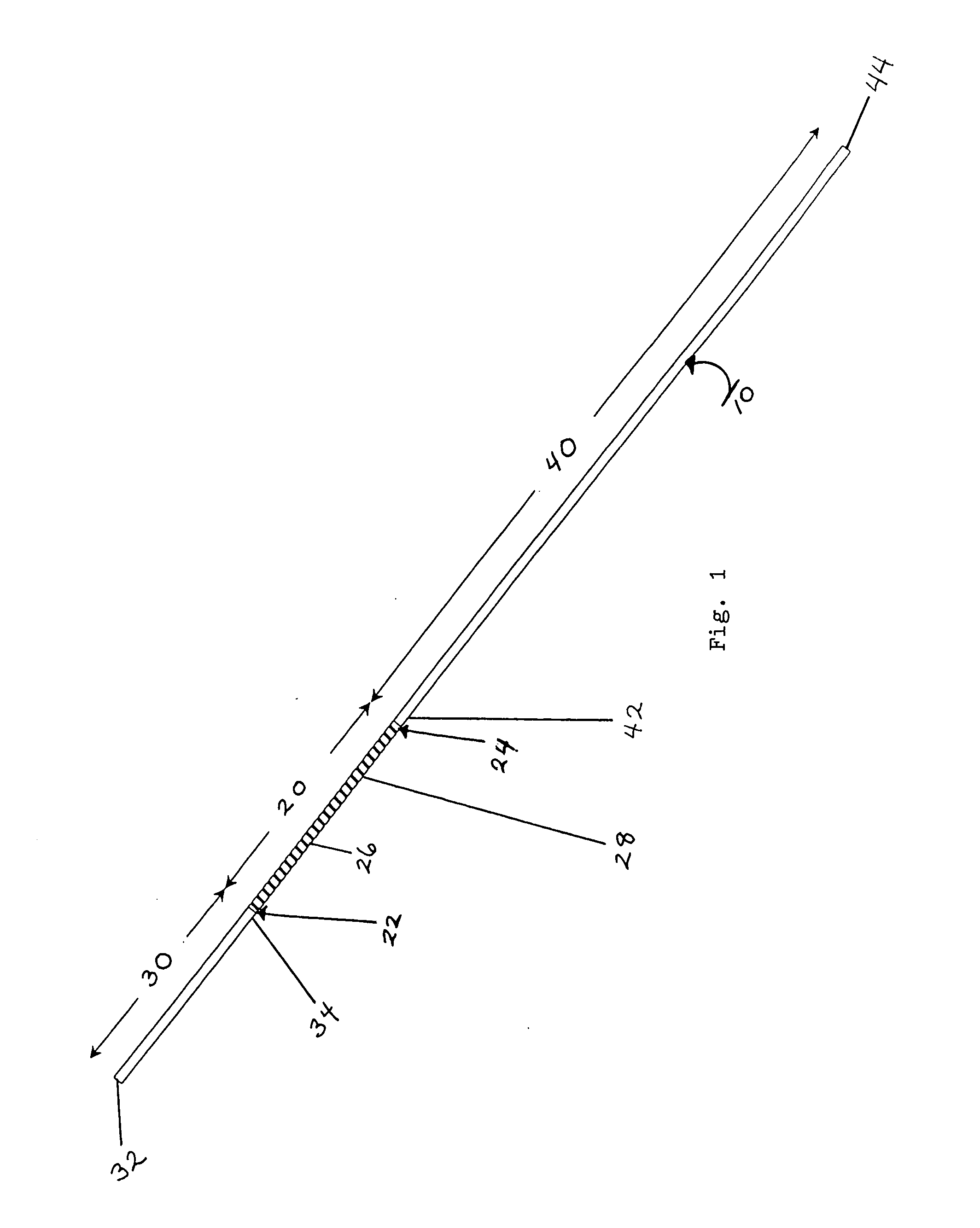
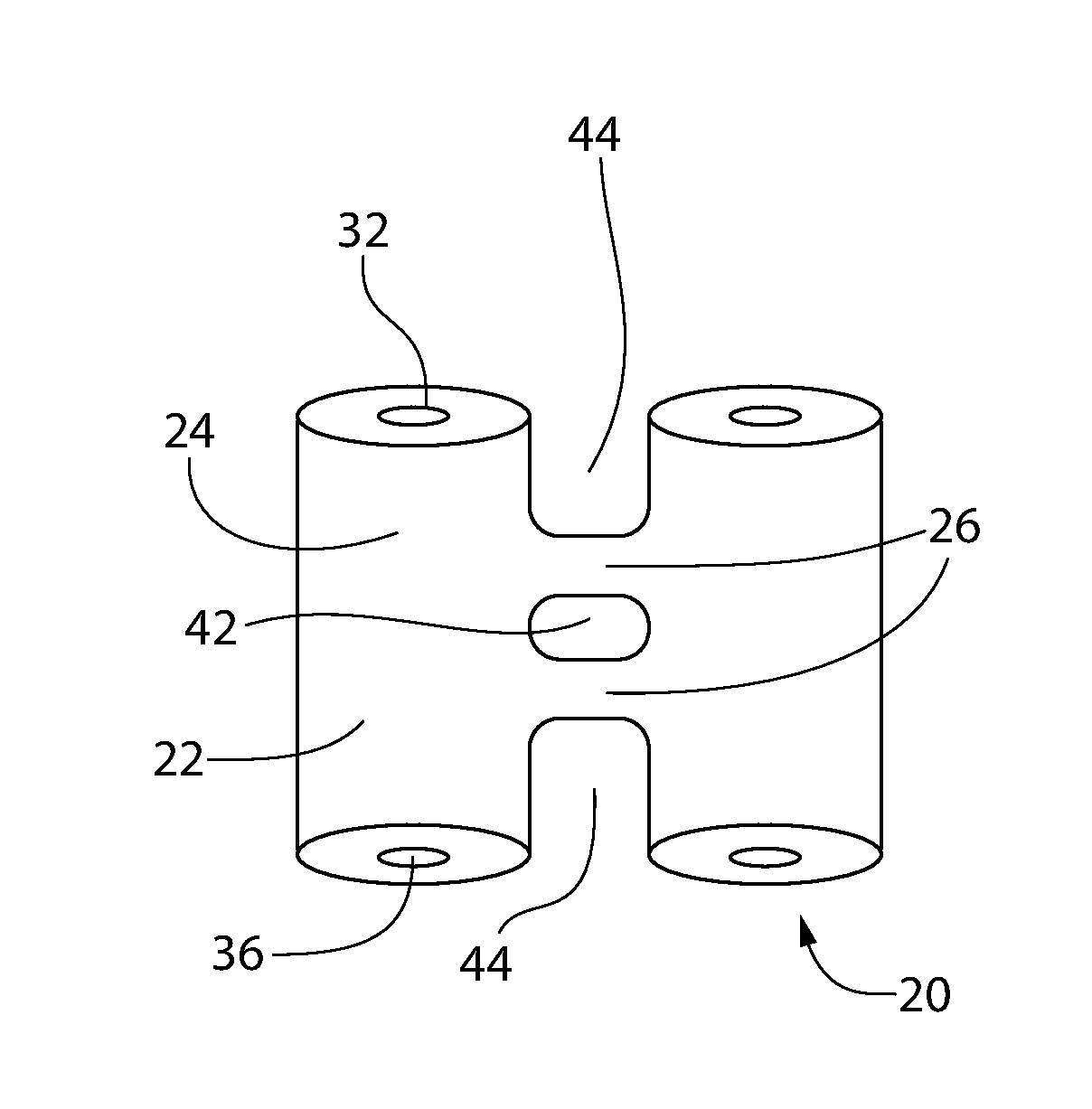
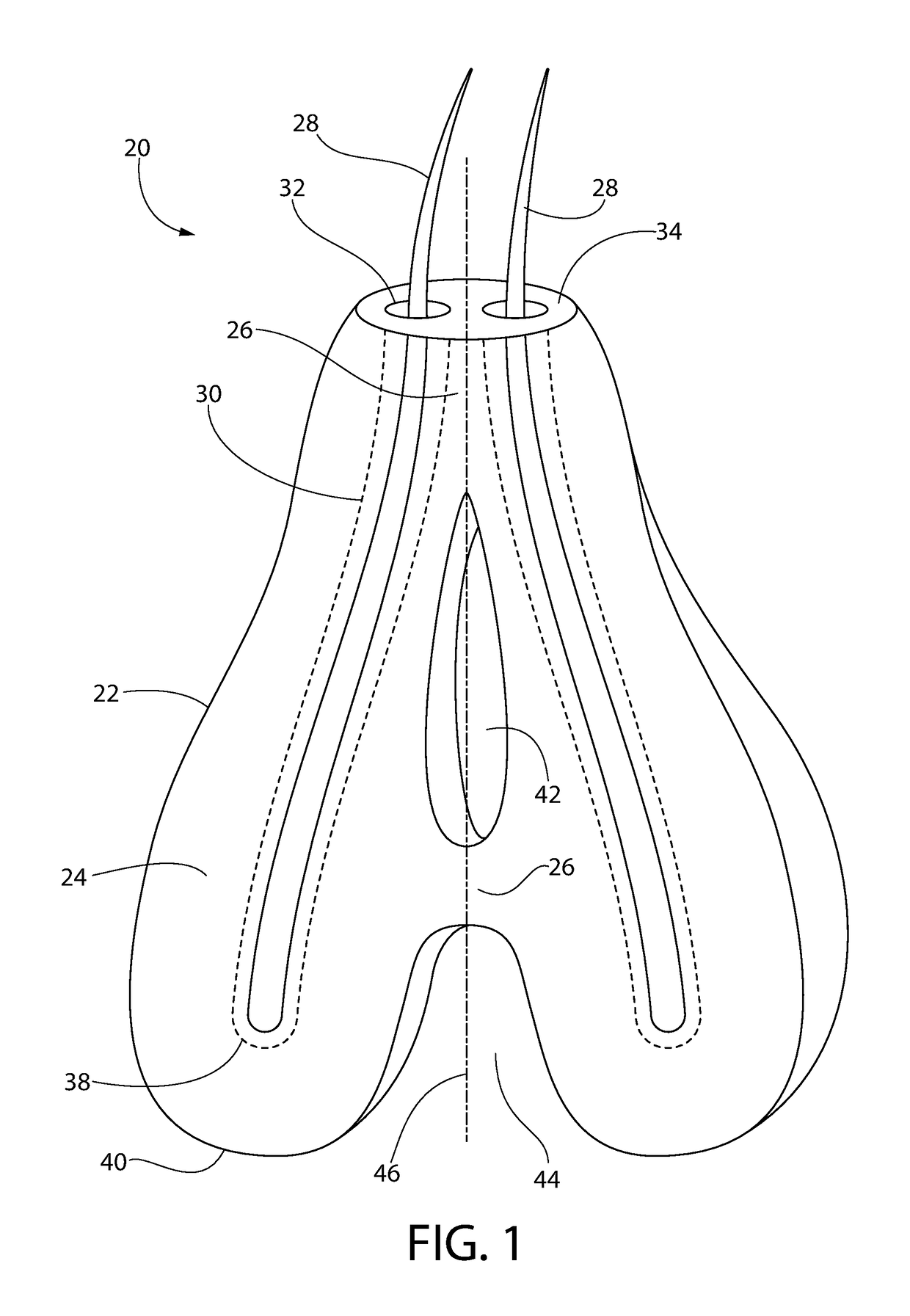
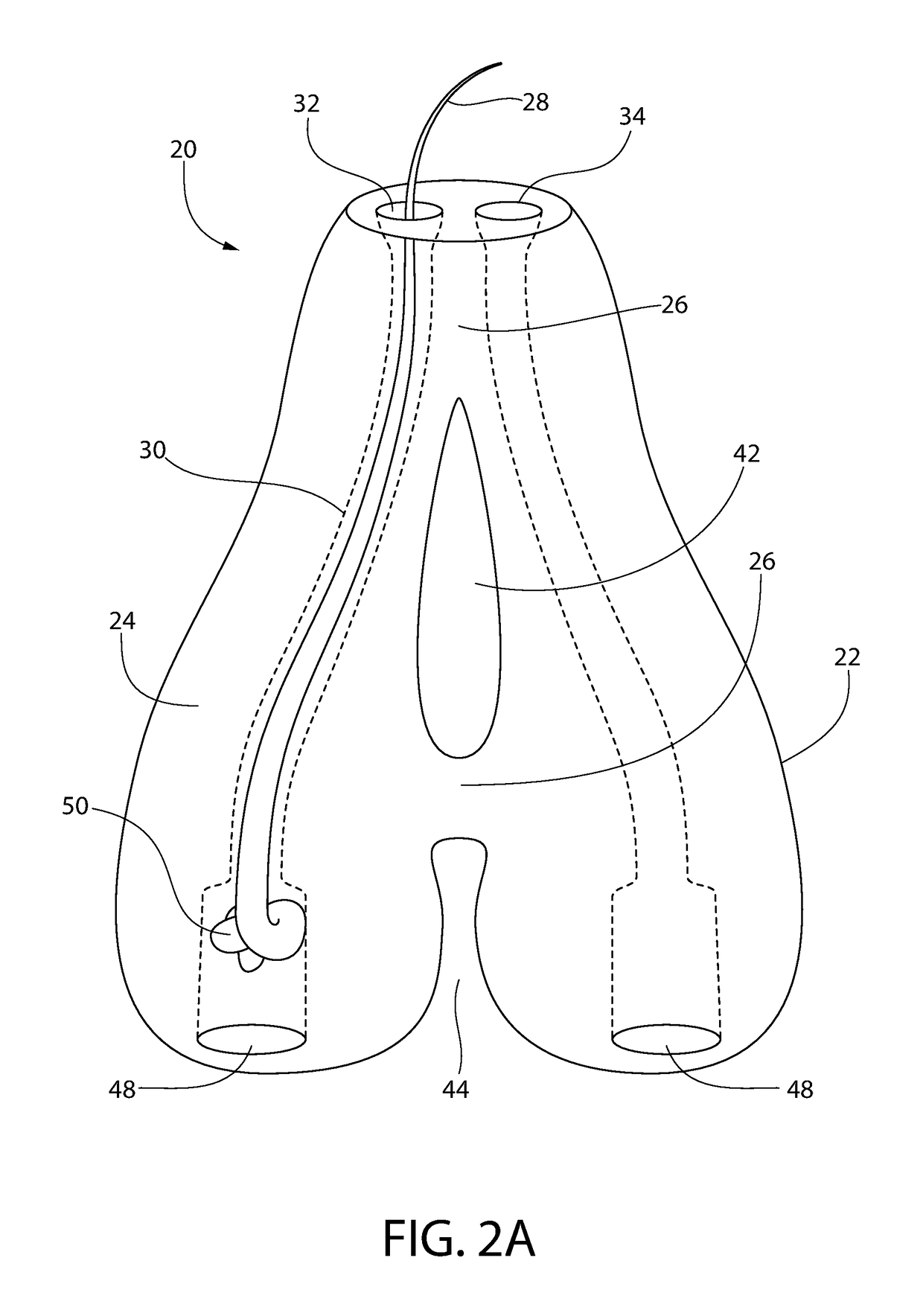
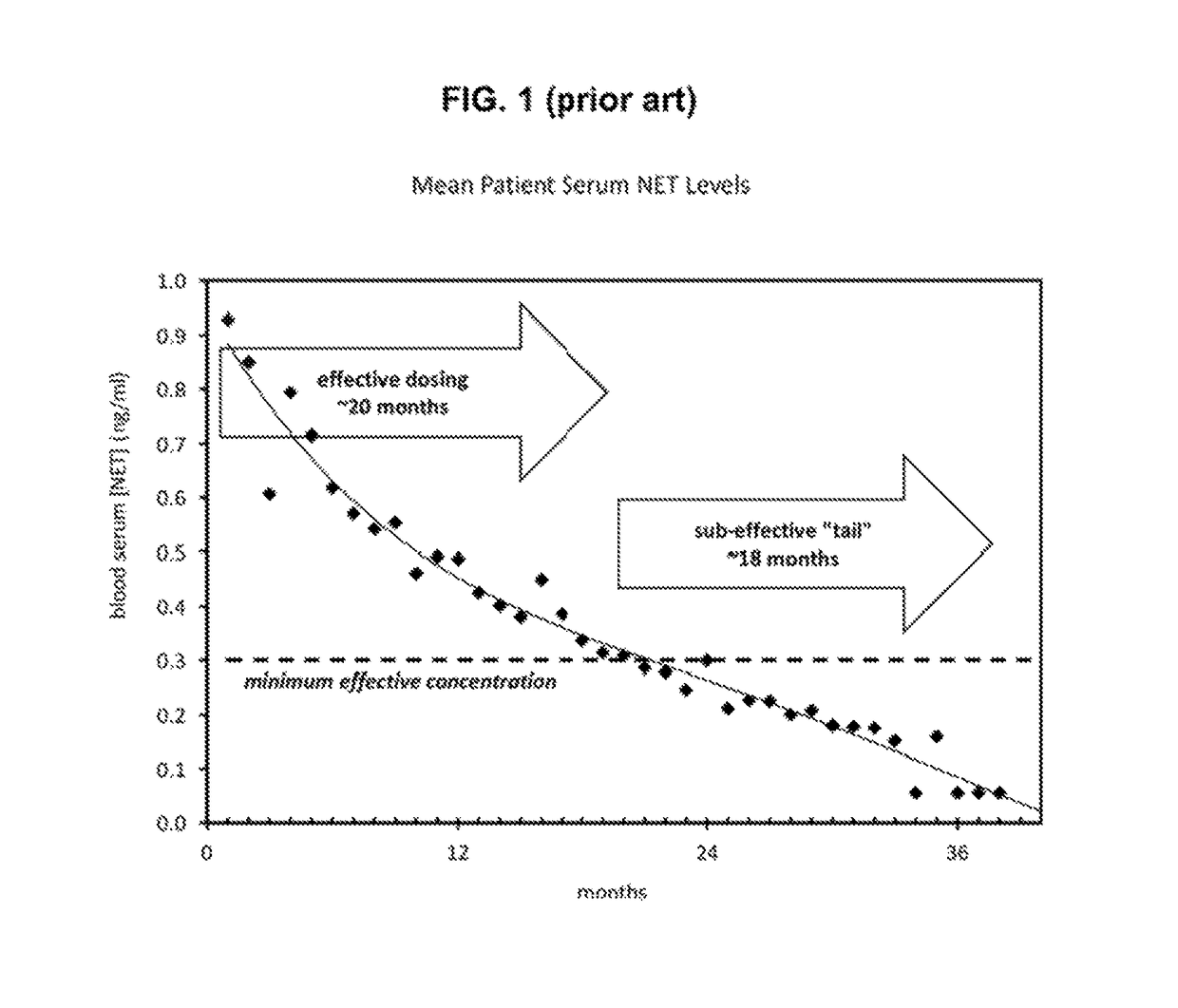
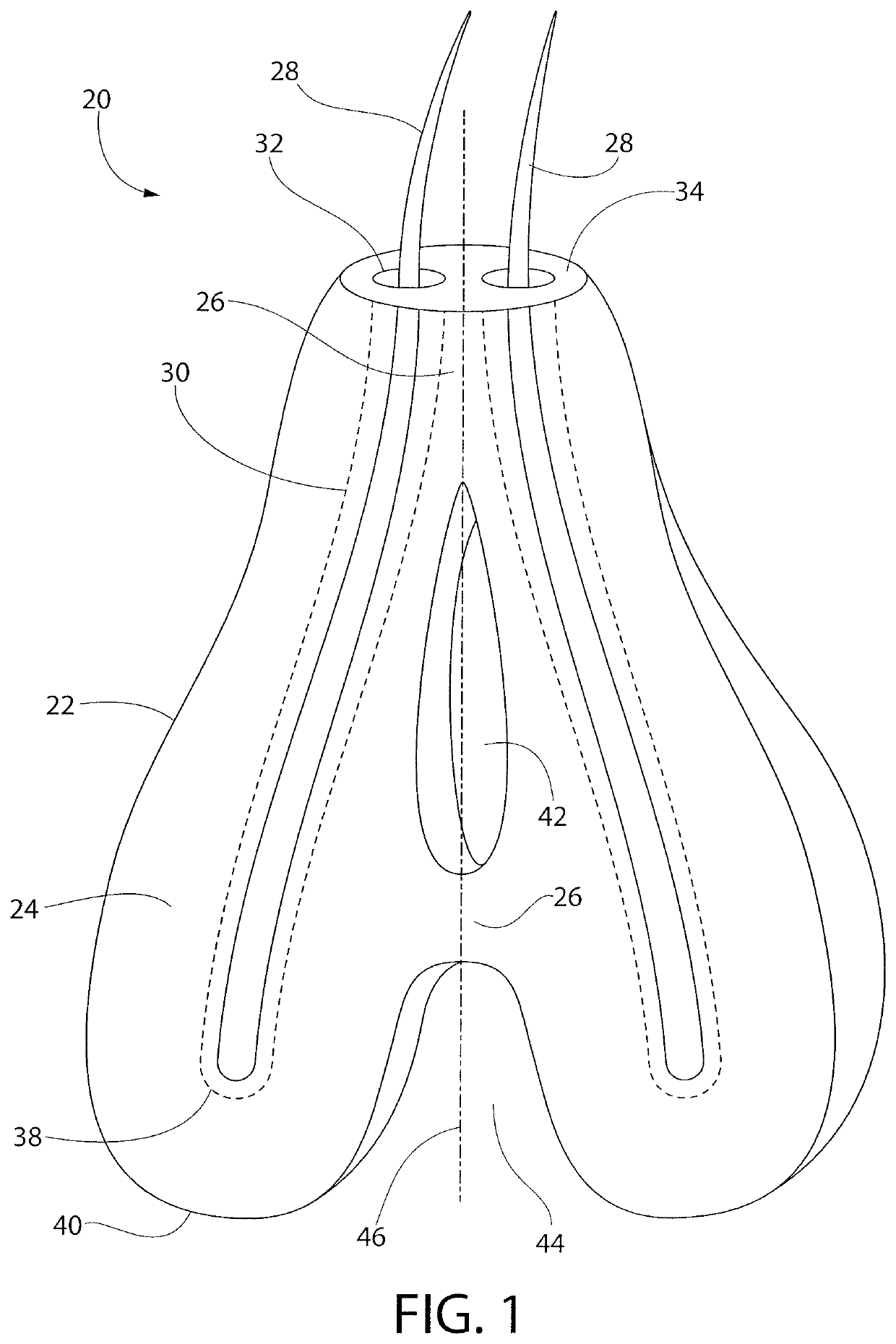
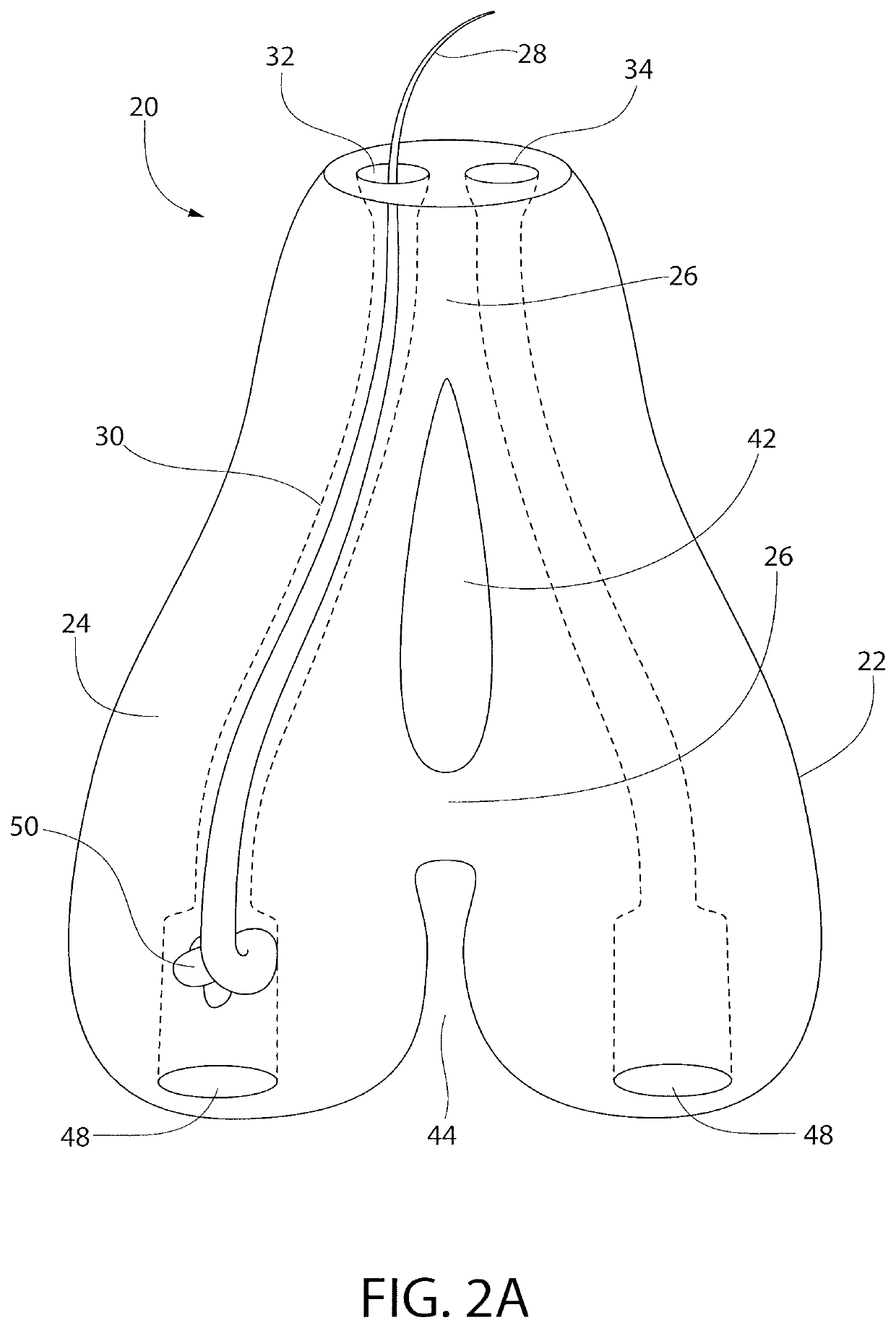
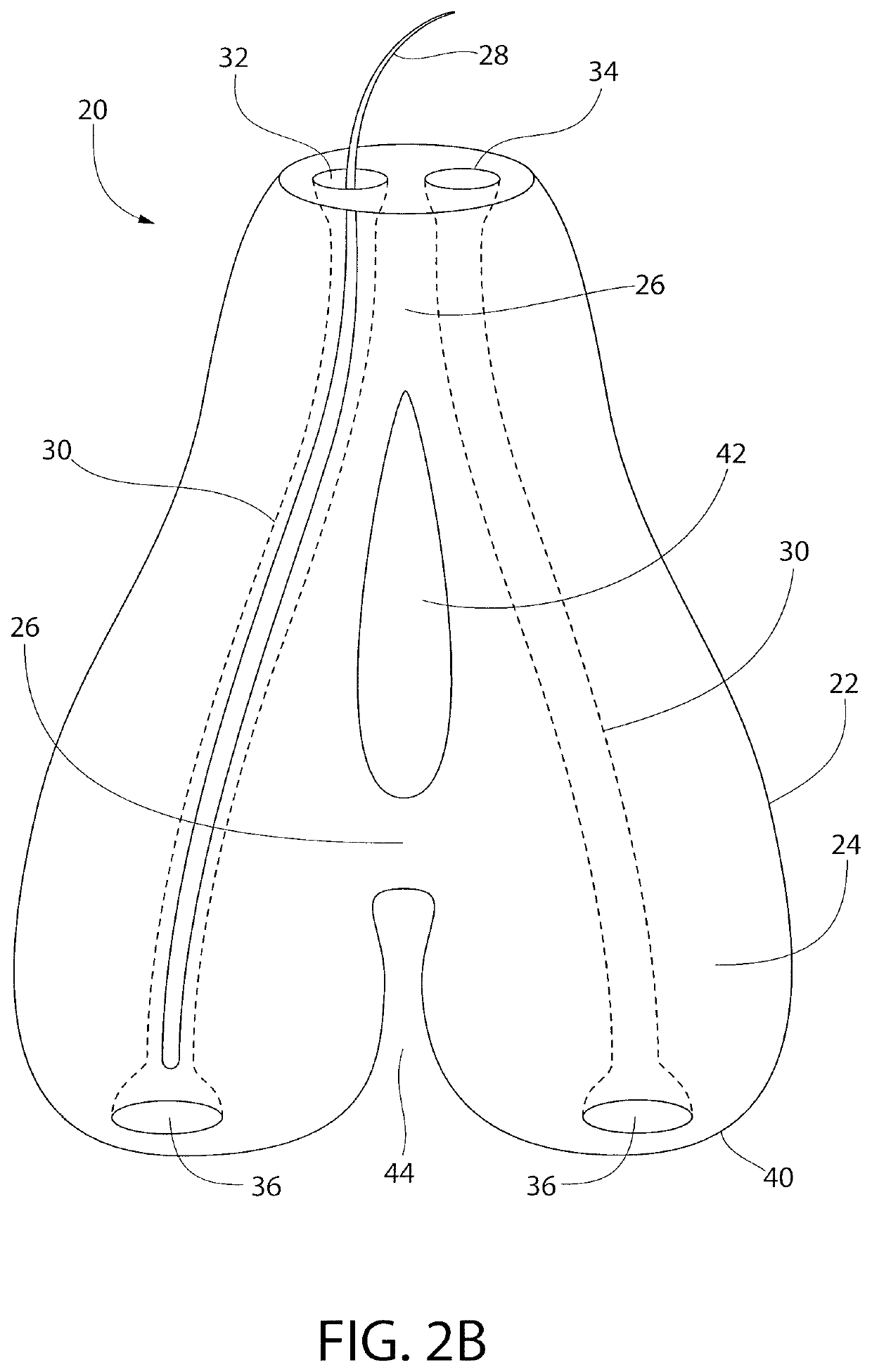
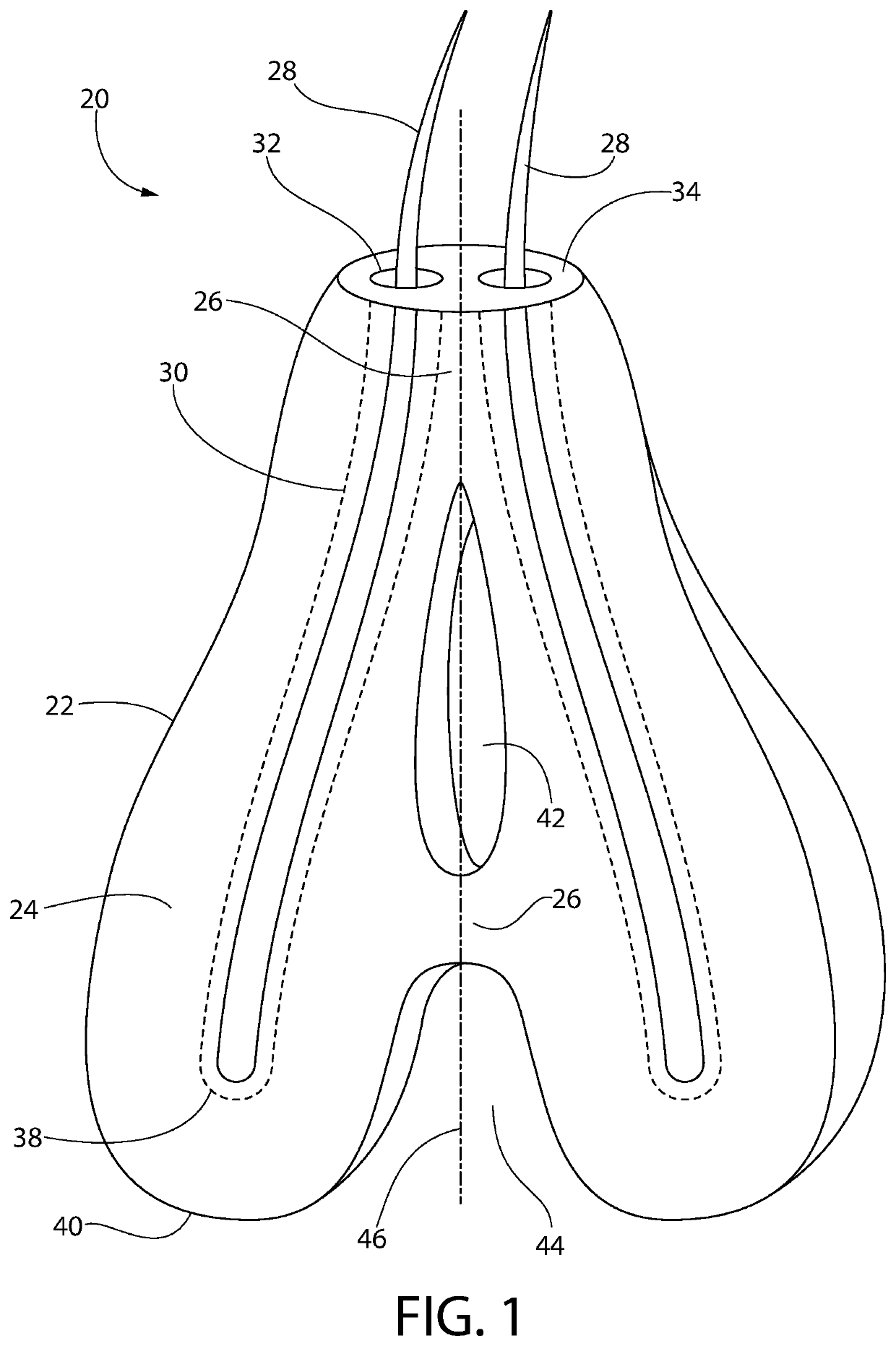
Subcutaneous implant
InactiveUS6126956AEasy to modifyEasy for flexibilityOrganic active ingredientsNervous disorderControl releaseSubcutaneous implant
A non-abusable, non-inflammatory, biocompatible, non-biodegradable, subcutaneous, polymeric implant for the prolonged, controlled release of hydromorphone with near zero-order kinetics is described. Methods of alleviating cancer pain and treating opioid drug addiction with the implant are also described.
