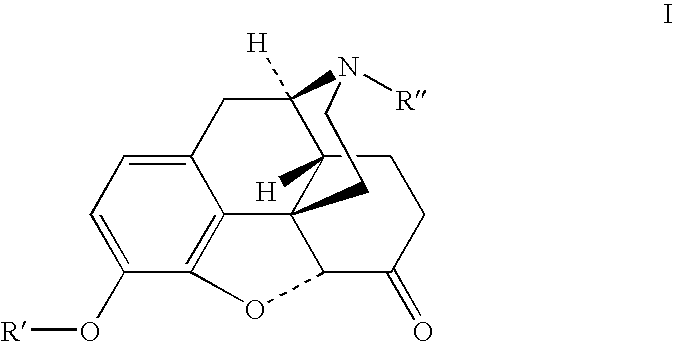
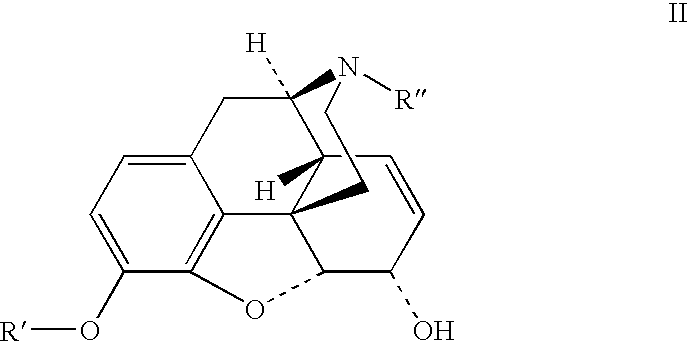
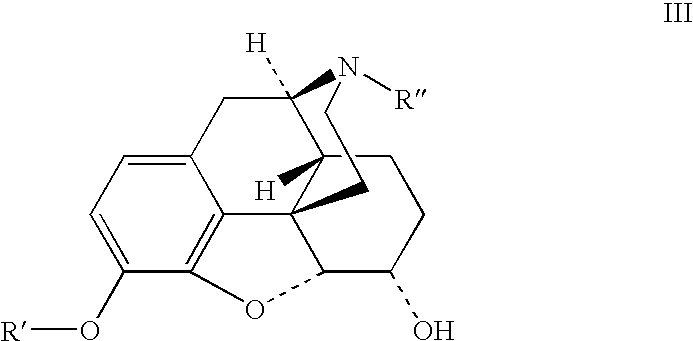
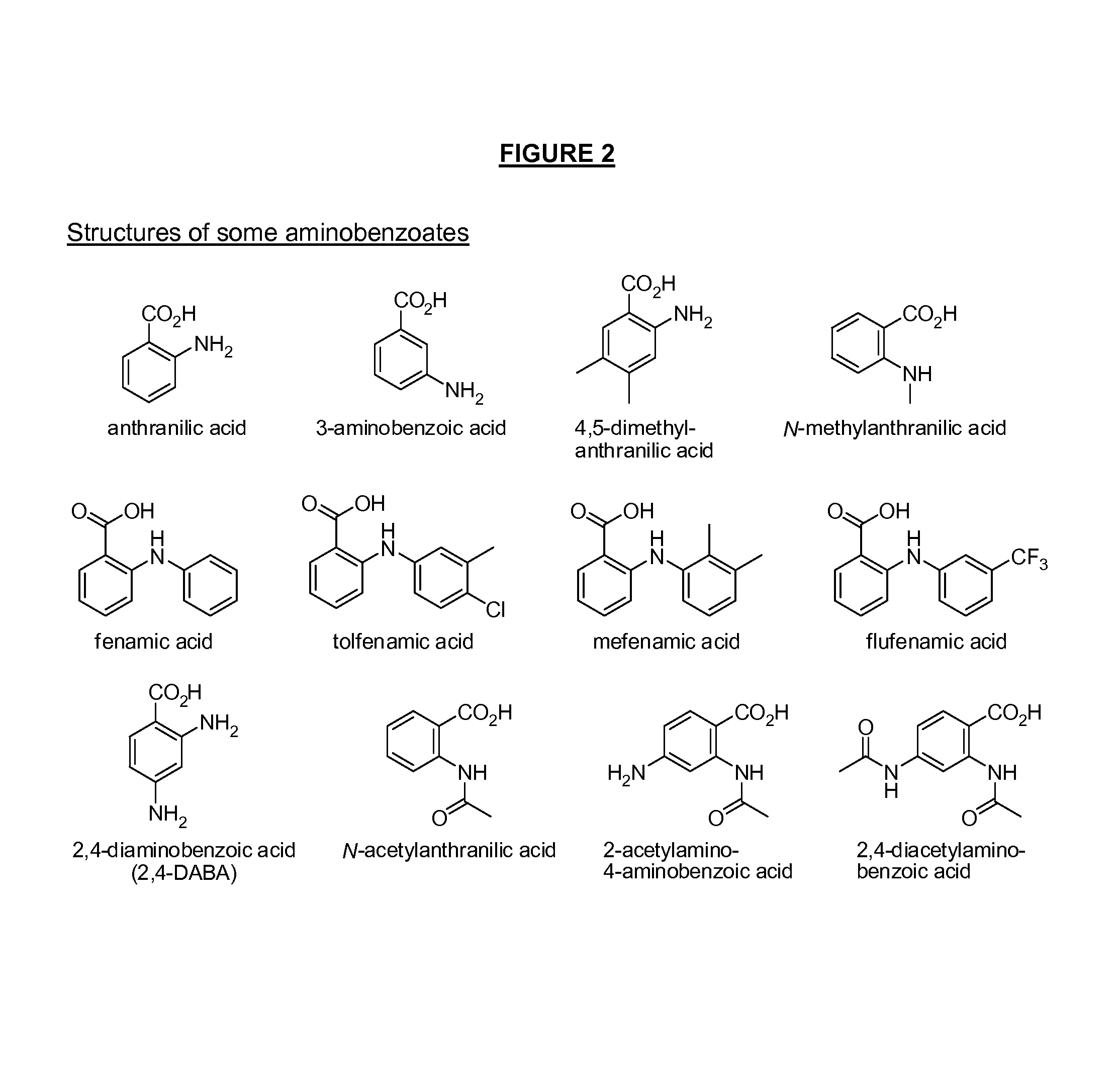
Patents
Literature
69 results about "Hydromorphone" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
This medication contains hydromorphone in a long-acting form. It is used to help relieve severe ongoing pain (such as due to cancer). Hydromorphone belongs to a class of drugs known as opioid (narcotic) analgesics.
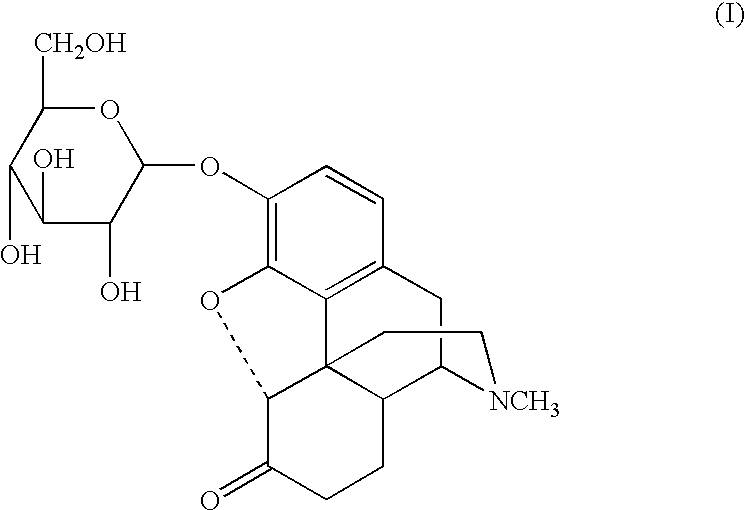
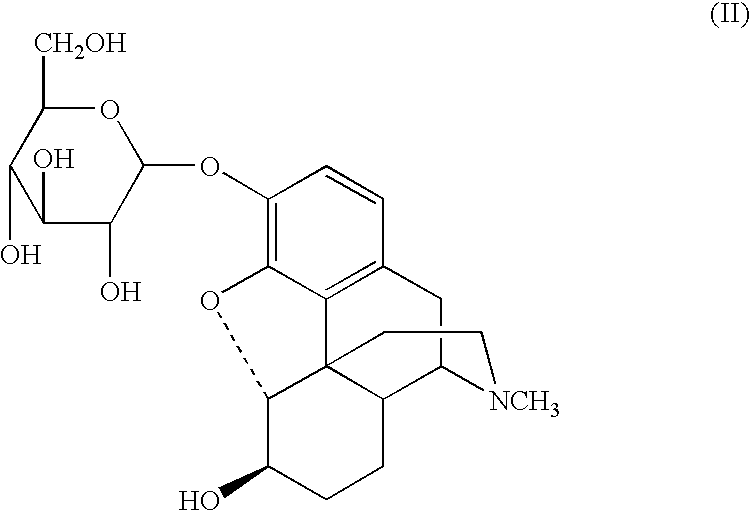
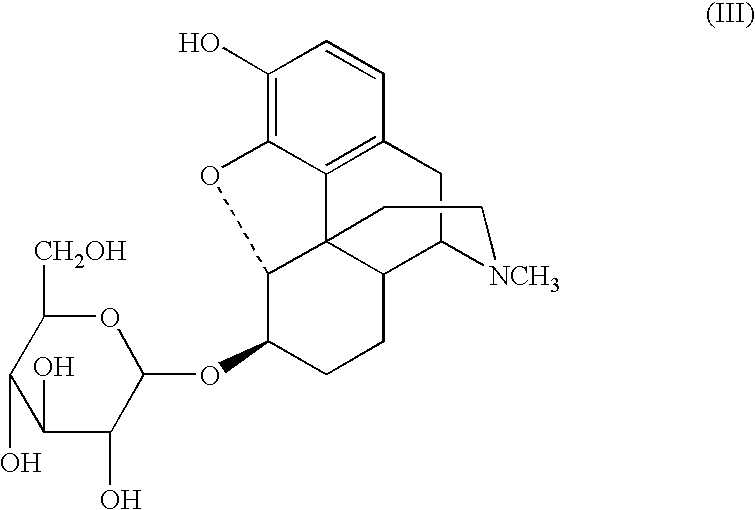
Sugar derivatives of hydromorphone, dihydromorphine and dihydroisomorphine,compositions thereof and uses for treating or preventing pain
Glucoside and glucuronide derivatives of hydromorphone, dihydromorphine, and dihydroisomorphine and pharmaceutically acceptable salts thereof; pharmaceutical compositions comprising a glucoside or glucuronide derivative of hydromorphone, dihydromorphine, or dihydroisomorphine or a pharmaceutically acceptable salt thereof; and methods for treating or preventing pain in a patient comprising administering to a patient in need thereof a glucoside or glucuronide derivative of hydromorphone, dihydromorphine, or dihydroisomorphine or a pharmaceutically acceptable salt thereof are disclosed.
Owner:PURDUE PHARMA LP
Opiod tannate compositions
A composition comprising the tannate of an opioid. Suitable opioids include alfentanil, buprenorphine, butorphanol, carfentanil, cocaine, codeine, dezocine, diacetylmorphine, dihydrocodeine, dihydromorphine, diphenoxylate, diprenorphine, etorphine, fentanyl, heroin, hydrocodone, hydromorphone, beta-hydroxy-3-methylfentanyl, levo-alpha-acetylmethadol, levorphanol, lofentanil, meperidine, methadone, morphine, nalbuphine, nalmefene, o-methylnaltrexone, naloxone, naltrexone, oxycodone, oxymorphone, pentazocine, pethidine, propoxyphene, remifentanil, sufentanil, tilidine and tramadol. The opioid tannate may be readily prepared by reacting an opioid free base with tannic acid, either neat or in the presence of up to about 30 wt. % water, at a temperature of about 60 to about 150° C. and thereafter recovering the resultant opioid tannate. The opioid tannate may also be prepared by an alternative process that involves reacting the opioid free base with water at a temperature such that not more than about 10 wt. % of the opioid tannate will be decomposed and thereafter removing the water by freeze-drying.