Owner:AXXIA PHARMA
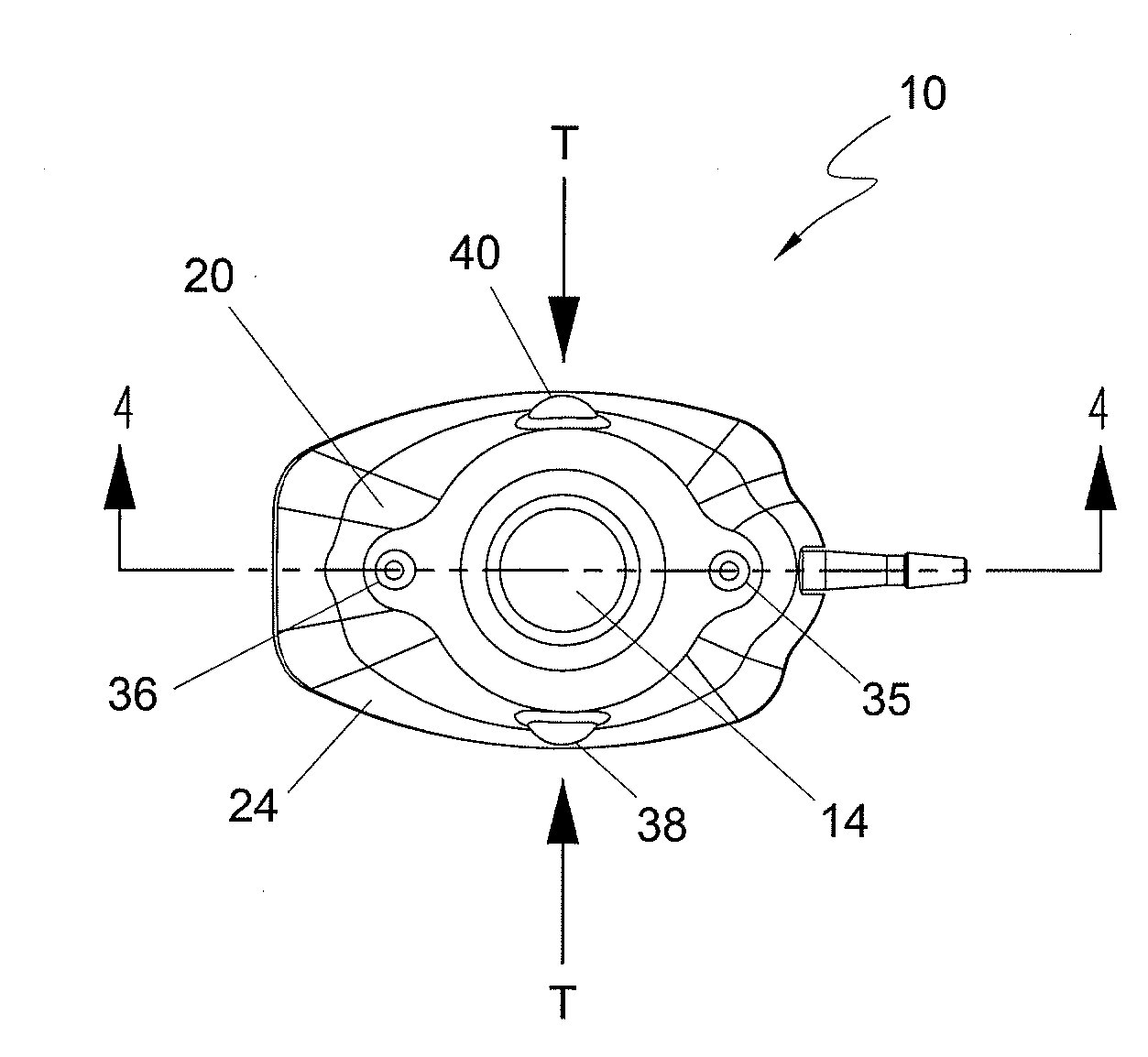
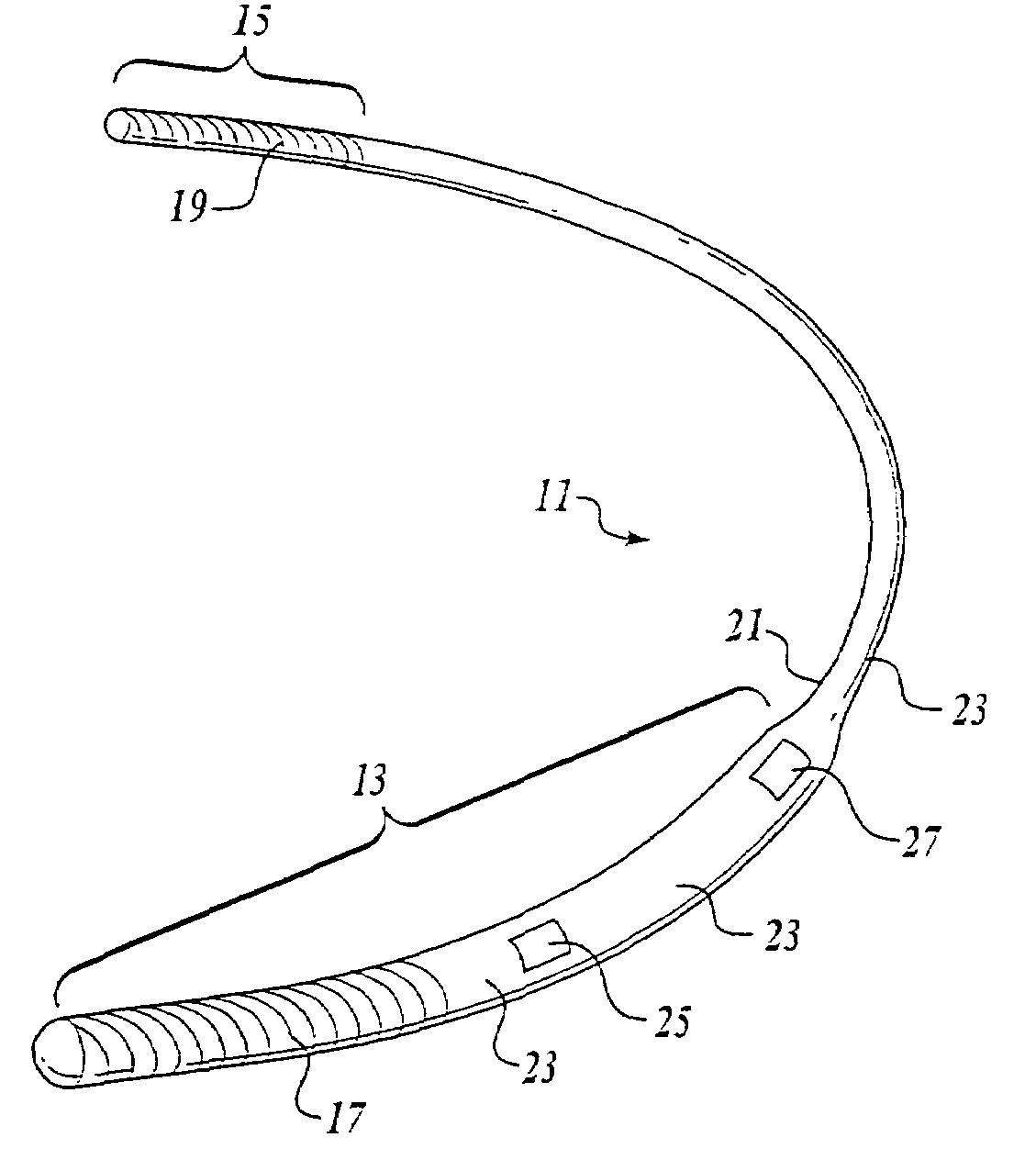
Subcutaneously implantable lead including distal fixation mechanism
InactiveUS7908015B2Transvascular endocardial electrodesSubcutaneous electrodesDistal fixationSubcutaneous implant
A subcutaneously implantable lead includes a coil disposed along a portion of the lead, and a lead tip coupled to a distal end of the lead. The lead tip includes at least one component that is movable relative to the distal end of the lead and configured to anchor the lead tip in subcutaneous tissue.
Owner:MEDTRONIC INC
Apparatus and method for creating an arterio-venous connection in hemodialysis maintenance
The present invention provides a kit apparatus and a methodology to prevent the primary causes of arterio-venous graft thrombosis; and provides a durable vascular access for successful long term use in hemodialysis. The invention employs a patient-customized prosthetic endograft as an subcutaneously implanted vascular access; and utilizes a surgical method for endovascular insertion of the prosthetic endograft into a pre-chosen vein, which does not require a distal anastomosis, and thus allows the distal outflow end of the implanted vascular access to remain unattached and freely floating at a precisely located anatomic position within the internal lumen the pre-chosen vein.
Owner:PERMAGRAFT +1