Owner:JAME FINE CHEM
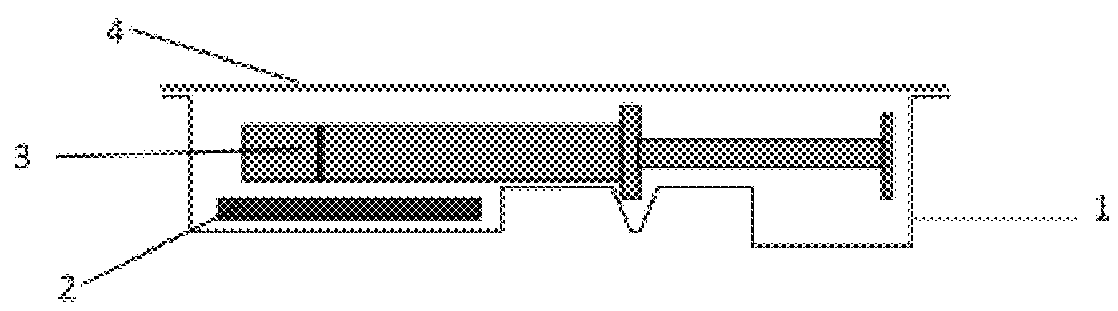
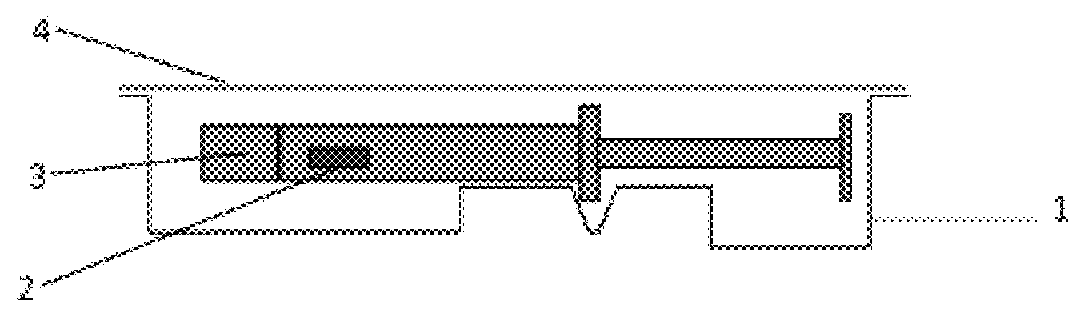
Subcutaneous implant
InactiveUS6126956AEasy to modifyEasy for flexibilityOrganic active ingredientsNervous disorderControl releaseSubcutaneous implant
A non-abusable, non-inflammatory, biocompatible, non-biodegradable, subcutaneous, polymeric implant for the prolonged, controlled release of hydromorphone with near zero-order kinetics is described. Methods of alleviating cancer pain and treating opioid drug addiction with the implant are also described.
Owner:AXXIA PHARMA
Process and compounds for the production of (+)opiates
The invention generally provides processes and intermediate compounds useful for the production of (+)-opiates. Non-limiting examples of (+) opiates that may be derived from one or more compounds of the invention include (+)-noroxymorphone, (+)-naltrexone, (+)-naloxone, (+)-N-cyclopropylmethylnorhydrocodone, (+)-N-cycloproylmethylnorhydromorphone, (+)-N-allylnorhydrocodone, (+)-N-allylnorhydromorphone, (+)-noroxycodone, (+)-naltrexol, (+)-naloxol, and (+)-3-O-methyl-naltrexone.
Owner:SPECGX LLC
Hydrophobic abuse deterrent delivery system for hydromorphone
Disclosed herein are oral dosage forms of hydromorphone that are resistant to abuse and methods of their formulation. In particular, oral dosage forms that are resistant to dissolution in aqueous solutions of ethanol are described.
Owner:LAB INT
Gastro-resistant and ethanol-resistant controlled-release formulations comprising hydromorphone
The invention provides a controlled-release composition comprising hydromorphone or a pharmaceutically acceptable form thereof in association with a gastro-resistant and ethanol-resistant compound and method of use thereof.
Owner:SPECTOR DONALD
Preparation of opioid analgesics by a one-pot process
A one-pot process for preparing opioid analgesics such as hydrocodone, hydromorphone, and analogues thereof by reacting codeine, morphine, and analogues thereof with hydrogen in a solvent system of benzophenone and neutral solvent in the presence of a metal catalyst followed by oxidation in the presence of potassium tert-alkylate.
Owner:ACURA PHARMA
Packaging system for oxygen-sensitive drugs
ActiveUS9248229B2Lower Level RequirementsOrganic active ingredientsFlexible coversPromethazineMorphine
Described herein are pharmaceutical packaging systems which prevent oxidative degradation of morphine, hydromorphone, promethazine and other oxygen-sensitive drugs, such systems including a syringe with an oxygen permeable tip cap, a hermetically sealed oxygen barrier blister packaging with very low permeability to oxygen and comprises ethylene vinyl, and an oxygen absorber.
Owner:FRESENIUS KABI DEUT GMBH
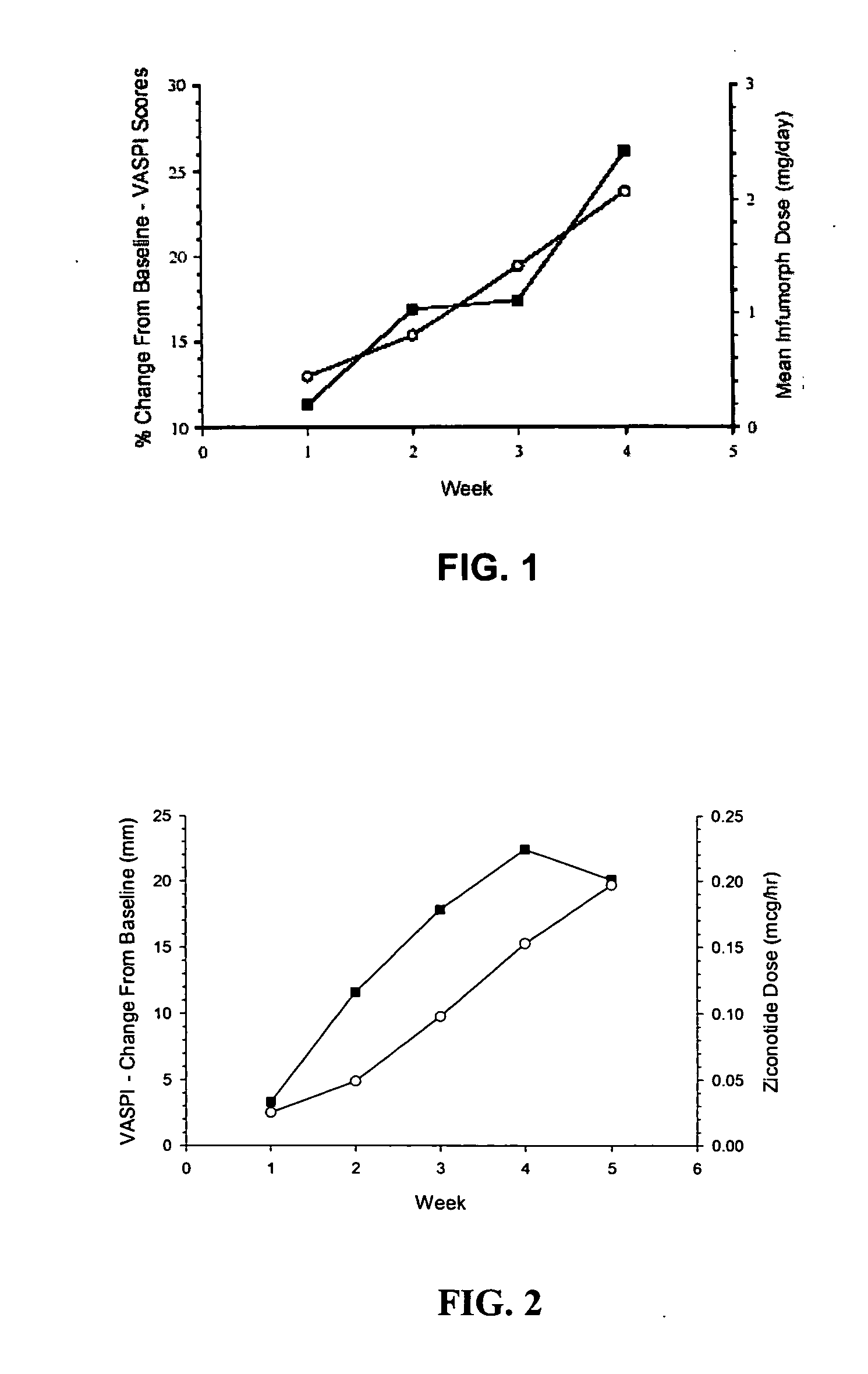
Method for reducing pain
ActiveUS20050192218A1Relieve painRetain potencyBiocideNervous disorderIntrathecal usePharmaceutical formulation
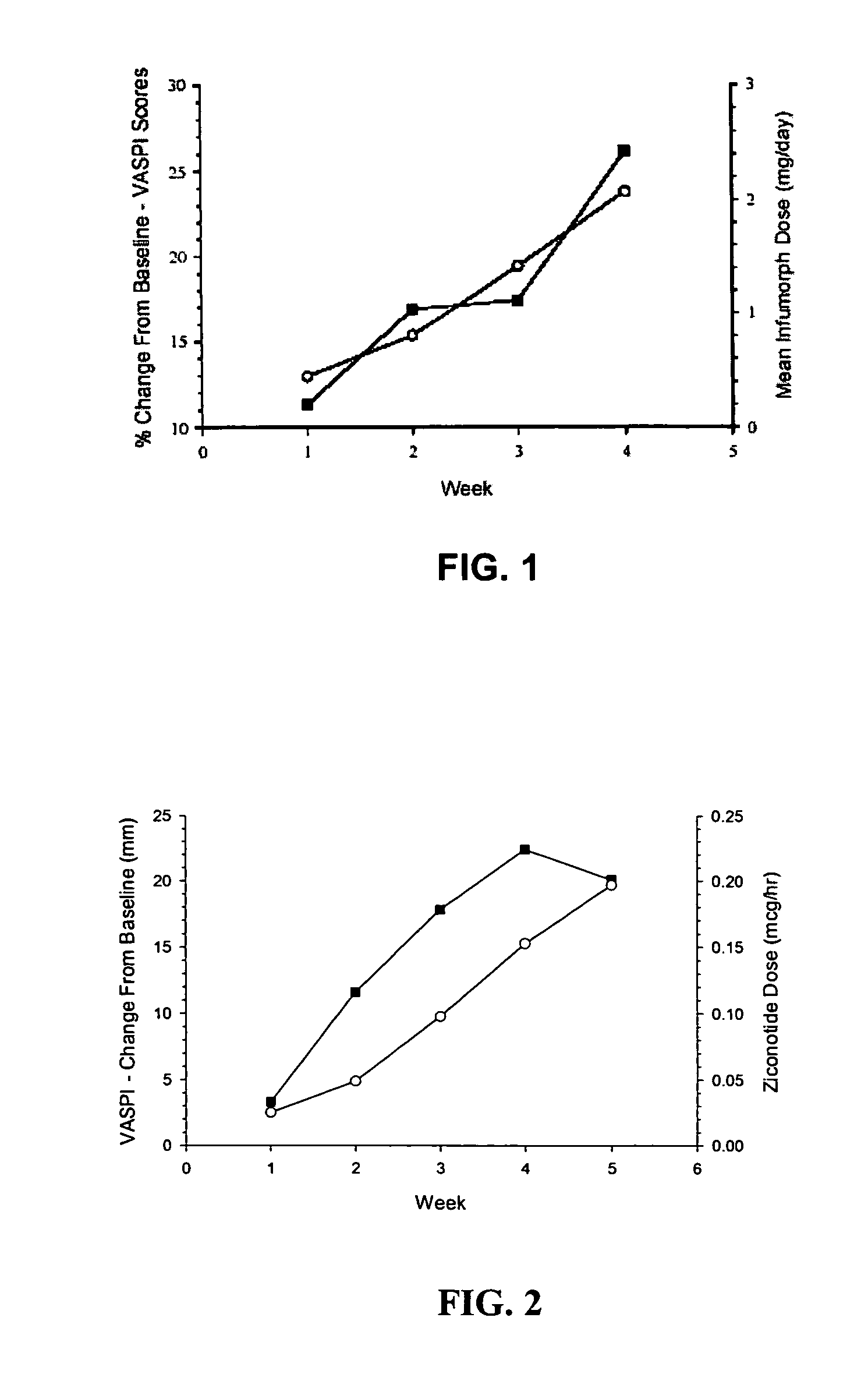
The present invention is direct to a method of producing analgesia in a mammalian subject. The method includes administering to the subject an omega conopeptide, preferably ziconotide, in combination with an analgesic selected from the group consisting of morphine, bupivicaine, clonidine, hydromorphone, baclofen, fentanyil, buprenorphine, and sufentanil, or its pharmaceutically acceptable salts thereof, wherein the ω-conopeptide retains its potency and is physically and chemically compatible with the analgesic compound. A preferred route of administration is intrathecal administration, particularly continuous intrathecal infusion. The present invention is also directed to a pharmaceutical formulation comprising an omega conopeptide, preferably ziconotide, in combination with an analgesic selected from the group consisting of morphine, bupivicaine, clonidine, hydromorphone, baclofen, fentanyl, buprenorphine, and sufentanil.