Hypodermic implant device
InactiveUS6802827B2Avoid spreadingReduce riskMedical devicesInfusion needlesImplanted deviceSubcutaneous implant
A hypodermic implant device, comprising a barrel; an upper piston segment engaged with an inner wall of the barrel; an axially aligned needle extendably distally out of the barrel for insertion into an object when the upper piston segment is moved distally within the body; a lower piston segment push rod in alignment with the needle, so that upon distal movement of the lower piston segment, the push rod moves distally through the needle expelling a releasable implant housed in the needle; and a means for retraction of the needle into a needle receptacle in the barrel.
Owner:GRAY PLANT MOOTY MOOTY & BENNETT PA
Subcutaneously implantable lead including distal fixation mechanism
InactiveUS20100030311A1Transvascular endocardial electrodesSubcutaneous electrodesDistal fixationSubcutaneous implant
A subcutaneously implantable lead includes a coil disposed along a portion of the lead, and a lead tip coupled to a distal end of the lead. The lead tip includes at least one component that is movable relative to the distal end of the lead and configured to anchor the lead tip in subcutaneous tissue.
Owner:MEDTRONIC INC
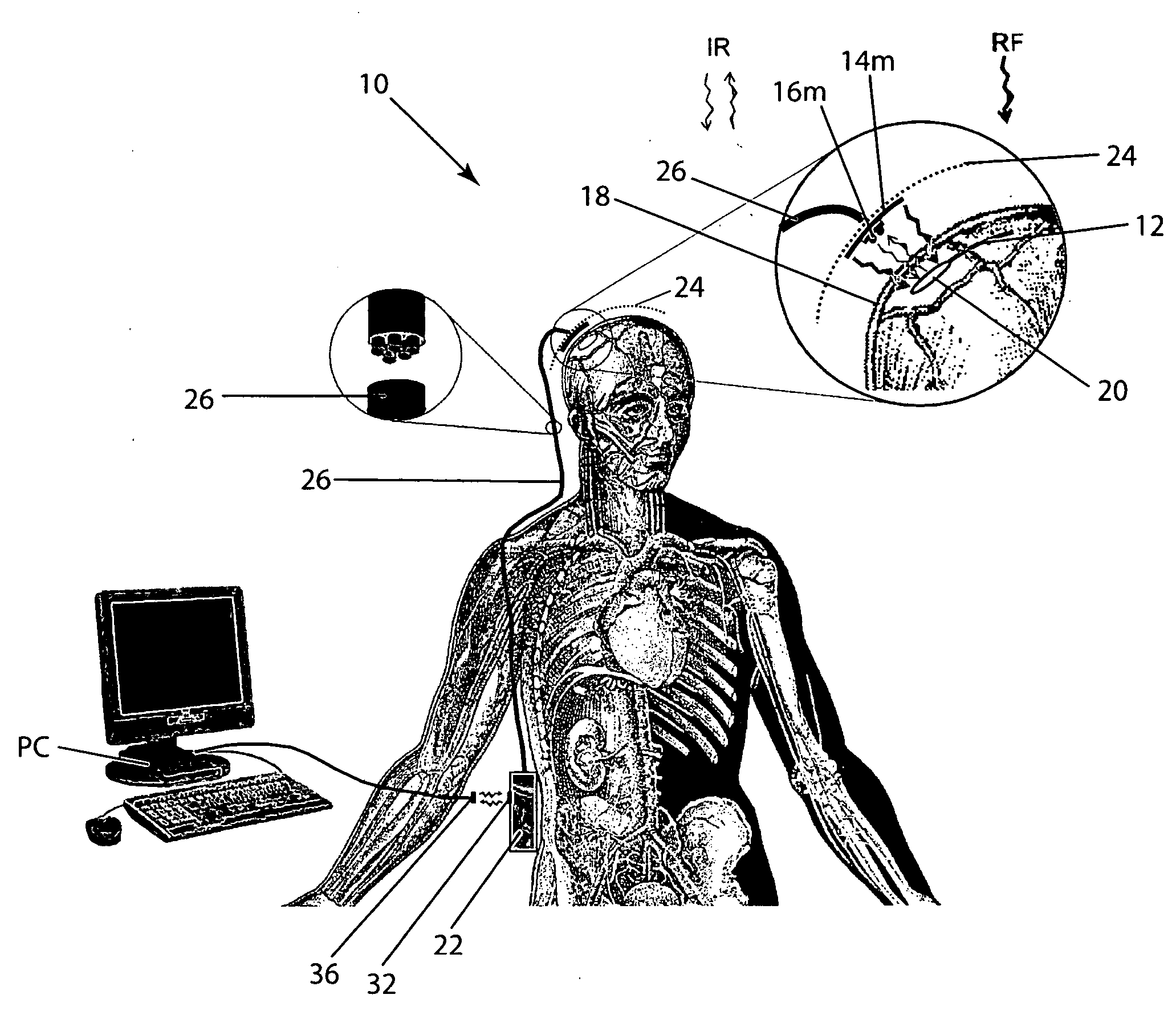
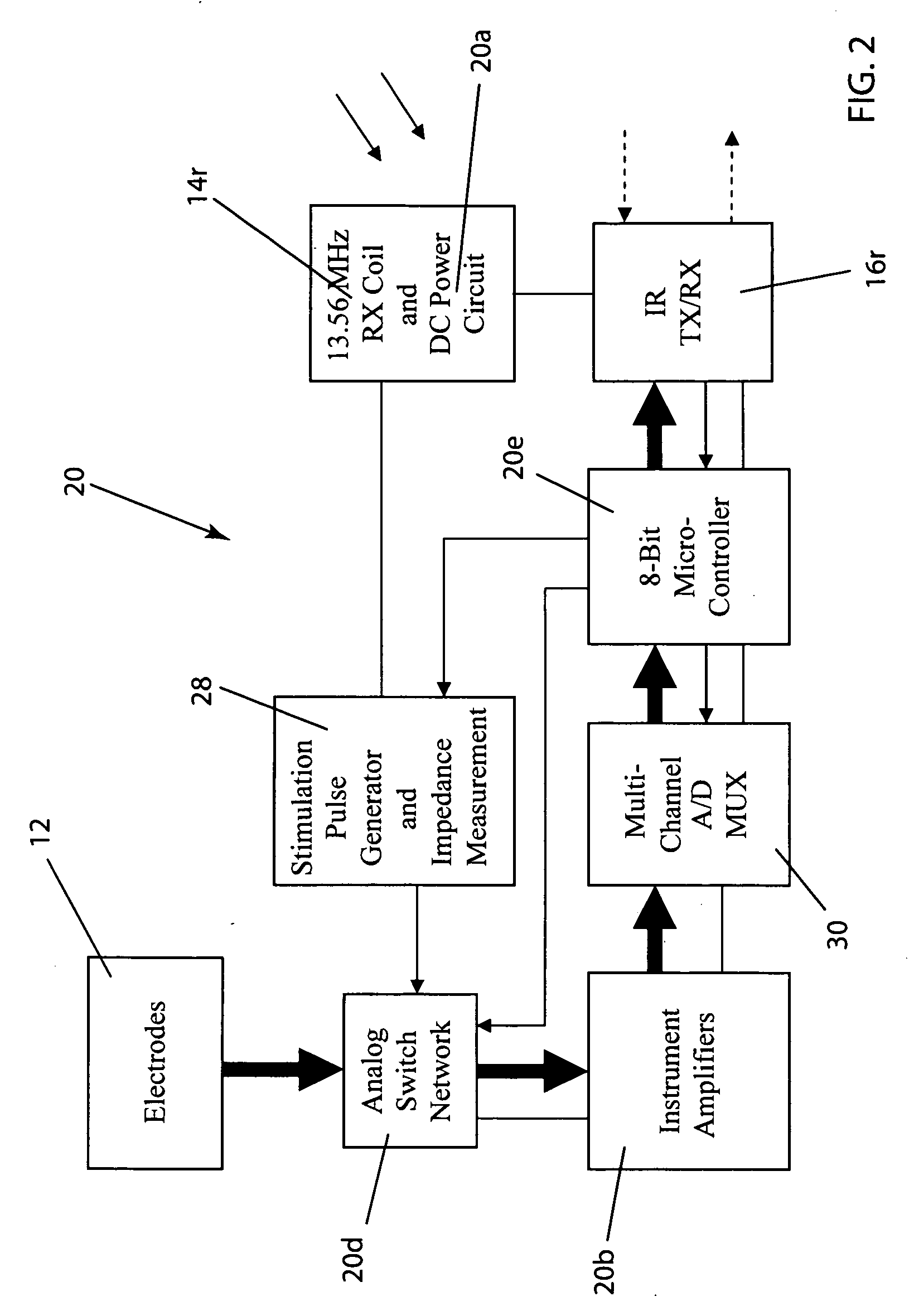
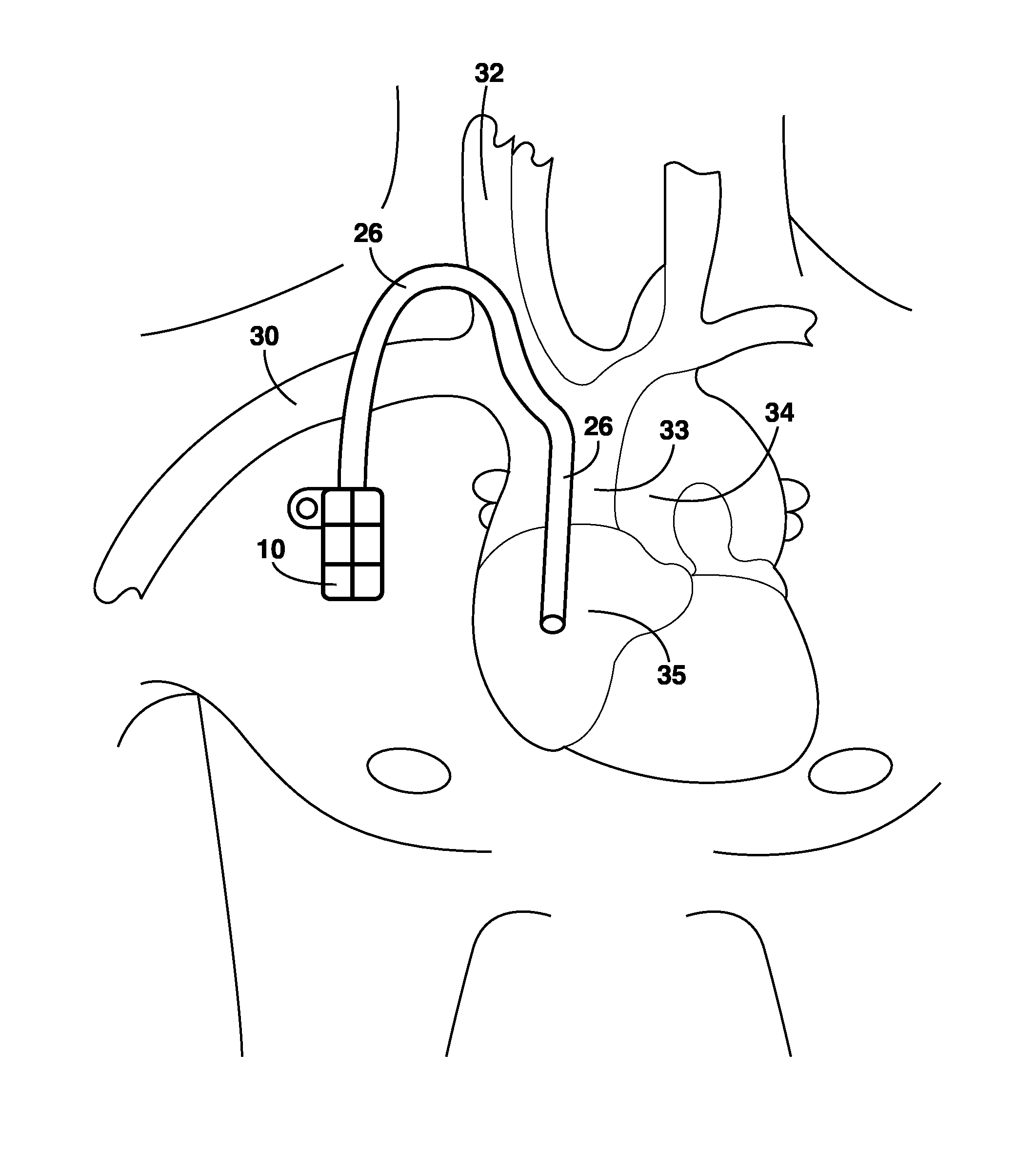
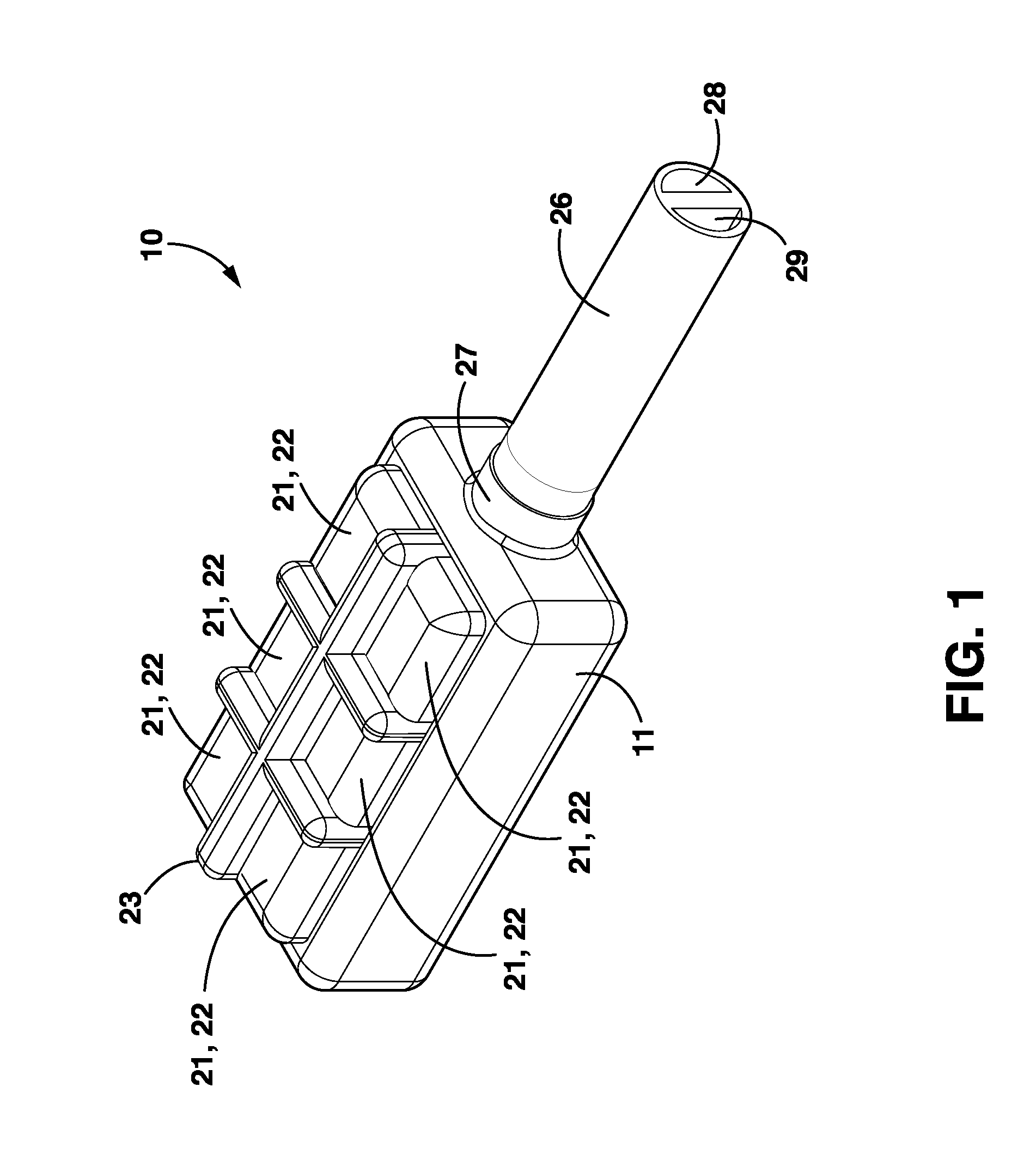
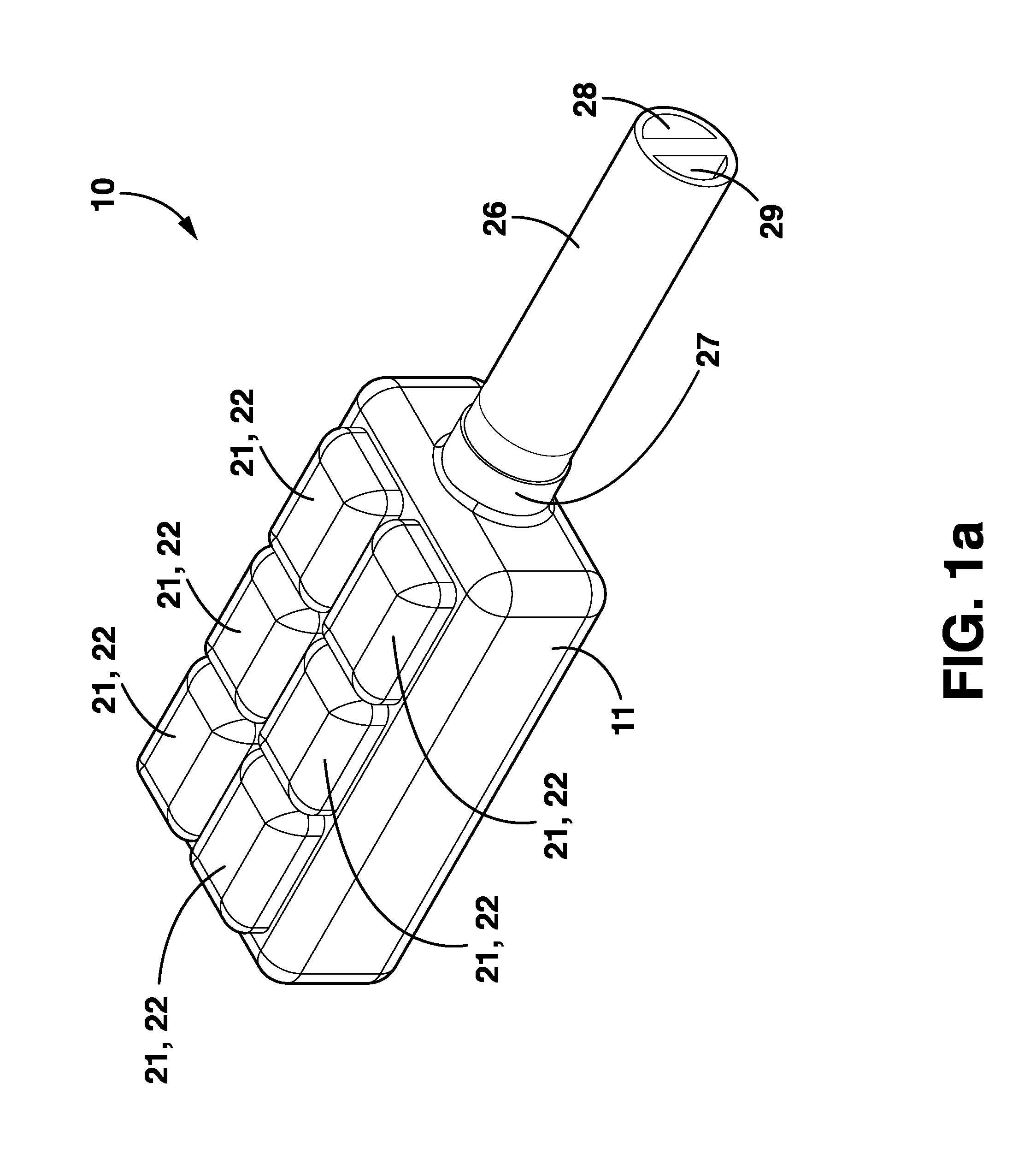
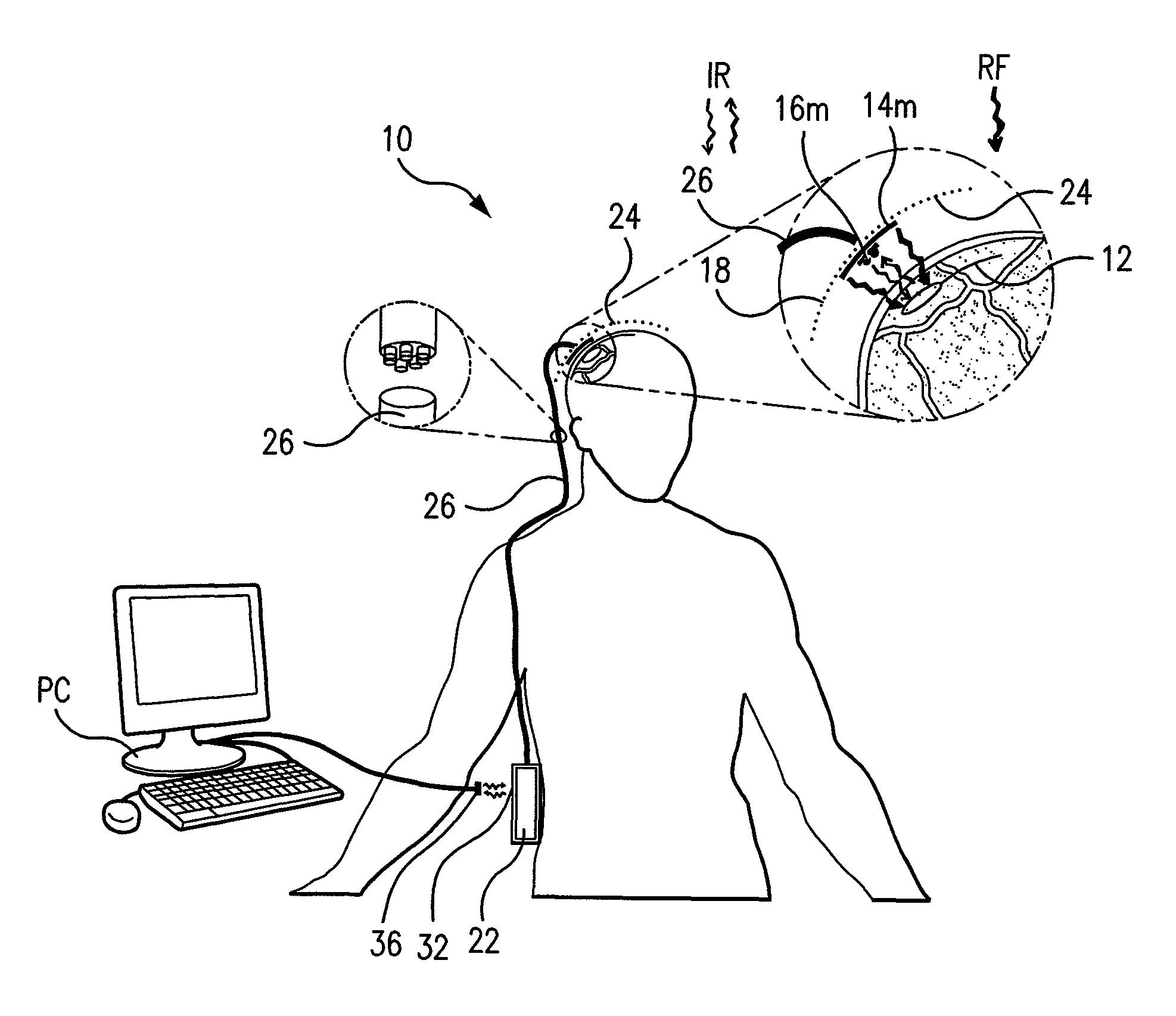
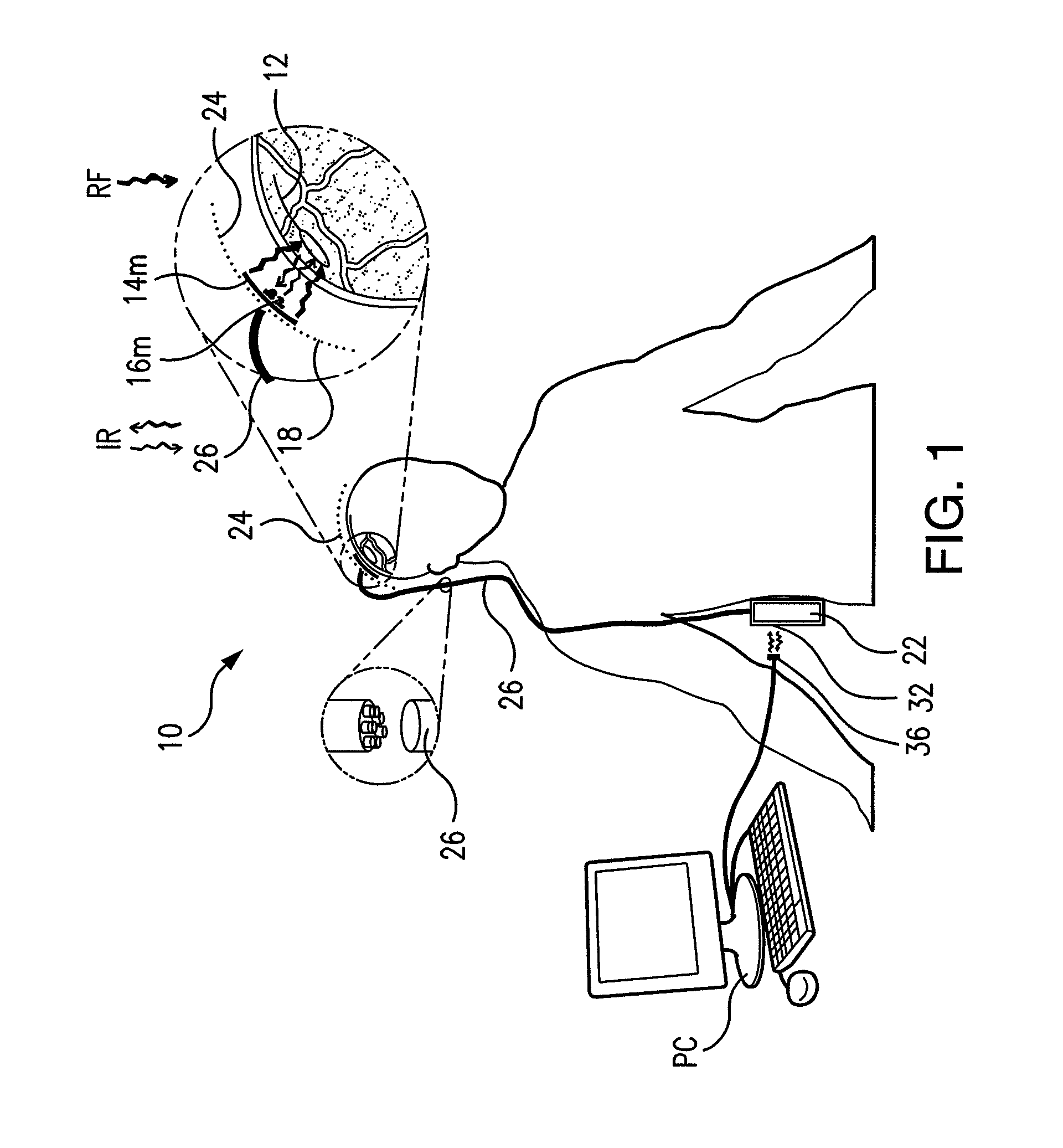
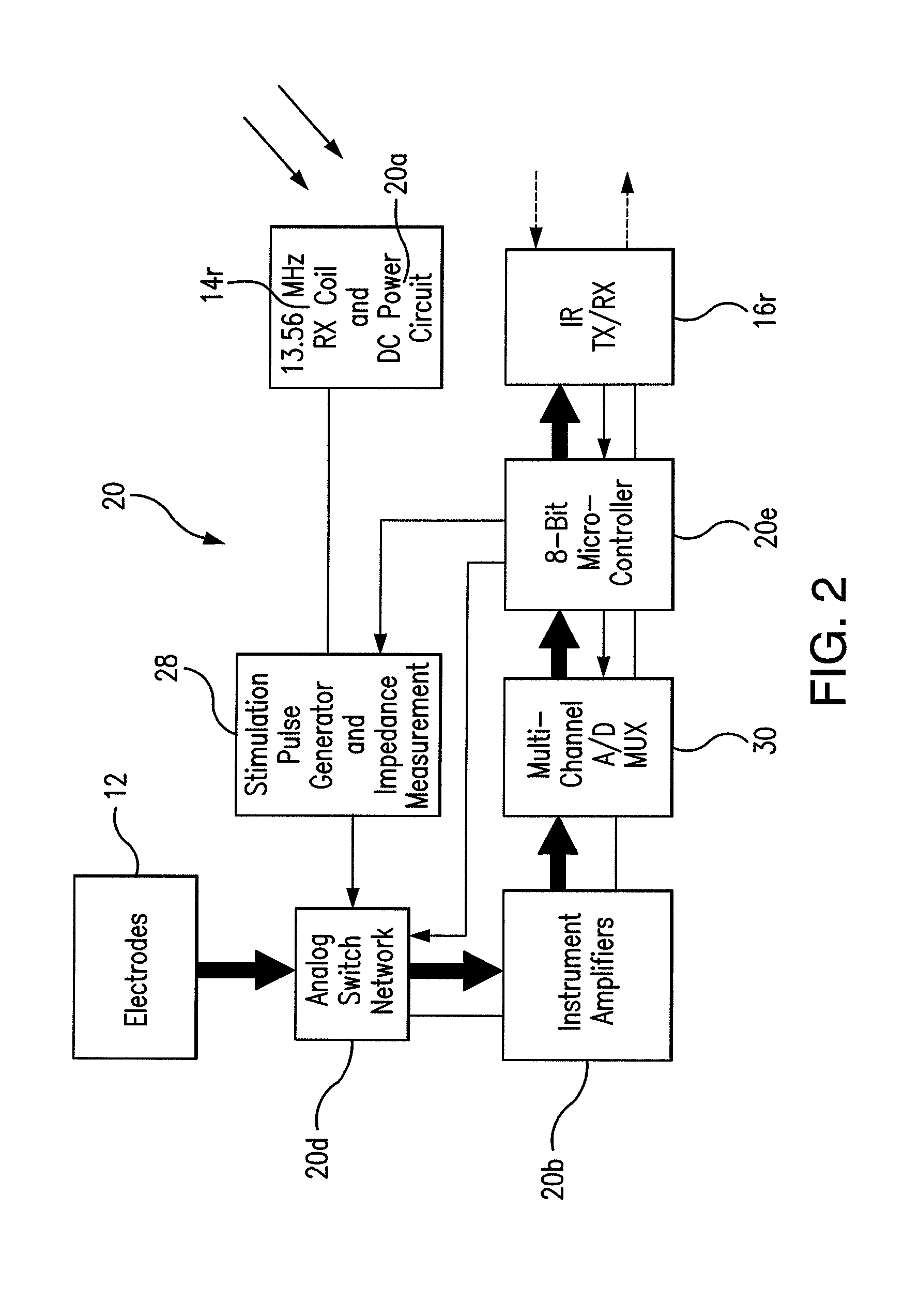
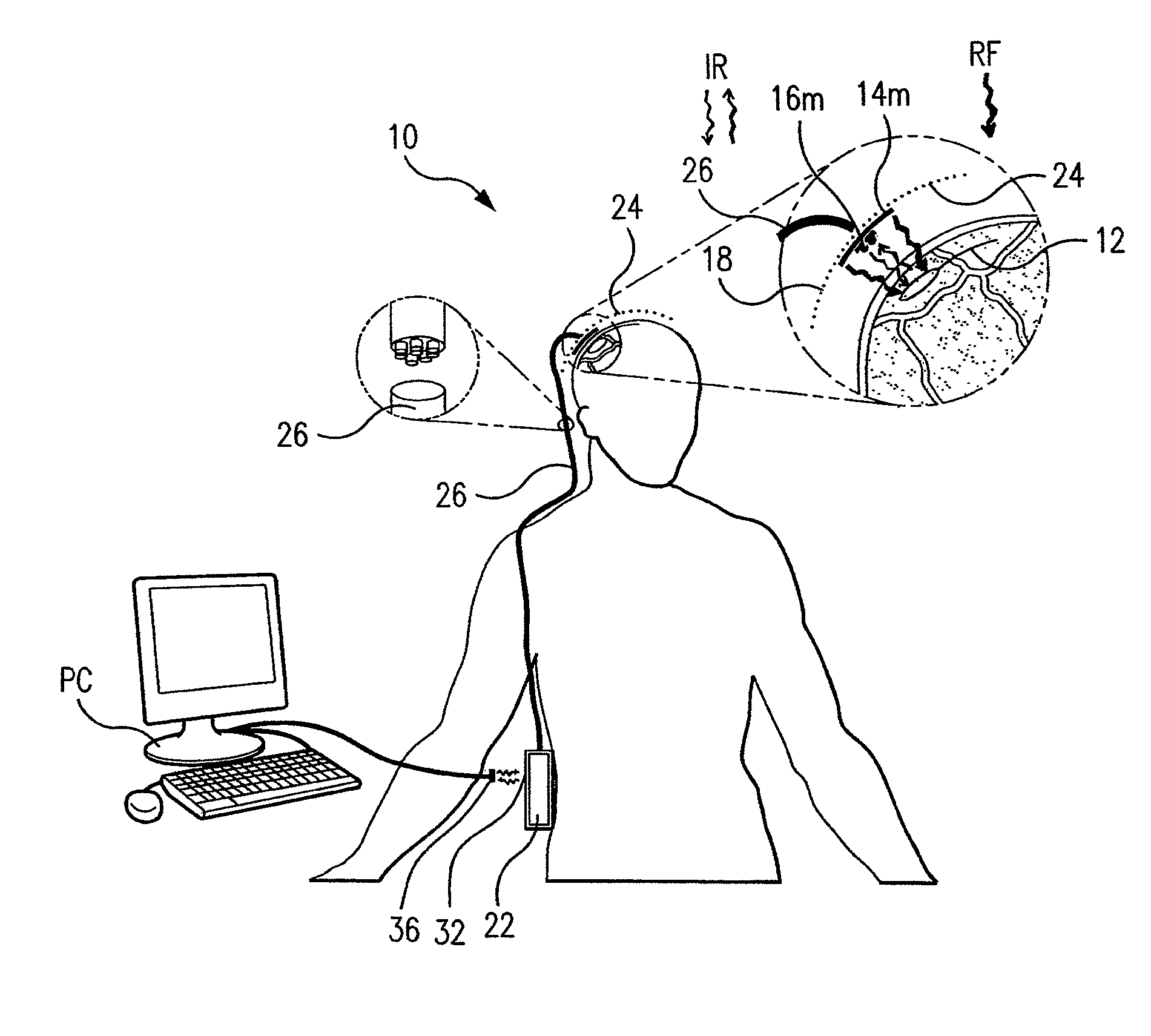
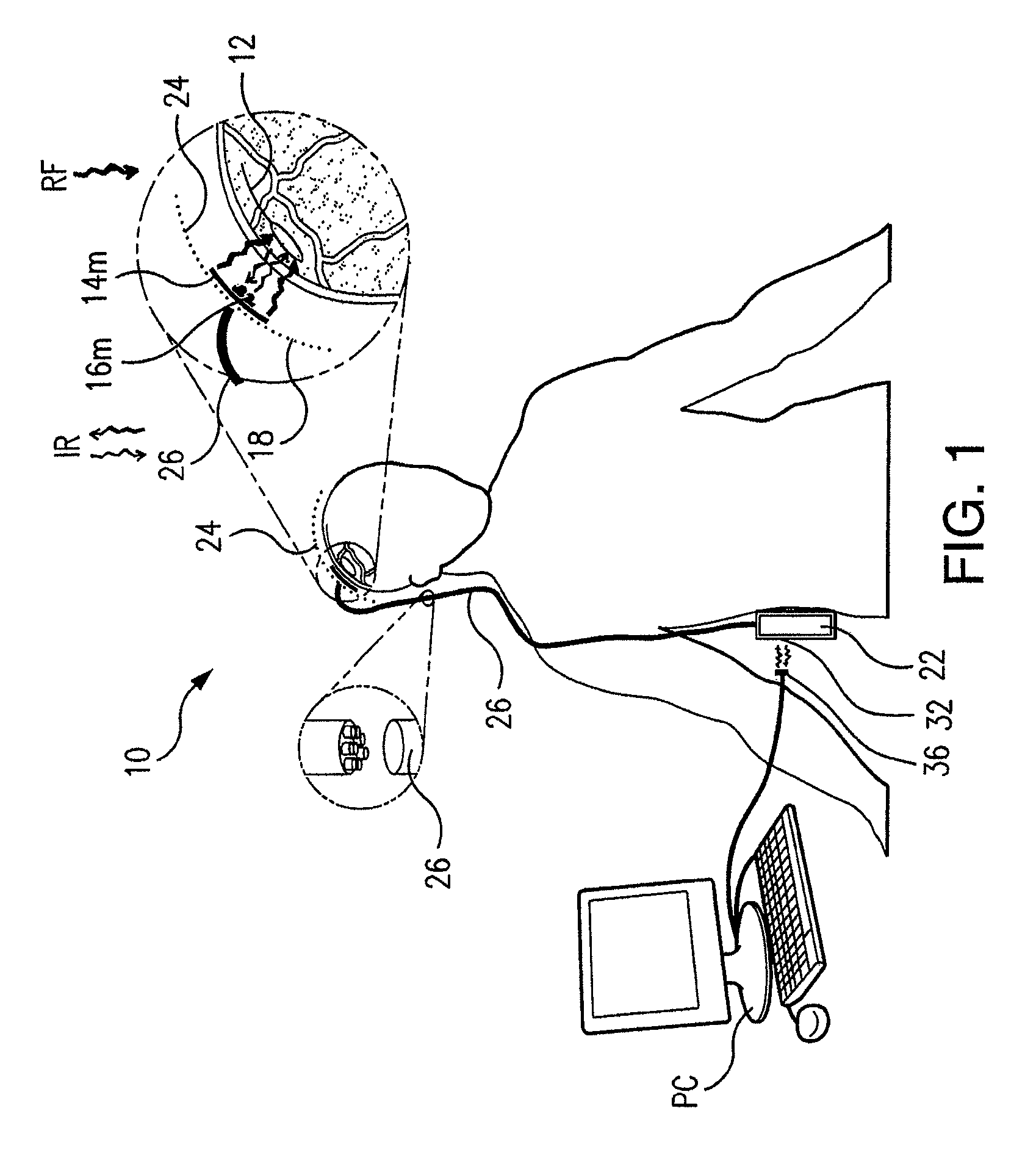
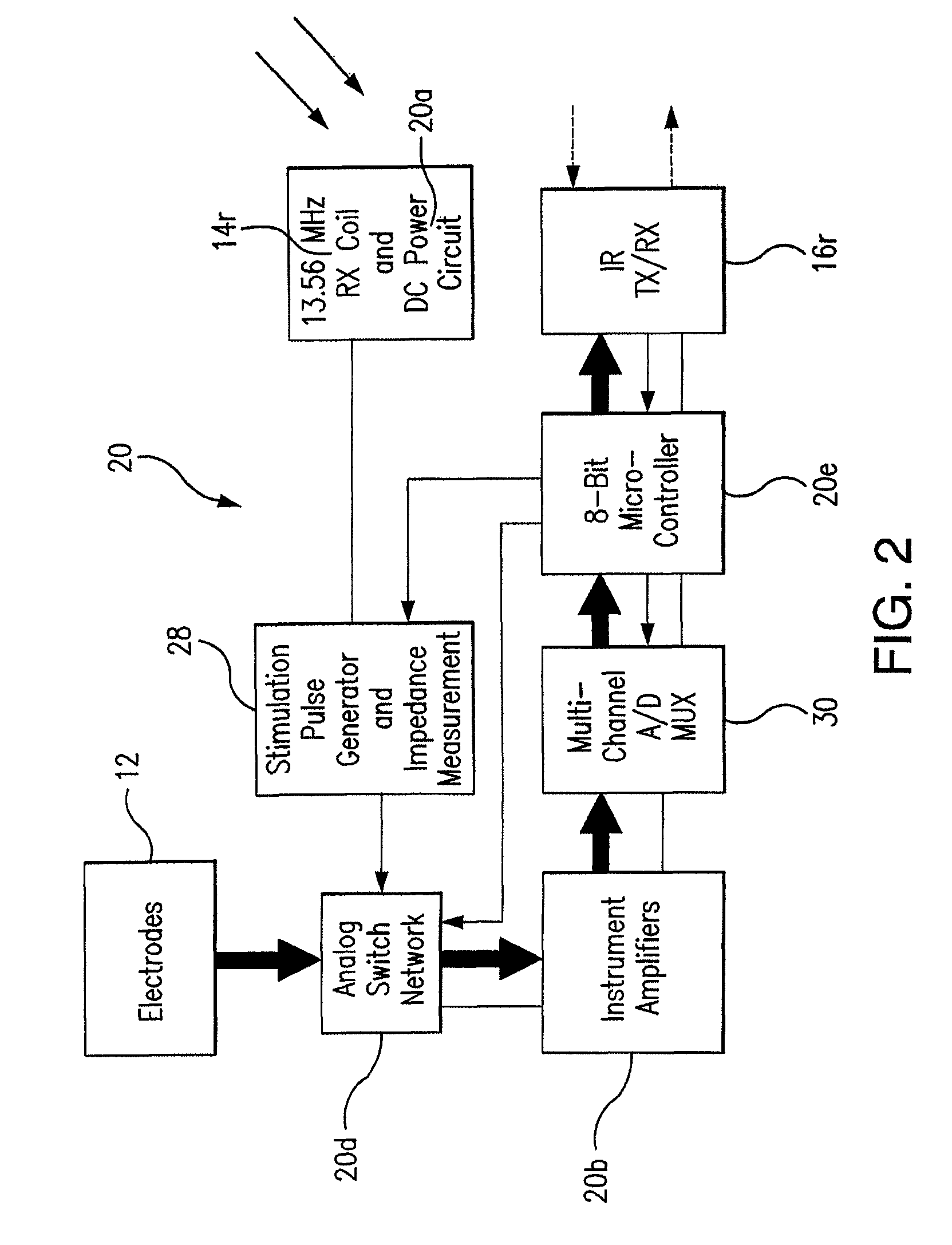
Wireless System for Epilepsy Monitoring and Measurement
ActiveUS20090149913A1Increase freedomReduce power consumptionElectroencephalographyElectrotherapyElectrical resistance and conductanceTransmitted power
A wireless system for monitoring a patient's brain tissue including (1) a plurality of electrodes abutting brain tissue, (2) main circuitry outside the patient's body to transmit power at radio frequencies and send / receive data using infrared energy, and (3) subcutaneously-implanted remote circuitry connected to the electrodes and configured to (a) receive transmitted RF power, (b) capture and digitize EEG signals from the electrodes, and (c) send / receive data to / from the main circuitry using IR energy, including sending digitized EEG signals from each electrode to capture the full bandwidth of each EEG signal. The system preferably includes circuitry to measure the electrical impedance of each electrode for real-time monitoring of the condition of the electrode / tissue interfaces to enhance interpretation of captured EEG signals.