Owner:TERSERA THERAPEUTICS LLC
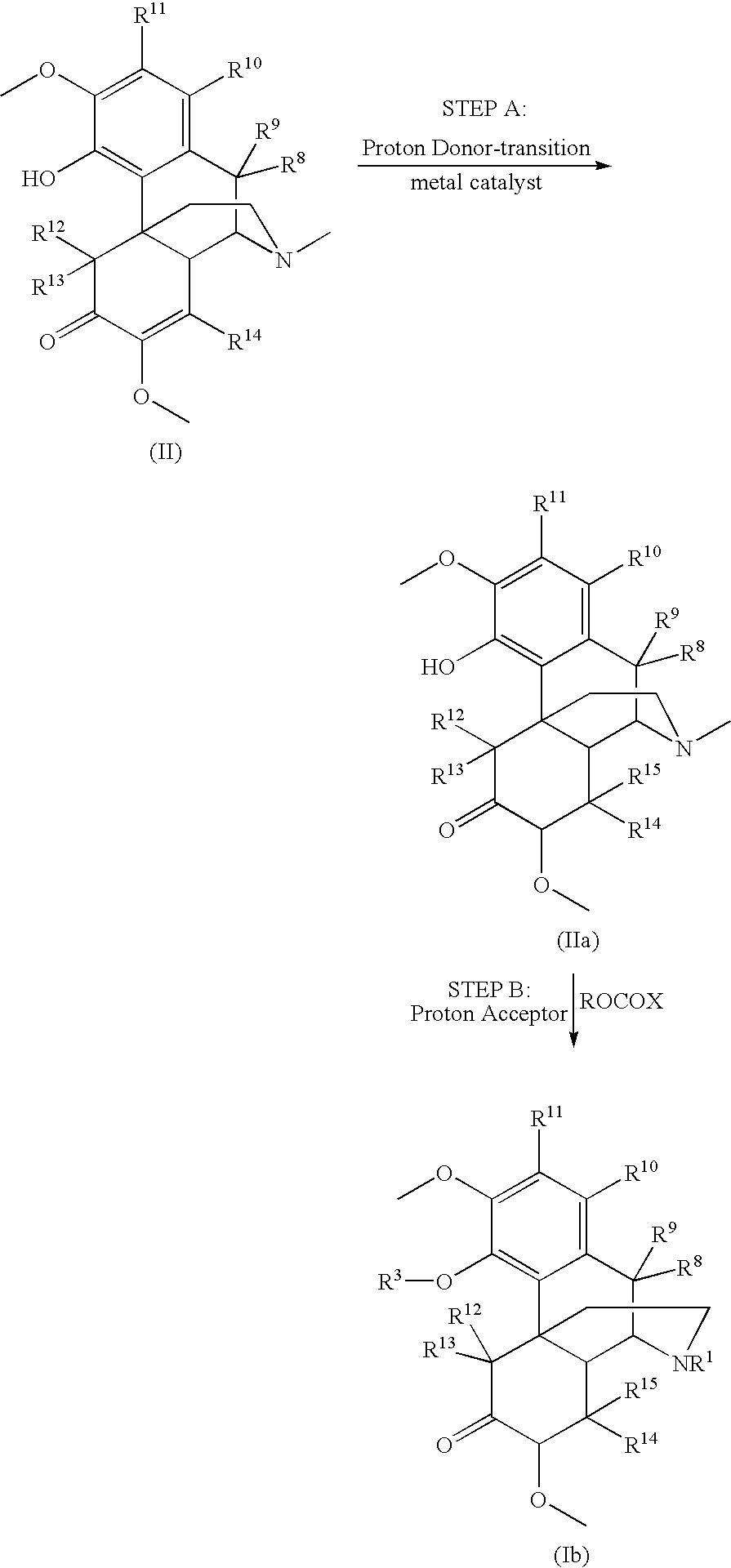
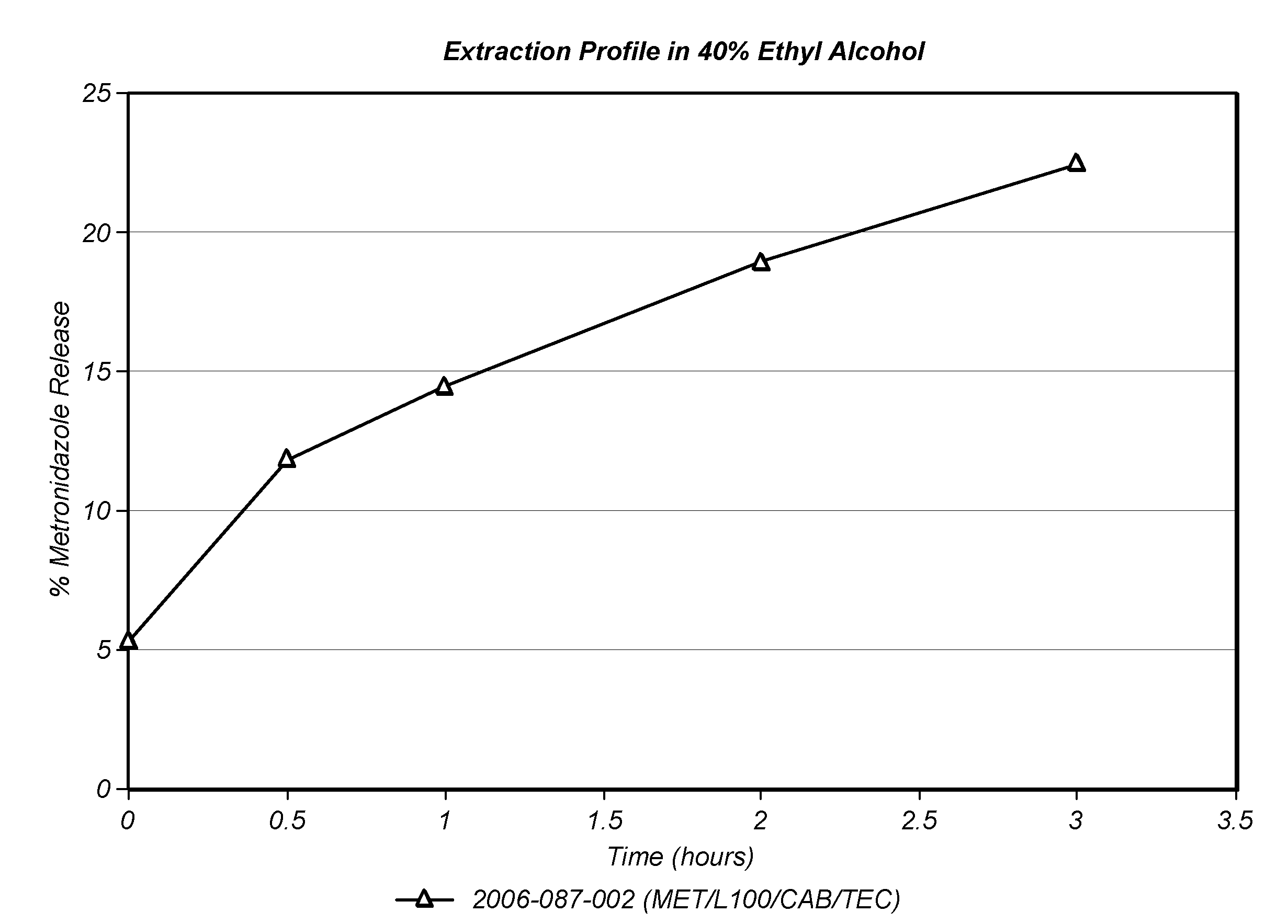
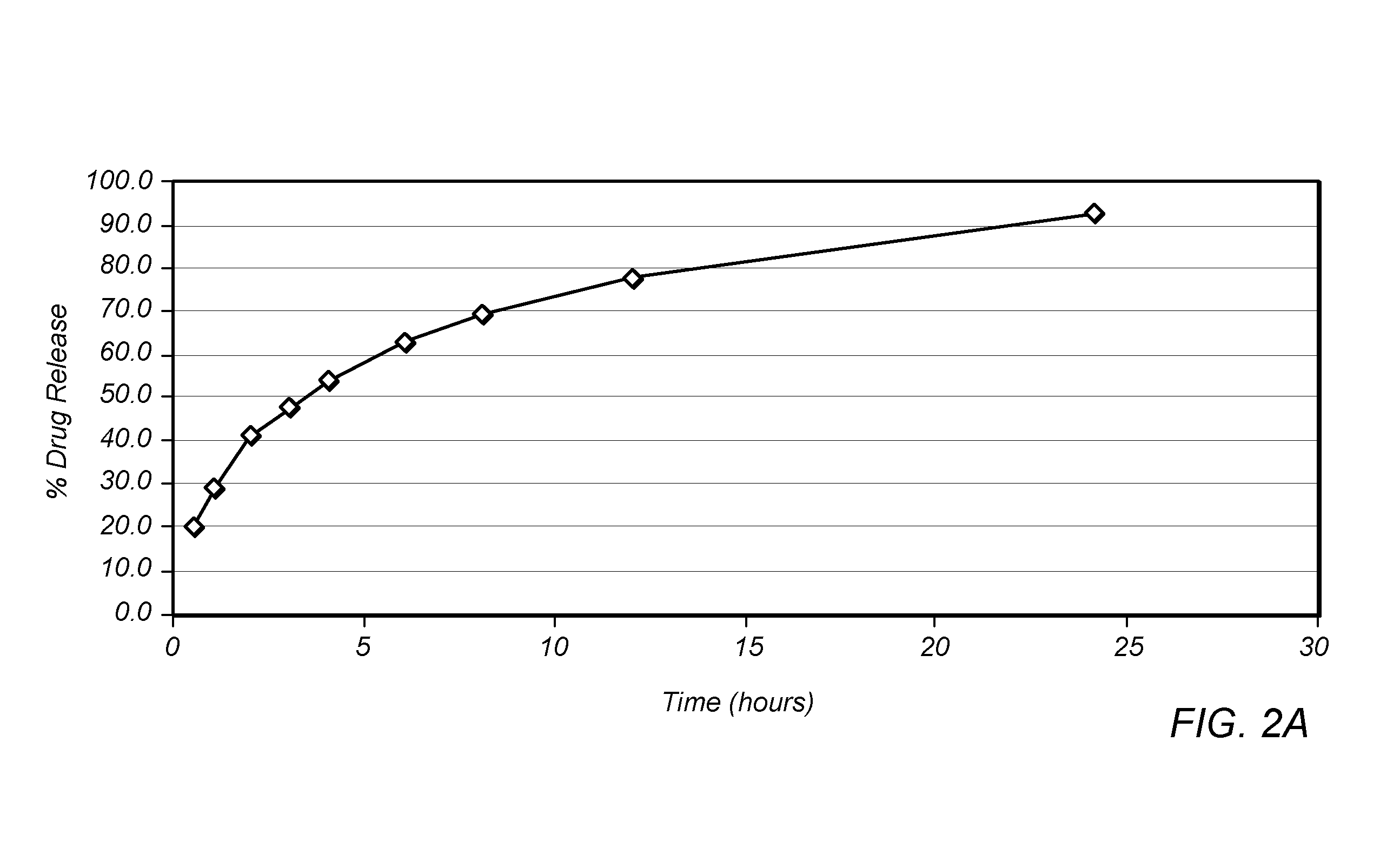
Methods of Reducing Alcohol-Induced Dose Dumping for Opioid Sustained Release Oral Dosage Forms
Disclosed are methods of sustained release administration of opioids, including but not limited to hydromorphone and oxycodone, that exhibit improved properties with respect to co-ingestion with aqueous alcohol.