Owner:TECH MEDICAL INSTR +1
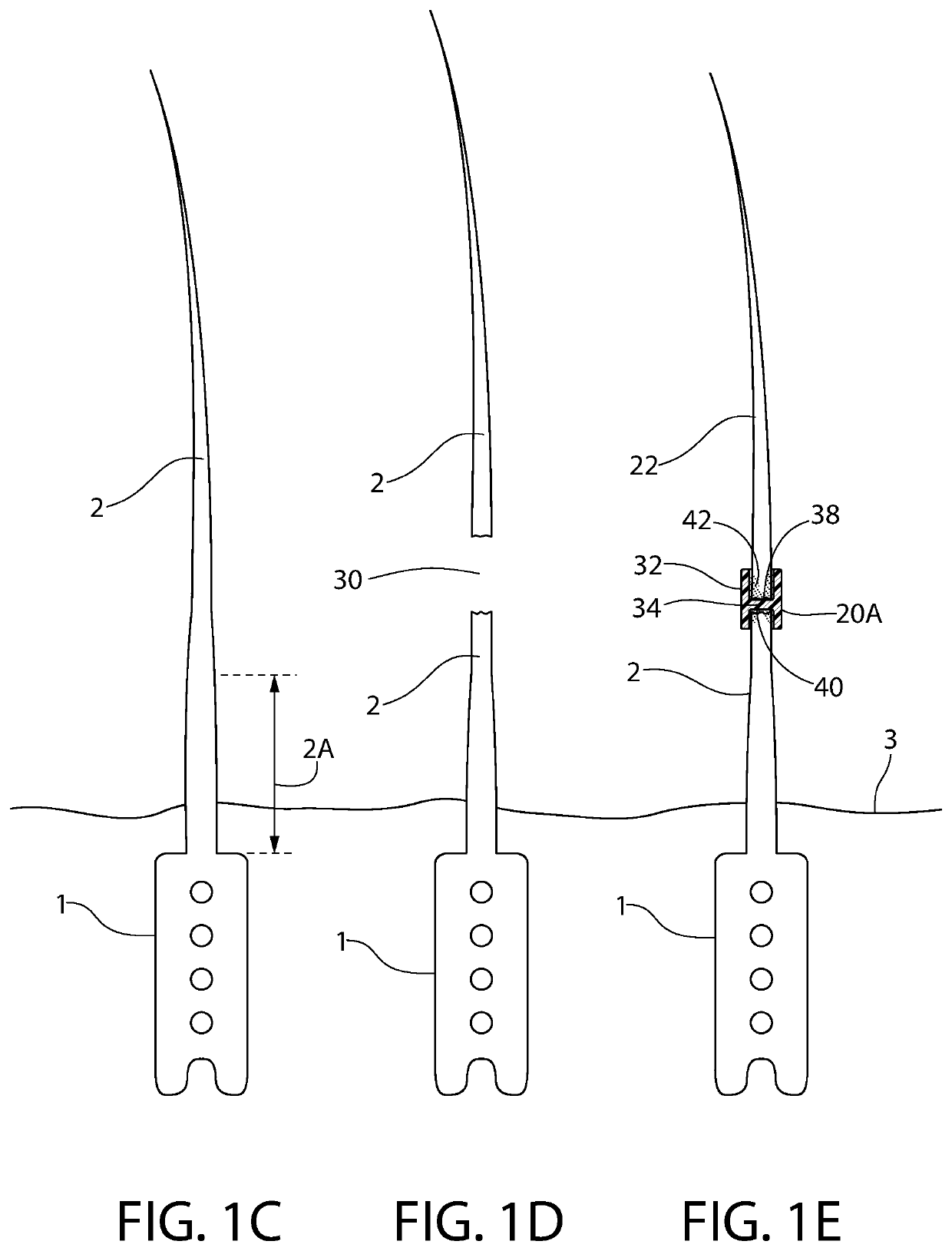
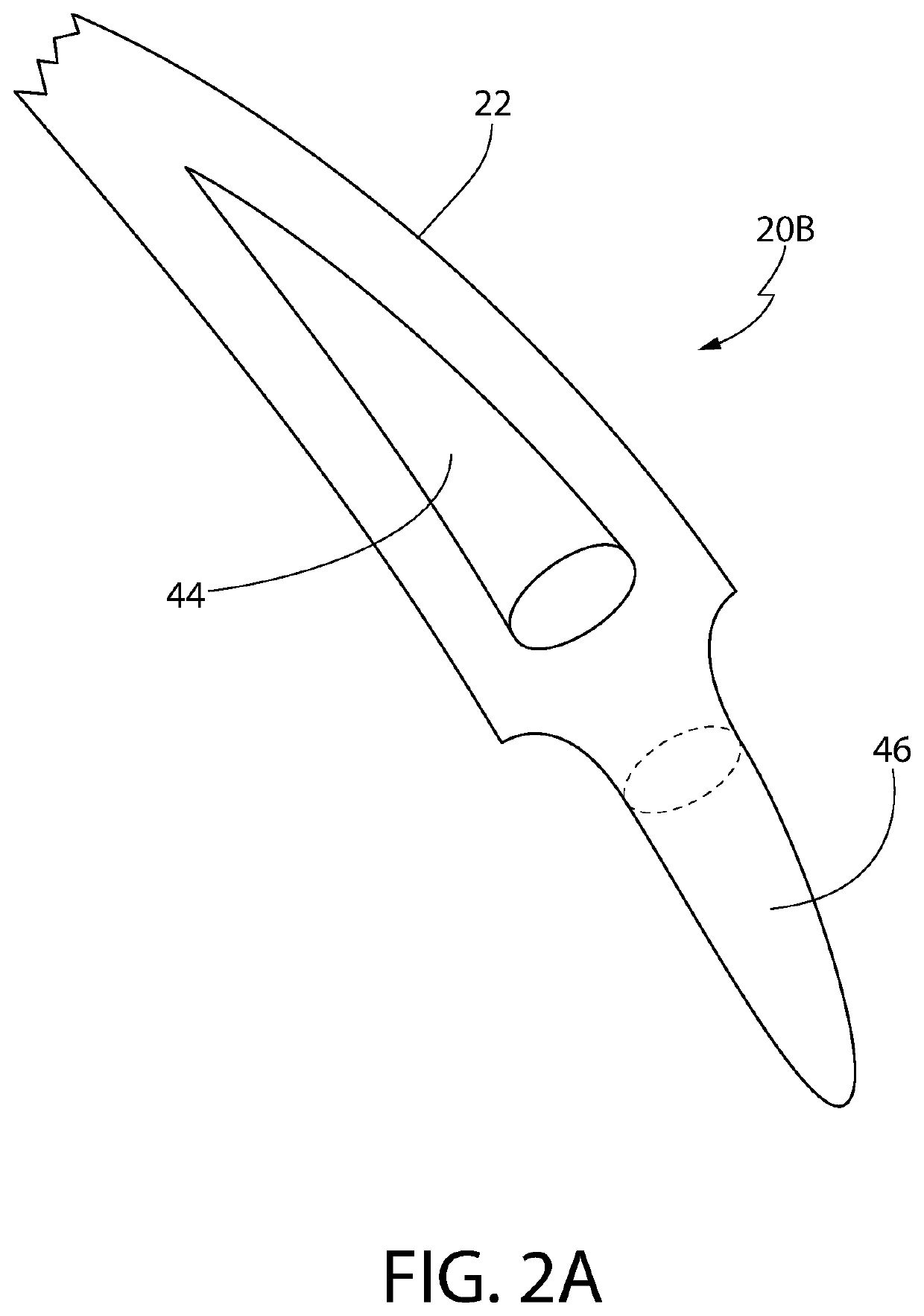
Hair implants comprising enhanced anchoring and medical safety features
ActiveUS9993334B1Avoid bacterial infectionInhibition is effectiveSuture equipmentsSkin implantsSubcutaneous implantCollagenan
A hair implant includes at least two strands including at least one of mammalian hair and synthetic hair; and an anchor, which: (a) includes silicone, (b) is configured for subcutaneous implantation, (c) includes a fracture line configured to facilitate fracturing of the anchor along the fracture line for ease of removal of the implant after subcutaneous implantation, and (d) is configured to provide a scaffold for collagen growth after subcutaneous implantation, wherein at least one of the at least two strands is joined to the anchor on one side of the fracture line and at least one of the at least two strands is joined to the anchor on an opposite side of the fracture line such that each fragment formed by fracturing the implant includes at least one of the at least two strands. Looped implants, a hair restoration method and an implant manufacturing method are also disclosed.
Owner:LORIA HAIR IMPLANT CO LLC
Hair implants comprising enhanced anchoring and medical safety features
ActiveUS10105212B1Avoid bacterial infectionInhibition is effectiveSuture equipmentsSkin implantsAnatomySubcutaneous implant
Owner:LORIA HAIR IMPLANT CO LLC
Unitary subcutaneous only implantable cardioverter-defibrillator and optional pacer
InactiveUS7289854B2Easy to identifyEliminating a significant impediment to broader scale prophylacetic useHeart defibrillatorsVeinElectrical cardioversion
A unitary subcutaneous implantable cardioverter-defibrillator has a long thin housing in the shape of a patient's rib. The housing contains a source of electrical energy, a capacitor, and operational circuitry that senses the presence of potentially fatal heart rhythms. Provided on the housing are cardioversion / defibrillation electrodes located to deliver electrical cardioversion-defibrillation energy when the operational circuitry senses a potentially fatal heart rhythm. The unitary subcutaneous implantable cardioverter-defibrillator does not have a transvenous, intracardiac, epicardial, or subcutaneous electrode.
Owner:CAMERON HEALTH
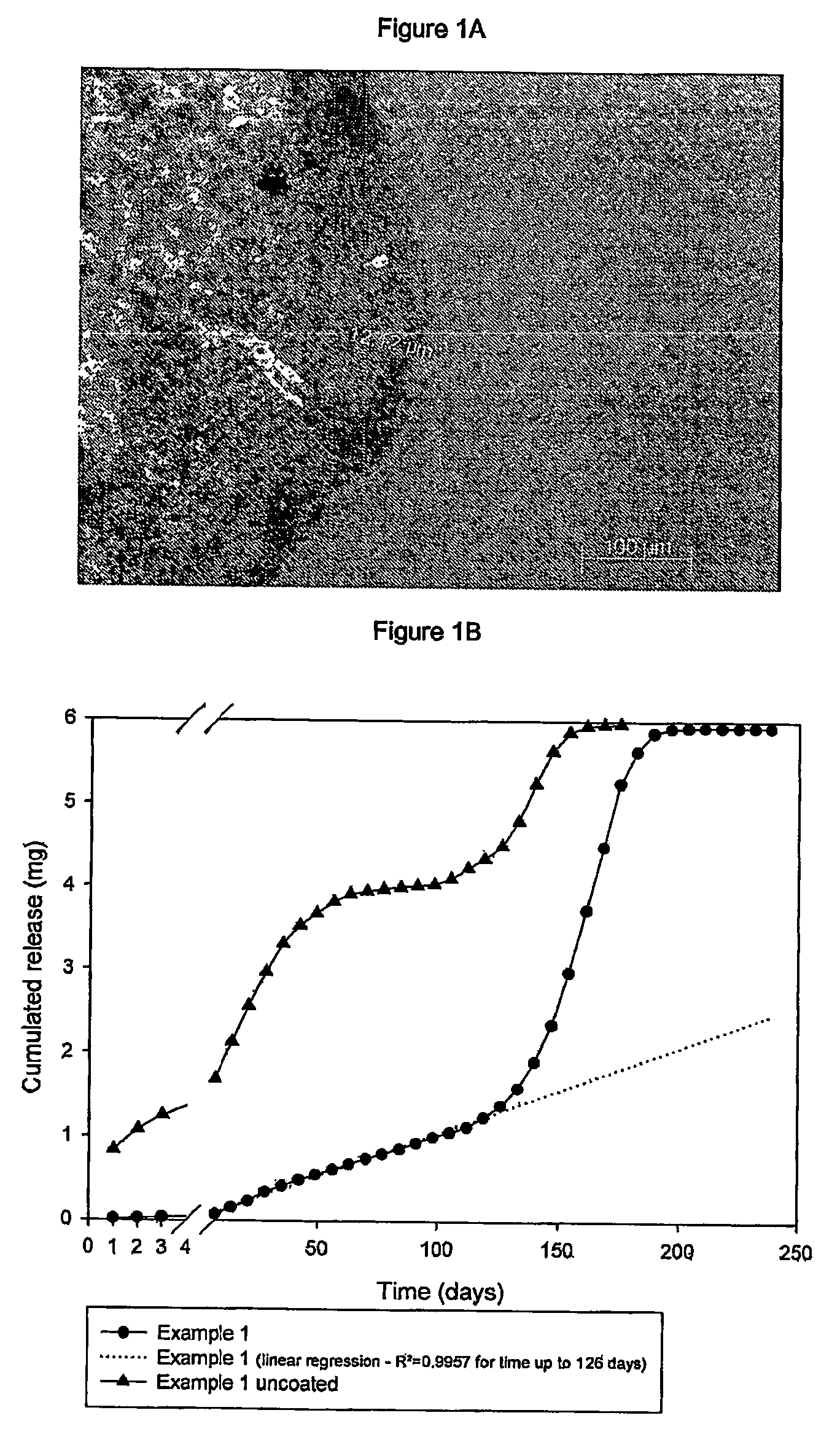
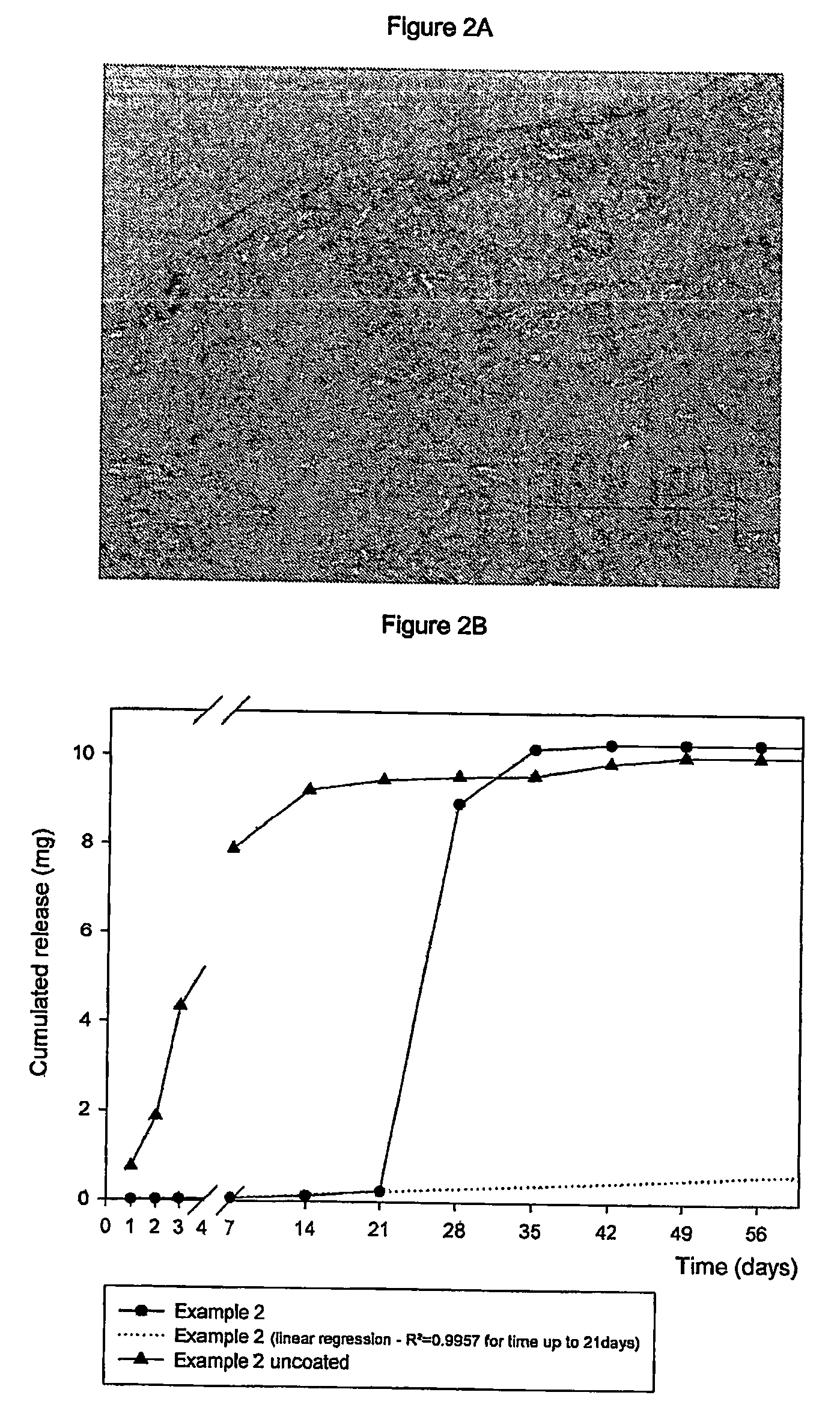
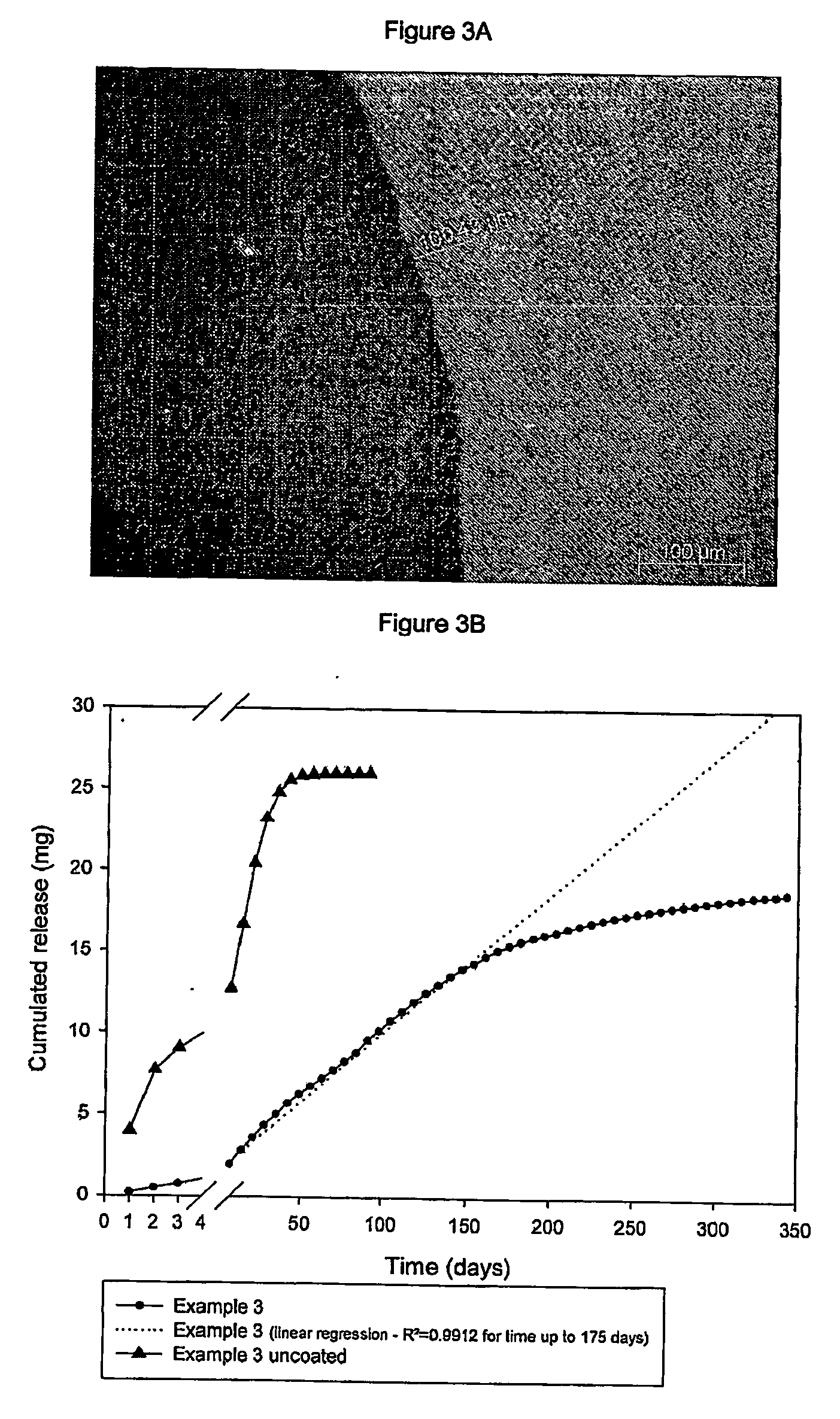
Subcutaneous implants having limited initial release of the active principle and subsequent linearly varying extended release thereof
InactiveUS20070116738A1Reduce the probability of spreadingMitigating burst releasePowder deliveryBiocideMedicineSubcutaneous implant
Subcutaneous implants having limited initial release of the active principle and subsequent linearly varying extended release thereof consisting of: a core (i) comprising an active principle dispersed in a polymeric matrix of polylactic-glycolic acid (PLGA) copolymer, a coating in film form (ii), comprising as the main component a lactic-glycolic acid copolymer, and the relative processes for preparing said implants.
Owner:MEDIOLANUM PHARMACEUTICALS LTD
Bioerodible contraceptive implant and methods of use thereof
ActiveUS9980850B2Eliminate needOrganic active ingredientsFallopian occludersControlled releaseGynecology
A contraceptive drug delivery system is provided in the form of a controlled release, bioerodible pellet for subdermal implantation. The pellet is bioerodible, and provides for the sustained release of a contraceptive agent over an extended time period. Bioerosion products are water soluble, bioresorbed, or both, obviating the need for surgical removal of the implant. Methods of using the drug delivery system, including in female contraception, are also provided.
Owner:GESEA BIOSCI INC
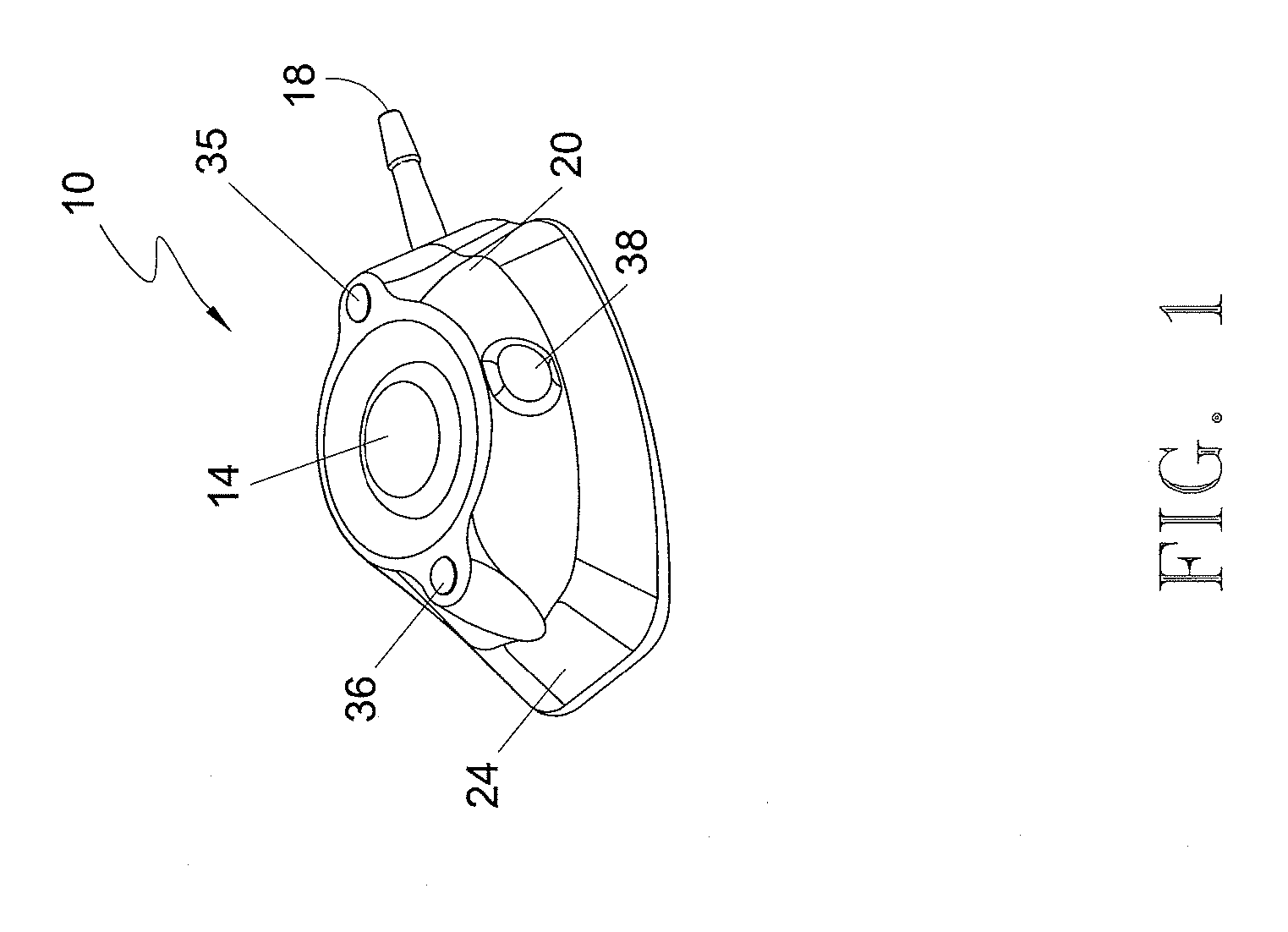
Implantable high flow multi-window vascular access port catheter
InactiveUS20150196704A1Reduce patient traumaReduce wearOther blood circulation devicesMedical devicesSkin breakdownBlood vessel
A subcutaneous implantable vascular access port reduces the risk of infection by creating a larger surface area for needle access with multiple palpable windows. These multiple needle access sites inhibit skin breakdown and thus reducing the risk of infection. This dual lumen port catheter is designed for both high pressure and high flow fluid volume blood flow rates. The port is suitable for dialysis, electrophoresis and a variety of other medical treatment functions. The interchangeable replaceable parts of the port assembly allow easier maintenance and more precise placement positions within the patient.
Owner:ADLER GRIT ARMSTRONG
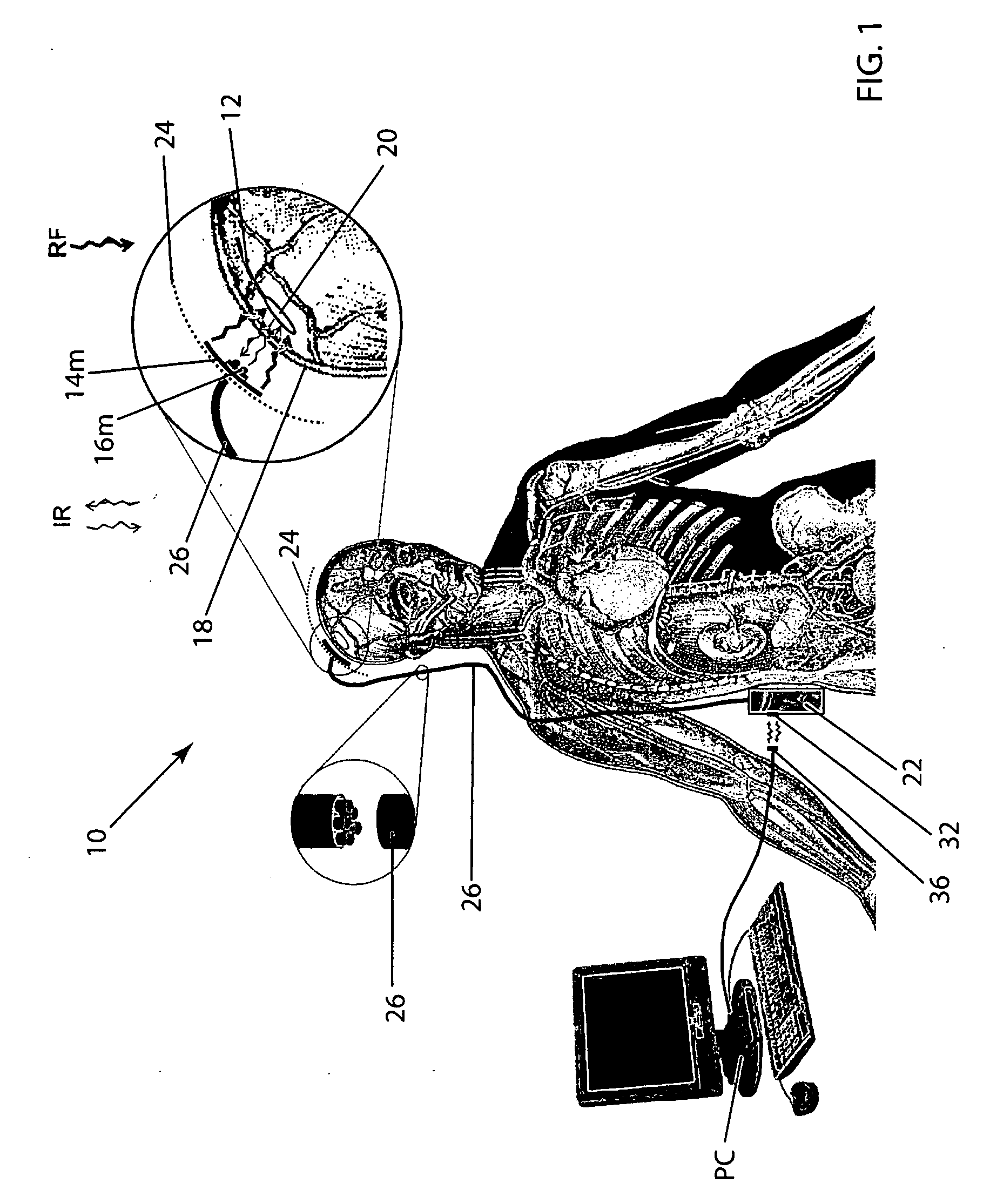
Wireless system for epilepsy monitoring and measurement
ActiveUS8165684B2Simple systemAccurate informationElectroencephalographyElectrotherapyTransmitted powerEngineering
A wireless system for monitoring a patient's brain tissue including (1) a plurality of electrodes abutting brain tissue, (2) main circuitry outside the patient's body to transmit power at radio frequencies and send / receive data using infrared energy, and (3) subcutaneously-implanted remote circuitry connected to the electrodes and configured to (a) receive transmitted RF power, (b) capture and digitize EEG signals from the electrodes, and (c) send / receive data to / from the main circuitry using IR energy, including sending digitized EEG signals from each electrode to capture the full bandwidth of each EEG signal. The system preferably includes circuitry to measure the electrical impedance of each electrode for real-time monitoring of the condition of the electrode / tissue interfaces to enhance interpretation of captured EEG signals.
Owner:TECH MEDICAL INSTR +1
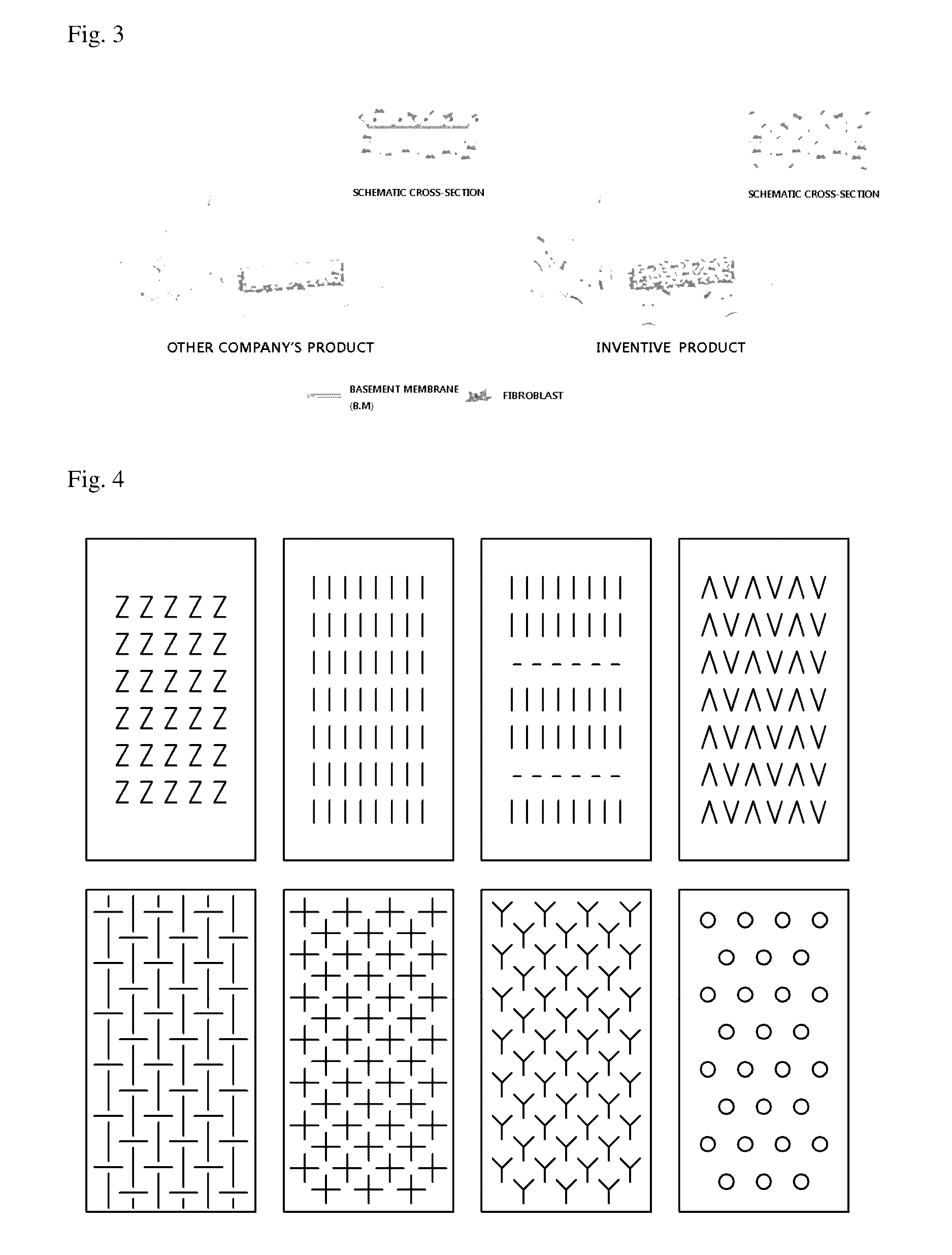
Acellular dermal graft
ActiveUS9532866B2Improves flexibility and extensibilityStablySkin implantsExtensibilityAutologous tissue
An acellular dermal graft is provided. The acellular dermal graft may be useful in minimizing side effects caused after transplantation since an environment favorable for formation of new blood vessels and proliferation of autologous tissues is provided by forming a multi-penetration structure in an acellular dermal tissue, removing a basement membrane layer and / or subjecting corners to slope cutting, and transplantation is stably performed within a short transplantation time due to improved extensibility and flexibility of tissues. The acellular dermal graft may be useful in reducing a time required to recover tissues after transplantation since the transplantation is stably performed due to improved grafting reaction with a host tissue by enhancing uptake of fibroblasts and promoting angiogenic activities. Also, the acellular dermal graft may be useful in maintaining smooth external appearance since hypodermic implantation is easily performed upon transplantation, and the skin at a graft site does not protrude after transplantation.