Owner:ALZA CORP
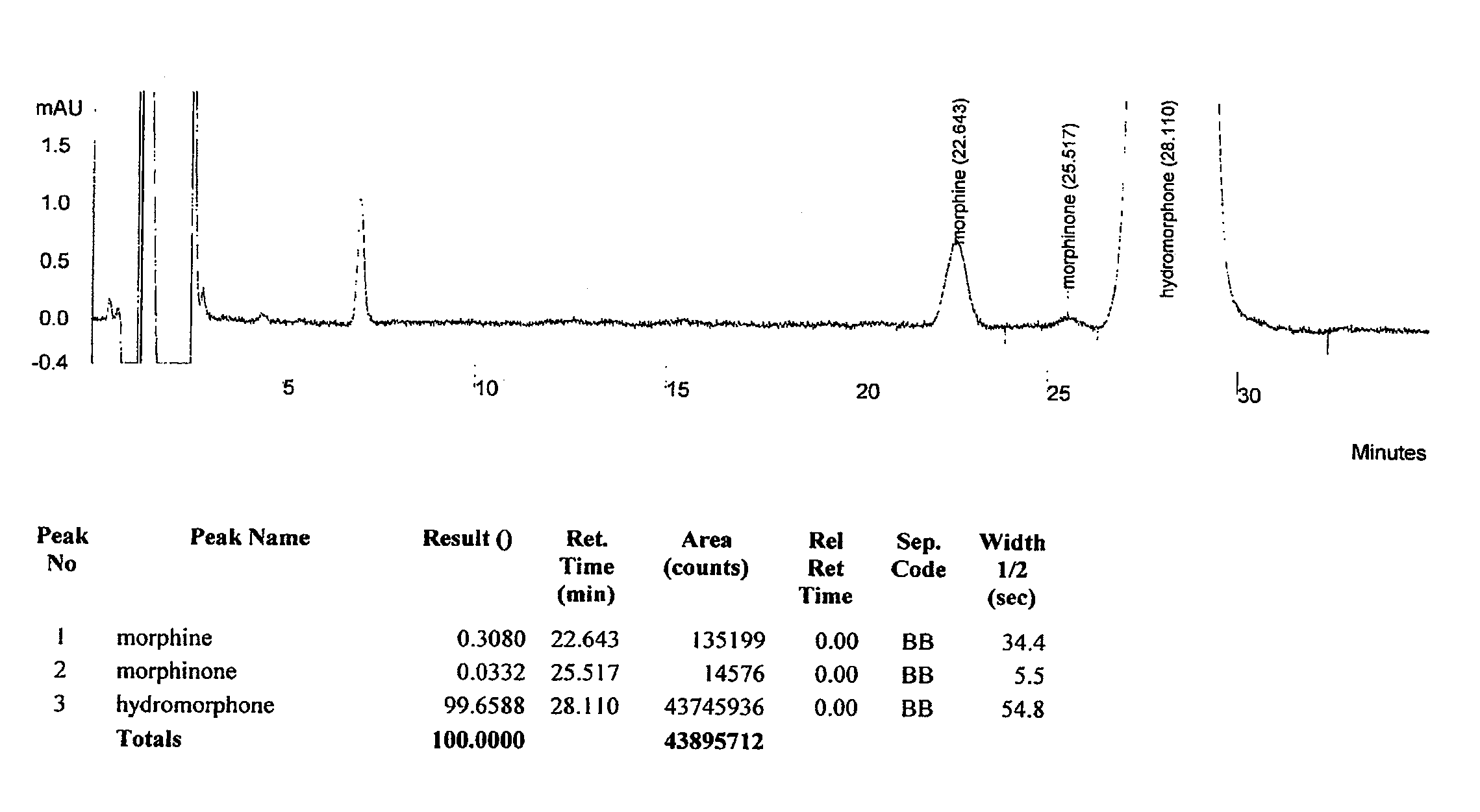
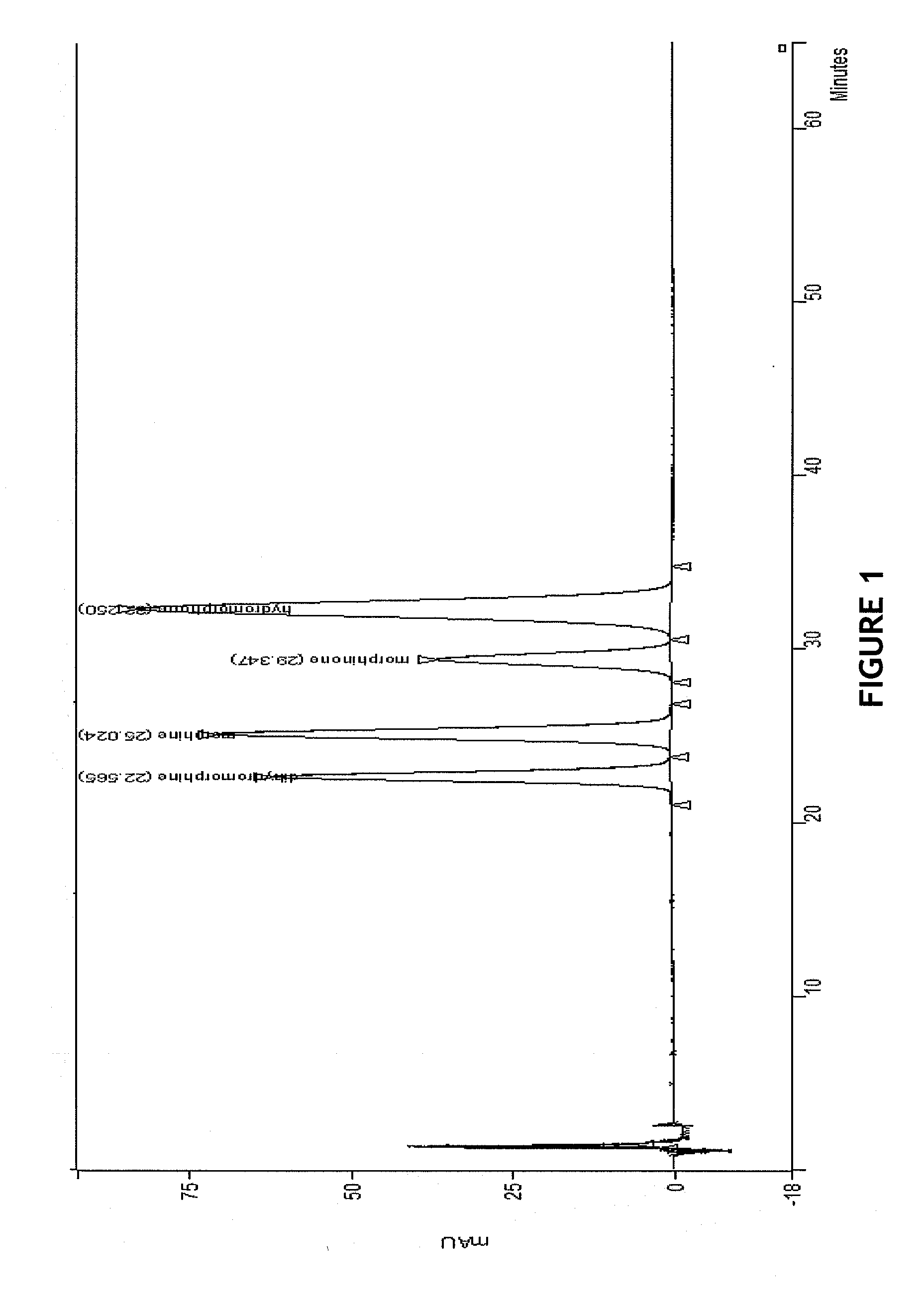
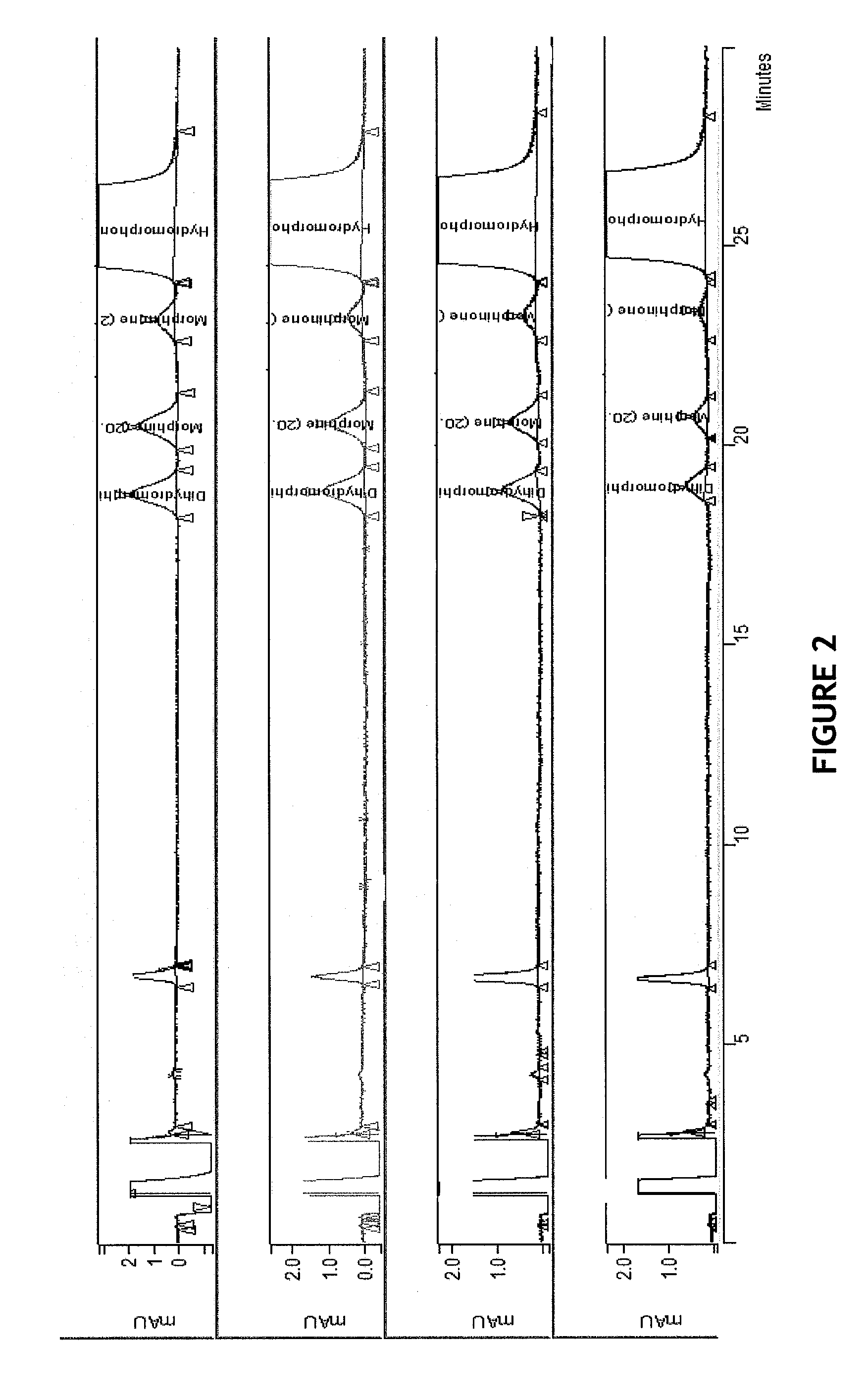
HPLC method for separation and detection of hydromorphone and related opioid pharmacophores
InactiveUS20080206883A1Component separationColor/spectral properties measurementsHplc methodPharmacophore
HPLC methods are provided to separate and detect morphinone, morphine, and dihydromorphine in the presence of hydromorphone. The isocratic HPLC methods employ ion-pair solute-solute ion-exchange mobile phase techniques in reversed phase chromatography. Method conditions in the disclosure provide separation and quantification of opioid pharmacophores in accordance with federal guidelines for obtaining resolution between analytes R≧2.0; tailing factor T≦2.0, capacity factor 2<k′≦50, and theoretical plate number N≧2000 for each opioid analyte peak.
Owner:CODY LAB
Pharmaceutical composition
A pharmaceutical composition comprising an analgesic or analgesic combination and a stool softener is disclosed. The analgesic is selected from morphine, meperidine, fentanyl, hydromorphone, oxymorphone, oxycodone, hydrocodone, methadone, propoxyphene, pentazocine, levorphanol, codeine, acetaminophen and combinations of these analgesics. The composition is formulated for oral administration as a liquid or solid dosage form for immediate, slow, delayed or sustained-release characteristics.
Owner:BRANDED PRODS FOR THE FUTURE
Hydromorphone therapy
A hydromorphone composition, a hydromorphone dosage form and a method for administering hydromorphone are disclosed, indicated for the management of pain.
Owner:MERRILL SONYA +3
Method for reducing pain
ActiveUS7268109B2Nervous disorderPeptide/protein ingredientsPharmaceutical formulationAnalgesic agents
The present invention is direct to a method of producing analgesia in a mammalian subject. The method includes administering to the subject an omega conopeptide, preferably ziconotide, in combination with an analgesic selected from the group consisting of morphine, bupivicaine, clonidine, hydromorphone, baclofen, fentanyil, buprenorphine, and sufentanil, or its pharmaceutically acceptable salts thereof, wherein the ω-conopeptide retains its potency and is physically and chemically compatible with the analgesic compound. A preferred route of administration is intrathecal administration, particularly continuous intrathecal infusion. The present invention is also directed to a