Owner:L&C BIO CO LTD
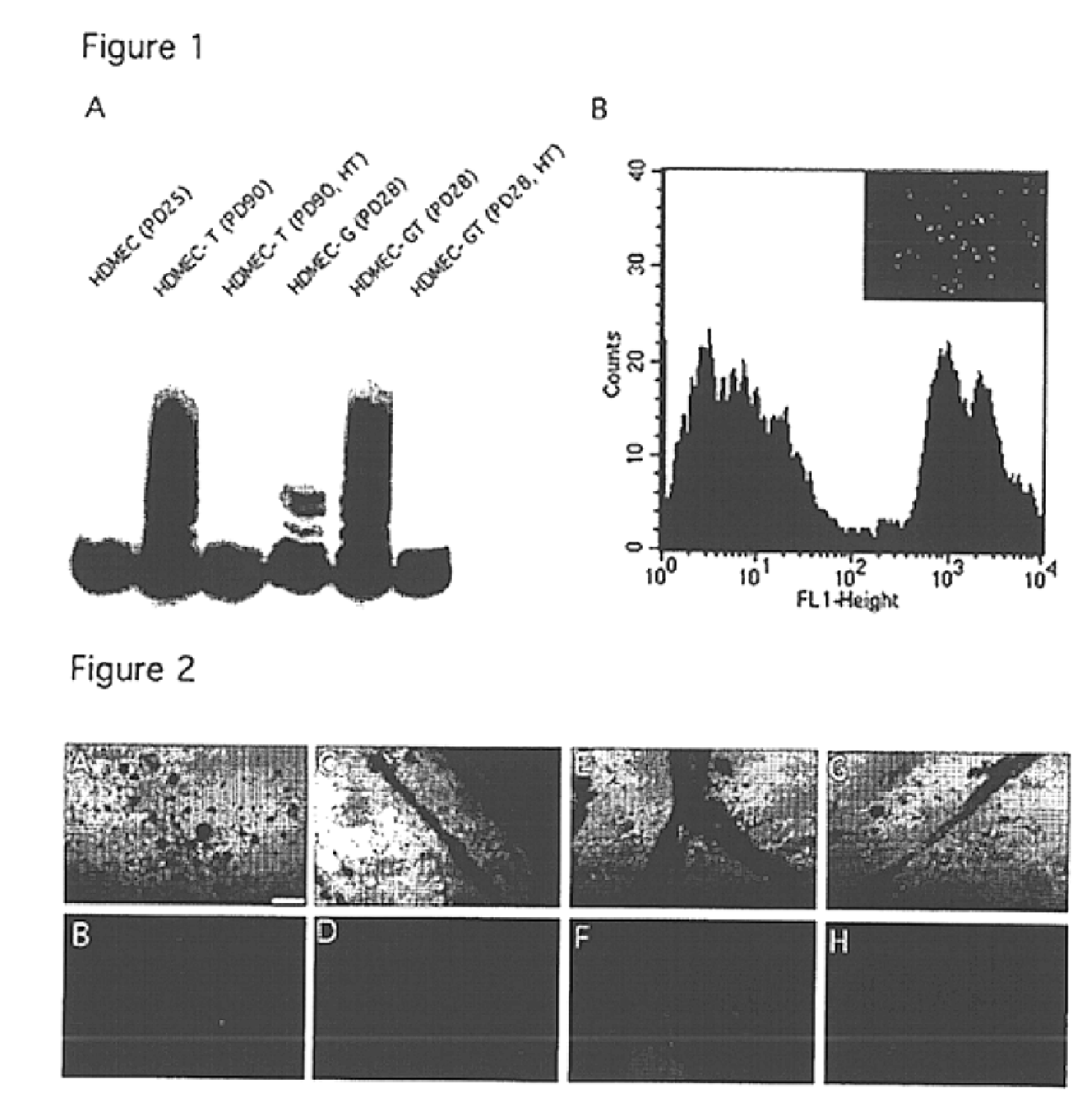
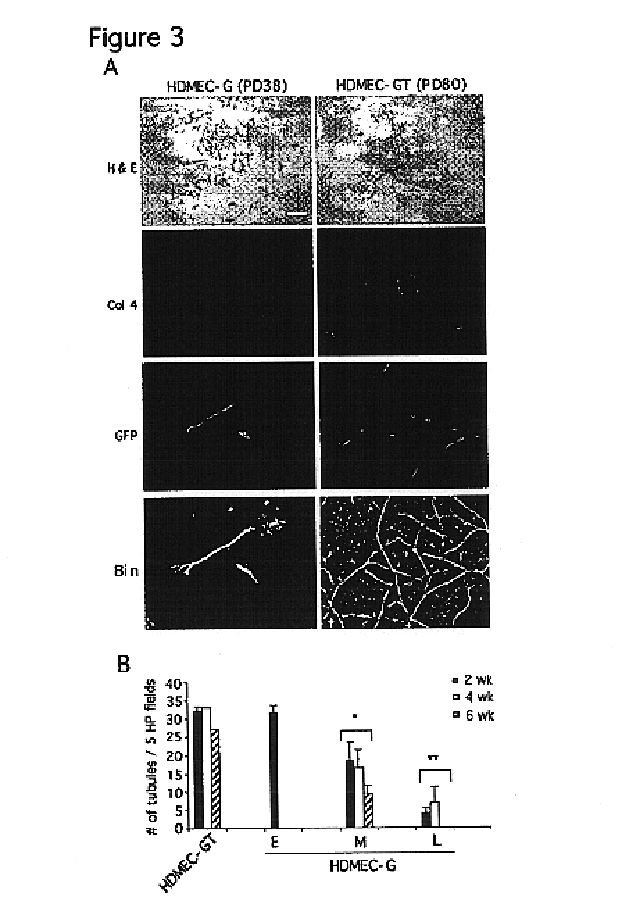
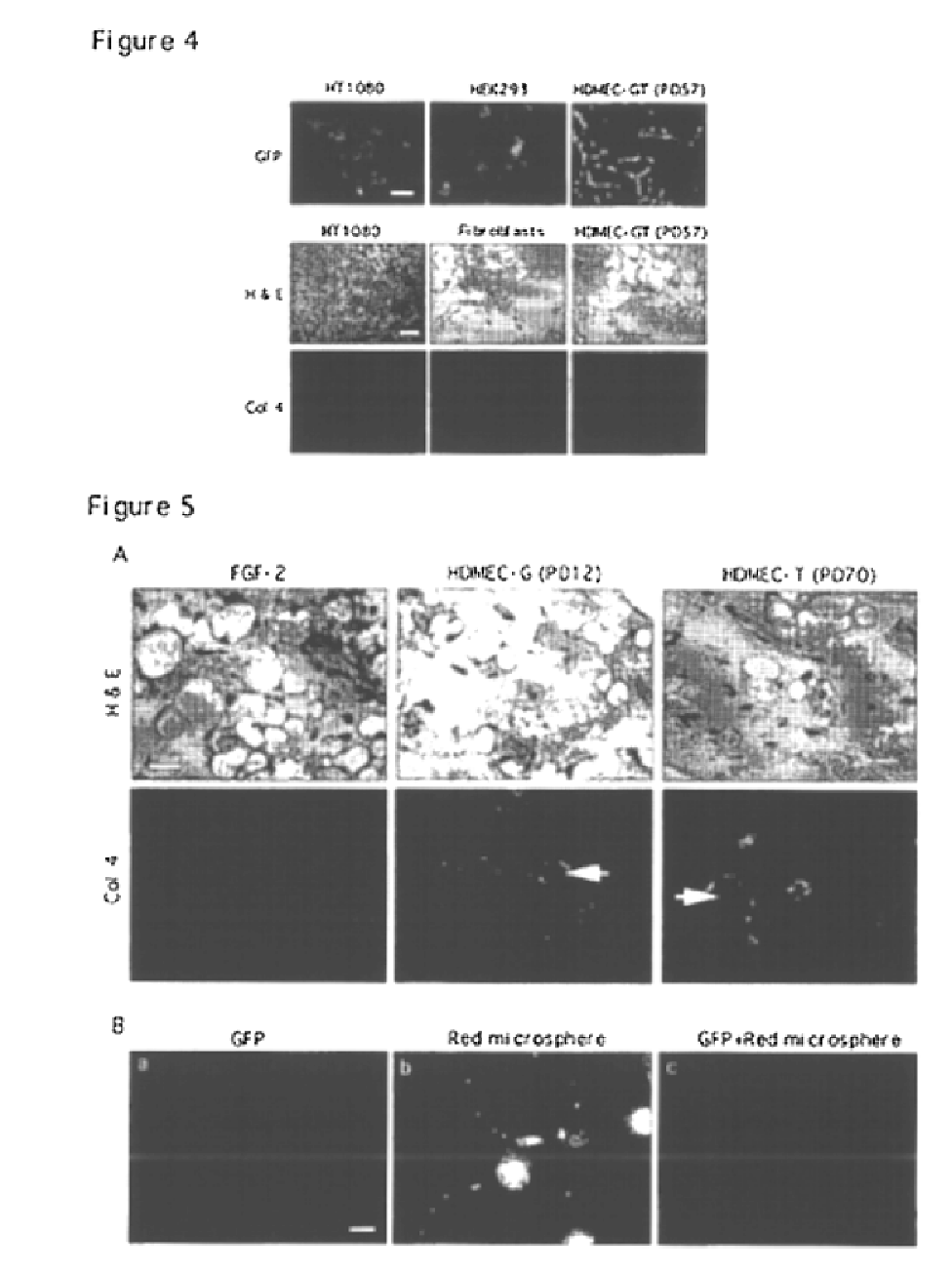
Vivo assay for anti angiogenic compounds
We report the use of telomerase-immortalized human microvascular endothelial cells in the formation of functional capillary blood vessels in vivo. Previously we showed the superior in vitro survival of human telomerase reverse transcriptase (hTERT)-transduced human endothelial cells. Here we show that retroviral-mediated transduction of hTERT in human dermal microvascular endothelial cells (HDMEC) results in cell lines that form microvascular structures when subcutaneously implanted in severe combined immunodeficiency (SCID) mice. The human origin of xenografted microvaculature was confirmed both by basement membrane immunoreactivity with anti-human type IV collagen staining and visualization of fluorescent vessels containing HDMEC that were co-transduced with hTERT and green fluorescent protein (eGFP). The lack of human vascular structures after implantation of HT1080 fibrosarcoma cells, 293 human embryonic kidney cells or human skin fibroblasts demonstrated the specificity of HDMEC at forming capillaries. Intravascular red fluorescent microspheres injected into the host circulation were found within green “telomerized” microvessels indicating functional murine-human vessel anastamoses. Whereas primary HDMEC-derived vessel density decreased steadily with time, telomerized HDMEC maintained durable vessels 6 weeks after xenografting. Modulation of implant vessel density by exposure to different angiogenic and angiostatic factors demonstrated the utility of this system for the study of human microvascular remodeling in vivo.
Owner:HERRON G SCOTT
Hair implants comprising enhanced anchoring and medical safety features
ActiveUS10682223B2Avoid bacterial infectionInhibition is effectiveSuture equipmentsSkin implantsAnatomyEngineering
A hair implant suitable for subcutaneous implantation is provided having an anchor comprising an anchor body, and at least one collagen receiving structure selected from the group consisting of at least one tunnel disposed through the anchor body and an external surface feature of the anchor body. The anchor further comprises at least one hair strand projecting from a distal end of the anchor body, wherein the at least one collagen receiving structure is configured to support collagen ligature growth after subcutaneous implantation of the hair implant so as to anchor the anchor to a hair implant recipient, and the collagen receiving structure is free of hair.
Owner:LORIA HAIR IMPLANT CO LLC
Hair implants comprising enhanced anchoring and medical safety features
ActiveUS10561490B2Avoid bacterial infectionInhibition is effectiveSkin implantsSubcutaneous implantCollagenan
A hair implant suitable for subcutaneous implantation is provided having an anchor comprising an anchor body, and at least one collagen receiving structure selected from the group consisting of at least one tunnel disposed through the anchor body and an external surface feature of the anchor body. The anchor further comprises at least one hair strand projecting from a distal end of the anchor body, wherein the at least one collagen receiving structure is configured to support collagen ligature growth after subcutaneous implantation of the hair implant so as to anchor the anchor to a hair implant recipient, and the collagen receiving structure is free of hair. A fracture line in the anchor body allows the body to fragment, thereby releasing collagen ligatures and allowing the implant fragments to “release” and fall out of the skin. The at least one hair strand may comprise a primary hair element with emerging hair elements.
Owner:LORIA HAIR IMPLANT CO LLC
Thermally reversible implant
InactiveUS20070110784A1Easily modulate physical property of gelImprove concentrationCosmetic preparationsHair cosmeticsWrinkle skinVascular implant
The invention relates to the use of a thermal reversible gel, such as a copolymer composition, as a biological filler or implant. The gel has a semi-solid form at body temperature, but upon cooling to a temperature below a threshold level, the gel is liquefied and can be re-shaped, re-sized, manipulated or removed from the body. The gel may be used as a subcutaneous implant, a biological filler, joint or tissue spacer, for wrinkle filling or other cosmetic implants, as a soft-tissue replacement for reconstructive surgery, or as a barrier within the lumen of a biological structure, such as a blood vessel. The implant may be used to provide reversible birth control by providing, for example, a reversible barrier to the cervix or a reversible blockage of the lumen of the vas deferens.
Owner:RIMON THERAPEUTICS
Heterogeneous implantable devices for drug delivery
ActiveUS20130195950A1Maintain good propertiesHigh strengthBiocideOrganic active ingredientsControl releaseSubcutaneous implantation
The present invention comprises compositions, methods and kits for delivering drugs. The invention provides an implantable device for delivery of a pharmaceutical substance to a patient, comprising a core comprising a core polymeric material optionally containing a core pharmaceutical substance, surrounded by a first layer comprising a first-layer pharmaceutical substance and a first-layer polymeric material, optionally surrounded by one or more additional layers comprising an additional pharmaceutical substance and an additional polymeric material, where the core, first, and optional additional polymeric materials may be the same or different, and where the optional core pharmaceutical substance, first-layer pharmaceutical substance, and optional additional pharmaceutical substances are the same or different. Implantation of the device allows a controlled release of drug for an extended period of time. The device may be implanted subcutaneously in an individual in need of continuous treatment with a drug.
Owner:FEDSON INC
Wireless system for epilepsy monitoring and measurement
ActiveUS8738139B2Simple systemAccurate informationElectroencephalographyElectrotherapyTransmitted powerRadio frequency
A wireless system for brain monitoring / mapping of neurological-disorder patients includes a plurality of electrodes each configured for surface abutment of brain tissue and main circuitry for placement outside a body of a patient and configured to transmit power at radio frequencies and send and receive data using infrared energy. Remote circuitry is provided for subcutaneous implantation in a head of the patient. The remote circuitry is connected to the plurality of electrodes and includes a multiplexer sampling signals from the plurality of electrodes. The multiplexer outputs electrode signals to an amplifier and A / D converter for transmission to the main circuitry. The remote circuitry is configured to (a) receive transmitted power at radio frequencies from the main circuitry, (b) capture and digitize full-bandwidth EEG signals from each of the electrodes, and (c) send data to and receive data from the main circuitry using infrared energy.
Owner:YALE UNIV +1
Extension apparatus for artificial hair implants
Owner:LORIA HAIR IMPLANT CO LLC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com