pharmaceutical formulation comprising an omega conopeptide, preferably ziconotide, in combination with an analgesic selected from the group consisting of morphine, bupivicaine, clonidine, hydromorphone, baclofen, fentanyl, buprenorphine, and sufentanil.
Owner:TERSERA THERAPEUTICS LLC
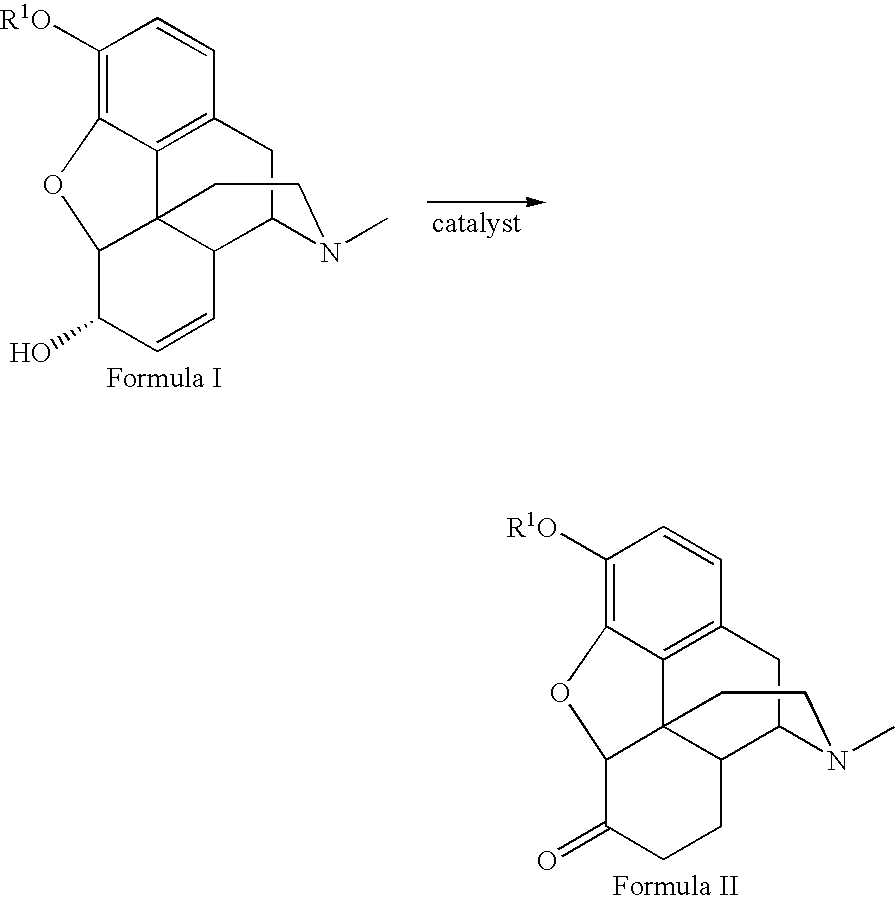
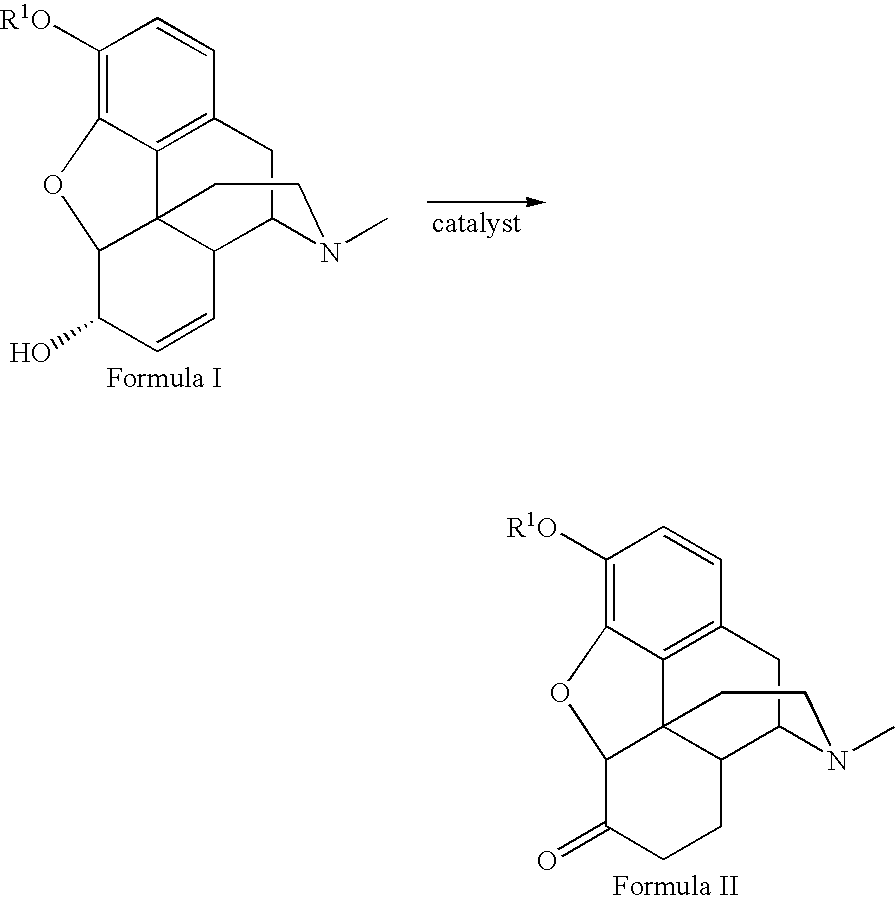
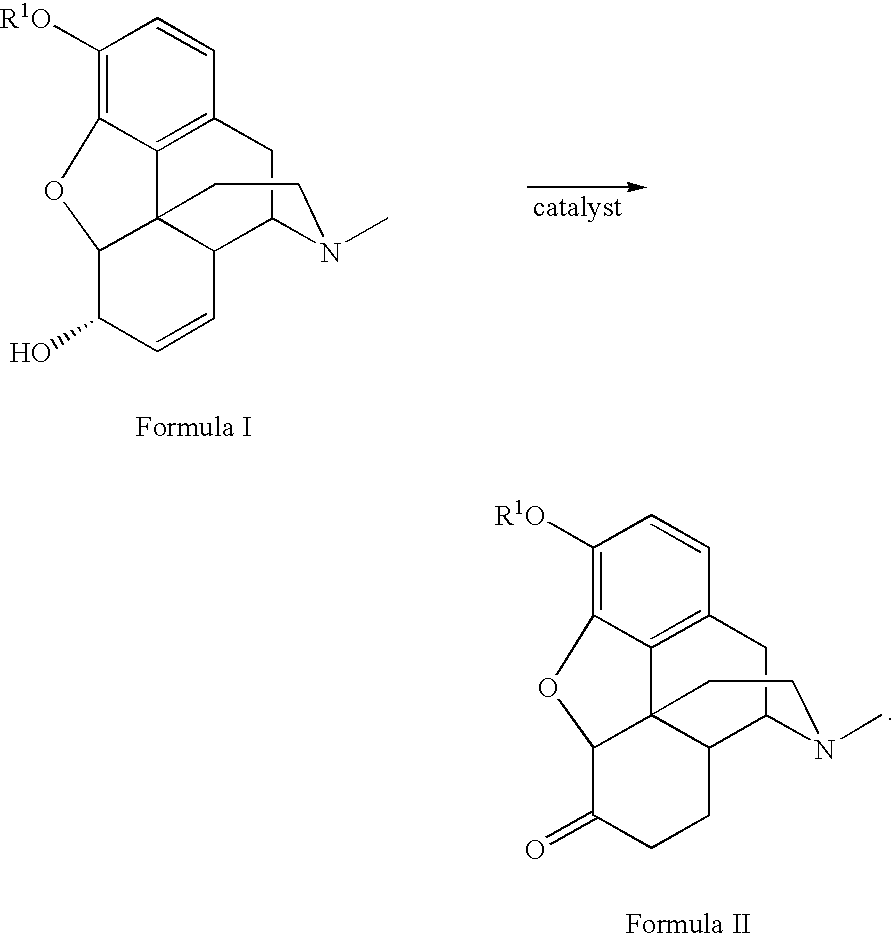
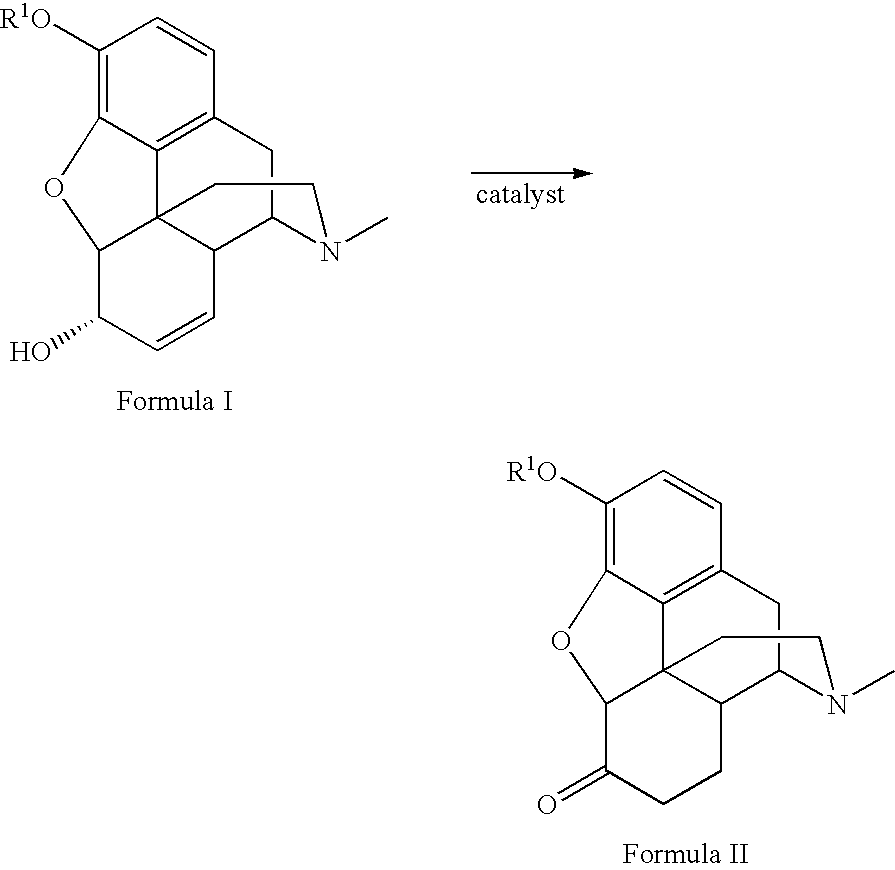
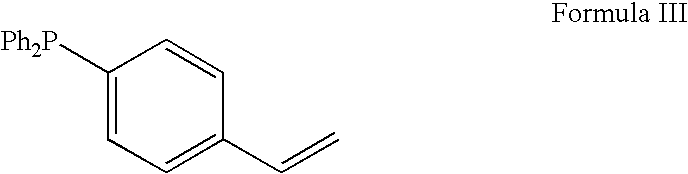
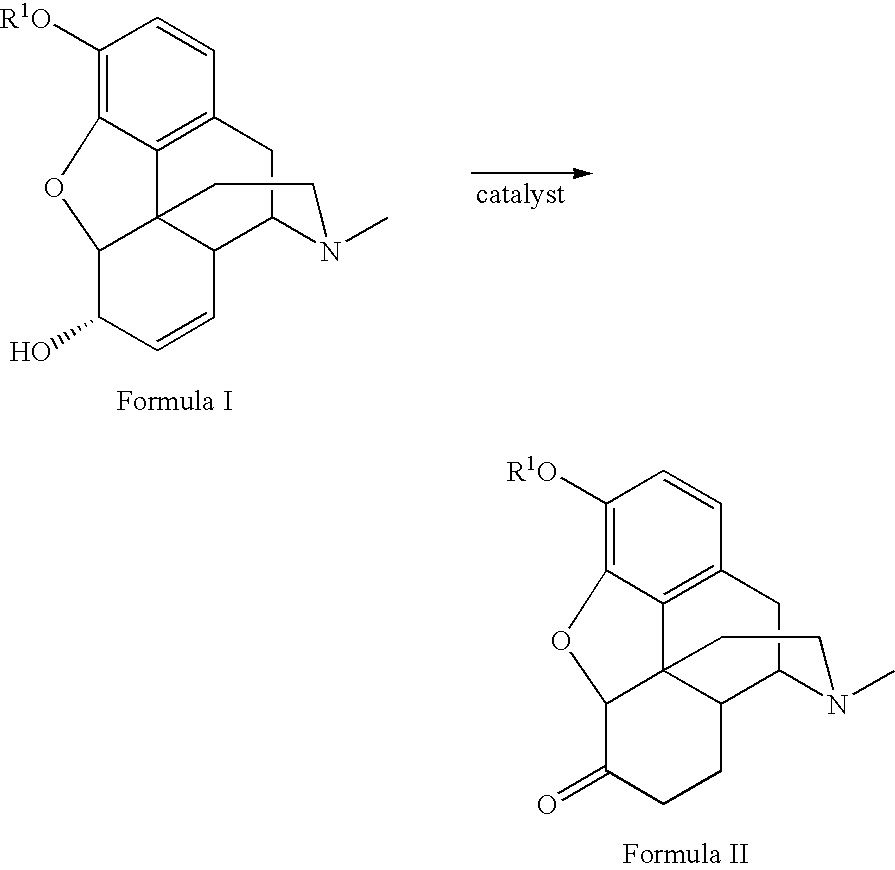
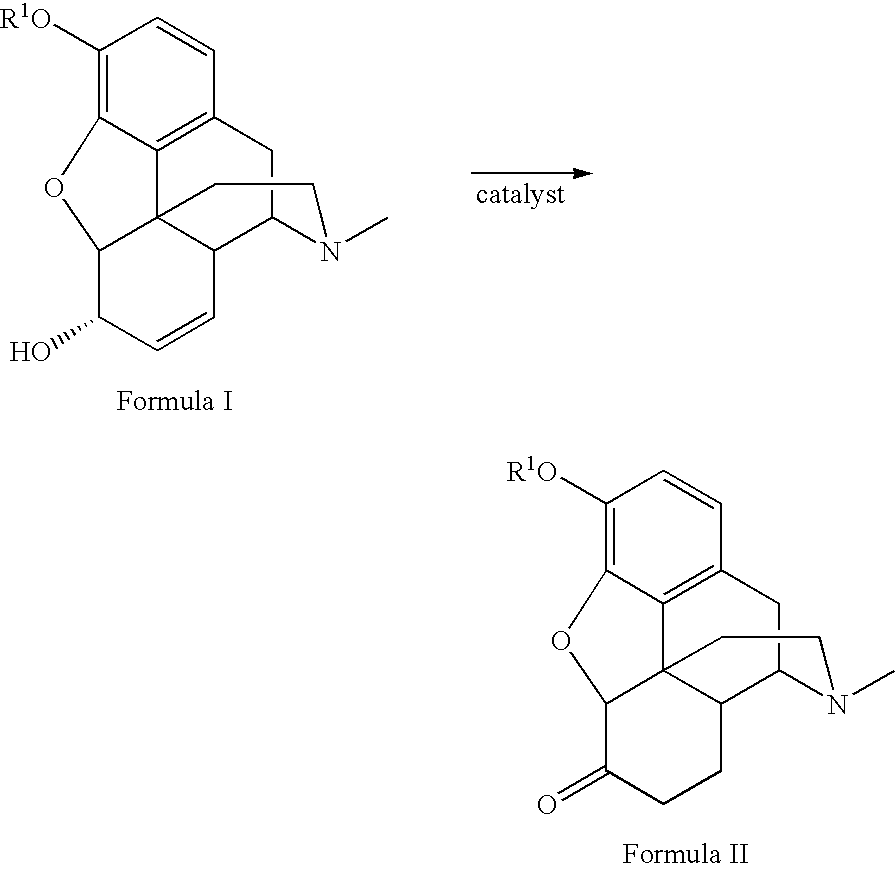
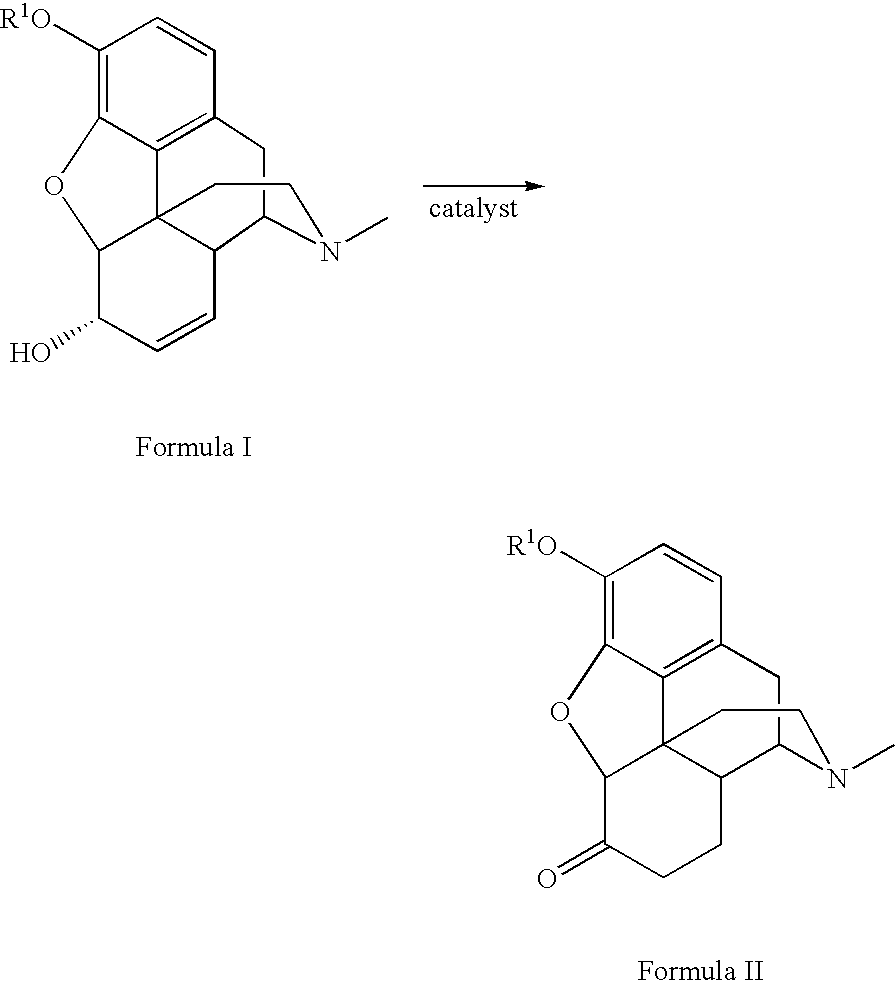
Method for the catalytic production of hydrocodone and hydromorphone
A method for the catalytic conversion of codeine, morphine or analogs thereof into hydrocodone, hydromorphone or analogs thereof utilizing a transition metal complex of a tertiary phosphine halide as catalyst.
Owner:MALLINCKRODT INC
Method for the catalytic production of hydrocodone and hydromorphone
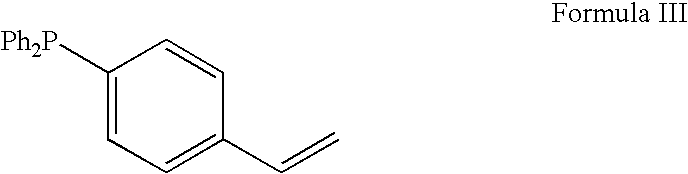
InactiveUS7321038B2Organic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsArylHydrocodone
A method for the catalytic conversion of codeine, morphine or analogs thereof into hydrocodone, hydromorphone or analogs thereof utilizing a transition metal catalyst of the formula [M(PR3R4R5)nXm]p; wherein R1 is H, alkyl, aryl or acyl; M is a Group VIII transition metal; R3, R4 and R5 are selected from the group consisting of alkyl, aryl, alkoxyl, phenoxyl and combinations thereof; X is a halide or an anion; n is 1, 2, 3 or 4; m is 1 or 2; and p is at least 1.
Owner:MALLINCKRODT INC
High Concentration Formulations of Opioids and Opioid Derivatives
InactiveUS20110136847A1Potent and concentratedReduce riskBiocideNervous disorderHigh concentrationMorphine
The present invention provides opioid formulations suitable for long-term delivery to a subject. The formulation of the invention comprises an opioid or opioid derivative (e.g., morphine, hydromorphone, fentanyl or a fentanyl congener), and an aqueous solvent comprising a low molecular weight carboxylic acid (e.g., C2-4, C2-7). The invention thus provides for formulations comprising morphine, hydromorphone, fentanyl or fentanyl congeners in concentrations significantly in excess of conventional aqueous formulations, e.g., on the order about 2-fold to about 10,000-fold greater than conventional formulations, e.g., currently commercially available formulations.
Owner:DURECT CORP
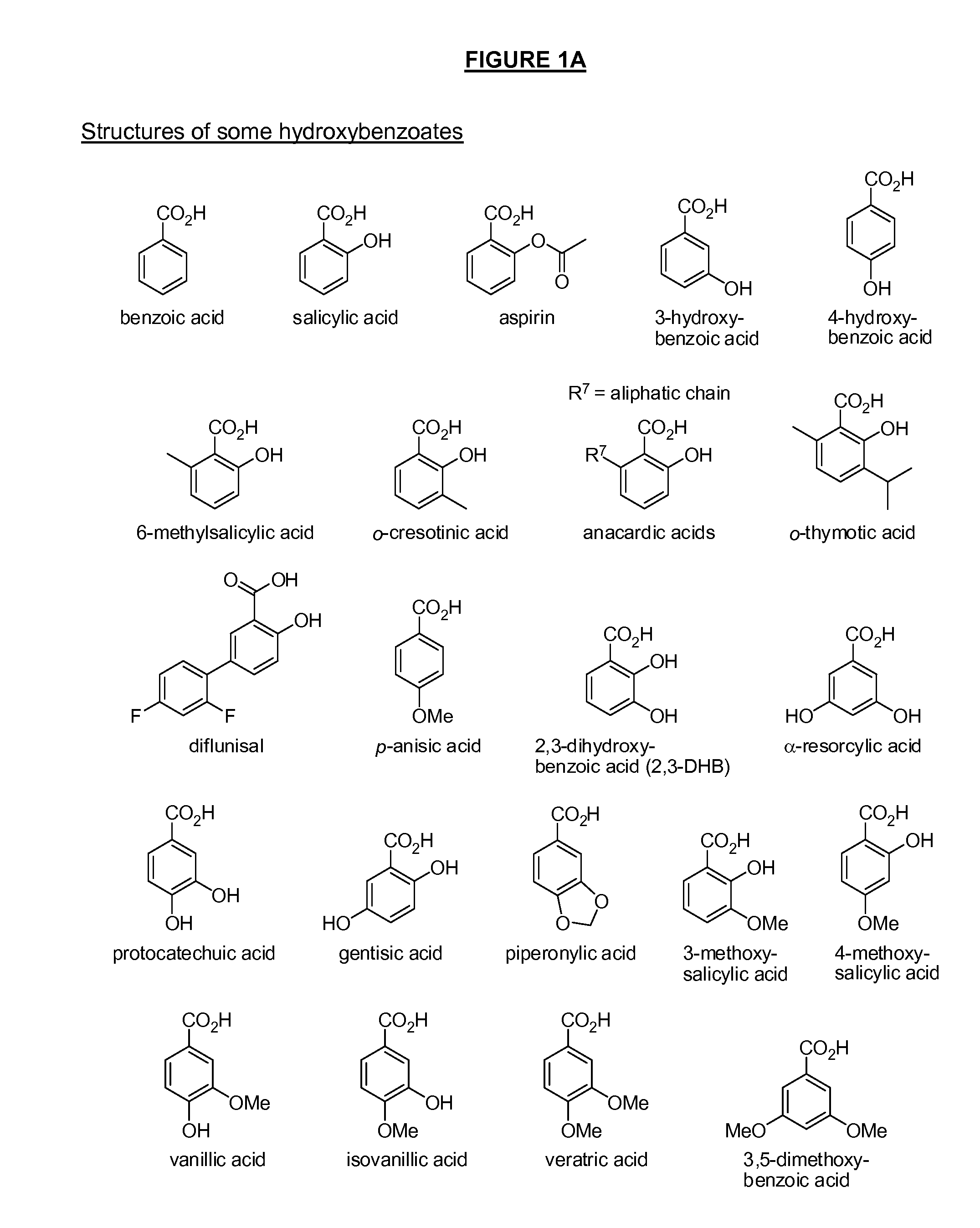
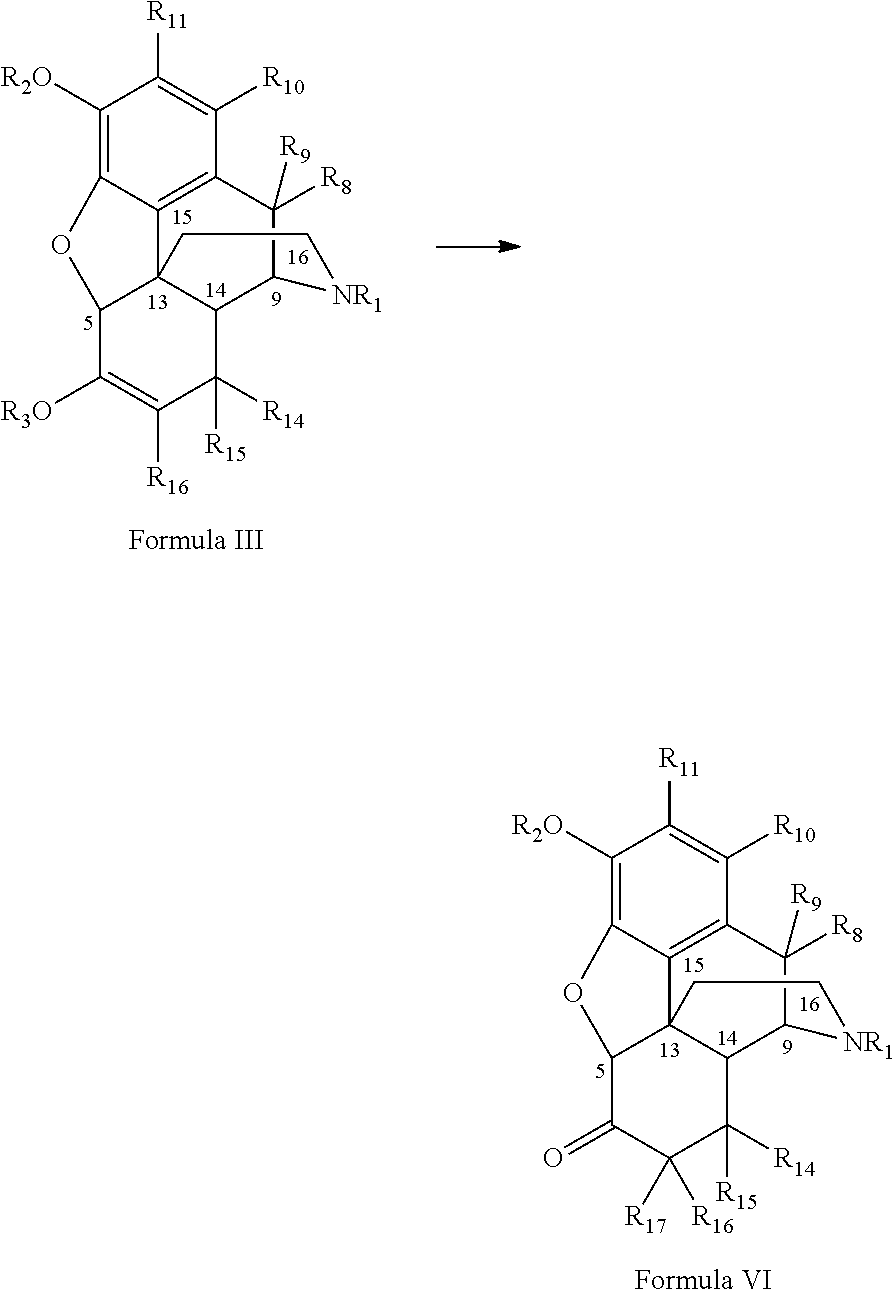
Benzoic acid, benzoic acid derivatives and heteroaryl carboxylic acid conjugates of hydromorphone, prodrugs, methods of making and use thereof
ActiveUS8816083B2Lower potentialReduced drug abuse potentialBiocideNervous disorderBenzoic acidEpoxy
The presently described technology provides compositions comprising aryl carboxylic acids chemically conjugated to hydromorphone (4,5-α-epoxy-3-hydroxy-17-methyl morphinan-6-one) to form novel prodrugs / compositions of hydromorphone. The hydromorphone prodrugs of the present technology have decreased side effects and decreased potential for abuse compared to unconjugated hydromorphone. The present technology also provides methods of treating patients, pharmaceutical kits and methods of synthesizing conjugates of the present technology.
Owner:KEMPHARM INC
Methods and compositions for reducing the risk associated with the administration of opioid analgesics in patients with diagnosed or undiagnosed respiratory illness
The present invention relates to methods for reducing the risk associated with the administration of opioid analgesics in patients diagnosed or undiagnosed with respiratory illness by administering an analgesic composition comprising a sub-analgesic dosage of a μ-opioid agonist selected from the group consisting of morphine, fentanyl, sufentanil, alfentanil, oxymorphone and hydromorphone, or a pharmaceutically acceptable salt thereof, and a sub-analgesic dosage of oxycodone which is a κ2-opioid agonist or a pharmaceutically acceptable salt thereof.
Owner:QRXPHARMA
Method for the catalytic production of hydrocodone and hydromorphone
InactiveUS20050124811A1Improve scalabilityOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsArylAcyl group
A method for the catalytic conversion of codeine, morphine or analogs thereof into hydrocodone, hydromorphone or analogs thereof utilizing a transition metal catalyst of the formula [M(PR3R4R5)nXm]p; wherein R1 is H, alkyl, aryl or acyl; M is a Group VIII transition metal; R3, R4 and R5 are selected from the group consisting of alkyl, aryl, alkoxyl, phenoxyl and combinations thereof; X is a halide or an anion; n is 1, 2, 3 or 4; m is 1 or 2; and p is at least 1.
Owner:MALLINCKRODT INC
Method for the catalytic production of hydrocodone, hydromorphone, and derivatives thereof
A method for the catalytic production of hydrocodone derivatives and hydromorphone derivatives, respectively, utilizing a transition metal catalyst of the formula [M(PR4R5R6)nXm]p; wherein M is a Group VIII transition metal; R4, R5 and R6 are selected from the group consisting of alkyl, aryl, alkoxyl, phenoxy and combinations thereof; X is a halide or an anion; n is 1, 2, 3 or 4; m is 1 or 2; and p is at least 1.
Owner:MALLINCKRODT INC
Methods and compositions for reducing the risk associated with the administration of opioid analgesics in patients with diagnosed or undiagnosed respiratory illness
The present invention relates to methods for reducing the risk associated with the administration of opioid analgesics in patients diagnosed or undiagnosed with respiratory illness by administering an analgesic composition comprising a sub-analgesic dosage of a p-opioid agonist selected from the group consisting of morphine, fentanyl, sufentanil, alfentanil, oxymorphone and hydromorphone, or a pharmaceutically acceptable salt thereof, and a sub-analgesic dosage of oxycodone which is a κ2-opioid agonist or a pharmaceutically acceptable salt thereof.
Owner:QRXPHARMA
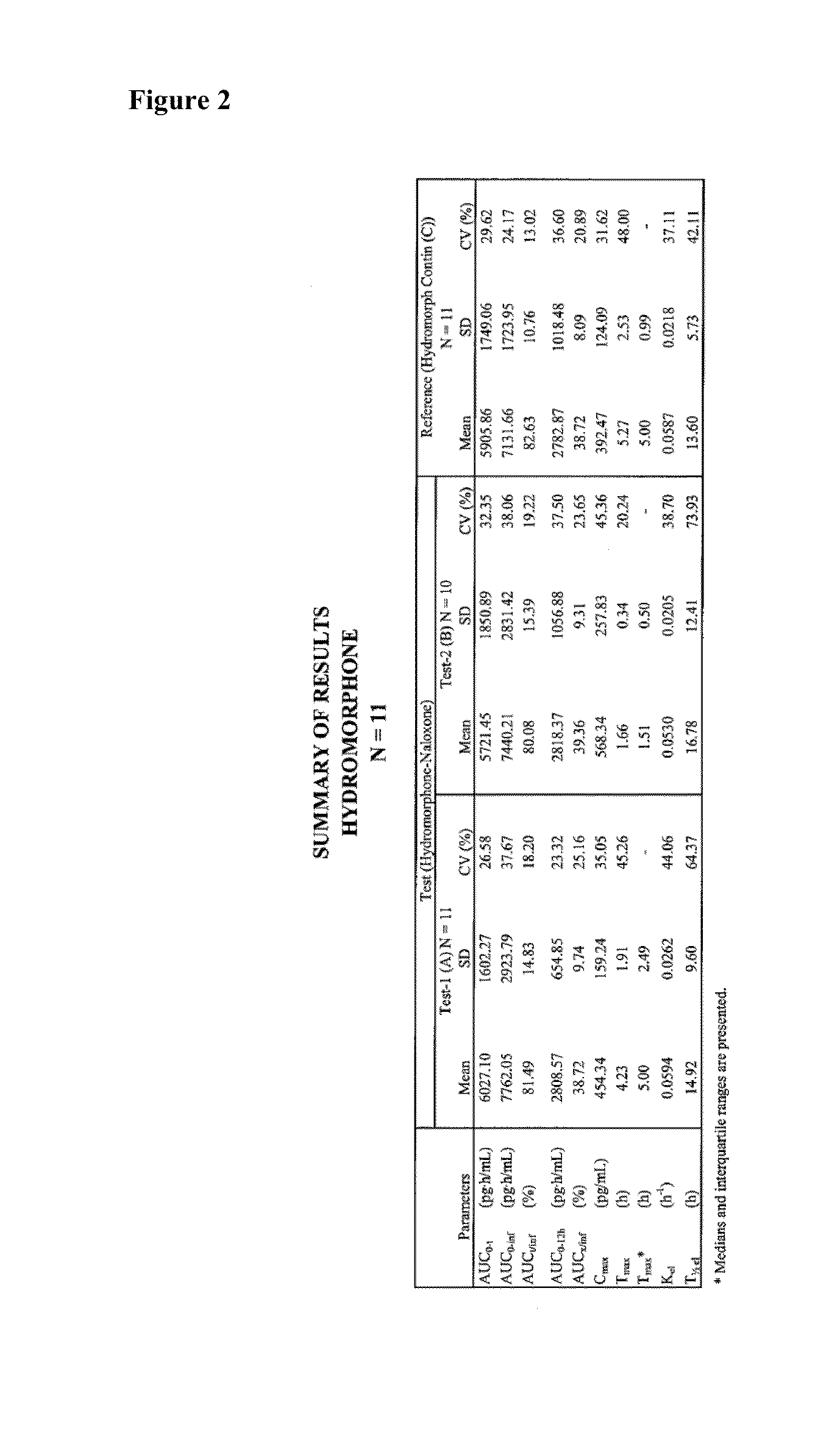
Pharmaceutical compositions comprising hydromorphone and naloxone
InactiveUS20150283091A1Improve stability and dissolution propertyImproved stability and dissolution propertyBiocideNervous disorderAcetic acidSodium metabisulfite
There is described a prolonged release pharmaceutical dosage form comprising a plurality of coated beads, each of the coated beads comprising: (a) a granule; (b) a first layer coated on the granule, the first layer comprising: (i) hydromorphone or a pharmaceutically acceptable salt thereof, (ii) naloxone or a pharmaceutically acceptable salt thereof, (iii) an antioxidant compound, and (iii) a chelating compound; and (c) a second layer coated on the first layer, the second layer comprising a prolonged release agent. The dosage form has improved stability and dissolution properties. Another aspect of the invention relates to use of a combination of an antioxidant (such as sodium metabisulfite) and a chelating agent (such as ethylenedinitrotetraacetic acid disodium salt dihydrate) to improve the stability and / or dissolution properties of a prolonged release dosage form comprising (i) hydromorphone or a pharmaceutically acceptable salt thereof and (ii) naloxone or a pharmaceutically acceptable salt thereof.
Owner:PURDUE PHARMA LP
Benzoic acid, benzoic acid derivatives and heteroaryl carboxylic acid conjugates of hydromorphone, prodrugs, methods of making and use thereof
ActiveUS20130150395A1Lower potentialReduced drug abuse potentialBiocideNervous disorderBenzoic acidEpoxy
The presently described technology provides compositions comprising aryl carboxylic acids chemically conjugated to hydromorphone (4,5-α-epoxy-3-hydroxy-17-methyl morphinan-6-one) to form novel prodrugs / compositions of hydromorphone. The hydromorphone prodrugs of the present technology have decreased side effects and decreased potential for abuse compared to unconjugated hydromorphone. The present technology also provides methods of treating patients, pharmaceutical kits and methods of synthesizing conjugates of the present technology.
Owner:KEMPHARM INC
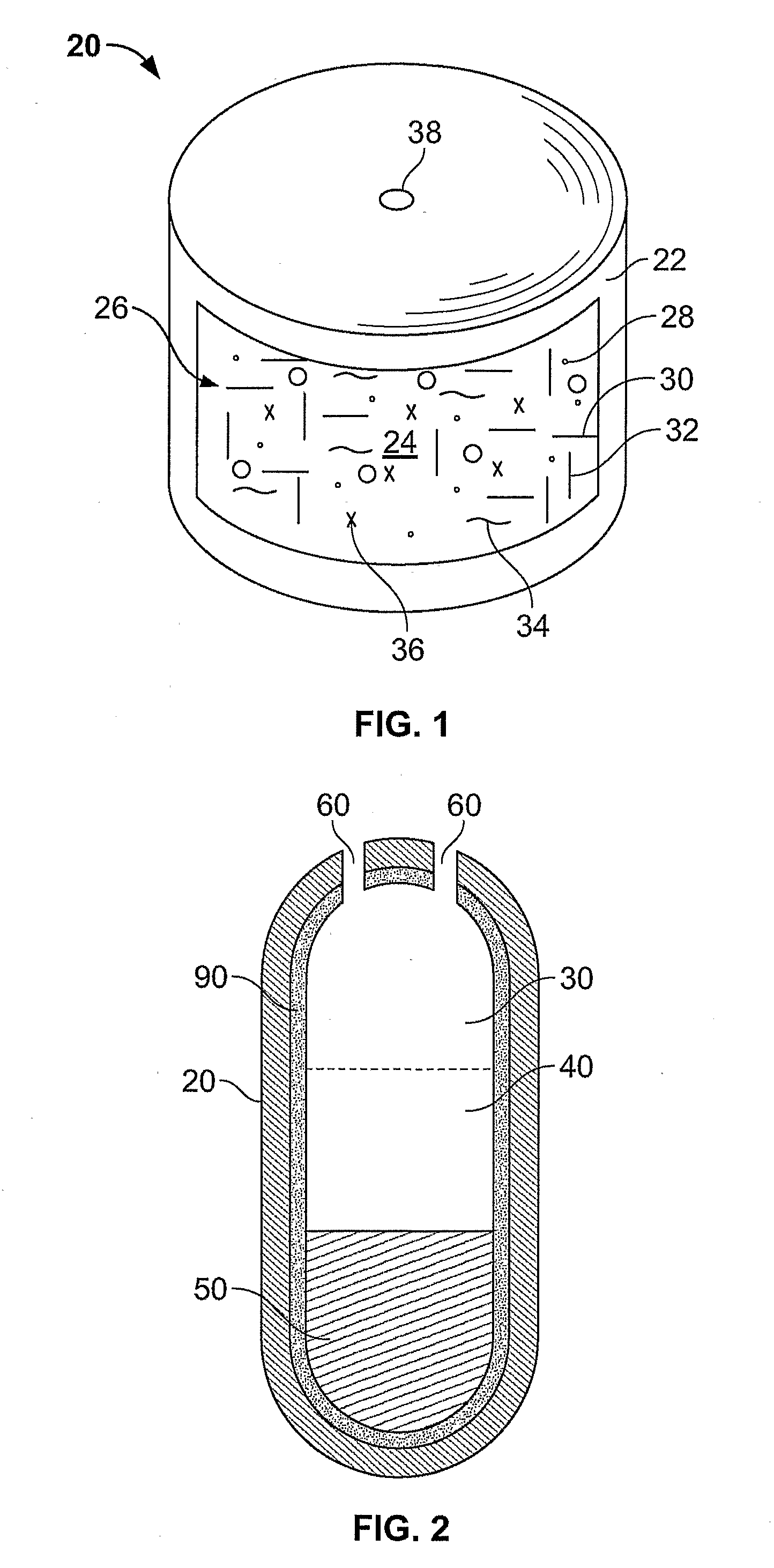
Methods of administering drugs in an implantable multi-chamber pump
InactiveUS20140296830A1Relieve painReducing severe and chronic painMedical devicesPressure infusionNeuropathic painZiconotide
One embodiment of the present invention is a method for reducing pain using a multi chamber pump to separately administer multiple drugs. For example, one chamber may contain an omega conopeptide, such as ziconotide, and the other chamber or chambers may contain one or more other drugs, which may include of morphine, hydromorphone, fentanyl, sufentanil, bupivacaine, baclofen, clonidine, and buprenorphine, or their pharmaceutically acceptable salts thereof. Other applications of the present invention include methods for treating severe chronic pain due to cancer, failed back syndrome, CRPS, neuropathic pain, mixed neuropathic and nociceptive pain.
Owner:JAZZ PHARMA
Hydromorphone therapy
InactiveUS20040234600A1Relief the painSuppress and apprehensionBiocideNervous disorderSurgeryHydromorphone
A hydromorphone composition, a hydromorphone dosage form and a method for administering hydromorphone are disclosed, indicated for the management of pain.
Owner:MERRILL SONYA +3
Method for the catalytic production of hydrocodone and hydromorphone
A method for the catalytic conversion of codeine, morphine or analogs thereof into hydrocodone, hydromorphone or analogs thereof utilizing a transition metal complex of a tertiary phosphine halide as catalyst.
Owner:MALLINCKRODT INC
Pharmaceutical compositions comprising hydromorphone and naloxone
The present invention relates to prolonged release pharmaceutical dosage forms, the manufacture thereof as well as their use for administration to human beings.
Owner:EURO-CELTIQUE SA
Pharmaceutical Composition
A pharmaceutical composition comprising an analgesic or analgesic combination and a stool softener is disclosed. The analgesic is selected from morphine, meperidine, fentanyl, hydromorphone, oxymorphone, oxycodone, hydrocodone, methadone, propoxyphene, pentazocine, levorphanol, codeine, acetaminophen and combinations of these analgesics. The composition is formulated for oral administration as a liquid or solid dosage form for immediate, slow, delayed or sustained-release characteristics.
Owner:BRANDED PRODS FOR THE FUTURE
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com